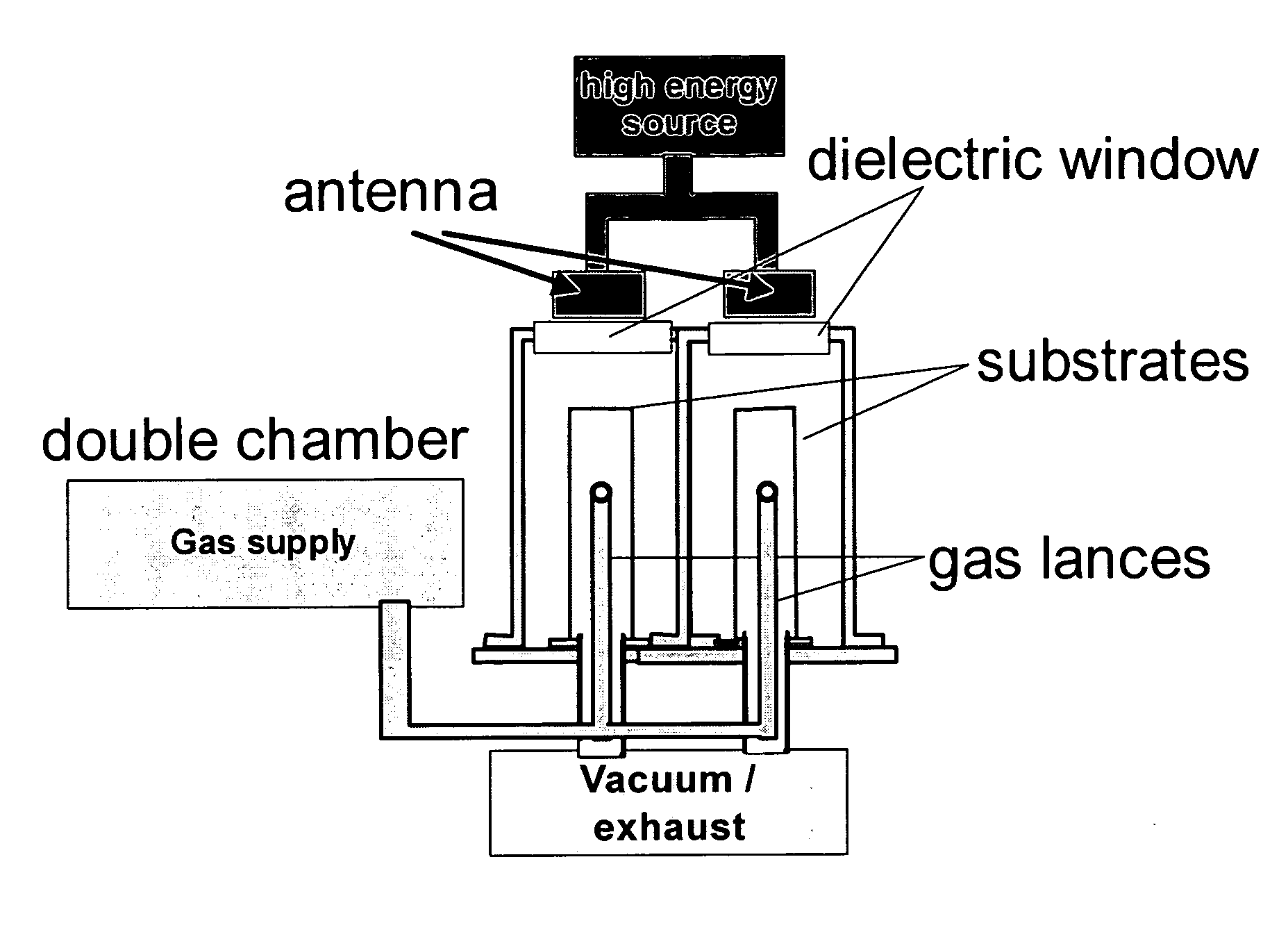
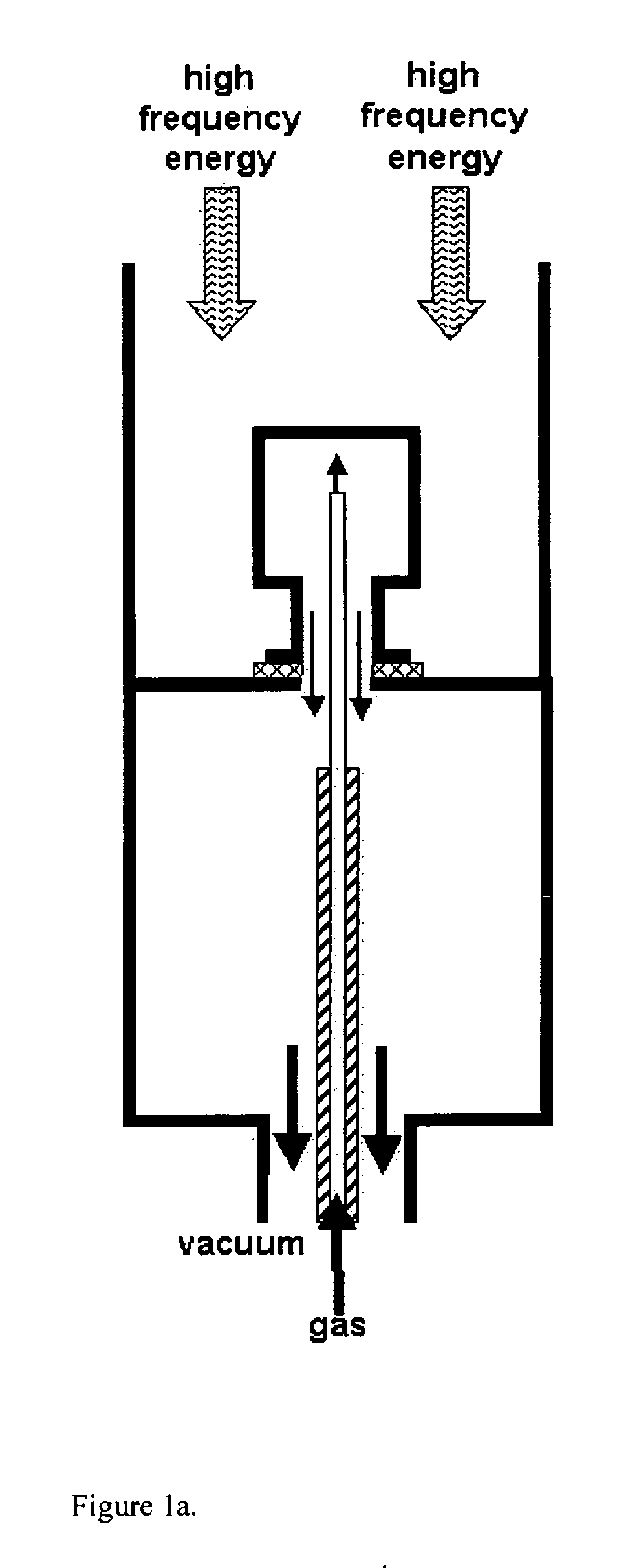
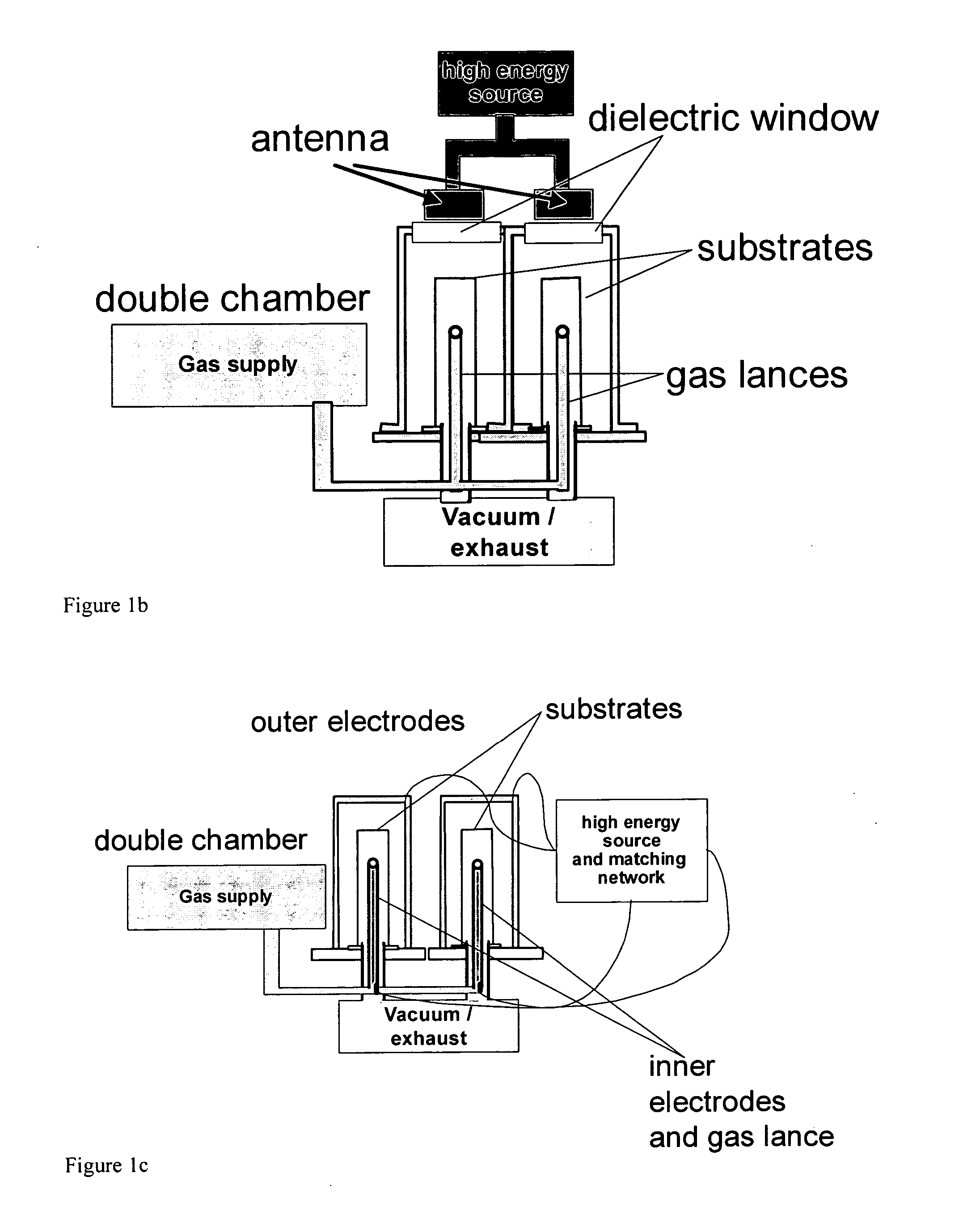
Patents
Literature
288 results about "Pharmaceutical packaging" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
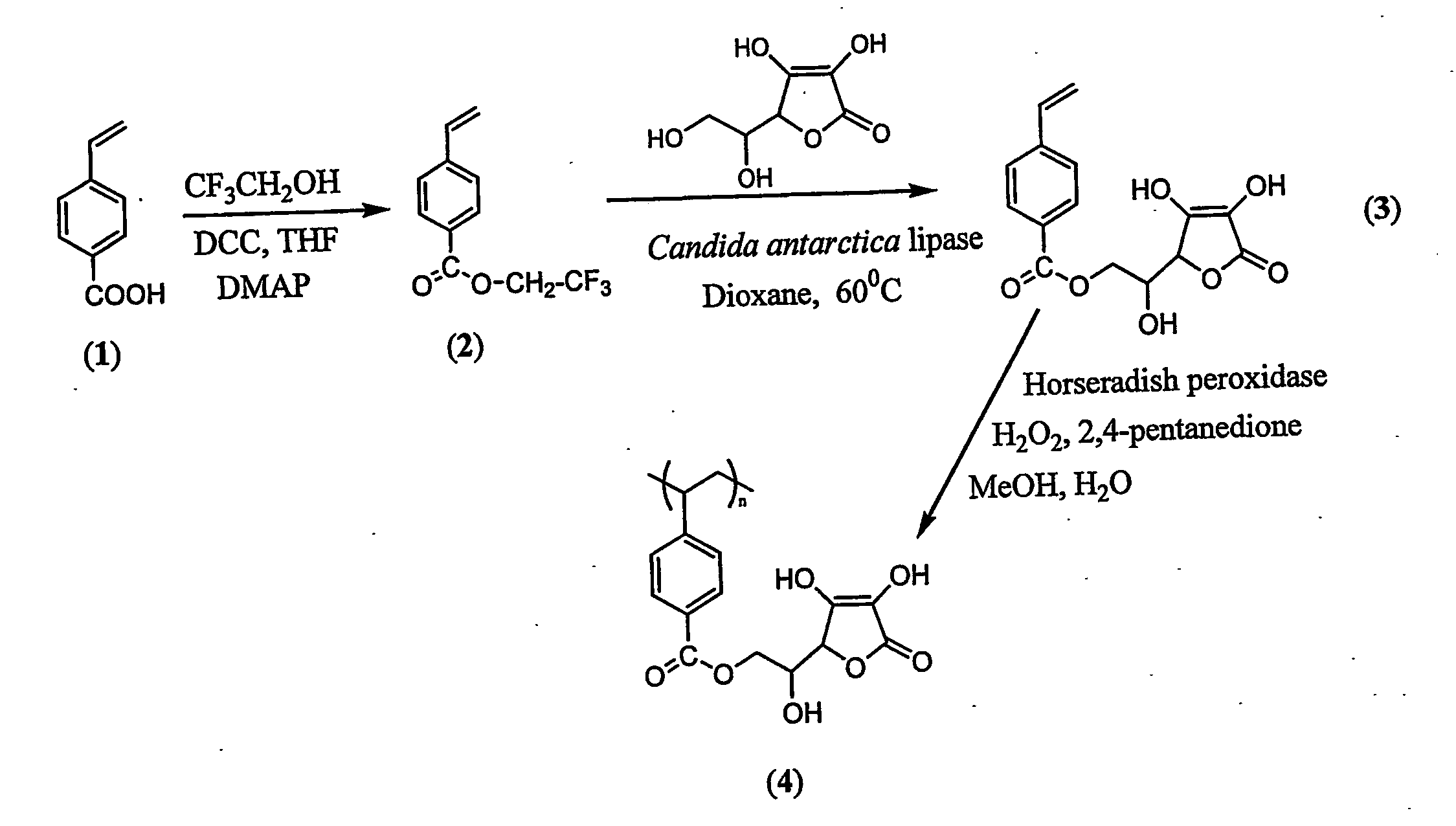
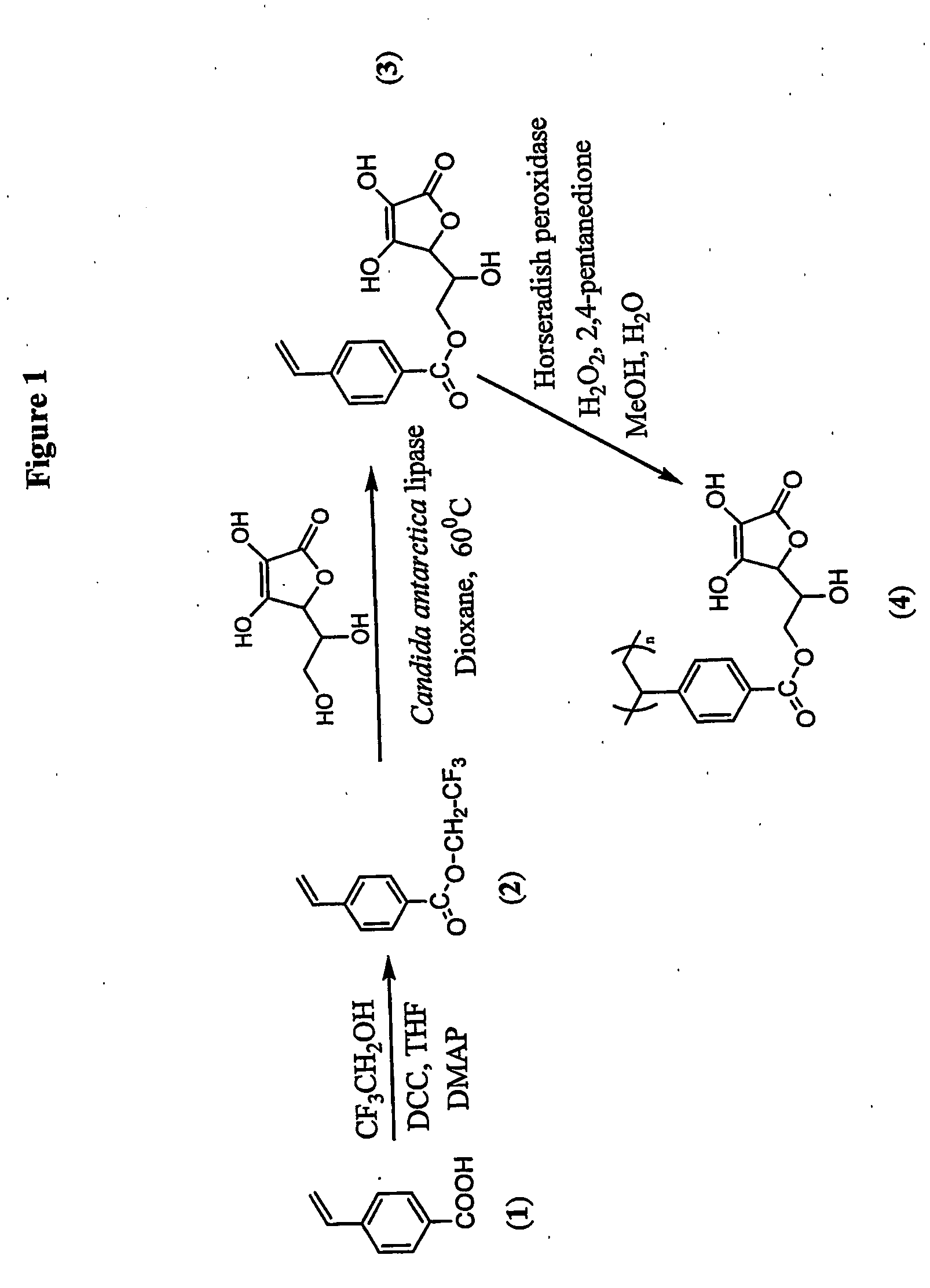
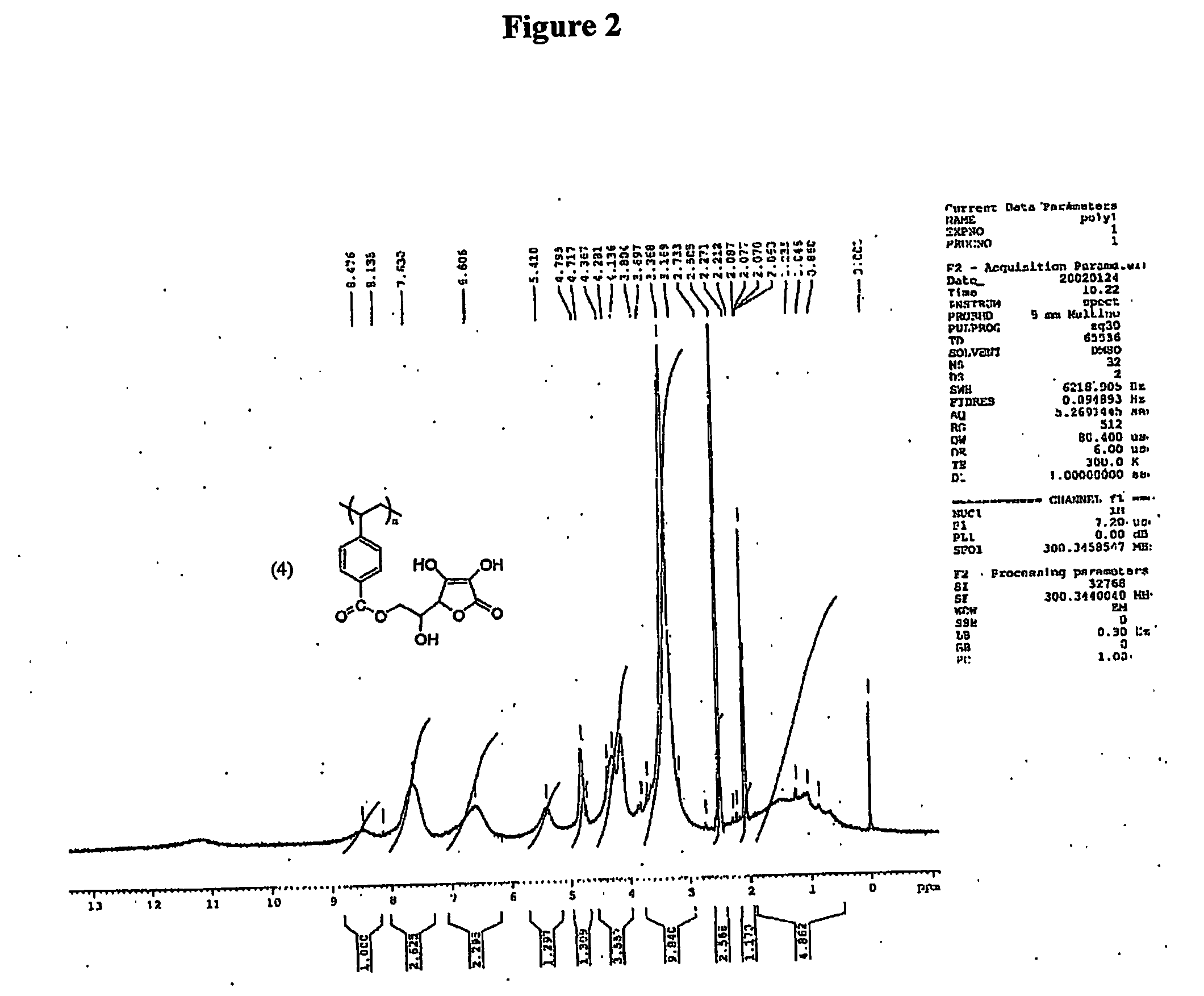
Antioxidant-functionalized polymers
InactiveUS20070010632A1Low yieldReduce sensitivitySuture equipmentsOrganic chemistryAntioxidantOxygen
Methods and compositions are disclosed for the preparation of free radical scavenging polymers and polymer films functionalized with antioxidants. Enzymatic and chemical tailoring of monomers with antioxidants followed by enzymatic polymerization is described. These antioxidant functionalized polymers can increase shelf life and quality of food products, as well as, increase effectiveness of pharmaceutical agents when used as packaging or as coatings on packaging for oxygen sensitive materials. The novel enzymatic covalent coupling of antioxidants to a polymer enhances the free radical scavenging ability of packaging while also inhibiting the escape of the antioxidants, and thus limiting exposure and / or absorption by an individual. In addition to its use in food or pharmaceutical packaging, methods are disclosed for using the antioxidant coupled polymers in a variety of applications including as coatings on the inside of medical devices, such as stents and catheters, which would substantially reduce free radical damage and / or oxygen depletion during medical procedures. Furthermore, through the coupling of antioxidants to biodegradable polymers, controlled delivery and sustained release of an antioxidant to a subject is possible.
