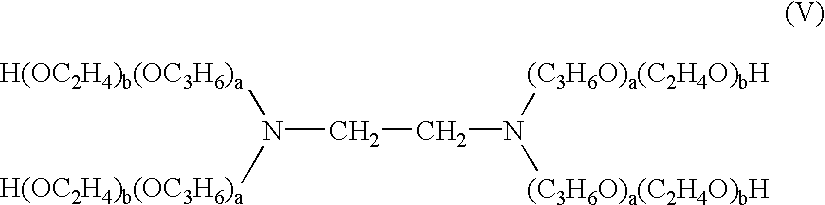Patents
Literature
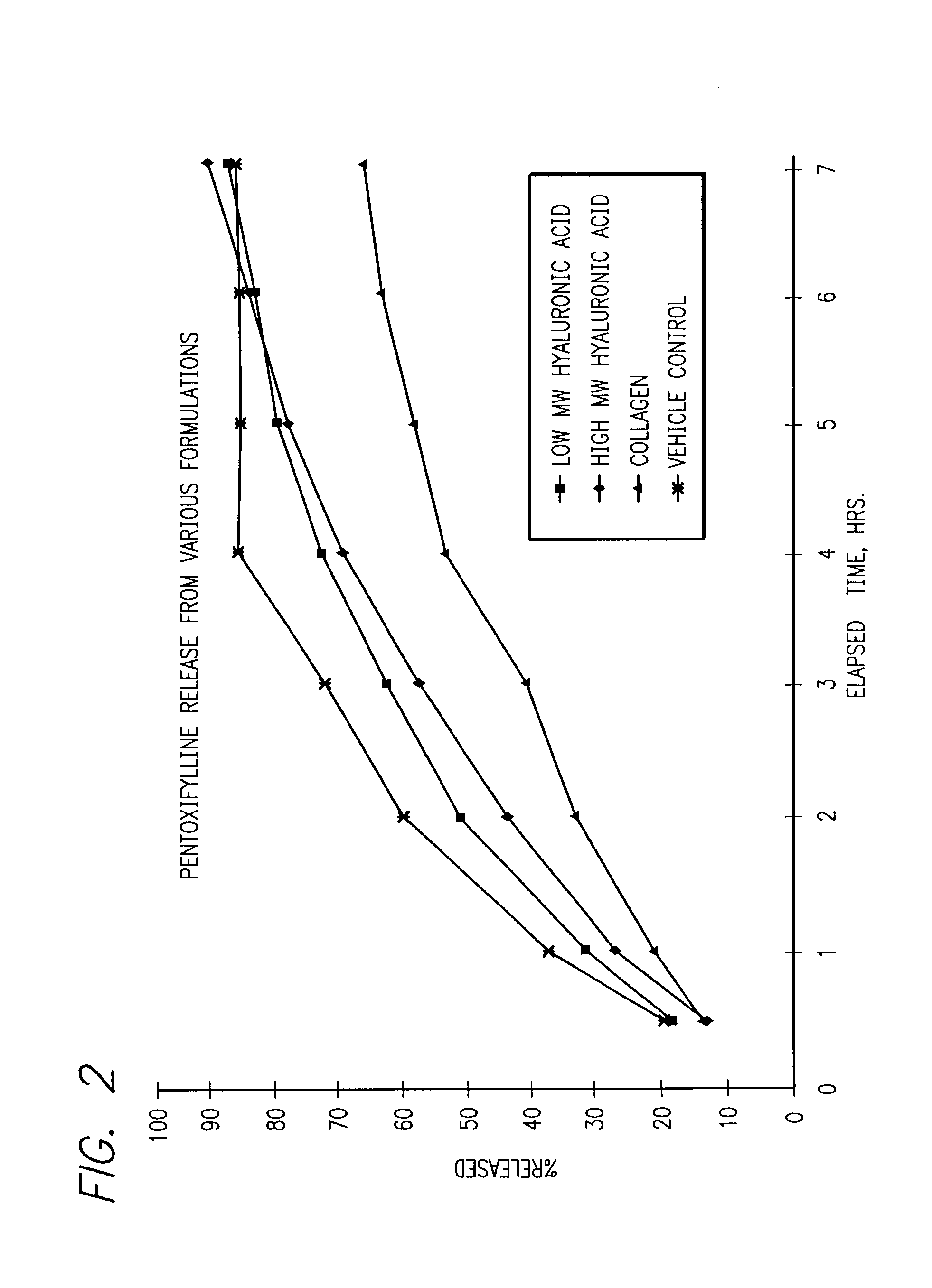
61 results about "Pentoxifylline" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
This medication is used to improve the symptoms of a certain blood flow problem in the legs/arms (intermittent claudication due to occlusive artery disease).
