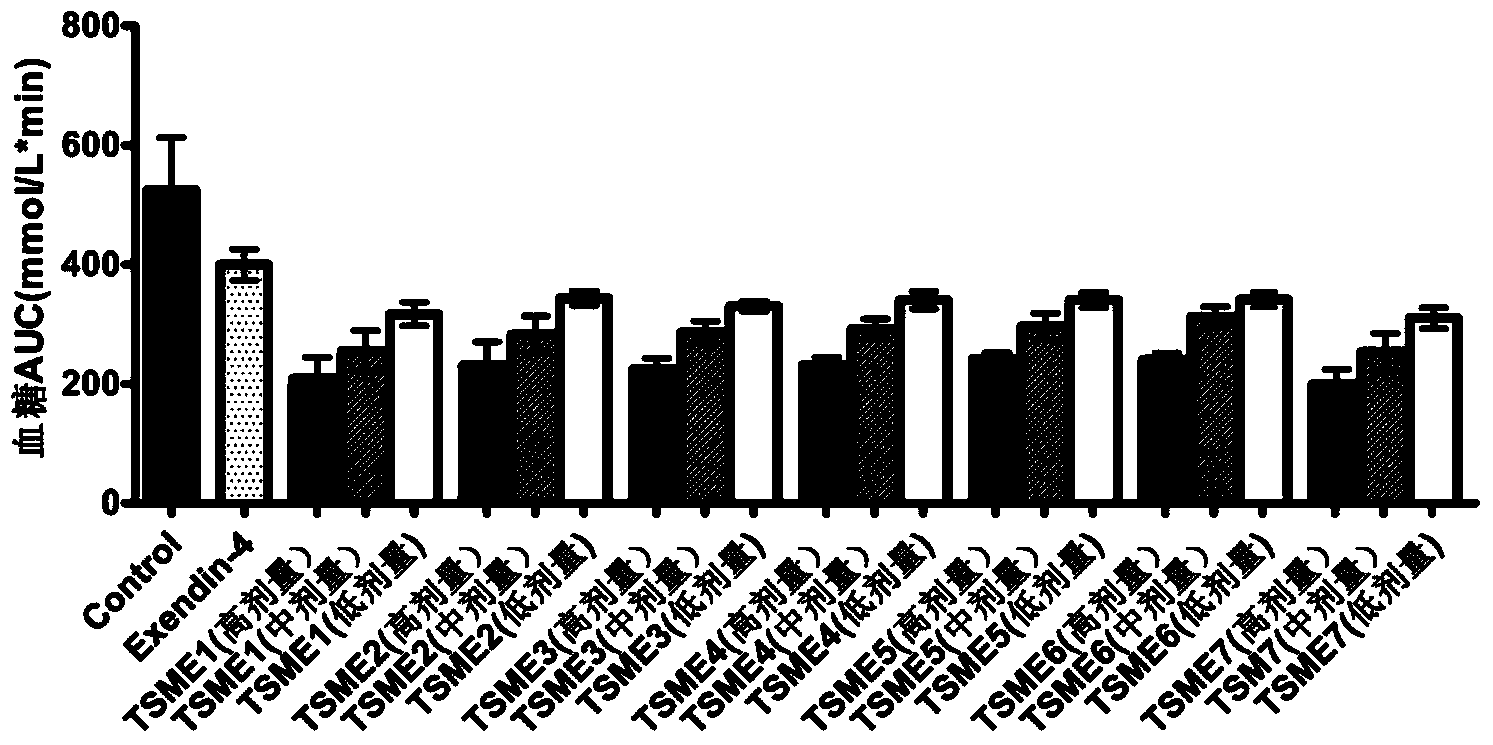Patents
Literature
321 results about "Oral dose" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Controlled release formulations of opioid and nonopioid analgesics
InactiveUS20050158382A1Reduce the maximumRapid rise in plasma concentrationBiocideNervous disorderImmediate releaseAnalgesic agents
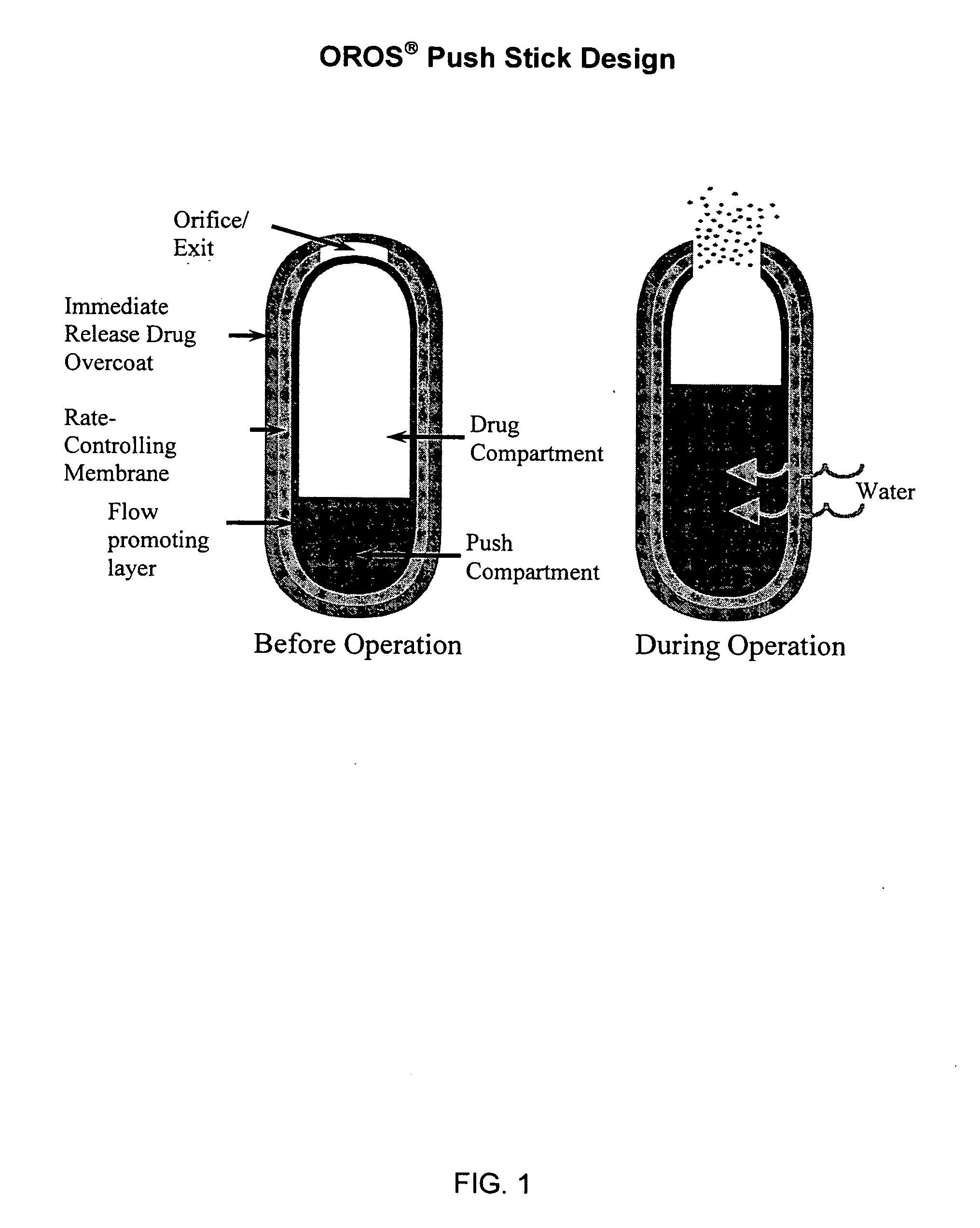
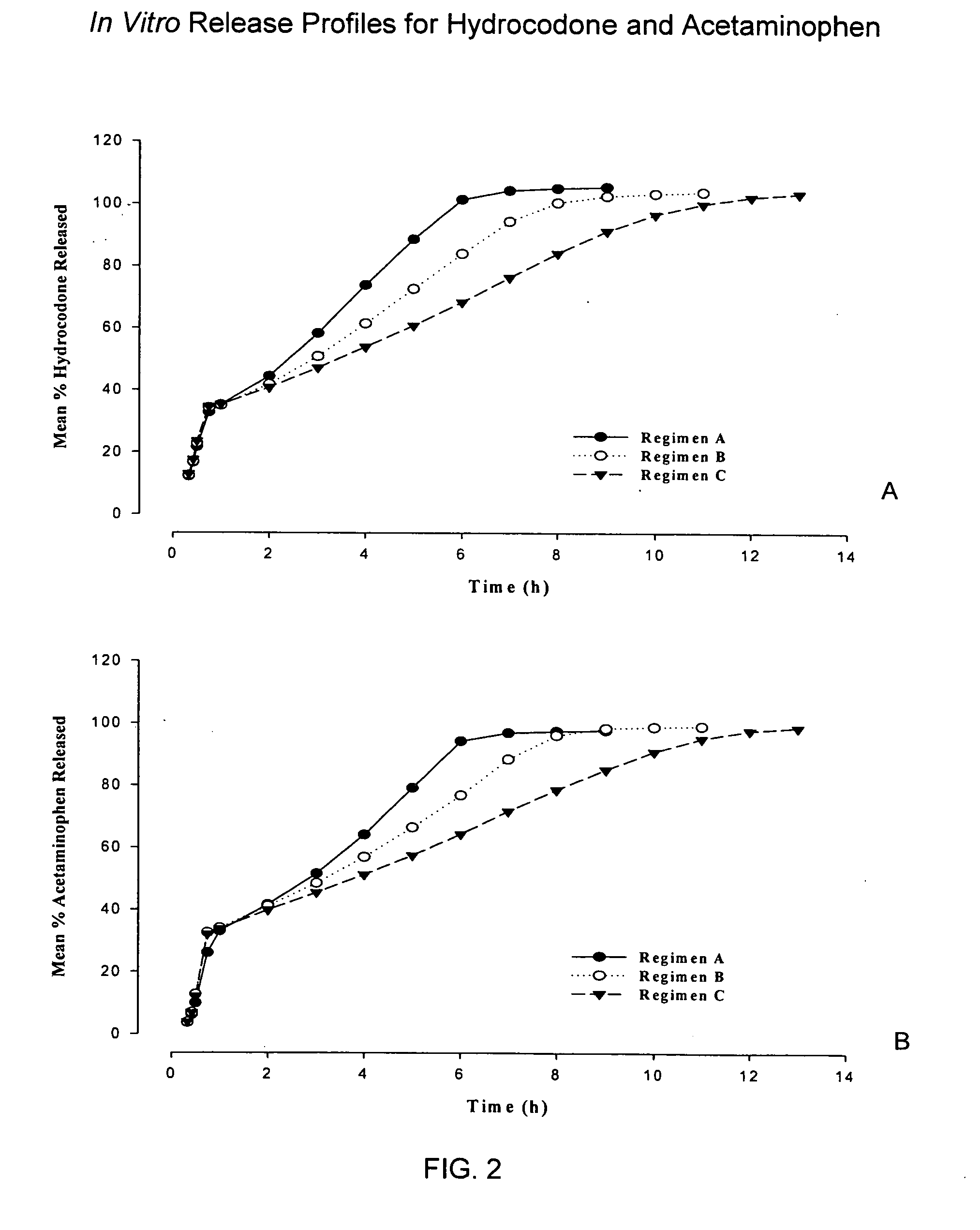
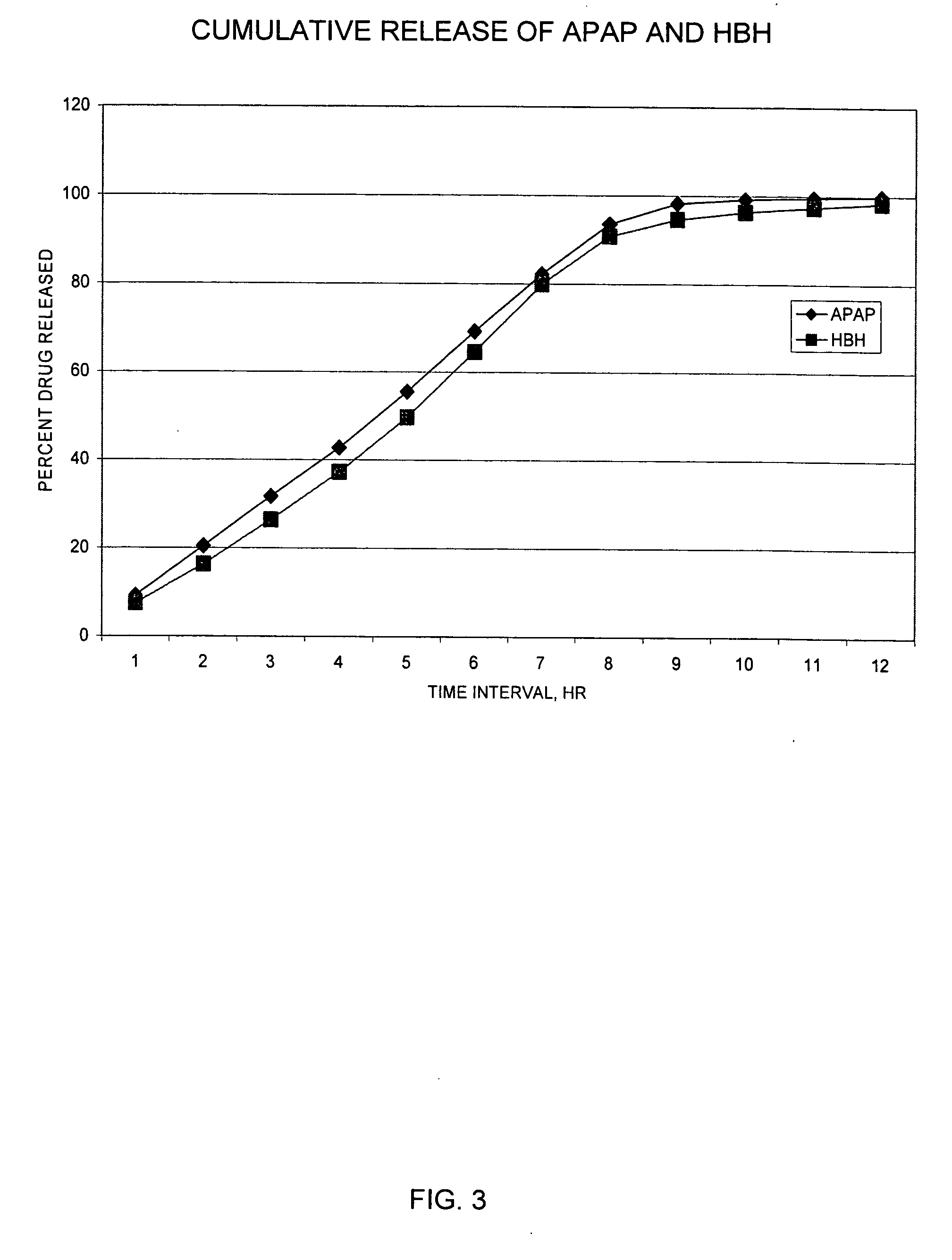
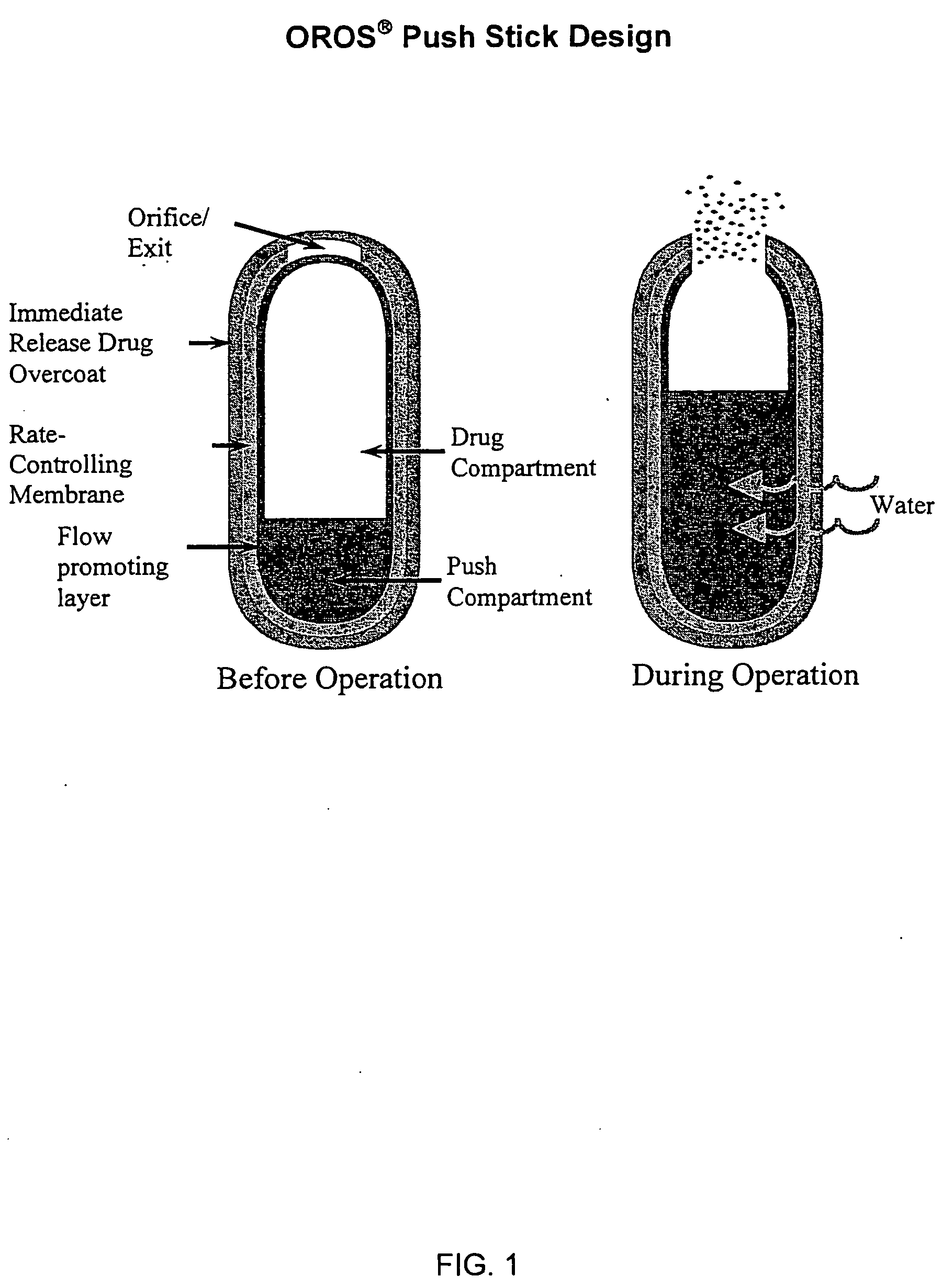
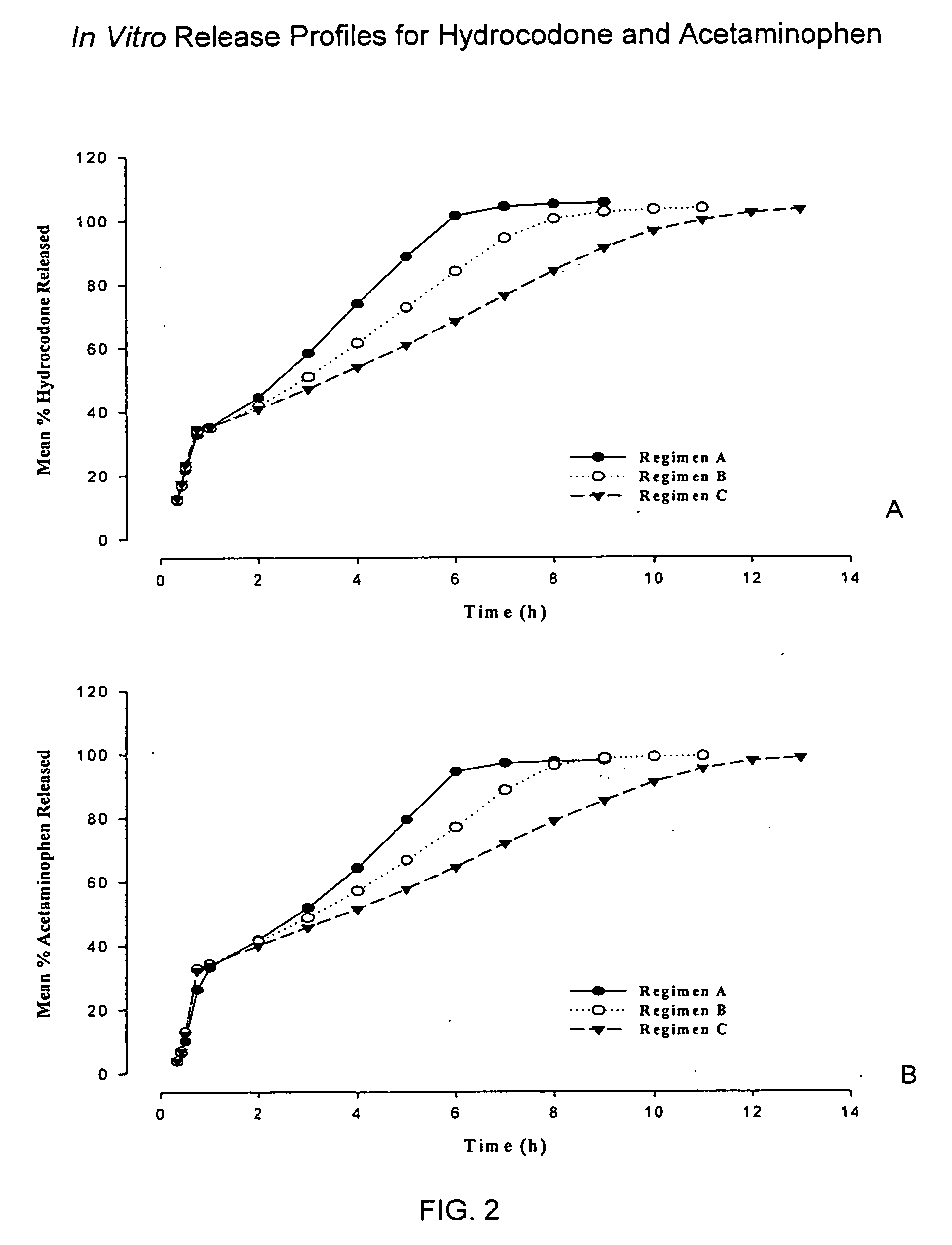
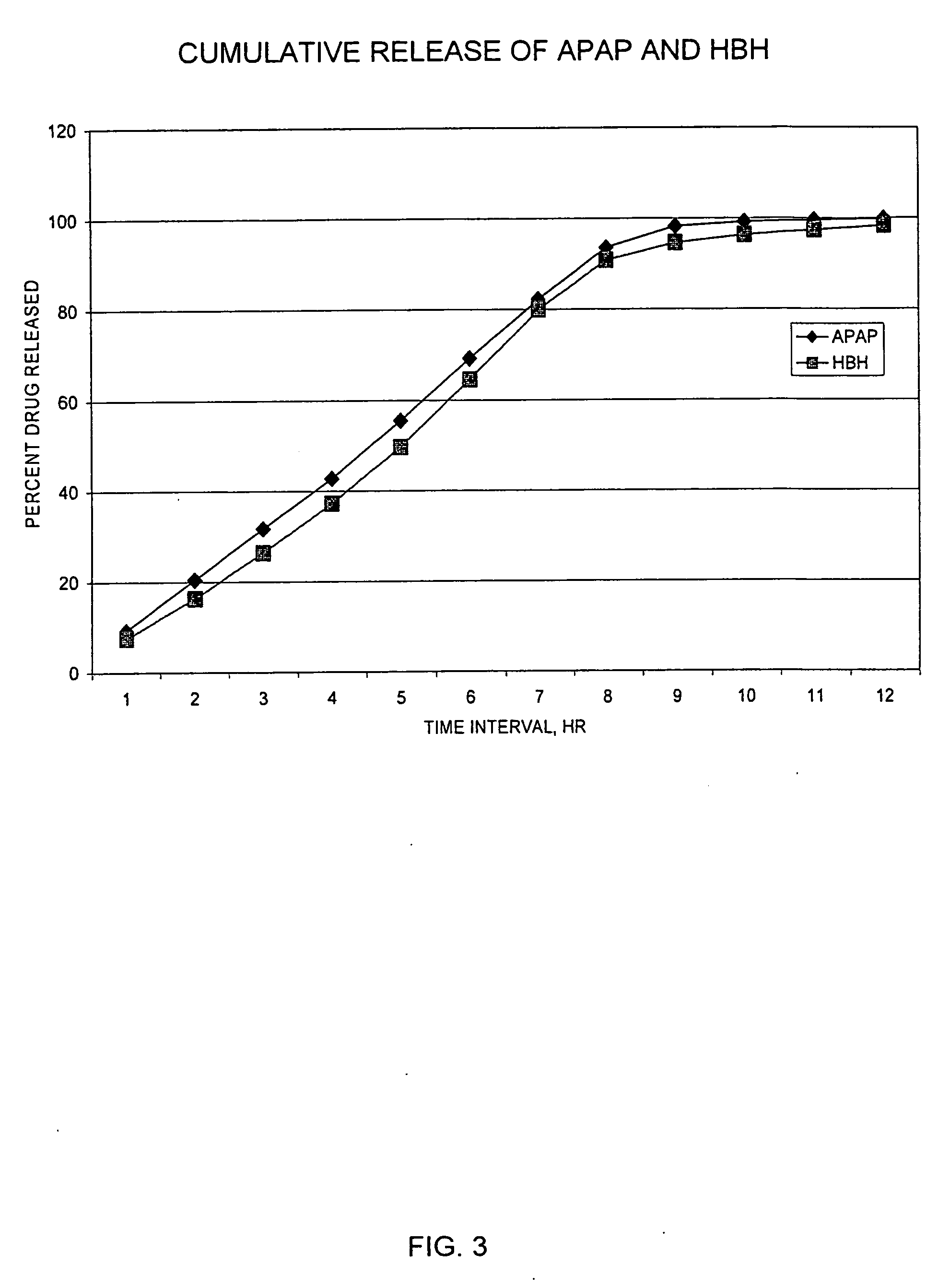
Sustained release dosage forms for twice daily oral dosing to a human patient for providing relief from pain are provided. The sustained release dosage form comprises an immediate release component and a sustained release component, wherein the immediate release component and the sustained release component collectively contain a therapeutically effective amount of an opioid analgesic and a therapeutically effective amount of nonopioid analgesic. In a preferred embodiment, the nonopioid analgesic is acetaminophen and the opioid analgesic is hydrocodone and pharmaceutically acceptable salts thereof, and in preferred embodiments, the pharmaceutically acceptable salt is bitartrate. The dosage forms produce plasma profiles in a patient characterized by a Cmax for hydrocodone of between about 0.6 ng / mL / mg to about 1.4 ng / mL / mg and an AUC for hydrocodone of between about 9.1 ng*hr / mL / mg to about 19.9 ng*hr / mL / mg (per mg hydrocodone bitartrate administered) and a Cmax for acetaminophen of between about 2.8 ng / mL / mg and 7.9 ng / mL / mg and an AUC for acetaminophen of between about 28.6 ng*hr / mL / mg and about 59.1 ng*hr / mL / mg (per mg acetaminophen administered) after a single dose.
