Patents
Literature
170 results about "Fixed dose" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
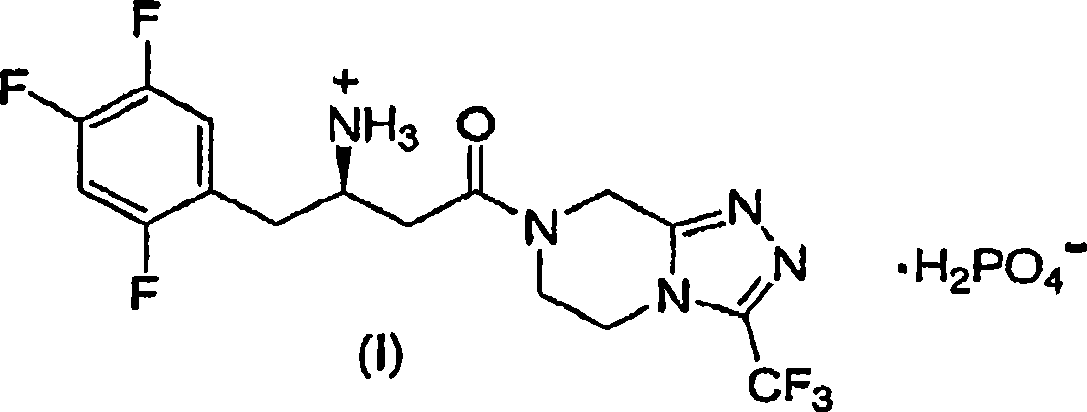
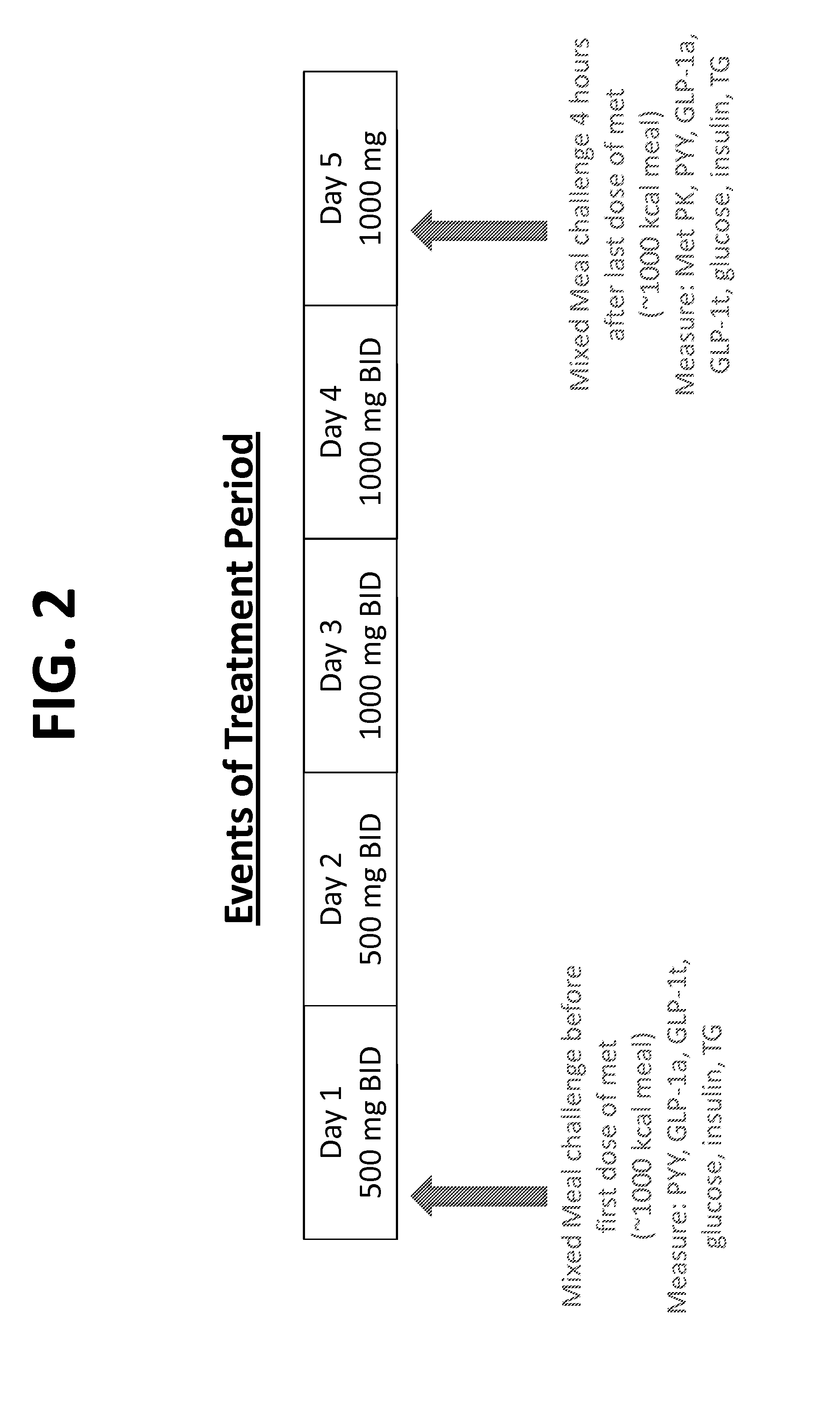
The fixed-dose procedure (FDP), proposed in 1984 by the British Toxicology Society, is a method to assess a substance's acute oral toxicity. In this procedure the test substance is given at one of the four fixed-dose levels (5, 50, 500, and 2000 mg/kg) to five male and five female rats.
Methods for treating juvenile idiopathic arthritis
ActiveUS20090258018A1Raise countIncrease the number ofOrganic active ingredientsPharmaceutical delivery mechanismDosing regimenMedicine
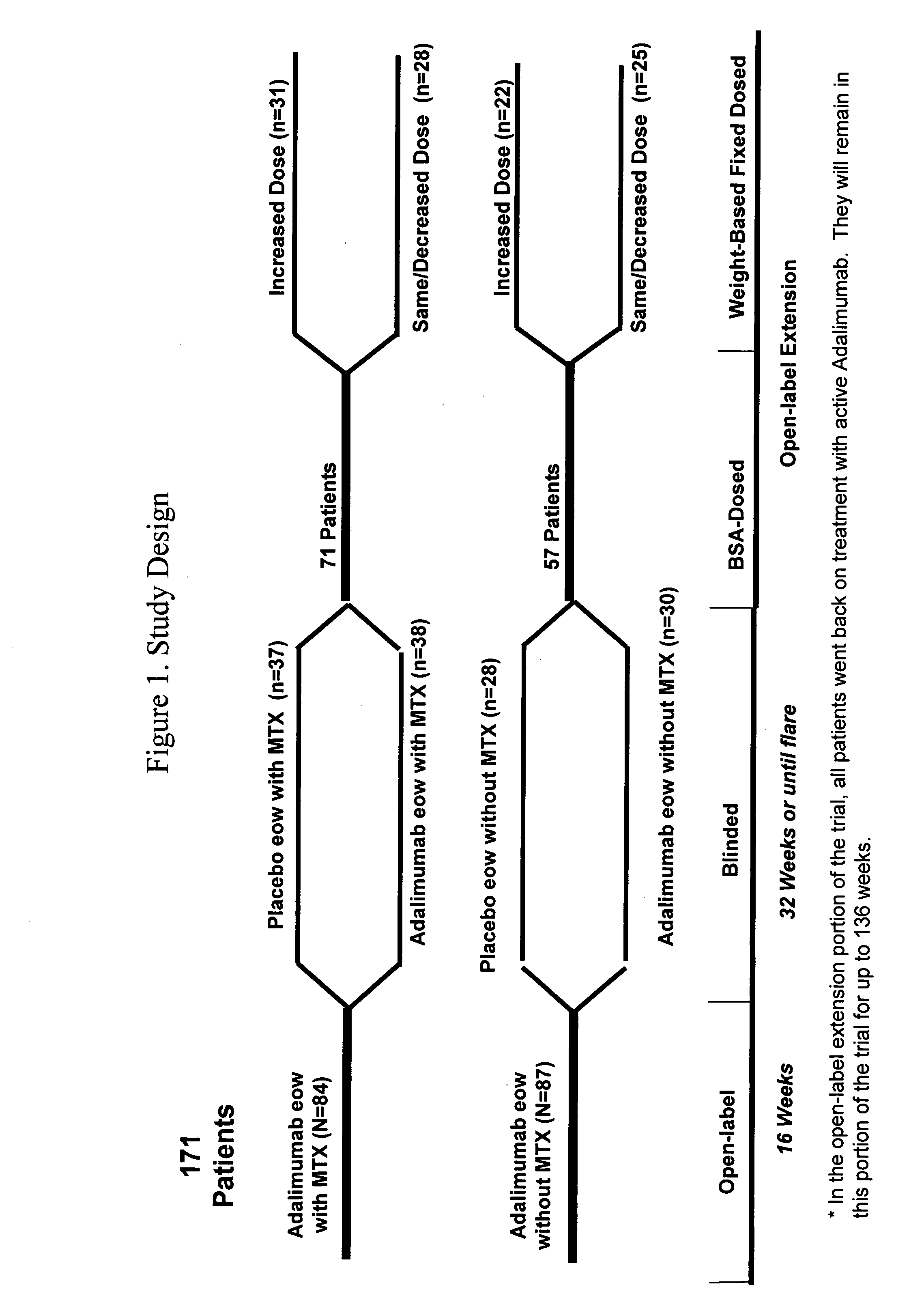
The invention provides methods and compositions for the treatment of juvenile idiopathic arthritis (JIA) where a TNFα inhibitor, such as a human TNFα antibody, or antigen-binding portion thereof, is used to treat JIA. In particular, the invention is directed to methods and compositions relating to a fixed dosing regimen for treating JIA with a TNFα inhibitor.
Owner:ABBVIE BIOTECHNOLOGY LTD
Fixed dosing of HER antibodies
Owner:GENENTECH INC
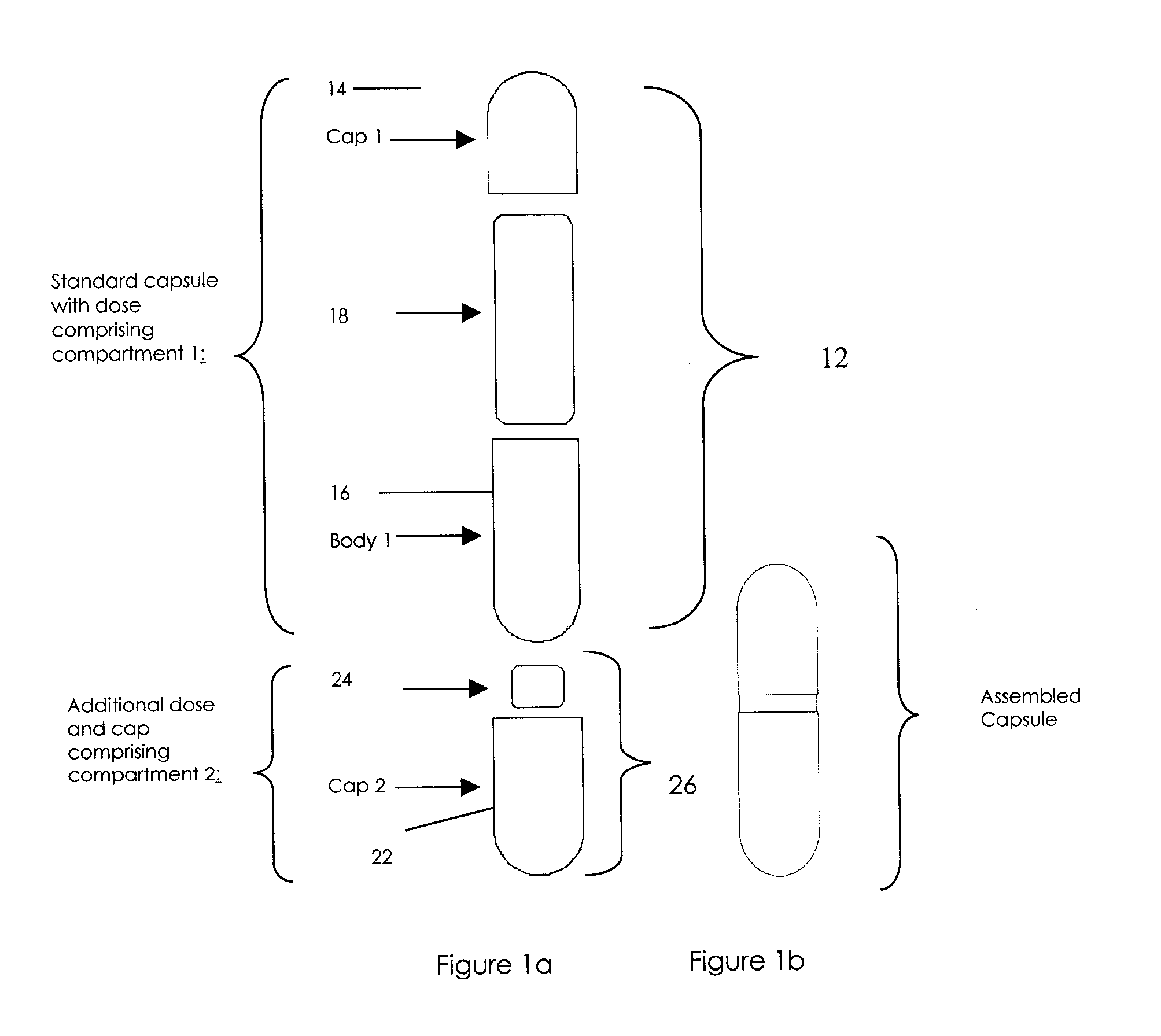
