Patents
Literature
103 results about "Donepezil Hydrochloride" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
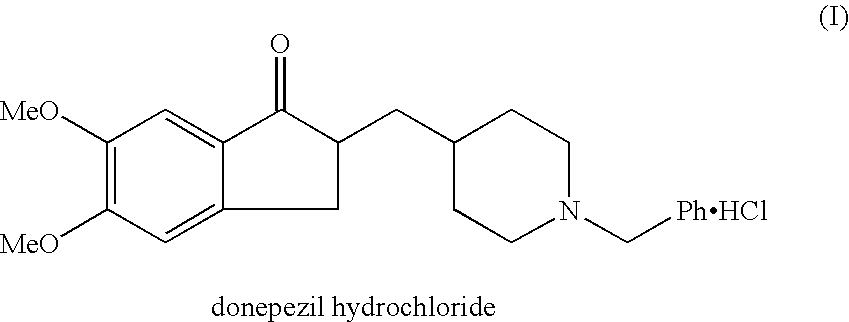
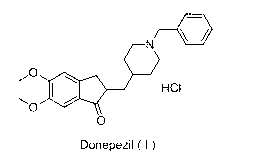
The hydrochloride salt of a piperidine derivative with neurocognitive-enhancing activity. Donepezil reversibly inhibits acetylcholinesterase, thereby blocking the hydrolysis of the neurotransmitter acetylcholine and, consequently, increasing its activity. This agent may improve neurocognitive function in Alzheimer's disease, reduce sedation associated with opioid treatment of cancer pain, and improve neurocognitive function in patients who have received radiation therapy for primary brain tumors or brain metastases.
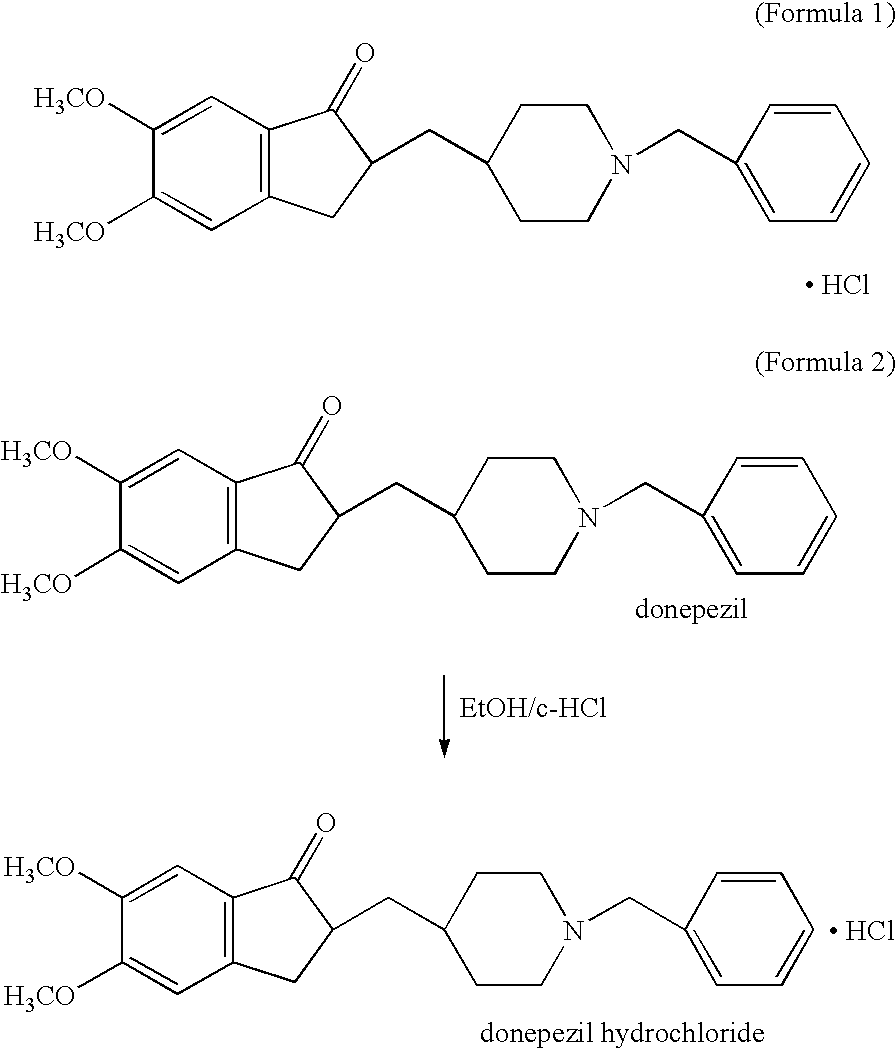
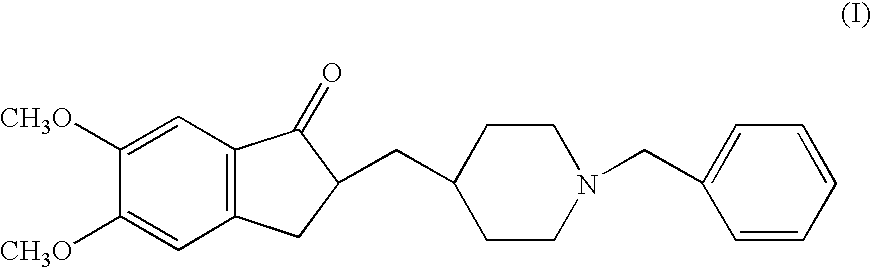
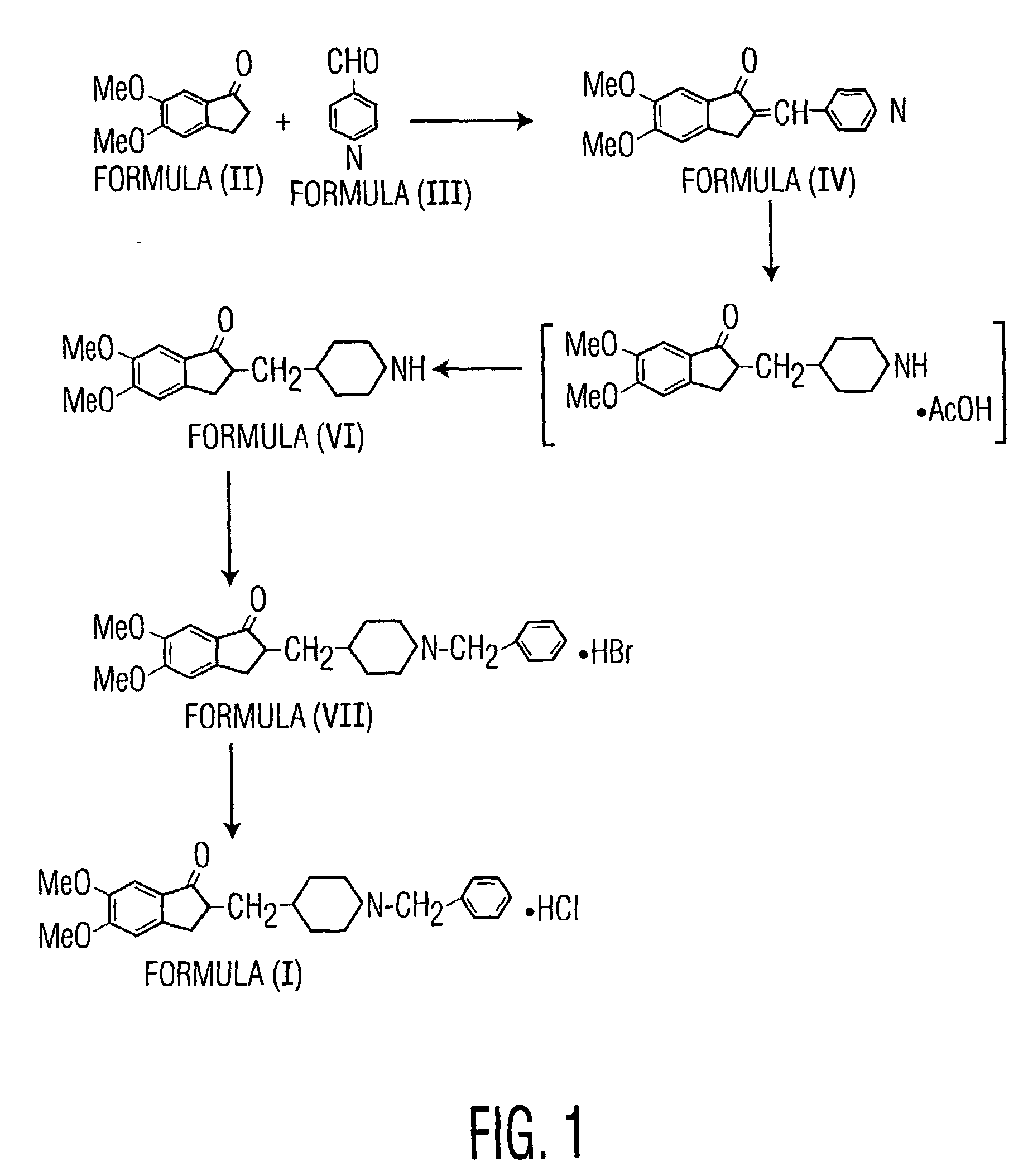
Donepezil polycrystals and process for producing the same
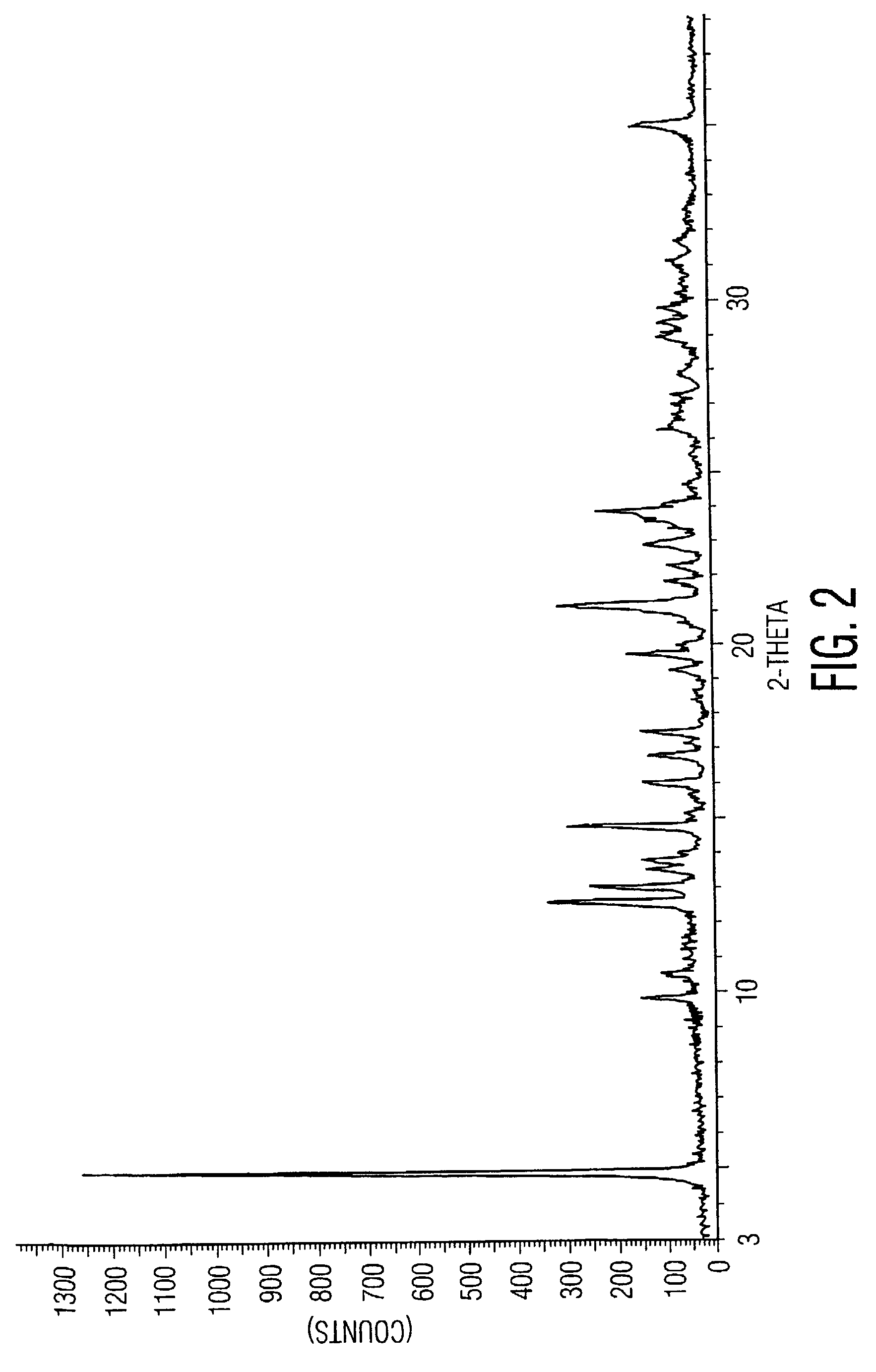
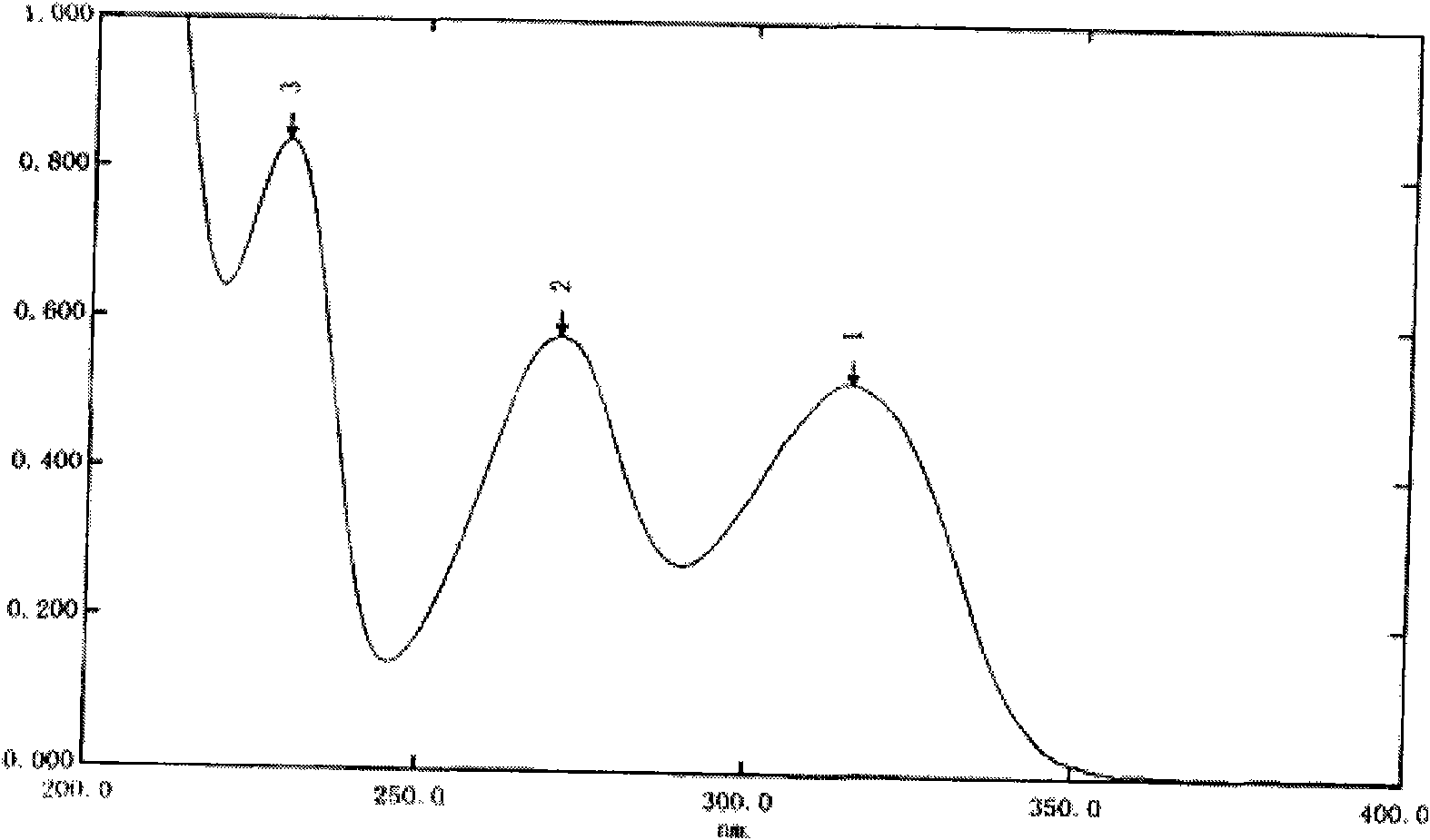
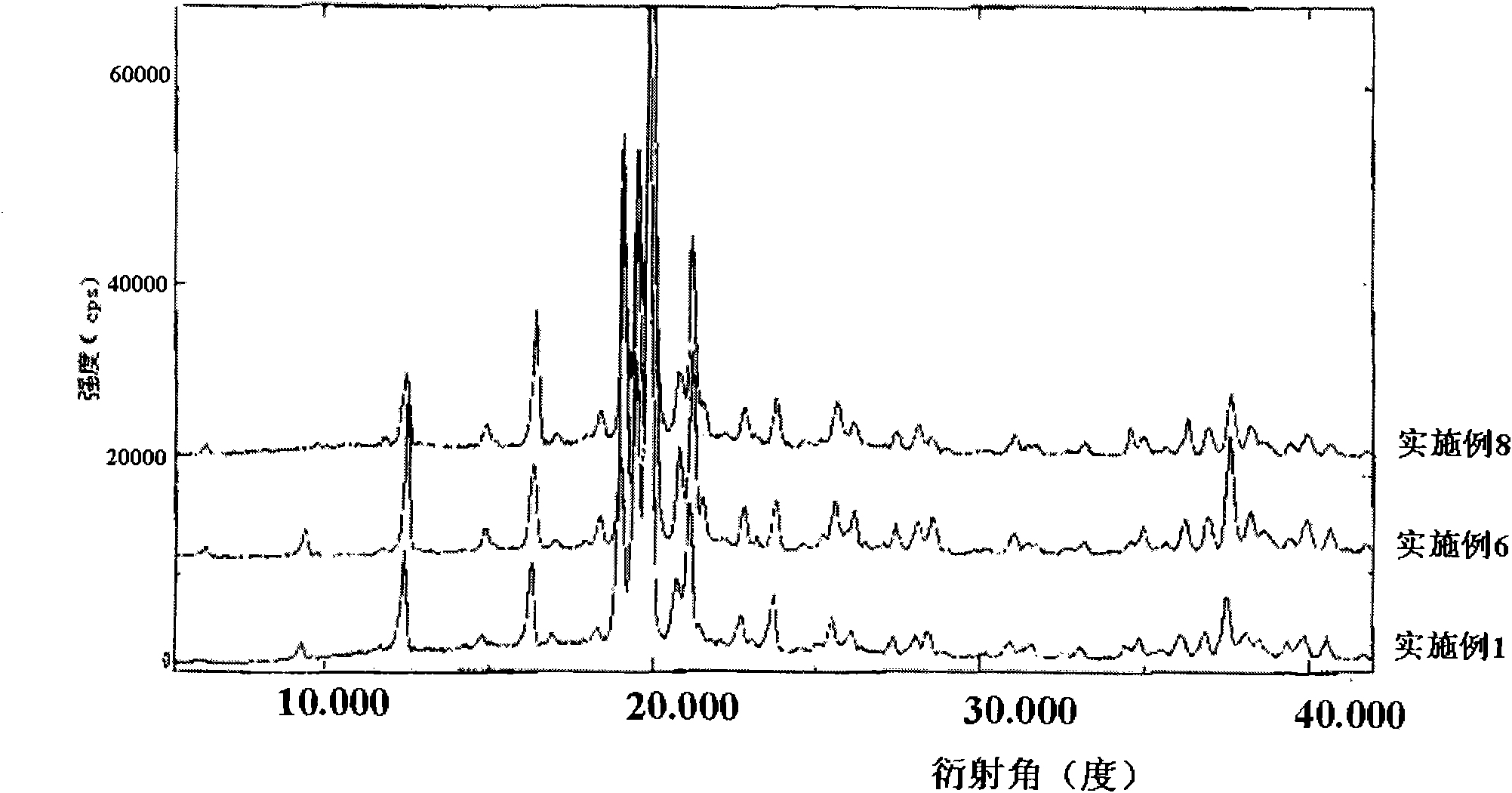
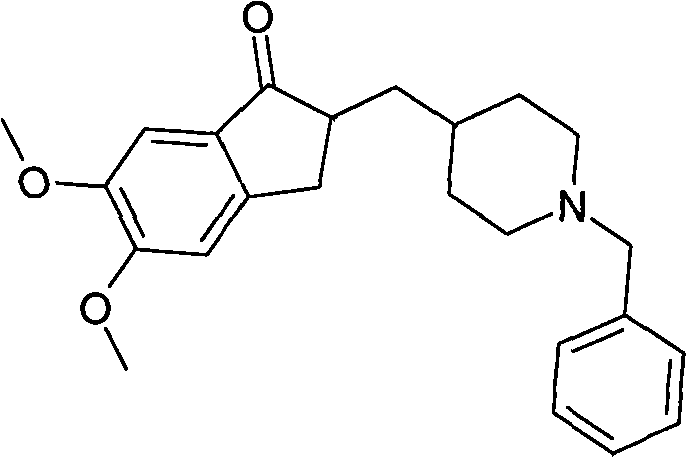
The present invention provides novel polymorphic crystals (A) to (C) having excellent handling properties and an extremely low content of residual solvent of donepezil used as a precursor for production of donepezil hydrochloride having an excellent action as a medicament, and an industrial process for producing the same. Further, the novel polymorphic crystals (A) to (C) according to the present invention are characterized by the powder X-ray diffraction pattern and / or IR absorption peaks of donepezil represented by the following formula:
