Patents
Literature
941 results about "High doses" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
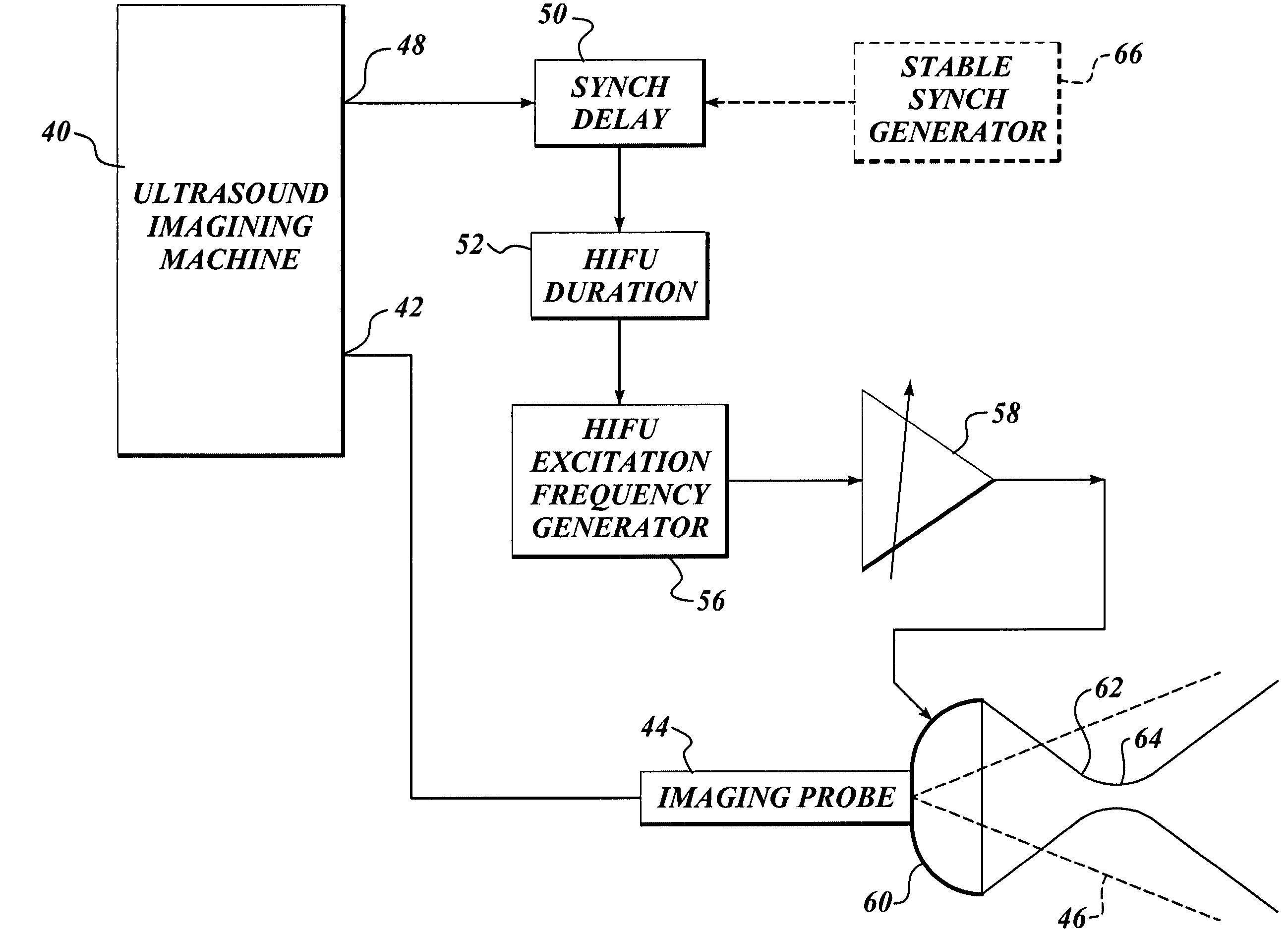
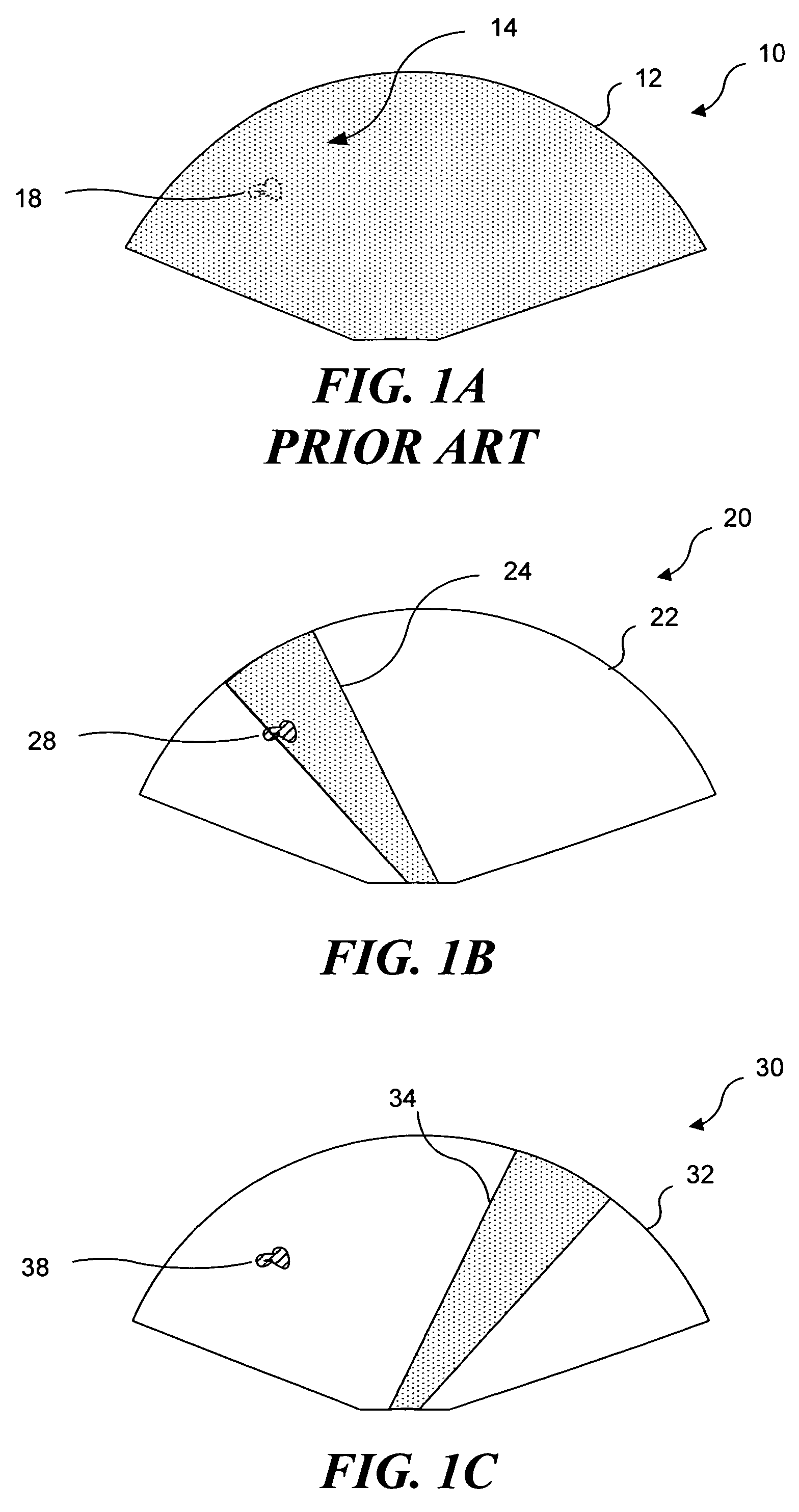
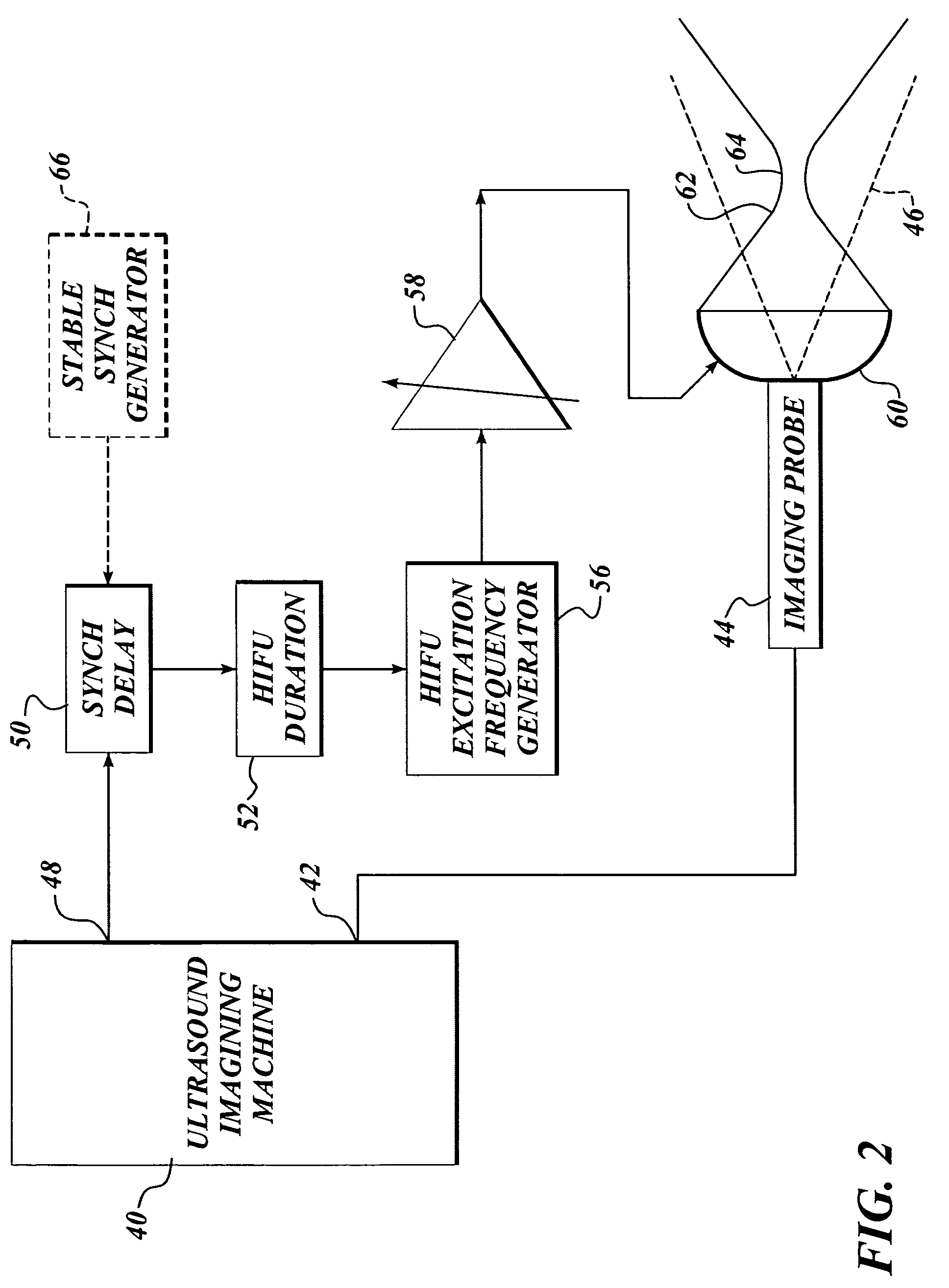
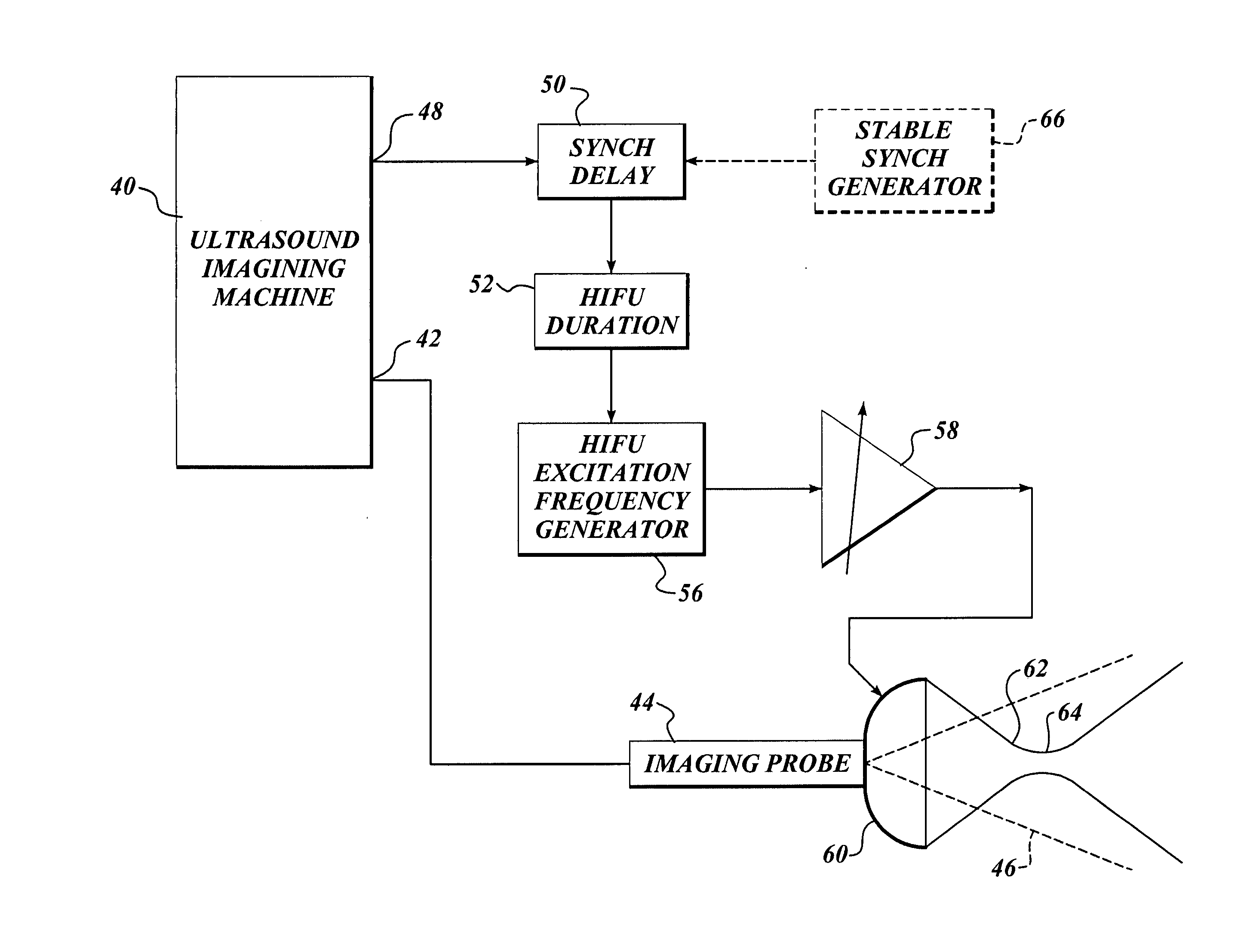
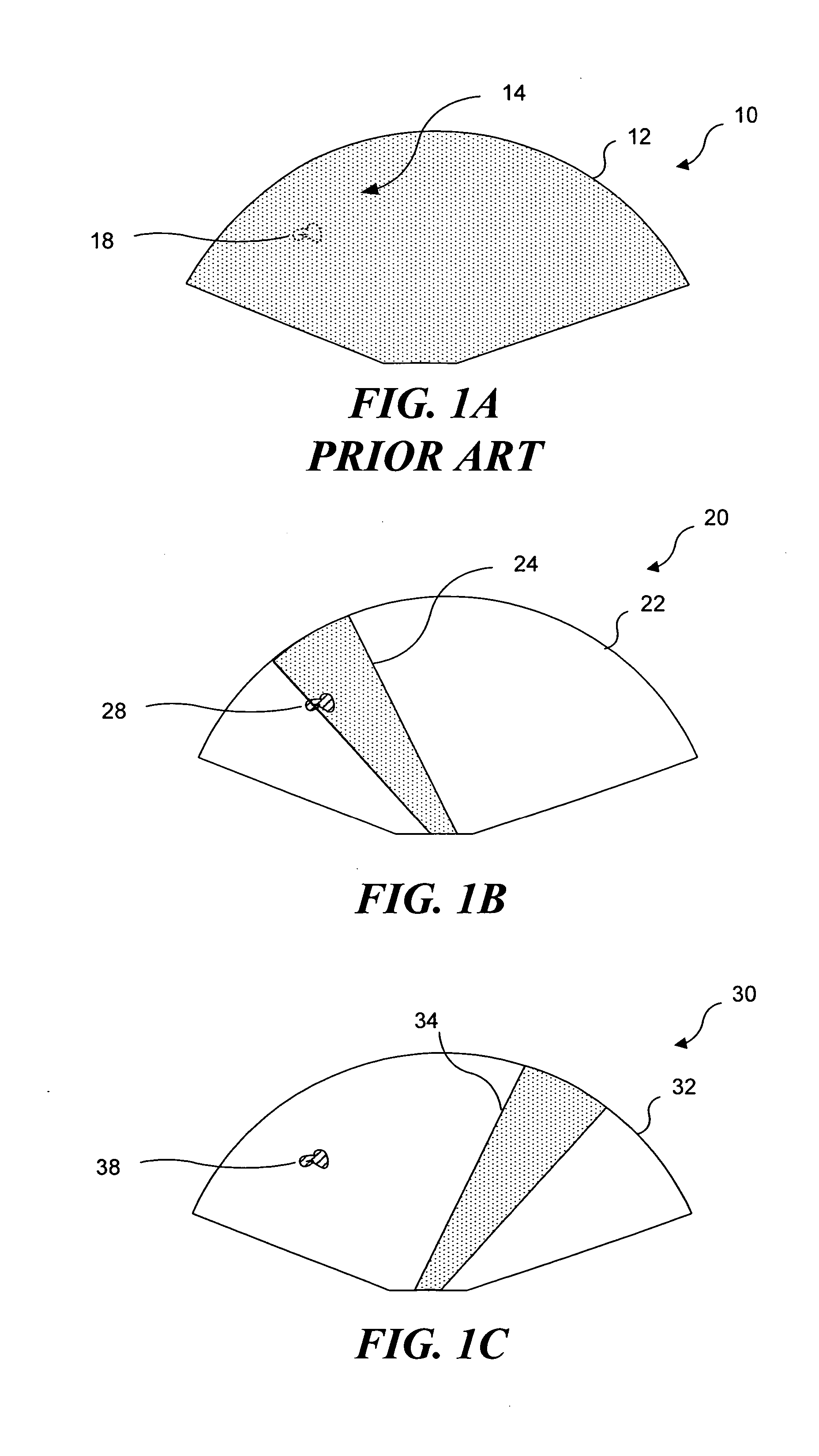
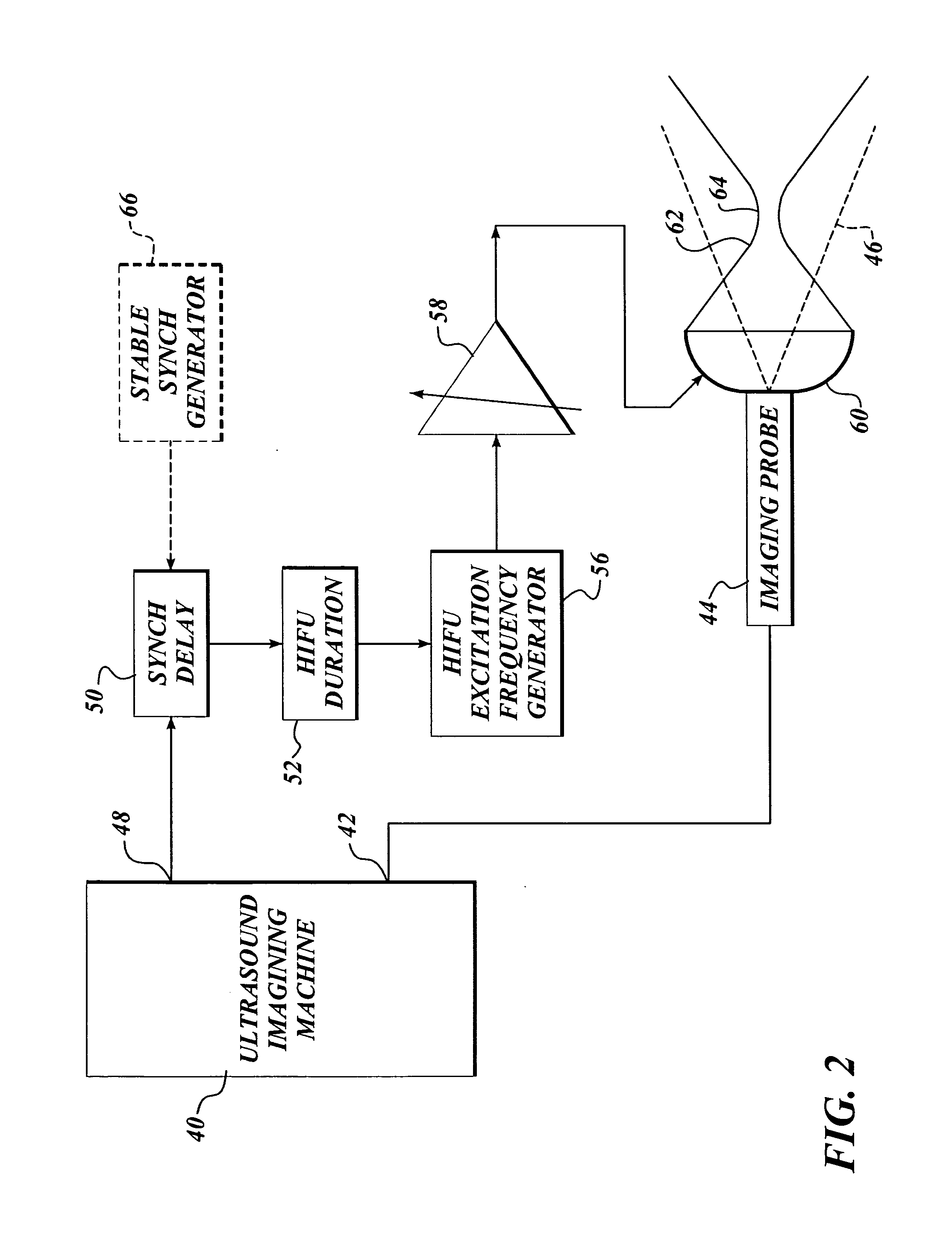
Ultrasound guided high intensity focused ultrasound treatment of nerves
InactiveUS7510536B2Relieve painEasy procedureUltrasound therapyBlood flow measurement devicesSonificationHigh doses
A method for using high intensity focused ultrasound (HIFU) to treat neurological structures to achieve a desired therapeutic affect. Depending on the dosage of HIFU applied, it can have a reversible or irreversible effect on neural structures. For example, a relatively high dose of HIFU can be used to permanently block nerve function, to provide a non-invasive alternative to severing a nerve to treat severe spasticity. Relatively lower doses of HIFU can be used to reversible a block nerve function, to alleviate pain, to achieve an anesthetic effect, or to achieve a cosmetic effect. Where sensory nerves are not necessary for voluntary function, but are involved in pain associated with tumors or bone cancer, HIFU can be used to non-invasively destroy such sensory nerves to alleviate pain without drugs. Preferably, ultrasound imaging synchronized to the HIFU therapy is used to provide real-time ultrasound image guided HIFU therapy of neural structures.
Owner:UNIV OF WASHINGTON
Ultrasound guided high intensity focused ultrasound treatment of nerves
InactiveUS20050240126A1Relieve painEasy procedureUltrasound therapyBlood flow measurement devicesAbnormal tissue growthHigh doses
A method for using high intensity focused ultrasound (HIFU) to treat neurological structures to achieve a desired therapeutic affect. Depending on the dosage of HIFU applied, it can have a reversible or irreversible effect on neural structures. For example, a relatively high dose of HIFU can be used to permanently block nerve function, to provide a non-invasive alternative to severing a nerve to treat severe spasticity. Relatively lower doses of HIFU can be used to reversible a block nerve function, to alleviate pain, to achieve an anesthetic effect, or to achieve a cosmetic effect. Where sensory nerves are not necessary for voluntary function, but are involved in pain associated with tumors or bone cancer, HIFU can be used to non-invasively destroy such sensory nerves to alleviate pain without drugs. Preferably, ultrasound imaging synchronized to the HIFU therapy is used to provide real-time ultrasound image guided HIFU therapy of neural structures.
