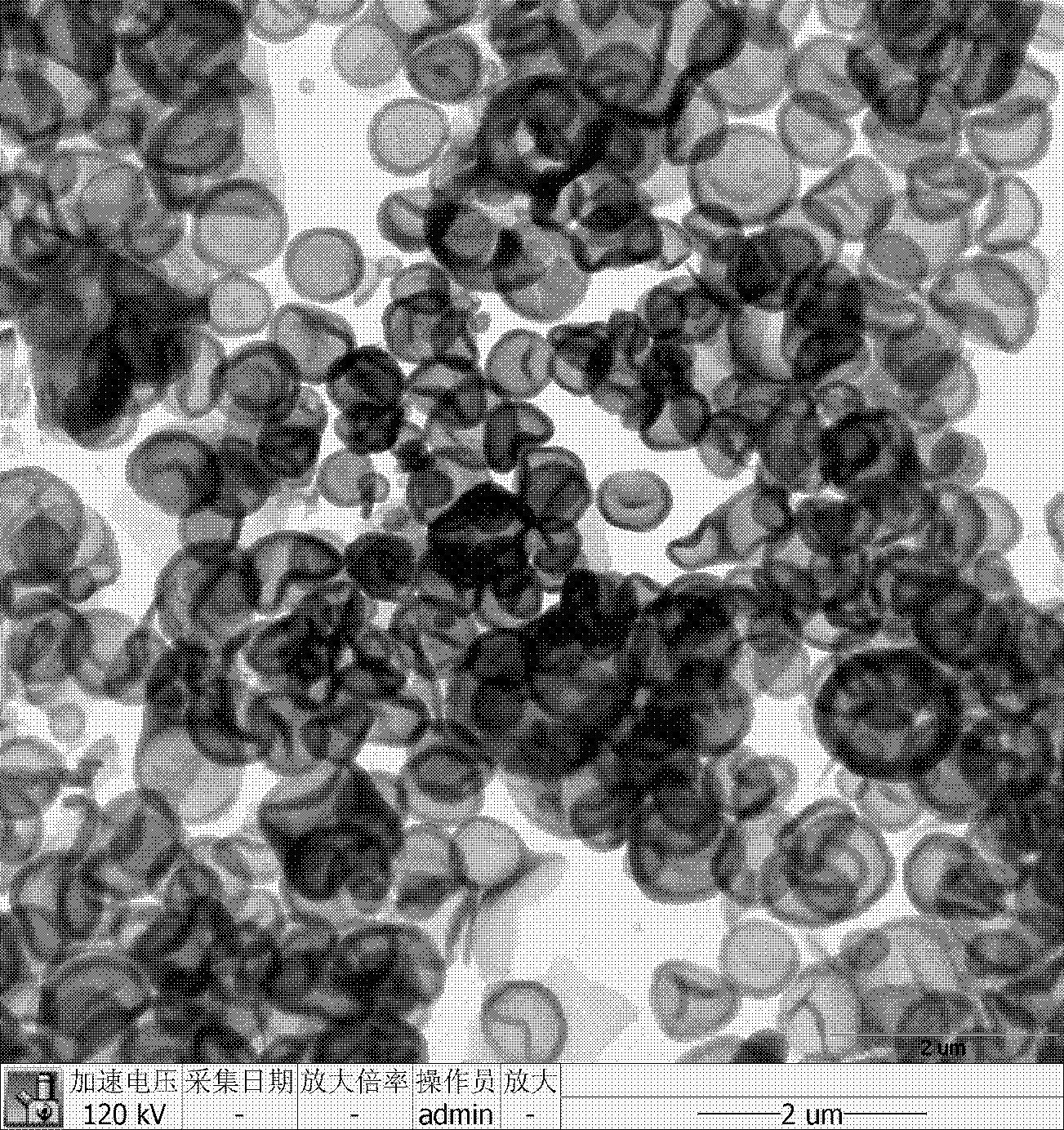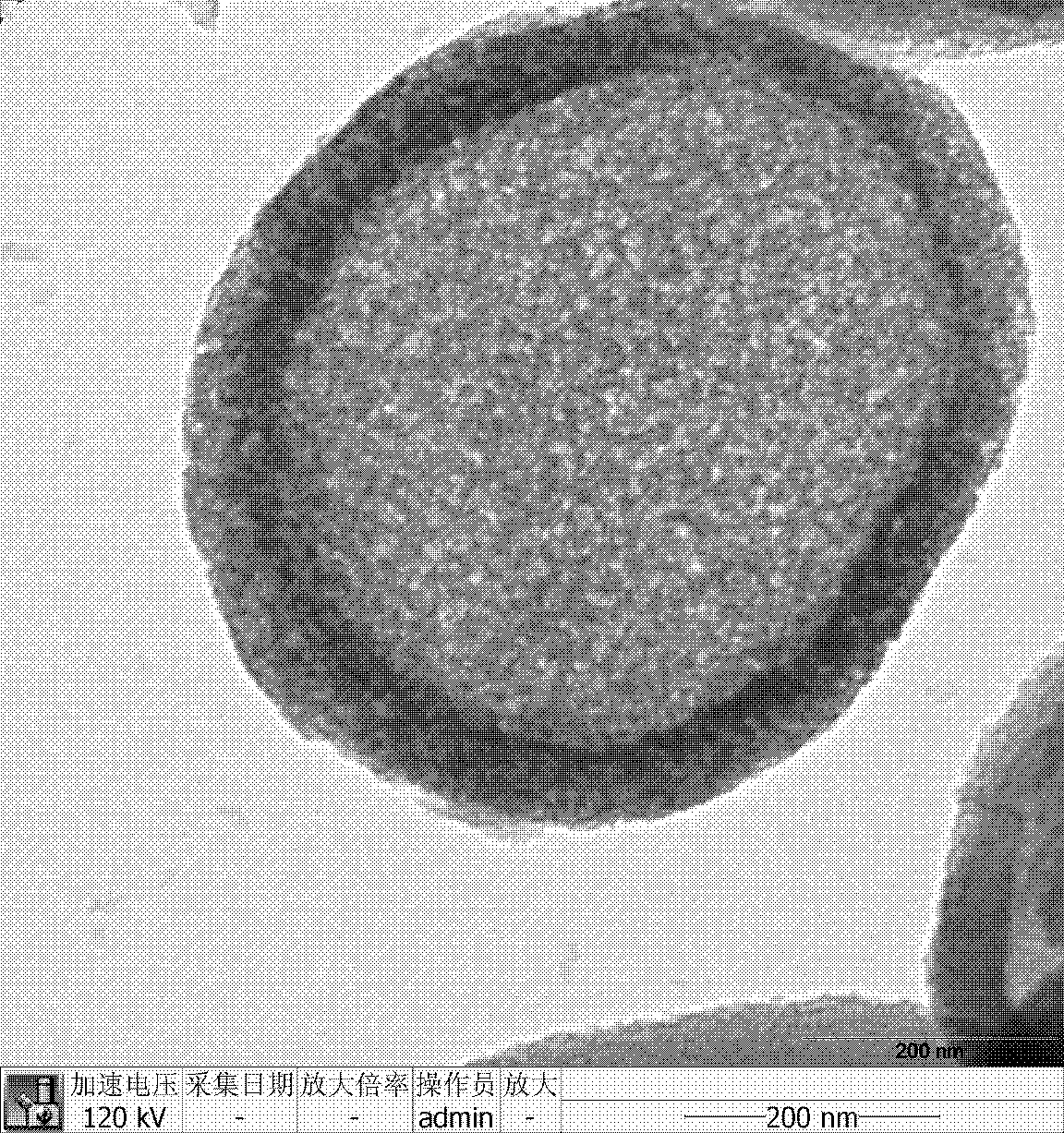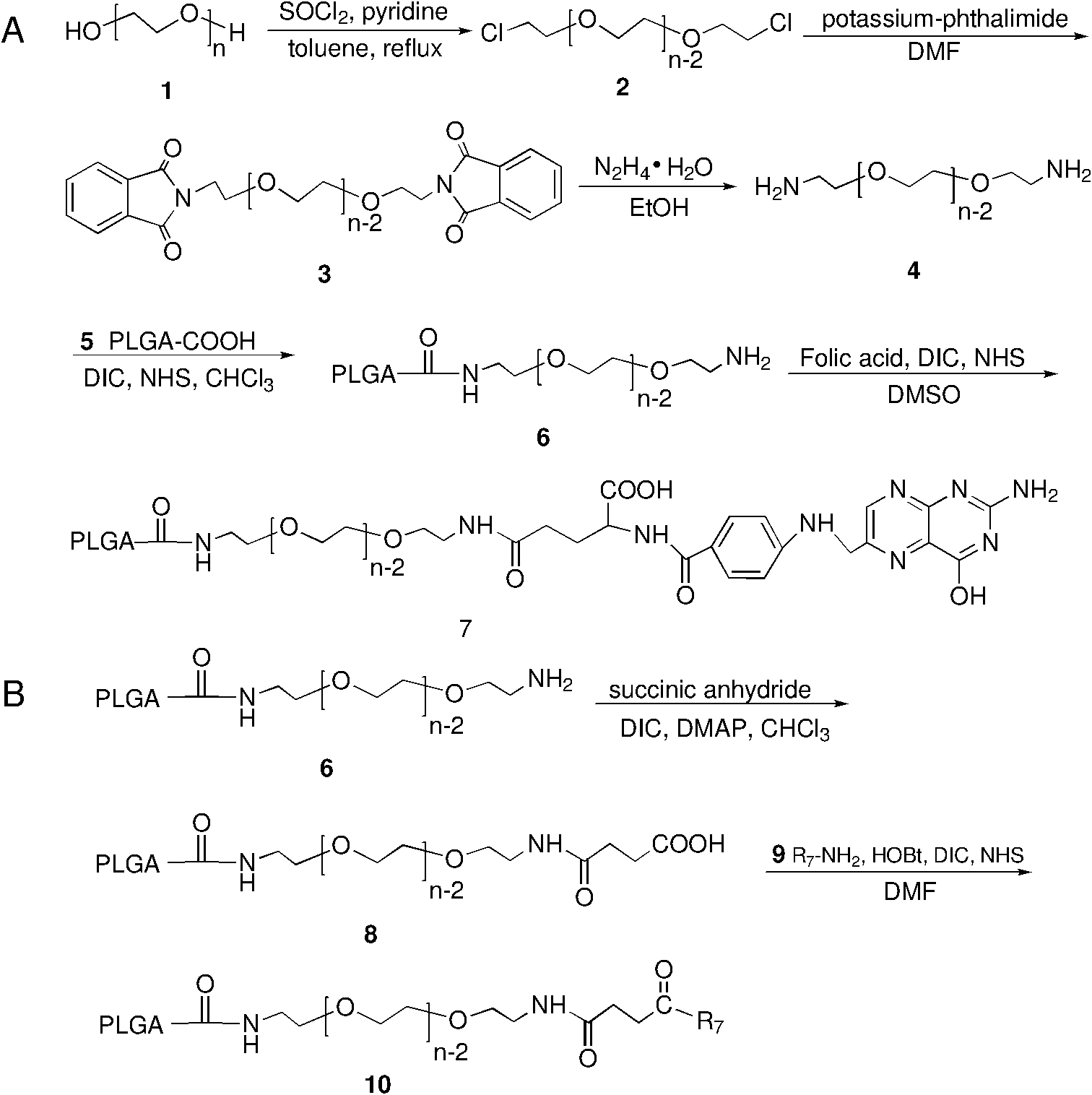Patents
Literature
853 results about "Drug loading dose" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
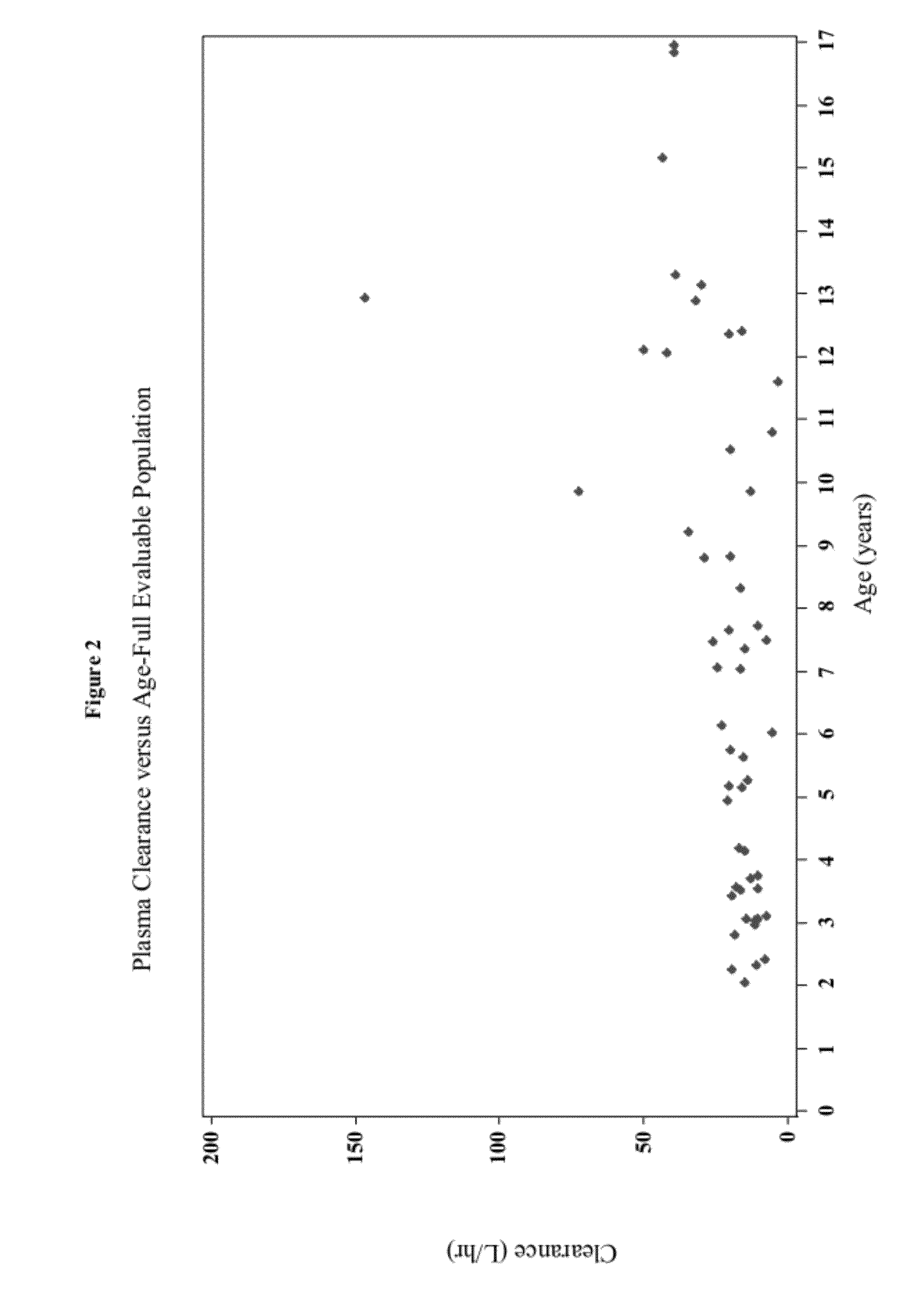
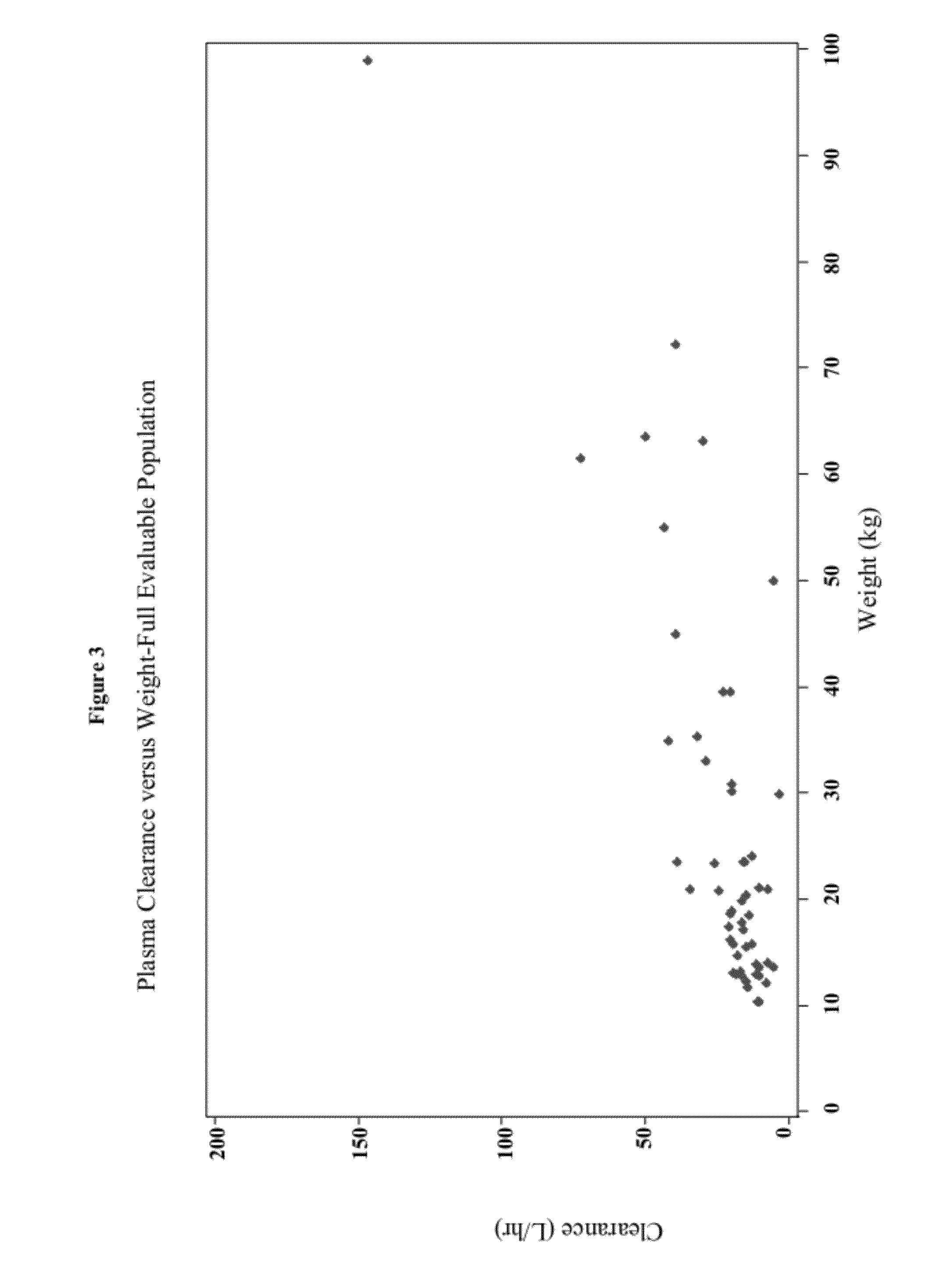
A loading dose is an initial higher dose of a drug that may be given at the beginning of a course of treatment before dropping down to a lower maintenance dose.
