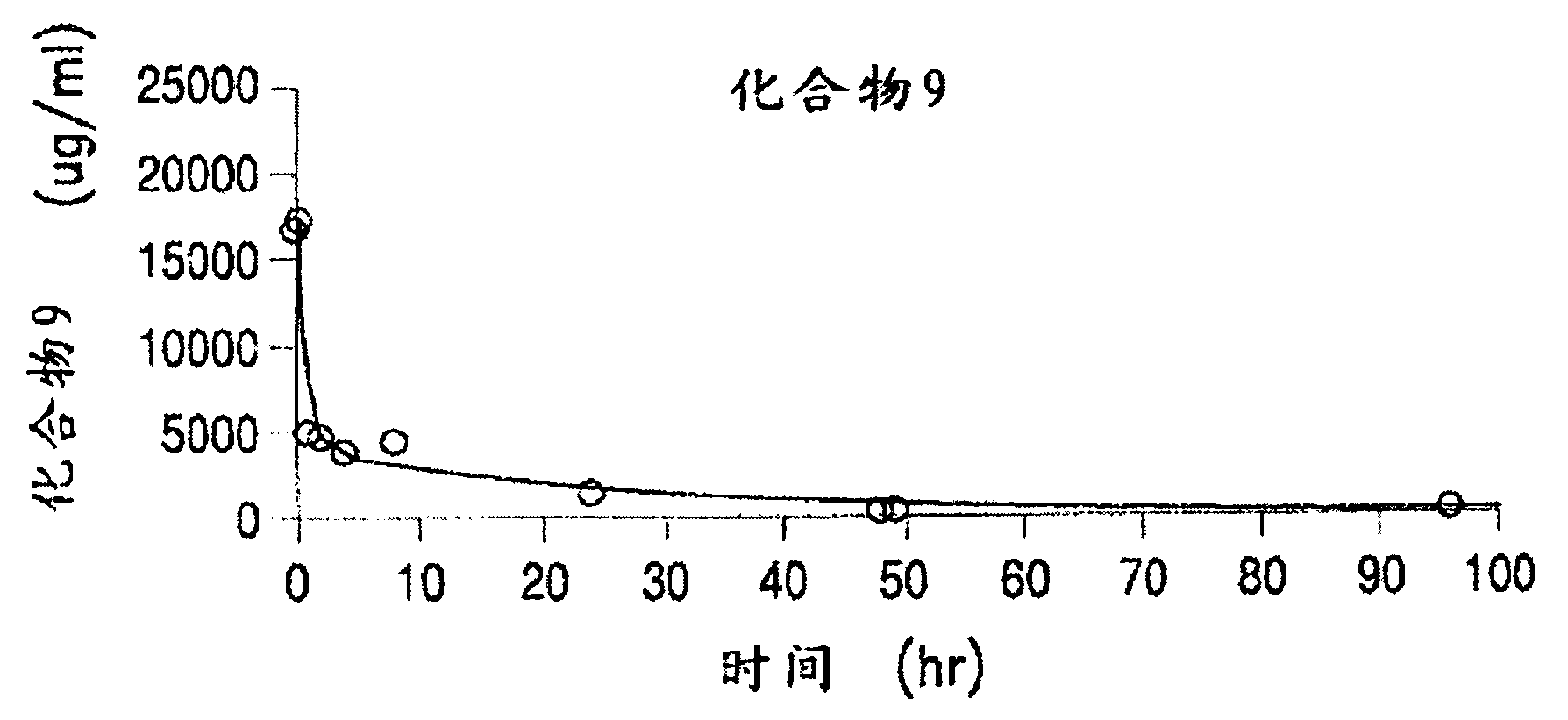
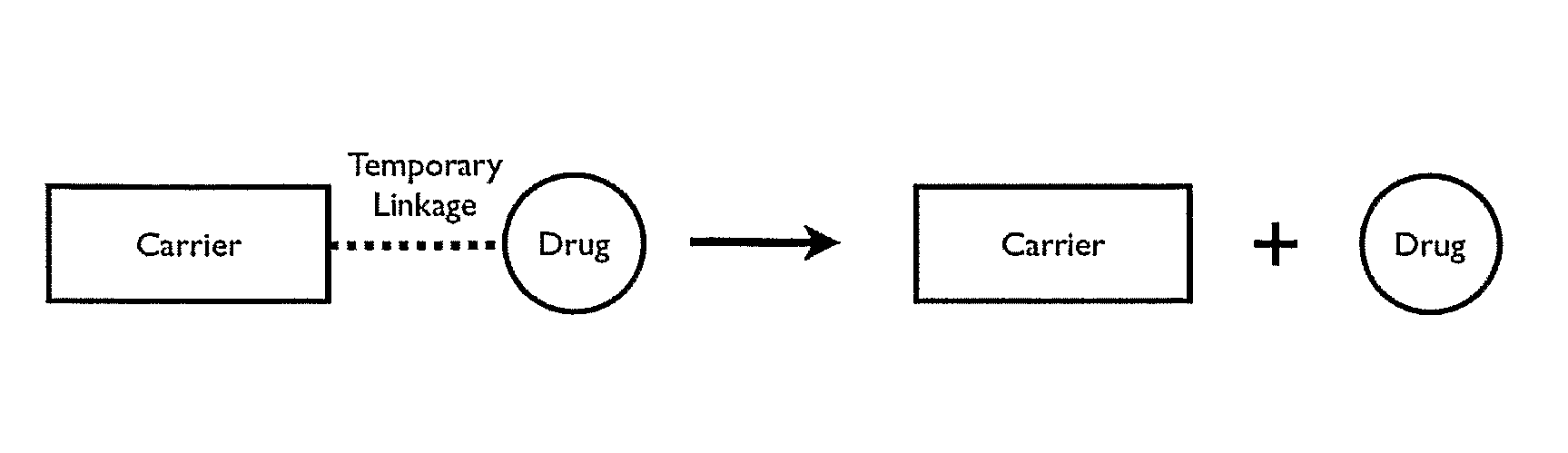
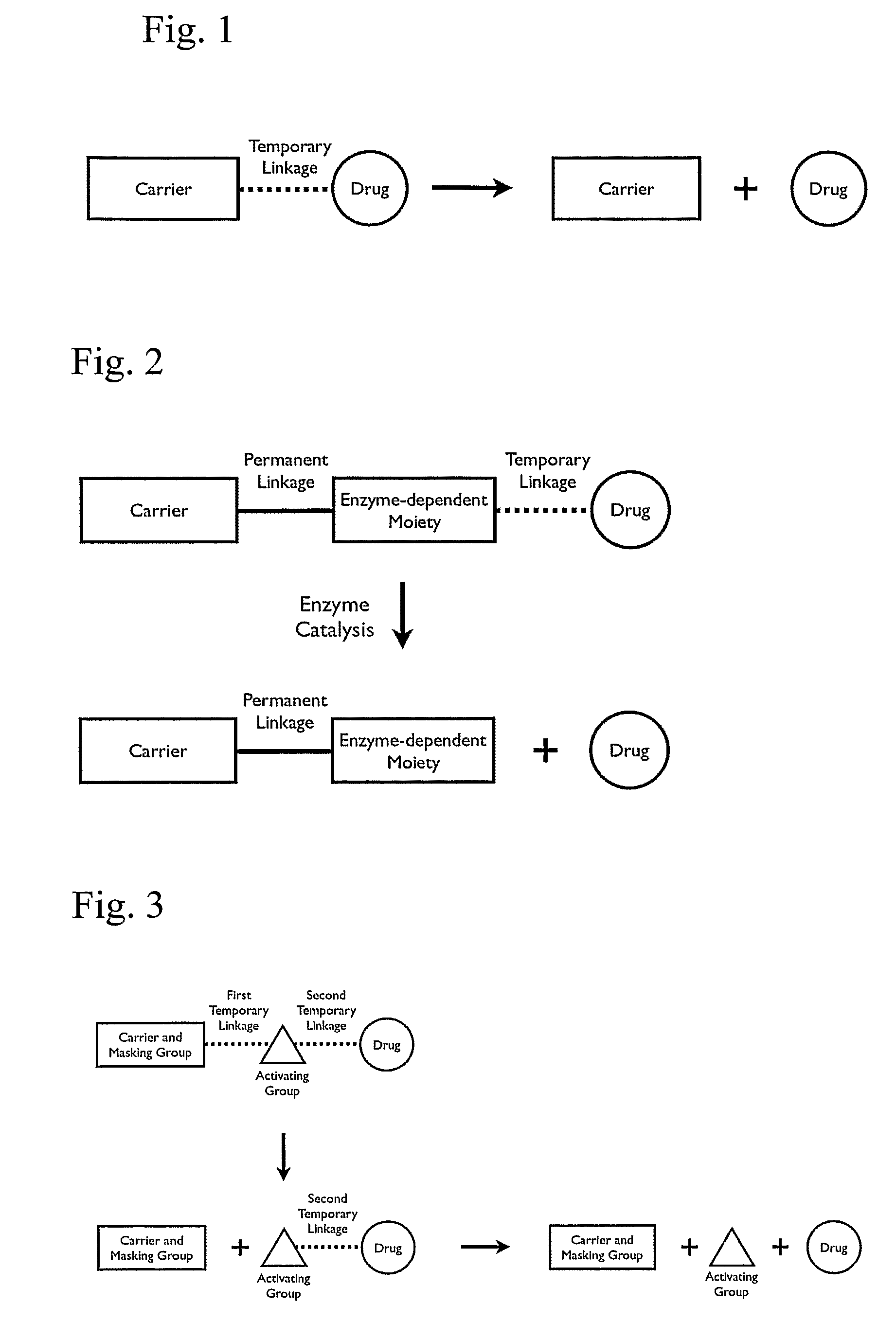
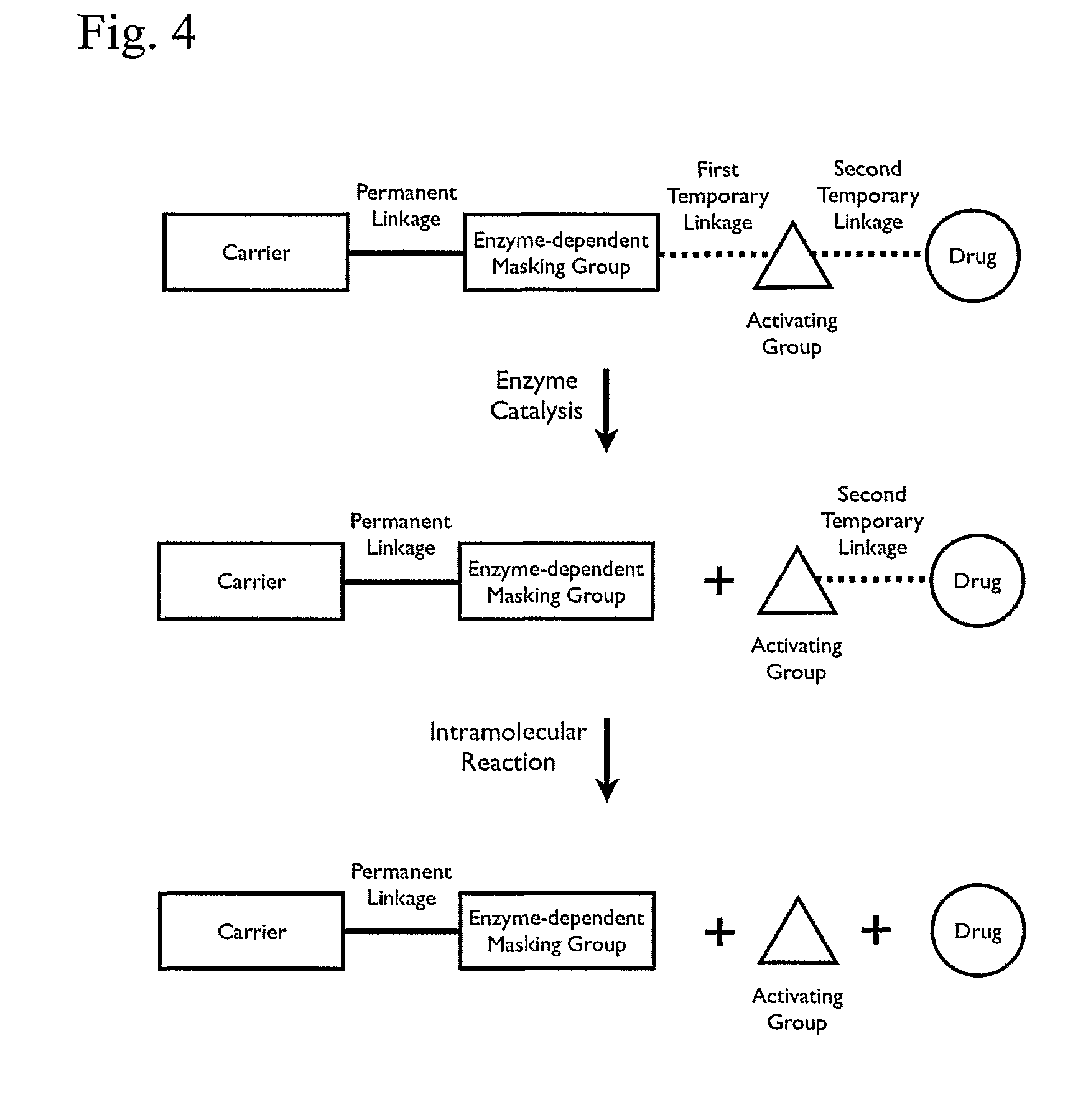
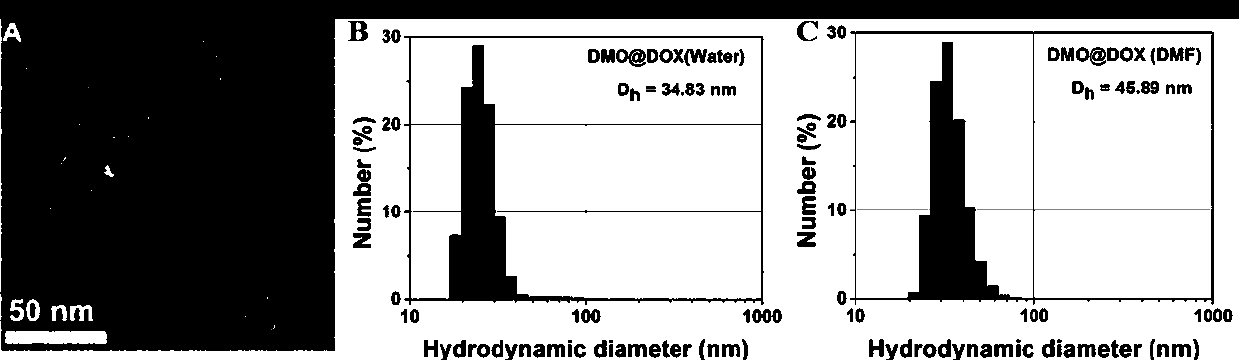
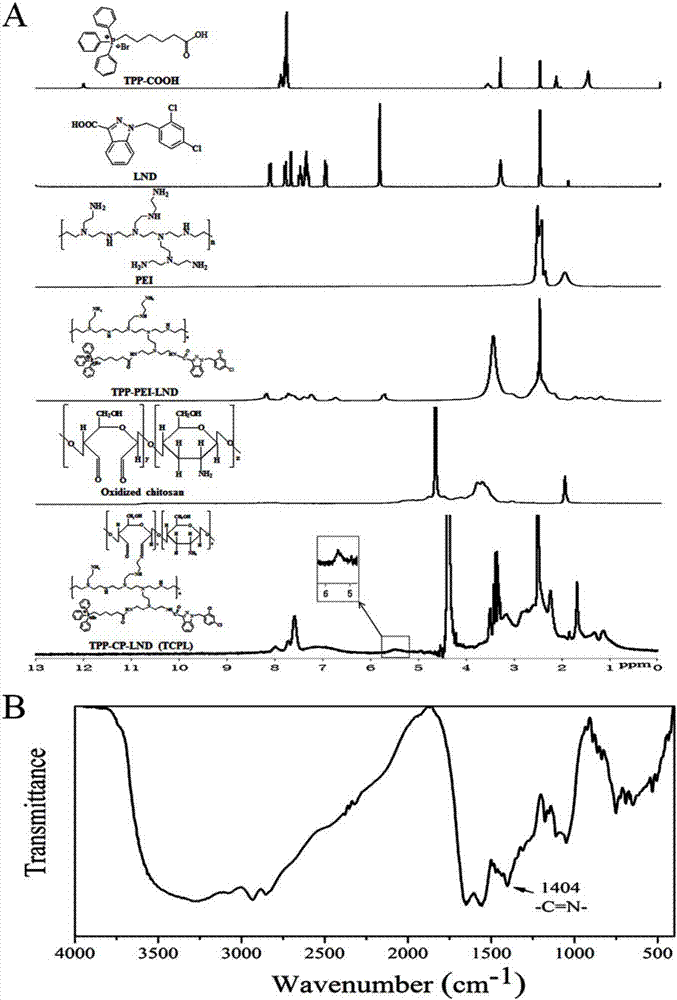
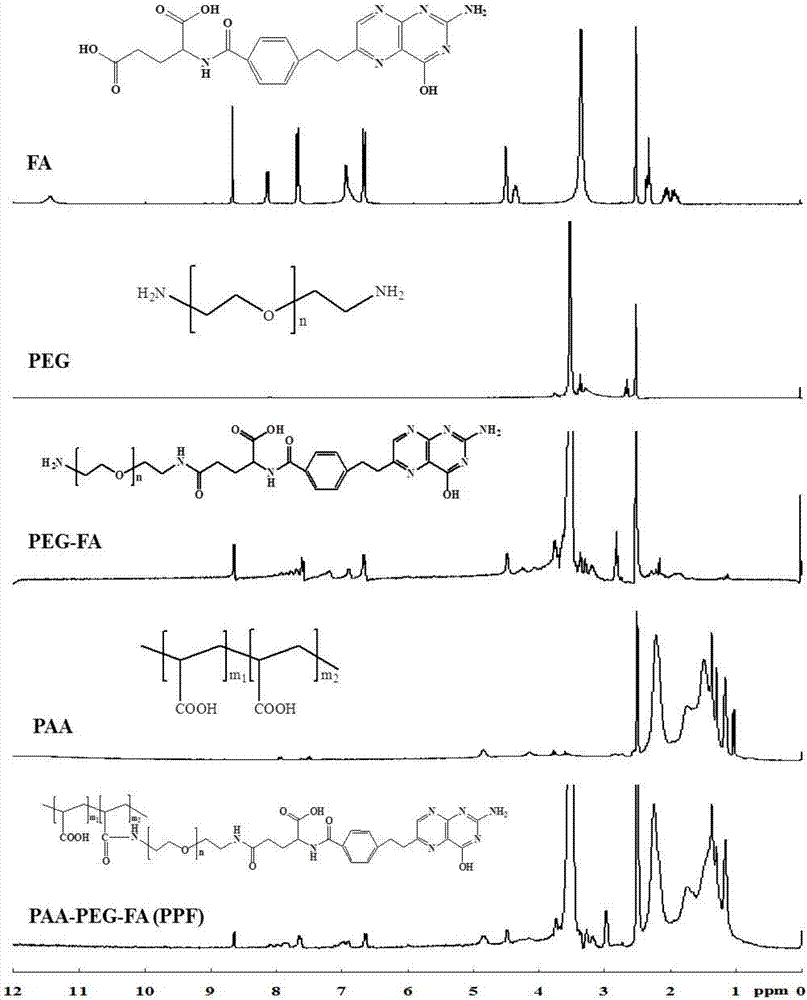
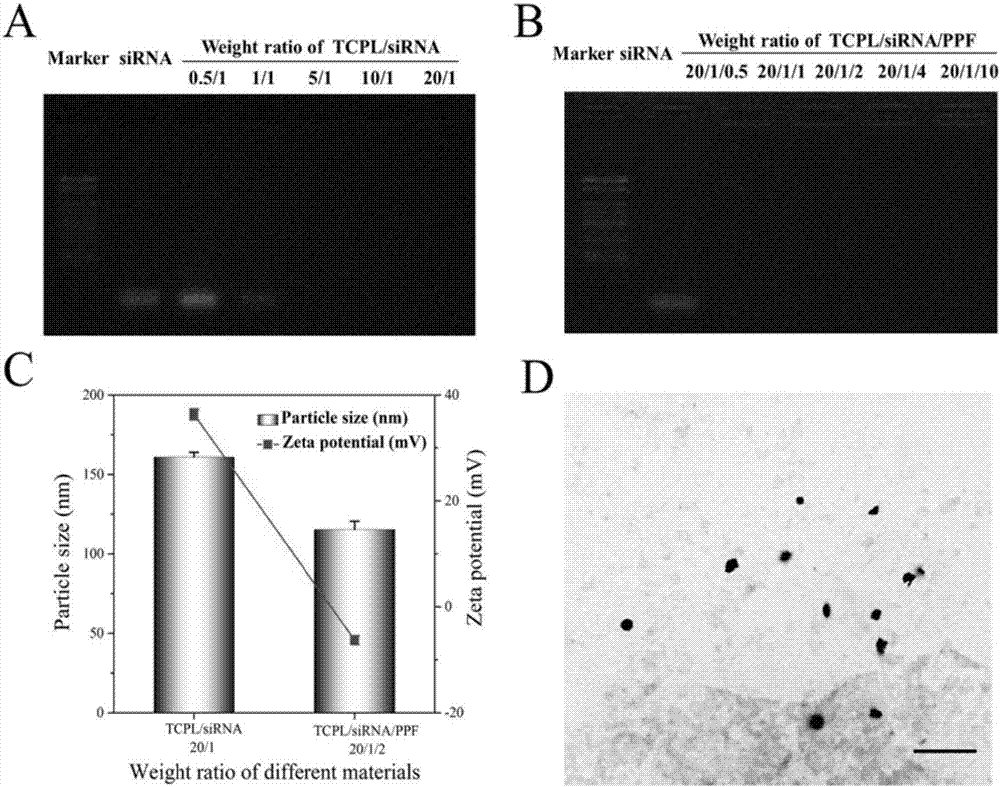
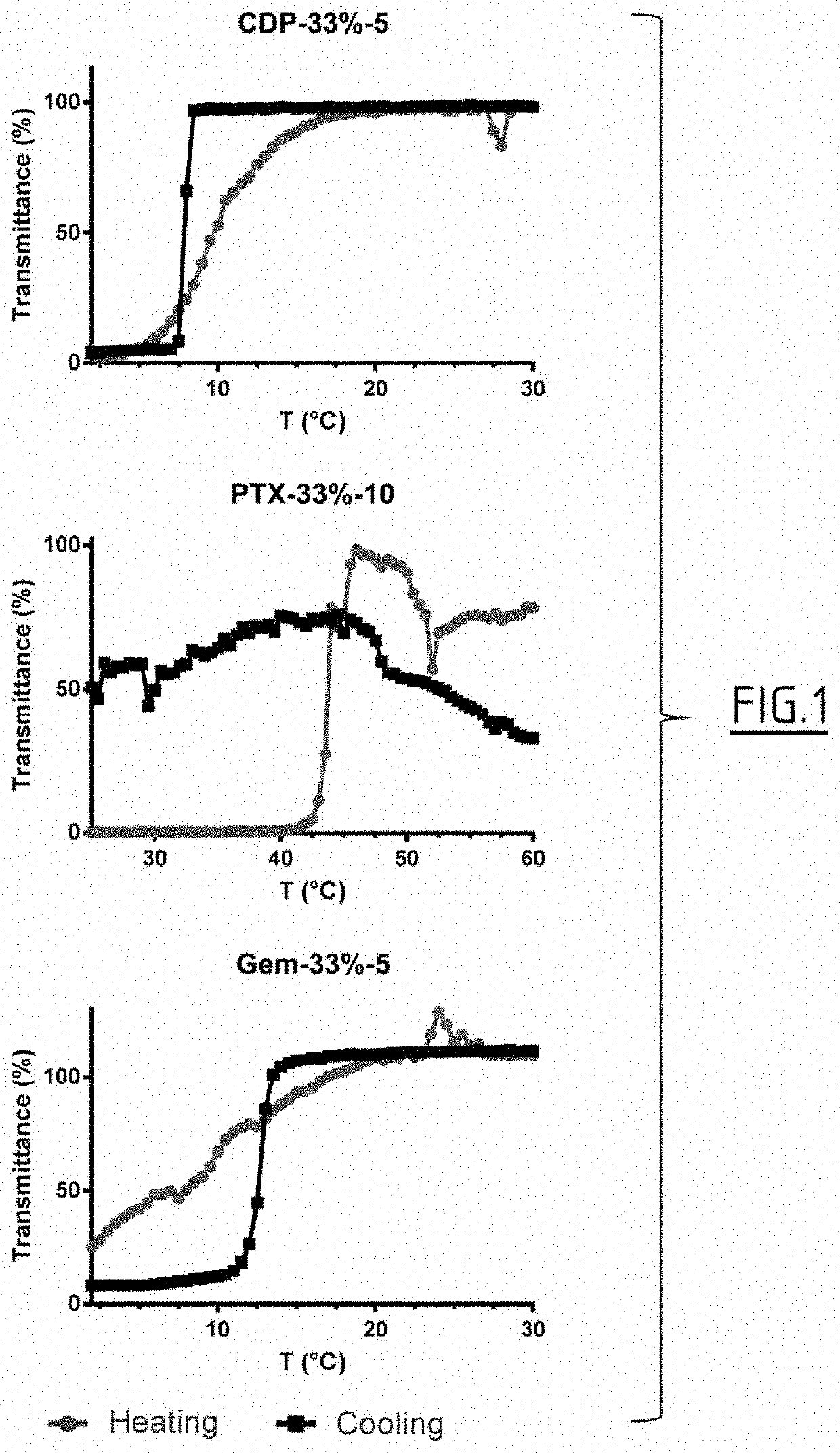
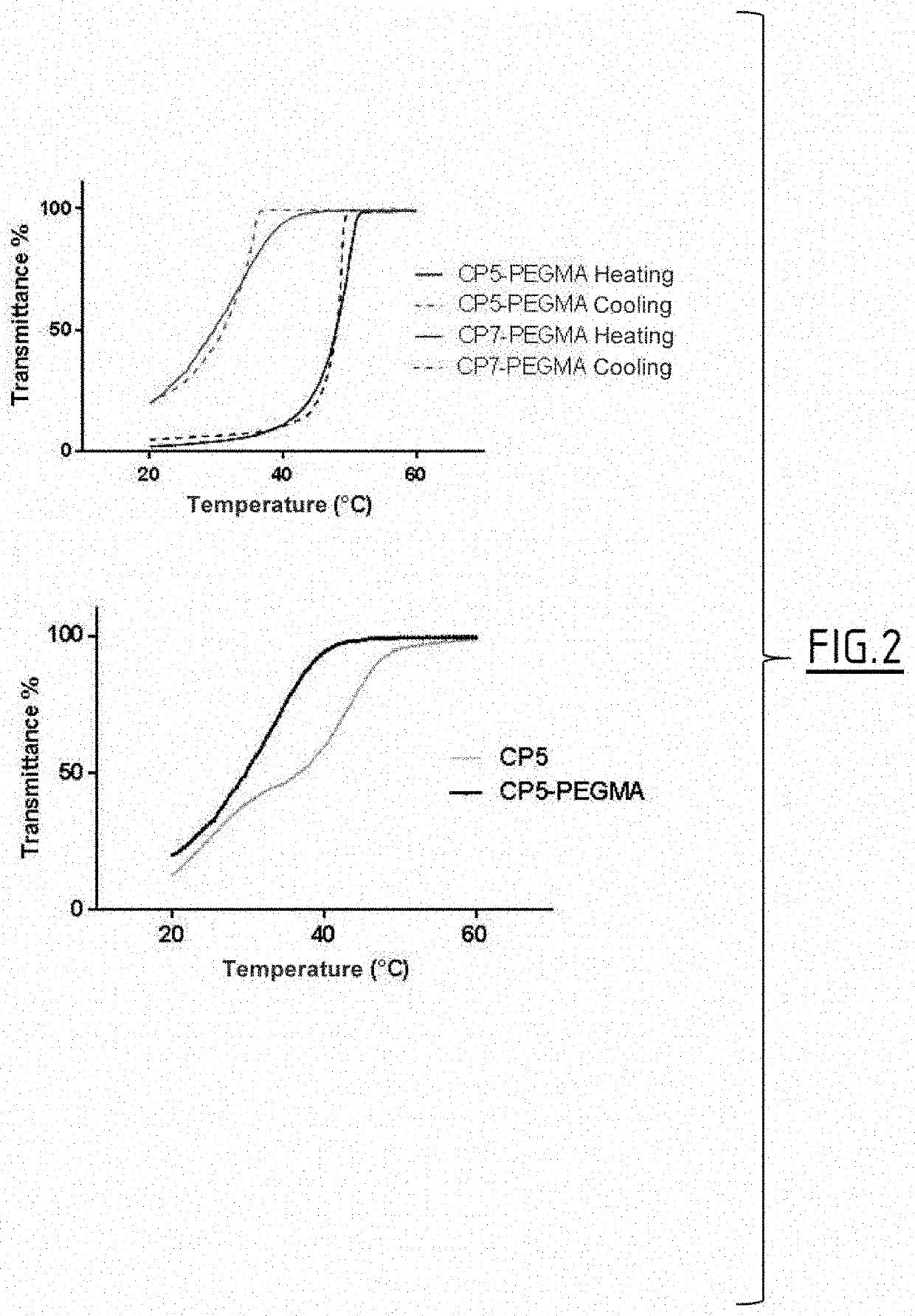
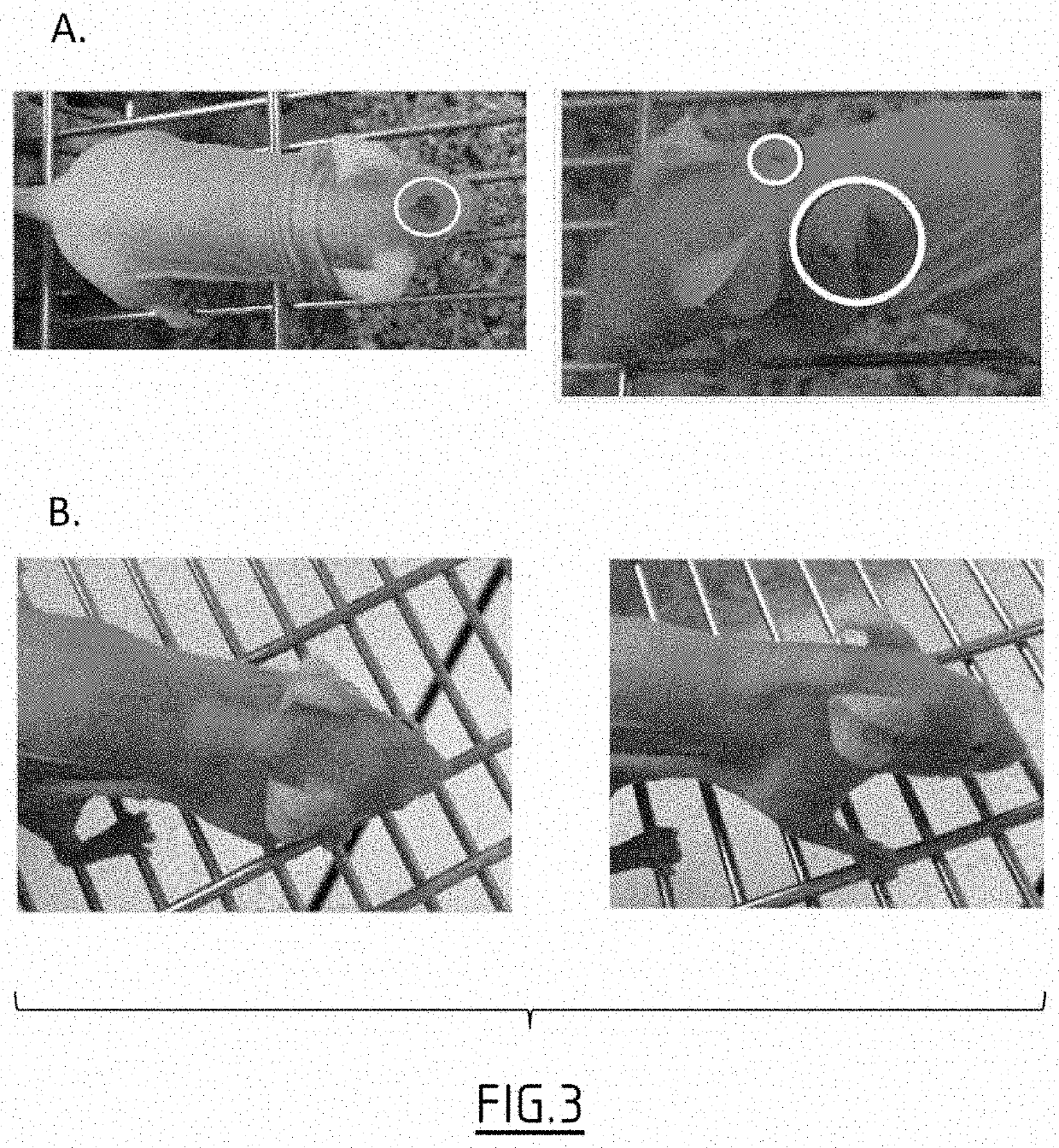
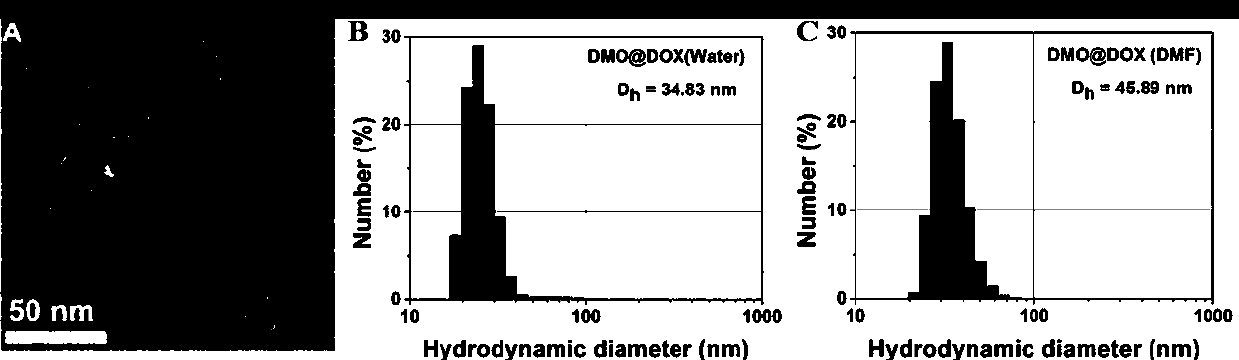
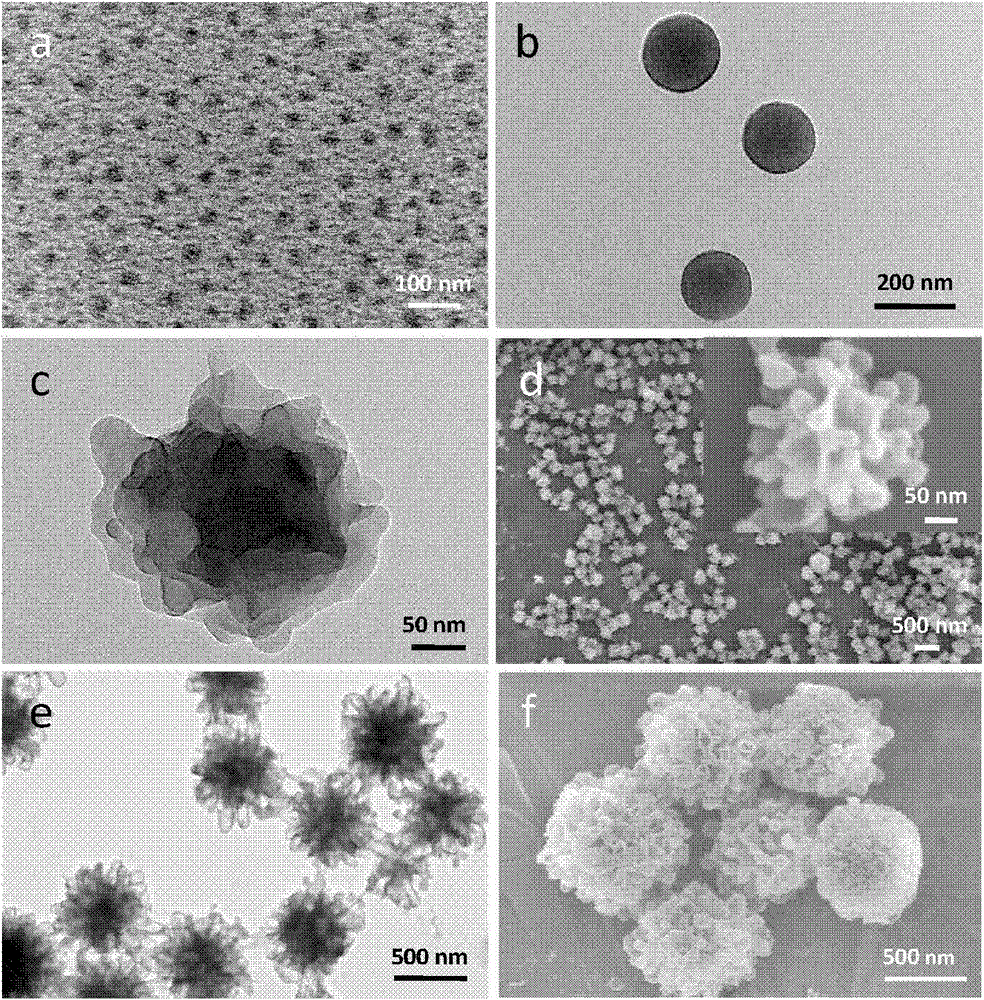
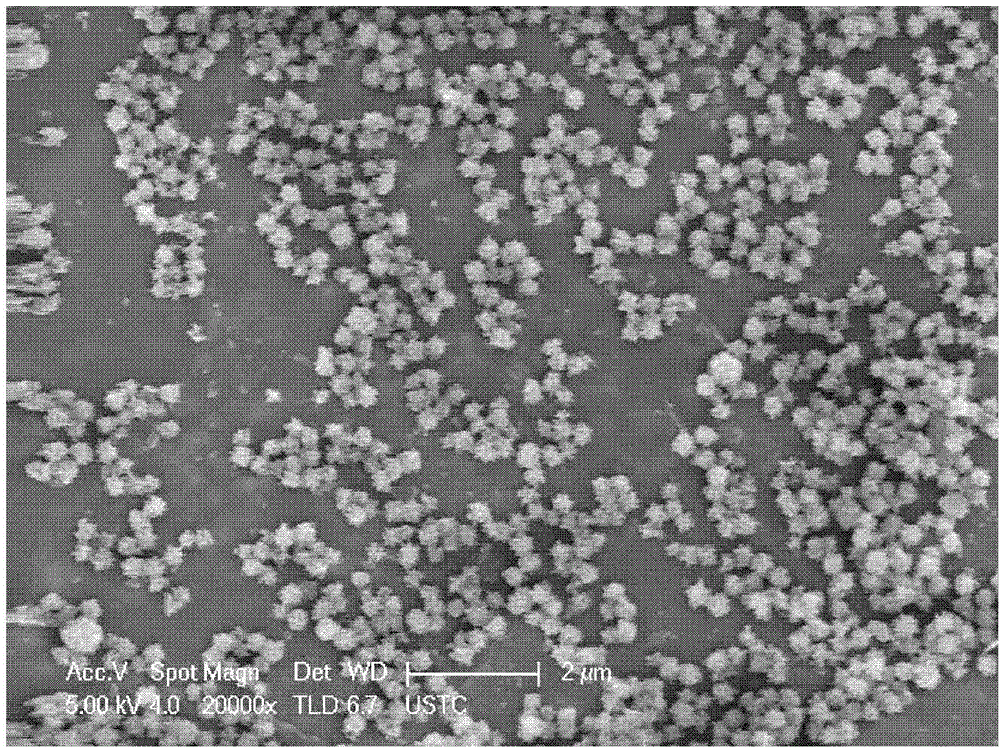
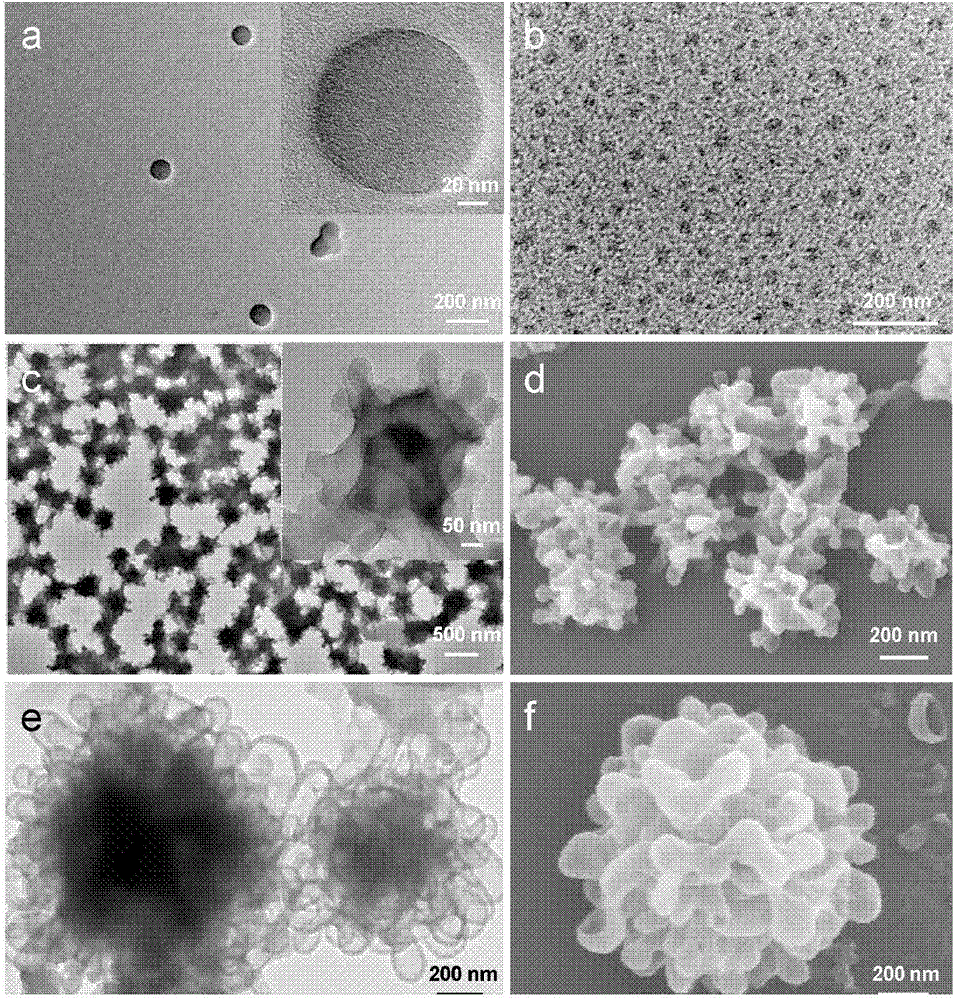
Patents
Literature
32 results about "Polymeric prodrug" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