Owner:UNIV OF WASHINGTON
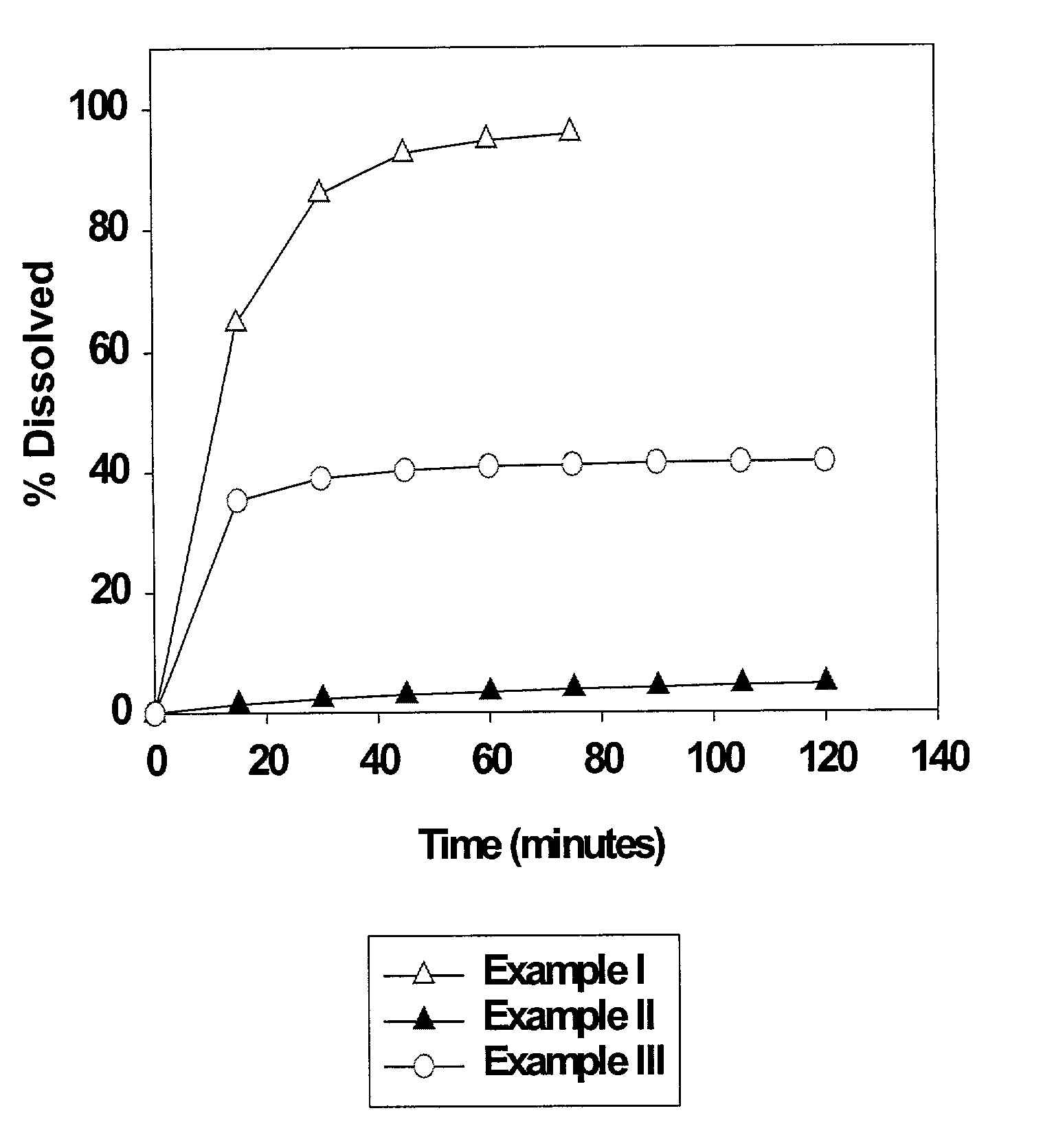
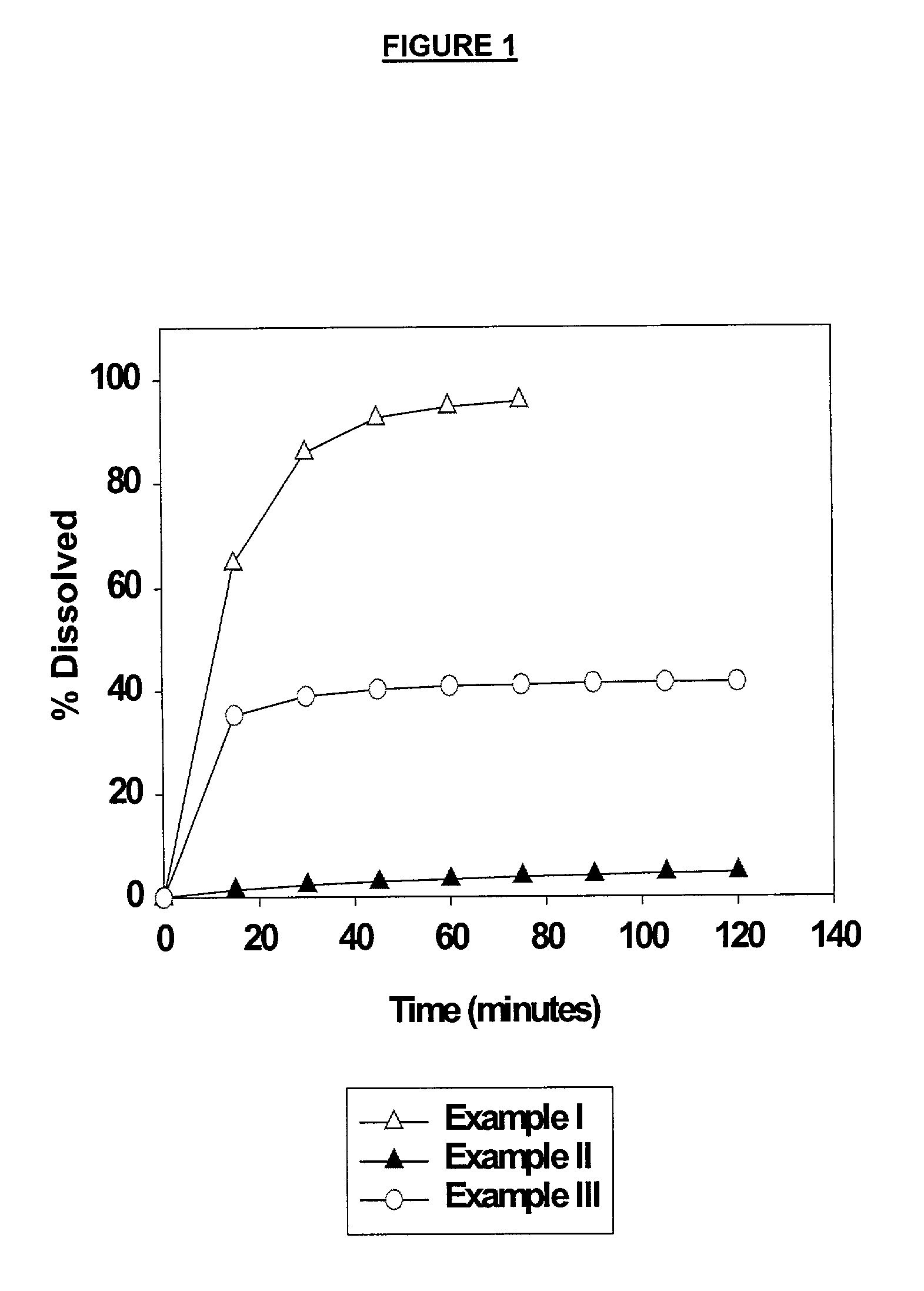
High dose solid unit oral pharmaceutical dosage form of amorphous nelfinavir mesylate and process for making same
InactiveUS7014866B2Satisfactory bioavailabilitySatisfactory dissolutionPowder deliveryBiocideHigh dosesNelfinavir mesylate
A solid unit oral pharmaceutical dosage form of amorphous nelfinavir mesylate is provided comprising amorphous nelfinavir mesylate in an amount of from about 400 mg to about 700 mg calculated as nelfinavir base, and a pharmaceutically acceptable water soluble, non-ionic synthetic block copolymer of ethylene oxide and propylene oxide, the copolymer having a melting point of at least about 45° C. and an HLB value at 25° C. of from about 18 to about 29, wherein the copolymer is present from about 40% to about 65% by weight of the nelfinavir mesylate. A hot melt granulation process for making the dosage form is provided.
Owner:F HOFFMANN LA ROCHE & CO AG
Sustained release pharmaceutical compositions for highly water soluble drugs
ActiveUS20070020335A1Reduce spreadReduce erosionPowder deliveryOrganic active ingredientsControlled releaseActive agent
The present invention provides pharmaceutical compositions for controlled release of pharmaceutically active agents, especially those with a high water solubility, high dose, and / or short half-life. In addition, the present application provides methods for preparing and using such pharmaceutical compositions.
Owner:FARNAM +1
Particle beam irradiation equipment and particle beam irradiation method
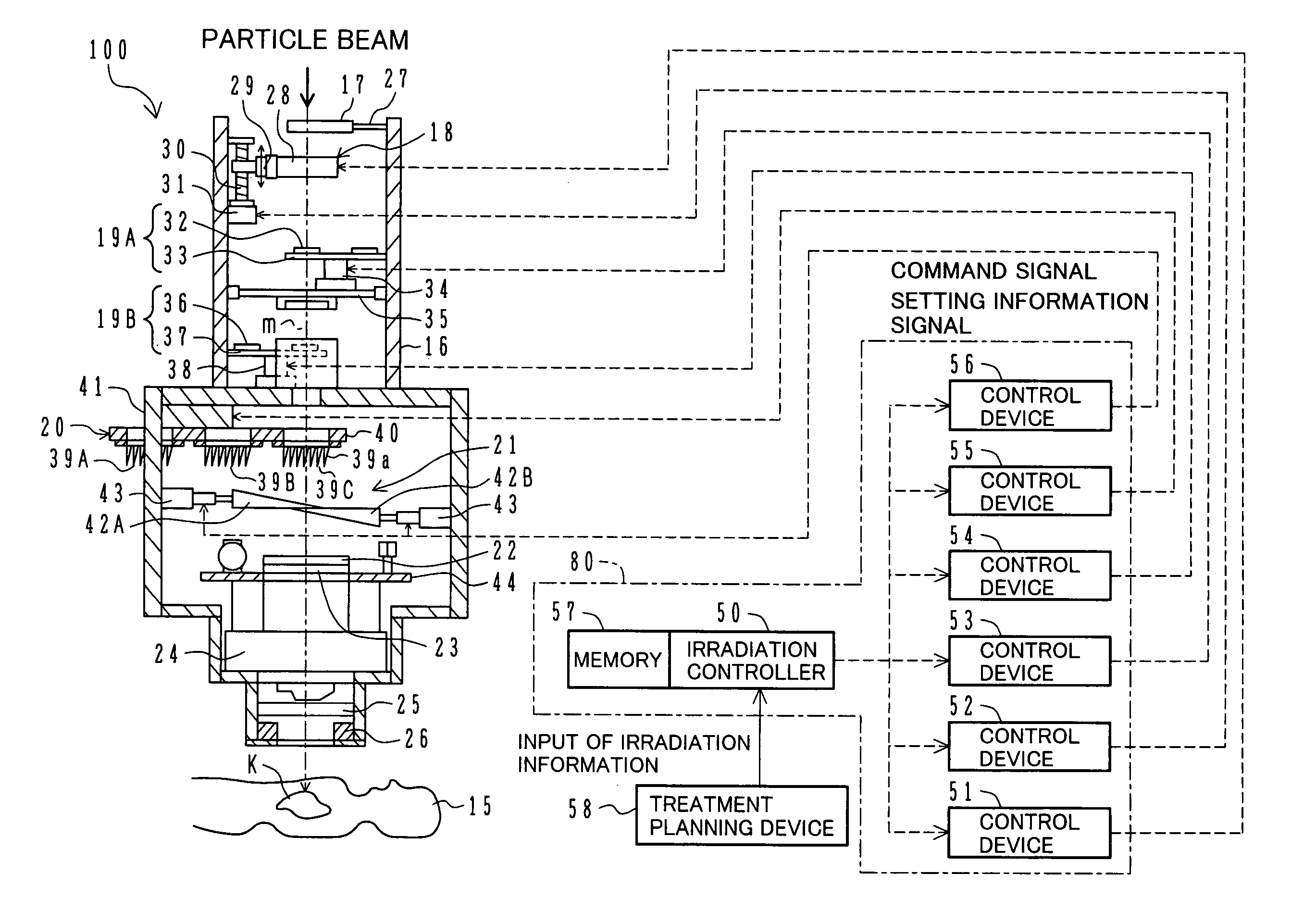
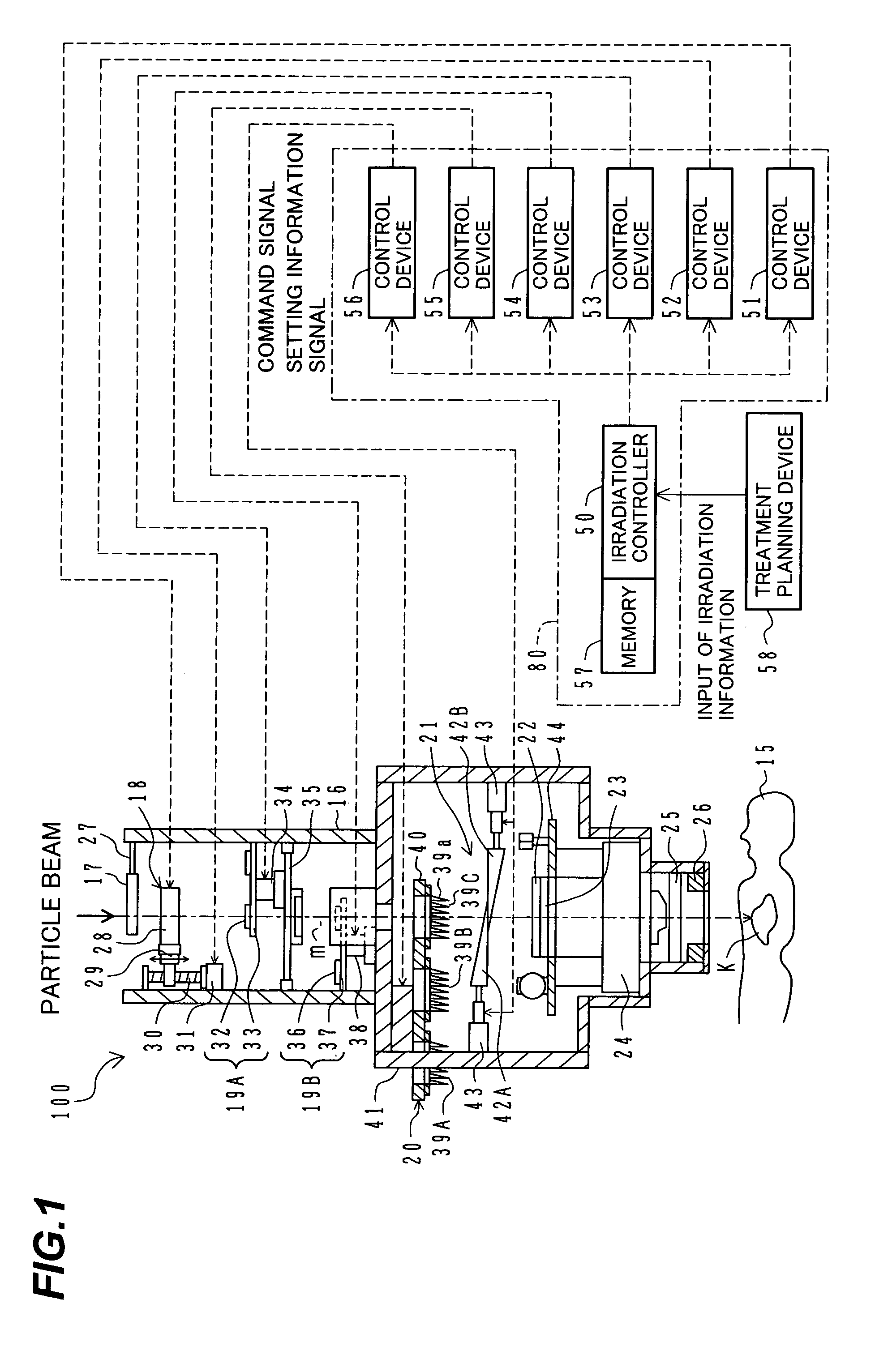
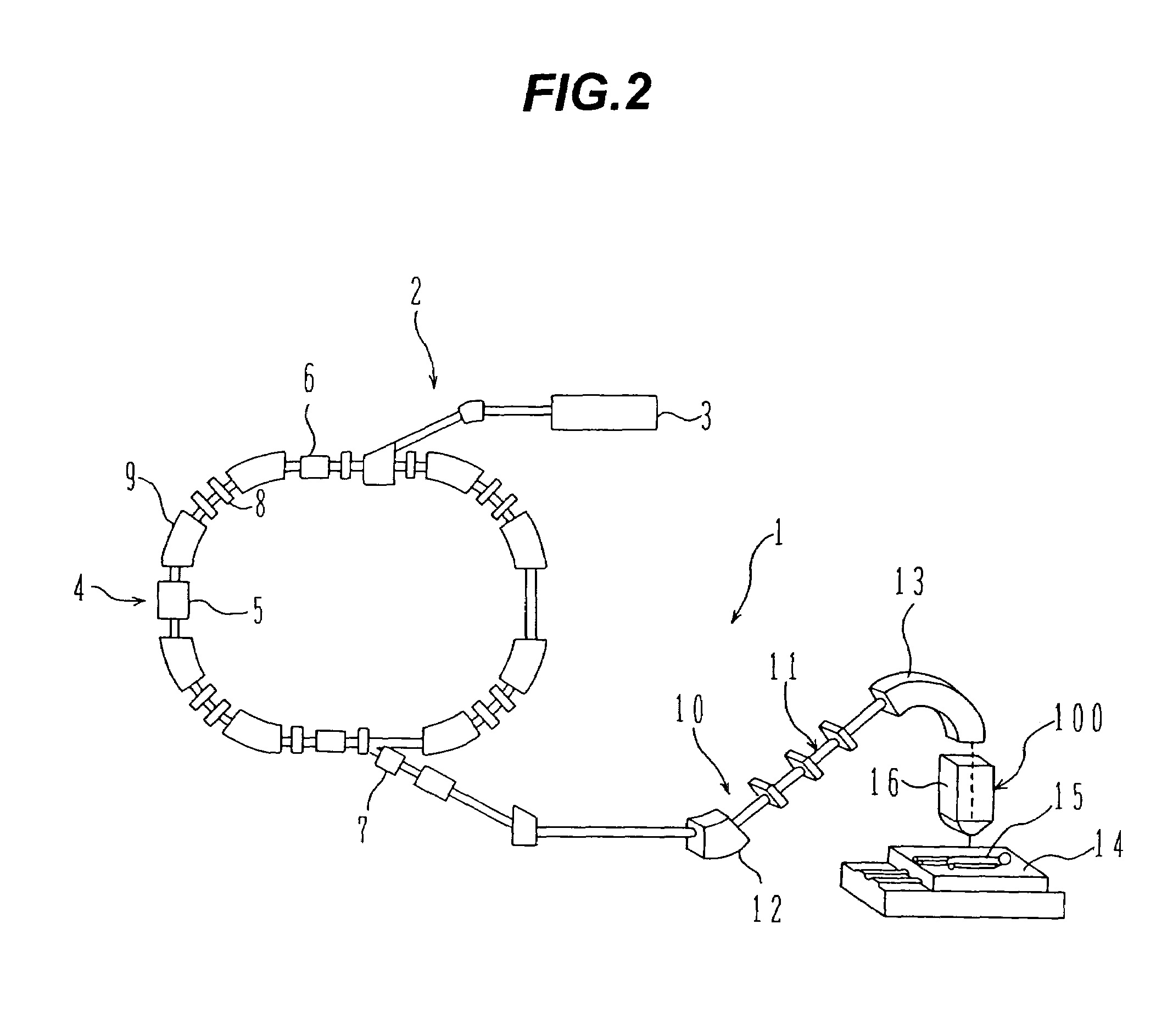
ActiveUS7449701B2Improve dose uniformityLong rangeRadiation/particle handlingElectrode and associated part arrangementsField sizeParticle beam
Particle beam irradiation equipment and a method of adjusting irradiation nozzle, which can ensure a long range and high dose uniformity at any field size are provided. The particle beam irradiation equipment comprises charged particle beam generation equipment and an irradiation nozzle for irradiating a charged particle beam extracted from the charged particle beam generation equipment to an irradiation target. The irradiation nozzle comprises a first scatterer device including a first scatterer for spreading out the charged particle beam into a Gaussian-like distribution, and multiple stages of second scatterer devices including second scatterers for producing a uniform intensity distribution of the charged particle beam having been spread out into a Gaussian-like distribution by the first scatterer. For forming irradiation fields having sizes different from each other, the second scatterer devices are disposed downstream of the first scatterer device in the direction of travel of the charged particle beam at the spacing depending on the difference in the field size.
Owner:HITACHI LTD
Use of umbilical cord blood to treat individuals having a disease, disorder or condition
The present invention provides methods of using cord blood and cord blood-derived stem cells in high doses to treat various conditions, diseases and disorders. The high-dose cord blood and cord blood-derived stem cells have a multitude of uses and applications, including but not limited to, therapeutic uses for transplantation and treatment and prevention of disease, and diagnostic and research uses. In particular, the cord blood or cord blood-derived stem cells are delivered in high doses, e.g., at least 3 billion nucleated cells per treatment, where treatment may comprise a single or multiple infusions. The invention also provides for the use of cord blood or cord blood-derived stem cells from multiple donors without the need for HLA typing.
Owner:CELULARITY INC
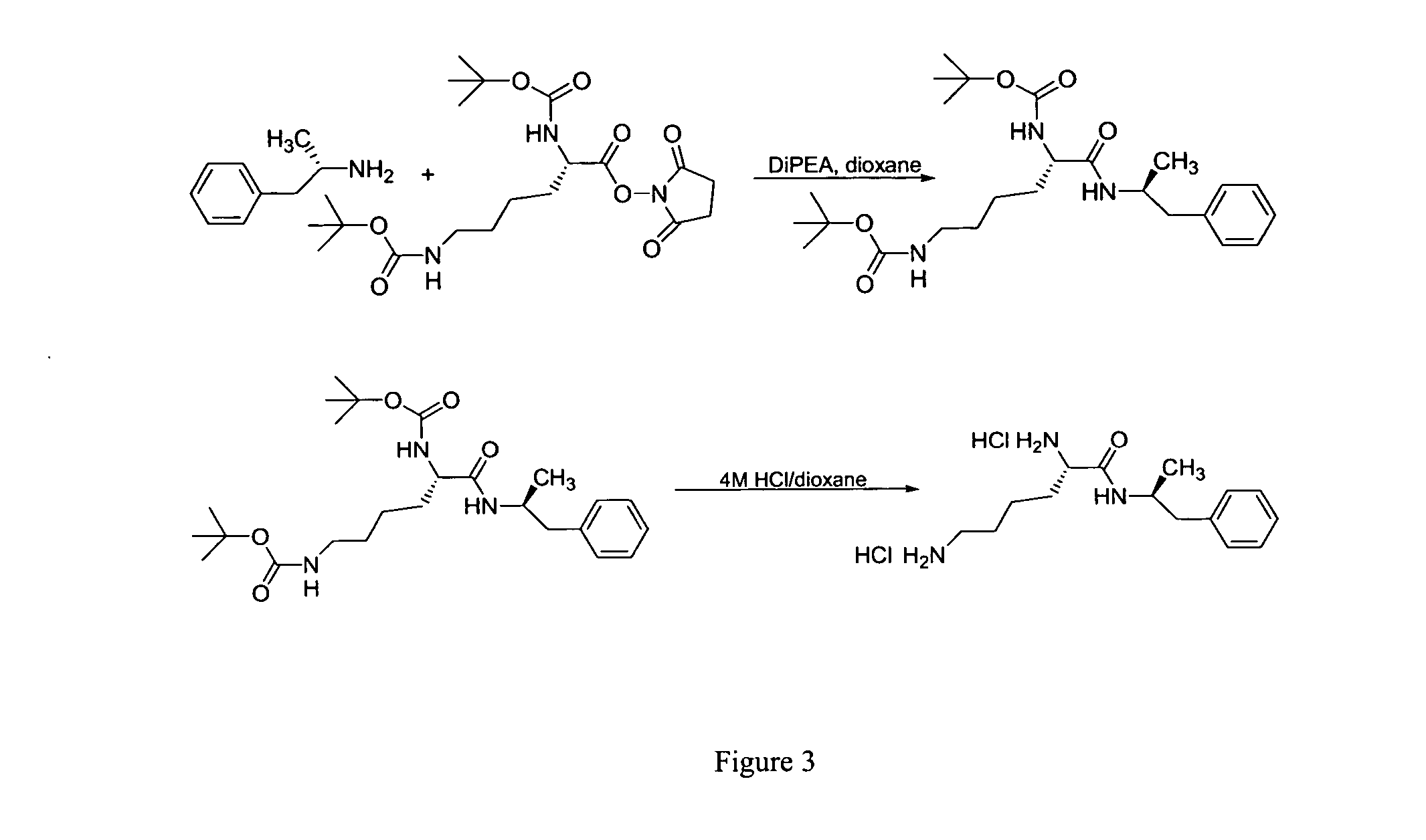
Abuse-resistant amphetamine compounds
InactiveUS7105486B2Reduced activityRelease is diminished and eliminatedOrganic active ingredientsPeptide/protein ingredientsChemical MoietyDisease
The invention describes compounds, compositions and methods of using the same comprising a chemical moiety covalently attached to amphetamine. These compounds and compositions are useful for reducing or preventing abuse and overdose of amphetamine. These compounds and compositions find particular use in providing an abuse-resistant alternative treatment for certain disorders, such as attention deficit hyperactivity disorder (ADHD), ADD, narcolepsy, and obesity. Oral bioavailability of amphetamine is maintained at therapeutically useful doses. At higher doses bioavailability is substantially reduced, thereby providing a method of reducing oral abuse liability. Further, compounds and compositions of the invention decrease the bioavailability of amphetamine by parenteral routes, such as intravenous or intranasal administration, further limiting their abuse liability.
