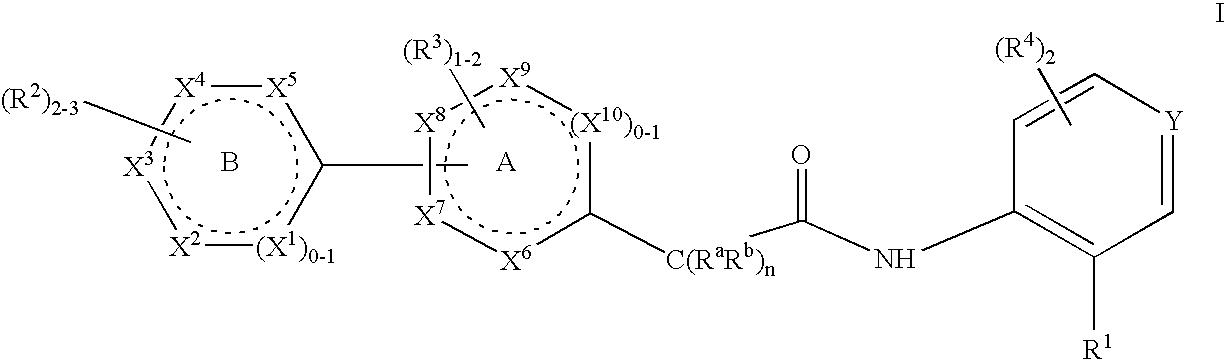
Patents
Literature
836 results about "Niacin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Niacin (nicotinic acid) is used to prevent and treat niacin deficiency (pellagra).
Nutraceutical composition and method of use for treatment / prevention of cancer
InactiveUS20070248693A1Function increaseAbility to createBiocideAlgae medical ingredients1,4-BenzoquinonePantothenic acid
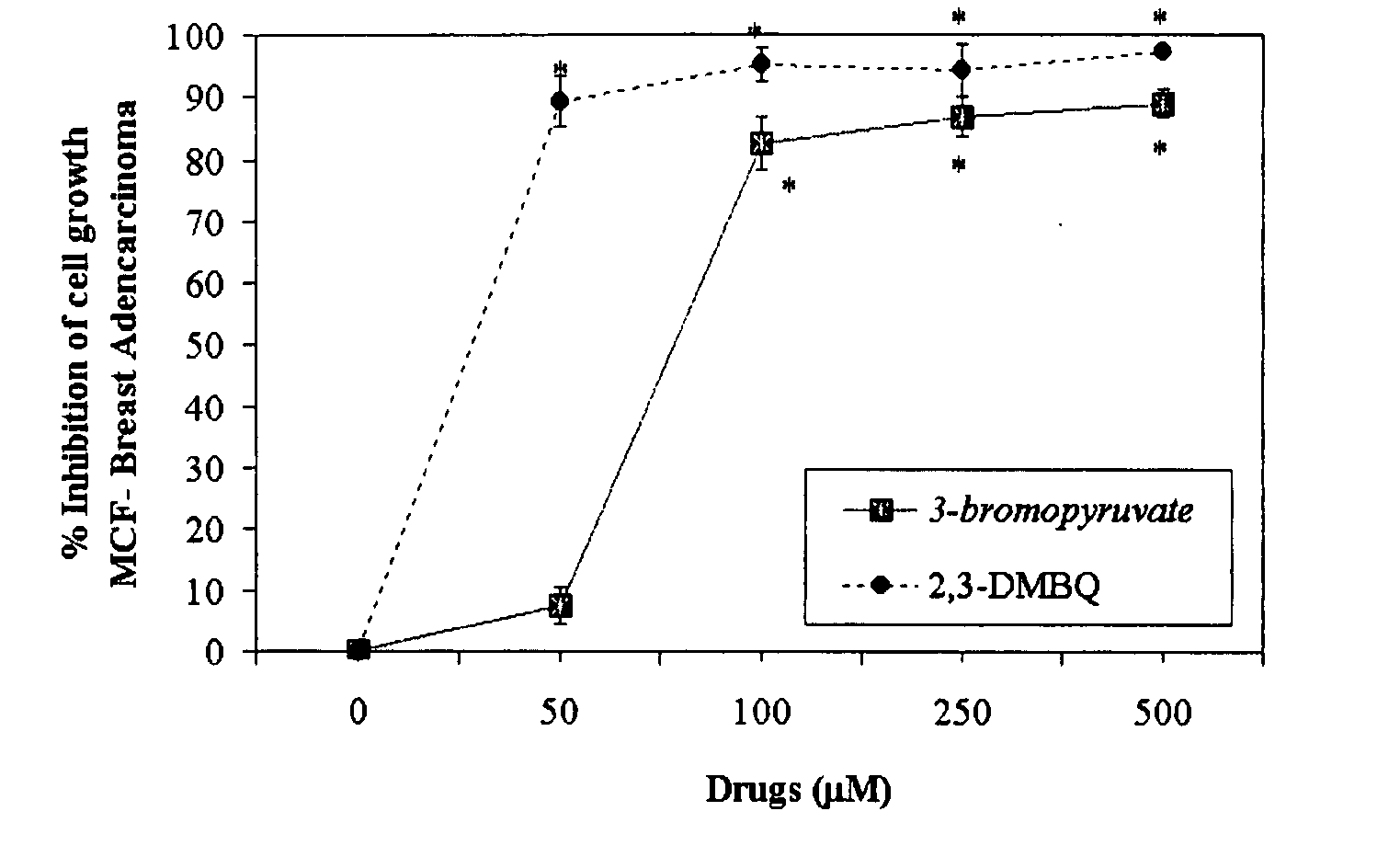
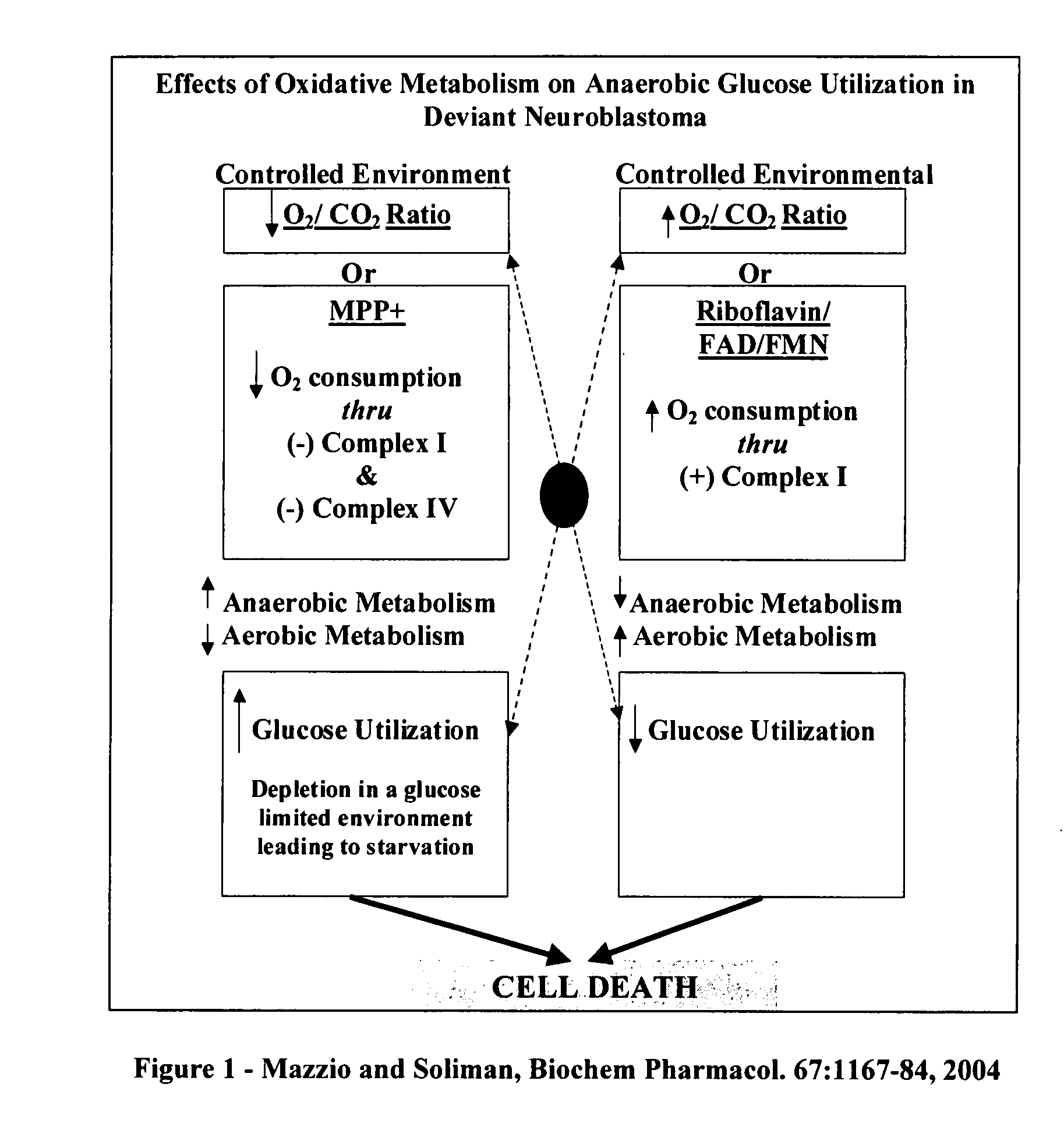
The invention describes a pharmaceutical composition and method for treating cancer comprised of A) 2,3-dimethoxy-5-methyl-1,4-benzoquinone and / or B) at least one of wild yam root, teasel root, balm of gilead bud, bakuchi seed, dichroa root, kochia seed, kanta kari, bushy knotweed rhizome, arjun, babul chall bark, opopanax and bhumy amalaki; optionally one or more of frankincense, garcinia fruit, vitex, dragons blood, mace, sage and red sandalwood with at least c) one compound capable of maximizing oxidative mitochondrial function preferably riboflavin or vitamin B2 derivatives, FAD, FMN, 5-amino-6-(5′-phosphoribitylamino)uracil, 6,7-Dimethyl-8-(1-D-ribityl)lumazine, ribitol, 5,6-dimethylbenzimidazole, tetrahydrobiopterin, vitamin B1, lipoic acid, biotin, vitamin B6, vitamin B12, folate, niacin, vitamin C and pantothenate and / or d) at least one lactic acid dehydrogenase inhibitor (preferably 2′,3,4′5,7-pentahydroxyflavone) and optionally f) an alkalizing agent (aloe vera, chlorella, wheat grass, sodium or potassium bicarbonate, potassium) g) an antiproliferative herb (speranskia or goldenseal) and h) a pharmaceutically acceptable carrier.
Owner:MAZZIO ELIZABETH +1
A combination of mitochondrial nutrients for relieving stress, preventing and improving stress-related disorders
InactiveUS20060257502A1Accelerated agingIncreasing oxidative metabolismBiocideCosmetic preparationsAlpha-TocopherolL-Carnosine
A dietary supplement of mitochondrial nutrients is designed for relieving stress, preventing and improving stress-related disorders, such as chronic fatigue syndrome, diabetes, age-associated cognitive dysfunction and diseases (Parkinson's and Alzheimer's disease). The supplement composition has the following nutrients: B vitamins (cyanocobalamin 2-1,000 ug, thiamin 1-1,000 mg, niacin 15-2,000 mg, pyridoxine 1-1,000 mg, Pantothenate 5-150 mg, folic acid 400-40,000 ug), alpha-tocopherol 10-800 mg, ascorbic acid 50-10,000 mg, calcium 20-2,000 mg, vitamin A 200-10,000 ug, alpha-lipoic acid 100-1,000 mg, N-acetyl cysteine 100-3,000 mg, L-carnosine 100-9,000 mg, tyrosine 100-9,000 mg, vanillin 10-100 mg, phosphatidylserine 10-800 mg, resveratrol 10-50 mg, dehydroepiandrosterone 1-50 mg, and melatonin 0.1-3 mg, all of which have been individually used experimentally or clinically for relieving stress, preventing and treating age- and stress-related disorders and diseases but no combination of these compounds has been used. Many embodiments also contain at least one adjunct ingredient such as coenzyme Q 10-200 mg, acetyl-L-carnitine 100-2,000 mg, choline 50-1,000 mg, and creatine 100-2,000 mg.
Owner:LIU JIANKANG
Free radical quenching composition and a method to increase intracellular and/or extracellular antioxidants
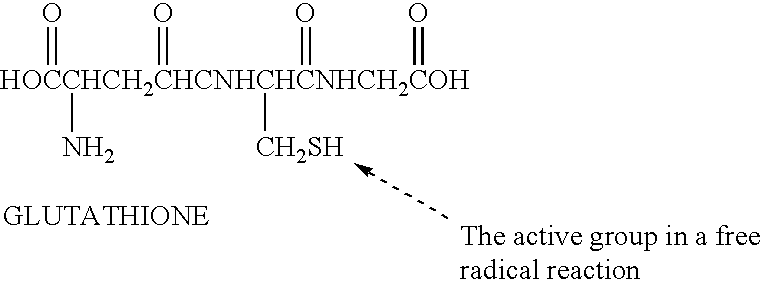
A free radical quenching composition is disclosed comprising a liposome containing at least two antioxidants selected from the following group: beta-carotene, vitamin E, vitamin C, glutathione, niacin, and optionally at least one trace metal (Zn, Se, Cr, Cu, Mn). Also disclosed is a method for reducing the undesirable side effects of free radicals in a mammal by administering to a mammal in need of such antioxidants an effective amount of liposomes containing at least two antioxidants.
Owner:AMAOX
Combination tablet with chewable outer layer
ActiveUS8404275B2Improve the level ofAbsorbed more rapidlyBiocideAnimal repellantsDosing regimenSide effect
A pharmaceutical composition in the form of a combination tablet is described. The tablet has a rapidly absorbed component that enters the circulation by traversing the buccal mucosa, oral mucosa and combinations thereof, and a more slowly absorbed component that is swallowed. The therapeutic agent in the swallowed portion is absorbed across the gastric mucosa. The combination tablet may be modified, by varying the specific combinations of excipients, fillers, and the like to effect distinct release rates. In addition, the rapid and slow components may have identical or different therapeutic agents depending on the application to a specific medical condition. One embodiment of the combination tablet includes a prostaglandin inhibitor in the rapidly absorbed component in order to mitigate the side effects of immediate release niacin that is in the slow absorbing component. Such combination compositions will increase patient compliance with various dosing regimens due to the resultant decrease in the number of tablets that a patient would need to take on a daily basis.
Owner:VITALS
Dietary supplement for supressing appetite, enhancing and extending satiety, improving glycemic control, and stimulant free
This invention relates to a nutritional intervention composition for enhancing satiety prior to a meal and extending satiety after a meal. The nutritional intervention composition decreases food intake producing weight loss over time. The composition consists of Niacin, Vitamin B6, Calcium, Phosphorous, Magnesium, Chromium, Chitosan, Fenugreek, Ginseng, White willow bark, Garcinia cambogia, Aloe Vera gel powder, Momordica charantia, Griffonia simplicifolia, Lagerstroemia speciosa and Vanadyl sulfate. The invention does not require stimulants or anabolic ingredients. There are three phases of activity within the composition. One, enhanced satiety through elevated serotonin. Two, improved carbohydrate metabolism, reduced blood glucose and slowed gastric emptying. Three, enhanced fiber binding of lipids and excess bile acids.
