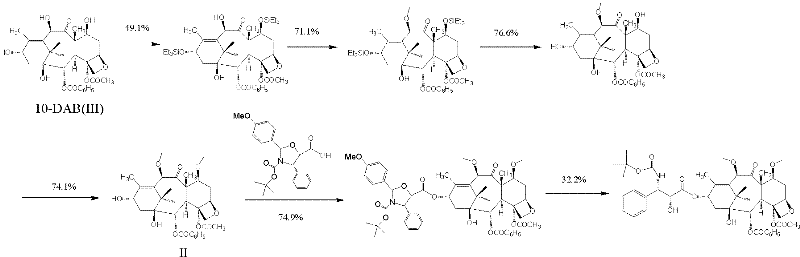
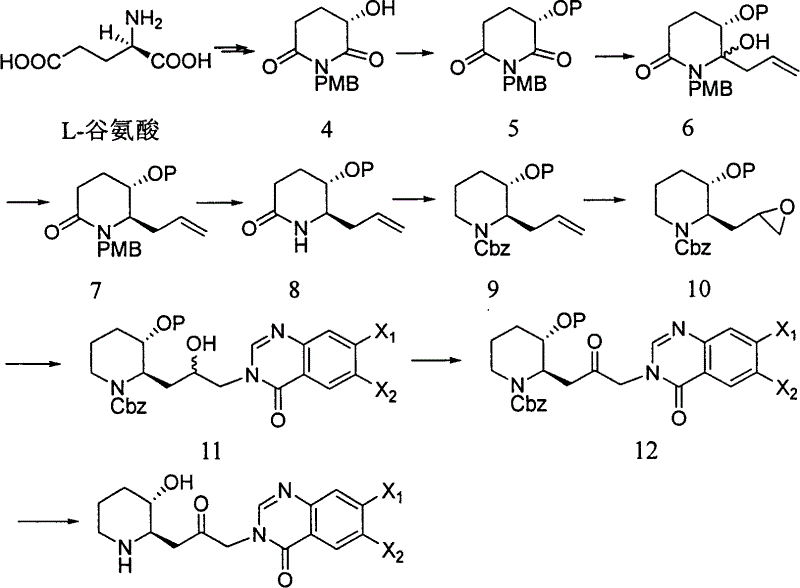
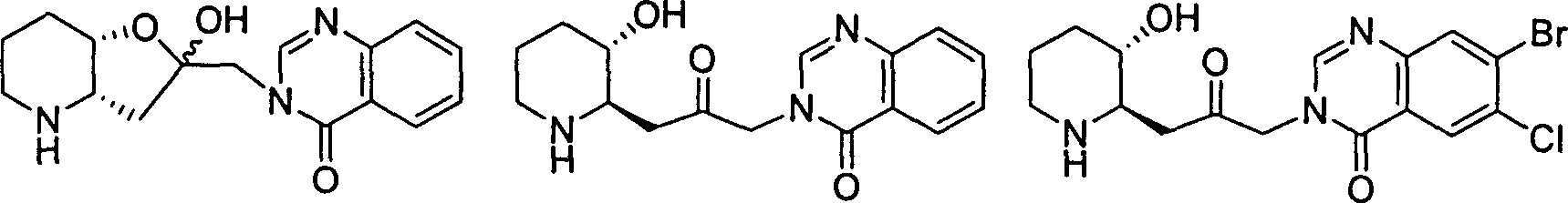
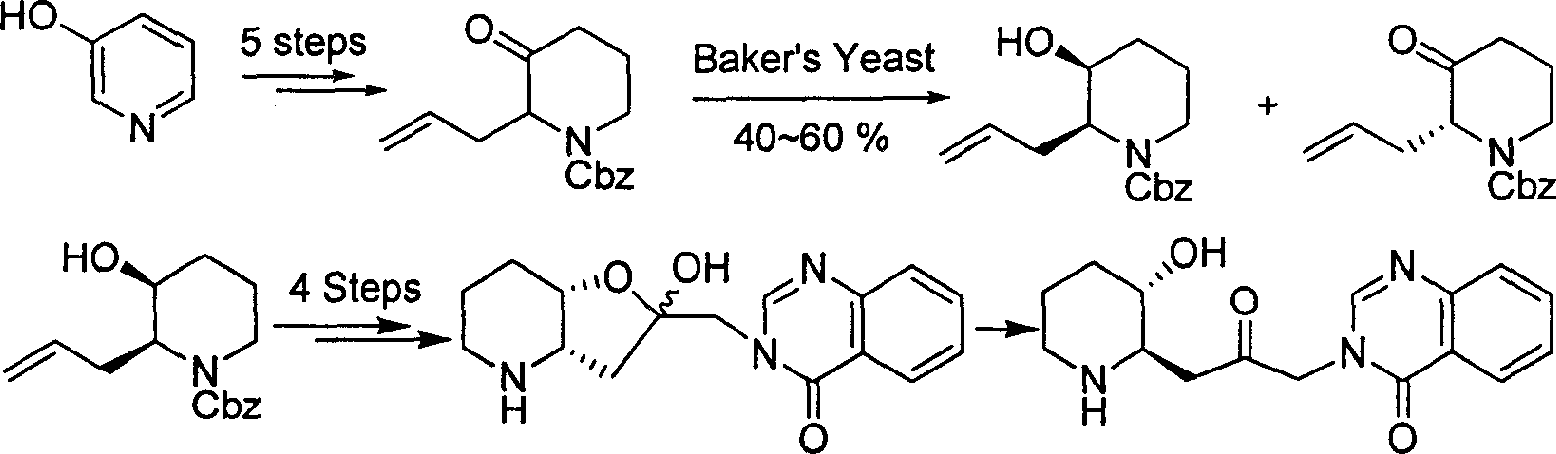
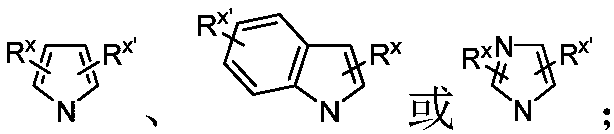
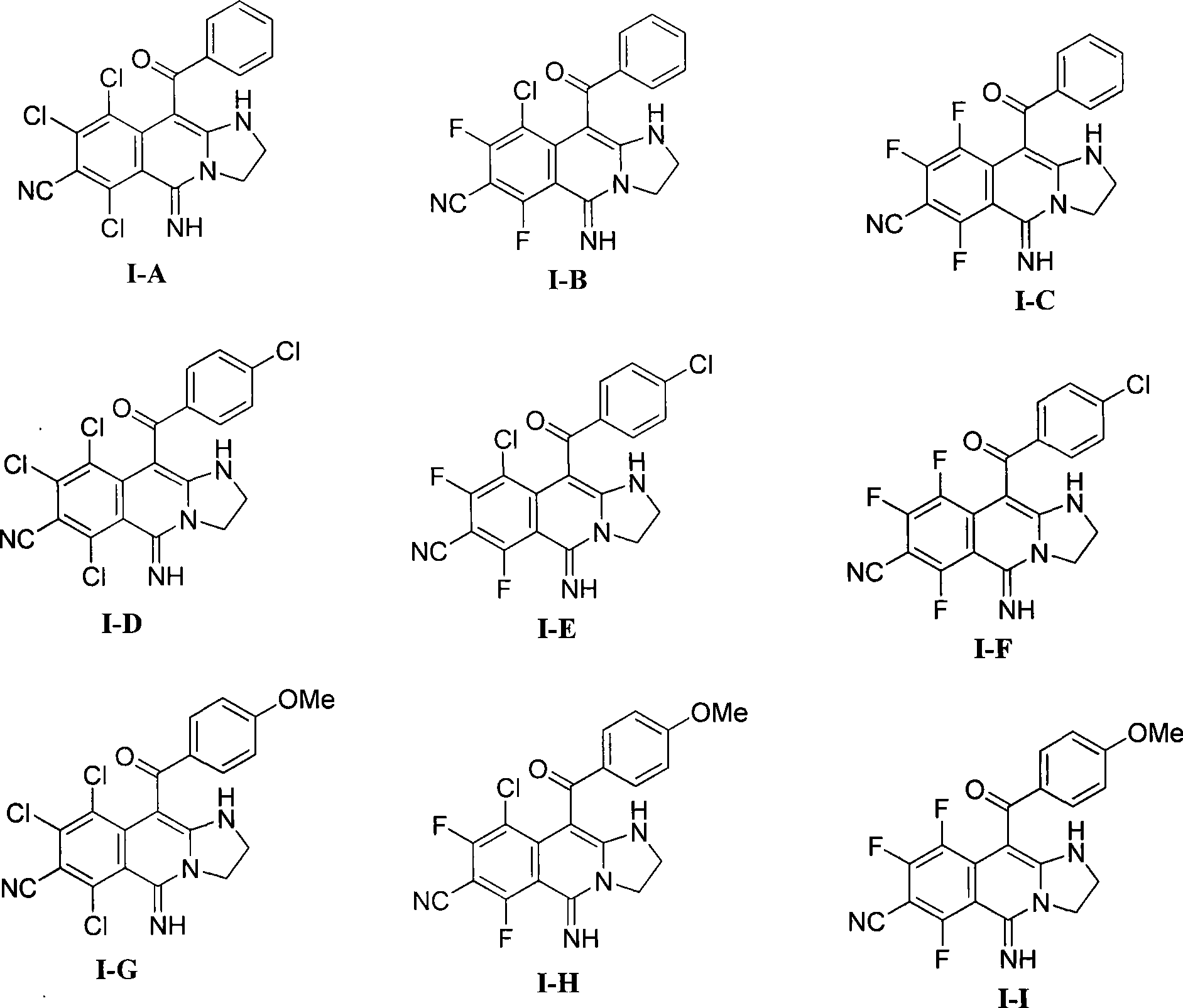
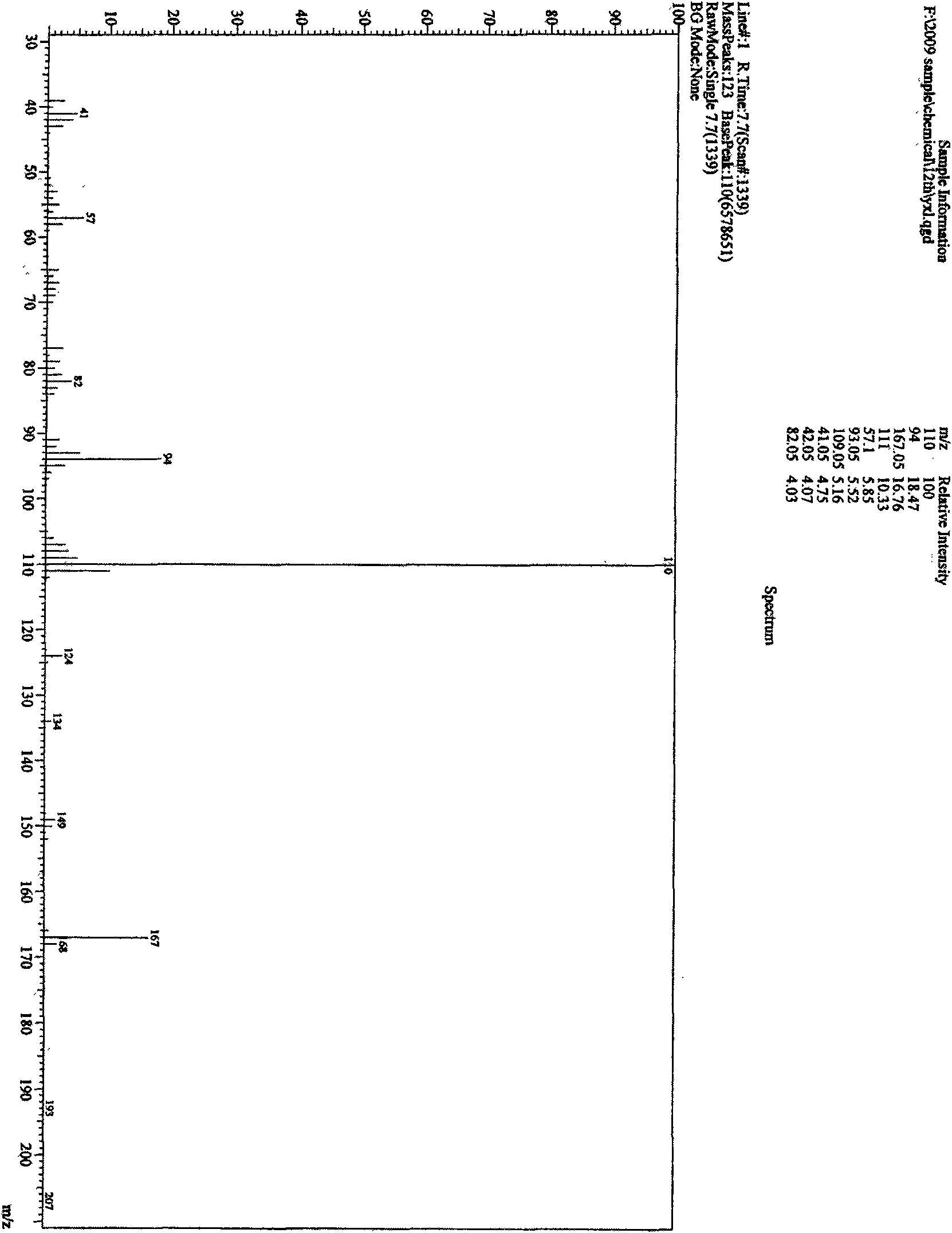
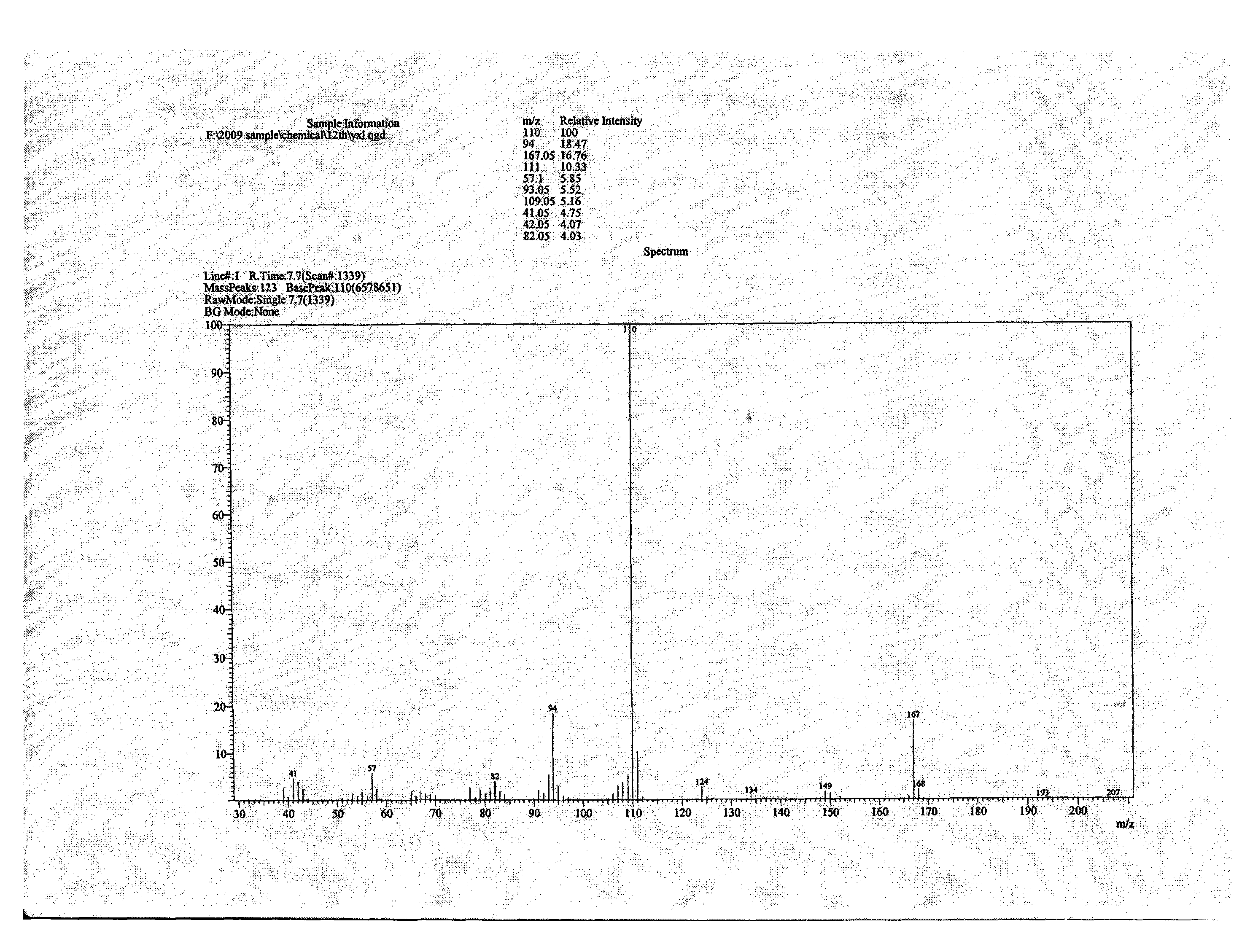
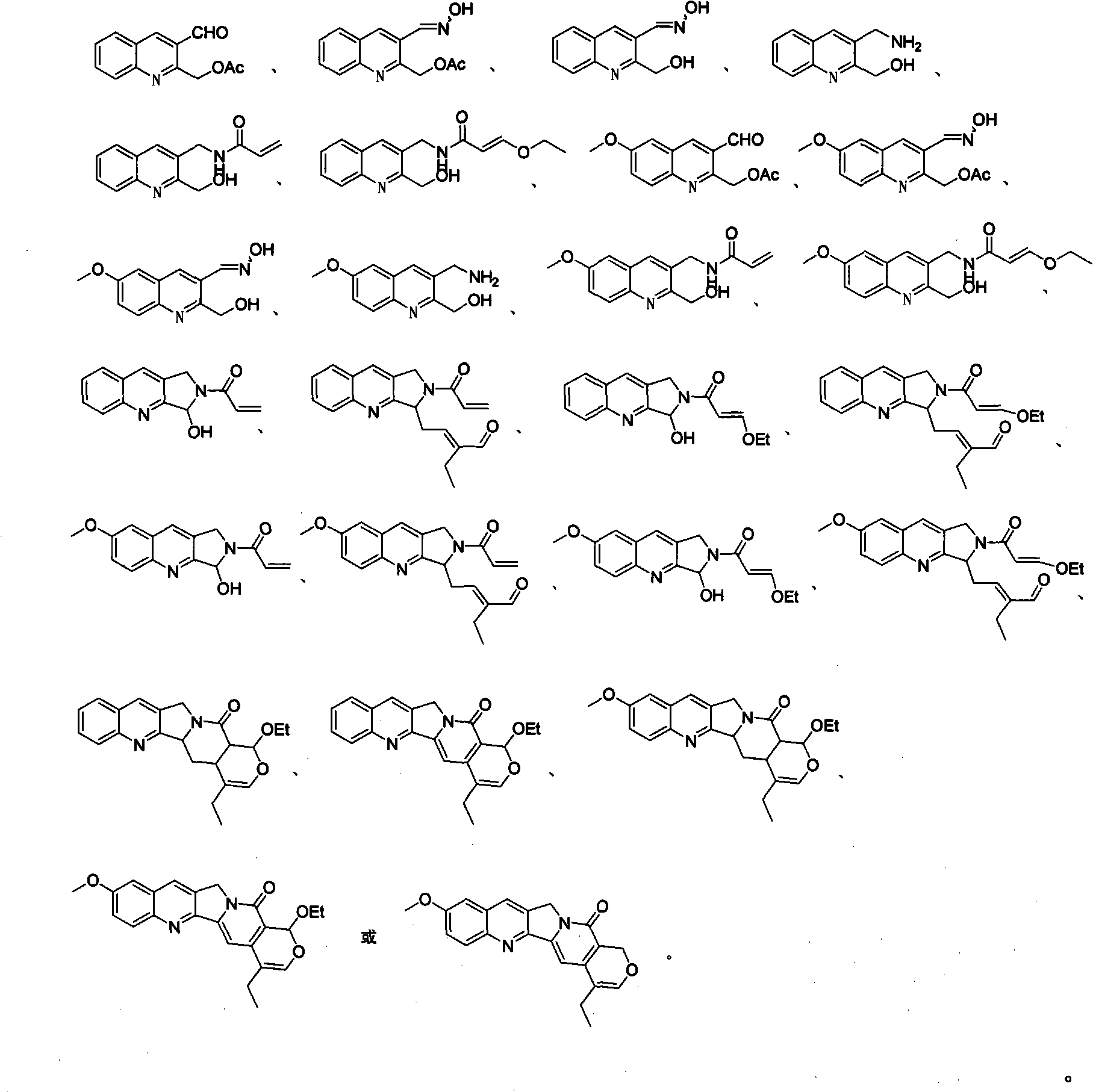Patents
Literature
558results about How to "Short route" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
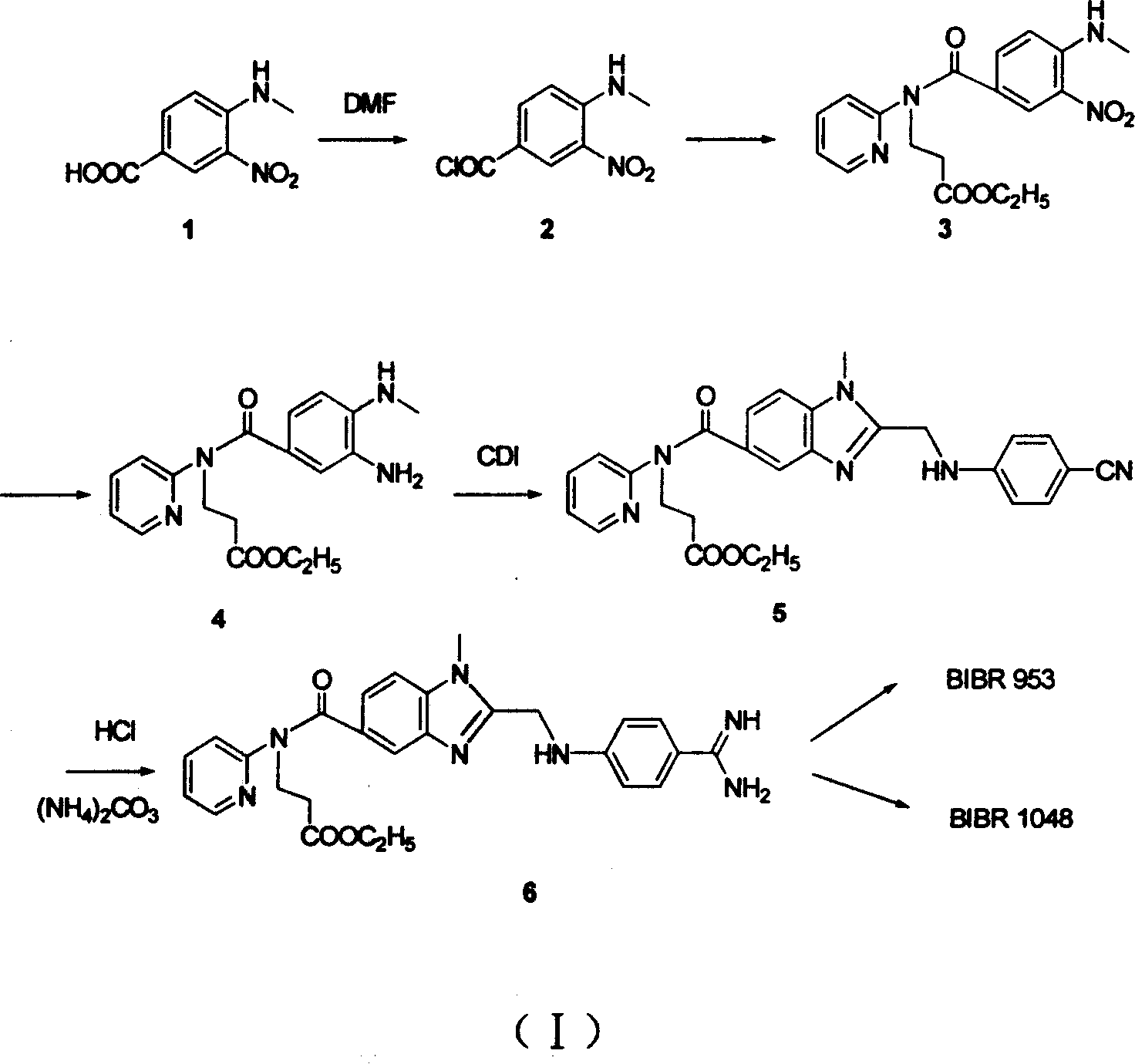
