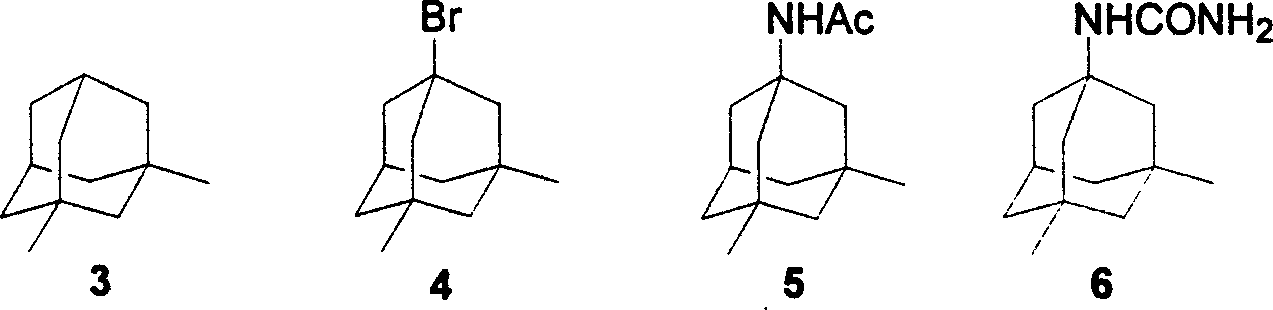Patents
Literature
119 results about "Memantine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
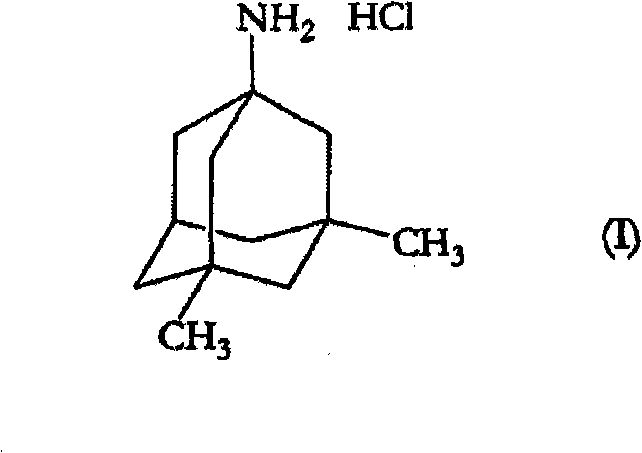
Memantine is used to treat moderate to severe confusion (dementia) related to Alzheimer's disease.
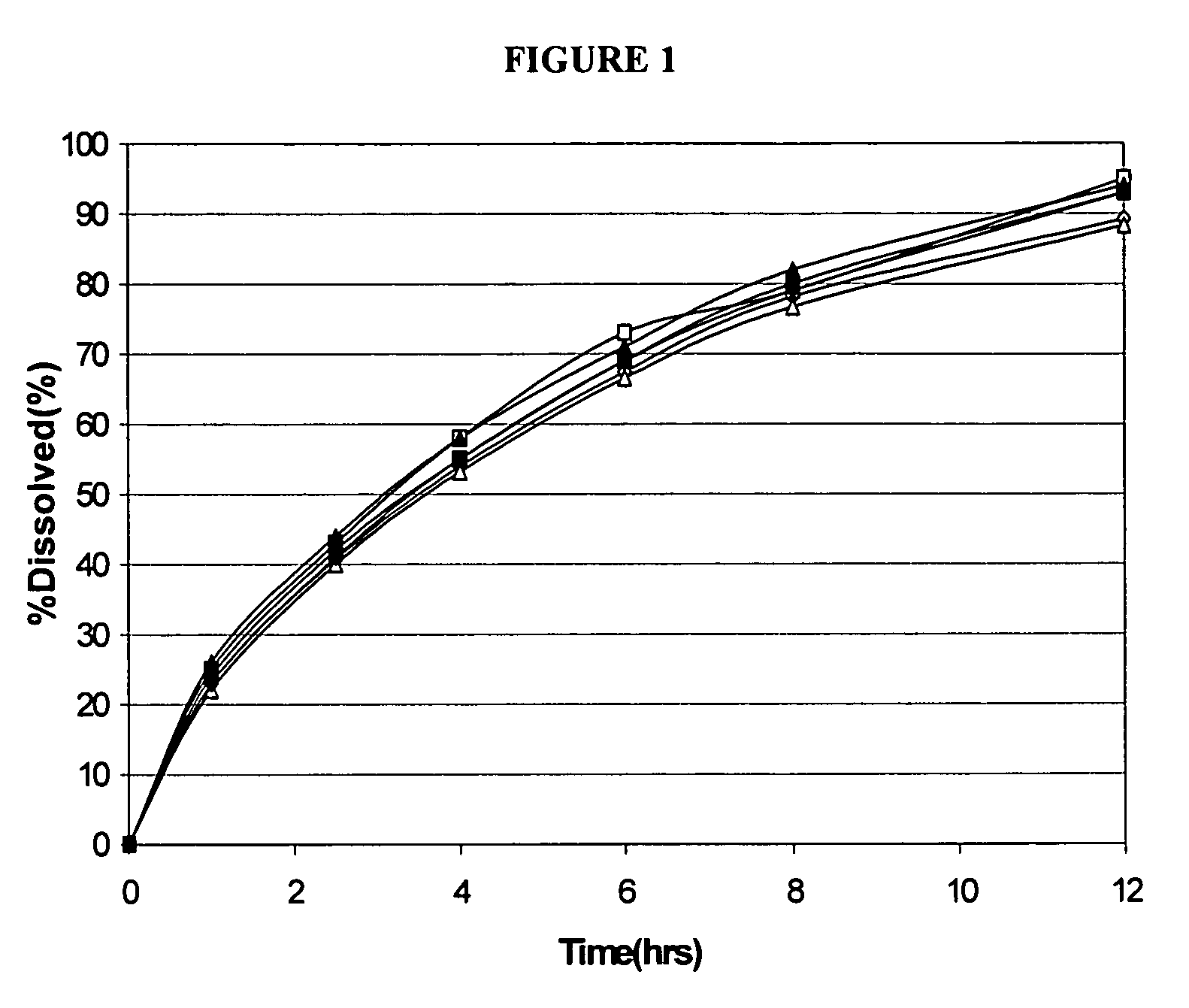
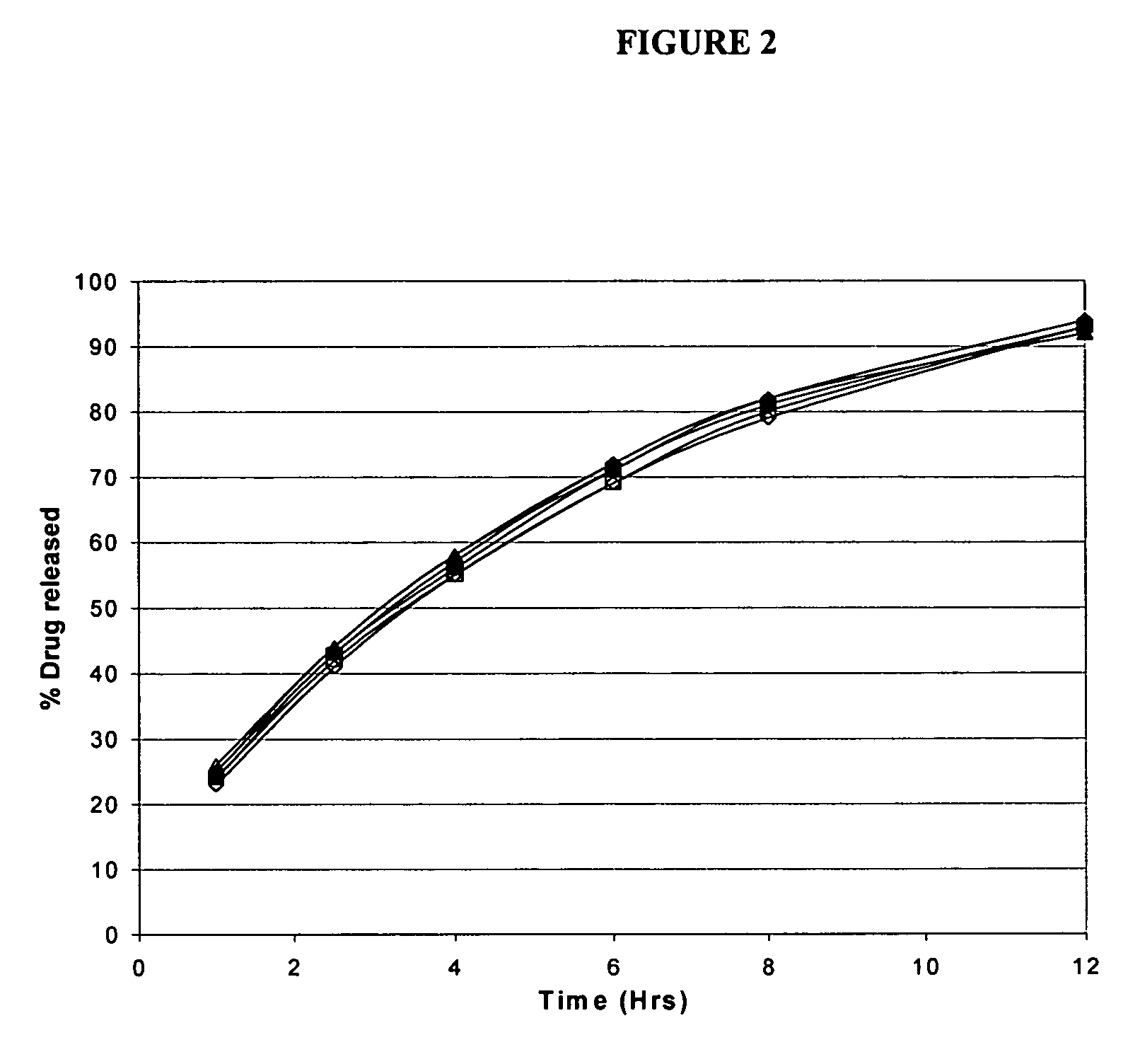
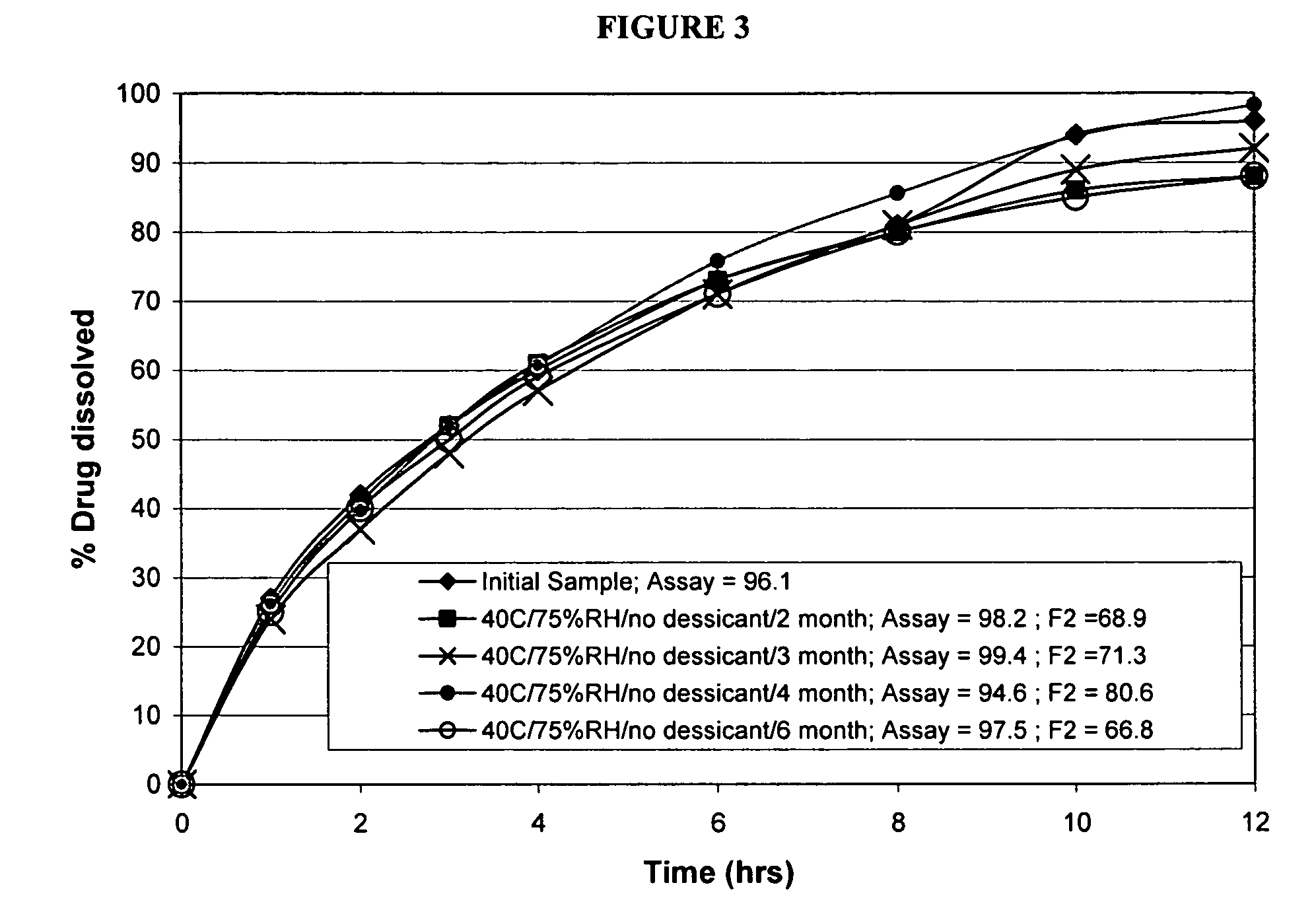
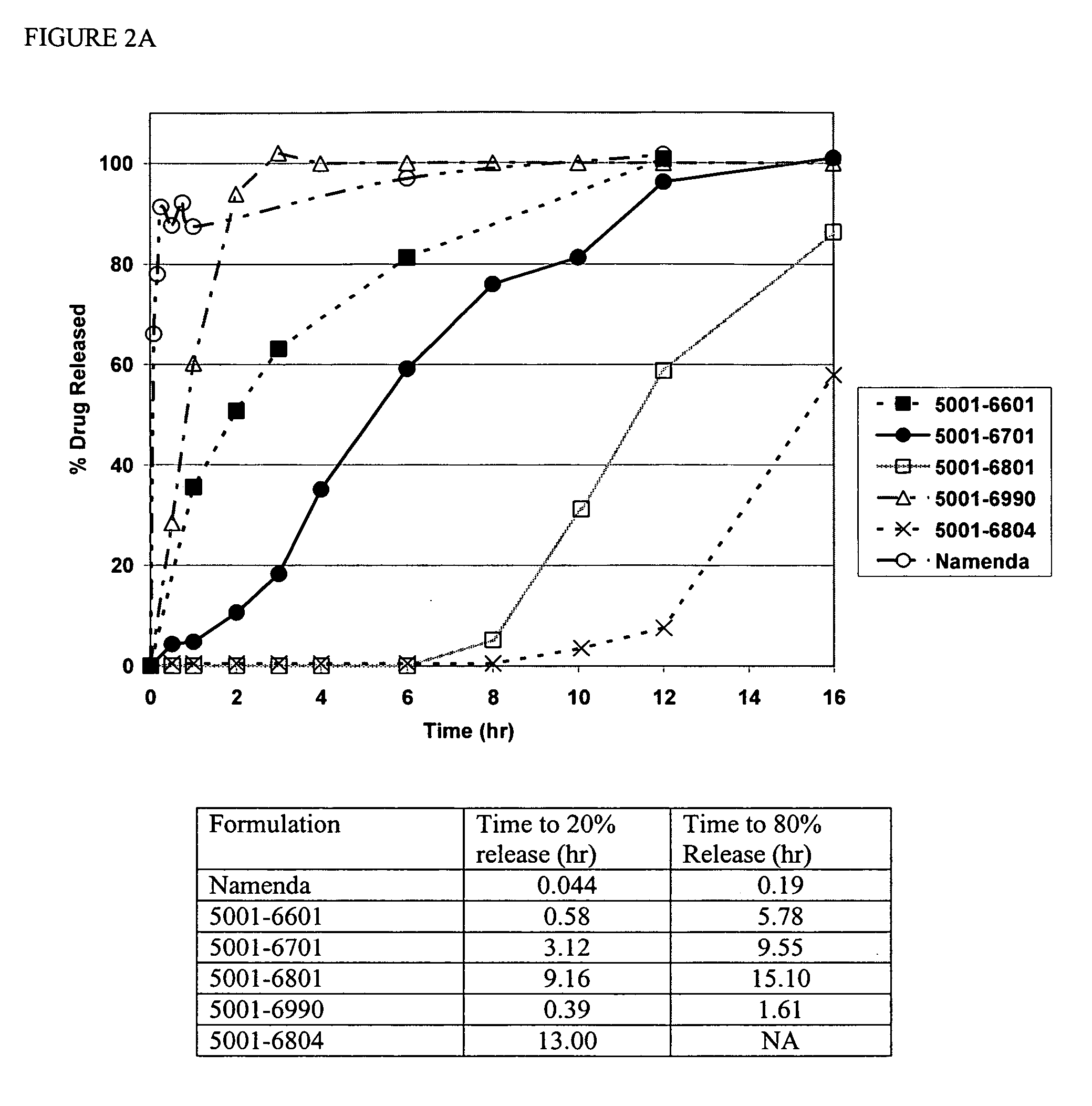
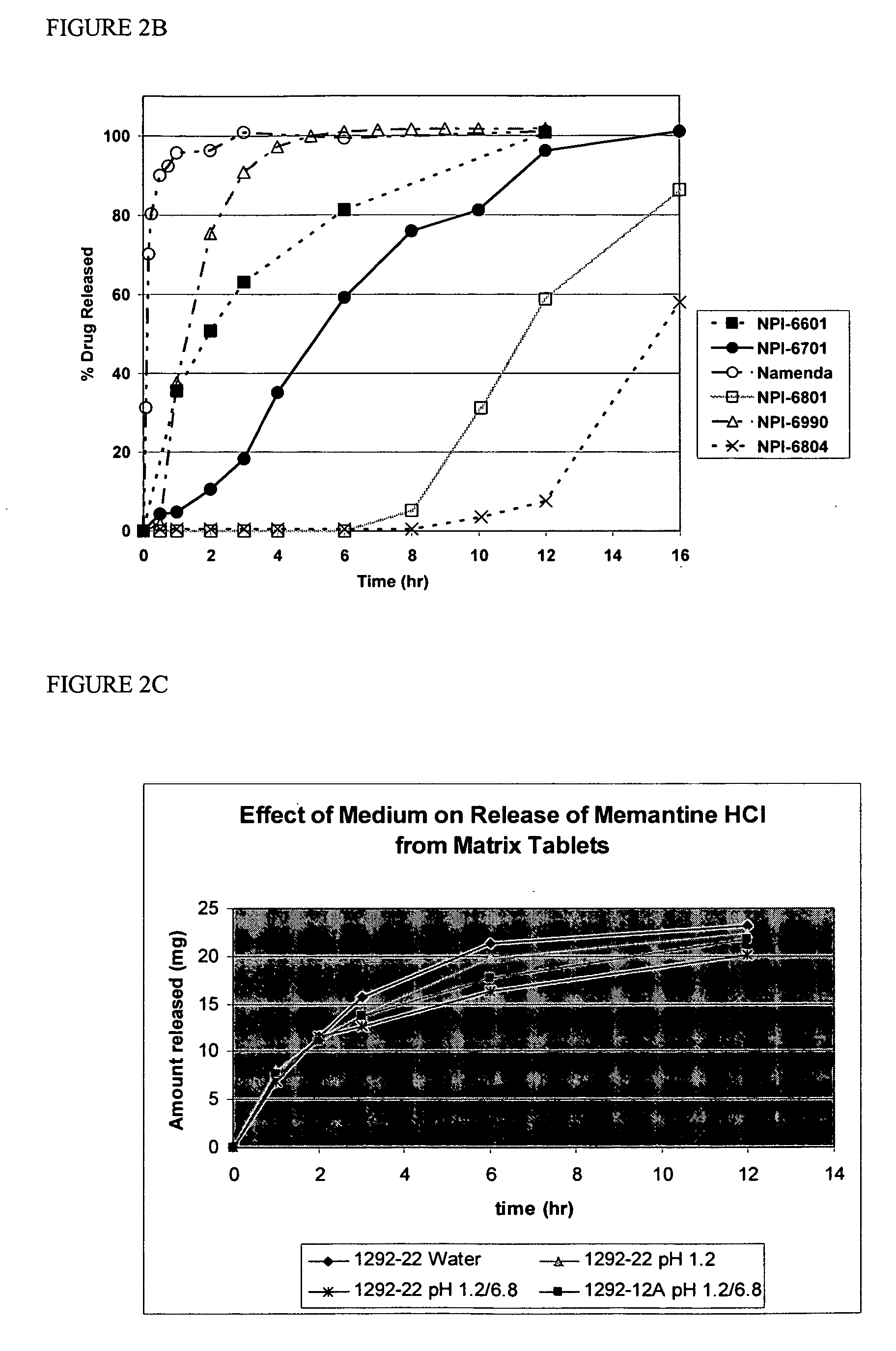
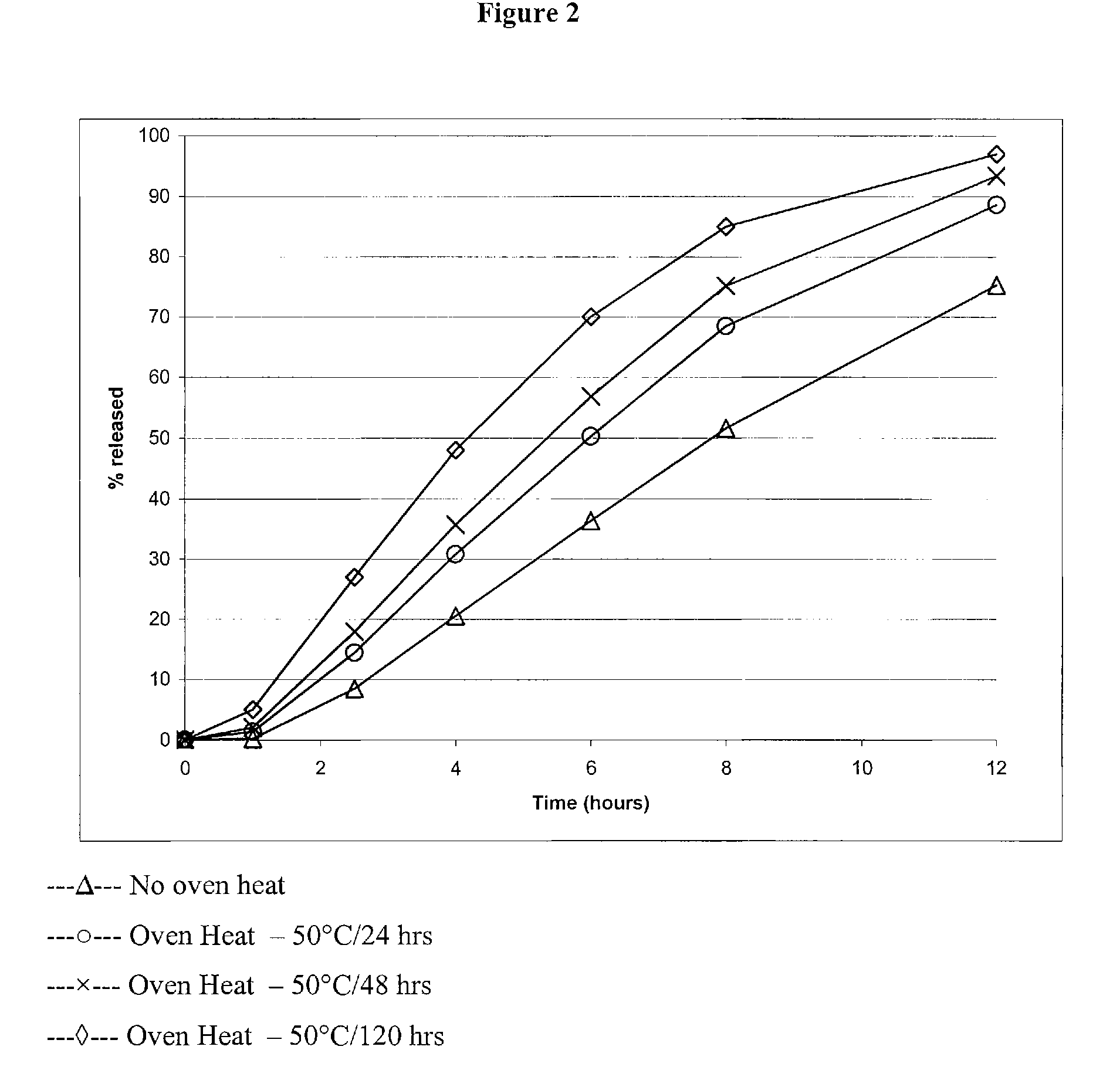
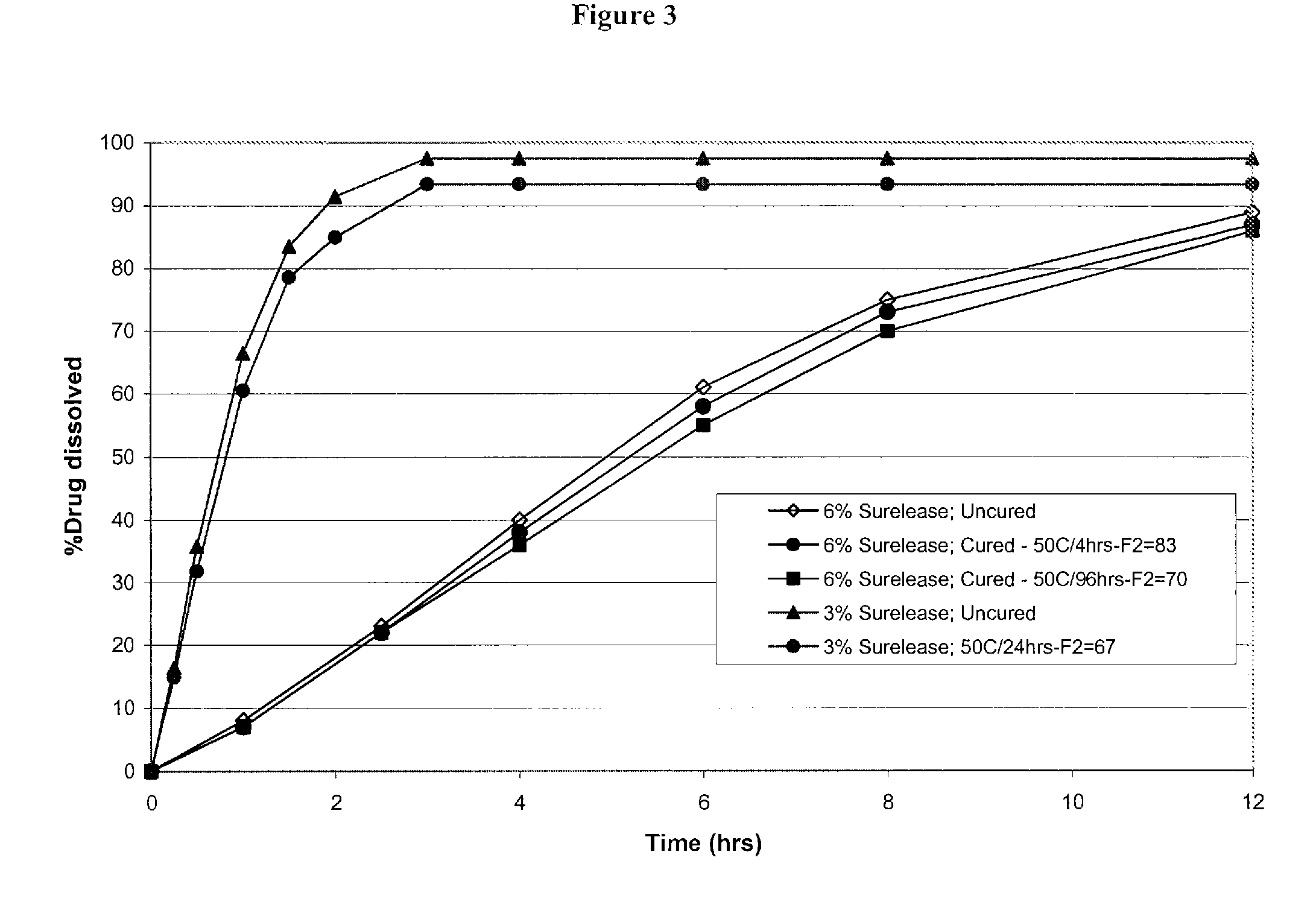
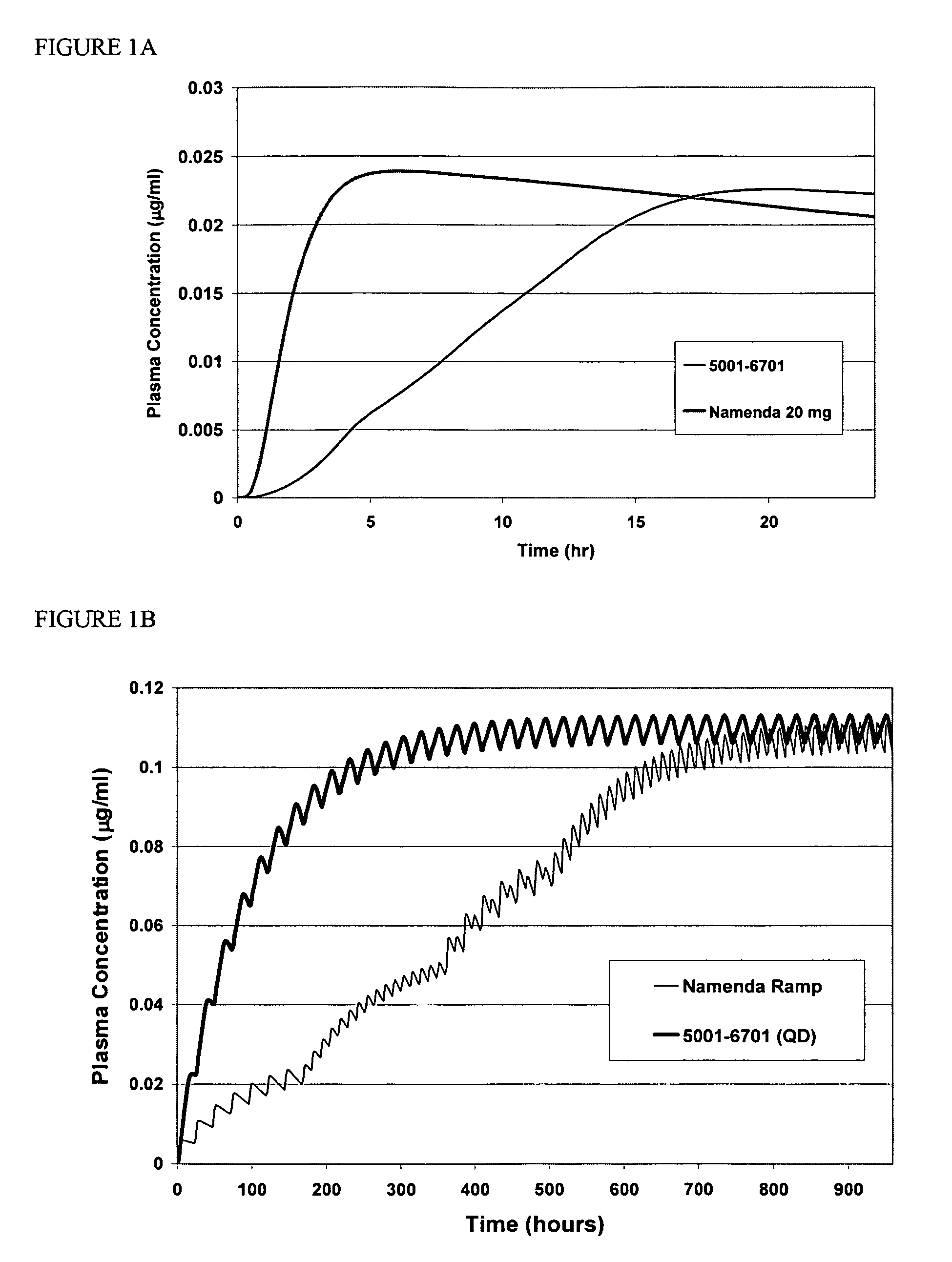
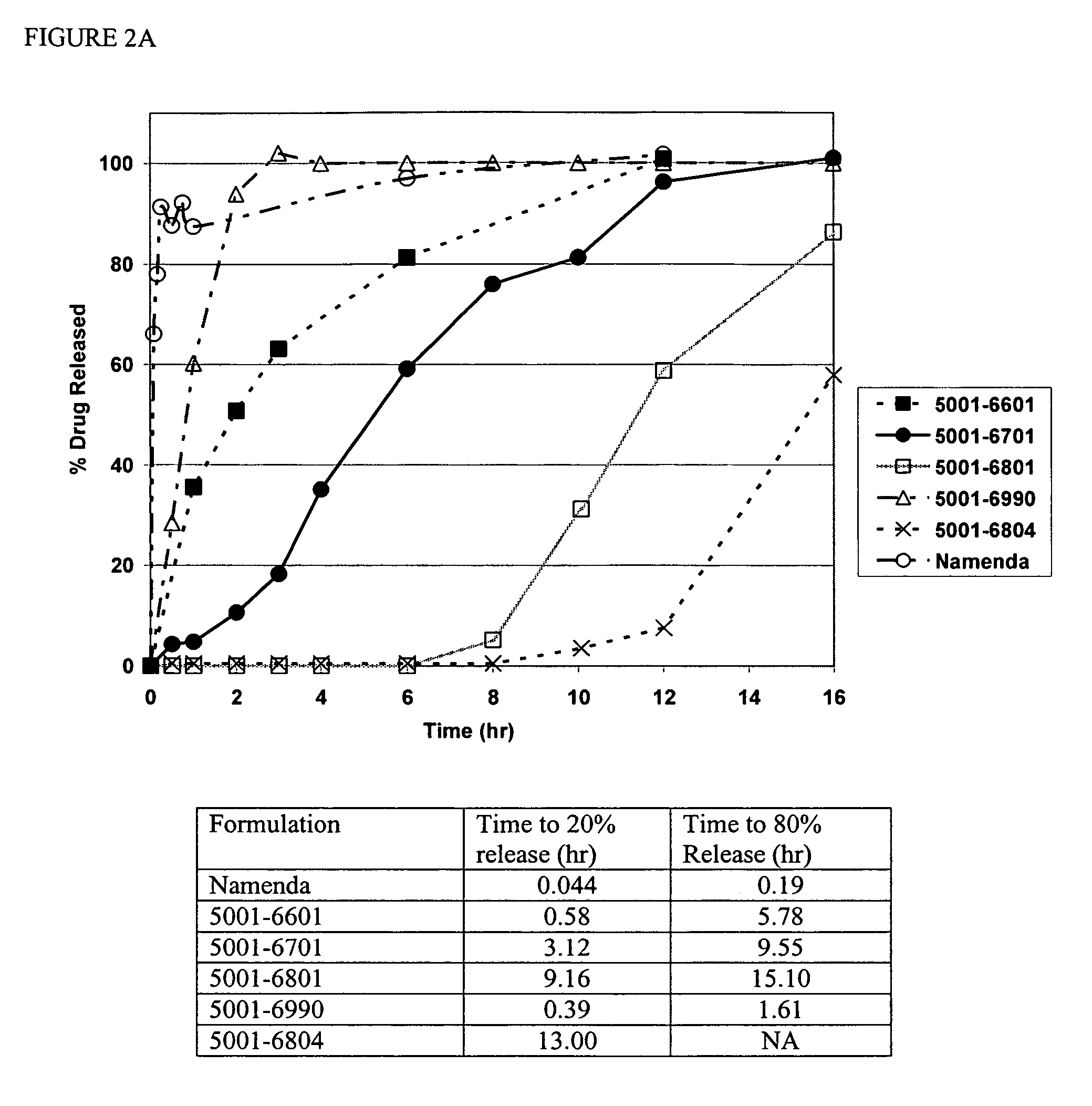
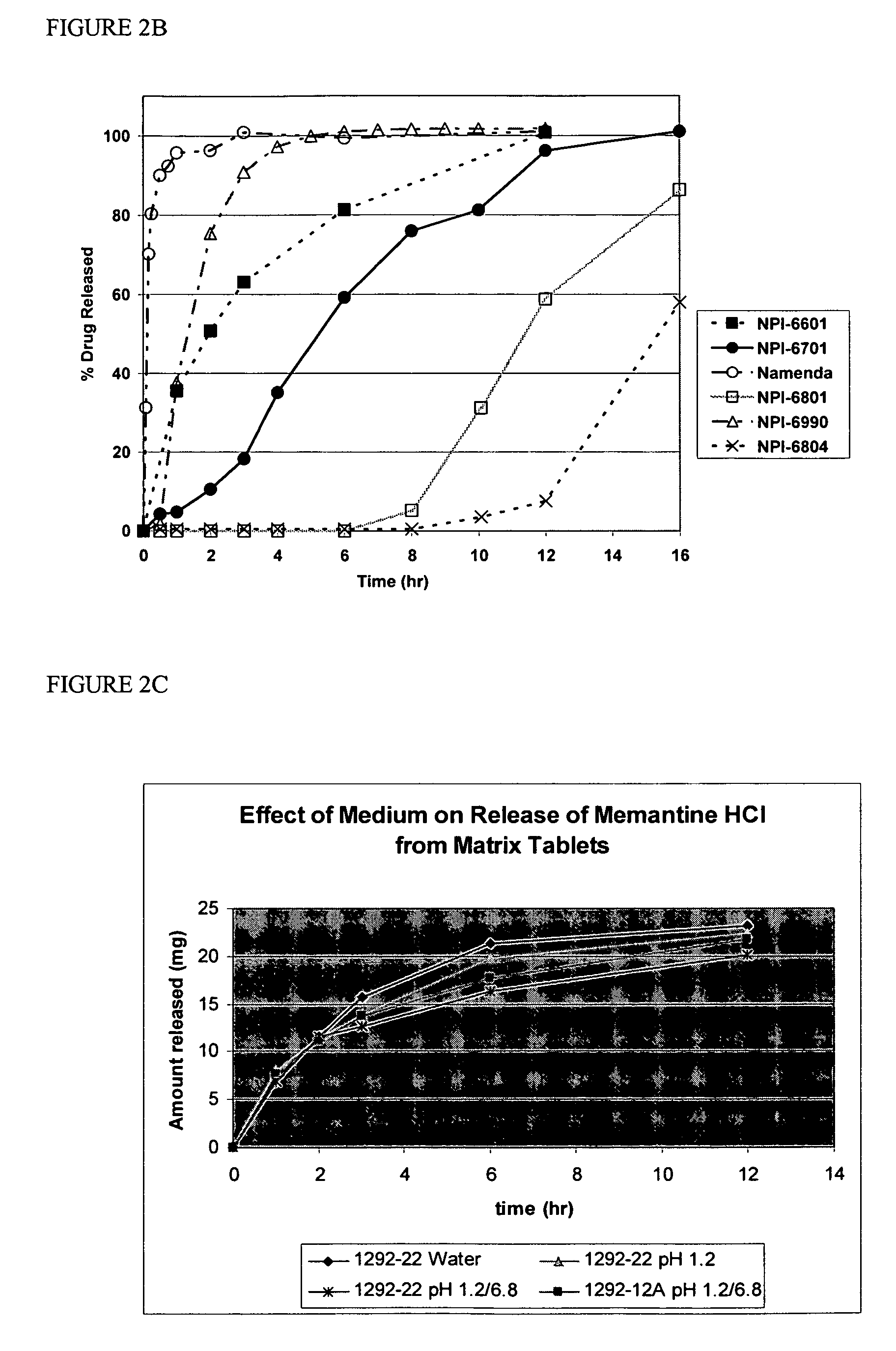
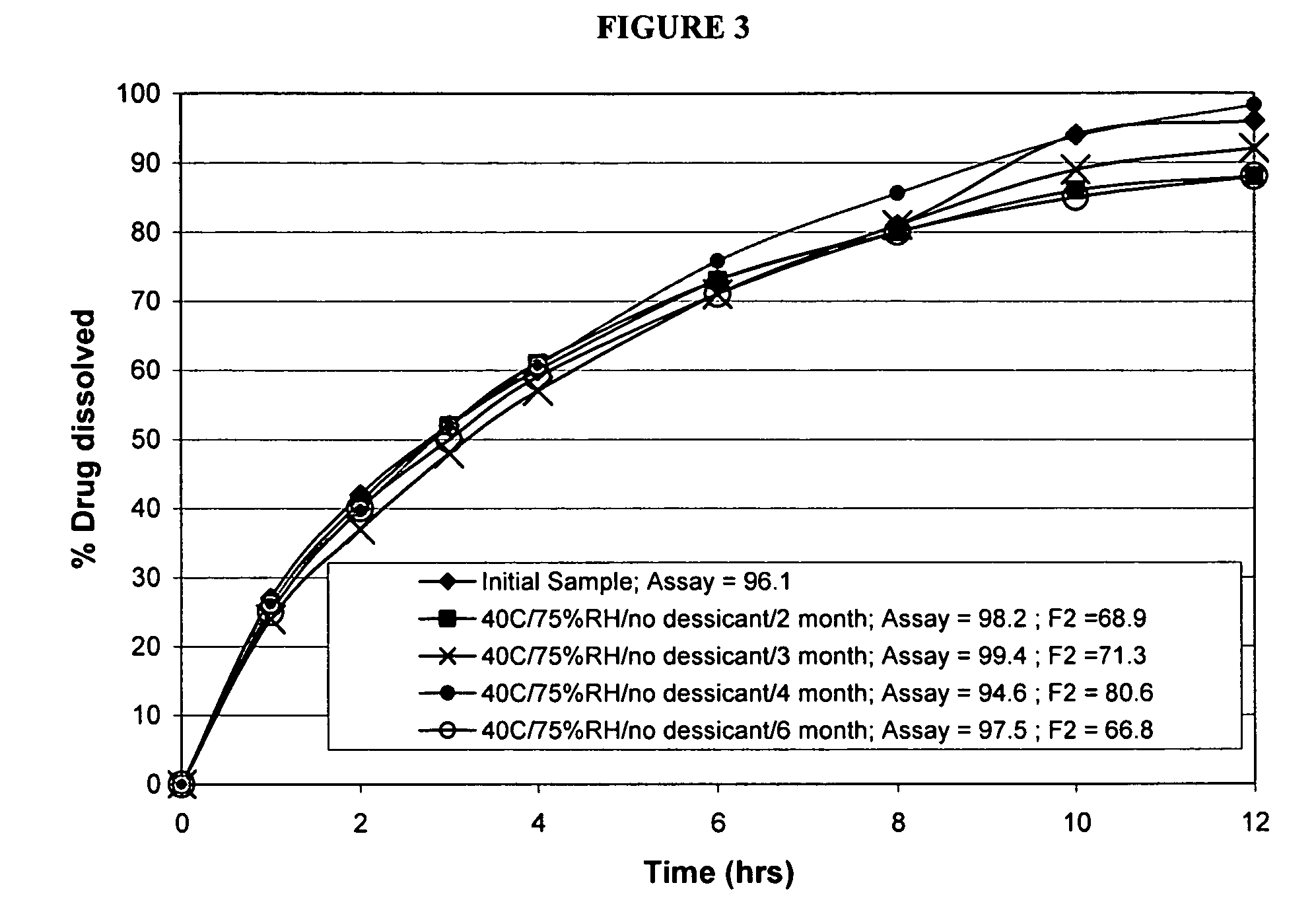
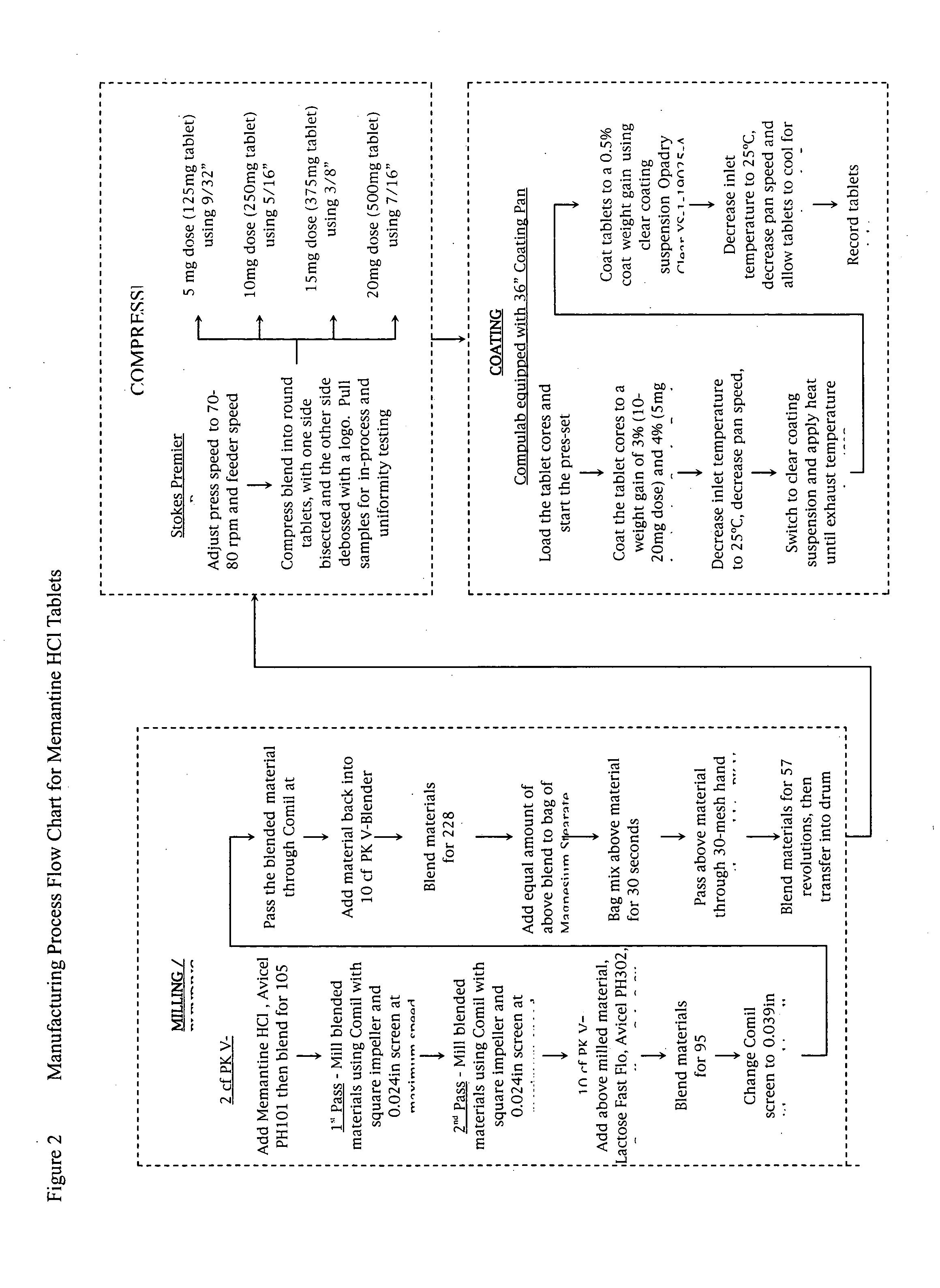
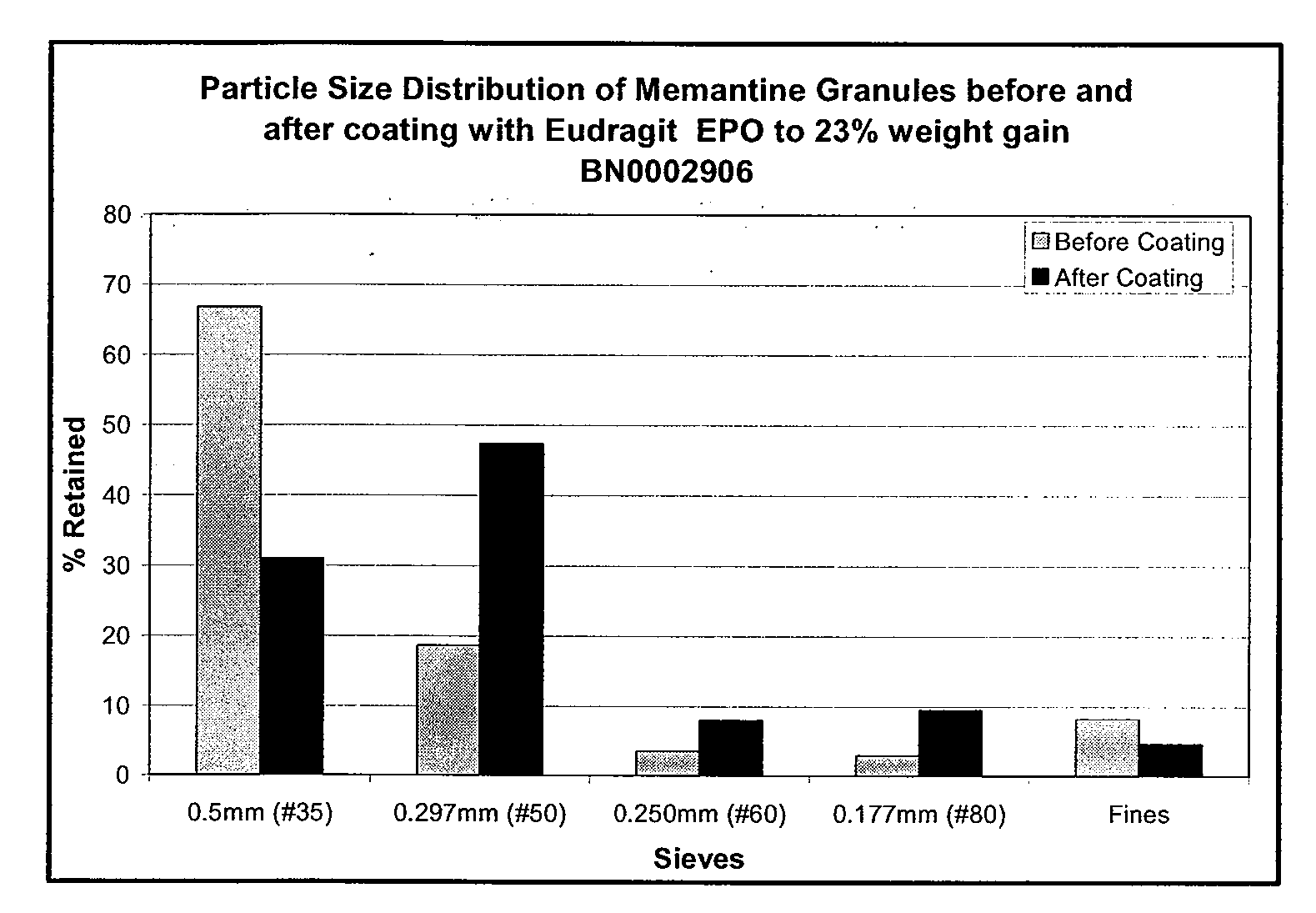
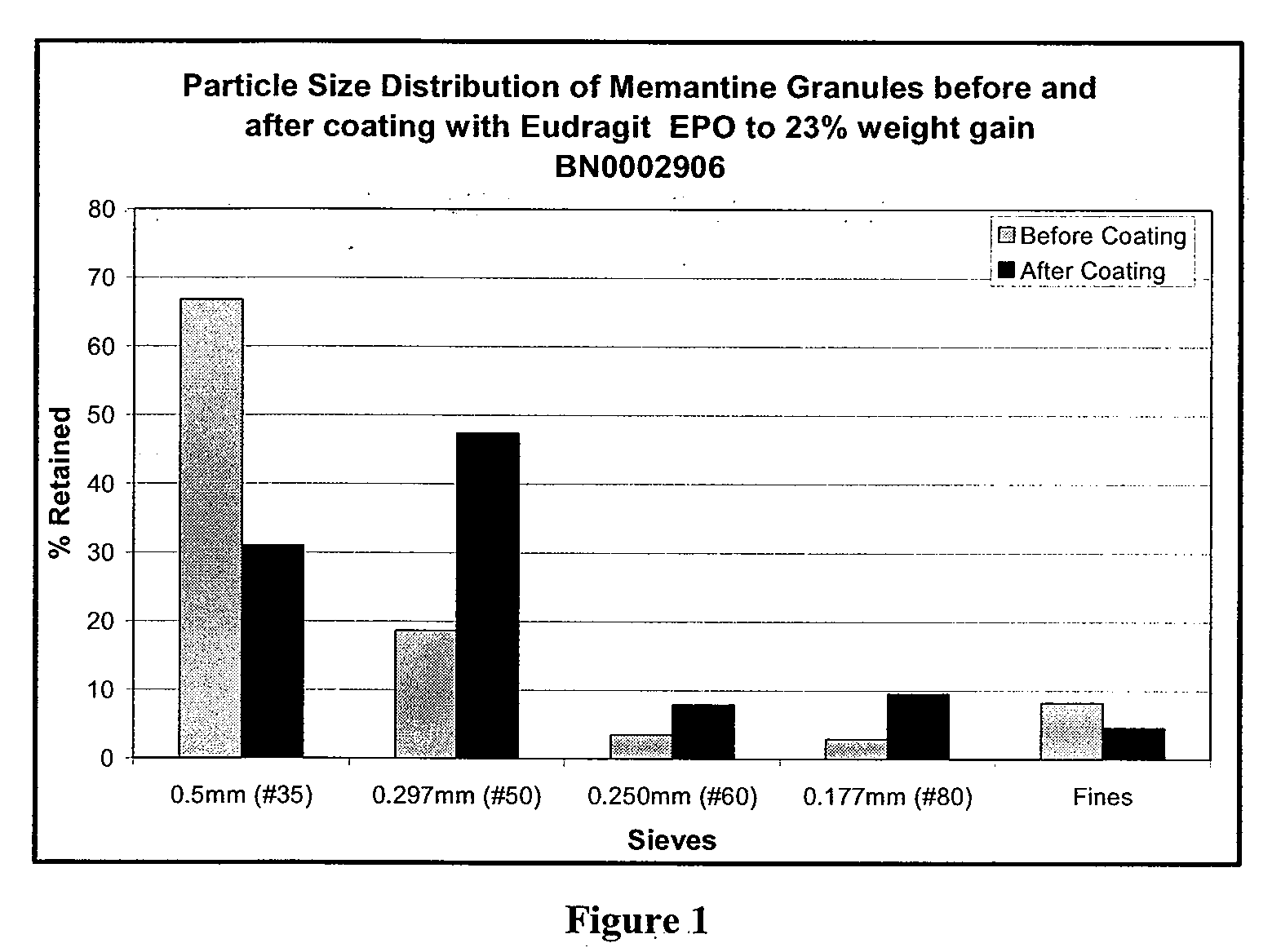
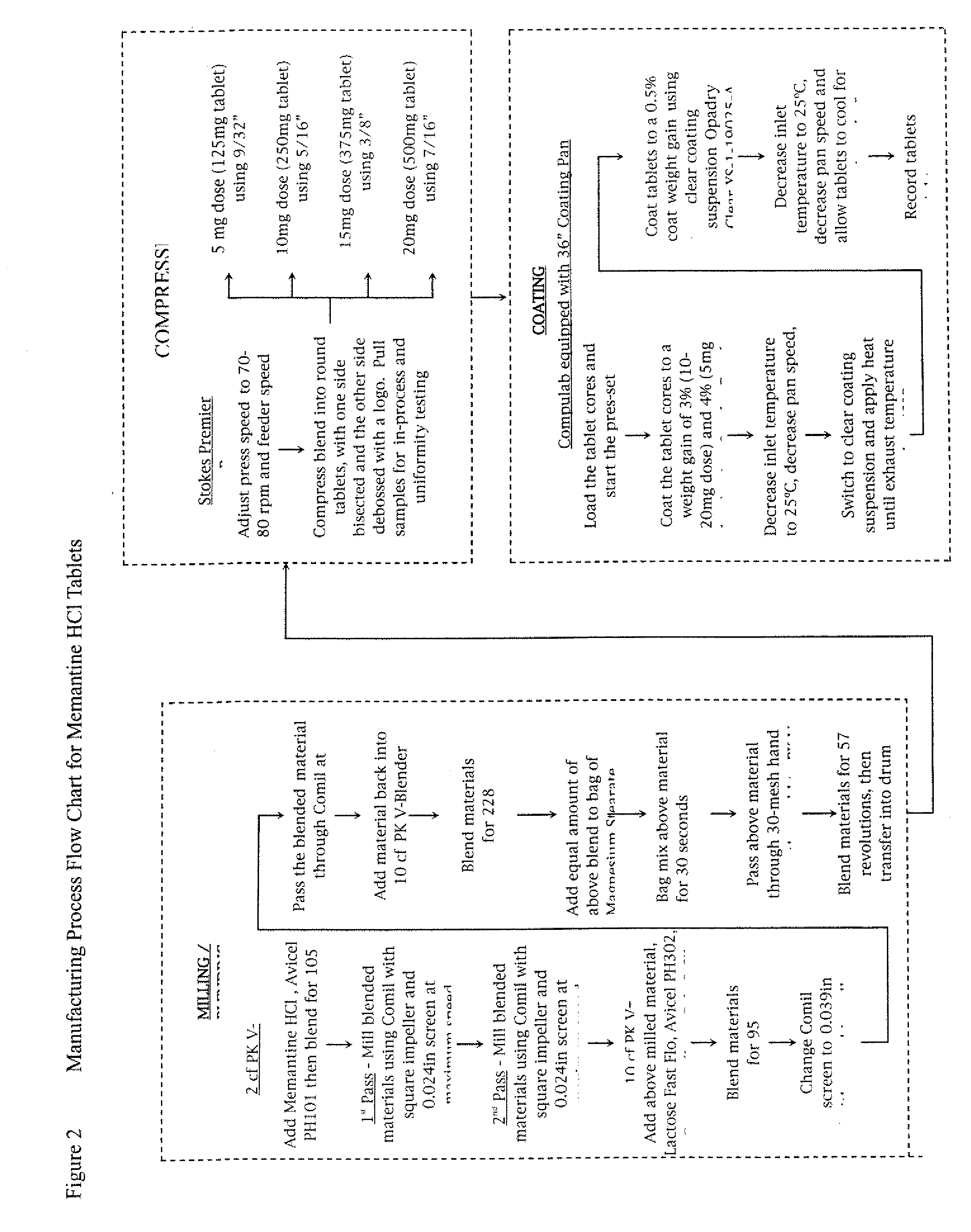
Modified release formulations of memantine oral dosage forms
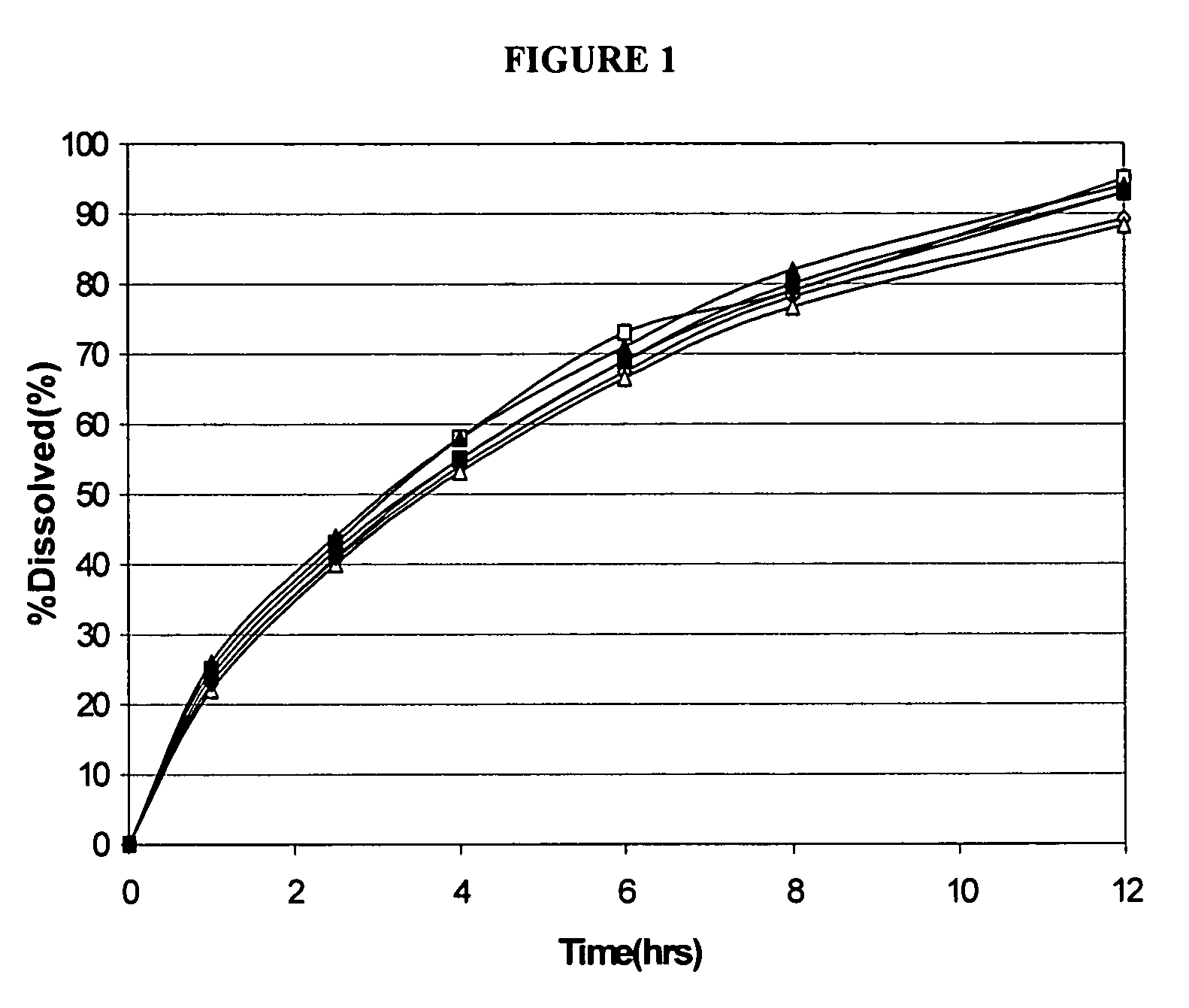