Owner:NEEDLEMAN ALVIN +1
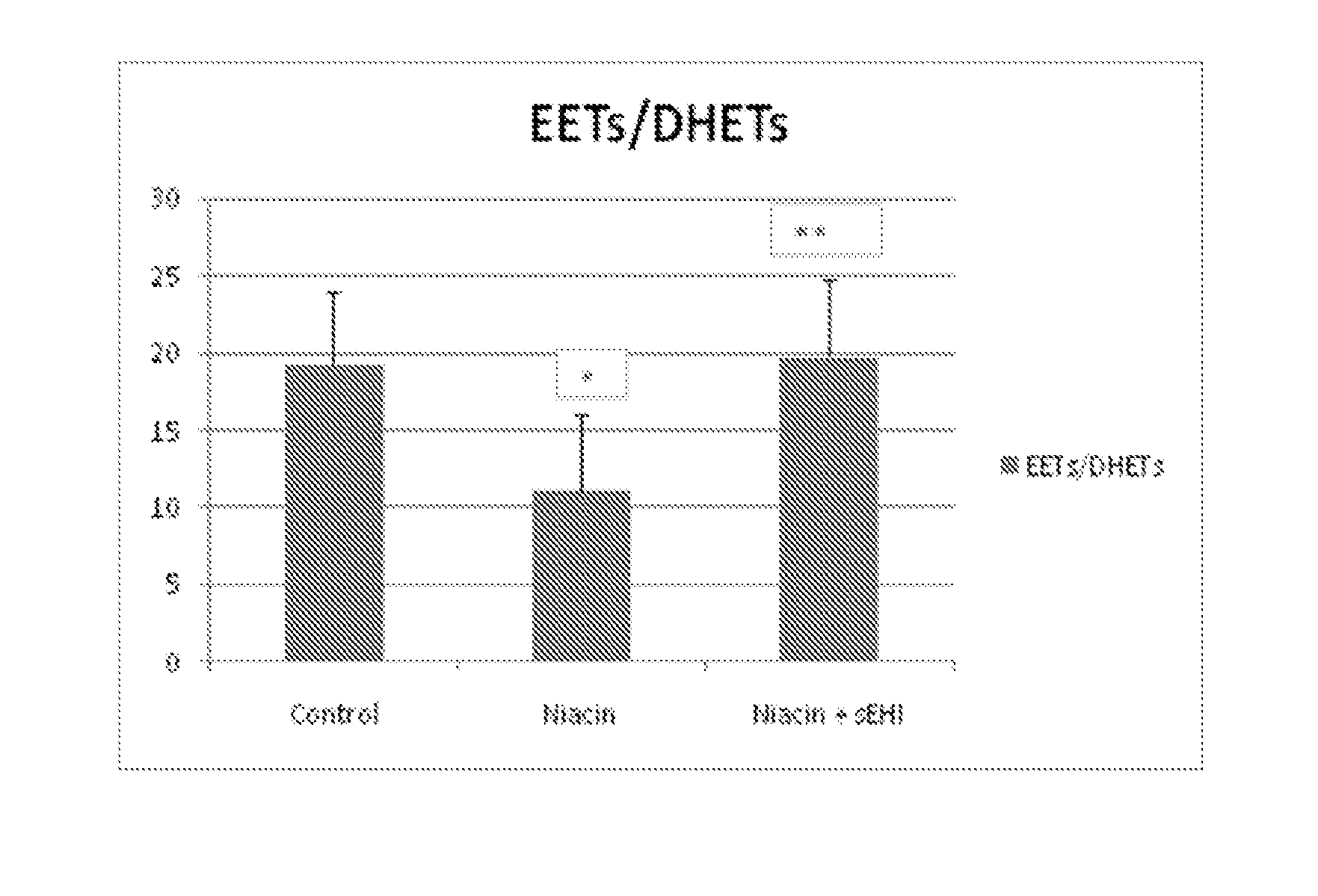
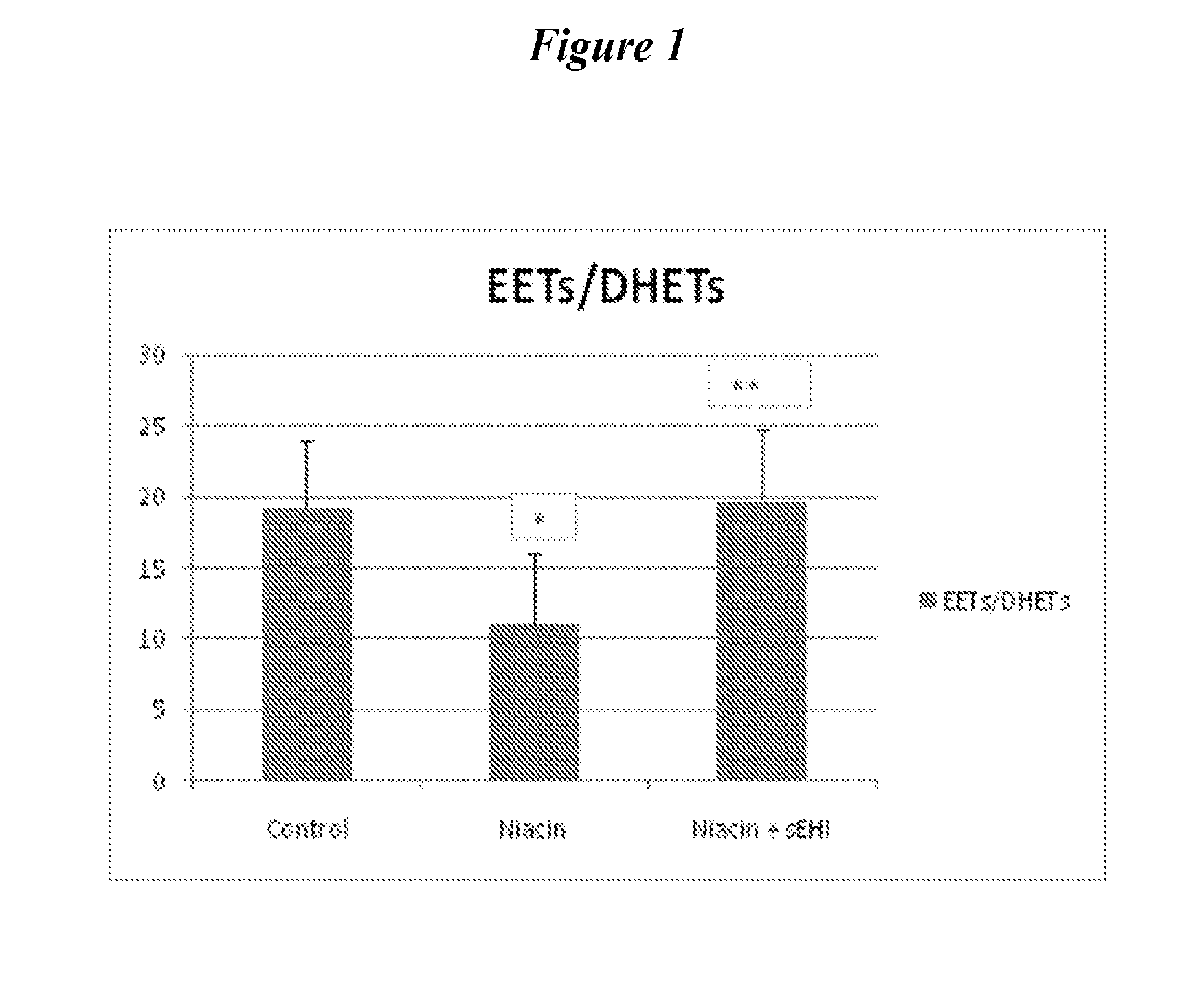
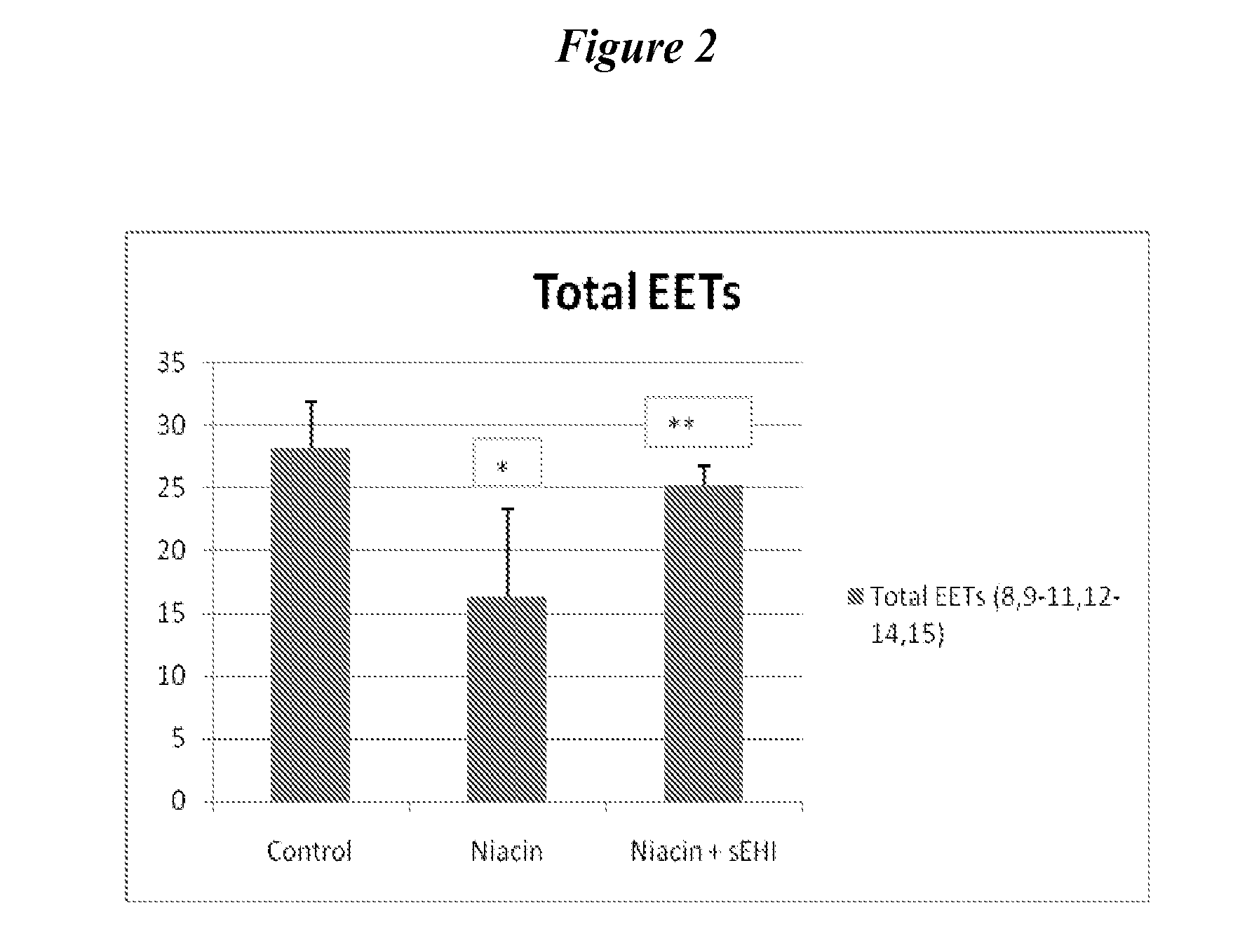
Inhibitors of soluble epoxide hydrolase to inhibit or prevent niacin-induced flushing
InactiveUS20120046251A1Prevent and reduce and block substantial flushingPrevent, reduce or block substantial flushingSalicyclic acid active ingredientsBiocideCutaneous vasodilationSide effect
The invention discloses methods of using cis-epoxyeicosantrienoic acids (“EETs”), inhibitors of soluble epoxide hydrolase (“sEH”), or a combination of an EET and an inhibitor of sEH, to reduce or prevent niacin-induced cutaneous vasodilation (“flushing”) in subjects suffering from this undesirable side effect of receiving therapeutic amounts of niacin.
Owner:SCHAEFER SAUL
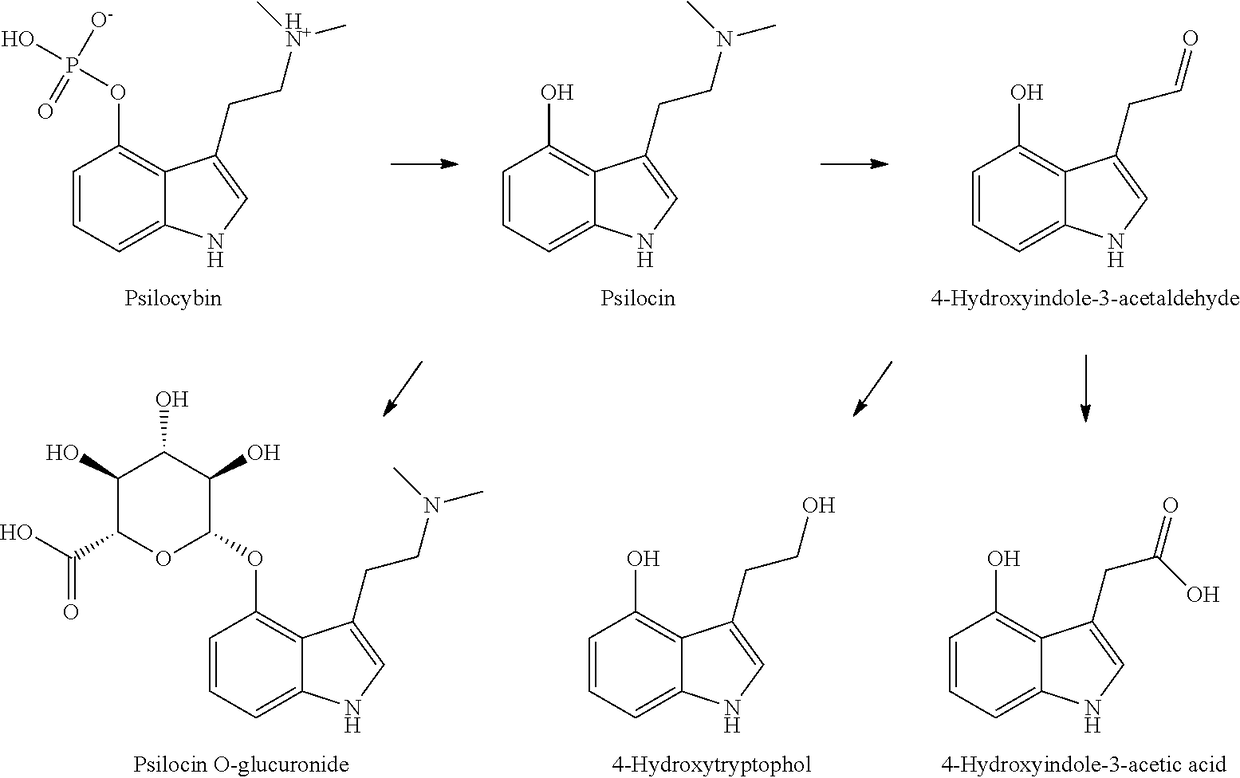
Compositions and methods for enhancing neuroregeneration and cognition by combining mushroom extracts containing active ingredients psilocin or psilocybin with erinacines or hericenones enhanced with niacin
InactiveUS20180021326A1Block neurotoxicity effectImprove cognitive functionOrganic active ingredientsFungi medical ingredientsBUTTON MUSHROOM EXTRACTNeurogenesis
Methods and compositions are disclosed for enhancing neurogenesis, resolving neuropathy and improving neurological health and functioning using fungal extracts and their active ingredients, including species of mushrooms and mycelia containing psilocybin and psilocin, combined with erinacines and hericenones or fungal extracts containing those active ingredients, with the addition of nicotinic acid. The compositions may optionally be combined with nervine plants.