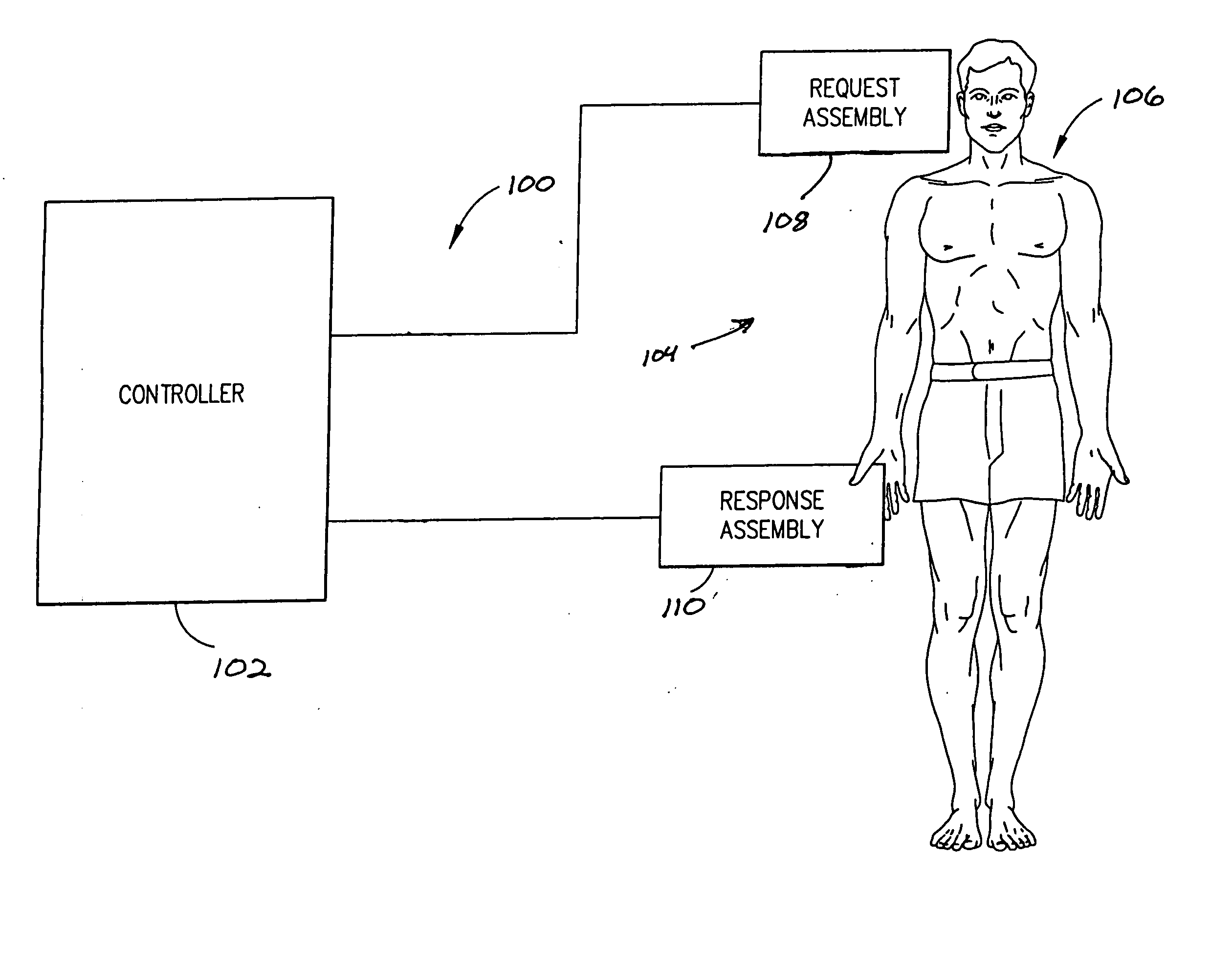
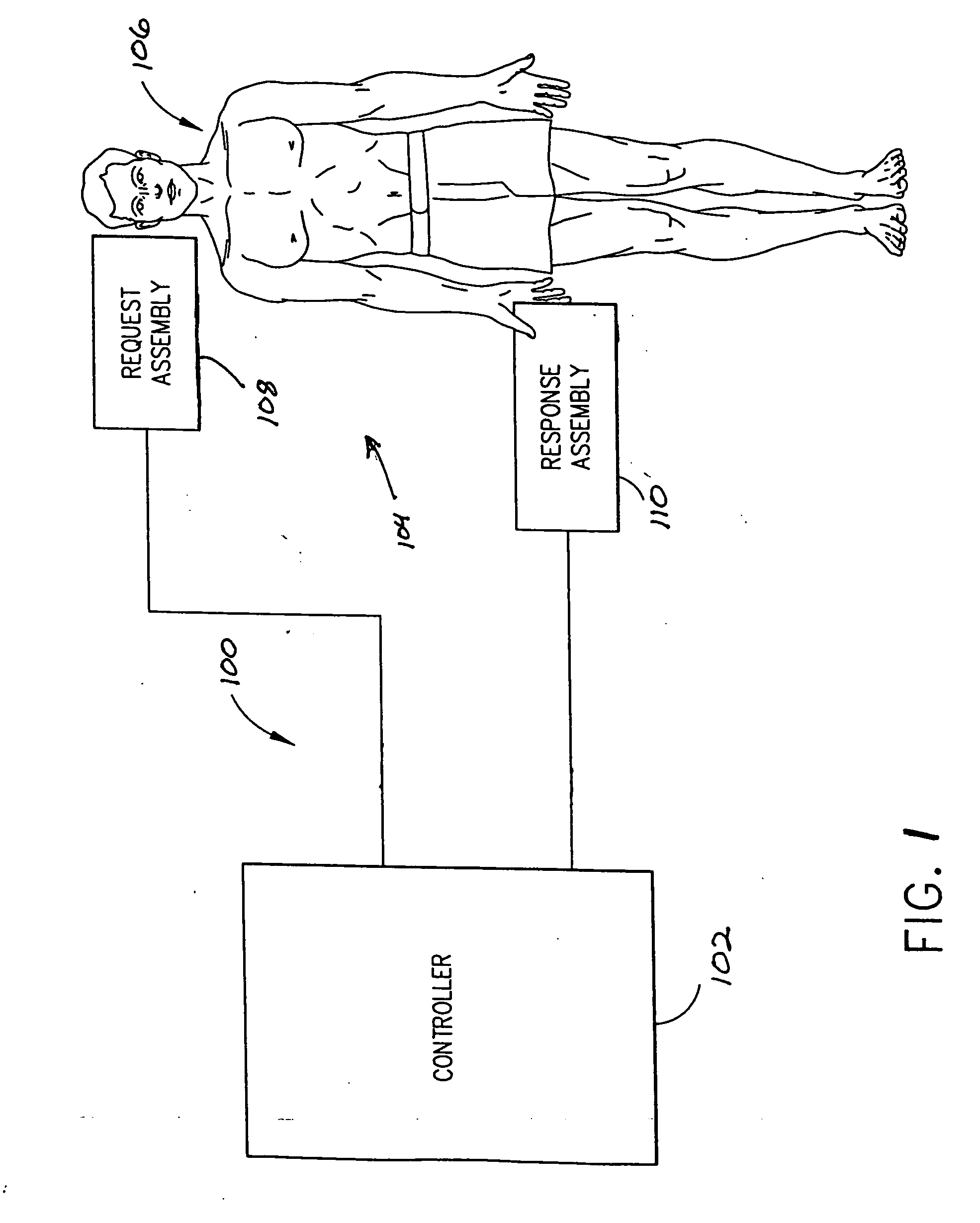
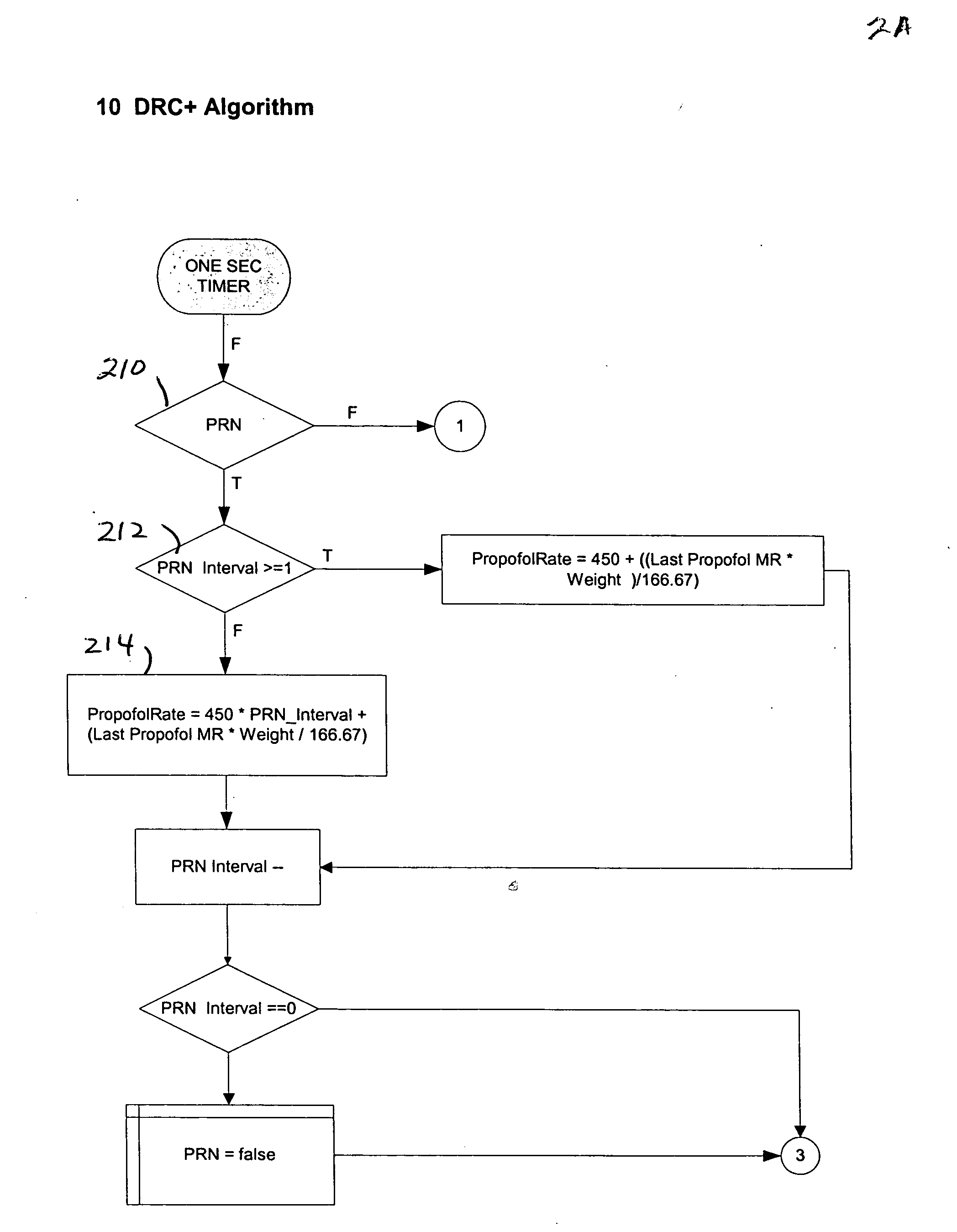
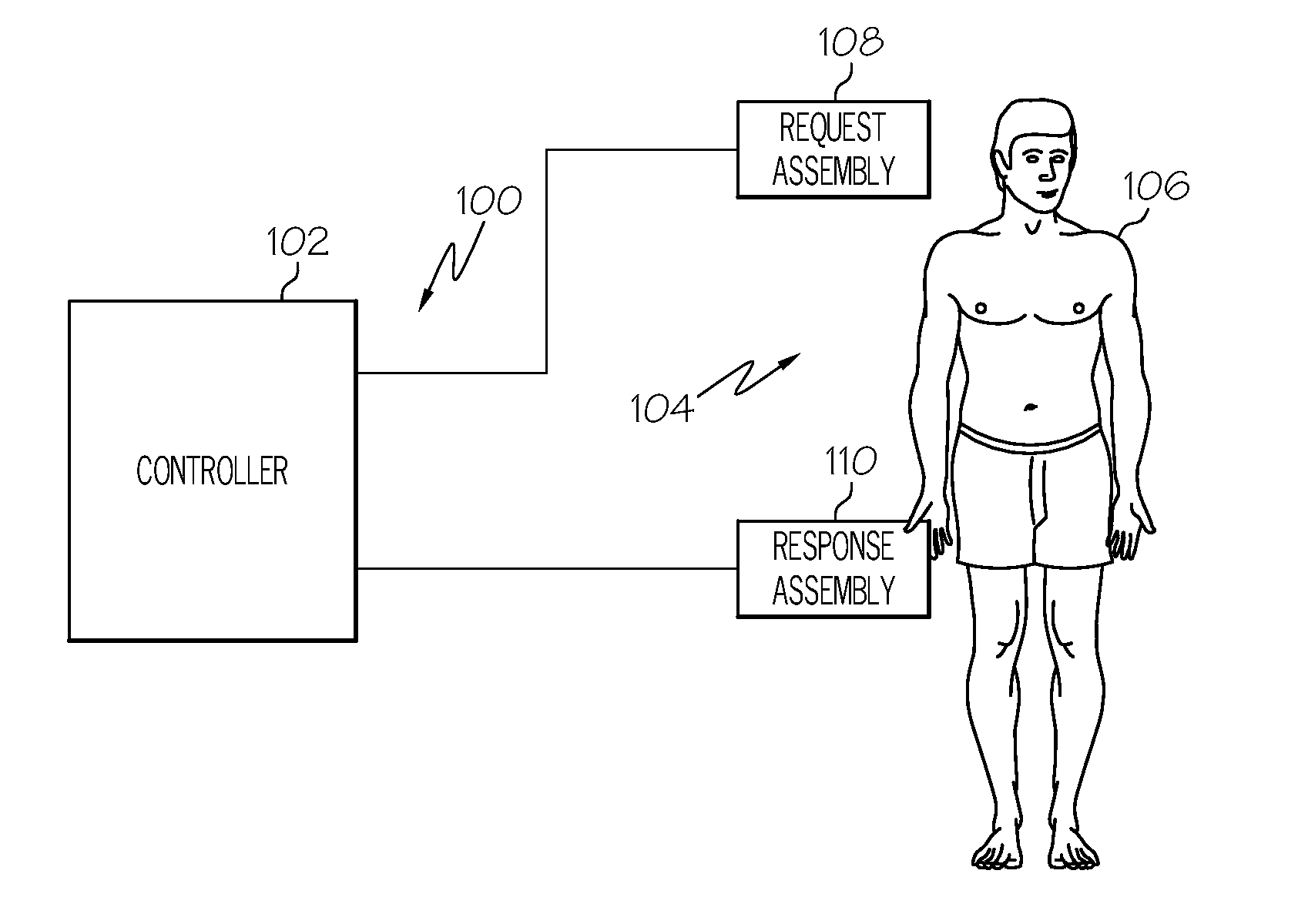
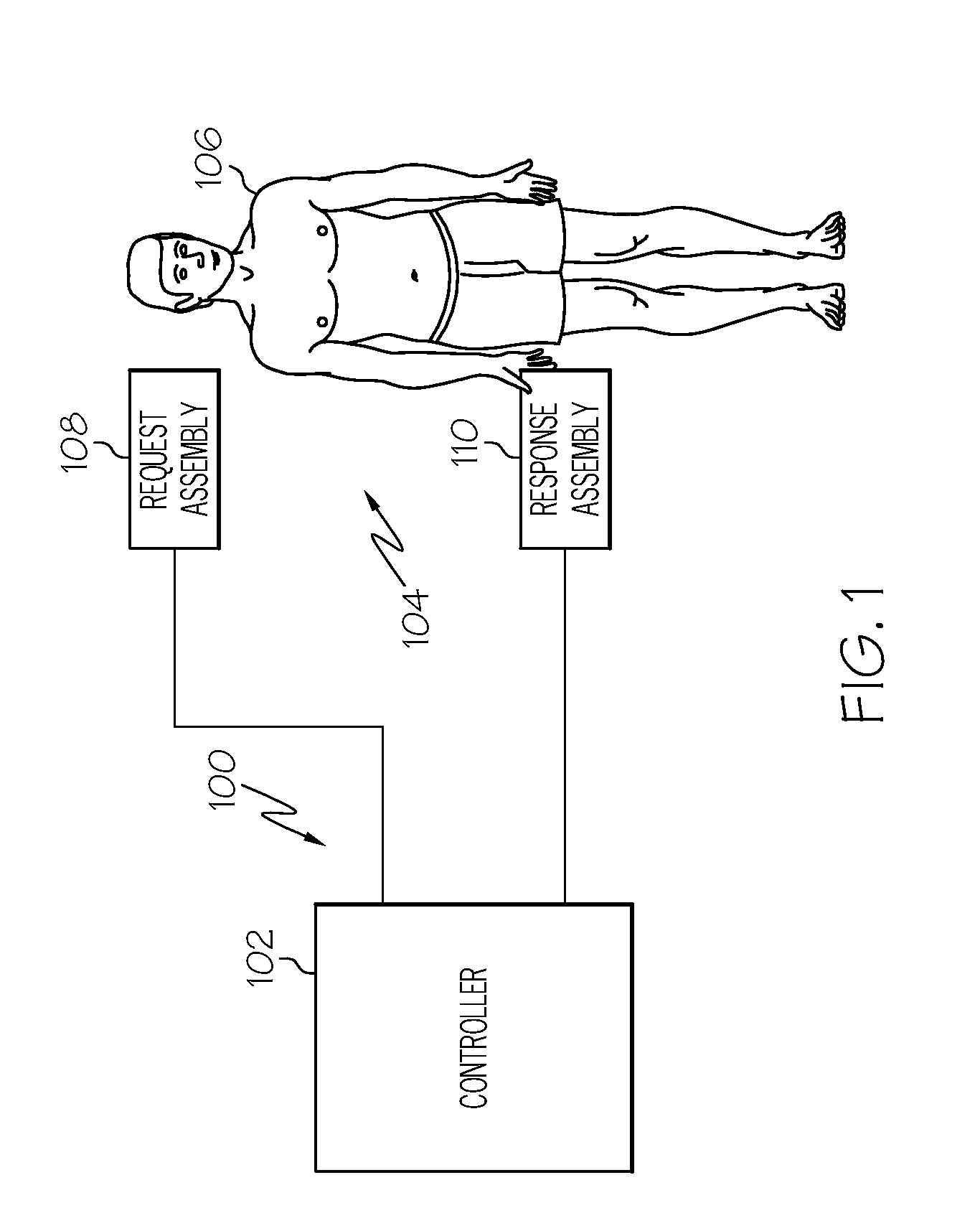
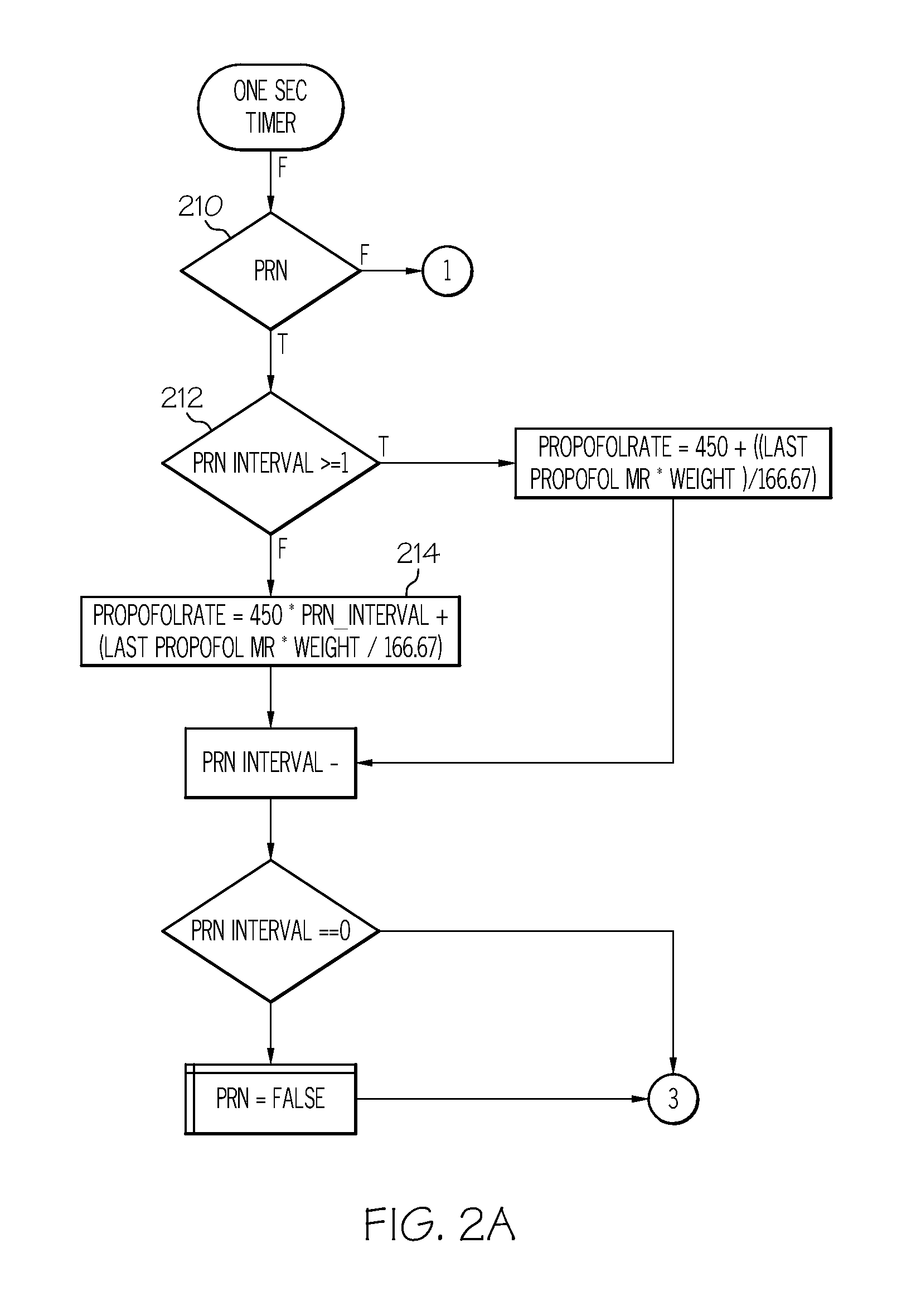
Dosage control for drug delivery system
A method for delivering intravenous drugs to a patient comprising programming a drug delivery system, including a controller and an infusion pump, with a maintenance rate or a loading dose for a drug and causing the drug delivery system to (a) calculate a loading dose based on the maintenance rate or a maintenance rate based on the loading dose, (b) administer the loading dose of the drug to the patient to rapidly achieve a desired level of effect, and (c) administer the drug at a first maintenance rate to maintain the level of effect.