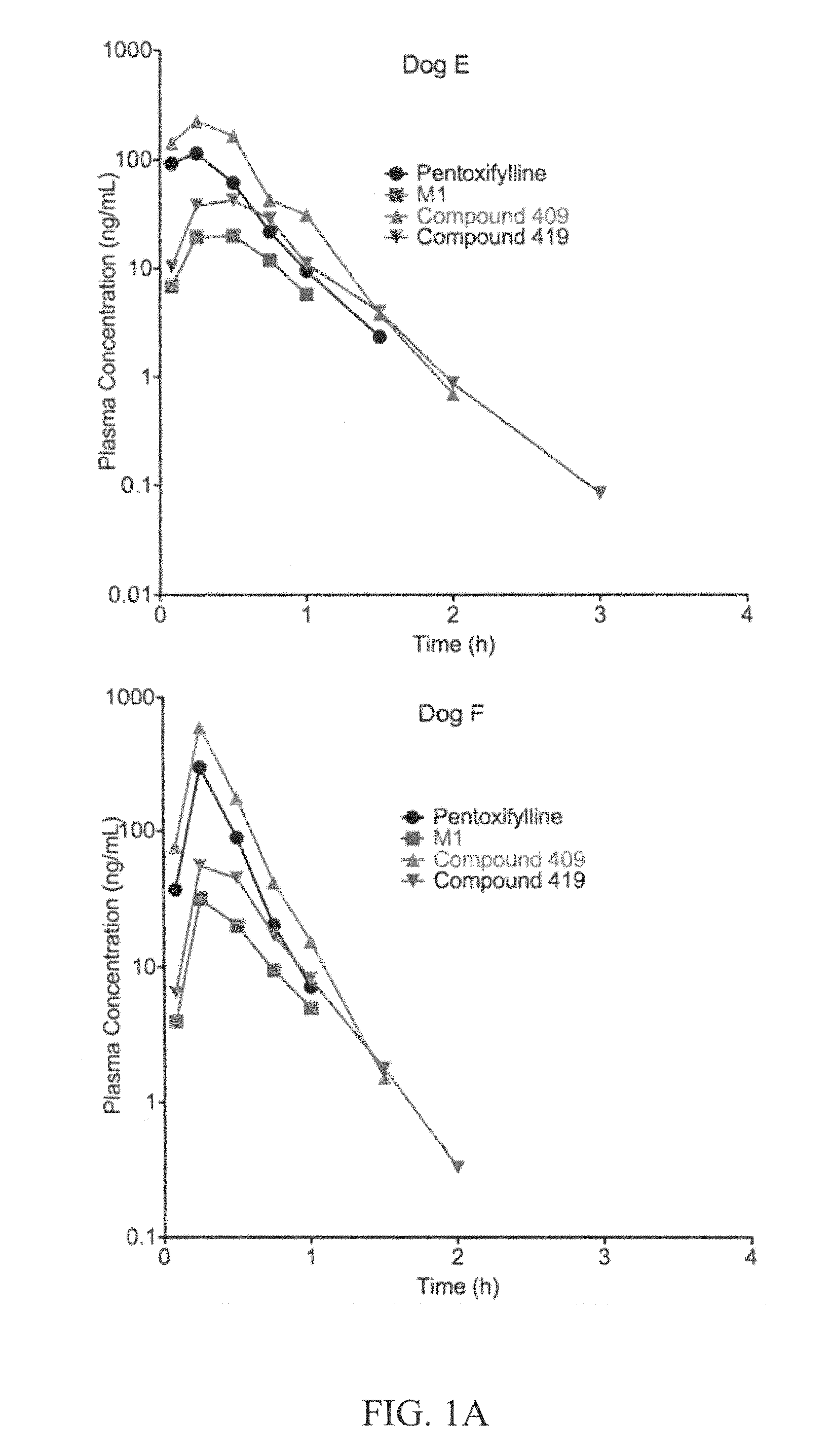
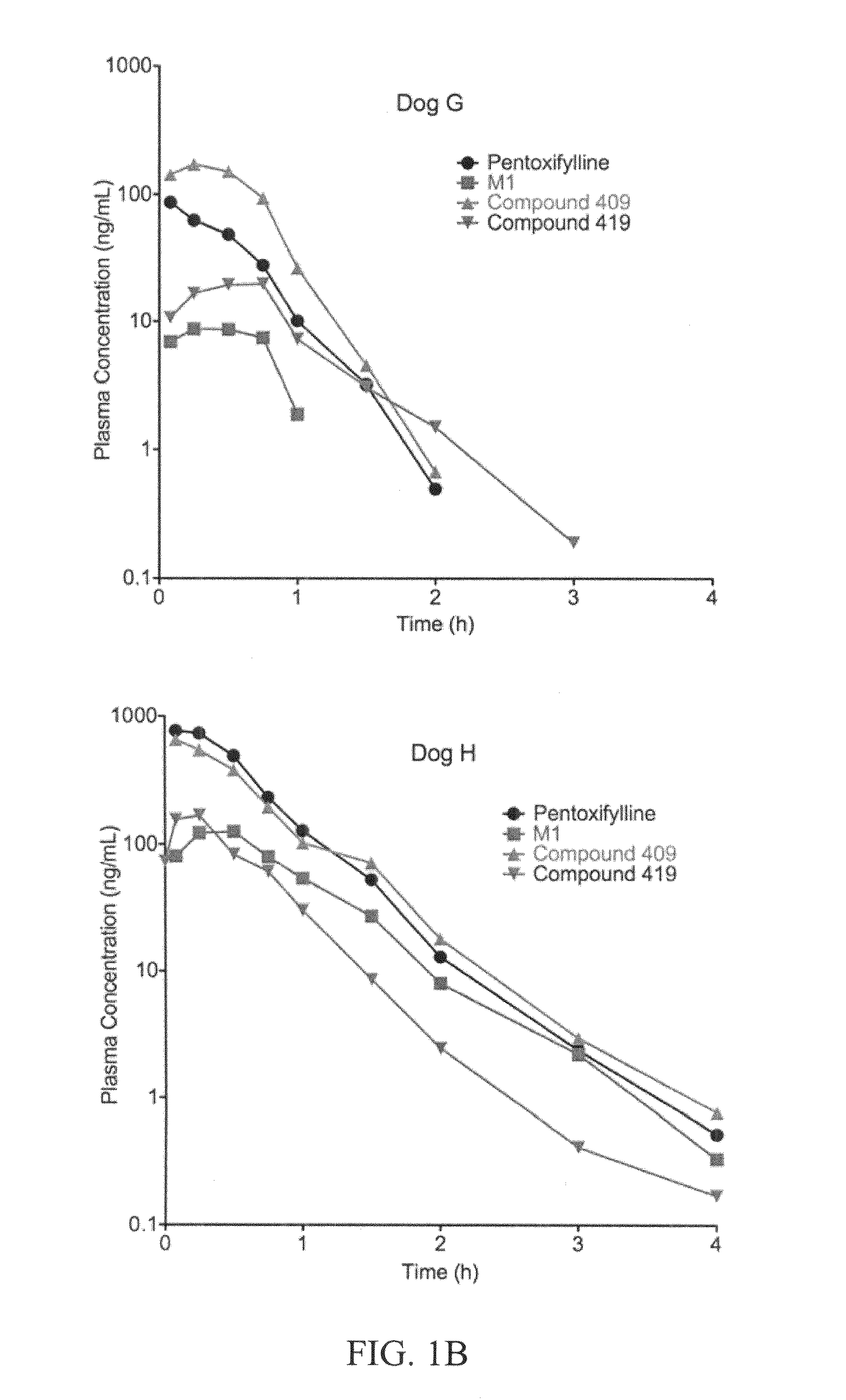
Substituted xanthine derivatives
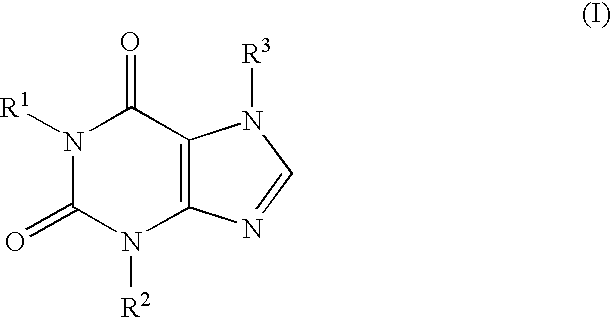
This invention relates to novel compounds that are substituted xanthine derivatives and pharmaceutically acceptable salts thereof. For example, this invention relates to novel substituted xanthine derivatives that are derivatives of pentoxifylline. This invention also provides compositions comprising one or more compounds of this invention and a carrier and the use of the disclosed compounds and compositions in methods of treating diseases and conditions for which pentoxifylline and related compounds are beneficial.