Owner:TURTLE BEAR HLDG LLC
Composition for improving blood cholesterol levels
A nutritional composition for improving blood cholesterol by jointly and simultaneously inhibiting cholesterol absorption, decreasing blood LDL levels, increasing blood HDL levels and interfering with HMG-CoA reductase synthesis or degradation in an individual comprising, therapeutically effective amounts of plant sterols or plant stanols or derivatives thereof, procyanidins, policosanol and niacin or derivatives of niacin is provided. Both a composition and a method are provided by the present disclosure.
Owner:IOMEDIX DEV INT
Refrigeration-shelf-stable ultra-pasteurized or pasteurized infant formula
InactiveUS6039985AMaintain qualityReduce degradationSugar food ingredientsVitamin food ingredientsPantothenic acidVitamin B6 synthesis
Refrigeration-shelf-stable ready-to-feed and concentrated infant formulas prepared through an ultra-pasteurization and / or pasteurization process, comprise per five fluid ounces from about 1.8 to about 6.3 grams of protein; from about 3.3 to about 15.9 grams of fat; from about 300 mg to about 3000 mg of linoleic acid; from about 250 to about 900 IU of Vitamin A; from about 40 to about 180 IU of Vitamin D; from about 0.7 to about 9 IU of Vitamin E; from about 4 to about 24 mcg of Vitamin K; from about 40 to about 300 mcg of Thiamine (Vitamin B1); from about 60 to about 450 mcg of Riboflavin (Vitamin B2); from about 35 to about 180 mcg of Vitamin B6; from