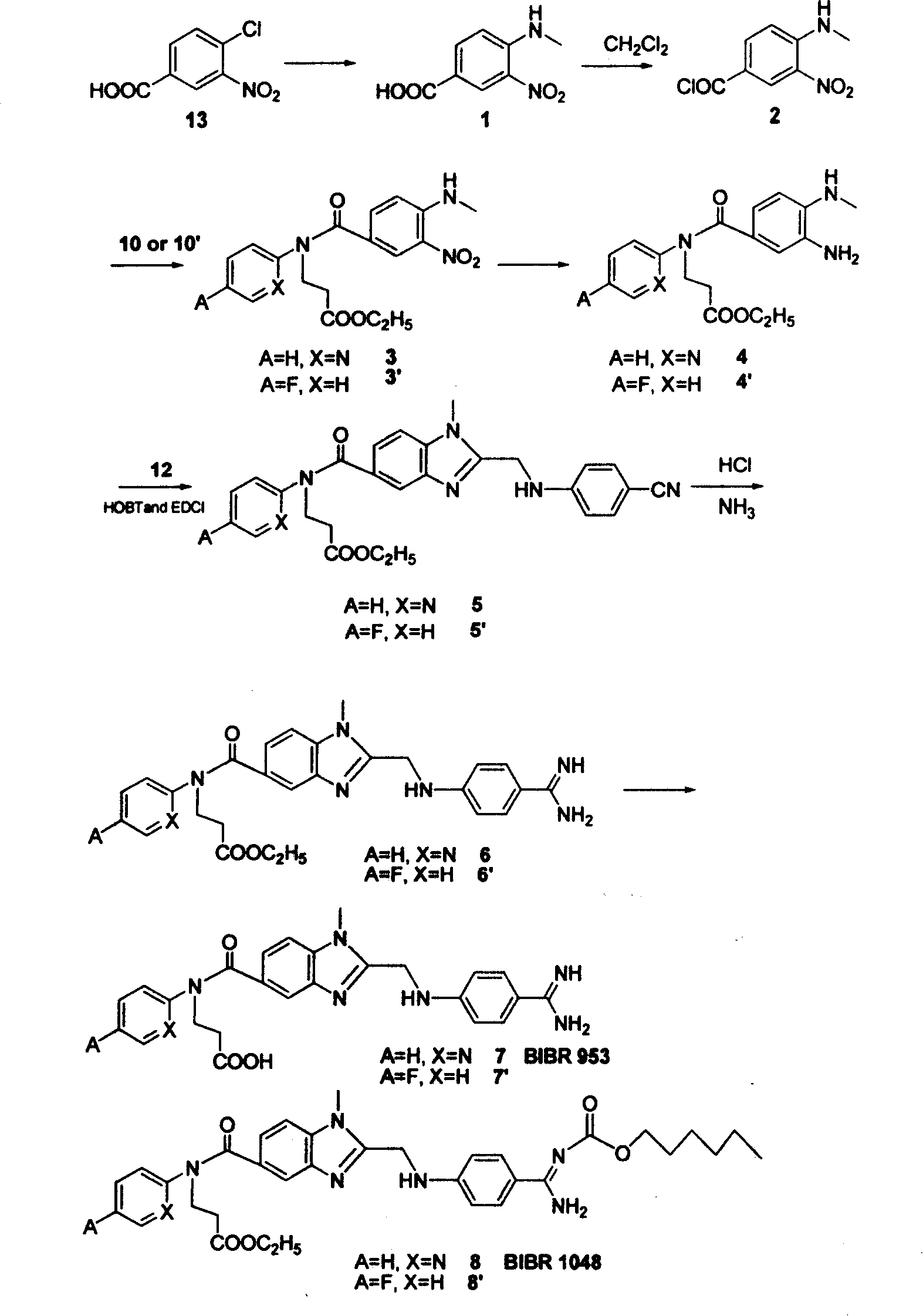
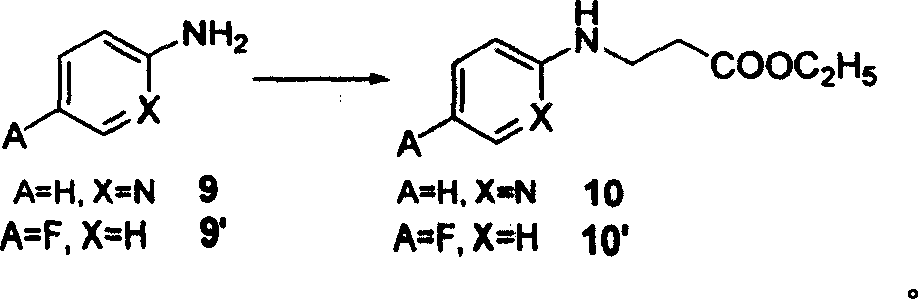
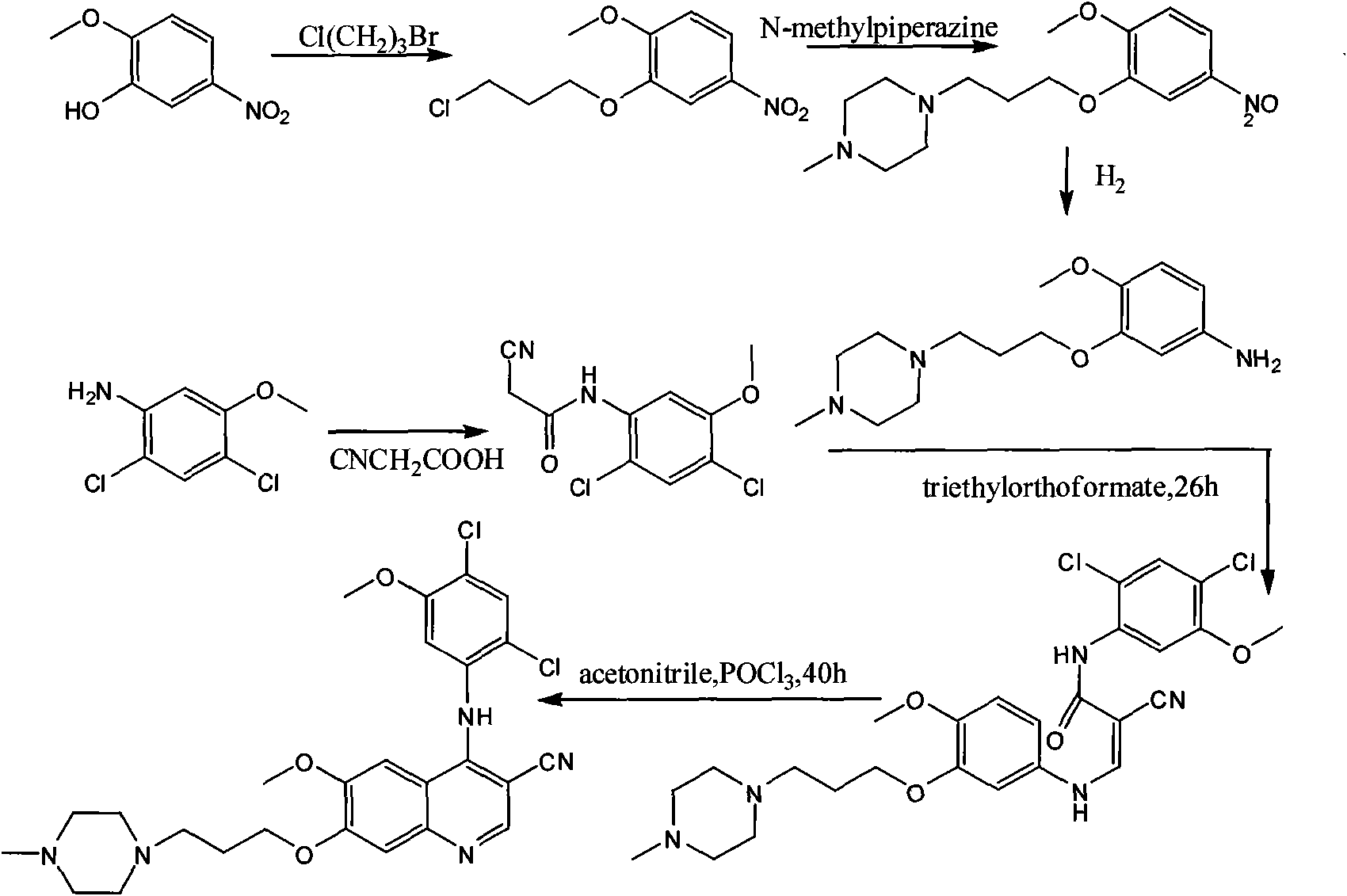
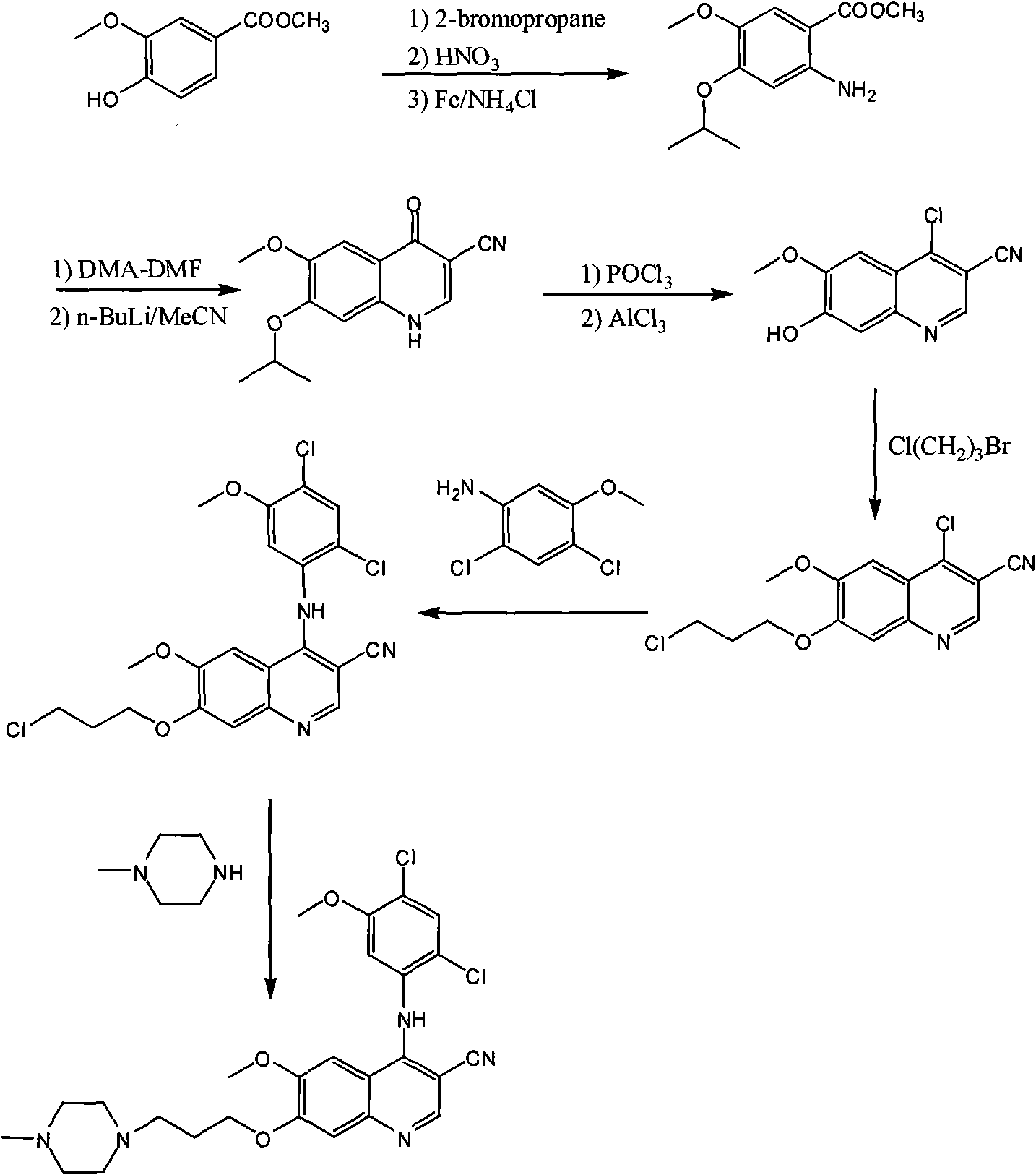
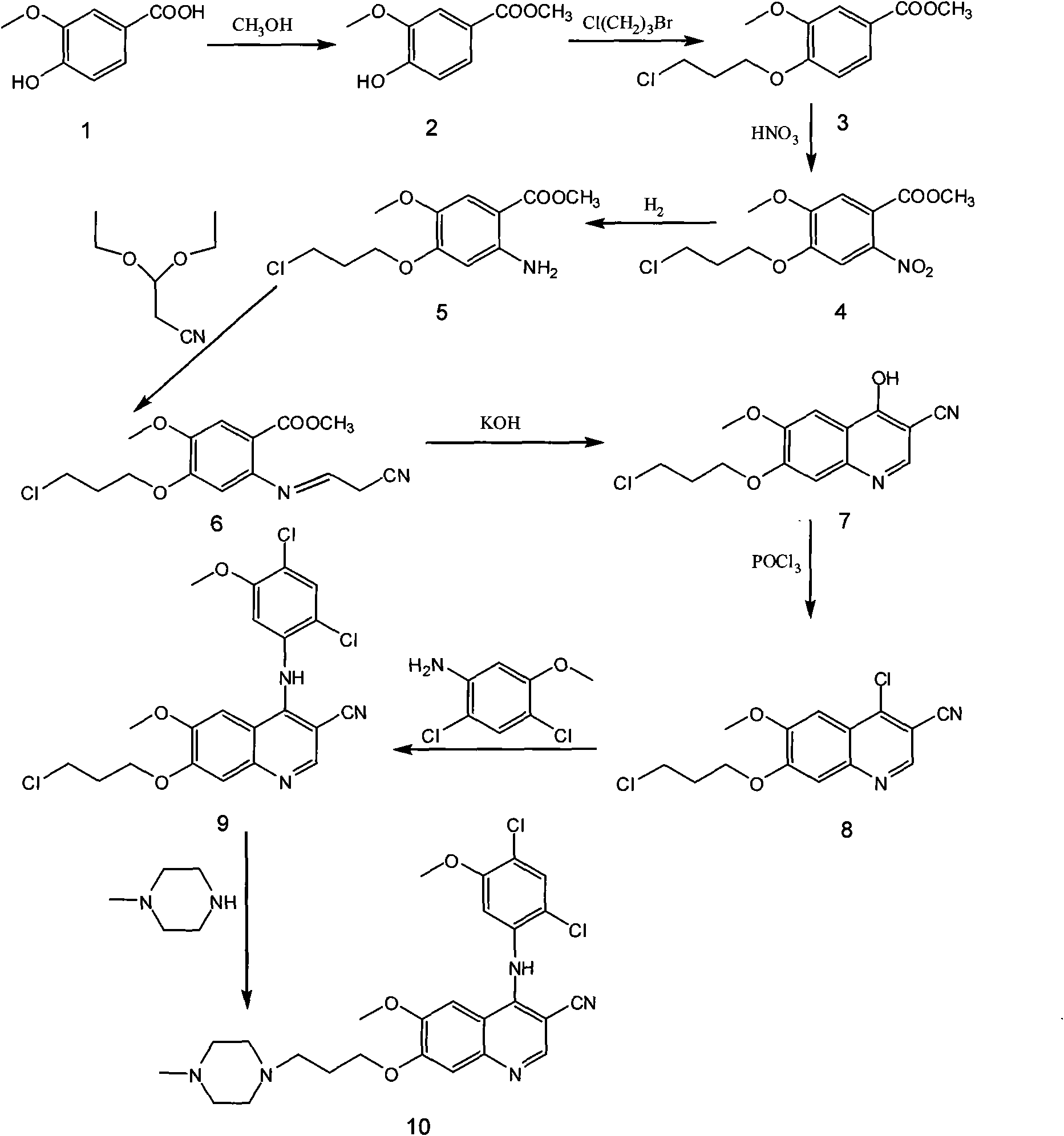
Process for synthesizing antithrombin inhibitor of non-asymmetric non-peptide kind
A process for preparing the non-chiral non-peptide antithrombase depressants BIBR-953, BIBR-1048, etc from 3-nitro-4-chlorophenyl formic acid is disclosed. Said depressant contains 1,2,5-trisubstituted benzimdazole as central skeleton or a F atom.
