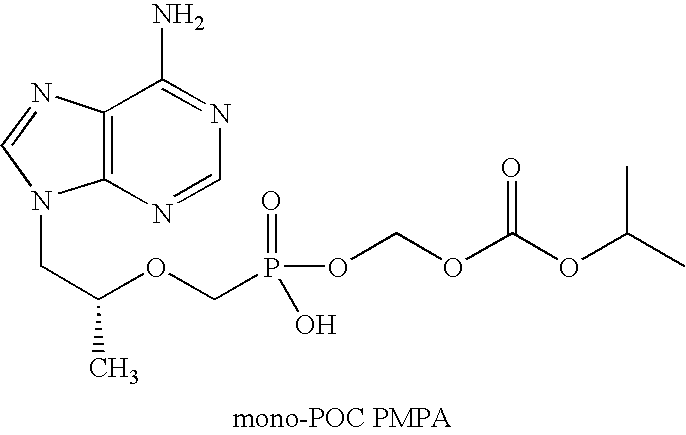
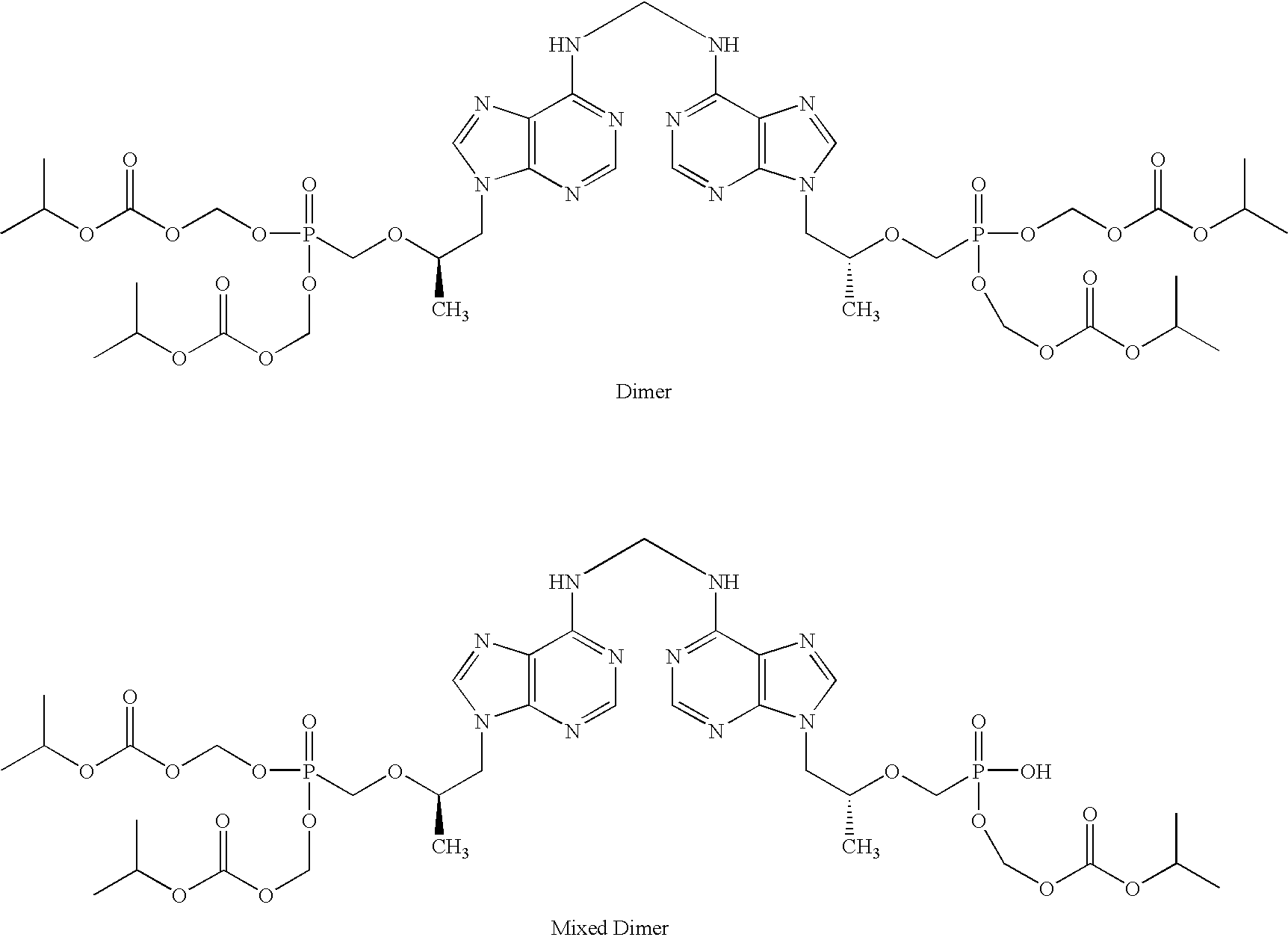
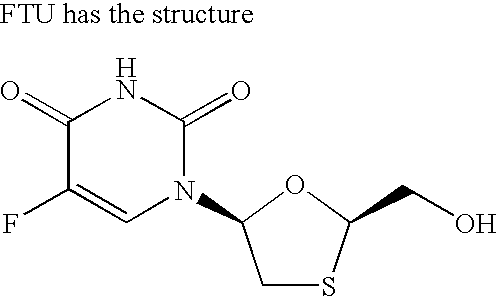
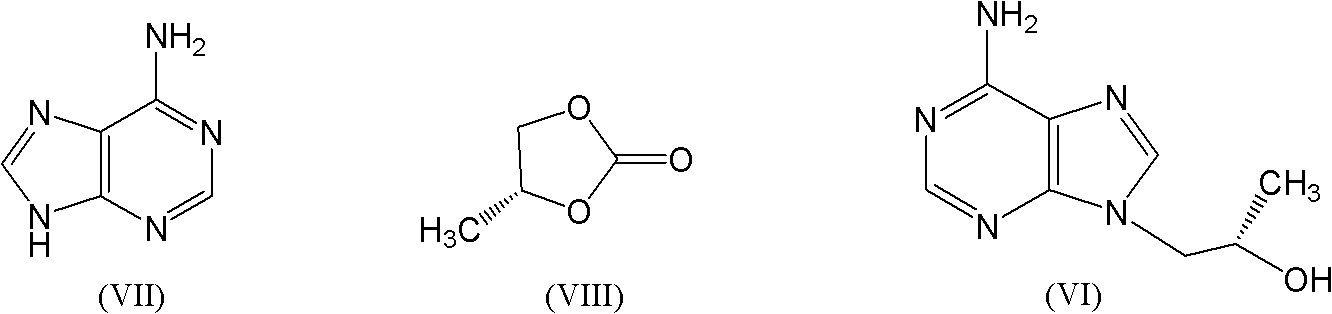
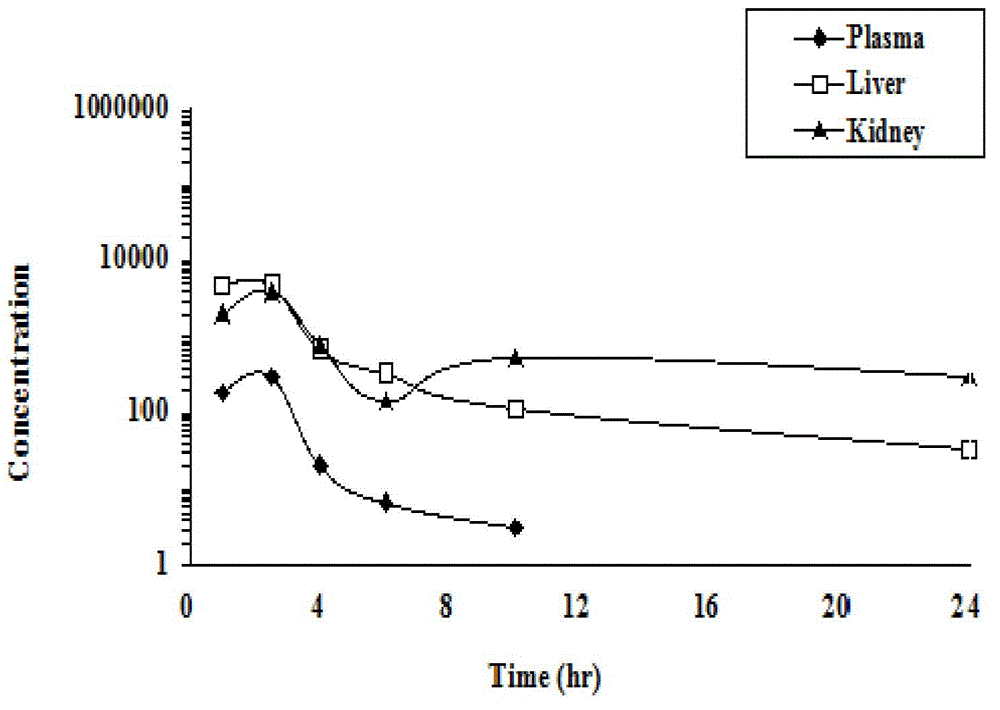
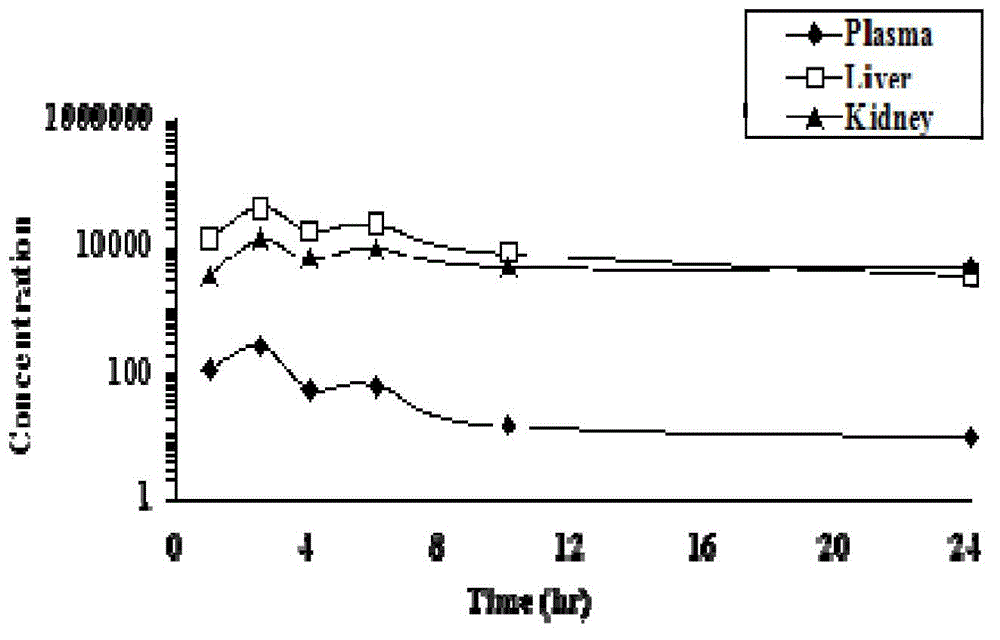
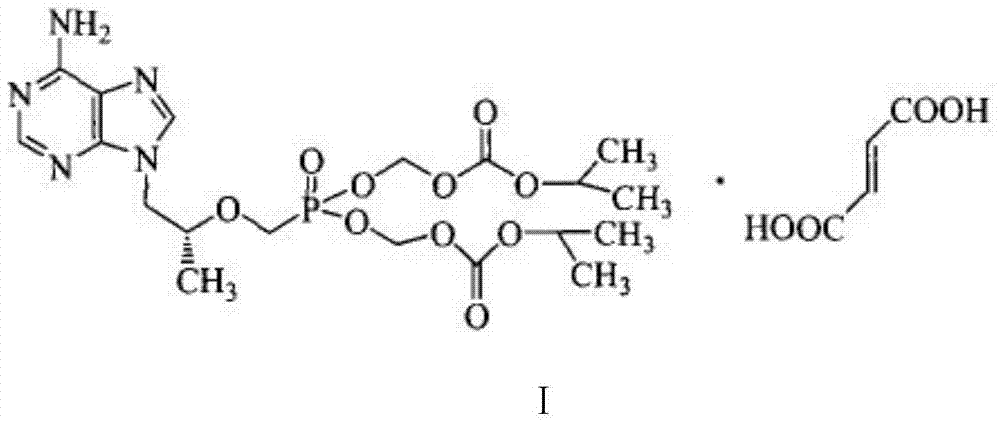
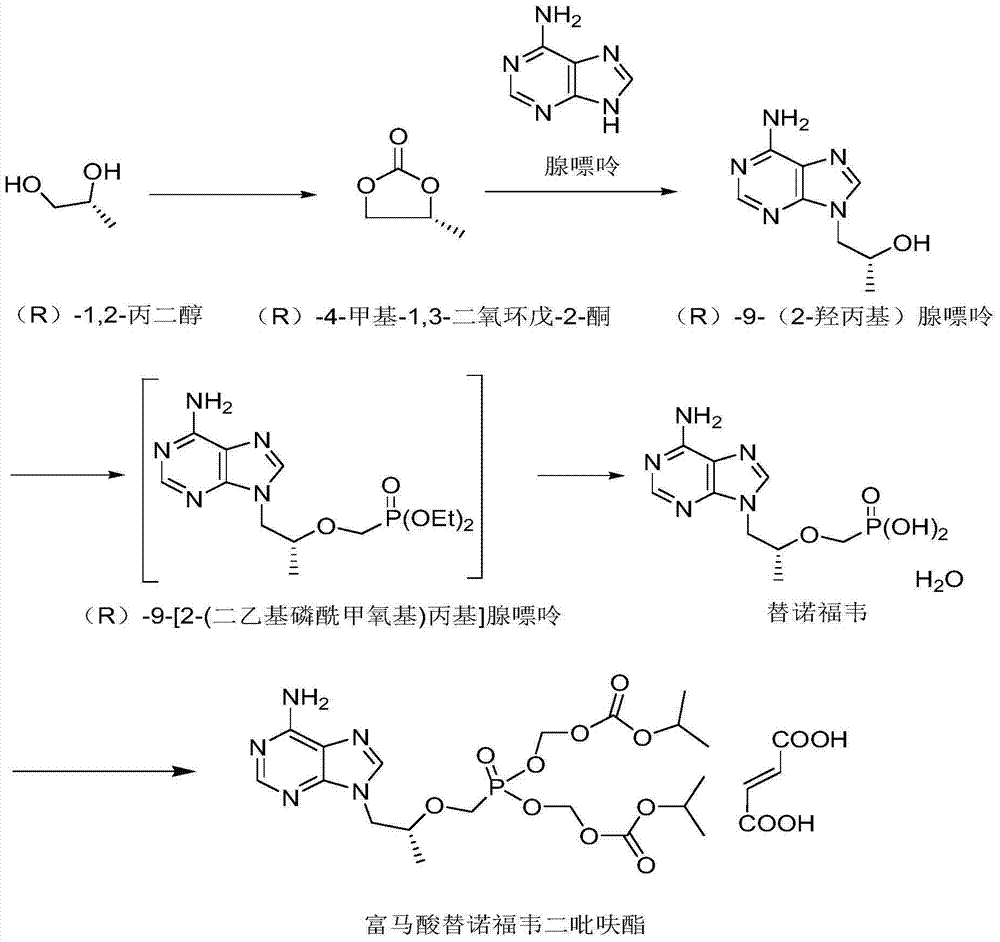
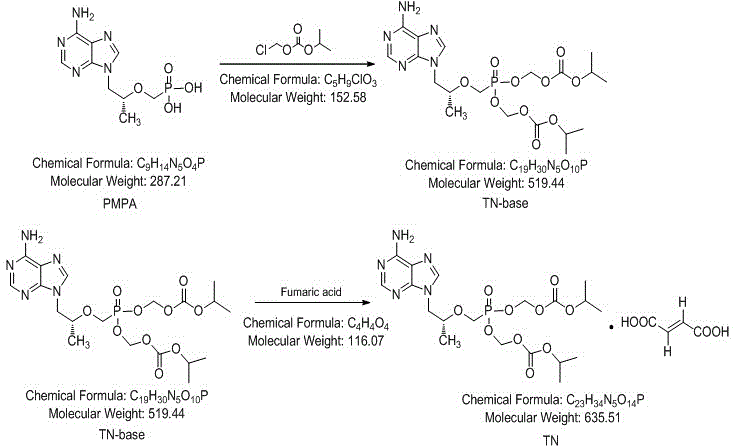
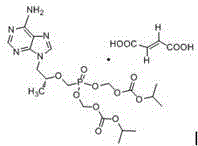