Owner:TAKEDA PHARMA CO LTD
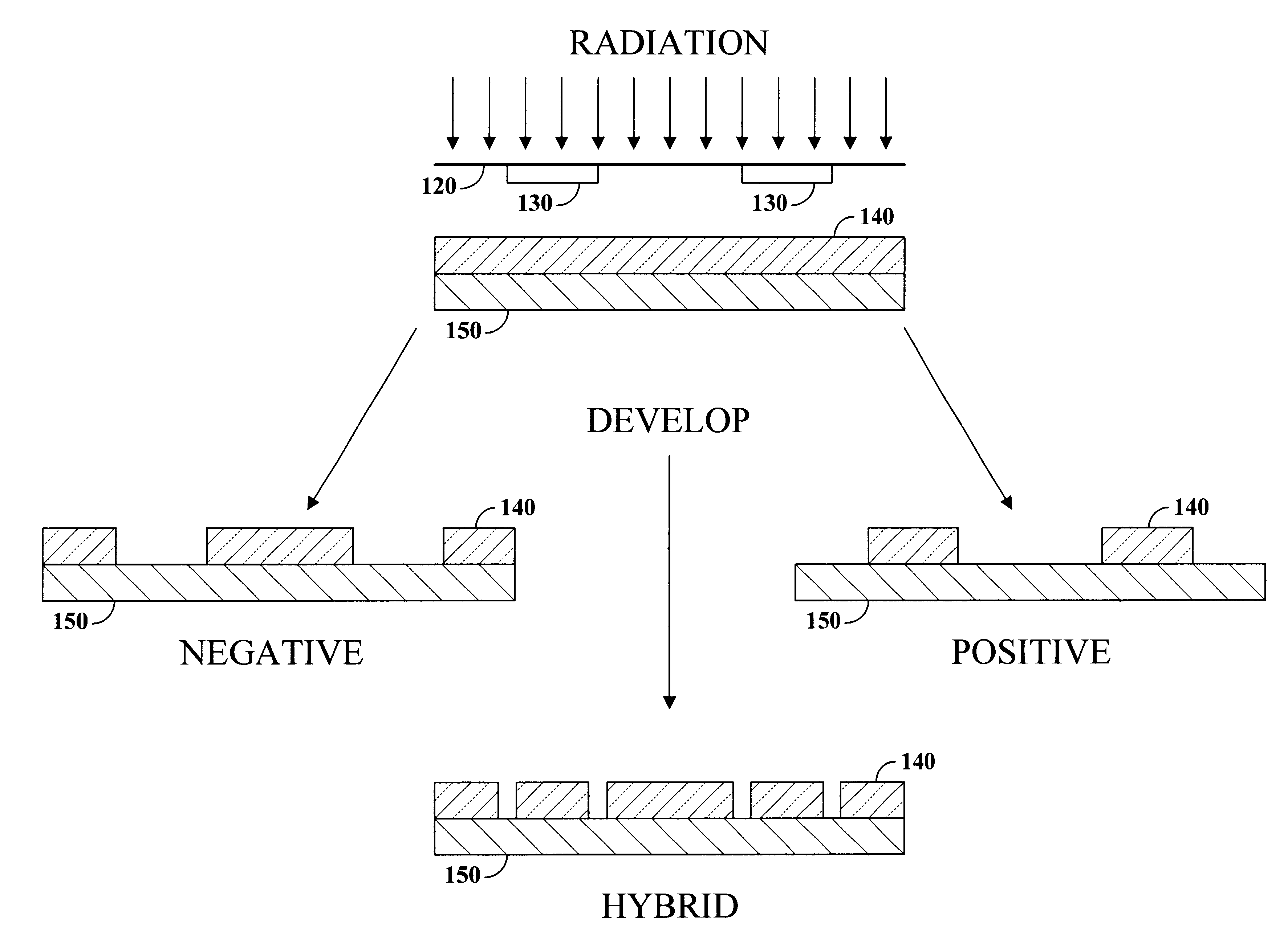
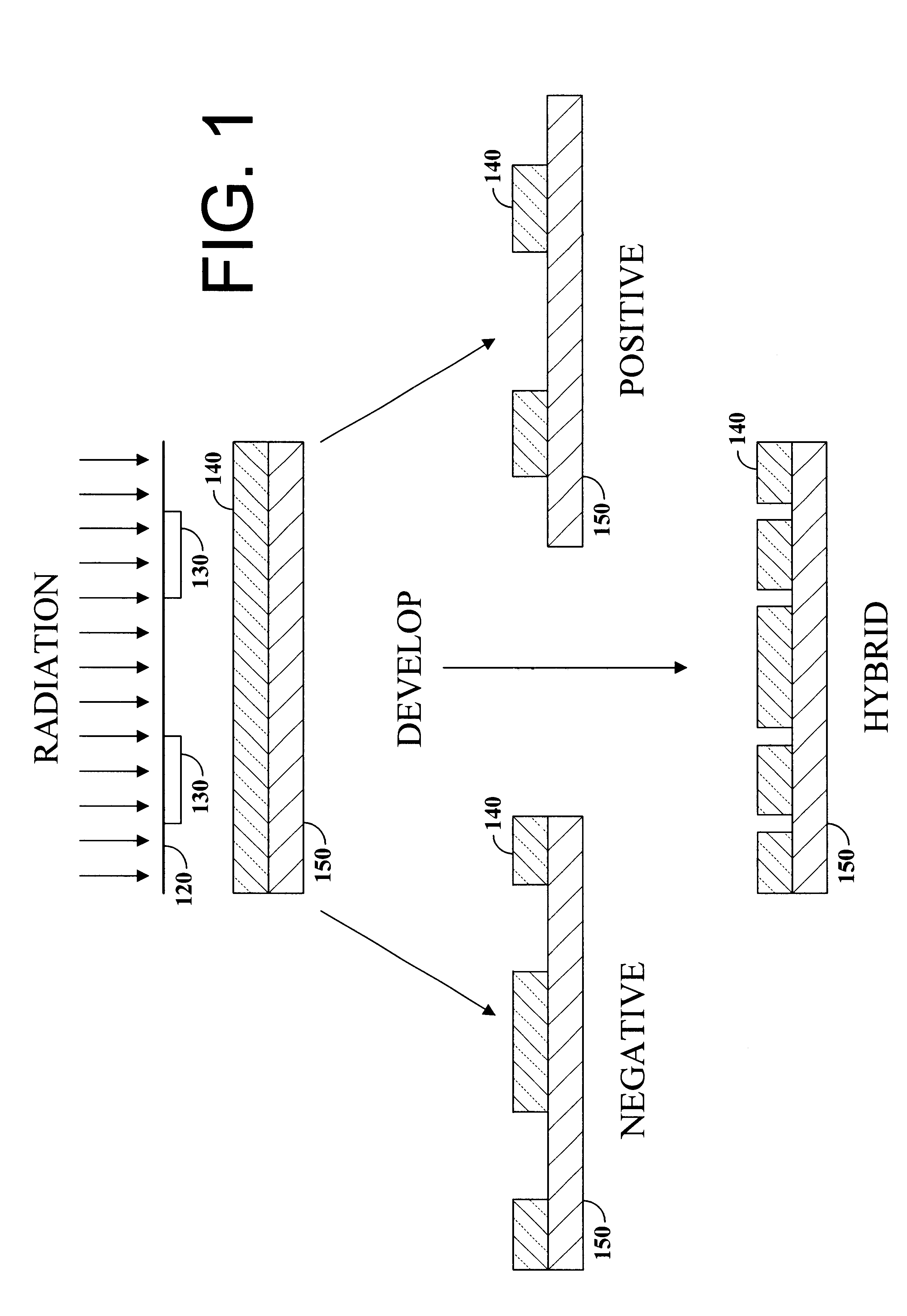
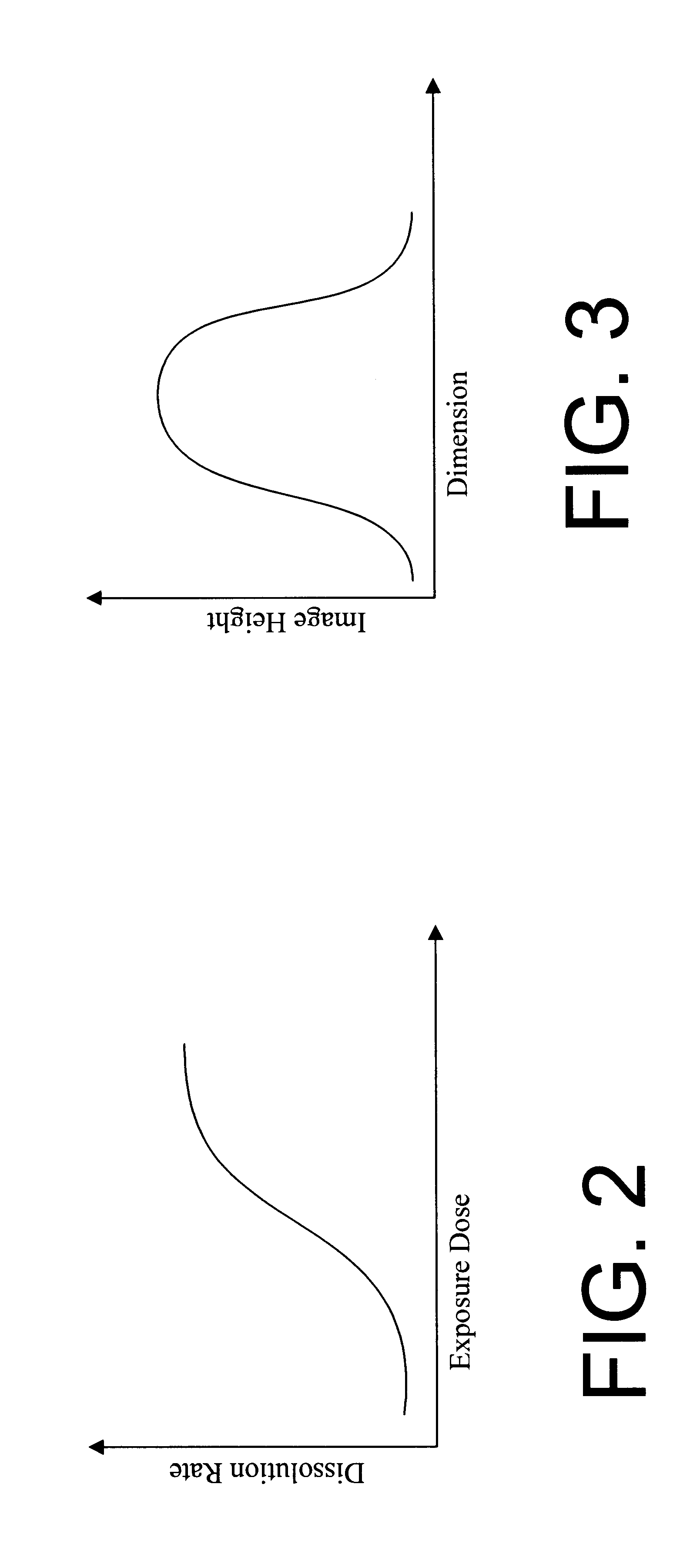
Hybrid resist based on photo acid/photo base blending
A photo resist composition contains a polymer resin, a first photo acid generator (PAG) requiring a first dose of actinic energy to generate a first photo acid, and a photo base generator (PBG) requiring a second dose of actinic energy, different from the first dose, to generate a photo base. The amounts and types of components in the photo resist are selected to produce a hybrid resist image. Either the first photo acid or photo base acts as a catalyst for a chemical transformation in the resist to induce a solubility change. The other compound is formulated in material type and loading in the resist such that it acts as a quenching agent. The catalyst is formed at low doses to induce the solubility change and the quenching agent is formed at higher doses to counterbalance the presence of the catalyst. Accordingly, the same frequency doubling effect of conventional hybrid resist compositions may be obtained, however, either a line or a space may be formed at the edge of an aerial image. Feature size may also be influenced by incorporating a quenching agent into the resist composition that does not require photo generation.
Owner:IBM CORP
Aerosol processing and inhalation method and system for high dose rate aerosol drug delivery
ActiveUS20070144514A1Increase dose rateRisk minimizationRespiratorsLiquid surface applicatorsSolvent vaporCounter flow
A method and system is disclosed which is capable of delivering at a high dose rate, respirable solid aerosols derived from aqueous- or nonaqueous-based solutions containing the desired therapeutic agent(s). The method and system comprises the integration of an aerosol generator, an aerosol evaporator, an aerosol concentrator, and an aerosol flow regulator. The aerosol generator generates 10-30 μm droplets, with a narrow size distribution. The aerosol jet is arrested by a coaxial counter-flow heated air jet, and evaporated rapidly by annular swirling heated air. Most of the air, together with the unwanted solvent vapor, is removed from the aerosol stream during the process of aerosol concentration. The output aerosol carries the dry particles to be inhaled by the patient. The respiratory-governed control of aerosol fluid generation system delivers fluid containing the test agent of interest (drug or toxin) to the aerosol generator throughout inhalation.
Owner:KAER BIOTHERAPEUTICS CORP
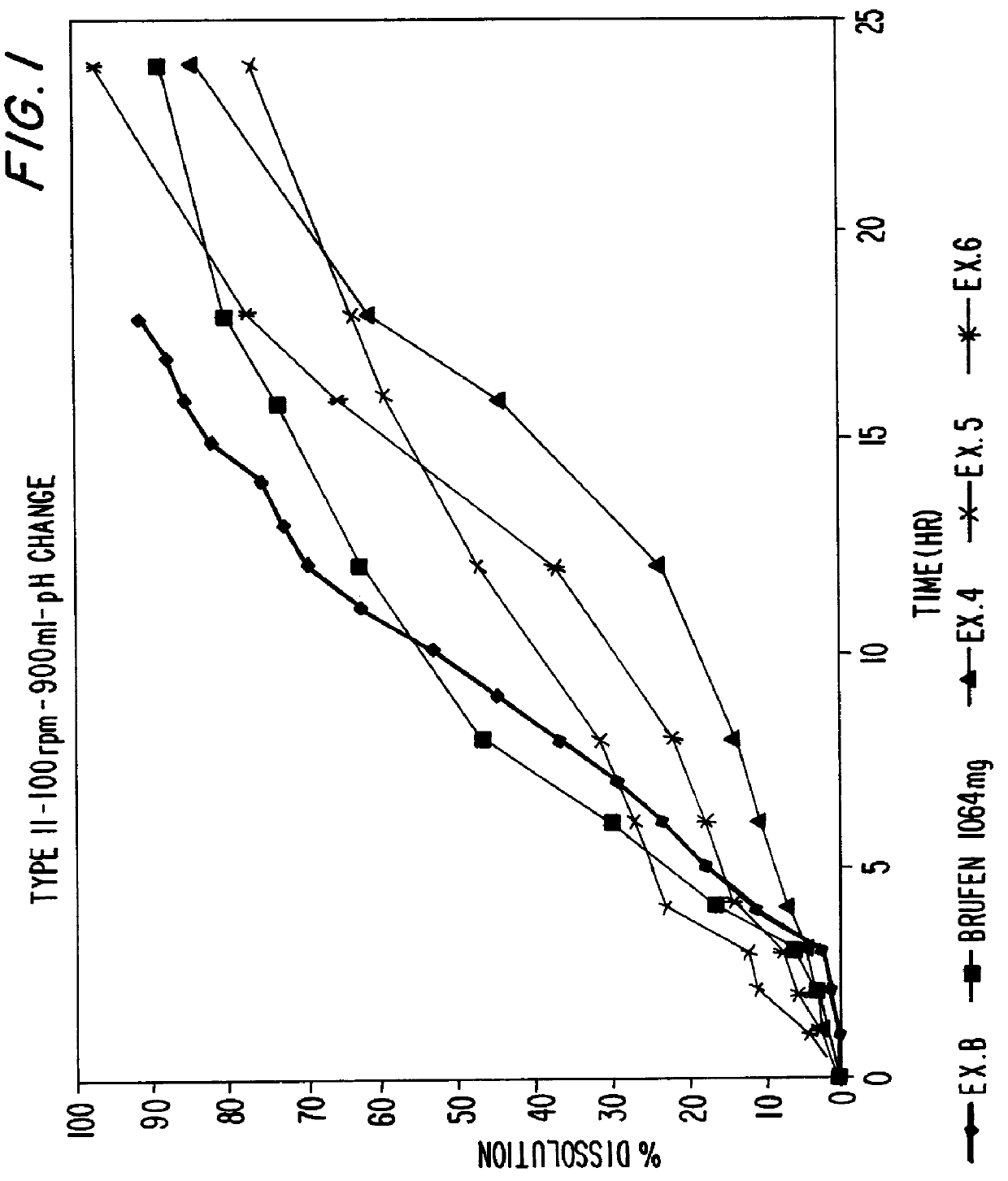
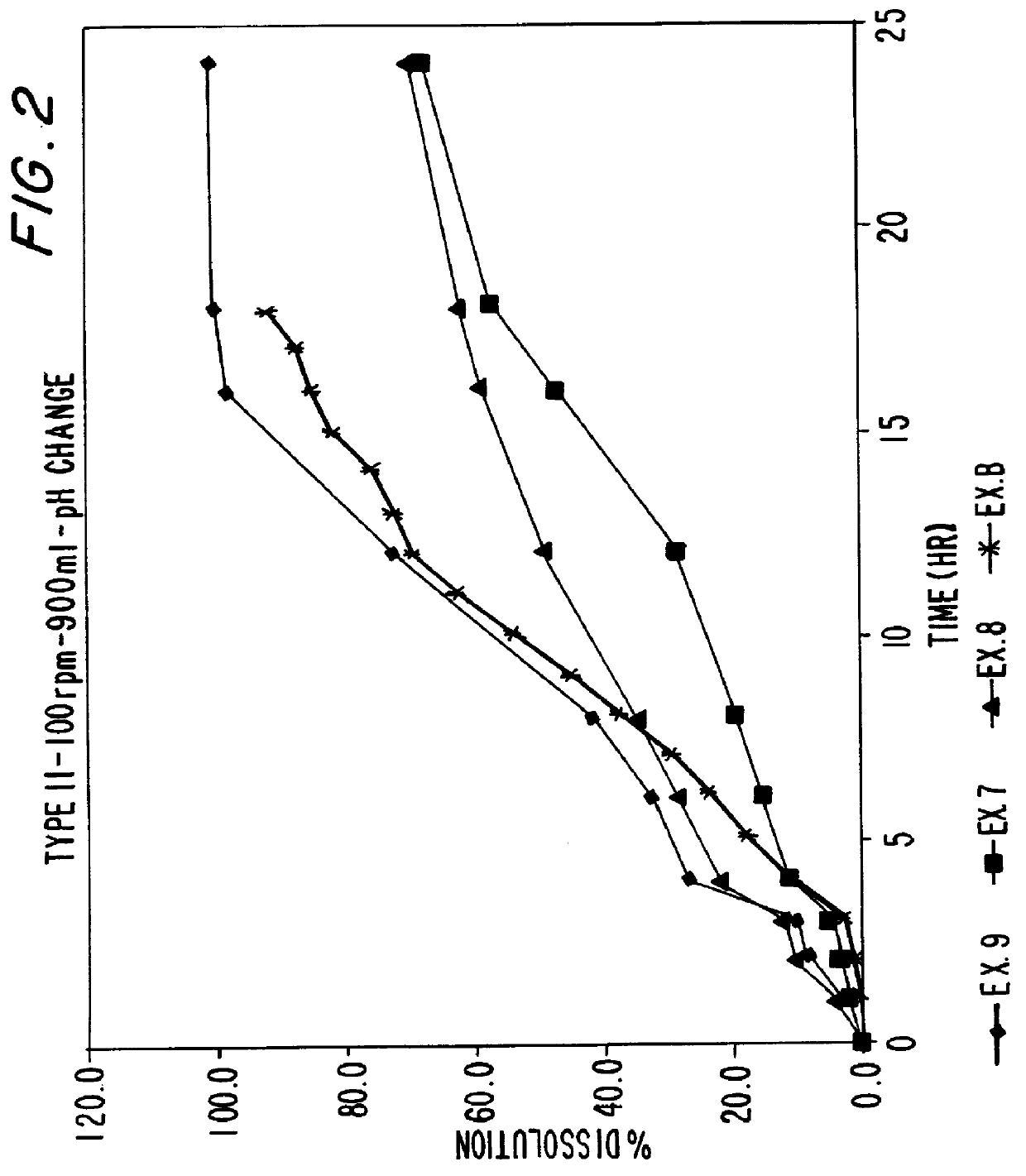
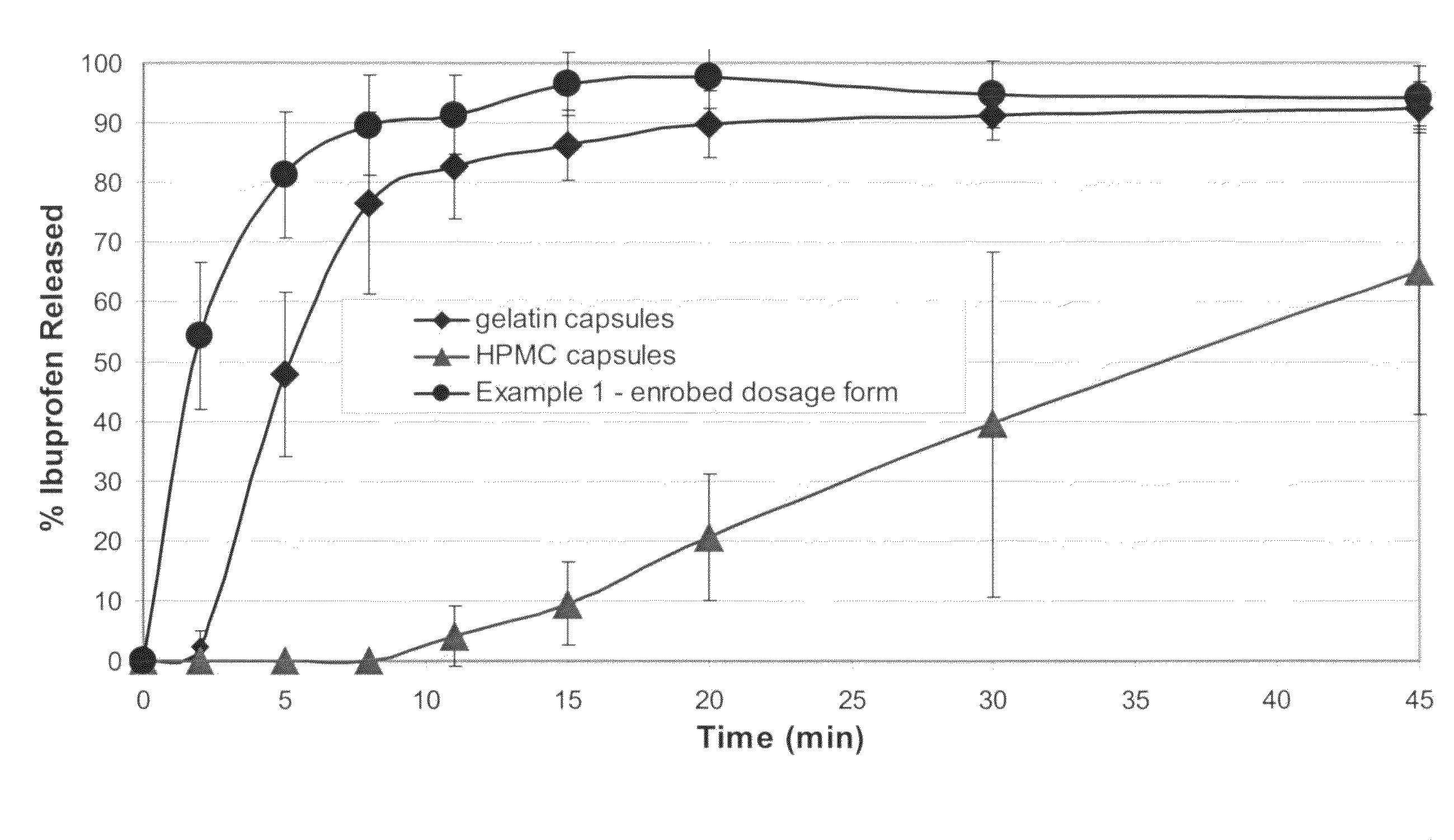
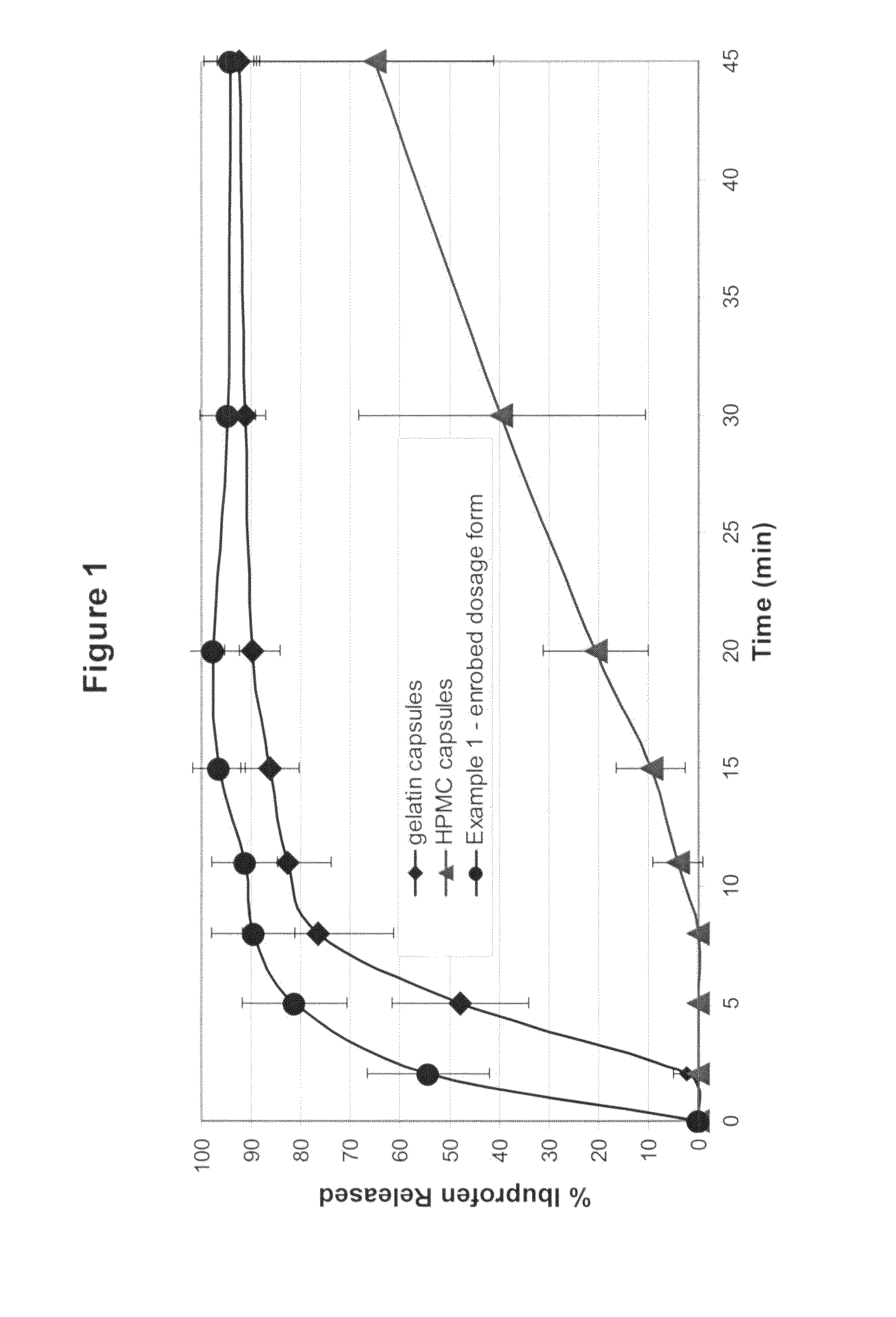
Sustained release matrix for high-dose insoluble drugs
Sustained release dosage forms of high dose insoluble drugs such as ibuprofen and methods for their manufacture are disclosed.