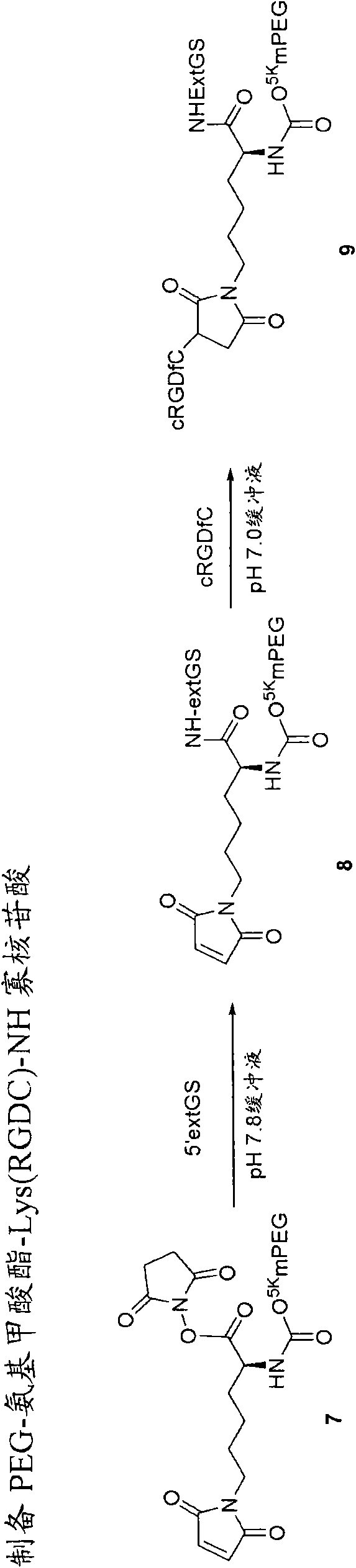
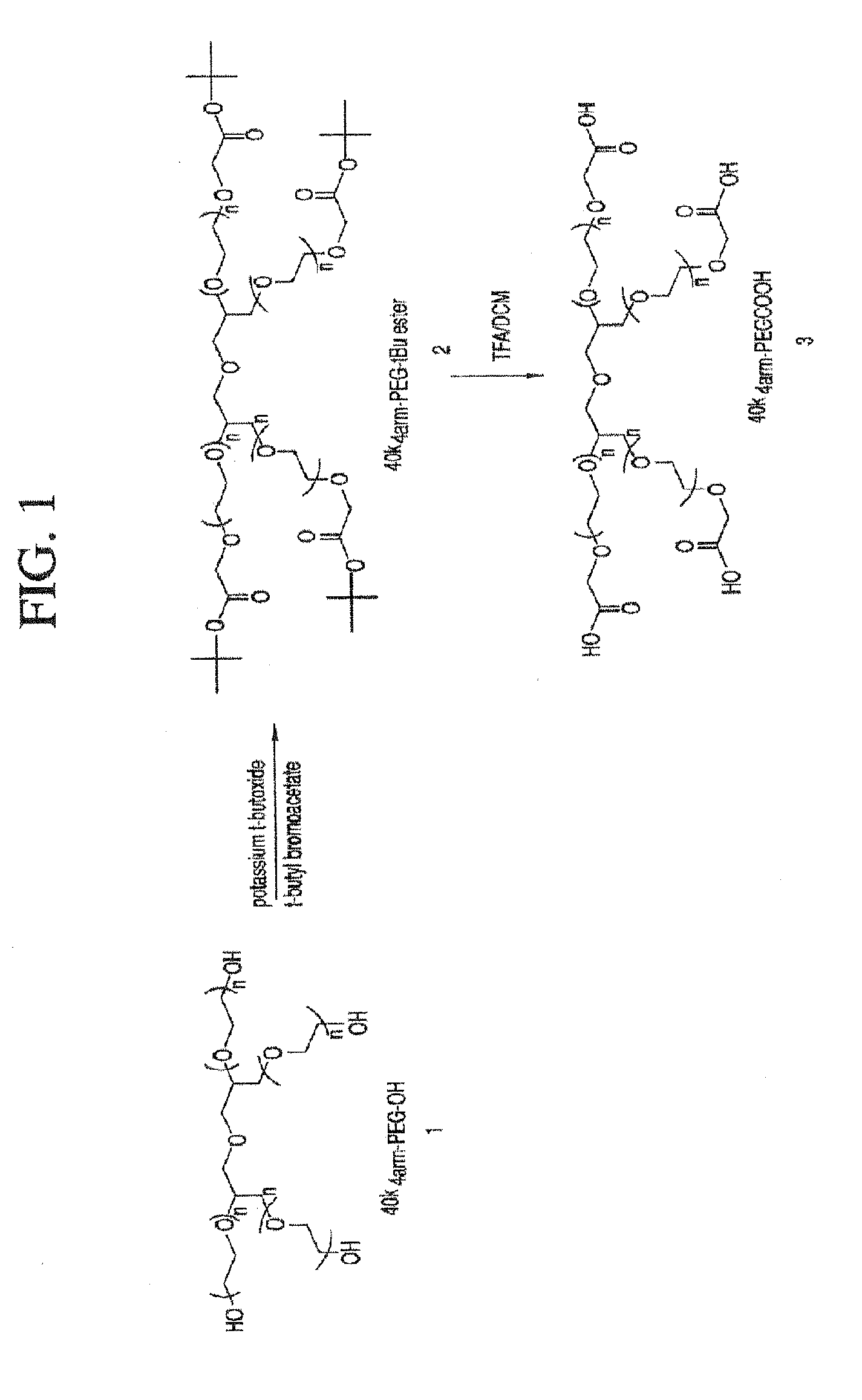
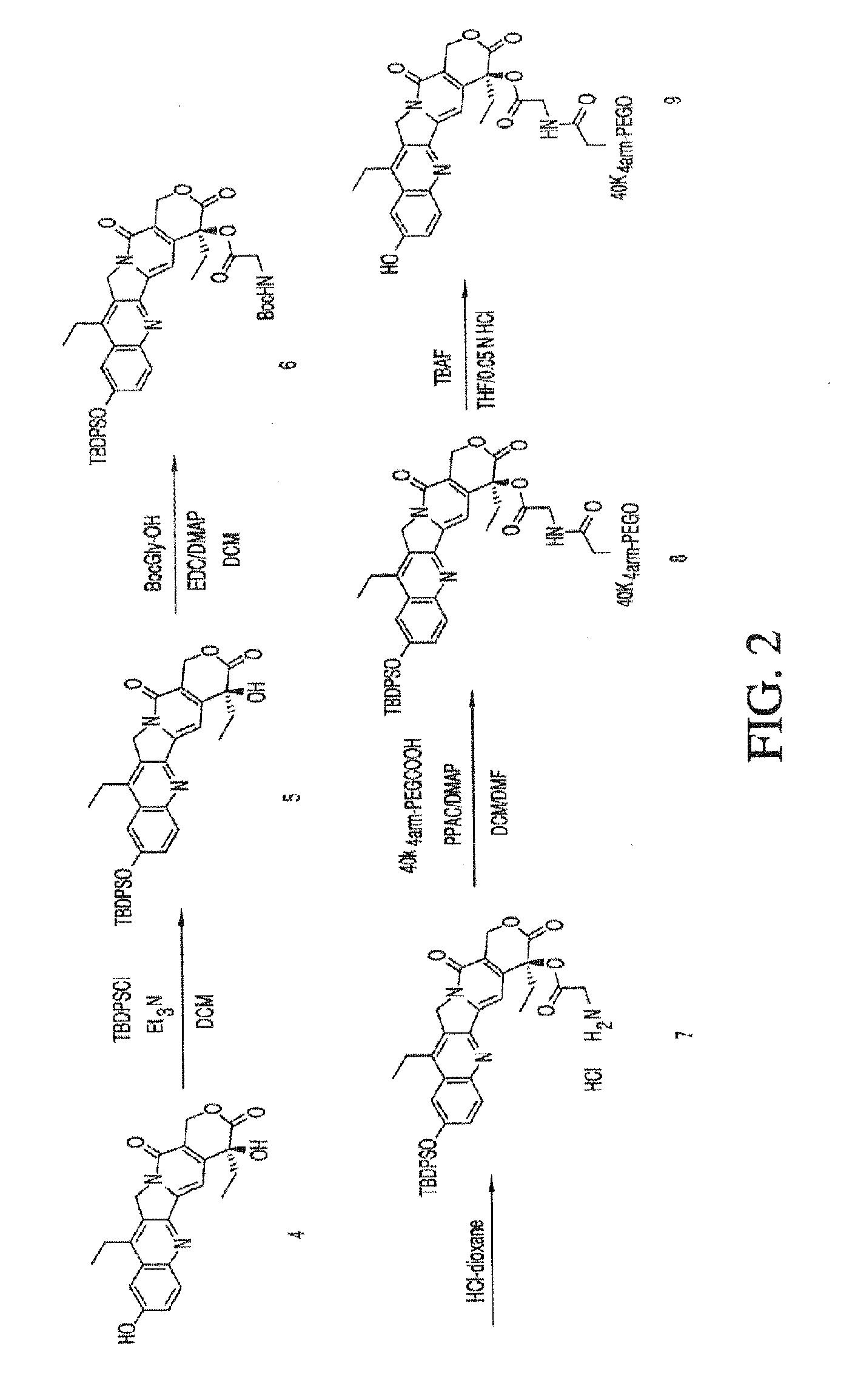
Acyl polymeric derivatives of aromatic hydroxyl-containing compounds
InactiveUS6011042APreferable balanceAccelerate the accumulation processBiocideSugar derivativesPolymeric prodrugAcylal
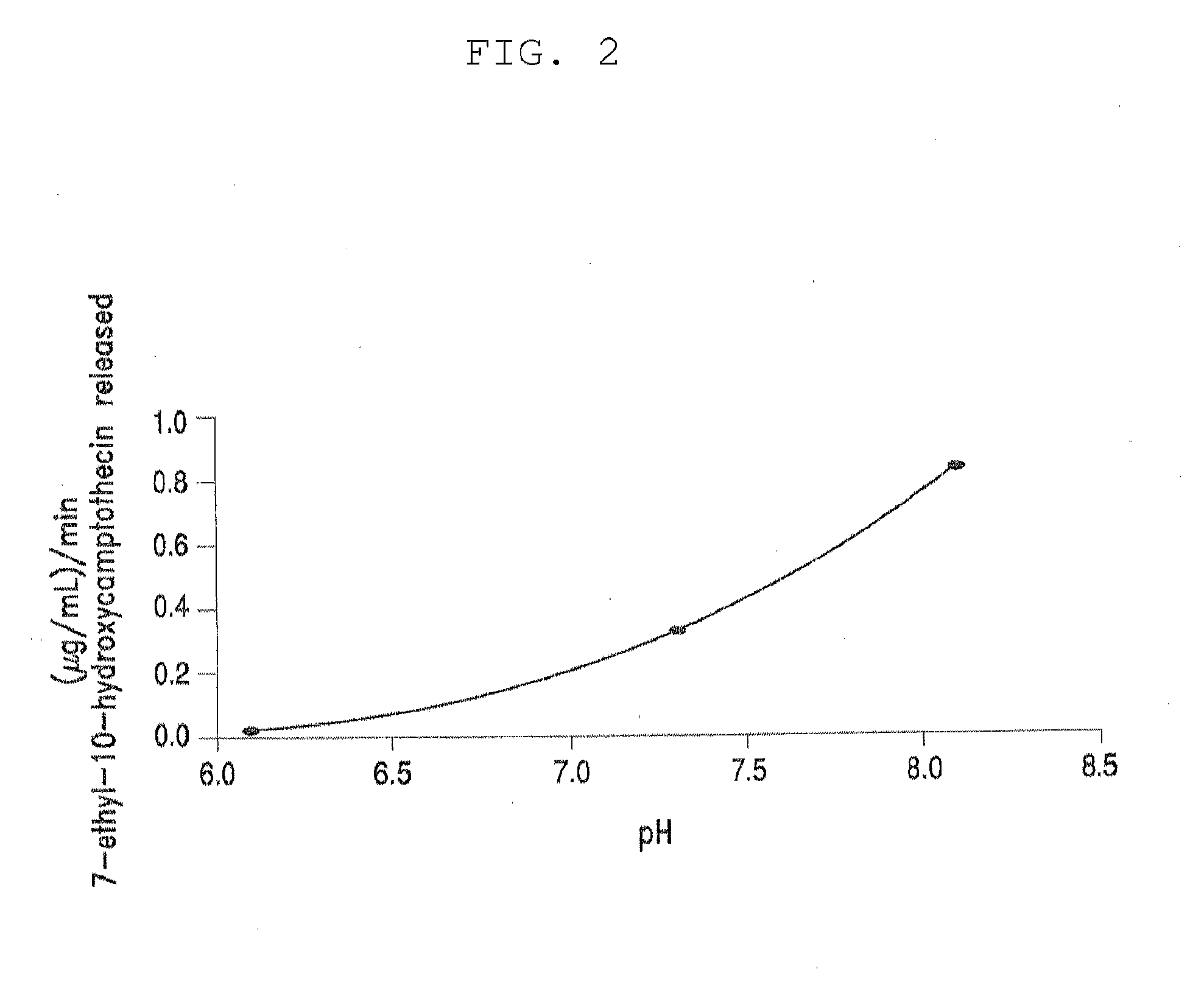
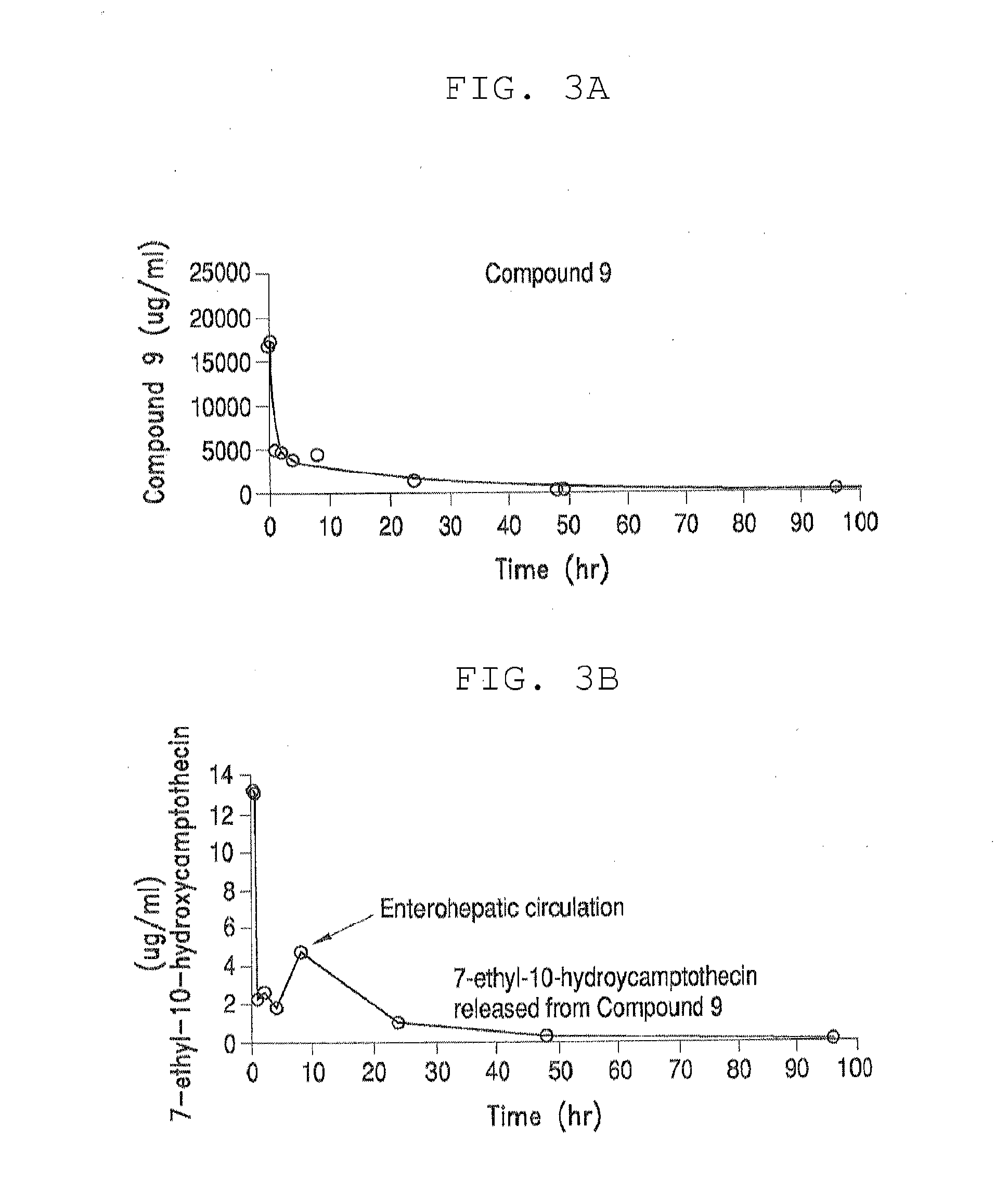
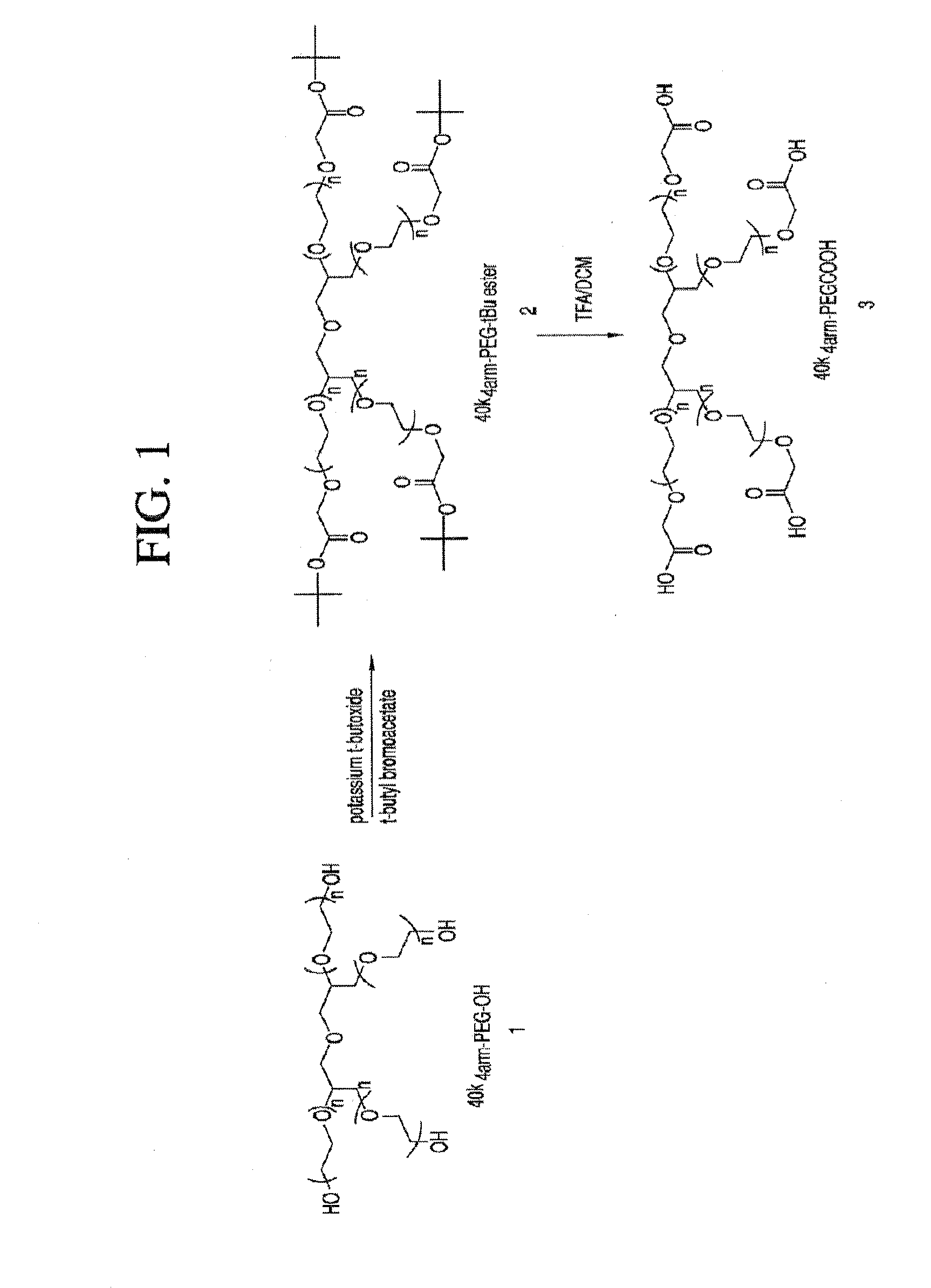
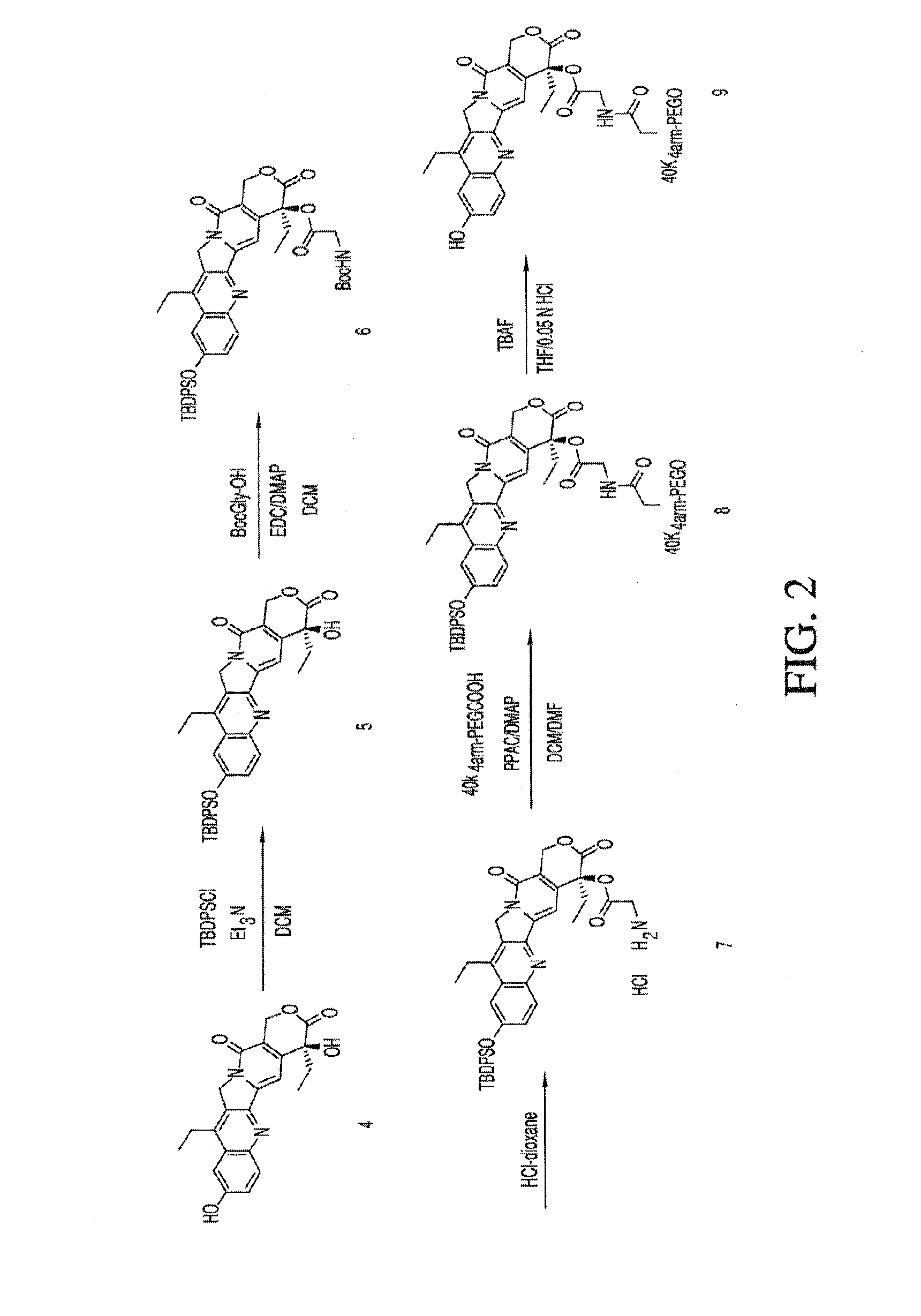
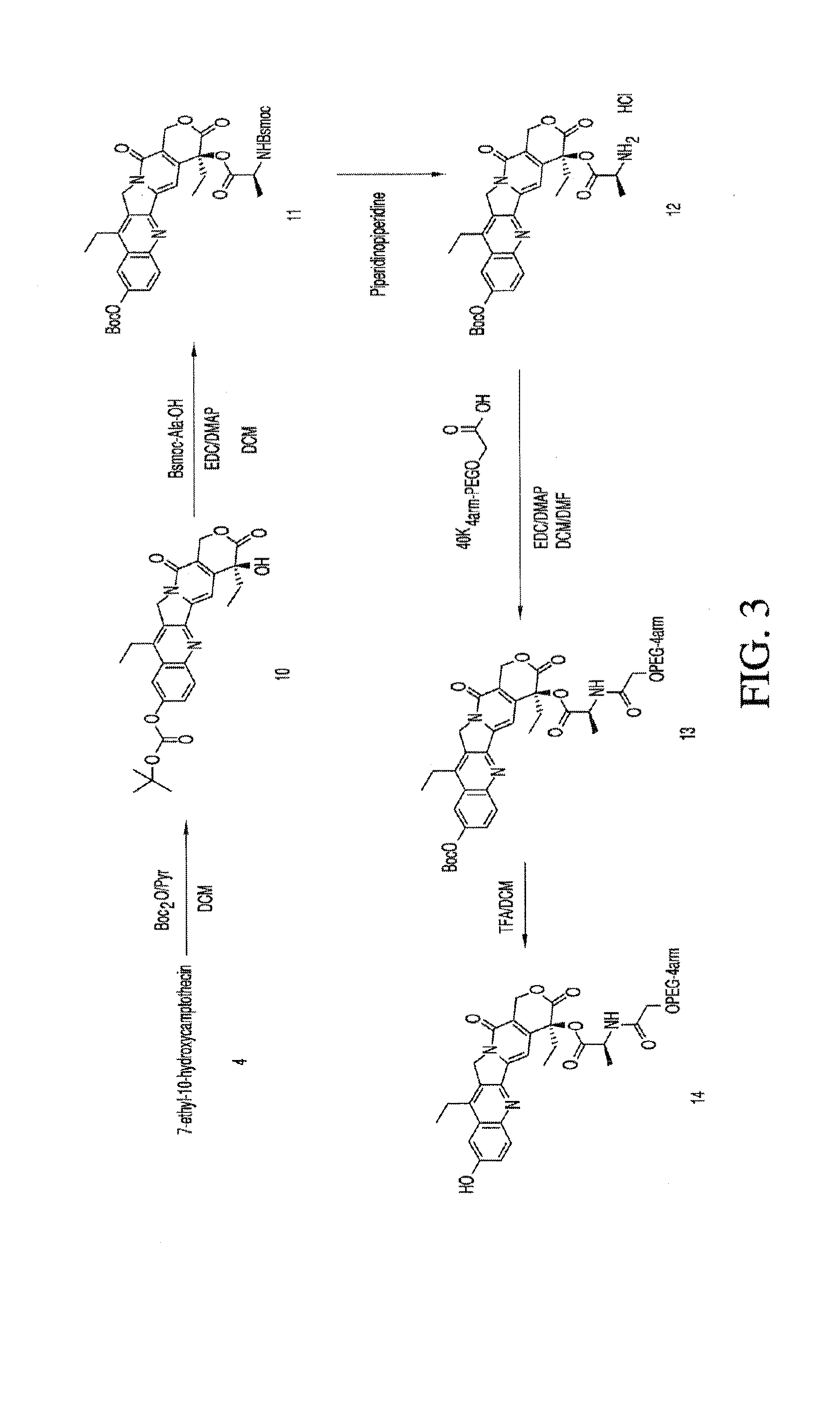
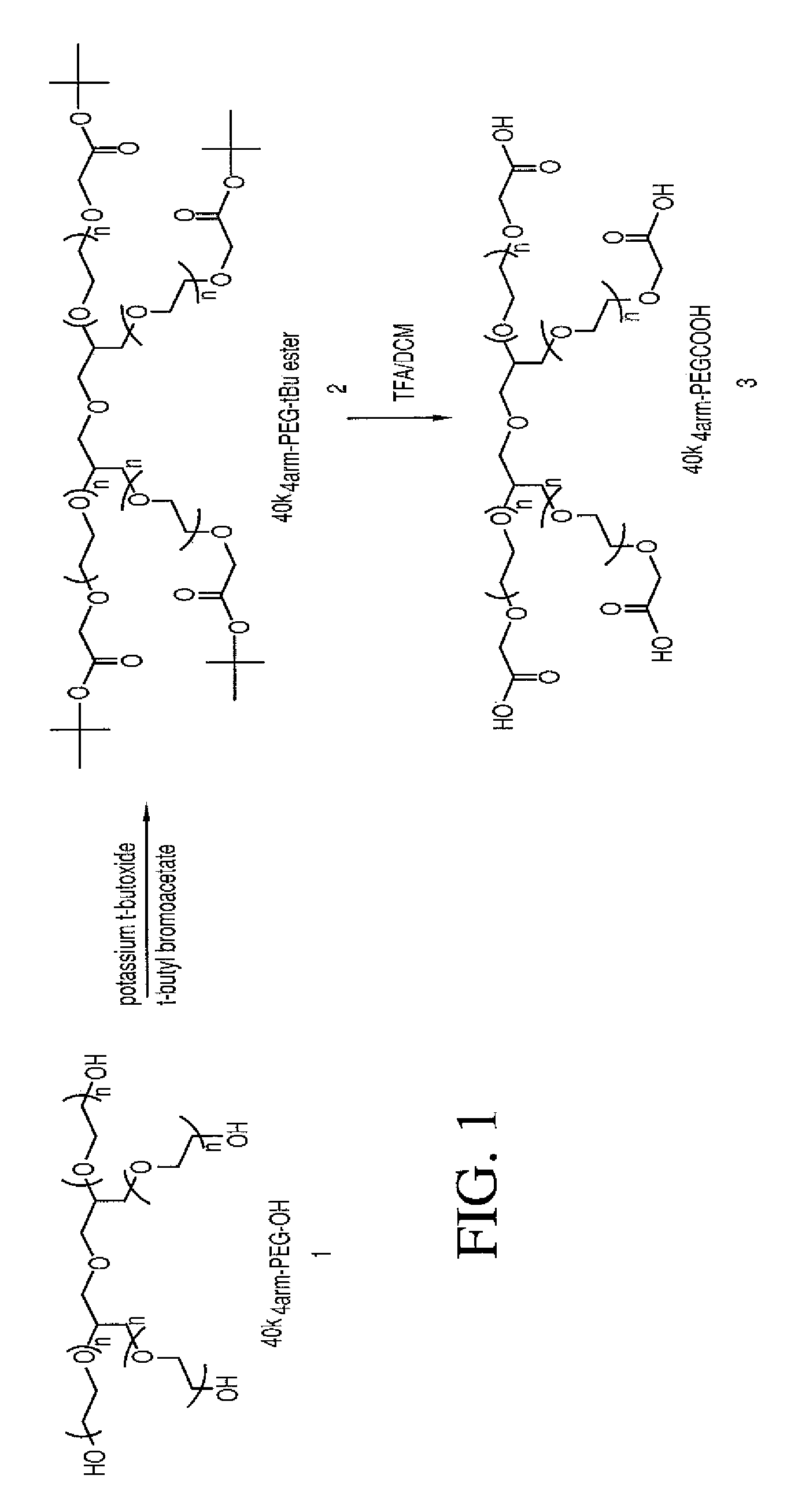
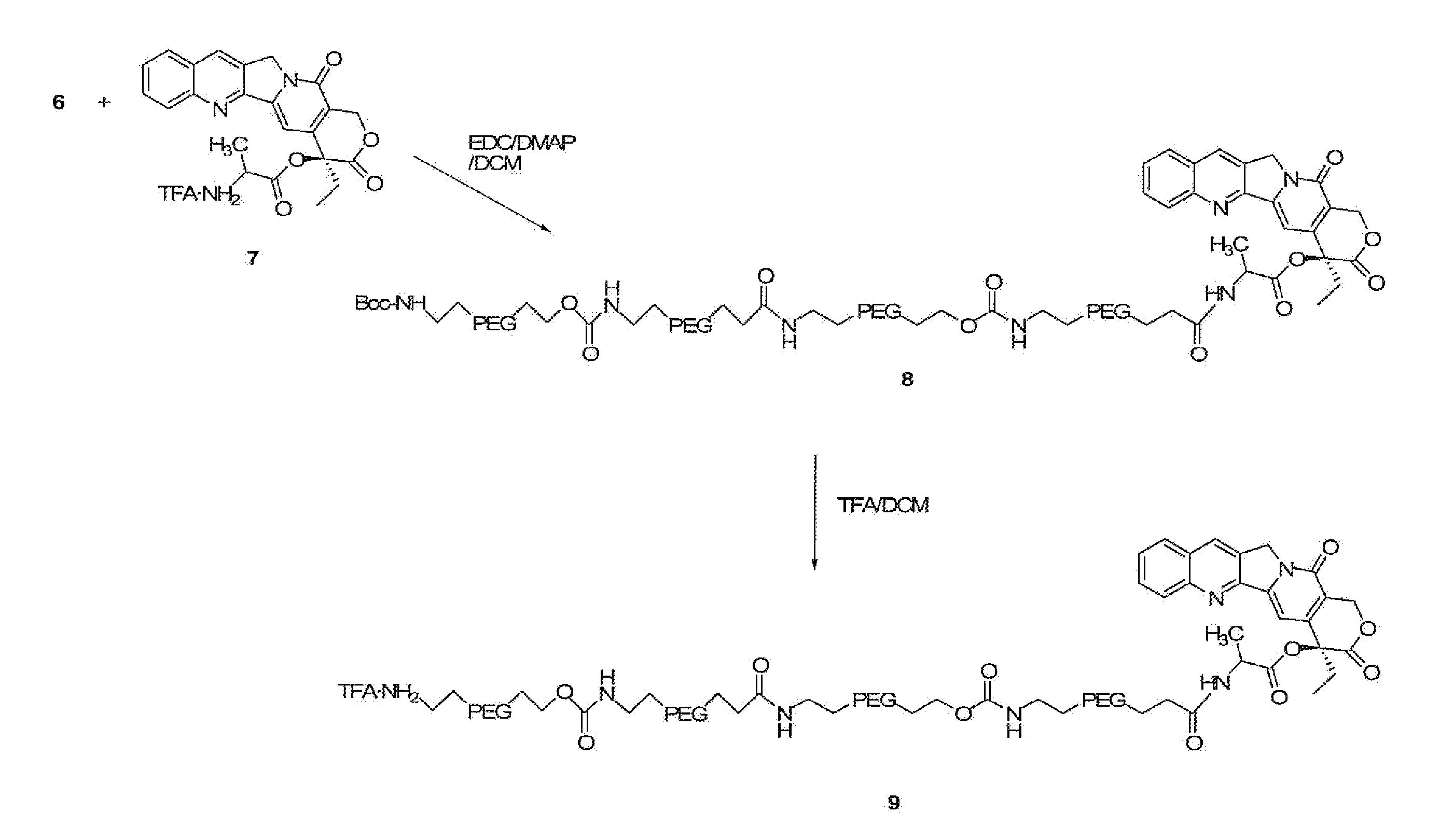
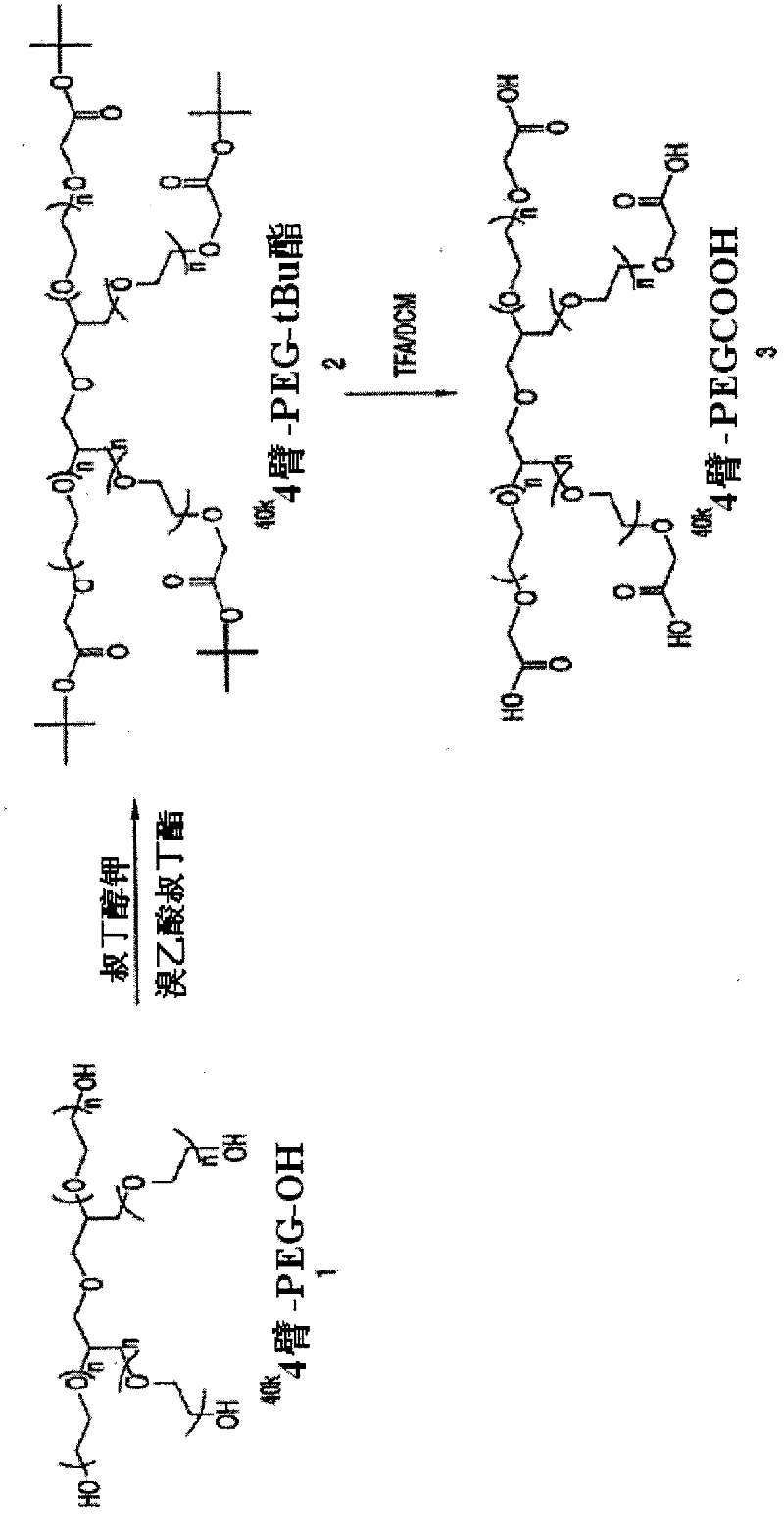