Owner:FUDAN UNIV
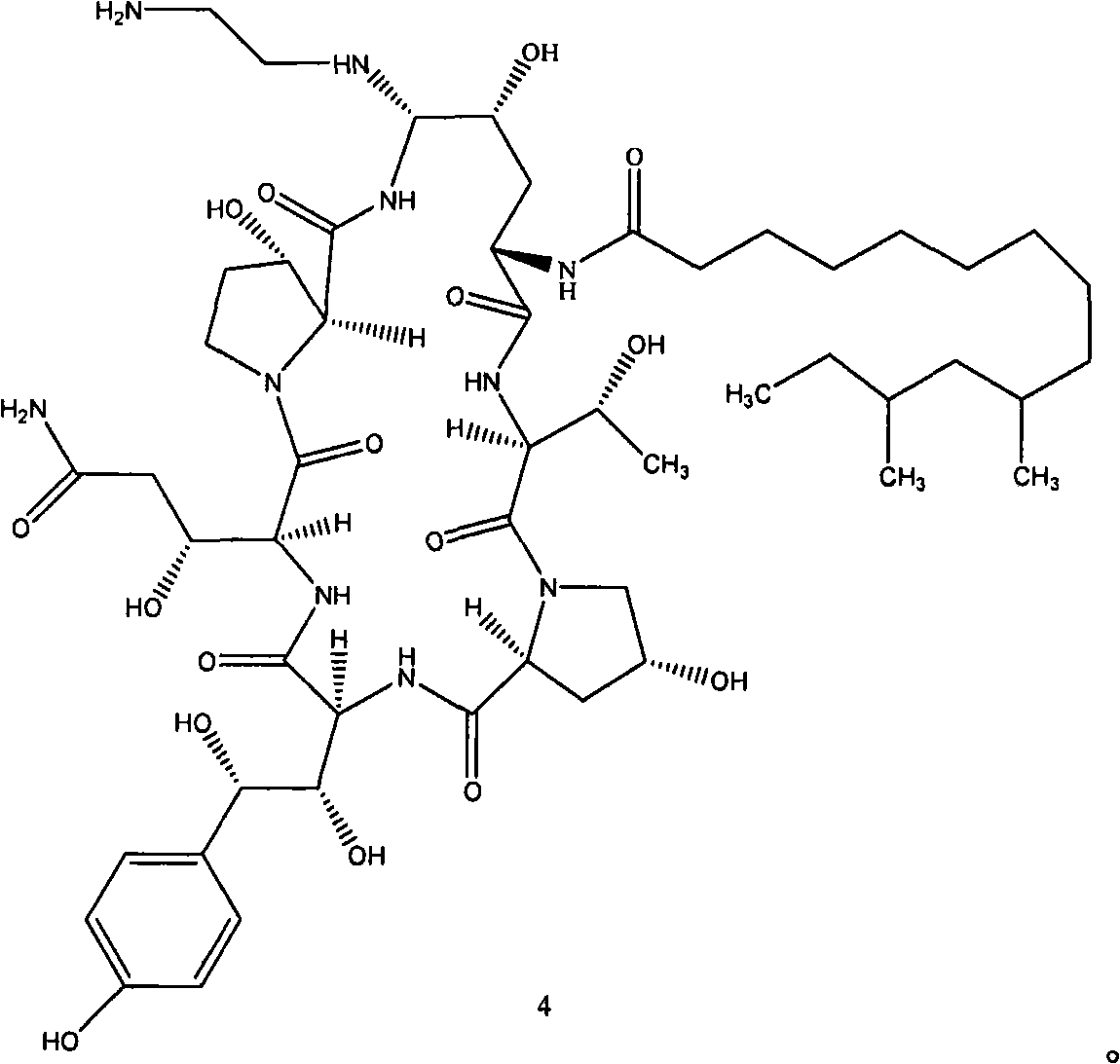
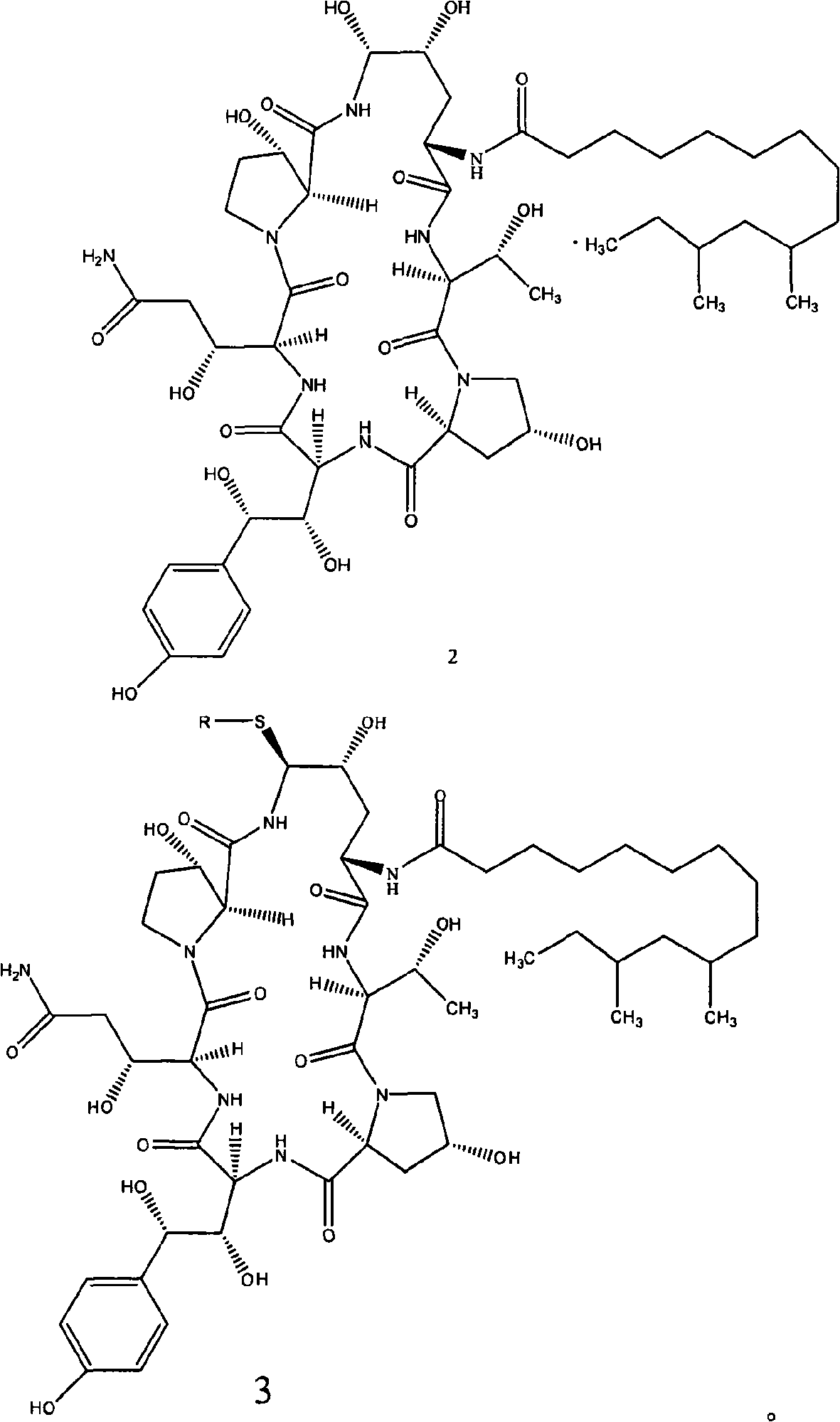
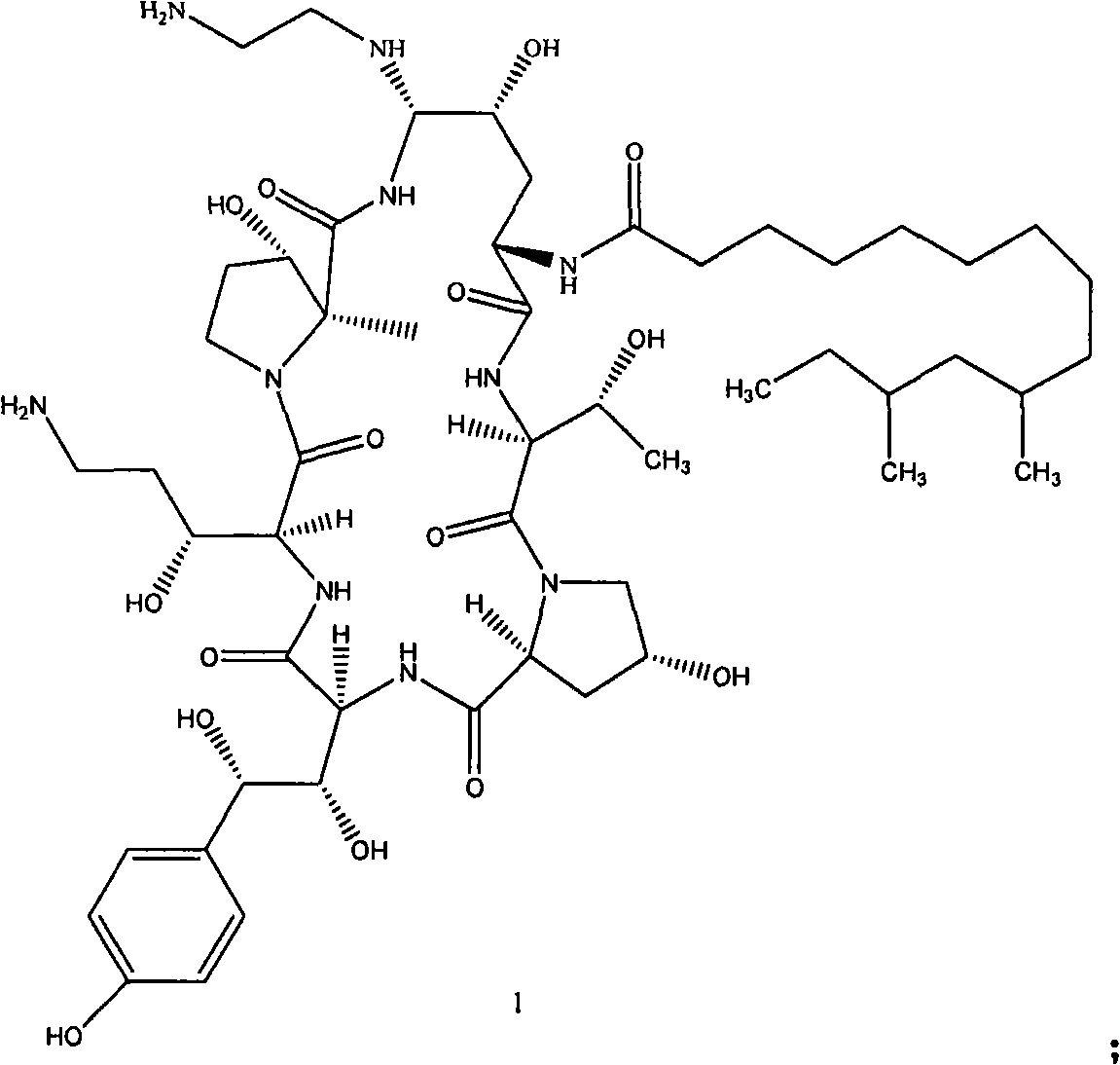
Azepine argireline or pharmaceutically acceptable salt thereof and preparation method and application thereof
Owner:SHANGHAI TECHWELL BIOPHARMACEUTICALS CO LTD
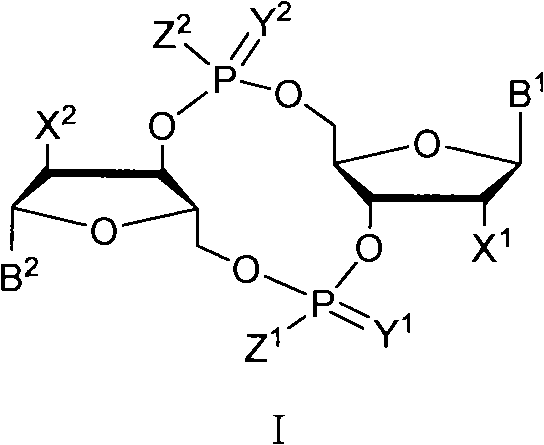
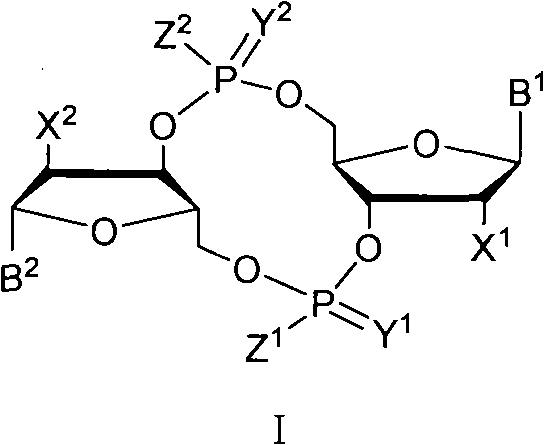
C-di-GMP, analogues thereof and preparation method thereof
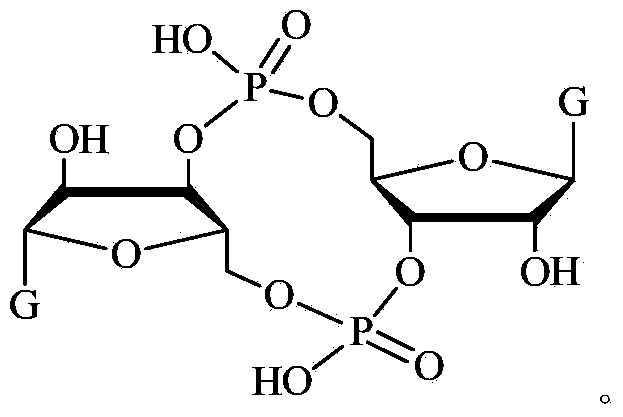
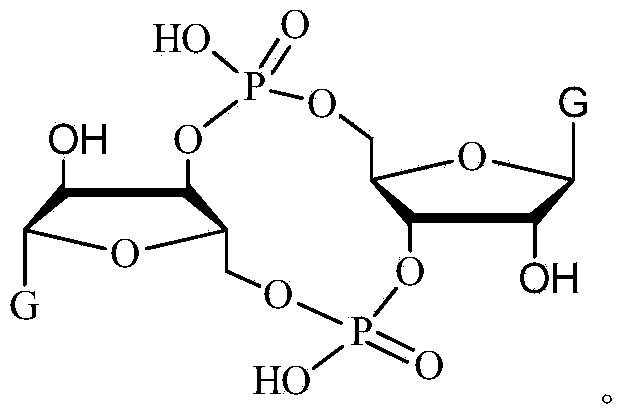
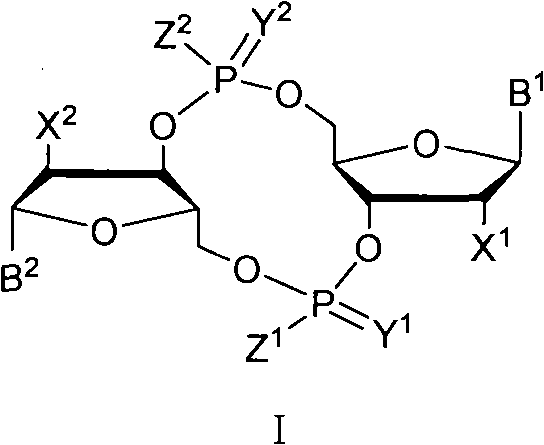
ActiveCN102199183AInhibition formationPrevent proliferationSugar derivativesSugar derivatives preparationDrug developmentBiological membrane
The invention discloses a c-di-GMP and analogues thereof which have structures of a general formula I. The invention also discloses a novel preparation method-a kettle phosphoramidite method which can be used for rapidly, simply and conveniently preparing c-di-GMP compounds with high yield and low cost on a large scale and at mild conditions. The c-di-GMP is prevalent in bacteria and is a novel second messenger molecular which takes part in regulating multiple physiological functions. The research shows that the c-di-GMP and the analogues thereof can inhibit the formation of bacterium biological membranes and the multiplication of eukaryotic cells; and thereof the c-di-GMP and the analogues thereof have good medicine development prospect.
Owner:PEKING UNIV
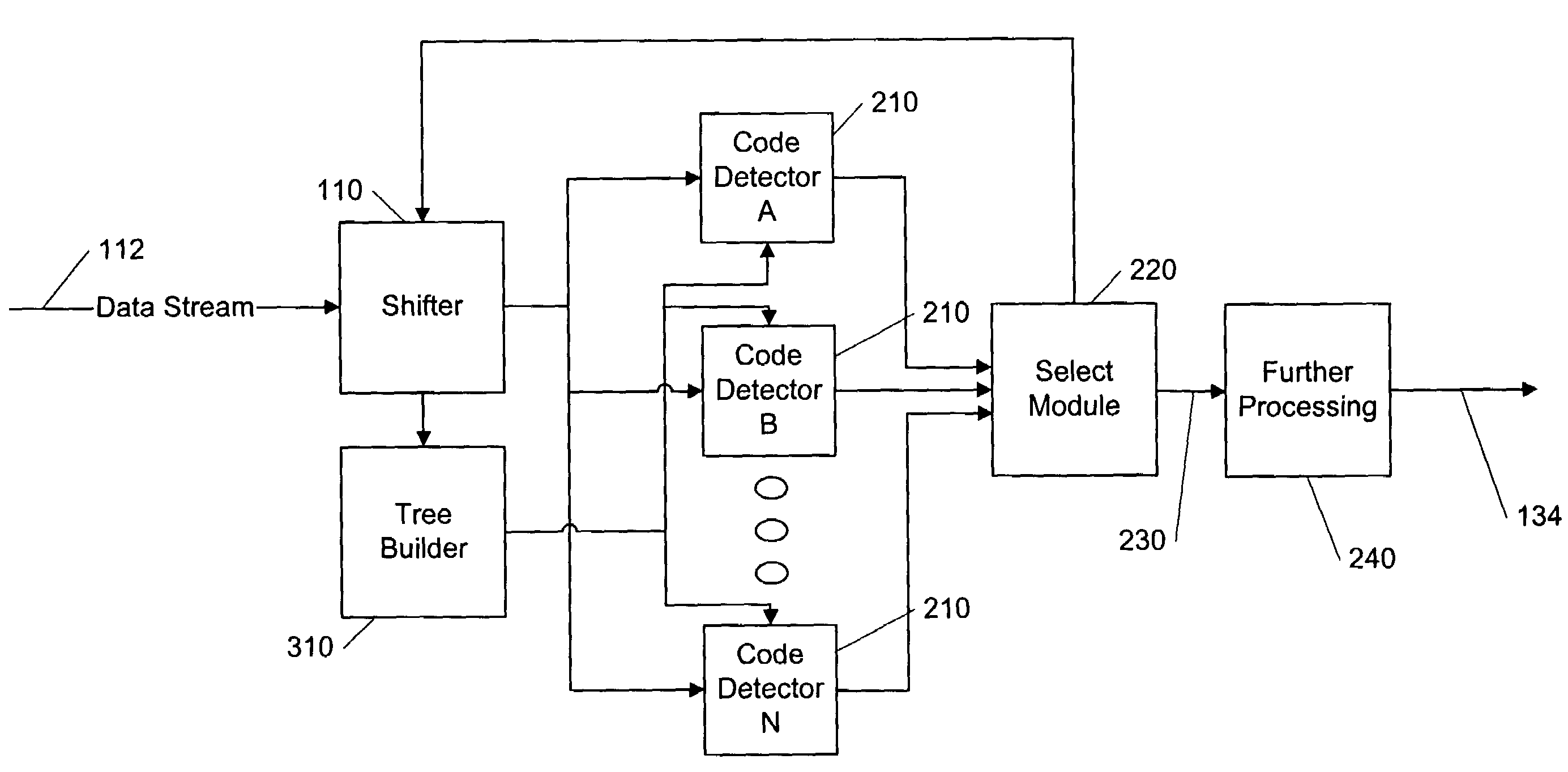
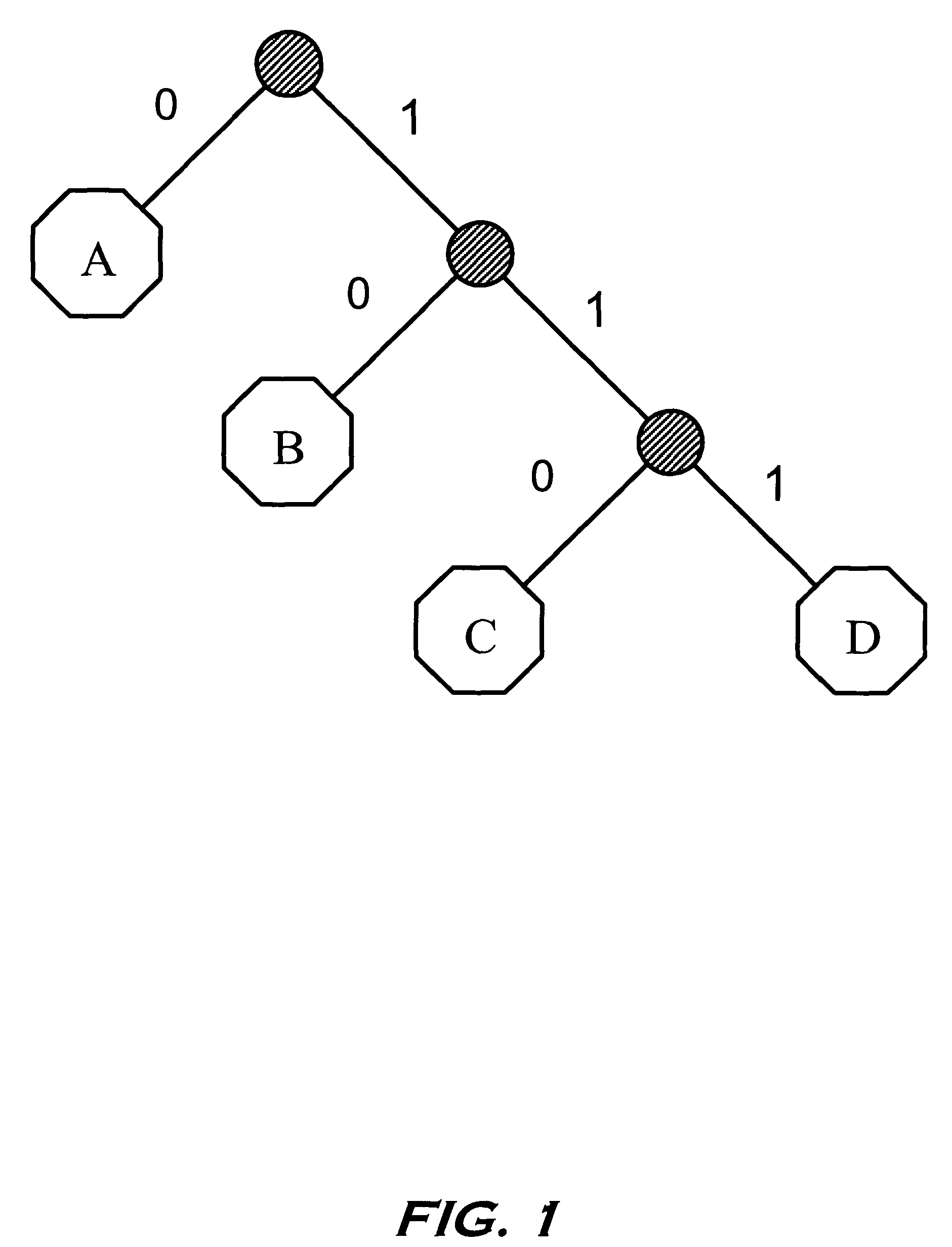
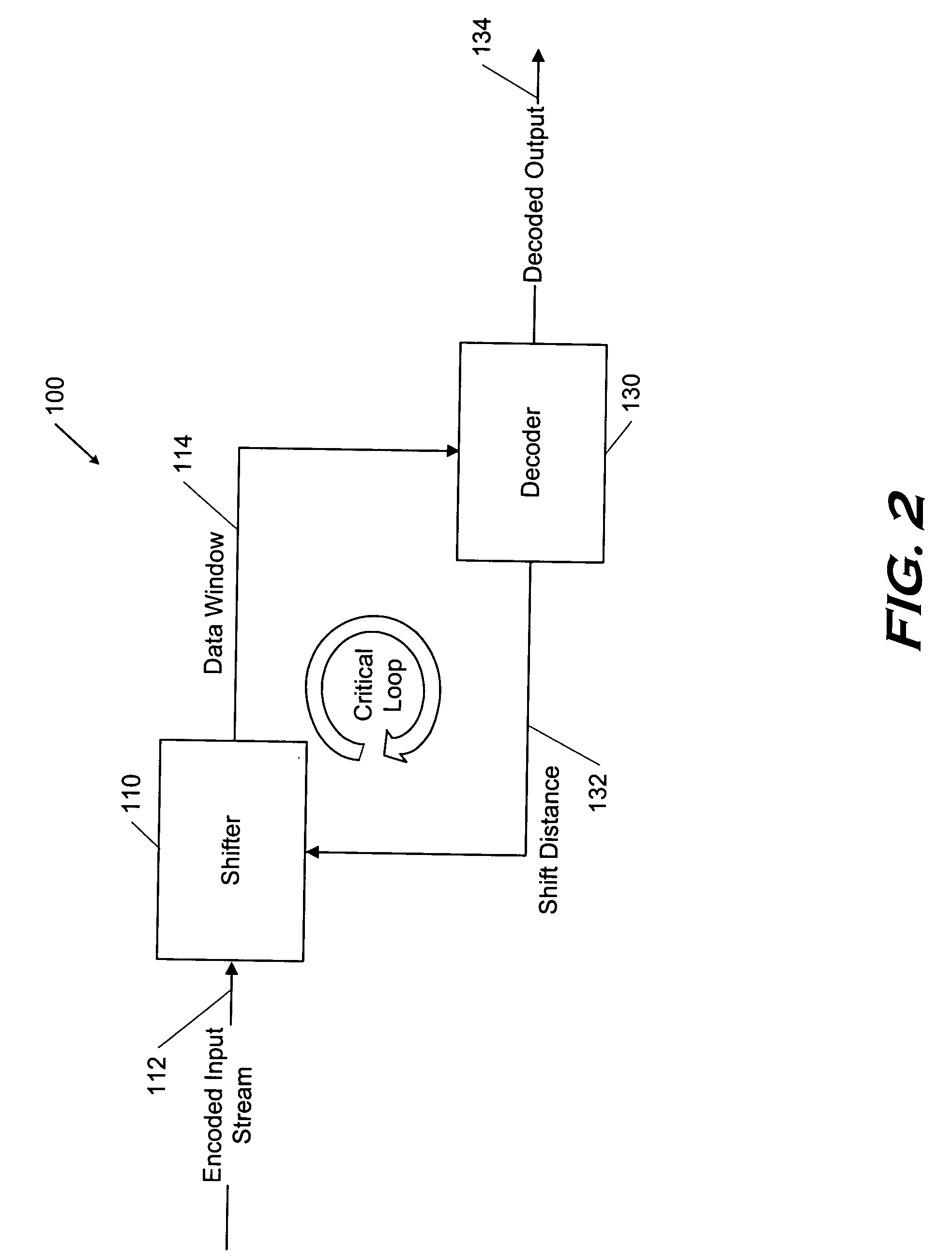
Parallelized dynamic Huffman decoder
InactiveUS7283591B2Overhead time spentManageable sizeColor television with pulse code modulationColor television with bandwidth reductionVariable-length codeProgramming language
Several code detectors in parallel simultaneously examine varying overlapping segments of a data stream containing variable length codes, referred to as a data window. The data window segments directly address memory structures within each of the code detectors without any previous logic stages. Each code detector is responsible for a range of code lengths, and ignores data window bits that are not relevant to its code length range. Each code detector outputs a possible result to a layer of logic that selects the possible result of the single code detector which contains result data corresponding to a variable length code in the data window.
