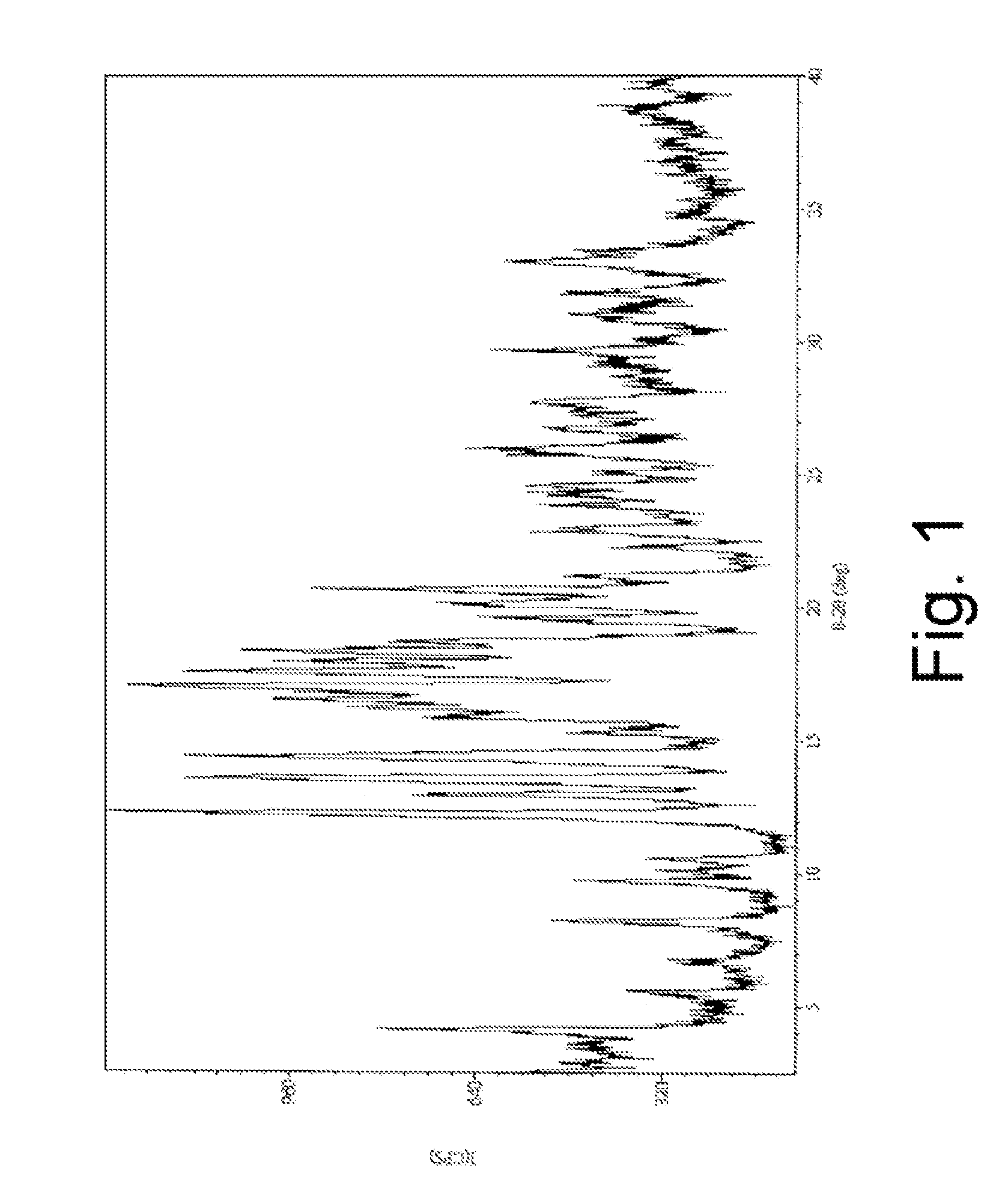
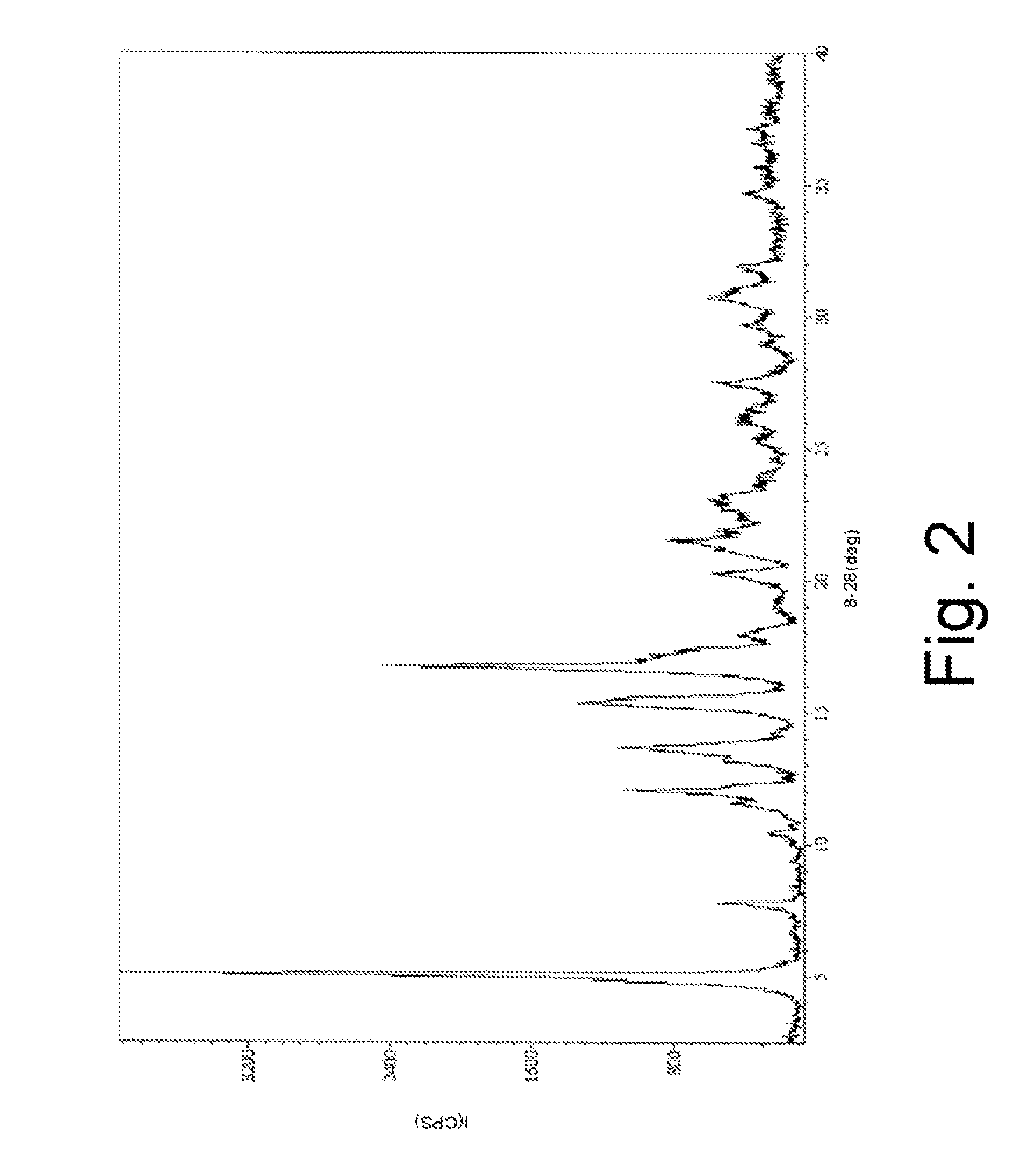
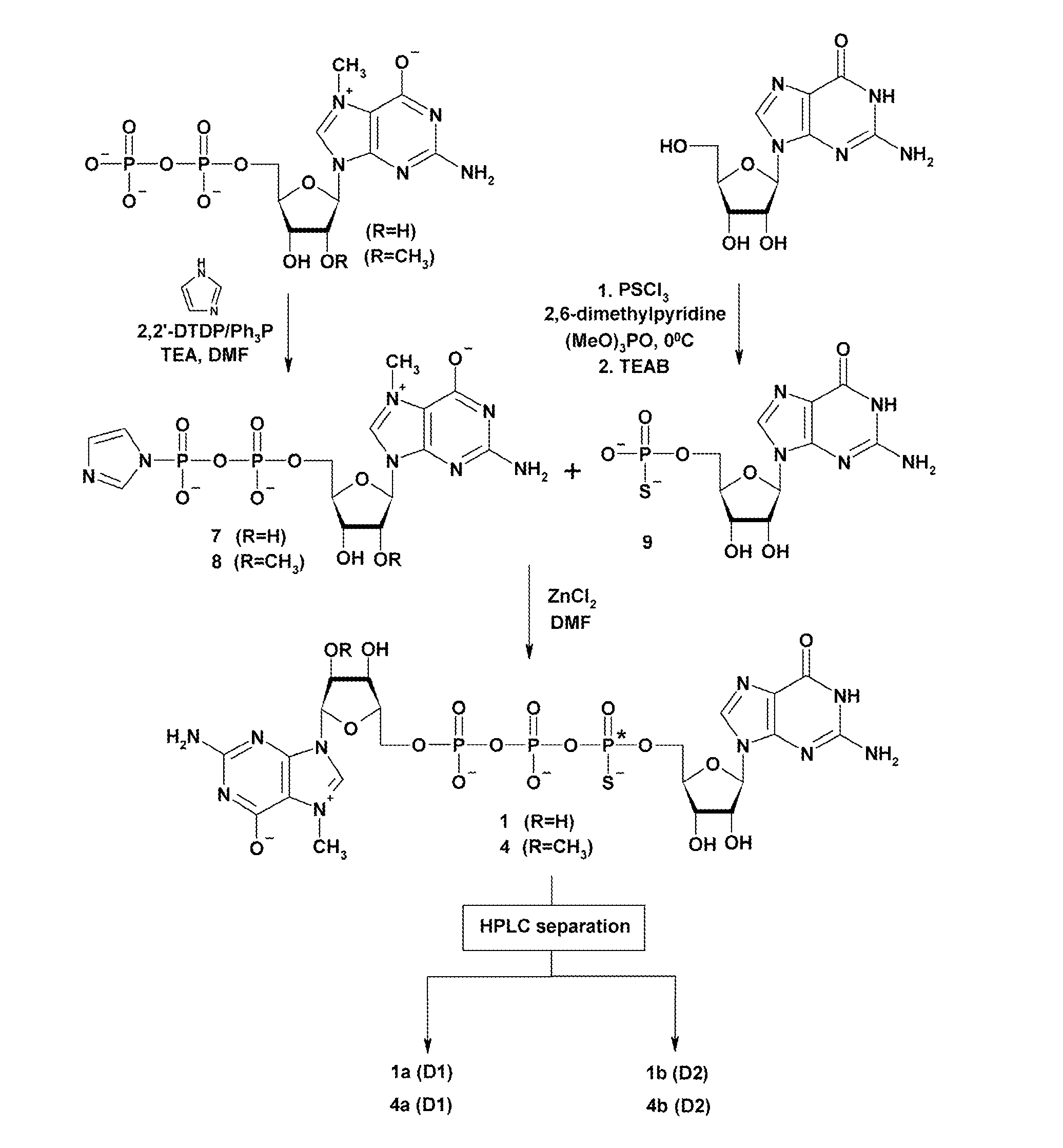
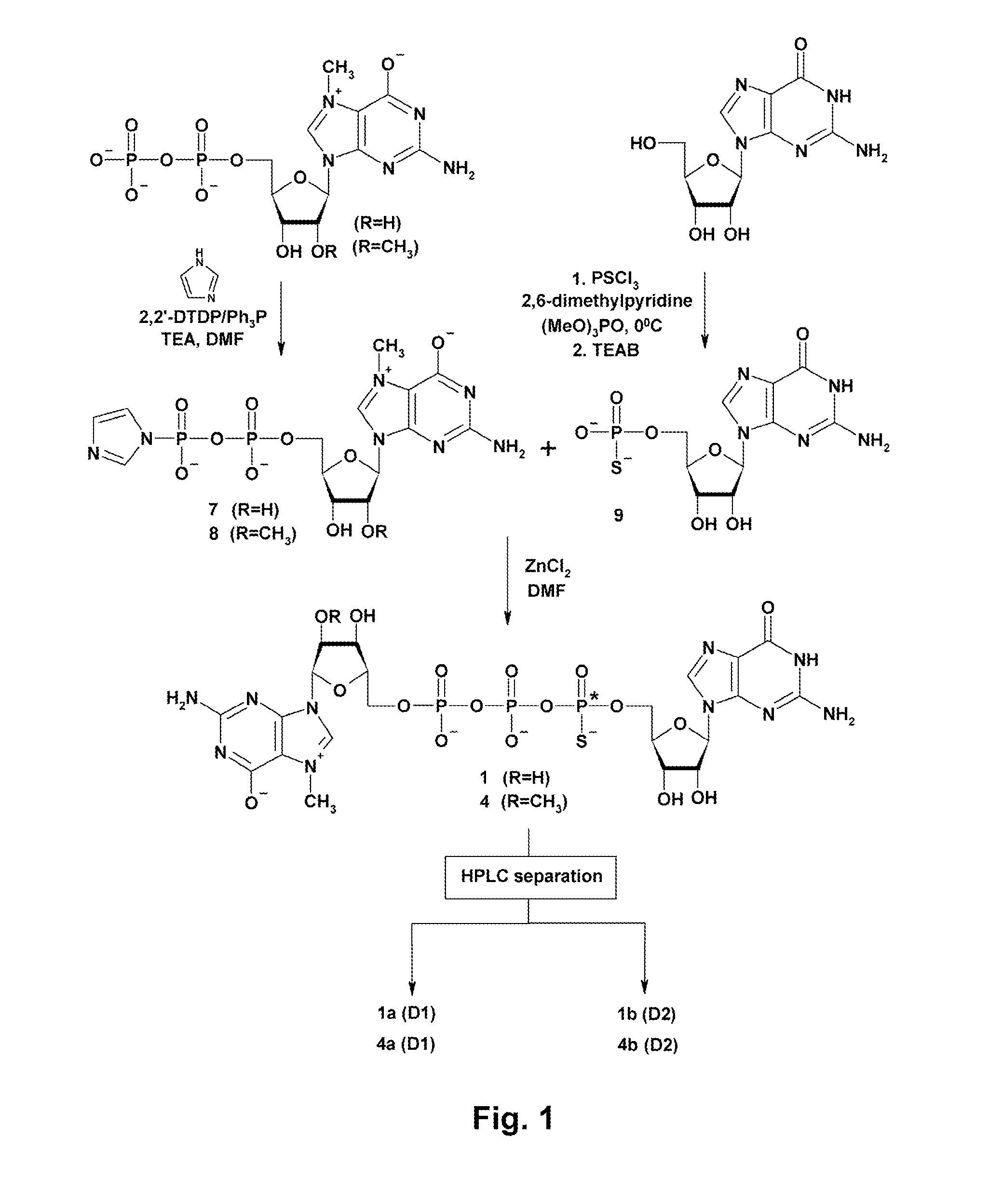
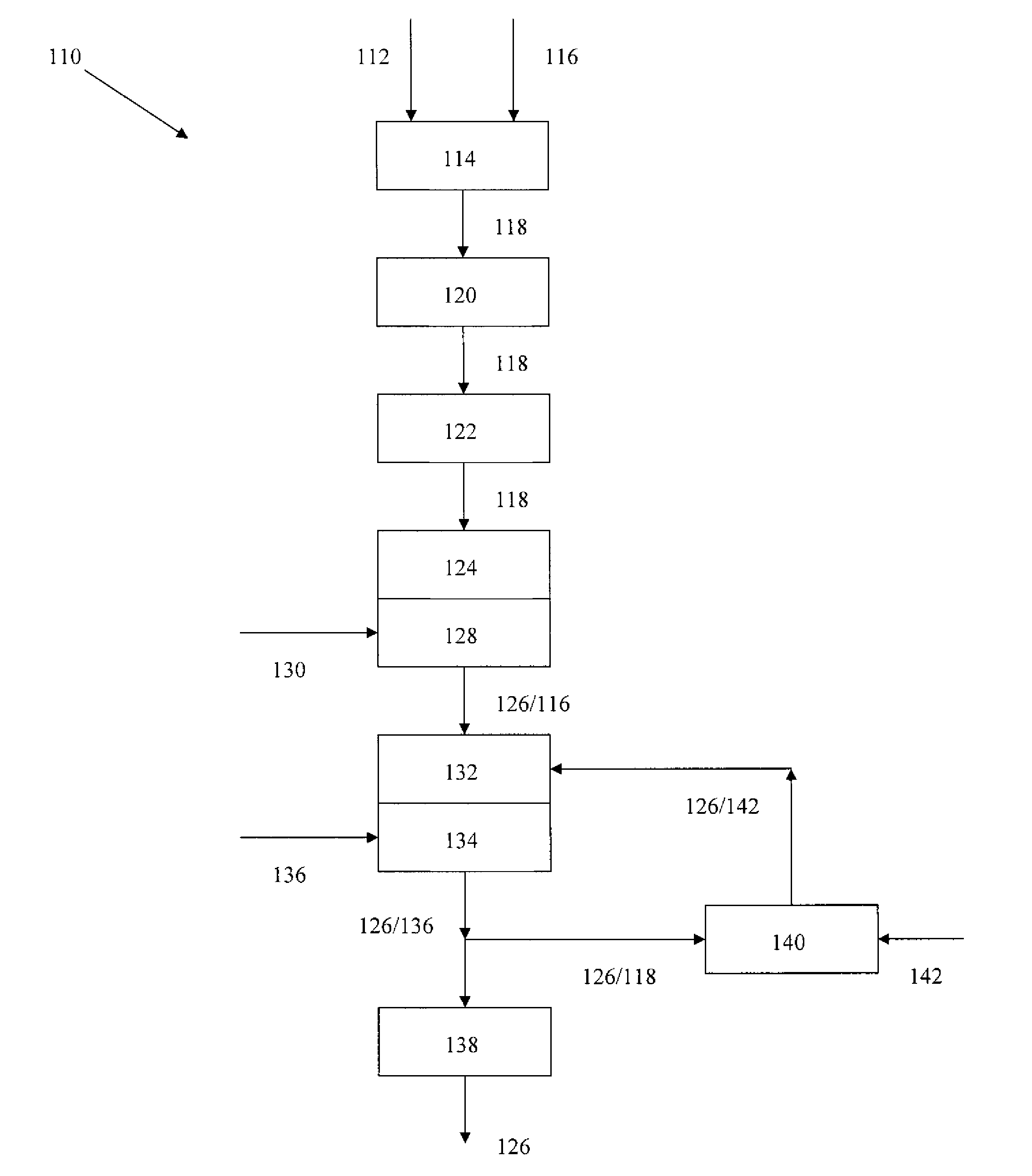Patents
Literature
18281results about "Sugar derivatives preparation" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
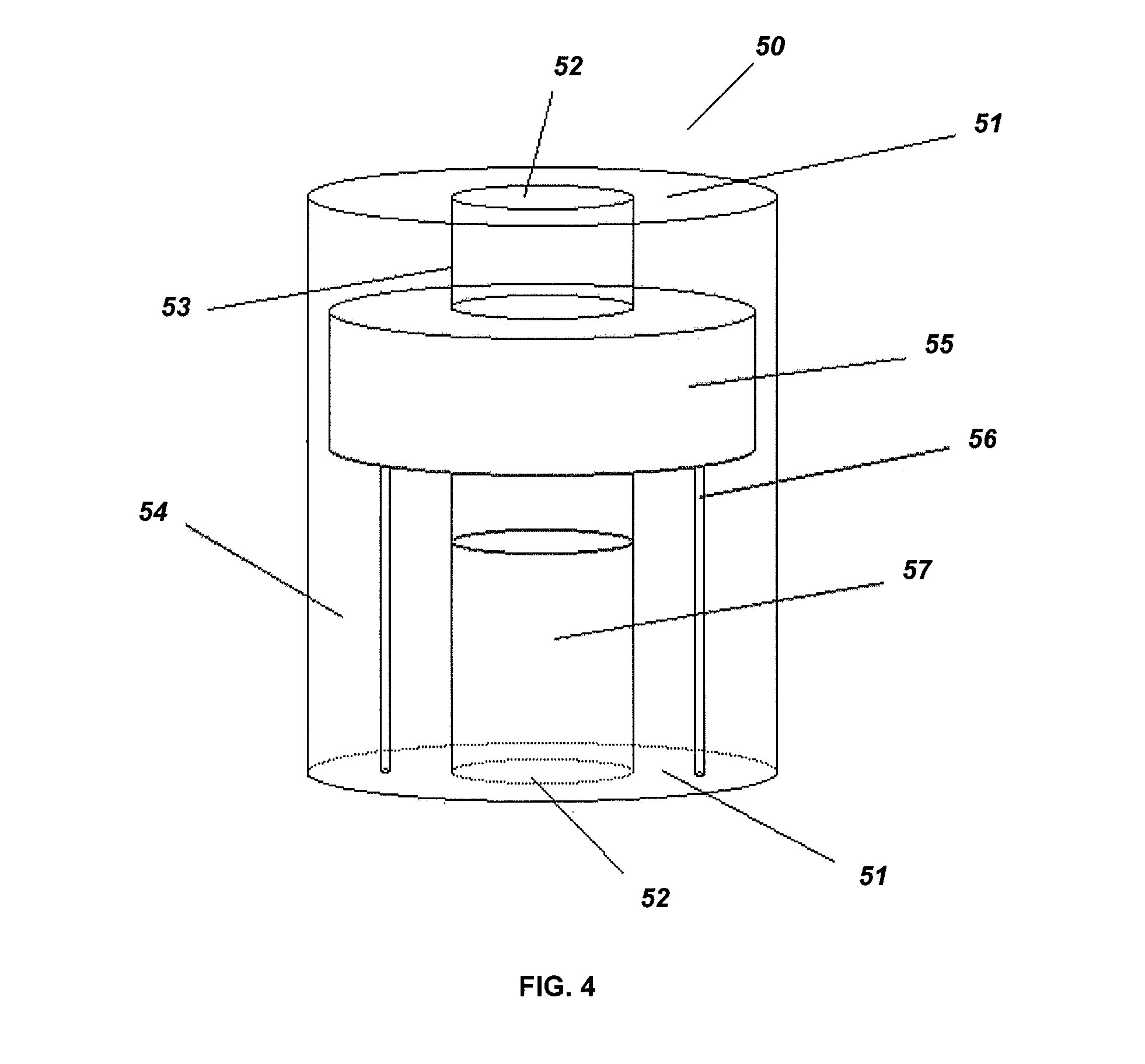
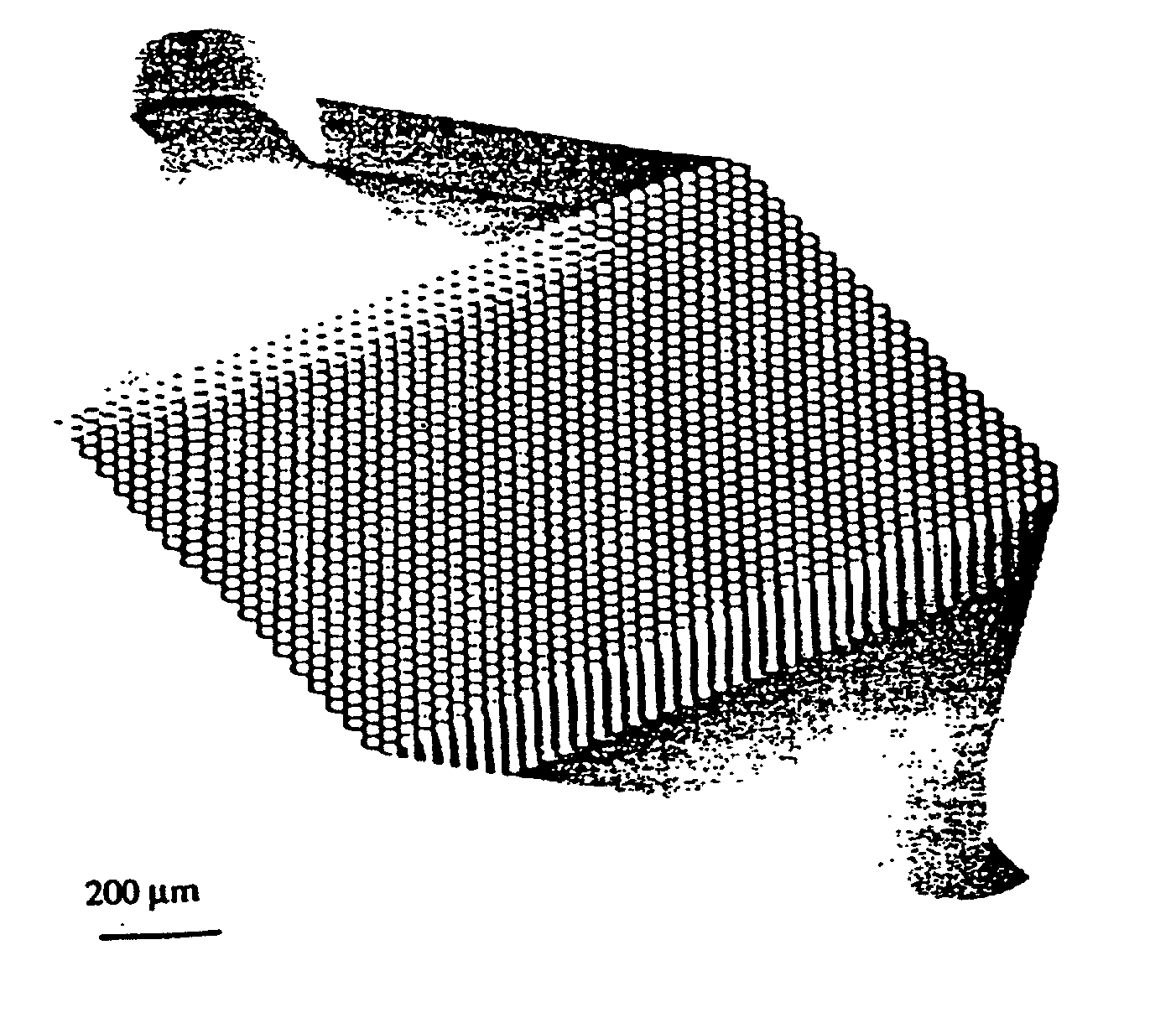
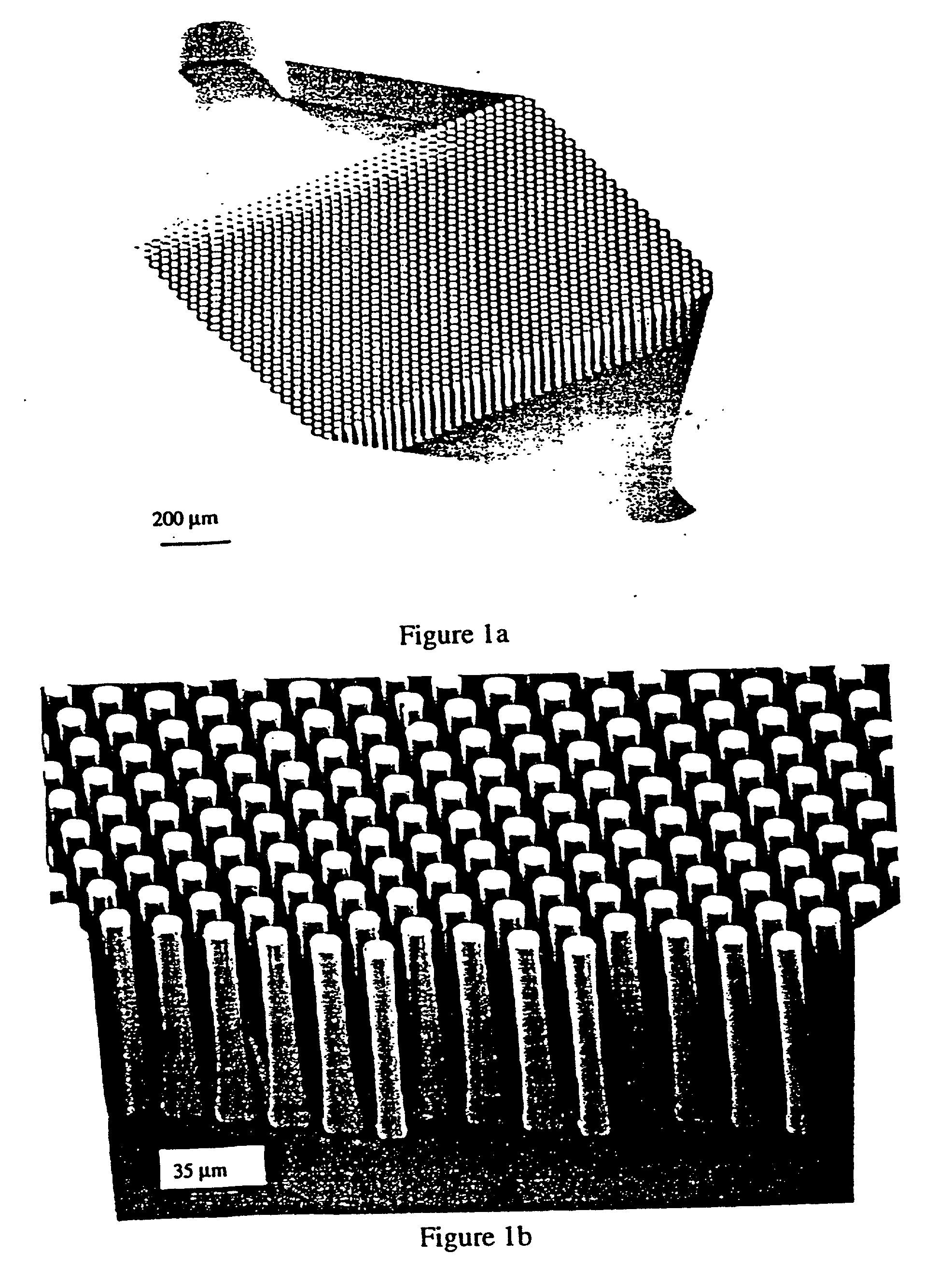
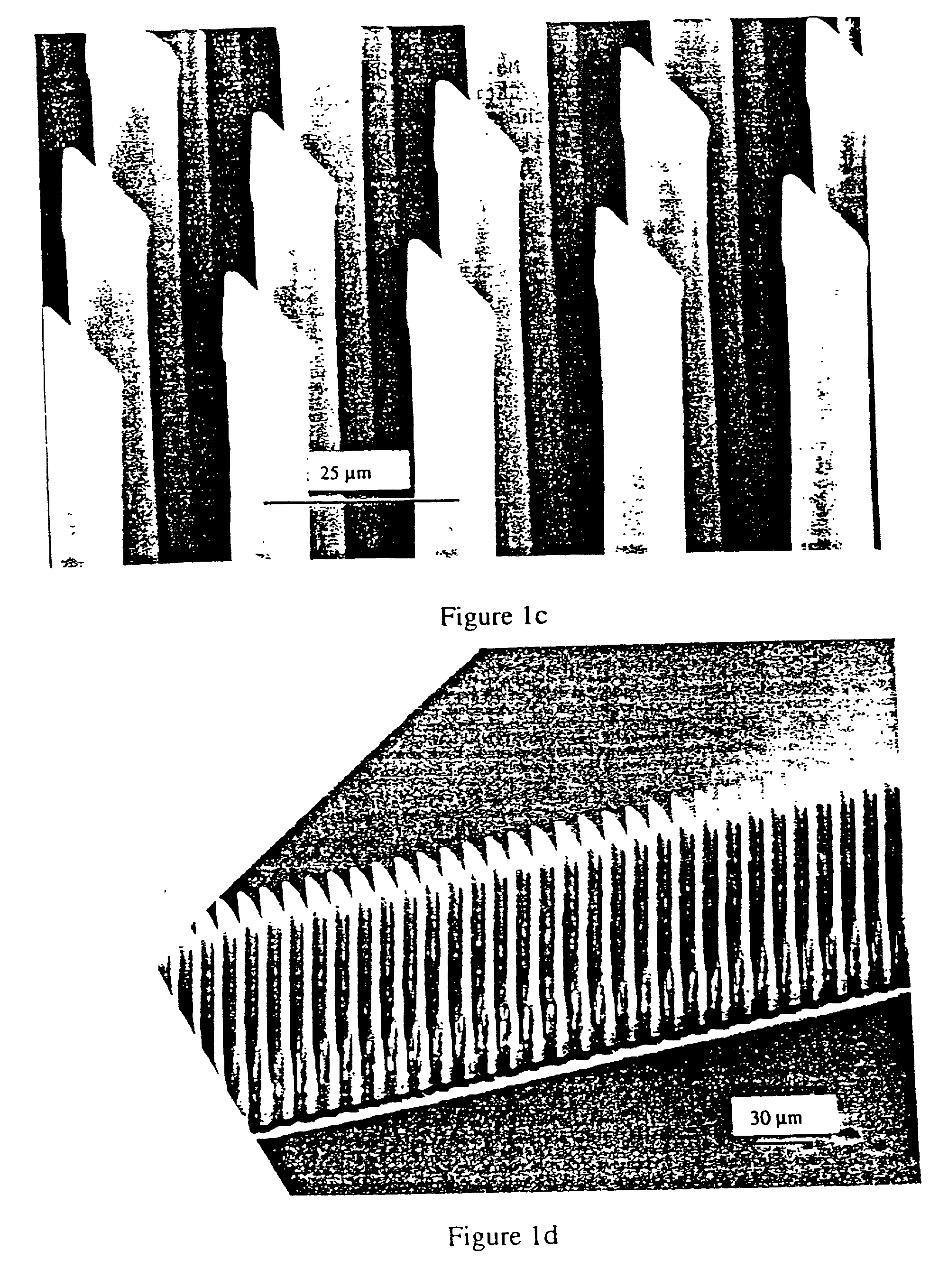
Non-planar microstructures for manipulation of fluid samples
This invention comprises an apparatus and method for the manipulation of materials, including particles, cells, macromolecules, such as proteins, nucleic acids and other moieties, in fluid samples. The apparatus comprises an enclosed chamber on a chip having an internal microstructure with surface area substantially greater than the facial surface area of the internal structure. Generally the internal microstructure comprises a continuous network of channels, each of which has a depth substantially greater than its width. The network may comprise a single channel, a single channel with multiple branches, multiple channels, multiple channels with multiple branches, and any combination thereof. The internal structure may present an inert, non-reactive surface, or be coated with a reactive ligand, or be electrically conductive and optionally be coated with an electrical insulator. Discrete portions of the internal structure may differ in structural and surface properties. Multiple chips may be linked together to create a multiplexed array of chambers, optionally linked to other analytical devices.
