Patents
Literature
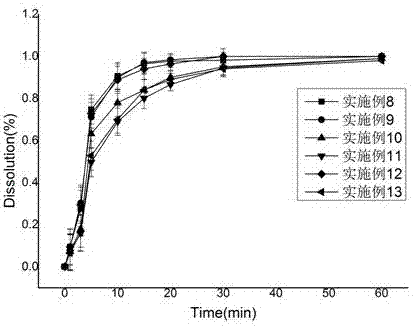
154 results about "Donepezil" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
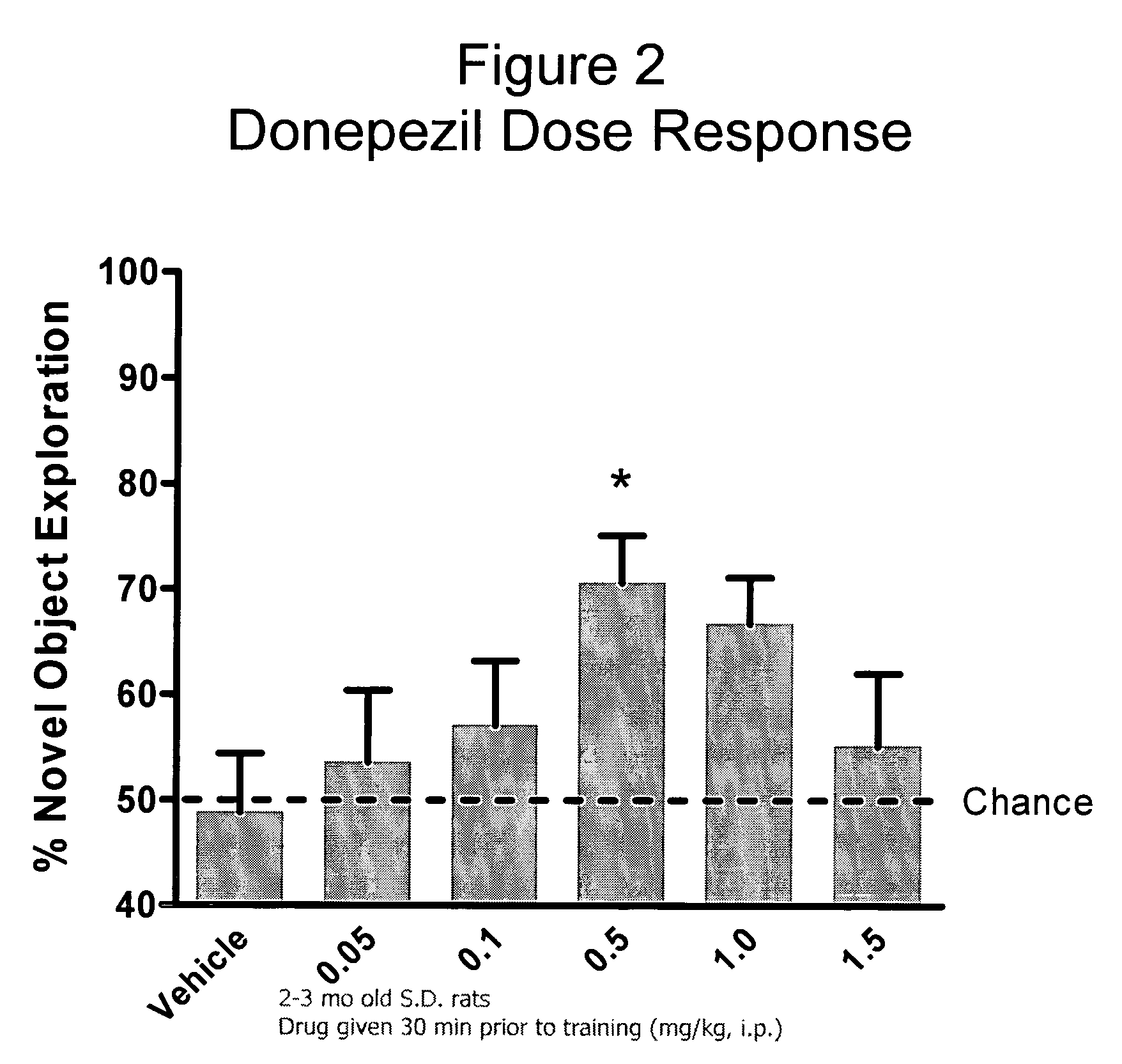
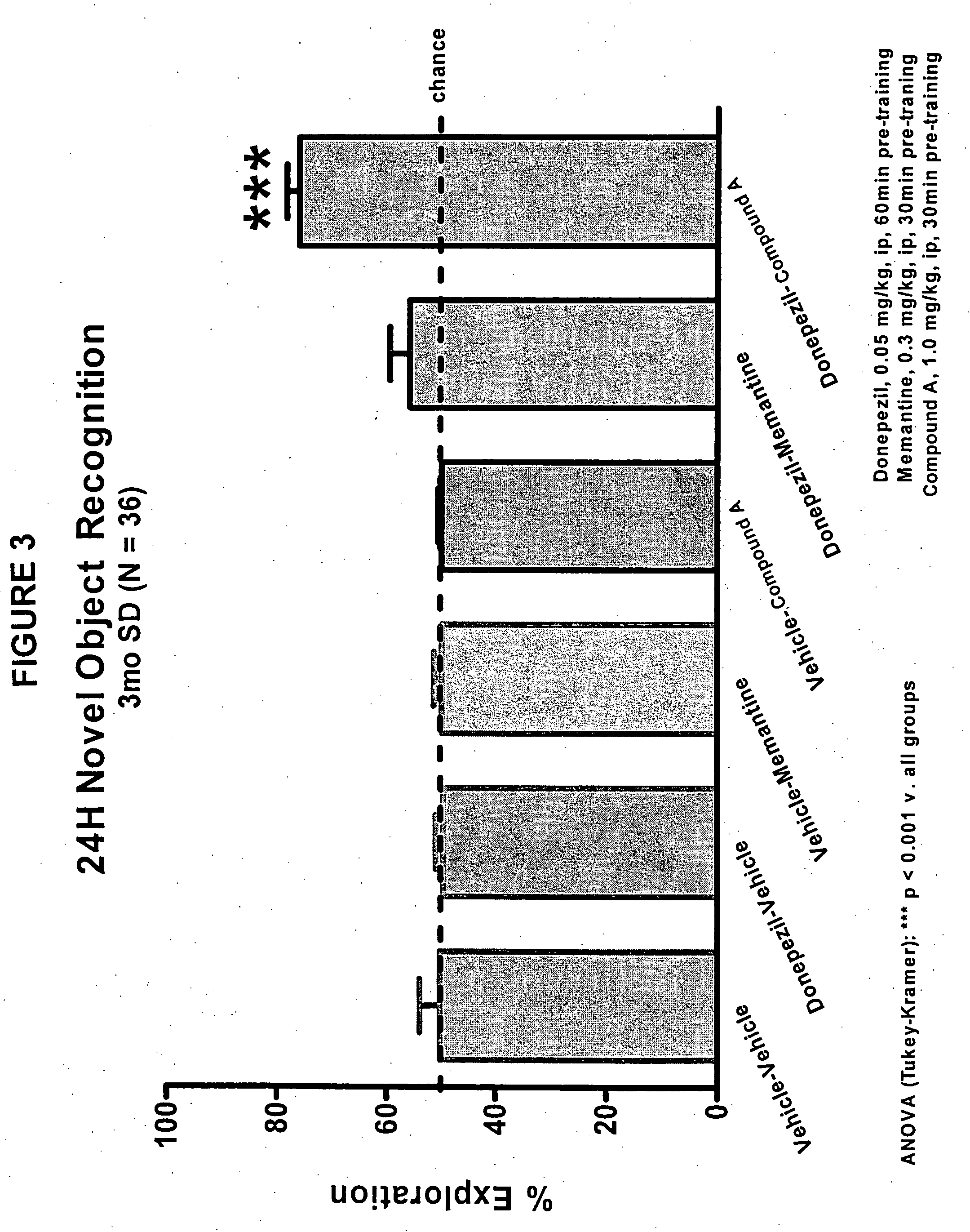
Donepezil is used to treat confusion (dementia) related to Alzheimer's disease.
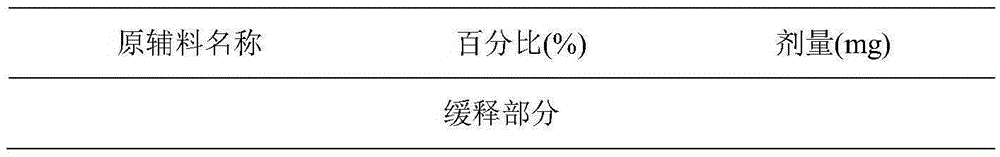
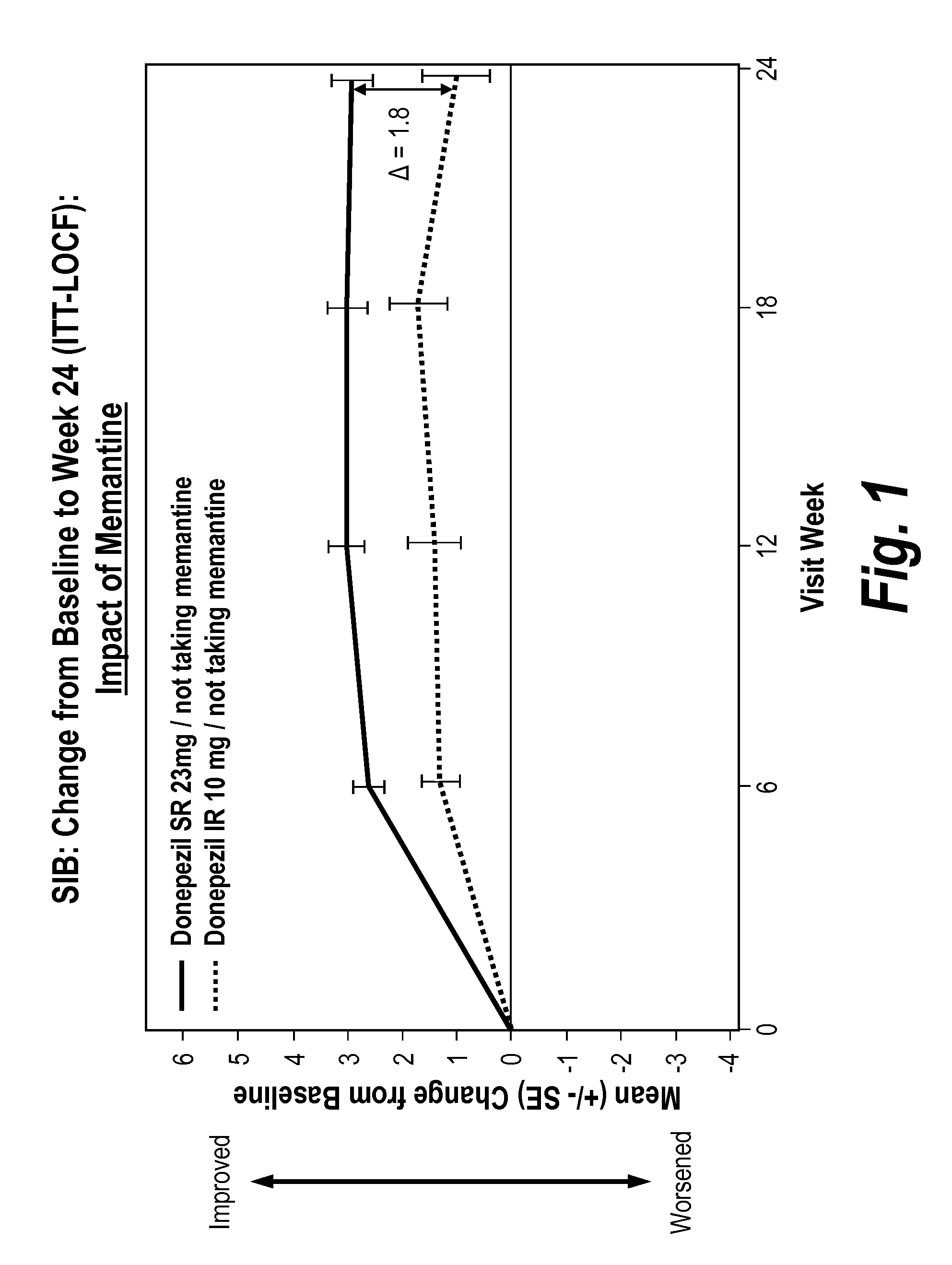
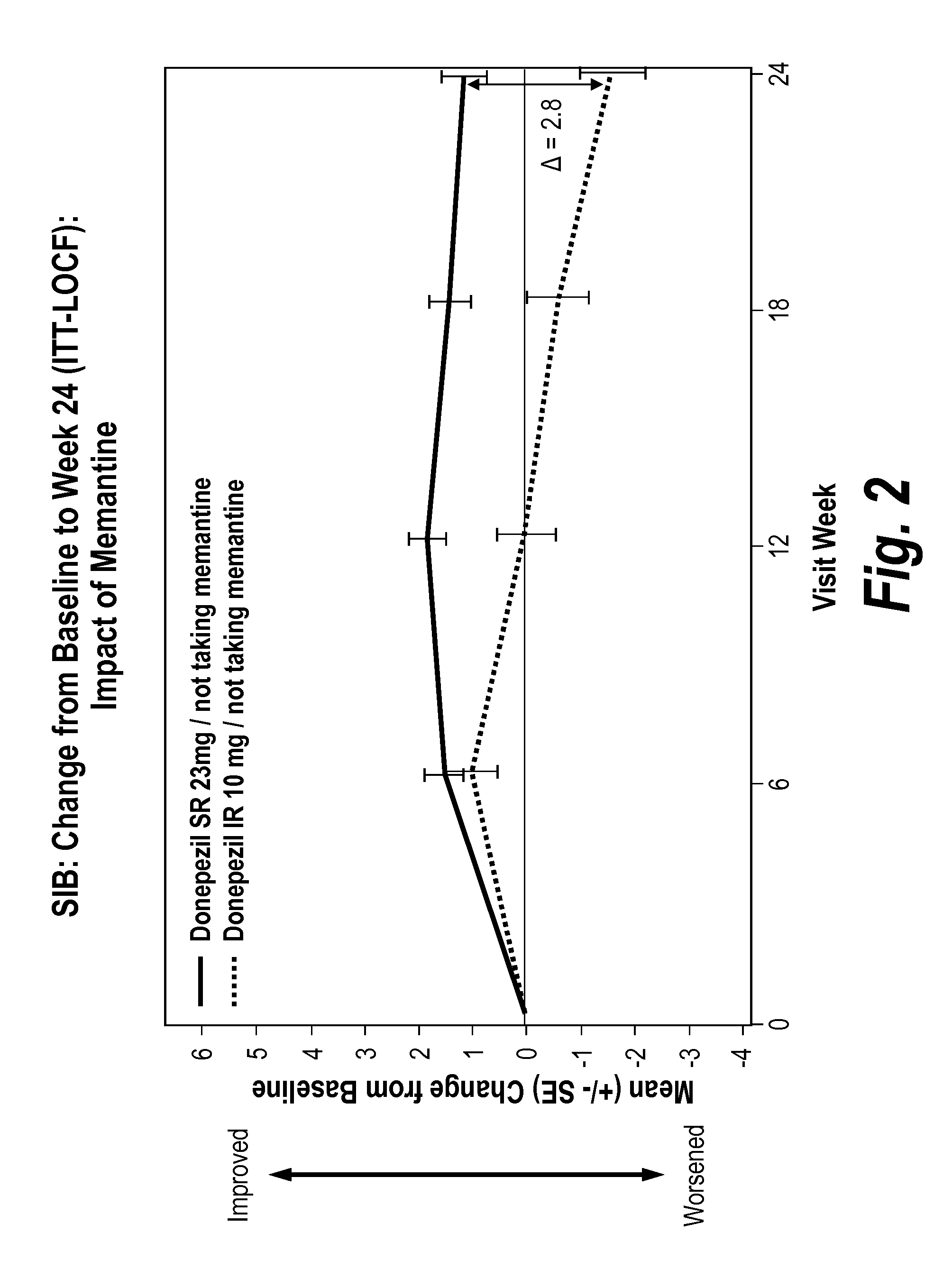
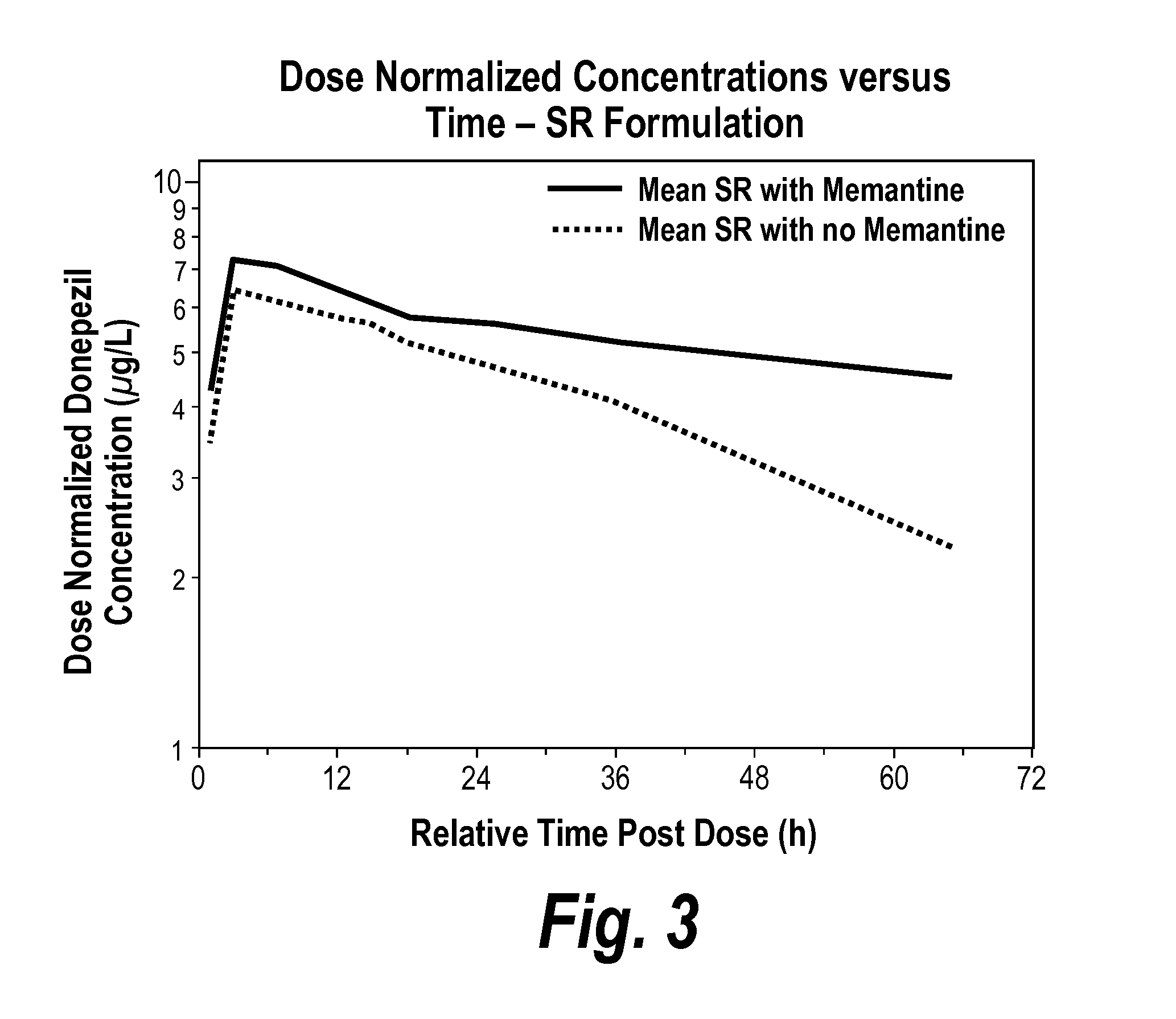
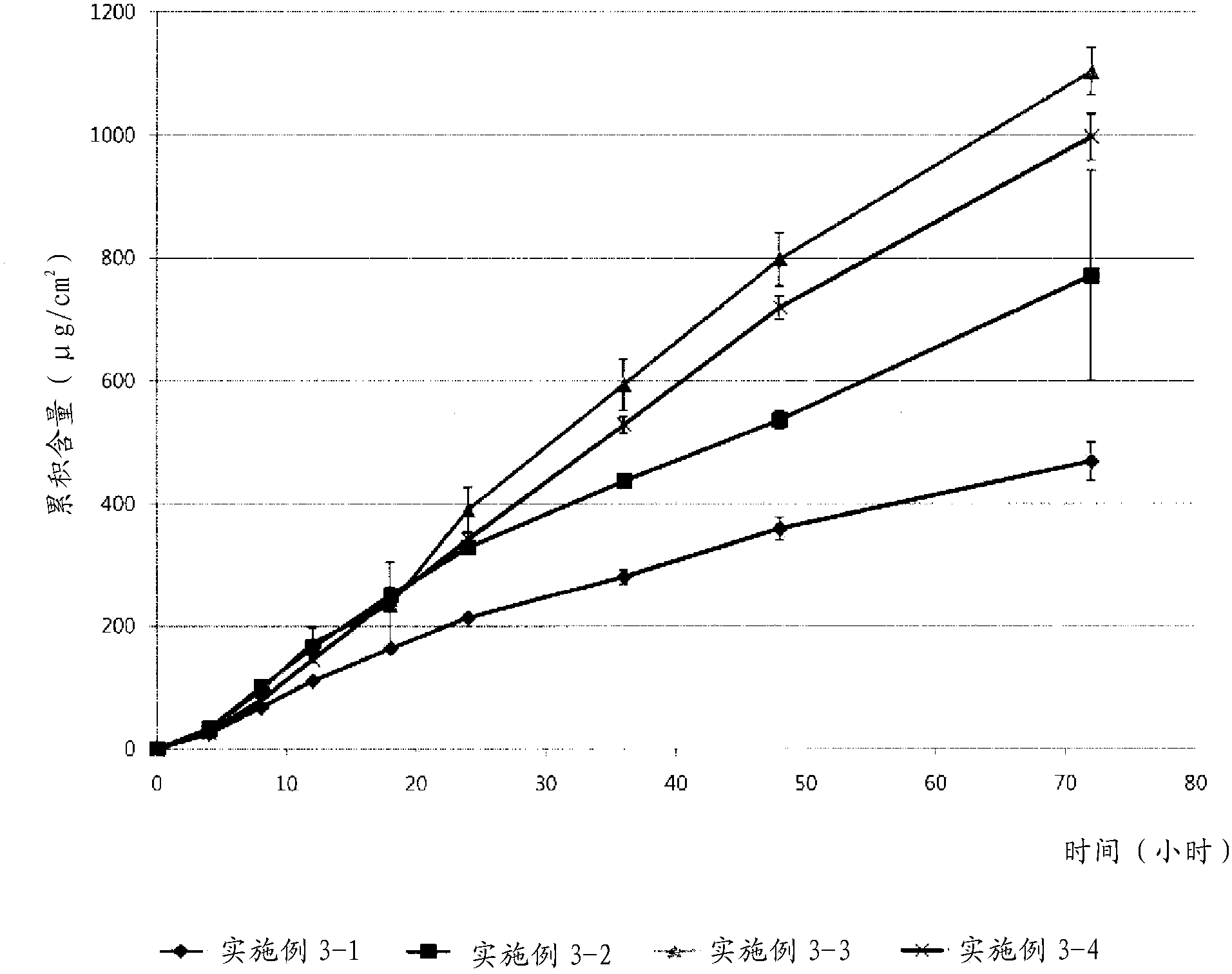
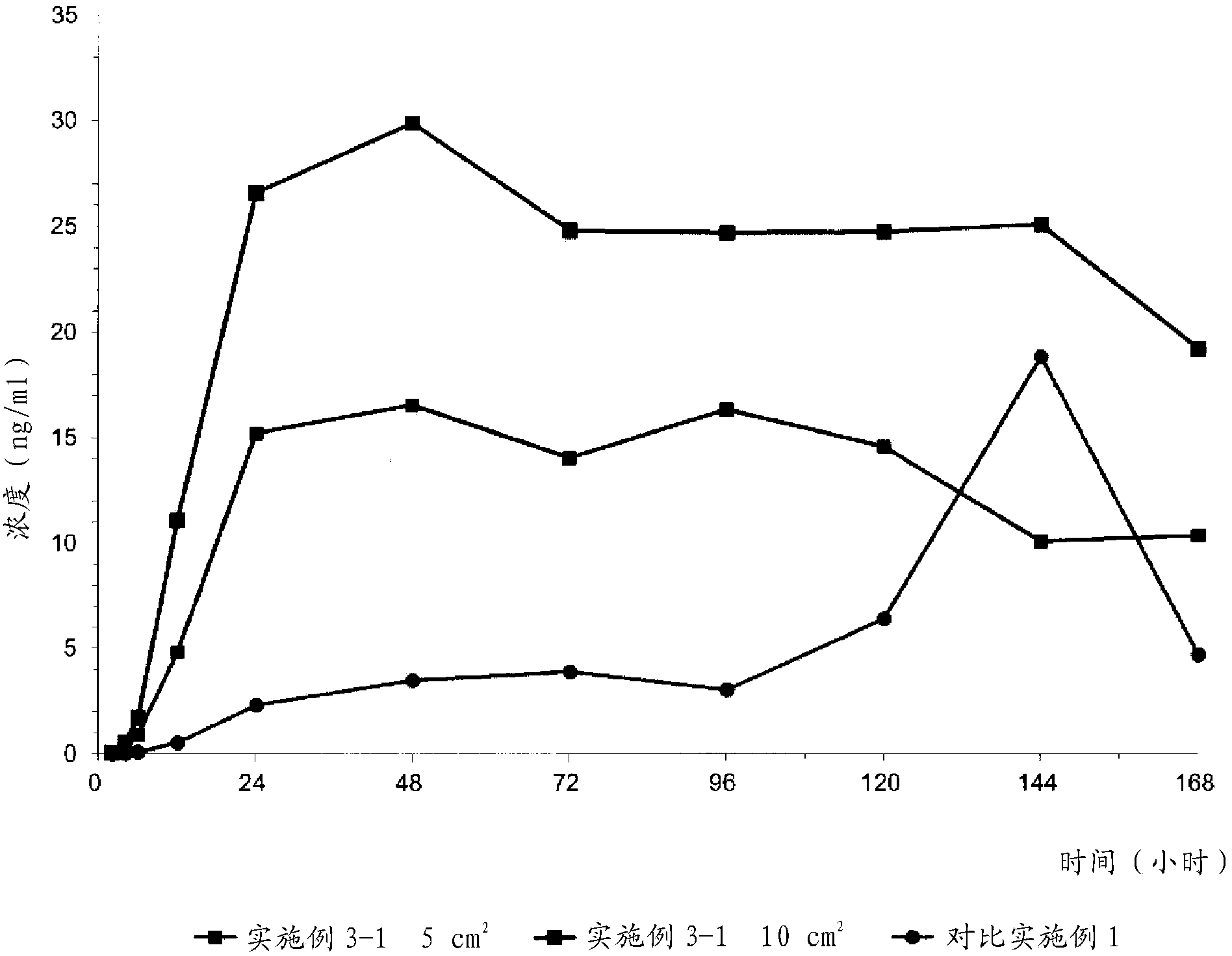
Donepezil formulations
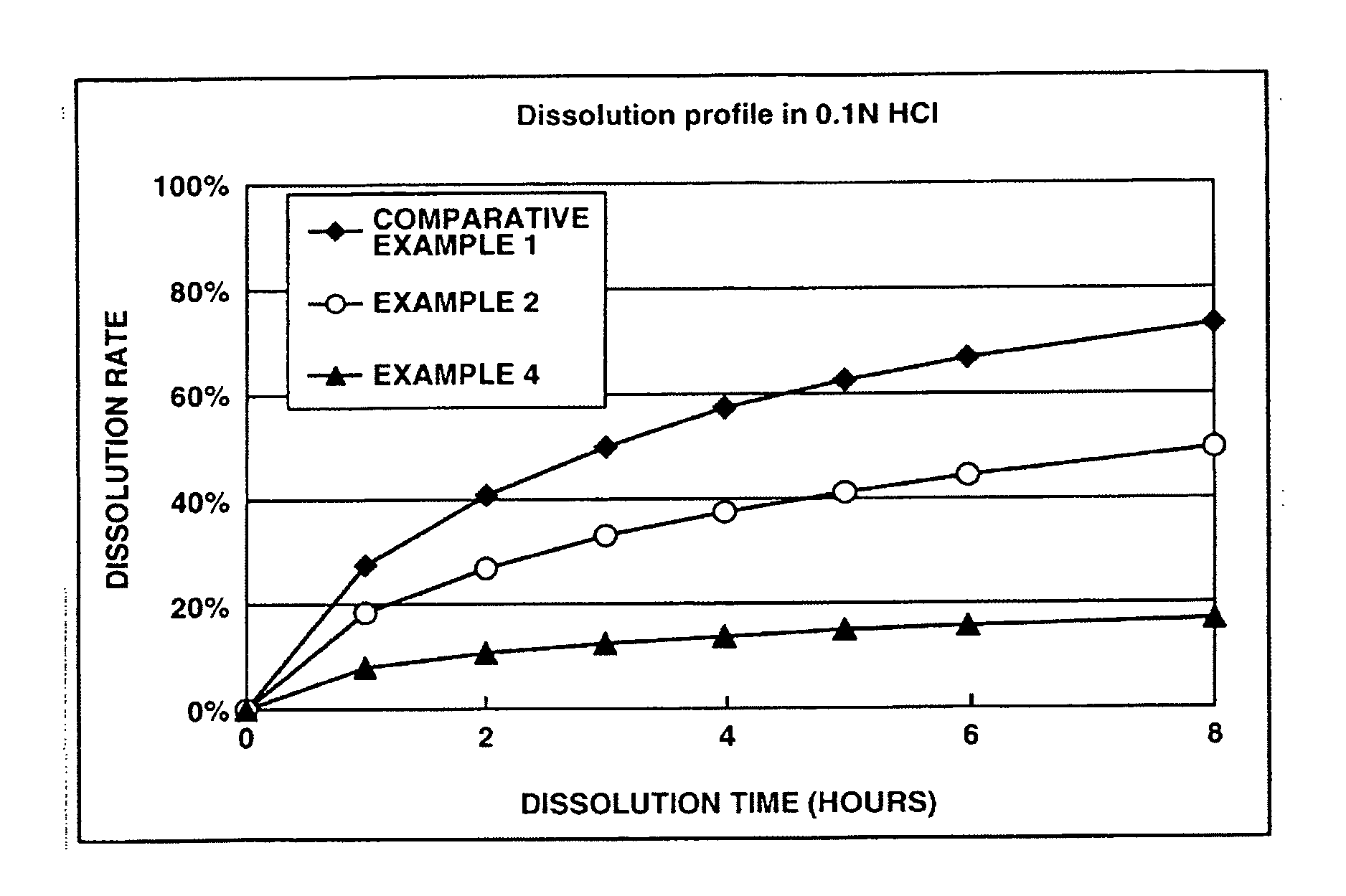
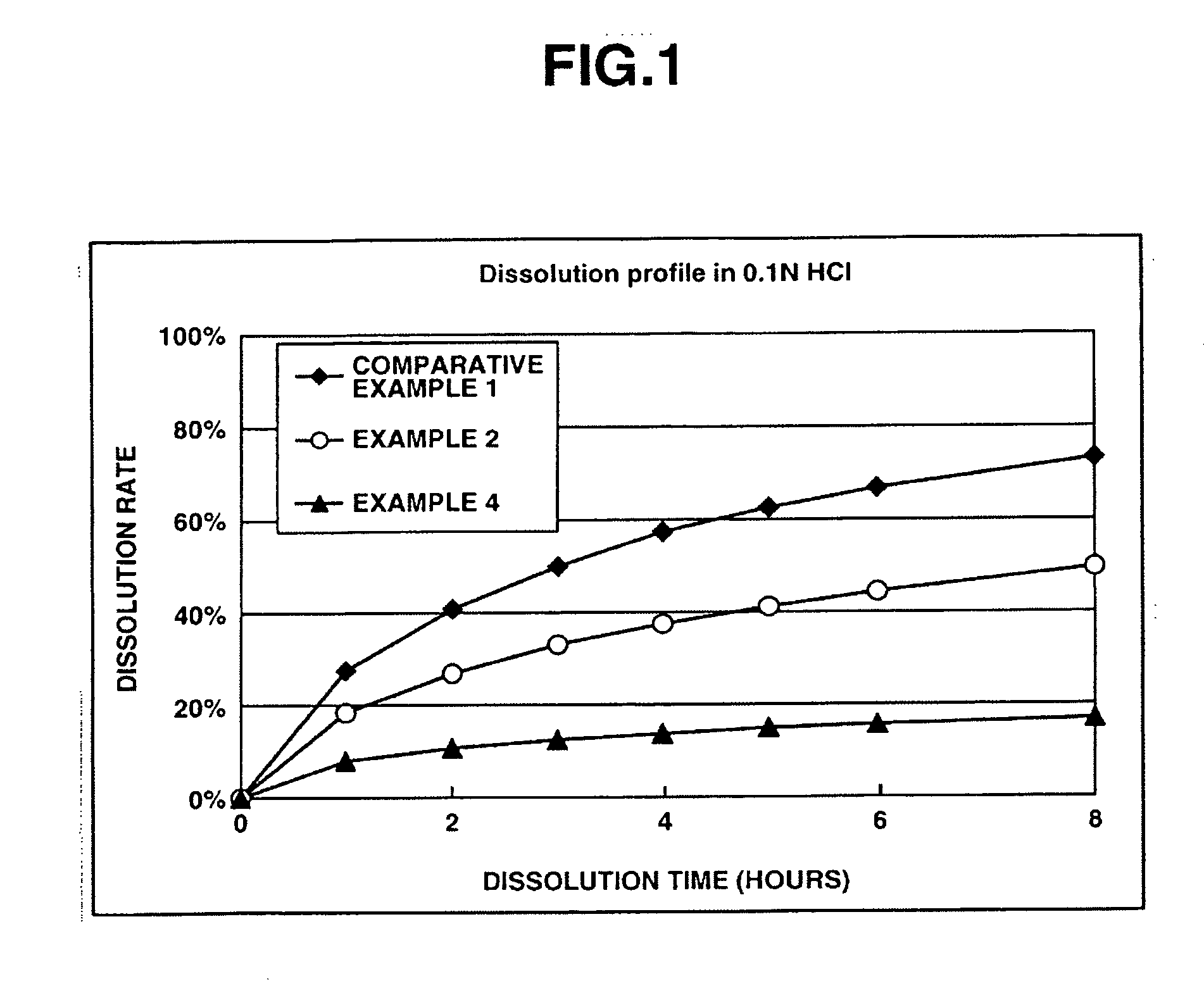
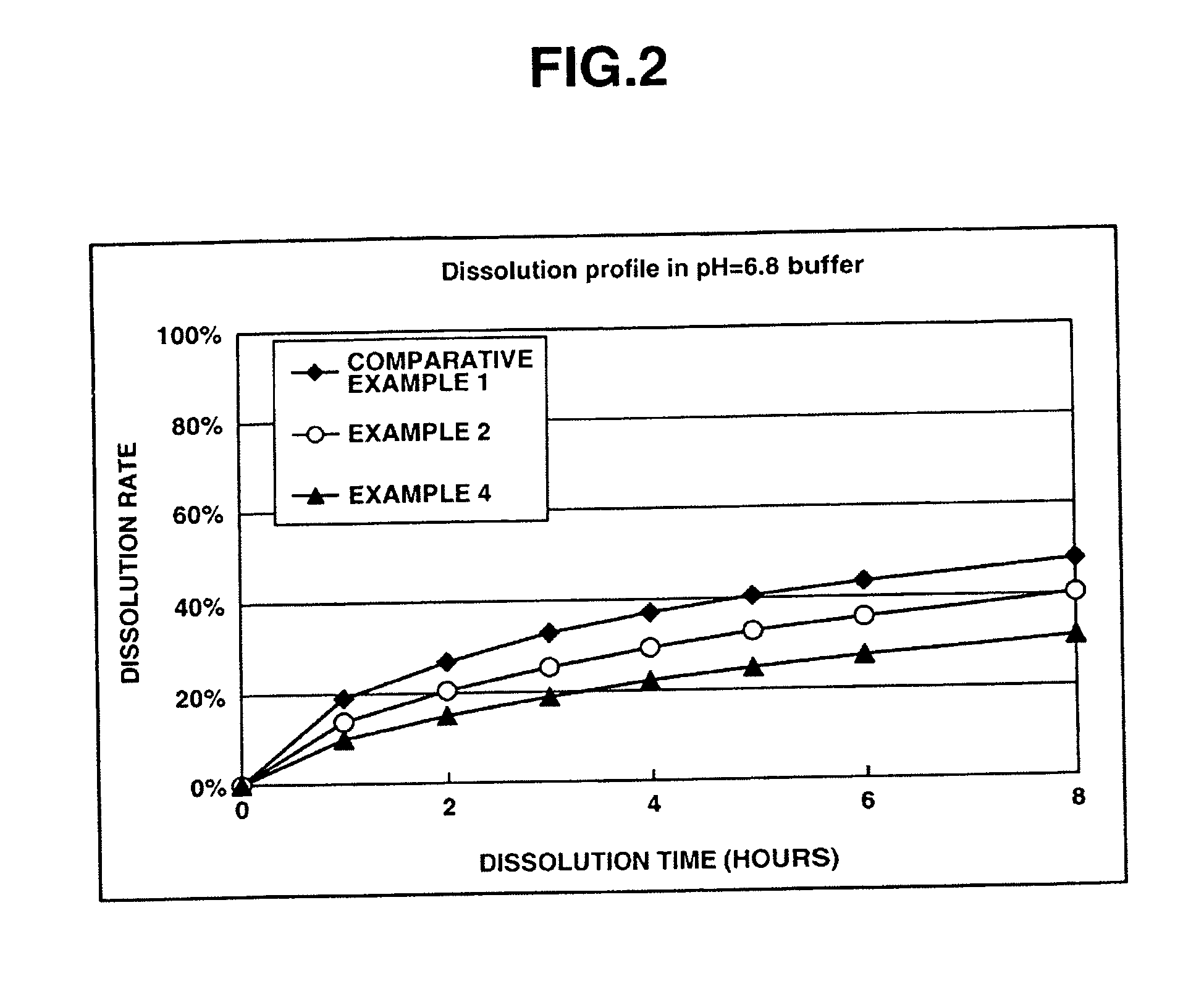
InactiveUS20050232990A1Highly preventive effectEasy to openPowder deliveryBiocideDonepezilSustained Release Formulations
Owner:ACTAVIS GRP PTC EHF
Extended release pharmaceutical composition of donepezil
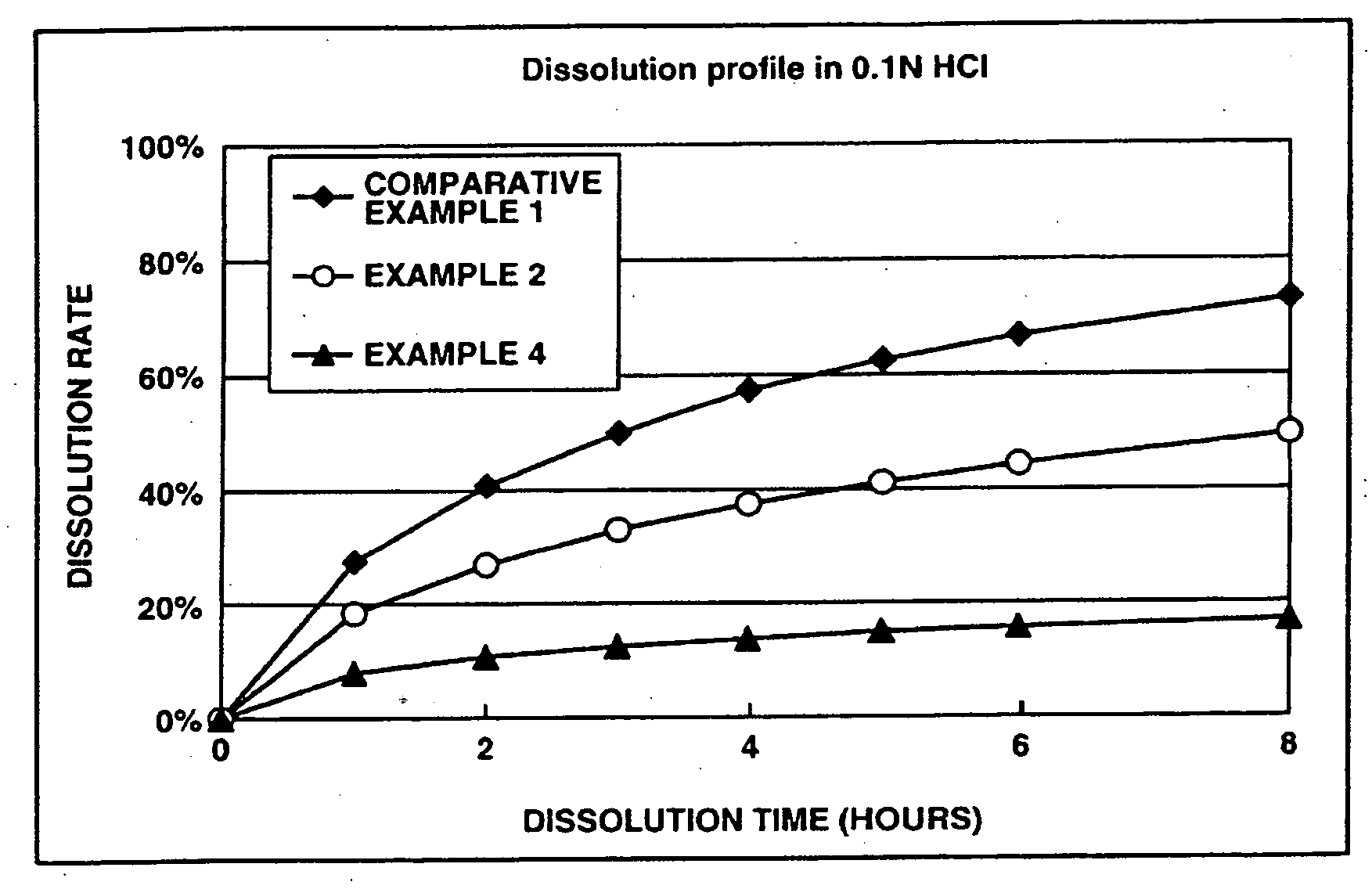
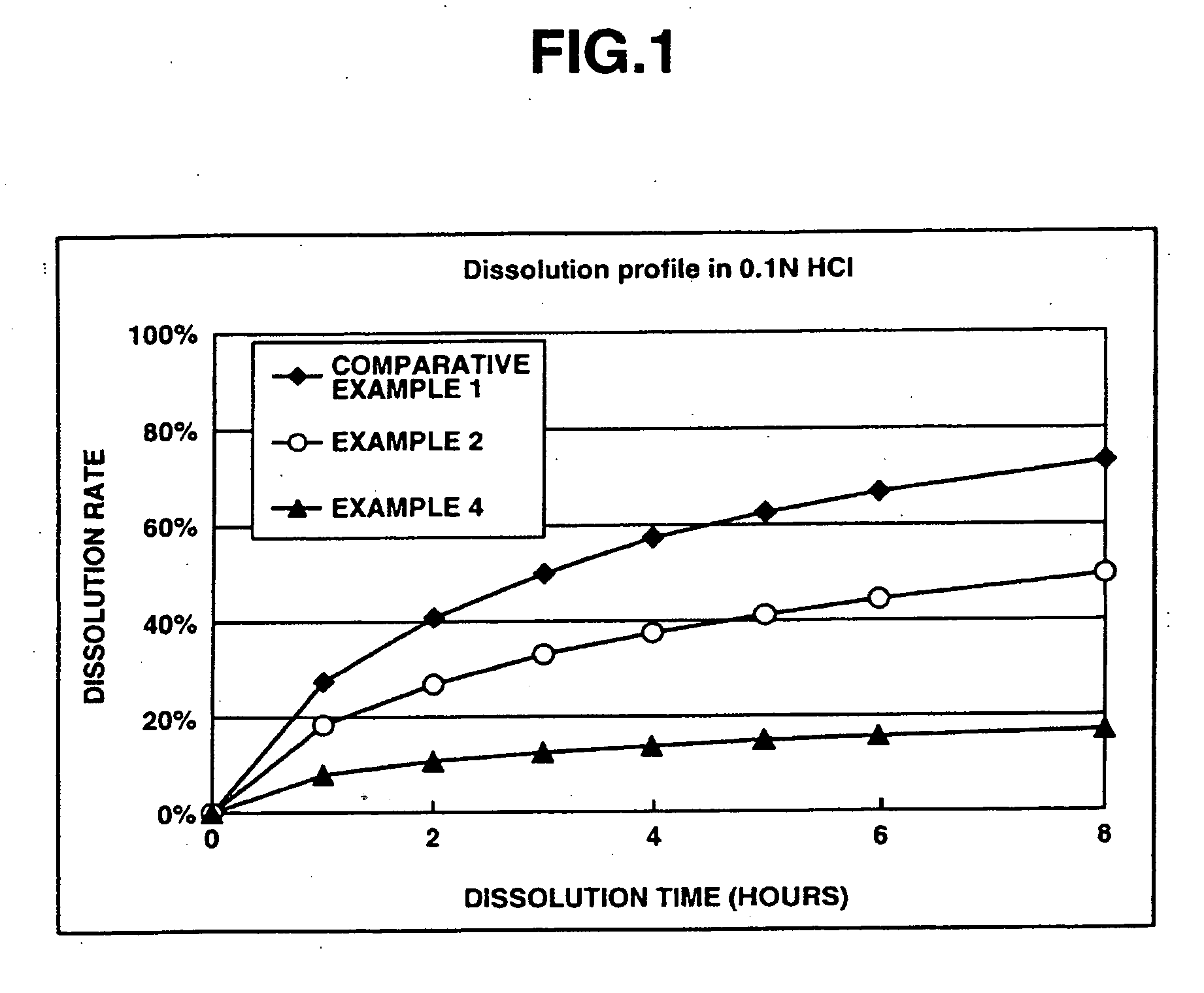
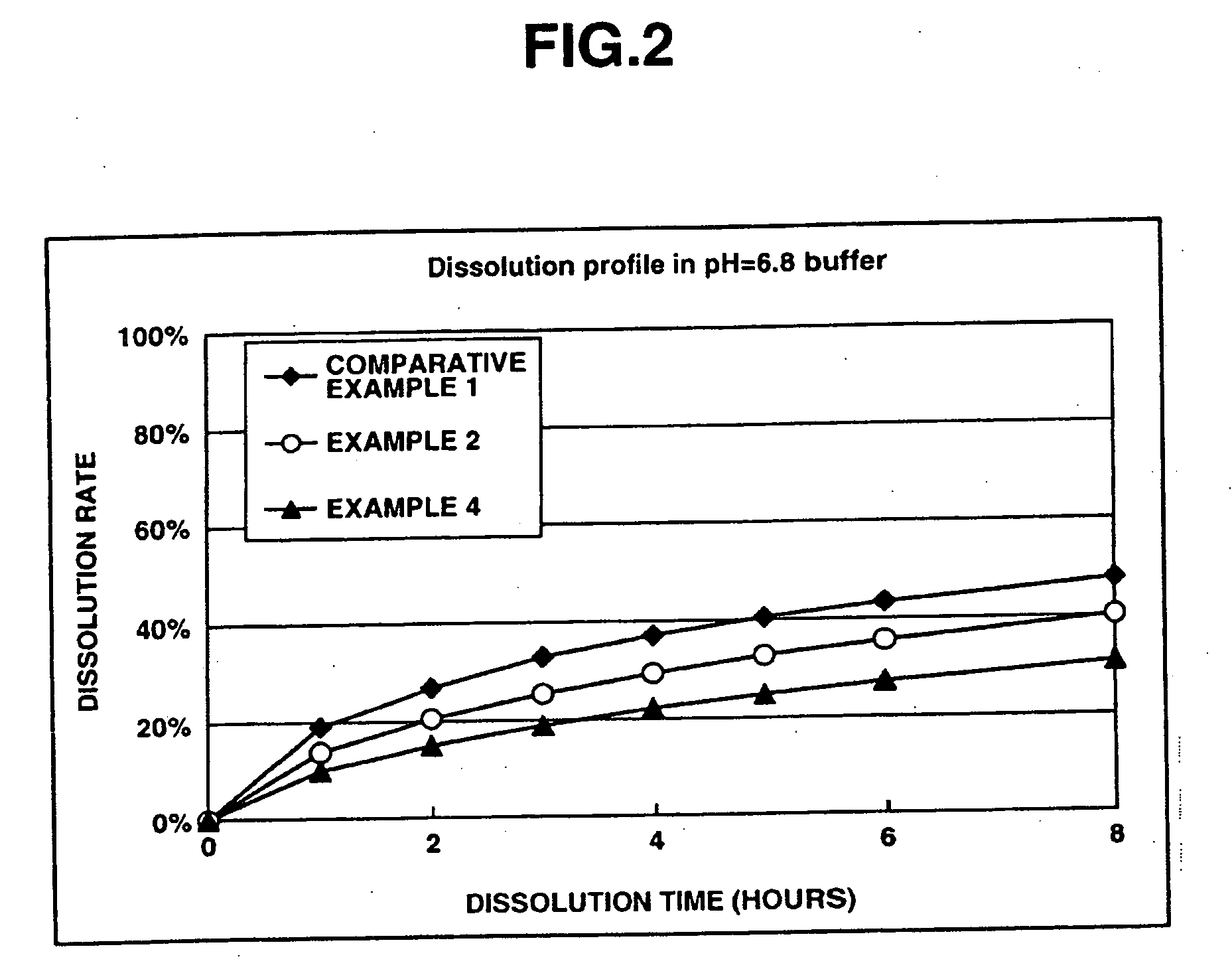
The present invention relates to an extended release pharmaceutical composition for oral administration comprising donepezil or pharmaceutically acceptable salt thereof and a release-controlling agent. Further, it relates to process for preparation of said compositions.
Owner:RANBAXY LAB LTD
Sustained release formulations
InactiveUS20060280789A1Reduce in quantityImprove complianceBiocidePill deliveryDonepezilCholinesterase
