Patents
Literature
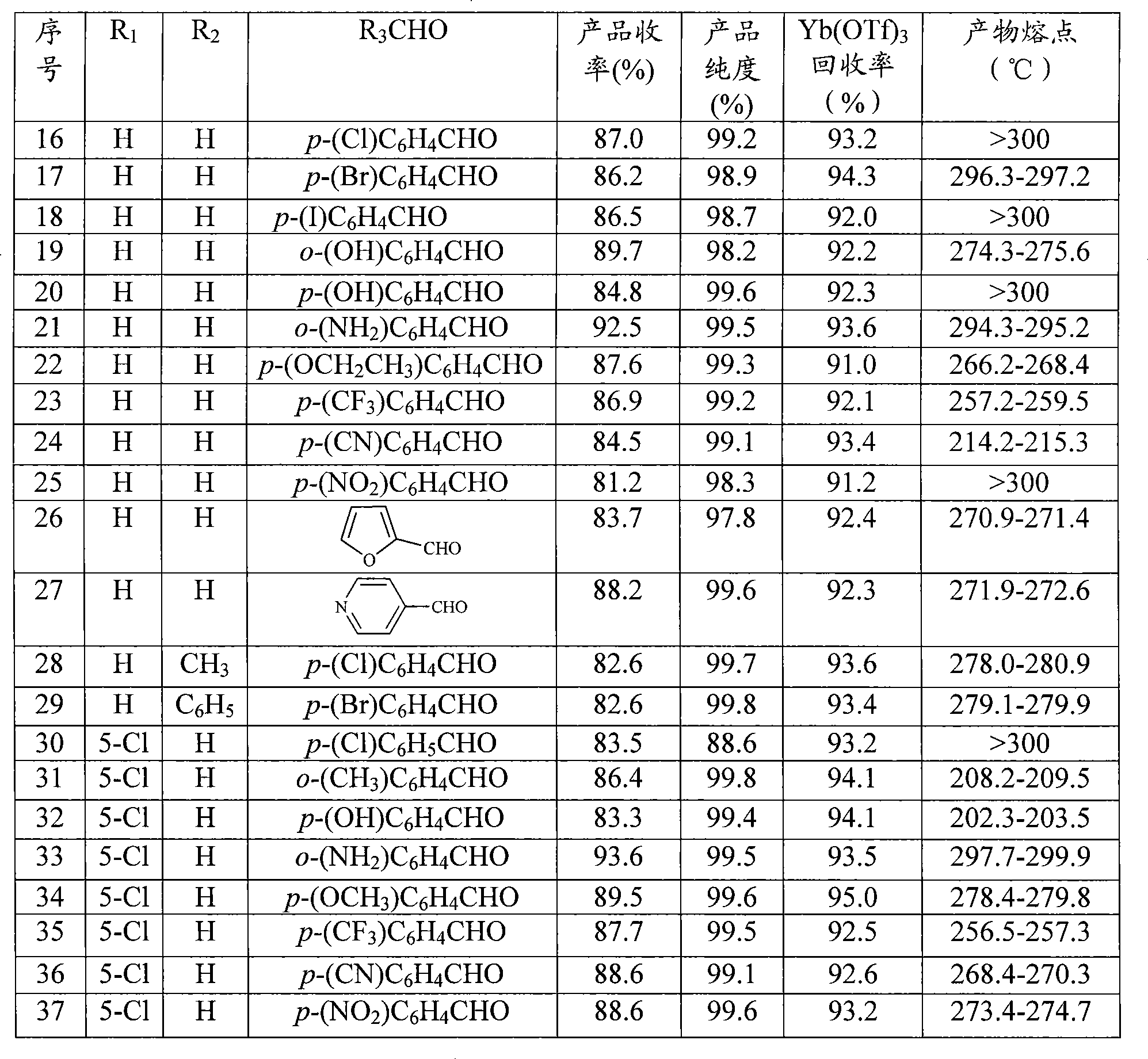
429 results about "Mesylate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
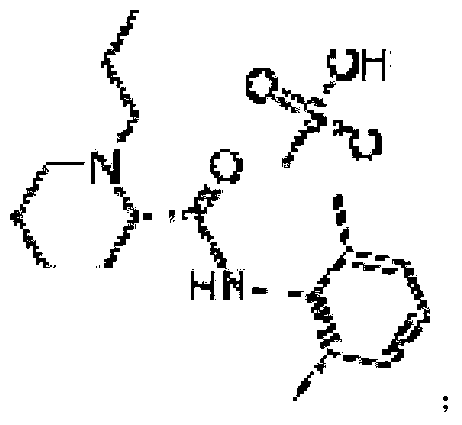
In chemistry, a mesylate is any salt or ester of methanesulfonic acid (CH₃SO₃H). In salts, the mesylate is present as the CH₃SO₃⁻ anion. When modifying the International Nonproprietary Name of a pharmaceutical substance containing the group or anion, the correct spelling is mesilate (as in imatinib mesilate, the mesylate salt of imatinib).
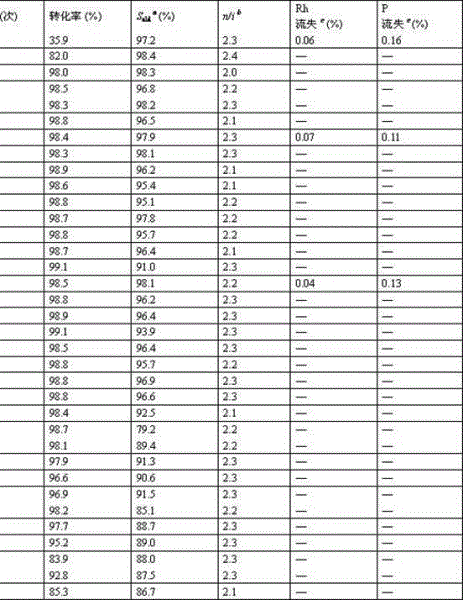
Olefin two-phase hydroformylation method
ActiveCN102617308AEffective fixed loadSeparation and easy handlingOrganic-compounds/hydrides/coordination-complexes catalystsPreparation by carbon monoxide reactionTPPTSPolymer science
The invention relates to an olefin two-phase hydroformylation method, which consists of three parts: polyether guanidine mesylate ionic liquid (PGMILs) with room temperature solidifiable characteristics, complex catalysts (Rh-TPPTS) formed by RhCl3.3H2O or dicarbonylacetylacetonato rhodium and triphenylphosphine sodium trithionate (TPPTS), and reactants of C6-C14 straight chain 1-olefin, wherein the Rh-TPPTS is dissolved in the PGMILs to form a lower layer catalyst phase, the C6-C14 straight chain 1-olefin or product aldehyde forms an upper layer organic phase, the selectivity of high-carbon aldehyde is 85 to 99 percent, the mol ratio of normal aldehyde to isomerism aldehyde is 2.0 to 2.4, the PGMILs phase containing Rh-TPPTS can be cyclically used for 35 times, the activity and the selectivity are unchanged, the accumulated conversion number (TON) reaches higher than 30000, rhodium flowing to the product phase is 0.04 percent to 0.07 percent, and ultra-long-period catalysis activity and selectivity can be realized.
Owner:山东聚强绿洲生物科技有限公司
[(indole-3-yl)pyrimidine-2-yl]aminophenylpropyl-2-eneamide derivative and its salt, preparation method of derivative, and application of derivative and salt
ActiveCN105153122AExtended half-lifeIncrease blood concentrationOrganic active ingredientsOrganic chemistryHigh concentrationSide effect
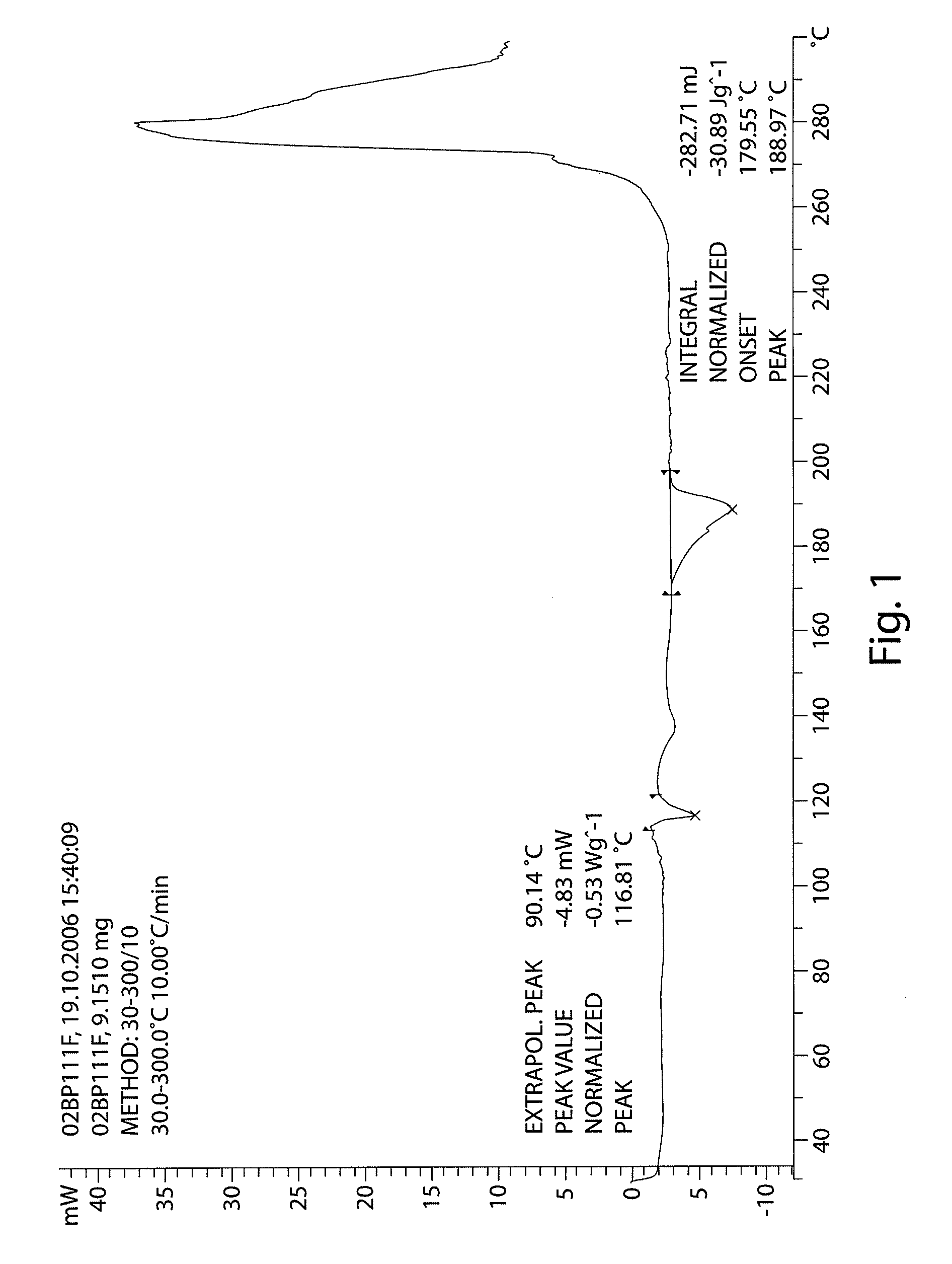
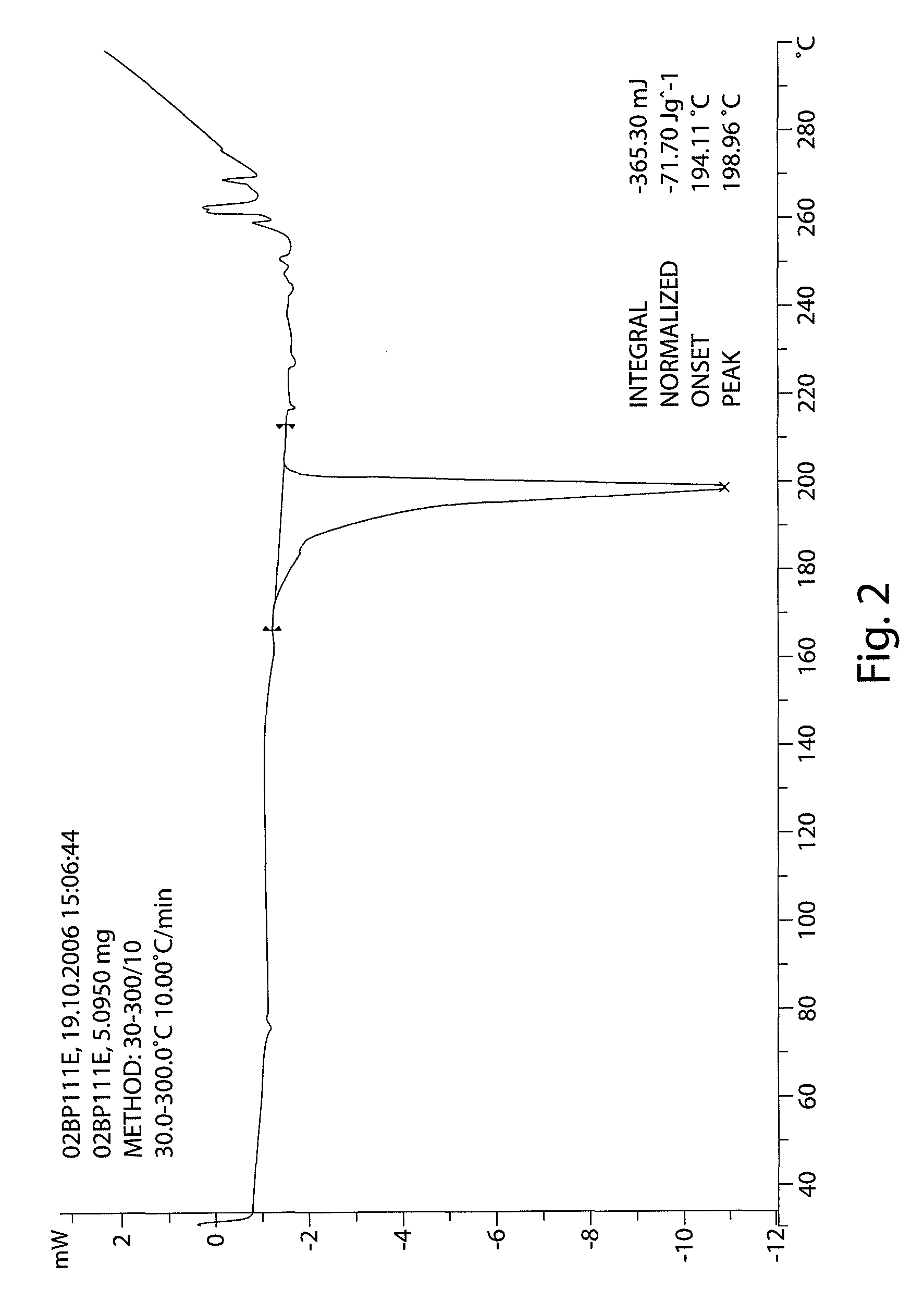
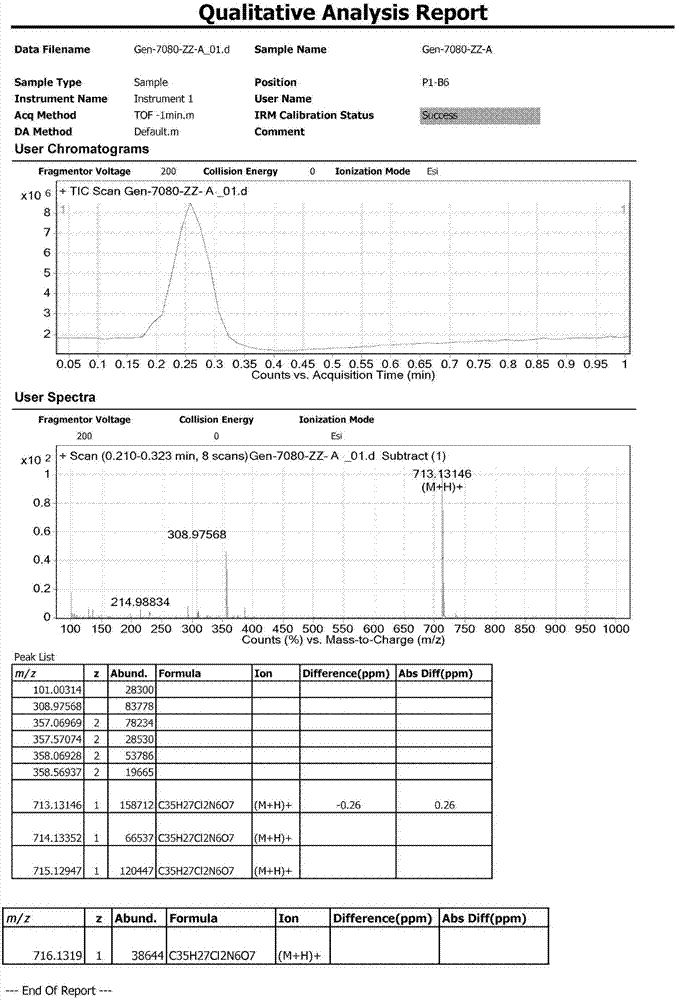
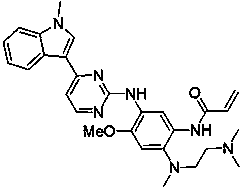
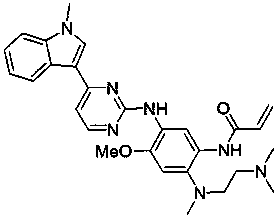
The invention provides a [(indole-3-yl)pyrimidine-2-yl]aminophenylpropyl-2-eneamide derivative and its salt, a preparation method of the derivative, and application of the derivative and the salt. The [(indole-3-yl)pyrimidine-2-yl]aminophenylpropyl-2-eneamide derivative has a structure shown in the formula I below. Deuterium-carbon bonds in the derivative enable the derivative to decompose slowly in a human body, a medicament of the derivative has a longer half-life period and a higher concentration in blood, the dosage of the medicament is finally reduced, and toxic and side effects of the medicament are decreased. Experiments show that compared with AZD 9291 mesylates, AZD 929-D9 mesylate of the deuterium-substituted derivative has Cmax which is 1.32 times as high as that of AZD 9291, exposed dose 1.41 times as high as that of AZD 9291, and elimination half-life 1.31 times as long as that of AZD 9291.
Owner:河南英诺唯医药科技有限公司
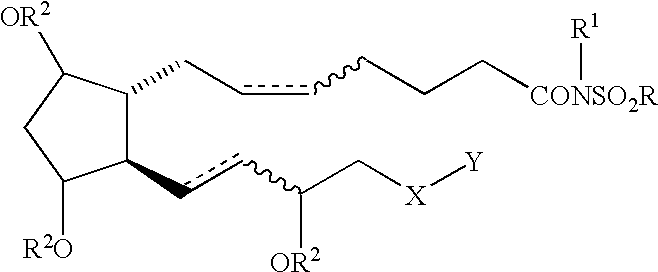
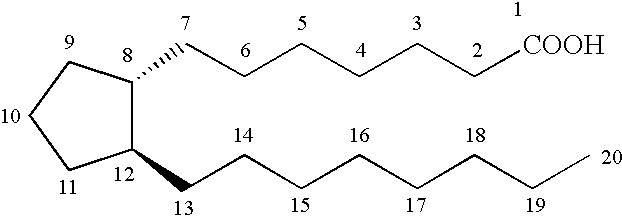
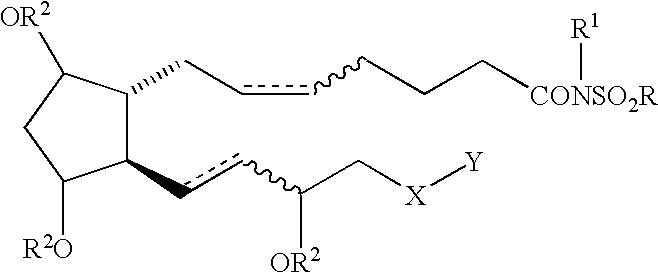
Cyclopentane heptan(ene) acyl sulfonamide, 2-alkyl or 2-arylalkyl, or 2-heteroarylalkenyl derivatives as therapeutic agents
The present invention provides a method of treating ocular hypertension or glaucoma which comprises administering to an animal having ocular hypertension or glaucoma therapeutically effective amount of a compound represented by the general formula I;wherein a hatched line represents the alpha configuration, a triangle represents the beta configuration, a straight line, e.g. at the 9, 11 or 15 position represents either the alpha or beta configuration, a dotted line represents the presence or absence of a double bond; a wavy line represents a cis or trans bond;X is O, S, NH or (CH2)n;n is 0 or an integer of from 1 to 4;Y is C1-C5 n-alkyl, C3-C7 cycloalkyl, phenyl, furanyl, thienyl, pyridinyl, thiazolyl, benzothienyl, benzofaranyl, naphthyl, or substituted derivatives thereof, wherein the substituents maybe selected from the group consisting of C1-C5 alkyl, halogen, CF3, CN, NO2, N(R2)2, CO2R2 and OR2;Z is (CH2)n or a covalent bond;R is C1-C6 lower alkyl or Z-CF3 or mesylate or triflate;R1 is H, R2 or COR2;andR2 is H or C1-C5 lower alkyl or 9, 11 or 15 esters thereof.