Owner:PENWEST PHARMA CO
System and method for high dose rate radiation intracavitary brachytherapy
InactiveUS20060116546A1Even doseEasy to installX-ray/gamma-ray/particle-irradiation therapyHigh dosesAdemetionine
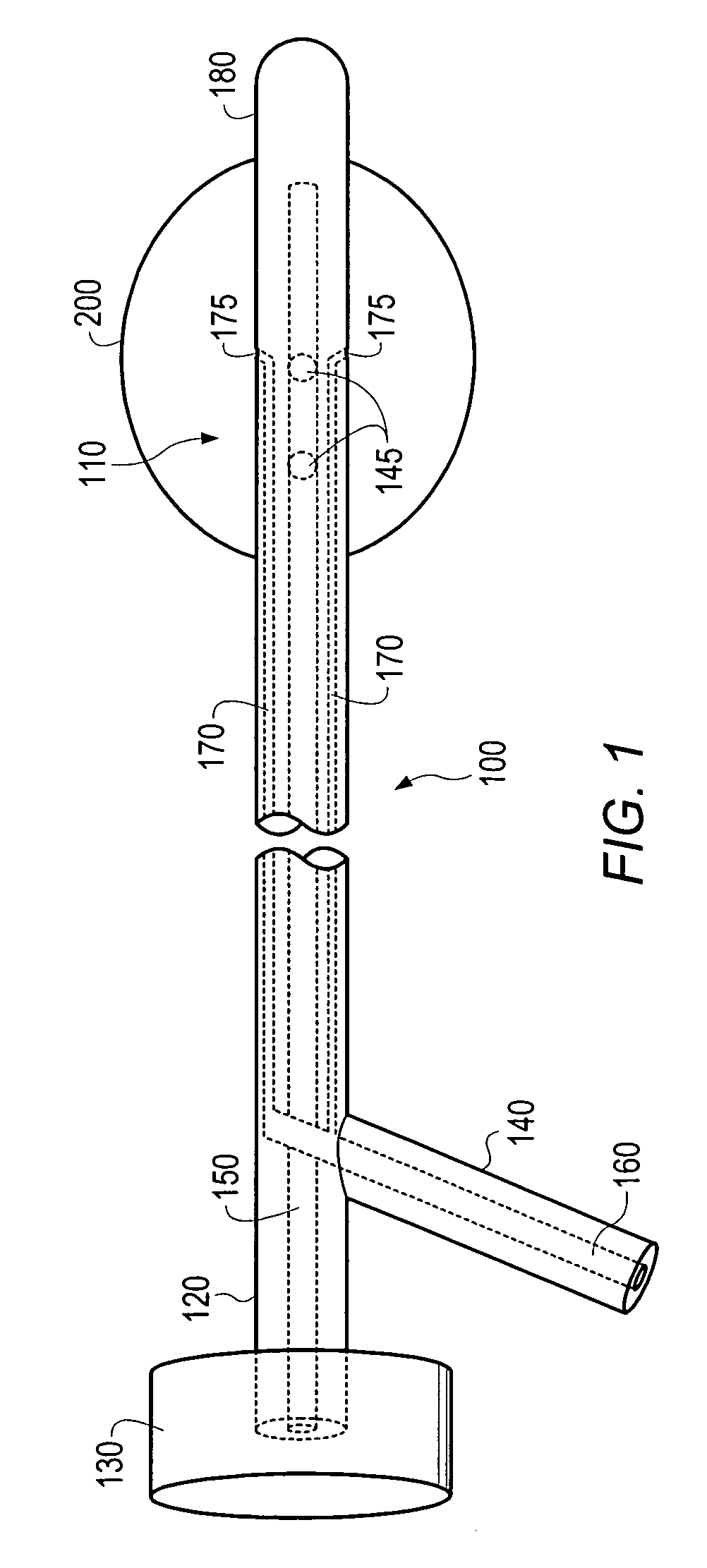
The apparatus, method and system for performing a high dose rate brachytherapy procedure in a body cavity may include an at least partially indwelling multi-lumen catheter with a plurality of inflatable balloons coupled thereto. A lumen may be adapted to receive radiation seeds. The balloons, when inflated, may be adapted to administer a substantially uniform radiation isodose to the body cavity. Some embodiments may be well suited to performing intrauterine brachytherapy for the treatment of endometrial carcinoma.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Resorbable polymeric device for localized drug delivery
InactiveUS20050177118A1Good adhesionMinimizes procedural discomfortMedical devicesMammal material medical ingredientsOsteoblastHigh doses
An implantable device for facilitating the healing of voids in bone, cartilage and soft tissue is disclosed. A preferred embodiment includes a cartilage region comprising a polyelectrolytic complex joined with a subchondral bone region. The cartilage region, of this embodiment, enhances the environment for chondrocytes to grow articular cartilage; while the subchondral bone region enhances the environment for cells which migrate into that region's macrostructure and which differentiate into osteoblasts. Another embodiment is arranged for the local delivery of therapeutic agent. A preferred embodiment is a porous resorbable implant, wherein the therapy delivery may be localized in nature, rather than systemic, such that higher doses at the target site may be allowed than would be tolerable by the body systemically.
Owner:KENSEY NASH CORP
Direct compression metformin hydrochloride tablets
InactiveUS6117451AGood compressibilityImproved flowabilityPowder deliveryBiocideMetformin HydrochlorideHigh doses
Metformin Hydrochloride (herein referred to as metformin HCl) that may be 98.5%-100% pure is a high dose drug capable of being directly compressed with specific excipients into tablets having desired, hardness, disintegrating ability, and acceptable dissolution characteristics. Metformin HCl is not inherently compressible and thus presents formulation problems. Excipients used in the formulation enhance the flow and compaction properties of the drug and tableting mix. Optimal flow contributes to uniform die fill and weight control. The binder used ensures sufficient cohesive properties that allow metformin HCl to be compressed using the direct compression method. The tablets produced provide an acceptable in-vitro dissolution profile.