The invention provides sustained release formulations of basic drugs, stereoisomers of basic drugs, pharmaceutically acceptable salts of basic drugs, and pharmaceutically acceptable salts of stereoisomers of basic drugs. The basic drugs may be anti-dementia drugs, such as cholinesterase inhibitors or memantine. In one embodiment, the cholinesterase inhibitor is donepezil.
Owner:EISAI CO LTD
Sustained release formulations
The invention provides sustained release formulations comprising donepezil, stereoisomers of donepezil, pharmaceutically acceptable salts of donepezil, or pharmaceutically acceptable salts of stereoisomers of donepezil. The formulations have desirable pharmacokinetic characteristics. Examples include AUC, Tmax, Cmax, dosage-corrected AUC, and dosage-corrected Cmax.
Owner:EISAI CO LTD
Compositions and methods of treatment using L-type calcium channel blockers and cholinesterase inhibitors
InactiveUS20050153953A1Good curative effectProlong the action timeBiocideNervous disorderDonepezilCholinesterase
The present invention relates to compositions comprising at least one L-type calcium channel blocker, particularly the compound (+)-isopropyl 2-methoxyethyl 4-(2-chloro-3-cyano-phenyl)-1,4-dihydro-2,6-dimethyl-pyridine-3,5-dicarboxylate, in combination with at least one cholinesterase inhibitor, particularly donepezil, and uses thereof in methods of treatment.
Owner:MEMORY PHARMA CORP
