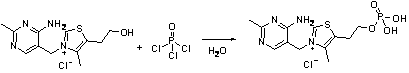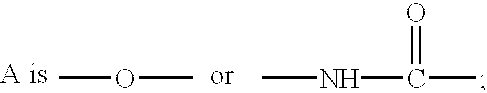Patents
Literature
180 results about "Antidiabetic agents" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Medical Definition of Antidiabetic agent. Antidiabetic agent: A substance that helps a person with diabetes control their level of glucose (sugar) in the blood. Antidiabetic agents include insulin and the oral hypoglycemic agents.
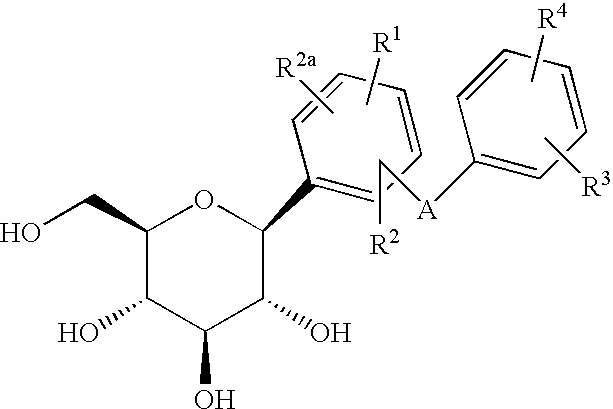
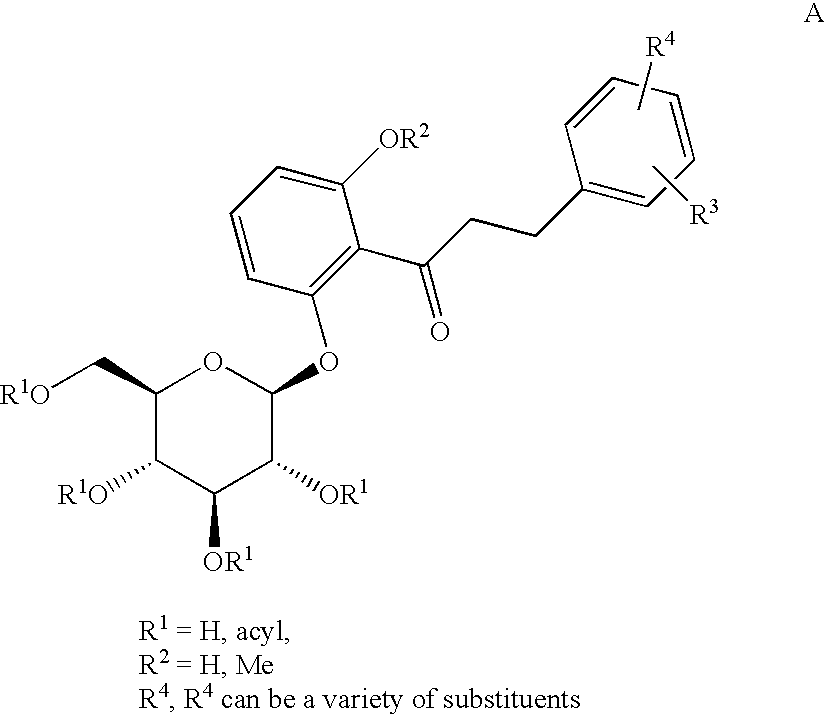
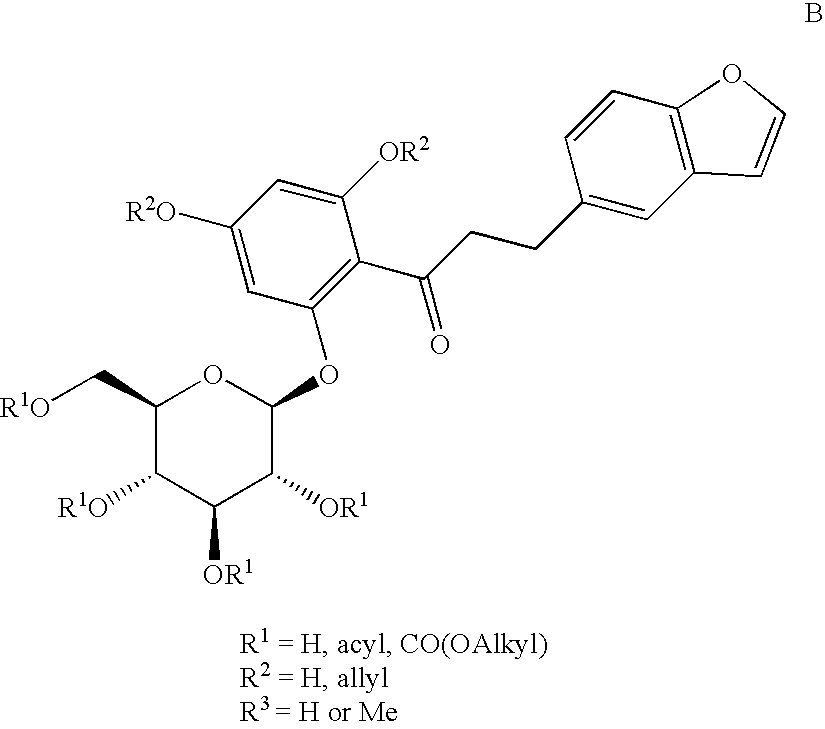
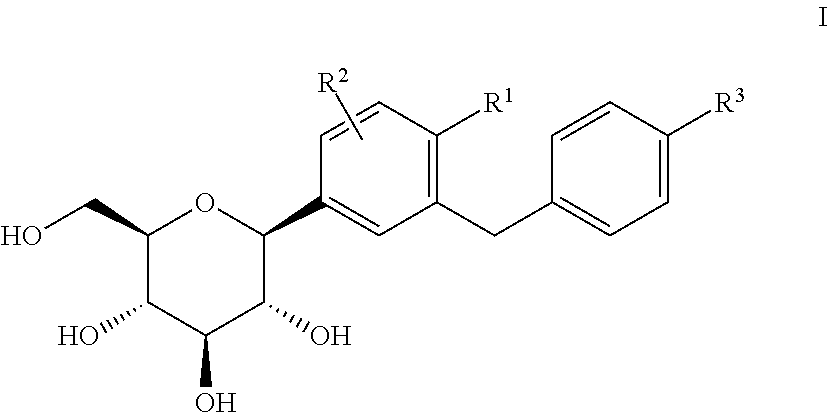
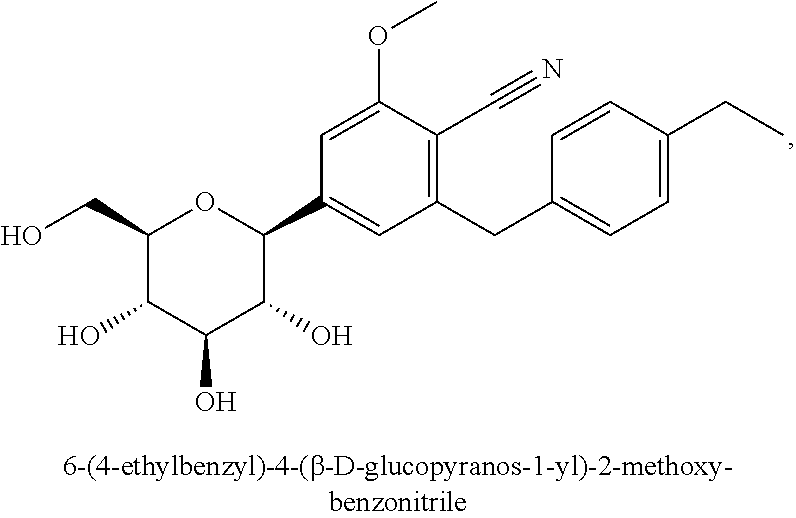
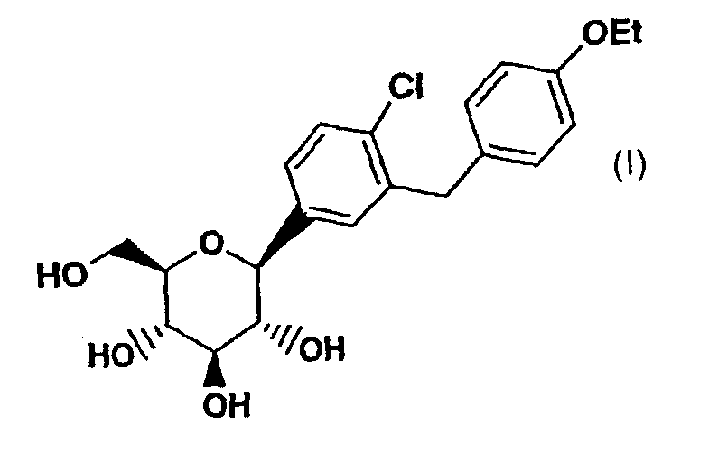
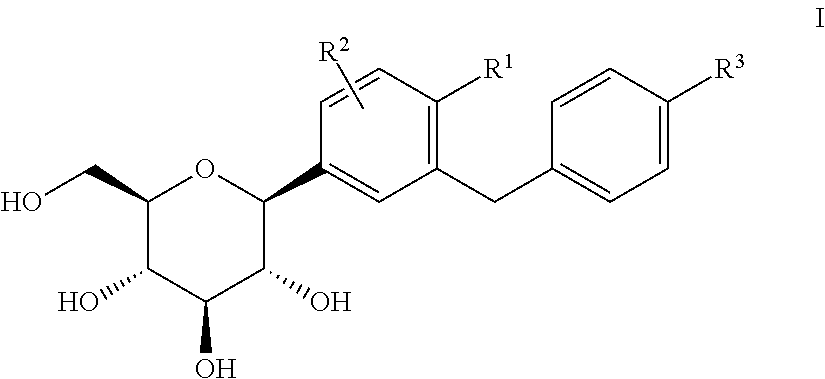
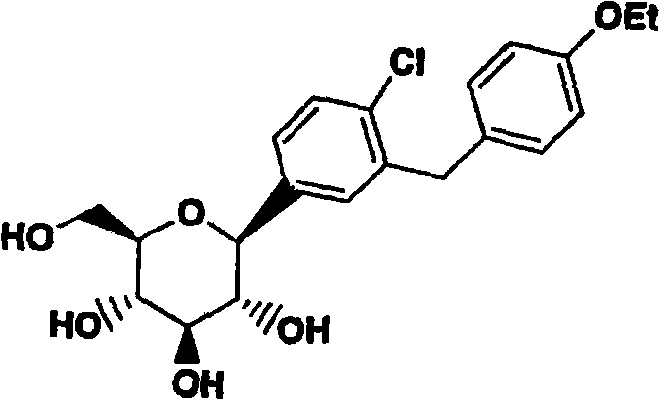
C-aryl glucoside SGLT2 inhibitors and method
Owner:ASTRAZENECA AB
Pharmaceutical composition, methods for treating and uses thereof
InactiveUS20110046076A1Improve blood sugar controlPrevent and slow progressionBiocideMetabolism disorderAcute hyperglycaemiaIGT - Impaired glucose tolerance
The invention relates to a pharmaceutical composition according to the claim 1 comprising an SGLT2 inhibitor, a DPPIV inhibitor and a third antidiabetic agent which is suitable in the treatment or prevention of one or more conditions selected from type 1 diabetes mellitus, type 2 diabetes mellitus, impaired glucose tolerance and hyperglycemia. In addition the present invention relates to methods for preventing or treating of metabolic disorders and related conditions.
Owner:BOEHRINGER INGELHEIM INT GMBH
Pyrazole compounds and their use as antidiabetes agents
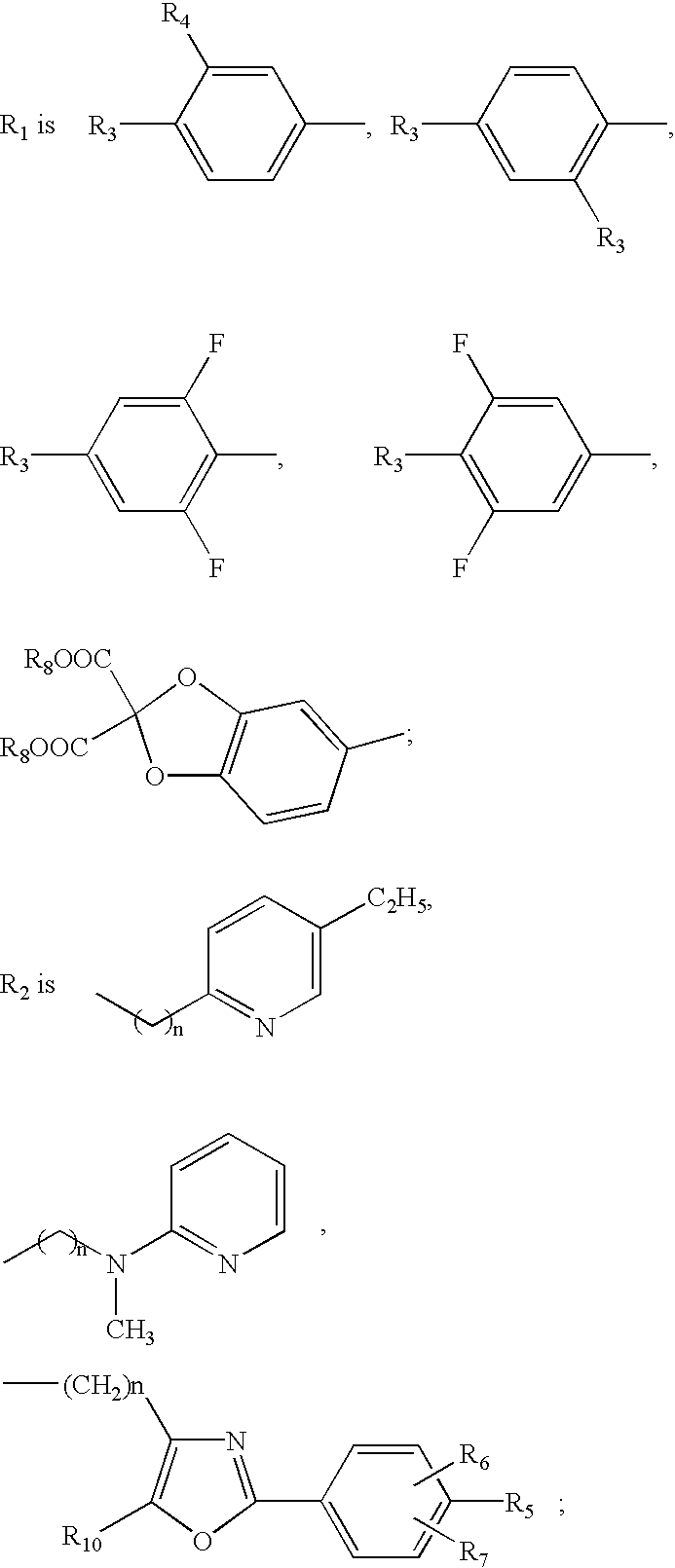
The present invention provides a pyrazole compound that has liver glycogen phosphorylase inhibitory activity and is useful as a therapeutic or prophylactic agent for diabetes, the pyrazole compound represented by the following general formula (I): wherein Ring Q represents an aryl or heteroaromatic group, R1 represents a hydrogen atom, a halogen atom, a C1-6 alkyl group or a C1-6 alkoxy group, R2 represents a halogen atom, a C1-6 alkyl group, a C1-6 alkoxy group or an azido group, R3 represents a halogen atom, a hydroxyl group, a C1-6 alkyl group, a halo C1-6 alkyl group, a C1-6 alkoxy group, an azido group, an amino group, an acylamino group or a C1-6 alkylsulfonylamino group, R4 and R5 are identical with or different from each other and represent a hydrogen atom, a substituted or unsubstituted C1-6 alkyl group, a C3-8 cycloalkyl group, a substituted or unsubstituted saturated heterocyclic group, a substituted or unsubstituted aryl group, a C7-14 aralkyl group, a heteroaromatic group, or the like, or a pharmacologically acceptable salt thereof.
