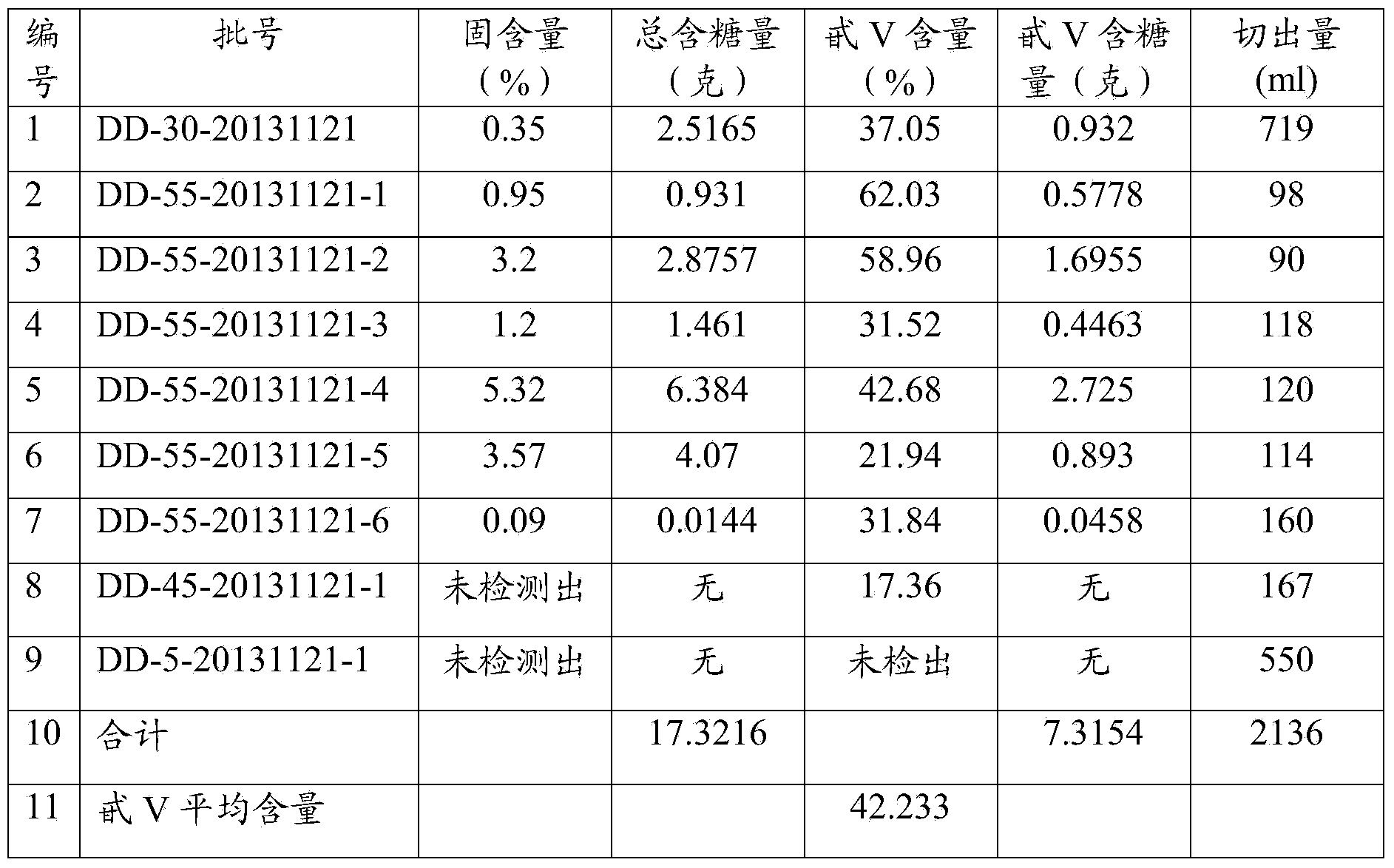
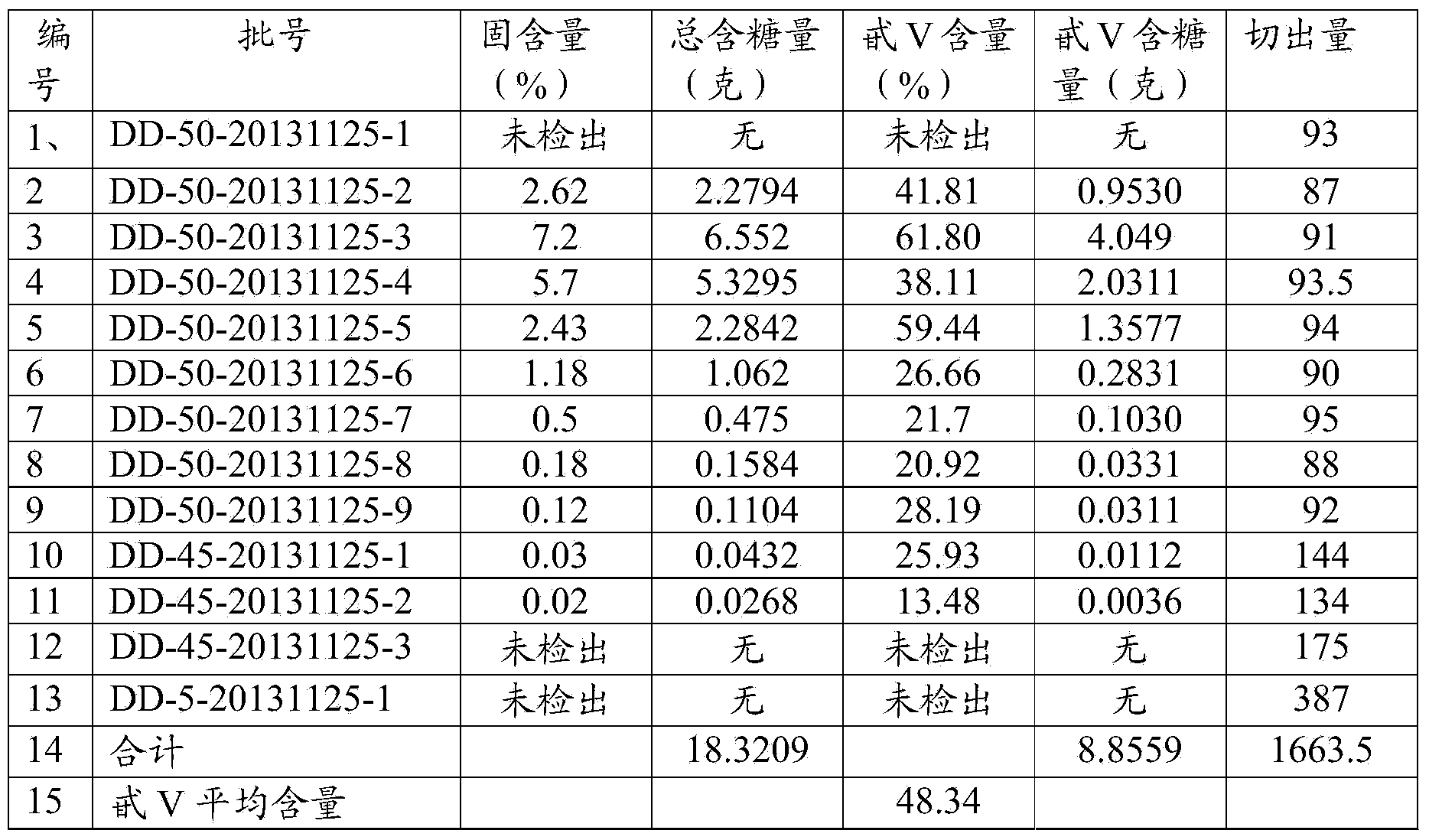
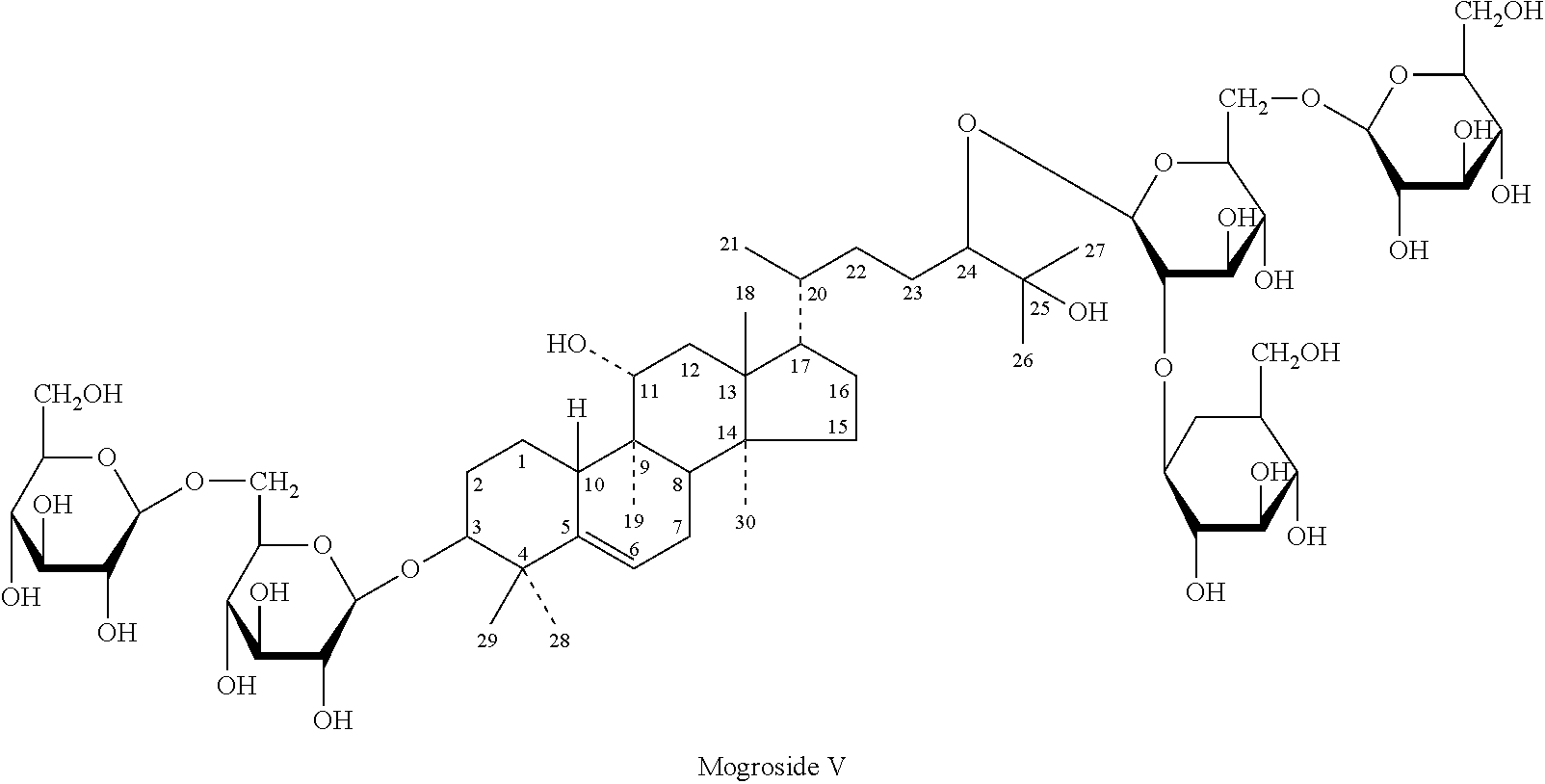
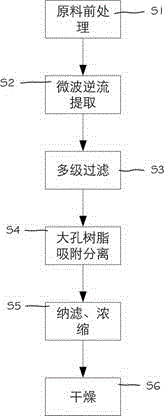
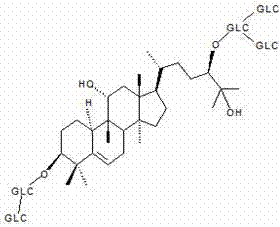
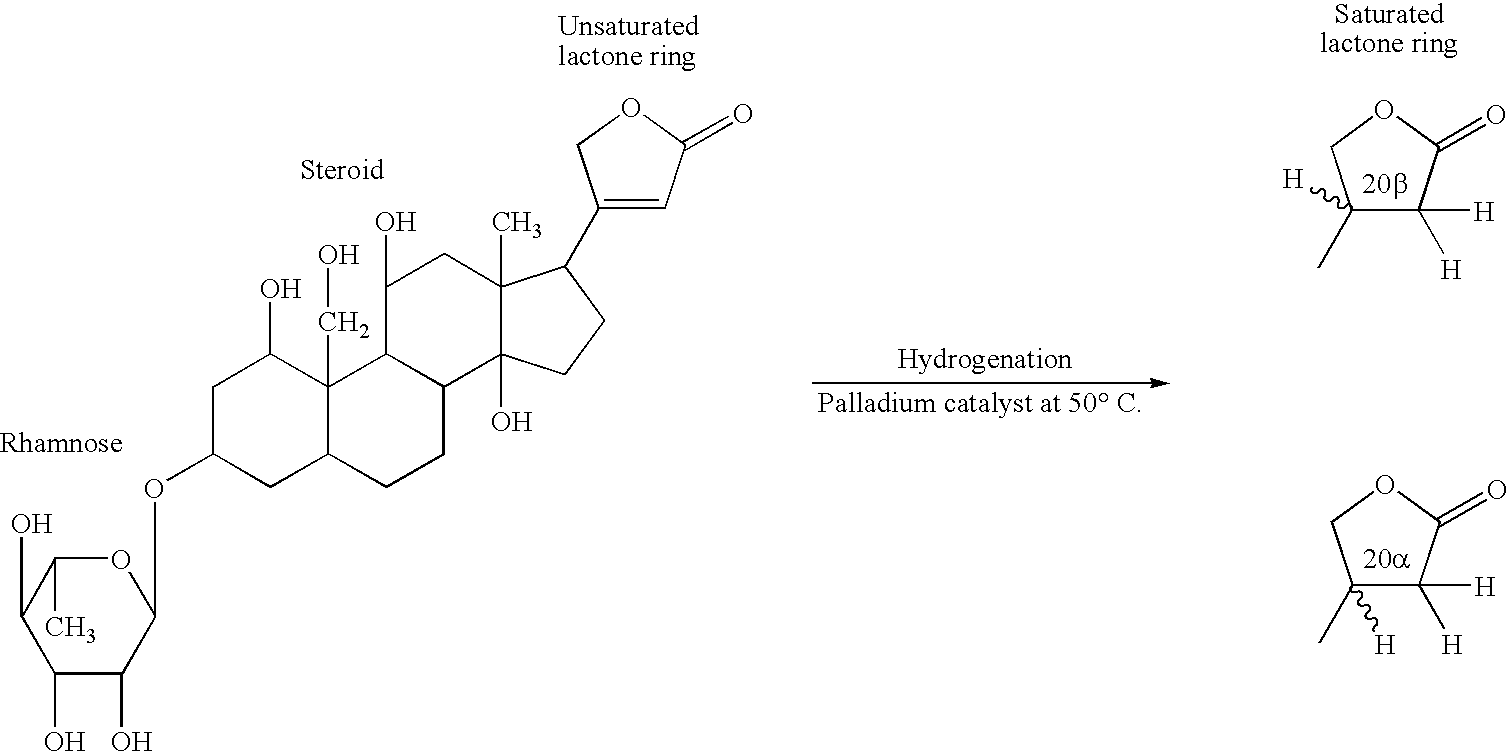
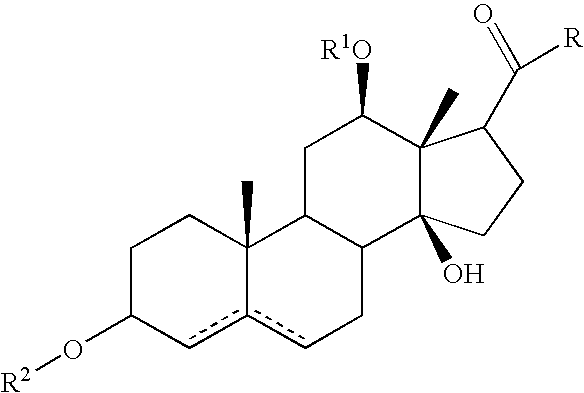
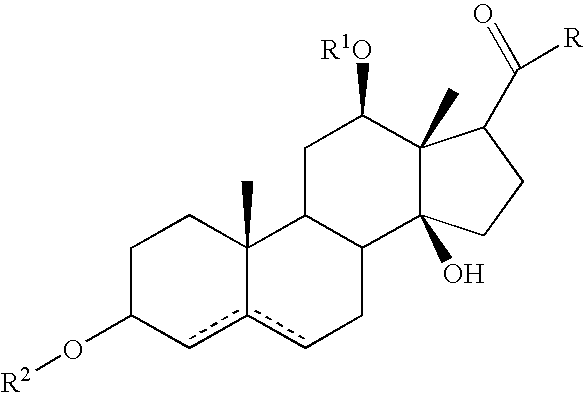
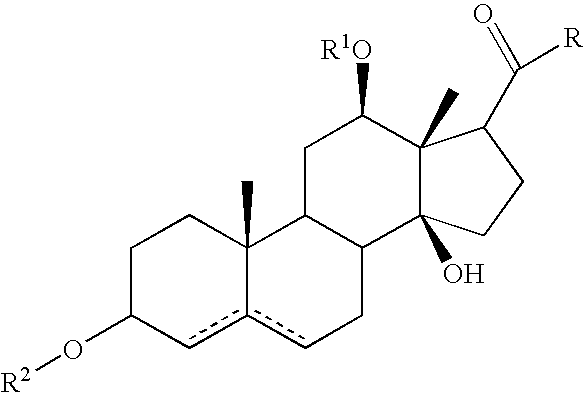
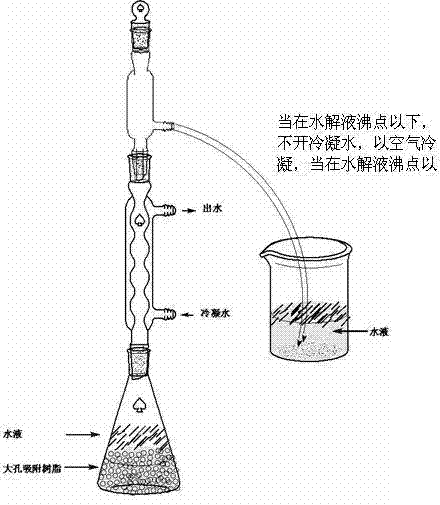
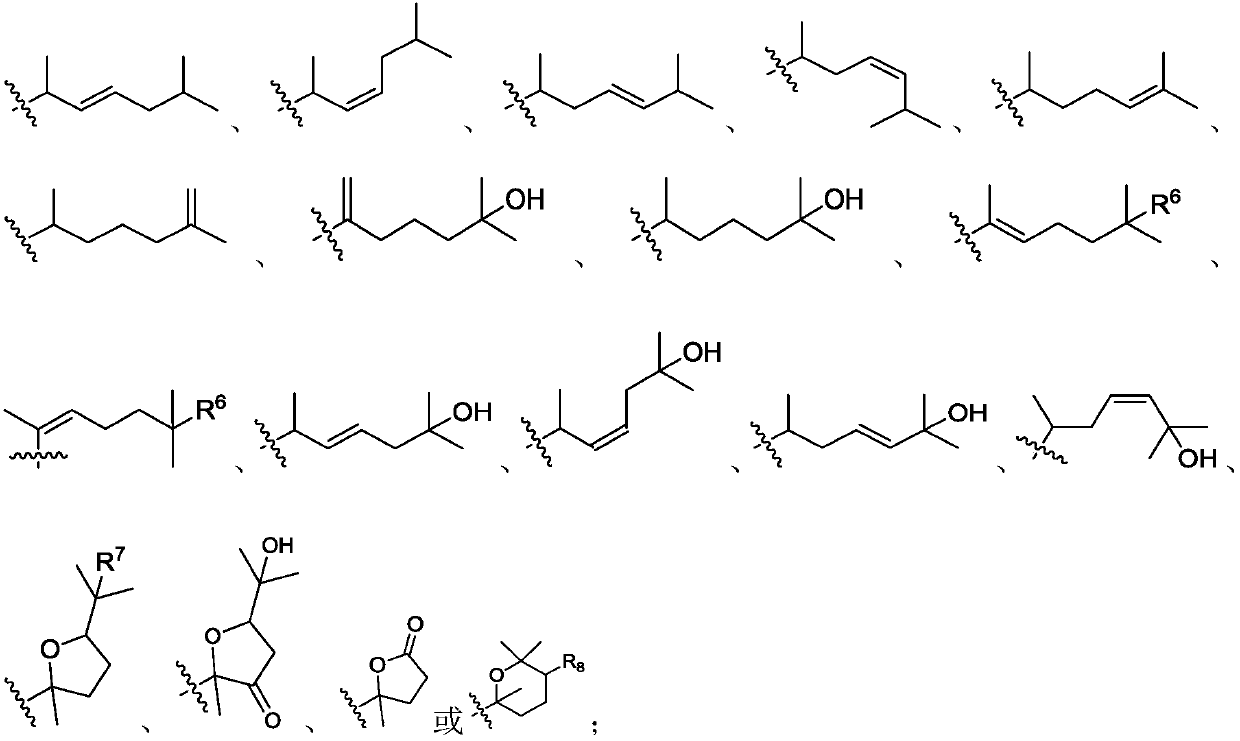
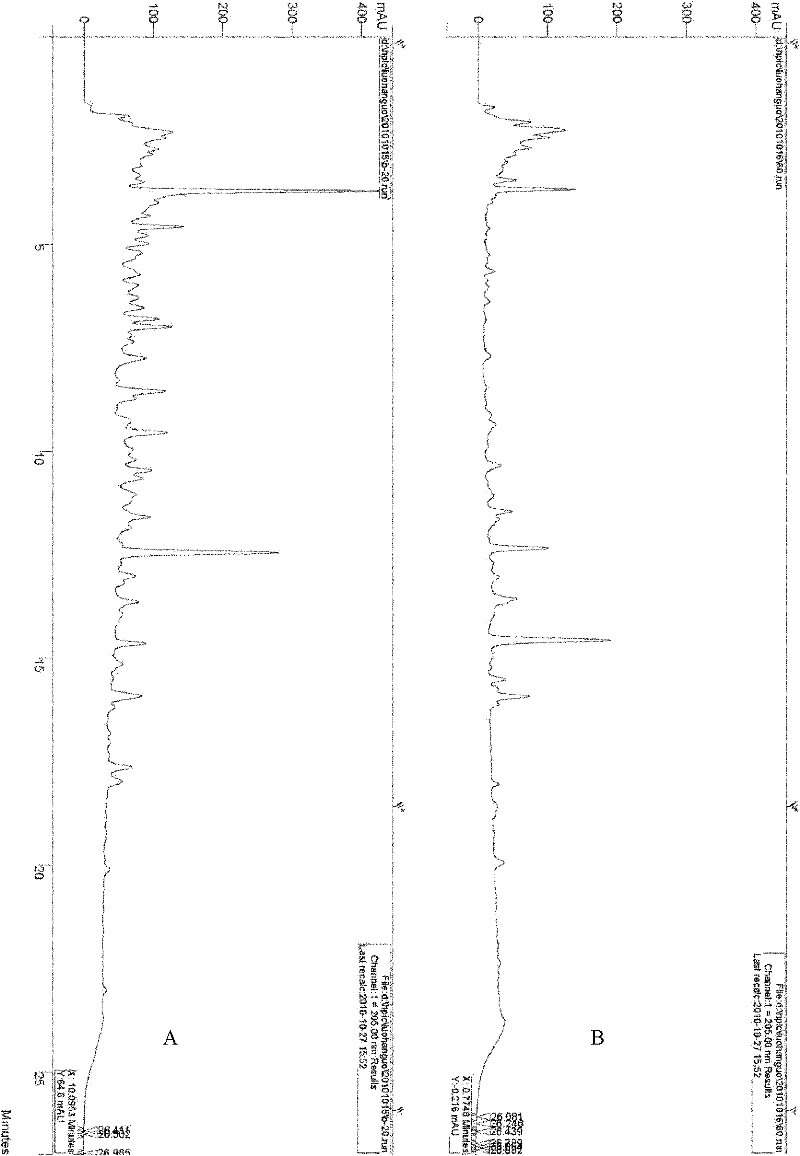
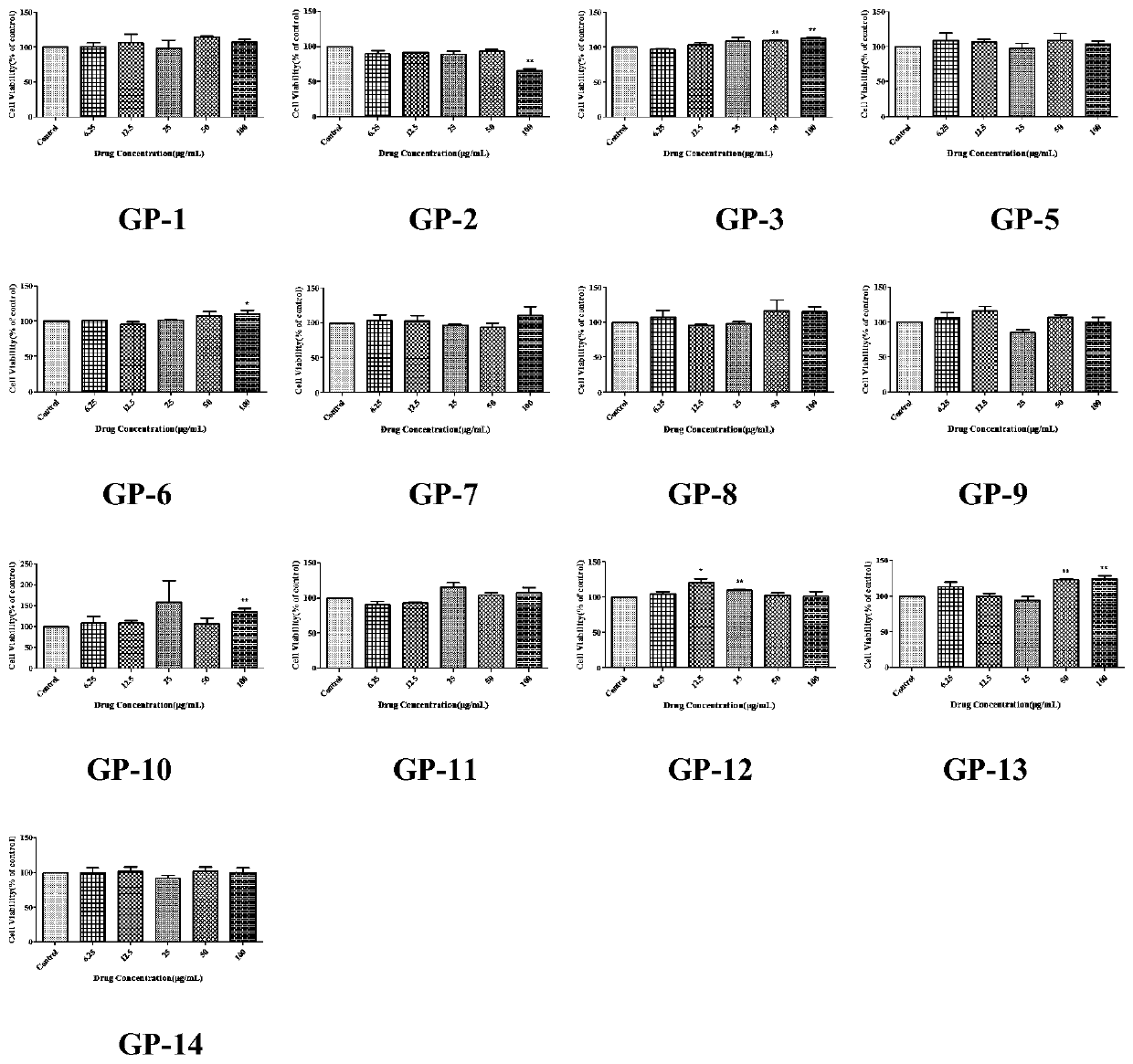
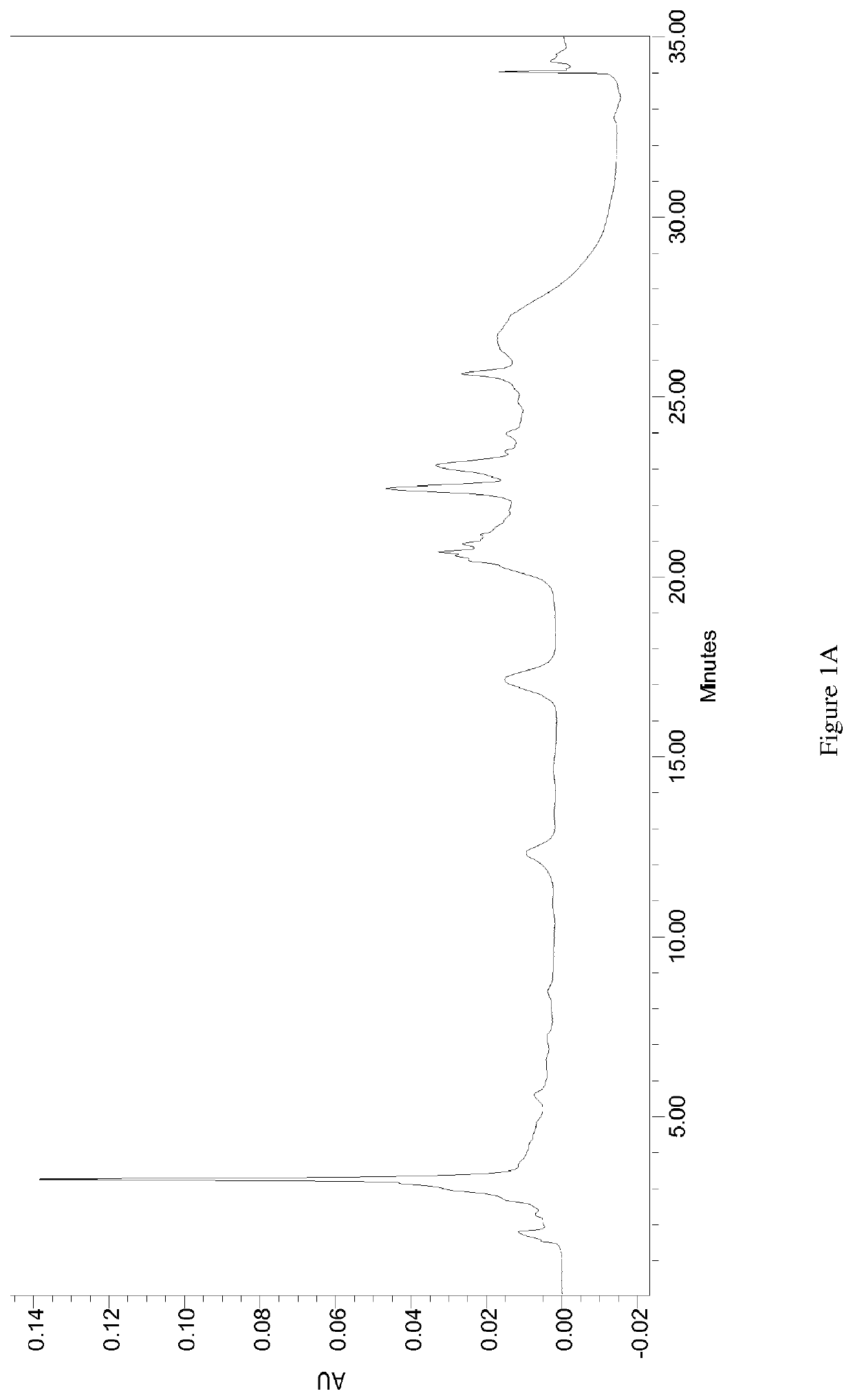
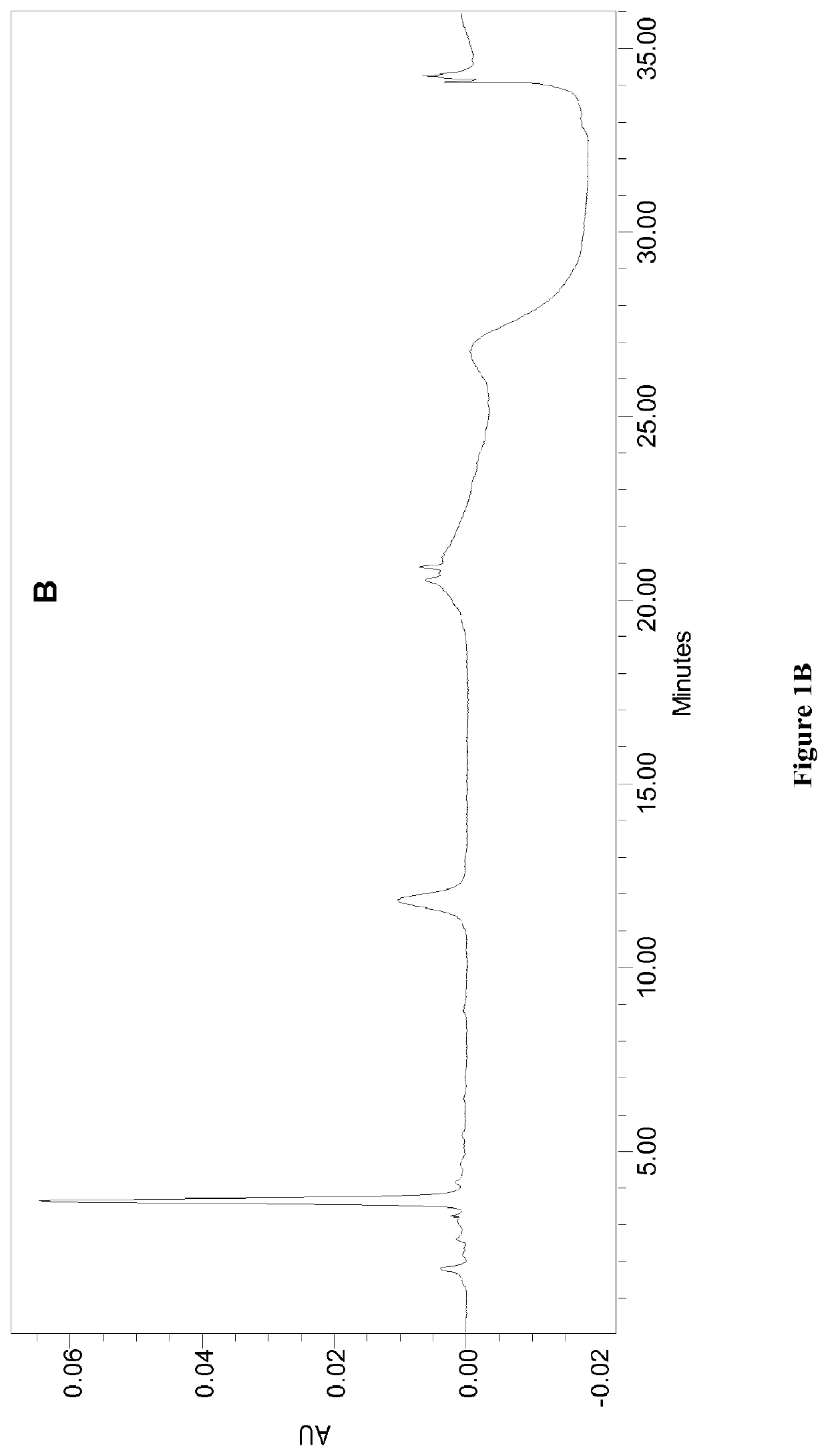
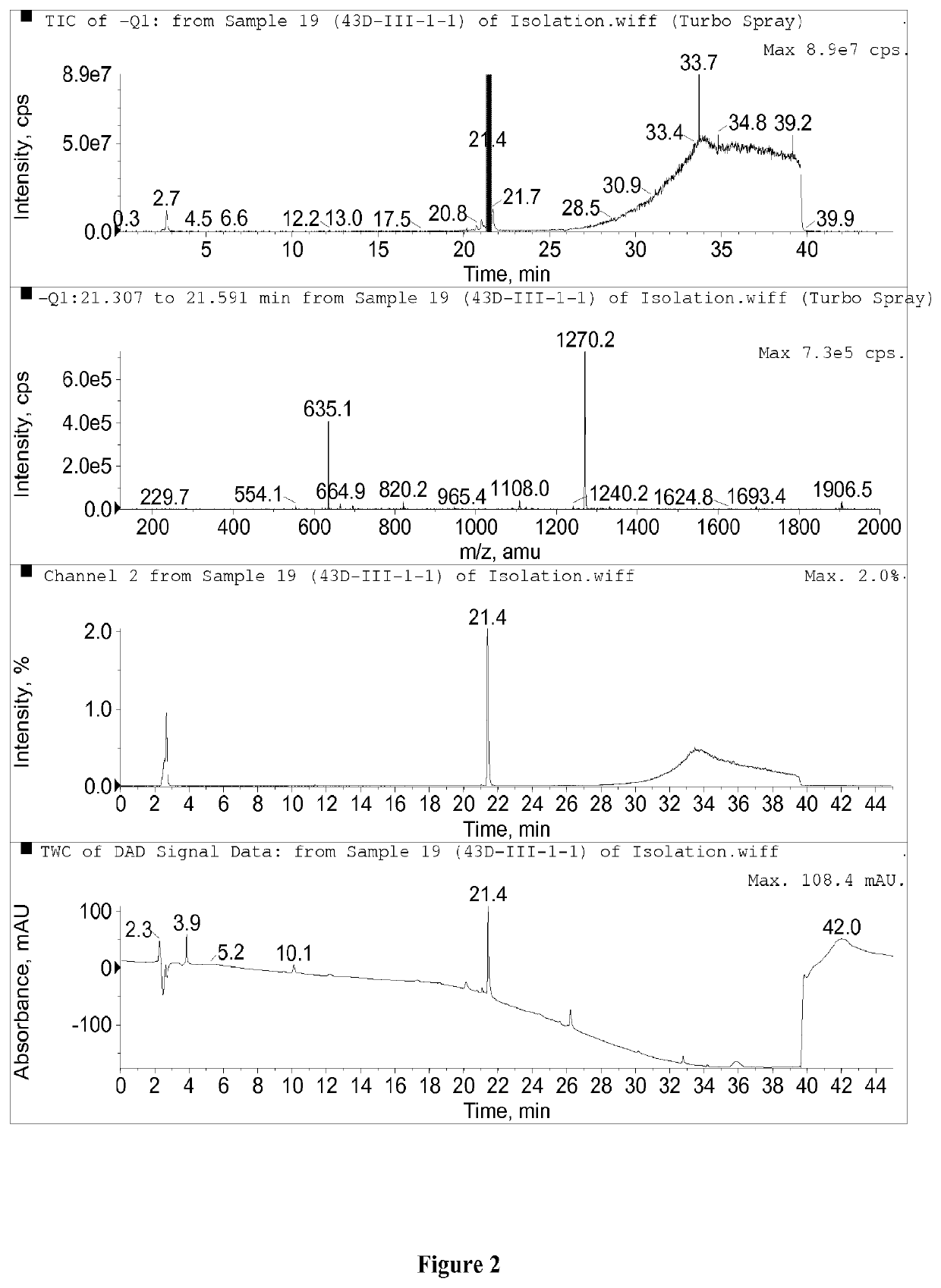
Patents
Literature
530results about "Glycoside steroids" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
High intensity sweeteners
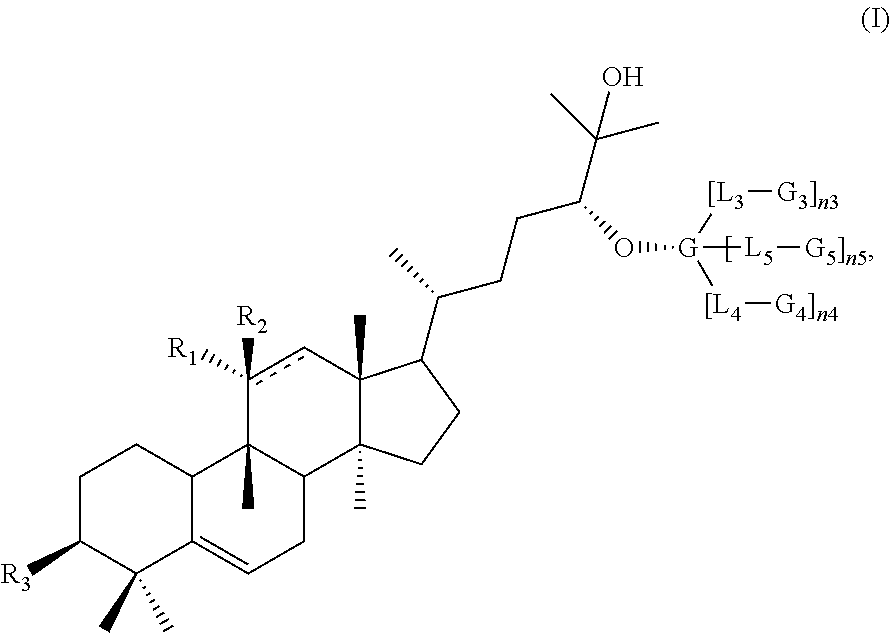
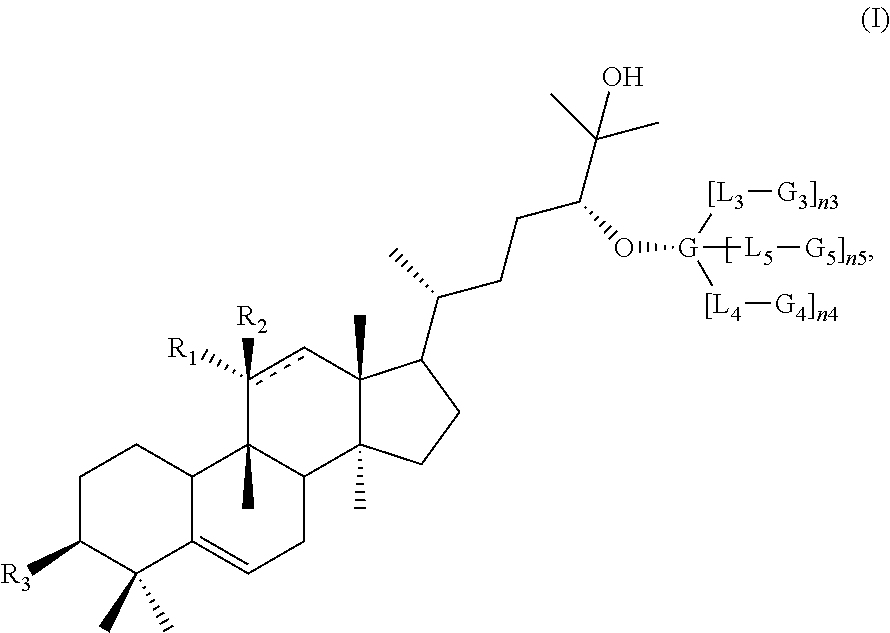