about 0.15 to about 0.9 mcg of Vitamin B12; from about 250 to about 3150 mcg of Niacin; from about 4 to about 48 mcg of Folic Acid (Folacin); from about 300 to about 1500 mcg of Pantothenic Acid; from about 1.5 to about 13.2 mcg of Biotin; from about 8 to about 36 mg of Vitamin C (Ascorbic Acid); from about 7 to about 48 mg of Choline; from about 4 to about 18 mg of Inositol; from about 60 to about 234 mg of Calcium; from about 30 to about 159 mg of Phosphorus; from about 6 to about 24 mg of Magnesium; from about 0.15 to about 5.4 mg of Iron; from about 0.5 to about 3 mg of Zinc; from about 5 to about 45 mcg of Manganese; from about 60 to about 270 mcg of Copper; from about 5 to about 75 mcg of Iodine; from about 20 to about 81 mg of Sodium; from about 80 to about 324 mg of Potassium; and from about 55 to about 195 mg of Chloride; wherein the total caloric content is from about 80 kilocalories to about 300 kilocalories per five fluid ounces.
Owner:KAMAREI A REZA +1
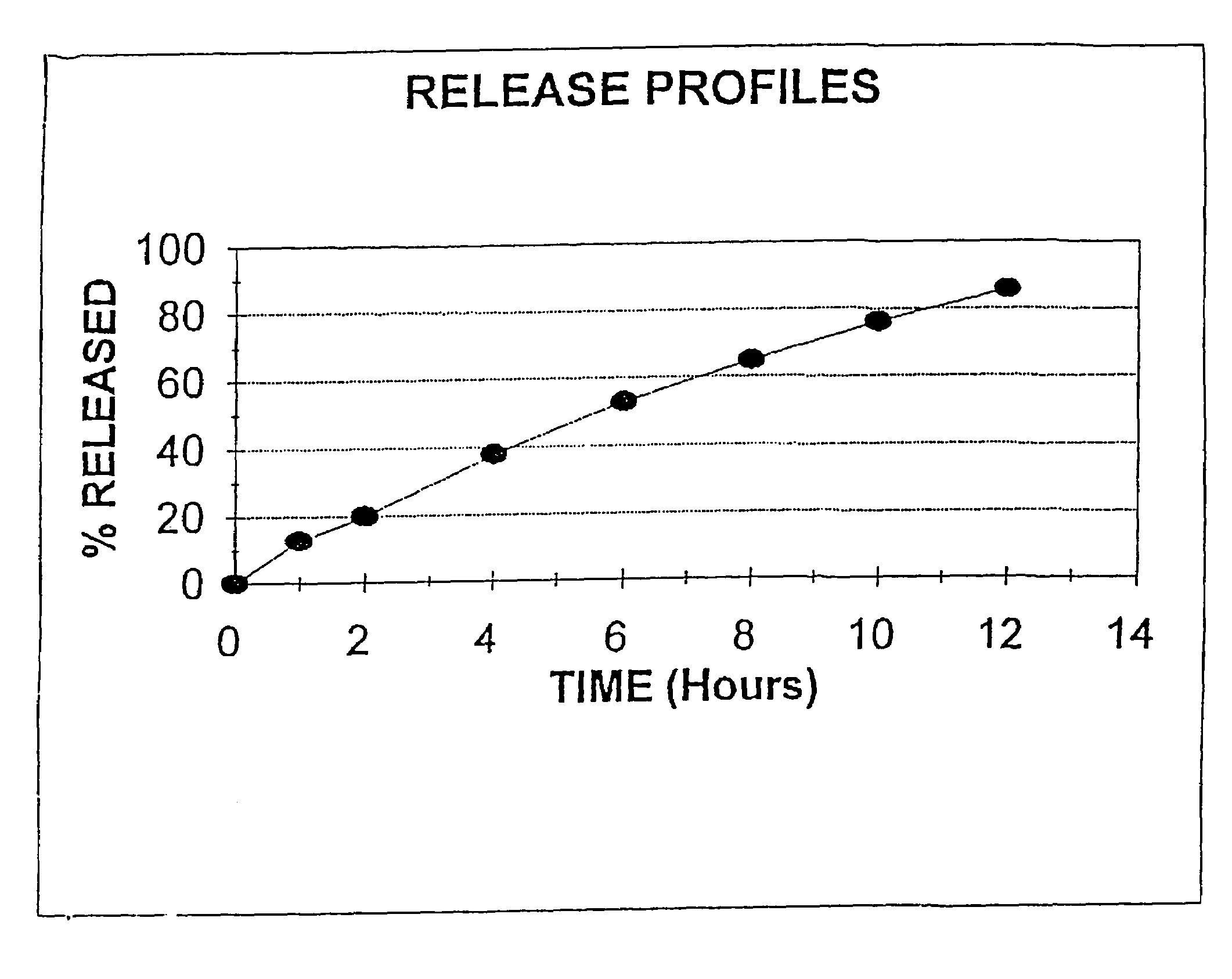
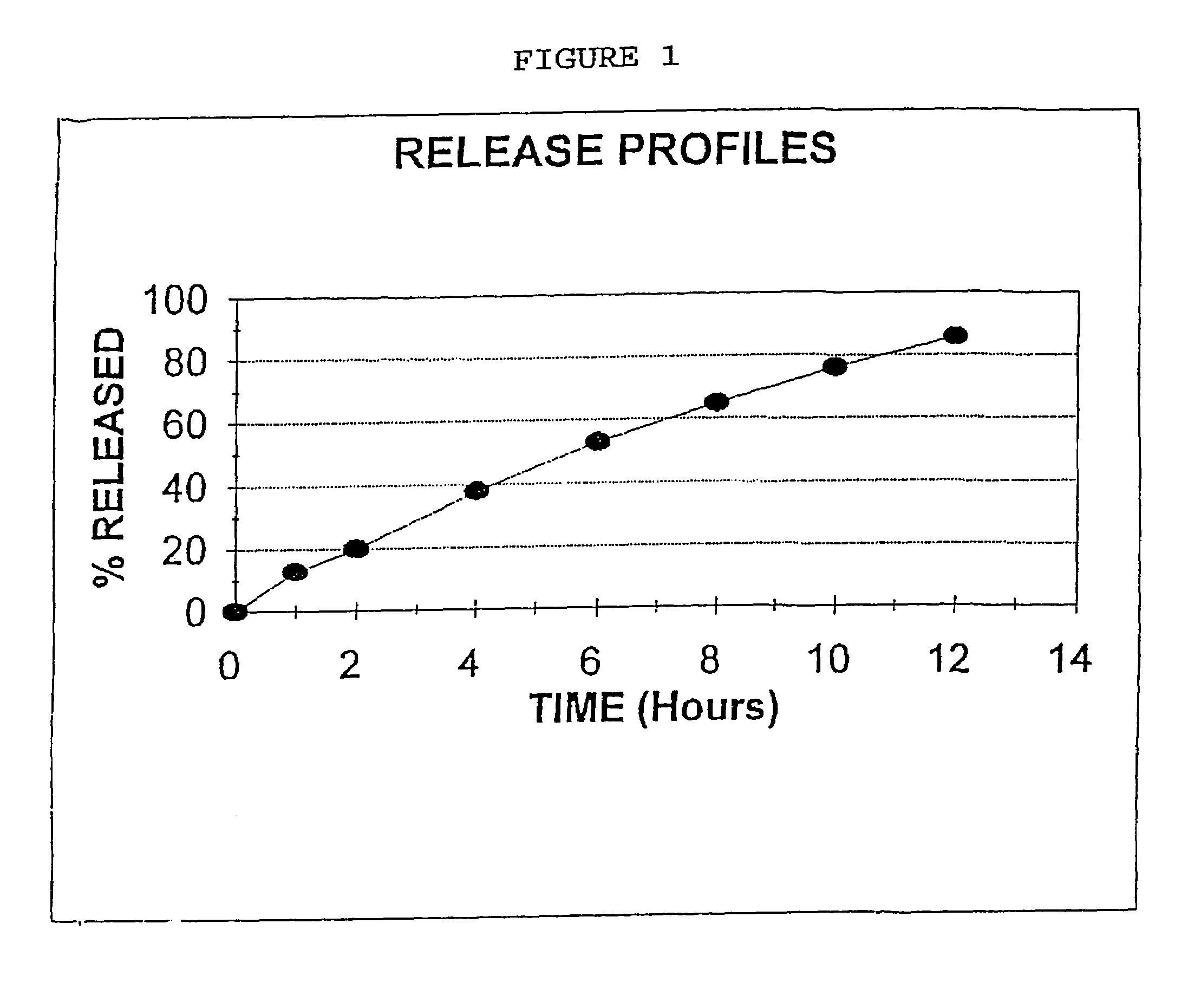
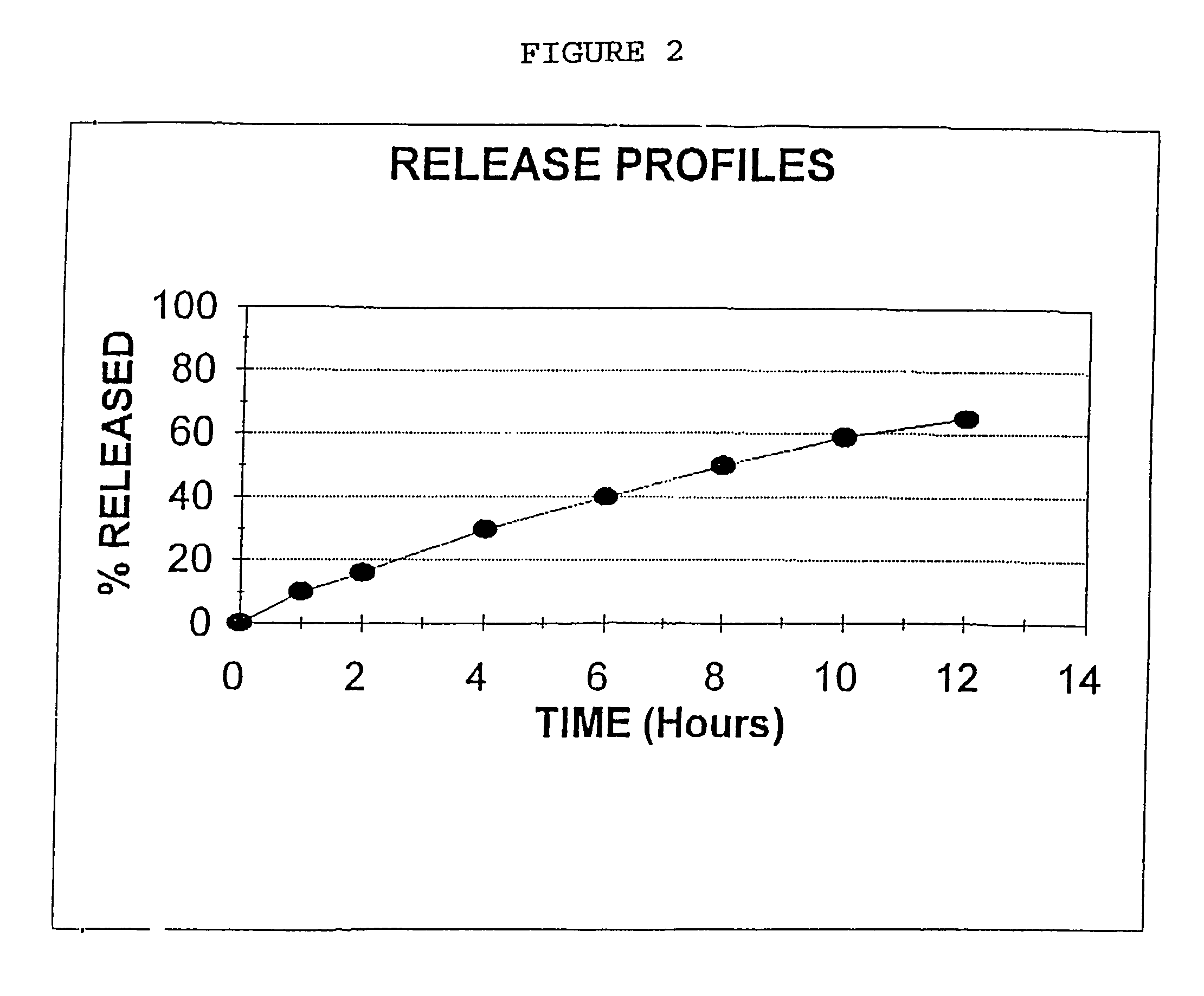
Process for preparing sustained release tablets
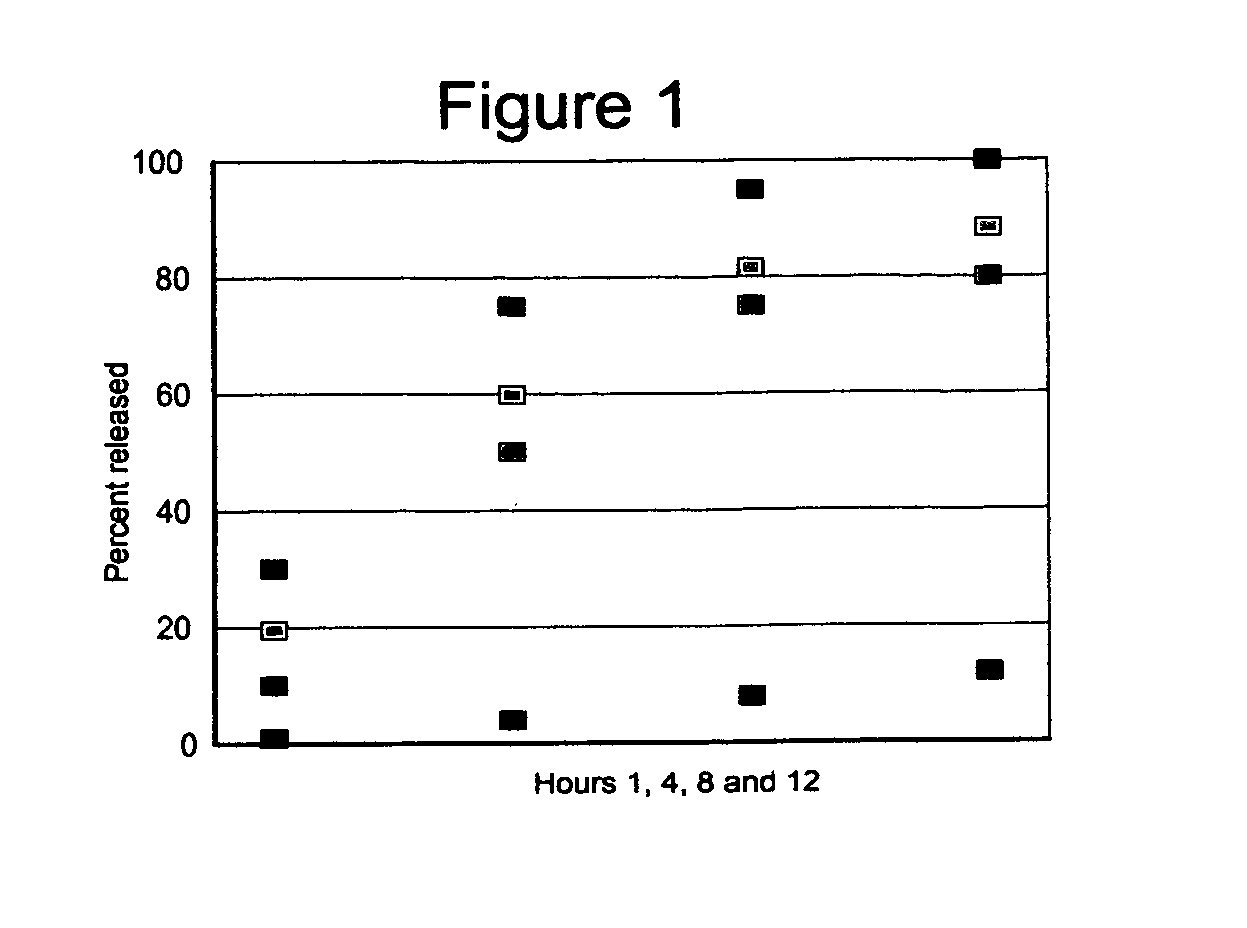
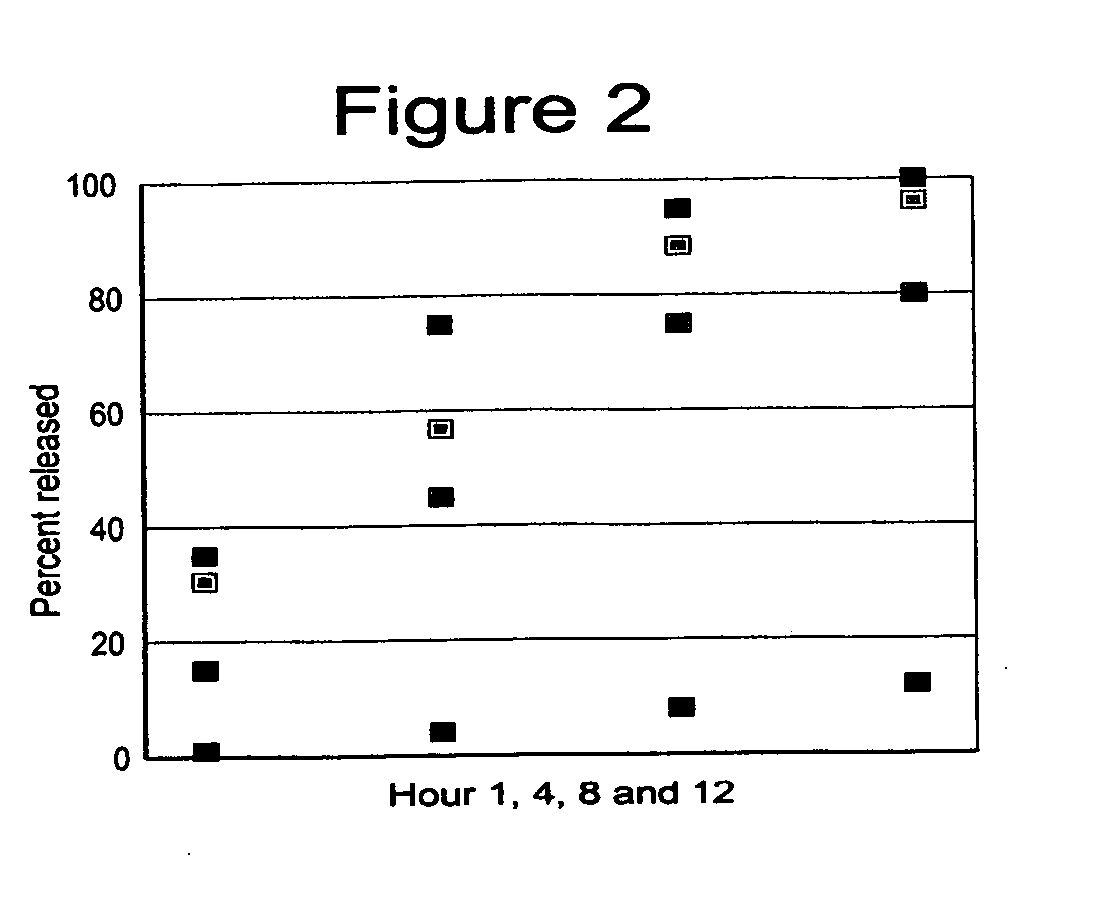
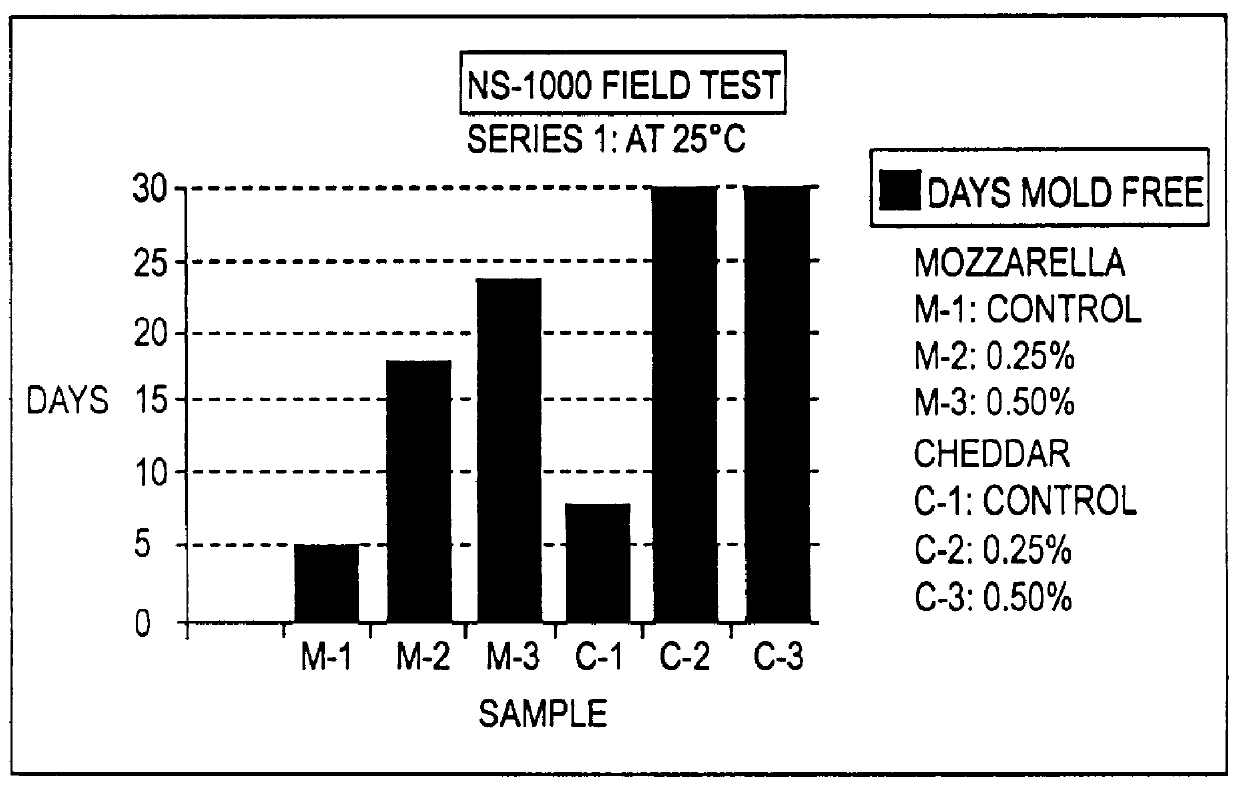
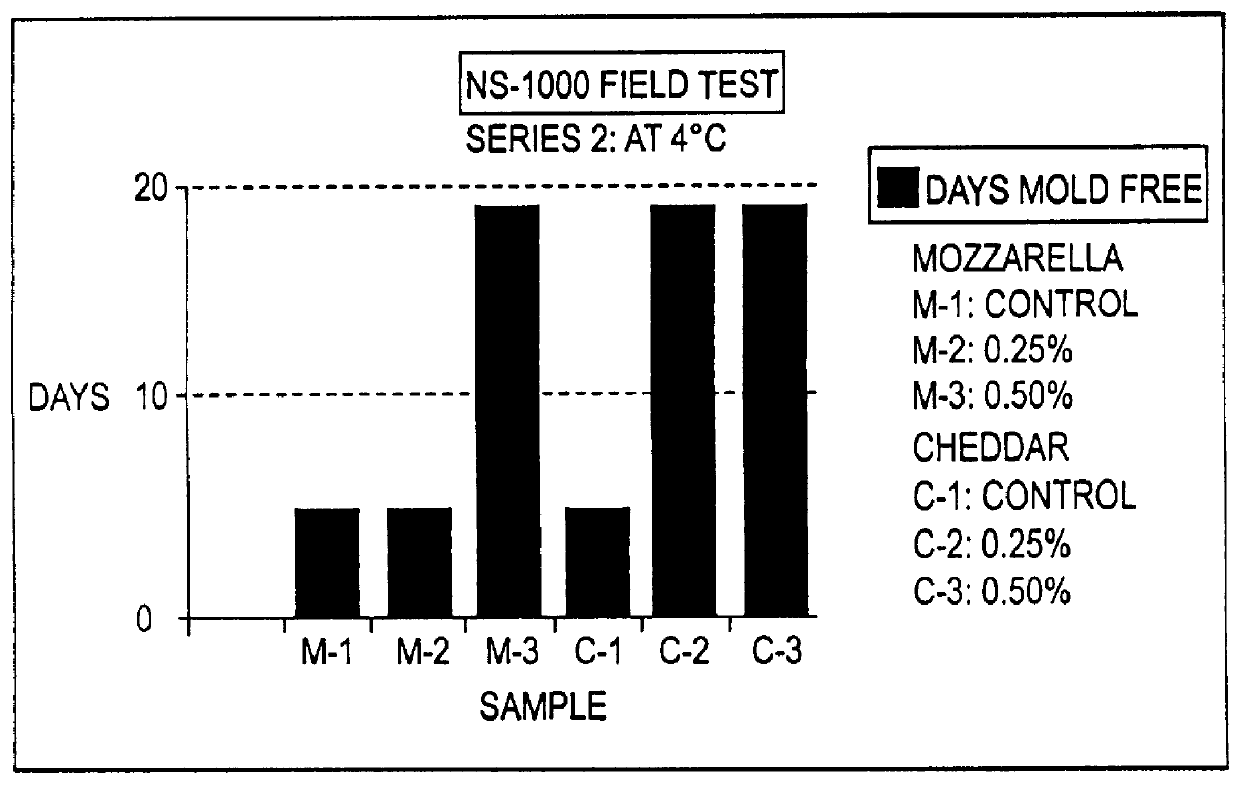
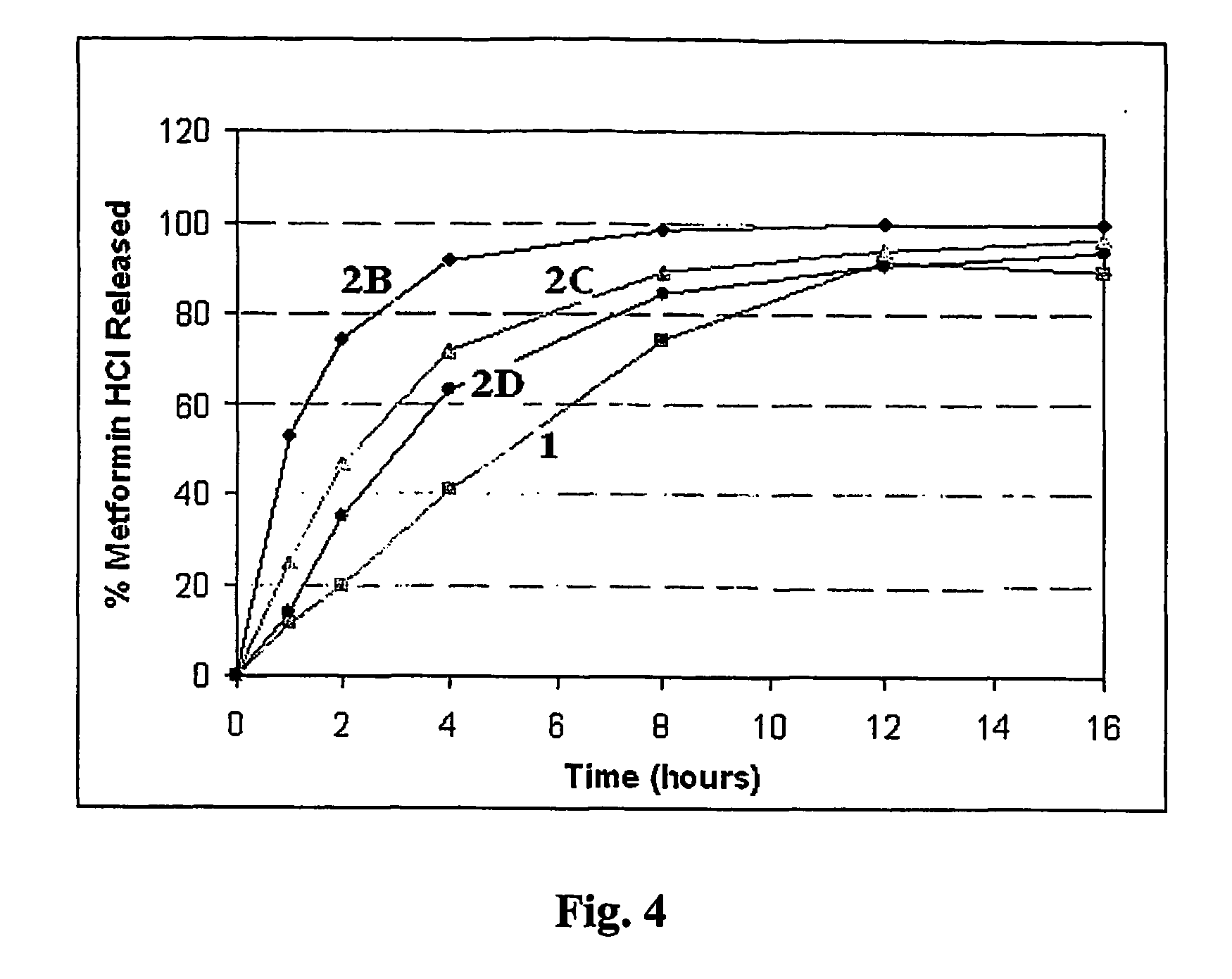
The present invention is directed to the process of preparing a sustained release niacin tablet and the product prepared therefrom.