Owner:JAPAN TOBACCO INC
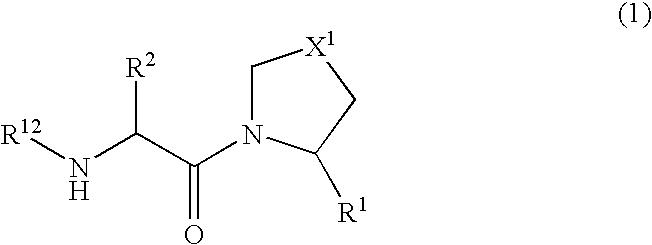
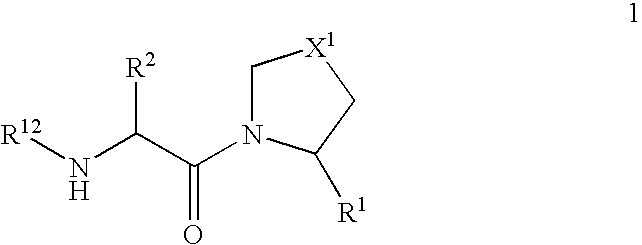
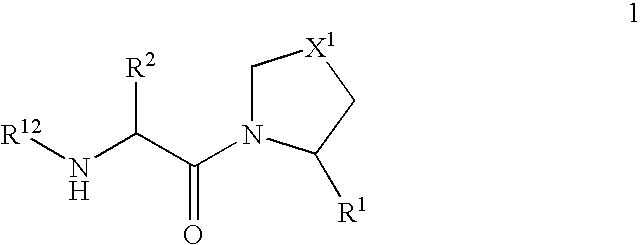
Novel dipeptidyl peptidase iv (dp-iv) inhibitors as anti-diabetic agents
The present invention relates to a series of prodrugs of inhibitors of DP-IV with improved properties. The compounds can be used for the treatment of a number of human diseases, including impaired glucose tolerance and type II diabetes. The compounds of the invention are described by general formula (1); wherein R1 is H or CN; R2 is selected from CH2R5, CH2CH2R5 and C(R3)(R4)—X2—(CH2)aR5; R3 and R4 are each independently selected from H and Me; R5 is selected from CON(R6)(R7), N(R8)C(=0)R9, N(R8)C(═S)R9, N(R8)SO2R10 and N(R8)R10; R6 and R7 are each independently R11(CH2)b or together they are —(CH2)2-Z-(CH2)2— or CH2-o-C6H4-Z-CH2—; R8 is H or Me; R9 is selected from R11(CH2)b, R11(CH2)bO and N(R6)(R7); R10 is R11(CH2)b; R11 is selected from H, alkyl, optionally substituted aryl, optionally substituted aroyl, optionally substituted arylsulphonyl and optionally substituted heteroaryl; R12 is selected from H2NCH(R13)CO, H2NCH(R14)CONHCH(R15)CO, C(R16)═C(R17)COR18 and R19OCO; R13, R14 and R15 are selected from the side chains of the proteinaceous amino acids; R16 is selected from H, lower alkyl (C1-C6) and phenyl; R17 is selected from H and lower alkyl (C1-C6); R18 is selected from H, lower alkyl (C1-C6), OH, O-(lower alkyl (C1-C6)) and phenyl; R19 is selected from lower alkyl (C1-C6), optionally substituted phenyl and R20C(=0)OC(R21)(R22); R20, R21 and R22 are each independently selected from H and lower alkyl (C1-C6); Z is selected from a covalent bond, —(CH2)c—, —O—, —SOd— and —N(R10)—; X1 is S or CH2; X2 is O, S or CH2; a is 1, 2 or 3; b is 0-3; c is 1 or 2; and d is 0, 1 or 2.