Owner:ALLERGAN INC
Process for the preparation of compositions for modulating a kinase cascade and methods of use thereof
The invention relates to compositions comprising 2-(5-(4-(2-morpholinoethoxy)phenyl)pyridin-2-yl)-N-benzylacetamide and its mesylate and dihydrochloride salts. More specifically, the invention provides an efficient process for the synthesis of 2-(5-(4-(2-morpholinoethoxy)phenyl)pyridin-2-yl)-N-benzylacetamide and its mesylate and dihydrochloride salts and methods for modulating one or more components of a kinase cascade using the compositions of the invention.
Owner:ATNX SPV LLC
Hydrate of medicinal salt of Fasudil
InactiveCN101092413AImprove stabilityImprove solubilityOrganic active ingredientsOrganic chemistryHydrobromideDisease
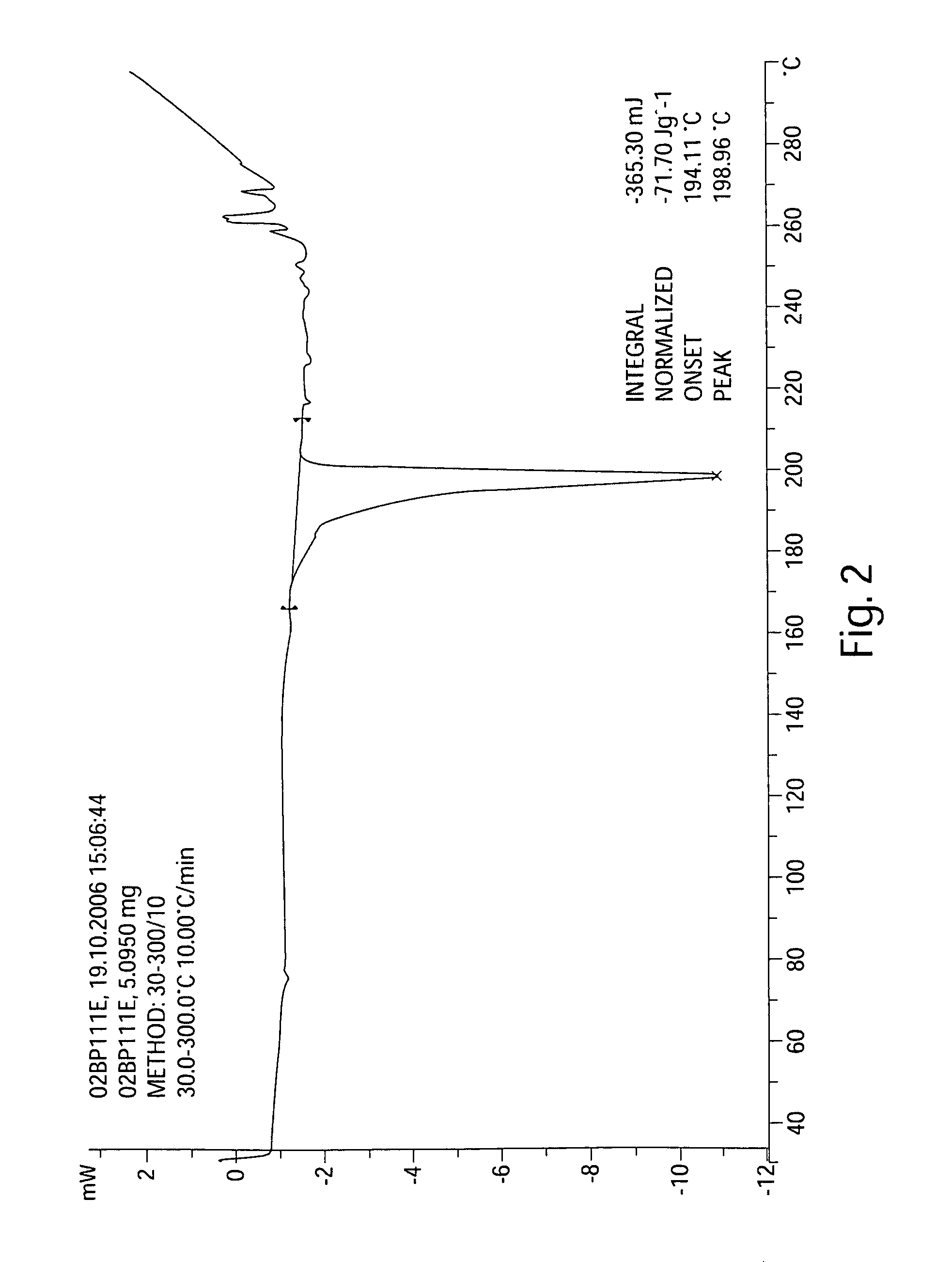
This invention discloses hydrates of pharmaceutical salts of hexahydro-1-(5-sulfonylisoquinoline)-1H-1,4-diaza, i.e., fasudil, especially semi-hydrates of nitrate, sulfate, hydrobromide and mesylate of fasudil, their preparation method, and their application in drugs for preventing and / or treating cardio-cerebrovascular diseases. The hydrates of pharmaceutical salts of fasudil have such advantages as high stability and high solubility, and can be manufactured into various preparations with other active components and / or pharmaceutically acceptable carriers. The method has such advantages as simple process, high drug purity, high yield, and stable product quality, and is suitable for industrial production.
Owner:SHANDONG XUANZHU PHARMA TECH CO LTD
Compositions for modulating a kinase cascade and methods of use thereof
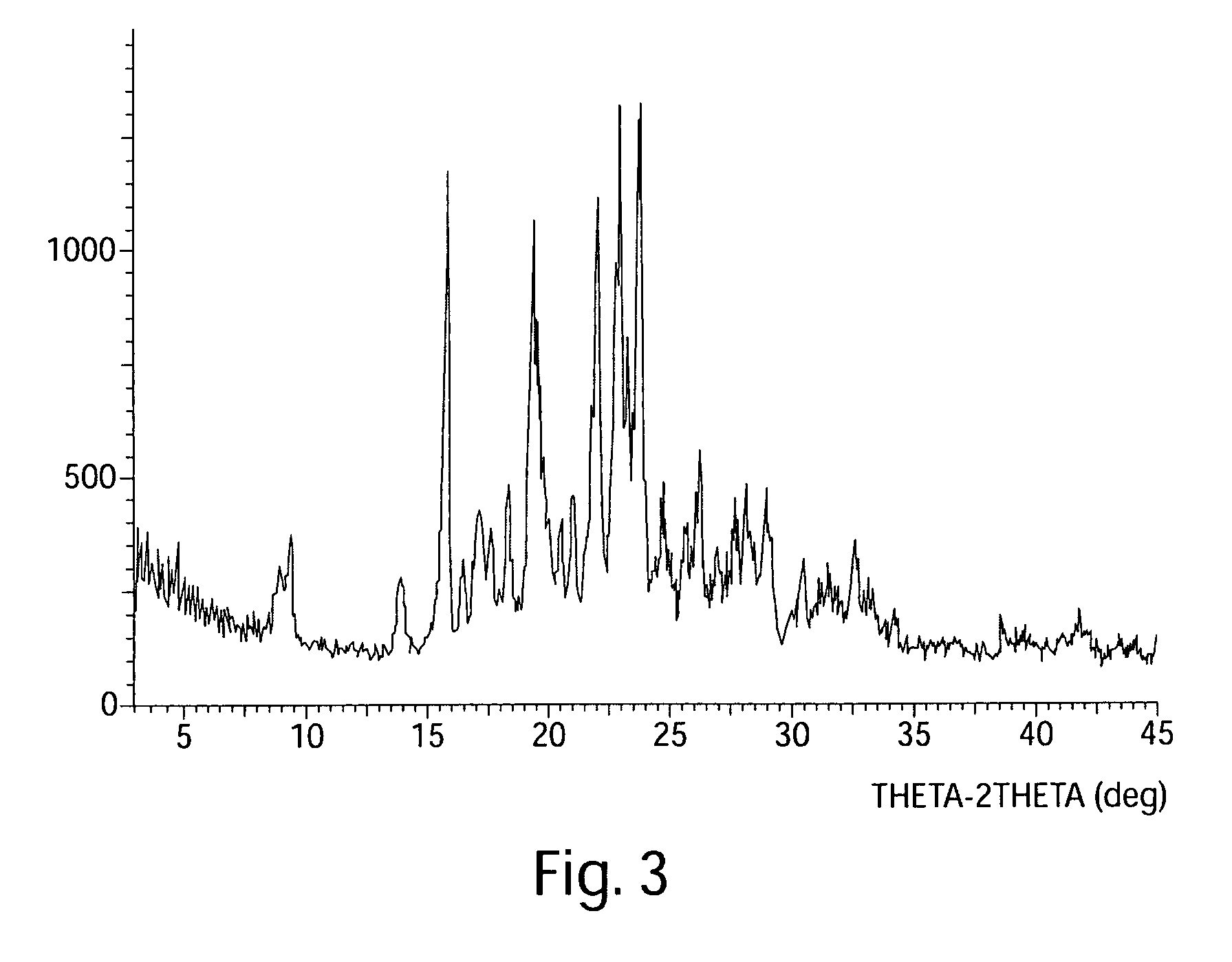
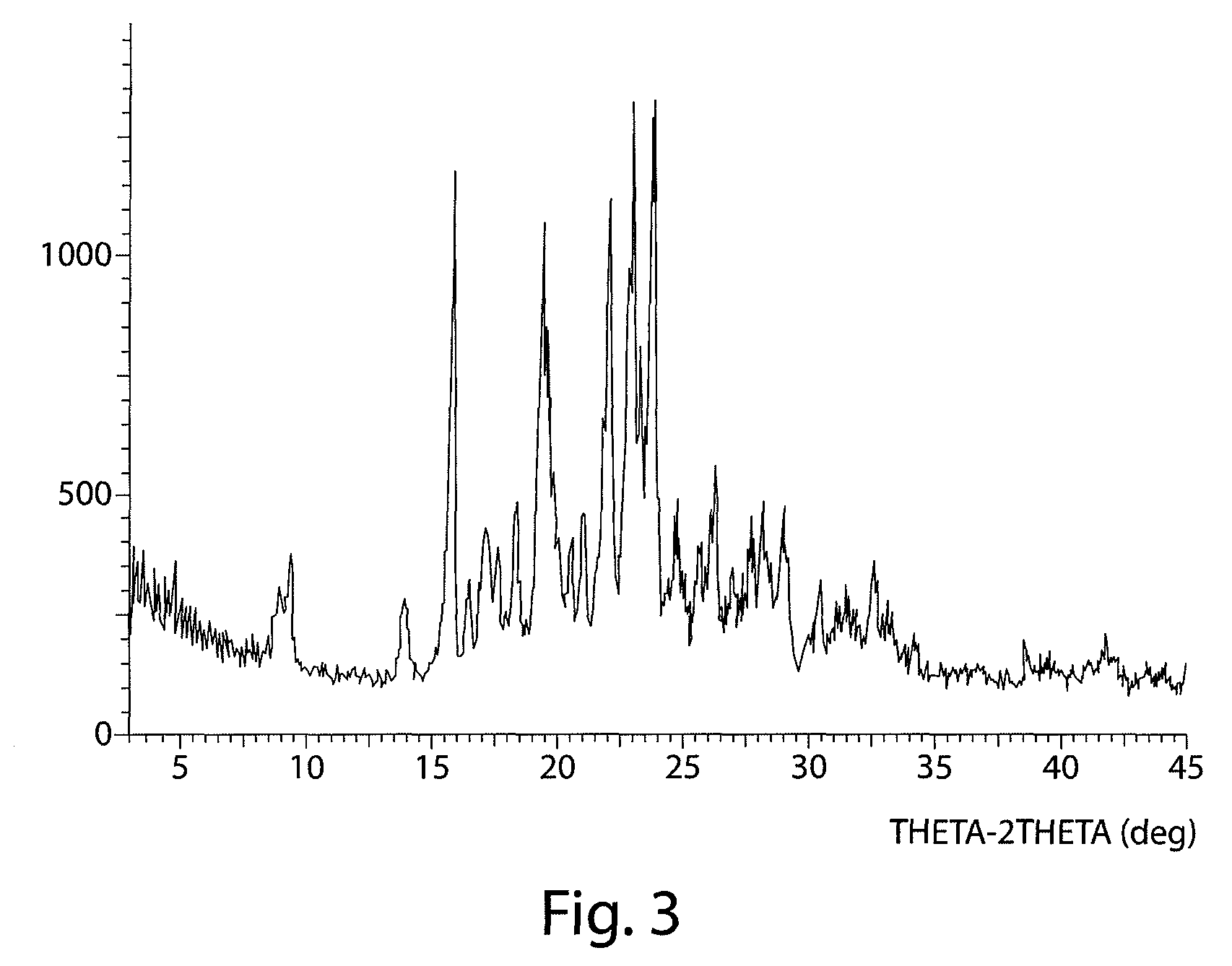
The invention relates to compositions comprising 2-(5-(4-(2-morpholinoethoxy)phenyl)pyridin-2-yl)-N-benzylacetamide and its mesylate and dihydrochloride salts. The invention provides an efficient process for the synthesis of 2-(5-(4-(2-morpholinoethoxy)phenyl)pyridin-2-yl)-N-benzylacetamide and its mesylate and dihydrochloride salts and methods for modulating one or more components of a kinase cascade using the compositions of the invention. The present invention also provides a novel polymorph of the mesylate salt of 2-(5-(4-(2-morpholinoethoxy)phenyl)pyridin-2-yl)-N-benzylacetamide (Form A), characterized by a unique X-ray diffraction pattern and Differential Scanning Calorimetry profile, as well as a unique crystalline structure.
Owner:ATNX SPV LLC
Synthesis of quinazoline ketone compounds
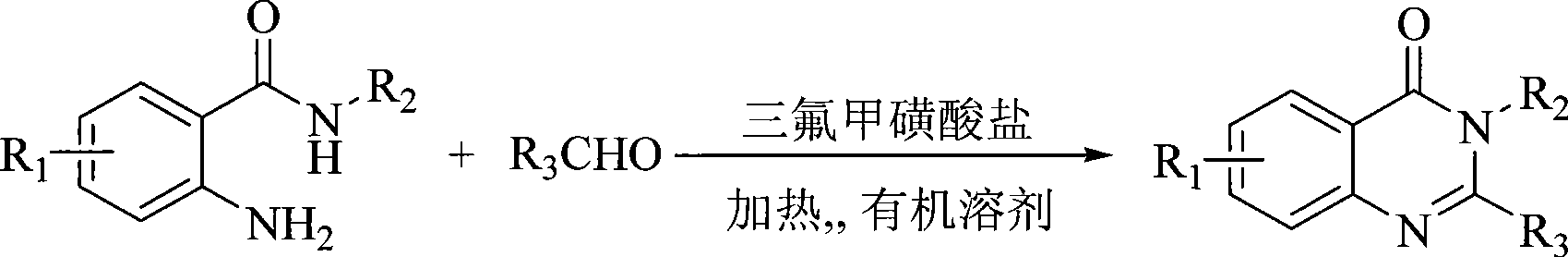
InactiveCN101429165AReduce dosageHigh reaction yieldOrganic chemistryChemical recyclingOrganic solventKetone
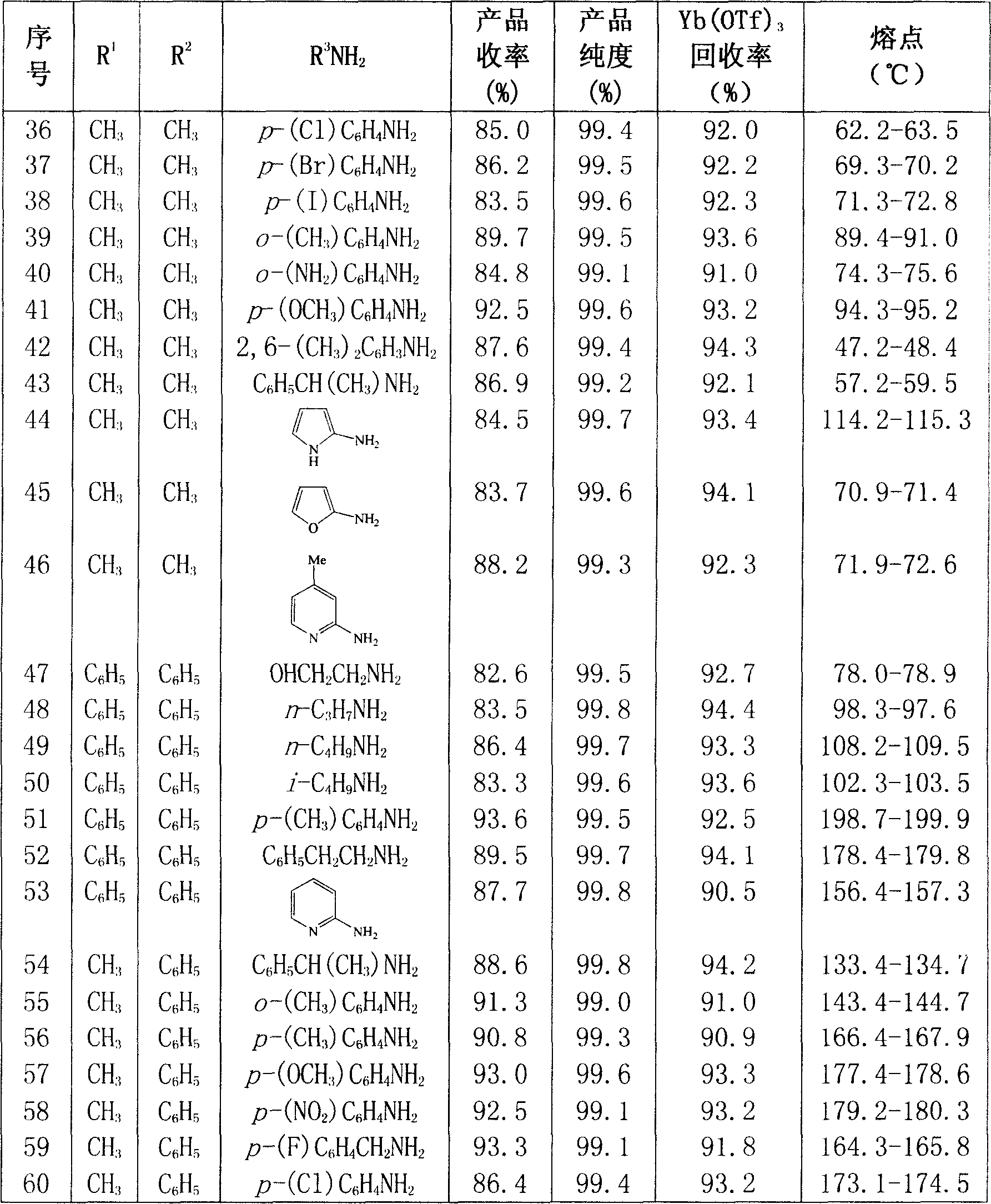
The invention provides a synthetic method for a quinazolinone compound. The structure of the quinazolinone compound is shown as formula (I). The method comprises the following steps: using o-amino benzamide compound shown in structural formula (II) and aldehyde shown in structural formula (III) as raw materials, using trifluoro mesylate as a catalyst, making the mixture reacted for 10 minutes to 24 hours in an inert organic solvent at a temperature of between 25 and 200 DEG C, and separating and purifying the reaction liquor after the reaction is finished to obtain the quinazolinone compound. The method has the advantages of high reaction yield up to more than 85 percent generally, advanced and reasonable process routes, mild reaction conditions, little dosage of the catalyst, reutilization, and substantially no three wastes.
