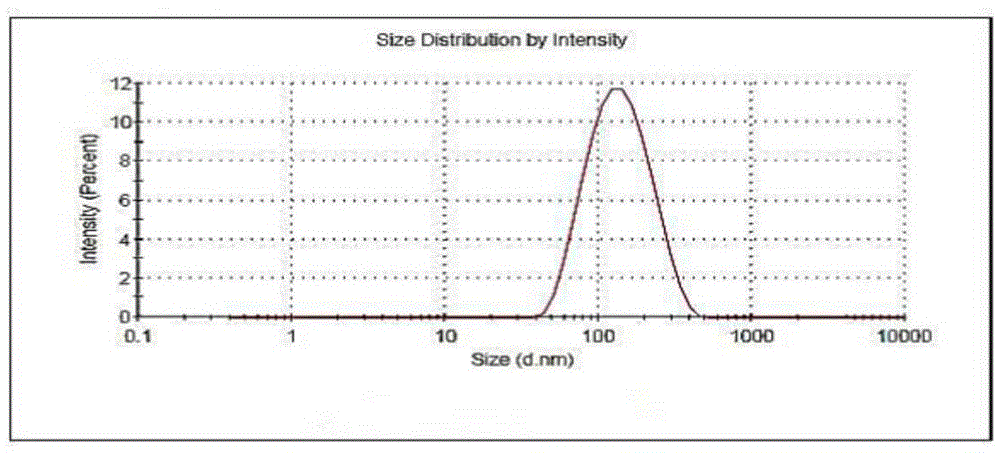
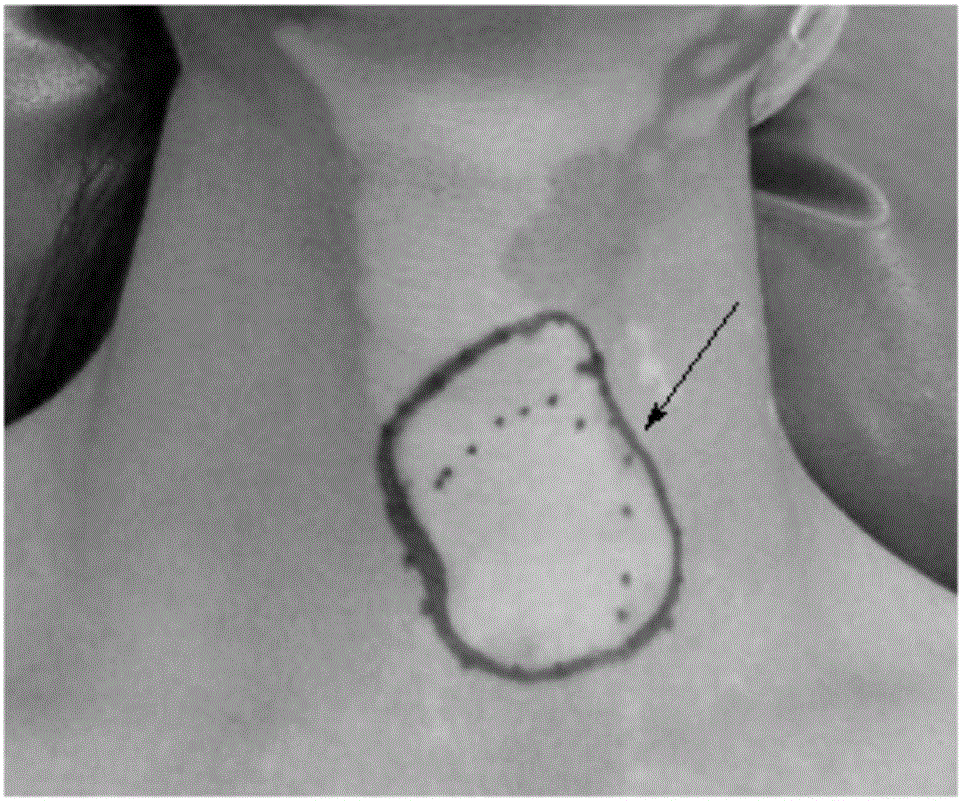
Patents
Literature
652 results about "Vitiligo" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A condition in which the melanocytes, the pigment cells of the skin, are destroyed causing affected areas to turn pale. Often seen as patches of white skin.
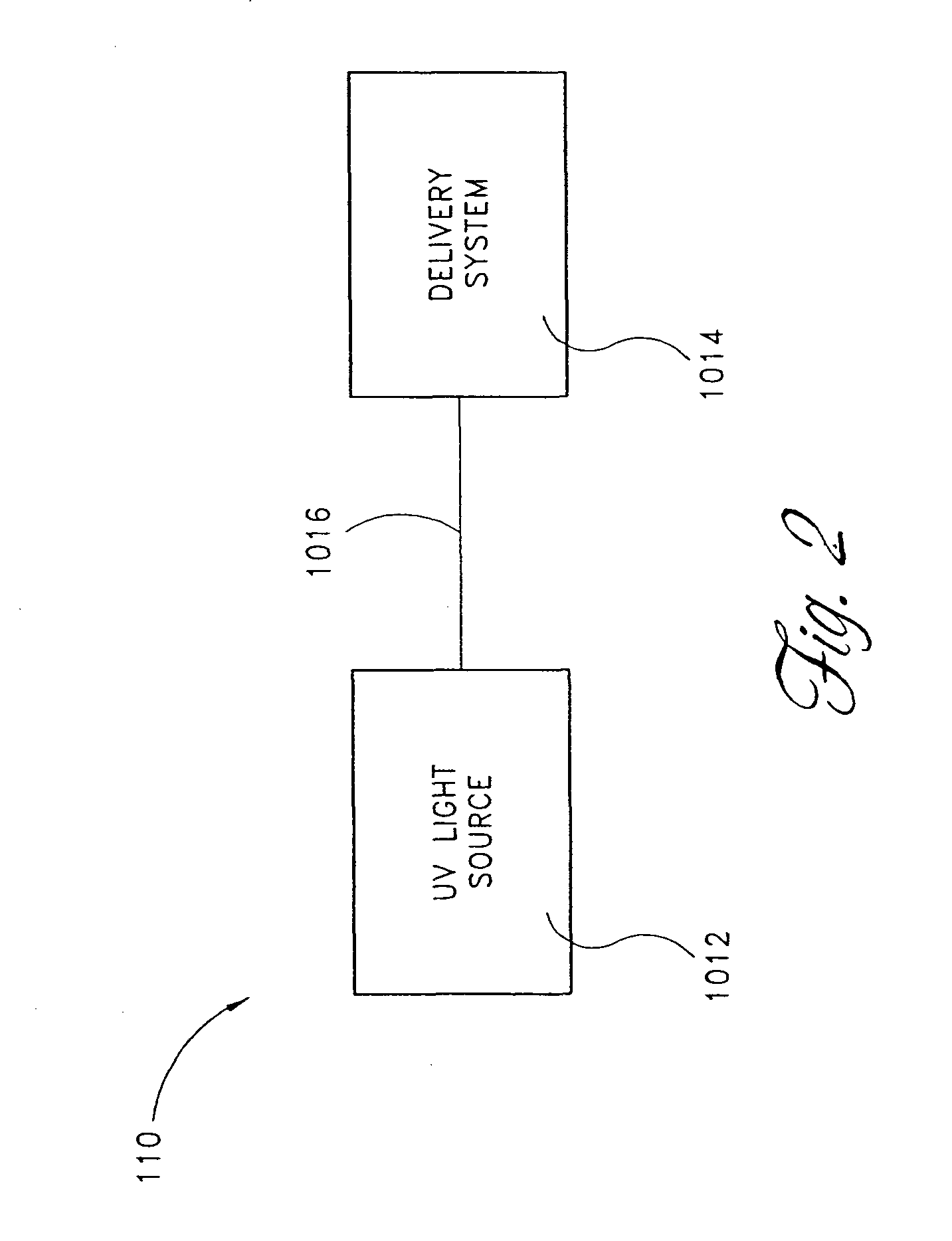
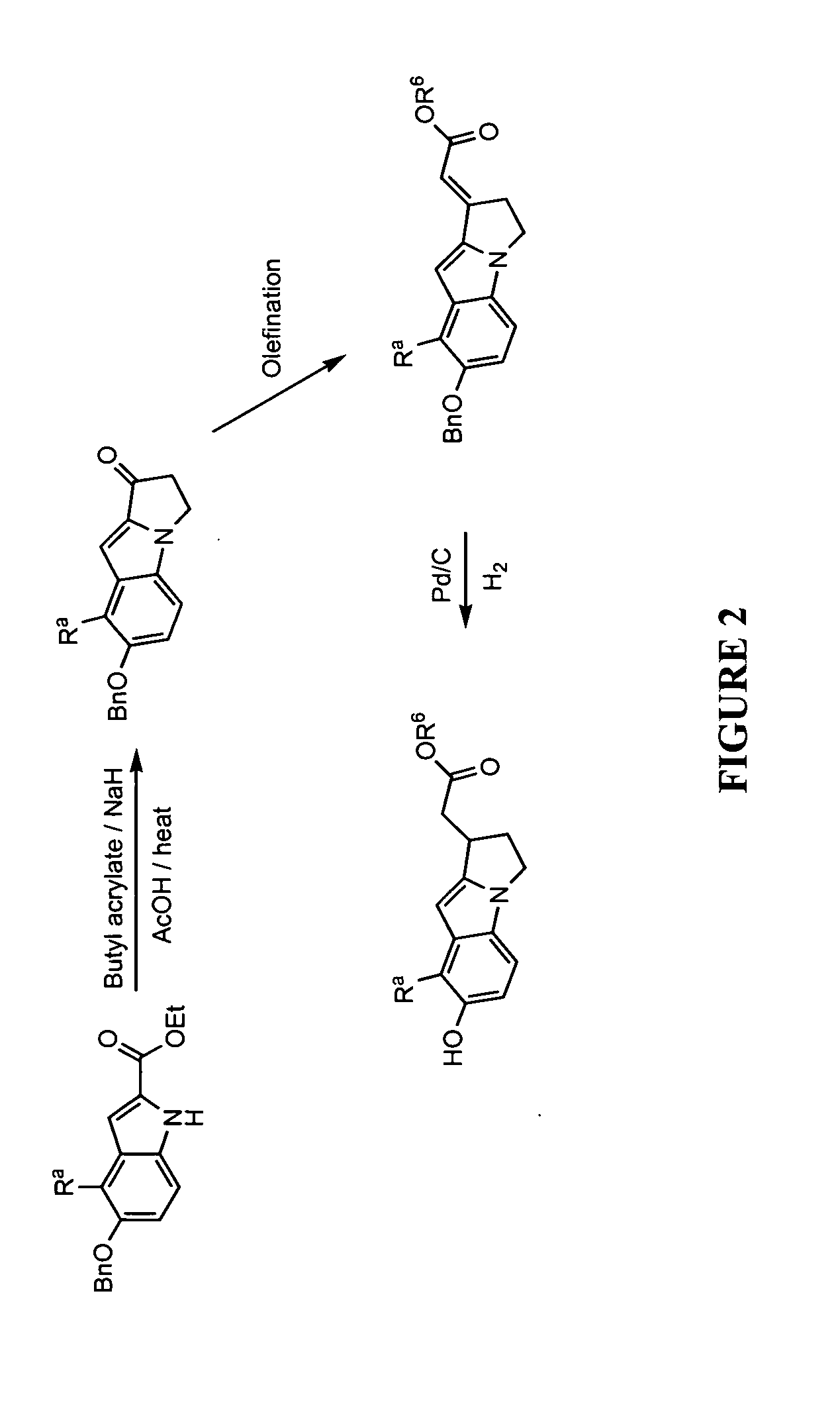
Device for oral UV photo-therapy
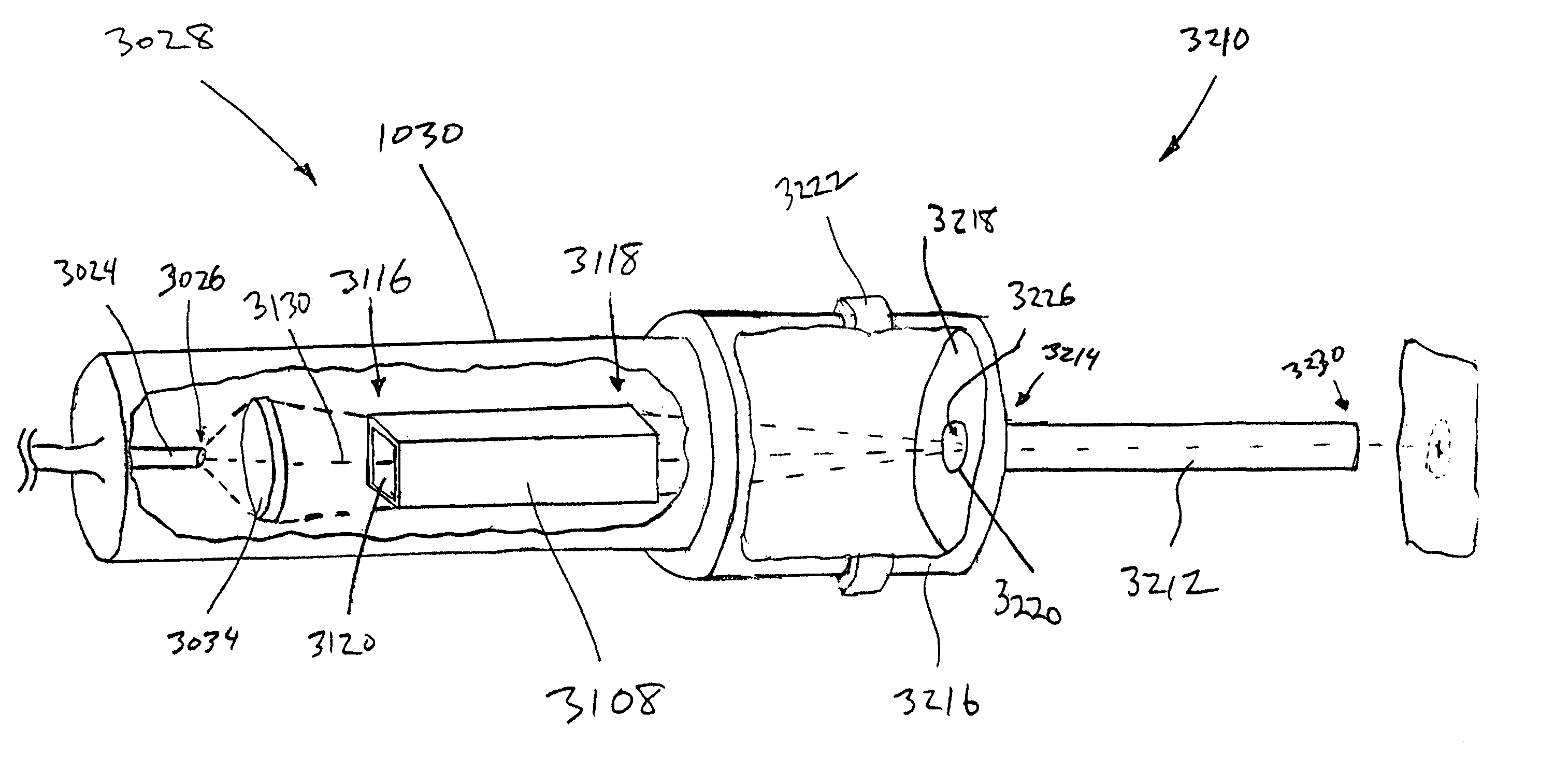
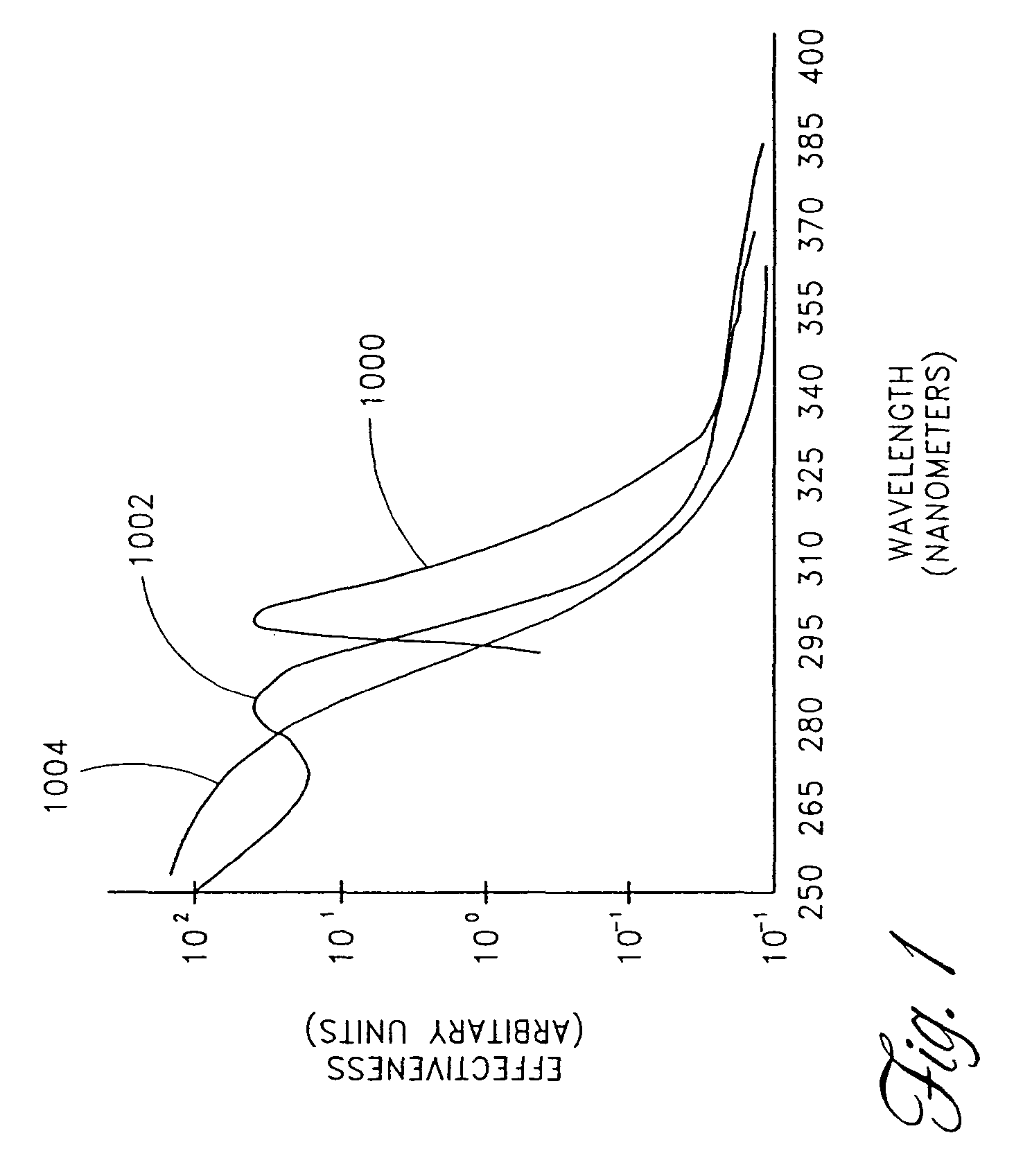
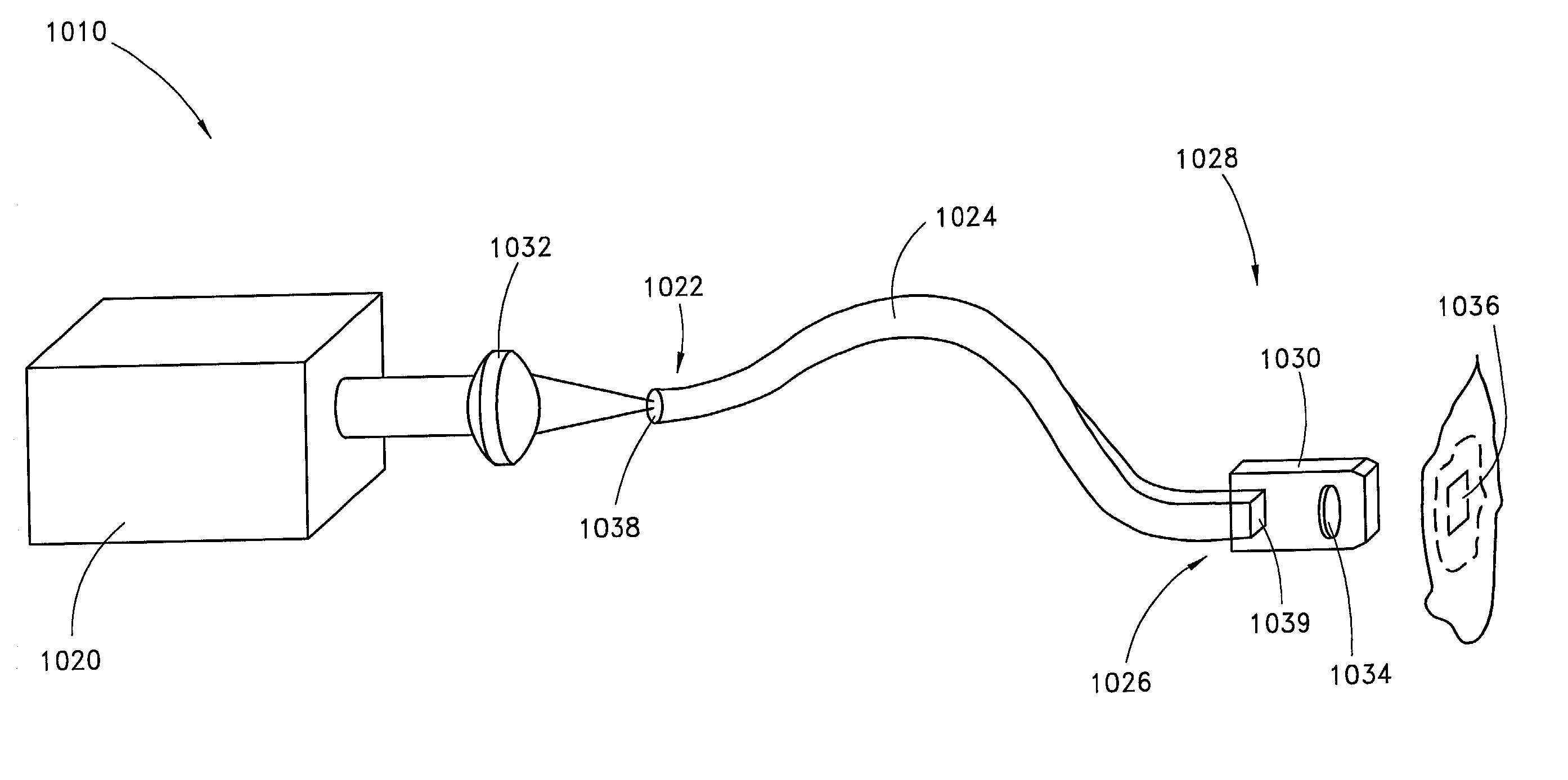
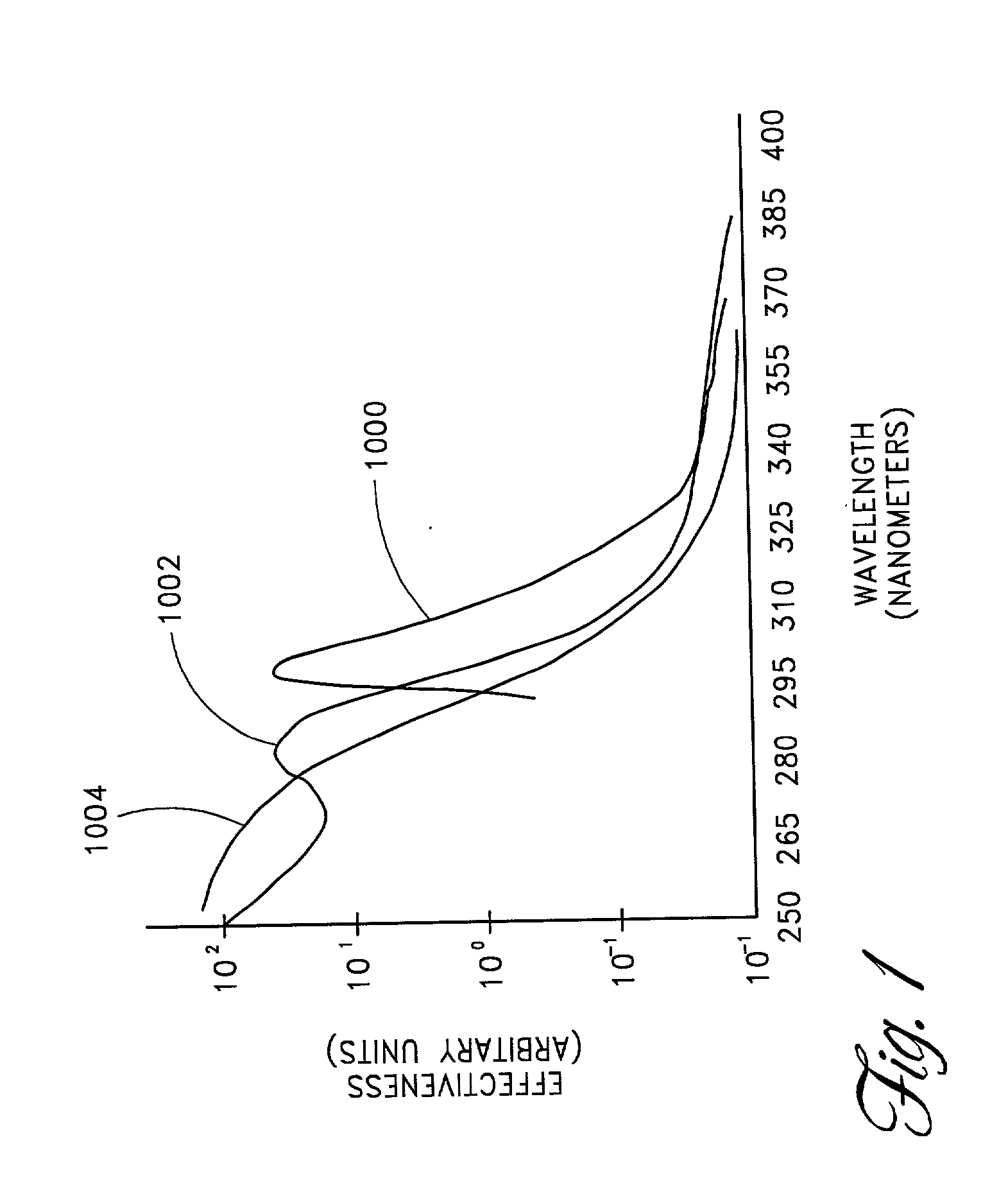
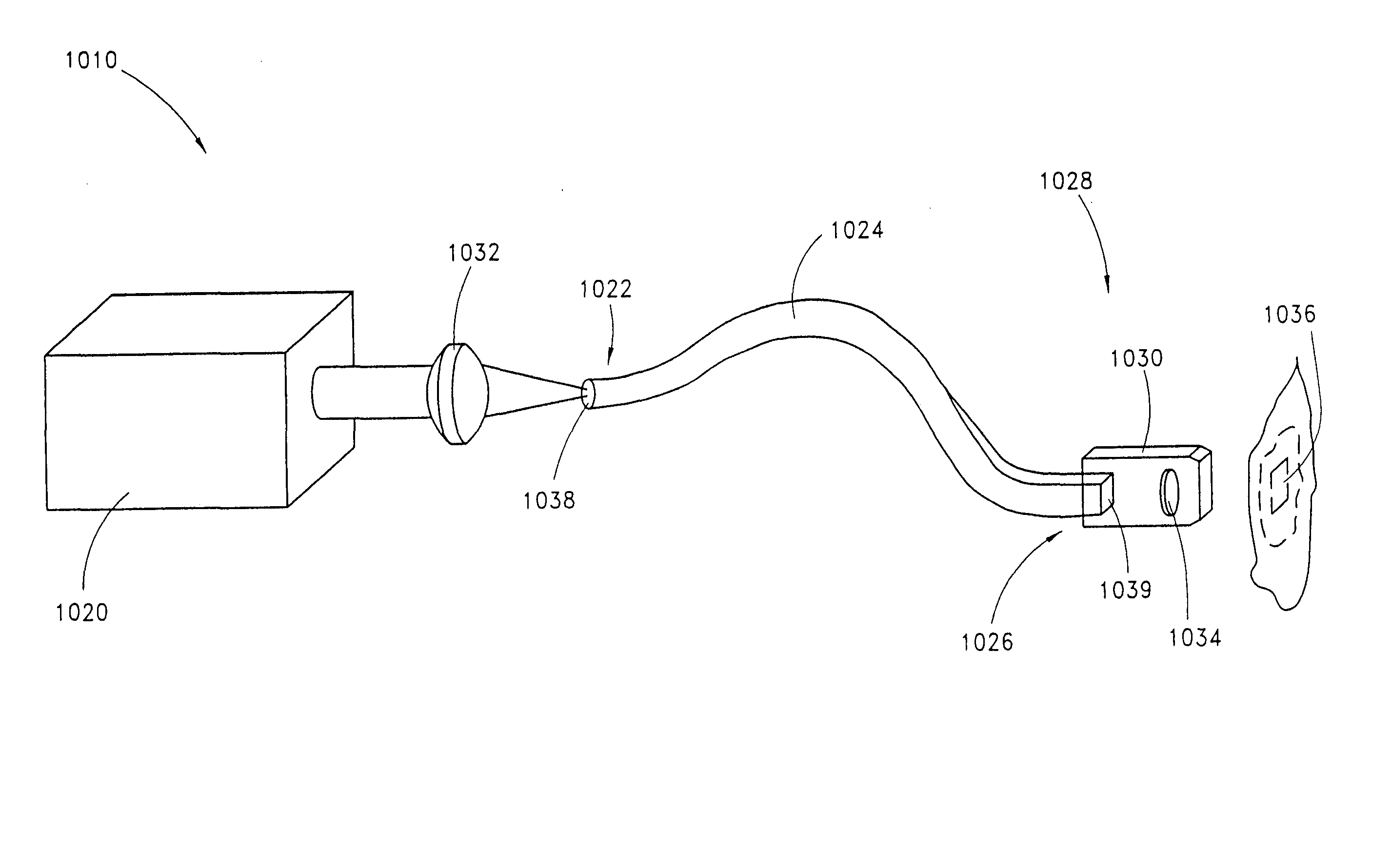
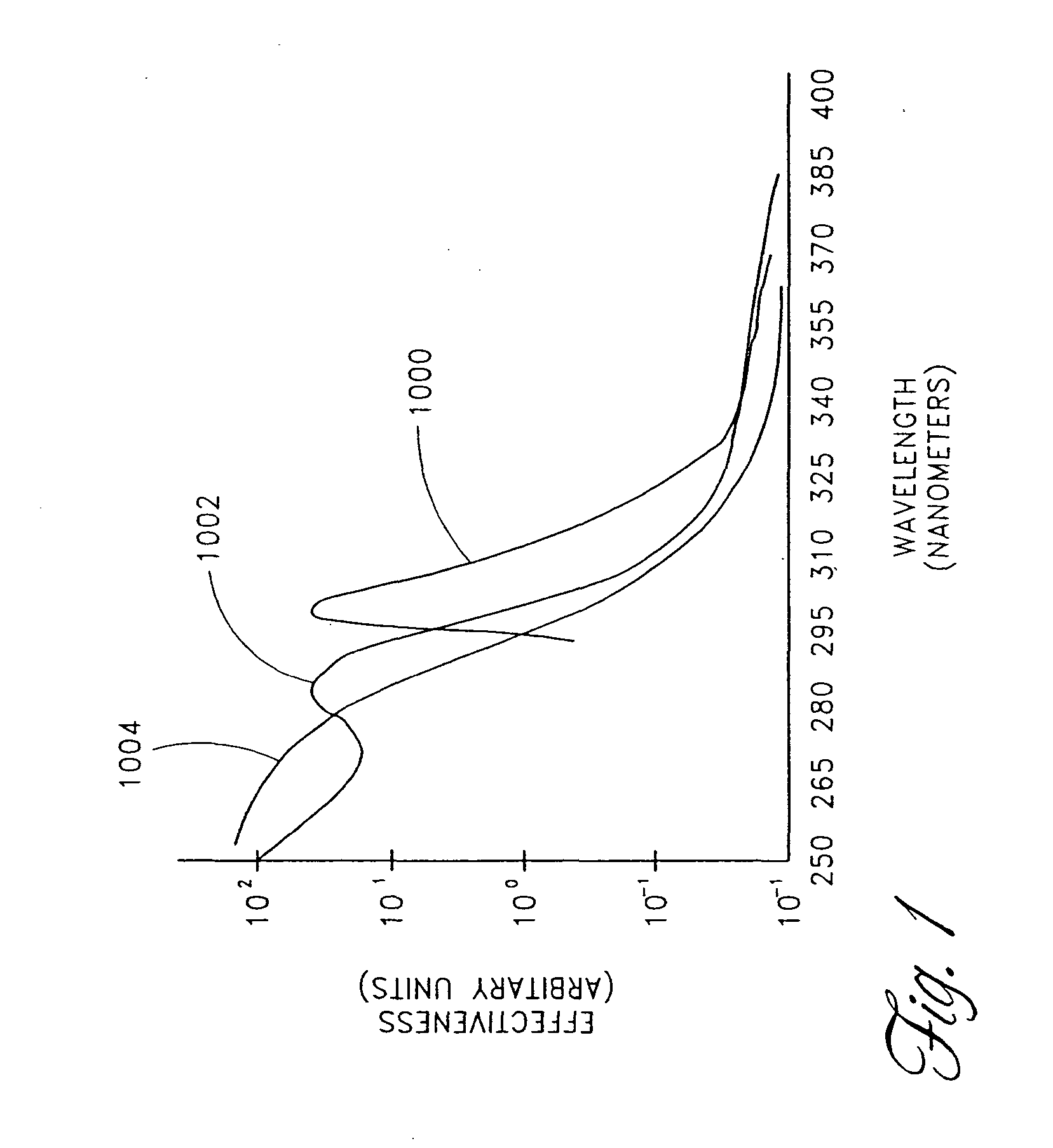
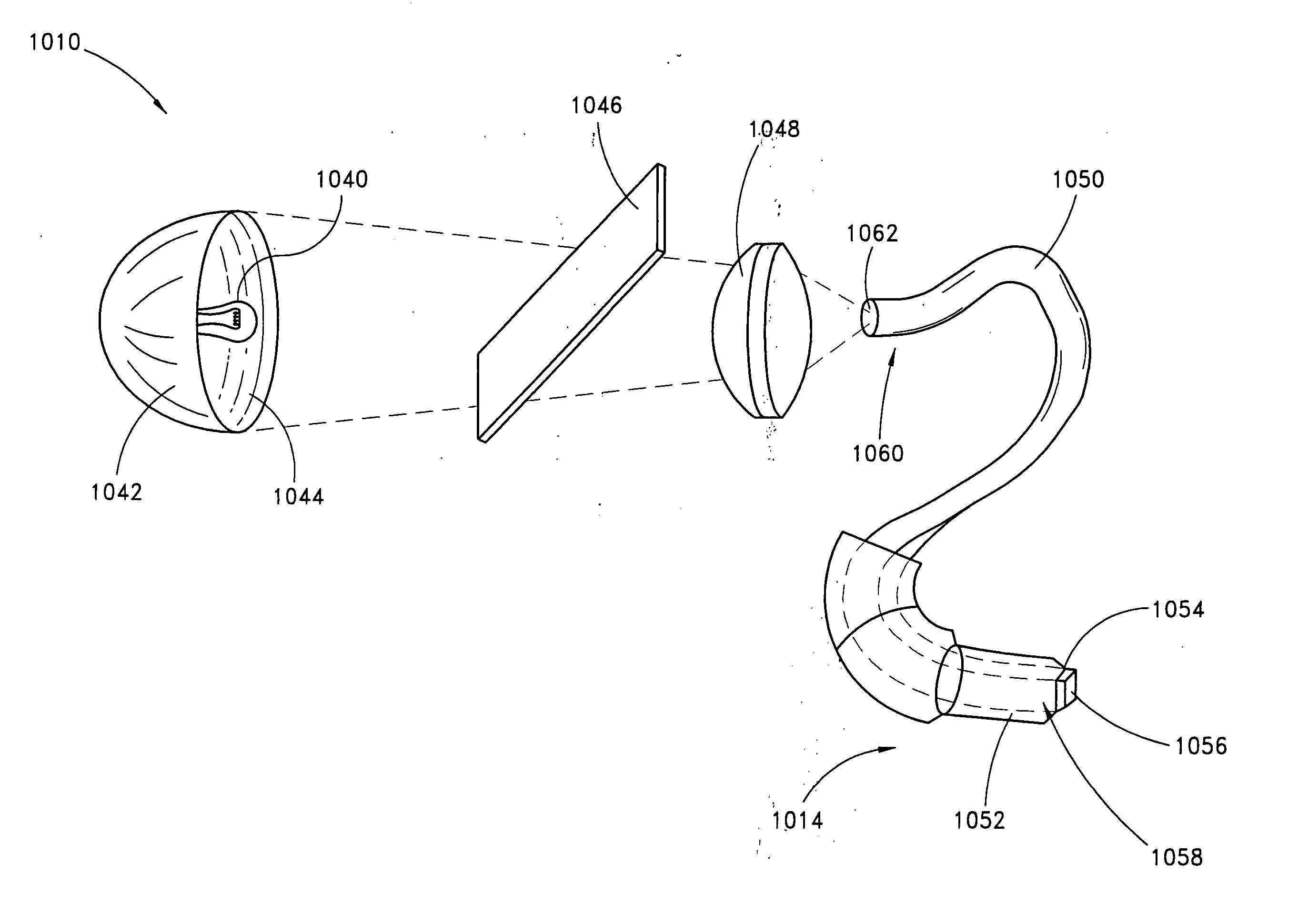
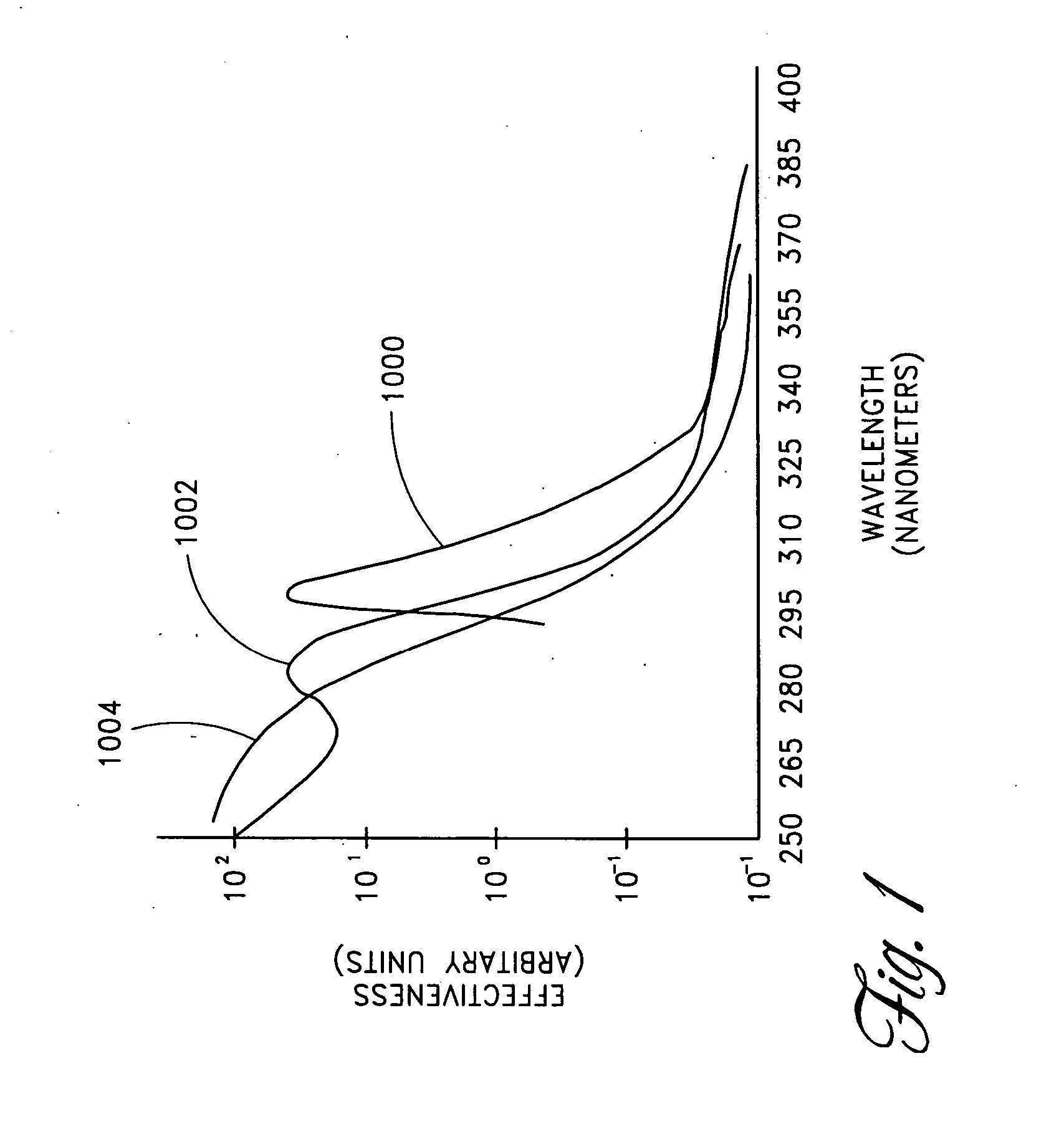
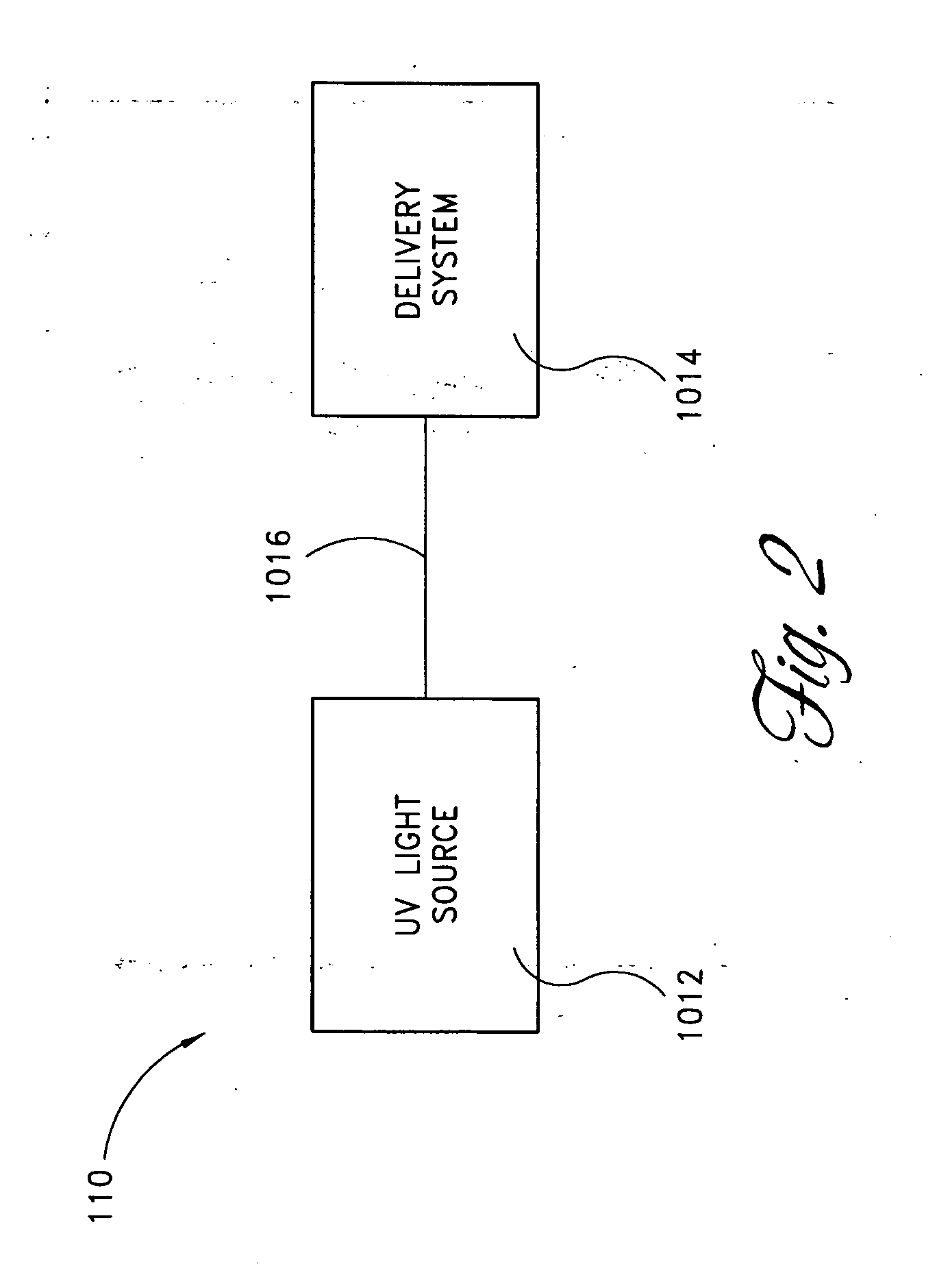
Skin disorders such as, for example, atopic dermatitis, dyshidrosis, eczema, lichen planus, psoriasis, and vitiligo, are treated by applying high doses of ultraviolet light to diseased regions of a patient's skin. The dosage exceeds 1 MED as determined for the particular patient and may range from about 1 MED to about 20 MED or higher. The ultraviolet light has a wavelength within the range of about 295 nanometers to about 320 nanometers. High doses of ultraviolet light are preferably restricted to diseased tissue areas. A specialized handpiece provides a beam profile especially suitable for application of controlled doses. A specialized delivery device is useful for UV treatment of tissue within the mouth.
Owner:MELA SCIENCES
Treatment of skin disorders with UV light and cooling
Skin disorders such as, for example, atopic dermatitis, dyshidrosis, eczema, lichen planus, psoriasis, and vitiligo, are treated by applying high doses of ultraviolet light to diseased regions of a patients skin. The dosage employed exceeds 1 MED, an MED being determined for the particular patient being treated, and may range from about 1 MED to about 20 MED or higher. The ultraviolet light has a