Patents
Literature
583 results about "Metformin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Metformin is used with a proper diet and exercise program and possibly with other medications to control high blood sugar. It is used in patients with type 2 diabetes.
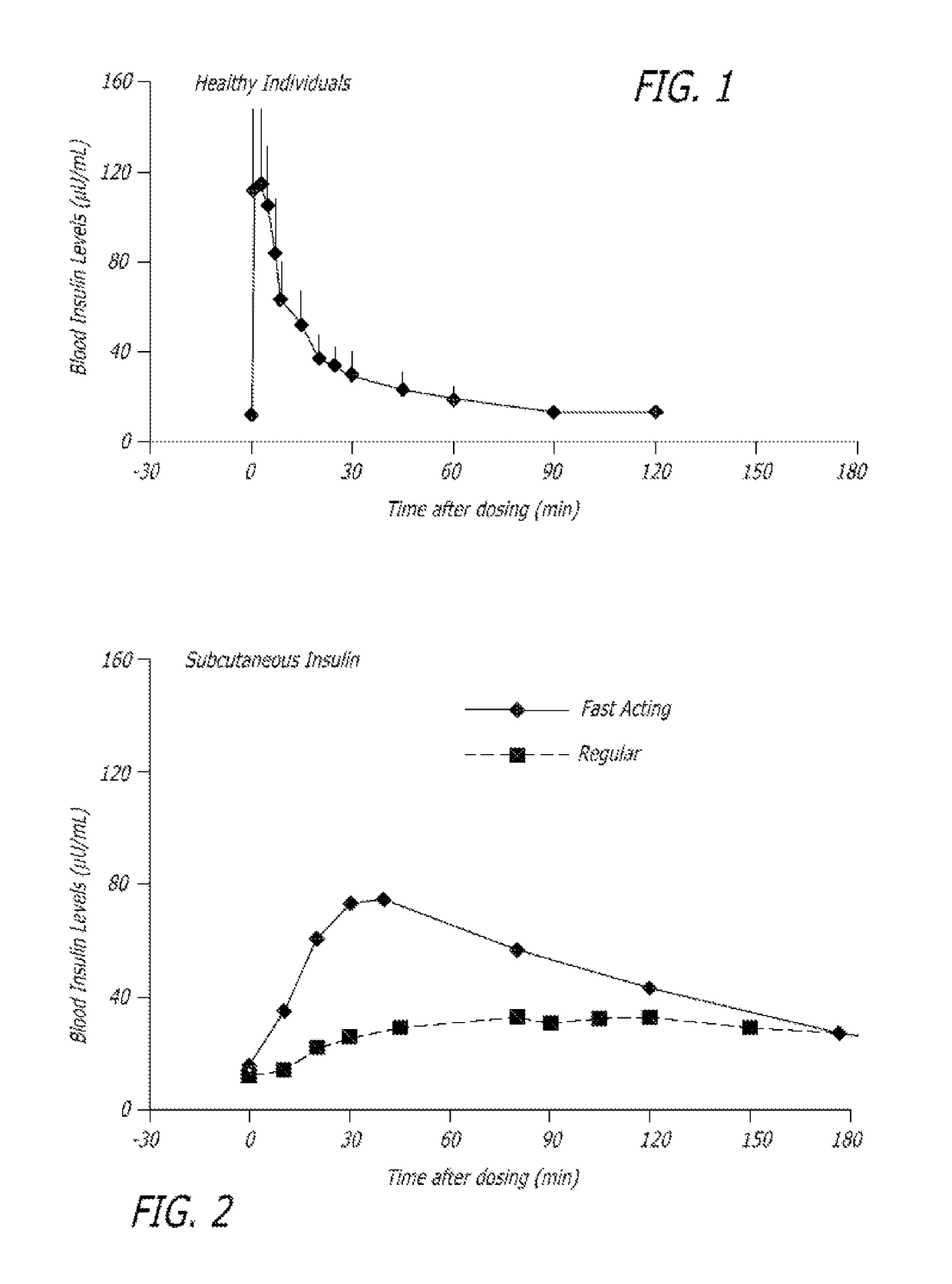
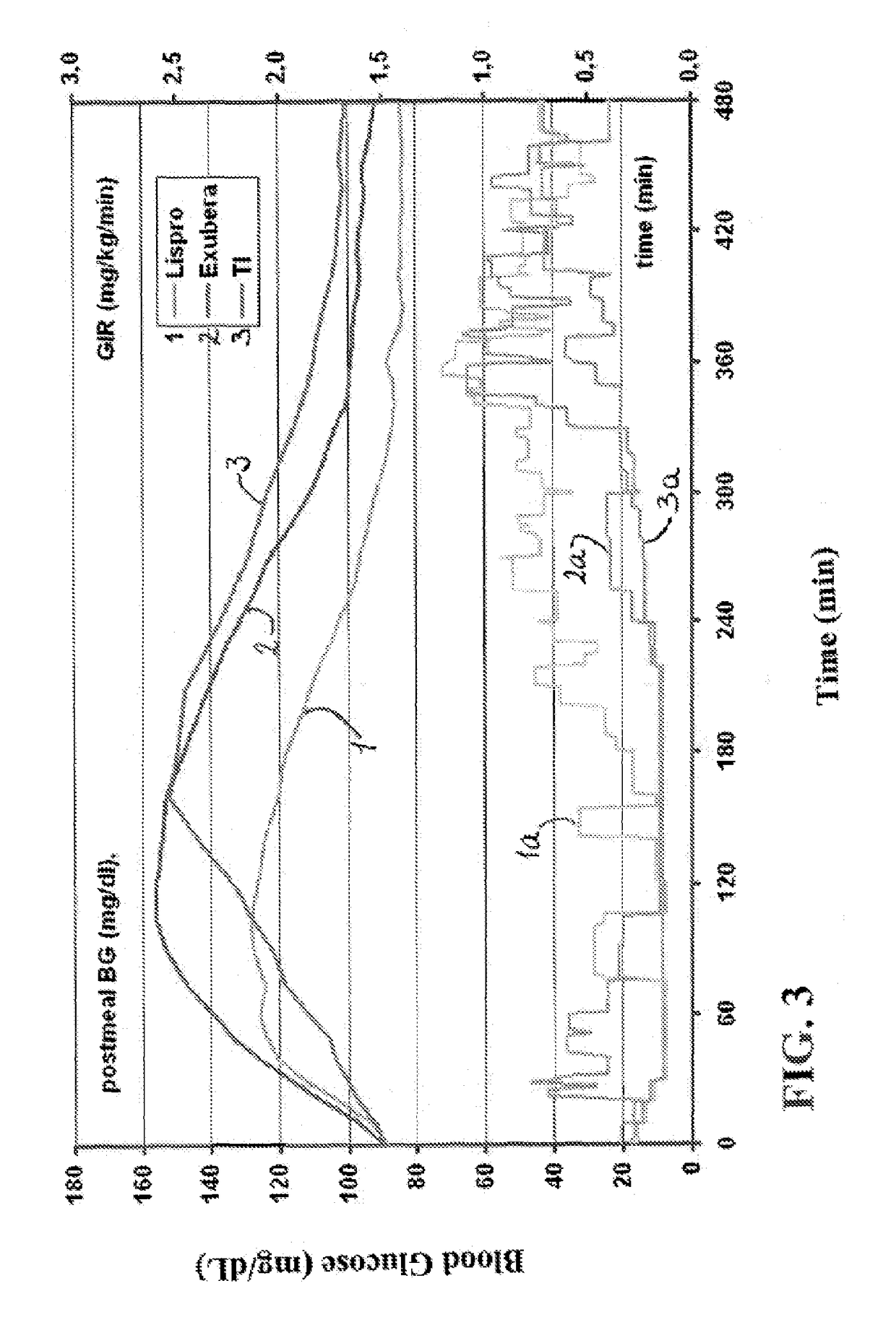
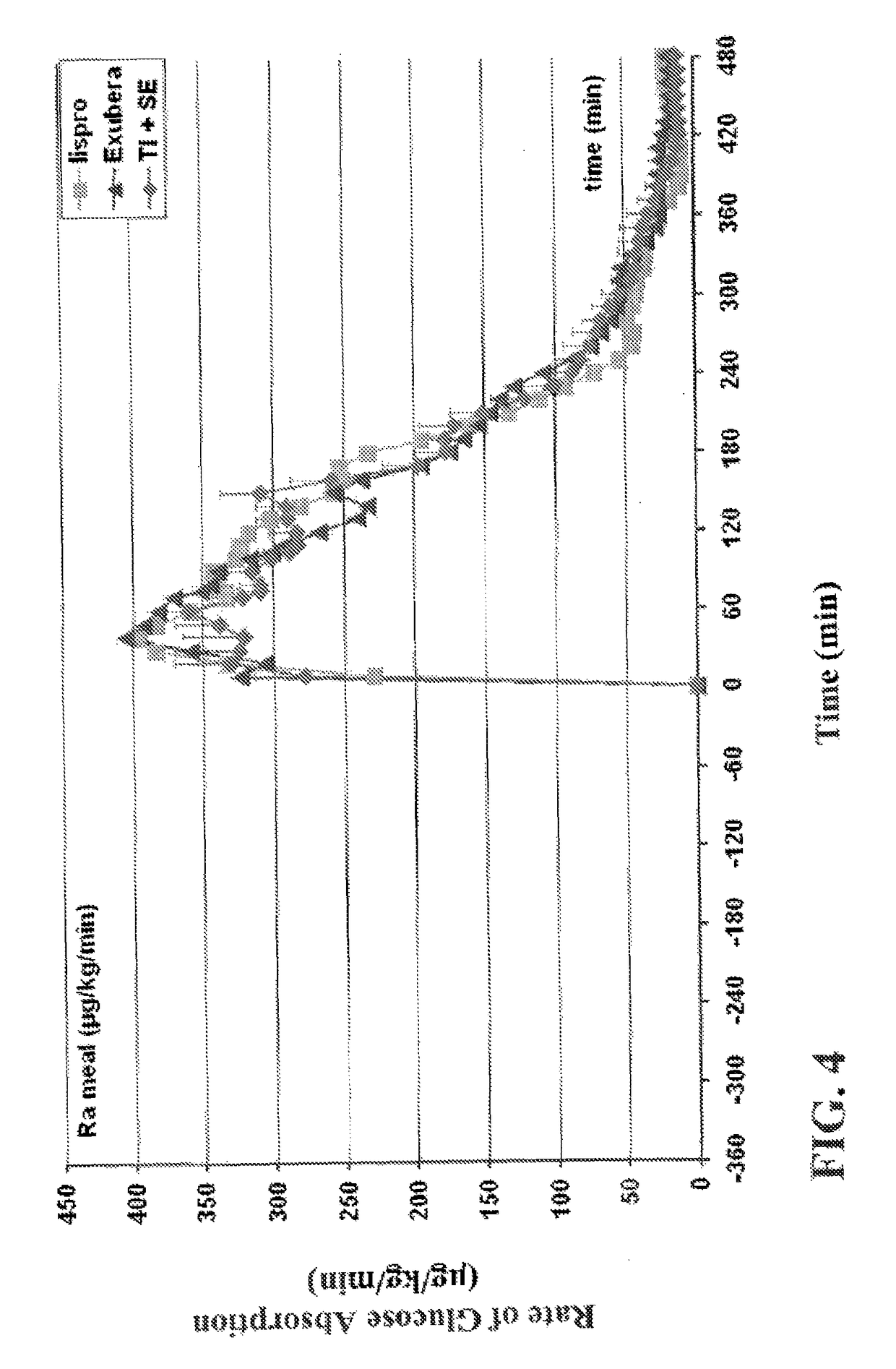
Method of treating diabetes type 2 by metformin and an ultrarapid acting insulin
Disclosed herein are improved methods of treating hyperglycemia with a combination of an ultrarapid acting insulin and insulin glargine comprising prandial administration of the ultrarapid insulin, and administration of a first dose of insulin glargine within 6 hours of waking for a day.
Owner:MANNKIND CORP
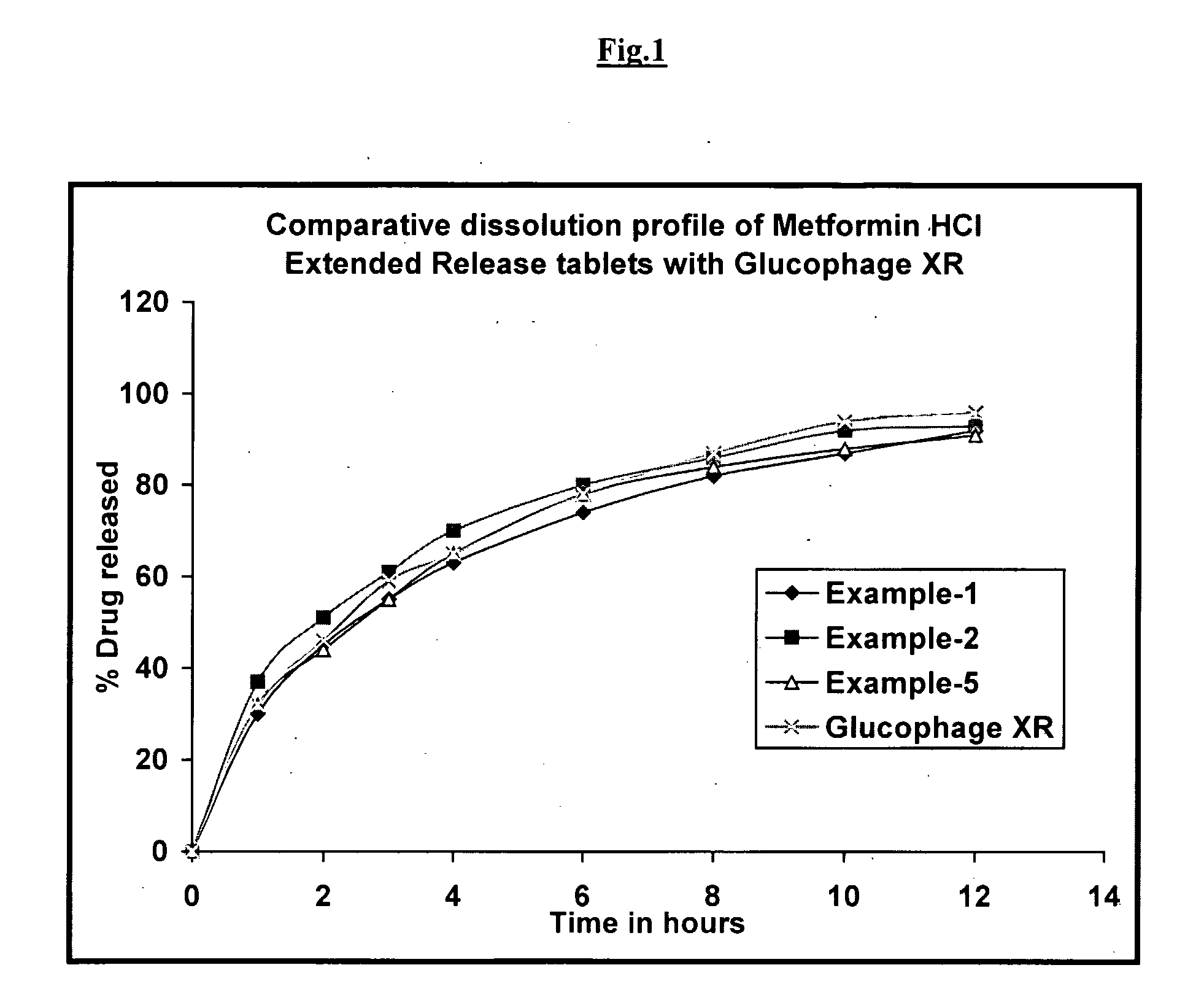
Controlled release metformin compositions
InactiveUS20060034922A1Effective controlImprove bioavailabilityCoatingsOsmotic deliveryControlled releaseHigh doses
Owner:ANDRX LABS
Compositions and methods for treating diabetes
InactiveUS20050137125A1Reducing and eliminating severityReducing and eliminating and intensityBiocideSenses disorderOral medicationGlucose polymers
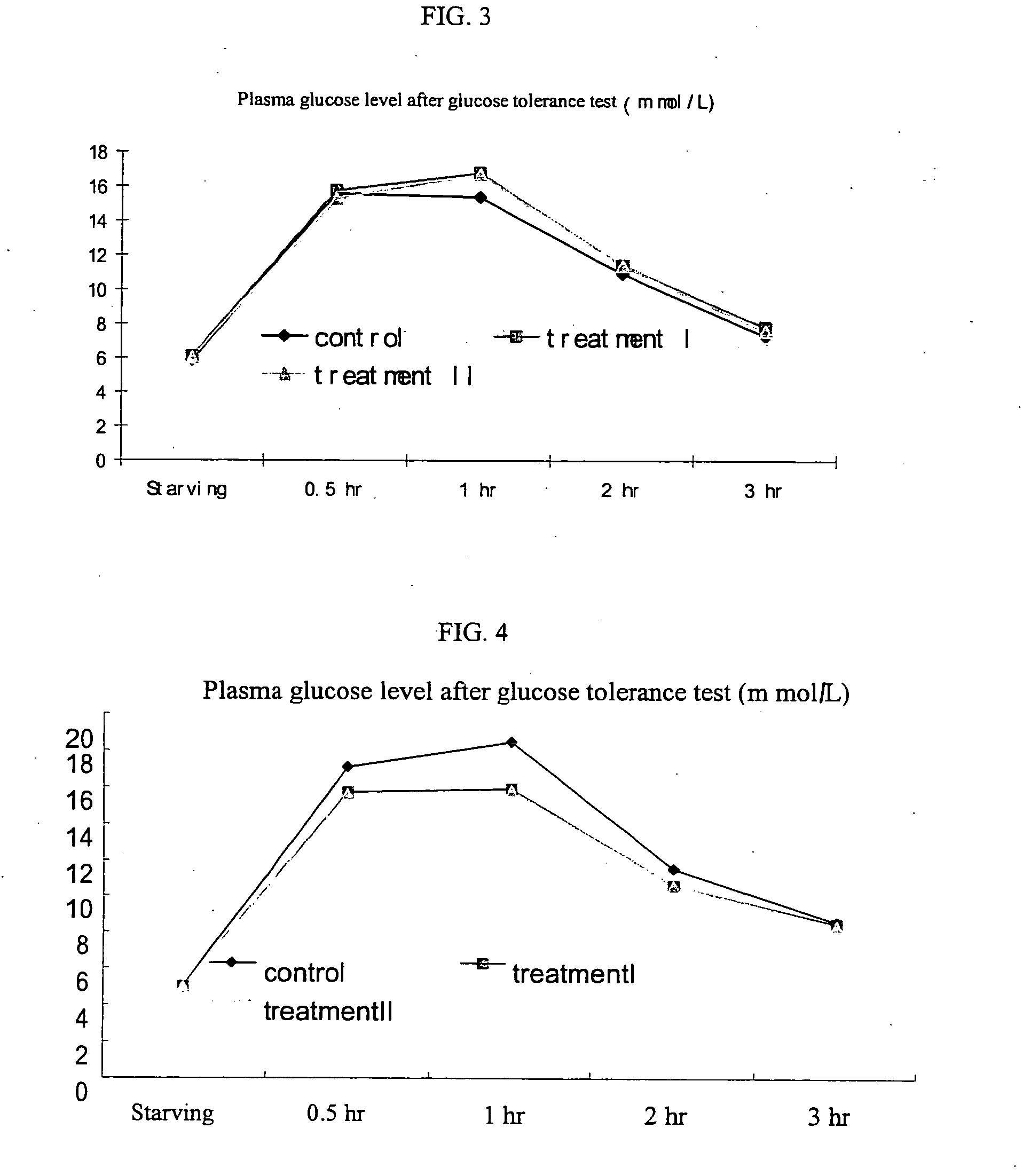
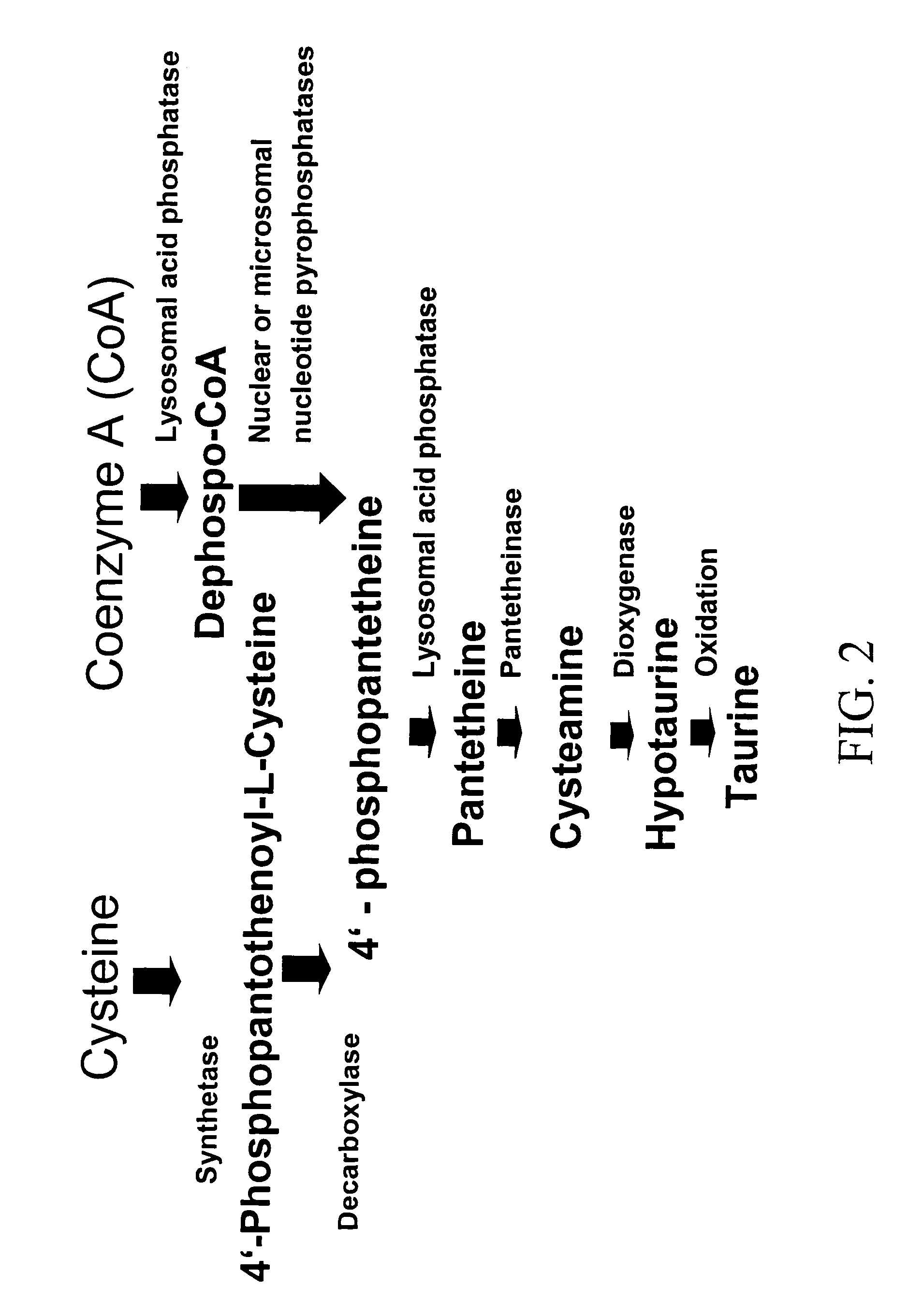
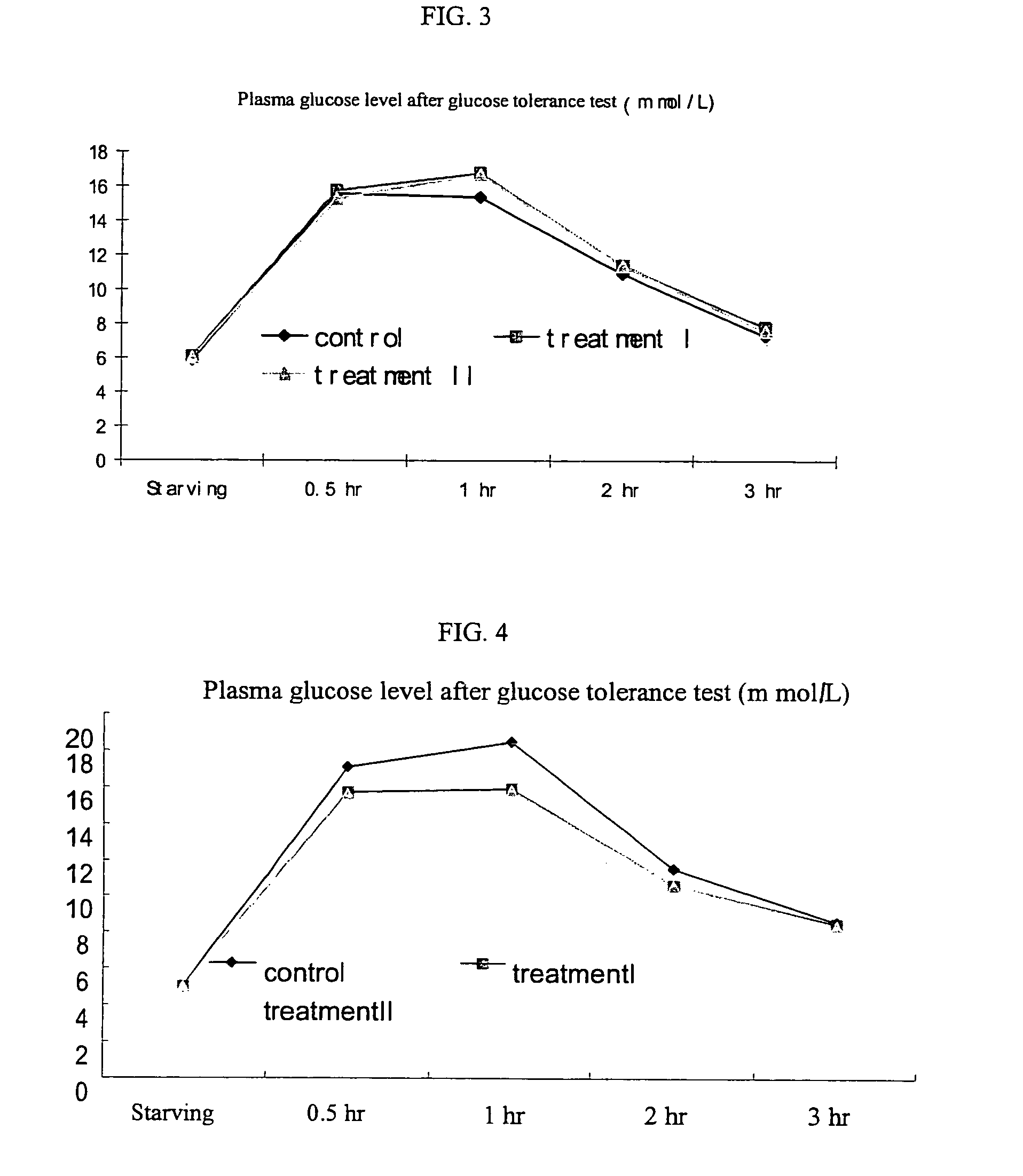