Owner:PHARMALOGIX
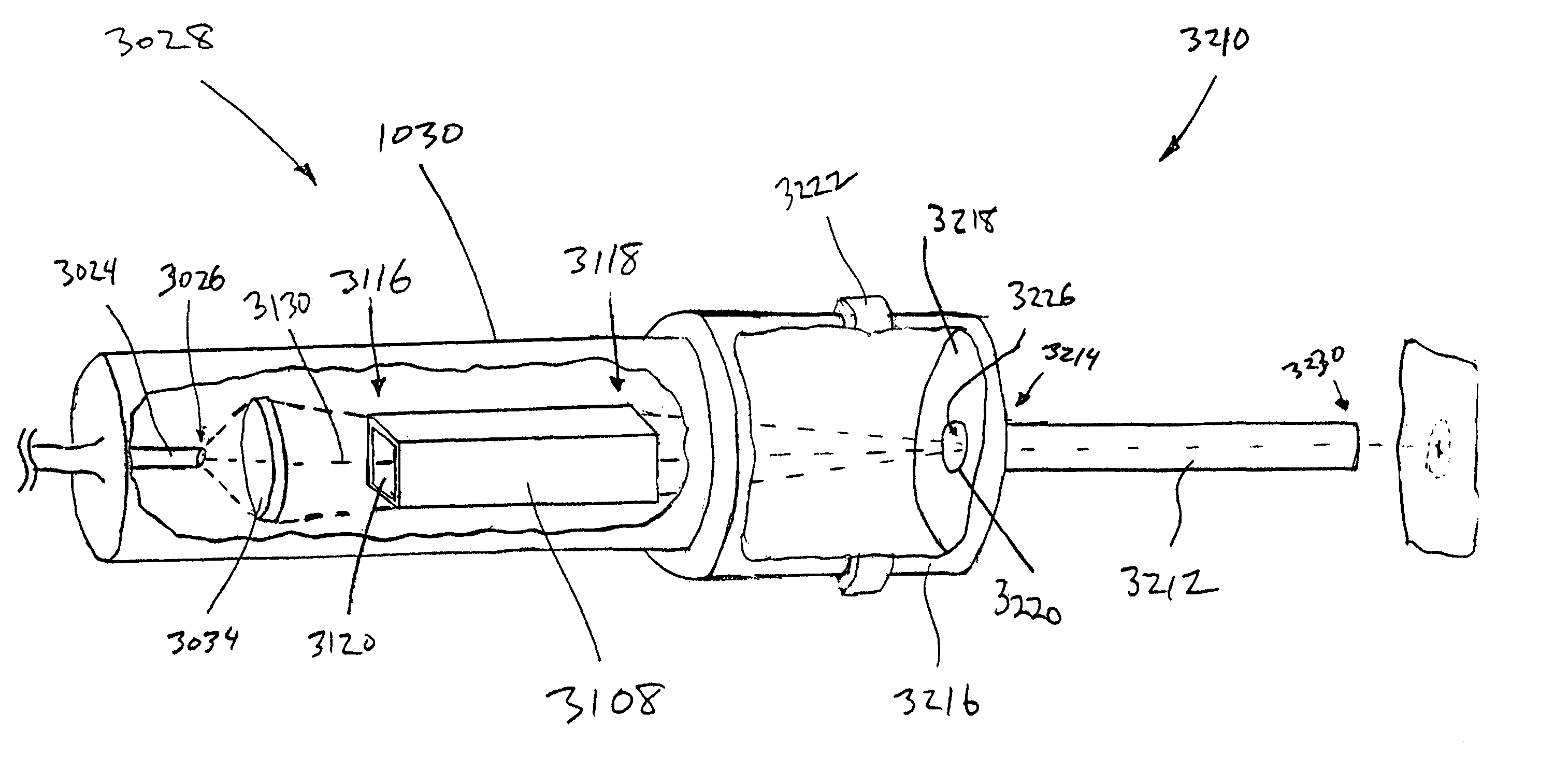
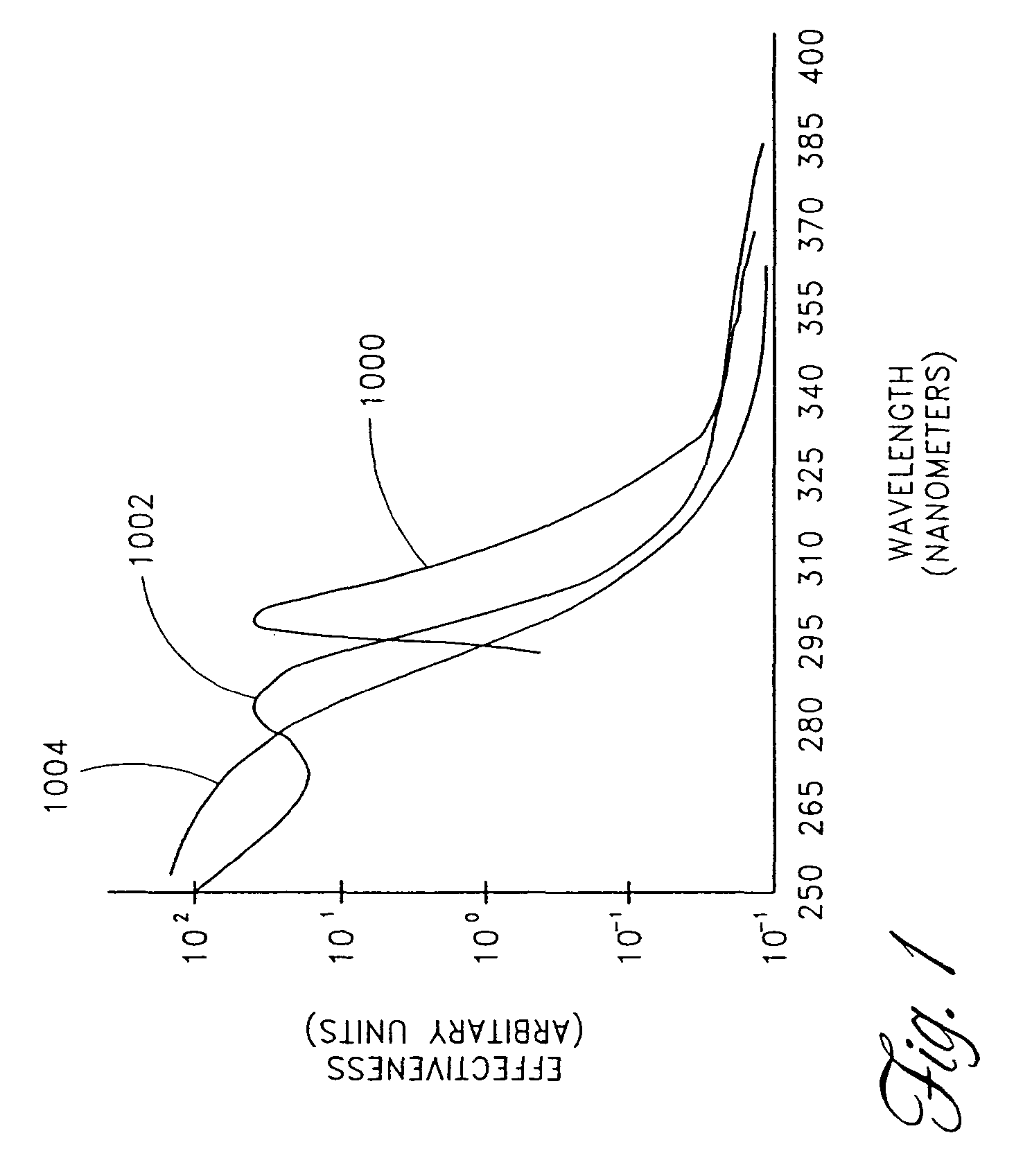
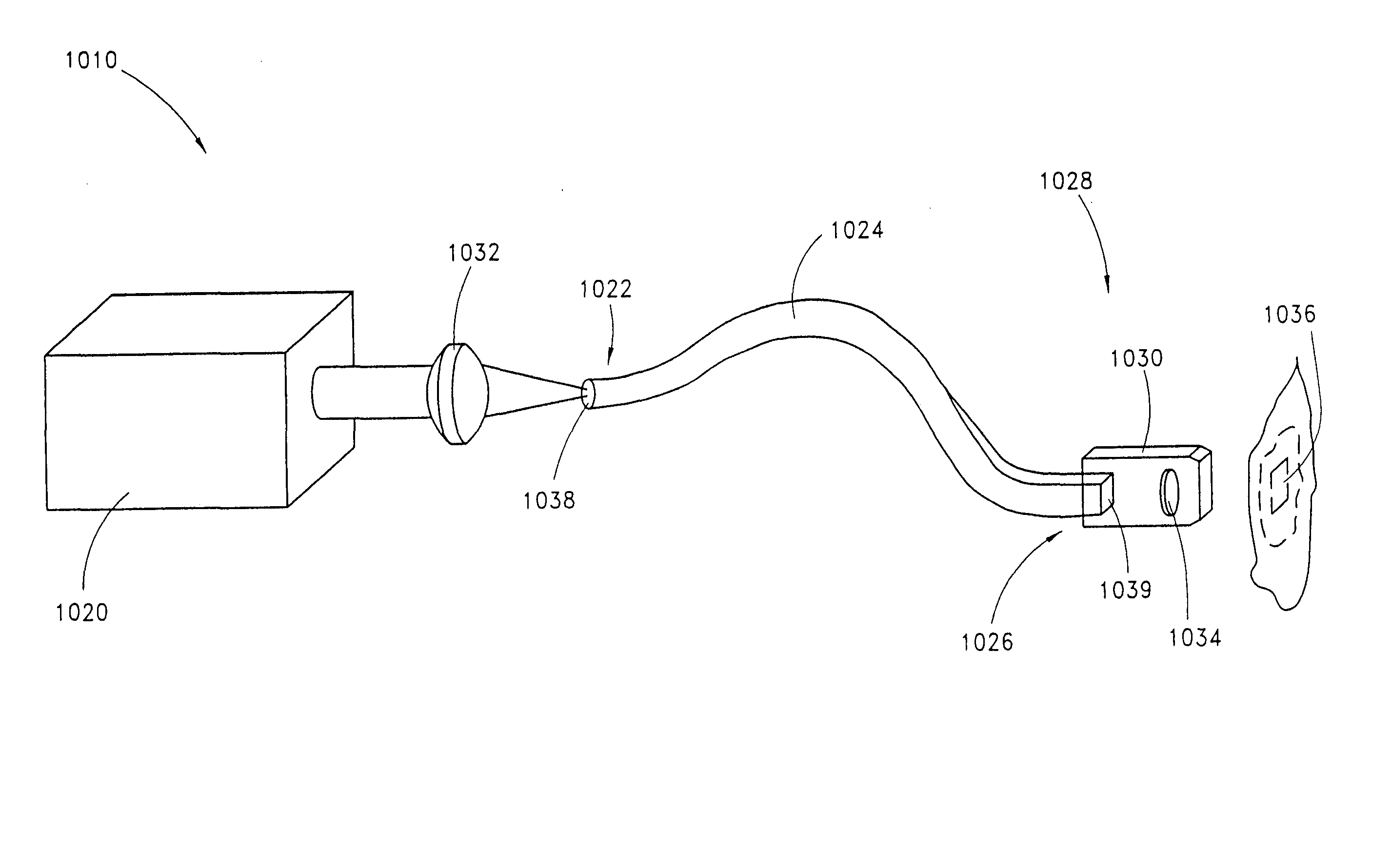
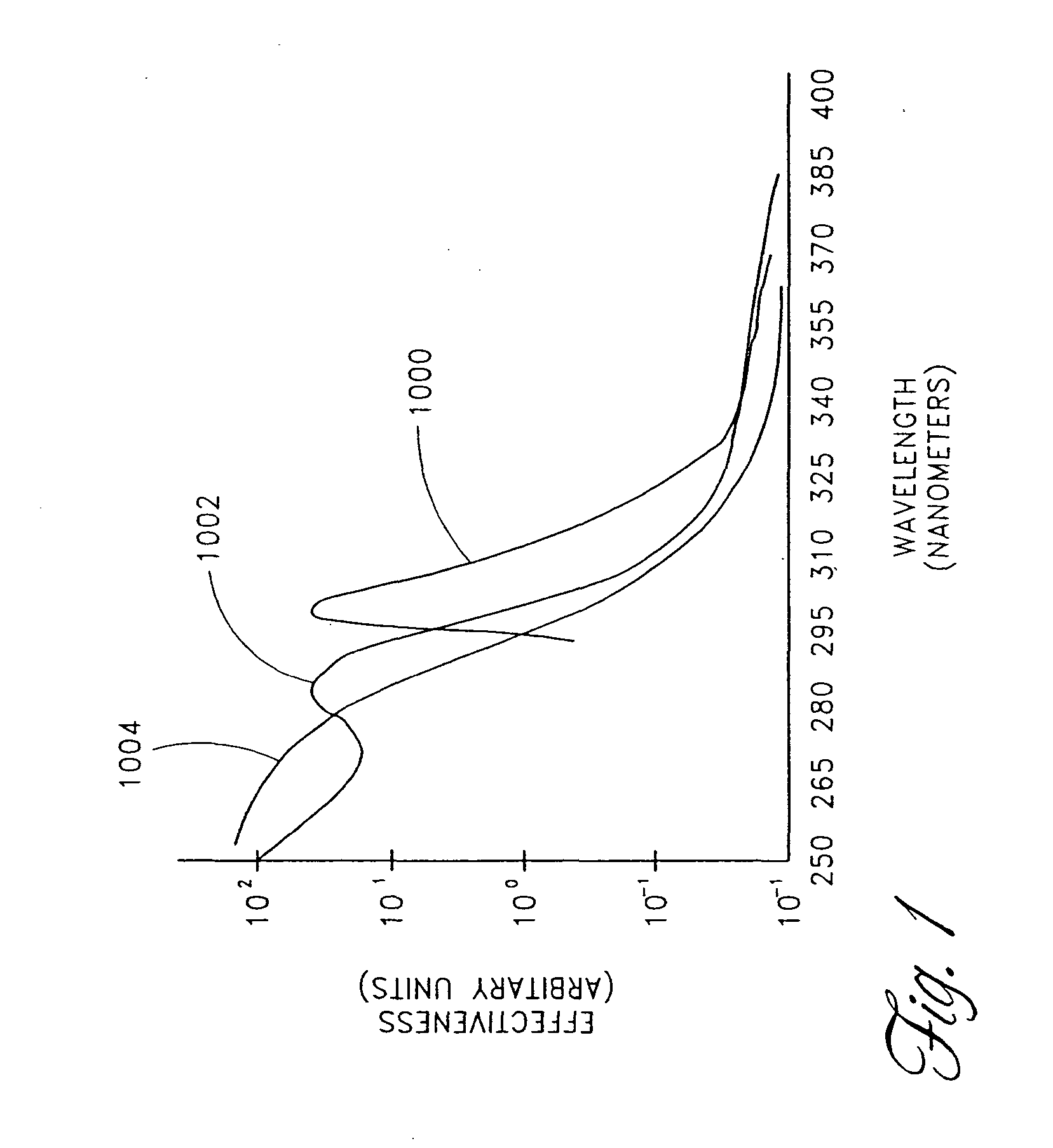
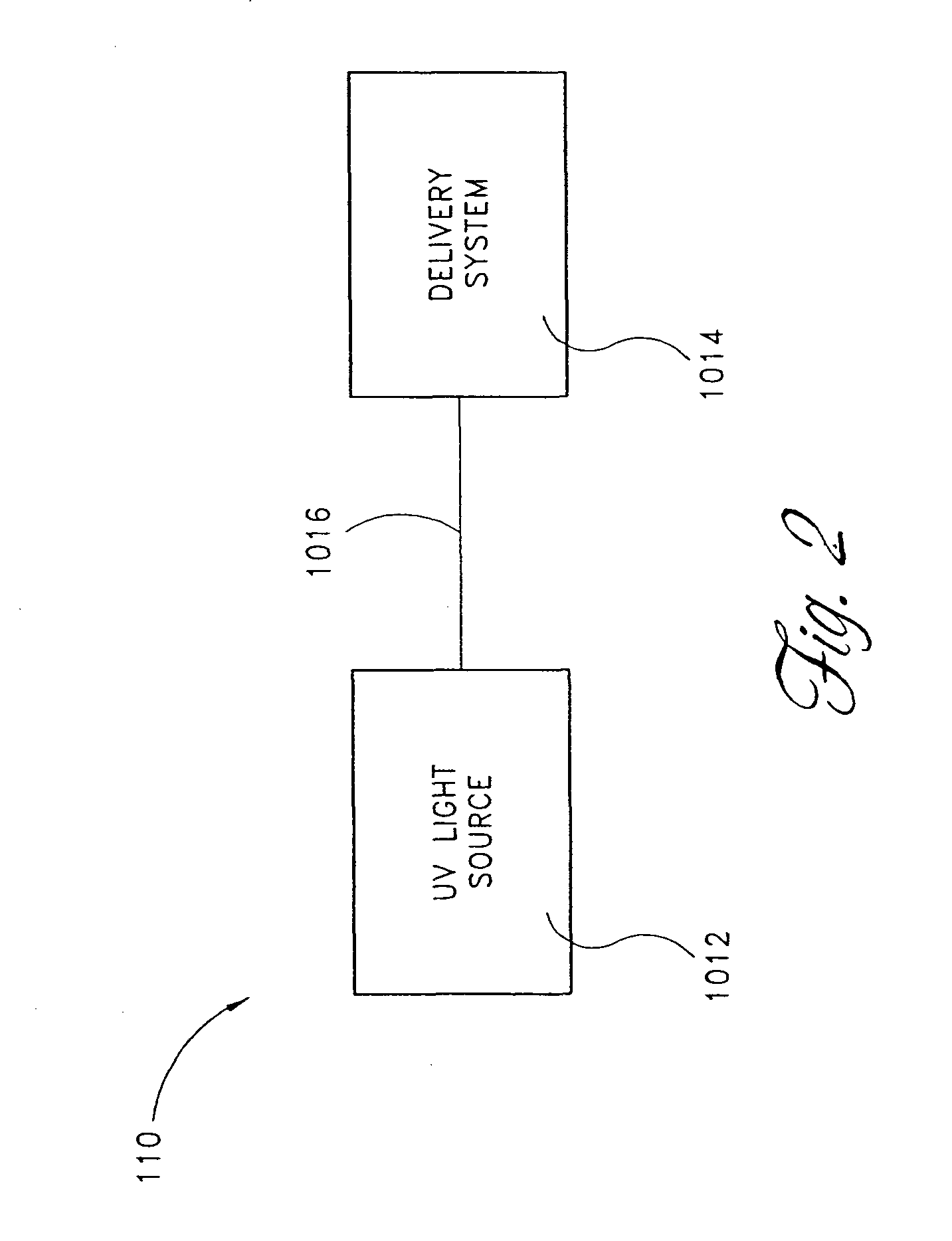
Device for oral UV photo-therapy
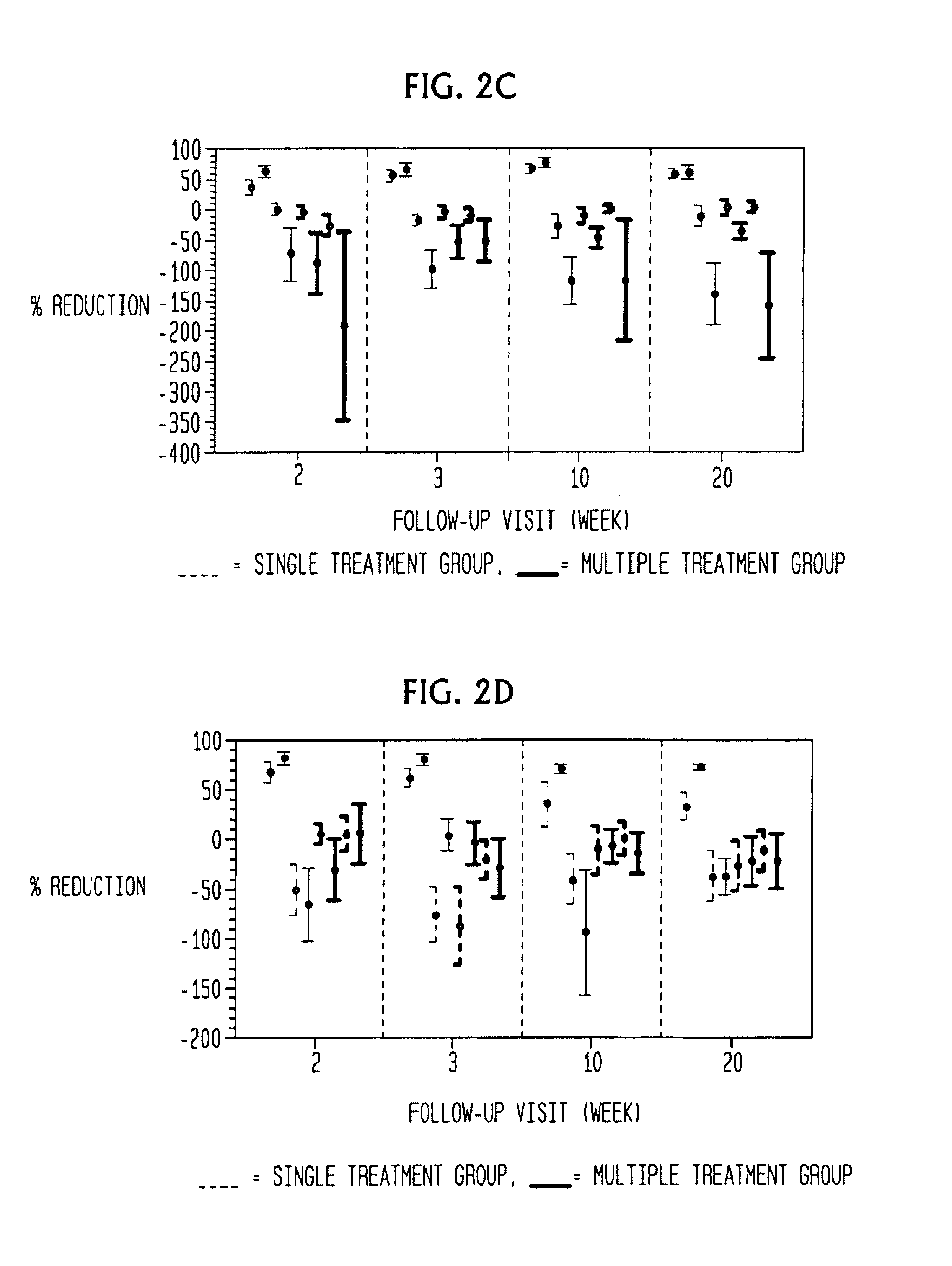
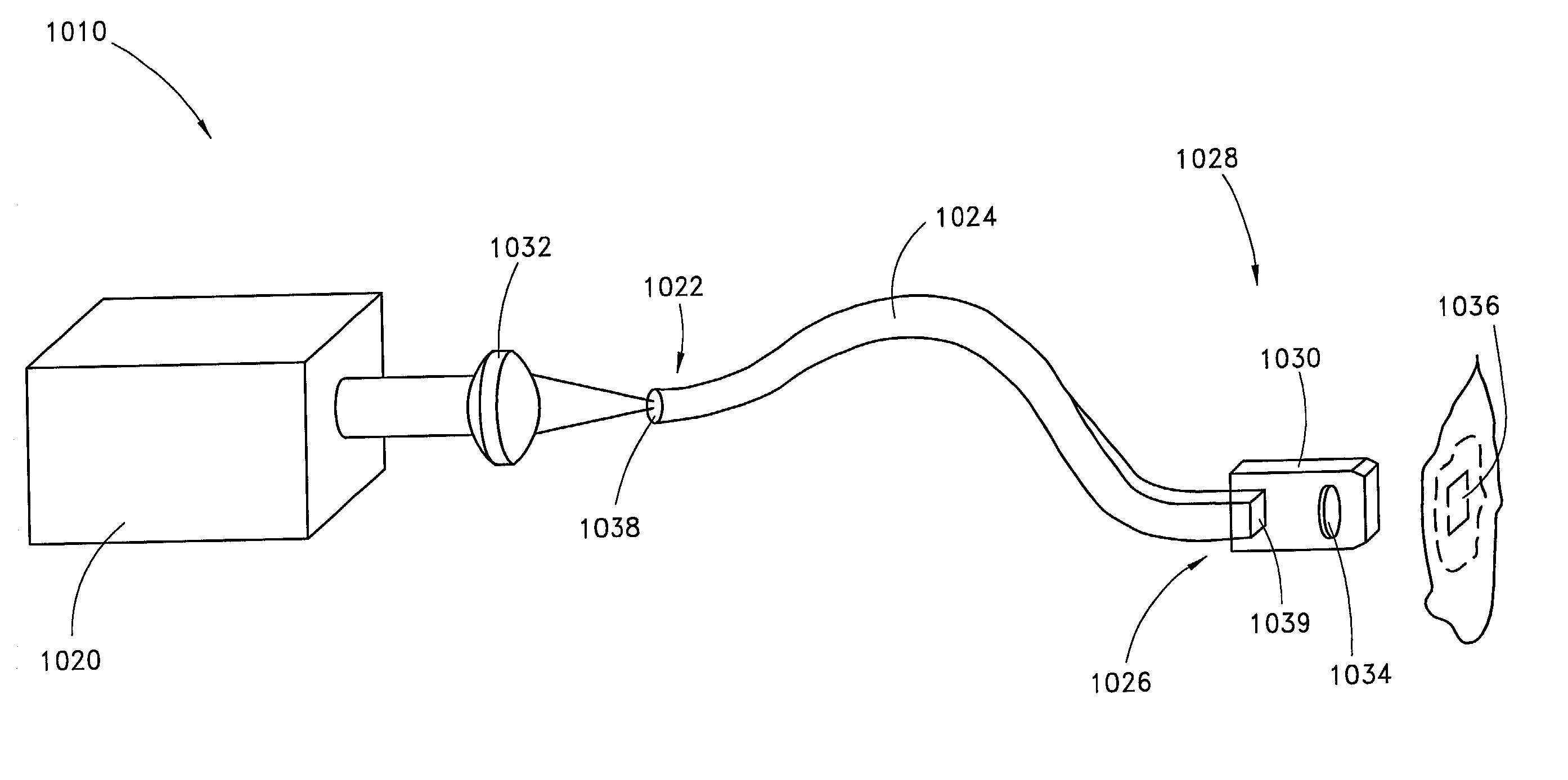
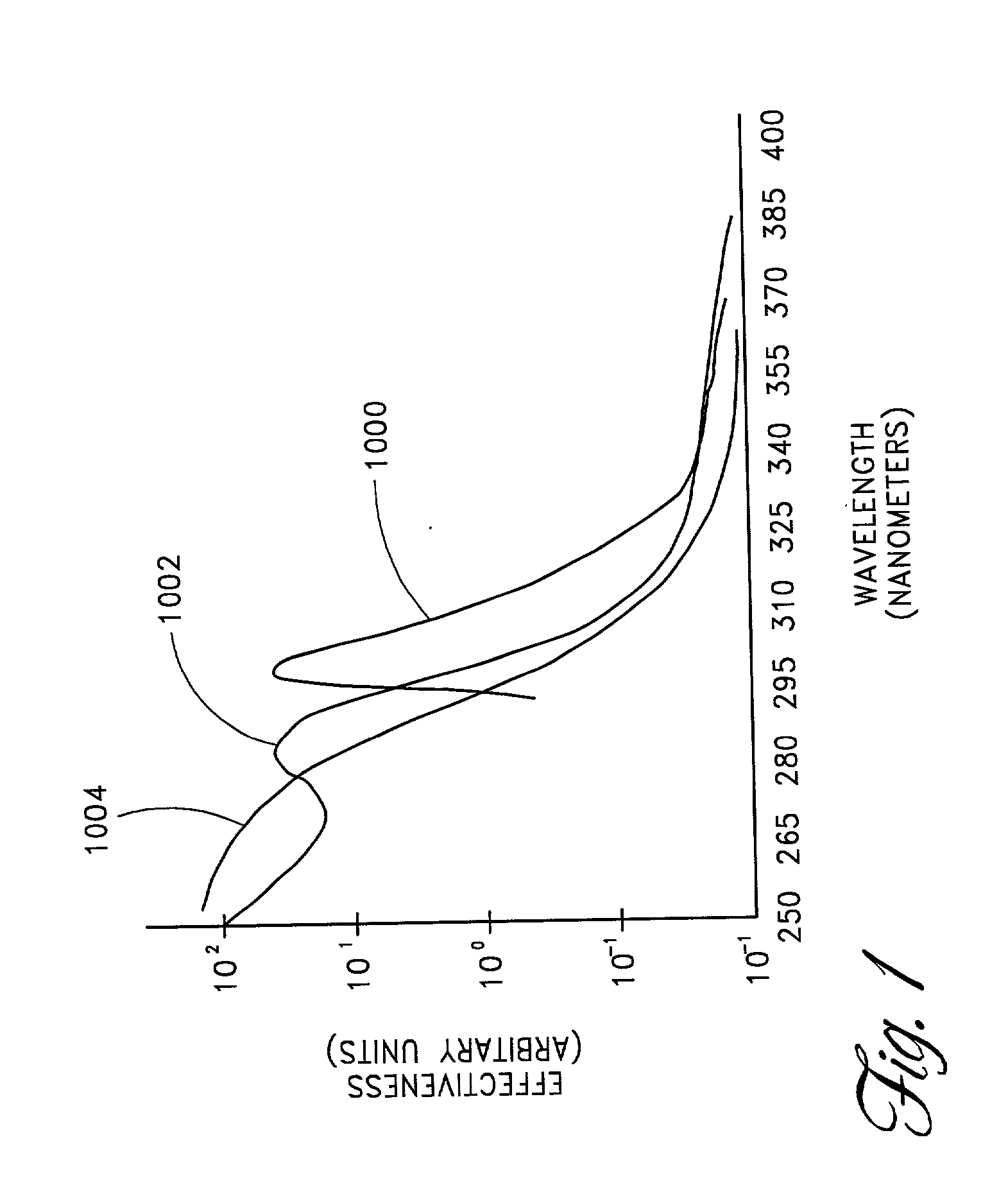
Skin disorders such as, for example, atopic dermatitis, dyshidrosis, eczema, lichen planus, psoriasis, and vitiligo, are treated by applying high doses of ultraviolet light to diseased regions of a patient's skin. The dosage exceeds 1 MED as determined for the particular patient and may range from about 1 MED to about 20 MED or higher. The ultraviolet light has a wavelength within the range of about 295 nanometers to about 320 nanometers. High doses of ultraviolet light are preferably restricted to diseased tissue areas. A specialized handpiece provides a beam profile especially suitable for application of controlled doses. A specialized delivery device is useful for UV treatment of tissue within the mouth.
Owner:MELA SCIENCES
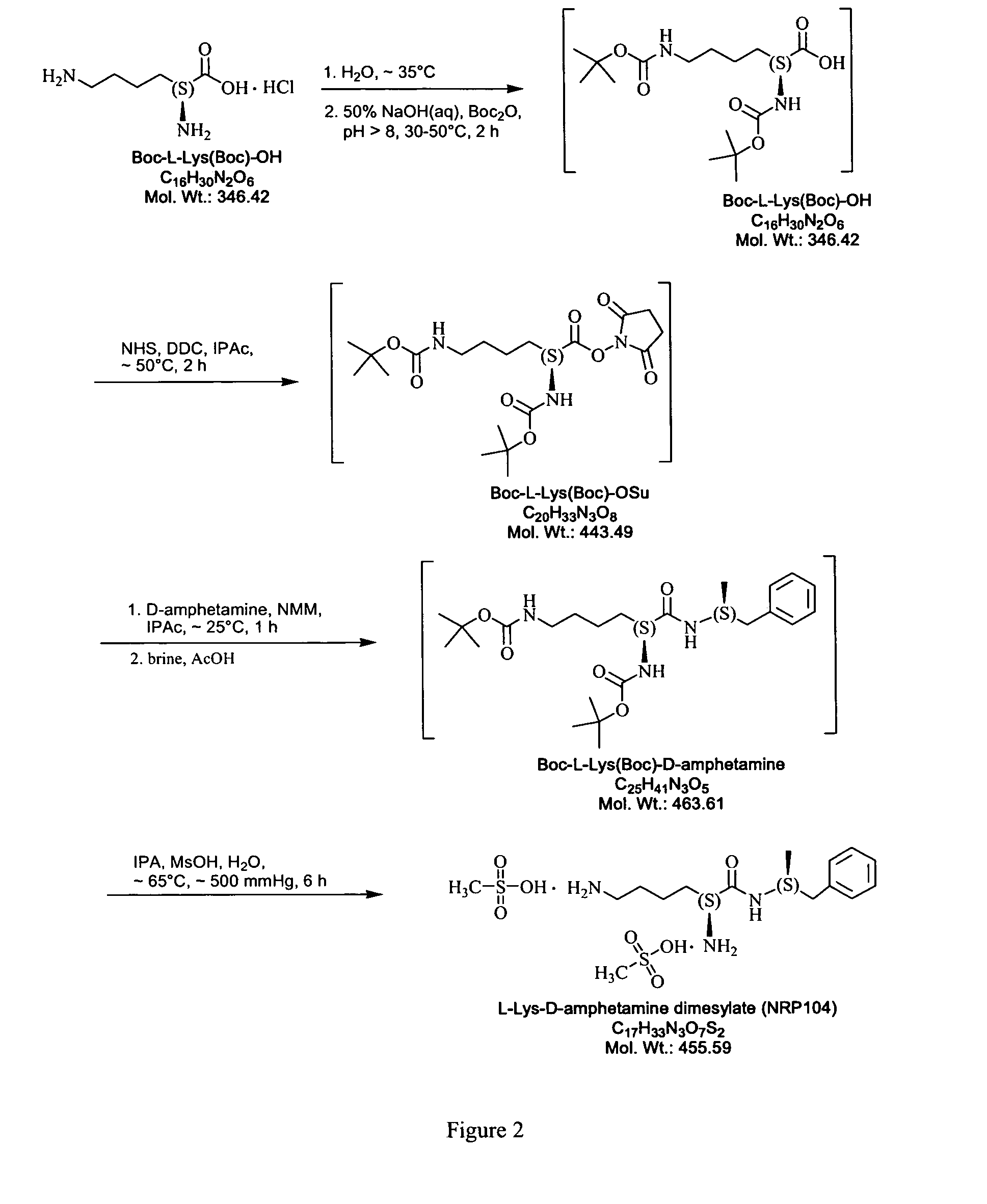
Abuse resistant lysine amphetamine compounds
ActiveUS20050038121A1Prevents euphoriaLower potentialOrganic active ingredientsBiocideDiseaseAlternative treatment
The present invention describes compounds, compositions and methods of using the same comprising lysine covalently attached to amphetamine. These compounds and compositions are useful for reducing or preventing abuse and overdose of amphetamine. These compounds and compositions find particular use in providing an abuse-resistant alternative treatment for certain disorders, such as attention deficit hyperactivity disorder (ADHD), ADD, narcolepsy, and obesity. Oral bioavailability of amphetamine is maintained at therapeutically useful doses. At higher doses bioavailability is substantially reduced, thereby providing a method of reducing oral abuse liability. Further, compounds and compositions of the invention decrease the bioavailability of amphetamine by parenteral routes, such as intravenous or intranasal administration, further limiting their abuse liability.