wavelength within the range of between about 295 nanometers to about 320 nanometers and preferably is between about 300 nanometers and about 310 nanometers. High doses of ultraviolet light are restricted to diseased tissue areas so as to avoid risk of detrimental side affects in healthy skin, which is more susceptible to damage from UV light. Cooling the skin prior to and / or while exposing the skin to the UV light can be used to minimize tissue damage resulting from exposure to the UV light. Higher doses of UV light can therefore be employed without injurious affects.
Owner:PHOTOMEDEX
Device for oral UV photo-therapy
Skin disorders such as, for example, atopic dermatitis, dyshidrosis, eczema, lichen planus, psoriasis, and vitiligo, are treated by applying high doses of ultraviolet light to diseased regions of a patients skin. The dosage employed exceeds 1 MED, an MED being determined for the particular patient being treated, and may range from about 1 MED to about 20 MED or higher. The ultraviolet light has a wavelength within the range of between about 295 nanometers to about 320 nanometers and preferably is between about 300 nanometers and about 310 nanometers. High doses of ultraviolet light are preferably restricted to diseased tissue areas so as to avoid risk of detrimental side affects in healthy skin, which is more susceptible to damage from UV light. Cooling the skin prior to and / or while exposing the skin to the UV light can be used to reduce tissue damage resulting from exposure to the UV light. Higher doses of UV light can therefore be employed without injurious affects. A specialized handpiece provides a beam profile especially suitable for application of controlled doses. A specialized delivery device is useful for UV treatment of tissue within the mouth.
Owner:MELA SCIENCES
Il-21 antagonists
InactiveUS20070122413A1Increasing in vivo serum half-lifeModulate antibody responseNervous disorderAntibody mimetics/scaffoldsAutoimmune conditionAutoimmune disease