Owner:CONCERT PHARMA INC
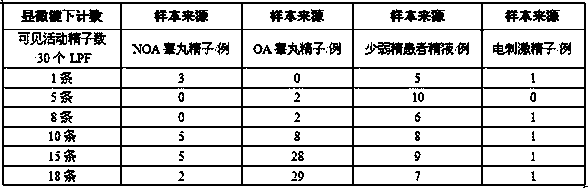
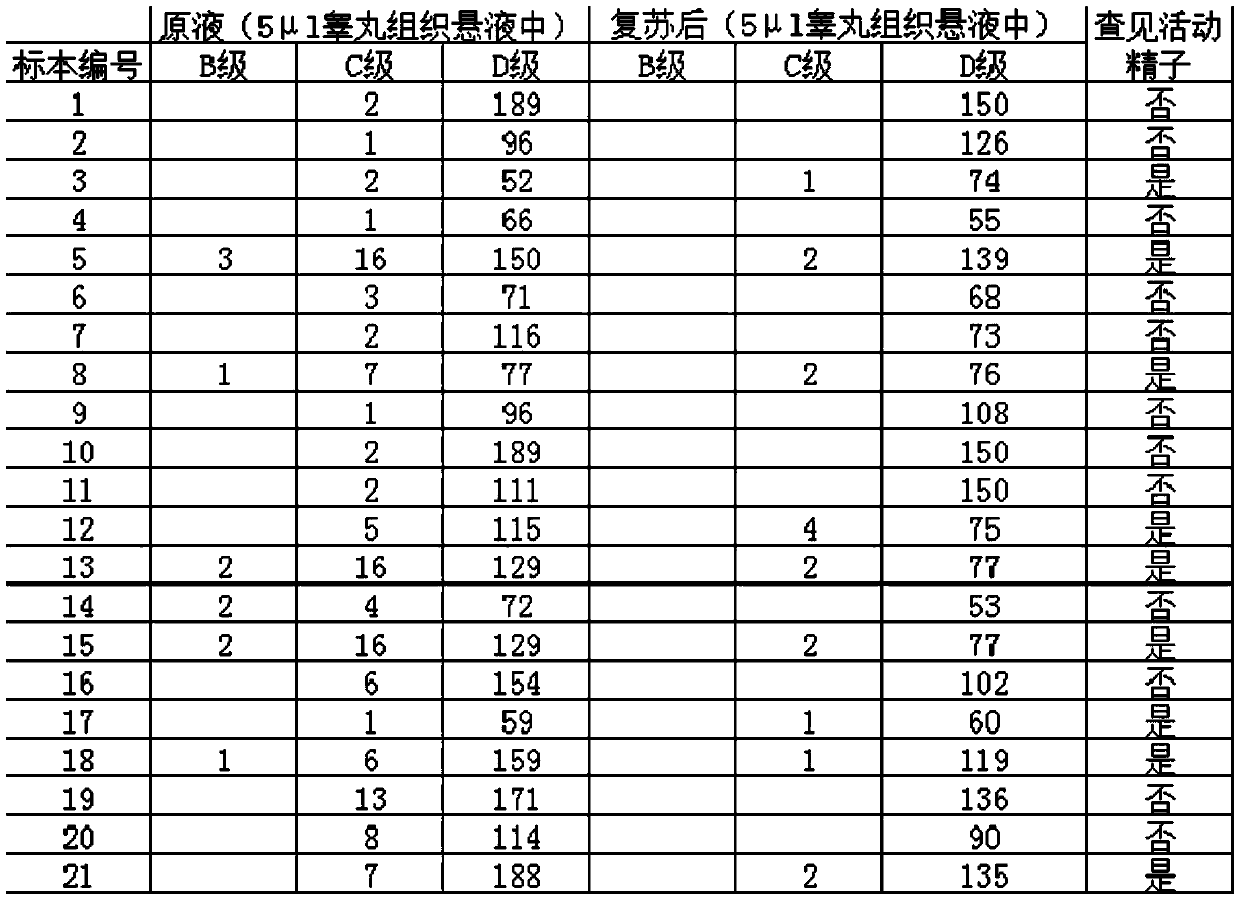
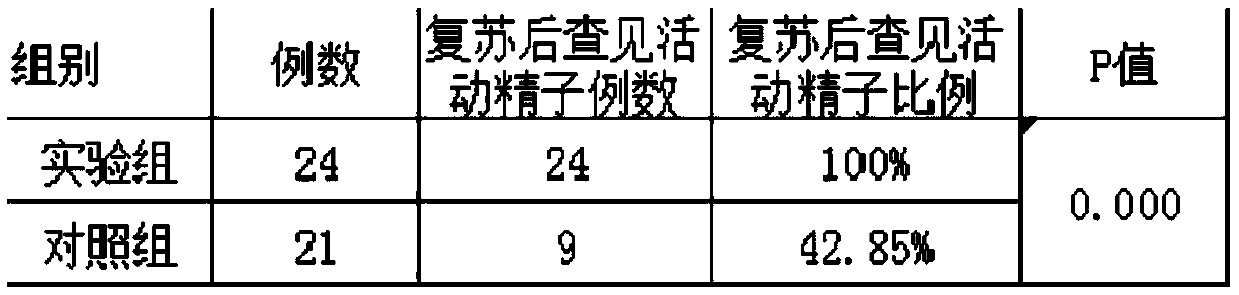
Extremely-low sperm freezing protective agent and application thereof
ActiveCN104365583AIncrease mobilityPhysiological impactDead animal preservationPhysiologyMonopotassium phosphate
The invention relates to an extremely-low sperm freezing protective agent and application thereof. The protective agent is prepared from the following ingredients: sodium chloride, potassium chloride, magnesium sulfate, monopotassium phosphate, calcium chloride, fructose, trehalose, sodium citrate, glycin, glutamine, pentoxifylline, vitamin E, HAS (human serum albumin), glycerin and gentamicin sulphate. The extremely-low sperm freezing protective agent can protect less sperms with low activity and without forward activity ability in seminal fluid, can remarkably improve the activity ability of the sperms which swing weakly and cannot move forwards in seminal fluid after freezing and thawing, has no obvious influence on the physiological function of the sperms, has high freezing anabiosis rate, can be used for cryopreservation of less and weak sperms, testicle sperms and epididymis sperms, and contributes to the successful development of assisted reproductive technology.
Owner:HAOHONG BIOTECH SHANGHAI
Substituted xanthine derivatives
This invention relates to novel compounds that are substituted xanthine derivatives and pharmaceutically acceptable salts thereof. For example, this invention relates to novel substituted xanthine derivatives that are derivatives of pentoxifylline. This invention also provides compositions comprising one or more compounds of this invention and a carrier and the use of the disclosed compounds and compositions in methods of treating diseases and conditions for which pentoxifylline and related compounds are beneficial.