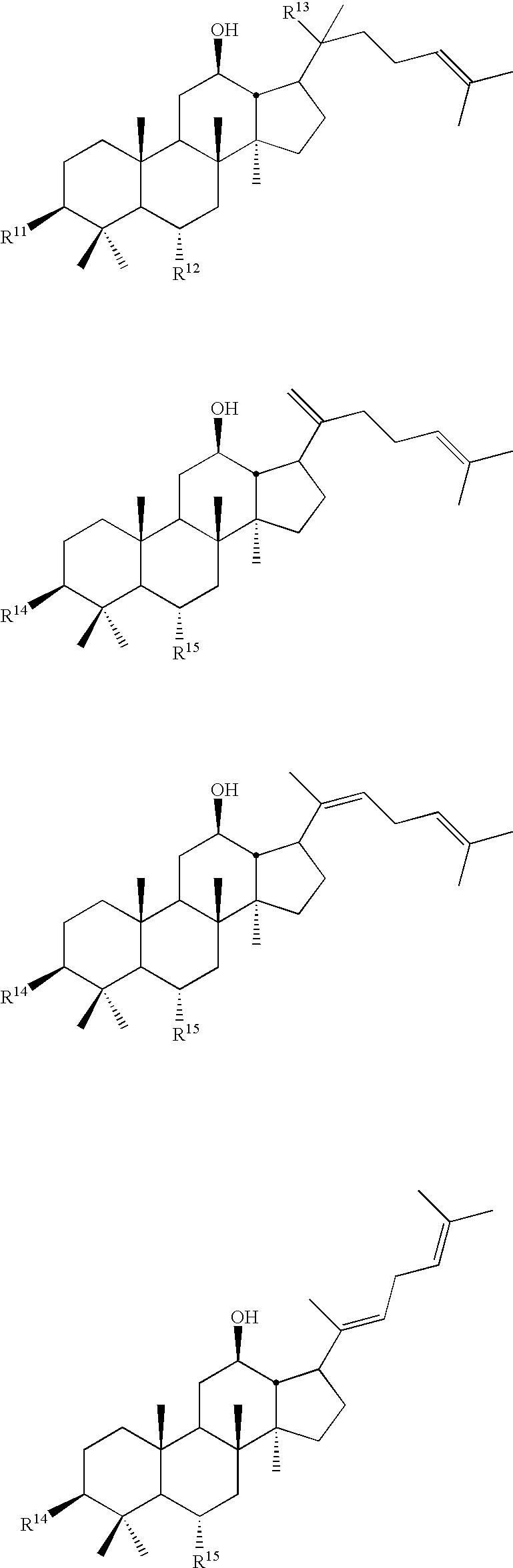
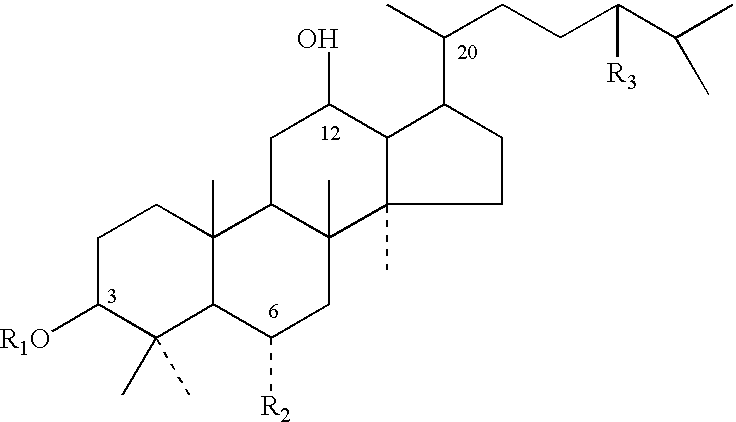
Disclosed herein are compounds having structural Formula (I), or salts thereof. These compounds are useful as sweet tasting agents and / or sweetness enhancers. Also disclosed are compositions comprising the present compounds and methods of increasing the sweet taste of ingestible compositions. Furthermore, methods for preparing the compounds are also disclosed.
Owner:SENOMYX INC
High-Purity Mogrosides And Process For Their Purification
The present invention provides a process for preparation of highly purified mogrosides mixture from low purity mogrosides mixture. The process comprises providing a mixture of low purity mogrosides, dissolving the low purity mogrosides mixture in water or an aqueous alcohol solution to form an initial solution of mogrosides, passing the initial solution through a column system, wherein the column system comprises a plurality of columns, and each column is packed with a sorbent having different affinities to impurities and mogrosides so that one or more columns retains more mogrosides than other columns, washing the columns to remove impurities with an acidic aqueous solution, a basic aqueous solution, and an aqueous alcoholic solution successively, eluting the columns with an aqueous alcohol solution that contains higher alcohol content than the aqueous alcohol solution used in the washing step, wherein the eluate from the columns with high content of mogrosides are combined, and drying the combined eluate to obtain high purity mogrosides with the content of the total mogrosides are more than 70% (w / w). The present invention also provides a sweetener mixture and product comprising high purity mogrosides.