Monoclonal antibodies are identified that bind the IL-21 protein. These antibodies are used to identify regions of the IL-21 protein to where binding neutralizes IL-21 activity. Hybridomas and methods of producing anti-IL-21 monoclonal antibodies are described. The monoclonal antibodies are useful in treating IL-21-mediated diseases, which may include autoimmune and inflammatory diseases such as pancreatitis, type I diabetes (IDDM), Graves Disease, inflammatory bowel disease (IBD), Crohn's Disease, ulcerative colitis, irritable bowel syndrome, multiple sclerosis, rheumatoid arthritis, diverticulosis, systemic lupus erythematosus, psoriasis, ankylosing spondylitis, scleroderma, systemic sclerosis, psoriatic arthritis, osteoarthritis, atopic dermatitis, vitiligo, graft vs. host disease (GVHD), cutaneous T cell lymphoma (CTCL), Sjogren's syndrome, glomerulonephritis, IgA nephropathy, graft versous host disease, transplant rejection, atopic dermatitis, anti-phospholipid syndrome, and asthma, and other autoimmune diseases.
Owner:ZYMOGENETICS INC
Compositions for reducing oxidative stress and uses thereof
InactiveUS20100286056A1Reduce oxidative stressGood dispersionCosmetic preparationsHair cosmeticsDiseaseMedicine
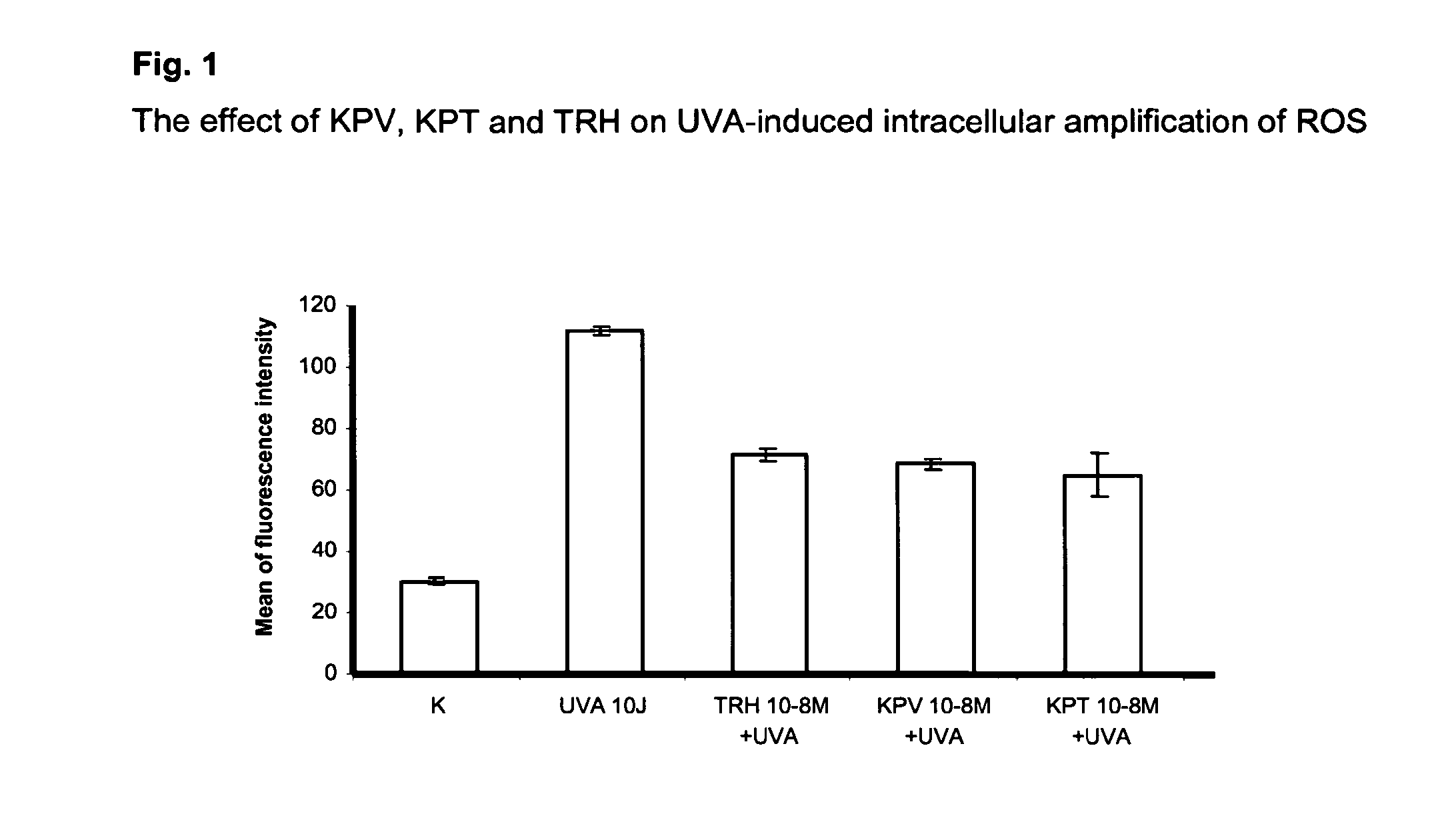
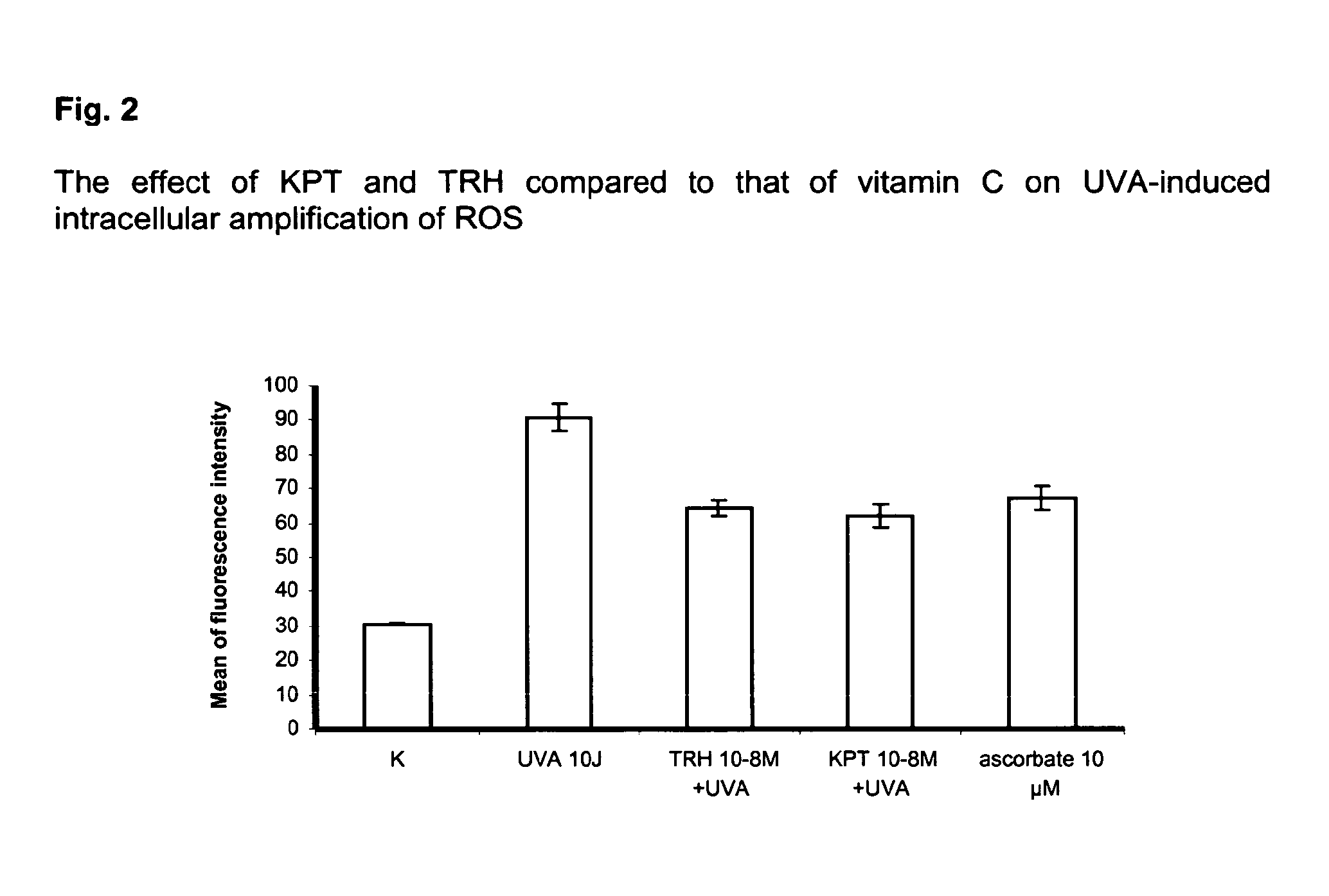
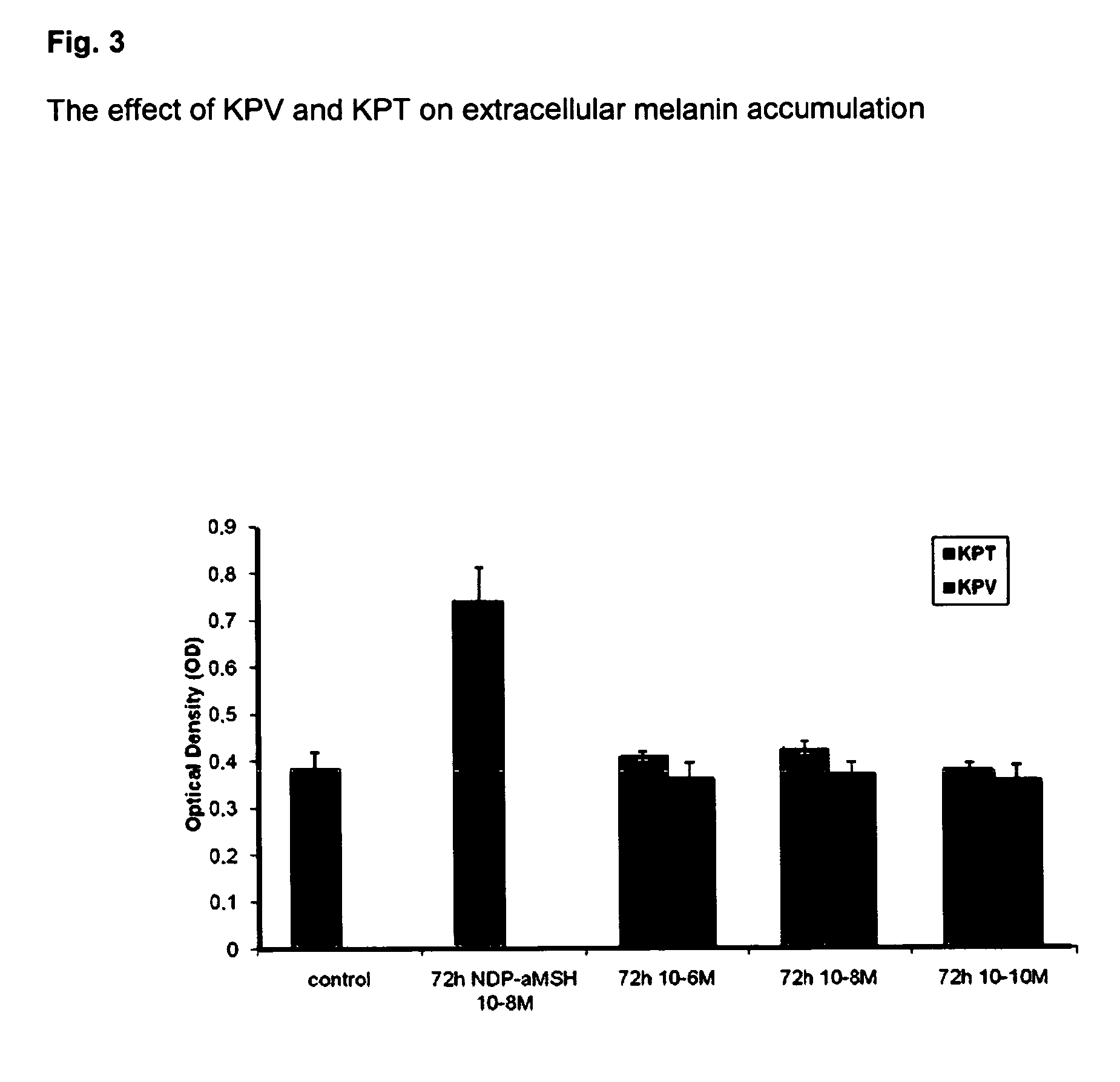
The present invention relates to the use of one or more tripeptides selected from the group consisting of NLys-Pro-ValC, NLys-Pro-ThrC and NpGLu-His-ProC for the reduction of oxidative stress. The above tripeptides are particularly useful for the treatment of a disease or damage caused by oxidative stress; such as vitiligo, scleroderma, necrosis, or erythema; furthermore, a disease or damage of the hair, like premature hair loss or premature formation of grey hair. Furthermore the invention relates the cosmetic use of the above tripeptides, in particular against skin aging. Further the invention relates cosmetic compositions containing at least one of said tripeptides.