Preparation method of donepezil oral fast dissolving film
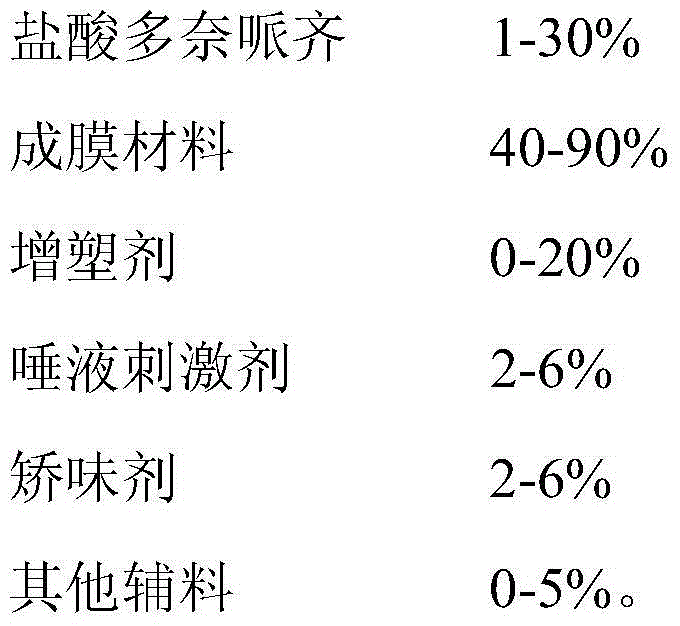
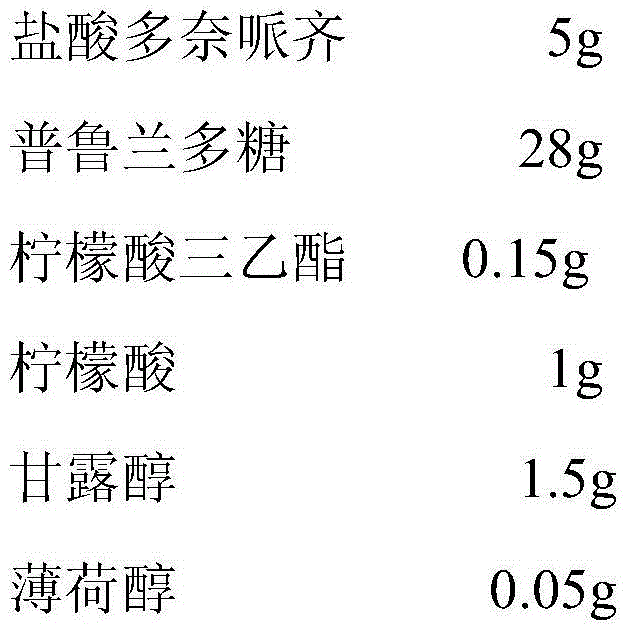
InactiveCN104940174ASimple processGood flexibilityNervous disorderPharmaceutical non-active ingredientsDiseaseDonepezil
The invention belongs to the field of medicinal preparations, and particularly relates to a donepezil oral fast dissolving film, a preparation method and medical application thereof. The film comprises the following components by weight percent: 1-30% of donepezil, 40-90% of a film-forming material, 0-20% of a plasticizer, 2-6% of a saliva irritant, 2-6% of a corrigent and 0-5% of other auxiliary materials. According to the donepezil oral fast dissolving film, water is not needed during medicine taking, and the film can be quickly melted in the mouth, so that the phenomenon that patients with alzheimer's disease hide medicine in the mouth and spilt medicine is avoided, and the medication compliance of patients is improved; the basic remedy, obtained from the preparation method provided by the invention, of the dosage form is uniformly dispersed in the film-forming material; the finished product is good in appearance; the basic remedy can be quickly dissolved out, and is good in stability; the product dosage is accurate; the production process is free of flying dust, so that the problems related to labor protection and environmental pollution can be solved.
Owner:HEFEI HUAFANG PHARMA SCI & TECH
Donepezils compound long-acting slow-releasing and controlled-releasing composition and preparation method thereof
InactiveCN101167697AReduce releaseImprove compliancePowder deliveryNervous disorderDonepezilProcess equipment
The invention discloses a long-acting sustained controlled release formulation of donepezil-like composition and a process for preparation. The composition is in the shape of micro-granular. According to the measurement by weight, the invention contains donepezil-like compounds 0.5-5 parts, carrier material of the sustained controlled release formulation 1-500 parts, and further contains other common excipient in pharmacy. The composition is used for anti senile dementia. The frequency of donepezil-like compounds is reduced, and the periodic time of administering medicament is prolonged, the compliance and conformability of the patient are improved, and the bioavailability and the therapeutic index of the donepezil are promoted. Meanwhile the invention discloses a plurality of processes for the preparation of long-acting sustained controlled release formulation of donepezil-like compounds. The process for preparation is flexible. Common processing equipment and commercial scale can be adopted. The production efficiency is high and the quality keeps steady. The invention can be directly or secondly used for making injection or medicament for oral administration.
