Saponin compounds or pharmaceutically acceptable salts thereof, compositions, and preparation method of saponin compounds or pharmaceutically acceptable salts therof, and application of saponin compounds or pharmaceutically acceptable salts thereof and compositions
A kind of technology of compounds and saponins, applied in the field of natural product medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
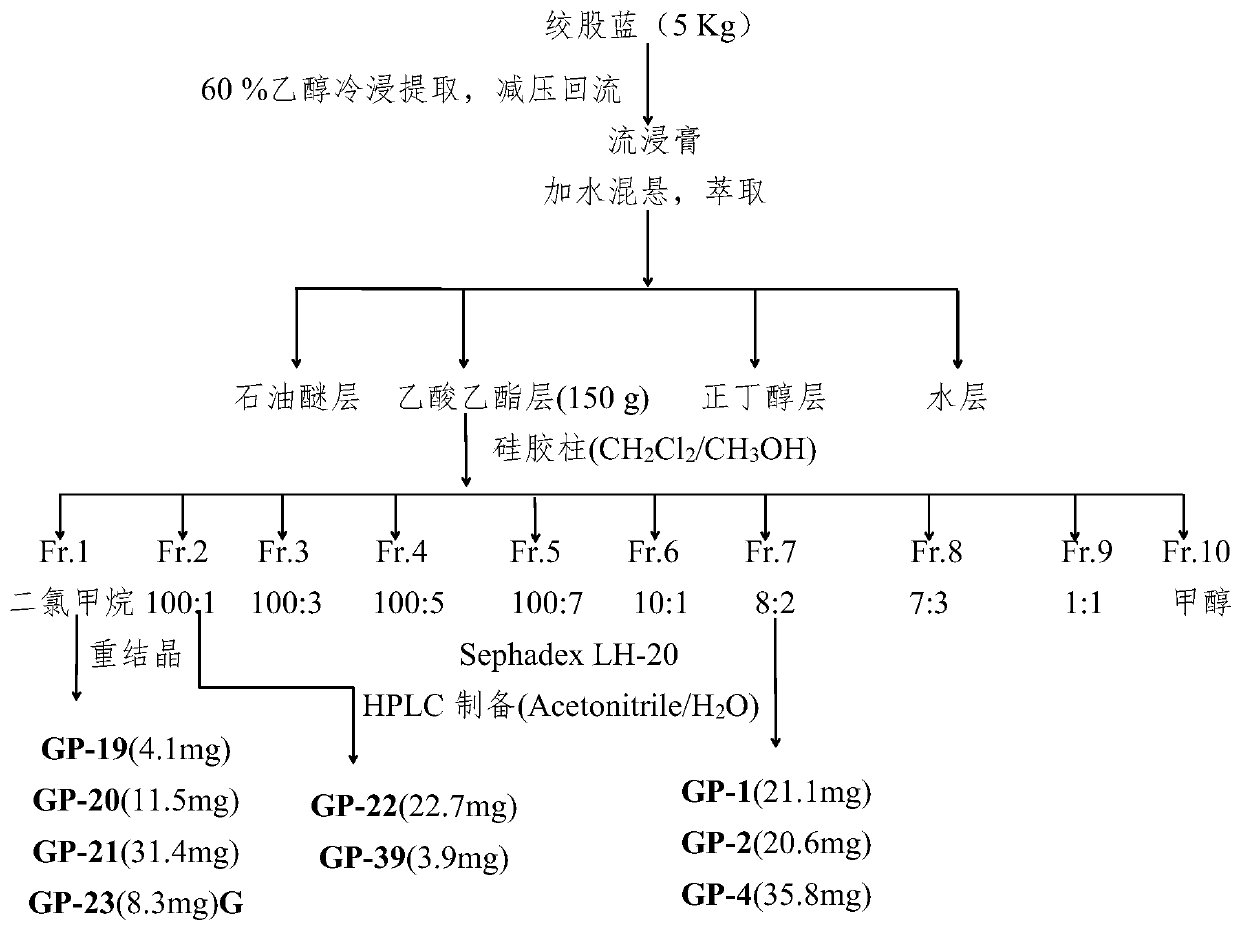
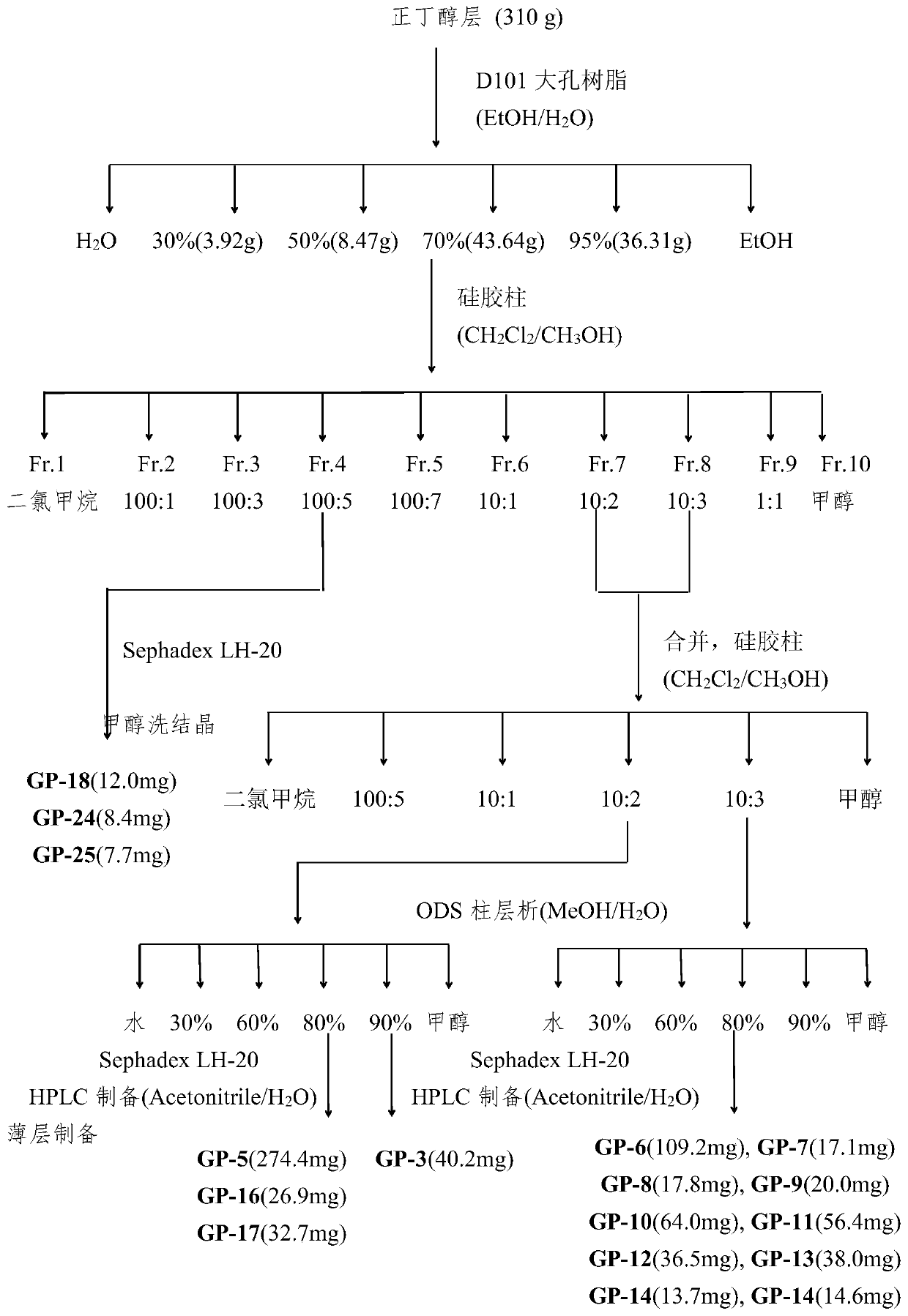
[0062] Such as figure 1 As shown, Gynostemma 5kg, 60% ethanol at room temperature soaked and extracted three times, once a week, filtered, concentrated under reduced pressure to obtain the extract extract, added an appropriate amount of water to suspend, and extracted three times with petroleum ether, ethyl acetate and n-butanol respectively . The combined extracts were concentrated under reduced pressure to obtain extracts. The ethyl acetate layer (150g) and n-butanol layer (310g) were taken for separation and purification. Using silica gel chromatographic column (100-200 mesh), gradient elution with dichloromethane-methanol system to obtain 10 fractions (Fr.1-10), each fraction was subjected to repeated column chromatography, recrystallization, and preparation of high-efficiency liquid phase Methods 30 compounds were obtained.
Embodiment 2
[0064] 1) Compound GP-1:
[0065] 3β,12β,20S-trihydroxy-24-hydroperoxydammar-25-ene-20-O-[β-D-glucopyranosyl(1→2)]-β-D-glucopyranoside.
[0066]
[0067] White powder (methanol), HR-ESI-MS gave m / z 861.4809 [M-H+HCOOH] - Peak (Calcd for: 861.4848), the molecular weight was determined to be 816. combine 1 H-NMR and 13 C-NMR confirms that the molecular formula is C 42 h 72 o 15 , 10% sulfuric acid-ethanol showed purple spots, the Liebermann-Burchard reaction was positive, and the Molish reaction was positive, suggesting that it was a triterpenoid saponin compound
[0068] 1 H-NMR (600MHz, pyridine-d 5 ) gives two groups of sugar terminal hydrogen signals: δ H 5.12 (1H, d, J = 7.8Hz), 5.80 (1H, d, J = 7.8Hz); 7 methyl proton signals: δ H 0.97(3H,s),1.03(3H,s),1.62(3H,s),1.96(3H,s),1.25(3H,s),0.87(3H,s),0.93(3H,s);1 Group ethylenic proton signal: δ H 5.23(1H,s), 5.07(1H,m).
[0069] 13 C-NMR (150MHz, pyridine-d 5 ) and DEPT-135 give 42 carbon signals, 2 sugar ter...