Owner:TRUSTEES OF TUFTS COLLEGE
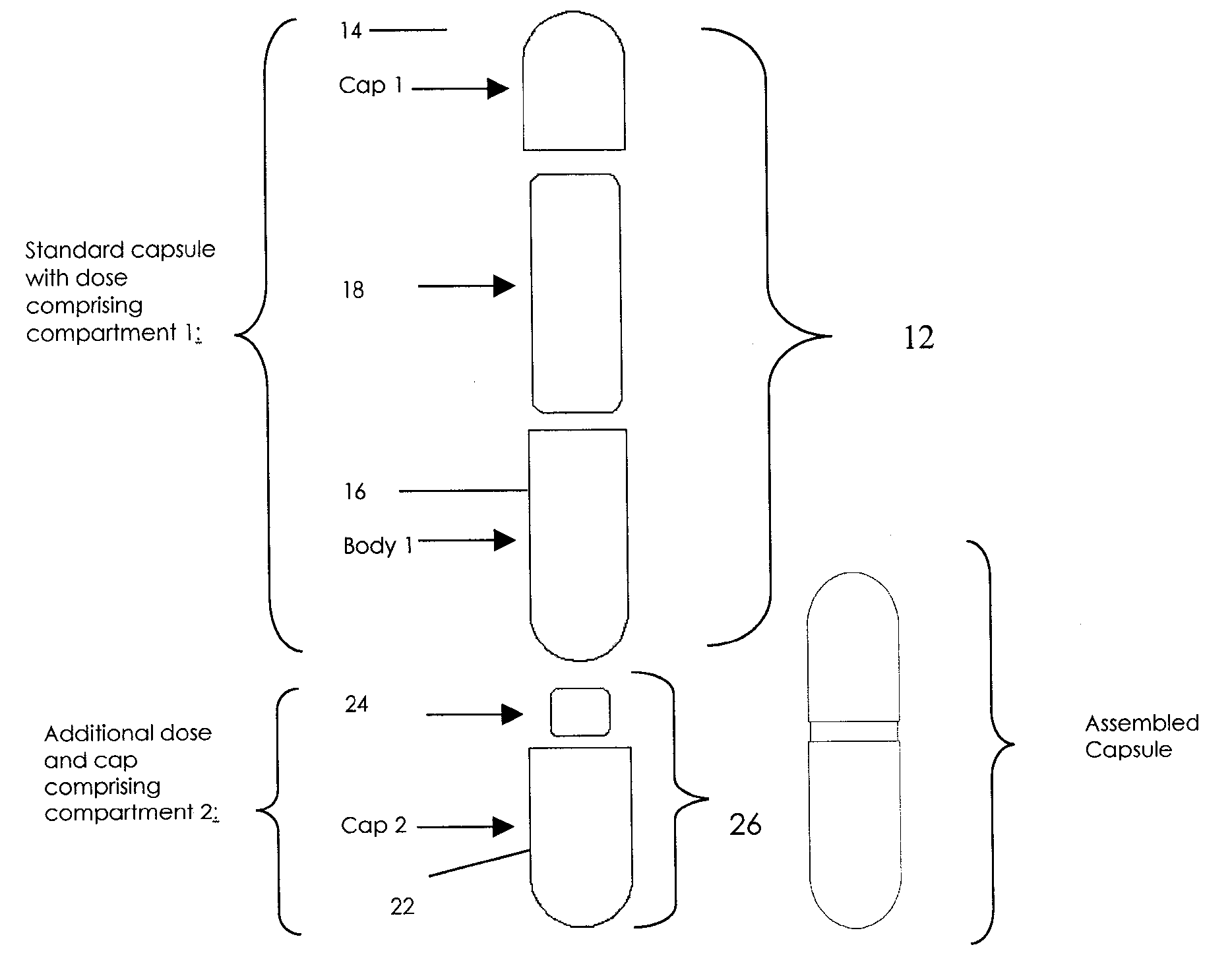
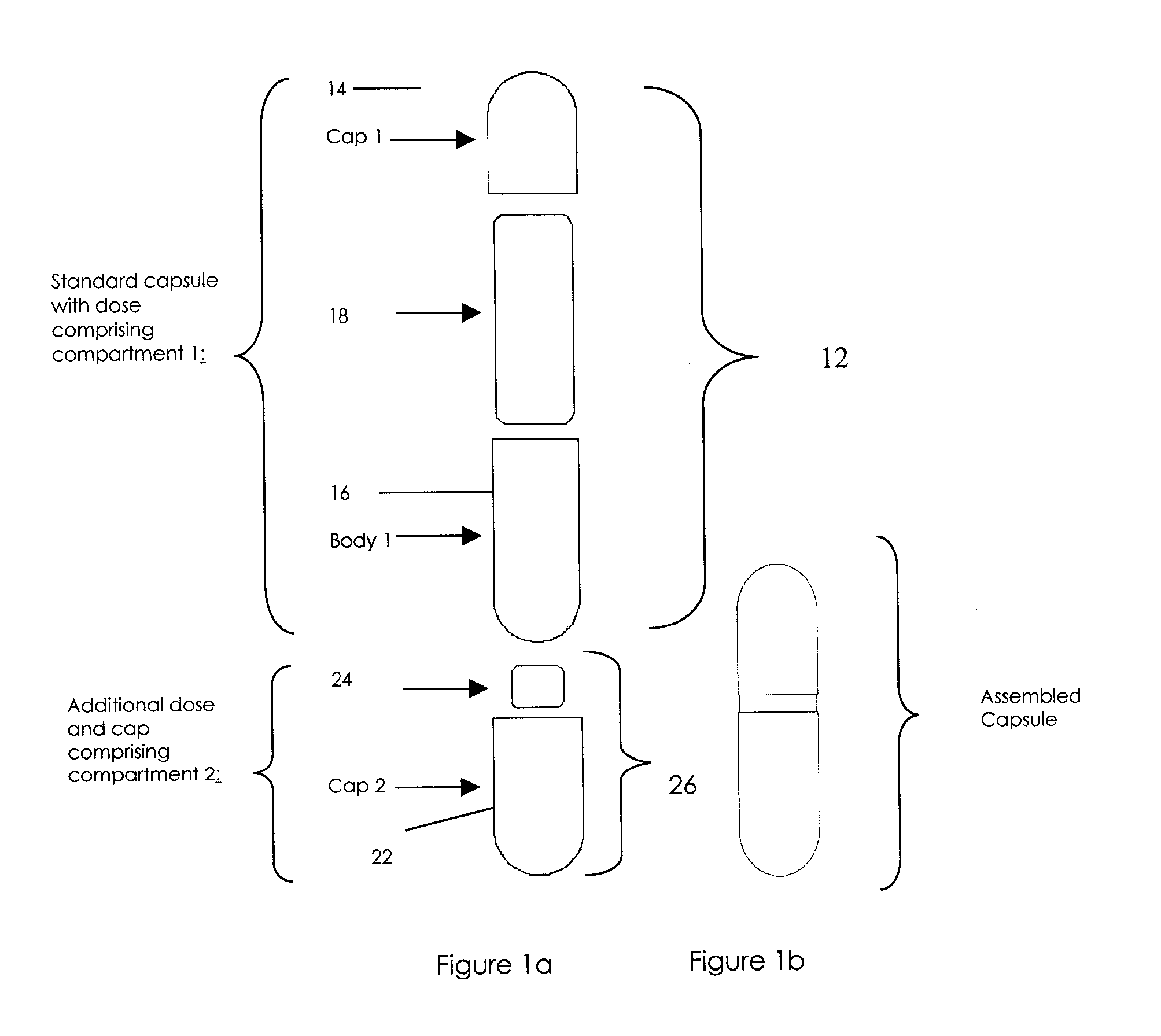
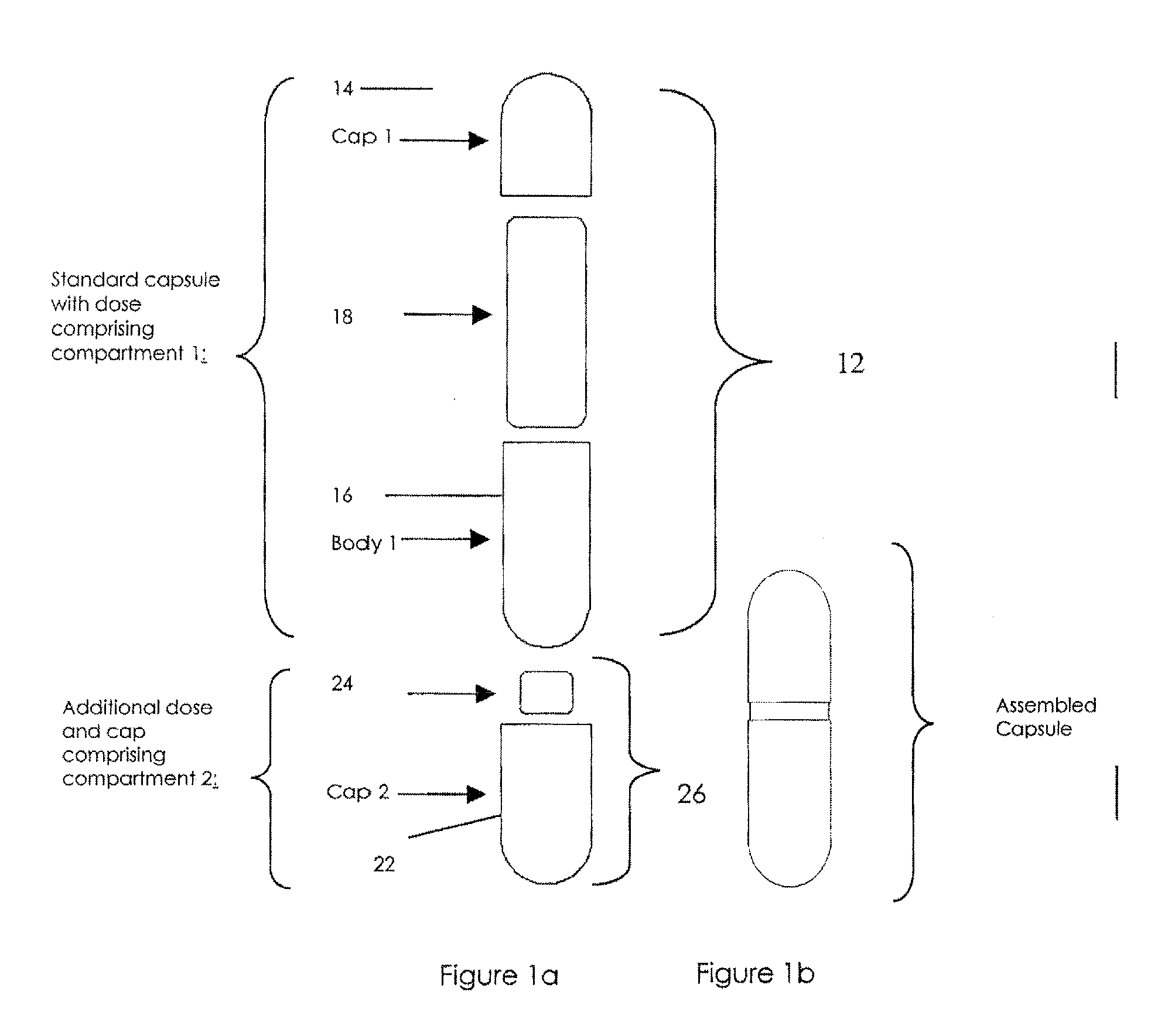
Oral dosage combination pharmaceutical packaging
InactiveUS20090087483A1Overall design flexibilityIncreased riskAntibacterial agentsBiocideMedicinePharmaceutical packaging
Pharmaceutical fixed dose combination products are formed by merging a fixed dose of a first pharmaceutical formulation from primary module, with a fixed dose of a second pharmaceutical formulation from a secondary module. In a preferred embodiment the first and second pharmaceutical formulations are separated from one another in a three piece capsule, a capsule-in-a-capsule or a tablet-in-a-capsule, and the primary and secondary modules are interchangeable.
Owner:MICRODOSE THERAPEUTX INC
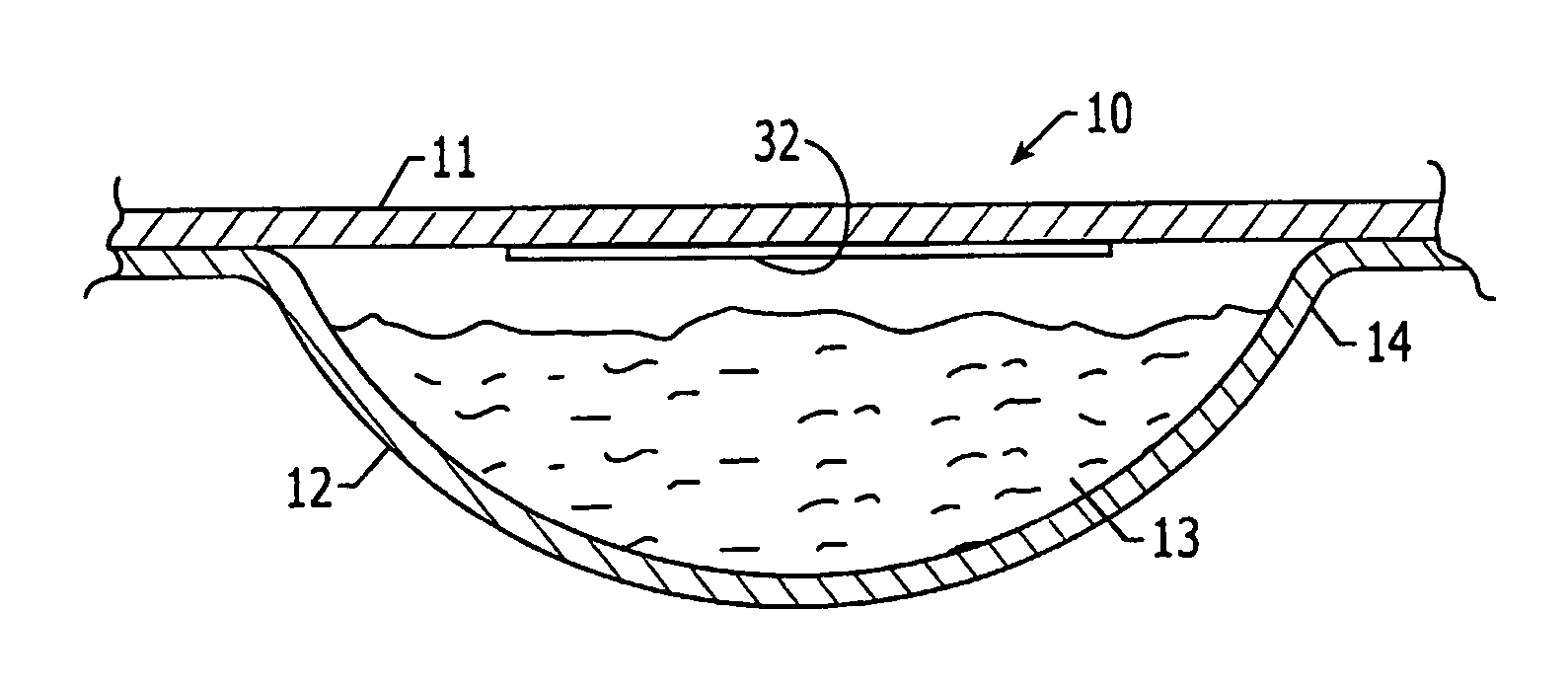
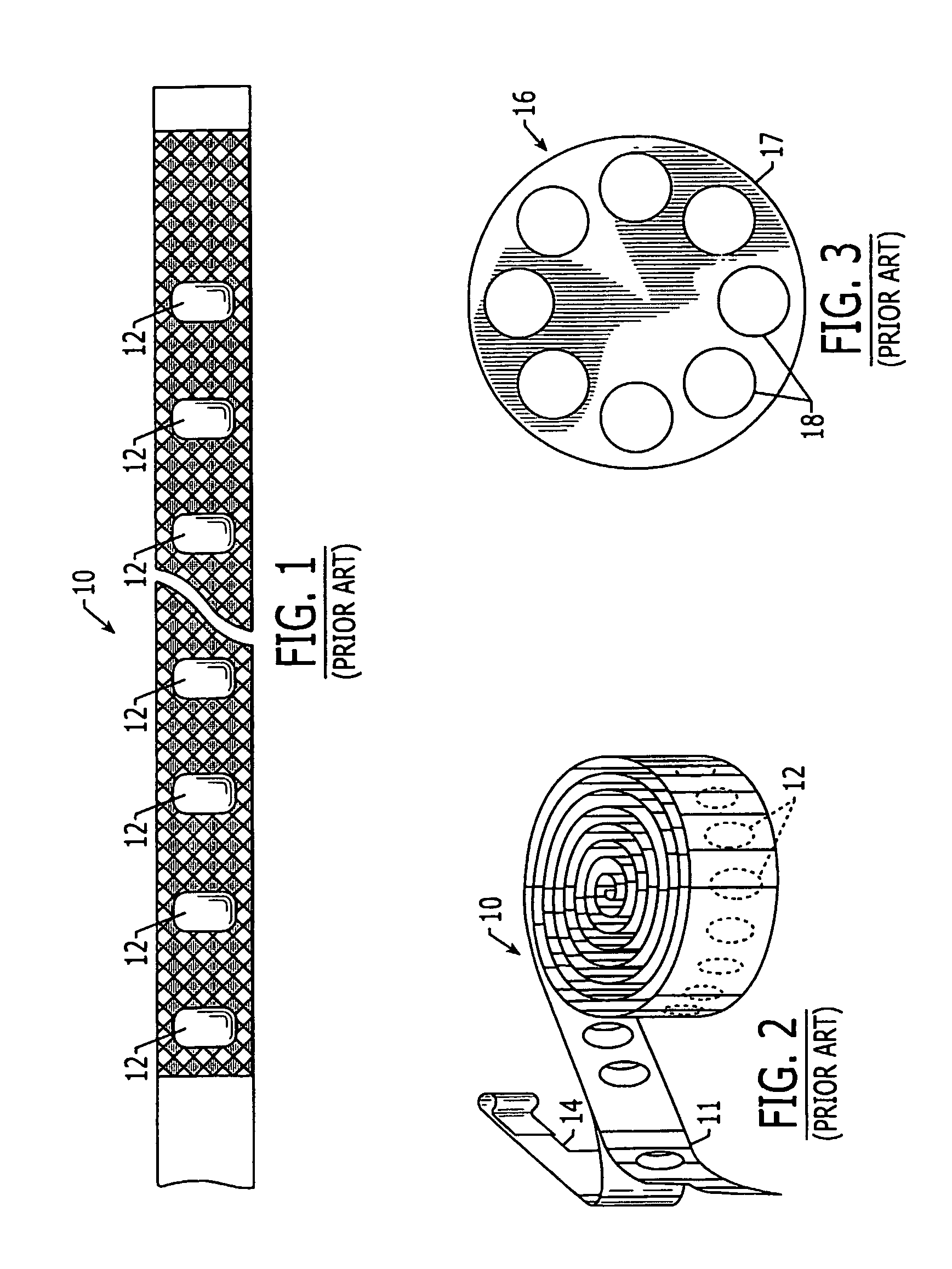
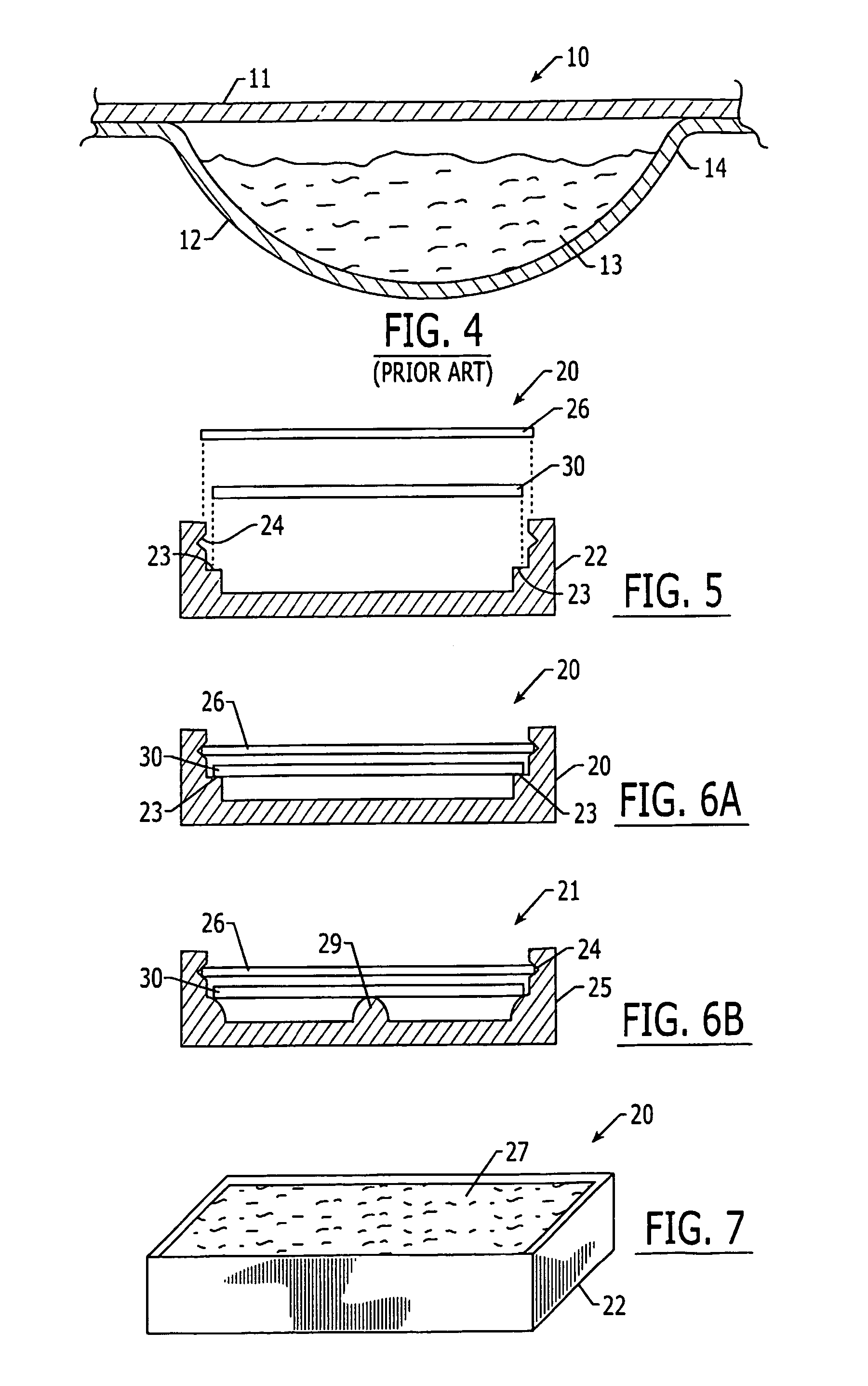
Oxygen scavenging pharmaceutical package and methods for making same
The present invention relates generally to a pharmaceutical packaging for increasing the product shelf life, reducing discoloration, and reducing degradation of pharmaceuticals by reducing the oxygen level present in the pharmaceutical package. The pharmaceutical package comprises a substantially oxygen impermeable container, at least one oxygen scavenging element disposed in the container, and at least one packaged pharmaceutical product disposed in the oxygen impermeable container.