Owner:SHANGHAI INST OF MATERIA MEDICA CHINESE ACAD OF SCI
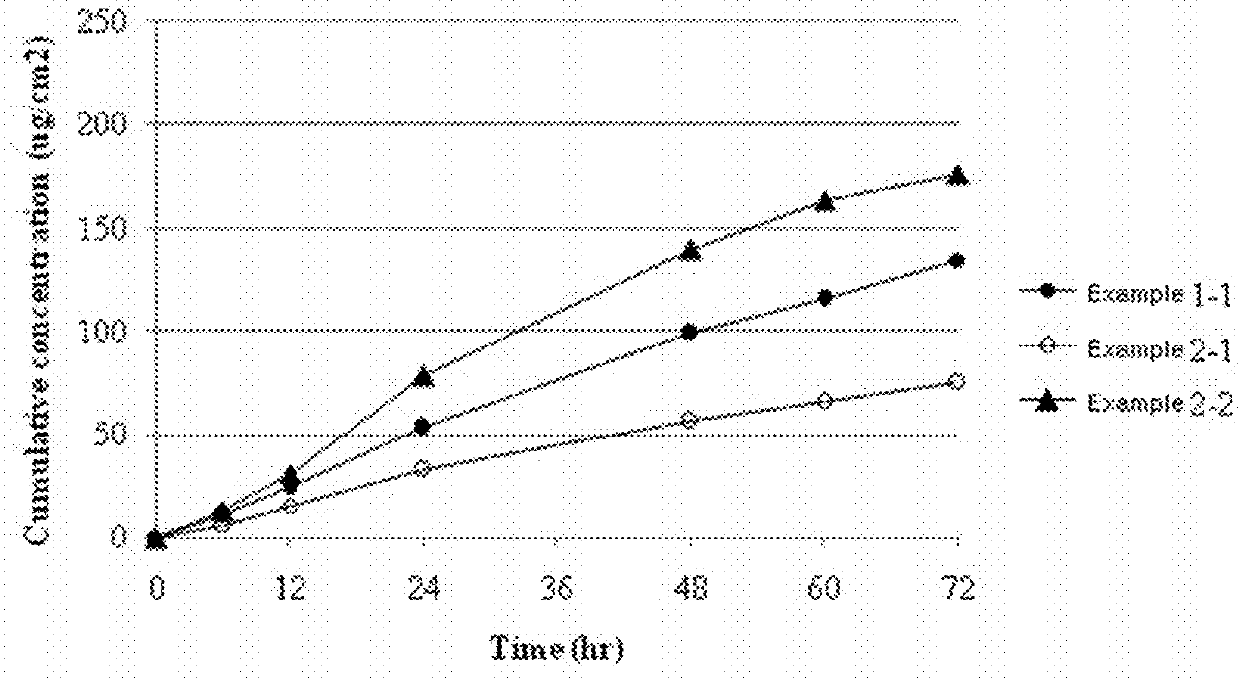
Transdermally absorbable donepezil-containing preparation
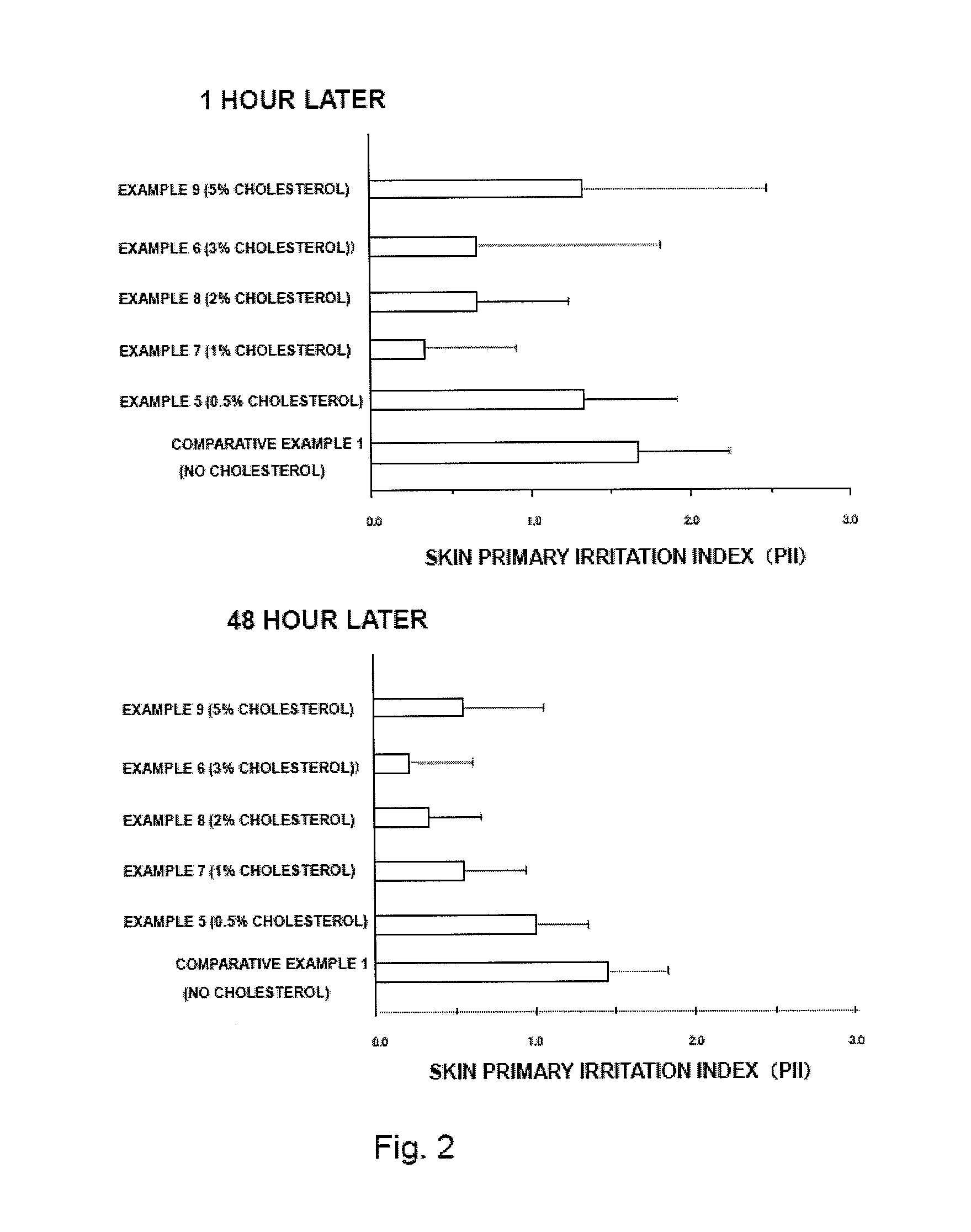
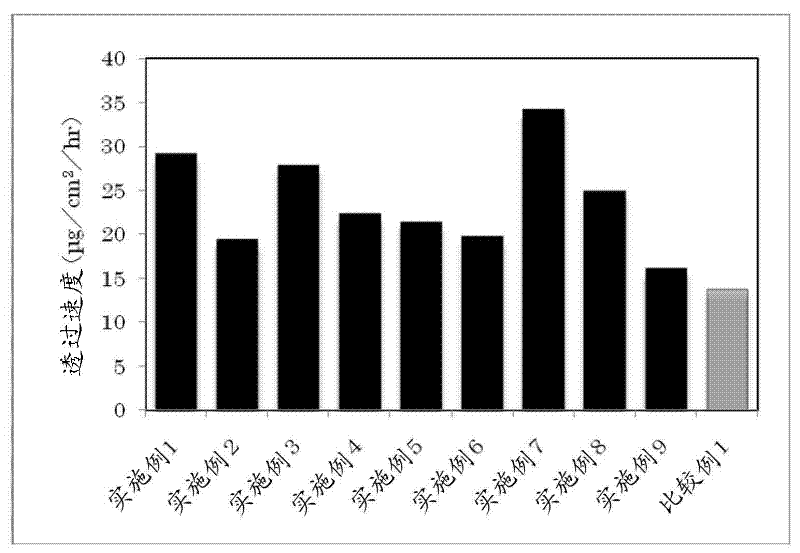
InactiveUS20130053358A1Reduce skin irritationSufficient rateBiocideNervous disorderDonepezilSkin permeability
Provided is a transdermal preparation having low skin irritation as well as a sufficient skin permeability rate of donepezil, which comprises one or more drugs selected from donepezil and pharmaceutically acceptable salts thereof. Also provided are a manufacturing method thereof, and a method for reducing skin irritation of a transdermally absorbable donepezil-containing preparation. An embodiment is a transdermal preparation comprising one or more drugs selected from donepezil and pharmaceutically acceptable salts thereof, and a skin irritation-reducing agent.
Owner:HISAMITSU PHARM CO INC
Gelled donepezil compositions and methods for making and using the same
Gelled donepezil compositions and methods for making and using the same are provided. The subject compositions include a donepezil active agent in a gelled oral pharmaceutically acceptable vehicle comprising an emulsion of water and oil. Also provided are kits of the subject compositions.
Owner:TEIKOKU PHARMA USA INC
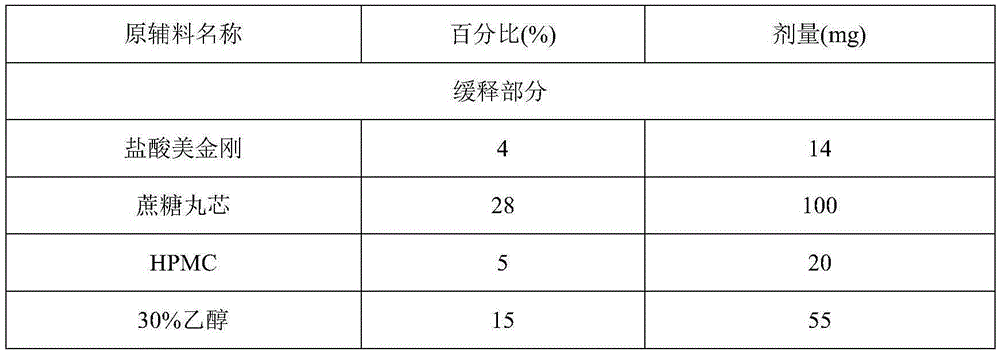
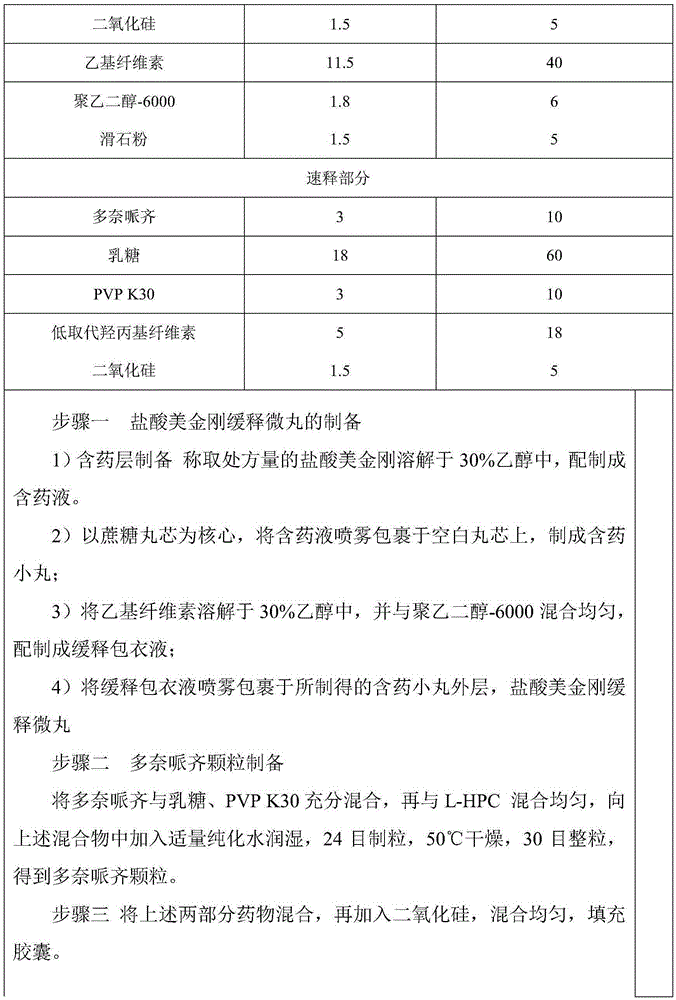
Memantine hydrochloride sustained release-donepezil quick release compound capsule
InactiveCN105326837AQuality is easy to controlHigh yieldNervous disorderPharmaceutical delivery mechanismSustained release pelletsMemantine Hydrochloride
Owner:BEIJING VENTUREPHARM BIOTECH
Donepezil Salts Suitable for the Preparation of Pharmaceutical Compositions
The invention relates to donepezil salts formed with organic acids and a process for the preparation thereof. Said salts can be used for the preparation of pharmaceutical compositions. The invention also relates to a process for the preparation of said salts, pharmaceutical compositions containing them and the use of said compounds for the treatment of diseases.
Owner:EGIS GYOGYSZERGYAR NYILVANOSAN MUKODO RESZVENY TARSASAG
Dihydropyridine compounds and compositions for headaches
InactiveUS20060270709A1Slow onsetBiocideNervous disorderHuntingtons choreaAmytrophic lateral sclerosis
The invention provides methods for treating and / or preventing cognitive impairments, dementia, or neurodegenerative diseases and disorders (e.g., Alzheimer's disease, Parkinson's disease, Huntington's disease, amyotrophic lateral sclerosis) in patients by administering therapeutically effective amounts of an AMPA receptor antagonist (e.g., 1,2-dihydropyridine compounds) and therapeutically effective amounts of nootropics (e.g., cholinesterase inhibitors) to patients. The invention also provides combinations, commercial packages, and pharmaceutical compositions comprising therapeutically effective amounts of AMPA receptor antagonists (e.g., 1,2-dihydropyridine compounds) and therapeutically effective amounts nootropics (e.g., cholinesterase inhibitors). The 1,2-dihydropyridine compound may be, for example, 3-(2-cyanophenyl)-5-(2-pyridyl)-1-phenyl-1,2-dihydropyridin-2-one. The cholinesterase inhibitor may be, for example, donepezil.
Owner:EISAI CO LTD
Memantine combinations and use
ActiveUS20180116979A1Simplify the management processNervous disorderPharmaceutical delivery mechanismDonepezilSolifenacin
A pharmaceutical combination of memantine and a non-anticholinergic antiemetic agent for the treatment of hypocholinergic disorders in further combination with high doses of donepezil and with solifenacin, and kits comprising said combination. A pharmaceutical combination of memantine and solifenacin for the treatment of hypocholinergic disorders, including Alzheimer type dementia, in further combination with high doses of donepezil, and kits comprising said combination.
Owner:CHASE PHARMA CORP
Transdermal delivery system comprising donepezil or its salt
ActiveUS20160051486A1Easily applied to industrial mass productionMinimize skin irritationNervous disorderSheet deliveryDonepezilAdhesive
Provided is a transdermal delivery system consisting of a backing layer, a drug-containing matrix layer, and a release layer, wherein the drug-containing matrix layer includes (a) donepezil or a pharmaceutically acceptable salt thereof as an active ingredient, (b) a mixture of high molecular weight polyisobutylene having a weight-average molecular weight ranging from 400,000 to 3,000,000 and low molecular weight polyisobutylene having a weight-average molecular weight ranging from 25,000 to 300,000 as an adhesive, and (c) a permeation enhancer in not more than 3% by weight based on the total weight of the drug-containing matrix layer.