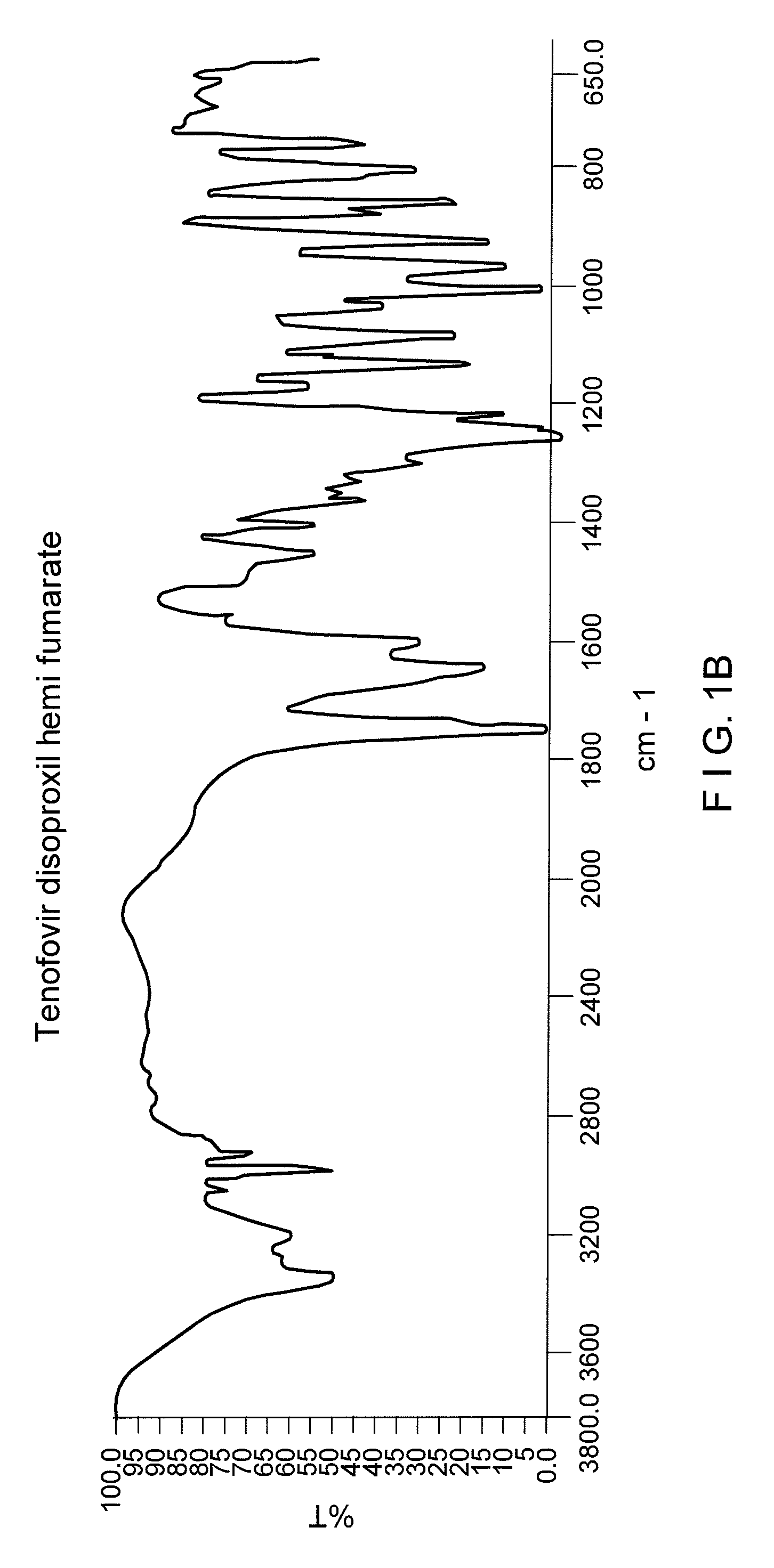
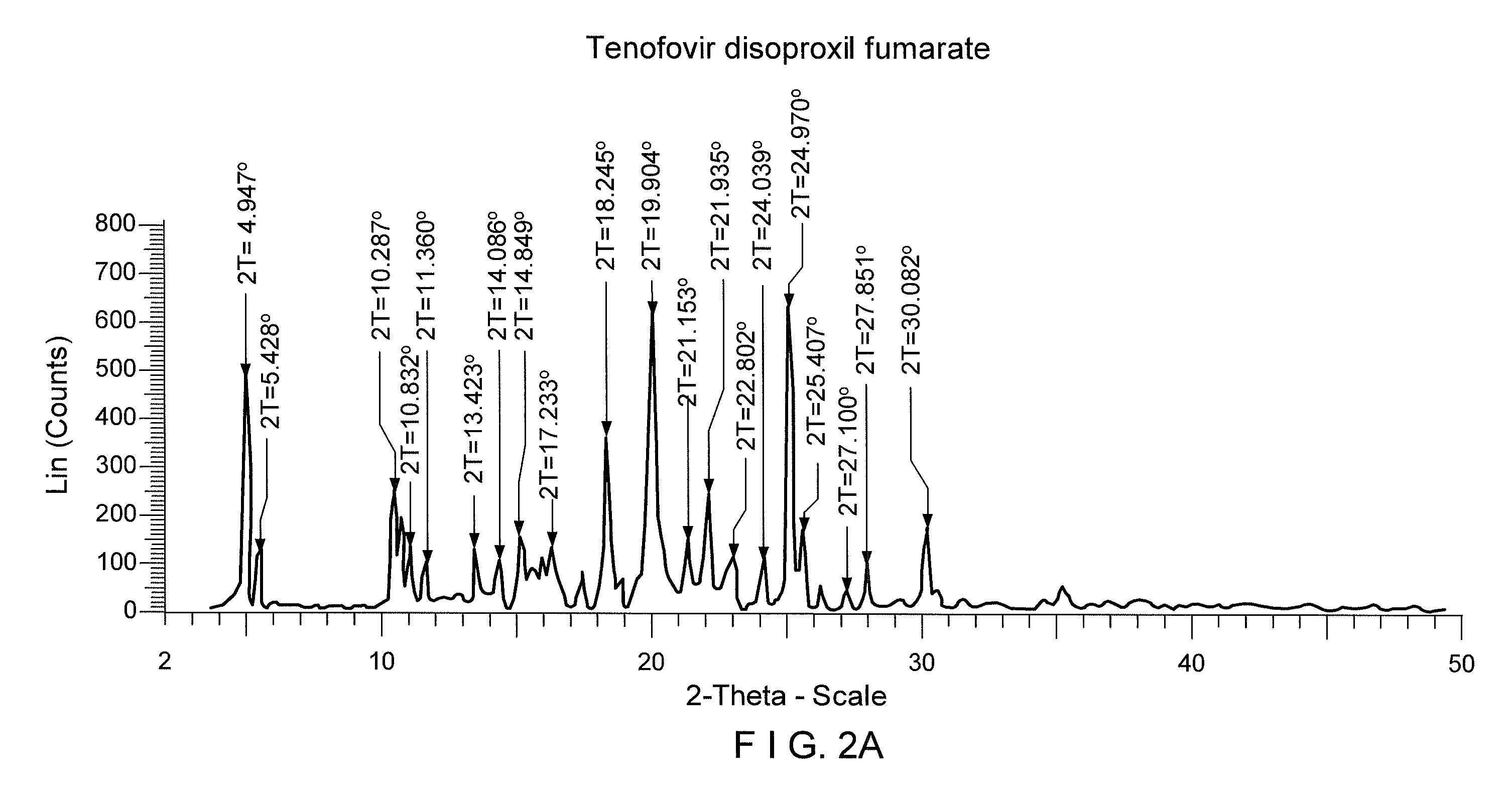
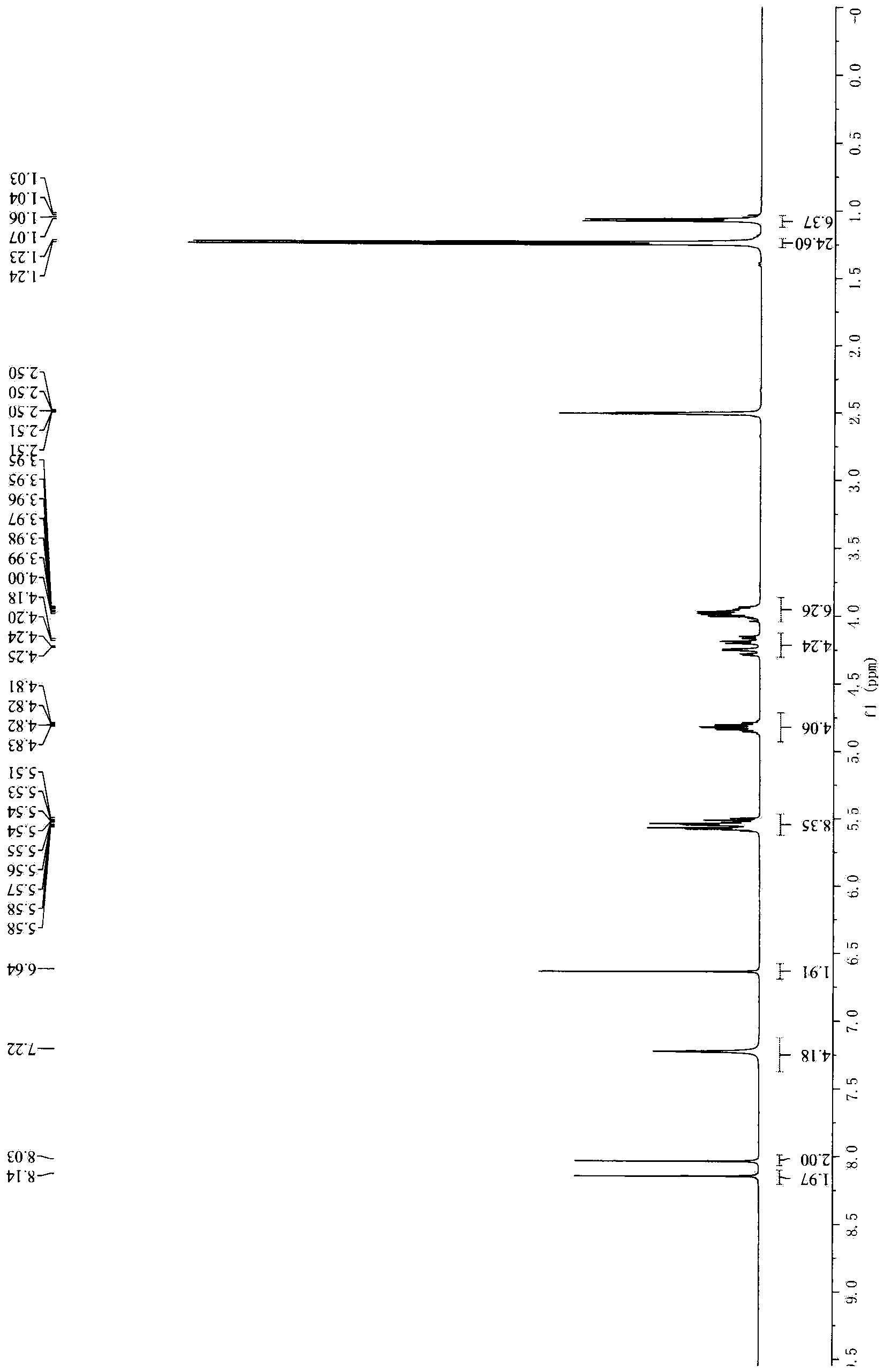
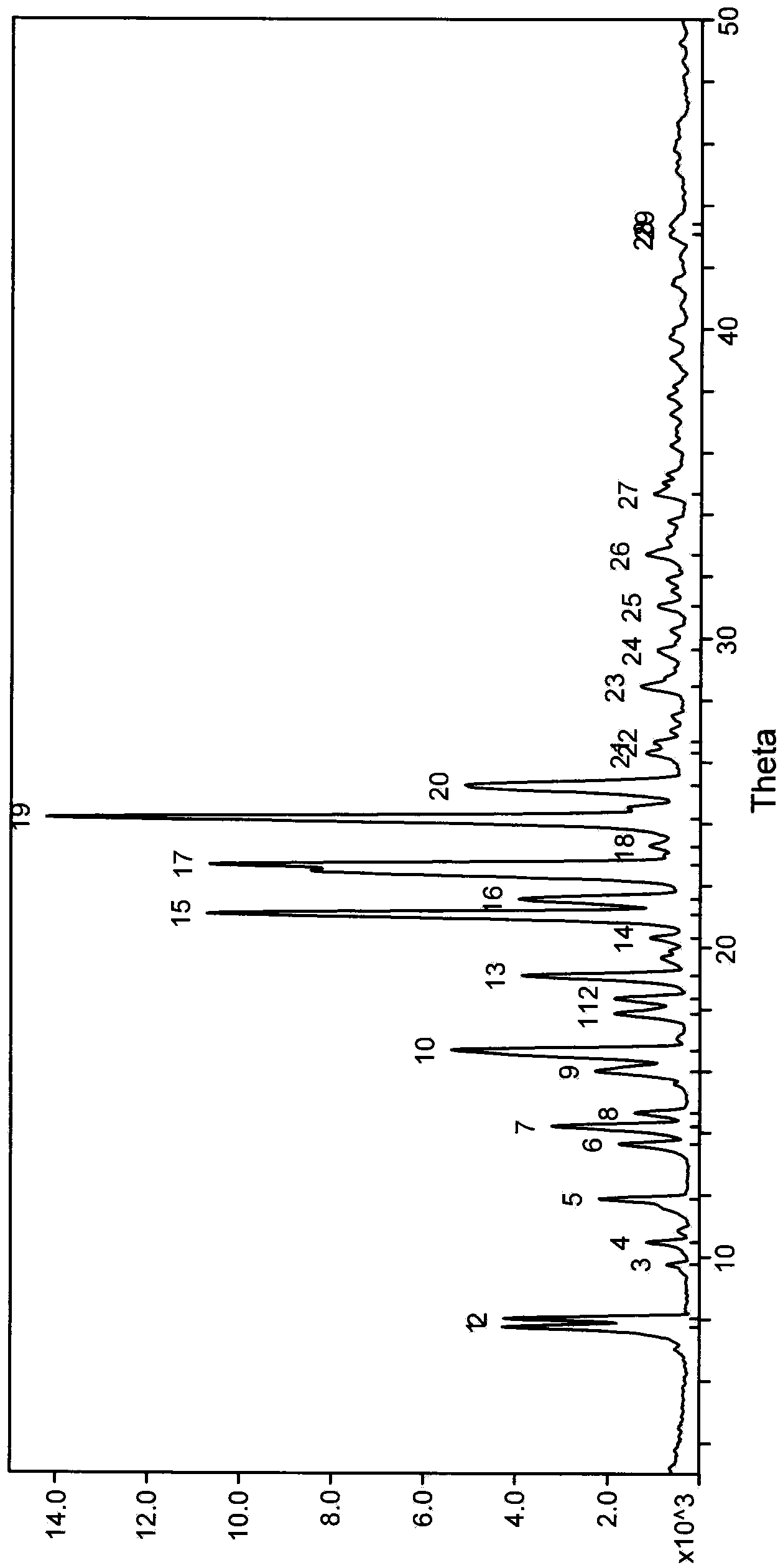
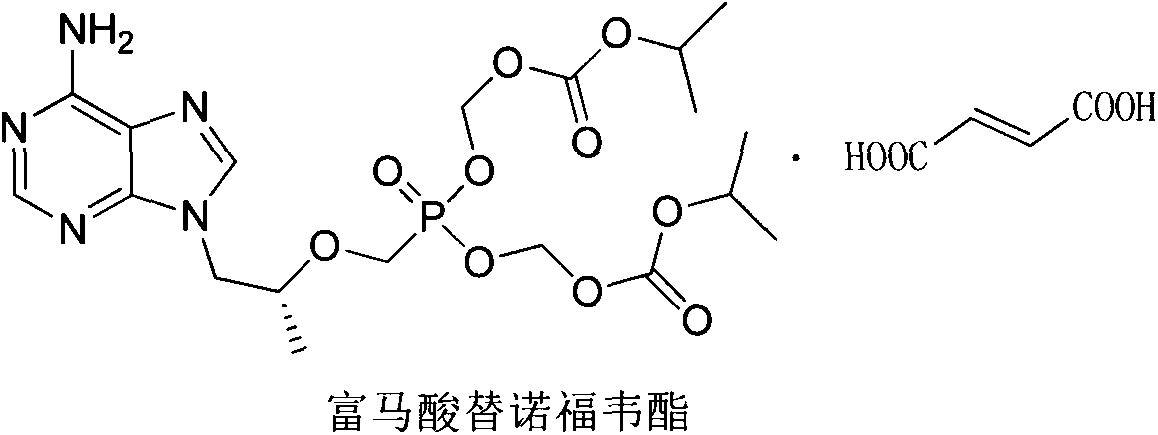
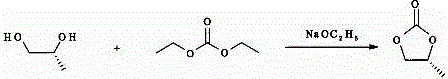
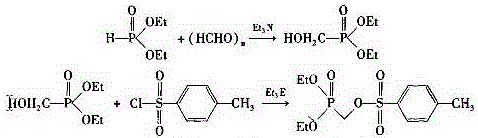
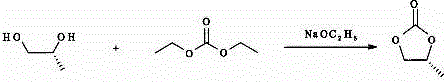
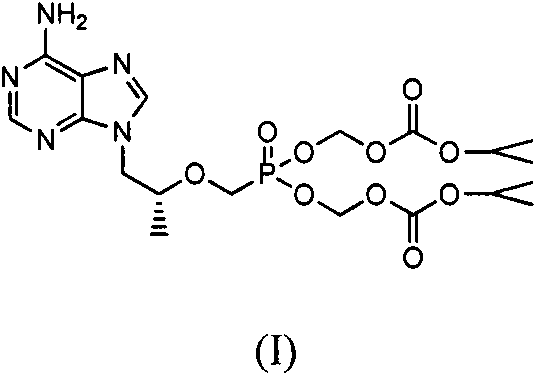
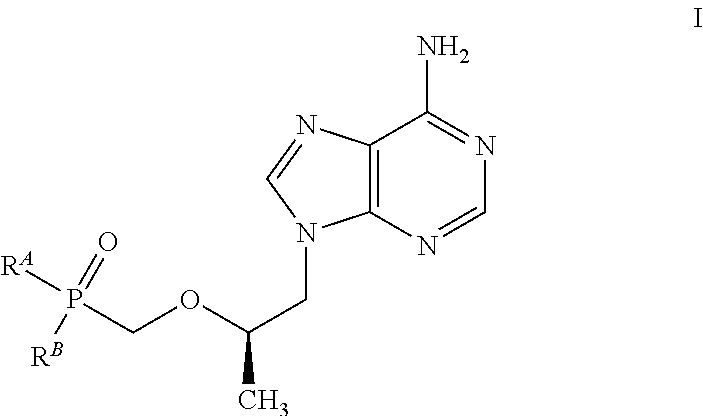