Owner:UNIVSKLINIKUM MUNSTER
Gel composition containing tacrolimu and its preparation method and medicinal application
InactiveCN101288643AEasy to cleanNo bad smellOrganic active ingredientsPharmaceutical delivery mechanismDiseaseAdditive ingredient
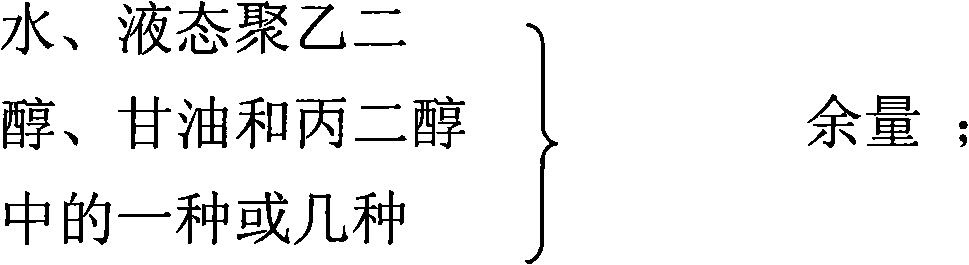
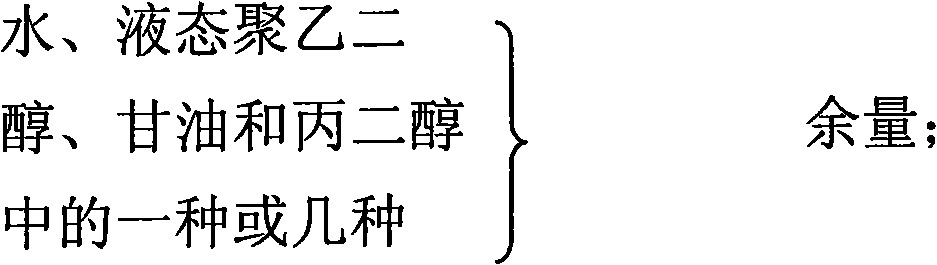
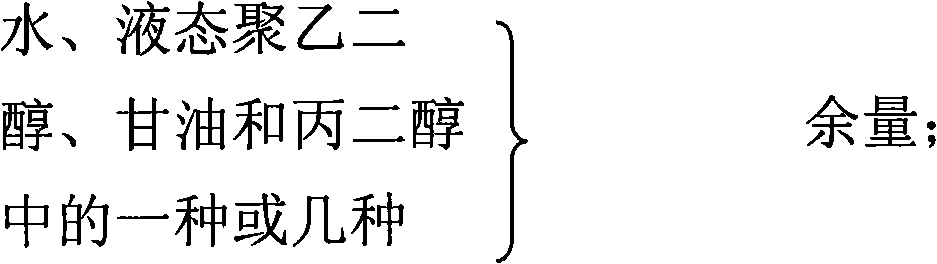
The invention relates to a gel composition containing tacrolimus, which contains the tacrolimus and ingredients of a matrix, wherein, the ingredients of the matrix contain one or more of liquid polyethylene glycol, glycerin and propylene glycol, the content of the tacrolimus in the gel is 0.01 percent to 0.5 percent, and the weight ratio of the tacrolimus to one or more of the liquid polyethylene glycol, the glycerin and the propylene glycol is 1: (50 to 3000). The invention further relates to a preparation method of the gel composition of the tacrolimus and the application of the gel composition in the preparation of drugs for the treatment of atopic dermatitis, vitiligo, psoriasis, hormone-dependent dermatitis, intractable neurodermatitis, lupus erythematosus, alopecia areata and other diseases.
Owner:杨喜鸿
Treatment of skin disorders with UV light and cooling
Skin disorders such as, for example, atopic dermatitis, dyshidrosis, eczema, lichen planus, psoriasis, and vitiligo, are treated by applying high doses of ultraviolet light to diseased regions of a patients skin. The dosage employed exceeds 1 MED, an MED being determined for the particular patient being treated, and may range from about 1 MED to about 20 MED or higher. The ultraviolet light has a wavelength within the range of between about 295 nanometers to about 320 nanometers and preferably is between about 300 nanometers and about 310 nanometers. High doses of ultraviolet light are restricted to diseased tissue areas so as to avoid risk of detrimental side affects in healthy skin, which is more susceptible to damage from UV light. Cooling the skin prior to and / or while exposing the skin to the UV light can be used to minimize tissue damage resulting from exposure to the UV light. Higher doses of UV light can therefore be employed without injurious affects.
Owner:MELA SCIENCES
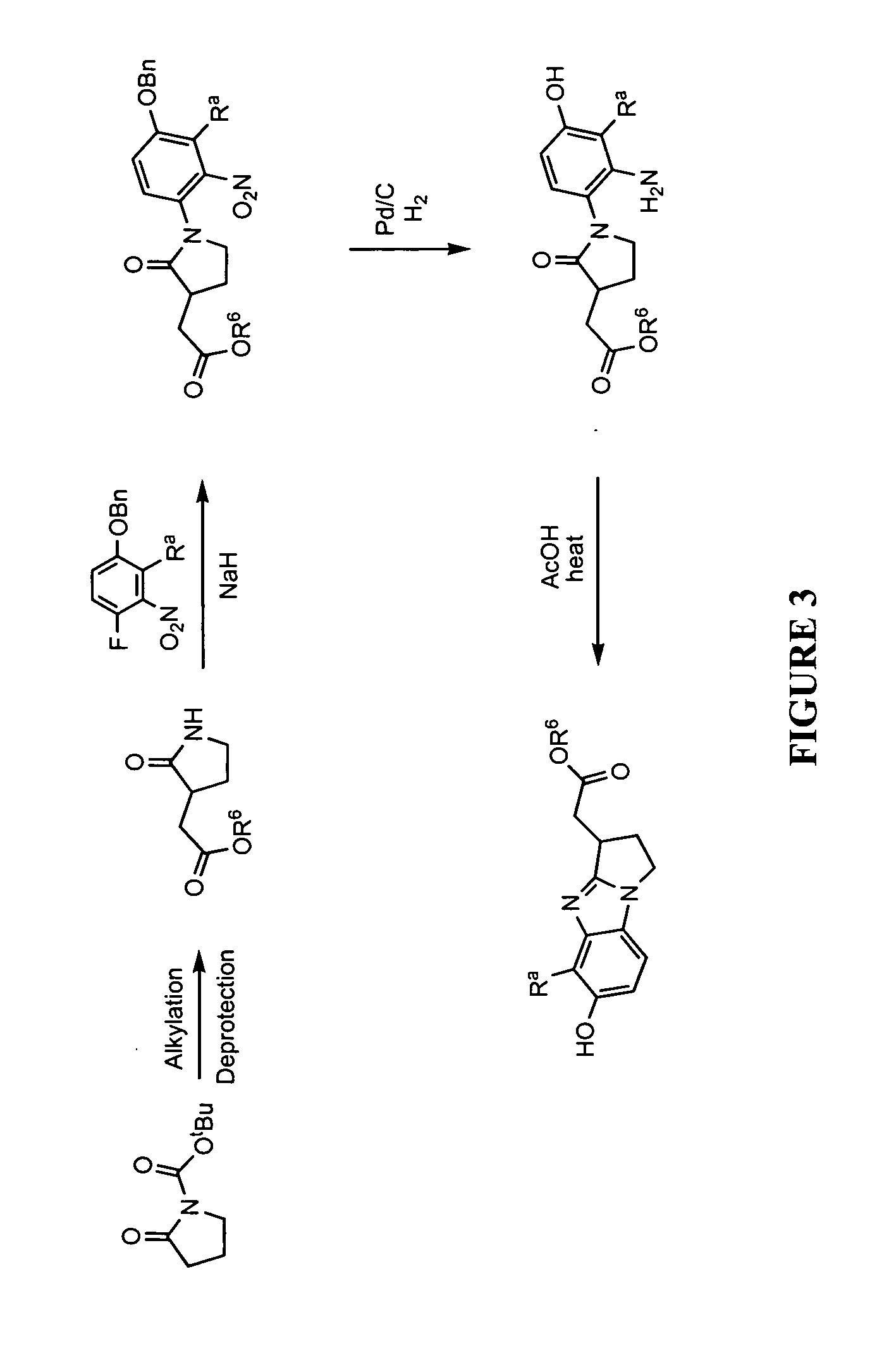
Method for treating vitiligo with a prostaglandin analogue
InactiveUS20120251613A1Improves pigmentationReduced maintenance dosageBiocidePeptide/protein ingredientsTransdermal patchSkin surface
A method of stimulating melanogenesis in a skin surface of a patient, by treating the skin surface with a topical formulation comprising an effective amount of 17-phenyl-18,19,20-trinor-PGF2α ethyl amide in a dermatologically acceptable carrier. The topical formulation may be a cream, a gel, a lotion, a spray, an ointment, an aqueous solution, a nonaqueous solution, or a transdermal patch. The dermatologically acceptable carrier may contain an oily carrier or an aqueous carrier.
Owner:AGILA SPECIALTIES PVT LTD +1
Treatment of vitiligo by micropore delivery of cells
InactiveUS20070225779A1Treating and preventing lossTreating and preventing pigmentation lossIn-vivo radioactive preparationsSurgical instrument detailsPigmentationsMelanin
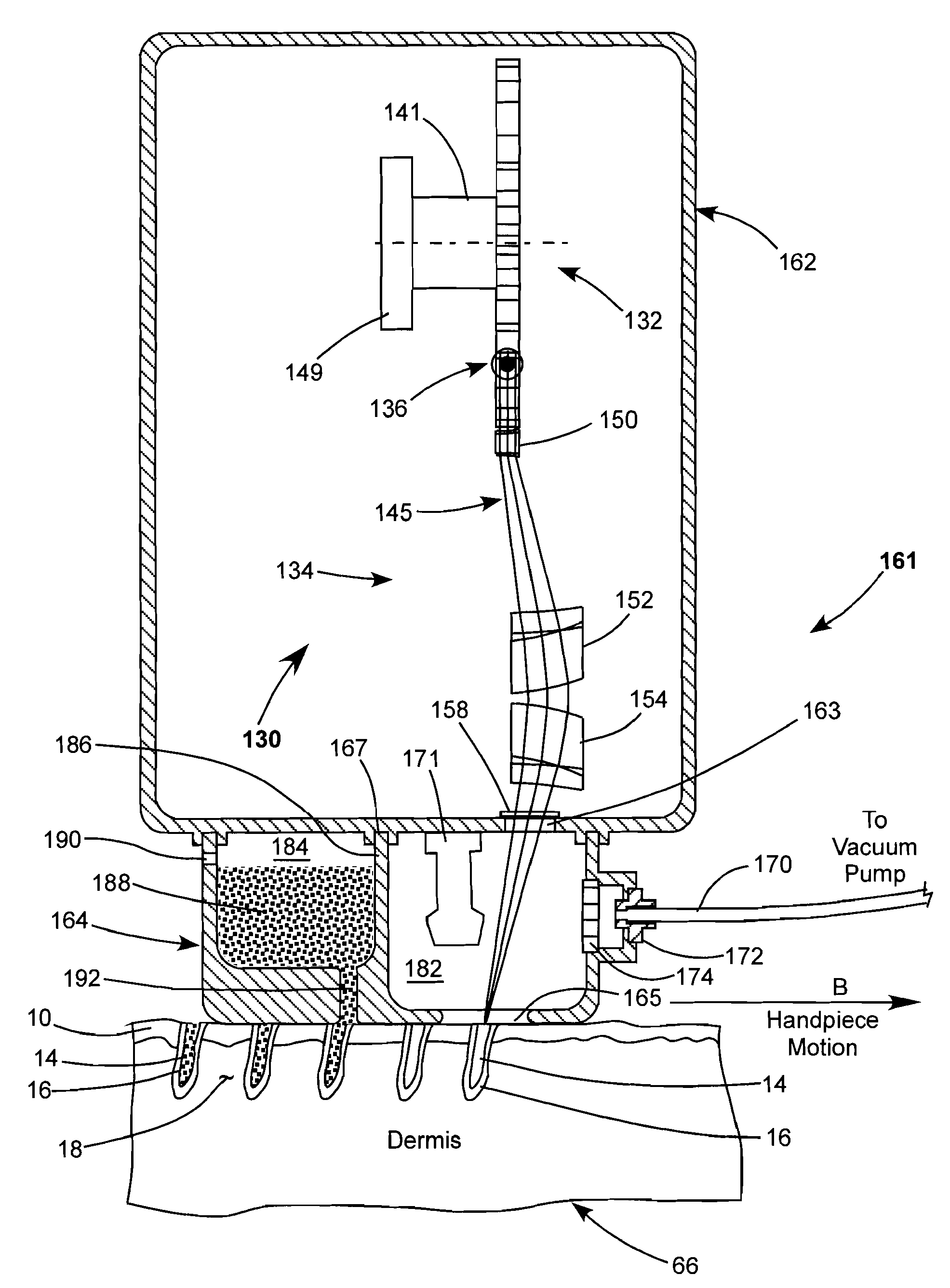
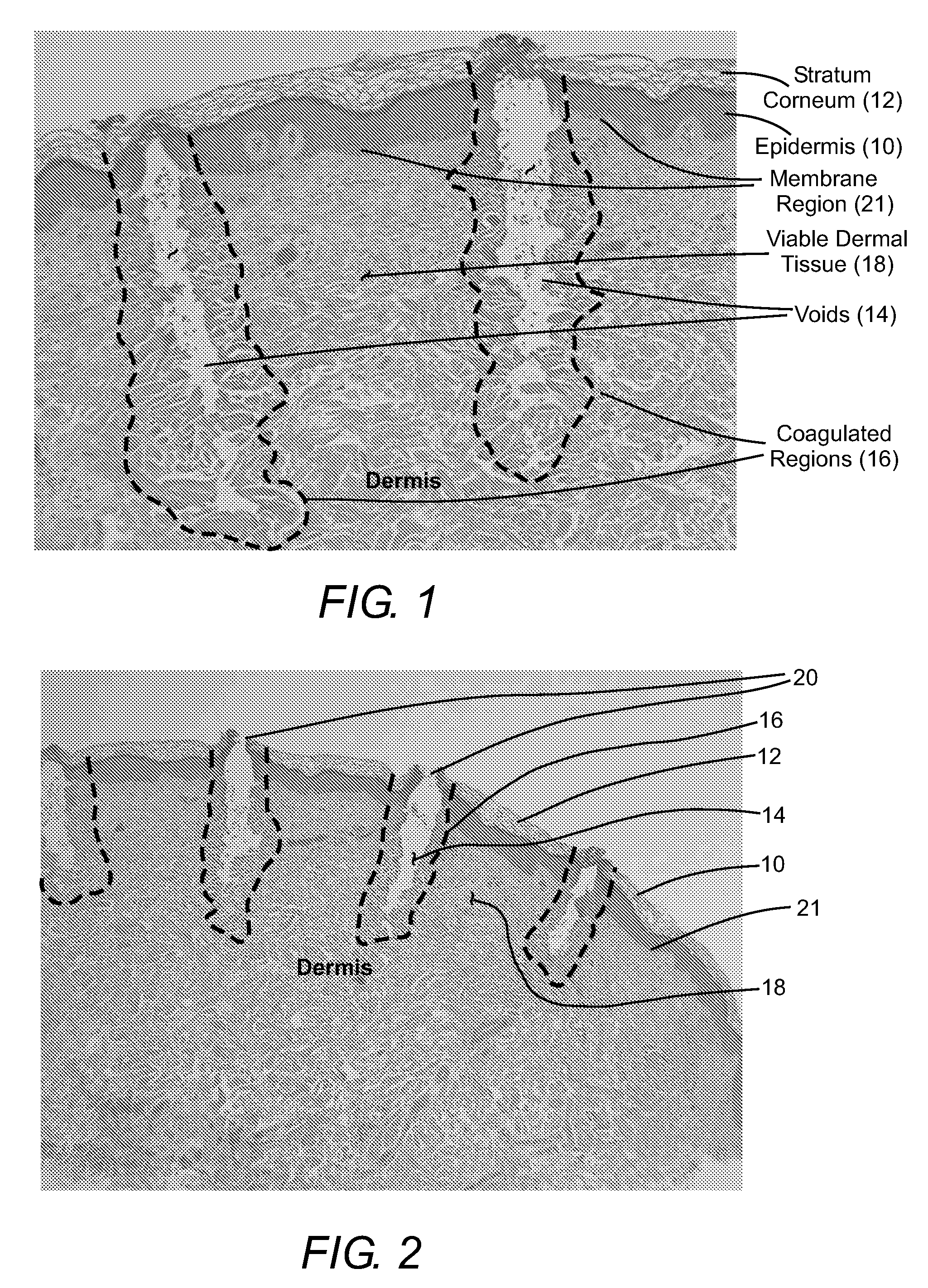
Methods, compositions and apparatus for restoring pigmentation to skin that has suffered pigment loss are described. The methods include creating a plurality of spaced-apart microchannels or voids in the skin and depositing into the micropore channels or voids a composition comprising at least one cell capable of producing melanin, a growth factor, and, optionally, a scaffolding material, a differentiation factor, a proliferation factor, and / or a pigment. Alternatively, a composition comprising a pigment can be deposited into the micropore channels or voids to restore pigmentation to the skin.