Owner:INTEL CORP
Storage switching mechanism for multi-vehicle-type vehicle body mixed line forming station clamps
ActiveCN105269187ASmooth switchingFacilitate transmissionWelding/cutting auxillary devicesAuxillary welding devicesProduction lineDelivery system
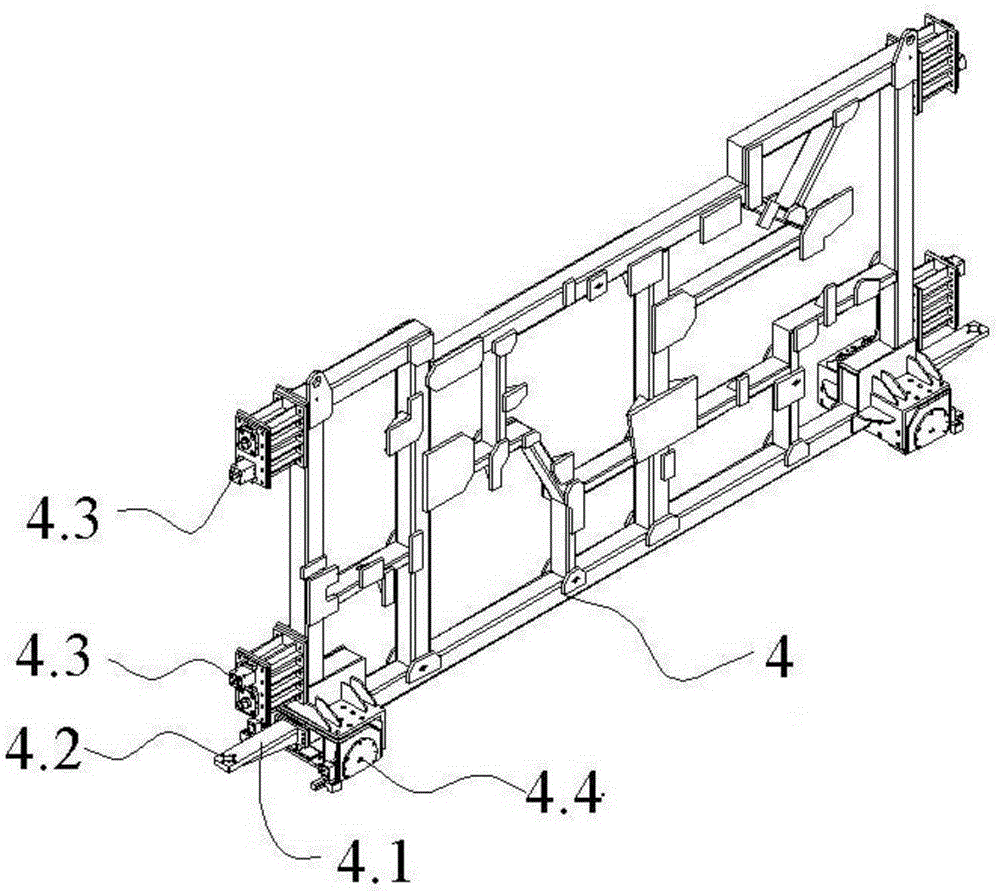
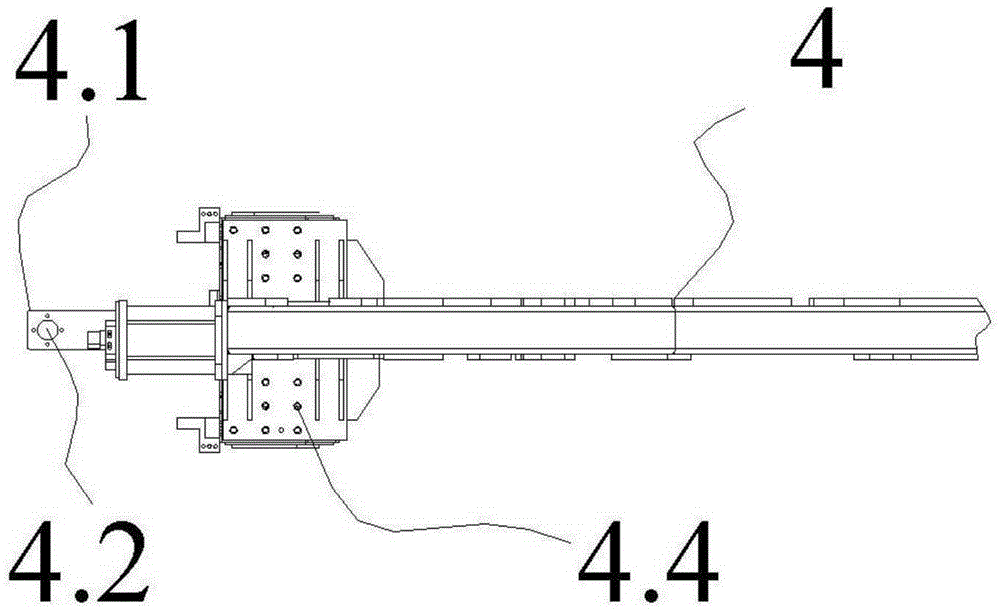
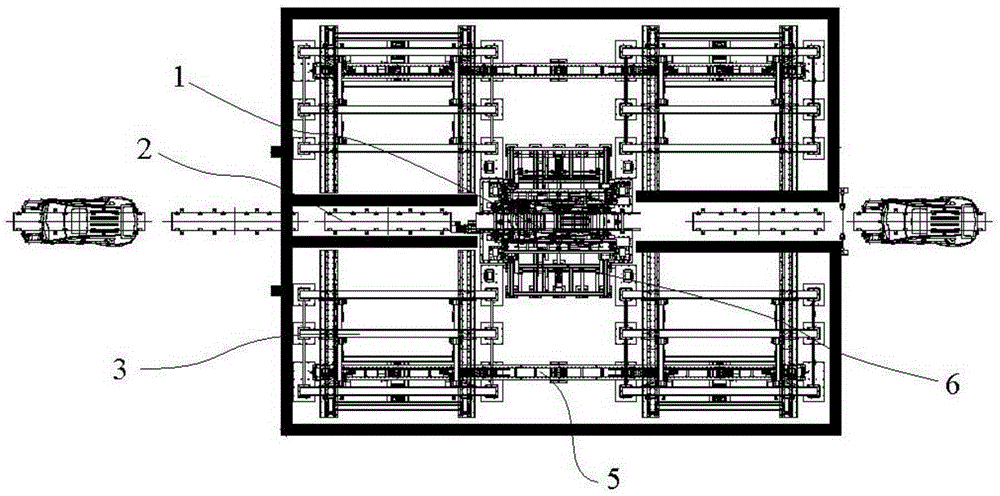
The invention relates to the field of vehicle welding in a vehicle production line, in particular to a storage switching mechanism for multi-vehicle-type vehicle body mixed line forming station clamps. The storage switching mechanism comprises four sets of clamp storage banks, and a clamp conveying device is installed below the clamp storage banks. The storage switching mechanism further comprises a clamp transfer device and a clamp in-place device. The clamp transfer device and the clamp in-place device are fixed between two sets of clamp storage banks located on the same side of a welding production line. The two ends of the clamp in-place device and the two ends of the clamp transfer device are connected with the clamp conveying device in the two sets of clamp storage banks on the same side respectively to form an annular clamp conveying system. The clamp storage banks are reasonable in arrangement structure, the occupied area of a factory is saved, the whole arrangement structure is more reasonable, the clamps can be well conveyed to designated positions through the cooperation of three-dimensional conveying mechanisms, the transfer device and the in-place device, the transfer efficiency of the clamps on the welding production line is improved, and high promotional value is achieved.
Owner:DONGFENG MOTOR CORP HUBEI
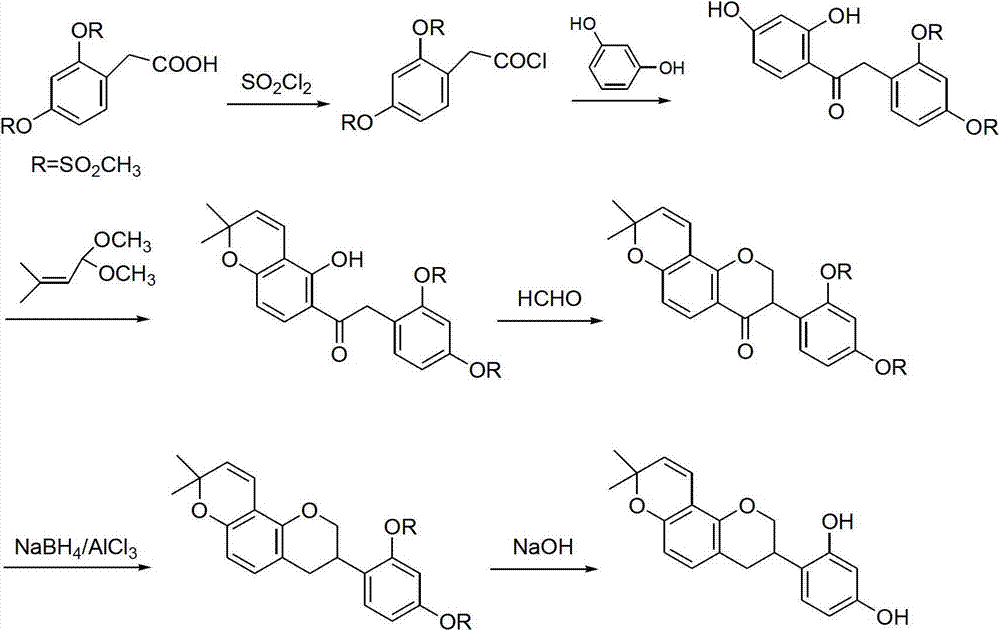
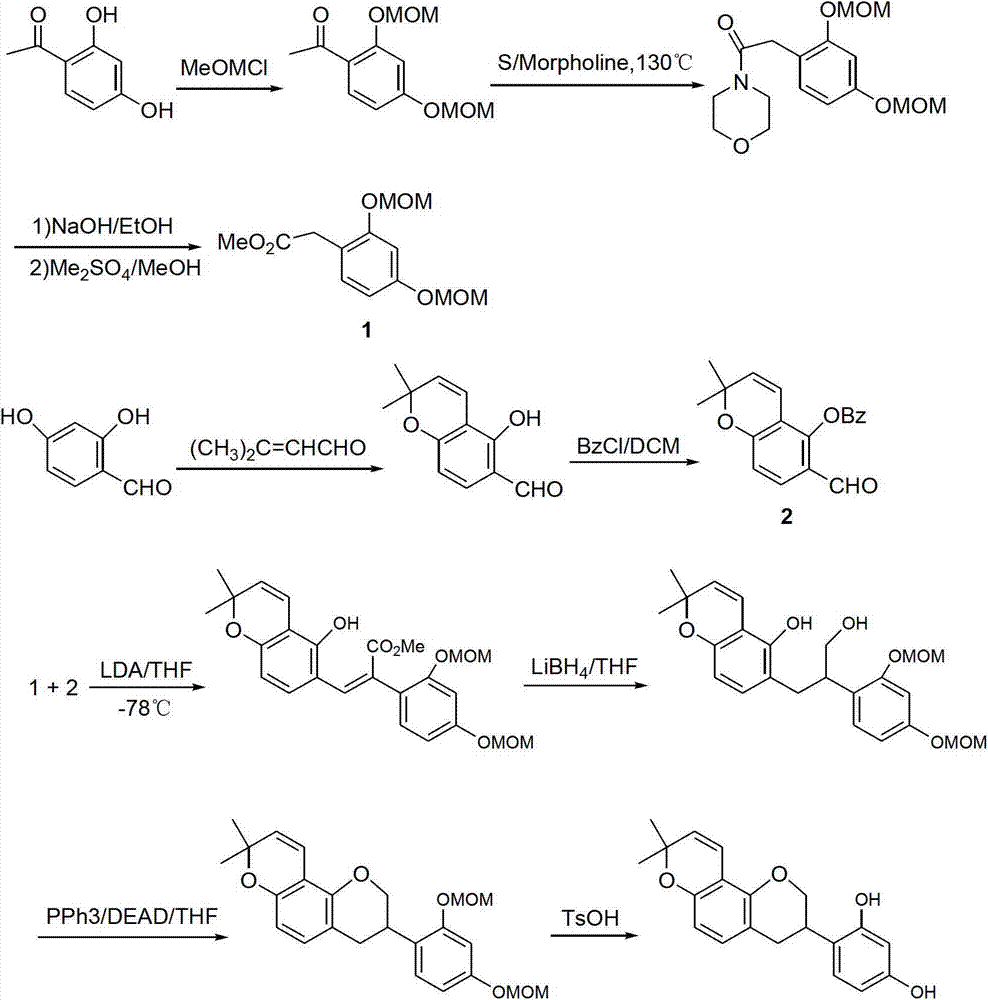
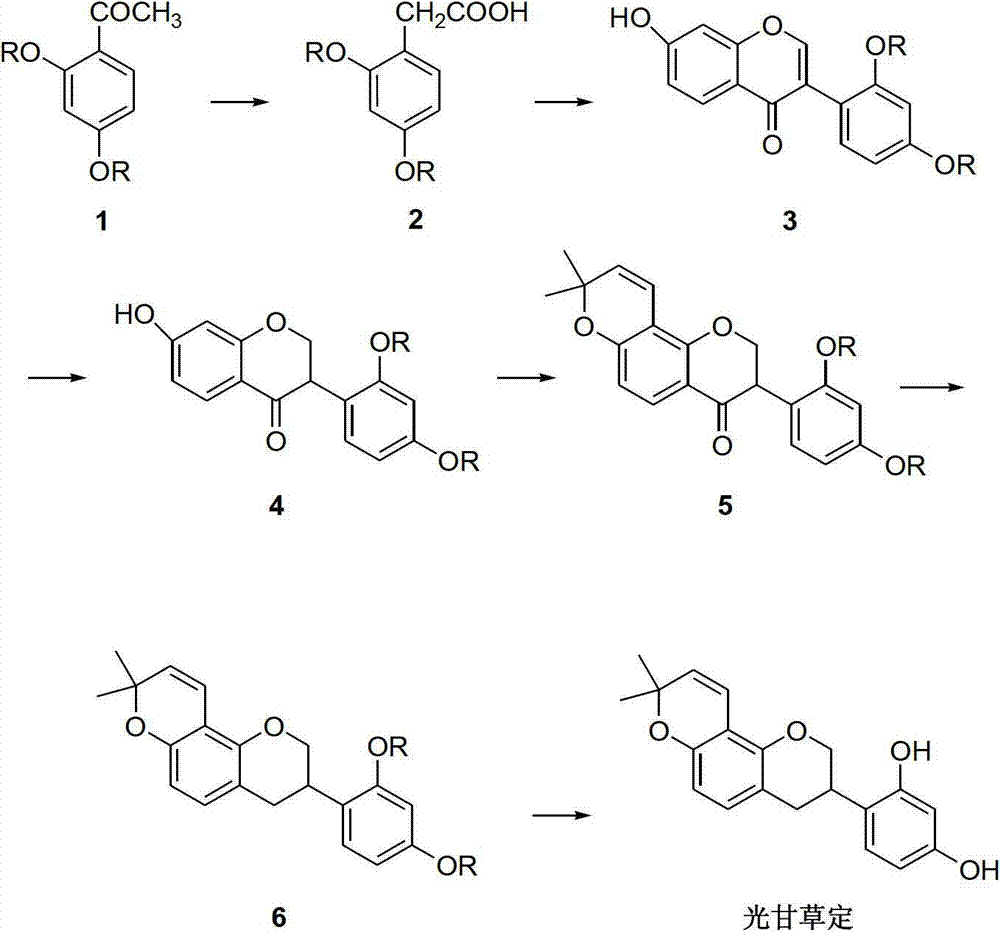
Method for synthesizing glabridin
The invention relates to a method for synthesizing glabridin. The method comprises the following steps: using acetophenone protected by phenol hydroxy as a raw material, carrying out a Willgerodt-Kindler reaction to obtain aryl phenylacetic acid, and carrying out a Friedel-Crafts reaction to obtain an isoflavones compound; causing the isoflavones compound to carry out Pd / C catalytic hydrogenation to obtain a isoflavanone compound; and causing the isoflavanone compound to carrying out a crclizationreaction, a conyl reduction reaction and a removing phenolic hydroxyl group protection group reaction to obtain the glabridin. The operation and the serparation of the steps of the method are simple, the yield is higher, the used reagents are common reagents, are cheap and are easily obtained, the path is shorter, and the tptal yield is not lower than 20 percent.