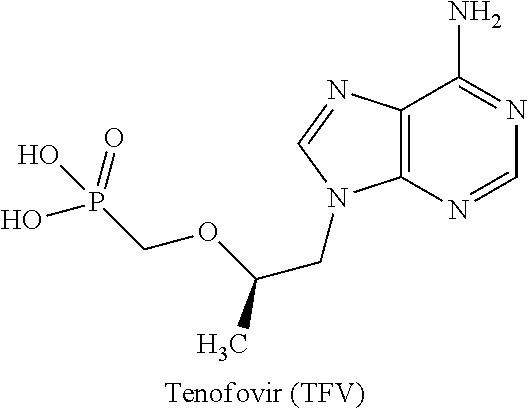
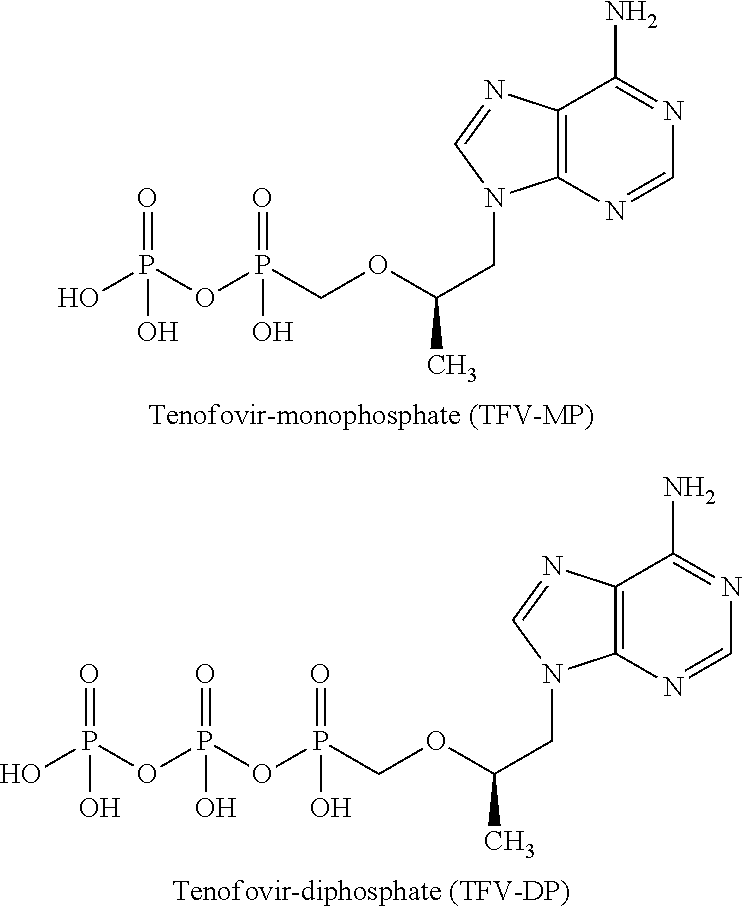
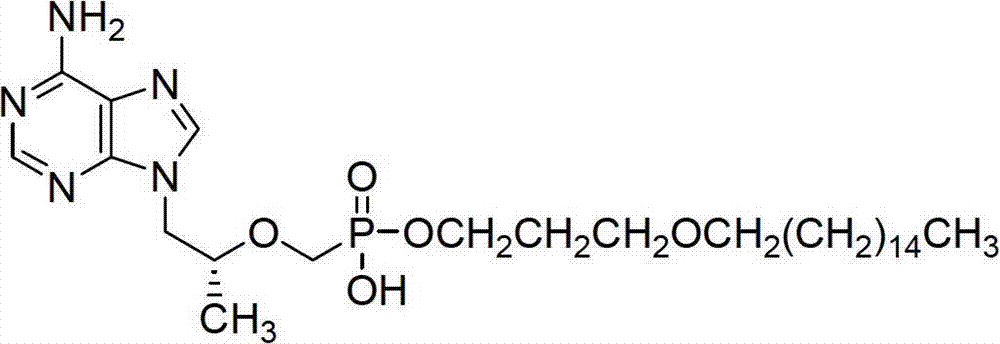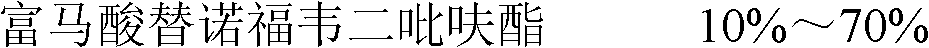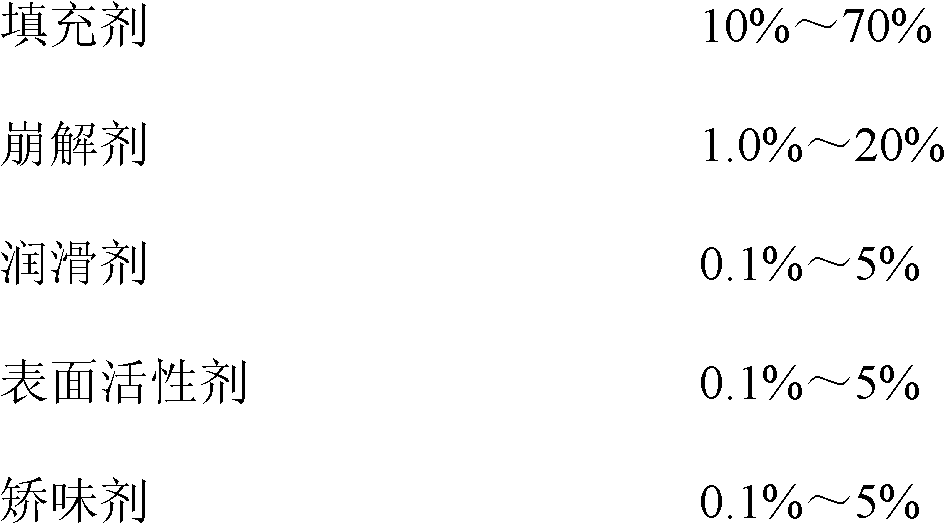Patents
Literature
209 results about "Tenofovir" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
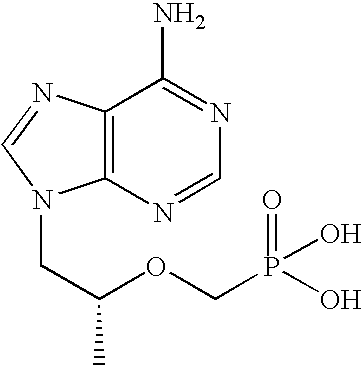
Tenofovir is used with other HIV medications to help control HIV infection.
