Donepezil-containing transdermal delivery system and process for preparing the same
A technology of donepezil and adhesive, which is applied in the field of transdermal drug delivery system, can solve problems such as difficulties, reduced adhesion strength, complex production process, etc., and achieve the effect of ensuring stability and inhibiting burst release effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0031] The present invention also provides a preparation method for a transdermal drug delivery system, the preparation method comprising (a) the step of dissolving an acrylic adhesive in a solution containing donepezil or a pharmaceutically acceptable salt thereof and polyvinylpyrrolidone; (b) a step of applying the solution obtained in step (a) to a film, followed by drying, to form a drug-containing matrix layer; and (c) forming a drug-containing matrix layer comprising a drug-containing matrix layer obtained in step (b). Steps for an adhesive layer of an acrylic adhesive or a silicone-based adhesive.
[0032] In step (a), the solution containing donepezil or its pharmaceutically acceptable salt and polyvinylpyrrolidone can be obtained by mixing a mixture of donepezil or its pharmaceutically acceptable salt and ethyl acetate with an ethanol solution of polyvinylpyrrolidone mixed.
[0033] Step (b) may be performed by coating the solution obtained in step (a) onto a membran...
Embodiment 1
[0037] Example 1: Evaluation of polymers
[0038] Preliminary experiments were performed to evaluate the compatibility between various polymers and adhesives. The effect on the solubility of donepezil base of polymers found to be relatively well compatible through preliminary experiments was evaluated. That is, donepezil (1 g) and ethyl acetate (1 g) were mixed with each other. In separate containers, other polymers (0.2 g each), i.e., polyvinylpyrrolidone (PVP K17, BASF), Eudragit TM E100 (Degussa) and Plastoid TM B (Evonik Industries AG) was dissolved in ethanol (0.8 g), which was then added to a mixed solution of donepezil and ethyl acetate, respectively. In the case of 1-1 (no polymer was added), ethanol (1.0 g) was added to the mixed solution of donepezil and ethyl acetate. Each solution was stirred for 2 h, and then the appearance of each solution was observed, the results are shown in Table 1 below.
[0039] Table 1
[0040]
