Low molecular weight carboxyl-reduced derivatives of fucosylated glycosaminoglycans and preparation method and applications of low molecular weight carboxyl-reduced derivatives
A glycated sugar amine, low molecular weight technology, applied in the field of medicine, can solve the problems of non-response to treatment, no low molecular weight derivatives of FGAG carboxyl group, toxic and side effects, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0073] [Example 1] Carboxyl Reduction of Fucosylated Glycosaminoglycans
[0074] 1.1 Materials
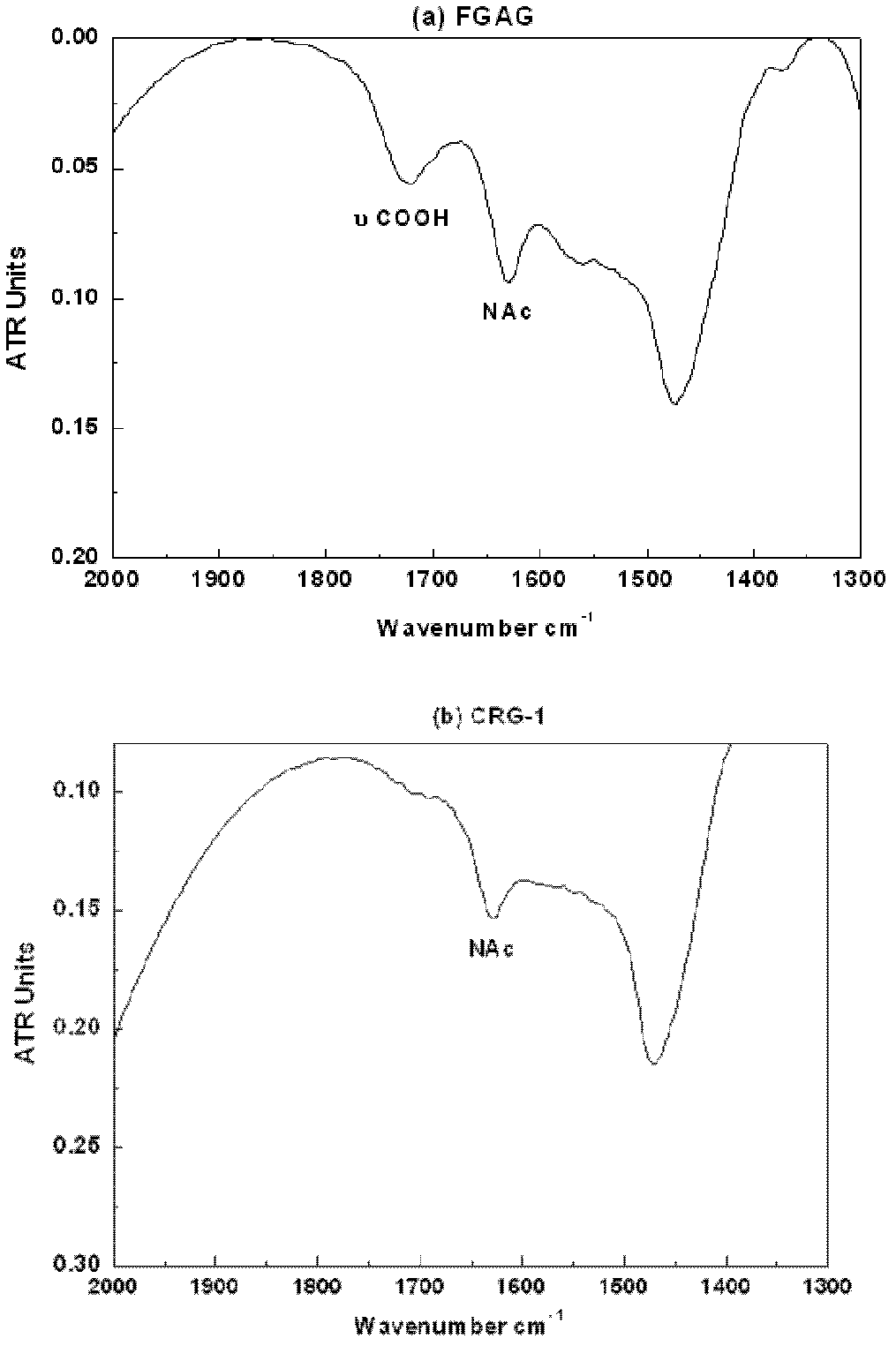
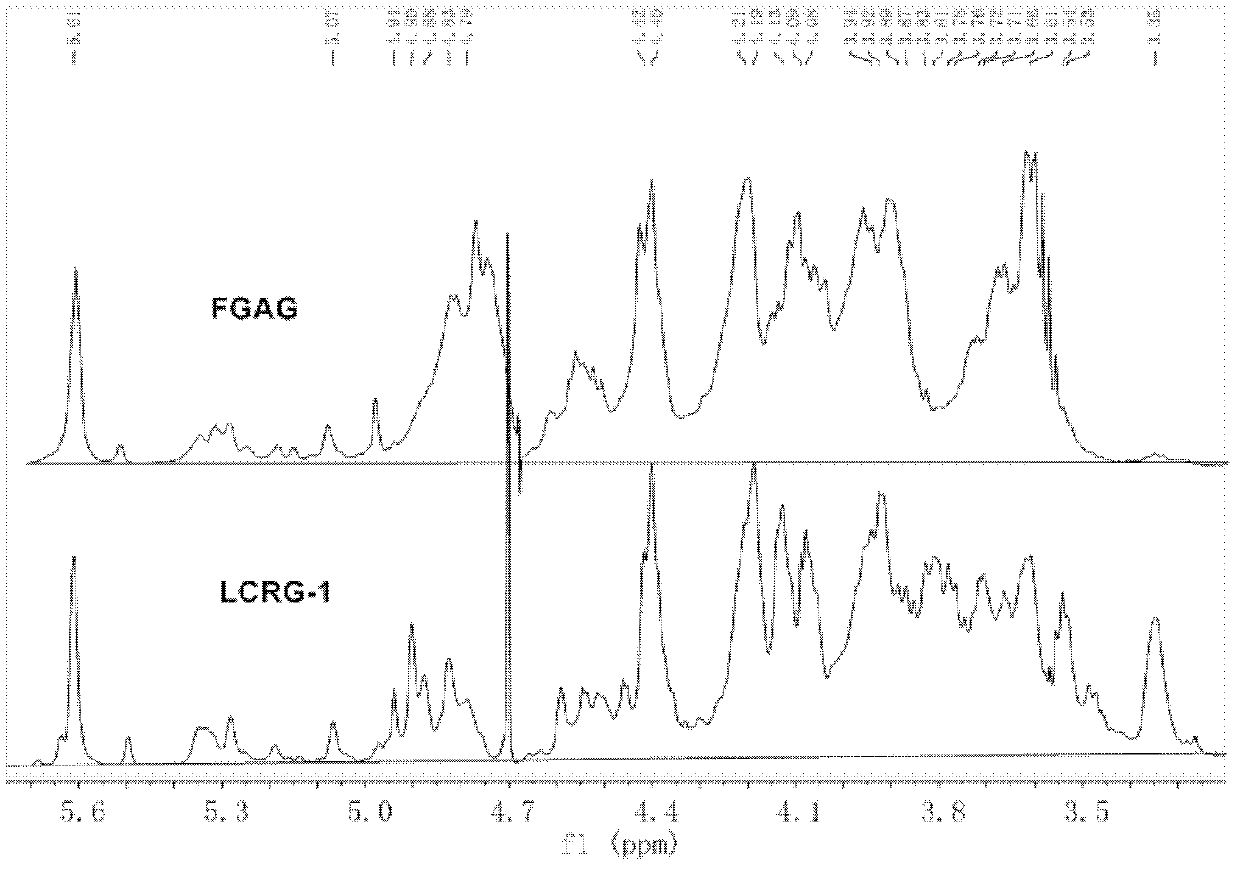
[0075] FGAG: FGAG derived from Meihua ginseng, sea cucumber and yuzu sea cucumber, extracted and prepared according to the literature method (J. Biol. Chem., 1991, 266(21): 13530-13536). The purity was greater than 98% (HPGPC, area normalization method).
[0076] 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDC), 1-phenyl-3-methyl-5-pyrazolone (PMP), HCl, NaBH 4 , NaCl and other reagents used: all commercially available analytical reagents; 4-chondroitin sulfate, glucose (Glc), glucuronic acid (GlcUA), acetamidogalactose (GalNAc) and fucose (Fuc) and other monosaccharides The reference substances are all products of sigma company; DCl and D 2 O is a product of Norell Company.
[0077] 1.2 Method
[0078] Weigh 300mg of FGAG and dissolve them in 48ml of water, adjust the pH to 4.75 with 0.1M HCl. 905 mg of solid EDC was added over the course of approximately 5 min and the pH...