Owner:山东济清科技服务有限公司
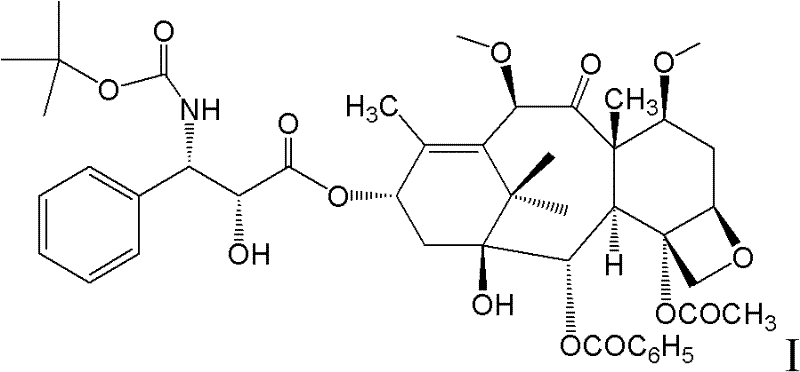
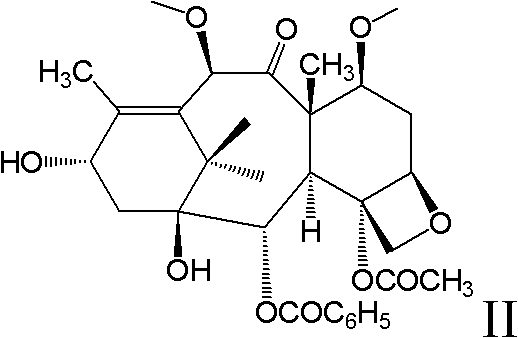
New taxane derivative and preparation method thereof
The invention provides a new taxane derivative and a preparation method thereof as well as a method of preparing antitumor medicaments (cabazitaxel, docetaxel and taxol) by utilizing new taxane isoserine ester.
Owner:SHANGHAI BIOMAN PHARMA
Method for synthetizing orixine and RU-19110 intermediate
A process for preparing dichroine and (2R, 3S)-1-benzyloxy-2-allyl-3-alkoxy piperidine as the intermediate of RU-19110 includes such steps as the reaction of imide on protecting agent to obtain compound E, reacting on allyl magnesium to obtain compound F, reacting on lewis acid and silane to obtain compound G, reacting on cerium ammonium nitrate to obtain compound H, reacting on litium aluminium hybrid to obtain protected (2R, 3S)-9, and reacting on meta-chloroperoxy benzoic acid to obtain compound J.
Owner:XIAMEN UNIV
Mercury ion fluorescence sensor as well as synthetic method and application thereof
InactiveCN103254891AThe synthesis steps are simpleRaw materials are easy to getOrganic chemistryFluorescence/phosphorescenceSynthesis methodsThiourea
The invention discloses a mercury ion fluorescence sensor shown in a formula (I). The sensor comprises a rhodamine b framework, anion bonding unit urea or thiourea and a signal element fluorescent molecular radical sensed. The invention further discloses a synthetic method of the mercury ion fluorescence sensor. The invention further provides an application of the mercury ion fluorescence sensor show in the formula (I) in mercury ion detection. Compared with the prior art, the raw materials used by the invention are available, the synthetic steps are simple and the post-treatment is convenient, and industrialized production is easy to realize.
Owner:EAST CHINA NORMAL UNIVERSITY
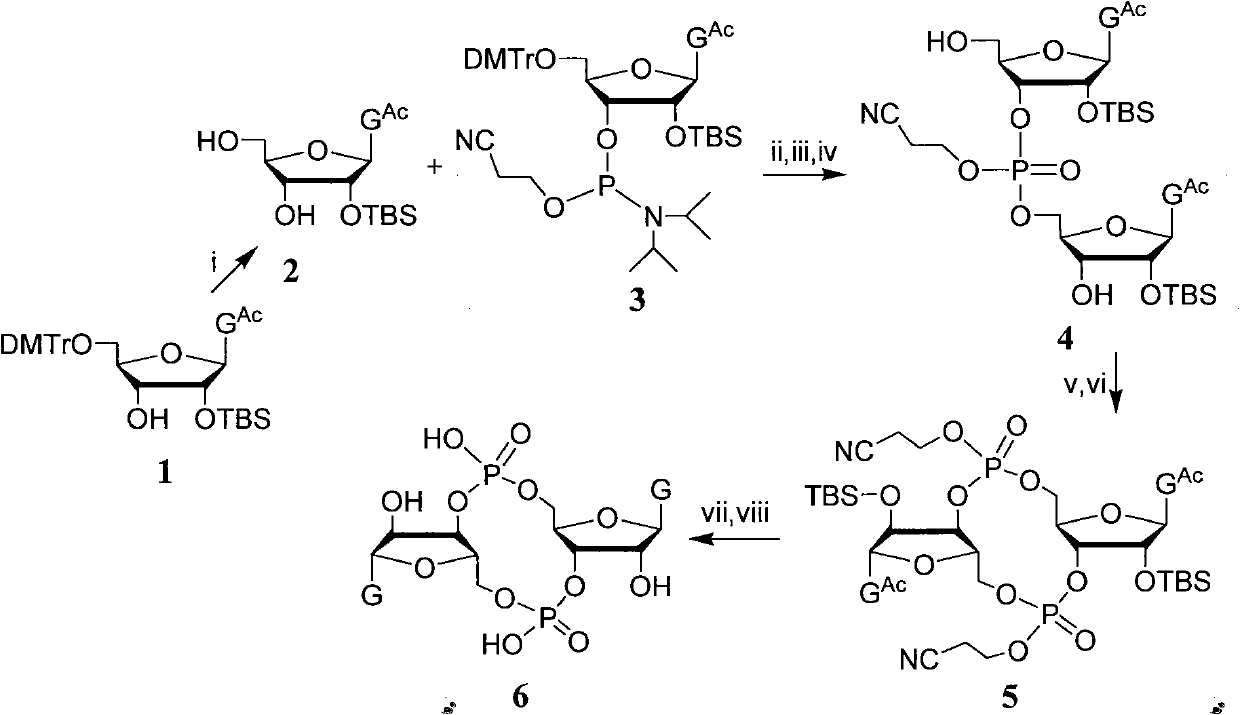
Preparation method of ribofuranose phosphate derivative
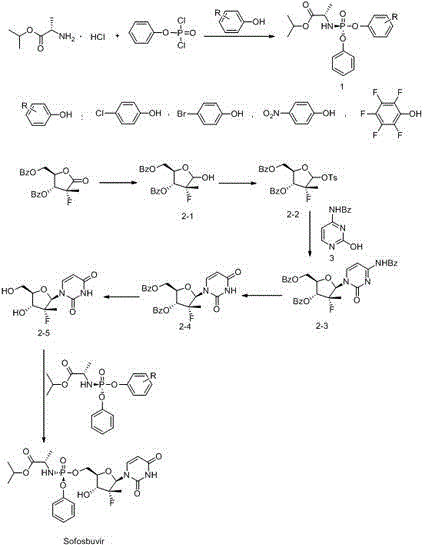
ActiveCN104610404APurity is easy to controlHigh yield of docking reactionSugar derivativesSugar derivatives preparationPhosphateGrignard reagent
The invention discloses a preparation method of a ribofuranose phosphate derivative. The preparation method comprises preparation steps as follows: L-alanine isopropyl ester hydrochloride, phenol dichlorophosphate and substituted phenol are taken as starting materials and have a docking reaction under the action of alkali; (2R)-2-deoxy-2-difluoro-2-methyl-D-erythropentonic acid GAMMA-lactone and 3,5-dibenzoate reduce carbonyl in a dichloromethane or ether solvent into an alcoholic hydroxyl group under the action of a strong reducing agent; an intermediate with a formula 2-1 has a reaction with paratoluensulfonyl chloride under the action of alkali to obtain p-toluenesulfonates; an intermediate with a formula 2-2 and a benzoyl cytosine derivative have a docking reaction under the action of a condensing agent; an intermediate with a formula 2-3 converts cytosine into uracil under the action of organic acid; benzoyl protection for an intermediate with a formula 2-4 is released under the action of an alkaline agent; an intermediate with a formula 2-5 and an intermediate with a formula 1 are docked under the action of a Grignard reagent to obtain Sofosbuvir.
Owner:NANTONG CHANGYOO PHARMATECH CO LTD
Method for directly producing adipic acid by cyclohexane catalytic oxidation
InactiveCN101337878BImprove stabilityAvoid pollutionPhysical/chemical process catalystsOrganic compound preparationCarbon nanotubeCatalytic oxidation
The invention discloses a method for directly producing adipic acid by catalytically oxidizing cyclohexane, which comprises the following steps: mixing cyclohexane, a solvent, an initiator and a carbon catalyst to form a mixed suspension, wherein the weight ratio of the solvent, the carbon catalyst, the initiator and cyclohexane is (0 to 99):(0.1 to 0.000125):(1 to 0.0005):1, and the carbon catalyst is carbon nanotube, graphite or active carbon; heating the mixed suspension to 50 to 250 DEG C; introducing oxygen or air as an oxidant; keeping the pressure in a reaction kettle at 0.1 to 5 MPa, and reacting for 0.1 to 20 h; and separating the reaction mixture to obtain the adipic acid product. The method can prevent environment pollution and corrosion of equipment due to the use of nitric acid, and can solve the problems such as the difficulties in recovering the homogeneous catalyst and deactivation of the homogeneous catalyst in the oxidization process of cyclohexane. No noble metal catalyst is used to reduce the catalyst cost, and the used catalyst can be recovered and used repeatedly.
Owner:SOUTH CHINA UNIV OF TECH
Process for preparing bosutinib
The invention discloses a process for preparing bosutinib, which simplifies reaction steps, optimizes reaction conditions, improves yield and paves the way for industrial production. The bosutinib is prepared from 4-hydroxy-3-methoxybenzoic acid serving as a raw material and the raw materials required in the reaction process have already been industrially produced, so the cost is low and can be saved; the process flow is relatively short and the yield is relatively high; and the cyclization conditions are mild and cyclization is only carried out at room temperature, so the process is suitable for industrial production.
Owner:NANJING MEDICAL UNIV
Process for producing light olefins
InactiveUS20080188701A1Short routingHigh olefin selectivityMolecular sieve catalystCatalystsMolecular sieveReactions stress
The present invention discloses a process for producing light olefins, comprising the steps of: i) contacting a feed comprising a monohalo-methane with a molecular sieve catalyst under the conditions: a reaction temperature in the range of from 350° C. to 600° C., a reaction pressure in the range of from 0.05 to 1.1 MPa (absolute), and a weight hourly space velocity of the monohalo-methane in the range of from 0.1 to 100 hour−1, to give an effluent comprising ethylene, propylene, and hydrogen halide; and ii) isolating ethylene, propylene and hydrogen halide from the effluent.
Owner:CHINA PETROCHEMICAL CORP +1
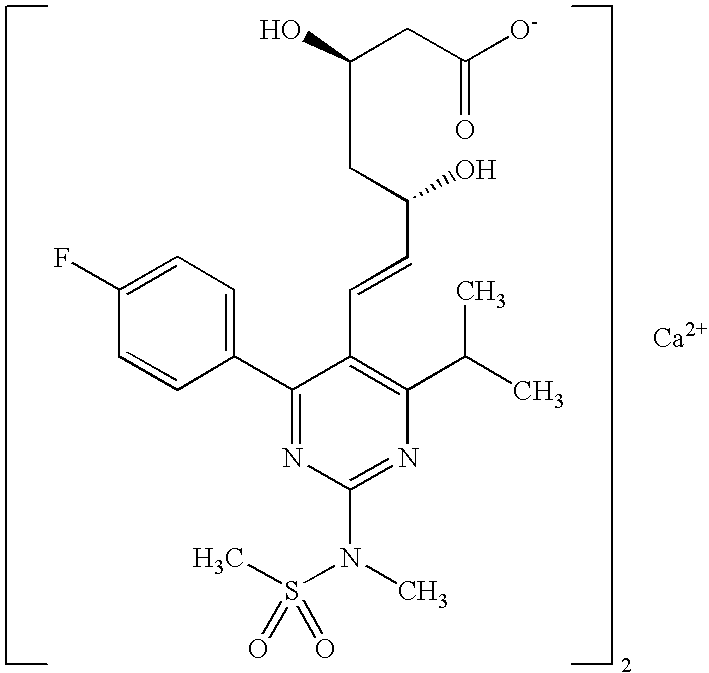
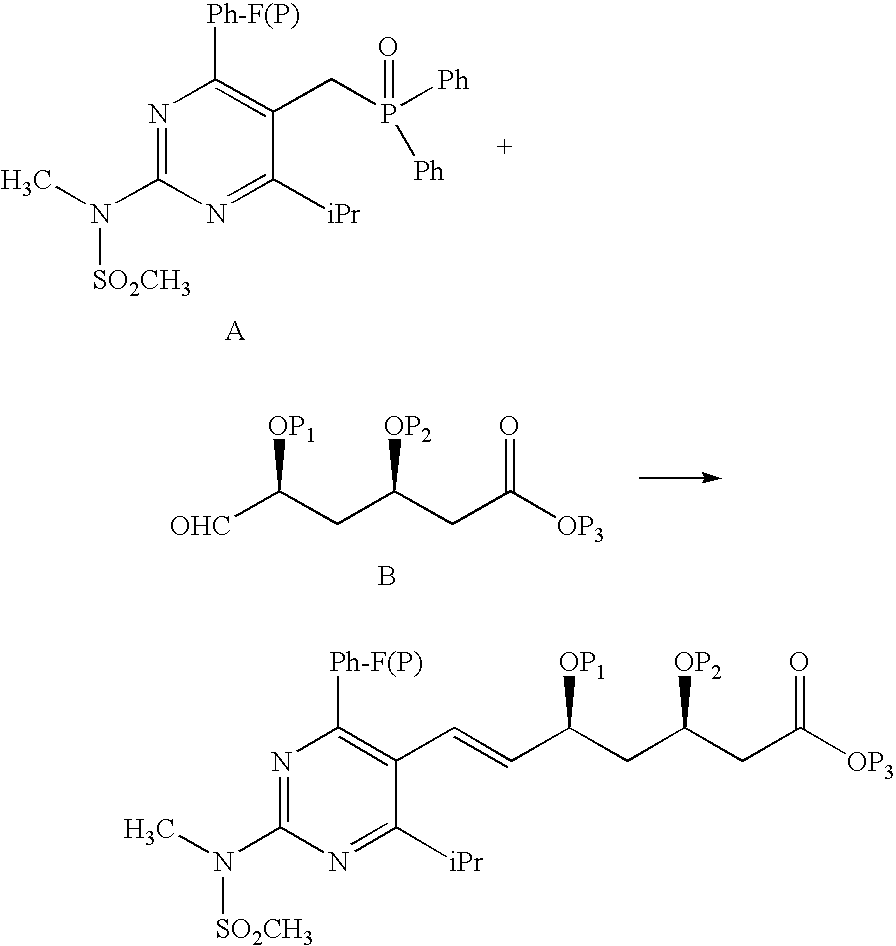
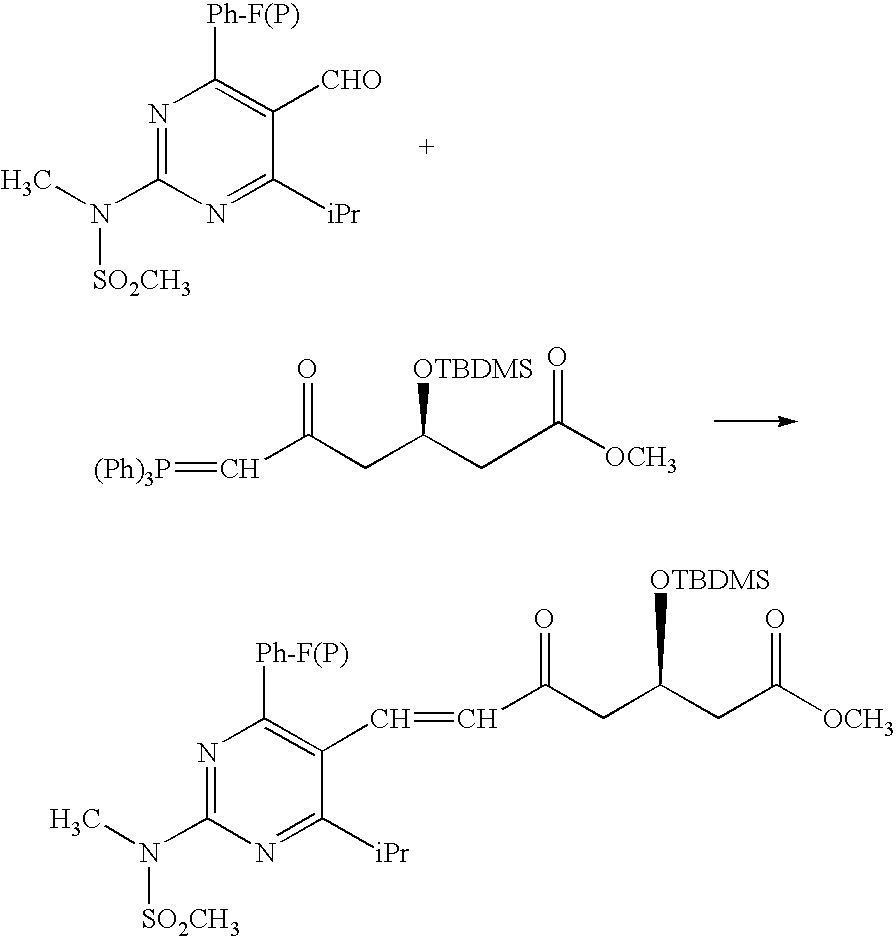
Synthetic Method and Intermediates of Rosuvastatin Calcium and Preparation Methods of Intermediates
The present invention publicly discloses a synthetic method and intermediates of rosuvastatin calcium and synthetic methods of the intermediates. The synthetic method uses 4-4′-fluorophenyl-6-isopropyl-2-(N-methyl-N-methylsulfonylamino)pyridine-5-formaldehyde as the raw material, includes 4-4′-fluorophenyl-6-isopropyl-2-(N-methyl-N-methylsulfonylamino)pyridine-5-acrylonitrile (intermediate I) from a nitrilized reaction, and 4-4′-fluorophenyl-6-isopropyl-2-(N-methyl-N-methylsulfonylamino)pyridine-5-acraldehyde (intermediate II) from an aldehydized reaction of the intermediate I, and further goes through such unit processes as side-chain extension, ketone-group reduction, ethyl-group hydrolysis and neutralization reaction or decomposition reaction to obtain rosuvastatin calcium. The nitrilized reagent can be phosphate diethylacetonitrile, acetonitrile, etc.; the aldehyde reductant can be diisobutyl aluminum hydride, red aluminum, etc.; and the ketone-group reductant can be diethylmethoxyborane, NaBH4, KBH4, etc.
