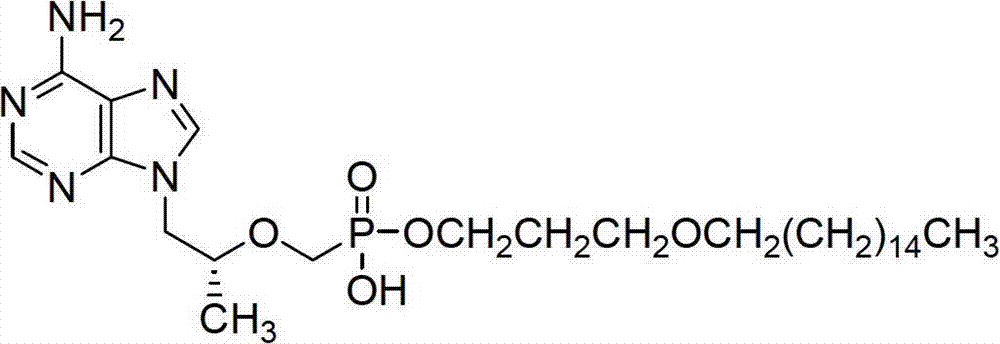Tenofovir diester compounds with activity of inhibiting HIV-1 (human immunodeficiency virus-1) virus replication and preparation method and pharmaceutical use thereof
A technology of tenofovir and ester compounds, which is applied in the field of nucleoside compounds, can solve the problems of maintaining sufficient concentration in infected parts, only human bioavailability, and no antiviral activity, so as to achieve high activity and improve bioavailability , improve the effect of the effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0061] L016 was synthesized by the above-mentioned preparation method of L114.
[0062] c) When m=4, n=12, the specific preparation process of L412:
[0063] Its reaction formula is:
[0064]
[0065] L412 was synthesized by the above-mentioned preparation method of L114.
[0066] The preparation process of (R)-9-[2-[[[(S)-1-(isopropoxycarbonyl)]ethyl]methoxyphosphoramidate]propyl]adenine (Ⅲ):
[0067] Its reaction formula is:
[0068]
[0069] In a 500ml round bottom flask, add commercially available tenofovir (7.2g, 25.1mmol) to 200ml of anhydrous acetonitrile solvent, under nitrogen protection, after heating up to 50°C, slowly add thionyl chloride dropwise (1.8ml, 25mmol), warm up to 80°C and stir for 2 hours after dripping; evaporate the solvent, add anhydrous dichloromethane (200ml), and add L-alanine isopropyl hydrochloride at -30°C (4.17g, 25mmol), slowly add triethylamine (16.7ml, 120mmol) dropwise, heat up to -10°C and react for 1h, wash the organic phase wi...
Embodiment 1
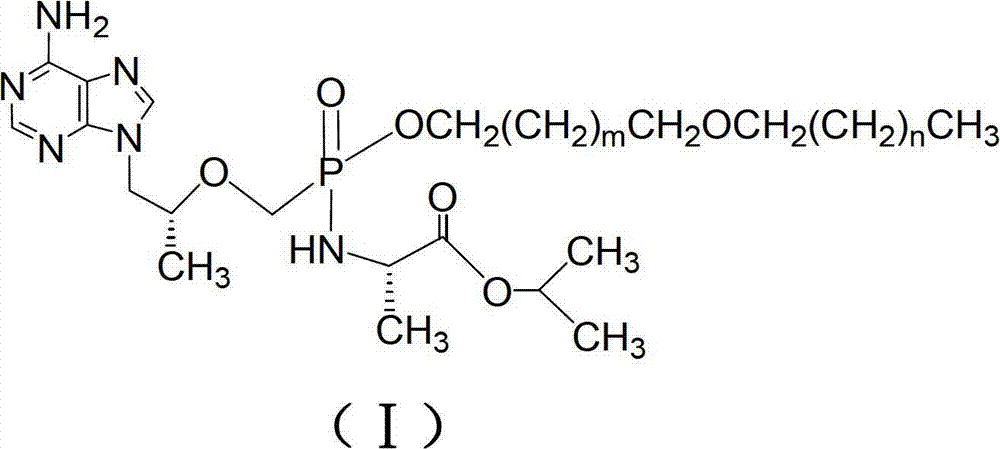
[0072] Embodiment 1: A kind of product of the present invention (R)-9-[2-[[hexadecyloxypropyl[(S)-1-(isopropoxycarbonyl)] ethyl] phosphoramidate methoxy] propyl ] adenine (compound 12 is m=1, the compound when n=14), its structural formula is:
[0073]
[0074] The preparation method of (R)-9-[2-[[hexadecyloxypropyl[(S)-1-(isopropoxycarbonyl)]ethyl]phosphoramidate methoxy]propyl]adenine:
[0075] In a 50ml round bottom flask, add (R)-9-[2-[[[(S)-1-(isopropoxycarbonyl)]ethyl]phosphoramidate methoxy]propyl]adenine prepared above in sequence (Ⅲ) (2.0g, 5.0mmol), N,N-dimethylformamide (25ml), L114 prepared above (1.8g, 5.0mmol) and triethylamine (0.85ml, 6.0mmol), at 80°C Stir for 6 hours, distill off the solvent, add ethyl acetate: ethanol 1:1 mixed solvent 100ml fully dissolved, filter, spin the filtrate through the column to obtain a white solid (R)-9-[2-[[hexadecyloxy Propyl[(S)-1-(isopropoxycarbonyl)]ethyl]phosphoramidatemethoxy]propyl]adenine (compound 12) (2.3g, 3.4mmo...
Embodiment 2
[0078] Example 2: Another product of the present invention (R)-9-[2-[[octadecyloxyethyl[(S)-1-(isopropoxycarbonyl)]ethyl]phosphoramidate methoxy]propane Base] adenine (compound 21 is m=0, the compound when n=16), its structural formula is:
[0079]
[0080] The preparation method of the above-mentioned product (R)-9-[2-[[octadecyloxyethyl[(S)-1-(isopropoxycarbonyl)]ethyl]phosphoramidate methoxy]propyl]adenine:
[0081] In a 50ml round bottom flask, add (R)-9-[2-[[[(S)-1-(isopropoxycarbonyl)]ethyl]phosphoramidate methoxy]propyl]adenine prepared above in sequence (Ⅲ) (2.0g, 5.0mmol), N,N-dimethylformamide (25ml), L016 prepared above (1.9g, 5.0mmol) and triethylamine (0.85ml, 6.0mmol), at 80°C Stir for 6 hours, evaporate the solvent, add ethyl acetate: ethanol 1:1 mixed solvent 100ml fully dissolved, filter, spin the filtrate through the column to obtain a white solid (R)-9-[2-[[octadecyloxy Ethyl[(S)-1-(isopropoxycarbonyl)]ethyl]phosphoramidatemethoxy]propyl]adenine (compo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com