Owner:PURECIRCLE SDN BHD
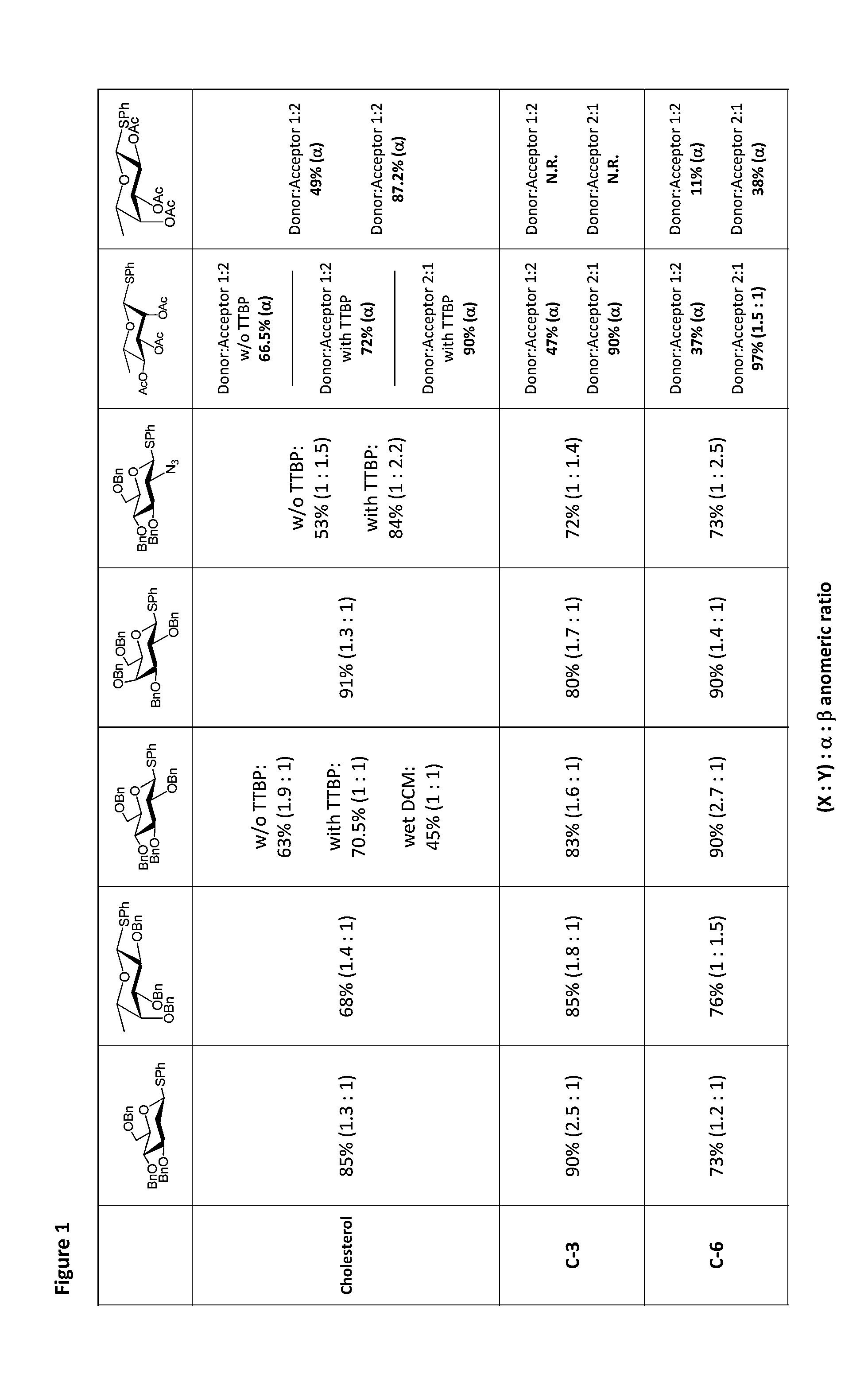
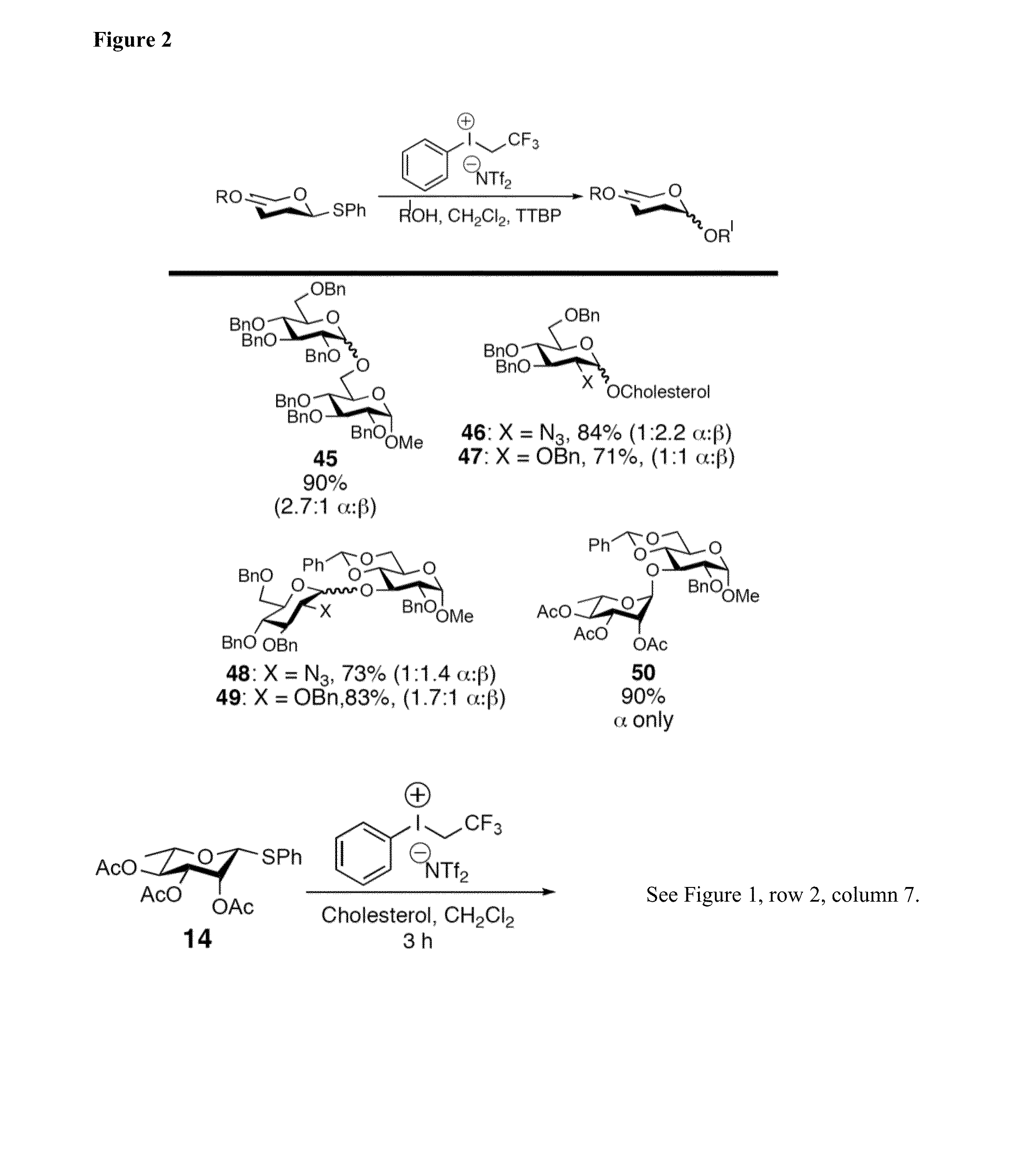
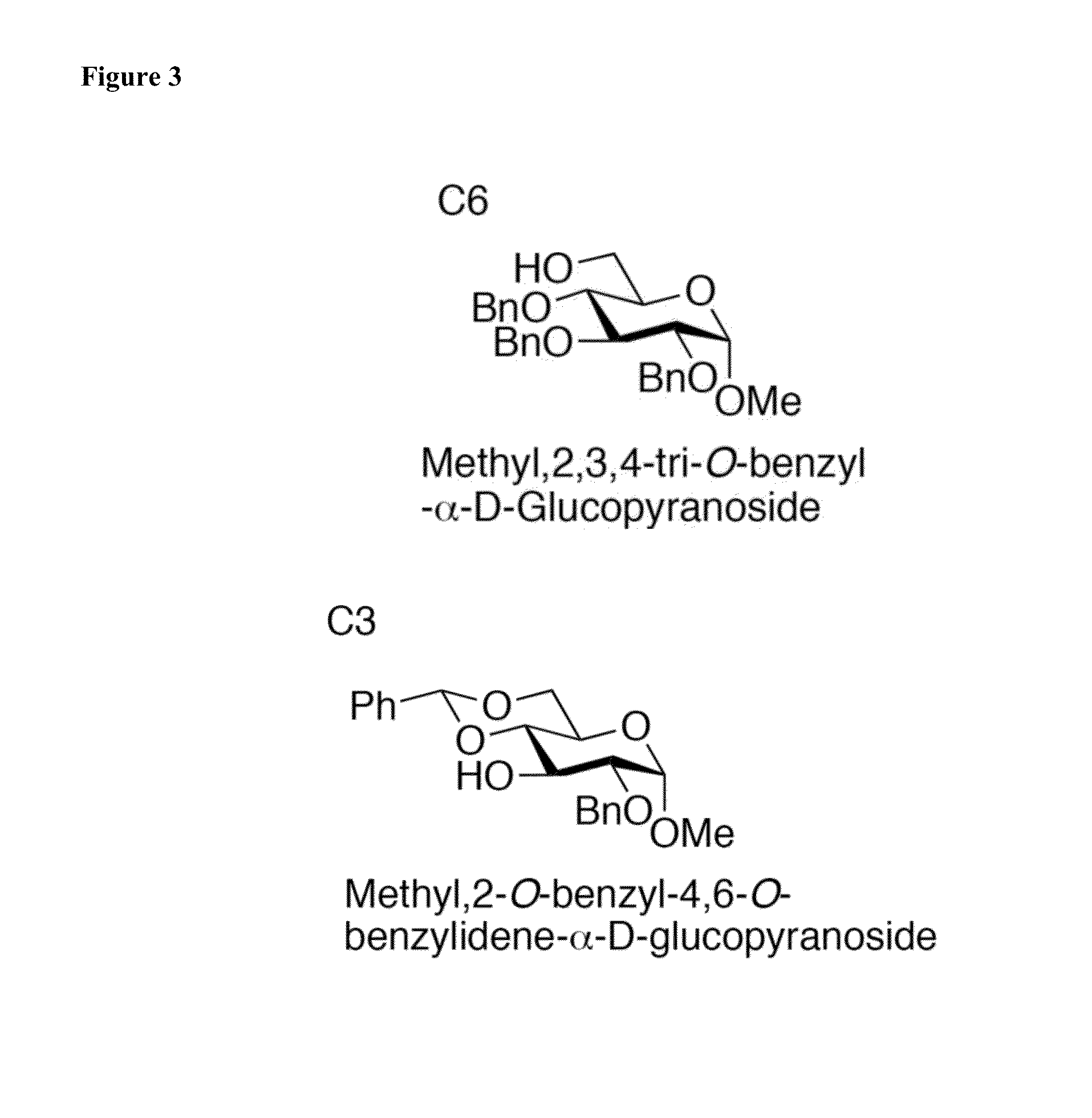
Glycosylation Reactions Using Phenyl(trifluoroethyl)iodonium Salts
Provided are methods for the preparation of glycosylation products, including those represented by formula I:Sugar-O—R′ Icomprising the step of combining R′—OH, a glycosyl sulfide glycosyl donor (“thioglycoside donor”), a hypervalent iodine alkyl-transfer activating reagent, and a base. In an embodiment, the hypervalent iodine alkyl-transfer activating reagent is (phenyl(trifluoroethyl)iodonium triflimide).
Owner:TRUSTEES OF TUFTS COLLEGE TUFTS UNIV
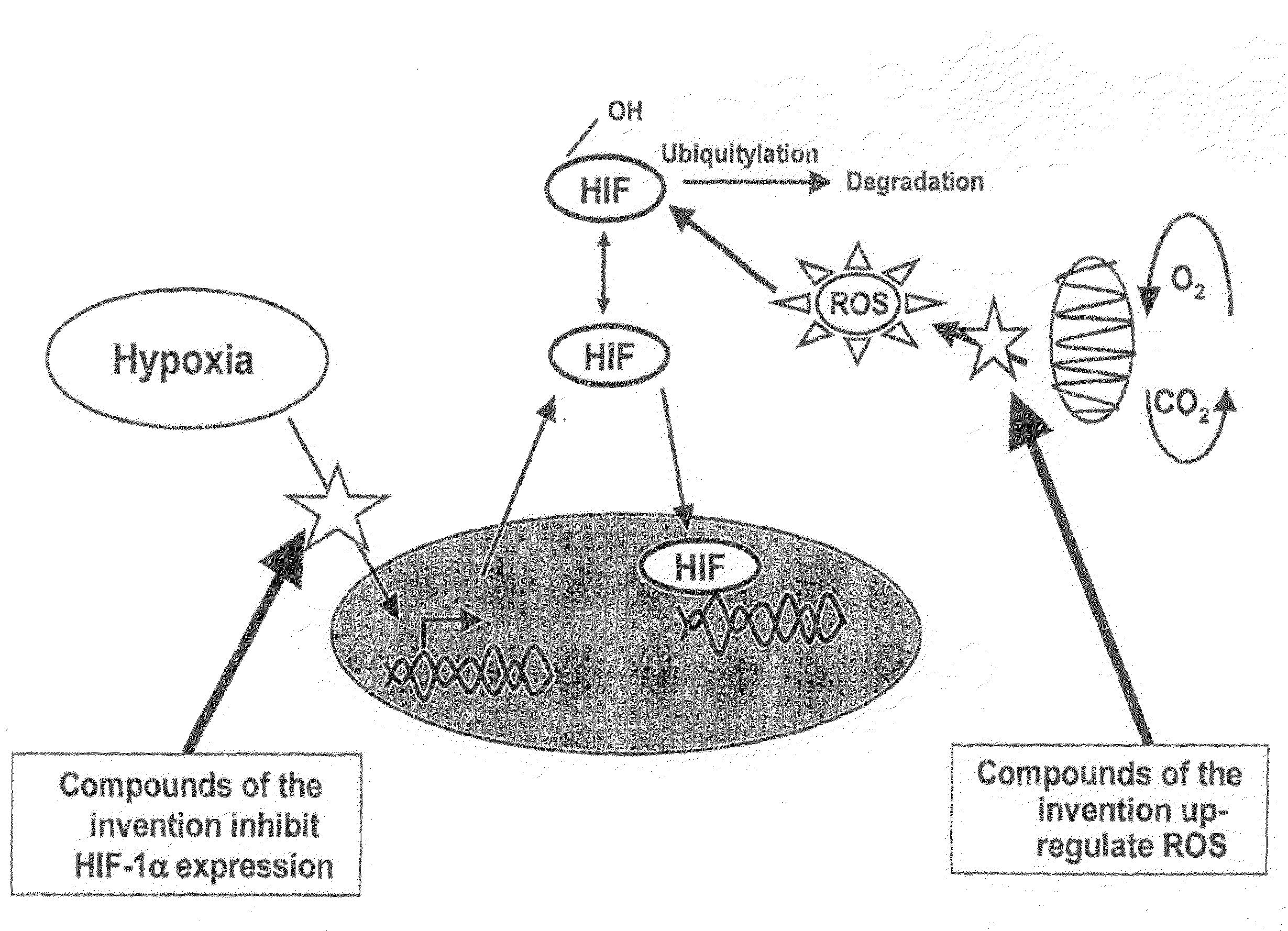
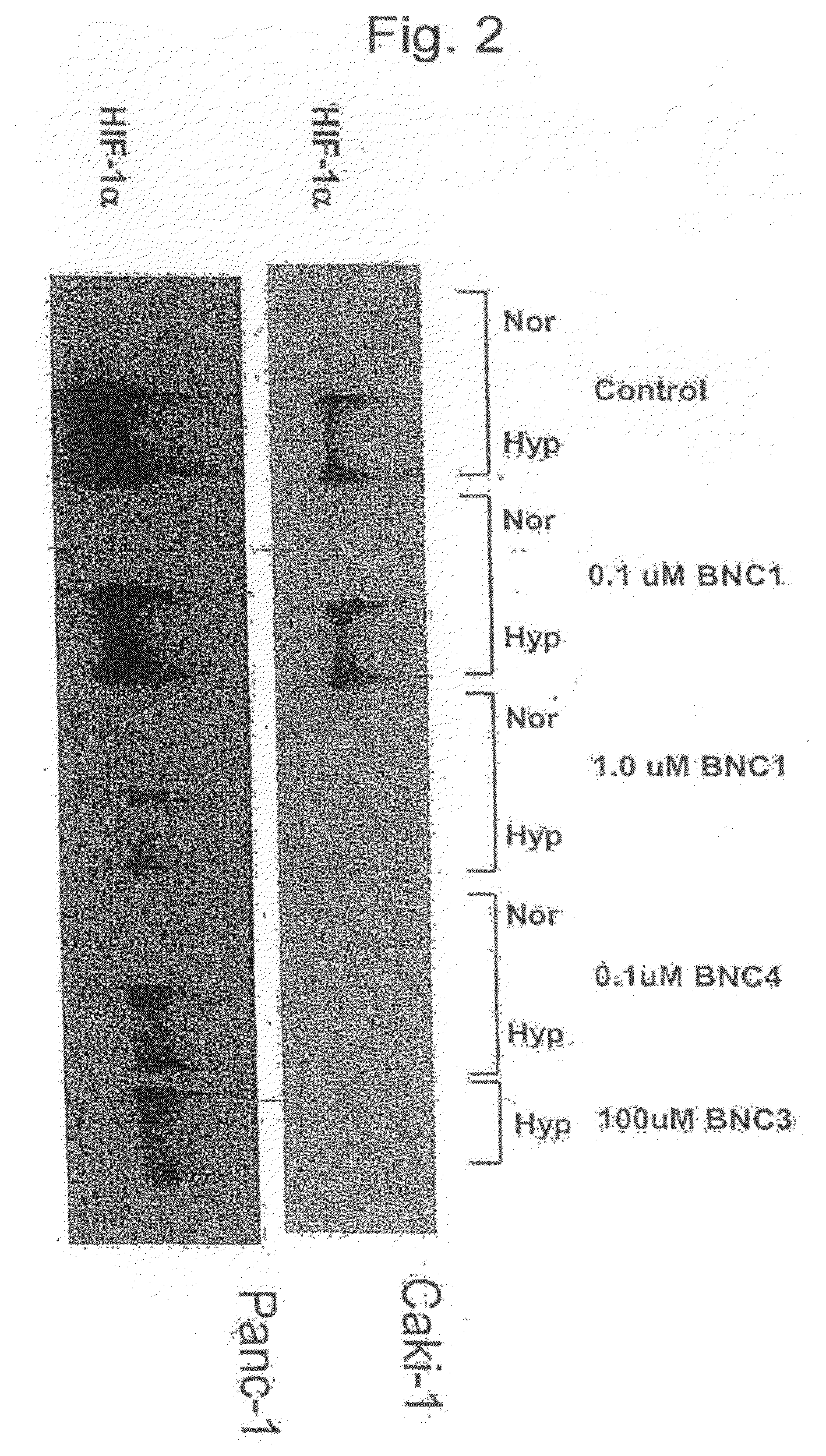
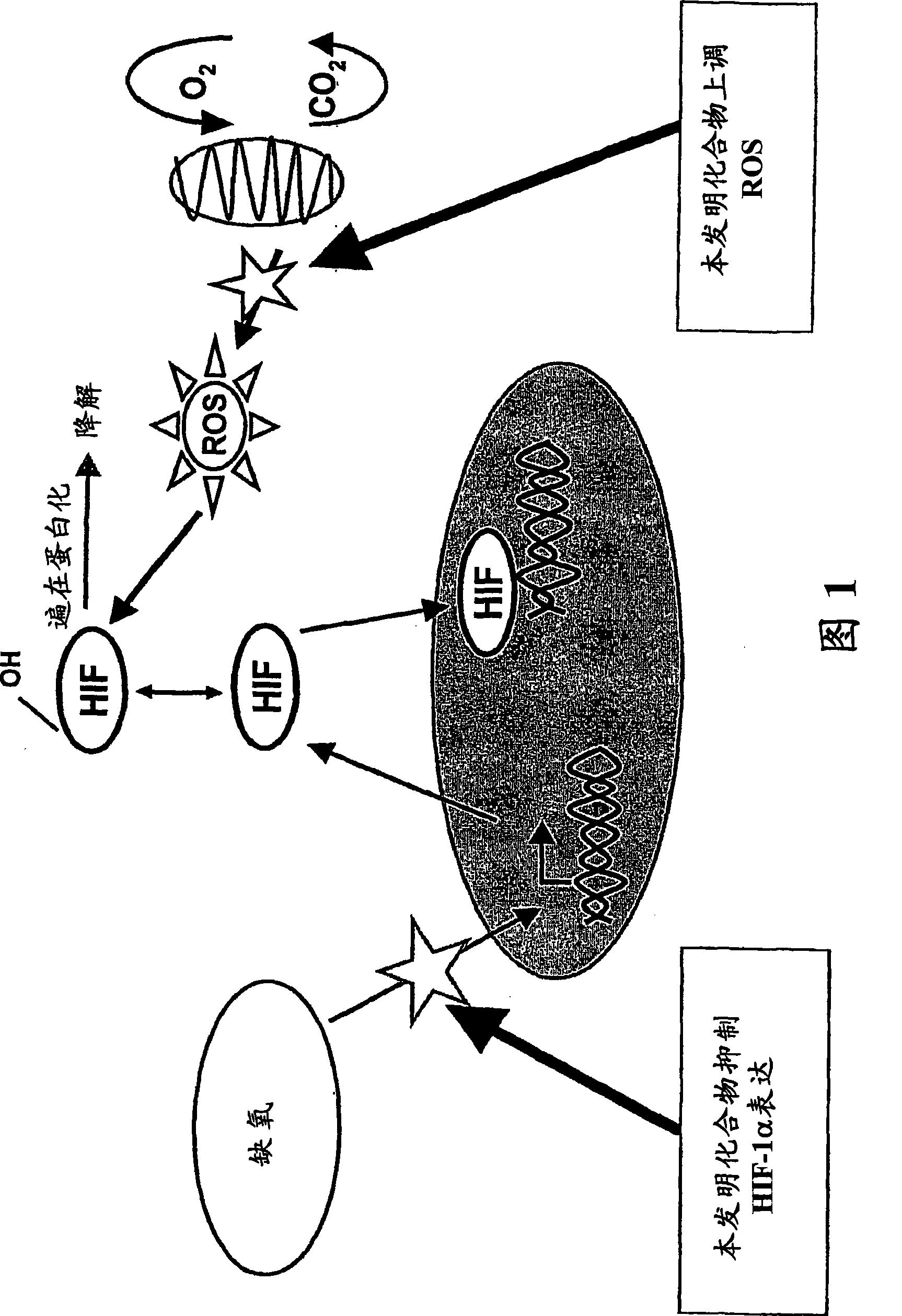
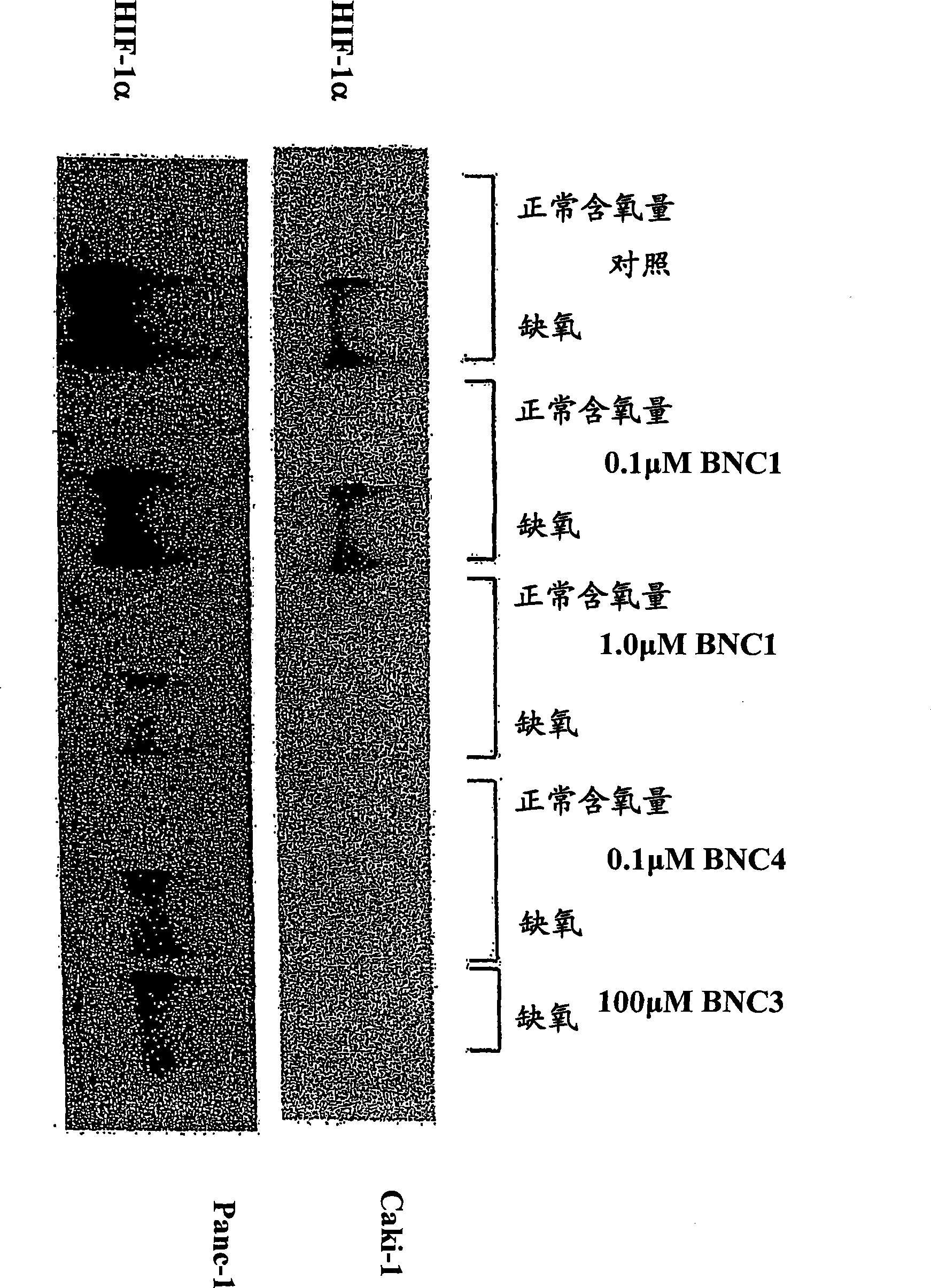
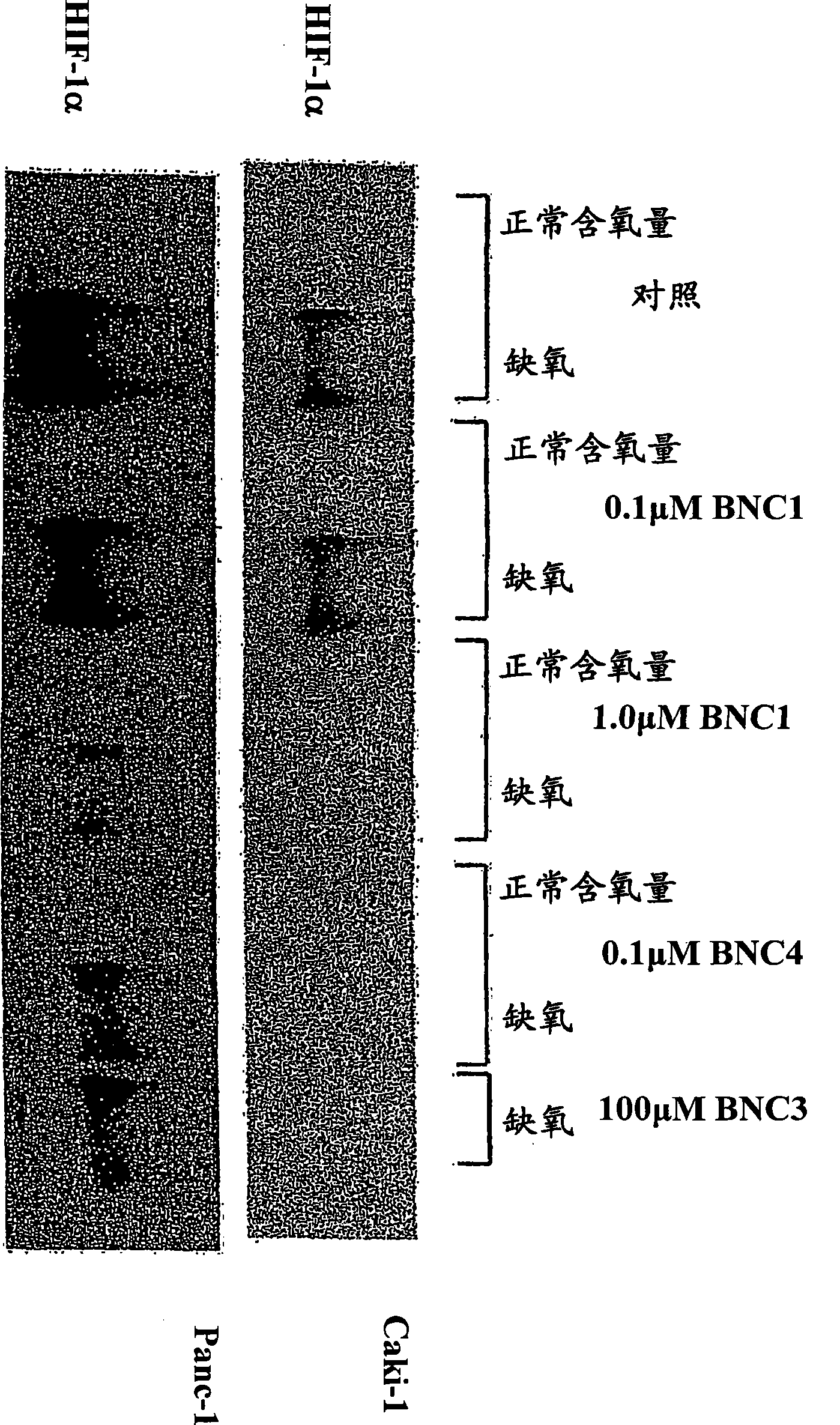
Modulators of Hypoxia Inducible Factor-1 and Related Uses
Owner:BIONAUT PHARMA
Method for extracting mogroside and water-soluble dietary fiber free of pesticide residue simultaneously
A method for extracting mogroside and water-soluble dietary fiber free of pesticide residue simultaneously comprises the following steps that (1) momordica grosvenori is washed and juiced, pomace is rinsed, and an extraction solution is obtained; (2) boiling, cooling and centrifugation are performed, and centrifugate is obtained; (3) an effluent is collected by aluminum oxide and an activated carbon chromatographic column which are connected in series front and back; (4) ultrafiltration membrane filtration is performed, and a trapped fluid and a permeate liquid are collected respectively; (5) nanofiltration and concentration are performed: an ultrafiltration liquid is filtered by a nanofiltration membrane, the trapped fluid and the permeate liquid are collected respectively, a nanofiltration trapped fluid is concentrated and dried, and the mogroside free of pesticide residue is obtained; the nanofiltration trapped fluid and the ultrafiltration trapped