Owner:ALZA CORP
Controlled release formulations of opioid and nonopioid analgesics
InactiveUS20060251721A1Improved ability to treat painLess attentionBiocideNervous disorderImmediate releasePharmaceutical medicine
Sustained release dosage forms for twice daily oral dosing to a human patient for providing relief from pain are provided. The sustained release dosage form comprises an immediate release component and a sustained release component, wherein the immediate release component and the sustained release component collectively contain a therapeutically effective amount of an opioid analgesic and a therapeutically effective amount of nonopioid analgesic. In a preferred embodiment, the nonopioid analgesic is acetaminophen and the opioid analgesic is hydrocodone and pharmaceutically acceptable salts thereof, and in preferred embodiments, the pharmaceutically acceptable salt is bitartrate. The dosage forms produce plasma profiles in a patient characterized by a Cmax for hydrocodone of between about 0.6 ng / mL / mg to about 1.4 ng / mL / mg and an AUC for hydrocodone of between about 9.1 ng*hr / mL / mg to about 19.9 ng*hr / mL / mg (per mg hydrocodone bitartrate administered) and a Cmax for acetaminophen of between about 2.8 ng / mL / mg and 7.9 ng / mL / mg and an AUC for acetaminophen of between about 28.6 ng*hr / mL / mg and about 59.1 ng*hr / mL / mg (per mg acetaminophen administered) after a single dose.
Owner:ALZA CORP
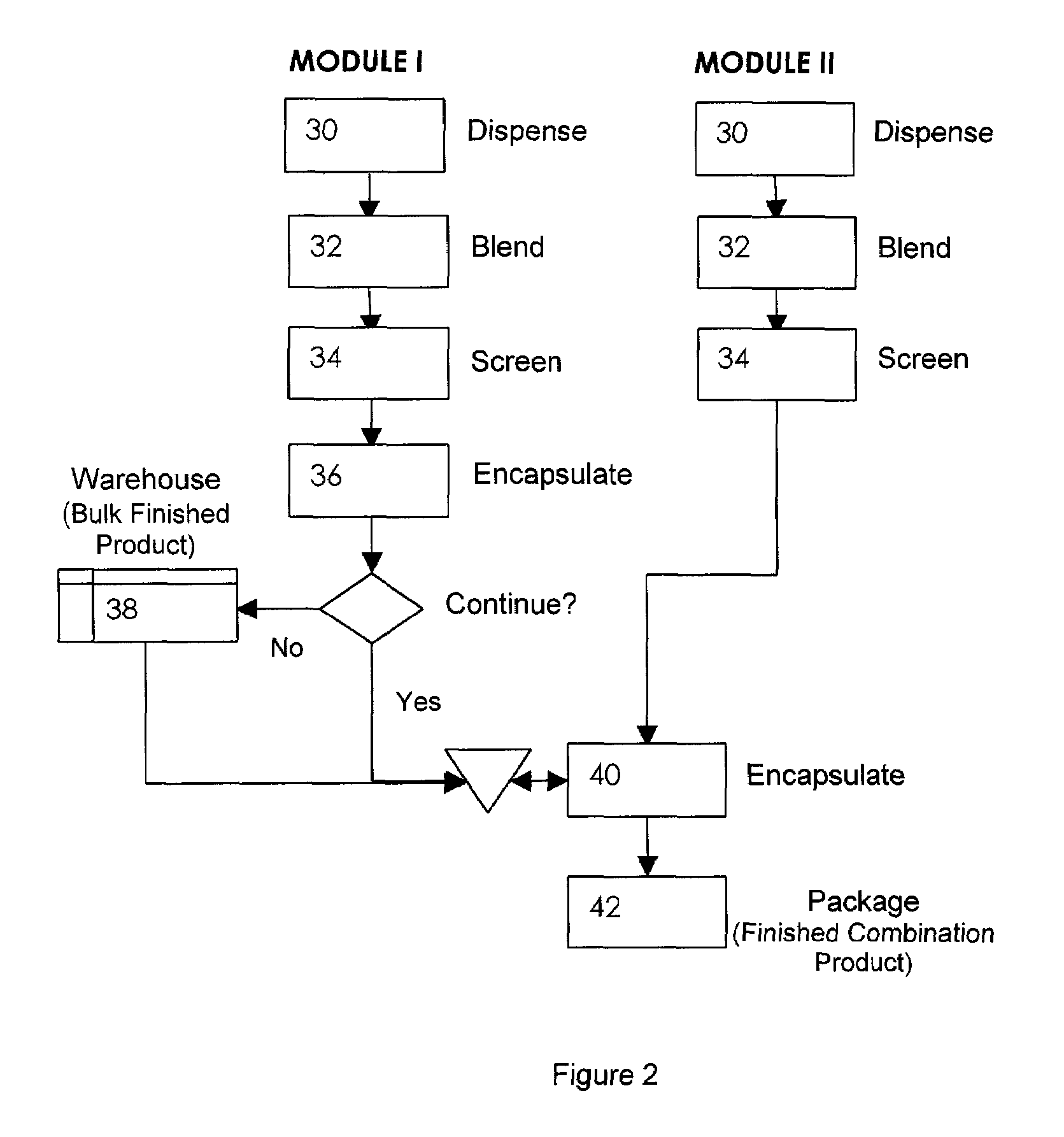
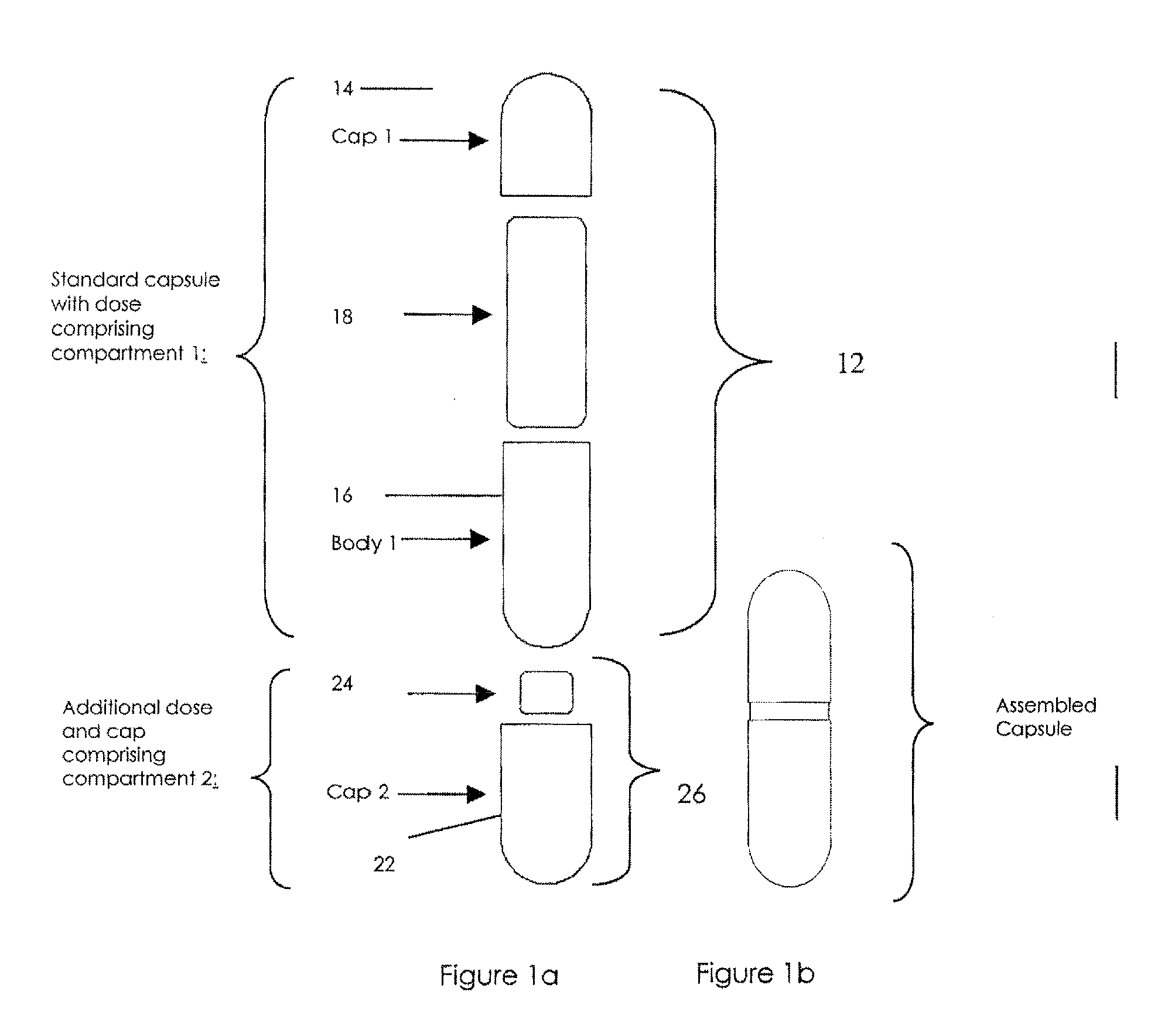
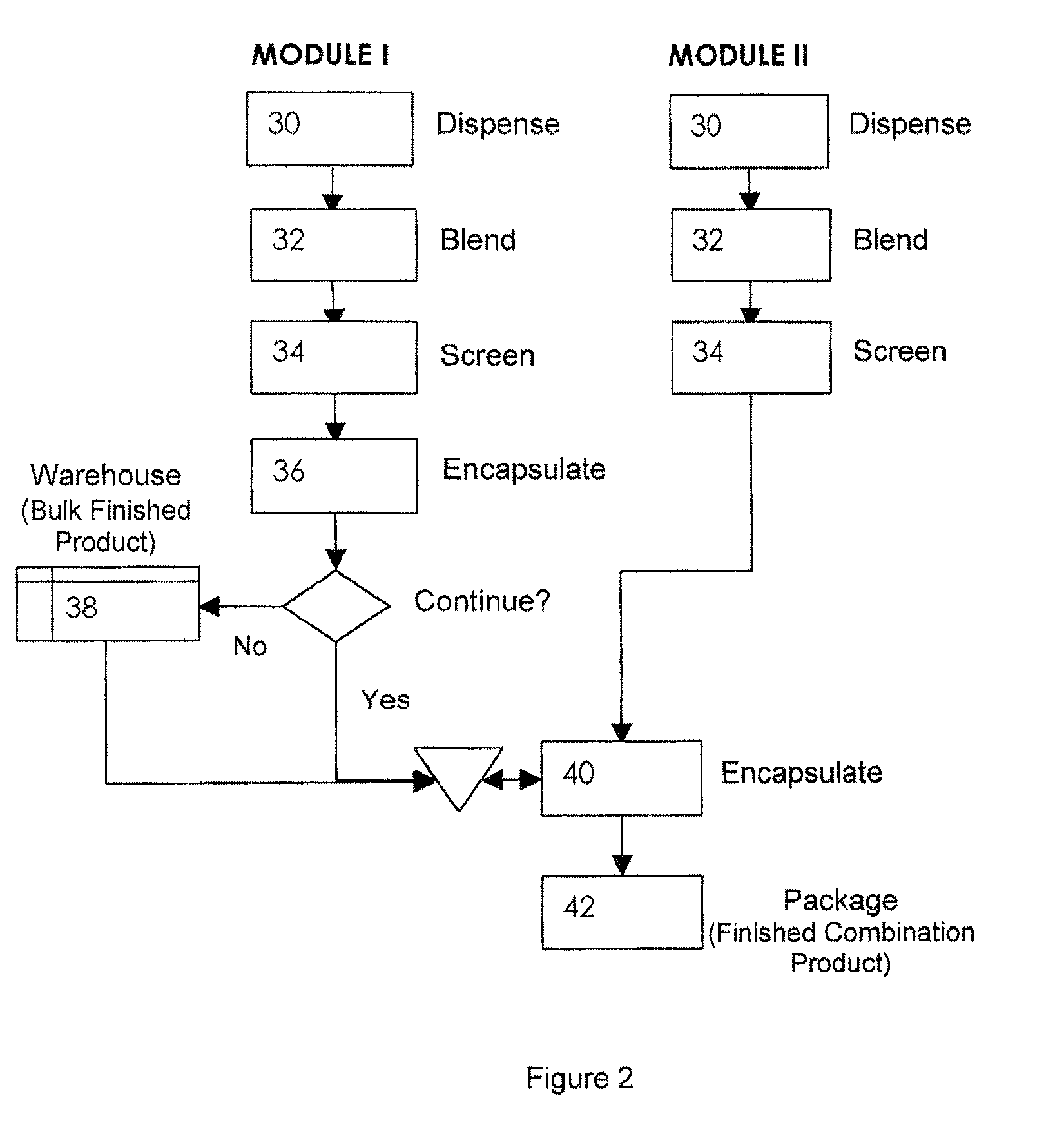
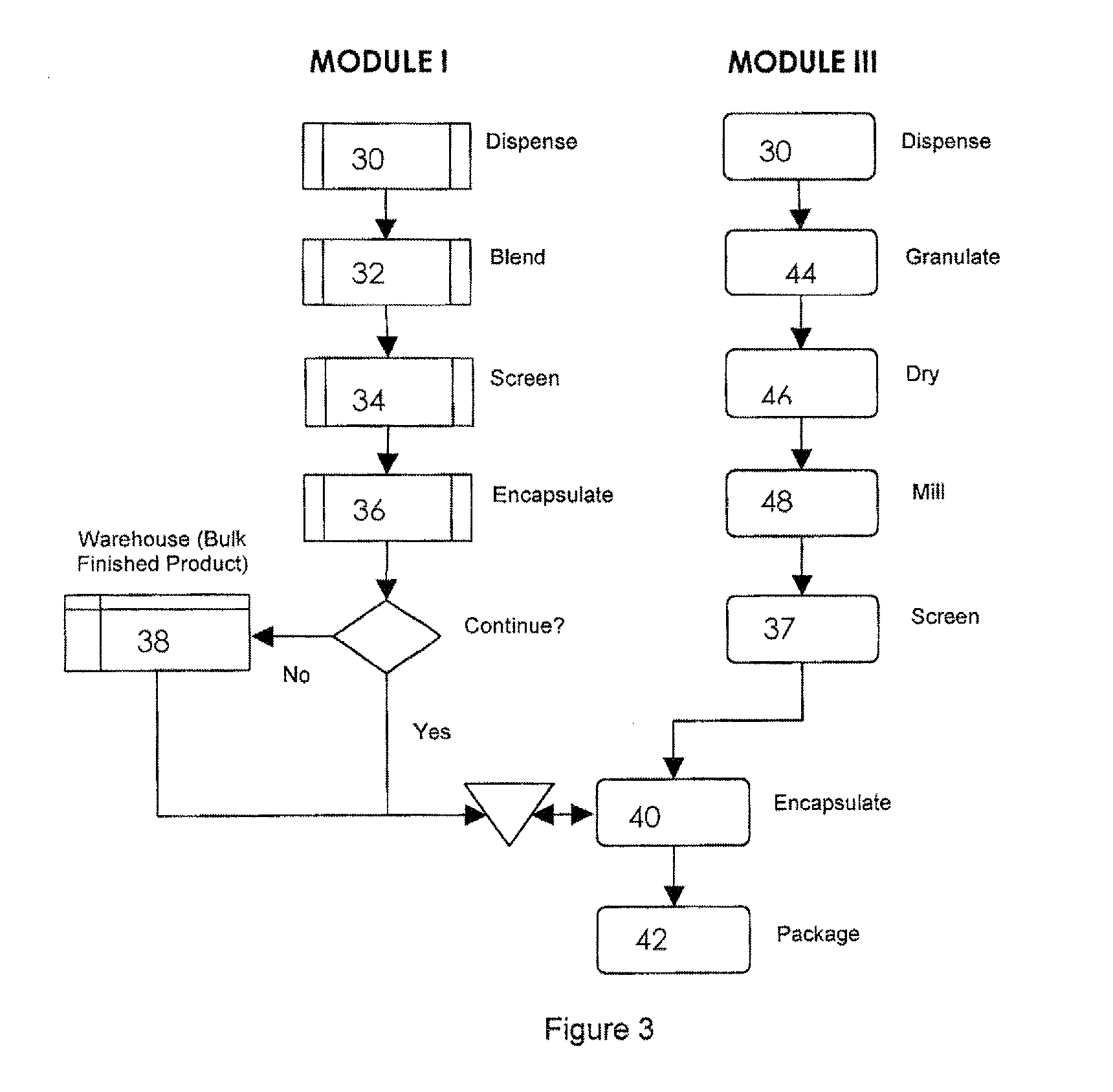
Oral dosage combination pharmaceutical packaging
InactiveUS20090087483A1Overall design flexibilityIncreased riskAntibacterial agentsBiocideMedicinePharmaceutical packaging
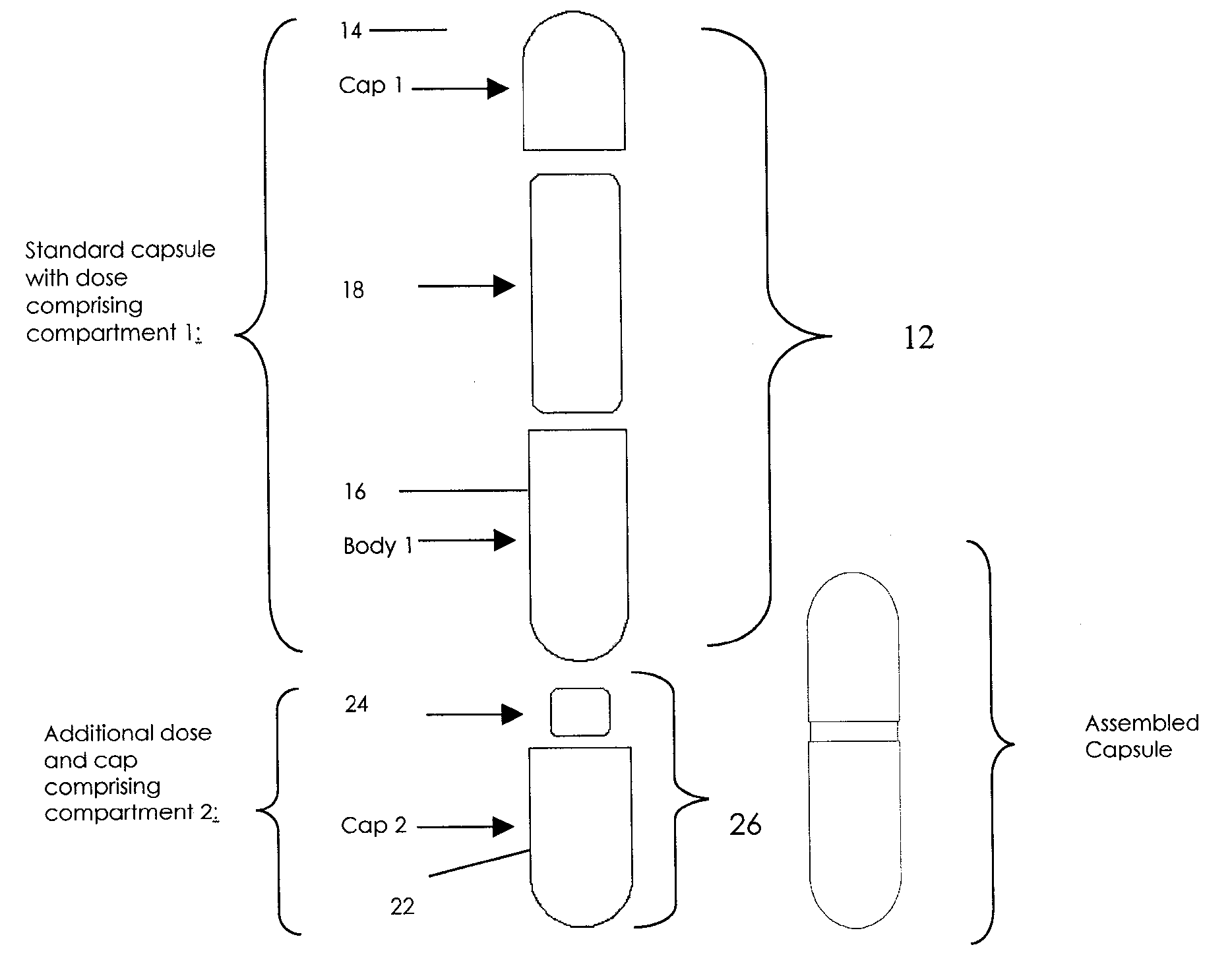
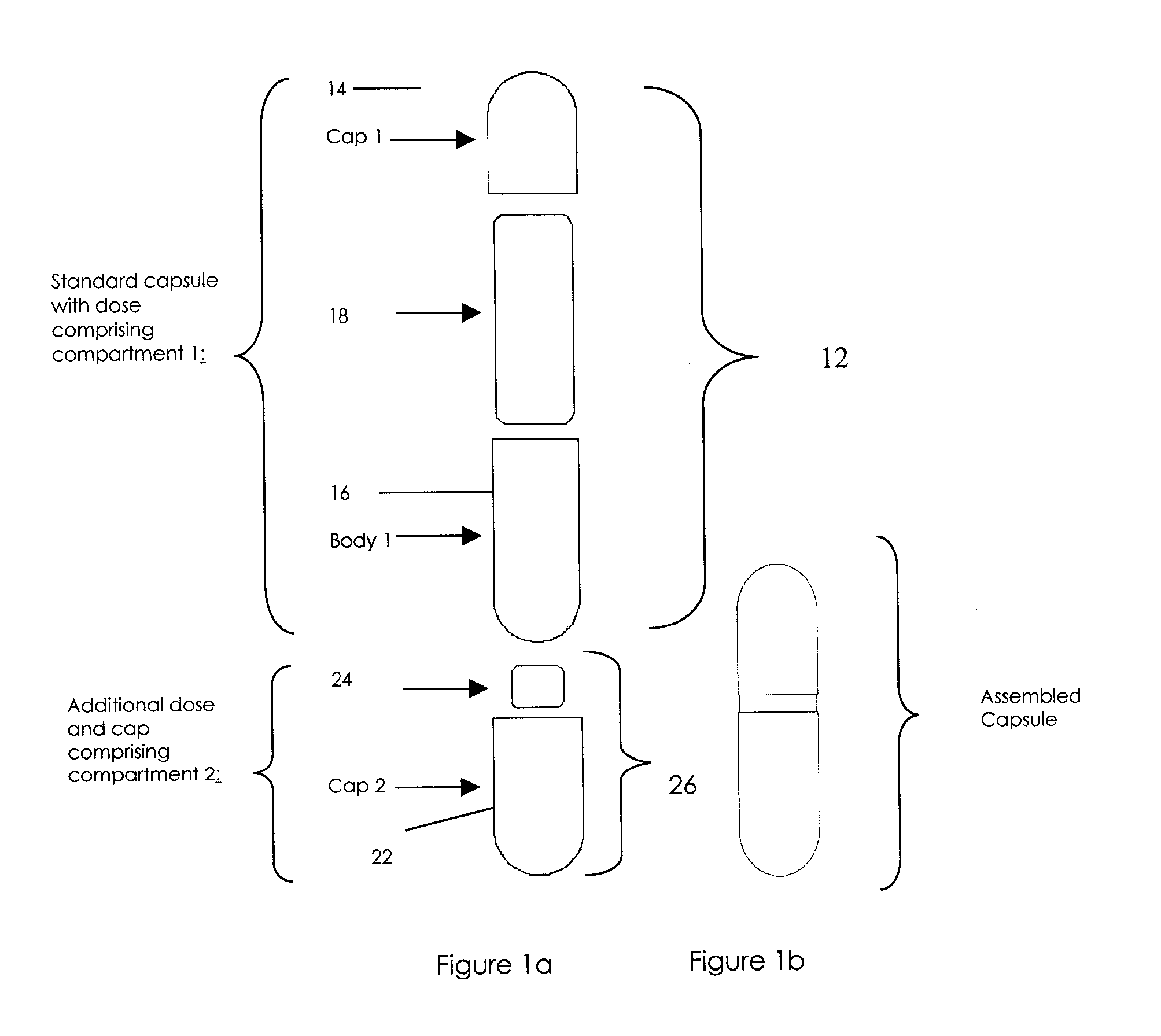
Pharmaceutical fixed dose combination products are formed by merging a fixed dose of a first pharmaceutical formulation from primary module, with a fixed dose of a second pharmaceutical formulation from a secondary module. In a preferred embodiment the first and second pharmaceutical formulations are separated from one another in a three piece capsule, a capsule-in-a-capsule or a tablet-in-a-capsule, and the primary and secondary modules are interchangeable.
Owner:MICRODOSE THERAPEUTX INC
Controlled Release Formulations
InactiveUS20080057123A1Modulate release of the active agentBiocideAnimal repellantsActive agentCentral layer
Controlled release oral dosage formulations containing one or more active agent, and methods of use thereof, are provided for the once-a-day treatment. The formulation can be in the form of a trilayer tablet containing a core or central layer and one or more barrier layers. The core may contain one or more enteric materials or polymeric materials which modulates the release of the active agent.
Owner:JAGOTEC AG
Azithromycin dosage forms with reduced side effects
ActiveUS6984403B2Reduce gastrointestinal side effectsAntibacterial agentsPowder deliveryOral suspensionsGLYCERYL MONOBEHENATE
The present invention is related to an oral dosage form comprising an effective amount of an alkalizing agent and an azithromycin multiparticulate wherein said multiparticulate comprises azithromycin, a glyceride which comprises glyceryl monobehenate, glyceryl dibehenate, glyceryl tribehenate, or a mixture thereof and a poloxamer. Typically, the oral dosage form includes any suitable oral dosing means such as a powder for oral suspension, a unit dose packet or sachet, a tablet or a capsule.
Owner:PFIZER INC
Bioadhesive Rate-Controlled Oral Dosage Formulations
InactiveUS20080260824A1Good bioadhesionImproving bioadhesion of polymersBiocidePowder deliveryDrug releasePolymer
The present invention relates to a bioadhesive drug delivery system (BIOadhesive Rate controlled Oral Dosage (BIOROD) formulation) in which a drug containing core either alone or coated with a rate controlling membrane system is enveloped on its circumference by a bioadhesive coating, thereby yielding a monolithic system that allows for drug release in a regulated manner. Also described herein are polymers with improved bioadhesive properties and methods for improving bioadhesion of polymers.