Owner:ETHICON ENDO SURGERY INC
Dosage control for drug delivery system
InactiveUS20070191817A1Drug and medicationsPharmaceutical delivery mechanismIntravenous drugDrug loading dose
A method for delivering intravenous drugs to a patient comprising programming a drug delivery system, including a controller and an infusion pump, with a maintenance rate or a loading dose for a drug and causing the drug delivery system to (a) calculate a loading dose based on the maintenance rate or a maintenance rate based on the loading dose, (b) administer the loading dose of the drug to the patient to rapidly achieve a desired level of effect, and (c) administer the drug at a first maintenance rate to maintain the level of effect.
Owner:ETHICON ENDO SURGERY INC
Methods and Kits For Administering Probiotics
InactiveUS20080241226A1Improve tolerability and perception of benefitBiocideNervous disorderMedicineDrug loading dose
Methods for administering probiotics comprising the steps of: administering a loading dose of a loading probiotic for a loading time period; and administering a dose of a botanical and / or additional materials for the loading time period are disclosed. The methods also include administering a maintenance dose of a maintenance probiotic, and / or a botanical and / or an additional material for a maintenance time period. Also disclosed are kits for use in administering probiotics.
Owner:THE PROCTER & GAMBLE COMPANY
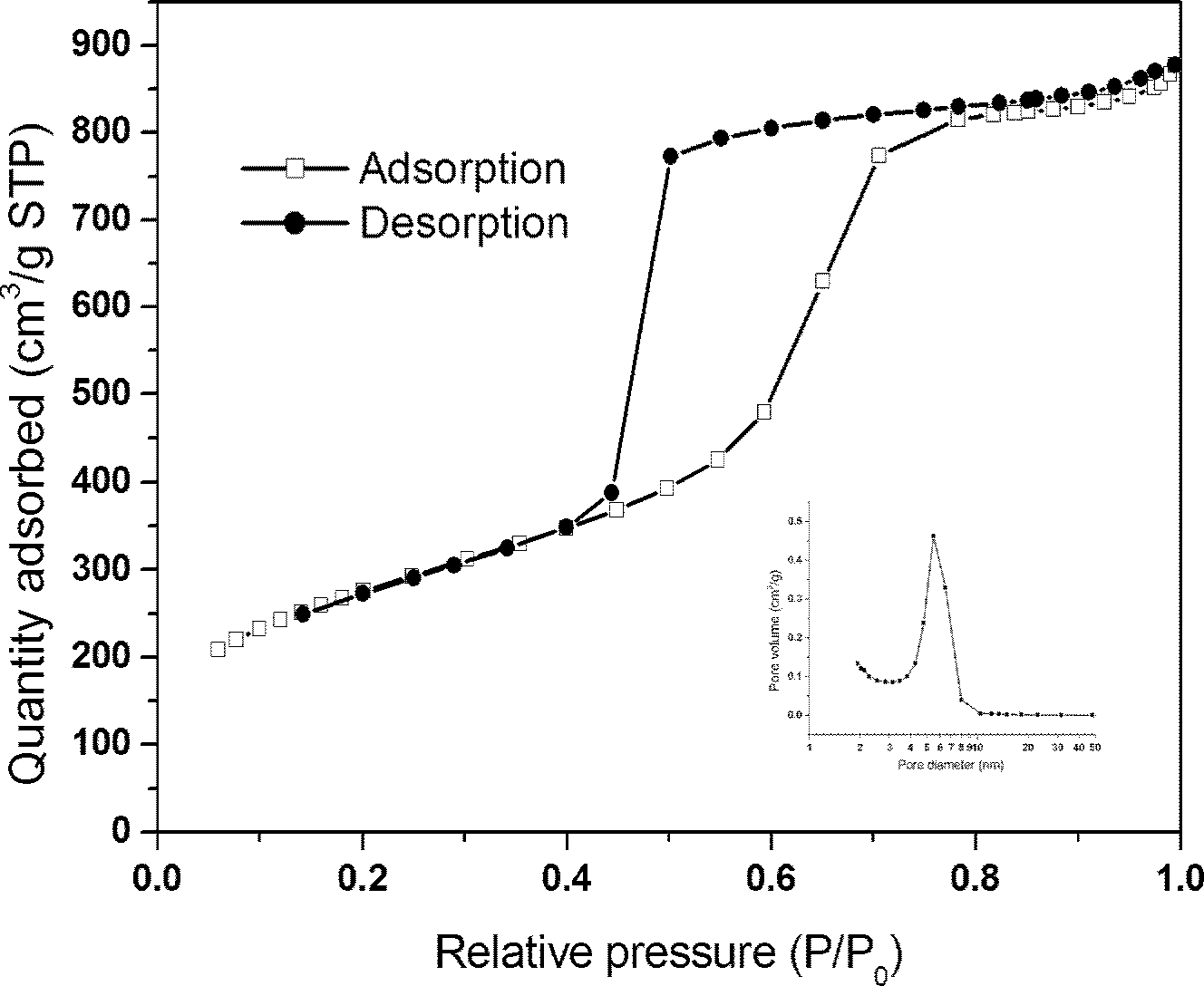
Hollow mesoporous silica microsphere, preparation method and application thereof
InactiveCN102432024ARealize internal and external transmissionIncrease dissolution rateSilicaPharmaceutical non-active ingredientsMicrosphereDrug carrier
The invention discloses a hollow mesoporous silica microsphere with hollow core and adjustable mesopore and penetrating through a shell, preparation method and application thereof. The hollow mesoporous silica microsphere is obtained by the following steps: under the condition of acid or alkaline solution, taking hexadecyl trimethyl ammonium bromide or PEO-PPO-PEO triblock copolymer as template, adding non-polar solvent, stirring at a certain temperature, emulsifying, then adding silica source, after hydrolysis and condensation, filtering, drying, and roasting to remove the template. The preparation method has simple technique and short time, easy operation and low cost, the prepared microsphere comprises both macropore and mesopore structures, the mesopore penetrates through the shell, the aperture thereof is larger than 5nm and is adjustable within the range of 5-20nm, and by relatively large mesopore channel, internal and external transmissions of large guest molecules can be realized. The microsphere can be used as drug carrier, the drug loading amount can exceed 50% (mass percentage), and the drug release can be controlled by adjusting aperture of the mesopore and dissolutionrate of indissolvable drug can be improved.
Owner:广州万泽医药科技有限公司
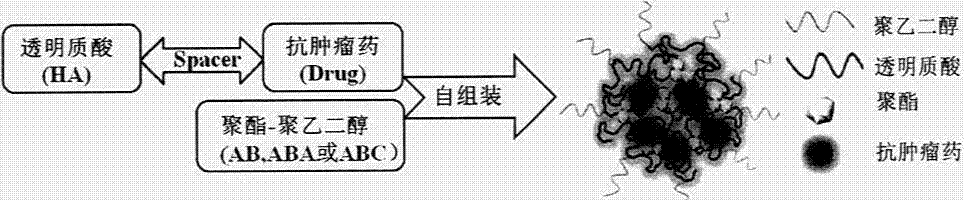
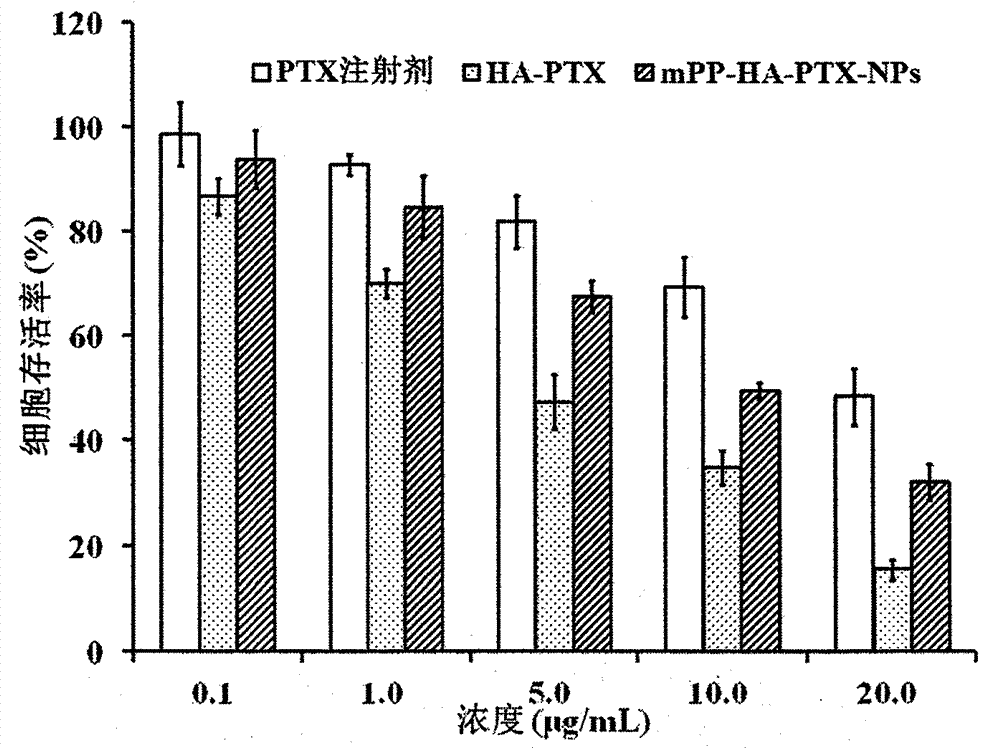
Preparation and application of hyaluronic acid-antitumor drug conjugate and composite nanoparticle composition
ActiveCN103751795AImprove solubilityAvoid devouringOrganic active ingredientsPharmaceutical non-active ingredientsTumor targetingEfficacy
The present invention relates to a preparation method and an application of a hyaluronic acid-antitumor drug conjugate and composite nanoparticle composition with characteristics of active targeting antitumor effect and biodegradability. The preparation method is characterized by comprising: (1) a synthesis method for conjugating an antitumor drug and a spacer and conjugating a targeting ligand hyaluronic acid or an ammonium salt thereof and the antitumor drug-spacer; and (2) a new technology for assembling the hyaluronic acid-antitumor drug conjugate and amphiphilic polyester block copolymer composite nanoparticles. The composition has effects of substantially increased drug loading, efficacy improving, in vivo long-circulating effect achievement, active tumor targeting property and drug toxic-side effect reduction. The macromolecular conjugate and complex nanoparticle composition can be used for injection administration, oral administration or mucosal administration. In addition, the preparation method has characteristics of mature process and high yield, and is suitable for industrial production.
Owner:CHINA PHARM UNIV
Microprojection array application with multilayered microprojection member for high drug loading
InactiveUS20070299388A1Improve permeabilityHigh drug loadingSurgeryMicroneedlesMedicineBiomedical engineering
A transdermal drug delivery system with microprojections for disrupting a body surface to an individual. At least some of the microprojections arise from a first microprojection layer and at least some of the microprojections arise from a second microprojection layer. The first and second microprojection layers are stacked together.
Owner:ALZA CORP
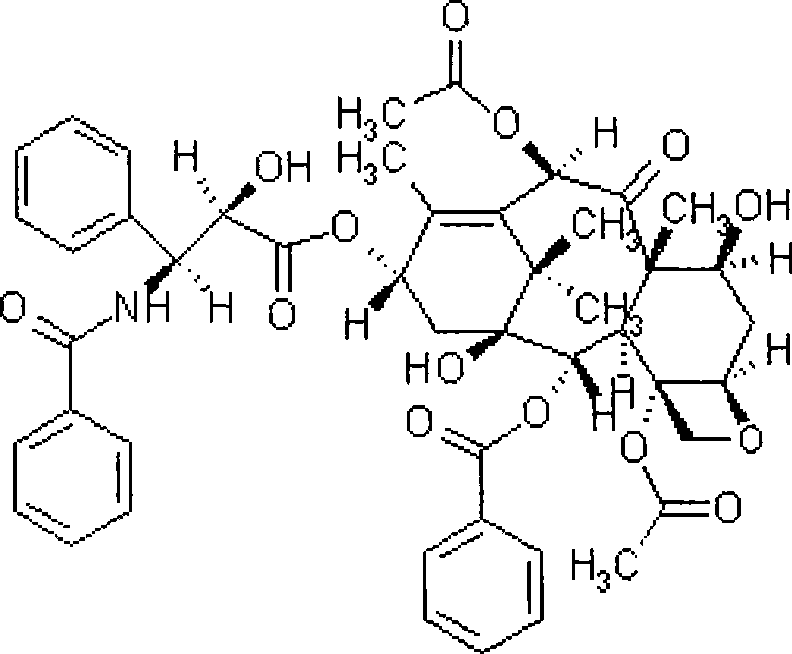
Paclitaxel lipid composite
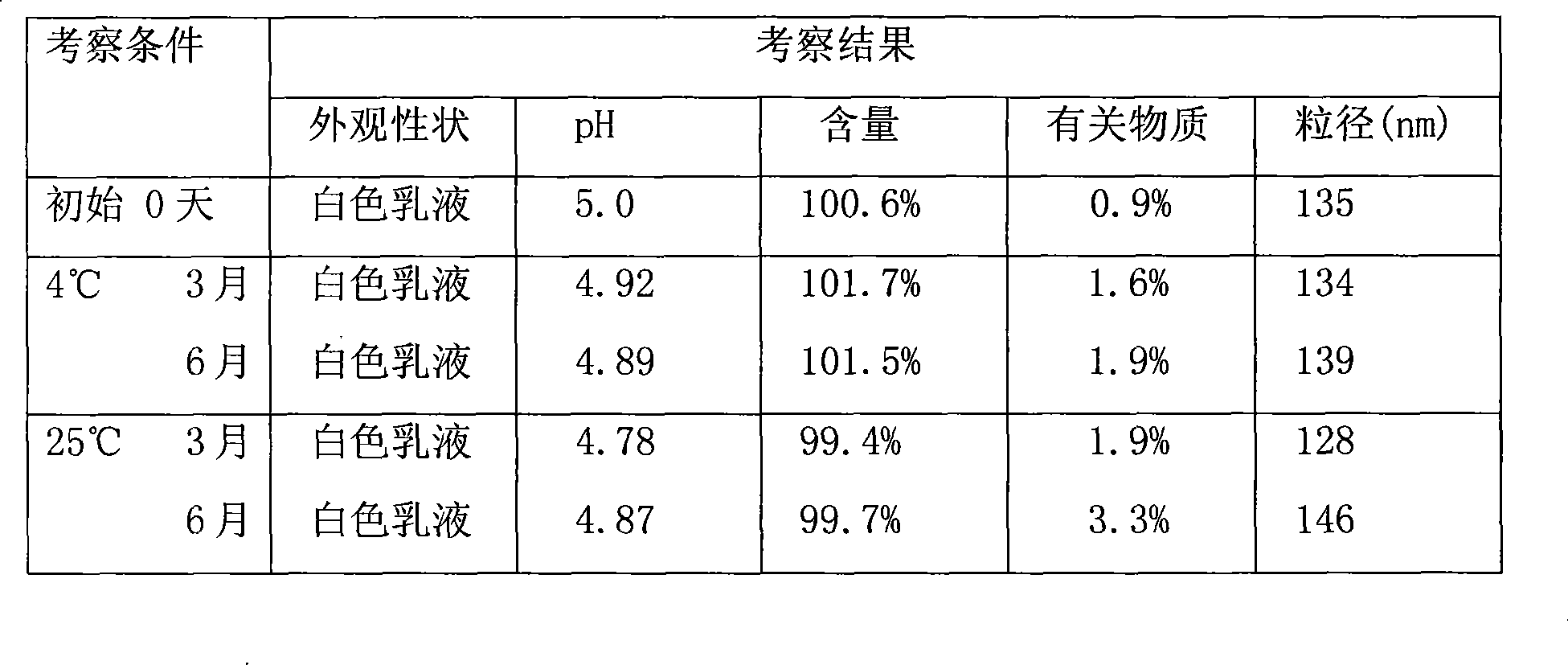
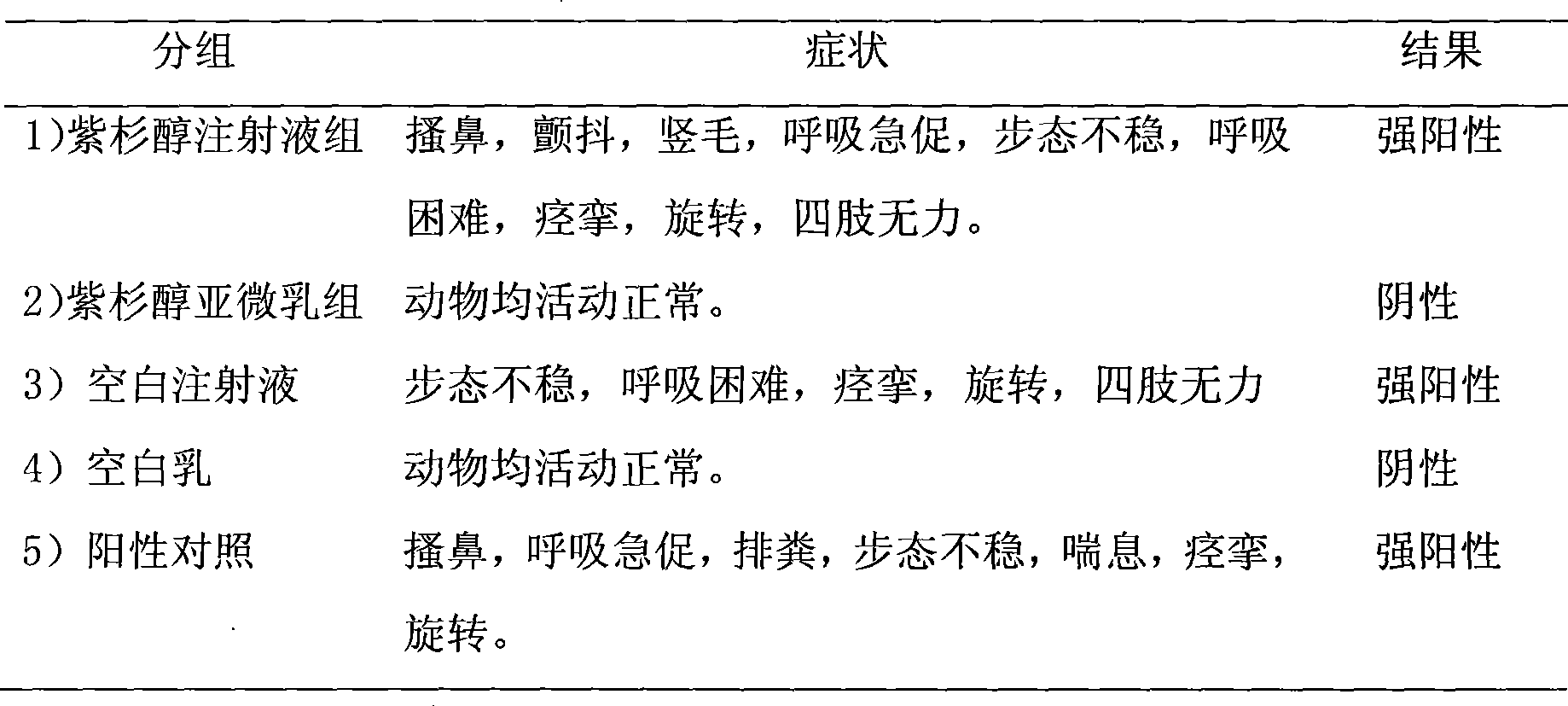
ActiveCN101396346AImprove solubilityGood dispersionOrganic active ingredientsPharmaceutical non-active ingredientsEmulsionDrug loading dose
The invention discloses a paclitaxel lipid complex. The paclitaxel lipid complex consists of paclitaxel and lipid material. The weight proportion of the paclitaxel and the lipid material is 1 to 1 - 19, the preferential proportion is 1 to 2 - 10, and the more preferential proportion is 1 to 3 - 6. The lipid material is selected from natural lipid and synthetic lipid or the mixture thereof. The paclitaxel lipid complex can also contain antioxidation stabilizer. The invention also discloses a preparation method of the paclitaxel lipid complex and the application in the preparation of injection submicron emulsion and dry emulsion. The paclitaxel lipid complex has high solubility in oil, and the prepared submicron emulsion has the advantages of high drug loading quantity, high stability, high safety and low irritability.