Owner:CEPHEID INC
Polysaccharide fibers
This invention pertains to novel fibers made of α(1→;3) polysaccharides, and a process for their production. The fibers of the invention have “cotton-like” properties but can be produced as continuous filaments on a year-round basis. The fibers are useful in textile applications.
Owner:DUPONT IND BIOSCIENCES USA LLC
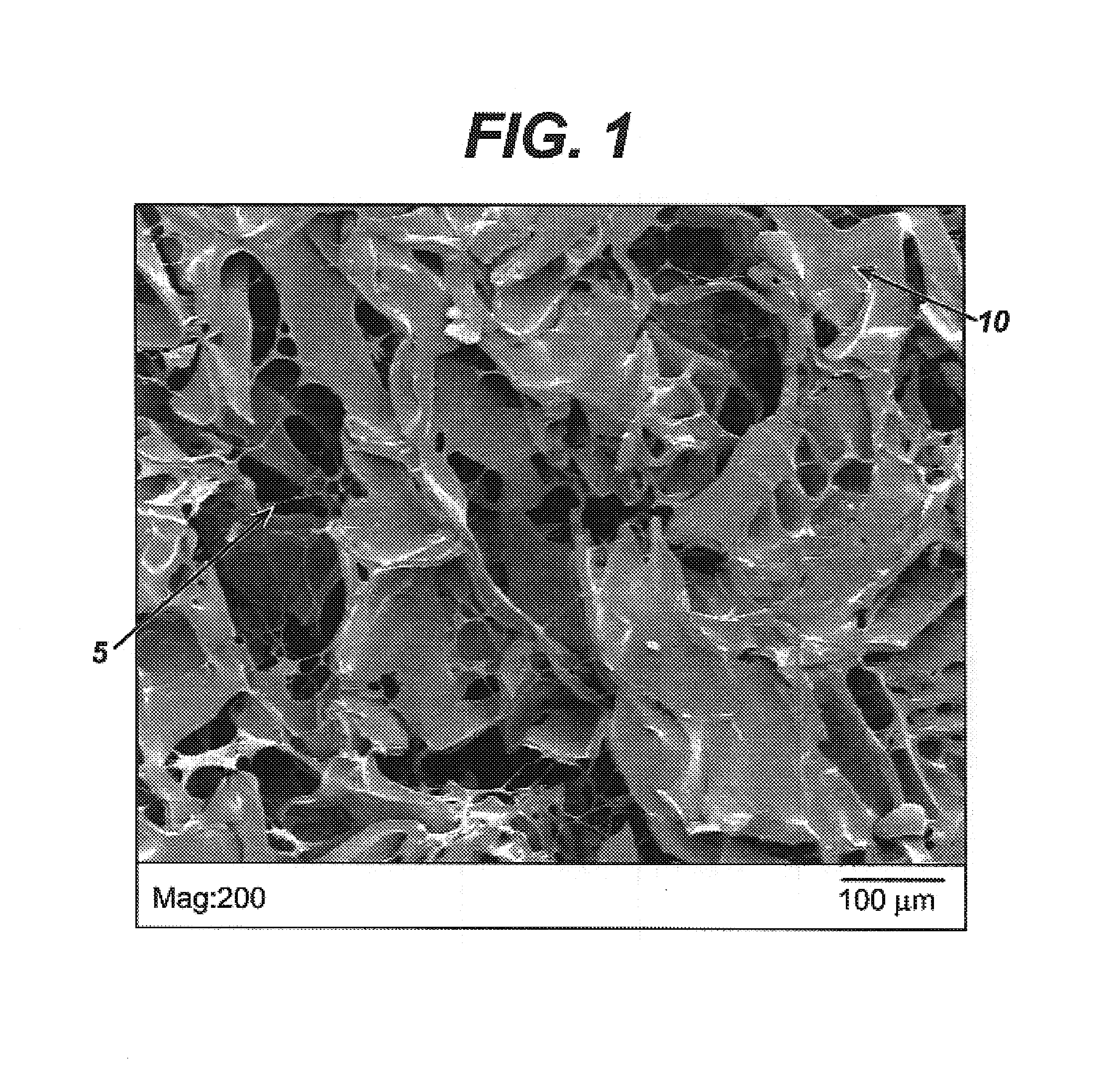
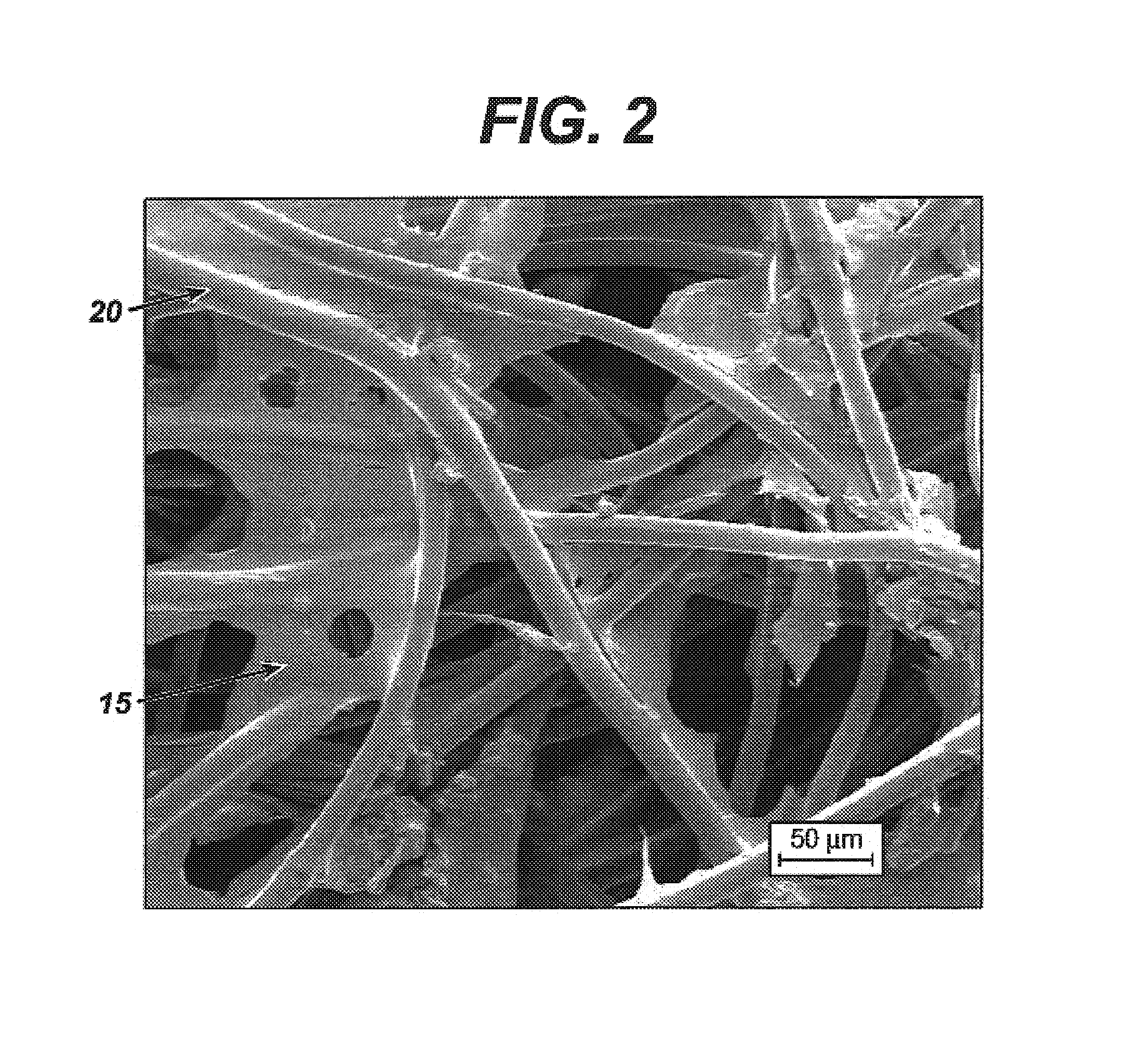
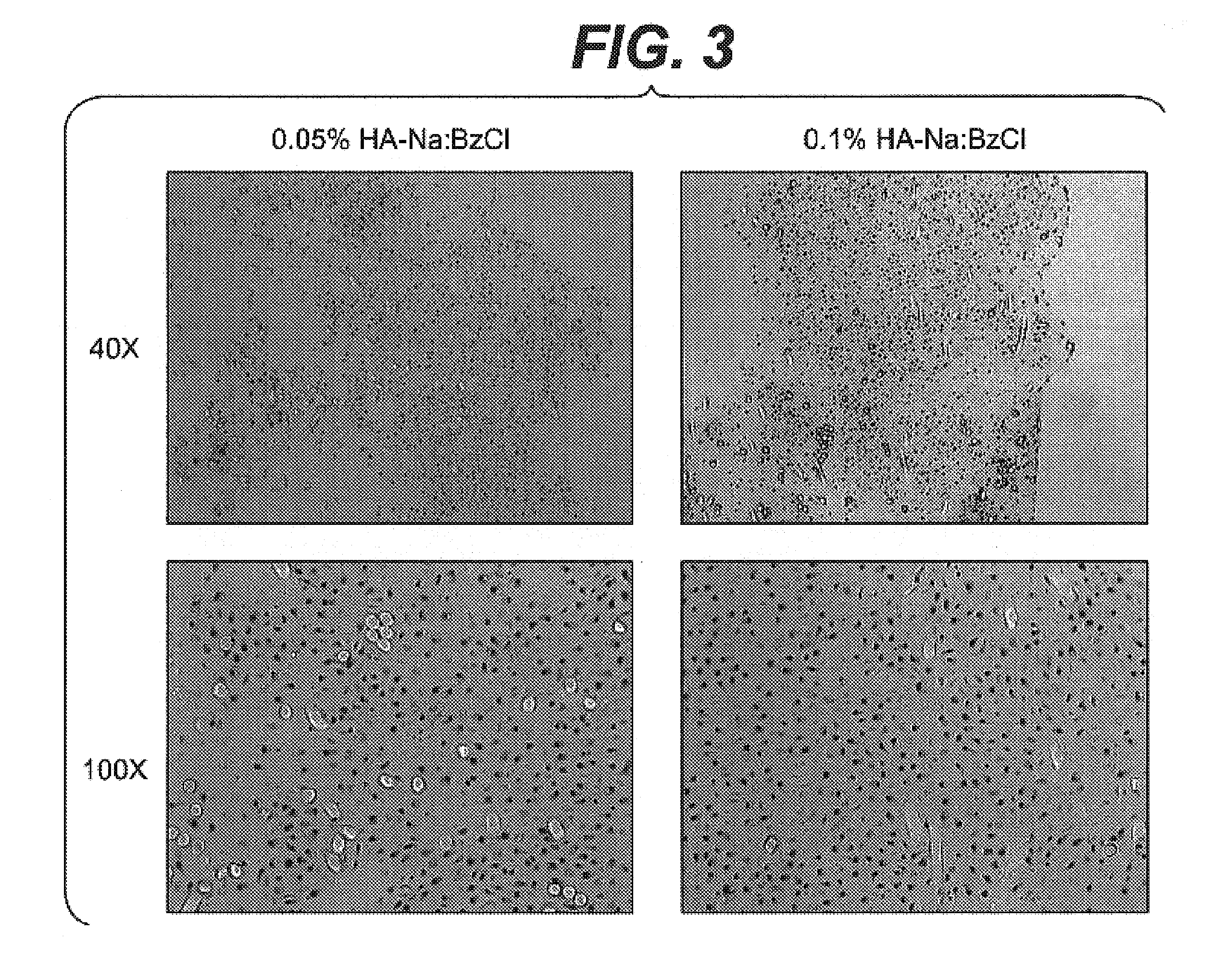
Modified hyaluronic acid for use in musculoskeletal tissue repair
The present invention includes hyaluronic acid complexes of a monovalent alkali metal salt of hyaluronic acid and a tetra alkyl ammonium halide that are suitable for incorporation with tissue scaffolds that are suitable for use in repair and / or regeneration of muscoloskeletal tissue and that include a biodegradable, porous substrate made from a biodegradable, hydrophobic polymer, where the hyaluronic acid complex is substantially insoluble in water at room temperature, yet soluble in mixtures of organic and aqueous solvents in which the selected hydrophobic polymer is soluble.
Owner:ADVANCED TECH & REGENERATIVE MEDICINE
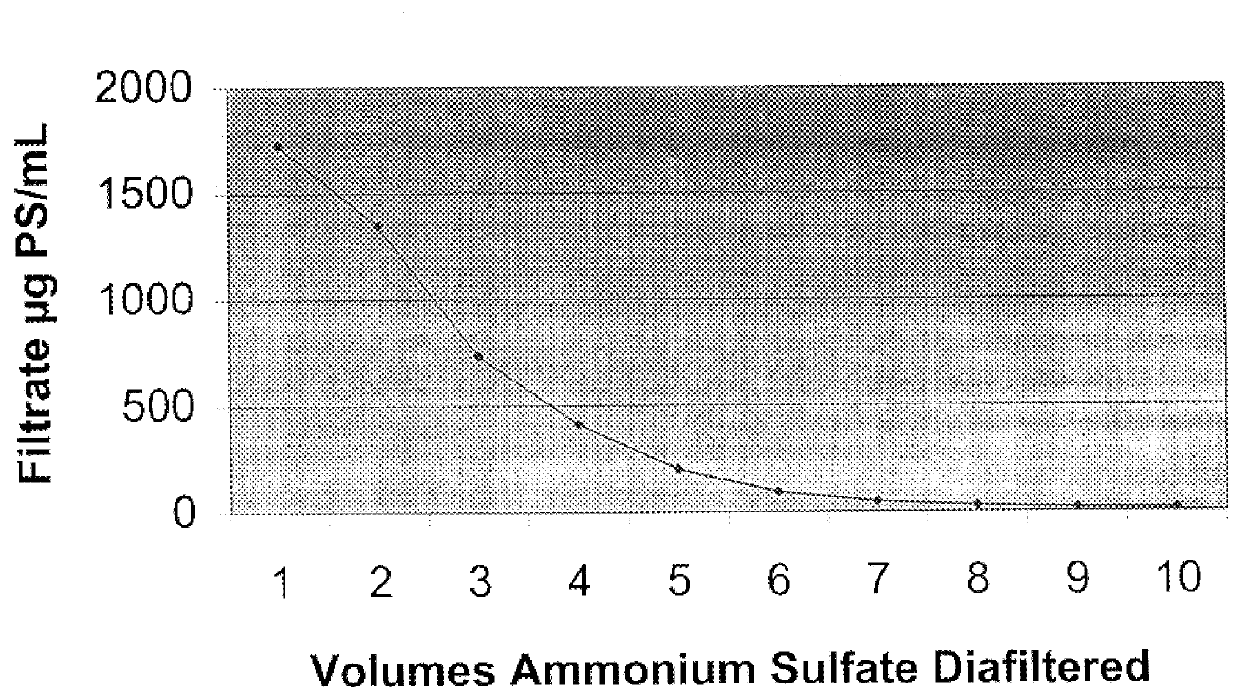
Purification of polysaccharide-protein conjugate vaccines by ultrafiltration with ammonium sulfate solutions
InactiveUS6146902AImprove scalabilityLevel of purityAntibacterial agentsSugar derivativesConjugate vaccineUltrafiltration
Disclosed and claimed are a method for the purification of polysaccharide-protein conjugate vaccines by ultrafiltration in a saturated solution of ammonium sulfate. The ultrafiltration method of the present invention provides an efficient, readily scalable method for removal of unbound polysaccharides from polysaccharide-protein vaccines, thereby improving the purity and consistency of the polysaccharide-protein vaccines.
Owner:AVENTIS PASTUER LTD
Acyl polymeric derivatives of aromatic hydroxyl-containing compounds
InactiveUS6011042APreferable balanceAccelerate the accumulation processBiocideSugar derivativesPolymeric prodrugAcylal
The present invention is directed to conjugates such as polymeric prodrugs of aromatic, hydroxyl-containing compounds and methods of making and using the same. These polymeric prodrugs are preferably esters of hydroxyl-containing aromatic compounds and are formed by reacting a desired aromatic, hydroxyl-containing compound with a substantially non-antigenic polymer so as to produce a transport form having an ester linkage between the aromatic compound and the polymer. Preferred aromatic hydroxyl-containing compositions include 10- and 11-hydroxycamptothecin derivatives. Methods of treatment are also disclosed.
Owner:ENZON PHARM INC
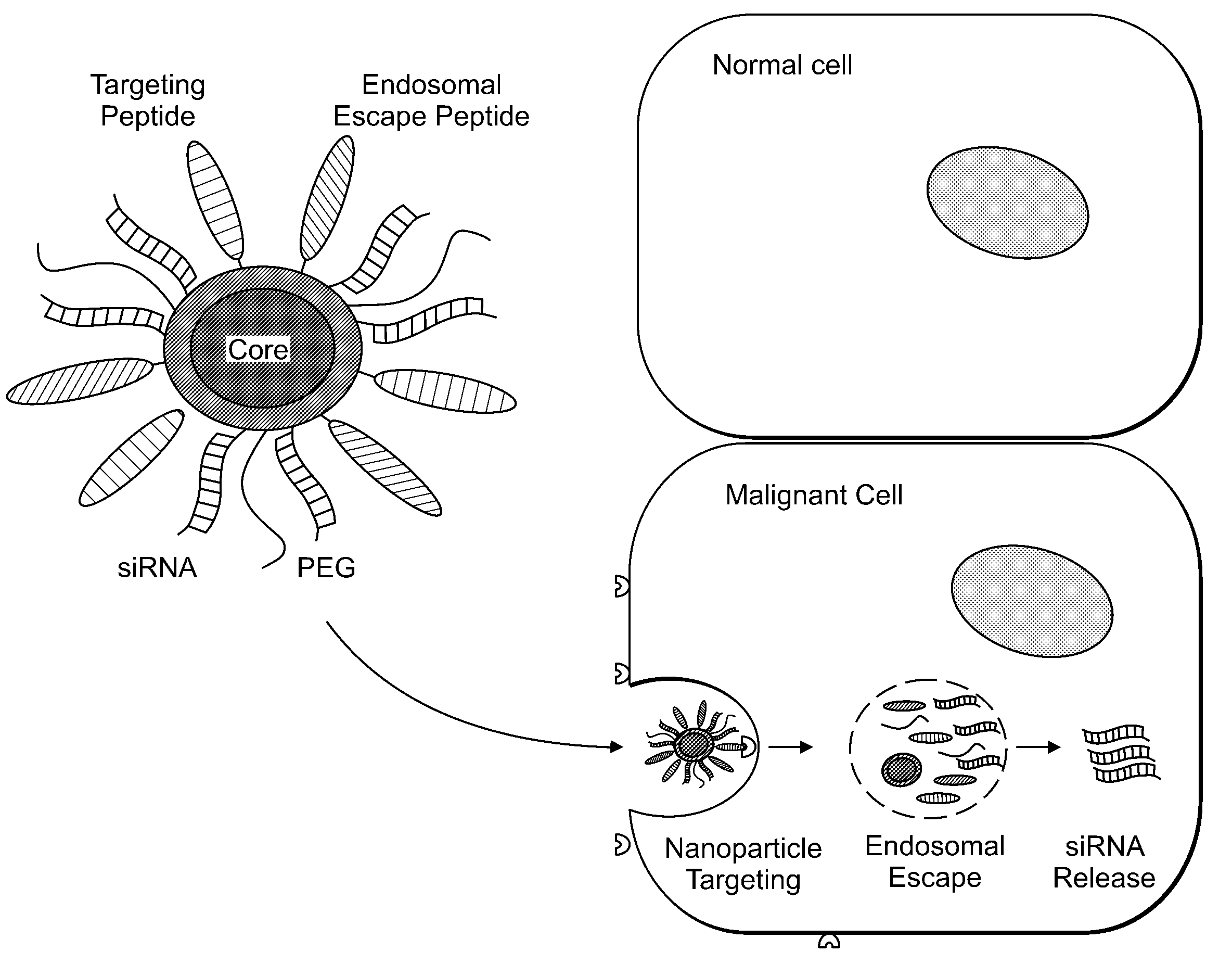
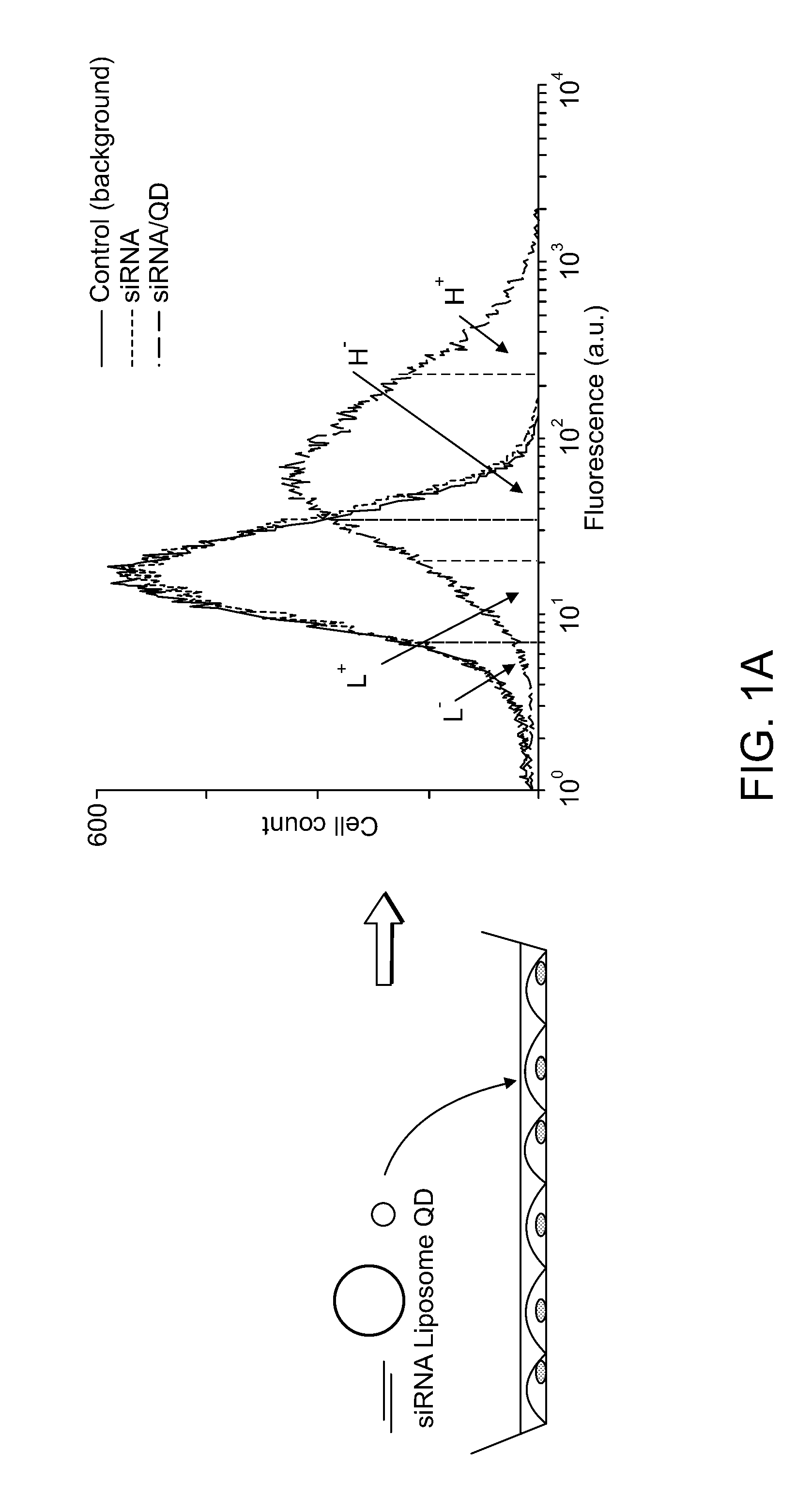
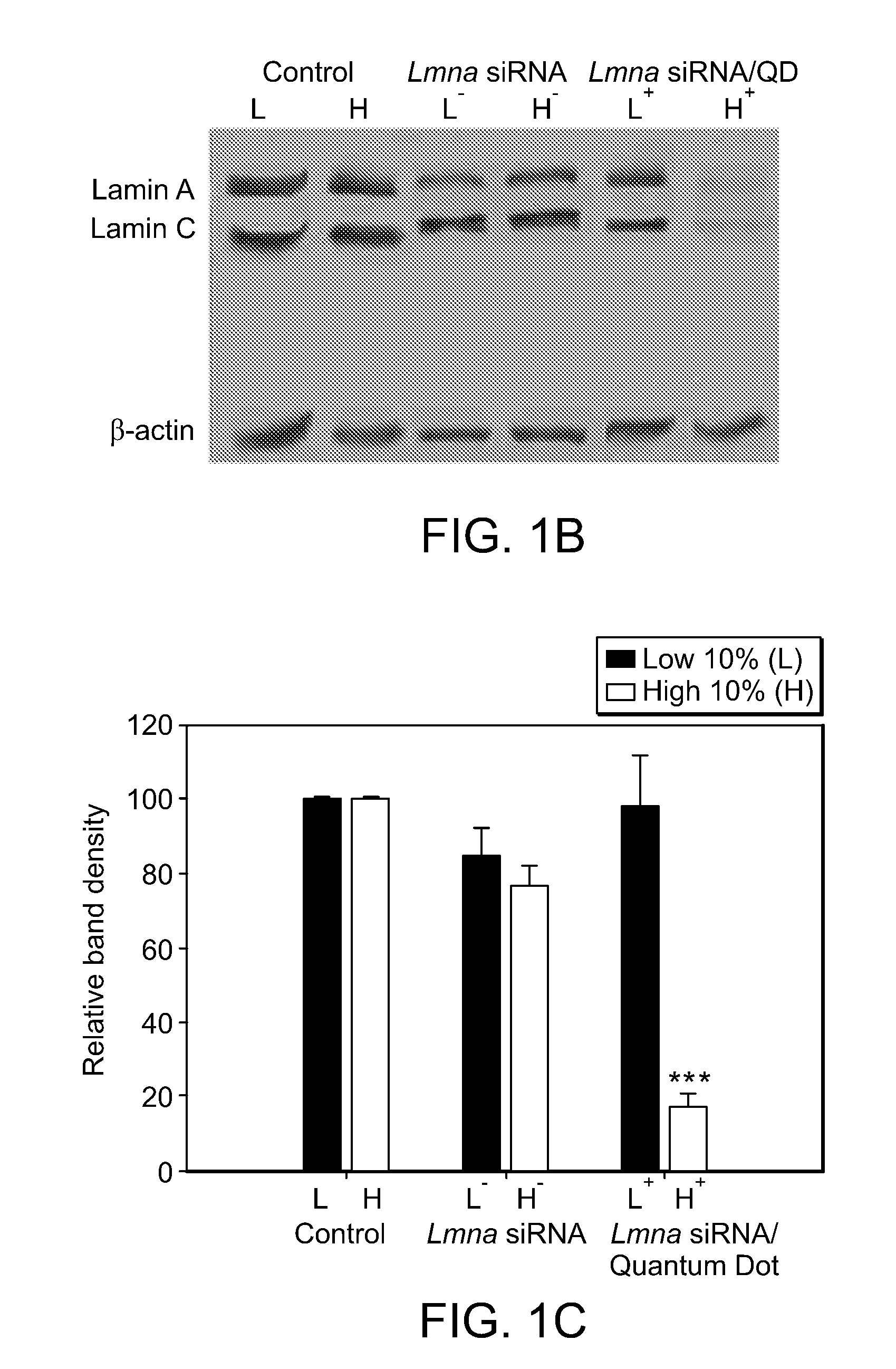
Delivery of Nanoparticles and/or Agents to Cells
InactiveUS20080213377A1Extended circulation timeReduce degradationPowder deliverySugar derivativesDiagnostic agentNanoparticle
The present invention provides systems, methods, and compositions for targeted delivery of nanoparticles and / or agents to tissues, cells, and / or subcellular locales. In general, compositions comprise a nanoparticle (e.g. quantum dot, polymeric particle, etc.), at least one modulating entity (such as a targeting moiety, transfection reagent, protective entity, etc.), and at least one agent to be delivered (e.g. therapeutic, prophylactic, and / or diagnostic agent). The present invention provides methods of making and using nanoparticle entities in accordance with the present invention.
