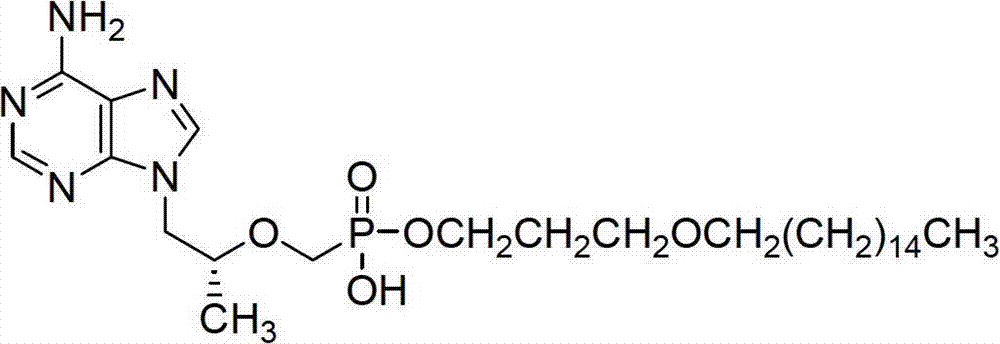Patents
Literature
532 results about "Therapy HIV" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Compressed tablet formulation
InactiveUS7060294B2Organic active ingredientsCapsule deliveryNucleoside Reverse Transcriptase InhibitorClinical study
This invention relates to a 50% drug loaded compressed tablet formulation for efavirenz. Efavirenz is a non-nucleoside reverse trancriptase inhibitor being studied clinically for use in the treatment of HIV infections and AIDS.
Owner:MERCK SHARP & DOHME CORP
HIV Integrase Inhibitors
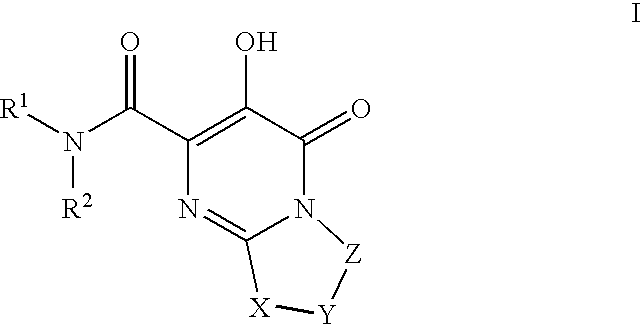
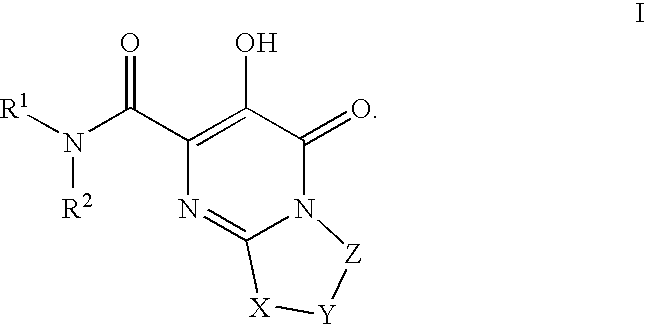
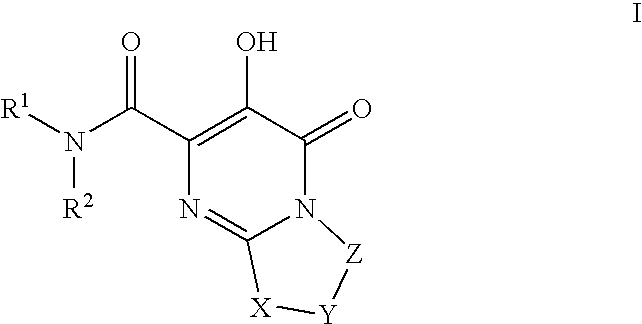
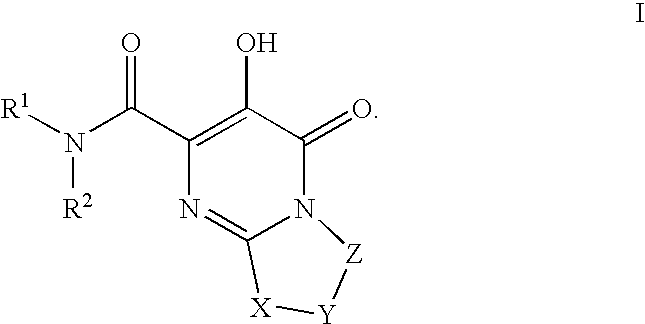
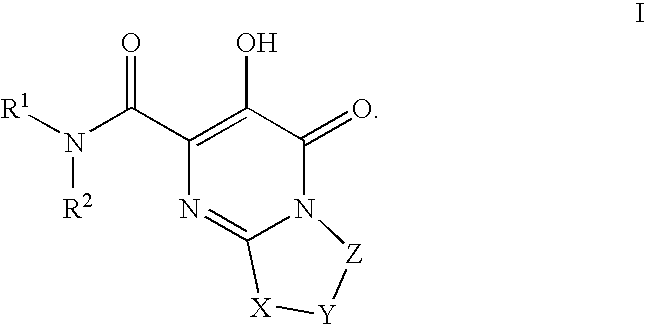
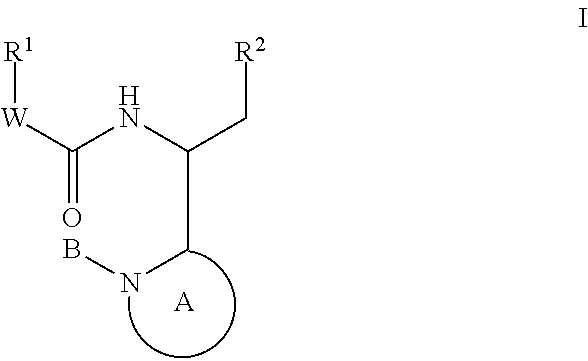
The invention encompasses series bicyclic pyrimidinone compounds of Formula I which inhibit HIV integrase and prevent viral integration into human DNA. This action makes the compounds useful for treating HIV infection and AIDS. The invention also encompasses pharmaceutical compositions and methods for treating those infected with HIV.
Owner:BRISTOL MYERS SQUIBB CO
Method for treating HIV
InactiveUS6620416B1Lower Level RequirementsIncrease CD4.sup.+ T cellsBiocidePeptide/protein ingredientsDiseaseHCG - Human chorionic gonadotropin
The present invention relates to peptides of one or more portions of the human chorionic gonadotropin beta-chain as well as methods for treatment and prevention of diseases, including HIV infection, using human chorionic gonadotropin, employing the beta-chain of human chorionic gonadotropin, peptides containing a sequence of one or more portions of the beta-chain of human chorionic gonadotropin and derivatives and analogues thereof. The invention further relates to fractions of sources and or preparations of human chorionic gonadotropin, such as fractions of human early pregnancy urine, which fractions have anti-HIV activity. The present invention further relates to pharmaceutical compositions for treating and / or preventing HIV infection.
Owner:NOBEL BIOSCI
HIV integrase inhibitors: cyclic pyrimidinone compounds
The invention encompasses a series of pyrimidinone compounds which inhibit HIV integrase and thereby prevent viral integration into human DNA. This action makes the compounds useful for treating HIV infection and AIDS. The invention also encompasses intermediates useful for making the pyrimidone compounds. Additionally, pharmaceutical compositions and methods for treating those infected with HIV are encompassed
Owner:BRISTOL MYERS SQUIBB CO
Bicyclic heterocycles as HIV integrase inhibitors
The invention encompasses a series cyclic bicyclic heterocyclic compounds of Formula I which are inhibitors of HIV integrase and prevent viral integration into human DNA. This action makes the compounds useful for treating HIV infection and AIDS. The invention also encompasses pharmaceutical compositions and methods for treating those infected with HIV
Owner:BRISTOL MYERS SQUIBB CO
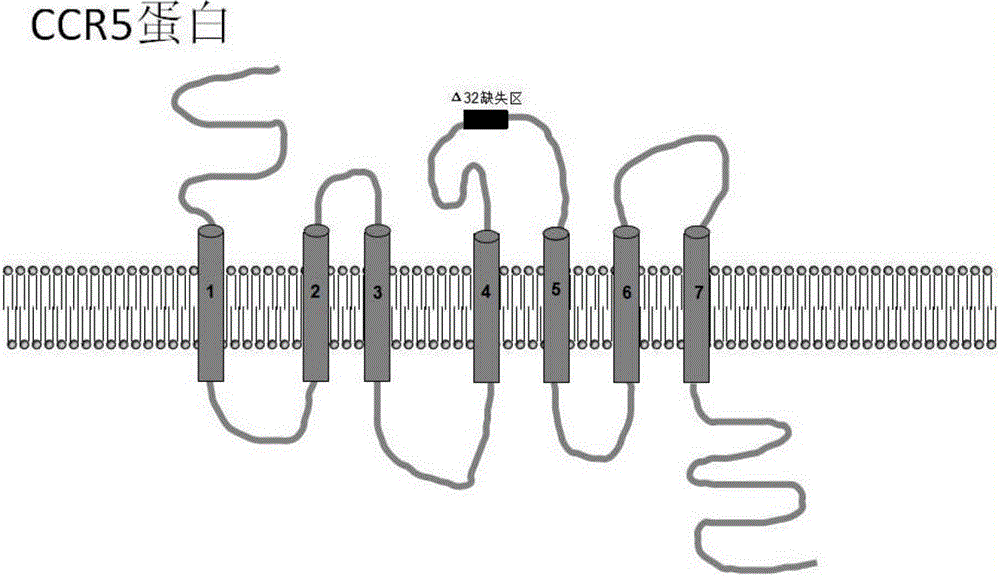
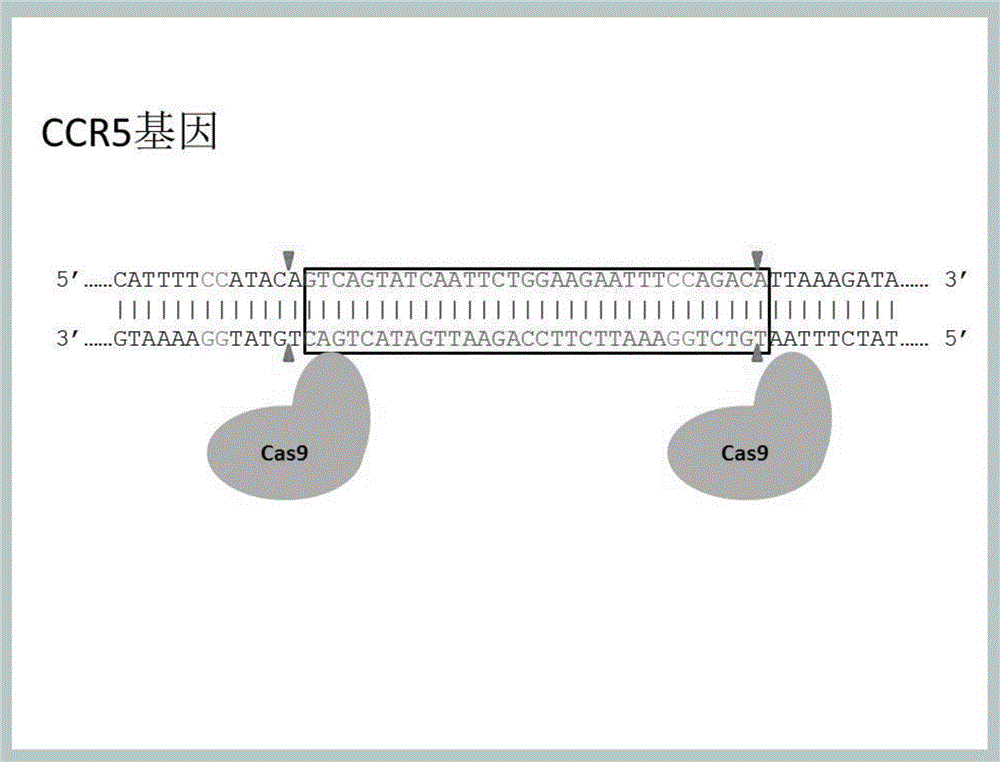
Method for inducing CCR5-delta32 deletion with genome editing technology CRISPR-Cas9
The invention relates to a method for successfully inducing cell chemokine receptor CCR5 genes to be mutated into CCR5-delta32 deletion-type genes with a new genome editing technology CRISPR-Cas9. CCR5 is an important receptor for human immunodeficiency viruses (HIV) to invade personal host cells. CCR5-delta32 deletion means deletion of 32 basic groups occurs in a CCR5 coding region, so that the sequence after the 185th amino acid is changed, and early termination occurs. CCR5-delta32 biallelic-gene homozygous deletion has natural resistance to HIV infection, and can not be infected by HIV. By means of the method, a slow virus packaging system and the CRISPR technology are used at the same time; as the slow virus infecting host range is wide, the method can be applied to cells such as bone marrow stem cells and CD4T cells, and the CCR5-delta32 deletion-type genes hopefully become medicine for treating acquired immune deficiency syndrome or other diseases.