Method and composition for pharmaceutical product
InactiveUS20070077295A1Improve stabilityFormulation stabilityOrganic active ingredientsBiocideMedicineEmtricitabine
This invention is directed to a composition comprising dry granulated tenofovir DF and emtricitabine, and a method for making same. Dry granulation was unexpectedly found to be important in preparing a tenofovir DF containing composition suitable for inclusion in a combination dosage form containing emtricitabine, efavirenz and tenofovir DF.
Owner:GILEAD SCI INC
Industrial production process for tenofovir disoproxil fumarate
InactiveCN101870713AImprove securitySolve the problem of excessive contentGroup 5/15 element organic compoundsAlcoholPropylene carbonate
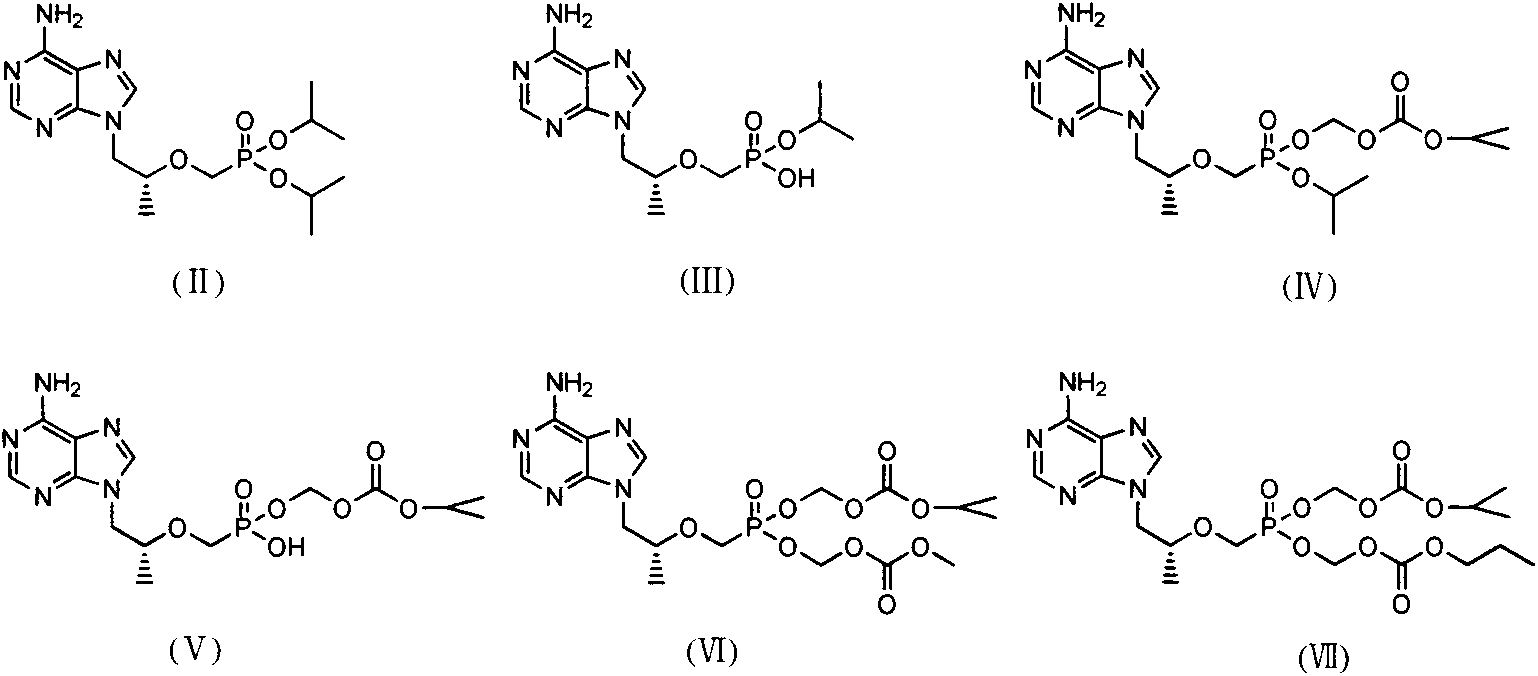
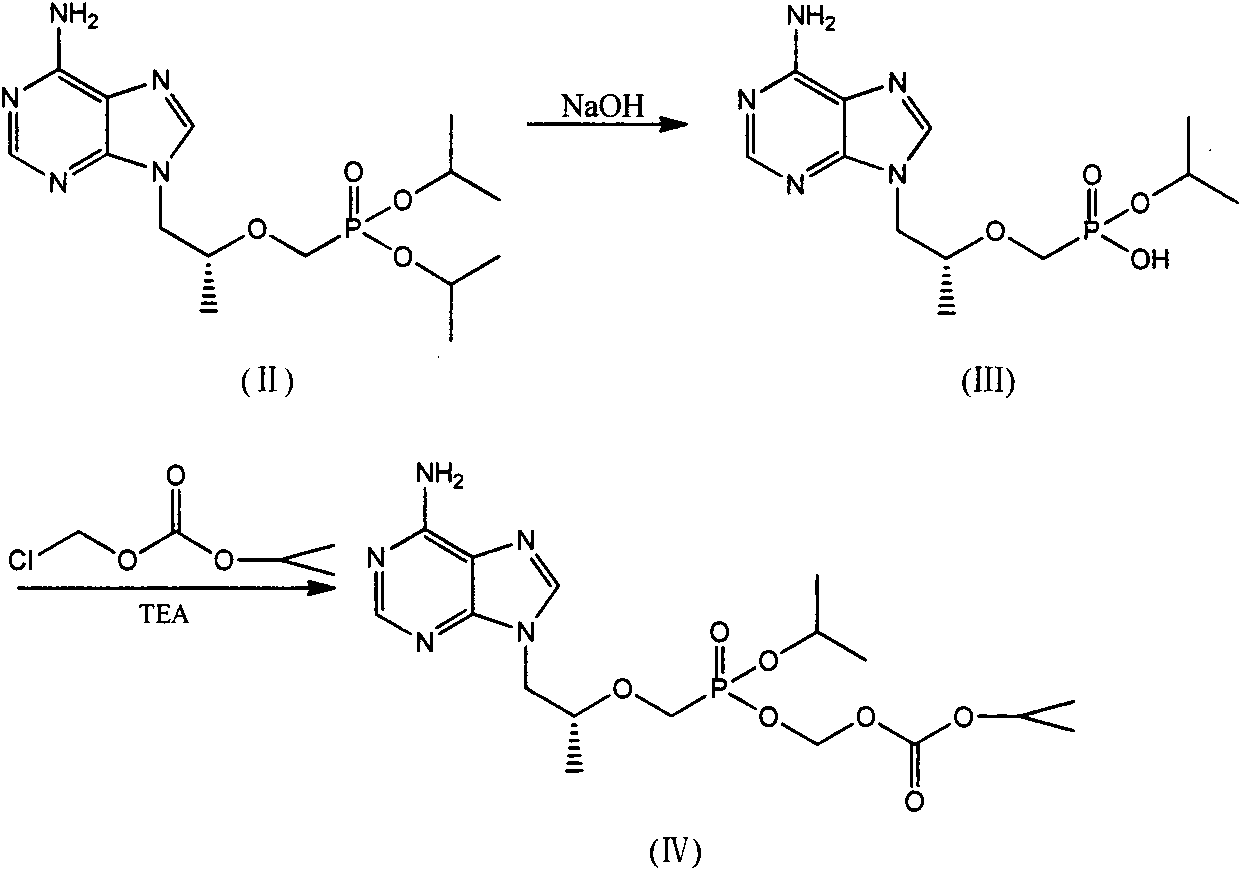
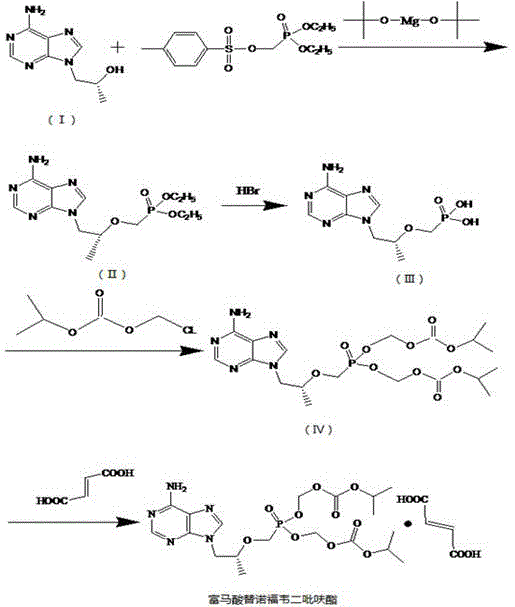
The invention discloses an industrial production process for tenofovir disoproxil fumarate represented by a structural formula (II). The production process comprises the following steps of: (1) preparing R-9-(2-hydroxypropyl)adenine by taking adenine and R-propylene carbonate as initial raw materials; (2) performing a condensation reaction on the obtained R-9-(2-hydroxypropyl)adenine and diethyl(tosyloxy)methylphosphonate under the catalytic action of magnesium alkoxide to prepare R-9[2-(diethyl-phosphonic acid methoxy)propyl]adenine; (3) hydrolyzing R-9[2-(diethyl-phosphonic acid methoxy)propyl]adenine to obtain tenofovir; and (4) performing condensation on the tenofovir and the chloromethyl isopropyl carbonate under the catalytic action of triethylamine to prepare the tenofovir disoproxil fumarate. The process of the invention has the characteristics of low cost, safe process, high product quality and suitability for industrial production.
Owner:杭州和素企业管理有限公司
Preparation method for tenofovir
ActiveCN102060876AReduce energy consumptionImprove securityGroup 5/15 element organic compoundsBenzeneAlcohol
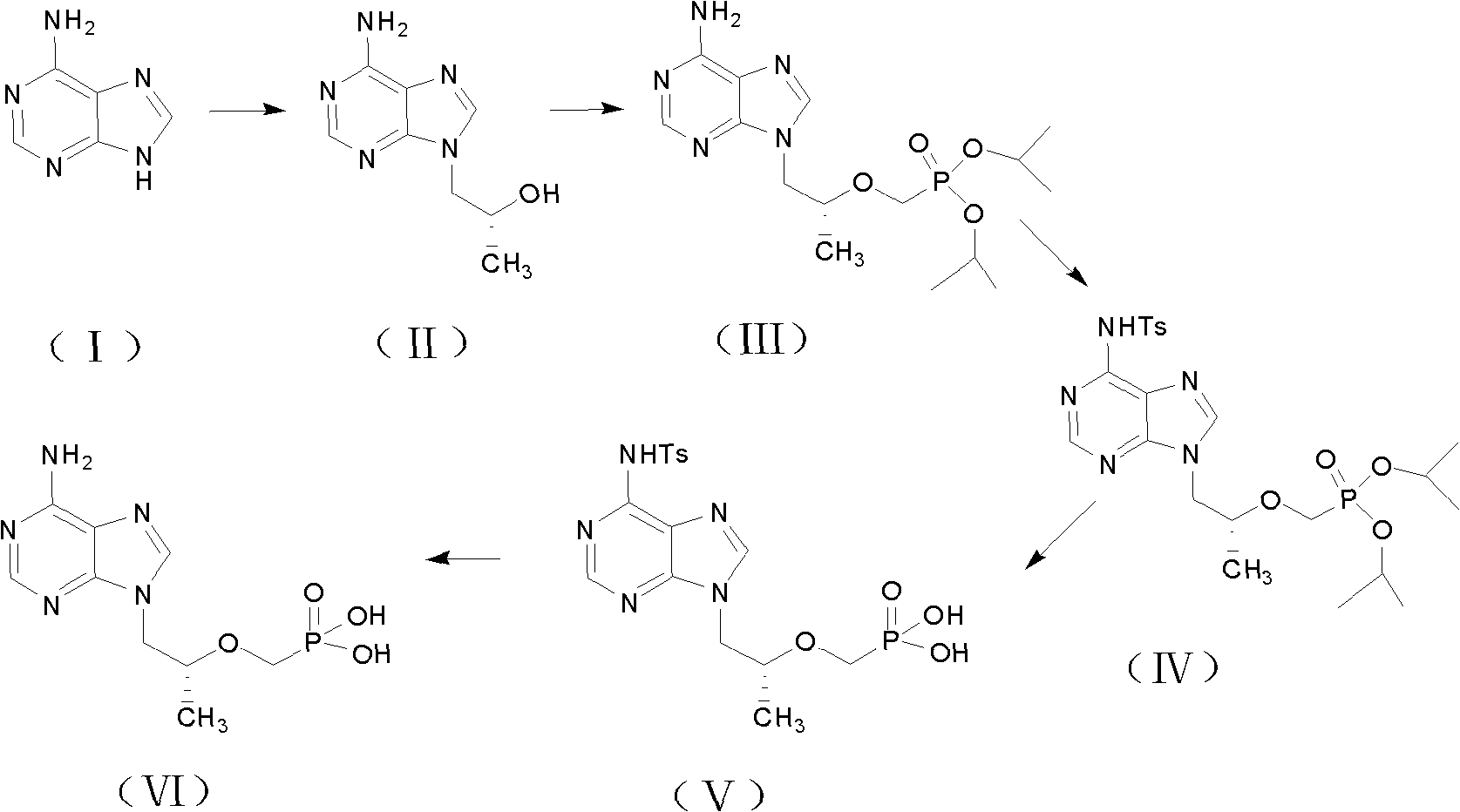
The invention discloses a preparation method for tenofovir disoproxil fumarate, which comprises the following steps of: A, performing condensation reaction adenine and (R)-propylene carbonate which serve as raw materials to generate (R)-9-(2-hydroxypropyl) adenine; B, performing condensation reaction on the (R)-9-(2-hydroxypropyl) adenine and p-methylphenyl mesyloxy diethyl phosphonate under the catalysis of potassium alcoholate to prepare (R)-9-[2-(diethyl phosphonyl methoxy) propyl] adenine; C, reacting the (R)-9-[2-(diethyl phosphonyl methoxy) propyl] adenine obtained by the step B with para-toluenesulfonate acyl chloride to protect an amino group at bit four to prepare (R)-4-(p-toluenesulfonyl)-9-[2-(diethyl phosphonyl methoxy) propyl] adenine; D, hydrolyzing the (R)-4-(p-toluenesulfonyl)-9-[2-(diethyl phosphonyl methoxy) propyl] adenine obtained by the step C under a strong acid condition to obtain (R)-4-(p-toluenesulfonyl)-9-[2-(dihydroxy phosphonyl methoxy) propyl] adenine; and E, reacting the (R)-4-(p-toluenesulfonyl)-9-[2-(dihydroxy phosphonyl methoxy) propyl] adenine obtained by the step D with mercapto-benzene under a weak alkaline condition to remove a para-toluenesulfonate group to obtain the tenofovir. The invention aims to provide the preparation method for the tenofovir, which is low in cost, safe in process and good in product quality, and is suitable for industrialization.