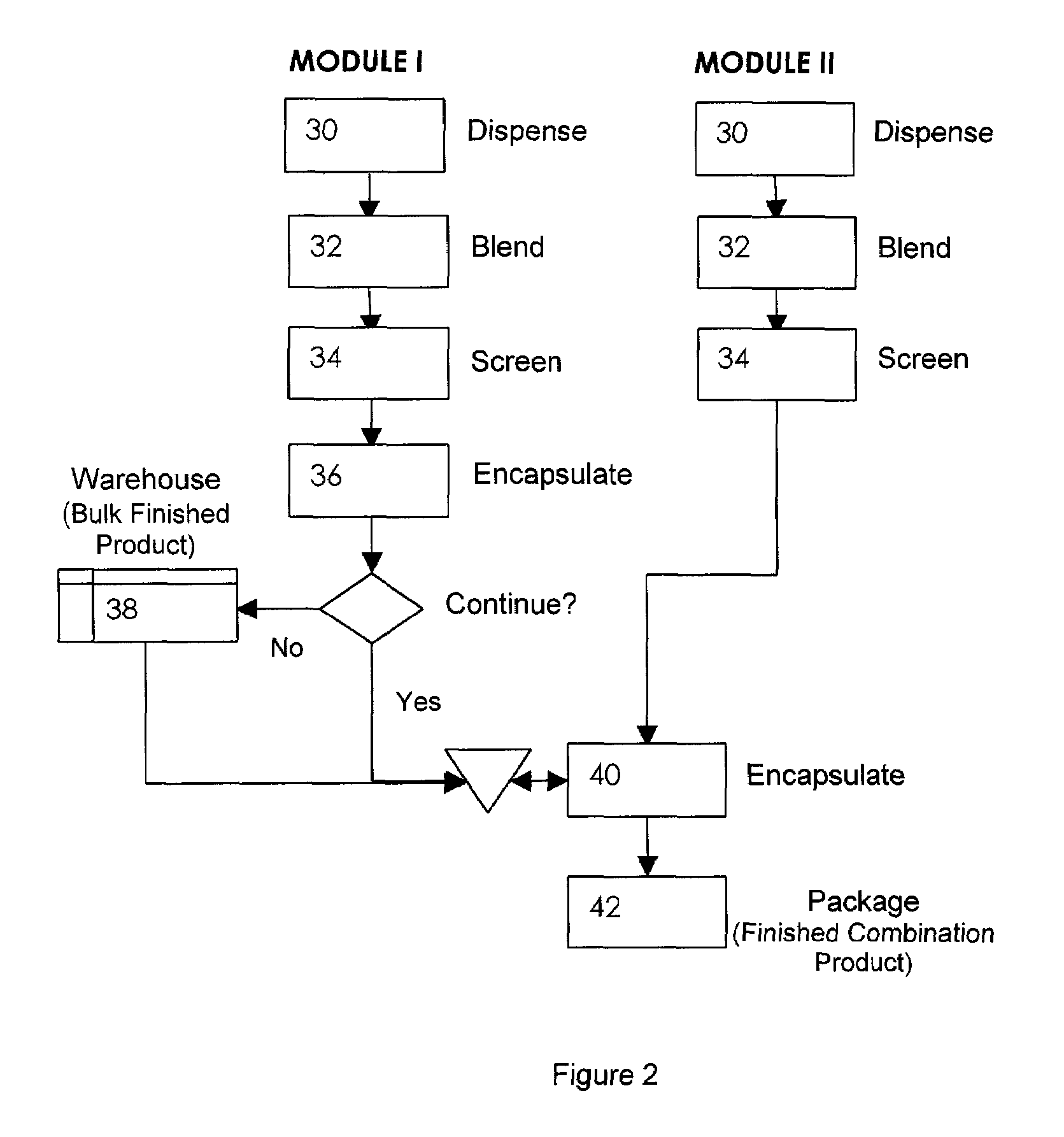
Oral dosage combination pharmaceutical packaging
InactiveUS20090087483A1Overall design flexibilityIncreased riskAntibacterial agentsBiocideMedicinePharmaceutical packaging
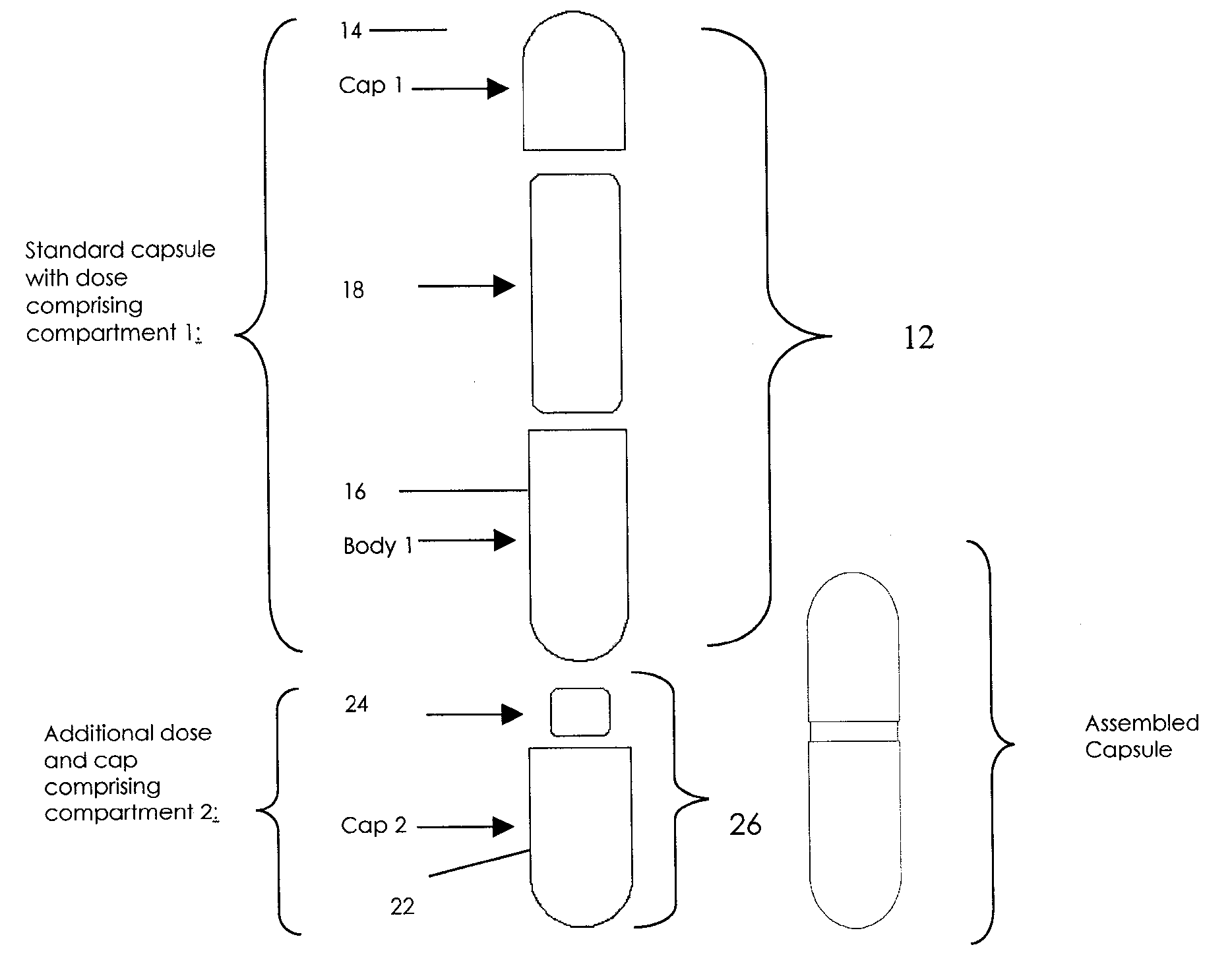
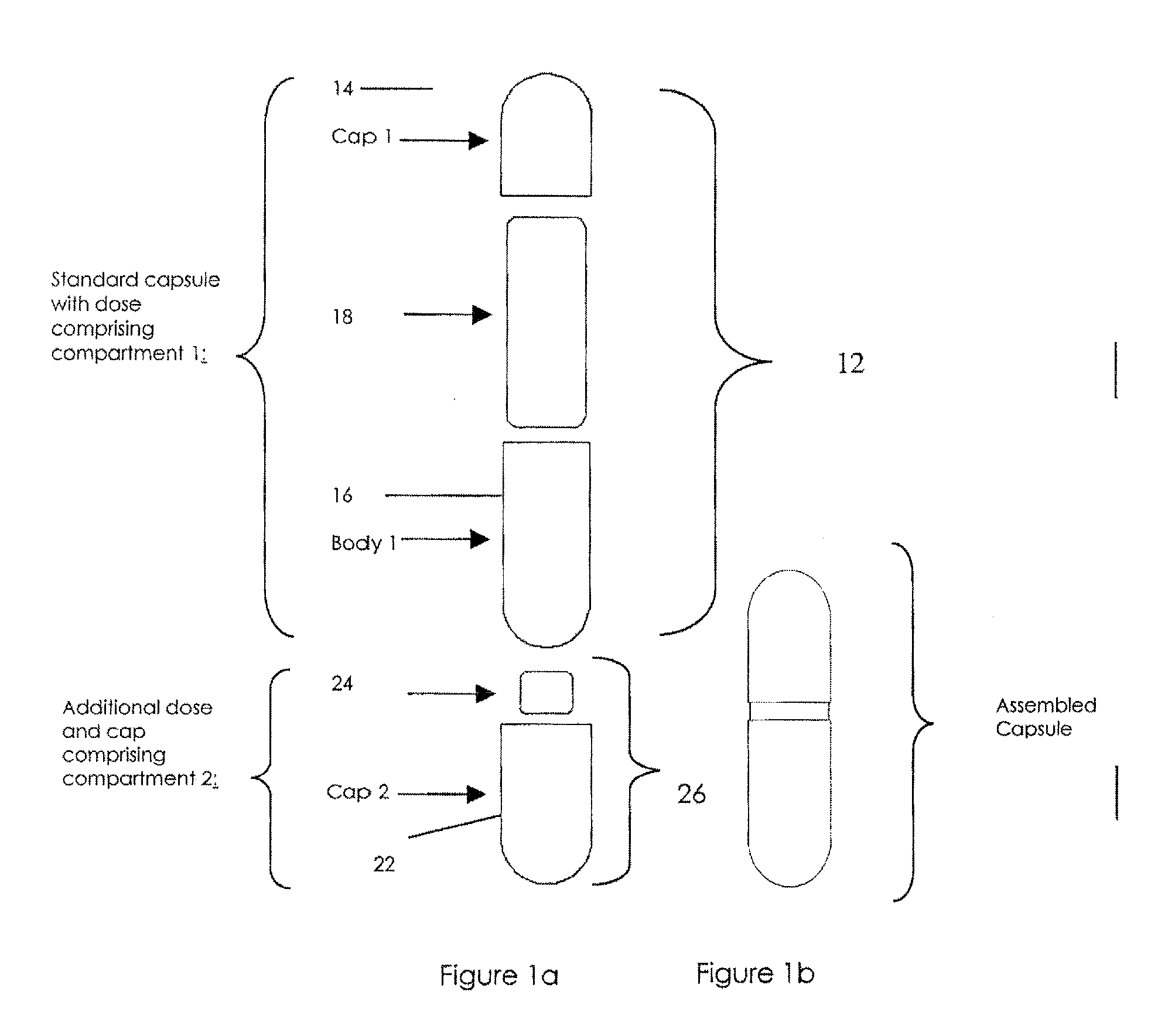
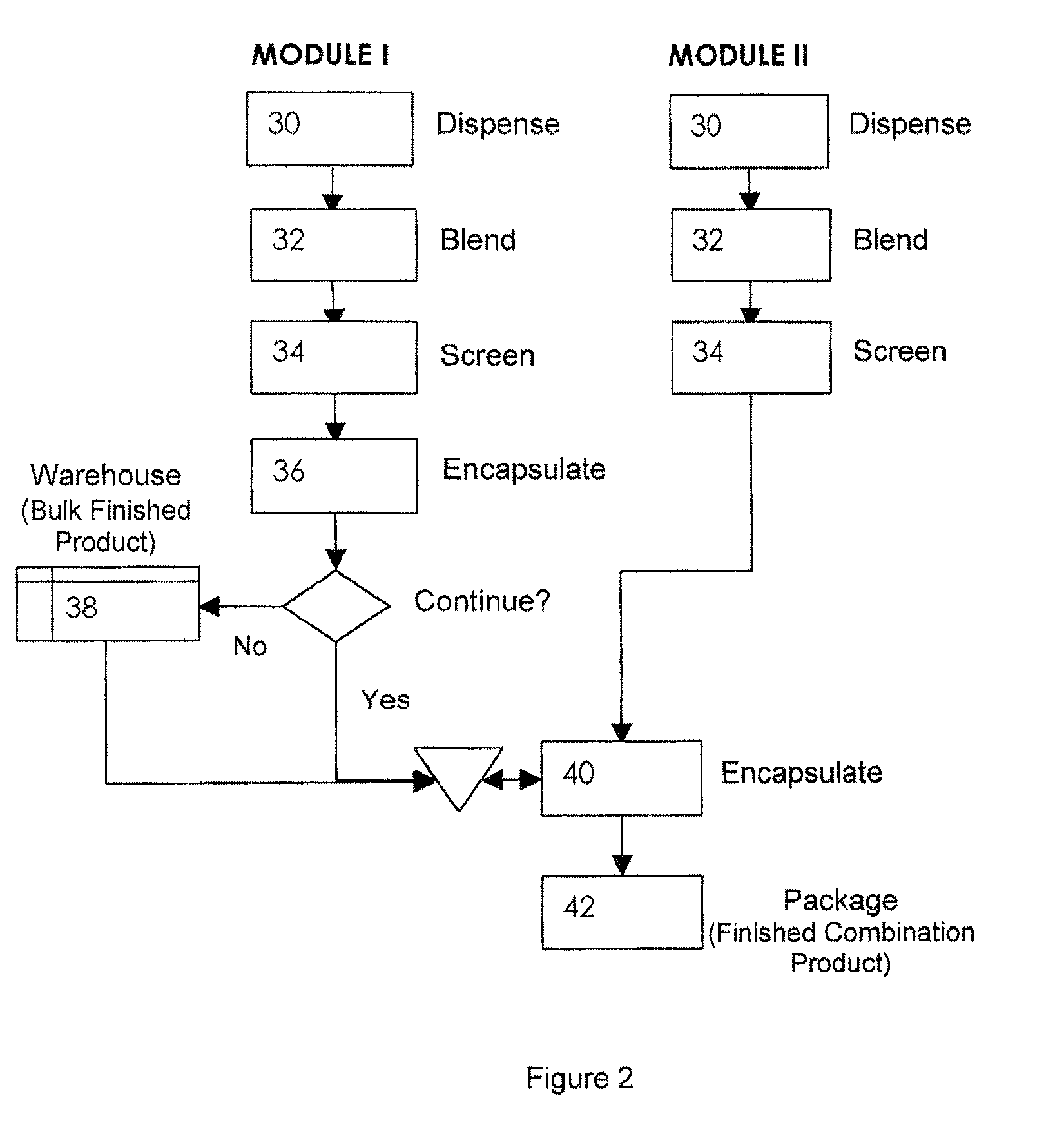
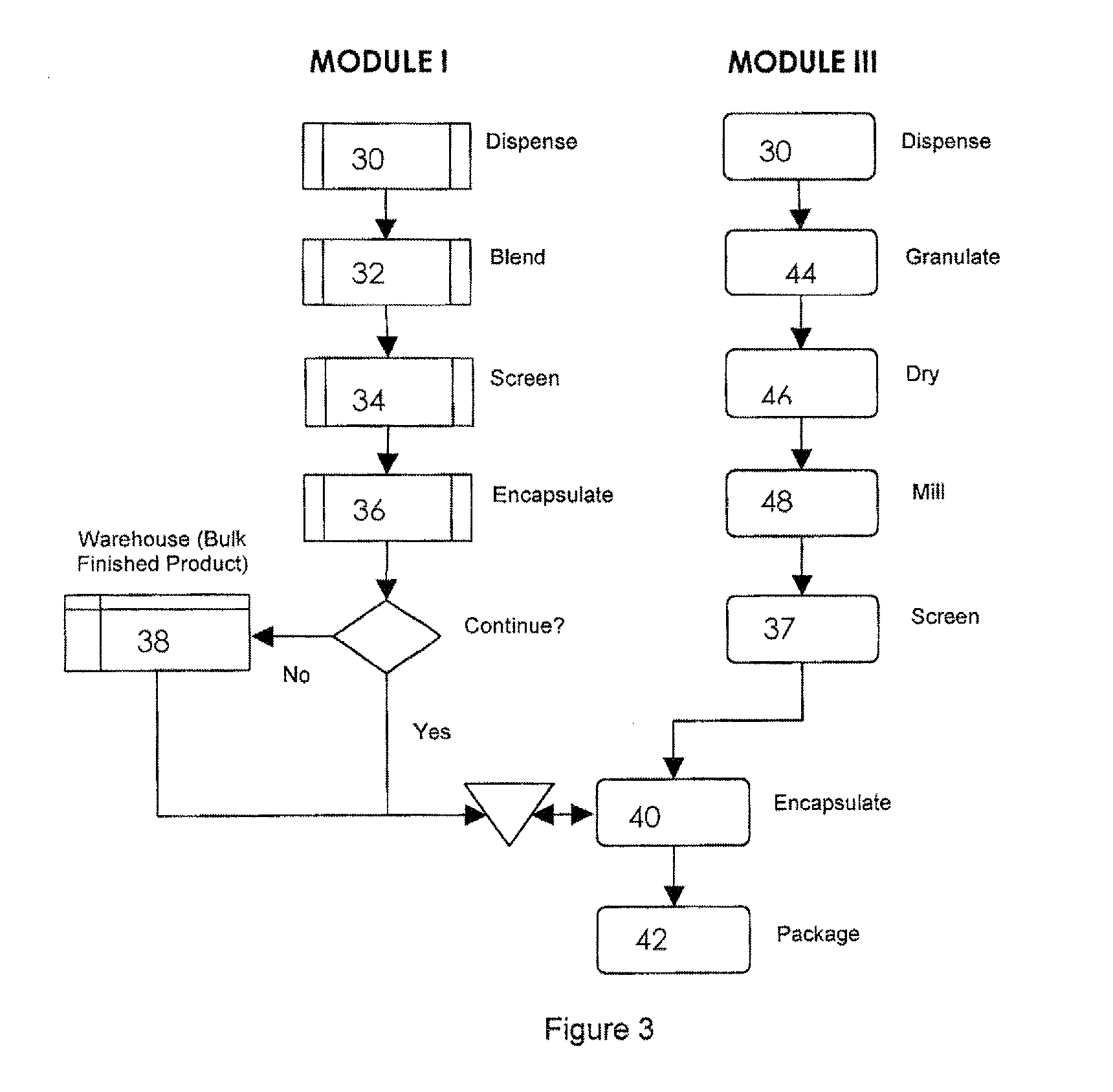
Pharmaceutical fixed dose combination products are formed by merging a fixed dose of a first pharmaceutical formulation from primary module, with a fixed dose of a second pharmaceutical formulation from a secondary module. In a preferred embodiment the first and second pharmaceutical formulations are separated from one another in a three piece capsule, a capsule-in-a-capsule or a tablet-in-a-capsule, and the primary and secondary modules are interchangeable.
Owner:MICRODOSE THERAPEUTX INC
Fixed dosing of HER antibodies
ActiveUS20060165702A1Effective treatmentOrganic active ingredientsPeptide/protein ingredientsAntibodyPertuzumab
Owner:GENENTECH INC
Subcutaneously administered Anti-il-6 receptor antibody
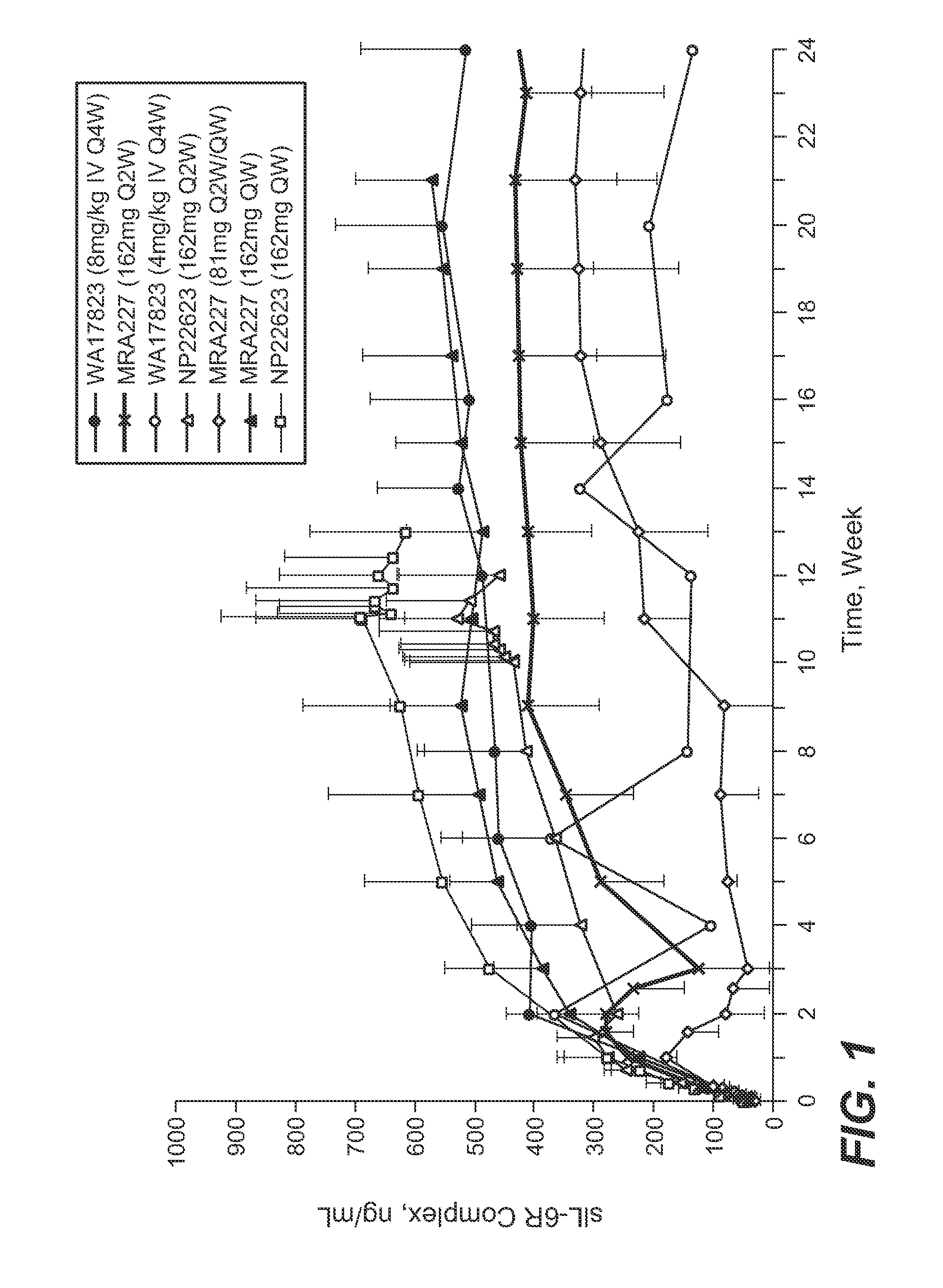
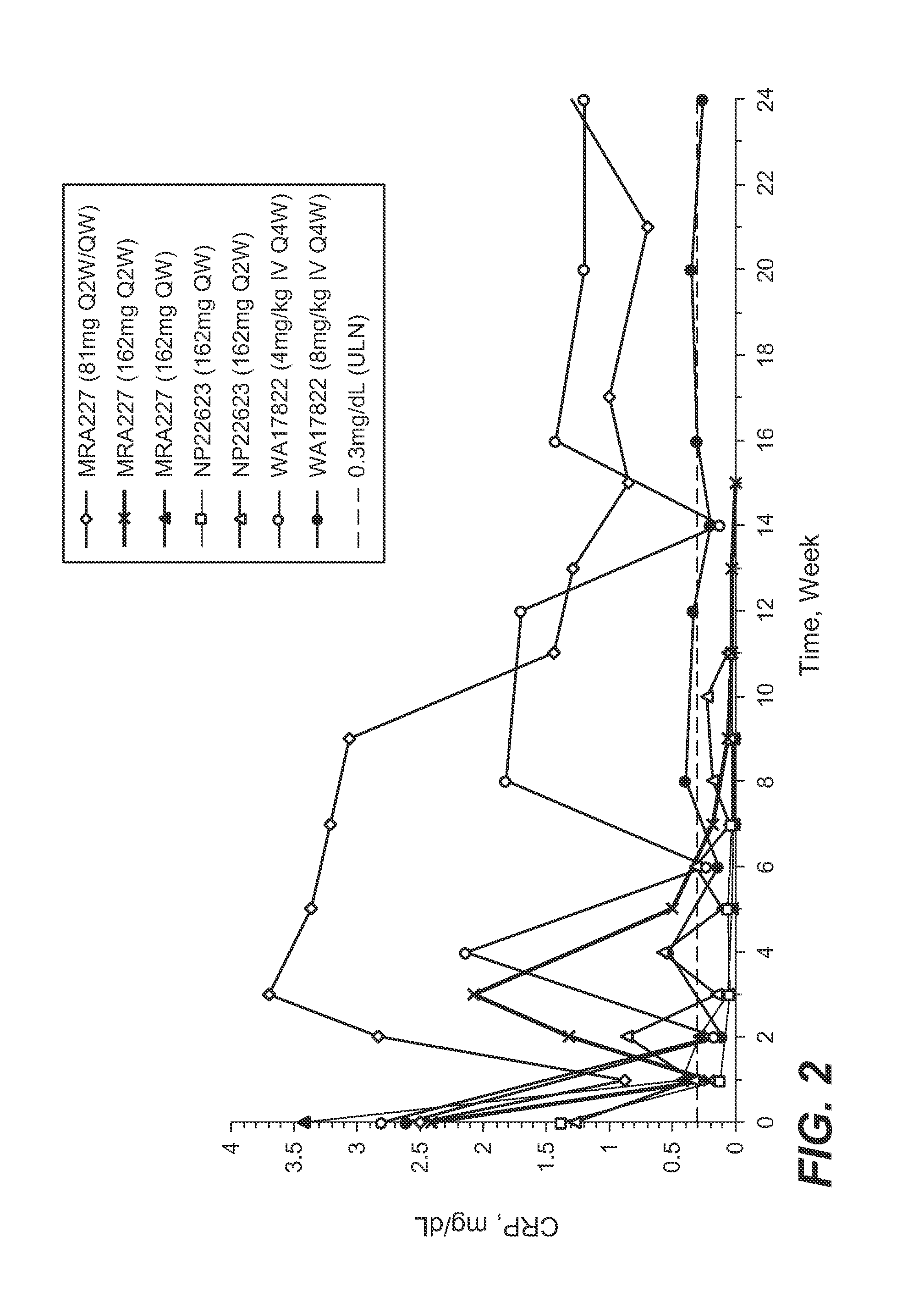