Owner:NANKAI UNIV
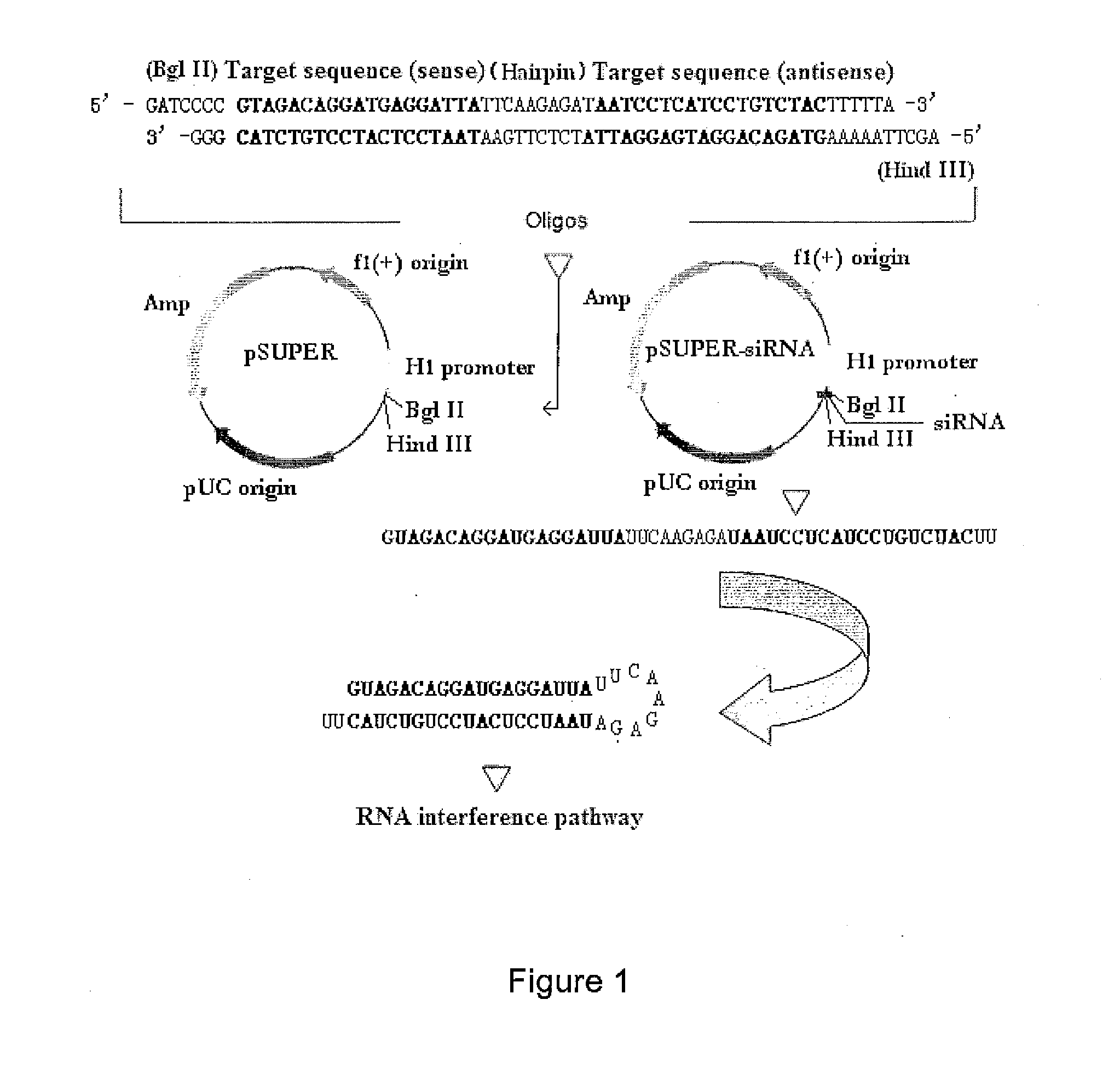
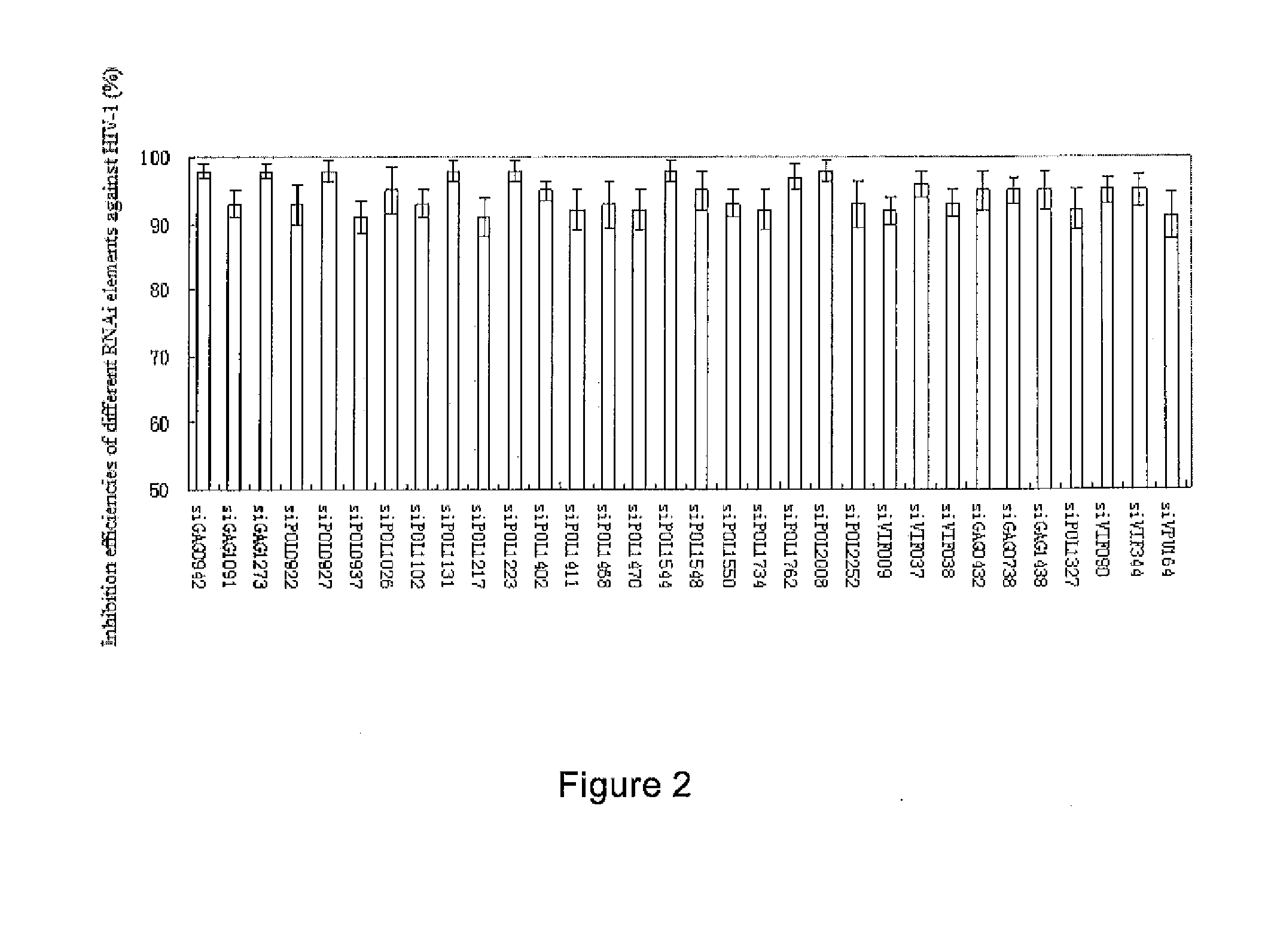
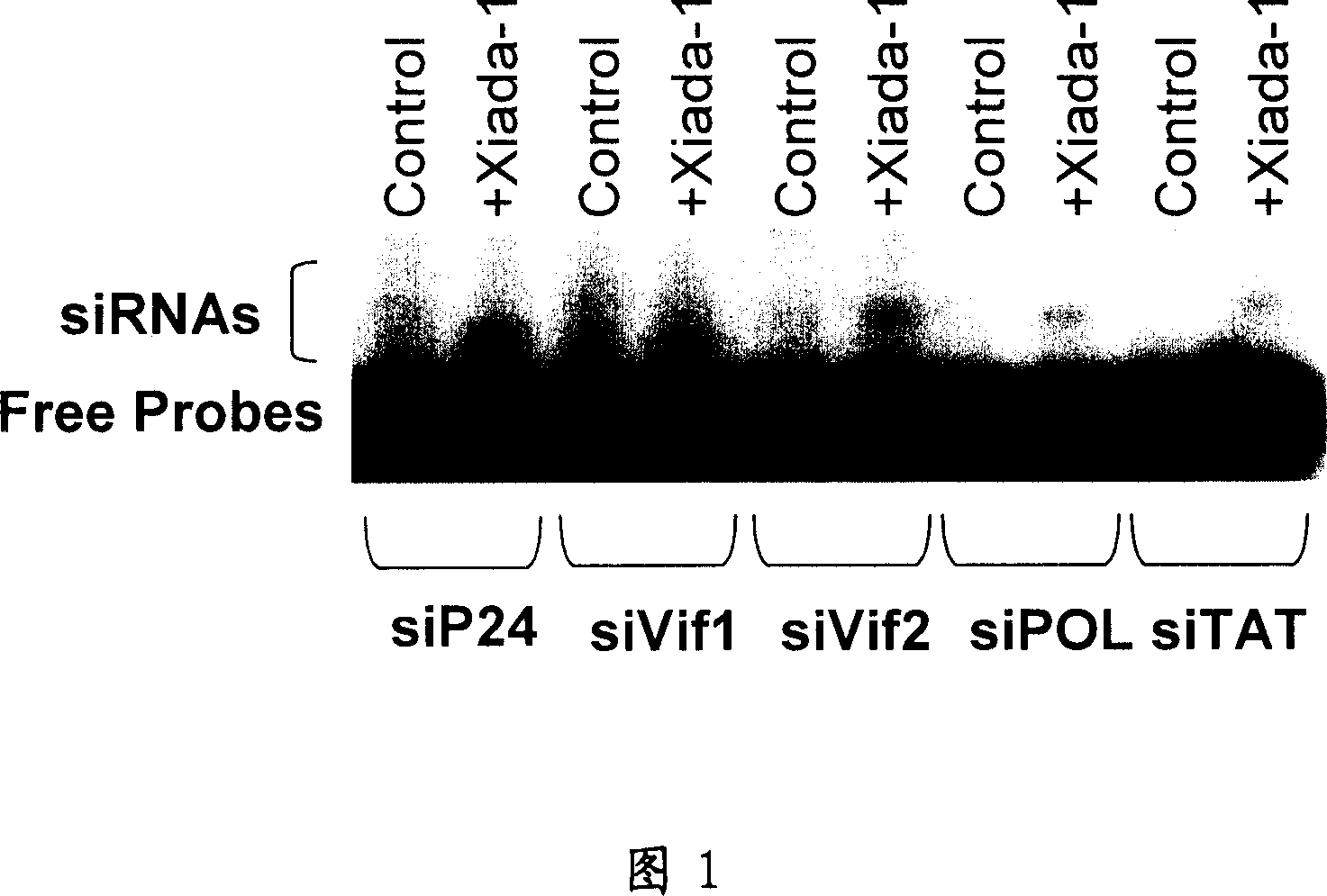
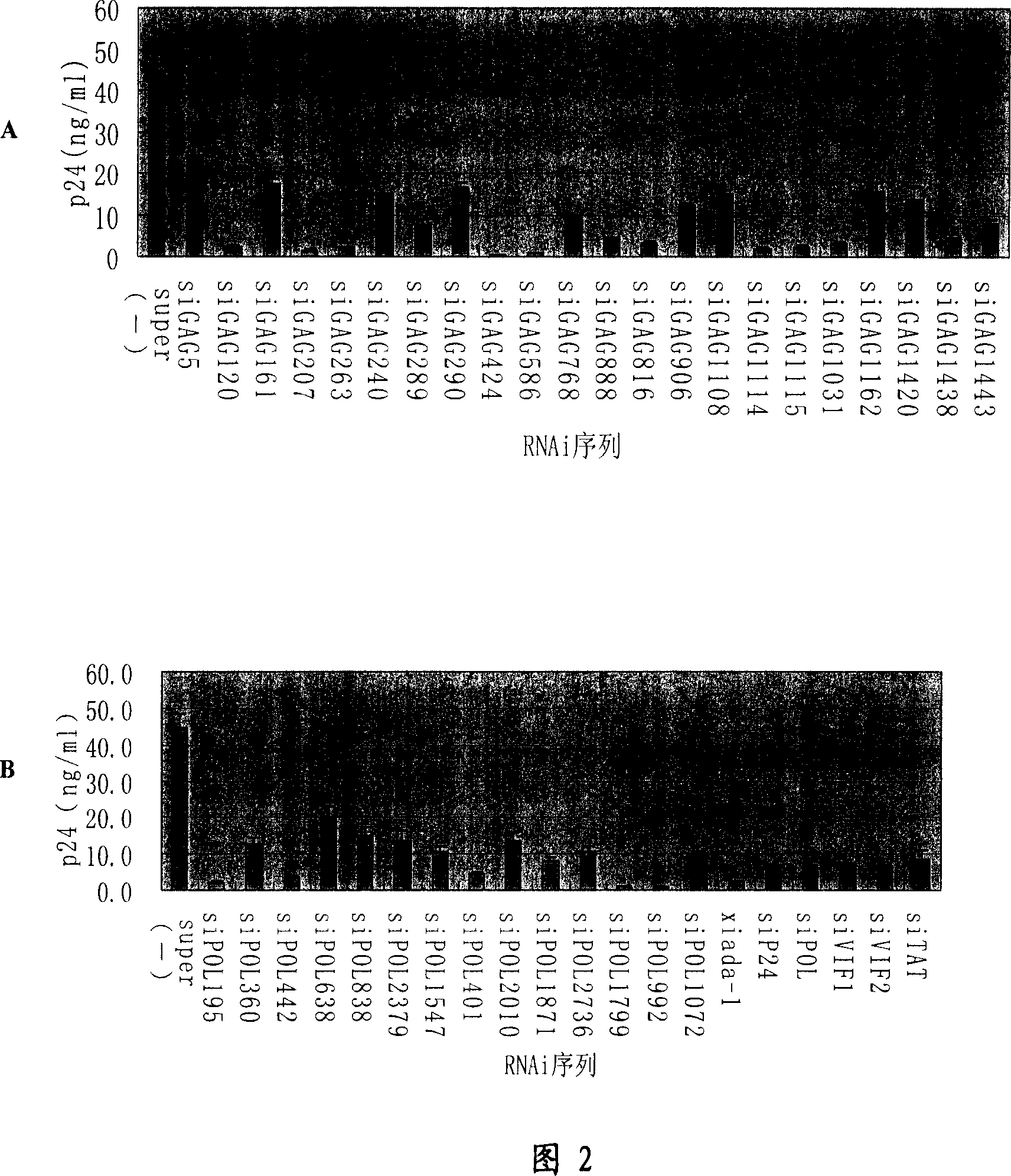
RNA interference target for treating aids
The present invention relates to RNA interference target sequence targeting HIV for the treatment of AIDS. Based on the target sequence, recombinant expression vectors, packaging vectors and cells were constructed, which express a siRNA and / or a miRNA and / or a ribozyme and / or an antisense oligonucleotide targeting HIV. Also provided is the use of the recombinant expression vectors, packaging vectors and recombinant cells in the manufacture of a medicament for the treatment of AIDS.