fluid are combined, concentrated and dried, and the momordica grosvenori water-soluble dietary fiber free of pesticide residue are obtained; the mogroside and water-soluble dietary fiber products are free of pesticide residue, the content of glucoside V reaches 90.6%, and the yield reaches 96.3%; the purity of the water-soluble dietary fiber reaches 95%, and the yield reaches 91%; the method is simple, the operation is simple and convenient, and the method is suitable for industrialization production.
Owner:HUNAN HUACHENG BIOTECH
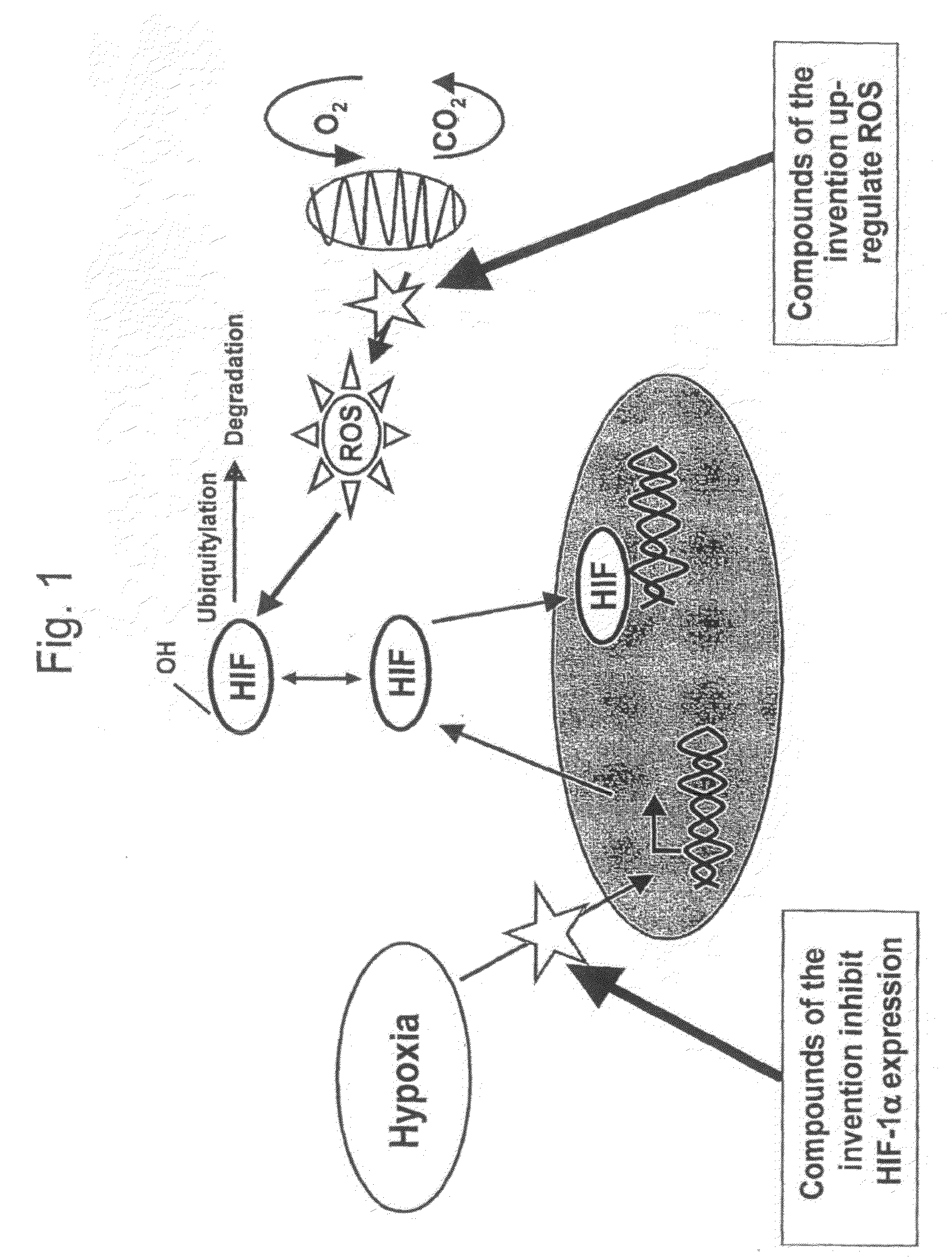
Modulators of hypoxia inducible factor-1 and related uses
InactiveCN101400690AEasy to administerOrganic active ingredientsSenses disorderMedicineHypoxia-inducible factors
Owner:BRITISH TECH GRP LTD
Method for extracting mogroside V
ActiveCN103923152AExtended service lifeTo achieve the effect of segmented enrichmentOrganic active ingredientsGlycoside steroidsFructoseAlcohol
The invention provides a method for extracting mogroside V. According to the method, fresh fructus momordicae serves as a raw material and is washed, smashed and extracted according to the following steps that (1) the fresh fructus momordicae is saccharifed; (2) aqueous extraction and concentration are conducted; (3) sedimentation and centrifugation are conducted; (4) adsorption and separation are conducted through macroporous resin, wherein multiple resin column groups are used for adsorption, resolution liquid is collected in sections, and according to different contents, combination is conducted in batches; (5) ion exchange resin is refined; (6) ethyl alcohol is concentrated and recycled; (7) silica gel is refined; (8) concentration and spray drying are conducted, so that mogroside V extract serving as a final product is obtained. According to the technology of refinement, various types of aglucone of fructose of the fructus momordicae are gathered in a bed layer in sections and the extracted mogroside V is high in purity, good in color and pure in taste.