Owner:WENZHOU UNIVERSITY
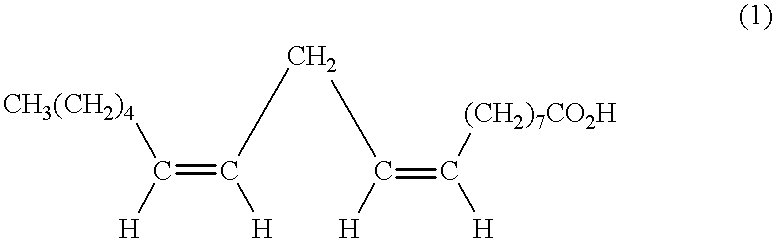
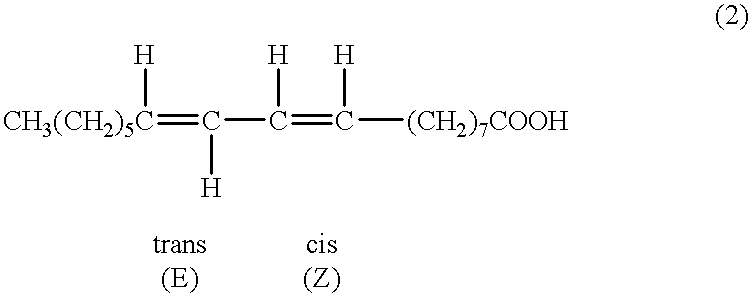
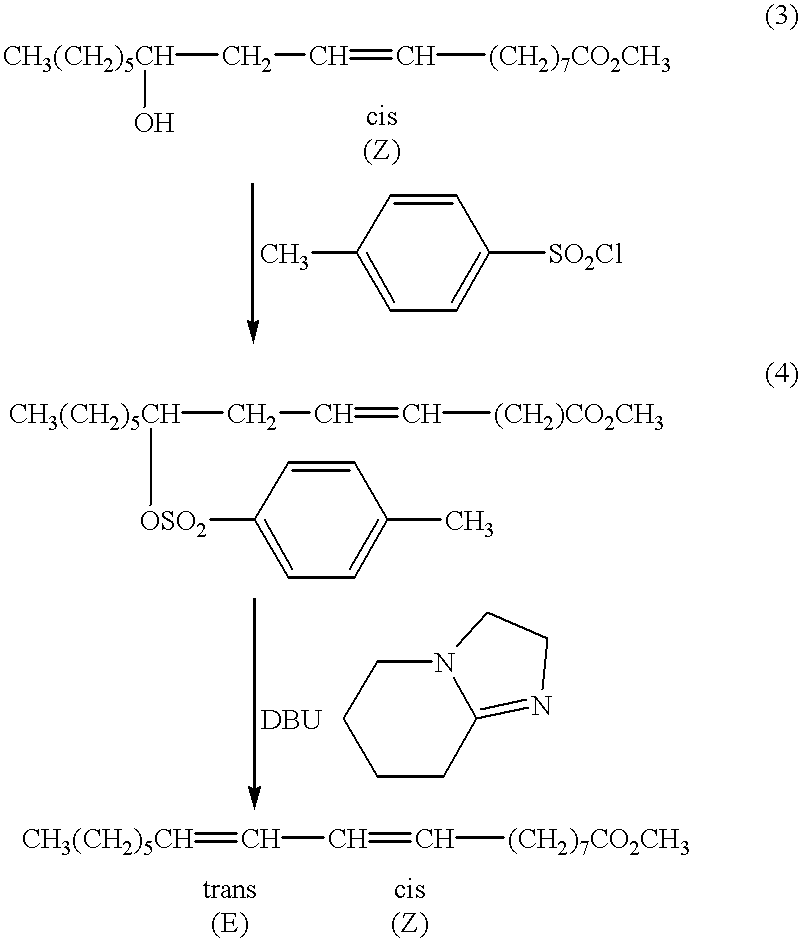
Suppression of carcinoma using high purity conjugated linoleic acid (CLA)
A method for the treatment of carcinoma in a human is disclosed, including administering to a human a therapeutically effective amount of 9-cis, 11-trans octadecadienoic acid formed by reacting an ester of ricinoleic acid with a tosyl chloride or a mesyl chloride to form a tosylate or mesylate of an ester of ricinoleic acid, and reacting the tosylate or mesylate of an ester of ricinoleic acid with diazabicyclo-undecene. The method includes administering to a human a purified conjugated linoleic acid (CLA) produced by a novel synthesis process for producing 9-cis, 11-trans octadecadienoic acid at room temperature in high yield including providing a tosylate or mesylate of a methyl ester of ricinoleic acid and providing a purified 9-cis, 11-trans octadecadienoic acid formed when the tosylate or mesylate reacts with diazabicyclo-undecene.
Owner:MATREYA
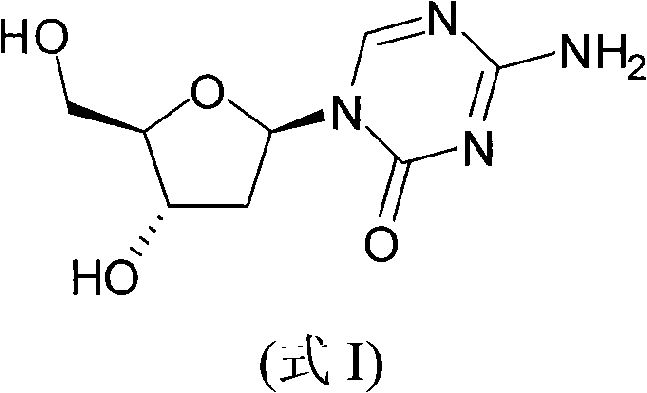
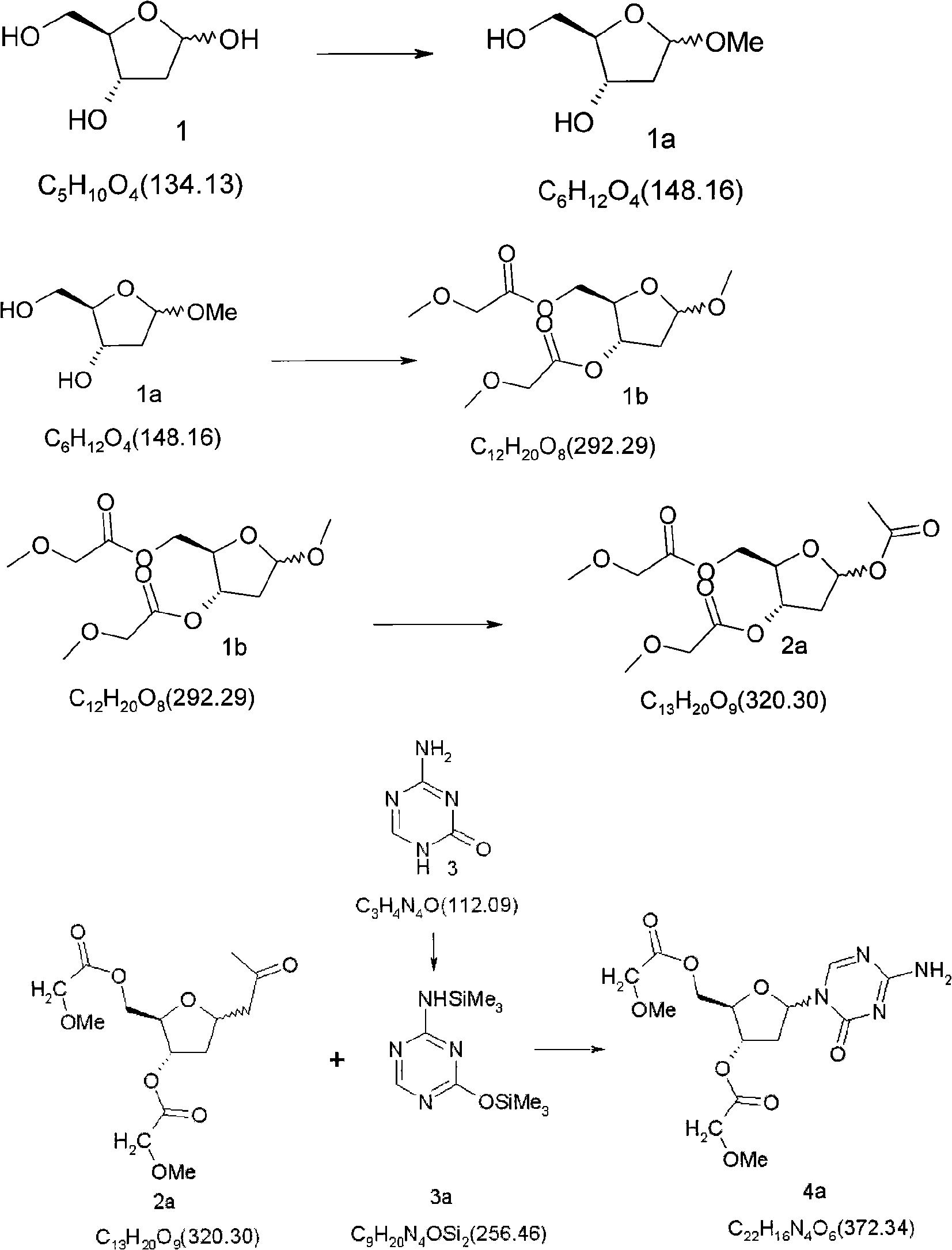
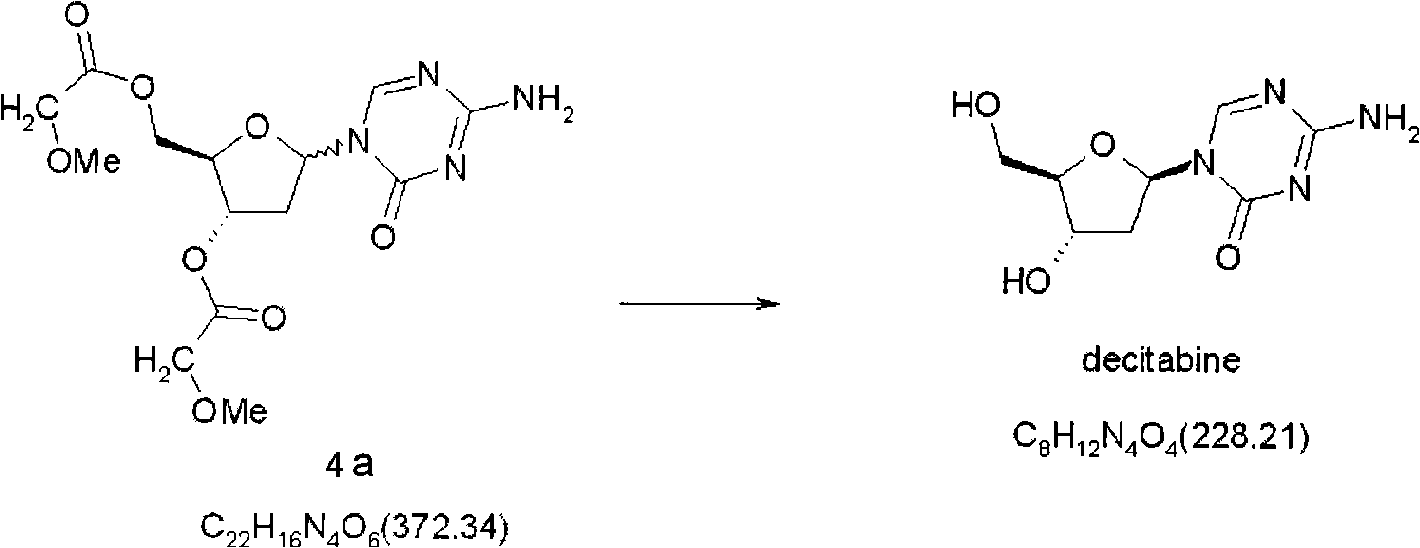
Synthetic process of decitabine
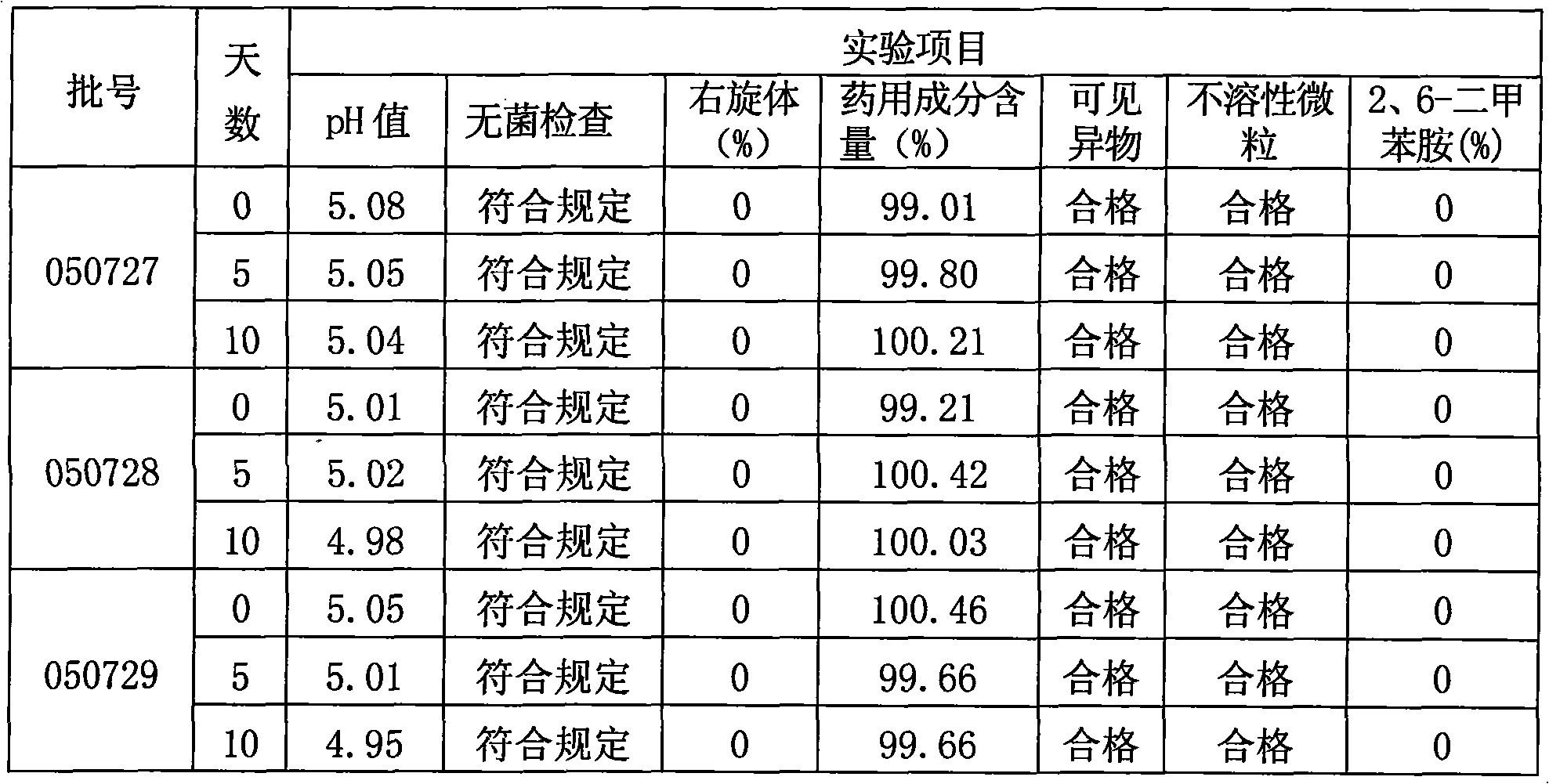
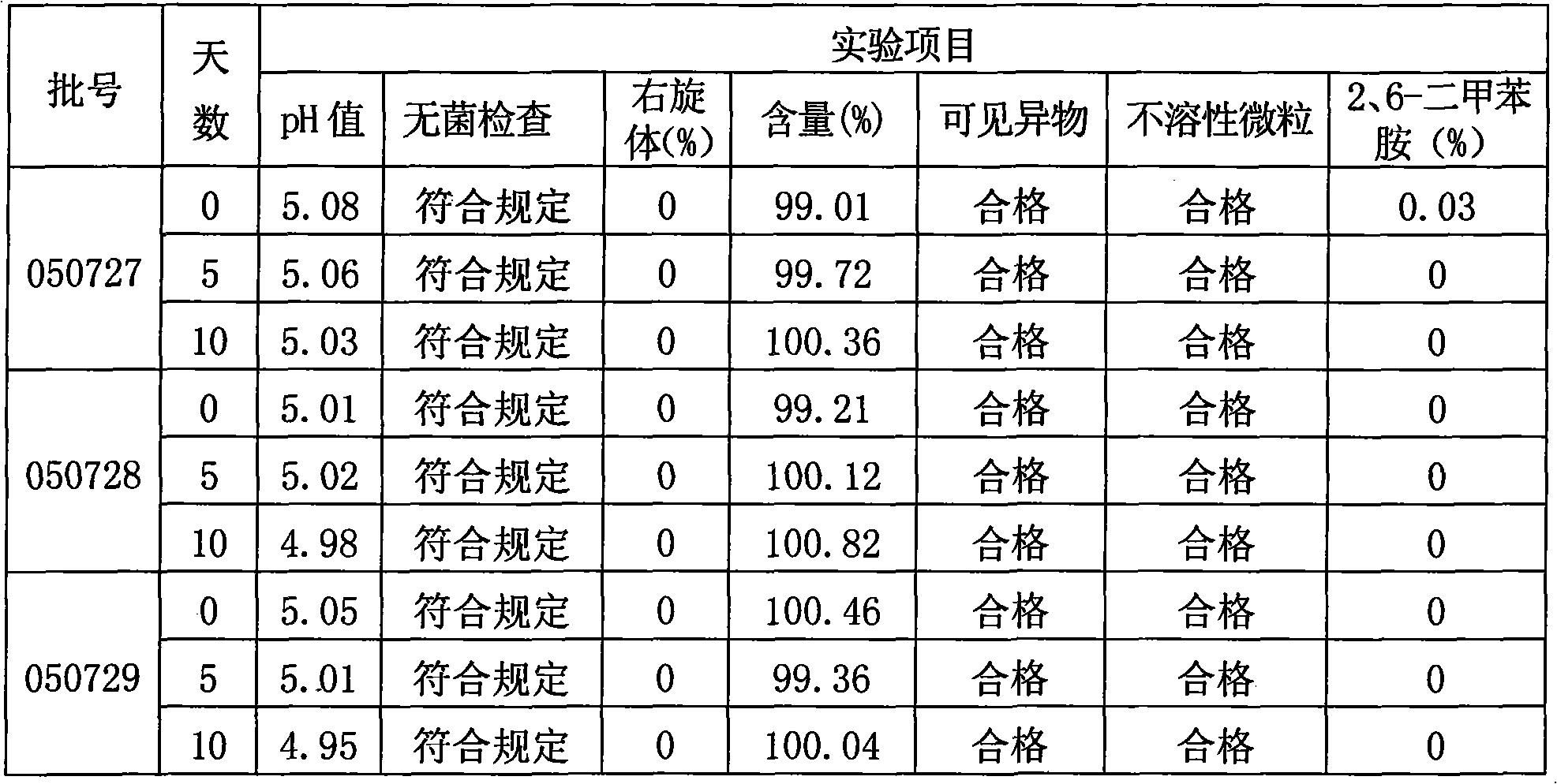
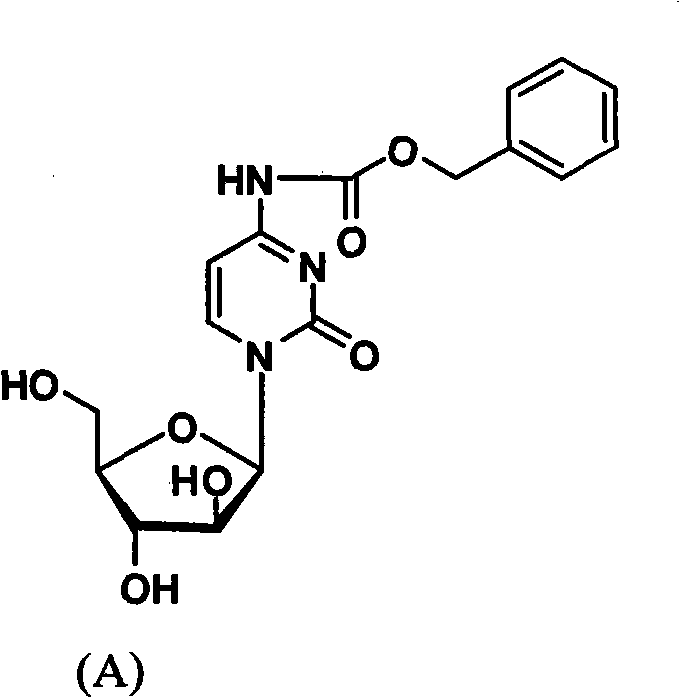
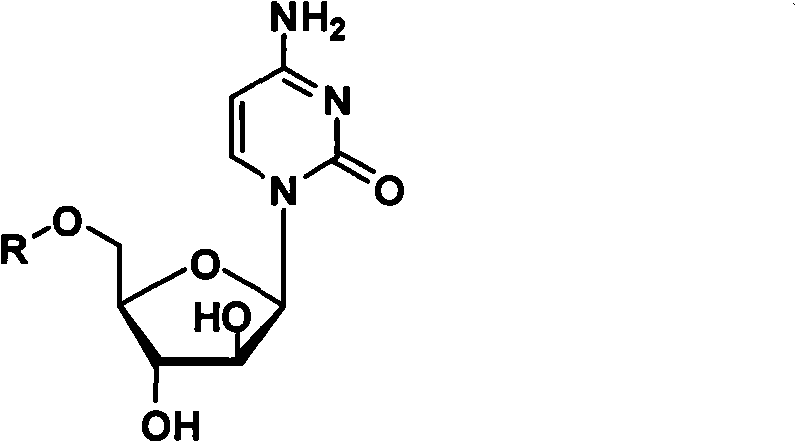
The invention relates to a method for preparing Decitabine. The particular proposal for solving the technical problem is as follows: 2-deoxidtion-D-ribose, 10 percent of HCL methanol solution, methoxyacetic acetic anhydride, HMDS, acetic anhydride, tri-silicyl tri-fluorine methane sulfonic acid ester, acetic acid amine, etc. are adopted as raw materials to synthesize the Decitabine; the target product of the Decitabine is obtained through the five steps of reactions, namely, methylation, acylation, trimethyl silication, ammoniation and deacylation with a total yield of above 18.4 percent and a product purity of above 99.7 percent.