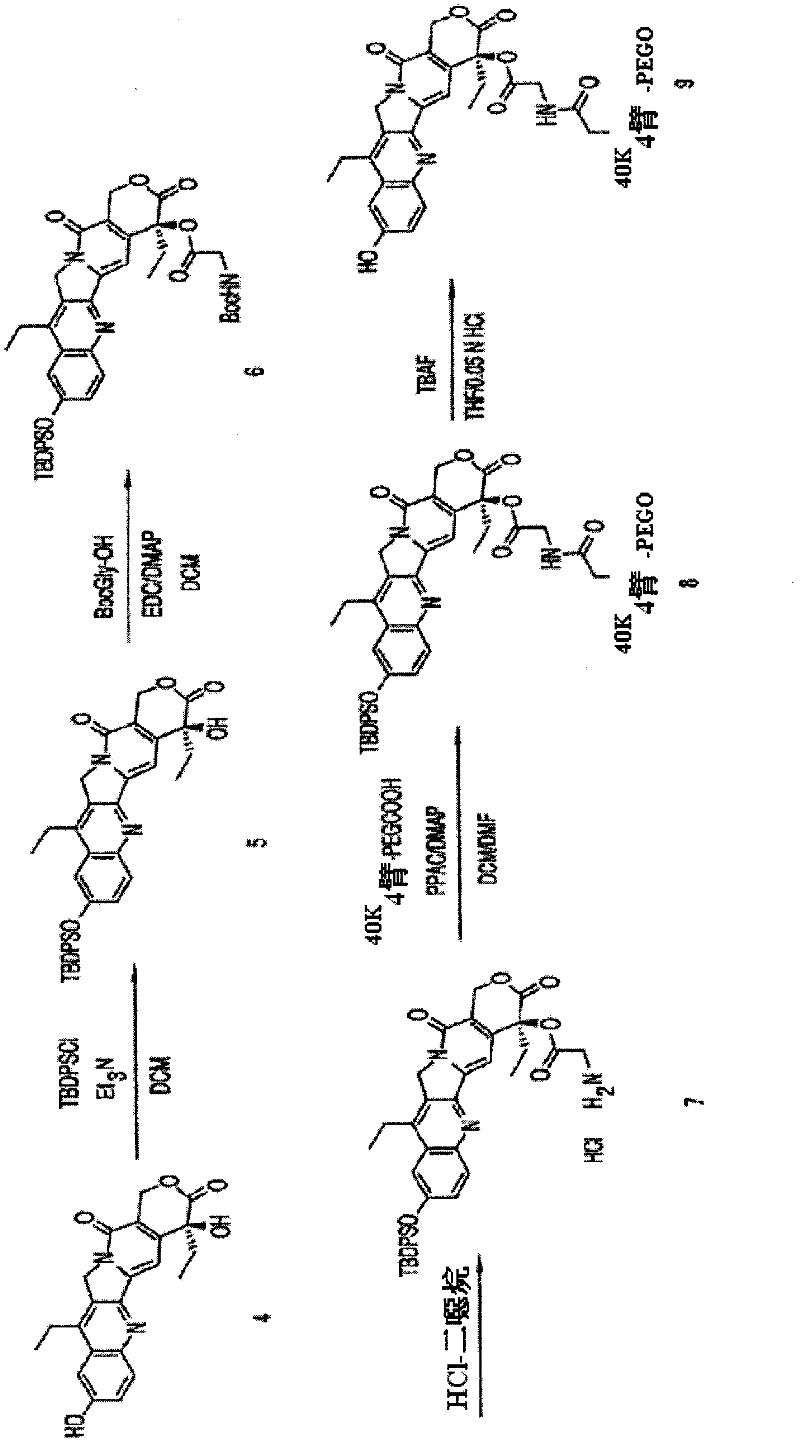
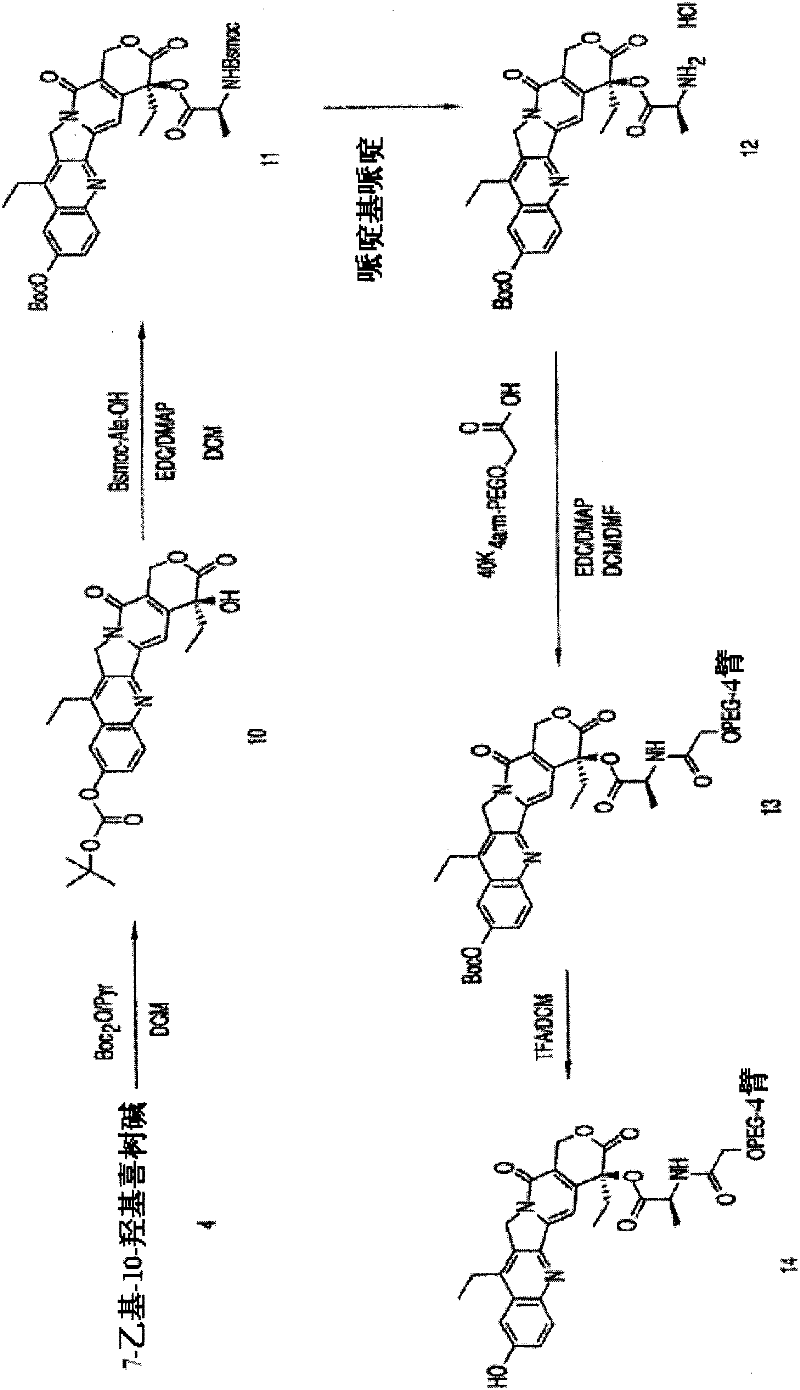
The present invention is directed to conjugates such as polymeric prodrugs of aromatic, hydroxyl-containing compounds and methods of making and using the same. These polymeric prodrugs are preferably esters of hydroxyl-containing aromatic compounds and are formed by reacting a desired aromatic, hydroxyl-containing compound with a substantially non-antigenic polymer so as to produce a transport form having an ester linkage between the aromatic compound and the polymer. Preferred aromatic hydroxyl-containing compositions include 10- and 11-hydroxycamptothecin derivatives. Methods of treatment are also disclosed.
