Preparation method of a class of pH-responsive amphiphilic rod-shaped doxorubicin polymer prodrug
A doxorubicin and amphiphilic technology, which is applied in the field of amphiphilic rod-shaped polymeric prodrugs and their preparation, can solve the problems of strong toxic and side effects, non-selectivity, low medical efficiency and the like, and achieve the effect of effective treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
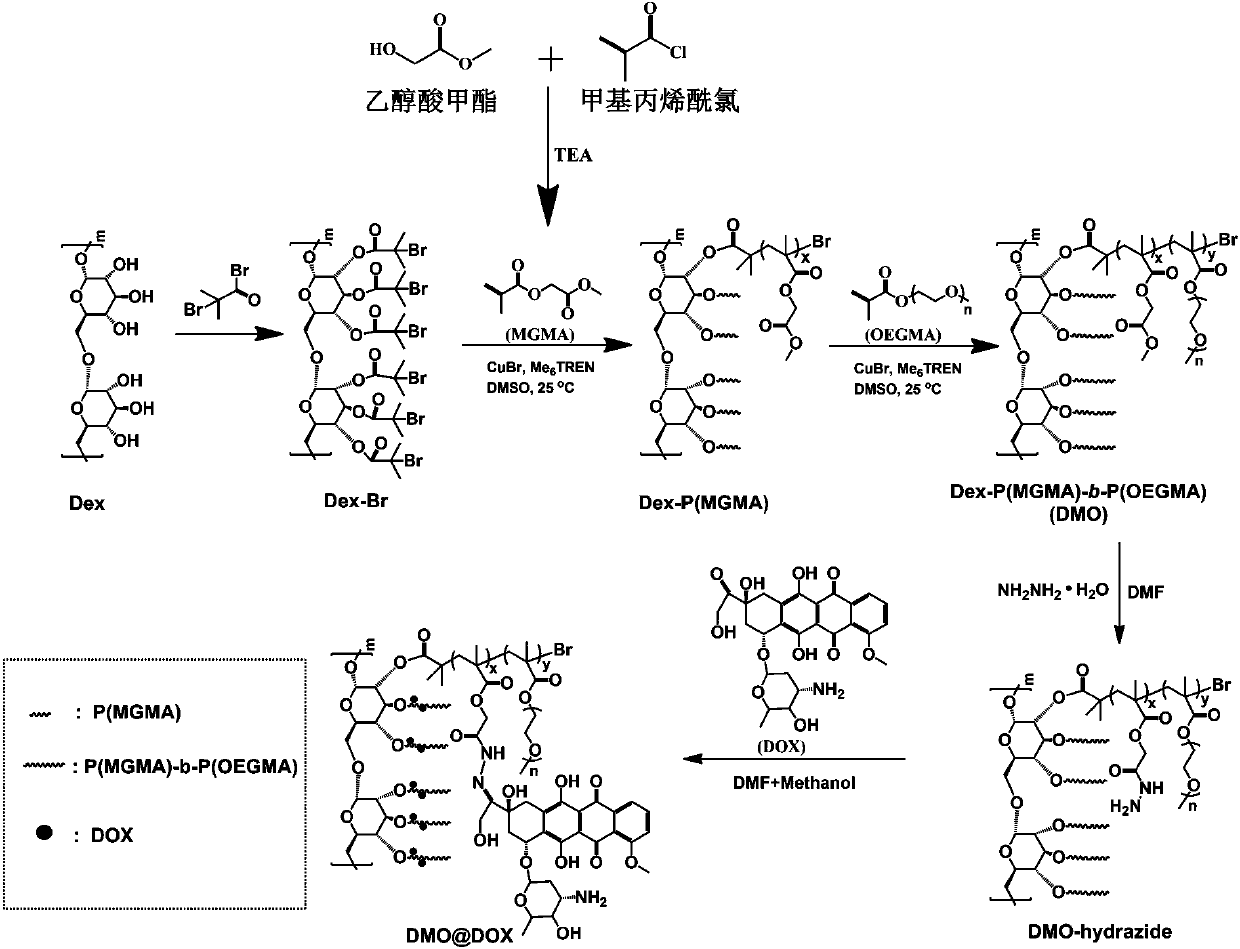
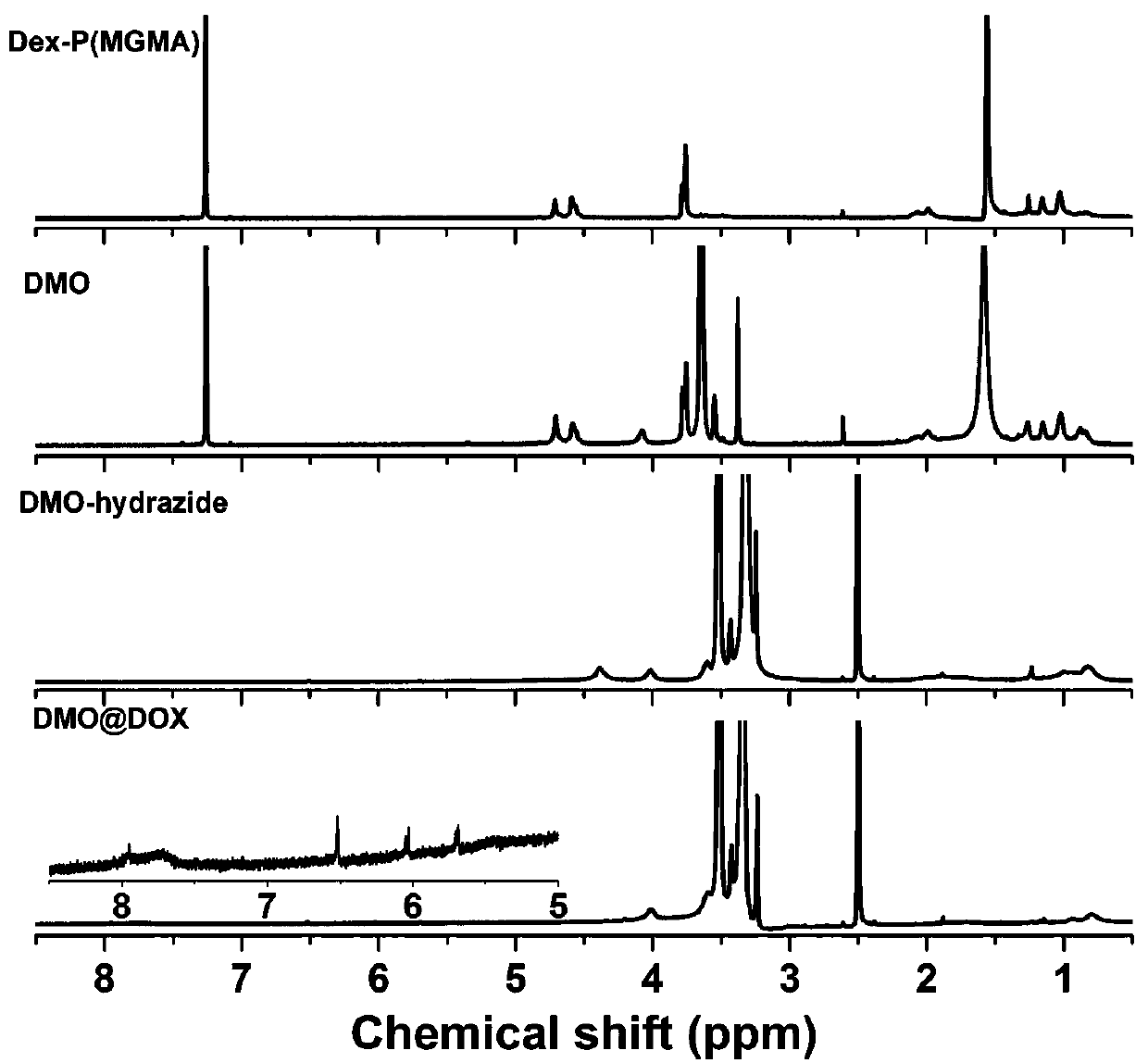
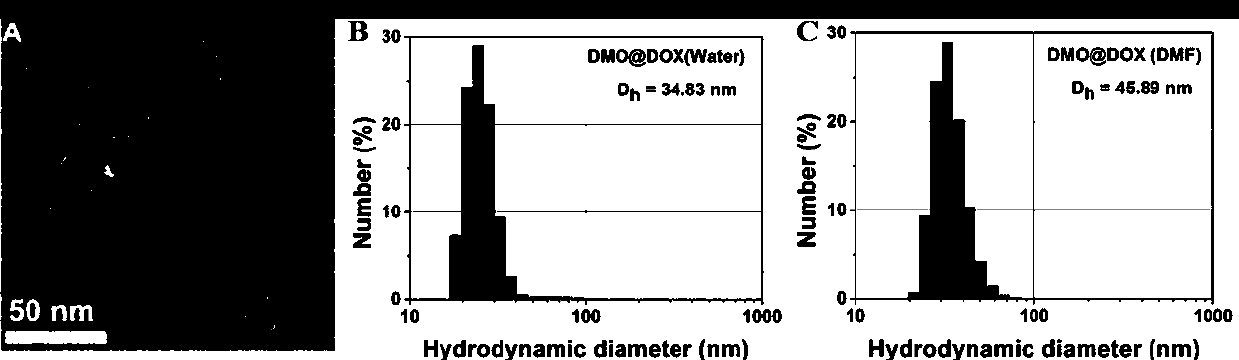
[0040] Example 1 Preparation of pH-responsive Dex-P(MGMA)-b-P(OEGMA) amphiphilic rod-like polymeric prodrug
[0041] (1) Preparation of Dex-Br: Under the condition of argon atmosphere, the dextran (Dex) dissolved in the ionic liquid (1-allyl-3-methylimidazole chloride) was cooled to 0°C, and then N - A mixed solution of methylpyrrolidone (NMP) and N,N-dimethylformamide (DMF), then slowly add 2-bromoisobutyryl bromide (BIBB), ice bath for 0.5-2h, and then rise to room temperature (25 ℃) and react in the dark for 12-72 hours, then precipitate in deionized water, dissolve the precipitate with acetone, and repeat the purification three times to obtain a light yellow intermediate product, which is dried in a vacuum oven (25-30°C), and then the resulting The light yellow intermediate product was dissolved in NMP, cooled to 0°C, then slowly added BIBB, ice-bathed for 0.5-2h, then raised to room temperature (25°C) and reacted in the dark for 12-72h, then precipitated in deionized wate...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com