The subject invention provides compositions and methods for treating diabetes in patients. In a preferred embodiment, the invention provides compositions methods for treating diabetes and / or preventing or alleviating complications associated with diabetes. Specifically exemplified herein is the concurrent administration of a cysteamine compound with at least one additional therapeutic agent to prevent and / or treat diabetes as well as prevent and / or treat complications associated with diabetes. In a preferred embodiment, oral administration of cysteamine hydrochloride with Metformin to a patient diagnosed with diabetes can substantially regulate the patient's glucose metabolism and insulin sensitivity.
Owner:OMEGA BIO PHARMA I P 3
Compositions and methods for treating diabetes
InactiveUS7442720B2Efficacious in lowering blood glucose levelReduce and eliminate and and durationAntibacterial agentsBiocideOral medicationGlucose polymers
The subject invention provides compositions and methods for treating diabetes in patients. In a preferred embodiment, the invention provides compositions methods for treating diabetes and / or preventing or alleviating complications associated with diabetes. Specifically exemplified herein is the concurrent administration of a cysteamine compound with at least one additional therapeutic agent to prevent and / or treat diabetes as well as prevent and / or treat complications associated with diabetes. In a preferred embodiment, oral administration of cysteamine hydrochloride with Metformin to a patient diagnosed with diabetes can substantially regulate the patient's glucose metabolism and insulin sensitivity.
Owner:OMEGA BIO PHARMA I P 3
Antidiabetic agent for control of diabetic hyperglycemia and diabetic complications