Owner:DAEWOONG PHARM CO LTD
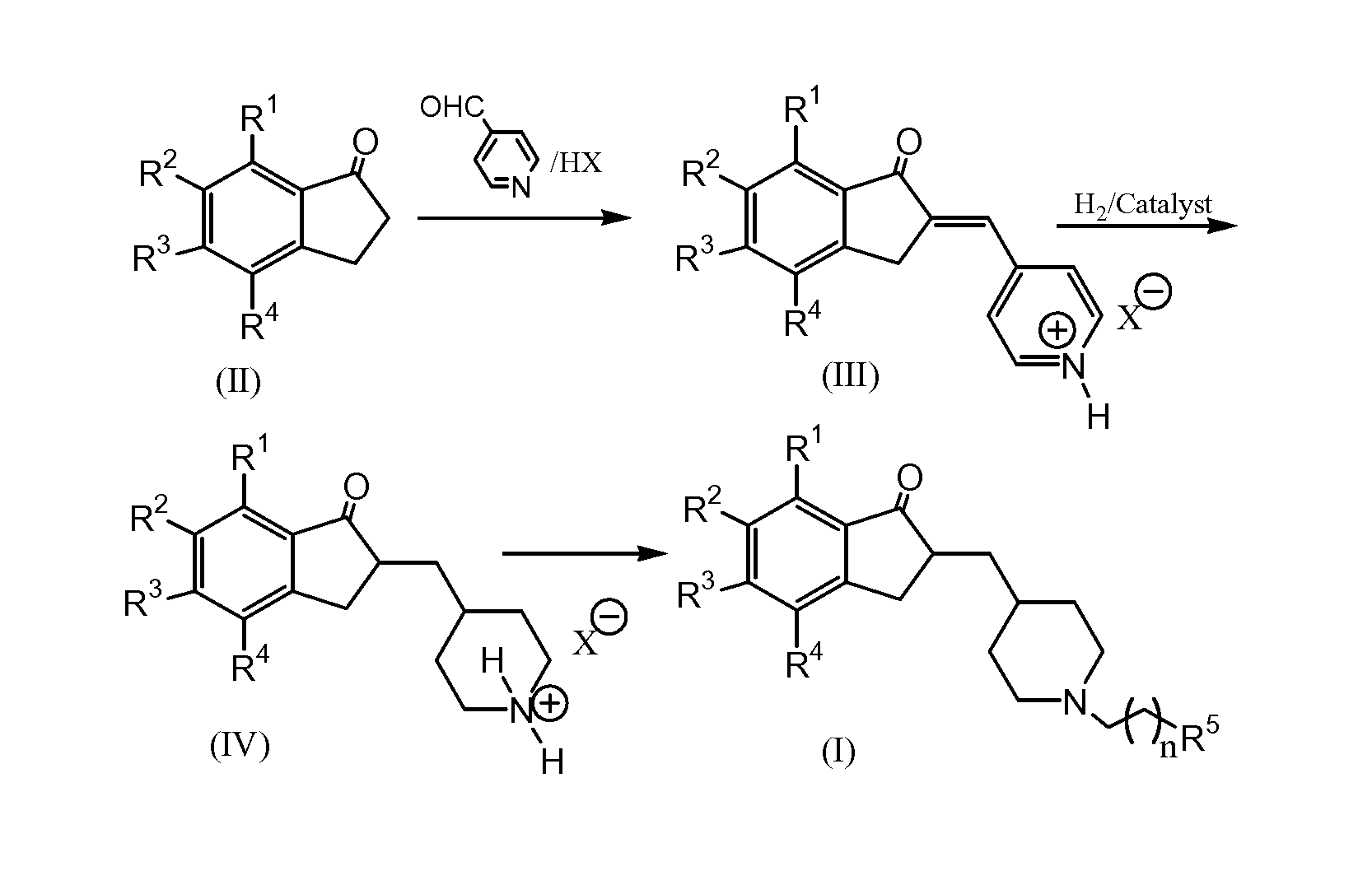
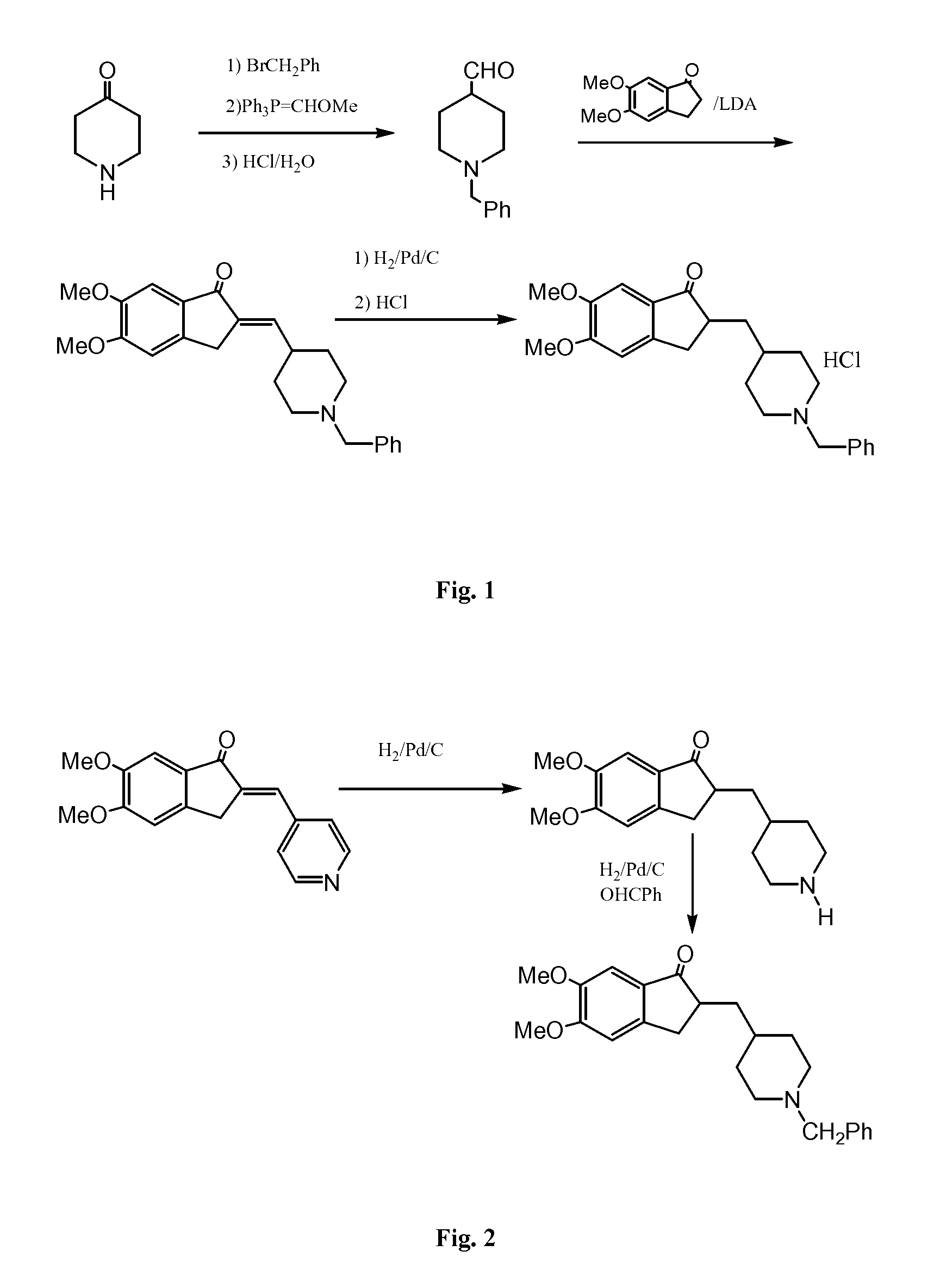
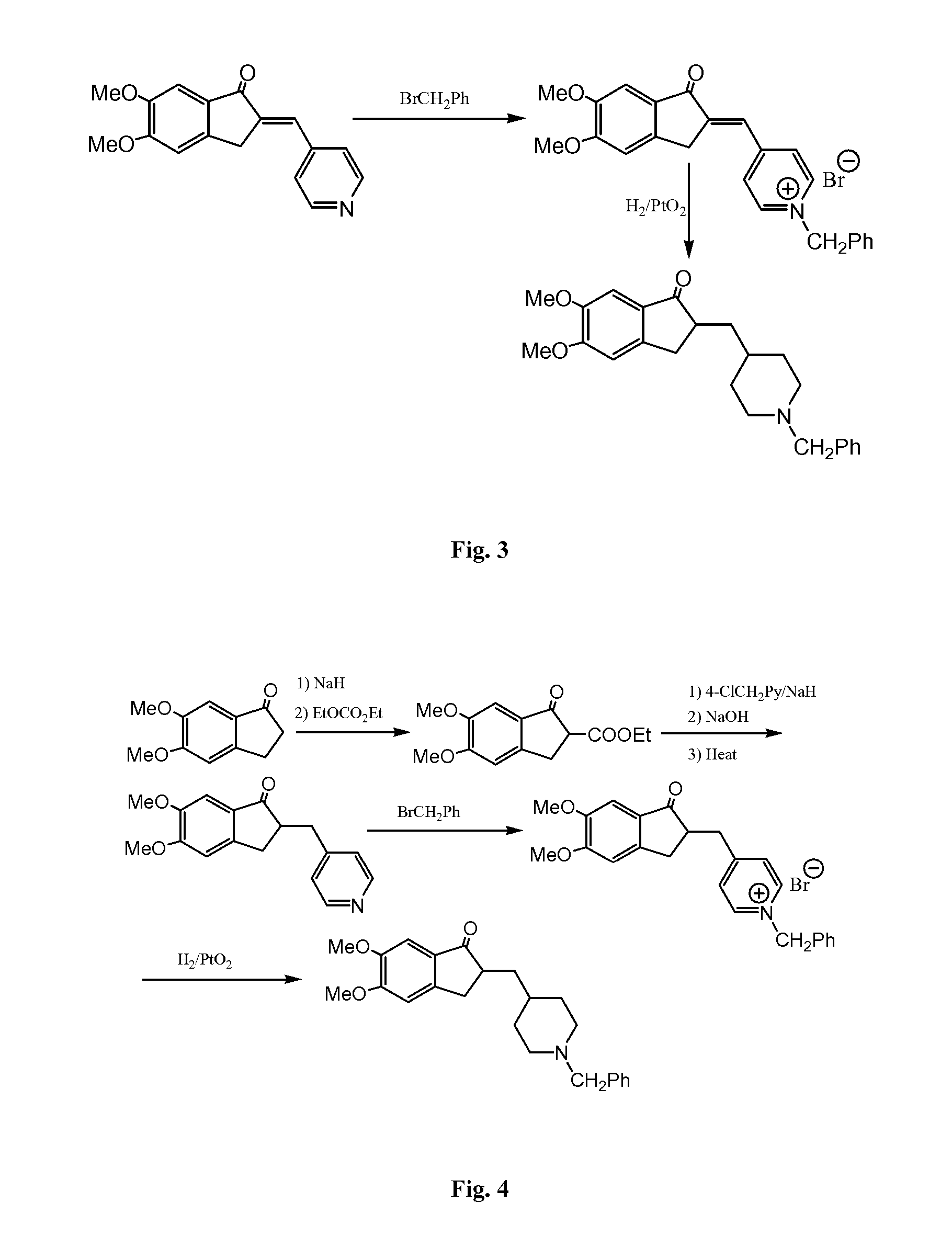
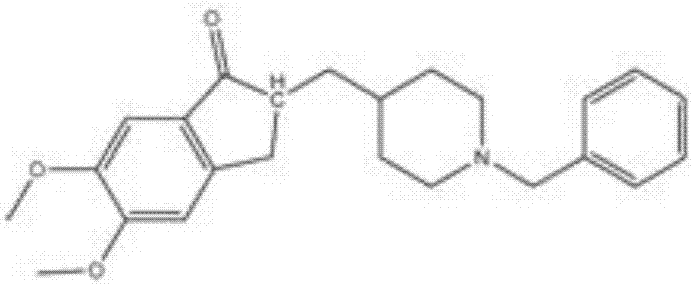
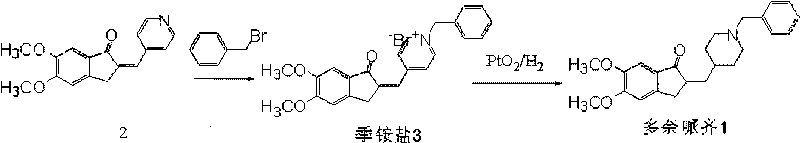
Process for preparing Donepezil and its derivatives
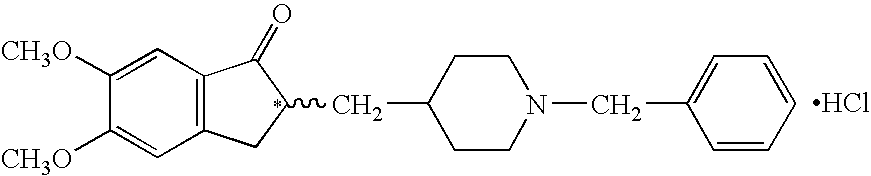
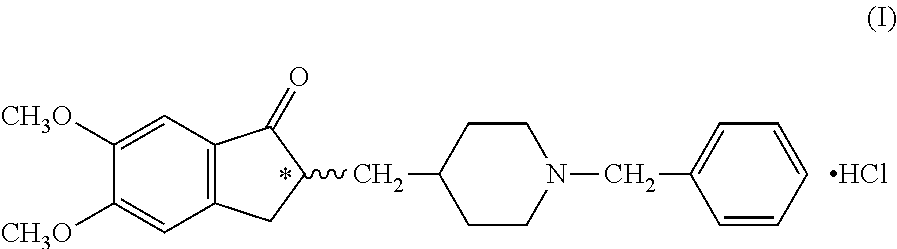
InactiveUS8318942B2Overcome disadvantagesReadily hydrogenatedBiocideOrganic chemistryAlkyl transferDonepezil
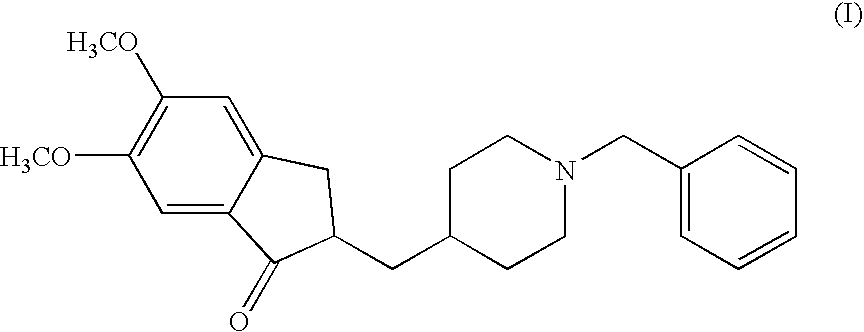
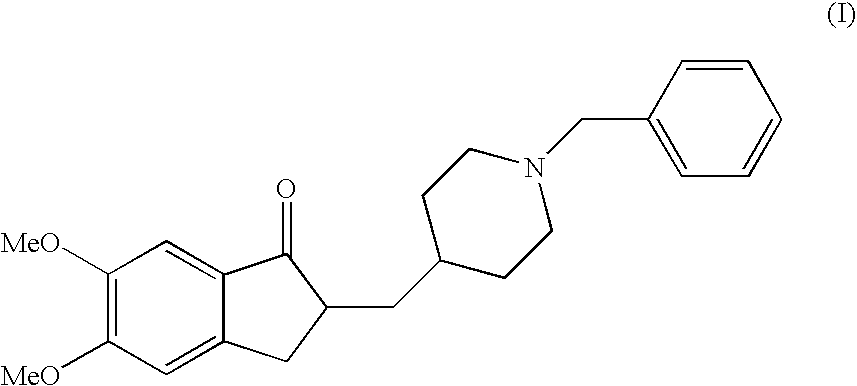
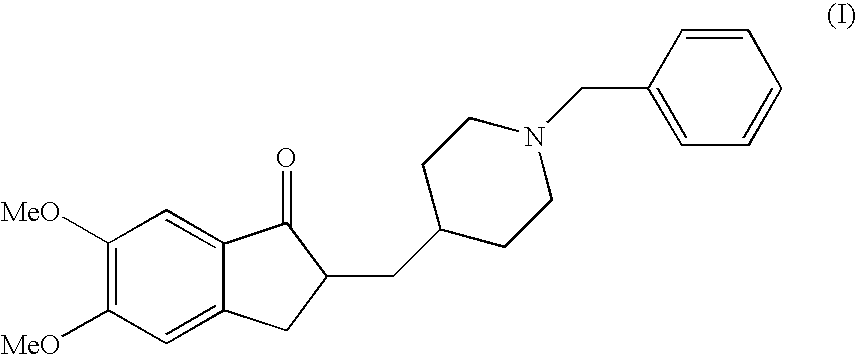
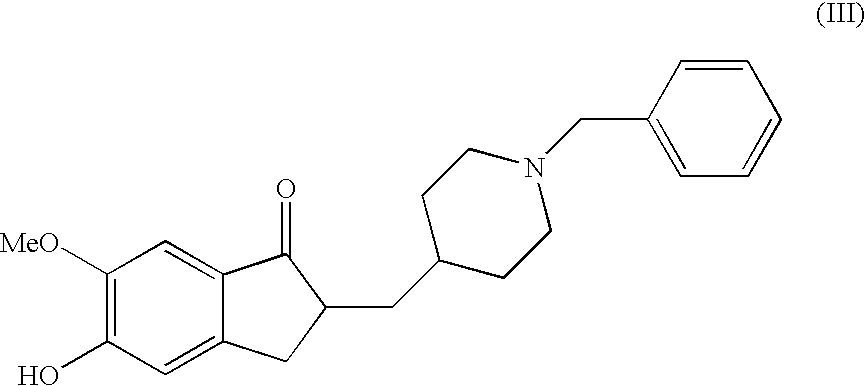
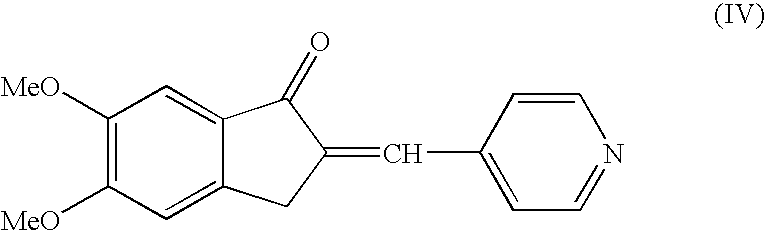
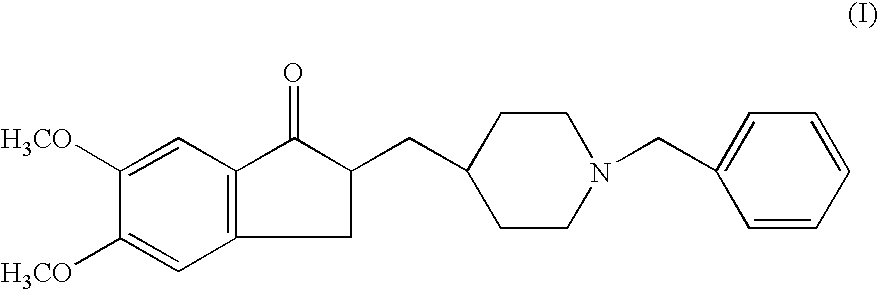
A process for producing a Donepezil derivative represented by the formula (I), wherein R1, R2, R3, and R4 each independently represents H, F, an alkyl having from 1 to 4 carbon atoms, or an alkoxy having from 1 to 4 carbon atoms; R5 represents a phenyl or a substituted phenyl; and n is an integer from 0 to 2, characterized in that the process comprises: (a) a reaction of 4-pyridinecarboxaldehyde with a compound of formula (II) in the presence of a strong acid HX to form a compound of formula (III); (b) a catalytic hydrogenation of a compound of formula (III) or a compound of formula (V) to yield a compound of formula (IV); and (c) an alkylation reaction of a compound of formula (IV) to yield a compound of formula (I).
Owner:TIANJIN HEMAY BIO TECH CO LTD
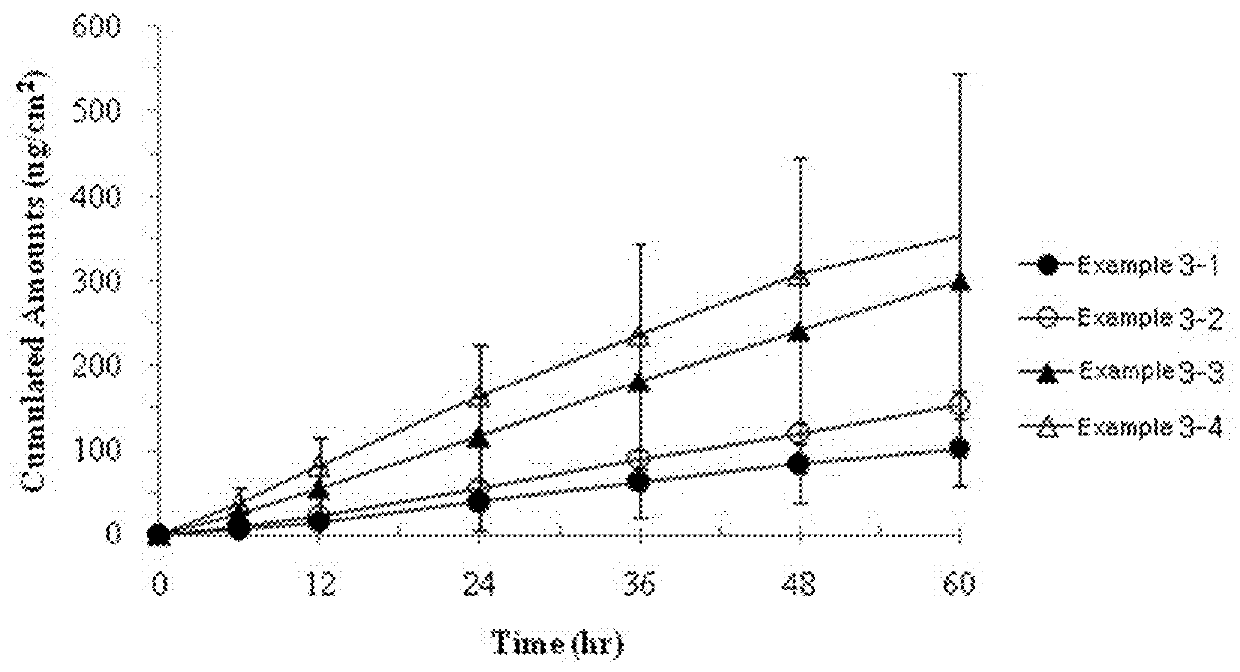
Transdermal medicament delivery system containing donepezil compound, preparation and preparation method
ActiveCN102188363AImprove complianceAvoid first pass effectNervous disorderMacromolecular non-active ingredientsDonepezilCross linker
The invention discloses a transdermal medicament delivery system containing a donepezil compound, a transdermal preparation and a preparation method. The transdermal medicament delivery system comprises the following components in percentage by weight: 0.1 to 50 percent of donepezil or acid radical salt thereof, 1 to 95 percent of skeleton polymer, 0.1 to 60 percent of transdermal penetration enhancer, 0 to 10 percent of cross linker, 0.5 to 60 percent of humectant, 0.02 to 10 percent of bacteriostatic agent, 0.02 to 30 percent of pH regulator and 0 to 90 percent of solvent. The system is used for treating light, medium and severe senile dementia, can maintain long-time stable medicament delivery of at least 3 days, has better performance, is convenient for medicament delivery, and can reduce the administration frequency and increase the compliance of patients; and meanwhile, the transdermal path avoids first-pass effect on gastrointestinal tracts and liver due to oral administration of medicaments, and the system has higher bioavailability and obvious advantages in medicinal application.
Owner:SHANGHAI MODERN PHARMA ENG INVESTIGATION CENT
Method for suppressing discoloration over time of adhesive preparation containing donepezil
InactiveUS20100048628A1Sufficient effectImprove stabilityBiocideNervous disorderPropanoic acidDonepezil
[Object] Discoloration, over time, of a donepezil-containing adhesive preparation is suppressed.[Solution] At least one species of stabilizer selected from the group consisting of ascorbic acid, a metal salt or an ester thereof, isoascorbic acid or a metal salt thereof, ethylenediamine tetraacetic acid or a metal salt thereof, 2-mercaptobenzimidazole, 3(2)-t-butyl-4-hydroxyanisole, 2,6-di-t-butyl-4-methylphenol, tetrakis[3-(3′,5′-di-t-butyl-4′-hydroxyphenyl)propionic acid]pentaerythritol, (±)-α-tocopherol, (±)-α-tocopherol acetate, rutin, hypophosphorous acid, a metal metabisulfite salt and a metal salt of hydroxymethanesulfinic acid, is blended in a pressure-sensitive adhesive layer containing a pressure-sensitive adhesive and donepezil.
Owner:NITTO DENKO CORP +1
New use of cape jasmine