Owner:TAKEDA PHARMA CO LTD
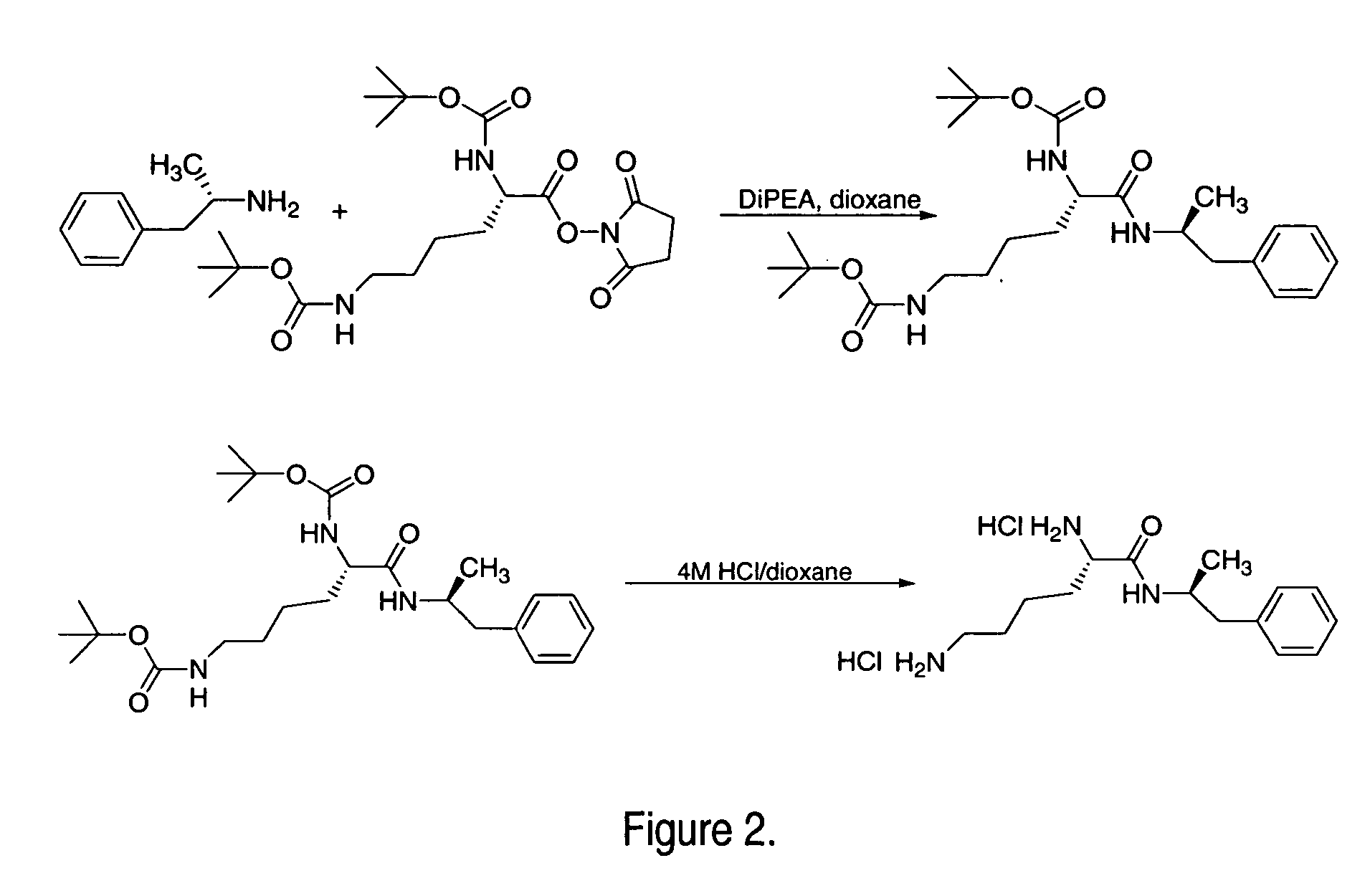
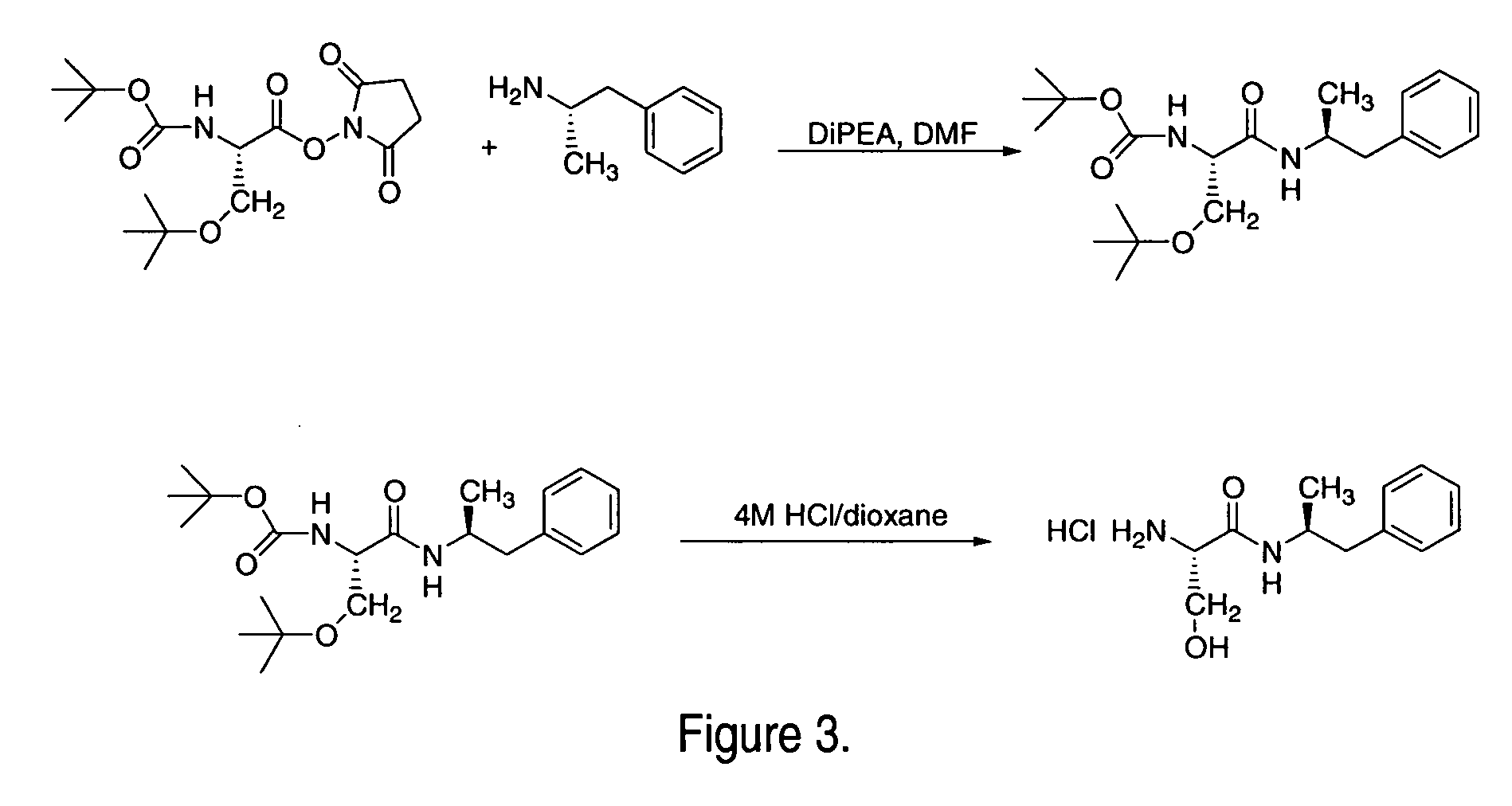
Solid form
InactiveUS20080014228A1Reduce deliveryImprove robustnessCosmetic preparationsNervous disorderFilling materialsImmediate release
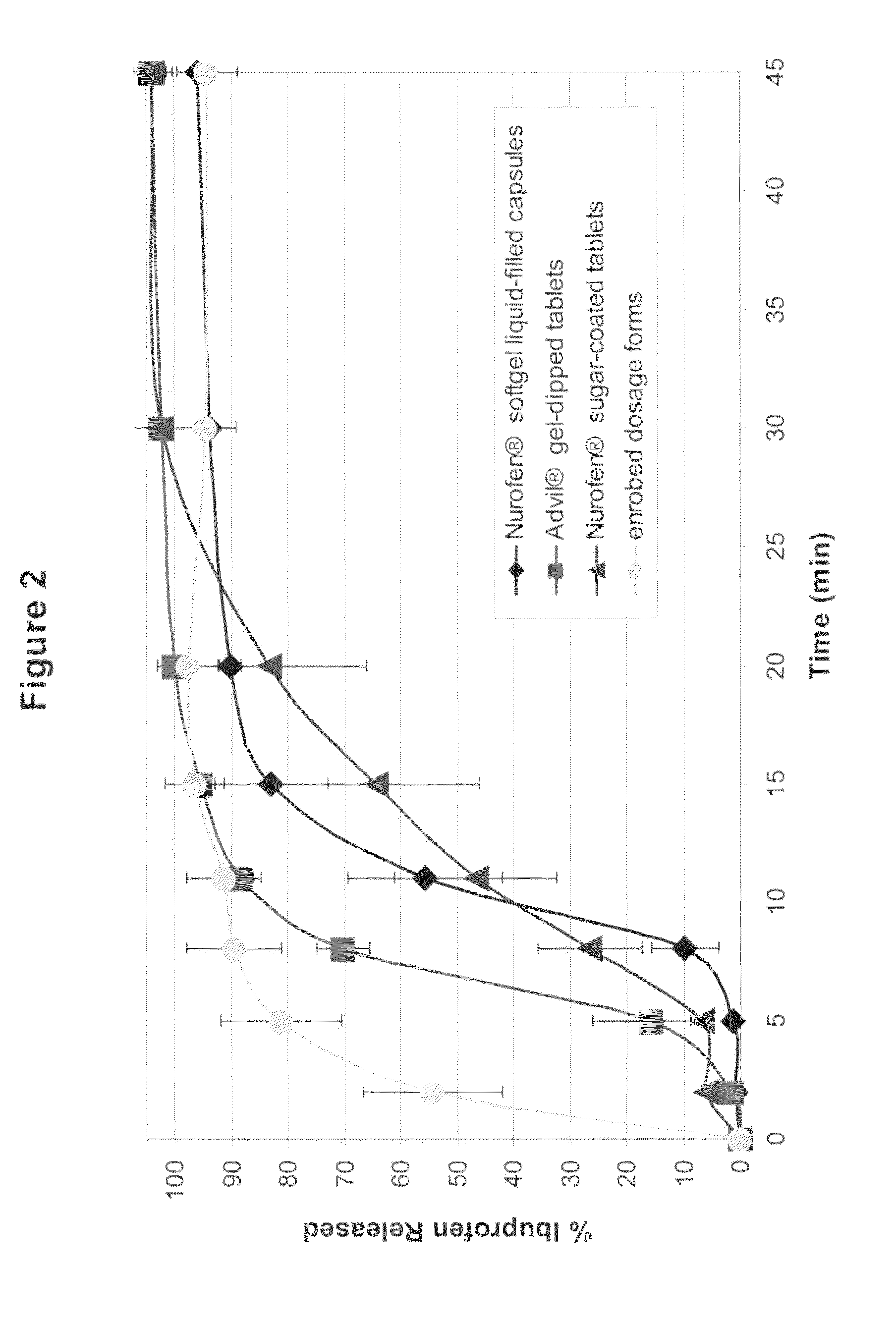
An enrobed solid form comprising a film enrobing a compacted fill material having at least one active material, the solid form shows a weight loss that is less than 1% during a 30 minutes USP Friability Test, the fill material having a density of at least 0.5 g / ml based on the total solid volume of the solid form and a tensile strength less than 0.9 MPa, and the at least one active material within the solid form has an immediate release profile. The solid form is useful in effective delivery of high dose levels of active material.
Owner:FMC CORP
Abuse-resistant amphetamine compounds
InactiveUS20050054561A1Prevents euphoriaLower potentialBiocidePeptide/protein ingredientsChemical MoietyDisease
The invention describes compounds, compositions and methods of using the same comprising a chemical moiety covalently attached to amphetamine. These compounds and compositions are useful for reducing or preventing abuse and overdose of amphetamine. These compounds and compositions find particular use in providing an abuse-resistant alternative treatment for certain disorders, such as attention deficit hyperactivity disorder (ADHD), ADD, narcolepsy, and obesity. Oral bioavailability of amphetamine is maintained at therapeutically useful doses. At higher doses bioavailability is substantially reduced, thereby providing a method of reducing oral abuse liability. Further, compounds and compositions of the invention decrease the bioavailability of amphetamine by parenteral routes, such as intravenous or intranasal administration, further limiting their abuse liability.
Owner:TAKEDA PHARMA CO LTD
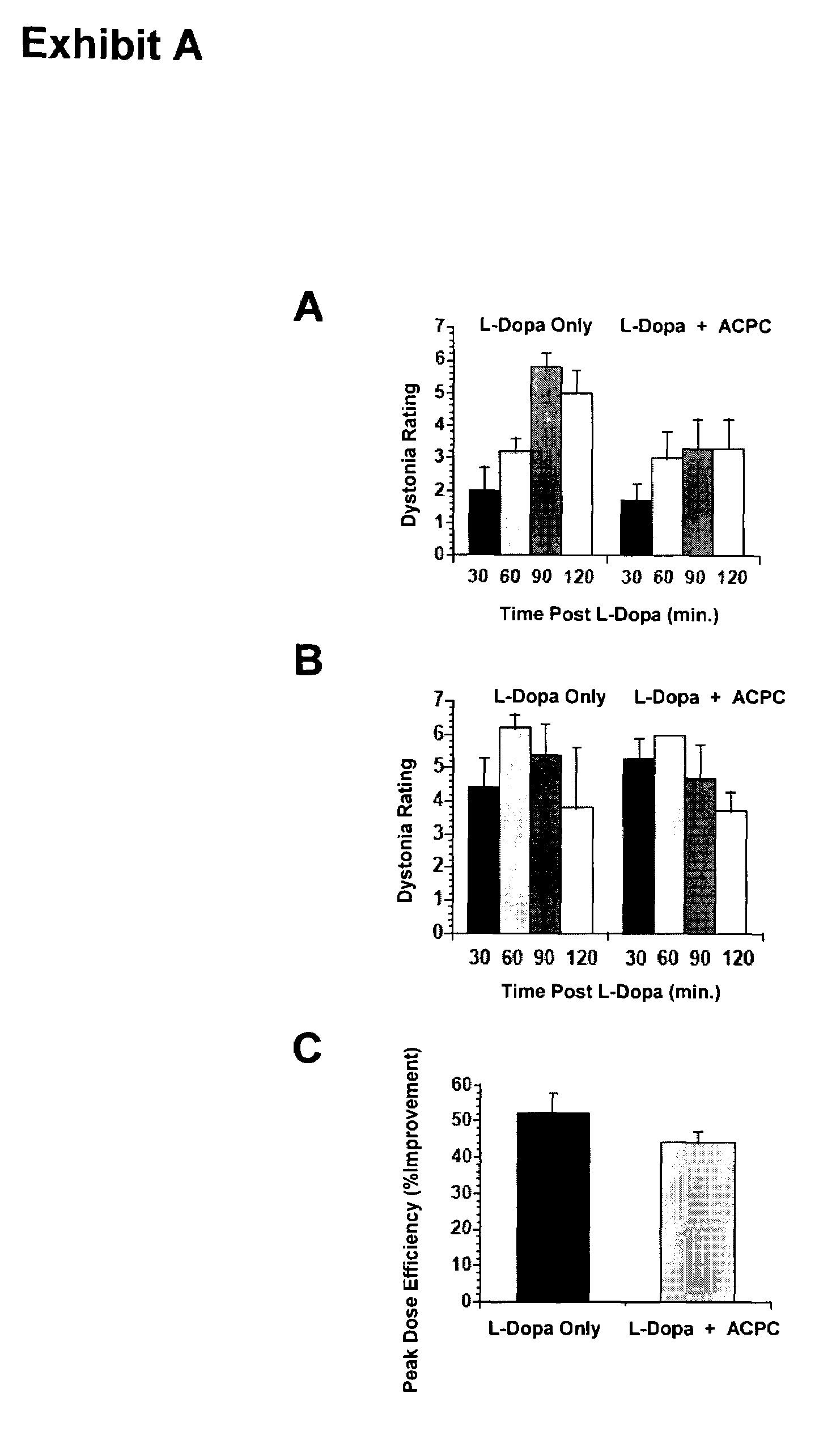
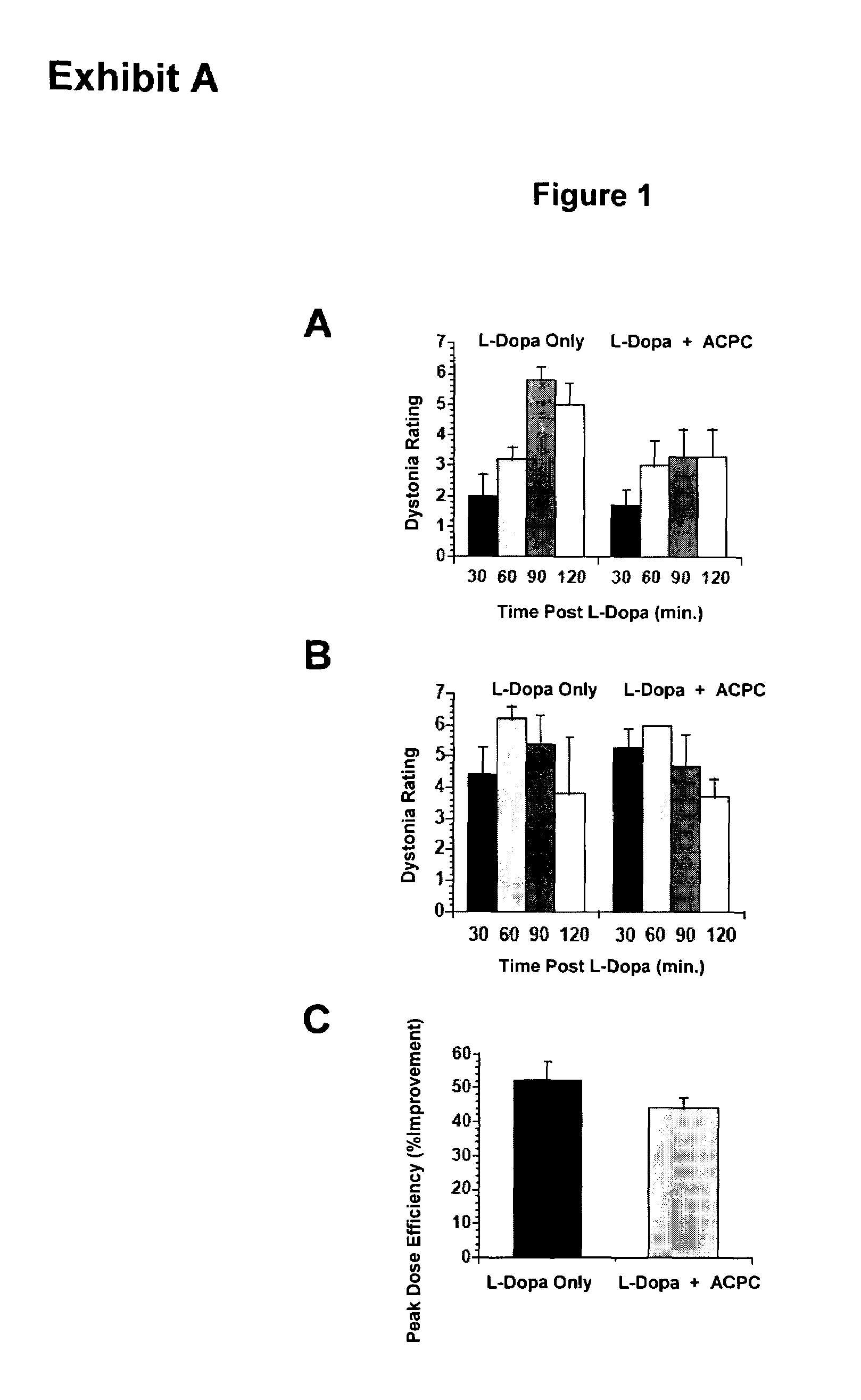
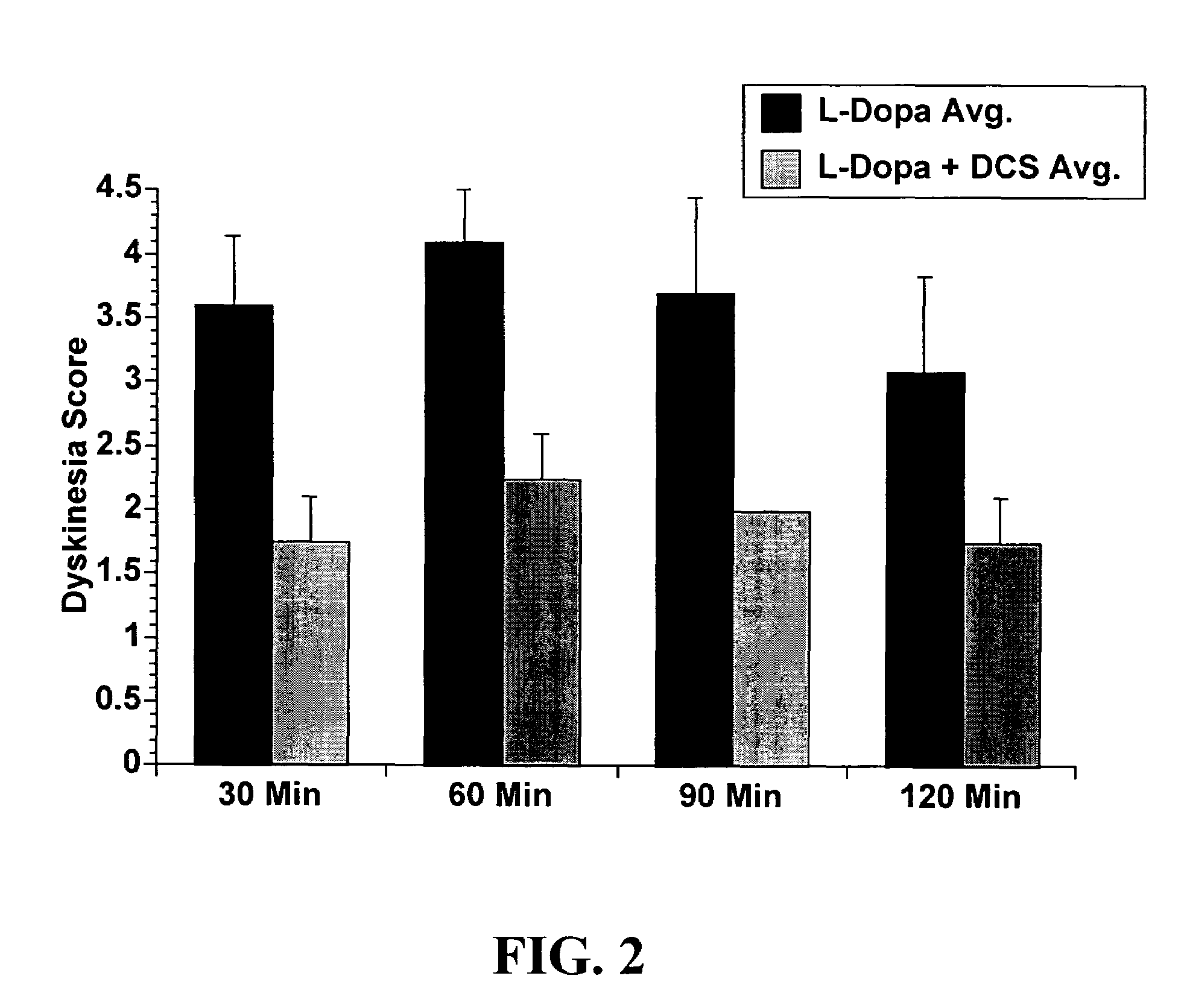
Methods and kit for treating Parkinson's disease
ActiveUS7160913B2Convenient treatmentReduce frequencyBiocidePeptide/protein ingredientsSide effectHigh doses
The efficacy of levodopa therapy in patients being treated for Parkinson's disease is enhanced by administering high doses of a partial glycine agonist. The frequency and severity of levodopa-induced side effects in Parkinson's disease patients are also reduced by administration of a partial glycine agonist.