Owner:ENZON PHARM INC
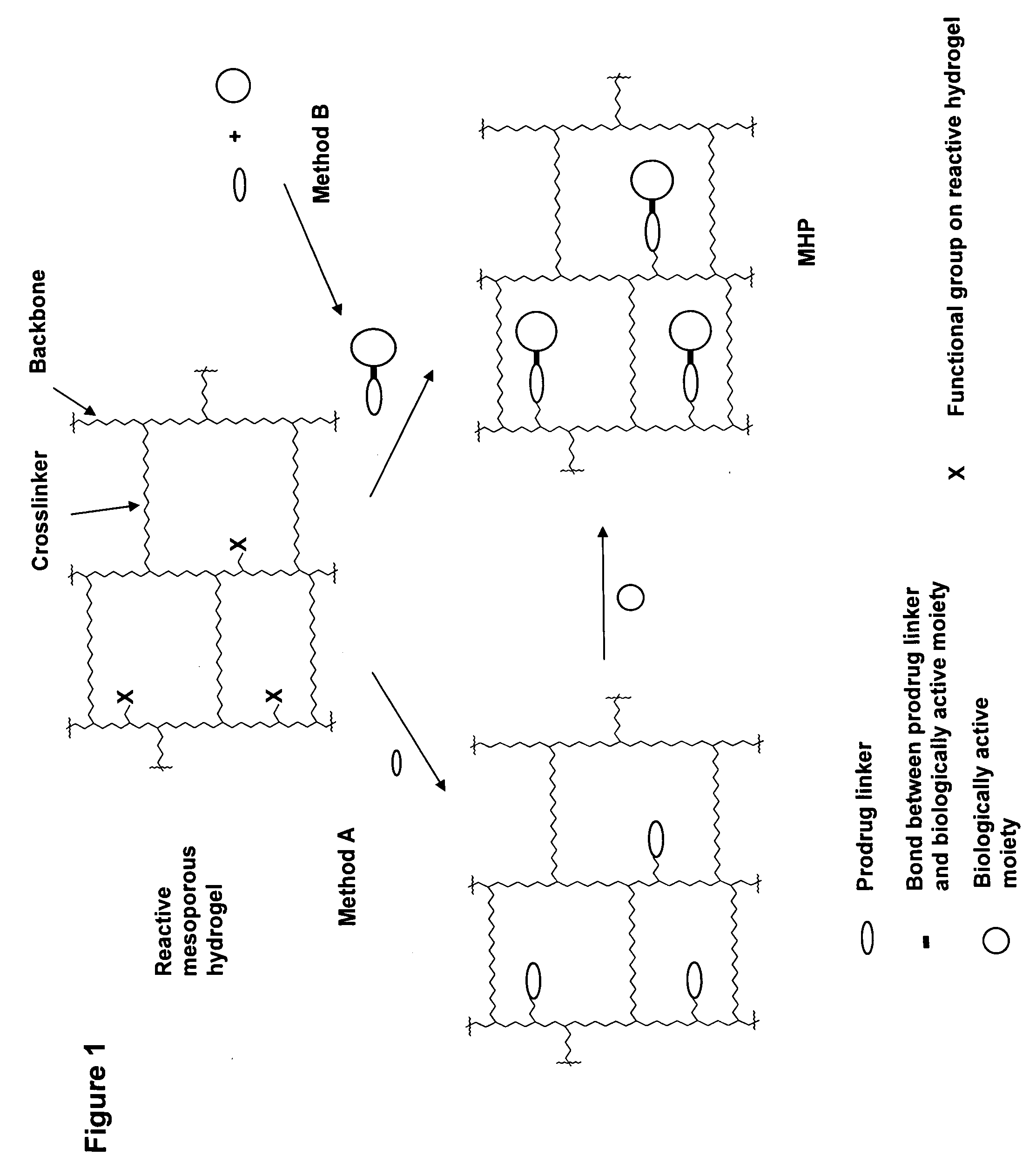
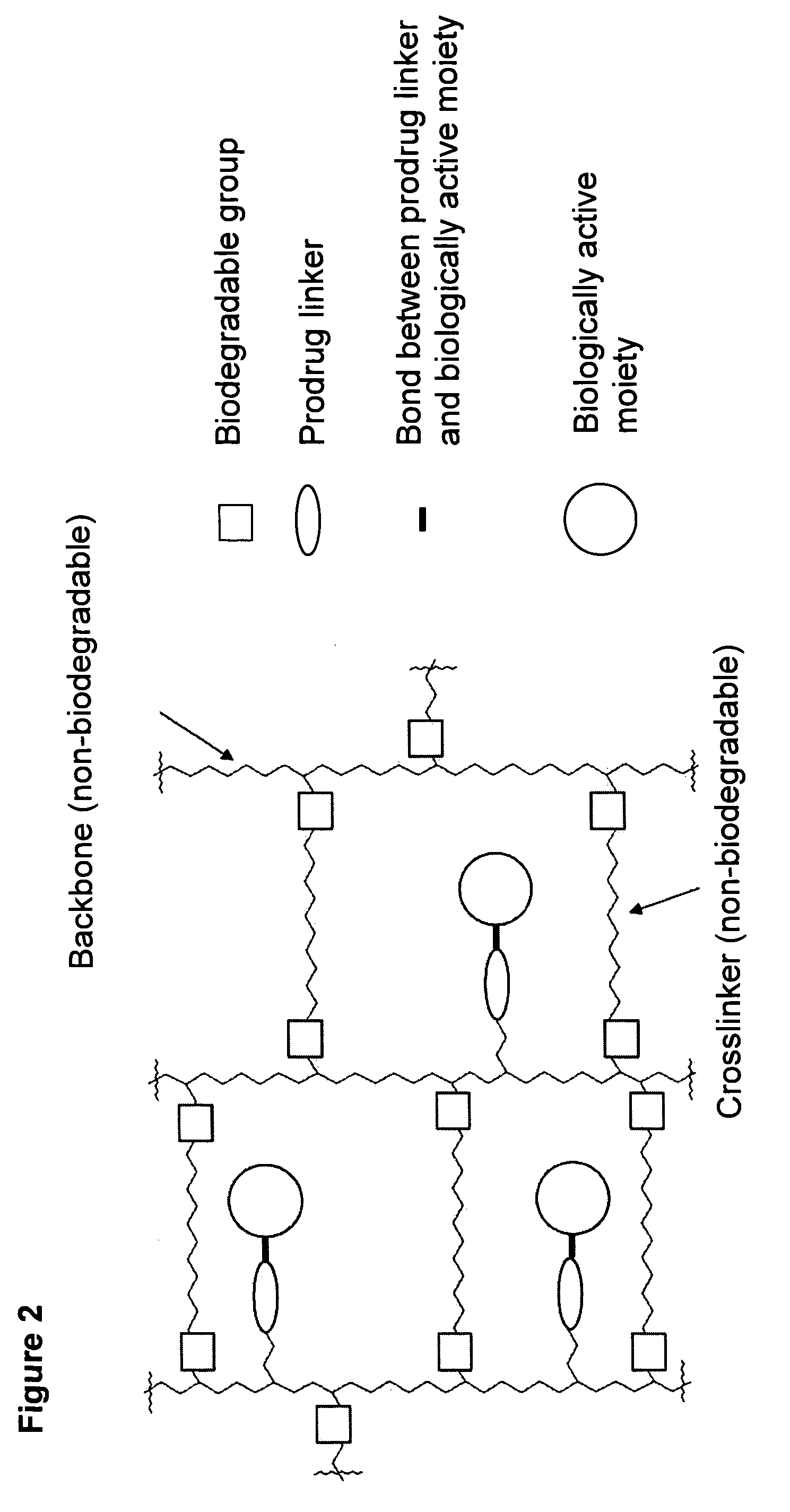
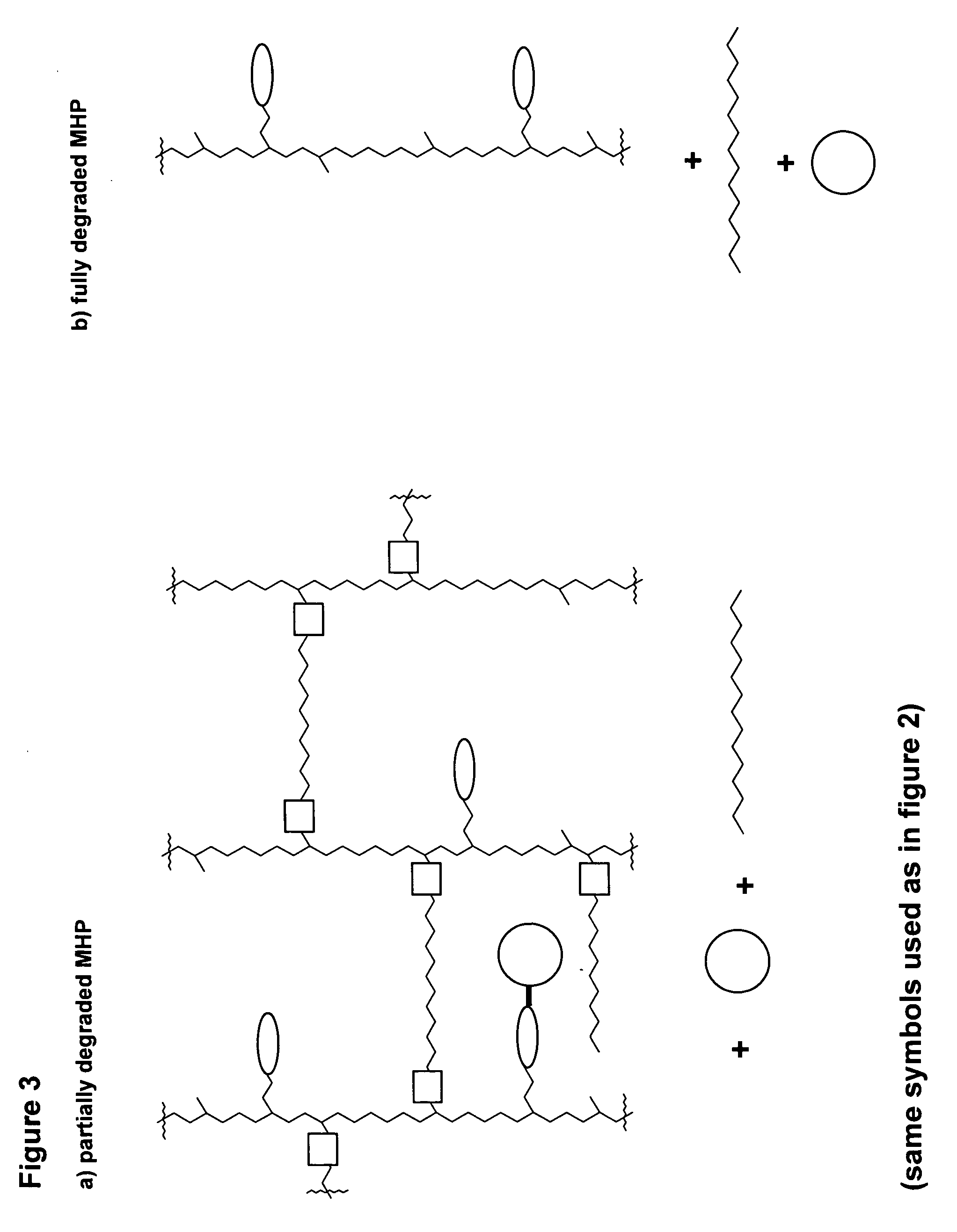
Hydrogel formulations
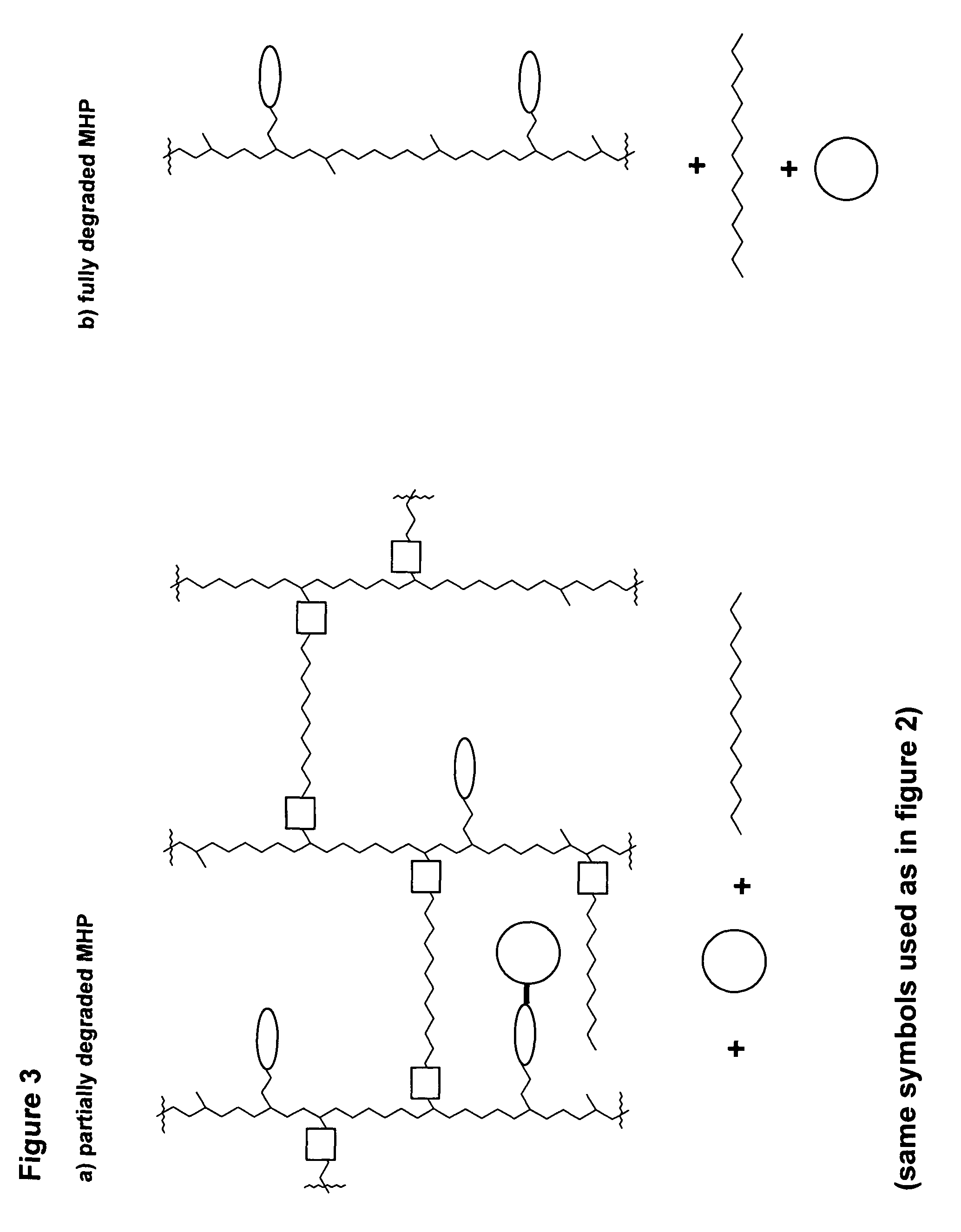
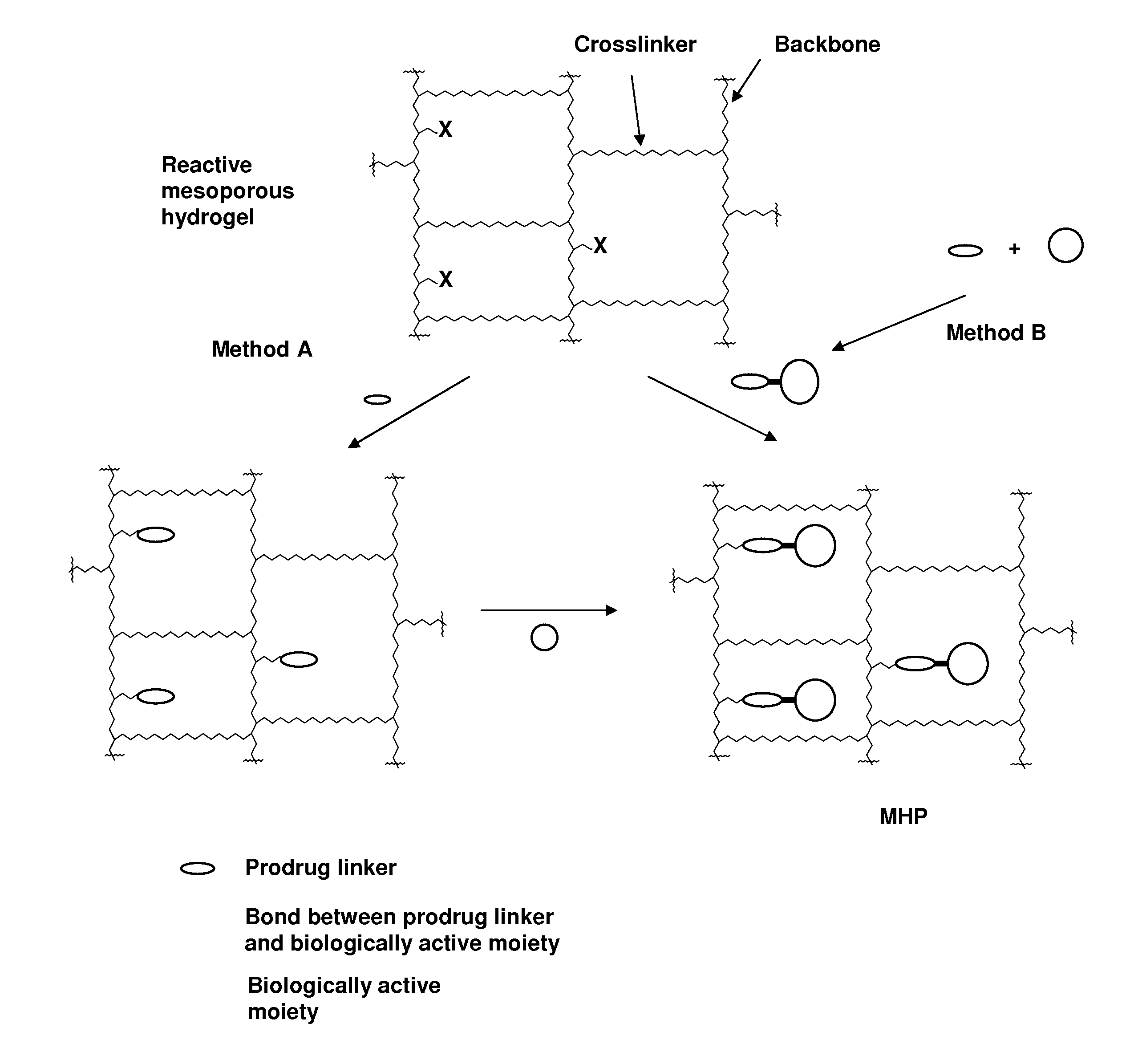
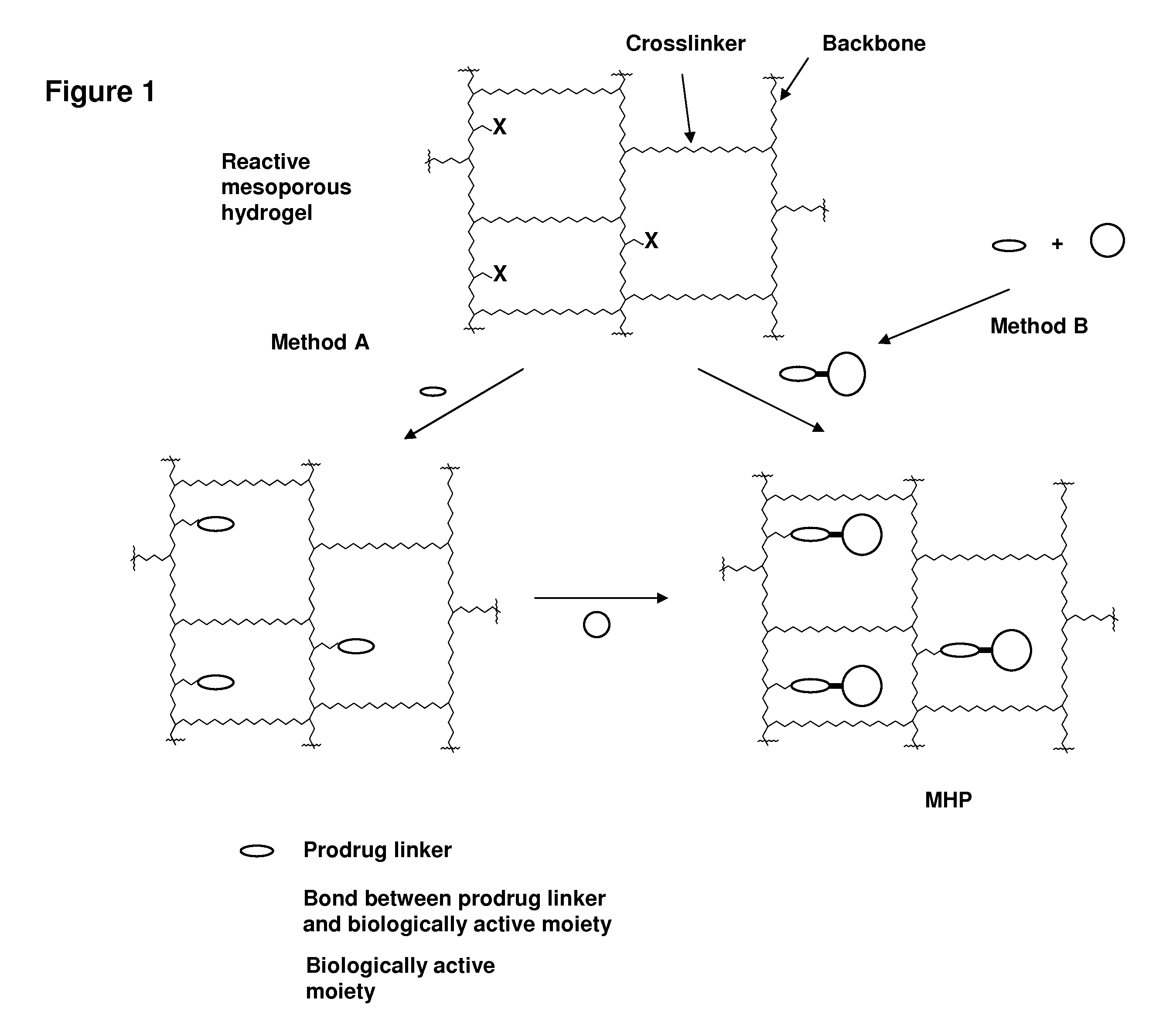
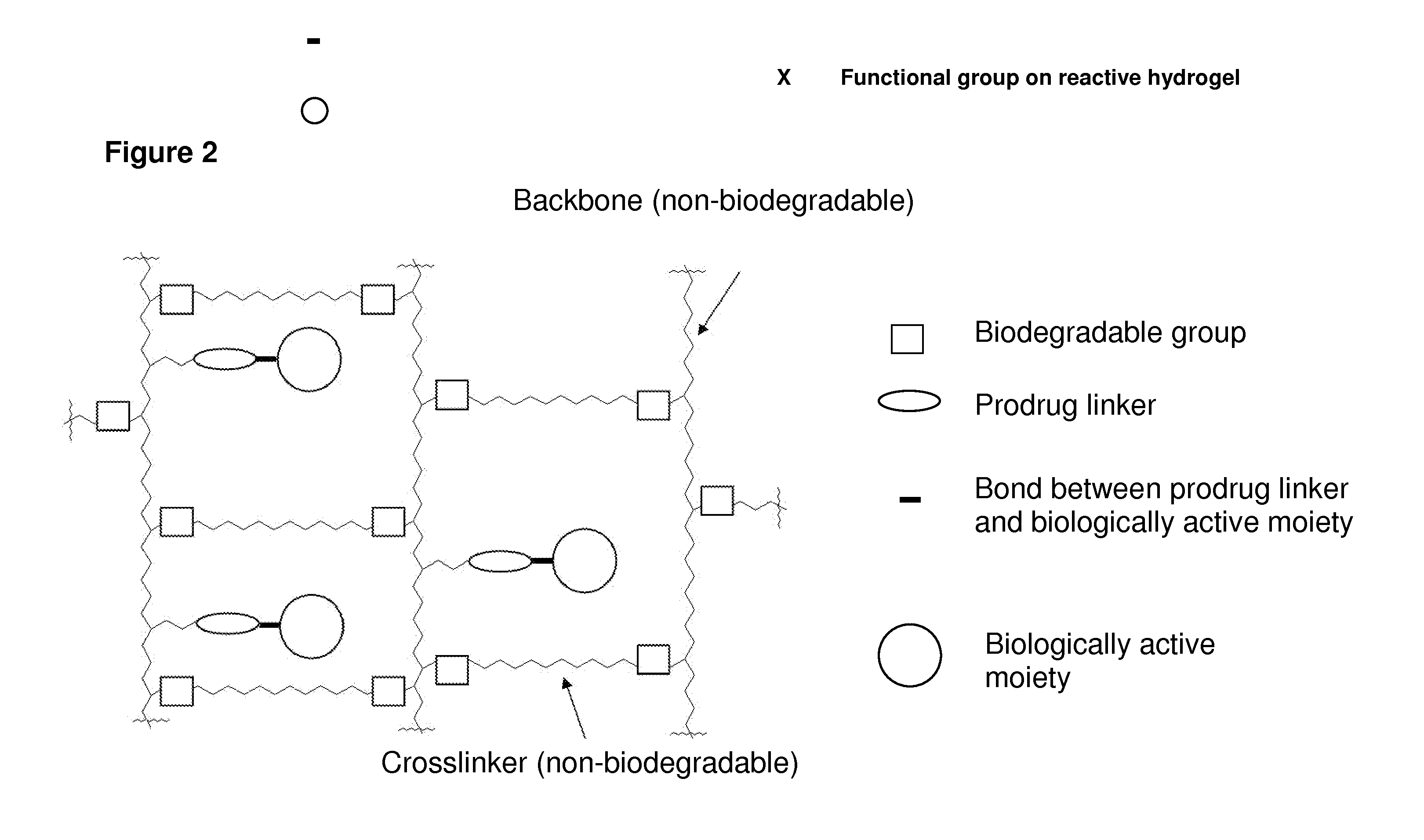
ActiveUS20060002890A1Reduce deliveryIncreased susceptibilityBiocidePeptide/protein ingredientsPolymeric prodrugBiological activity
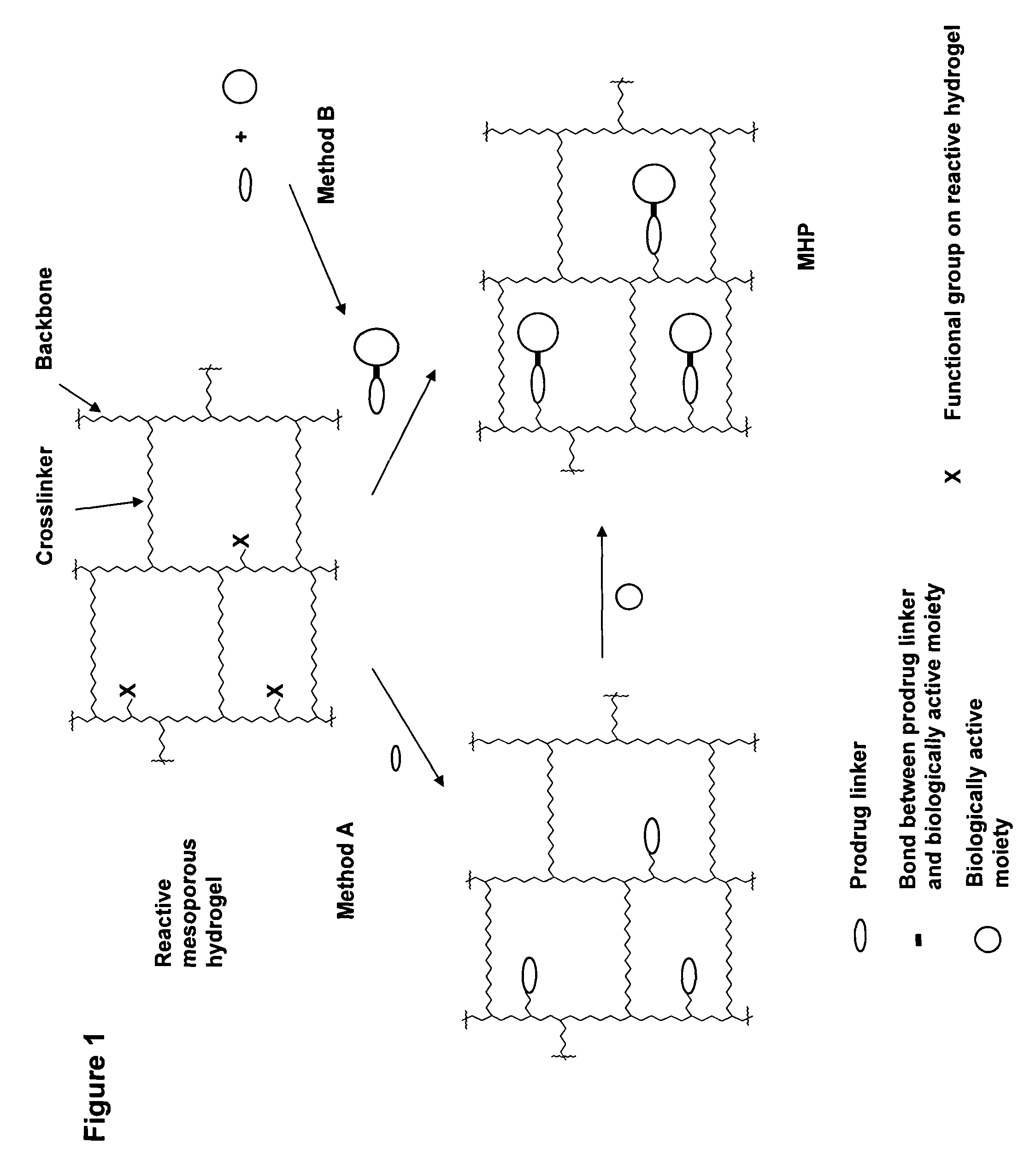
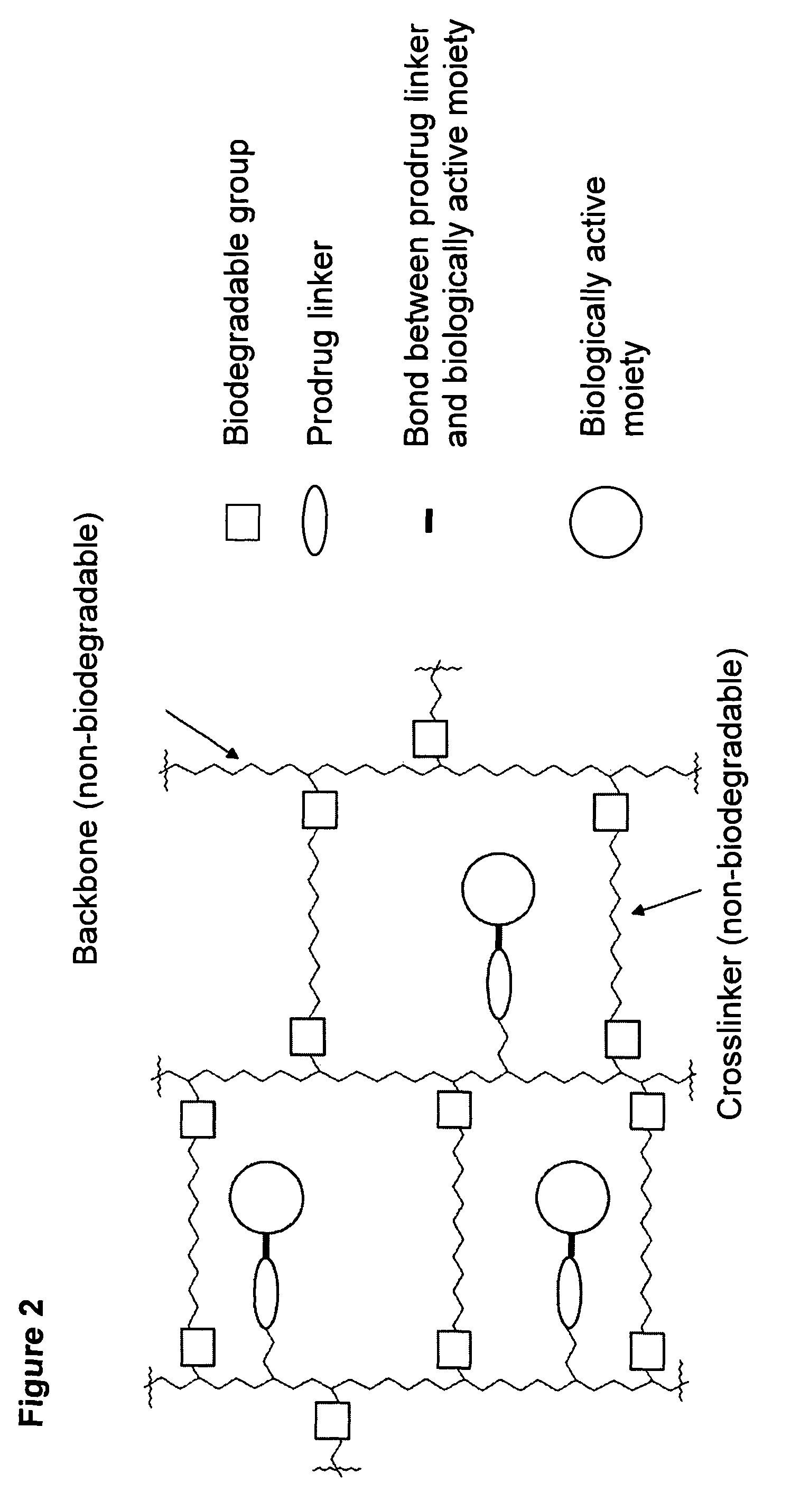
A polymeric prodrug composition including a hydrogel, a biologically active moiety and a reversible prodrug linker. The prodrug linker covalently links the hydrogel and the biologically active moiety at a position and the hydrogel has a plurality of pores with openings on its surface. The diameter of the pores is larger than that of the biologically active moiety at least at all points of the pore between at least one of the openings and the position of the biologically active moiety.