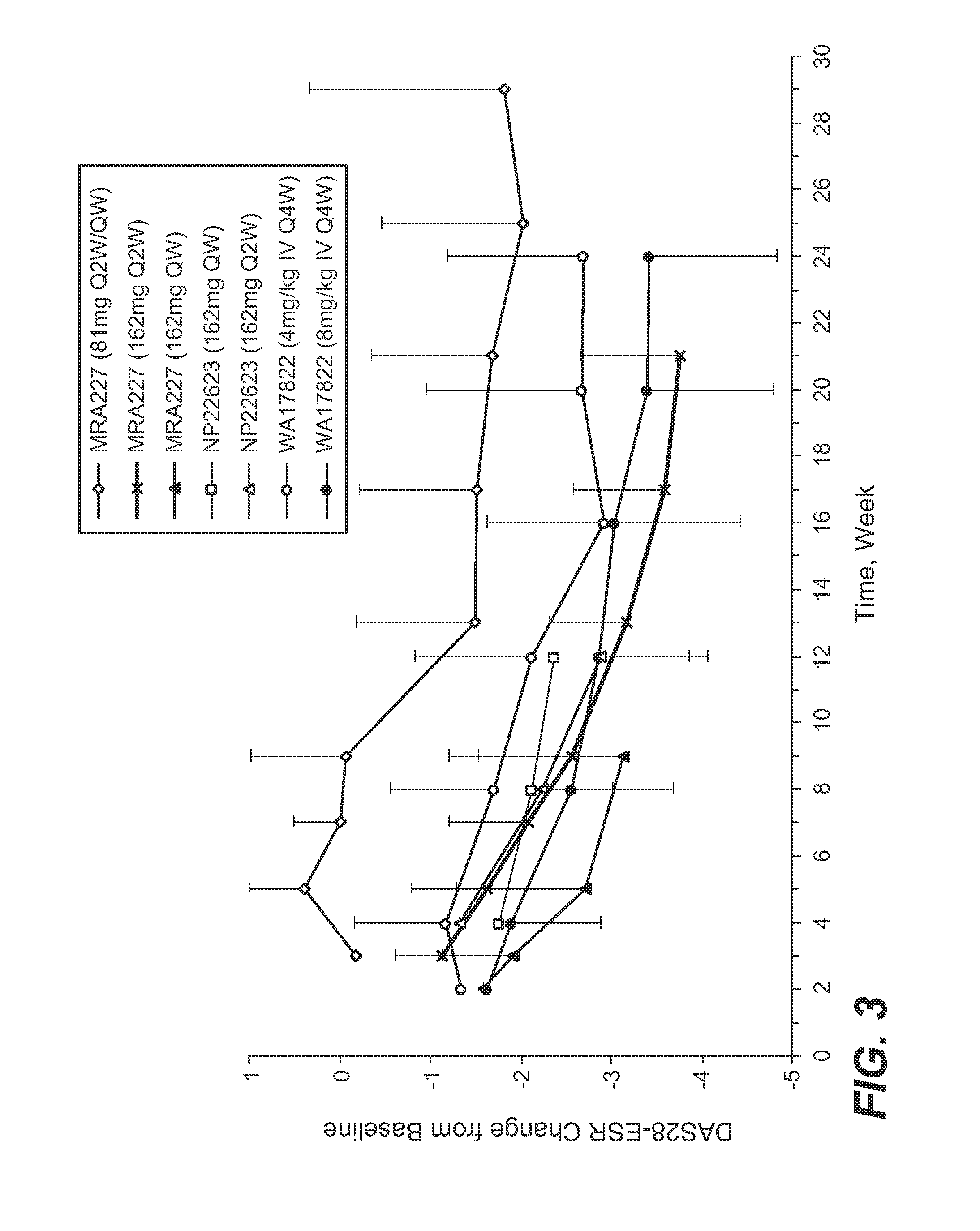
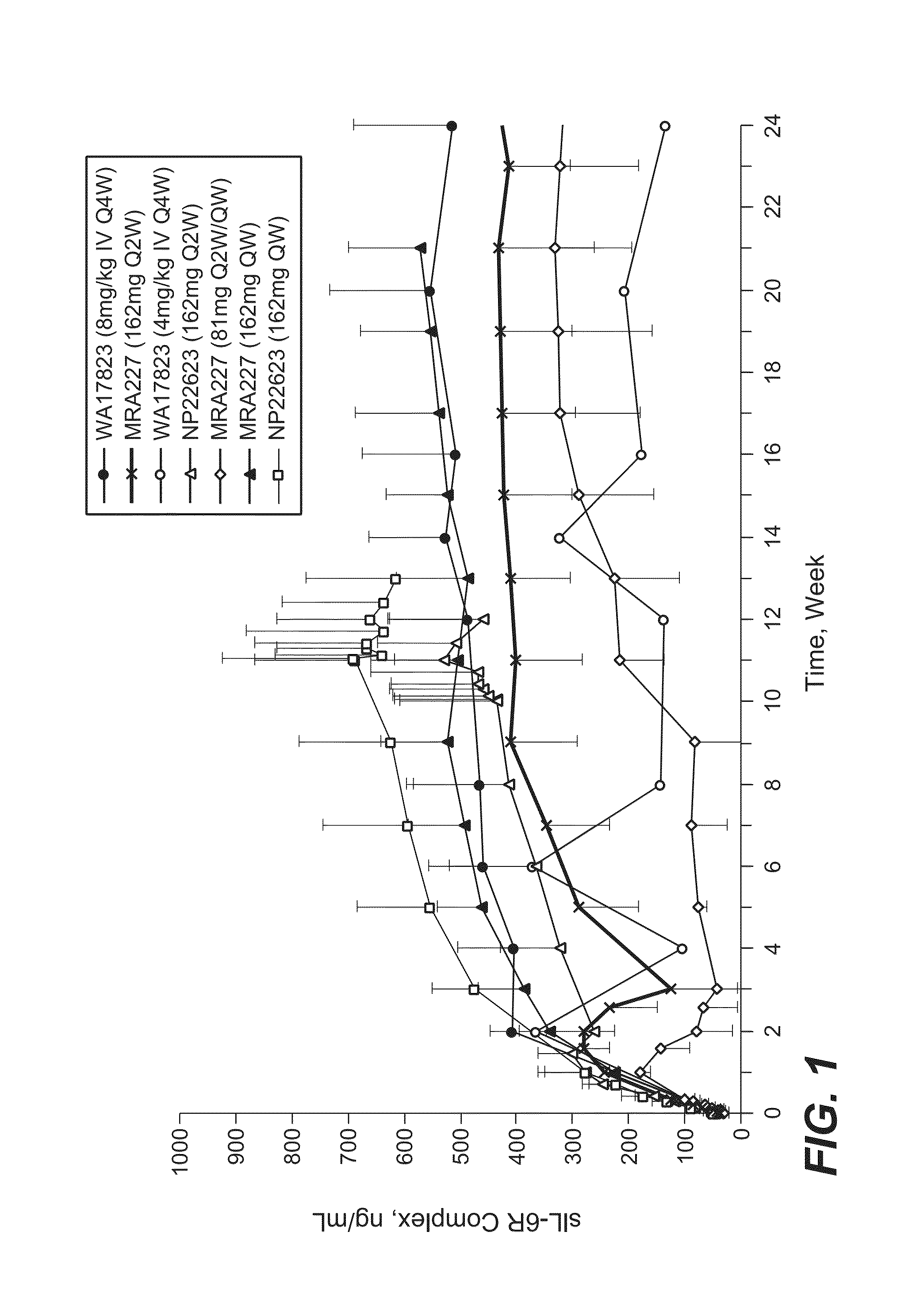
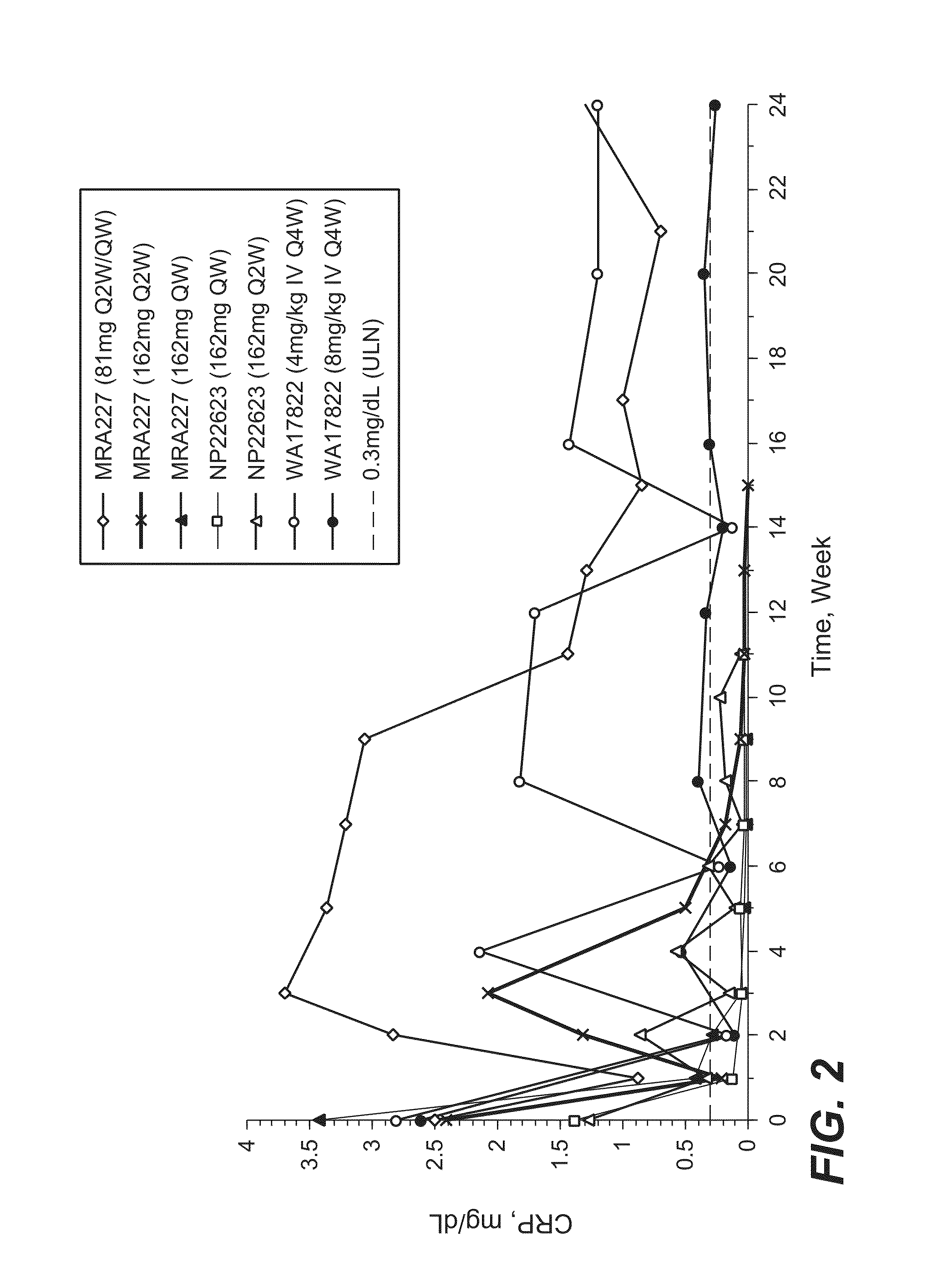
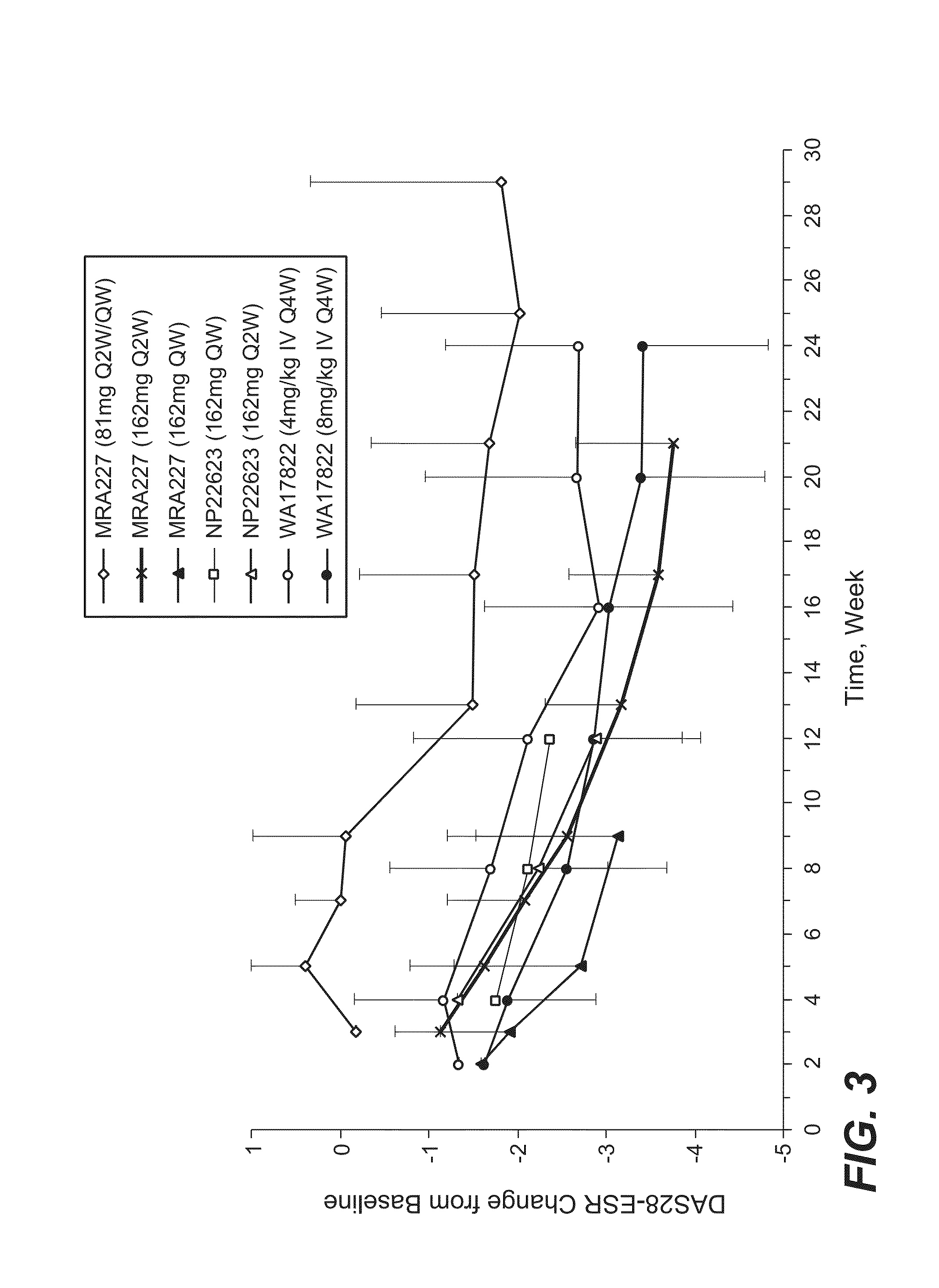
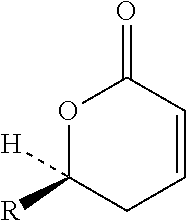
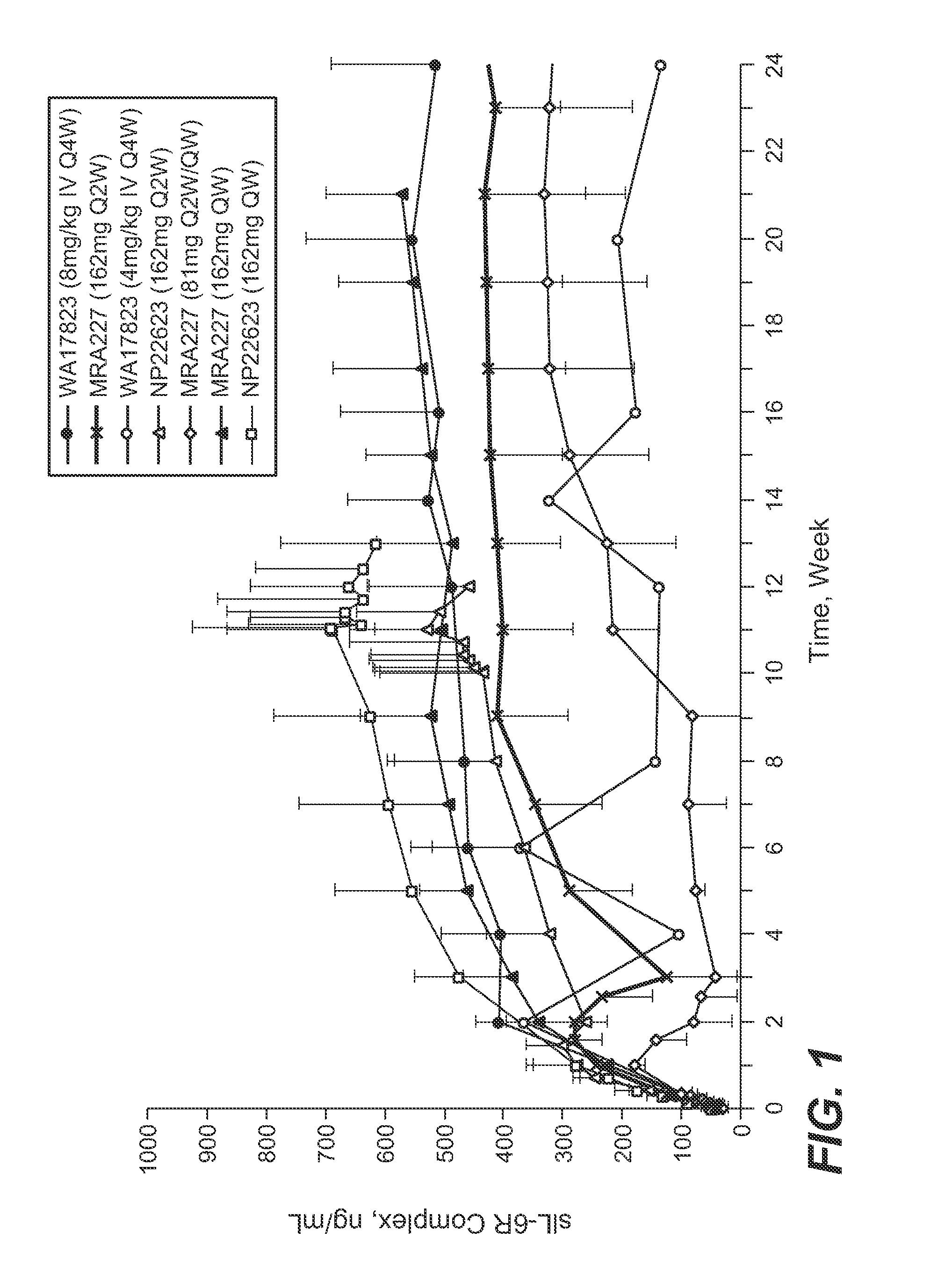
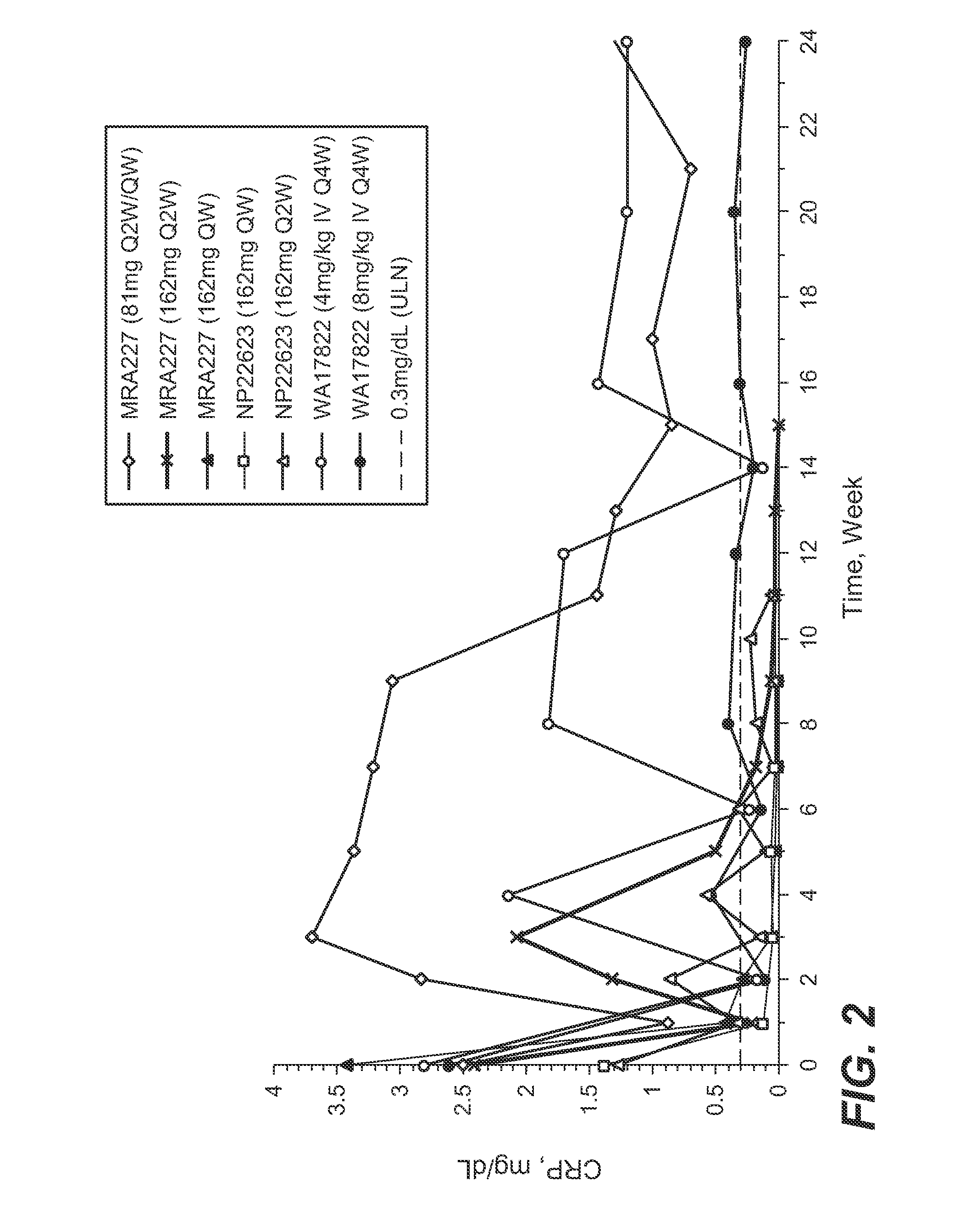
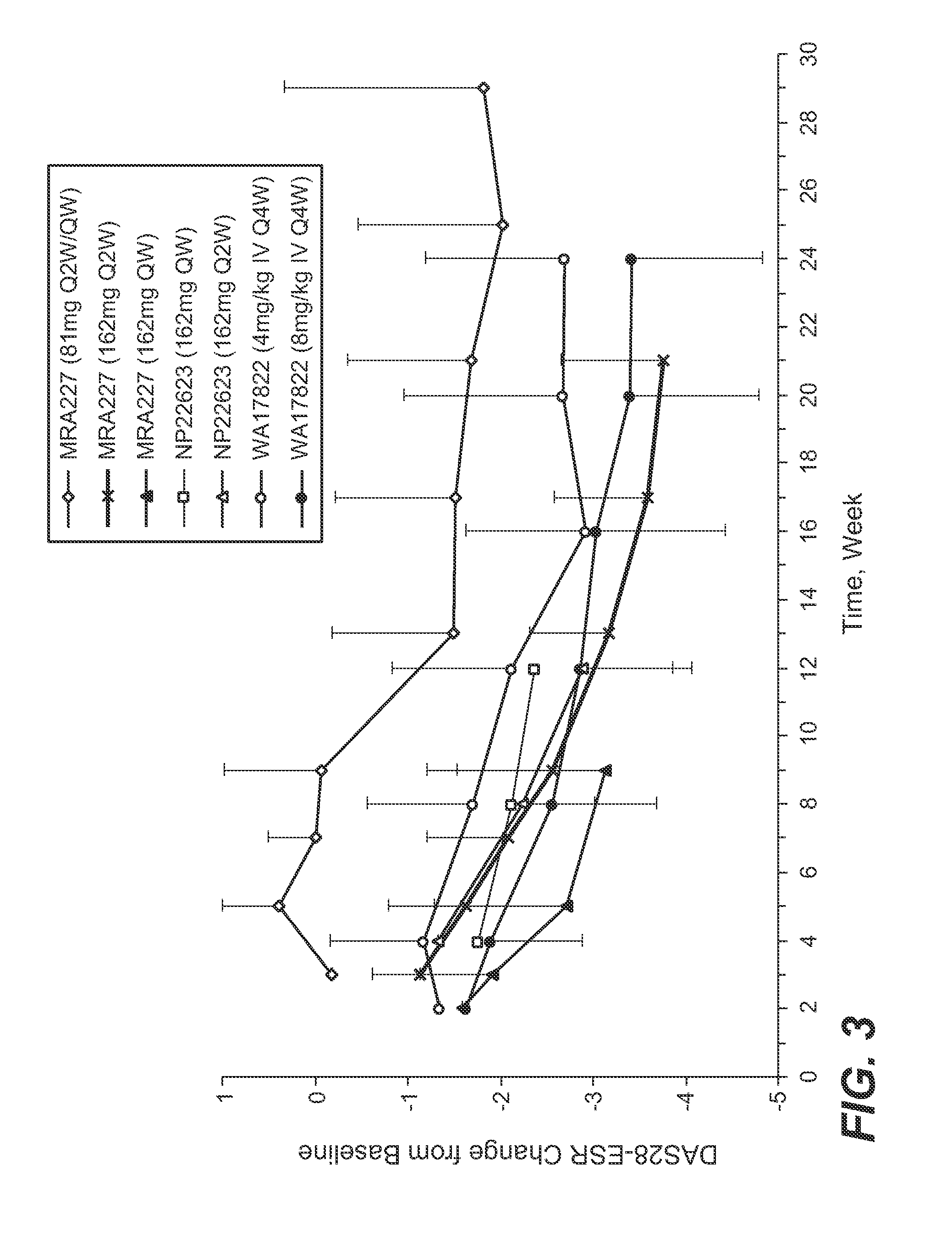
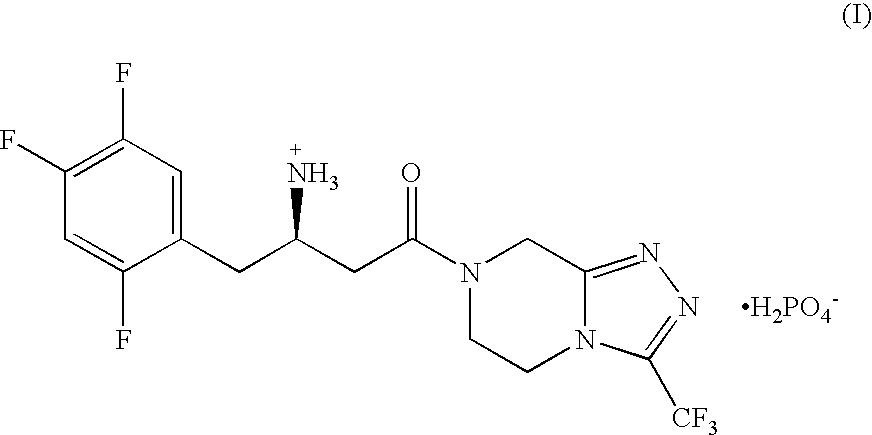
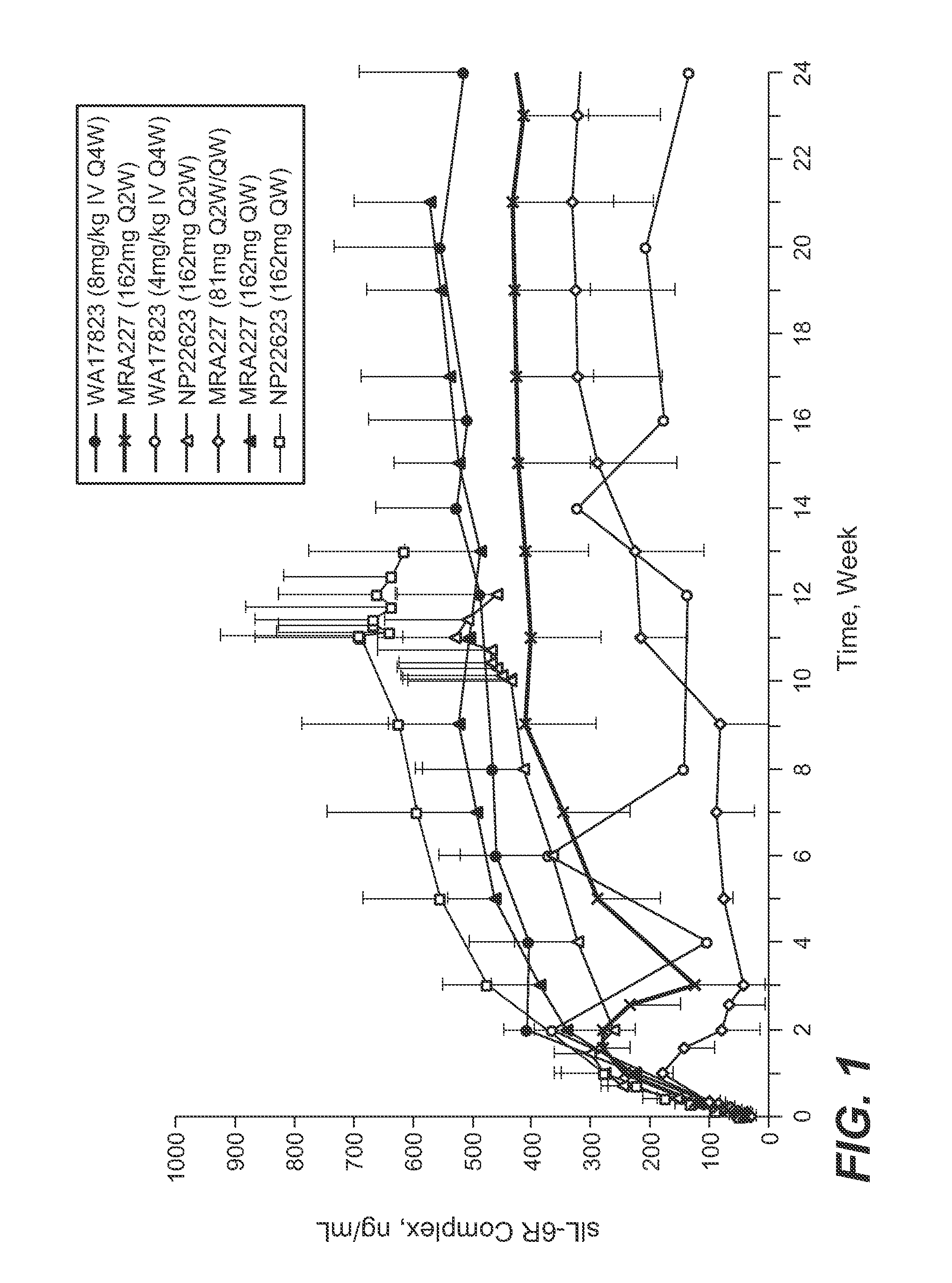
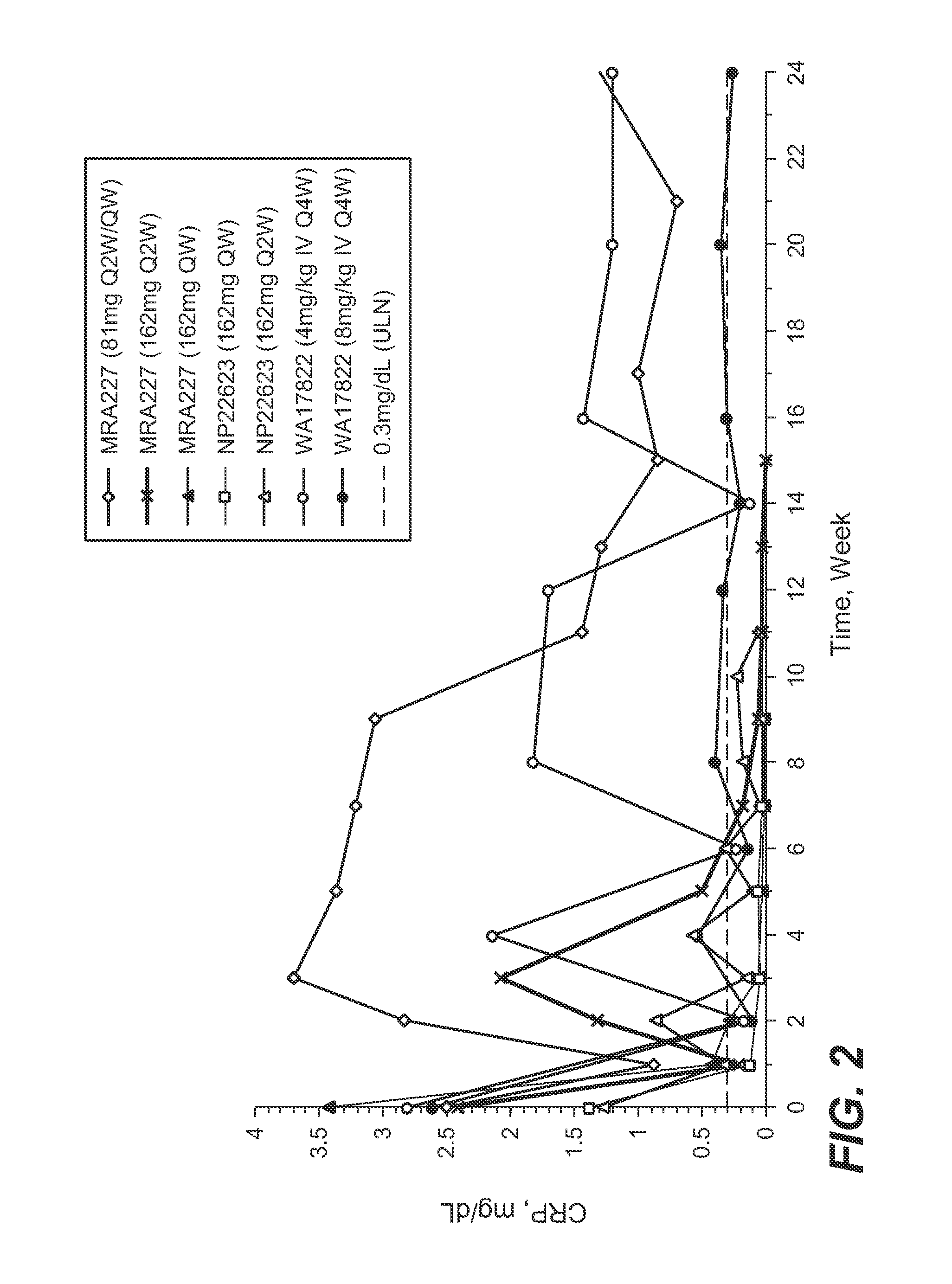
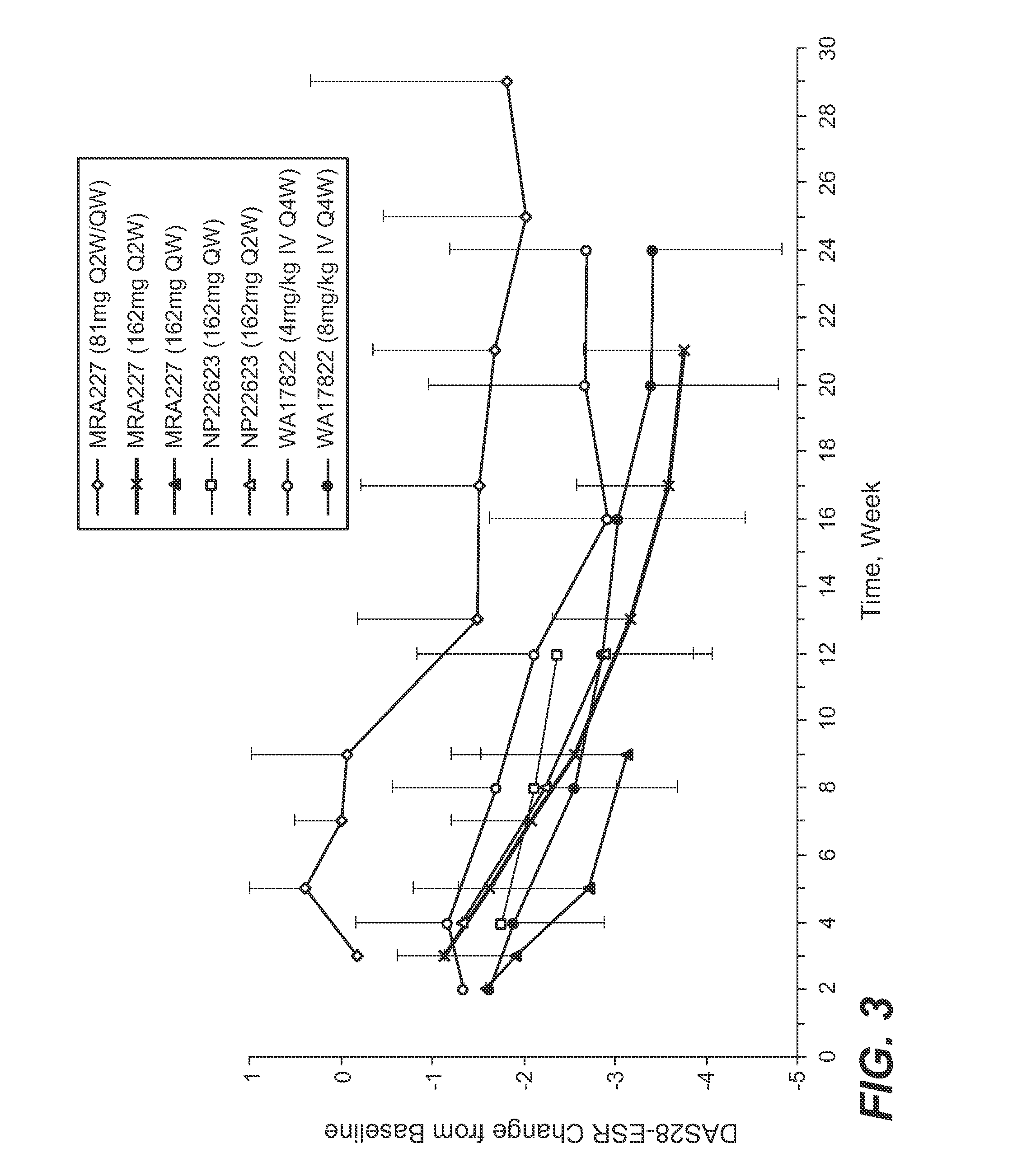
The present application discloses methods for treating an IL-6-mediated disorder such as rheumatoid arthritis (RA), juvenile idiopathic arthritis (JIA), systemic JIA (sJIA), polyarticular course JIA (pcJIA), systemic sclerosis, or giant cell arteritis (GCA), with subcutaneously administered antibody that binds interleukin-6 receptor (anti-IL-6R antibody). In particular, it relates to identification of a fixed dose of anti-IL-6R antibody, e.g. tocilizumab, which is safe and effective for subcutaneous administration in patients with IL-6-mediated disorders. In addition, formulations and devices useful for subcutaneous administration of an anti-IL-6R antibody are disclosed.