InactiveUS20070293562A1High blood levelBiocideOrganic active ingredientsAcute hyperglycaemiaDiabetic complication
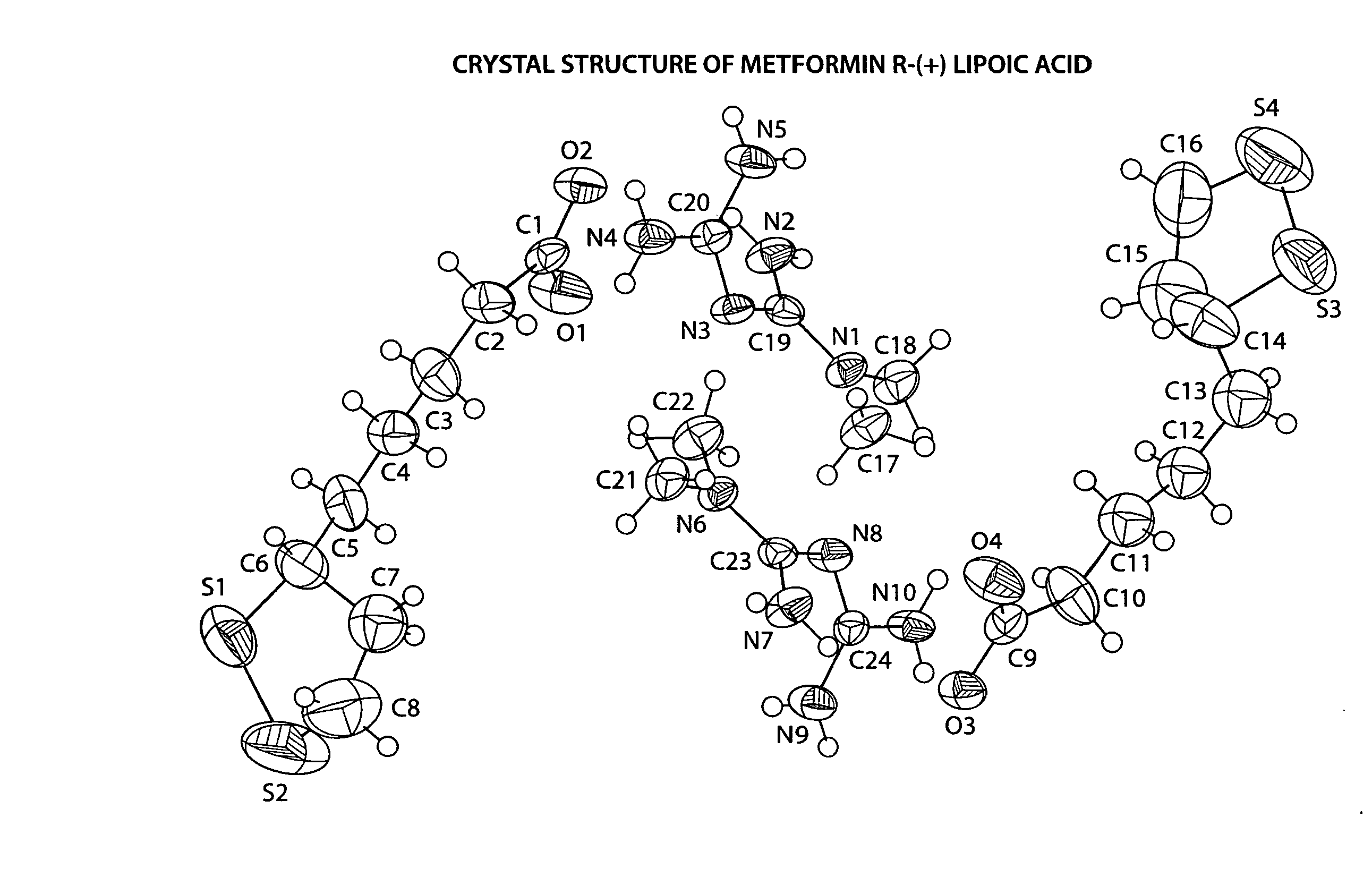
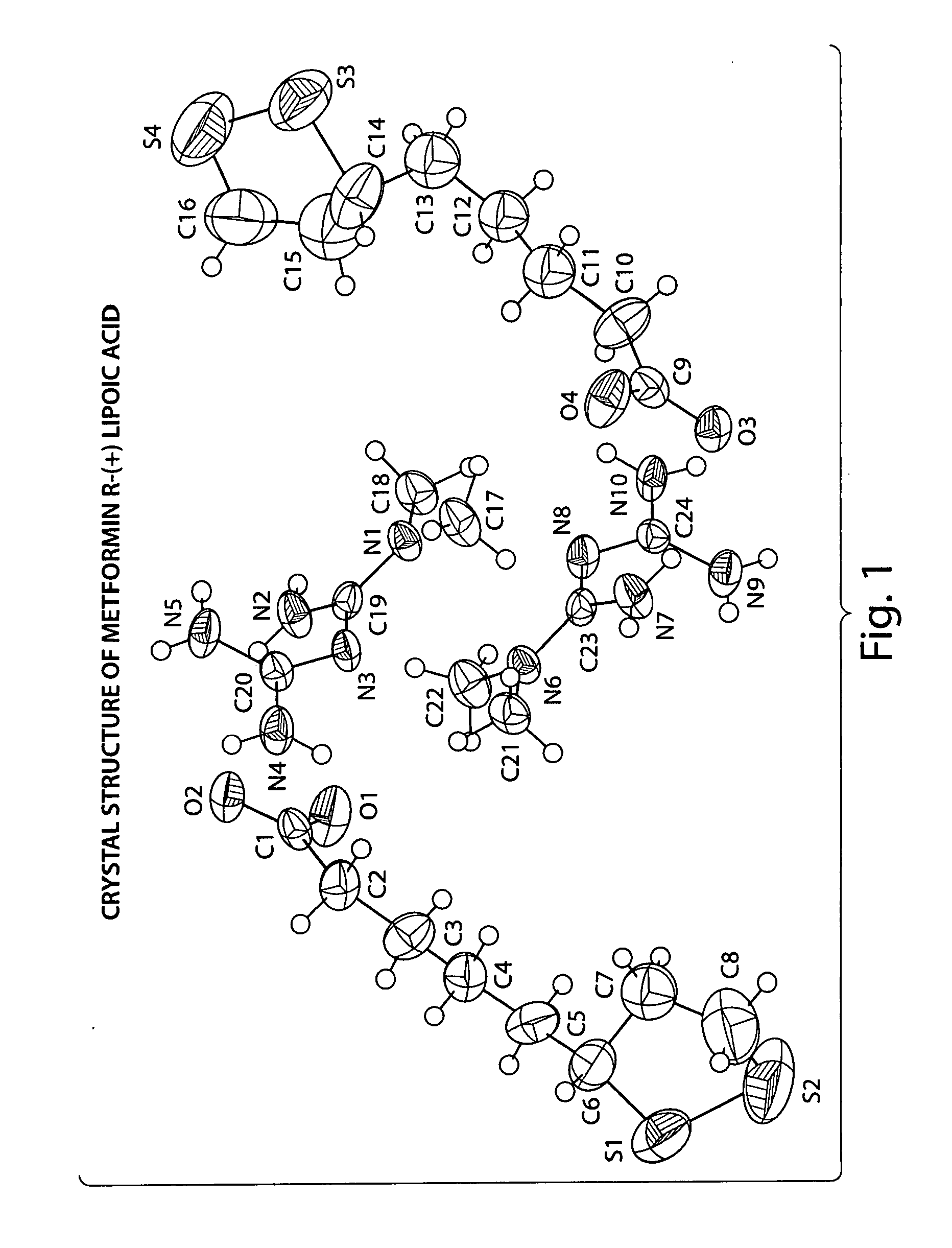
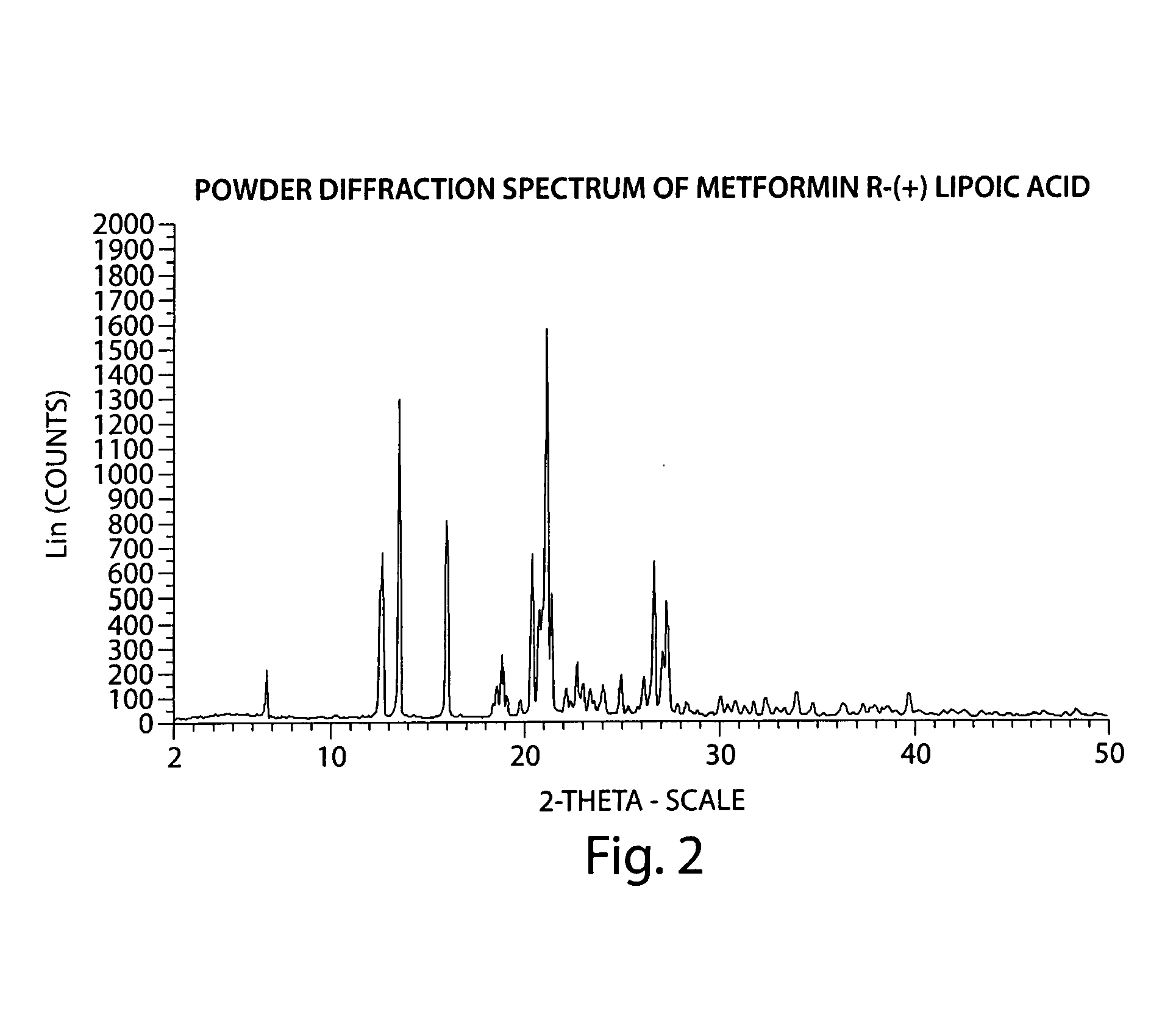
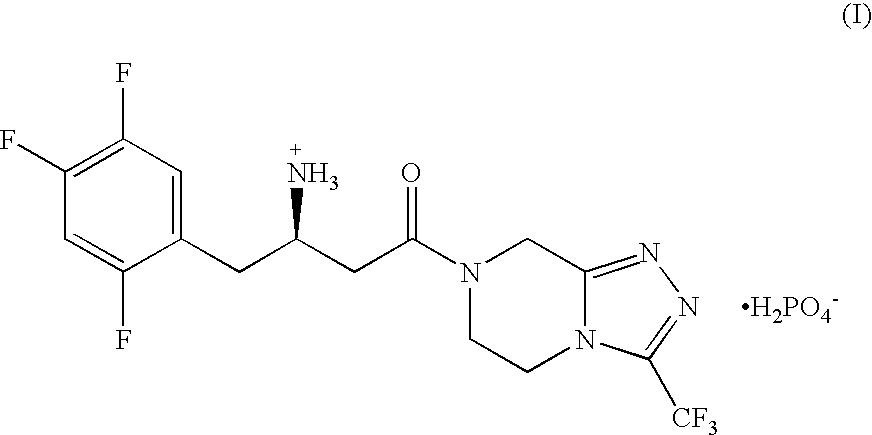
Described herein is a compound of Formula I, which is the metformin salt of the naturally occurring endogenous biological compound, (R)-(+) α lipoic acid, pharmaceutical compositions containing the compound of Formula I, and methods of treatment of diabetes or diabetic complications with the compound of Formula I.
Owner:INDIGENE PHARMA
Controlled release metformin formulations
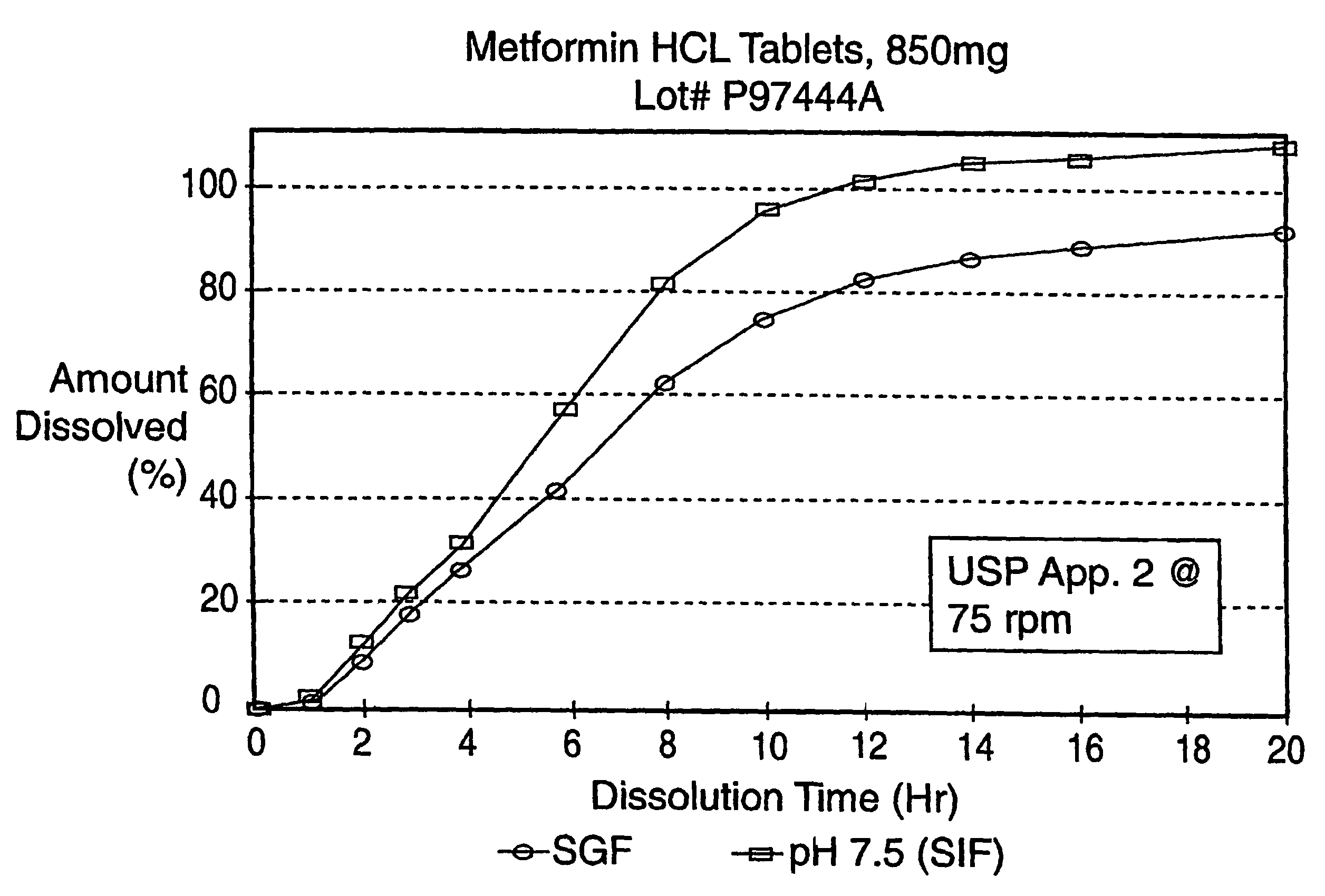
InactiveUS7919116B2Reduced bioavailabilityImprove bioavailabilityOrganic active ingredientsBiocideControlled releaseSustained release drug
Sustained release pharmaceutical formulations comprising an antihyperglycemic drug or a pharmaceutically acceptable salt thereof are disclosed. The formulations provide therapeutic plasma levels of the antihyperglycemic drug to a human patient over a 24 hour period after administration.
Owner:ANDRX LABS
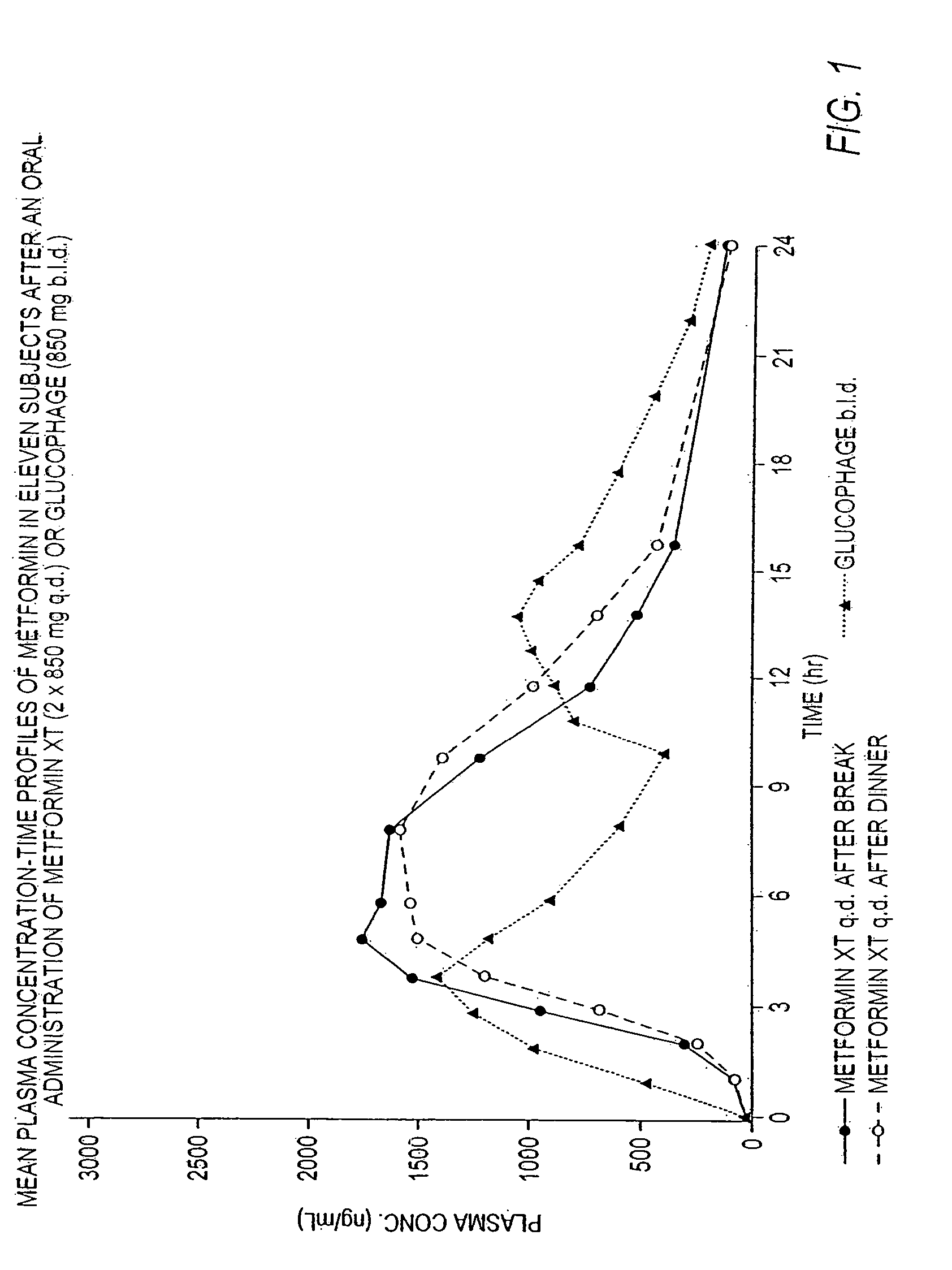
Sustained release formulations of metformin
The invention provides sustained release formulations of metformin or a pharmaceutically acceptable salt thereof, and methods of treating diabetes by administering to a patient a therapeutically effective amount of a sustained release formulation of metformin or a pharmaceutically acceptable salt thereof.