Owner:GUIZHOU UNIV
Preparation method of ropivacaine mesylate injection packed by soda-lime glass bottle
InactiveCN101658490AGuaranteed stabilityGood acid and alkali resistanceAmpoule syringesPharmaceutical delivery mechanismFiltrationAdditive ingredient
The invention relates to a preparation method of ropivacaine mesylate injection packed by a soda-lime glass bottle, which comprises the following processing steps of ingredient preparation, liquor preparation, soda-lime glass bottle and rubber plug cleaning, filling, capping, sterilization, lamp test, labeling, packing and finished product inspection. The injection is subject to a filtration sterilization processing step once and a flowing steam sterilization processing step once, wherein microorganisms larger than 0.22 microns in liquid medicine are removed by a filter method, microorganismssmaller than 0.22 microns are killed by flowing steam sterilization, and insoluble particles in the liquid medicine are removed during filtration sterilization simultaneously, thereby greatly reducingthe occurrence of phlebitis; and the invention is packed by the soda-lime glass bottle which has the advantages of heat resistance, acid and alkali resistance, large strength, and the like, thereby ensuring the stability of the medicine. Clinic comparison tests show that the ropivacaine mesylate injection packed by a soda-lime glass bottle has the advantages of quicker effect, long action time, reliable anesthetic effect, small toxicity to heart, separate blocking pf sensory and motor nerves, and the like, thereby being suitable for anesthesia in surgeries and postoperative analgesia.
Owner:陕西吉尾斯美业有限公司
Cytarabine 5'-O-amino-acid ester, salts thereof and preparation method thereof
InactiveCN101812105AGood membrane permeabilityIncrease Absolute BioavailabilityOrganic active ingredientsSugar derivativesCytarabineSodium bicarbonate
The invention belongs to the technical field of medicines and discloses cytarabine 5'-O-amino-acid ester, pharmaceutically acceptable salts thereof and a preparation method thereof. The preparation method comprises the following steps of: slowly dropping carbobenzoxy chloride into a solution formed by cytarabine, sodium bicarbonate and N,N-dimethylacetylamide, and obtaining a compound A after reacting at room temperature; using the compound A and N-butyloxy formoxyl-amino acid as raw materials; adding a reagent to the solution to carry out an esterification reaction to obtain the cytarabine 5'-O-amino-acid ester; and then adding acid to obtain a finished product. The pharmaceutically acceptable salts comprise hydrochlorides, sulfates, formates, acetates, mesylates, propionates, butyrates, p-toluene sulphonates, phosphates, bisulfates, maleates, lactates, carbonates, bicarbonates, malonates, and salts formed with acidic amino acids, and the like. The invention can obviously improve the membrane permeability of the cytarabine so as to improve the bioavailability of the cytarabine.
Owner:SHENYANG PHARMA UNIVERSITY
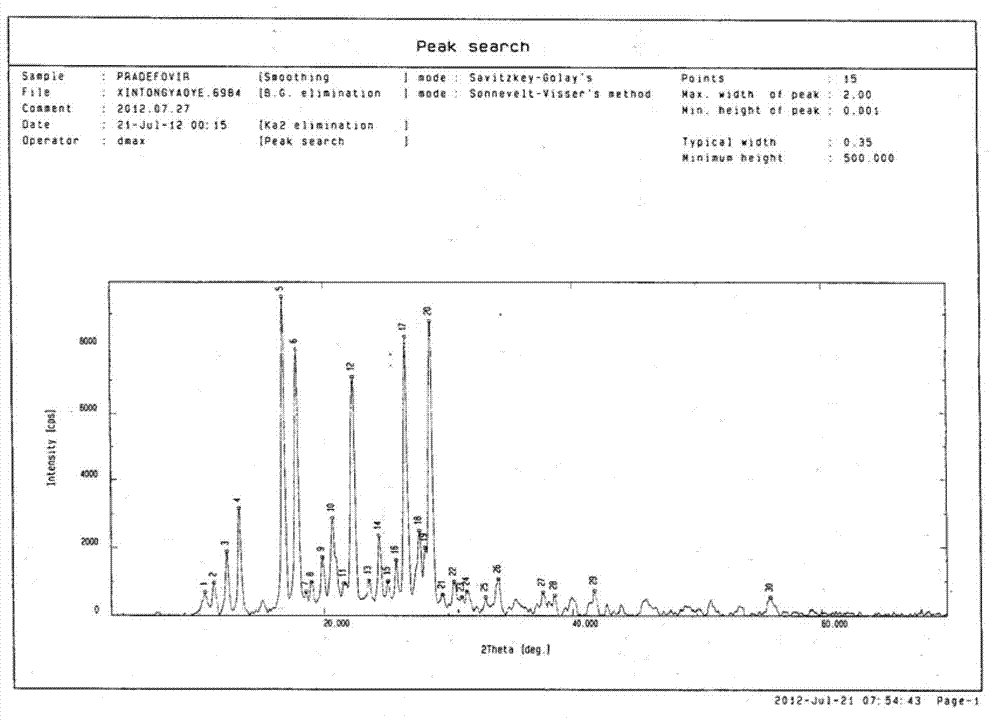
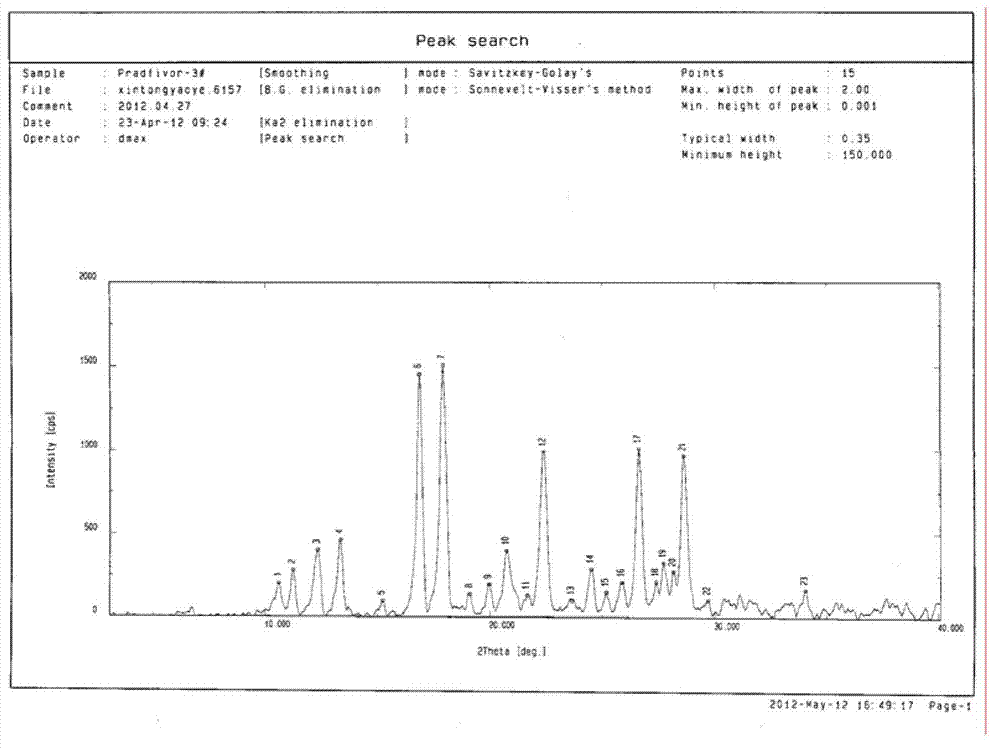
Pradefovir crystal
ActiveCN102827206AGood natureImprove adaptabilityOrganic active ingredientsOrganic compound preparationHEXAPhosphorus
Owner:XIAN XINTONG PHARM RES CO LTD
Anti infectious compound and usage
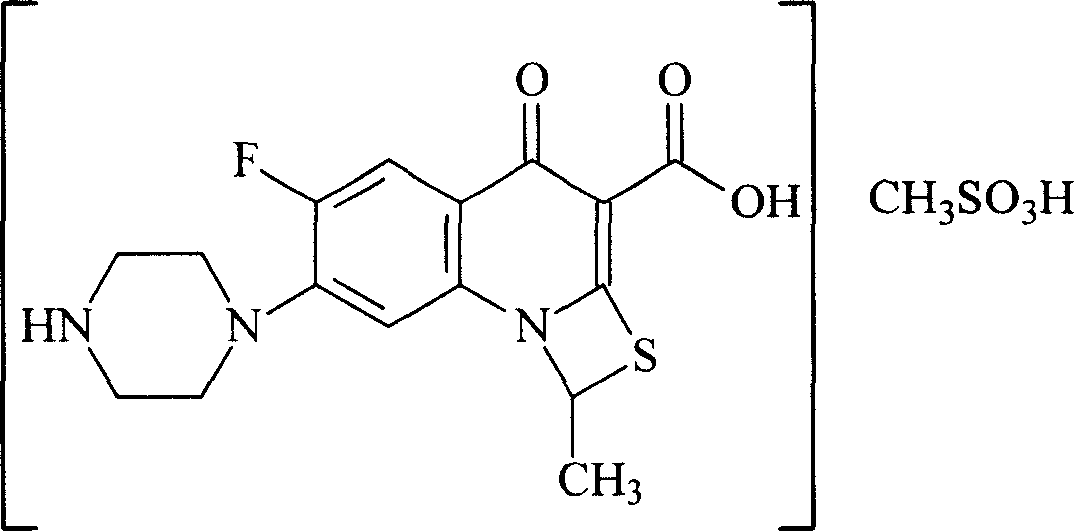
InactiveCN101003540AStrong anti-infection effectStructure determinationPowder deliveryOrganic active ingredientsQuinolineCarboxylic acid
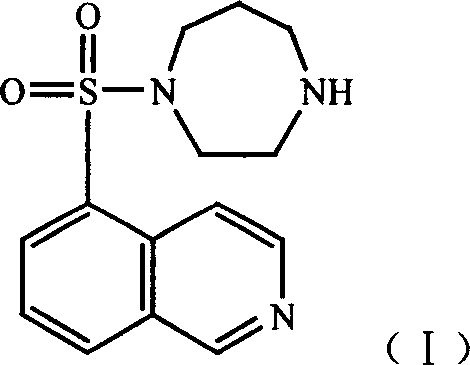
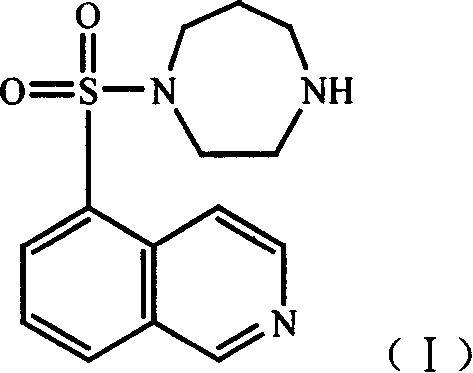
This invention relates to an anti-infective compound, more specifically, 6-fluoro-1-methyl-4-oxo-7-(1-piperazine)-4H-[1, 3] thiazine [3, 2-a] quinoline-3-carboxylic mesylate (compound I), its preparation method and its application. Compound I is prepared by reaction of 6-fluoro-1-methyl-4-oxo-7-(1-piperazine)-4H-[1,3]thiazine[3,2-a]quinoline-3-carboxylic acid and methyl sulfonic acid. Compound I can be used as effective component and mixed with normal pharmaceutical carrier to manufacture anti-infective drug composition, which can be tablets, capsules, granules, injection, eye preparations, ear preparations, gynecological preparations, and external use preparations. Compound I has stable properties, and can be easily dissolved in water, thus can be easily manufactured into various preparations used in clinical treatment. The method has such advantages as simple and reasonable process.
Owner:GUANGZHOU BAIYUNSHAN PHARMA HLDG CO LTD BAIYUNSHAN PHARMA GENERAL FACTORY +1
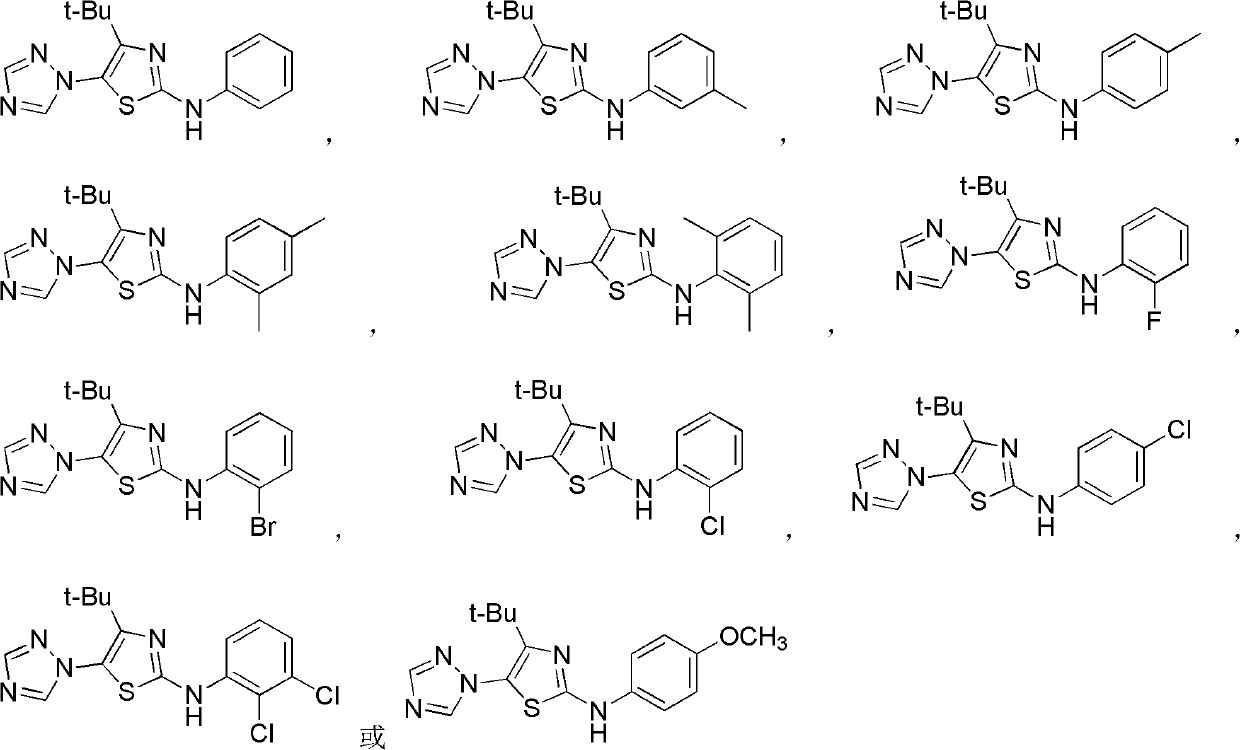
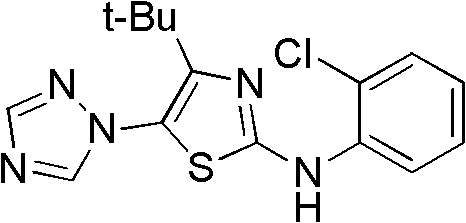
4-alkyl-2-arylamino-5-(1,2,4-triazole-1-group) thiazole and application thereof to preparation of medicaments for resisting cancer
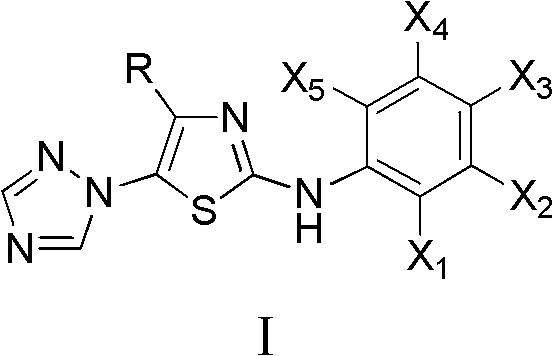
InactiveCN102675303AAnti-cervical cancerCtiveOrganic chemistryAntineoplastic agentsHydrobromidePhosphate
The invention discloses 4-alkyl-2-arylamino-5-(1,2,4-triazole-1-group) thiazole and salts thereof shown in a formula I, wherein R is selected from H, alkyl groups of C1-C2 and straight-chain alkyl groups or branched-chain alkyl groups of C3-C4; X1, X2, X3, X4 and X5 are selected from hydrogen, alkyl groups of C1-C2, hydroxide group, methoxy group, oxyethyl group, trifluoromethyl, fluorine, chlorine, bromine, nitryl, amino group, acetyl amino group, methanesulfamide, ethoxycarbonyl or carboxyl; and the salts of the 4-alkyl-2-arylamino-5-(1,2,4-triazole-1-group) thiazole are selected from hydrochloride, hydrobromide, nitrate, sulfate, phosphate, mesylate, p-toluene sulfonate, tartrate, lactate or malate. The 4-alkyl-2-arylamino-5-(1,2,4-triazole-1-group) thiazole is applied to preparation of medicaments for resisting cervical cancer or lung neoplasms.
Owner:HUNAN UNIV
Preparation methods of lenvatinib mesylate drug impurities
The invention belongs to the field of pharmaceutical synthesis, and relates to impurities in a raw medical material production process and preparation methods of the impurities, in particular to preparation methods of process impurities A, B and C of lenvatinib mesylate, namely, 4-[3-chloro-4-(N'-cyclopropylureido) phenoxy]-7-methoxyquinoline-6-carboxamide mesylate), as a drug for treating radioiodine-refractory thyroid cancer and an application of the impurities to quality research of lenvatinib mesylate. With adoption of the methods, the process impurities A, B and C are obtained through chemical synthesis for the first time, and the target compounds shown in the description can be obtained through efficient and rapid separation.