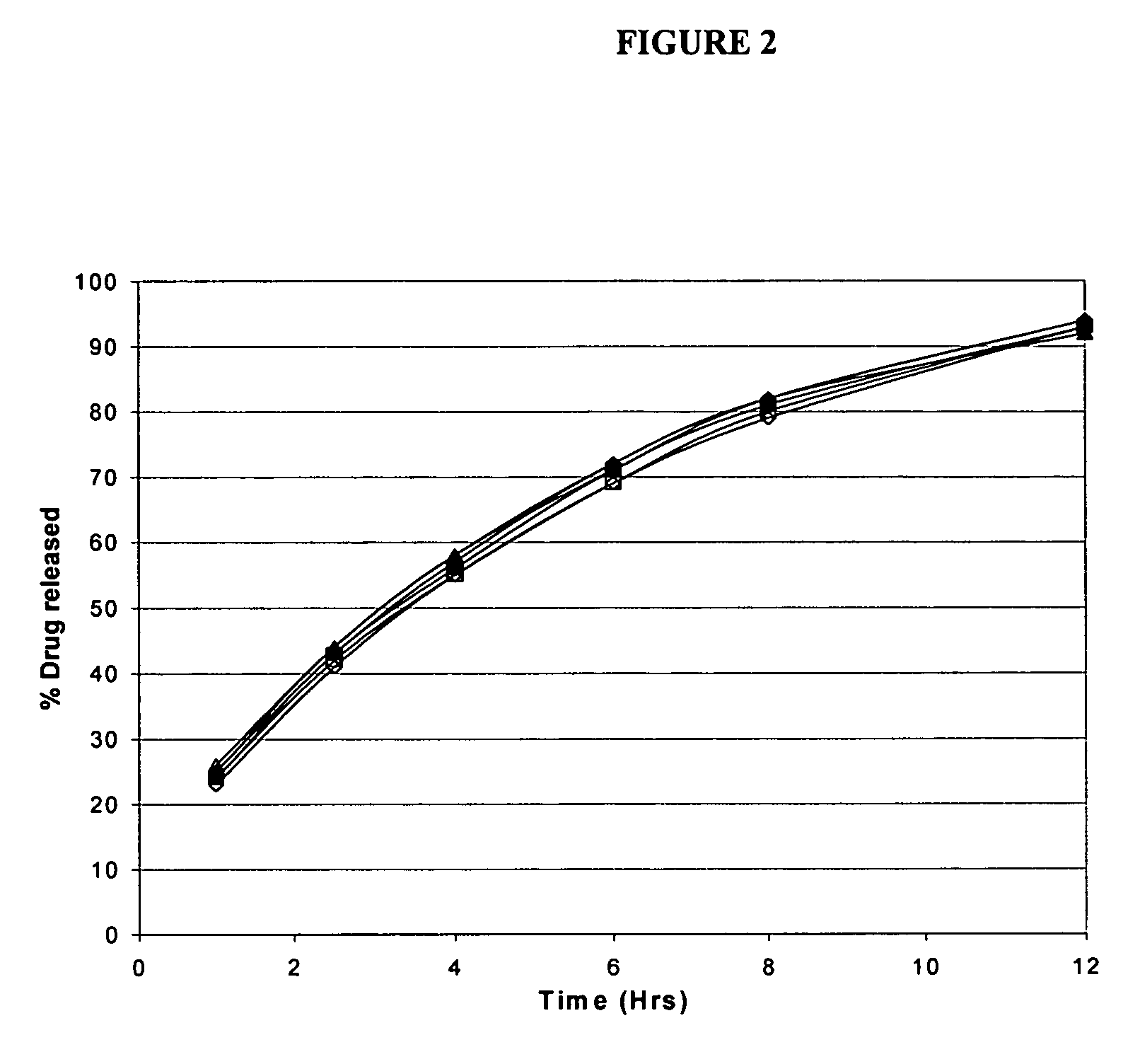
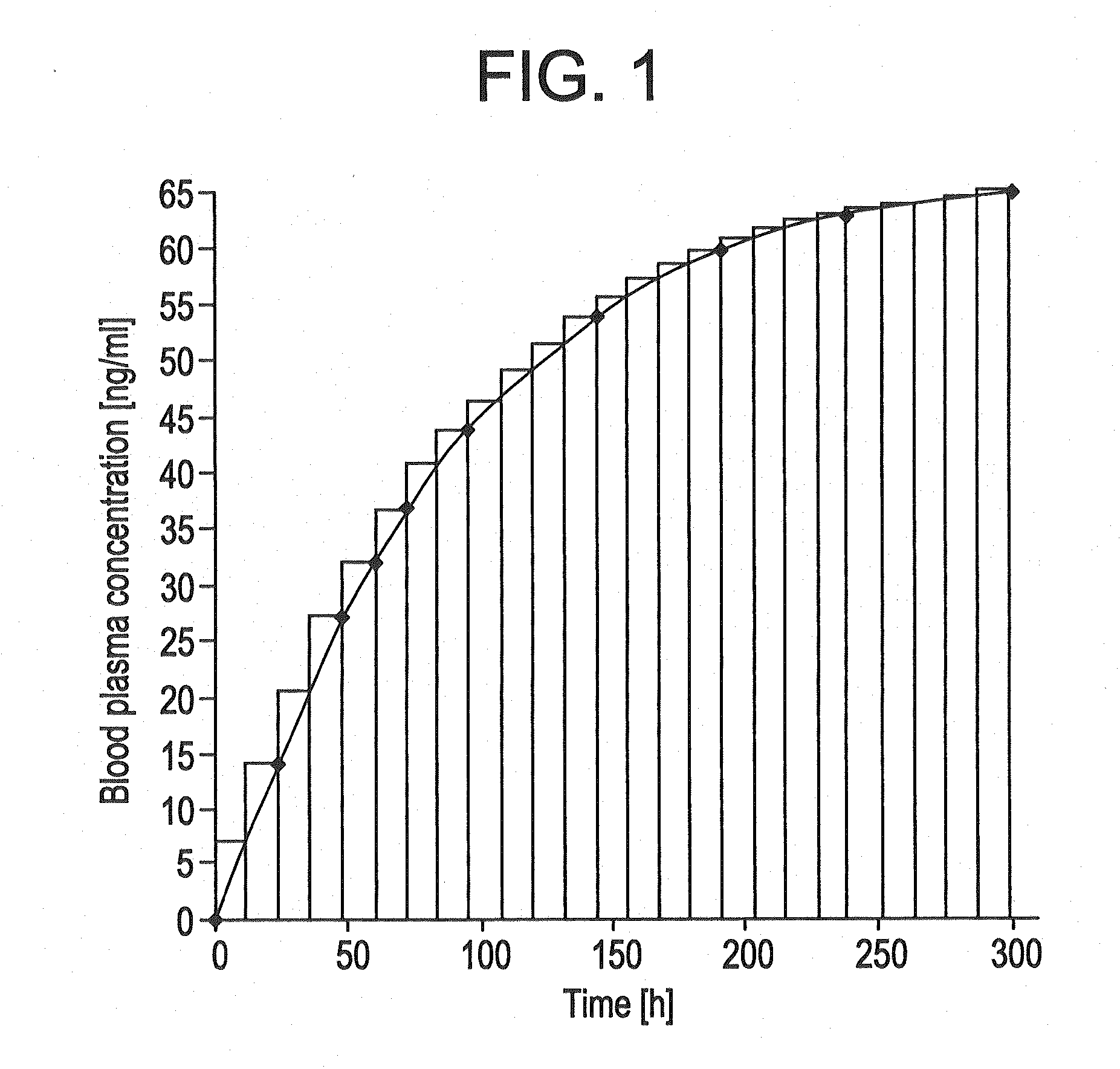
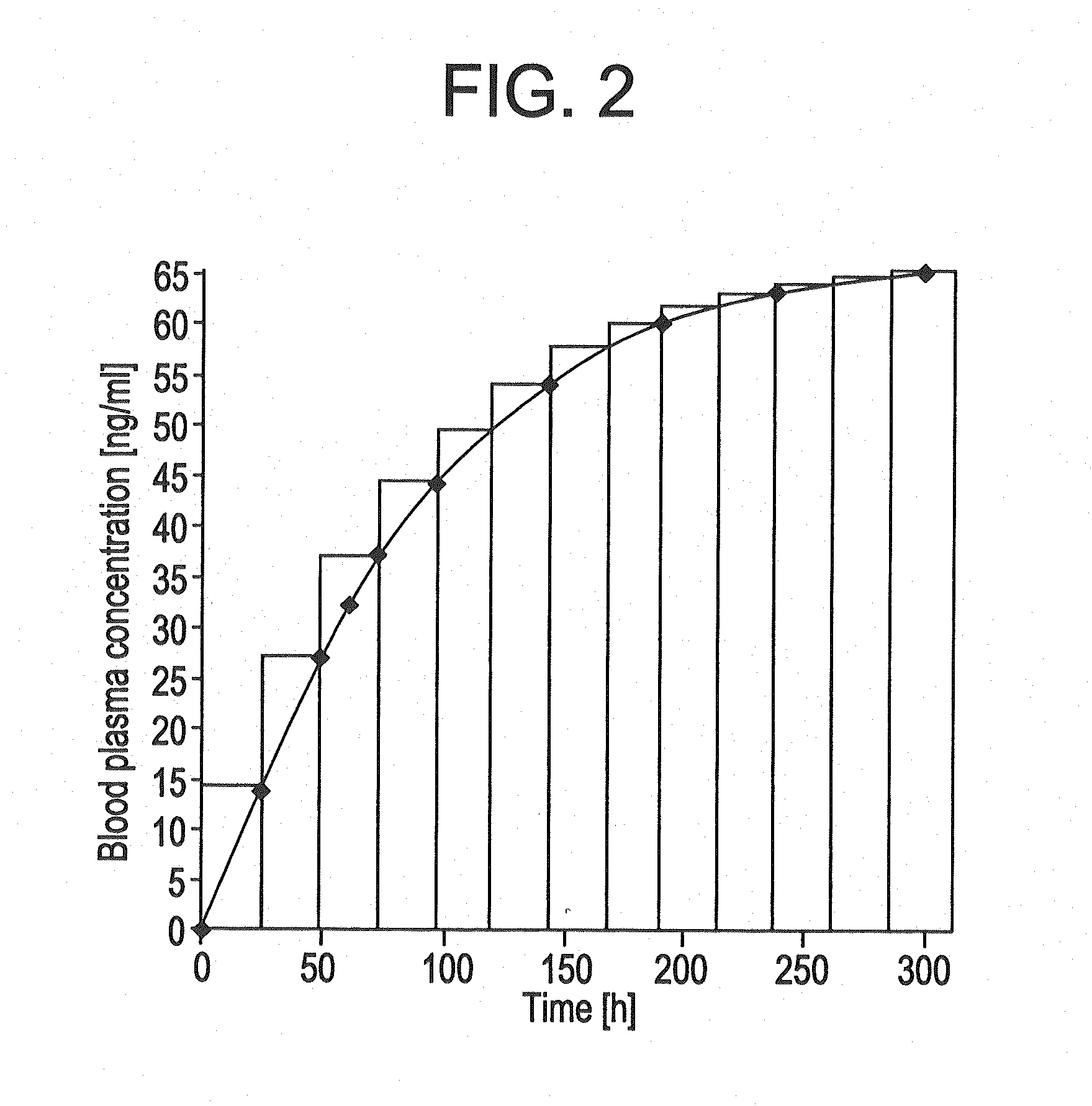
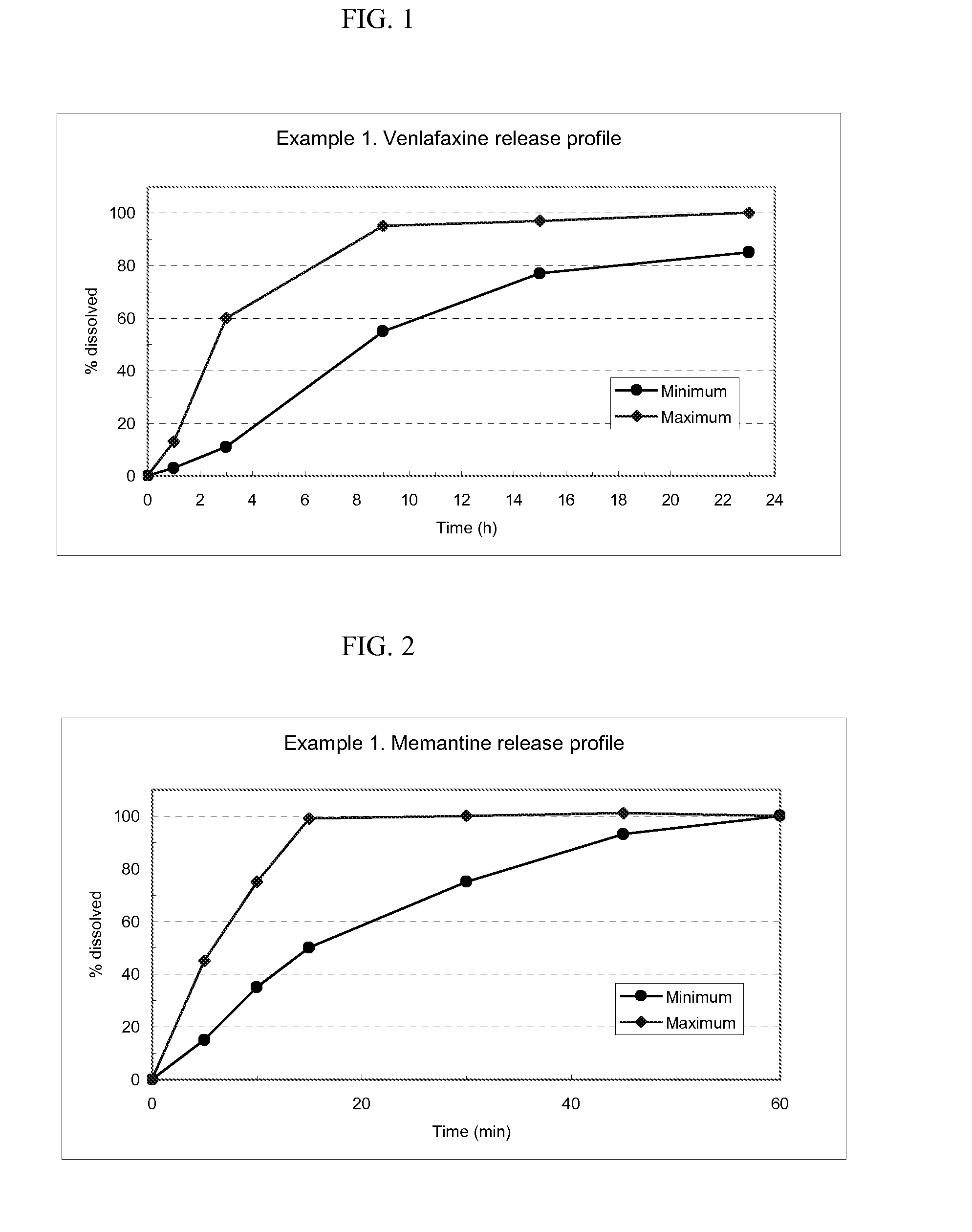
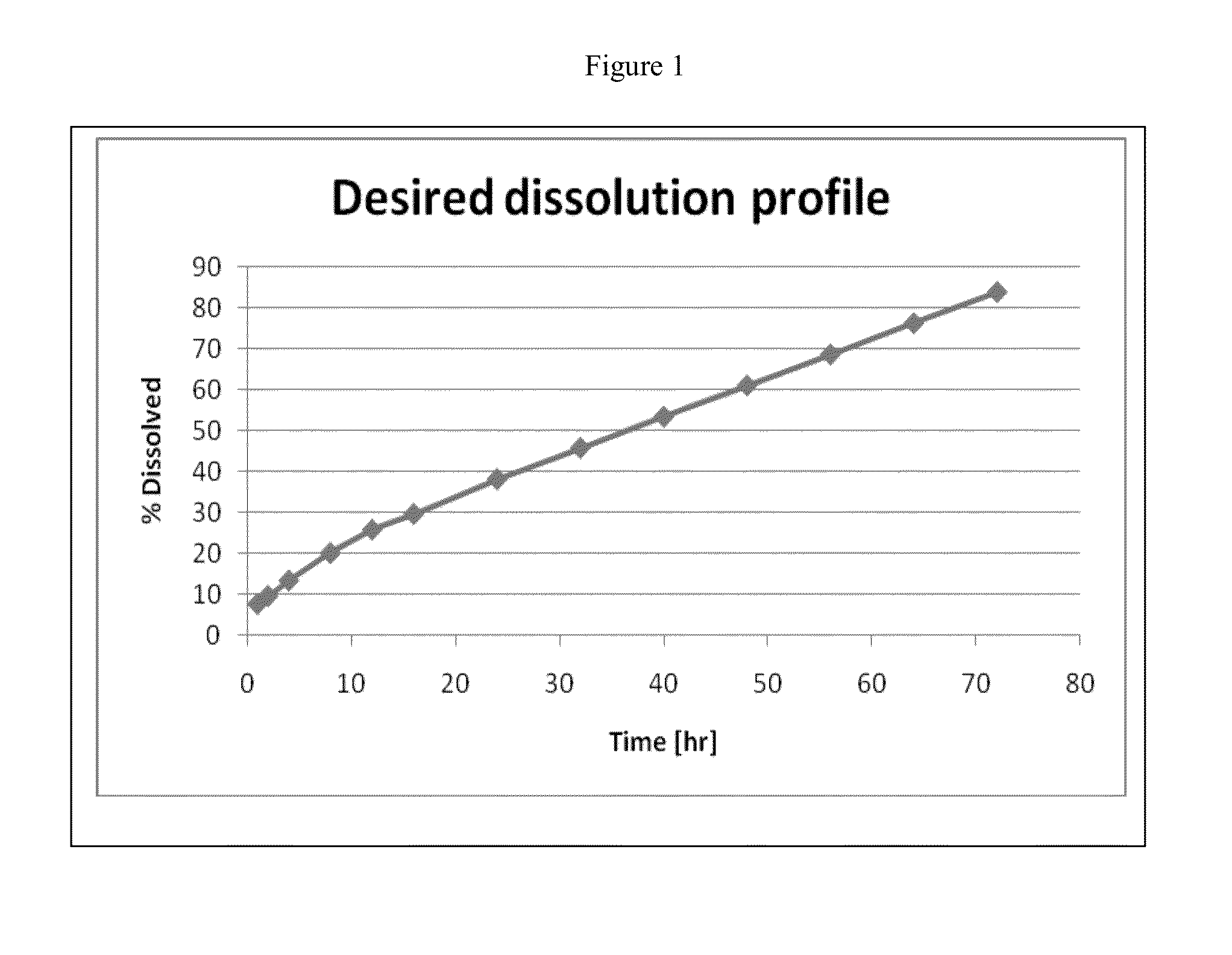
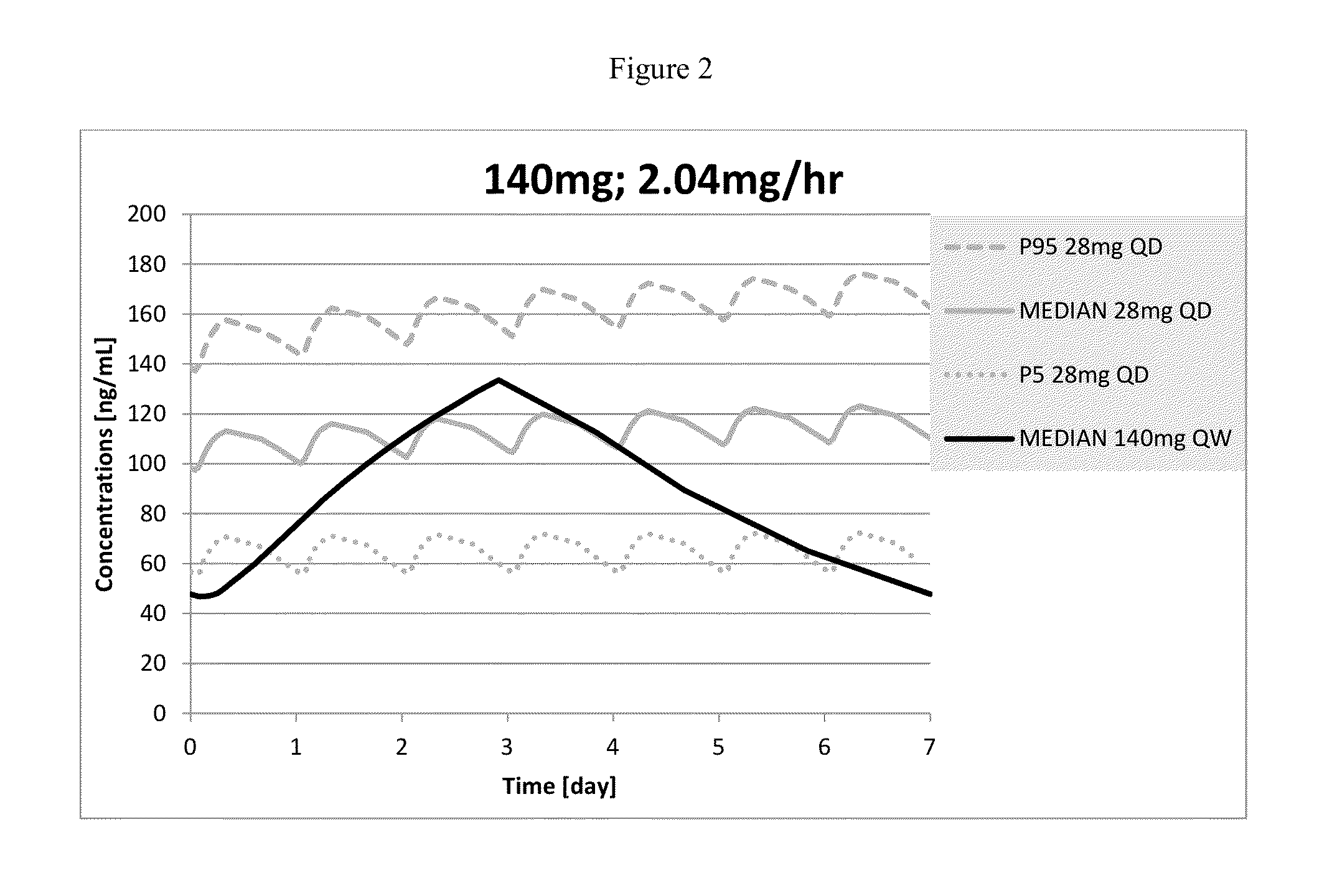
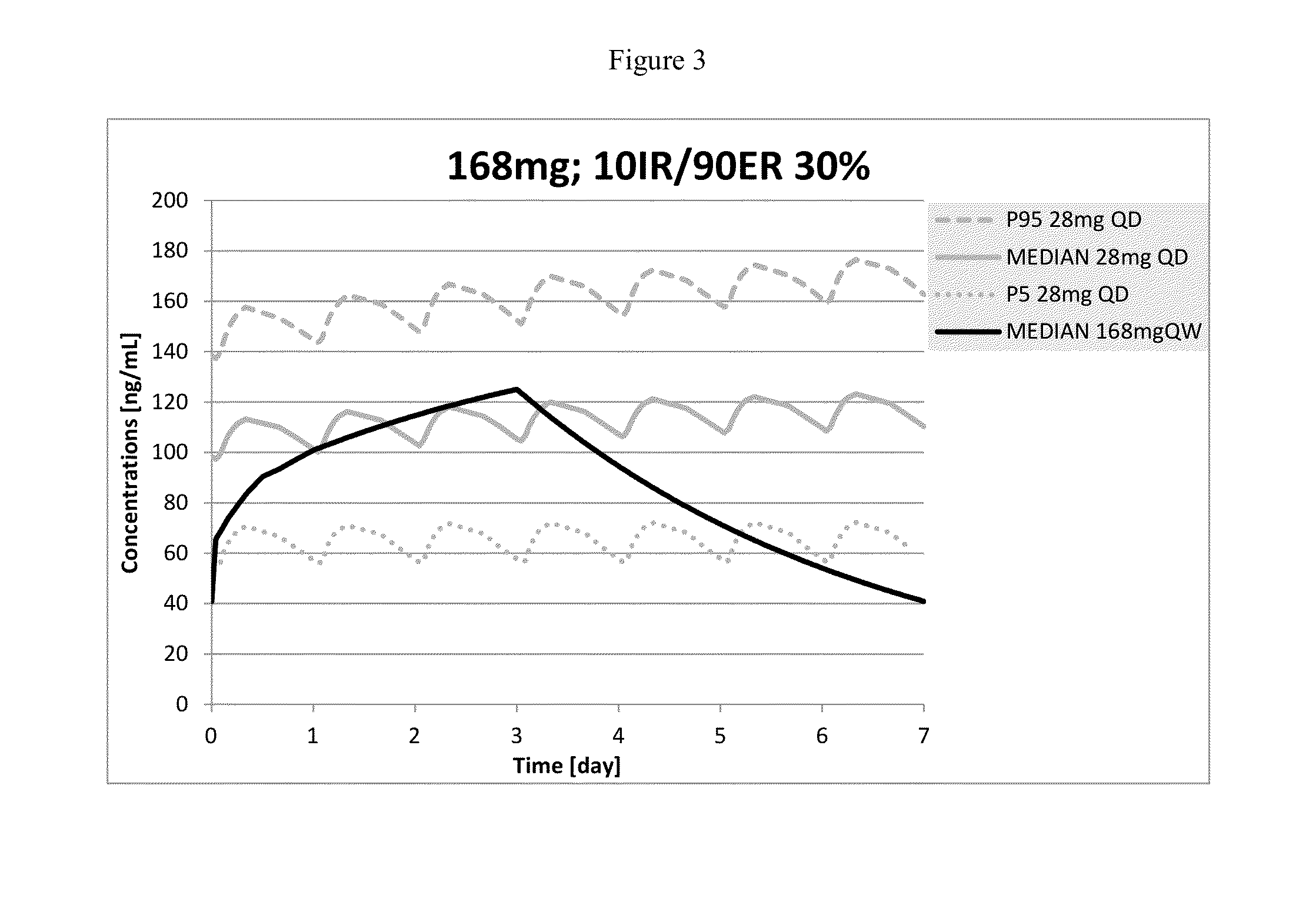
The present invention provides pharmaceutical compositions given once daily containing at least one therapeutically active ingredient selected from the group consisting of memantine and a pharmaceutically acceptable salt of memantine, and a pharmaceutically acceptable polymeric matrix carrier. The dosage forms of the invention sustain the release of the therapeutically active agent from about 4 to about 24 hours when said dosage form is exposed to aqueous solutions. following entry of said form into a use environment, wherein said dosage form has a dissolution rate of more than about 80% after passage of about 6 hours to about 12 hours following said entry into said use environment.
Owner:FOREST LAB HLDG LTD