Owner:ENDO PHARMA INC
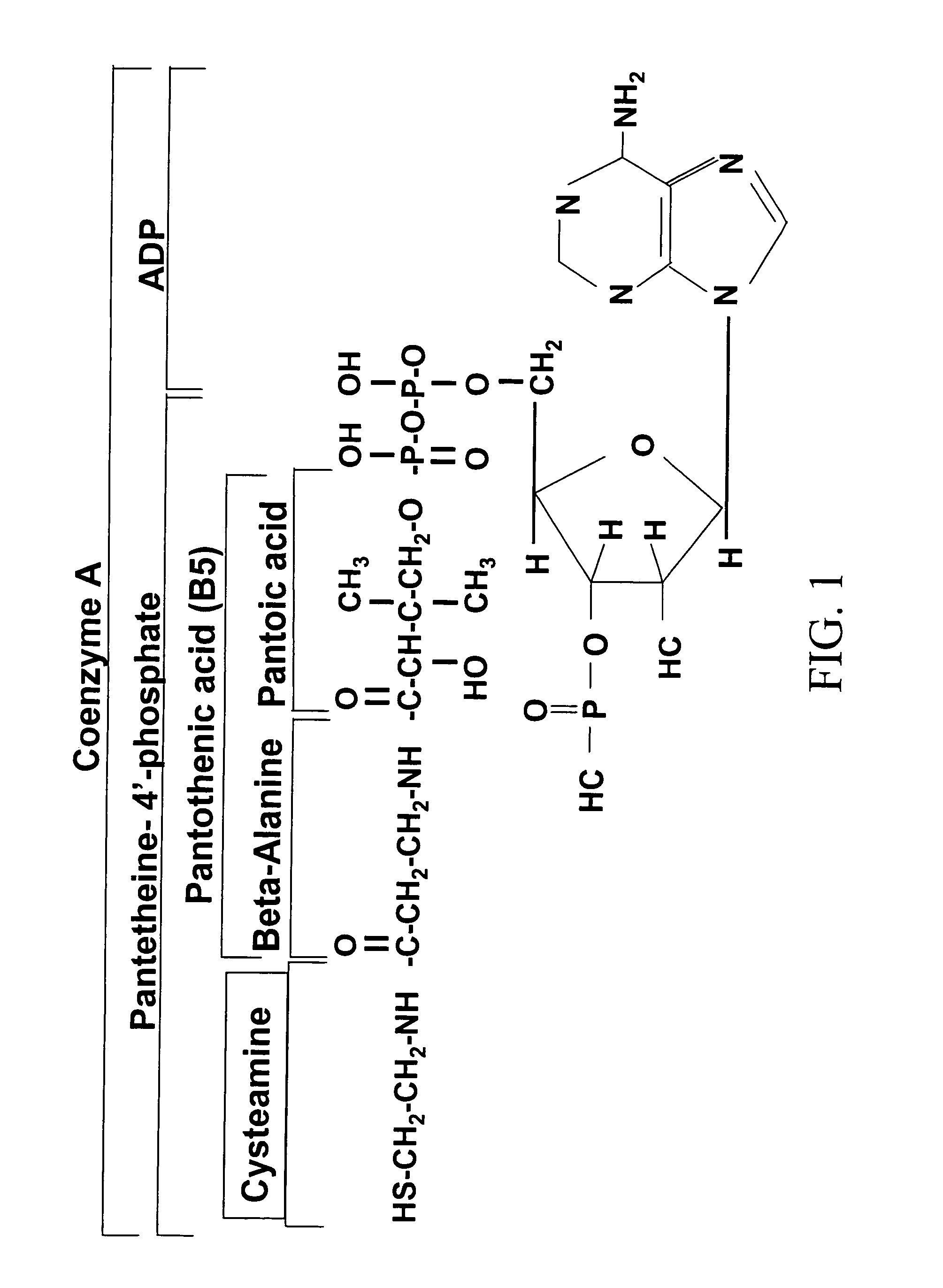
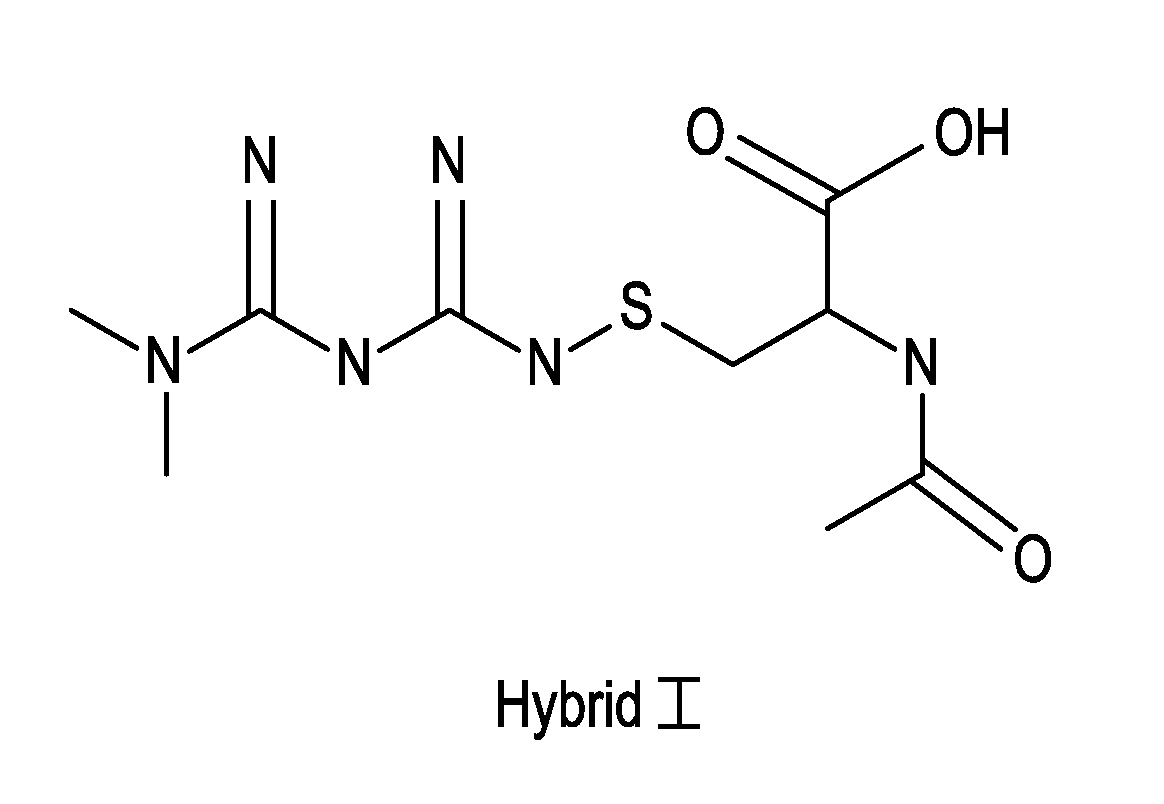
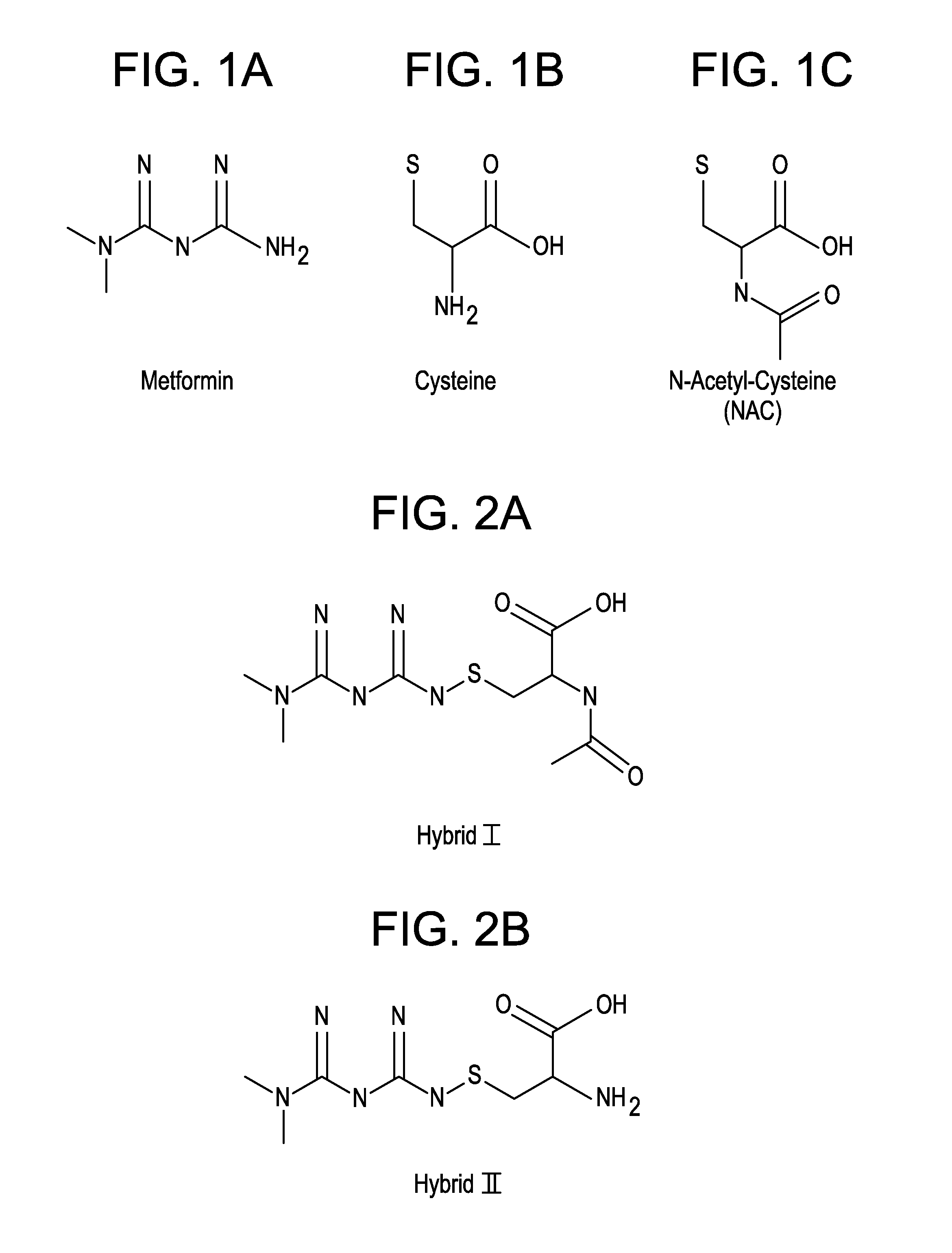
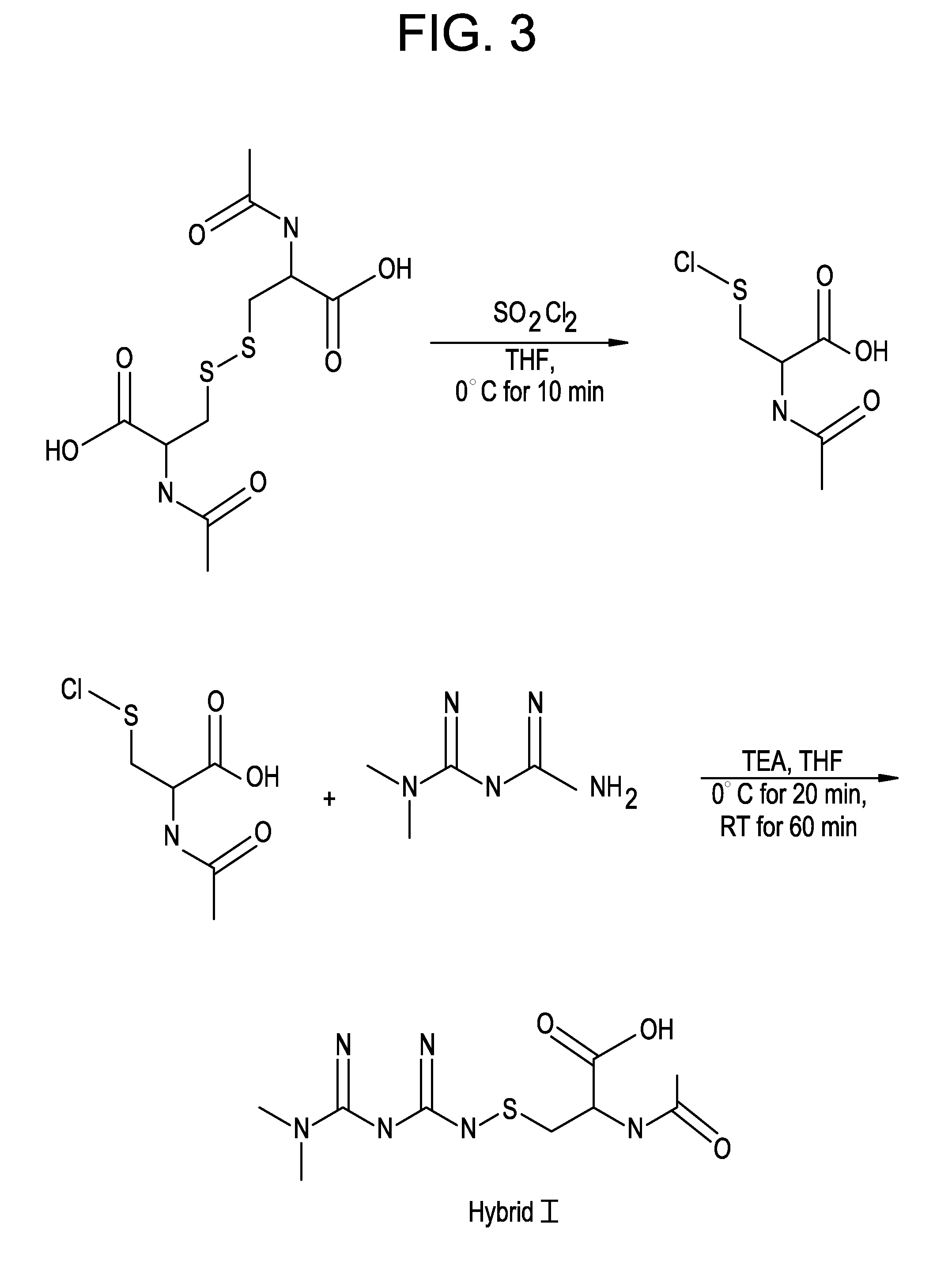
Metformin-Cysteine Prodrug
ActiveUS20110257432A1Promote intestinal absorptionEffective absorptionOrganic chemistrySmall intestinePlasma concentration
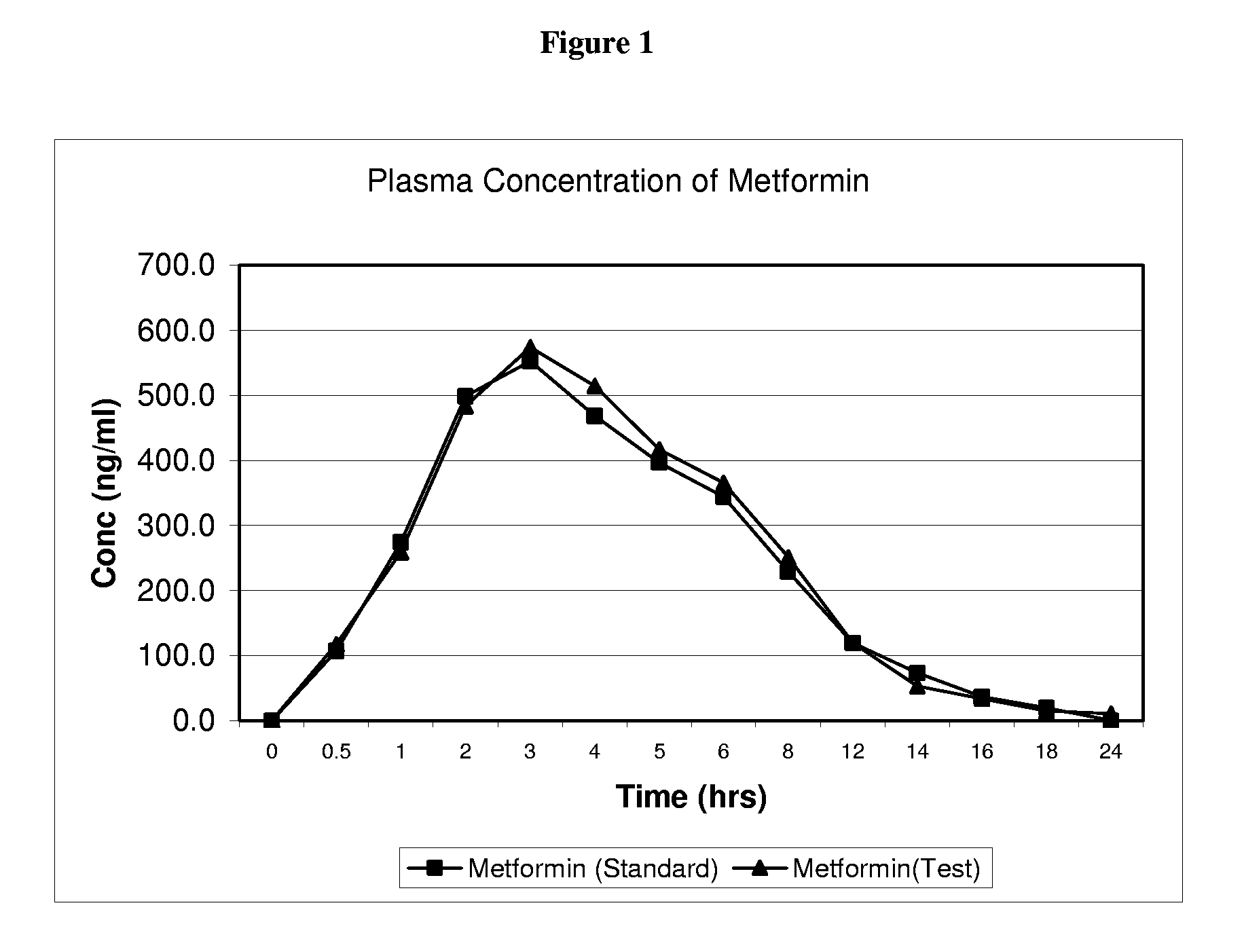
A metformin-cysteine prodrug. It is believed that the prodrug of the present invention will transport in the LAT1 and LAT2 transporter system. Because the LAT1 and LAT2 transporters are important and effective transporters of amino acids in both the small intestine and colon, it is believed that the LAT-transportable prodrugs of the present invention will be effectively absorbed both in small intestine and in the colon. The increased absorption window provided by the present invention should result in highly sustained plasma concentrations of metformin, thereby increasing the effectiveness of the medication and allowing for a single daily dose.
Owner:CODMAN & SHURTLEFF INC
Mucoadhesive Oral Formulations of High Permeability, High Solubility Drugs
Solid oral dosage formulations, such as tablet, mini-tab, multiparticulates or osmotic delivery systems, are coated with a mucoadhesive polymeric coating or formed of a mucoadhesive polymer to increase oral bioavailability of Biopharmaceutical Classification System (BCS) Class I drugs. Representative BCS I drugs include valacyclovir, gabapentin, furosemide, levodopa, metformin, and ranitidine HCl. The inclusion of mucoadhesives in the solid oral dosage form brings the dosage form into close proximity with the target epithelium and facilitates diffusion of drug into intestinal tissue. The mucoadhesive polymer may be either dispersed in the matrix of the tablet or applied as a direct compressed coating to the solid oral dosage form. Preferred mucoadhesive polymers include poly(adipic)anhydride “P(AA)” and poly(fumaric-co-sebacic)anhydride “P(FA:SA)”. Other preferred mucoadhesive polymers include non-erodable polymers such as DOPA-maleic anhydride co polymer; isopthalic anhydride polymer; DOPA-methacrylate polymers; and DOPA-cellulosic based polymers.
Owner:JACOB JULES S +4
Lipid-lowering antidiabetic agent
InactiveUS20120178813A1Improve bioavailabilityHigh degreeBiocideOrganic chemistryTriglyceridePrediabetes
A composition which includes a salt of metformin and the use of the composition for treatment of or use in prediabetes, diabetes, lowering triglycerides and / or other conditions in mammals.