Embodiment 3
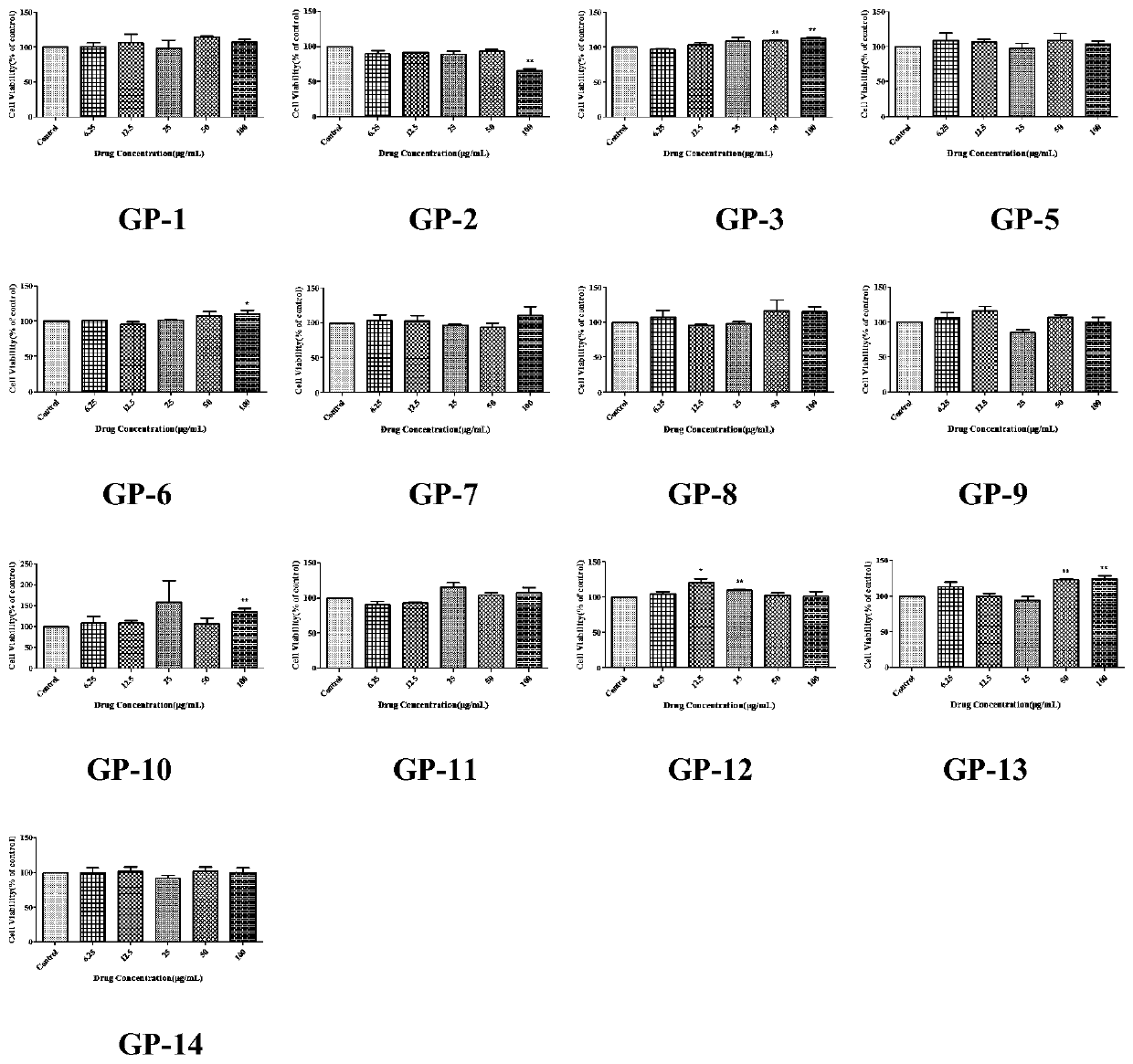
[0167] Cytotoxicity detection of compounds in Gynostemma pentaphyllum:
[0168] Dissolve the 14 saponins isolated from Gynostemma pentaphyllum: GP-1 to GP-3, GP-5 to GP-13, and GP15 respectively in DMSO, and prepare a stock solution with a concentration of 1 mg / mL and store at -20°C Store in the refrigerator and dilute to the desired concentration before use.
[0169] DMEM (high glucose) medium (Hyclone, USA); fetal bovine serum (PBS, Hyclone, USA); double antibody (100U / mL penicillin + 10mg / mL streptomycin); Cell Counting Kit-8 kit was purchased from Dojindo company.
[0170] Take 45 mL of DMEM / High Gluose basal medium, add 5 mL of fetal bovine serum (final concentration 10%) and 500 μL double antibody solution (final concentration of penicillin 100 U / mL, final concentration of streptomycin 0.1 mg / mL), store in 4°C refrigerator for later use.
[0171] Weigh 0.2g KCl, 8g NaCl, 0.27g KH2PO4, 1.42gNa2HPO4, add ultrapure water and stir it with a magnetic stirrer to dissolve it...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com