Owner:TEVA PHARM USA INC
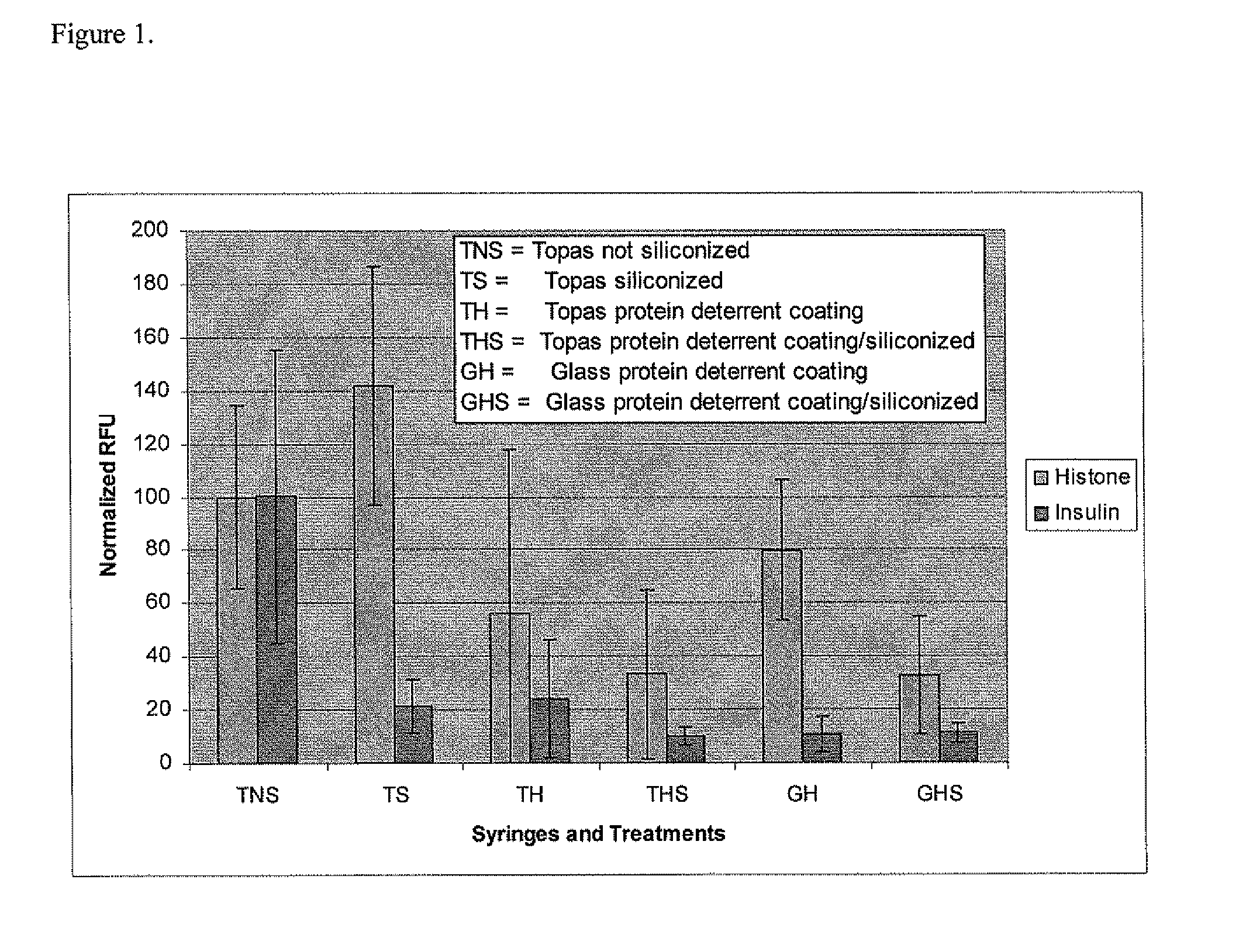
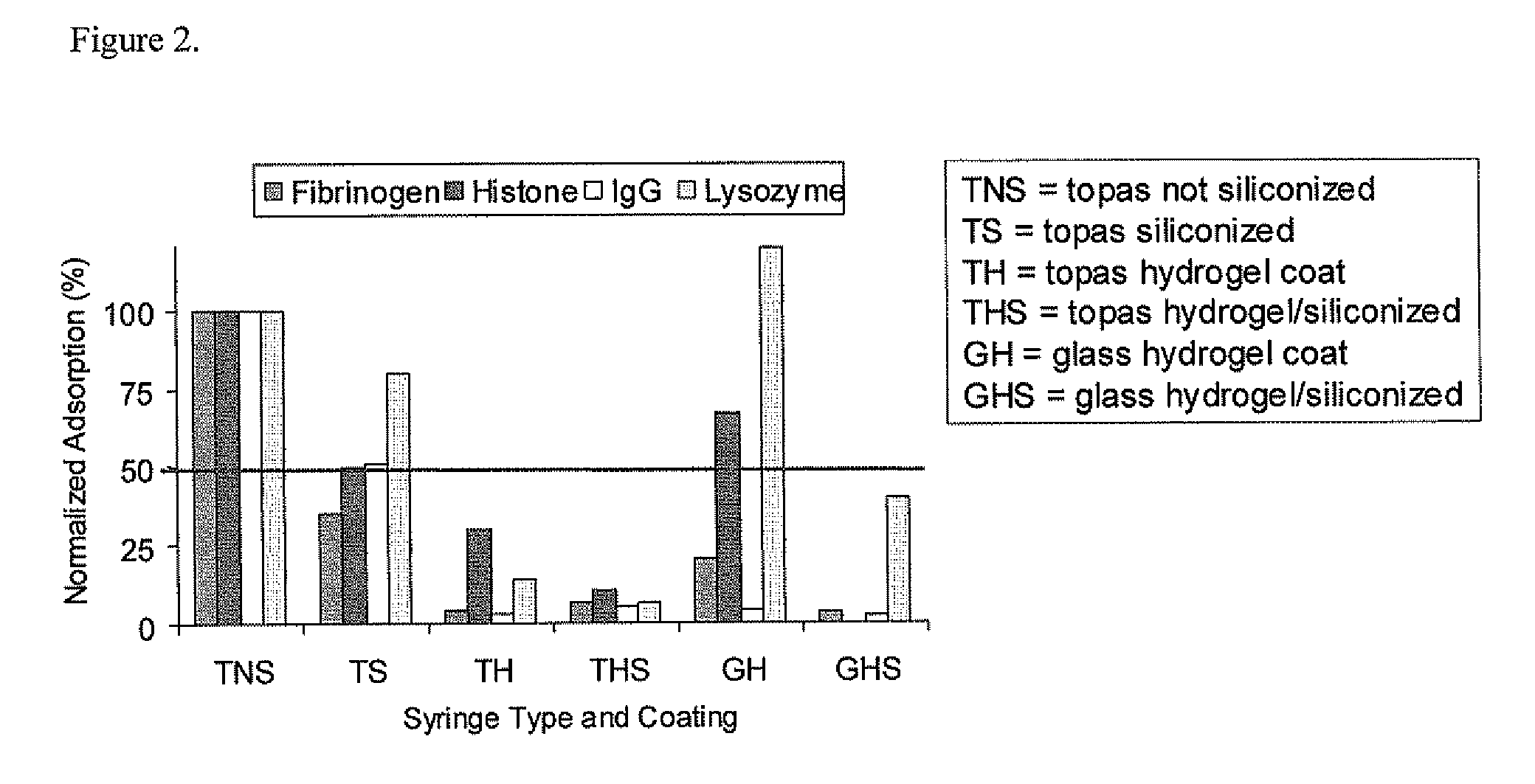
Method of preparing a macromolecule deterrent surface on a pharmaceutical package
ActiveUS20070187280A1Reduce adsorptionReduced ion exchangeSmall article dispensingSynthetic resin layered productsProtein solutionPharmaceutical packaging
A method of preparing a macromolecule deterrent surface on a pharmaceutical package. In particular, the present invention relates to a method of preparing a protein deterrent surface on a pharmaceutical package by applying a coating or coatings directly to the pharmaceutical package that reduces the adsorption of proteins onto pharmaceutical packaging while not affecting the activity of the protein solution contained.
Owner:SCHOTT PHARMA AG & CO KGAA
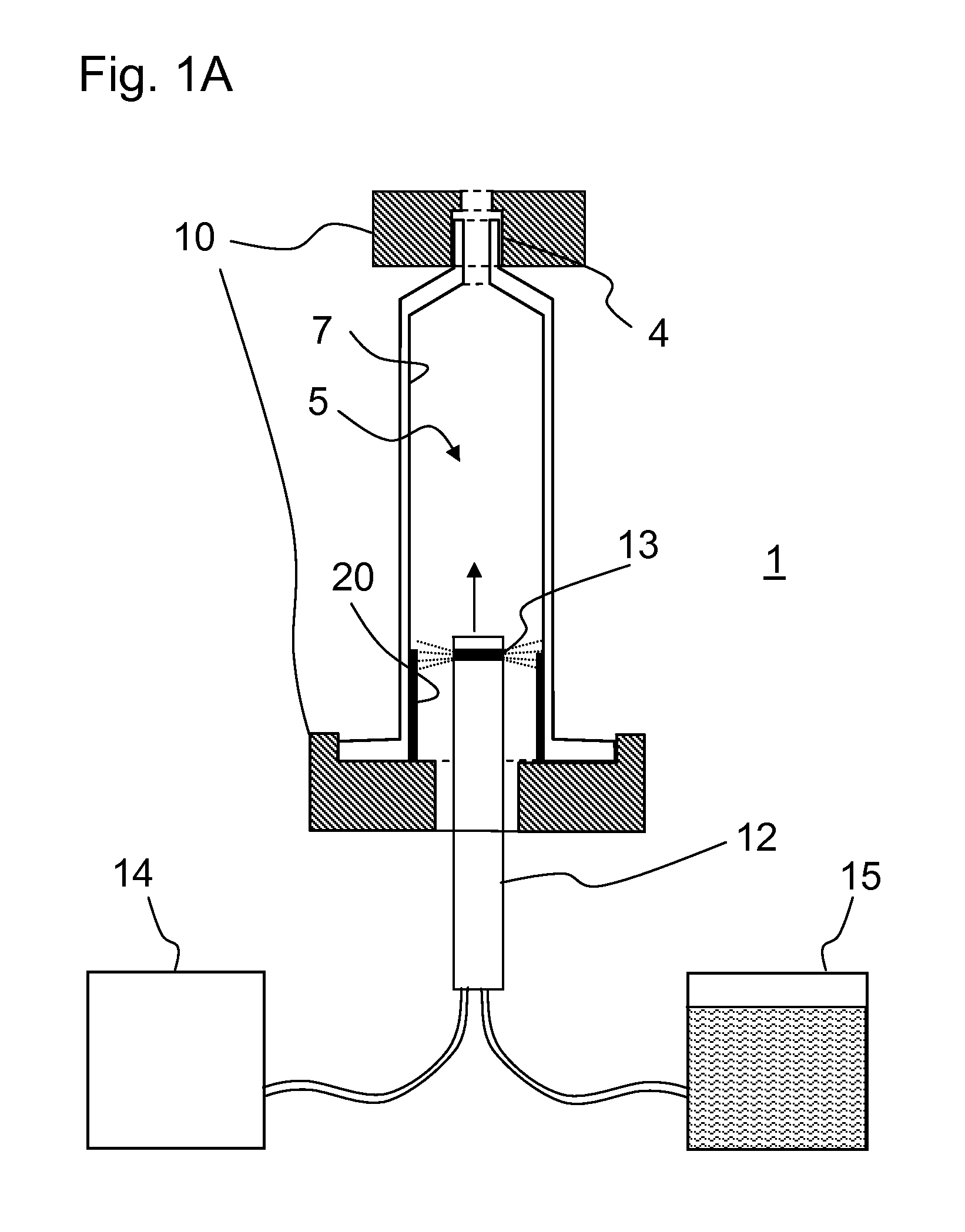
Fused quartz tubing for pharmaceutical packaging
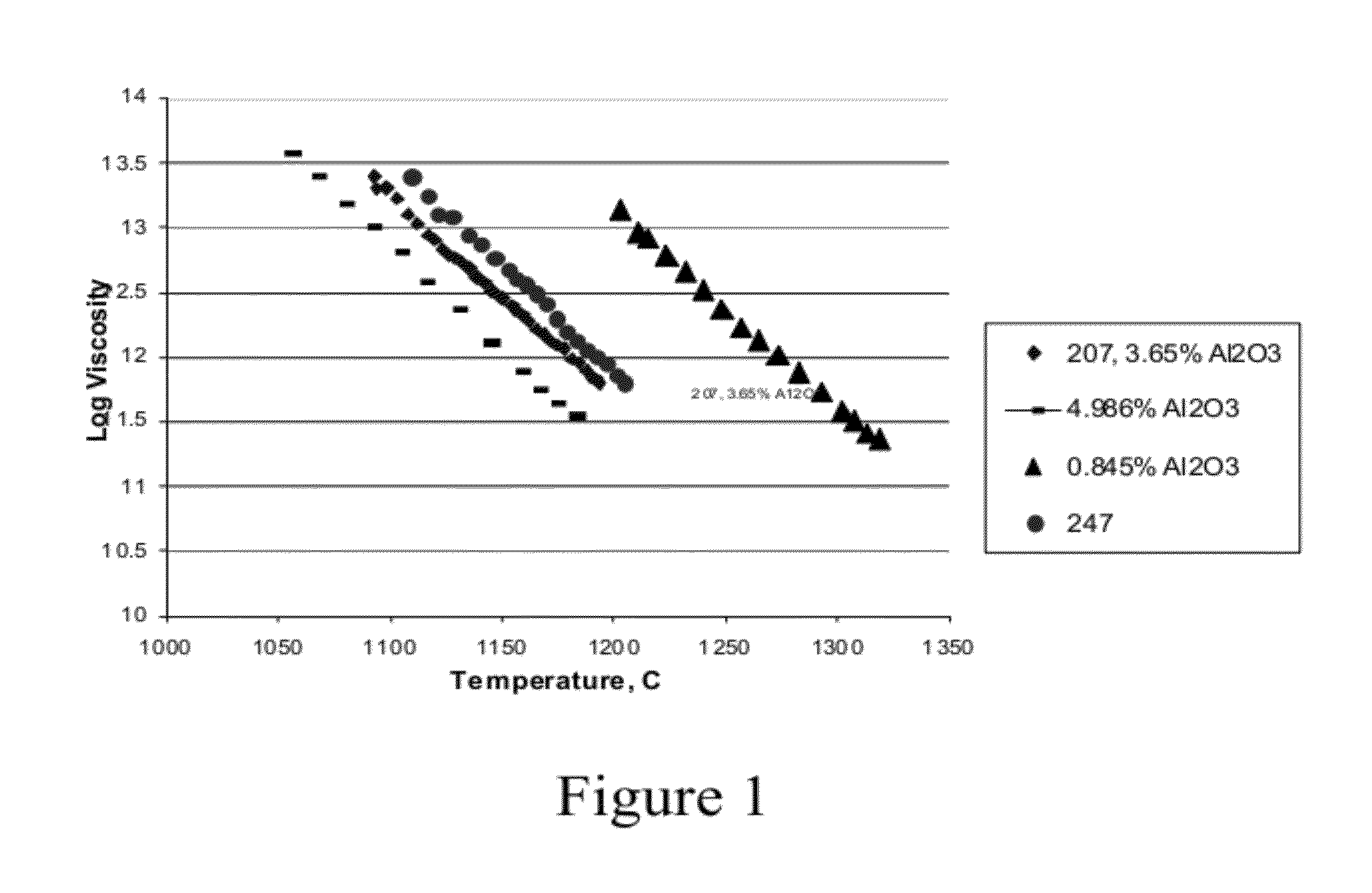
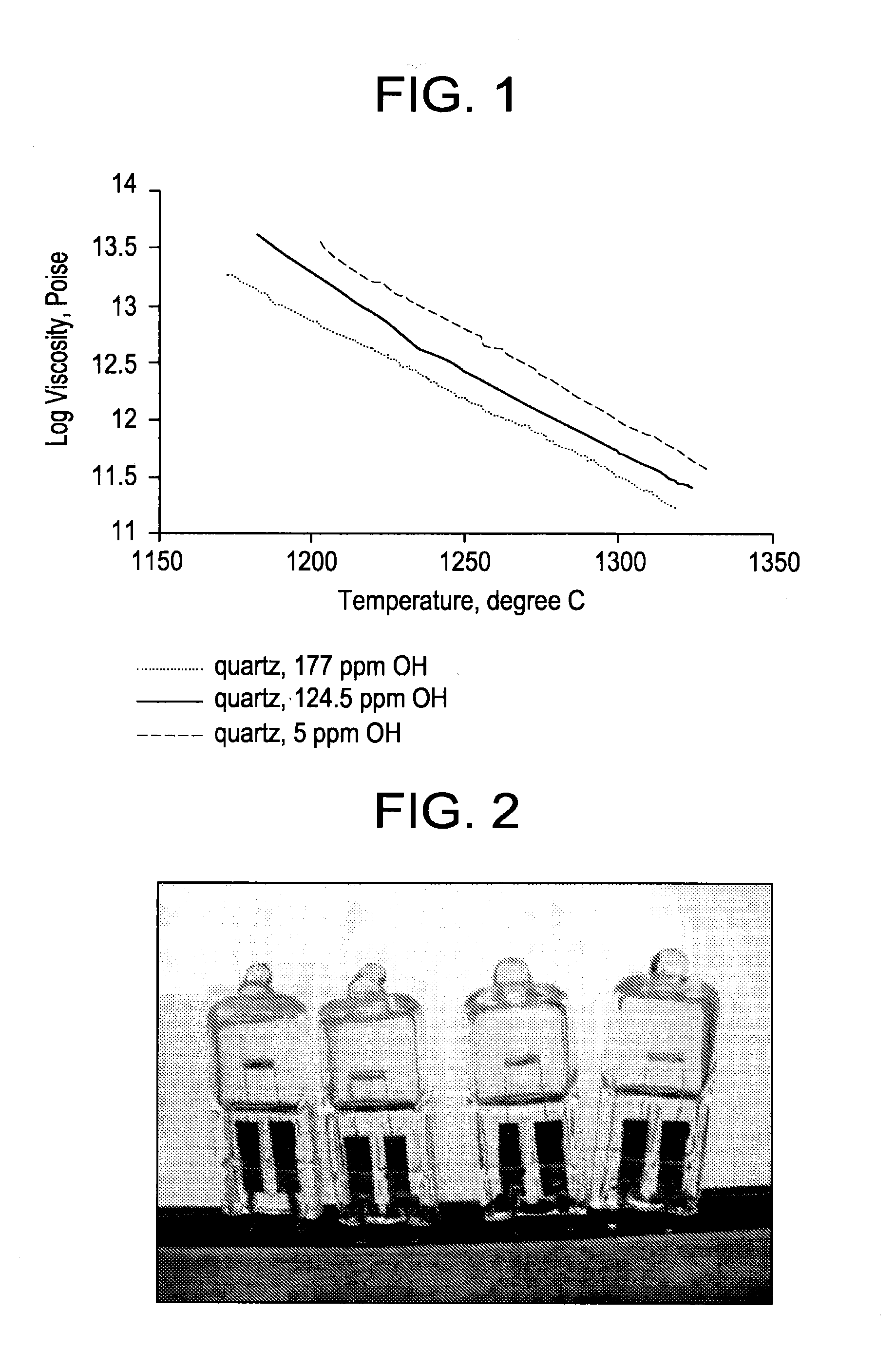
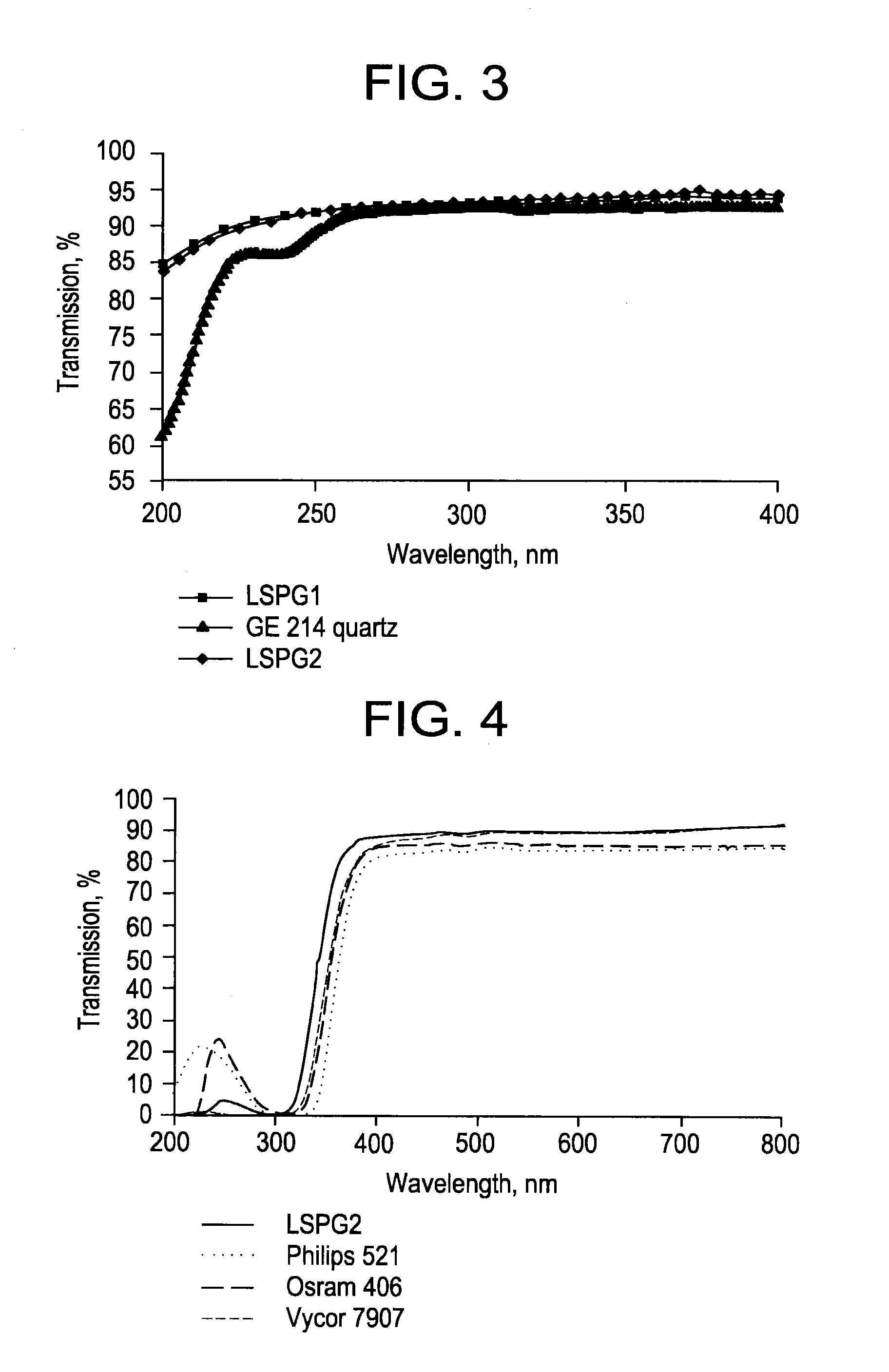
InactiveUS20120148770A1Reduce softeningLow working point temperatureLayered productsPharmaceutical containersRare earthWorking temperature
A high silica glass composition comprising about 82 to about 99.9999 wt. % SiO2 and from about 0.0001 to about 18 wt. % of at least one dopant selected from Al2O3, CeO2, TiO2, La2O3, Y2O3, Nd2O3, other rare earth oxides, and mixtures of two or more thereof. The glass composition has a working point temperature ranging from 600 to 2,000° C. These compositions exhibit stability similar to pure fused quartz, but have a moderate working temperature to enable cost effective fabrication of pharmaceutical packages. The glass is particularly useful as a packaging material for pharmaceutical applications, such as, for example pre-filled syringes, ampoules and vials.