Owner:HANGZHOU HUADONG MEDICINE GRP PHARMA RES INST +2
Preparation method of trifluoro methanesulfonic anhydride
ActiveCN102911086AHigh yieldReduce contentOrganic chemistryOrganic compound preparationTrifluoromethanesulfonic anhydrideSulfonyl chloride
The invention provides a preparation method of trifluoro methanesulfonic anhydride. The method comprises the following steps of: firstly reacting trifluoro methanesulphonyl fluoride with alkali metal hydroxide to prepare trifluoro mesylate, purifying trifluoro mesylate by recrystallization by utilizing an organic solvent, reacting trifluoro methane sulfonyl chloride with trifluoro mesylate to generate a trifluoro methanesulfonic anhydride crude product, and finally purifying trifluoro methanesulfonic anhydride by atmospheric distillation. The preparation method of trifluoro methanesulfonic anhydride can be used for not only effectively simplifying reaction steps so that the operation process is simple and convenient and the operation is safe, but also avoiding byproducts generated in the process of the traditional method for producing trifluoro methanesulfonic anhydride, and effectively reducing the contents of F<-> and SO4<2-> in the product; by utilizing recrystallization, atmospheric distillation and other methods for purification, the product purity is up to 99.5%; and more importantly, the yield of anhydride is greatly increased and raised to 88% from original 60%.
Owner:JIANGXI GUOHUA IND CO LTD
Pharmaceutical salt of AZD9291 and preparation method thereof
ActiveCN107915725AOvercome deliquescence and other problemsImprove solubilityOrganic chemistryRespiratory disorderGluconic acidSuccinic acid
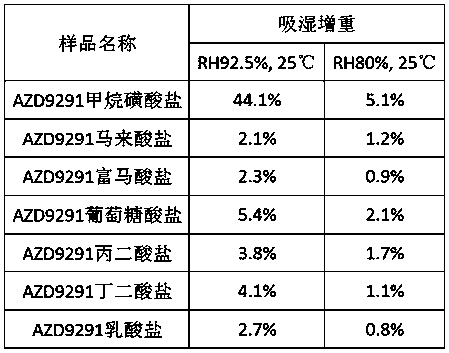
The invention belongs to the technical field of medicines, and particularly relates to a pharmaceutical salt of AZD9291 and a preparation method thereof. The novel pharmaceutical salt of AZD9291 disclosed by the invention is maleate, fumarate, gluconate, malonate, succinate or lactate. AZD9291 reacts with maleic acid, fumaric acid, gluconic acid, malonic acid or succinic acid in a ketone or alcohol solvent, and by crystallization, the pharmaceutical salt is obtained. Compared with AZD9291 mesylate, the pharmaceutical salt of AZD9291 provided by the invention has lower hygroscopicity, overcoming the problem that the conventional AZD9291 mesylate can easily deliquesce, and therefore is more suitable for medicine development.
Owner:FUDAN UNIV
Novel environment friendly preparation method for prasugrel
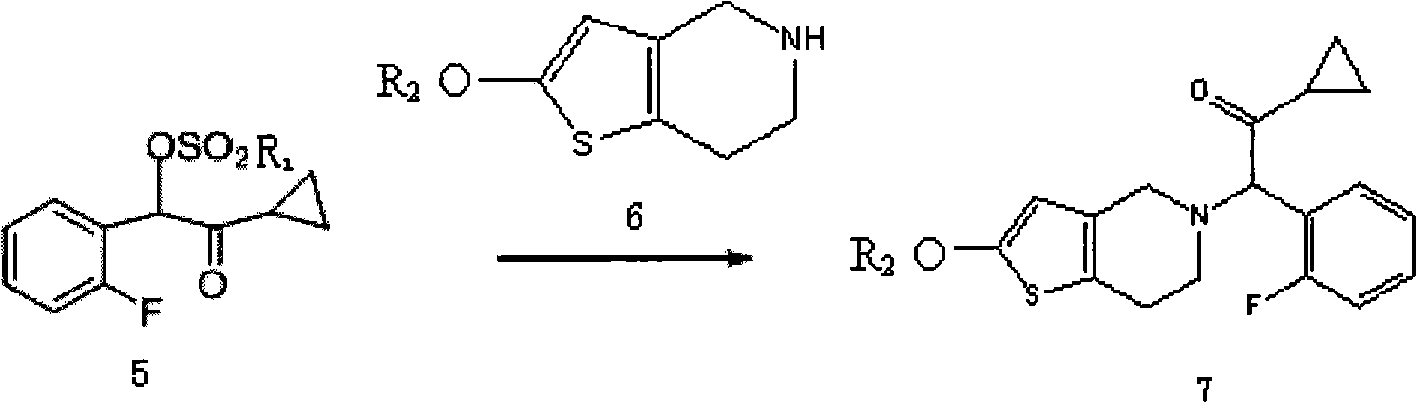
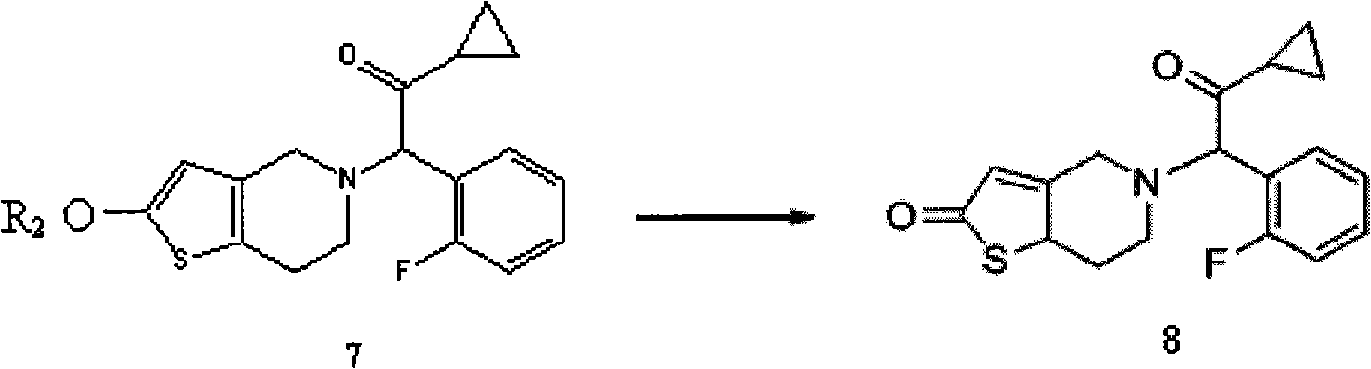
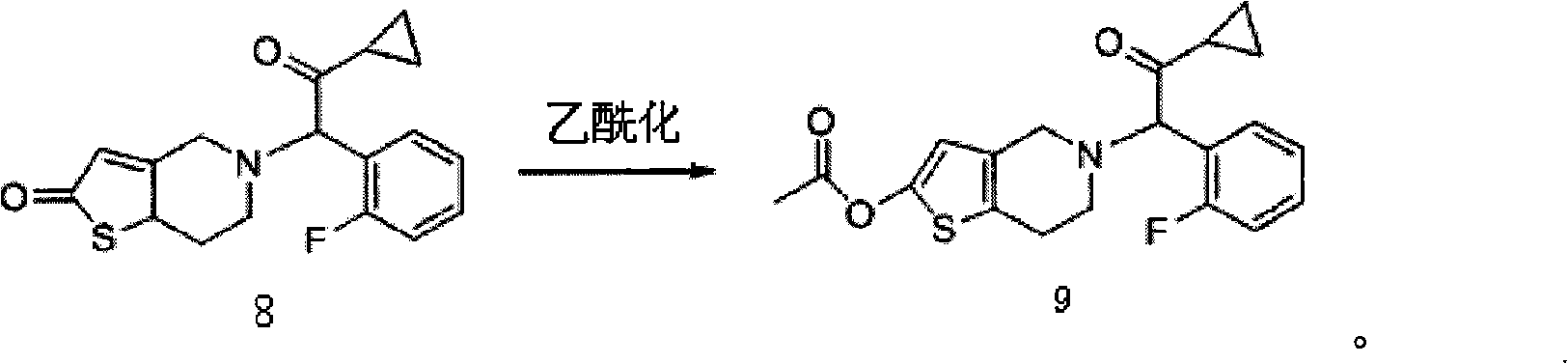
ActiveCN101402642ANo pollution in the processNo pollutionOrganic chemistryBlood disorderEthyl esterPyridine
The invention relates to the technical field of a preparation method of an antithrombotic medicament Prasugrel. The preparation method condenses and synthesizes 2-cyclopropyl-1-(2-fluorophenyl)-2- carbonyl ethyl ester mesylate and 2-methoxyl-4, 5, 6, 7-tetrahydro-thieno (3, 2-c) pyridine to form the Prasugrel. Compared with the prior art, the preparation method provided by the invention has low toxicity in raw materials and reagents used, low requirements in labor protection, low prices of and easy access to raw materials and reagents, less emission of three wastes (waste gas, waste water andwaste residues) and no corrosion to equipment during the production; simultaneously, the preparation method also has tender reaction conditions, simple operation and greater yield compared with the prior art and is applicable to industrial mass production.
Owner:SHANGHAI SHYNDEC PHARMA CO LTD +1
Salts of clopidogrel and process for preparation
Disclosed are new salts of Clopidogrel viz. Clopidogrel mesylate, Clopidogrel besylate and Clopidogrel tosylate, methods for their preparation and pharmaceutical compositions containing them and their use in medicine.
Owner:CADILA HEALTHCARE LTD
Process for the preparation of compositions for modulating a kinase cascade and methods of use thereof
The invention relates to compositions comprising 2-(5-(4-(2-morpholinoethoxy)phenyl)pyridin-2-yl)-N-benzylacetamide and its mesylate and dihydrochloride salts. More specifically, the invention provides an efficient process for the synthesis of 2-(5-(4-(2-morpholinoethoxy)phenyl)pyridin-2-yl)-N-benzylacetamide and its mesylate and dihydrochloride salts and methods for modulating one or more components of a kinase cascade using the compositions of the invention.
Owner:ATNX SPV LLC
Compositions for modulating a kinase cascade and methods of use thereof
The invention relates to compositions comprising 2-(5-(4-(2-morpholinoethoxy)phenyl)pyridin-2-yl)-N-benzylacetamide and its mesylate and dihydrochloride salts. The invention provides an efficient process for the synthesis of 2-(5-(4-(2-morpholinoethoxy)phenyl)pyridin-2-yl)-N-benzylacetamide and its mesylate and dihydrochloride salts and methods for modulating one or more components of a kinase cascade using the compositions of the invention. The present invention also provides a novel polymorph of the mesylate salt of 2-(5-(4-(2-morpholinoethoxy)phenyl)pyridin-2-yl)-N-benzylacetamide (Form A), characterized by a unique X-ray diffraction pattern and Differential Scanning Calorimetry profile, as well as a unique crystalline structure.
Owner:ATNX SPV LLC
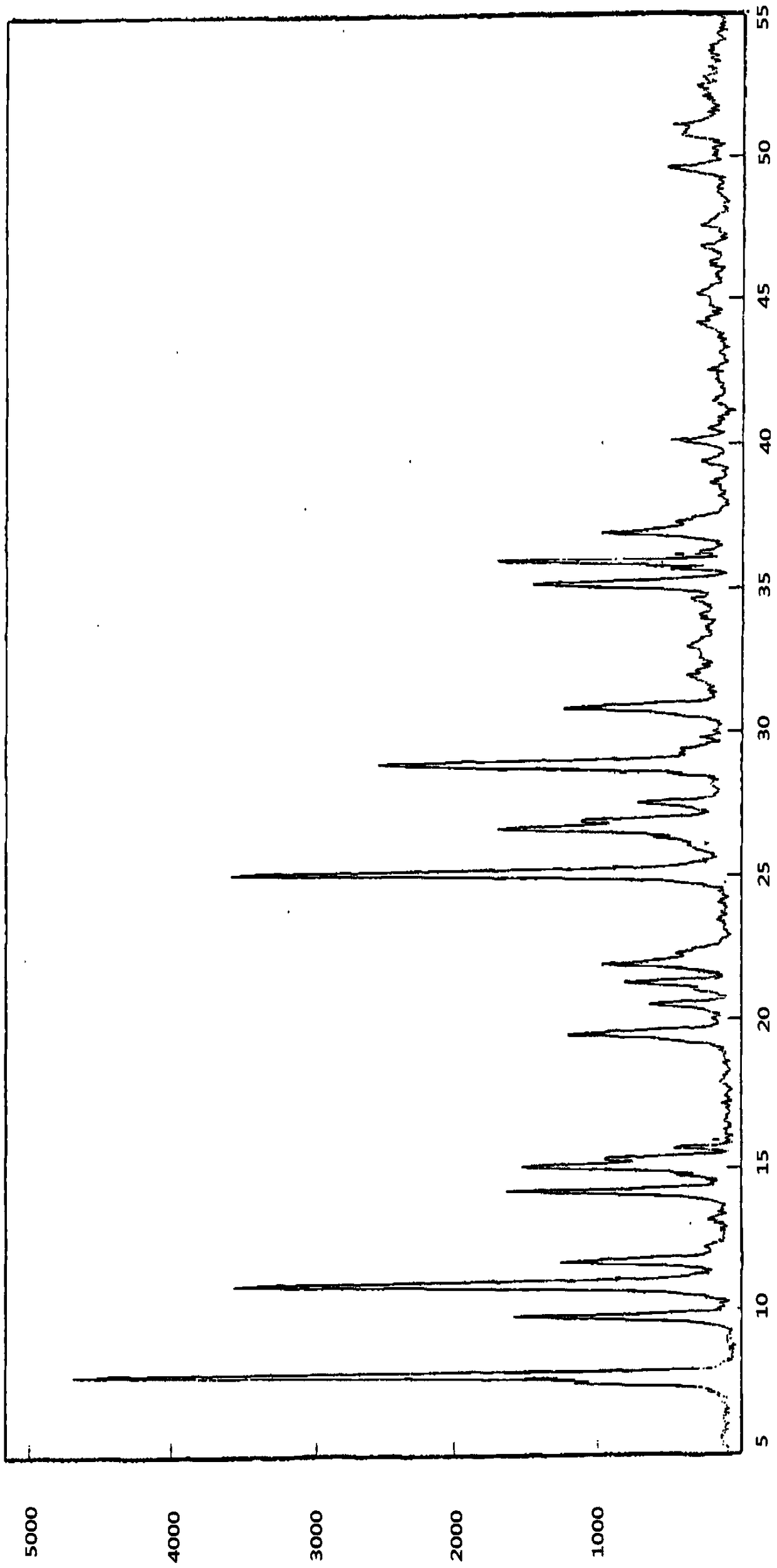
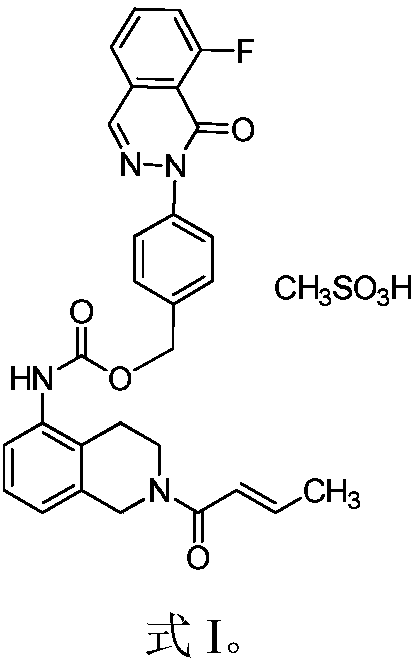
Medicinal composition combining paclitaxel with novel phthalazinone compound
InactiveCN109288830AGood anticancer effectImprove biological activityOrganic active ingredientsOrganic chemistry methodsCrystalCarbamic acid
A medicinal composition combining paclitaxel with a novel phthalazinone compound, provided by the invention, comprises an active component and a pharmaceutically-acceptable auxiliary material, the active component is composed of paclitaxel and the phthalazinone compound with an N crystal form, represented by formula I, and a mass ratio of the paclitaxel to the phthalazinone compound of the formulaI in the active component is (0.1-0.3):1, wherein the chemical name of the phthalazinone compound of the formula I is [2(1H)-butyl-2-enoyl-3,4-dihydroisoquinolin-5-yl]-carbamic acid-4-[8-fluoro-(2H)-phthalazin-1-one]benzyl ester mesylate. The medicinal composition has a good anticancer effect. It is found that the paclitaxel and the phthalazinone compound with an N crystal form, represented by formula I, are combined to greatly enhance the antitumor growth bioactivity and achieve antitumor proliferation synergistic effects, so the medicinal composition is expected to be developed into clinically anti-pancreatic cancer first-line drugs.
Owner:NANJING ADVANCED BIOLOGICAL MATERIALS & PROCESS EQUIP INST CO LTD
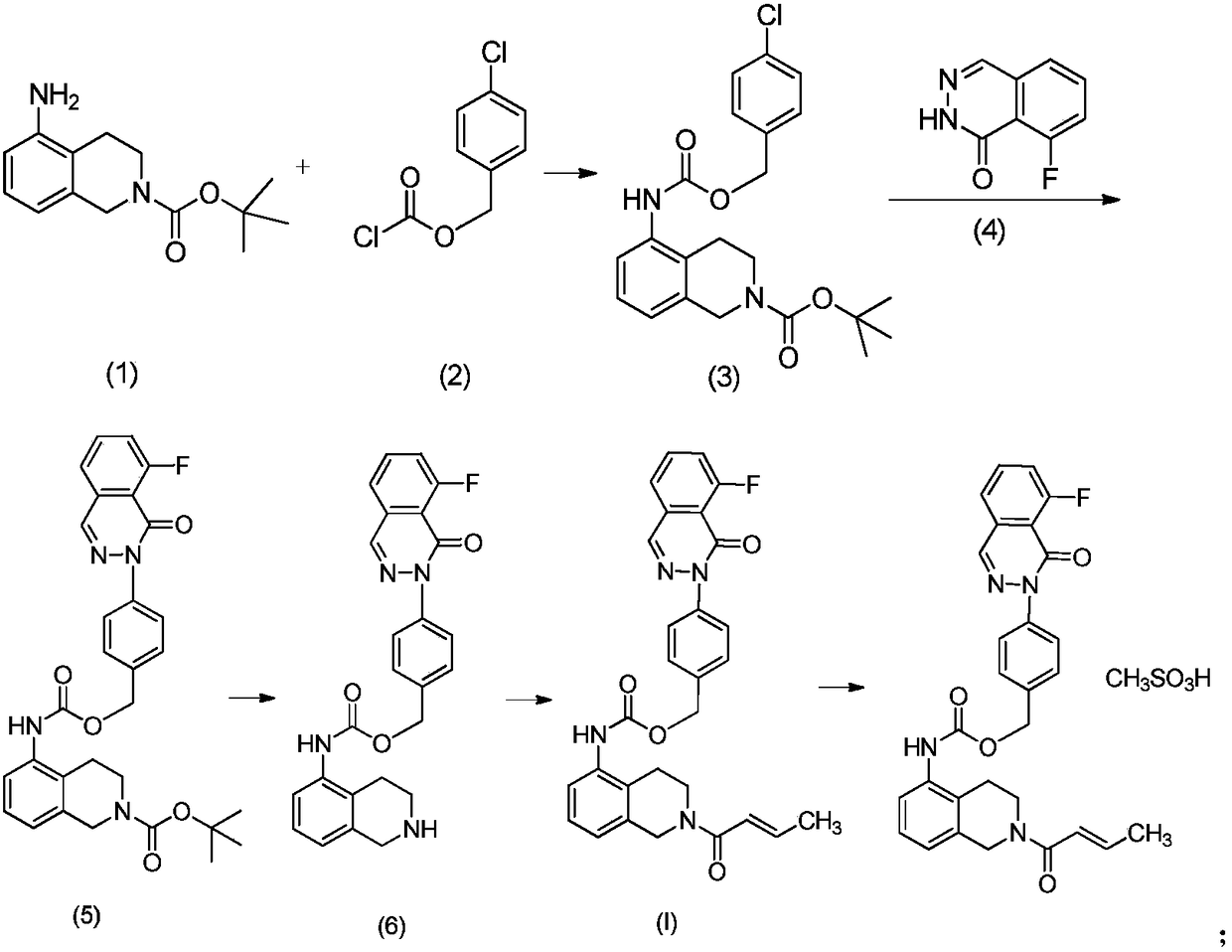
Synthesis process of N-substituted pyrrole
InactiveCN1931839AAdvanced and reasonable process routeMild reaction conditionsOrganic chemistryRare-earth elementDiketone
The present invention is green synthesis process of N-substituted pyrrole as shown in expression I. The N-substituted pyrrole is prepared with diketone as shown in expression II and amine as shown in expression III as materials, the trifluoro mesylate of RE element or transition element as catalyst, and the reactant as solvent or added organic solvent, and through reaction at 0-150 deg.c for 10 min to 24 hr, filtering the reacted liquid to obtain filter cake, dissolving the filter cake in water and further filtering out the insoluble matter as the N-substituted pyrrole compound. The present invention has high reaction yield over 85 %, reasonable technological path, mild reaction condition, reusable catalyst consumption and no wastes produced basically.