Owner:EISAI CO LTD
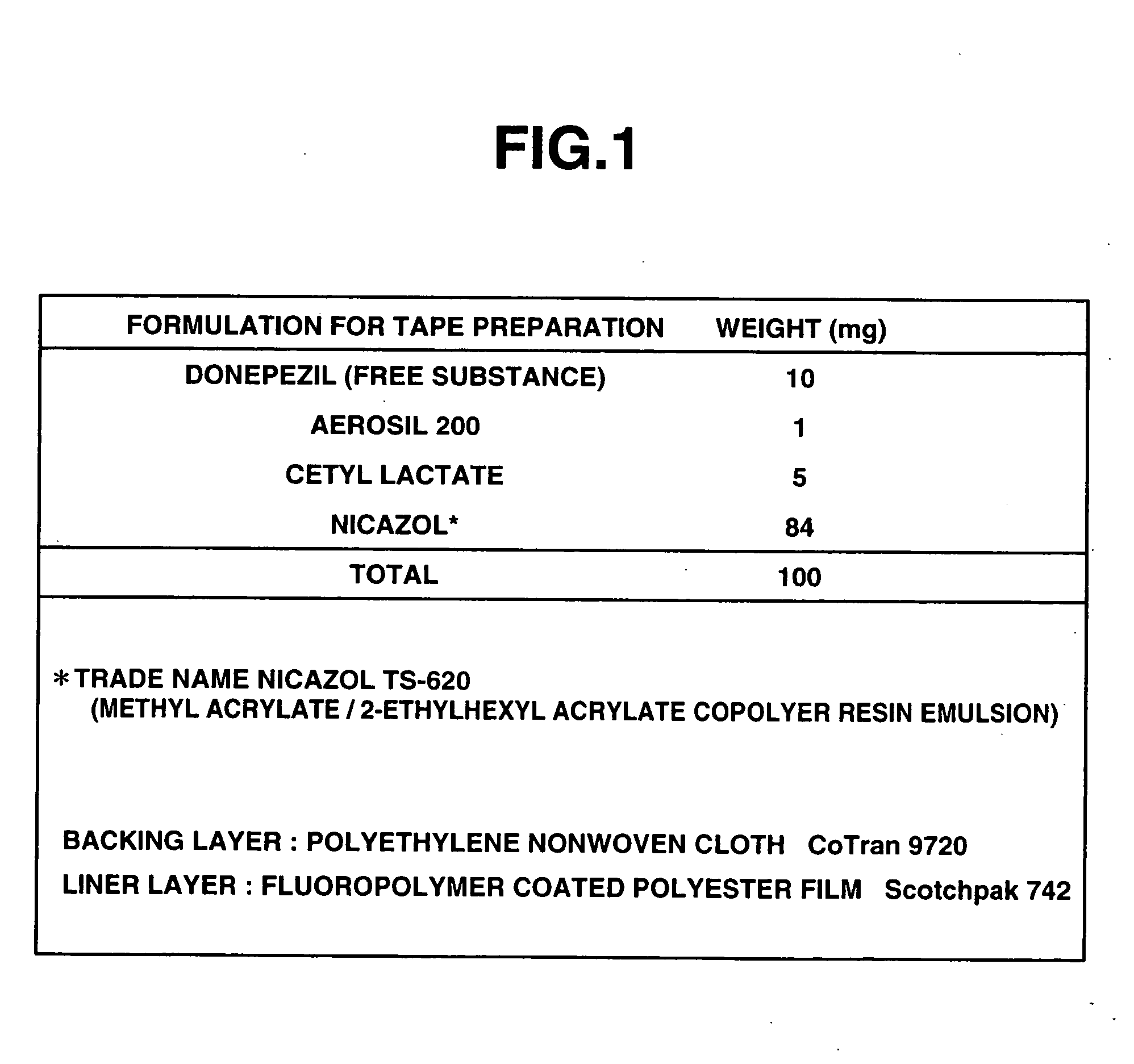
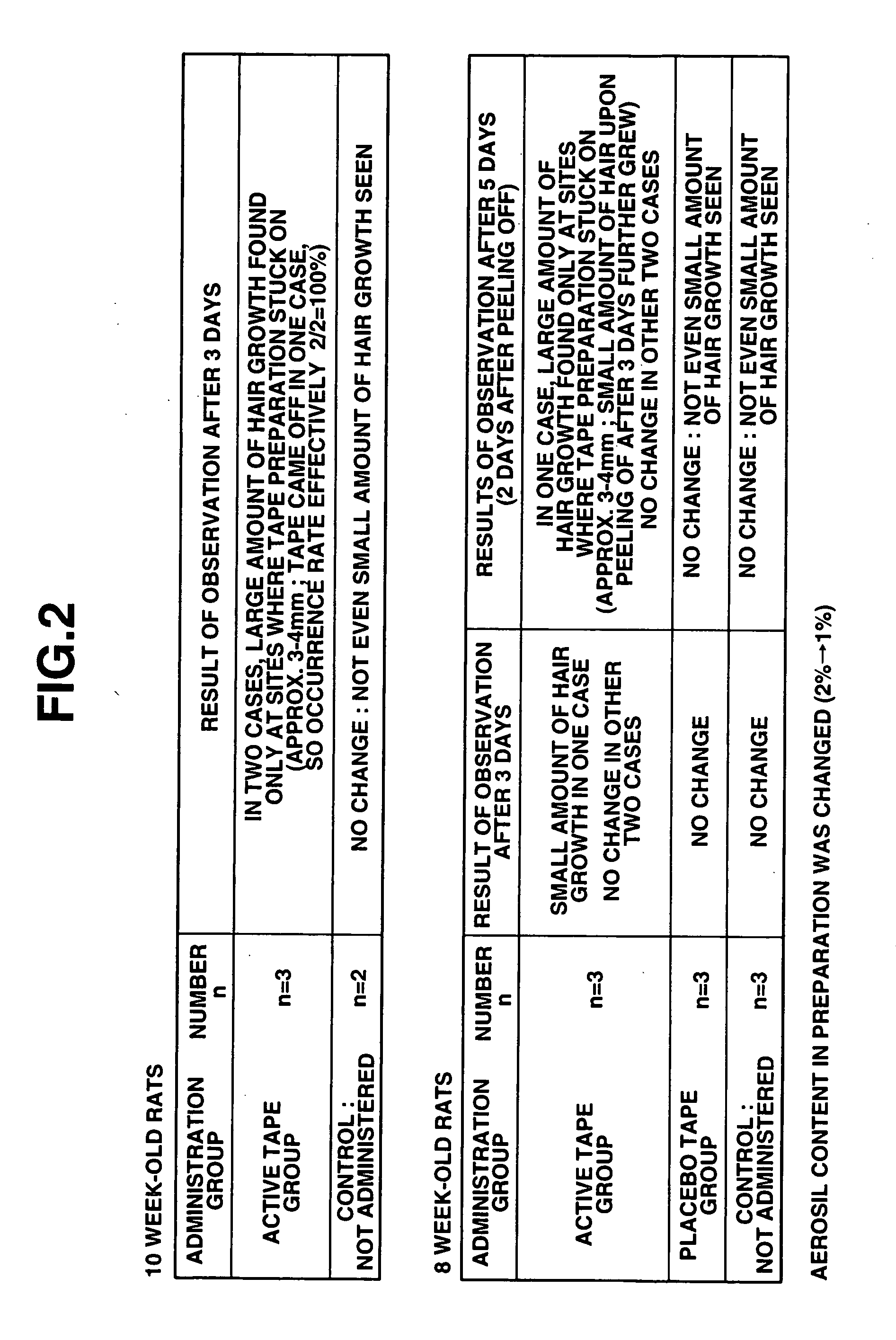
Hair growth stimulants, percutaneous preparations and method of stimulating hair growth
InactiveUS20050142088A1High elongationCosmetic preparationsHair removalCholinesteraseCholinesterase inhibition
According to the present invention, a compound having a cholinesterase inhibiting action has a hair growth promoting action, and there is provided a hair growth promoting agent containing such a compound having a cholinesterase inhibiting action. Moreover, a compound having an acetylcholinesterase inhibiting action as the cholinesterase inhibiting action is preferable, and in particular donepezil hydrochloride has a good hair growth promoting effect. Furthermore, according to the present invention, there is provided a percutaneously applied preparation having a hair growth promoting action, containing a compound having a cholinesterase inhibiting action such as donepezil; the percutaneously applied preparation is preferably a liquid preparation, a cream, an ointment, a plaster or a tape preparation. Moreover, according to the present invention, there is also provided a scalp hair growth promoting method comprising the step of applying donepezil onto the scalp.