The invention belongs to the medicinal field, in particular to a new application of Gardenia. The technological problem needed to be solved is to provide the new medicinal application of the Gardenia, namely the application in preparing medicines for curing vascular dementia. Proved by experiments, after the inventor adopts a permanent bilateral common carotid artery ligation method and establishes animal models of the vascular dementia and cures the animal models for a month by giving different dosages of geniposide and positive control drug, the inventor carries out behavior and pathological observation for observing the influence of behavior and pathological brain tissue and related biochemical parameters of the geniposide on dementia rats. The inventor finds out that the geniposide with different dosages has functions of improving and recovering cognitive dysfunction of the vascular dementia rats and the rats have dose-dependency, wherein, the geniposide with medium and high dosages has the function of curing the dementia close to donepezil. Therefore, the Gardenia or the geniposide can be adopted for curing the vascular dementia.
Owner:WEST CHINA HOSPITAL SICHUAN UNIV +1
Pharmaceutical composition for parenteral administration, containing donepezil
InactiveCN105338966AStable drug releaseImprove Medication AdherencePowder deliveryNervous disorderDonepezilParenteral nutrition
The present invention relates to a composition for parenteral administration, containing donepezil as an active ingredient, and a preparation method therefor. Donepezil, which has been conventionally used for oral or transdermal administration, is prepared as microparticles comprising a biodegradable and biocompatible polymer and a release controller so as to be provided as a pharmaceutical composition for sustained release parenteral administration, thereby enabling in vivo sustained release continuously for 2-12 weeks or more. Therefore, it is possible to reduce the frequency of administration to a patient and maintain an effective concentration in the blood for a long time.
Owner:DONG KOOK PHARMA CO LTD
Method for suppressing coloring of adhesive prepartion containing donepezil and method for reducing amounts of donepezil-related substances formed
InactiveUS20100062045A1Appealing appearanceInhibit coloringBiocideNervous disorderDonepezilAscorbic acid
[Object] By suppressing coloring of an adhesive preparation containing donepezil, a naturally colored appearance is realized.[Solution] In a method for suppressing coloring of an adhesive preparation having a support and a pressure-sensitive adhesive layer, which comprises a pressure-sensitive adhesive and donepezil in the pressure-sensitive adhesive layer, ascorbic acid, a metal salt or an ester thereof and metal metabisulfite salt are contained in the pressure-sensitive adhesive layer. This method preferably has: a step of preparing a mixture comprising the pressure-sensitive adhesive, the donepezil, the ascorbic acid, a metal salt or an ester thereof, and the metal metabisulfite salt; and a step of film-forming the pressure-sensitive adhesive layer by applying the mixture to the surface of the support.
Owner:NITTO DENKO CORP +1
Combination therapy for the treatment of dementia
InactiveUS20110251239A1Increase exposureAdditional impairmentBiocideNervous disorderDonepezilCombined Modality Therapy
Owner:EISAI INC
Process for preparation of donepezil
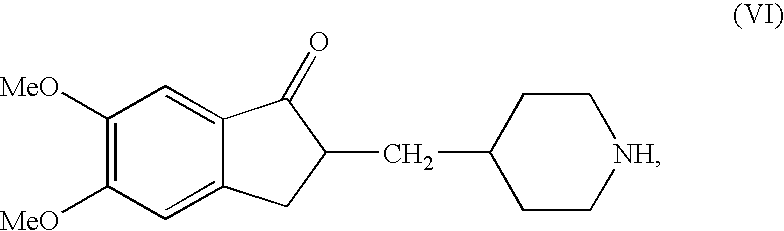
An efficient process for preparation of donepezil is provided. In one embodiment, the process for preparation of donepezil includes suspending a catalyst, which is palladium metal on carbon and the compound of the structurein an alcoholic solvent and hydrogenating the suspension at the hydrogen pressure of from about 1 to about 5 and a temperature of from about 40 to about 90° C. till the hydrogenation reaction is substantially complete to obtain a compound of the formula (VI):which then converted to donepezil. The processes of the invention are believed to be simple, eco-friendly, and commercially viable.
