Treatment composition containing amlodipine series salt and pril medicament
A kind of technology of amlodipine salt and composition, which is applied in the field of combined medicine kits, can solve the problems such as lack of solubility, intractable benzenesulfonic acid, slow onset of amlodipine and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0088] Example 1 General Preparation Method of Benazepril Hydrochloride / Amlodipine Niacin Common Tablets
[0089] Benazepril hydrochloride and nicotinic acid amlodipine were crushed separately, passed through a 80-mesh sieve, and set aside; lactose, microcrystalline cellulose, pregelatinized starch, sodium carboxymethyl starch, povidone K30, cross-linked carboxymethyl cellulose Sodium plain and magnesium stearate are passed through 80 mesh sieves respectively, and set aside.
[0090] Make povidone K30 or pregelatinized starch into a suitable viscosity adhesive with pure water. In addition to magnesium stearate, mix other raw materials and auxiliary materials according to the equal incremental dilution method, and then use the prepared adhesive to make a soft material, dry below 80°C, granulate, and add stearic acid in the prescribed amount Magnesium, mixed evenly, compressed into tablets. Or mix the raw materials and auxiliary materials of the prescribed amount, ...
Embodiment 2
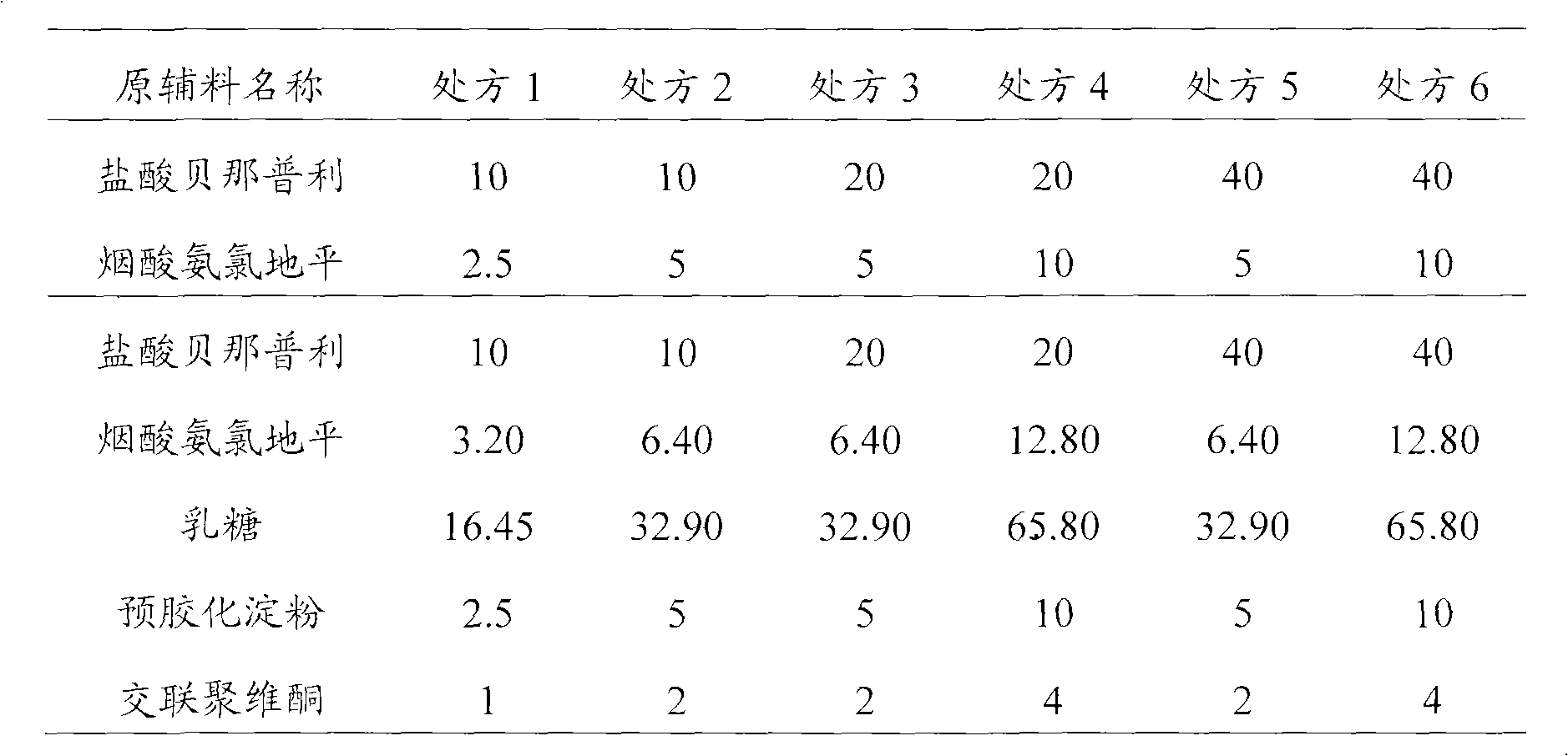
[0091] Example 2 General Preparation Method of Benazepril Hydrochloride / Amlodipine Series Salt Compound Capsules
[0092] The compound capsules include 6 dosage combinations of benazepril hydrochloride and amlodipine nicotinate, respectively including amlodipine nicotinate (calculated as amlodipine base) and benazepril hydrochloride (calculated as benazepril hydrochloride) Total) 2.5mg / 10mg, 5mg / 10mg, 5mg / 20mg, 10mg / 20mg, 5mg / 40mg and 10mg / 40mg. Its preparation method is as follows (preparation amount is 1000):
[0093]
[0094]
[0095] The capsule core of benazepril hydrochloride is firstly prepared, and the preparation method is as follows: pulverizing and mixing benazepril hydrochloride, lactose and pregelatinized starch, and then adding water to make granules. The wet granules are then sieved and dried. Then mix and pulverize with crospovidone and microcrystalline cellulose. The resulting mixture is then compressed into capsule cores. The capsule core is...