Owner:EISIA R&D MANAGEMENT CO LTD
Novel process for production of highly pure polymorph (I) donepezil hydrochloride
InactiveCN101341122APromote formationReduce the temperatureNervous disorderOrganic chemistryPyridineSolvent
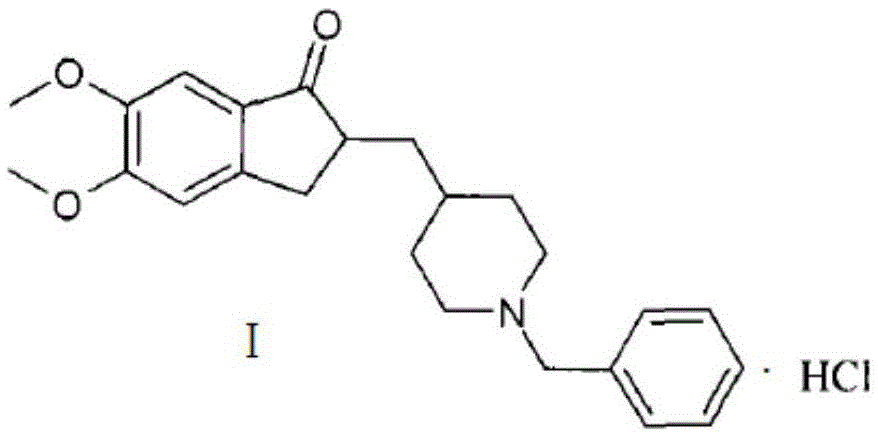
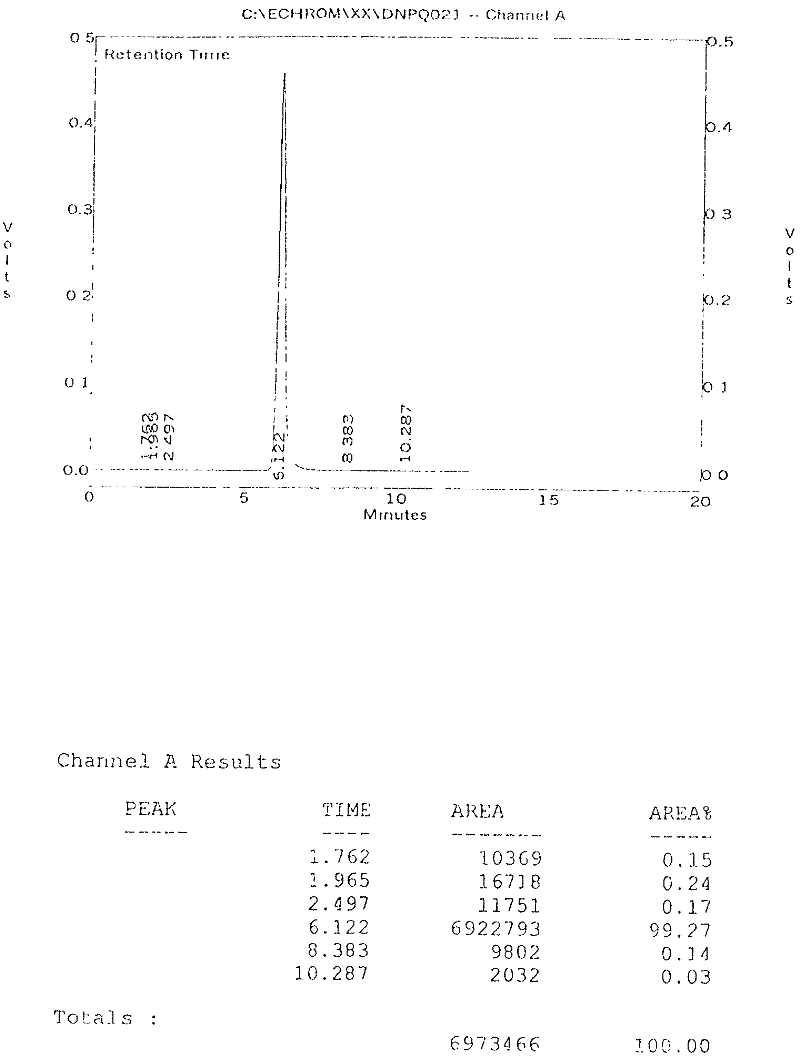
The present invention provides a novel, industrially realisable and economically preferable process for production of highly pure l-benzyl-4-[(5,6-dimethoxy-l-indanon)-2-yl]methyl piperidine hydrochloride, i.e., donepezil hydrochloride shown in the following reaction scheme, in Polymorph (I) morphological crystal form. (I) In one of the key steps of the process, during the hydrogenation 5,6-dimethoxy-2-(pyridine-4-yhnethylene)indan-l-one hydrochloride is saturated using Pd carbon to get 4-[(5,6-dimethoxy-l-indanon)-2-yl]-methyl piperidine at more than 97 % HPLC purity. In the crystallization step donepezil-hydrochloride is crystallized from an aqueous alcoholic solvent to get Polymorph (I) in at least 99.95 % HPLC purity.
Owner:RICHTER GEDEON NYRT
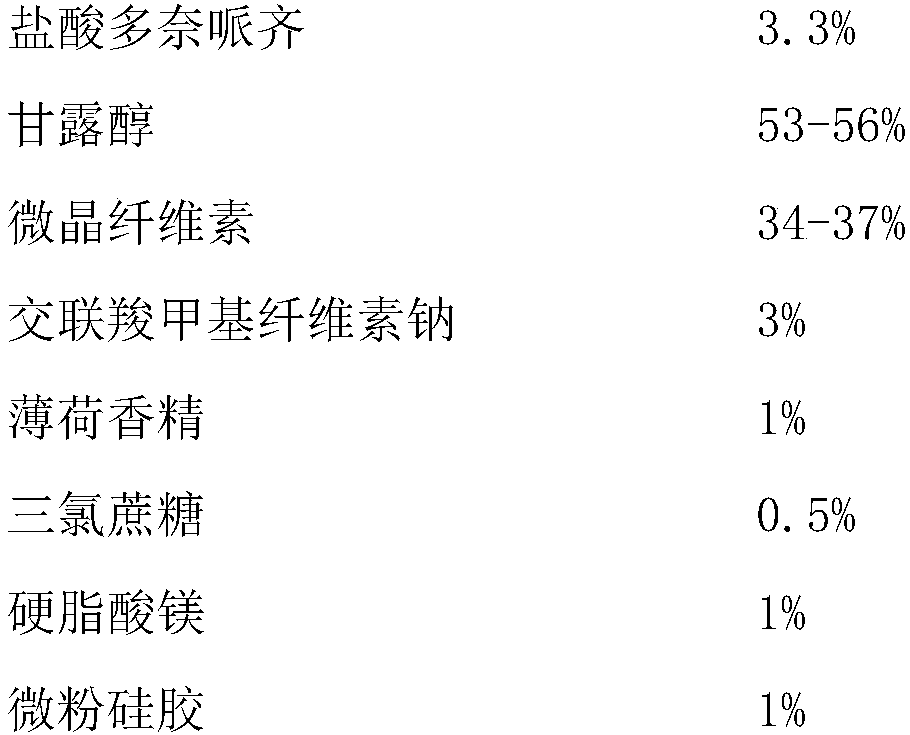
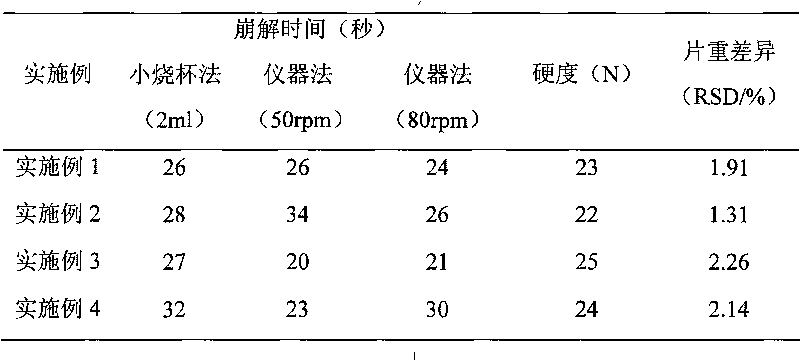
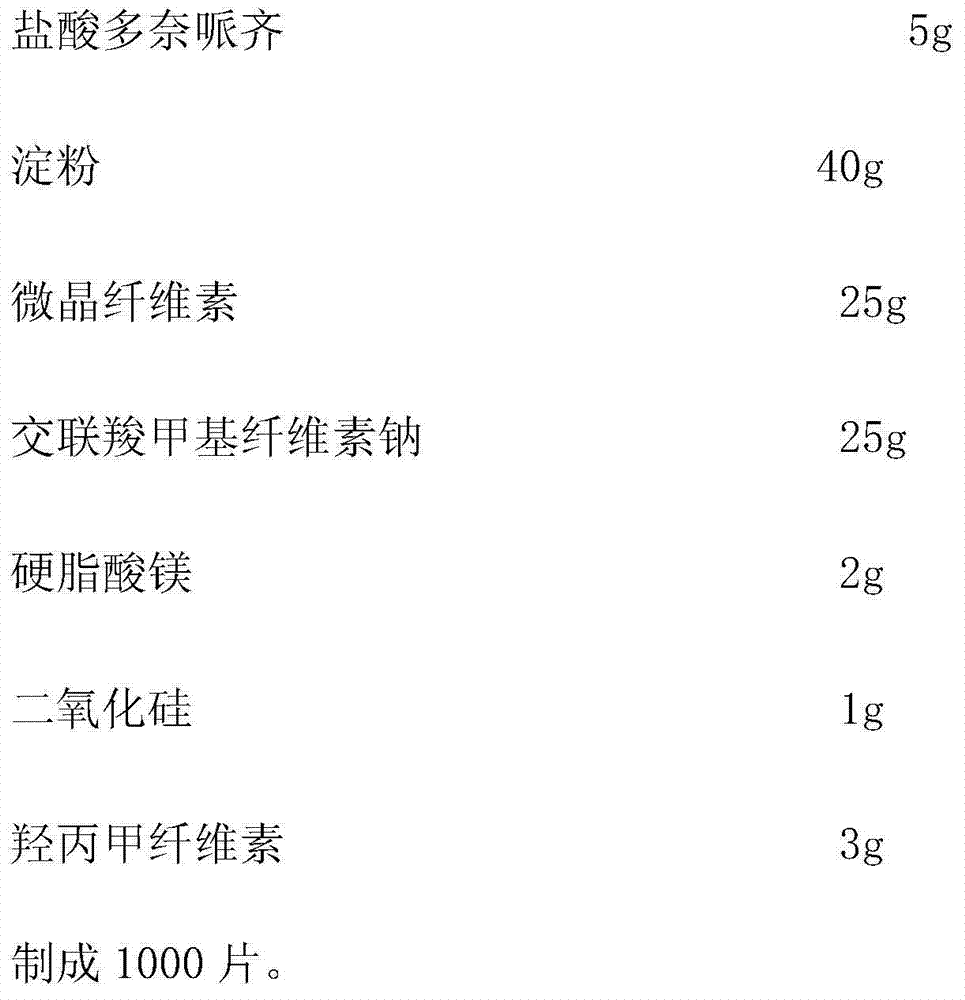
Donepezil hydrochloride orally disintegrating tablet and preparation method thereof
The invention relates to a donepezil hydrochloride orally disintegrating tablet and a preparation method thereof, in particular to an orally disintegrating tablet taking donepezil hydrochloride as main medicine and a preparation method thereof. The donepezil hydrochloride orally disintegrating tablet can be used for treating Alzheimer's Association.
Owner:COSCI MED TECH CO LTD
Medicament for prophylactic and/or therapeutic treatment of alzheimer-type dementia
InactiveUS20100035927A1Prolonged therapeutic periodAvoid symptomsBiocideNervous disorderValproic AcidTherapeutic treatment
A medicament for prophylactic and / or therapeutic treatment of Alzheimer-type dementia, which comprises donepezil hydrochloride and valproic acid or a salt thereof in combination.
Owner:NAGOYA CITY UNIVERSITY +1
Process for the preparation of donepezil hydrochloride
InactiveUS20070191610A1Easy to prepareSimple processOrganic chemistryHydrochlorideDonepezil Hydrochloride
The present invention relates to an improved process for the preparation of 1-benzyl-4-[(5,6-dimethoxy-1-indanon)-2-yl]methylpiperidine hydrochloride of Formula I.
Owner:AUROBINDO PHARMA LTD
Composition for covering flavor and preparation of containing the composition
InactiveCN1795857AImprove featuresMask irritationNervous disorderMacromolecular non-active ingredientsFlavorMANNITOL/SORBITOL
A taste-masking composition for donepezil hydrochloride is prepared from mannitol, trichlorosucrose, gelatin and xanthan gum. It can be mixed with donepezil for masking the irritative taste of donepezil, and can be used to prepare the oral disintegrating tablet.
Owner:ZHEJIANG WANBANG PHARMA
Use of purified donepezil maleate for preparing pharmaceutically pure amorphous donepezil hydrochloride
InactiveUS7592459B2Cheap and efficient and straightforwardPromote precipitationBiocideNervous disorderCrystallizationStereochemistry
The present invention provides a crystalline donepezil maleate, which is used as an intermediate in the preparation of donepezil hydrochloride. Also provided are novel processes for producing same in substantially pure form and a process for producing pharmaceutically pure amorphous donepezil hydrochloride therefrom.
