Drug composition for curing tumour diseases
A composition and drug technology, applied in the direction of drug combination, antineoplastic drugs, pharmaceutical formulations, etc., can solve the problems of strong toxic side effects, poor selectivity, easy to produce drug resistance, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
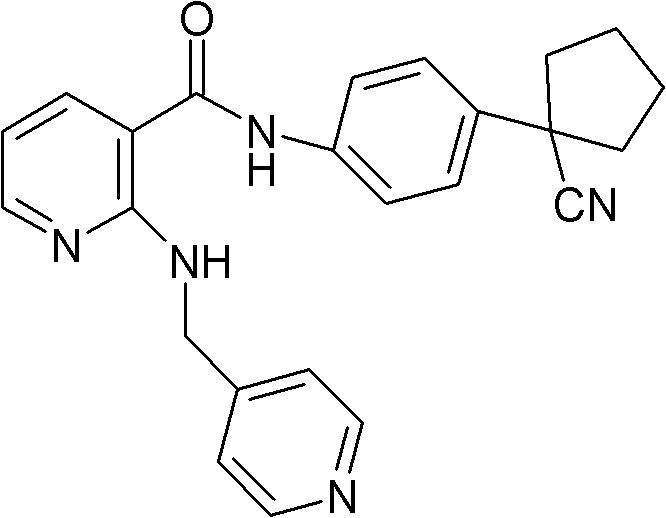
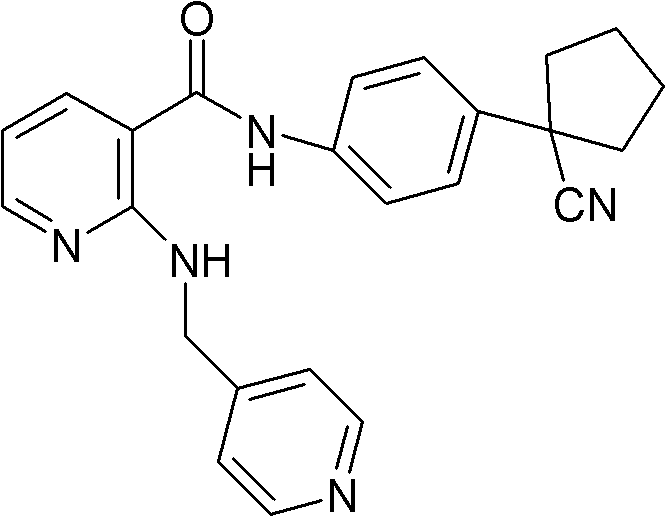
[0048] Embodiment 1, the preparation of compound A mesylate
[0049] In a 5L reaction flask, put 170g (0.428mol) of compound A, 42.5g (0.442mol) of methanesulfonic acid, and 2.55L of 95% isopropanol aqueous solution, stir and heat under nitrogen protection and light-shielding conditions until completely dissolved, to obtain light The yellow transparent solution was filtered while it was hot, cooled and crystallized to room temperature, filtered, washed with isopropanol, and dried in vacuum to obtain 180.2 g (0.365 mol) of white needle-like crystals, with a yield of 85.4%.
[0050] In a 5L reaction flask, put 180.2g of compound A, 2.52L of 95% isopropanol aqueous solution, stir and heat until completely dissolved under nitrogen protection and dark conditions, filter while it is hot, and cool the filtrate to room temperature for crystallization, filter, isopropanol Wash and dry in vacuo to obtain 161.5 g of white needle-like crystals, with a yield of 89.6%. Melting range: 193.5...
Embodiment 2
[0051] Embodiment 2, compound A maleate and erlotinib hydrochloride compound tablet
[0052] Prescription Each tablet contains:
[0053]
[0054] Preparation method: Mix compound A maleate, erlotinib hydrochloride and microcrystalline cellulose, and wet granulate with 2% starch slurry. Dry, add magnesium stearate, mix well and press into tablets.
Embodiment 3
[0055] Embodiment 3, Compound A mesylate and erlotinib hydrochloride compound tablet
[0056] Prescription Each tablet contains:
[0057]
[0058] Preparation method: Mix compound A mesylate, erlotinib hydrochloride and microcrystalline cellulose, and wet granulate with 2% starch slurry. Dry, add magnesium stearate, mix well and press into tablets.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com