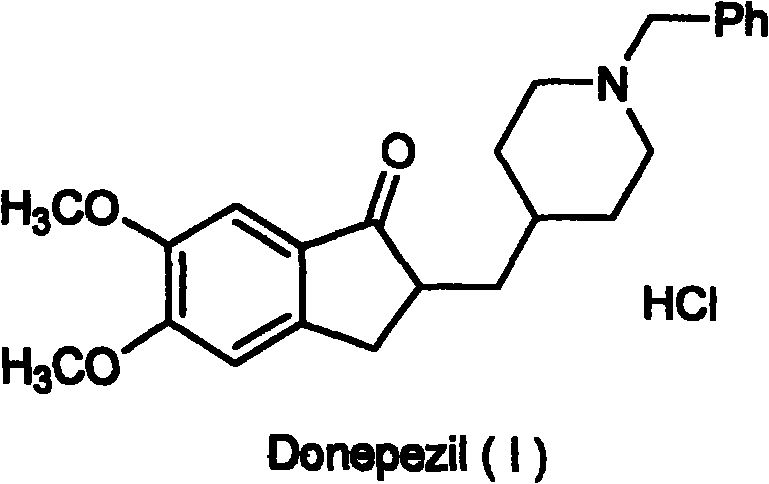
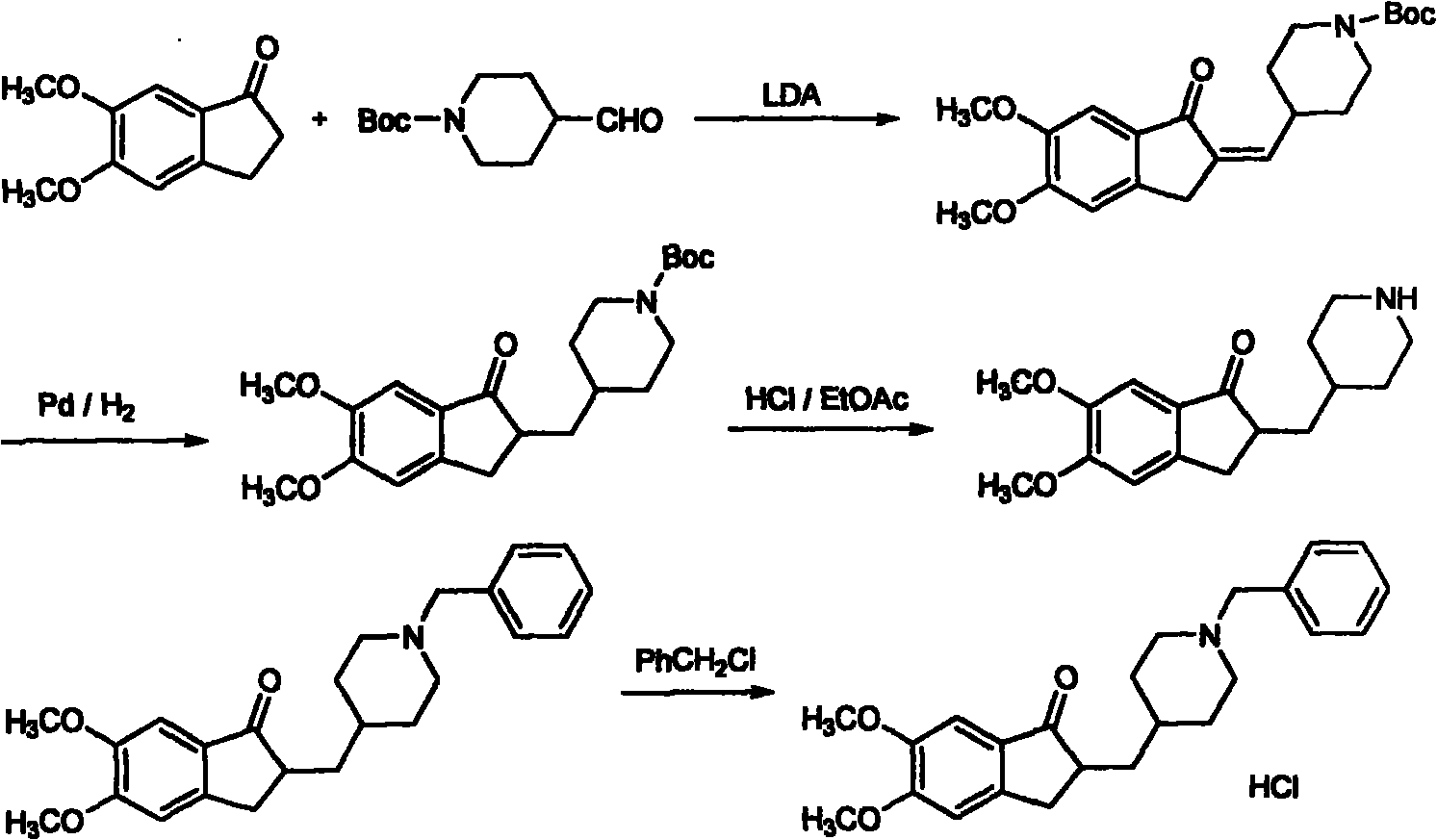
Novel donepezil synthesis process
A technique for the synthesis of donepezil hydrochloride, which is applied in the direction of organic chemistry, can solve problems such as difficulty in industrialized large-scale production, and achieve the effects of low price, easy control, and good reaction selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
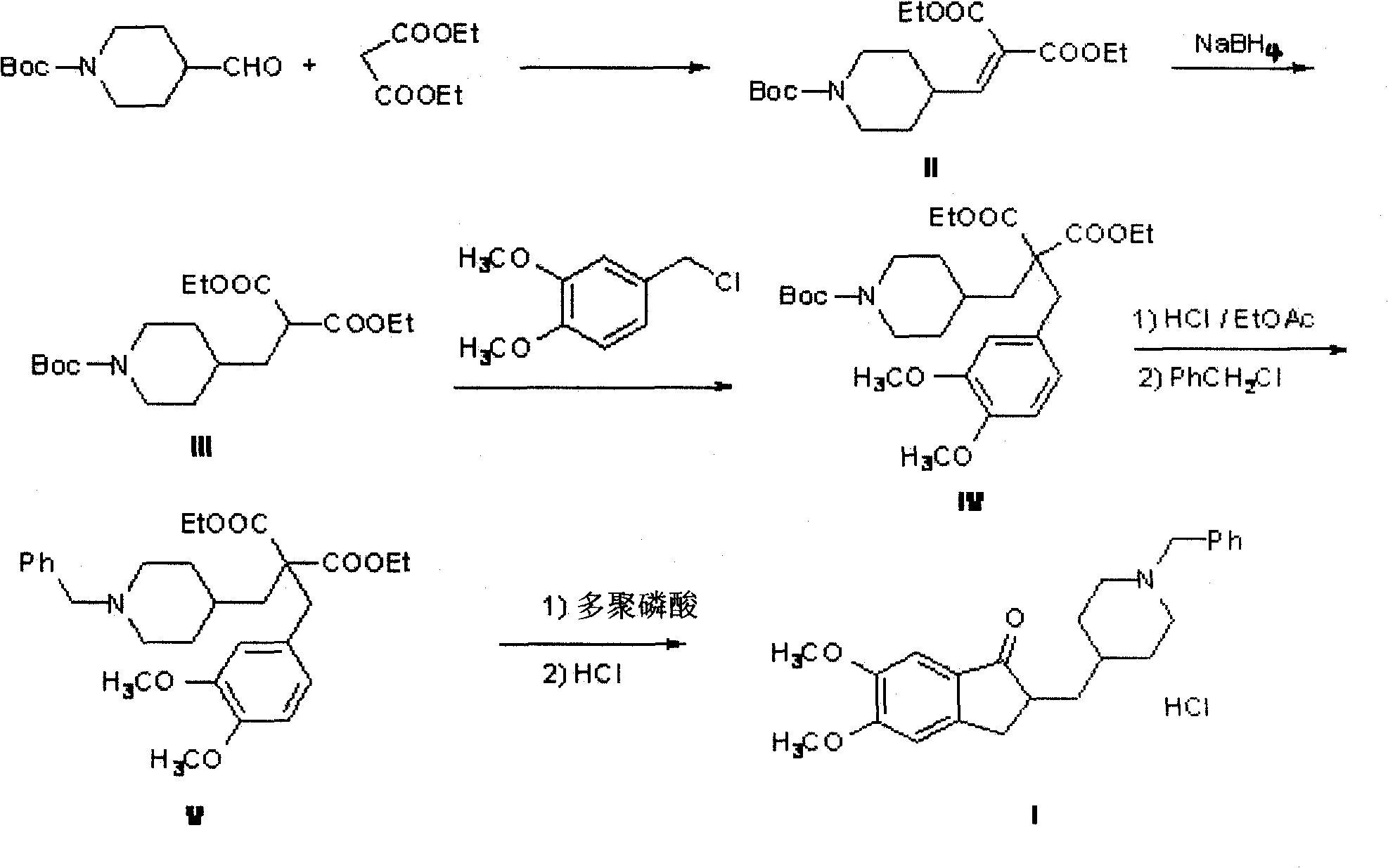
[0032] The preparation of compound 4'-(1'-Boc-piperidinyl) methylene malonate diethyl ester (II):
[0033] Dissolve 3.4g (0.014mol) of 1-Boc piperidine formaldehyde in 50mL of toluene, add 2.3g (0.014mol) of diethyl malonate, 0.17g (0.002mol) of piperidine and 0.12g (0.002mol) of glacial acetic acid , 140 ° C water reaction 8h. 50 mL of water and 50 mL of ethyl acetate were added to the reaction solution, and the organic