Owner:ANHUI QINGYUN PHARMA & CHEM
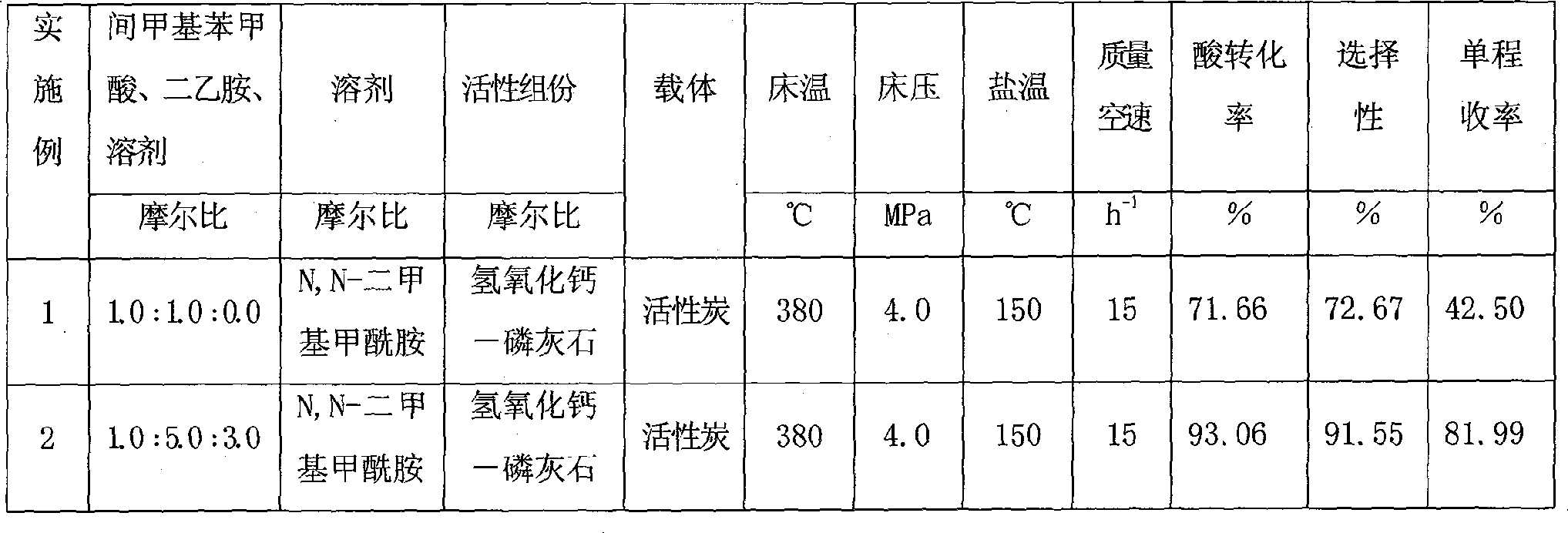
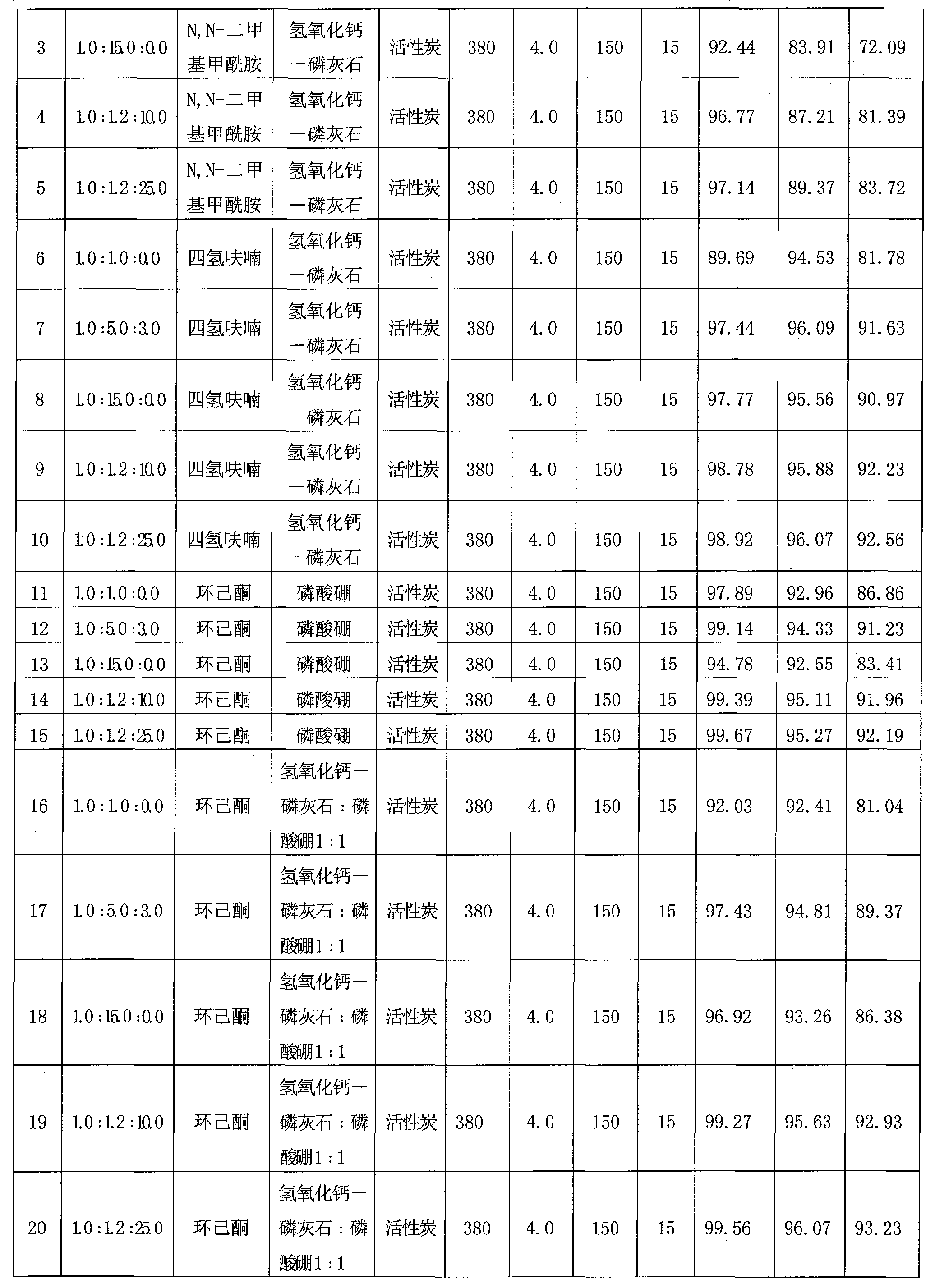
Method for one-step synthesizing N,N-diethyl-m-methyl benzamide in fixed bed
InactiveCN101362707ALow costIncrease profitOrganic compound preparationCarboxylic acid amides preparationRecyclable catalystBenzoic acid
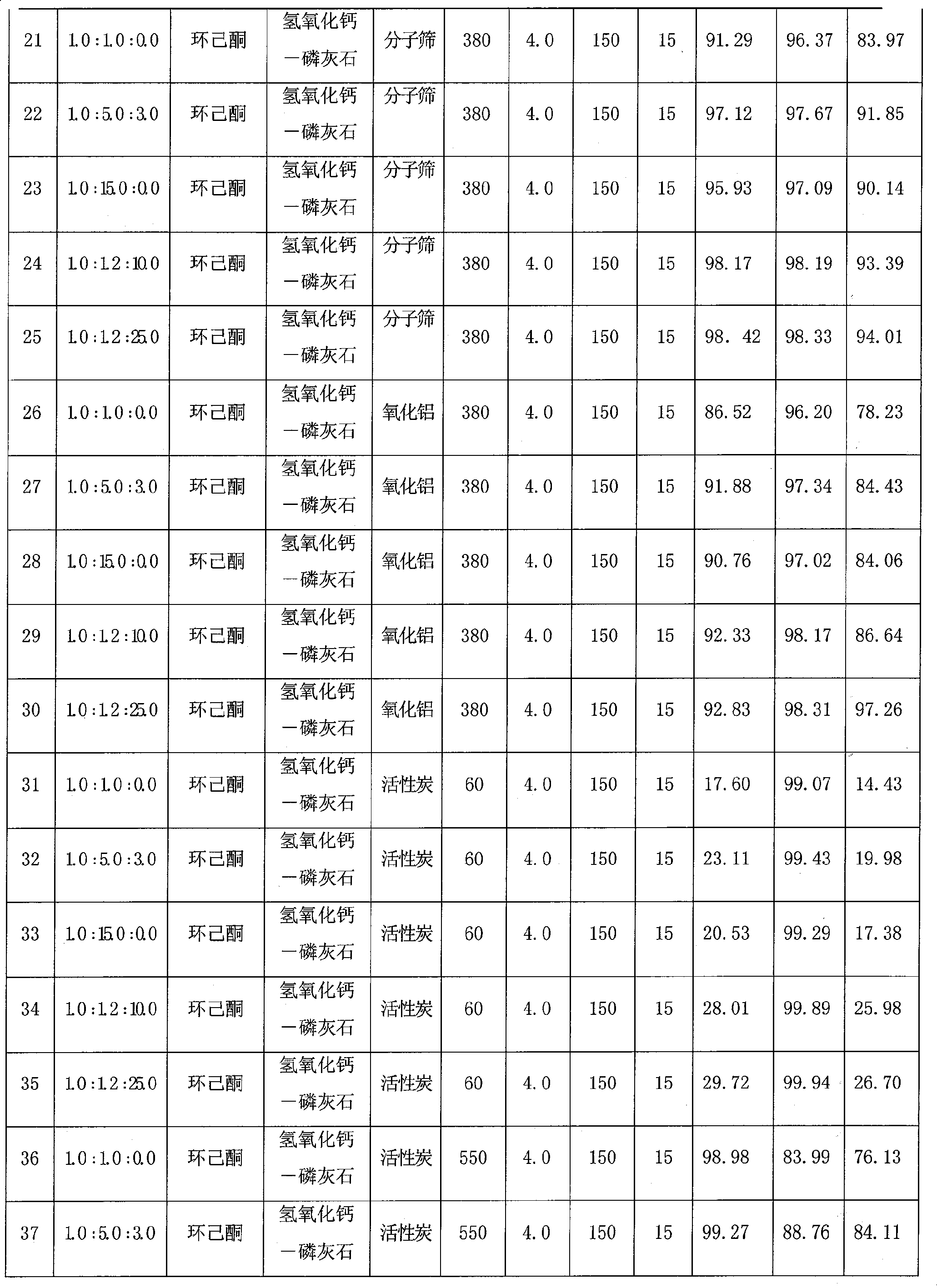
The invention discloses a one-step synthesis method of N, N-diethyl-3-methyl-benzamide with a fixed bed, which comprises the steps that: 3-methyl benzoic acid and diethylamine are stirred under a solvent system for reaction to generate a complex salt compound of the 3-methyl benzoic acid and diethylamine, and then materials are fed to the catalyzing fixed bed continuously under constant temperature and constant pressure, then dehydrated continuously to obtain a crude product; the solvent is recovered by distillation under ordinary pressure; finally the pure product of the N, N-diethyl-3-methyl-benzamide is obtained after rectification under vacuum. The molar ratio of the 3-methyl benzoic acid to the diethylamine to the solvent is 1.0: (1.0-15.0): (0-25.0), the mass velocity is 0.1 h<-1> to 20.0 h<-1>, the reaction temperature of the fixed bed is 60 DEG C to 550 DEG C and the reaction pressure of the fixed bed is 0.1 MPa to 5.0 MPa. The synthesis technology has the advantages of short technological process, high conversion ratio, good selectivity, high yield rate, small amount of waste gas, waste water and waste residues, reproducible and recyclable catalyst and low cost.
Owner:JIANGSU PANOXI CHEM
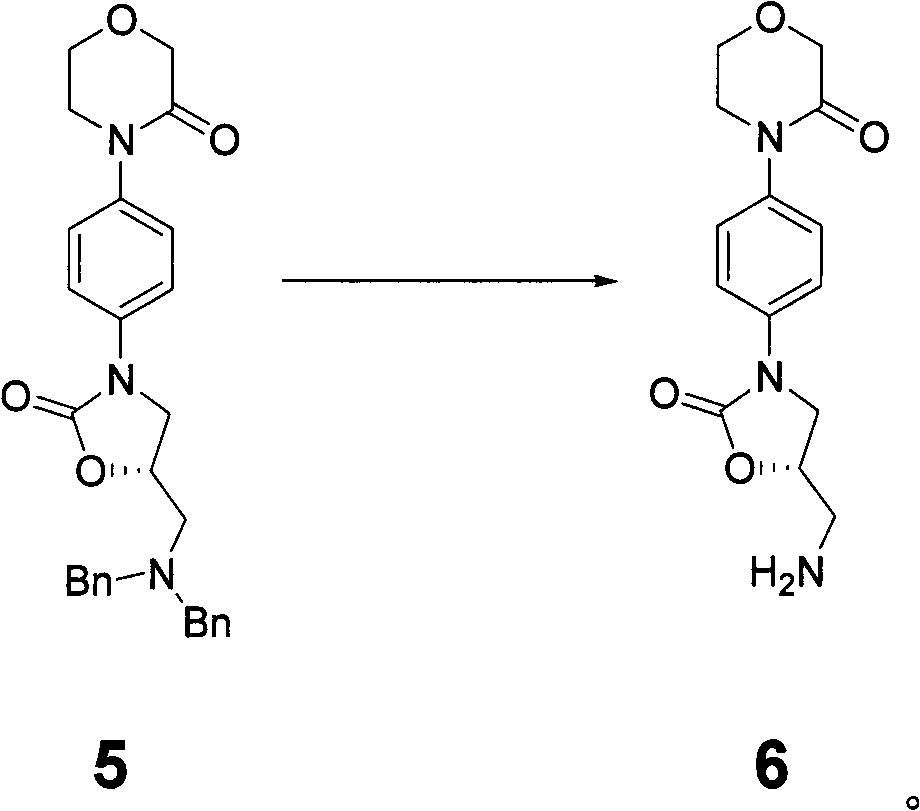
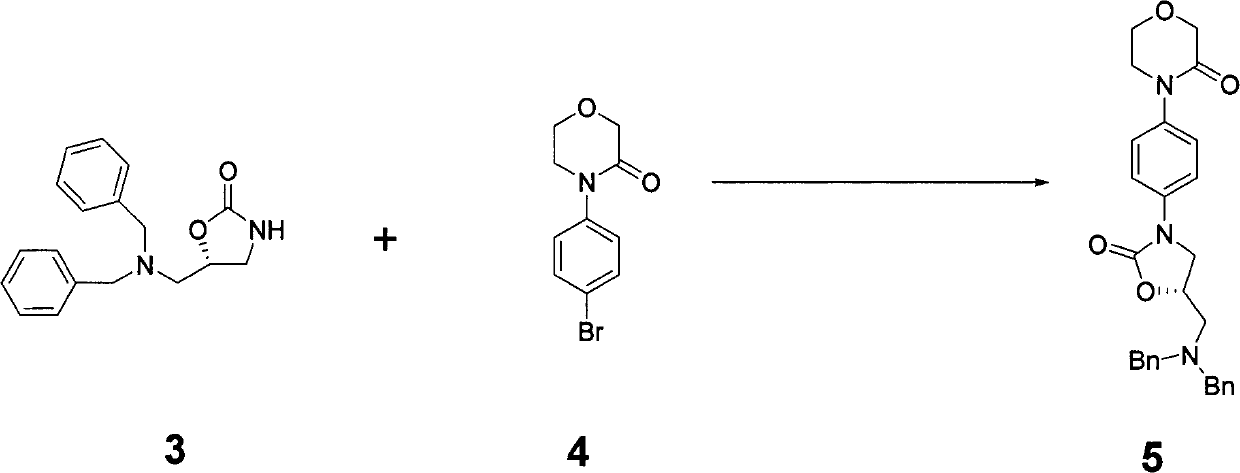
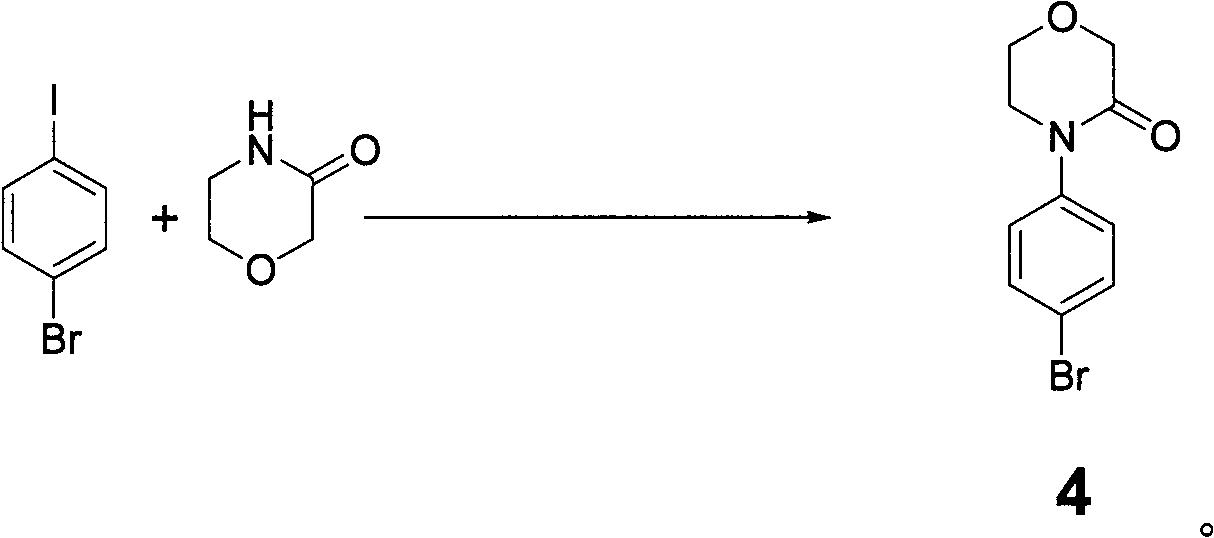
Preparation method of rivaroxaban and intermediate thereof and intermediate compound
InactiveCN102408420AShort routeLow costOrganic compound preparationBulk chemical productionProduction rateRivaroxaban
The invention discloses a preparation method of intermediate of rivaroxaban of formula 6, which comprises the following steps of: carrying out reaction of removing benzyl protecting group of amino to the compound 5 in a solvent. The invention also discloses a preparation method of rivaroxaban and an intermediate compound thereof. The preparation method disclosed by the invention has the advantages of shorter process, lower cost, easily acquired material and high production rate, and the prepared product is suitable for industrial production.
Owner:汕头经济特区鮀滨制药厂
Enzyme for catalyzing formaldehyde to synthesize hydroxyl acetaldehyde and application thereof
ActiveCN106916794AEfficient aggregationNo inputFermentationCarbon-carbon lyasesPhosphate acetyltransferasePhosphoric acid
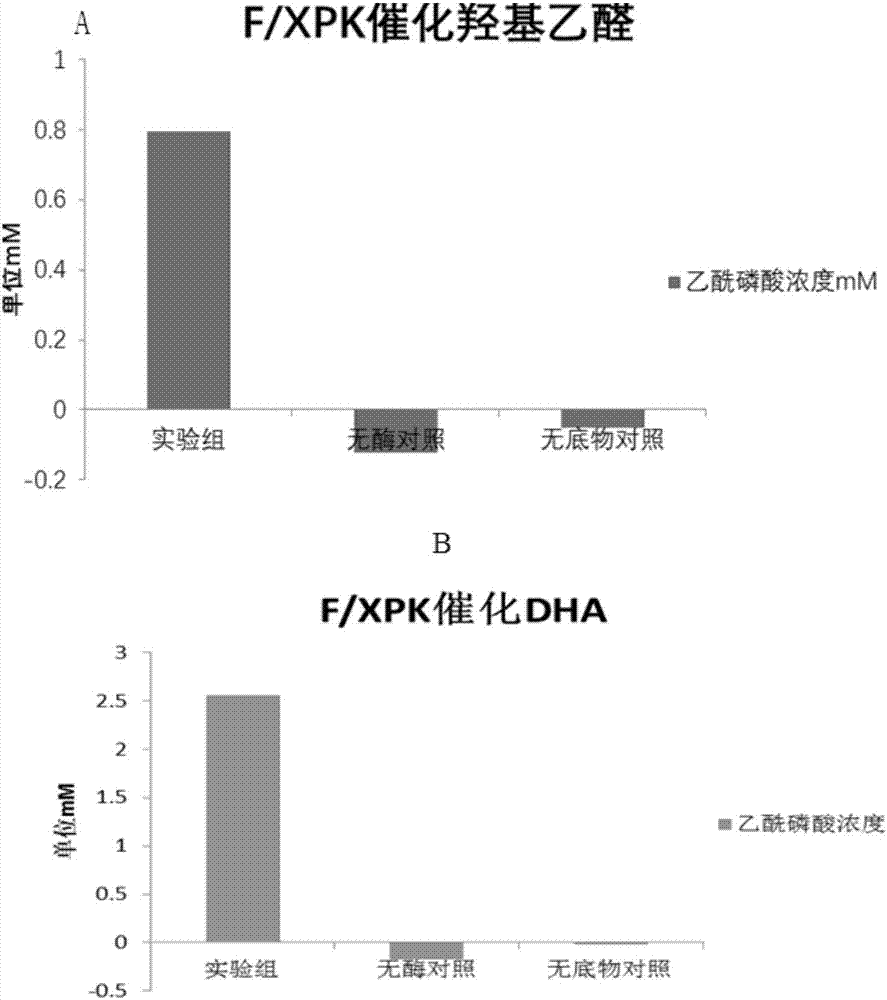
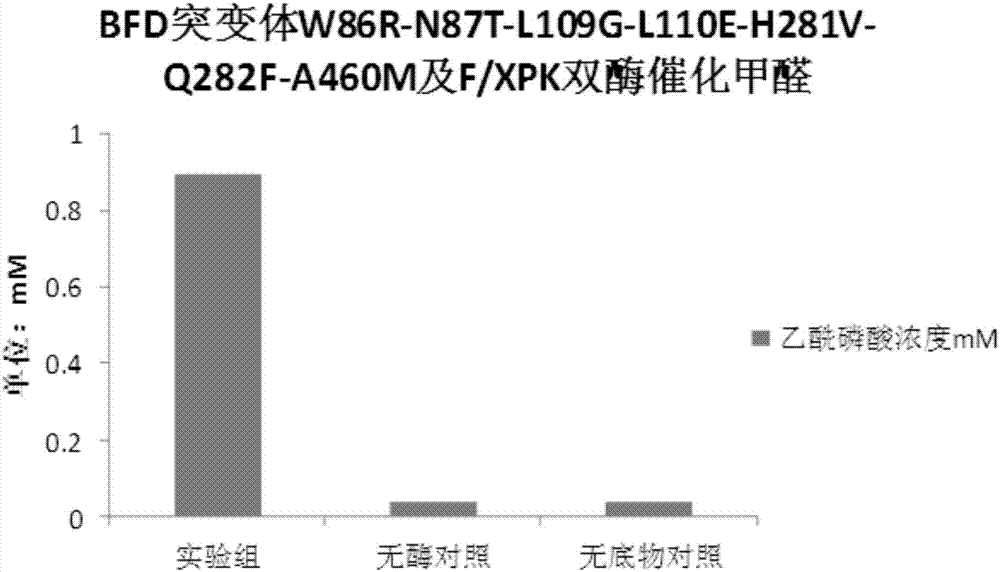
The invention discloses an enzyme for catalyzing formaldehyde to synthesize hydroxyl acetaldehyde and an application thereof. In the invention, through site-directed mutation of BFD, a mutant of the enzyme is found; and by means of the mutant of the enzyme, high-effect polymerization of the formaldehyde is achieved; meanwhile, through F / XPK, generation of acetyl phosphoric acid from the hydroxyl acetaldehyde or 1,3-dihydroxyacetone is achieved; with combination of phosphotransacetylase (Pta), a route from the formaldehyde to acetyl coenzyme A is achieved in three steps with the enzyme, thereby creating a new formaldehyde assimilation route, namely, synthesizing the acetyl coenzyme A from the formaldehyde in three steps. The route is short and is free of carbon loss and ATP input.