Owner:THETIS PHARMA
Extended Release Pharmaceutical Composition of Metformin and a Process for Producing It
InactiveUS20080274180A1Extended retention timeReliably controllable mannerOrganic active ingredientsMetabolism disorderControlled drugsOral medication
A pharmaceutical composition in the form of tablets constitutes an orally administered, controlled drug delivery system that will provide increased retention time of the device in the stomach over conventional dosage forms and release metformin or its pharmaceutically acceptable salt in a controllable manner, and further that is easy and inexpensive to manufacture.
Owner:ABBOTT HEALHCARE PROD BV
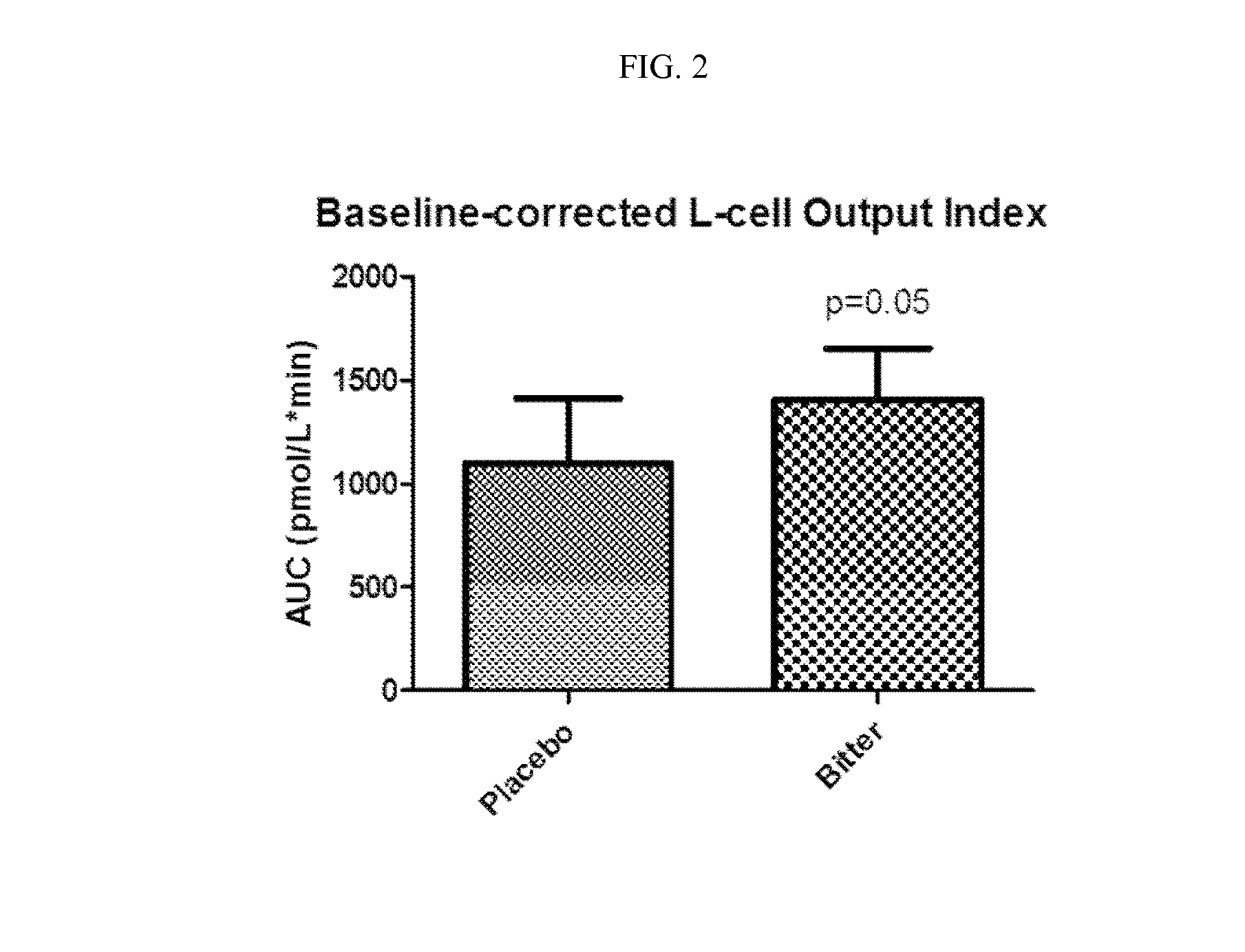
Chemosensory Receptor Ligand-Based Therapies
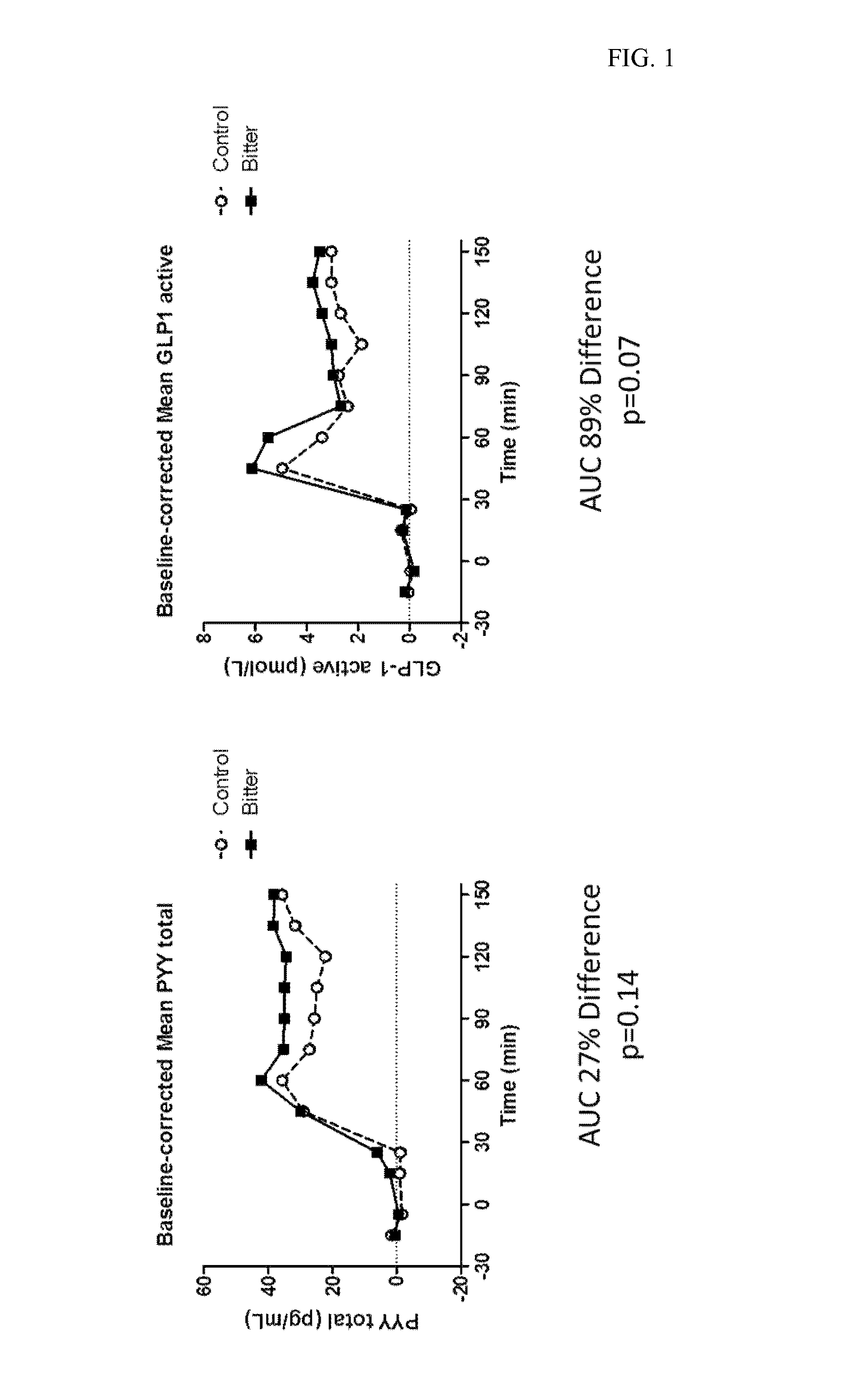
Provided herein are methods for treating conditions associated with a chemosensory receptor, including diabetes, obesity, and other metabolic diseases, disorders or conditions by administrating a composition comprising a chemosensory receptor ligand, such as a bitter receptor ligand. Also provided herein are chemosensory receptor ligand compositions, including bitter receptor ligand compositions, and methods for the preparation thereof for use in the methods of the present invention. Also provided herein are compositions comprising metformin and salts thereof and methods of use.
Owner:ANJI PHARMA INC
Solid Pharmaceutical Dosage Form
InactiveUS20110028456A1High drug loadingEasy to manufacturePowder deliveryBiocideValsartanTrenbolone
A pharmaceutical composition comprising a solid unit dosage form comprising: one or more of pharmaceutically active ingredients selected from valacyclovir, olanzapine, voriconazole, topotecan, artesunate, amodiaquine, guggulosterone, ramipril, telmisartan, tibolone, atorvastatin, simvastatin, amlodipine, ezetimibe, fenofibrate, tacrolimus, valgancyclovir, valsartan, clopidrogel, estradiol, trenbolone, efavirenz, metformin, pseudoephedrine, verapamil, felodipine, valproic acid / sodium valproate, mesalamine, hydrochlorothiazide, levosulpiride, nelfinavir, cefixime and cefpodoxime proxetil in combination with a water insoluble polymer and / or a water soluble polymer. Methods for making the pharmaceutical composition are also disclosed.
Owner:CIPLA LTD
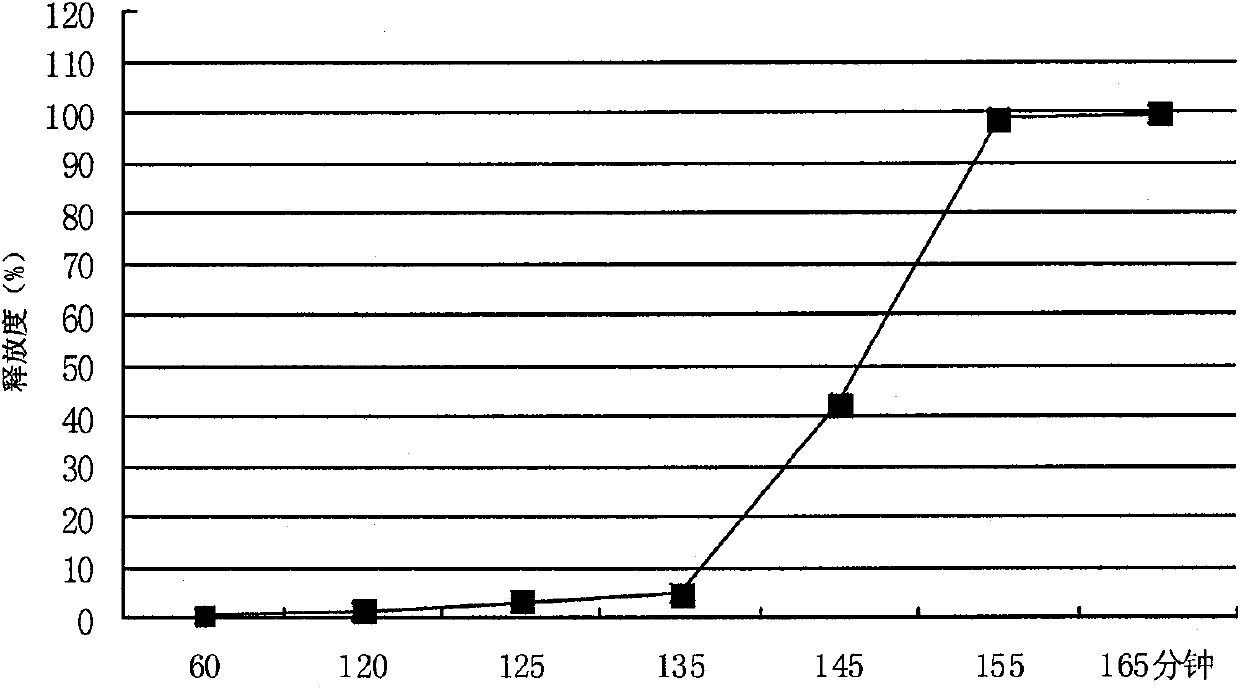
Metformin hydrochloride enteric-coated sustained release tablet and preparation method thereof
ActiveCN101785763AOrganic active ingredientsMetabolism disorderPatient complianceSustained-Release Preparations
The invention discloses a metformin hydrochloride enteric-coated sustained release tablet which is prepared by enteric coating the metformin hydrochloride sustained release tablet. Compared with the prior art, the sustained release tablet integrates with the enteric coating technology to prepare a new form of the metformin hydrochloride enteric-coated sustained release tablet. By using the enteric coating technology, the metformin hydrochloride does not disintegrate in the stomach or stimulate the gastric mucosa, and the adverse reaction of nausea, stomachache and diarrhea caused by medicine taking can be avoided; meanwhile, the metformin hydrochloride is prevented from being damaged by gastric juice, and the bioavailability is improved. The product is a sustained release preparation, the medicine can stably release in vivo, the effective blood concentration can be maintained for a long time, the toxic and side effects caused by over-high blood concentration in a short time are avoided, the medicine taking frequency is decreased, and the patient compliance is improved as well.
Owner:贵州天安药业股份有限公司
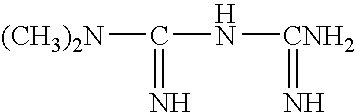
Preparation method of metformin hydrochloride
ActiveCN103435518AHigh recovery rateImprove securityOrganic chemistryOrganic compound preparationMetformin hclPharmacology
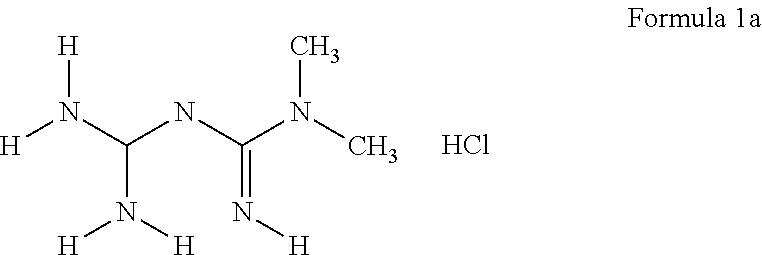
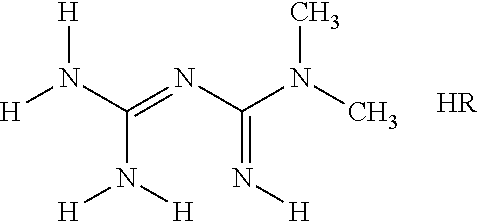
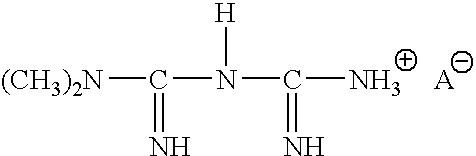
The invention discloses a preparation method of metformin hydrochloride. The preparation method is characterized by using dicyandiamide and dimethylamine hydrochloride as raw materials, feeding the materials in a mole ratio of (1:1)-(1:1.2), using N,N-dimethylacetamide or dimethyl sulfoxide 2-4 times dicyandiamide by weight as a solvent, and reacting for 4-8 hours at 140+ / -5 DEG C to prepare a crude metformin hydrochloride product; recrystallizing the crude product with ethanol, regulating the pH value to be 5-6, decoloring the crystal, cooling the crystal to minus 10-0 DEG C while stirring, precipitating the crystal, obtaining a refined metformin hydrochloride product through filtering and drying, and recovering the solvent from filtrate. The qualified product has yield of 80-85% and high purity. The preparation method has the advantages that as the selected reaction solvent has a relatively high boiling point, the recovery rate of the solvent is high; the phenomenon of material surging can be effectively avoided, so that the preparation method has the advantages of mild reaction conditions, simplicity in operation and high safety.