Owner:SPHERICS
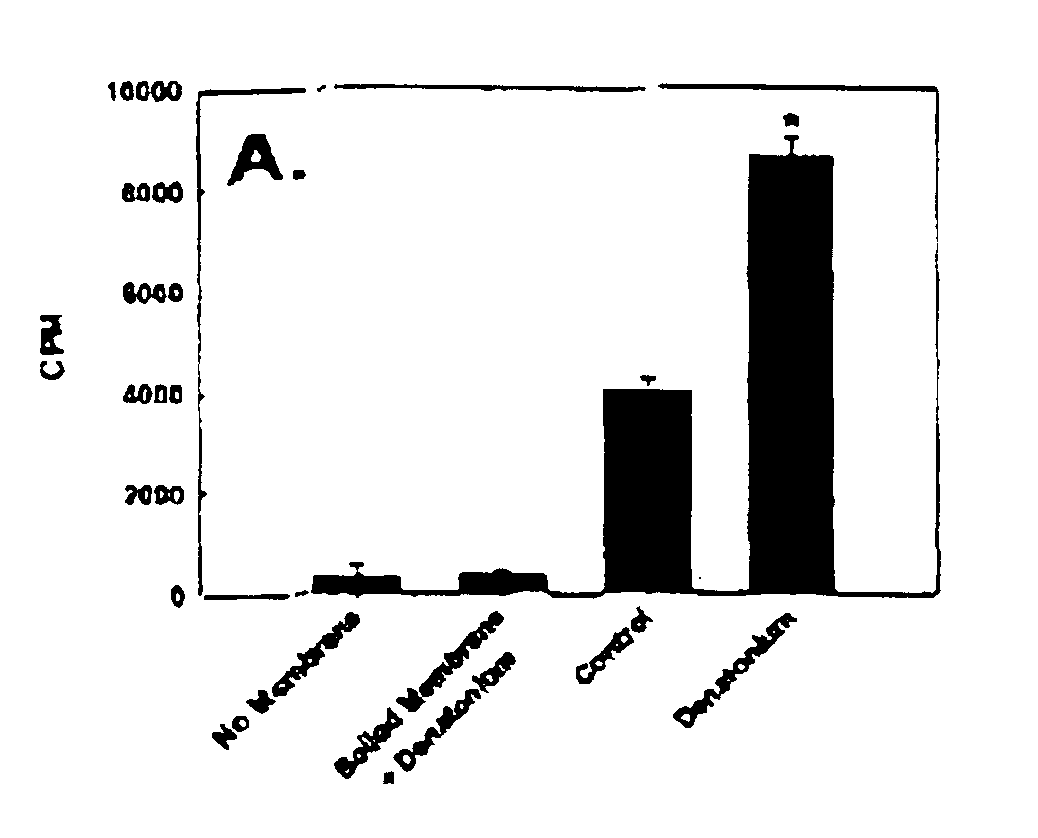
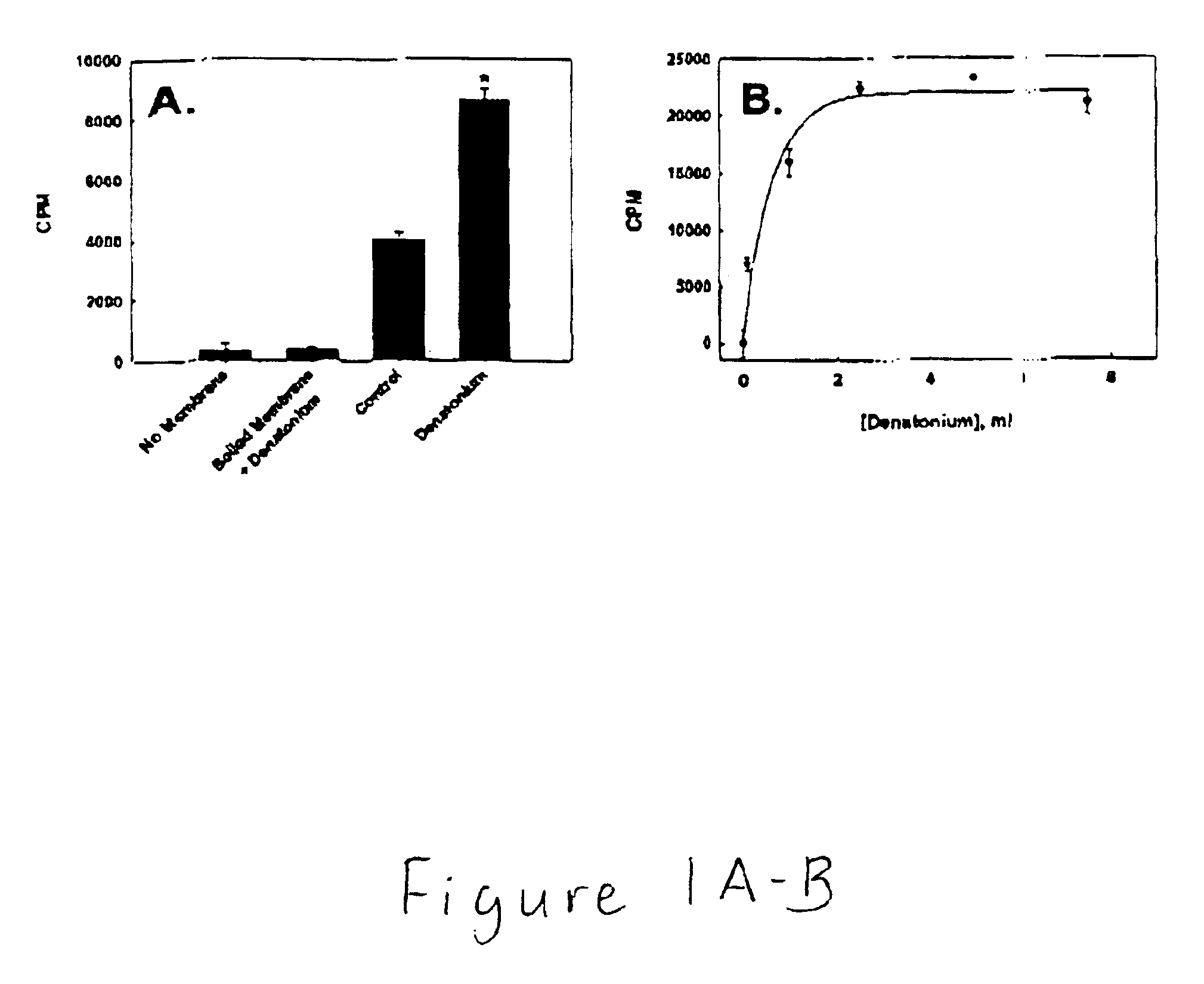
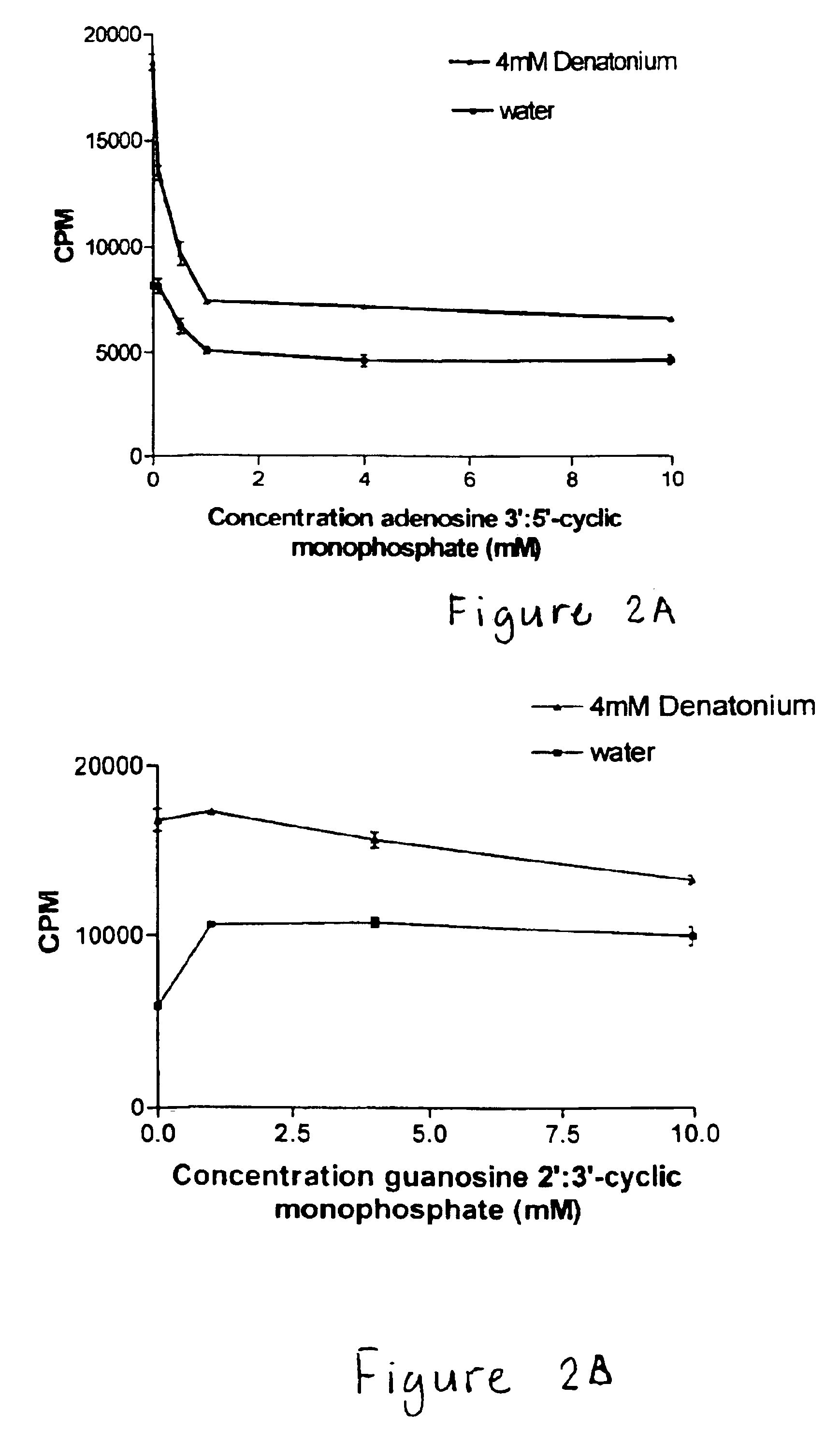
Nucleotide compounds that block the bitter taste of oral compositions
Nucleotides that block the bitter taste of foods, beverages, pharmaceutically active oral dose preparations, cosmetics and other bitter compounds that come into contact with taste tissue. The nucleotides consist of a purine or pyrimidine group, or derivative thereof, and an ionizable phosphate or other anionic organic molecule.
Owner:REDPOINT BIO CORP
Flashmelt oral dosage formulation
There is provided granules for the production of flash-melt pharmaceutical oral dosage forms. In addition to one or more medicaments, the granules are composed of an excipient combination consisting of a superdisintegrant, a dispersing agent, a distributing agent, and a binder and may also include other conventional ingredients such as sweetening and flavoring agents. The subject granules are advantageous in that they are stable and can be prepared without the aid of solvents and without the need for special environments or handling. Dosage forms, especially tablets, prepared therefrom on conventional equipment disintegrate in the mouth in under about twenty five seconds.
Owner:KOTHARL SANJEEV +1
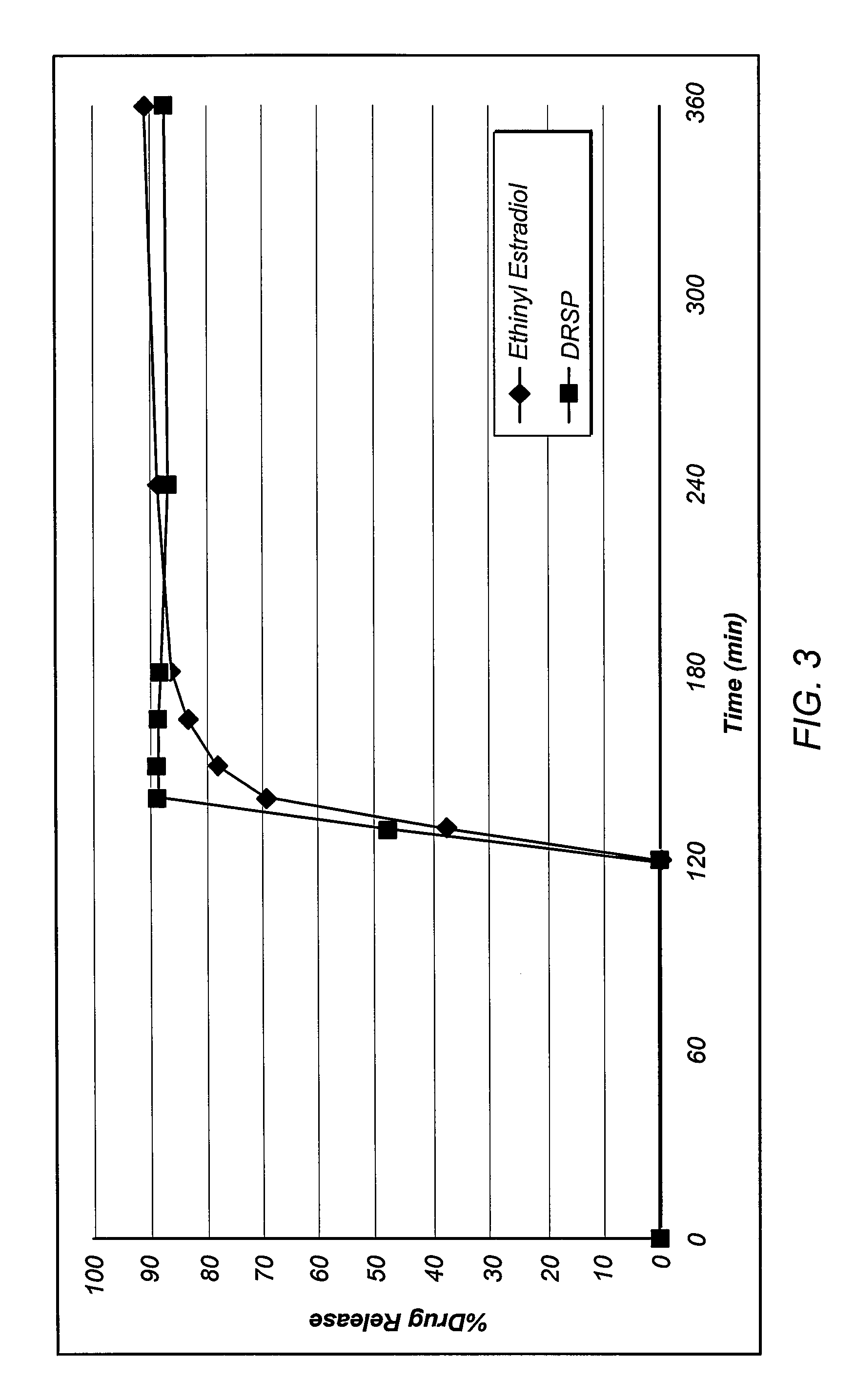
Oral contraceptive dosage forms and methods of making such dosage forms
Disclosed herein are oral dosage forms and methods of their use, in particular oral dosage systems for the delivery of drugs for use as a female oral contraceptive. In an embodiment, an oral dosage form includes a progestogen dispersed in an enteric polymer and an estrogen.
Owner:EVESTRA
Rate-Controlled Oral Dosage Formulations
InactiveUS20100226855A1Good bioadhesionMaintain good propertiesPowder deliveryBiocideDrug releaseMonolithic system
The present invention relates to a drug delivery system, in which a drug containing core, either alone or coated with a rate controlling membrane system, is enveloped on its circumference by an optionally bioadhesive coating, thereby yielding a monolithic system that allows for drug release in a regulated manner.
Owner:VAUNNEX
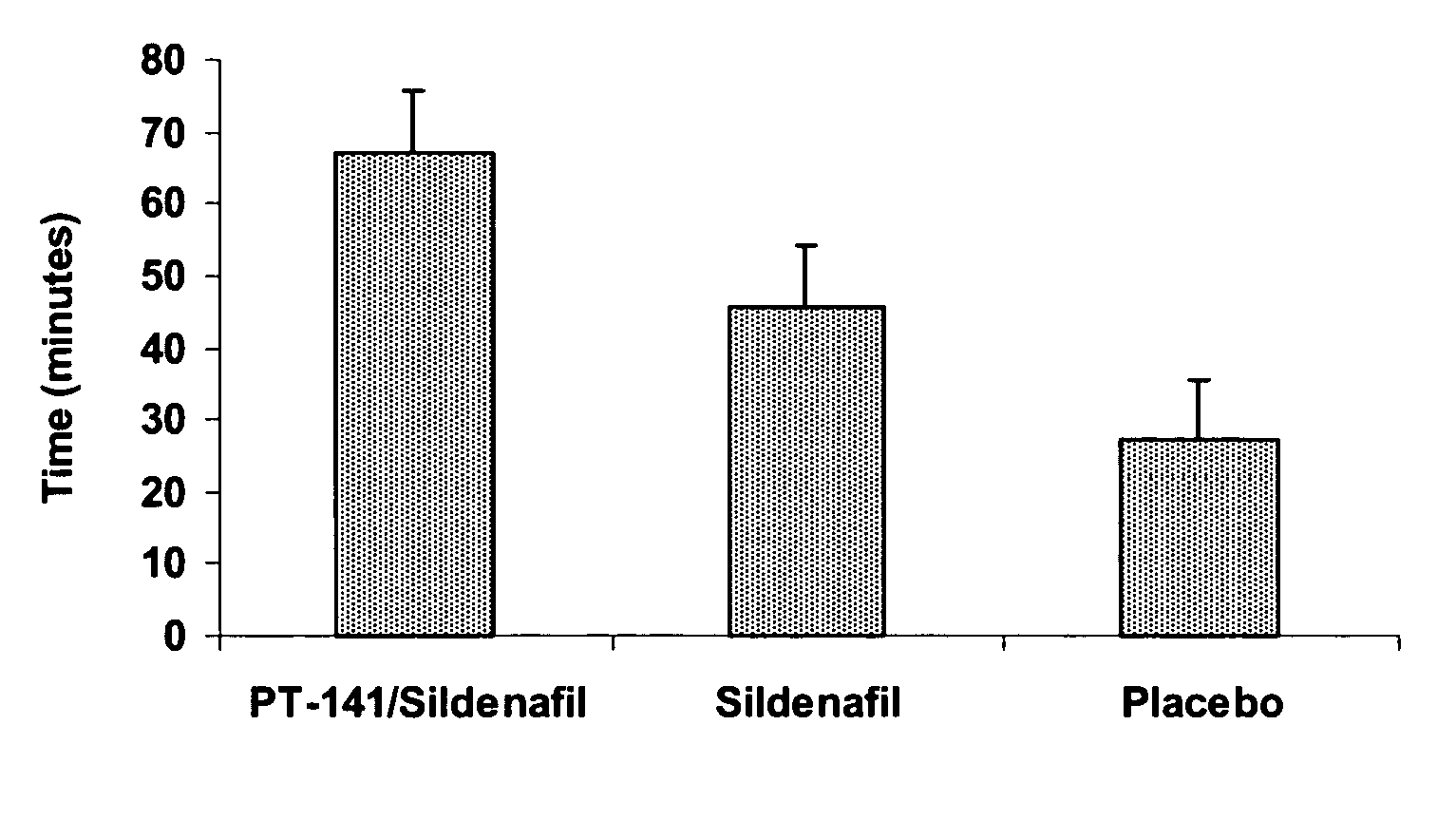
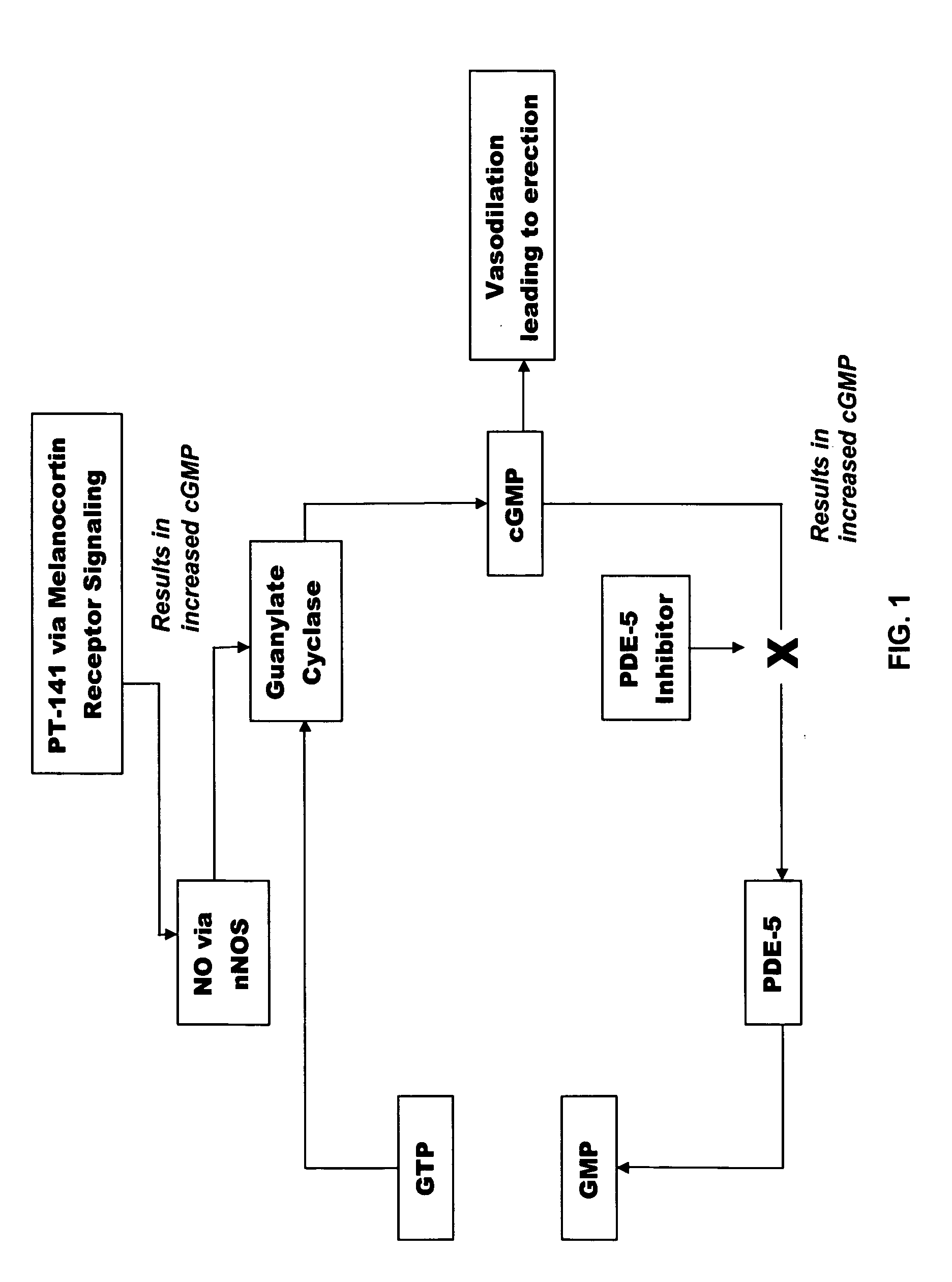
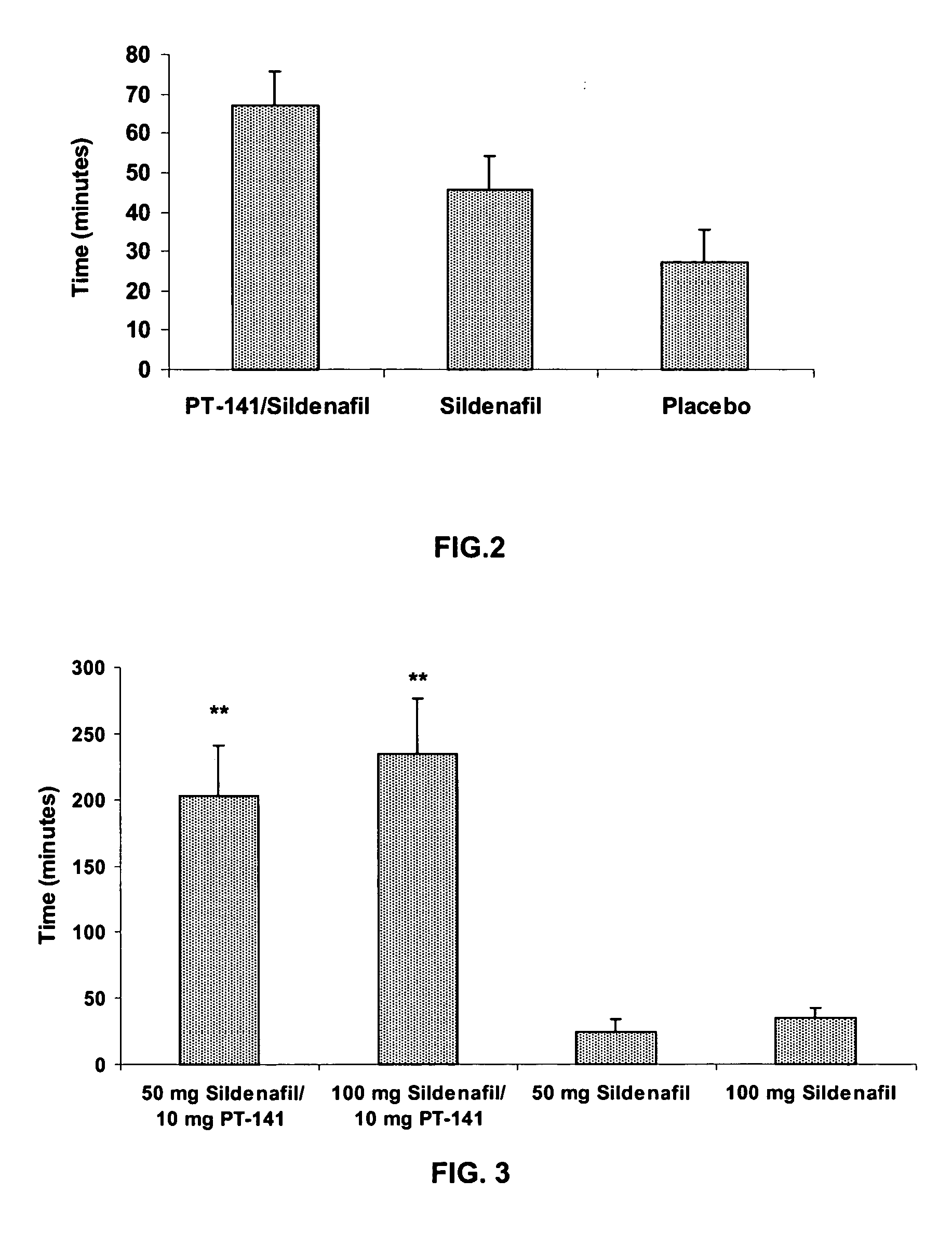
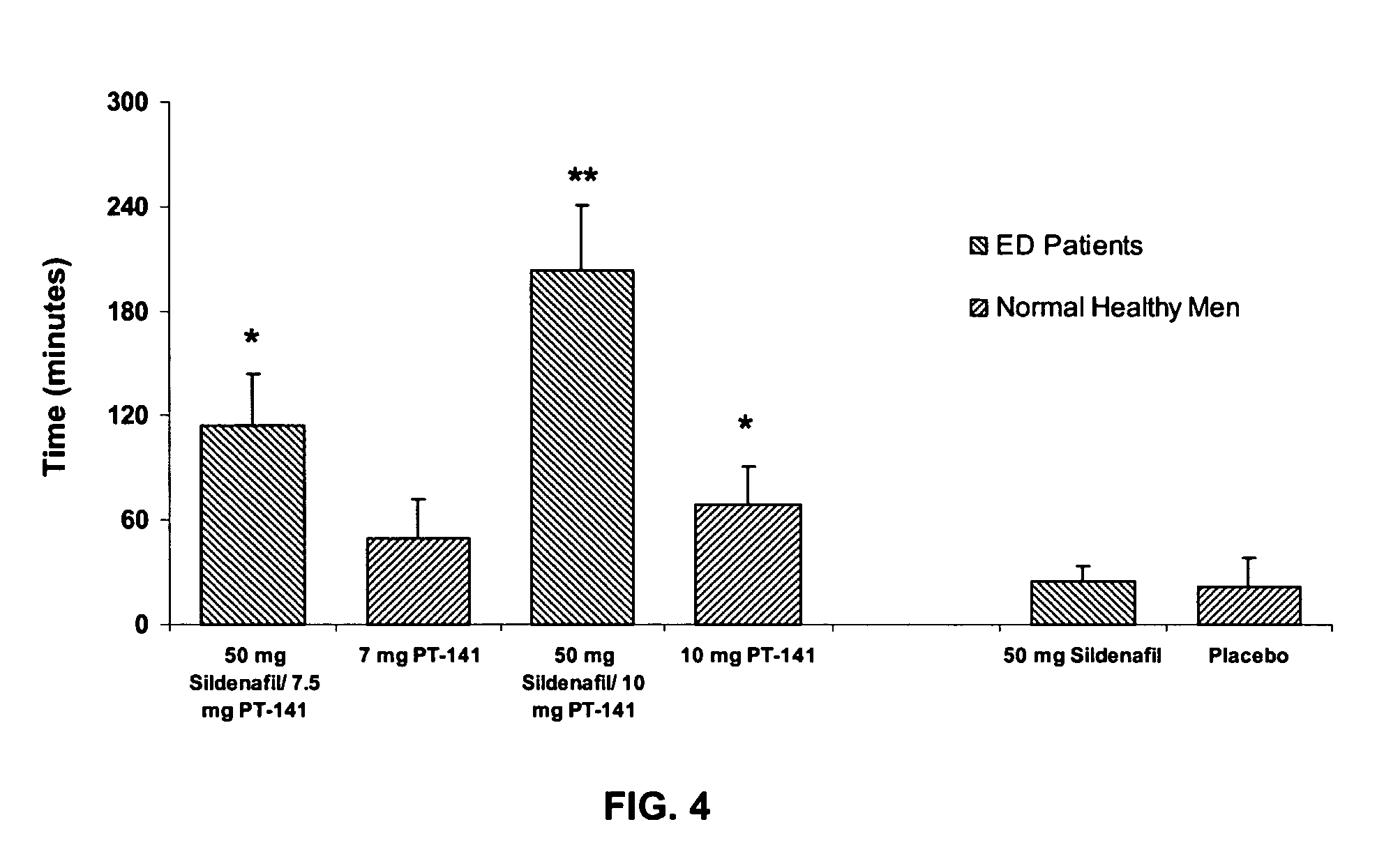
Multiple agent therapy for sexual dysfunction
InactiveUS20050222014A1Improve reliabilityIncreased temporal durationBiocideHeavy metal active ingredientsSexual dysfunctionAgonist
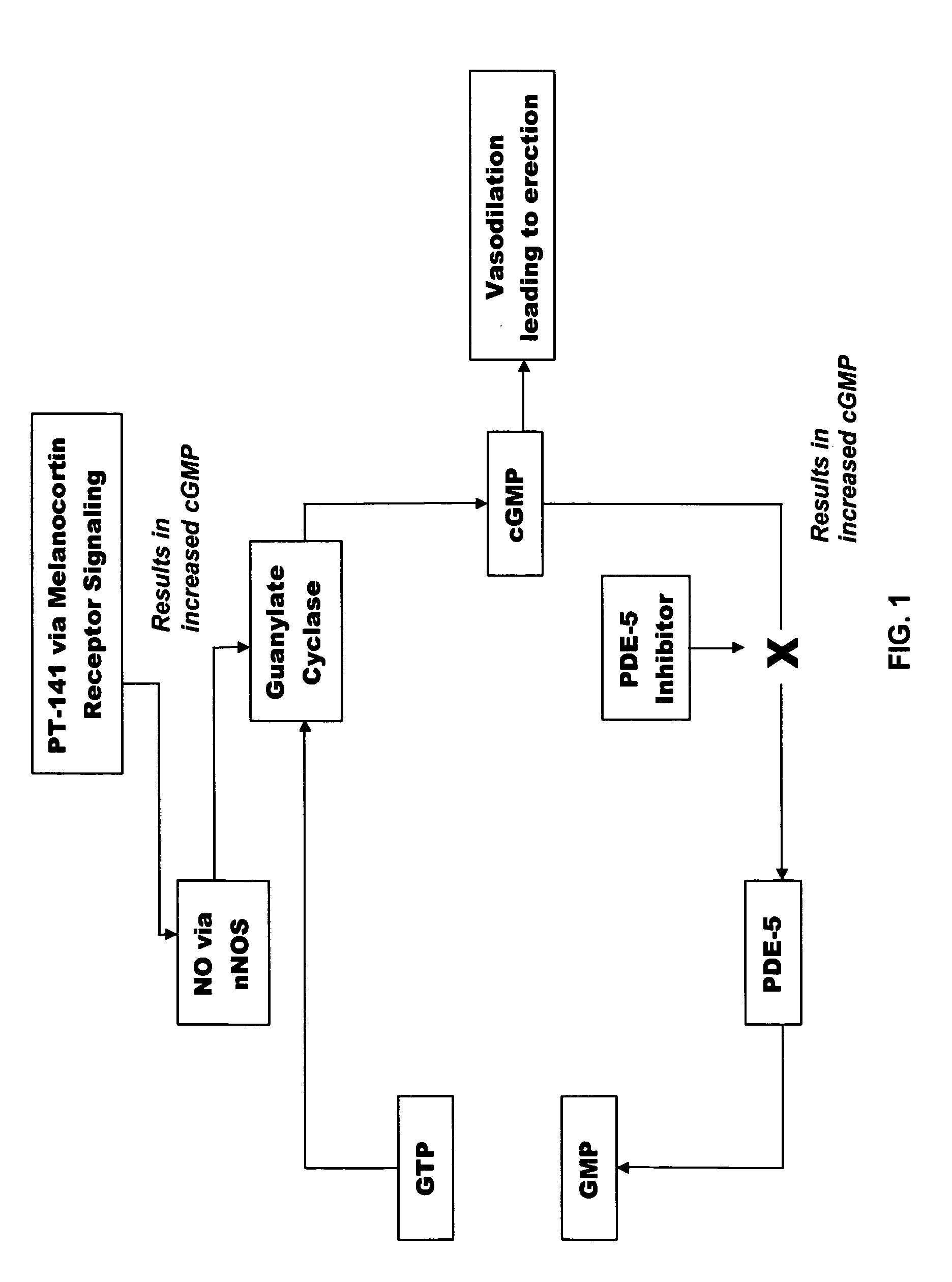
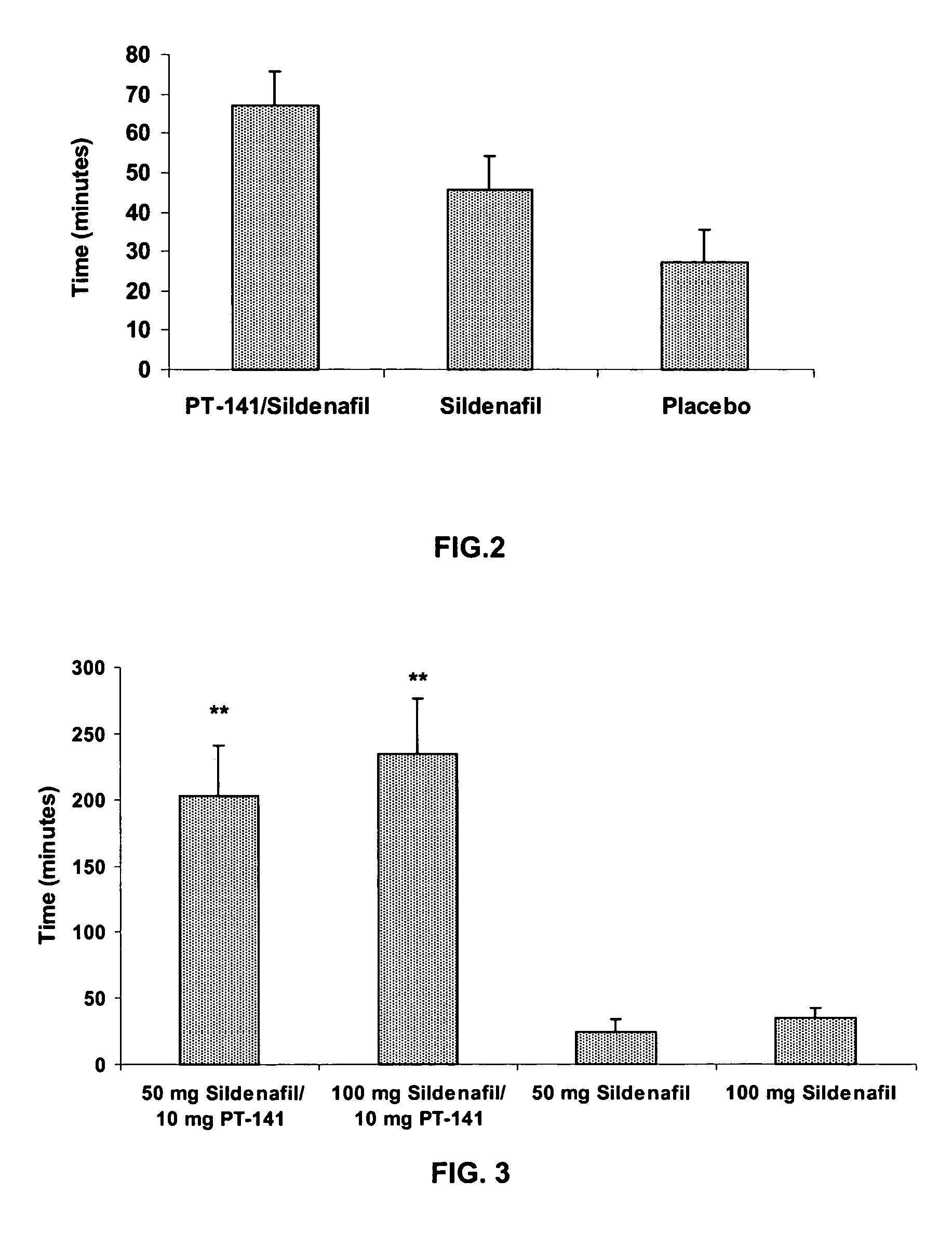