layer was separated. The organic layer was washed twice with 60 mL of saturated NaCl, dried over anhydrous sodium sulfate, and the solvent was evaporated under reduced pressure to obtain 4.3 g of light yellow oil (II). Yield 75%.
Embodiment 2
[0035] The preparation of compound 4'-(1'-Boc-piperidinyl) methylene malonate diethyl ester (II):
[0036] Dissolve 3.4g (0.014mol) of 1-Boc piperidine formaldehyde in 50mL of benzene, add 2.3g (0.014mol) of diethyl malonate, 0.17g (0.002mol) of piperidine and 0.12g (0.002mol) of glacial acetic acid , 90 ° C water reaction for 12h. 50 mL of water and 50 mL of ethyl acetate were added to the reaction solution, and the organic layer was separated. The organic layer was washed twice with 60 mL of saturated NaCl, dried over anhydrous sodium sulfate, and the solvent was evaporated under reduced pressure to obtain 3.7 g of light yellow oil (II). Yield 66%.
Embodiment 3
[0038] The preparation of compound 4'-(1'-Boc-piperidinyl) diethyl methylmalonate (III):
[0039] Dissolve 3.8g (0.011mol) of the above oil (II) in 25mL of methanol, add NaBH in batches under ice-cooling 4 0.45g (0.012mol), stirred and reacted for 3h, after the reaction was completed, 1mL of concentrated hydrochloric acid and 25mL of water were added, extracted with 50mL of ethyl acetate, the ethyl acetate layer was separated, dried over anhydrous sodium sulfate, concentrated to give a light yellow oil (III )3.6g, yield 94.2%.
[0040] 1 HNMR (300MHz, CDCl 3 )δ: 4.19 (q, J=7.1Hz, 4H, CH 2 ), 4.09-4.05 (m, 2H, NCH 2 ), 3.45-3.40 (t, J=7.5Hz, 1H, CH), 2.68-2.59 (m, 2H, NCH 2 ), 1.84(t, J=7.3Hz, 2H, CH 2 ), 1.67-1.65 (m, 3H, piperidine ring), 1.44 (s, 9H, CH 3 ), 1.28-1.24 (t, J=7.5Hz, 6H, CH 3 ), 1.16-1.07 (m, 2H, piperidine ring).
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com