Owner:RELIANT TECH INC
Use of locally applied DNA fragments
Methods of treatment or prevention of hyperproliferative diseases or pre-cancerous conditions affecting epithelial cells, such as psoriasis, vitiligo, atopic dermatitis, or hyperproliferative or UV-responsive dermatoses, hyperproliferative or allergically mediated diseases of other epithelia and methods for reducing photoaging, or oxidative stress or for prophylaxis against or reduction in the likelihood of the development of skin cancer, are disclosed.
Owner:TRUSTEES OF BOSTON UNIV
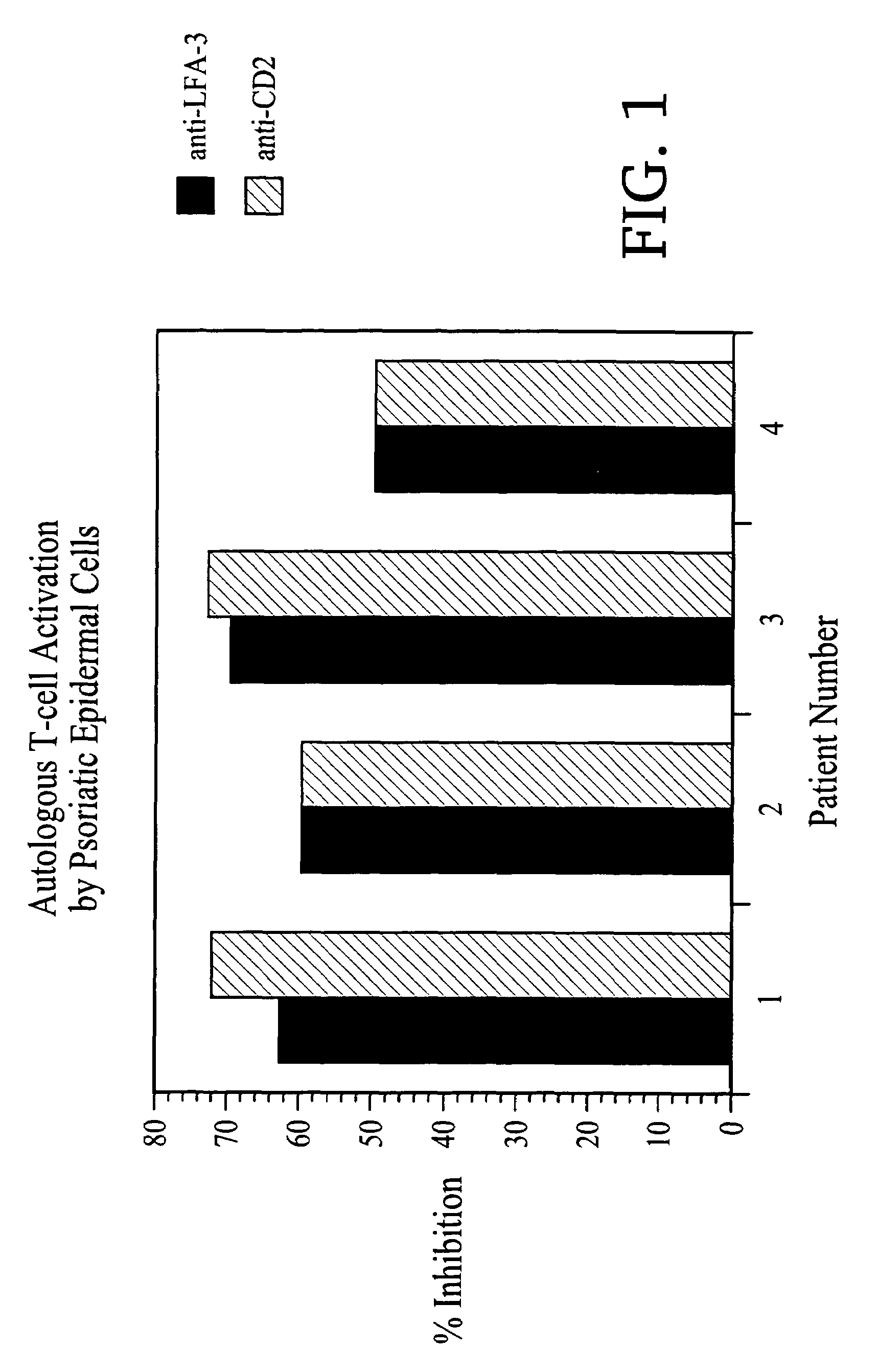
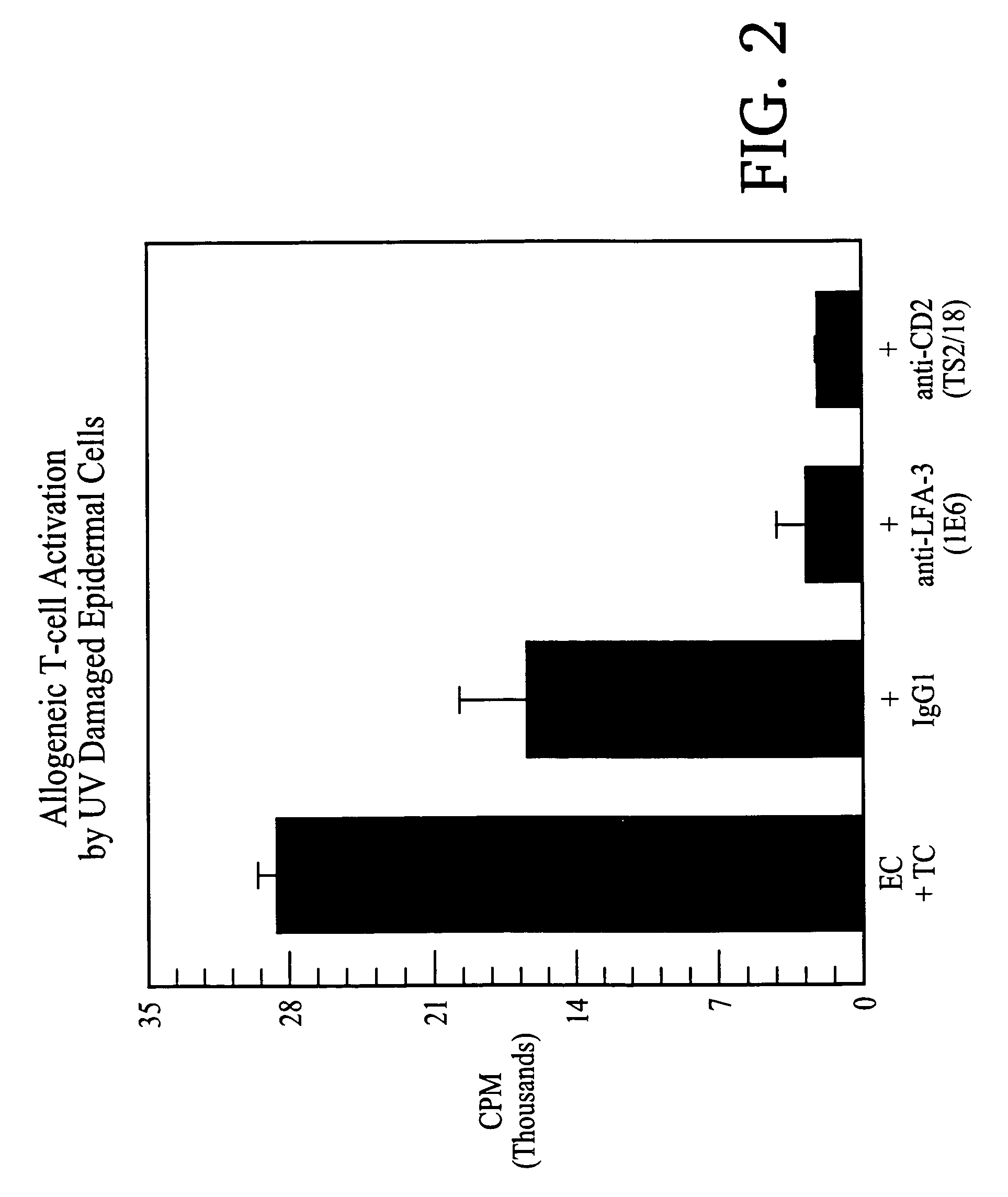
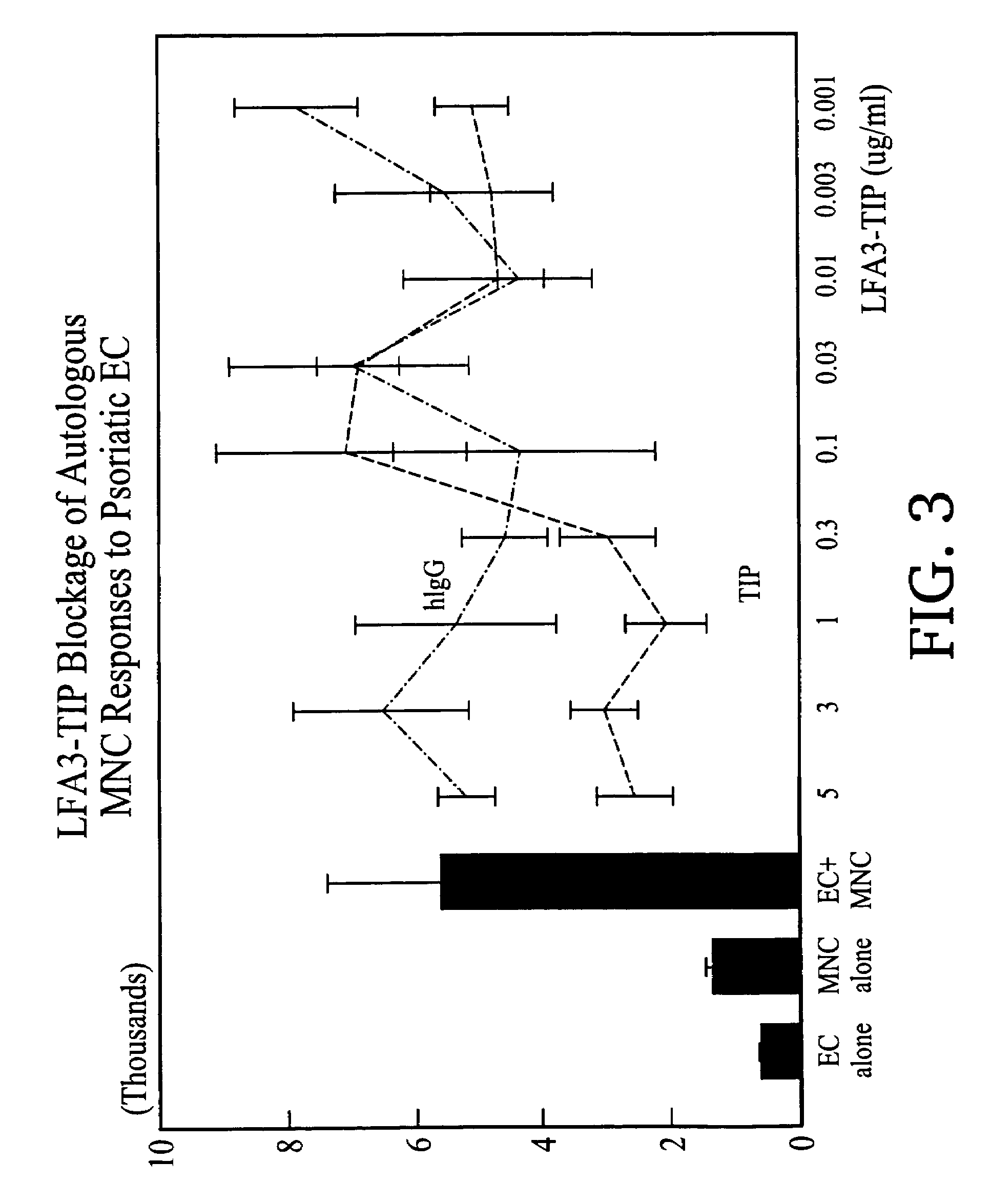
Methods of treating skin conditions using inhibitors of the CD2/LFA-3 interaction
InactiveUS7323171B2Increased activationImprove presentationCompound screeningApoptosis detectionAntigenMammal
Methods of using inhibitors of the CD2 / LFA-3 interaction in treating skin conditions characterized by increased T cell activation and abnormal antigen presentation in the dermis and epidermis in mammals, including humans. Such conditions include psoriasis, UV damage, e.g., photoaging, atopic dermatitis, cutaneous T cell lymphoma such as mycosis fungoides, allergic and irritant contact dermatitis, lichen planus, alopecia areata, pyoderma gangrenosum, vitiligo, ocular cicatricial pemphigoid, and urticaria.
Owner:ASTELLAS US
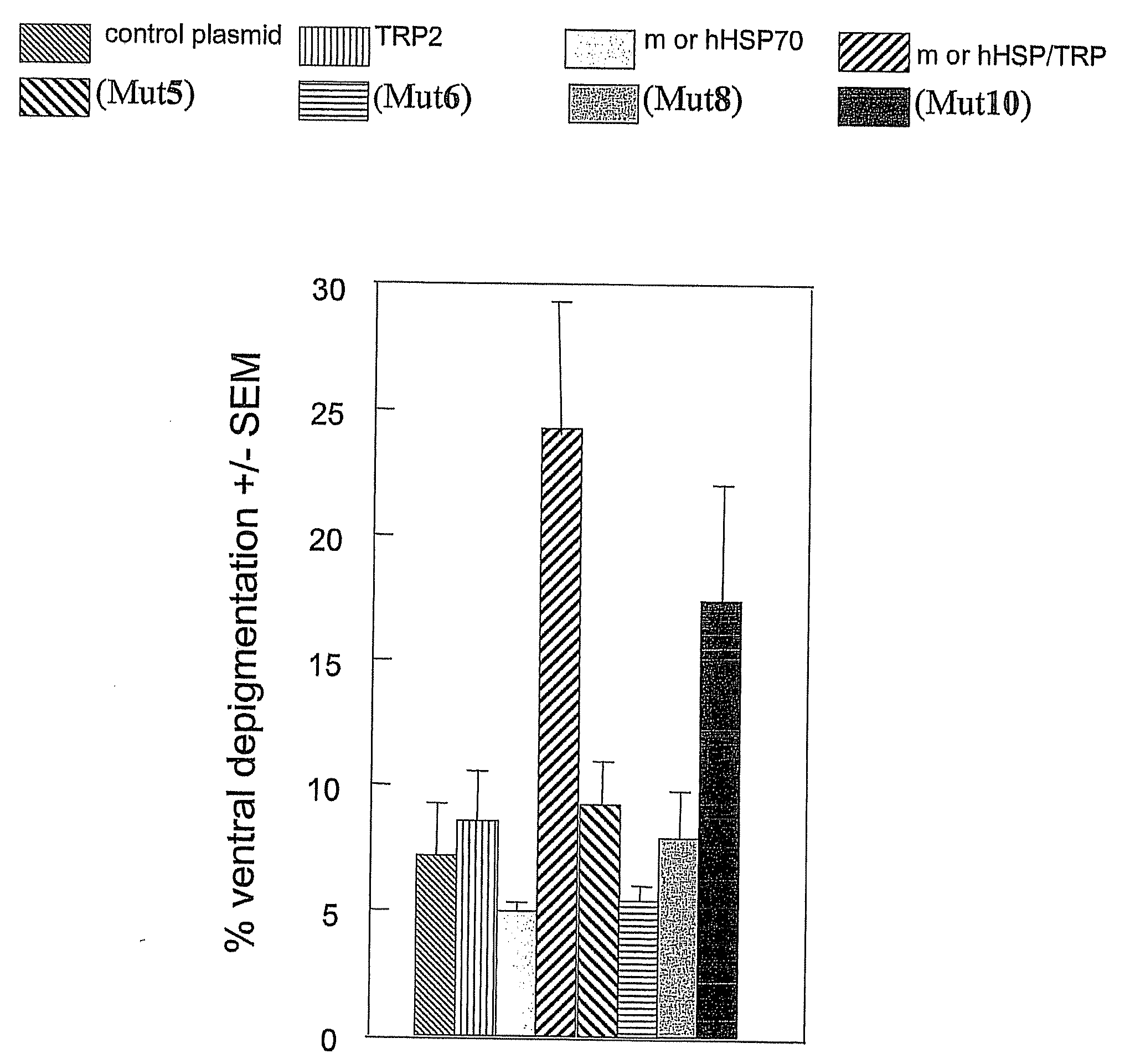
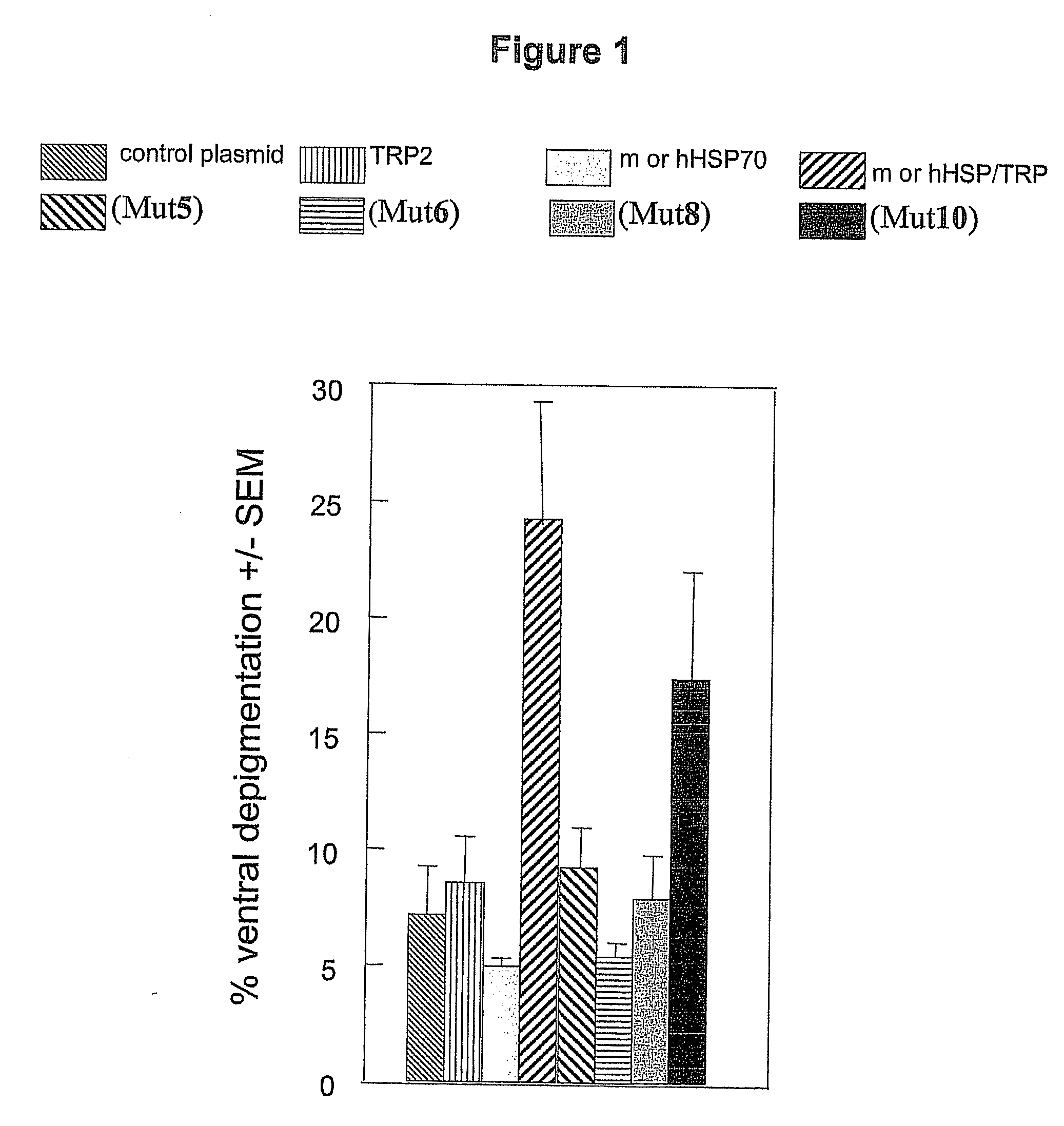
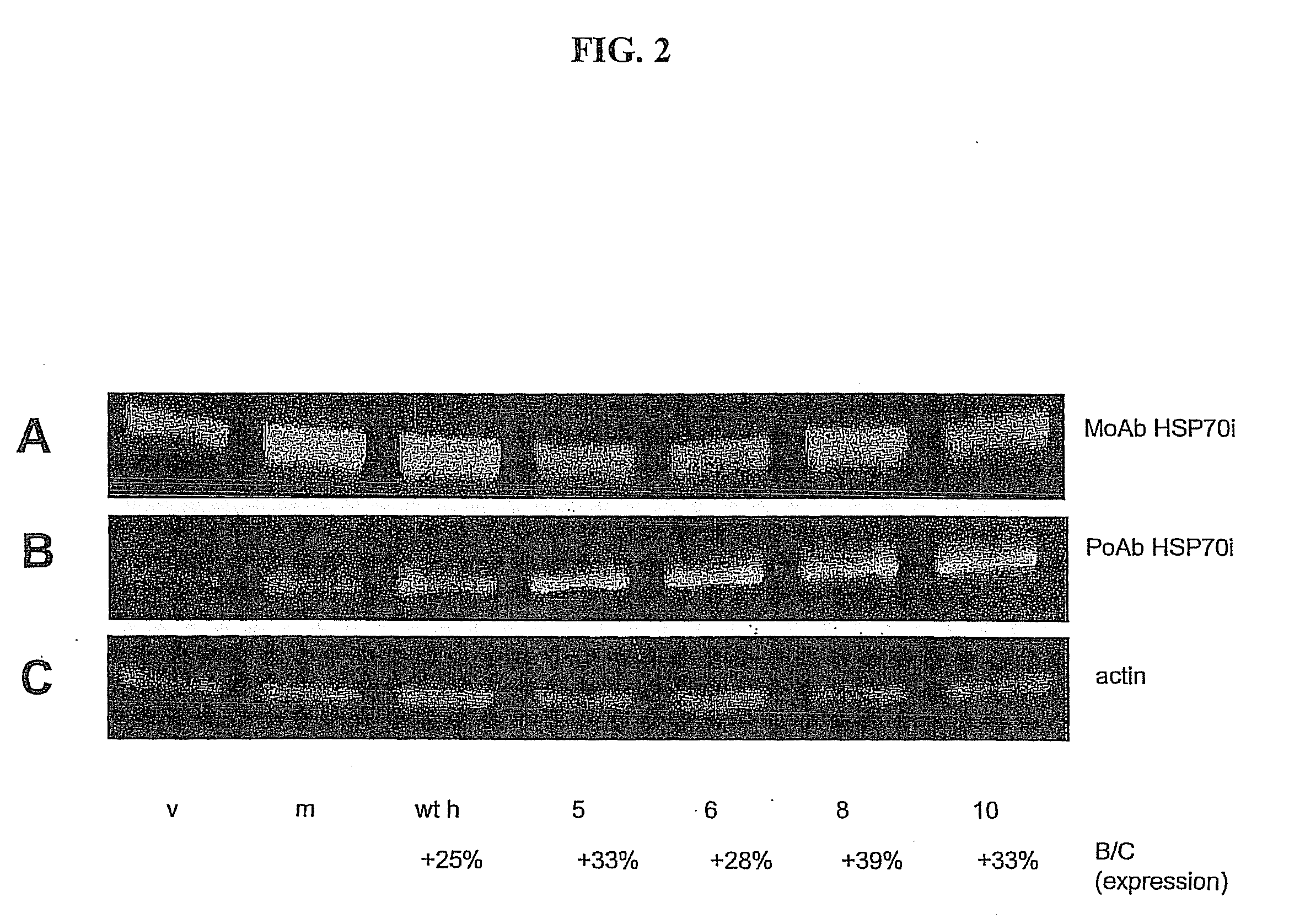
HSP70-Based Treatment for Autoimmune Diseases and Cancer
InactiveUS20110028403A1Inhibition of activationPeptide/protein ingredientsAntibody mimetics/scaffoldsDiseaseDendritic cell
A non-natural HSP70 activating region that activates dendritic cells. Polypeptides that bind to the HSP70 activating region can be used to treat autoimmune diseases, such as vitiligo, by binding to HSP70 and preventing HSP70 form activating dendritic cells. The HSP70 binders can be constructed in the form of fusions proteins with a trimerizing structural element that may associate to form a trimeric complex. Pharmaceutical compositions and methods for treating vitiligo using the HSP70 binding proteins, fusion proteins and complexes.