Owner:XIAMEN UNIV +1
Methods of treating HIV infection
Owner:VIIV HEALTHCARE UK (NO 5) LTD
Indole, azaindole and related heterocyclic 4-alkenyl piperidine amides
This invention provides compounds having drug and bio-affecting properties, their pharmaceutical compositions and method of use. In particular, the invention is concerned with new piperidine 4-alkenyl derivatives that possess unique antiviral activity. More particularly, the present invention relates to compounds useful for the treatment of HIV and AIDS. The compounds of the invention for the general Formula I: wherein: Z is Q is selected from the group consisting of: -W- is
Owner:VIIV HEALTHCARE UK (NO 5) LTD
Vaccine for prevention and treatment of HIV-infection
ActiveUS7612173B2Promote humoral and cellular responseAntibody mimetics/scaffoldsVirus peptidesImmunogenicityPolynucleotide
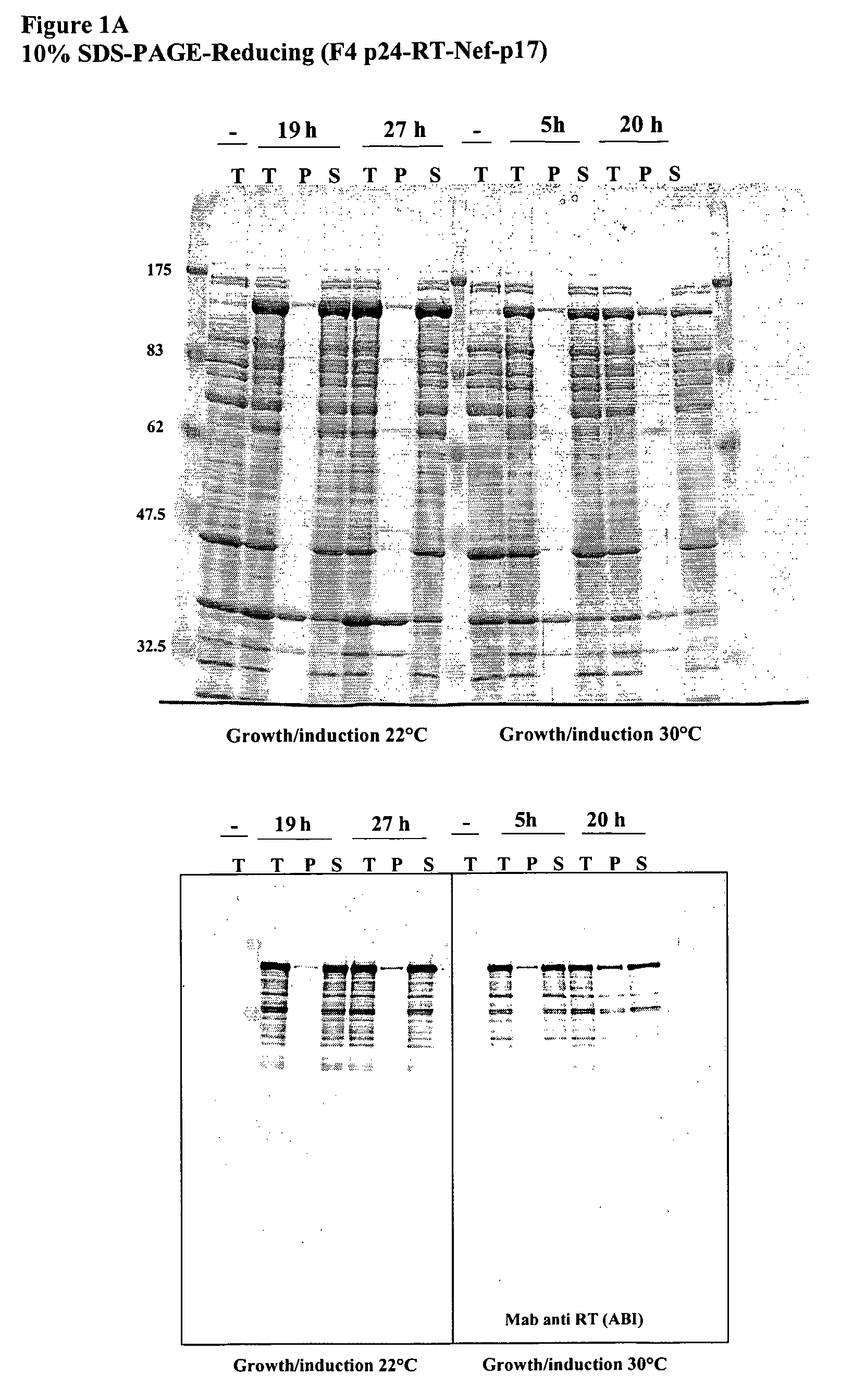
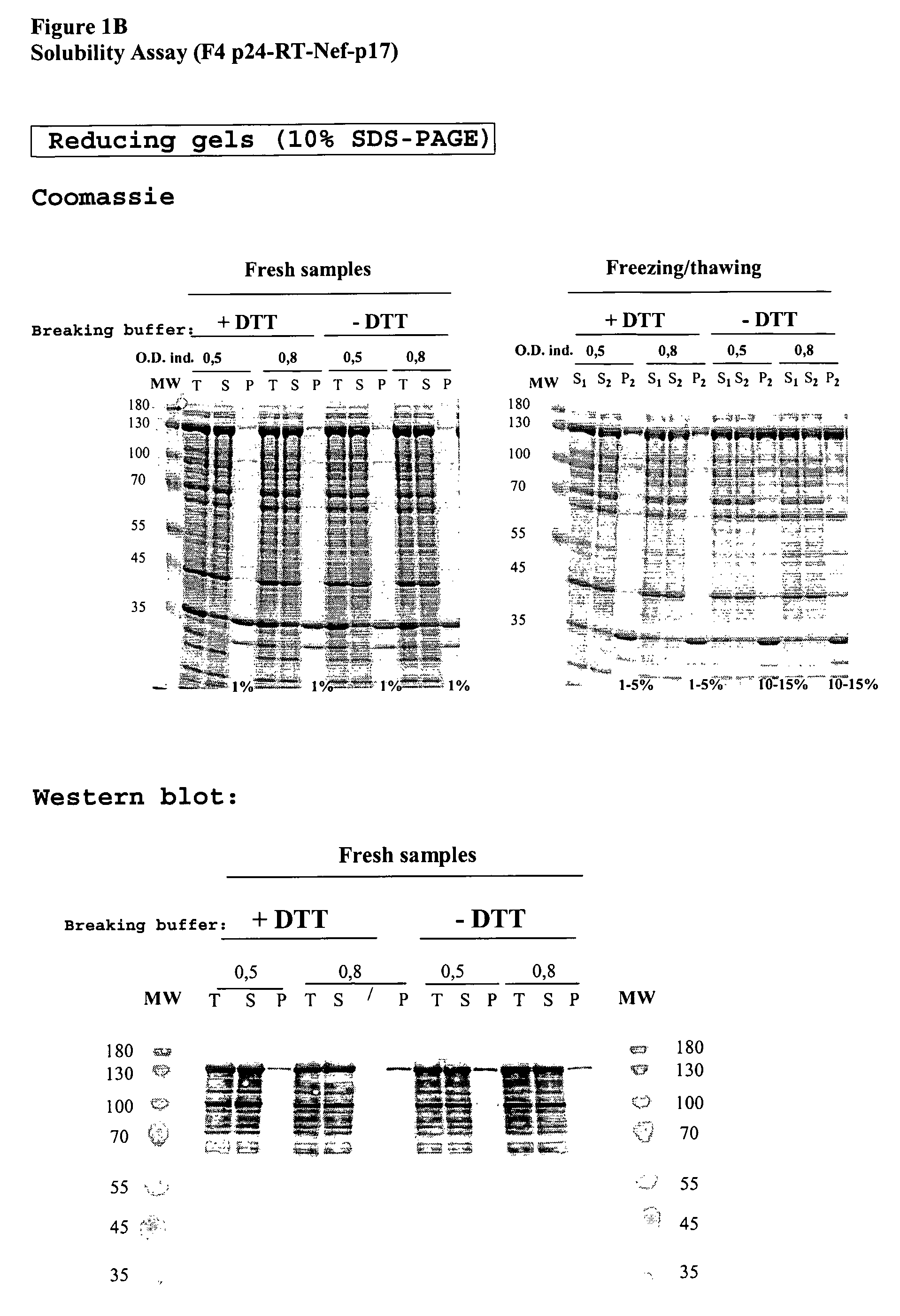
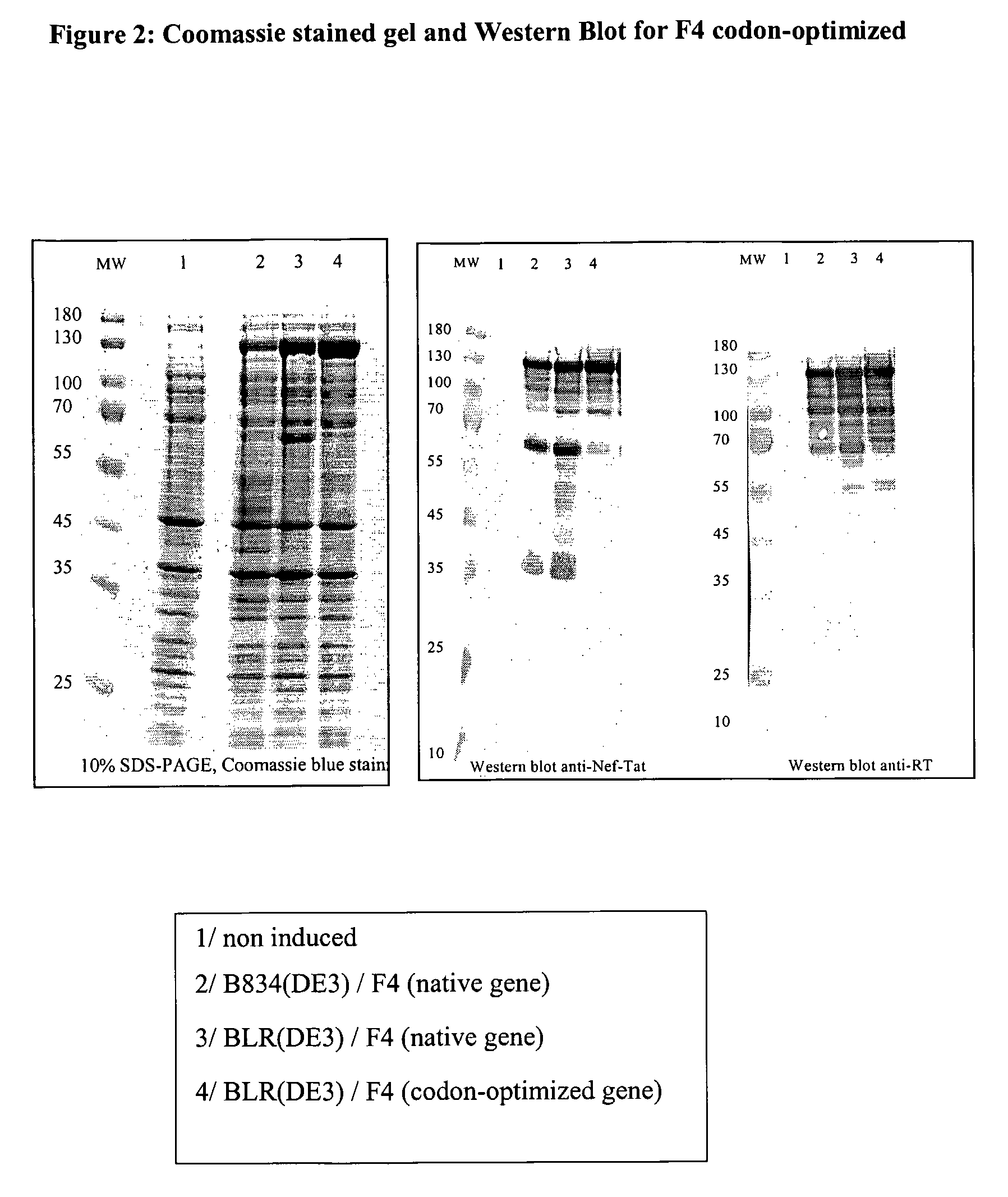
This invention relates to novel HIV polypeptide and polynucleotide fusions of Gag, Pol and Nef which are useful in immunogenic compositions and vaccines. The invention relates in particular to a polypeptide which comprises Nef or an immunogenic fragment thereof, and p17 Gag and / or p24 Gag or immunogenic fragments thereof, wherein when both p17 and p24 Gag are present there is at least one HIV antigen or immunogenic fragment between them. The polypeptide may also comprise Pol or RT or an immunogenic fragment thereof.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Compounds Useful in the Treatment of Hiv
There is provided inter alia use of 2′,3′-dideoxy-3′-hydroxymethylcytosine or a prodrug or salt thereof in the manufacture of a medicament for the treatment of HIV infection wherein the reverse transcriptase of the HIV bears at least one mutation that allows an obligate chain terminating nucleoside- or nucleotide phosphate to be excised from the nascent DNA strand by ATP- or pyrophosphate-mediated excision.