Owner:QINGDAO RUNDE BIOTECH
Method for extracting high-purity mogroside V from siraitia grosvenorii
ActiveCN106008645AReduce water consumptionReduce extraction timeMembranesSemi-permeable membranesOrganic solventMicrowave
The invention discloses a method for extracting high-purity mogroside V from siraitia grosvenorii, and relates to the extraction method of the mogroside V. The method includes the specific steps: raw material pretreatment, extraction, centrifugation, enzymolysis, ultrafiltration, nanofiltration, decolorization, concentration, microwave drying, and crushing. The membrane technique is adopted for separation and purification, only pure water is used as a solvent, an organic solvent is discarded, and the method has the advantages of simple operation, safe and environmental-protection products, simple process, high quality and low price, and can realize the continuous large-scale industrial production.
Owner:JIANGXI HAIFU BIOENG
Sweetening compositions and processes for preparing them
InactiveUS20110021456A1Increase ethanol concentrationOrganic active ingredientsBiocideGlycosideSolid content
The invention relates to sweetening compositions containing from about 16% to about 75% mogroside V and from about 30% to about 95% total terpene glycosides on a dry weight basis, and wherein a filtered (0.2 μm) solution of the composition in water having a solids content of 1% w / v has an absorbance at 420 nm of about 0.55 or below. The invention also relates to methods of preparing such compositions.
Owner:GUILIN GFS MONK FRUIT CORP
Method for extracting mogroside V from momordica grosvenori
InactiveCN104558088AImprove qualityTo achieve the purpose of concentrationGlycoside steroidsChemistryEnvironmentally friendly
The invention provides a method for extracting mogroside V from momordica grosvenori. The method comprises the steps of carrying out pretreatment on raw materials, carrying out microwave countercurrent extraction, carrying out multistage filtering, carrying out macroreticular resin adsorption separation, carrying out nanofiltration, concentrating and drying. The method integrates the advantages of multiple extracting ways, and is used for continuously and efficiently extracting the mogroside V. Products obtained by adopting the method are proved that the mogroside V is 63-65% and the final yield of the mogroside V is 70-74% after being detected by adopting a high performance liquid chromatography. Except ethyl alcohol, in the method, other organic solvents which are harmful to the human body are not used, thus the products are environmentally friendly; the products contain less than 10ppm of ethyl alcohol residue, thus being safe and high in quality.
Owner:JIANGXI HAIFU BIOENG
Cardiac glycoside compounds and antitumor application thereof
InactiveCN102219821AStrongly inhibits proliferative activityOrganic active ingredientsGlycoside steroidsBenzoic acidO-Phosphoric Acid
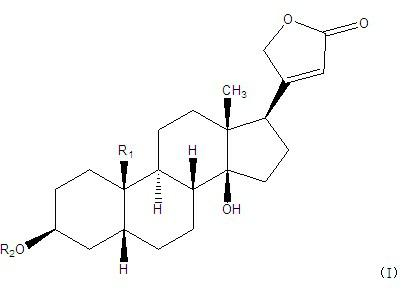
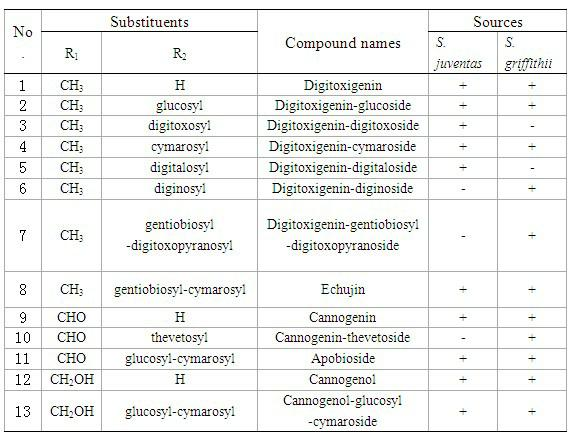
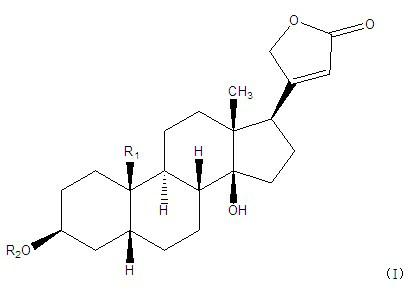
The invention belongs to the technical field of medicaments and in particular relates to cardiac glycoside compounds with a general formula (I), as well as derivatives, stereoisomers, a racemic mixture or a non-racemic mixture of the stereoisomers and pharmaceutically acceptable acid addition salts or solvates, wherein R1 is CH3, CHO or CH2OH; R2 is H or a linear saccharide chain or a branched saccharide chain, the linear saccharide chain or the branched saccharide chain is formed by saccharides; and these acids comprise inorganic acids like hydrochloric acid, sulfuric acid, hydrobromic acid, phosphoric acid, nitric acid, carbonic acid and the like as well as organic acids like methanoic acid, acetic acid, succinic acid, citric acid, lactic acid, fumaric acid, tartaric acid, benzoic acid, p-methyl-benzenesulfonic acid, methylsulphonic acid, naphthalenesulfonic acid, gluconic acid and the like. The cardiac glycoside compounds can be obtained by separation from plants, in particular Streptocaulon plant juventas or Streptocaulon griffithii, by using multiple conventional separating means or obtained through synthesis, semisynthesis or bioconversion means. These compounds have excellent inhibiting and treatment effects on multiple tumor cells.
Owner:SHENYANG PHARMA UNIVERSITY
Sweetening compositions and processes for preparing them
The invention relates to sweetening compositions obtained from the Luo Han Guo fruit, a member of the Cucurbiticeae family. The compositions are free of bitter-tasting impurities, have a light colour and contain about 16-75% mogroside V and about 30-95% total terpene glycosides on a dry weight basis. A filtered (0.2 μm) solution of the composition in water with a solids content of 1% w / v has an absorbance at 420 nm of about 0.55 or below. Also disclosed is a method of preparing such compositions which includes a heating step to encourage the formation of melanoidins, highly coloured impurities, thereby permitting their removal by filtration providing a lighter coloured product.
Owner:GUILIN GFS MONK FRUIT CORP
Method for simultaneously extracting Mogroside V and VI and 11-O-based glucoside V
ActiveCN106967142AIncrease profitIncrease added valueGlycoside steroidsHigh concentrationMogroside V
The invention discloses a method for simultaneously extracting Mogroside V and VI and 11-O-based glucoside V. The method comprises the following steps: (1) dissolving Mogroside V refining mother solution, extracting by n-butyl alcohol to obtain a n-butyl alcohol layer; (2) concentrating, cooling, crystallizing, and filtering to obtain a crystal and crystallization mother solution, and drying the crystal to obtain the Mogroside VI; (3) concentrating the crystallization mother solution until the alcohol is not existent, dissolving, and passing through a polyamide chromatography column, collecting effluent, concentrating, and drying to obtain the Mogroside V; (4) eluting the chromatography column by using the low-concentration alcohol and the high-concentration alcohol, collecting high-concentration alcohol eluant, concentrating, cooling, crystallizing, filtering, and drying to obtain Momordica grosvenori 11-O-based glucoside V. The purity of the Mogroside VI obtained by the method is greater than or equal to 97%, and the yield is greater than or equal to 83%; the purity of the Mogroside V is greater than or equal to 89%, and the yield is greater than or equal to 89%; and the purity of the 11-O-based glucoside V is greater than or equal to 96%, and the yield is greater than or equal to 85%. The condition of the method is moderate, safe and environmental, and the method is suitable for the industrial production.
Owner:HUNAN HUACHENG BIOTECH
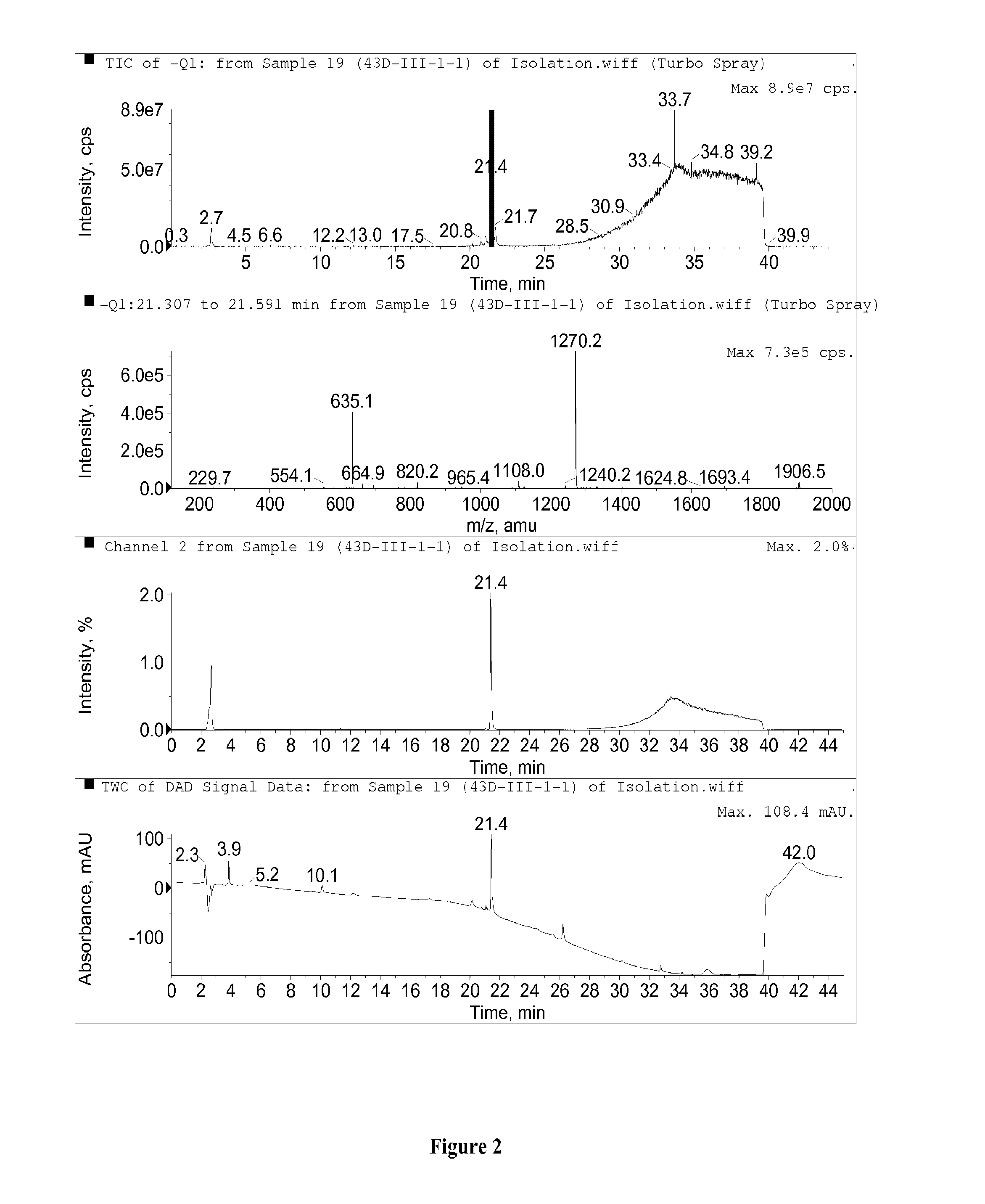
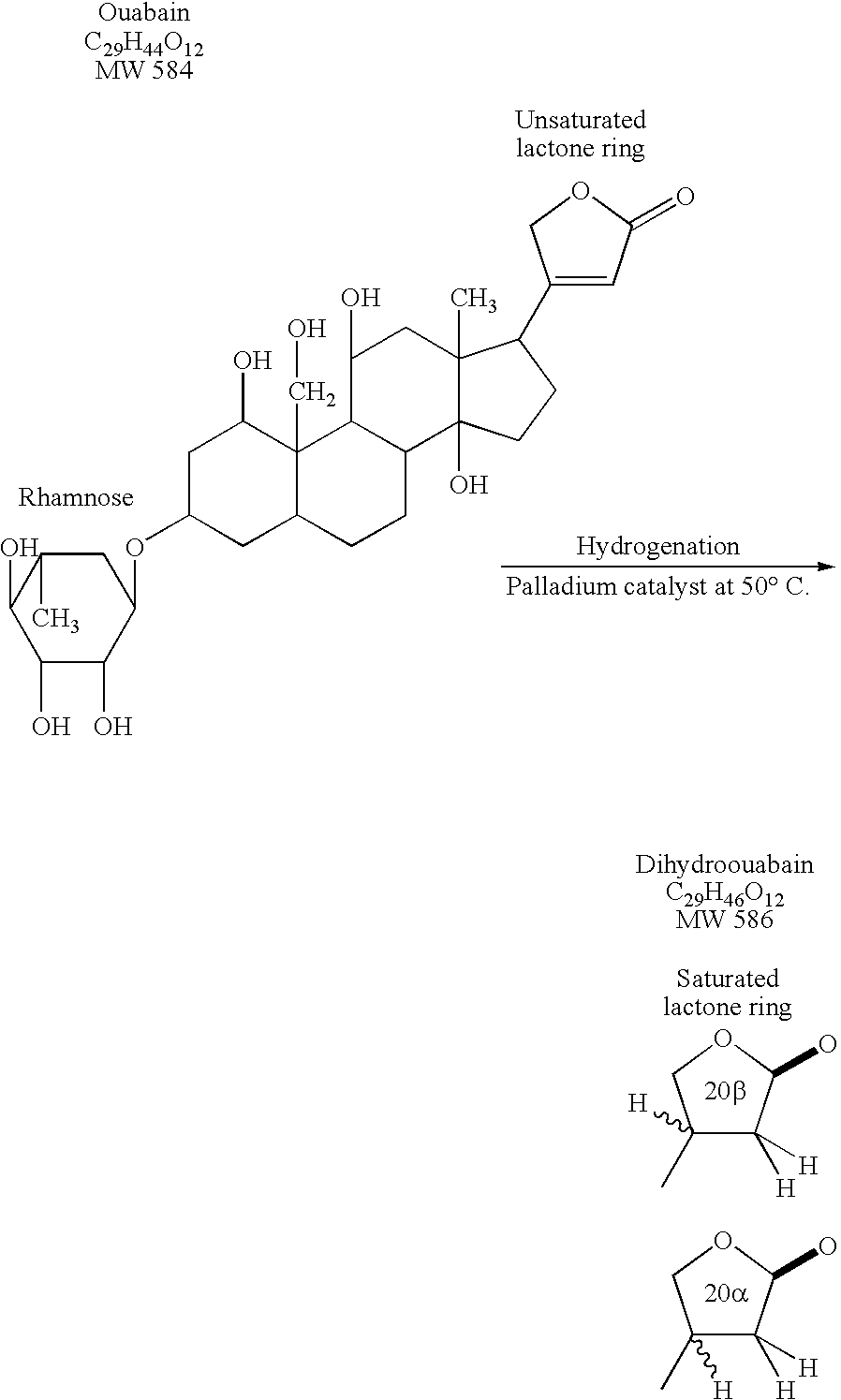
Mammalian dihydroouabain-like factor and therapeutic compositions
A novel mammalian dihydroouabain-like factor is disclosed which substantially fails to cross-react with mammalian ouabain-like factor (OLF) for binding to anti-OLF antibody, but cross-reacts with plant-related dihydroouabain (dho) for binding to anti-dho antibody, has maximal u.v. absorbance at 196 nm, has a non-peptidic, non-lipidic chemical structure and a fully hydrogenated lactone ring, has a concentration-dependent Na<+>,K<+>-ATPase (sodium pump) catalytic inhibitory activity which is 10-fold lower than OLF and 3-fold higher than plant-related dihydroouabain, and a high pressure liquid chromatography elution time about the same as dho. This factor is useful for therapy for congestive heart failure. An antibody and antibody fragments having affinity for mammalian Dh-OLF but not for OLF, and diagnostic and therapeutic methods comprise the antibody and means for quantifying the antibody and are useful for treating a condition caused by high level of OLF or Dh-OLF. Two isomers of plant-related dihydroouabain have been isolated. These compositions and methods are suitable for characterizing a variety of diseases and conditions associated with reduced sodium pump activity.
Owner:UNIV OF LOUISVILLE RES FOUND INC
Novel mogrosides, compositions and their purification
ActiveUS20160039864A1High strengthSugar derivativesGlycoside steroidsCombinatorial chemistryMogroside V
Novel mogrosides and methods for heir purification are provided herein. In addition, compositions comprising said novel mogrosides and methods for preparing the same are provided. The present invention relates generally to novel mogrosides, as well as compositions comprising such novel mogrosides, including consumables. The present invention further extends to methods of purifying such novel mogrosides, methods for preparing compositions (e.g., consumables) comprising such novel mogrosides and methods of enhancing the flavor or sweetness of consumables using these novel mogrosides.
