Method for preparing aglycone and secondary glucoside through various glycoside hydolysis assisted by macroporous adsorption resin
A technology of pore adsorption and secondary glycosides, applied to secondary glycosides and aglycones, can solve the problems of high cost, lack of industrialization, and limited usage, and achieve the effect of increasing yield and improving stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
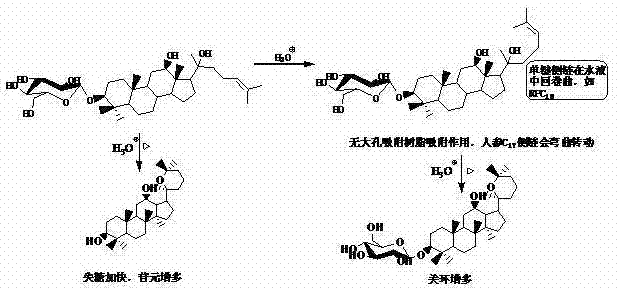
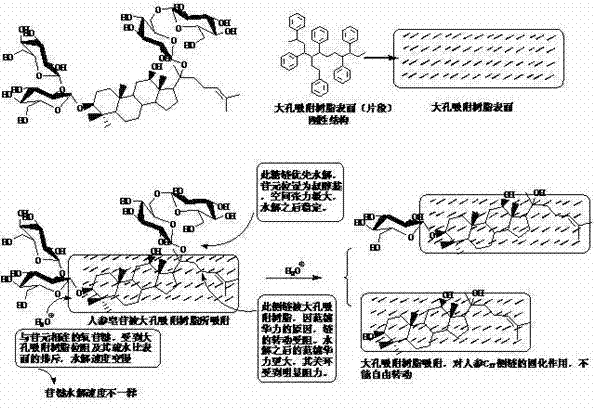
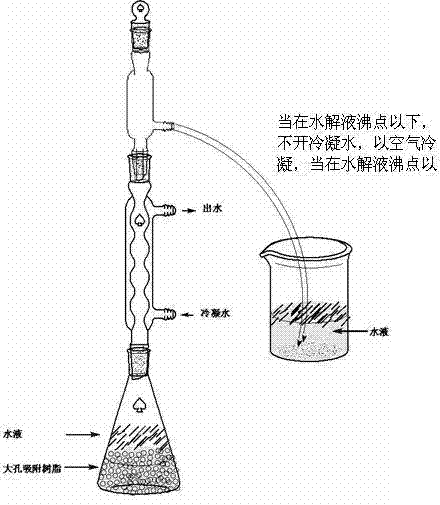
[0028] Due to the glycoside compounds involved, there are many types and a large number. Therefore, each glycoside compound cannot be illustrated, but in order to further understand the present invention, the role played by the macroporous adsorption resin in the hydrolysis process of glycosides is classified and described, and the examples are as follows:
[0029] The first category: for easily oxidized aglycone, the macroporous adsorption resin can absorb, disperse and settle to prevent its oxidation.
[0030] Example 1:
Embodiment 1
[0032] Example 2:
Embodiment 2
[0034] Example 3:
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com