Owner:DR REDDYS LAB LTD +1
Acid Addition Salt of Donepezil and Pharmaceutical Composition Thereof
Disclosed is an acid addition salt of donepezil, wherein acid counterion is selected from the group consisting of pamoic acid, cypionic acid, camphor sulfonic acid, enanthic acid, fusidic acid, gluceptic acid, gluconic acid, lactobionic acid, lauric acid, valeric acid, Dibenzoyl-D-Tartaric acid and terephthalic acid. Disclosed is a process for the preparation and pharmaceutical composition comprising the same. More specifically, disclosed is concerned with the pamoate acid addition salts of donepezil. Disclosed also is long acting formulation comprising the acid addition salt of donepezil and process for the preparation thereof.
Owner:TORRENT PHARMA LTD
Transdermally absorbable donepezil-containing preparation
Disclosed is a transdermally absorbable donepezil-containing preparation which enables the administration of donepezil in a sustained manner for a long period and can achieve both the rapid increase in blood donepezil level and the sustained release of donepezil. The transdermally absorbable donepezil-containing preparation comprises an adhesive patch base material comprising a hydrophobic polymer and an absorption-enhancing agent and donepezil (an active ingredient) dissolved in the adhesive patch base material, wherein the absorption-enhancing agent comprises at least one component selected from lauryl alcohol, triethyl citrate, isopropyl myristate, cetyl lactate, oleyl alcohol, sorbitan monooleate, polyethylene glycol monostearate, lauromacrogol, N-methyl-2-pyrrolidone and triacetin.
Owner:TEIKOKU SEIYAKU KK TEIKOKU SEIYAKU CO LTD
Sustained release donepezil formulations
InactiveUS20130059003A1Reduce incidenceStable and desired blood levelBiocideNervous disorderSustained Release FormulationsDonepezil
Sustained release formulations comprising donepezil, or its pharmaceutically acceptable salts, and methods of preparing the sustained release formulations.
Owner:DR REDDYS LAB LTD +1
Donepezil cyclodextrin inclusion compound and oral instant membrane containing same
ActiveCN107375945AGreat tasteDisintegrate fastNervous disorderPharmaceutical non-active ingredientsDonepezilAdditive ingredient
The invention relates to a taste-masked donepezil cyclodextrin inclusion compound and an oral instant membrane containing the same, belonging to the technical field of medicine. The oral instant membrane comprises the donepezil cyclodextrin inclusion compound, a film-forming material, a plasticizer, a disintegrating agent and other excipients. Specifically, the oral instant membrane is prepared by including donepezil free alkali in cyclodextrin or a derivative thereof, then dissolving the excipients such as the film-forming material in distilled water, adding an appropriate amount of the donepezil cyclodextrin inclusion compound and preparing a homogeneous solution. The donepezil cyclodextrin inclusion compound provided by the invention completely masks the bad taste of donepezil; the oral instant membrane prepared from the donepezil cyclodextrin inclusion compound has good taste, is rapid in drug release and greatly improves patient compliance; moreover, the taste-masked donepezil cyclodextrin inclusion compound can be applied to other oral dosage forms such as tablets and solutions.
Owner:SHENYANG PHARMA UNIVERSITY
Transdermal Adhesive Composition Comprising A Poorly Soluble Therapeutic Agent
Methods, compositions, and devices for transdermally administering an active agent such as donepezil are provided.
Owner:CORIUM LLC
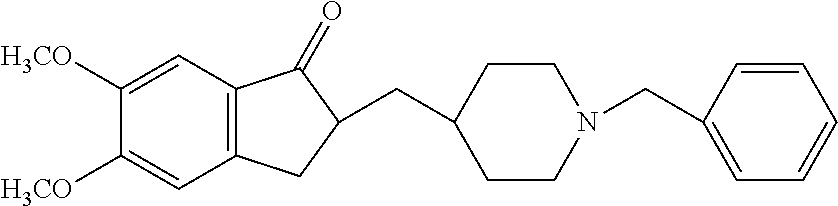
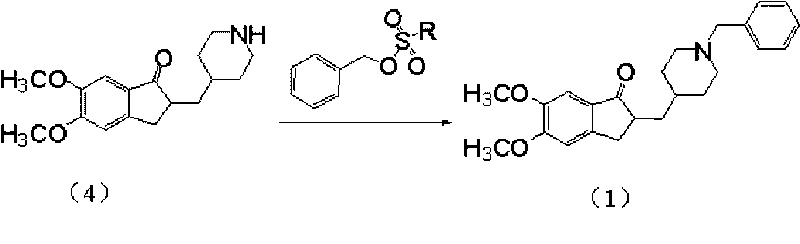
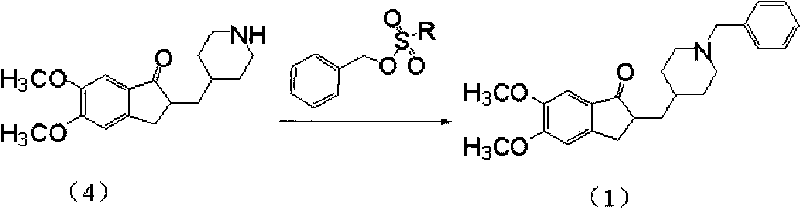
Preparation method of donepezil
InactiveCN101723878AHigh purityShort reaction timeNervous disorderOrganic chemistryDonepezilBenzyl group
The invention provides a preparation method of donepezil. The method comprises the following steps: using hydride (4) and substituted benzyl ester of substituted sulfuric acid to perform the condensation reaction, and then collecting donepezil (1) from the reaction product. The method of the invention can not generate impurities, the purity of the obtained product is greatly increased, the yield is up to 85%, the reaction time is as short as 2-5 hours, the operation is simple, the stability is good, the equipment investment is small and the method is applicable to industrial production. The reaction formula is shown below.
Owner:浙江东亚药业股份有限公司 +1
Novel donepezil synthesis process
InactiveCN100436416CLow priceSynthetic operation is simpleOrganic chemistryDonepezilCombinatorial chemistry
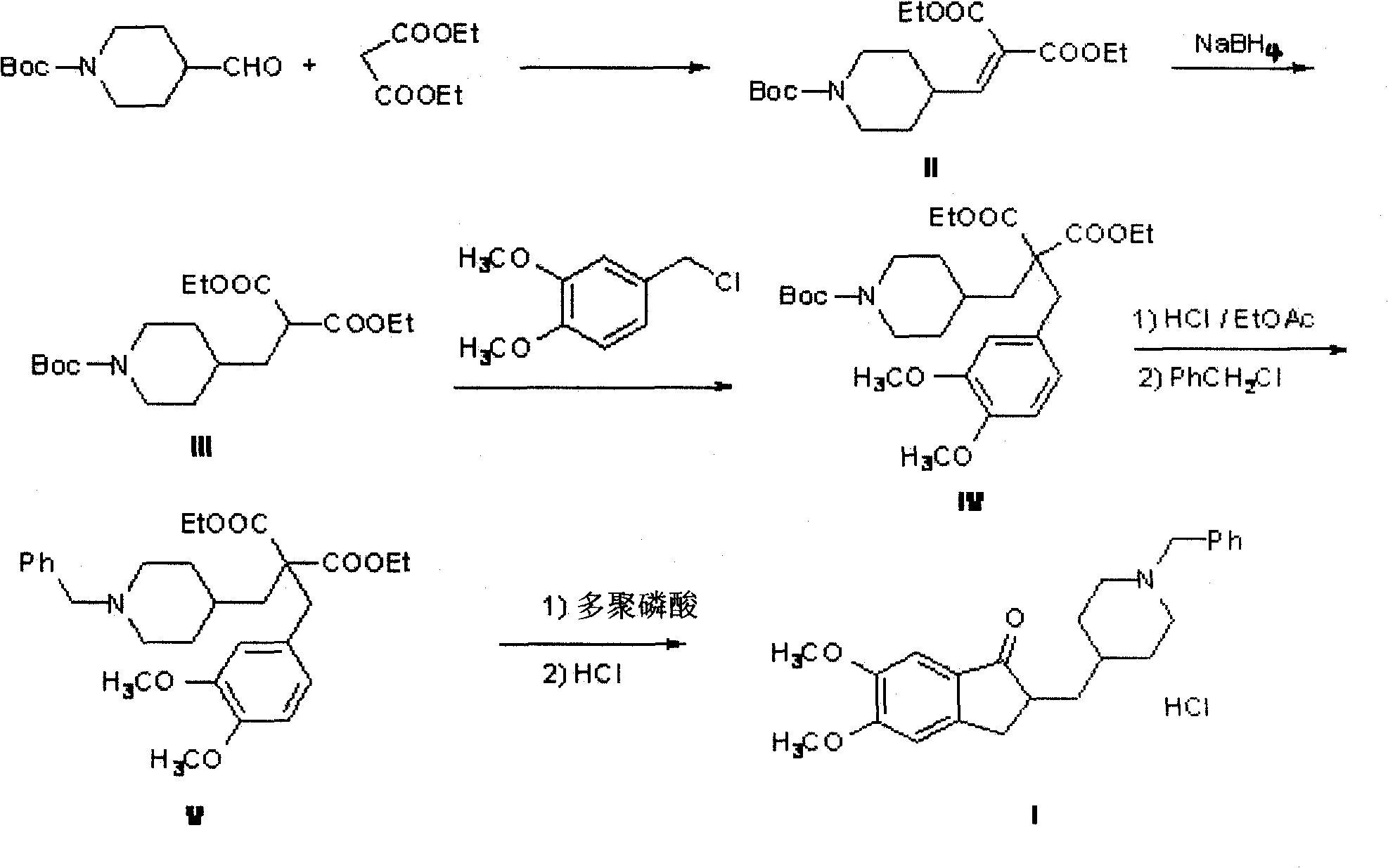
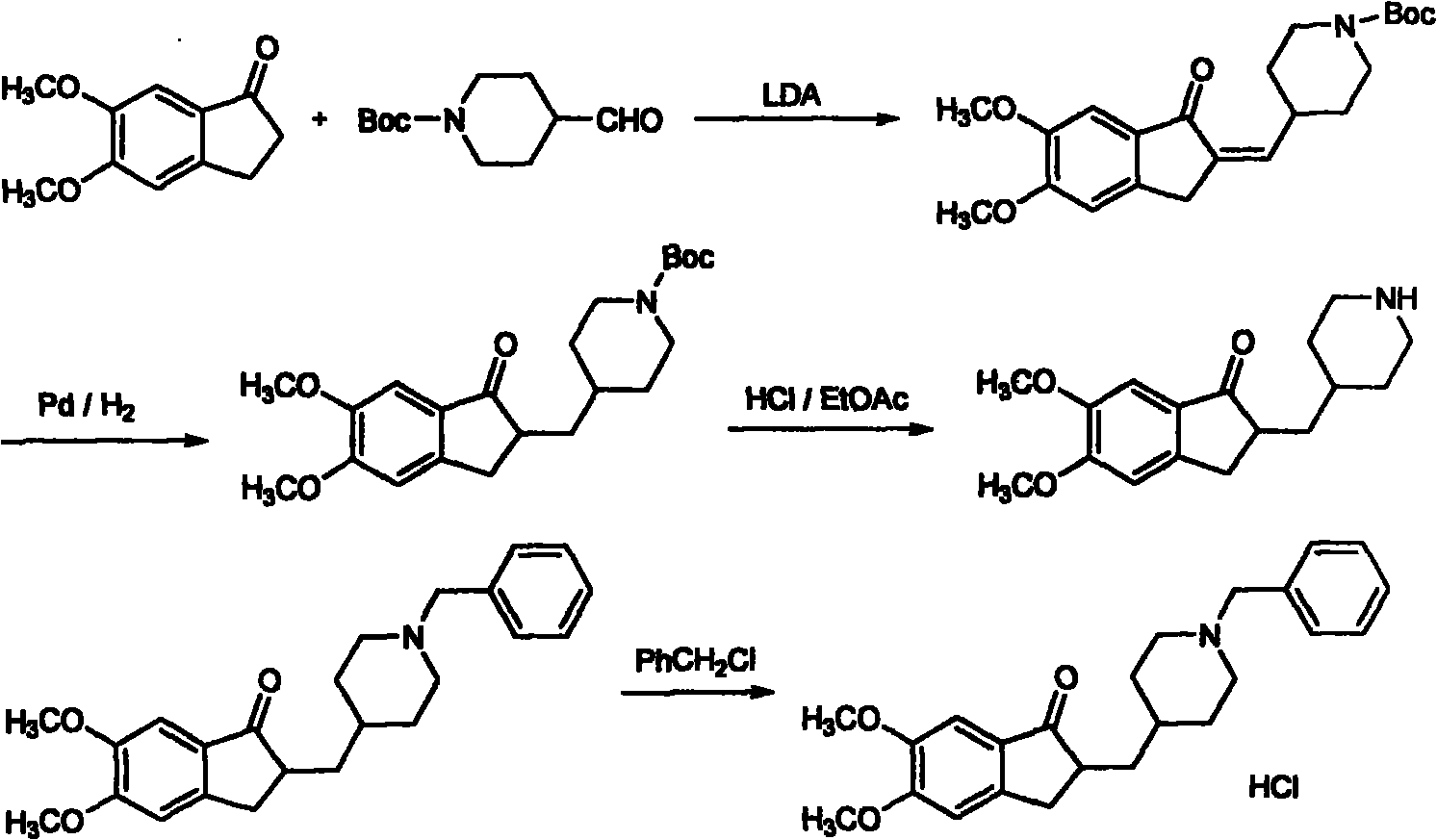
The invention discloses a novel process for synthesizing Donepezil by using diethyl malonate as the starting raw material, which comprises condensation, reduction, substitution, cycling reaction and decarboxylation.
Owner:PKUCARE PHARMA R&D CENT +1
Donepezil-containing transdermal delivery system and process for preparing the same
InactiveCN104144684AGuaranteed stabilityInhibition of burst effectNervous disorderSheet deliveryDonepezilAdhesive
The present invention relates to a percutaneous absorption preparation such as one provided in the form of a patch, and to a method for preparing same, wherein the percutaneous absorption preparation has a double-layer structure including an adhesive layer and a matrix layer containing a drug which is formulated with an adhesive after completely dissolving donepezil using a specific polymer. According to the present invention, the percutaneous absorption preparation does not exhibit the eduction of donepezil within the preparation, and can release donepezil at a constant rate for a long time, as well as prevent the release dumping phenomenon that occurs in a percutaneous absorption preparation having a single-layer structure.
Owner:SK CHEM CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com