Owner:THE COCA-COLA CO
Dihydroouabain-like factor and diagnostic & therapeutic compositions and methods
A novel mammalian dihydroouabain-like factor is disclosed which substantially fails to cross-react with mammalian ouabain-like factor (OLF) for binding to anti-OLF antibody, but cross-reacts with plant-related dihydroouabain (dho) for binding to anti-dho antibody, has maximal u.v. absorbance at 196 nm, has a non-peptidic, non-lipidic chemical structure and a fully hydrogenated lactone ring, has a concentration-dependent Na+,K+-ATPase (sodium pump) catalytic inhibitory activity which is 10-fold lower than OLF and 3-fold higher than plant-related dihydroouabain, and a high pressure liquid chromatography elution time about the same as dho. This factor is useful for therapy for congestive heart failure. An antibody and antibody fragments having affinity for mammalian Dh-OLF but not for OLF, and diagnostic and therapeutic methods comprise the antibody and means for quantifying the antibody and are useful for treating a condition caused by high level of OLF or Dh-OLF. Two isomers of plant-related dihydroouabain have been isolated. These compositions and methods are suitable for characterizing a variety of diseases and conditions associated with reduced sodium pump activity.
Owner:UNIV OF LOUISVILLE RES FOUND INC
Separation and purification method of high-purity mogroside V
ActiveCN106279339ASimple processEasy to operateGlycoside steroidsPurification methodsUltrafiltration
The invention discloses a separation and purification method of high-purity mogroside V. The separation and purification method comprises the following steps: firstly, dissolving and centrifuging: taking a momordicagrosvenori crude product, and adding water for dissolving and centrifuging to obtain centrifugal liquid; secondly, ultrafiltration, nanofiltration and concentration: performing ultrafiltration, nanofiltration and vacuum concentrationon on the centrifugal liquid to obtain a concentrated solution; thirdly, sample mixing of silica gel, eluting and concentrating: performing mixing and sample mixing on the concentrated solution and the silica gel, drying, loading into a silica gel chromatography column, eluting, collecting objective fractions in a segmented manner, and concentrating and drying to obtain mogroside V dry powder; fourthly, crystallizing, washing and drying: dissolving the mogroside V dry powder, filtering, crystallizing, washing a crystal and drying to obtain a high-purity mogroside V product. The purity of the high-purity mogroside V product obtained by the method disclosed by the invention is greater than or equal to 98.4 percent, and a final yield of the high-purity mogroside V product is greater than 90 percent; the method disclosed by the invention breaks limitations of the prior art and is suitable for industrial production; a process flow is simplified; the method has the advantages of high operability, safety, environmental protection and low cost.
Owner:HUNAN HUACHENG BIOTECH
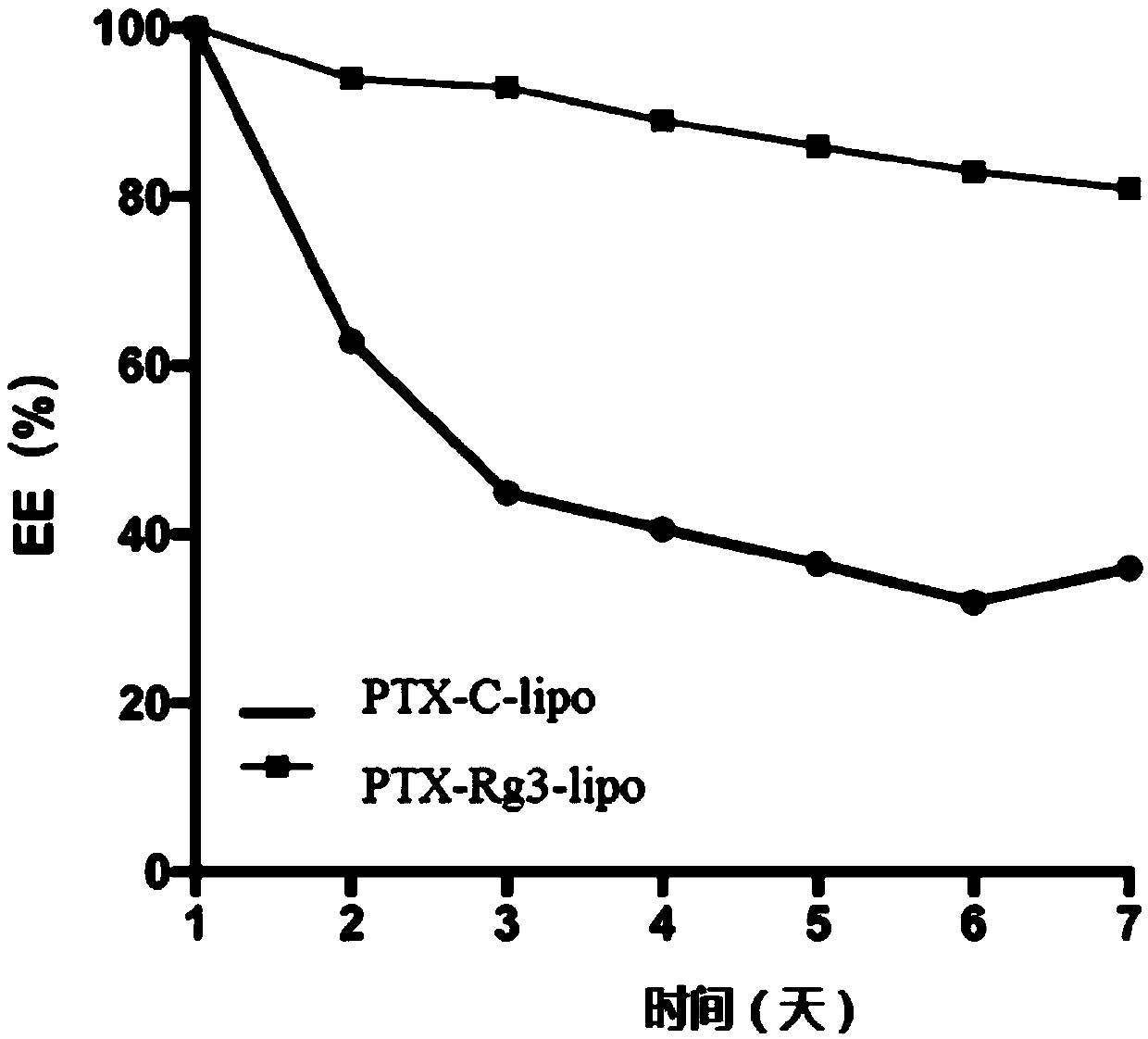
Blank liposome taking ginsenoside Rg3 or analogue thereof as membrane material, and preparation method and application thereof
ActiveCN111228219AHigh activityImprove uniformityOrganic active ingredientsOrganic compounds purification/separation/stabilisationLipid formationNucleotide
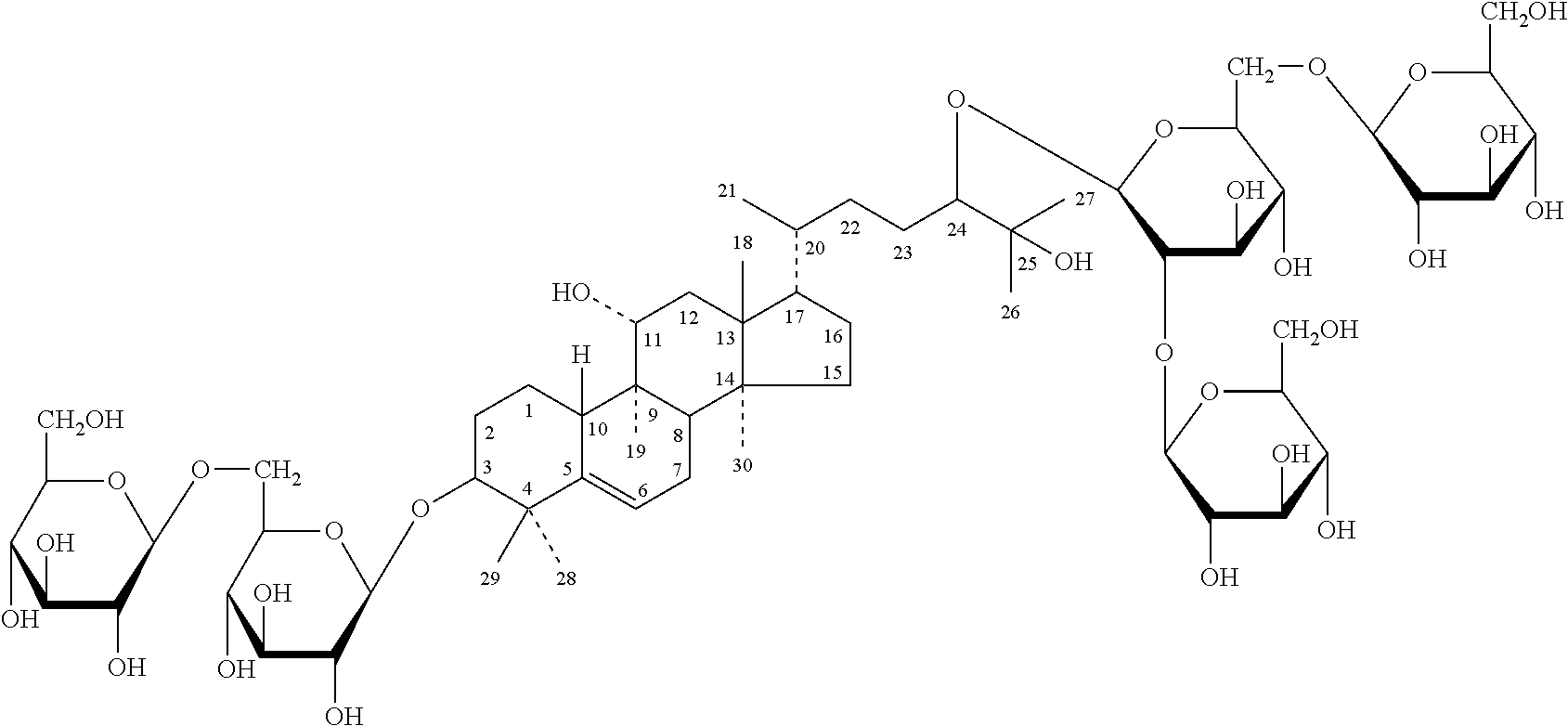
The invention discloses a blank liposome taking ginsenoside Rg3 or an analogue thereof as a membrane material, and a preparation method and application thereof. The ginsenoside as shown in the formulaI is blank liposome of a membrane material, wherein the blank liposome is provided with a membrane, and the membrane comprises lipid substances and the ginsenoside as shown in the formula I. The blank liposome disclosed by the invention has the characteristics of good film-forming property, encapsulation efficiency, targeting property, long circulation, stability, safety and uniformity, is used for encapsulating active substances, biological agents, polynucleotides or oligonucleotides in medicines and cosmetics to form the liposome loaded with the active substances, and is simple and convenient in preparation process.
Owner:XIAMEN GINPOSOME PHARM CO LTD
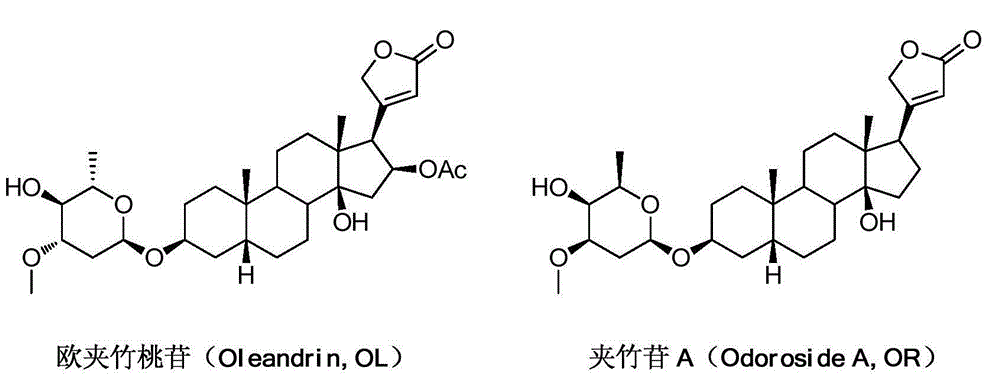
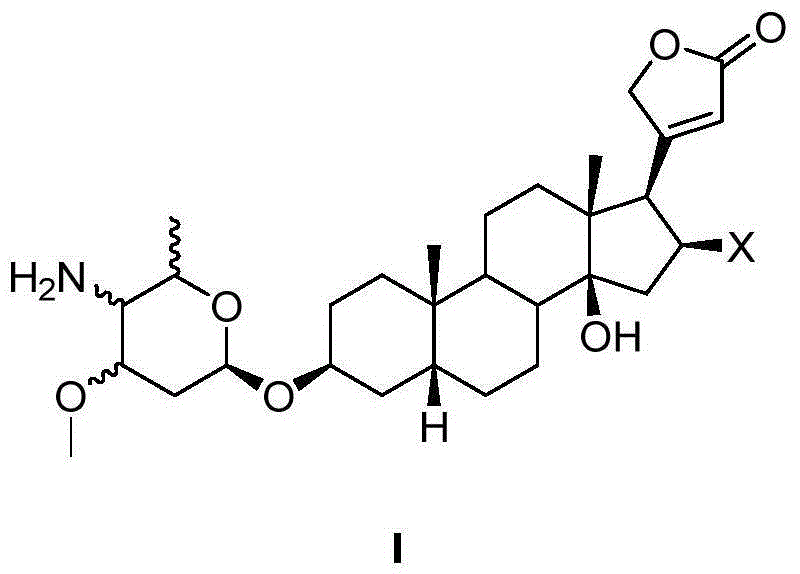
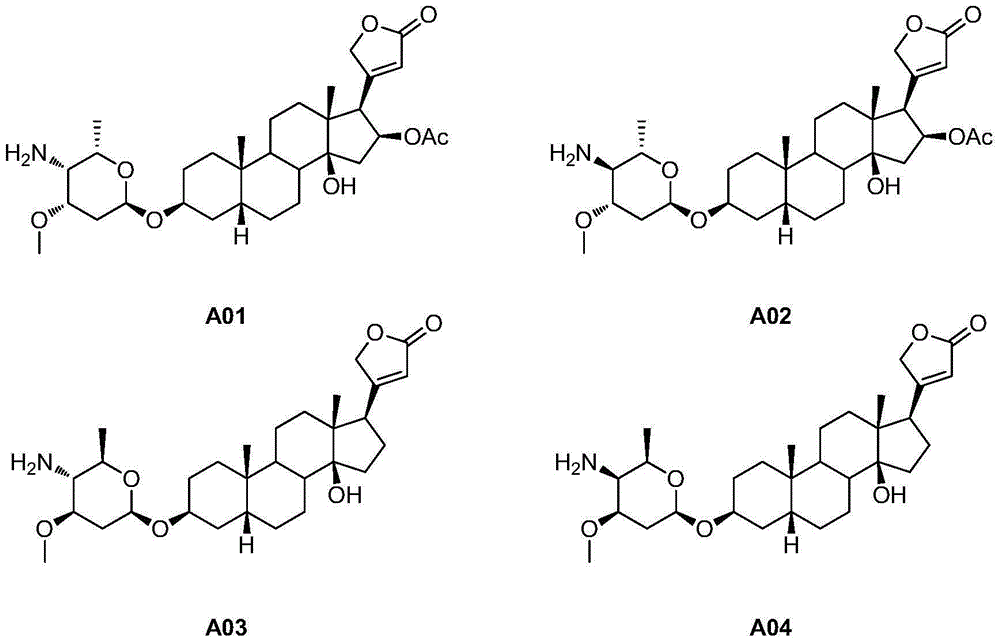
4'-amino-4'-dehydroxyl-oleandrin and 4'-amino-4'-dehydroxyl-odoroside A and use thereof
InactiveCN105037474AEasy to synthesizeEasy to manufactureOrganic active ingredientsGlycoside steroidsOleandrinTumor cells
The invention relates to 4'-amino-4'-dehydroxyl-oleandrin and 4'-amino-4'-dehydroxyl-odoroside A and use thereof. The invention provides a compound represented by the following general formula I shown in the description or pharmaceutically acceptable salts thereof, a preparation method therefor, a pharmaceutical composition containing the compound represented by the following general formula I shown in the description or pharmaceutically acceptable salts thereof and use thereof. The compound has inhibitory activity to the propagation of humanized tumor cell lines and can serve as a drug for treating malignant tumors.
Owner:SHANGHAI INST OF MATERIA MEDICA CHINESE ACAD OF SCI
Process for preparing an edible composition comprising steroidal glycosides
A process for preparing an edible aqueous dispersion of one or more steroidal glycosides, the edible dispersion comprising one or more steroidal glycoside, and a food product comprising the dispersion, as well as methods of controlling body weight or obesity by ingesting the food product.
Owner:CONCOPCO INC
Dicarboxylic acid ester derivatives of ginsenoside, pharmaceutical preparations containing the same, and preparation thereof
InactiveUS20060234956A1Increase concentrationBiocideOrganic active ingredientsProtopanaxadiolSuccinic acid
The present invention relates to a series of dicarboxylic acid ester derivatives of ginsenosides such as succinate and glutarate derivatives of 20-O-β-D-glucopyranosyl-protopanaxadiol (compound K, abbreviated as CK), preparation thereof and pharmaceutical uses thereof. The dicarboxylic acid ester derivatives of ginsenosides of the present invention can be used to form pharmaceutical acceptable salts thereof having a high water solubility or can be directly dissolved in an aqueous solution of metal salt, and retain the pharmaceutical activities of ginsenosides such as tumor growth inhibition and cancer preventive cytotoxicity. The dicarboxylic acid ester derivatives of ginsenosides of the present invention are thus suitable for use in the manufactures of various pharmaceutically and cosmetically acceptable dosage forms of preparations, such as peripheral, oral, and topical dosage forms.