Method and composition for adminstering an NMDA receptor antagonist to a subject
ActiveUS20060142398A1Prevent adverse side effectsPatient compliance is goodBiocideNervous disorderNR1 NMDA receptorPharmacology
The invention provides methods and compositions for administering an NMDA receptor antagonist (e.g., memantine) to a subject.
Owner:ADAMAS PHARMA LLC
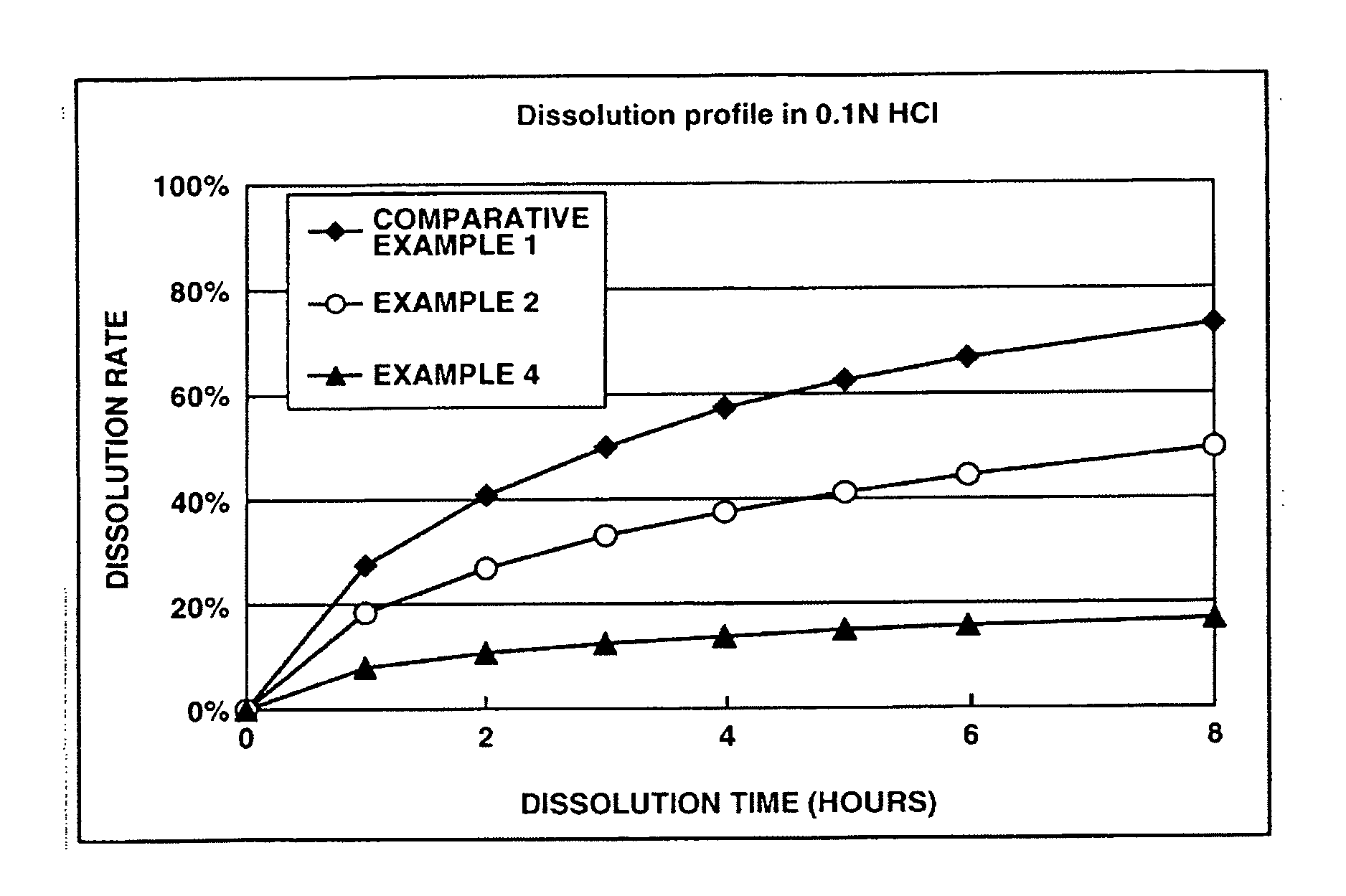
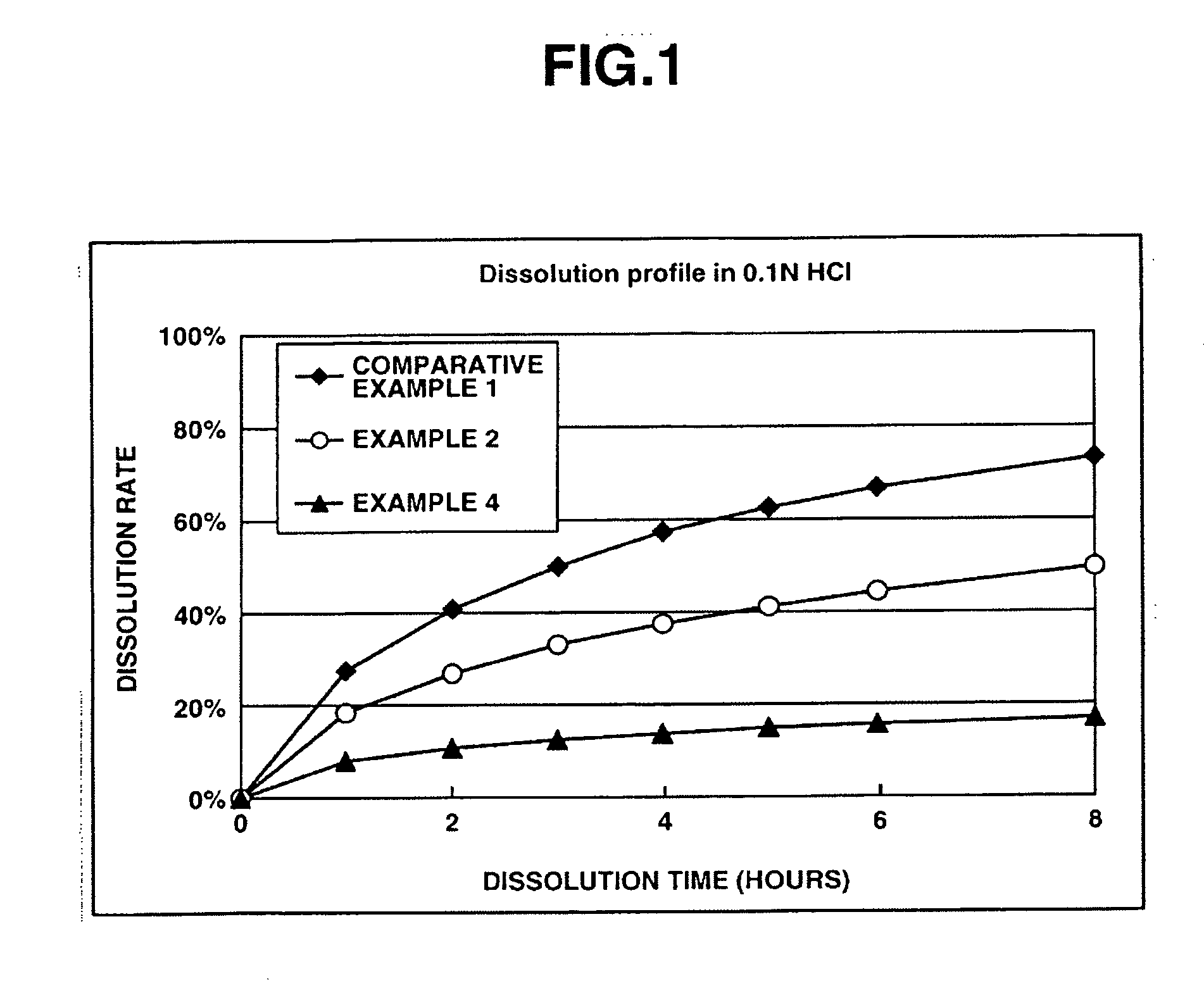
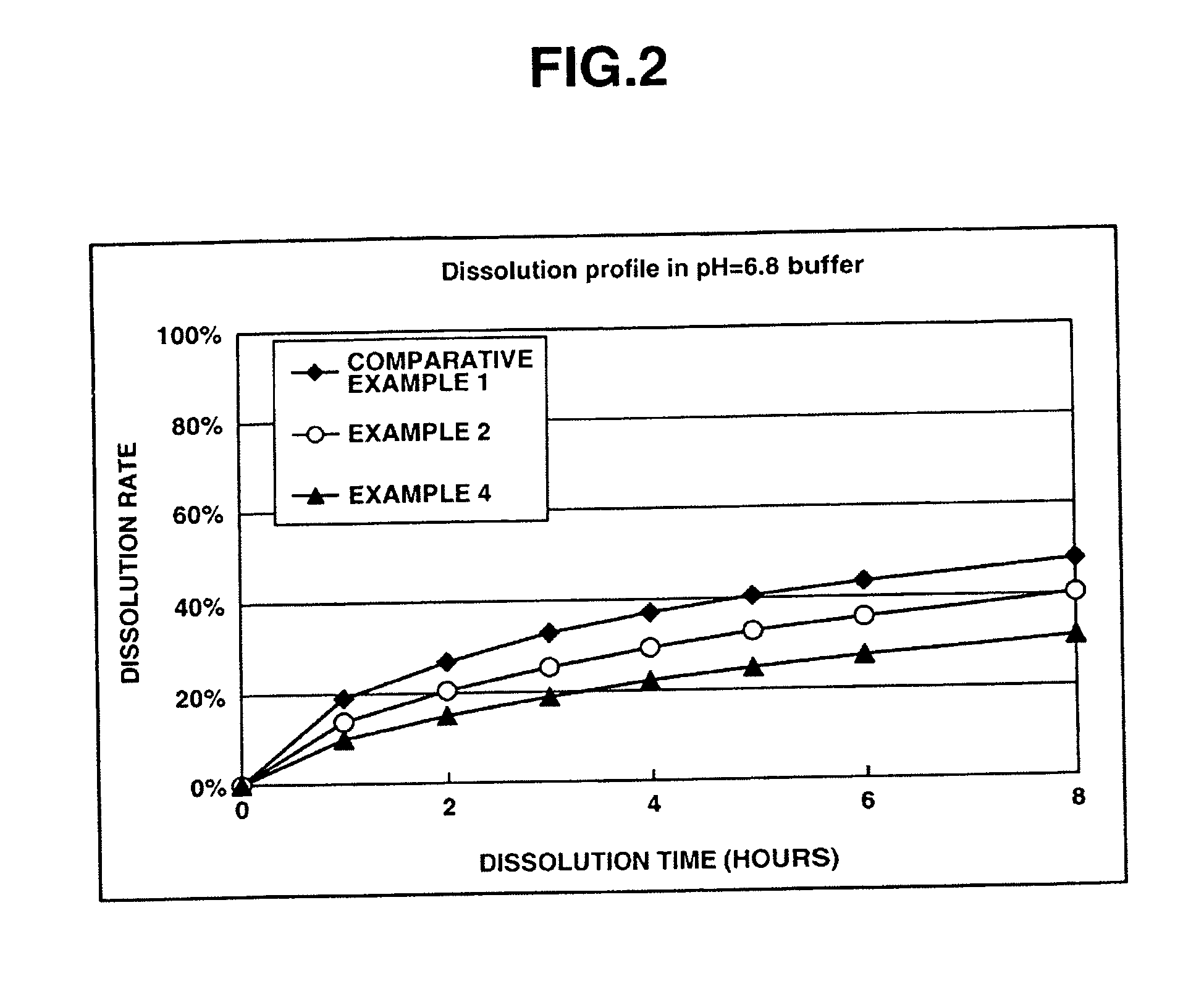
Modified and immediate release formulations of memantine
InactiveUS20070065512A1Reliable absorptionImprove toleranceBiocideNervous disorderImmediate releaseNeuropathic pain
The present invention provides immediate release and modified release oral dosage forms. Specifically, the invention provides modified and immediate release pharmaceutical dosage forms containing memantine that exhibit an enhanced release profile and provide reliable absorption. The dosage forms may be used to treat mild, moderate or severe Alzheimer's disease or neuropathic pain.
Owner:FOREST LAB HLDG LTD
Combination therapy for treatment of demyelinating conditions
InactiveUS20080089861A1Relieve symptomsEfficacious, safe and toleratedBiocidePeptide/protein ingredientsNR1 NMDA receptorAutoimmune condition
The invention provides compositions and methods for treating autoimmune diseases such as multiple sclerosis. The compositions include a combination of an NMDA Receptor antagonist, such as memantine or rimantadine, and a fumarate agent, such as dimethyl fumarate.
Owner:ADAMAS PHARMA INC
Sustained release formulations
InactiveUS20060280789A1Reduce in quantityImprove complianceBiocidePill deliveryDonepezilCholinesterase
The invention provides sustained release formulations of basic drugs, stereoisomers of basic drugs, pharmaceutically acceptable salts of basic drugs, and pharmaceutically acceptable salts of stereoisomers of basic drugs. The basic drugs may be anti-dementia drugs, such as cholinesterase inhibitors or memantine. In one embodiment, the cholinesterase inhibitor is donepezil.