Owner:ASCENDIS PHARM AS
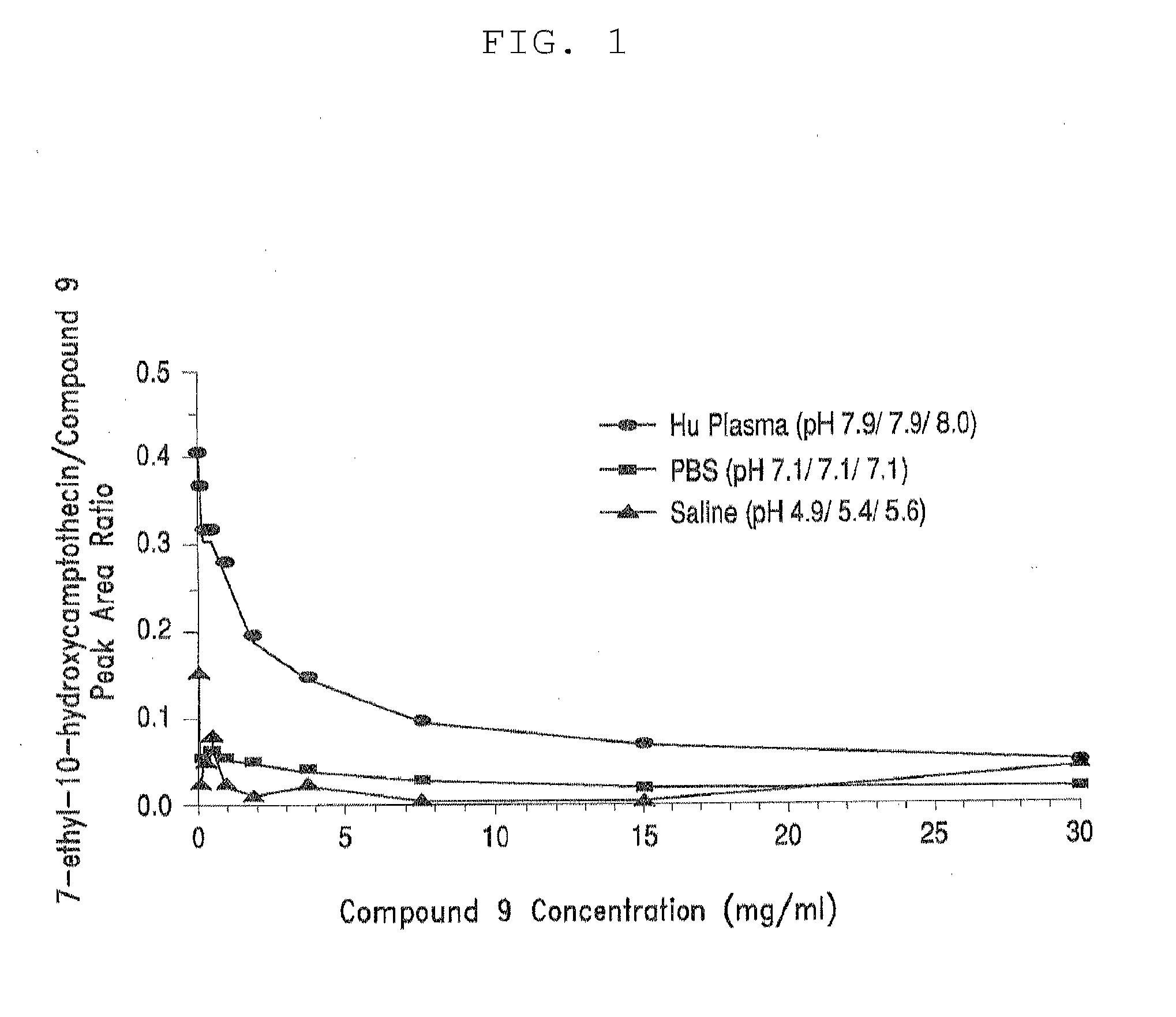
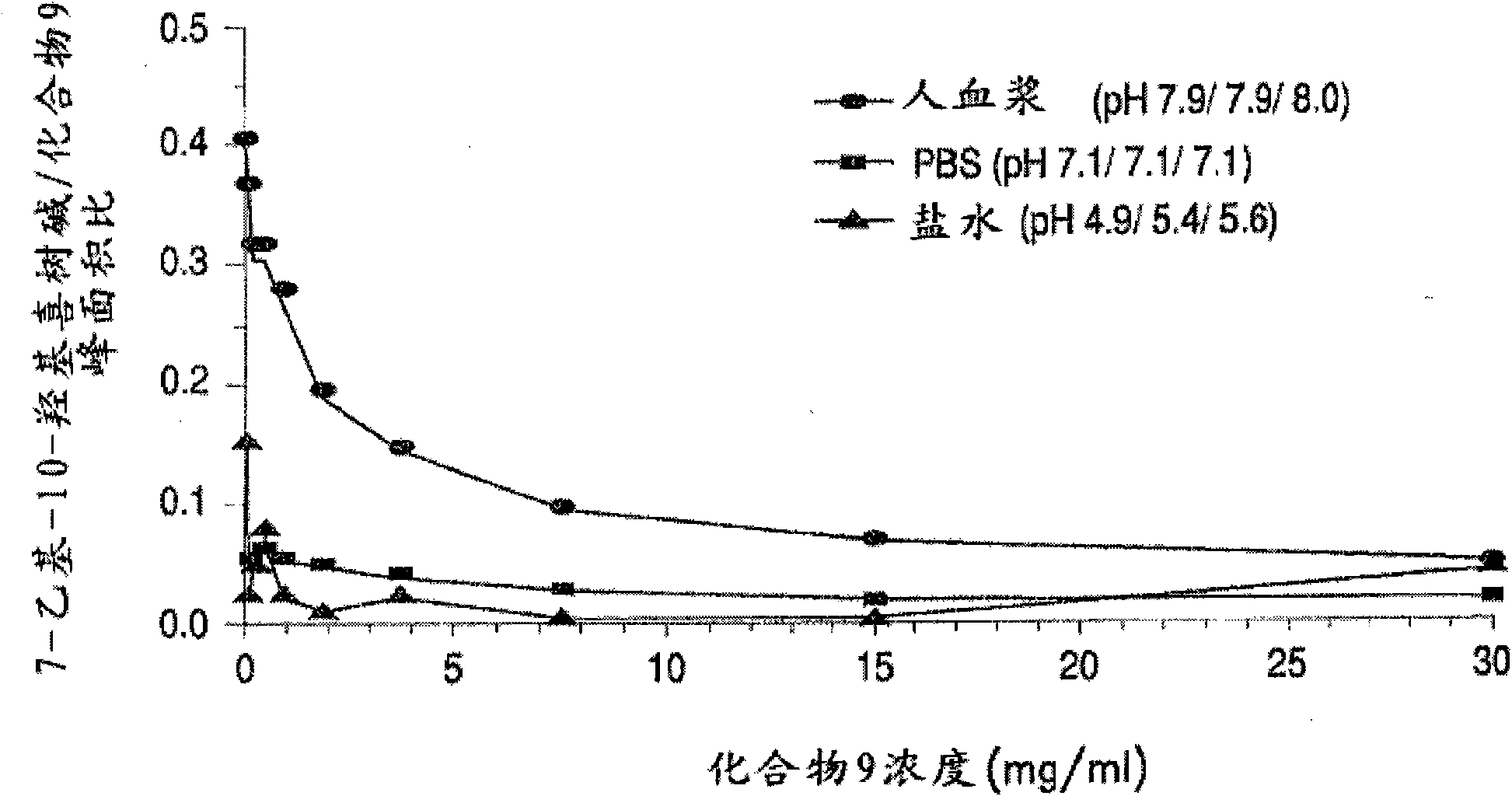
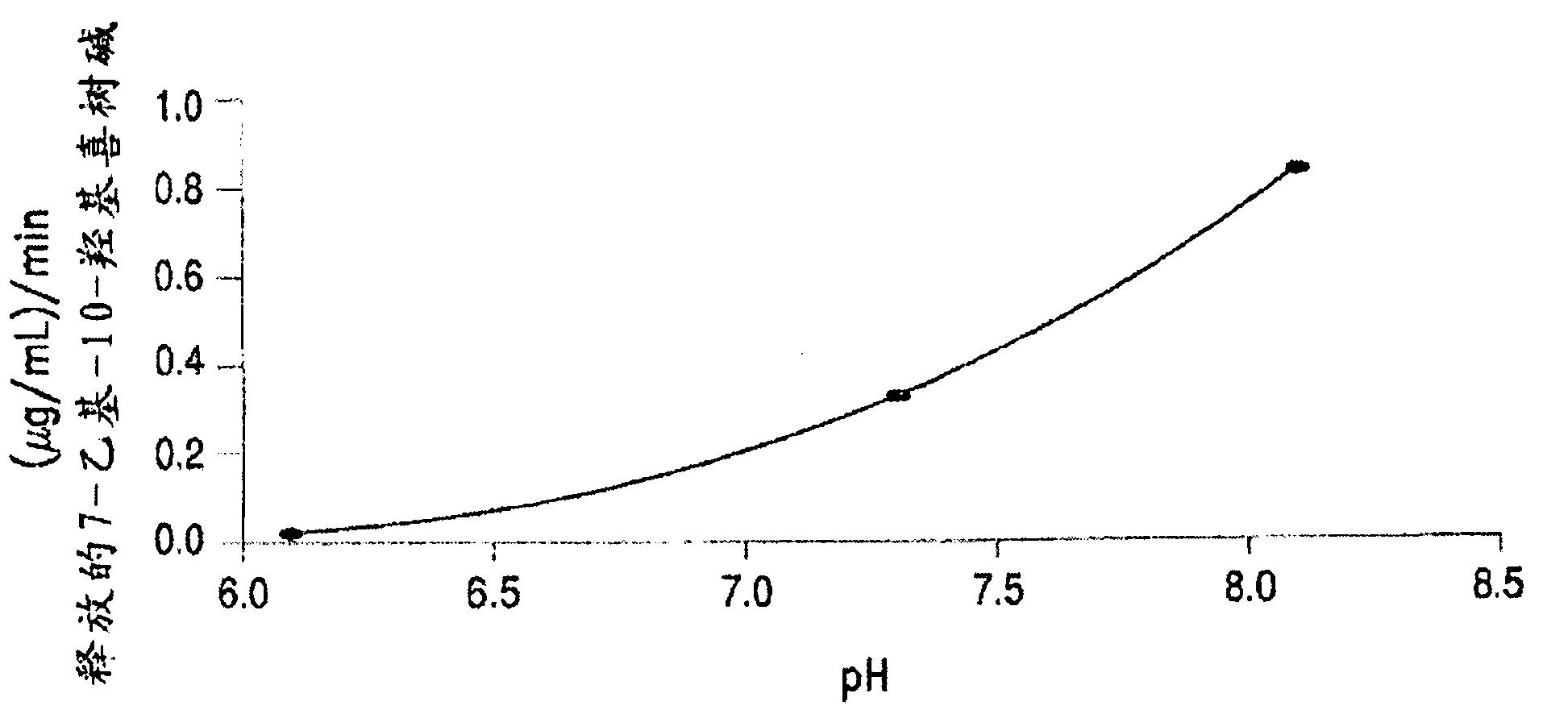
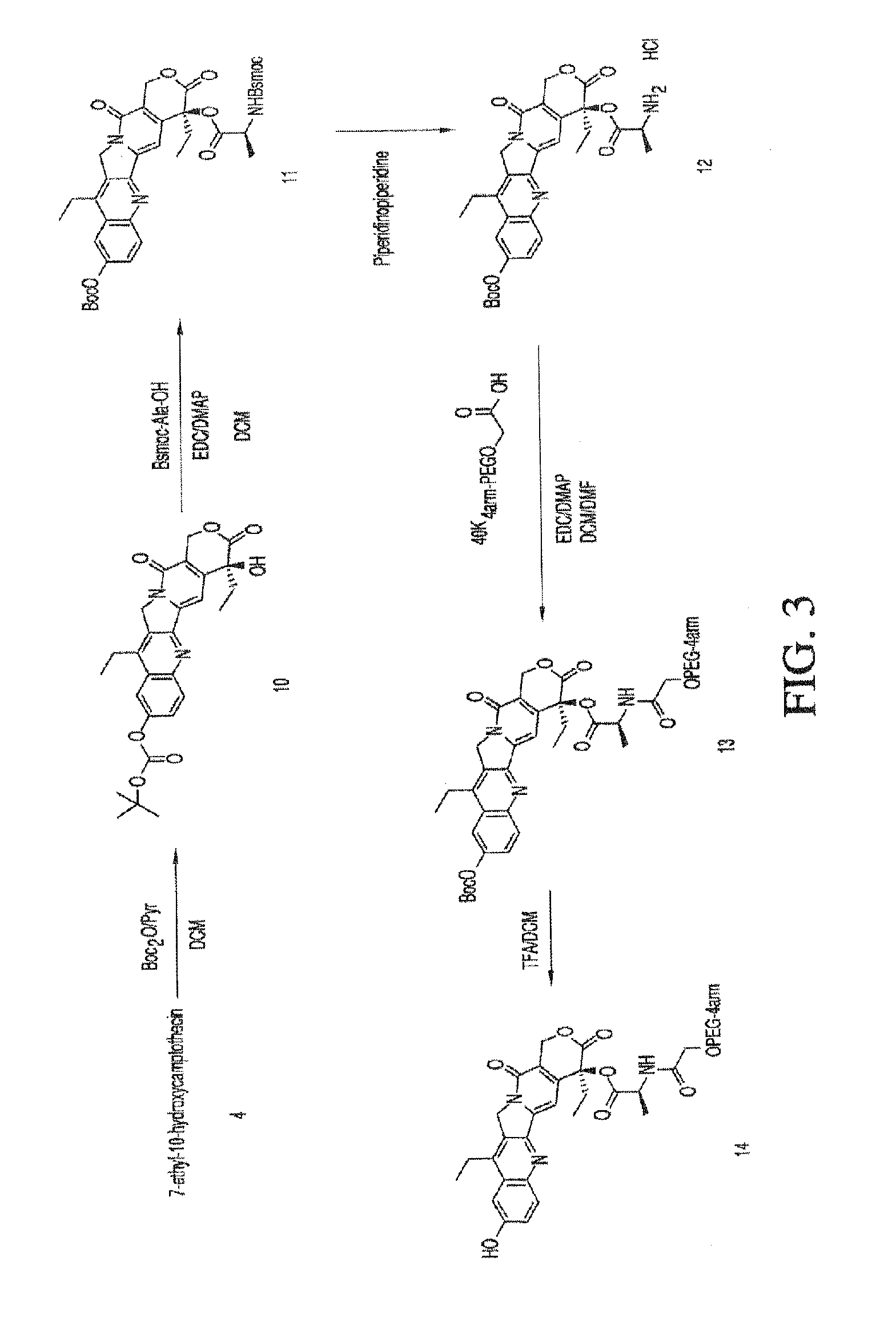
Methods of treating her2 positive cancer with her2 receptor antagonist in combination with multi-arm polymeric conjugates of 7-ethyl-10-hydroxycamptothecin
InactiveUS20120171201A1Reduce resistanceTherapeutic utilityAntibody ingredientsImmunoglobulinsPolymeric prodrugMedicine
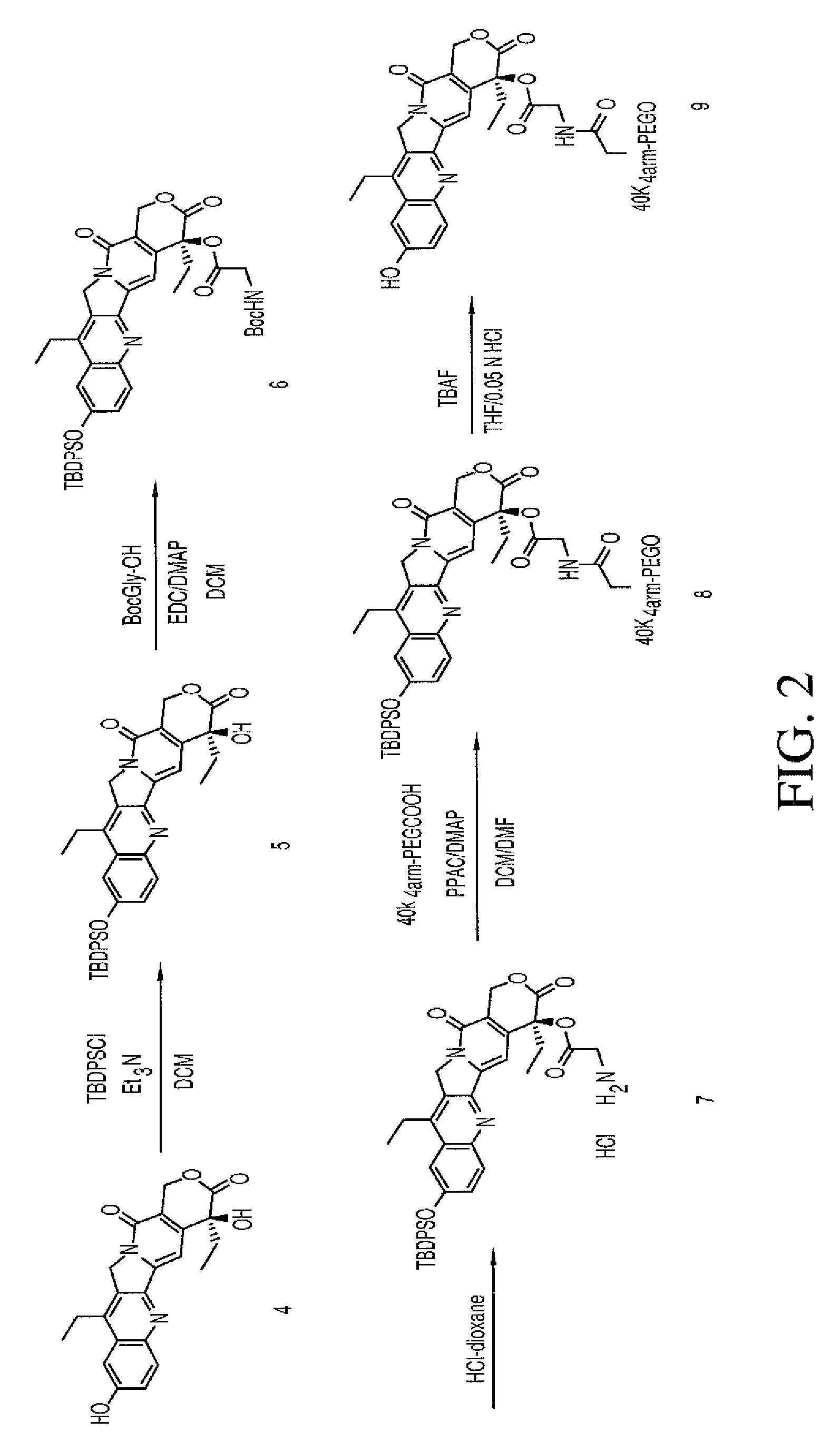
The present invention relates to methods of treating a HER2 positive cancer in mammals. The present invention includes administering a HER2 antagonist in combination with a polymeric prodrug of 7-ethyl-10-hydroxycamptothecin to the mammals in need thereof.
Owner:BELROSE PHARMA
Hydrogel formulations
ActiveUS7968085B2Reduce deliveryIncreased susceptibilityBiocideGenetic material ingredientsPolymeric prodrugChemistry
A polymeric prodrug composition including a hydrogel, a biologically active moiety and a reversible prodrug linker. The prodrug linker covalently links the hydrogel and the biologically active moiety at a position and the hydrogel has a plurality of pores with openings on its surface. The diameter of the pores is larger than that of the biologically active moiety at least at all points of the pore between at least one of the openings and the position of the biologically active moiety.
Owner:ASCENDIS PHARM AS
Hydrogel formulations
ActiveUS20110223230A1Reduce deliveryIncreased susceptibilityOrganic active ingredientsPowder deliveryPolymeric prodrugChemistry
A polymeric prodrug composition including a hydrogel, a biologically active moiety and a reversible prodrug linker. The prodrug linker covalently links the hydrogel and the biologically active moiety at a position and the hydrogel has a plurality of pores with openings on its surface. The diameter of the pores is larger than that of the biologically active moiety at least at all points of the pore between at least one of the openings and the position of the biologically active moiety.
Owner:ASCENDIS PHARM AS
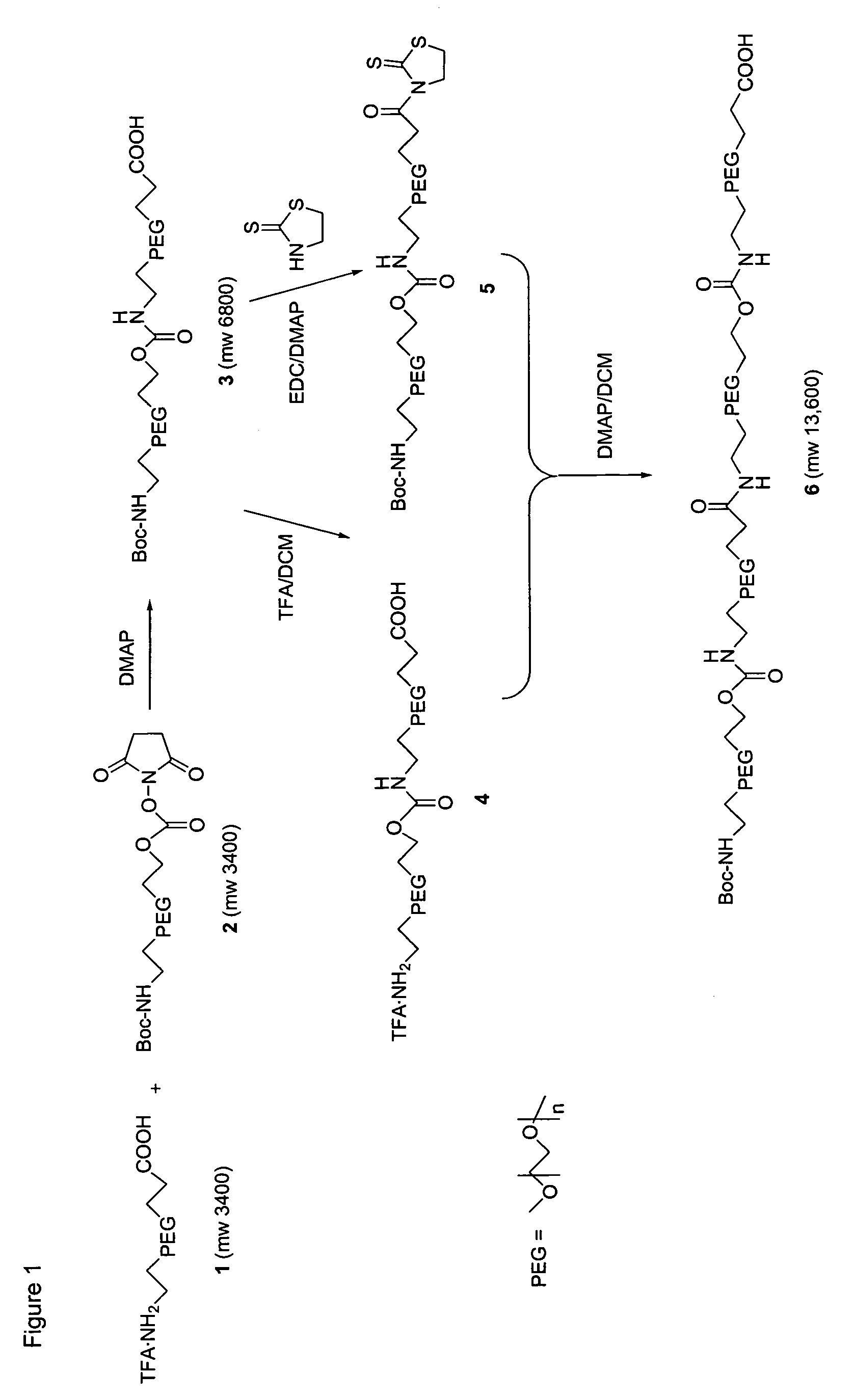
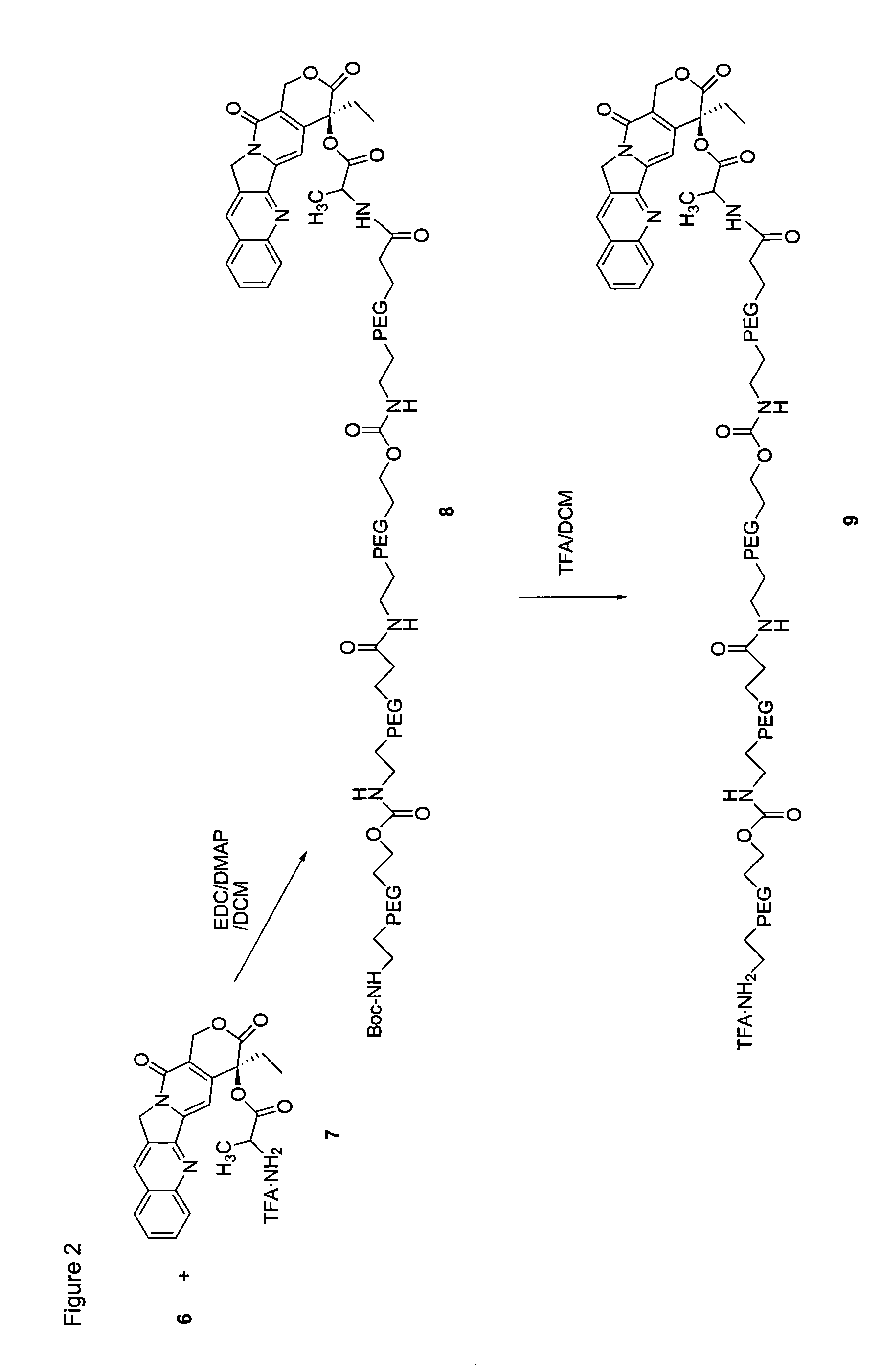
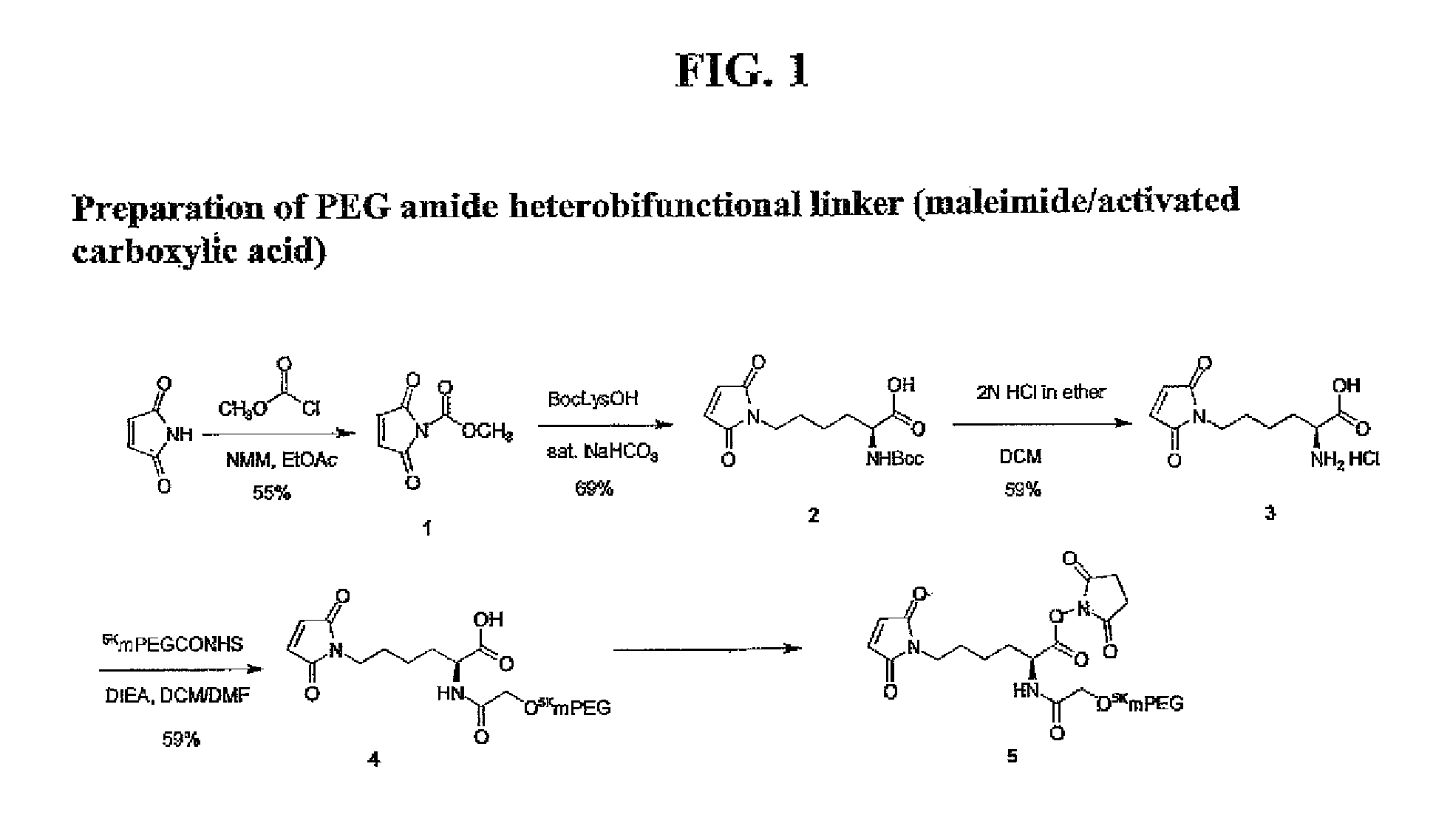
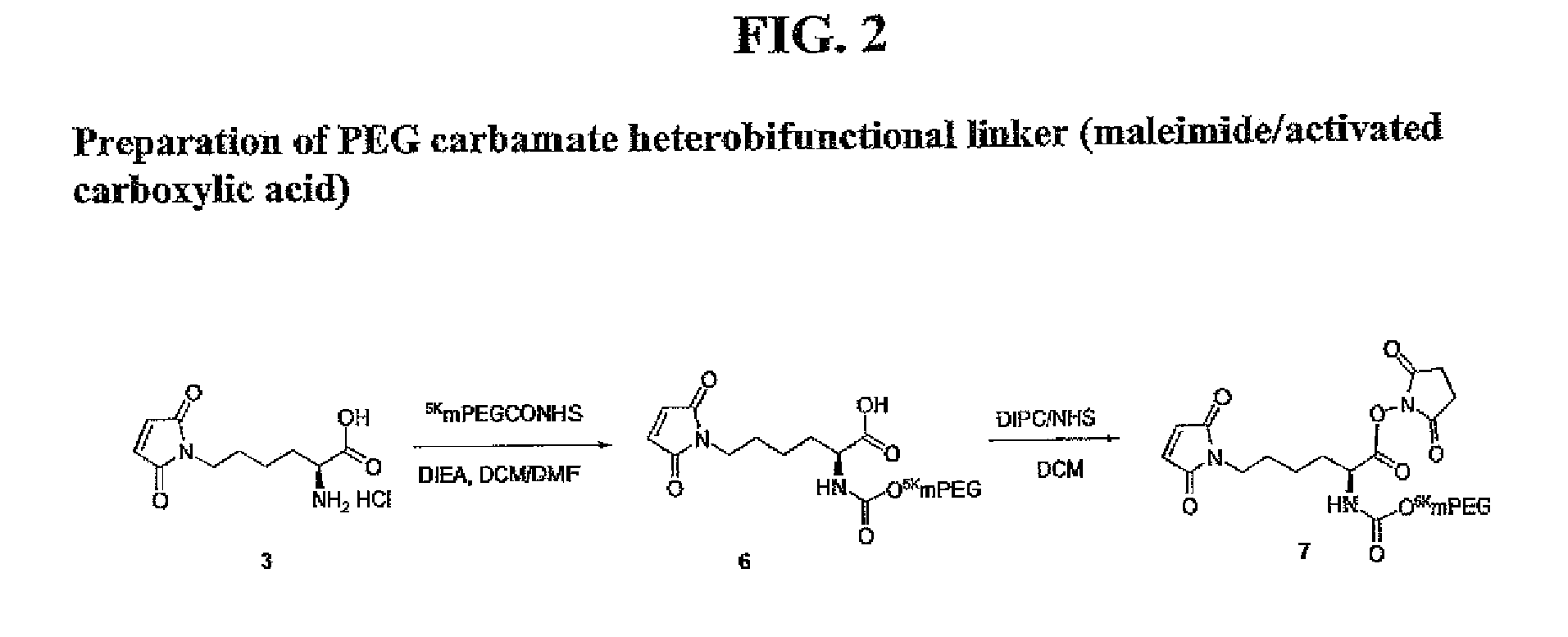
Heterobifunctional polymeric bioconjugates
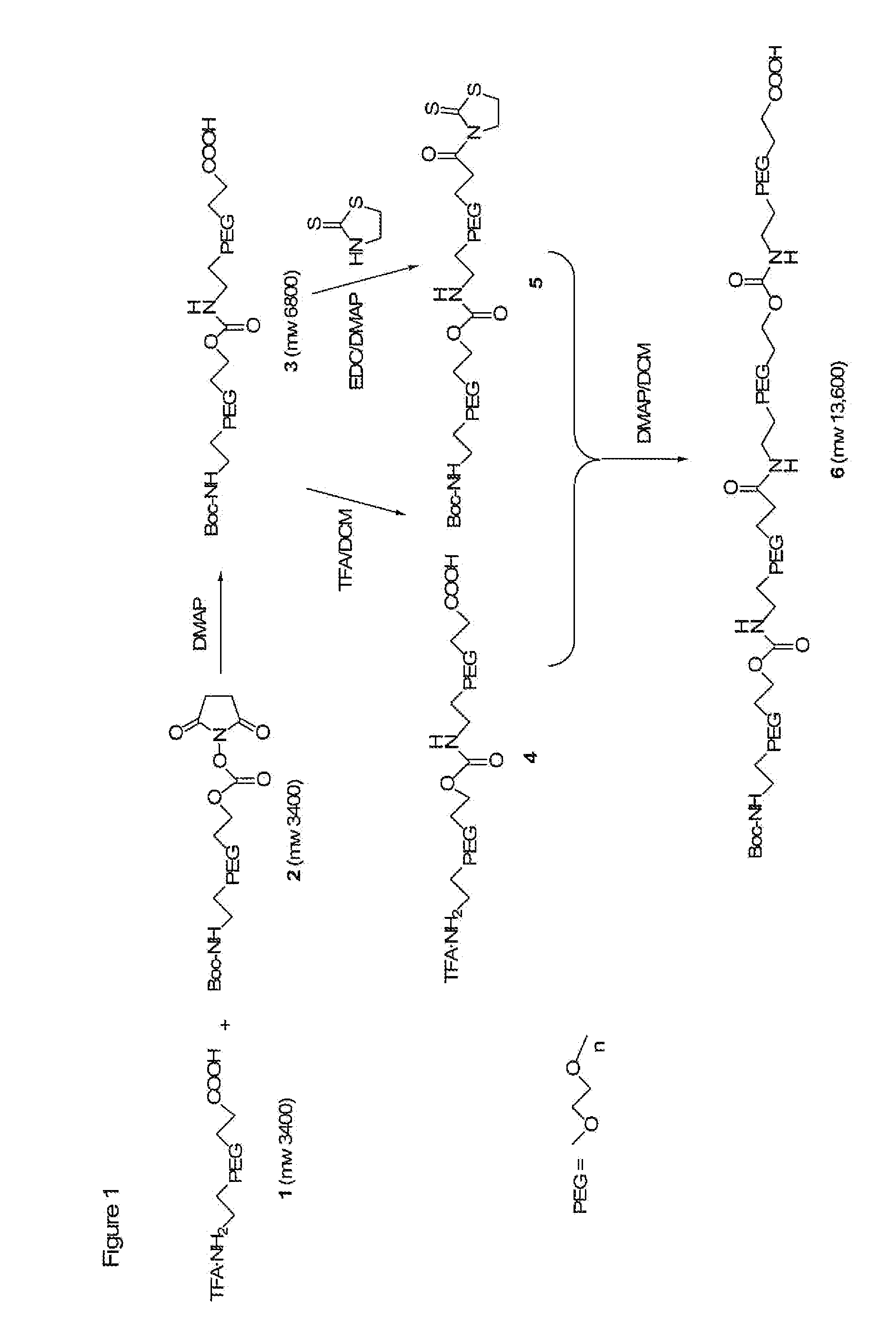
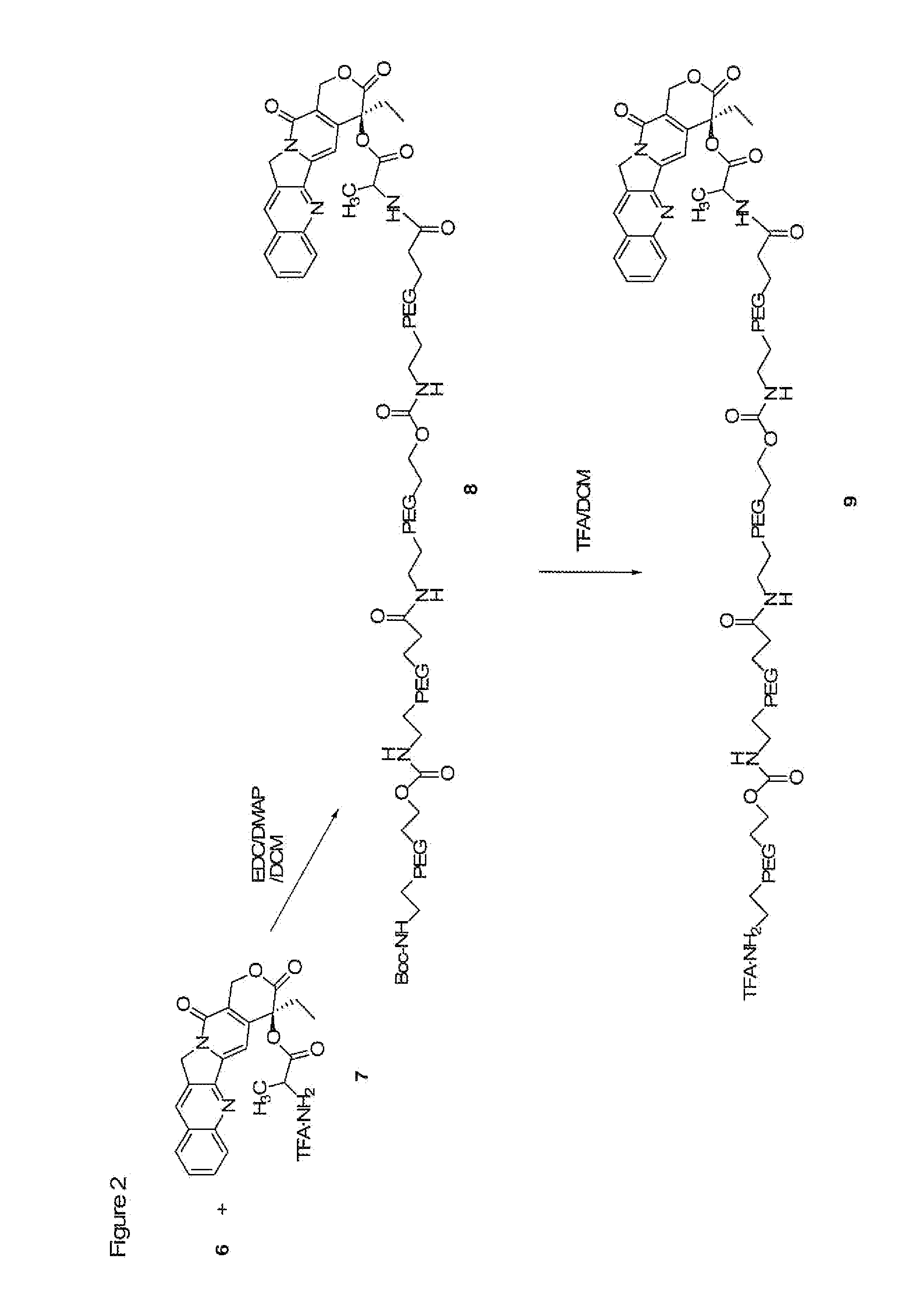
InactiveUS7332164B2Extended half-lifeImprove solubilityBiocideCarbamic acid derivatives preparationPolymeric prodrugActive component
Heterobifunctional polymeric prodrug platforms for delivering biologically active compounds, including proteins, monoclonal antibodies and the like are disclosed. One preferred compound isMethods of making and using the compounds and conjugates described herein are also provided.