Owner:FERRING BV
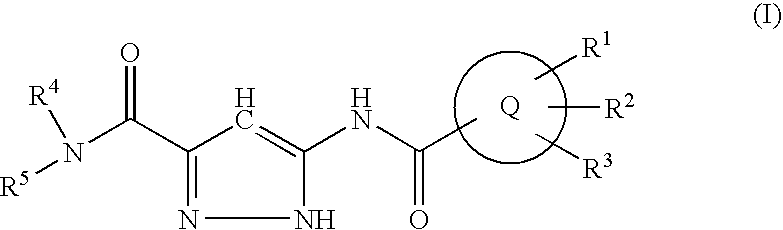
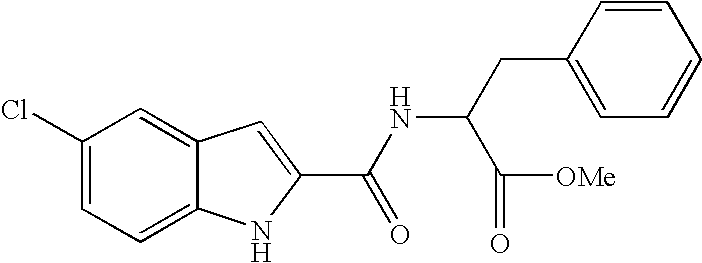
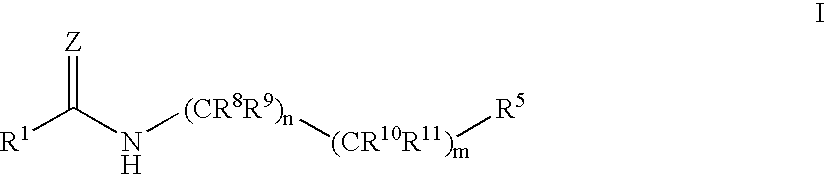
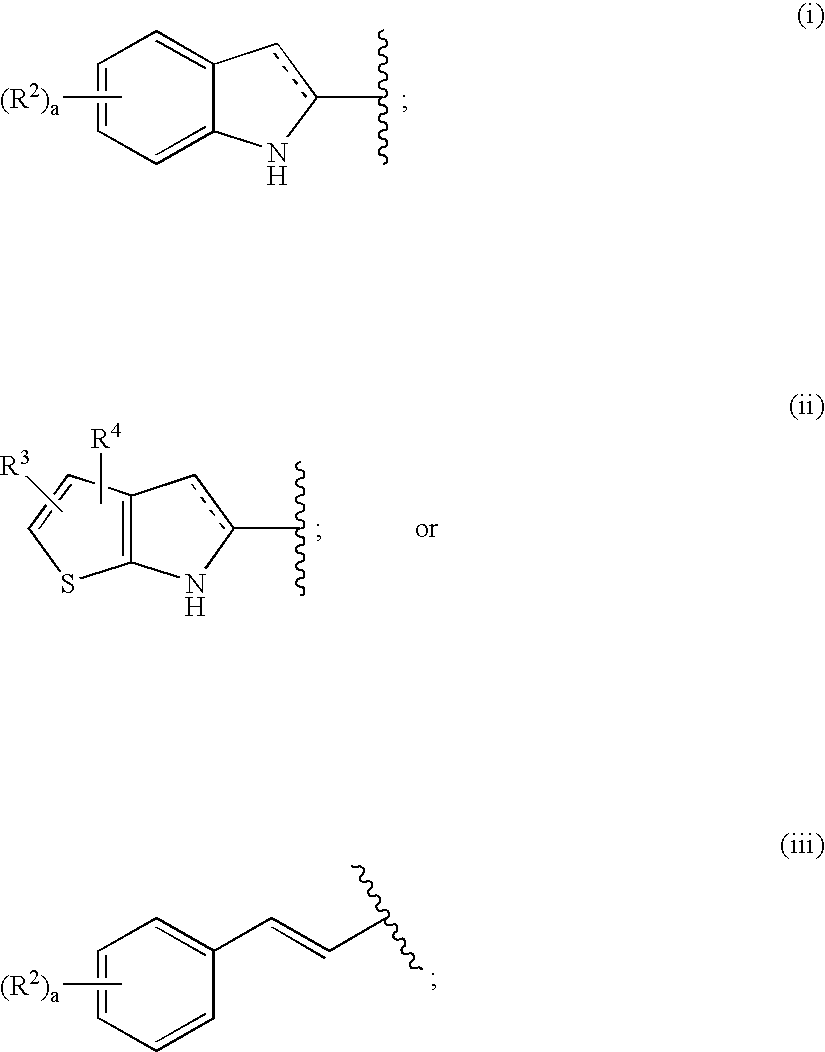
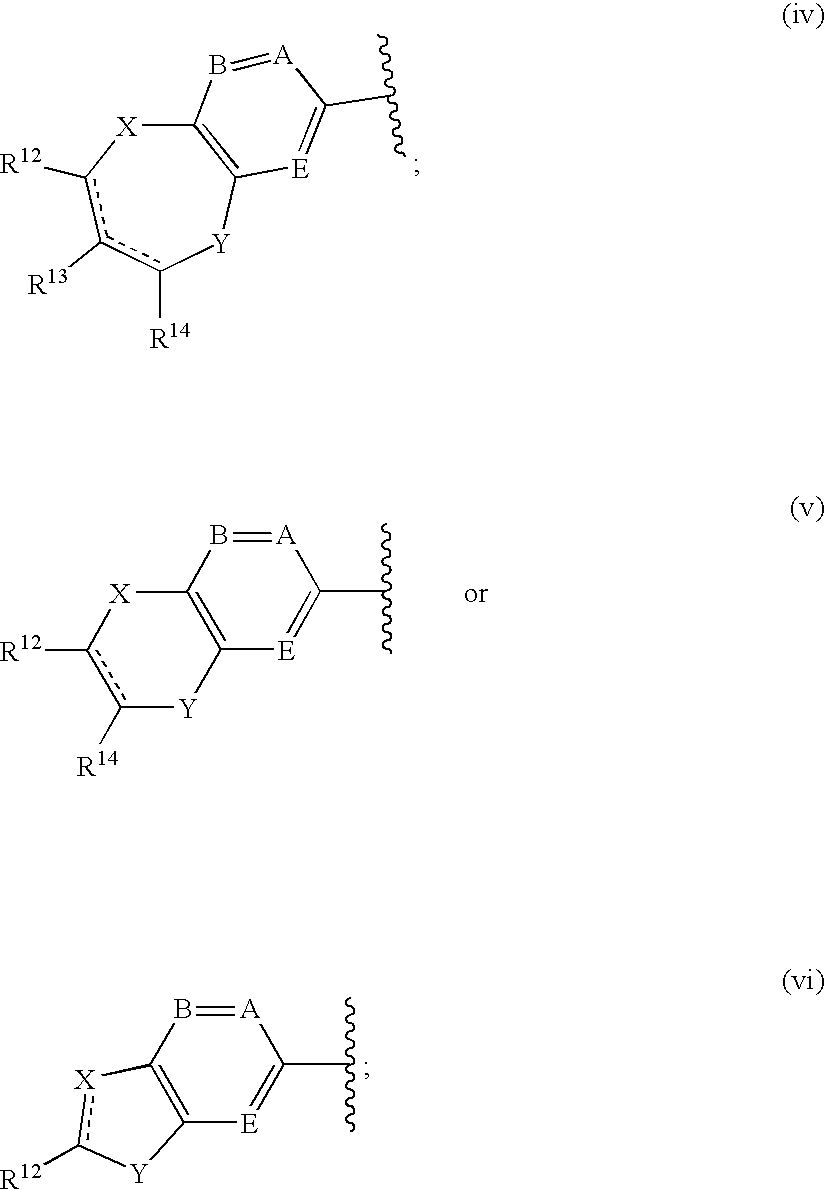
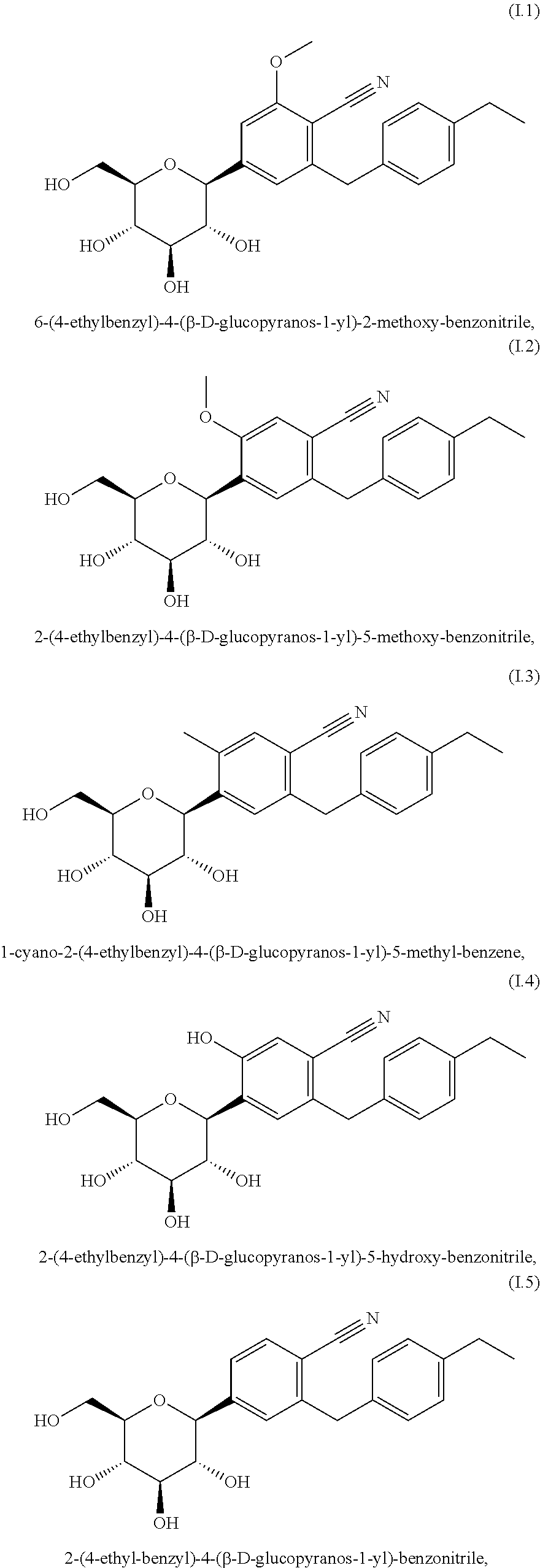
Antidiabetic agents
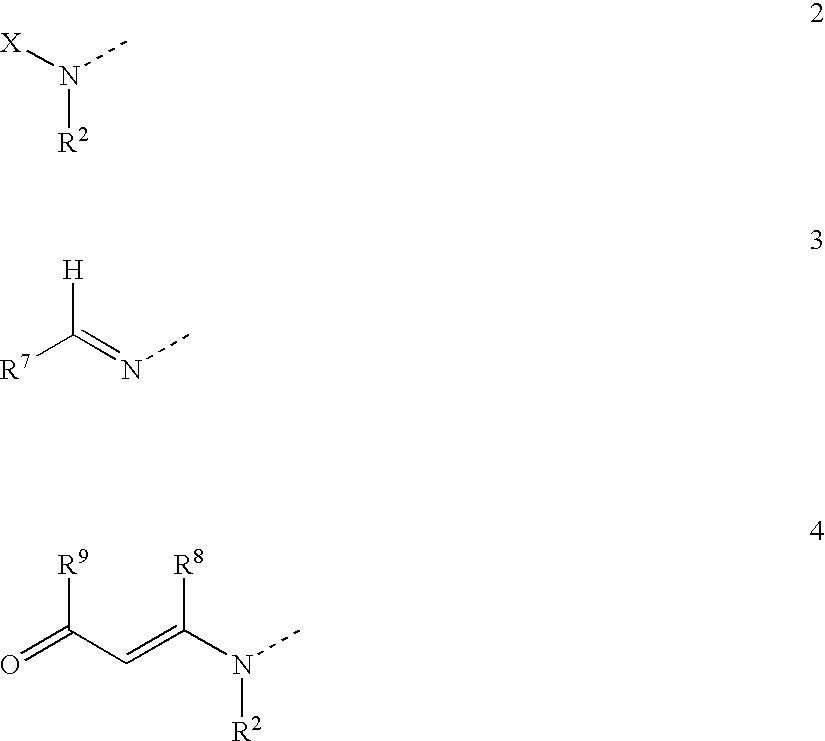
A compound of the formula wherein R1 is: R5 is: and n, m, Z, R8, R9, R10 and R11 are as defined herein, useful in the treatment of diabetes, insulin resistance, diabetic neuropathy, diabetic nephropathy, diabetic retinopathy, cataracts, hyperglycemia, hypercholesterolemia, hypertension, hyperinsulinemia, hyperlipidemia, atherosclerosis, and tissue ischemia, particularly, myocardial ischemia.
Owner:GAMMILL RONALD B
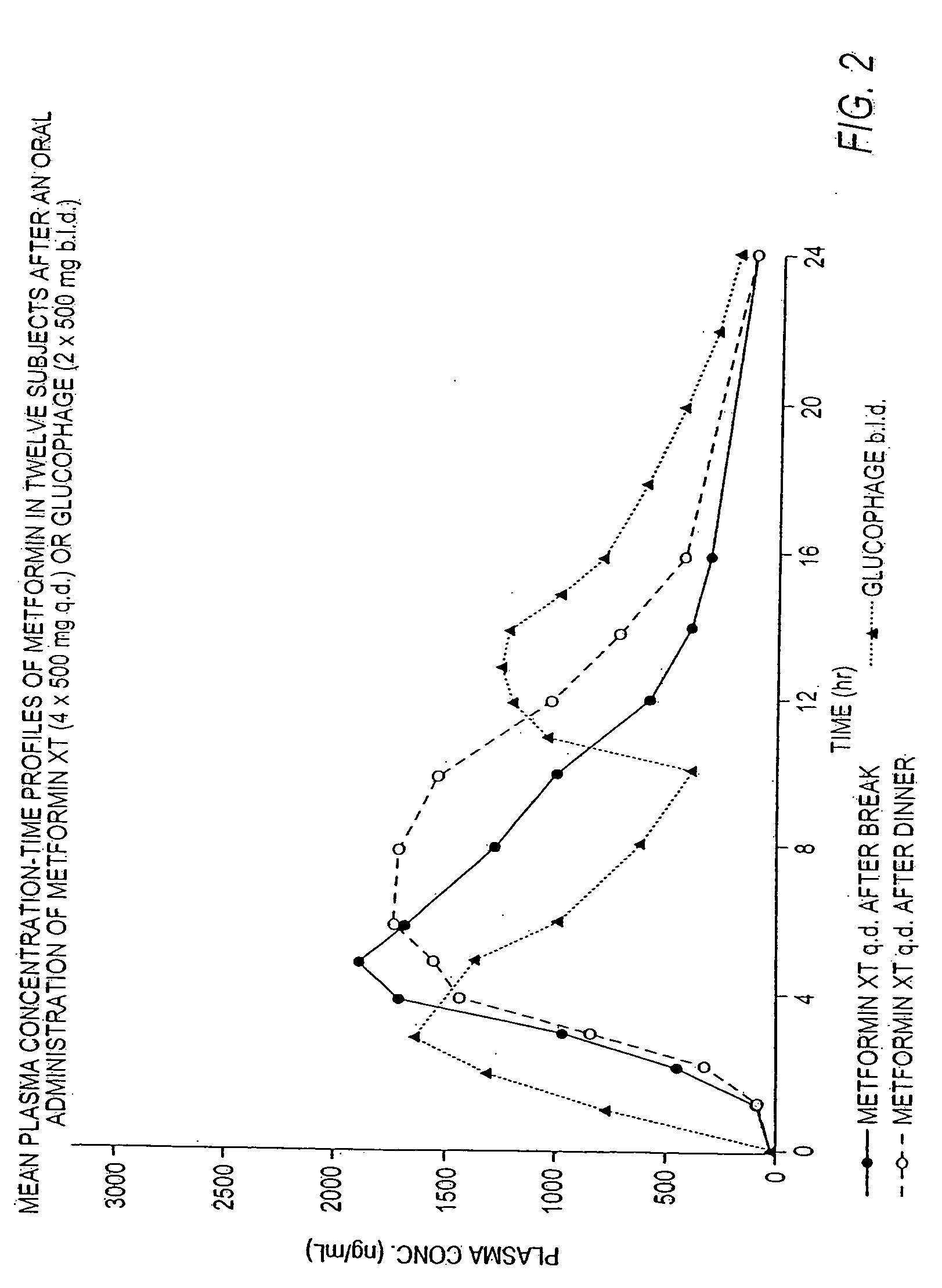
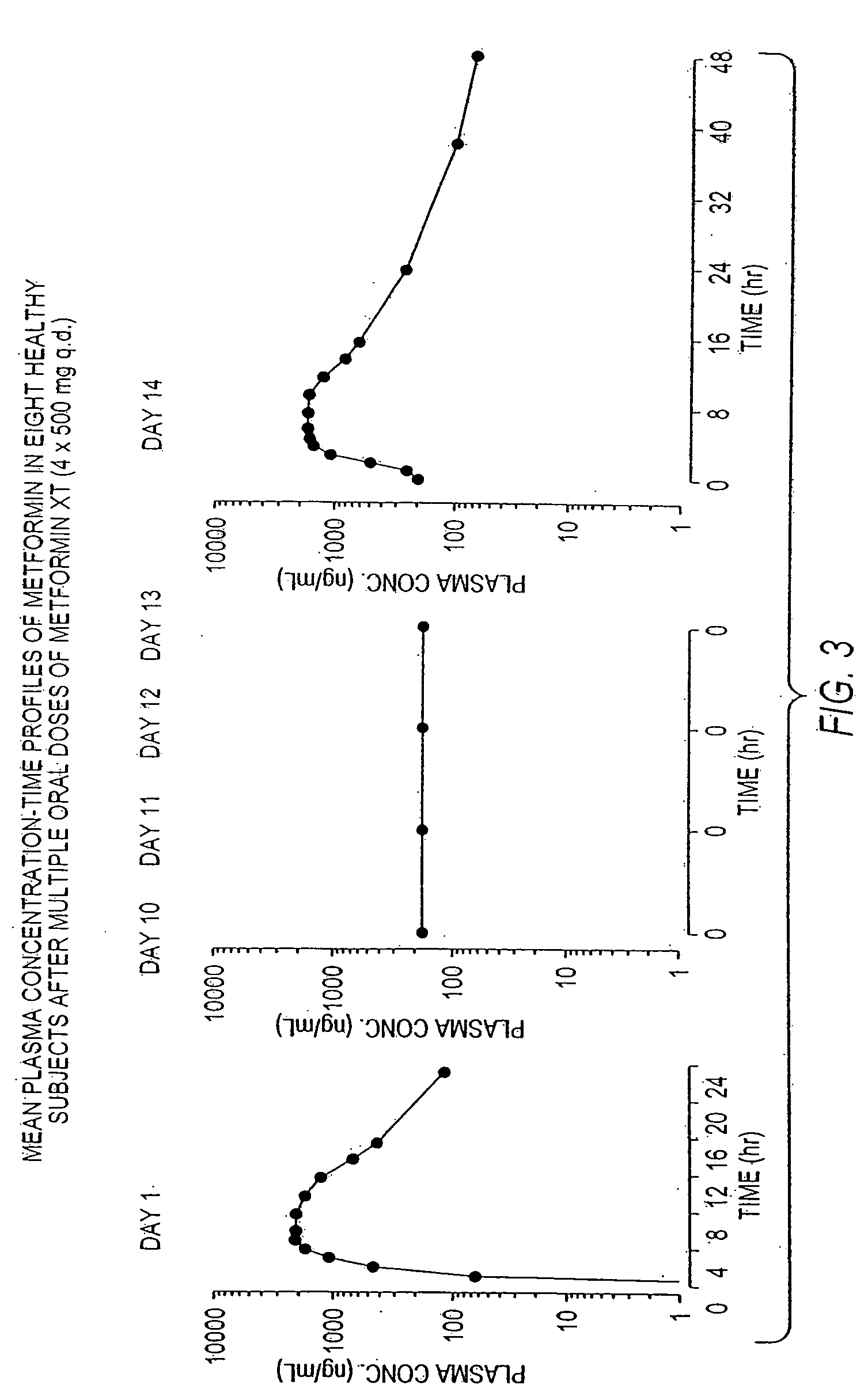
Controlled release metformin compositions
InactiveUS20060034922A1Effective controlImprove bioavailabilityCoatingsOsmotic deliveryControlled releaseHigh doses
Owner:ANDRX LABS
Combination of an aldosterone receptor antagonist and an anti-diabetic agent
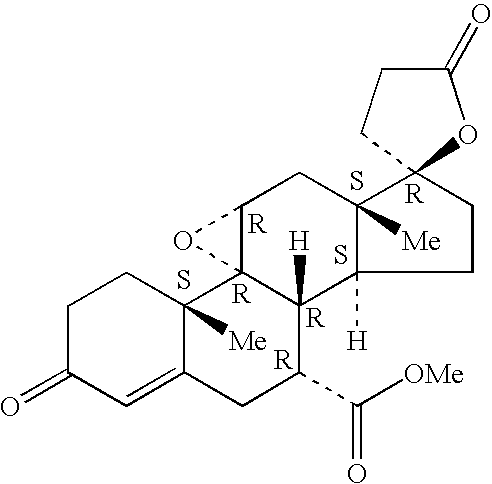
A combination therapy comprising a therapeutically-effective amount of an aldosterone receptor antagonist and a therapeutically-effective amount of an anti-diabetic agent is described for treatment of circulatory disorders, including cardiovascular disorders such as hypertension, congestive heart failure, cirrhosis and ascites. Preferred antidiabetic agents are those compounds having high potency and oral or parenteral bioavailability. Preferred aldosterone receptor antagonists are 20-spiroxane steroidal compounds characterized by the presence of a 9α,11α-substituted epoxy moiety.