Embodiment 3
[0096] Embodiment 3 Light fastness experiment
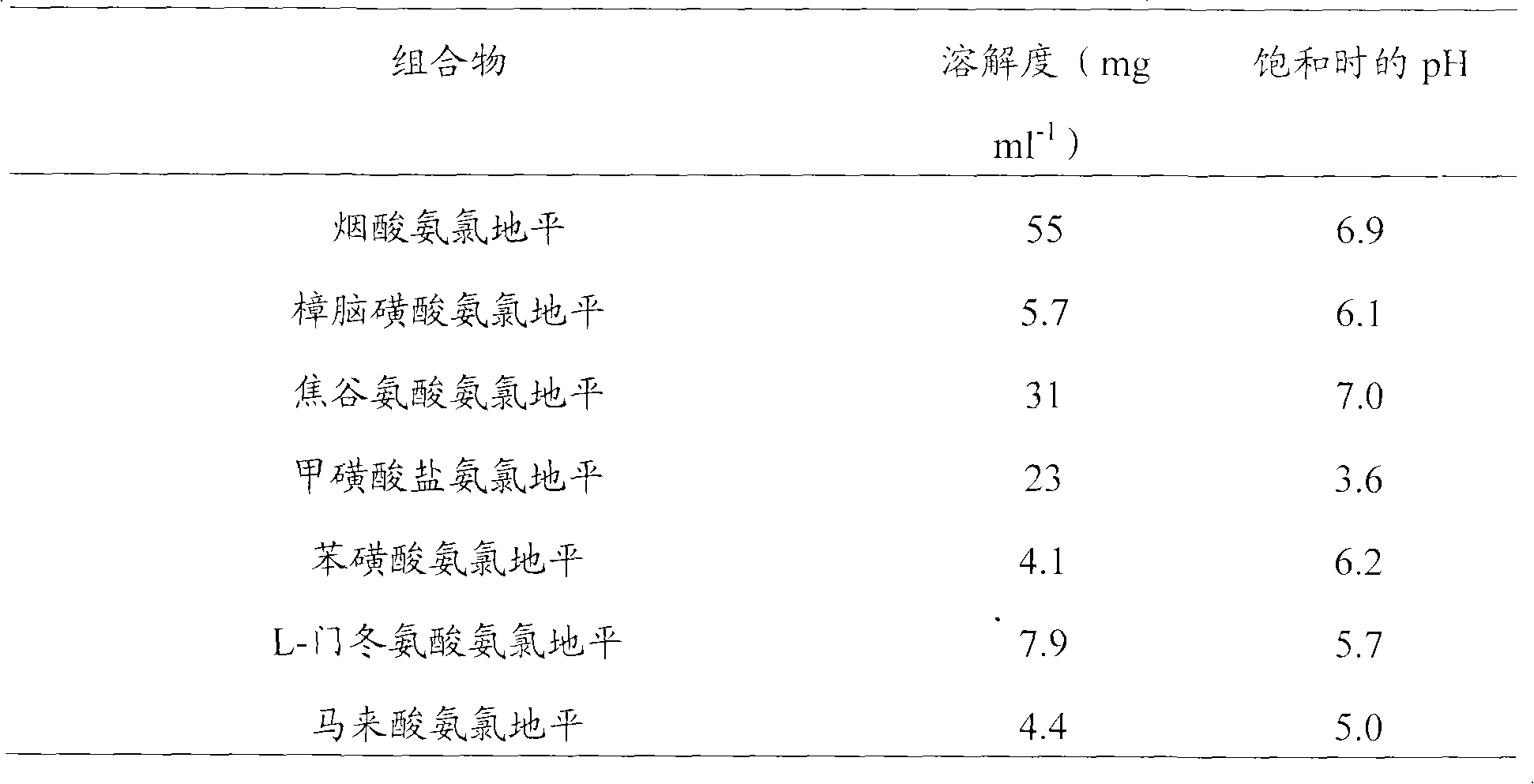
[0097] The pharmaceutical composition of the research product amlodipine series salts (5 mg) and benazepril hydrochloride (80 mg) is as described in Examples 1-4 (specifically the following table). The research article and the reference article (amlodipine besylate / benazepril hydrochloride composition) were exposed to an incandescent lamp (220V, 100W) at 50°C and placed 30cm above the sample for 4 weeks, and then each combination was measured related substances. From the above results, it is obvious that, compared with the reference substance, the study product exhibits the same effect except the L-amlodipine aspartic acid / benazepril hydrochloride and amlodipine maleate / benazepril hydrochloride compositions. Improved light fastness (arranged from high stability to low stability in the following table according to the stability of the related substance-related compositions produced).
[0098] Table 2....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com