Owner:BELROSE PHARMA
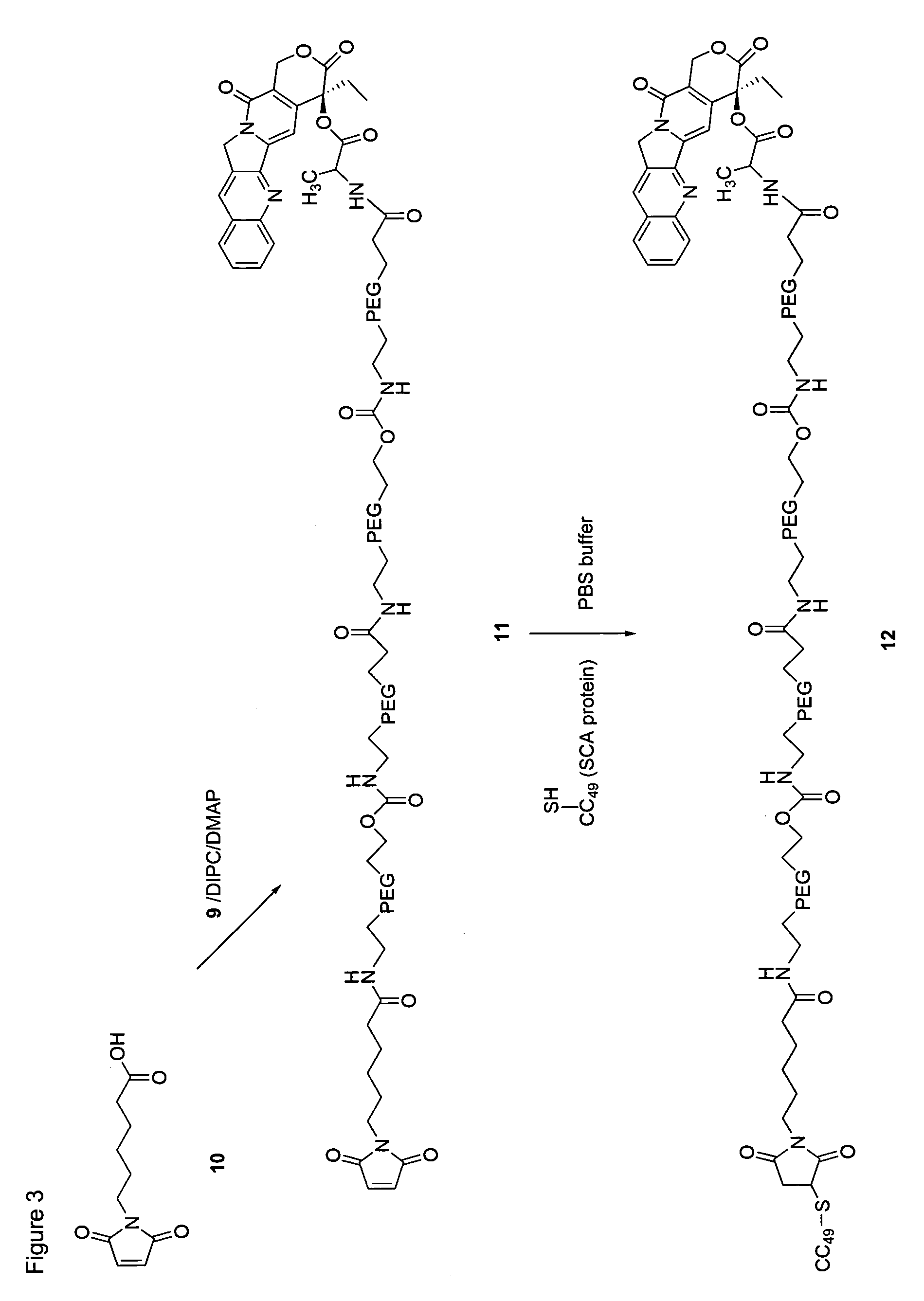
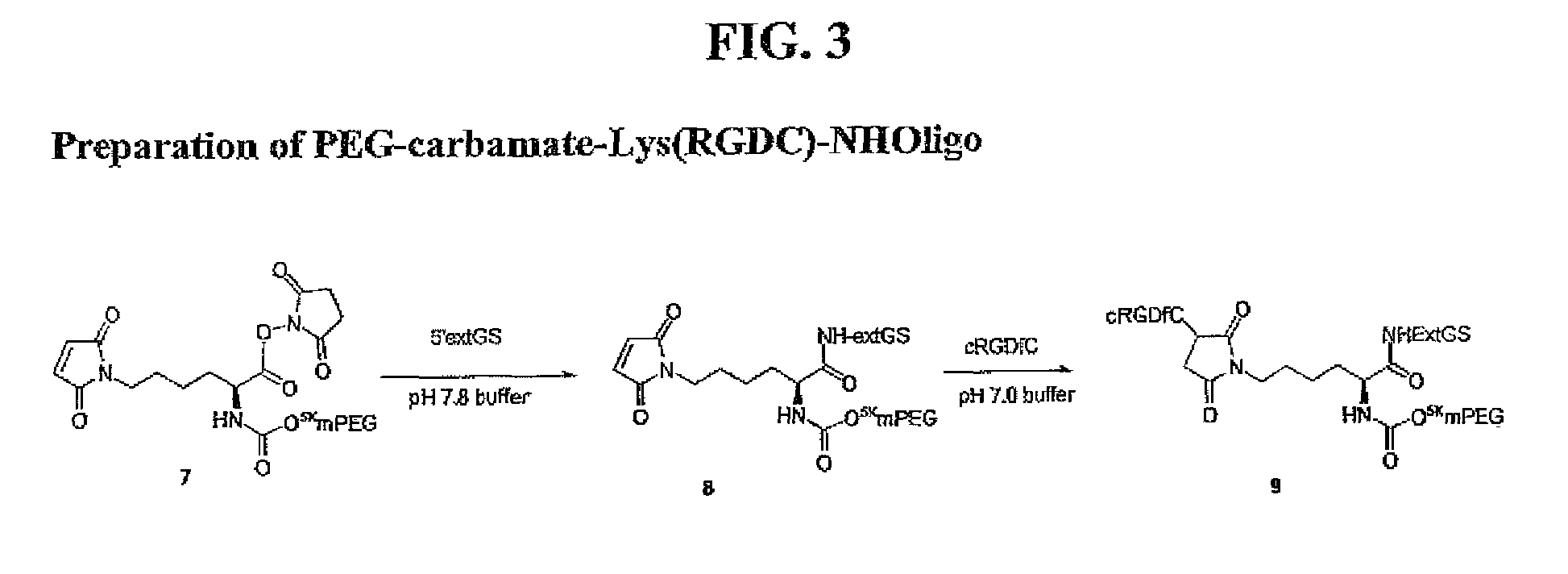
Targeted polymeric prodrugs containing multifunctional linkers
InactiveUS8367065B2Small molecular weightBiocidePeptide/protein ingredientsPolymeric prodrugSingle-Chain Antibodies
Owner:BELROSE PHARMA
Methods of treating her2 positive cancer with her2 receptor antagonist in combination with multi-arm polymeric conjugates of 7-ethyl-10-hydroxycamptothecin
The present invention relates to methods of treating a HER2 positive cancer in mammals. The present invention includes administering a HER2 antagonist in combination with a polymeric prodrug of 7-ethyl-10-hydroxycamptothecin to the mammals in need thereof.
Owner:ENZON PHARM INC
Polymeric prodrug with a self-immolative linker
ActiveUS8377917B2Good predictability and controlLow variabilityBiocideCarbamic acid derivatives preparationPolymeric prodrugMedicinal chemistry
A cascade carrier linked prodrug is described which comprises a biologically active moiety and a masking group having at least one nucleophile and being distinct from the carrier.
Owner:ASCENDIS PHARM GMBH
Prodrugs of anticancer agents employing substituted aromatic acids
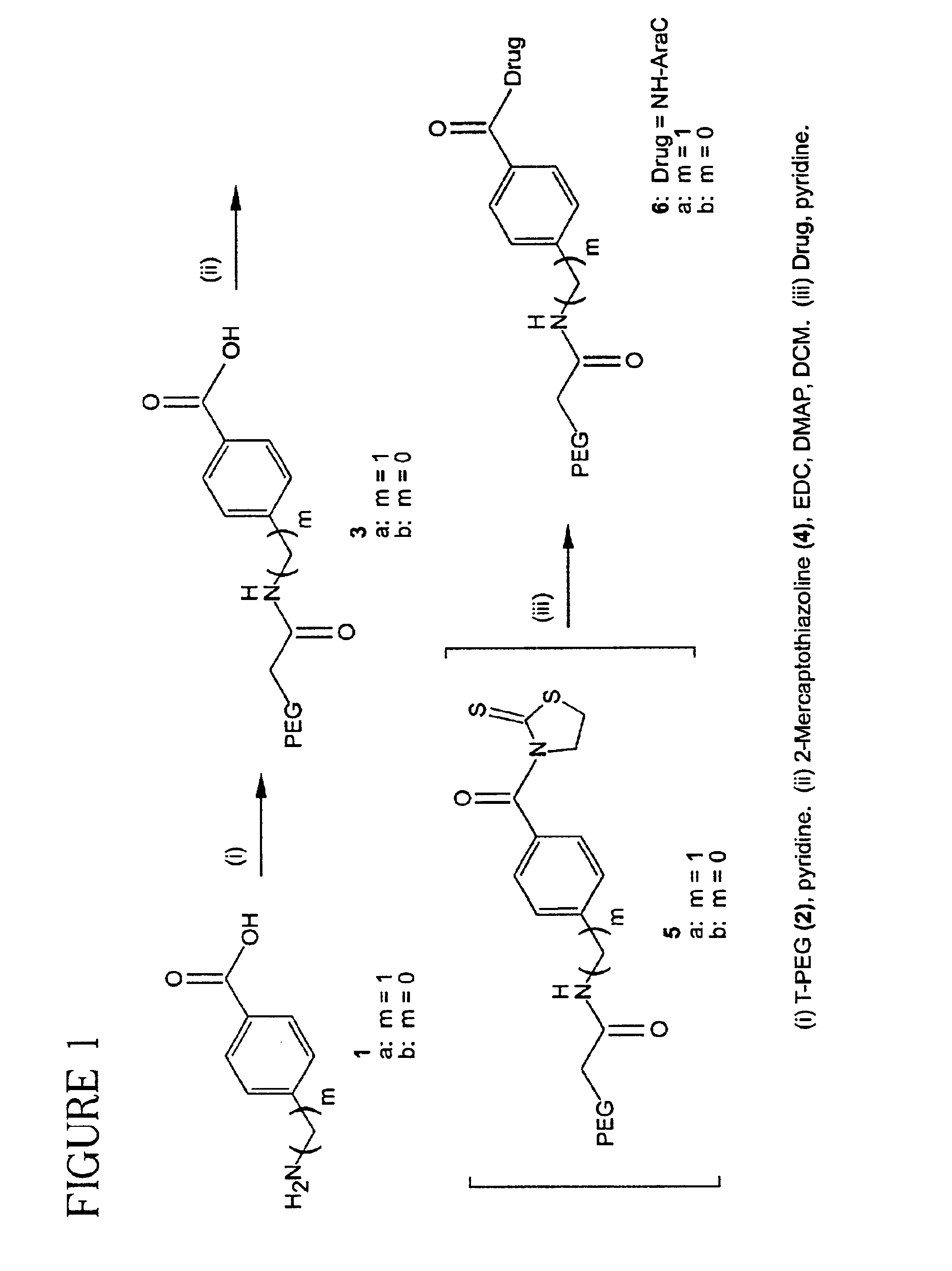
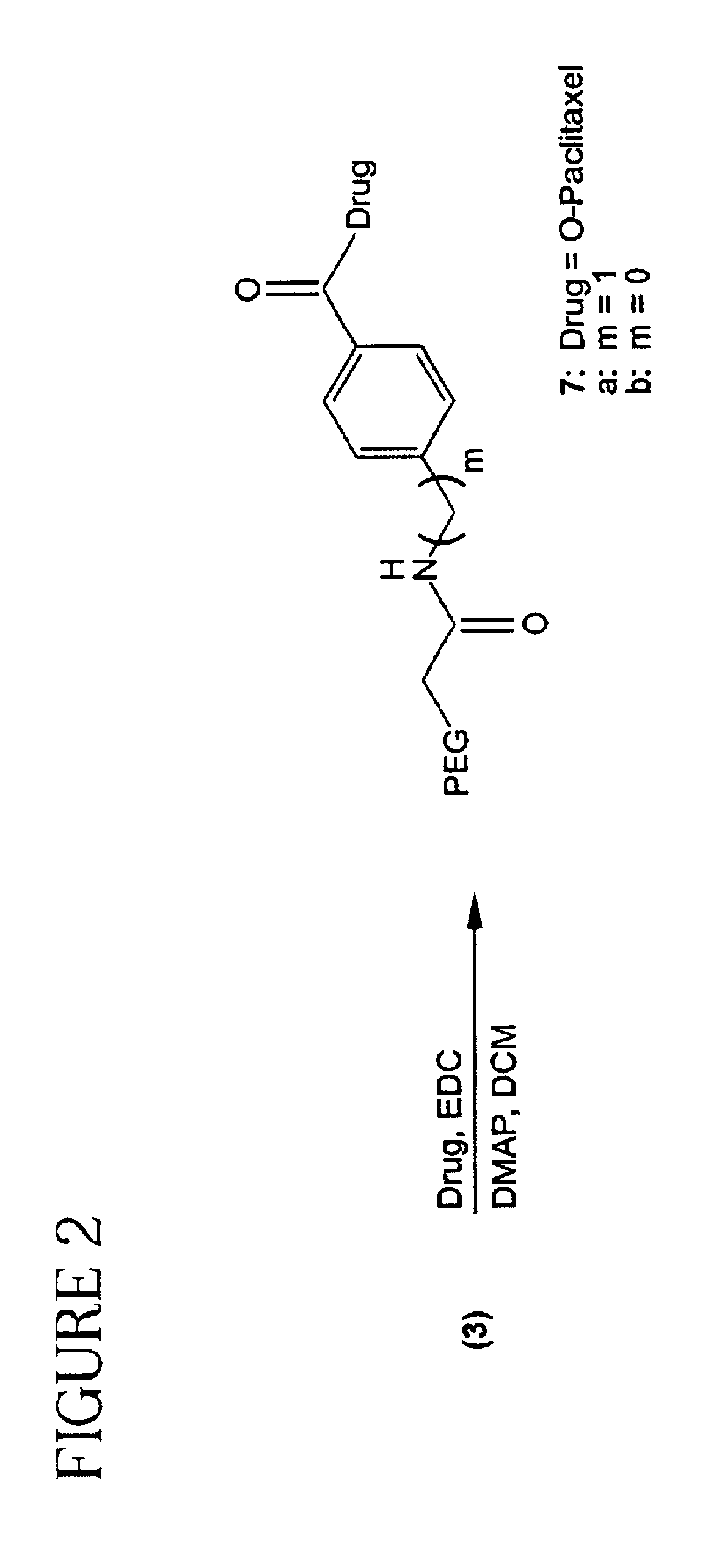
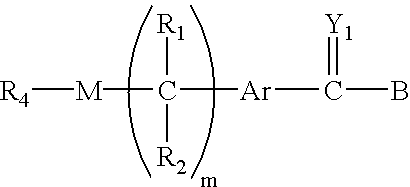
Polymeric prodrugs of the formula: whereinB is selected from the group consisting of OH, leaving groups, residues of amine-containing moieties and a residues of hydroxyl-containing moieties;Y1 is selected from the group consisting of O, S, and NR5;M is NR3, O or S;Ar is a moiety which when included in Formula I forms a multi-substituted aromatic or heteroaromatic hydrocarbon or a multi-substituted heterocyclic group;(m) is zero or a positive integer;R1-3 and R5 are independently selected from the group consisting of hydrogen, C1-6 alkyls, C3-12 branched alkyls, C3-8 cycloalkyls, C1-6 substituted alkyls, C3-8 substituted cycloalkyls, aryls, substituted aryls, aralkyls, C1-6 heteroalkyls, substituted C1-6 heteroalkyls, C1-6 alkoxy, phenoxy and C1-6 heteroalkoxy; andR4 is a polymeric residue;as well as methods of making and using the same are disclosed.
Owner:RESENIUS USA +1
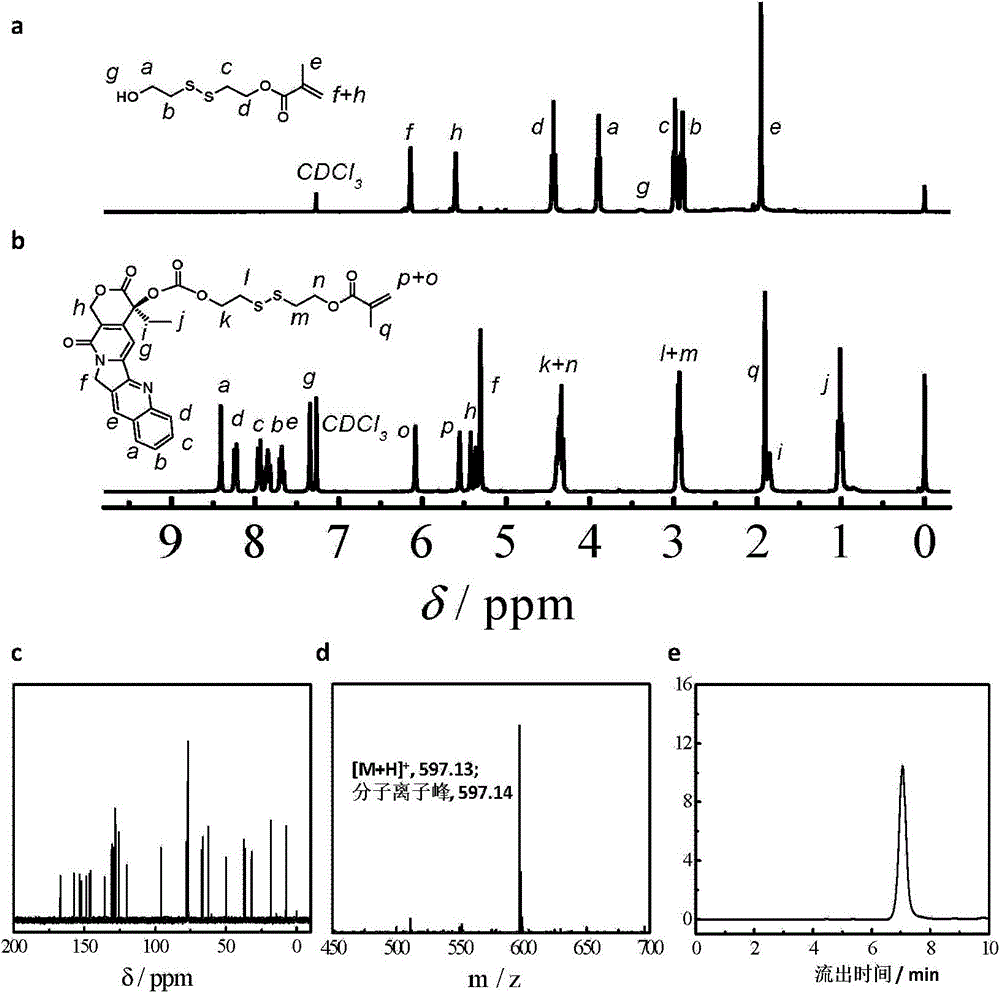
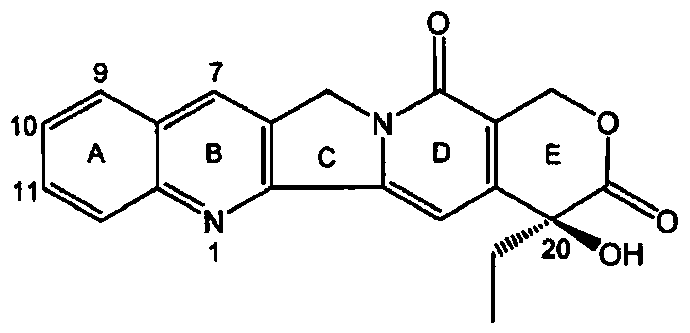
Camptothecin prodrug monomer and polymeric prodrug amphipathic molecules thereof as well as preparation method and application of camptothecin prodrug monomer and polymeric prodrug amphipathic molecules
ActiveCN103524519AGood water solubilityImprove stabilityOrganic active ingredientsOrganic chemistryDisulfide bondingPolymeric prodrug
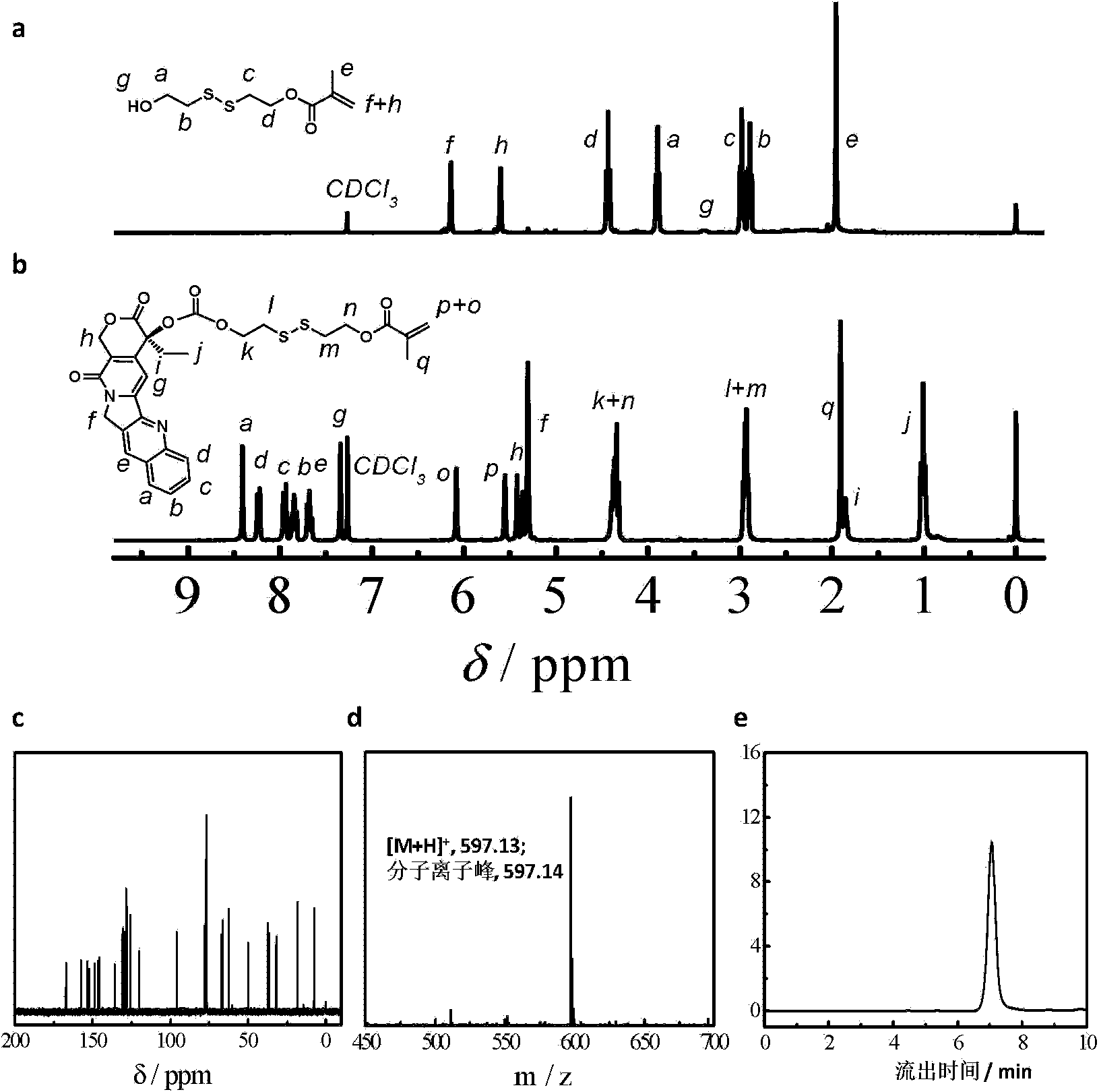
The invention relates to a reduced response camptothecin prodrug monomer as shown in a formula I, reduced response camptothecin polymeric prodrug amphipathic molecules of the reduced response camptothecin prodrug monomer, and a preparation method and application of the reduced response camptothecin prodrug monomer and the reduced response camptothecin polymeric prodrug amphipathic molecules. In the formula I, each i is 2 or 3 independently, each X is O or NH independently, and R is H, CH3 or CH2CH3. According to the preparation method, raw materials including camptothecin, triphosgene and the like are used for modifying 20 bits of hydroxyls of camptothecin to prepare a camptothecin prodrug monomer containing disulfide bonds, then the camptothecin prodrug monomer is subjected to high-conversion-rate polymerization by using active free radical polymerization method to obtain an amphiphilic polymer which contains a camptothecin drug repetitive unit and has an extremely high drug loading capacity (more than 50wt%), and in a tumor cell reducing environment, a side chain disulfide bond is broken to perform molecular rearrangement and release camptothecin prodrug molecules, so that the amphiphilic polymer has characteristics of a reduced response controlled-release raw drug. The polymeric prodrug amphipathic molecules improve the water solubility and stability of the drug and have a controlled release characteristic.
Owner:UNIV OF SCI & TECH OF CHINA
Targeted polymeric prodrugs containing multifunctional linkers
InactiveCN101541332AImprove bioavailabilityFor full releasePeptide/protein ingredientsPeptidesPolymeric prodrugSingle-Chain Antibodies
The present invention provides single chain antibody-directed polymeric prodrugs containing multifunctional linkers. Methods of making the polymeric delivery systems and methods of treating mammals using the same are also disclosed.