Owner:MEDIVIR AB
Herbal pharmaceutical composition for treatment of HIV/AIDS patients
Owner:WU TZU SHENG
Combination therapy comprising the use of protein kinase C modulators and Histone Deacetylase inhibitors for treating HIV-1 latency
InactiveUS20100166806A1Adverse propertyPrevent HIV-1-induced cytotoxicityBiocideOrganic chemistryReverse transcriptaseHydroxamic acid
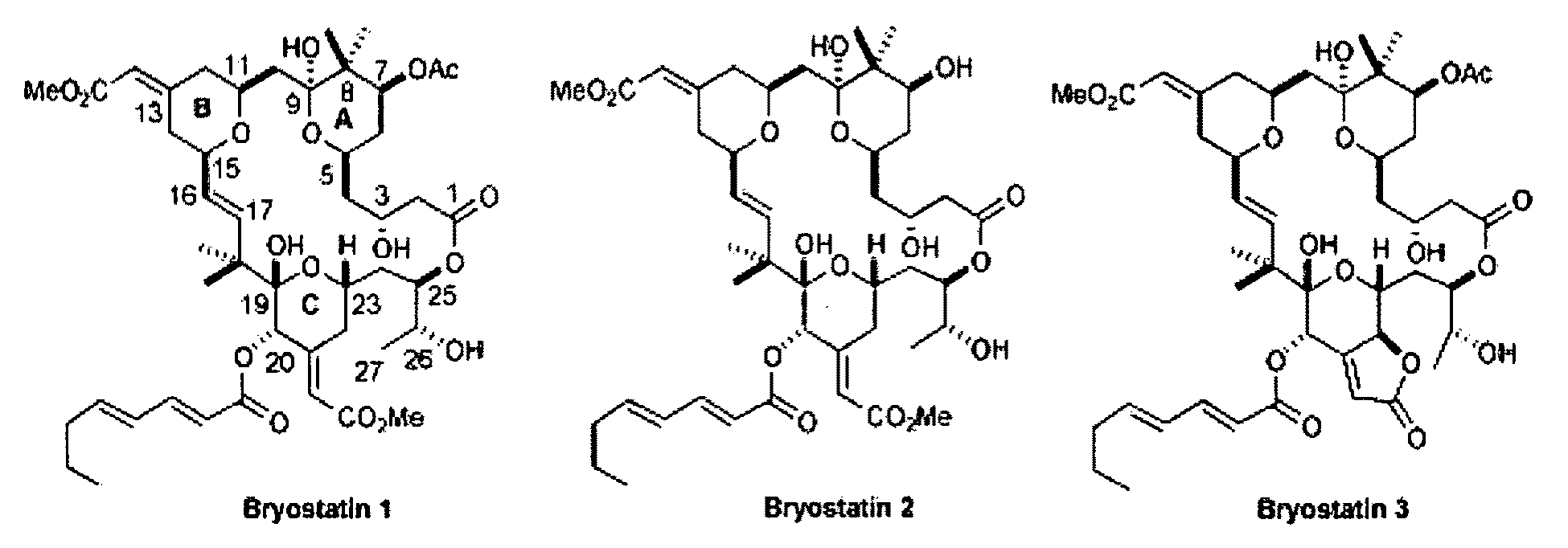
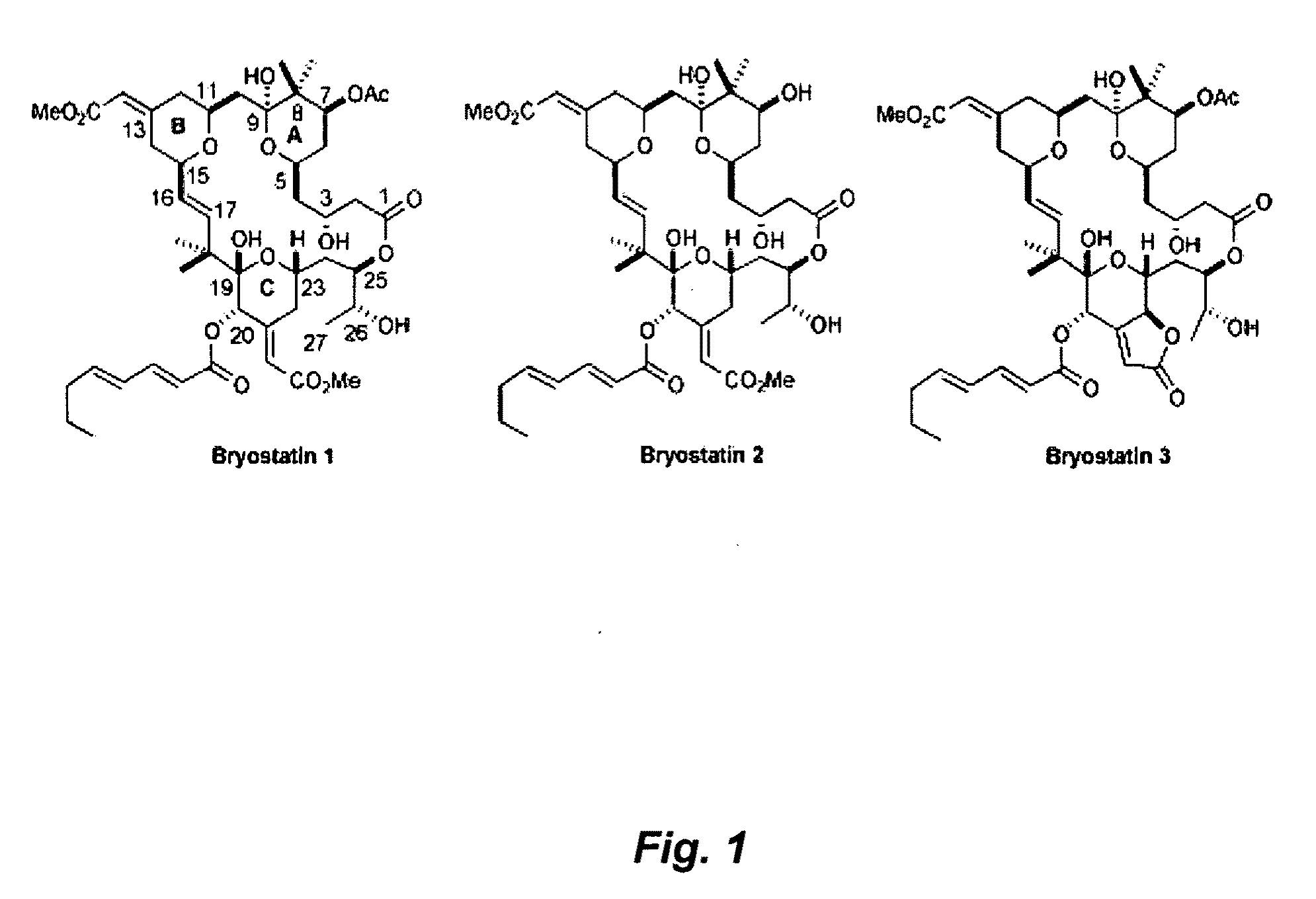
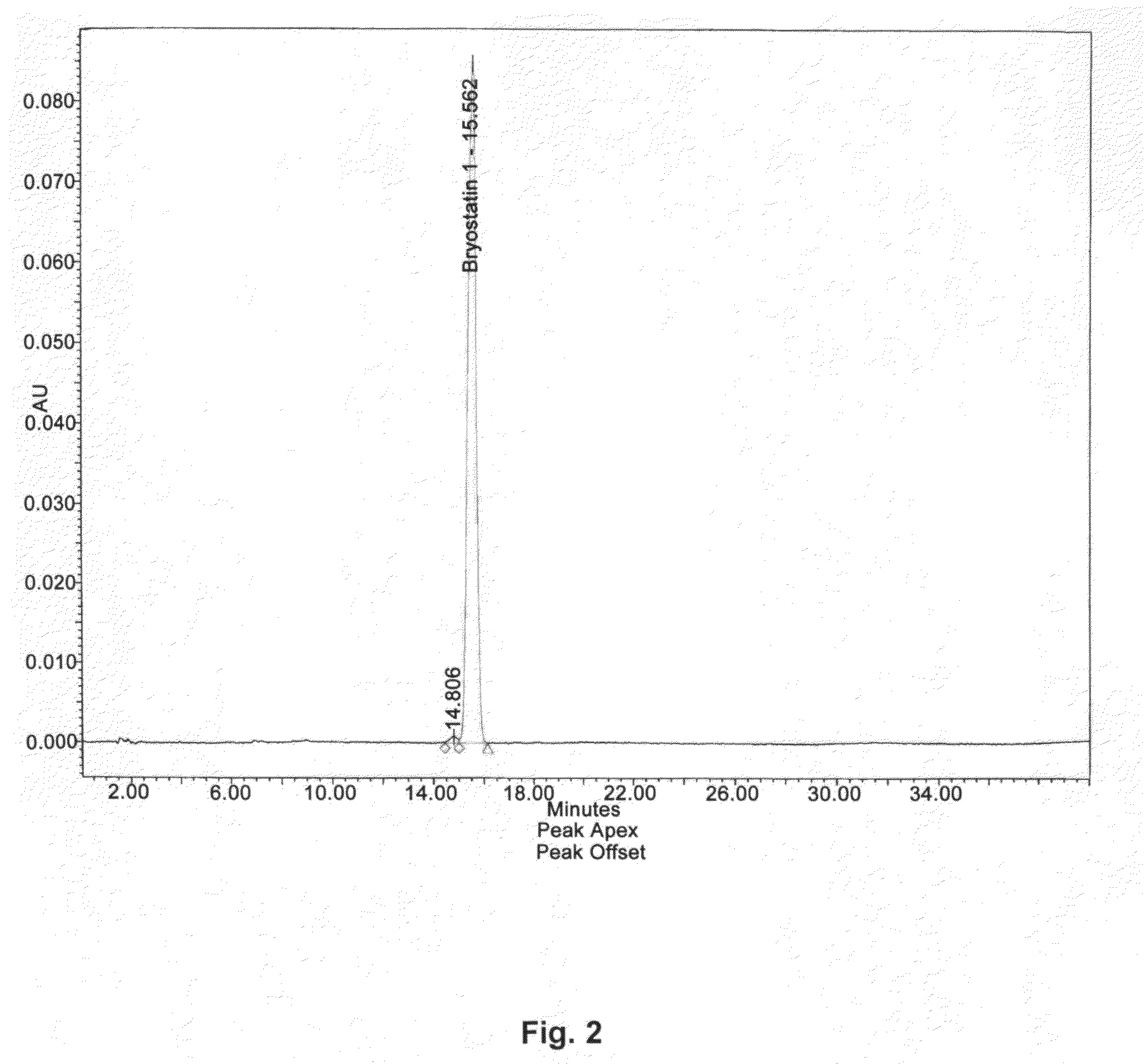
The invention relates to a combination of treatments, more particularly a combination treatment for HIV-1 infection. The present invention is directed to the use of bryostatin-1 and their natural and synthetic derivatives for AIDS therapy, in particular to the use of bryostatins in combination with other active drugs such as Histone Deacetylases (HDACs) inhibitors and anti-retrovirals, for the treatment of HIV-1 latency. According to the present invention, we provide a combination therapy for the treatment of HIV-1 latency which employs bryostatin-1 (and analogues) and one of the following HDAC inhibitors; valproic acid, butyrate derivatives, hydroxamic acids and benzamides. While HDACi can be used in continuous dosing protocol, bryostatins can be used following a cyclical dosing protocol. Bryostatins can be formulated in pharmaceutical acceptable carriers including nanoparticles, phospholipids nanosomes and / or biodegradable polymer nanospheres. This combination therapy needs to be used in patients treated with antiretroviral therapy (HIV-1 protease inhibitors, HIV-1 reverse transcriptase inhibitors, HIV-1 integrase inhibitors, CCR5 co-receptor inhibitors and fusion inhibitors).