Owner:MASSACHUSETTS INST OF TECH
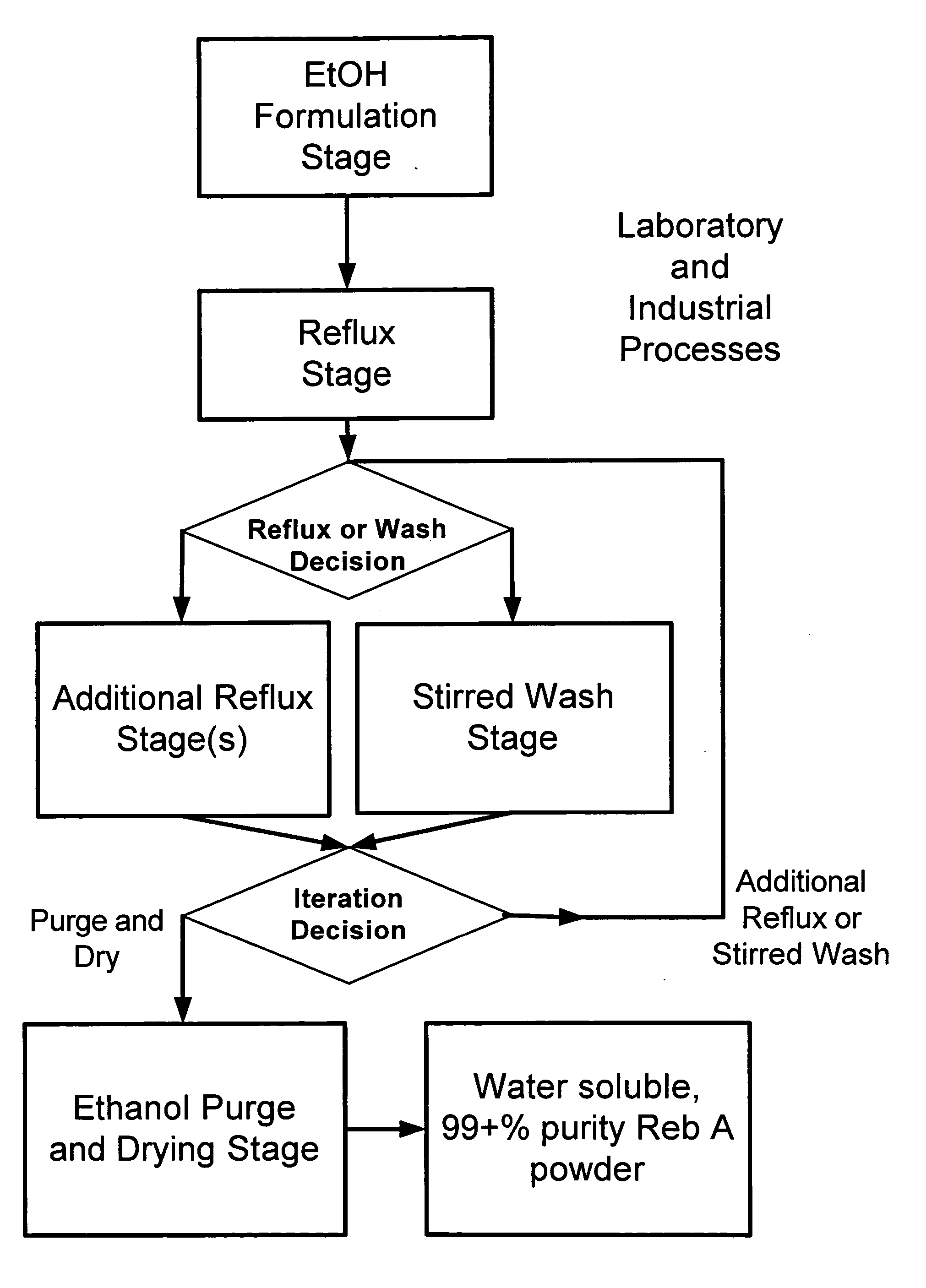
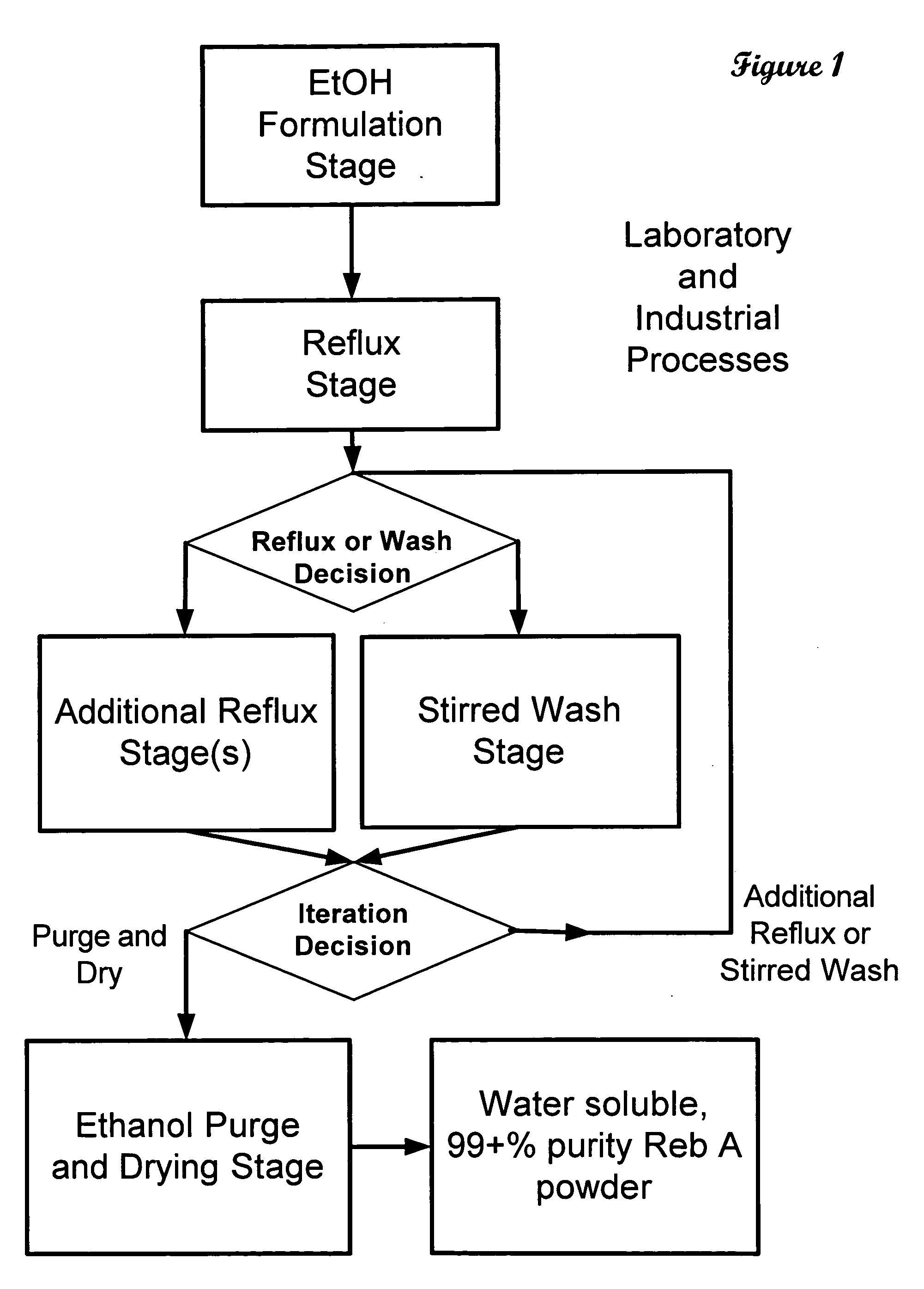
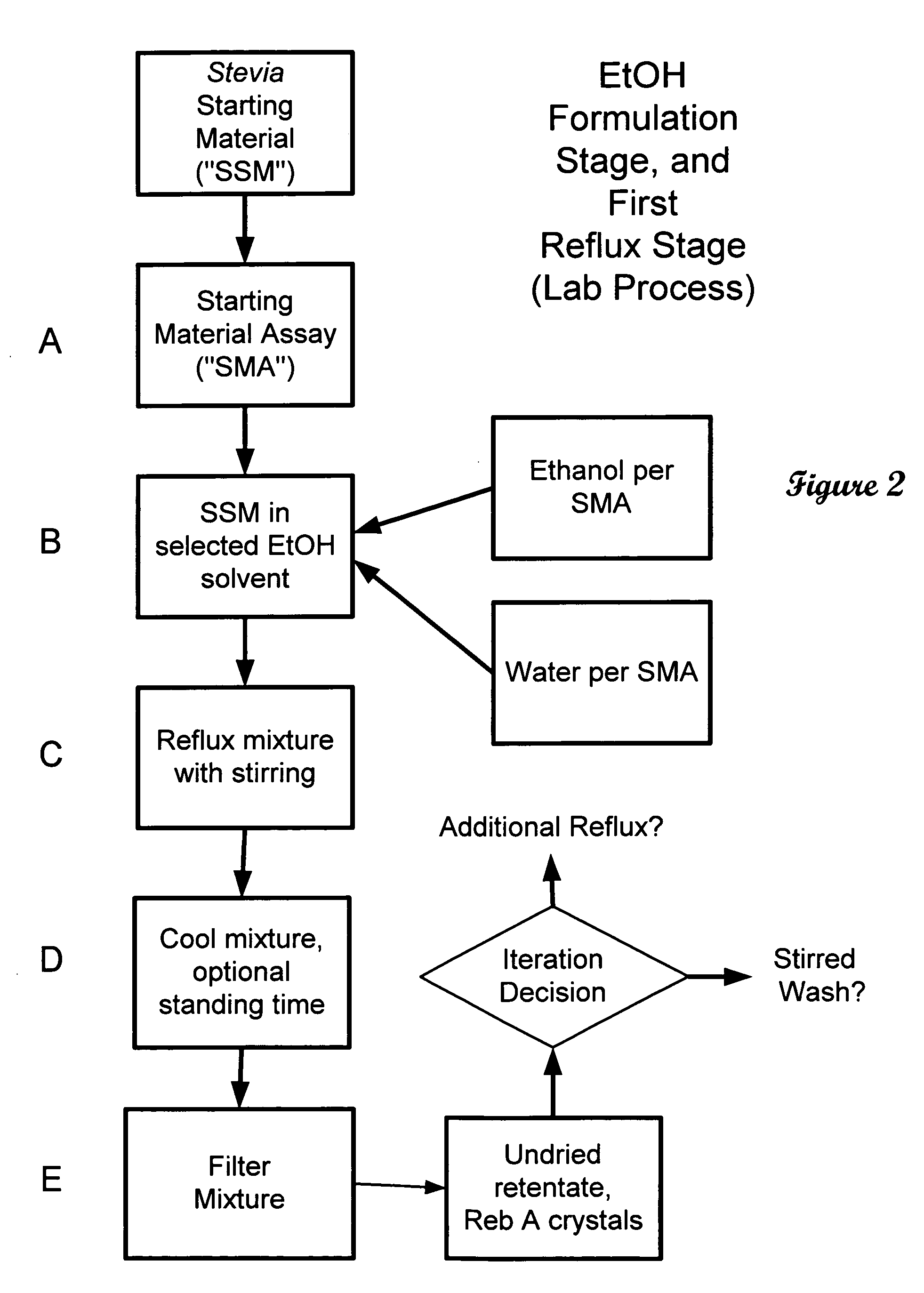
High yield method of producing pure rebaudioside A
ActiveUS20060083838A1Reduction in yieldQuality improvementSugar derivativesMetabolism disorderSolubilityAdditive ingredient
The invention provides a high throughput, high purity, high yield system and method of isolating and purifying rebaudioside A (“Reb A”), with acceptable water solubility for all commercial uses, from commercially available Stevia rebaudiana starting material. The invention also provides a means of maximizing yields of 99+% purity Reb A based on the attributes of a given batch of Stevia starting material. The Reb A produced by the invention is water soluble, devoid of bitterness heretofore associated with rebaudioside sweeteners, non-caloric, and suitable for use as a reagent and as an ingredient in orally consumed products, e.g., as a sweetener, flavor enhancer, and flavor modifier.
Owner:SWEET GREEN FIELDS INT CO LTD
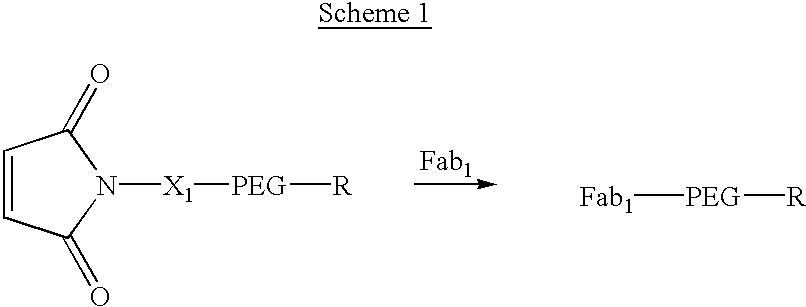
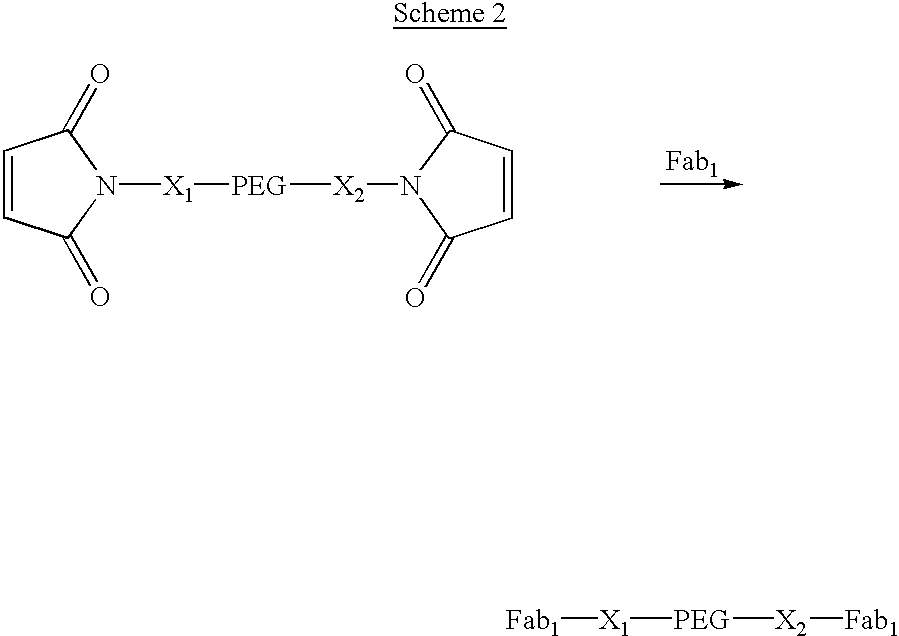
Pseudo-antibody constructs
InactiveUS20030211078A1Reduce productionInhibit synthesisOrganic active ingredientsBiocideHalf-lifeIn vivo
This invention relates to novel pharmaceutically useful compositions that bind to a biological molecule, having improved circulatory half-life, increased avidity, increased affinity, or multifunctionality, and methods of use thereof. The present invention provides a pseudo-antibody comprising an organic moiety covalenty coupled to at least two target-binding moieties, wherein the target-binding moieties are selected from the group consisting of a protein, a peptide, a peptidomimetic, and a non-peptide molecule that binds to a specific targeted biological molecule. The pseudo-antibody of the present invention may affect a specific ligand in vitro, in situ and / or in vivo. The pseudo-antibodies of the present invention can be used to measure or effect in an cell, tissue, organ or animal (including humans), to diagnose, monitor, modulate, treat, alleviate, help prevent the incidence of, or reduce the symptoms of, at least one condition.
Owner:CENTOCOR
Linking sequence reads using paired code tags
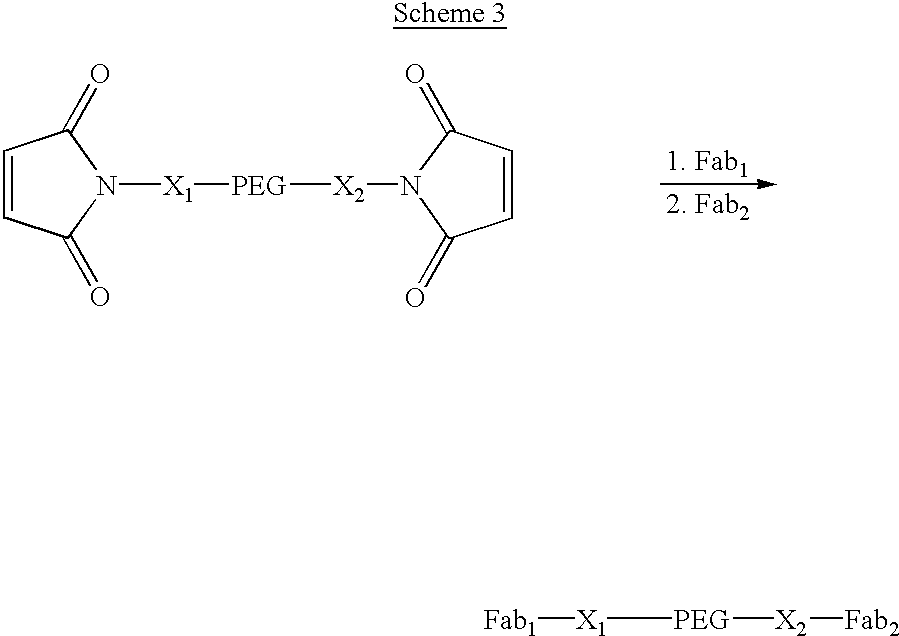
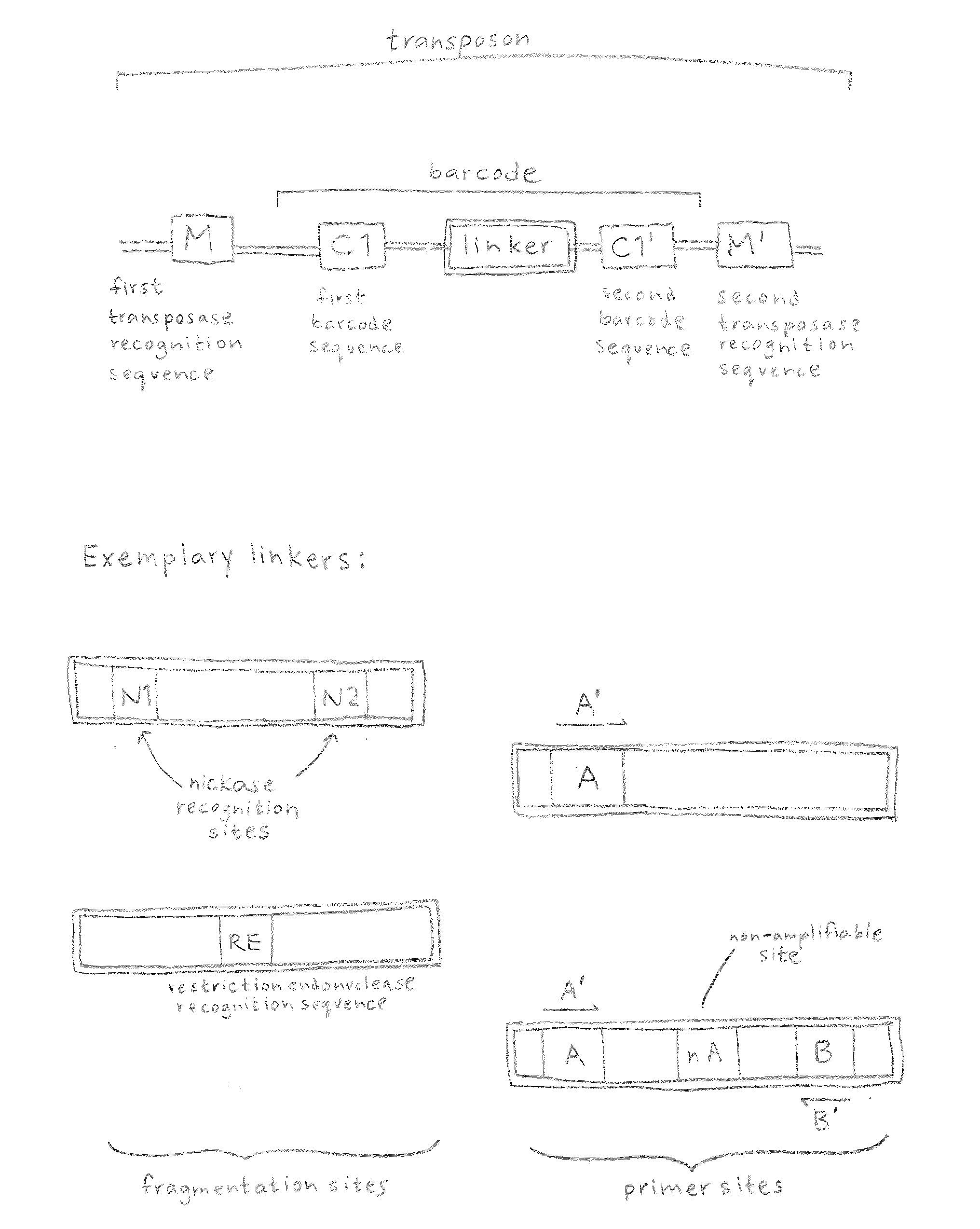
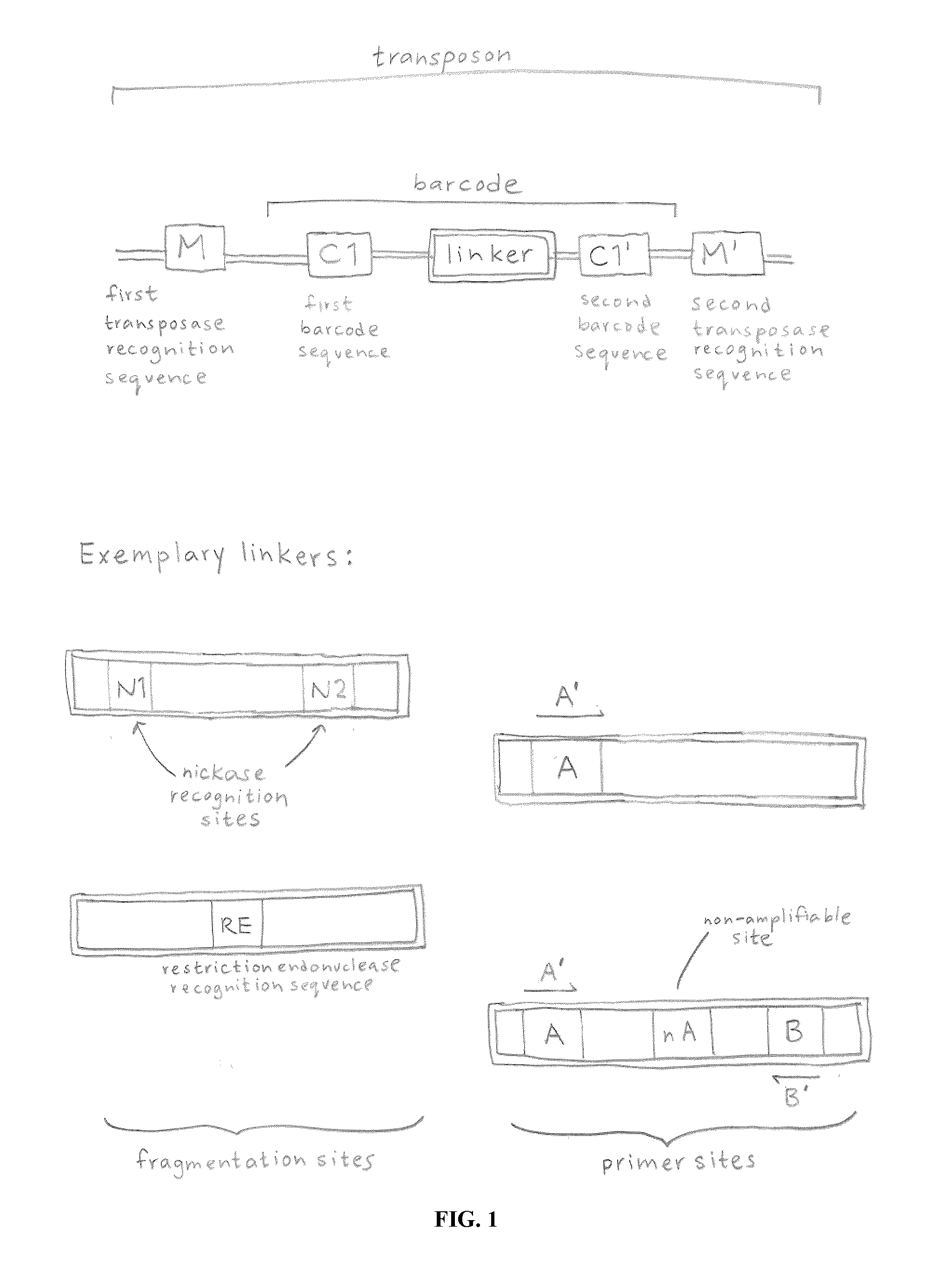
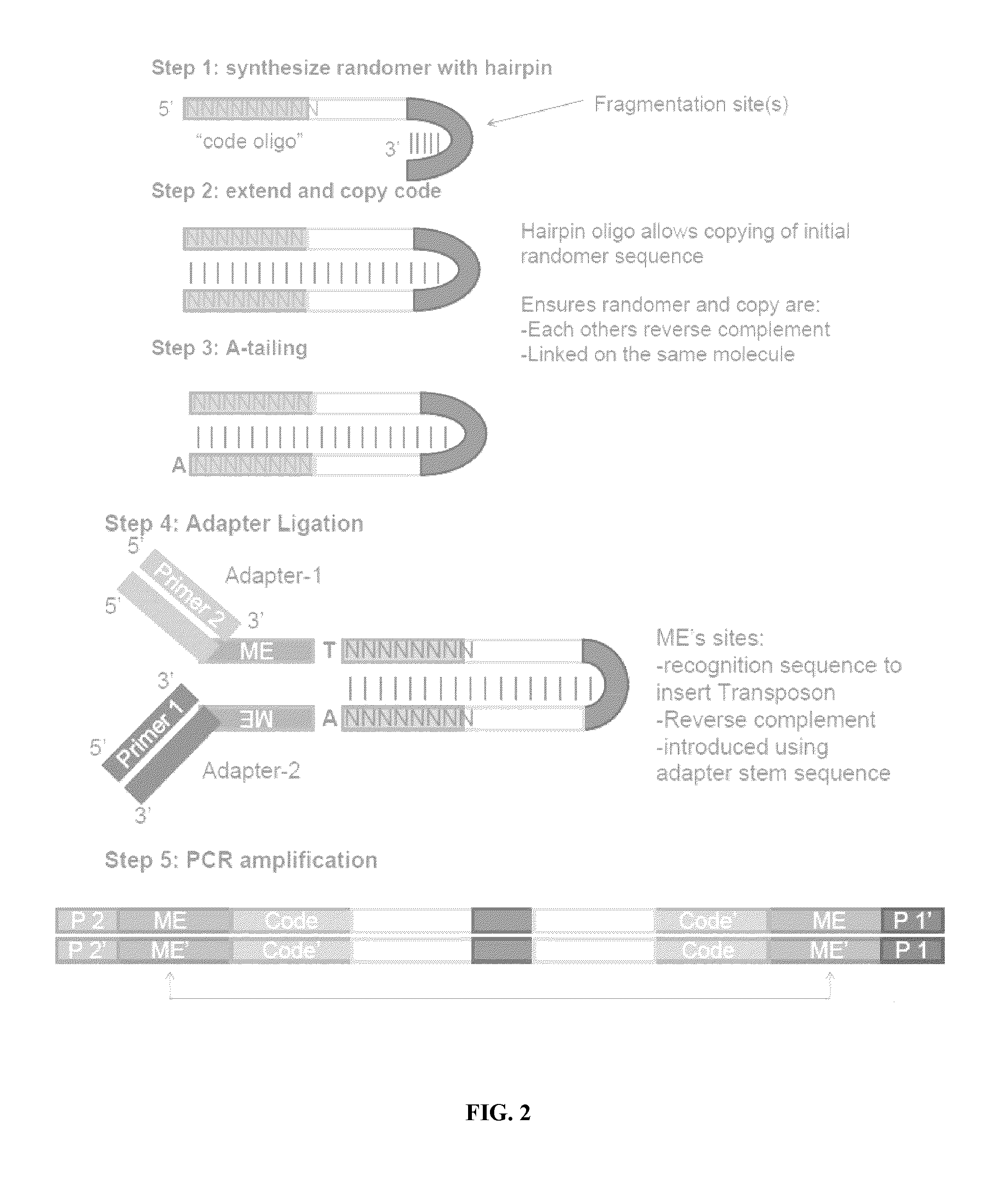
ActiveUS20120208705A1Reduce in quantitySugar derivativesMicrobiological testing/measurementComputational biologyTransposon element
Owner:ILLUMINA INC
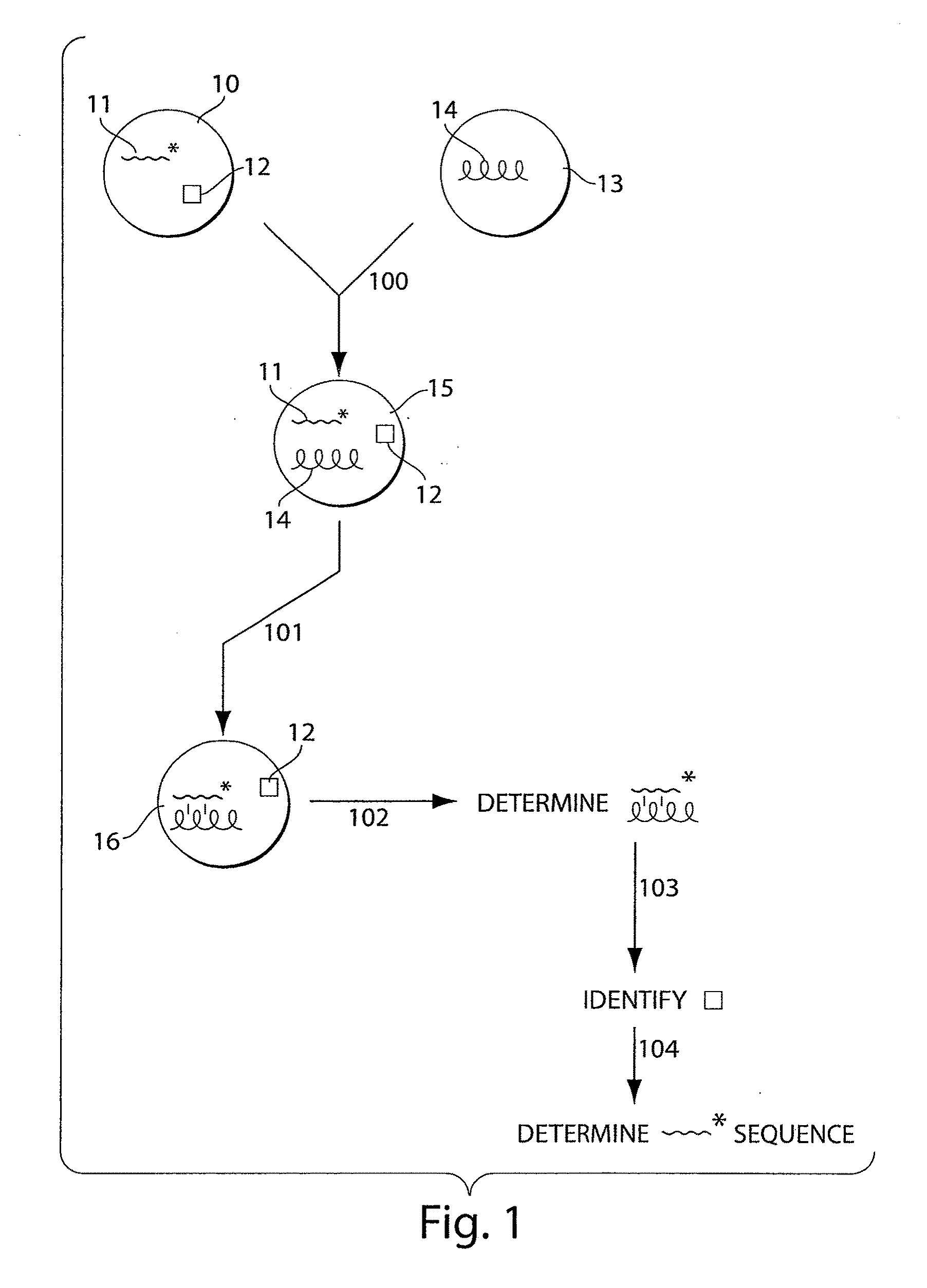
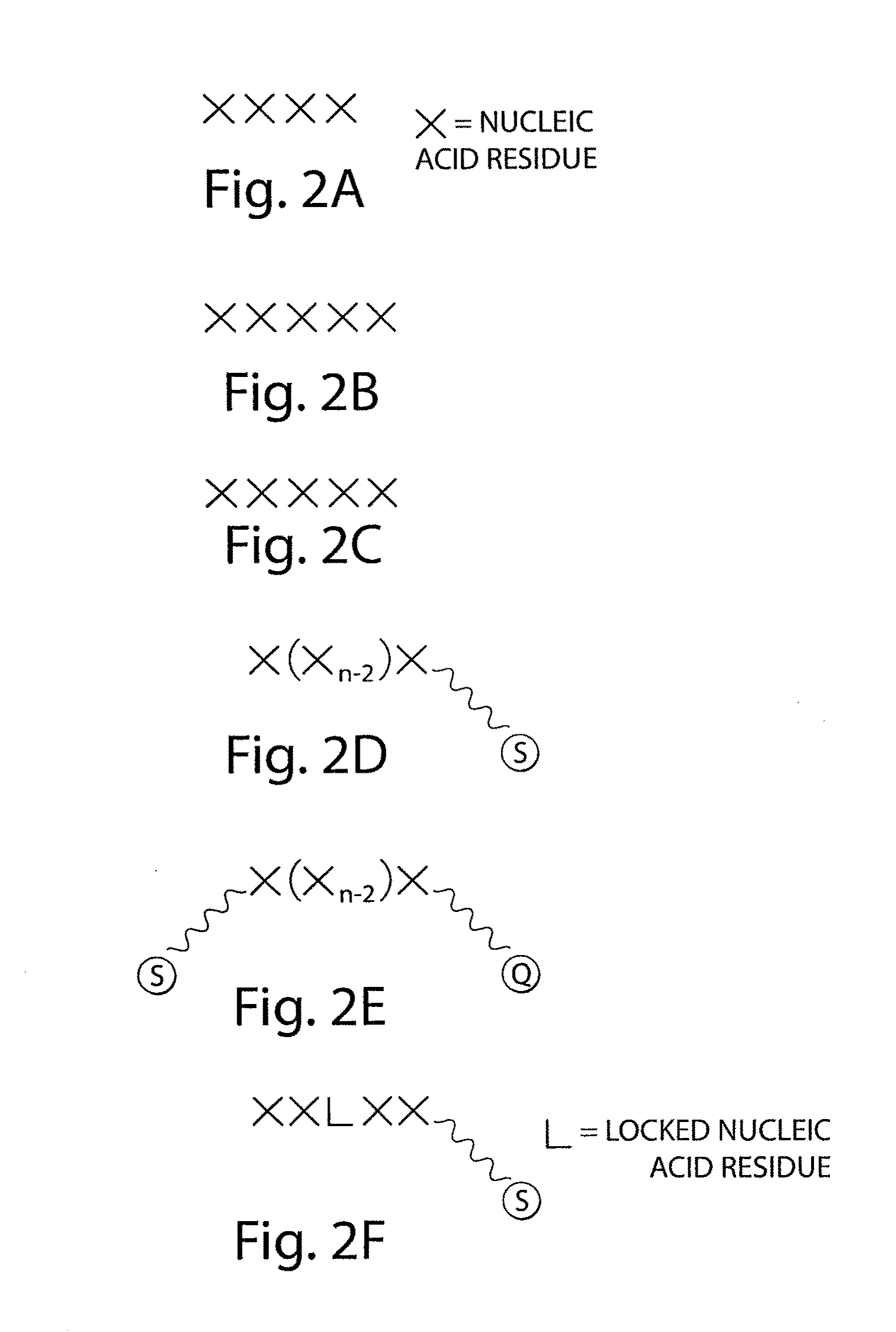
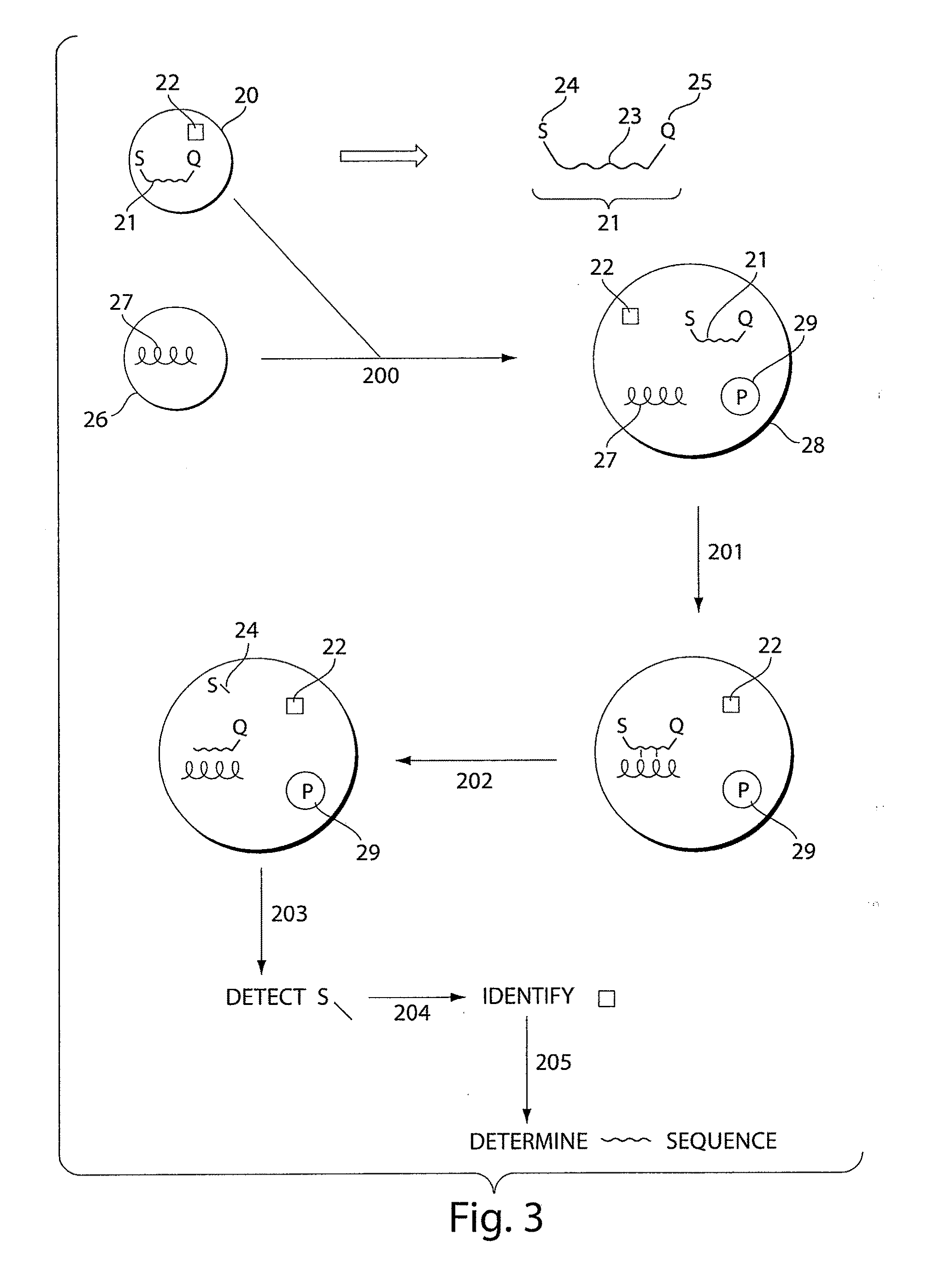
Systems and methods for nucleic acid sequencing
ActiveUS20110267457A1Sugar derivativesMicrobiological testing/measurementNucleic Acid ProbesNucleic acid sequencing
The present invention relates to systems and methods for sequencing nucleic acids, including sequencing nucleic acids in fluidic droplets. In one set of embodiments, the method employs sequencing by hybridization using droplets such as microfluidic droplets. In some embodiments, droplets are formed which include a target nucleic acid, a nucleic acid probe, and at least one identification element, such as a fluorescent particle. The nucleic acid probes that hybridize to the target nucleic acid are determined, in some instances, by determining the at least one identification element. The nucleic acid probes that hybridize to the target nucleic acid may be used to determine the sequence of the target nucleic acid. In certain instances, the microfluidic droplets are provided with reagents that modify the nucleic acid probe. In some cases, a droplet, such as those described above, is deformed such that the components of the droplets individually pass a target area.
