Enzyme for catalyzing formaldehyde to synthesize hydroxyl acetaldehyde and application thereof
A formaldehyde and acetyl coenzyme technology, applied in the biological field, can solve problems that are difficult to apply
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Embodiment 1, the preparation of BFD mutant
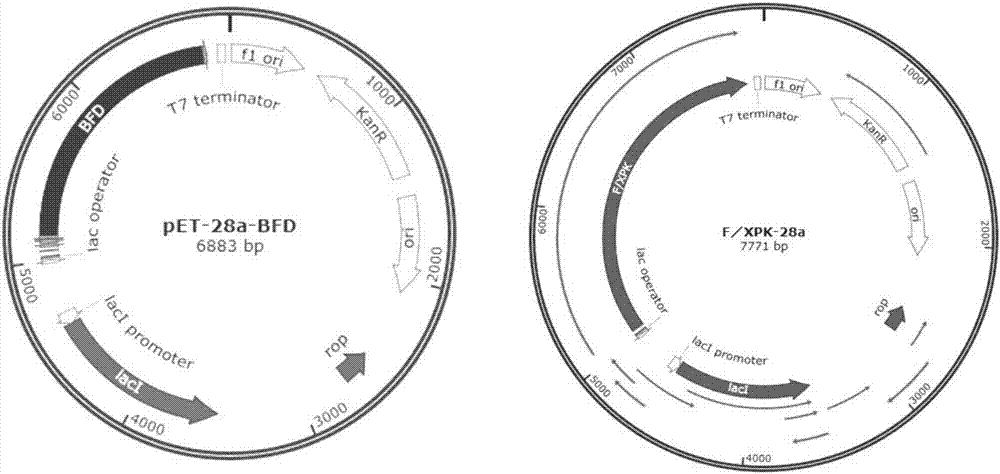
[0035] The species source of the gene encoding the BFD enzyme is Pseudomonas putida, the amino acid of the BFD enzyme is shown in sequence 1, and the nucleotide sequence of the gene encoding the BFD enzyme is shown in sequence 2.
[0036] Single-point or multiple-point mutations were performed on the BFD enzyme to obtain the BFD mutants shown in Table 1.
[0037] The BFD mutant W86R-N87T is the mutation of tryptophan (Trp) at position 86 to arginine (Arg) in the BFD amino acid sequence (sequence 1), and the mutation of asparagine (Asn) at position 87 to threonine. Amino acid (Thr), other amino acid residues remain unchanged, the resulting sequence.
[0038] The coding gene of BFD mutant W86R-N87T is that the tgg at the 256-258th position of the BFD enzyme coding gene nucleotide sequence (sequence 2) is mutated into cgt, and the aac at the 259-261st position is mutated into acc, other bases Basis remains the same, the resul...