Owner:PHARMACIA CORP
Antidiabetic agent for control of diabetic hyperglycemia and diabetic complications
InactiveUS20070293562A1High blood levelBiocideOrganic active ingredientsAcute hyperglycaemiaDiabetic complication
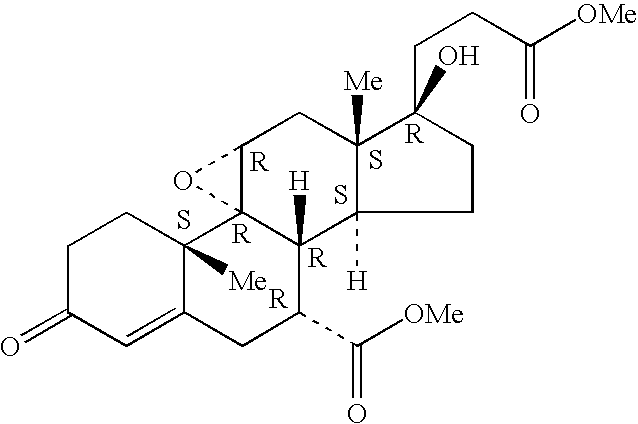
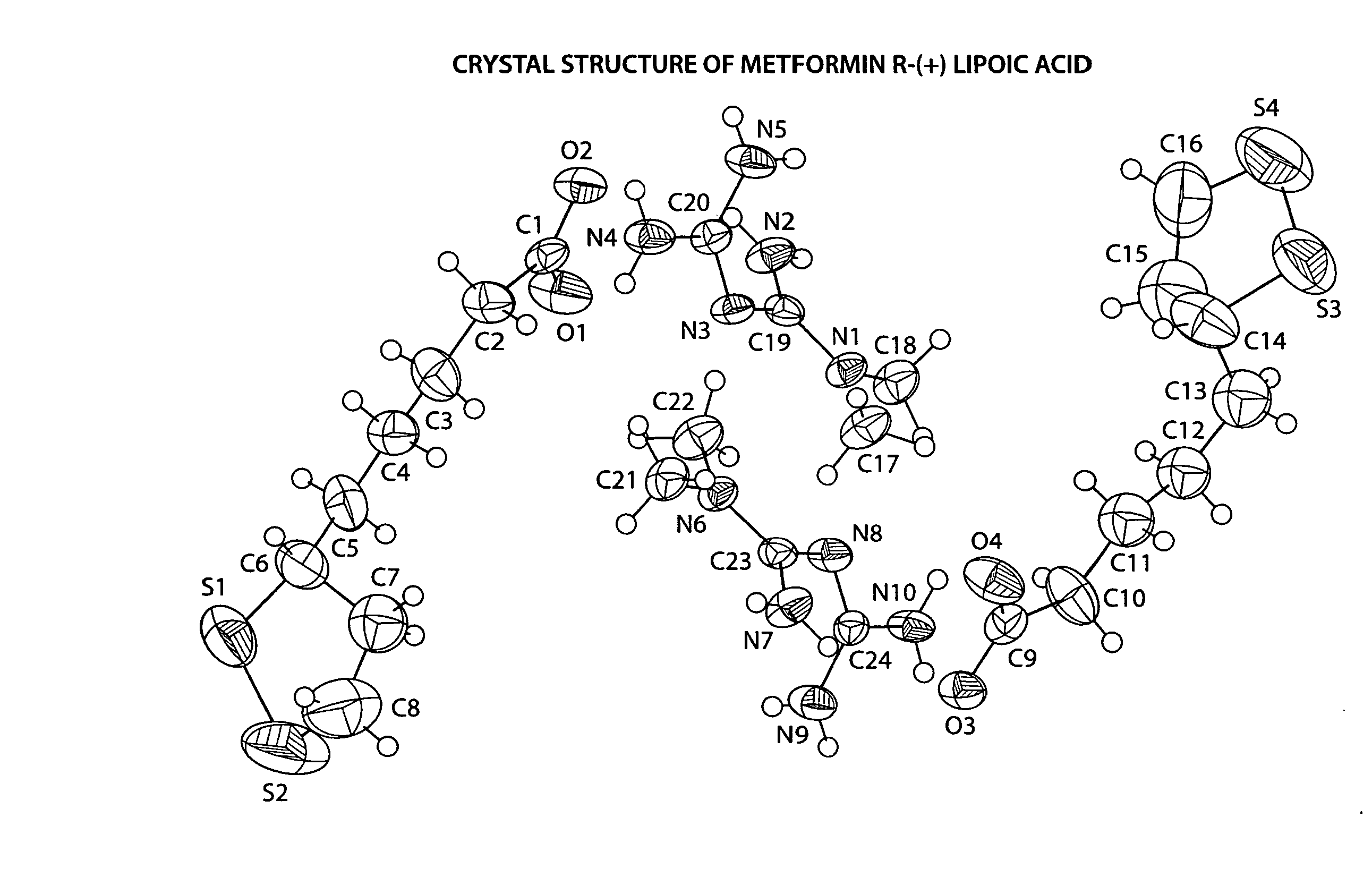
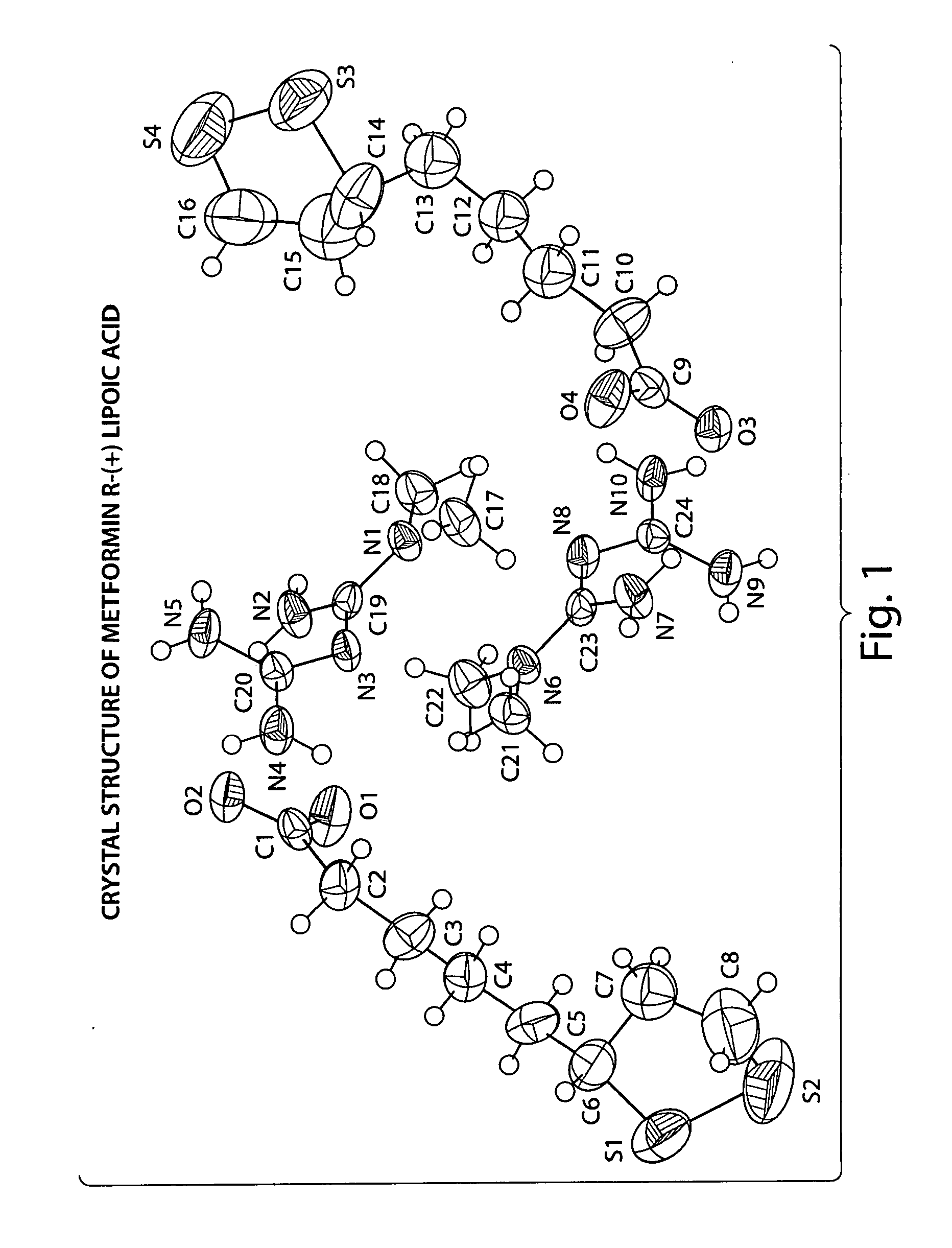
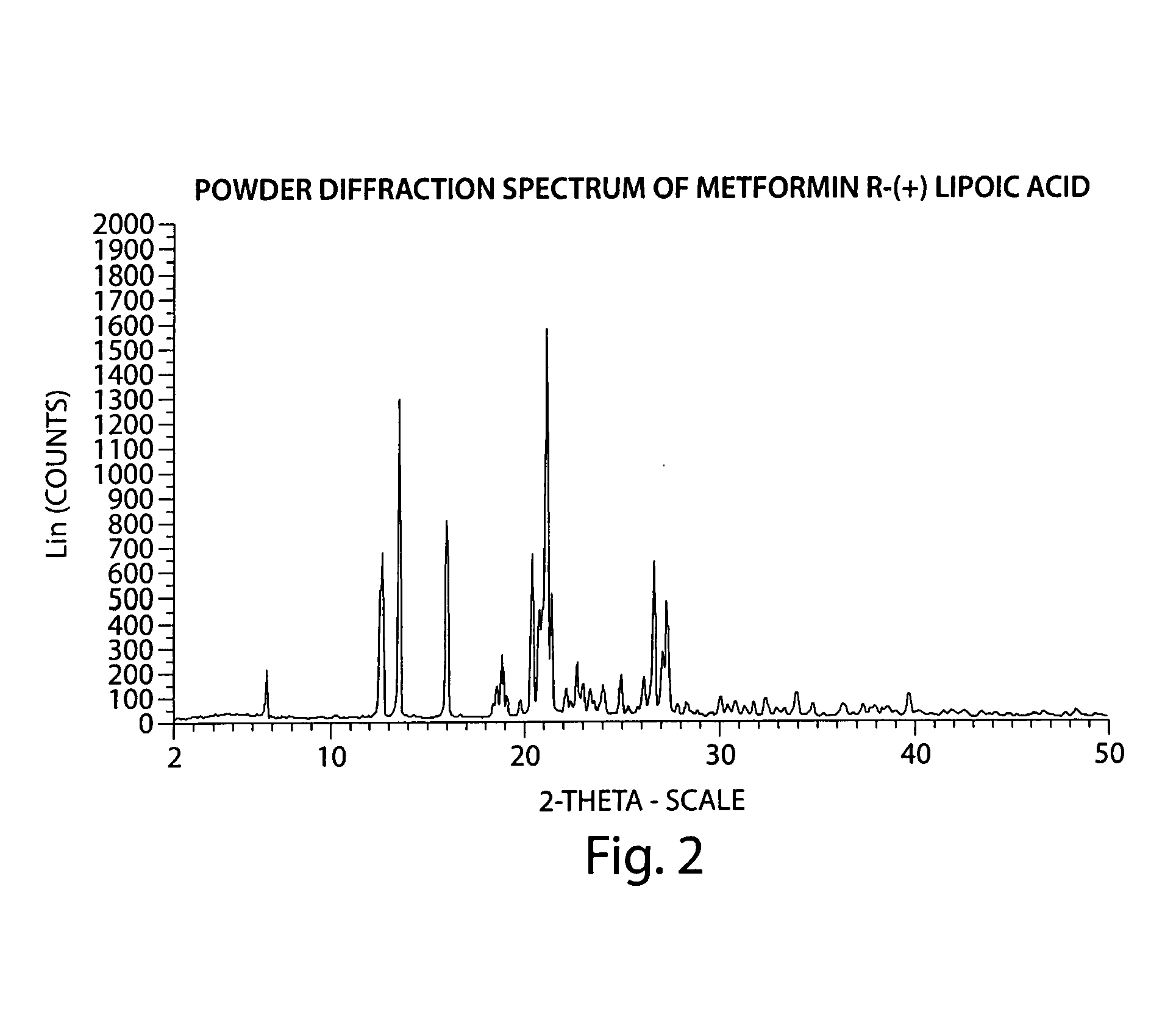
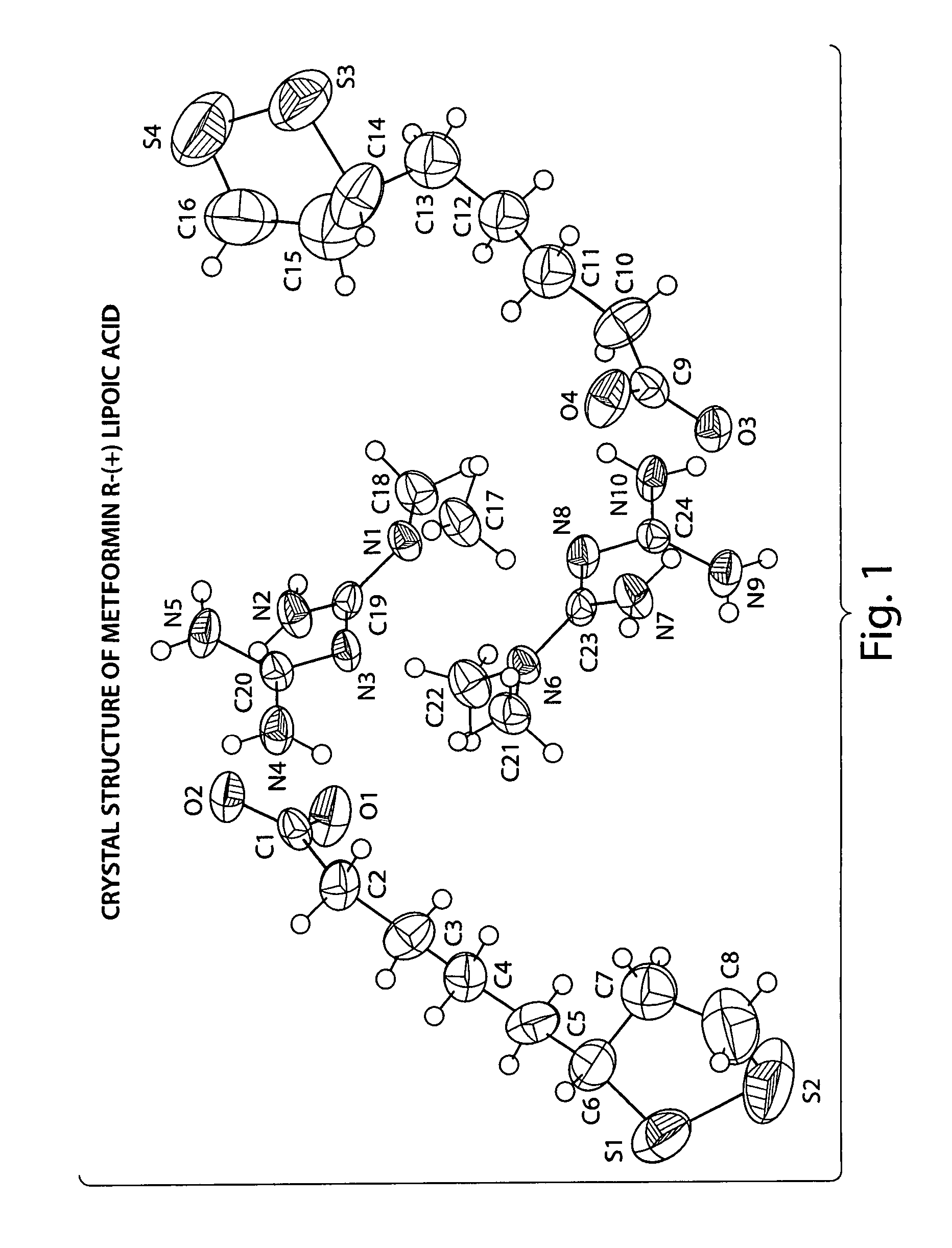
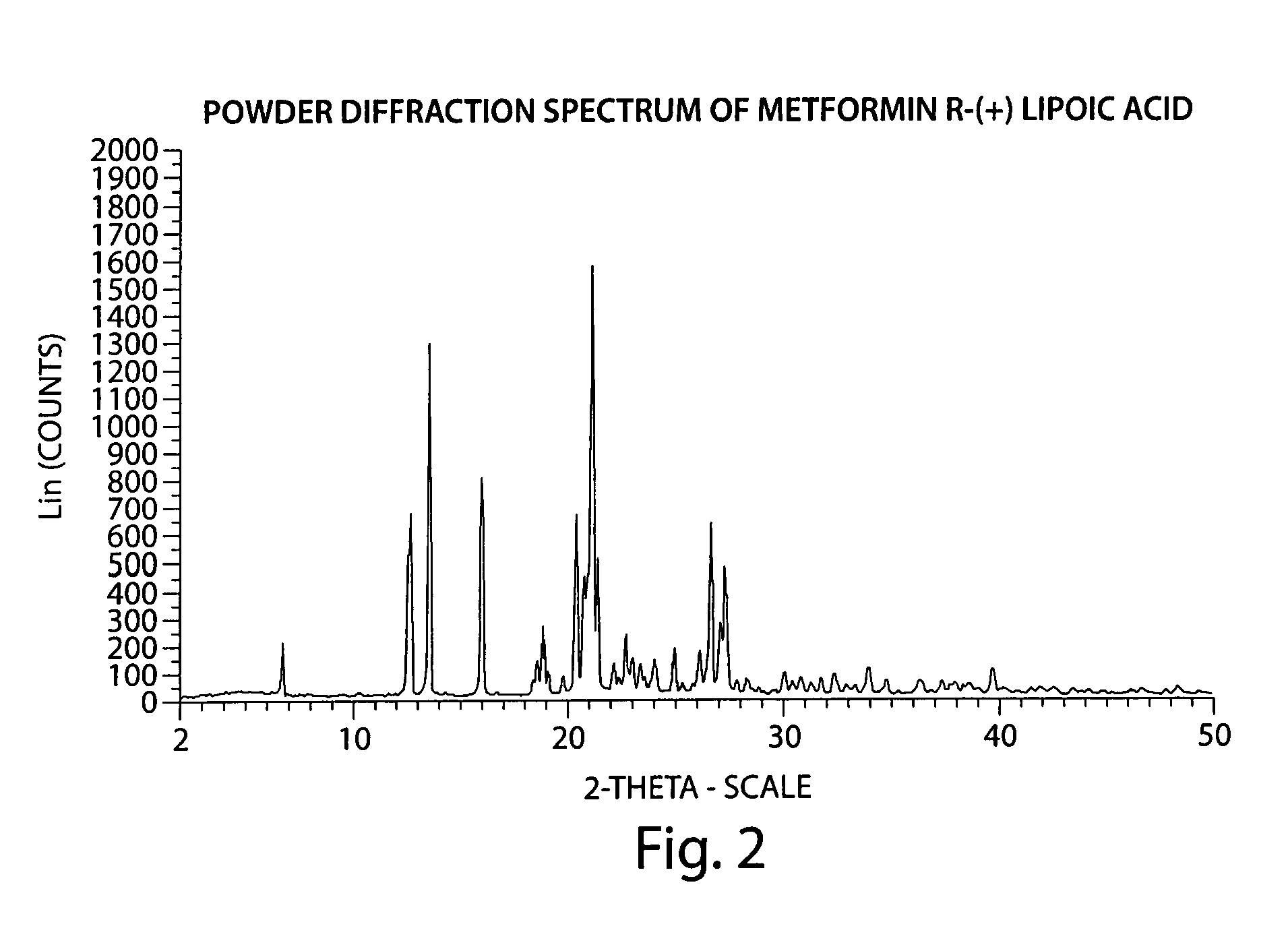
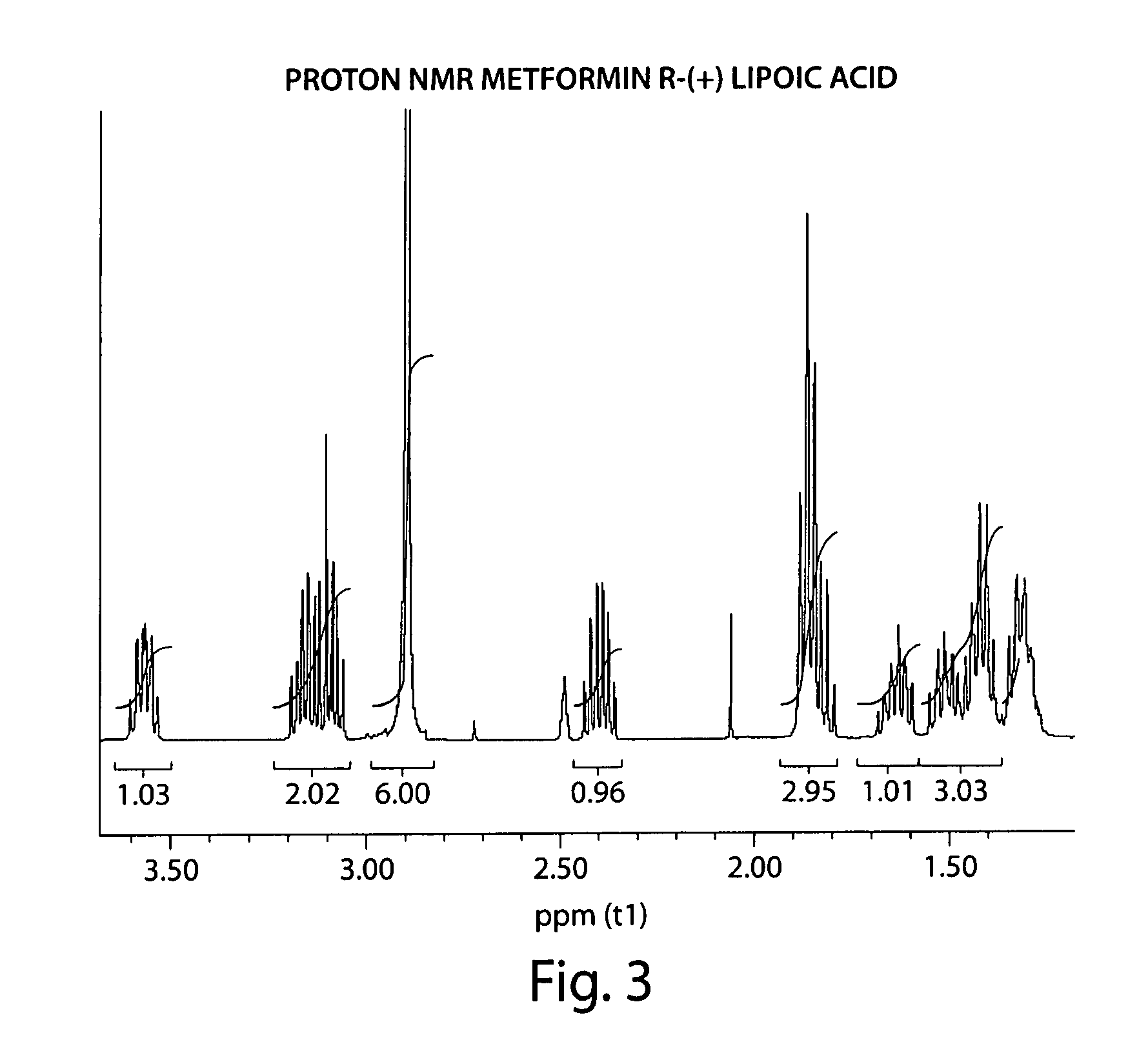
Described herein is a compound of Formula I, which is the metformin salt of the naturally occurring endogenous biological compound, (R)-(+) α lipoic acid, pharmaceutical compositions containing the compound of Formula I, and methods of treatment of diabetes or diabetic complications with the compound of Formula I.
Owner:INDIGENE PHARMA
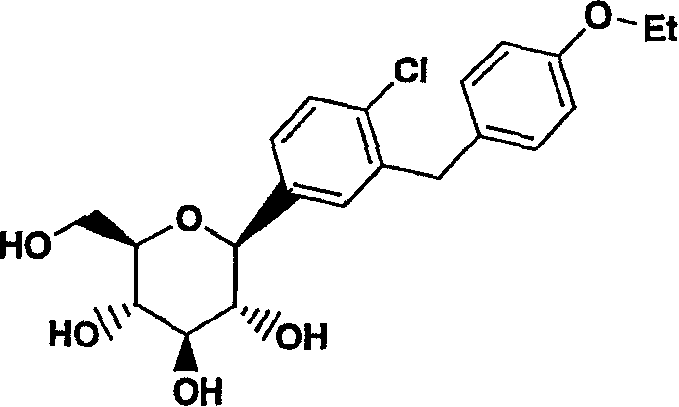
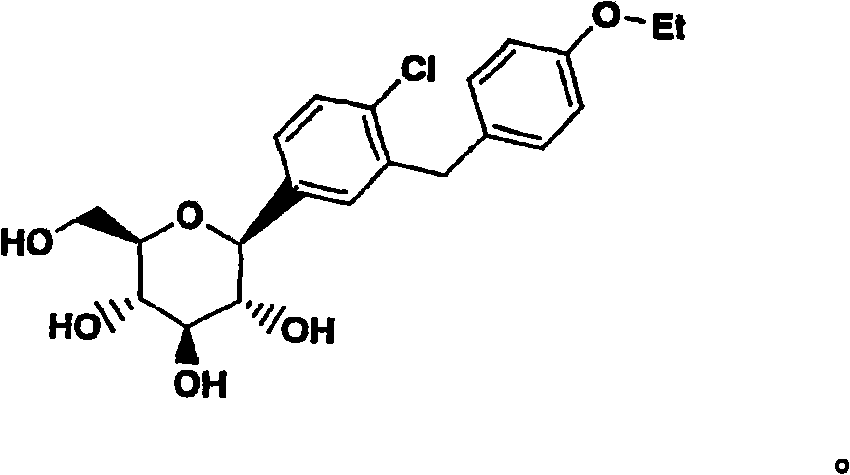
C-aryl glucoside SGLT2 inhibitors and method
Owner:ASTRAZENECA AB
Lipid-lowering antidiabetic agent
InactiveUS20120178813A1Improve bioavailabilityHigh degreeBiocideOrganic chemistryTriglyceridePrediabetes
A composition which includes a salt of metformin and the use of the composition for treatment of or use in prediabetes, diabetes, lowering triglycerides and / or other conditions in mammals.
Owner:THETIS PHARMA
2-hydroxypropionic acid derivative and its manufacturing method
InactiveUS6030993ARemarkable antidiabetic activityLess side effectsBiocideOrganic compound preparationGlucose loweringMedicinal chemistry