Multiple agent therapy for treatment of sexual dysfunction, including male erectile dysfunction, with sequential administration a type V phosphodiesterase inhibitor (PDE-5), such as sildenafil, preferably wherein the PDE-5 inhibitor is administered by oral dose means, and a melanocortin 3 and / or 4 receptor agonist, such as Ac-Nle-cyclo(-Asp-His-D-Phe-Arg-Trp-Lys)-OH (PT-141) preferably wherein the PT-141 is formulated for and administered by intranasal means, and further preferably wherein the PDE-5 inhibitor is administered prior to PT-141.
Owner:PALATIN TECH INC
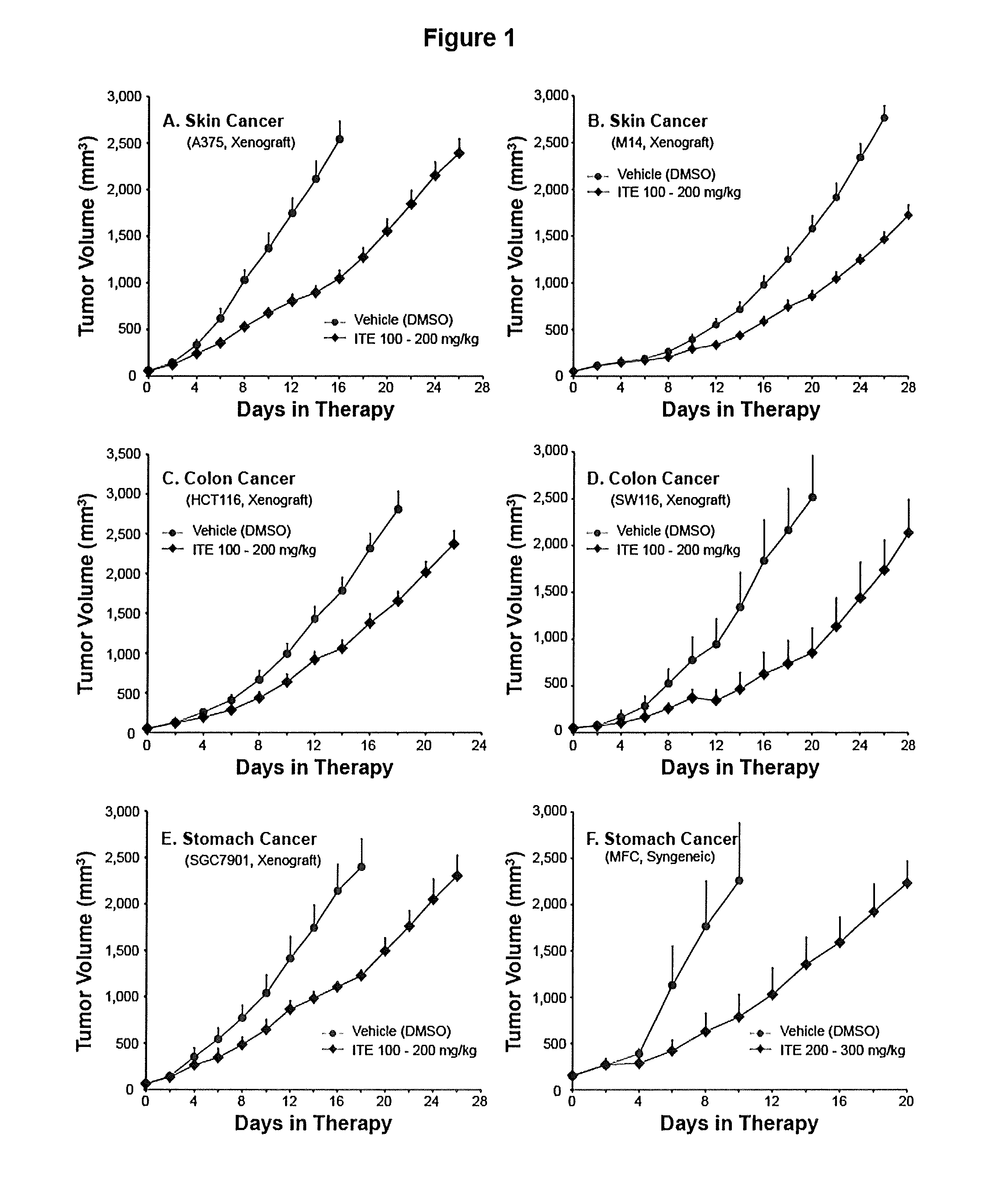
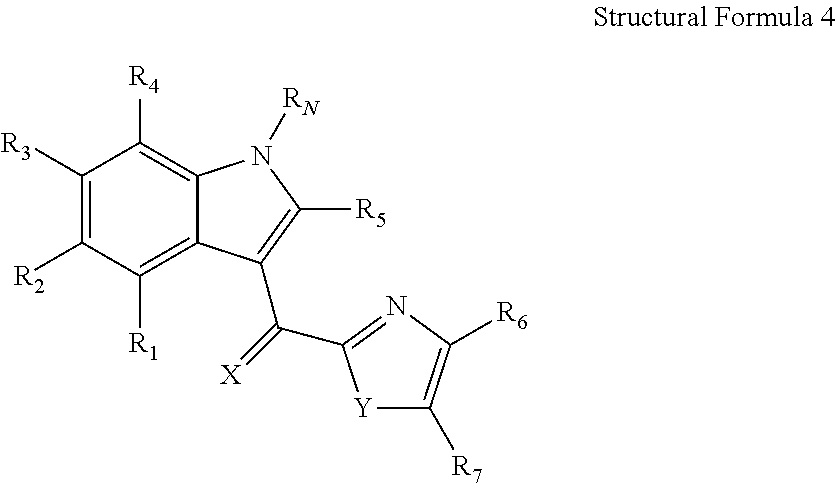
Method of Cancer Treatment with 2-(1H-Indole-3-Carbonyl)-Thiazole-4-Carboxylic Acid Methyl Ester
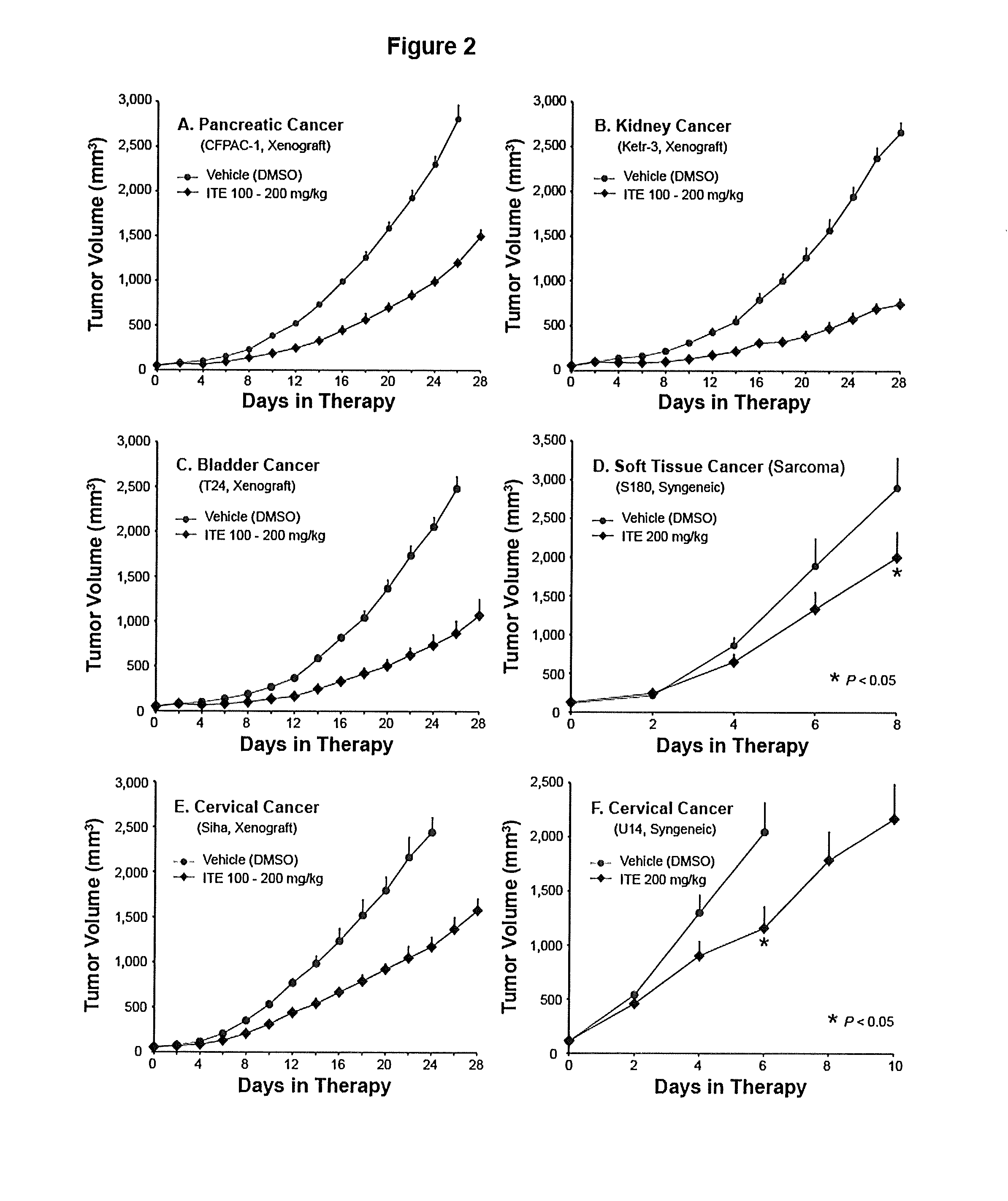
A method of cancer treatment is provided that includes administering an effective amount of an endogenous ligand for the aryl hydrocarbon (Ah) receptor (AhR) named ITE or one of its structural analogs (the active ingredient) to a subject with cancer is disclosed. An effective dose and dosing frequency of the active ingredient are determined by measuring its blood levels of the subject after dosing. The active ingredient formulated with a carrier system is applied topically, enterally, or parenterally to the subject. An oral dose of water, in addition to normal water drinking, is administered to help alleviate feces hardening, a complication of ITE dosing. Subjects with cancers of skin, colon (or rectum), stomach, pancreas, kidney, bladder, soft tissue, and cervix, are preferably accepted for treatment or intervention.
Owner:ARIAGEN INC
Multiple agent therapy for sexual dysfunction
Owner:PALATIN TECH INC
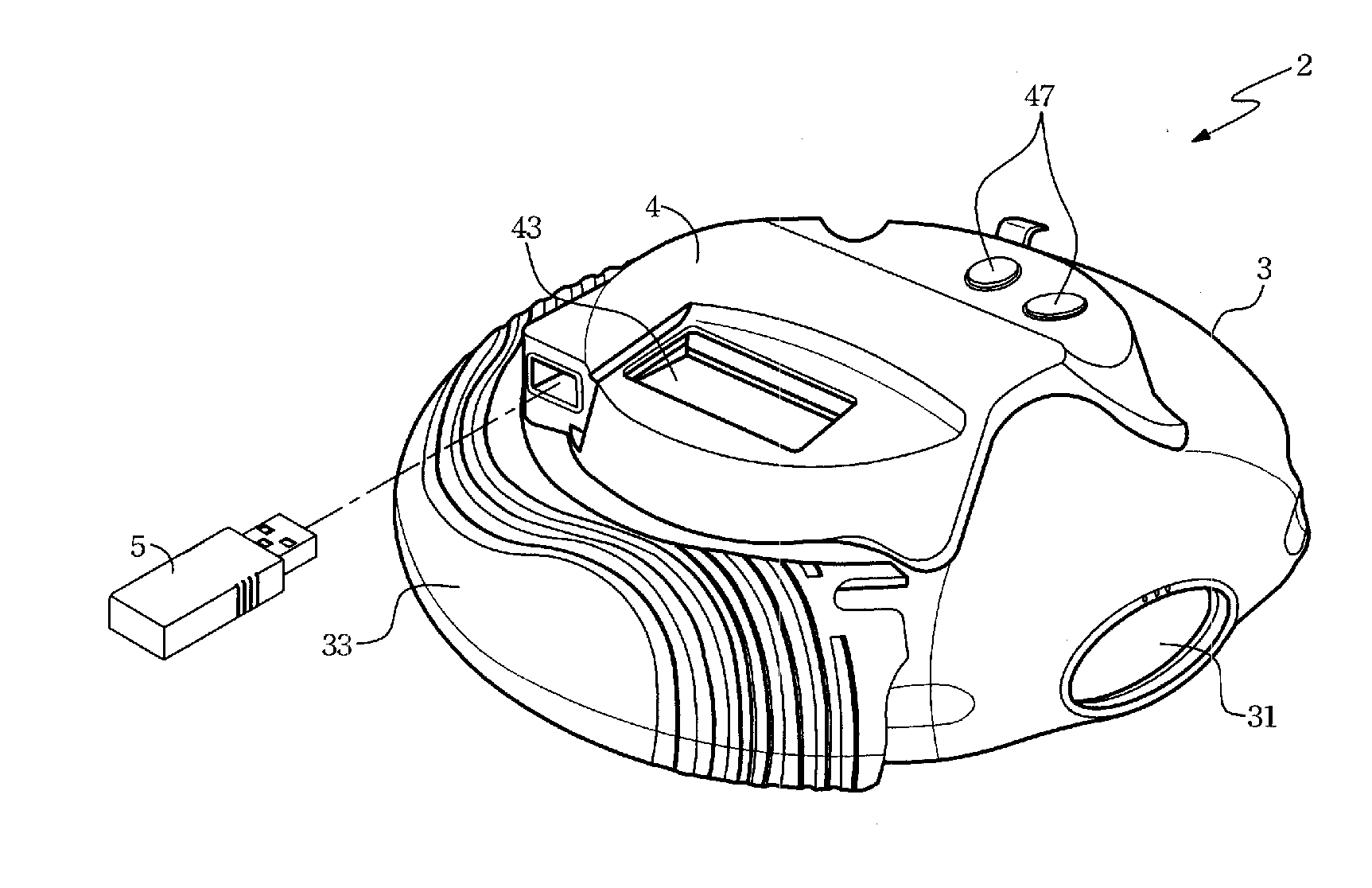
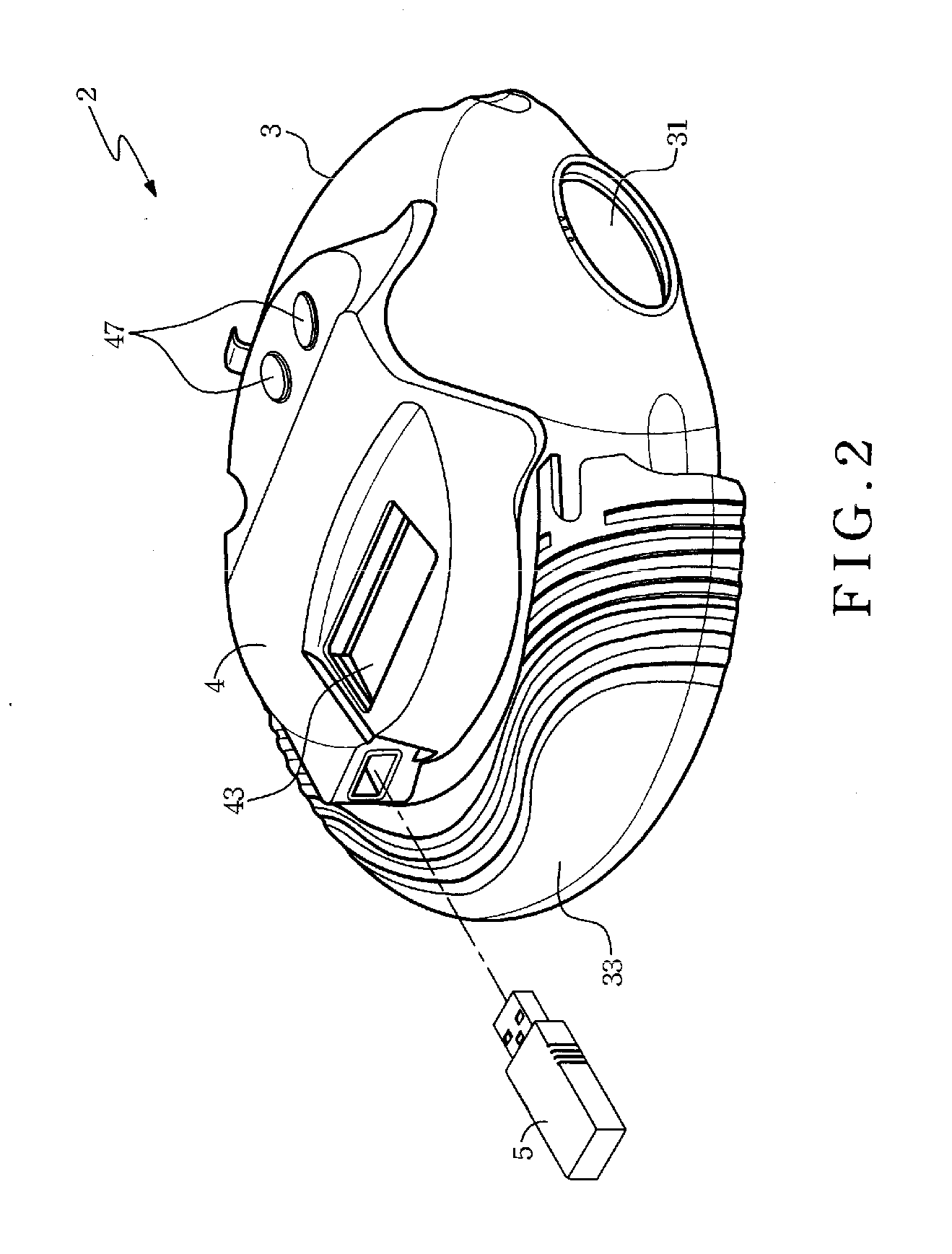
Dose inhalation monitor
InactiveUS20070125372A1Accurate doseAccurate diagnosisMedical devicesMedical atomisersInhalationIntensive care medicine
A dose inhalation monitor includes a main body for containing medicine therein, a dose monitoring unit and a transmission device. The main body has a feeding mouth and a bypass communicated spatially with the feeding mouth. The dose monitoring unit is disposed on the main body, and includes a detector capable of detecting and converting a pressure within the bypass into a voltage level and a processor capable of processing the voltage level in order to generate an oral dose of medicine corresponding to the voltage level. The transmission device is connected to the dose monitoring unit for transmitting the oral dose of medicine to a distal receiver after receipt thereof.