Owner:ZHONGSHAN BAILING BIOTECHNOLOGY CO LTD
Tenofovir diester compounds with activity of inhibiting HIV-1 (human immunodeficiency virus-1) virus replication and preparation method and pharmaceutical use thereof
ActiveCN102786549AHigh activityLow cytotoxicityOrganic active ingredientsGroup 5/15 element organic compoundsTherapy HIVHuman immunodeficiency virus 1
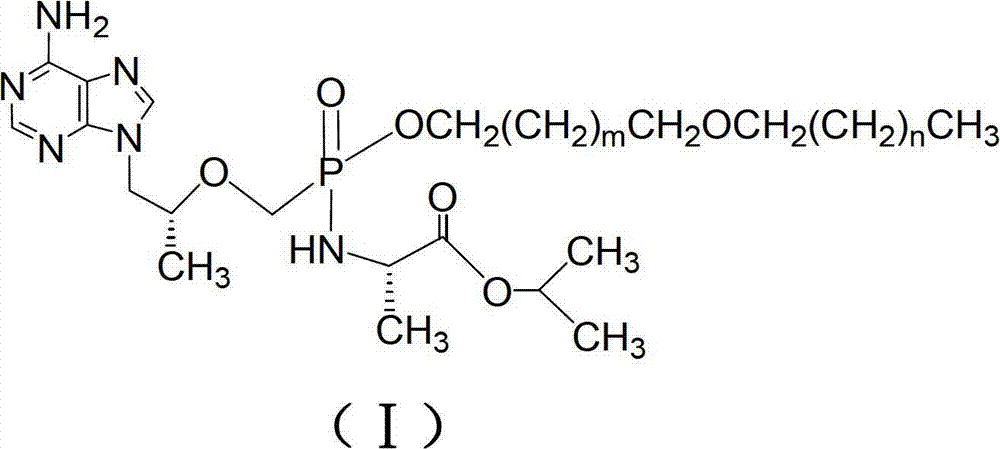
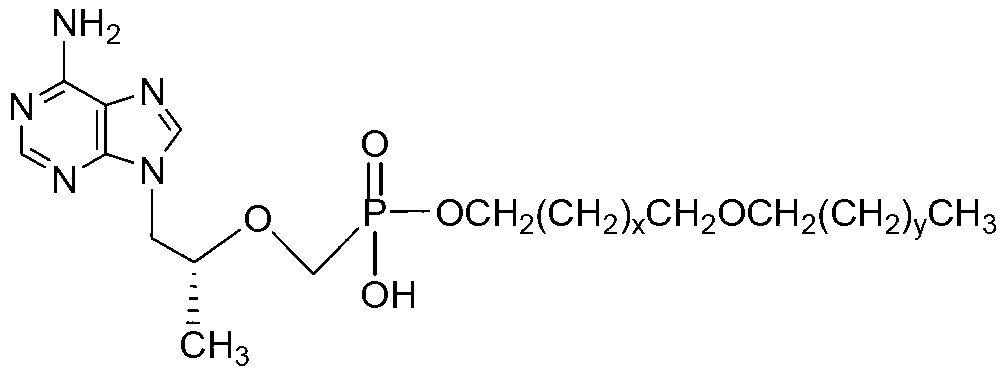
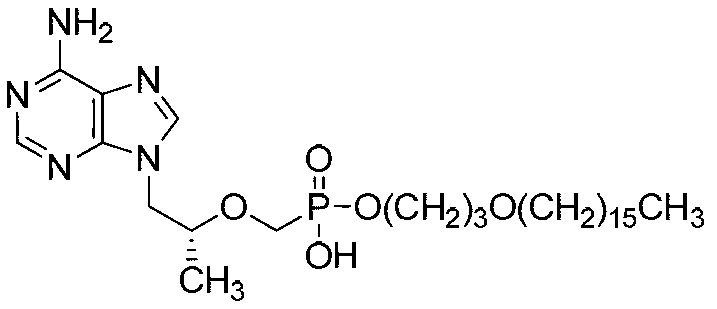
The invention discloses tenofovir diester compounds with activity of inhibiting HIV-1 (human immunodeficiency virus-1) virus replication and a preparation method and pharmaceutical use thereof. The structure of the compounds is shown in Formula (I), wherein m is 0-4, n is 12-16, and the structural formula is fixed when m is 1 and n is 14. The invention also discloses a preparation method of the compounds shown in the structural formula (I) and a pharmaceutical composition with the compounds. Tests show that the compounds have the activity of inhibiting HIV-1 replication and also much higher lipophilicity than the current HIV treatment drug tenofovir fumarate, and can be applied in development of drugs for treatment of HIV infection.
Owner:洛阳聚慧新材料科技有限公司 +2
Process for the preparation of tenofovir
ActiveUS20130165413A1Improve production yieldQuality improvementOrganic active ingredientsBiocideTenofovirChemistry
Owner:LAURUS LABS
Novel crystal of tenofovir prodrug
ActiveCN103333209AGood natureImprove adaptabilityOrganic active ingredientsGroup 5/15 element organic compoundsProdrugCrystal
Owner:XIAN XINTONG PHARM RES CO LTD
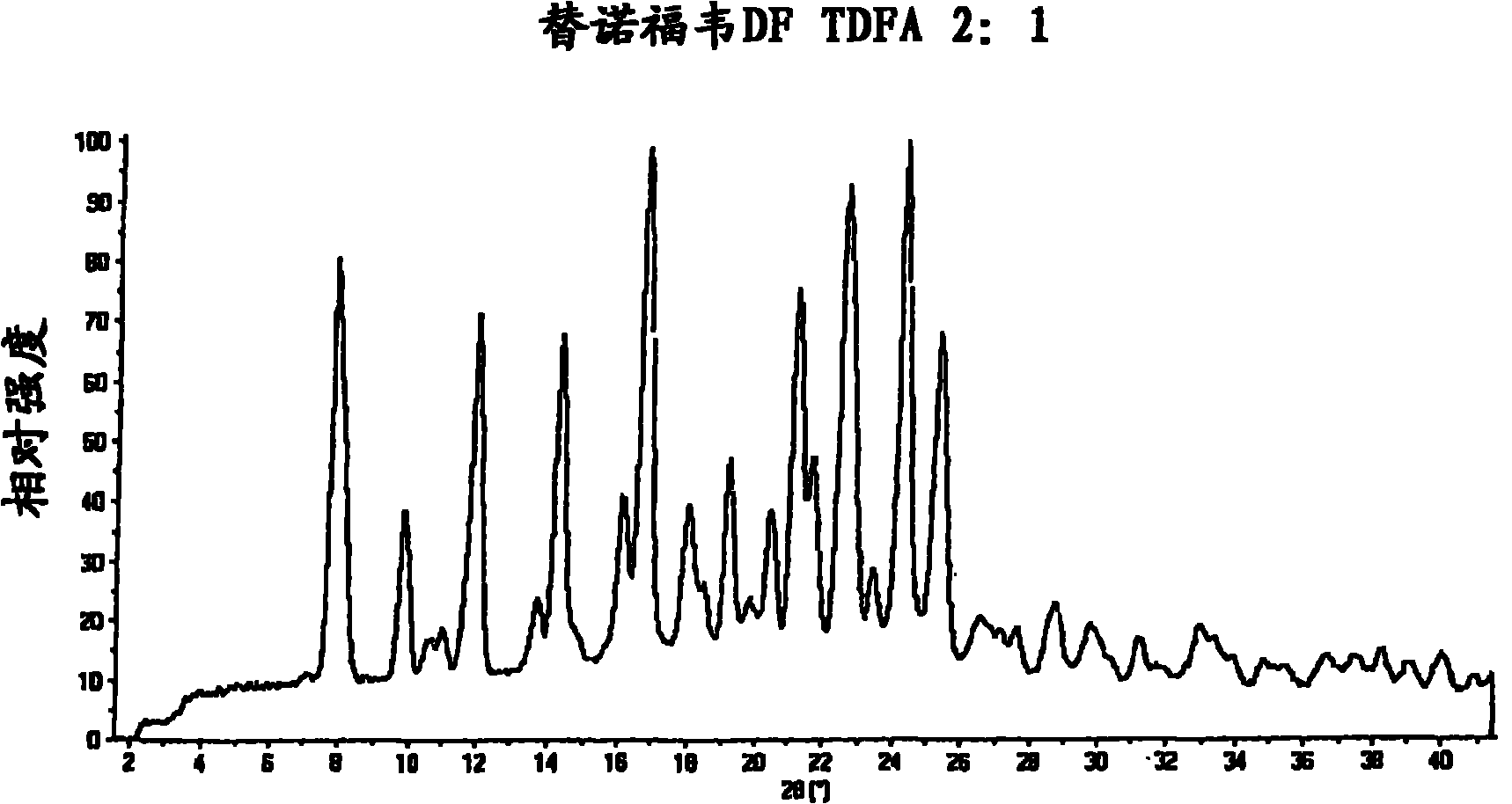
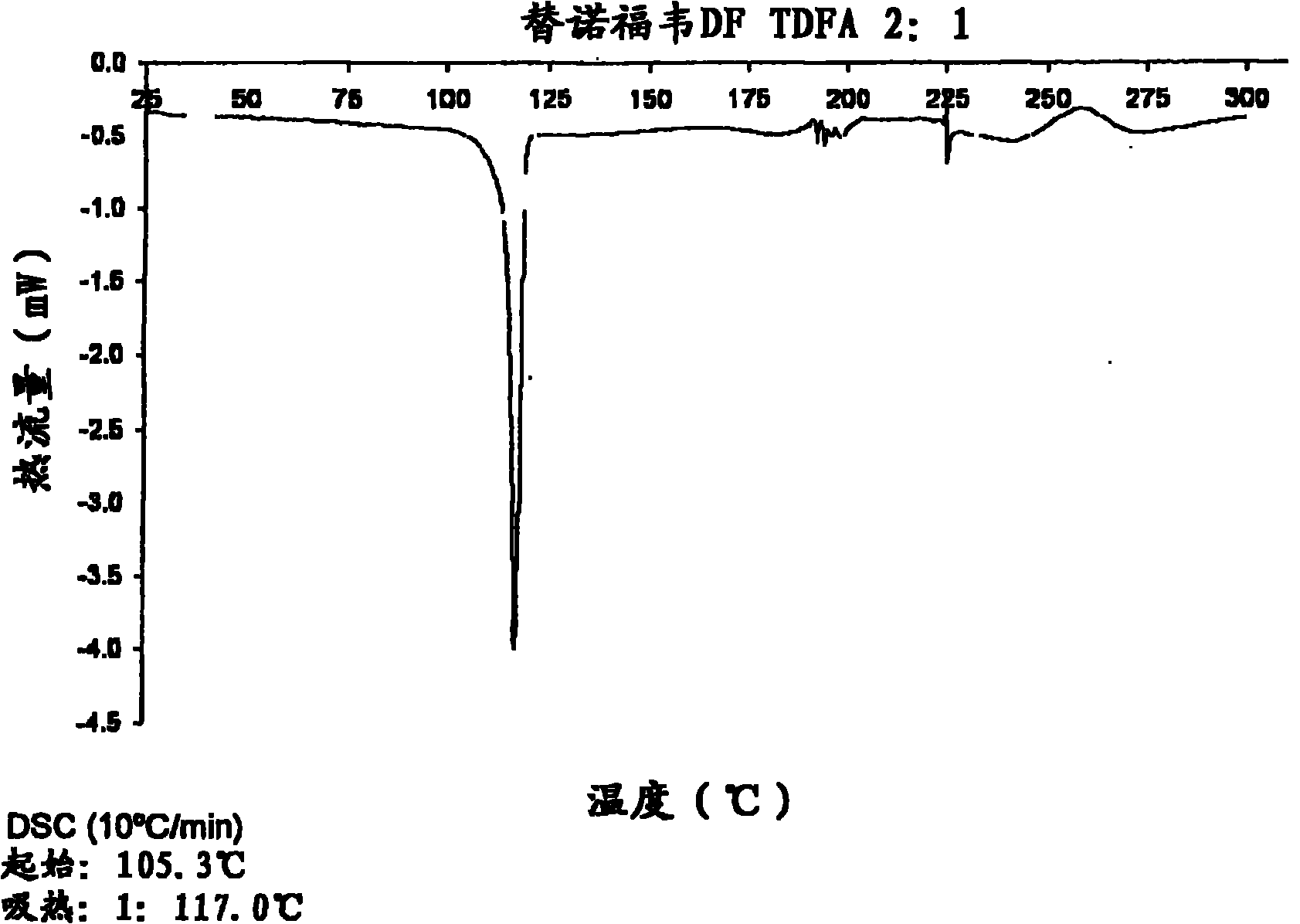
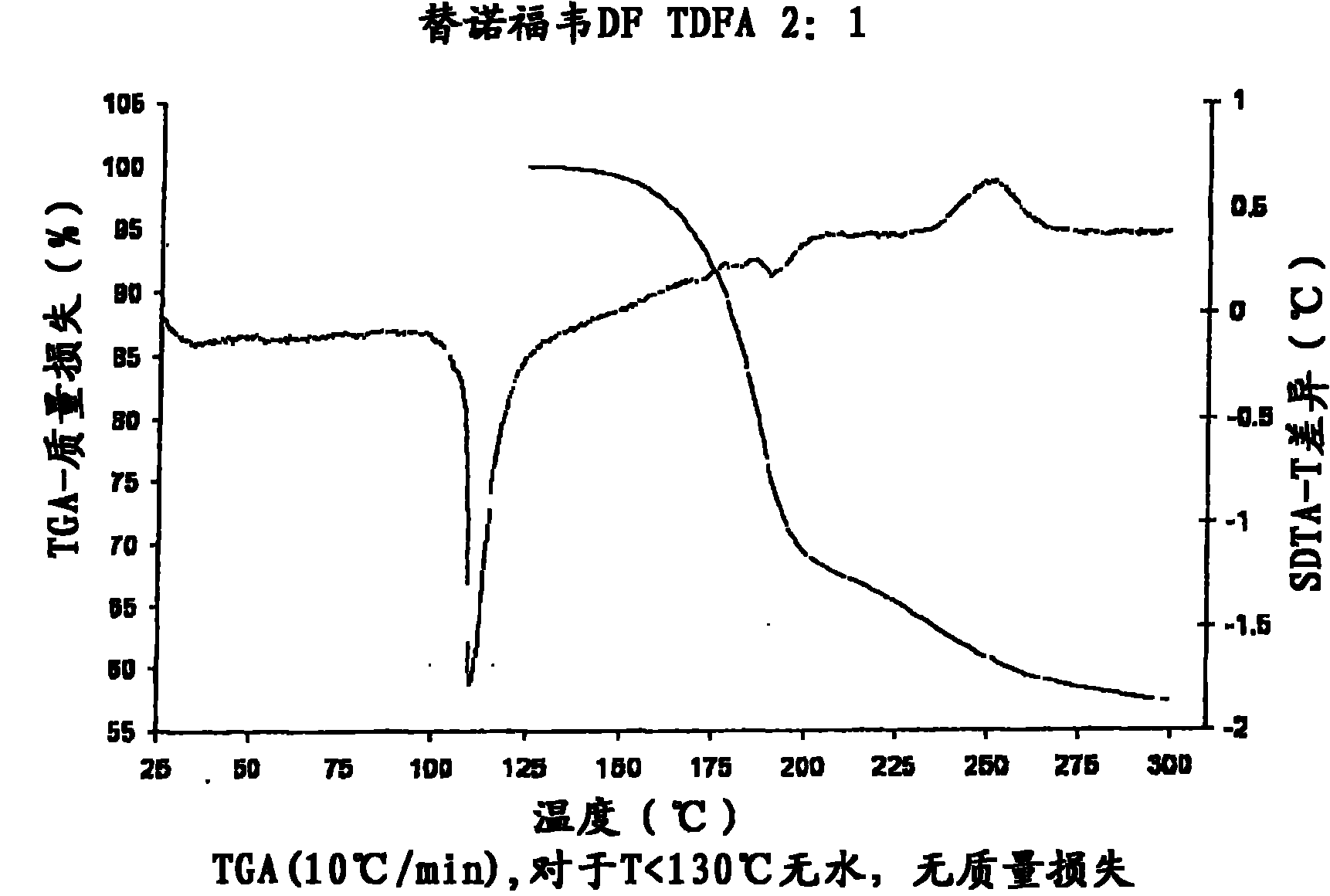
Tenofovir disoproxil hemi-fumaric acid co-crystal
InactiveCN101778855AOrganic active ingredientsGroup 5/15 element organic compoundsEmtricitabineTenofovir DF
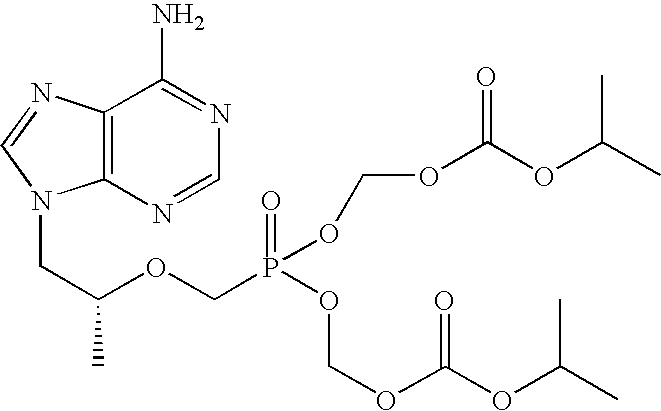
The present invention provides a novel crystalline form of Tenofovir disoproxil fumarate (Tenofovir DF), designated Co-crystal TDFA 2:1, methods for the preparation thereof and its use in pharmaceutical applications, in particular in anti-HIV medicaments. The crystalline form TDFA 2:1 can be used in combination with other anti-HIV medicaments such as Efavirenz, Emtricitabine, Ritonavir and / or TMC114.
Owner:ULTIMORPHIX TECH
Compositions and methods for combination antiviral therapy
InactiveUS20060246130A1Eliminate side effectsPatient compliance is goodBiocideOrganic active ingredientsMedicineEmtricitabine
The present invention relates to therapeutic combinations of [2-(6-amino-purin-9 yl)-1-methyl-ethoxymethyl]-phosphonic acid diisopropoxycarbonyloxymethyl ester (tenofovir disoproxil fumarate, Viread®) and (2R,5S,cis)-4-amino-5-fluoro-1-(2 hydroxymethyl-1,3-oxathiolan-5-yl)-(1H)-pyrimidin-2-one (emtricitabine, Emtriva™, (−)-cis FTC) and their physiologically functional derivatives. The combinations may be useful in the treatment of HIV infections, including infections with HIV mutants bearing resistance to nucleoside and / or non-nucleoside inhibitors. The present invention is also concerned with pharmaceutical compositions and formulations of said combinations of tenofovir disoproxil fumarate and emtricitabine, and their physiologically functional derivatives, as well as therapeutic methods of use of those compositions and formulations.
Owner:GILEAD SCI INC
Solid of tenofovir disoproxil, and preparation method and application thereof
ActiveCN103626803AEasy to prepareCrystal form controllableOrganic active ingredientsGroup 5/15 element organic compoundsMedicineHepatitis B virus
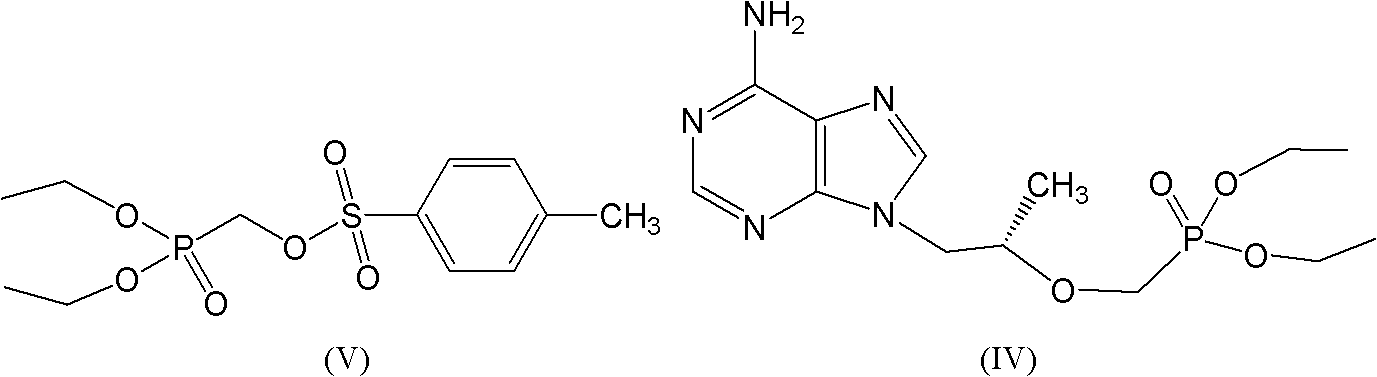
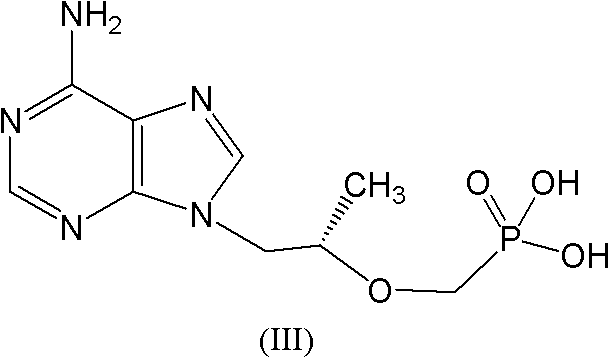
The invention relates to a solid of tenofovir disoproxil. The solid is (1) a tenofovir disoproxil compound represented by a formula IV or (2) a tenofovir disoproxil cocrystal or salt represented by a formula V. The invention further relates to a preparation method for the solid of tenofovir disoproxil, a pharmaceutical composition containing the solid and application of the solid in preparation of drugs used for preventing and / or treating virus infection, especially hepatitis b virus (HBV) and / or human immunodeficiency virus (HIV) infection.