Owner:BEIJING WEHAND BIO PHARMA CO LTD
In-vivo degradable and absorbable artificial medical tissue repairing film
The invention provides an in-vivo degradable and absorbable artificial medical tissue repairing film and a preparation method thereof. The biodegradable repairing film is composed of a porous layer and a reinforcing layer and is a sponge-like substance which is of a two-layer, three-layer or multi-layer overlapped structure, wherein the layers are closely combined. The porous layer has a certain thickness so that the biodegradable repairing film has a certain shape and a certain drug loading capacity so as to meet the needs of tissue repair; the reinforcing layer is compact and has reinforcing and anti-tearing effects; each layer of structure can be prepared from different raw materials at different ratios and loaded with different drugs according to treatment requirements; the degradation speed and drug release can be controlled by controlling the quantity and size of holes. The porous layer is higher than the reinforcing layer in degradation speed, so that the repairing film keeps a certain mechanical strength within a certain time and the drug is released slowly and directionally. The repairing film is excellent in operability and suturing property, nontoxic, degradable and absorbable in vivo, good in biocompatibility, free of injuries to tissues, applicable to various surgical repair operations and suitable for popularization and implementation.
Owner:SHANDONG BRANDEN MEDICAL DEVICE
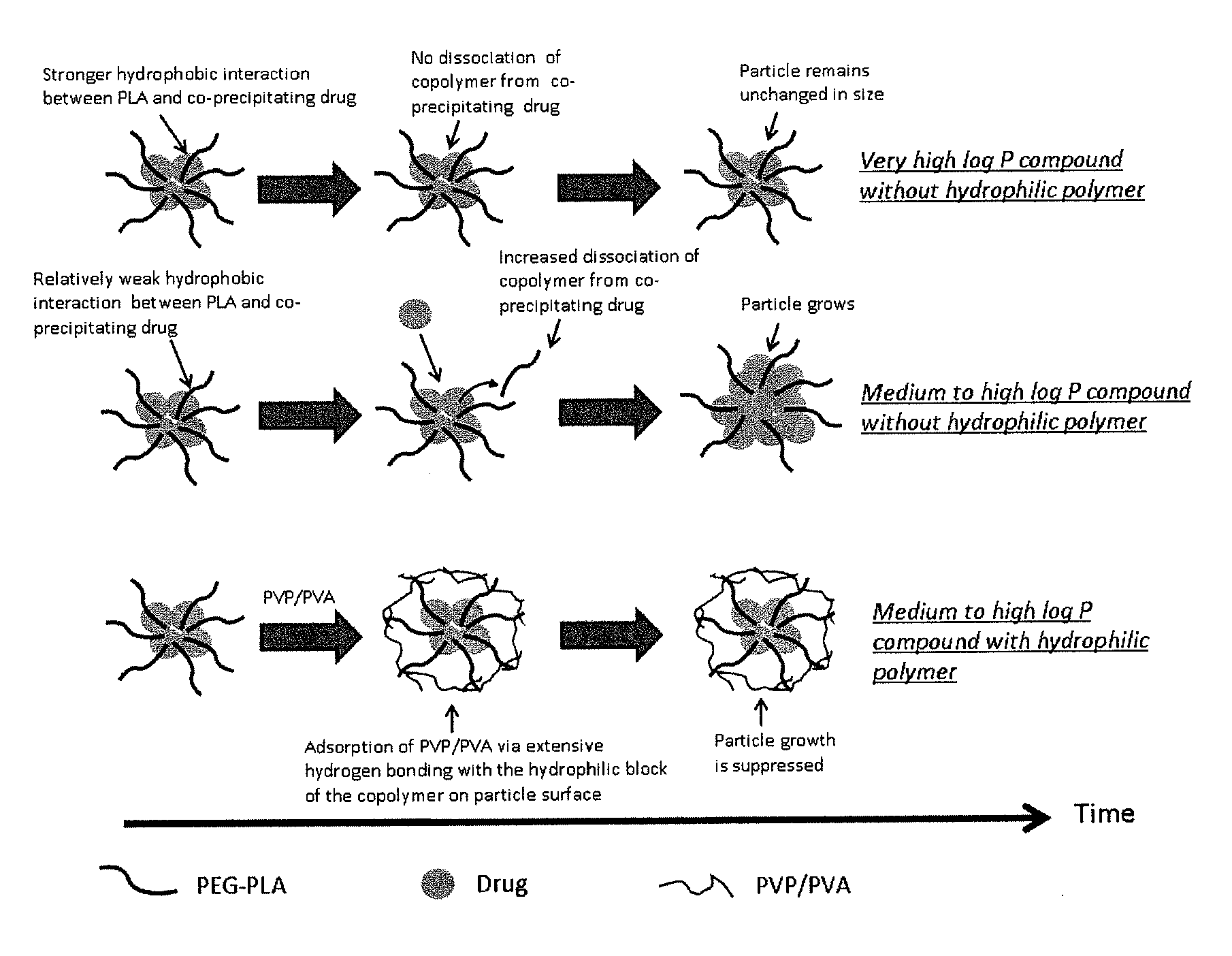
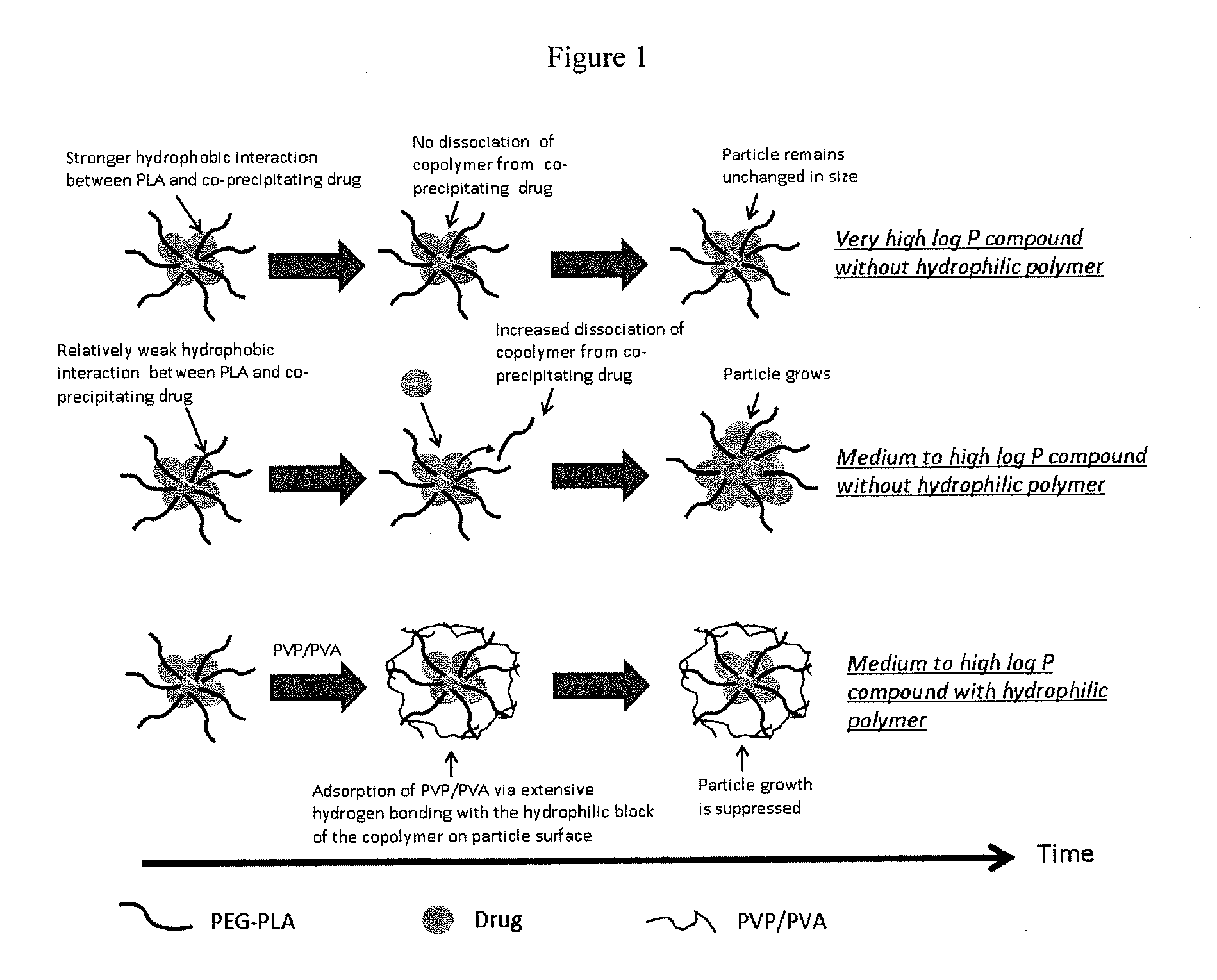
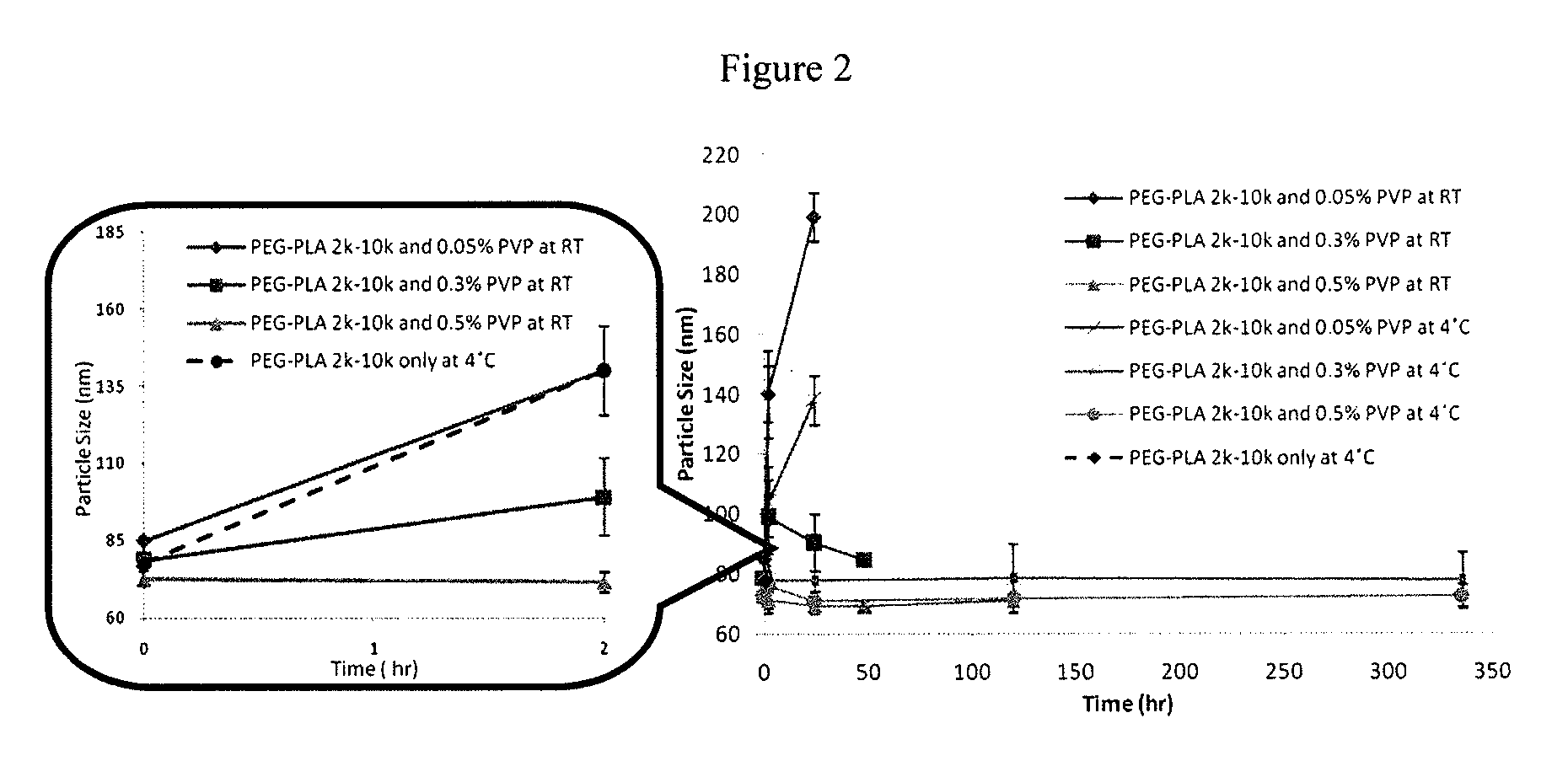
Engineering of polymer-stabilized nanoparticles for drugs with log p values below 6 by controlled antisolvent precipitation
The present invention provides organic nanoparticles that include a molecule having a Log P value of about 3 or above, an amphiphilic diblock copolymer or a surfactant, and a pharmaceutically-acceptable hydrophilic polymer. The present invention also provides methods of making these nanoparticles, e.g., by flash nanoprecipitation, with control over particle size and surface properties. The methods of the present invention provide a means for co-precipitating a water-insoluble compound with an amphiphilic stabilizer within a few milliseconds. The nanoparticles of the present invention exhibit high drug loading, e.g., 50% w / w, and can be produced with a mean particle size less than 200 nm and with a narrow particle size distribution.
Owner:THE CHINESE UNIVERSITY OF HONG KONG
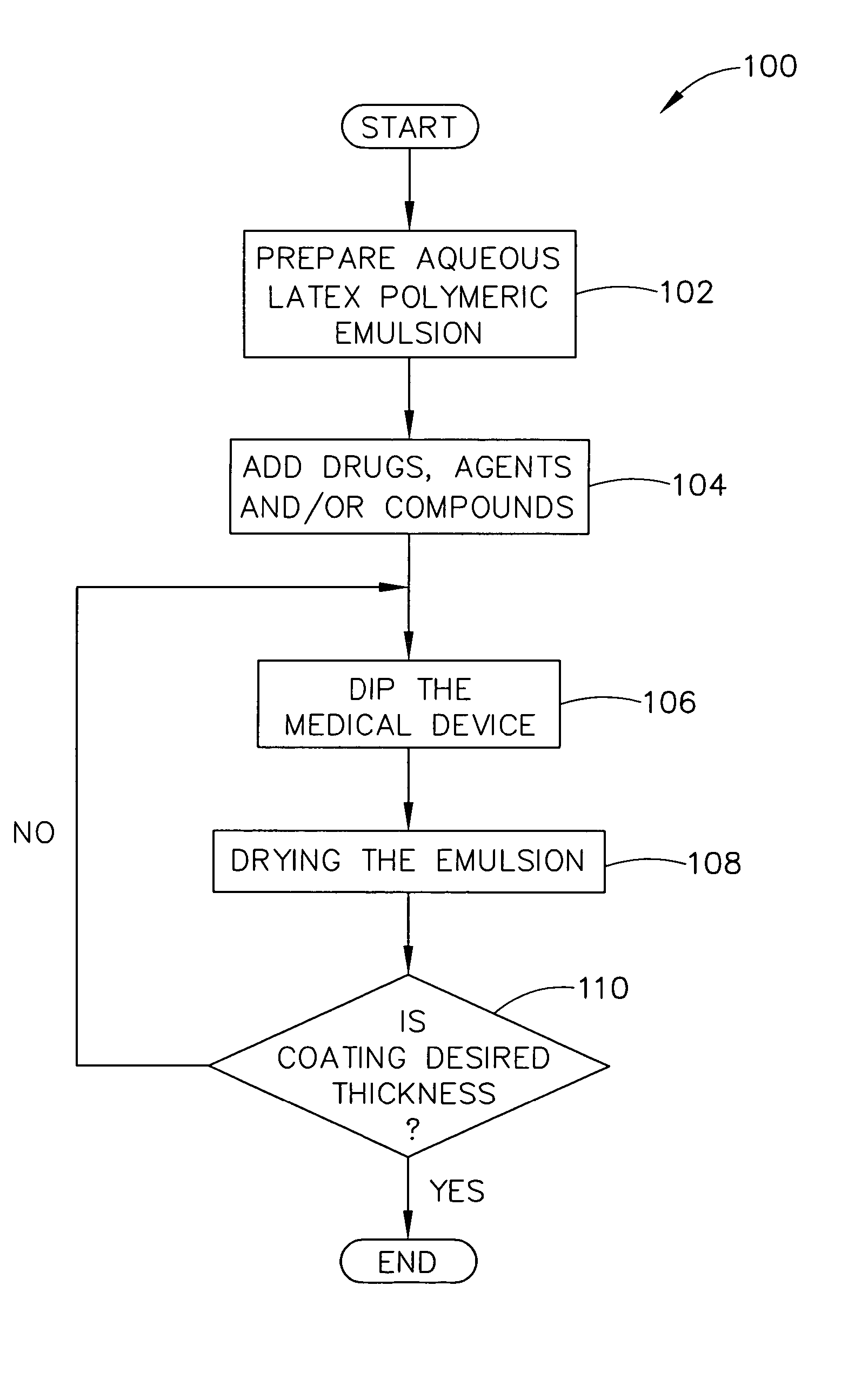
Method for applying drug coating to a medical device in surgeon room
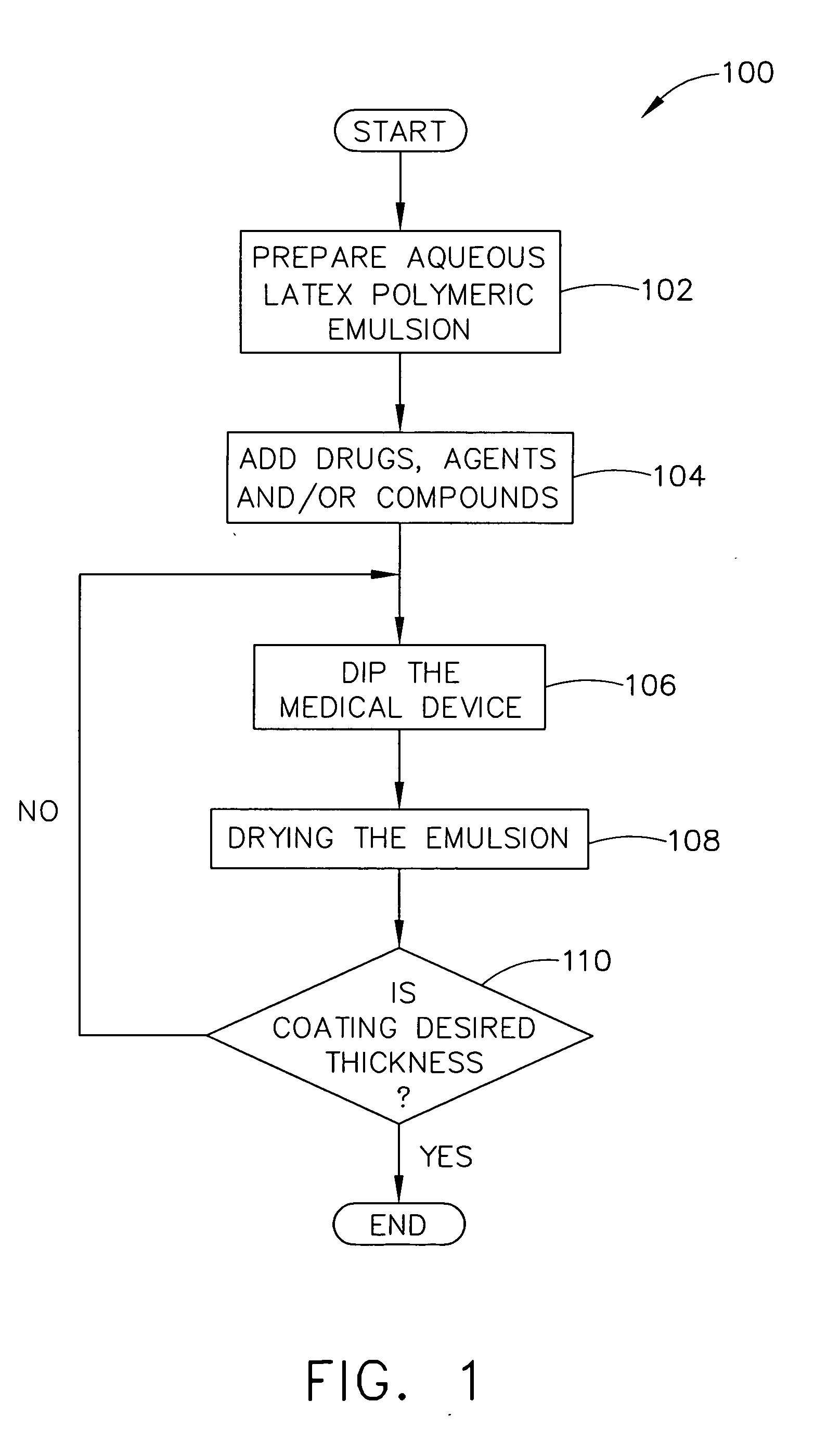
ActiveUS20050037133A1Safe and efficient and effective processSimple and complex configurationOrganic active ingredientsSurgeryDip-coatingDelivery system
Medical devices, and in particular implantable medical devices such as stents and stent delivery systems including catheters, may be coated to minimize or substantially eliminate a biological organism's reaction to the introduction of the medical device to the organism or to treat a particular condition. A dip coating process is utilized to minimize waste and to customize coating thickness and drug loading directly at the clinical site just prior to therapeutic use on a patient. An aqueous latex polymeric emulsion is utilized to coat any medical device to a desired thickness by allowing for successive dipping and drying cycles at the clinical site. In addition, aqueous latex polymeric emulsions pose less of a chance of the bridging phenomenon associated with organic solvent based polymers.