Owner:CHEMAGIS
Donepezil hydrochloride dispersible tablet and preparation method thereof
InactiveCN103877046AGood effectPromote dissolution and absorptionNervous disorderPill deliveryDiseaseAdhesive
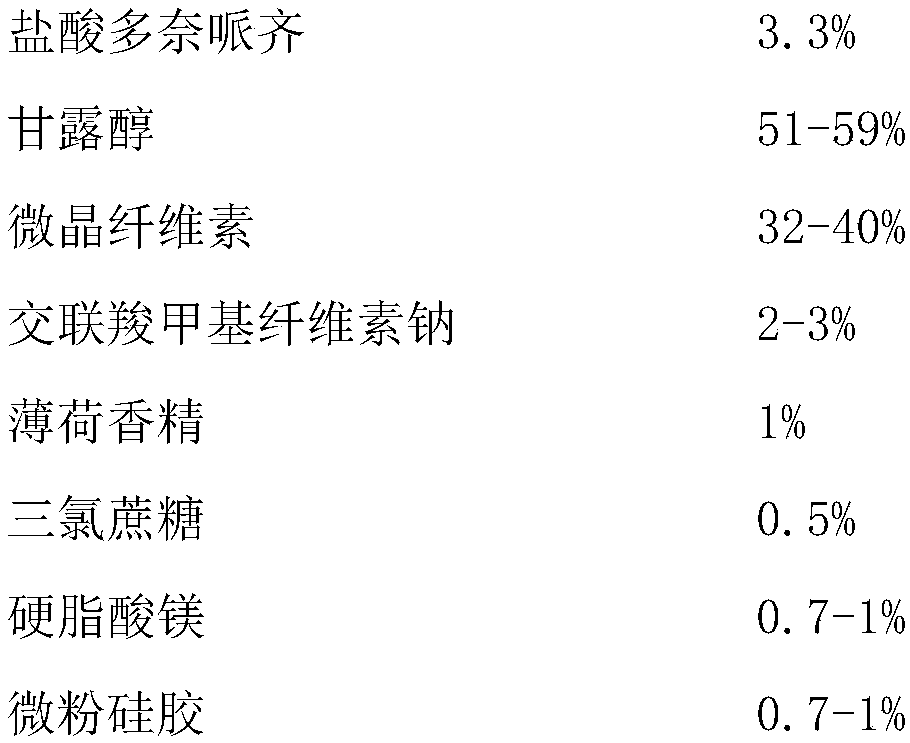
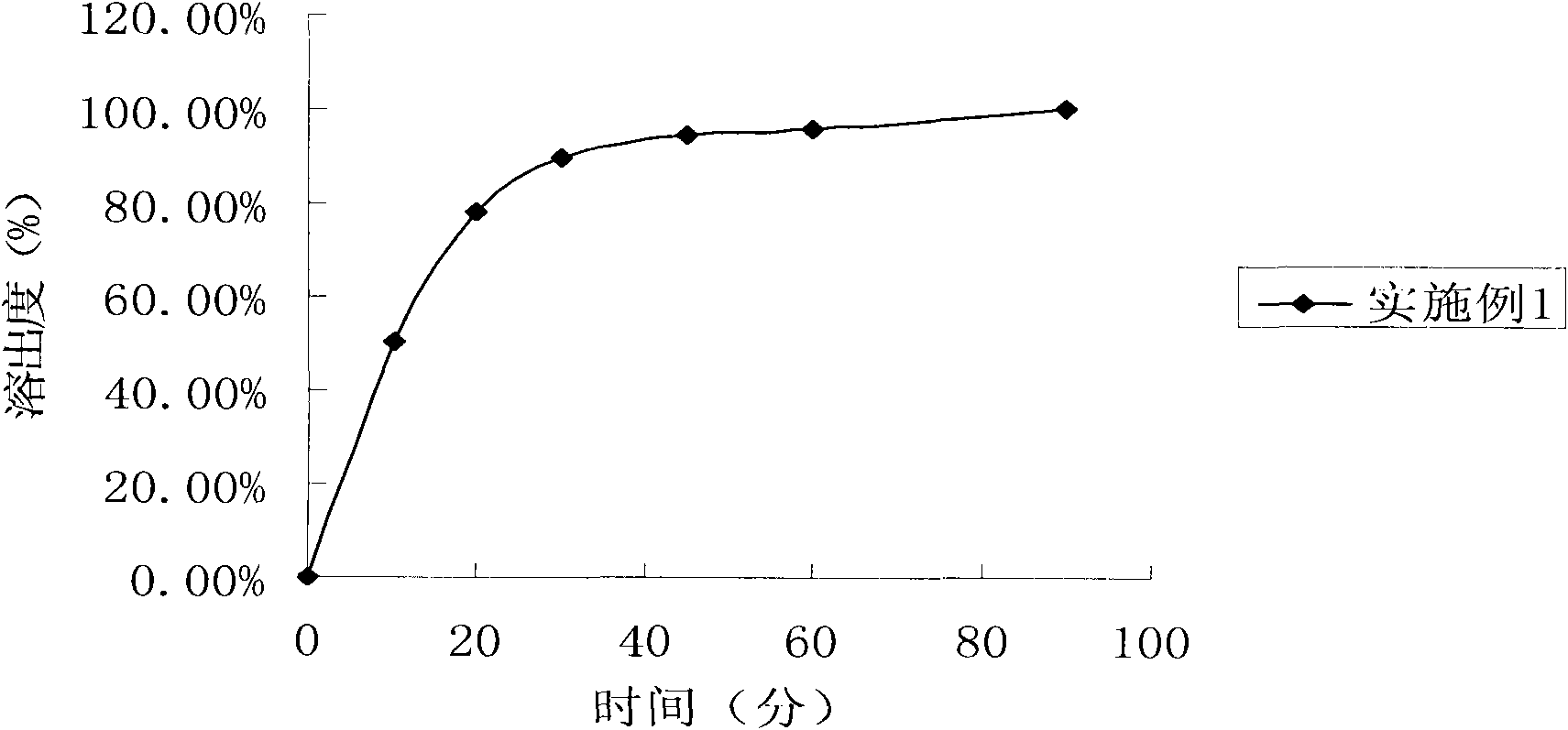
The invention relates to a donepezil hydrochloride dispersible tablet and a preparation method thereof, and belongs to the technical field of medicines. The donepezil hydrochloride dispersible tablet comprises the following components in percentage by weight: 1-5 percent of donepezil hydrochloride, 40-90 percent of disintegrating agent, 1-15 percent of lubricant and glidant and 1-15 percent of adhesive. The preparation method comprises the following steps: screening the components; uniformly mixing the donepezil hydrochloride and the disintegrating agent; adding the adhesive to prepare a soft material; granulating, baking and straightening granulating; adding the disintegrating agent, the lubricant and the glidant, and uniformly mixing; determining tablet weight, and tabletting to obtain the oxycodone hydrochloride dispersible tablet. The medicine is mainly used for treating senile dementia, especially mild or moderate alzheimer disease in clinical application, has stable quality, is beneficial to drug dissolution and absorption, is quick in response and convenient to take, can be taken orally after being dispersed with water, and also can be sucked in the mouth or swallowed.
Owner:张绪伟
Crystalline Form of Donepezil Hydrochloride
InactiveUS20080114173A1Easy to formulate into pharmaceutical productImprove stabilityOrganic chemistryHydrobromideBenzoyl bromide
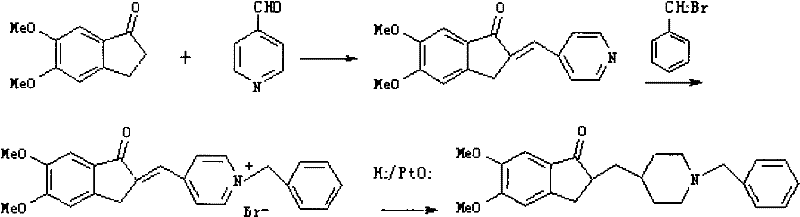
A process for preparing crystalline form I of donepezil hydrochloride comprises: a) condensing 5,6-dimethoxy-2-piperidin-4-yl-methyl-indan-1-one with benzyl bromide and reacting a condensation product with hydrobromic acid to form donepezil hydrobromide; and b) hydrolyzing donepezil hydrobromide, followed by reacting with aqueous hydrochloric acid.
Owner:DR REDDYS LAB LTD
Memantine hydrochloride slow release-donepezil quick release compound capsule
InactiveCN106727439AReduce the peak and valley phenomenon of blood drug concentrationReduce the frequency of takingNervous disorderAmine active ingredientsMemantine HydrochlorideFluidized bed
The invention provides a memantine hydrochloride slow release-donepezil quick release compound capsule. The compound capsule is prepared by jointly filling a capsule with memantine hydrochloride slow release pellets and donepezil quick release granules; the memantine hydrochloride slow release pellets are prepared by using medicinal empty pellet cores as original cores, dissolving memantine hydrochloride serving as main drug and proper excipients to form coating liquid, spraying the coating liquid to the bottom of a fluidized bed to form a drug-containing layer and sequentially coating the fluidized bed with an isolating layer and a slow release layer from inside to outside; the donepezil quick release granules are formed by subjecting donepezil hydrochloride serving as main drug and proper excipients to wet-process granulation, and the slow release pellets and quick release granules are filled into one same capsule according to a certain proportion of the main drug.
Owner:ZHONGSHUAI PHARMA SCI & TECH CO LTD
Synthesizing technology of donepezil hydrochloride
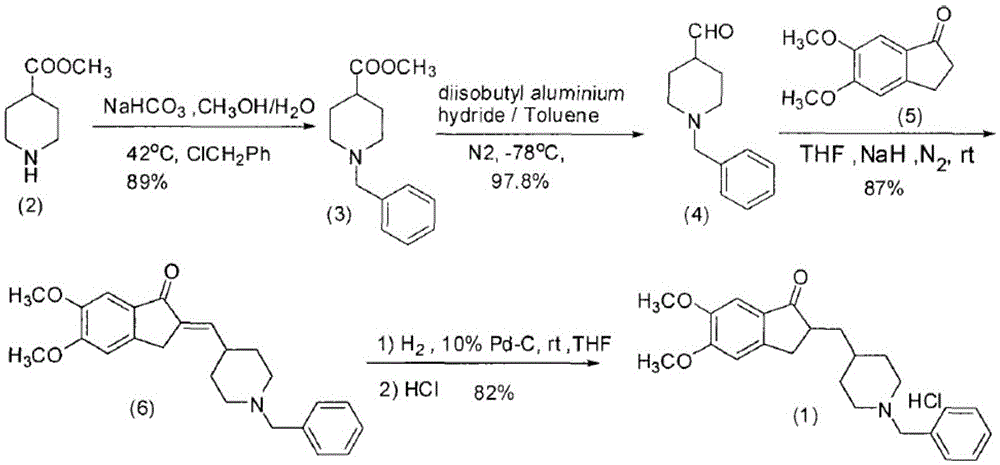
The invention relates to the synthesis of donepezil hydrochloride. According to a published patent CN100436416 which relates to a synthesizing technology of donepezil hydrochloride, diethyl malonate is adopted as an initial material, and 5 steps of condensation, reduction, substitution, ring-closing, and decarboxylation are adopted, such that donepezil hydrochloride is obtained. The steps are complicated, and the total yield is not high. The invention aims at providing a donepezil hydrochloride synthesizing technology employing 5,6-dimethoxy-2-(4-pyridyl)methylene-indan-1-one as an initial material. The technical scheme of the invention comprises steps that: the raw material is processed through hydrogenation, cooling, and filtration; a solvent glacial acetic acid is removed by reduced-pressure distillation; the obtained solution is processed through neutralization and extraction; a filtrate is condensed, and is dissolved in dichloromethane; the mixture is stirred, and triethylamine and benzyl chloride are dropped into the solution; the solution is cooled, the filtrate is condensed, and the obtained material is dissolved in methanol; a methanol solution of hydrogen chloride is dropped into the solution, such that a salt is formed; and the solution is cooled, crystallized, filtered, and dried, such that donepezil hydrochloride is obtained. The synthesizing technology provided by the invention is advantaged in short synthetic route and improved total yield. With the technology, the donepezil hydrochloride content is higher than 99%. The technology is suitable for industrialized productions.
Owner:陕西方舟制药有限公司
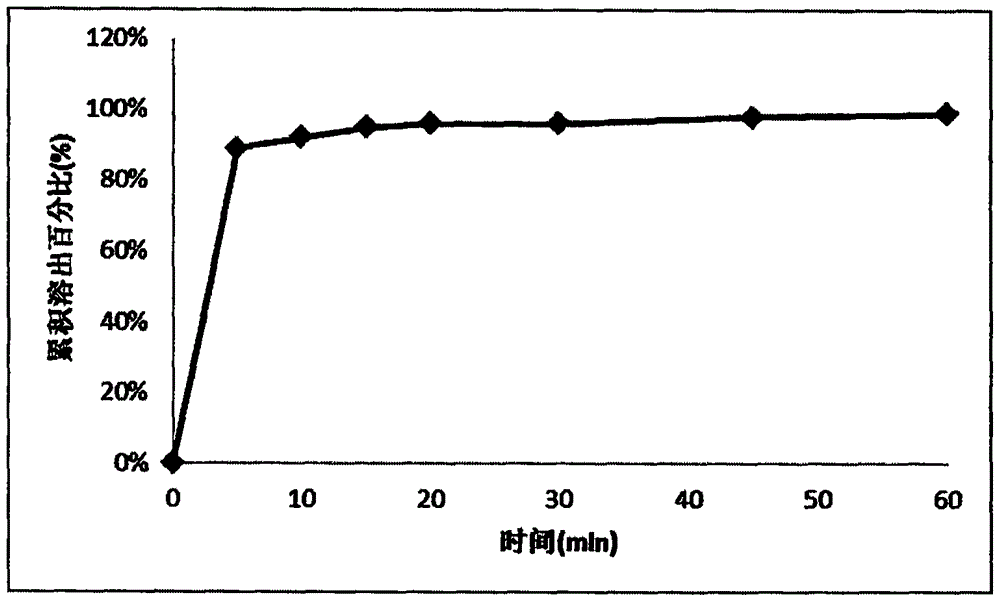
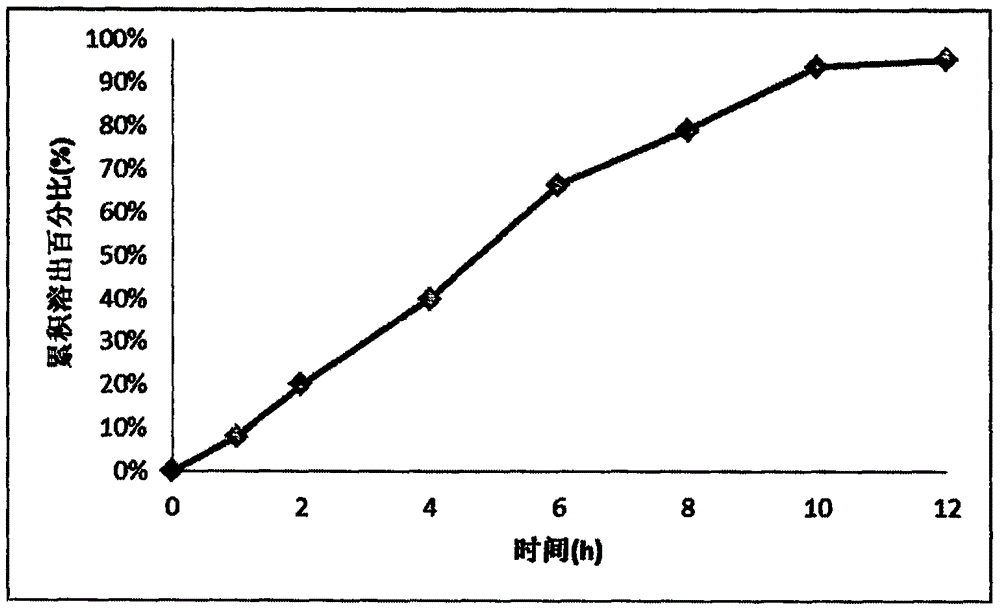
Sustained release tablet containing donepezil hydrochloride active component as well as preparation method and application thereof
InactiveCN102309465AGood reproducibilityImprove consistencyNervous disorderPharmaceutical delivery mechanismSustained Release TabletSide effect
The invention discloses a sustained release tablet containing a donepezil hydrochloride serving as an active component. The sustained release tablet provided by the invention comprises the donepezil hydrochloride serving as an active component, a sustained-release material and other auxiliary materials, wherein the weight ratio of the donepezil hydrochloride serving as an active component to the sustained-release material is 1:(0.2-20), preferably 1:(0.5-10) and further preferably 1:(0.7-8). The sustained release tablet containing donepezil hydrochloride prepared by the invention is released steadily, which is favorable for the reduction of the fluctuation of the in-vivo medicament, thus the side effect is reduced, and the sustained release tablet is more suitable for treating patients suffering from Alzheimer-type dementia.