Owner:APHIOS
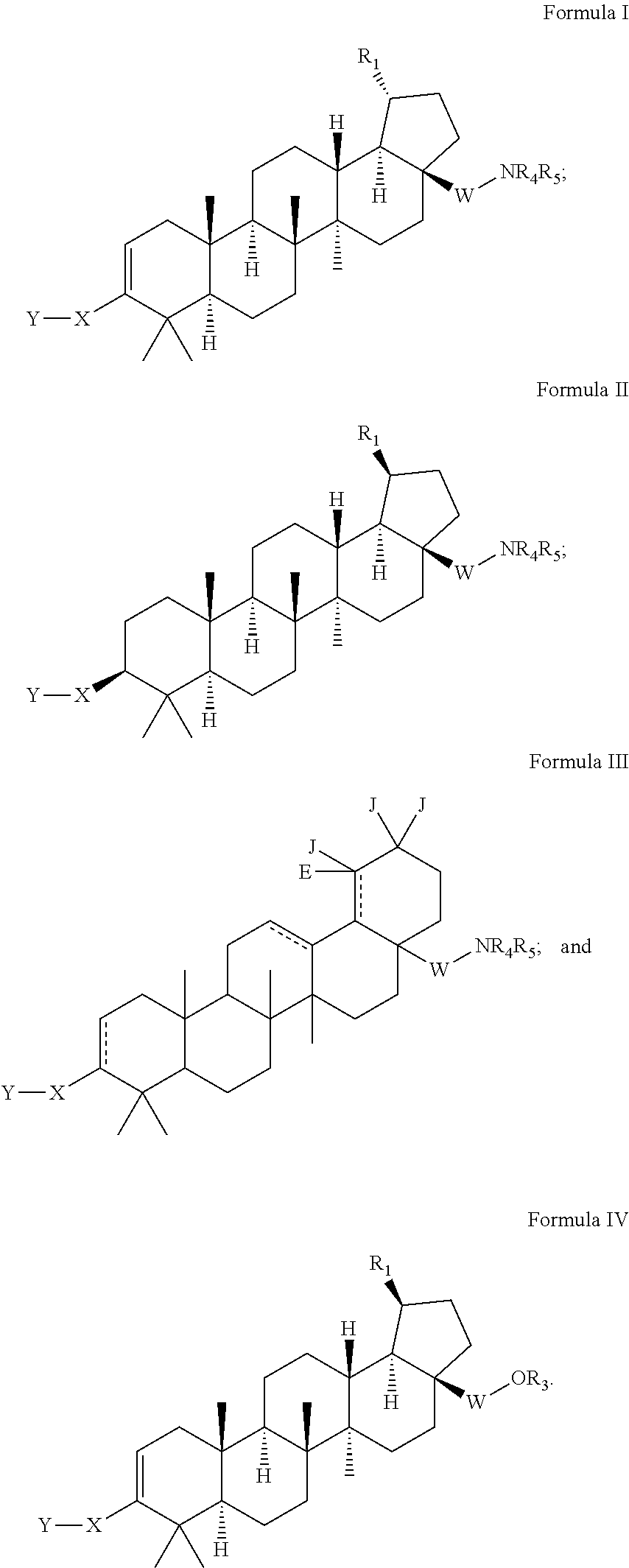
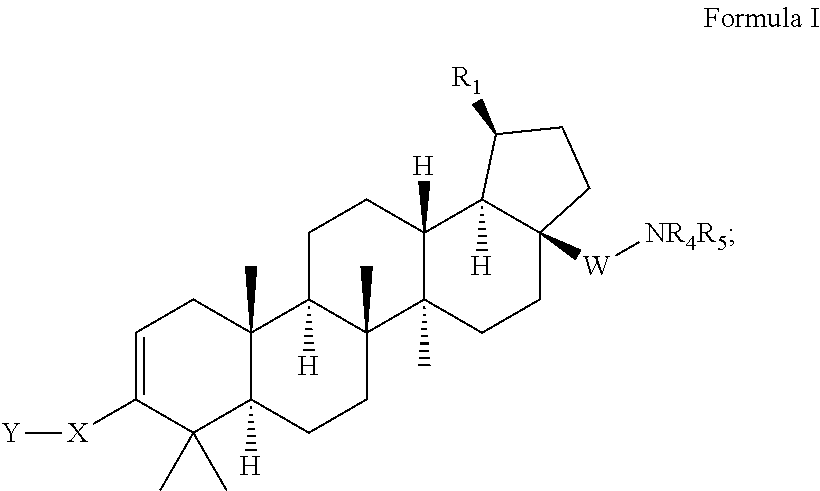
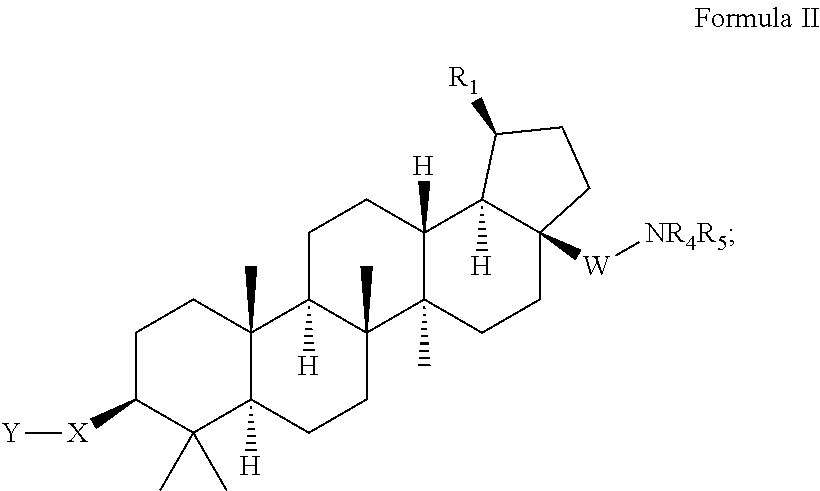
C-3 cycloalkenyl triterpenoids with HIV maturation inhibitory activity
Compounds having drug and bio-affecting properties, their pharmaceutical compositions and methods of use are set forth. In particular, C-3 cycloalkenyl triterpenoids that possess unique antiviral activity are provided as HIV maturation inhibitors, as represented by compounds of Formulas I, II, III and IV:wherein X can be a C4-8 cycloalkyl, C4-8 cycloalkenyl, C4-9 spirocycloalkyl, C4-9 spirocycloalkenyl, C4-8 oxacycloalkyl, C4-8 dioxacycloalkyl, C6-8 oxacycloalkenyl, C6-8 dioxacycloalkenyl, C6-9 oxaspirocycloalkyl, or C6-9 oxaspirocycloalkenyl ring. These compounds are useful for the treatment of HIV and AIDS.
Owner:VIIV HEALTHCARE UK (NO 5) LTD
Medicament for treating acquired immune deficiency syndrome (AIDS)
InactiveCN102210837ARelieve spasmsReduce the burden onAmphibian material medical ingredientsAnthropod material medical ingredientsPollenForsythia
The invention provides a completely oral medicament for treating acquired immune deficiency syndrome (AIDS), comprising the following components: hedyotis diffusa, honeysuckle, meadowrueleaf corydalis root, dandelion, weeping forsythia, asparagus, ophiopogon root, figwort root, fresh rehmannia root, peach kernel, chuanxiong rhizome, zedoary, achyranthes root, dahurian patrinia herb, giant knotweed, stiff silkworm, isatis root, white peony root, polygonatum, bitter caramon, mistletoe, tortoise plastron, glossy privet fruit, oyster, pollen, salvia miltiorrhiza, tree peony bark, bupleurum, bitter orange, cimicifuga foetida, dried orange peel, amomum fruit, officinal magnolia bark, rose, songaria cynomorium herb, self-heal, medicine terminalia fruit, gromwell, licorice, chrysanthemum, castor bean, bullfrog gallbladder, baikal skullcap root, medicated leaven, malt, rice sprout, chicken's gizzard-skin, radish seed, scorch-fried crataegus, Chinese yam, astragalus root, bighead atractylodes rhizome, sealwort, dangshen, prepared rehmannia root, cornus fruit, tuckahoe, jujube, Chinese wolfberry, cistanche, dragon bone, oyster, epimedium, curculigo root, deerhorn glue, ginseng, donkey-hide glue, white fungus, angelica, spatholobus stem and turtle shell. The medicament provided by the invention not only can cure AIDS and has good therapeutic effect on AIDS, but also can prevent infection of AIDS.
Owner:肖嘉惠
CAR-T cell for treating AIDS-associated lymphoma, and preparation method and application thereof
PendingCN107904259ALow storm riskSmall storm riskGenetically modified cellsAntiviralsAIDS-related lymphomaCord blood stem cell
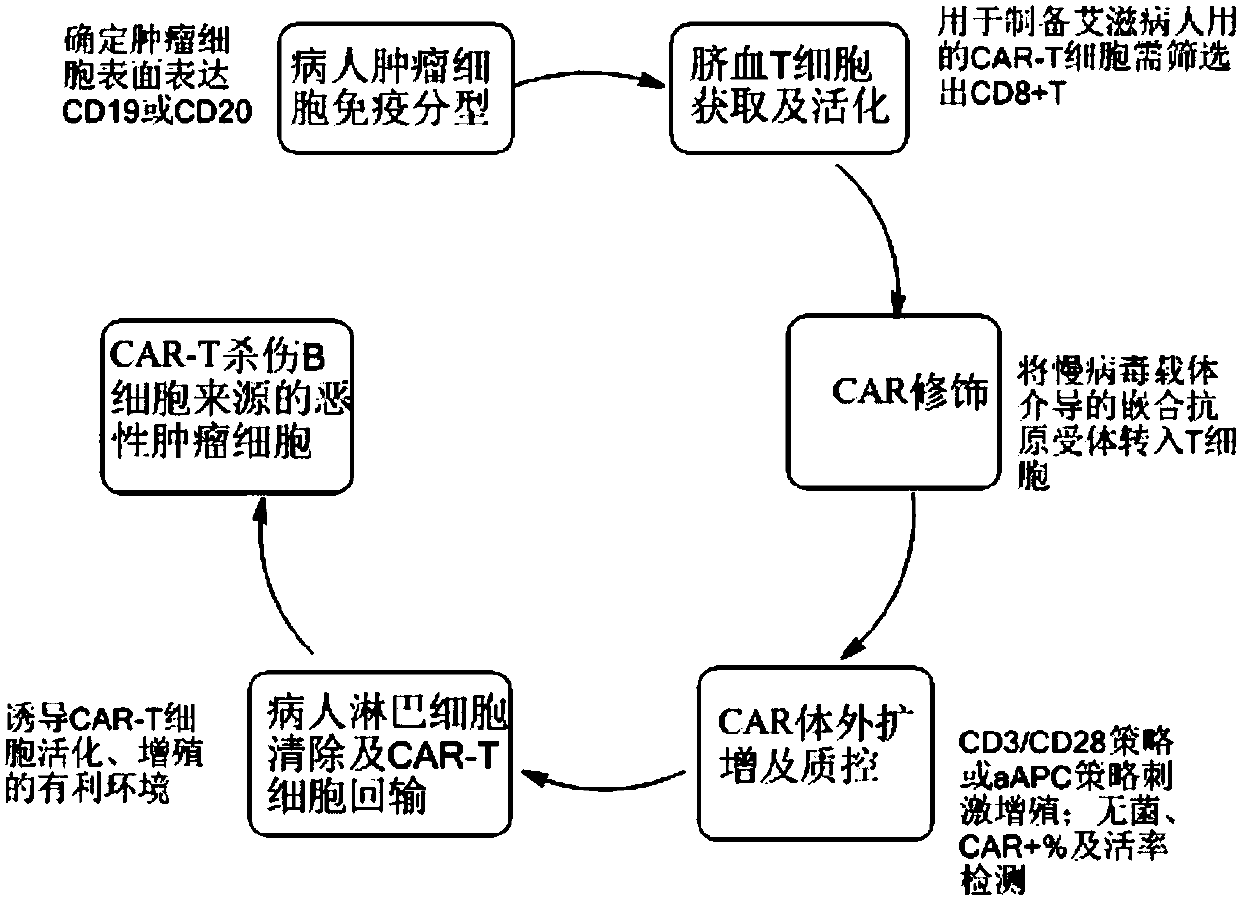
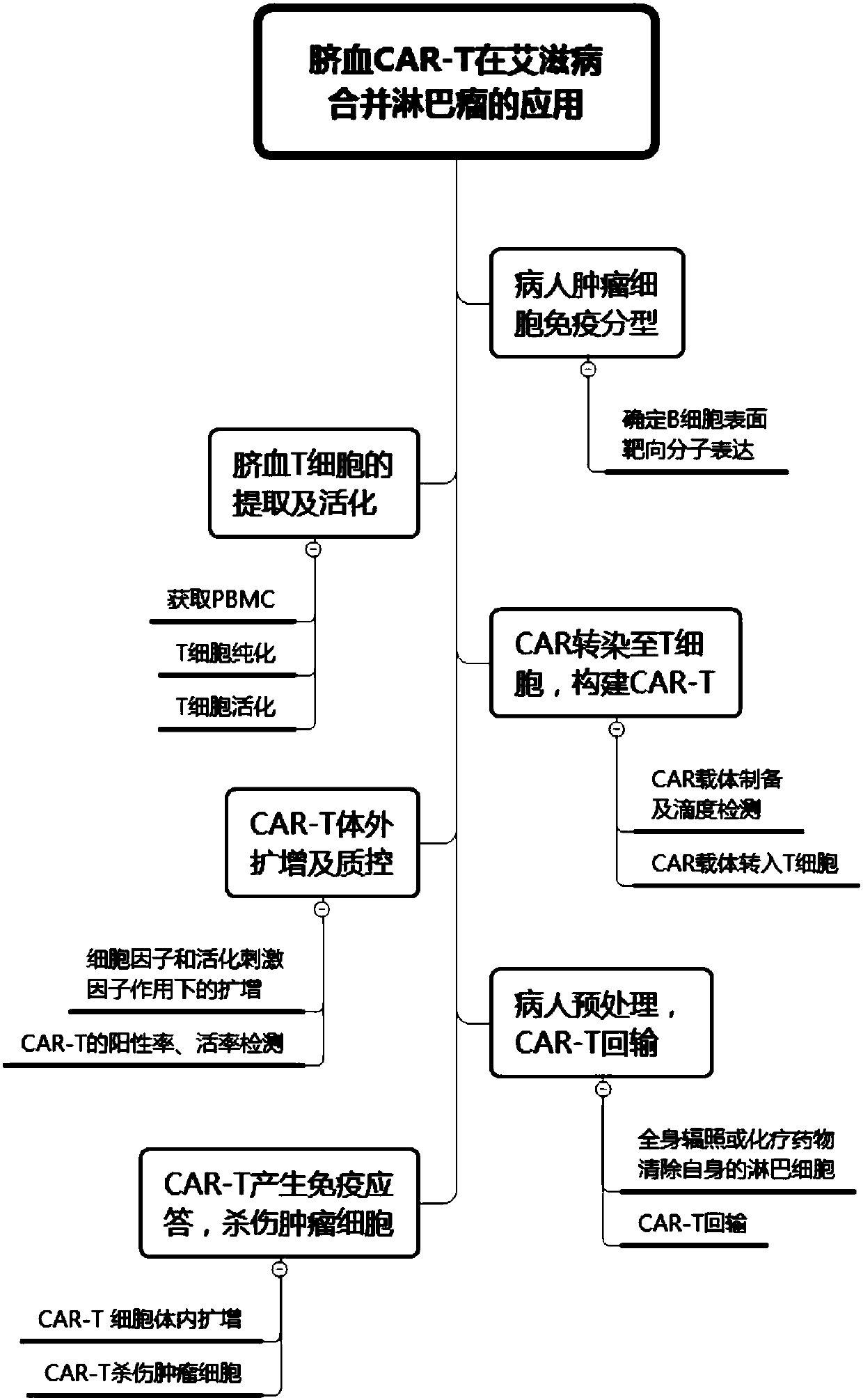
The invention discloses a preparation method of a CAR-T cell for treating AIDS-related lymphoma. A CD8+ T cell is used to produce the CAR-T cell, and the CD8-T cell derives from a cord blood T cell. The method includes the following steps: preparing cord blood mononuclear cells from cord blood, removing tumors and other cells by using a human T cell purification kit to obtain T cells, and carryingout separation by using magnetic beads to obtain the CD8+ T cell; activating the CD8+ T cell by using an appropriate medium and appropriate stimulation conditions; transferring CAR to the CD8+ T cellby using lentivirus to prepare the CAR-T cell; and amplifying the CAR-T cell in vitro by using cytokines and activating stimulators to achieve the desired effective dose. The invention also relates to the CAR-T cell prepared by the method, and an application thereof. The CAR-T cell prepared in the invention has a good cell activity and a high proliferation speed, and reduces the attack risk of GVHD; and only the CD8+T cell of the umbilical blood T cells of AIDS patients is used to prepare the CAR-T cell for, so the risk of input CAR-T infected with in-vivo HIV is reduced.
