Lipid-lowering antidiabetic agent
a lipid-lowering and anti-diabetic agent technology, applied in the field of poly unsaturated fatty acids, can solve the problems of limiting the use of hypoglycemic agents, affecting and abnormally elevated glucose levels, etc., to achieve the effect of improving the bioavailability of their component moities and high water solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 2
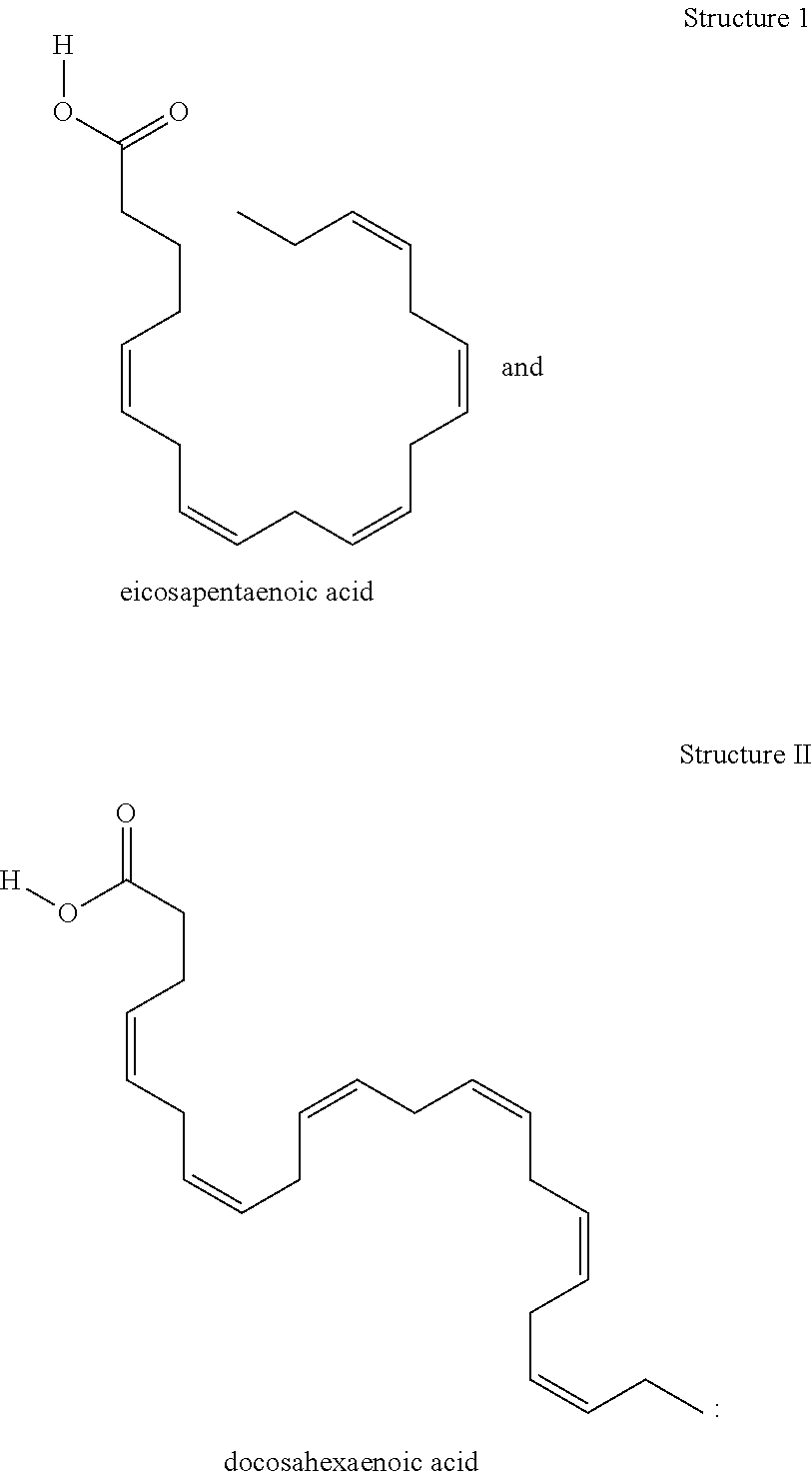
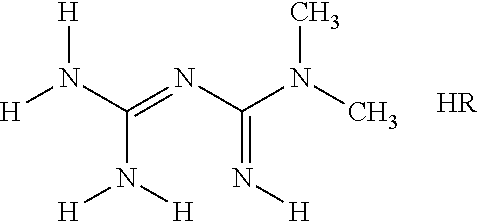
Preparation of {[amino(imino)methyl]amino } (dimethylamino)methaniminium (4Z,7Z,10Z,13Z,16Z,19Z)-docosa-4,7,10,13,16,19-hexaenoate
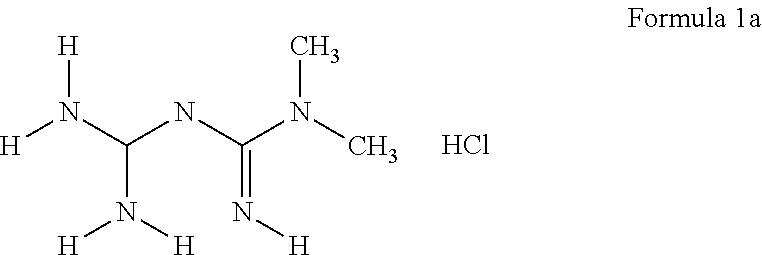
[0044]N,N-dimethylimidodicarbonimidic diamide. N,N-dimethylimidodicarbonimidic diamide hydrochloride (4.06 g, 24.5 mmol) was dissolved in IN sodium hydroxide (24.5 mL, 24.5 mmol) and stirred at room temperature for 30 minutes. The solution was concentrated in vacuum and to the residue was added ethanol (80 mL). The mixture was carefully concentrated to azeotropically remove water. To the resulting solid was added (60 mL) and the suspension was filtered to remove precipitated sodium chloride. The filtrate was concentrated and the resulting solid was placed on high vaccum overnight to yield 3.22 g (102%) of N,N-dimethylimidodicarbonimidic diamide as a white solid.
{[Amino(imino)methyl] amino}(dimethylamino)methaniminium (4Z,7Z,10Z,13Z,16Z,19Z)-docosa-4,7,10,13,16,19-hexaenoate
[0045]N,N-Dimethylimidodicarbonimidic diamide (968 mg, 7.61 mmol) was dissolved in ...
example 3
Preparation of {[amino(imino)methyl]amino}(dimethylamino)methaniminium (5Z,8Z,11Z,14Z,17Z)-eicosa-5,8,11,14,17-pentaenoate
[0046]N,N-dimethylimidodicarbonimidic diamide.