Owner:NOSTRUM PHARMA INC
Compositions and methods for timed release of water-soluble nutritional supplements, green coffee extract
InactiveUS20050181044A1High energyAppetite suppressantPill deliveryGranular deliveryAmylase inhibitorsNiacinamide
Owner:ROMERO JAIME
Methods and related systems and formulations to normalize and improve human body chemistry and healing ability
InactiveUS20080260708A1Improve the situationImprove abilitiesBiocidePeptide/protein ingredientsSodium BentoniteOlive leaf
Methods, systems and formulations for normalizing and improving human body chemistry and the body's natural ability to heal itself. In one embodiment a system including effective amounts of a digestive enzyme, soluble and insoluble fiber, laxative, probiotics, vitamin C, potassium, protease enzymes, lipase, lysine, taurine, proline, choline, inositol, inositol hexaphosphate, policosanol, charcoal, bentonite clay, thyme, ascorbic acid, magnesium citrate, calcium citrate, methylsulfonyl methane, cayenne pepper, magnesium, potassium, ester-c, ginger and niacin, lysine calcium, stevia leaf, citric acid, a tincture of bayberry bark, juniper berries, yam root, cramp bark, golden seal root, fennel seed, uva ursi leaves, ginger root, lobelia herb, catnip herb, and peppermint leaf, golden seal root, Echinacea angustifolia root, ginger root, and licorice root, a tincture of black walnut hulls, venus fly trap, chaparral, wormwood, licorice root, slippery elm, cloves and comfrey root, burdock root, sheep sorrel, rhubarb root, slippery elm, olive leaf and yarrow flower is provided.
Owner:HALL MICKEY A
Dietary nutritional supplements for persons consuming alcohol products
A dietary nutritional supplement is designed specifically to address the needs of moderate to heavy alcohol product consumers. The nutritional supplement contains effective amounts of essential components vitamin B2, vitamin B6, vitamin B12 (Cobalamin), vitamin B3 (Niacin), vitamin B1 (Thiamin), Calcium, magnesium, vitamin A, vitamin C (ascorbic acid), folic acid, L-cysteine, L-methionine, Milk Thistle, selenium, dandelion, and Molybdenum.
Owner:LAK ZAHRAMEHRAN SALARI
Dietary Supplement Cognitive Support System
The present invention relates to a nutritional supplement composition, comprising a therapeutically effective amounts of Vitamin C, Vitamin D3, Thiamin, Riboflavin, Niacin, Vitamin B6, Folic acid, Vitamin B12, Pantothenic acid, Calcium, Magnesium, Zinc, Chromium, Sugar, Protein, Acetyl-L-Carnitine, Dimethylaminoethanol complex, Phosphatidylserine complex, L-Glutamine, N-Acetyl-L-Tyrosine, L-Phenylalanine, Taurine, Methionine, Valine, Isoleucine, 5 Hydroxytryptophan, L-Taurine, N-Acetyl-Tyrosine, N-Acetyl-L-Cysteine, Alpha Lipoic Acid, Alpha Glycerylphosphoricholine complex, Bacopa Monnieri extract, Gingko Biloba extract, Passion flower, Lemon Balm, Gotu Kola, Ashwagandha, Choline Bitartrate complex, Panax Ginseng extract, Turmeric, Organic freeze dried fruit juice blends (concord grape, red raspberry, pineapple, cranberry, acai, pomegranate, acerola cherry, bilberry, lingonberry, black currant, aronia, sour cherry, black raspberry), Organic freeze dried greens blends (barley grass, broccoli, beet, carrot, alfalfa, oat), and Protein digestive enzyme blends (Protease 4.5, peptidase, bromelain, protease 6.0, protease 3.0, L planatrum, B bifidum) in a mixture to provide optimal cognitive function.
Owner:FANTZ DAVID R
Methods of preparation and using antimicrobial products
InactiveUS6123973AEliminate bad tasteLower decomposition temperatureBiocideDough treatmentPropanoic acidSodium sorbate
Novel antimicrobial products and methods of making and using the same are shown, whereby the products can be used in the same or greater percentages as conventional microbial growth inhibitors without imparting an off-flavor, taste, color or odor to the products in which they are used. The antimicrobial products are formed by reacting azodicarbonamide or an ammonia gas with a compound selected from the group consisting of benzoic acid, sodium benzoate, calcium benzoate, potassium benzoate, acetic acid, sodium diacetate, paraben, niacin, calcium acetate, calcium diacetate, citric acid, lactic acid, fumaric acid, sorbic acid, sodium sorbate, calcium sorbate, potassium sorbate, propionic acid, sodium propionate, calcium propionate, potassium propionate and mixtures thereof. In one embodiment, the product is prepared by placing a layer of azodicarbonamide on a substrate and covering the layer with a gas permeable separator. The antimicrobial compound is then added on top of the separator, and the combination is heated to form the final product. In another embodiment, the product is prepared by exposing the antimicrobial compound to an ammonia gas. The ammonia gas reacts with free acids in the antimicrobial compound to convert the free acids into ammonium salts, thereby eliminating off-flavor and off-odor of the resulting antimicrobial product. The antimicrobial products prepared according to the present invention are suitable for use in foodstuffs, sanitation products, cosmetics, pharmaceuticals, and so forth.
Owner:TILLIN
Mineral fortification systems
InactiveUS7279187B2High redox potentialReduce concentrationReady-for-oven doughsFruit and vegetables preservationVitamin B6 synthesisLiquid composition
A mineral-fortification system that has a bottle cap, a pouch and a pouch opener. A powder is contained within the pouch, and the powder contains at least one mineral and a redox modulating compound. When the cap is secured onto the opening of a bottle containing a liquid and when the pouch opener is activated, the powder is released from the pouch and mixes with the liquid to form a mineral fortified liquid composition that is fortified with at least one mineral and has a pH between about 2.5 and 9.5. Moreover, the mineral fortified liquid composition has a redox potential that satisfies the following equation:0≧RP−(A−B*pH).In this equation RP is the redox potential in millivolts of the mineral-containing liquid composition, pH is the pH of the mineral-containing liquid composition, A is 400 and B is 20. The mineral is preferably selected from calcium, iron, zinc, copper, manganese, iodine, magnesium, and mixtures of these. Moreover, the mineral-fortified liquid composition may preferably be substantially free of flavor or sweetener compounds. Even more preferably, the liquid composition has no metallic taste or after-taste, a Hunter colorimetric “b” reading of less than 5.0, and an NTU turbidity value of less than 5.0. The mineral-fortified liquid composition may optionally contain other nutrients and vitamins, for example, vitamin A, vitamin C, vitamin E, niacin, thiamin, vitamin B6, vitamin B2, vitamin B 12, folic acid, selenium, pantathonic acid, and iodine.
Owner:THE PROCTER & GAMBLE COMPANY
Krill Oil Compositions
InactiveUS20080166420A1Low densityHigh densityCrustacean material medical ingredientsEster active ingredientsSignificant riskNiacin
The present disclosure provides for novel krill oil-based compositions, method of administration and method of manufacture which provide for the treatment and prevention of cardiovascular disease, including the reduction of one or more significant risk factors involved with cardiovascular disease. The active ingredients of the composition include krill oil, combined in one embodiment with niacin, and combined in an alternate embodiment with polymethoxylated flavones (PMFs), and combined in yet another embodiment with Cissus quadrangularis, and combined in a further embodiment with Gynostemma pentaphyllum.
Owner:SONES SCOTT F
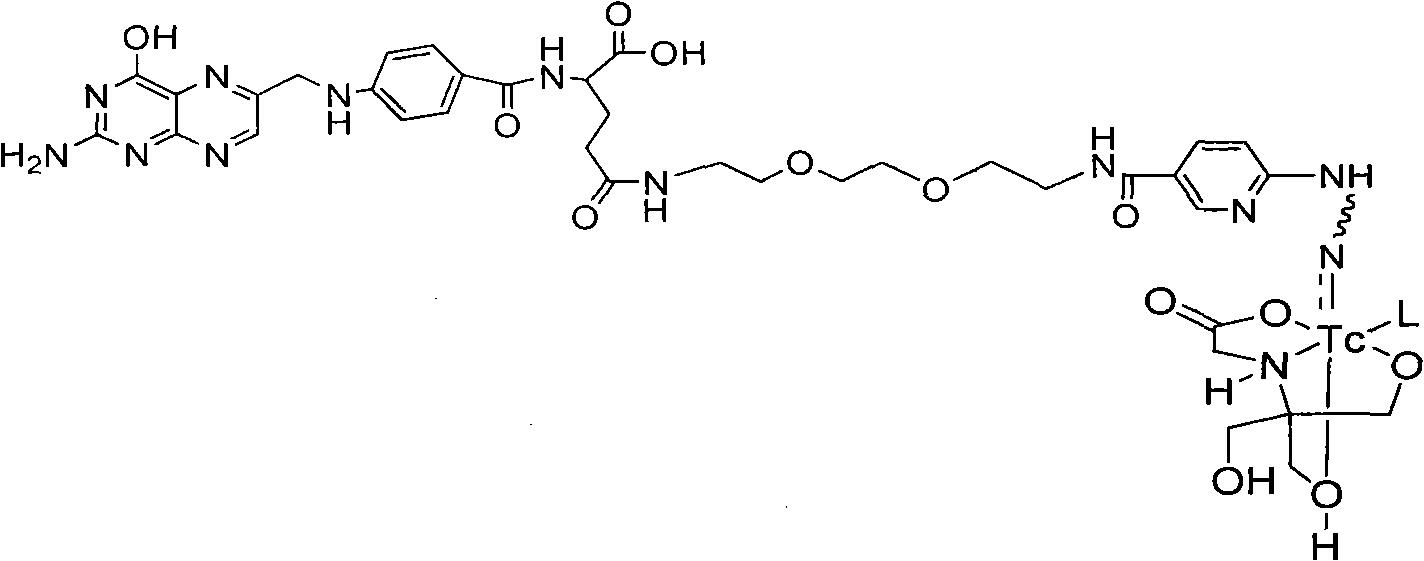
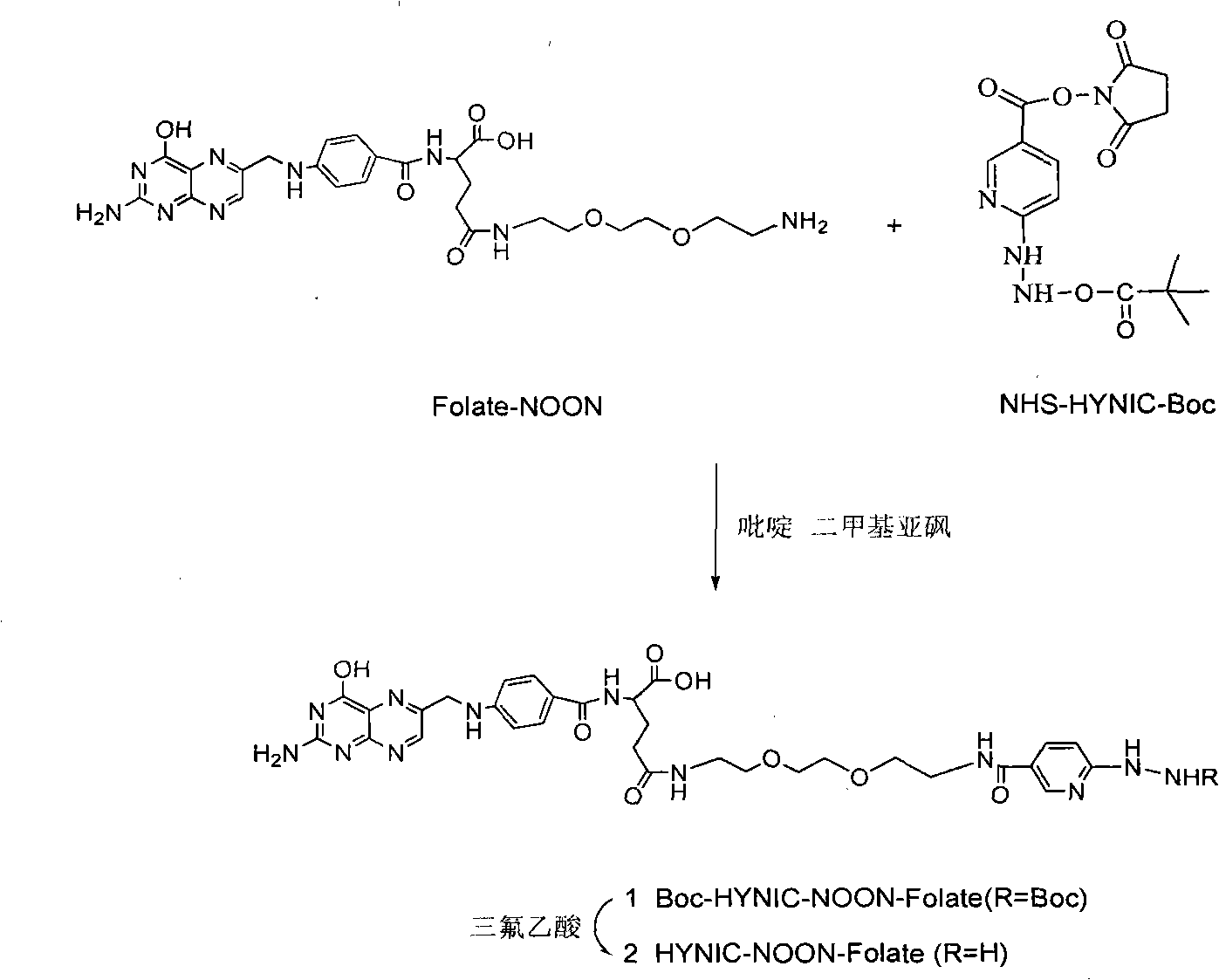
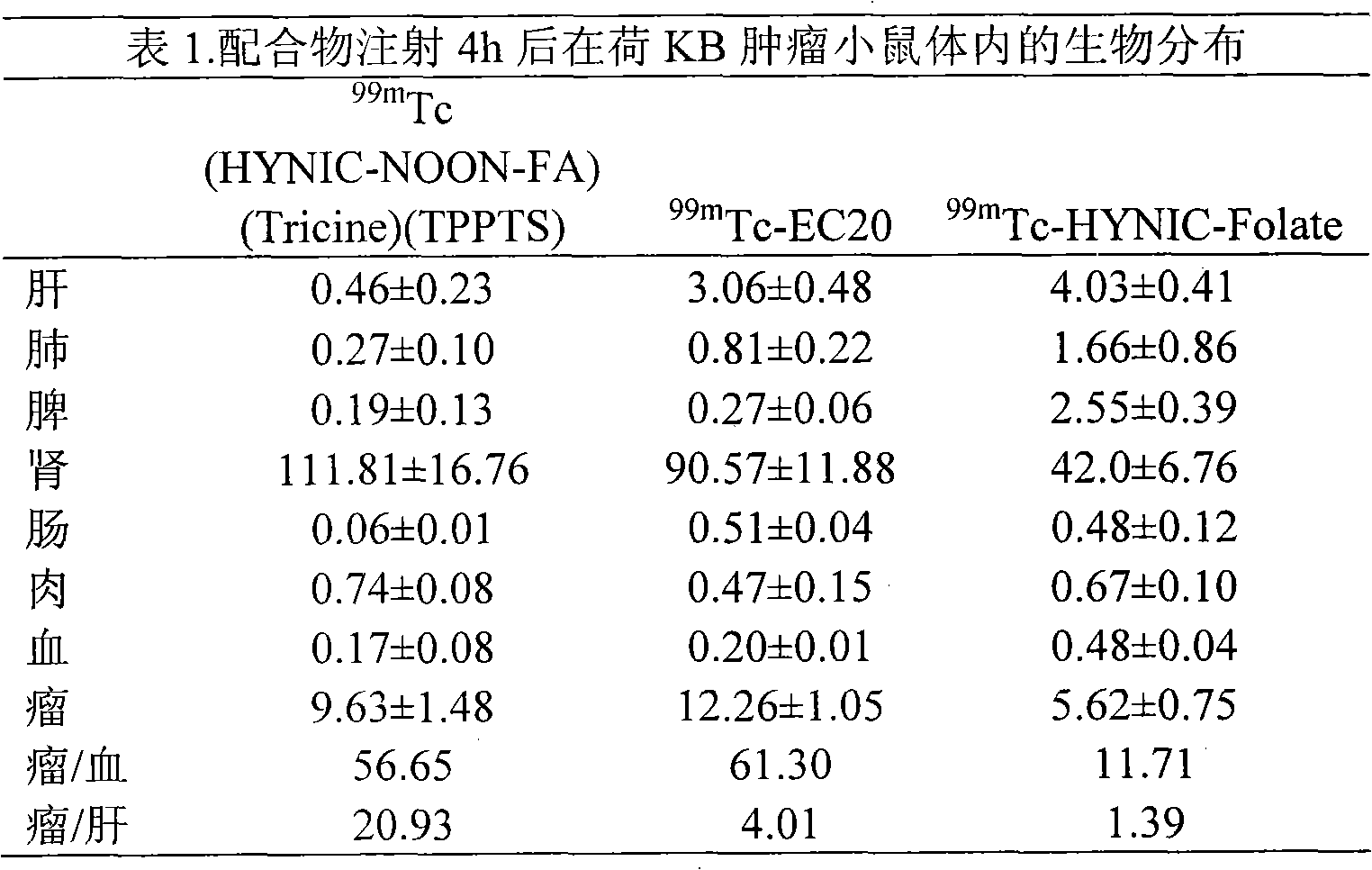
Labeled 99mTc hydrazino-nicotinamide-dioxodecoyl-folic acid coordination compound and preparation method
InactiveCN101863924AImprove performanceRadioactive preparation carriersGroup 7/17 element organic compoundsSodium phosphatesNiacin
The invention discloses a labeled 99mTc hydrazino-nicotinamide-dioxodecoyl-folic acid coordination compound with a general formula of 99mTc(HYNIC-NOON-FA)(Tricine)(L). In the structural formula, L is triphenyl sodium phosphate or triphenyl sodium photrisulfonic acid, wherein 1,8-diamido-3,6-octane dioxide is used as a connecting chain for generating a hydrazino-nicotinamide-3,6-dioxodecoyl-folic acid coupler respectively with folic acid and hydrazino-niacin through amido bonds and coordinating with oxygen atoms and phosphorus atoms in a co-ligand Tricine and an L molecule and 99mTc, and the 99mTc(HYNIC-NOON-FA)(Tricine)(L) coordination compound is obtained through two steps of: (a) synthesizing the hydrazino-nicotinamide-3,6-dioxodecoyl-folic acid coupler used as a ligand; and (b) labeling the 99mTc-hydrazino-nicotinamide-dioxodecoyl-folic acid coordination compound. The coordination compound has the advantages of high radiochemical purity, good stability, high tumor intake, good retention, low non-target organ background and clear tumor SPECT (Single Photon Emission Computed Tomography) development and can be prepared into a novel 99mTc labeled folic acid receptor tumor developer widely applied to the technical field of radioactive pharmaceutical chemistry and nuclear medicine.
