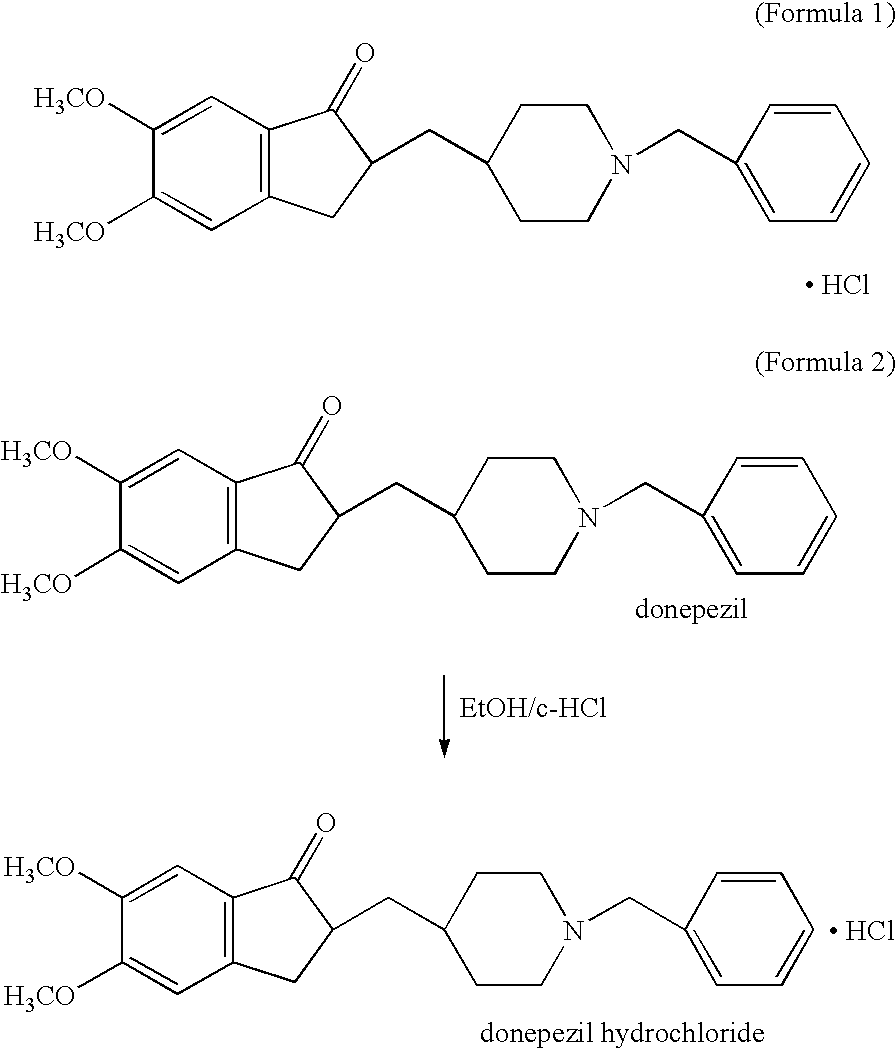
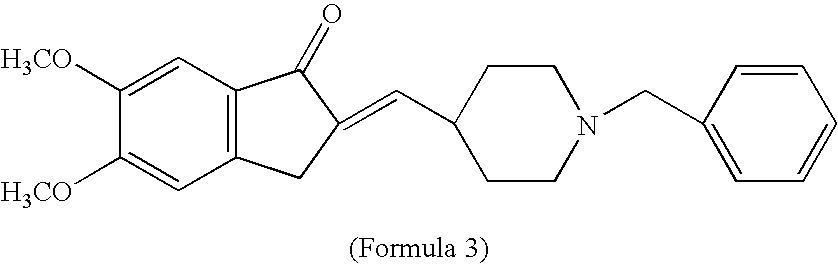
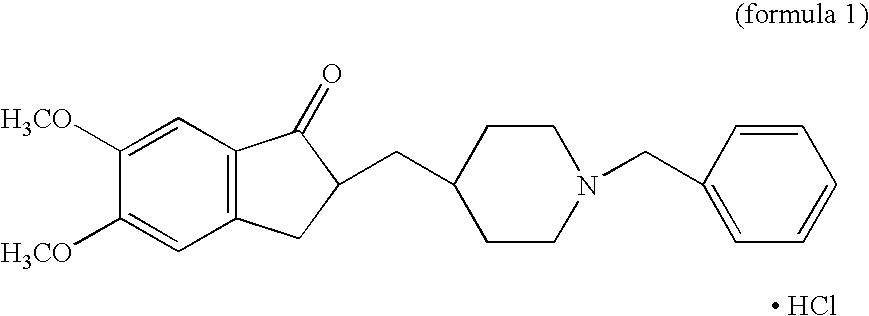
Process for producing multiform crystal of donepezil hydrochloride
a technology of donepezil hydrochloride and multi-form crystals, which is applied in the direction of biocide, cardiovascular disorder, drug compositions, etc., can solve the problems of high volatility of organic solvents, inflammability, and safety to the environment and health, and achieve the same methods and problems, and achieve difficult to secure chemical stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0050] 50 g of donepezil was dissolved by stirring in 315 g of ethanol under heating, followed by adding 15.1 g of concentrated hydrochloric acid thereto at the internal temperature of 30.degree. C. Then, it was cooled and continued stirring while keeping the internal temperature at 30.degree. C. Crystals started precipitating about 30 minutes after the concentrated hydrochloric acid was poured into the mixture. After the precipitation started, the mixture was stirred at the internal temperature of 15.degree. C. for 18 hours and then cooled to the internal temperature of 9.degree. C. The crystals were collected by filtration and dried about 4 hours after the cooling was started, to give 52.32 g of polymorphic crystal (III) of donepezil hydrochloride (yield: 95.5%).
example 2
[0051] 50 g of donepezil was dissolved by stirring in 240 g of ethanol under heating, followed by adding 15.1 g of concentrated hydrochloric acid thereto at the internal temperature of 30.degree. C. Then, it was cooled and continued stirring while keeping the internal temperature at about 25.degree. C. Crystals started precipitating about 1 hour after the concentrated hydrochloric acid was poured into the mixture. After the precipitation started, the mixture was stirred at the internal temperature of 25.degree. C. for 17 hours and then cooled to the internal temperature of 9.degree. C. The crystals were collected by filtration and dried about 3 hours after the cooling was started, to give 52.25 g of polymorphic crystal (III) of donepezil hydrochloride (yield: 95.3%).
example 3
[0052] 50 g of donepezil was dissolved by stirring in 320 g of ethanol under heating, followed by adding 15.1 g of concentrated hydrochloric acid thereto at the internal temperature of 30.degree. C. After concentrated hydrochloric acid was poured, 100 mg of seed crystals were added. The mixture was cooled and continued stirring while keeping the internal temperature at about 20.degree. C. Crystals started precipitating immediately after the seed crystals were added. After the precipitation started, the mixture was stirred at the internal temperature of 20.degree. C. for 18 hours and then cooled to the internal temperature of 9.degree. C. The crystals were collected by filtration and dried about 3 hours after the cooling was started, to give 52.90 g of polymorphic crystal (III) of donepezil hydrochloride (yield: 96.5%).
PUM
| Property | Measurement | Unit |
|---|---|---|
| Length | aaaaa | aaaaa |
| Length | aaaaa | aaaaa |
| Length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com