Owner:ANAPHORE INC
Substituted tricyclic acid derivatives as S1P1 receptor agonists useful in the treatment of autoimmune and inflammatory disorders
Owner:ARENA PHARMA
Methods of treating diseases which are mediated by cutaneous lymphocyte antigen positive cells
ActiveUS8388964B2Improves and prevents and inhibits and reduces skinImproves and prevents and inhibits and reduces pruritisCompounds screening/testingLuminescence/biological staining preparationDiseaseContact dermatitis
The present invention relates to methods of treating patients suffering from itching and puritis mediated by cutaneous lymphocyte antigen positive T cell. In particular, diseases or disorders including contact dermatitis, drug induced delayed type cutaneous allergic reactions, toxic epidermal necrolysis, cutaneous T cell lymphoma, bullous pemphigoid, alopecia aereata, vitiligo, acne rosacea, prurigo nodularis, and herpes simplex virus, or combination thereof will benefit from the administration of an IL-31 antagonist. The invention also includes methods of predicting a therapeutically responsive patient population.
Owner:ZYMOGENETICS INC +1
Methods and mechanisms involving hyperpigmentation particularly for african american skin
Sets of genes are identified that show modulated activity in hyperpigmented sun-exposed (HE) and non-hyperpigmented sun-exposed (NHE) skin, when compared to non-hyperpigmented non-exposed (NHNE) skin. The modulated sets of genes reveal important information about the genetic changes that take place in skin as a result of environmental exposure and damage. The modulated sets of genes may be used to fabricate custom DNA microarrays for evaluating patients with skin diseases or disorders. The microarrays may also be used to screen new substances for treating skin diseases and disorders. The modulated gene sets, and substances that target them, may also be used to develop therapies for individuals who suffer from hypopigmentation, such as those with Fitzpatrick type I skin or vitiligo.
Owner:HAMPTON UNIVERSITY
An External use medicine for treating skin pigment reduction and its preparing method
This invention relates to an external use medicine for treating skin pigment reduction and its preparing method. This external use medicine is prepared by using alcohol, radices polygoni multiflori, fructus psoraleae, flos sophorae immaturus, dioxyacetonem through refining. The medicine has the strongpoint of fast effectiveness, high curative effect, innocuity, no side-effect, convenience in use and low price, especially suitable for treatment of vitiligo.
Owner:成中田
Dermatological pharmaceutical composition for the treatment of skin inflammation diseases, such as dermatitis, atopic dermatitis, vitiligo, alopecia areata, acne, psoriasis, pruritus or combinations of same
InactiveUS20110129546A1Improve antioxidant capacityGood anti-inflammatory effectBiocideAntimycoticsDiseaseIsoflavones
The invention relates to a dermatological pharmaceutical composition for the treatment of skin inflammation diseases, such as dermatitis, atopic dermatitis, vitiligo, alopecia areata, acne, psoriasis and pruritus. The invention comprises a base anti-inflammatory agent, such as indometacin; one or more optional active ingredients selected alternatively from among at least a corticoid and an antibiotic; and a combination of topical antioxidants used to potentiate the anti-inflammatory effect, selected from among green tea, lipoic acid, curcumin, ascorbyl palmitate, Coenzyme Q10, resveratrol, Pycnogenol™, L-camosine, taurine, vitamin E, vitamin C, papaya extract, isoflavones, manganese, lycopene and quercetin. At least one of the topical antioxidants is a peroxisome proliferator-activated receptor-gamma (PPAR-γ) activator. The invention also includes at least one antioxidant substance with an antiproliferative effect on keratonocytes, e.g. manganese, and at least one substance that blocks tumour necrosis factor-alpha (TNF-α) or other cytokines that provoke the acute phase of the inflammatory reaction, also with an antiproliferative effect, e.g. pentoxifylline.
Owner:UMBERT MILL IGNACIO
Leucoderma covering agent
ActiveCN102525847AEffective waterproofEffective anti-sweatCosmetic preparationsBody powdersVitamin E AcetateWear resistant
The invention discloses a leucoderma covering agent. The leucoderma covering agent is a covering liquid or covering cream, wherein the covering liquid mainly comprises 1,2-propyleneglycol, jojoba oil, a preservative, a surfactant, vitamin E acetate, 1,3-dihydroxyacetone, an edible pigment and deionized water; and the covering cream mainly comprises magnesium aluminum silicate, 1,2-propyleneglycol, methyl parahydroxybenzoats, propyl p-hydroxybenzoate, imidazolidinyl urea, talcpowder, titanium dioxide, iron oxide, jojoba oil, vegetable fat, vitamin E acetate, stearyl alcohol, stearic acid, decapolyglycerol monooleate, diolein, triethanolamine and deionized water. The covering liquid has waterproof, sweatproof and wear-resistant effects, and is durable; the covering cream is quick in coloration and natural in color formation, and is convenient to use; and the covering liquid and the covering cream are combined to provide comprehensive covering for leucoderma patients.
Owner:刘飞
Substituted tricyclic acid derivatives as s1p1 receptor agonists useful in the treatment of autoimmune and inflammatory disorders
The present invention relates to certain substituted tricyclic acid derivatives of Formula (I) and pharmaceutically acceptable salts thereof, which exhibit useful pharmacological properties, for example, as agonists of the S1P1 receptor. Also provided by the present invention are pharmaceutical compositions containing compounds of the invention, and methods of using the compounds and compositions of the invention in the treatment of S1P1-associated disorders, for example, psoriasis, rheumatoid arthritis, Crohn's disease, transplant rejection, multiple sclerosis, systemic lupus erythematosus, ulcerative colitis, type I diabetes, acne, myocardial ischemia-reperfusion injury, hypertensive nephropathy, glomerulosclerosis, gastritis, polymyositis, thyroiditis, vitiligo, hepatitis, biliary cirrhosis, microbial infections and associated diseases, viral infections and associated diseases, diseases and disorders mediated by lymphocytes, auto immune diseases, inflammatory diseases, and cancer.
Owner:ARENA PHARMA
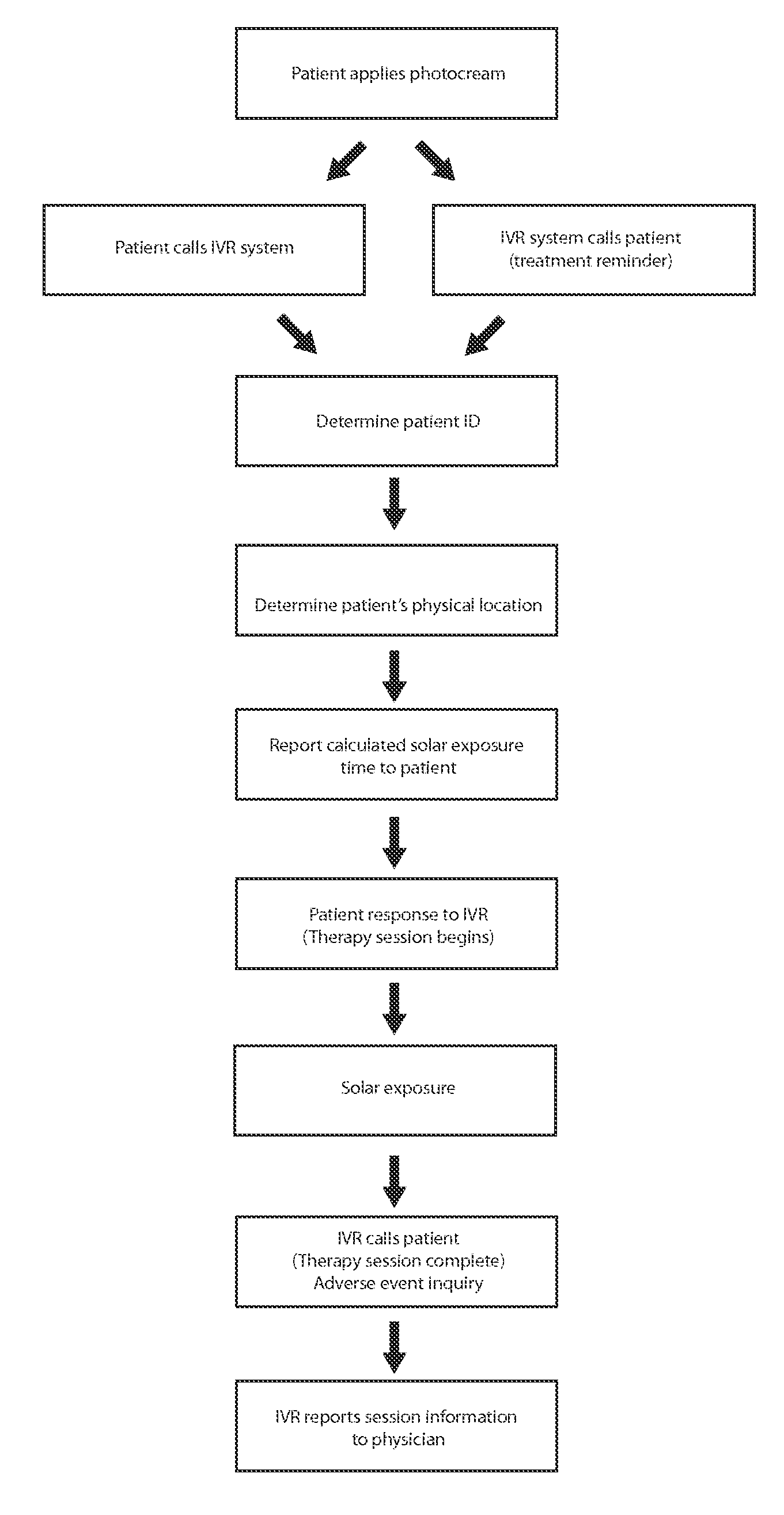
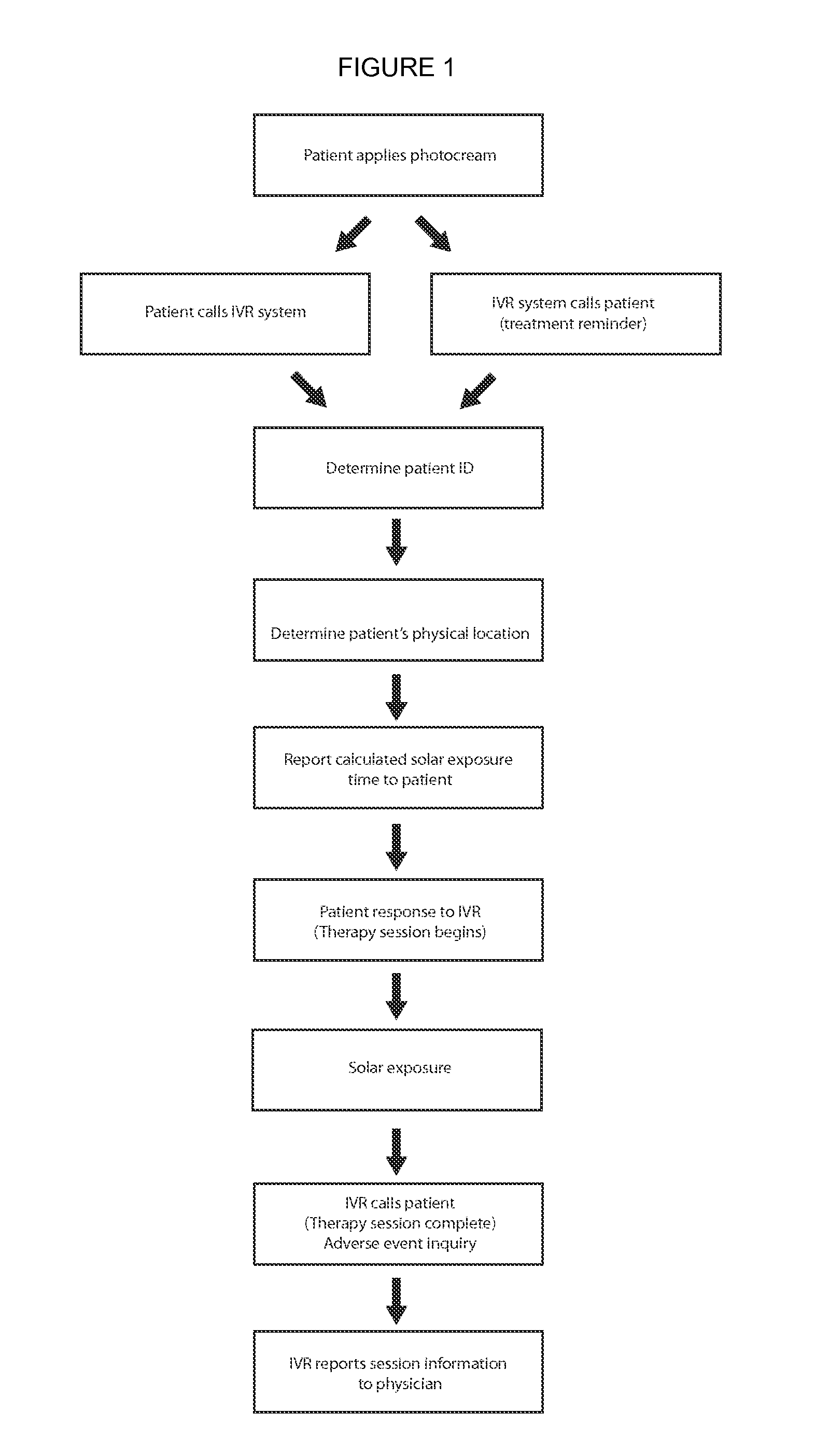
Methods and compositions for administering a specific wavelength phototherapy
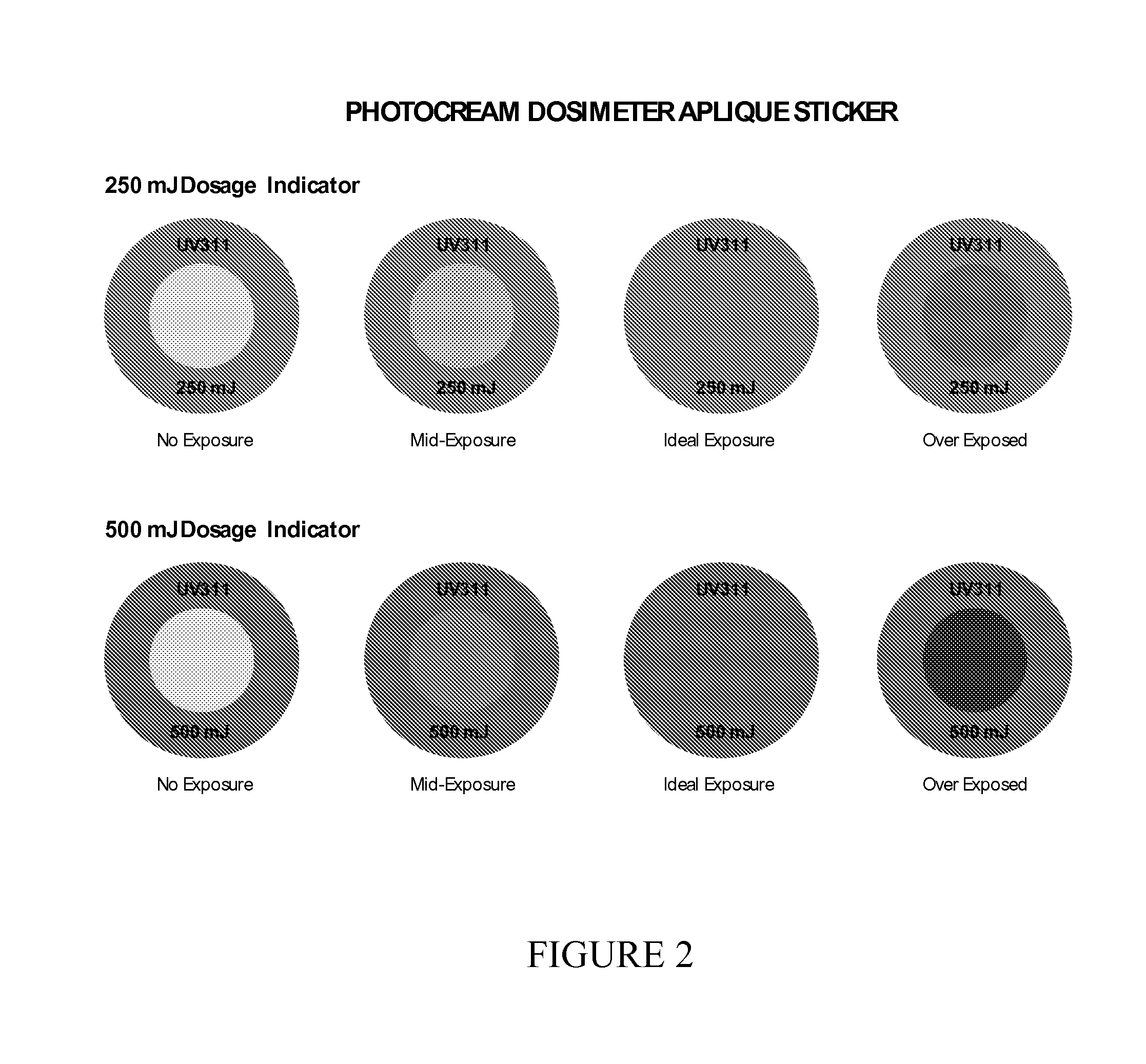
ActiveUS20140121732A1Avoid lightCosmetic preparationsHair cosmeticsDosimeterElectromagnetic radiation
Methods are disclosed for administering electromagnetic radiation (EMR), which may include filtering EMR from part of the EMR spectrum while allowing passage of EMR at a desired wavelength range. Uses may include the treatment of such conditions as psoriasis, vitiligo, pruritus, acne, vitamin-D deficiency and atopic dermatitis. Topical creams, sprays, or other compositions may be used, with or without dosimeters, or in conjunction with an application, which calculates local UV exposure and other factors relating to dosage.