Owner:SICHUAN HAISCO PHARMA CO LTD
Tenofovir disoproxil compounds, and preparation method and application thereof in anti-virus aspects
ActiveCN103224530AInhibition of replication activityImprove attributesOrganic active ingredientsGroup 5/15 element organic compoundsSolubilityAnti virus
The invention discloses a group of tenofovir disoproxil compounds with activity for inhibiting HIV-1 / HBV virus replication and pharmaceutically acceptable salts thereof, and a preparation method and pharmaceutical applications thereof. The group of the compounds have a general formula I, wherein X=H, Y=H, R1=-CH2(CH2)mCH2O(CH2)nCH3, m=0-4, n=10-20, and R2, R3 and R4 are respectively described in the specification. The invention also discloses a pharmaceutical composition containing the group of the compounds. Experiments show that one of the compounds has the advantages that an activity for inhibiting HIV-1 virus replication is 20 times that of a positive control medicine zidovudine (AZT), 1,000 times that of TDF that is the best medicine for treating Aids and about 9 times that of CMX157 in a clinical stage, and lipid solubility is about 2 times that of CMX157. Experiments also show that the compounds provided by the invention have the activity for inhibiting HBV virus replication, and can be used for development of drugs for treating the Aids and hepatitis B.
Owner:洛阳聚慧新材料科技有限公司 +2
Preparation methods of tenofovir disoproxil and fumarate thereof
ActiveCN106008603AAvoiding Disadvantages of DecompositionEasy to storeOrganic compound preparationGroup 5/15 element organic compoundsRefluxOrganic solvent
The invention discloses preparation methods of tenofovir disoproxil and fumarate thereof, and belongs to the field of preparation of compounds. The methods comprise the following steps: carrying out a condensation reaction of a tenofovir anhydrous substance or tenofovir hydrate with chloromethyl isopropyl carbonate to obtain a reaction liquid, pouring the reaction liquid obtained from the condensation reaction into supersaturated salt water, stirring, filtering, washing, and drying to obtain a tenofovir disoproxil crude product; adding the tenofovir disoproxil crude product into a certain amount of a nonpolar organic solvent with low boiling point, heating up to a reflux temperature, carrying out beating reflux, then carrying out gradient cooling and stirring, filtering, washing the obtained product with a certain amount of the nonpolar organic solvent with low boiling point, and drying, to obtain powdered tenofovir disoproxil; and in the presence of isopropyl alcohol, carrying out a reaction of the powdered tenofovir disoproxil with fumaric acid to obtain the tenofovir disoproxil fumarate. The method has the advantages of simple post treatment and high yield, and the obtained product has the characteristics of high purity, low impurity content and the like.
Owner:NORTHEAST PHARMA GRP
Tenofovir disoproxil fumarate dispersible tablets and preparation method thereof
ActiveCN102198110AModerate hardnessSmall weight differenceOrganic active ingredientsAntiviralsDissolutionLubricant
The invention discloses tenofovir disoproxil fumarate dispersible tablets and a preparation method thereof. The tenofovir disoproxil fumarate dispersible tablets are prepared from tenofovir disoproxil fumarate as an effective pharmaceutical ingredient and pharmaceutically acceptable auxiliary ingredients, wherein the pharmaceutically acceptable auxiliary ingredients comprise a filler, a disintegrant, a lubricant, a surfactant and a flavoring agent. The tenofovir disoproxil fumarate dispersible tablets prepared by the invention have appropriate hardness, small weight difference, bright and clean tablet surfaces and good taste, fully meet the requirements on the disintegration time and dispersion uniformity of dispersible tablets, and notably improve the pharmaceutical bioavailability, and in addition, the tenofovir disoproxil fumarate dispersible tablets have quick dissolution rate, the dissolution percentage of the product is about 80-90% in 2 minutes, and the product is almost completely dissolved in 5 minutes.