PCT No. PCT / KR97 / 00133 Sec. 371 Date May 4, 1999 Sec. 102(e) Date May 4, 1999 PCT Filed Jul. 2, 1997 PCT Pub. No. WO98 / 00389 PCT Pub. Date Jan. 8, 1998This invention relates to a novel 2-hydroxypropionic acid derivative and its manufacturing method. Based on its mechanism to inhibit the CPT I, 2-hydroxypropionic acid derivative of this invention has blood glucose lowering effects so that the derivative may be effectively used as an antidiabetic agent having remarkable antidiabetic activity and fewer side effects.
Owner:JEW SANG SUP +2
Combination therapeutic compositions and method of use
The present invention provides pharmaceutical compositions and methods for the treatment of diabetes mellitus using combination therapy. The compositions relate to a compound of Formula I selected from one or more of betaines, lipidic betaines, betaine lipids and an antidiabetic agent such as sulfonylureas, biguanides, glitazones, .alpha.-glucosidase inhibitors, potassium channel antagonists, aldose reductase inhibitors, glucagon antagonists, activators of RXR, insulin therapy or other anti-obesity agent. The methods include the administration of the combination of compound of Formula I with antidiabetic agent where the two components are delivered in a simultaneous manner, where the compound of Formula I is administered first, followed by the antidiabetic agent, as well as wherein the antidiabetic agent is delivered first followed by the compound of Formula I.