Owner:CHUGAI PHARMA CO LTD
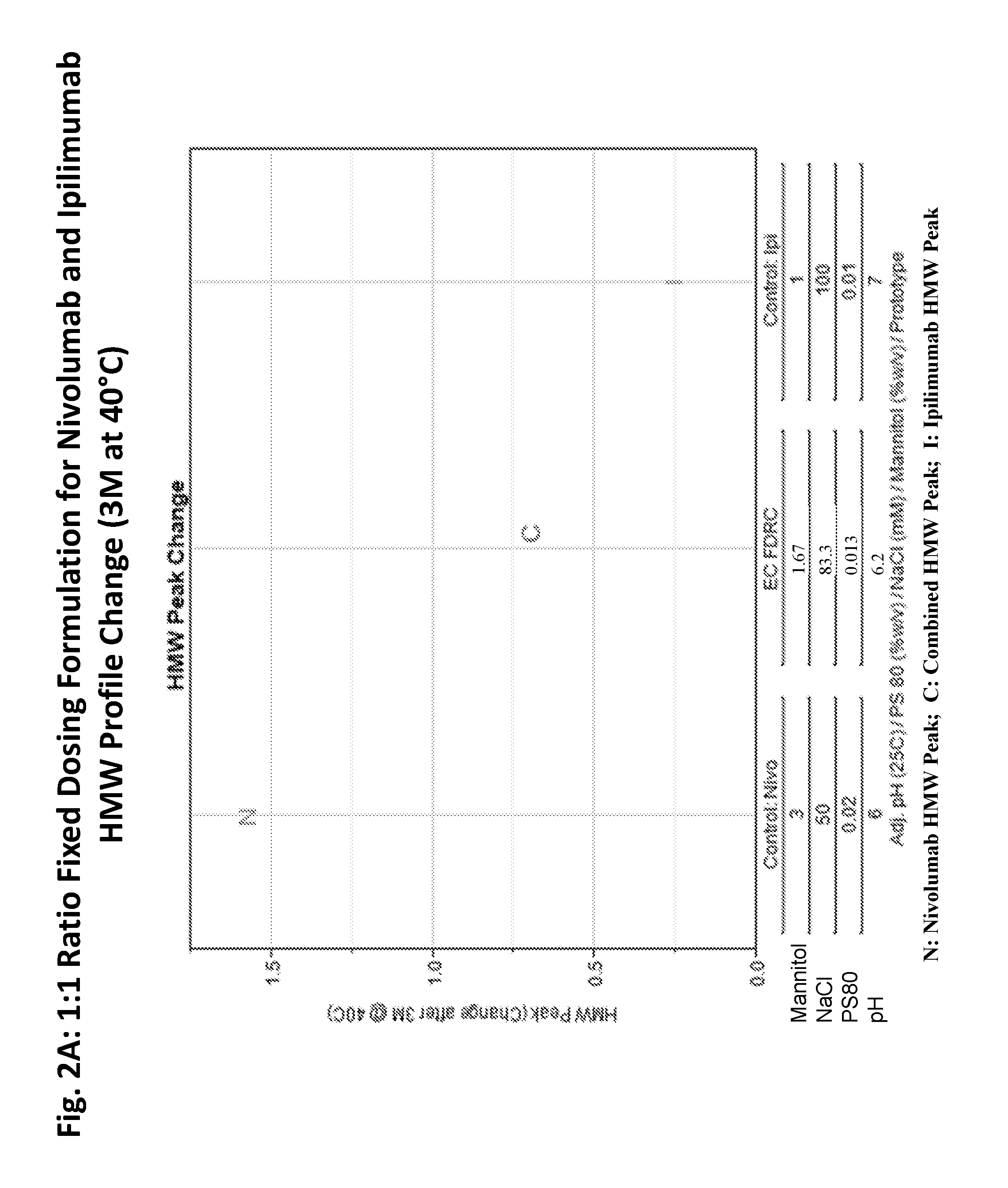
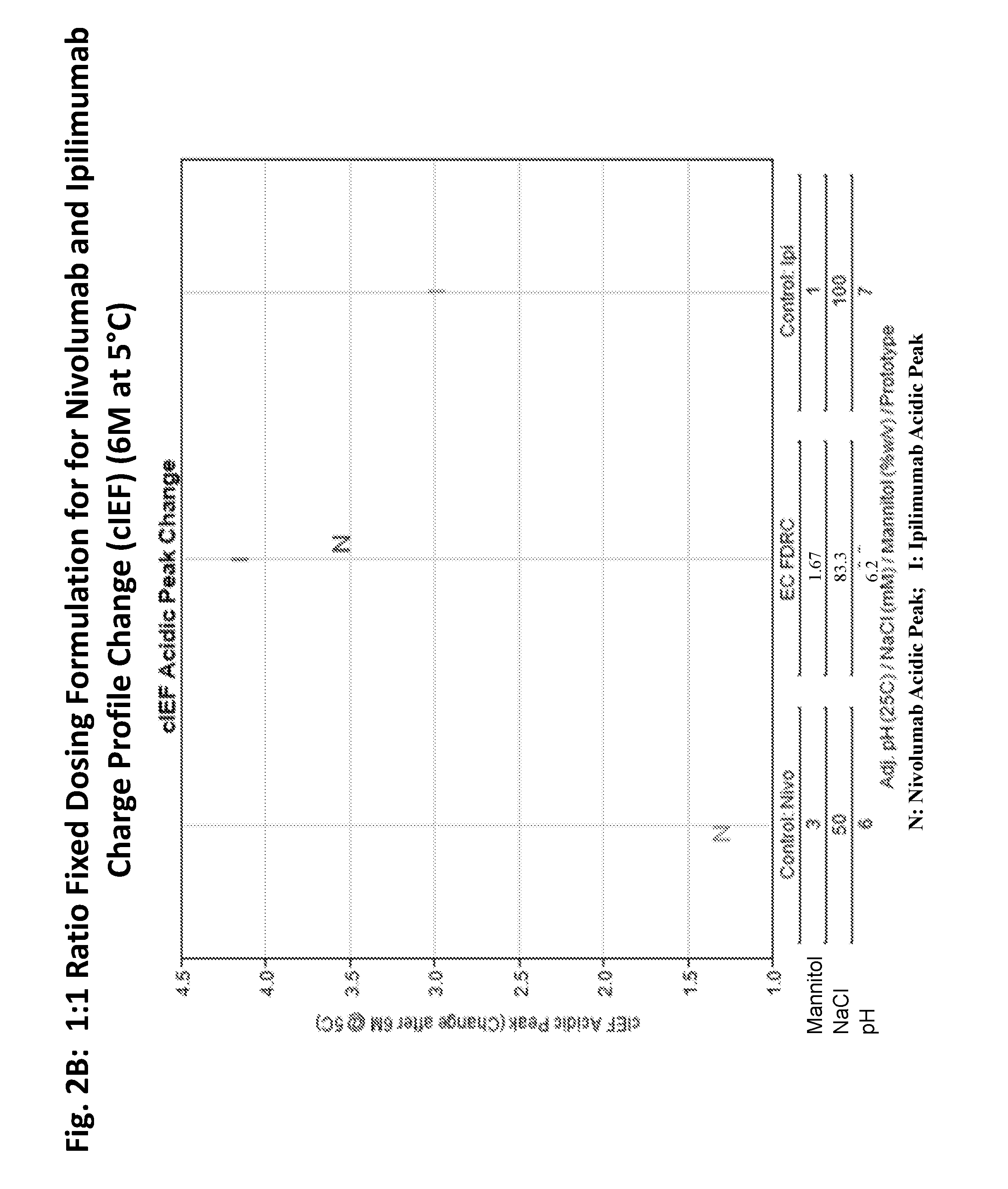
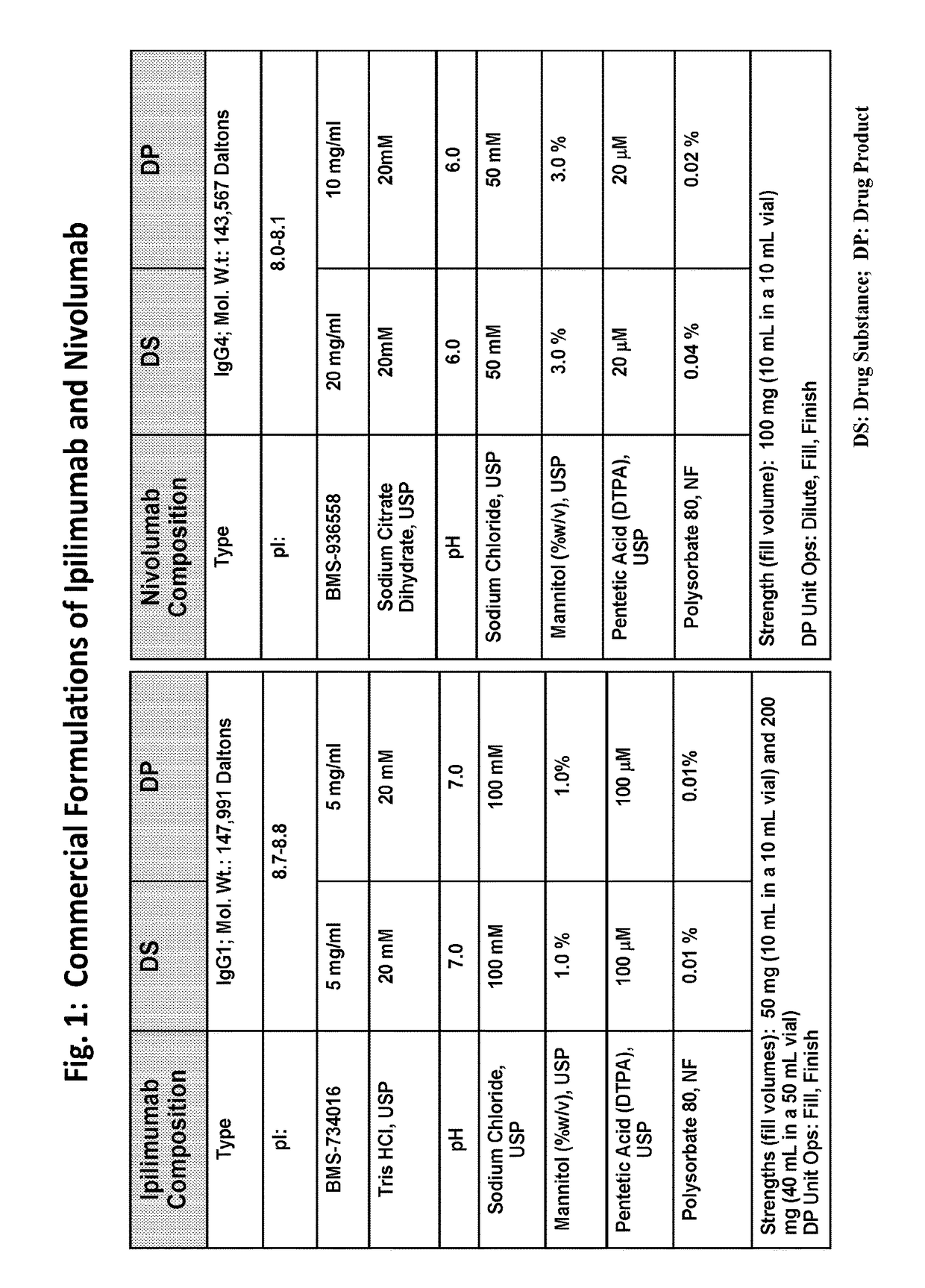
Compositions comprising a combination of an Anti-pd-1 antibody and another antibody
InactiveUS20160304607A1Change is minimalSenses disorderNervous disorderAnticarcinogenProgrammed death
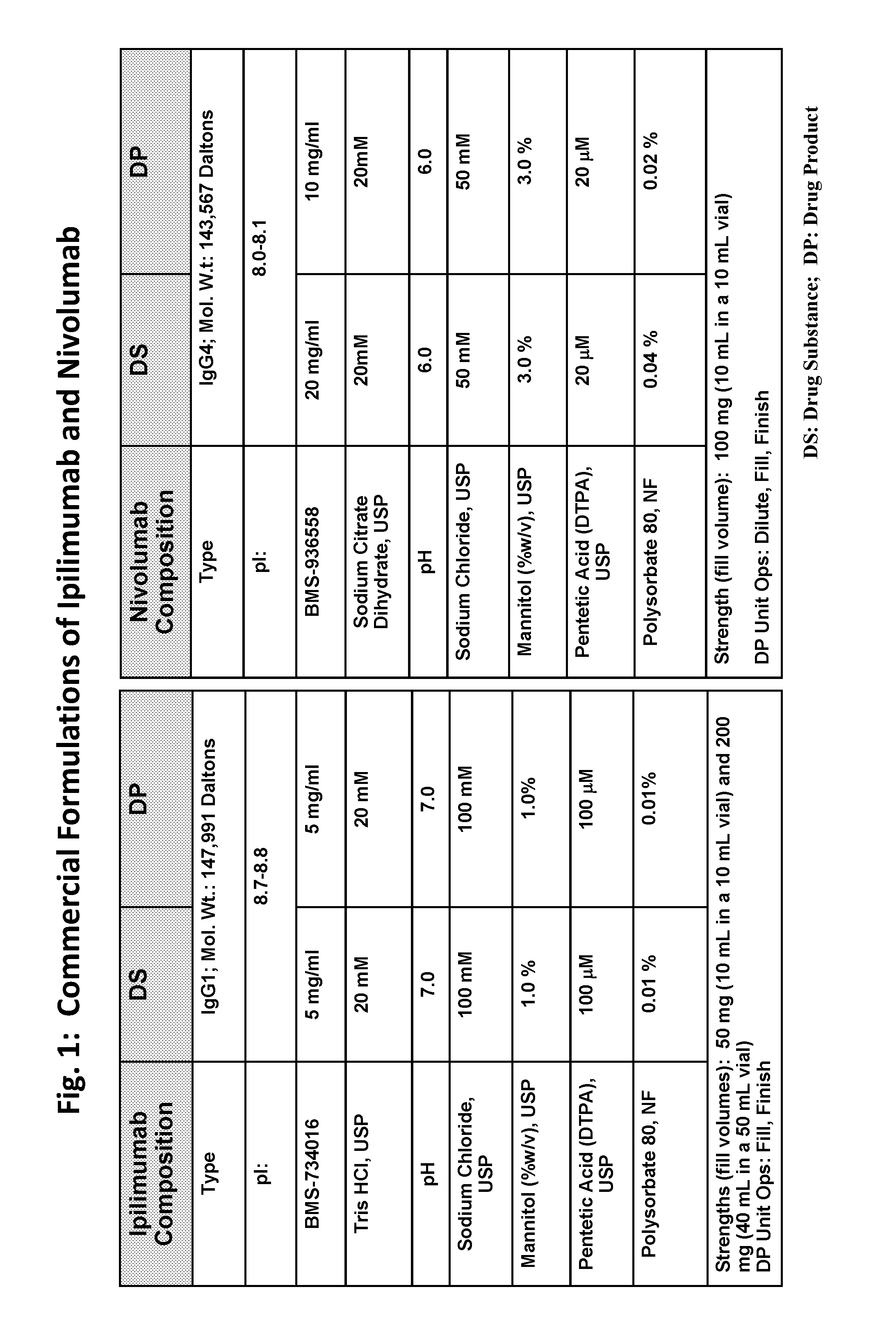
This provides pharmaceutical compositions that comprise a combination of an anti-cancer agent which is an first antibody and a second antibody. In some embodiments, the first antibody is an anti-Programmed Death-1 (PD-1) antibody. In certain embodiments, the composition is a fixed dose formulation. In certain embodiments, the composition is administered as a flat-dose. The disclosure also provides a kit for treating a subject afflicted with a disease, the kit comprising a dosage of any composition disclosed herein and instructions for using the composition in any of the disclosed methods for treating a disease.