Owner:EISAI CO LTD
Method and composition for administering an NMDA receptor antagonist to a subject
ActiveUS7619007B2Prevent adverse side effectsIncrease profitBiocideNervous disorderNR1 NMDA receptorNK1 receptor antagonist
The invention provides methods and compositions for administering an NMDA receptor antagonist (e.g., memantine) to a subject.
Owner:ADAMAS PHARMA LLC
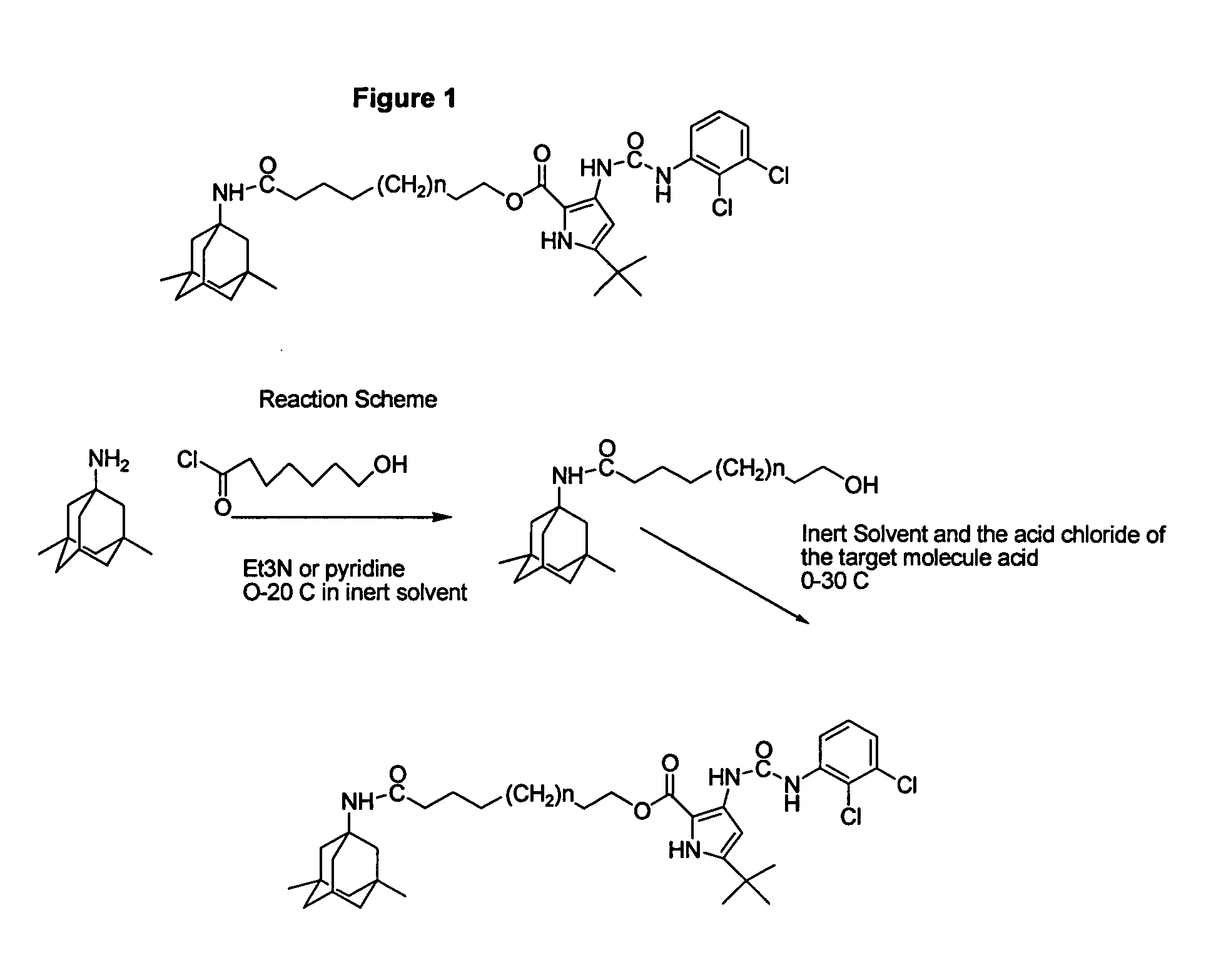
Method of targeting a therapeutic agent
Disclosed are conjugates in which an aminoadamantane derivative, such as amantadine, memantine, or rimantadine is linked to a therapeutic agent. The conjugate can then be used to target the therapeutic agent to an injured neuron.
Owner:NEUROMOLECULAR INC
Modified release formulations of memantine oral dosage forms
The present invention provides pharmaceutical compositions given once daily containing at least one therapeutically active ingredient selected from the group consisting of memantine and a pharmaceutically acceptable salt of memantine, and a pharmaceutically acceptable polymeric matrix carrier. The dosage forms of the invention sustain the release of the therapeutically active agent from about 4 to about 24 hours when said dosage form is exposed to aqueous solutions. following entry of said form into a use environment, wherein said dosage form has a dissolution rate of more than about 80% after passage of about 6 hours to about 12 hours following said entry into said use environment.
Owner:FOREST LAB HLDG LTD
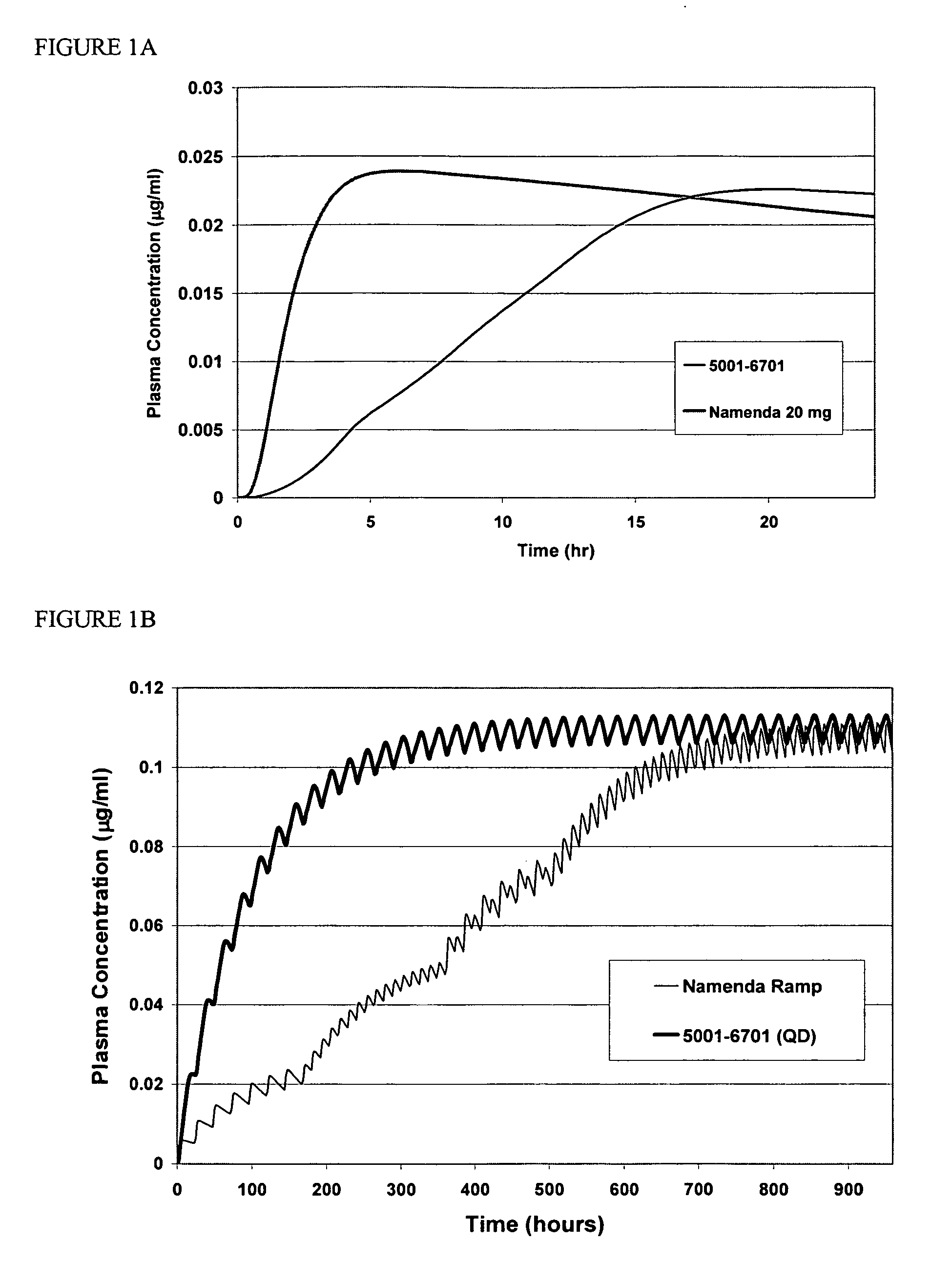
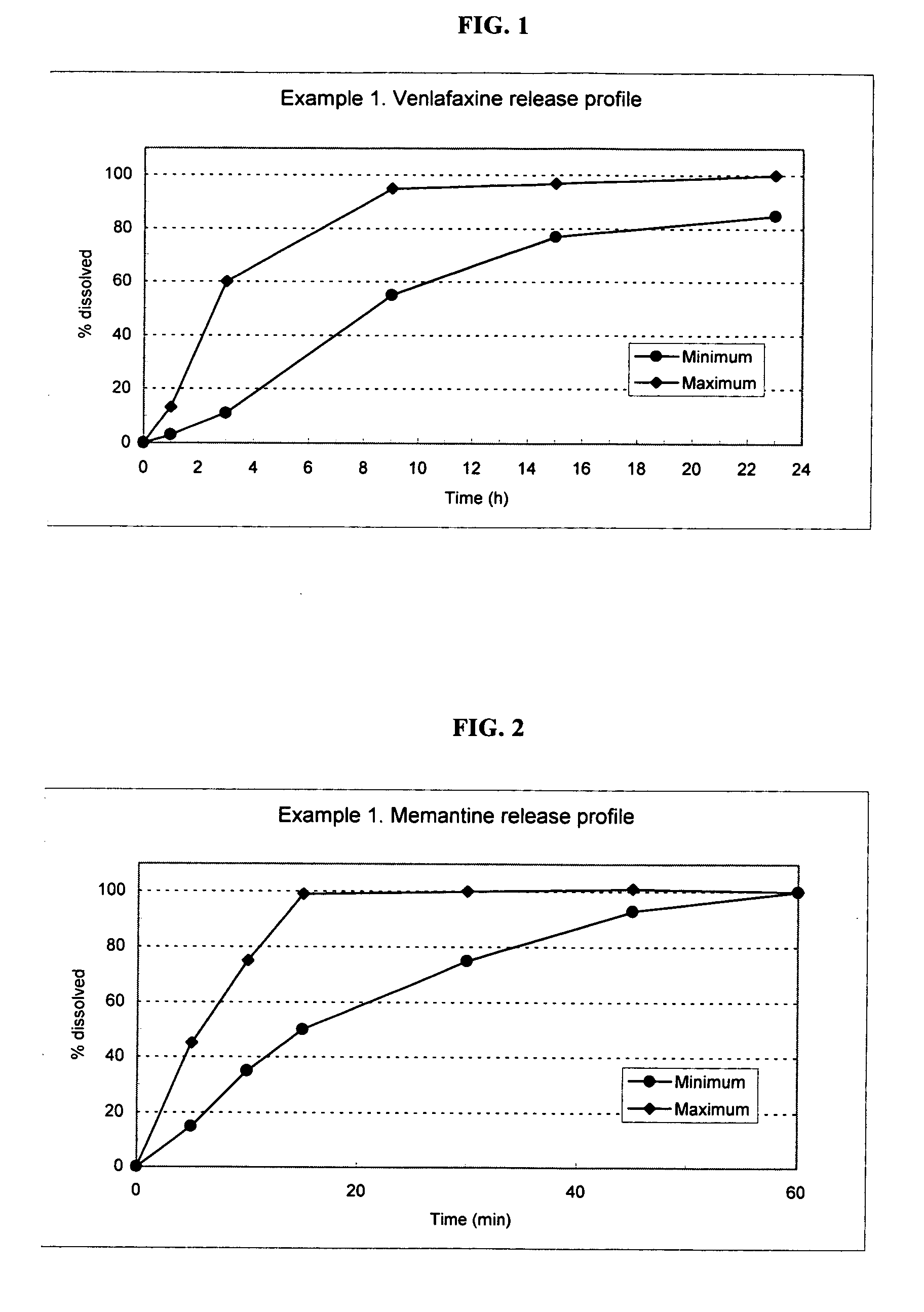
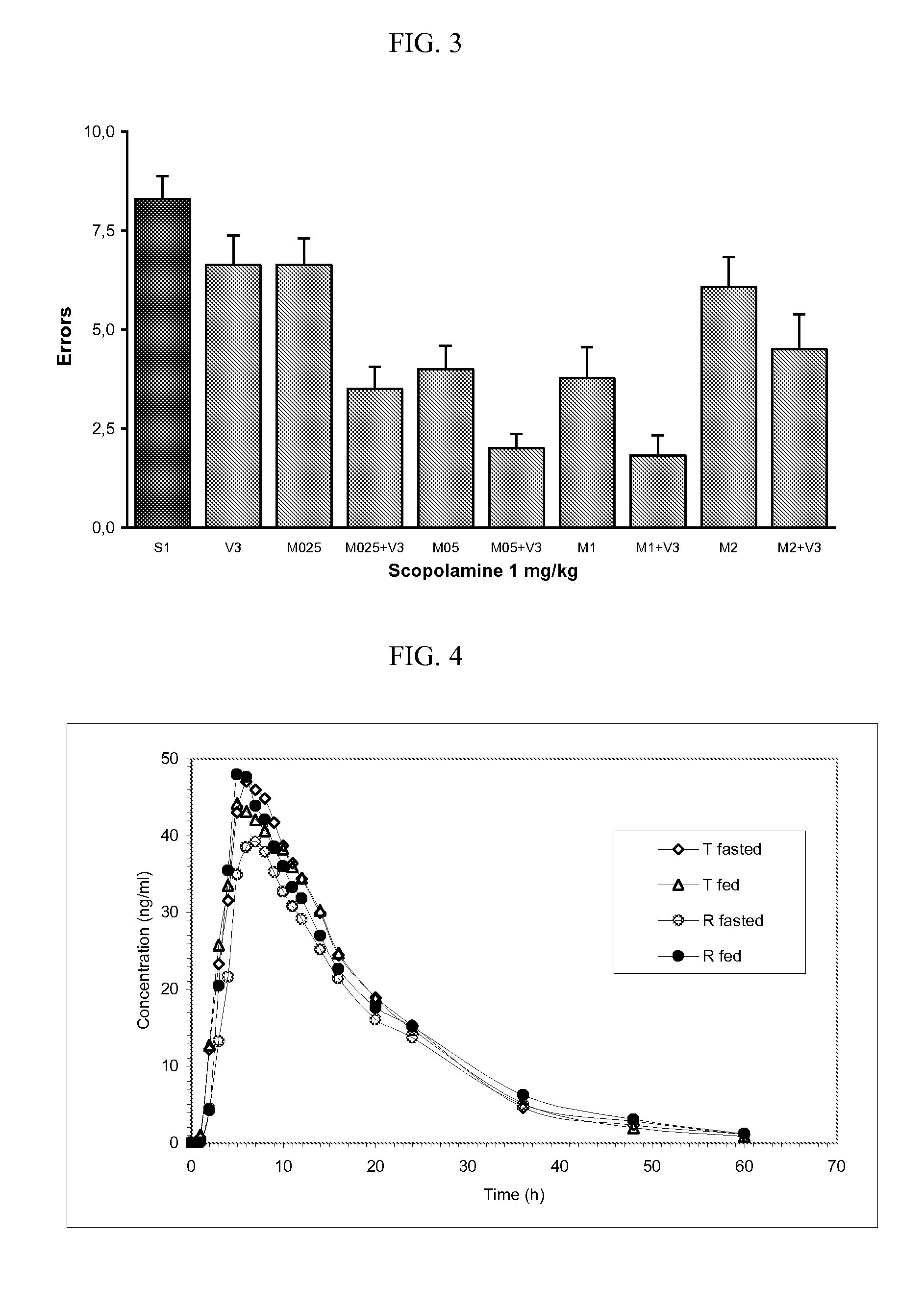
Delivery device containing venlafaxine and memantine and methods of use thereof
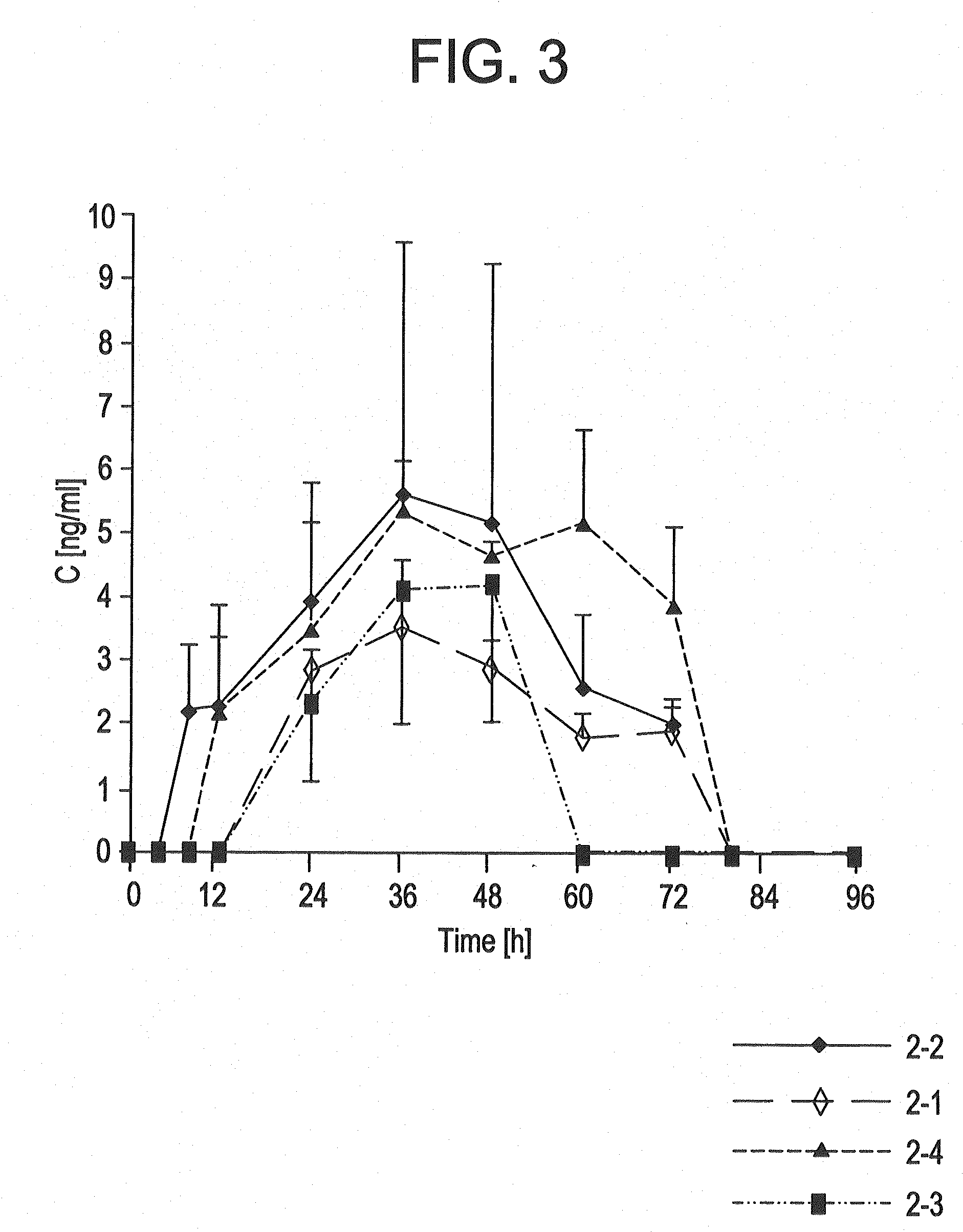
The present invention provides an osmotic device containing controlled release venlafaxine in the core in combination with an anti-Alzheimer's or an anti-Parkinson's drug in a rapid release external coat. Memantine is used as an anti-Alzheimer's drug or an anti-Parkinson's drug. Particular embodiments of the invention provide osmotic devices having predetermined release profiles. One embodiment of the osmotic device includes an external coat that has been spray-coated rather than compression-coated onto the device. The device is useful for the treatment of depression in Alzheimer's disease and / or Parkinson's disease patients. The device and method can also be used to treat or ameliorate other symptoms associated with Alzheimer's disease, Parkinson's disease or any other neurological disorder. Other dosage forms that provide a controlled, sustained or extended release of venlafaxine in combination with a rapid or immediate release of memantine are useful in the invention.
Owner:OSMOTICA CORP
Use of 1-aminocyclohexane derivatives to modify deposition of fibrillogenic a-beta peptides in amyloidopathies
InactiveUS20050113458A1Reduce riskSlow onsetBiocideNervous disorderNR1 NMDA receptorGreek letter beta
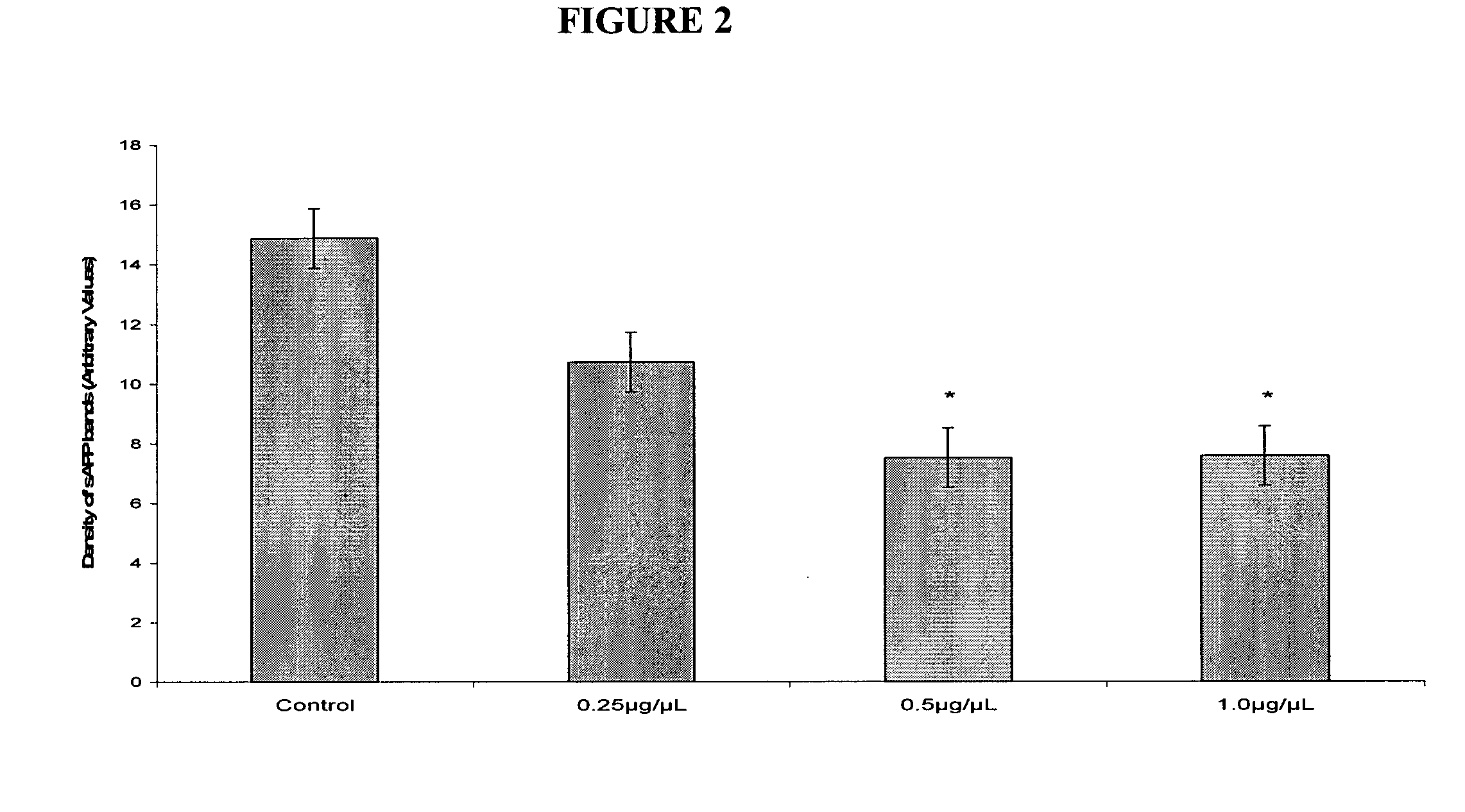
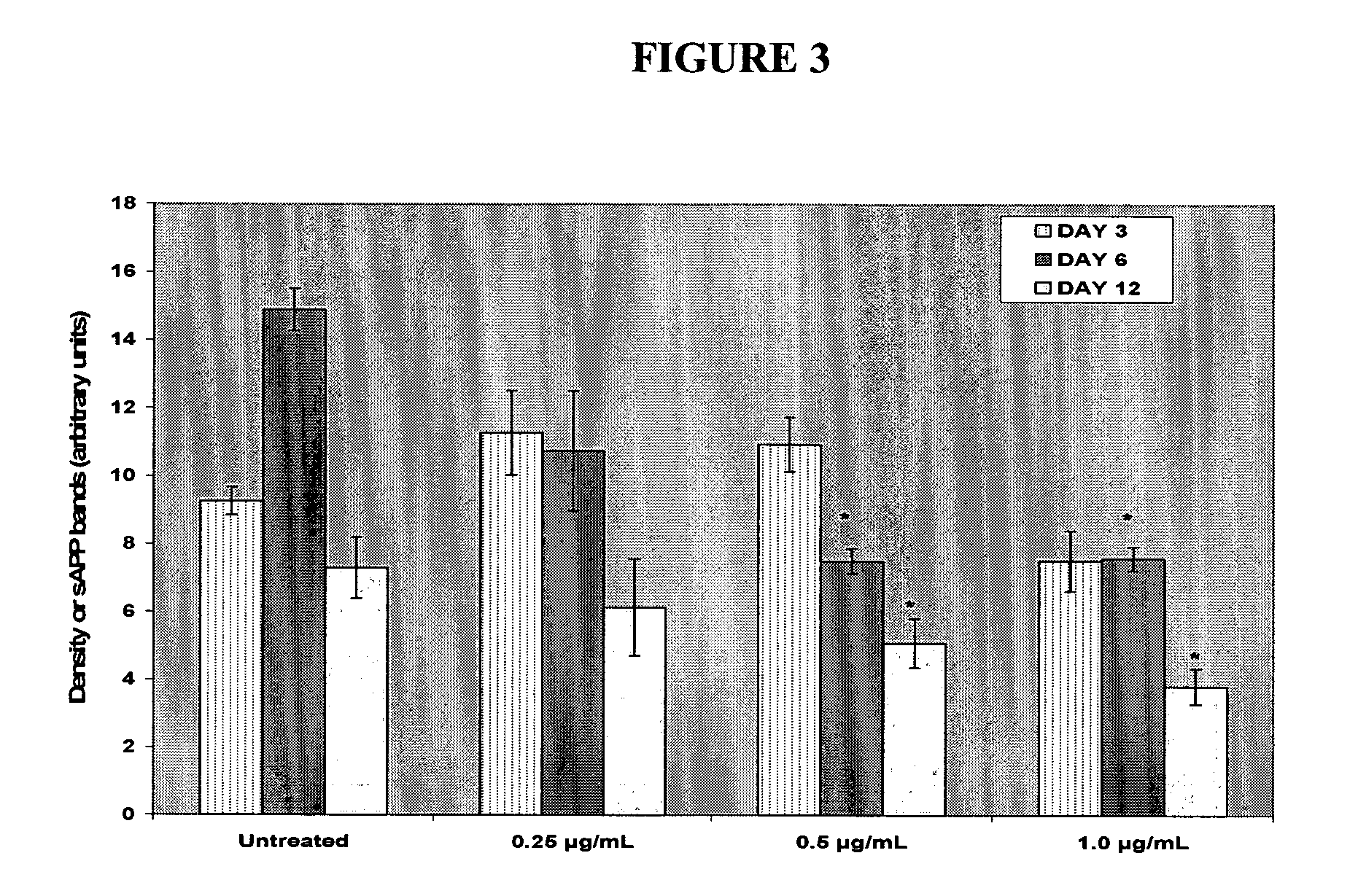
The invention relates to the use of NMDA receptor antagonists such as 1-aminocyclohexane derivatives to modify deposition of potentially toxic and fibrillogenic Aβ peptides in amyloidopathies. Specifically, the invention relates to the ability of memantine to intervene in the processing of APP and decrease the levels of fibrillogenic Aβ peptides.