Owner:杭州康本医药科技有限公司 +2
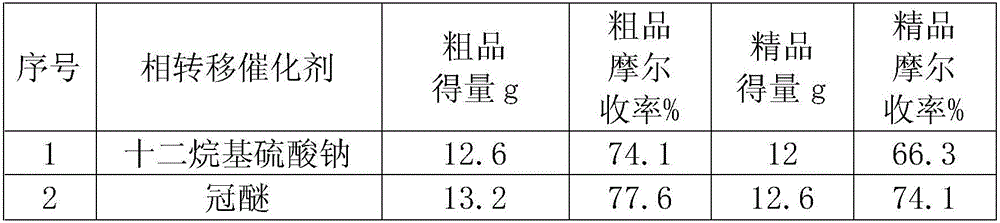
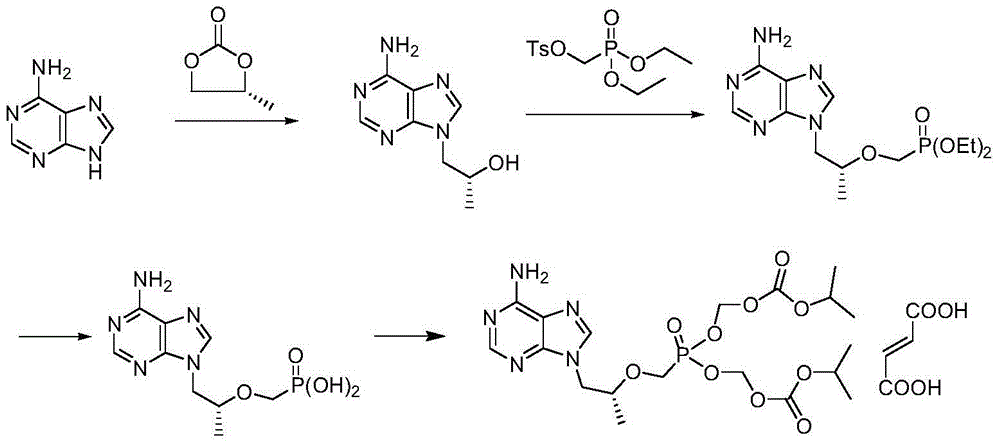
Method for preparing tenofovir disoproxil fumarate
The invention relates to a method for preparing high-purity tenofovir disoproxil fumarate. The method comprises the following steps: preparing (R)-4-methyl-1,3-dioxolan-2-one, synthesizing (R)-9-(2-hydroxypropyl)adenine, synthesizing a tenofovir hydrate, refining by virtue of dehydrating and synthesizing tenofovir disoproxil fumarate. The corresponding product is refined and the target product is detected by dehydrating through tenofovir and using alkali-soluble acid-analysis manners, the reaction, separation and purification processes in the subsequent steps are facilitated and the purity and yield of the product are increased. The technical scheme disclosed by the invention has the advantages of simple operation, relatively inexpensive selected reagents, small side effects, high yield and less three wastes produced in the reaction and the environmental protection is facilitated.
Owner:SHANDONG NEWTIME PHARMA
Tenofovir disoproxil fumarate synthesis method
InactiveCN104725423AHigh yieldHigh purityGroup 5/15 element organic compoundsSynthesis methodsReaction intermediate
The present invention belongs to the technical field of medicine, and relates to a tenofovir disoproxil fumarate synthesis method, wherein (R)-1,2-propanediol and carbonate are adopted as raw materials, and condensation, etherification, hydrolysis, esterification salification and other steps are performed to prepare the finished product. According to the present invention, the process improving and the reaction intermediate treatment are performed so as to easily achieve the reactions in the subsequent steps and the separation and purification, improve the yield and the corresponding purity of the target product, and obtain the tenofovir disoproxil fumarate meeting the medical standard.
Owner:SHANDONG NEWTIME PHARMA
Preparation method of tenofovir disoproxil fumarate
ActiveCN104447868AHigh yieldHigh purityGroup 5/15 element organic compoundsCarboxylic acid salt preparationSide reactionOrganic chemistry
The invention discloses a preparation method of tenofovir disoproxil fumarate, belonging to the technical field of synthesis of tenofovir disoproxil fumarate. The preparation method comprises the following steps: preparing tenofovir disoproxil, preparing a tenofovir disoproxil fumarate crude product and refining the tenofovir disoproxil fumarate, wherein in the step of refining the tenofovir disoproxil fumarate, by virtue of a 65% ethanol solution which is used as a tenofovir disoproxil fumarate recrystallizing solvent, the tenofovir disoproxil fumarate is kept in an environment at 5-10 DEG C for 3-6h until the tenofovir disoproxil fumarate is naturally separated out and crystallized. The preparation method disclosed by the invention can be used for solving the problems of high contents of organic residue and side reactant in the process of preparing the tenofovir disoproxil fumarate, and the total yield can reach more than 40%; in addition, the tenofovir disoproxil fumarate occupies a high content in a final product, and the preparation method is simple and convenient to operate in the recrystallizing process, low in cost, and free from toxic and harmful side reactions or products.
Owner:山东世博金都药业有限公司
Salt compound of tenofovir disoproxil fumarate and preparation method and medicinal application thereof
ActiveCN101781334AImprove bioavailabilityGood effectOrganic active ingredientsGroup 5/15 element organic compoundsPhosphoric acidTenofovir
The invention relates to a salt compound of tenofovir disoproxil fumarate and a preparation method and medicinal application thereof. The preparation method comprises the following steps: heating to dissolve the tenofovir disoproxil fumarate with isopropanol in an amount which is 5 to 20 times the weight of the tenofovir disoproxil fumarate, stirring, regulating pH with phosphoric acid to between 2 and 3, and performing reaction for 30 minutes; and cooling to 0 DEG C, standing for crystallization, filtering, washing twice with a small amount of isopropanol, and performing vacuum drying.
Owner:FUJIAN COSUNTER PHARMA
Pharmaceutical antiretroviral composition
InactiveUS20140193491A1Easy to manufactureBiocideOrganic active ingredientsNucleoside Reverse Transcriptase InhibitorEmtricitabine
The present invention relates to a pharmaceutical antiretroviral composition comprising (i) a nucleoside reverse-transcriptase inhibitor selected from lamivudine and emtricitabine, (ii) extended release nevirapine, and (iii) tenofovir; a process for preparing such composition and the use of such composition in medicine, particularly for the prophylaxis and / or treatment of diseases caused by retroviruses.
Owner:CIPLA LTD
Tenofovir disoproxil fumarate and preparation method thereof
ActiveCN103641858AAdvantages and Notable ImprovementsSignificant progressGroup 5/15 element organic compoundsAntiviral drugDimethyl methylphosphonate
The invention relates to an antiviral drug tenofovir disoproxil fumarate and a novel preparation method thereof, belonging to the field of medicines. Content of related substances in the tenofovir disoproxil fumarate, especially content of impurity K is effectively lowered by taking toluenesulfonyloxy diethyl methylphosphonate as a starting material through hydrolysis, condensation and salifying. Moreover, the preparation method is gentle in reaction condition, low in production cost, simple and convenient to operate, and suitable for large-scale industrial production.
Owner:湖南千金湘江药业股份有限公司
Process for the preparation of Tenofovir
ActiveUS8049009B2Increase productionPromote crystallizationPhosphorus organic compoundsMedicinal chemistryCrystallization
The present invention relates to novel Tenofovir disoproxil hemifumarate salt, which is prepared by dealkylation of Phosphonate esters by using mineral acids followed by esterification and crystallization to give crystalline Tenofovir disoproxil. The Tenofovir disoproxil is further converted to fumarate salts with improved yield.
Owner:MYLAN LAB
Preparation method of tenofovir disoproxil fumarate hemifumarate
InactiveCN104045667AThe process operation is convenient and controllableHigh yieldGroup 5/15 element organic compoundsCarboxylic acid salt preparationCombinatorial chemistrySolvent
The invention relates to the field of pharmaceutical chemistry and provides a preparation method of tenofovir disoproxil fumarate hemifumarate. The tenofovir disoproxil fumarate hemifumarate is a eutectic composed of tenofovir disoproxil fumarate and fumaric acid with the ratio of tenofovir disoproxil fumarate to fumaric acid being 2:1. According to the preparation method, (R)-9-(2-hydroxy propyl) adenine is used as a starting material, and condensation, hydrolysis and esterification are carried out to prepare free tenofovir disoproxil fumarate; and the free tenofovir disoproxil fumarate and fumaric acid in a solvent undergo salt formation and crystallization to prepare the tenofovir disoproxil fumarate hemifumarate.
Owner:SHANGHAI SCIENCHEM CO LTD
Therapeutic compositions comprising rilpivirine hcl and tenofovir disoproxil fumarate
ActiveUS20130243857A1Decrease exposureCompromise complianceOrganic active ingredientsBiocideRilpivirine HClPharmacology
The invention provides multilayer tablets that contain rilpivirine hydrochloride, emtricitabine, and tenofivir disoproxil fumarate. The tablets are useful for the treatment of HIV.
Owner:JANSSEN SCI IRELAND UC
Industrialization production technology for tenofovir disoproxil fumarate
InactiveCN105859781AHigh yieldHigh purityGroup 5/15 element organic compoundsDiethyl phosphateDiethyl methylphosphonate
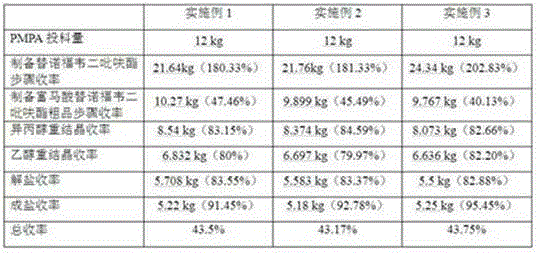
An industrial production process of tenofovir, the production process steps are as follows: add R-1,2-propylene glycol, diethyl carbonate and sodium ethylate into the reaction kettle; add absolute ethanol and diphosphite Ethyl ester, stirring, after the stirring is completed, put in paraformaldehyde and triethylamine, after the reaction is complete, add anhydrous sodium sulfate, dry, filter, and distill out, the distilled product is diethyl p-toluenesulfonyloxymethylphosphonate Fine product; add adenine, R-propylene carbonate, DMF and NaOH in the reaction kettle, after the reaction is complete, add magnesium tert-butoxide, drop diethyl p-toluenesulfonyl phosphate, after the reaction is complete, add acetic acid, Concentrate under reduced pressure, add hydrochloric acid, filter, filter out the solid and dry under normal pressure to obtain PMPA fine product. The method has the advantages of high yield, high product purity and low impurity content, and can be fully industrialized.
Owner:JINGMEN SHUAIBANG CHEM SCI & TECHCO
Preparation method of tenofovir disoproxil fumarate impurities
The invention relates to a novel synthesis method of three impurities of tenofovir disoproxil fumarate, and the method has an important significance for synthesizing high-quality tenofovir disoproxil fumarate. The invention mainly aims at researching the synthesis of a tenofovir disoproxil fumarate isopropyl ester impurity (R)-9-(2-phosphonomethoxy propyl) adenyl isopropyl oxycarbonyl methoxy isopropyl ester (IV), a tenofovir disoproxil fumarate methoxycarbonyl methoxy ester impurity (R)-9-(2-phosphonomethoxy propyl) adenyl isopropyl oxycarbonyl methoxy ester methoxycarbonyl methoxy ester (VI) and a tenofovir disoproxil fumarate n-propyl oxycarbonyl methoxy ester impurity (R)-9-(2-phosphonomethoxy propyl) adenyl isopropy oxycarbonyl methoxy ester n-propyl oxycarbonyl methoxy ester (VII). The synthetic routes of the three impurities of tenofovir disoproxil fumarate are as shown in the specification.