Owner:BRISTOL MYERS SQUIBB CO
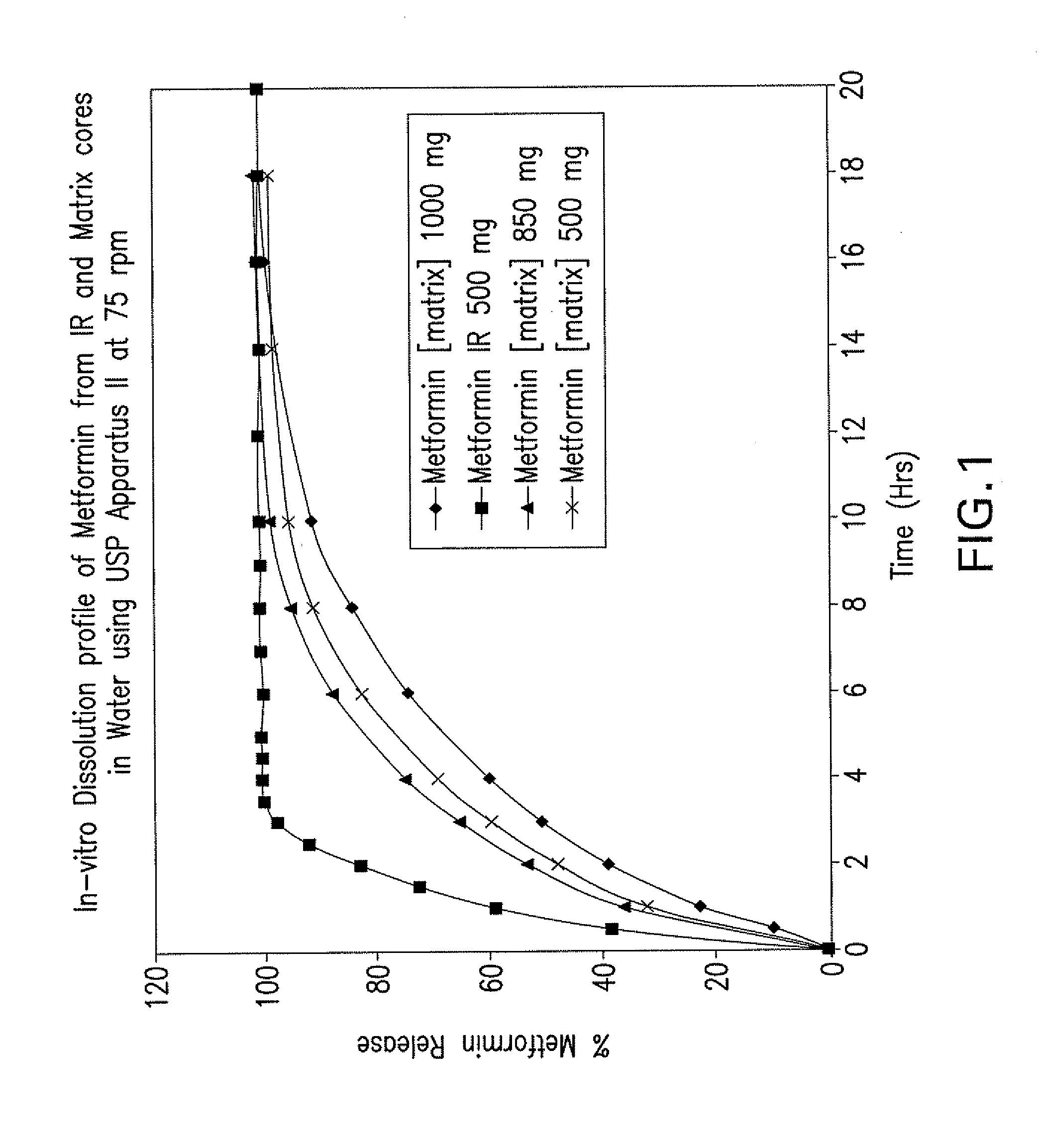
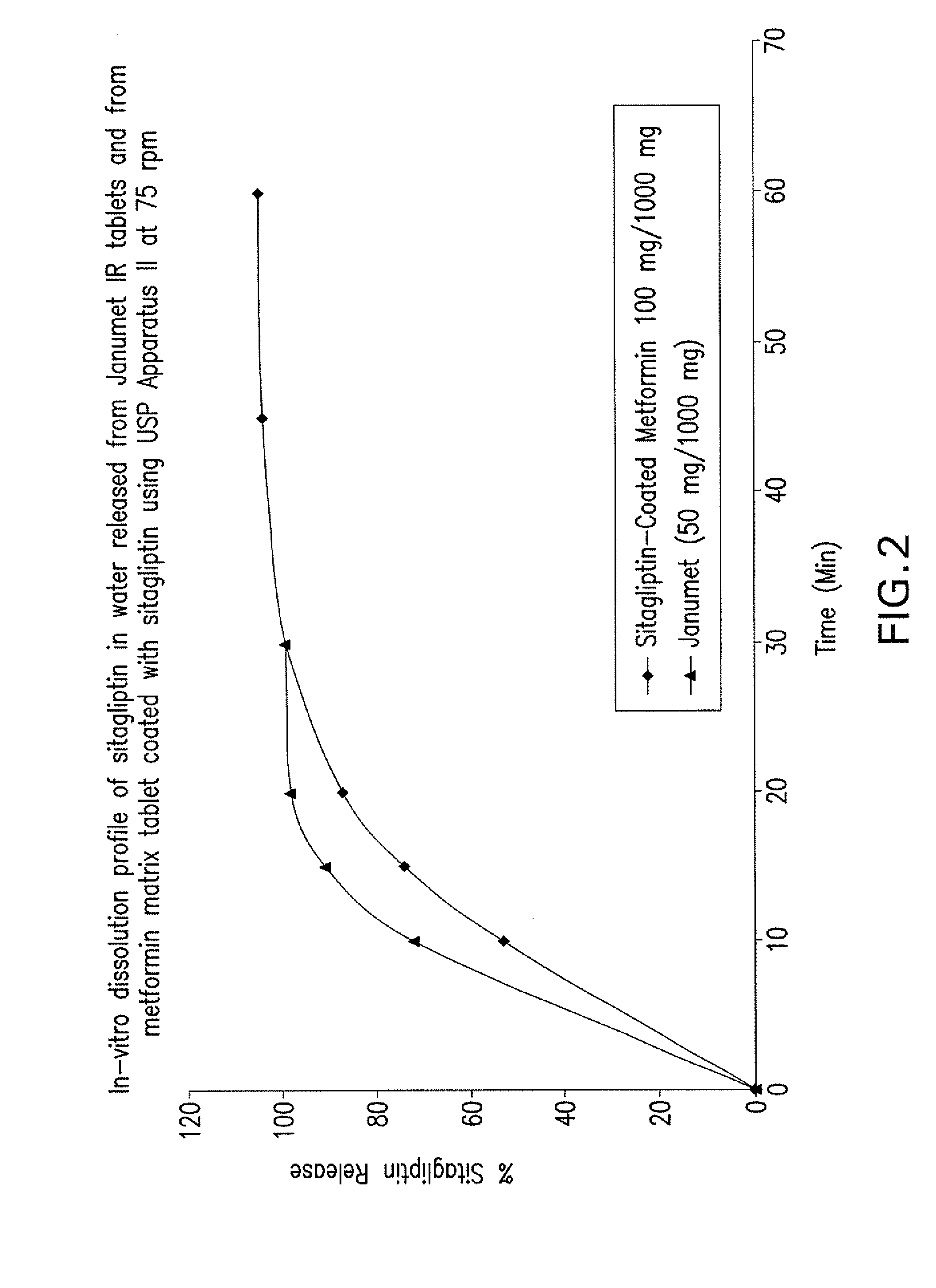
Pharmaceutical compositions of a combination of metformin and a dipeptidyl peptidase-iv inhibitor
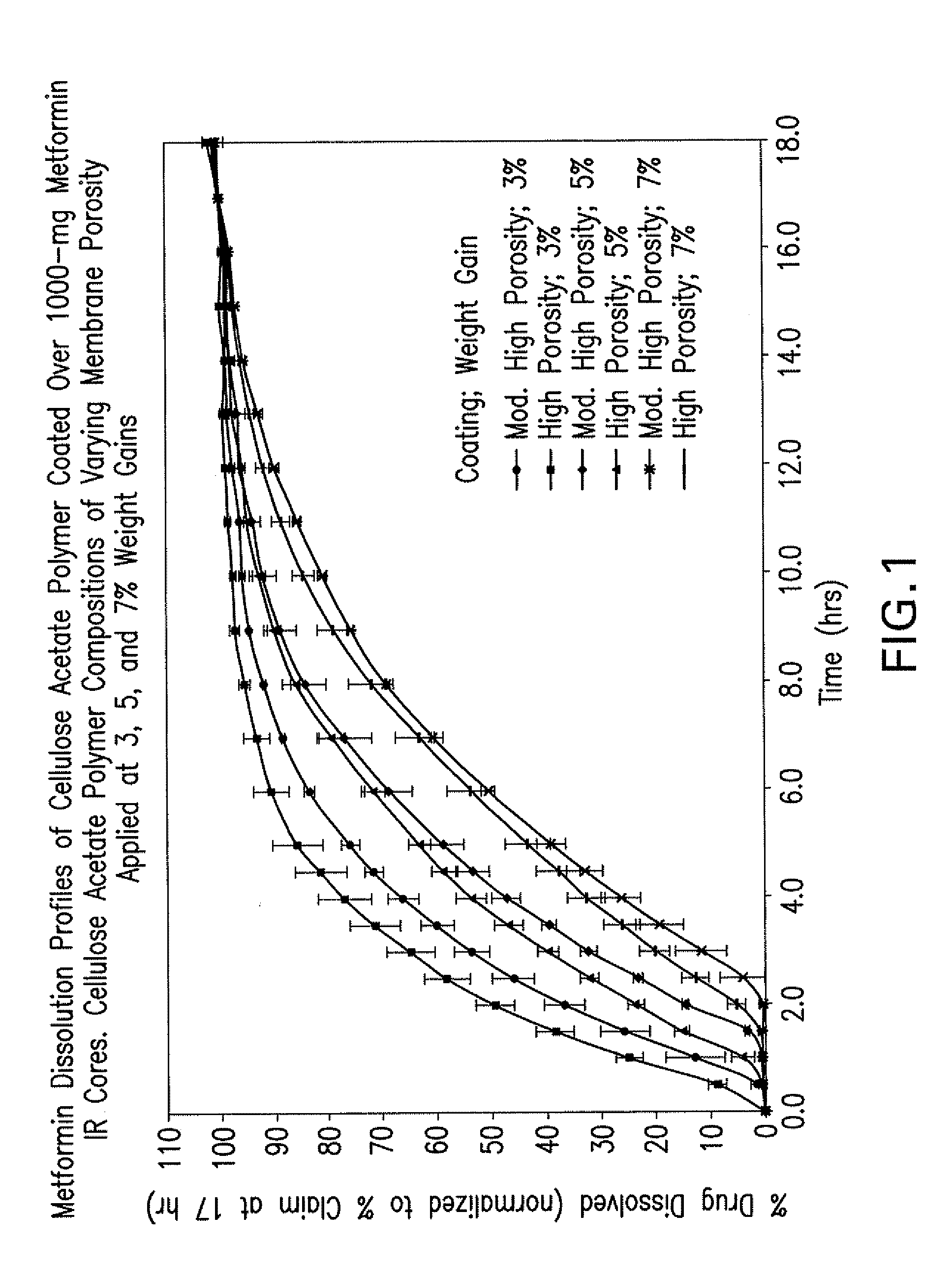
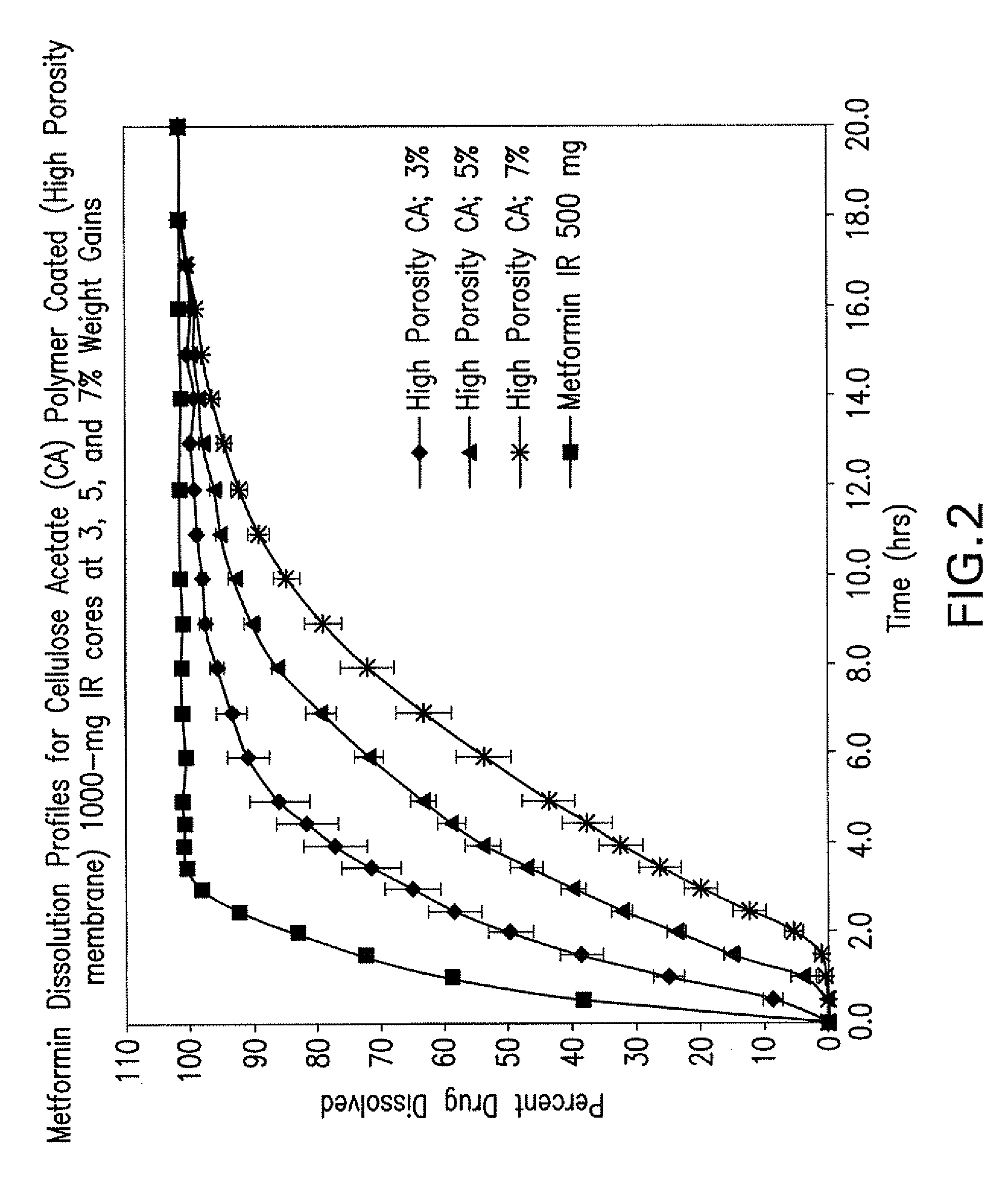
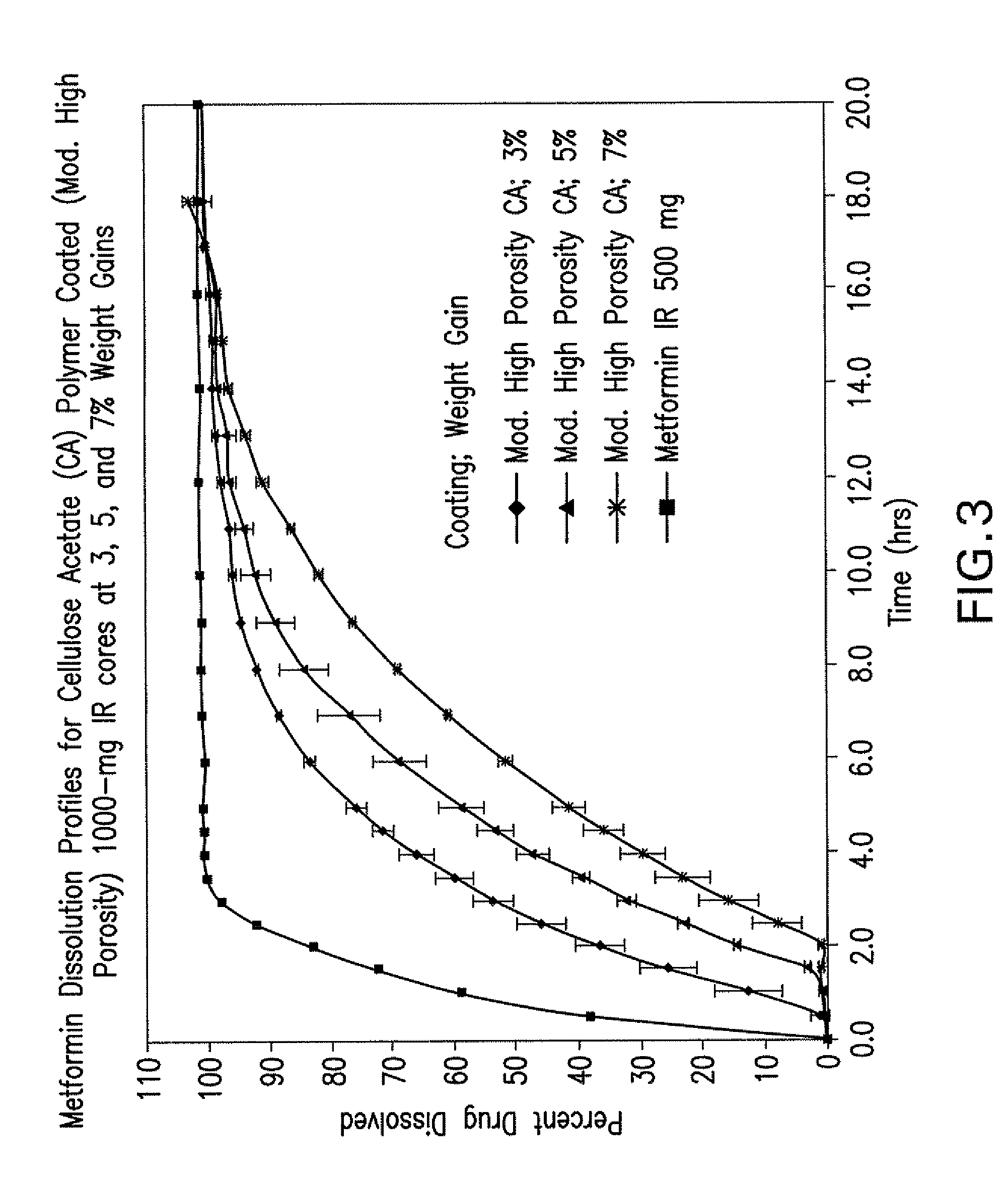
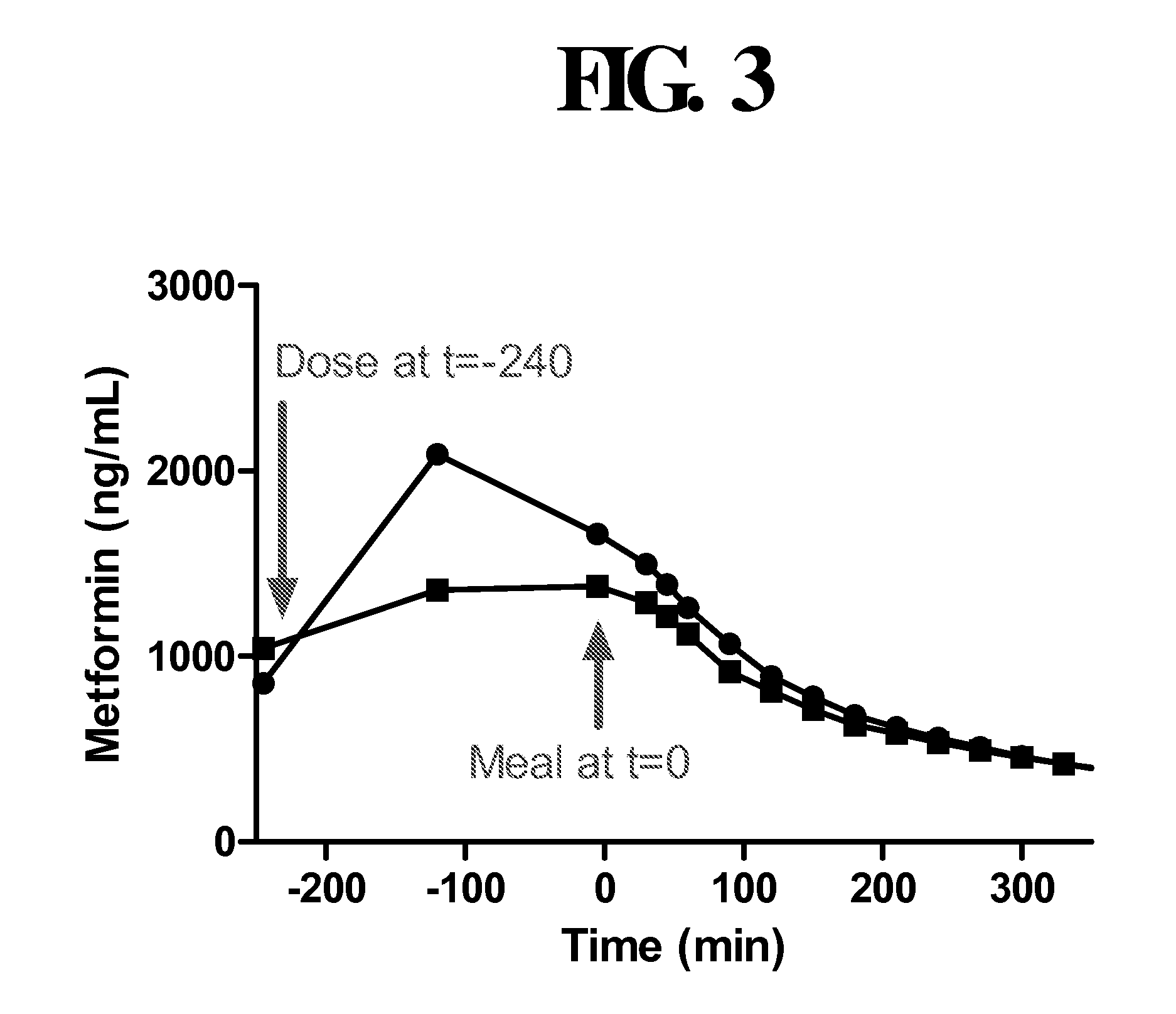
Disclosed are pharmaceutical compositions comprising fixed-dose combinations of an extended-release form of metformin, or a pharmaceutically acceptable salt thereof, coated with an immediate-release form of the DPP-4 inhibitor sitagliptin, or a pharmaceutically acceptable salt thereof.
Owner:MERCK SHARP & DOHME CORP
Pharmaceutical composition, pharmaceutical dosage form, process for their preparation, methods for treating and uses thereof
The present invention relates to pharmaceutical compositions comprising fixed dose combinations of a SGLT-2 inhibitor drug and a partner drug, processes for the preparation thereof, and their use to treat certain diseases.
Owner:BOEHRINGER INGELHEIM INT GMBH
Device for delivering liquid medicament
InactiveUS8663167B2Easy to installSpace minimizationJet injection syringesAutomatic syringesNeedle freeNeedle Free Injection
The present invention relates to a device for delivering a fixed dose or an adjustable dose of liquid medicament to a patient. Characterising for the present invention is that it is equipped with a planetary gear with the purpose of increasing the power needed to deliver liquid medicament out of this type of devices. On almost all of the devices of this type appearing on the market with the purpose of delivering a fixed dose or an adjustable dose of liquid medicament, the dose volume can be set in separated steps. With assistance of a planetary gear these steps, which each represent an increase of the volume of the medicament to be delivered, get smaller, which increases the possibility to set an optimum dose volume of the medicament. In its primary field of use the invention is intended to be used together with an injection needle. The invention facilitates to create high fluid pressure in the medicament and can be used to operate an inhaler or a needle free injector at the same time as the invention will keep a compact outside measurement.
Owner:SANOFI AVENTIS DEUT GMBH
Stable Pharmaceutical Composition Comprising a Fixed Dose Combination of Fenofibrate and an Hmg-Coa Reductase Inhibitor
InactiveUS20080131503A1Avoid interactionImprove stabilityBiocideDrug compositionsHMG-CoA reductaseAdditive ingredient
Owner:VELOXIS PHARMA
Subcutaneously administered anti-IL-6 receptor antibody
The present application discloses methods for treating an IL-6-mediated disorder such as rheumatoid arthritis (RA), juvenile idiopathic arthritis (JIA), systemic JIA (sJIA), polyarticular course JIA (pcJIA), systemic sclerosis, or giant cell arteritis (GCA), with subcutaneously administered antibody that binds interleukin-6 receptor (anti-IL-6R antibody). In particular, it relates to identification of a fixed dose of anti-IL-6R antibody, e.g. tocilizumab, which is safe and effective for subcutaneous administration in patients with IL-6-mediated disorders. In addition, formulations and devices useful for subcutaneous administration of an anti-IL-6R antibody are disclosed.
Owner:CHUGAI PHARMA CO LTD
Oral dosage combination pharmaceutical packaging
InactiveUS20090232886A1Increased riskDevelopment costAntibacterial agentsBiocidePharmaceutical packagingPharmaceutical formulation
Pharmaceutical fixed dose combination products are formed by merging a fixed dose of a first pharmaceutical formulation from primary module, with a fixed dose of a second pharmaceutical formulation from a secondary module In a preferred embodiment the first and second pharmaceutical formulations are separated from one another in a three piece capsule, a capsule-in-a-capsule or a tablet-in-a-capsule, and the primary and secondary modules are interchangeable.
Owner:SISON RAYMUNDO A
Pharmaceutical compositions of a combination of metformin and a dipeptidyl peptidase-iv inhibitor
Disclosed are pharmaceutical compositions comprising fixed-dose combinations of an extended-release form of metformin, or a pharmaceutically acceptable salt thereof, coated with an immediate-release form of the DPP-4 inhibitor sitagliptin, or a pharmaceutically acceptable salt thereof.