Owner:MOMENTIVE PERFORMANCE MATERIALS INC
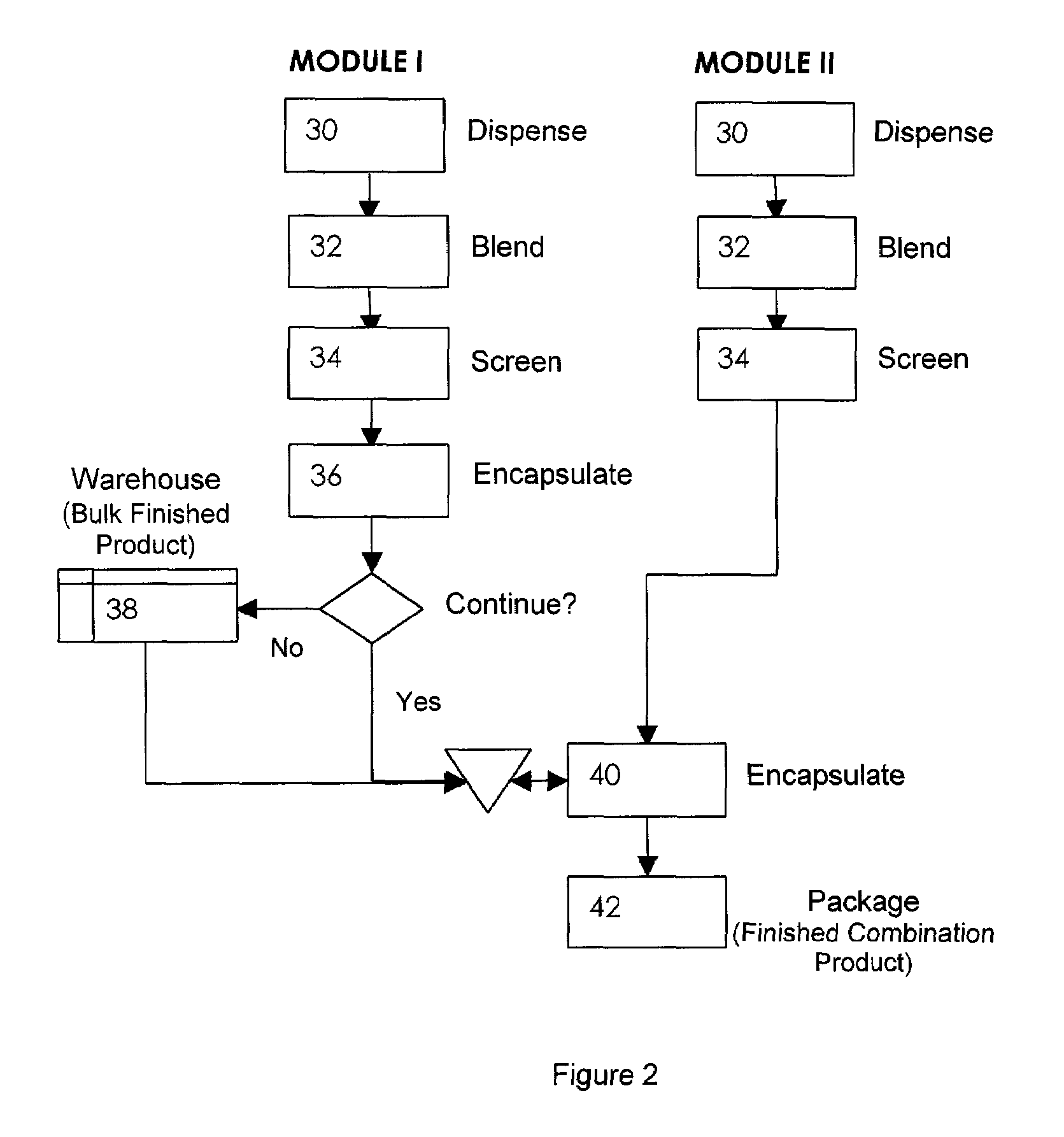
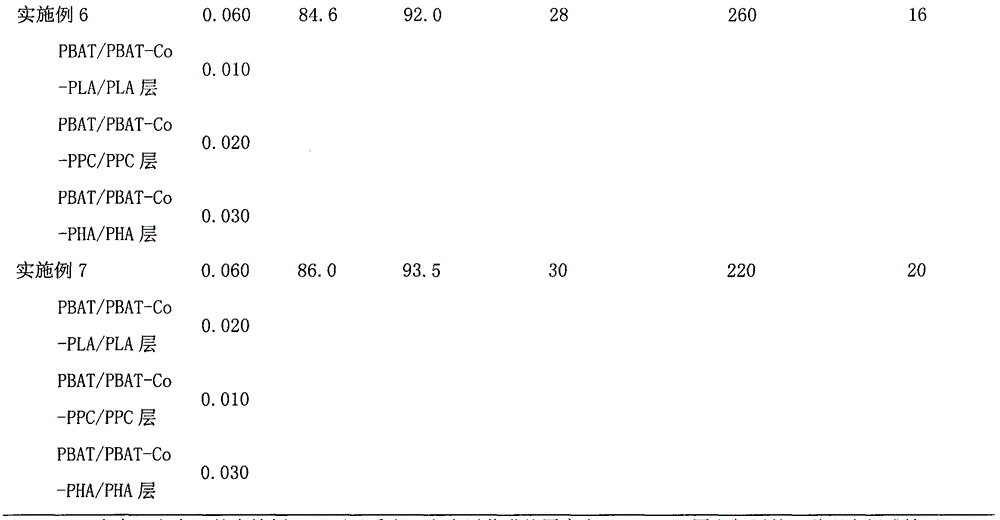
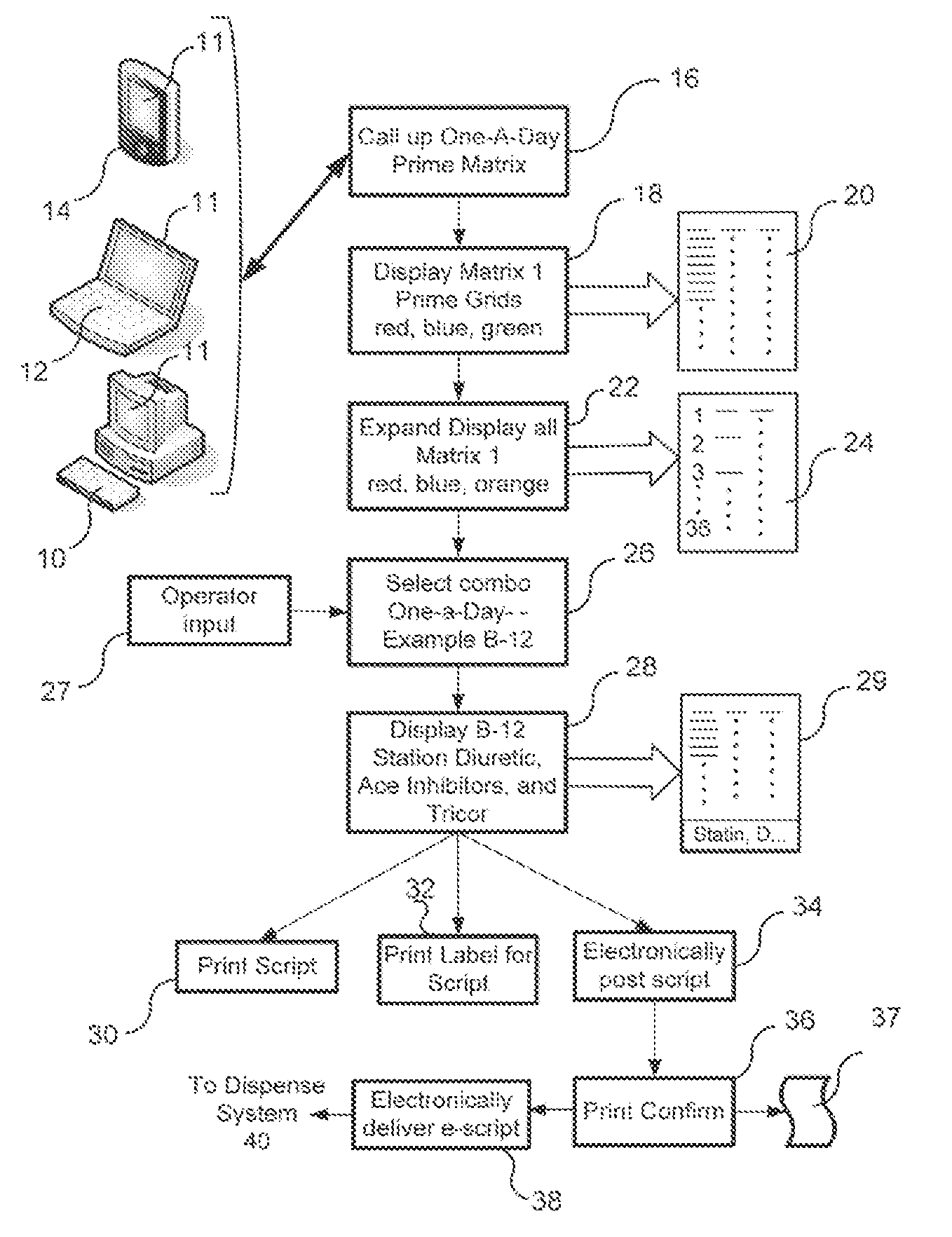
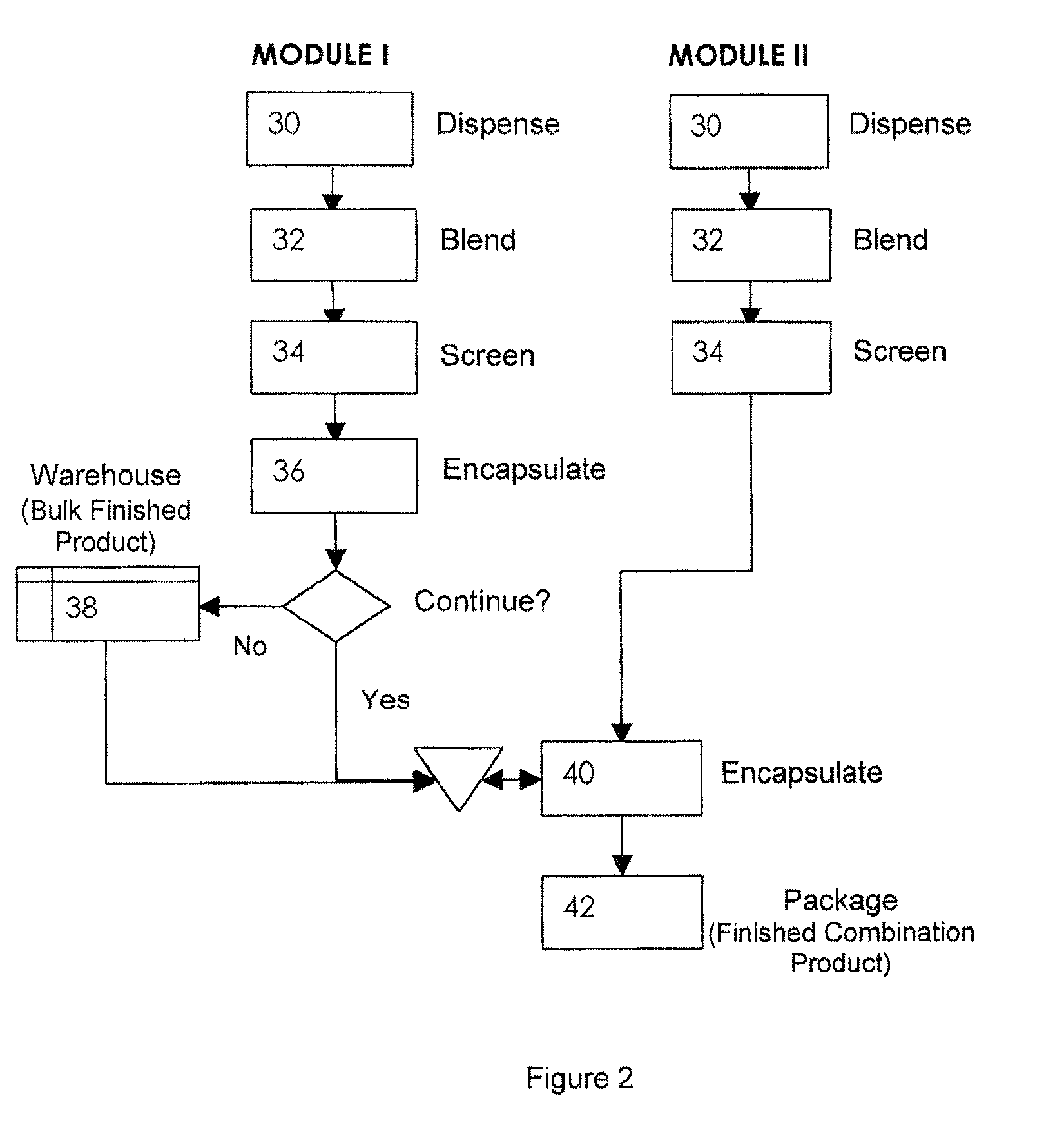
Pharmaceutical packaging and method for delivery of same
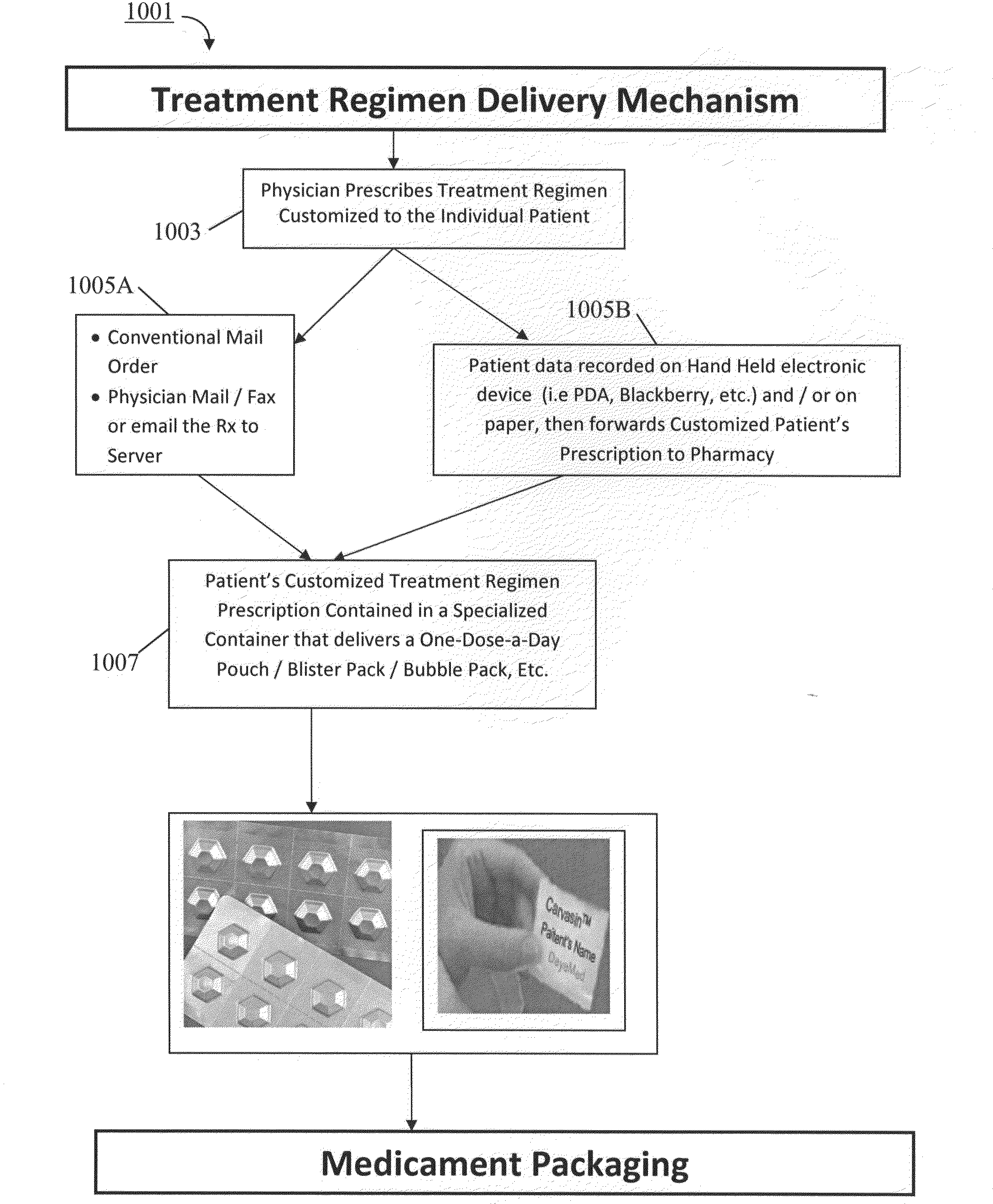
InactiveUS20100100391A1Easy to changeEasy selectionSmall article dispensingDrug and medicationsGuidelinePatient management
A disease management system including: a Diagnostic Module, which provides access to patient information and scientific guidelines for patient treatment; a Diagnostic Interpretive Module, which provides tools to evaluate risk of particular diseases or conditions based on patient information and an evaluative methodology; a Prescriptive Module, which is used to recommend, select, and / or evaluate one or more treatment regimens based on patient information and guidelines; a Dispensing Module, which evaluates a patient's compliance with a treatment regimen; and / or a Feedback and Patient Management Module, which gathers compliance information and evaluates efficacy of a treatment regimen for a patient. In embodiments of the subject invention, some or all of the modules described can communicate to manage a disease, medical condition, and / or health problem in a patient.
Owner:DAYA MEDICALS
Pharmaceutical package having a multi-functional surface and a method of preparing a multi-functional surface on a pharmaceutical package
ActiveUS20100044268A1Reduce protein adsorptionReduce adsorptionSmall article dispensingLiquid surface applicatorsProtein solutionPharmaceutical packaging
The present invention relates to a multi-functional pharmaceutical package surface and a method of preparing a multi-functional pharmaceutical package surface. In particular, the present invention relates to a pharmaceutical package having a protein deterrent and lubricious surface and methods of preparing said surface by applying coatings directly to the pharmaceutical package that (a) reduce the adsorption of proteins onto pharmaceutical packaging while not affecting the activity of the protein solution and (b) provide a lubricious surface. The pharmaceutical package surface may also contain a barrier coating. Coatings can be deposited on a variety of pharmaceutical packaging materials and configurations by various methods.
Owner:SCHOTT AG
Edible collagen food packaging film and preparation method thereof
InactiveCN102093722AEasy to degradeEnvironmental protection is goodFlexible coversWrappersSide effectPlasticizer
The invention provides an edible collagen food packaging film and a preparation method thereof. The preparation method comprises the following steps: firstly, stirring a 5-30wt% collagen solution and a 0.1-3wt% modifier solution at the temperature of 30-60 DEG C to react for 30-60 minutes, thus obtaining a compound solution; then adding a plasticizer and an auxiliary additive into the compound solution, then continuing to stir for 20-40 minutes at the temperature of 30-60 DEG C so as to obtain a mixed solution; and pouring the mixed solution in a die, molding, drying for 5-15 hours at the temperature of 40-80 DEG C, so as to obtain the edible collagen food packaging film. The edible collagen food packaging film has the advantages of better mechanical property, better heat stability, better moisture barrier property, safety, effectiveness and no side effect; wastes can be degraded and do not cause environment pollution; and the edible collagen food packaging film can be widely applied to the fields of various food packaging and medicament packaging, and has a good market application prospect.