Owner:ENZON PHARM INC
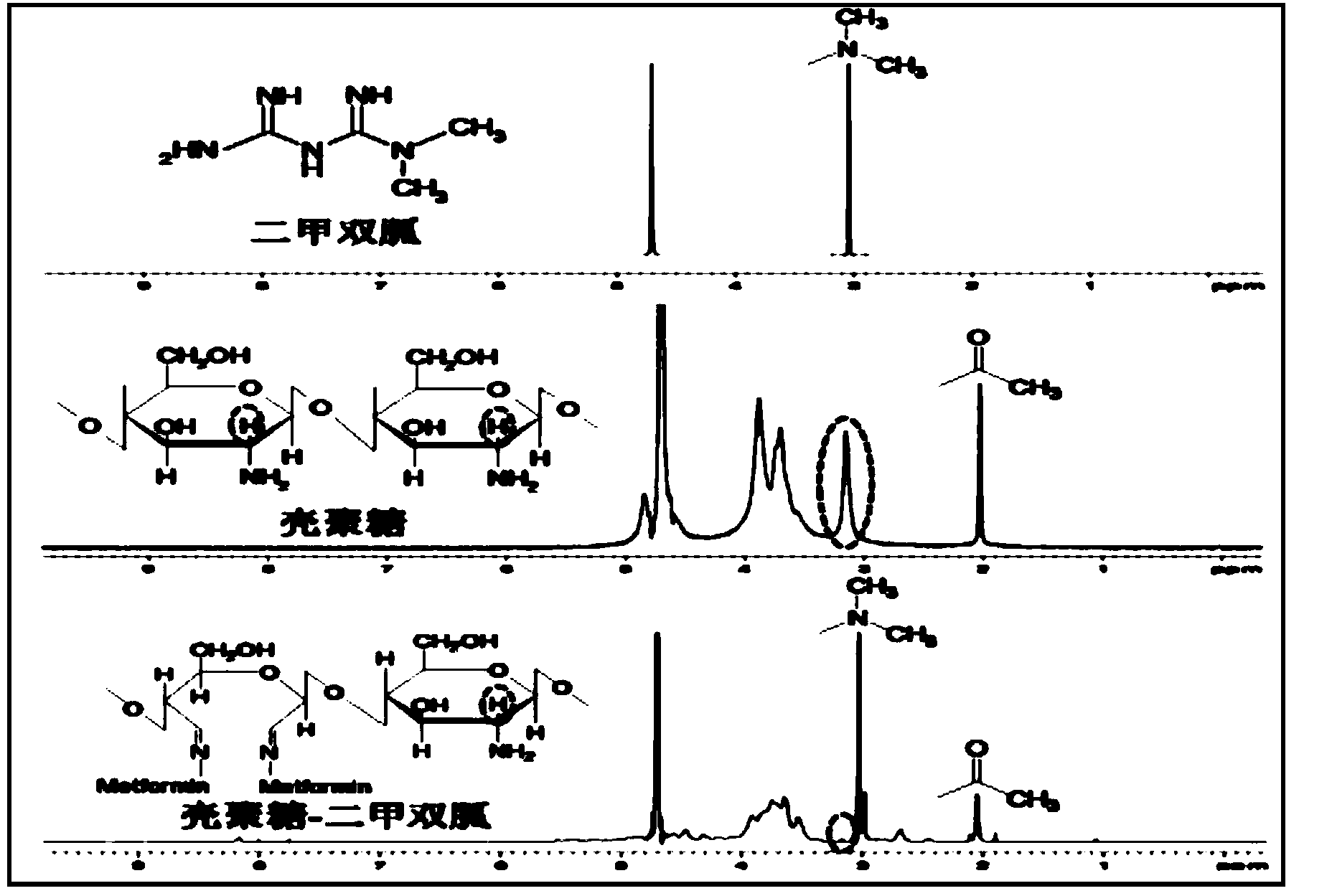
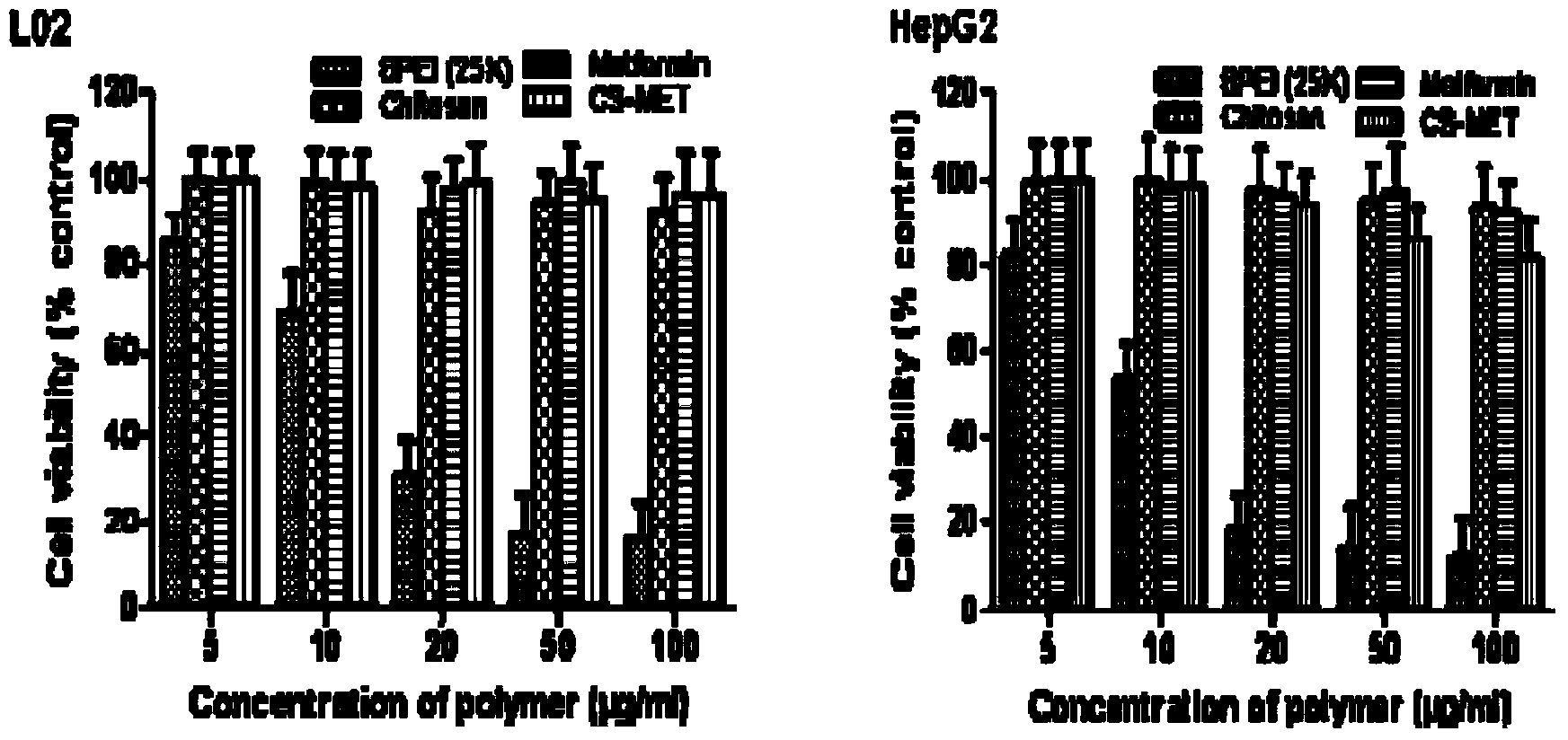
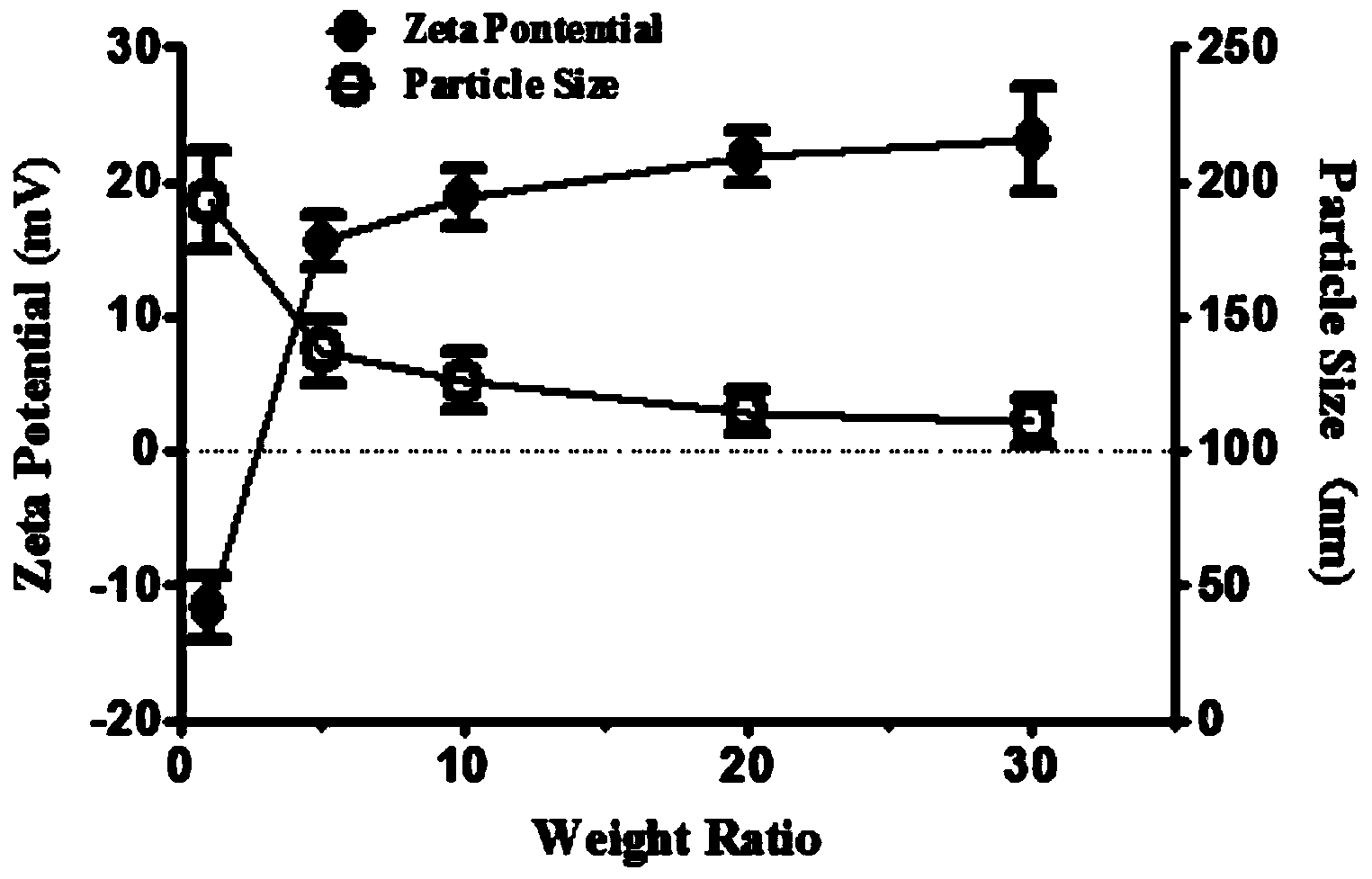
Guanidine hypoglycemic drug-polysaccharide conjugate, as well as preparation method and application thereof
ActiveCN103977422AThe synthesis method is simpleFew reaction stepsPowder deliveryOrganic active ingredientsPolymeric prodrugPharmacologic action
The invention discloses a guanidine hypoglycemic drug-polysaccharide conjugate, as well as a preparation method and application thereof. The conjugate is formed by connecting primary amine on a guanidine drug and the aldehyde group on oxidative polysaccharide through a schiff base bond. Compared with an existing guandine hypoglycemic drug, on the one hand, the conjugate can be used as a polymeric prodrug for reducing blood glucose, so that the pharmacologic action is strengthened, few adverse responses exist, and the safety is higher; on the other hand, the conjugate can be combined with therapeutic genes for treating diabetes mellitus, in particular obese diabetic, and the curative effect of the conjugate on cellular level and animal pattern are better than those of active compounds and therapeutic gene. The results prove that the guanidine hypoglycemic drug-polysaccharide conjugate has tremendous potential in the aspect of drug combined gene therapy of diabetes mellitus due to the excellent biocompatibility and effective gene transfer efficiency; the synthesizing and preparing method is simple, the process is mature, and the yield is high.
Owner:CHINA PHARM UNIV
Treatment of neuroblastoma with multi-arm polymeric conjugates of 7-ethyl-10-hydroxycamptothecin
InactiveUS20100098654A1Difficulty of therapyEliminate and significantly reduce immune responseNervous disorderPharmaceutical non-active ingredientsPolymeric prodrugMedicine
The present invention relates to methods of treatment of neuroblastoma. The present invention includes administering polymeric prodrugs of 7-ethyl-10-hydroxycamptothecin to patients in need thereof.
Owner:ENZON PHARM INC
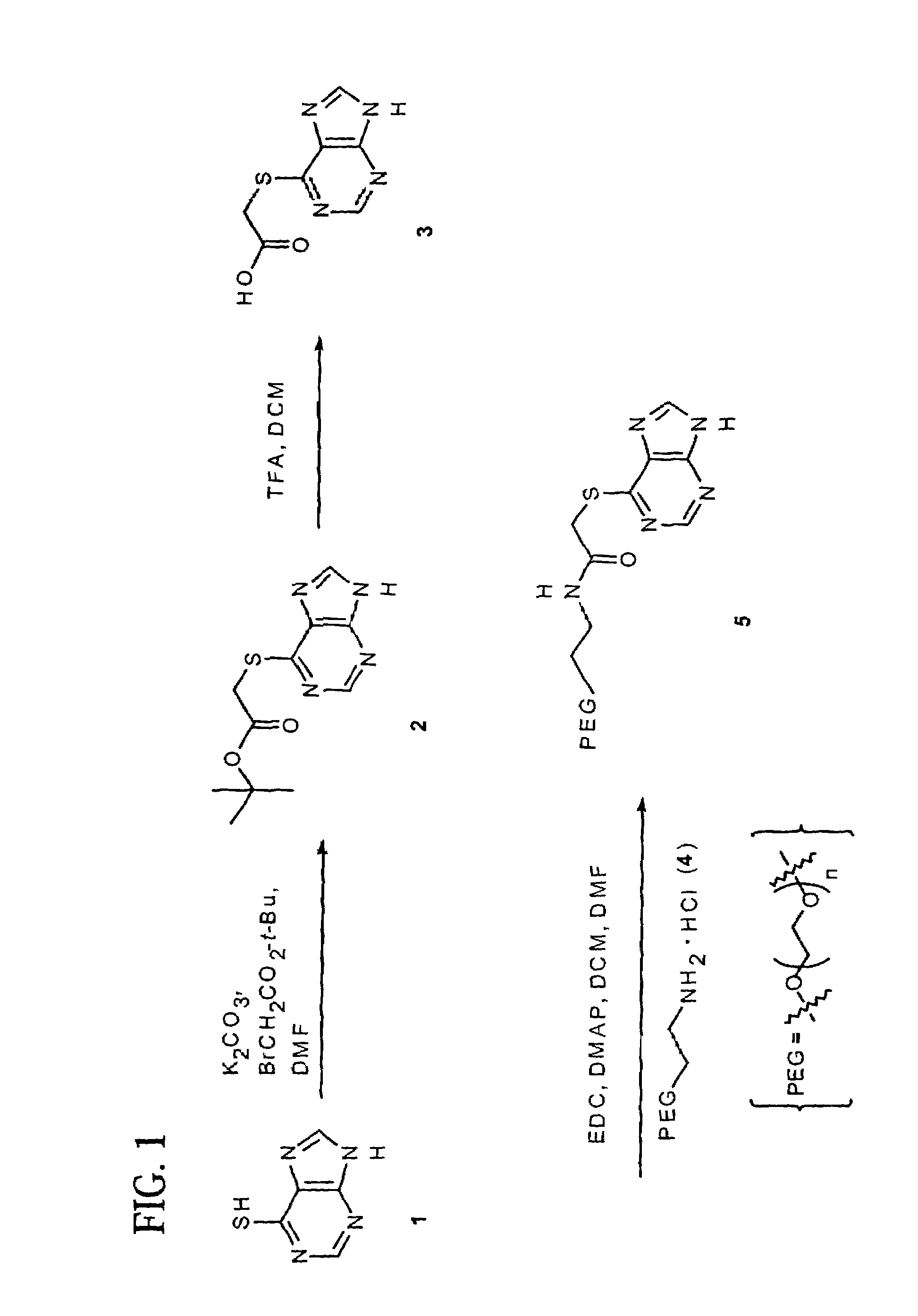
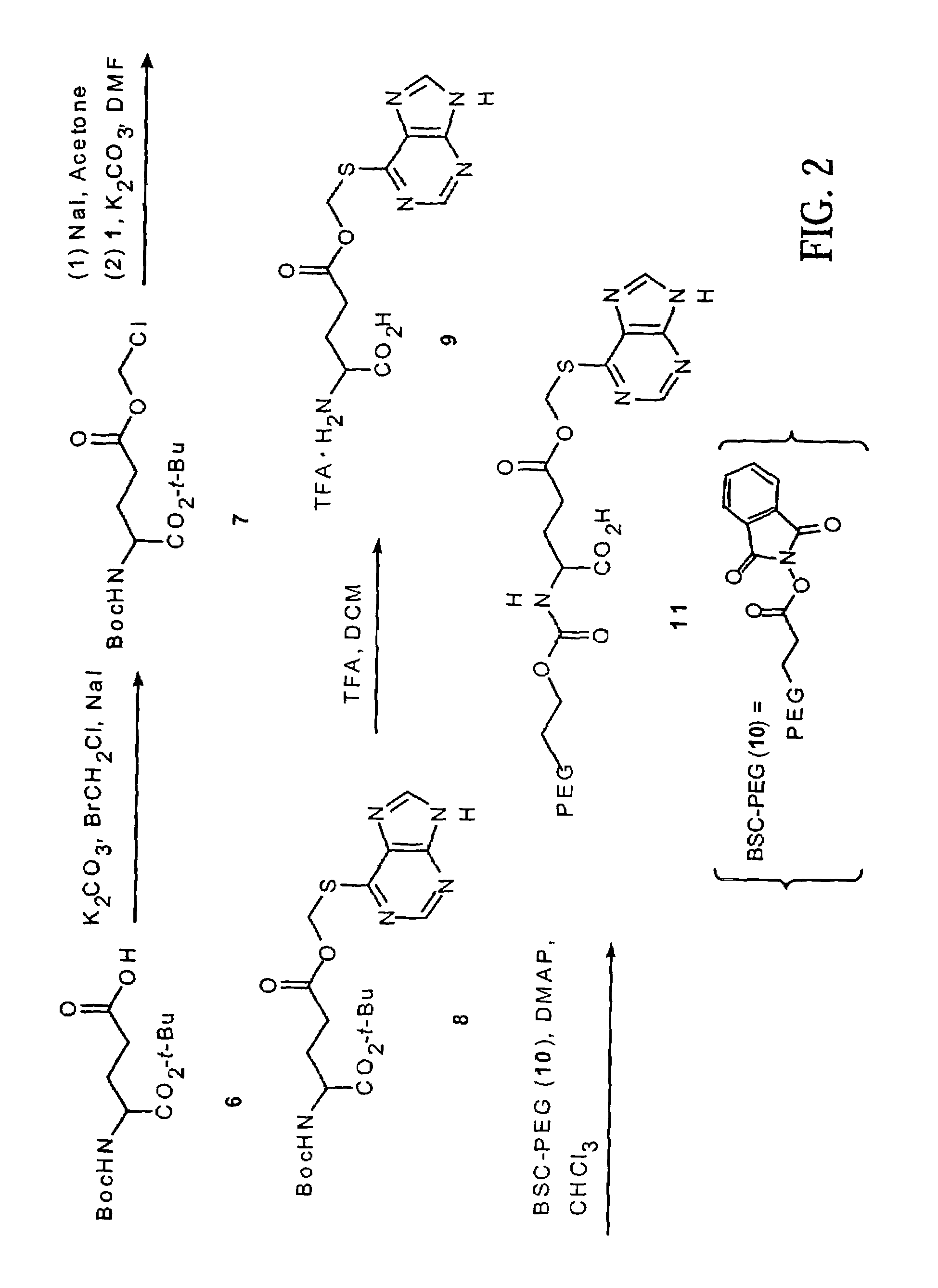
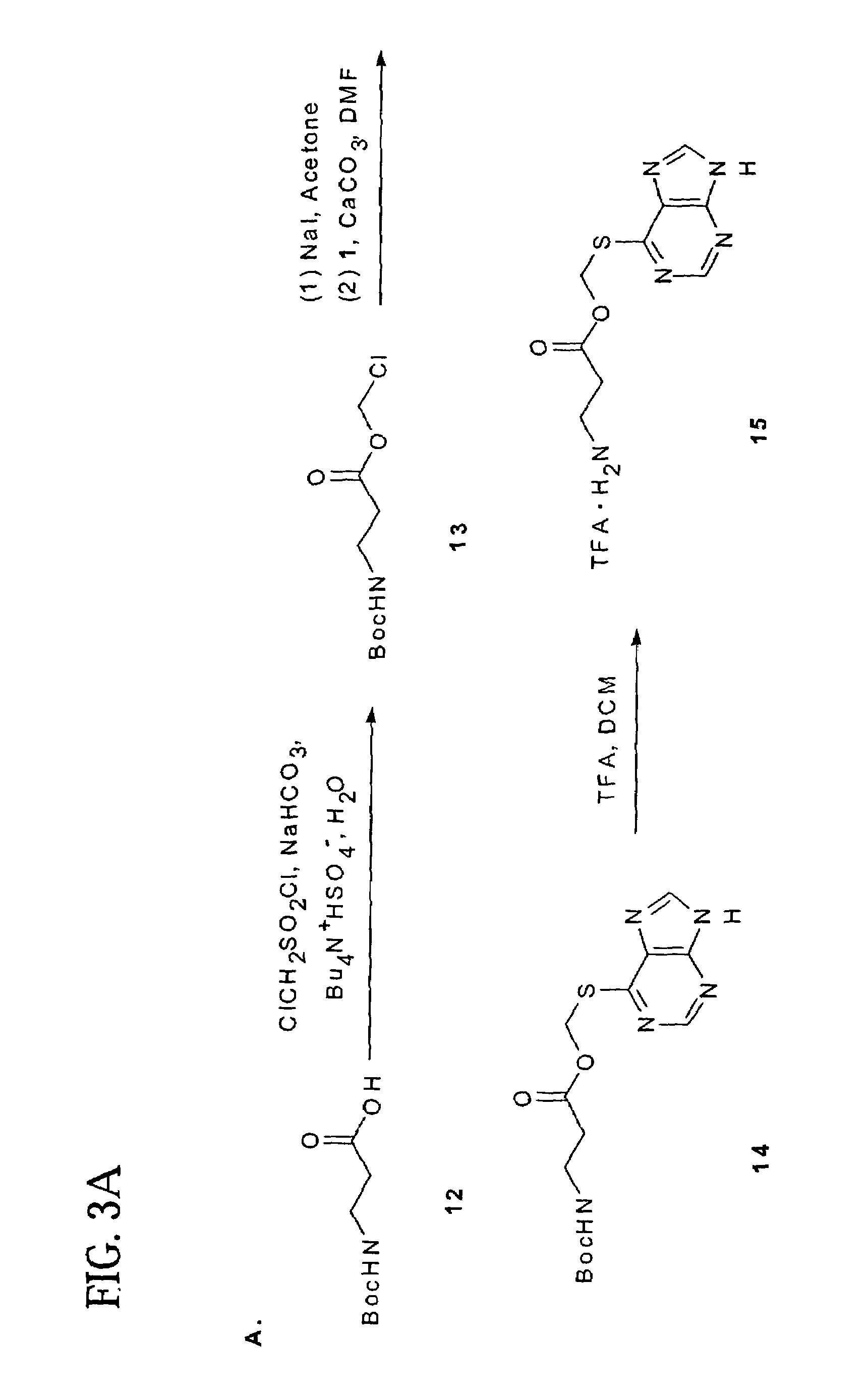
Polymeric thiol-linked prodrugs
InactiveUS7033583B2Pharmaceutical non-active ingredientsSynthetic polymeric active ingredientsPolymeric prodrugThiol
Thiol-linked polymeric prodrugs and methods of making and using the same are disclosed. The use of a sulfhydryl bond as the basic link for linking the polymer to the drug allows a prodrug to be formed which takes advantage of plasma enzymes in vivo. A preferred conjugate is Methods of preparing and treatment are also disclosed.
Owner:BELROSE PHARMA
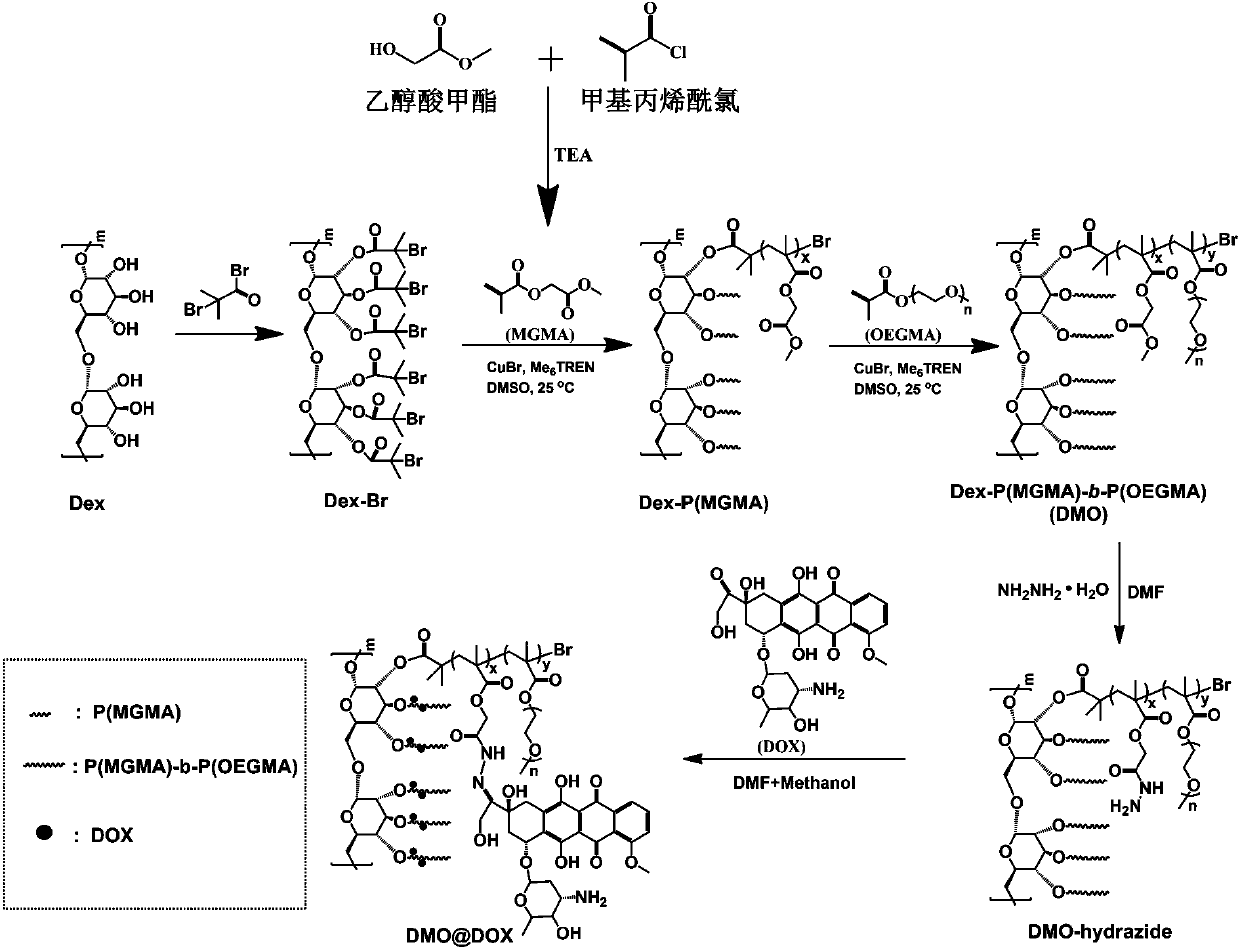
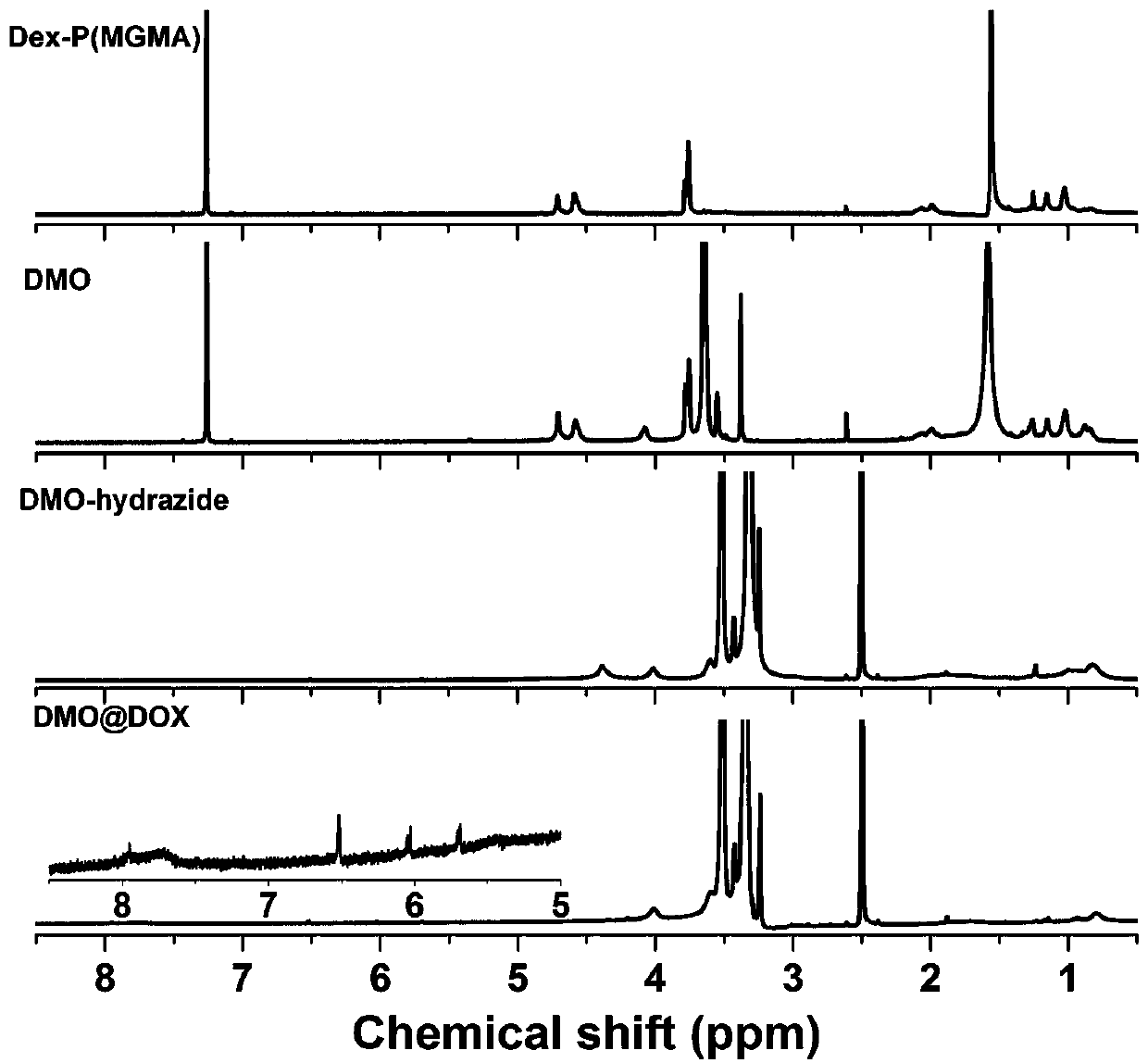
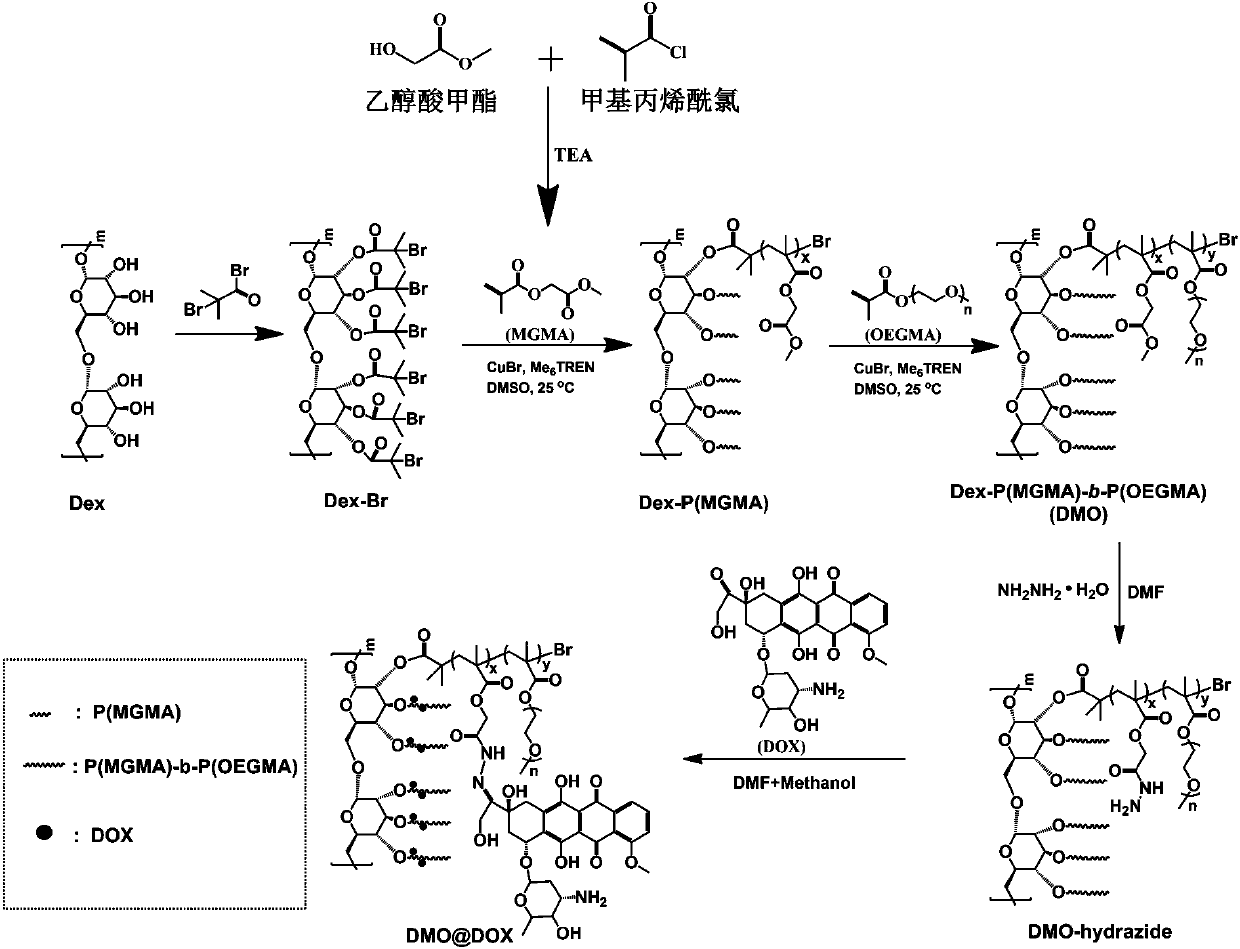
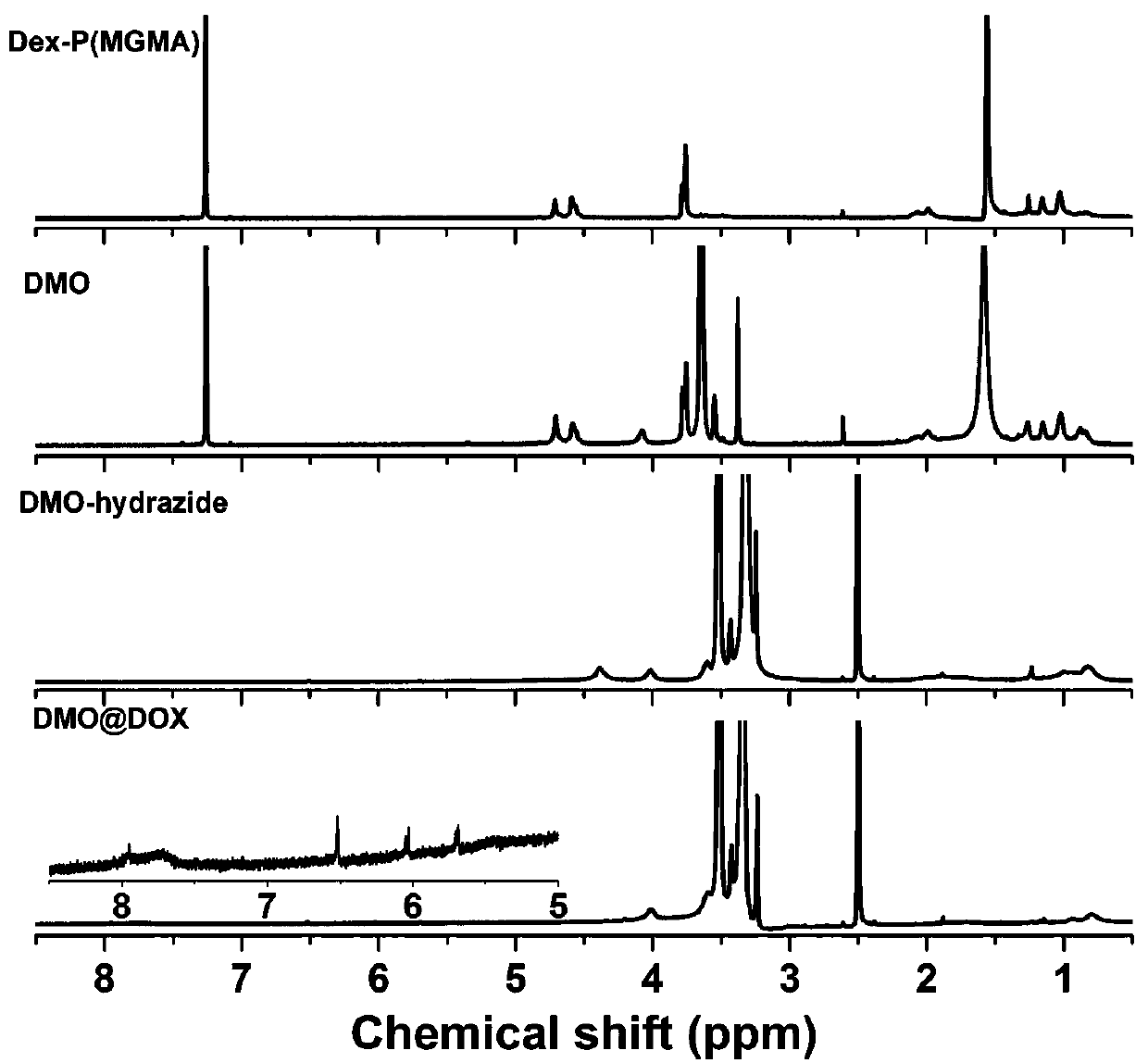
Preparation method of pH-responsive amphiphilic rod-like adriamycin polymer prodrug
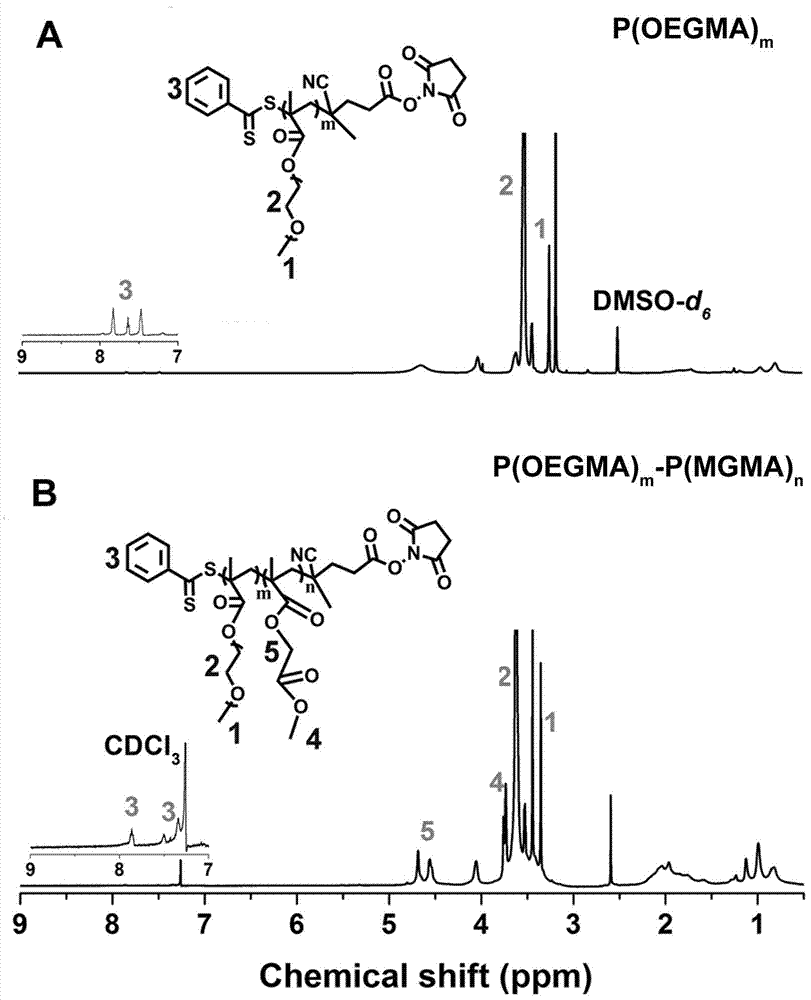
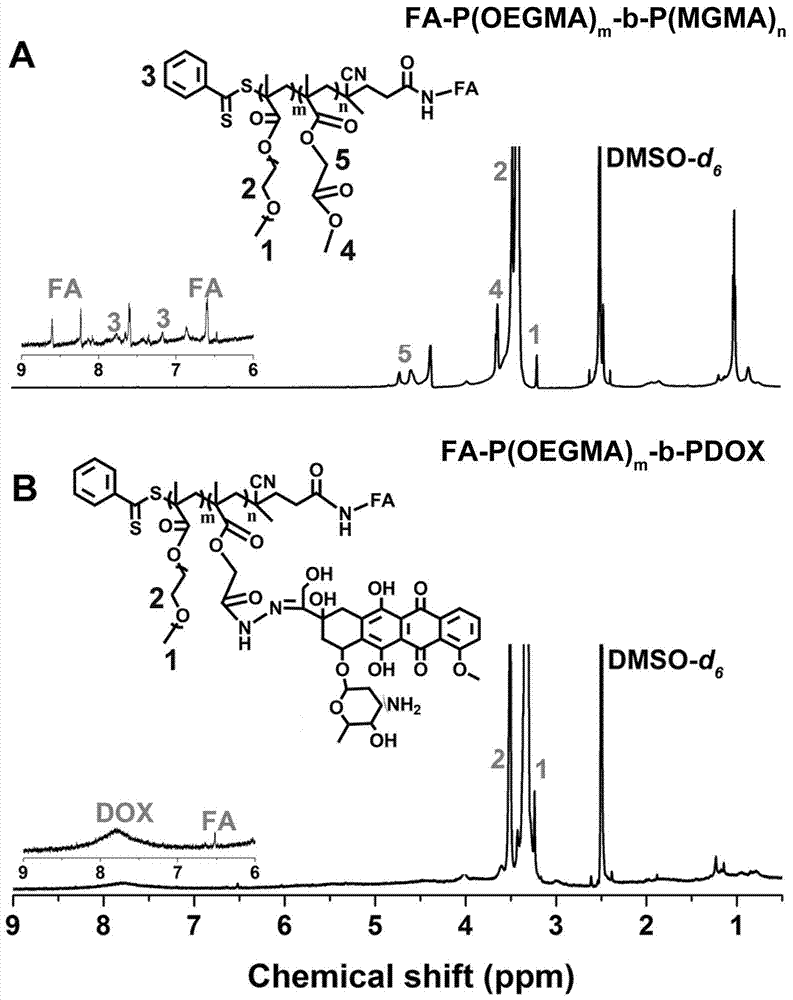
ActiveCN107596383AEasily brokenEffective treatmentOrganic active ingredientsPharmaceutical non-active ingredientsChemical synthesisPolymeric prodrug
The invention relates to the fields of chemical synthesis and biological representation, and more particularly, provides a preparation method and an application of a pH-responsive amphiphilic rod-likepolymer prodrug, which is represented as the drawing in the specification. The preparation method of the amphiphilic rod-like polymer material includes the following steps: 1) synthesizing a rod-likeATRP initiator on the basis of glucan; 2) introducing a pH-responsive hydrophobic block on the basis of ATRP reaction; 3) introducing a hydrophilic block on the basis of ATRP reaction to obtain an amphiphilic polymer material; 4) substituting an ester group on the terminal of MGMA by means of hydrazine hydrate, thus producing a pH-responsive precursor; 5) forming a hydrazone bond by means of a carbonyl group on adriamycin and an amino group on the polymer material, thus producing the pH-responsive polymer prodrug. By means of weak acidity of interior of cancer cells, the amphiphilic rod-likepolymer prodrug can selectively release a drug by means of pH-stimulating response. The polymer prodrug is high in micelle stability, is high in drug carrying capacity and enables drug release to be under stimulating response control.