Owner:TRIAD TECH
Oral dosage combination pharmaceutical packaging
InactiveUS20090232886A1Increased riskDevelopment costAntibacterial agentsBiocidePharmaceutical packagingPharmaceutical formulation
Pharmaceutical fixed dose combination products are formed by merging a fixed dose of a first pharmaceutical formulation from primary module, with a fixed dose of a second pharmaceutical formulation from a secondary module In a preferred embodiment the first and second pharmaceutical formulations are separated from one another in a three piece capsule, a capsule-in-a-capsule or a tablet-in-a-capsule, and the primary and secondary modules are interchangeable.
Owner:SISON RAYMUNDO A
Beta-elemi alkene bulk medicament and method of preparing its preparations
InactiveCN101402543AHydrocarbon active ingredientsDistillation purification/separationVolatilesSelf emulsifying
The invention provides a method for preparing high-purity beta elemene from natural plants containing the beta elemene such as curcuma zedoary (earthnuts or tubers of the curcuma zedoary), cedronella (fresh leaves of the cedronella), yellowtop (roots, stems, leaves, flowers and seeds of the yellowtop) and so on, which can improve the production efficiency from starting materials to the high-purity beta elemene and reduce production cost. Compared with the prior art, the method is mainly different in that the roots, stems, leaves, flowers and seeds of the natural plants are taken as raw materials; oleum volatile of specific parts of the natural plants is obtained by methods for extracting different oleum volatiles, and is distilled by molecular distillation method to remove compositions with high boiling points and non-volatile compositions; impurity compositions are removed by the ethanol extraction method and silver nitrate complex extraction method; and finally the beta elemene with the content between 95.0 and 99.9 percent is obtained through reduced pressure distillation or rectification. The bulk pharmaceutical chemicals not only can be prepared into oral dosing preparation such as emulsion oral liquid, self-emulsifying / self-microemulsifying capsules, soft capsules and so on, but also can be prepared into non-alimentary dosing preparation such as emulsion injection, liquid drugs injection, transdermal absorbent, lung sprays, suppository and so on. The method has the advantages of novel design, concise operating steps, mild operating conditions and improvement of the production efficiency of the beta elemene.
Owner:沈阳万爱普利德医药科技有限公司
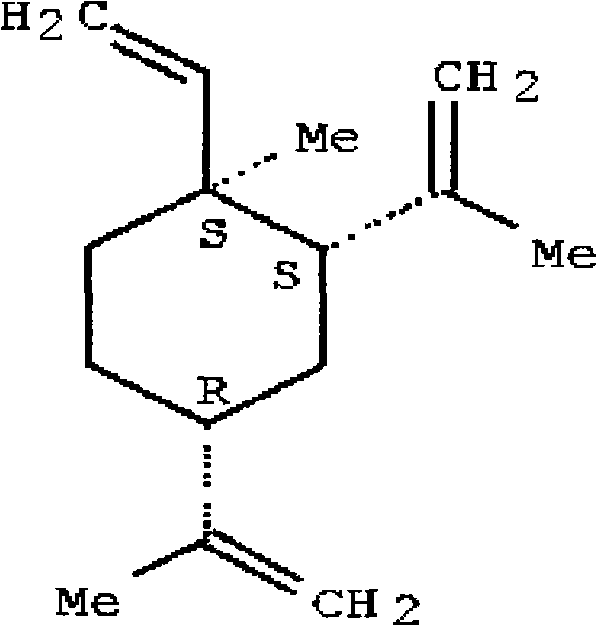
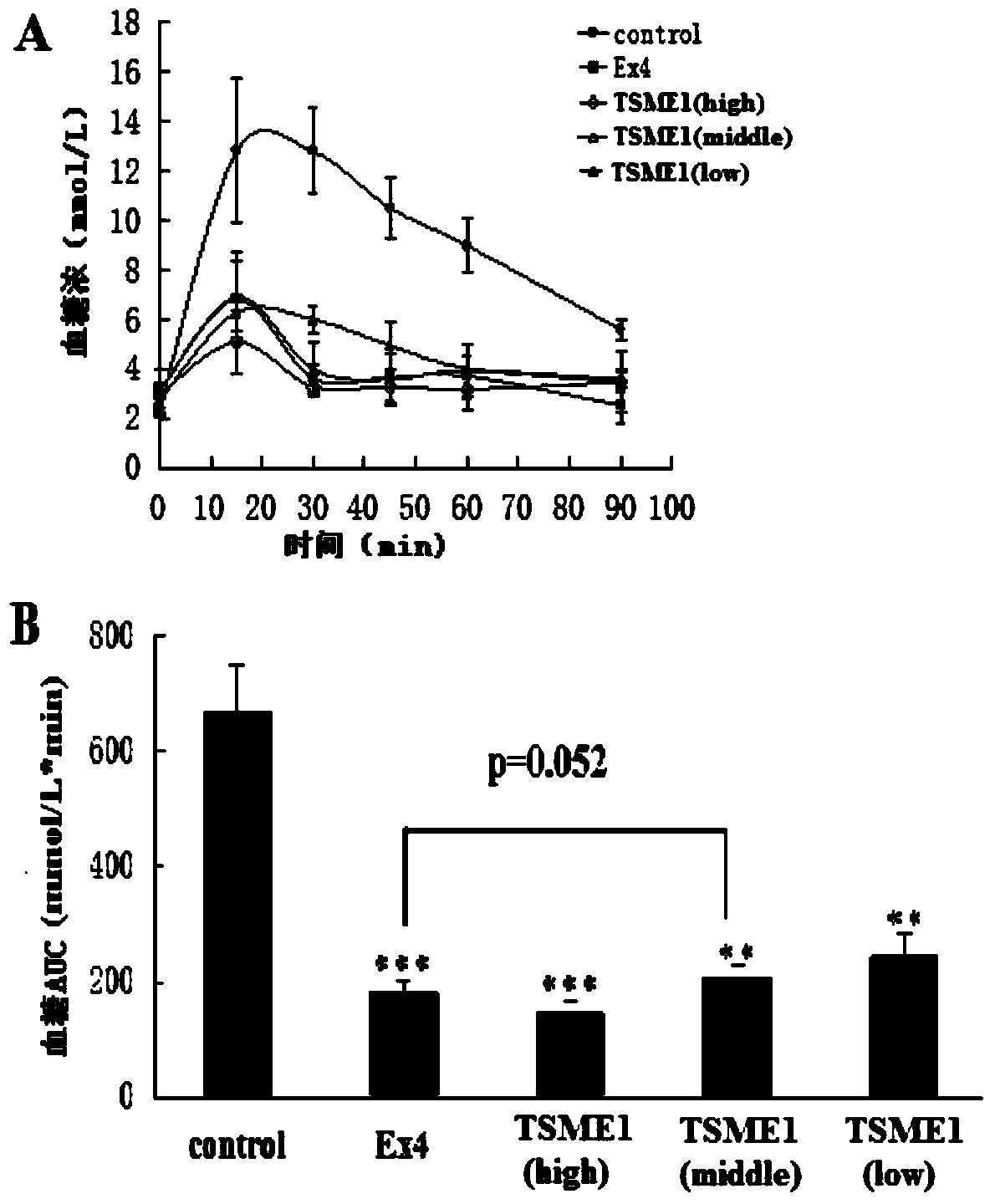
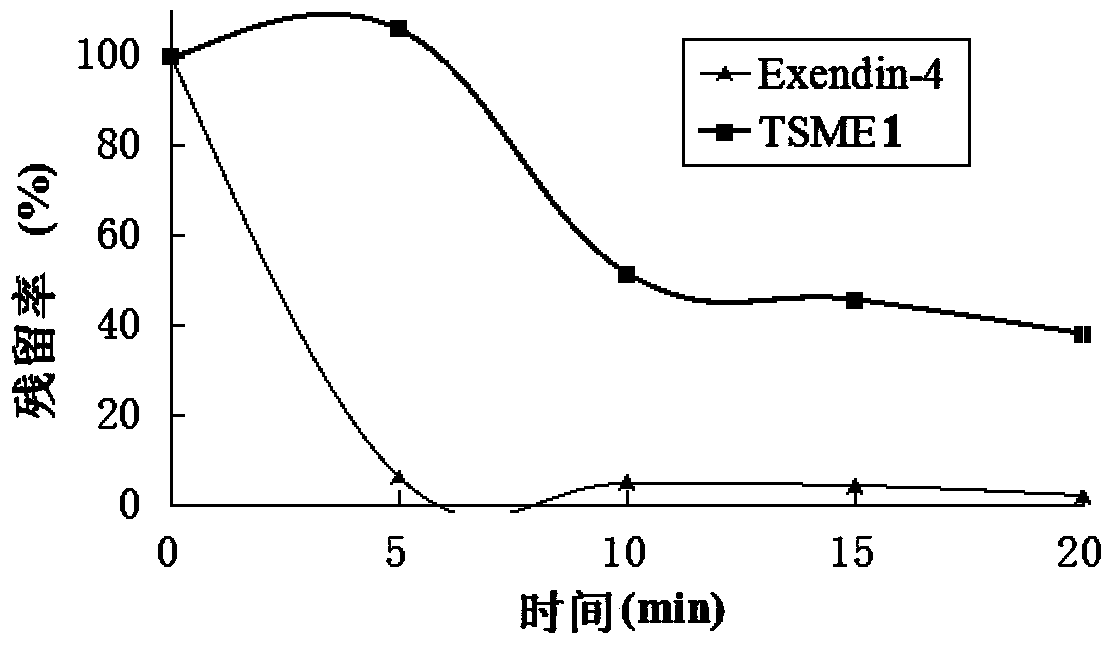
Oral dosing hypoglycemic polypeptide as well as preparation method and application thereof
ActiveCN103665148AImprove resistance to enzymatic hydrolysisPeptide/protein ingredientsMetabolism disorderEnzymatic hydrolysisPharmacology
Oral hypoglycemic polypeptide is an Exendin-4 analogue and is obtained by replacing twelfth, twentieth and twenty-seventh amino acids in an amino acid sequence of the Exendin-4 with non basic amino acids. Compared with an Exendin-4 archetype, the enzymatic hydrolysis resistance of the oral hypoglycemic polypeptide is remarkably improved, and the hypoglycemic polypeptide can be orally taken and has a good application prospect in treatment of type II diabetes mellitus.
Owner:CHINA PHARM UNIV
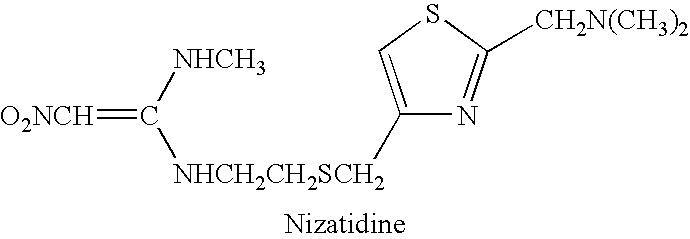
Composition, system and method of treatment of gastrointestinal disorders with nizatidine oral solution
InactiveUS20060094760A1Faster gastric secretionMore responsiveBiocideAnimal repellantsAlcohol freeMedicine
An alcohol-free, oral solution of nizatidine treats gastric and intestinal disorders. Oral doses of solution, which are equivalent to 150 mg twice daily, or 300 mg once daily, pill form of conventional nizatidine are orally administered and have a bioequivalency greater than 70%. The oral solution allows a wider population to obtain nizatidine treatment, particularly children, and the elderly, who have difficulty ingesting pills, can take the oral solution. Also, adolescents and younger children, in particular, can be treated with an alcohol-free oral solution.
Owner:BRAINTREE LAB
Nutritional composition and a container for the convenient transport and storage of the nutritional composition
InactiveUS20060193961A1Minimize oxidation of fatty acidAvoid contactFood ingredientsFood preparationOctadecadienoic AcidLipid oxidation
The present invention relates to a combination of a hermetically sealed, substantially air-tight container and a nutritional composition. The container of the present invention is adapted for the convenient transport, and storage of a single daily oral dose of a nutritional composition containing a fatty acid, for example, diacyl glycerol, an octadecatrienoic acid, an octadecadienoic acid, linolenic acid, linoleic acid, conjugated linoleic acid (CLA) or a combination thereof. In a preferred embodiment, the present invention relates to the use of the c9, t11 and t10, c12 isomers of CLA in a nutritional composition. The wide range of health benefits associated with consumption of these CLA fatty acids is well known. Furthermore, the invention relates to a container that substantially prevents CLA lipid oxidation caused by exposure to air.
Owner:SHASTRI SIDDHARTH +1
Nucleotide compounds that block the bitter taste of oral compositions
Nucleotides that block the bitter taste of foods, beverages, pharmaceutically active oral dose preparations, cosmetics and other bitter compounds that come into contact with taste tissue. The nucleotides consist of a purine or pyrimidine group, or derivative thereof, and an ionizable phosphate or other anionic organic molecule.
Owner:REDPOINT BIO CORP
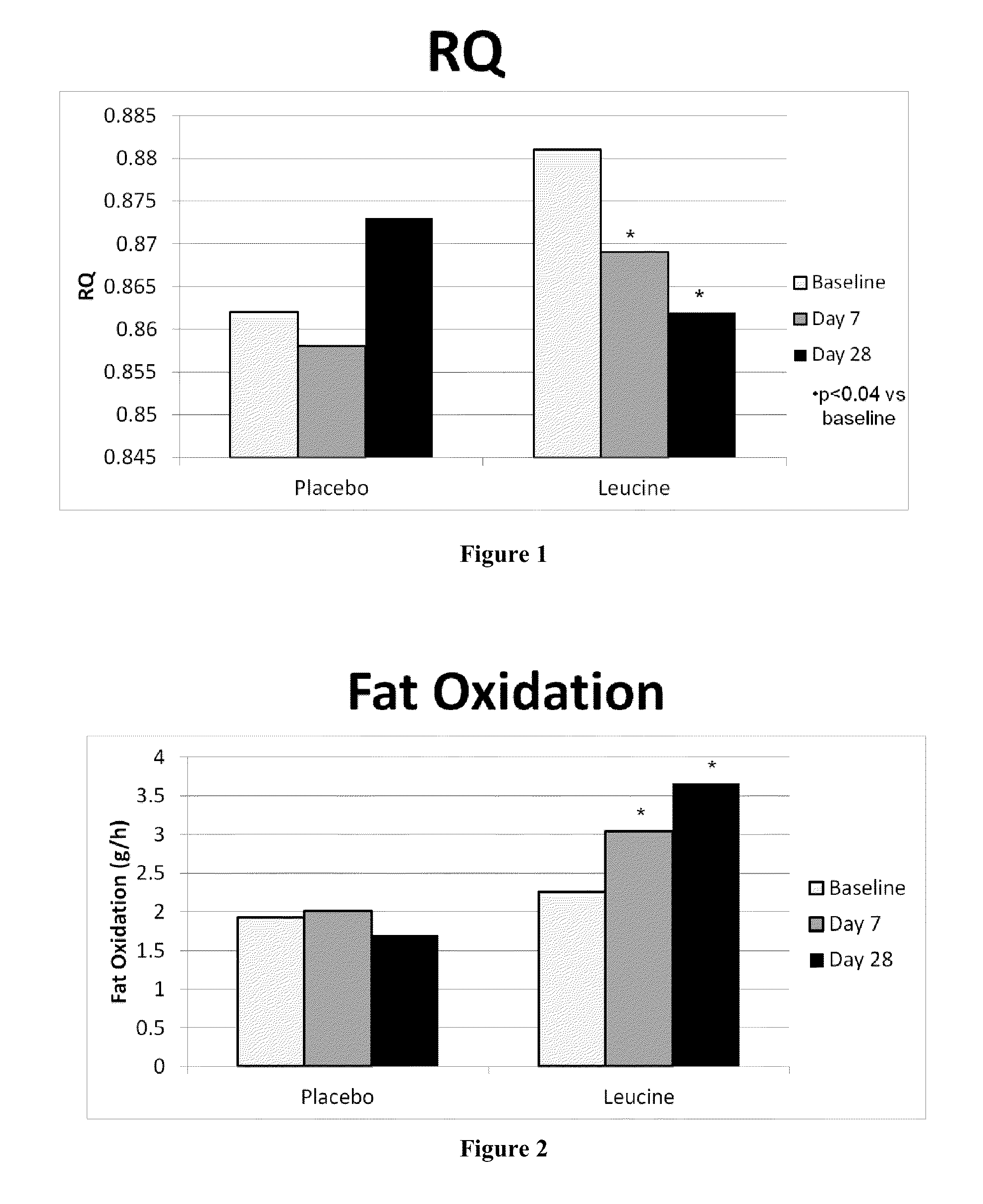
Compositions, methods, and kits for regulating energy metabolism
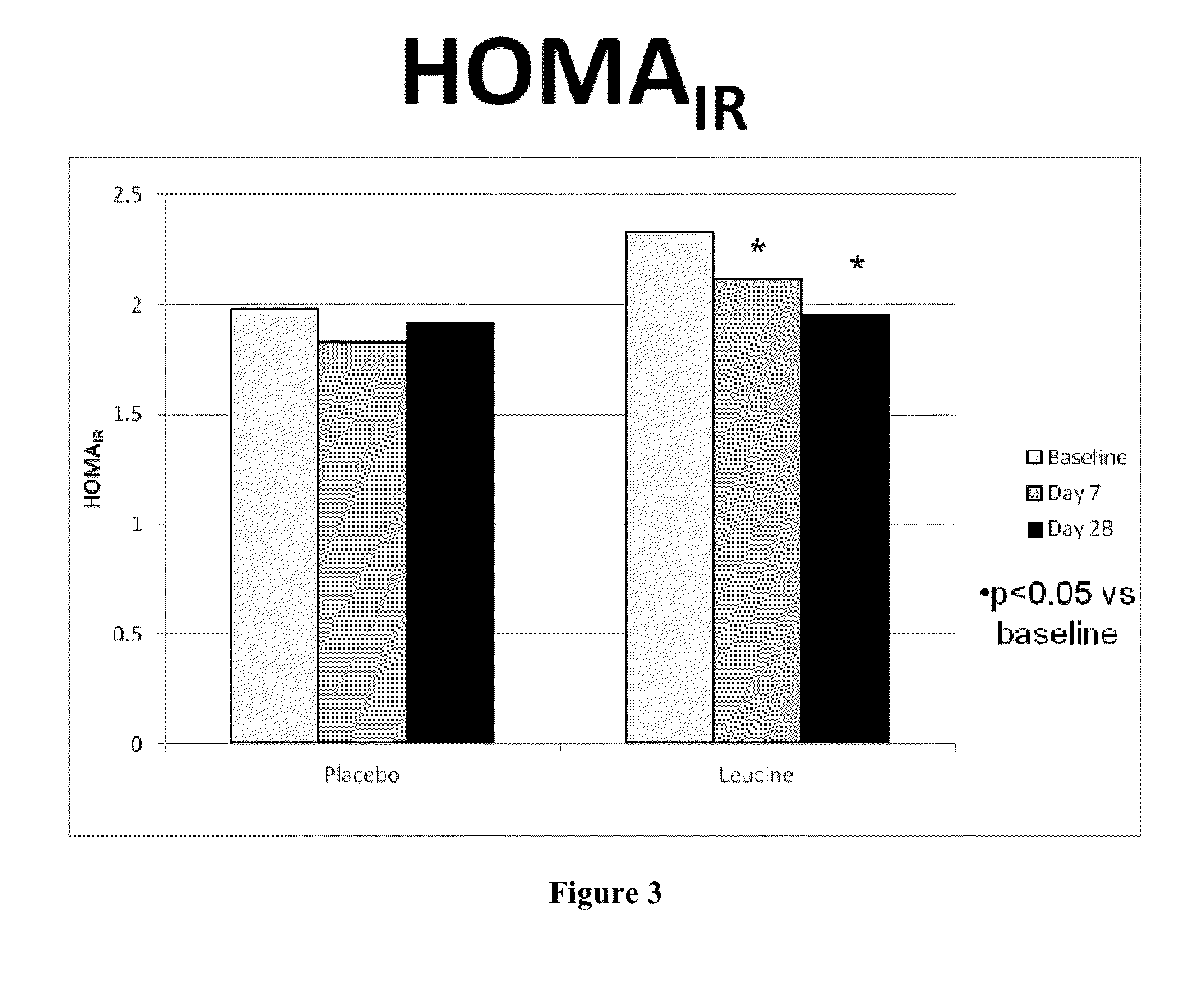
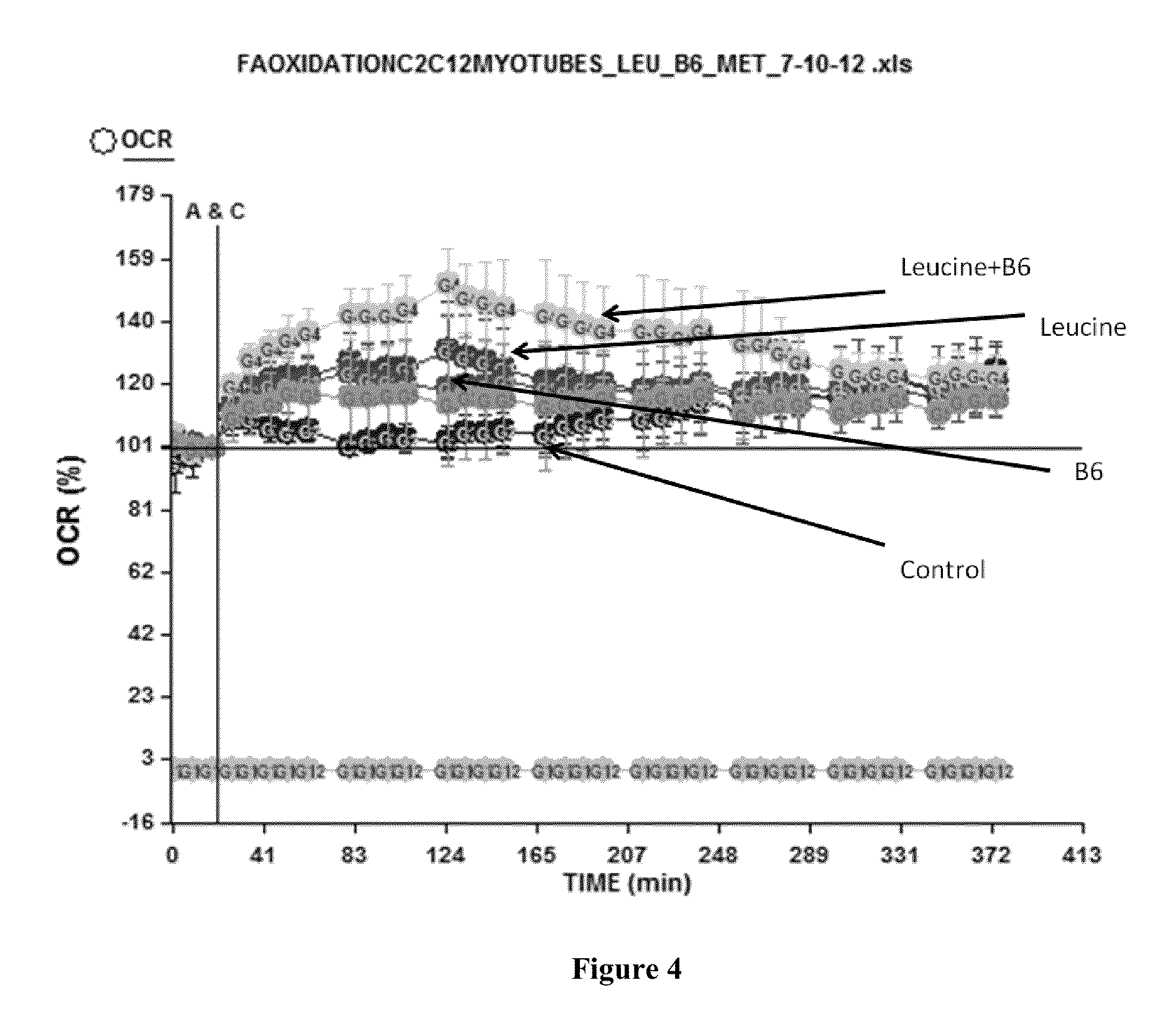
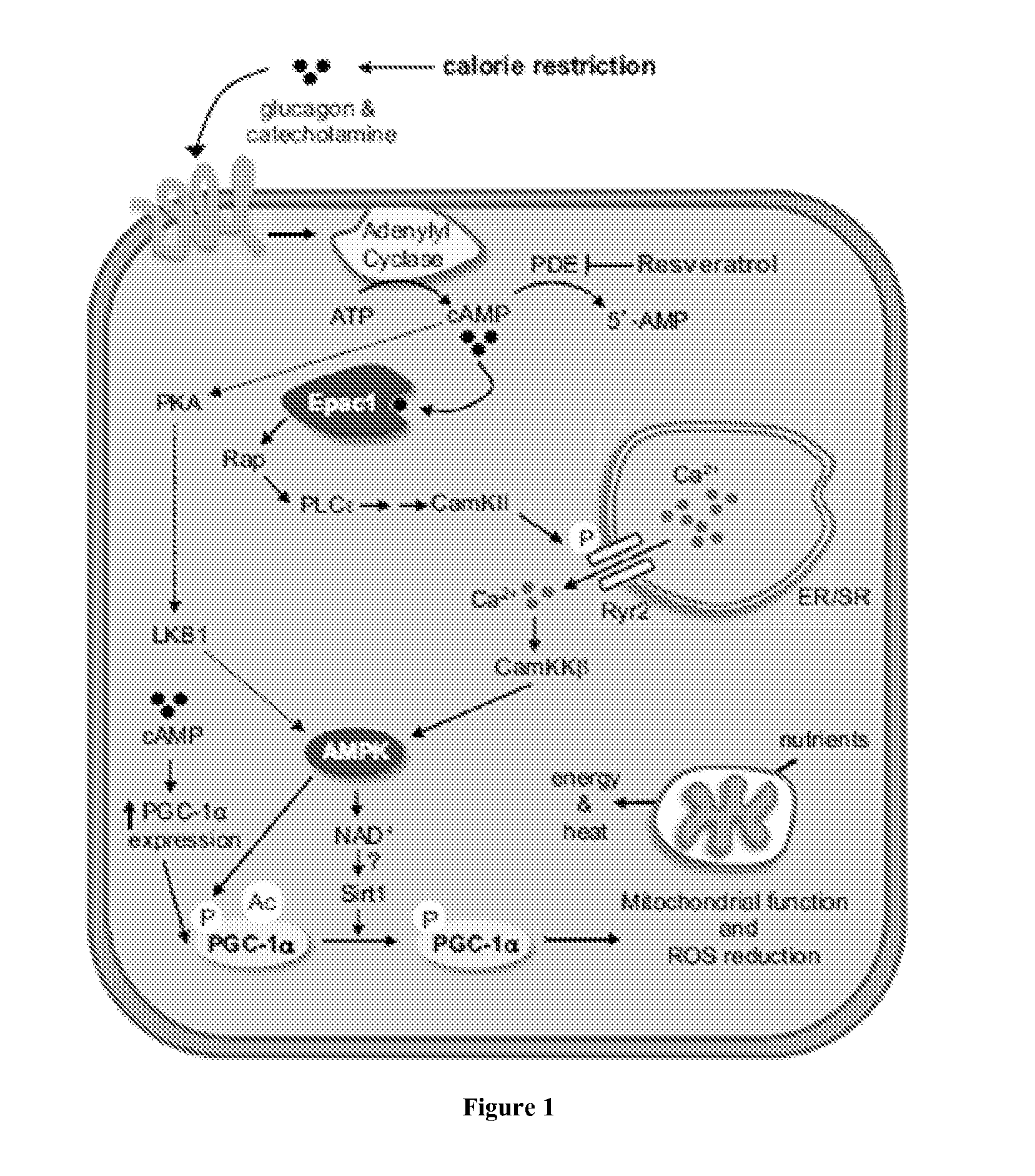
ActiveUS20130237605A1Increase energy metabolismIncreasing fatty acid oxidationBiocideOrganic active ingredientsVitamin b6Metabolite
The present invention provides for compositions, methods and kits for regulating energy metabolism. In one aspect, the invention provides for compositions that comprise a combination of (a) branched chain amino acids, such as leucine, and (b) vitamin B6, or any precursors or metabolites of (a) or (b). These combinations may be synergistic and / or effective for reducing weight or adipose volume. In another aspect, the invention provides for methods of regulating energy metabolism by the administration of one or more compositions comprising branched chain amino acids and vitamin B6. The invention also provides for kits comprising compositions of branched chain amino acids and vitamin B6 packaged in an oral dose form with usage instructions.