[0047]Metformin hydrochloride (331.25 g, 2 moles) was weighed into a 4000 mL beaker containing a stir bar. 1N KOH (1980 mL, 1.998 moles) was added, the beaker was covered, and the mixture was stirred for 2 h. The solids were collected by vacuum filtration, and the filtrate was concentrated to a damp solid. Isopropanol (500 mL) was added and after brief swirling, the mixture was concentrated. The residual white solid was dried for 16 h in a vacuum oven (yield: 269.08 g).
{[Amino(imino)methyl]amino}(dimethylamino)methaniminium (5Z,8Z,11Z,14Z,17Z)-eicosa-5,8,11,14,17-pentaenoate
[0048]Metformin free base (50.10 g, 0.366 mole) was weighed into a 4000 mL beaker containing a stir bar. CH3CN (2000 mL) was added and the mixture was rapidly stirred until all metformin had dissolved. A fine white solid was removed by vacuum filtr...
example 4
[0049]The solubility of the compound of Example 1 in water was compared with that of eicosapentaenoic acid (EPA).
[0050]Measurement of the water solubility of the test compounds is accomplished by using methods well known to those skilled in the art. Specifically, to a weighed amount of the test compound of Example 1 distilled water is added in small portions until a clear solution is obtained. The total-volume of the solution is measured. The water solubility is calculated by dividing the weight of the salt, in mg, by the volume of the solution, in mL. The water solubility of the compound of Example 1 when measured using the above technique, was determined to be 50 mg / ml. Likewise, the water solubility of EPA was found to be <0.2 mg / mL. The compound of Example 1 is therefore, at least, 250 times more soluble in water than EPA itself. This is a clear indication of an unexpectedly high degree of bioavailability of the compositions of the invention. Highly water soluble medicinal prepa...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| flow rate | aaaaa | aaaaa |
| flow rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com