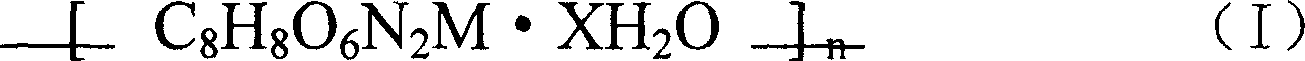SOD simulated compound with short peptide as ligand and preparation method thereof
A compound and amino acid technology, which is applied in the field of simulated SOD compounds with aspartic acid short peptide as ligands and their preparation, can solve the problem of low biological activity, low yield, poor activity stability and catalytic specificity, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Weigh 30.0g of manganese aspartate, pour it into a 250ml Erlenmeyer flask with 120ml of distilled water, stir at 80°C until it completely dissolves into a transparent solution, and carry out polycondensation reaction at 180-205°C for 4~ 5h. Eventually a white powdery solid was formed. Yield 93.92%.
[0017] C H N
[0018] Elemental analysis (%) Calculated value 30.11 3.79 8.78
[0019] Measured value 29.75 4.15 8.69
[0020] Determination of biological activity (chlorination NBT method) (see Table 1, 2)
[0021] Infrared spectrum measurement 3151~3424cm -1 Strong absorption (-NH stretching vibration, hydrogen bond)
[0022] 1579.63cm -1 Strong absorption (amide C=O, -NH deformation vibration)
[0023] 1395.78cm -1 (-COO-stretching vibration)
[0024] Gel Chromatography Analysis Weight Average Molecular Weight M W =1906
Embodiment 2
[0026] Weigh 60.0g of L-aspartic acid, pour it into a 500ml three-necked bottle with 240ml of distilled water preheated to 60°C, stir, heat up to 85°C, add 18.81g of manganese oxide, and keep it warm for 4.5 hours. It is light red, PH=6.8. After appropriate refining treatment, conduct polycondensation reaction at 180-210°C for 5.5 hours to obtain a white powdery solid. Yield 90.78%.
[0027] C H N
[0028] Elemental analysis (%) Calculated value 30.11 3.79 8.78
[0029] Measured value 30.03 3.97 8.74
[0030] Determination of biological activity (chlorination NBT method) (see Table 1, 2)
[0031] Infrared spectrum measurement 3134~3424cm-1 strong absorption (-NH stretching vibration, H bond)
[0032] Strong absorption at 1585.30cm-1 (amide C=O, -NH deformation vibration)
[0033] 1400.41cm-1 (-COO- stretching vibration)
[0034] Gel Chromatography Analysis Weight Average Molec...
Embodiment 3
[0036] Weigh 27.0g of L-aspartic acid, pour it into a 1L three-necked bottle filled with 200ml of distilled water, raise the temperature to 80-90°C, add 11.7g of manganese carbonate in batches and keep it warm at 90°C, react for 4-5 hours, and refine it appropriately After treatment, it becomes a transparent liquid, and undergoes polycondensation reaction at 180-220°C for 3-5 hours to obtain light yellow powdery solid. Yield 93.03%.
[0037] C H N
[0038] Elemental analysis (%) Calculated value 33.92 2.83 9.90
[0039] Measured value 33.12 3.24 9.31
[0040] Determination of biological activity (chlorination NBT method) (see Table 1, 2)
[0041] Infrared spectrum measurement 3265~3419cm-1 strong absorption (-NH stretching vibration, H bond)
[0042] 1587.76cm-1 strong absorption (amide C=O, -NH deformation vibration)
[0043] 1402.20cm-1 (-COO-stretching vibration)
[0044] ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com