Owner:SICHUAN UNIV
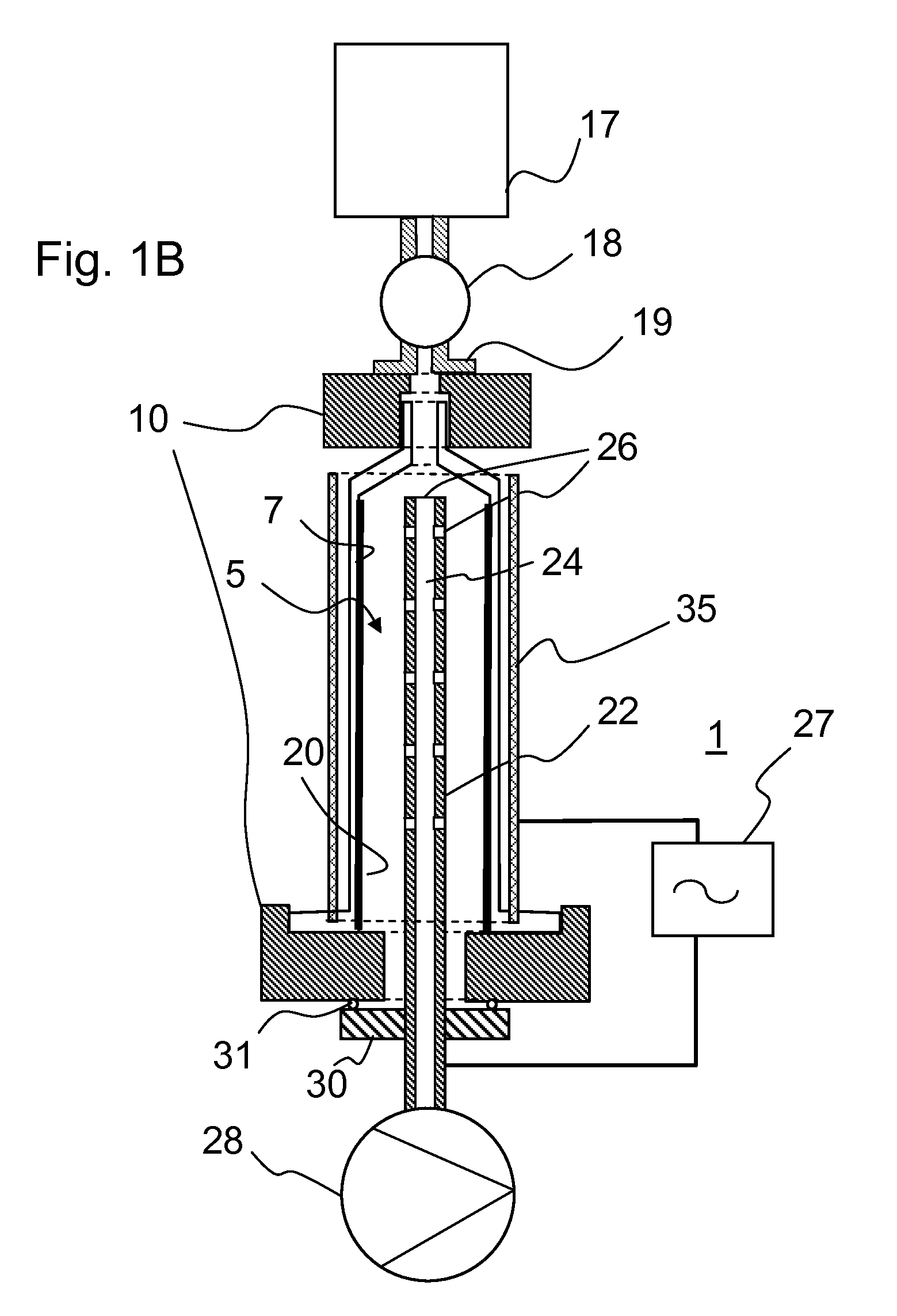
Method for the production of pharmaceutical packaging
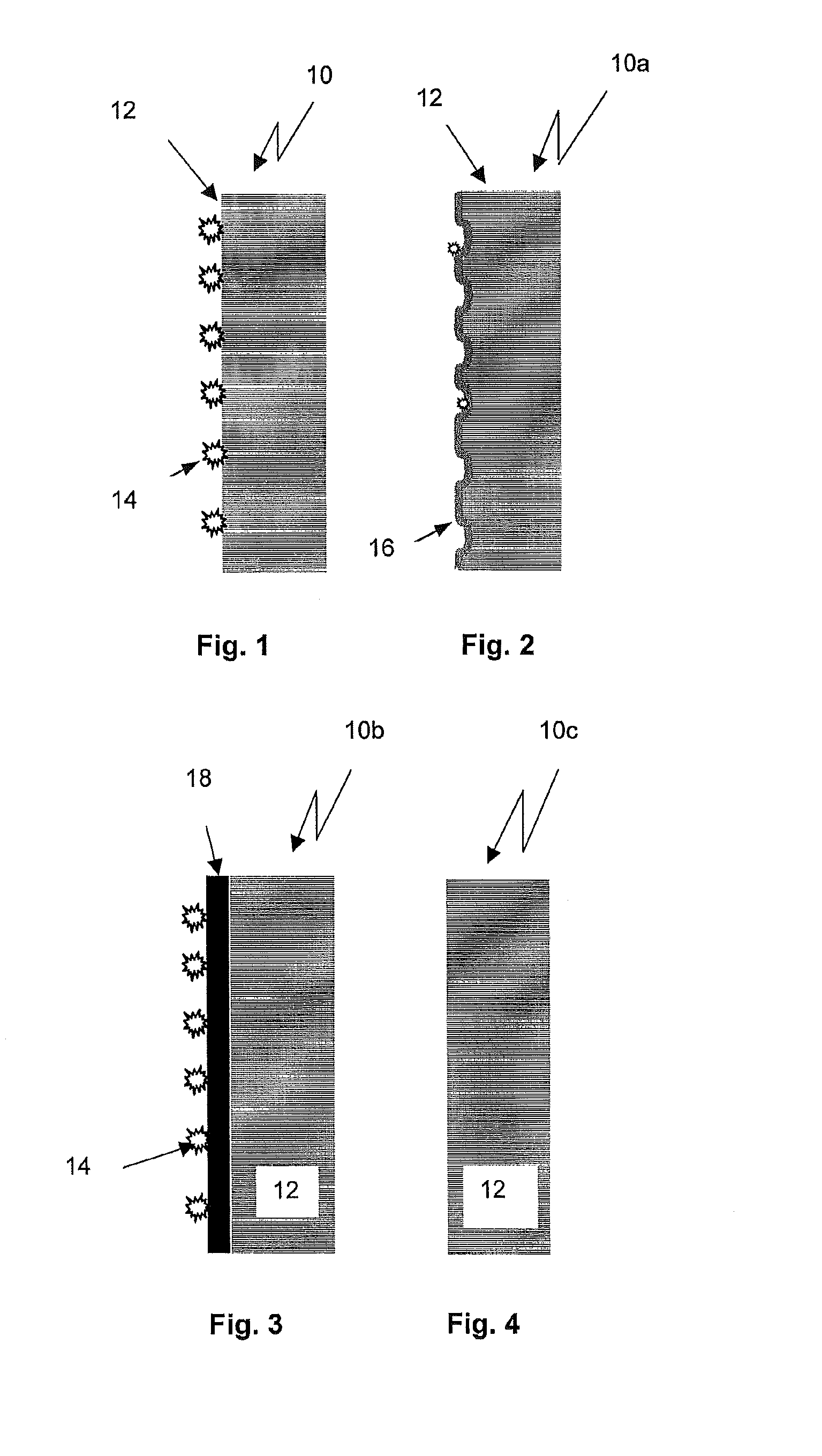
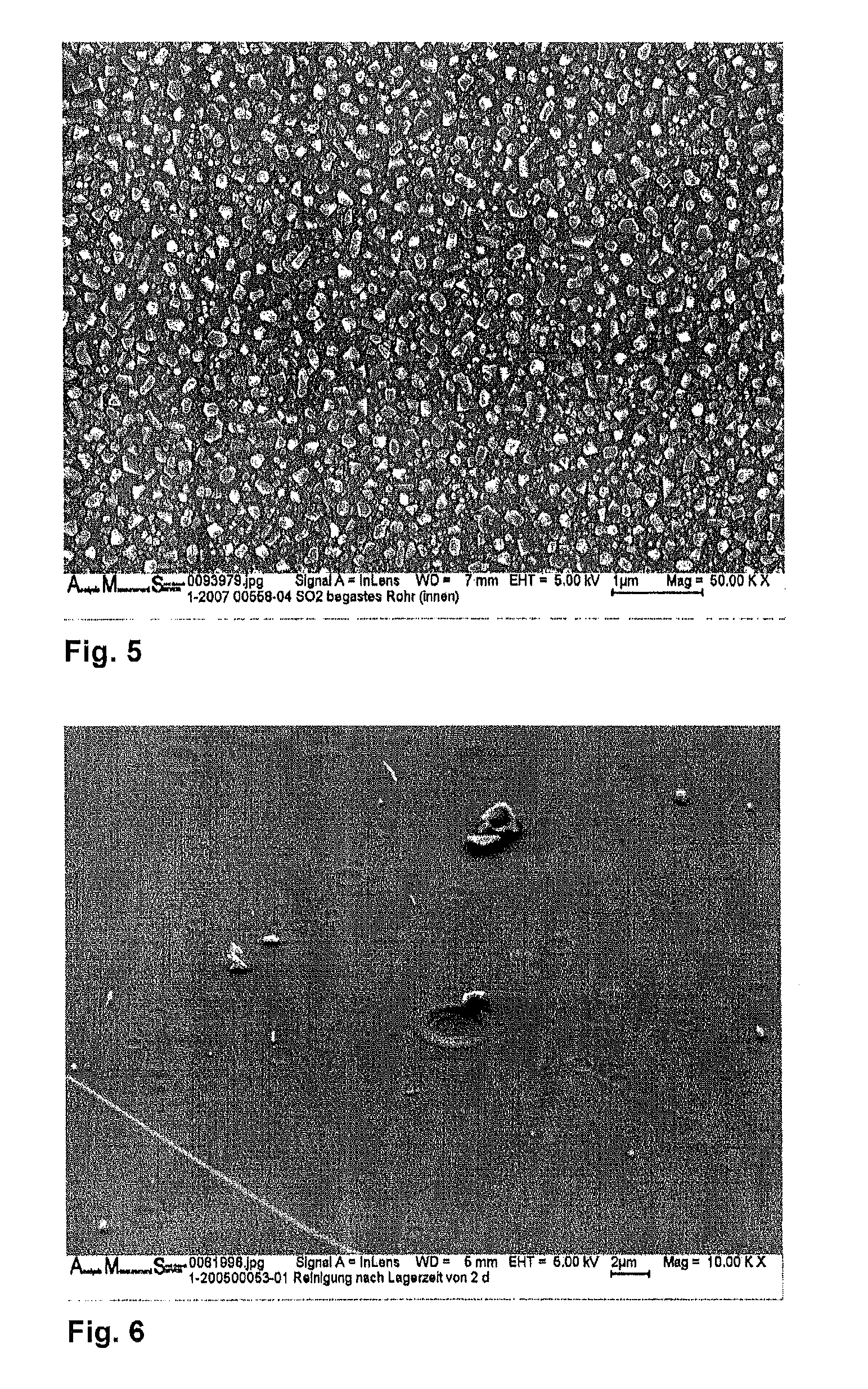
InactiveUS20100089097A1Increased susceptibilityDeterioration of surface qualityGlass drawing apparatusGlass reforming apparatusRoom temperatureMedical product
The invention discloses a method for the production of packaging made from borosilicate glass for pharmaceutical products and medical products comprising the steps of: providing a glass tube made from a borosilicate base glass, generating a temporary interface layer on an inner surface of the glass tube, hot-forming the glass tube at a temperature above Tg, and cooling down the glass tube to room temperature.
Owner:SCHOTT AG
Fused quartz tubing for pharmaceutical packaging
ActiveUS20130095261A1Low working point temperatureHigh wt % contentDiagnosticsLayered productsDopantWorking temperature
A high silica glass composition comprising about 92 to about 99.9999 wt. % SiO2 and from about 0.0001 to about 8 wt. % of at least one dopant selected from Al2O3, CeO2, TiO2, La2O3, Y2O3, Nd2O3, other rare earth oxides, and mixtures of two or more thereof. The glass composition has a working point temperature ranging from 600 to 2,000° C. These compositions exhibit stability similar to pure fused quartz, but have a moderate working temperature to enable cost effective fabrication of pharmaceutical packages. The glass is particularly useful as a packaging material for pharmaceutical applications, such as, for example pre-filled syringes, ampoules and vials.
Owner:MOMENTIVE PERFORMANCE MATERIALS QUARTZ INC
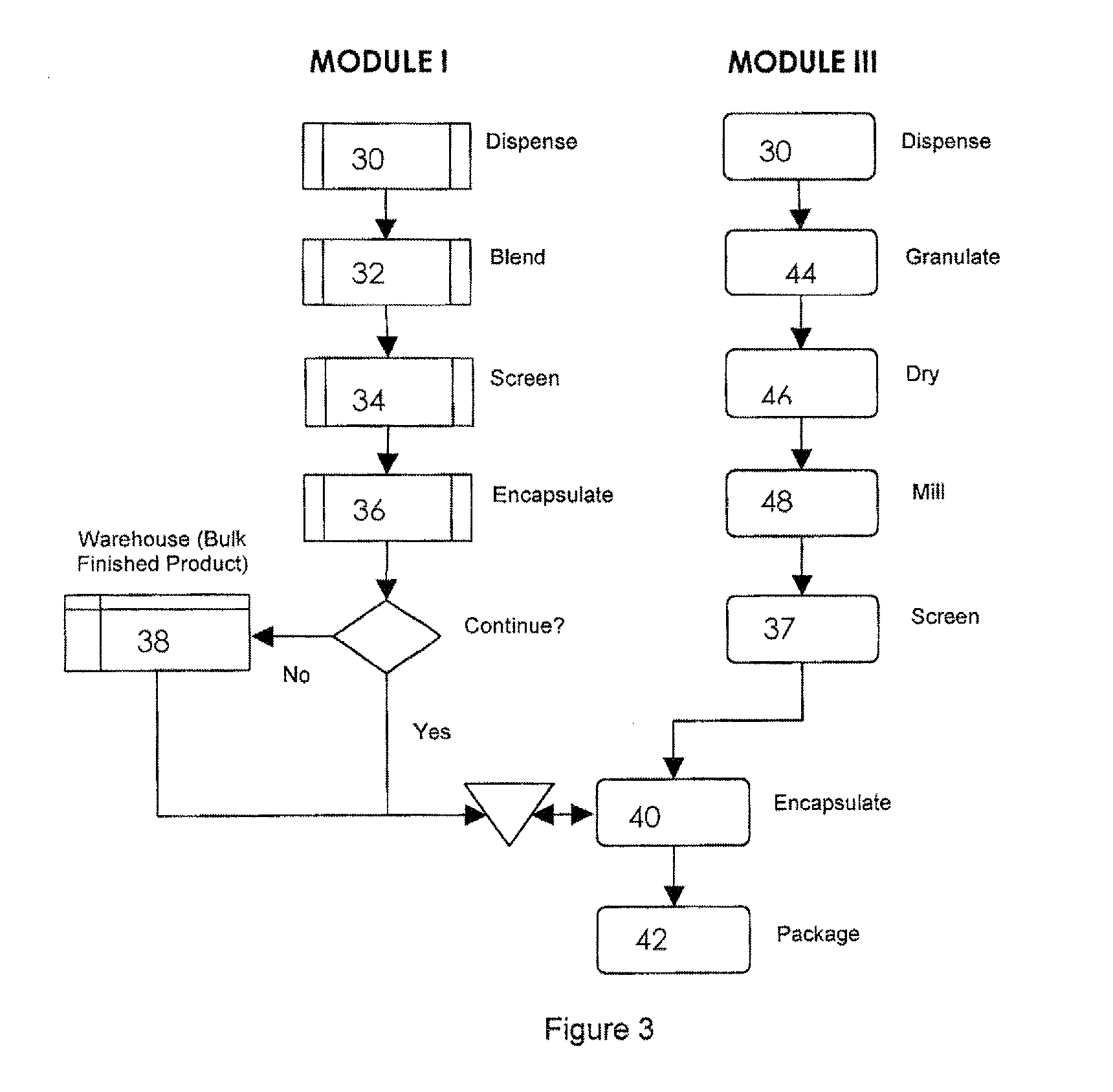
Automated solid pharmaceutical product packaging machine
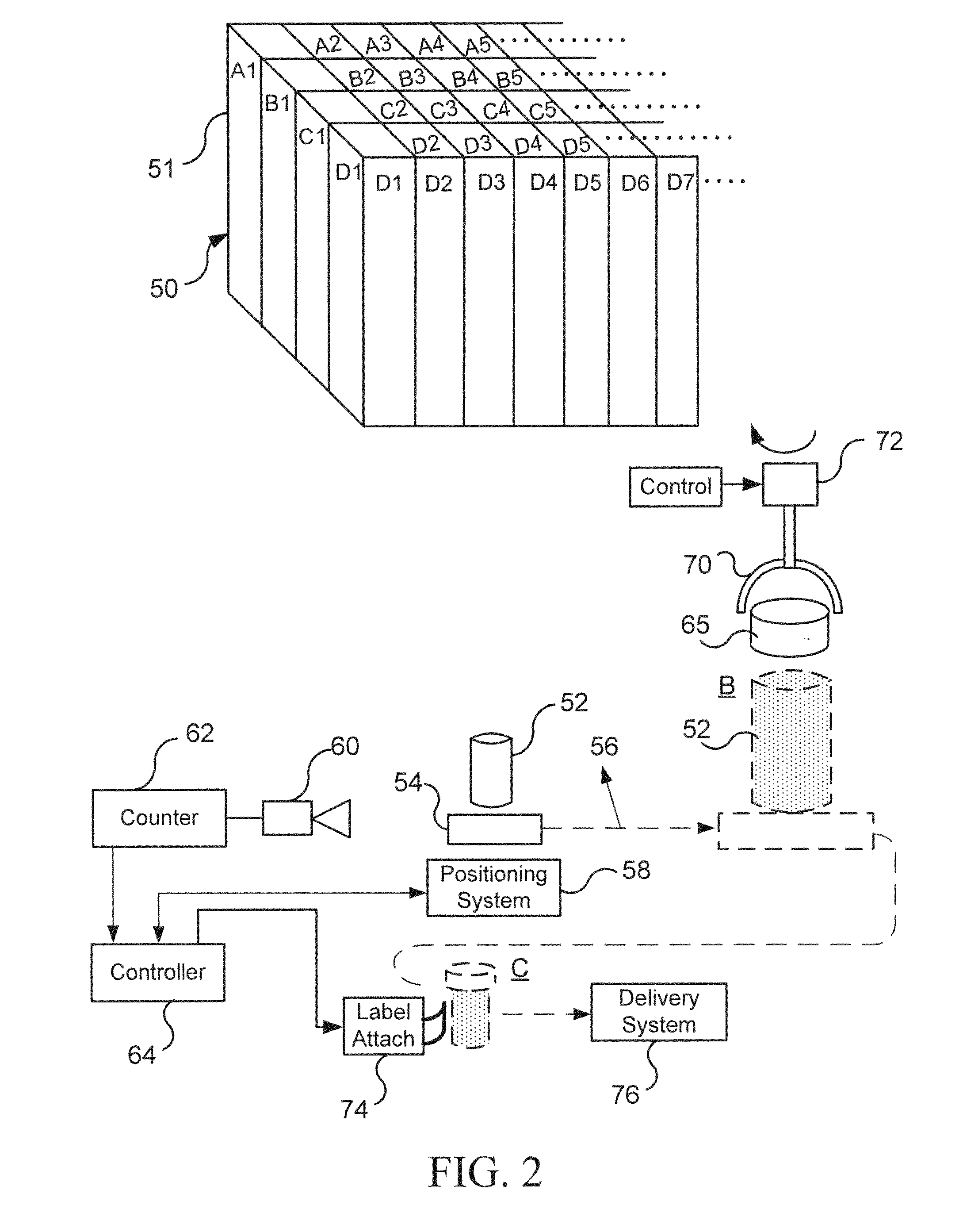
InactiveUS20070084150A1Improve versatilityImprove efficiencyPackaging automatic controlSolid materialPharmaceutical packagingBiomedical engineering
An automated pharmaceutical product packaging machine simultaneously fills a plurality of product package templates in parallel with desired pharmaceutical dosing requirements. The templates are subsequently positioned over a temporary storage template having cavities for receiving solid pharmaceutical doses. A collector member is subsequently placed beneath the temporary storage template for receiving the pharmaceuticals which in turn is positioned over a solid pharmaceutical product package having a plurality of cavities which correspond to openings on the templates and wherein each of the templates fills a pharmaceutical package.
Owner:MTS MEDICATION TECH
Pharmaceutical packaging and method for delivery of same
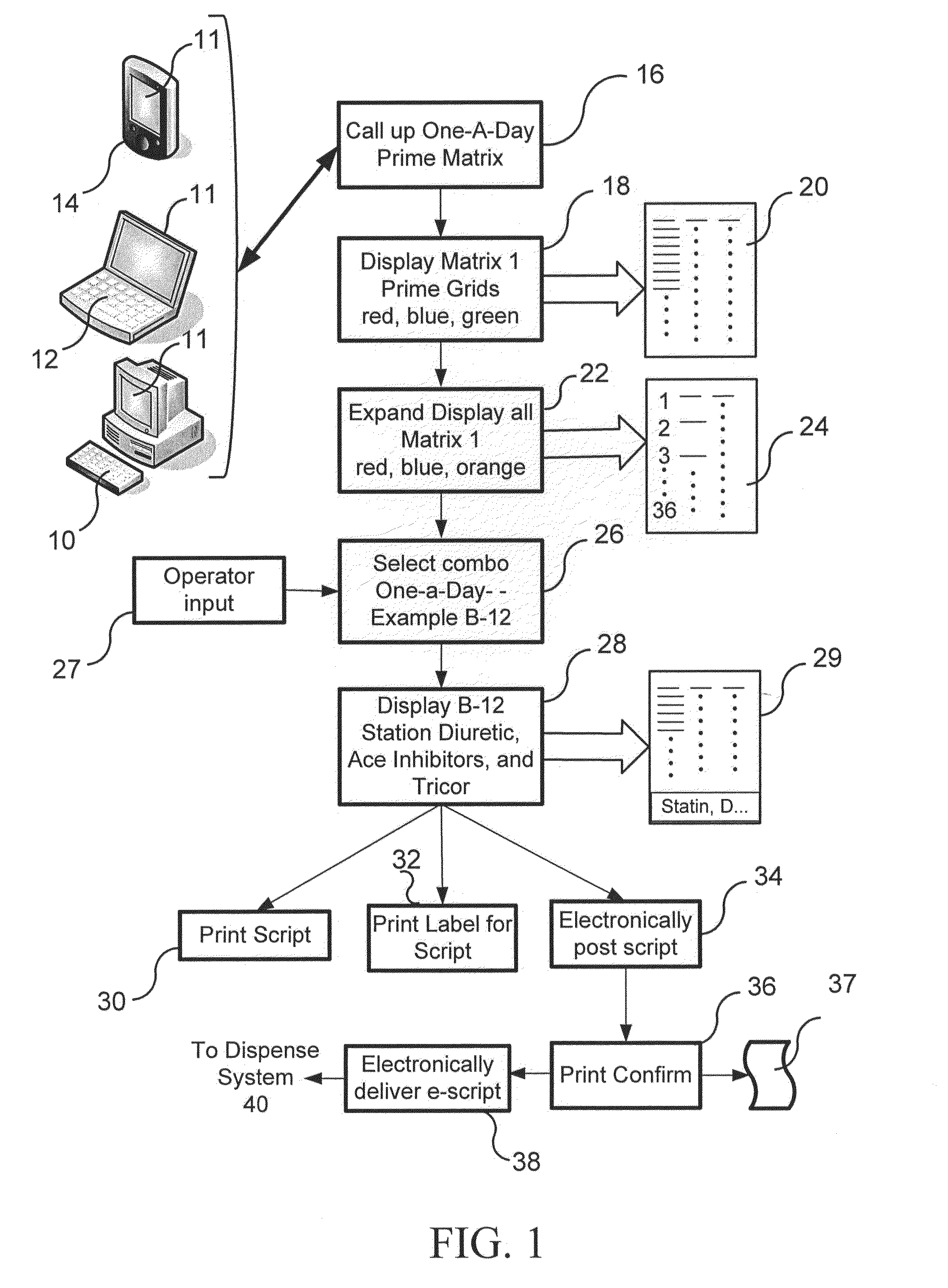
InactiveUS20120101630A1Motivate patientEasy to changeFinanceDrug and medicationsCompliance MonitoringPharmaceutical packaging
A disease management system and therapeutic hub are provided. In certain embodiments, a dispensing apparatus used as part of a compliance monitoring system for the disease management system can function as a therapeutic hub that interacts with a plurality of peripheral devices to accumulate, communicate, and analyze a variety of medical and non-medical related data of the patient.
Owner:DAYA MEDICALS
Gene detection assay for improving the likelhood of an effective response to an ErbB antagonist cancer therapy
InactiveUS20060228745A1Great likelihoodChoose accuratelyOrganic active ingredientsBiocideFhit geneTumor cells
The invention provides a method for more effective treatment of patients susceptible to or diagnosed with tumors overexpressing ErbB, as determined by a gene amplification assay, with an ErbB antagonist. Such method comprises administering a cancer-treating dose of the ErbB antagonist, preferably in addition to chemotherapeutic agents, to a subject in whose tumor cells ErbB has been found to be amplified e.g., by fluorescent in situ hybridization. ErbB antagonists described include an anti-HER2 antibody. Pharmaceutical packaging for providing the components for such treatment is also provided.