Owner:MERCK SHARP & DOHME CORP
Dose setting mechanism and method of setting a dose
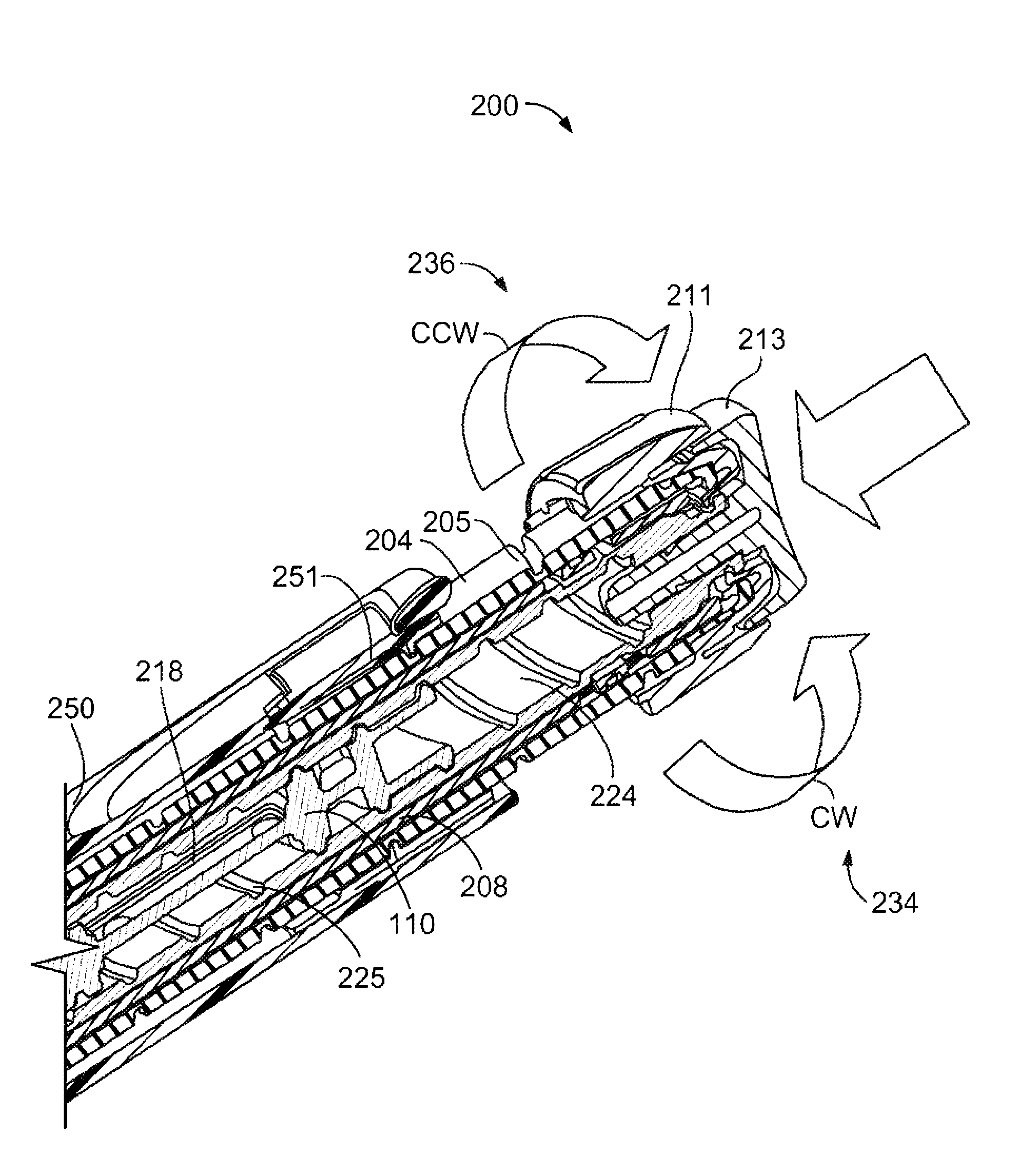
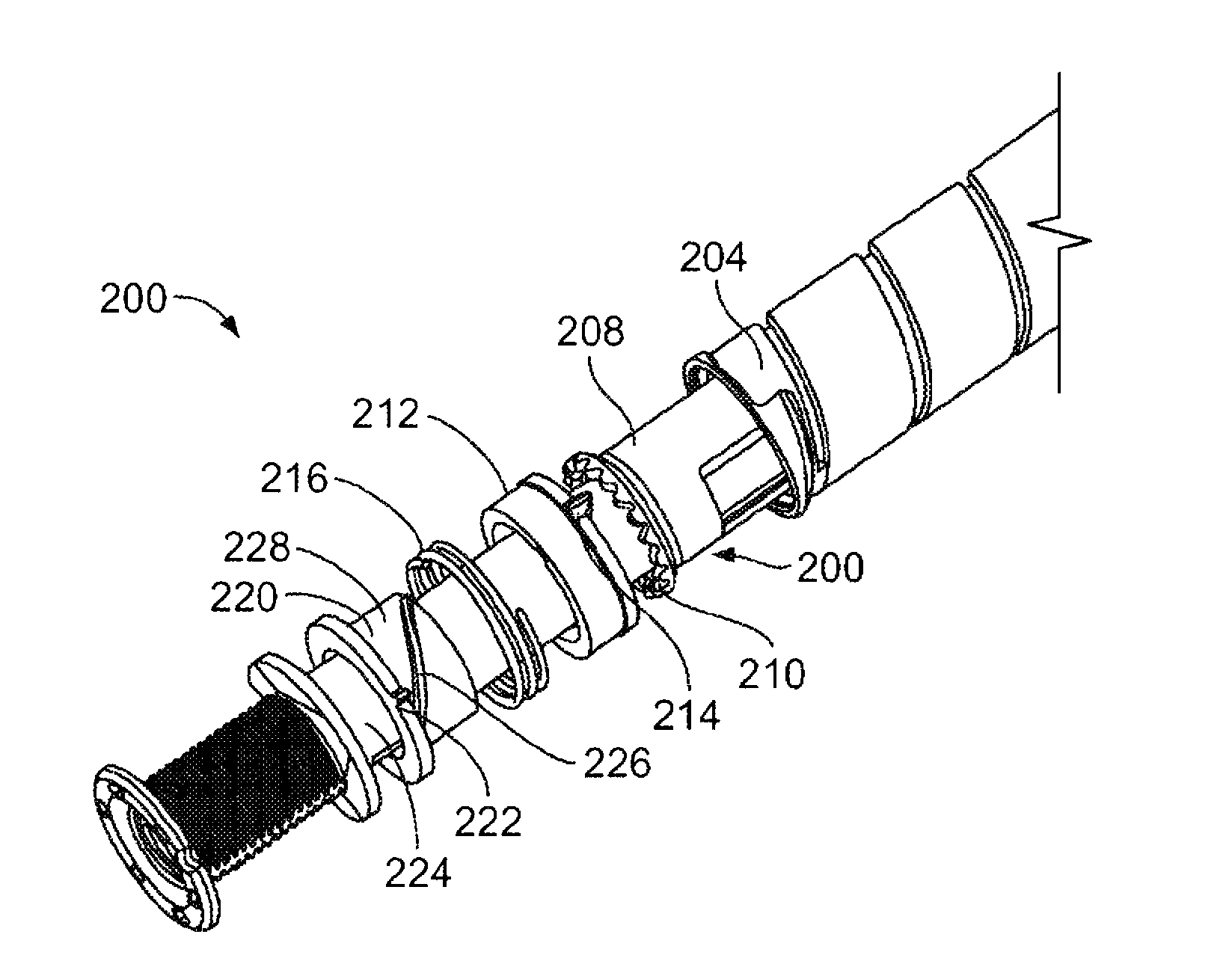
A dose setting mechanism (200) comprises a drug delivery device housing (250) and a dual state track (262) provided within the housing that is axially and rotationally fixed with respect to the housing. A dose dial component (204) is positioned in the housing and rotatable during dose setting and dose delivery. A clutch (208) is rotatable during dose setting and non-rotatable during dose delivery. A clutch plate (212) is rotationally fixed relative to the housing; and a clutch blocker (220) is in threaded engagement with the clutch plate and has a radial key (222) engaged with the dual state track. The mechanism may comprise a biasing member (216) positioned between the clutch blocker and clutch plate.
Owner:SANOFI AVENTIS DEUTSCHLAND GMBH
Compositions comprising a combination of an Anti-pd-1 antibody and another antibody
This provides pharmaceutical compositions that comprise a combination of an anti-cancer agent which is an first antibody and a second antibody. In some embodiments, the first antibody is an anti-Programmed Death-1 (PD-1) antibody. In certain embodiments, the composition is a fixed dose formulation. In certain embodiments, the composition is administered as a flat-dose. The disclosure also provides a kit for treating a subject afflicted with a disease, the kit comprising a dosage of any composition disclosed herein and instructions for using the composition in any of the disclosed methods for treating a disease.
Owner:BRISTOL MYERS SQUIBB CO
Fixed dose drug combination formulations
InactiveUS20120045505A1Stable pharmaceutical compositionReduce riskBiocideAntipyreticPharmaceutical formulationCardiovascular disorder prevention
Pharmaceutical formulations comprising multiple drugs, for treating or preventing cardiovascular disease. Embodiments are capsules containing individual drugs, or combinations of drugs, in the form of small tablets.
Owner:DR REDDYS LAB LTD +1
Subcutaneously administered Anti-il-6 receptor antibody
Owner:CHUGAI PHARMA CO LTD +1
Pharmaceutical Compositions of Combinations of Dipeptidyl Peptidase-4 Inhibitors With Metformin
Disclosed are pharmaceutical compositions comprising fixed-dose combinations of a dipeptidyl peptidase-4 inhibitor and metformin, methods of preparing such pharmaceutical compositions, and methods of treating Type 2 diabetes with such pharmaceutical compositions.
Owner:MERCK SHARP & DOHME LLC
Subcutaneously administered Anti-il-6 receptor antibody
The present application discloses methods for treating an IL-6-mediated disorder such as rheumatoid arthritis (RA), juvenile idiopathic arthritis (JIA), systemic JIA (sJIA), polyarticular course JIA (pcJIA), systemic sclerosis, or giant cell arteritis (GCA), with subcutaneously administered antibody that binds interleukin-6 receptor (anti-IL-6R antibody). In particular, it relates to identification of a fixed dose of anti-IL-6R antibody, e.g. tocilizumab, which is safe and effective for subcutaneous administration in patients with IL-6-mediated disorders. In addition, formulations and devices useful for subcutaneous administration of an anti-IL-6R antibody are disclosed.
Owner:F HOFFMANN LA ROCHE INC +1
Pharmaceutical compositions of combinations of dipeptidyl peptidase-4 inhibitors with metformin
ActiveCN101365432APeptide/protein ingredientsMetabolism disorderDipeptidyl peptidaseDipeptidyl peptidase-4 inhibitor
Disclosed are pharmaceutical compositions comprising fixed-dose combinations of a dipeptidyl peptidase-4 inhibitor and metformin, methods of preparing such pharmaceutical compositions, and methods of treating Type 2 diabetes with such pharmaceutical compositions.
Owner:MERCK SHARP & DOHME BV
Pharmaceutical formulations comprising nsaid and proton pump inhibitor drugs
InactiveUS20100305163A1Increased riskAvoid erosionBiocideAntipyreticTherapeutic intentPharmaceutical formulation
Aspects of the invention relate to pharmaceutical formulations comprising an NSAID and acid reducer drug for therapeutic purposes, and methods of preparing the same. Further aspects of the invention relate to fixed dose pharmaceutical formulations comprising naproxen, or pharmaceutically acceptable salts thereof, and esomeprazole, or pharmaceutically acceptable salts thereof.
Owner:DR REDDYS LAB LTD +1
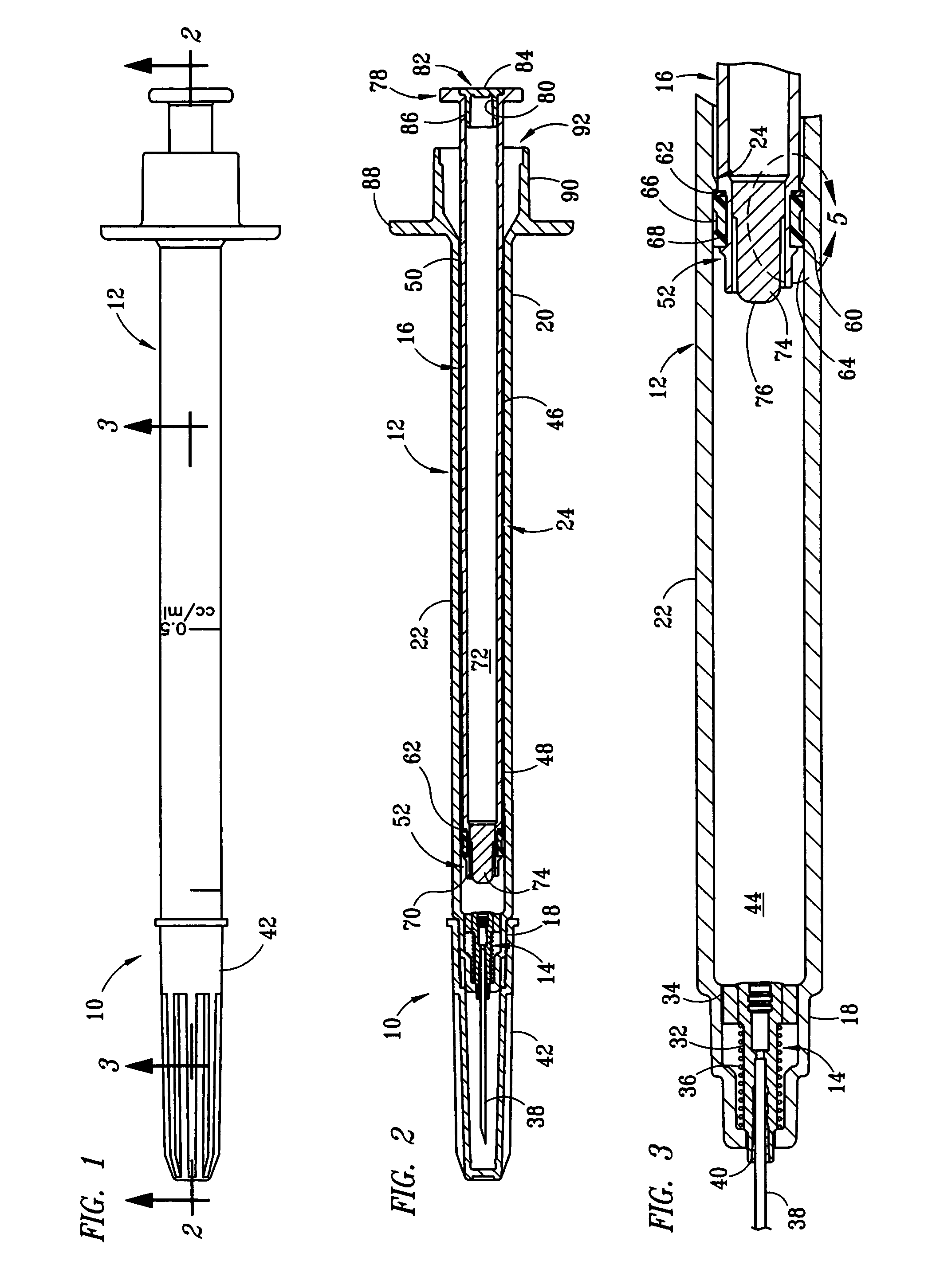
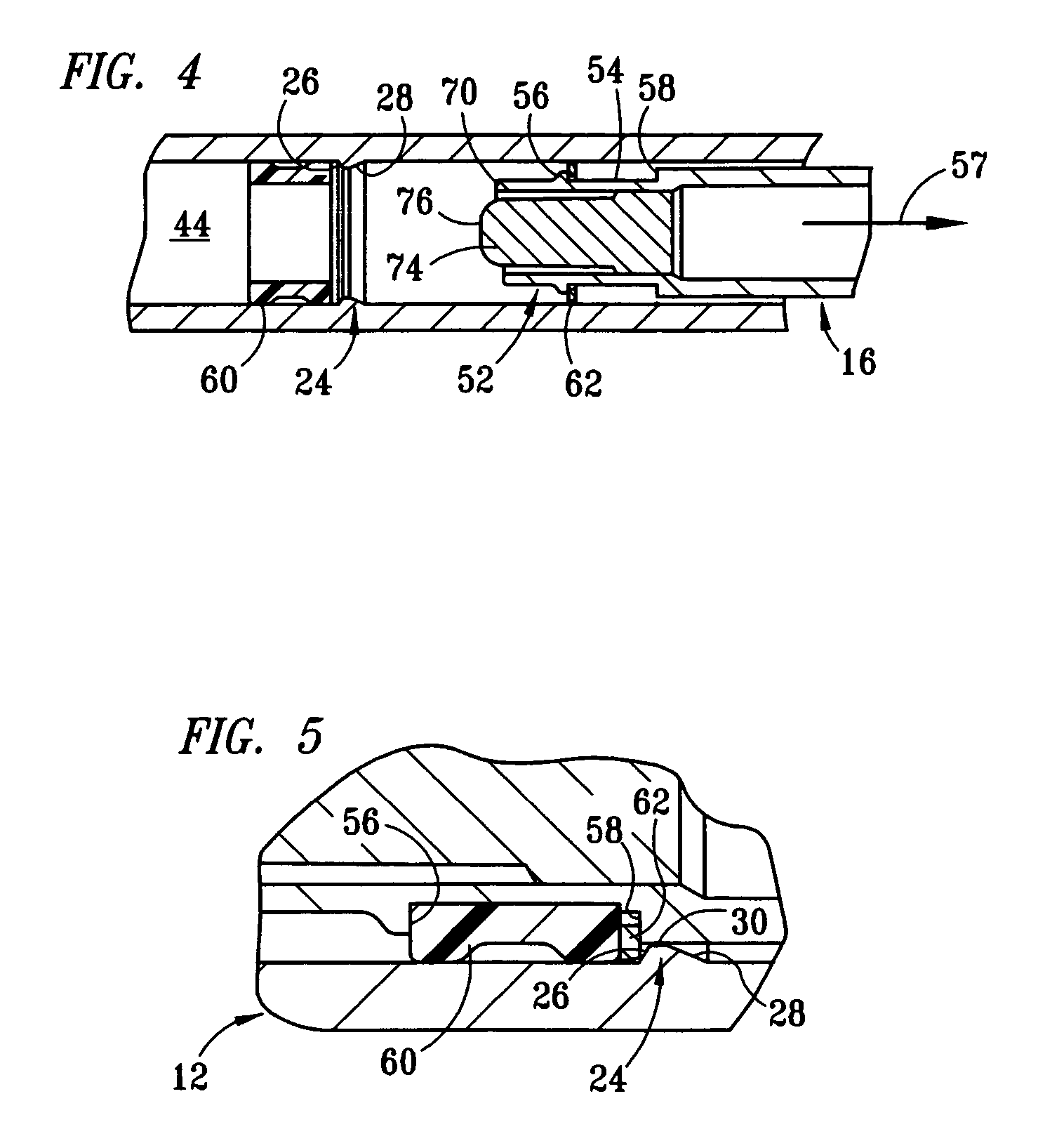
Fixed-dose syringe with limited aspiration
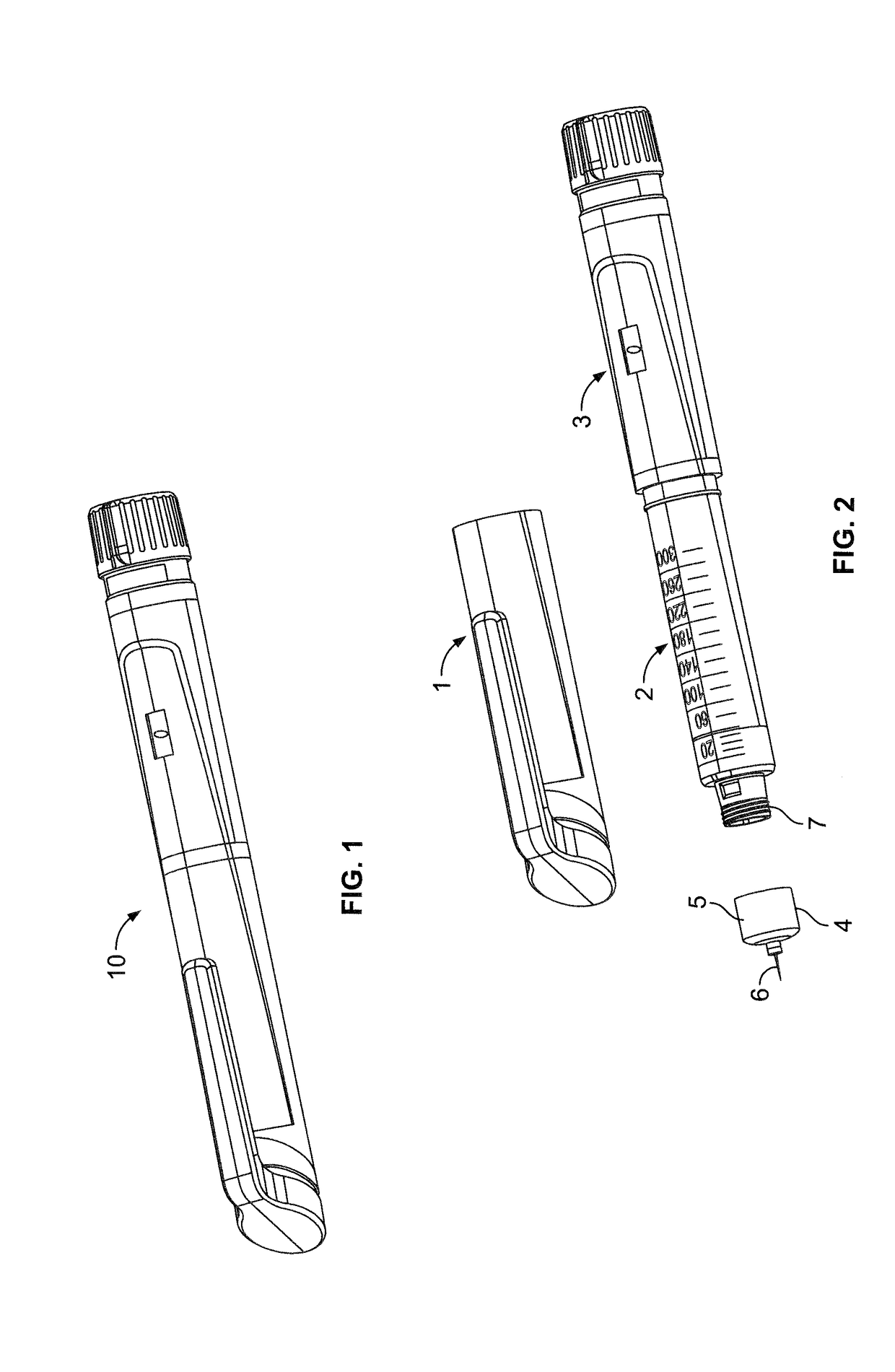
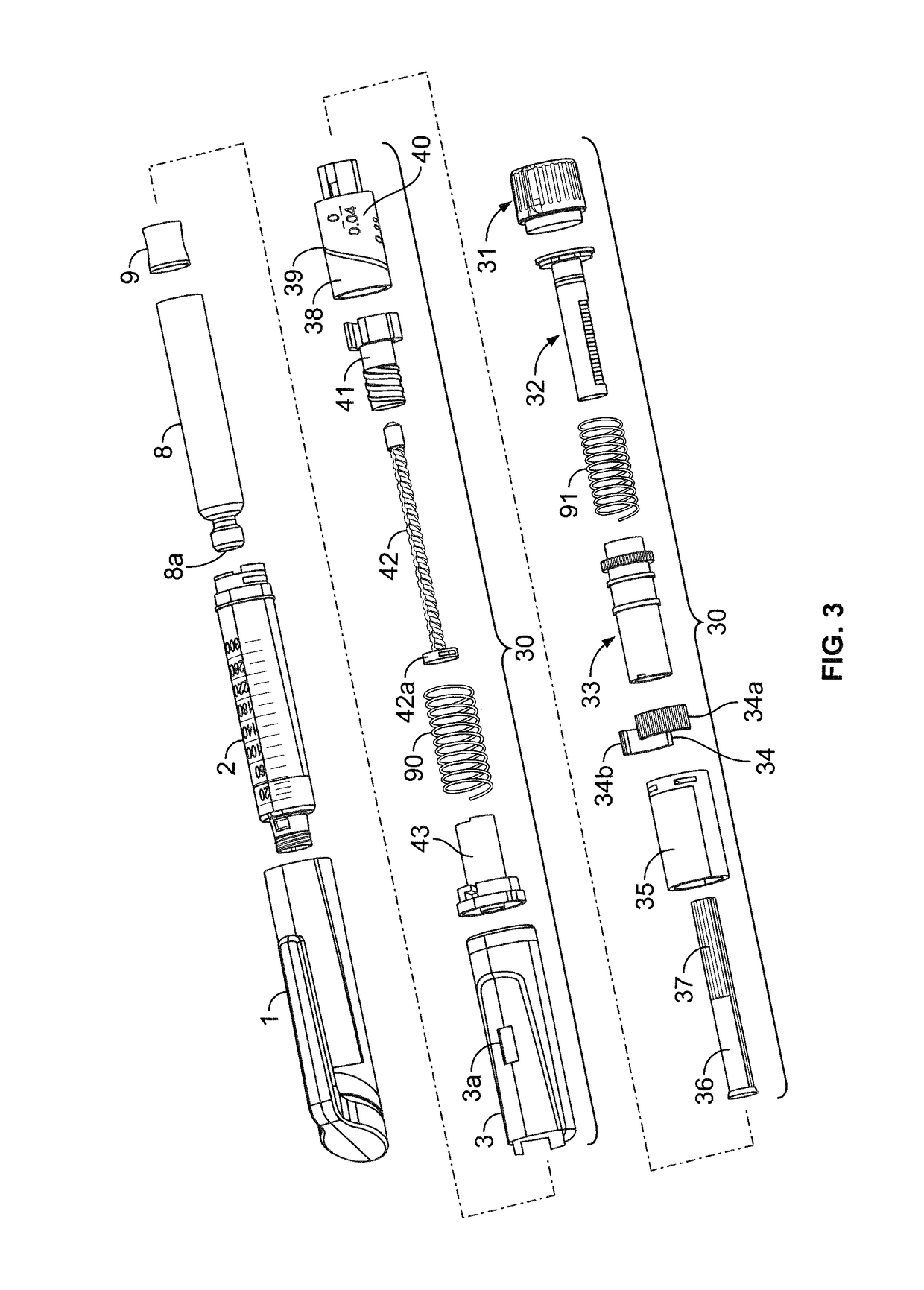
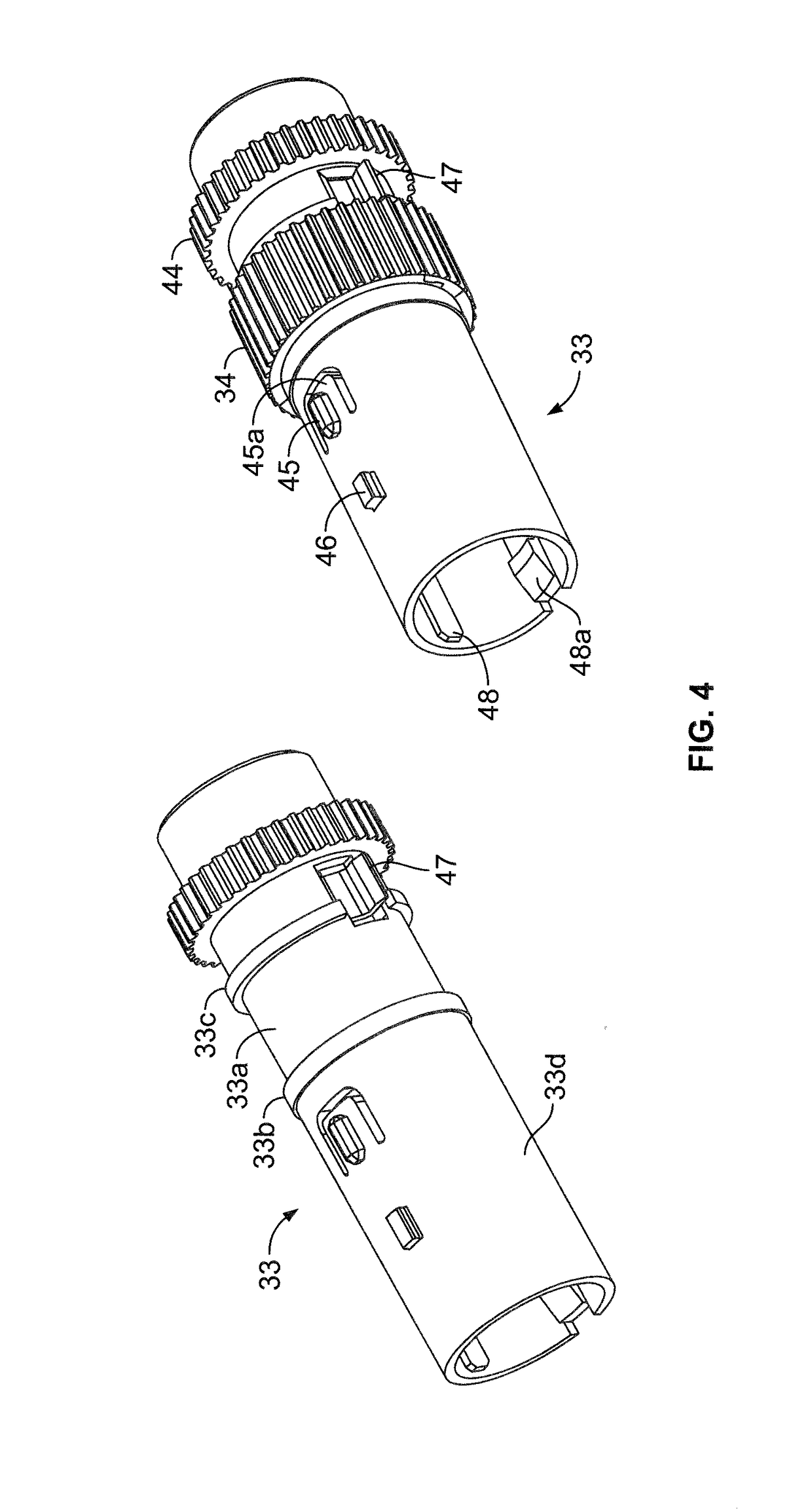
InactiveUS20060084919A1Prevent reuseReduce the possibilityMedical devicesIntravenous devicesEngineeringSyringe needle
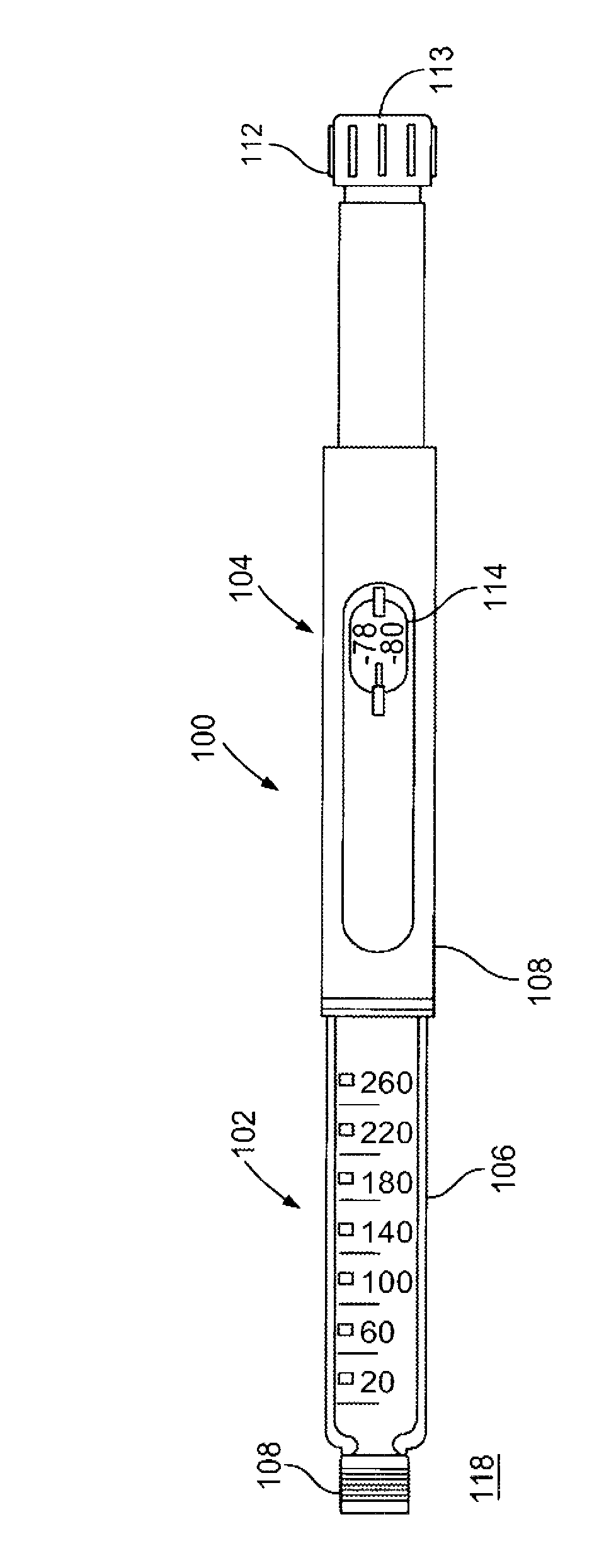
A syringe configured with a limited maximum usable capacity. The syringe of the invention desirably has a retractable needle to prevent reuse. In the preferred embodiment, a dose-limiting structure includes a stop-ring member on the head of the plunger that abuts a constriction in the housing when the plunger is moved away from the needle to prevent the further rearward movement of the plunger. Preferably, the syringe of the invention is configured such that a user is tactilely signaled when the plunger has reached a position corresponding to a nominal fixed-dose. If the user attempts to force the stop-ring member beyond the constriction, the plunger seal is stripped off or removed from the plunger head and the syringe rendered inoperable. The features of the invention can also be applied to a nonretracting syringe.
