Transdermally absorbable donepezil-containing preparation
A donepezil and absorption-type technology, which is applied in the direction of medical preparations containing active ingredients, medical preparations with inactive ingredients, organic active ingredients, etc., can solve the problem of insufficient and difficult to take anti-dementia drugs, drug percutaneous absorption Increased blood drug concentration and other problems to achieve the effect of avoiding side effects, reducing side effects, and effective treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0087] (prescription)
[0088] SIS 15%
[0089] Liquid Paraffin 24%
[0090] BHT 1%
[0091] Glycerides of Hydrogenated Rosin 35%
[0092] Triacetin 5%
[0093] Donepezil 20%
[0094] Total 100%
[0095] After previously dissolving donepezil in a mixture of triacetin and toluene, mix with the remaining ingredients dissolved in toluene. After the mixture was coated on a release film, the toluene was dried and removed, and then bonded to a PET film backing layer to obtain the transdermal preparation of the present invention.
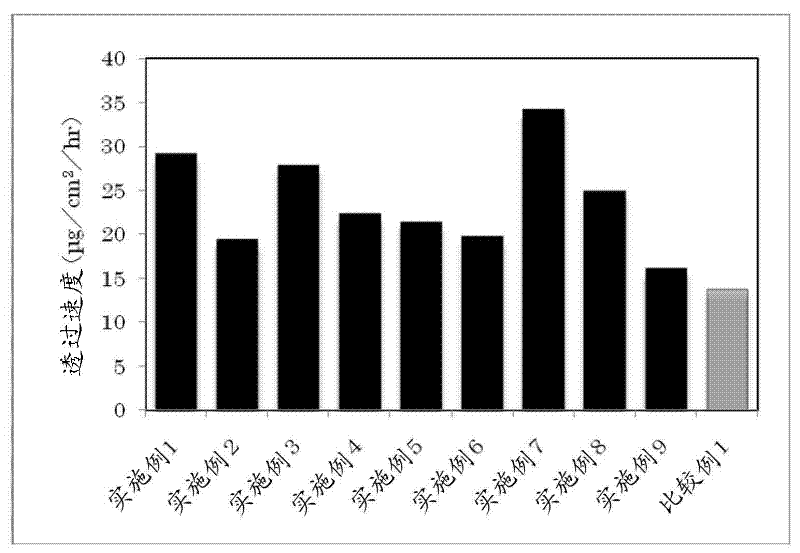
Embodiment 2~ Embodiment 10
[0097] According to the compounding shown in Table 1 below, the transdermal preparations of Examples 2 to 10 of the present invention were obtained according to the method described in Example 1 above.
[0098] 【Table 1】
[0099]
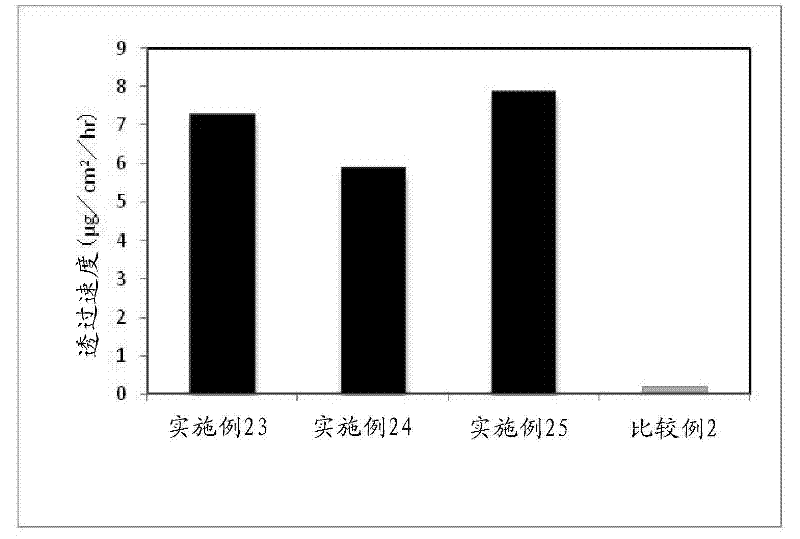
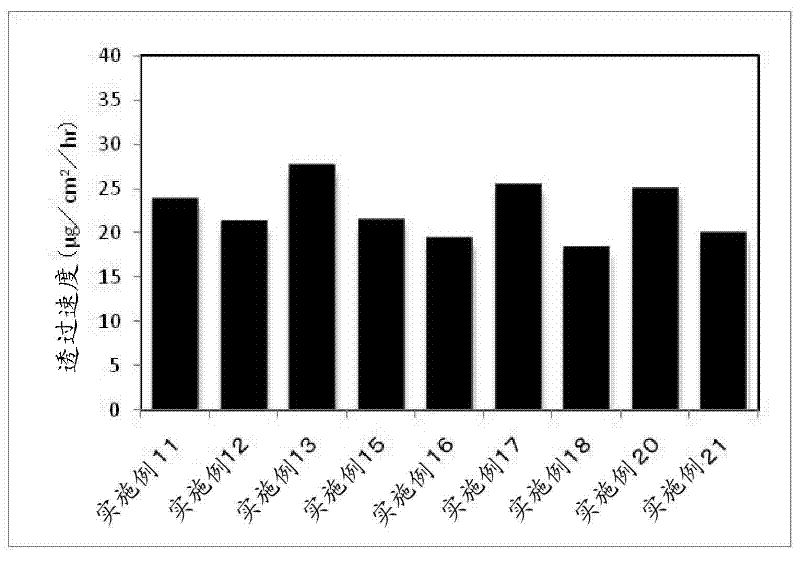
Embodiment 11~ Embodiment 21
[0104] According to the compounding shown in the following table 2, the compounding amount of donepezil and the compounding amount of triacetin, lauromacrogol, and lauryl alcohol as the absorption enhancer are changed, and the method of embodiment 1 is obtained according to the method of embodiment 1. 11 to the percutaneous absorption type preparation of Example 21.
[0105] 【Table 2】
[0106]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com