Owner:TIANJIN INSTITUTE OF PHARMA RESEARCH
Method for preparing donepezil hydrochloride crystal form I
ActiveCN101906066AGuaranteed stabilityShorten drying timeOrganic chemistryIsopropyl etherDonepezil Hydrochloride
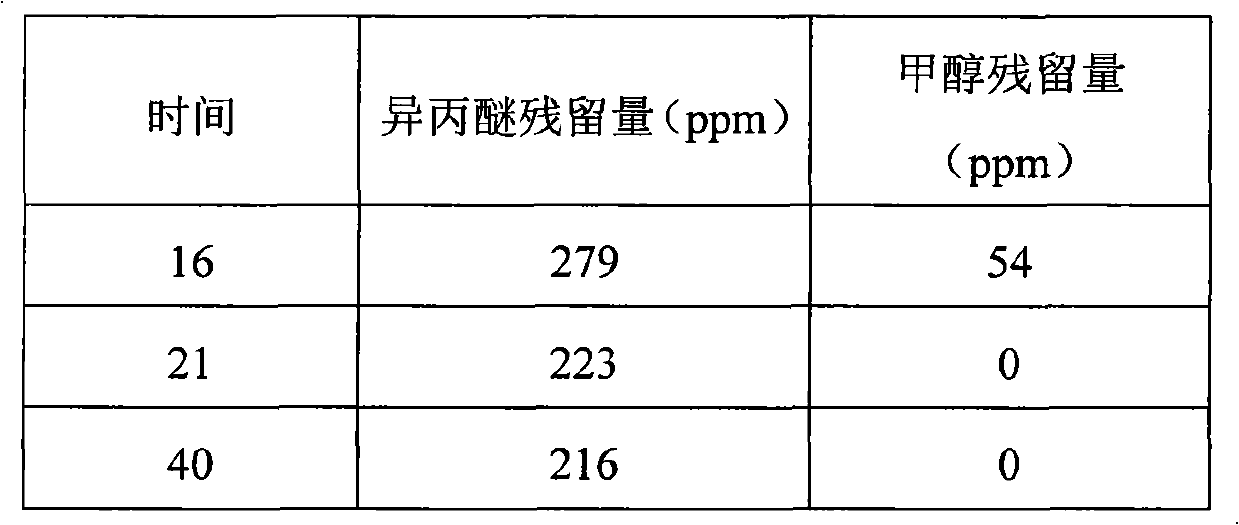
The invention relates to a method for preparing a donepezil hydrochloride crystal form I. The method comprises the following steps of: separating the donepezil hydrochloride crystal form I from a solvent system which contains methanol and isopropyl ether; and separating and drying the donepezil hydrochloride crystal form I to obtain a finished product. The method is characterized in that: the drying temperature is controlled in the range of between 25 and 60 DEG C by stepped temperature rise. The scheme provided by the invention can effectively control the residual quantity of the isopropyl ether in the donepezil hydrochloride crystal form I to be less than 100 ppm.
Owner:ZHEJIANG HUAHAI PHARMA CO LTD
Method for producing high-purity donepezil hydrochloride anhydrous I type crystal form and product thereof
The invention relates to a method for producing a high-purity donepezil hydrochloride anhydrous I type crystal form and a product thereof, belonging to the technical field of drug synthesis. The method comprises the following steps of: re-crystallizing a donepezil hydrochloride polycrystal type crude product or an amorphous donepezil hydrochloride crude product with a lower alcohol solvent to obtain a donepezil hydrochloride I type crystal form wet product; and drying in vacuum and cooling to the room temperature to obtain a high-purity donepezil hydrochloride anhydrous I type crystal form finished product under the protection of nitrogen, wherein KF is less than 0.5%; the purity reaches more than 99.5%; and single impurity is less than 0.1%. The invention has the advantages of simple production method and fewer steps, is suitable for industrial production, has better crystal form of the product and great significance to pharmacy, sale and storage of the product, and overcomes technology barriers.
Owner:浙江东亚药业股份有限公司
Method for preparing donepezil hydrochloride
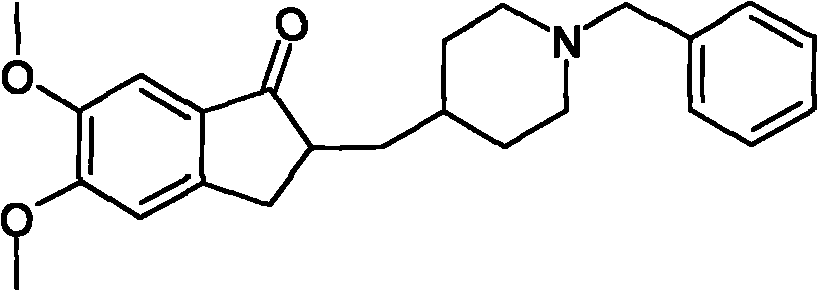
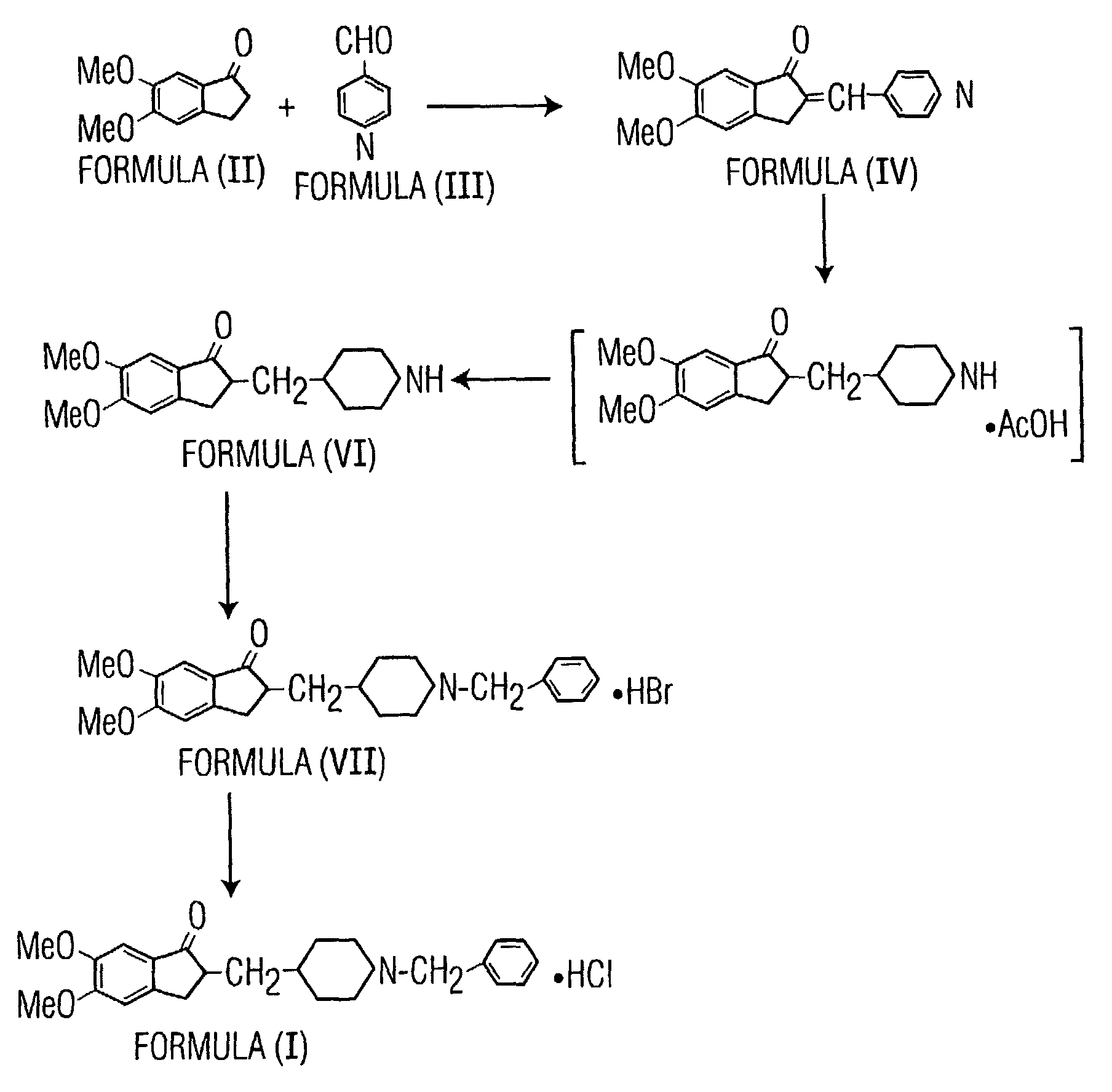
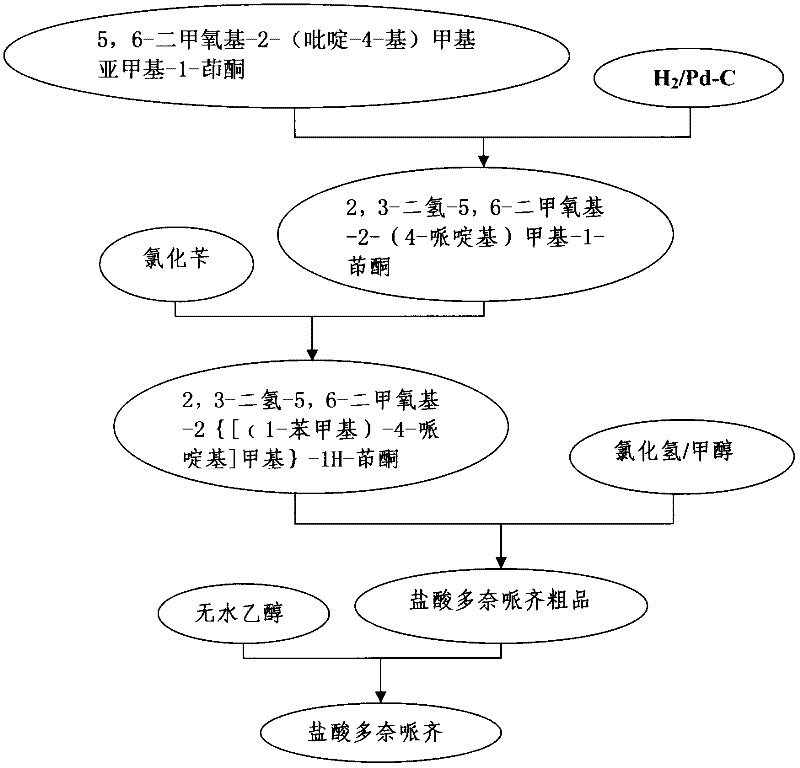
The invention provides a method for preparing donepezil hydrochloride, which comprises the steps as follows: (1) 5,6-dimethoxy-2-(4-pyridyl)methylene-1-idenone (II) is taken as a starting material, and is processed through catalytic hydrogenation under the action of platinum dioxides, so as to generate compounds that are 5,6-dimethoxy-2-(4-piperidyl)methylene-2,3-dihydro-1-idenone (III); (2) the obtained compounds (III) and benzaldehyde are processed through condensation, so as to generate 1-benzyl-4-[(5,6-dimethoxy-1-idenone)-2-methylene]piperidine (IV); and (3) the obtained compounds (IV) and hydrogen chloride are processed through salifying, so as to generate 1-benzyl-4-[(5,6-dimethoxy-1-idenone)-2-methylene]piperidine hydrochloride that is donepezil hydrochloride (I). In the method for preparing donepezil hydrochloride, 5,6-dimethoxy-2-(4-pyridyl)methylene-1-idenone is taken as the starting material, and three-step reaction comprising catalytic hydrogenation, condensation and salifying are adopted to prepare donepezil hydrochloride. The method is simple and efficient to operate, has the advantages of mild reaction conditions, high safety and higher yield coefficient, is easy to control, and is suitable for industrialized production.
Owner:JIANGSU HAICI BIOLOGICAL PHARMA CO LTD OF YANGTZE RIVER PHARMA GRP
Donepezil hydrochloride dripping pills
InactiveCN1175813CEasy to divideLittle side effectsNervous disorderPill deliveryPolyethylene glycolWater soluble
Donepezil hydrochloride guttate pills contains Donepezil hydrochloride 6-16 wt%, water soluble supplementary material polyglycol-4000 0-94 wt% and polyethenoxy searate 0-94 wt%. The present invention is easy in separating dosage and has high biological utilization, stable quality and easy-to-control production condition.
Owner:SHANDONG NEWTIME PHARMA
Donepezil hydrochloride orally disintegrating tablet and preparation method thereof
InactiveCN102038653AOvercome the disadvantages of single-actingGood disintegrationNervous disorderPill deliverySodium bicarbonateOrally disintegrating tablet
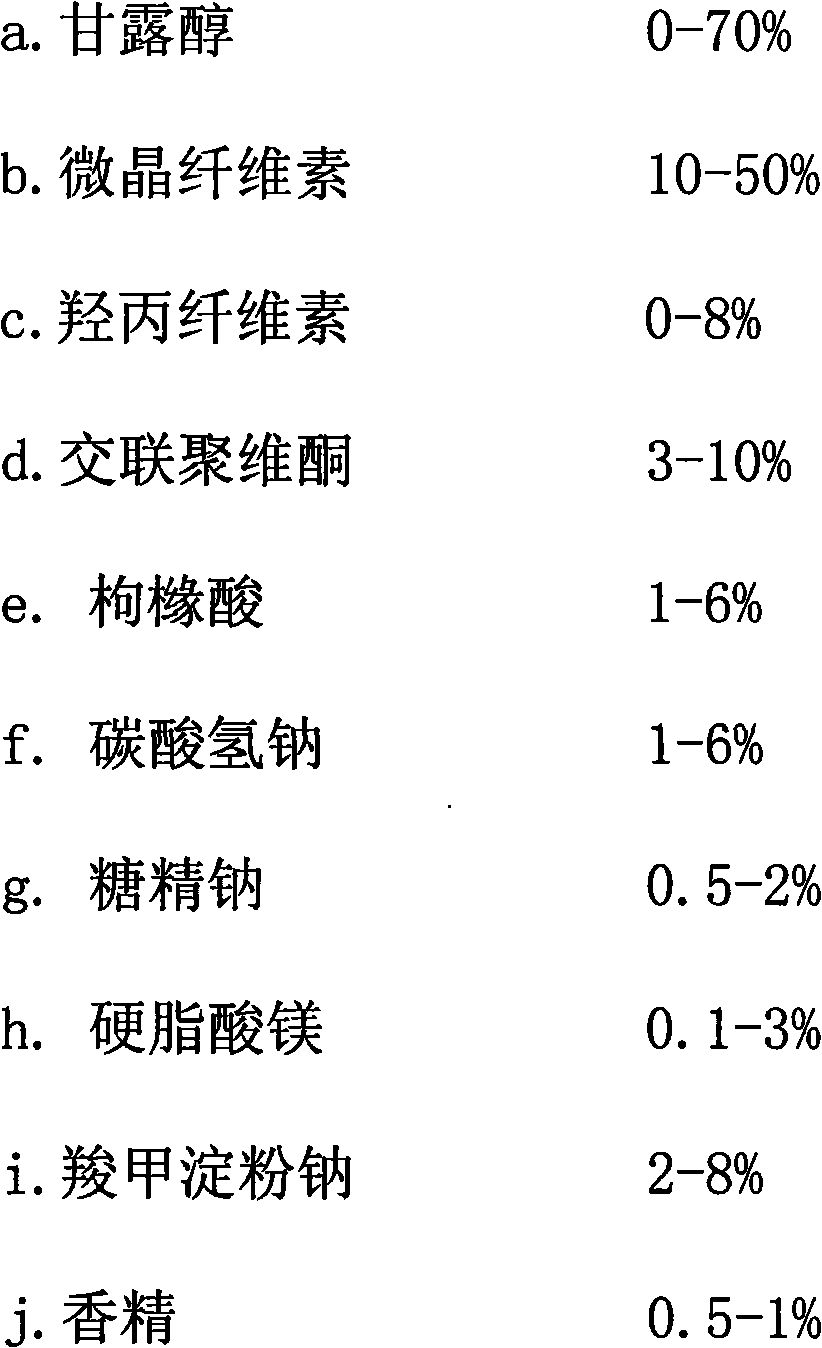
The invention discloses a donepezil hydrochloride orally disintegrating tablet. The main medicine of the donepezil hydrochloride orally disintegrating tablet is donepezil hydrochloride; and the auxiliary medicines of the donepezil hydrochloride orally disintegrating tablet comprise mannitol, microcrystalline cellulose, hyprolose, crospovidone, sodium bicarbonate, citric acid, soluble saccharin, carboxymethyl starch sodium, magnesium stearate and essence. The invention can preferably treat the low-degree or moderate-degree alzheimer type dementia, can preferably improve the cognitive dysfunction, the mental and behavioral abnormalities and the daily self-care ability of the patient who suffers from the alzheimer type dementia (AD), can alleviate the dementia degree, is convenient to take, can fast disintegrate, and is convenient for the old or the patient who has medication obstacles or is hard to take water.