Owner:PRESIDENT & FELLOWS OF HARVARD COLLEGE
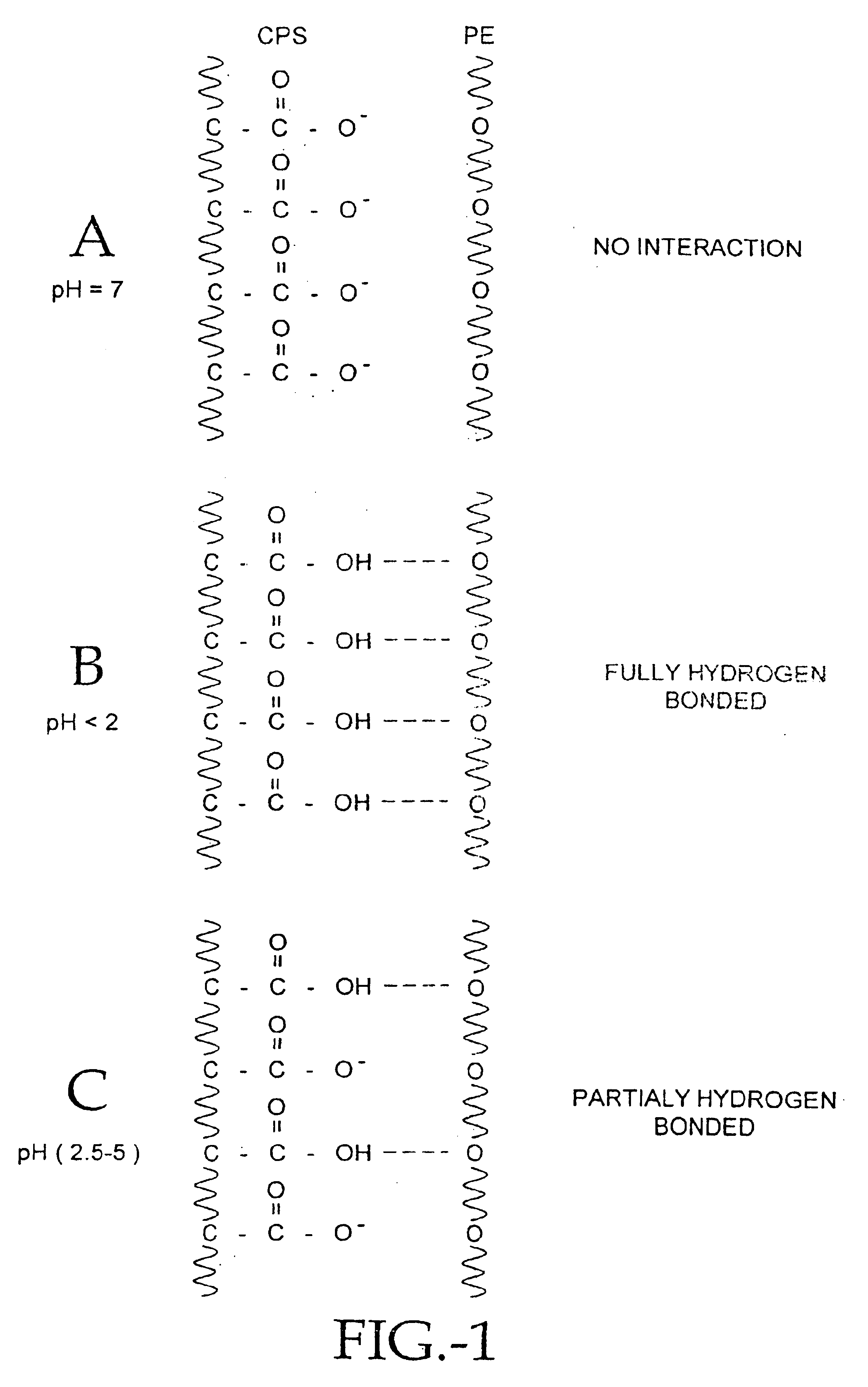
Compositions of polyacids and polyethers and methods for their use in reducing adhesions
InactiveUS6869938B1Improve anti-adhesion performancePrevent reformationBiocideSurgeryMicrosphereThrombogenicity
The present invention relates to improved methods for making and using bioadhesive, bioresorbable, anti-adhesion compositions made of intermacromolecular complexes of carboxyl-containing polysaccharides, polyethers, polyacids, polyalkylene oxides, multivalent cations and / or polycations. The polymers are associated with each other, and are then either dried into membranes or sponges, or are used as fluids or microspheres. Bioresorbable, bioadhesive, anti-adhesion compositions are useful in surgery to prevent the formation and reformation of post-surgical adhesions. The compositions are designed to breakdown in-vivo, and thus be removed from the body. Membranes are inserted during surgery either dry or optionally after conditioning in aqueous solutions. The anti-adhesion, bioadhesive, bioresorptive, antithrombogenic and physical properties of such membranes and gels can be varied as needed by carefully adjusting the pH and / or cation content of the polymer casting solutions, polyacid composition, the polyalkylene oxide composition, or by conditioning the membranes prior to surgical use. Multi-layered membranes can be made and used to provide further control over the physical and biological properties of antiadhesion membranes. Membranes and gels can be used concurrently. Antiadhesion compositions may also be used to lubricate tissues and / or medical instruments, and / or deliver drugs to the surgical site and release them locally.
Owner:FZIOMED
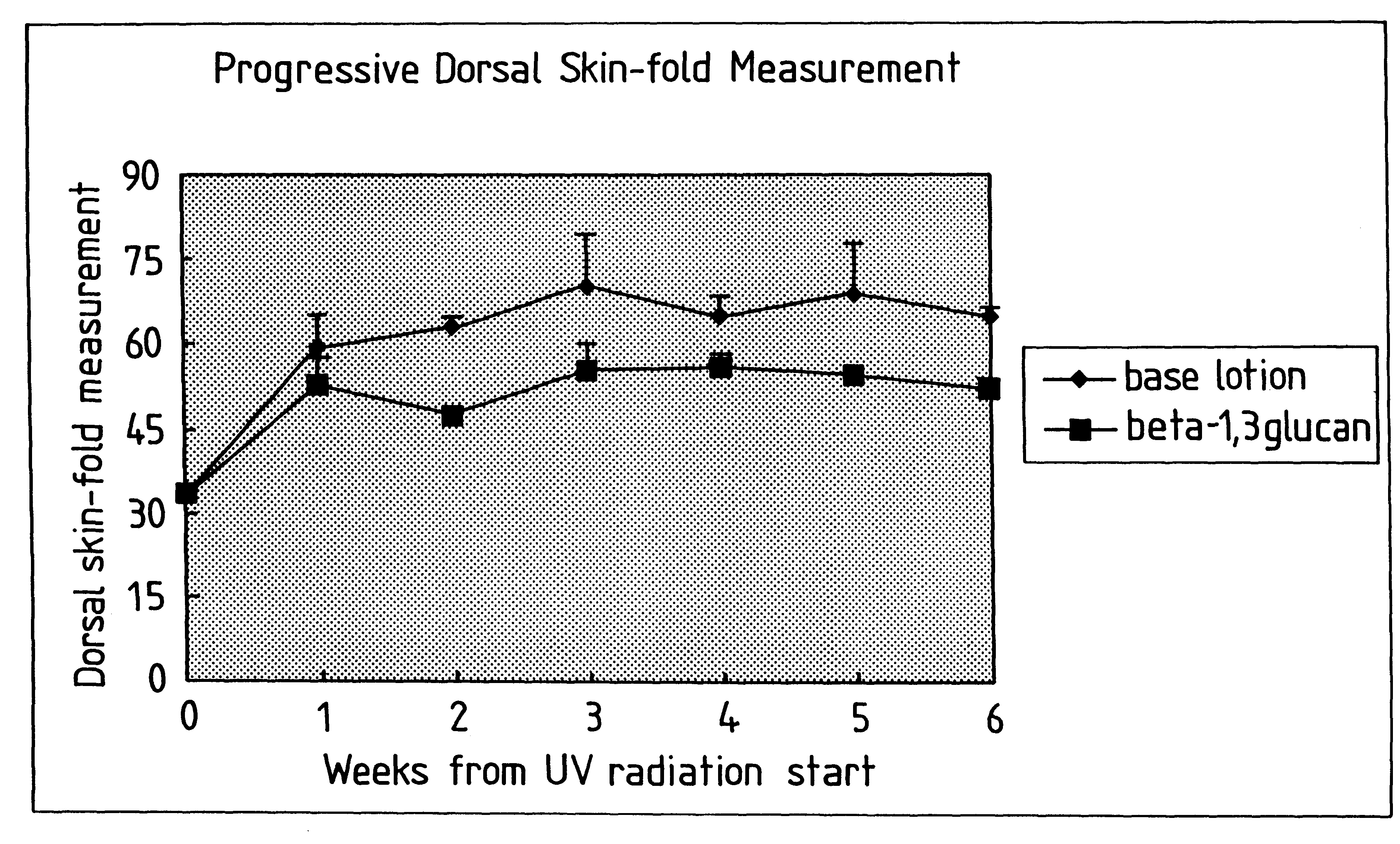
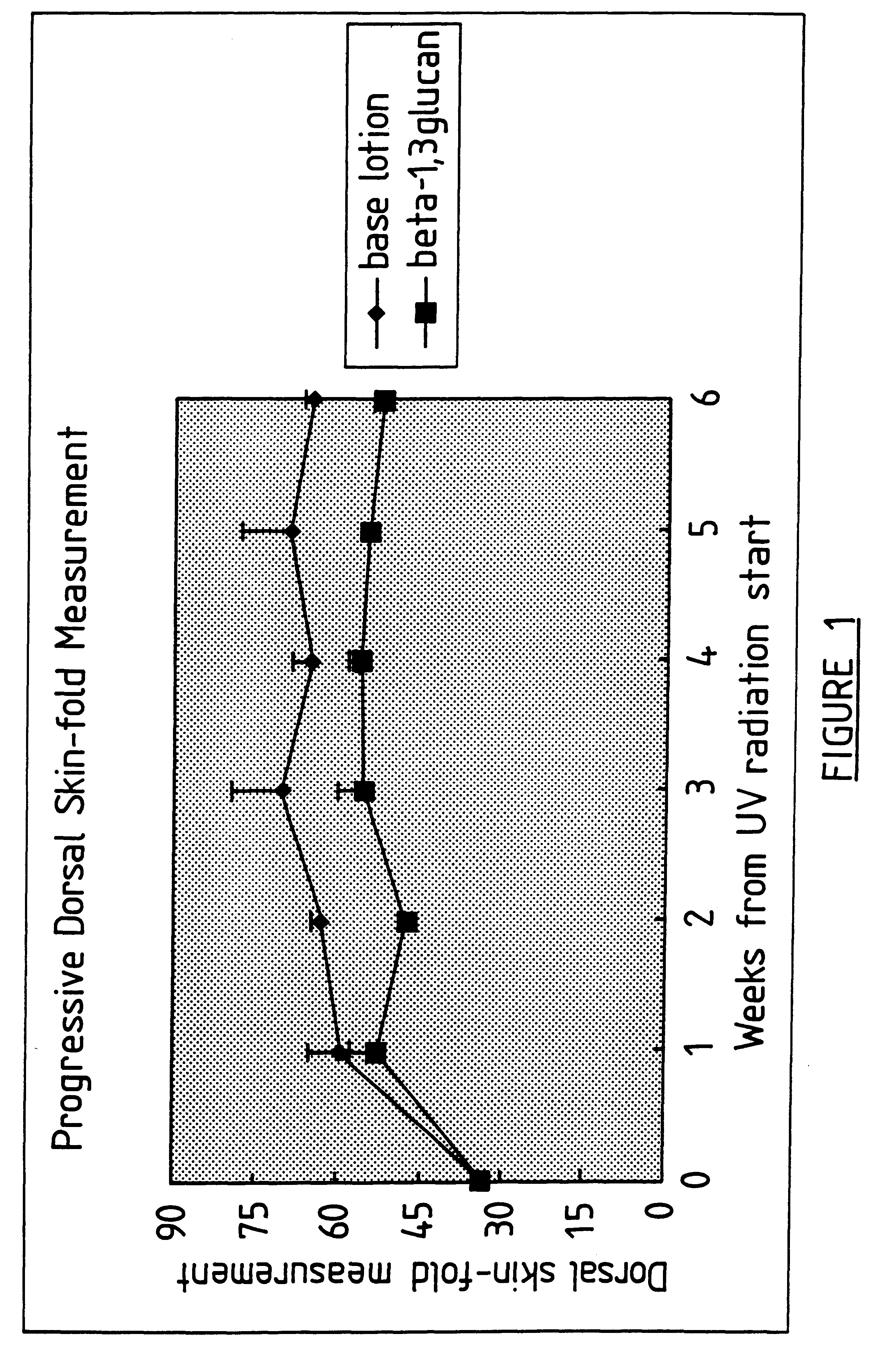
Process for glucan preparation and therapeutic uses of glucan
InactiveUS6242594B1Low costSuitable solubility characteristicAntibacterial agentsOrganic active ingredientsOrganic solventMicroparticle
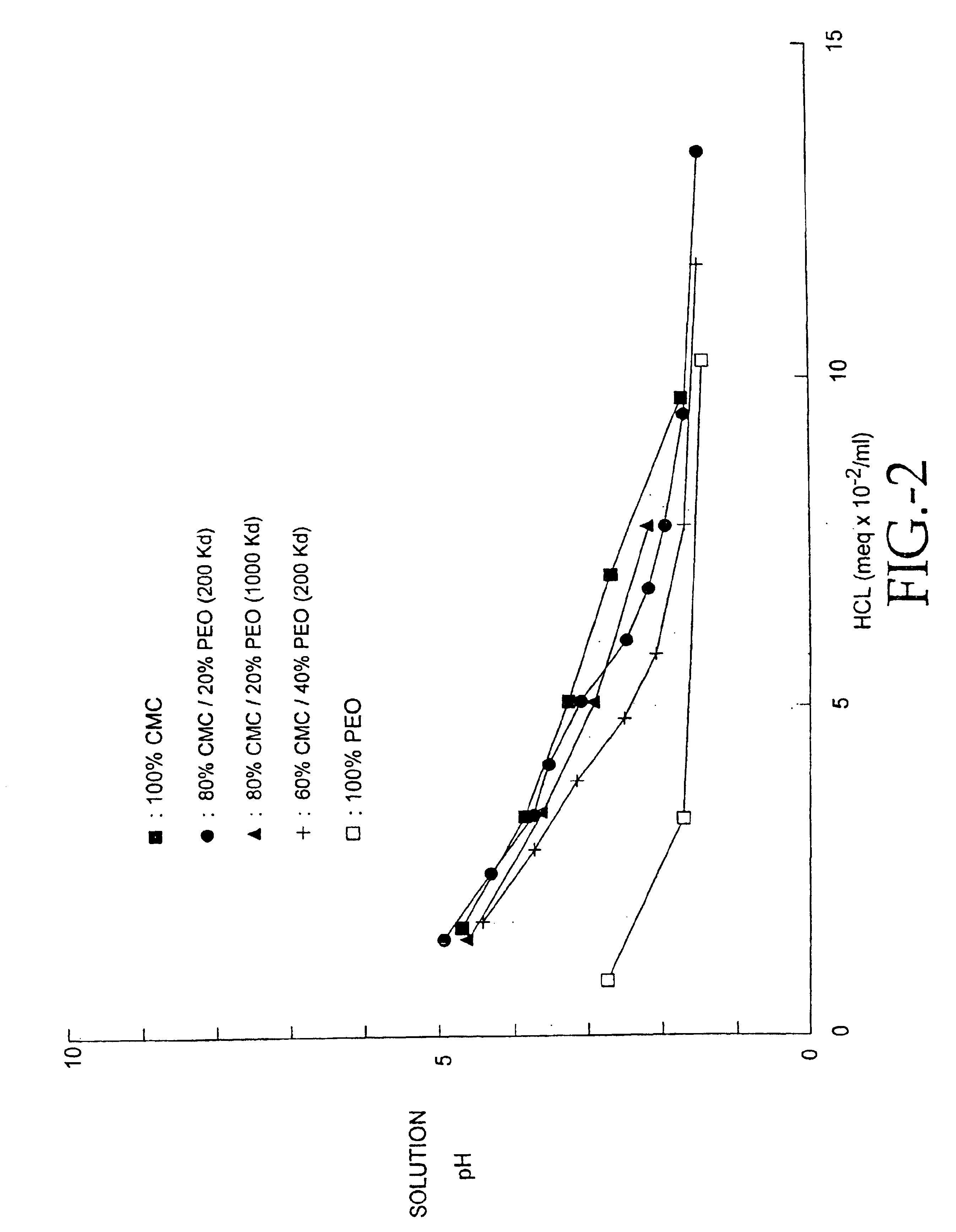
A process for the production of beta-3-(1,3)(1,6) glucan from a glucan containing cellular source is described, together with compositions and uses / methods of treatment involving glucan. The process of the invention comprises the steps of: (a) extracting glucan containing cells with alkali and heat, in order to remove alkali soluble components; (b) acid extracting the cells of step (a) with an acid and heat to form a suspension; (c) extracting the suspension obtained of step (b) or recovered hydrolyzed cells with an organic solvent which is non-miscible with water and which has a density greater than that of water separating the resultant aqueous phase, solvent containing phase and interface so that substantially only the aqueous phase comprising beta-(1,3)(1,6) glucan particulate material remains; wherein the extraction with said organic solvent provides separation of glucan subgroups comprising branched beta-(1,3)(1,6)-glucan, and essentially unbranched beta-(1,3) glucan which is associated with residual non-glucan contaminents; and (d) drying the glucan material from step (c) to give microparticulate glucan.