Embodiment 2
[0067] Example 2, Functional detection of BFD mutant protein
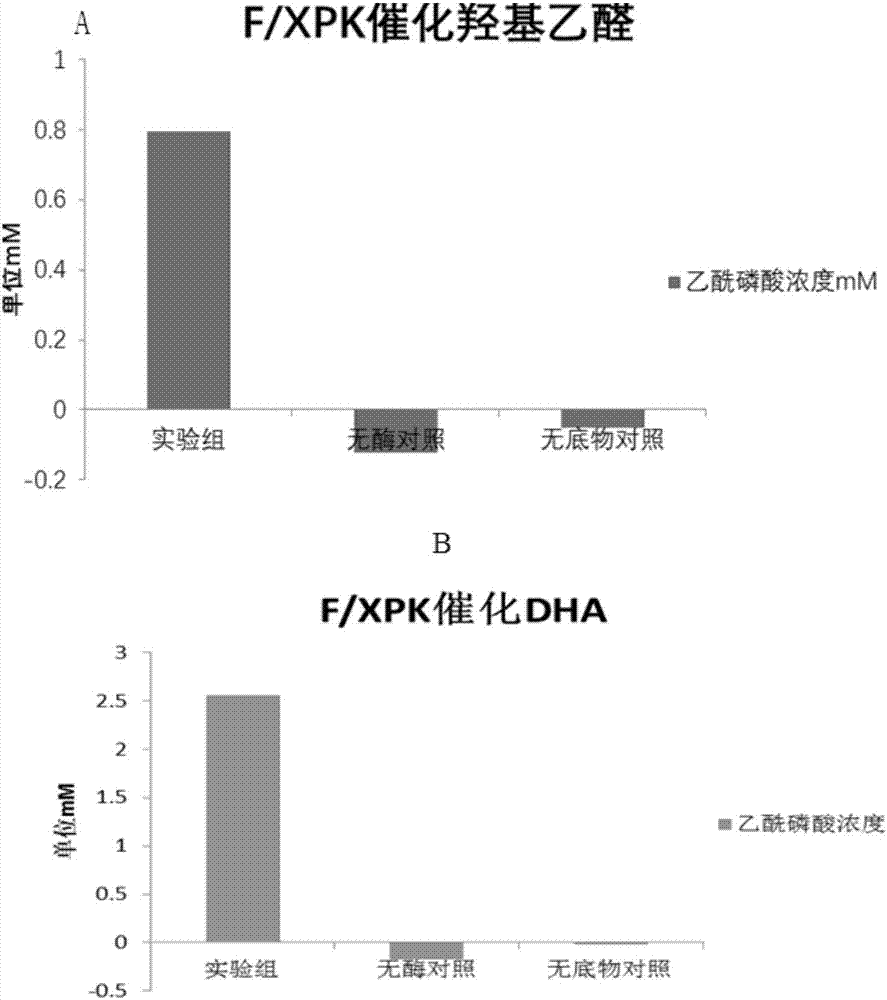
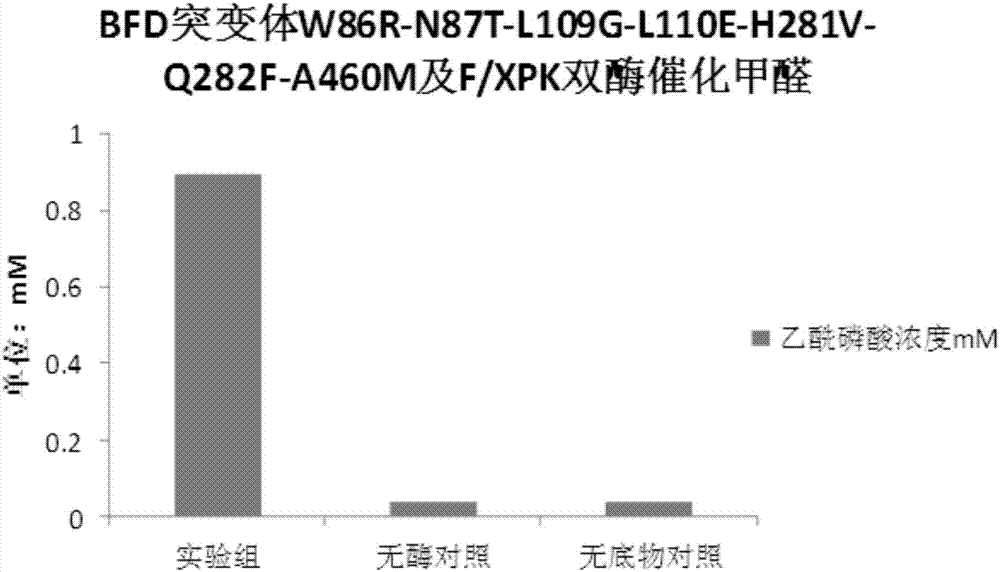
[0068] 1. BFD mutant protein catalyzes the condensation of formaldehyde to glycolaldehyde and 1,3-dihydroxyacetone
[0069] Detection method: The recombinant bacteria expressing BFD enzyme prepared in Example 1, the recombinant bacteria expressing BFD mutants and the recombinant bacteria expressing F / XPK were cultured in 200ml 2YT 37°C to OD 600 =0.6, 0.5mM IPTG induced at 16°C for 18h, centrifuged at 3500rpm for 15min, the medium was washed away with protein buffer, the cells were collected, and then resuspended in 20ml of protein buffer added with 0.5mM TPP. Take 500ul of resuspended bacteria and add 500ul of formaldehyde solution containing 0.5mM TPP with a final concentration of 5g / L prepared from protein buffer, react at 37°C and 750rpm for 2h, centrifuge to take the supernatant, and perform liquid phase detection. AMINEXHPX-87H, 300X7.8MM column, 5mM sulfuric acid was used as the mobile phase for liquid phas...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com