Owner:SHANDONG FANGMING PHARMACEUTICAL CO LTD
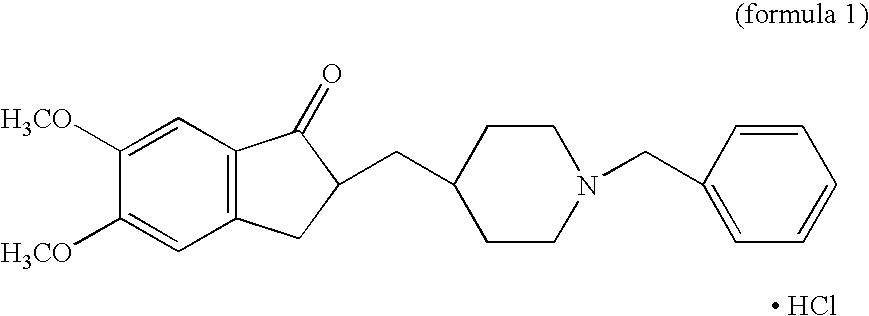
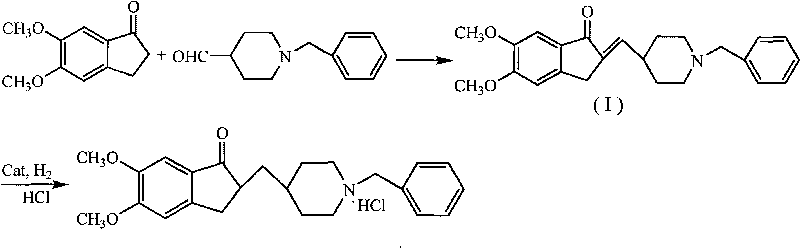
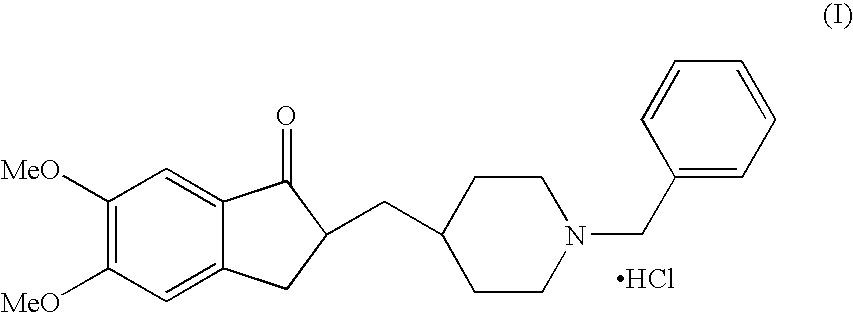
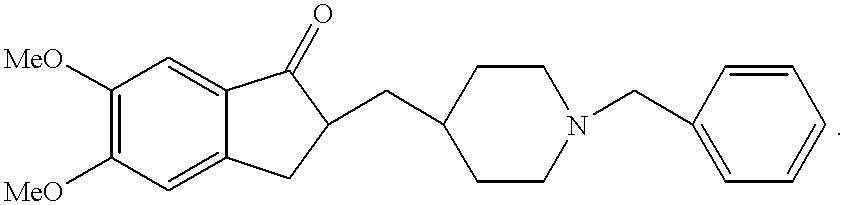
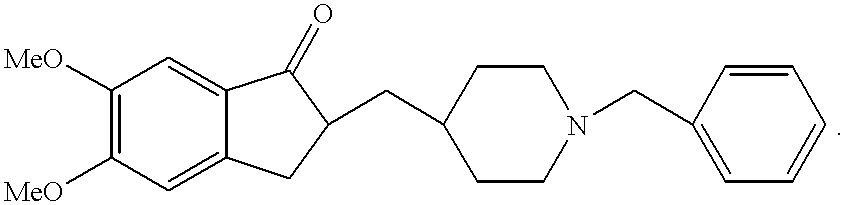
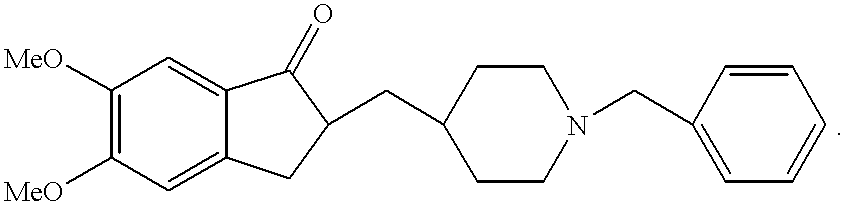
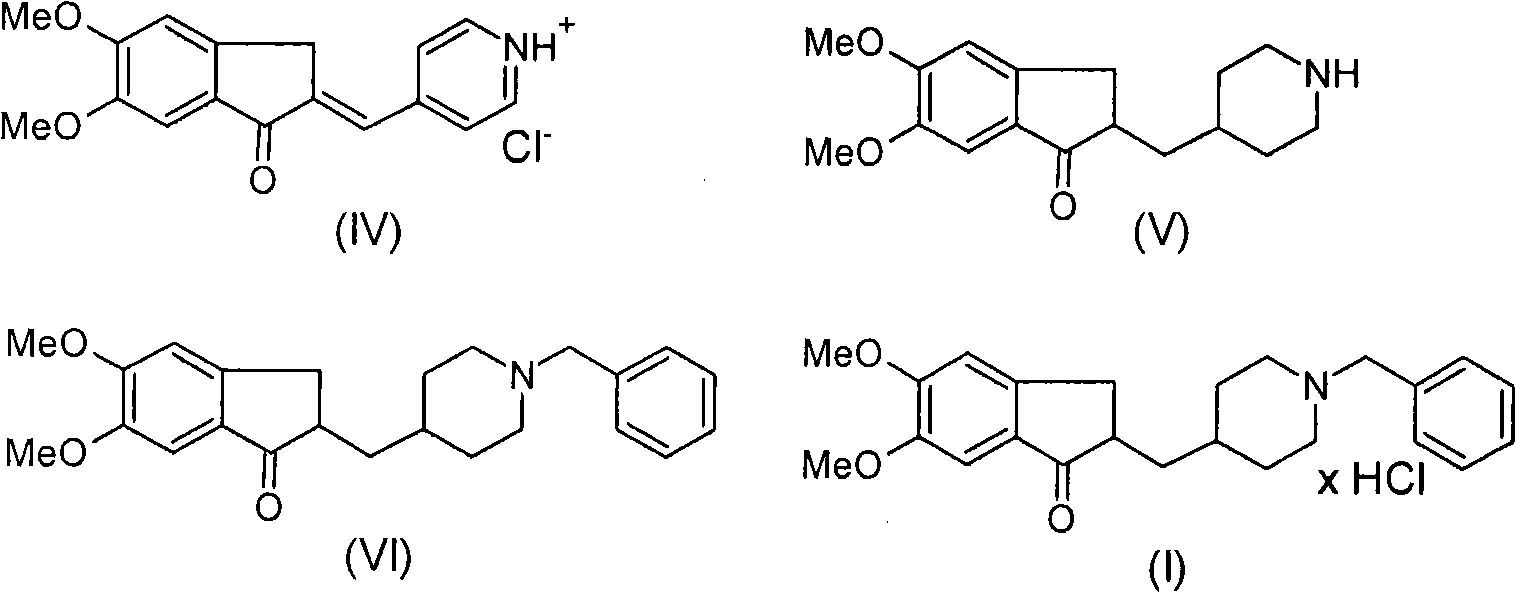
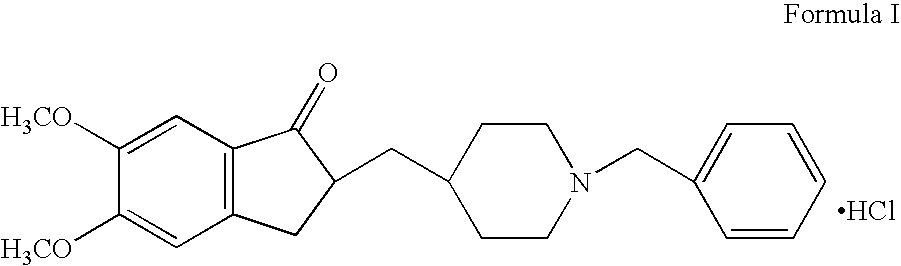
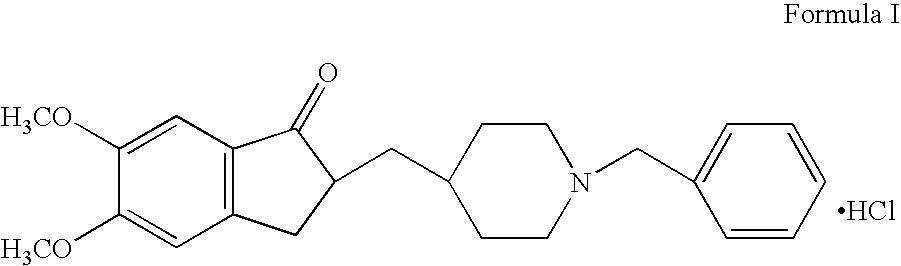
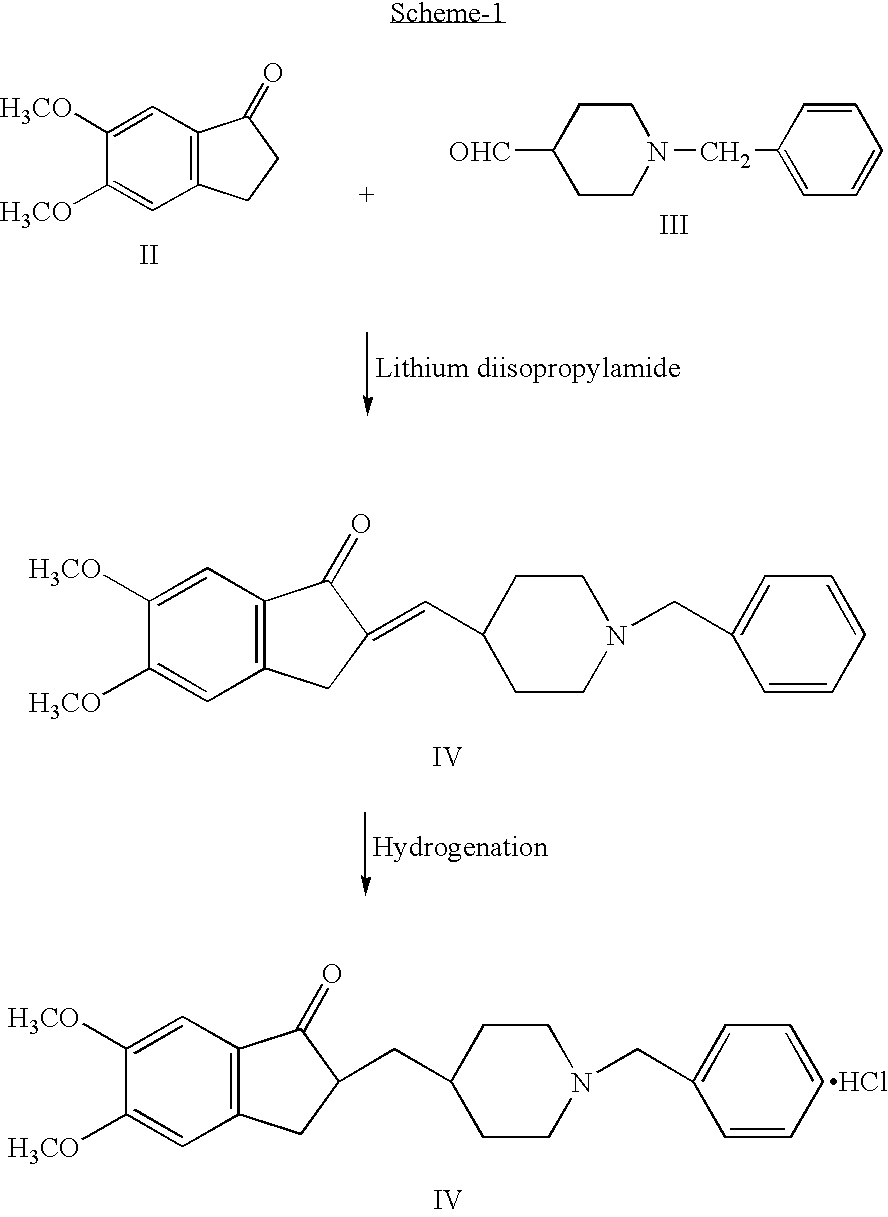
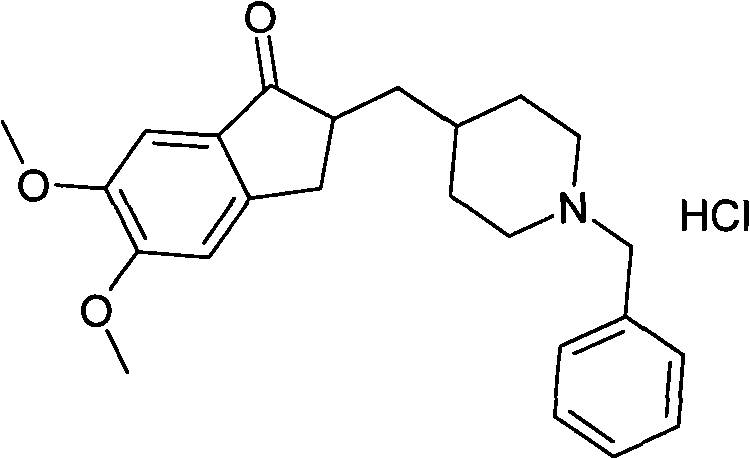
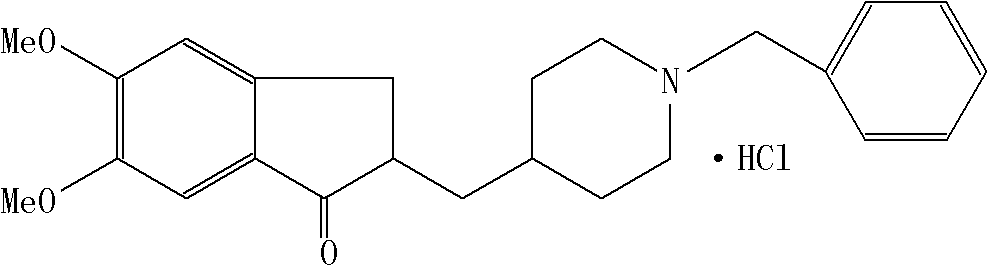
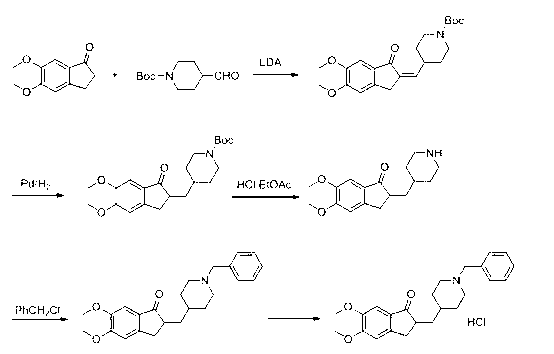
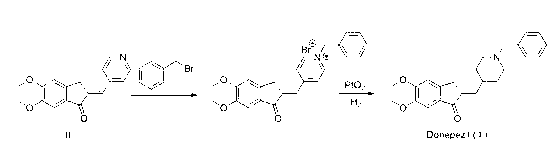
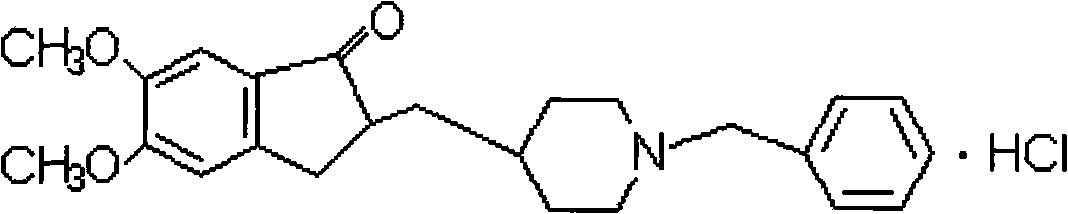
Process for producing a polymorphic form of (1-Benzyl-4-[(5,6-dimethoxy-1-indanone)-2-yl] methyl piperidine hydrochloride (donepezil hydrochloride)
InactiveUS20050288330A1Suitable for mass manufacturingLarge scaleBiocideOrganic chemistryHydrochlorideDonepezil Hydrochloride
The present invention discloses a novel, stable polymorph of 1-benzyl-4-[(5,6-dimethoxy-1-indanone)-2-yl]methyl piperidine hydrochloride commonly known as Donepezil hydrochloride. Further the present invention discloses a process for producing Donepezil HCl amorphous and it's polymorph Form (VI).
