Industrial production process for tenofovir disoproxil fumarate
A technology of tenofovir disoproxil and production process, applied in the field of industrialized production technology of tenofovir disoproxil, can solve the problems of high cost and price of the final product, and achieve the effects of simple operation of the production process and improved safety.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
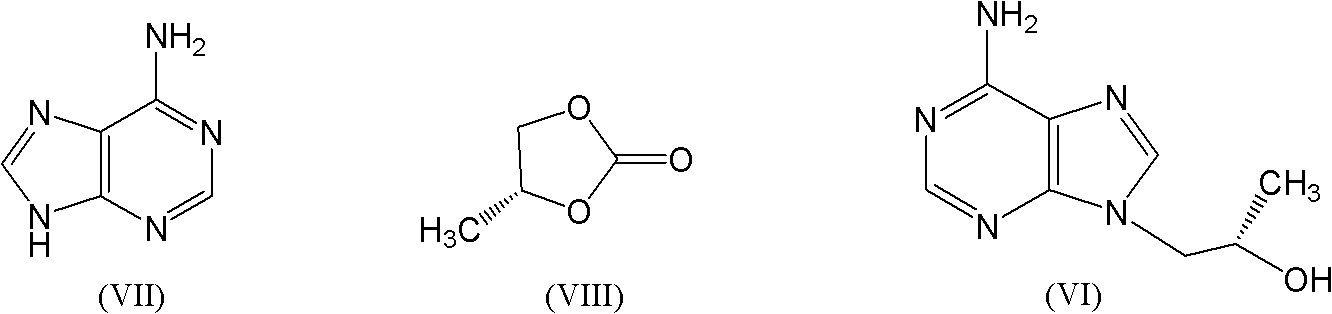
[0067] Add adenine 100g (0.74mol) in a 2L three-necked flask, N 2 Protect, then add 500ml of DMF, start stirring, add, add R-propylene carbonate 95g (0.92mol), add NaOH 3g (0.075mol), stir at room temperature for 10 minutes, after the system is uniform, start to heat up to 100-130°C, Insulation reaction, sampling test after 12 hours, (the reaction is clear and transparent); when the content of adenine in the reaction system is below 1%, the reaction can be stopped; slowly lowered to below 90°C, start to add 600ml of toluene, a large amount of white solids are precipitated After cooling down to room temperature, continue to drop to 0-5°C, keep stirring for 2 hours; filter with suction, soak and wash the filter cake with 200ml ice toluene to obtain about 180g of wet filter cake, about 130-140g of dry product, HPLC purity 85%- 90%, melting point 188-192°C;
[0068] Refining: add 130g of the product obtained in the previous step to 600ml of ethanol and 100ml of toluene, heat up t...
Embodiment 2
[0070] Add adenine 100g (0.74mol) in a 2L three-necked flask, N 2 Protect, then add 700ml of DMSO, start stirring, add, add R-propylene carbonate 95g (0.92mol), add KOH 4g (0.071mol), stir at room temperature for 10 minutes, after the system is uniform, start to heat up to 110-125°C, Insulation reaction, sampling test after 8 hours, the reaction is basically clear or with a little floc; when the content of adenine in the reaction system is below 1%, the reaction can be stopped; after slowly cooling to room temperature, continue to drop to 0-5°C , keep stirring for 2 hours; filter with suction, soak and wash the filter cake with 200ml ice toluene to obtain about 170g of wet filter cake, about 125-130g of dry product, HPLC purity of 82%-88%, melting point of 182-188°C;
[0071] Refining: Add 130g of the product obtained in the previous step to 500ml of methanol and 100ml of toluene, heat up to dissolve, add 5g of activated carbon, reflux for half an hour and remove the activated...
Embodiment 3
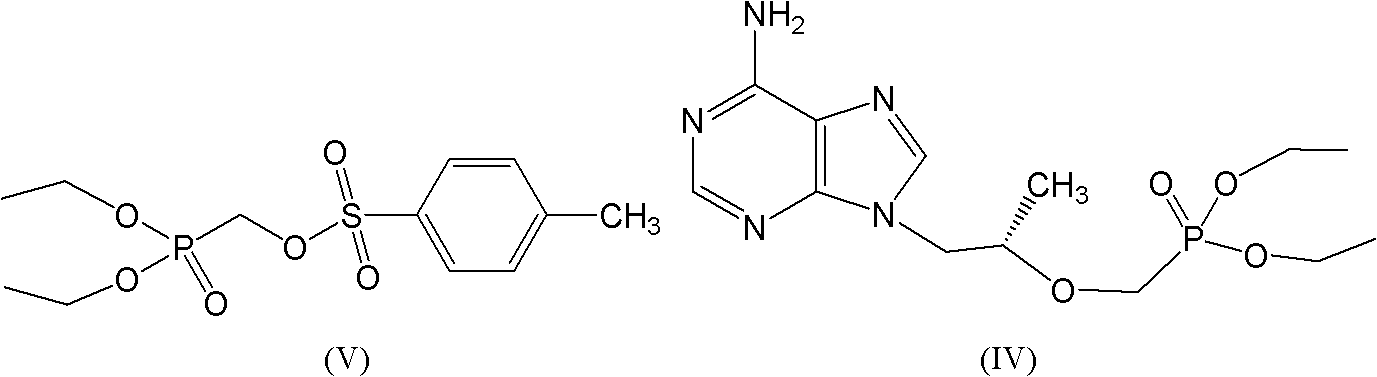
[0073] In a 1L four-necked flask, add R-9-(2-hydroxypropyl) adenine 100g (0.52mol), N 2 For protection, add 200ml of DMF, start stirring; add 90g (0.53mol) of magnesium tert-butoxide, the whole reaction system is grayish white and turbid, (magnesium tert-butoxide decomposes with water, it is necessary to ensure that the whole system is anhydrous and oxygen-free);
[0074] Slowly raise the temperature to 50-70°C, and keep warm at this temperature for 1 hour; then raise the temperature to 60-80°C, and start to add 250g of DESMP (diethyl p-toluenesulfonyloxyphosphonate) (0.77mol) dropwise;
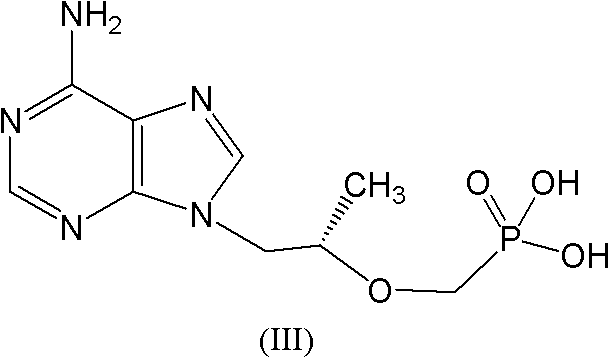
[0075]With the dropwise addition of DESMP, the reaction solution will release heat, control the rate of addition, about 1.5hrs drop, when DESMP drops about 75%, the reaction solution will become clear gelatinous transparent orange yellow; dropwise 2 Check the HPLC after 1 hour, when the raw material in the HPLC is below 2%, the reaction can be stopped; slowly drop to room temperature, add 75g...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com