Owner:FOREST LAB HLDG LTD
1-Aminocyclohexane derivatives for the treatment of multiple sclerosis, emotional lability and pseudobulbar affect
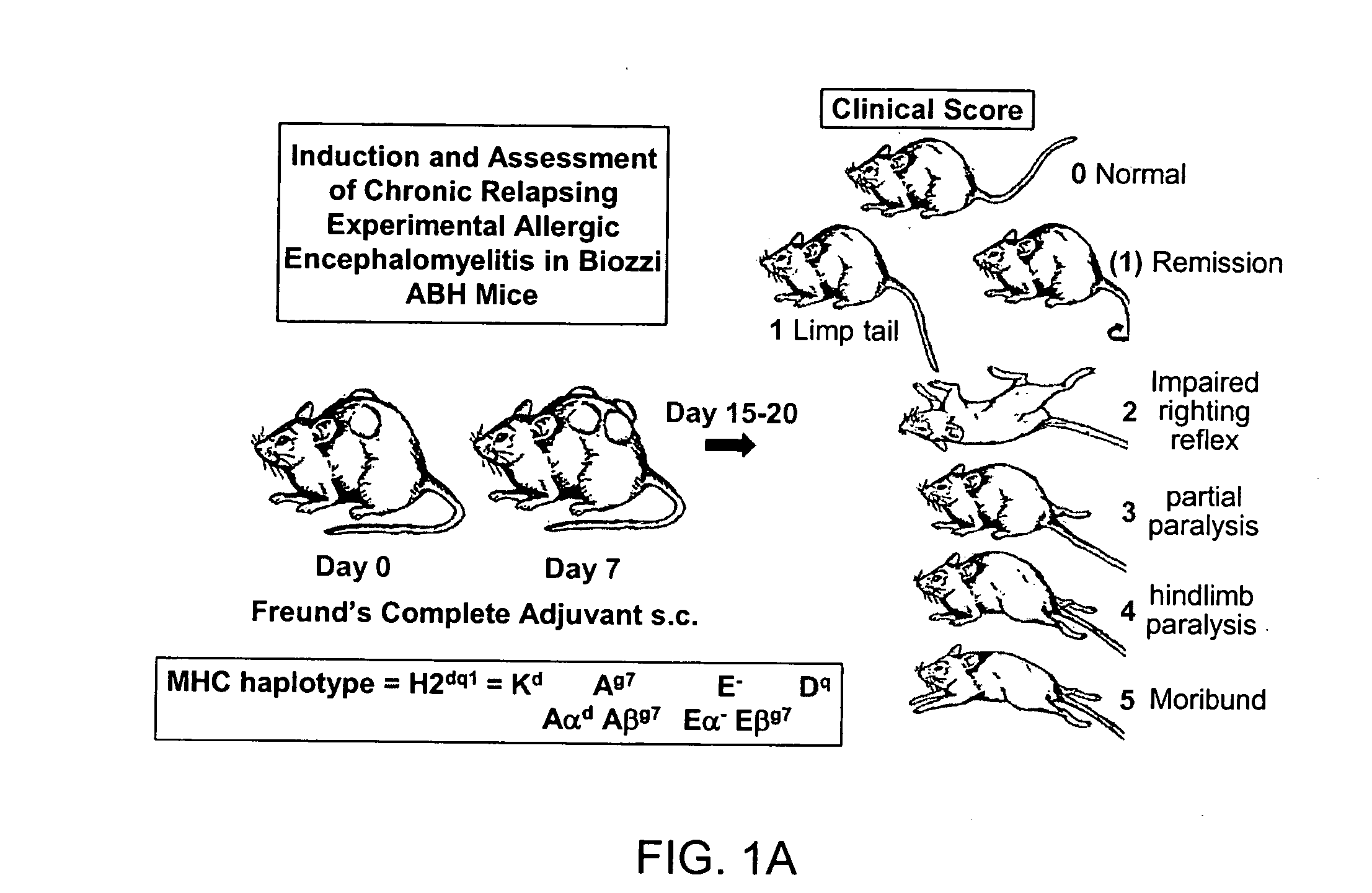
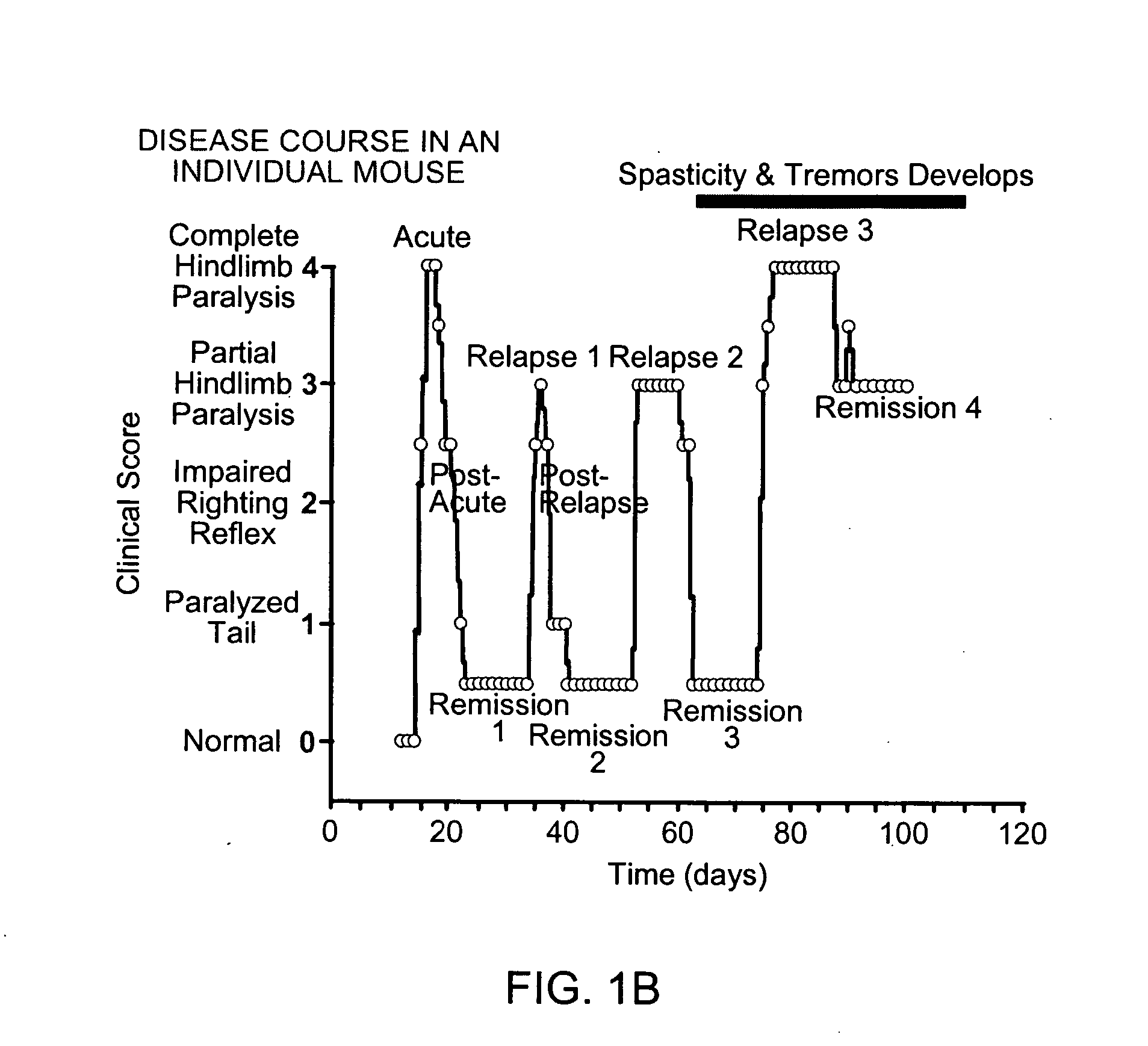
The present invention relates to the treatment of individuals diagnosed with multiple sclerosis, emotional lability or pseudobulbar affect comprising administering to said individual an effective amount of a 1-aminocyclohexane derivative, namely memantine or neramexane.
Owner:FOREST LAB HLDG LTD
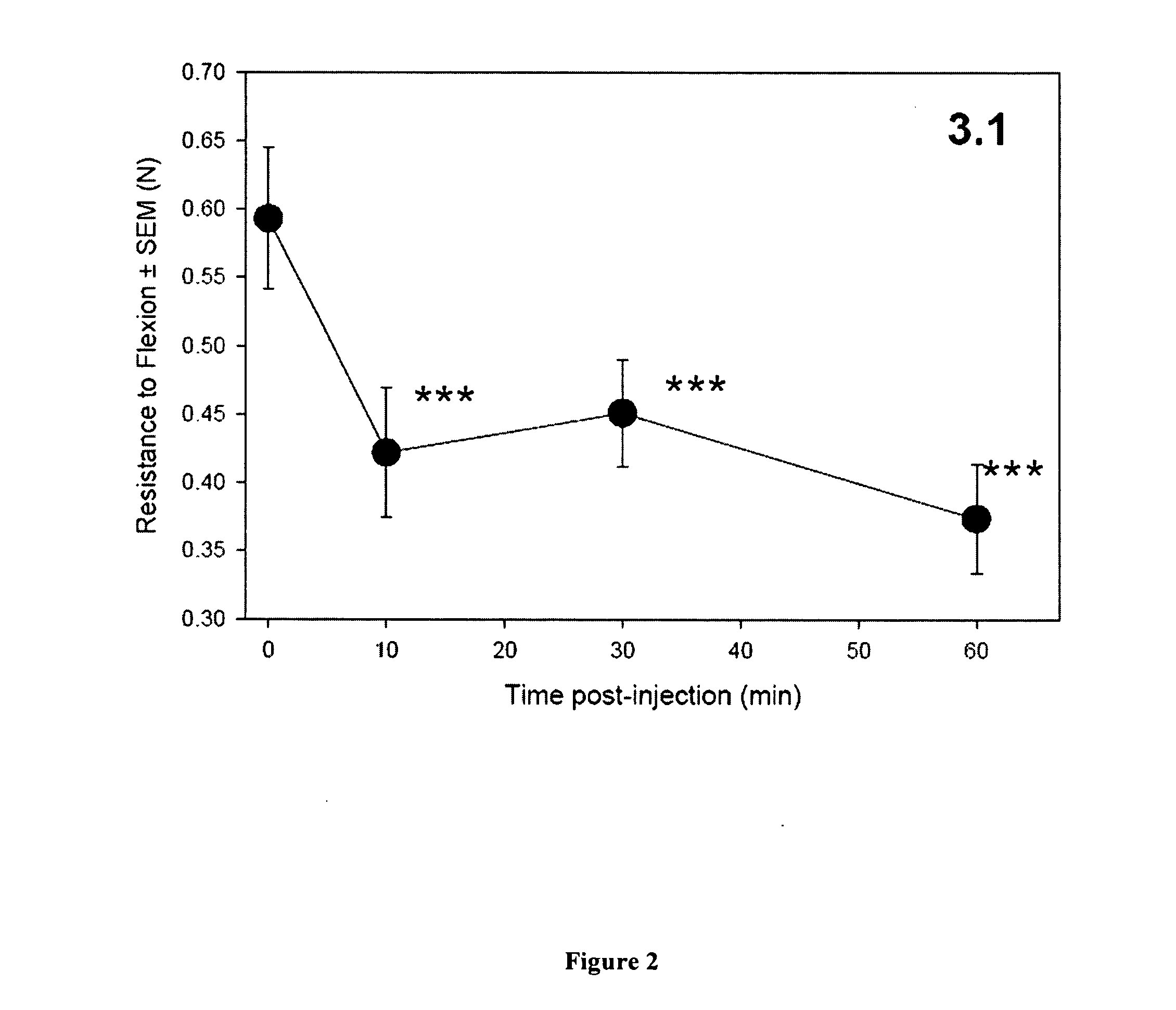
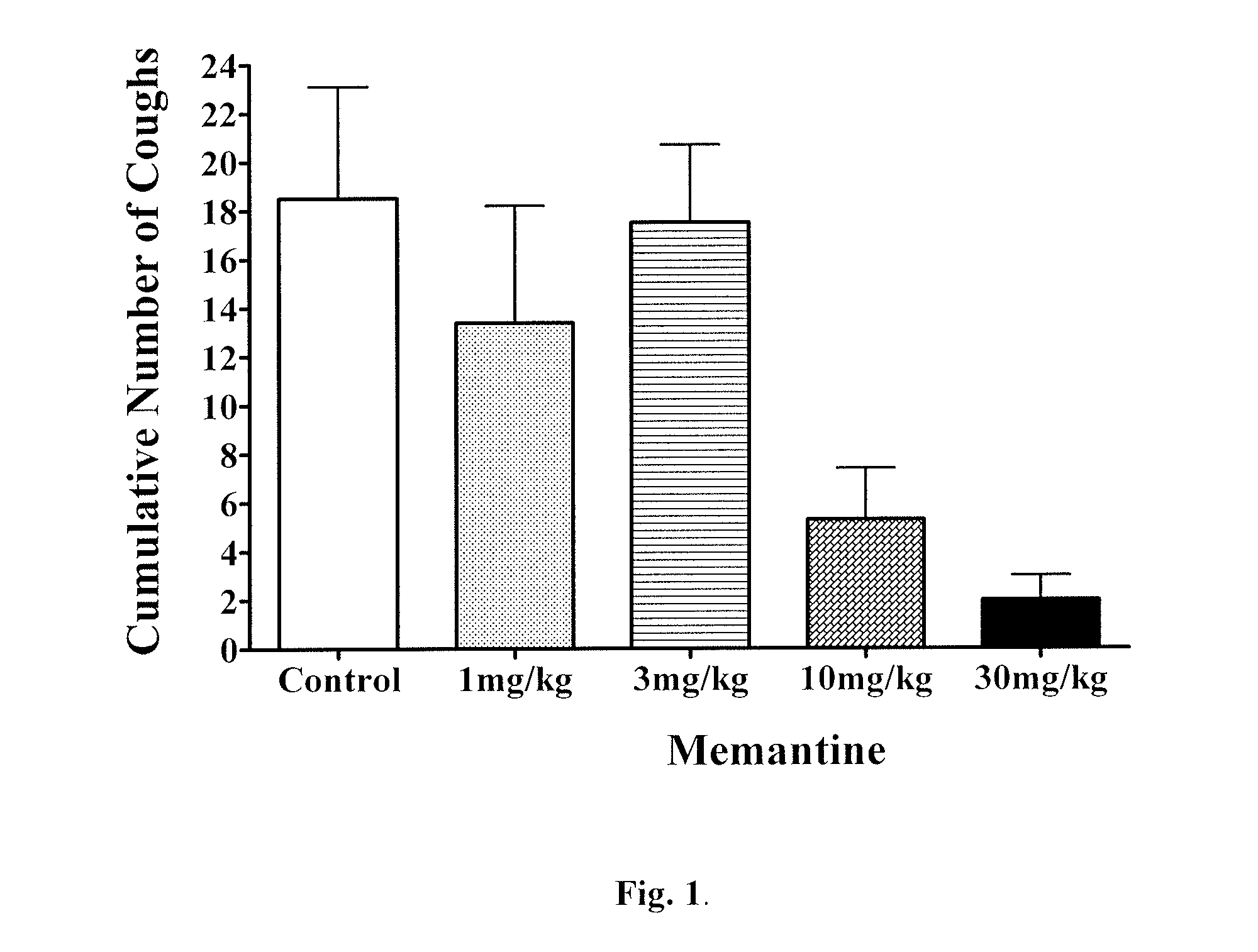
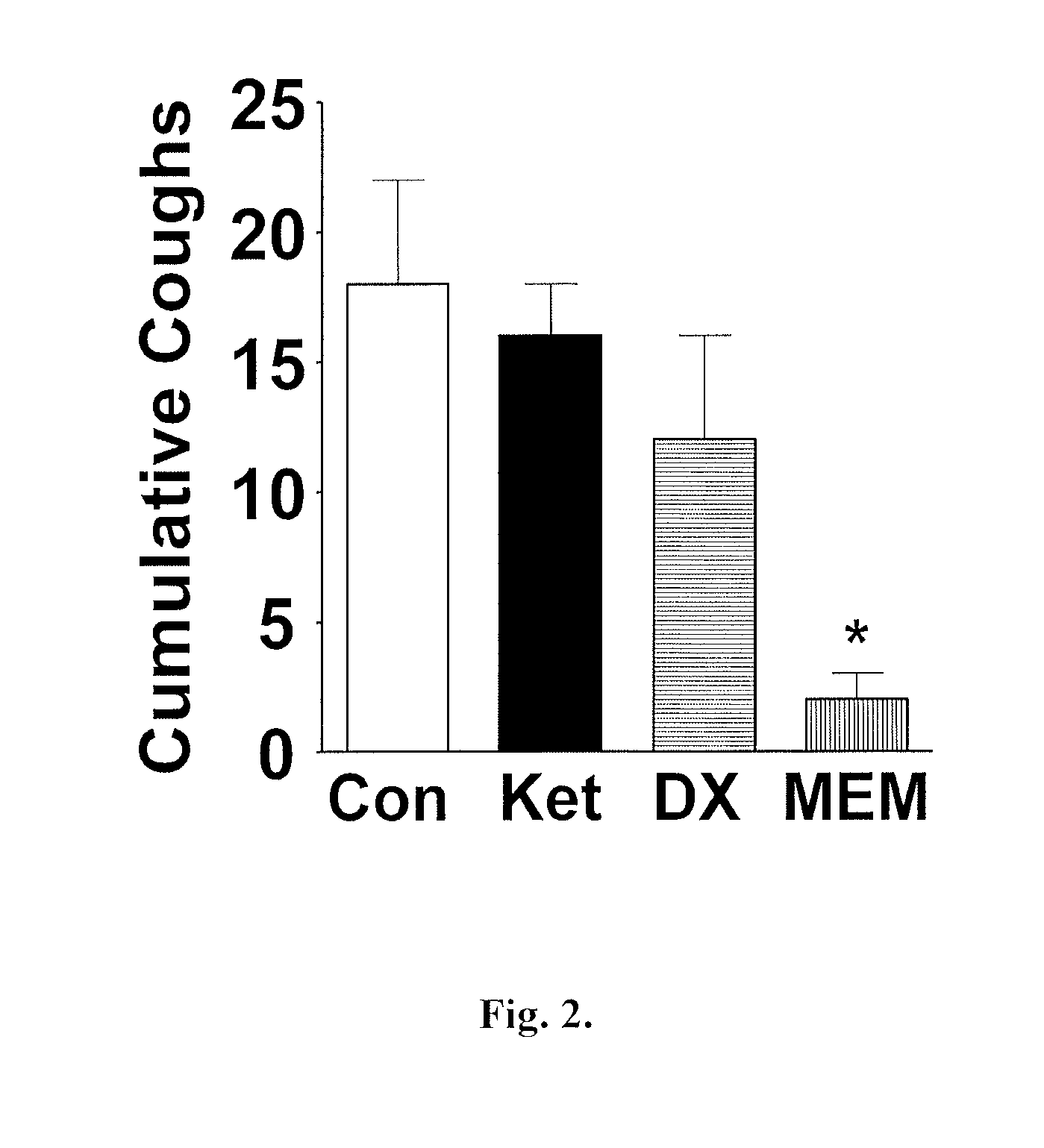
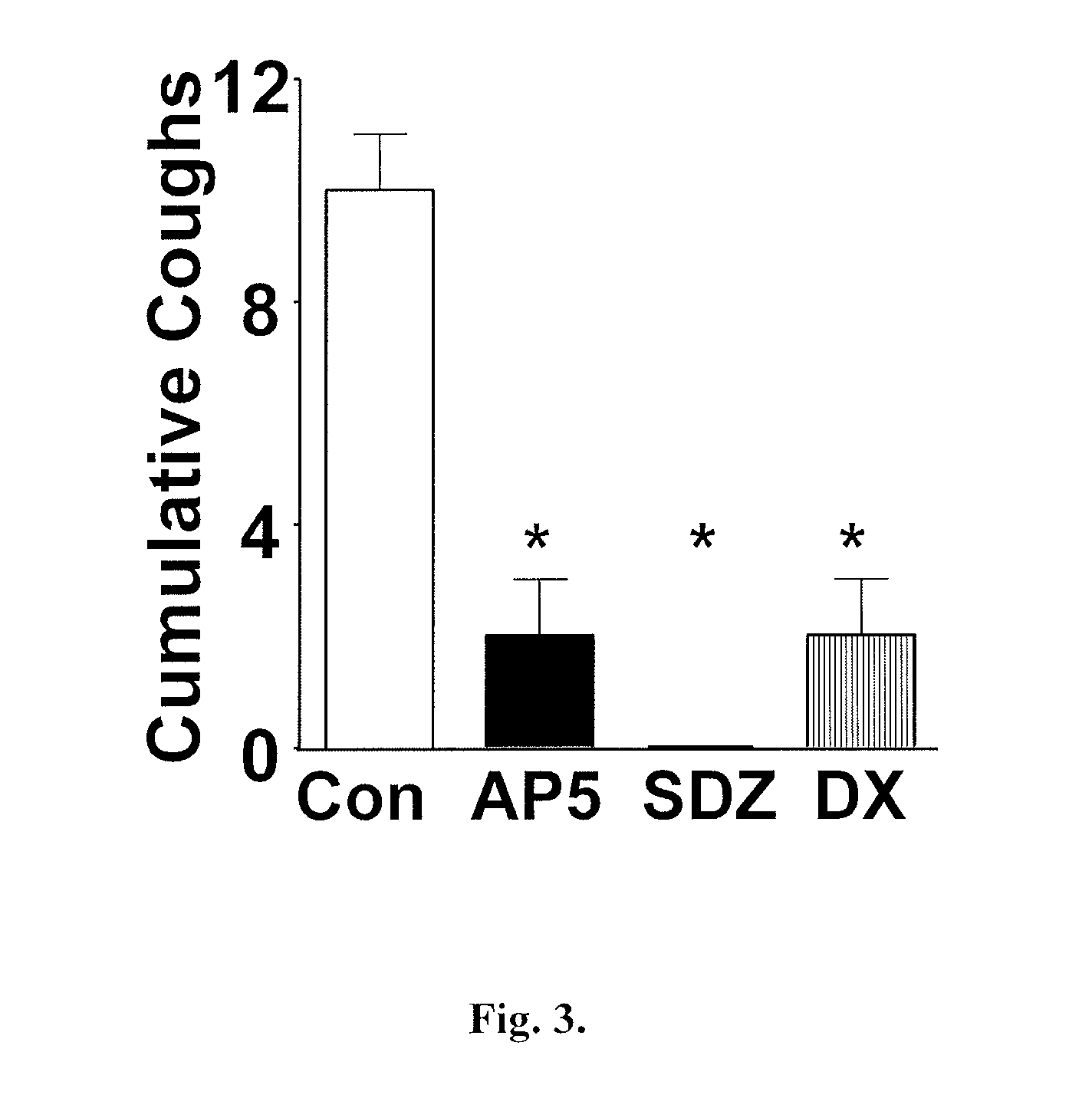
Antitussive compositions comprising memantine
Memantine compositions and methods of use are described herein. In some embodiments, the compositions comprise memantine and an absorption enhancer, or memantine and an elimination enhancer, or memantine and an absorption enhancer and an elimination enhancer.
