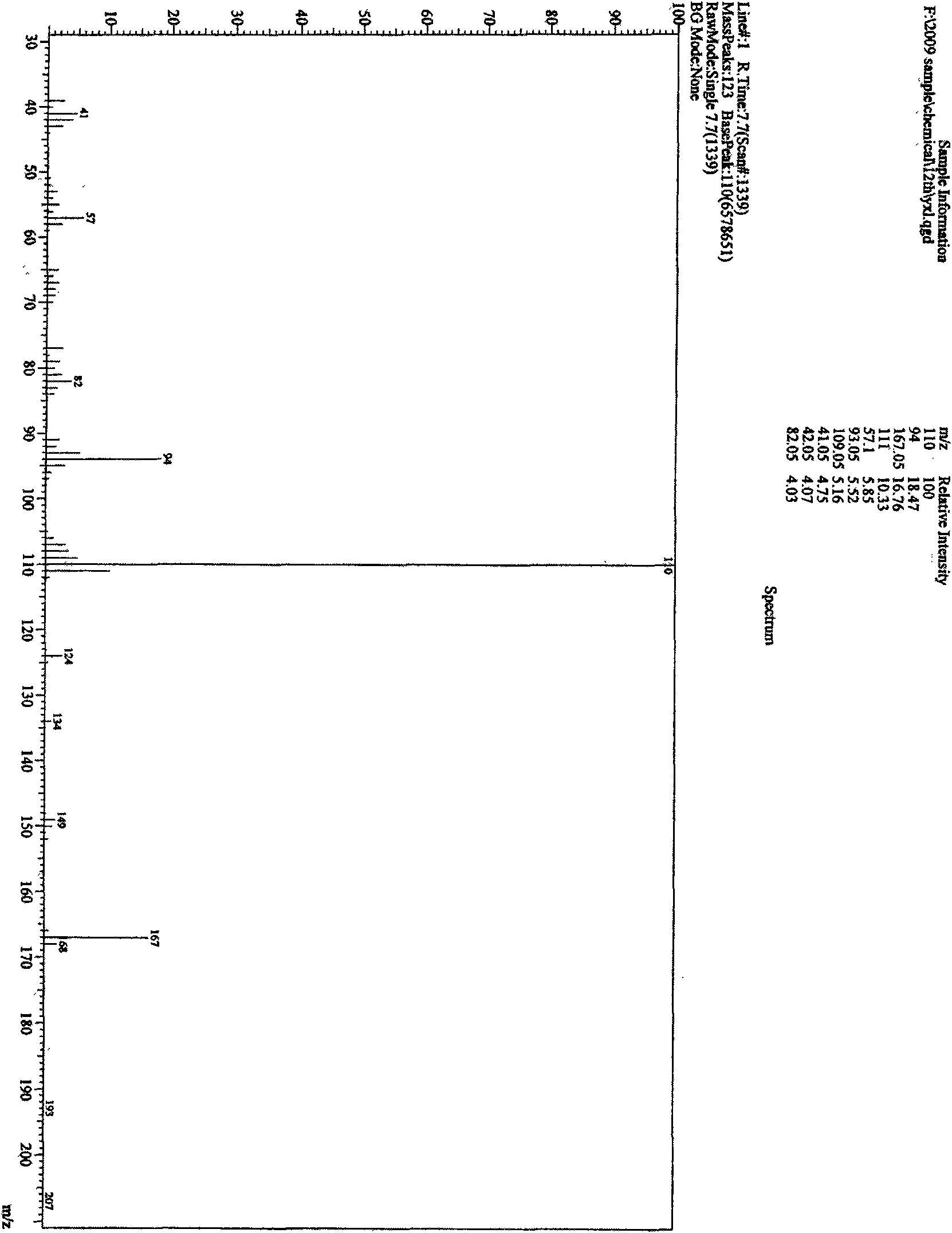
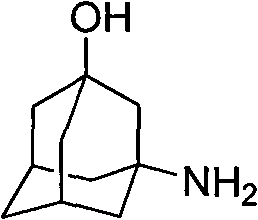
Method for preparing 3-amino-1-adamantane alcohol
A technology for amantadine alcohol and amantadine hydrochloride is applied in the preparation of aminohydroxy compounds, the preparation of organic compounds, chemical instruments and methods, etc. The effect of three waste treatment, reducing consumption and reducing cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] (1) 3-nitro-1-adamantanamine
[0027]
[0028] Take 14.0mL (0.21mol) HNO 3 Put it into an ice-water bath, then add 12mL (0.21mol) of sulfuric acid, 21.1g (0.21mol) of potassium nitrate, stir and cool to -5°C, add 20.0g (0.11mol) of amantadine hydrochloride in batches within 30min, and react in an ice-water bath After 1 hour, continue to react at room temperature for 30 hours to obtain a light yellow liquid; then pour it into 100 mL of ice water and continue stirring for 30 minutes to obtain a blue-green liquid;
[0029] (2) 3-Amino-1-adamantanol
[0030]
[0031] Under mechanical stirring, add 48.0 g (0.85 mol) KOH in batches to the blue-green solution prepared in step (1), keep the temperature below 80 ° C, continue stirring for 1 h after the addition, filter with suction, wash with dichloromethane and extract two Next, the organic layer was taken, dried over anhydrous sodium sulfate, and evaporated to dryness with a rotary evaporator to obtain a white solid, w...
Embodiment 2
[0039] (1) 3-nitro-1-adamantanamine
[0040] Take nitric acid 20.0mL (0.28mmol) HNO 3Add it into an ice-water bath, then add 30mL (0.56mmol) of sulfuric acid, add a solution of 23.8g (0.28mol) of sodium nitrate and 40mL of water, stir and cool to -5°C, add 52.4g (0.28mL) of amantadine hydrochloride in batches within 30min mol), after 2 hours of reaction in an ice-water bath, the reaction was continued at room temperature for 30 hours to obtain a light yellow liquid; then poured into 150 mL of ice water and continued to stir for 30 minutes to obtain a blue-green liquid;
[0041] (2) 3-Amino-1-adamantanol
[0042] Under mechanical stirring, add 47.0 g (0.84 mol) KOH in batches to the blue-green solution obtained in (1), keep the temperature below 80 ° C, continue stirring for 1 h after the addition, filter with suction, wash with dichloromethane and extract two Next, the organic layer was taken, dried over anhydrous sodium sulfate, and evaporated to dryness with a rotary evapo...
Embodiment 3
[0044] Potassium nitrate was used to replace sodium nitrate in step (1) of Example 2, and nitration and hydroxylation reactions were carried out to obtain 3-amino-1-adamantanol with a yield of 69%, mp: 266-269°C.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com