Owner:AMERSEN BIOSCI INT
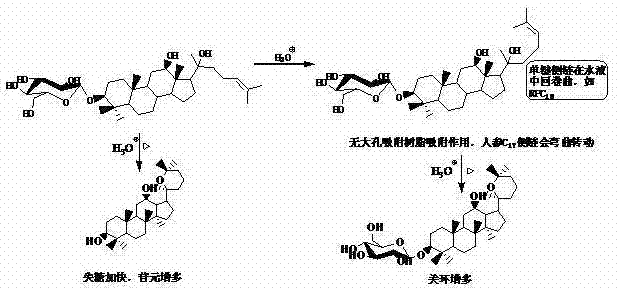
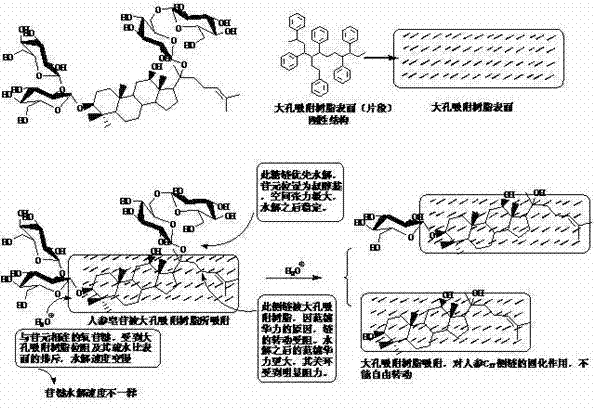
Method for preparing aglycone and secondary glucoside through various glycoside hydolysis assisted by macroporous adsorption resin
InactiveCN103772337AHigh purityHigh yieldSugar derivativesGlycoside steroidsProtopanaxadiolSide chain
Various glycoside compounds and particularly glycoside compounds which are unstable, easy to oxidize and difficult to dissolve in water and an organic solvent are hydrolyzed under the actions of adsorption, dispersion, curing and settlement of macroporous adsorption resin to generate aglycone or a mixture of the aglycone and secondary glucoside, and the macroporous adsorption resin is secondarily eluted or primarily eluted by using an organic solvent to obtain aglycone, secondary glucoside and a mixture of the aglycone and the secondary glucoside. The invention provides a universal method for preparing the aglycone and the secondary glucoside by using the various glycoside compounds, the method is simple in operation, almost integrated in hydrolysis and separation, high in product purity, good in product yield, free of expensive reagent, capable of realizing repeated utilization of hydrolysate and the macroporous adsorption resin, environment-friendly, easy for realizing industrial production and low in cost and has great advantages as comparison with an enzyme hydrolysis method, a fermentation method and the like. By taking the hydrolysis of the total saponin of panax ginseng as an example, through the hydrolysis of acetic acid and other acids, more prosapogenins Rh2 of panax ginseng and protopanaxadiol aglycones are obtained, but a C17 side chain cyclization product is not obvious.
Owner:闻永举
Novel blank liposomes taking ginsenoside derivatives as membrane materials, and preparation method and application thereof
ActiveCN109833298AHigh activityLow hemolyticOrganic active ingredientsCosmetic preparationsLiposome membraneNiosome
The invention discloses novel blank liposomes taking ginsenoside derivatives as membrane materials as well as a preparation method and application thereof. The ginsenoside derivatives used in the novel blank liposomes taking the ginsenoside derivatives as the membrane materials are high in activity and low in hemolytic activity, and can be used as liposome membrane materials for preparing blank liposomes; moreover, structures of some of the compounds are novel. In addition, the novel blank liposomes prepared by using the ginsenoside derivatives meet the requirements for hemolytic activity; andthus, the liposomes are higher in safety, better in film-forming properties and more excellent in stability. Therefore, the liposomes are of important application values.
Owner:XIAMEN GINPOSOME PHARM CO LTD
Method for preparing mogroside IV
InactiveCN102477455AIncrease contentSimple processGlycoside steroidsFermentationAlgluceraseBeta-Glucosidases
The invention which relates to a method for preparing mogroside IV belongs to the traditional Chinese medicinal field. A technical problem to be solved in the invention is to provide a low cost method for preparing the mogroside IV. The method for preparing the mogroside IV in the invention is characterized in that mogroside V, Momordica grosvenori, or a Momordica grosvenori extract containing the mogroside V is hydrolyzed with beta-glucosidase. The method of the invention, which has the advantages of simple technology and low cost, allows the content of the mogroside IV in Momordica grosvenori powder to be improved to above 1.5% from below 0.05%; and the method allows the content of the mogroside IV in the Momordica grosvenori extract to be improved to above 20%, wherein the content of the mogroside V in the Momordica grosvenori extract is 40%, and the original content of the mogroside IV in the Momordica grosvenori extract is 1.5%. The purity of the mogroside IV can reach above 98% after separation and purification. The method of the invention, which provides a new approach for the preparation of the mogroside IV, has a wide application prospect.
Owner:成都普瑞法科技开发有限公司
Saponin compounds or pharmaceutically acceptable salts thereof, compositions, and preparation method of saponin compounds or pharmaceutically acceptable salts therof, and application of saponin compounds or pharmaceutically acceptable salts thereof and compositions
ActiveCN111187331ANovel side chain structureActiveOrganic active ingredientsAntipyreticSide chainPharmaceutical medicine
The invention relates to saponin compounds or pharmaceutically acceptable salts thereof, compositions, and a preparation method of the saponin compounds or the pharmaceutically acceptable salts thereof, and application of the saponin compounds or the pharmaceutically acceptable salts therof and the compositions. Chemical components of gynostemma pentaphylla are subjected to systematic extraction,separation and purification by using various column chromatographic separation methods, and the compound having a dammarane tetracyclic triterpenoid structures and containing a glycosyl group in a side chain is obtained through separation. The compounds obtained by separation according to a technical scheme in the invention have anti-inflammatory, anti-tumor, antioxidant and lipid-lowering activities and the like.
Owner:TIANJIN UNIV OF TRADITIONAL CHINESE MEDICINE
Mogrosides, compositions and their purification
Novel mogrosides and methods for heir purification are provided herein. In addition, compositions comprising said novel mogrosides and methods for preparing the same are provided. The present invention relates generally to novel mogrosides, as well as compositions comprising such novel mogrosides, including consumables. The present invention further extends to methods of purifying such novel mogrosides, methods for preparing compositions (e.g., consumables) comprising such novel mogrosides and methods of enhancing the flavor or sweetness of consumables using these novel mogrosides.
Owner:THE COCA-COLA CO
Industrialization preparation method of mogroside V
The invention discloses an industrialization preparation method of mogroside V. The industrialization preparation method comprises the following steps: I, preparing a crude mogroside product by using a digestion and dehydration method; II, preparing the crude mogroside product into a solution by using water, and filtering off undissolved substances; III, pumping the mogroside solution into a dynamic axial compression column preparative chromatographic system, wherein the column is filled with reversal phase special silica gel as packing, the feeding amount of the packing is 0.1-3%, and an organic solvent solution is adopted as the flowing phase in the column; firstly, eluting for 30-50 minutes when controlling the initial gradient of the organic solution to (2:8) to (4:6) and the flow speed to be 100ml / minute, further eluting to 50-60 minutes when adjusting the gradient to be greater than or equal to 1, adjusting the detection wavelength of an ultraviolet luminosity detector to be 203nm, injecting the flow phase into the dynamic axial compression column preparative chromatographic system for liquid chromatogram detection, and collecting eluant corresponding to a chromatographic peak on line, thereby obtaining mogroside V of which the purity is greater than 95%. By adopting the industrialization preparation method disclosed by the invention, a treatment process is simplified, on-line real-time detection is achieved, the security is improved, the cost is saved, and ideal purity of mogroside V is achieved.
Owner:黄晓 +1
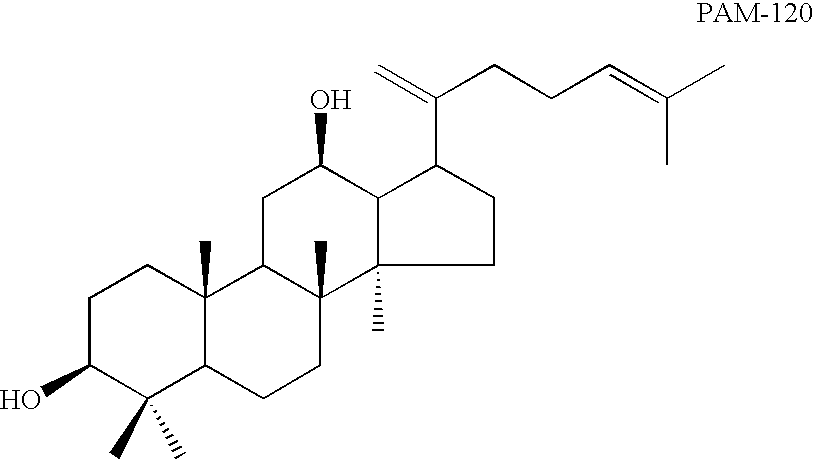
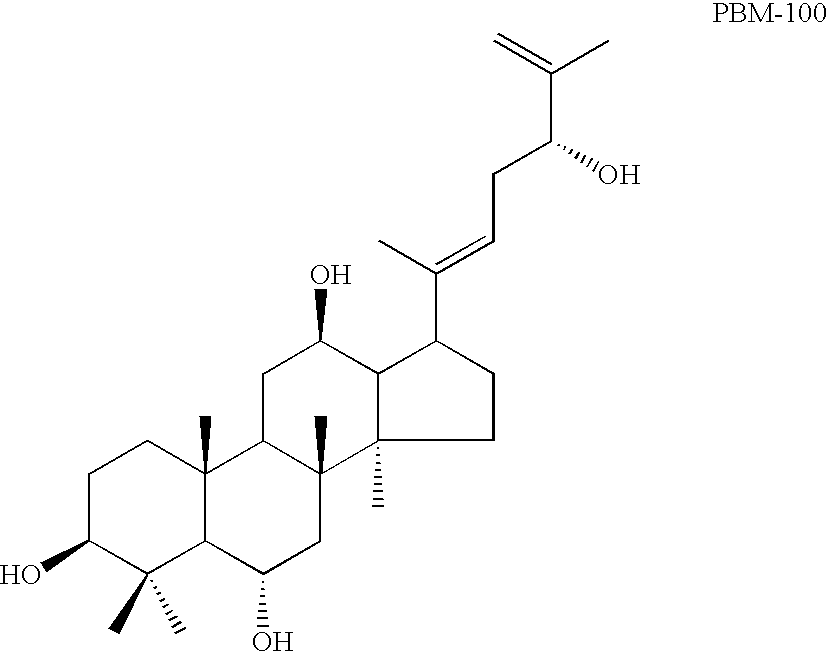
Novel dammarane sapogenins, their use as anti-cancer agents, and a process for producing same
This invention relates to a group of novel sapogenins, their use in anti-cancer applications, and to a process for their production. More particularly, this invention pertains to a novel group of dammarane sapogenins, PAM-120, PBM-110 and PBM-100 (the dammarance sapogenine structure is specifically clean of any sugar moieties (glycons) at any position and hydroxyl at C-20) and PAN-20 and PAN-30 (the dammarance sapogenin structure has sugar moieties but is free of hydroxyl at C-20), obtained by chemical cleavage of dammarane saponins. The invention also includes a novel application of the said sapogenins for anti-cancer treatment by using them separately or together, and / or jointly with other drugs, as well as to the process of producing these novel sapogenins. Said novel dammarane sapogenins show surprising anti-cancer effect when applied, particularly against multi-drug resistant cancers.
Owner:PANAGIN PHARMA INC
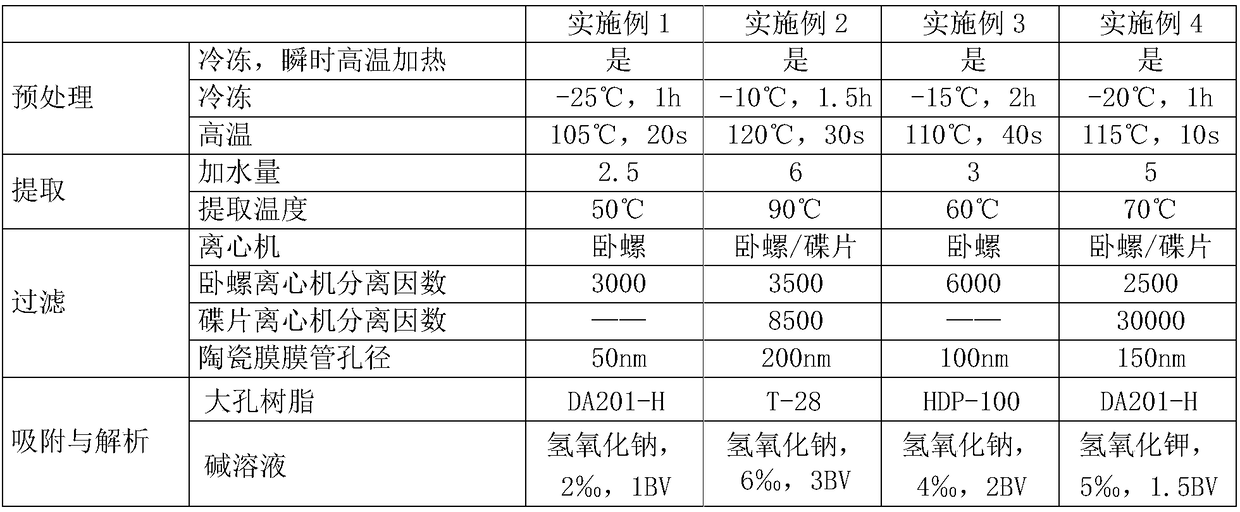
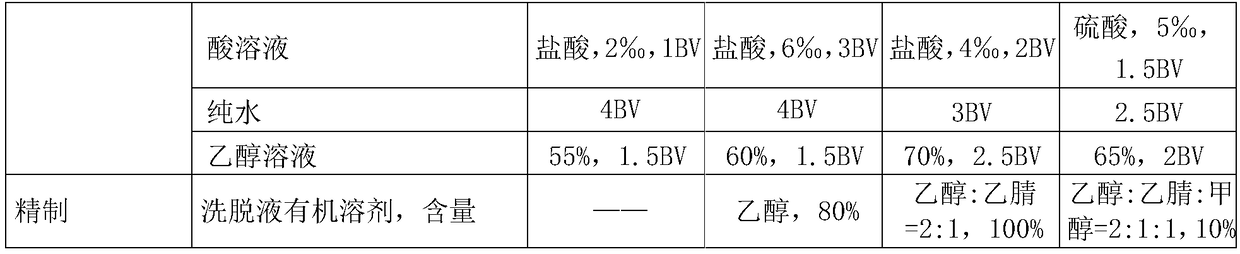
Production method for high-content mogroside V
The invention discloses a production method for high-content mogroside V, which belongs to the field of natural plant extract production. Grosvenor momordica fruits which are immediately treated by instantaneous high-temperature heating after being frozen are adopted as a raw material. The invention has the advantages of low extraction cost, high yield, high product purity, little water consumption, simple process, capability of realizing continuous industrial mass production and the like.
Owner:CHENGUANG BIOTECH GRP CO LTD
Extraction process of ginseng saponin Rd
ActiveCN104610410APerfect purification stepsTrial production results showGlycoside steroidsN-ButanolSilica gel
The invention discloses an extraction process of ginseng saponin Rd. The extraction process comprises the following steps: S1, grinding ginseng saponin Rd-containing herbal materials into herbal powder, performing reflux extraction by using 70-95% ethanol, recycling the ethanol, and concentrating to obtain a total extract; S2, taking the total extract, enabling the total extract to pass through D101 macroporous adsorption resin, eluting by using 40-80% ethanol, recycling the ethanol, and performing deconperssion concentration to obtain total saponin; S3, taking the total saponin, enabling the total saponin to pass through a silica gel chromatography column, performing isocratic elution by using a mixture of n-butanol, ethyl acetate and water, performing thin layer chromatography, combining fractions to obtain crude ginseng saponin Rd; S4, taking the crude ginseng saponin Rd, enabling the crude ginseng saponin Rd to pass through an Rp-C18 silica gel column, performing isocratic elution by using a mixture of methanol and water, performing thin layer chromatography, combining required components, separating out precipitate to obtain the ginseng saponin Rd. By the extraction process, the ginseng saponin Rd separating and purifying effects can be improved, use of a toxic organic reagent is avoided, the operation is simple and convenient and a production process is easy to control; therefore, the extraction process is suitable for industrial production.
Owner:GUIZHOU XINBANG PHARMACEUTICAL CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com