Owner:TIANJIN INST OF IND BIOTECH CHINESE ACADEMY OF SCI
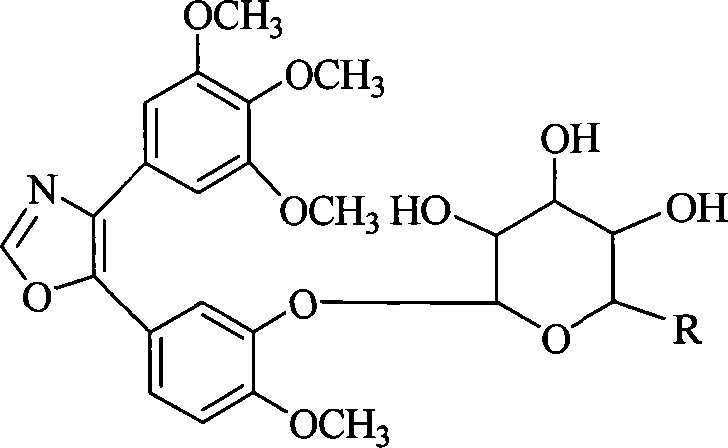
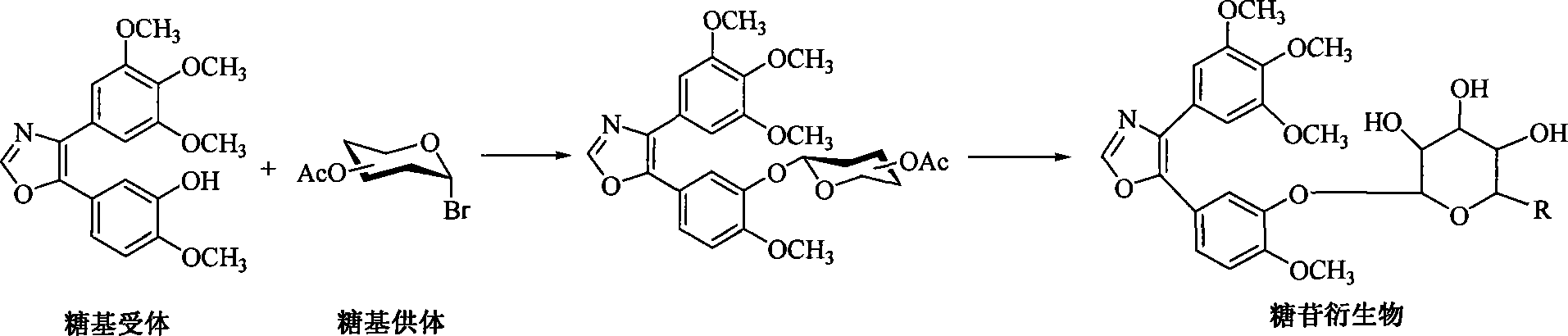
1,2-glycoside transderivative of oxazole compounds and preparation method thereof
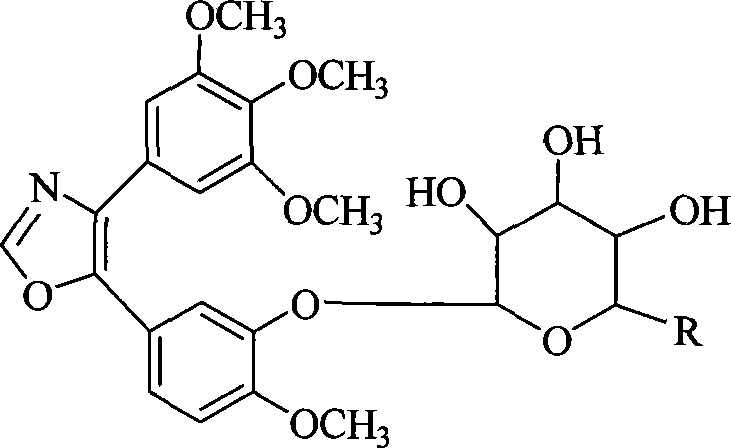
InactiveCN101230079AImprove bioavailabilityImprove targeted tumor vasculatureOrganic active ingredientsSugar derivativesSolubilitySodium methoxide
The invention discloses 1, 2-anti form glucoside derivative of oxazole compound. During the preparation, 4-(3, 4, 5-trimethoxy phenyl)-5-(3-hydroxyl-4-methoxy phenyl oxazole is used as the acceptor of glycosyl, bromo sugar of D-glucose, D-galactose, L-arabinose, D-xylose, L-fucose or lactose with full acetyl protection, or trichlorine imine ester of L-rhamnose or D-mannose with full acetyl protection is adopted as the donator of glycosyl, glycosylation is performed with the catalysis of alkali and tetrabutyl ammonium bromide or lewis acid, to obtain glucoside derivative containing acetyl protecting group; and then the acetyl protecting group is removed by utilizing methyl alcohol and sodium methoxide, to obtain 1,2 anti-form glucoside derivative of oxazole compound. The preparation method is simple, highly effective and universal, the route is short, the water-solubility of the product is good, the bioavailability is high, thereby the glucoside derivative can be applied as antineoplastic medicine inhibiting microtubule assembly and selectively targeting tumor blood vessels.
Owner:OCEAN UNIV OF CHINA
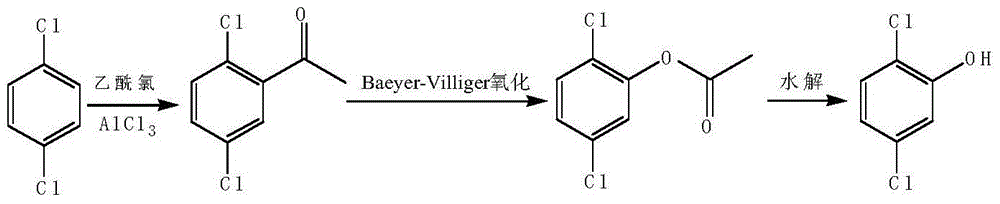
Preparation method of 2,5-dichlorophenol
InactiveCN104591973AThe synthesis process is simpleShort routeOrganic compound preparationCarboxylic acid esters preparationHydrolysisPeroxide
The invention discloses a preparation method of 2,5-dichlorophenol and relates to the technical field of pesticide intermediate synthesis. The preparation method comprises the following steps: with p-dichlorobenzene as a start raw material, performing a Friedel-Crafts acylation reaction between the p-dichlorobenzene and acetyl chloride in the presence of aluminum trichloride to obtain 2,5-dichloroacetophenone; performing a Baeyer-Villiger oxidation reaction between the 2,5-dichloroacetophenone and a peroxide in the presence of a catalyst at room temperature to obtain 2,5-dichlorobenzene acetate; and performing a hydrolysis reaction between the 2,5-dichlorobenzene acetate and inorganic aqueous alkali in a reflux condition to obtain 2,5-dichlorophenol. The preparation method disclosed by the invention has the characteristics of simple synthesis process, short line, low production cost and high yield; and moreover, with less quantity of generated three wastes and high environmental protection property, the preparation method is more suitable for large-scale industrial production.
Owner:ANHUI XUELANG BIOTECHNOLOGY CO LTD
Chiral single phosphorus ligand PC-Phos based on xanthene framework, preparation method of full structure of ligand and application
ActiveCN107417726AAvoid expensiveHigh reactivityGroup 5/15 element organic compoundsOrganic-compounds/hydrides/coordination-complexes catalystsAlleneDiastereomer
The invention discloses a novel single phosphorus ligand PC-Phos of a xanthene framework. The ligand is a compound as shown in a formula (1) or an enantiomer, racemate or diastereoisomer of the compound as shown in the specification. The invention further discloses a preparation method of the ligand. A formula 2 and a formula 4 as shown in the specification serve as raw materials, and substitution reaction, addition reaction, condensation reaction and reduction reaction are performed to prepare the ligand. Alternatively, a formula 6 and the formula 4 as shown in the specification serve as raw materials, are subjected to condensation reaction and subjected to addition reaction with a formula as shown in the specification to prepare the ligand. Chiral sulfonamide 4 with two structures and different types of metallic reagents are subjected to addition reaction, and optical voidness of four full structures 1 (S, Rs), 1 (R, Rs), 1 (S, Ss) and 1 (R, Ss) of the chiral single phosphorus ligand can be obtained. The invention further discloses an application of the ligand to asymmetric cyclization reaction in catalytic allene amine molecules. The ligand has quite high reaction activity, stereo-selectivity and wide application values.
Owner:EAST CHINA NORMAL UNIV
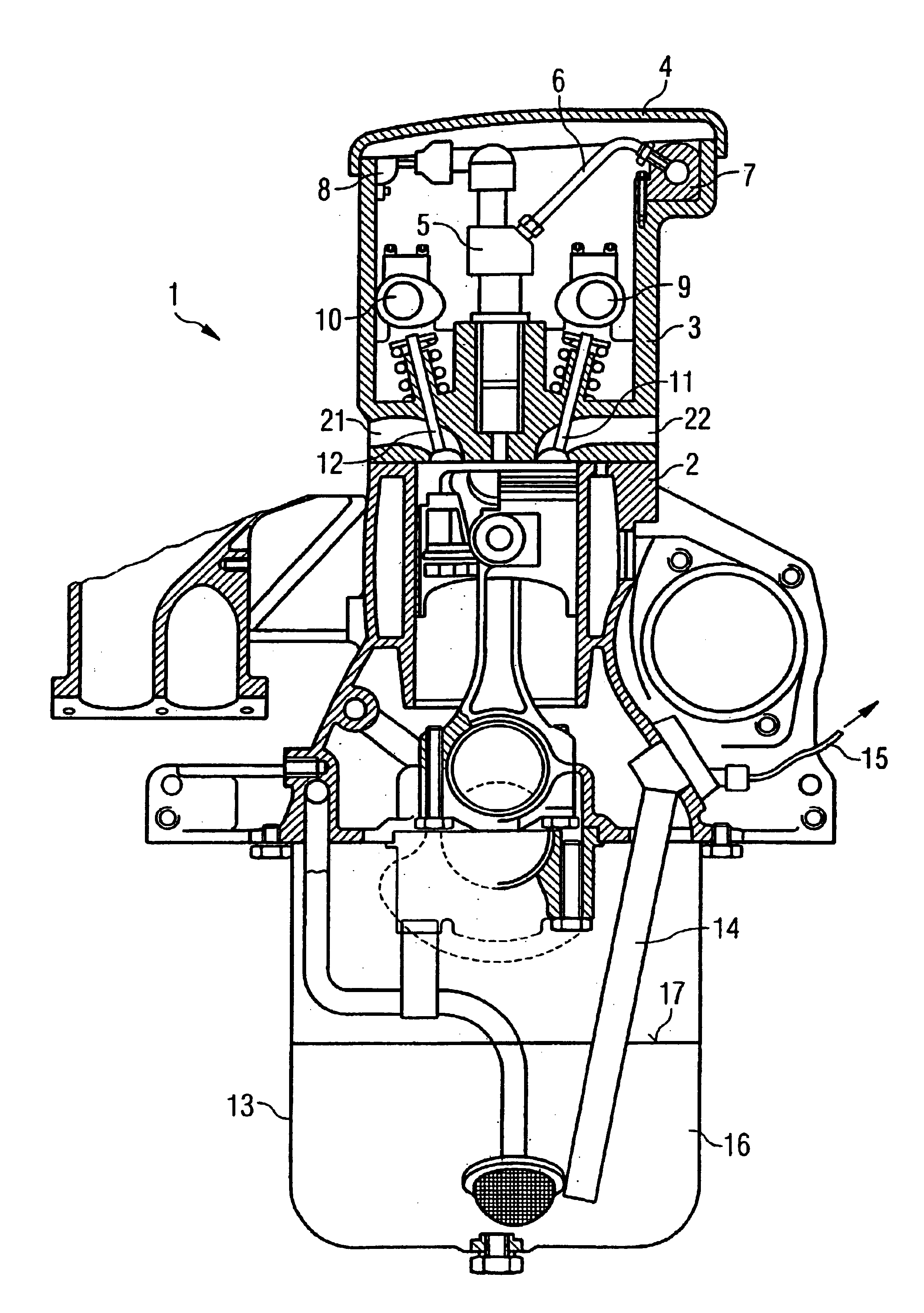
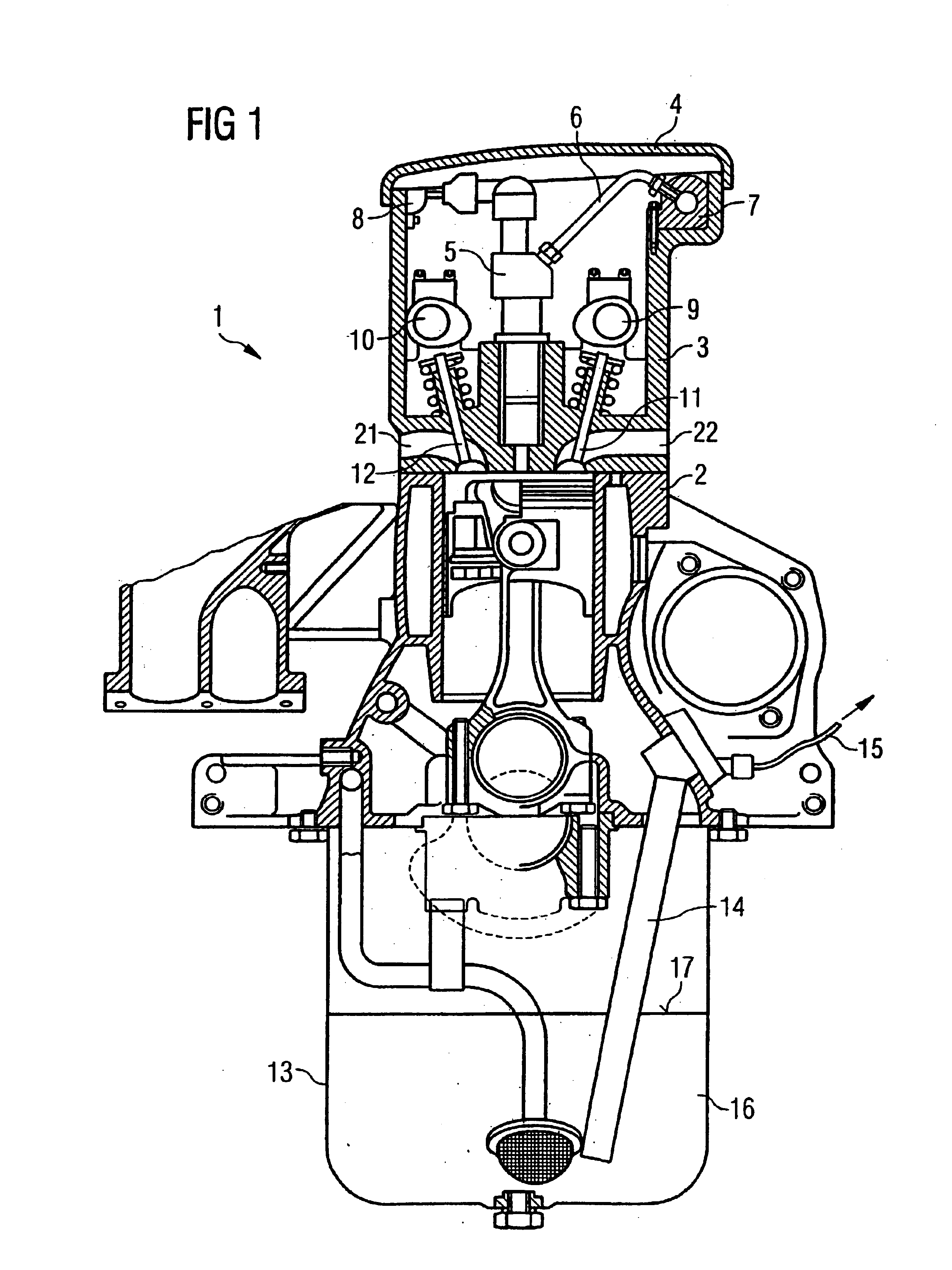
Cylinder-head-integrated diesel injection system with oil sensor
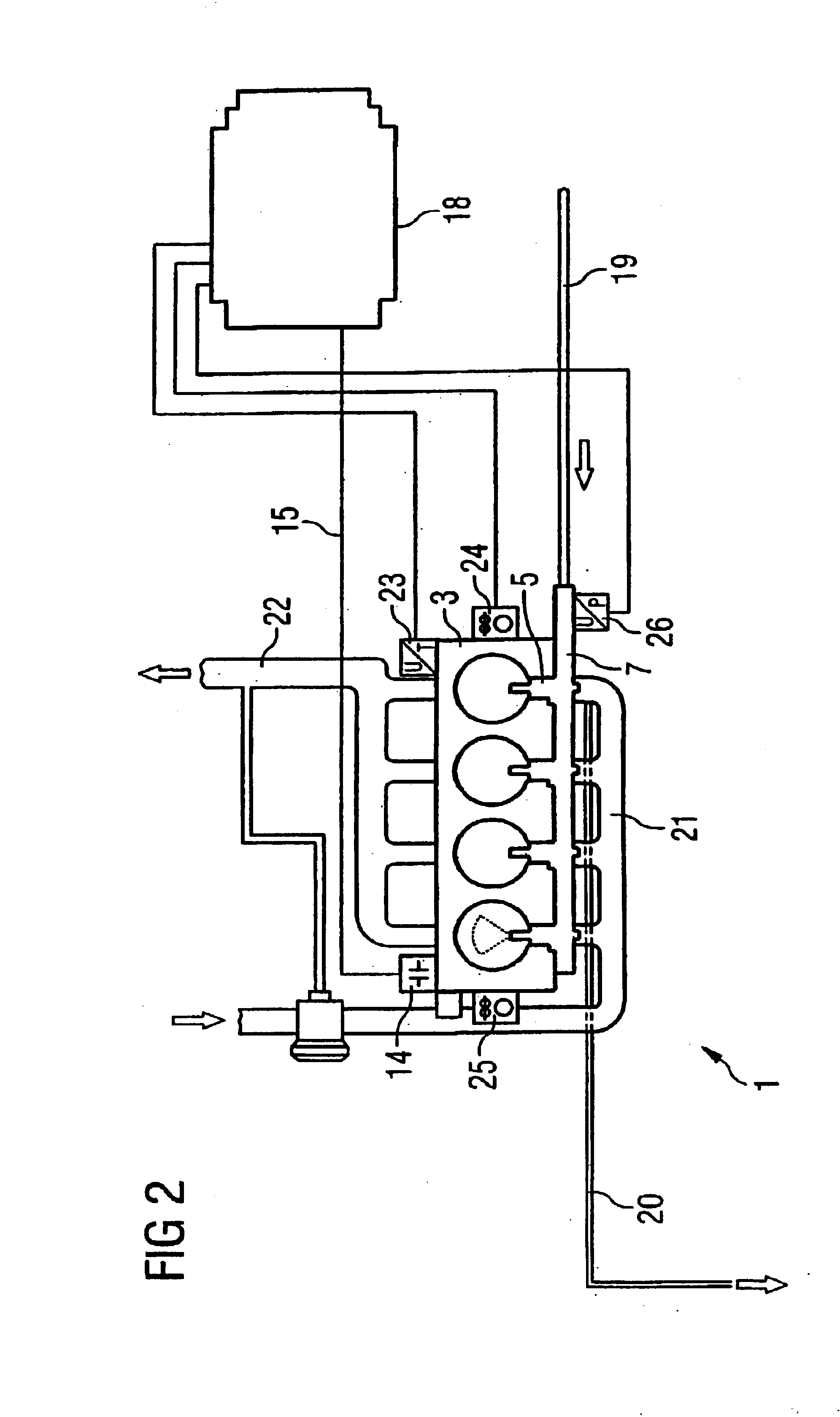
InactiveUS6880528B2Reduce noiseCompact designElectrical controlInternal combustion piston enginesCombustion chamberCylinder head
The present invention relates to an accumulator-type injection system for injecting fuel from an accumulator into a combustion chamber of an internal combustion engine by means of injectors, the injectors being arranged completely within the cylinder head of the internal combustion engine.