Owner:TR THERAPEUTICS
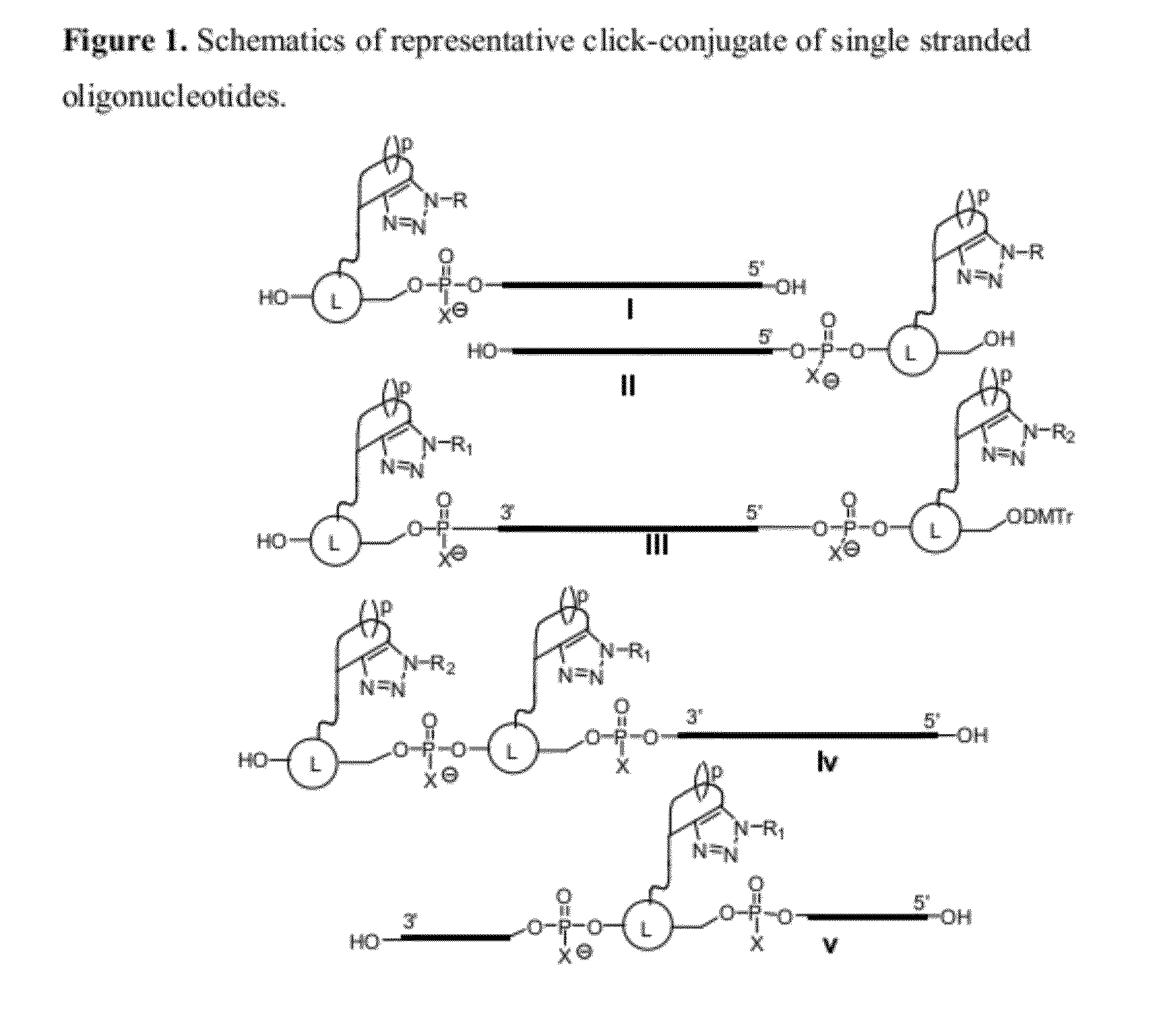
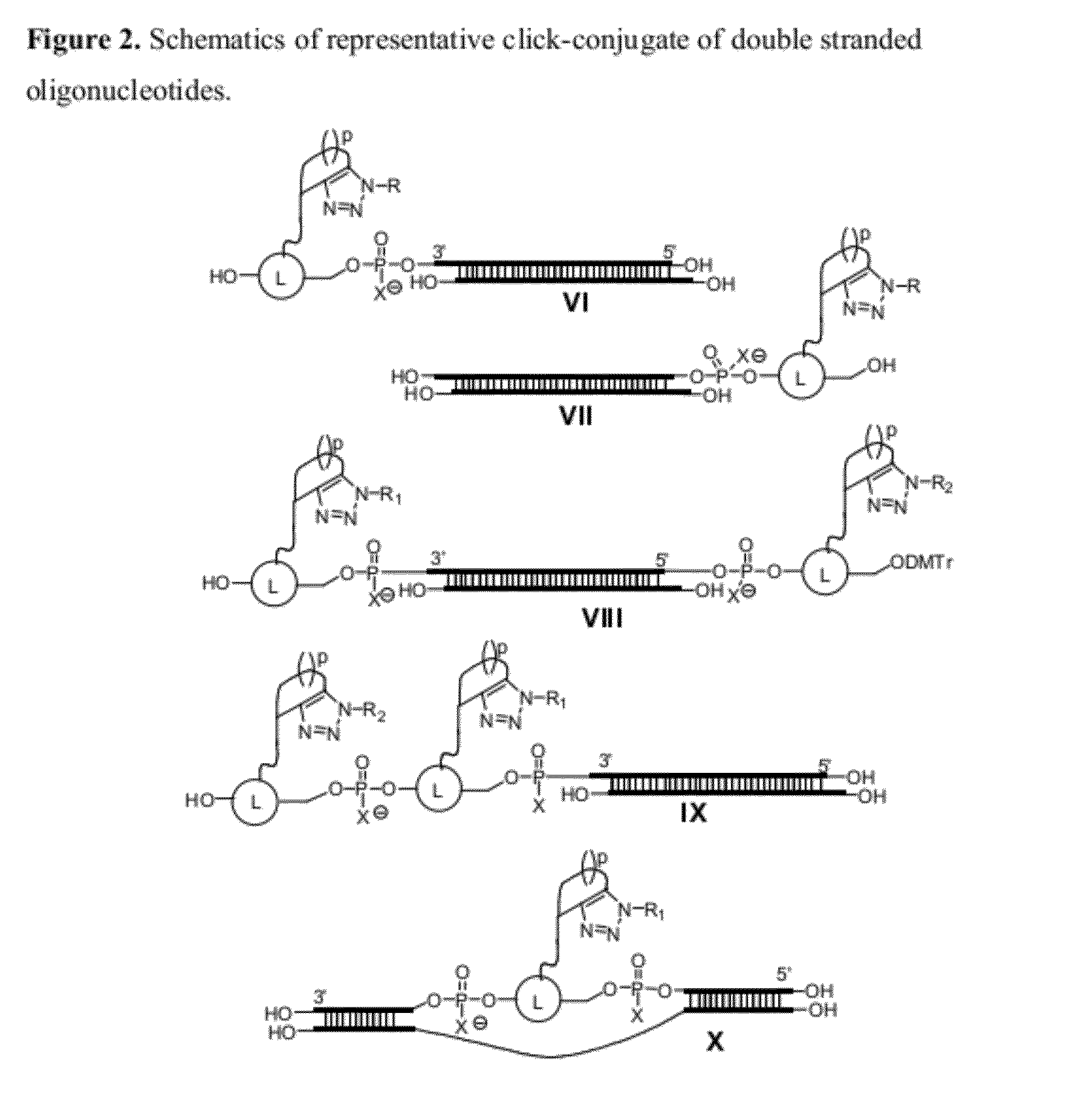
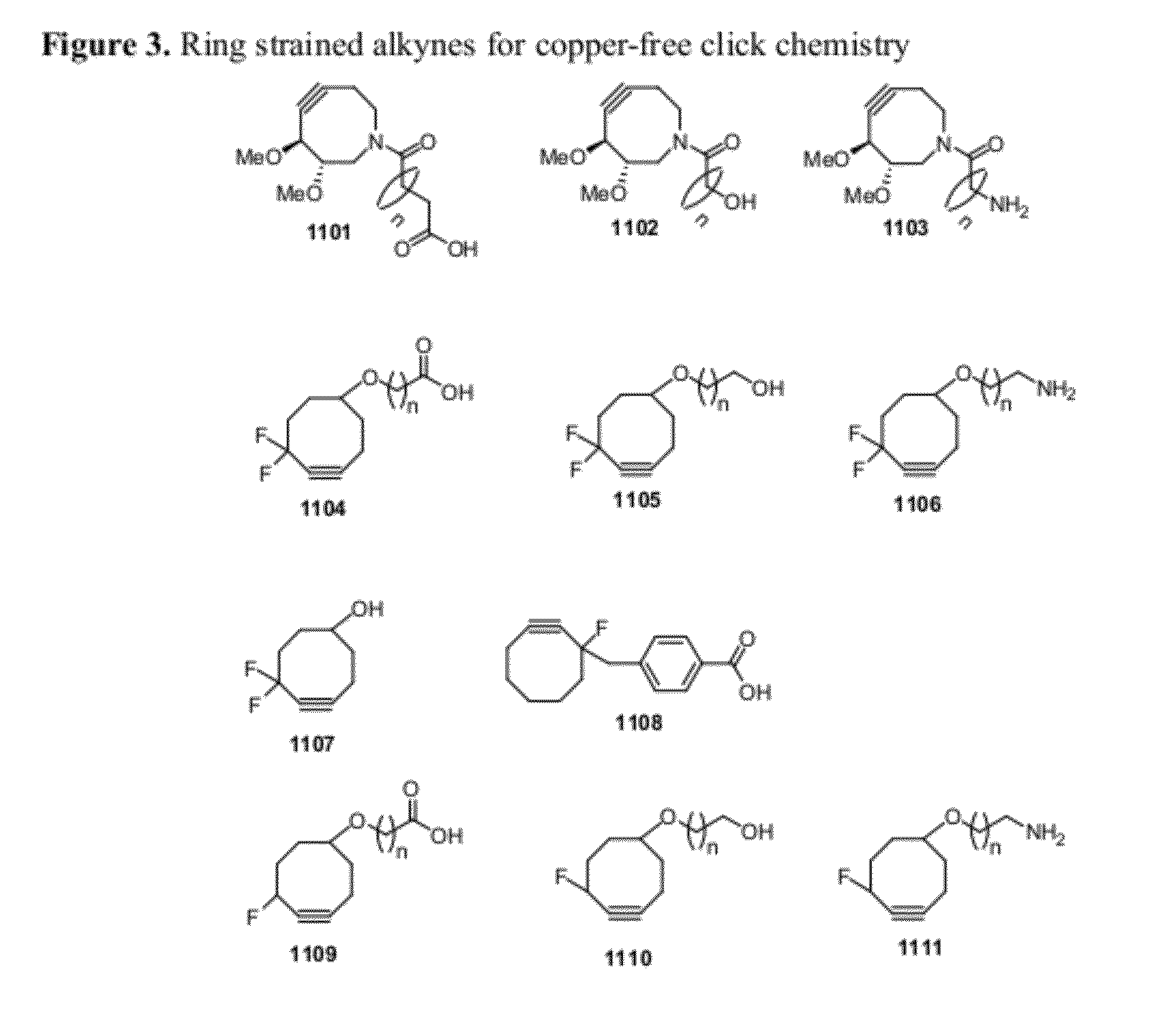
Chemical modifications of monomers and oligonucleotides with cycloaddition
The invention features compounds of formula I or II:In one embodiment, the invention relates compounds and processes for conjugating ligand to oligonucleotide. The invention further relates to methods for treating various disorders and diseases such as viral infections, bacterial infections, parasitic infections, cancers, allergies, autoimmune diseases, immunodeficiencies and immunosuppression.
Owner:ALNYLAM PHARMA INC
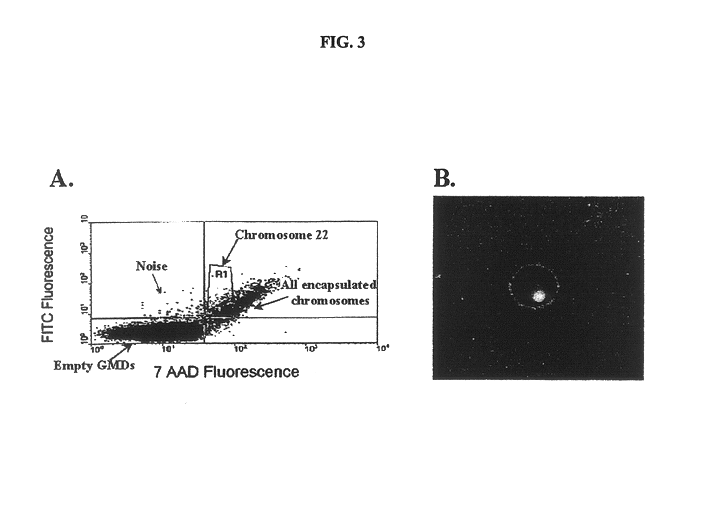
Gel microdrops in genetic analysis
InactiveUS6586176B1Bioreactor/fermenter combinationsBiological substance pretreatmentsSingle entityVirus
The invention provides methods of nucleic acid analysis. Such methods entail forming a population of gel microdrops encapsulating a population of biological entities, each entity comprising a nucleic acid, whereby at least some microdrops in the population each encapsulate a single entity. The population of gel microdrops is then contacted with a probe under conditions whereby the probe specifically hybridizes to at least one complementary sequence in the nucleic acid in at least one gel microdrop. At least one gel microdrop is then analyzed or detected. The biological entities can be cells, viruses, nuclei and chromosomes.
Owner:CELLAY
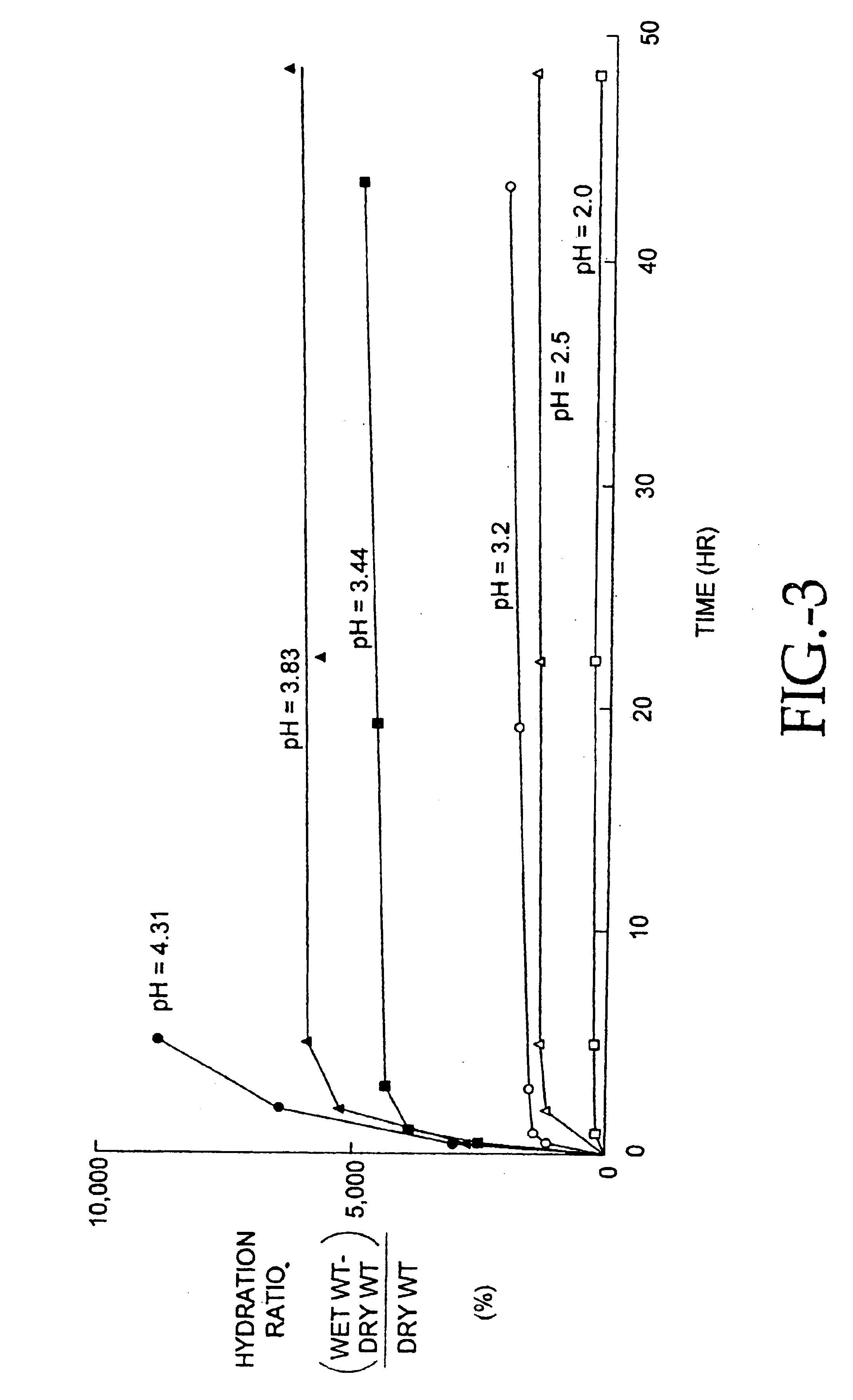
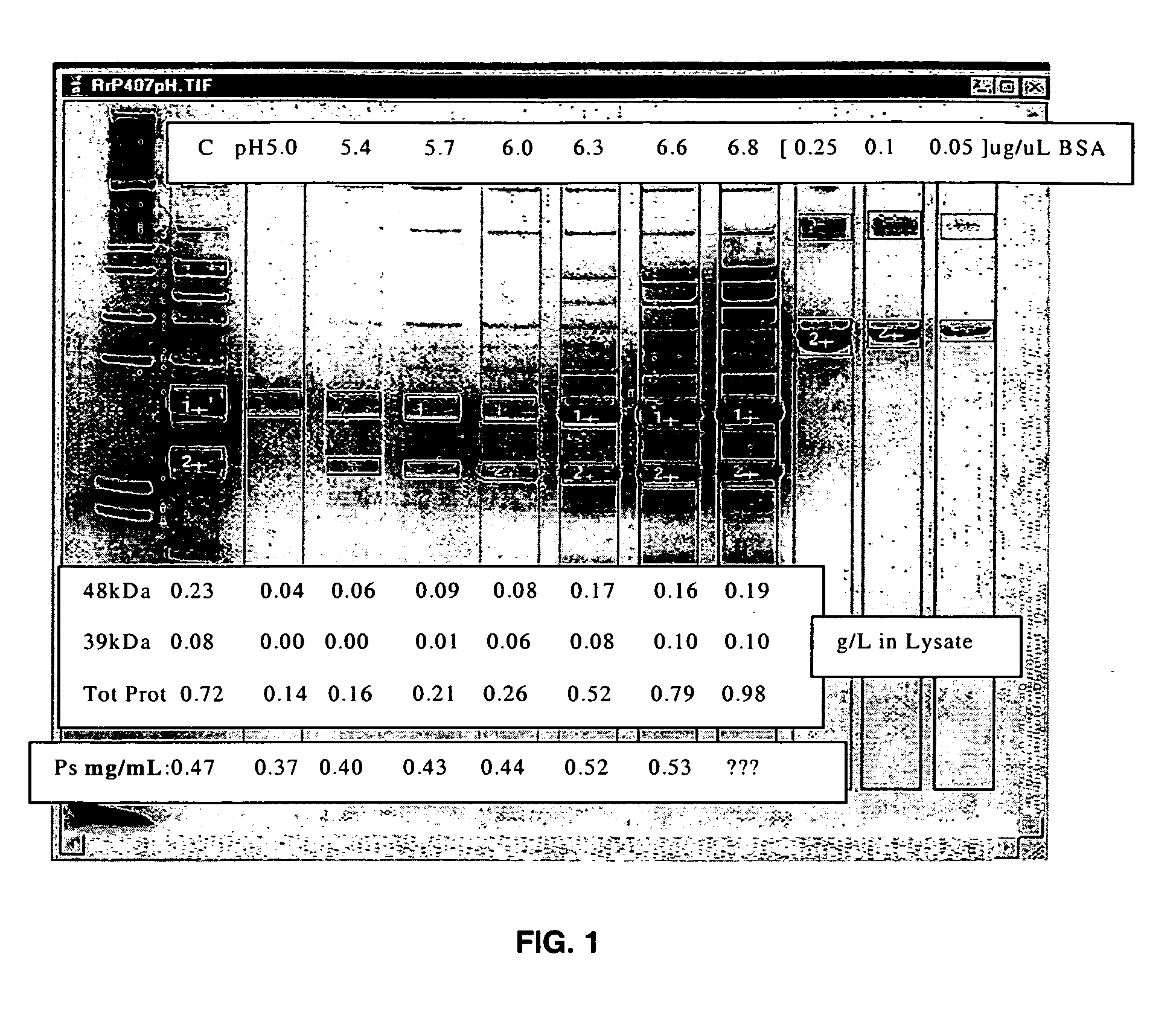
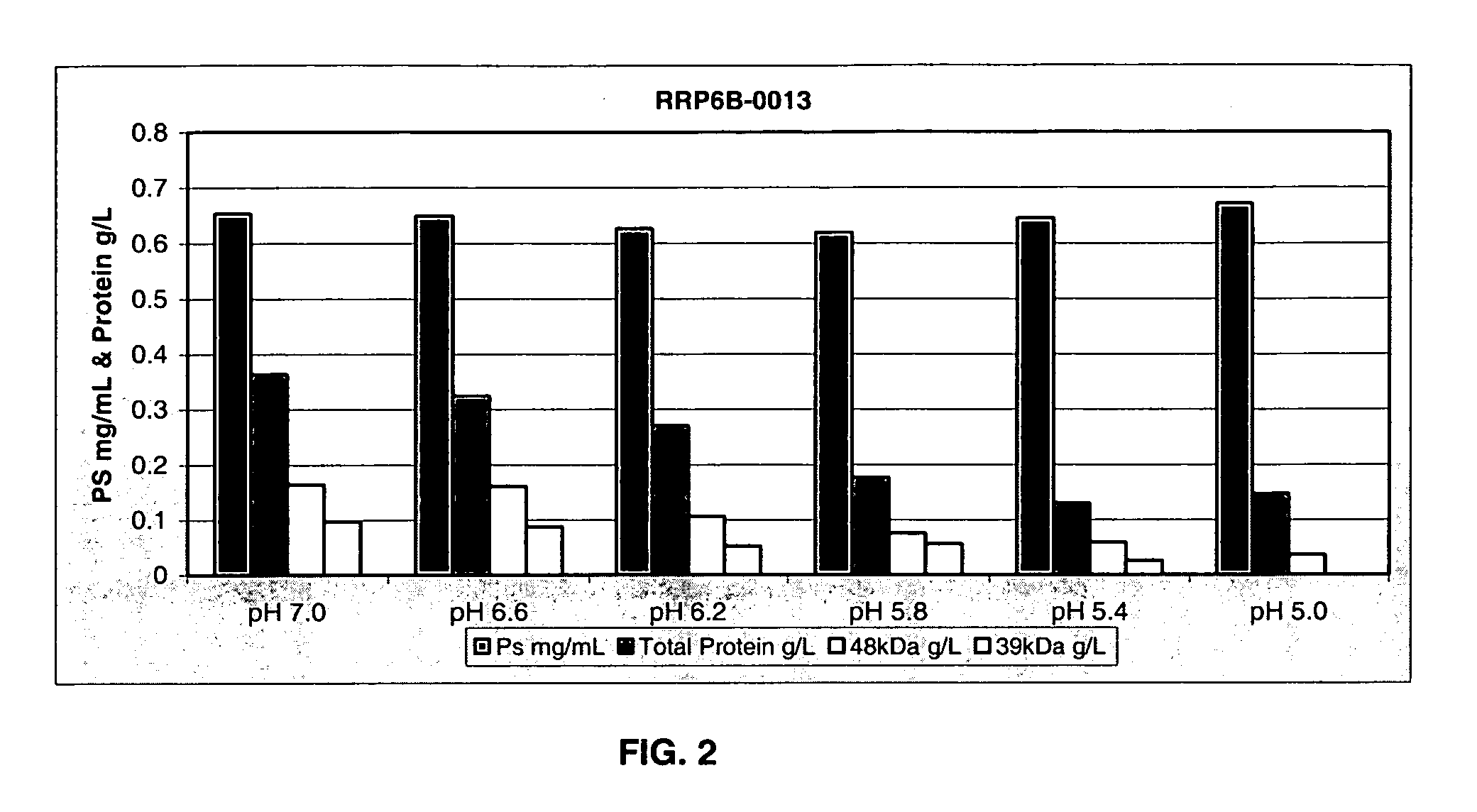
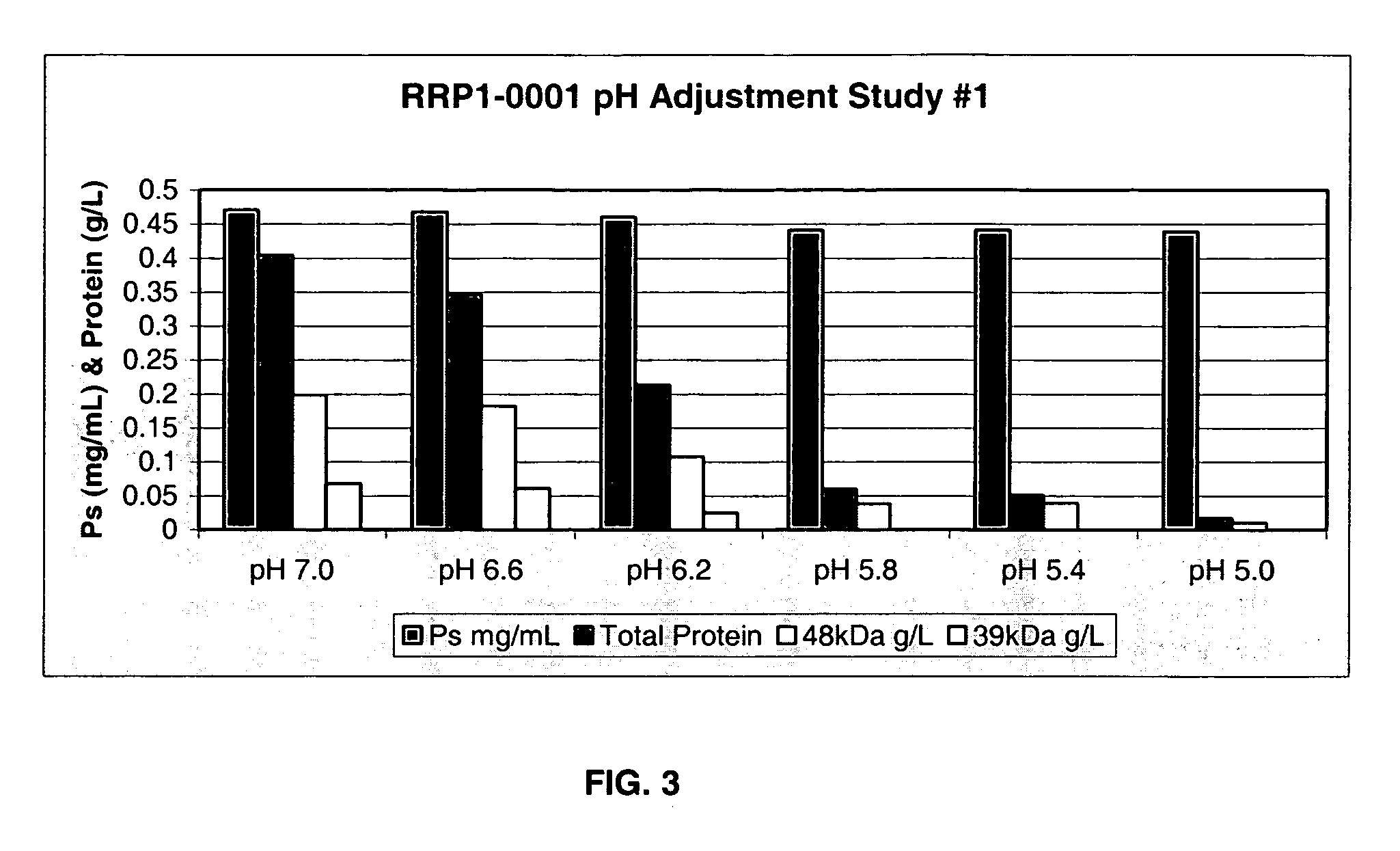
Separation of contaminants from Streptococcus pneumoniae polysaccharide by pH manipulation
ActiveUS20060228381A1Soluble protein is effectively reducedBacterial antigen ingredientsSugar derivativesStreptococcus pneumoniaeLysis
A process for reducing the protein content and preserving the capsular polysaccharide content in a complex cellular Streptococcus pneumoniae lysate broth prior to purification is described. Utilizing pH reduction after cellular lysis has resulted in a purified polysaccharide that consistently meets the protein specification, and higher recovery yields of polysaccharide during the purification process.