Owner:WENZHOU UNIVERSITY
Pazufloxacin mesylate tablet and preparation method and detection method thereof
ActiveCN102125533ANice appearanceHigh dissolution rateAntibacterial agentsOrganic active ingredientsSide effectAdhesive
The invention relates to a pazufloxacin mesylate tablet and a preparation method and a detection method thereof, which belong to the technical field of medicines. A medicinal oral tablet with high dissolvability and high stability is prepared by the following steps of: screening the formula components of the pazufloxacin mesylate tablet; and selecting suitable adhesives and adopting specific coating materials. Clinical trials show that the pazufloxacin mesylate tablet has an exact curative effect, a few side effects and high safety.
Owner:BEIJING SIHUAN KEBAO PHARM CO LTD
Ropivacaine mesylate freeze-dried powder injection
ActiveCN102038651AAvoid the risk of safety accidentsGood freeze-dried appearancePowder deliveryPharmaceutical product form changeFreeze-dryingPhysical chemistry
The invention relates to a ropivacaine mesylate freeze-dried powder injection which consists of ropivacaine mesylate and a PH regulator and is prepared by using the following freeze drying method: (1) a section quick-freezing stage: keeping bulked ropivacaine mesylate solution at the temperature of 0 DEG C for 10-30 minutes, and then keeping the bulked ropivacaine mesylate solution at the temperature of minus 35 DEG C-minus 45 DEG C for 1-2 hours; (2) a lyophilization stage: heating to 0 DEG C under the vacuum degree of 10-20 Pa and at the speed of 2-10 DEG C / h, and then keeping the temperature for 1-3 hours; and (3) a desorption drying stage: heating to 30 DEG C under the vacuum degree of 0-10 Pa and at the speed of 5-10 DEG C / h and keeping the temperature for 2-5 hours. The freeze-dried powder injection provided by the invention has the advantages of high yield, good re-dissolubility, more stable quality and the like.
Owner:鲁南新时代生物技术有限公司
Preparation method of fulvestrant
InactiveCN103788164AHigh yieldEasy to prepareSteroidsBulk chemical productionProtecting groupFulvestrant
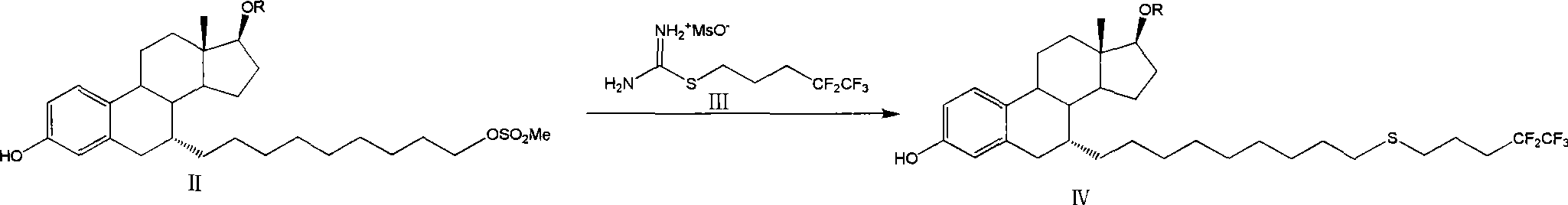
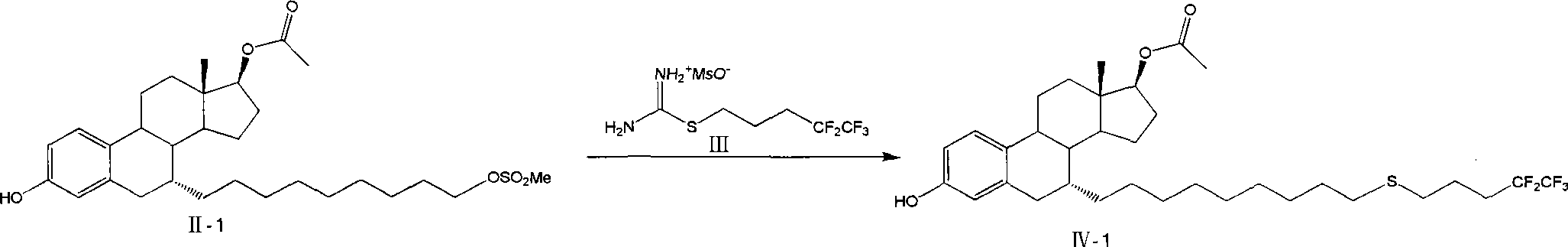
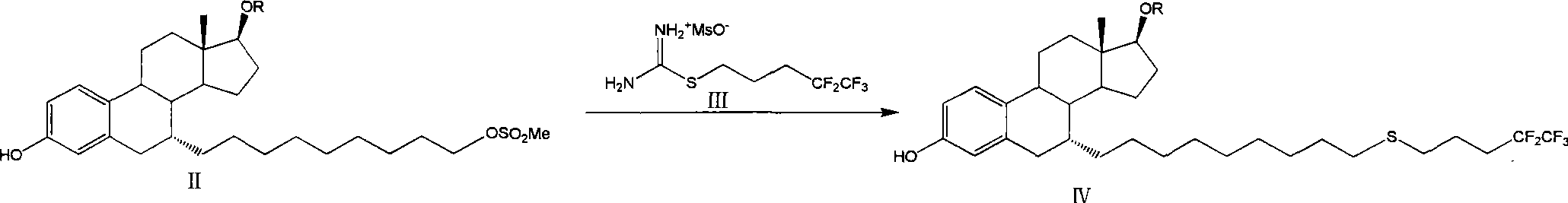
The invention relates to a preparation method of fulvestrant. The preparation method comprises a step of implementing reaction on a fulvestrant intermediate body (represented as formula II) and S-(4,4,5,5,5-pentafluoro amyl) isothiourea mesylate to introduce a pentafluoro amyl sulfenyl group, and further comprises a step (ii) of removing a protecting group of hydroxyl and / or a step iii) of oxidizing thioether into sulfoxide according to demand. According to the preparation method disclosed by the invention, raw materials are convenient to prepare and the post-processing is relatively simple, the yield of a target product is increased, the production cost is reduced, and the fulvestrant is applicable to large-scale industrial production.
Owner:CHIA TAI TIANQING PHARMA GRP CO LTD
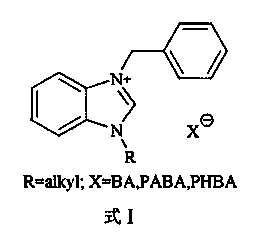
Halogen-free flame retardant gel coat resin and preparation method thereof
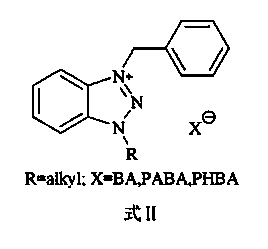
The invention discloses a halogen-free flame retardant gel coat resin which comprises a matrix resin, a thixotropic agent, a liquid additive, color paste, and a flame retardant, wherein the flame retardant is composed of more than three of 1-alkyl-3-benzylbenzimidazole mesylate ionic liquid or 1-alkyl-3-benzyl benzotriazole ionic liquid, and aluminium hydroxide, magnesium hydroxide, ammonium polyphosphate, phosphate and derivatives thereof, silicon nitride, nano montmorillonite, barium sulfate, metal oxides and nano hydrotalcite. According to the halogen-free flame retardant gel coat resin disclosed by the invention, the ionic liquids are used in a flame retardant gel coat, and the addition amount of the ionic liquids is less, but the flame retardant properties of the coat are significantly enhanced. The prepared gel coat resin has the advantages of moderate viscosity, high thixotropy, high solid content, high strength, high flame retardant properties and the like, and is applicable to the surfaces of base resins or laminating materials with high flame retardant requirements for chemical engineering, yachts, textiles and rail transit and the like.
Owner:CHANGZHOU TIANMA GROUP CO LTD
Synthesis of pharmaceutically useful pyridine derivatives
A process is provided for the preparation of compounds of formula I, useful in the preparation of compounds such as Omeprazole, Lansoprazole and Pantoprazole, wherein R1=H or CH3, R2=H or CH3, R3=Alkoxy (1-4C), OCH2CF3, Cyano, Hydrogen, Halogen, Acetoxy or Aryloxy, any electron withdrawing group or salts (organic or inorganic) of electron donating groups, R=Alkoxy, Hydroxy, Halogen, Activated ester, Tosylate, Mesylate, Thiol or Xanthyl, wherein the process for the preparation of compound of formula I employs a free radical reaction to functionalize the 2-position.
Owner:PDI RES LAB
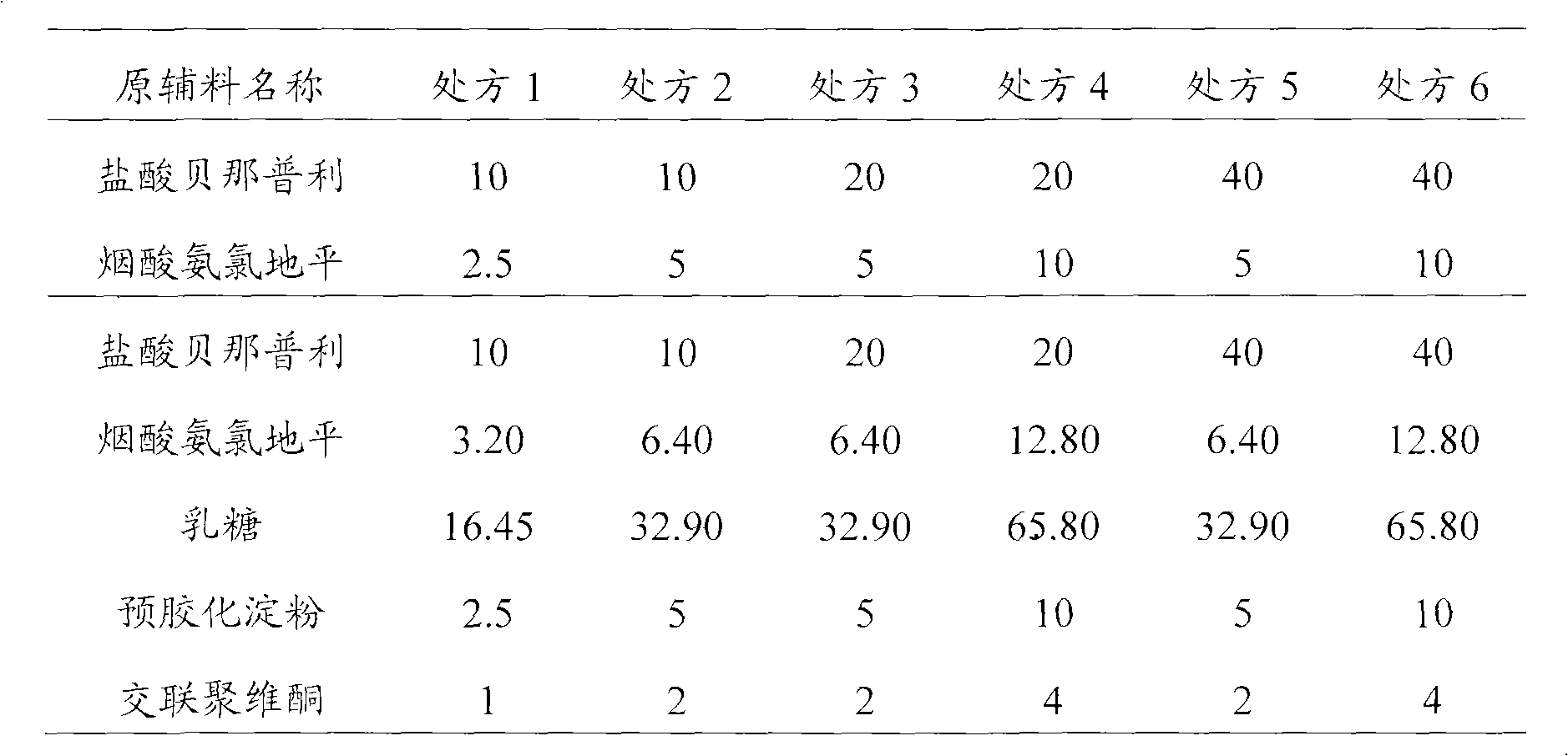
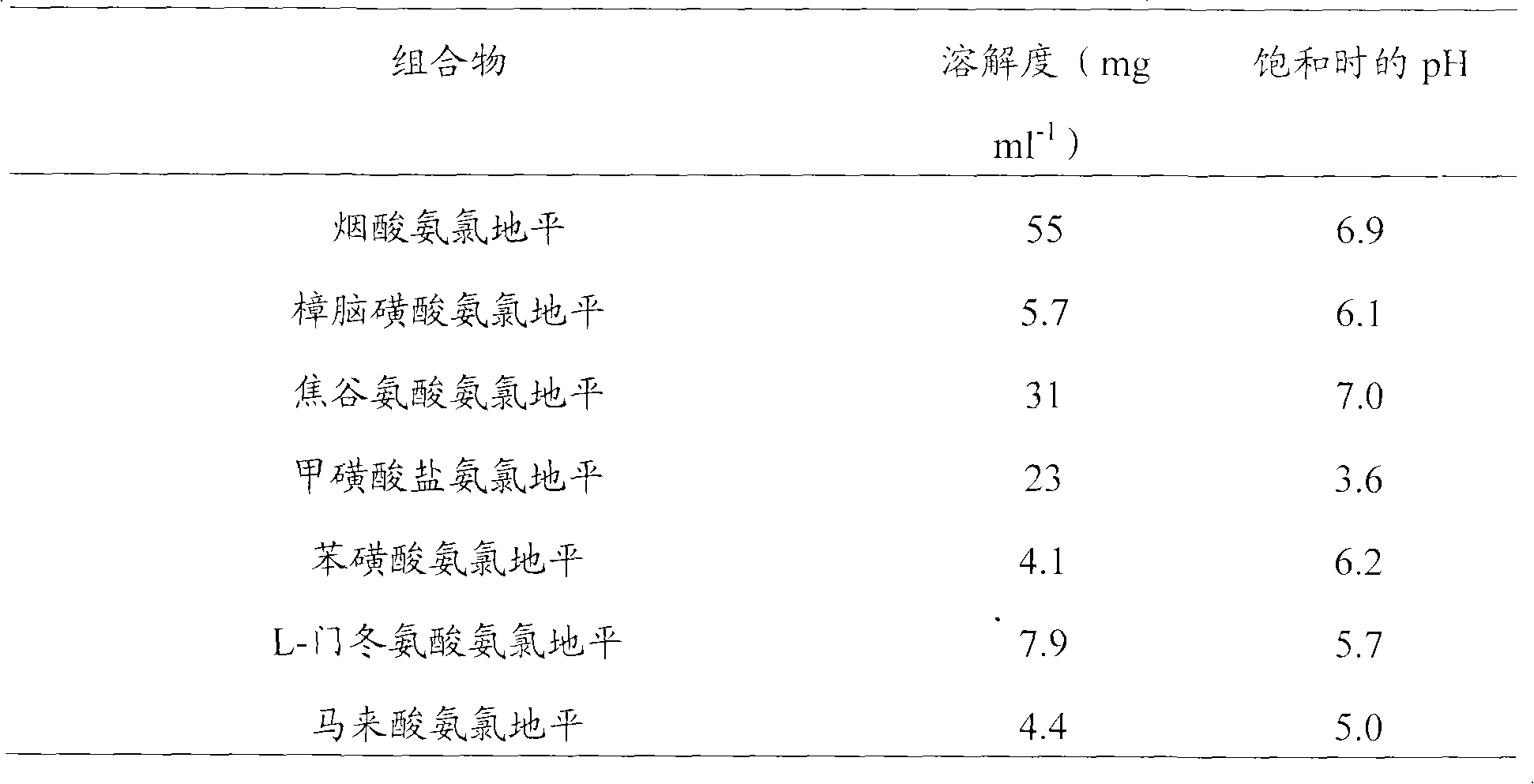
Treatment composition containing amlodipine series salt and pril medicament
InactiveCN101653440AMetabolism disorderHeterocyclic compound active ingredientsL-AspartateAmlodipine
The invention relates to an amlodipine series salt, which particularly comprises nicotinate, camsilate, pyroglutamate, L-aspartate, maleate and mesylate. The invention also relates to a pril compoundor a pharmaceutical composition of a medicinal salt of the pril compound and a preparation method thereof, and a drug combination medicine box containing a composition of the amlodipine series salt and a composition of sartan compounds. The composition of the invention comprises the following components: a) a certain amount of an amlodipine salt; b) a certain amount of the pril compound or a medicinal salt of the pril compound; and c) a medicinal carrier or a diluting agent. The composition or the medicine box can be used for treating the patients suffering from hypertension, angina pectoris,atherosclerosis, and / or hypersphyxia and treating the patients (including the human beings) having heart danger symptoms.