Owner:SHANGHAI LONGYAO BIOTECH CO LTD
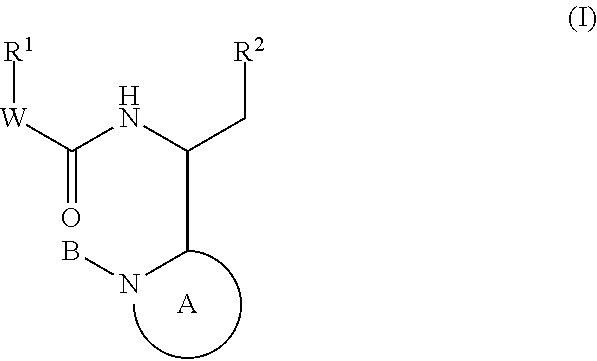
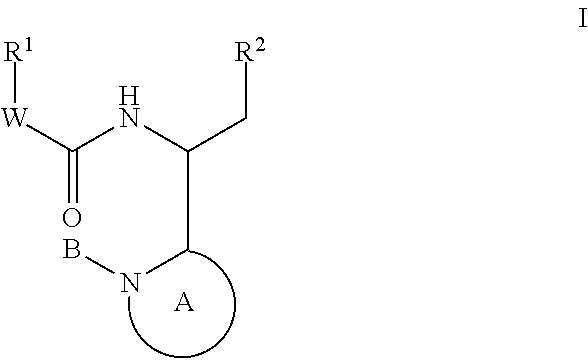
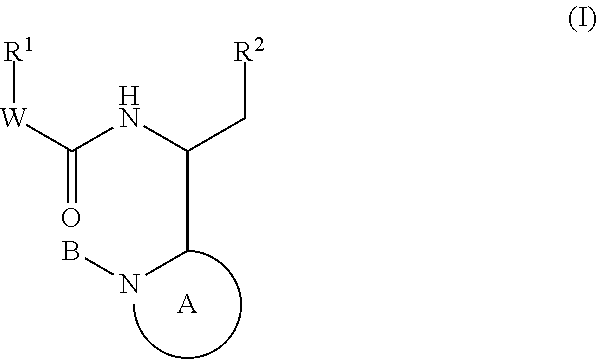
Compounds for the treatment of HIV
The invention provides compounds of formula (I): or a salt thereof as described herein. The invention also provides pharmaceutical compositions comprising a compound of formula (I), processes for preparing compounds of formula (I), intermediates useful for preparing compounds of formula I and therapeutic methods for treating a Retroviridae viral infection including an infection caused by the HIV virus.
Owner:GILEAD SCI INC
Combined therapy for treatment of HIV infection
InactiveUS7094413B2Good curative effectReduce resistanceBiocideSugar derivativesImmunodeficiency virusGastrointestinal complications
The present invention relates to pharmaceutical preparations and methods for treating individuals infected with the human immunodeficiency virus (HIV). The pharmaceutical preparations comprise an immunomodulating agent and a anti-retroviral compound. The pharmaceutical preparations are used to treat HIV infected patients, particularly for gastrointestinal complications arising from viral infection. In addition, the pharmaceutical preparations of the present invention have the effect of raising the levels of CD4+ single positive and CD4+ and CD8+ double positive T cells, thus promoting restoration and normalization of the immune system following HIV infection.
Owner:SANGSTAT MEDICAL +1
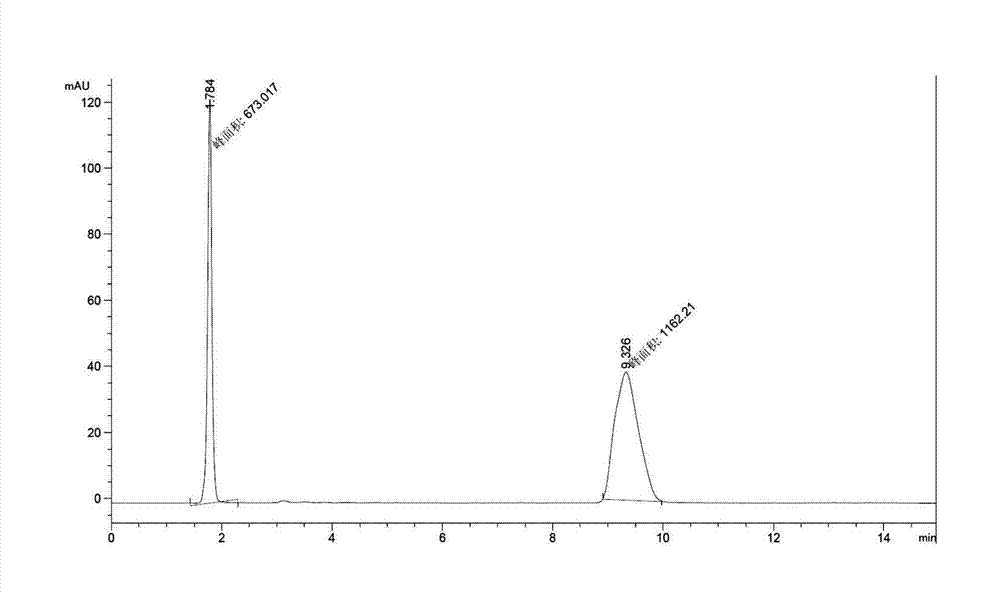
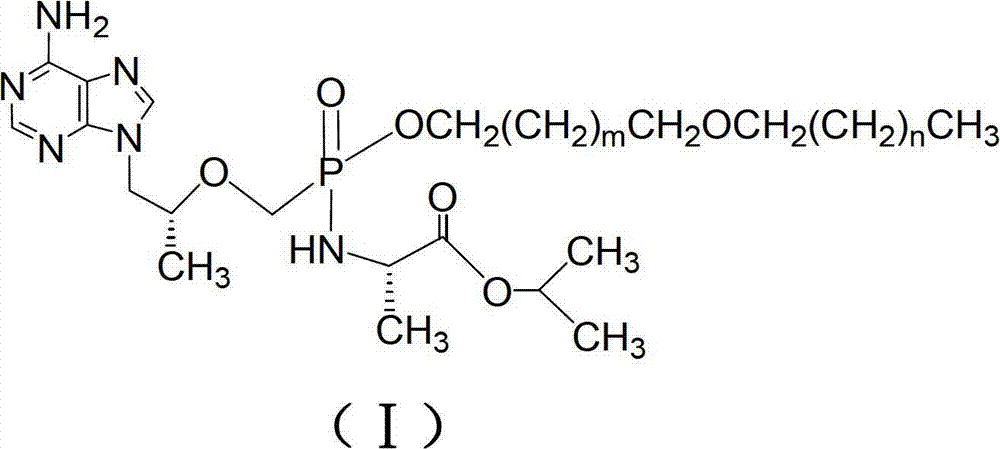
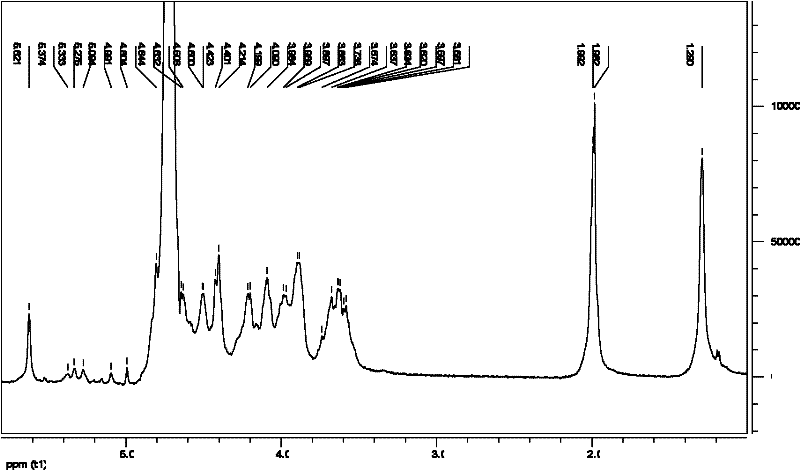
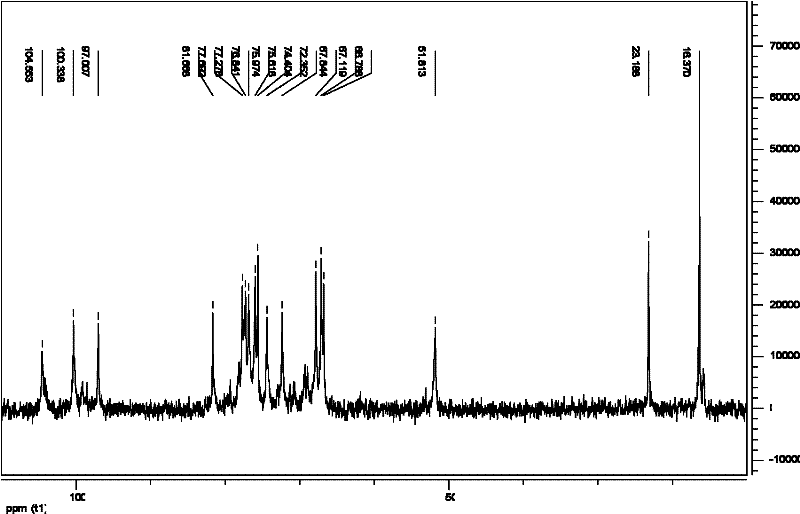
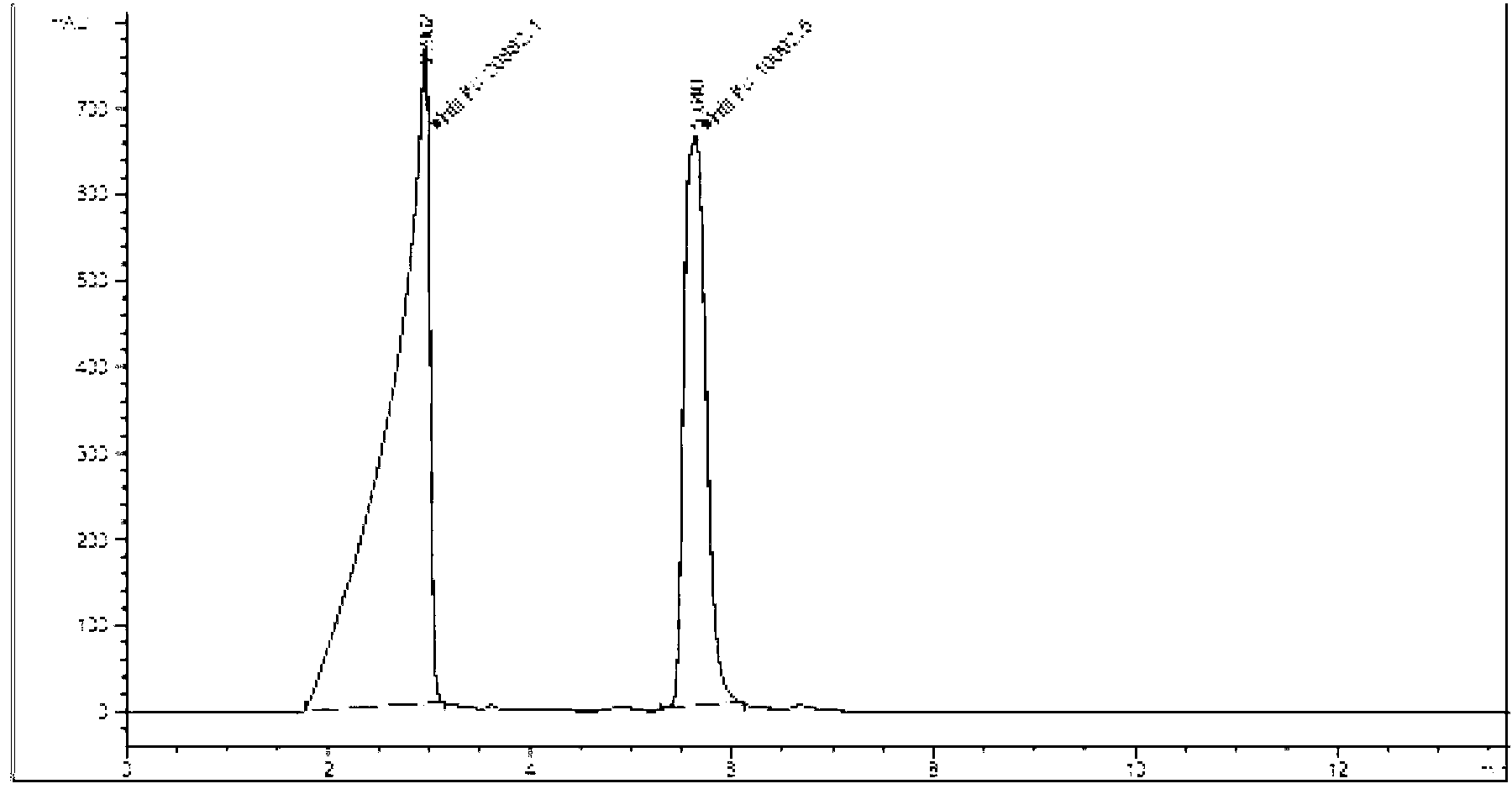
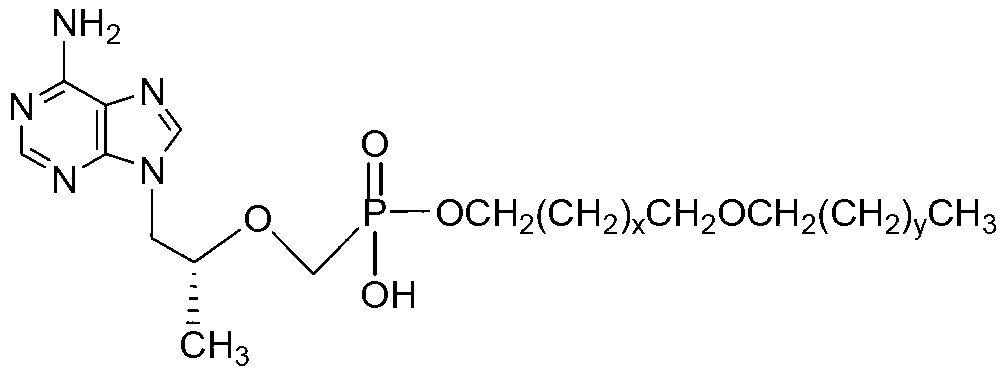
Tenofovir diester compounds with activity of inhibiting HIV-1 (human immunodeficiency virus-1) virus replication and preparation method and pharmaceutical use thereof
ActiveCN102786549AHigh activityLow cytotoxicityOrganic active ingredientsGroup 5/15 element organic compoundsTherapy HIVHuman immunodeficiency virus 1
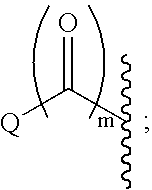
The invention discloses tenofovir diester compounds with activity of inhibiting HIV-1 (human immunodeficiency virus-1) virus replication and a preparation method and pharmaceutical use thereof. The structure of the compounds is shown in Formula (I), wherein m is 0-4, n is 12-16, and the structural formula is fixed when m is 1 and n is 14. The invention also discloses a preparation method of the compounds shown in the structural formula (I) and a pharmaceutical composition with the compounds. Tests show that the compounds have the activity of inhibiting HIV-1 replication and also much higher lipophilicity than the current HIV treatment drug tenofovir fumarate, and can be applied in development of drugs for treatment of HIV infection.