Owner:QINGDAO HUANGHAI PHARM CO LTD
Metformin salts of lipophilic acids
InactiveUS20050182029A1Promote absorptionSustained control of blood glucose levelBiocideOrganic active ingredientsAcute hyperglycaemiaPharmaceutical formulation
Owner:SONUS PHARM INC
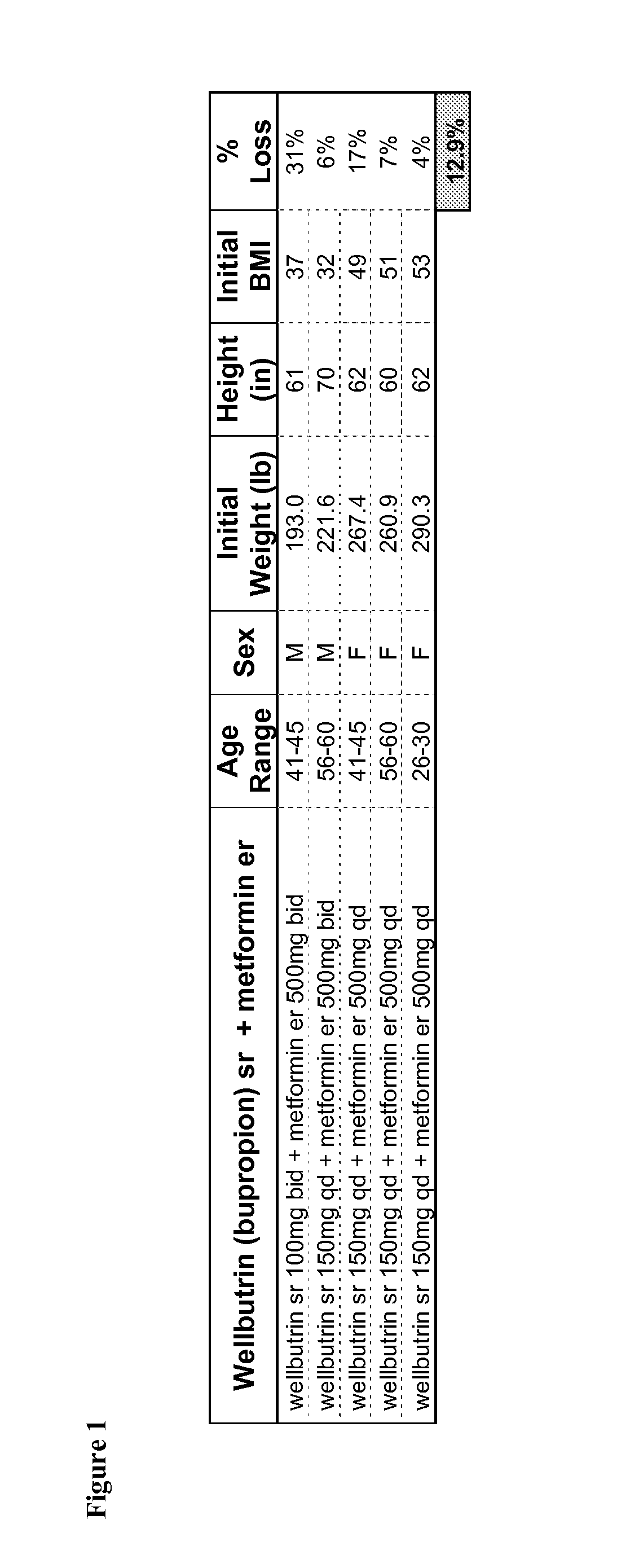
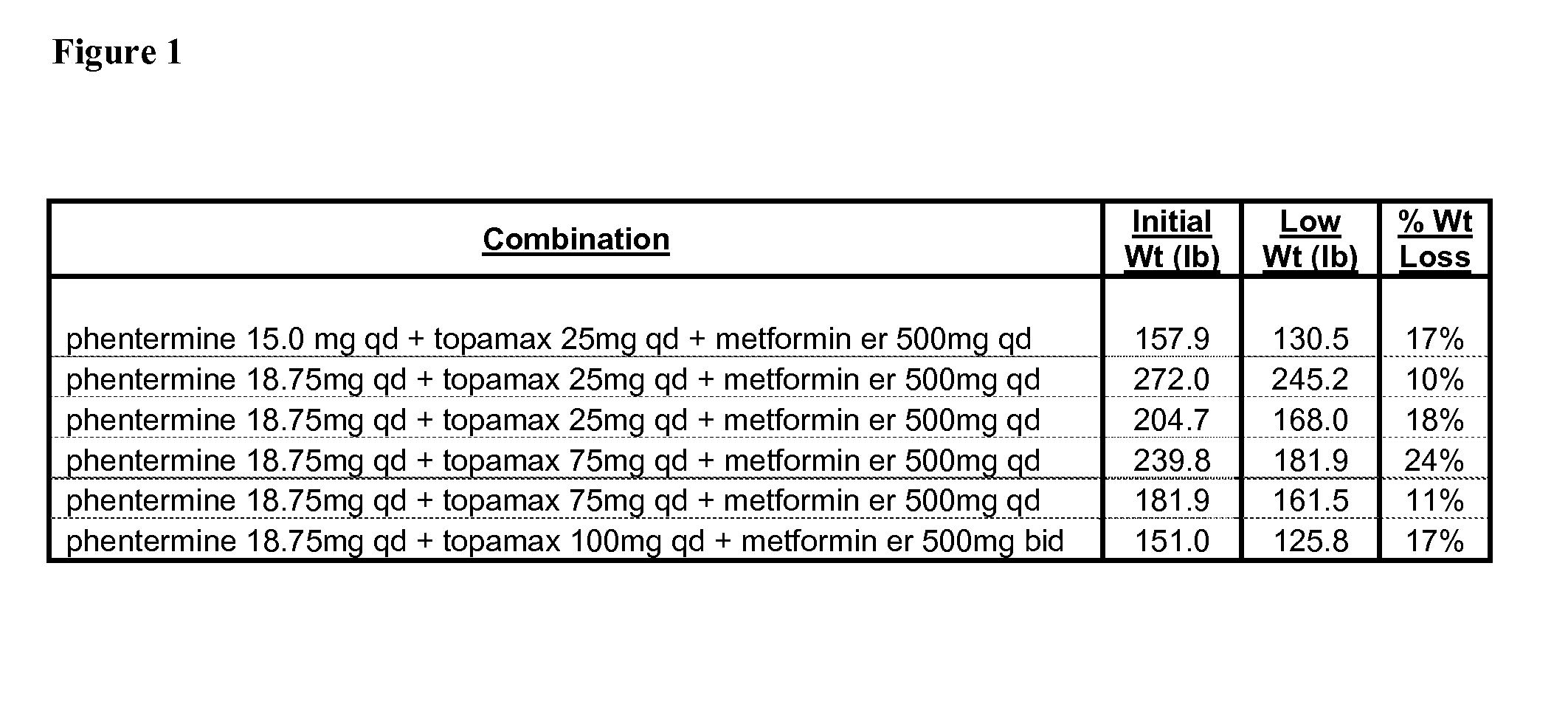
Combination Therapies for the Treatment of Obesity
InactiveUS20100331419A1Reduce weightOrganic active ingredientsBiocideCombined Modality TherapyObesity
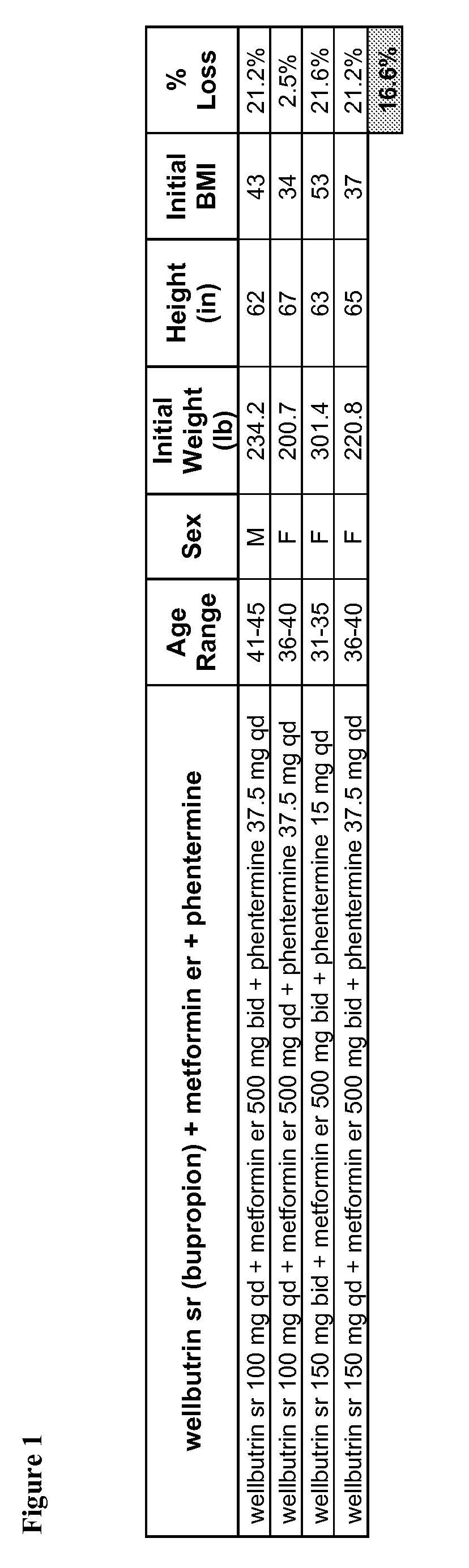
Described are pharmaceutical compositions comprising bupropion, metformin, and at least one pharmaceutically acceptable carrier or excipient. Another aspect of the present invention relates to a method of treating a patient suffering from obesity or needing to lose weight, comprising the step of co-administering to said patient a therapeutically effective amount of bupropion and metformin. In certain embodiments, an aforementioned method is practiced in conjunction or tandem with a medical procedure or the use of a medical device or both.
Owner:METABOLOUS PHARMA
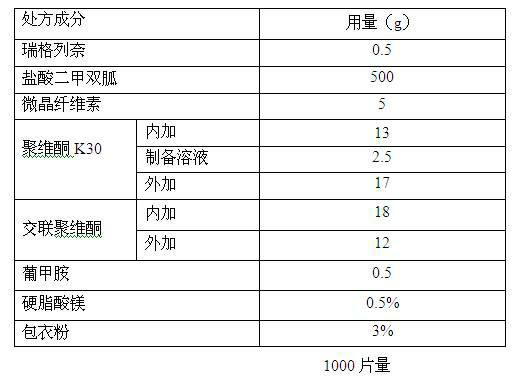
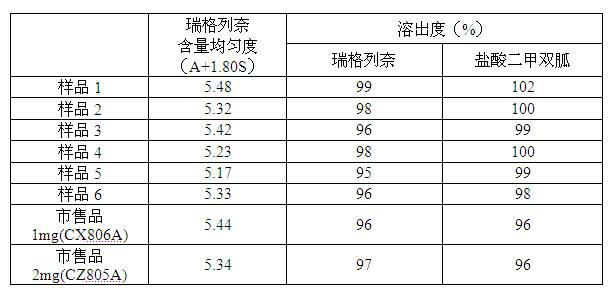
Composition containing repaglinide and metformin hydrochloride and preparation thereof
ActiveCN102319245AReduce dosageFacilitated releaseOrganic active ingredientsMetabolism disorderMetformin hclPharmaceutical Aids
The invention relates to a composition containing repaglinide and metformin hydrochloride and a preparation method thereof, belonging to the technical field of medicines. The composition containing repaglinide and metformin hydrochloride consists of repaglinide, metformin hydrochloride, a filling agent, a disintegrating agent, a bonding agent, a latent solvent and a lubricant, wherein the repaglinide is added into the metformin hydrochloride and auxiliary materials in the form of aqueous solution for wet granulation. The preparation method has the advantages of simple and efficient process, small using quantity of auxiliary materials, capability of solving the problem of poor content uniformity caused by small using amount of the repaglinide and increase in the dissolution rate; and moreover, a preparation has a smaller unit quantity and can be applied to industrial mass production.
Owner:HANGZHOU HUADONG MEDICINE GRP PHARMA RES INST
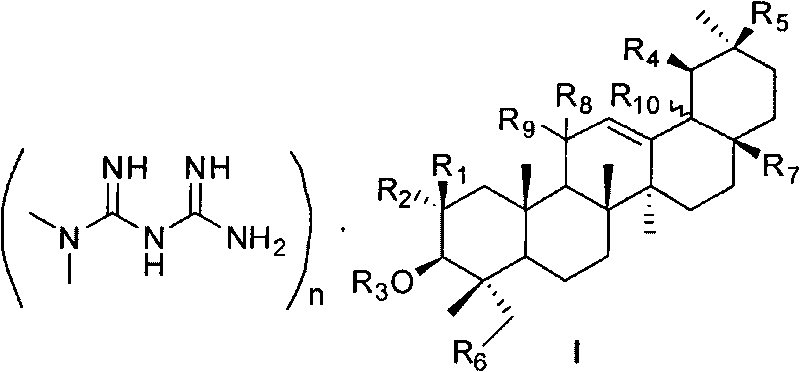
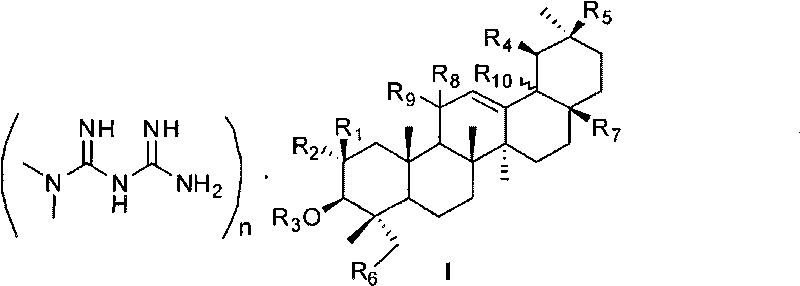
Pentacyclic triterpene and melbine salt of derivative thereof, preparation method and medical application of pentacyclic triterpene
The invention relates to field of natural medicine and medicinal chemistry, in particular to novel pentacyclic triterpene and melbine salt I of the derivative thereof. The salt compound can be used for preparing medicaments curing diabetes mellitus and complicating disease thereof, cerebral ischemia, angiocardiopathy, atherosclerosis, hepatitis, fatty liver and metabolic syndrome or tumour, descendens blood fat drugs and anti-obesity drugs. The invention also relates to the preparation method of the salt compound.