Owner:BEIJING NORMAL UNIVERSITY +1
Co-formulations or kits of bioactive agents
Provided, among other things, is a formulation or kit comprising: (a) a pharmaceutically effective dosage of one or more a glucose-level-controlling bioactive agents selected from an α-glucodase inhibitor, sulfonylurea, meglitinide, thiazolidinediones, biguanide, insulin, dual PPARα / γ agonist, PPARγ agonist or insulin secretagogue; and (b) a pharmaceutically effective dosage of (i) one or more of an antihypertensive bioactive agent selected from an ACE inhibitor, calcium channel blocker, beta blocker, angiotension II receptor antagonist or diuretic, or (ii) one or more of an anti-dyslipidemia bioactive agent selected from a HMG-CoA reductase inhibitor, bile acid sequestrant, fibric acid derivative, sterol, cholesterol absorption inhibitor, MTP inhibitor or nicotinic acid derivative; wherein: in the case of (i) a combination of a first bioactive agent of group (a) that is metformin with a second bioactive agent of group (b), or (ii) a combination of a first bioactive agent of group (a) that is a thiazolidinedione or dual PPARα / γ agonist with an angiotension II receptor antagonist, one or more of the following applies: (I) one of the first bioactive agent or the second bioactive agent is formulated for sustained release, and the other is formulated for immediate release, each formulated for once-a-day dosing; or (II) the co-formulation or kit comprises (A) a biguanide and a thiazolidinedione and (B) one or more group (b) bioactive agents.
Owner:ABEILLE PHARMA
Novel pig feed formula
InactiveCN101683127AFix security issuesReduce material consumption and weight gain ratioFood processingAnimal feeding stuffWeight gainingPhytase
The invention provides a novel pig feed formula which relates to an animal feed formula, in particular to a pig feed formula. The feed comprises the following ingredients in proportions: 40-75% of corn, 5-28% of soybean meal, 0-6% of peanut meal, 0.05-0.45% of table salt, 0-0.4% of sodium sulfate, 0-0.7% of lysine hydrochloride, 0-1.1% of lysine sulfate, 0-1.2% of powdered 50% choline chloride, 0.05-5% of mineral trace element premix, vitamin premix 50-1000grams / ton, phytase feed additive 0-1000grams / ton, bacillus licheniformis powdered preparation 50-2000grams / ton, and niacin / chromium picolinate feed level / food level / medicine level additive 50-500grams / ton. By adopting the novel pig feed formula, the back fat of the fattened pigs for sale is lower than 1cm, the daily weight gain of the fattened pigs above 60-100kg for sale reaches or exceeds 1000 grams in the late fattening stage, and the consumption-weight gain ratio is lower than 2.7.
Owner:刘显富
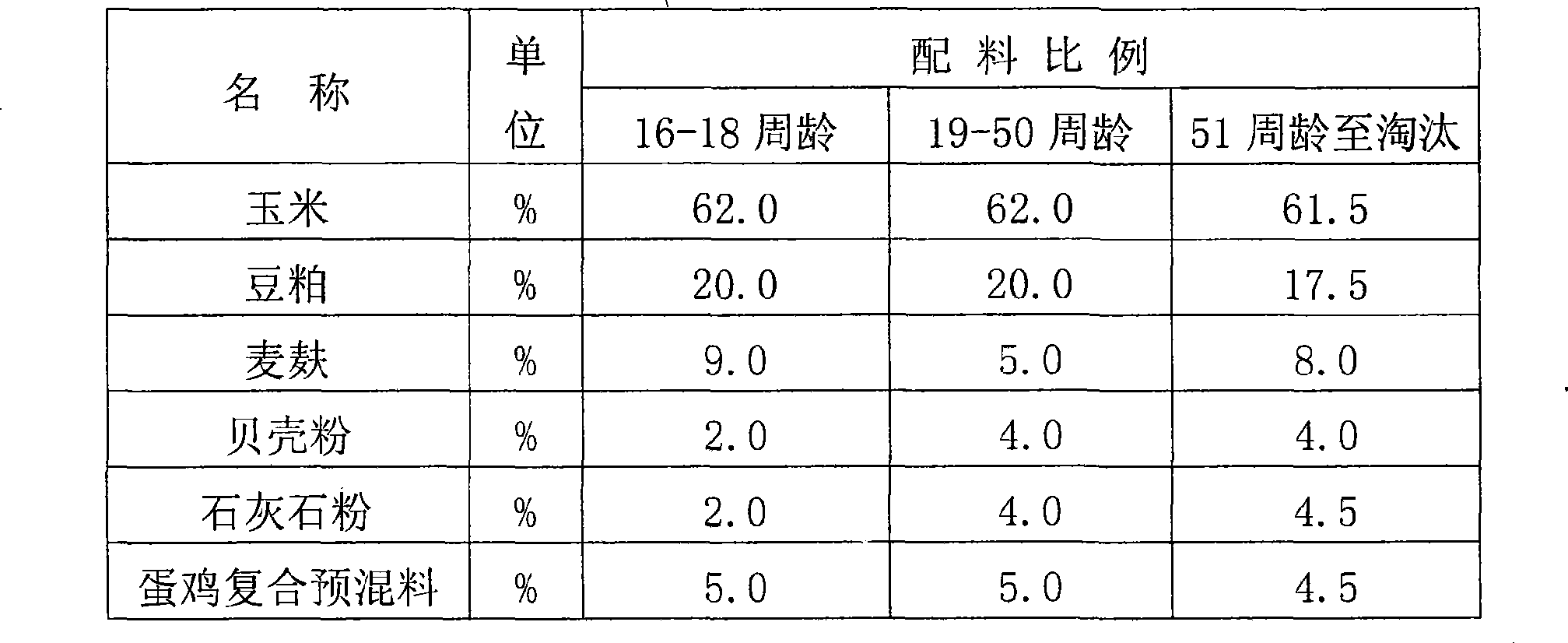
Composite premix feed for laying hen and processing technique thereof
ActiveCN101485398AMaximize your genetic potentialNutritionally balanced and richFood processingAnimal feeding stuffVitamin b6Phytase
The invention relates to compound premix feed for laying hens, which comprises 3 to 9 kg of manganous sulfate, 3 to 7 kg of zinc sulfate, 3 to 7 kg of ferrous sulfate, 0.6 to 1.4 kg of copper sulphate, 12 to 18 kg of choline chloride, 7 to 11 kg of lysine, 26 to 34 kg of methionine, 1.6 to 2.4 kg of phytase, 400 to 461 kg of calcium carbonate, 80 to 120 kg of calcium hydrophosphate, 300 to 360 kg of fish meal, 45 to 75 kg of common salt, 0.20 to 0.44 kg of vitamin A, 0.06 to 0.14kg of vitamin D3, 0.52 to 1.12 kg of vitamin E, 0.047 to 0.1 kg of vitamin K3, 0.026 to 0.056 kg of vitamin B1, 0.177 to 0.33 kg of vitamin B2, 0.078 to 0.145 kg of vitamin B6, 0.025 to 0.058 kg of vitamin B12, 0.02 to 0.03 kg of folic acid, 0.008 to 0.018 kg of biotin, 0.3 to 0.674 kg of nicotinic acid, 0.11 to 0.245 kg of calcium pantothenate, and 1.2 to 3.83 kg of rice hull powder. The laying hens fed by the premix feed have high laying rate, and keep long period of peak time of laying eggs reaching 6-9 months. The ratio of the feed to the egg is as low as 2.0-2.1:1.