Owner:洛阳聚慧新材料科技有限公司 +2
Pramipexole for the treatment of HIV dementia
InactiveUS20030166696A1Reduce formationReduce in quantityBiocideOrganic active ingredientsPramipexoleTherapy HIV
The invention relates to the use of pramipexole and the pharmacologically acceptable acid addition salts thereof as well as hydrates and solvates thereof, for preparing a pharmaceutical composition for the prevention and / or treatment of HIV encephalopathy.
Owner:BOEHRINGER INGELHEIM PHARM KG
Compounds for the treatment of HIV
The invention provides compounds of formula (I): or a salt thereof as described herein. The invention also provides pharmaceutical compositions comprising a compound of formula (I), processes for preparing compounds of formula (I), intermediates useful for preparing compounds of formula I and therapeutic methods for treating a Retroviridae viral infection including an infection caused by the HIV virus.
Owner:GILEAD SCI INC
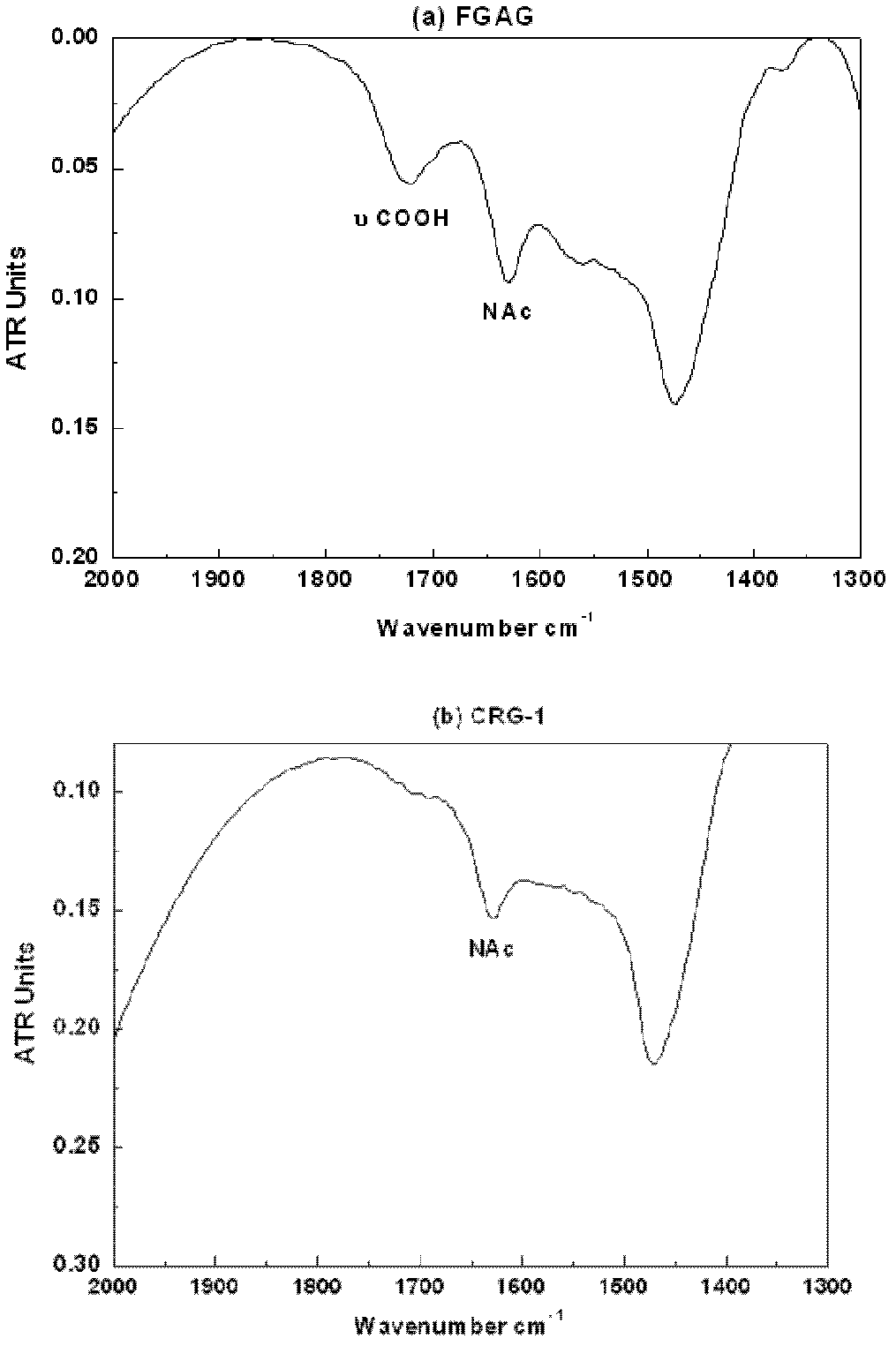
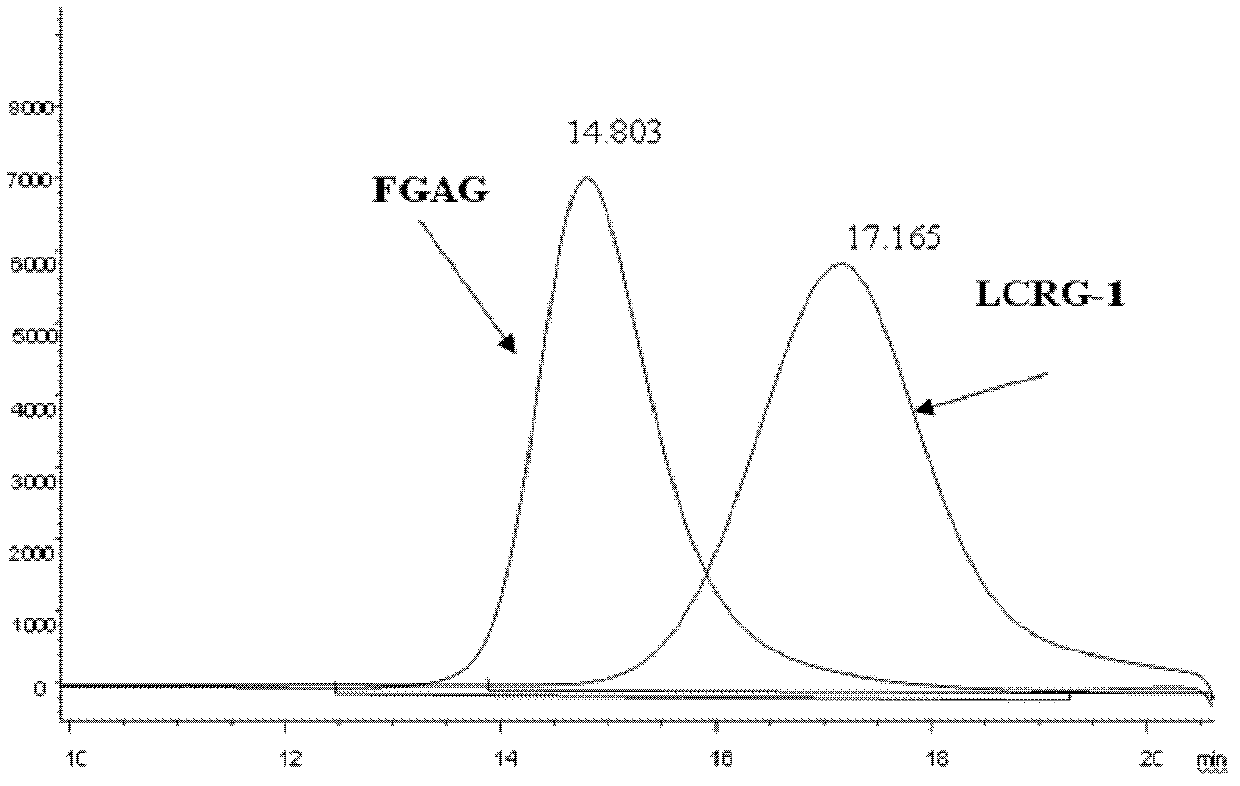
Low molecular weight carboxyl-reduced derivatives of fucosylated glycosaminoglycans and preparation method and applications of low molecular weight carboxyl-reduced derivatives
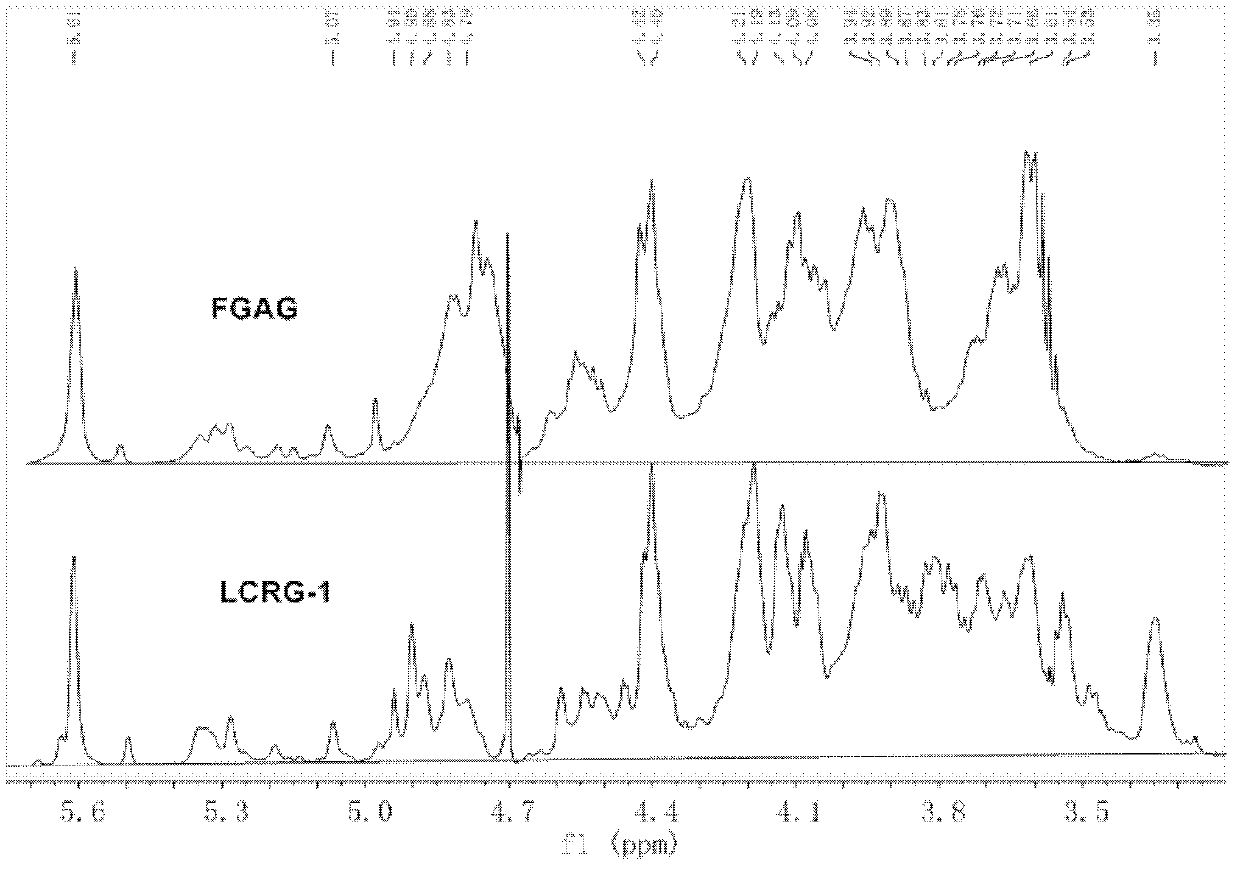
The invention discloses low molecular weight carboxyl-reduced derivatives of fucosylated glycosaminoglycans (LCRG). The extent of carboxyl reduction is not less than 20%. Weight-average molecular weight of the LCRG is about 3000-20000Da, and monosaccharides comprise acetyl galactosamine (GalNAc), glucose (Glc) or glucuronic acid (GlcUA) and fucose (Fuc) or sulfates of fucose (shown as -OSO3-). The mole ratio of GalNAc, Glc (containing GlcUA), Fuc and -OSO3- is about 1: (1+-0.3): (1+-0.3): (3.0+-1.0). The LCRG is a potent human immunodeficiency virus (HIV) Type 1 entry inhibitor which acts on conserved regions and has the advantages of high activity against HIV Type 1, high therapeutic index and no-drug-resistance, and the LCRG can be used for preventing or curing HIV. The invention further provides a preparation method of the LCRG. The carboxyl-reduced derivatives of fucosylated glycosaminoglycans and medicinal compositions of the carboxyl-reduced derivatives can be prepared into injection agents, lyophilized powder or suppository and the like.