Owner:JUPITER WELLNESS INC
Tissue engineering material-based culture method and applications of melanophore
ActiveCN101856517ASuitable for growthEasy to shapeArtificial cell constructsVertebrate cellsDiseaseBiocompatibility Testing
The invention discloses a tissue engineering material-based culture method and applications of melanophore. In the method of the invention, the tissue engineering technology is used to build carrier material with good biocompatibility and perform culture (co-culture) and transfer of melanophore or melanophore, fibroblast and keratinocyte and the method can be used in depigmentation diseases such as vitiligo and for the regulation of skin color. The invention relates to the preparation of the carrier material with biocompatibility and the building and transplanting of the cell-carrier composite material. The carrier material can be used to provide good carrier for cell culture, maintain cellular activity and solve the problem that the cell suspension is easy to lose in the cellular transplantation process; the inoculum density and culture time of cells can be regulated at the same time, the regulation to the skin colors of different individuals can be realized; and the carrier materialhas good performances such as damage resistance, tear resistance and easy transfer, has good function of promoting wound healing, and satisfies the use requirements of the large-area depigmentation disease and the regulation of skin color.
Owner:HANGZHOU THIRD HOSPITAL +1
Application of 20(R)-ginsenoside Rg3 in preparation of medicines for treating leucoderma
InactiveCN103845349AReduce sizeIncrease the number ofOrganic active ingredientsDermatological disorderSide effectCurative effect
The invention discloses a novel application of 20(R)-ginsenoside Rg3 in preparation of medicines for relieving or / and treating leucoderma. Tests show that the 20(R)-ginsenoside Rg3 is significant in treating effects for the leucoderma, quick in effectiveness, low in toxic and side effects, safe, efficient, stable, simple in preparation process, suitable for industrial production, and prone to popularization. The application provides a novel medicine source for preventing and treating the leucoderma and complications thereof.
Owner:富力
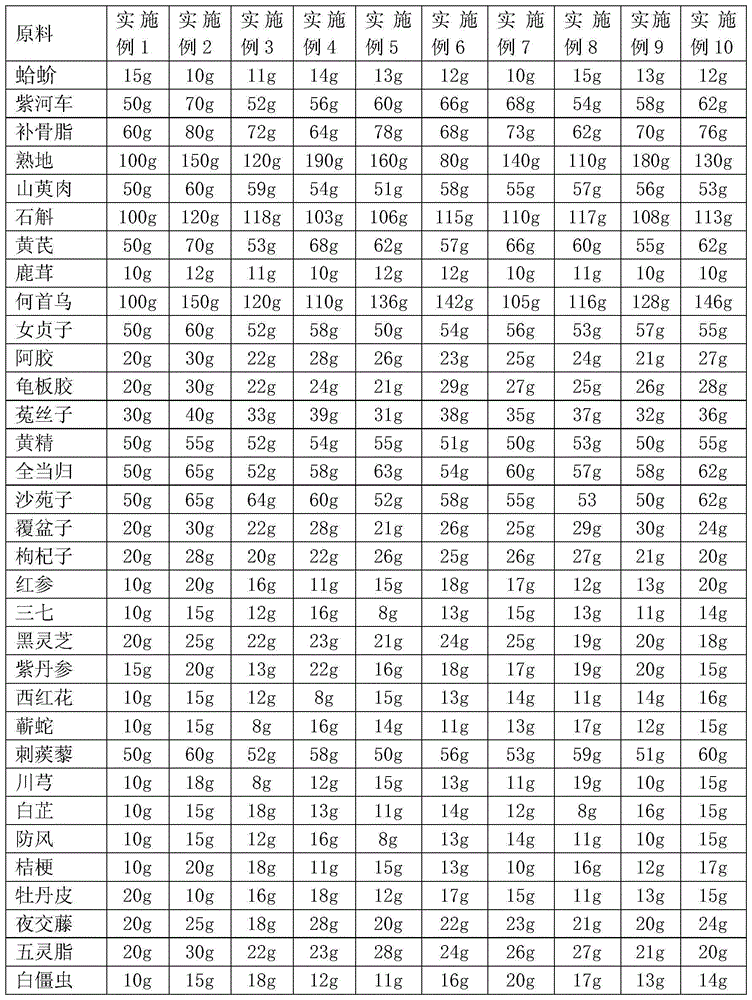
Chinese patent medicine for treating leucoderma
InactiveCN102416079APromote absorptionEnhance physical fitnessDermatological disorderPlant ingredientsLiver and kidneyCutaneous microcirculation
The invention relates to a Chinese patent medicine for treating leucoderma. The Chinese patent medicine consists of eclipta alba, tuber fleeceflower root, Chinese angelica, szechuan lovage rhizome, astragalus, largehead atractylodes rhizome, tuckahoe, glossy privet fruit, black sesame, suberect spatholobus stem, divaricate saposhnikovia root, fineleaf schizonepeta herb, red paeony root, flowery knotweed stem, knotweed, saffron, tribulus fruit, black soybean hull, malaytea scurfpea fruit and liquoric root, wherein various formulations for oral administration are prepared from the Chinese medicines according to the conventional preparation method. Compared with the prior art, the Chinese patent medicine has the effects of warming and invigorating liver and kidney, dispelling wind and removing rheumatism, regulating qi and blood, clearing and activating channels and collaterals, promoting blood circulation and removing blood stasis, regulating an immunologic function and improving skin microcirculation, and has the outstanding substantial characteristics and obvious improvement of definite curative effects on the treatment and prevention of leucoderma, convenience for administration and the like.
Owner:渠淑敏
External preparation containing sirolimus as well as preparation method and application thereof
ActiveCN105663027APromote absorptionImprove stabilityOrganic active ingredientsAerosol deliveryIrritationTherapeutic effect
The invention provides a new route of administration for sirolimus, namely that sirolimus is prepared into an external preparation for treating skin diseases. The external preparation is a prescribed preparation or compound preparation containing sirolimus, and the dosage form can be any one of pharmaceutical skin external dosage forms including cream, ointment, gel, spray, coating agent and the like, and can be any one of new external dosage forms including solid lipid nanoparticles and the like. The research shows that the external preparation containing sirolimus can be used for treating multiple immune and inflammatory skin diseases including atopic dermatitis, eczema, dermatitis, lichen planus, psoriasis, vitiligo, rosacea, sarcoidosis and the like, and is good in curative effect, high in safety and free of skin irritation. The external preparation is expected to become an important pharmaceutical preparation for clinically treating skin diseases following corticosteroids.
Owner:WUHAN GENERAL HOSPITAL OF GUANGZHOU MILITARY
Mixed cultivation method of keratinocyte and melanocyte and application
InactiveCN105112353AEliminate the effects ofImprove proliferative abilityArtificial cell constructsVertebrate cellsDiseaseCutin
The invention provides a mixed cultivation method of keratinocyte and melanocyte comprising: breaking the skin sample reserving epidermal layer and at least part of corium layer, cold digesting by pancreatin; collecting deposition organization; heat digesting by I type collagenase, collecting cells; primary inoculating, culturing in KGM-Gold medium; subcultring to the cells merge, culturing in KGM-Gold medium to the cells differentiate and stratify; collecting the formed skin sheet. The mixed cultivation method of keratinocyte and melanocyte uses the medium only for skin keratinocyte and melanocyte with which can avoid pollution of the other cells, and can get skin sheet including functional melanocyte and distributing uniformly. The mixed cultivation method of keratinocyte and melanocyte can get large area of skin sheet from quite small area of autologous sample, solve the problem of shortage of the skin source at skin grafting for the patient with large-area skin diseases, and apply in skin diseases of pigment deficiency such as leucoderma and so on.
Owner:赫柏慧康生物科技无锡有限公司 +1
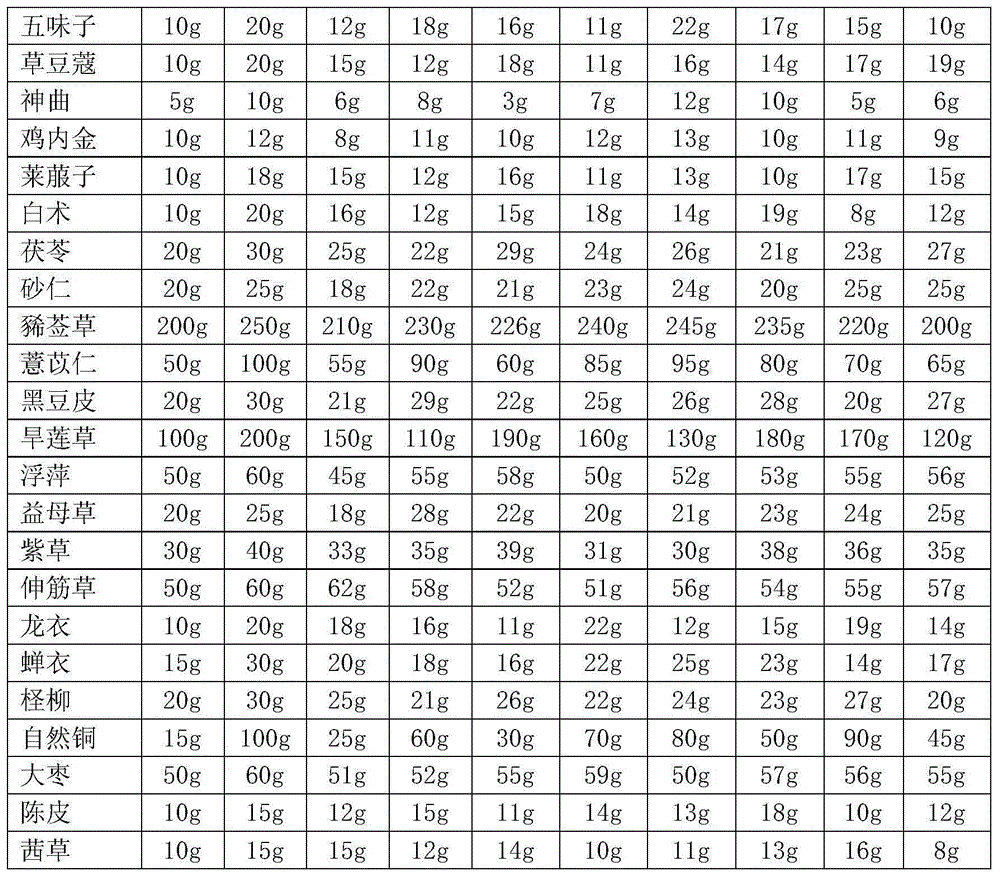
Drug for treating leucoderma and preparation method thereof
InactiveCN104147496AGood effectCompletely curedAnthropod material medical ingredientsInorganic active ingredientsSaposhnikoviaSemen
The invention discloses a drug for treating leucoderma and a preparation method thereof. The drug is prepared from gecko, human placenta, fructus psoraleae, prepared rehmannia root, pulp of cornus officinalis, dendrobium, radix astragali, pilose antler, fleece-flower root, glossy privet fruit, donkey-hide gelatin, tortoise-plastron glue, semen cuscutae, rhizoma polygonati, root of Angelicasinensis (Oliv)Diels, semen astragali complanati, raspberry, wolfberry, red ginseng, pseudo-ginseng, ganoderma atrum, radix salviae miltiorrhizae, stigma croci, long-nosed pit viper, tribulus terrestris, ligusticum wallichii, dahurian angelica root, divaricate saposhnikovia root, balloonflower root, tree peony bark, tuber fleeceflower stem, trogopterus dung, white-stiff silkworm, fruit of Chinese magnoliavine, katsumadai seed, medicated leaven, chicken's gizzard membrane, radish seed, bighead atractylodes rhizome, poria cocos, fructus amomi, herba siegesbeckiae, coix seed, black bean hull, esliptae herba, duckweed, motherwort, lithospermum, common clubmoss herb, periostracum serpentis, periostracum cicada, tamarix chinensis, native copper, Chinese-date, dried orange peel and madder. The drug does not damage the human body, realizes treatment on both symptoms and root causes and thoroughly cures leucoderma.
Owner:徐辉
Traditional Chinese medicine for treating leucoderma
InactiveCN102362950APromote absorptionEnhance physical fitnessDermatological disorderPlant ingredientsSalvia miltiorrhizaCutaneous microcirculation
The invention relates to traditional Chinese medicine for treating leucoderma, which is characterized in that eclipta alba, angelica root, angelica, polygonum multiflorum, astragalus campanulatus R.Brown, ligusticum, tribulus terrestris, ligustrum lucidum, nepeta, lithospermum, paris polyphylla, divaricate saposhnikovia root, saffron crocus, purple salvia miltiorrhiza, radix sophorae flavescentis and fructus psoraleae are prepared into various oral preparations according to the conventional pharmacy method. The traditional Chinese medicine has the advantages that the functions of warming and nourishing the liver and the kidney, dispelling the wind, eliminating dampness, regulating qi and blood, clearing and activating the channels and collaterals, activating blood, dissolving stasis and regulating the immunity are realized, the effect of improving the skin micro circulation is reached, the curative effect on the prevention and the treatment of the leucoderma is definite, and the taking is convenient.