Owner:GENENTECH INC
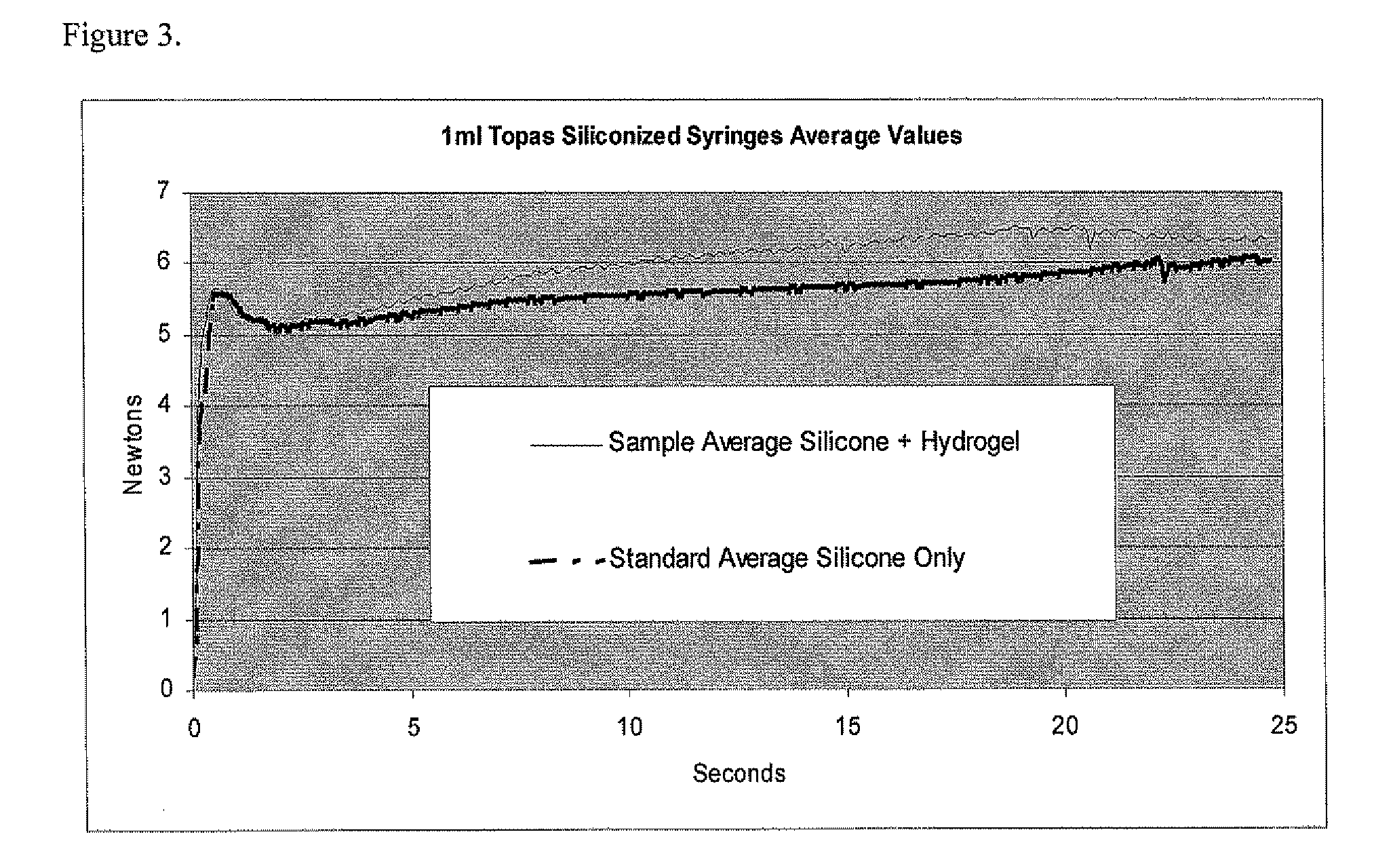
Pharmaceutical Packaging with Lubricating Film and Method for Producing Same
ActiveUS20120171386A1Reduce dispersionSurface interaction can be avoidedPretreated surfacesCoatingsBiochemical engineeringPharmaceutical packaging
Owner:SCHOTT AG
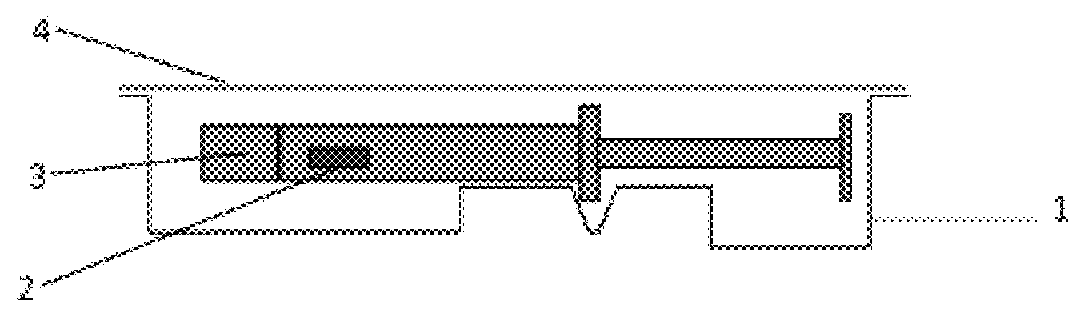
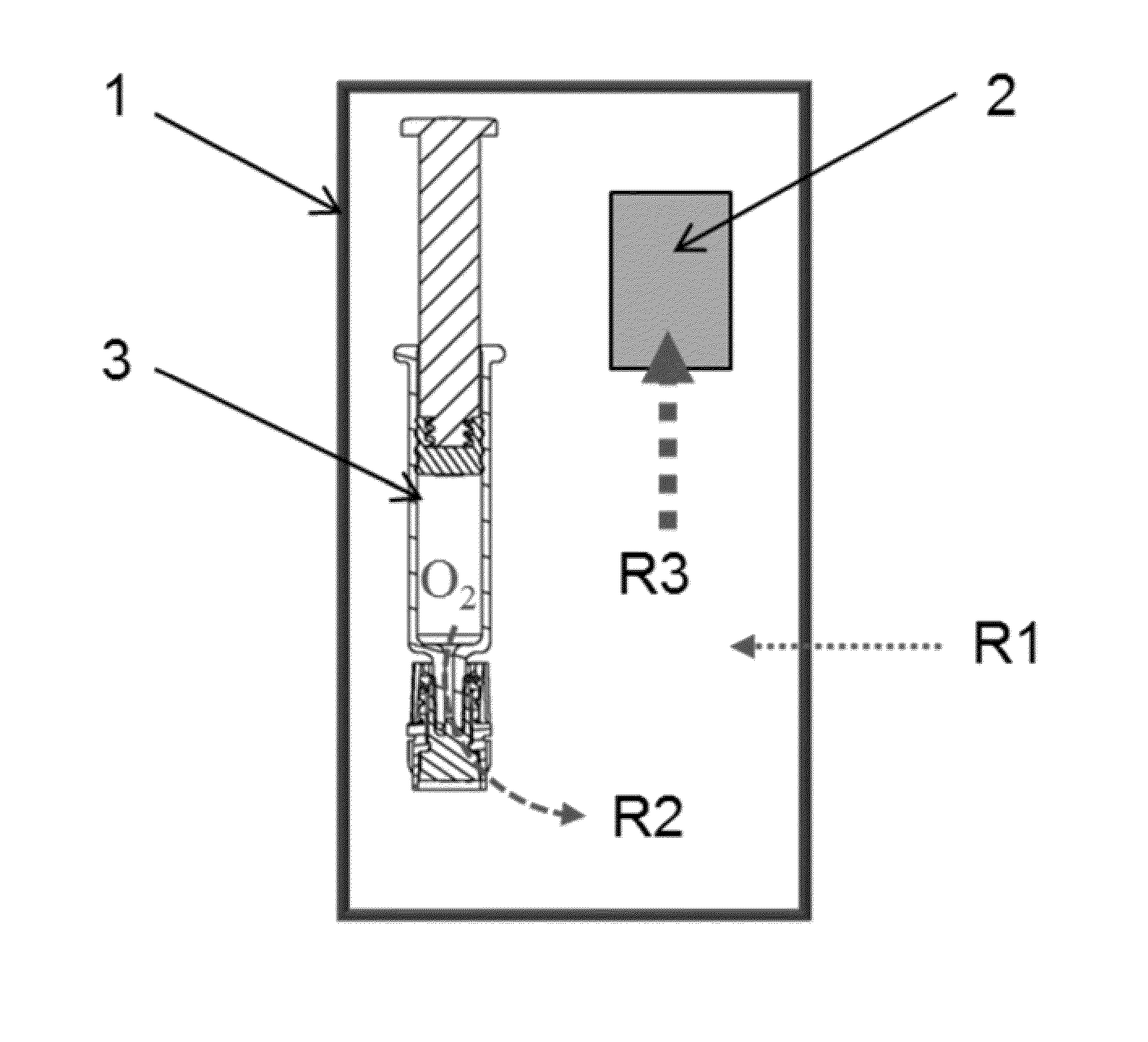
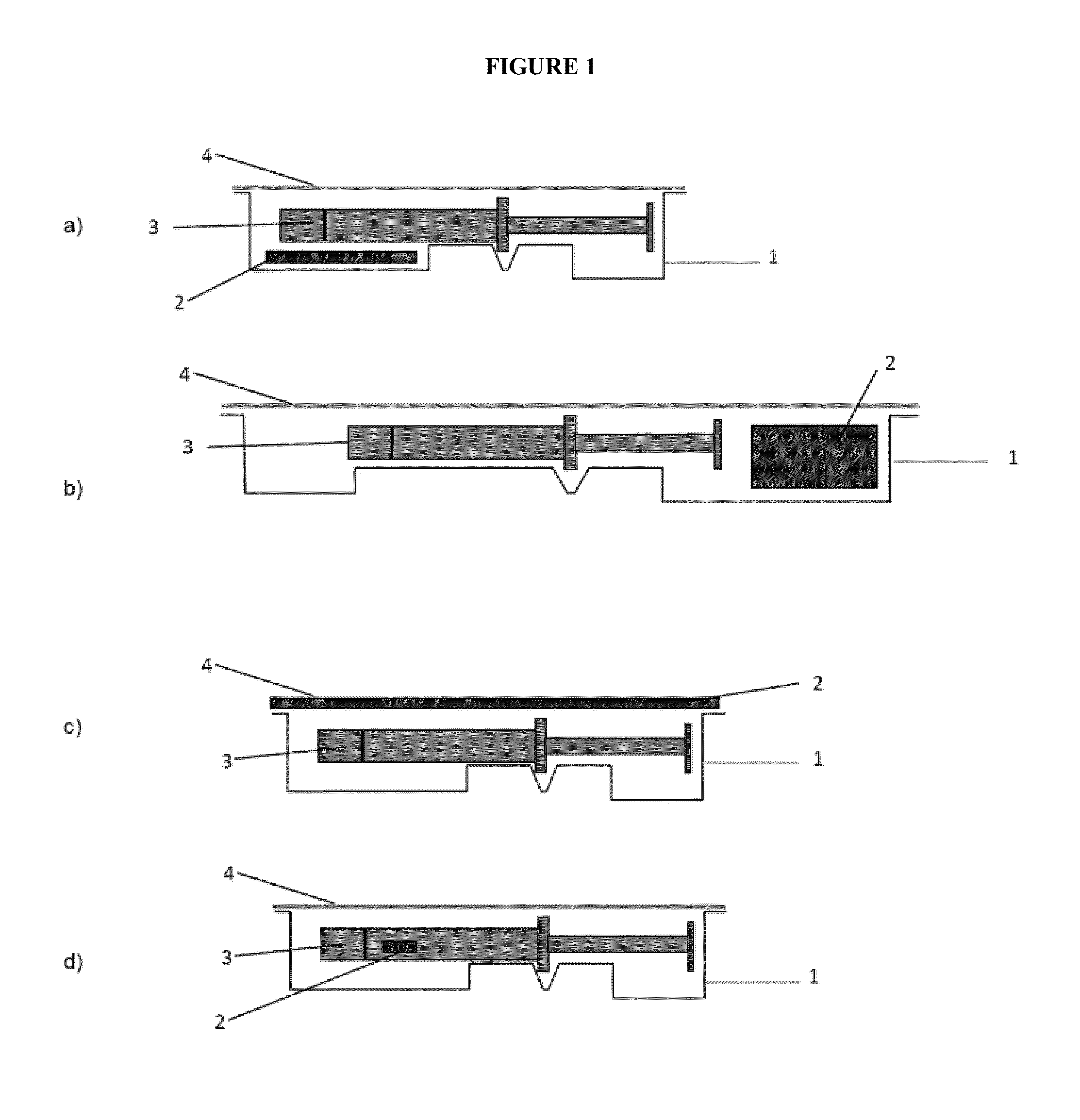
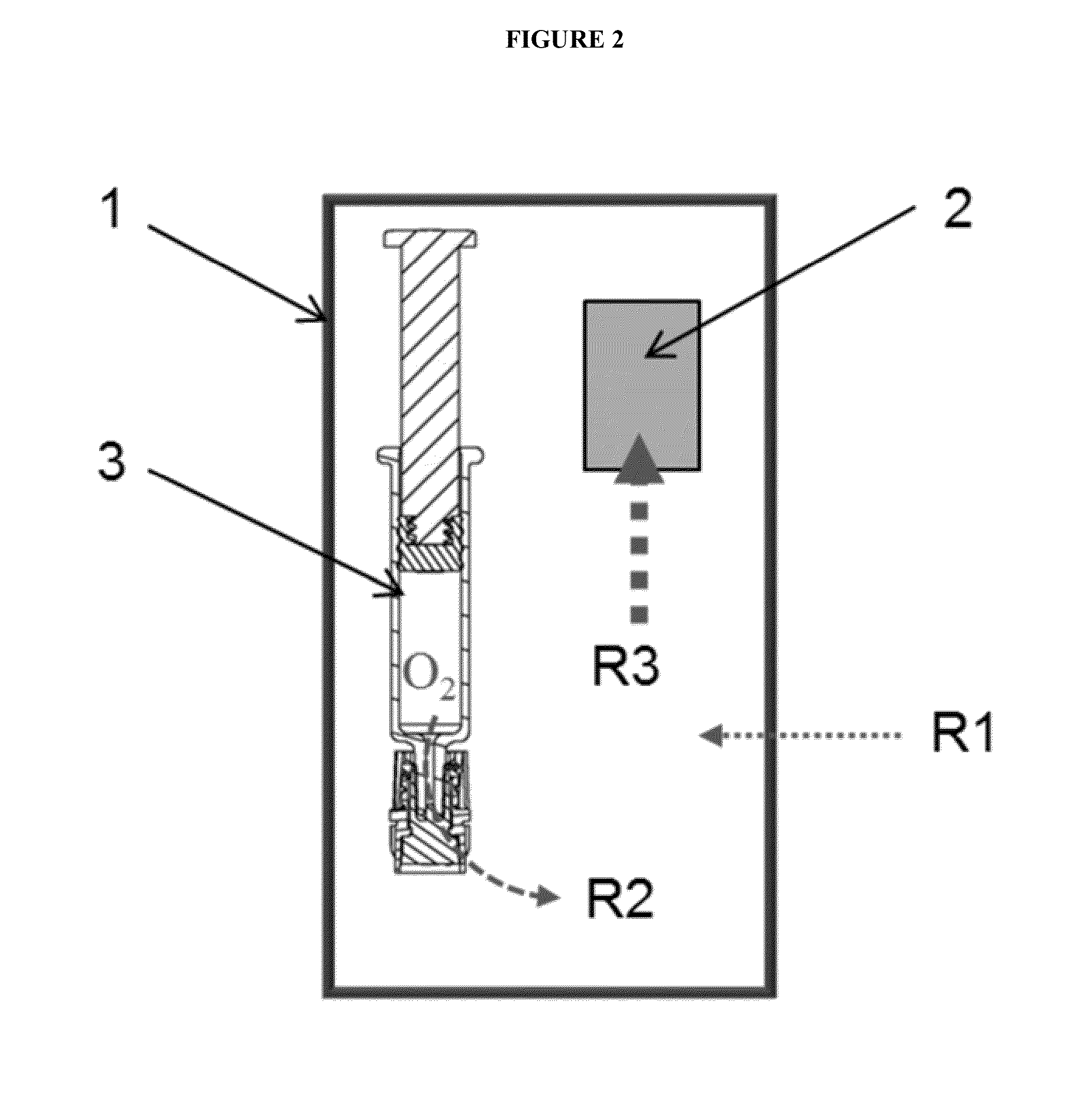
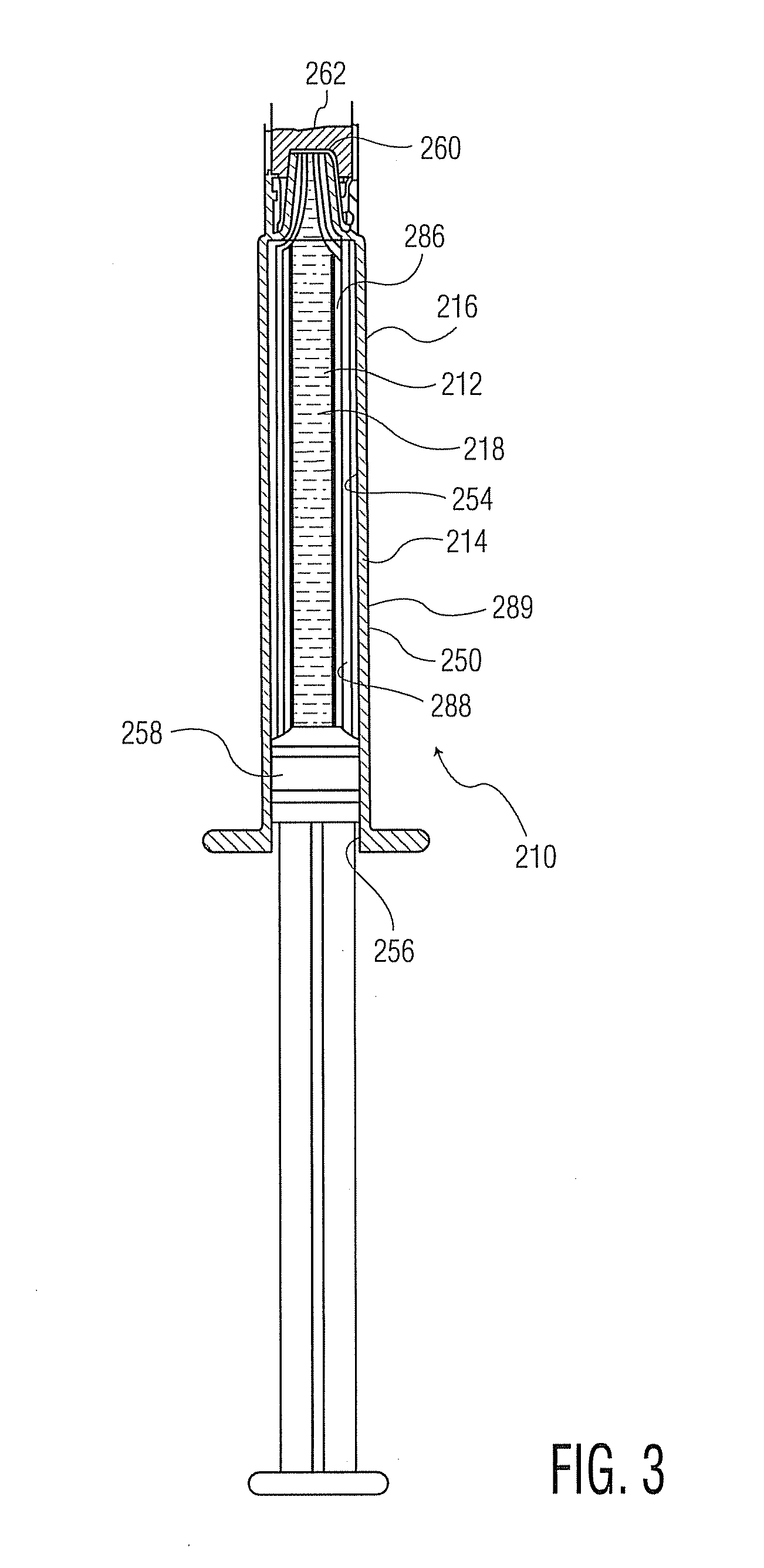
Packaging system for oxygen-sensitive drugs
ActiveUS9248229B2Lower Level RequirementsOrganic active ingredientsFlexible coversPromethazineMorphine
Described herein are pharmaceutical packaging systems which prevent oxidative degradation of morphine, hydromorphone, promethazine and other oxygen-sensitive drugs, such systems including a syringe with an oxygen permeable tip cap, a hermetically sealed oxygen barrier blister packaging with very low permeability to oxygen and comprises ethylene vinyl, and an oxygen absorber.
Owner:FRESENIUS KABI DEUT GMBH
Packaging system for oxygen-sensitive drugs
ActiveUS20140262883A1Lower Level RequirementsOrganic active ingredientsFlexible coversOxygenPharmaceutical packaging
Described herein are pharmaceutical packaging systems which prevent oxidative degradation of oxygen-sensitive drugs, such systems including a primary packaging container with an oxygen permeable component, a secondary packaging with very low permeability to oxygen and an oxygen absorber.
Owner:FRESENIUS KABI DEUT GMBH
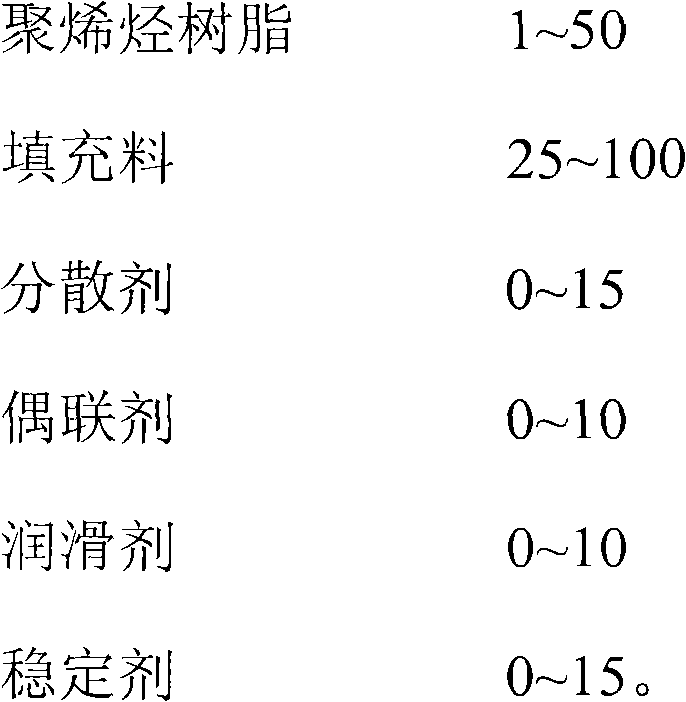
High-filling master batch composition for food and medicine packaging polyolefin
The invention discloses a high-filling master batch composition for food and medicine packaging polyolefin. A carrier resin is a polyolefin resin which comprises a metallocene polyolefin resin. The composition also comprises filler and a functional aid which comprises a dispersing agent, a coupling agent, a lubricating agent and a stabilizing agent, or a mixture of two of the dispersing agent, the coupling agent, the lubricating agent and the stabilizing agent. The metallocene polyolefin resin is introduced and is used as the carrier resin, and the conventional functional aid and the filler are added, so the obtained filling master batch composition is high in whiteness, dispersibility, melt fluidity, and processability, and good in normal-temperature and low-temperature impact resistance performance, and a product meets the contact and control requirements of food and medicines, can be used as the filling master batch special for the packaging plastic of the foods and medicines, and has the function of coloring master batch.
Owner:GUANGZHOU EASTERN RAINBOW MASTERBATCH FACTORY
Pharmaceutical identification
ActiveUS20040166063A1Avoid diversionIncreased complexityContainer decorationsNervous disorderBiochemical engineeringPharmaceutical packaging
Disclosed are methods for marking a pharmaceutical product, container or pharmaceutical packaging system with a scent to establish the identity and / or source of the pharmaceutical.
Owner:PURDUE PHARMA LP
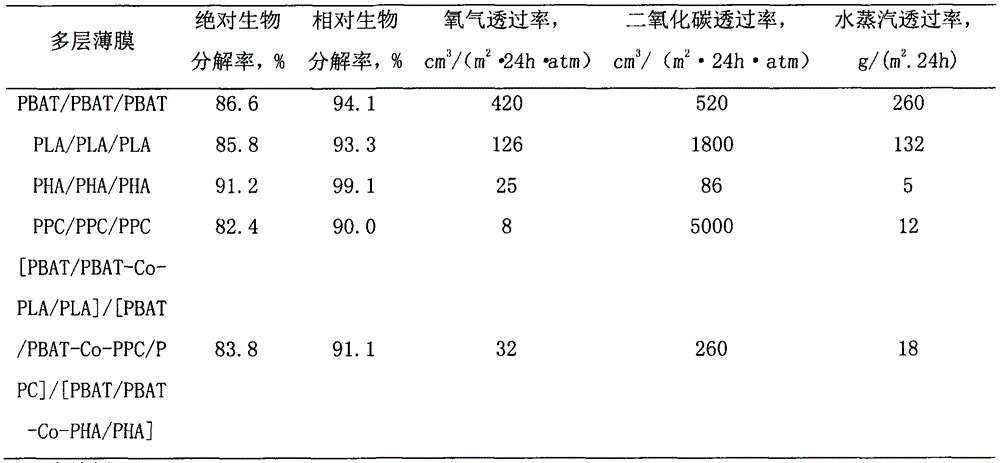
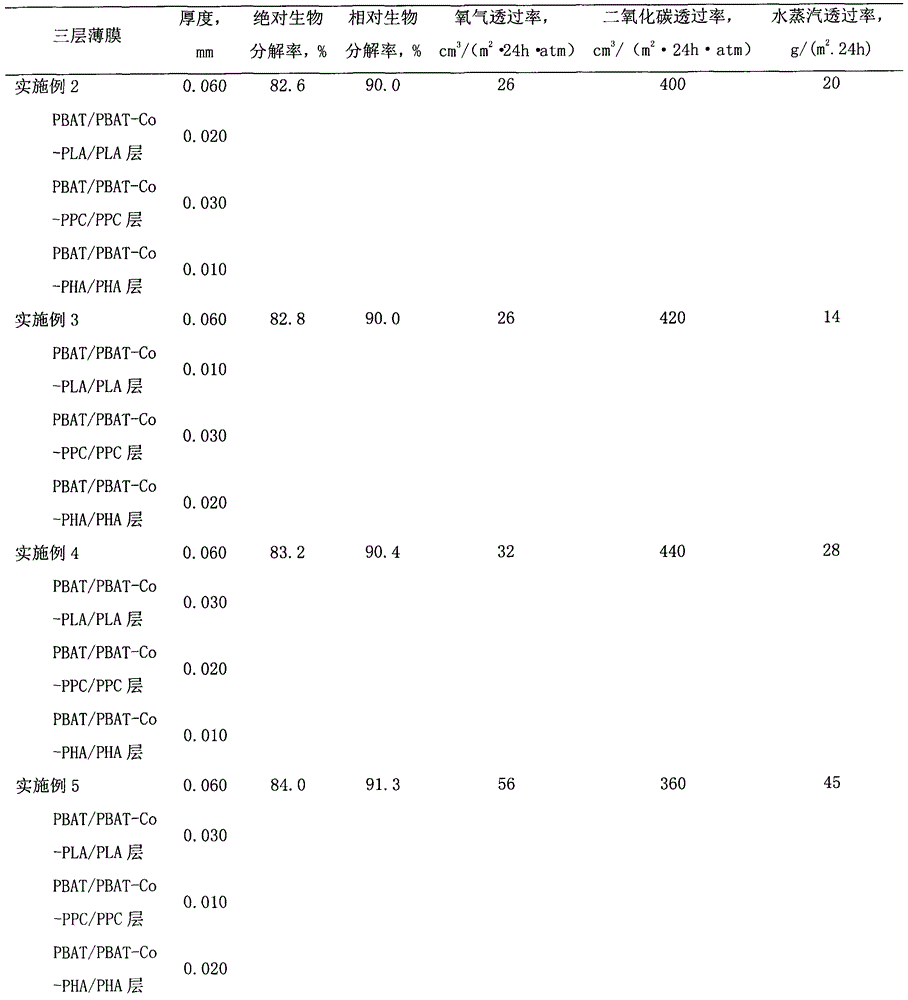
Biodegradable multilayer material with adjustable gas transmission rate and preparation method and applications thereof
ActiveCN104691067AStable physical propertiesNot easy to layerFlexible coversWrappersWater vaporOxygen
The invention relates to a biodegradable multilayer material, which can be a thin film material or a sheet material. The biodegradable multilayer material at least comprises one of an adipic butanediol terephthalate copolymer (PBAT) / PBAT-chain extender-polypropylene carbonate (PBAT-Co-PPC) / PPC blend layer, a PBAT / PBAT-chain extender-polylactic acid (PBAT-Co-PLA) / PLA blend layer and a PBAT / PBAT-chain extender-polyhydroxyalkanoate (PBAT-Co-PHA) / PHA blend layer, and blend resins are respectively prepared through twin-screw reactive extrusion. The multilayer material is produced by using extrusion and coextrusion methods, has a function that the transmission rates of oxygen, carbon dioxide and water vapor can be adjusted, and can be applied to the packaging field and the agriculture field, and especially applied to food packaging, pharmaceutical packaging, and ground film mulching.