Owner:CHINA PHARM UNIV
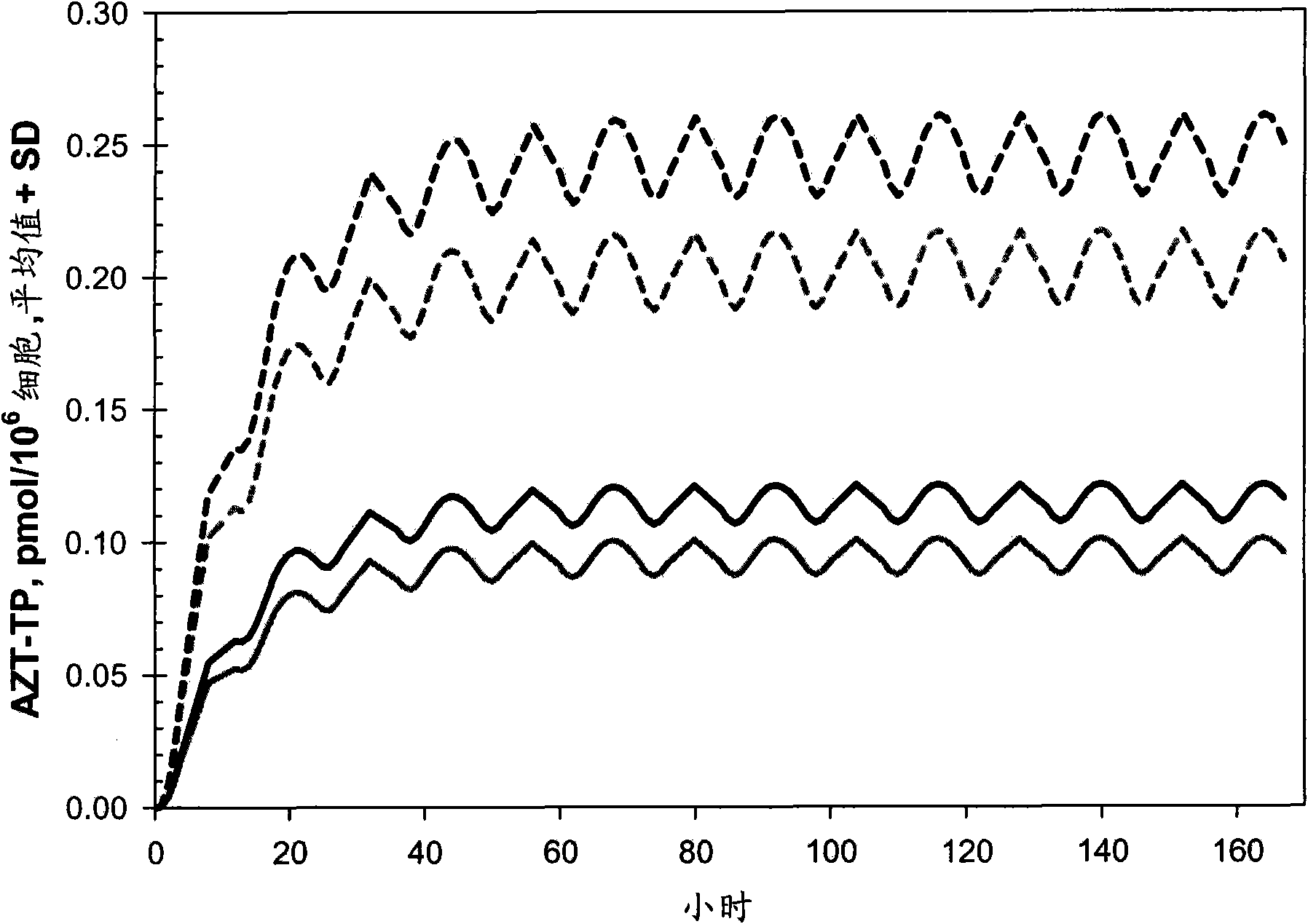
Potent combinations of zidovudine and drugs that select for the K65R mutation in the HIV polymerase
InactiveCN101878032ALow toxicityImprove absolute antiviral effectAntiviralsCarbohydrate active ingredientsNucleoside Reverse Transcriptase InhibitorRetroviral infection
Owner:EMORY UNIVERSITY
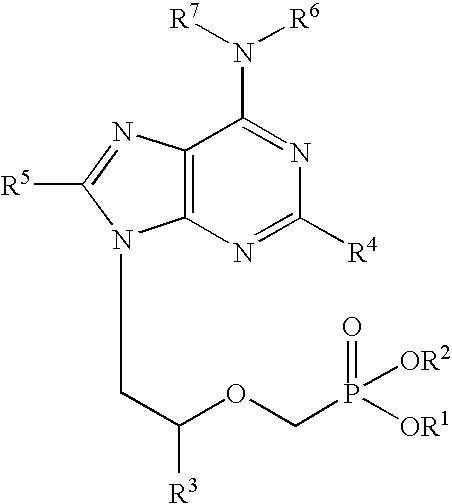
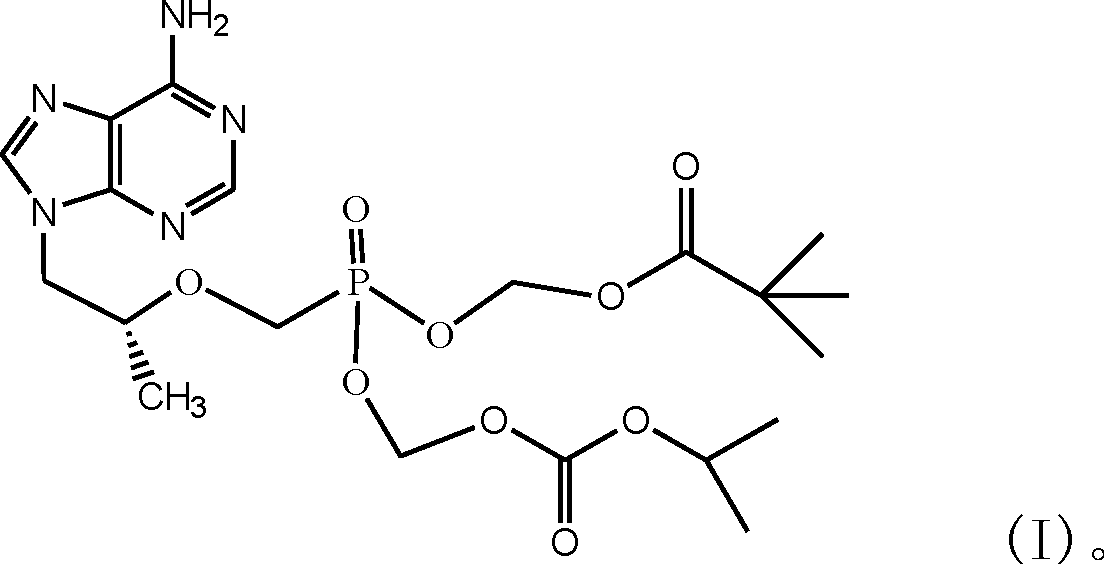
Antiviral aliphatic ester prodrugs of tenofovir
ActiveUS20180179208A1Group 5/15 element organic compoundsPharmaceutical delivery mechanismEster prodrugReverse transcriptase
Compounds of Formula I:and their pharmaceutically acceptable salts are useful for the inhibition of HIV reverse transcriptase. The compounds may also be useful for the prophylaxis or treatment of infection by HIV and in the prophylaxis, delay in the onset or progression, and treatment of AIDS. The compounds and their salts can be employed as ingredients in pharmaceutical compositions, optionally in combination with other antiviral agents, immunomodulators, antibiotics or vaccines
Owner:INDENIX PHARM LLC +1
Tenofovir disoproxil fumarate fine granules
ActiveCN103330683ATaste-masked bitternessReduce exposureOrganic active ingredientsPowder deliveryHot meltPolymer
The invention relates to tenofovir disoproxil fumarate fine granules. The tenofovir disoproxil fumarate fine granules are formed by the following steps of: mixing tenofovir and a sweetening agent according to a mass ratio of 1: (0-6); mixing the mixture with a polymer for hot melting extrusion according to a mass ratio of 1: (2-15); then carrying out hot melting extrusion through a hot melting extruder; and crushing into the fine granules with the grain diameter less than or equal to 30 meshes. The tenofovir disoproxil fumarate fine granules disclosed by the invention overcome the disadvantages that the taste of tenofovir disoproxil fumarate is very bitter and the taste masking effect of an existing preparation is not enough, and the medicine-taking compliance of a patient is greatly improved.
Owner:SHINEWAY PHARMA GRP LTD
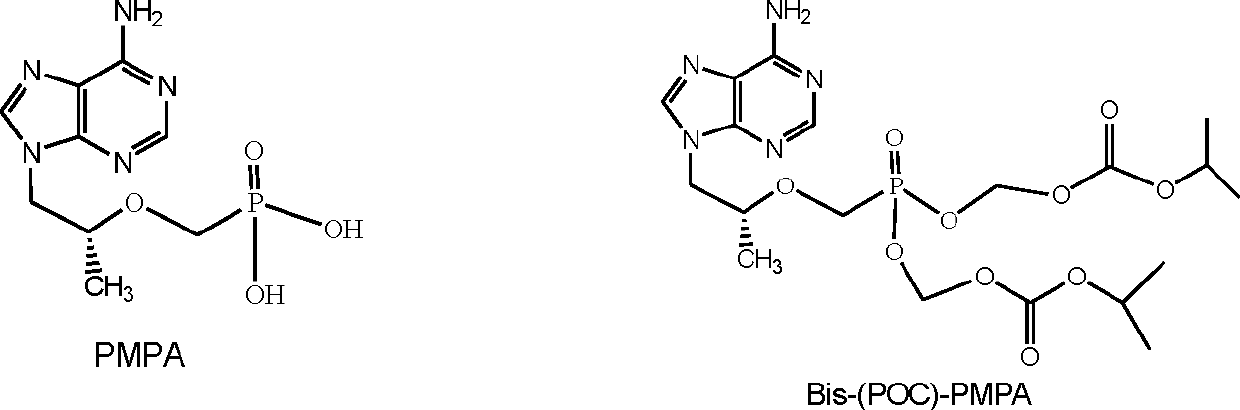
Adenine derivative and inclusion compound thereof
ActiveCN102977146AStrong antiviral activityImprove securityOrganic active ingredientsGroup 5/15 element organic compoundsAdenine derivativesPurine
The invention discloses the technical field of medicines and particularly discloses a new prodrug, namely (R)-9-(2-(phosphonyl methoxyl)propenyl) adenine, a cyclodextrin inclusion compound of the prodrug and a non-toxic and pharmaceutically acceptable salt of the prodrug. The prodrug disclosed by the invention can be metabolized into PMPA in vivo, and has the bioavailability of about 39%, and the bioavailability of the prodrug is better than that of tenofovir disoproxil fumarate (Bis-(POC)-PMPA); and the prodrug has more excellent antiviral activity and better safety compared with the tenofovir disoproxil fumarate.
Owner:STAR LAKE BIOSCI CO INC ZHAOQING GUANGDONG
Preparing method for realizing industrial mass production of tenofovir disoproxil fumarate
InactiveCN105622671AEasy to buyEasy to transportOrganic compound preparationGroup 5/15 element organic compoundsSide reactionReagent
The invention relates to a preparing method for realizing industrial mass production of tenofovir disoproxil fumarate.The preparing method includes the following steps of preparing (R)-9-[2-(diethoxyphosphonylmethoxy) propyl] adenine, synthesizing tenofovir, refining tenofovir, dewatering tenofovir, synthesizing tenofovir disoproxil and synthesizing tenofovir disoproxil fumarate.The technical scheme is easy to operate, selected reagents are low in price, the number of side reactions is small, the yield is high, few three wastes are generated in the reaction process, and the preparing method is beneficial for environment protection and suitable for industrial mass production.
Owner:广东京豪生物制药有限公司
Novel process for acyclic phosphonate nucleotide analogs
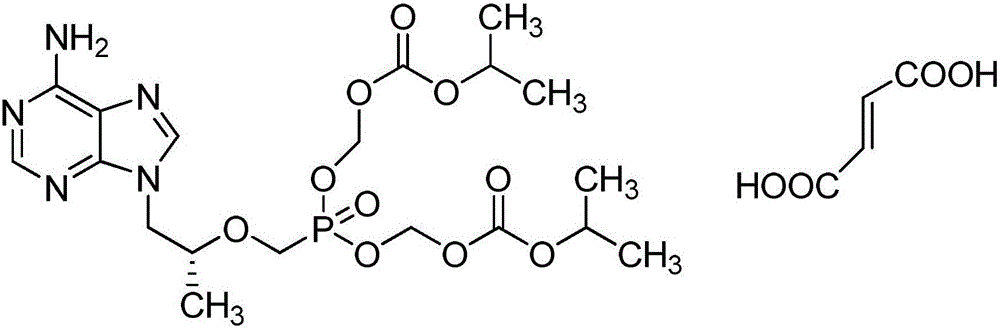
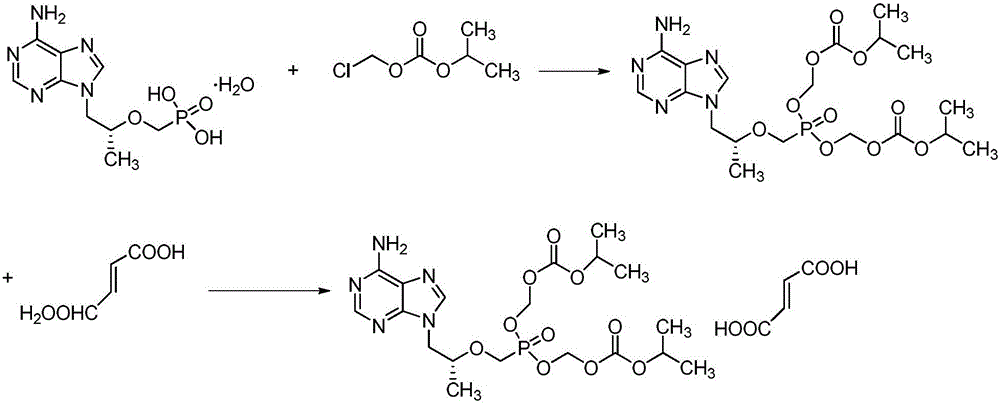
The present invention provides a novel process for the preparation of acyclic phosphonate nucleotide analogs using novel intermediates. Thus, for example, (R)-9-(2-phosphonomethoxypropyl)adenine is reacted with dimethylformamide dimethylacetal to give N4-dimethylaminomethyledino-9-(2-phosphonomethoxy ethyl) adenine, which is then reacted with chloromethyl-2-propyl carbonate in presence of triethylamine to give (R)—N4-Dimethylaminomethyledino-9-(2-phosphono methoxypropyl) adenine disoproxil, followed by deprotection with acetic acid to get tenofovir disoproxil. Tenofovir disoproxil is then treated with fumaric acid to give tenofovir disoproxil fumarate.
Owner:HETERO DRUGS LTD
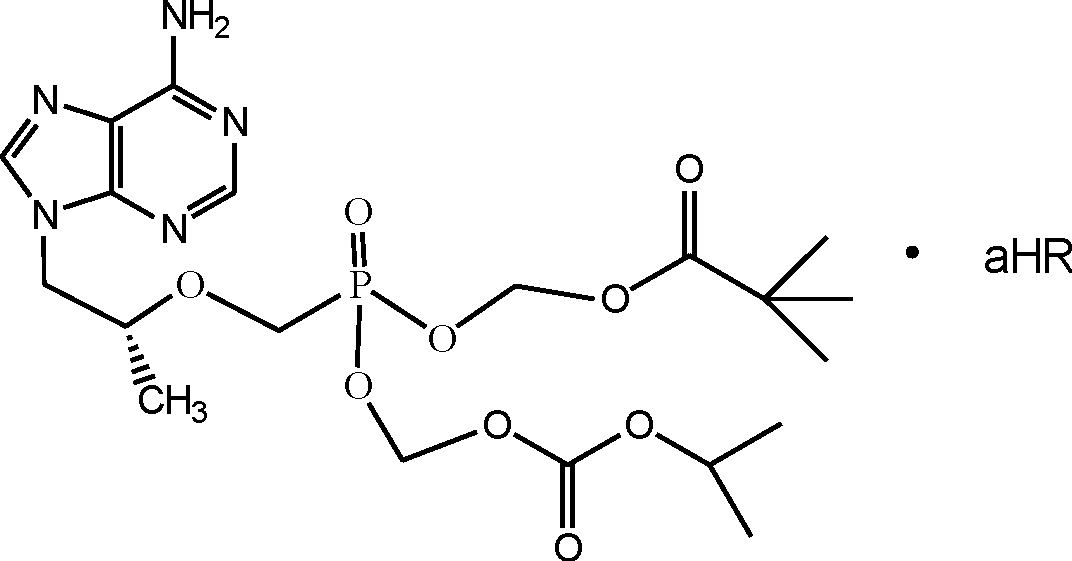
Antiviral phosphodiamide prodrugs of tenofovir
ActiveUS20180362562A1Organic active ingredientsGroup 5/15 element organic compoundsReverse transcriptaseMedicine
Compounds of Formula I and pharmaceutically acceptable salts and co-crystals thereof are useful for the inhibition of HIV reverse transcriptase. The compounds may also be useful for the prophylaxis or treatment of infection by HIV and in the prophylaxis, delay in the onset or progression, and treatment of AIDS. The compounds and their salts can be employed as ingredients in pharmaceutical compositions, optionally in combination with other antiviral agents, immunomodulators, antibiotics or vaccines.
Owner:MERCK SHARP & DOHME LLC +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com