Owner:CERECOR INC +1
Transdermal administration of memantine
ActiveUS20110313372A1Low variabilityLeveling precisionBiocideNervous disorderDiseaseNR1 NMDA receptor
The invention relates to transdermal therapeutic systems (TTS) which include, as active ingredient, the NMDA receptor antagonist memantine or one of its physiologically compatible salts. The TTSs can be produced and used for treating diseases of the central nervous system.
Owner:LTS LOHMANN THERAPIE-SYST AG
Use of memantine (namenda) to treat autism, compulsivity and impulsivity
The present invention relates to the treatment of compulsive, impulsive and pervasive developmental disorders. More particularly, the methods described herein comprise administration of memantine to an individual suffering from such a disorder in an amount effective to relieve one or more symptoms of said disorder. In particularly preferred aspects, the invention is directed to the use of memantine for the treatment of autism.
Owner:MT SINAI SCHOOL OF MEDICINE
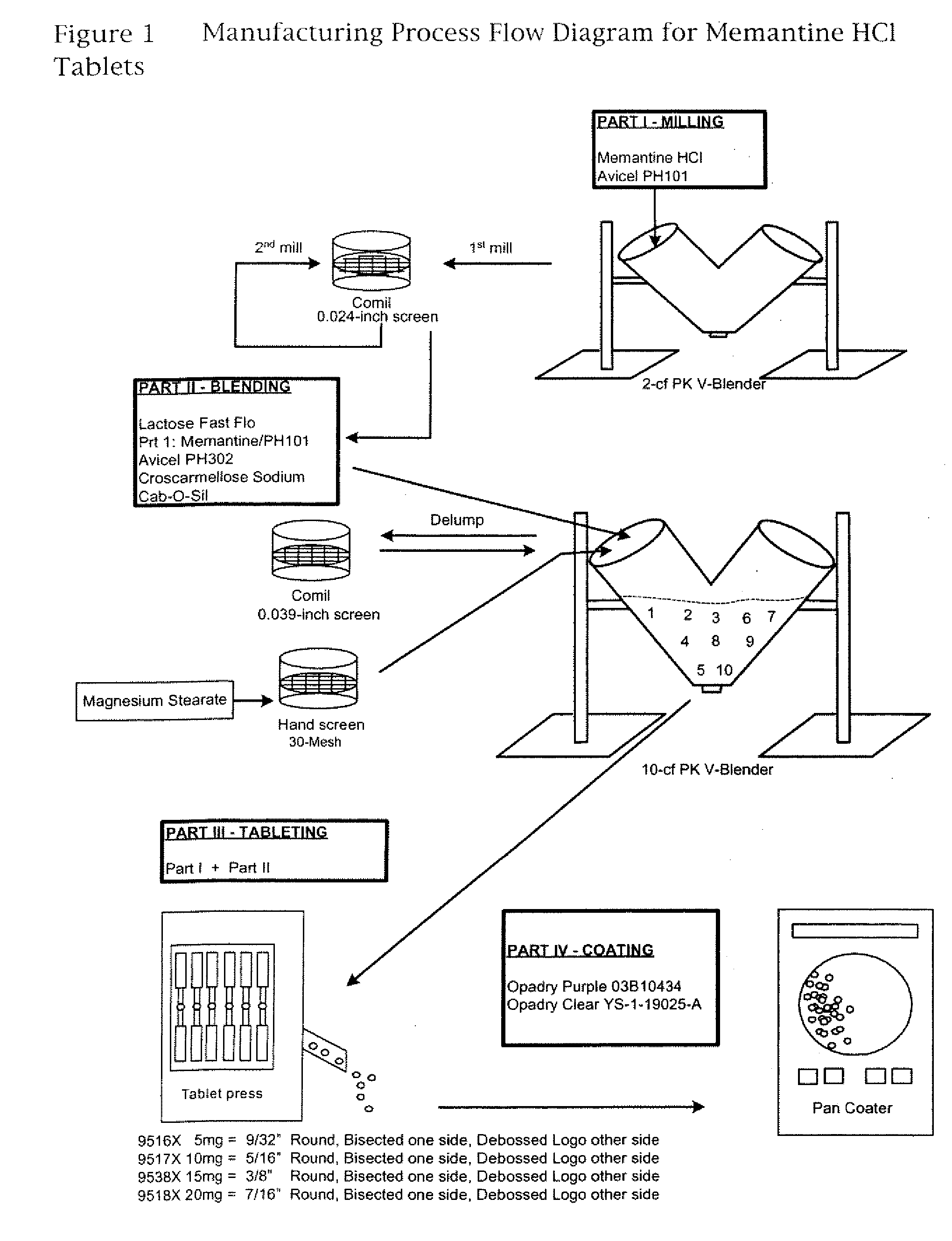
Memantine oral dosage forms
This invention relates to an oral dosage form containing between 1 mg and 100 mg of memantine, wherein said dosage form does not contain 10 mg of memantine or 20 mg of memantine, and wherein said dosage form is not prepared by the patient or a person administering the drug to the patient who divides the dosage form containing a larger dose of memantine. Other aspects of this invention relate to pharmaceutical products comprising said dosage forms and methods of administering memantine and treating disease with said dosage form.
Owner:ALLERGAN INC
Memantine for the treatment of mild and mild to moderate Alzheimer's disease
InactiveUS20060020042A1Preventing decrease in glucose metabolismBiocideNervous disorderGlucose polymersCerebrum
The present invention provides a method for the treatment, prevention, or delay of progression of mild, or mild-to-moderate Alzheimer's disease, by administering an effective dose of memantine. The present invention also provides a method for preventing the decrease in glucose metabolism in the cortical and sub-cortical regions of the brain in subjectes with mild, or mild-to-moderate Alzheimer's disease.
Owner:FOREST LAB HLDG LTD
Memantine as adjunctive treatment to atypical antipsychotic in schizophrenia patients
The present invention provides a method for treating schizophrenia in a patient in need thereof, the method comprising administering to the patient a therapeutically effective amount of memantine, or a pharmaceutically acceptable salt thereof, and a therapeutically effective amount an atypical antipsychotic. The method of the present invention embodies both the co-administration of memantine with an atypical antipsychotic, and the use of memantine as an adjunctive treatment to treatment with an atypical antipsychotic.
Owner:FOREST LAB HLDG LTD
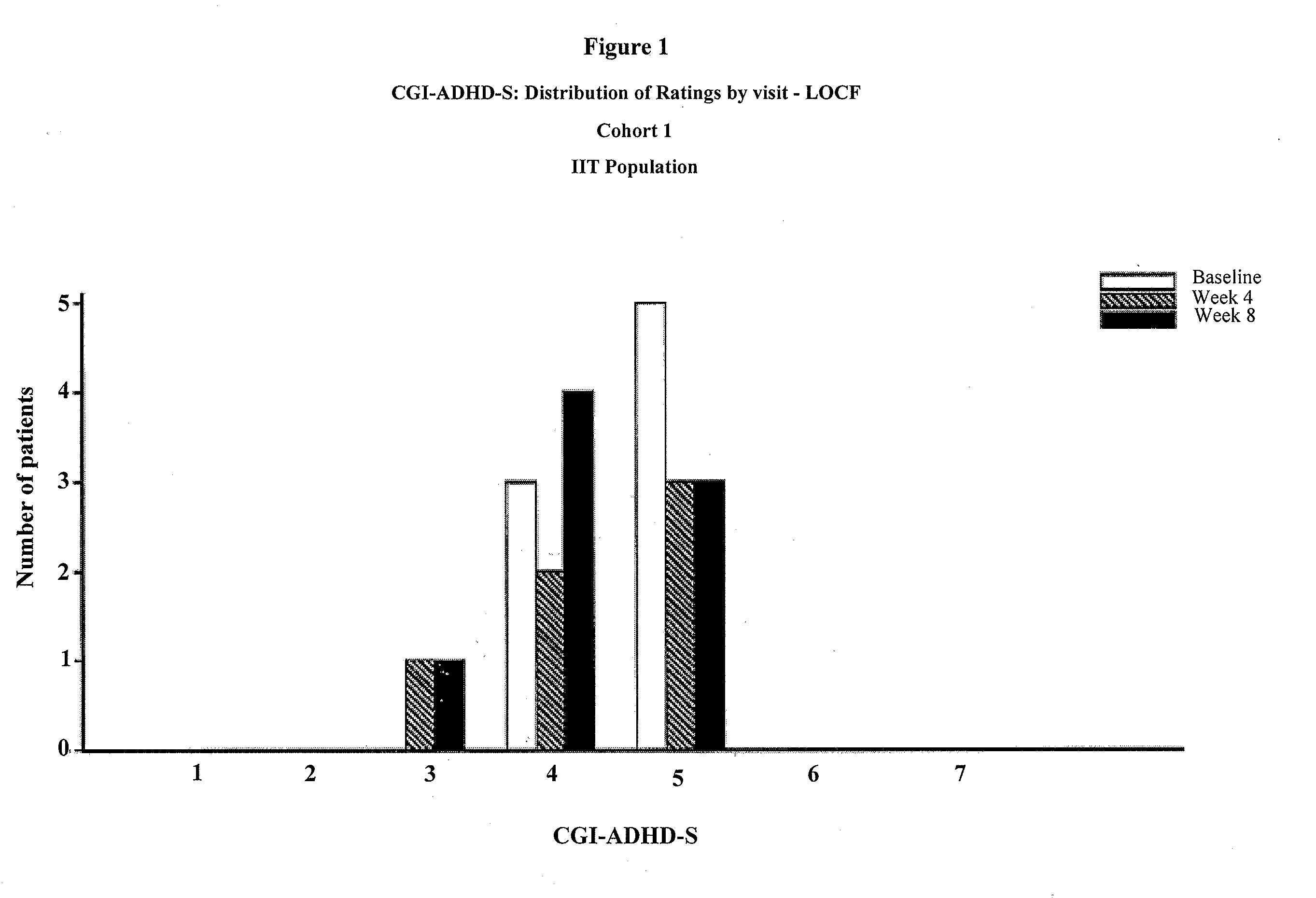
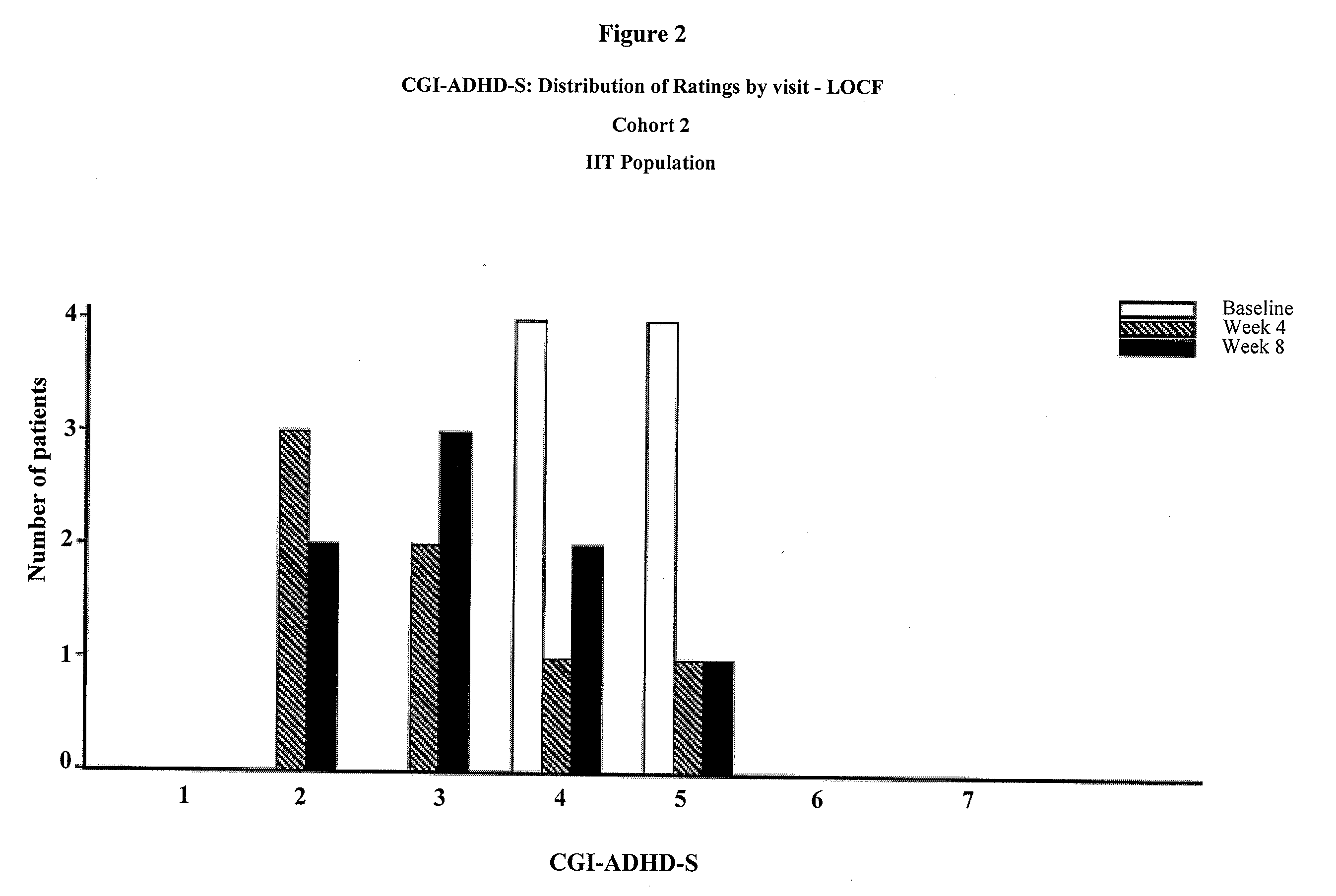
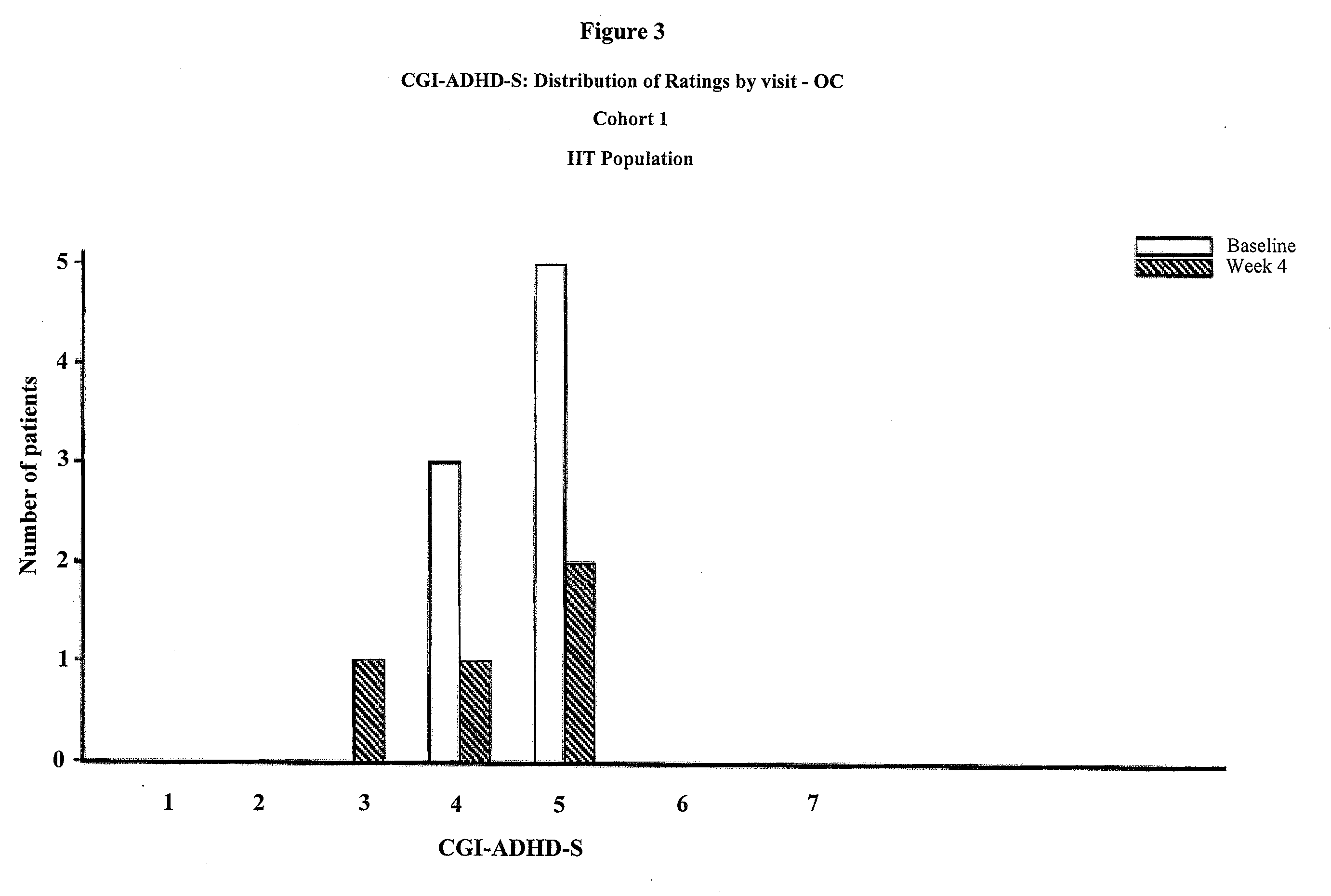
Memantine For The Treatment Of Childhood Behavioral Disorders
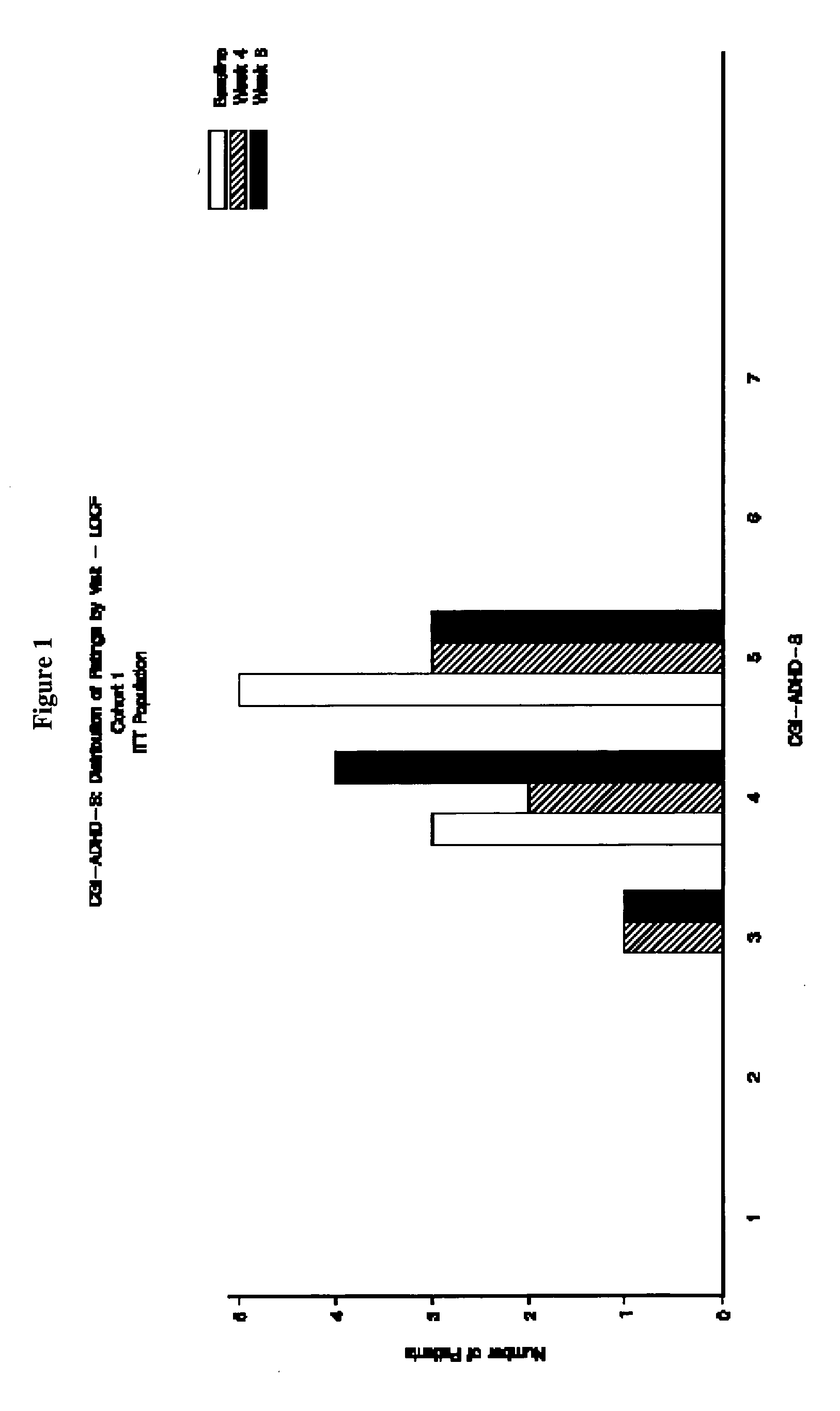
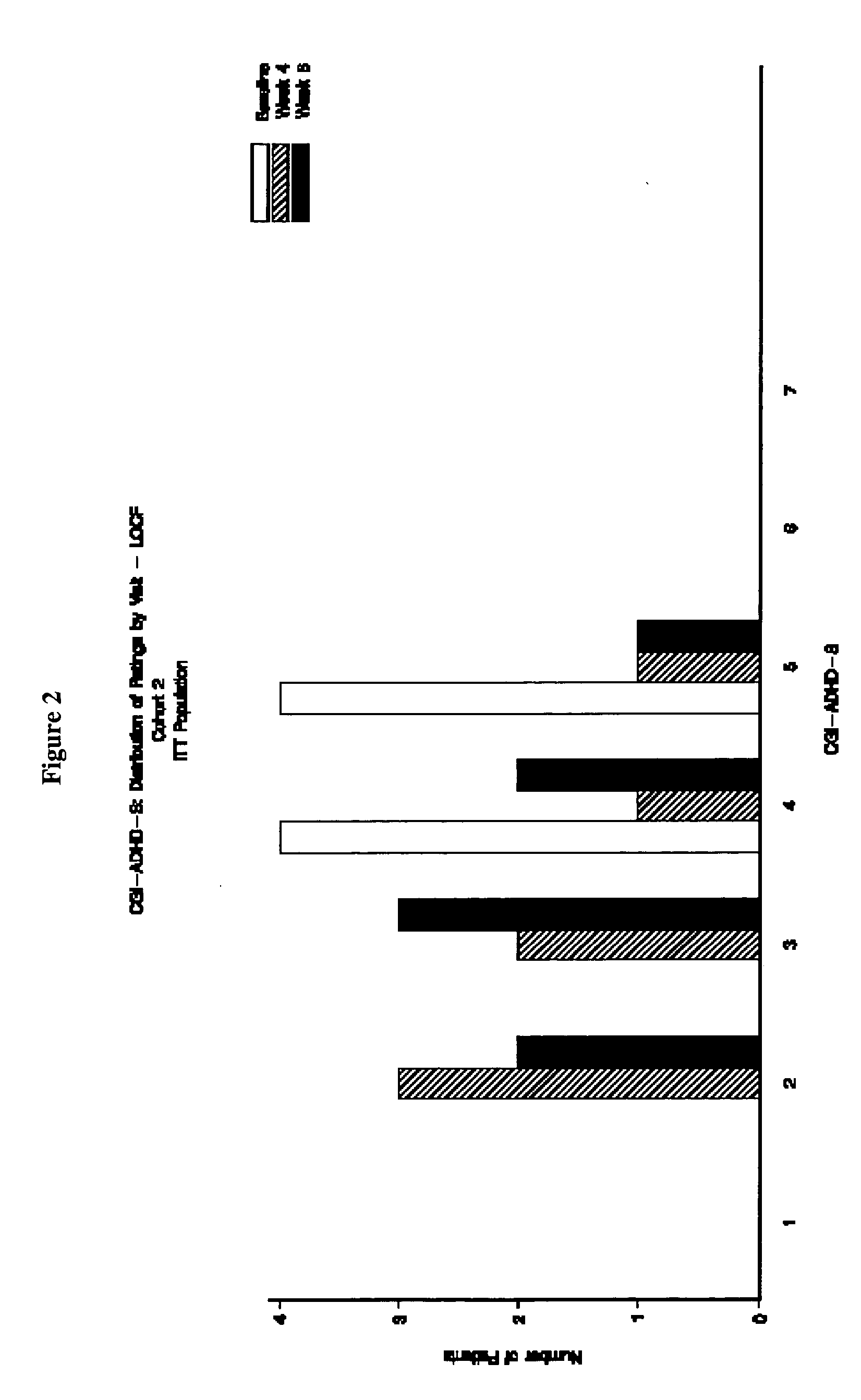
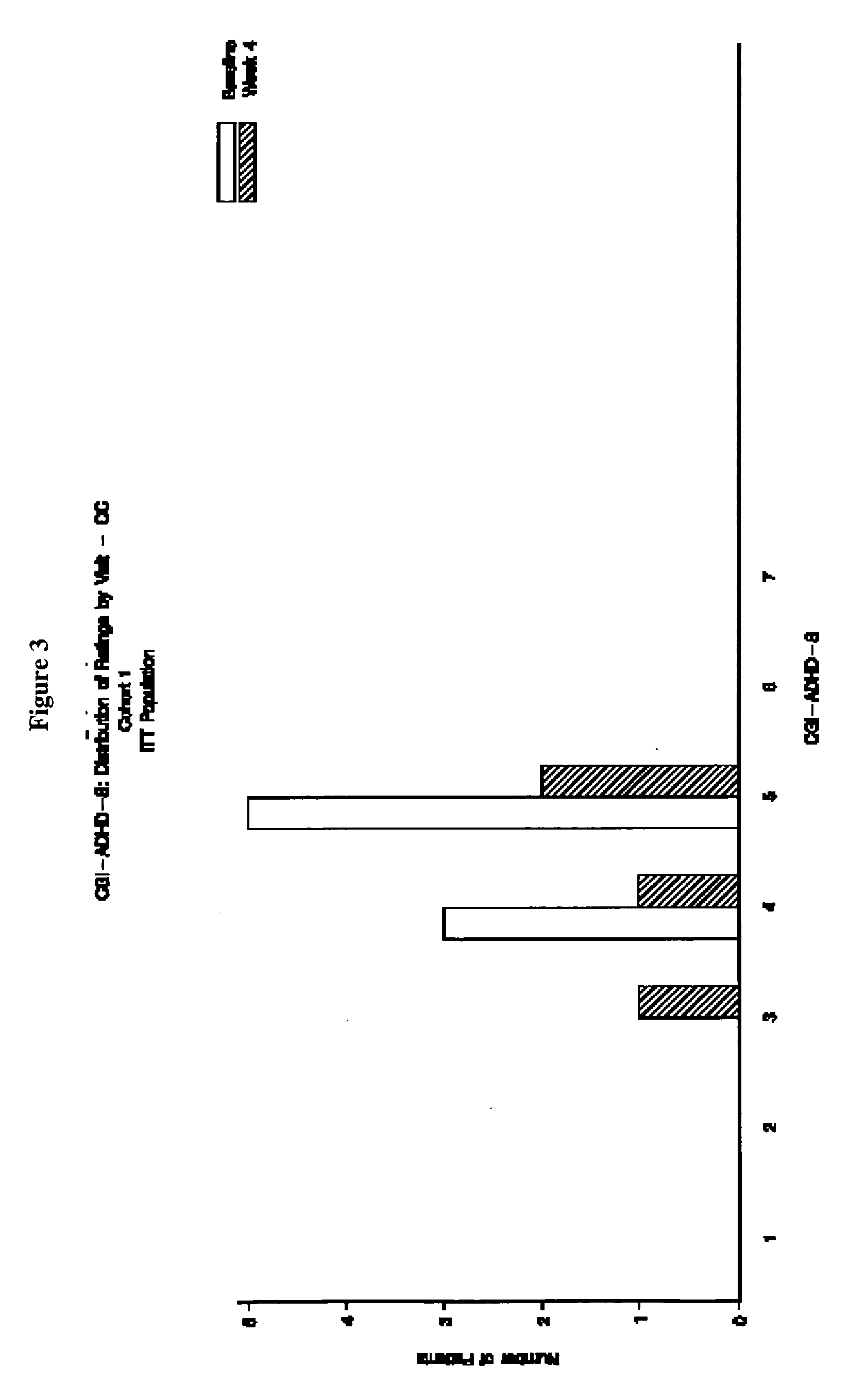
The present invention provides a method for the treatment of individuals diagnosed with a childhood behavioral disorder such as autistic spectrum disorders or combined type Attention-Deficit / Hyperactivity Disorder (ADHD) by administering an effective amount of memantine.