Owner:RETRACTABLE TECH INC
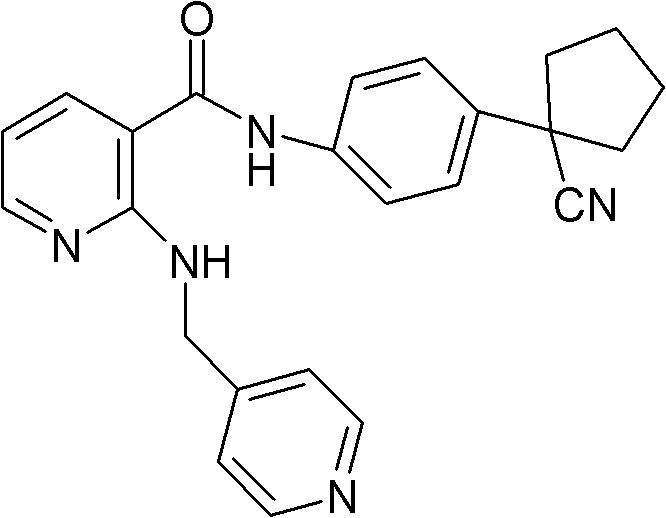
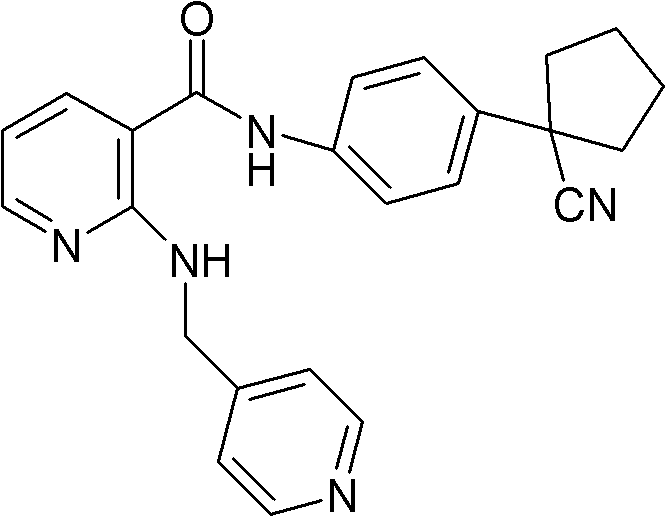
Drug composition for curing tumour diseases
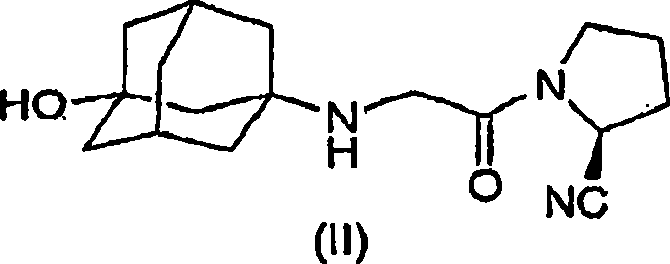
The invention discloses a drug composition for curing tumour diseases, which comprises fixed dose of N-(4-(1-cyanocyclopentyl) phenyl)-2-(4-picolyl) amino-3-pyridinecarboxamide or pharmaceutically acceptable salt of the N-(4-(1-cyanocyclopentyl) phenyl)-2-(4-picolyl) amino-3-pyridinecarboxamide and erlotinib or pharmaceutically acceptable salt of the erlotinib or comprises fixed dose of N-(4-(1-cyanocyclopentyl) phenyl)-2-(4-picolyl) amino-3-pyridinecarboxamide or pharmaceutically acceptable salt of the N-(4-(1-cyanocyclopentyl) phenyl)-2-(4-picolyl) amino-3-pyridinecarboxamide and gefitinib or pharmaceutically acceptable salt of the gefitinib. A method for preparing the drug composition and an application of the drug composition in preparing drugs for curing tumour diseases are further provided.
Owner:JIANGSU HENGRUI MEDICINE CO LTD
Injection device with flexible dose selection
ActiveUS20190015595A1Easily and efficiently designPrevent rotationAmpoule syringesMedical devicesFlexible dosingDose delivery
An injection device incorporating a dose setting mechanism is presented where the dose setting mechanism contains a dose selector having one or more dose stops corresponding to a finite set of predetermined fixed doses, where the set of finite predetermined fixed doses includes a lowest fixed dose and one or more higher fixed doses, and where at least one of the one or more higher fixed doses is equal to the lowest fixed dose plus a fractional amount of the lowest fixed dose. The dose setting mechanism can further include a floating spline that is rotationally engaged with a snap element such that the snap element can rotate relative to the floating spline during both dose setting and dose delivery.
Owner:MEDMIX SWITZERLAND AG
Compositions Comprising Statins, Biguanides and Further Agents for Reducing Cardiometabolic Risk
ActiveUS20150196509A1Reduced gastrointestinal complicationReduce riskBiocideOrganic chemistryVascular diseaseActive agent
Compositions and methods comprising at least one biguanide compound and at least one statin combined with at least one additional active agent in fixed dose combinations are provided for reducing cardiometabolic risk, and for the treatment of cardiovascular disease, wherein the biguanide compound is formulated for delayed release.
Owner:ANJI PHARMA INC
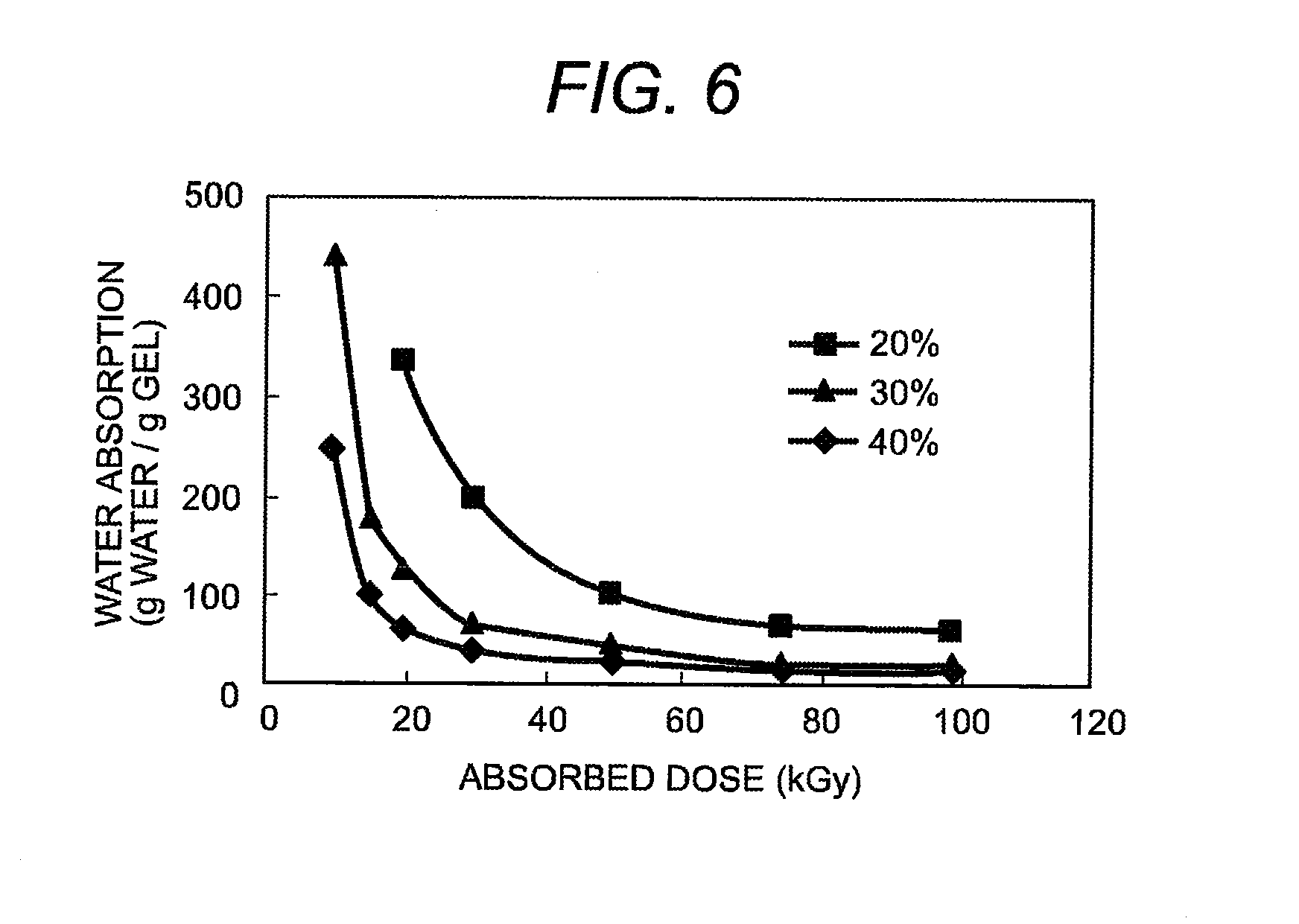
Method of manufacturing gel using polysaccharides as raw materials
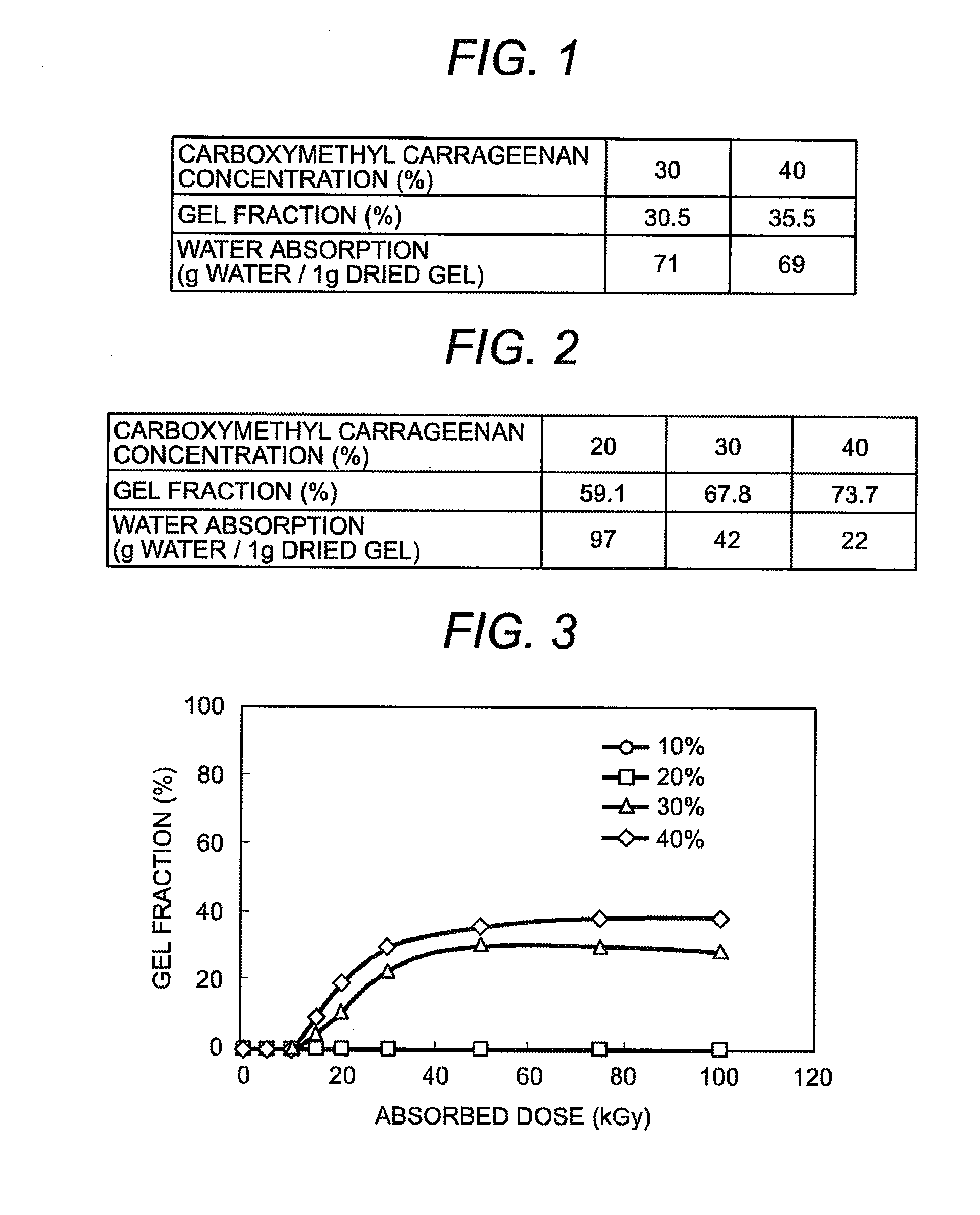
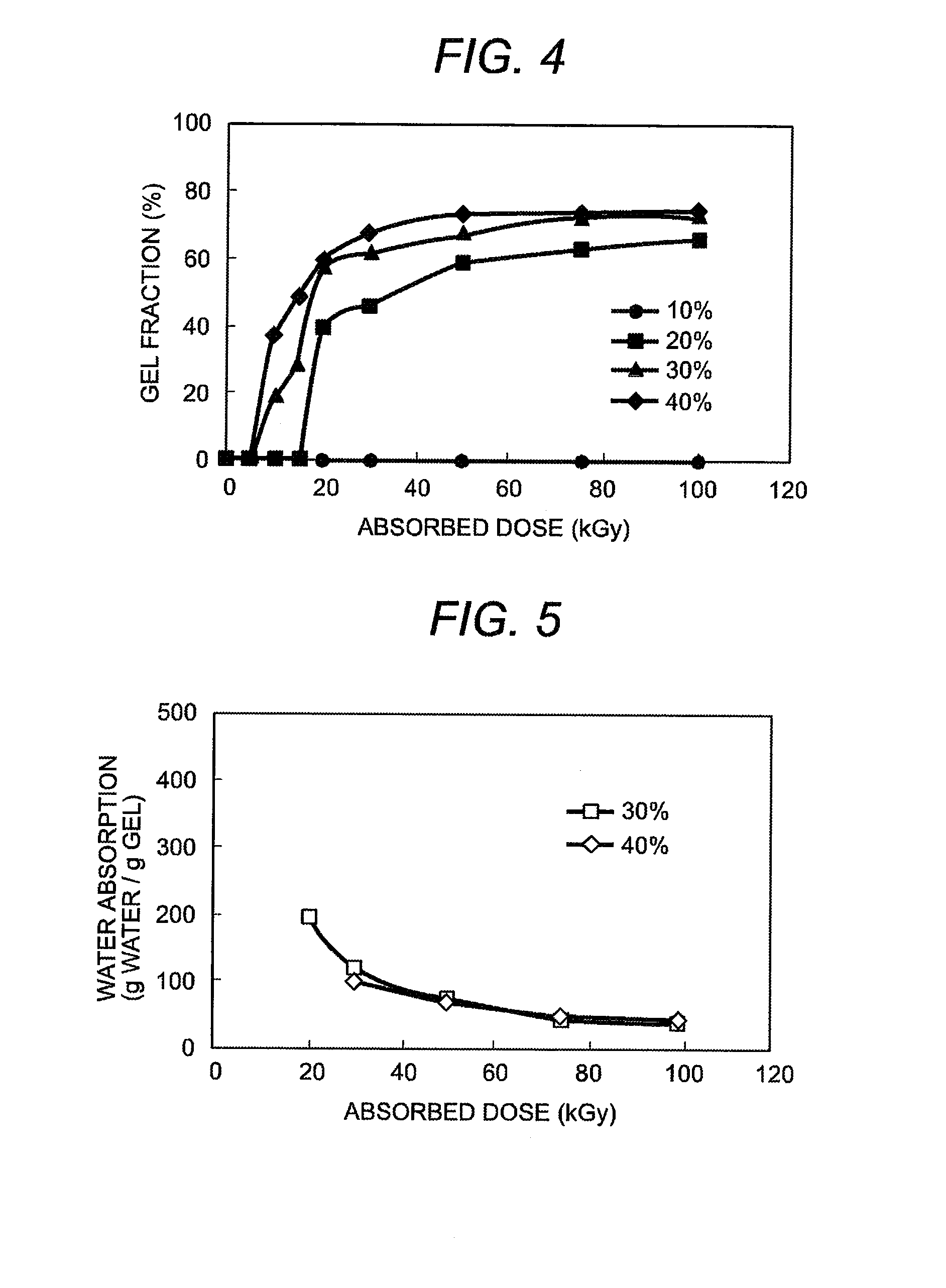
InactiveUS20080139796A1Increase in carbon dioxideEnvironmental load increaseSugar derivativesSugar derivatives preparationCarrageenanHeat resistance
After adding water to the carboxymethyl carrageenan which is raw material and mixing them well, the ionizing radiation of more than fixed dose is irradiated to the obtained paste sample of fixed concentration. As a result, an excellent hydrogel in heat resistance which does not dissolve at 50° C. or more can be obtained. The manufactured gel can be used for many kinds of products. Because this gel has a biodegradation characteristic, it is possible to dispose by composting.
Owner:JAPAN ATOMIC ENERGY AGENCY INDEPENDANT ADMINISTRATIVE CORP
Levodopa/carbidopa/entacapone pharmaceutical preparation
The invention relates to an oral solid fixed dose composition comprising pharmacologically effective amounts of entacapone, levodopa, and carbidopa, or a pharmaceutically acceptable salt or hydrate thereof, and comprising at least one pharmaceutically acceptable excipient. The composition of the invention can be used e.g. for the treatment of Parkinson's disease.
Owner:ORION CORPORATION
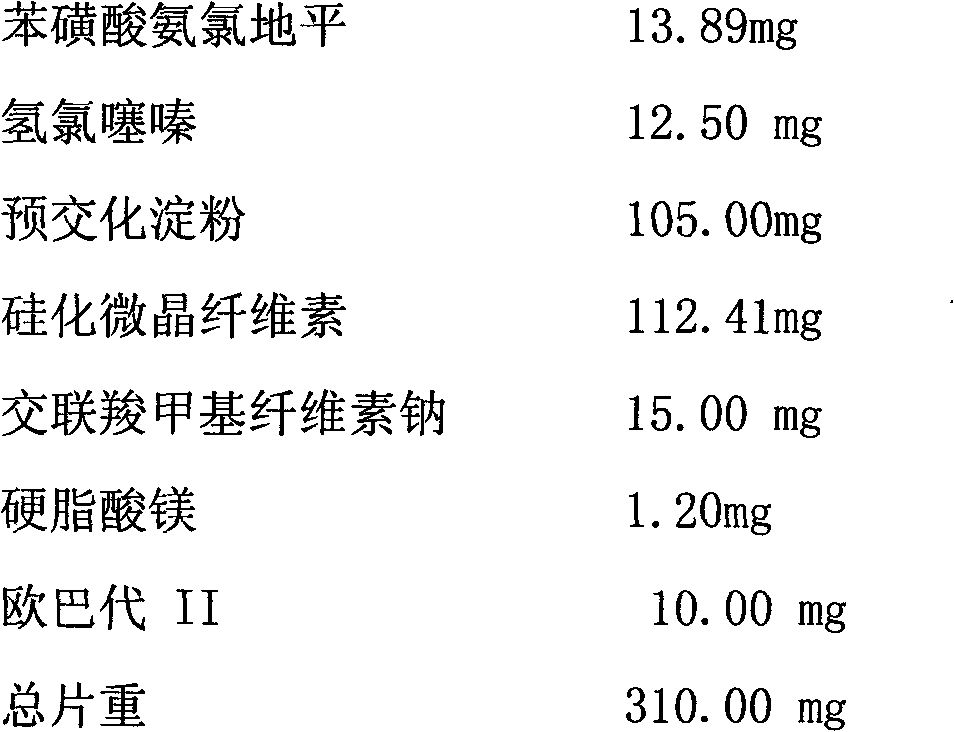
Medicament compound preparation formed by mixing olmesartan medoxomil with benzene sulfonic acid amlodipine and hydrochlorothiazide
The invention relates to a medicament compound preparation formed by mixing olmesartan medoxomil with benzene sulfonic acid amlodipine and hydrochlorothiazide. Both the olmesartan medoxomil and amlodipine are unstable compounds, and are difficultly prepared into mixed medicaments with stable active ingredients. According to the invention, the adjuvant of the olmesartan medoxomil and amlodipine compound preparation is regulated, so that the amounts of degradation products and impurities of the olmesartan medoxomil are effectively reduced. The invention provides a method for preparing the fixed-dose compound preparation which takes the olmesartan medoxomil, the amlodipine and hydrochlorothiazide as the active ingredients.
