Total cedar polysaccharide and application of total cedar polysaccharide in preparation of immunosuppressive drug
A technology of total polysaccharide and cedar, applied in the field of preparing anti-complement and anti-inflammatory drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0014] Example 1 Preparation of Cedar Cedar Total Polysaccharides
[0015] Grind the cedar branches and leaves, and use 95% ethanol to heat and reflux under reduced pressure at a ratio of 1:30 to liquid (55°C, 3 times, 4 hours each time). Ratio of material to liquid is subjected to decompression heating and reflux extraction (70°C, divided into 3 times, each time for 5h). Concentrate the water extract to 0.4g crude drug / ml, add 4 times the volume of 95% ethanol, filter after alcohol precipitation for 24h, and dilute with water. The total polysaccharides were obtained by freeze-drying after precipitation and reconstitution; according to this process, three batches of total polysaccharides of cedar cedar SP1, SP2 and SP3 were prepared respectively.
Embodiment 2
[0016] Example 2 Structural characterization of cedarwood total polysaccharides
[0017] (1) Molecular weight distribution:
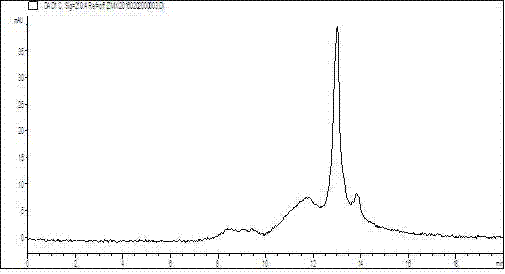
[0018] Cedar cypress total polysaccharide SP3 was formulated into 0.3mg / ml aqueous solution for HPLC analysis: TSK-GEL GMPWXL gel column (300×7.6 mm) was used, the mobile phase was deionized water, the flow rate was 0.8 mL / min, and the column temperature was 25°C , the detection wavelength is 210nm; the polysaccharide standard is analyzed by the same method, and the comparison of the spectrum shows that the molecular weight of cedar polysaccharide is basically less than 25000 Da;
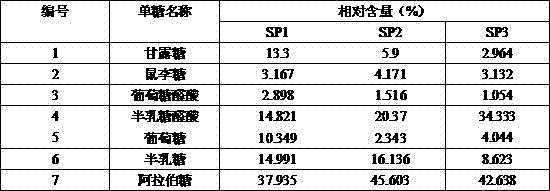
[0019] (2) Monosaccharide composition:
[0020] Take 8 mg polysaccharide sample and add 2ml of 1M TFA, heat at 110°C for 5h, centrifuge, evaporate the supernatant to dryness, and wash the precipitate three times with methanol; 0.3 M NaOH, heated at 70°C for 2h for hydrolysis. After cooling, add 1ml 0.3M HCl to neutralize, add chloroform to extract 3 times, discard the chlo...
Embodiment 3
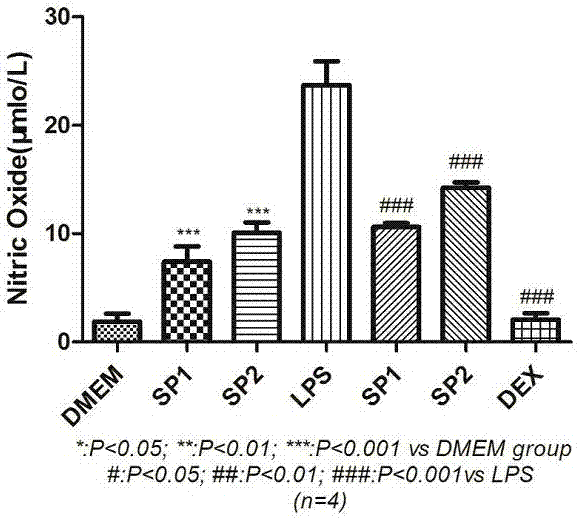
[0033] Example 3 Determination of anti-complement activity of total polysaccharides of cedar cypress by cell hemolysis
[0034] Take about 2.0mg total polysaccharides of cedar cedar, accurately weighed, dissolve in 0.8ml BBS (barbiturate buffer solution), and double-dilute to obtain 2.5, 1.25, 0.625, 0.3125, 0.1563, 0.0781, 0.0391, 0.0195mg / Each 0.4ml of sample solution in 1ml was drawn into 0.2ml to 0.4ml of BBS as a blank control; 0.2ml of complement, 0.1ml of hemolysin and 0.1ml of 2% sheep red blood cell solution were added to the remaining sample solution, and centrifuged Then take the supernatant and measure the absorbance at 405nm, and calculate the IC of the hemolysis inhibition rate of the three batches of cedarwood polysaccharide after deducting the absorbance of the blank control 50 SP1: 25.04μg / ml, SP2: 35.40μg / ml, SP3: 23.82μg / ml respectively; IC of positive control drug heparin sodium 50 It was 27.81 μg / ml.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com