Embodiment 2
[0085] [Example 2] Preparation of low molecular weight products by peroxide depolymerization
[0086] 2.1 Materials
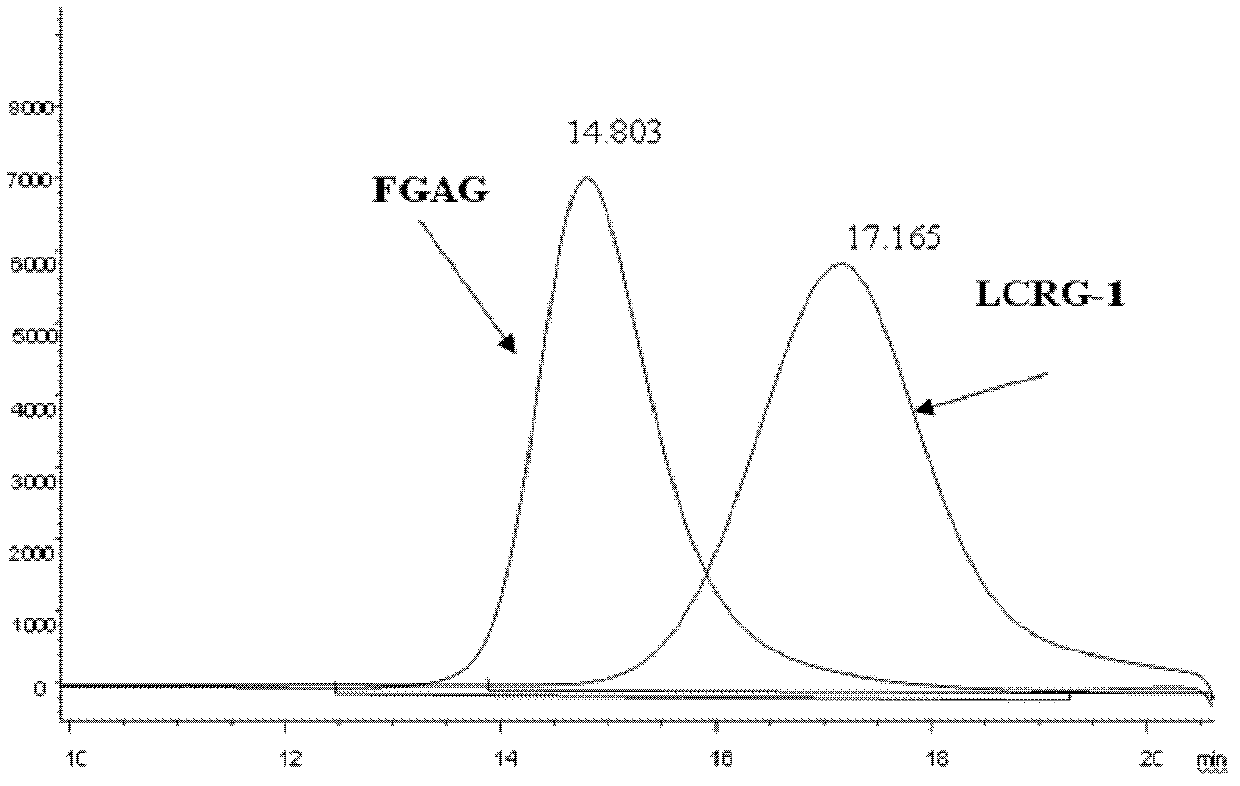
[0087] CRG-1: FGAG carboxyl reduction product derived from plum flower ginseng, prepared according to the method of [Example 1]. Purity 98% (HPGPC, area normalization method), weight average molecular weight (Mw), 68,840.
[0088] h 2 o 2 , CH 3 COONa·3H 2 O, NaCl, NaOH, Cu(CH 3 COO) 2 ·H 2 Reagents used in O, etc.: All commercially available analytical reagents.
[0089] 2.2 Method
[0090] Preparation of oligomeric carboxyl reduction products: Dissolve 5.0 g of ginseng CRG-1 in 180 ml of aqueous solution in a round-bottomed flask, keep warm in a water bath at 35° C. and continue to stir evenly, then add copper chloride (Cu 2+ ) solution, and then drop 15% H at a rate of 10ml / h within 2 hours 2 o 2 , During the reaction process, 1N NaOH solution was used to control the pH value range from 7.2 to 7.8. After continuously stirring and reacting for 4 ...
Embodiment 3
[0103] [Example 3] Anti-HIV activity of low molecular weight fucosylated glycosaminoglycan carboxyl reduction product LCRG-1
[0104] 3.1 Materials
[0105] (1) Test sample: LCRG-1 (Mw12930Da) prepared in [Example 2] was dissolved in water for injection, prepared as a storage solution with a concentration of 25 mg / ml, and stored at 4°C.
[0106](2) Reagents: MTT (3-(4,5)-dimethylthiahiazo-2-y1)-2,5-diphenyltetrazoliumbromide), SDS (SodiumDodecylSulfate), DMSO (Dimethylsulfoxide) and other reagents are commercially available and analytically pure. Coagulation quality control plasma, activated partial thromboplastin time (APTT) assay kit, thrombin time (TT) assay kit, prothrombin time assay kit (PT-dry powder), all produced by TECO GmbH in Germany; all others Reagents were commercially available analytically pure.
[0107] Instrument: MC-4000 Hemagglutination Analyzer (Metron, Germany); ELx800ELISAReader Microplate Reader (Bote, USA)
[0108] (3) Cells and viruses: C8166, HIV...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Weight average molecular weight | aaaaa | aaaaa |
| Weight average molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com