Owner:WYETH LLC
Self-assembled nano-system of unsaturated fatty acid-anti-tumor drug conjugates as well as preparation method and application thereof
InactiveCN105617394AImprove bioavailabilityProlong circulation time in the bodyHeavy metal active ingredientsOrganic active ingredientsUnsaturated fatty acidDspe peg
The invention relates to a self-assembled nano-system of unsaturated fatty acid-anti-tumor drug conjugates as well as a preparation method and application thereof. (1) Unsaturated fatty acid-anti-tumor drug conjugates can be self-assembled in water to form spherical nano-particles with uniform particle sizes and good stability; and (2) in a preparation process, an amphiphilic polymer material such as DSPE-PEG can be optionally added, so that the particle sizes of the self-assembled nano-particles can be further reduced, and the stability of the self-assembled nano-particles can be further enhanced. The self-assembled nano-system of unsaturated fatty acid-anti-tumor drug conjugates, provided by the invention, is simple in preparation method and easy in industrial production and has the advantages of high drug loading capacity, strong stability, good safety and the like; and growth inhibition tests of tumor cells in vitro prove that the self-assembled nano-system has favorable anti-tumor effects on multiple tumor cells.
Owner:PEKING UNIV
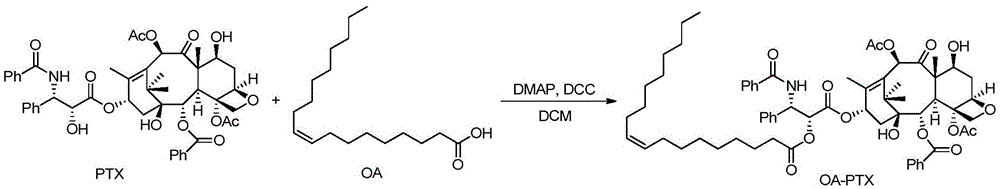
Construction of paclitaxel-oleic acid small-molecular prodrug self-assembled nanoparticles
ActiveCN105833284AAchieve specific drug releasePromote enrichmentPowder deliveryOrganic active ingredientsSide effectPolyethylene glycol
The invention designs and synthesizes a series of paclitaxel-oleic acid small-molecular prodrugs; with the application of a chemical connecting arm which is sensitive to an oxidation-deoxidation environment, the rapid release of the drugs in tumor cells is promoted. On the basis, small-molecular prodrug self-assembled nano-drug delivery systems are prepared. The small-molecular prodrug self-assembled nano-drug delivery systems have the advantages that by virtue of a one-step nano-precipitation method, nano-drug self-assembled nanoparticles are simple in preparation process and easy for industrialization; the nano-drug self-assembled nanoparticles are small and uniform in grain size (to 100nm), and the nano-drug self-assembled nanoparticles are enriched in a tumor part by virtue of an EPR (enhanced permeability and retention) effect; an ultrahigh drug-loading capacity is guaranteed, which is beneficial for reducing adverse reactions caused by auxiliary materials and biological materials; surface modification is easy to implement, and the intake of a reticuloendothelial system can be effectively avoided and the intake of the tumor cells to the nanoparticles can be improved by virtue of PEG (polyethylene glycol) and active targeting modification; and on the basis of the sensitivity of the chemical connecting arm to the oxidation-deoxidation microenvironment of the tumor cells, the specific drug release of paclitaxel in the tumor part is achieved, a curative effect is improved and toxic and side effects are reduced.
Owner:SHENYANG PHARMA UNIVERSITY +1
Preparation and application of insoluble drug-entrapped poloxamer/amphiphilic polysaccharide mixed micelle
InactiveCN102626518AOvercome the problems of high critical micelle concentration and low drug loadingImprove oral bioavailabilityPharmaceutical delivery mechanismPharmaceutical non-active ingredientsMixed micelleCytochrome p450 enzyme
The invention discloses preparation and application of an insoluble drug-entrapped poloxamer / amphiphilic polysaccharide mixed micelle. The insoluble drug-entrapped poloxamer / amphiphilic polysaccharide mixed micelle is prepared through a dialysis method or a solvent evaporation method. The mixed micelle is low in critical micelle concentration, is high in drug-loading rate, is capable of obviously prolonging the stabilization time and has the long-circulation function of a nanomicelle and has dual functions of restraining the metabolism of P-glycoprotein and cytochrome P450 enzyme and is capable of increasing the bioavailability of oral administration. The mixed micelle is simple in preparation method, is mature in process and is high in yield and can be prepared into preparations for the oral administration, such as tablets, capsules, pills and syrups.
Owner:CHINA PHARM UNIV
Protection-sleeve-carrying paclitaxel drug balloon and preparation method thereof
The invention relates to a protection-sleeve-carrying paclitaxel drug balloon and a preparation method thereof. The protection-sleeve-carrying paclitaxel drug balloon comprises a drug balloon and a paclitaxel-containing degradable drug coating which is coated on a balloon work section. The drug balloon can be used as a pre-expansion balloon, a conveying balloon and a post-expansion balloon and the drug balloon comprises a HUB protection-sleeve handle, a near-end push rod, a transition section, a far-end push rod and a head end. The coating, which is configured by the paclitaxel and a contrast agent, or which includes the paclitaxel, a degradable polymer and a solvent of trichloroethane, acetone or tetrahydrofuran, can be layeredly coated on the balloon work section. Through the arrangement of a protection sleeve, the drug balloon is, during conveying processes from entering blood vessels to reaching diseased regions, in a protection state, so that drugs on the drug balloon may not be scoured, ineffective loss is avoided, initial drug loading capacity of the drug balloon can be reduced, the treatment effect can be meet and at the same time safety of the drug balloon is greatly improved.
Owner:BIOVAS (WUHAN) MEDICAL TECH CO LTD
Paclitaxel loaded sustained release nano fiber and preparation method and use thereof
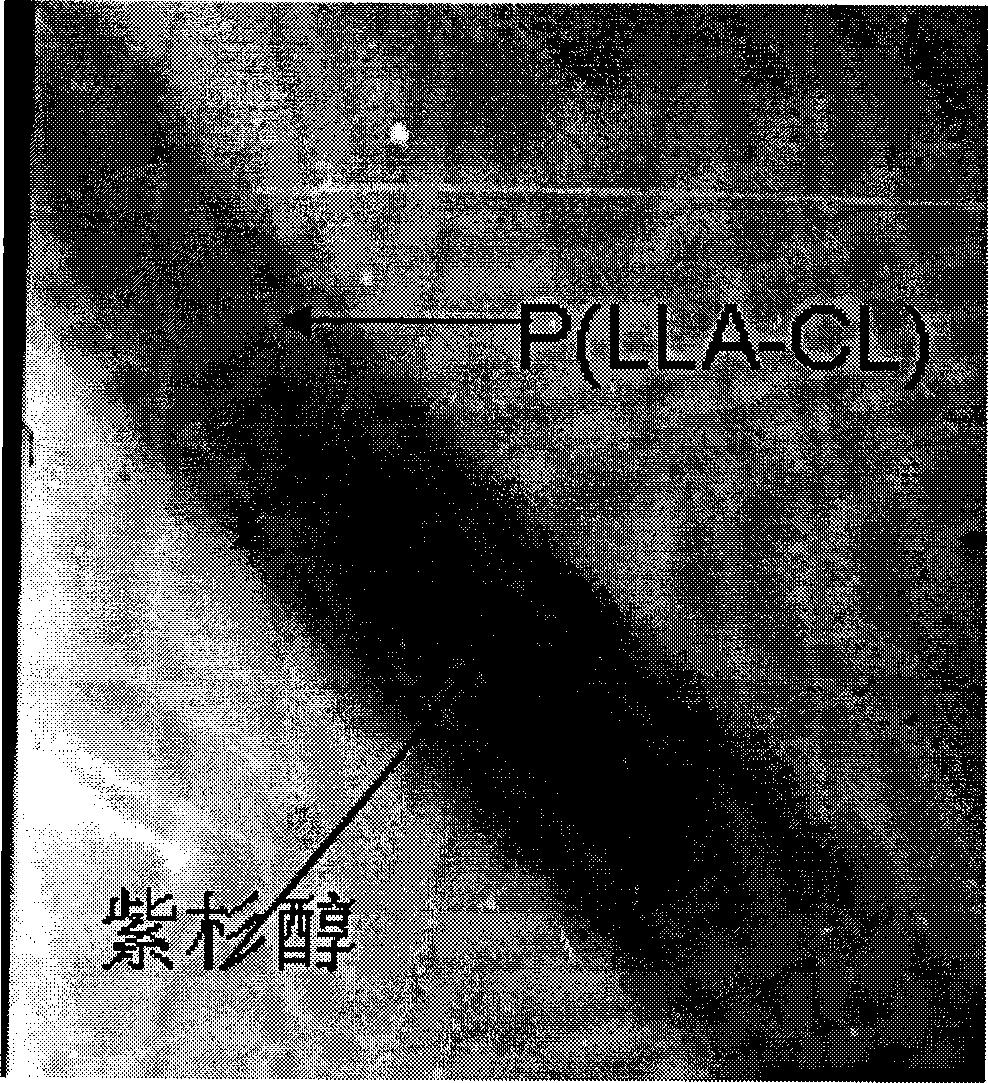
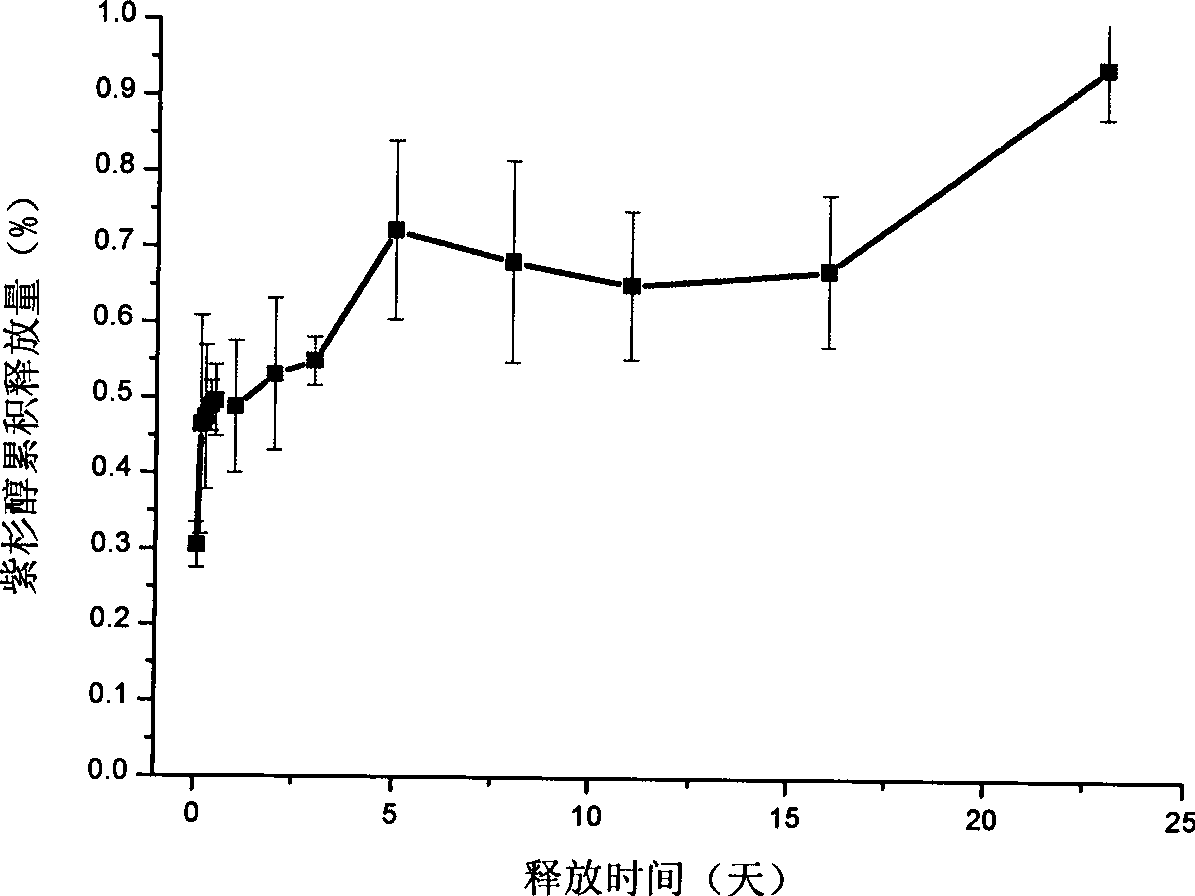
InactiveCN101396337AControlled slow releaseInhibition of value addedPharmaceutical delivery mechanismUnknown materialsFiberMicroinjections
The invention relates to a paclitaxel-loaded sustained release nano-fibre material which comprises the components of bio-degradable polymer material and pure paclitaxel, the mol ratio of which is 1.33 to 10. The diameter of the nano-fibre is 90nm to 1.44micrometer, and the drug-loading rate can be regulated within the range of 0 percent to 100 percent. The preparation of the nano-fibre comprises the steps as follows: (1) the bio-degradable polymer material is solved in organic solvent and stirred to be solved completely to obtain lamella spinning solution A; (2) the white pure paclitaxel powder is solved in trifluoroethanol and stirred to be solved completely to obtain core spinning solution B; (3) clear transparent solutions A and B are respectively added into two injectors; the speed of a microinjection pump, the voltage of a static generator and the receiving distance between a grounded aluminum foil and a spinning needle are adjusted, and unordered drug-loaded nano-fibre is obtained by a coaxial electrostatic spinning technology. A nanometer control release system composed by the drug-loaded nano-fibre effectively controls the sustained release of the drug and is applied to the preparation of the drug for remedying malignant tumor.
Owner:DONGHUA UNIV
Oral disintegrating tablet and preparation method thereof
InactiveCN102614138AIncrease disintegration rateSolve the problem of incomplete dissolutionCosmetic preparationsToilet preparationsLarge doseActive component
The invention discloses an oral disintegrating tablet and a preparation method thereof, and especially discloses an oral disintegrating tablet containing a bicomponent as a binder, and a preparation method thereof. The oral disintegrating tablet of the invention is prepared through adopting a lyophilizing process, and components of the tablet comprise effective doses of drug active components, a skeleton supporting agent, the binder, and a suspending aid, wherein the binder comprises pullulan and an auxiliary binder. The preparation method which utilizes the compound use of pullulan and the auxiliary binder in a specific application amount range solves problems of slow disintegration and incomplete dissolving-out when the lyophilized oral disintegrating tablet loads a large dose of the drug. Compared with lyophilized oral disintegrating tablets which are prepared with the prior art and have large drug loading doses, the lyophilized oral disintegrating tablet prepared in the invention, which has the advantages of rapid disintegration and complete dissolving-out, has a very good application value.
Owner:QUANTUM HI TECH BEIJING RES INST
Aripiprazole sustained-release microspheres and preparation method thereof
ActiveCN105310997AImprove complianceGood treatment effectOrganic active ingredientsNervous disorderAcetic acidMicrosphere
The invention relates to aripiprazole sustained-release microspheres and a preparation method thereof. The sustained-release microspheres include aripiprazole and a bio-degradable pharmaceutical high-molecular material PLGA, wherein the ratio of lactic acid to hydroxyacetic acid in the PLGA is 75:50-25:50. The PLGA is 25000-35000 Dolton in molecular weight. The addition weight ratio of the aripiprazole to the PLGA is 1:1-20. The aripiprazole accounts for 3.01-21.09% of total weight of the microsphere. The aripiprazole sustained-release microspheres have high drug embedding rate, is high in drug loading capacity, is smooth and round in surface and can release more than 90% of the drug in 30 days.