Owner:CHINA PHARM UNIV
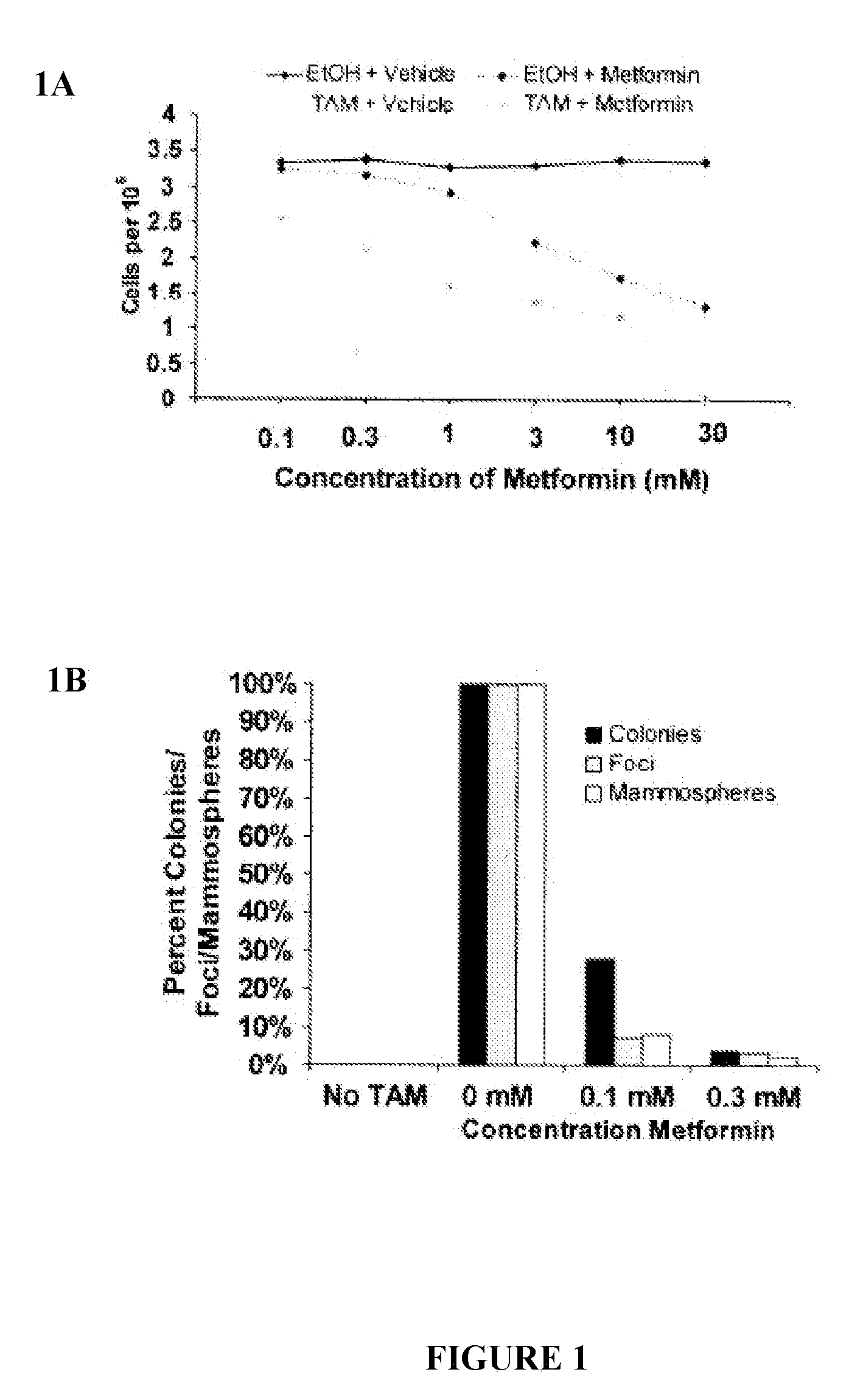
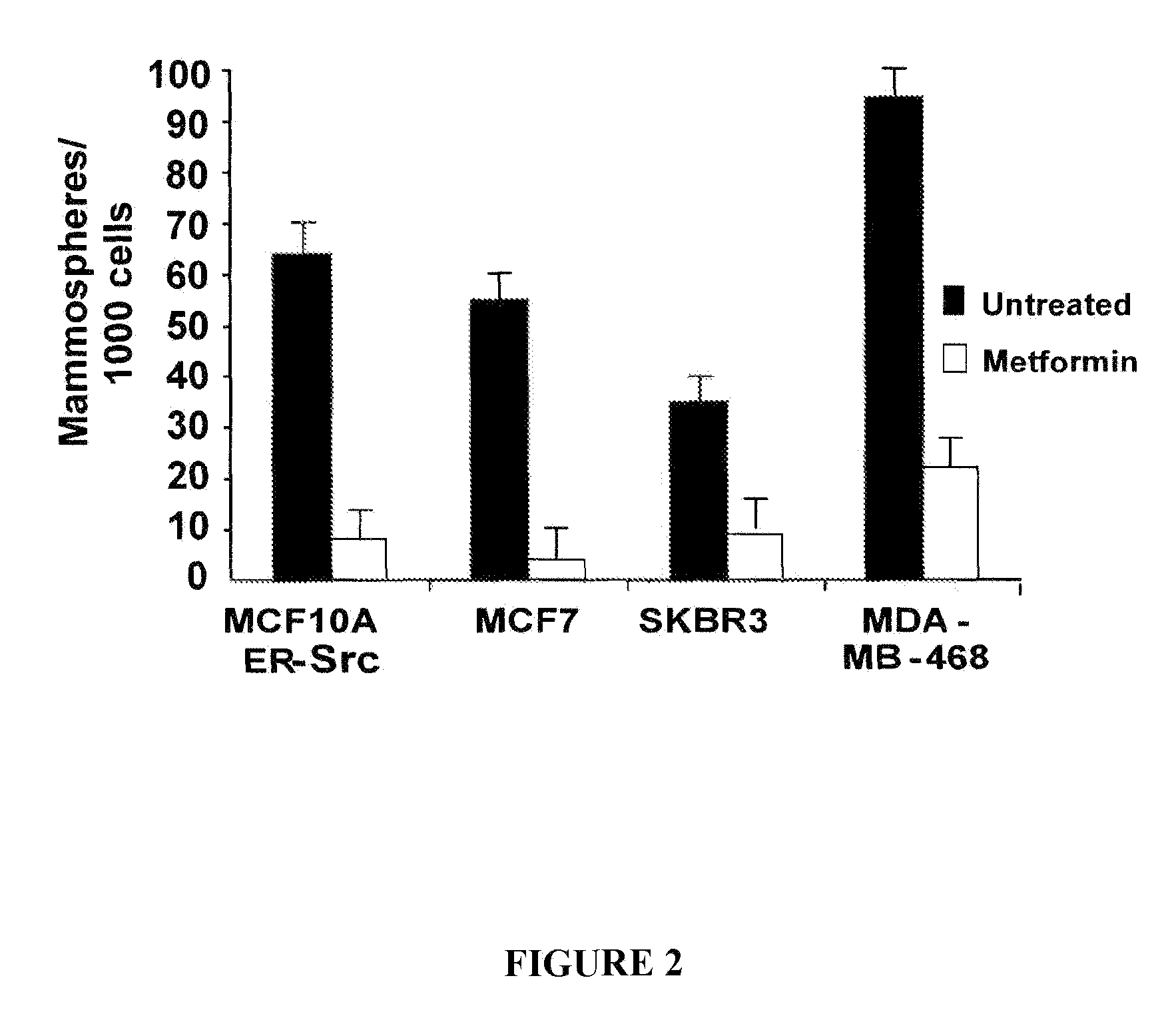
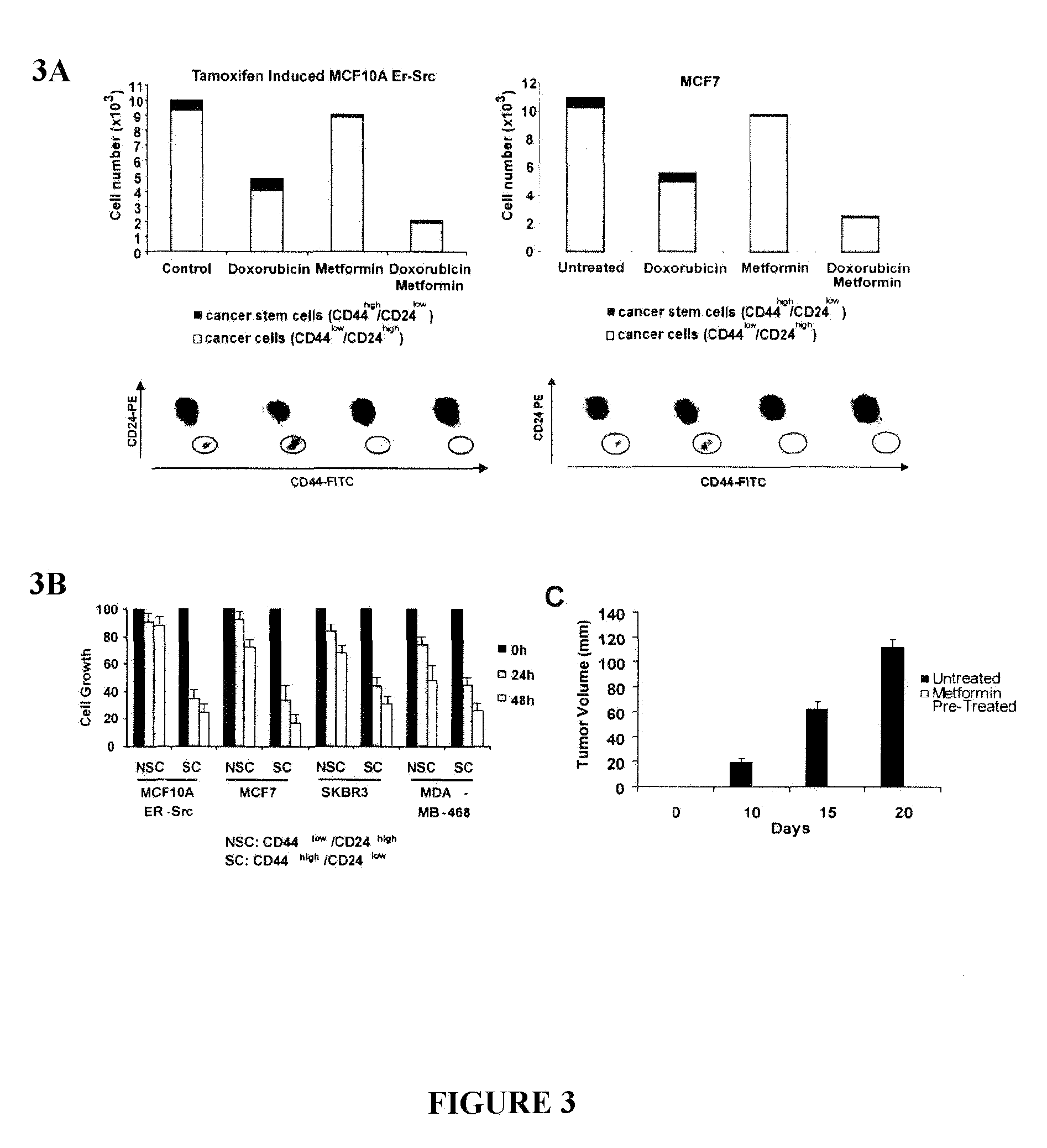
Use of metformin in cancer treatment and prevention
InactiveUS20120220664A1Increase volumeExcessive amountBiocideAmide active ingredientsCancer preventionCancer treatment
Disclosed herein is a method for treating a tumor in a subject in need thereof comprising administering an enhancing amount of metformin and a reduced amount of one or more chemotherapeutic agents. One example of an enhancing amount of metformin is about 250 mg / day. Also disclosed is a Untreated method for preventing cancer or delaying the recurrence of cancer in a subject comprising administering an effective amount of metformin to the subject. In one example of such a method, the amount of metformin is about 75 mg / day. Also disclosed is a composition comprising an enhancing amount of metformin, and a reduced amount of one or more chemotherapeutic agents and a pharmaceutically acceptable carrier. Kits comprising metformin and one or more chemotherapeutic agents are also disclosed.
Owner:PRESIDENT & FELLOWS OF HARVARD COLLEGE
Combination Therapies for the Treatment of Obesity
Described are pharmaceutical compositions comprising topiramate, phentermine, and metformin, and at least one pharmaceutically acceptable carrier or excipient. Another aspect of the present invention relates to a method of treating a patient suffering from obesity or needing to lose weight, comprising the step of co-administering to said patient a therapeutically effective amount of topiramate, phentermine, and metformin. In certain embodiments, an aforementioned method is practiced in conjunction or tandem with a medical procedure or the use of a medical device or both.
Owner:METABOLOUS PHARMA
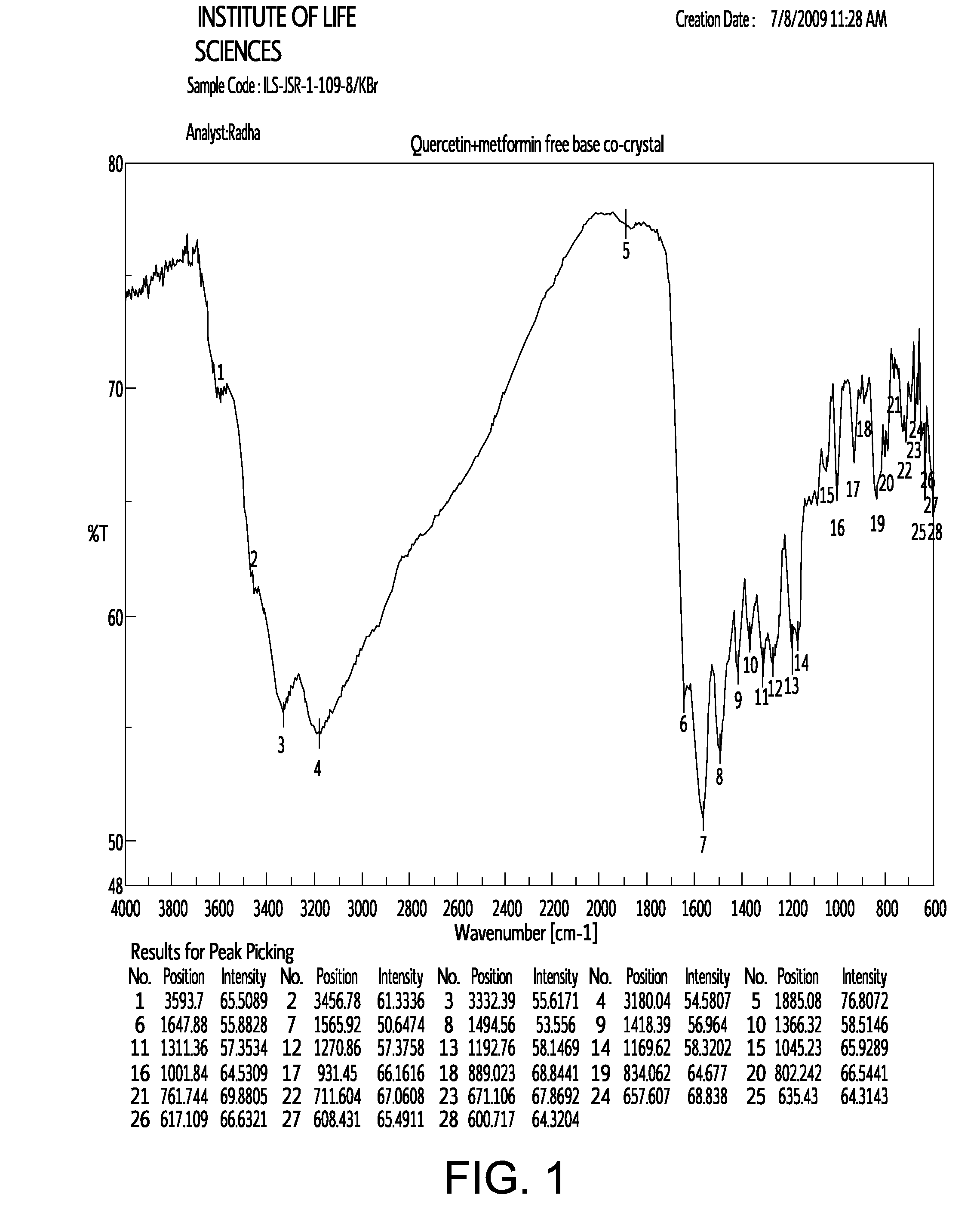
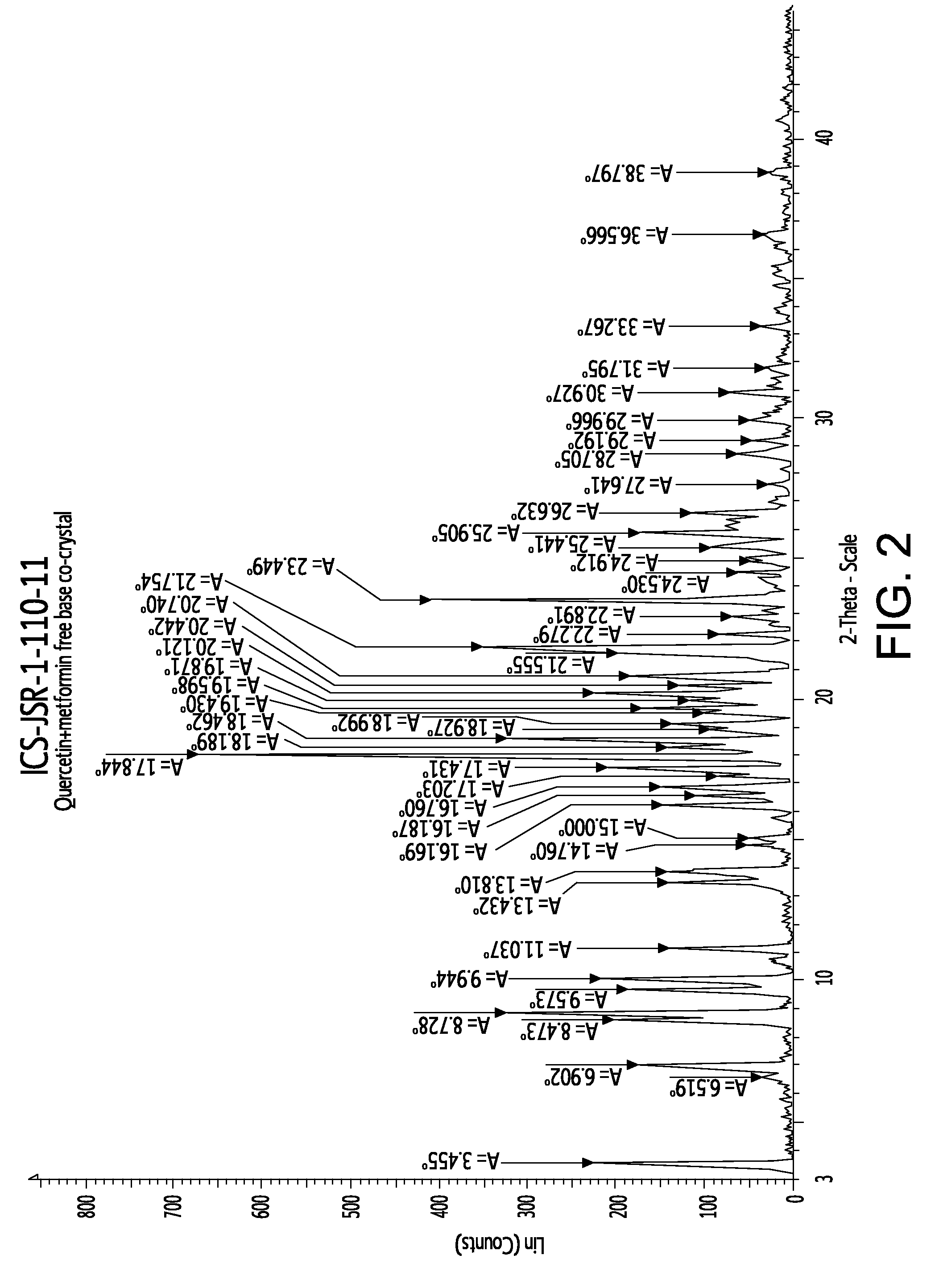
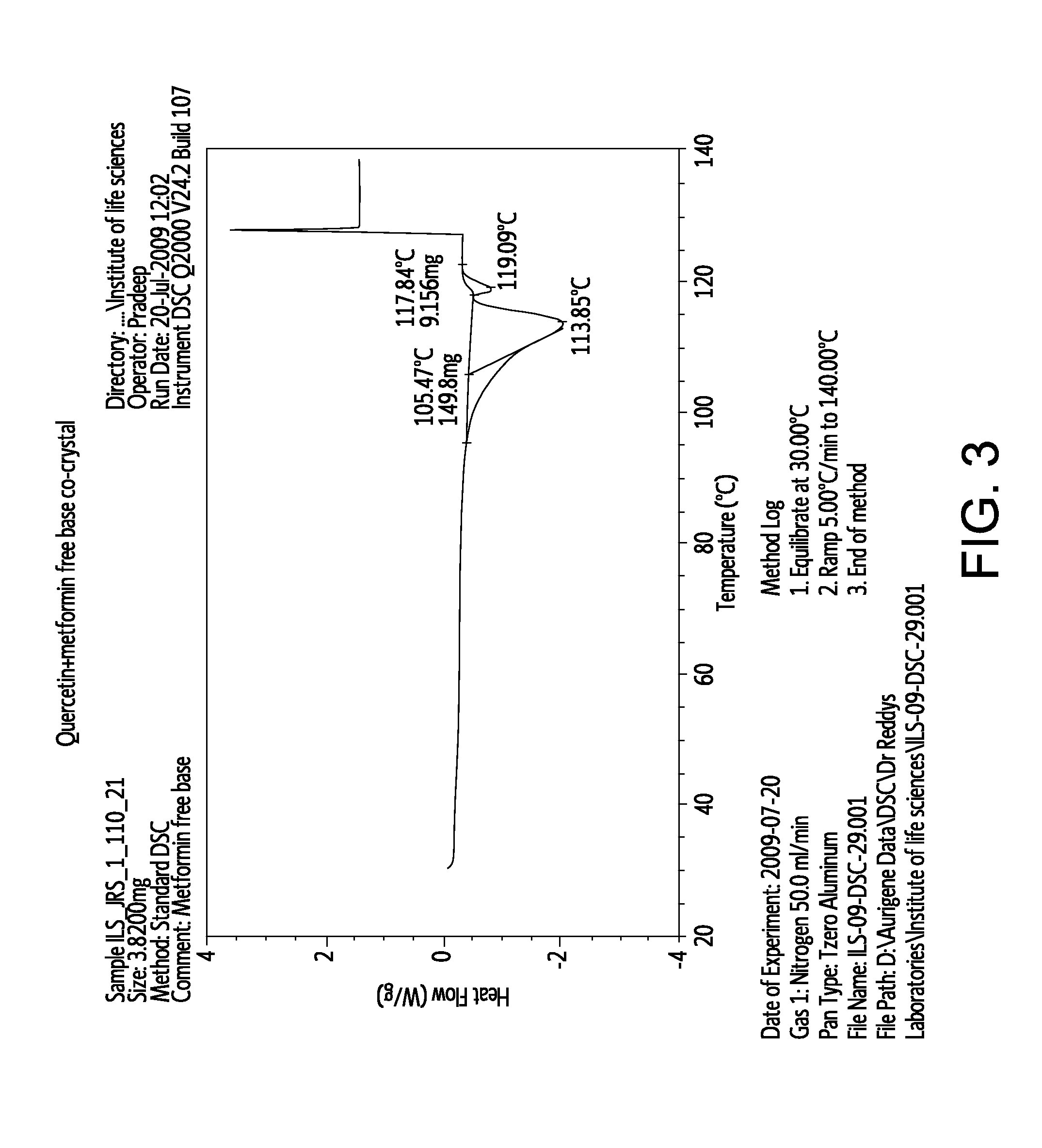
Pharmaceutical co-crystals of quercetin
InactiveUS20120258170A1Improve solubilitySuitable for preparationBiocidePowder deliveryDrug biological activityMetformin
A co-crystal composition comprised of Quercetin and at least one antidiabetic agent acts as a combination drug having unique physical properties and biological activity, which differ from both Quercetin in pure form and the at least one antidiabetic agent in pure form. The co-crystal composition may comprise quercetin and metformin. The co-crystals of quercetin and metformin may be prepared by grinding the compounds, and used in pharmaceutical compositions comprising these co-crystals. Co-crystal compositions of quercetin and Metformin may be used in combination with other anti-diabetic agents, including DPP-IV inhibitors.