Owner:WYETH LLC
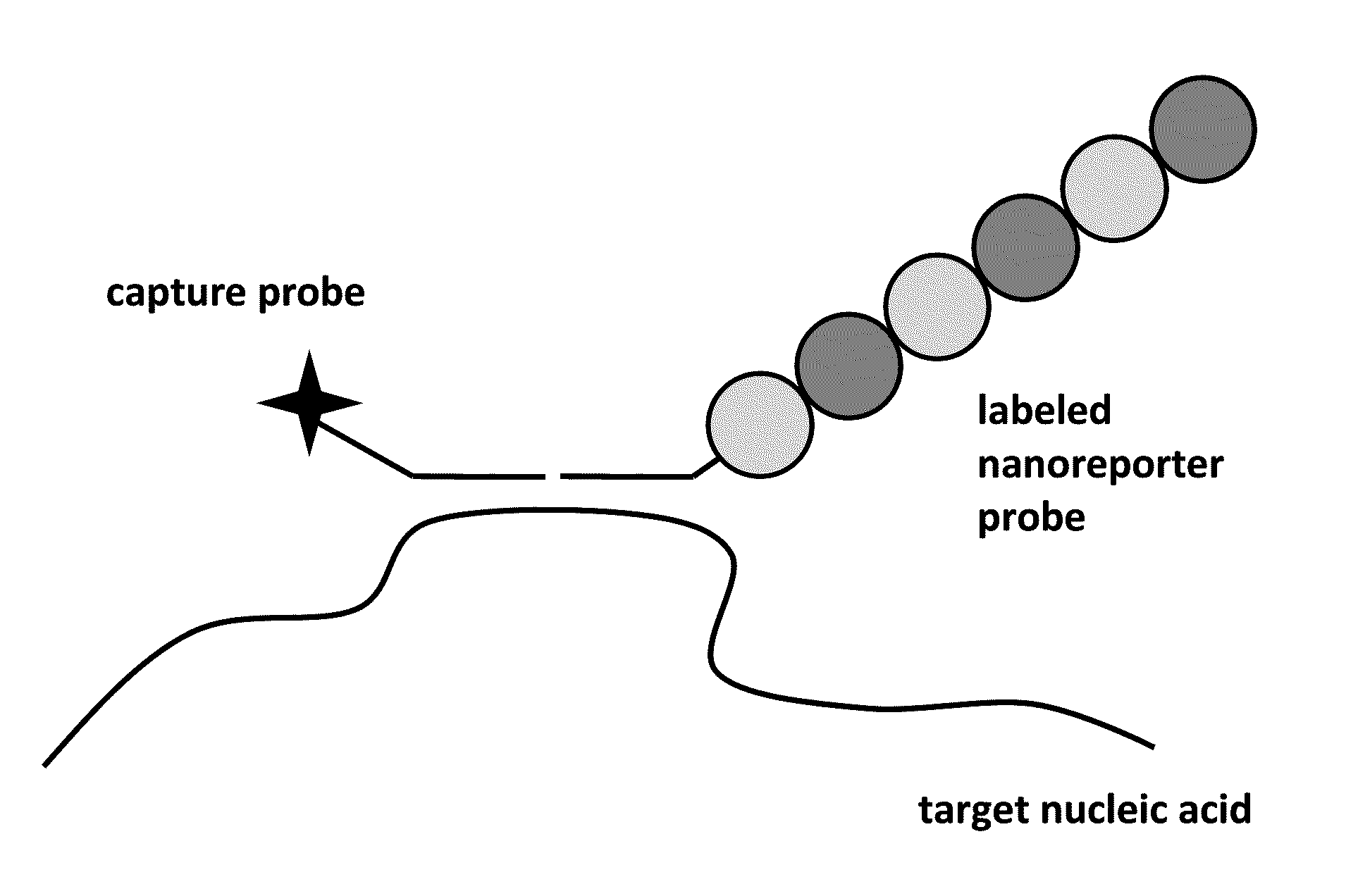
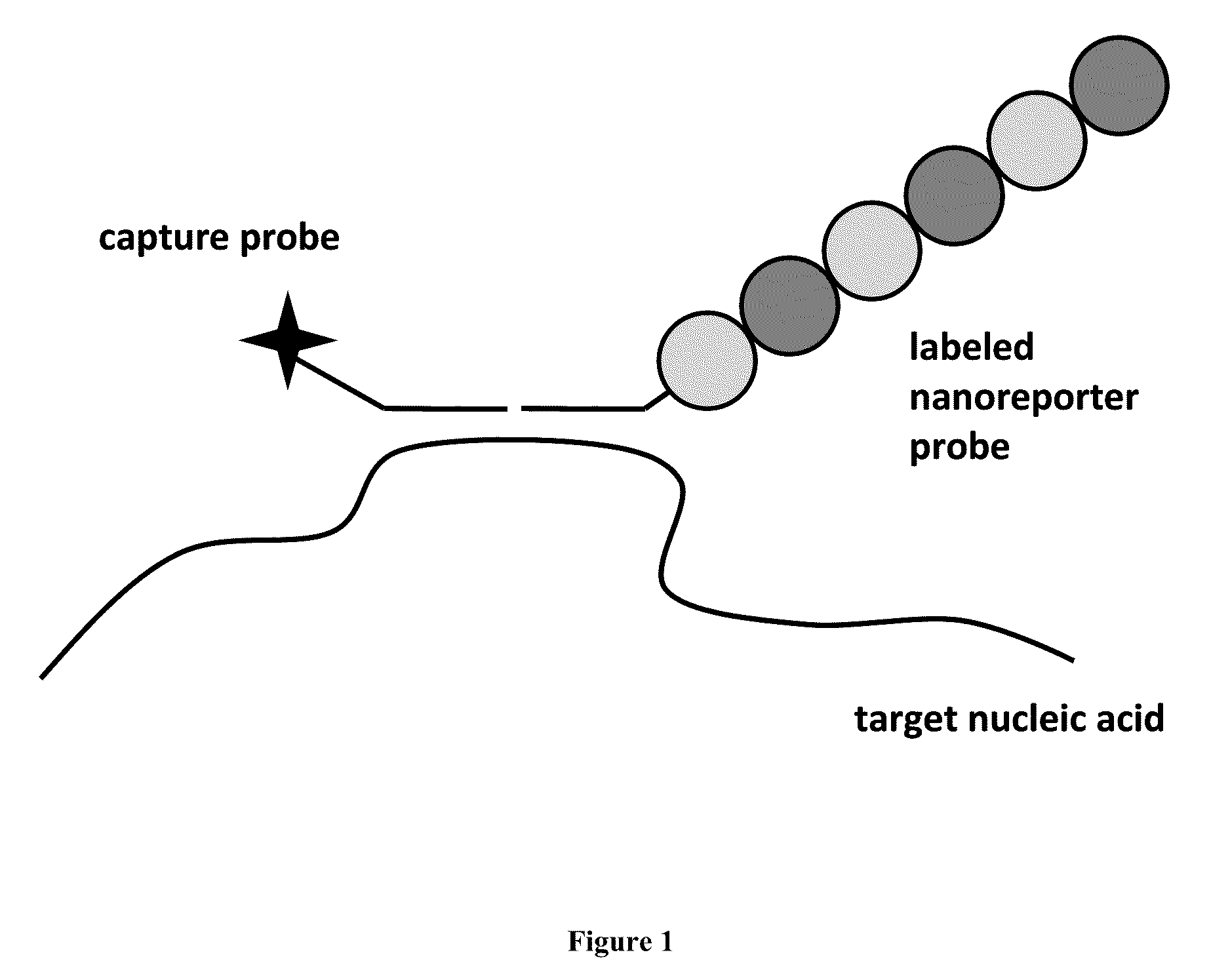
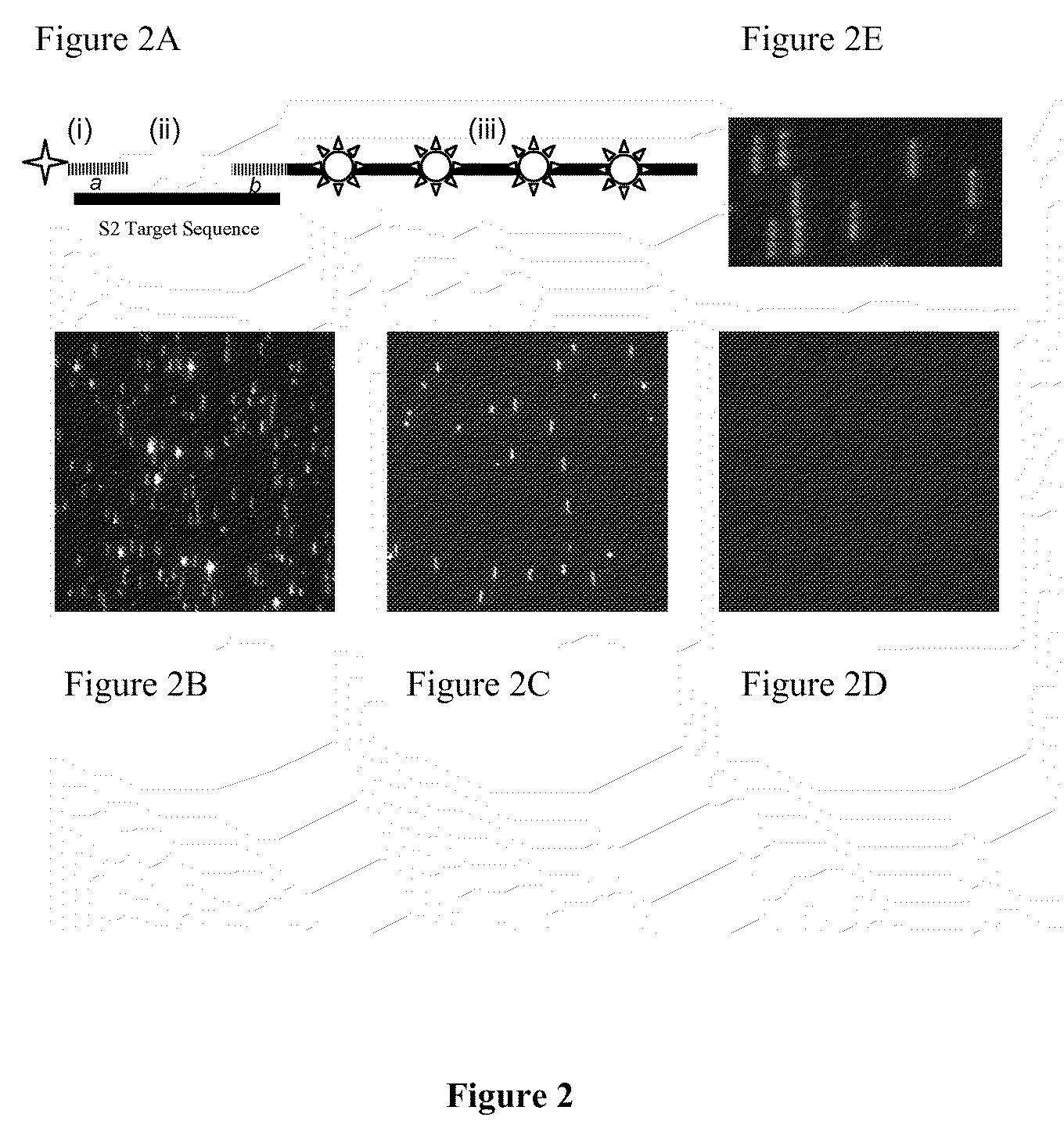
Stable nanoreporters
ActiveUS20100047924A1Stable populationSugar derivativesNucleotide librariesBio moleculesPolynucleotide
The present invention relates to compositions and methods for detection and quantification of individual target molecules in biomolecular samples. In particular, the invention relates to improved, stable nanoreporter probes that are capable of binding to and identifying target molecules based on the probes' uniquely detectable signal. Methods for identifying target-specific sequences for inclusion in the probes are also provided, as are methods of making and using such probes. Polynucleotide sequences of certain nanoreporter components are also provided. The probes can be used in diagnostic, prognostic, quality control and screening applications.
Owner:NANOSTRING TECH INC
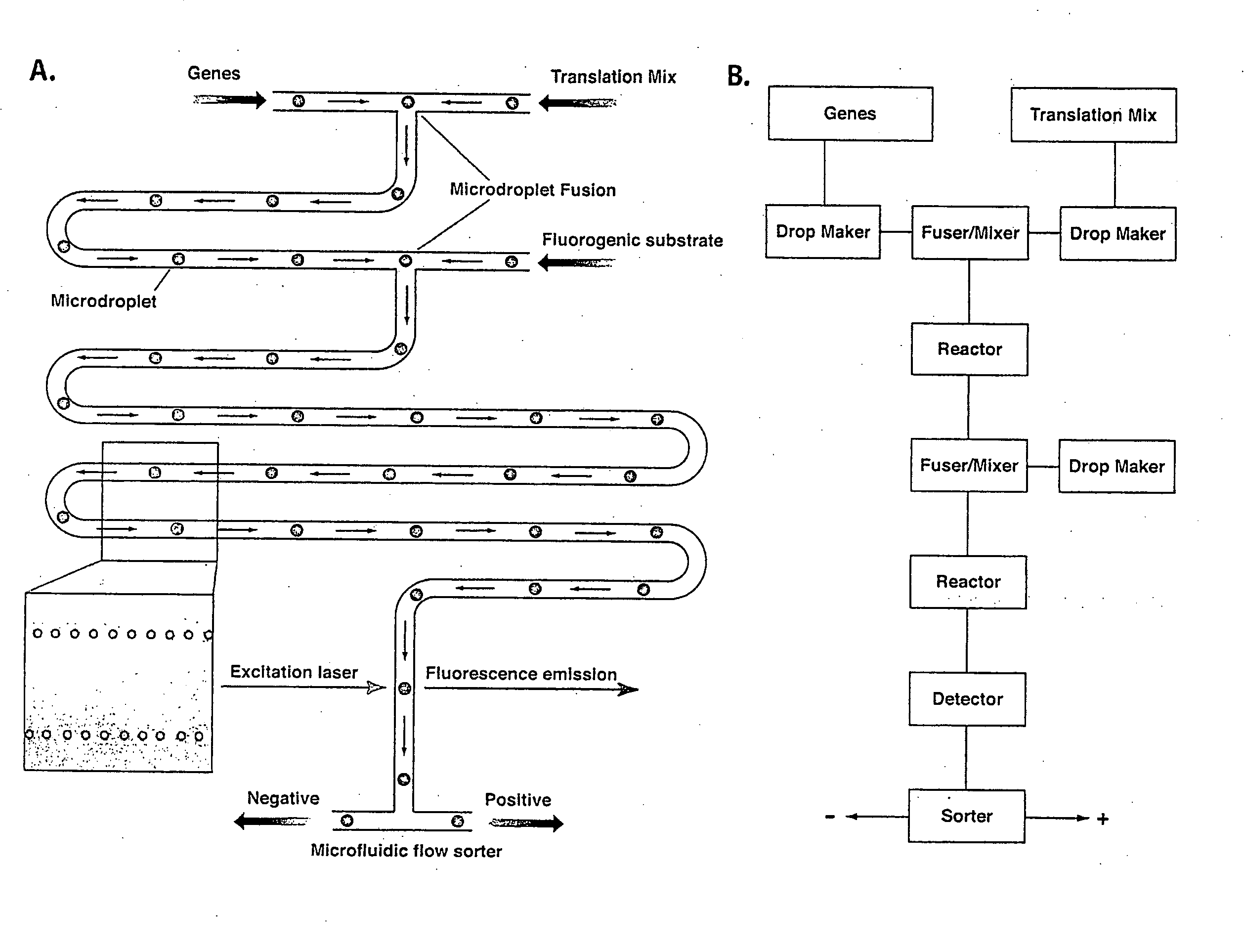
Vitro evolution in microfluidic systems
ActiveUS20090197248A1High activitySpeed up the processSequential/parallel process reactionsSugar derivativesGene productGenetic element
The invention describes a method for isolating one or more genetic elements encoding a gene product having a desired activity, comprising the steps of: (a) compartmentalising genetic elements into microcapsules; and (b) sorting the genetic elements which express the gene product having the desired activity; wherein at least one step is under microfluidic control. The invention enables the in vitro evolution of nucleic acids and proteins by repeated mutagenesis and iterative applications of the method of the invention.
Owner:PRESIDENT & FELLOWS OF HARVARD COLLEGE +1
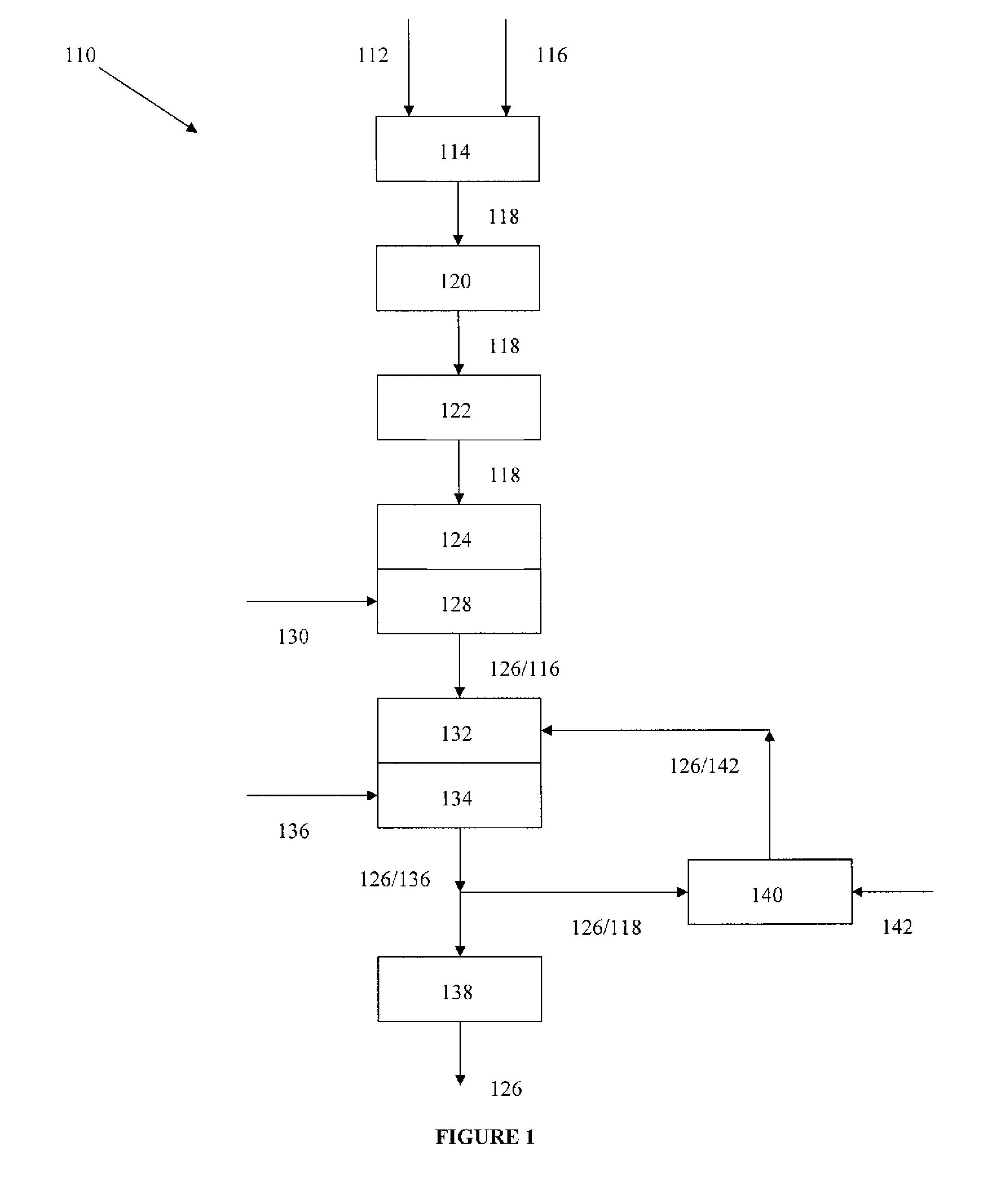
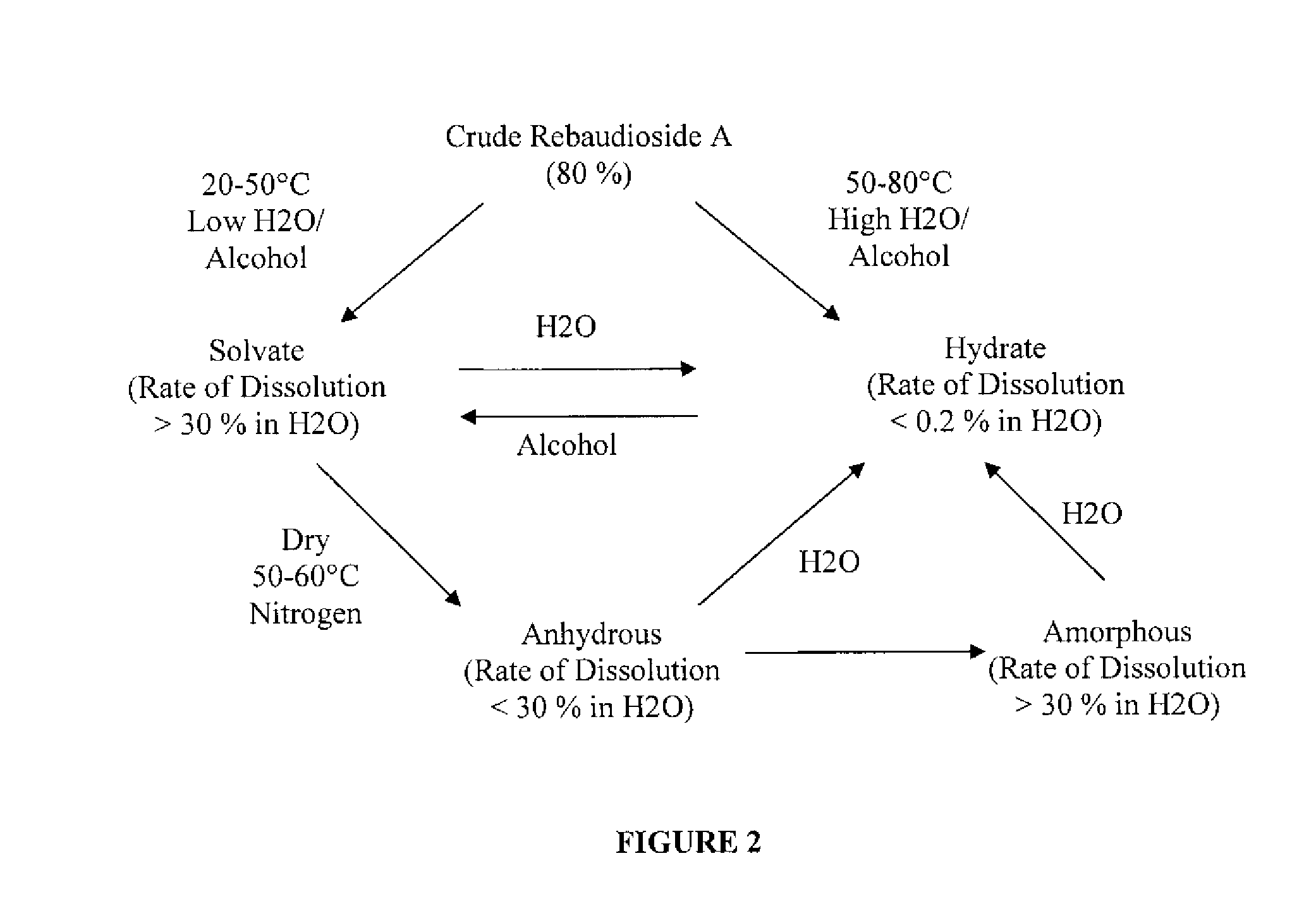
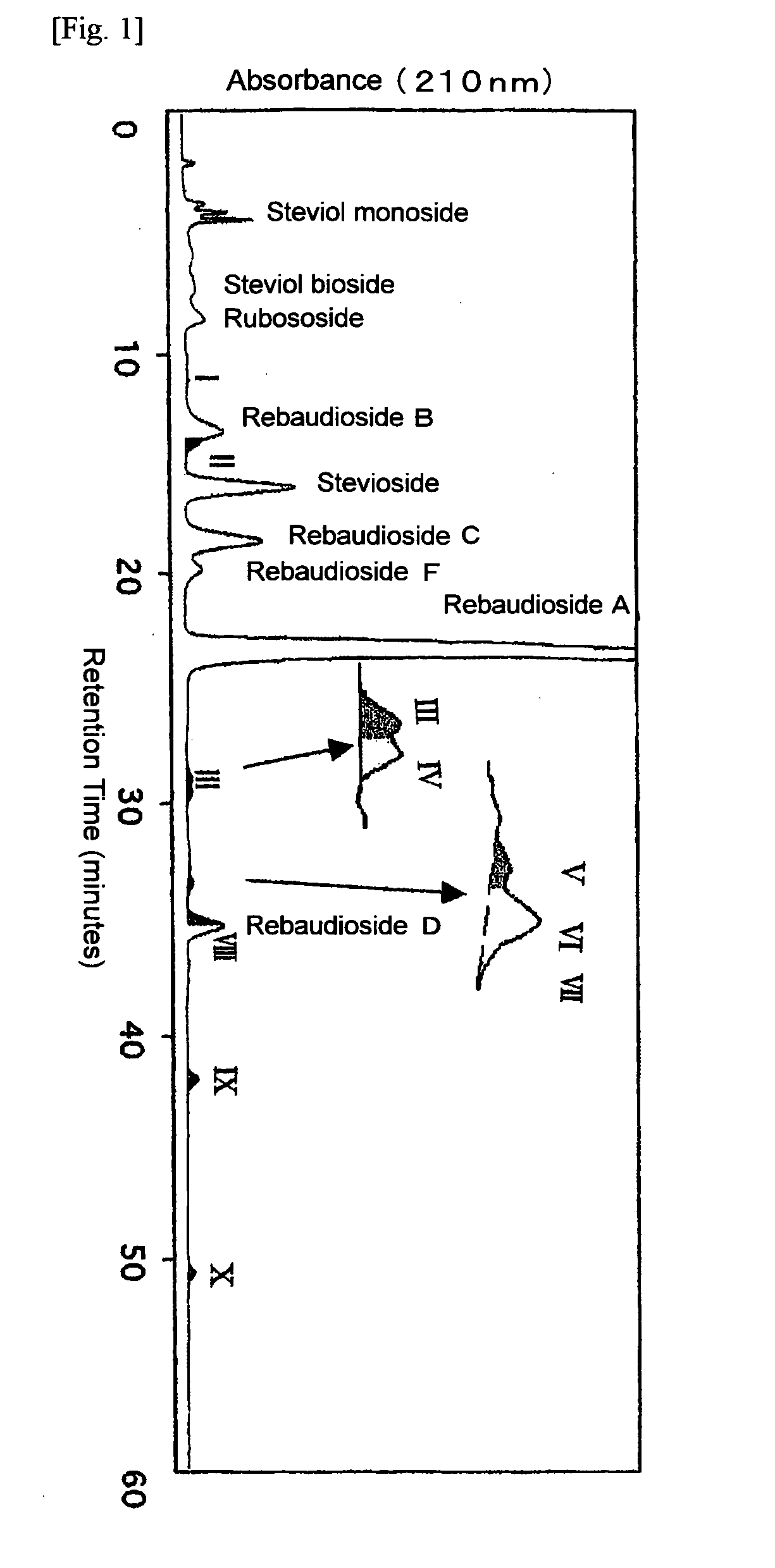
Rebaudioside A Composition and Method for Purifying Rebaudioside A
ActiveUS20070292582A1Simple crystallizationSugar derivativesFood ingredientsBiochemistryCrystallization
Exemplary embodiments of this invention encompass a method for purifying crude rebaudioside A. In particular, this invention relates to a method for purifying crude rebaudioside A compositions comprising purities from approximately 40% to approximately 95% rebaudioside A to obtain a substantially pure rebaudioside A product with a single crystallization step. Resulting polymorph and amorphous forms of rebaudioside A and methods for preparing polymorph and amorphous forms of rebaudioside A from crude rebaudioside A compositions and substantially pure rebaudioside A compositions also are disclosed.