Owner:CHONGQING PHARMA RES INST
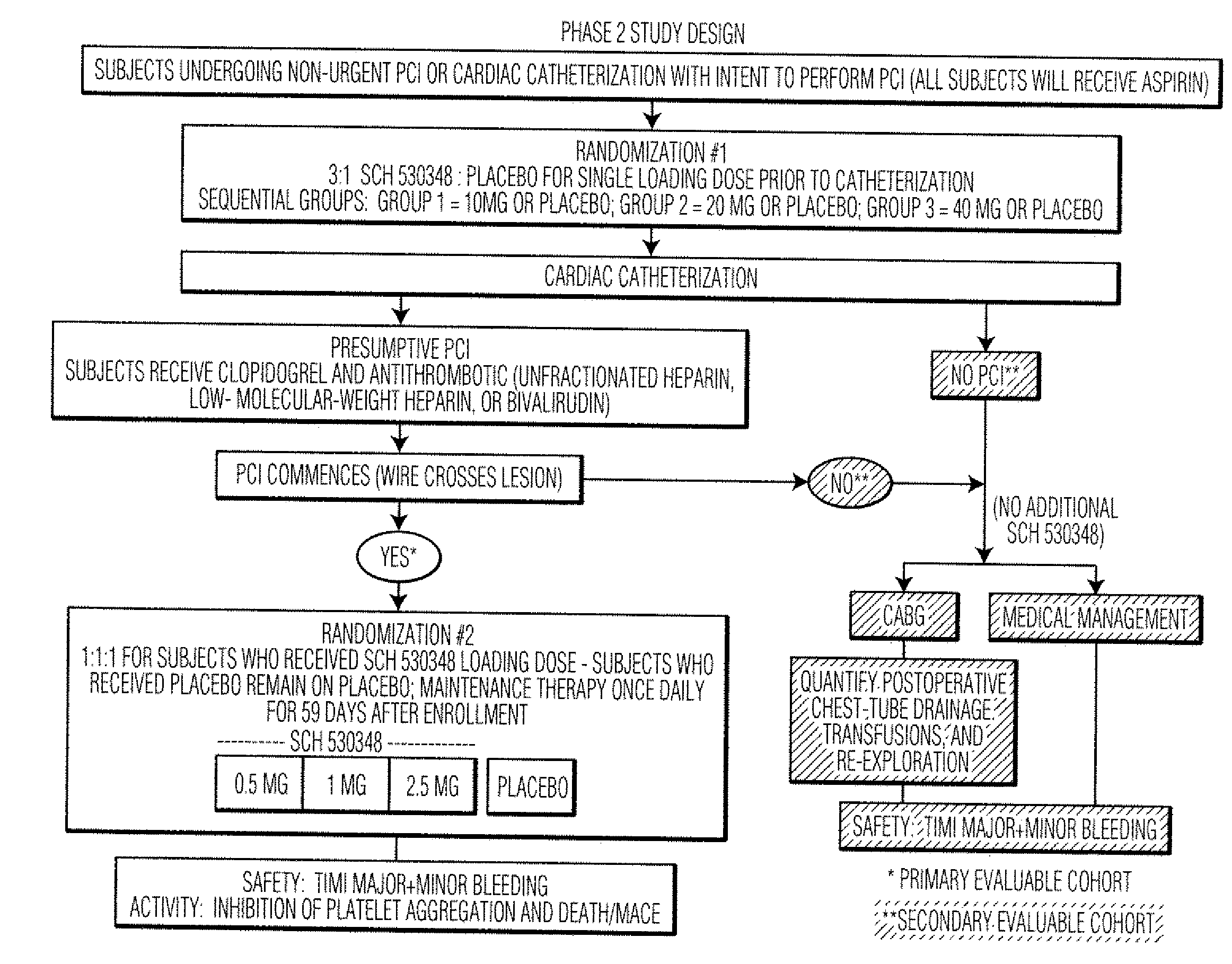
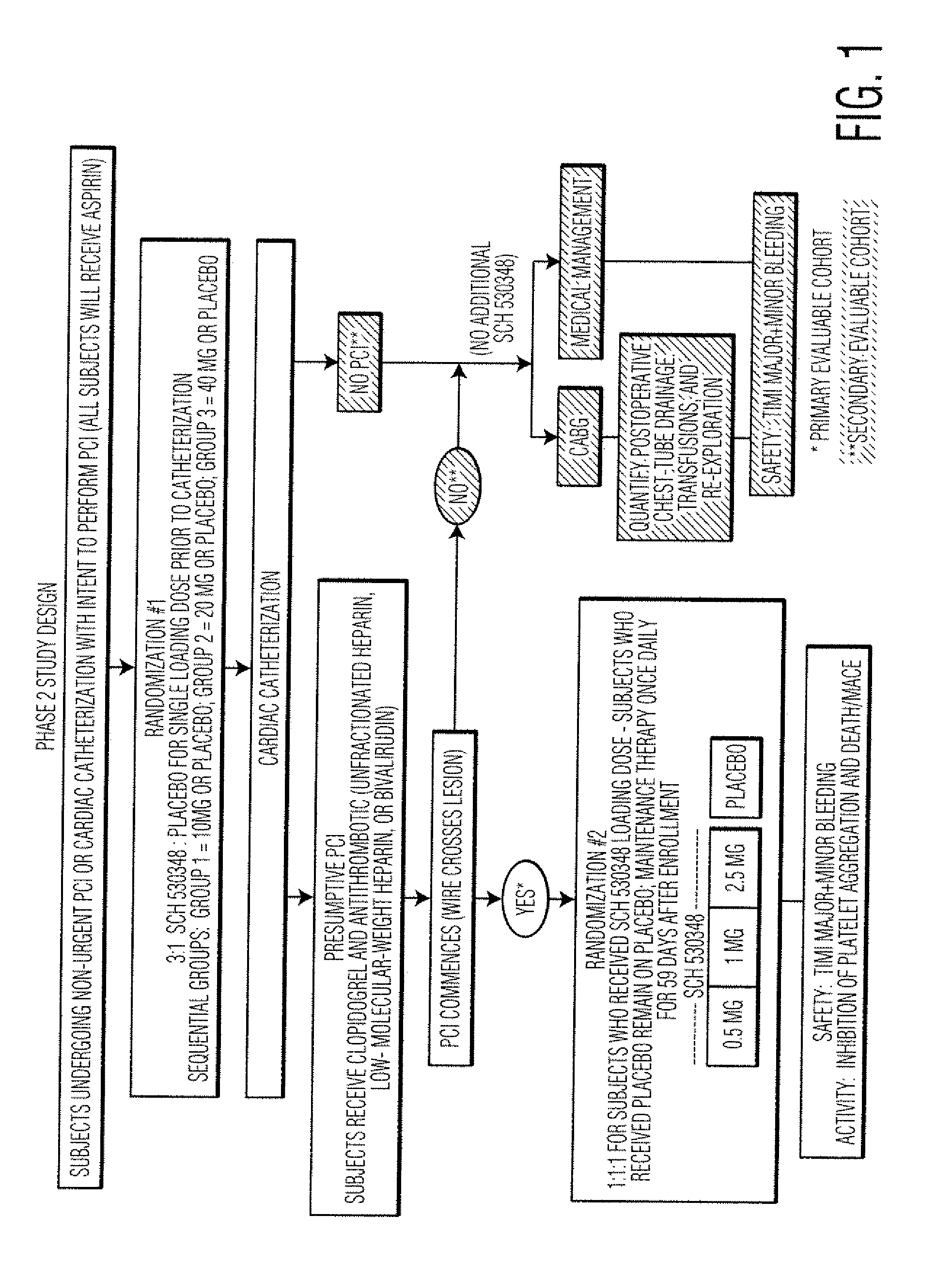
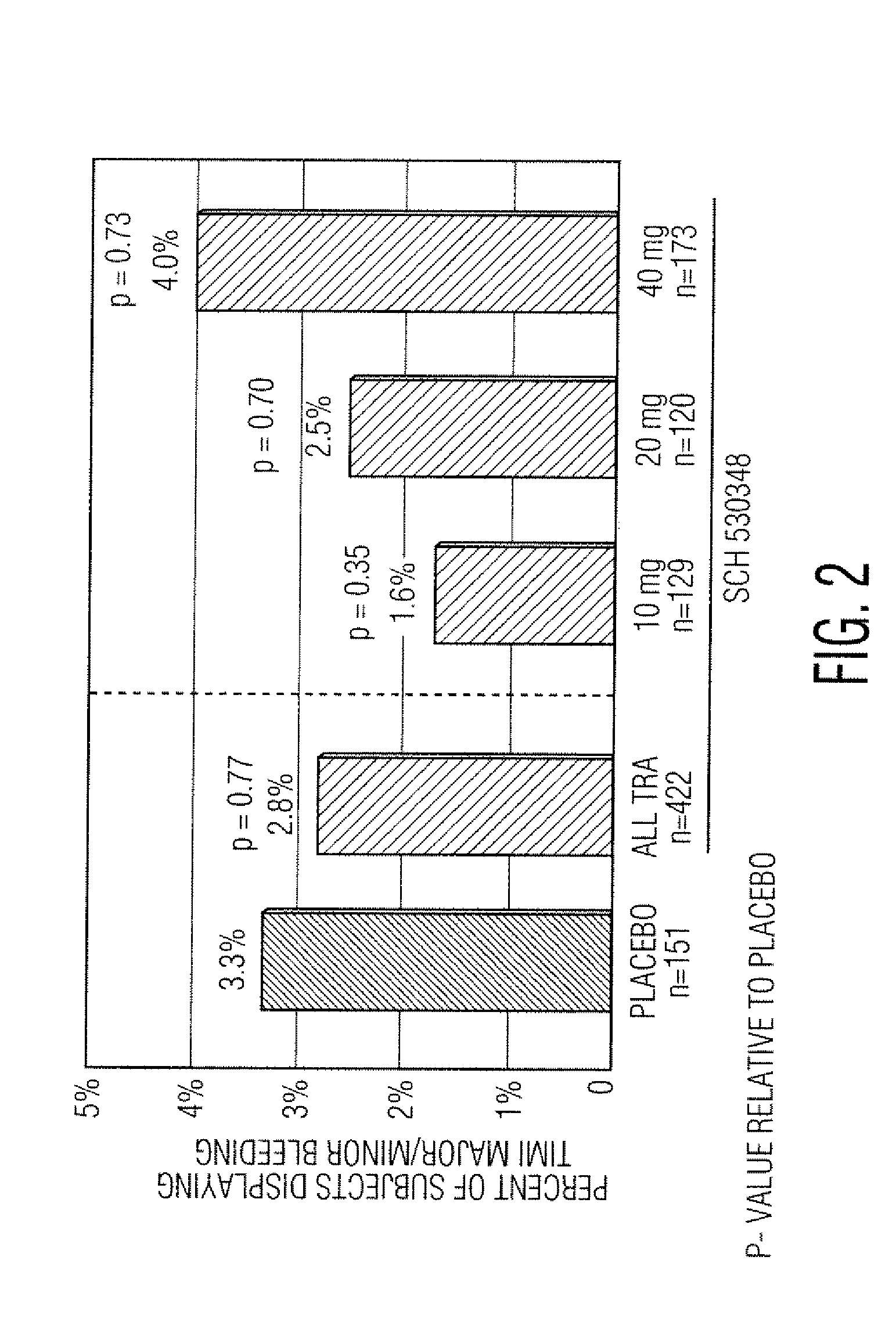
Reduction of adverse events after percutaneous intervention by use of a thrombin receptor antagonist
InactiveUS20080234236A1Preventing an adverse clinical eventSalicyclic acid active ingredientsBiocideDrug loading doseNK1 receptor antagonist
Disclosed are methods of preventing adverse clinical events in a patient undergoing a percutaneous coronary intervention procedure or a peripheral percutaneous interventional procedure comprising administering a therapeutically effective amount of a thrombin receptor antagonist, such as SCH 530348, to the patient. Administration of a loading dose of about 40 mg of SCH 530348 in as little as one hour prior to the procedure can result in therapeutically effective levels of platelet aggregation.
Owner:SCHERING CORP
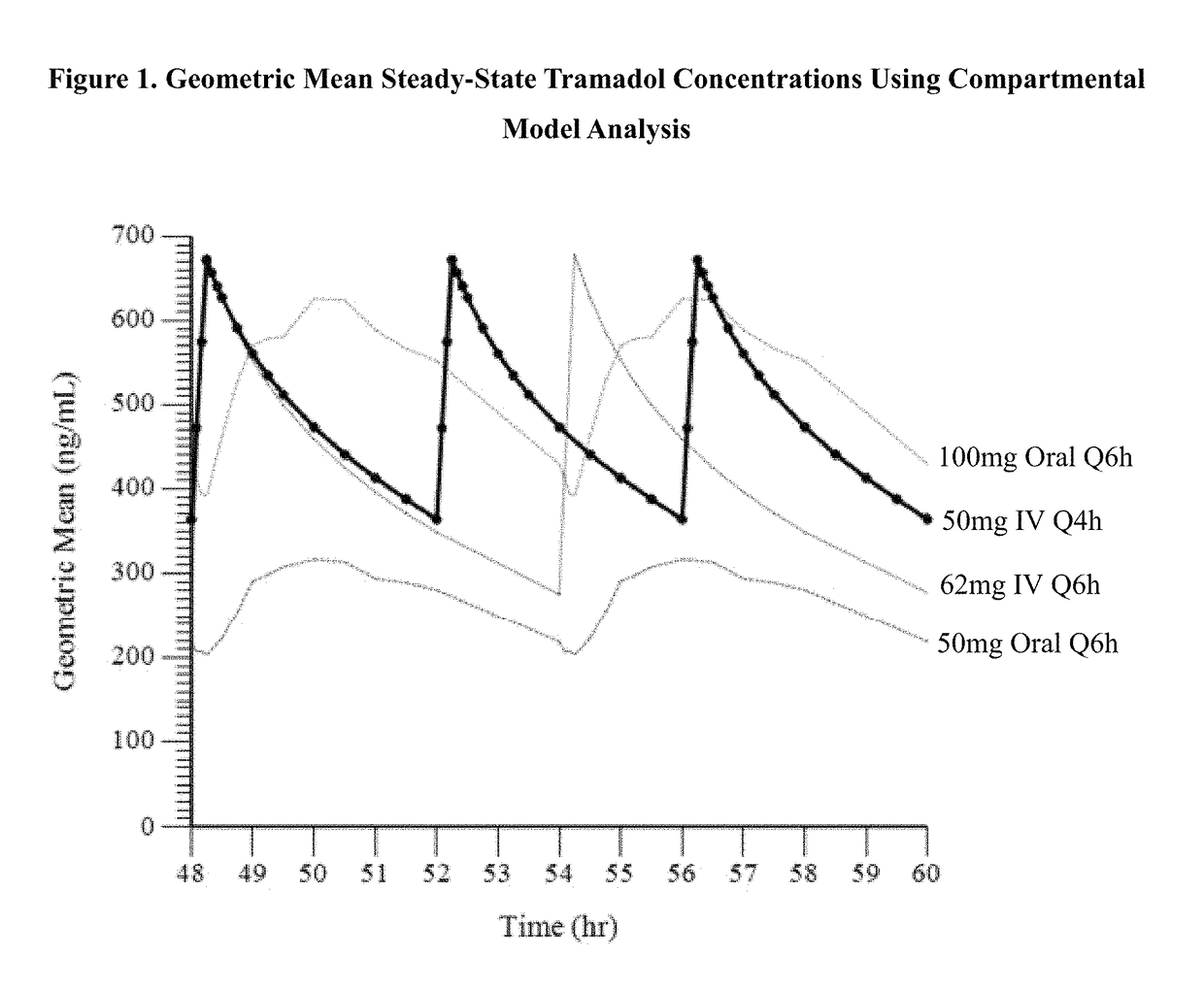
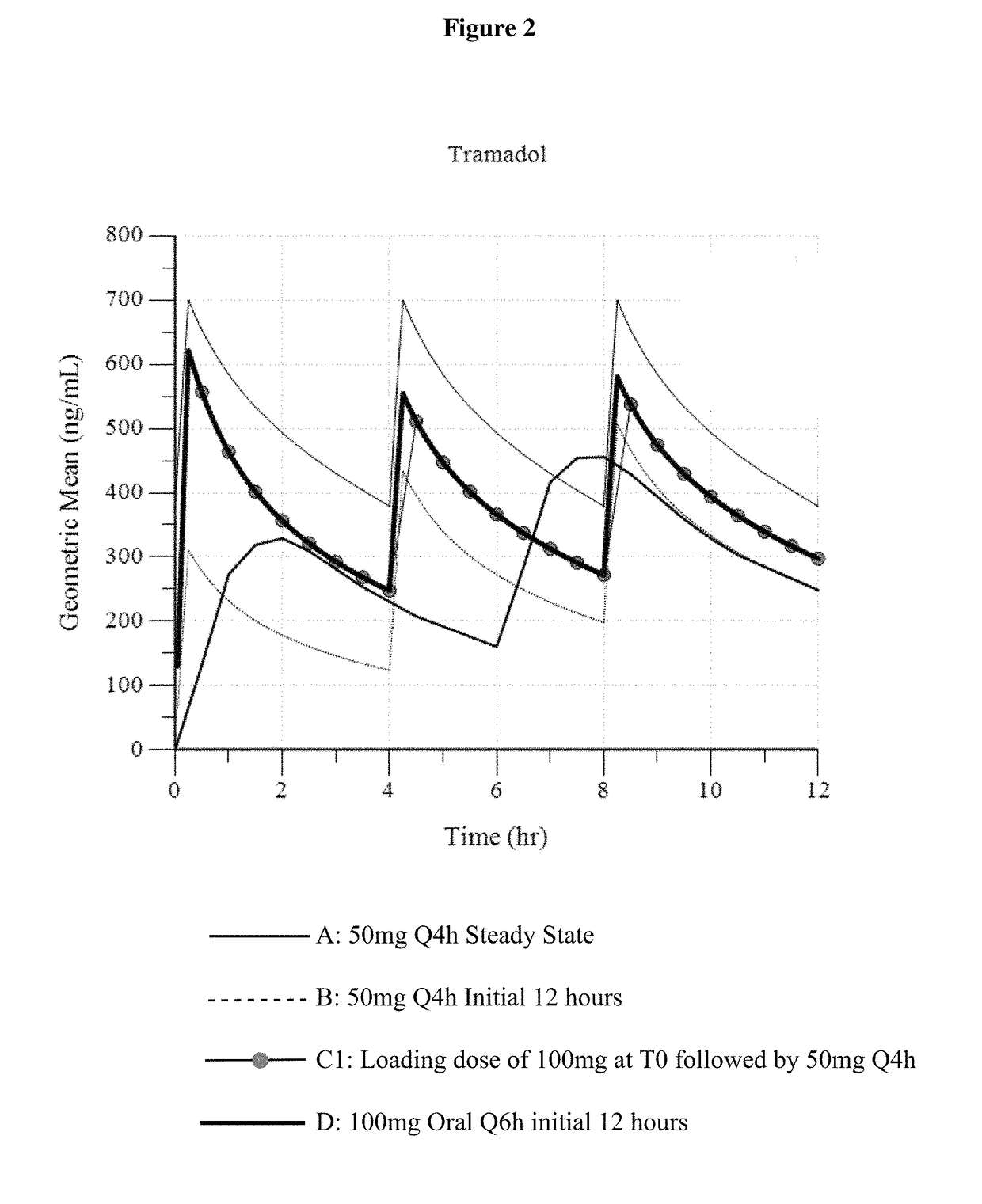
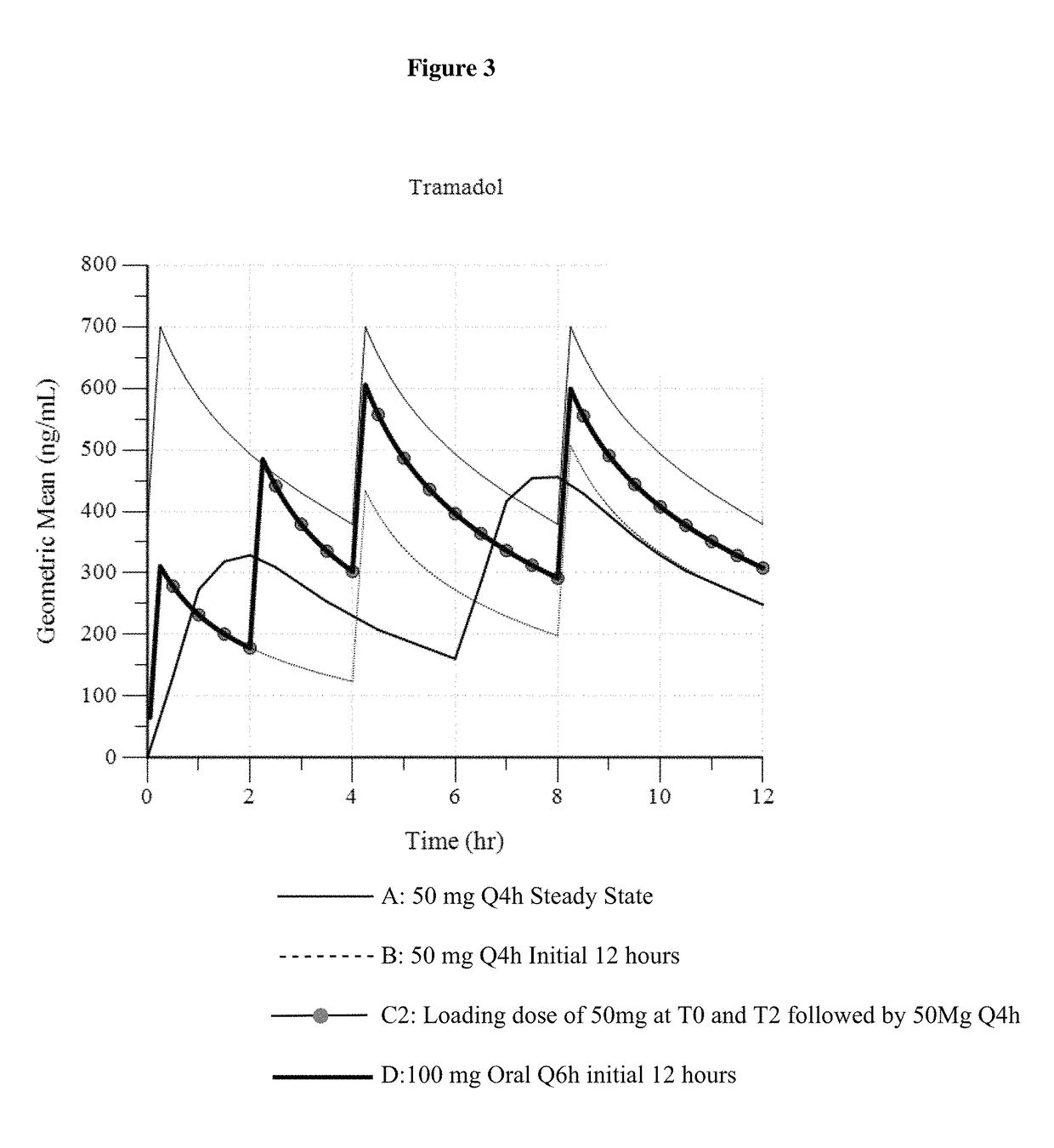
Intravenous administration of tramadol
ActiveUS9693949B1InhibitionRelieve painOrganic active ingredientsNervous disorderDosing regimenRegimen
A method of treating pain, e.g., acute post-operative pain, by administering to a human patient(s) a therapeutically effective dose of tramadol intravenously in a dosing regimen which includes one or more loading doses administered at shortened intervals as compared to dosing at steady-state is disclosed. In certain embodiments, the dose of tramadol is from about 45 mg to about 80 mg and the second (and optionally) third doses are intravenously administered at intervals of from about 2 to about 3 hours, and thereafter the tramadol is intravenously administered at a dosing interval of about 4 to about 6 hours, until the patient no longer requires treatment with tramadol. In preferred embodiments, the intravenous dosing regimen provides a Cmax and AUC of tramadol is similar to the Cmax and AUC of an oral dose of 100 mg tramadol HCl given every 6 hours. In certain preferred embodiments, the dosing regimen comprises 50 mg IV tramadol at Hour 0, followed by 50 mg at Hour 2, 50 mg at hour 4, and 50 mg every 4 hours thereafter (e.g., until the patient no longer requires treatment with tramadol).