Owner:SOUTHWEST UNIVERSITY
Nano-carrier for drug and gene co-delivery and preparation method and application thereof
ActiveCN106957436AIncrease savingsReduced effectOrganic active ingredientsPharmaceutical non-active ingredientsPolymeric prodrugNanocarriers
The invention discloses a nano-carrier for drug and gene co-delivery and a preparation method and application thereof, and particularly relates to a co-delivery nano-carrier TCPL-siRNA-PPX for simultaneously carrying a chemotherapeutic drug and a gene-based drug. A polymeric prodrug carrier TCPL, siRNA and a multifunctional polyanionic polymer PPX are self-assembled by electrostatic adsorption between ingredients to form the drug delivery system; a drug and a gene can be targeted to be delivered to the same tumor cell, and moreover, siRNA is released in cytoplasm to silence the Bc1-2 protein, promoting apoptosis and releasing the inhibition of Bcl-2 on lonidamine; the chemotherapeutic drug acting on mitochondria, lonidamine, is delivered to the mitochondria; both cooperatively trigger the apoptosis of the mitochondrial pathway, jointly killing the tumor cell. By in-vitro and in-vivo activity evaluation, the invention proves that the system is better than the simultaneous delivery of each single component, can remarkably increase anti-cancer activity, and has a definite synergistic treatment effect.
Owner:CHINA PHARM UNIV
Treatment of non-hodgkin's lymphomas with multi-arm polymeric conjugates of 7-ethyl-10-hydroxycamtothecin
The present invention relates to methods of treatment of non-Hodgkin's lymphomas. The present invention includes administering polymeric prodrugs of 7-ethyl-10-hydroxy-camptothecin to patients in need thereof.
Owner:BELROSE PHARMA
Preparation method of linear diblock polymeric prodrugs with pH simulative responsibility
ActiveCN107236100AIncrease upload volumeAchieve targeted deliveryOrganic active ingredientsPharmaceutical non-active ingredientsSolubilitySide effect
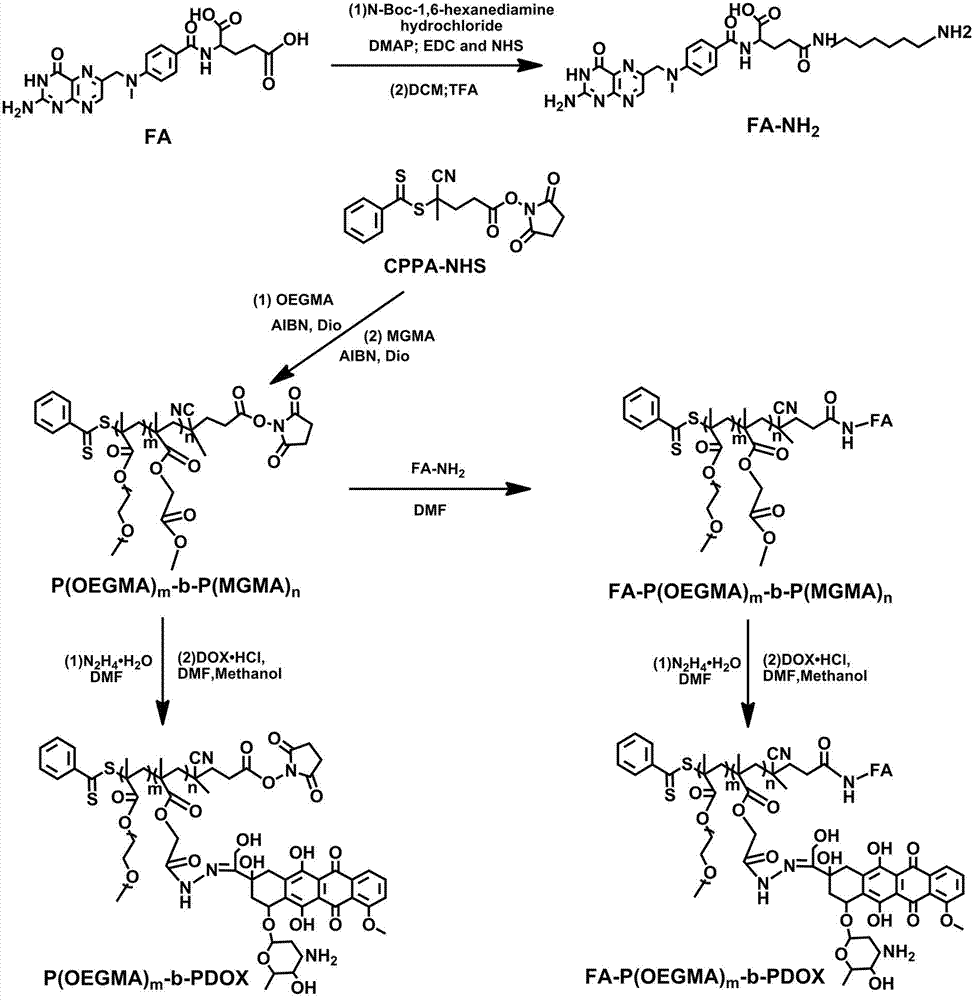
The invention discloses a preparation method of linear diblock polymeric prodrugs with pH simulative responsibility and in-vitro activity of the prodrugs with pH simulative responsibility. The preparation method is characterized in that with an RAFT (reversible addition and fragmentation chain transfer) polymerization reaction as a main reaction, different polymeric prodrugs are synthesized by changing the proportion of a hydrophilic block and a hydrophobic block, and folic acid is further used to partially modify and synthesize a drug delivery system with pH simulative responsibility and targeting performance. An experiment proves that the system has high drug load capacity, good water solubility and low toxic and side effects and has accurate and efficient cancer treatment potential, and the utilization rate of the drugs is effectively increased.
Owner:SOUTHWEST UNIVERSITY
Methods for inhibiting angiogenesis with multi-arm polymeric conjugates of 7-ethyl-10-hydroxycamptothecin
InactiveUS20120122956A1Reduce adverse effectsSure easyBiocidePharmaceutical non-active ingredientsDiseasePolymeric prodrug
The present invention relates to methods of inhibiting angiogenesis in mammals. The present invention includes administering polymeric prodrugs of 7-ethyl-10-hydroxycamptothecin to the mammals in need thereof. The present invention also relates to methods of treating a disease associated with angiogenesis in mammals by administering polymeric prodrugs of 7-ethyl-10-hydroxycamptothecin to the mammals in need thereof.
Owner:BELROSE PHARMA
Camptothecin prodrug monomer and polymeric prodrug amphipathic molecules thereof as well as preparation method and application of camptothecin prodrug monomer and polymeric prodrug amphipathic molecules
ActiveCN103524519BGood water solubilityImprove stabilityOrganic active ingredientsOrganic chemistryDisulfide bondingPolymeric prodrug
Owner:UNIV OF SCI & TECH OF CHINA
Heterobifunctional Polymeric Bioconjugates
InactiveUS20080076792A1Extended half-lifeImprove solubilityBiocideCarbamic acid derivatives preparationPolymeric prodrugMonoclonal antibody
Heterobifunctional polymeric prodrug platforms for delivering biologically active compounds, including proteins, monoclonal antibodies and the like are disclosed. One preferred compound is Methods of making and using the compounds and conjugates described herein are also provided.
Owner:BELROSE PHARMA
Polymeric thiol-linked prodrugs employing benzyl elimination systems
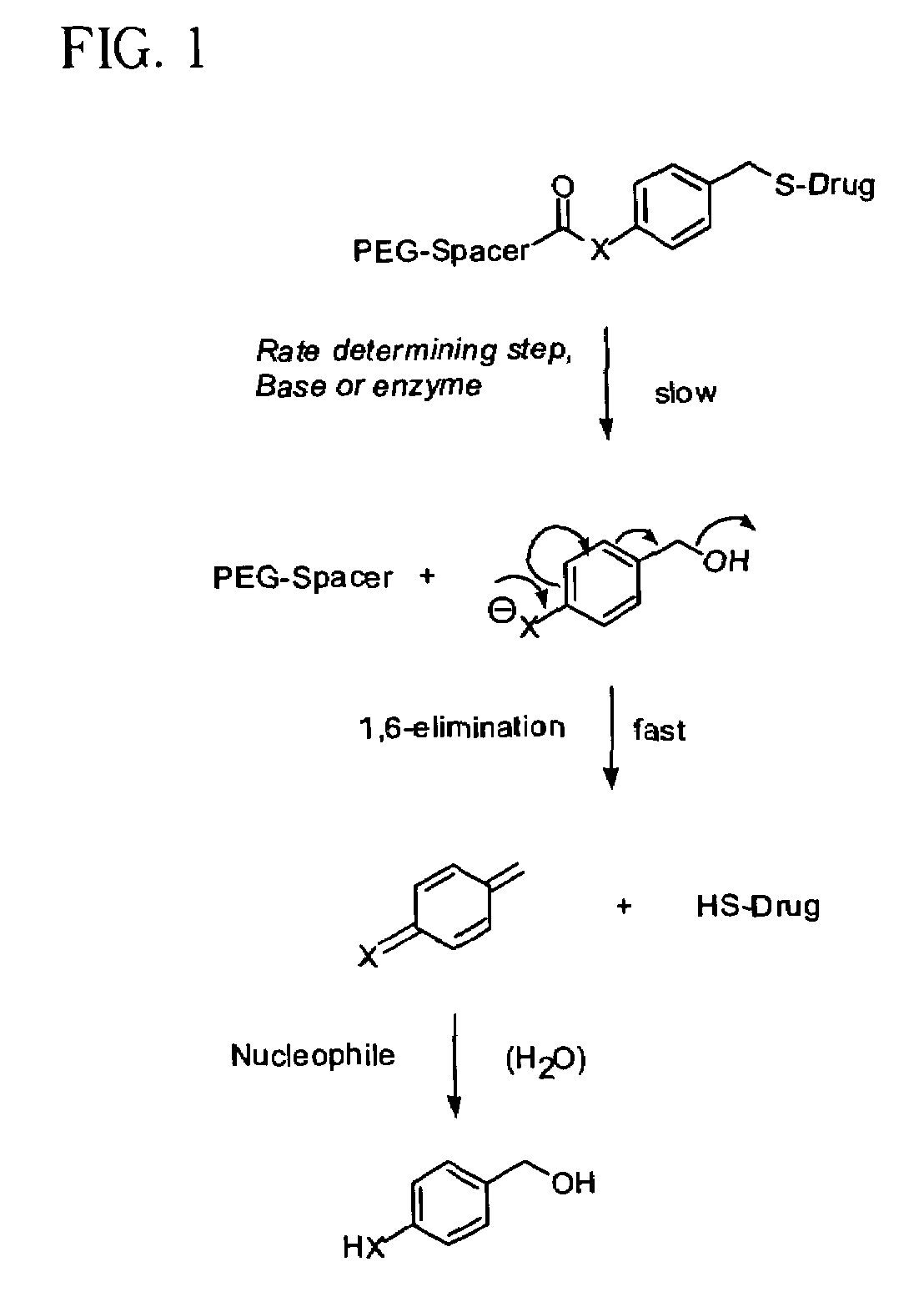
InactiveUS7262164B2Increase loadHigh payload per unitNervous disorderOrganic chemistryPolymeric prodrugThiol
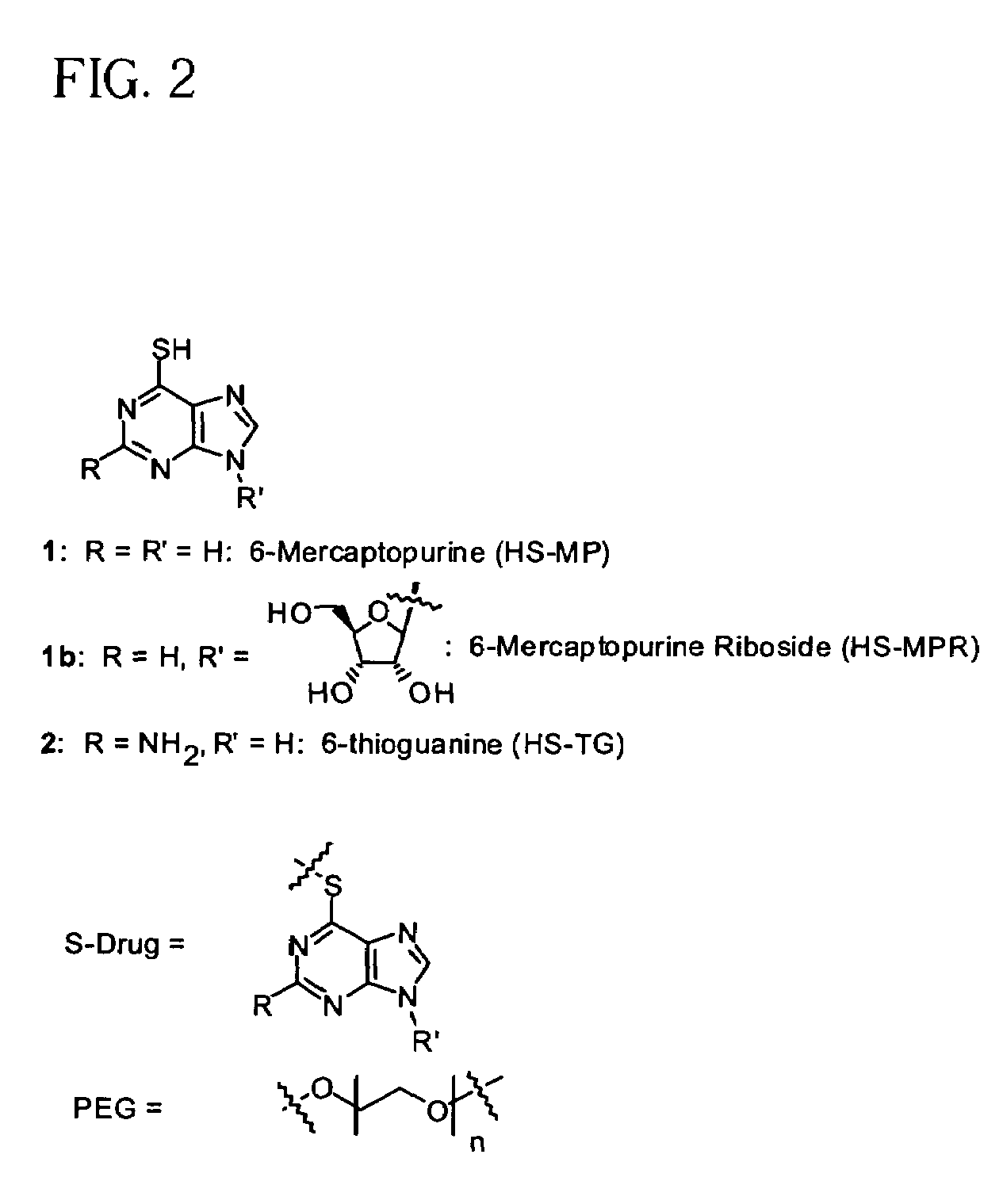
Thiol-linked polymeric prodrugs, methods of making and using the same are disclosed. The use of a sulfhydryl bond in combination with a benzyl elimination system results in the formation of prodrugs which can take advantage of plasma enzymes in vivo for regeneration of the parent molecule. A preferred prodrug in accordance with the invention is:where S-MP is 6-mercaptopurine.
Owner:BELROSE PHARMA
Multi-branched polymeric prodrugs and their applications
ActiveCN104650342BImprove solubilityExtended half-lifeOrganic active ingredientsPharmaceutical non-active ingredientsPolymeric prodrugCancer cell
The present invention relates to multi-branched polymeric prodrugs and applications thereof. Specifically, the present invention relates to a multi-branched polymeric prodrug having a structure of formula (I) or a pharmaceutically acceptable salt thereof, which is effective for topoisomerase I-related diseases in mammalian subjects, In particular, solid tumors in mammalian subjects have very good therapeutic effects. In the case of the same in vitro activity as inhibiting the proliferation of cancer cells, it has a more reasonable half-life and residence time of the drug in the body, greatly reduces the clearance rate, is more conducive to determining the way of drug administration, and reduces the number of administrations. It is expected to be developed into a new drug. A generation of antineoplastic drugs.
Owner:JIANGSU HANSOH PHARMA CO LTD +1
Chitosan-based multi-environment-responsive type polymeric prodrug micelle and preparation method thereof
ActiveCN111419805AHigh drug loadingImprove solubilityPowder deliveryOrganic active ingredientsPolymer sciencePolymeric prodrug
The invention discloses a chitosan-based multi-environment-responsive type polymeric prodrug micelle and a preparation method thereof. The preparation method of the chitosan-based multi-environment-responsive type polymeric prodrug micelle comprises the steps that, a folic acid-chitosan conjugate and amino-terminated poly(N-isopropylacrylamide) are used to prepare folic acid-chitosan-poly(N-isopropylacrylamide); a grafted polymer of gambogic acid and the folic acid-chitosan-poly(N-isopropylacrylamide) is prepared; and the grafted polymer is dissolved in dimethyl sulfoxide and slowly dripped into water, and the solution is subjected to dialysis and freeze-drying to obtain the micelle. Chitosan is the main component, respectively links with folic acid and the poly(N-isopropylacrylamide) which is a thermosensitive polymer material via chemical bonding, and links with the gambogic acid which is an antitumor medicine via an ester bond. A formed polymeric prodrug is multi-environment-responsive to pH, temperature and esterase.
Owner:XUZHOU MEDICAL UNIV
Polymeric prodrugs and subcutaneous and/or intramuscular administration thereof
PendingUS20200353090A1Increase viscosity of formulationControl quantityOrganic active ingredientsPharmaceutical delivery mechanismPolymeric prodrugThermosensitive polymer
The invention relates to new prodrugs of active molecules. These prodrugs allow, in particular, the subcutaneous or intramuscular administration of active molecules of which the subcutaneous or intramuscular administration is problematic or impossible, in particular because of the toxicity at the injection site. The prodrugs according to the invention comprise an active ingredient, covalently linked with a polymer chain, preferably a hydrophilic and / or thermosensitive polymer chain. The invention relates, in particular, to polymeric prodrugs comprising a polymer chain formed at least in part by acrylamide monomer or one of its derivatives, the polymer comprising a proximal part and a terminal part; a first pharmaceutically active molecule covalently coupled to the proximal part of the polymer; possibly a second pharmaceutically active molecule covalently coupled to the terminal part of the polymer.
Owner:CENT NAT DE LA RECHERCHE SCI +1
A redox-responsive hyperbranched polyprodrug nanomicelle and its preparation method and application
ActiveCN110859966BQuick releaseLow cytotoxicityAntipyreticAnalgesicsDisulfide bondingPolymeric prodrug
The invention provides a redox-responsive hyperbranched polyprodrug nano-micelle and its preparation method and application. In the invention, the hydrophobic drug is connected with the disulfide bond that can be cut by high GSH, and then the redox-responsive amphiphilic hyperbranched polyprodrug nano-micelle is synthesized through two-step RAFT polymerization. The redox-responsive hyperbranched polyprodrug nano-micelle of the present invention has the advantages of simple preparation process, good stability, prolonged blood circulation time, improved bioavailability of drugs, and the like. Moreover, the nano-micelle can break under the reducing environment of high GSH concentration in tumor cells, release hydrophobic drugs rapidly, and inhibit the proliferation of tumor cells. The redox-responsive hyperbranched polyprodrug nanomicelles of the present invention provide a new option for tumor therapy.
Owner:FUZHOU UNIV
Preparation method of a class of pH-responsive amphiphilic rod-shaped doxorubicin polymer prodrug
ActiveCN107596383BIncrease upload volumeImprove stabilityOrganic active ingredientsPharmaceutical non-active ingredientsChemical synthesisPolymeric prodrug
The invention relates to the fields of chemical synthesis and biological representation, and more particularly, provides a preparation method and an application of a pH-responsive amphiphilic rod-likepolymer prodrug, which is represented as the drawing in the specification. The preparation method of the amphiphilic rod-like polymer material includes the following steps: 1) synthesizing a rod-likeATRP initiator on the basis of glucan; 2) introducing a pH-responsive hydrophobic block on the basis of ATRP reaction; 3) introducing a hydrophilic block on the basis of ATRP reaction to obtain an amphiphilic polymer material; 4) substituting an ester group on the terminal of MGMA by means of hydrazine hydrate, thus producing a pH-responsive precursor; 5) forming a hydrazone bond by means of a carbonyl group on adriamycin and an amino group on the polymer material, thus producing the pH-responsive polymer prodrug. By means of weak acidity of interior of cancer cells, the amphiphilic rod-likepolymer prodrug can selectively release a drug by means of pH-stimulating response. The polymer prodrug is high in micelle stability, is high in drug carrying capacity and enables drug release to be under stimulating response control.
Owner:SOUTHWEST UNIV
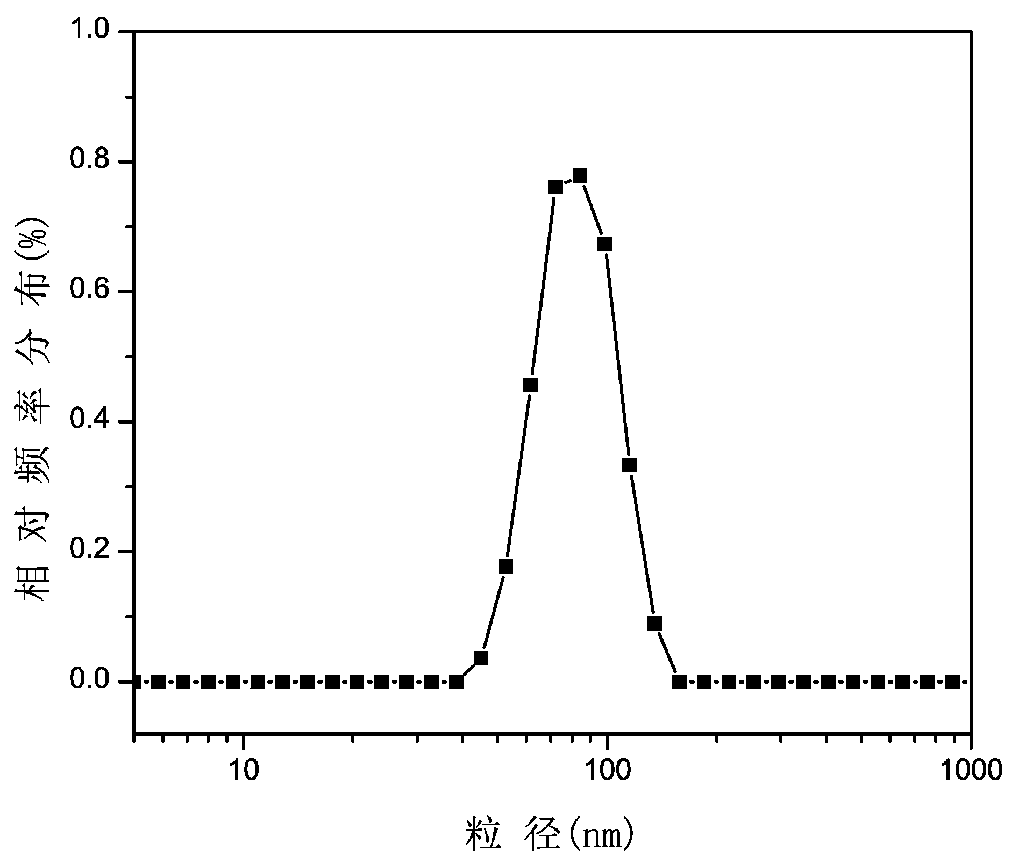
Method for preparing nano particles of camptothecin polymeric prodrug amphipathic molecules as well as product and application of nano particles
ActiveCN103520110BHigh drug loadingToxicPowder deliveryOrganic active ingredientsPolymeric prodrugDrug loading dose
The invention relates to a preparation method for different nano particles of reducing response camptothecin polymeric prodrug amphipathic molecules represented in a formula I as well as a product and an application of the nano particles, wherein morphology of a polymeric prodrug amphipathic molecule final assembly can be controlled just by regulating the kind of a solvent and through a way of adding water and regulating a water adding speed, so as to obtain four typical nano structures: spherical structure, disc-shaped structure, flower-shaped compound vesicle and staggered accumulated lamellar nano particles. The nano structures have high drug loading rate (more than 50wt%); compared with the most studied spherical nano particle at present, an assembly of the staggered accumulated lamellar structure is the longest in blood circulation time, followed by the disc-shaped nano particles. In addition, non-spherical particle is greater in tumor cytotoxicity compared with the spherical nano particles. In the formula I, various is independently represent 2 or 3; various Xes independently represent O or NH; R is H, CH3 or CH2CH3; m ranges from 4 to 400; and n ranges from 2 to 300; and number-average molecular weight of the reducing response camptothecin polymeric prodrug amphipathic molecule is 1200-220000.
Owner:UNIV OF SCI & TECH OF CHINA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com