Owner:BEIJING ROCK PHARMA
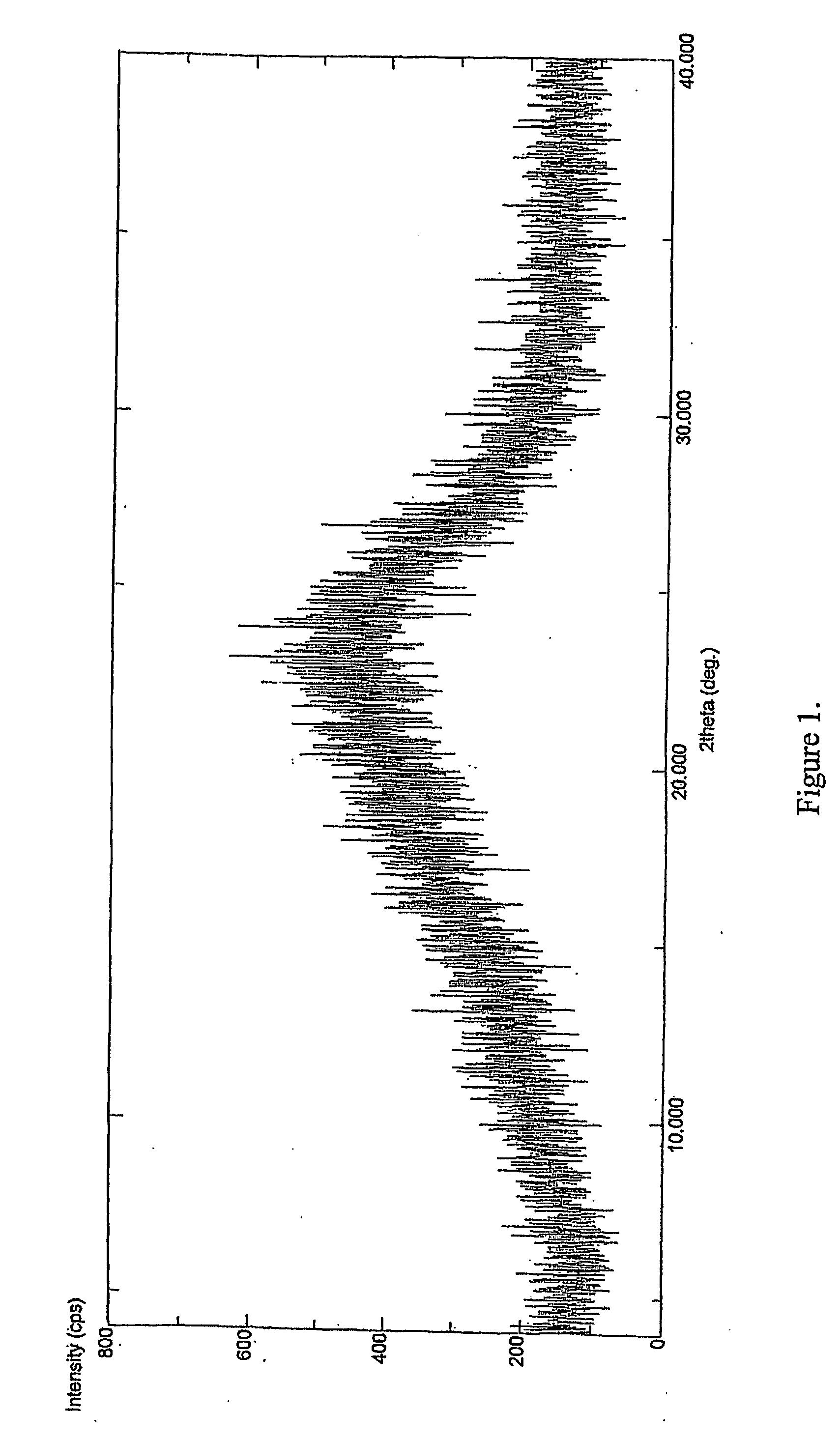
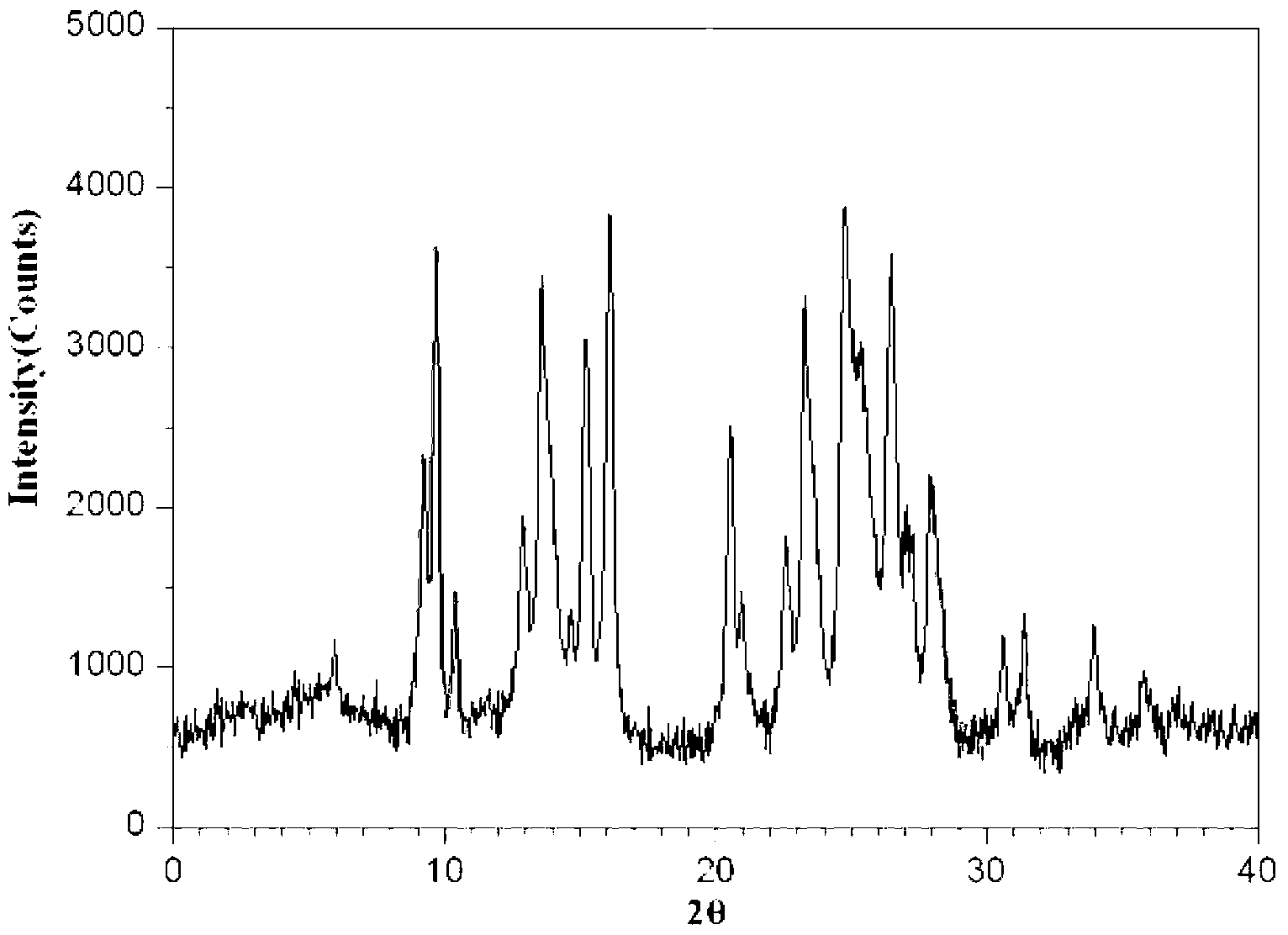
Ropivacaine mesylate compound, preparation process thereof and pharmaceutical composition thereof
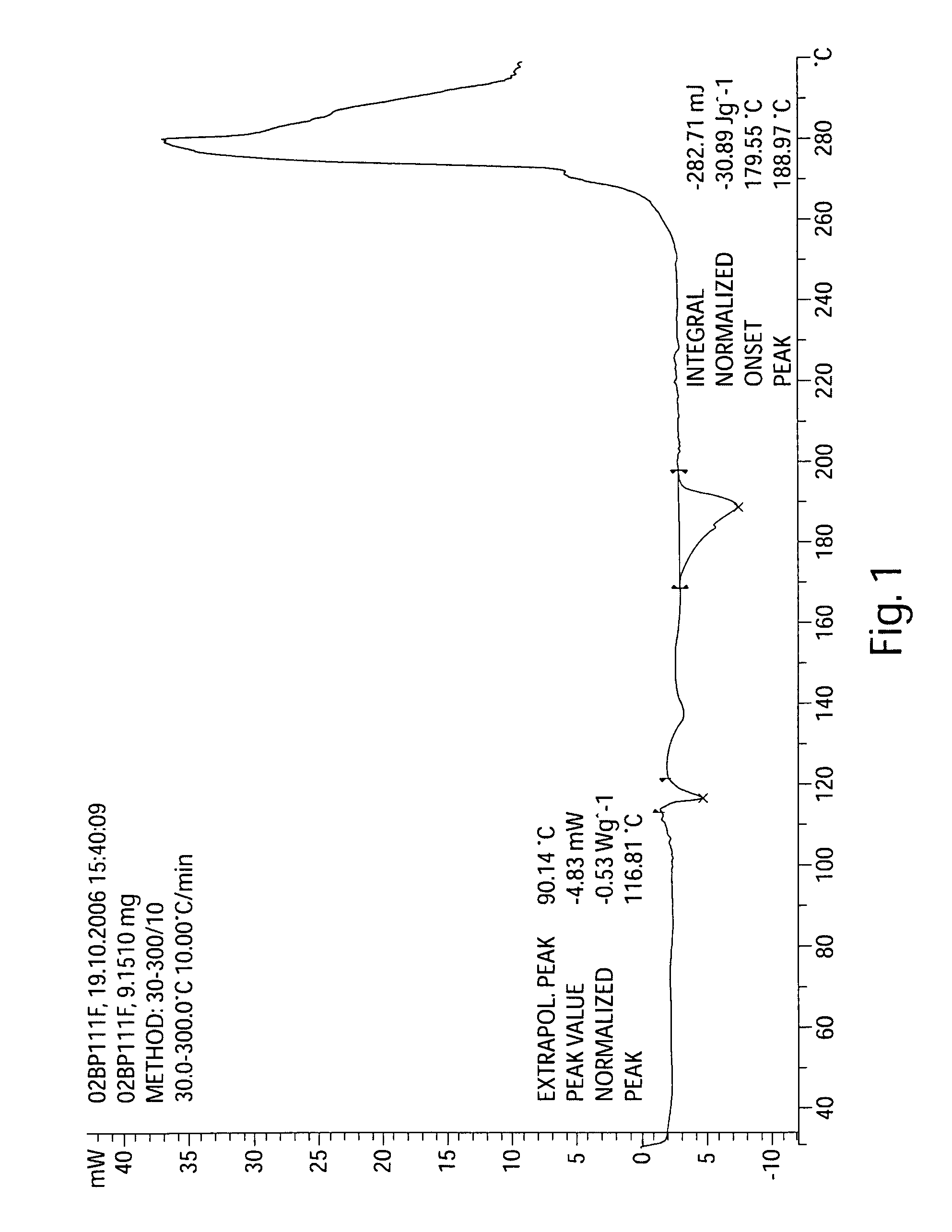
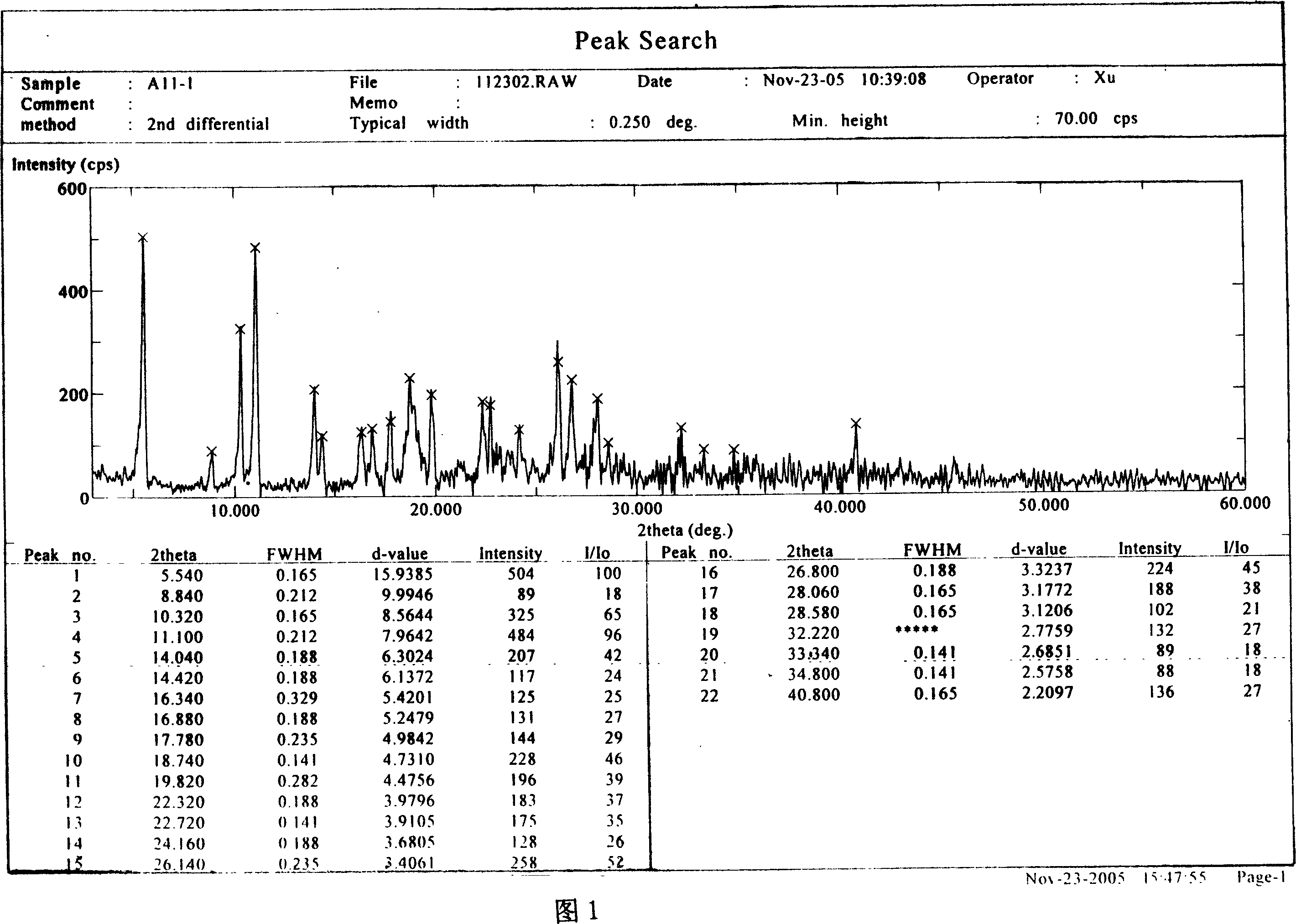
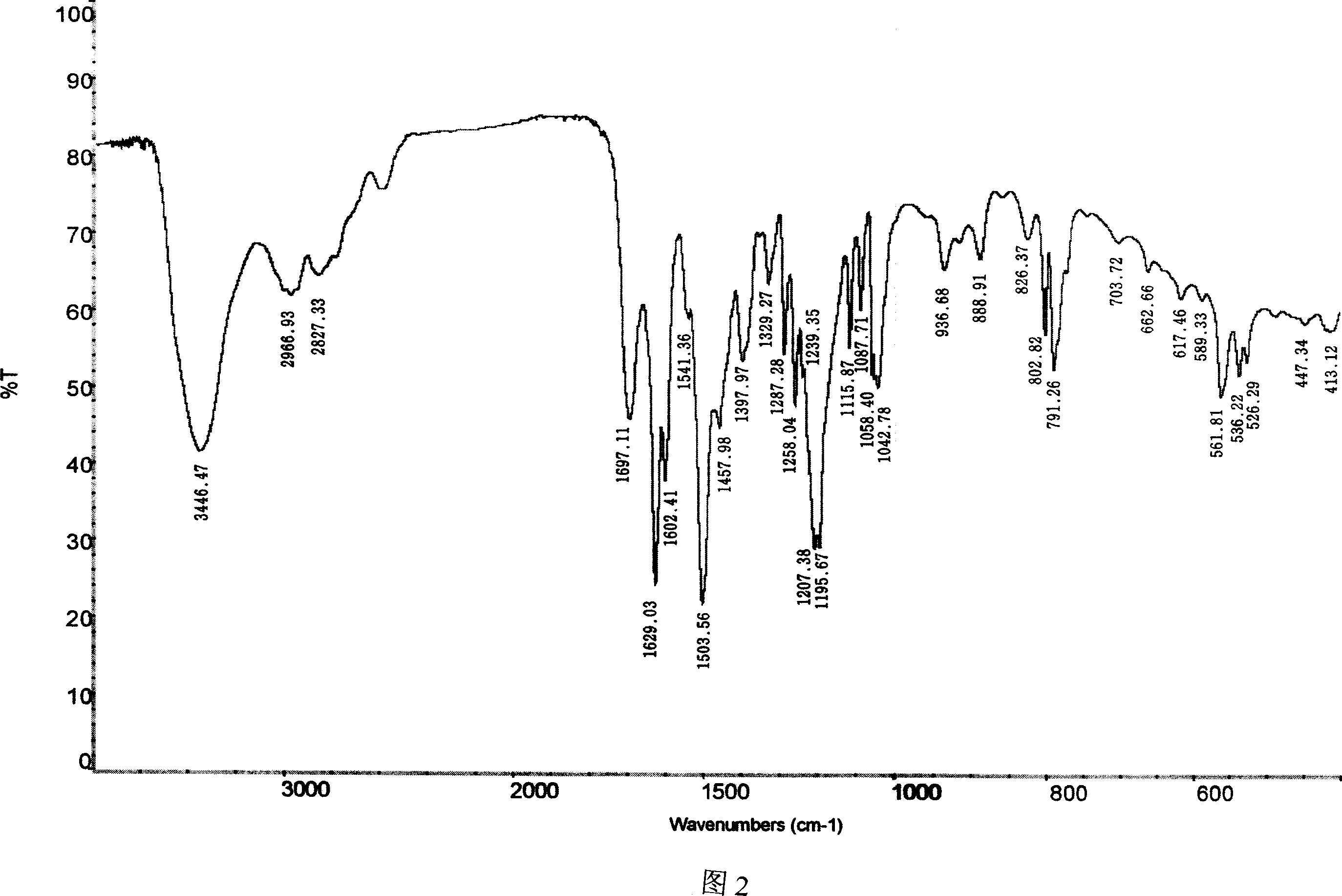
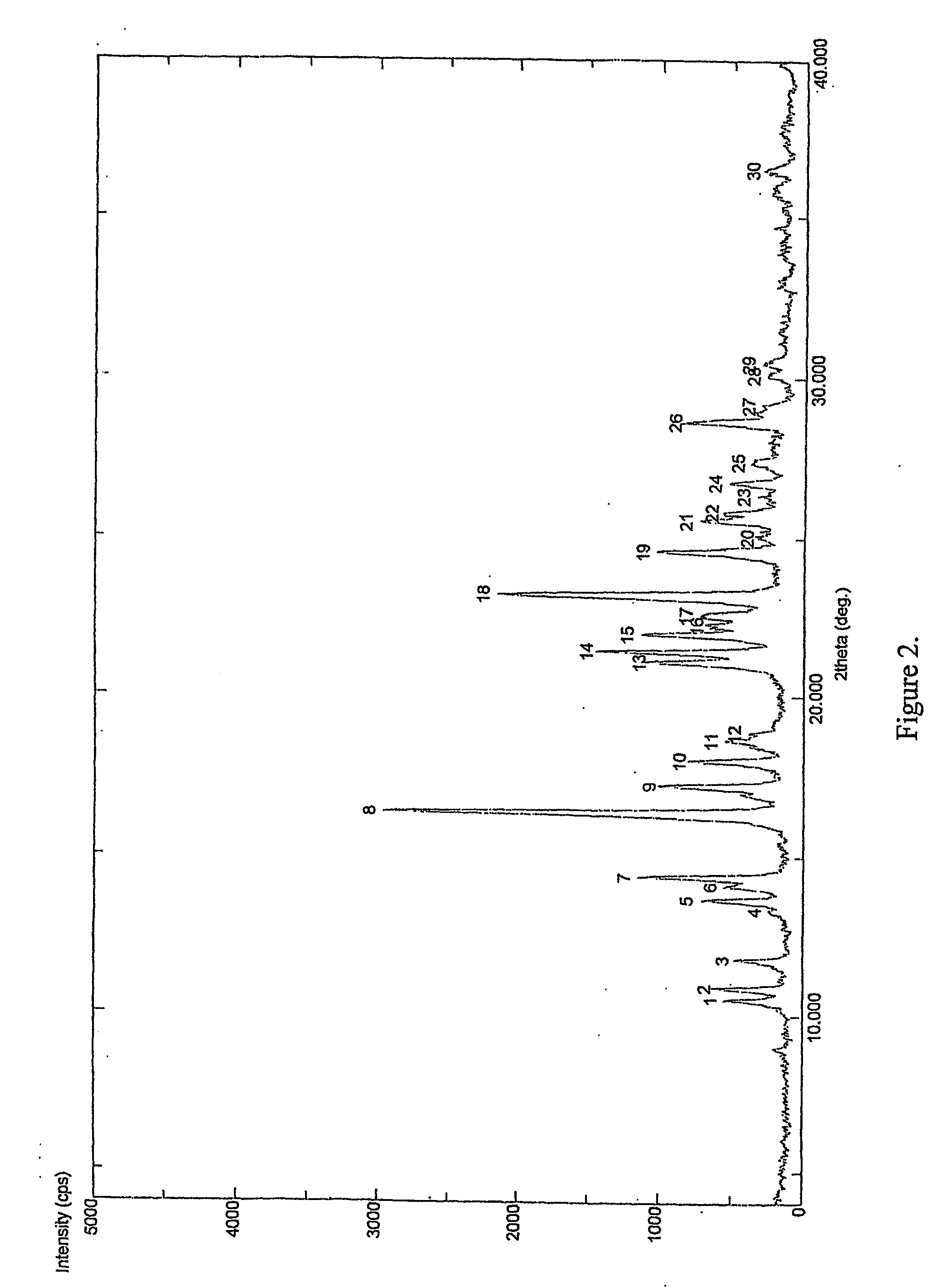
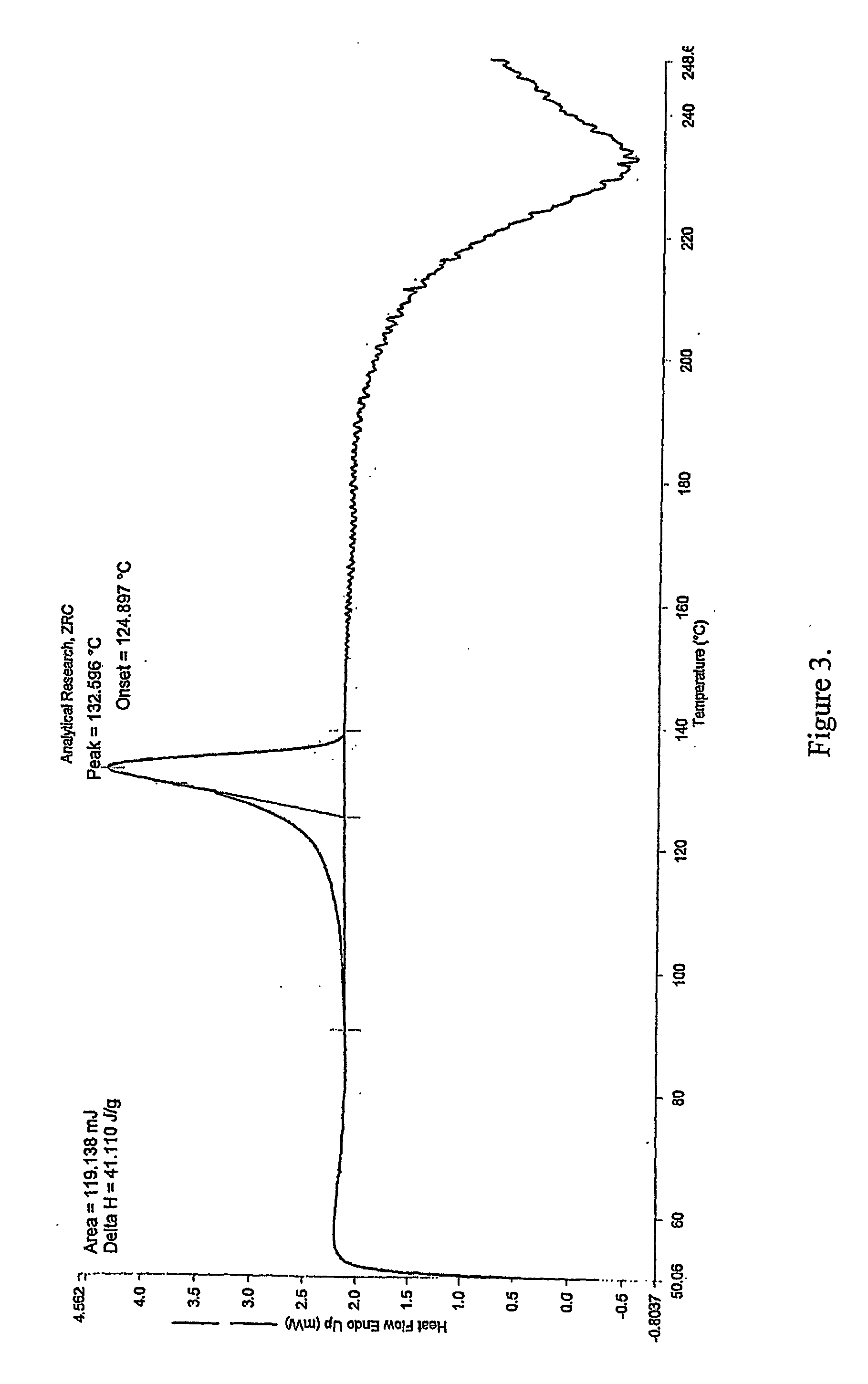
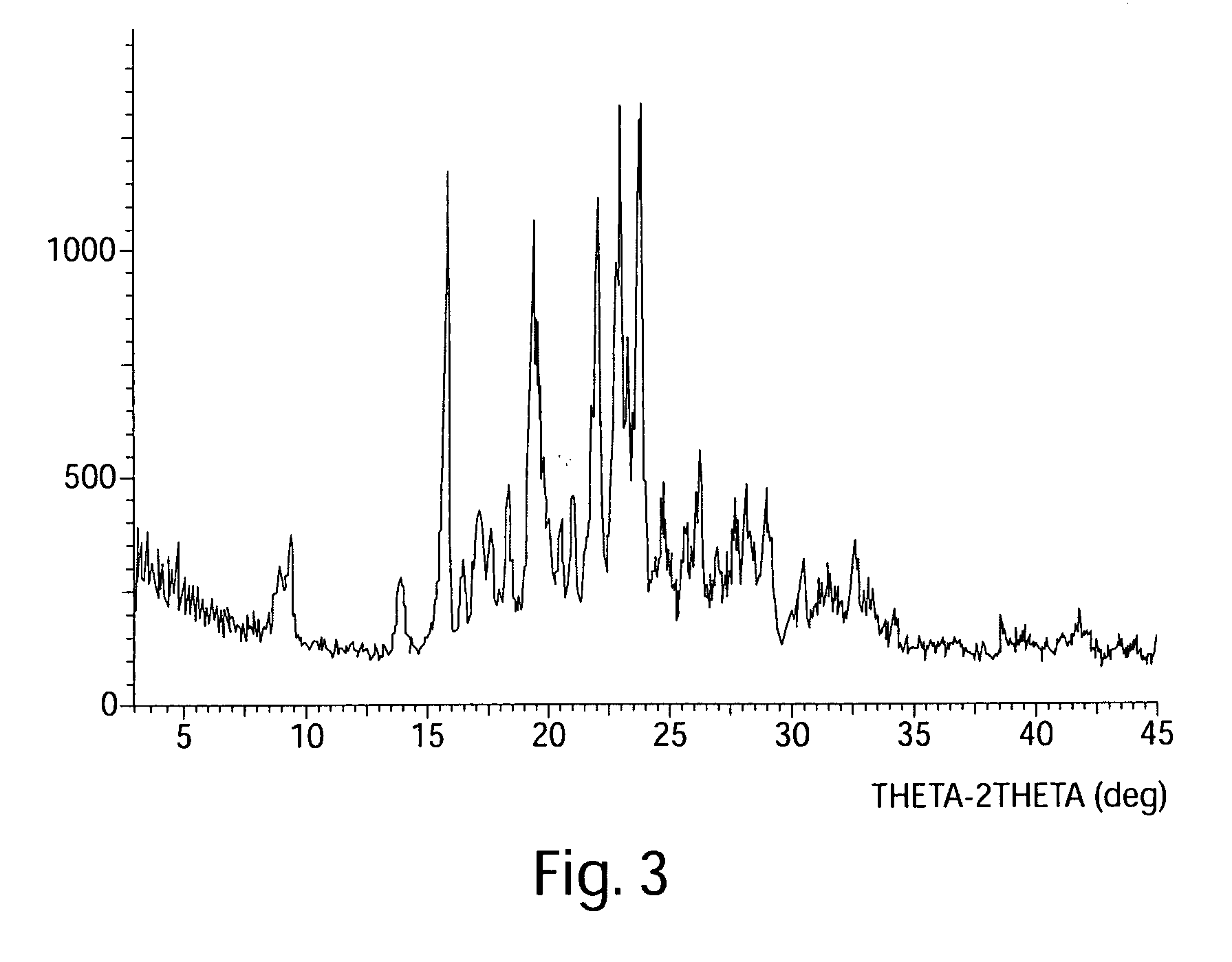
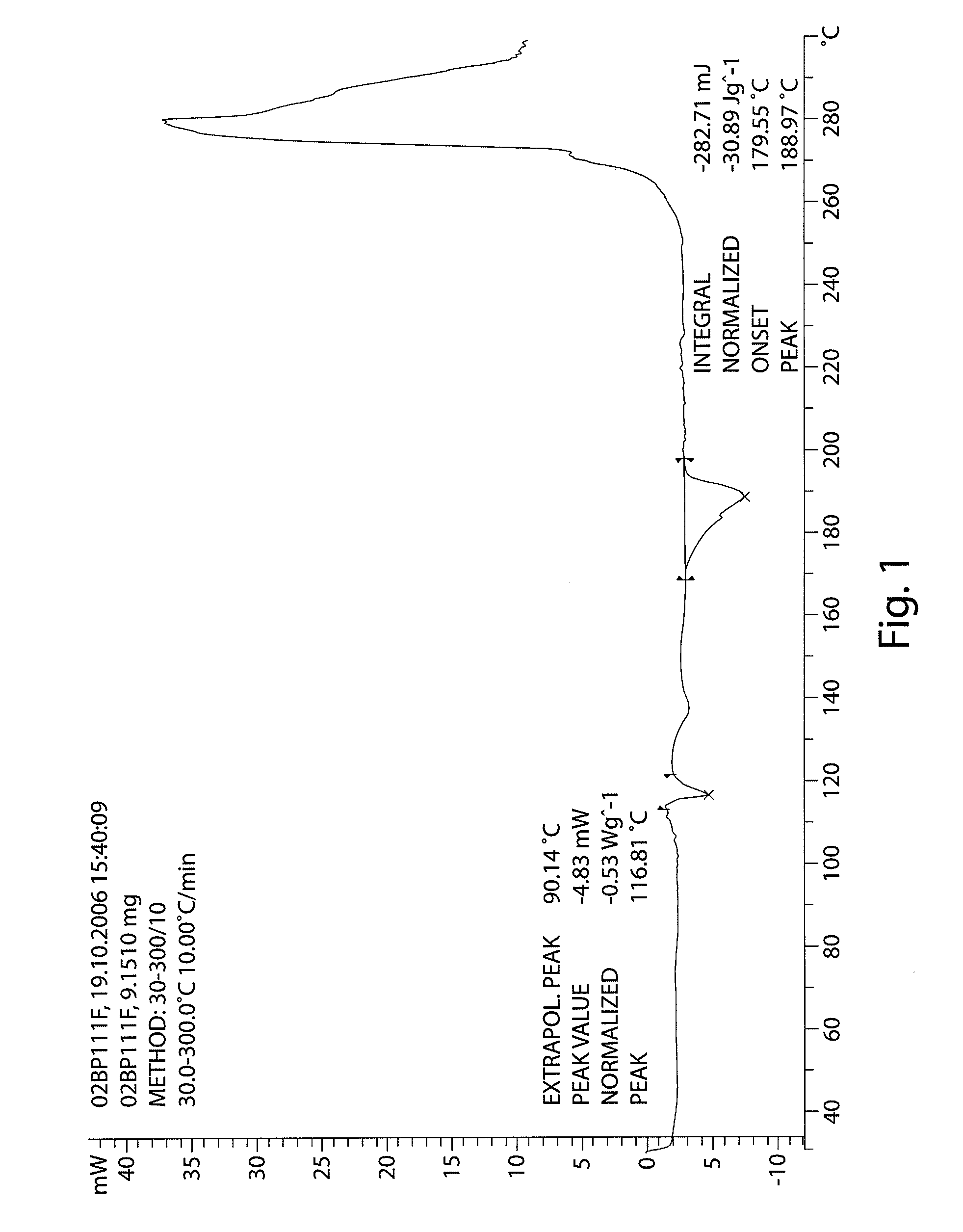
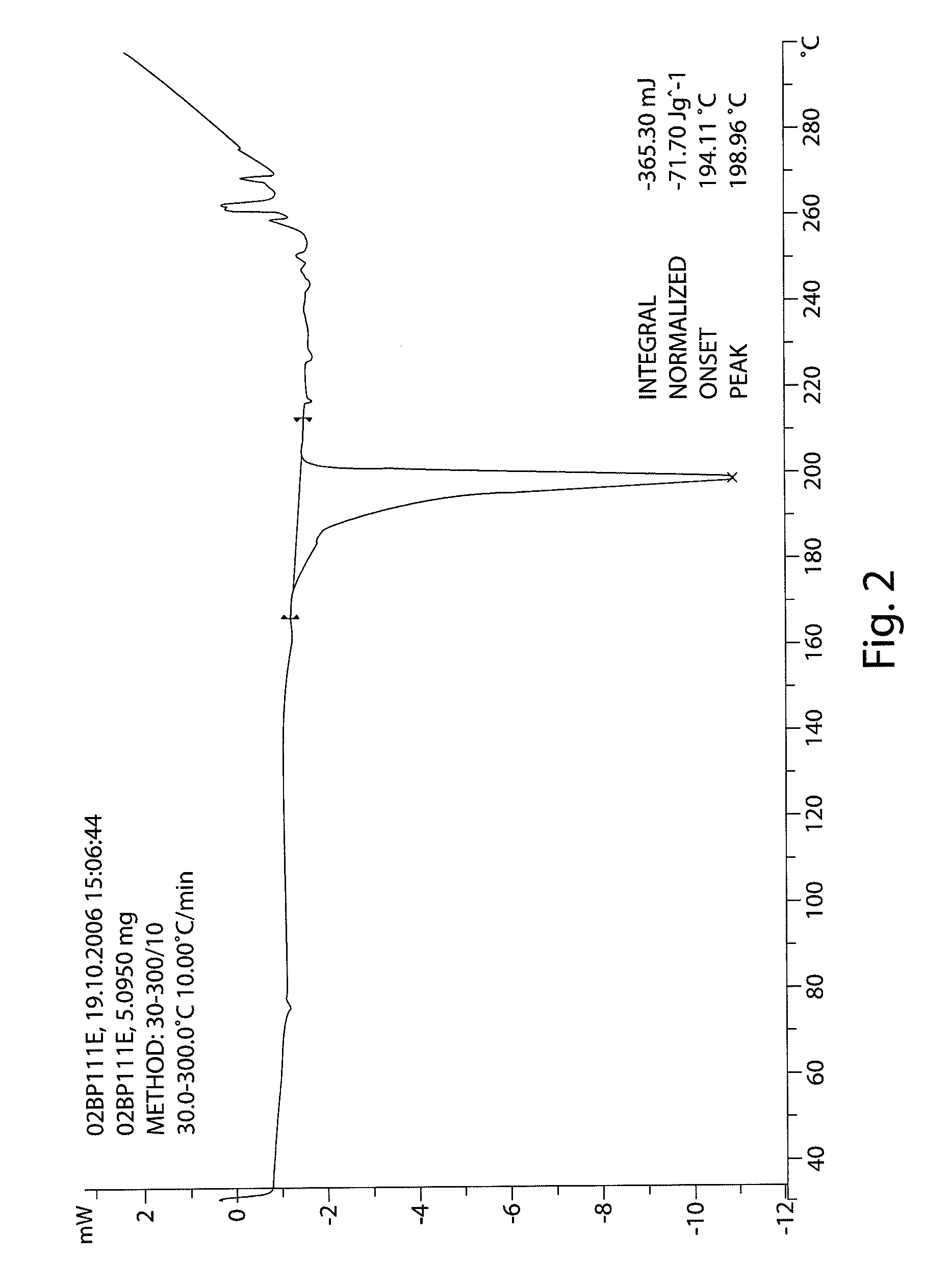
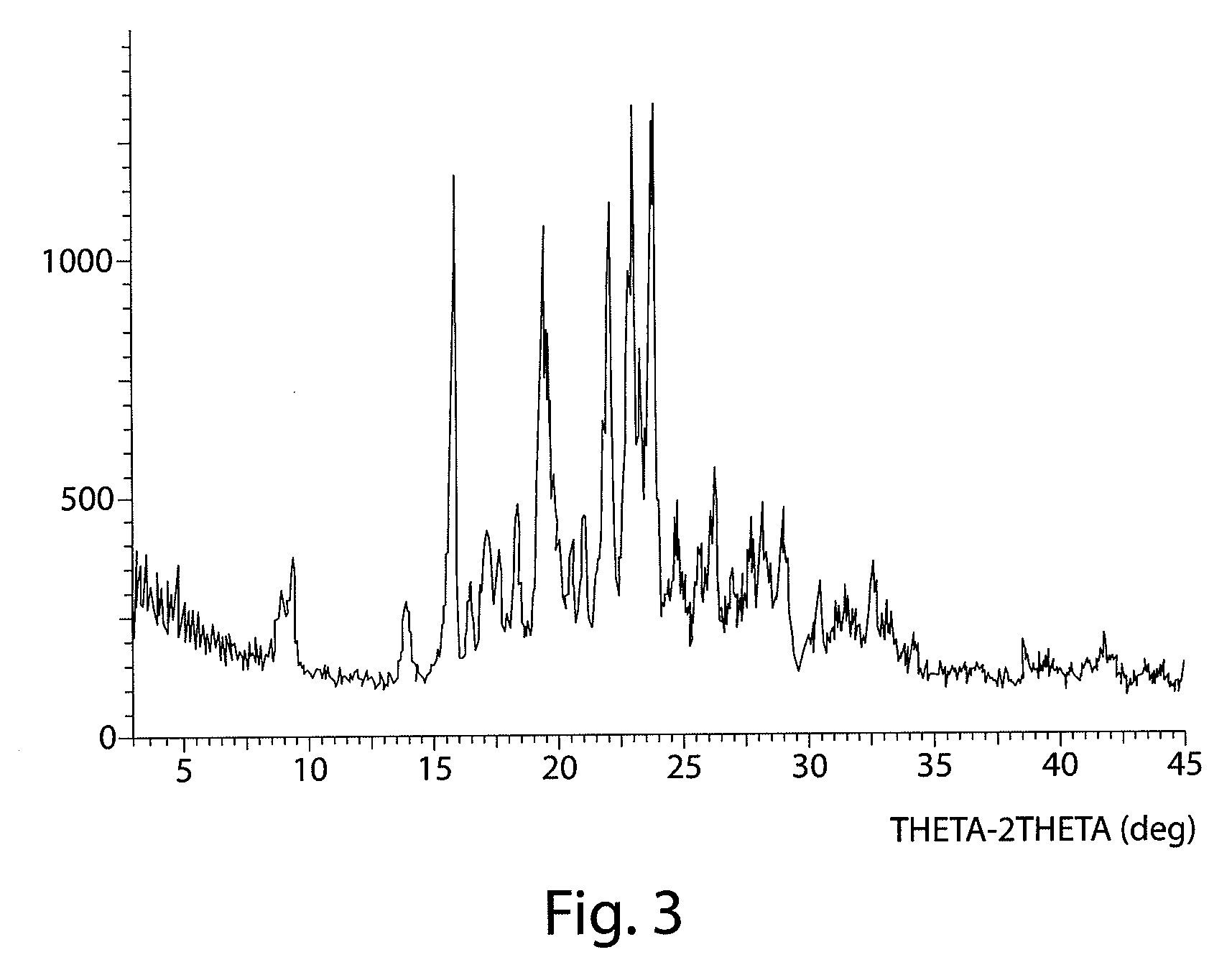
InactiveCN103304471AImprove solubilityFast dissolution rateSulfonic acids salts preparationHeterocyclic compound active ingredientsSolubilityX-ray
The invention belongs to the technical field of medicines and particularly relates to a ropivacaine mesylate compound. The structural formula of the ropivacaine mesylate compound is shown in the description, and an X-ray powder diffraction spectrogram obtained by measuring the ropivacaine mesylate compound with Cu-K alpha rays is shown in figure 1. The invention also provides a preparation process of the ropivacaine mesylate compound, a pharmaceutical composition containing the ropivacaine mesylate compound and a preparation process of the pharmaceutical composition. Dosage forms of the ropivacaine mesylate pharmaceutical composition are powder injections, small volume injections and large volume injections. The ropivacaine mesylate compound is high in solubility and high in dissolution rate, and the pharmaceutical composition of the ropivacaine mesylate compound is good in dissolubility, easy to dissolve and composite and high in bioavailability.