Owner:NUSIRT SCI
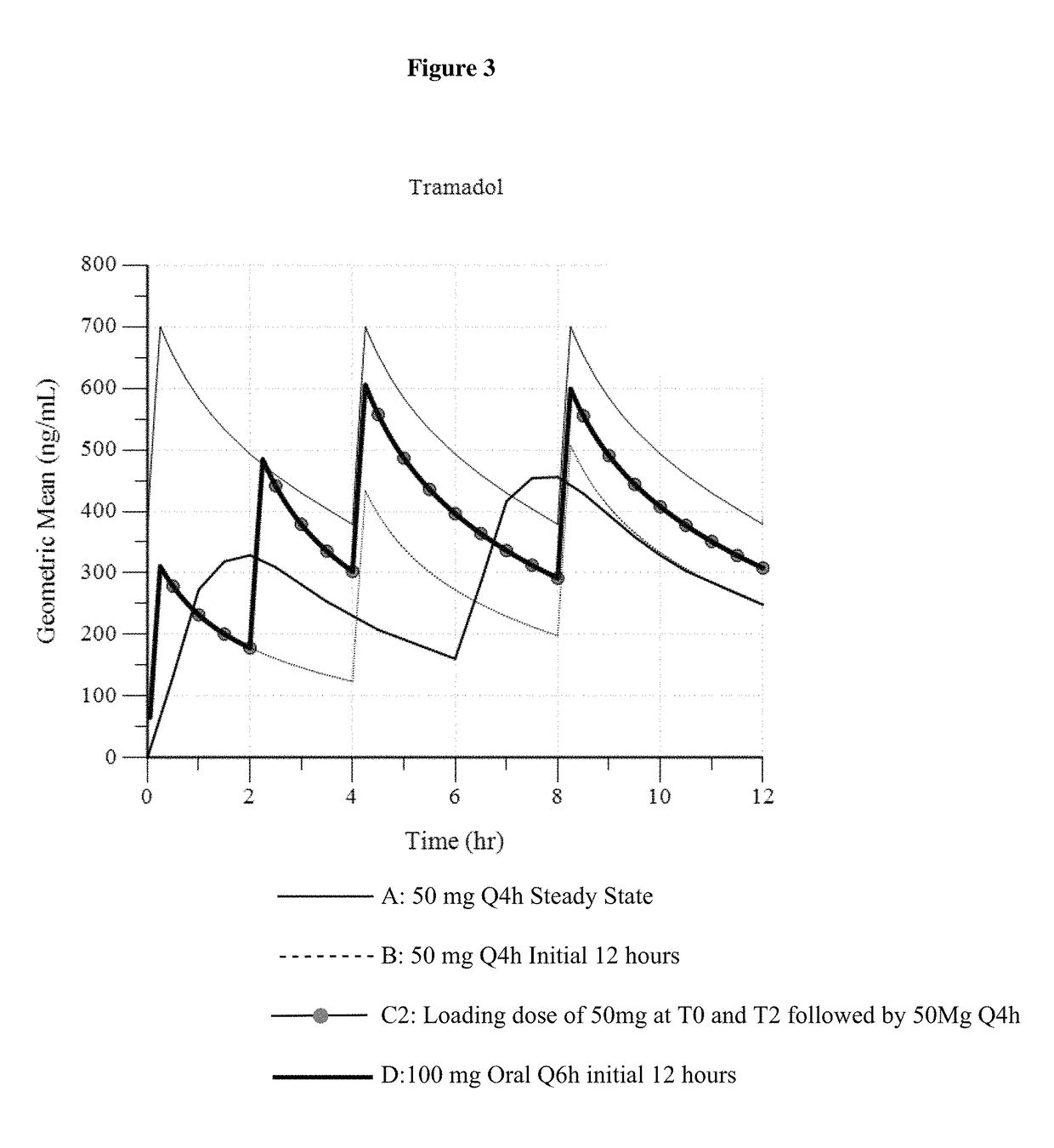
Intravenous administration of tramadol
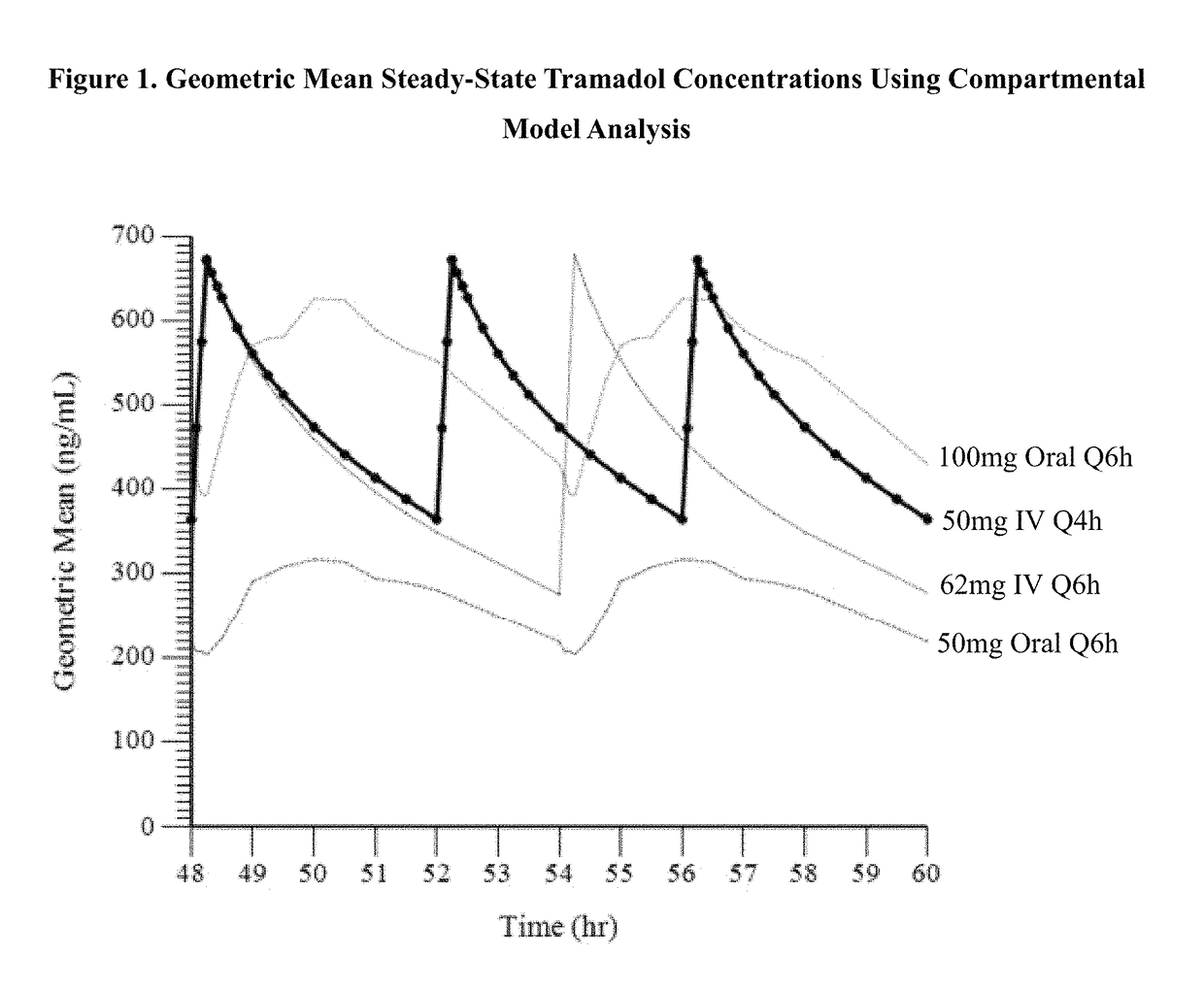
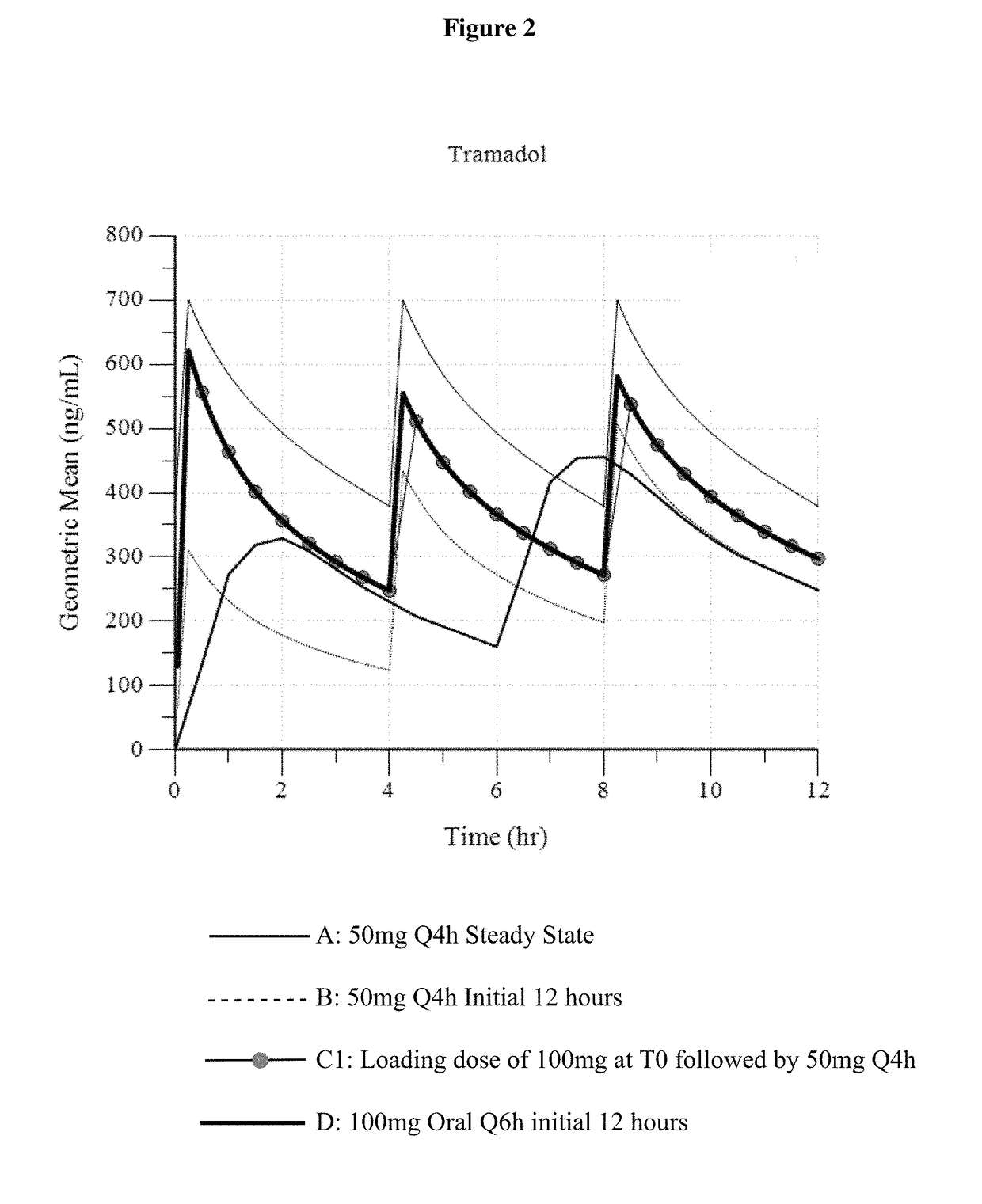
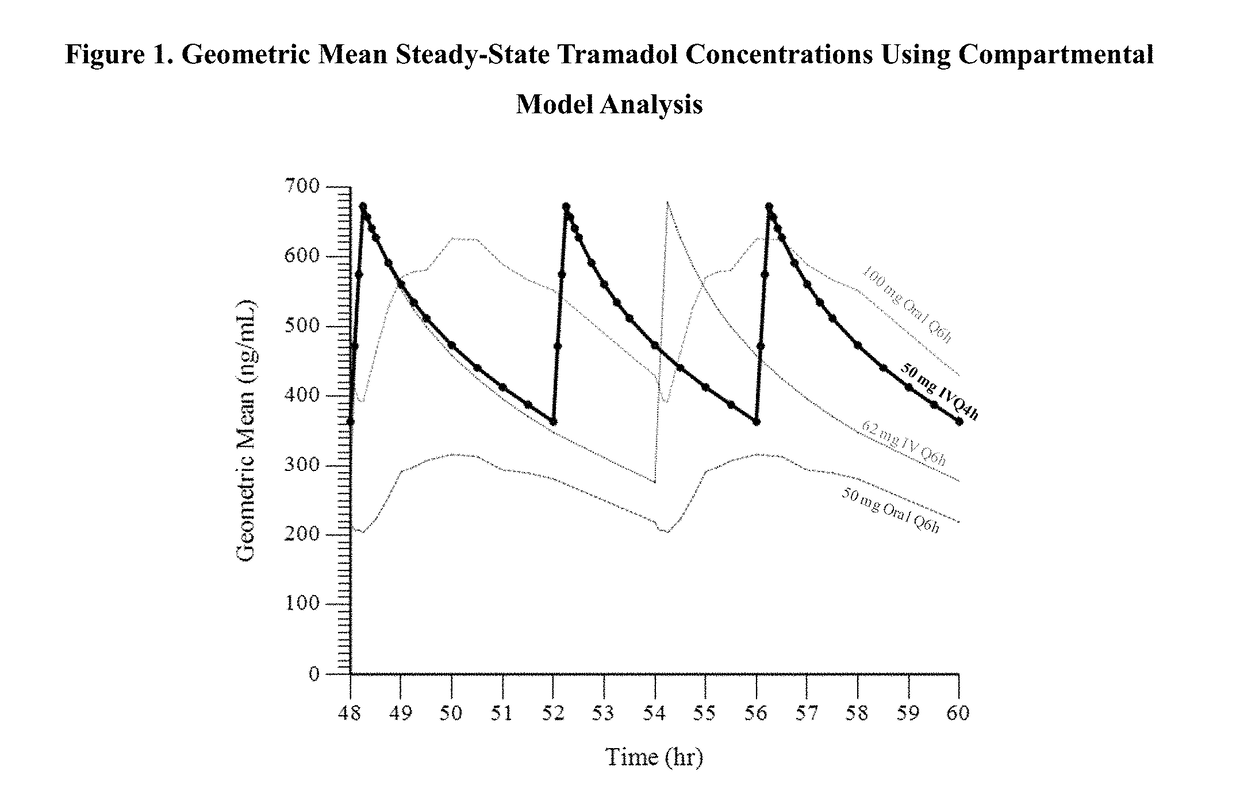
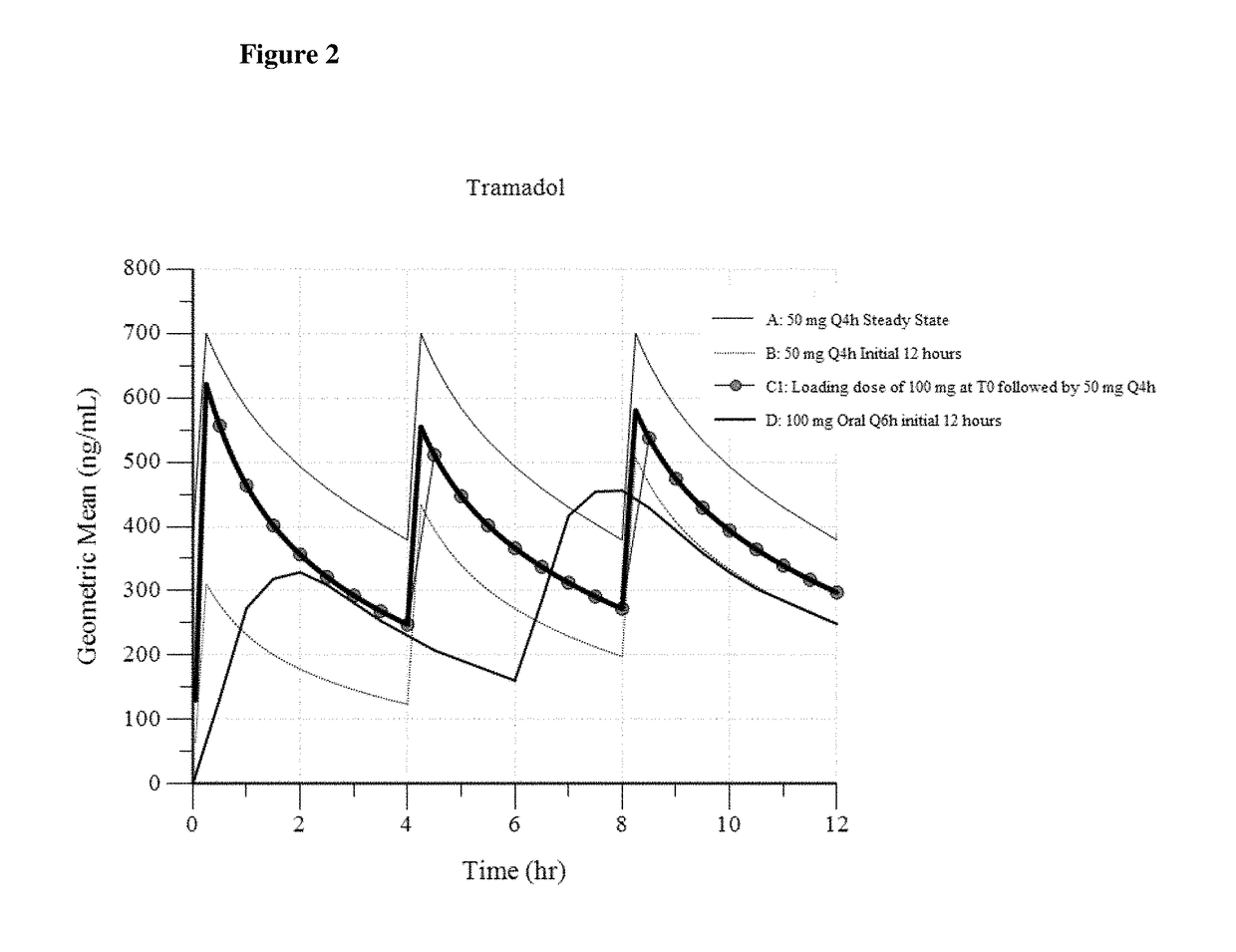
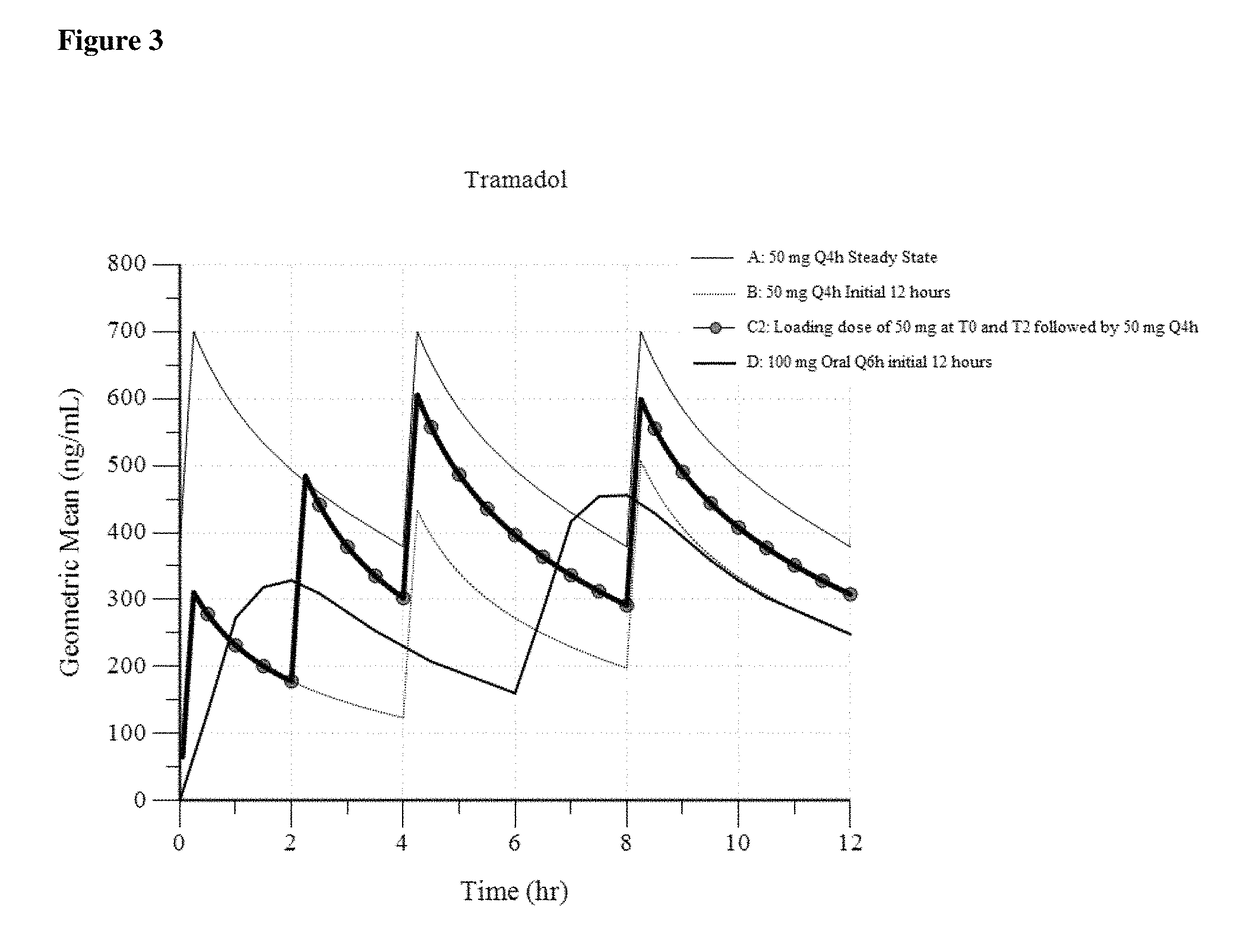
ActiveUS9693949B1InhibitionRelieve painOrganic active ingredientsNervous disorderDosing regimenRegimen
A method of treating pain, e.g., acute post-operative pain, by administering to a human patient(s) a therapeutically effective dose of tramadol intravenously in a dosing regimen which includes one or more loading doses administered at shortened intervals as compared to dosing at steady-state is disclosed. In certain embodiments, the dose of tramadol is from about 45 mg to about 80 mg and the second (and optionally) third doses are intravenously administered at intervals of from about 2 to about 3 hours, and thereafter the tramadol is intravenously administered at a dosing interval of about 4 to about 6 hours, until the patient no longer requires treatment with tramadol. In preferred embodiments, the intravenous dosing regimen provides a Cmax and AUC of tramadol is similar to the Cmax and AUC of an oral dose of 100 mg tramadol HCl given every 6 hours. In certain preferred embodiments, the dosing regimen comprises 50 mg IV tramadol at Hour 0, followed by 50 mg at Hour 2, 50 mg at hour 4, and 50 mg every 4 hours thereafter (e.g., until the patient no longer requires treatment with tramadol).