Owner:THOMAS JEFFERSON UNIV
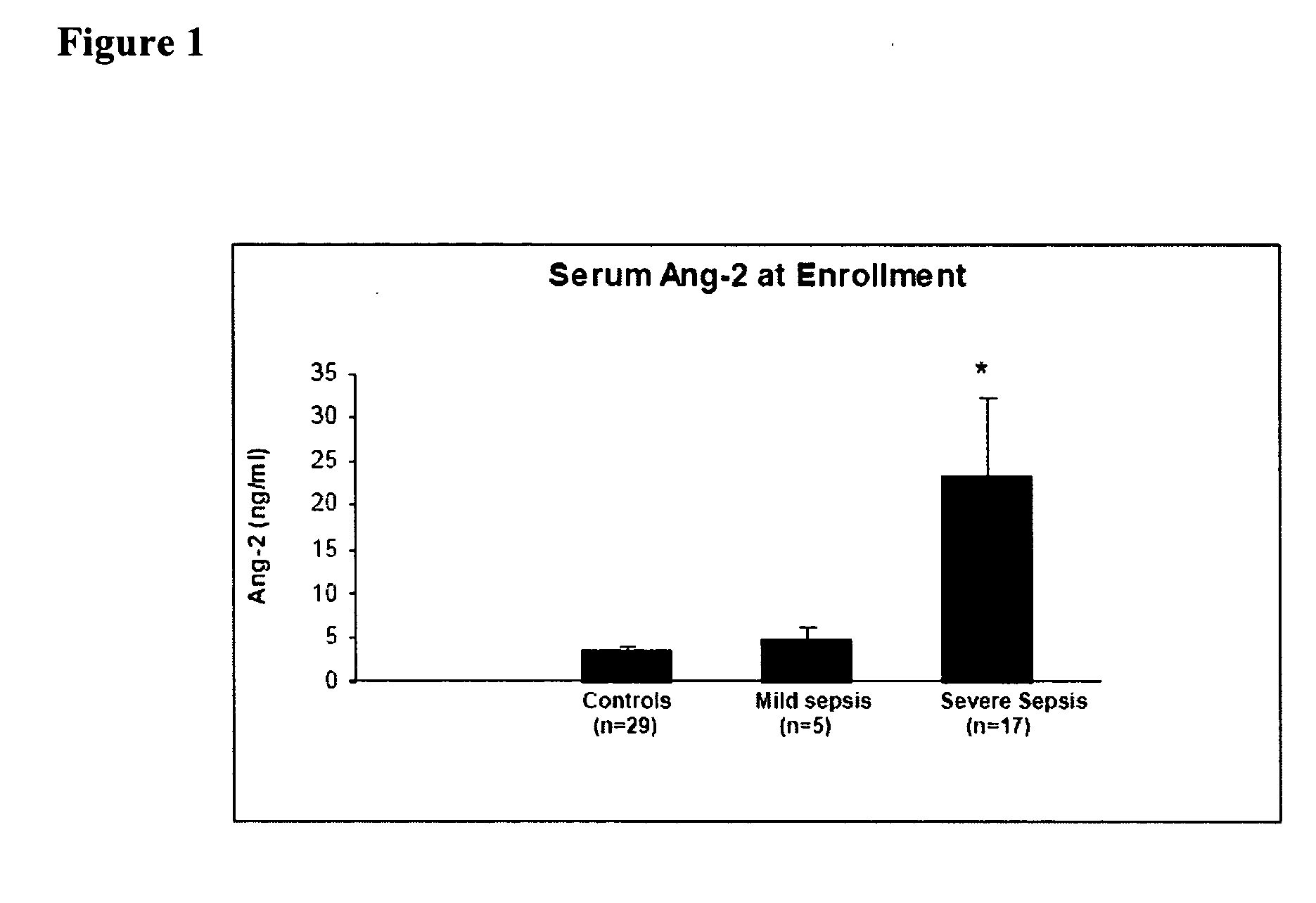
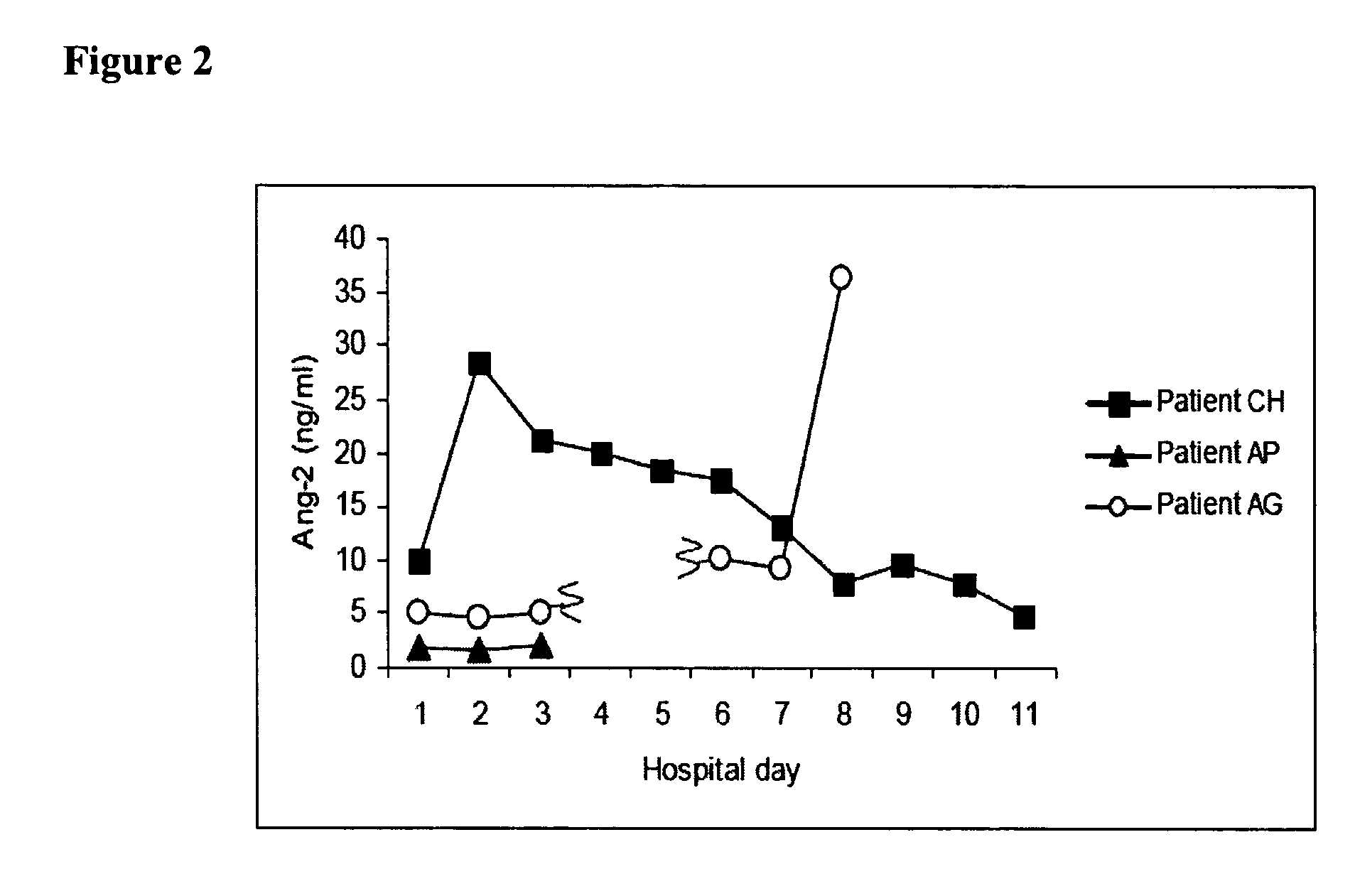
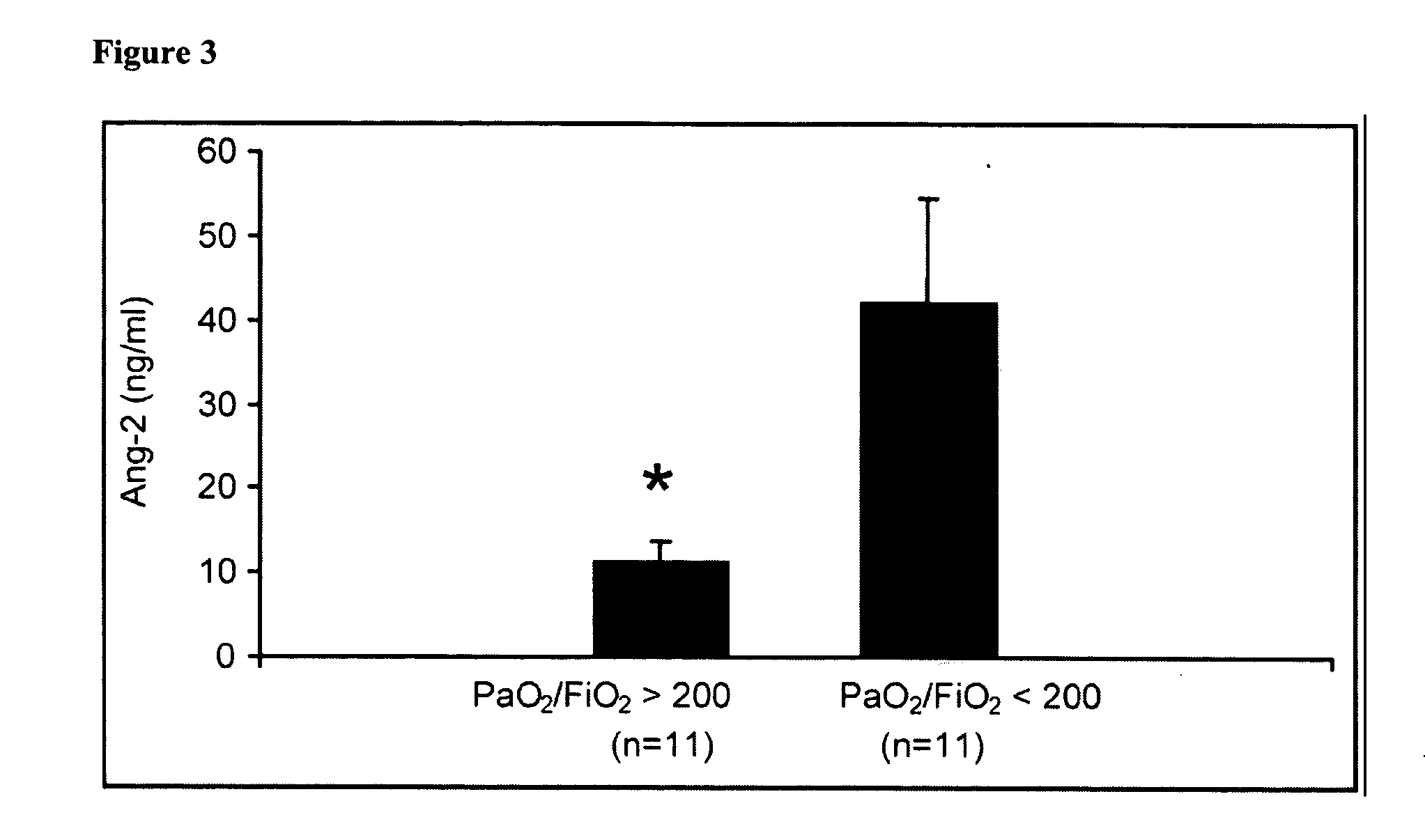
Methods and compositions for the treatment and diagnosis of diseases characterized by vascular leak, hypotension, or a procoagulant state
InactiveUS20070154482A1Improve subject 's conditionIncrease cardiac outputCompounds screening/testingOrganic active ingredientsHigh dose therapyHigh doses
Disclosed herein are methods for treating a vascular leak disorder, hypotension, or a procoagulant state using angiopoietin-2 (Ang-2) antagonist compounds. Also disclosed are methods for treating a vascular leak disorder associated with high dose IL-2 therapy using angiopoietin-2 antagonist compounds. Methods for diagnosing and monitoring vascular leak disorders, hypotension, or a procoagulant state that include the measurement of Ang-2 polypeptide or nucleic acid levels are also disclosed. Methods for inducing a vascular leak using an Ang-2 agonist are also disclosed.
Owner:BETH ISRAEL DEACONESS MEDICAL CENT INC
Topical aminolevulinic acid-photodynamic therapy for the treatment of acne vulgaris
InactiveUS6897238B2Reduce sebum productionSmall sizeBiocideOrganic active ingredientsBacteroidesDisease
Light treatments of sebaceous gland disorders with 5-aminolevulinic acid and photodynamic therapy are disclosed. A preferred treatment includes topical application of 5-aminolevulinic acid to the skin followed by light exposures with repeated treatment at various intervals. At low doses of ALA and photodynamic therapy (PDT) in single or multiple treatments, improvement in the sebaceous gland disorder, e.g., acne, provides the discovery that diminishment in sebum secretion and the eradication of bacteria occurs. At high doses of ALA and a single high energy PDT treatment, permanent changes to the sebaceous gland and sebum secretion have been discovered.
Owner:THE GENERAL HOSPITAL CORP
Use of umbilical cord blood to treat individuals having a disease, disorder or condition
The present invention provides methods of using cord blood and cord blood-derived stem cells in high doses to treat various conditions, diseases and disorders. The high-dose cord blood and cord blood-derived stem cells have a multitude of uses and applications, including but not limited to, therapeutic uses for transplantation and treatment and prevention of disease, and diagnostic and research uses. In particular, the cord blood or cord blood-derived stem cells are delivered in high doses, e.g., at least 3 billion nucleated cells per treatment, where treatment may comprise a single or multiple infusions. The invention also provides for the use of cord blood or cord blood-derived stem cells from multiple donors without the need for HLA typing.
Owner:CELULARITY INC
Treatment of skin disorders with UV light and cooling
Skin disorders such as, for example, atopic dermatitis, dyshidrosis, eczema, lichen planus, psoriasis, and vitiligo, are treated by applying high doses of ultraviolet light to diseased regions of a patients skin. The dosage employed exceeds 1 MED, an MED being determined for the particular patient being treated, and may range from about 1 MED to about 20 MED or higher. The ultraviolet light has a wavelength within the range of between about 295 nanometers to about 320 nanometers and preferably is between about 300 nanometers and about 310 nanometers. High doses of ultraviolet light are restricted to diseased tissue areas so as to avoid risk of detrimental side affects in healthy skin, which is more susceptible to damage from UV light. Cooling the skin prior to and / or while exposing the skin to the UV light can be used to minimize tissue damage resulting from exposure to the UV light. Higher doses of UV light can therefore be employed without injurious affects.
Owner:PHOTOMEDEX
Few seconds beam on time, breathing synchronized image guided all fields simultaneous radiation therapy combined with hyperthermia
This invention relates to single session image guided all field simultaneous radiation therapy combined with hyperthermia. Hyperthermia renders the radiation resistant cells as more radiation sensitive cells. The high and super-high dose rate radiation greatly improves the RBE of the photon radiation. It also minimizes photon radiation therapy's OER and cell cycle dependent tumor cell kill by minimizing the repair capacity of cell after photon radiation. Single session hyperthermia and radiation therapy overcomes the thermotolerance-associated inefficiency of hyperthermia treatment as it is when hyperthermia is combined with fractionated, lower dose rate radiation. The synergetic effects of sublethal damage repair inhibiting single session hyperthermia-combined with high dose and dose rate single session radiation therapy, and combined chemotherapy brings the photon radiation therapy's tumor cure and control capabilities closer to high LET radiation therapy.
Owner:SAHADEVAN VELAYUDHAN
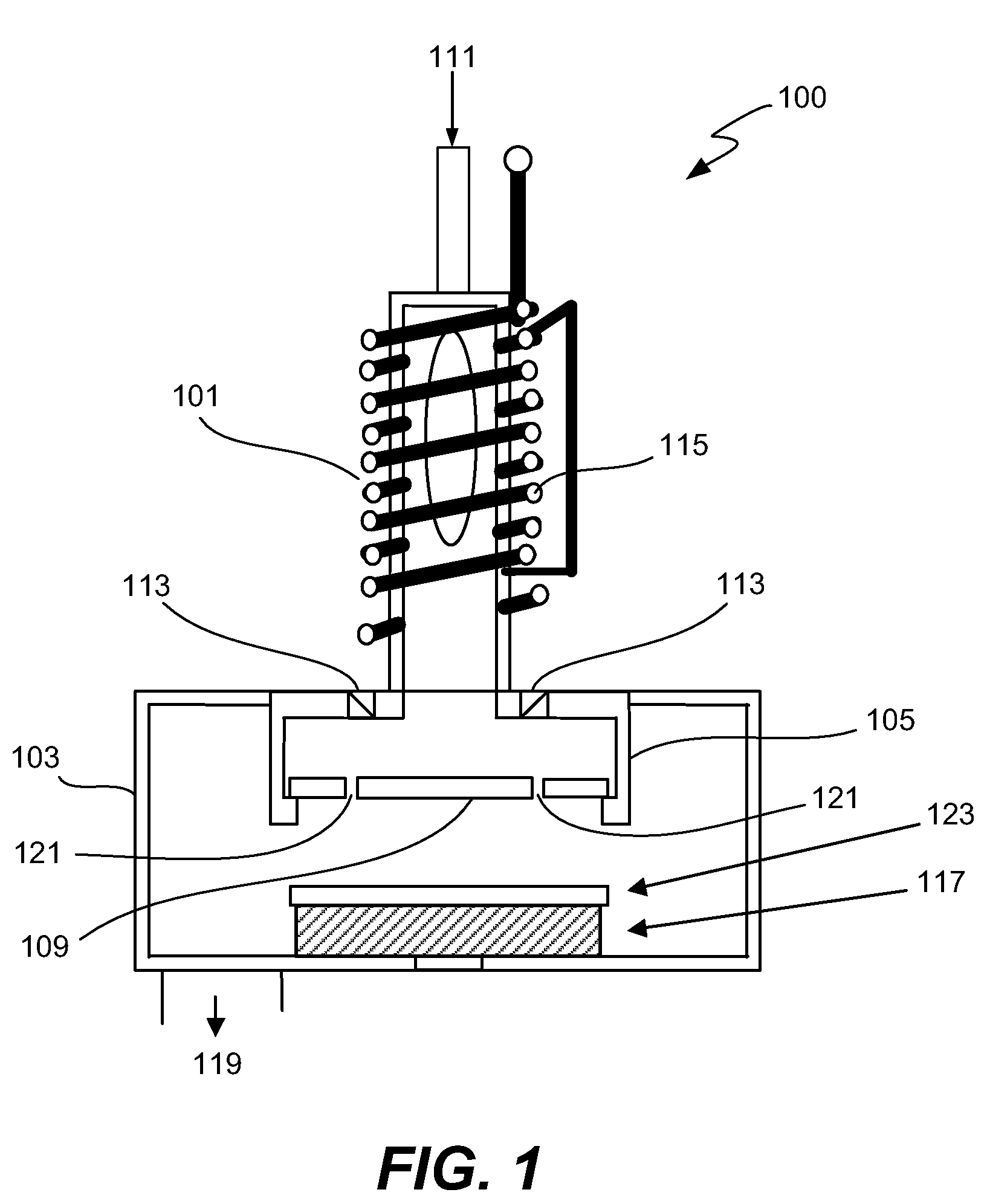
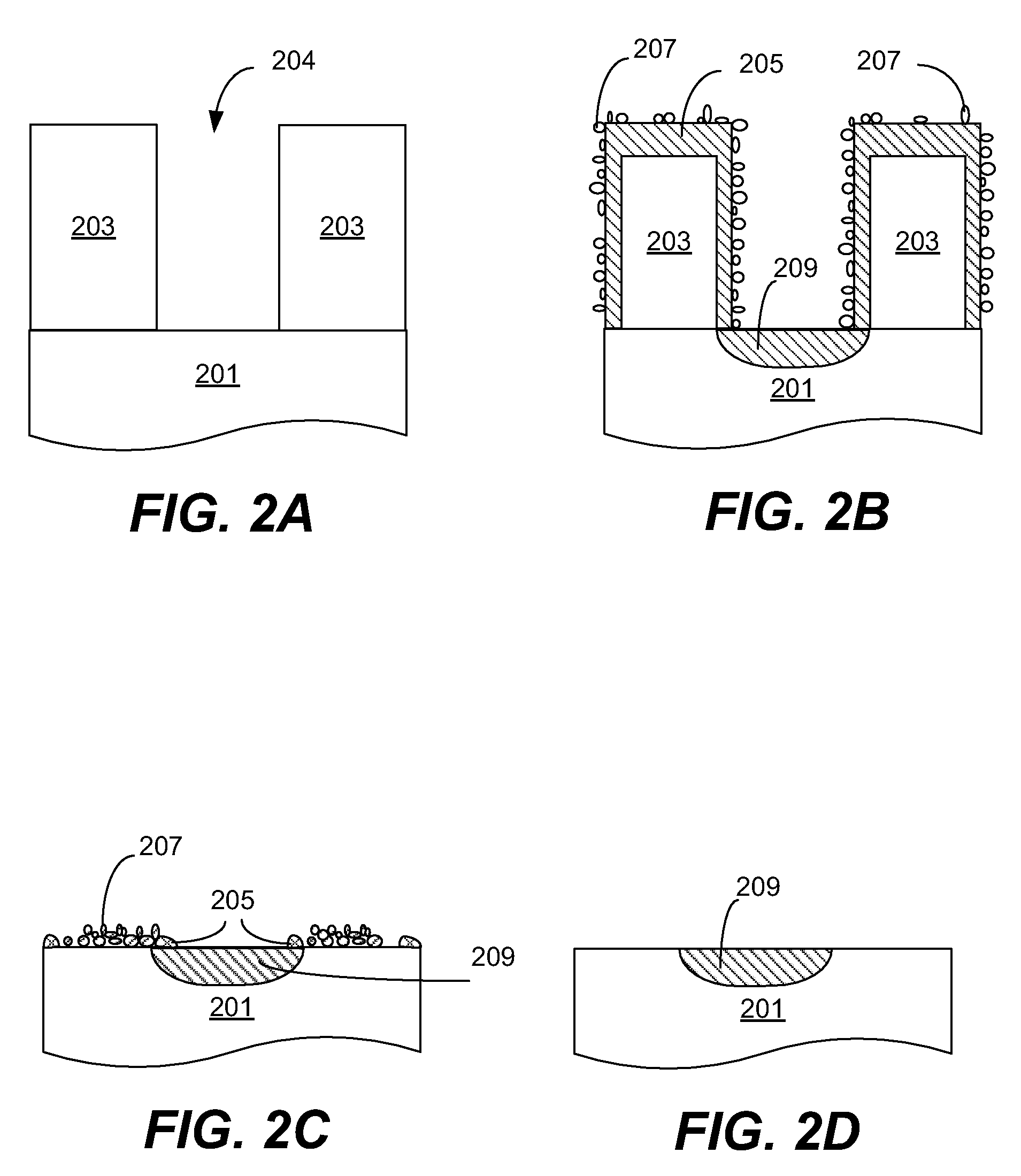
High dose implantation strip (HDIS) in h2 base chemistry
Plasma is generated using elemental hydrogen, a weak oxidizing agent, and a fluorine containing gas. An inert gas is introduced to the plasma downstream of the plasma source and upstream of a showerhead that directs gas mixture into the reaction chamber where the mixture reacts with the high-dose implant resist. The process removes both the crust and bulk resist layers at a high strip rate, and leaves the work piece surface substantially residue free with low silicon loss.
Owner:NOVELLUS SYSTEMS
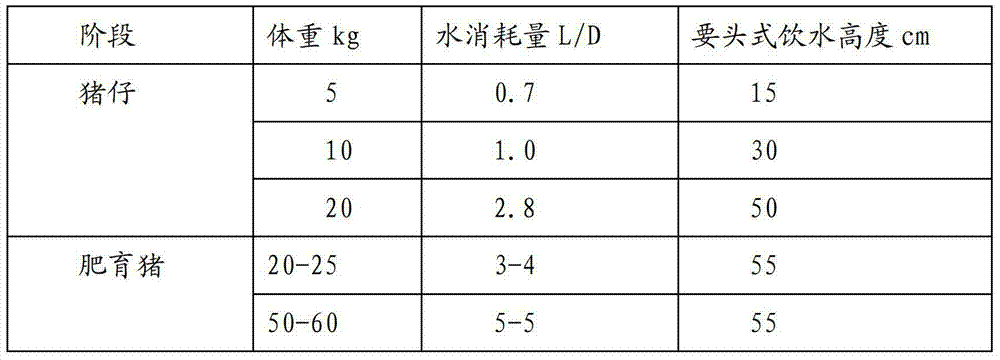
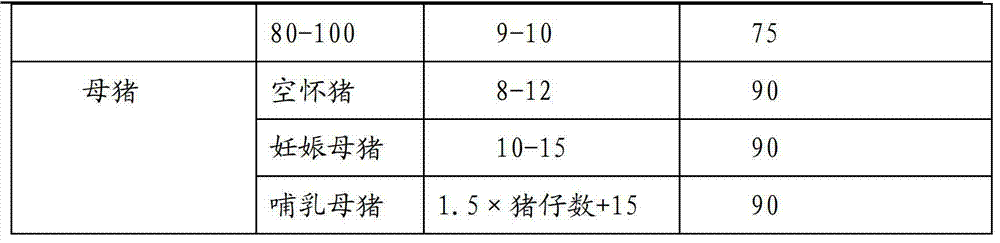
Healthy efficient pig raising method
ActiveCN103238562AImprove righteousnessImprove internal and external environmentAnimal feeding stuffAnimal scienceHigh doses
The invention discloses a healthy efficient pig raising method. The healthy efficient pig raising method includes steps of 1) selecting a pigsty and a site; 2) feeding reserved sows and boars; 3) feeding pregnant sows; 4) feeding milking sows; 5) feeding nonpregnant sows; 6) managing the pigsty of the sows and managing temperature and humidity; 7) feeding piglets; 8) feeding in care period; 9) feeding in growing period, and 10) feeding in fattening period and specially preparing fodder for the pregnant sows, breast-feeding fodder, milk replacers, care fodder and fodder for medium and large pigs in different stages. By the healthy efficient pig raising method, annual survival piglet number of the sows can be increased, and simultaneously, daily ration can be sufficiently converted by complex phosphoesterasum, and feed conversion rate of pigs is increased. By adopting feed formula without antibiotics and additives containing copper, phosphorous and zinc in high dose, the pigs are healthier.