Owner:MESSADEK JALLAL
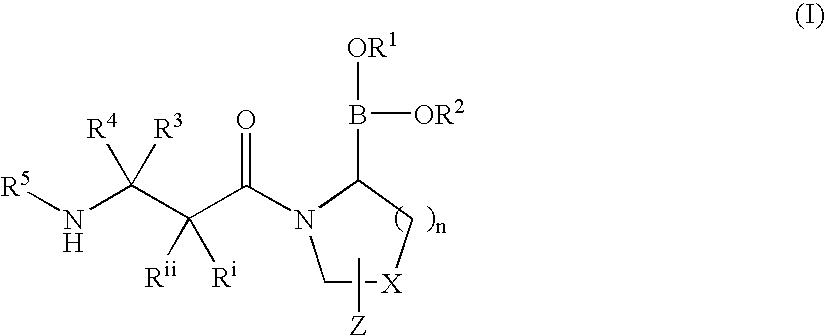
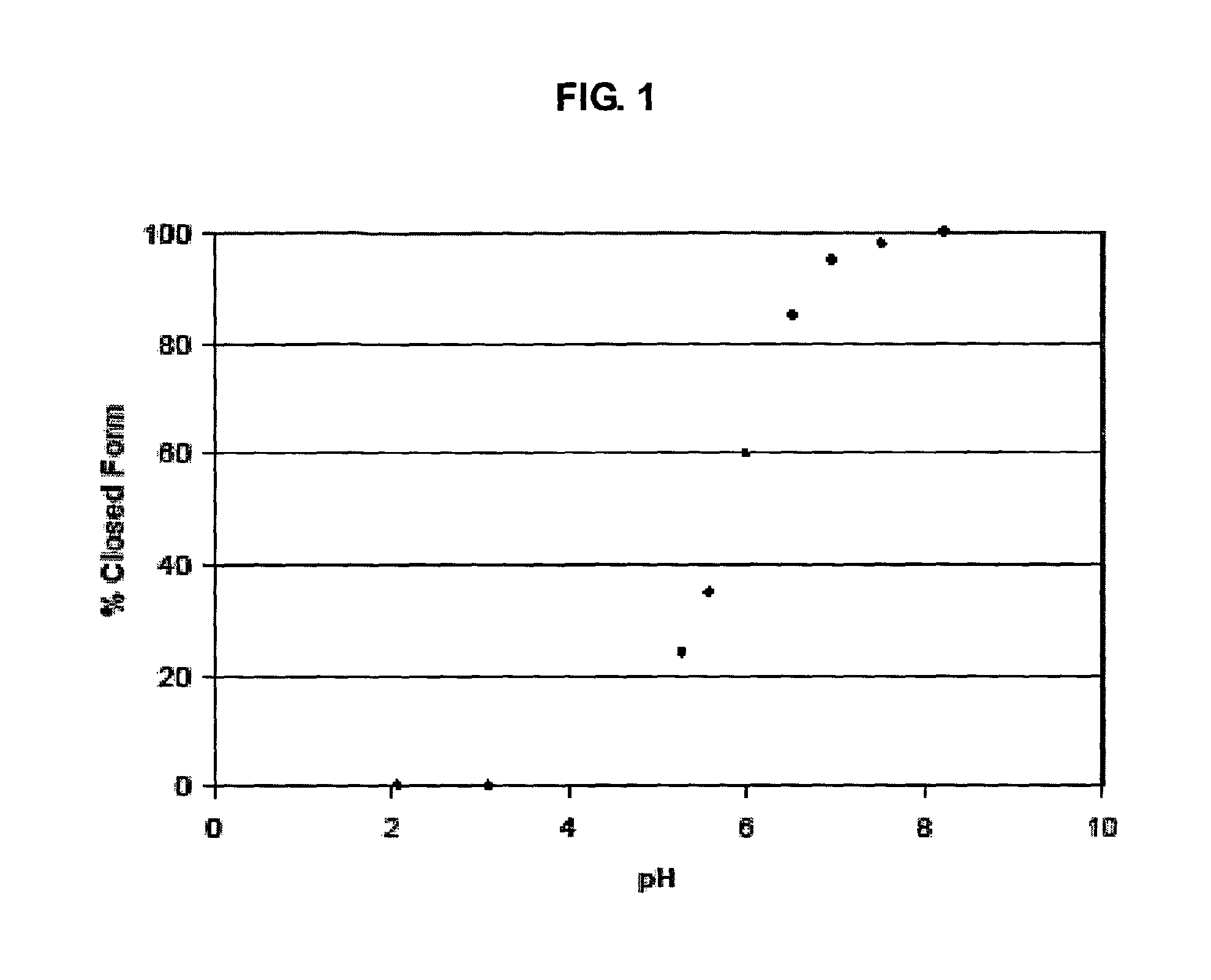
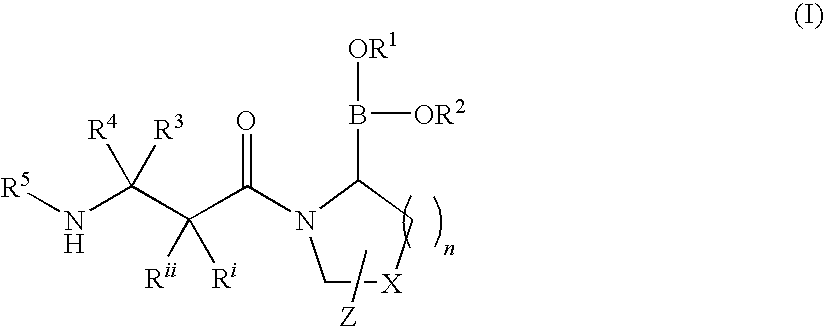
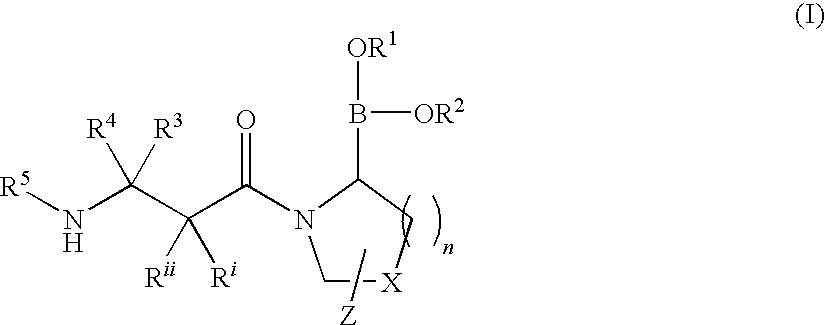
Heterocyclic boronic acid compounds
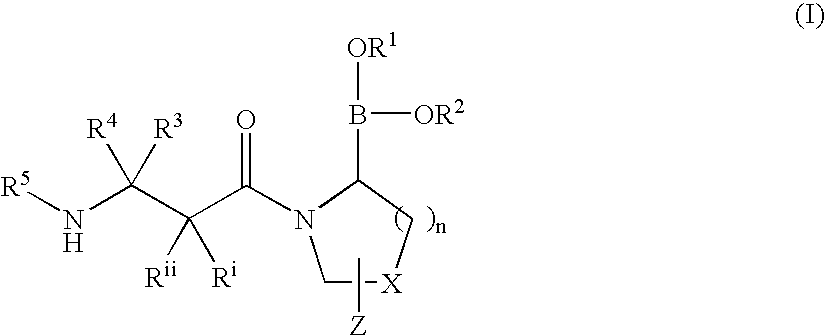
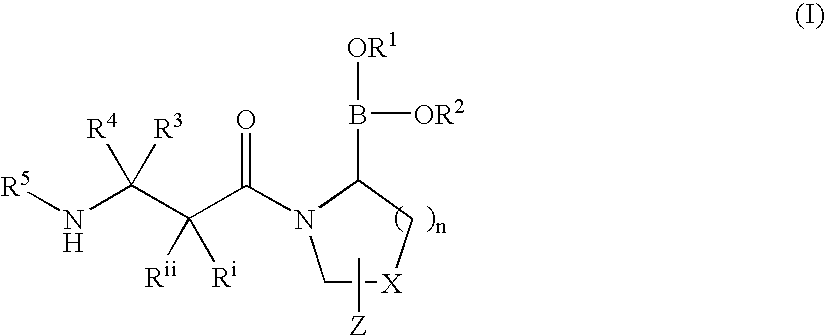
Dipeptidyl peptidase IV (DPP-IV)-inhibiting compounds are provided that have formula I: wherein n is 1 to 3; X is CH2; S; O; CF2 or C(CH3)2; Z is H; halogen; hydroxyl; (C1-6)alkoxy; (C1-12)alkyl; (C3-12)cycloalkyl; phenyl; or heteroaryl; where the phenyl and heteroaryl groups are optionally mono- or independently plurisubstituted with R7; optionally, X together with an adjacent ring carbon and Z form a fused cyclopropyl; and optionally, one of the bonds in the ring containing X is a double bond; and CriRii, R1, R1, R3, R4 and R5 are as described herein. Methods for preparing these compounds, and methods for treating diabetes, especially Type II diabetes, and other related diseases are described using the compounds of formula I in pharmaceutical compositions which contain these compounds. Pharmaceutical compositions which contain combinations of these compounds with other antidiabetic agents are also described herein.
Owner:SINO MED INT ALLIANCE
Heterocyclic boronic acid compounds
Dipeptidyl peptidase IV (DPP-IV)-inhibiting compounds are provided that have formula I: wherein n is 1 to 3; X is CH2; S; O; CF2 or C(CH3)2; Z is H; halogen; hydroxyl; (C1-6)alkoxy; (C1-12)alkyl; (C3-12)cycloalkyl; phenyl; or heteroaryl; where the phenyl and heteroaryl groups are optionally mono- or independently plurisubstituted with R7; optionally, X together with an adjacent ring carbon and Z form a fused cyclopropyl; and optionally, one of the bonds in the ring containing X is a double bond; and CriRii, R1, R1, R3, R4 and R5 are as described herein. Methods for preparing these compounds, and methods for treating diabetes, especially Type II diabetes, and other related diseases are described using the compounds of formula I in pharmaceutical compositions which contain these compounds. Pharmaceutical compositions which contain combinations of these compounds with other antidiabetic agents are also described herein.
Owner:SINO MED INT ALLIANCE
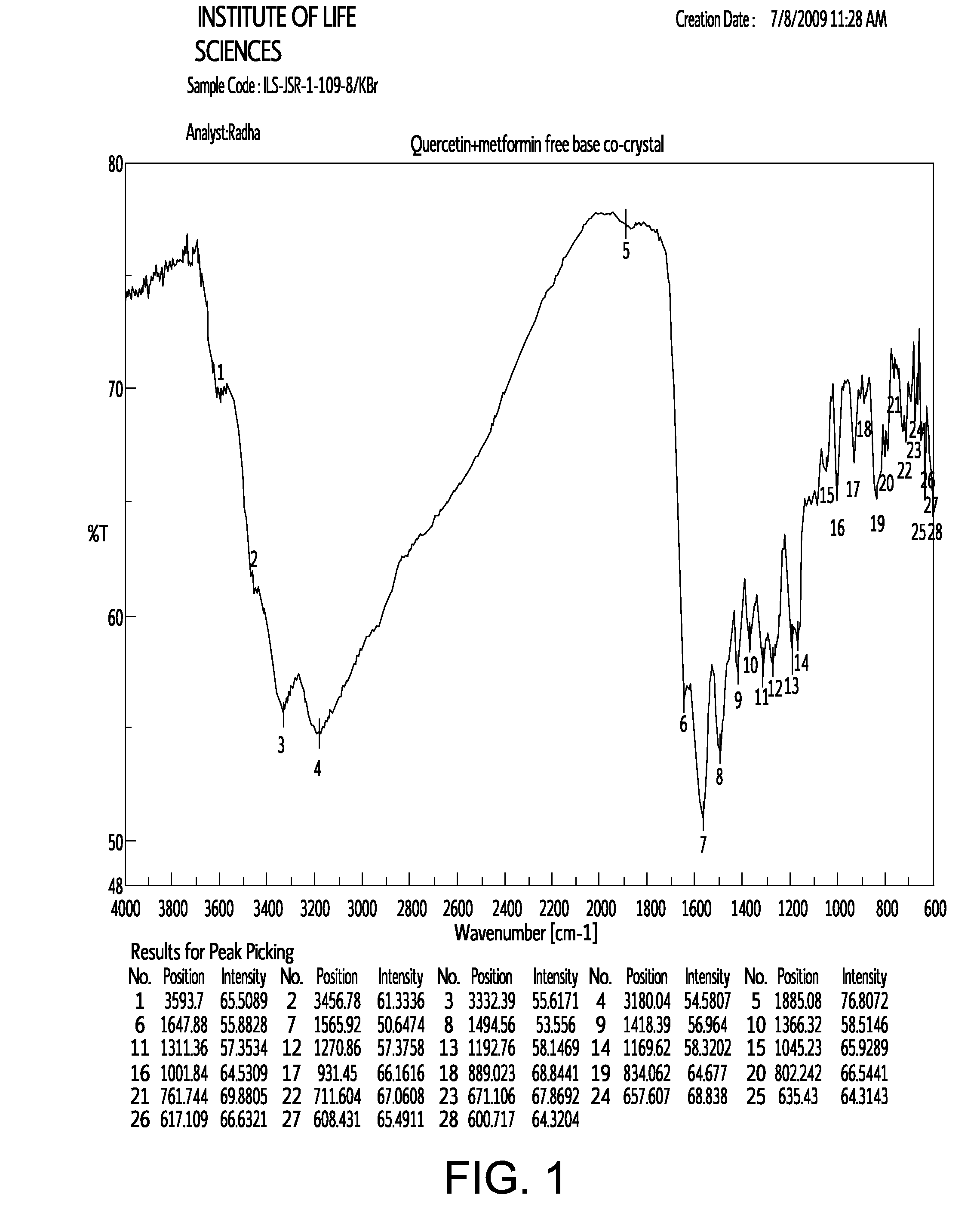
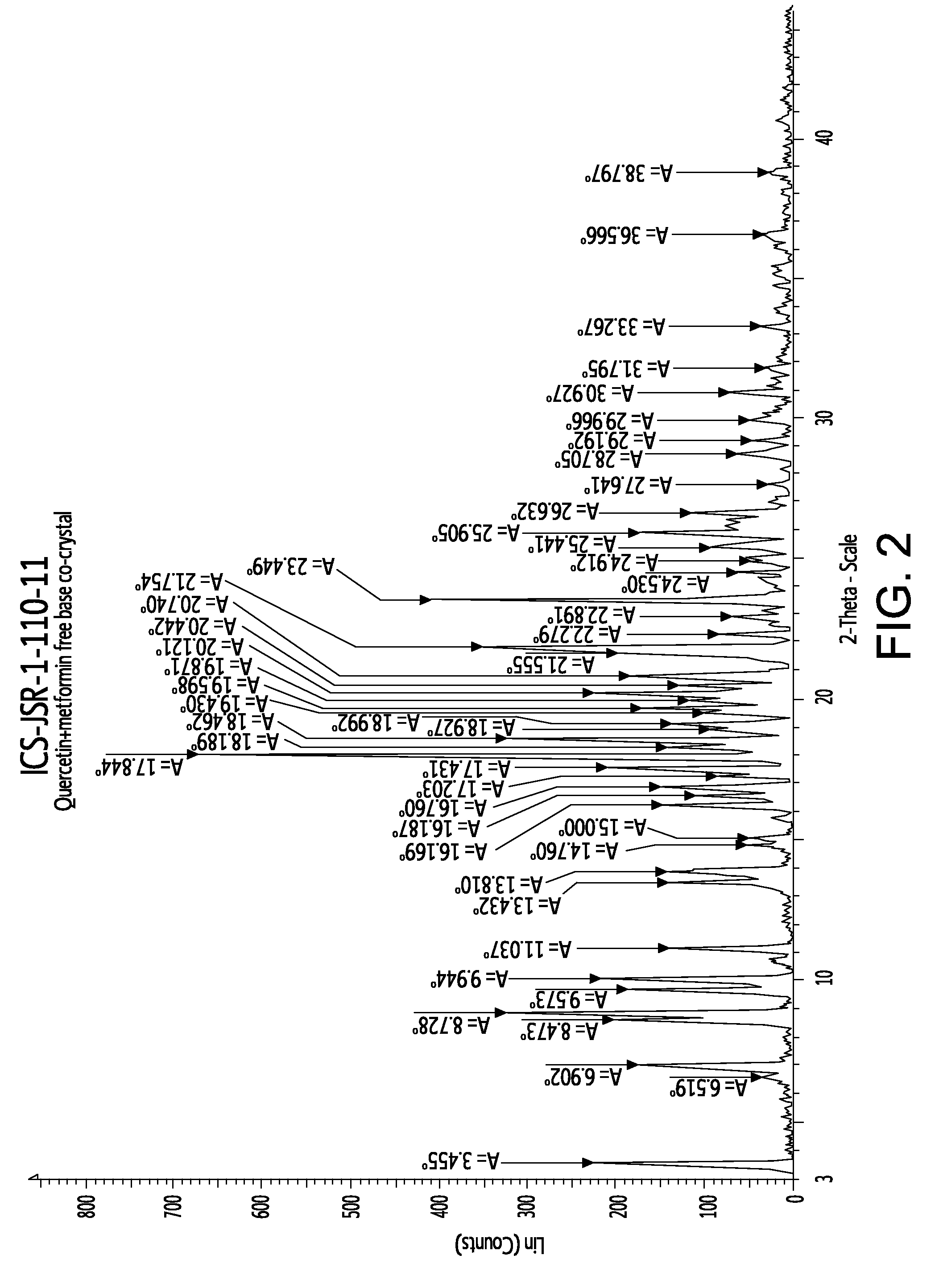
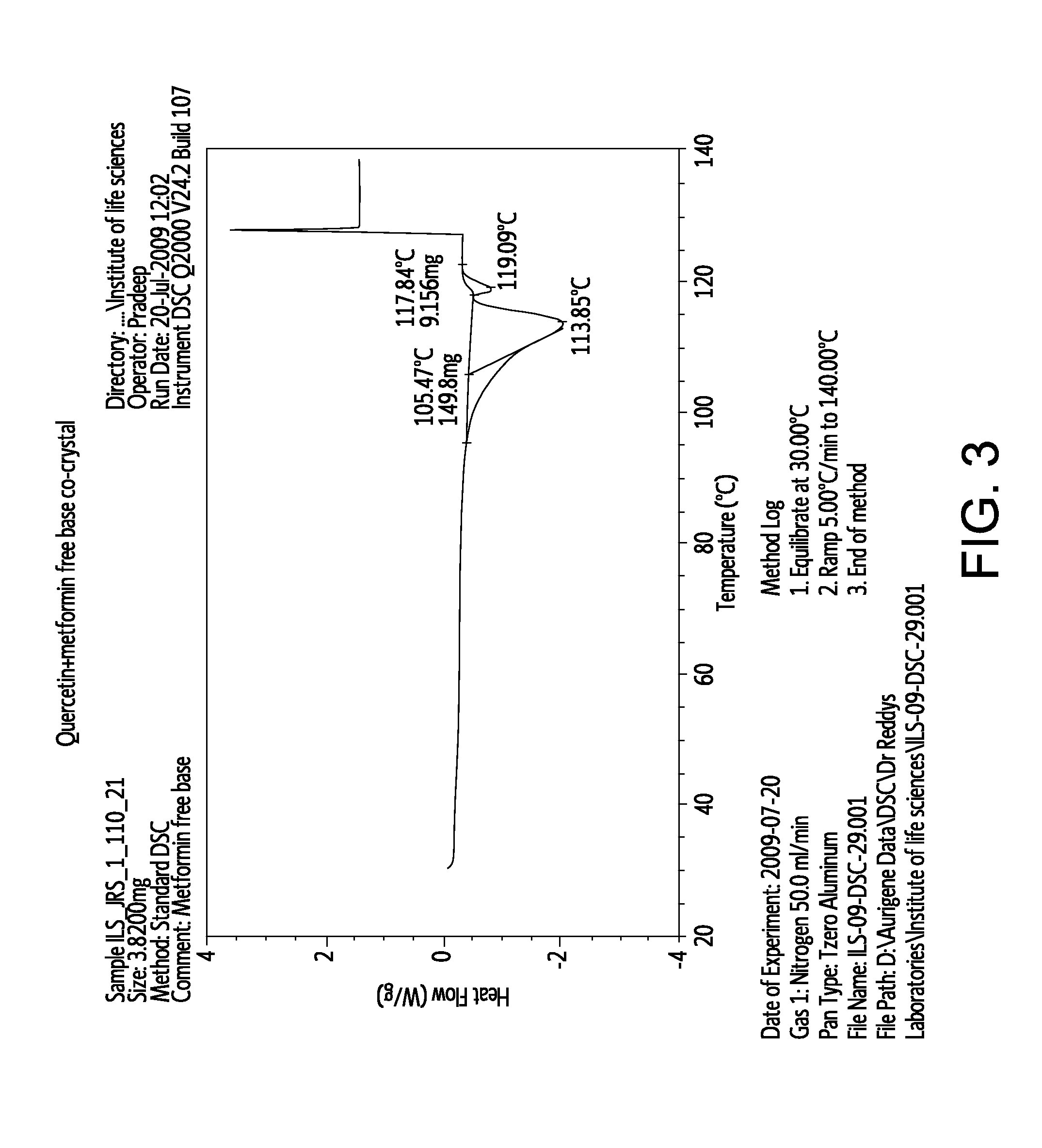
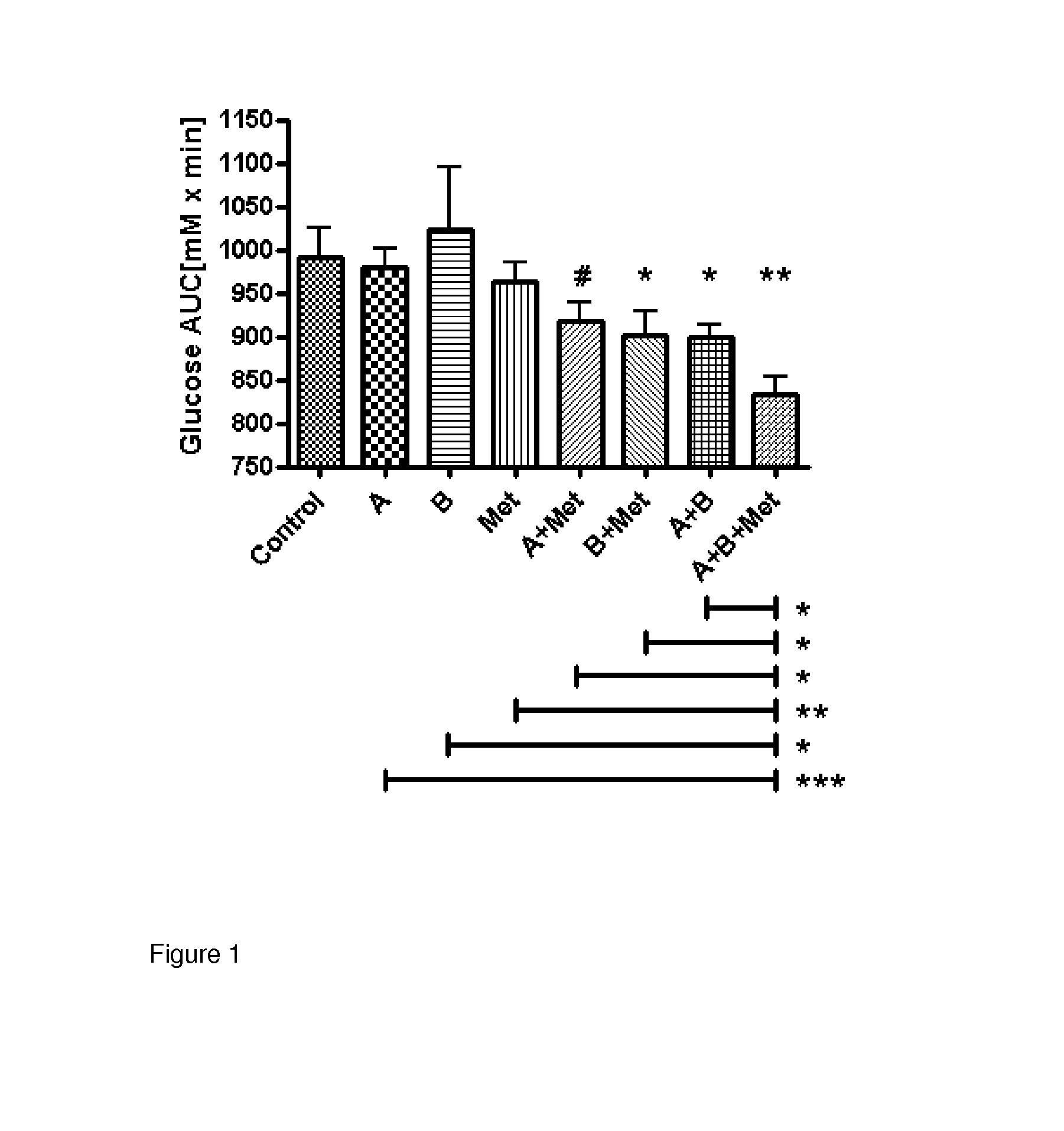
Pharmaceutical co-crystals of quercetin
InactiveUS20120258170A1Improve solubilitySuitable for preparationBiocidePowder deliveryDrug biological activityMetformin
A co-crystal composition comprised of Quercetin and at least one antidiabetic agent acts as a combination drug having unique physical properties and biological activity, which differ from both Quercetin in pure form and the at least one antidiabetic agent in pure form. The co-crystal composition may comprise quercetin and metformin. The co-crystals of quercetin and metformin may be prepared by grinding the compounds, and used in pharmaceutical compositions comprising these co-crystals. Co-crystal compositions of quercetin and Metformin may be used in combination with other anti-diabetic agents, including DPP-IV inhibitors.