Owner:REVOGENEX IRELAND
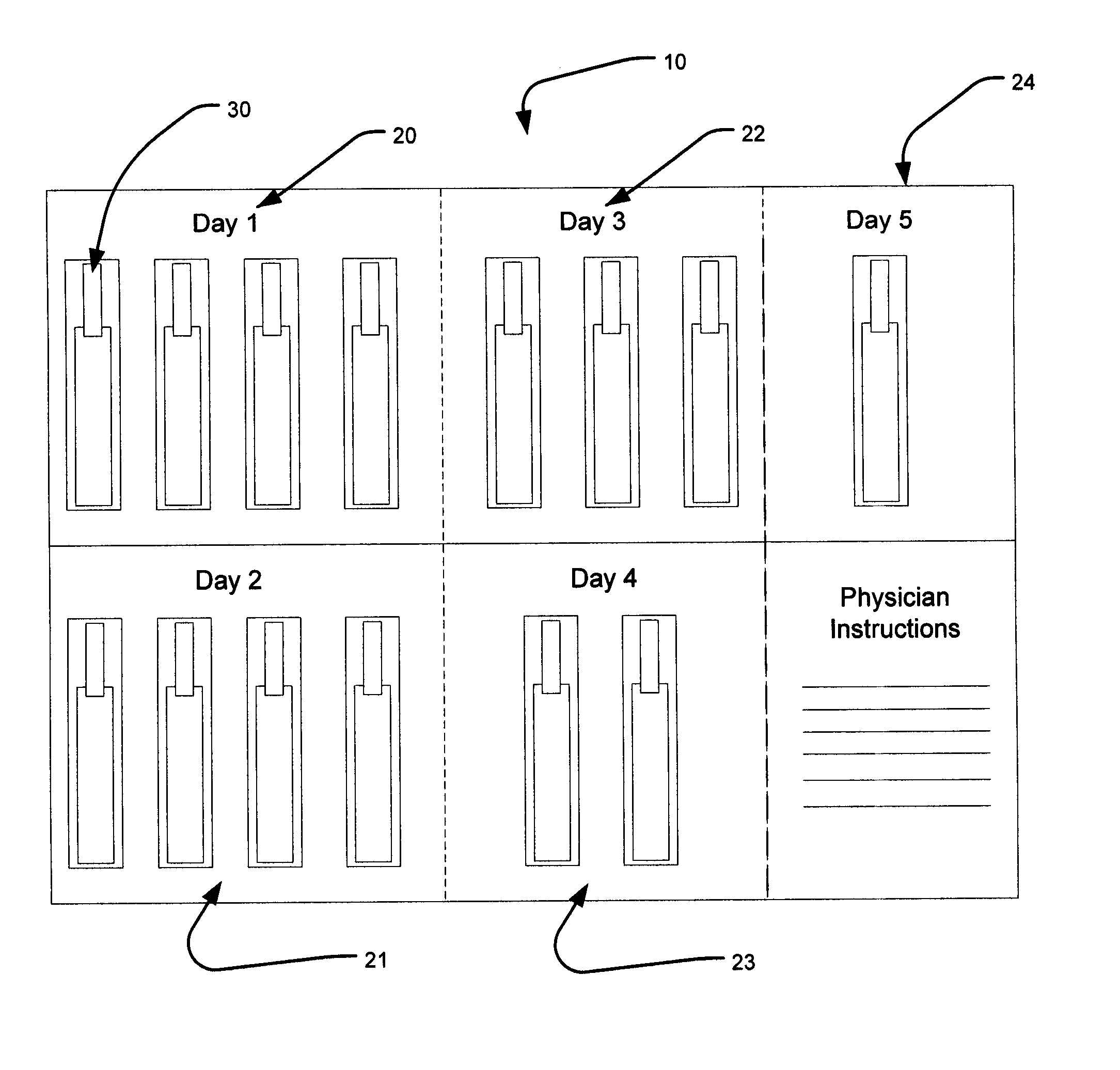
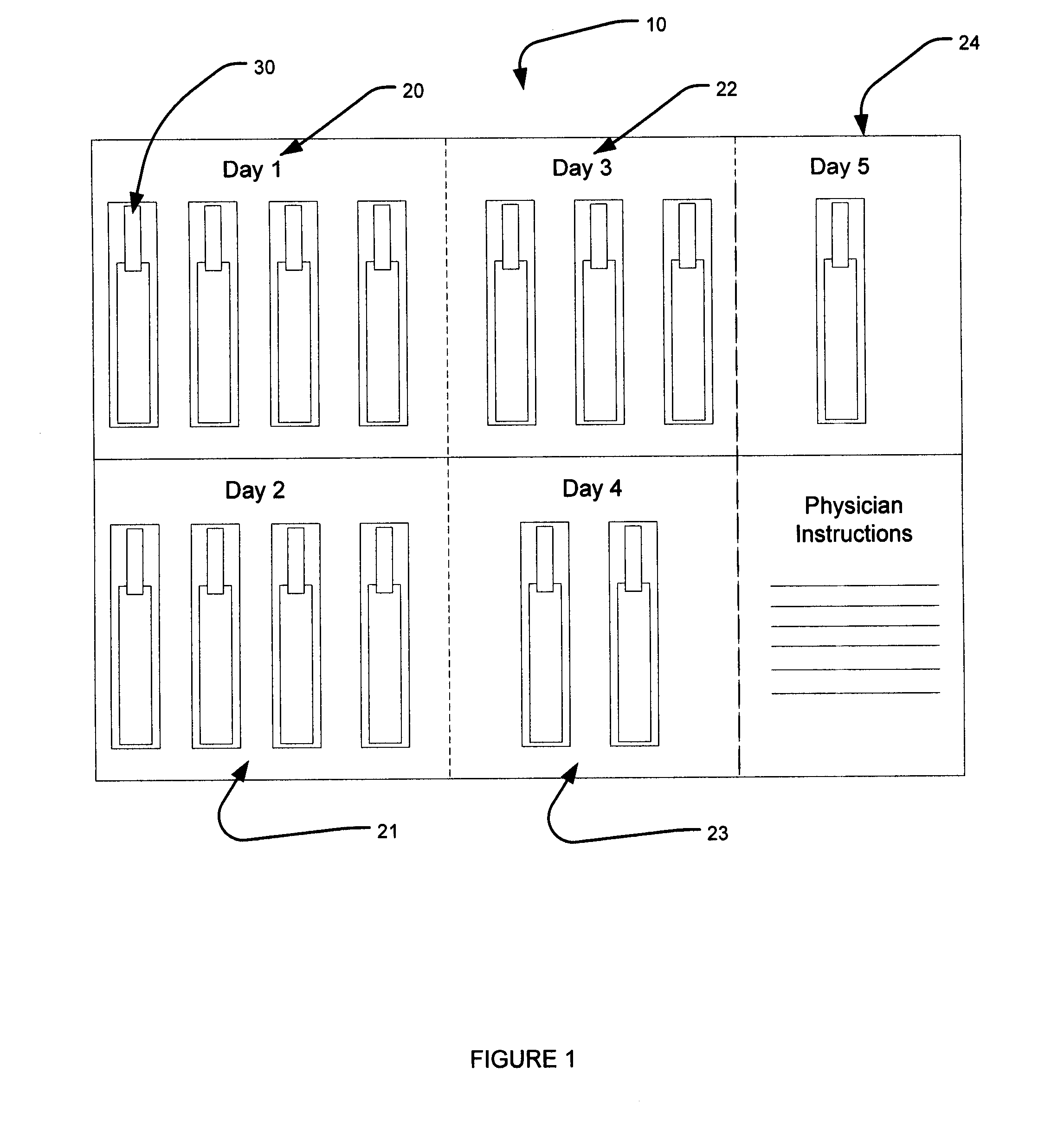
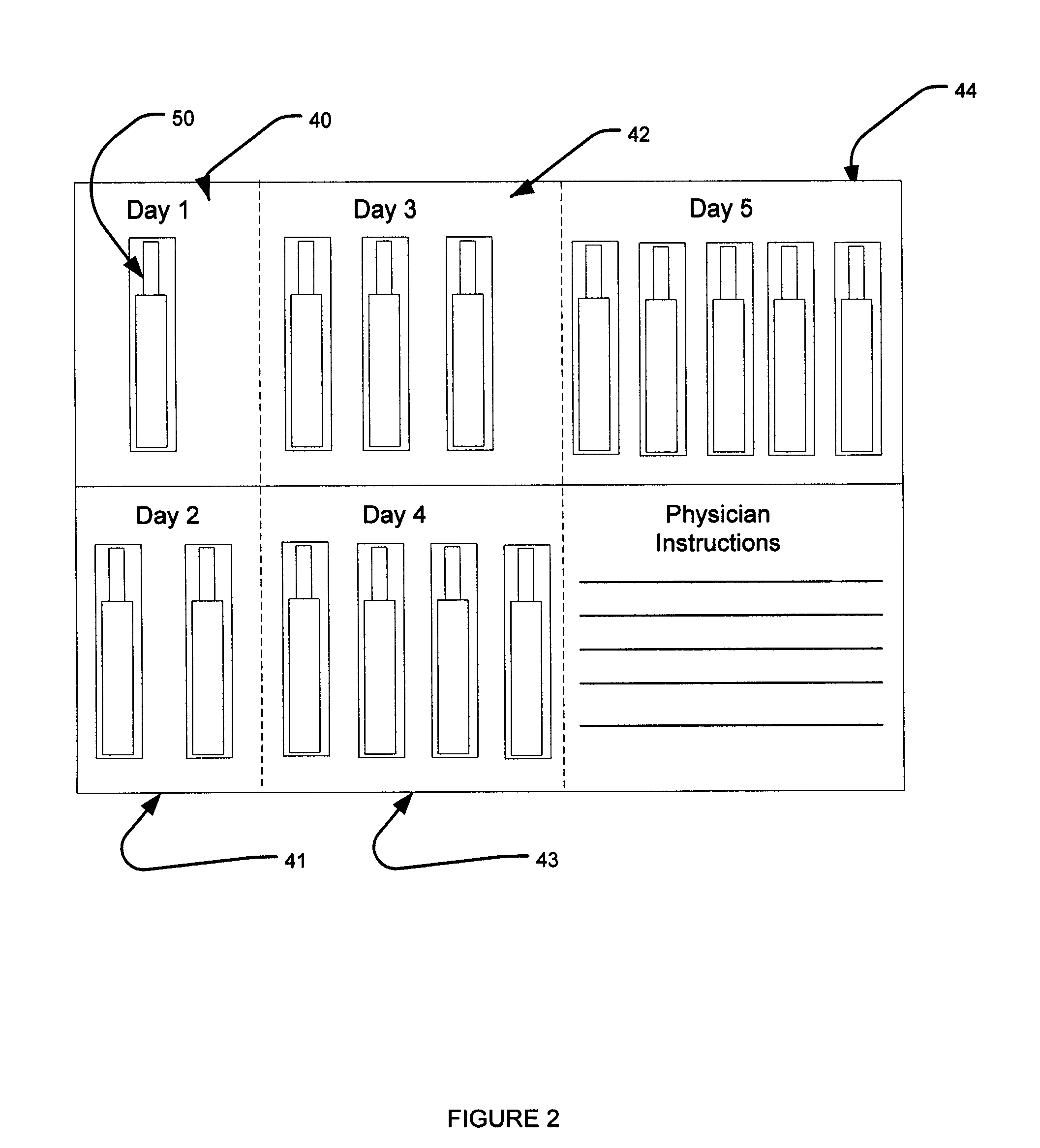
Dose packaging system for load-dose titration administration of a liquid formulation
The invention generally relates to a dose packaging system for the administration of a liquid formulation to a patient requiring load-dose titration therapy of a pharmaceutically active drug, such as steroid therapy. More particularly, the invention relates to a dose packaging system for administering titration therapy of liquid formulations to a patient which eliminates the disadvantages that are associated with cups, dosing spoons, calibrated oral syringes and bottles.
Owner:WARNER W RANDLOPH
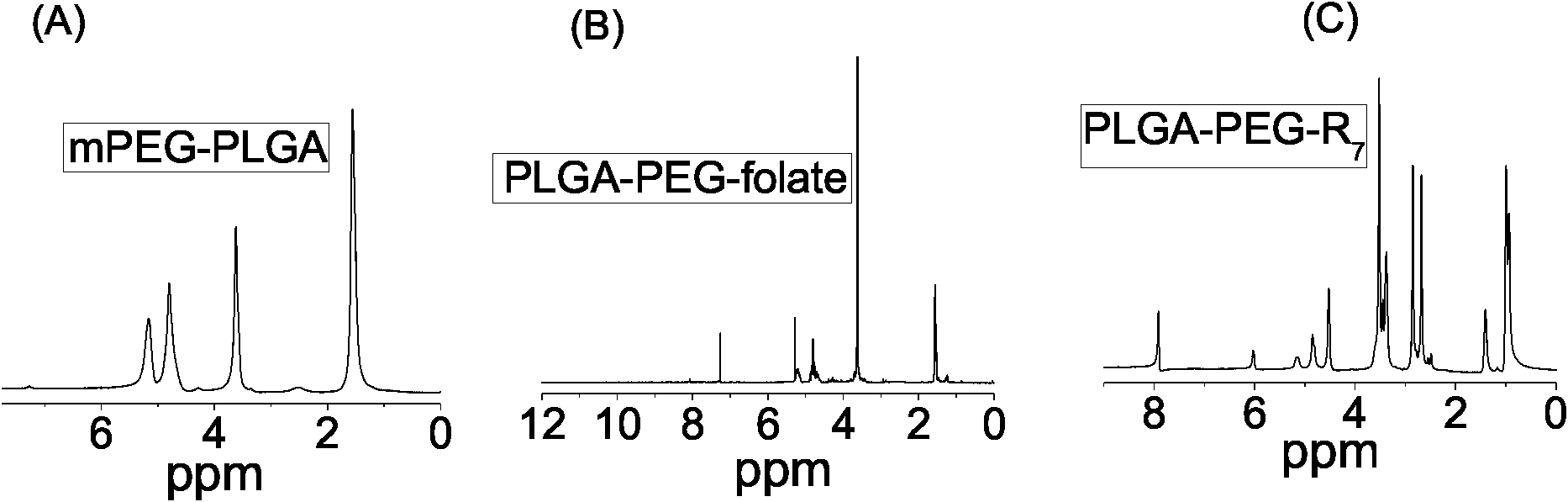
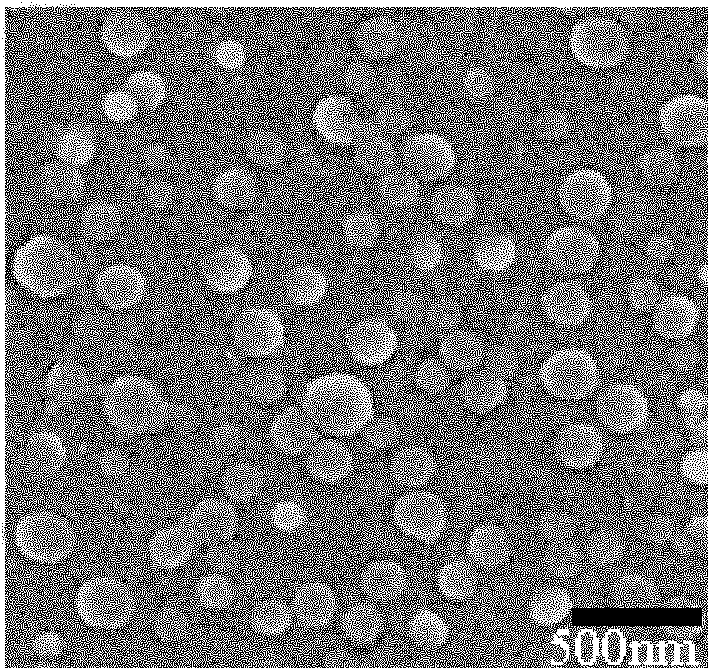
Preparation and application methods of difunctional naonparticle preparation entrapping vincristine sulphate
The invention relates to a preparation method of a difunctional naonparticle preparation entrapping vincristine sulphate, which belongs to the technical field of medicine. The difunctional naonparticle preparation is prepared by entrapping the vincristine sulphate in a PLGA-PEG polymer carrier modified by folic acid / cell-penetrating peptide through a multiple emulsion method. The difunctional naonparticle preparation shows favorable pharmacokinetic behaviors in vitro and vivo. The diameter of the prepared PLGA-PEG difunctional naonparticle modified by folic acid / cell-penetrating peptide is 287.2+ / -0.8nm, and the difunctional naonparticle preparation has high drug loading rate, high entrapment rate and good stability.
Owner:SHANGHAI JIAO TONG UNIV
Traditional Chinese medicine asiaticoside carrying core/shell structure nanometer fiber film preparation method and wound dressing use
The invention discloses a preparation method and use of a traditional Chinese medicine asiaticoside carrying core / shell structure nanometer fiber film. The traditional Chinese medicine asiaticoside carrying core / shell structure nanometer fiber film is prepared from a lactic acid / glycollic acid copolymer (PLGA) as a shell and polycaprolactone (PCL) as an inner core through a coaxial electrospinning method. The preparation method has the advantages of use of easily available raw materials, operation easiness, good controllability and large scale industrial production easiness. The traditional Chinese medicine asiaticoside carrying core / shell structure nanometer fiber film can be used for wound dressing and has the advantages of controllable drug loading amount, high drug utilization rate and excellent wound healing promoting effect.
Owner:杭州珈晟生物数字技术有限公司
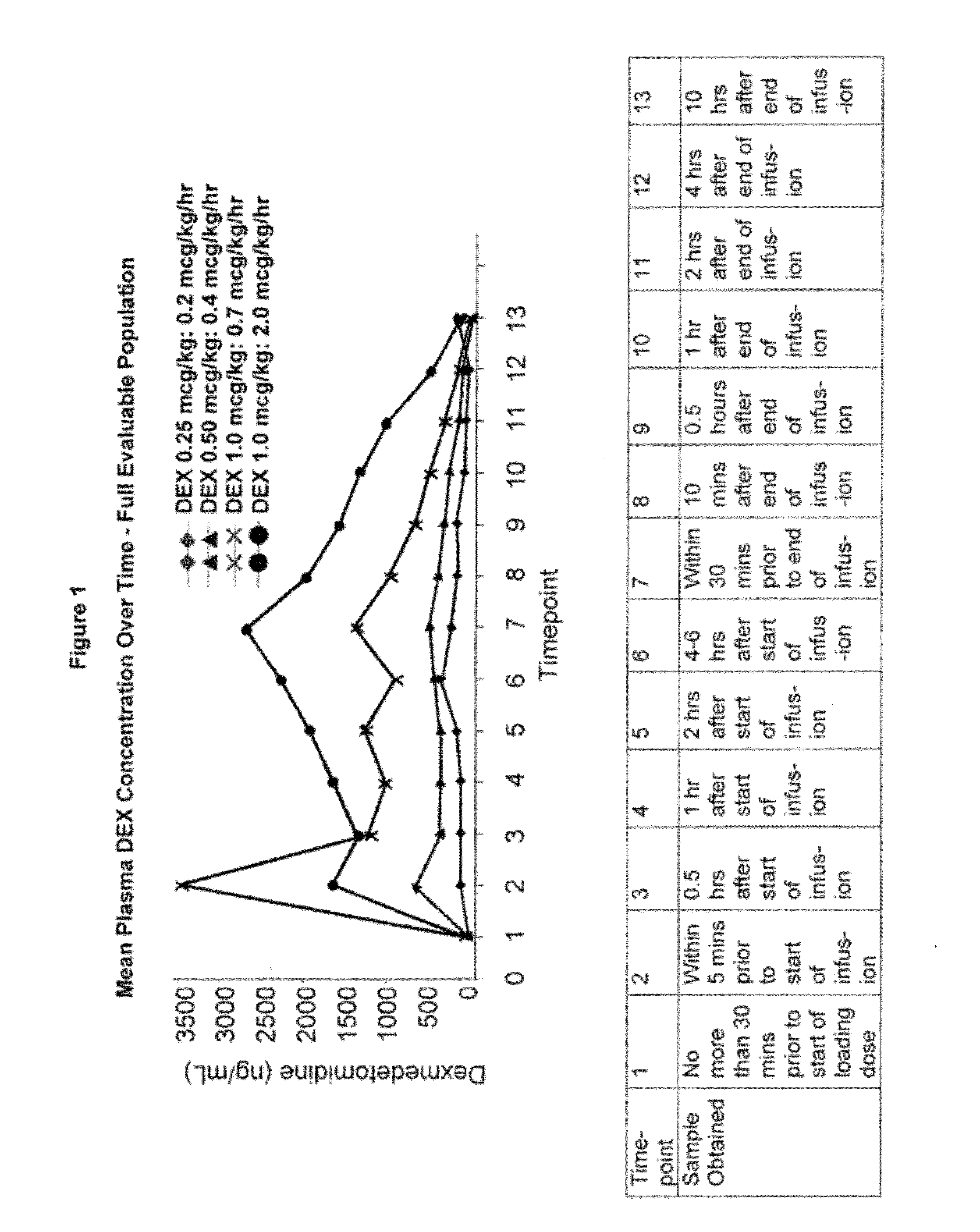
Methods of treating pediatric patients using dexmedetomidine
ActiveUS8324260B1Reduce morbidityReduce needBiocideOrganic active ingredientsPediatric patientSubject matter
The presently disclosed subject matter relates to methods of administering an effective amount of dexmedetomidine to a pediatric patient in order to reduce the incidence of neurological damage. More particularly, the presently disclosed subject matter relates to methods of providing sedation or analgesia to a pediatric patient by administering a dexmedetomidine infusion and optionally a loading dose. The dexmedetomidine can be administered before, during, or after surgery.