Owner:李云杨
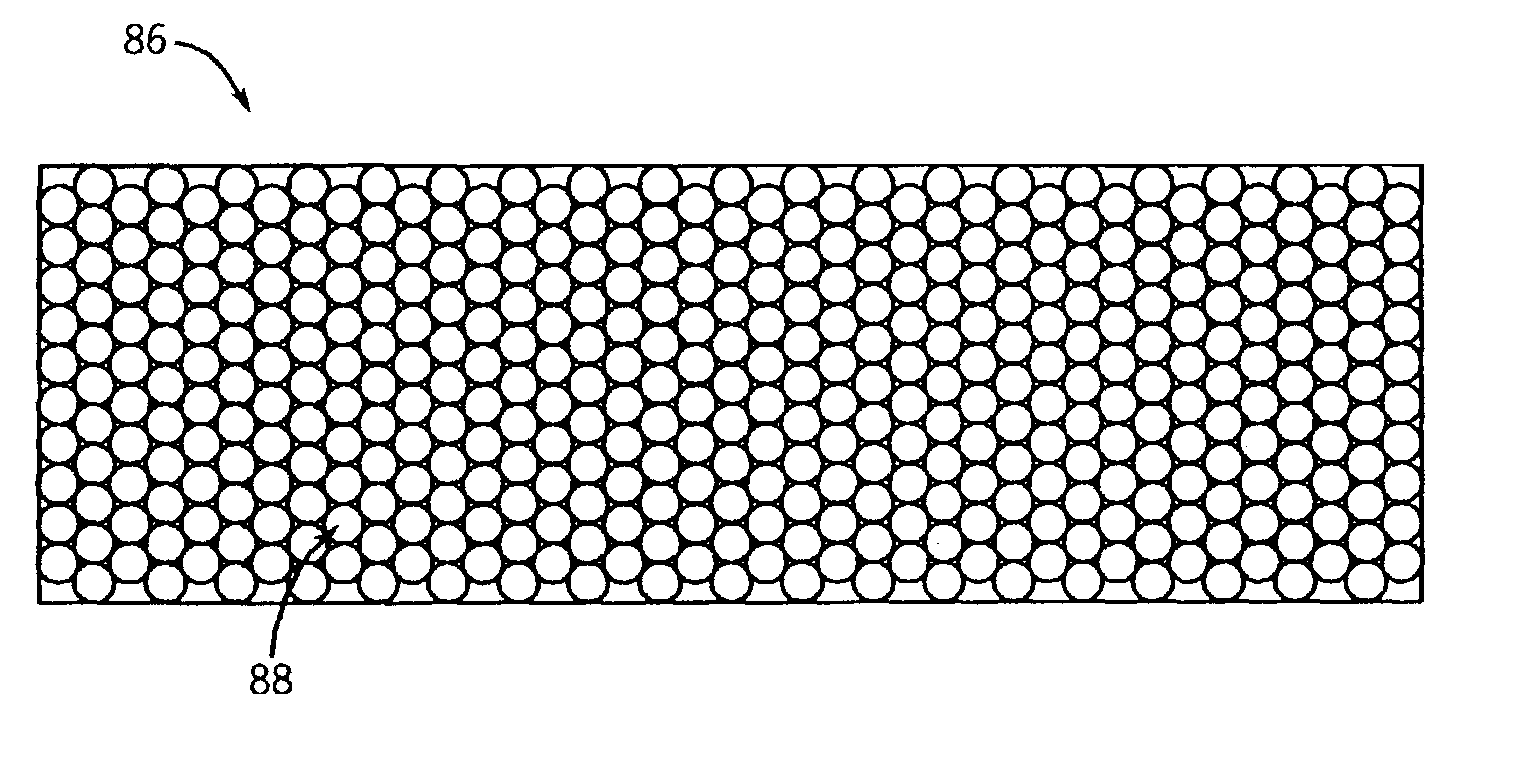
CT detector array having non pixelated scintillator array
InactiveUS7054408B2Improve efficiencyImprove light outputSolid-state devicesHandling using diaphragms/collimetersImage resolutionScattering loss
Owner:GENERAL ELECTRIC CO
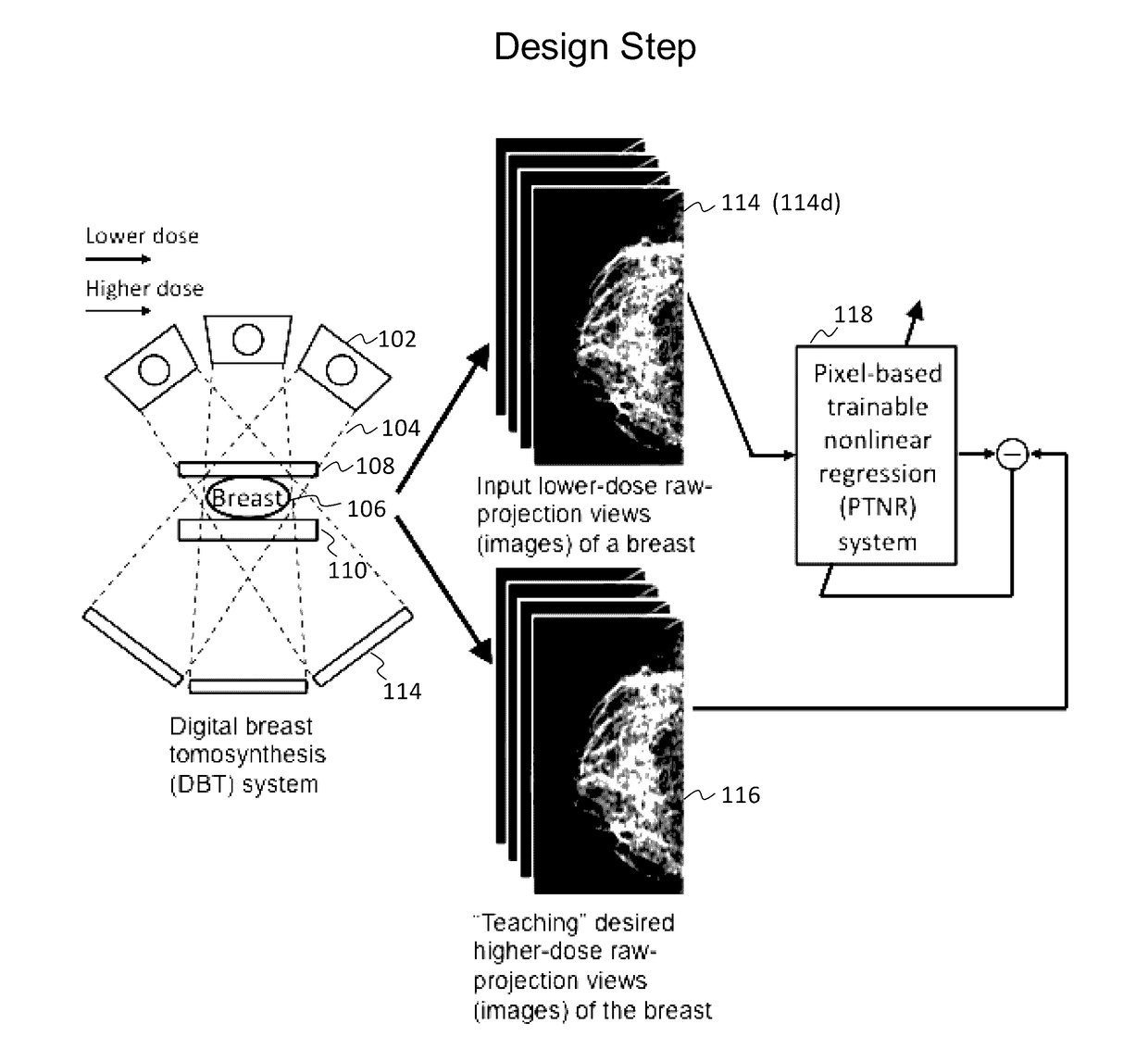
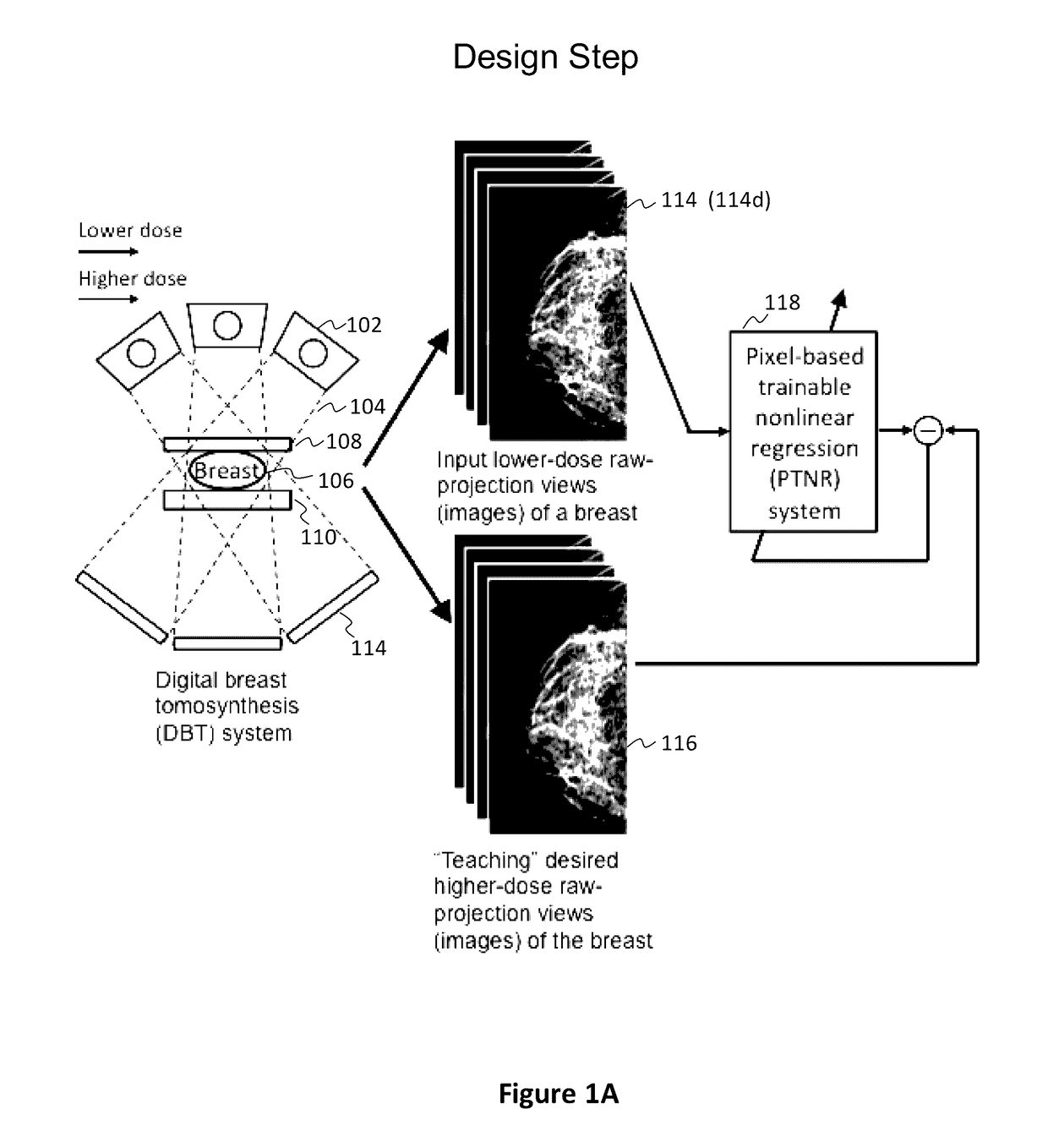
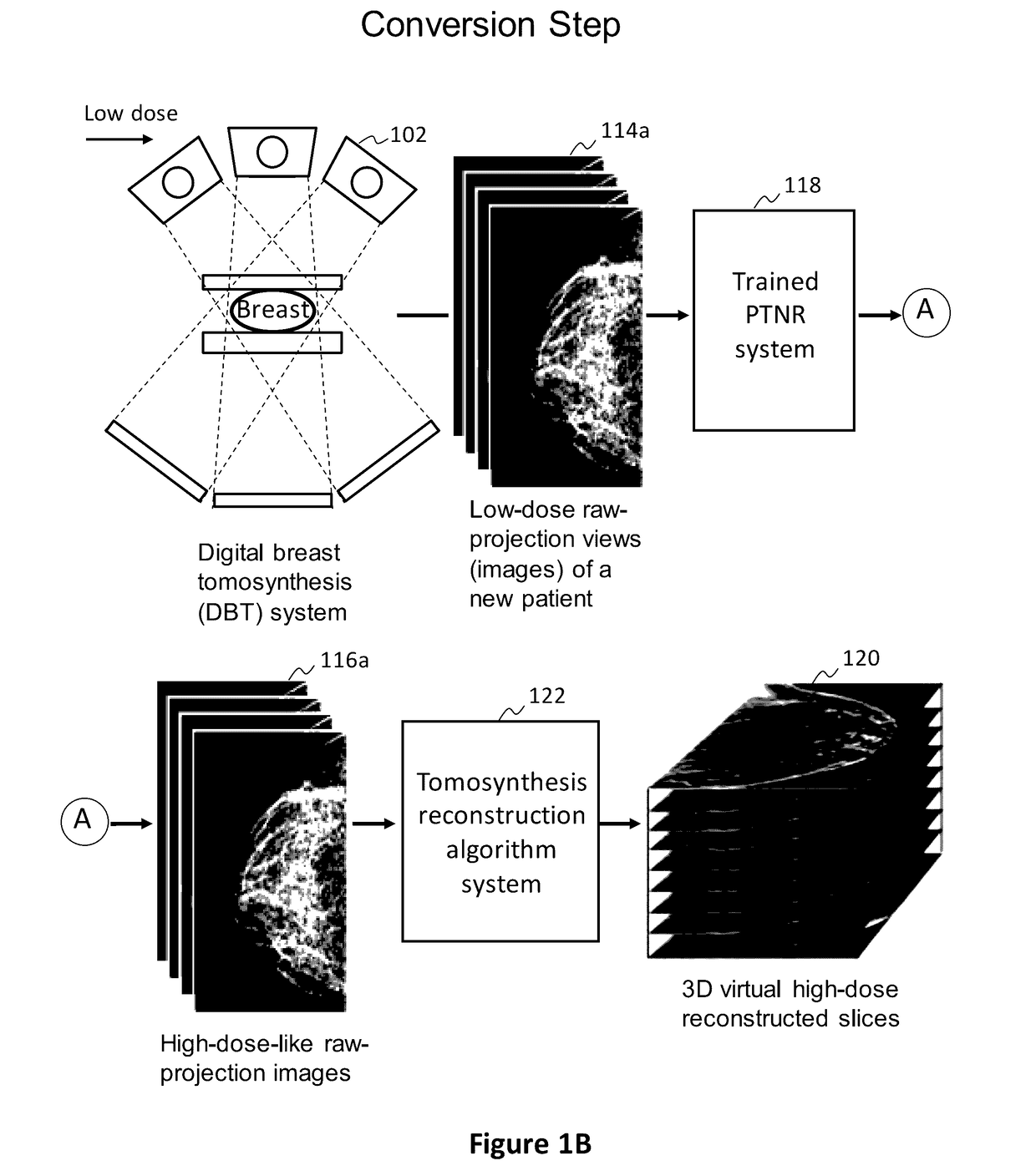
Converting low-dose to higher dose 3D tomosynthesis images through machine-learning processes
ActiveUS20170071562A1Quality improvementReduce noiseImage enhancementReconstruction from projectionTomosynthesisImaging quality
A method and system for converting low-dose tomosynthesis projection images or reconstructed slices images with noise into higher quality, less noise, higher-dose-like tomosynthesis reconstructed slices, using of a trainable nonlinear regression (TNR) model with a patch-input-pixel-output scheme called a pixel-based TNR (PTNR). An image patch is extracted from an input raw projection views (images) of a breast acquired at a reduced x-ray radiation dose (lower-dose), and pixel values in the patch are entered into the PTNR as input. The output of the PTNR is a single pixel that corresponds to a center pixel of the input image patch. The PTNR is trained with matched pairs of raw projection views (images together with corresponding desired x-ray radiation dose raw projection views (images) (higher-dose). Through the training, the PTNR learns to convert low-dose raw projection images to high-dose-like raw projection images. Once trained, the trained PTNR does not require the higher-dose raw projection images anymore. When a new reduced x-ray radiation dose (low dose) raw projection images is entered, the trained PTNR outputs a pixel value similar to its desired pixel value, in other words, it outputs high-dose-like raw projection images where noise and artifacts due to low radiation dose are substantially reduced, i.e., a higher image quality. Then, from the “high-dose-like” projection views (images), “high-dose-like” 3D tomosynthesis slices are reconstructed by using a tomosynthesis reconstruction algorithm. With the “virtual high-dose” tomosynthesis reconstruction slices, the detectability of lesions and clinically important findings such as masses and microcalcifications can be improved.
Owner:ALARA SYST
Device for oral UV photo-therapy
Skin disorders such as, for example, atopic dermatitis, dyshidrosis, eczema, lichen planus, psoriasis, and vitiligo, are treated by applying high doses of ultraviolet light to diseased regions of a patients skin. The dosage employed exceeds 1 MED, an MED being determined for the particular patient being treated, and may range from about 1 MED to about 20 MED or higher. The ultraviolet light has a wavelength within the range of between about 295 nanometers to about 320 nanometers and preferably is between about 300 nanometers and about 310 nanometers. High doses of ultraviolet light are preferably restricted to diseased tissue areas so as to avoid risk of detrimental side affects in healthy skin, which is more susceptible to damage from UV light. Cooling the skin prior to and / or while exposing the skin to the UV light can be used to reduce tissue damage resulting from exposure to the UV light. Higher doses of UV light can therefore be employed without injurious affects. A specialized handpiece provides a beam profile especially suitable for application of controlled doses. A specialized delivery device is useful for UV treatment of tissue within the mouth.
Owner:MELA SCIENCES
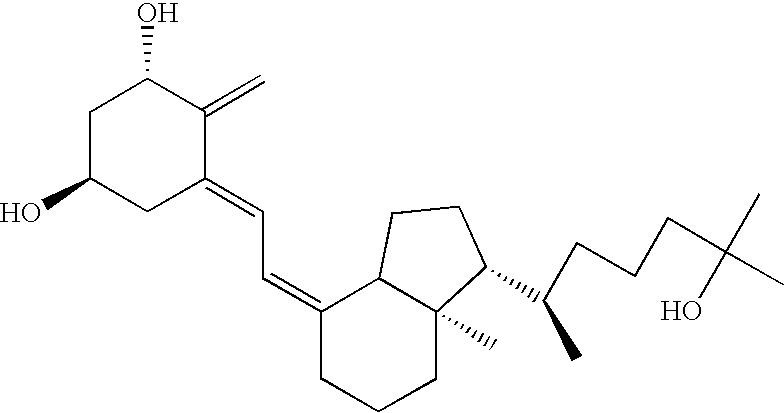
Methods of using vitamin D compounds in the treatment of myelodysplastic syndromes
InactiveUS20050101576A1Minimizing and avoiding effectUseful in treatmentOrganic active ingredientsBiocideActive agentHigh doses
Methods of treating MDS, or ameliorating a symptom thereof, are disclosed. Specific methods encompass the administration of one or more vitamin D compounds, or a pharmaceutically acceptable salt, solvate, hydrate, stereoisomer, clathrate, or prodrug thereof, alone or in combination with one or more additional active agents. Other methods include intermittent administration of a high dose of one or more vitamin D compounds, or a pharmaceutically acceptable salt, solvate, hydrate, stereoisomer, clathrate, or prodrug thereof, alone or in combination with one or more additional active agents. Such intermittent administration allows high doses of the vitamin D compounds to be administered while minimizing or eliminating hypercalcemia.
Owner:NOVACEA INC
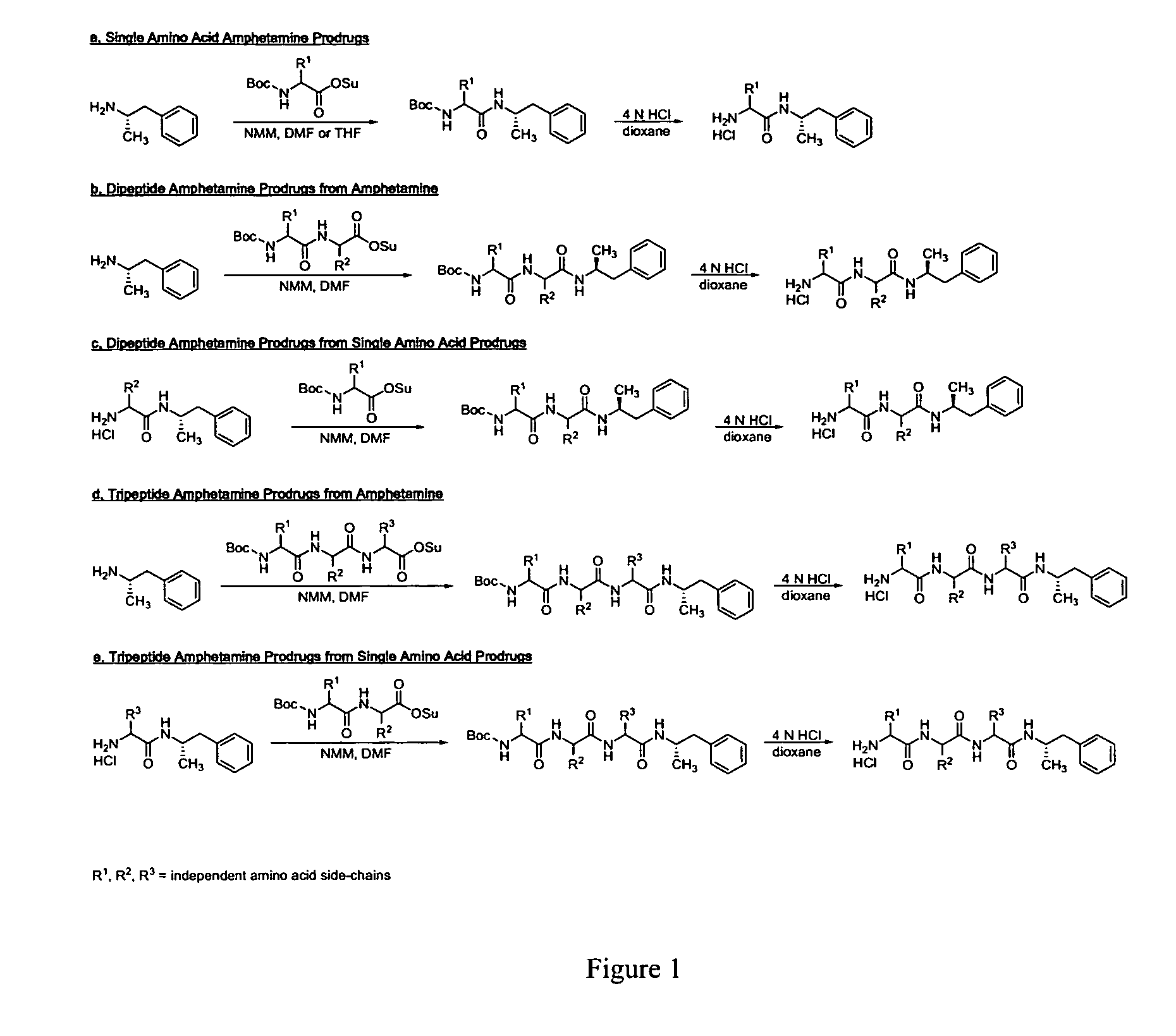
Abuse-resistant amphetamine prodrugs
The invention describes compounds, compositions, and methods of using the same comprising a chemical moiety covalently attached to amphetamine. These compounds and compositions are useful for reducing or preventing abuse and overdose of amphetamine. These compounds and compositions find particular use in providing an abuse-resistant alternative treatment for certain disorders, such as attention deficit hyperactivity disorder (ADHD), ADD, narcolepsy, and obesity. Oral bioavailability of amphetamine is maintained at therapeutically useful doses. At higher doses bioavailability is substantially reduced, thereby providing a method of reducing oral abuse liability. Further, compounds and compositions of the invention decrease the bioavailability of amphetamine by parenteral routes, such as intravenous or intranasal administration, further limiting their abuse liability.
Owner:TAKEDA PHARMA CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com