Owner:NUTRACRYST THERAPEUTICS PRIVATE
Spaced drug delivery system
InactiveUS20040086562A1Reduce adverse effectsImprove welfareMetabolism disorderSulfonylurea active ingredientsDiseaseCo administration
The present invention provides to a method of administration of two or more therapeutically active agents comprising orally administering to a patient a spaced drug delivery system, wherein the time of release of the two or more therapeutically active agents is designed to provide desired control on the disease condition. The present invention also provides a method of administration of two or more therapeutically active agents comprising orally administering to a patient a spaced drug delivery system at a specified time prior to food intake by the patient. The present invention further provides a spaced drug delivery system that releases two or more antidiabetic agents at different times after oral administration, for the treatment of diabetes mellitus or conditions associated with diabetes mellitus. More particularly, the present invention provides a spaced drug delivery system that immediately releases one or more antidiabetic agents after oral administration of the system, and releases as a pulse one or more antidiabetic agents in a reliable manner at about a predetermined time after oral administration of the system.
Owner:SUN PHARMA INDS
Pharmaceutical composition, methods for treating and uses thereof
ActiveUS20140038911A1Good effectFew complianceBiocideMetabolism disorderAcute hyperglycaemiaIGT - Impaired glucose tolerance
The invention relates to a pharmaceutical composition according to the claim 1 comprising an SGLT2 inhibitor, a DPPIV inhibitor and a third antidiabetic agent which is suitable in the treatment or prevention of one or more conditions selected from type 1 diabetes mellitus, type 2 diabetes mellitus, impaired glucose tolerance and hyperglycemia. In addition the present invention relates to methods for preventing or treating of metabolic disorders and related conditions
Owner:BOEHRINGER INGELHEIM INT GMBH
Antidiabetic agents
InactiveUS6911467B2BiocidePeptide/protein ingredientsIGT - Impaired glucose toleranceStereochemistry
Owner:FERRING BV
Antidiabetic agent for control of diabetic hyperglycemia and diabetic complications
Described herein is a compound of Formula I, which is the metformin salt of the naturally occurring endogenous biological compound, (R)-(+) α lipoic acid, pharmaceutical compositions containing the compound of Formula I, and methods of treatment of diabetes or diabetic complications with the compound of Formula I.