Owner:THE COCA-COLA CO
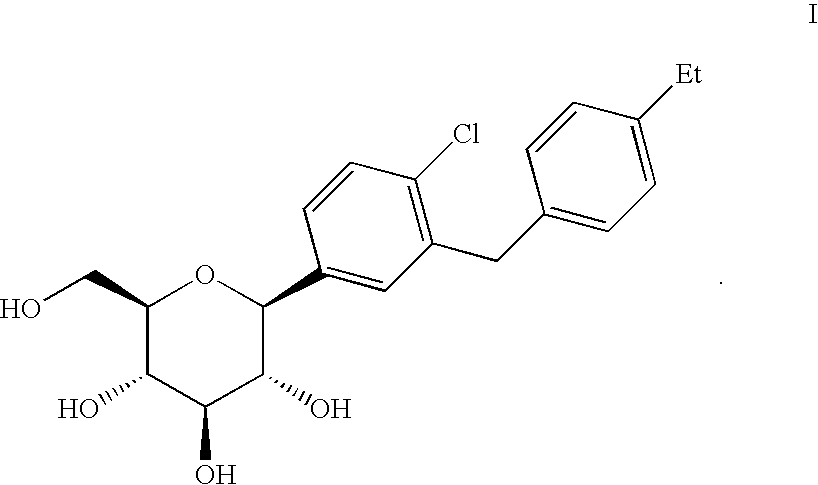
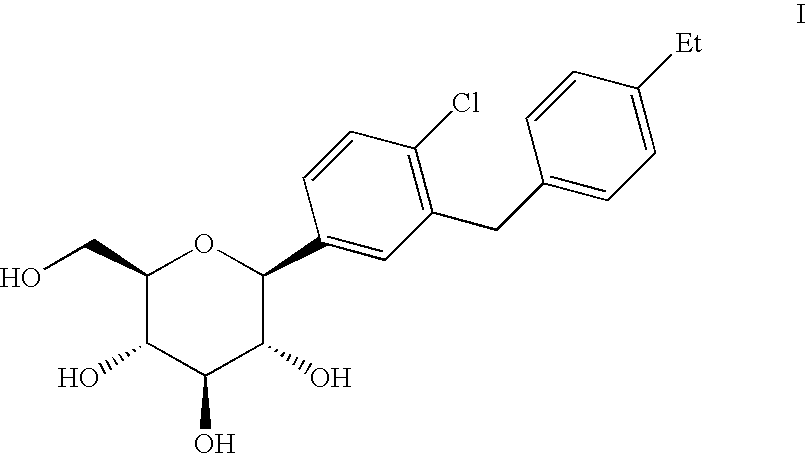
C-aryl glucoside SGLT2 inhibitors and method
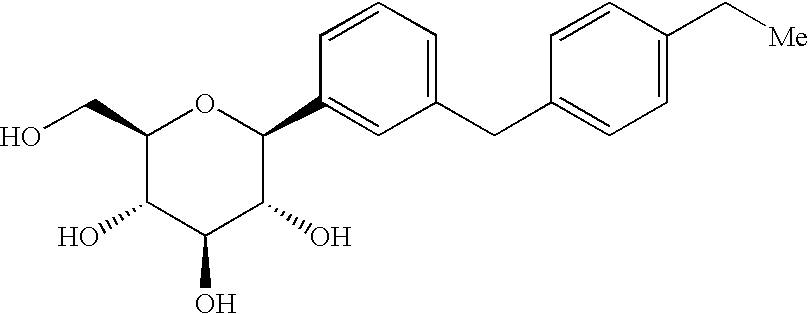
Owner:BRISTOL MYERS SQUIBB CO
Nucleoside phosphoramidates
Disclosed herein are nucleoside phosphoramidates and their use as agents for treating viral diseases. These compounds are inhibitors of RNA-dependent 5 RNA viral replication and are useful as inhibitors of HCV NS5B polymerase, as inhibitors of HCV replication and for treatment of hepatitis C infection in mammals.
Owner:GILEAD SCI INC
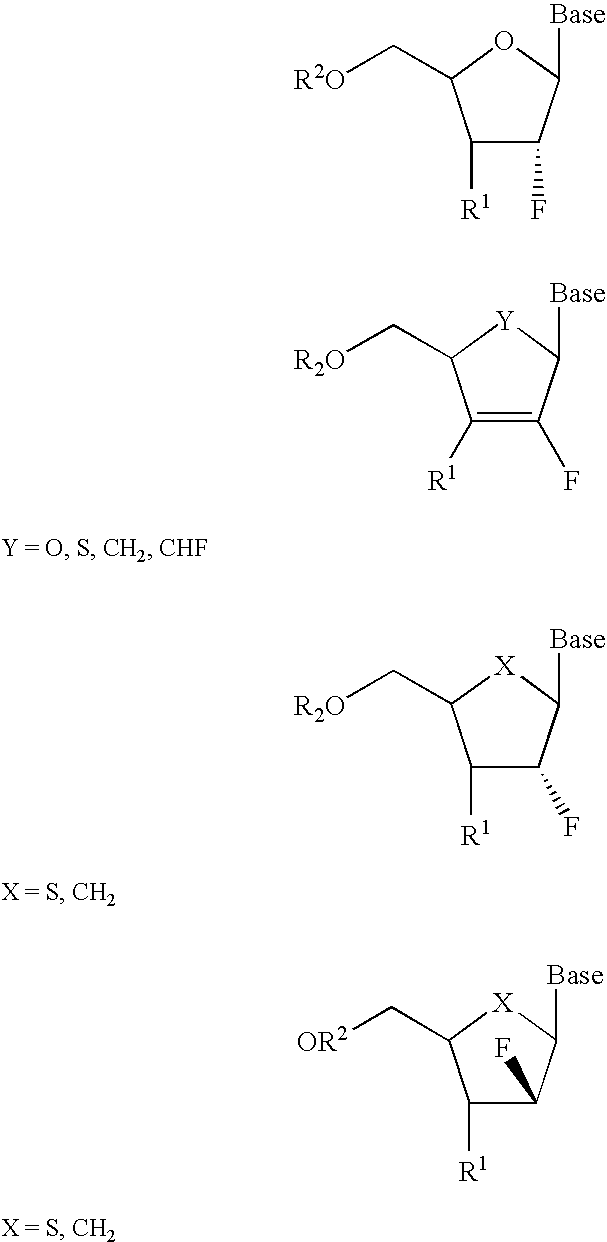
2′-fluoronucleosides
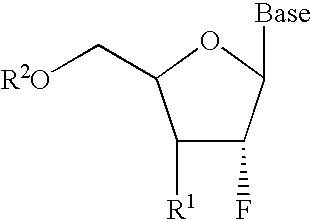
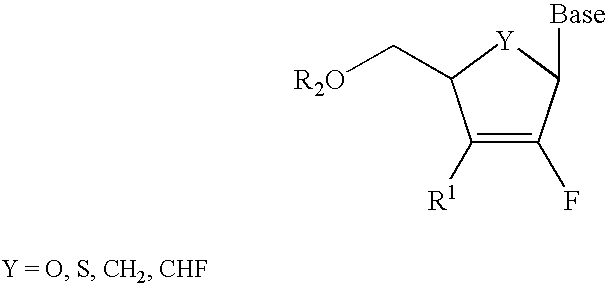
InactiveUS6911424B2Sure easyUseful in treatmentBiocideGroup 5/15 element organic compoundsPhosphoric Acid EstersPurine
A class of 2′-fluoro-nucleoside compounds are disclosed which are useful in the treatment of hepatitis B infection, hepatitis C infection, HIV and abnormal cellular proliferation, including tumors and cancer. The compounds have the general formulae: wherein[0001]Base is a purine or pyrimidine base;[0002]R1 is OH, H, OR3, N3, CN, halogen, including F, or CF3, lower alkyl, amino, loweralkylamino, di(lower)alkylamino, or alkoxy, and base refers to a purine or pyrimidine base;[0003]R2 is H, phosphate, including monophosphate, diphosphate, triphosphate, or a stabilized phosphate prodrug; acyl, or other pharmaceutically acceptable leaving group which when administered in vivo, is capable of providing a compound wherein R2 is H or phosphate; sulfonate ester including alkyl or arylalkyl sulfonyl including methanesulfonyl, benzyl, wherein the phenyl group is optionally substituted with one or more substituents as described in the definition of aryl given above, a lipid, an amino acid, peptide, or cholesterol; and[0004]R3 is acyl, alkyl, phosphate, or other pharmaceutically acceptable leaving group which when administered in vivo, is capable of being cleaved to the parent compound, or a pharmaceutically acceptable salt thereof.
Owner:EMORY UNIVERSITY
Superabsorbent surface-treated carboxyalkylated polysaccharides and process for producing same
Owner:ARCHER DANIELS MIDLAND CO
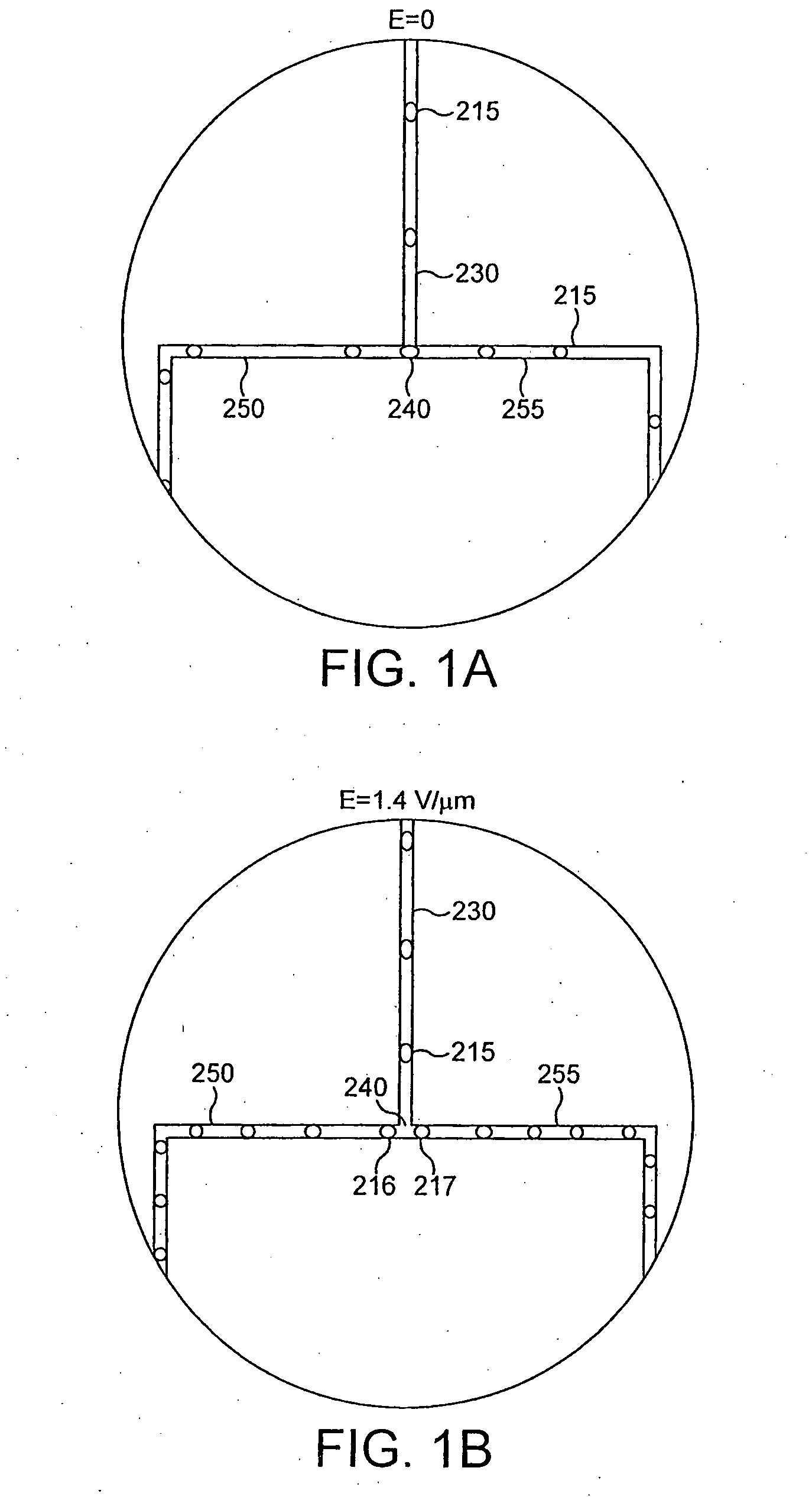
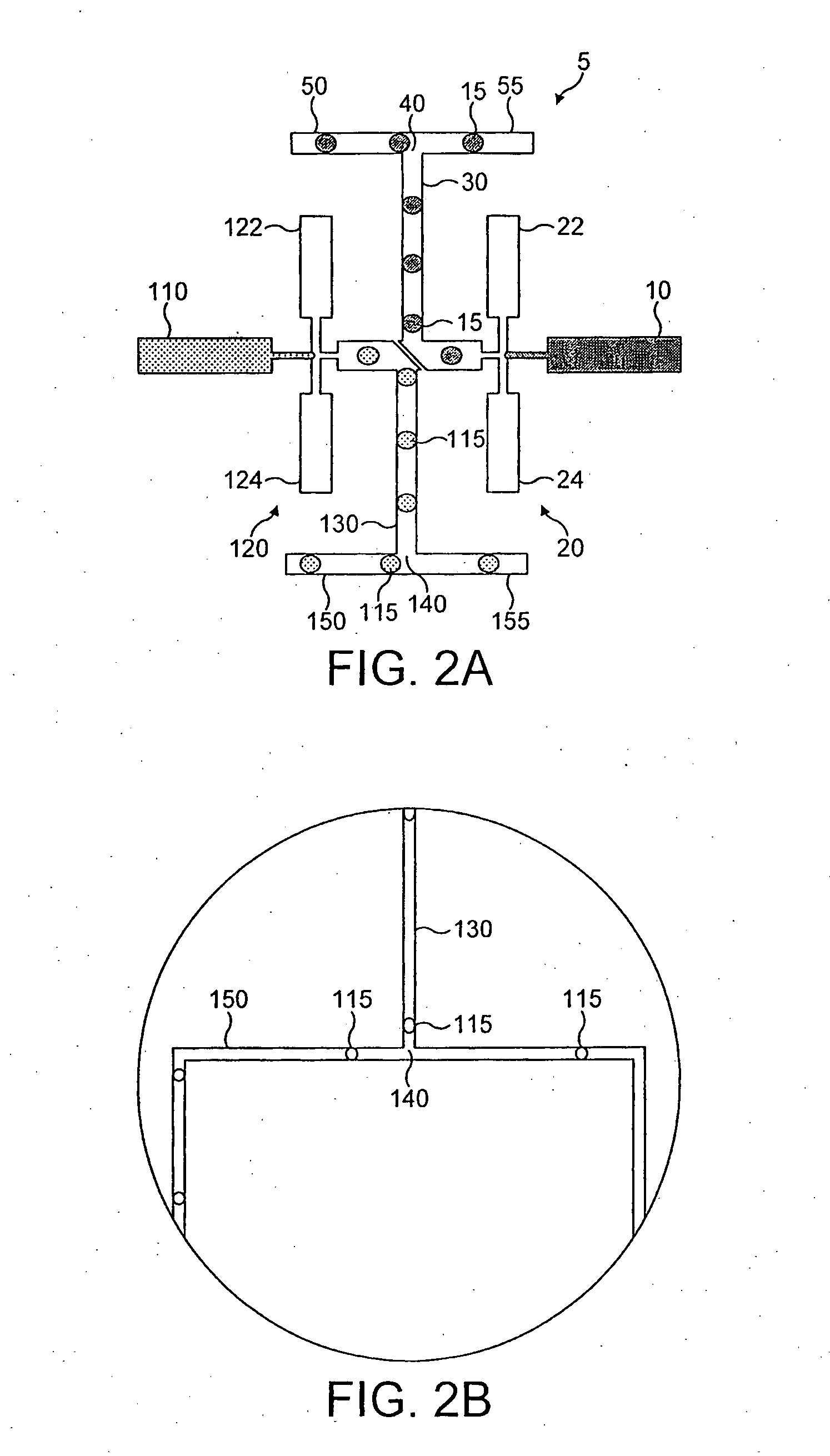
Device and method for the manipulation of a fluid sample
The invention provides a device and method for the manipulation of materials (e.g., particles, cells, macromolecules, such as proteins, nucleic acids or other moieties) in a fluid sample. The device comprises a substrate having a plurality of microstructures and an insulator film on the structures. Application of a voltage to the structures induces separation of materials in the sample. The device and method are useful for a wide variety of applications such as dielectrophoresis (DEP) or the separation of a target material from other material in a fluid sample.
Owner:CEPHEID INC
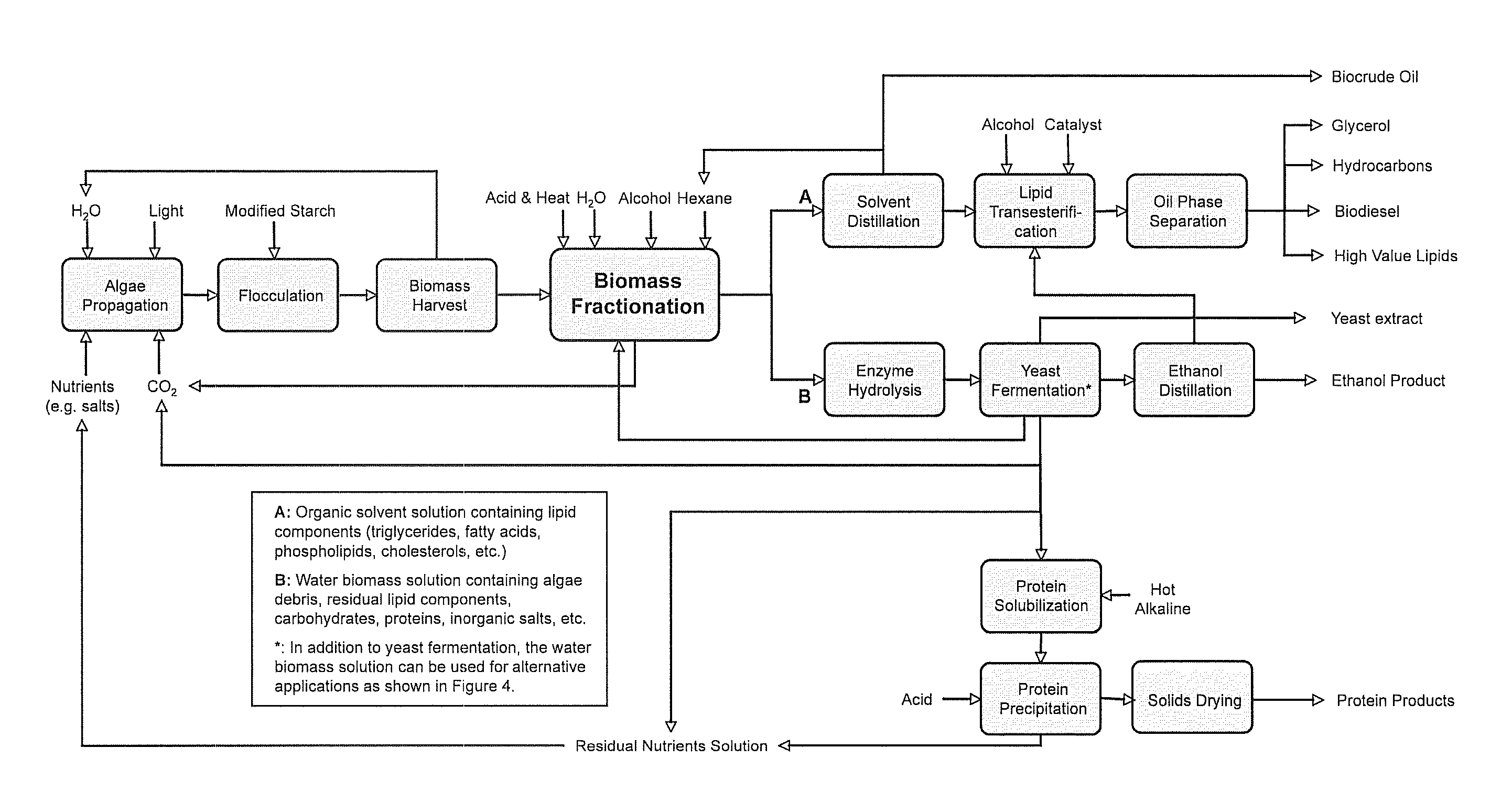
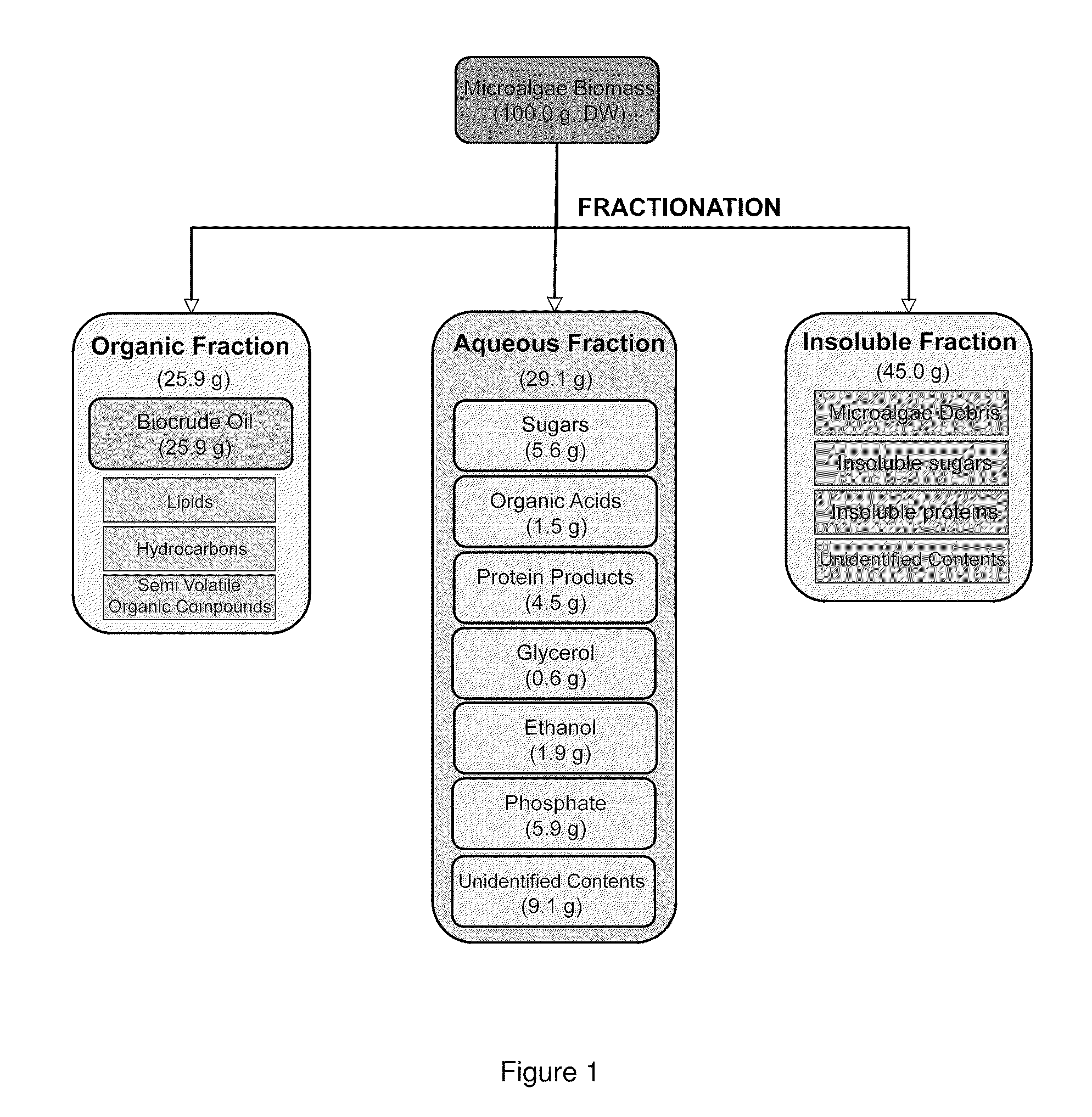
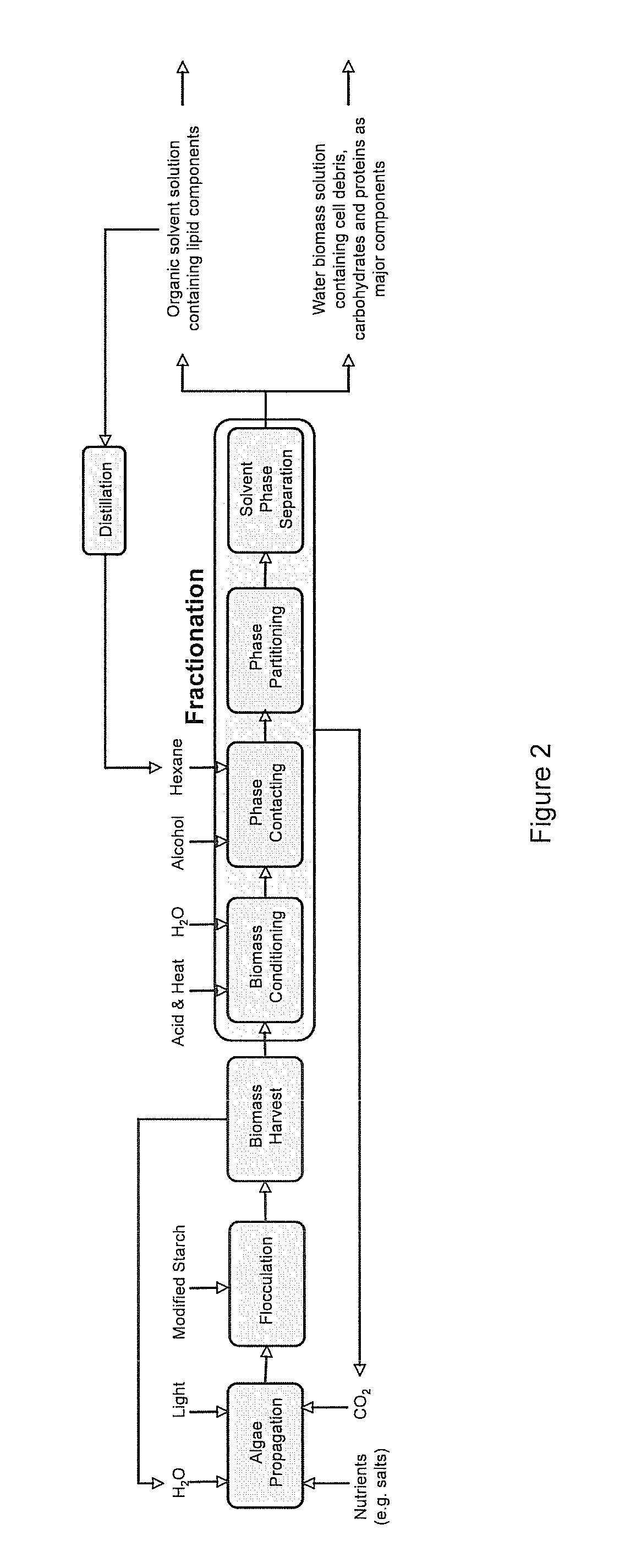
Algae biomass fractionation
A method of fractionating biomass, by permeability conditioning biomass suspended in a pH adjusted solution of at least one water-based polar solvent to form a conditioned biomass, intimately contacting the pH adjusted solution with at least one non-polar solvent, partitioning to obtain an non-polar solvent solution and a polar biomass solution, and recovering cell and cell derived products from the non-polar solvent solution and polar biomass solution. Products recovered from the above method. A method of operating a renewable and sustainable plant for growing and processing algae.
Owner:VALICOR
Non-invasive detection of fetal genetic traits
ActiveUS20080071076A1Facilitates non-invasive detectionSugar derivativesOther chemical processesPregnancyNon invasive
Blood plasma of pregnant women contains fetal and (generally>90%) maternal circulatory extracellular DNA. Most of said fetal DNA contains .Itoreq.500 base pairs, said maternal DNA having a greater size. Separation of circulatory extracellular DNA of .Itoreq.500 base pairs results in separation of fetal from maternal DNA. A fraction of a blood plasma or serum sample of a pregnant woman containing, due to size separation (e.g. by chromatography, density gradient centrifugation or nanotechnological methods), extracellular DNA substantially comprising .Itoreq.500 base pairs is useful for non-invasive detection of fetal genetic traits (including the fetal RhD gene in pregnancies at risk for HDN; fetal Y chromosome-specific sequences in pregnancies at risk for X chromosome-linked disorders; chromosomal aberrations; hereditary Mendelian genetic disorders and corresponding genetic markers; and traits decisive for paternity determination) by e.g. PCR, ligand chain reaction or probe hybridization techniques, or nucleic acid arrays.
Owner:SEQUENOM INC
Pharmaceutical Composition with High-Potency Sweetener
InactiveUS20070116829A1Improve flavor profileImproving temporal profile profileSugar derivativesPharmaceutical delivery mechanismSweetnessFood flavor
The present invention relates generally to pharmaceutical compositions comprising non-caloric or low-caloric high-potency sweeteners and methods for making and using them. In particular, the present invention relates to different pharmaceutical compositions comprising at least one non-caloric or low-caloric natural and / or synthetic high-potency sweetener, at least one sweet taste improving composition, and a pharmaceutically active substance. The present invention also relates to pharmaceutical compositions and methods that can improve the tastes of non-caloric or low-caloric natural and / or synthetic,, high-potency sweeteners by imparting a more sugar-like taste or characteristic. In particular, the pharmaceutical compositions and methods provide a more sugar-like temporal profile, including sweetness onset and sweetness linger, and / or a more sugar-like flavor profile.