Owner:BEIJING TECHNOLOGY AND BUSINESS UNIVERSITY
Pharmaceutical Packaging and Method for Delivery of Same
InactiveUS20100305975A1Easy to changeEasy selectionDrug and medicationsLarge containersPatient managementGuideline
A disease management system including: a Diagnostic Module, which provides access to patient information and scientific guidelines for patient treatment; a Diagnostic Interpretive Module, which provides tools to evaluate risk of particular diseases or conditions based on patient information and an evaluative methodology; a Prescriptive Module, which is used to recommend, select, and / or evaluate one or more treatment regimens based on patient information and guidelines; a Dispensing Module, which evaluates a patient's compliance with a treatment regimen; and / or a Feedback and Patient Management Module, which gathers compliance information and evaluates efficacy of a treatment regimen for a patient. In embodiments of the subject invention, some or all of the modules described can communicate to manage a disease, medical condition, and / or health problem in a patient.
Owner:DAYA MEDICALS
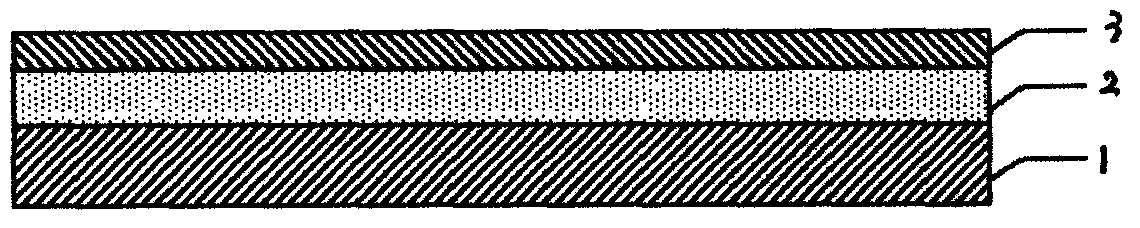
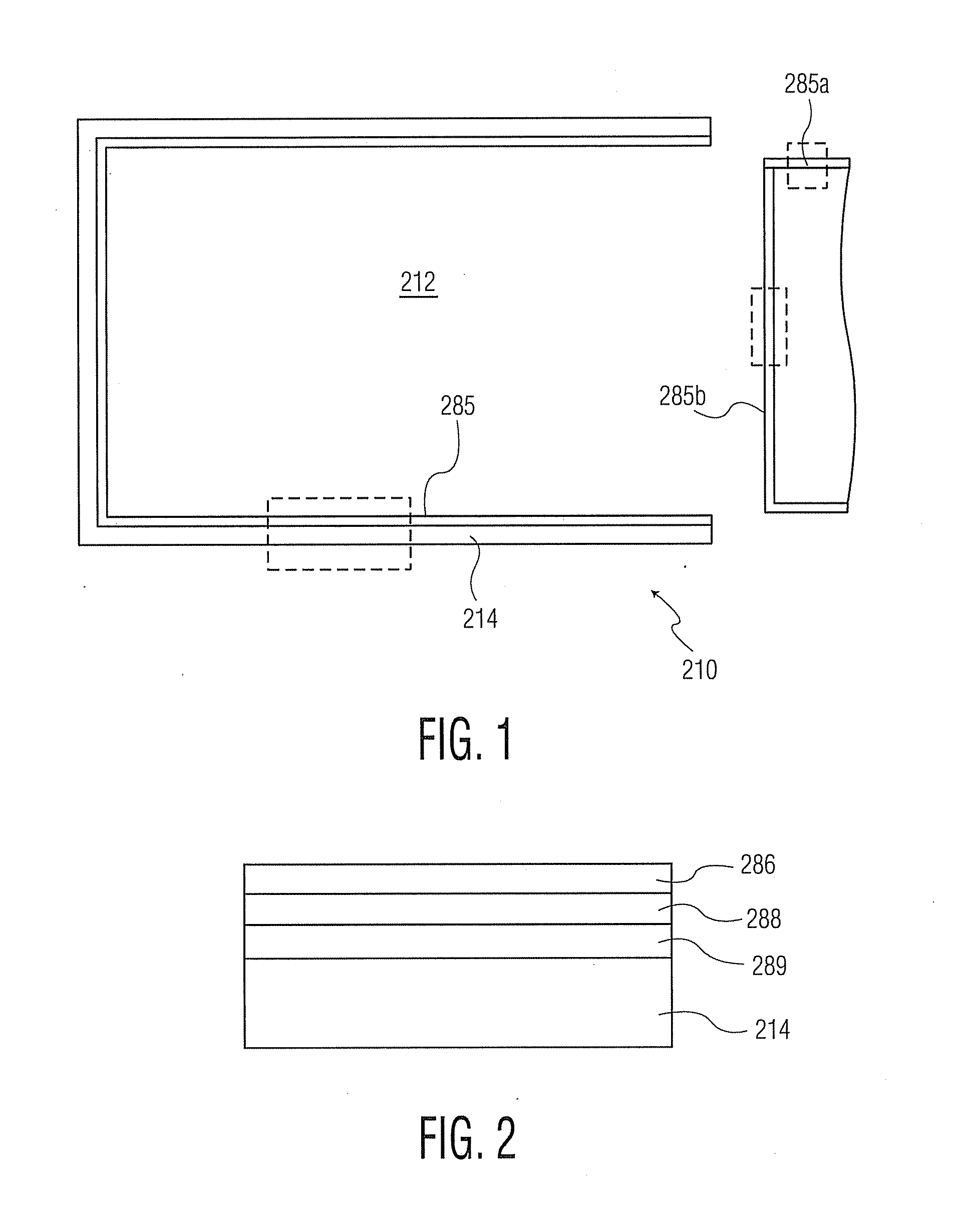
Trilayer coated pharmaceutical packaging
An article is described including an article surface and a coating set comprising a tie coating or layer of SiOxCy or Si(NH)xCy applied to the article surface, a barrier coating or layer of SiOx, and a pH protective layer of SiOxCy or Si(NH)xCy. The respective coatings or layers can be applied by chemical vapor deposition of a polysiloxane or polysilazane precursor in the presence of oxygen. Examples of such an article are a prefilled thermoplastic syringe or thermoplastic pharmaceutical vial with a coated interior portion containing a pharmaceutical preparation or other fluid with a pH of 4 to 8, alternatively 5 to 9. The barrier coating or layer prevents oxygen from penetrating into the thermoplastic syringe or vial, and the tie coating or layer and pH protective coating or layer together protect the barrier layer from the contents of the syringe or vial.
Owner:SI02 MEDICAL PRODS
Magnetoacoustic sensor system and associated method for sensing environmental conditions
InactiveUS7429127B2Thermometer detailsAnalysing fluids using sonic/ultrasonic/infrasonic wavesAudio power amplifierEngineering
A remote sensor system (60) and method for passively sensing environmental conditions, such as temperature and humidity, uses a magnetic impulse from an AC interrogation coil (68) to stimulate a magnetoelastic sensor (62) to generate an acoustic signal (AE) at the resonant frequency of the magnetoelastic sensor (62). The acoustic signal (AE) is received by amplifier (74), detected and displayed (76). The systems and methods are particularly suited for detecting environmental conditions in commercial pharmaceutical packaging, such as blister packs.
Owner:GLAXO GRP LTD
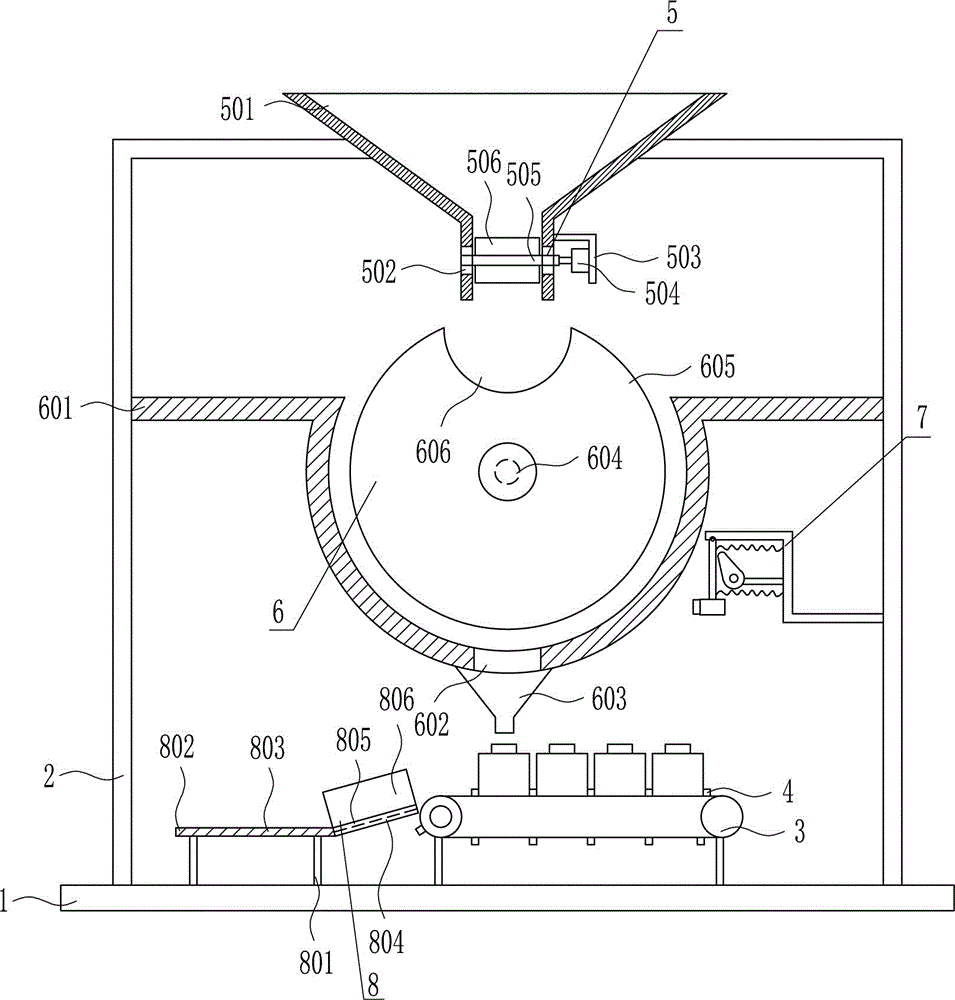
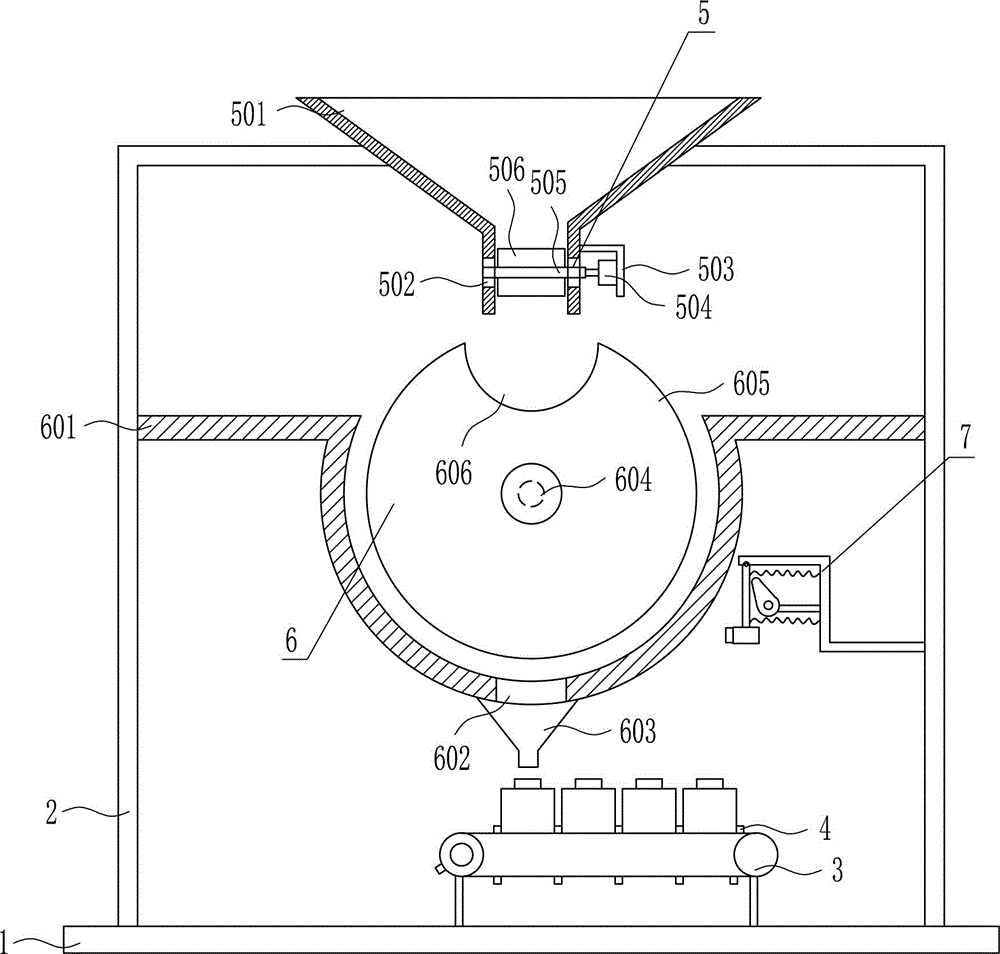
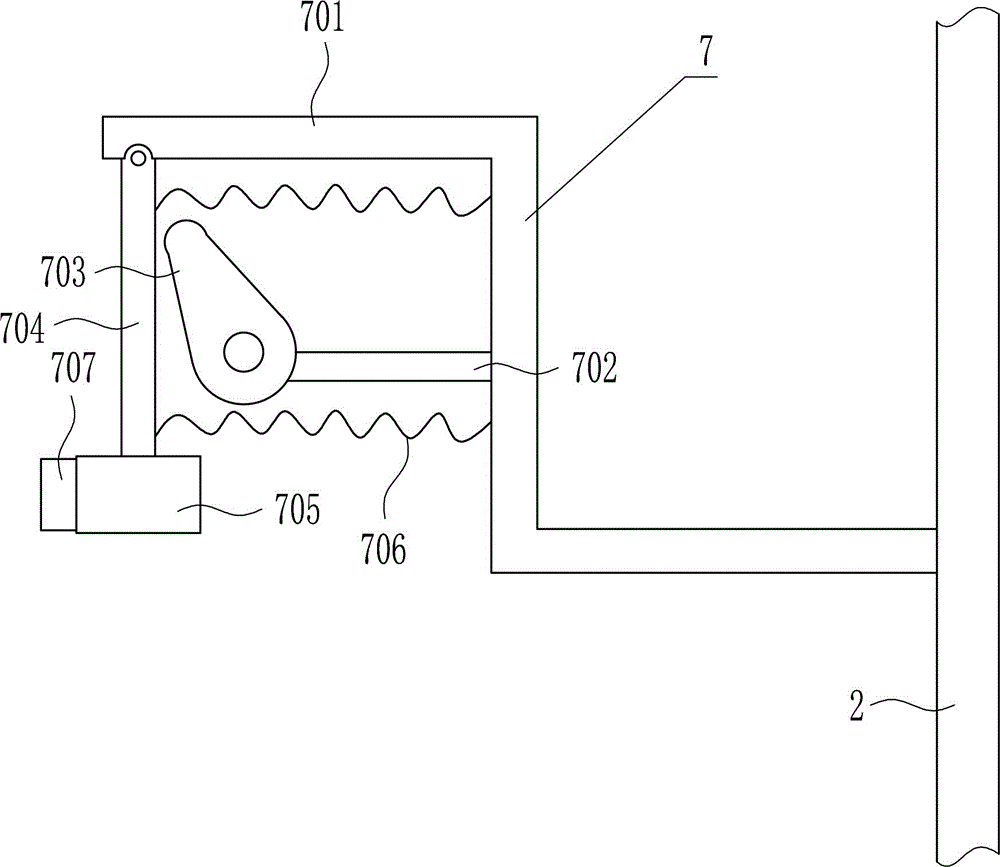
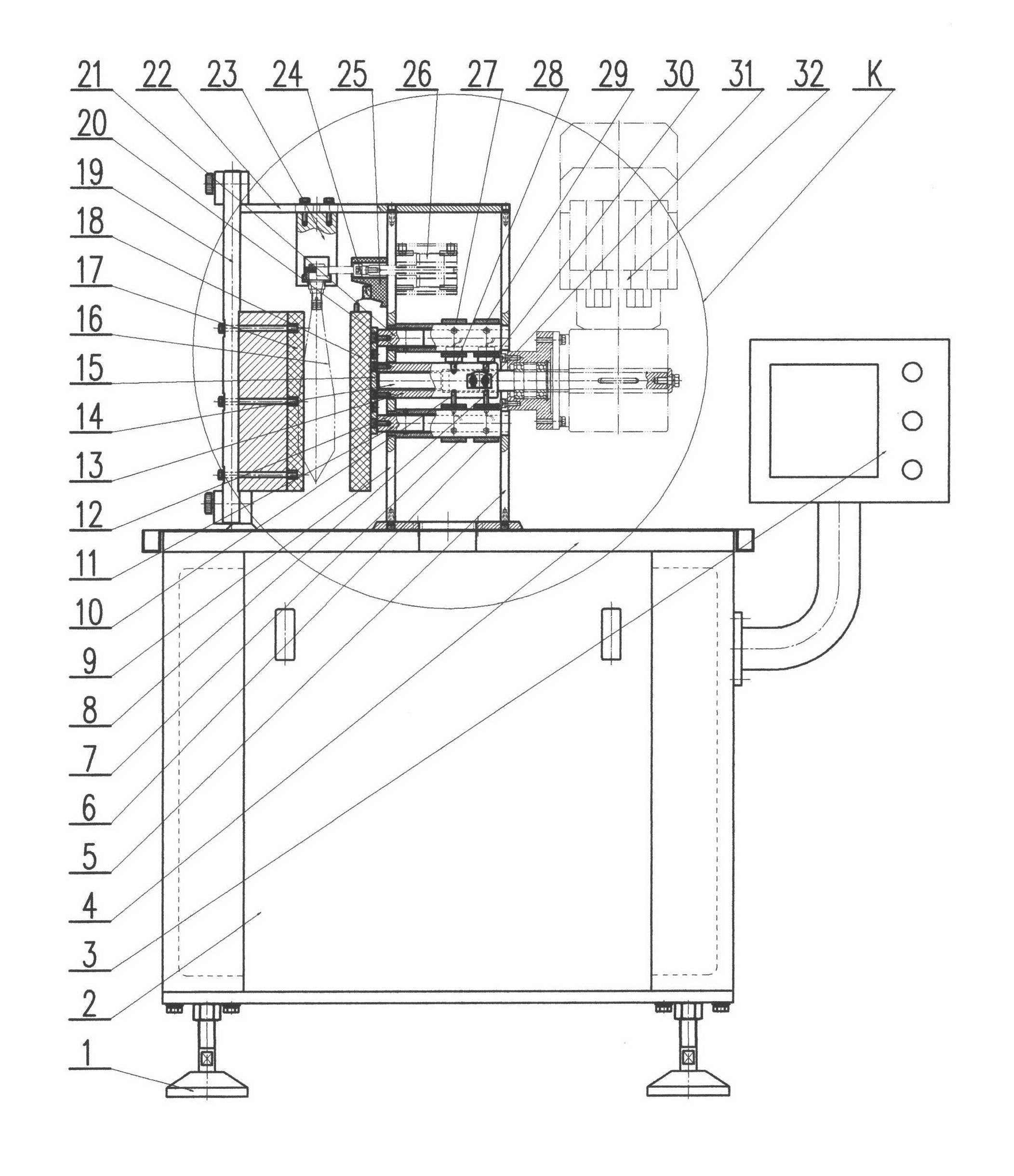
Quantitative feeding device for solid medicine for pharmaceutical packaging
The invention relates to a feeding device for pharmaceutical packaging, and in particular relates to a quantitative feeding device for a solid medicine for pharmaceutical packaging. The technical problem to be solved by the invention is to provide a quantitative feeding device for a solid medicine for pharmaceutical packaging that can save time and labor, achieve quantitative feeding, and reduce the manufacturing cost, and is simple to operate. To solve the above technical problem, the invention provides such a quantitative feeding device for a solid medicine for pharmaceutical packaging that comprises a baseplate and the like. The top of the baseplate is vertically connected to an n-shaped rack by means of a bolt. A conveyor is arranged on the top of the baseplate at the right side within the n-shaped rack. A plurality of fixed blocks are connected to a conveying part of the conveyor at uniform intervals. A first feeding device is arranged on the upper part of the n-shaped rack. According to the invention, quantitative feeding to a second feeding device is achieved by means of the first feeding device, and then the solid medicine is loaded into medicine bottles by means of the second feeding device. The operation is simple, and the manufacturing cost is low.
Owner:高永玲 +1
Oral dosage combination pharmaceutical packaging
InactiveUS20090232886A1Increased riskDevelopment costAntibacterial agentsBiocidePharmaceutical packagingPharmaceutical formulation
Pharmaceutical fixed dose combination products are formed by merging a fixed dose of a first pharmaceutical formulation from primary module, with a fixed dose of a second pharmaceutical formulation from a secondary module In a preferred embodiment the first and second pharmaceutical formulations are separated from one another in a three piece capsule, a capsule-in-a-capsule or a tablet-in-a-capsule, and the primary and secondary modules are interchangeable.