Owner:HOSPIRA
Paclitaxel lipid complexes and micelle composition thereof for injection
InactiveCN101439032AAddressing Particle Size IncreaseSolve the degradabilityOrganic active ingredientsPharmaceutical delivery mechanismChemistrySurface-active agents
The invention provides a paclitaxel lipid compound and a micellar compound used in the injection of the paclitaxel lipid compound. The paclitaxel liposome compound is composed of paclitaxel with a therapeutic dose, phospholipid, cholesterol sulfate or / and similar cholesterol derivative, additive and injection water. By means of the lipidization of the paclitaxel, the problem about organic menstruum of an injection and the problem of hypersusceptibility of a surface active agent are solved. A paclitaxel lipid compound injection provided by the invention has the advantages of small side effect, low blood vessel simulation, high drug-loading rate, narrow particle size distribution, capability of filtering and degerming, good pharmaceutical stability, etc.
Owner:SHENYANG WOSEN PHARMA INST
Drug-loaded soluble microneedle patch and preparation method thereof
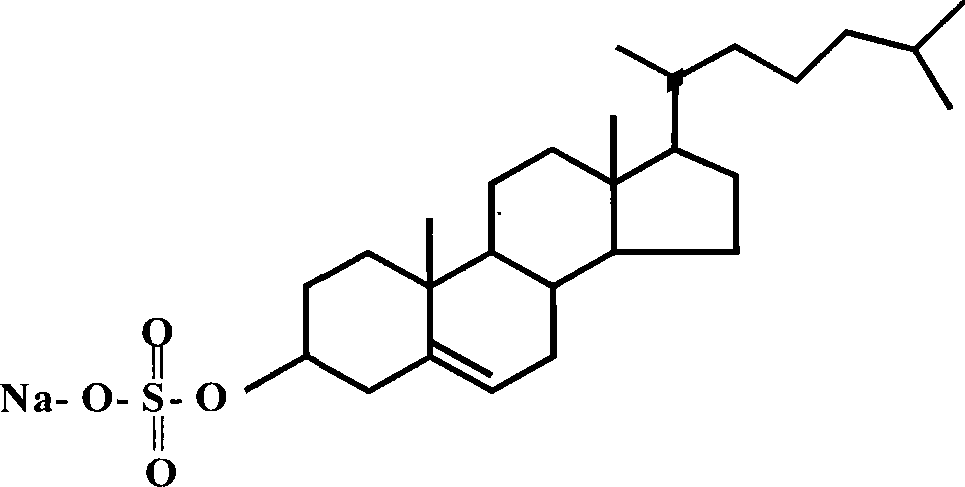
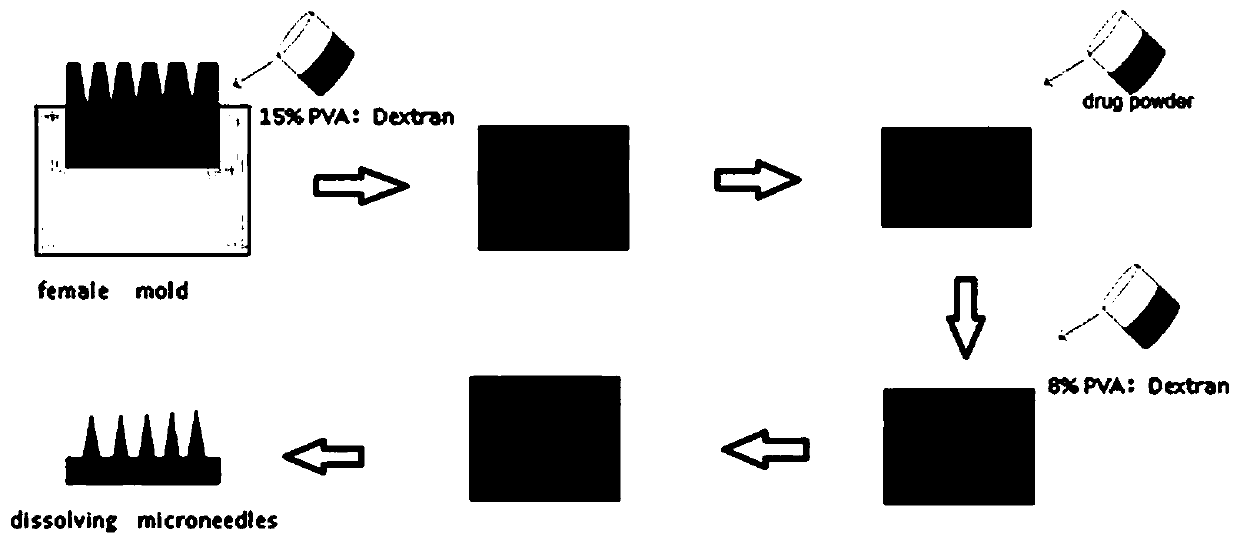
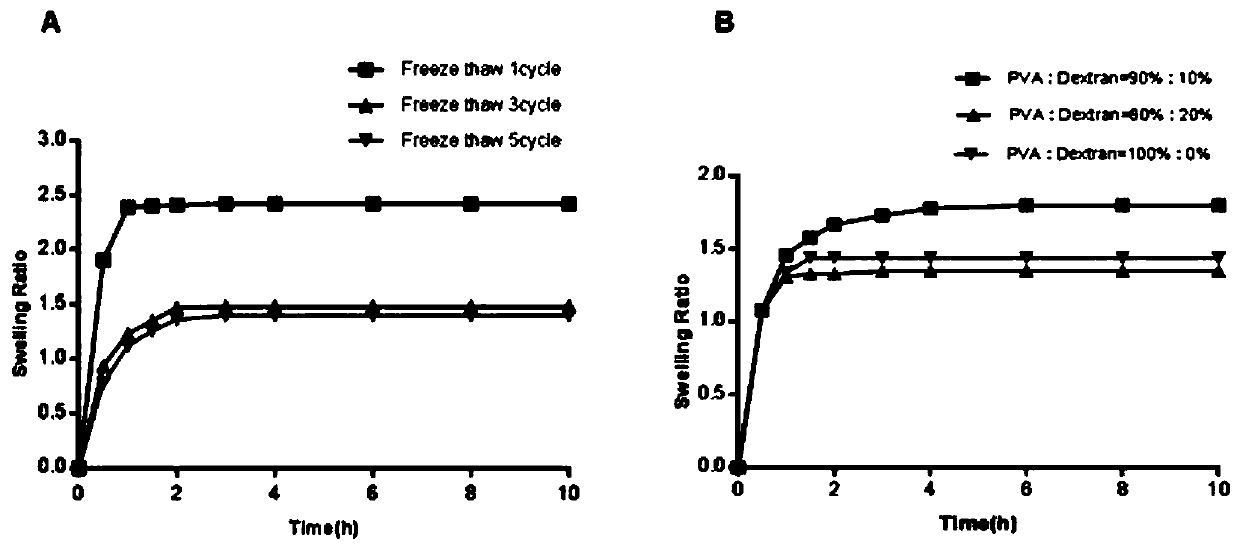
InactiveCN109701152ALarge storage volumeStable and sustained release effectMedical devicesPharmaceutical delivery mechanismSolubilityPolyvinyl alcohol
The invention belongs to the technical field of a microneedle, and concretely relates to a drug-loaded soluble microneedle patch and a preparation method thereof. The microneedle patch of the presentinvention comprises a needle and a base patch, and the needle and the base patch are prepared by centrifuging, freezing and thawing a polyvinyl alcohol-glucan solution with different mass fractions, the drug powder is placed between the needle and the base patch to form the drug-loaded soluble microneedle patch. The microneedle patch has high safety, good solubility, good mechanical properties andlarge drug loading; the invention also provides the preparation method of the microneedle patch, the method is simple and easy, does not require special equipment and preparation processes, and is suitable for popularization. In the microneedle patch of the invention, a sandwich layer between the tip of the needle and a substrate can be loaded with a large amount of solid powder medicine, which greatly increases the drug loading amount of the microneedle patch, and achieves the purpose of transdermal sustained release of the drug or vaccine.
Owner:ZHEJIANG UNIV OF TECH
Microprojection Array Application with Grouped Microprojections for High Drug Loading
InactiveUS20070293816A1Good skin permeabilityImprove permeabilitySurgeryMicroneedlesBiomedical engineeringBody surface
A transdermal drug delivery system with microprojections for disrupting a body surface to an individual. At least some of the microprojections form groups in a microprojection array. There are repeated units of such groups in the microprojection array.
Owner:ALZA CORP
Paeonol microemulsion preparation and preparation method thereof
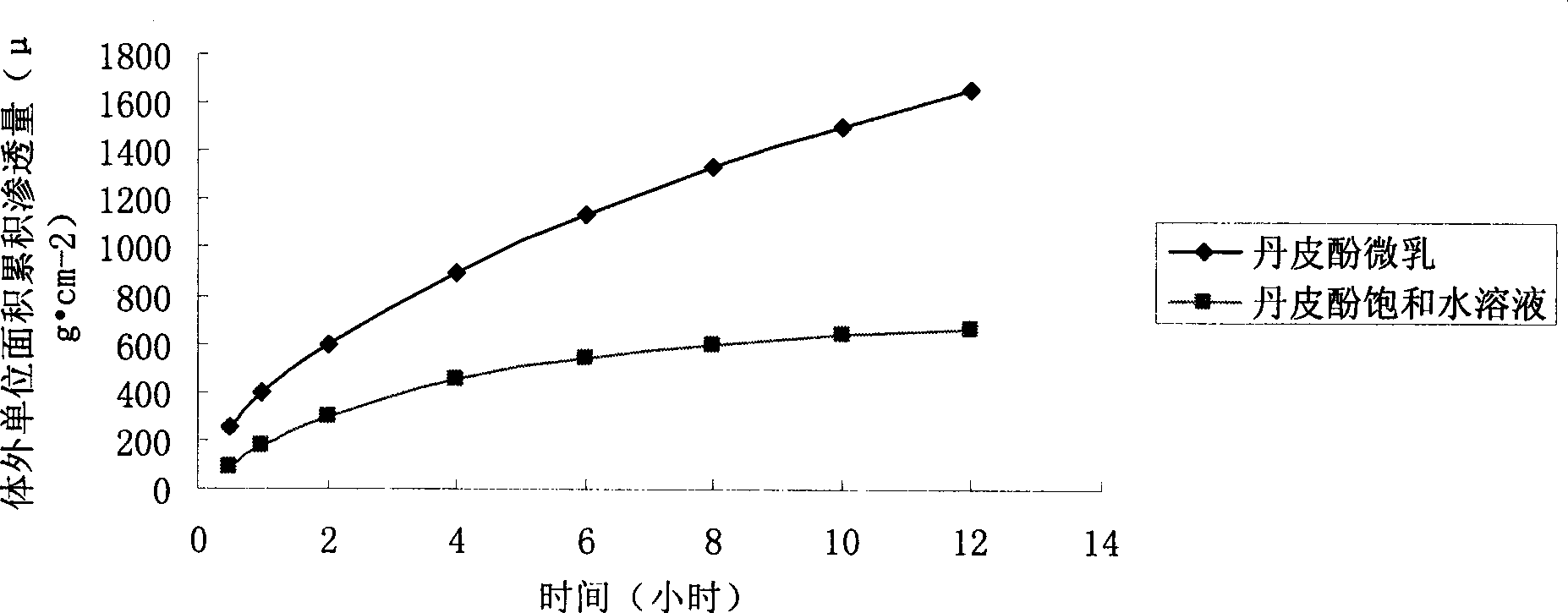
The invention relates to the medical technical field, in particular to a paeonol micro-emulsion preparation and a preparation method thereof. The paeonol micro-emulsion preparation consists of components with the weight percentage as follows: 0.1 percent to 2 percent of paeonol, 10 percent to 25 percent of surfactant, 5 percent to 30 percent of cosurfactant, 5 percent to 15 percent of oil phase and 40 percent to 70 percent of deionized water. The mouse percutaneous experiment in vitro shows that the paeonol micro-emulsion prepared by the invention has obviously higher capacity of penetrating through the horny layer than the saturated aqueous solution of paeonol (p is less than 0.05), and the paeonol micro-emulsion can penetrate into the skin deeply to take curative effect within a short time and also has certain sustained and controlled release function. At the same time, the paeonol micro-emulsion improves the solubility of the paeonol obviously, increases the drug loading quantity of the preparation and reduces the drug administration times and the dosage. The paeonol micro-emulsion preparation has simple preparation method and uniform grain diameter of the preparation, has the advantages of being safe, stable and efficient and, can be used for preparing dermal medication preparation or cosmetic.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Nanoparticle for embedding medicinal Adriamycin as well as preparation method and application thereof
InactiveCN101785759AImprove packaging efficiencyImprove stabilityOrganic active ingredientsPowder deliveryTreatment fieldBiocompatibility Testing
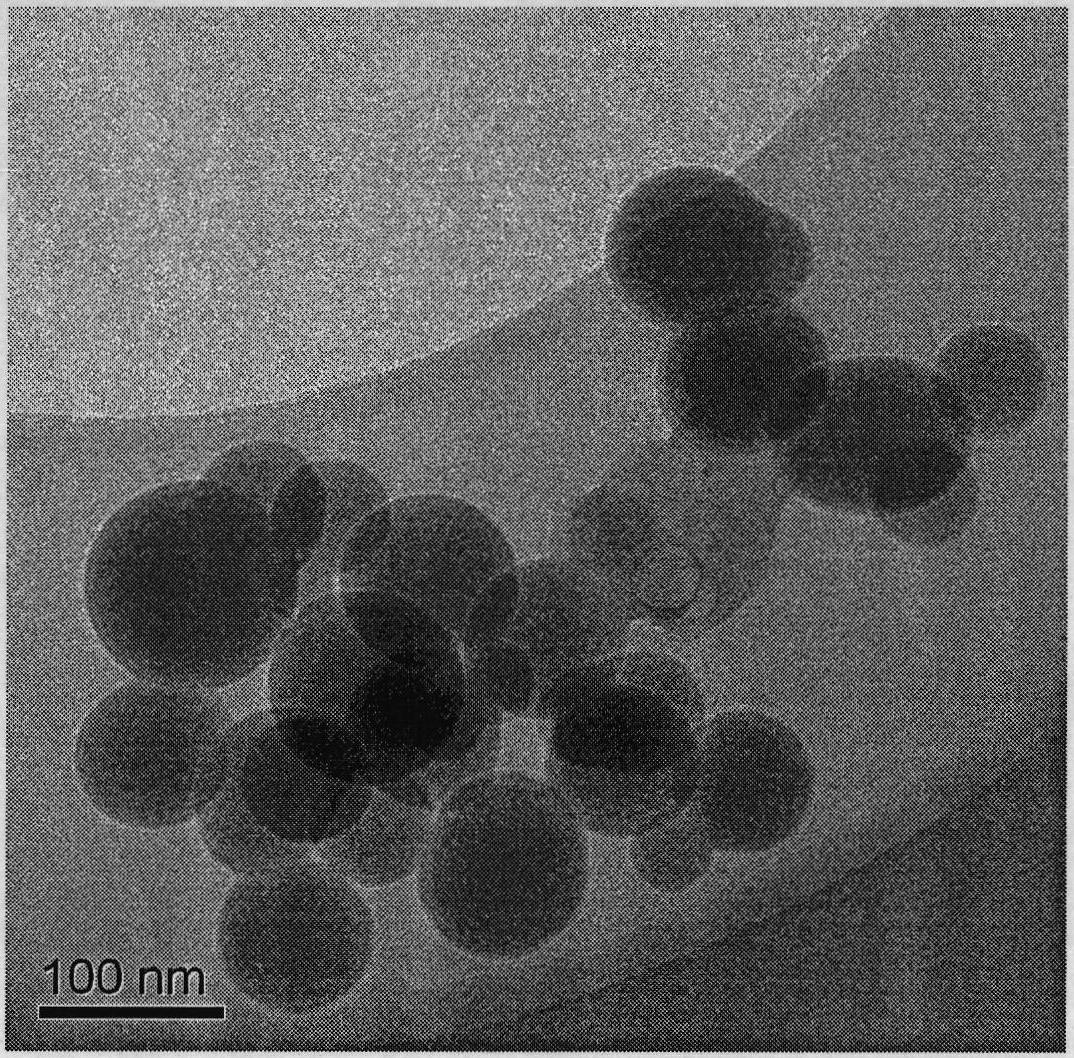
The invention belongs to the technical field of composite medicine materials as well as a preparation method and application thereof, more particularly discloses a nanoparticle for embedding medicinal Adriamycin. The nanoparticle has a core-shell type structure with an inner core embedded by an outer shell, wherein the inner core is embedded medicinal adriamycin, and the material for the outer shell is silicon dioxide; and the preparation method of the nanoparticle comprises the following steps of: evenly mixing cyclohexane, a surfactant and n-hexylalcohol, adding a sodium fluoride solution into the mixed solution after being evenly mixed to form a reverse-phase microemulsion; adding adriamycin and tetraethoxysilane into the reverse-phase microemulsion, and reacting to obtain a nanoparticle microemulsion system for embedding the adriamycin; adding a silylanization reagent containing functional groups into the microemulsion system, stirring for reacting, adding ethanol and demulsifying, centrifuging and then preparing the nanoparticle for embedding the medicinal adriamycin and modifying the functional groups. The nanoparticle embedding for the adriamycin has good stability, good biocompatibility, long slow-release time, large drug-loading rate, high medicine packaging rate, and the like, and has application prospect in the fields of tumor imaging and treatment.
Owner:HUNAN UNIV
Ultrathin calcium silicate nanosheet with ultrahigh specific surface area and preparation method thereof
ActiveCN102923725AImprove adsorption capacityHigh drug loadingMaterial nanotechnologyAlkaline-earth metal silicatesCalcium silicateMaterials science
The invention provides an ultrathin calcium silicate nanosheet with an ultrahigh specific surface area and a preparation method thereof. According to the ultrathin calcium silicate nanosheet with the ultrahigh specific surface area, the mole ratio of calcium to silicon is 0.4-1.5, the BET specific surface area is 200-550m<2> / g, and the thickness of the nanosheet is 1-10nm. The ultrathin calcium silicate hydrate nanosheet and an ultrathin non-crystal water calcium silicate nanosheet prepared by the method are uniform in shape and size and ultrathin in thickness, and the specific surface areas of the ultrathin calcium silicate hydrate nanosheet and the ultrathin non-crystal water calcium silicate nanosheet are larger than those of other calcium silicate nanosheets. Compared with the other methods in the prior art, the method provided by the invention has the advantages that the prepared ultrathin calcium silicate hydrate nanosheet and the prepared ultrathin non-crystal water calcium silicate nanosheet are very large in specific surface area, can be used as drug carriers, have extremely high drug loading capacity and good drug slow release performance on drugs difficult to dissolve in water, have the extremely strong capability of absorbing protein and heavy metal ions, and have a good application prospect in the fields of biological medicine and water treatment.
Owner:SHANGHAI INST OF CERAMIC CHEM & TECH CHINESE ACAD OF SCI
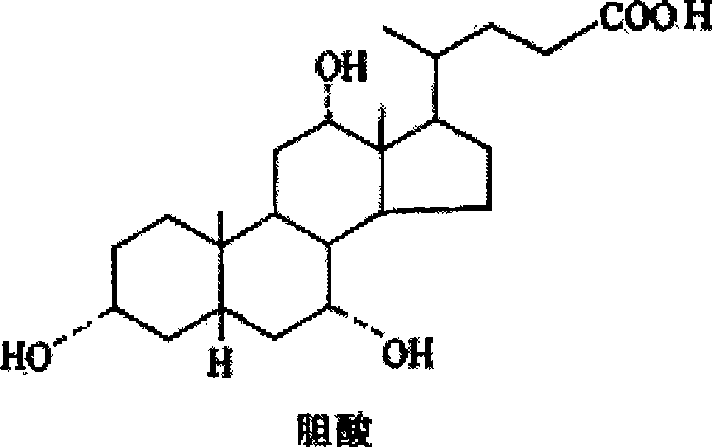
Bicyclo-ethanol submicron emulsion and preparation method thereof
ActiveCN101524329AImprove solubilityGood chemical stabilityDigestive systemEmulsion deliveryOrganosolvOil phase
The invention discloses a bicyclo-ethanol submicron emulsion and a preparation method thereof. the preparation method comprises the steps of dissolving bicyclo-ethanol and emulsifying agent in an oil phase, adding an assistant for emulsifying agent, a stabilizing agent, other additive and a water phase, adopting a cutting dispersing and high-pressure homogeneous emulsification process to prepare an oil-in-water(O / W) submicron emulsion with the average grain diameter below 500 nm and drug loading dosage between 0.01mg / ml and 5mg / ml. The bicyclo-ethanol submicron emulsion is injected through vein and used for treating medium and serious hepatitis. The prepared submicron emulsion does not contain solubilizer such as Tween-80 or organic solvent, can be mixed with glucose injection, physiological saline or distilled water according to random proportion, and can not easily generate insoluble particulates when stored, used or matched with other components, thereby having high security and good stability. The invention also relates to various preparations of the bicyclo-ethanol submicron emulsion.
Owner:INST OF MATERIA MEDICA CHINESE ACAD OF MEDICAL SCI +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com