Owner:REVOGENEX IRELAND
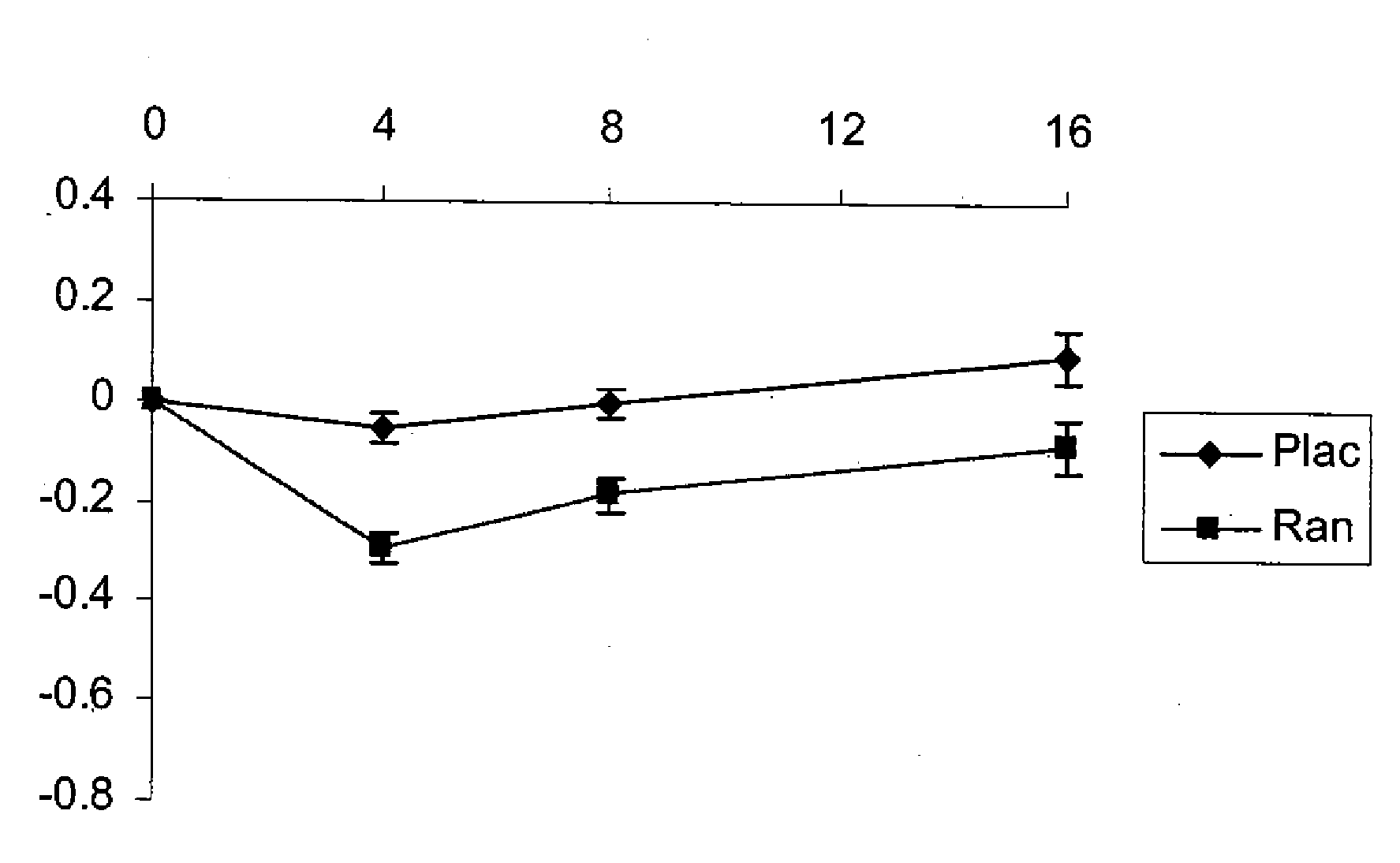
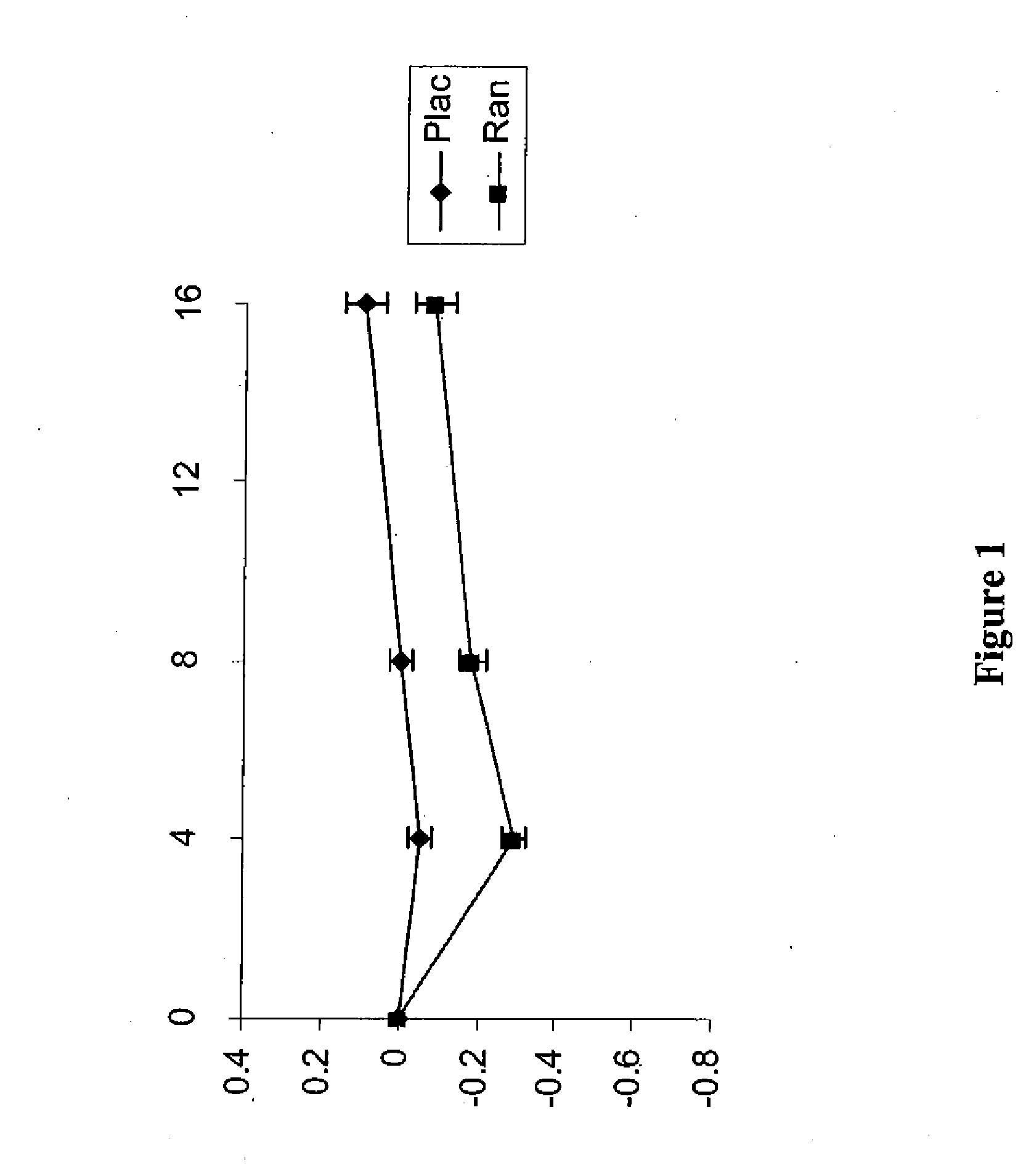
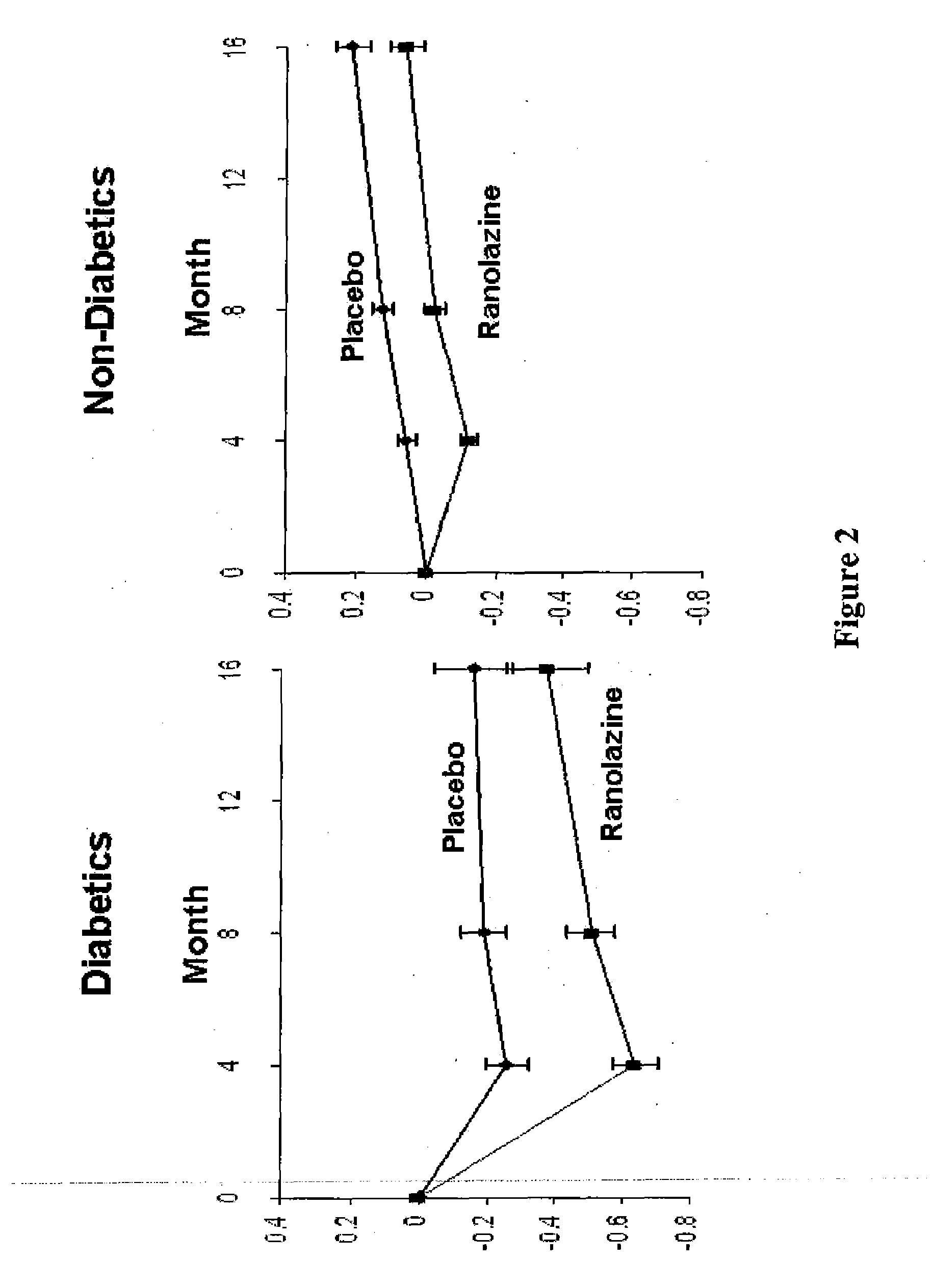
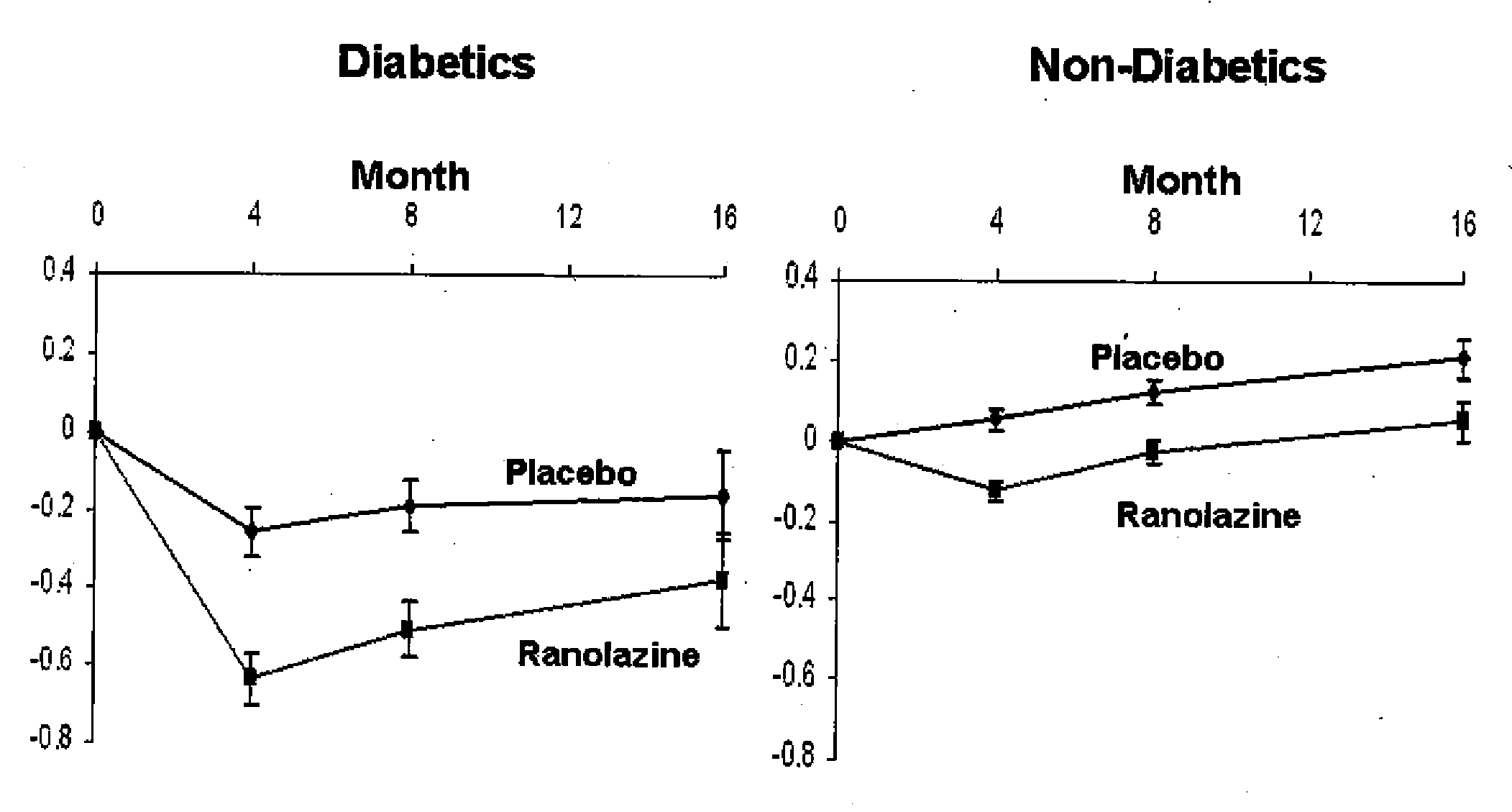
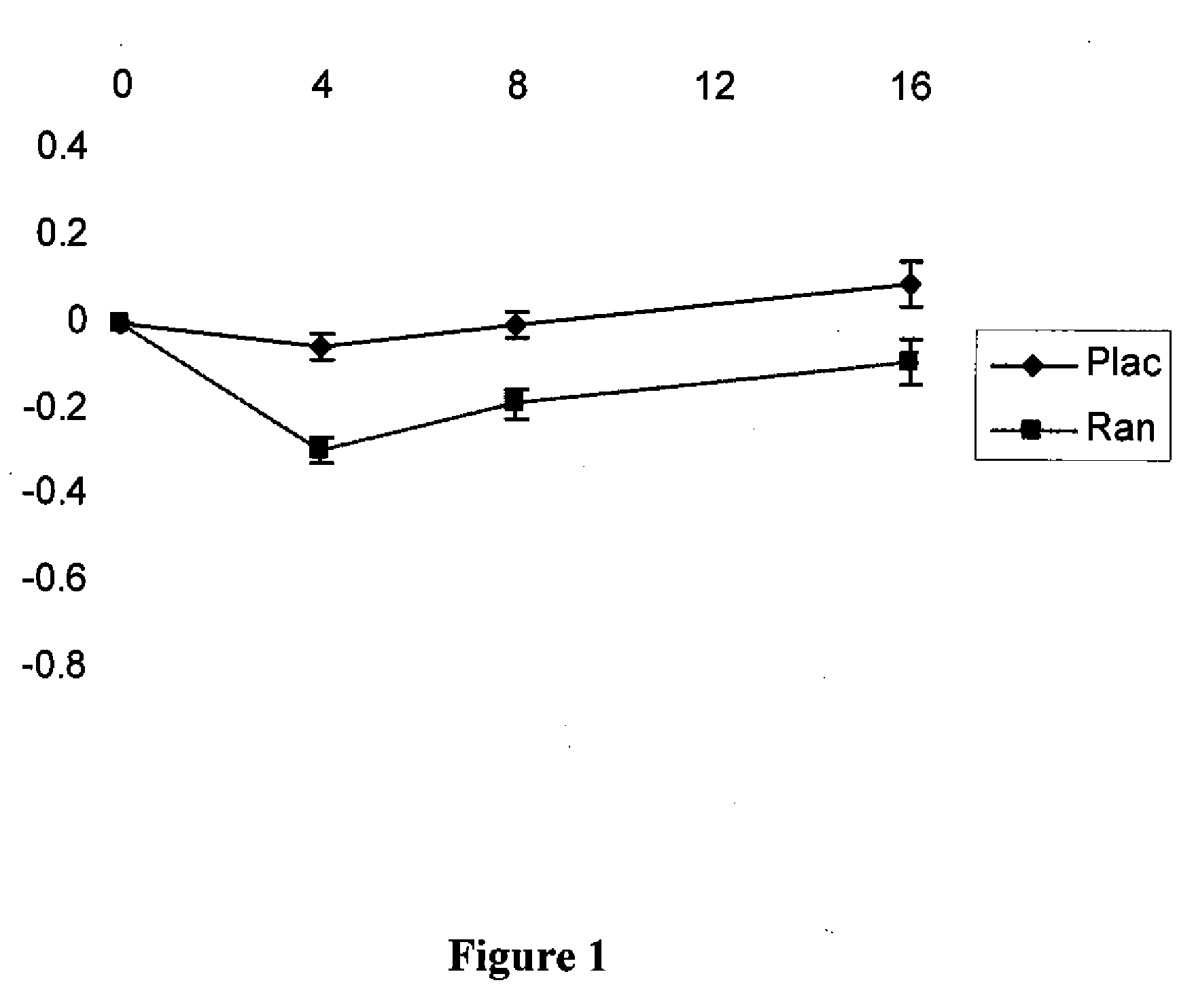
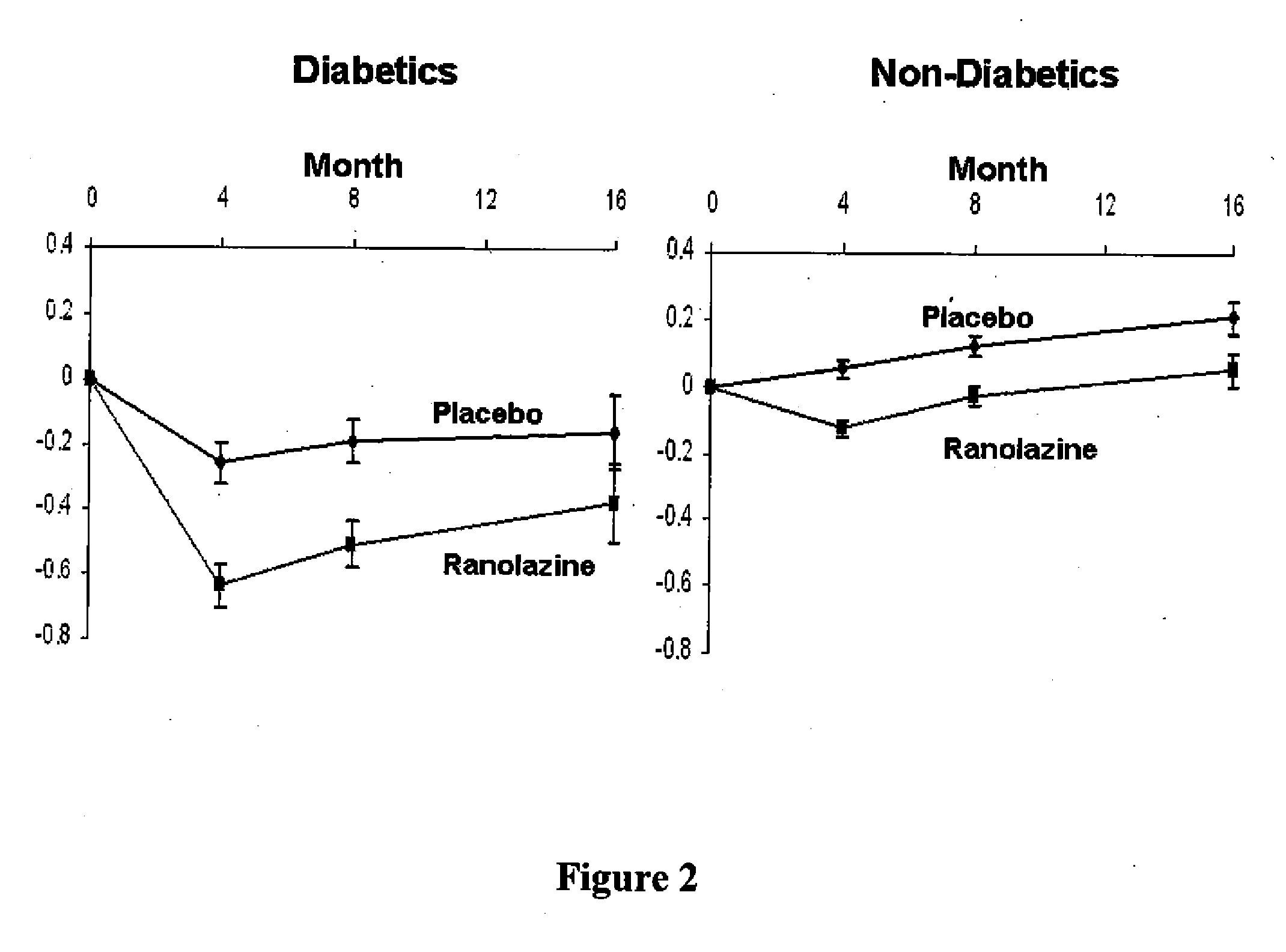
Use of ranolazine for the treatment of coronary microvascular diseases
Disclosed are methods for treating patients suffering from coronary microvascular disease comprising administering ranolazine to the patient. In one embodiment, ranolazine is administered as an oral dose
Owner:GILEAD SCI INC
Use of ranolazine for the treatment of non-coronary microvascular diseases
Disclosed are methods for treating patients suffering from non-coronary microvascular disease comprising administering ranolazine to the patient. In one embodiment, ranolazine is administered as an oral dose.
Owner:GILEAD SCI INC
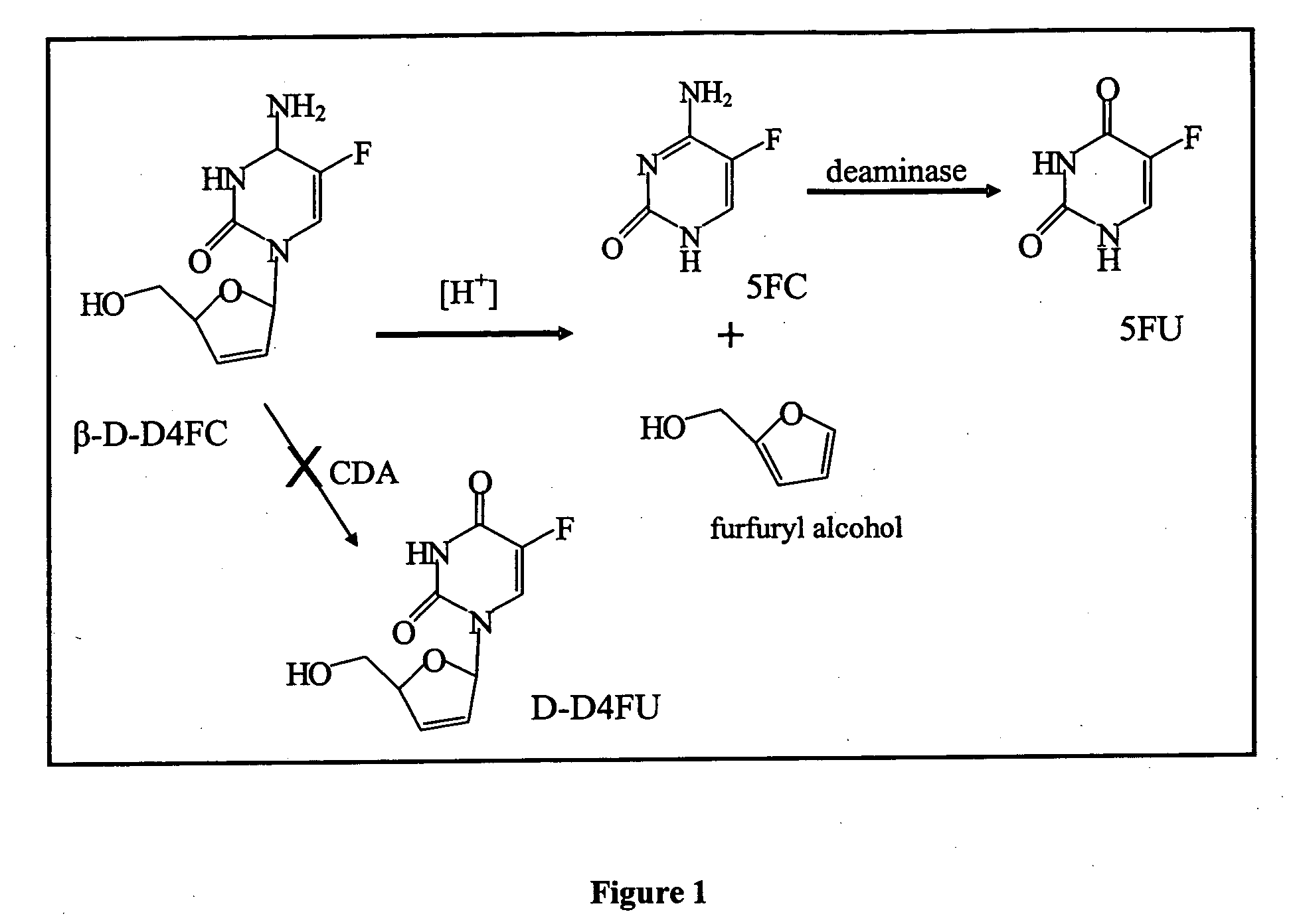
Dosing methods for beta-D-2',3'-dideoxy-2',3'-didehydro-5-fluorocytidine antiviral therapy
InactiveUS20050244490A1Improve oral bioavailabilityLow pill burdenBiocideCarbohydrate active ingredients5-fluorocytidineIn vivo
The disclosed invention is a composition for and a method of treating a HIV infection in a host, such as a human, using a single, once a day, oral dose of β-D-D4FC in an enteric-coated tablet. The enterically coated β-D-D4FC increases the amount of the drug that remains in active form for use in inhibiting the HIV virus in vivo.