Owner:SISON RAYMUNDO A
Plastic-free environment-friendly isolation paper, plastic-free environment-friendly release paper as well as preparation method of plastic-free environment-friendly isolation paper and plastic-free environment-friendly release paper
ActiveCN102817281AImprove flatnessImprove isolationLamination ancillary operationsPaper coatingLogistics managementIsolation layer
The invention belongs to the field of papermaking technology, and particularly relates to a plastic-free environment-friendly isolation paper and a plastic-free environment-friendly release paper used for the industry fields of food hygiene, pharmaceutical packaging, electronic appliances, household products, cosmetics, supermarket logistics, post express and the like and a preparation method of the plastic-free environment-friendly isolation paper and the plastic-free environment-friendly release paper. The technical scheme is as follows: the isolation paper is composed of a 30-60g / m<2> low-weight tissue paper or a 50-200g / m<2> cultural paper, and a precoating layer and an isolation layer coated in sequence on the tissue or cultural paper surface, wherein a layer of precoating is coated on the front face of the base paper firstly and then a layer of isolation layer is coated into the precoating layer; the release paper is formed by coating a solvent-free silicone oil onto the isolation paper and comprises the base paper layer, the precoating layer, the isolation layer and the silicone oil layer from top to bottom in sequence. The paper prepared by the method provided by the invention has the characteristics of low requirements for base paper performance, good isolation (release) effect, high paper smoothness, no pollution, easiness of recycling, stable properties and the like. The production process has the advantages of low manufacture cost, high production efficiency, easiness of mass production and the like.
Owner:GUANGDONG GUANHAO HIGH TECH
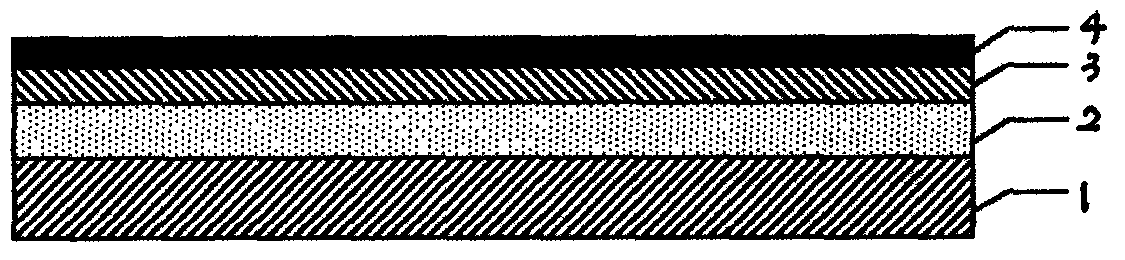
Trilayer coated pharmaceutical packaging with low oxygen transmission rate
An article is described including an article surface and a coating set comprising an optional tie coating or layer, a barrier coating or layer, and a pH protective layer. The respective coatings or layers can be applied by chemical vapor deposition of a polysiloxane or polysilazane precursor in the presence of oxygen. Examples of such an article are a prefilled thermoplastic syringe or thermoplastic pharmaceutical vial with a coated interior portion containing a pharmaceutical preparation or other fluid with a pH of 4 to 8, alternatively 5 to 9. The barrier coating or layer prevents oxygen from penetrating into the thermoplastic syringe or vial. The tie coating or layer, if present, and the pH protective coating or layer protect the barrier layer from the contents of the syringe or vial.
Owner:SI02 MEDICAL PRODS
Device for counting the number of medicines in medicine packaging envelope
InactiveUS20120002042A1Small sizeDifficult to eliminateSurveying instrumentsColor television detailsInfraredMedicine
A device for counting the number of medicines in a medicine packaging envelope the device includes: an illumination part which is configured to irradiate near infrared rays toward a sheet surface of the medicine packaging envelope; an imaging part which is arranged at a position where the near infrared rays which pass through the medicine packaging envelope are received, and is configured to image the medicine packaging envelope; and a medicine counting unit which is configured to count the number of medicines based on a gray image from the imaging part, wherein the imaging part includes a visible light cut filter in a light receiving part thereof, and the visible light cut filter is configured to prevent a reflection light which is formed by the reflection of the visible light on the medicine packaging envelope from being incident on the imaging part.
Owner:OOKUMA ELECTRONICS
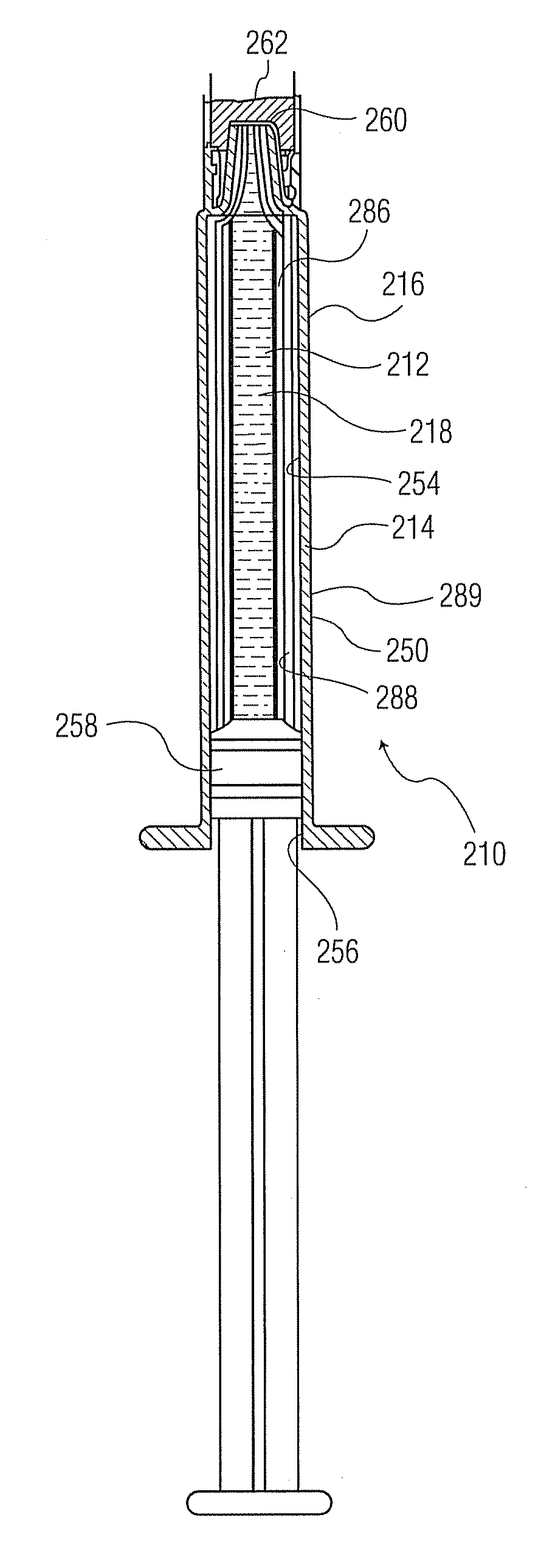
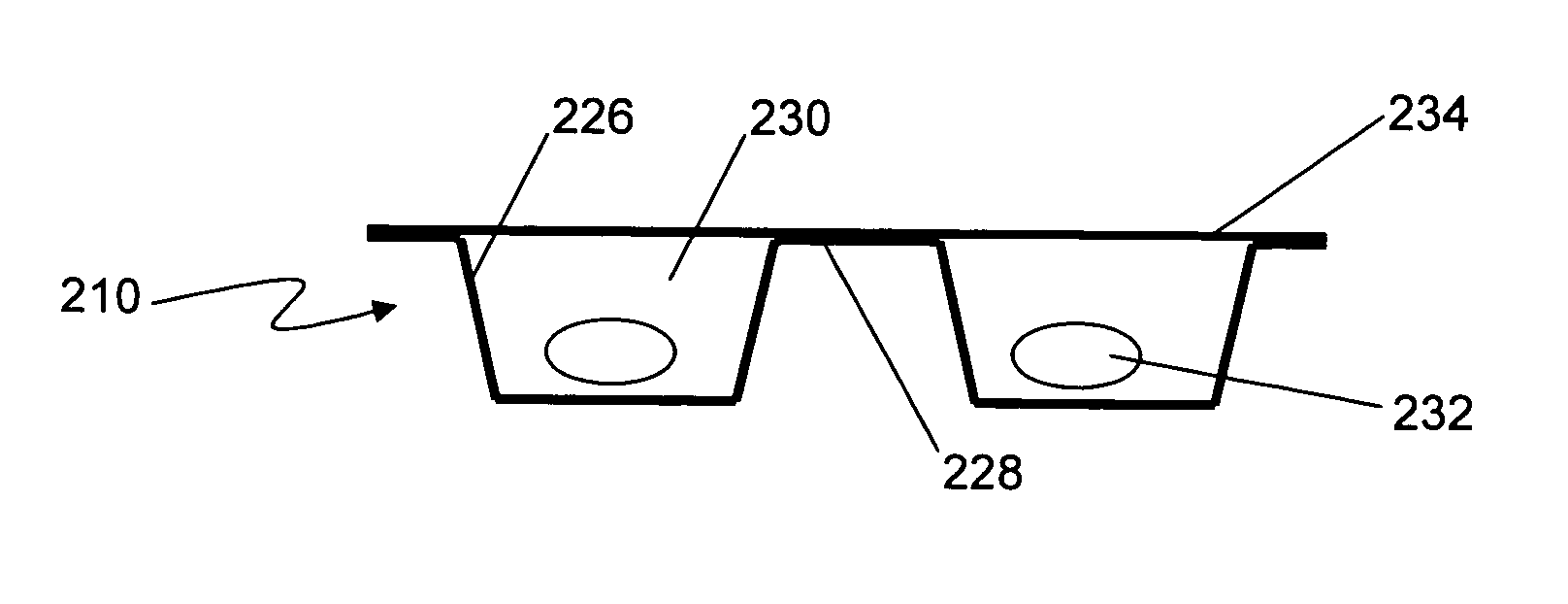
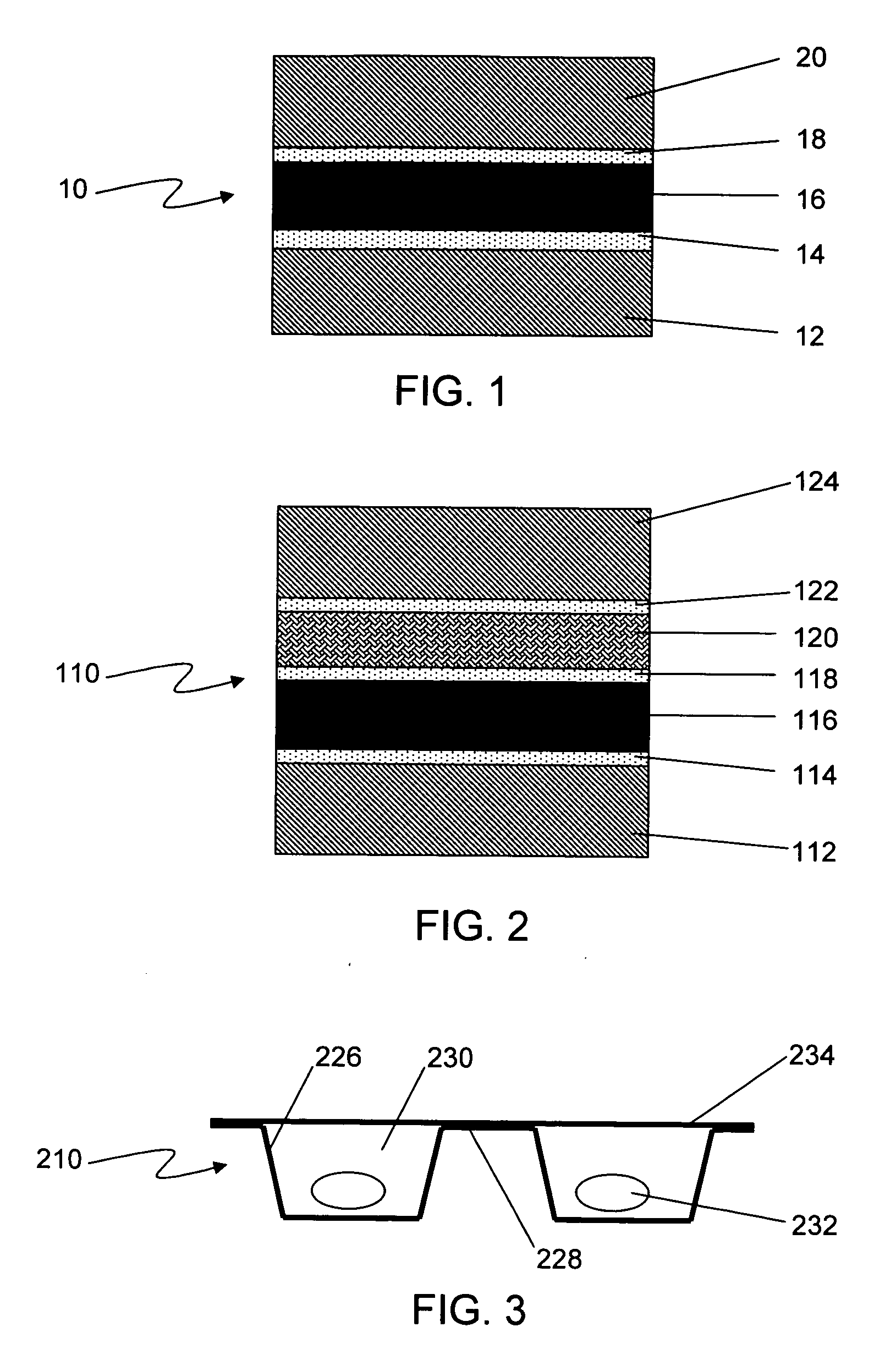
Formable film for cold-form, blister-type pharmaceutical packaging
Cold-formable film composite structures useful for pharmaceutical blister packaging may be constructed from a first polyester surface layer that has a low extractables level, an aluminum layer adhered to the first surface layer, and an additional layer adhered to the aluminum layer. A second surface layer may be adhered to the additional layer. Blister packs may be prepared from such composite structures by cold-forming the structures such that the first polyester surface layer is inside the blister, facing the pharmaceutical. The use of a low-extractables material on the inside surface of the blister minimizes the potential for contamination of the pharmaceutical within the package.
Owner:DUPONT TEIJIN FILMS U S LLP
Neutral borosilicate glass and application thereof
ActiveCN104261676APharmaceutical containersMedical packagingNational standardPharmaceutical packaging
The invention relates to a pharmaceutical neutral borosilicate glass. The pharmaceutical neutral borosilicate glass comprises the following components (in weight percentage, based on oxides): 70.0-80.0% of SiO2, 7.0-12.0% of B2O3, 1.5-5.0% of Al2O3, 5.5-8.0% of Na2O, 0-1.0% of K2O, 0-1.5% of Li2O, 1.5-3.0% of CaO, 0-3.5% of BaO, 0-2.0% of MgO, 0.3-0.5% of NaCl, 6.0-9.5% of Na2O+K2O+Li2O and 1.5-6% of CaO+BaO+MgO. The pharmaceutical neutral borosilicate glass is in line with a national standard of pharmaceutical packaging containers (materials) YBB00022005-2, and is suitable for being used as pharmaceutical primary packaging materials, such as ampoules, portable bottles, penicillin bottles and the like.
Owner:RUYUAN YAO AUTONOMOUS COUNTY DONGYANGGUANG FORMED FOIL CO LTD
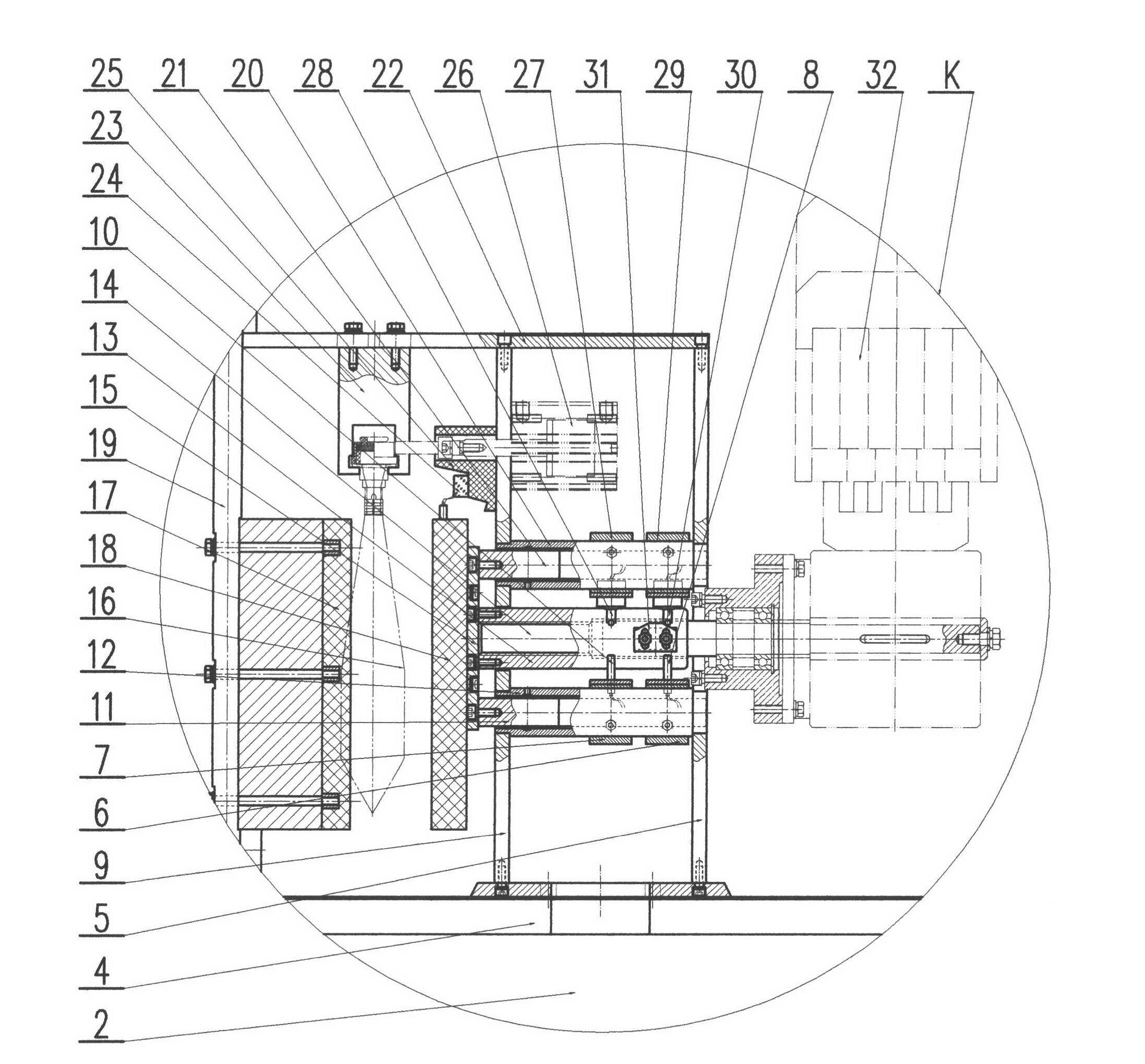
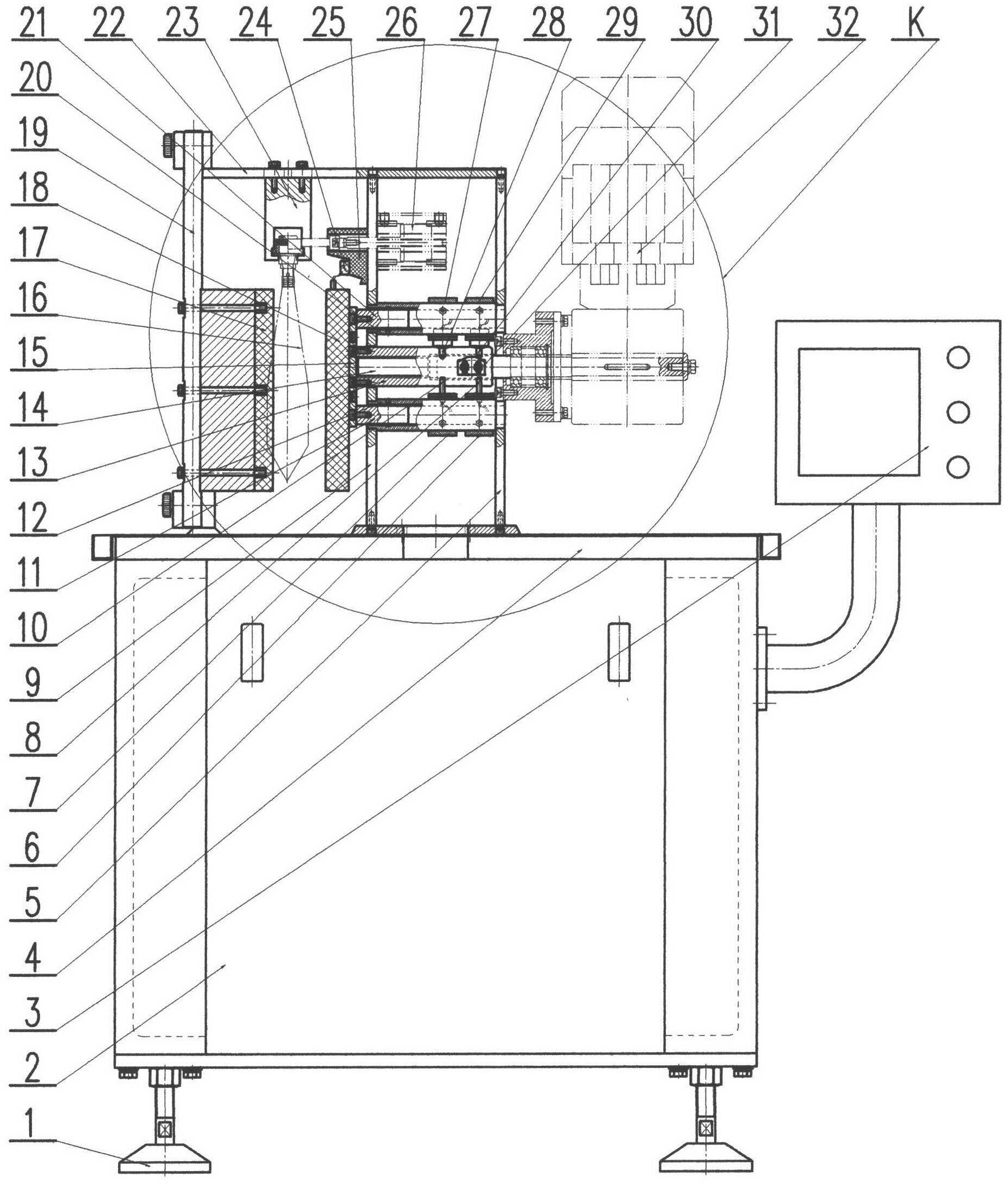
Leakage detection method and leakage detection device of large soft transfusion package bags or soft bottles
InactiveCN102310055AReduce intensityReduce chanceMeasurement of fluid loss/gain rateSortingProgrammable logic controllerEngineering
The invention discloses a leakage detection device of large soft transfusion package bags or soft bottles, and relates to the technical field of pharmaceutical packaging machinery. The leakage detection device is composed of a rack (4), a programming logic controller (3), a vertical beam (19), a horizontal beam (22), an extrusion fixed plate (17), a fixture (23), a recycling bin (2), a motor (32) with a brake and a deceleration component, and a leakage detection component. The device has the characteristics of high detection precision, low working intensity, safety and reliability and the like, is suitable for the leakage detection of non-PVC (poly vinyl chloride) film large soft transfusion package bags in various specifications or PP large soft transfusion bottles in various specifications, and can be suitable for the leakage detection of large soft transfusion package bags or soft bottles in various specifications, which are made of other materials.
Owner:HUNAN FE PHARM MASCH CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com