Owner:四川省惠达药业有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
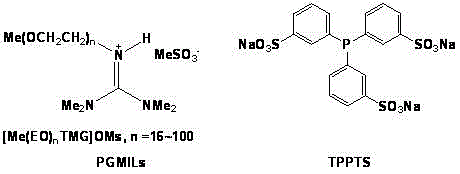
![[(indole-3-yl)pyrimidine-2-yl]aminophenylpropyl-2-eneamide derivative and its salt, preparation method of derivative, and application of derivative and salt [(indole-3-yl)pyrimidine-2-yl]aminophenylpropyl-2-eneamide derivative and its salt, preparation method of derivative, and application of derivative and salt](https://images-eureka.patsnap.com/patent_img/eb66cca8-32dc-4f07-9f38-12677107a7ba/HDA0000790432580000011.PNG)
![[(indole-3-yl)pyrimidine-2-yl]aminophenylpropyl-2-eneamide derivative and its salt, preparation method of derivative, and application of derivative and salt [(indole-3-yl)pyrimidine-2-yl]aminophenylpropyl-2-eneamide derivative and its salt, preparation method of derivative, and application of derivative and salt](https://images-eureka.patsnap.com/patent_img/eb66cca8-32dc-4f07-9f38-12677107a7ba/HDA0000790432580000012.PNG)
![[(indole-3-yl)pyrimidine-2-yl]aminophenylpropyl-2-eneamide derivative and its salt, preparation method of derivative, and application of derivative and salt [(indole-3-yl)pyrimidine-2-yl]aminophenylpropyl-2-eneamide derivative and its salt, preparation method of derivative, and application of derivative and salt](https://images-eureka.patsnap.com/patent_img/eb66cca8-32dc-4f07-9f38-12677107a7ba/HDA0000790432580000021.PNG)