Owner:NAIDU AVINASH +3
Preparation method of 3,4-dimethoxy phenylpropionic acid
ActiveCN105601496AReduce usageReduce pollutionOrganic compound preparationCarboxylic acid esters preparationChemical synthesisAcetic acid
The invention belongs to the technical field of chemical synthesis and in particular relates to a preparation method of a donepezil hydrochloride key intermediate 3,4-dimethoxy phenylpropionic acid. The method comprises the following steps: taking 3,4-dimethoxybenzaldehyde as a raw material to react with ethyl acetate under the action of sodium ethoxide to obtain an intermediate 3,4-dimethoxy ethyl cinnamate; hydrolyzing the 3,4-dimethoxy ethyl cinnamate under an alkaline condition, and carrying out hydrogenation reduction to obtain the 3,4-dimethoxy phenylpropionic acid. The preparation method provided by the invention has the advantages of environmental friendliness, high product purity, high yield and low raw material cost and the like.
Owner:山东诚汇双达药业有限公司
Fine purification method for key intermediate of Donepezil Hydrochloride
ActiveCN101343248ALow impurity contentHigh purityOrganic chemistryPurification methodsOrganic solvent
The invention discloses a refining method of a key intermediate of 2-(1-benzyl-piperidine-4-methyl alkenyl)-5, 6 dimethoxy-indanone of Donepezil, namely, a compound (I), and the method is to obtain the high purity compound (I) by agitating a crude product of the compound (I) in the presence of inorganic base in a benzenoid aromatic hydrocarbon-containing organic solvent of C6 to C12 at a certain temperature. The method has advantages of simple operation, short reaction time, single solvent and low cost, and is suitable for the industrialized production.
Owner:ZHEJIANG HUAHAI PHARMACEUTICAL CO LTD +1
Orally disintegrating tablet containing donepezil hydrochloride and preparation method thereof
ActiveCN107789328AFast disintegrationGood compressibilityNervous disorderPharmaceutical non-active ingredientsOrally disintegrating tabletMannitol
The invention provides an orally disintegrating tablet containing donepezil hydrochloride and a preparation method thereof. The orally disintegrating tablet comprises donepezil hydrochloride, mannitol, microcrystalline cellulose, and a disintegrating agent. By controlling the using amounts of mannitol and microcrystalline cellulose, the orally disintegrating tablet having the advantages of rapid disintegration, low friability, good taste, and simple technology is obtained.
Owner:CHENGDU KANGHONG PHARMA GRP
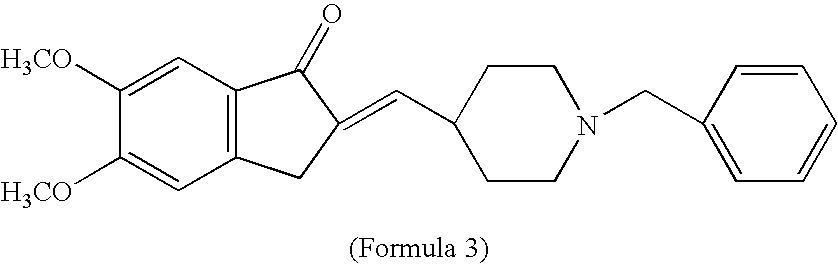
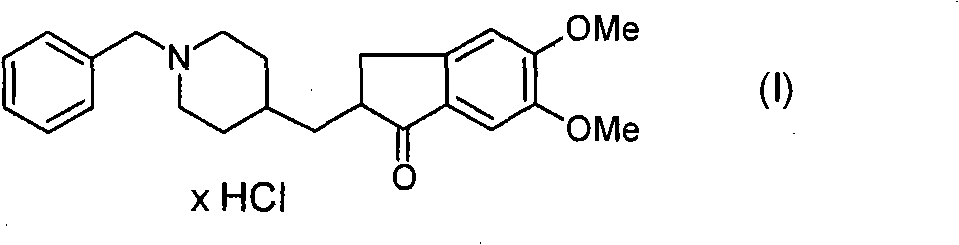
Process for producing multiform crystal of donepezil hydrochloride
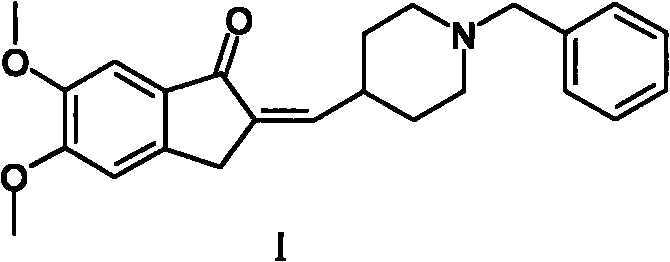
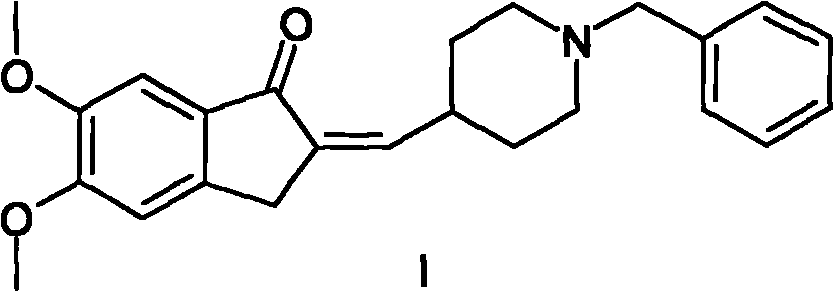
The present invention provides a simple method of producing polymorphic crystal (III), which has high safety to environment and the bodies of operators; is gentle to environment; and can produce at low costs; and has a high refining effect. It is a method of producing polymorphic crystal (III) of donepezil hydrochloride (chemical name: 1-benzyl-4-[(5,6-dimethoxy-1-indanon)-2-yl)methylpiperidine.monohydrochloride) represented by the following structural formula (formula (I)), which comprises dissolving donepezil (chemical name: 1-benzyl-4-[(5,6-dimethoxy-1-indanon)-2-yl]methylpiperidine) in ethanol; and adding hydrochloric acid or hydrogen chloride thereto, followed by stirring.
Owner:EISAI CO LTD
Preparation method of donepezil hydrochloride
ActiveCN105418488AReduce purificationStarting materials are readily availableOrganic chemistryKetoneIonic liquid
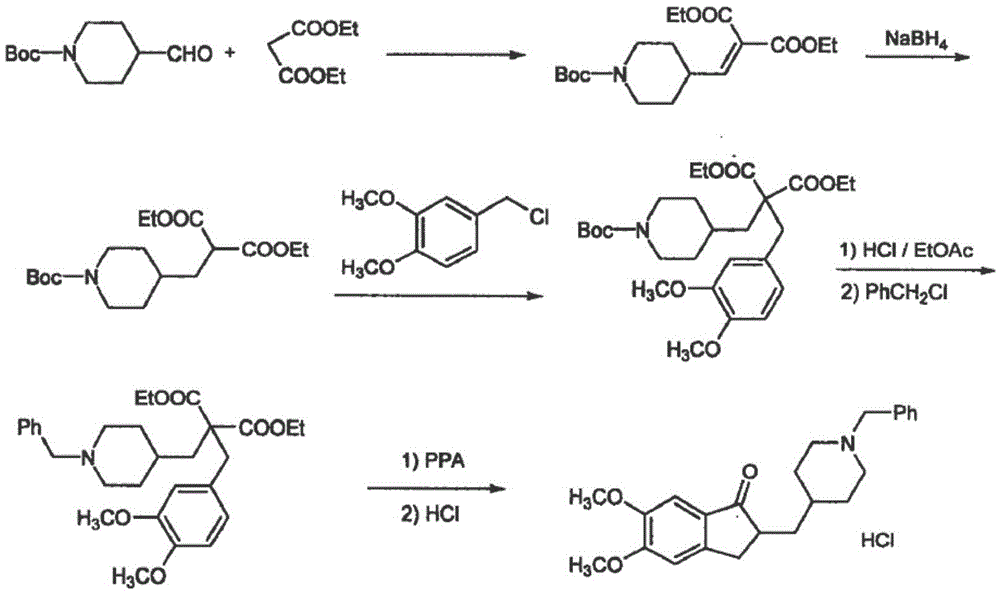
The invention discloses a preparation method of donepezil hydrochloride. The preparation method comprises the steps that 3-chlorine-1-(3, 4-dimethoxy phenyl) propane-1-ketone (II) is made to react with N-benzyl-4-formyl-piperidine (III) under the condition of a lewis acid ionic liquid catalyst, and 1-benzyl-4-(5, 6dimethoxy-1-indanone-2-methylene)-piperidine (IV) is obtained through a one-pot method, and then the donepezil hydrochloride (I) is obtained through reduction and salt formation. The preparation method of the donepezil hydrochloride has the advantages of being simple in process, low in production cost, environmentally friendly and suitable for industrialized production.