Owner:潍坊中基饲料有限公司
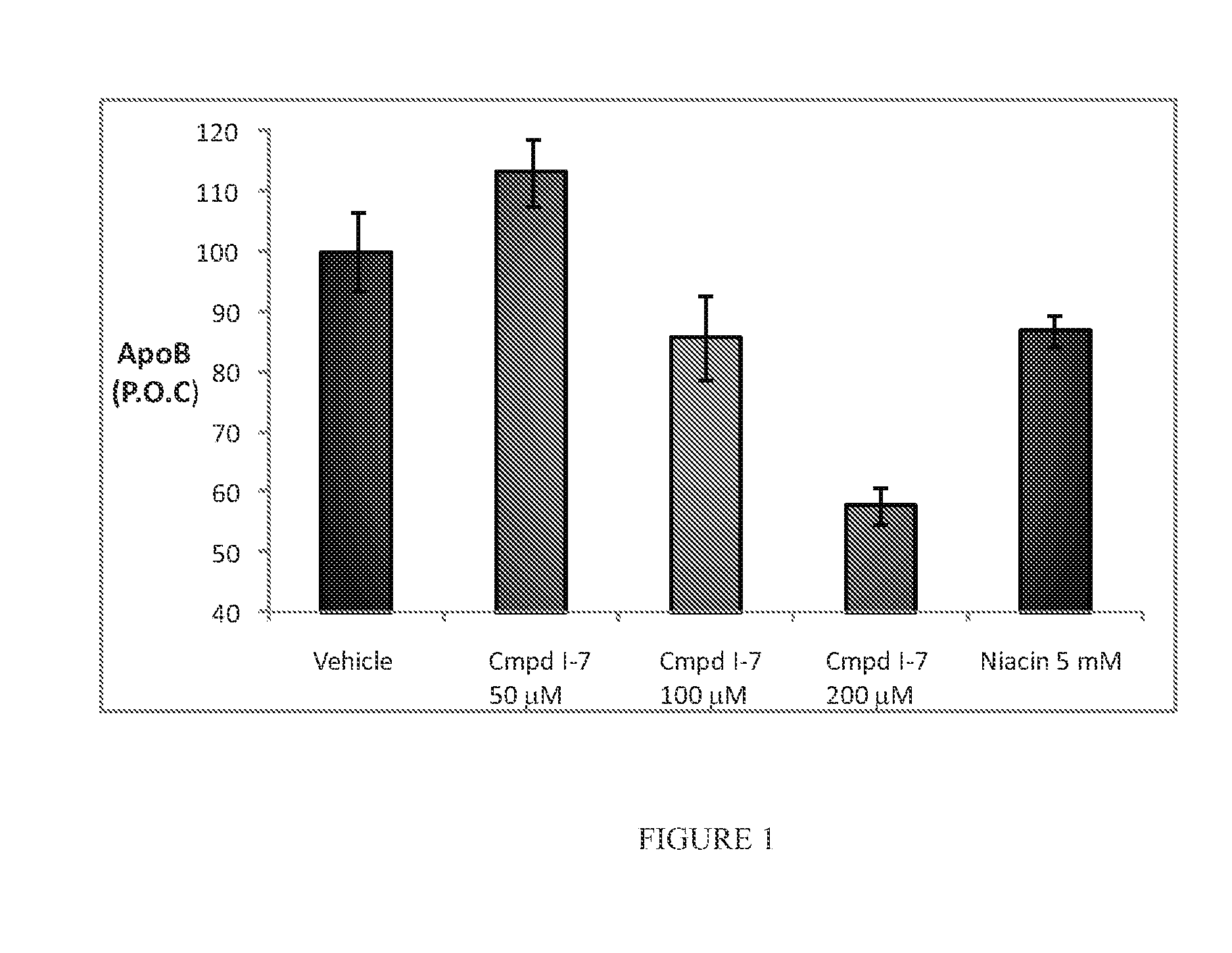
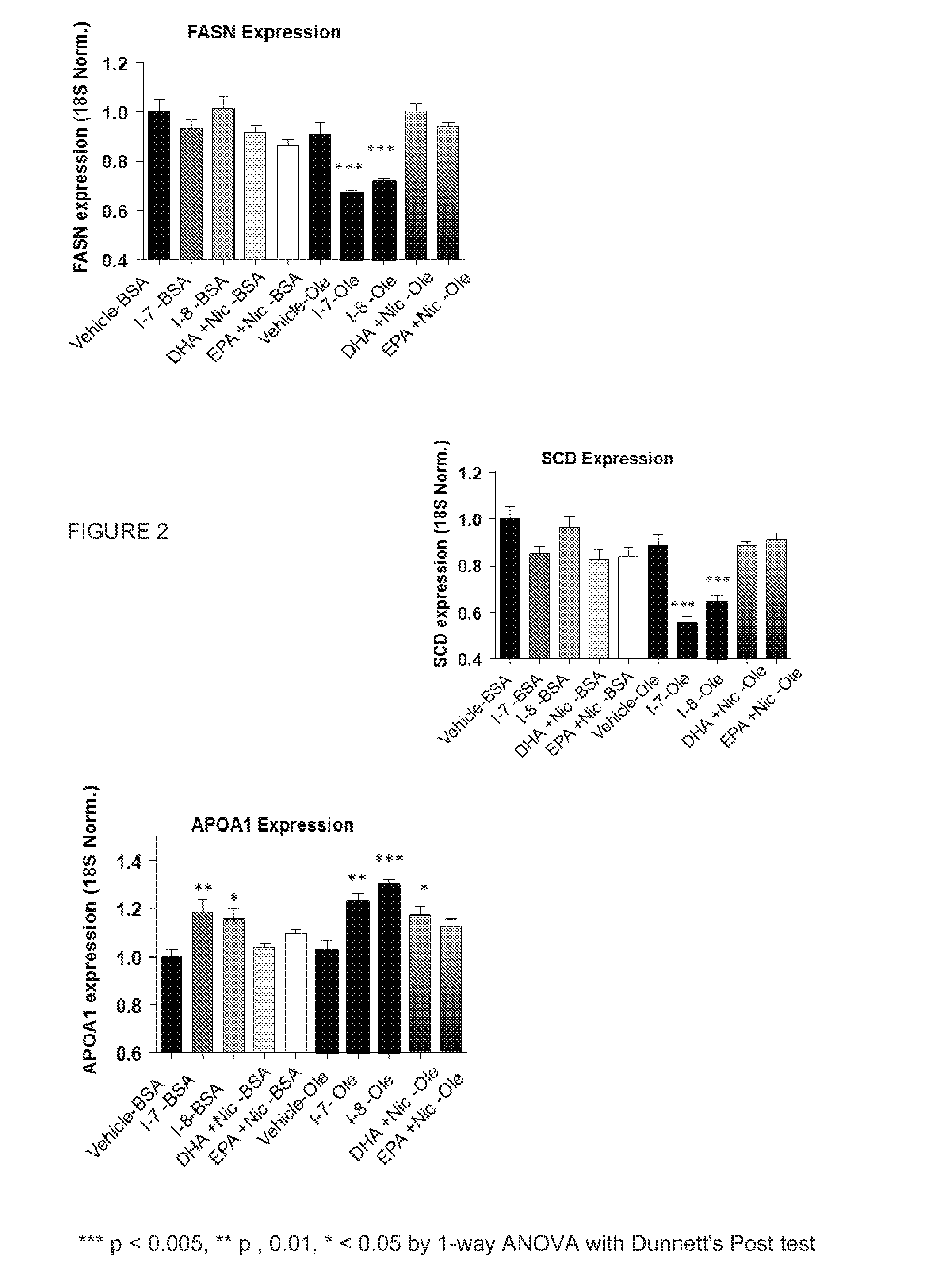
Compositions containing policosanol and niacin and/or niacin derivatives and their pharmaceutical uses
InactiveUS20050267091A1BiocideHydroxy compound active ingredientsCholesterol bloodCoronary heart disease
A composition is provided which contains policosanol and niacin and / or niacin derivatives and which may be used for treating and or reducing hypercholesterolemic diseases, total cholesterol, LDL-cholesterol, LDL / HDL ratio, Lp(a), triglycerides, coronary heart disease (heart attacks and strokes), inflammation, immunoregulatory diseases, cardiovascular diseases, deep vein thrombosis, anxiety, depression and / or neurodegenerative disorders, and / or raise HDL cholesterol in humans and animals. The method comprises administering policosanol and niacin and / or niacin derivatives which together effectively lower the LDL / HDL cholesterol ratio. Typically, the administered composition includes about 0.1-10:1 parts by weight of policosanol to niacin and / or niacin derivatives.
Owner:WYETH LLC
Fatty acid niacin conjugates and their uses
The invention relates to fatty acid niacin conjugates; compositions comprising an effective amount of a fatty acid niacin conjugate; and methods for treating or preventing an metabolic disease comprising the administration of an effective amount of a fatty acid niacin conjugate.
Owner:CATABASIS PHARMA
Compositions and methods for prophylactic and therapeutic supplementation of nutrition in subjects
InactiveUS6863904B2Improves oxidative stressElevated level of homocysteineHeavy metal active ingredientsBiocideVitamin CPhysiology
The present invention relates to compositions without added iron and methods for prophylactic nutritional supplementation and therapeutic nutritional supplementation. Specifically, the method involves administering to an individual a composition comprising carotenoids, vitamin E, vitamin D, vitamin C, thiamine, riboflavin, niacin, folic acid, pyridoxine, biotin, pantothenic acid, cobalamin, magnesium, manganese, zinc, selenium, chromium, copper, alpha lipoic acid, and lutein, wherein the composition is free of added iron.
Owner:EVERETT LAB
Krill oil compositions
InactiveUS20080166419A1Low densityHigh densityEster active ingredientsCrustacean material medical ingredientsSignificant riskNiacin
The present disclosure provides for novel krill oil-based compositions, method of administration and method of manufacture which provide for the treatment and prevention of cardiovascular disease, including the reduction of one or more significant risk factors involved with cardiovascular disease. The active ingredients of the composition include krill oil, combined in one embodiment with niacin, and combined in an alternate embodiment with polymethoxylated flavones (PMFs), and combined in yet another embodiment with Cissus quadrangularis.
Owner:SONES SCOTT F
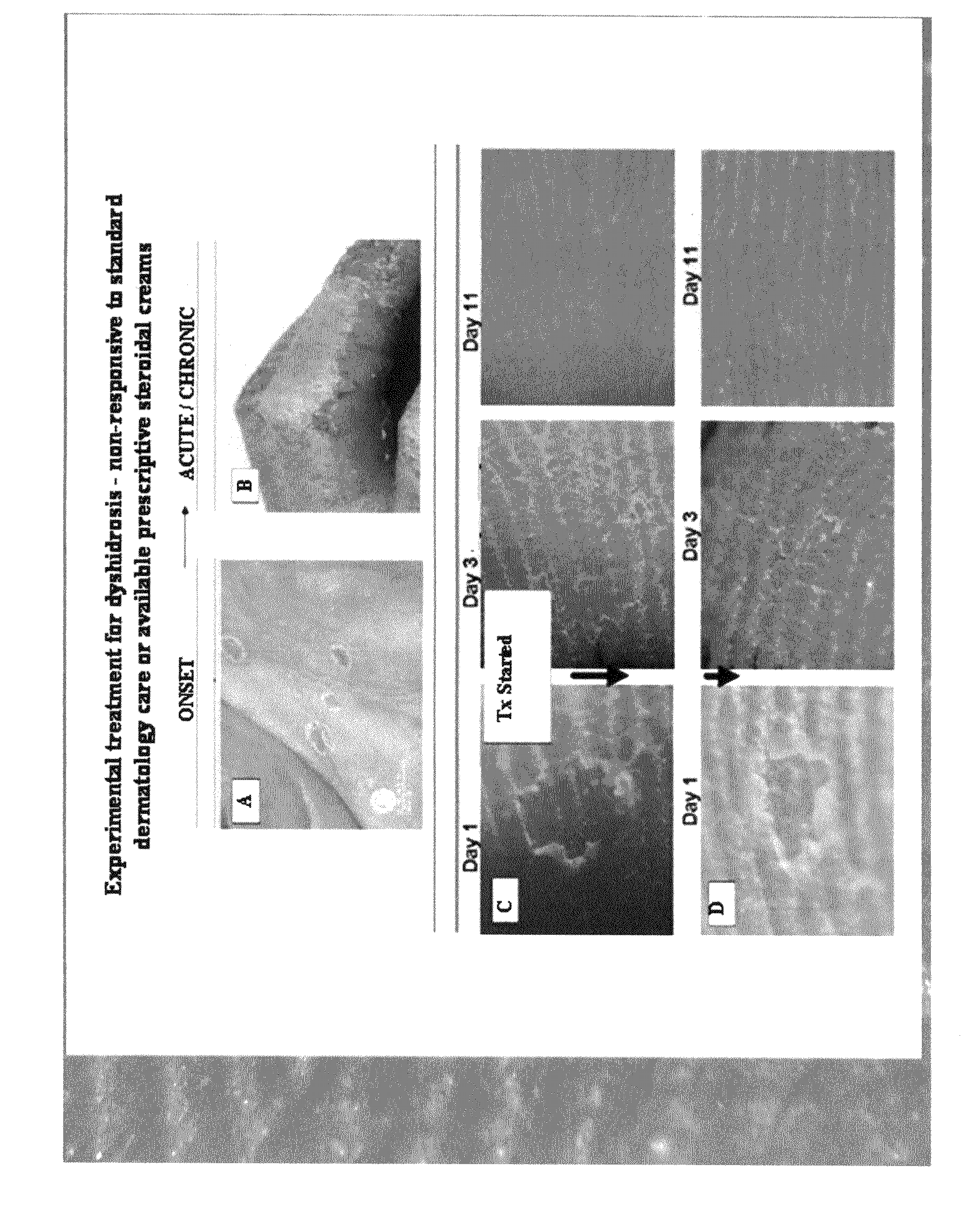
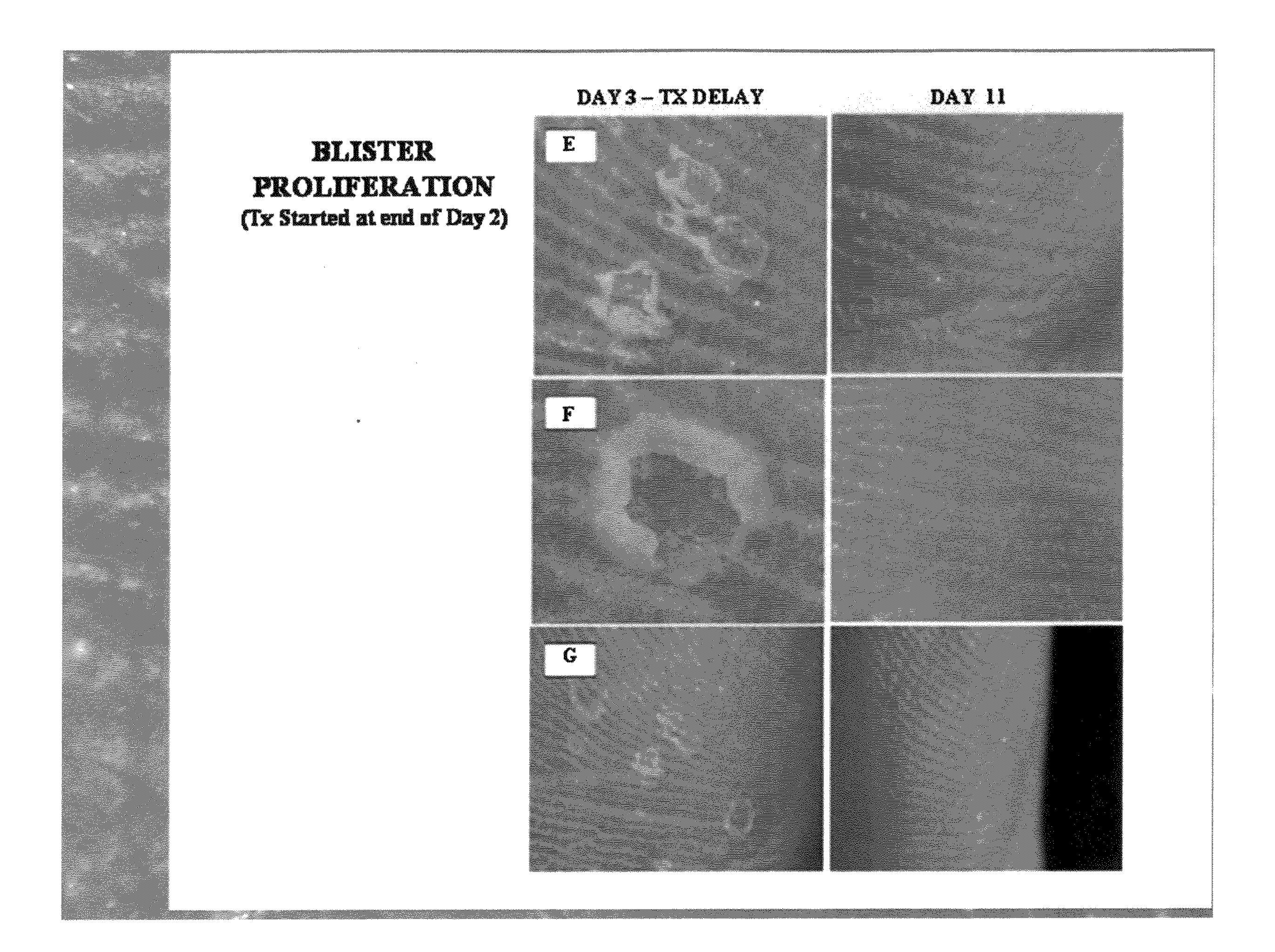
Method of treating dyshidrosis(pompholyx) and related dry skin disorders
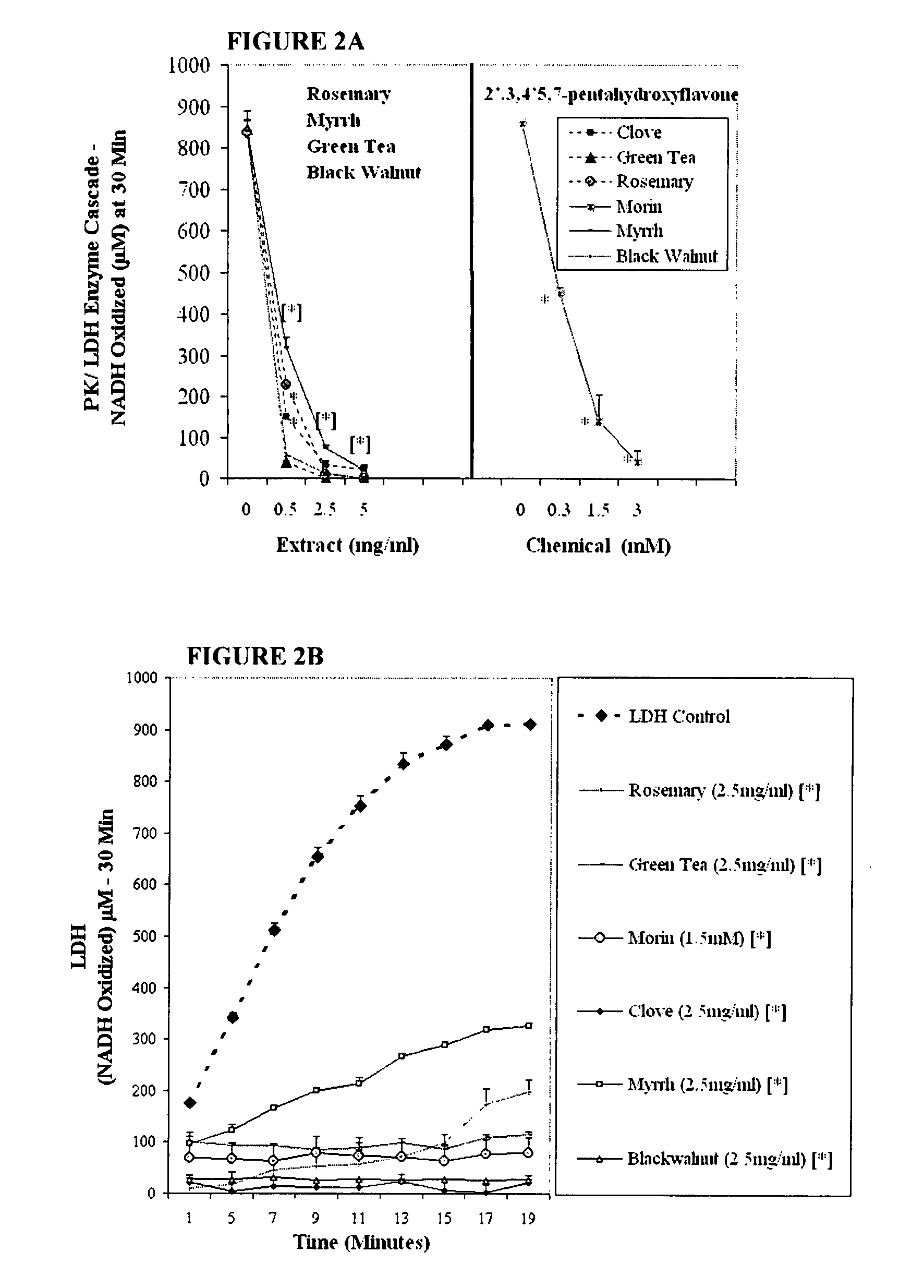
This invention discloses a method of use for a topical herbal formulation alone or in combination with oral administration of niacin (preferably a flush preparation) to prevent and / or treat dyshidrosis (pompholyx) and related skin diseases. The formulation may also be used to treat contact dermatitis, eczema, palmoplantar pustulosis and skin infections incurred by invasive pathogens such as mold, fungus and bacteria. The formulation is comprised of plant extracts and niacin, that when combined yield an effective multi-faceted pharmaceutical approach to treating dry skin disorders. The active ingredients within the formula include a combination of dry, aqueous, acid and alcohol extracts of black walnut hull (Juglans nigra), wormwood (Artemisia absinthium), tumeric rhizome (Curcuma longa), garlic (Allium sativum), two or more herbal antibacterial agents from the group consisting of chamomile (Matricaria Chamomile), licorice root (Glycyrrhiza glabra), St Johns wort (Hypericum perforatum), clove (Syzygium aromaticum), nutmeg (Myristica fragans), ginger (Zingiber officinale), frankincense (Boswellia carteri) and myrrh (Commiphora molmol), further combined with aloe vera and niacin.