[0041] From the results in Table...
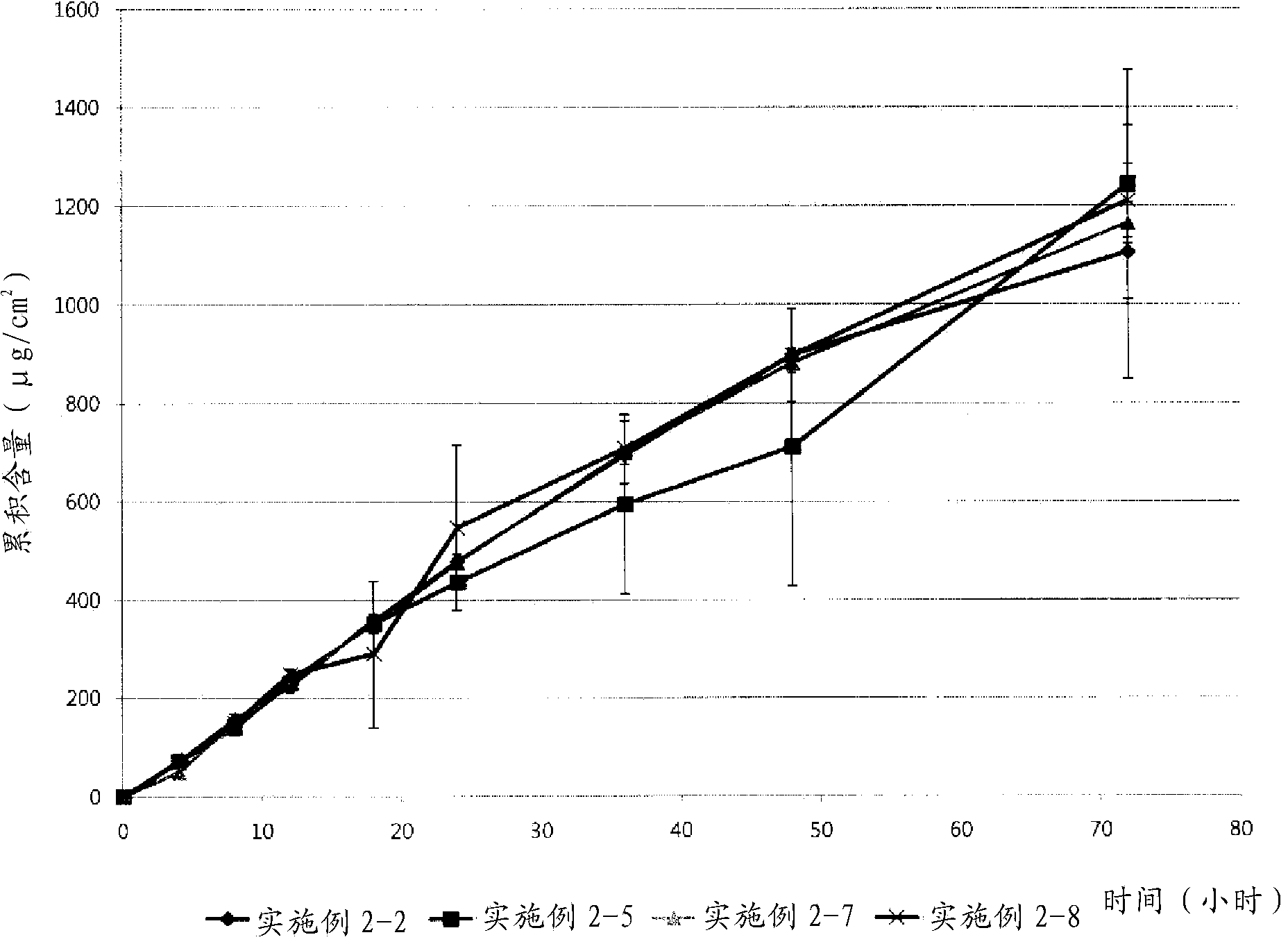
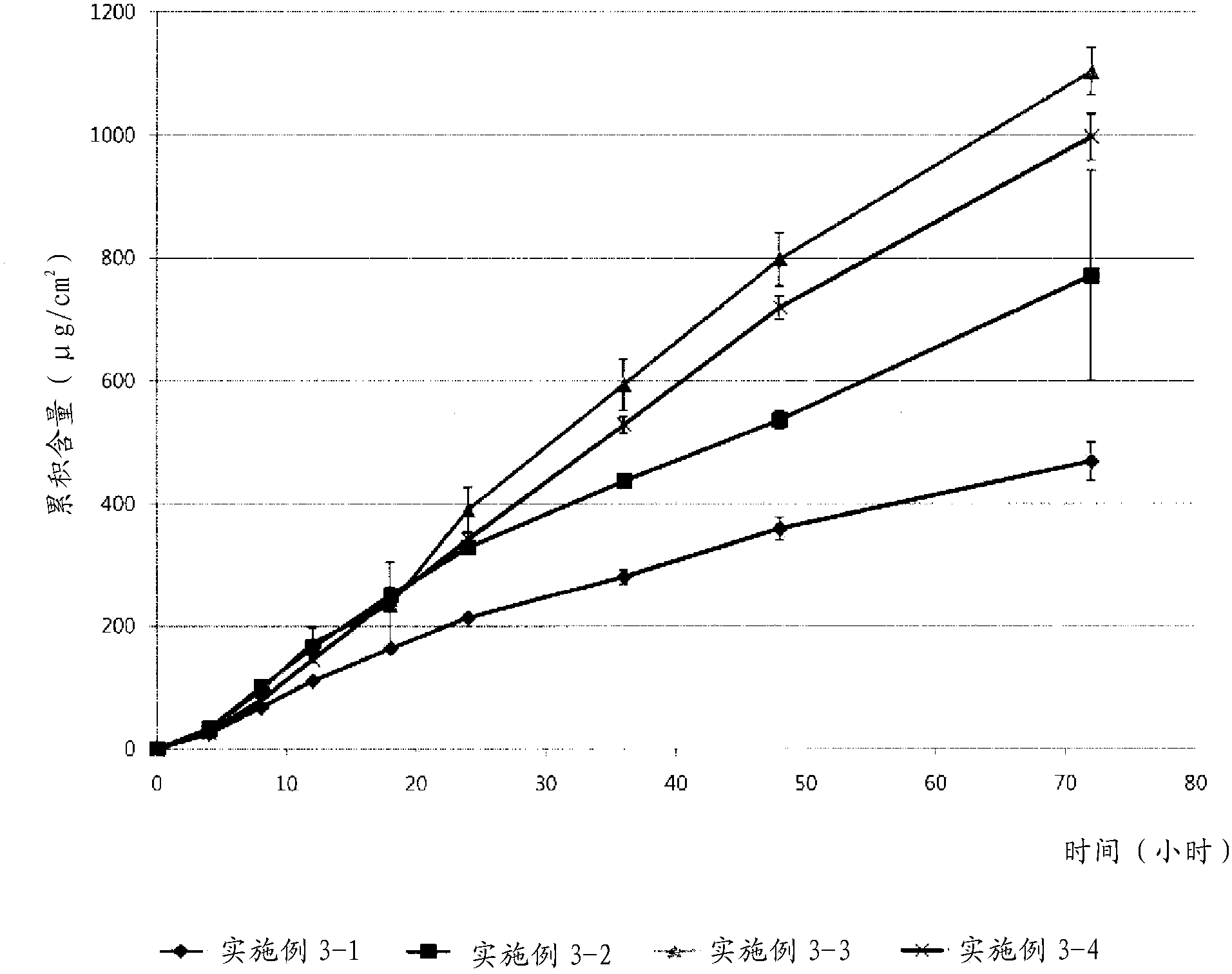
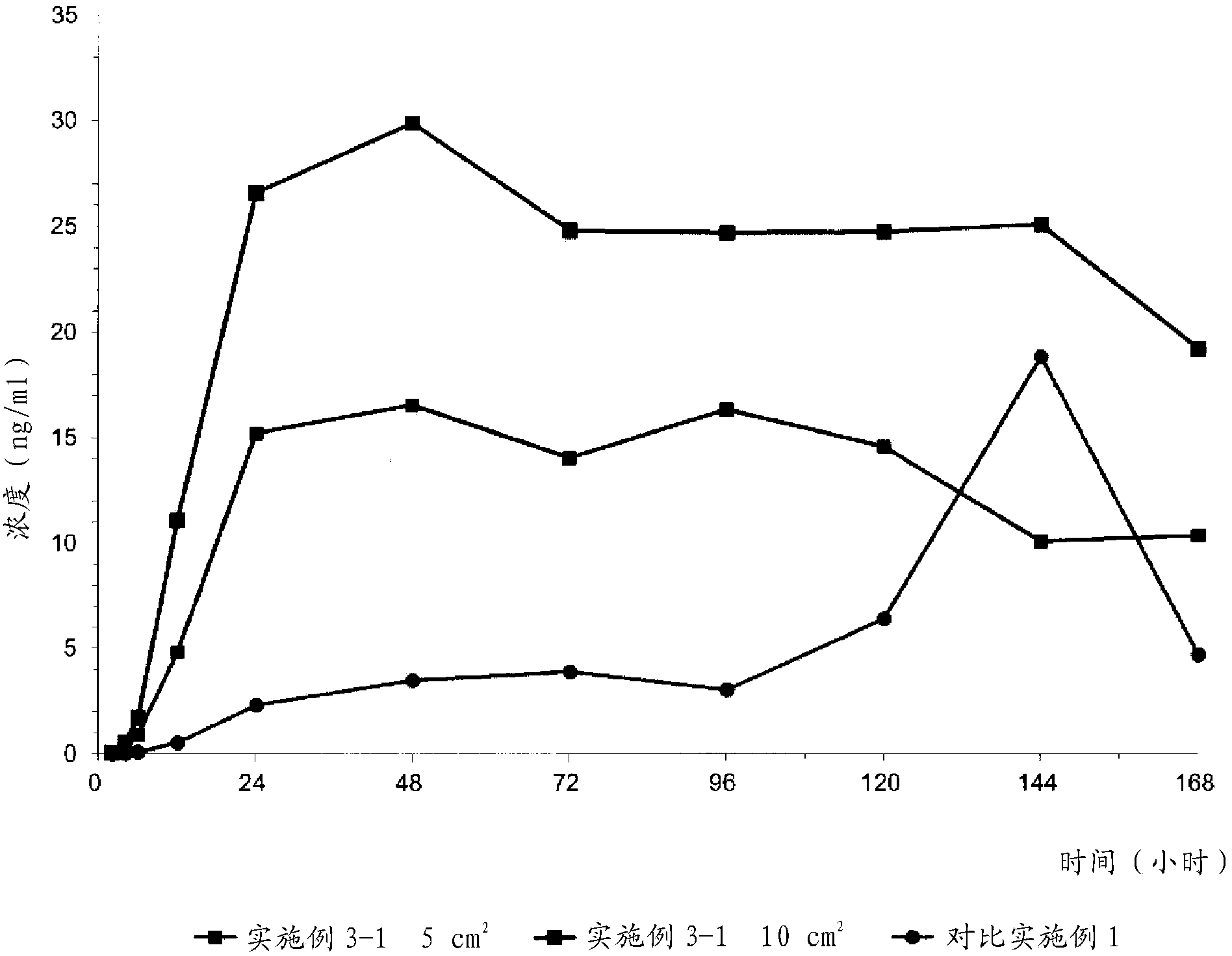
Embodiment 2
[0042] Example 2: Evaluation of Formulations Based on Molecular Weight of Polyvinylpyrrolidone
[0043] The effect of the molecular weight of polyvinylpyrrolidone on the formulation of transdermal drug delivery systems was evaluated. According to the components in Table 2 below (in the case of 2-1, only ethanol (1 g) was added), a solution containing donepezil base and polyvinylpyrrolidone was prepared according to the procedure of Example 1. The acrylic binder was added to the solution, which was then homogenized. Each of the resulting solutions was coated onto a silicone-coated PET film. The resulting individual films were dried in a 70°C oven for 15 minutes and then laminated to a backing film. Evaluate the possibility of formulation. In Table 2, as the polyethylene pyrrolidone; and Durotak 87-235A (Henkel) was used as the acrylic adhesive.
[0044] Table 2
[0045]
[0046]
[0047] Solution 2-1, which did not contain polymer, could not be coated because its vi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com