Owner:王莲芬
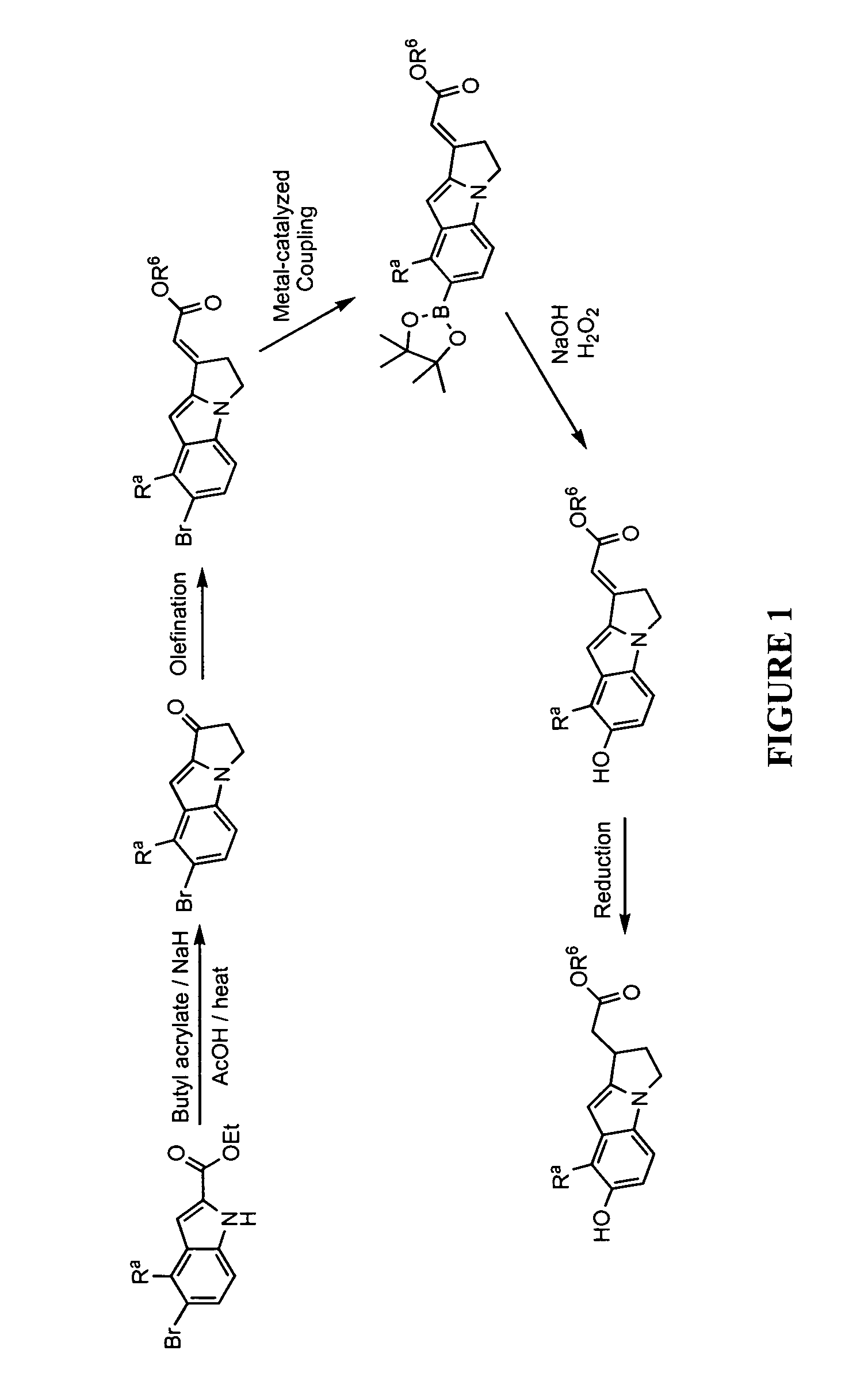
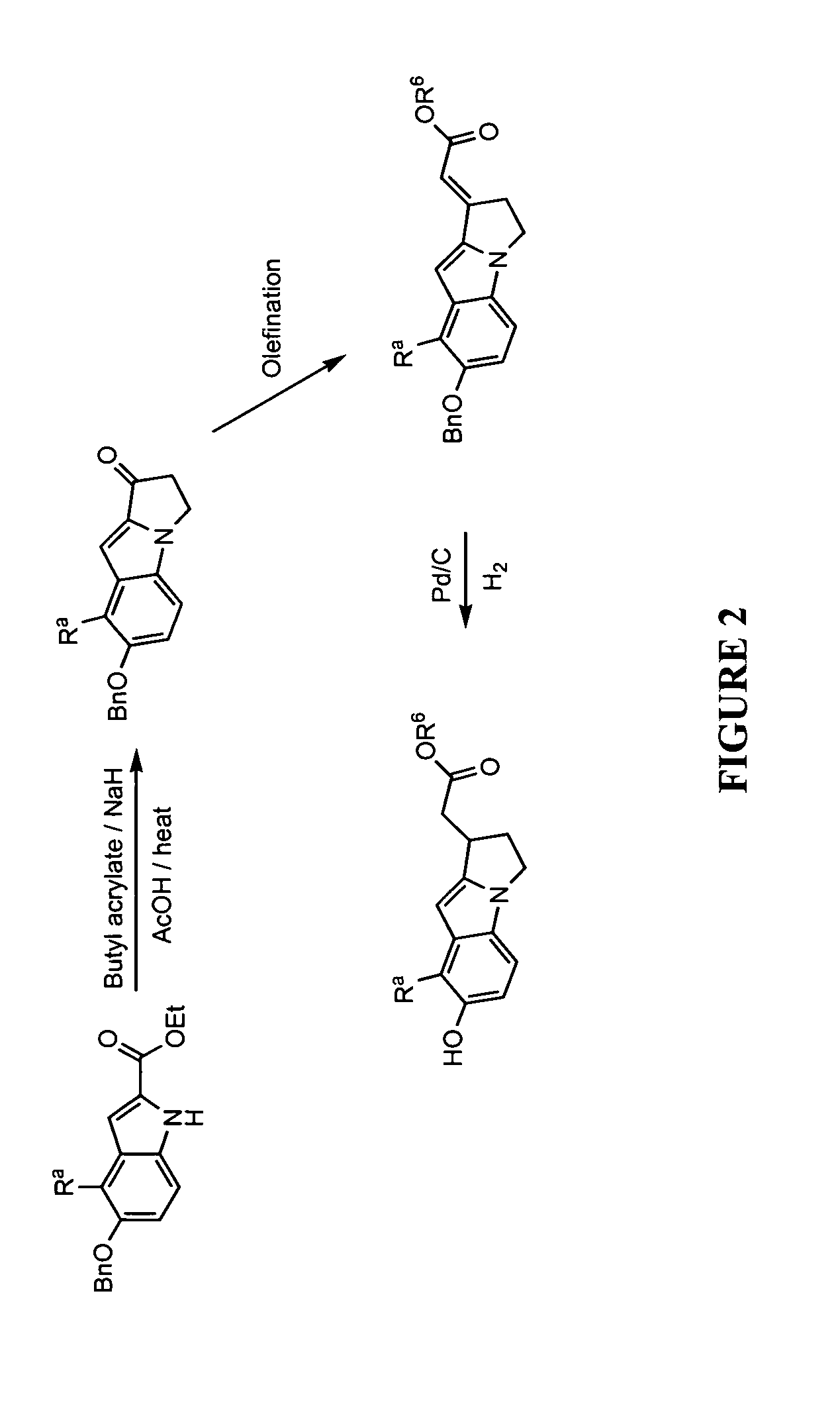
Compositions and methods for treatment of vitiligo
InactiveUS20150202187A1Reduce T-cell induced destructionReduce adverse effectsOrganic active ingredientsPeptide/protein ingredientsMedicineVitiligo
Compositions and methods are disclosed for treating hypomelanotic conditions such as vitiligo and promoting melanogenesis.
Owner:0901911 B C UNLIMITED LIABILITY
Photoelectric device for vitiligo treatment
InactiveCN105920742ALow costReduce work intensityLight therapyOptical elementsVisual technologyUltraviolet lights
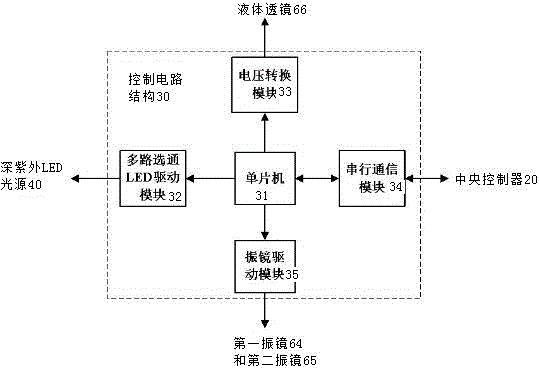
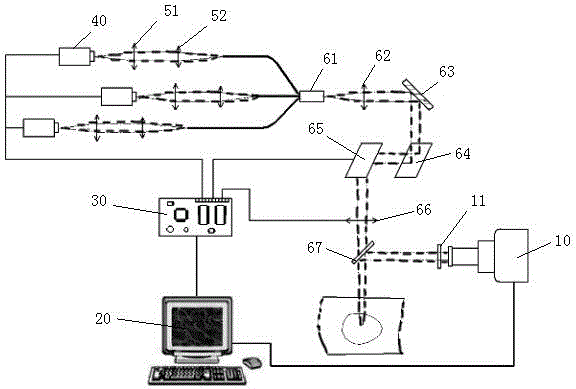
The invention discloses a photoelectric device for vitiligo treatment. Low-price deep ultraviolet LED light sources are adopted; beam combination and light intensity graded adjustment of ultraviolet light are realized through photoelectric devices; a computer vision technology is utilized to realize intelligent detection on a plurality of main parameters of affected skin; feedback control on the deep ultraviolet LED light sources, galvanometers and a liquid lens can be completed; and therefore, the cost of the vitiligo treatment equipment is greatly decreased, and the intelligent degree of the vitiligo treatment equipment is improved, and the work intensity of doctors can be reduced.
Owner:FOSHAN UNIVERSITY
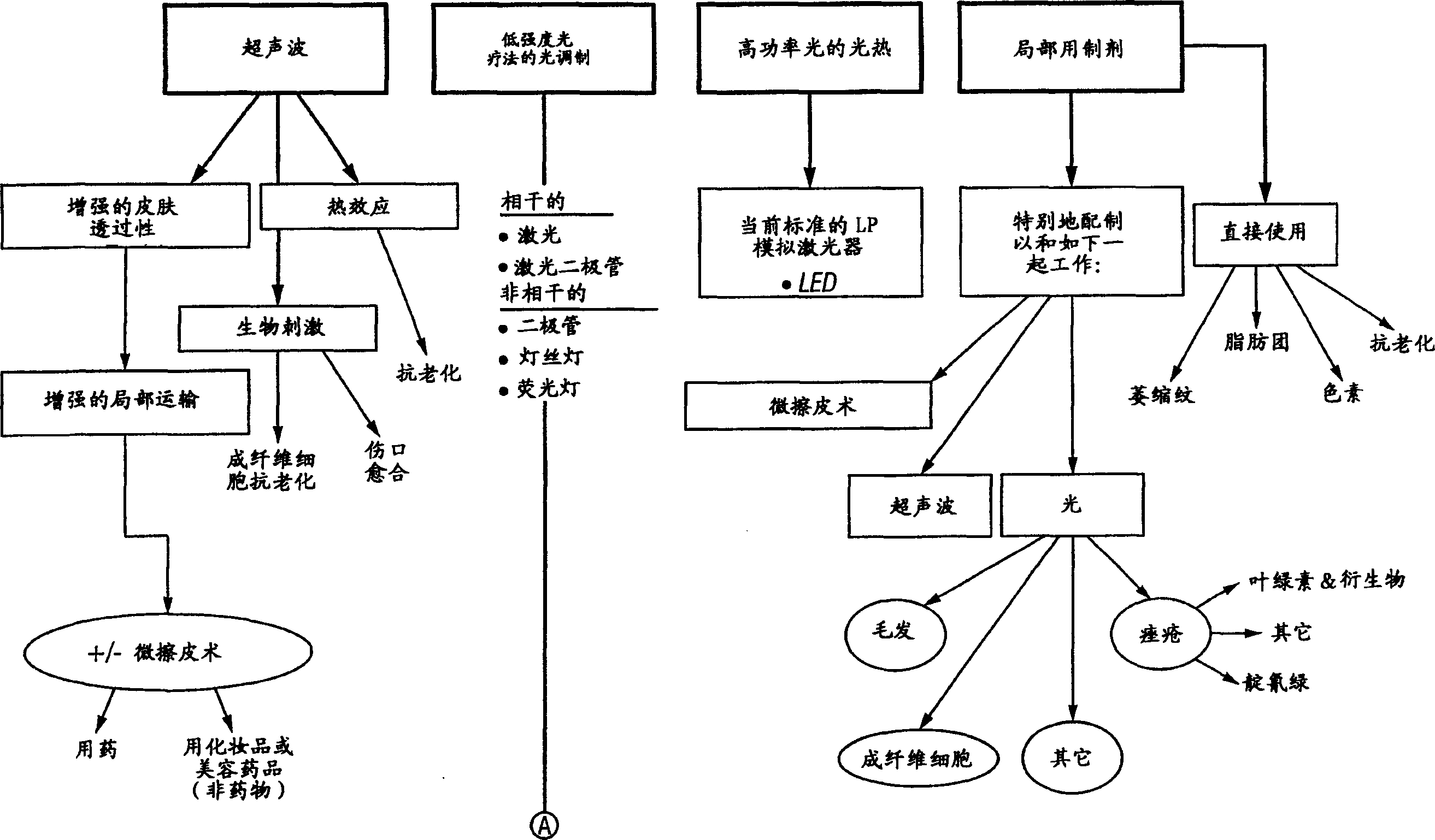
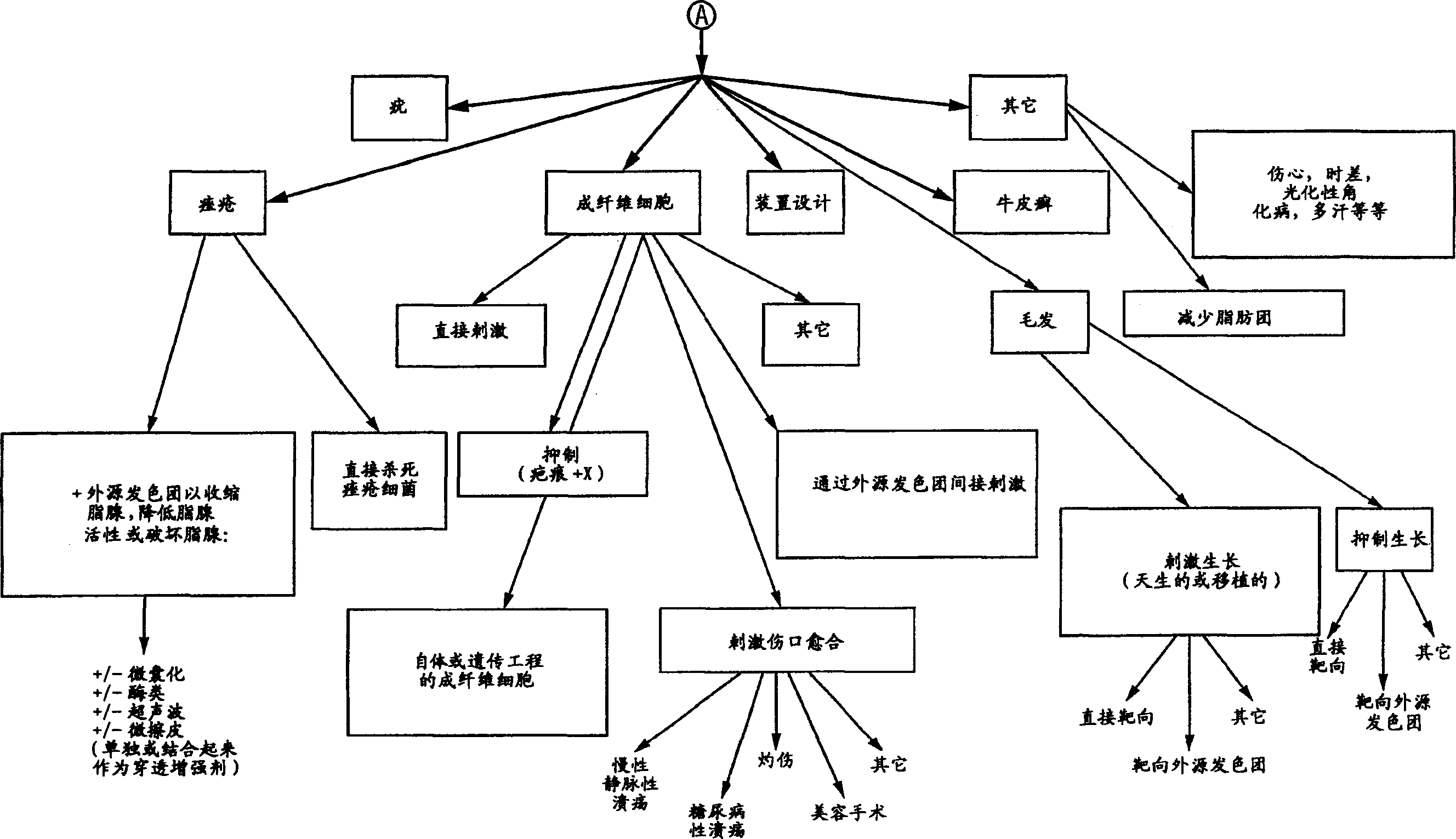
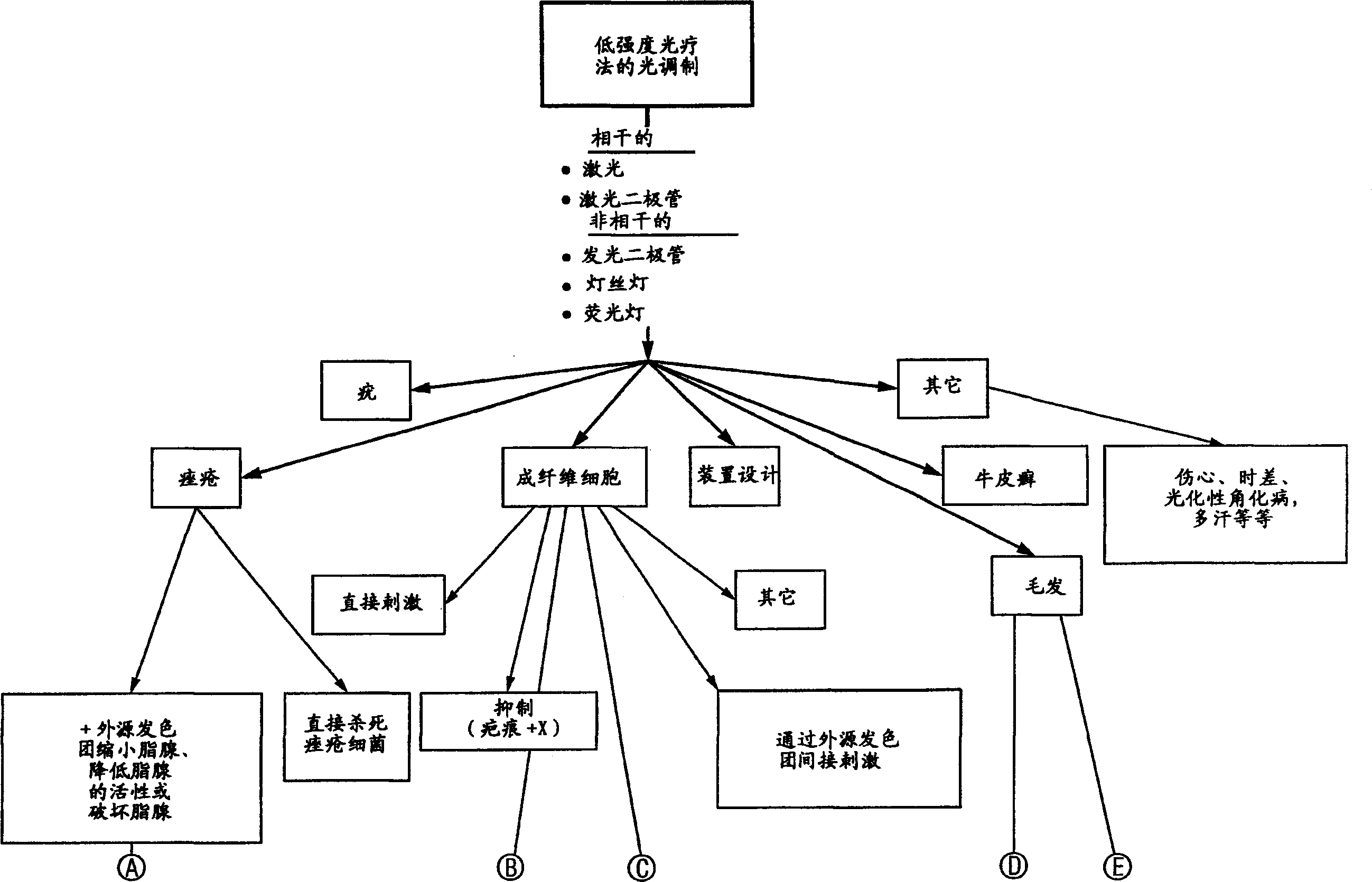
Method and apparatus for the photomodulation of living cells
The present invention relates to a system and method for the photomodulation of living tissue. When photomodulated, living tissue will exhibit bioactivation or bioinhibition according to the present invention and, when using the disclosed sources of narrowband multichromatic radiation can cause significant dermatologic advantages such as hair removal, hair growth stimulation, wrinkle reduction, acne reduction and scar removal, vitiligo, etc. The present invention has application to non-dermatological medical treatments including tumor growth inhibition, cell regeneration, the stimulation of tissue in organs, etc.
Owner:轻微波浪有限责任公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com