Owner:THE COCA-COLA CO
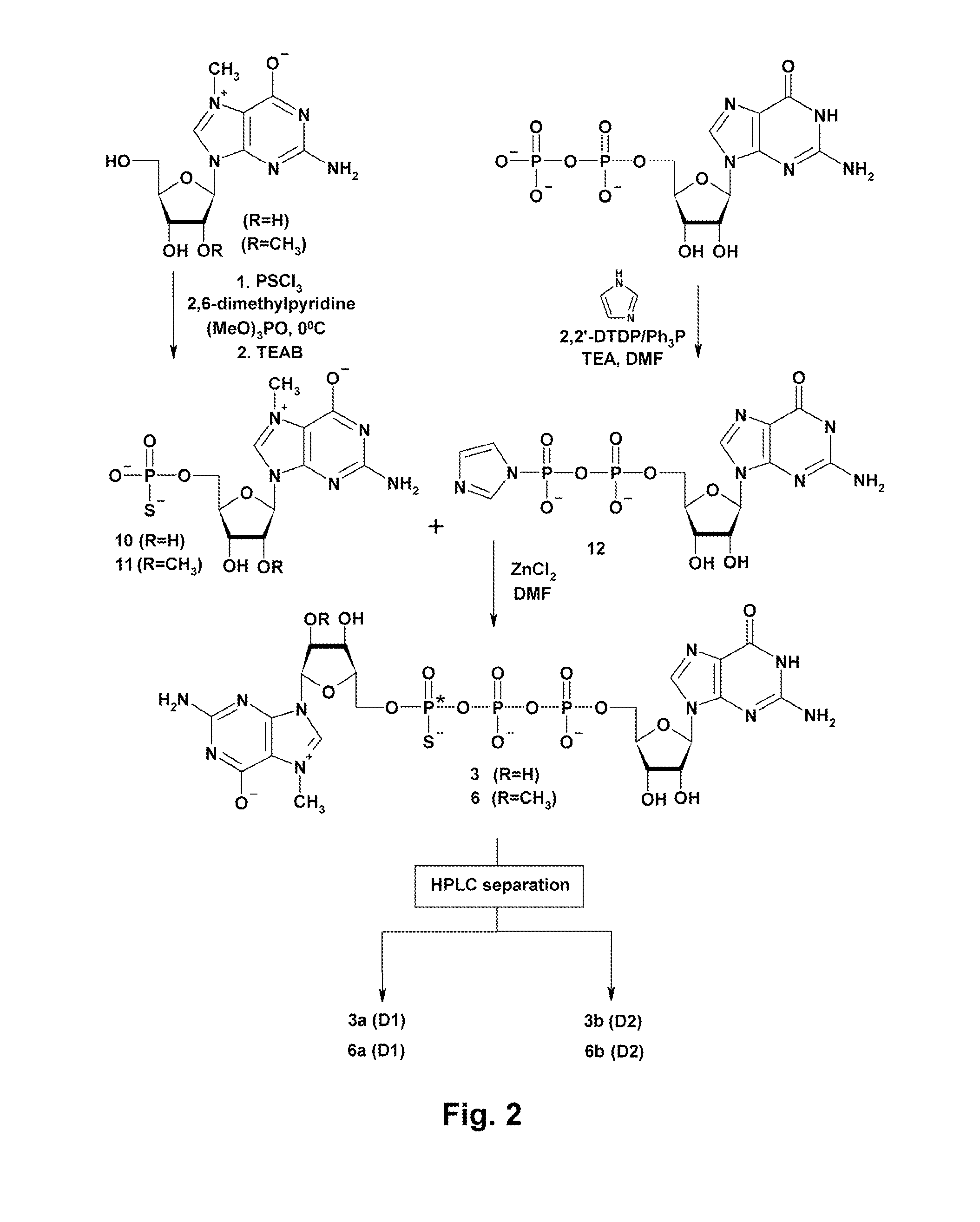
Synthesis and use of anti-reverse phosphorothioate analogs of the messenger RNA cap
ActiveUS8153773B2High affinityHigh productSugar derivativesPeptide/protein ingredients7-MethylguanosineEIF4E
New RNA cap analogs are disclosed containing one or more phosphorothioates groups. The analogs also contain modifications at the 2′-O position of 7-methylguanosine that prevent them from being incorporated in the reverse orientation during in vitro synthesis of mRNA and that hence are “anti-reverse cap analogs” (ARCAs). The ARCA modification ensures that the S atom is precisely positioned within the active sites of cap-binding proteins in both the translational and decapping machinery. The new S-ARCA analogs are resistant to in vivo decapping enzymes. Some S-ARCAs have a higher affinity for eIF4E than the corresponding analogs not containing a phosphorothioate group. When mRNAs containing the various S-ARCAs are introduced into cultured cells, some are translated as much as five-fold more efficiently than mRNAs synthesized with the conventional analog m7GpppG.
Owner:BOARD OF SUPERVISORS OF LOUISIANA STATE UNIV & AGRI & MECHANICAL COLLEGE +1
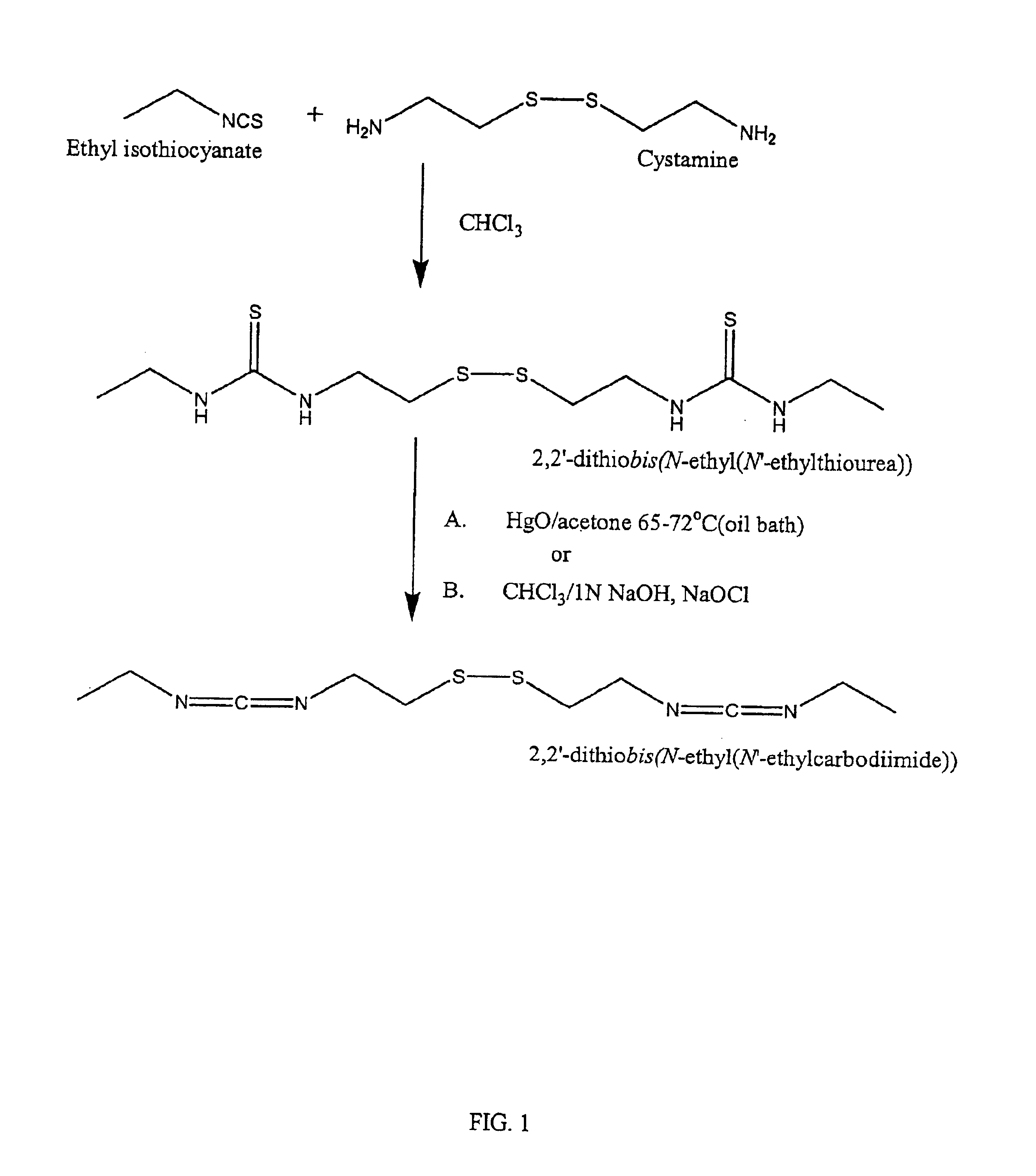
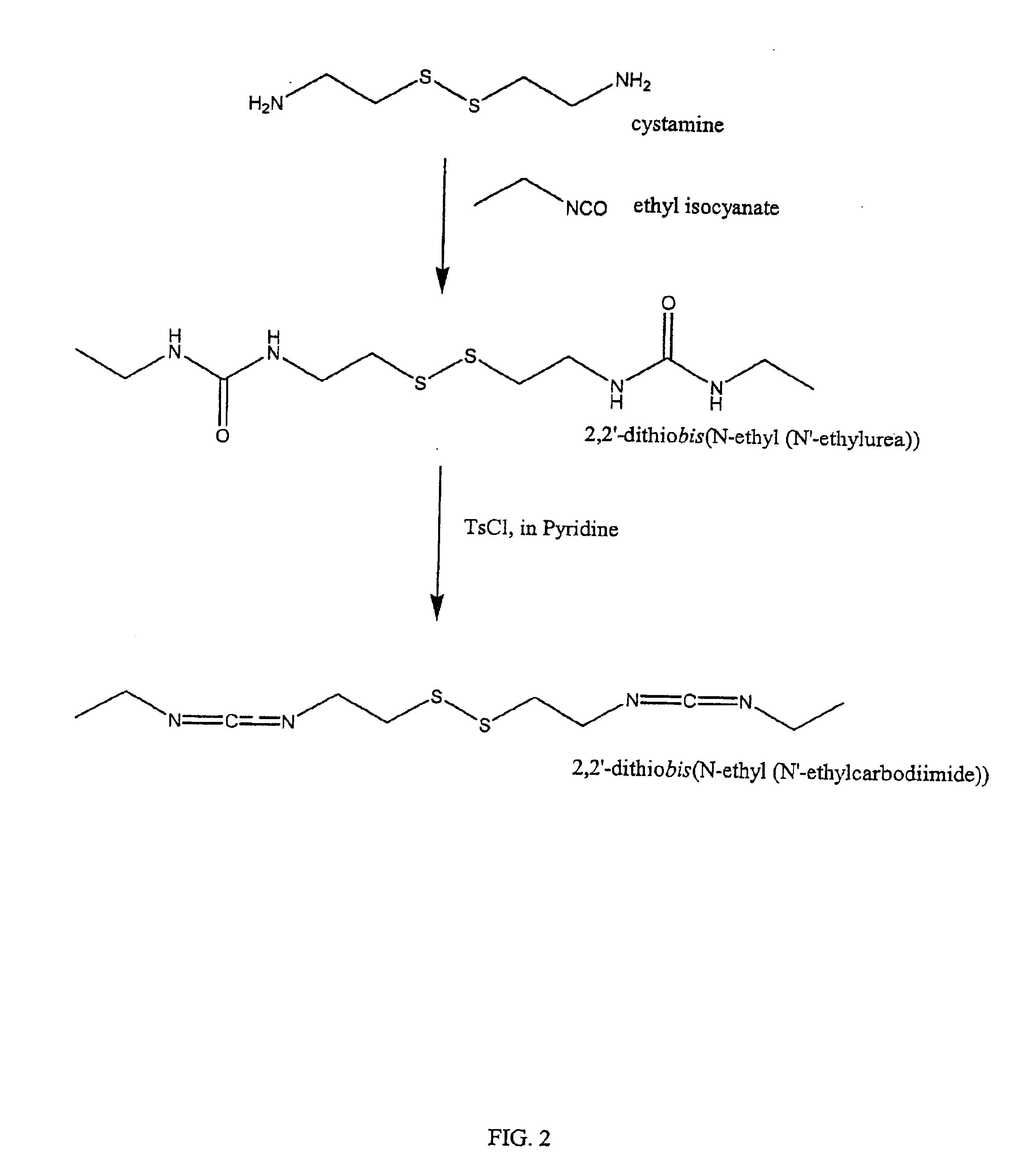
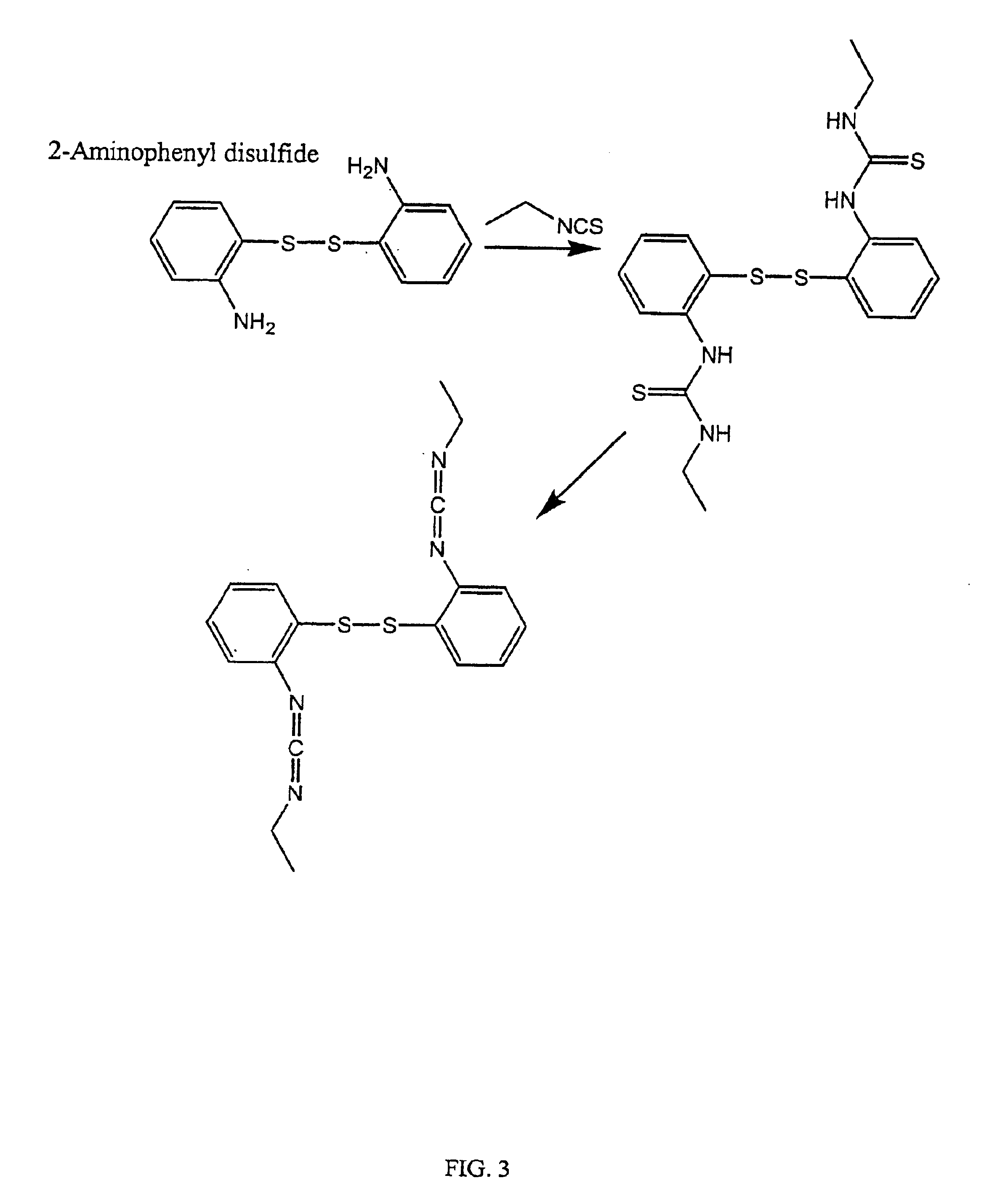
Thiol-modified hyaluronan
InactiveUS6884788B2High activityControl moreBiocideOrganic active ingredientsUrea derivativesCross-link
The present invention relates to biscarbodiimides, thiourea derivatives, urea derivatives, and cross-linked hyaluronan derivatives having at least one intramolecular disulfide bond, and methods of preparation thereof. The invention also includes thiolated hyaluronan derivatives and salts thereof having at least one pendant thiol group or a modified pendant thiol group, and methods of preparation thereof. An example of a modified pendant thiol group is a sulfhydryl group linked to a small molecule such as a bioactive agent, for example a drug or pharmaceutically active moiety. A hyaluronan derivative having a sulfhydryl group linked to a pharmaceutically active moiety is useful as a sustained or controlled release drug delivery vehicle. Compositions containing the hyaluronan derivatives of the invention can reversibly viscosify in vivo or in vitro, in response to mild changes in condition, and are thus useful in ophthalmic surgery and in tissue engineering.
Owner:ANIKA THERAPEUTICS INC
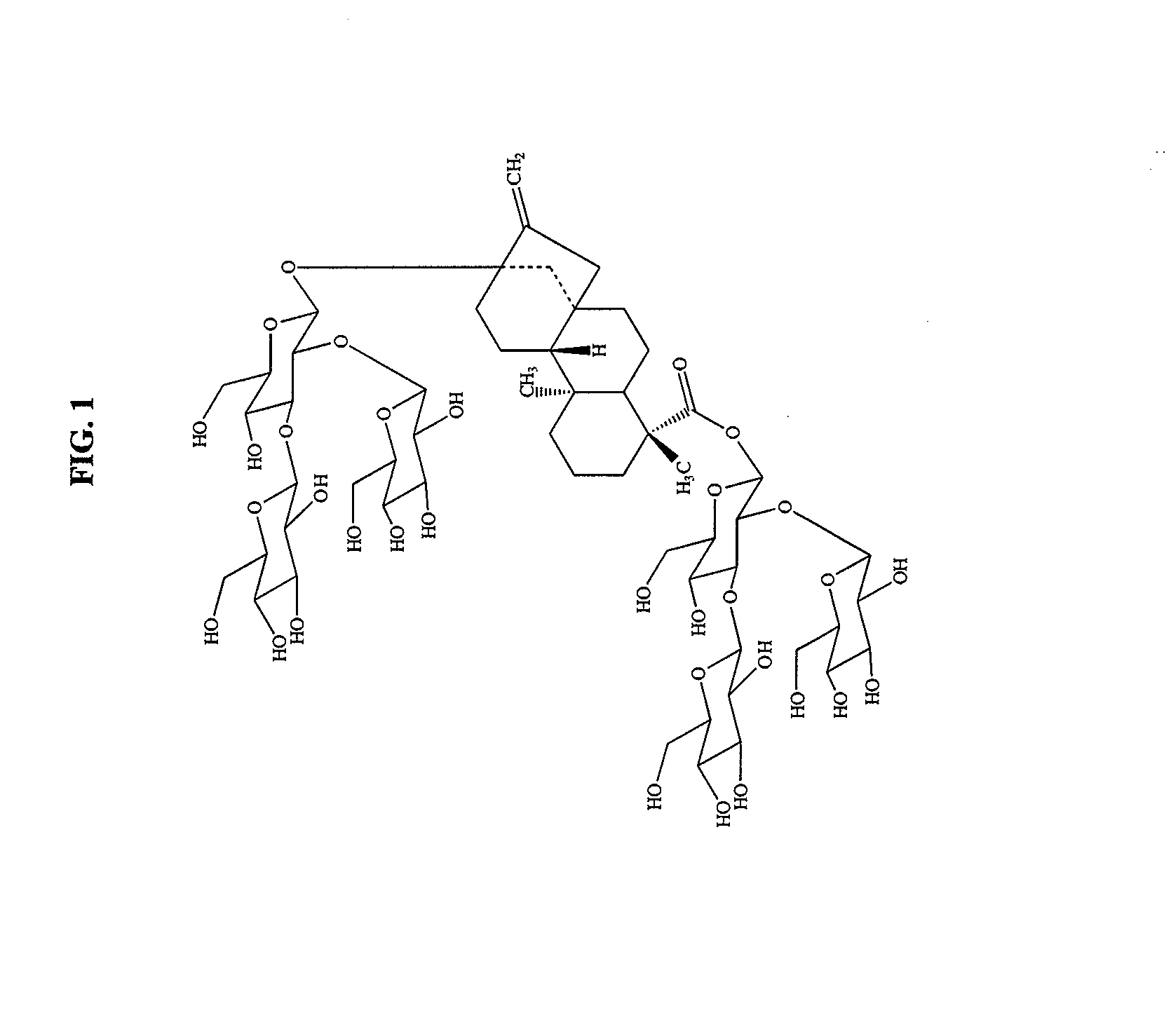
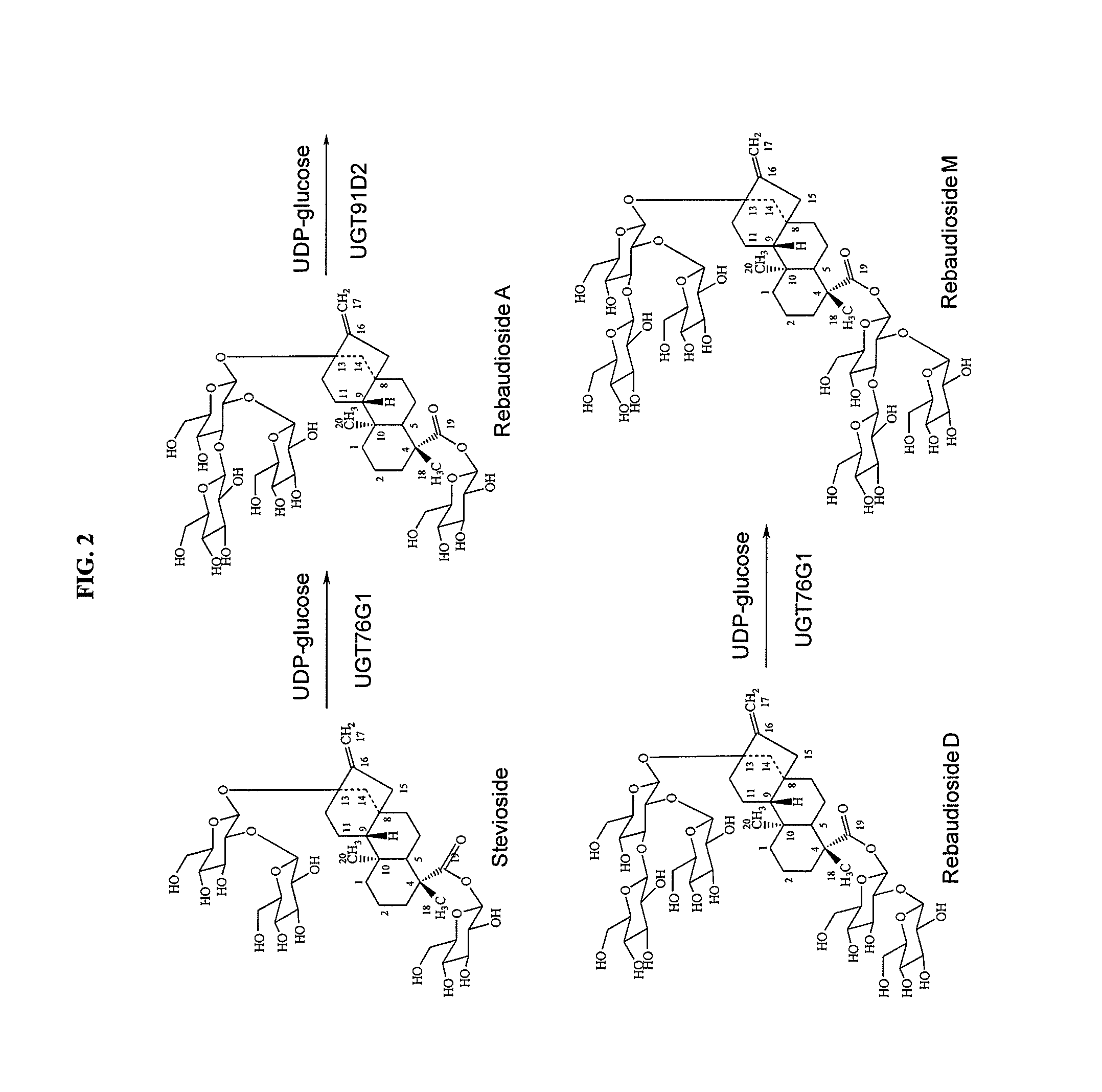
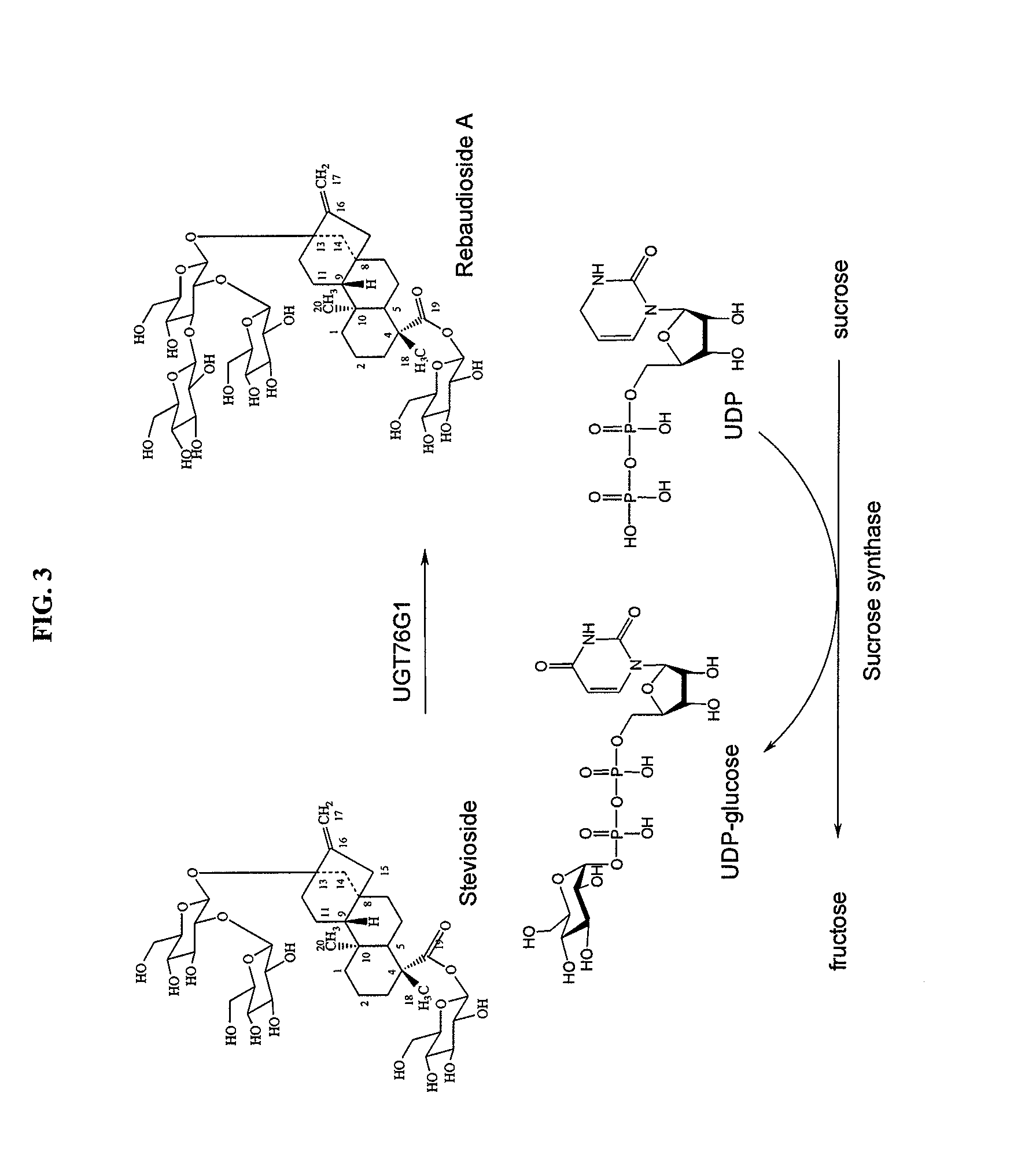
High-purity steviol glycosides
Methods of preparing highly purified steviol glycosides, particularly rebaudiosides A, D and M are described. The methods include utilizing recombinant microorganisms for converting various staring compositions to target steviol glycosides. In addition, novel steviol glycosides reb D2 and reb M2 are disclosed, as are methods of preparing the same. The highly purified rebaudiosides are useful as non-caloric sweetener in edible and chewable compositions such as any beverages, confectioneries, bakery products, cookies, and chewing gums.
Owner:PURECIRCLE SDN BHD
New steviol glycoside
ActiveUS20110183056A1Excellent strong tasteStrong tasteSugar derivativesComponent separationQuality controlSweetness
Nobel Stevia Sweetening components are provided. Through the analysis of the components of the nobel Steviol Glycoside included in the stevia extract and / or crystals, not only the quality control of sweeteners, but judgment on the correctness of indication of origin or infringement of right are facilitated since the raw material of the sweetener can be identified.
Owner:MORITA KAGAKU KOGYO CO LTD
Popular searches
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com