Owner:SIEMENS AG
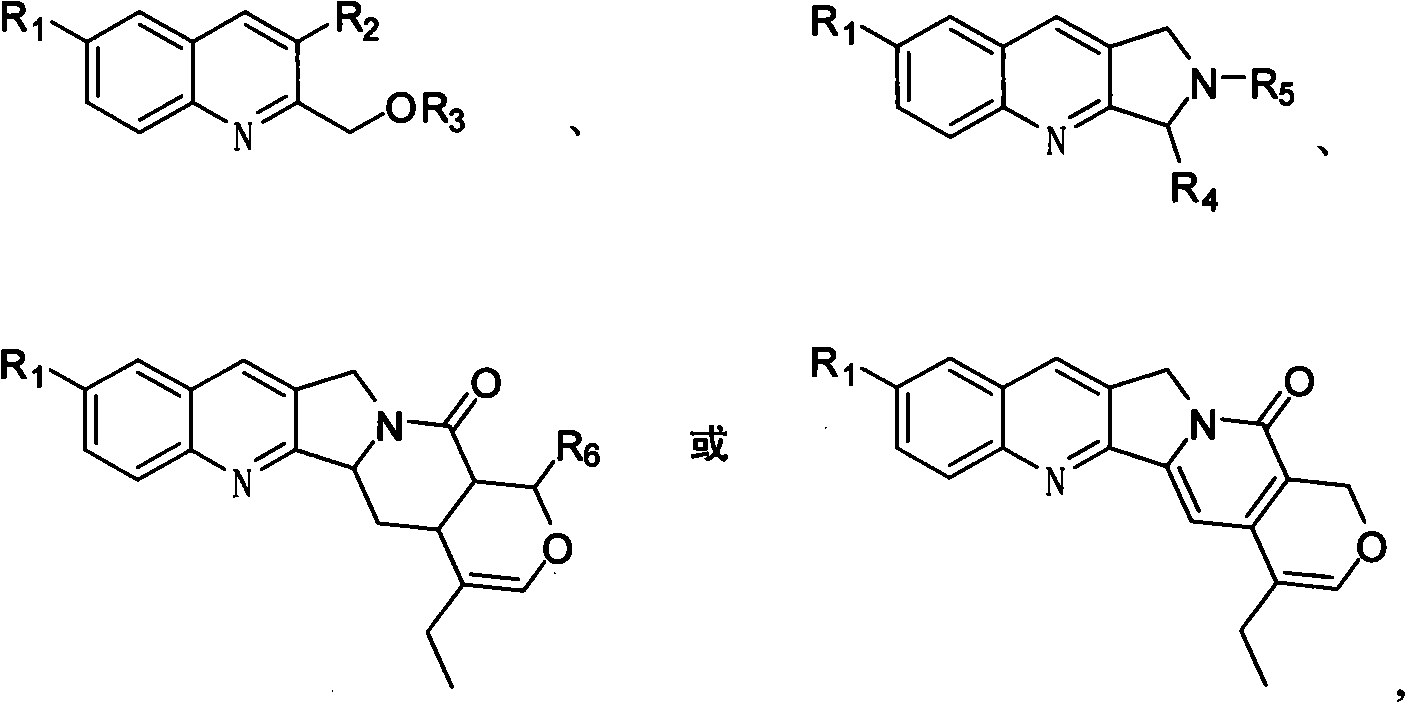
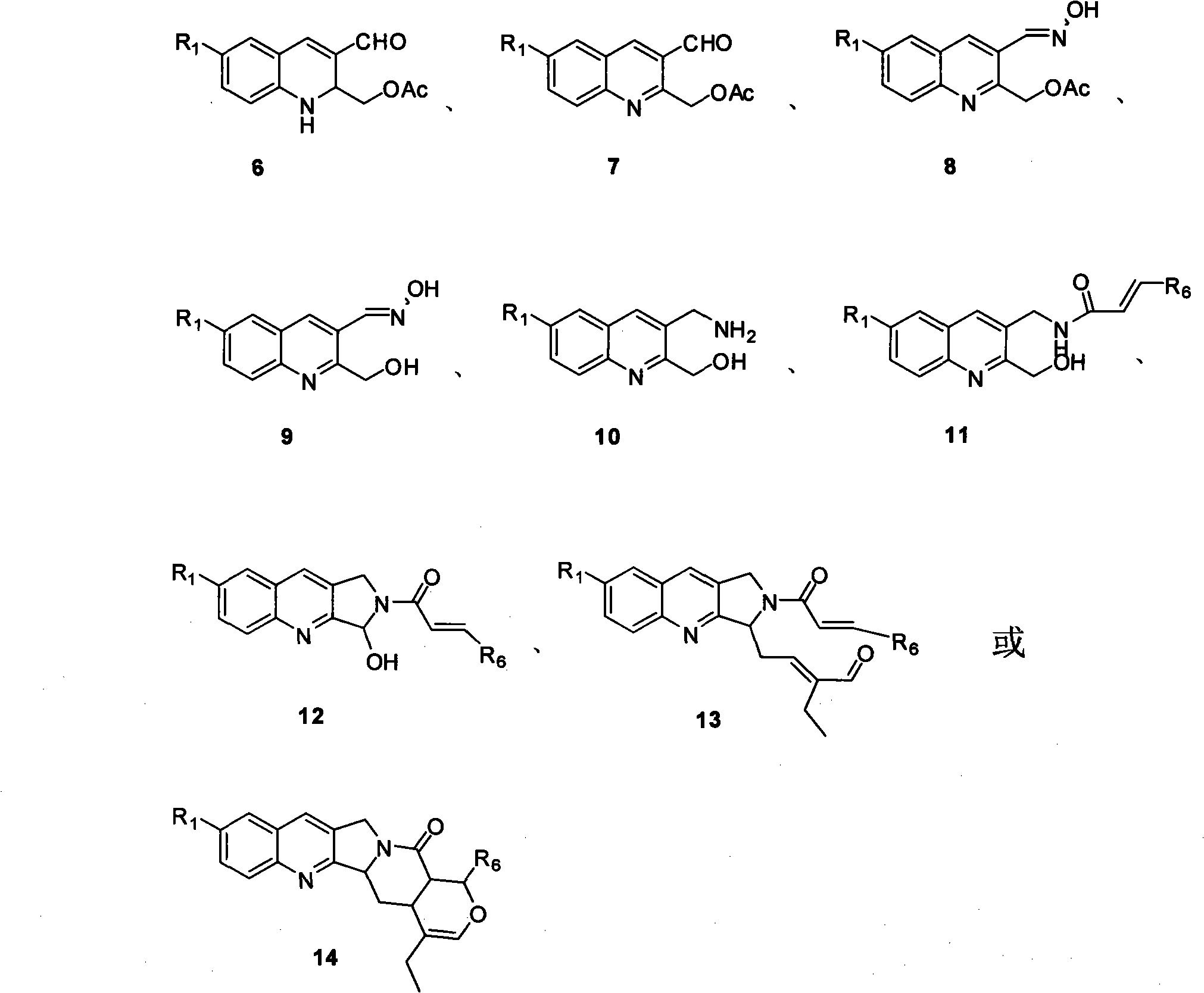
Quinoline compounds, synthesizing method, applications in synthesis of alkaloid of camptothecins
InactiveCN101337928AReduce usageShort routeOrganic chemistryAntineoplastic agentsSynthesis methodsQuinoline
The invention relates to quinoline compound, a synthesis method and the application for preparing medicines of camptothecin alkaloid. The syntheticroute is concise, high-efficient and economical, and can play a great role in promoting the later industrialized production of camptothecin alkaloid.
Owner:SHANGHAI INST OF ORGANIC CHEMISTRY - CHINESE ACAD OF SCI
Process for preparing glycyl gtutamine
InactiveCN1532204AReduce pollutionShort routePeptide preparation methodsDipeptidesGlycyl glutamineAlcohol
The present invention belongs to the field of medicine chemical technology. The preparation process of glycyl glutamine includes: dissolving carbobenzoxy Gly and N-maloylimine in organic solvent, adding solution of dicyclohexylcarbodiimide to obtain activated N-carbobenzoxy Gly-N-maloylimine solution, reacting the activated ester solution with glutamine under alkali condition to obtain carbobenzoxy glycyl glutamine; hydrogenolyzing the obtained carbobenzoxy glycyl glutamine in mixed water-alcohol solvent and under the catalysis of Pd catalyst, and re-crystallization to obtain glycyl glutamine product. The advantages includes short path, simple technological process, strong operability, low cost and less environmental pollution.
Owner:四川三高生化股份有限公司
Method for preparing adipate
ActiveCN102001931ALow costHigh reactivityChemical recyclingCarboxylic preparation by oxidationAdipic acidSolvent
The invention discloses a method for preparing adipate, comprising the following steps of: (1) adding cyclohexane, solvent, an initiator and a solid catalyst into a reactor and mixing to form a mixed suspension; (2) introducing enough amount of oxygen into the mixed suspension to be used as an oxidant; and reacting under the conditions that the pressure is 0.5-5MPa and the temperature is 100-200DEG C for 1-20h; (3) separating the reaction mixture in the step (2) to obtain the solid catalyst and a liquid phase mixture; and (4) separating the adipate from the liquid phase mixture in the step (3). The invention prevents nitric acid from corroding the environment and the equipment, can solve the problems that a homogeneous catalyst is difficult to recover and is deactivated in the oxidation process of the cyclohexane, is free from using noble metals and has high reaction selectivity and favorable activity. The used catalyst can be recycled.
Owner:SOUTH CHINA UNIV OF TECH
Ursodesoxycholic acid preparation method
The invention discloses an ursodesoxycholic acid preparation method. The method comprises the following steps: 1, adding chenodeoxycholic acid and a solvent A to a reaction container, stirring for dissolving, adding 7-alphaHSDH, 7-betaHSDH and a coenzyme II, and carrying out a reaction at a controlled temperature at a controlled pH value to convert chenodeoxycholic acid into ursodesoxycholic acid in order to obtain a conversion liquid; 2, heating the conversion liquid obtained in step 1 to denaturalize the 7-alphaHSDH and the 7-betaHSDH, centrifuging through a high speed centrifuge, removing proteins, adding a sodium hydroxide solution to the above obtained solution, distilling to remove the solvent A, adding water to dissolve obtained distillation residues, adding an acid, and crystallizing to obtain crude ursodesoxycholic acid; and 3, adding the crude ursodesoxycholic acid obtained in step 2 and a solvent B to the reaction container, heating and refluxing the crude ursodesoxycholic acid and the solvent B for 1h, cooling the obtained reaction product to normal temperature, and filtering the cooled product to obtain ursodesoxycholic acid with the purity being greater than 99%. The ursodesoxycholic acid preparation method has the advantages of simple technology, short synthesis route, high conversion rate, easy post-treatment and environmental protection.
Owner:ZHONGSHAN BAILING BIOTECHNOLOGY CO LTD
C-di-GMP, analogues thereof and preparation method thereof
ActiveCN102199183BInhibition formationPrevent proliferationSugar derivativesSugar derivatives preparationDrug developmentBiological membrane
The invention discloses a c-di-GMP and analogues thereof which have structures of a general formula I. The invention also discloses a novel preparation method-a kettle phosphoramidite method which can be used for rapidly, simply and conveniently preparing c-di-GMP compounds with high yield and low cost on a large scale and at mild conditions. The c-di-GMP is prevalent in bacteria and is a novel second messenger molecular which takes part in regulating multiple physiological functions. The research shows that the c-di-GMP and the analogues thereof can inhibit the formation of bacterium biological membranes and the multiplication of eukaryotic cells; and thereof the c-di-GMP and the analogues thereof have good medicine development prospect.
Owner:PEKING UNIV
Preparation method of triallyl phosphate
InactiveCN103467513AToxic reductionSimple processGroup 5/15 element organic compoundsPhosphateDistillation
The invention relates to a preparation method of triallyl phosphate, which comprises the following steps that allyl alcohol, an acid-binding agent and an ether solvent are mixed in an inert gas atmosphere; phosphorus oxychloride is dropwise added to a mixed liquid in the inert gas atmosphere; then stirring and reaction are performed; a liquid obtained after the reaction is filtered; a filtrate obtained after filtering is subjected to atmospheric distillation and solvent recovery; the residual filtrate after the solvent recovery is subjected to rectification under vacuum; and a fraction of triallyl phosphate is collected. The method has the following advantages that 1, the method is simple in technology, short in route, cheap in material and very small in toxic property, and is suitable for industrial production; 2, the method is few in byproduct and high in yield; and 3, the purity of a rectified and purified product is high, and can reach 99.5%, and the method is particularly suitable for the fields of lithium ion battery electrolyte additives and the like.
Owner:SHENZHEN CAPCHEM TECH CO LTD
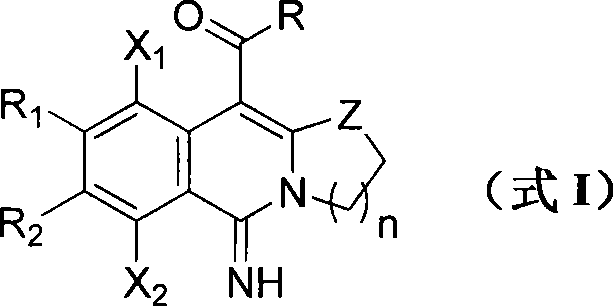
Polyhalogenated isoquinoline class derivate and synthetic method thereof
The invention discloses a novel polyhalogenated isoquinoline class derivate and a synthetic method thereof. The structure of the polyhalogenated isoquinoline class derivate is as a formula I, wherein Z=NH, NR', O, S; n=1,2,3; the R' is alkyl and aryl; R is the alkyl, the aryl, heteroaryl, condensed aryl, alkoxyl and alkylthio group; R1 is a halogen atom, a hydrogen atom, the alkyl, the aryl, alkylamino radical, arylamine, alkylthio group and arylthio; R2 is cyano-group, nitryl, ester group, the halogen atom, the hydrogen atom, the alkyl, the aryl, the alkylamino radical, the arylamine, the alkylthio group and the arylthio; X1 is the halogen atom; and the X2 is also the halogen atom. The synthetic method of the polyhalogenated isoquinoline class derivate including the steps of porphyrizing and heating polyhalogenated cyanobenzene and heterocyclic ketene aminals derivates in a mortar. When raw materials are basically completely disappeared, and the polyhalogenated cyanobenzene and the heterocyclic ketene aminals derivates are all transferred to a round bottom flask by menstruum and then reacted to synthesize the polyhalogenated isoquinoline class derivate with potential medicine activity in the formula I after a catalyst is added into the round bottom flask. The invention has simple synthesis technology, high productive rate, stable products, simple routes, rapidness, and the like, realizes a parallel high-efficiency heterocyclic compound library and really realizes the molecular diversity.
Owner:YUNNAN UNIV
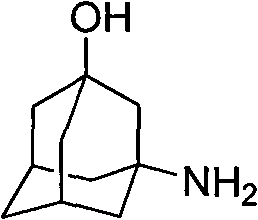
Method for preparing 3-amino-1-adamantane alcohol
InactiveCN101798270AReduce dosageReduce processing costsOrganic compound preparationAmino-hyroxy compound preparationWater bathsIce water
The invention relates to a method for preparing 3-amino-1-adamantane alcohol. The method comprises the following steps of: adding amantadine hydrochloride into a nitrating agent in batches, performing reaction for 1 to 2 hours in an ice-water bath and performing reaction for 1 to 30 hours at room temperature to obtain yellowish liquid; pouring the yellowish liquid into ice, continuously reacting for 0.5 to 2 hours with stirring to obtain blue-green liquid; and adding solid base into solution obtained by the step 2 with stirring, keeping temperature below 80 DEG C, regulating pH to be between 10 and 12, performing reaction for 30 minutes with stirring, leaching, extracting reaction liquid by using dichloromethane, drying the obtained product with anhydrous sodium sulfate, steaming off the dichloromethane and performing recrystallization by using ethyl acetate to obtain white solid. The preparation method has the advantages of readily available starting raw materials, simple reaction operation, short route, environmental friendliness, easy industrial production and good application prospect, and also reduces cost for the synthesis of Vildagliptin serving as a medicament for treatingdiabetes; and the yield of products reaches 75 percent.
Owner:DONGHUA UNIV
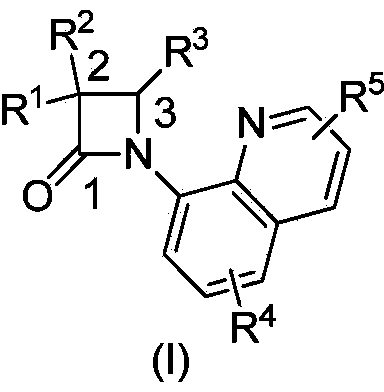
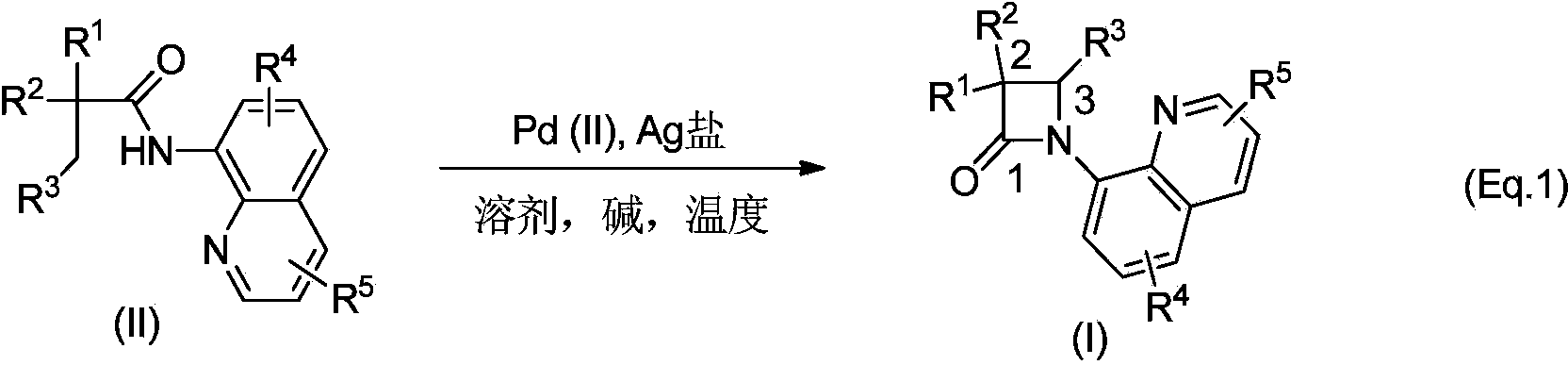
N-quinolyl substituted beta-lactam compound, as well as pharmaceutical composition, synthetic method and application of compound
InactiveCN103524486AEasy to operateThe reaction equipment is simpleAntibacterial agentsOrganic active ingredientsArylHalogen
The invention discloses an N-quinolyl substituted beta-lactam compound as shown in a general formula (I), wherein R<1> and R<2> refer to mutually independent H or C1-12 straight-chain or branched-chain hydrocarbonyl, aryl, alkoxy, acyloxy or amino; R<3> refers to H or C1-25 straight-chain or branched-chain hydrocarbonyl or aryl, or R<3> together with R<1> or R<2> forms a substituted or non-substituted five-eight-membered ring containing or without containing heteroatoms, and the heteroatoms can be N, O and S; R<4> and R<5> refer to mutually independent H or C1-12 straight-chain or branched-chain hydrocarbonyl, aryl, alkoxy, acyloxy or amino, nitryl, sulfonyl and halogen, and halogen refers to F, Cl, Br and I. The invention also provides a synthetic method of the N-quinolyl substituted beta-lactam compound, a pharmaceutical composition containing the compound, as well as application of the compound in medicine preparation of beta-lactamase inhibitors.
Owner:KUNMING INST OF BOTANY - CHINESE ACAD OF SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com