Owner:KUNMING INST OF BOTANY - CHINESE ACAD OF SCI +1
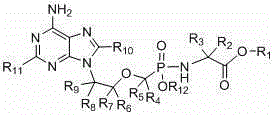
Preparation of non-cyclic nucleotide phosphoamides and salts thereof and application of non-cyclic nucleotide phosphoamides and salts thereof in aspect of antivirus
ActiveCN105669751AIntellectual property enhancementReduce exposureOrganic active ingredientsGroup 5/15 element organic compoundsViral infectious diseaseSolvent
The invention belongs to the field of antivirus in medical chemistry, and relates to preparation of non-cyclic nucleotide phosphoamides and salts thereof and application of the non-cyclic nucleotide phosphoamides and salts thereof in treating human immunodeficiency virus (HIV), hepatitis B, hepatitis B virus (HBV) and other virus infection diseases. The non-cyclic nucleotide phosphoamides, and isomers, pharmaceutically acceptable salts, hydrates, solvates or crystals thereof have the general formula I of which the structure is disclosed in the specification. The invention also provides a pharmaceutical composition containing the compounds and isomers, pharmaceutically acceptable salts, hydrates, solvates or crystals thereof, and application of the compounds or composition in treating and / or preventing HIV and HBV infection. The compounds provided by the invention have the advantages of favorable anti-HIV and HBV activities, low oral dosage and low toxicity.
Owner:洛阳聚慧新材料科技有限公司
Recombination expression carrier for treating Aids, reformed blood building stem cell and method
InactiveCN1948475AMammal material medical ingredientsImmunological disordersTherapy HIVCancer research
This invention involves a new treatment for AIDS recombinant expression vector, a modified cells (especially hematopoietic stem cells), and methods. The invention provides recombinant expression vector and modified cells (especially hematopoietic stem cells), its expression: (1) MGMT (P140K) gene; and (2) various (especially targeting HIV) siRNA and / or miRNAs and / or ribozymes, and / or anti - oligonucleotides. The invention also provides treatment of HIV infection, including (1) Preparation of the cell of this invention, then transplant to their patients with HIV infection; and (2) BG / BCNU appropriate treatment.
Owner:XIAMEN UNIV
Low molecular weight glycosylated chondroitin sulfate and its purpose in preparation of anti-HIV-1 medicament
InactiveCN102247401AAvoid drug resistanceDepolymerization speed is stableOrganic active ingredientsAntiviralsMonosaccharide compositionUltrafiltration
The invention discloses a low molecular weight glycosylated chondroitin sulfate, whose weight average molecular weight is 3000-15000Da. The monosaccharide composition comprises acetyl galactosamine (D-GalNAc), glucuronic acid (D-GlcUA), fucose (L-Fuc), or its sulfuric ester (expressed in -OS03<->), wherein the mole ratio of D-GalNAc to D-GlcUA to L-Fuc to -OS03<-> is 1: (1+ / -0.3): (1+ / -0.3): (3.5+ / -0.5). The low molecular weight glycosylated chondroitin sulfate has a strong anti-HIV-1 virus activity, is a gp120 entry inhibitor, and can be used for preventing and / or treating AIDS. The invention also provides a method for preparing the low molecular weight glycosylated chondroitin sulfate and its composition preparation. Glycosylated chondroitin sulfate is depolymerized by the peroxide method to obtain a low molecular weight product, and then low molecular and / or high-molecular impurities of the product are removed by gel separation or ultrafiltration method. The low molecular weight glycosylated chondroitin sulfate and its medicinal composition can be prepared in the form of an injection, a lyophilized powder or a suppository.
Owner:KUNMING INST OF BOTANY - CHINESE ACAD OF SCI +1
Tenofovir disoproxil compounds, and preparation method and application thereof in anti-virus aspects
ActiveCN103224530AInhibition of replication activityImprove attributesOrganic active ingredientsGroup 5/15 element organic compoundsSolubilityAnti virus
The invention discloses a group of tenofovir disoproxil compounds with activity for inhibiting HIV-1 / HBV virus replication and pharmaceutically acceptable salts thereof, and a preparation method and pharmaceutical applications thereof. The group of the compounds have a general formula I, wherein X=H, Y=H, R1=-CH2(CH2)mCH2O(CH2)nCH3, m=0-4, n=10-20, and R2, R3 and R4 are respectively described in the specification. The invention also discloses a pharmaceutical composition containing the group of the compounds. Experiments show that one of the compounds has the advantages that an activity for inhibiting HIV-1 virus replication is 20 times that of a positive control medicine zidovudine (AZT), 1,000 times that of TDF that is the best medicine for treating Aids and about 9 times that of CMX157 in a clinical stage, and lipid solubility is about 2 times that of CMX157. Experiments also show that the compounds provided by the invention have the activity for inhibiting HBV virus replication, and can be used for development of drugs for treating the Aids and hepatitis B.
Owner:洛阳聚慧新材料科技有限公司 +2
Medicine for curing AIDS
The present invention discloses a medicine for resisting virus, raising immunity of human body and curing AIDS. Said medicine is a pure Chinese medicine preparation, and is made up by using 37 Chinese medicinal materials of licorice, radix trichosanthis, salvia root, dandelion herb, scutellaria root, coptis, phellodendron bark and others.
Owner:张在春
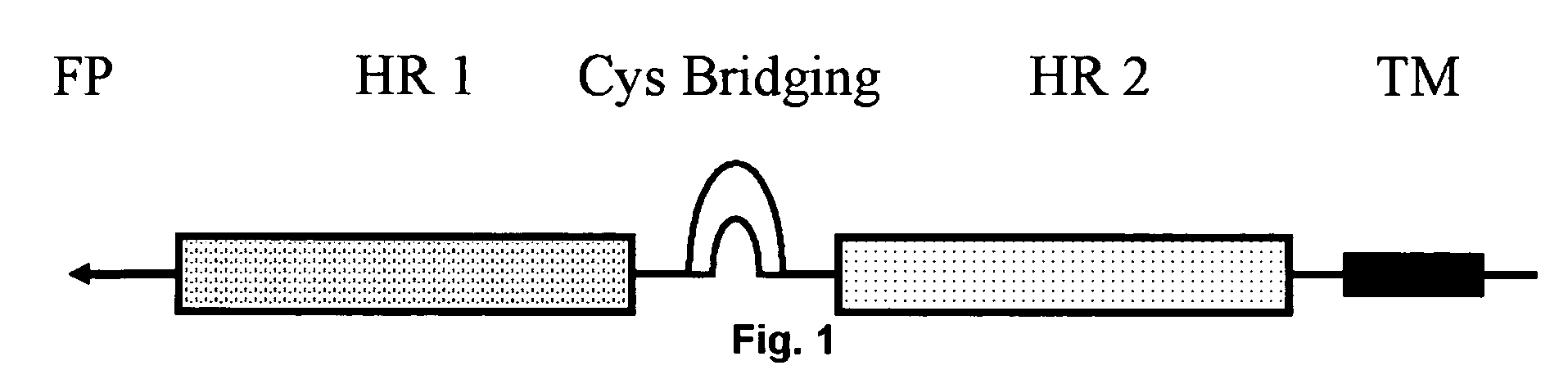
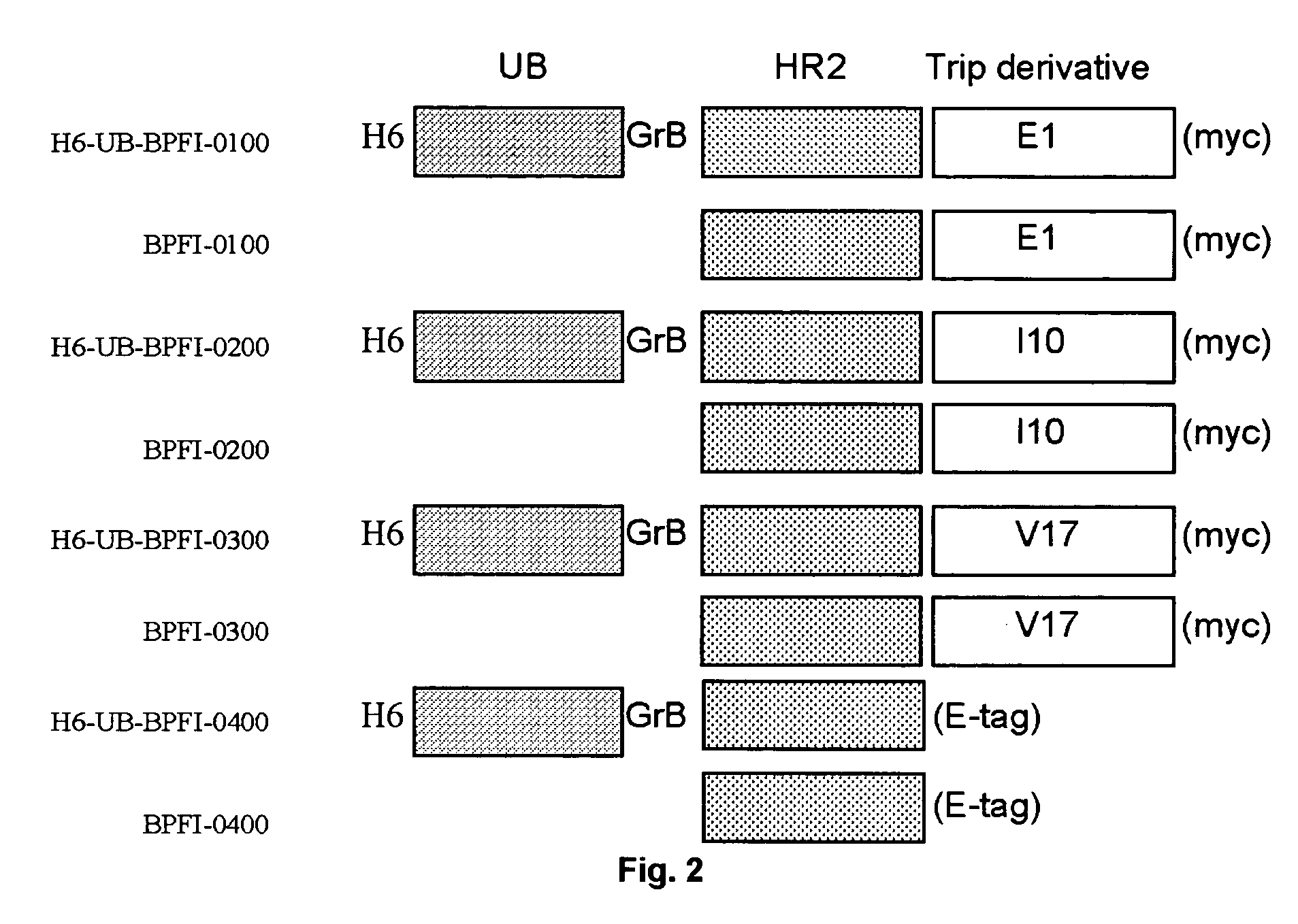
Multimerised HIV fusion inhibitors
InactiveUS20050202043A1Improve isolationHigh protein yieldPeptide/protein ingredientsAntibody mimetics/scaffoldsImmunodeficiency virusTherapy HIV
There are provided multimeric fusion proteins exhibiting anti-viral activity. The fusion proteins comprise the HR2 region of the ectodomain of the human immunodeficiency virus gp41 protein which is fused to a multimerisation domain peptide such as a trimerisation domain derived from tetranectin. The multimerised fusion proteins may be used as HIV fusion inhibitors in the treatment of AIDS.
Owner:BOREAN PHARMA APS
Compounded medicine for anti pathogeny and immunoenhancement
InactiveCN1539441AImprove autoimmunityControl intrusionAntiviralsUnknown materialsDiseaseTherapy HIV
A Chinese medicine in the form of decoction, tincture, powder, capsule, or pill for softening blood vessel, improving immunity and treating the diseases caused by virus, chlamydia, or mycoplasma, such as AIDS and SARS is disclosed. Its advantages are high curative effect, broad spectrum and no toxic by-effect.
Owner:黄益新
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com