Owner:SHANDONG LUOXIN PHARMA GRP CO LTD
Preparation method of donepezil hydrochloride impurities
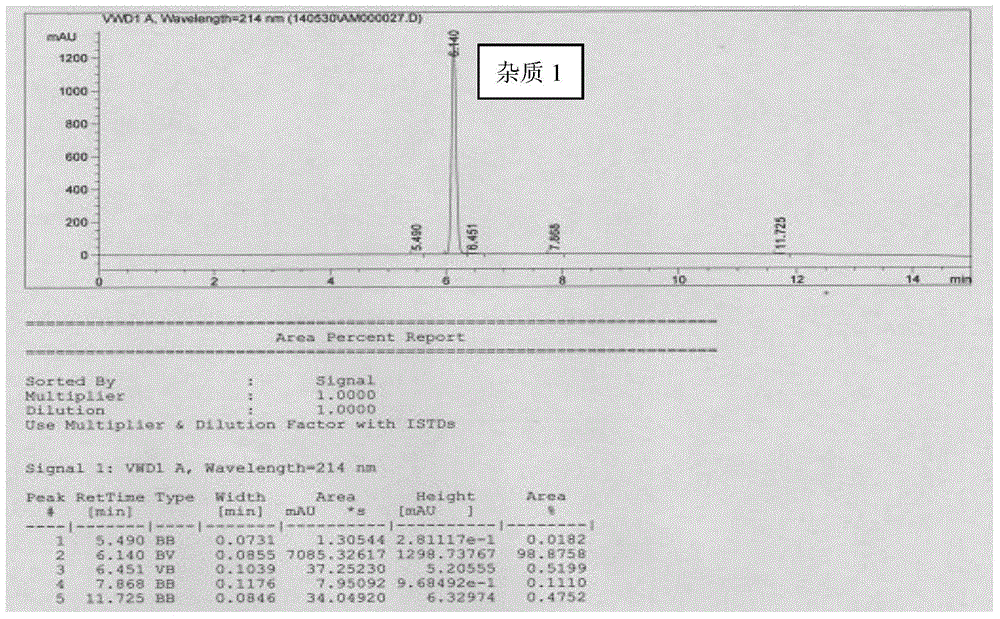
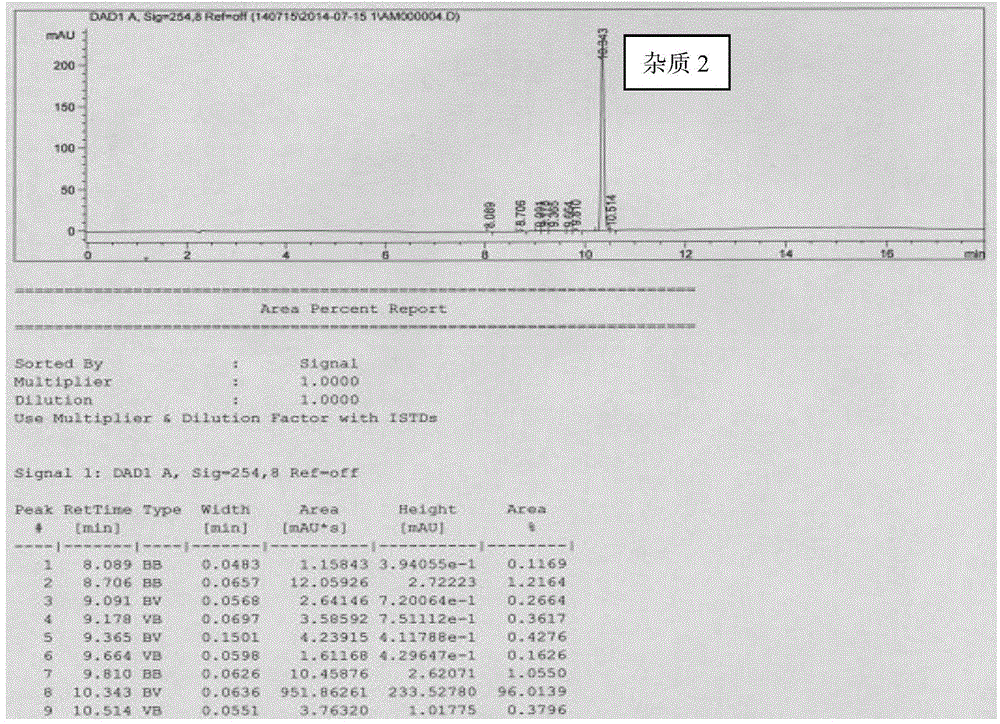
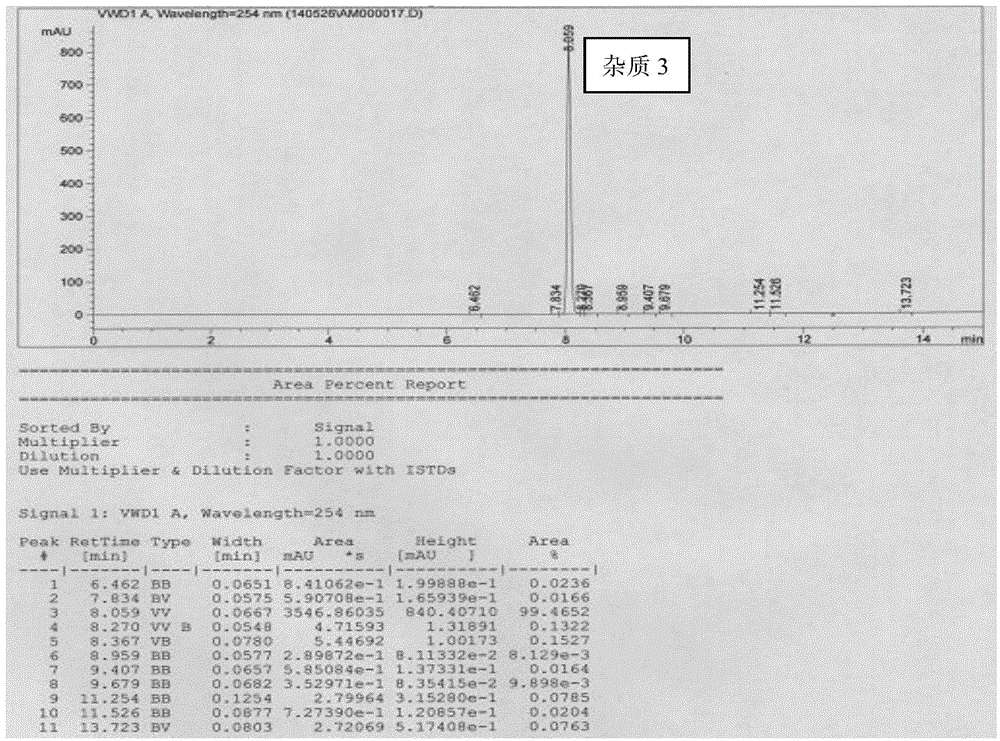
ActiveCN104892489ASimple and fast operationMild reaction conditionsOrganic chemistryHydrogenSodium borohydride
The invention provides a preparation method of three donepezil hydrochloride impurities, which comprises the following steps: 1) impurity 1: by using 5,6-dimethoxy-2-(4-piperidylmethylene)-1-indeneone (SM) as an initial raw material, generating 4-(5,6-dimethoxy-1-indene-2-methylene)piperidine (I) under the action of sodium borohydride, carrying out Pd / C reduction to generate 4-(2,3-dihydro-5,6-dimethoxy-1-indene-2-methylene)piperidine (II), and finally, carrying out formylation to generate 4-(2,3-dihydro-5,6-dimethoxy-1-indene-2-methylene)piperidine-1-formaldehyde (1); 2) impurity 2: by using the 4-(5,6-dimethoxy-1-indene-2-methylene)piperidine (I) as the raw material, carrying out formylation to generate 4-(5,6-dimethoxy-1-indene-2-methylene)piperidine-1-formaldehyde (2); and 3) impurity 3: by using the 5,6-dimethoxy-2-(4-piperidylmethylene)-1-indeneone (SM) as the initial raw material, carrying out formylation to generate 4-(2,3-dihydro-5,6-dimethoxy-1-indeneone-2-methylene)piperidine-1-formaldehyde (3). The preparation of the donepezil hydrochloride impurities has important quality monitoring meanings for industrial production of the donepezil hydrochloride active pharmaceutical ingredient.
Owner:JIANGSU HAICI BIOLOGICAL PHARMA CO LTD OF YANGTZE RIVER PHARMA GRP
Catalyst for hydrogenation reaction of donepezil hydrochloride key intermediate and application thereof
ActiveCN101693195ALow costHigh activityOrganic chemistryMetal/metal-oxides/metal-hydroxide catalystsHydrogenation reactionActive component
The invention discloses a catalyst for hydrogenation reaction of a donepezil hydrochloride key intermediate and application thereof. The key intermediate is 2-(1-benzyl-4-piperidine methylene)-5,6-dimethoxy-1-indenone. The catalyst takes Al2O3 as a carrier and comprises composite active components selected from one or several simple substances or the simple substances and oxides of Pd, Cu, Co, Ni, Zn, Fe and Cr, wherein the mass content of each active component is 1-15 percent of the total mass of the catalyst and the total content of the active components is 5-30 percent of the total mass of the catalyst. The catalyst is prepared by utilizing a conventional catalyst preparation method and has the characteristics of low cost, stable activity and cycle use. The hydrogenation reaction by applying the catalyst has the characteristics of easy control of operation conditions, good catalyst performance and high product yield.
Owner:CANGZHOU SENARY CHEM SCI TEC
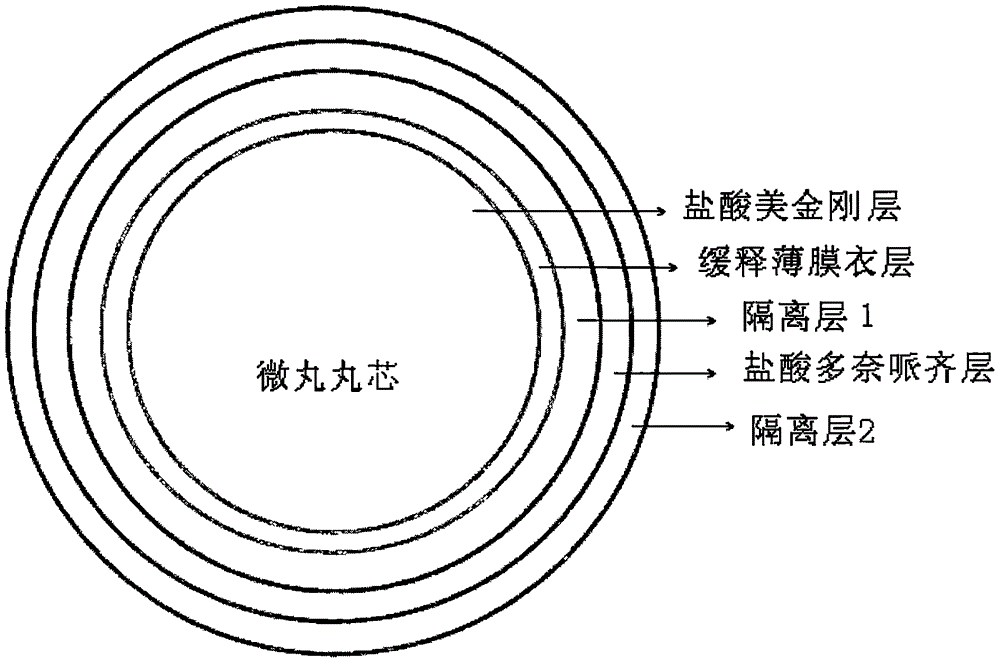
Memantine hydrochloride and donepezil hydrochloride composite preparation
InactiveCN105816441AQuick releaseAvoid uneven fillingNervous disorderAmine active ingredientsMaillard reactionMemantine Hydrochloride
The present invention adopts memantine hydrochloride and donepezil hydrochloride as the active ingredients of the medicine, and co-wraps the memantine hydrochloride and donepezil hydrochloride on the pellets, and controls the slow release of memantine hydrochloride and the rapid release of donepezil hydrochloride through multi-layer coating, It is finally filled in capsules, which also avoids the problem of uneven filling that may exist in capsules mixed with pellets and granules, and is easier to realize industrial production. There is no lactose and other excipients that can undergo Maillard reactions with memantine hydrochloride, ensuring formulation stability.
Owner:BEIJING KANG LISHENG PHARMA TECH DEV
Chinese-Western compound preparation for treating senile dementia and preparation method thereof
InactiveCN108904739AImprove circulationImprove insomnia and forgetfulnessNervous disorderCapsule deliveryAmnesiaSide effect
The invention discloses a Chinese-Western compound preparation for treating senile dementia and a preparation method thereof. The Chinese-Western compound preparation is formed by compounding traditional Chinese medicine components and Western medicine components. The traditional Chinese medicine components are prepared from the following raw materials in parts by weight: 10-20 parts of rhizoma gastrodiae, 10-15 parts of radix salviae miltiorrhizae, 10-15 parts of folium ginkgo, 5-10 parts of cortex albiziae, 5-10 parts of fructus alpiniae oxyphyllae, 2-5 parts of dark plum, and 5-10 parts oflumbricus; the western medicines are composed of the following components: 1-1.5 parts of piracetam, 1-1.5 parts of donepezil hydrochloride and 0.5-1 part of vitamin B1. The method of combining Chinese and Western medicines can effectively improve the circulation functions of brain nerves, brain blood vessels and brain blood circulation, improves and treats insomnia, amnesia, hypomnesia and cognitive decline caused by senile dementia, has the advantages of short course of treatment, quick effect and low side effects, and has broad medical application prospects.
Owner:SHAANXI UNIV OF CHINESE MEDICINE
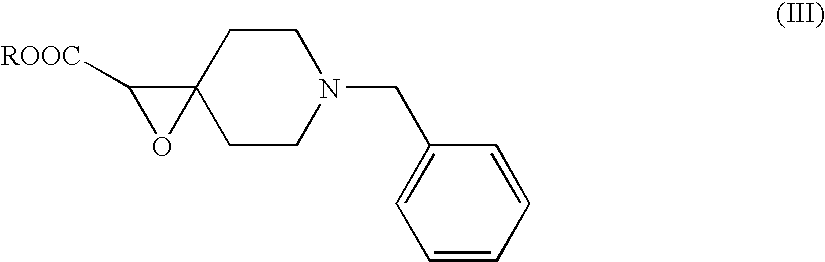
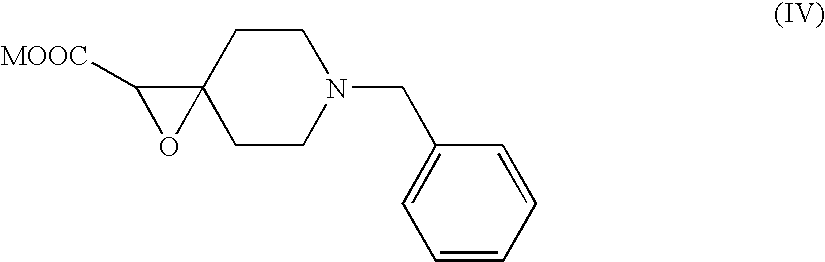
Process And Intermediate For Preparation Of Donepezil
The invention relates to new compounds of formula (III):wherein R is a C1-C4 linear or branched alkyl group.The invention also relates to new compounds of formula (IV)wherein M is a metal.The invention also relates to methods of making compounds of formulas (III) and (IV) and to methods of making donepezil and pharmaceutically acceptable salts thereof, such as donepezil hydrochloride, using the compounds.
Owner:CIPLA LTD
Inhalation-type pharmaceutical composition for the treatment of alzheimer's disease and preparation method thereof
ActiveUS20150272867A1Promote absorptionGood curative effectBiocidePowder deliveryDiseaseMemantine Hydrochloride
The present invention provides an inhalation-type pharmaceutical composition for Alzheimer's disease and preparation method thereof, comprising a first gas and an atomized medicine. The first gas comprises hydrogen. The gas volume concentration of hydrogen in the inhalation-type pharmaceutical composition is between 2 to 96%. The atomized medicine is selected from a group comprising rivastigmine hydrogen tartrate, donepezil hydrochloride, galantamine hydrobromide, memantine hydrochloride, and any combination thereof. The inhalation-type pharmaceutical composition of the present invention can provide the convenience of taking medicine and removing harmful radicals in the body of the patient through the use of hydrogen while also increases the absorption effect of the medicine for the patient by using an atomized medicine. At the same time, because the use of the small amount of the vaporized pharmaceutical liquid can indirectly reduce the side effects on the user.
Owner:LIN HSIN YUNG
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com




![Process for producing a polymorphic form of (1-Benzyl-4-[(5,6-dimethoxy-1-indanone)-2-yl] methyl piperidine hydrochloride (donepezil hydrochloride) Process for producing a polymorphic form of (1-Benzyl-4-[(5,6-dimethoxy-1-indanone)-2-yl] methyl piperidine hydrochloride (donepezil hydrochloride)](https://images-eureka.patsnap.com/patent_img/3c657139-c9a3-4eff-8d3e-ccf08180bfc7/US20050288330A1-20051229-D00001.png)
![Process for producing a polymorphic form of (1-Benzyl-4-[(5,6-dimethoxy-1-indanone)-2-yl] methyl piperidine hydrochloride (donepezil hydrochloride) Process for producing a polymorphic form of (1-Benzyl-4-[(5,6-dimethoxy-1-indanone)-2-yl] methyl piperidine hydrochloride (donepezil hydrochloride)](https://images-eureka.patsnap.com/patent_img/3c657139-c9a3-4eff-8d3e-ccf08180bfc7/US20050288330A1-20051229-D00002.png)
![Process for producing a polymorphic form of (1-Benzyl-4-[(5,6-dimethoxy-1-indanone)-2-yl] methyl piperidine hydrochloride (donepezil hydrochloride) Process for producing a polymorphic form of (1-Benzyl-4-[(5,6-dimethoxy-1-indanone)-2-yl] methyl piperidine hydrochloride (donepezil hydrochloride)](https://images-eureka.patsnap.com/patent_img/3c657139-c9a3-4eff-8d3e-ccf08180bfc7/US20050288330A1-20051229-C00001.png)