Owner:INDIGENE PHARMA
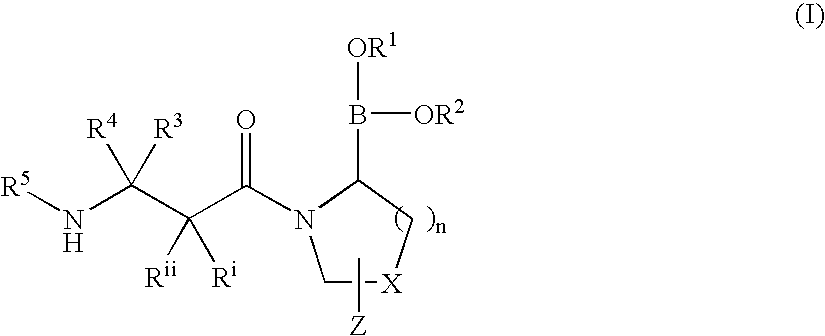
Heterocyclic boronic acid compounds
Dipeptidyl peptidase IV (DPP-IV)-inhibiting compounds are provided that have formula I: wherein n is 1 to 3; X is CH2; S; O; CF2 or C(CH2)2; Z is H; halogen; hydroxyl; (C1-6)alkoxy; (C1-12)alkyl; (C3-12)cycloalkyl; phenyl; or heteroaryl; where the phenyl and heteroaryl groups are optionally mono- or independently plurisubstituted with R7; optionally, X together with an adjacent ring carbon and Z form a fused cyclopropyl; and optionally, one of the bonds in the ring containing X is a double bond; and CriRii, R1, R1, R3, R4 and R5 are as described herein. Methods for preparing these compounds, and methods for treating diabetes, especially Type II diabetes, and other related diseases are described using the compounds of formula I in pharmaceutical compositions which contain these compounds. Pharmaceutical compositions which contain combinations of these compounds with other antidiabetic agents are also described herein.
Owner:SINO MED INT ALLIANCE
Combinations of statins and anti-obesity agent and glitazones
Co-therapy of an anti-obesity agent, a statin, and a glitazone is disclosed along with fixed combinations thereof. Atorvastatin, rosiglitazone, and orlistat are preferred as the various components. Non-glitazone antidiabetic agents may be optionally added to the therapy and / or to the fixed combination product.
Owner:PALEPU NAGESWARA R
Lipid-lowering antidiabetic agent
InactiveUS20130281535A1Improve bioavailabilityHigh degreeBiocideOrganic chemistryLipid formationTG - Triglyceride
A composition which includes a salt of metformin and the use of the composition for treatment of or use in prediabetes, diabetes, lowering triglycerides and / or other conditions in mammals.
Owner:THETIS PHARMA
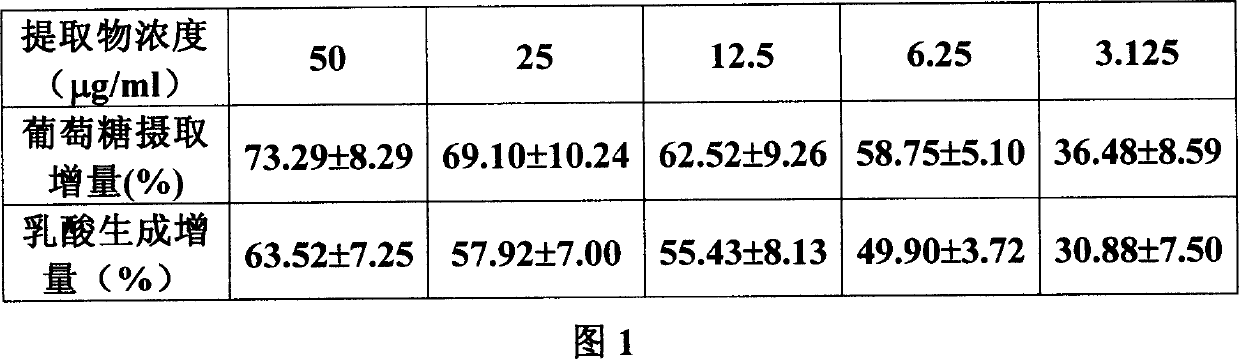
Preparation method and application of total flavone and total polysaccharide in cyclocarya paliurus leaves
PendingCN110051726AHigh purityLow priceMetabolism disorderNatural extract food ingredientsGlucose uptakeFood material
The invention provides a preparation method and application of total flavone and total polysaccharide in cyclocarya paliurus leaves. The method comprises the steps of taking cyclocarya paliurus leavesas raw materials, executing extraction by an ethanol-water solvent, executing extraction by an organic solvent, executing elution by a chromatographic column and by macroporous resin to obtain totalflavone, conducting extraction on cyclocarya paliurus leaf dregs by hot water, executing precipitation, removing protein, and adsorbing impurities by macroporous resin to obtain total polysaccharide.Studies show that the total triterpenes, the total flavones, the total polysaccharides and their composition prepared by the method can effectively increase glucose uptake of 3T3-L1 adipocytes, obviously reduce the fasting blood glucose level of the hereditary diabetes db / db mice, has antidiabetic activity, and can be used for preparing products such as antidiabetic medicines, health foods, functional foods and the like for reducing blood sugar. The invention develops the comprehensive preparation method of total flavones and total polysaccharides in cyclocarya paliurus leaves and applicationof the mehtod in resisting diabetes, and provides a basis and a new application direction for popularization, utilization and deep processing of traditional Chinese medicinal materials and new food raw materials.
Owner:ZHEJIANG UNIV
Sugarapple plant extract with action of resisting diabetes, medicinal application and preparation method
The present invention relates to a plant extract with the action of resisting diabetes, its application and preparation method. The extract is a kind of acetogenins extracted from branck, leaf, trunk, bark, root, seed and fruit skin of annona squamosa plant as raw material. The compound acetogenins can be made into various dosage forms for curing diabetes, and its preparation method can adopt oneor several processes of solvent extraction process, resin adsorption process, supercritical CO2 extraction process and conventional drying process.
Owner:CHINESE MEDICINE & NATURAL MEDICINE RES CENT SHENZHEN
Pharmaceutical compositions containing abiguanide-glitazone combination
The present invention relates to an orally administered pharmaceutical composition that is a combination of two or more antidiabetic agents in which one of the antidiabetic agents is present in an extended release form and the other antidiabetic agent is present in an immediate release form.
Owner:RANBAXY LAB LTD
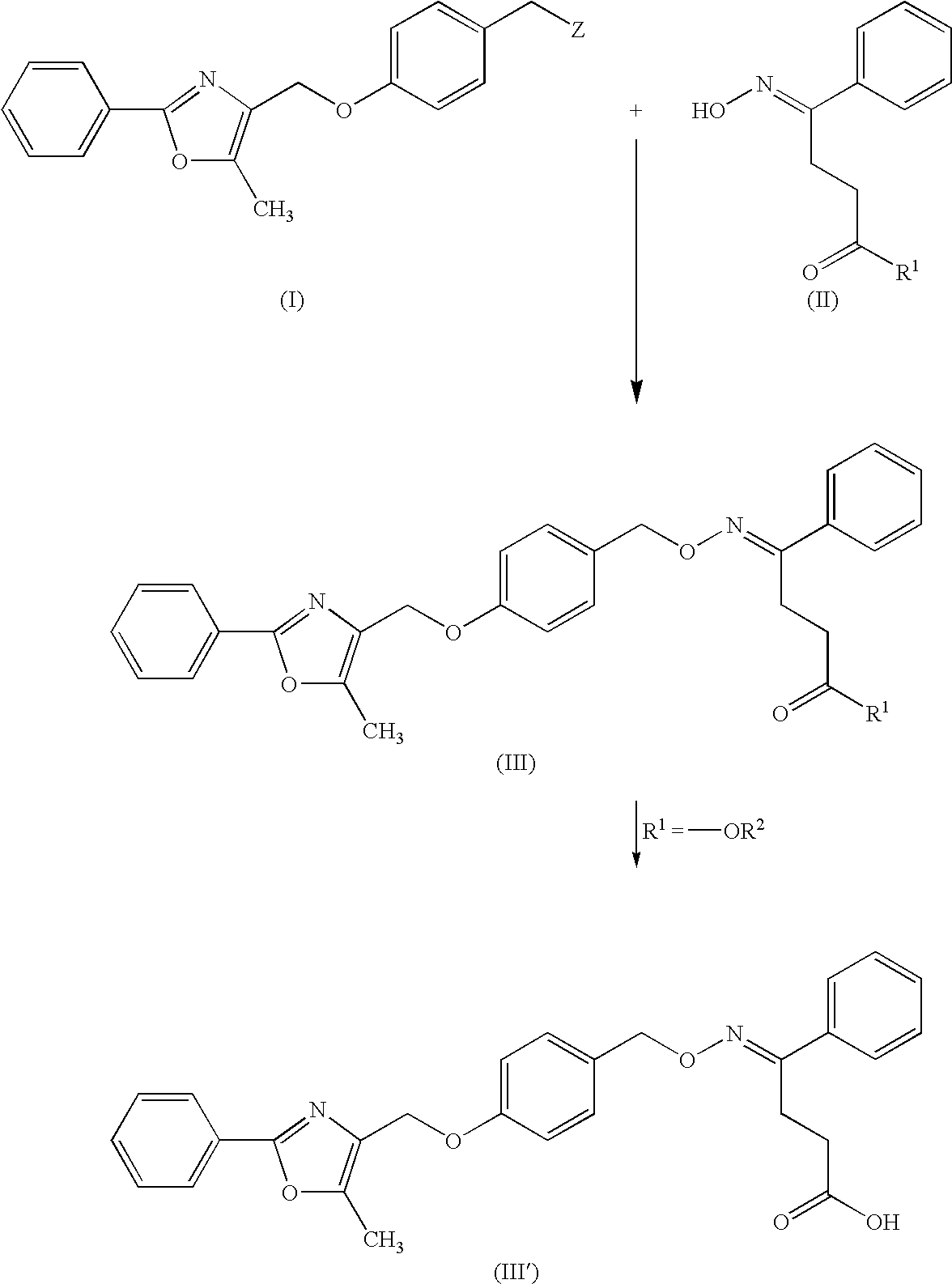
Crystals of an oxyiminoalkanoic acid derivative and their use as antidiabetics
Crystals of (E)-4-[4-(5-methyl-2-phenyl-4-oxazolylmethoxy)benzyloxyimino]-4-phenylbutyric acid (provided that crystals having a melting point of 126° C. to 127° C. are excluded), which have an excellent anti-diabetic action.
Owner:TAKEDA PHARMA CO LTD
Method for synthesizing benfotiamine
InactiveCN102911208ALow priceHigh reactivityGroup 5/15 element organic compoundsPhosphoric Acid EstersPhosphorylation
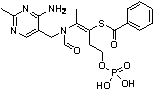
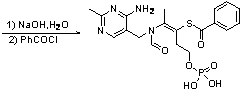
The invention relates to a method for synthesizing benfotiamine, in particular to a process for synthesizing benfotiamine which is an anti-diabetic medicament by the phosphorylation of thiamine which severs as the raw material, the opening of the thiazole ring and the reaction of multiple steps. Benfotiamine is subjected to phosphorylation under the control of appropriate temperature and other conditions in a short time by taking phosphorus oxychloride as a phosphorylation reagent to obtain monophosphothiamine, and finally the steps of ring opening and benzoylation are carried out to obtain benfotiamine. The process has the advantages of high conversion rate of raw materials, low-cost, high product purity and greatly reduced production cycle and is suitable for industrial production.
Owner:TONGJI UNIV
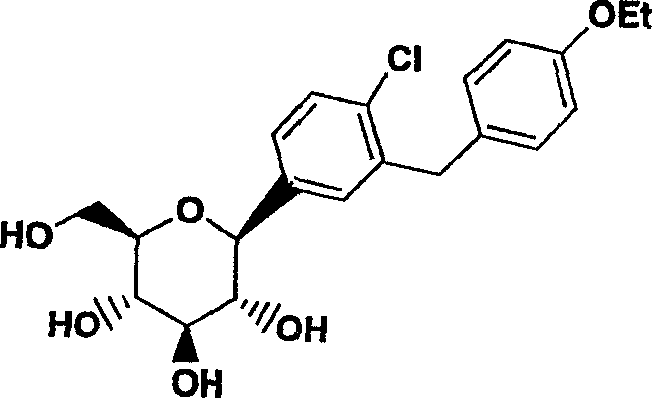
C-aryl glucoside sglt2 inhibitors and method
An SGLT2 inhibiting compound is provided having the formula[Chemical structure] A method is also provided for treating diabetes and related diseases employing an SGLT2 inhibiting amount of the above compound alone or in combination with another antidiabetic agent or other therapeutic agent.
Owner:ASTRAZENECA AB
Pharmaceutical dosage forms of biguanide-sulfonylurea combinations
The present invention relates to orally administered pharmaceutical compositions that include a combination of antidiabetic agents wherein one agent is present in an extended release form and the other agent is present in an immediate release form. For example, in one embodiment the dosage form includes an extended release layer that includes a biguanide; and an immediate release layer that includes a sulfonylurea.
Owner:RANBAXY LAB LTD
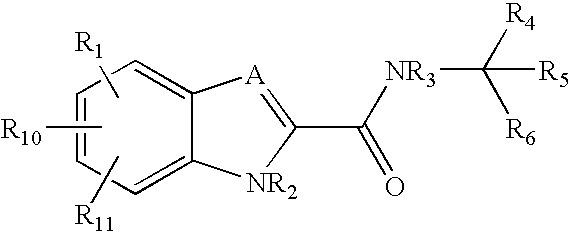
Ether and amide compounds preparation thereof composition containing same and use thereof as antidiadetics
InactiveUS6414001B2Enhance insulin actionLow toxicityBiocideOrganic chemistryPharmaceutical medicineTert butyl
Ether and amide derivatives are disclosed, which are represented by the following formula (I) and its pharmaceutical acceptable salt, and which are useful for the treatment of diabetes.wherein(with the provisos that (i) when A is -O-, then n is 2 or 3 (ii) whenthenn is 1 or 2. R3 is OH-, CH3SO2NH-, CF3SO2NH-, CH3SO2NHCH2-, CF3SO2NHCH2-, HOOC-, CH3OOC-,HOOC-CH2SO2NH-, CF3-CH2SO2NH-, R8-NHSO2-,R8-NHSO2-CH2-, HOOC-CH2-O-, HSO3N=CH-, or R9-SO2NHCO-;R4 is H, OH, O-alkyl or O-CH2OCH3;R5 is H, halogen atom, -CH2COOH or OH;R6 and R7 are hydrogen, t-butyl or pyrolidyl;R8 is hydrogen or lower alkyl;R9 is alkyl or thienyl;R10 is lower alkyl)or a pharmaceutically acceptable salt.
Owner:KOTOBUKI PHARMA CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com