Owner:NUTRACRYST THERAPEUTICS PRIVATE
Pharmaceutical Compositions of Metformin
InactiveUS20090124702A1Solve the lack of hardnessGood reproducibilityOrganic active ingredientsBiocideDiabrezideWater soluble
The present invention relates to an extended release dosage form of highly water-soluble antidiabetic drug metformin or its pharmaceutically acceptable salts. This invention also relates to methods for preparing the extended release dosage forms of metformin or its pharmaceutically acceptable salts.
Owner:SIVA SATYA KRISHNA BABU PECHETTI +3
Liquid formulation of metformin
The present invention is directed to a liquid formulation of metformin or its pharmaceutically acceptable salts thereof. The liquid pharmaceutical composition comprises a therapeutically effective amount of metformin or its pharmaceutically acceptable salt, in a liquid carrier, which may also include a sweetener that does not increase the blood glucose level of a subject after ingestion thereof. In one embodiment, it may also include alkyl hydroxyethylcellulose, and / or a polyhydroxy alcohol. In another embodiment, the carrier may contain a sweetener, mineral acid, and bicarbonate salt maintained at a pH of 4.0 to 9.0. It is useful for treating hyperglycemia and diabetes.
Owner:RANBAXY SIGNATURE
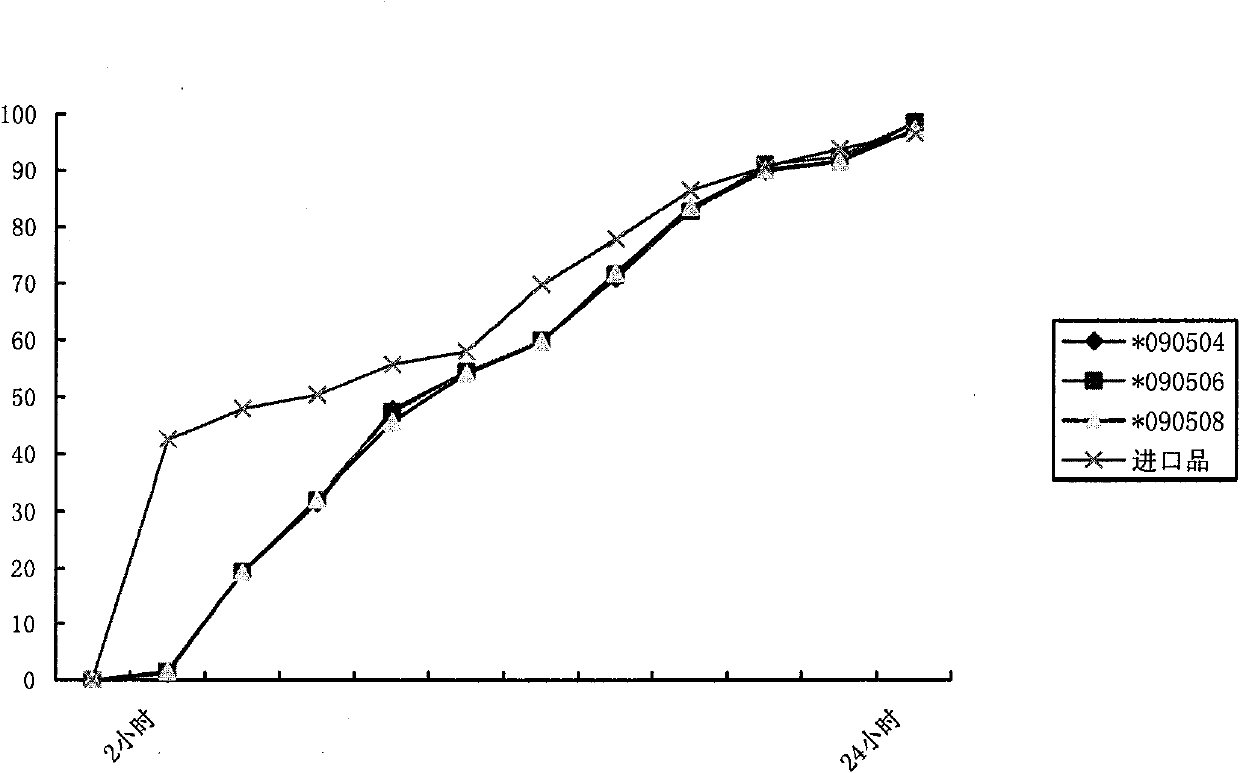
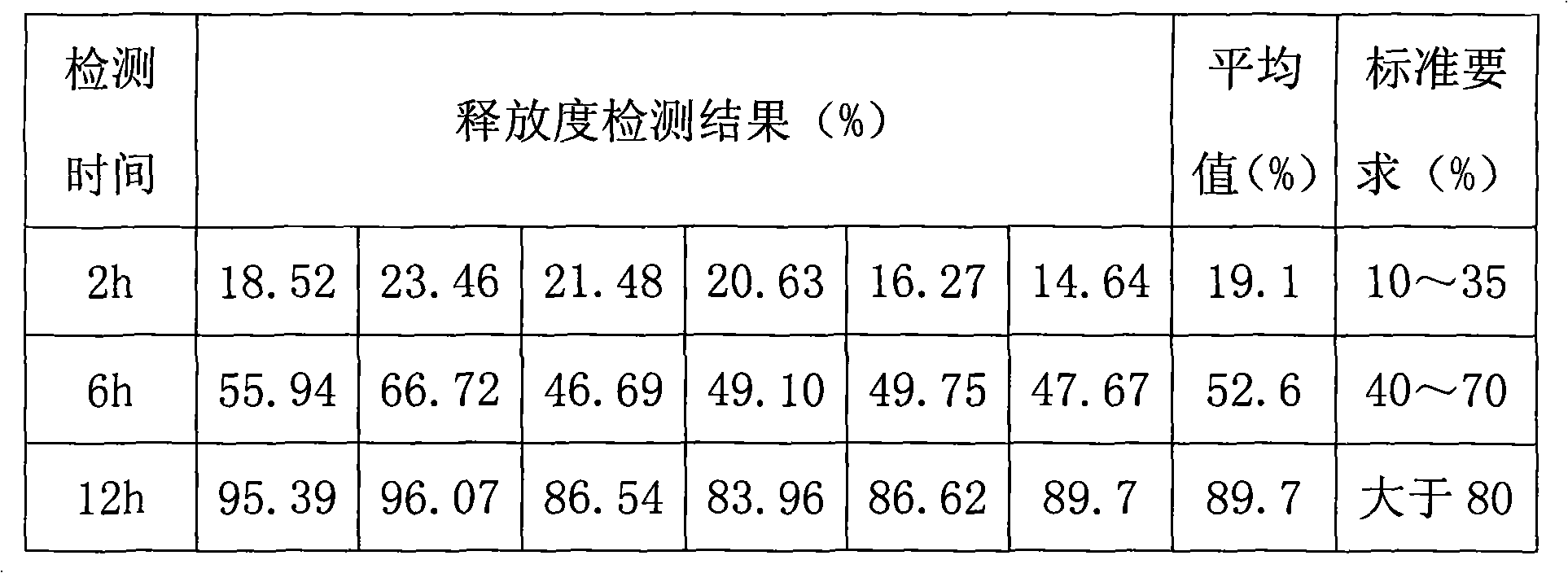
Metformin hydrochloride controlled-release tablet and preparation method thereof
ActiveCN101579325ALess weight gainIncrease production capacityOrganic active ingredientsMetabolism disorderPharmaceutical industryMetformin Hydrochloride
The invention provides a metformin hydrochloride controlled-release tablet, which comprises a metformin hydrochloride controlled-release tablet with effective dose and pharmaceutical accessories and is characterized in that the metformin hydrochloride controlled-release tablet uses the common tabletting, and the controlled-release effect is controlled by totally depending on the film coating technique. The coating adopted by the invention is a releasing system comprising the prescription which can cause the medicine to reach release degree standard in vitro. The preparation method in the invention is simple and convenient; the process conditions are easy to control and suitable for batch production, can use conventional production equipment in pharmaceutical industries for economically and conveniently producing the metformin hydrochloride controlled-release tablet on a large scale, can effectively and stably cause the release degree of the metformin hydrochloride controlled-release tablet in the second hour to be 10% to 35%, the release degree to be 40% to 70% in the sixth hour and the release degree to be more than 80% in the twelfth hour.
Owner:CHONGQING CONQUER PHARML
Combination therapies for the treatment of obesity
Described are pharmaceutical compositions comprising bupropion, metformin, phentermine, and at least one pharmaceutically acceptable carrier or excipient. Another aspect of the present invention relates to a method of treating a patient suffering from obesity or needing to lose weight, comprising the step of co-administering to said patient a therapeutically effective amount of bupropion, metformin, and phentermine. In certain embodiments, an aforementioned method is practiced in conjunction or tandem with a medical procedure or the use of a medical device or both.
Owner:METABOLOUS PHARMA
Antidiabetic formulation and method
InactiveUS20070141154A1Addressing Insufficient ControlBiocideMetabolism disorderPharmaceutical formulationMetformin
An antidiabetic pharmaceutical formulation is provided, especially adapted for treating Type II diabetes, which includes a combination of metformin and glipizide in a manner to control moisture in the formulation so that the glipizide does not hydrolyze, yet the metformin is compressible, if necessary. A method for treating diabetes is also provided employing the above formulation.
Owner:BRISTOL MYERS SQUIBB CO
Pharmaceutical Compositions of Combinations of Dipeptidyl Peptidase-4 Inhibitors With Metformin
Disclosed are pharmaceutical compositions comprising fixed-dose combinations of a dipeptidyl peptidase-4 inhibitor and metformin, methods of preparing such pharmaceutical compositions, and methods of treating Type 2 diabetes with such pharmaceutical compositions.
Owner:MERCK SHARP & DOHME LLC
Slow release capsule of compound metformin pyrrolidone and preparation method
A slow-releasing capsule of compound fluamine pyrrolinone for treating diabetes is composed of the central slow-releasing fluamine microball or small tablet and the coated fast-releasing pyrrolinone layer. Its advantages are high curative effect and no toxic by-effect.
Owner:TIANJIN INSTITUTE OF PHARMA RESEARCH
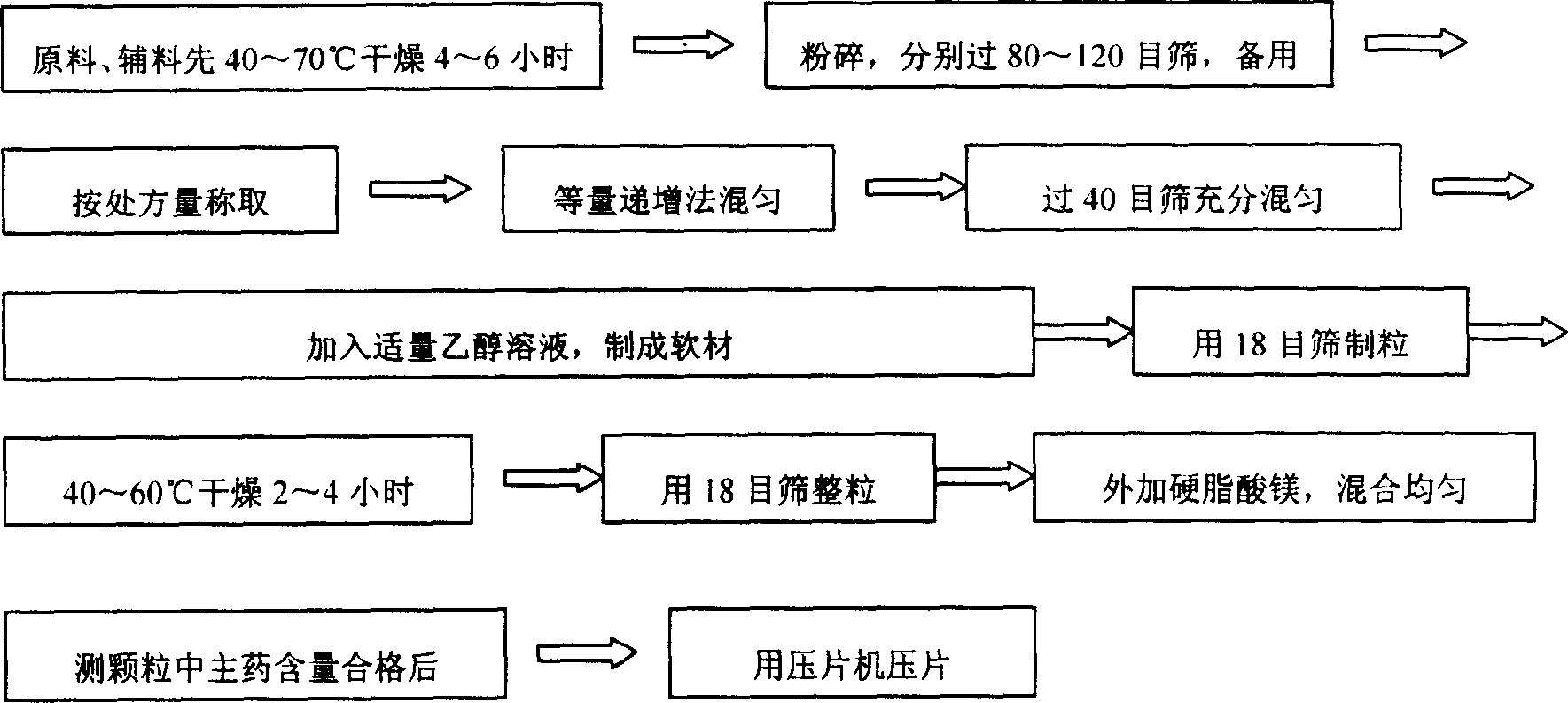
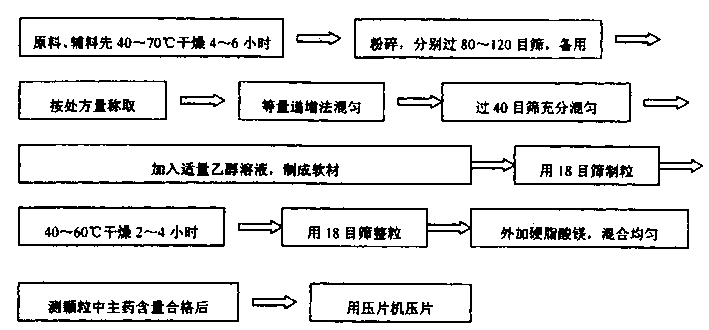
Metformin hydrochloride slowly released tablet and its preparation method
InactiveCN1415288APromote aerobic metabolismIncrease intakeOrganic active ingredientsMetabolism disorderExtended release tabletsAlcohol
A slowly-releasing mellitin tablet is prepared from fluamine, excipient, adhesive and lubricant through drying at 40-70 deg.C for 4-6 hrs, pulverizing, sieving by 80-120 meshes, proportionally mixing, mixing with alcohol solution, granularating by 18-mesh sieving, drying at 40-60 deg.C for 2-4 hrs, mixing with lubricant, and tabletting. Its advantages are long half-life (0.9-2.6 hr), low dosage and low by-effect.
Owner:NANJING CHANGAO PHARMA SCI & TECH CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com