Owner:MAZZIO ELIZABETH ANNE +1
Cinnamon formulation for reducing cholesterol and/or glucose levels
InactiveUS20050147620A1Good curative effectImprove efficiencyBiocidePeptide/protein ingredientsDiabetes mellitusRed yeast rice
A formulation comprising cinnamon and an active compound such as creatine, a statin drug, niacin, lipoic acid and / or Red Yeast Rice is disclosed. The cinnamon aids in moving the active compound into cells making the active compound more effective as compared to its administration in the absence of cinnamon. Methods of treatment including methods of reducing cholesterol levels, building muscle and treating diabetes are enhanced by the co-administration of cinnamon with another compound.
Owner:BOZICEVIC KARL
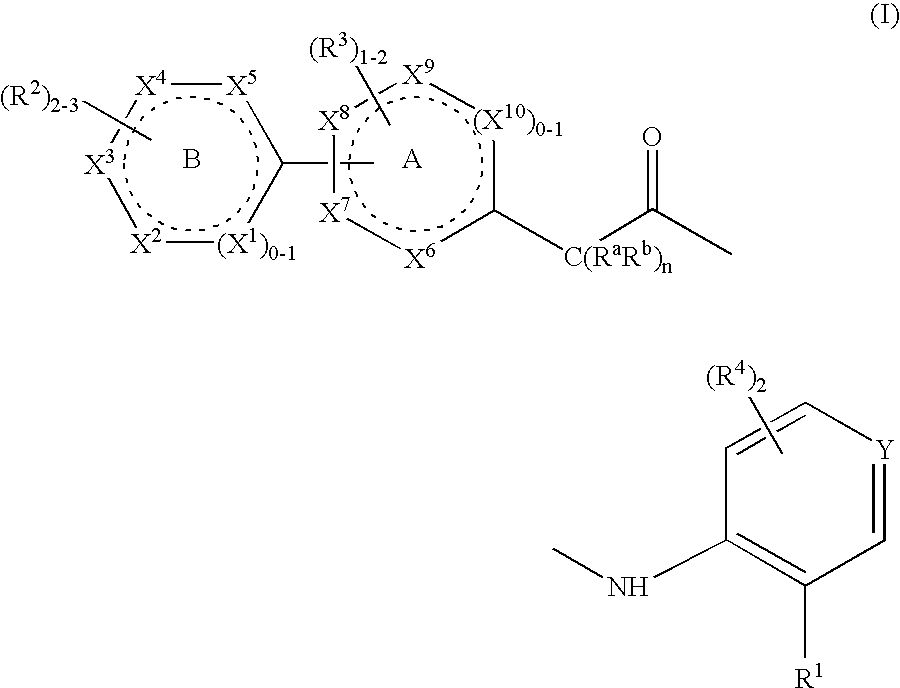
Niacin Receptor Agonists, Compositions Containing Such Compounds and Methods of Treatment
Owner:MERCK SHARP & DOHME CORP
Pig fattening feed
InactiveCN101965922AIncrease profitIncrease economic incomeFood processingAnimal feeding stuffVitamin K3Corn meal
The invention relates to a pig fattening feed and belongs to the technical field of animal feeds. The pig fattening feed mainly solves the technical problems of overmuch fatty pork, high cost and low income which are caused by adopting concentrated feed to fast fatten pigs when the pigs are fattened before being taken to markets in the conventional feedlots. The pig fattening feed is prepared by mixing the following raw materials in a certain proportion: corn flour, soybean meal, bran, rock flour, calcium hydroxide, salt and premix, wherein the premix consists of vitamin A, vitamin B1, vitamin B2, vitamin B6, vitamin B12, vitamin D3, vitamin E, vitamin K3, biotin, niacin, calcium pantothenate, folic acid, choline chloride, ferrous sulfate, copper sulfate, manganese sulfate, zinc sulfate, potassium iodide and sodium selenite. The pig fattening feed has the advantages of high feed utilization efficiency, low cost and high income.
Owner:沁源县灵兴养殖专业合作社
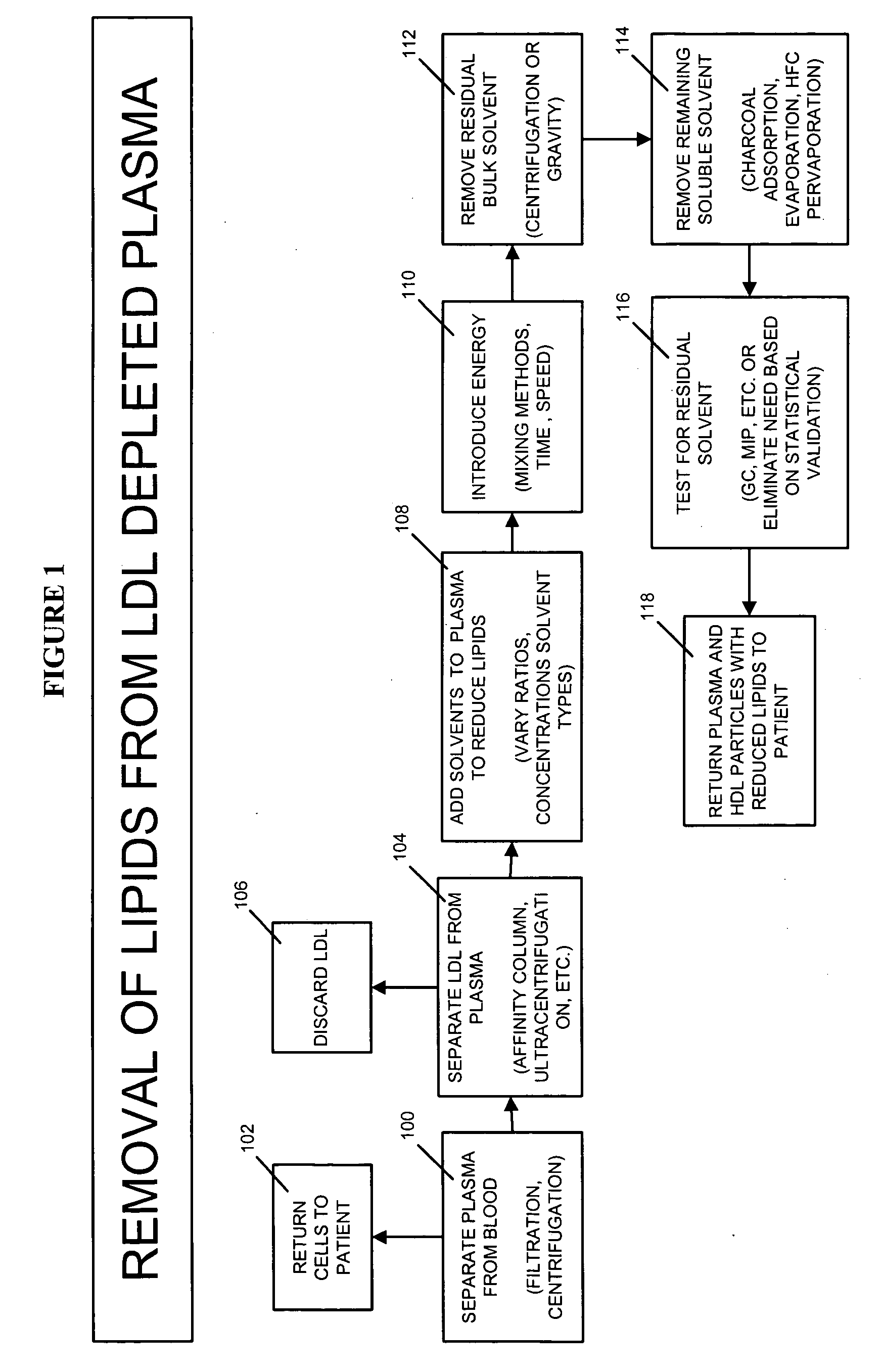
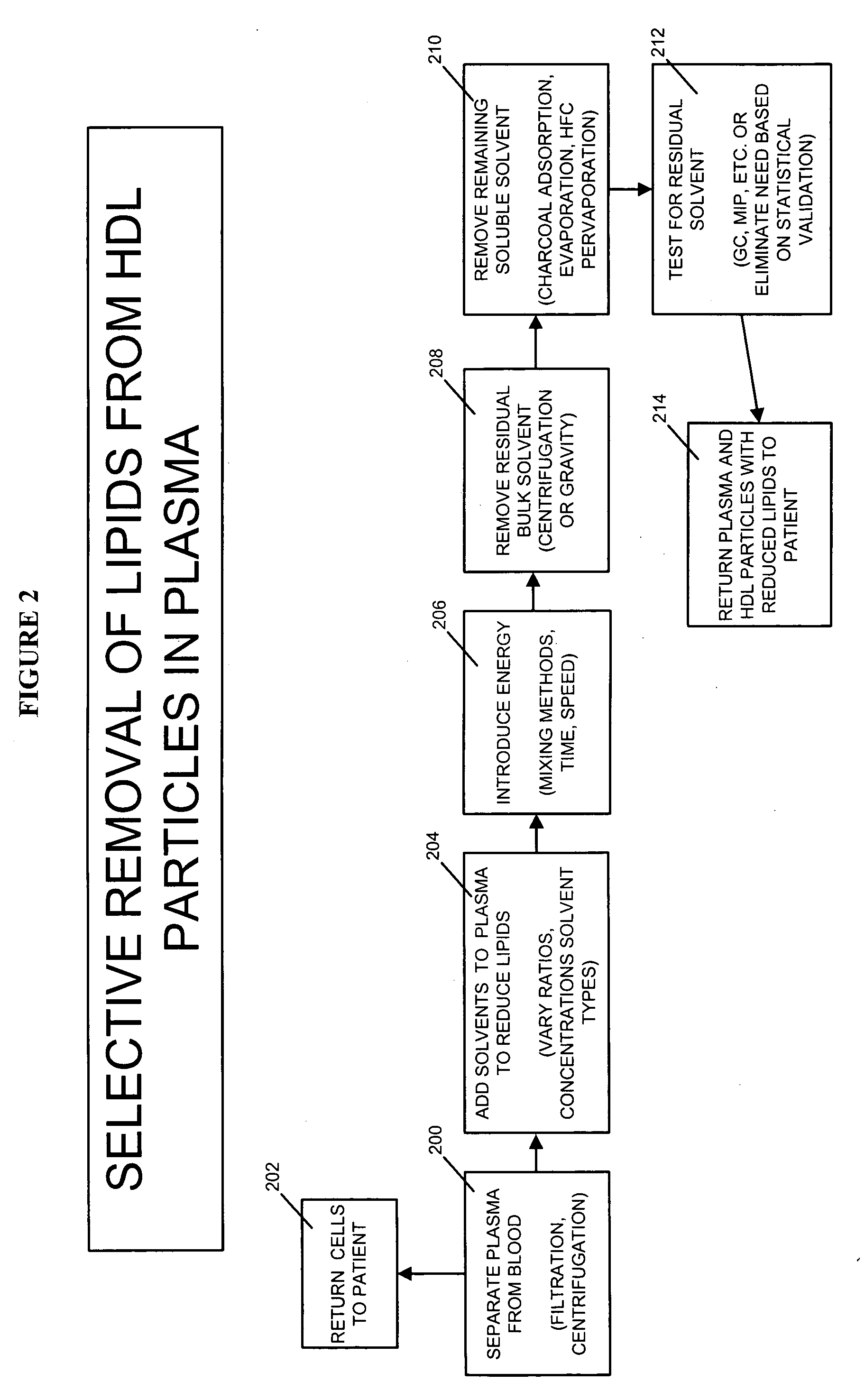
Methods and apparatus for creating particle derivatives of HDL with reduced lipid content
ActiveUS20050004004A1Low in lipidsEnhancing cellular cholesterol effluxNervous disorderPeptide/protein ingredientsPresent methodCellular cholesterol
The present invention is directed to systems, apparatus and methods for creating derivatives of at least one form of HDL without substantially affecting LDL. These derivatives of HDL are particles with reduced lipid content, particularly reduced cholesterol content. These particles have the capacity to bind cholesterol and are administered to a patient to enhance cellular cholesterol efflux and reduce cholesterol levels in cells, tissues, organs, and blood vessels. The present method is useful for treating atherogenic vascular disease and may be combined with other therapies such as statins, inhibitors of cholesterol absorption, niacin, anti-inflammatories, exercise and dietary restriction.
Owner:LIPID SCI +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com