Owner:FOREST LAB HLDG LTD
Use of Memantine (Namenda) to Treat Autism, Compulsivity and Impulsivity
The present invention relates to the treatment of compulsive, impulsive and pervasive developmental disorders. More particularly, the methods described herein comprise administration of memantine to an individual suffering from such a disorder in an amount effective to relieve one or more symptoms of said disorder. In particularly preferred aspects, the invention is directed to the use of memantine for the treatment of autism
Owner:MT SINAI SCHOOL OF MEDICINE
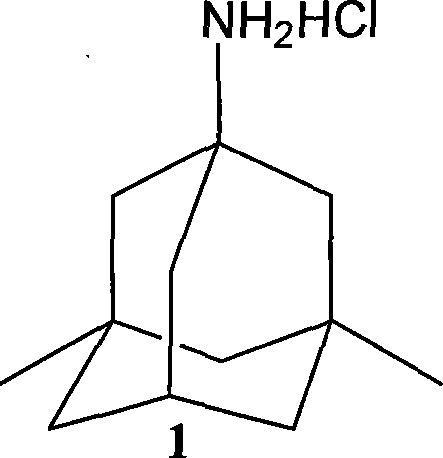
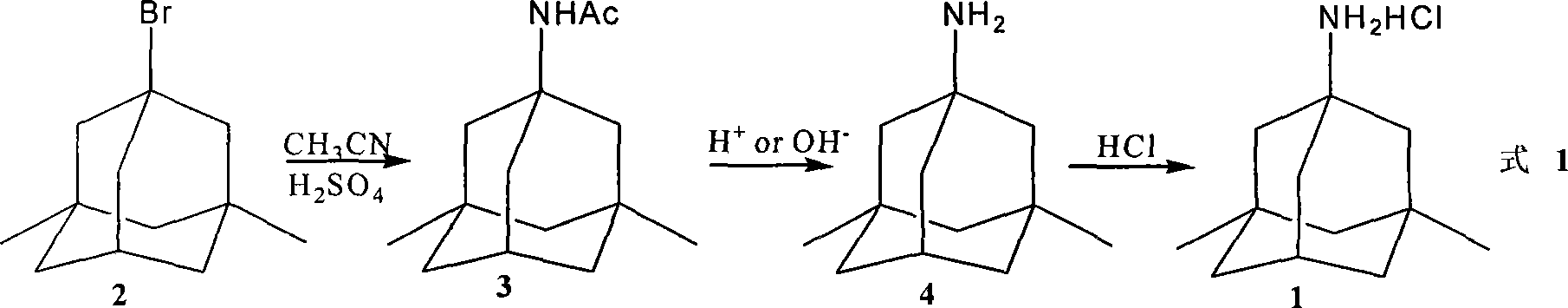
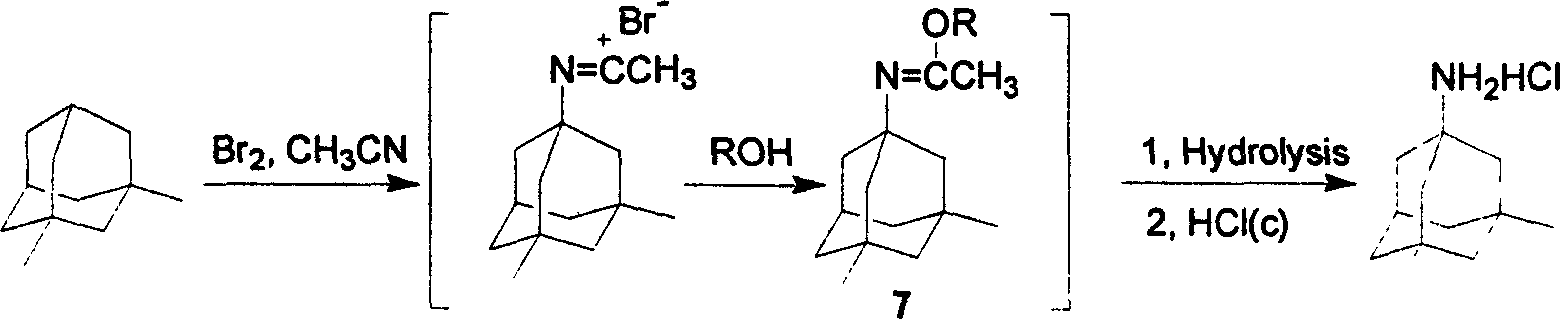
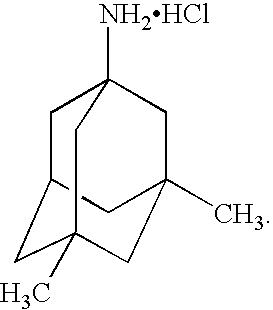
Preparation method of memantine salt
ActiveCN101041622ALess impuritiesProduct quality is easy to controlOrganic compound preparationAmino compound preparationRitter reactionMemantine
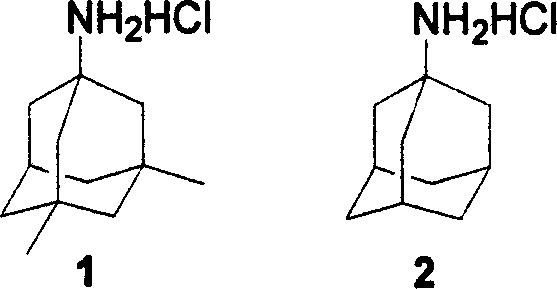
The invention discloses a new making method of amantadine, which comprises the following steps: adopting 1, 3-dimethyl adamantane as raw material to do halogenation and Ritter reaction; separating and purifying the make 1-subtituted-amido-3, 5-dimethyl diamantine as intermediate; hydrolyzing the intermediate into diamantine; extracting; obtaining rough product; recrystallizing the rough product to obtain the fined product; fitting for industrial manufacturing.
Owner:ZHUHAI UNITED LAB
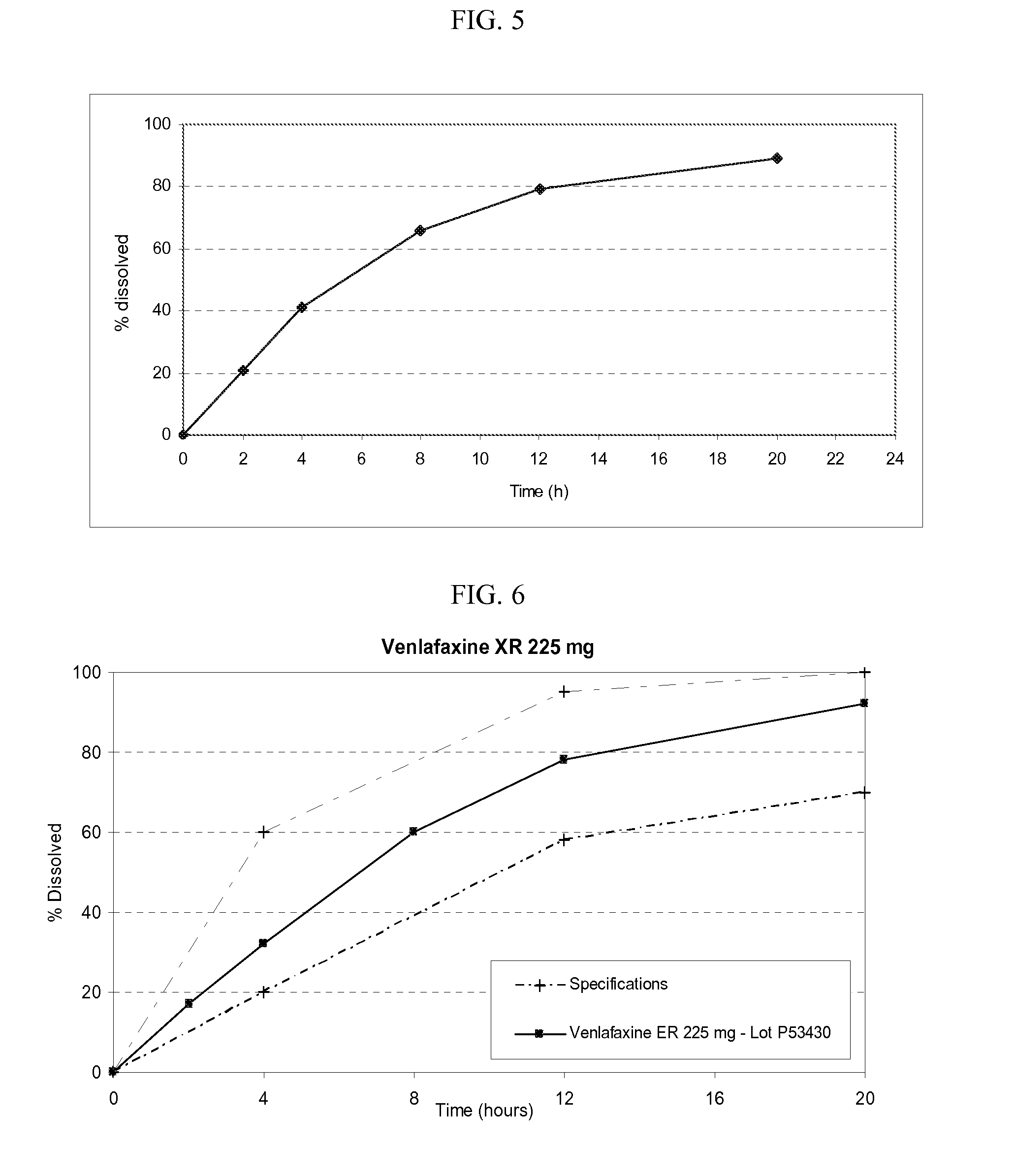
Venlafaxine osmotic device formulation
InactiveUS20070077301A1Reduced food effectReducing food effectBiocideOrganic active ingredientsImmediate releaseNeurological disorder
The present invention provides an osmotic device containing controlled release venlafaxine in the core, wherein the osmotic device exhibits a reduced food effect as compared to a reference controlled release capsule formulation. Some embodiments include venlafaxine in controlled release form in combination with an anti-Alzheimer's or an anti-Parkinson's drug in a rapid release external coat. Memantine is used as an anti-Alzheimer's drug or an anti-Parkinson's drug. Particular embodiments of the invention provide osmotic devices having predetermined release profiles. One embodiment of the osmotic device includes an external coat that has been spray-coated rather than compression-coated onto the device. The device is useful for the treatment of depression in Alzheimer's and / or Parkinson's patients. The device and method can also be used to treat or ameliorate other symptoms associated with Alzheimer's disease, Parkinson's disease or any other neurological disorder. Other dosage forms that provide a controlled, sustained or extended release of venlafaxine in combination with a rapid or immediate release of memantine are useful in the invention.
Owner:OSMOTICA KERESKEDELMI & SZOLGALTATO
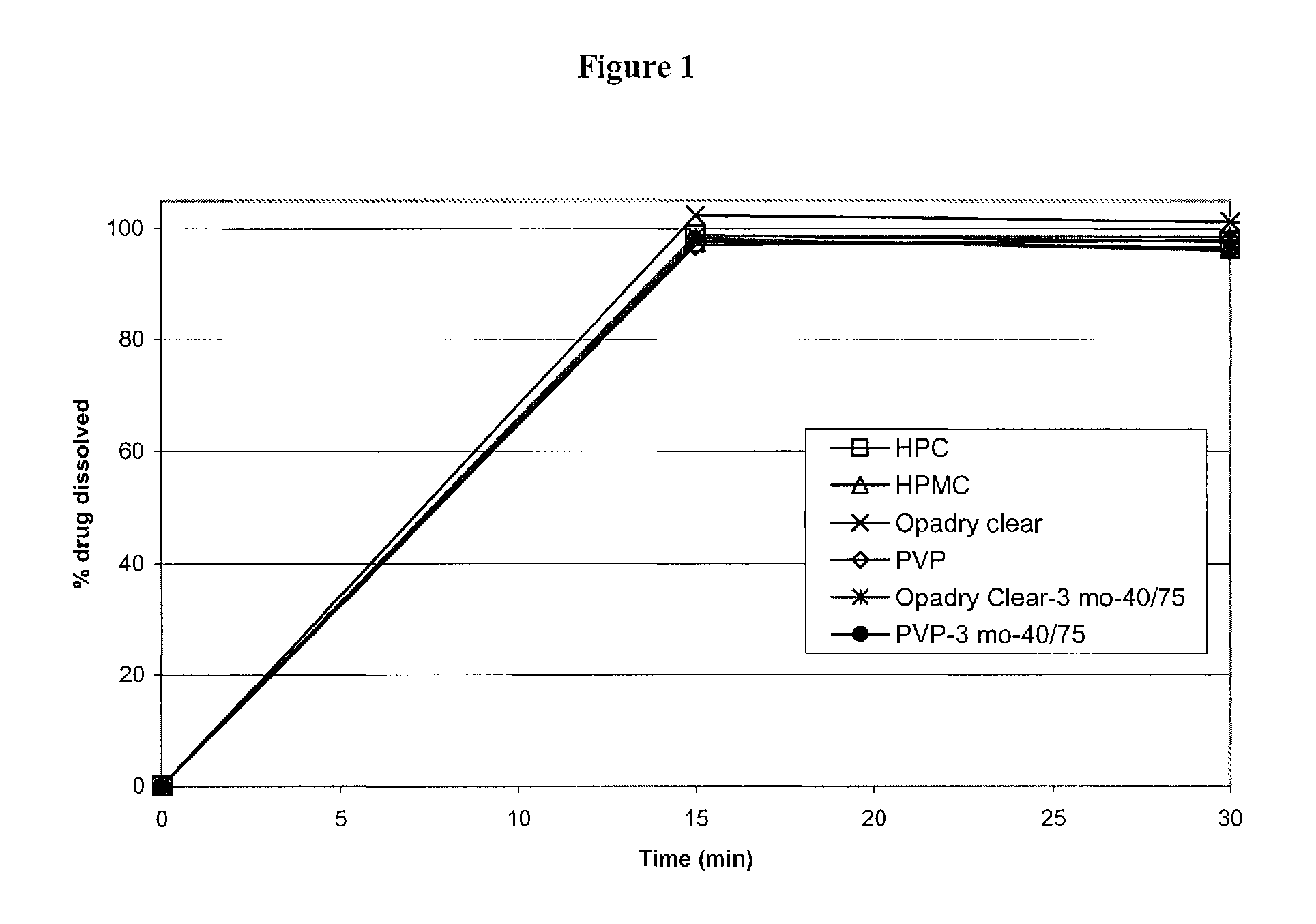
Pharmaceutical compositions of memantine
InactiveUS20140050784A1Prevent adverse side effectsMaintain securityBiocidePill deliveryNeuropathic painPharmaceutical formulation
The present invention relates to oral dosage forms comprising Memantine or a pharmaceutically acceptable salt thereof, pharmaceutical formulations comprising the oral dosage forms, and methods for treating mild, moderate or severe Alzheimer's dementia, or neuropathic pain comprising the oral dosage forms and formulations.
Owner:TEVA PHARM USA INC +1
Method of synthesizing amantadine hydrochloride
InactiveCN1556094ASmall side effectsMild reaction conditionsOrganic compound preparationAmino compound preparationAlcoholBoiling point
A process for synthesizing adamantanamine hydrochloride from 1,3-dimethyl adamantane include browo reaction while Ritter reaction, adding the resultant mixture in the mixture of polar proton solvent and alcohol solvent, stirring while alcoholyzing, regulating pH, alkaline hydrolyzing to generate adamantanamine, extracting in arylhydrocarbon solvent, water washing, drying, filtering, vacuum concentrating of filtrate, reacting on concentrated hydrochloric acid to obtain coarse product, and recrystallizing in the mixed solvent of alcohol and arylhydrocarbon.
Owner:NANJING UNIV
Orally Dissolving Formulations of Memantine
Orally dissolving formulations, e.g., tablets (ODTs) and films (ODFs) comprising memantine and methods of treating conditions, including childhood behavioral disorders and Alzheimer's disease, by administering orally dissolving formulations are provided. The orally dissolving formulations of the present invention may be used to treat various conditions, but is particularly suited to treat childhood behavioral disorders, such as autistic spectrum disorders or combined type Attention-Deficit / Hyperactivity Disorder (ADHD) and also to treat elderly patients suffering from Alzheimer's disease.
Owner:FOREST LAB HLDG LTD
Memantine for the treatment of childhood behavioral disorders
The present invention provides a method for the treatment of individuals diagnosed with a childhood behavioral disorder such as autistic spectrum disorders or combined type Attention-Deficit / Hyperactivity Disorder (ADHD) by administering an effective amount of memantine.
Owner:FOREST LAB HLDG LTD
Memantine combinations and use
ActiveUS20180116979A1Simplify the management processNervous disorderPharmaceutical delivery mechanismDonepezilSolifenacin
A pharmaceutical combination of memantine and a non-anticholinergic antiemetic agent for the treatment of hypocholinergic disorders in further combination with high doses of donepezil and with solifenacin, and kits comprising said combination. A pharmaceutical combination of memantine and solifenacin for the treatment of hypocholinergic disorders, including Alzheimer type dementia, in further combination with high doses of donepezil, and kits comprising said combination.
Owner:CHASE PHARMA CORP
Memantine Oral Dosage Forms
This invention relates to an oral dosage form containing between 1 mg and 100 mg of memantine, wherein said dosage form does not contain 10 mg of memantine or 20 mg of memantine, and wherein said dosage form is not prepared by the patient or a person administering the drug to the patient who divides the dosage form containing a larger dose of memantine. Other aspects of this invention relate to pharmaceutical products comprising said dosage forms and methods of administering memantine and treating disease with said dosage form.
Owner:FIRESTONE BRUCE A +7
Pharmaceutical compositions of memantine
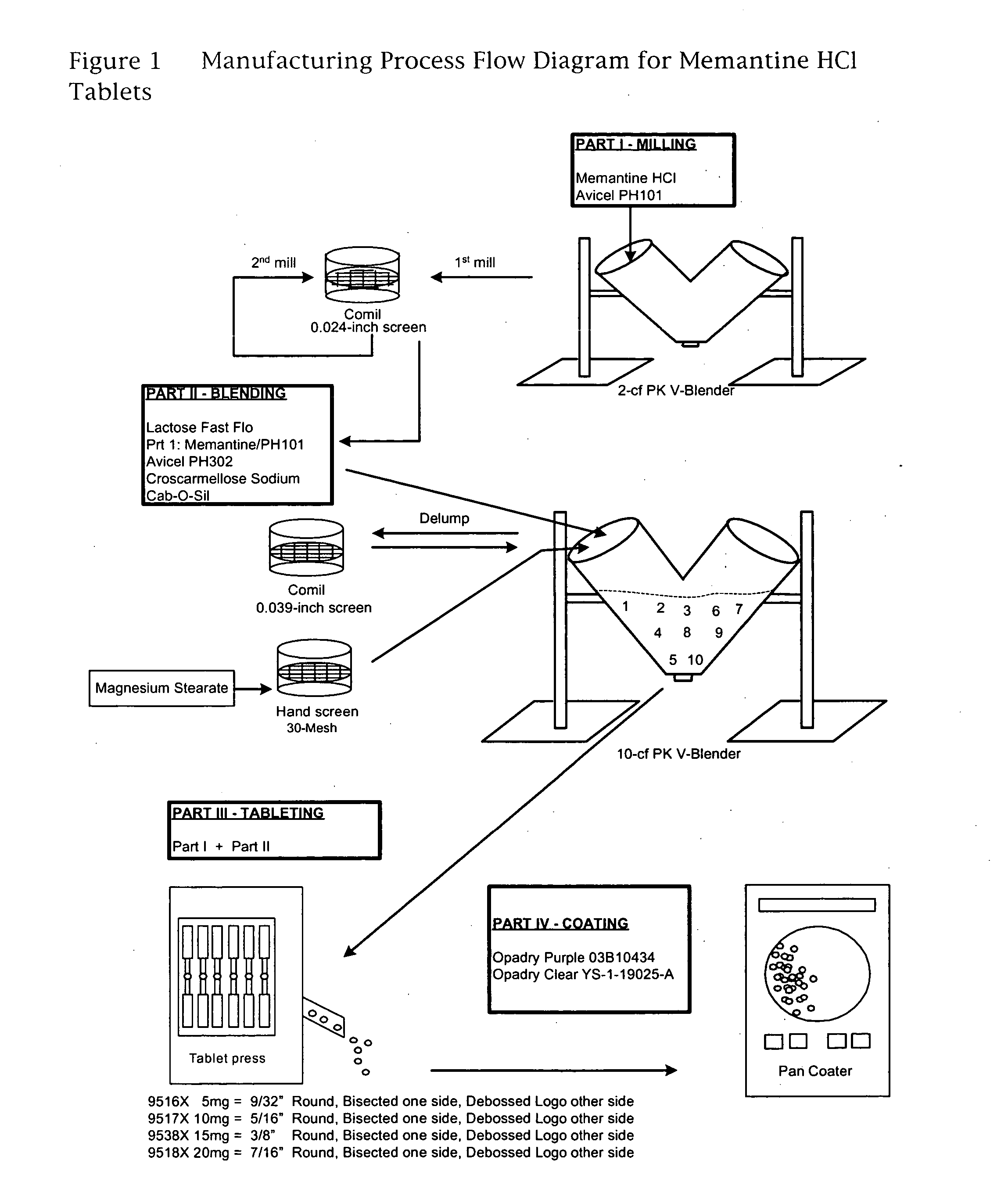
The invention is directed to easily dissolved, stable dose proportional pharmaceutical compositions, comprising granulated memantine and methods of preparing the same. In particular, the invention is directed to granulated memantine pharmaceutical compositions in the form of film coated tablets.
Owner:TEVA PHARM USA INC
Memantine For The Treatment Of Mild And Mild To Moderate Alzheimer's Disease
InactiveUS20100048726A1Preventing decrease in glucose metabolismBiocideNervous disorderDiseaseMidbrain
Owner:FOREST LAB HLDG LTD
Assay methods for memantine
InactiveCN101801911AAmino compound purification/separationOrganic compound preparationMemantineImmunology
Owner:GENERICS UK LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com