Owner:PHARMASSET
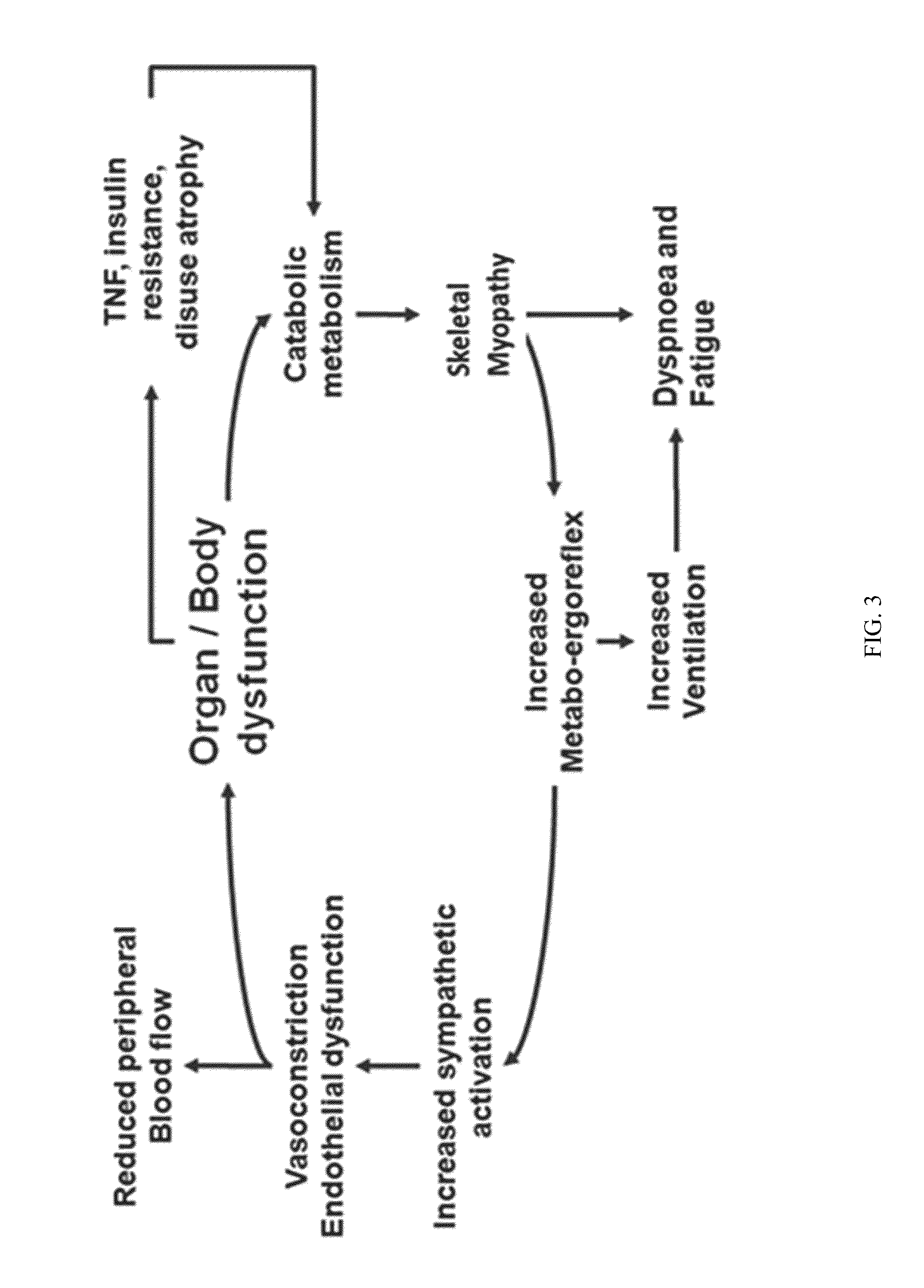
Method of Treating Cachexia and Sarcopenia
InactiveUS20140194484A1Prolong lifeImproved prognosisBiocideOrganic active ingredientsSarcopeniaPharmaceutical formulation
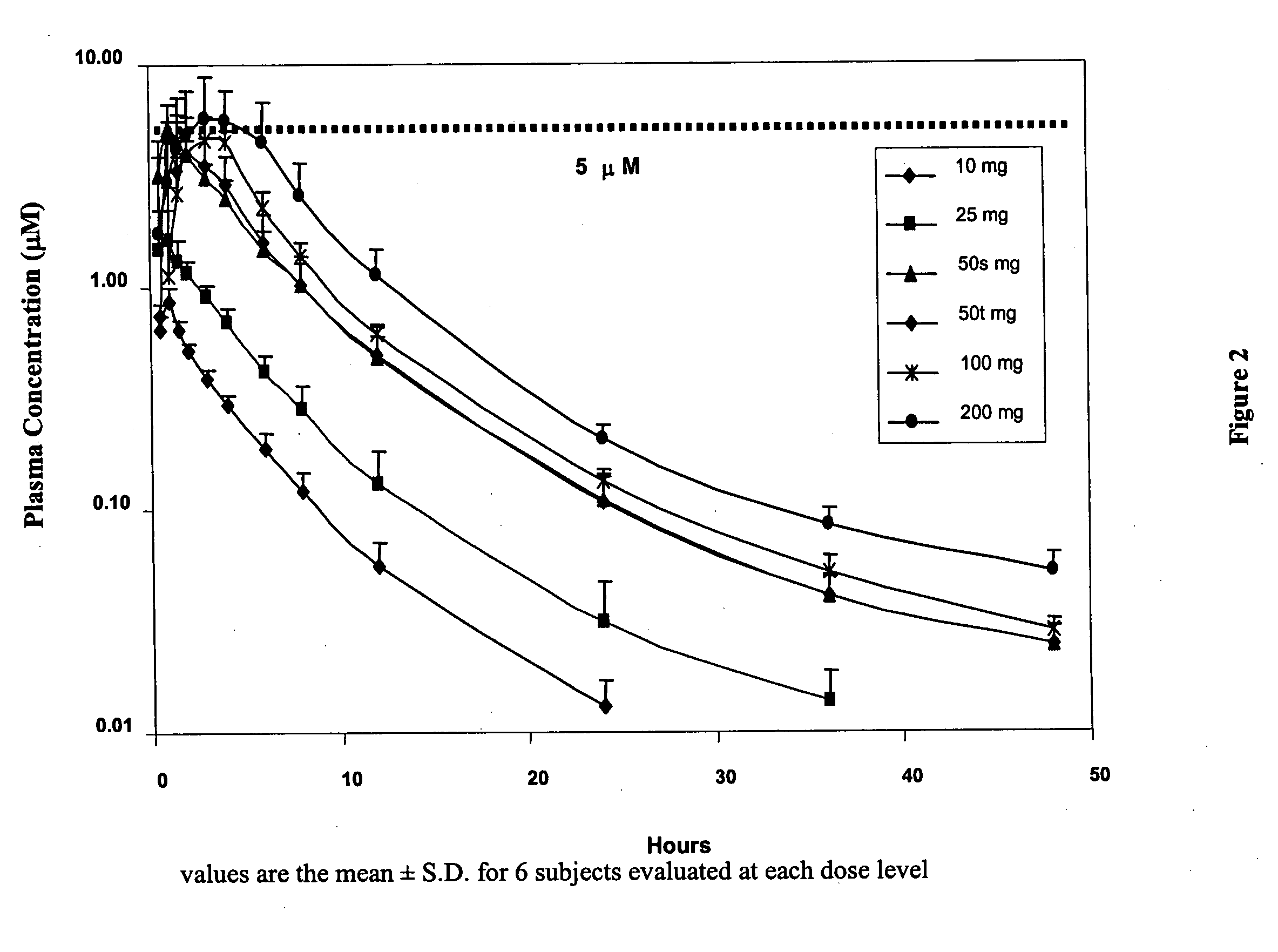
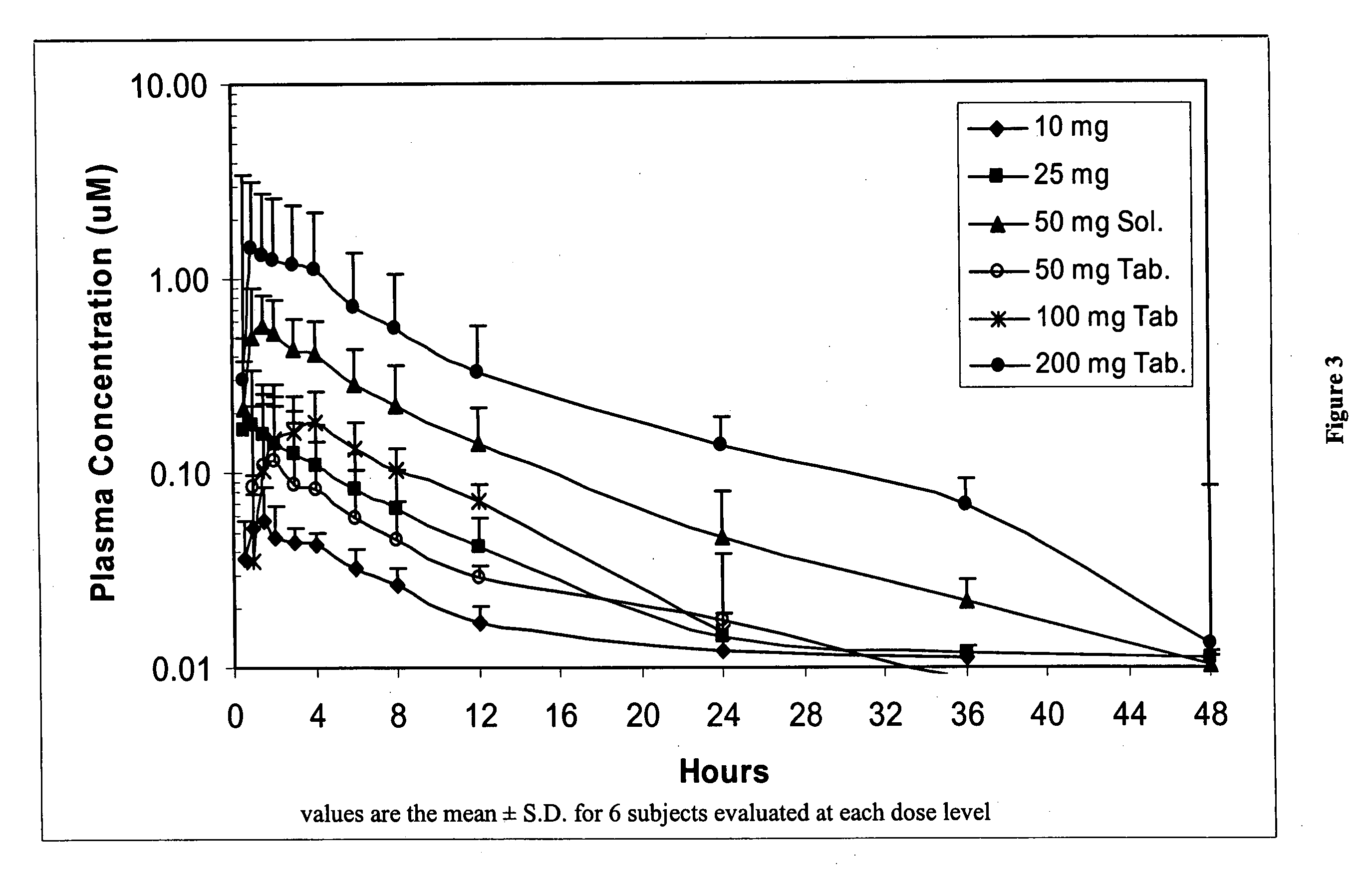
The present invention relates to a method of treating a cachexia and / or sarcopenia with an oral dose of S-pindolol or a pharmaceutical formulation thereof and to an oral formulation for use in said method of treatment. The method and oral formulation comprise administering a total daily dose of 2.5 to 20 mg of S-pindolol or a pharmaceutical formulation comprising the same.
Owner:COATS ANDREW +2
Intravenous administration of tramadol
ActiveUS9980900B2Rapid onsetRelieve painOrganic active ingredientsNervous disorderDosing regimenRegimen
Owner:REVOGENEX IRELAND
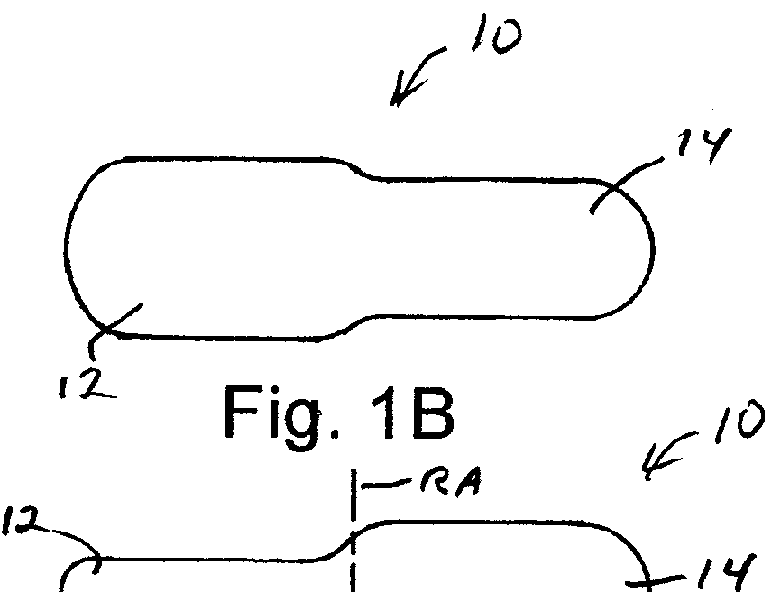
Solid Dosage Form That Promotes Reliable Oral, Esophageal and GI Transit
InactiveUS20120141544A1Rapid and reliableRapid and reliable esophagealBiocideAntipyreticRotational axisGastrointestinal transit
A solid dosage form designed to facilitate rapid and reliable oral, esophageal and GI transit has a surface area of the contact patch, i.e., the area of contact between the dosage form and the bodily surface reduced. The dosage form can be an asymmetric oral dosage unit containing a bioactive, the dosage unit being asymmetric with respect to a rotational axis perpendicular to a longitudinal axis of the dosage form, the rotational axis being located substantially at a mid point along the longitudinal axis. The dosage unit may have an outer surface ridged or dimpled or have at least one annular ring so as to reduce the contact patch of the dosage unit with a flat surface compared to non-ridged dosage unit of the same size and shape. The oral dosage unit can also have a spherical shape with or without ridges and / or dimples. Dies for forming these oral dosage units have, in a closed state, a cavity having a shape corresponding to the oral dosage unit.
Owner:FUISZ RICHARD C +1
Treatment of pets with sirtuin activators
InactiveUS20160000737A1Regulating energy metabolismReduce weightBiocideLiquid surface applicatorsMetaboliteReady to use
The present invention provides for systems, compositions, methods and kits for regulating energy metabolism in pets. The systems, compositions, and kits can comprise, and methods can make use of, pet foods, pet treats, pet supplements, and pet drinks. In one aspect, the invention provides for pet food, treat, supplement, and drink compositions that comprise a combination of (a) leucine, and (b) a sirtuin activator, or any precursors or metabolites of (a) or (b). These combinations may be effective for reducing weight or adipose volume in the pet. In another aspect, the invention provides for methods of regulating energy metabolism by the administration of one or more compositions comprising leucine, a leucine metabolite, and / or a sirtuin activator. The invention also provides for kits comprising compositions of leucine, a leucine metabolite, and / or a sirtuin activator packaged in an oral dose form with usage instructions.
Owner:NUSIRT SCI
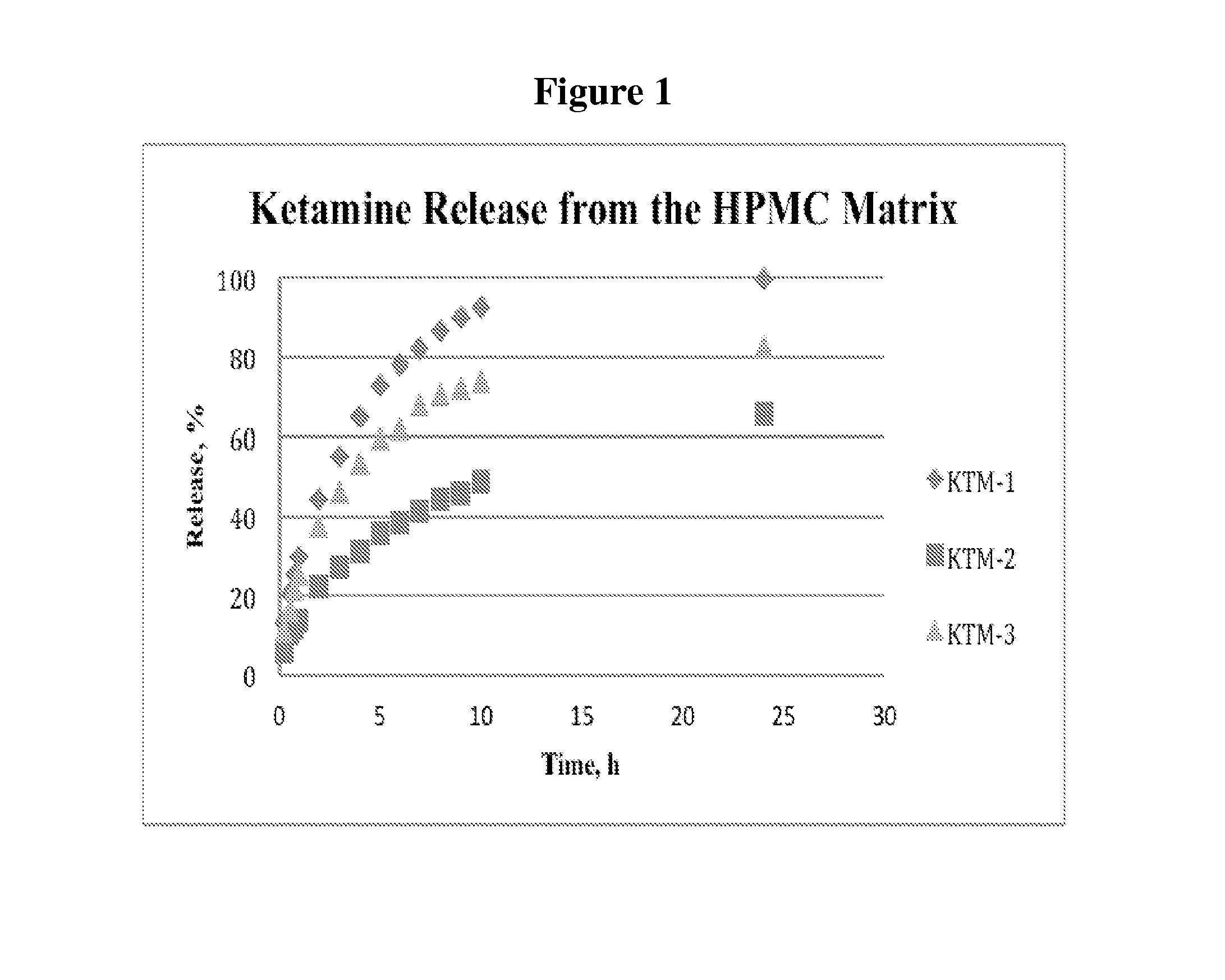
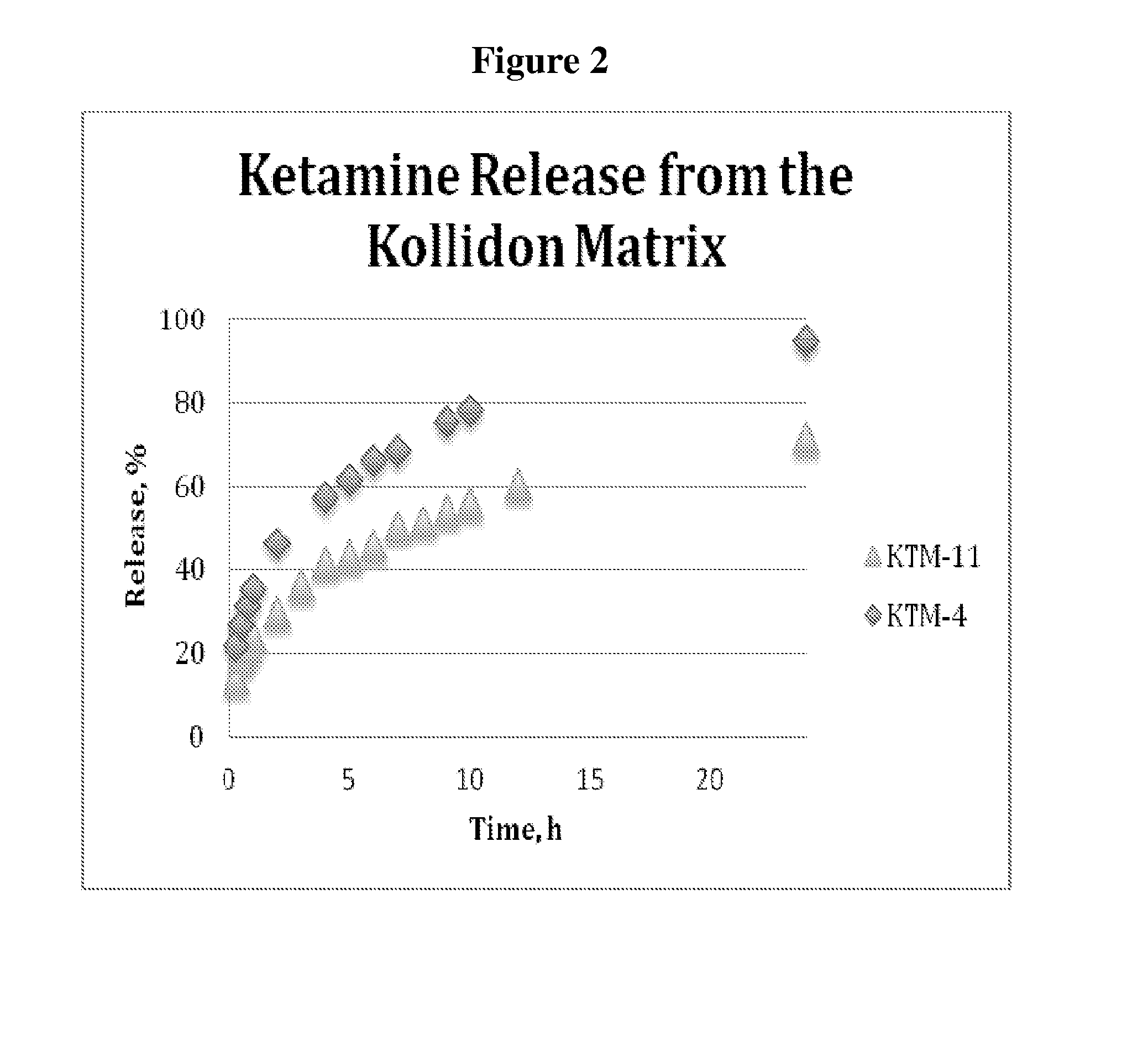
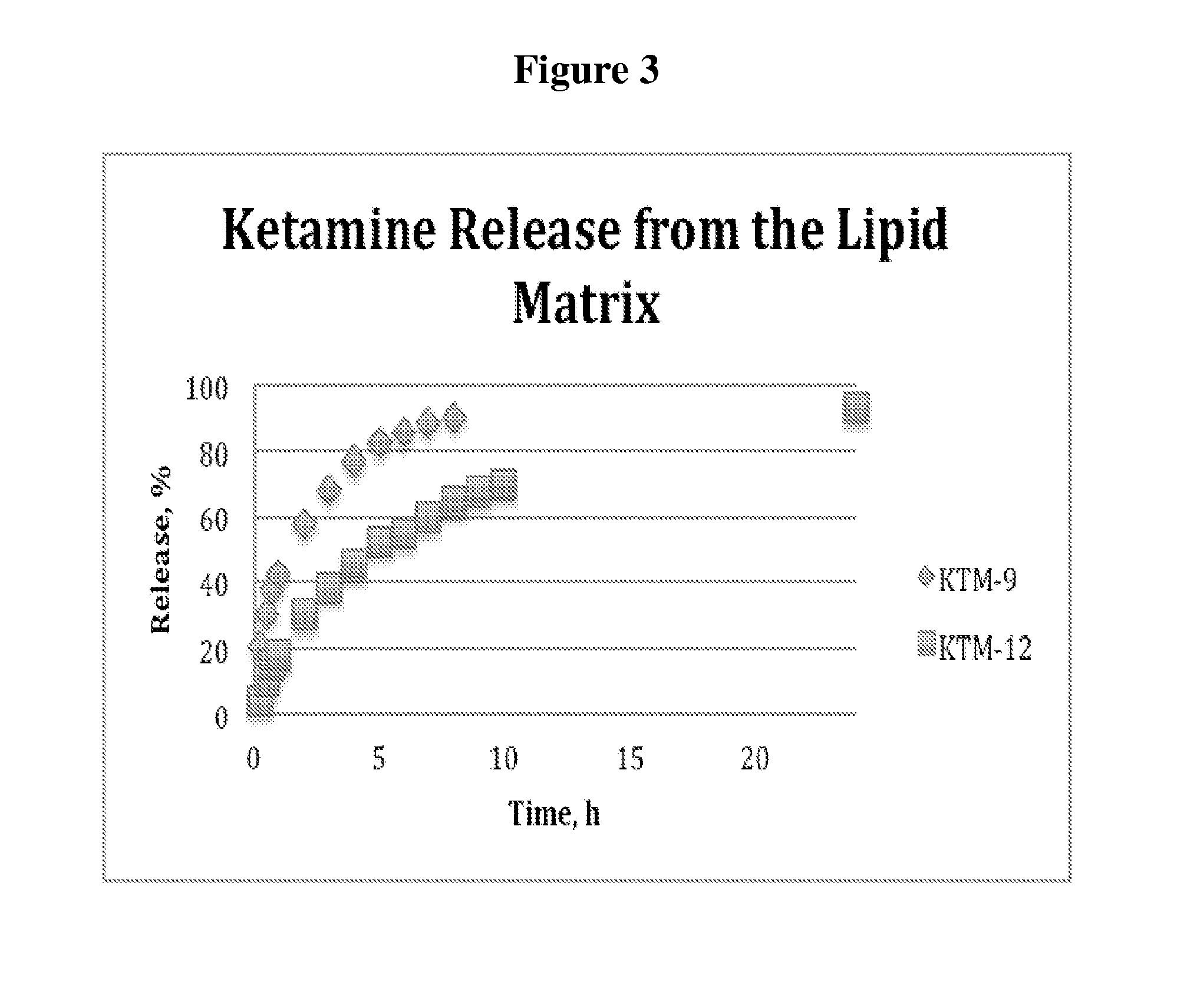
Single-layer oral dose of neuro-attenuating ketamine
The present invention is directed to oral neuro-attenuating ketamine (NAKET) tablet formulations, and methods of administration, which ensure the steady release of a therapeutically effective concentration of ketamine from an oral tablet without neurologically toxic spikes in ketamine concentration. In particular, the present invention provides single layer oral tablet formulation of NAKET. In a specific embodiment, the NAKET tablet formulation, and methods of administration provide steady administration of NAKET to a subject for 24 hours or greater, for example, up to 36 hours, after a single administration event.
Owner:AMORSA THERAPEUTICS
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com