Owner:ZHUHAI EBANG PHARMA
Subcutaneous her2 antibody formulations
ActiveUS20180296470A1Good dispersionOrganic active ingredientsPeptide/protein ingredientsCancer therapyPertuzumab
Fixed dose HER2 antibody formulations for subcutaneous administration are provided along with their use in the treatment of cancer. The formulations include fixed dose subcutaneous formulations of pertuzumab and subcutaneous co-formulations of pertuzumab and trastuzumab, and their use in the treatment of cancer.
Owner:F HOFFMANN LA ROCHE & CO AG
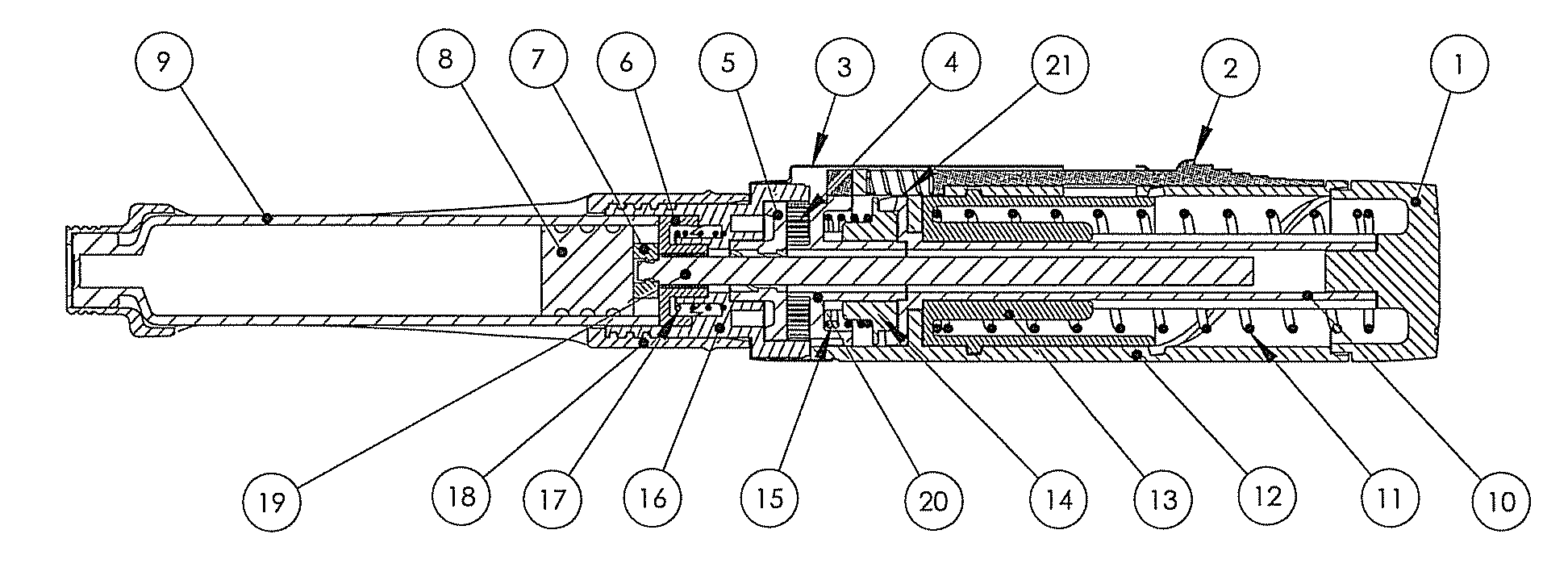
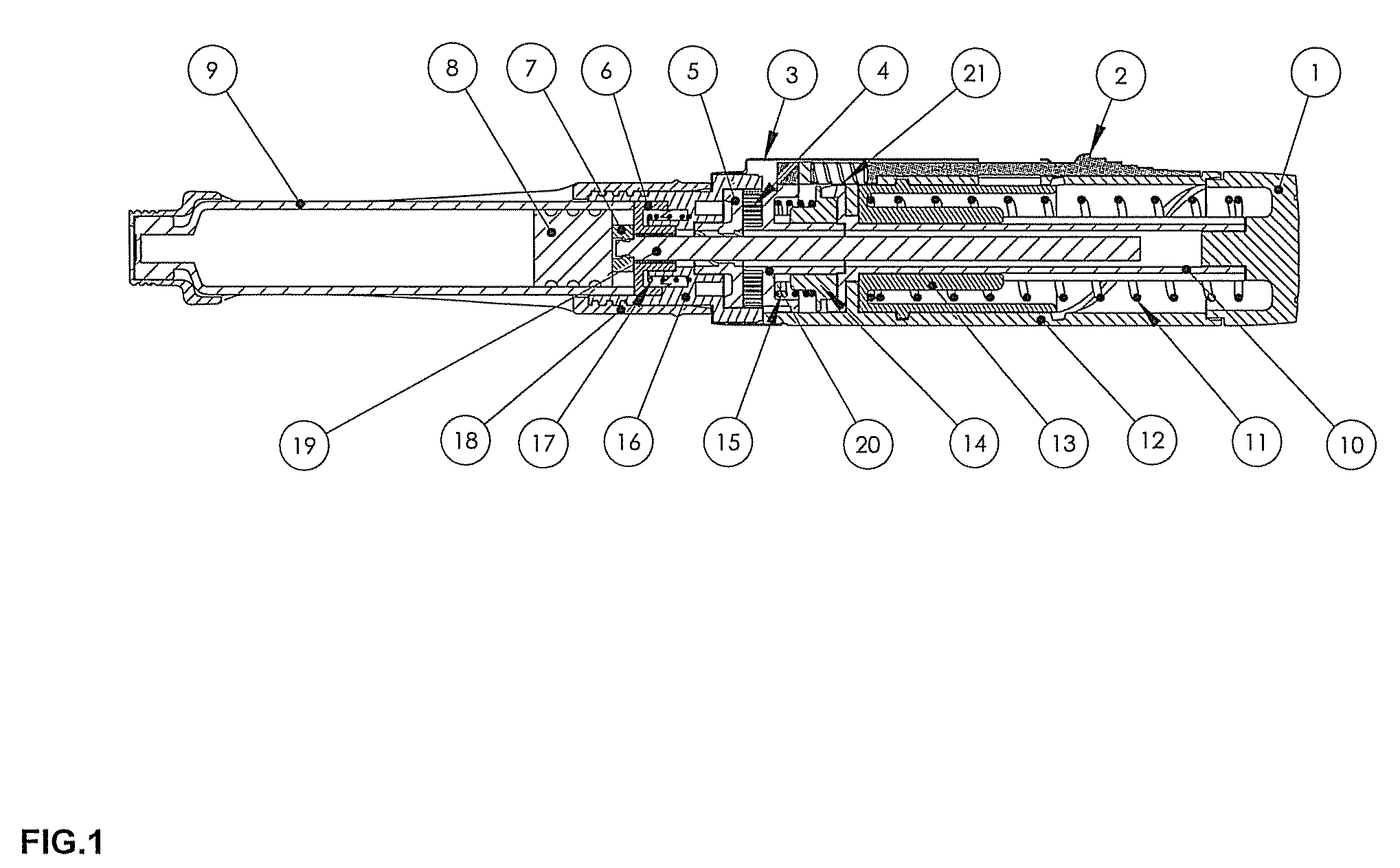
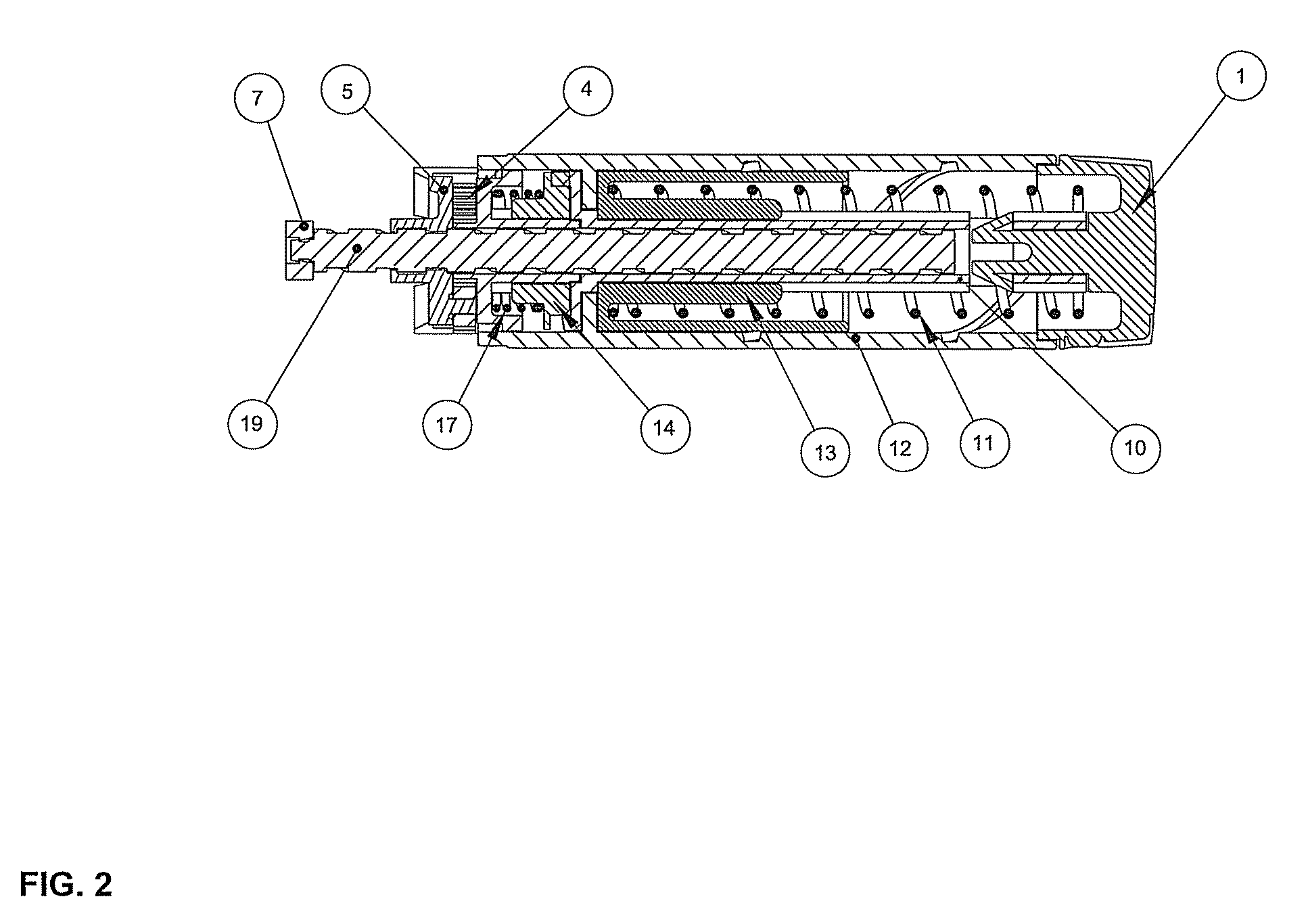
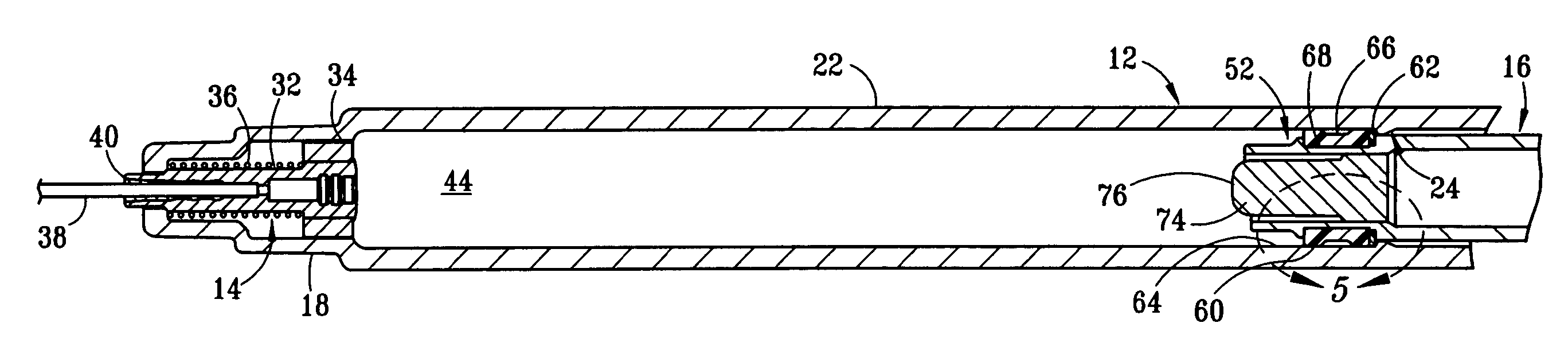
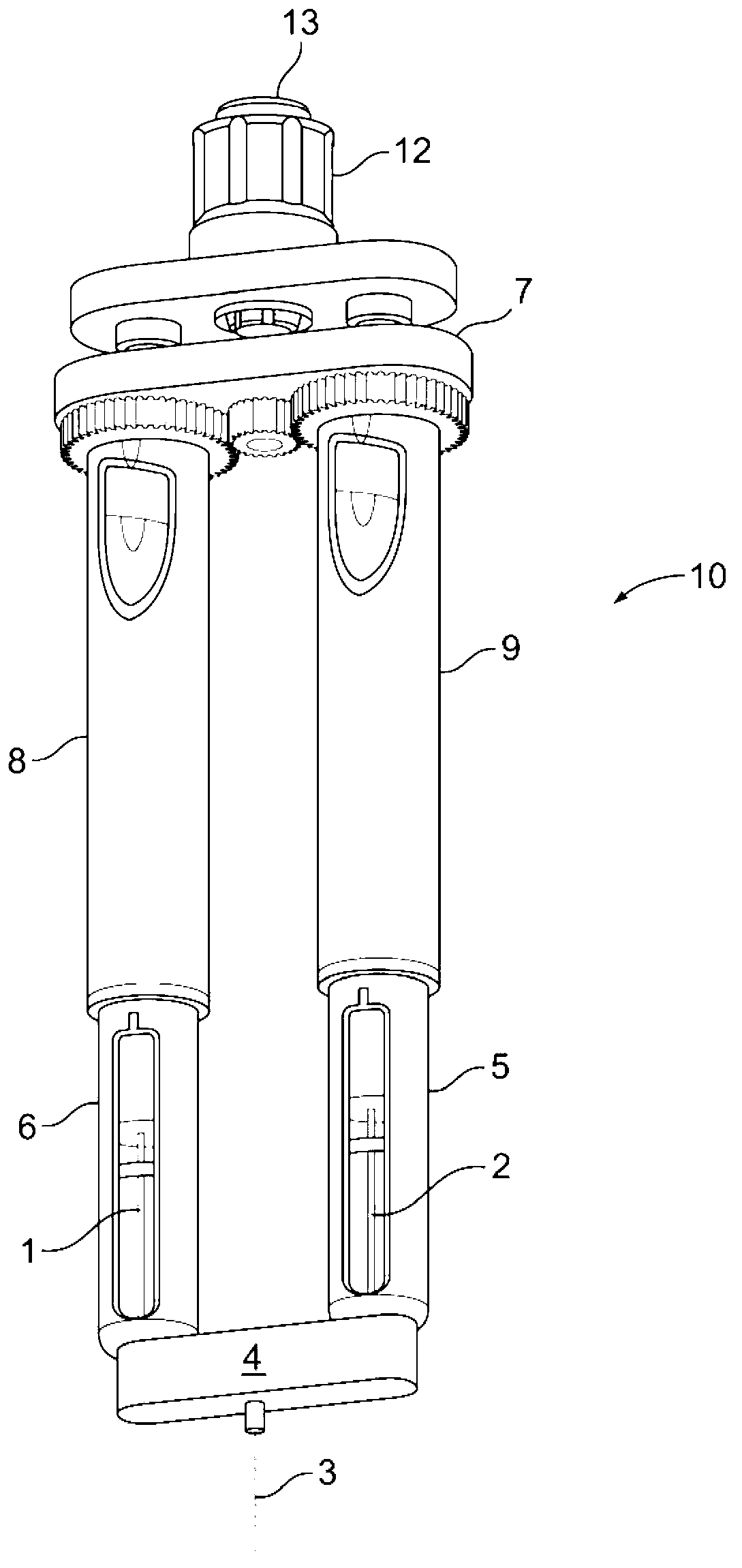
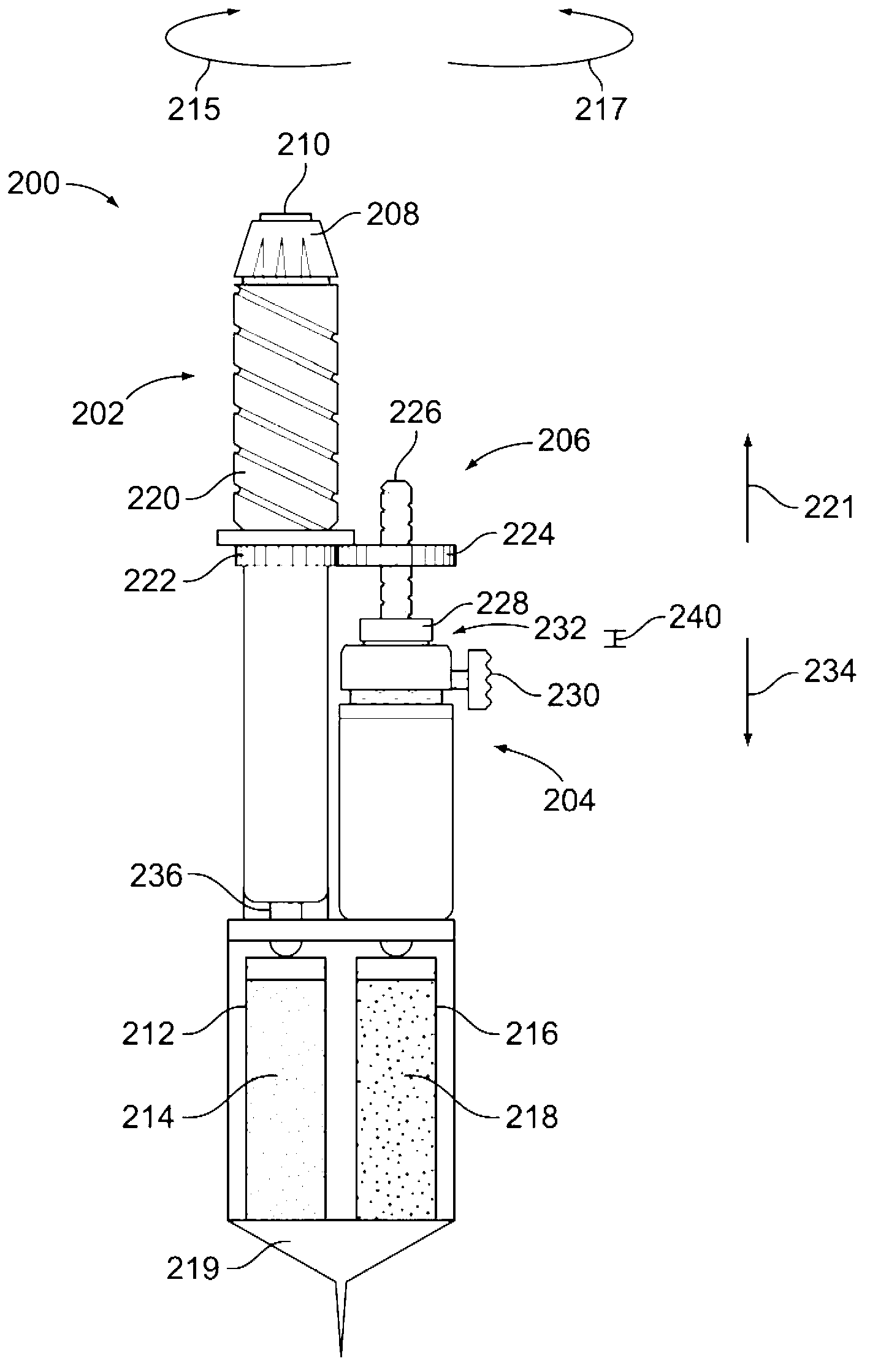
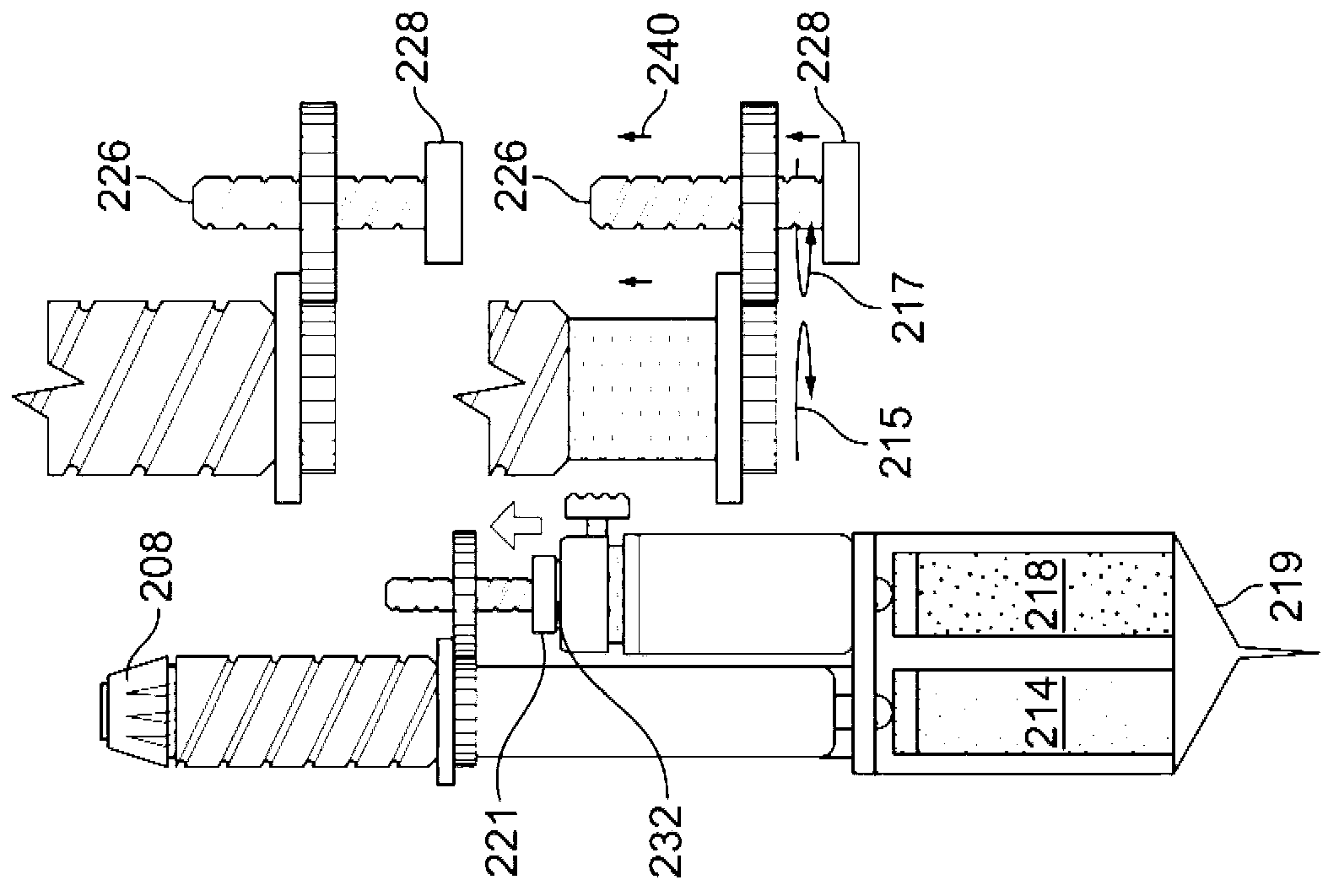
Drug delivery device
The invention relates to a drug delivery device having a dose limiting system. The drug delivery device includes a first dose setting mechanism (205) operably coupled to a primary reservoir (212) holding a first medicament (214). The first dose setting mechanism includes a first dose setter (208) and is a variable dose setting mechanism. The device further includes a second dose setting mechanism (204) operably coupled to a secondary reservoir (216) holding a second medicament, and the second dose setting mechanism includes a second dose setter (230). Still further, the device includes a dose limiting system (206). The dose limiting system operably couples the variable dose setting mechanism and the fixed dose setting mechanism. Further, the dose limiting system is configured to limit a settable amount of a dose of the second medicament a user can set using the second dose setter based on an amount of a variable dose that a user sets using the first dose setter.
Owner:SANOFI AVENTIS DEUT GMBH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com