Owner:CONCERT PHARMA INC
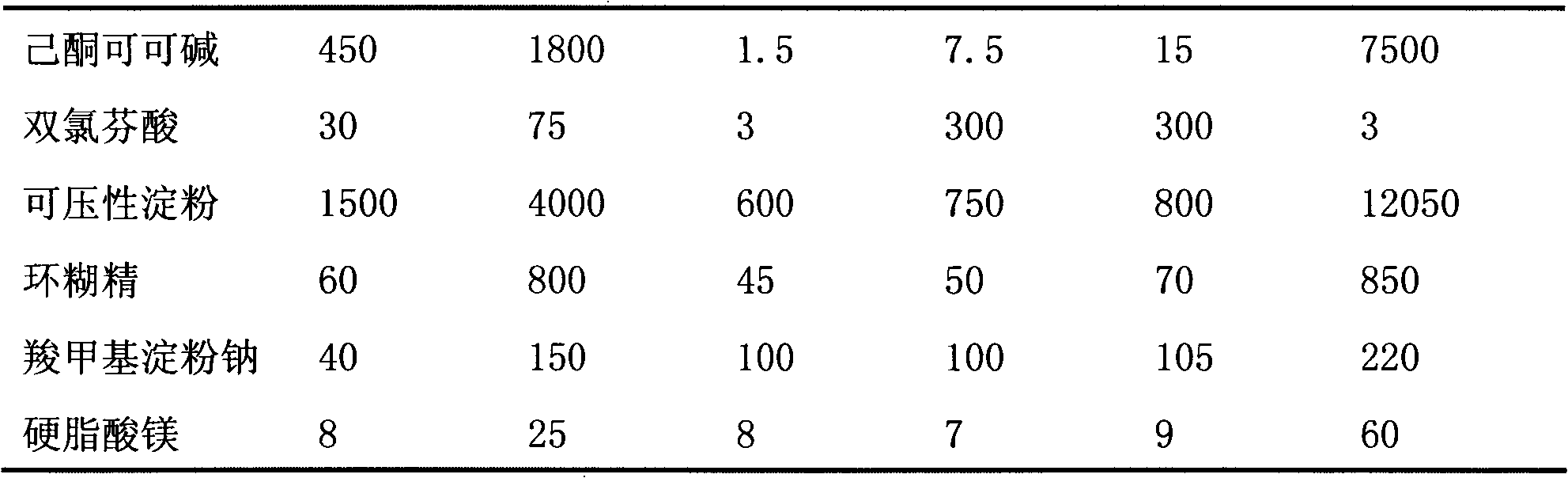
Pentoxifylline sustained release tablets and preparation thereof
InactiveCN101239050AImprove stabilityDigestive systemPharmaceutical delivery mechanismTheobromineActive component
The invention relates to a slow release formulation of hexanone theobromine, which contains hexanone theobromine used as the active components and slow release materials, filling agents, stabilizing agents, lubricants and bonds all used as accessories; wherein, each of the slow release tablet contains hexanone theobromine for 100 to 800mg, the slow release materials at least contain hydroxypropyl methylcellulose. The medicine releasing is controlled by adding slow release materials hydroxypropyl methylcellulose and other slow release materials; simultaneously the stabilizing agents are added in order to elevate the stability of the hexanone theobromine in the slow release formulation.
Owner:沈阳双鼎制药有限公司
Topical composition and method for treating occlusive wounds
The present invention provides a topical composition comprising about 6% to about 15% nifedipine and about 6% to about 15% pentoxifylline for treating severe vascular occlusive wounds. The present invention also provides a method and a kit for treating the vascular occlusive wound by applying the composition to the open wound, and cleaning and dressing the wound at least once daily.
Owner:FOOTE MARY ANN +1
Substituted xanthine derivatives
This invention relates to novel compounds that are substituted xanthine derivatives and pharmaceutically acceptable salts thereof. For example, this invention relates to novel substituted xanthine derivatives that are derivatives of pentoxifylline. This invention also provides compositions comprising one or more compounds of this invention and a carrier and the use of the disclosed compounds and compositions in methods of treating diseases and conditions for which pentoxifylline and related compounds are beneficial.
Owner:SUN PHARMA IND INC
Method of treating erectile dysfunction
An improved method of treating male erectile dysfunction by enteral administration of a combination of sildenafil citrate and papavarine hydrochloride. An alternate mode of treatment indicated for individuals with known vascular disease includes the contemporaneous administration of pentoxifylline. Further embodiments include administration of zinc monomethionine aspartate.
Owner:HINES ROBERT +1
Method for inducing hormone soaked loaches
InactiveCN102177863ASave the arrestSave injection timeClimate change adaptationPisciculture and aquariaHormone analogSaline water
The invention relates to a method for inducing hormone soaked loaches. The method comprises the following steps: (1) selecting a mature loach parent in water at the temperature of between 21 and 29 DEG C; and (2) taking a mixture of domperidone maleate, luteotropin releasing hormone d-ala analog and chorionic gonadotropin as oxytocic hormones, and soaking the loach parent with a soaking solution which is prepared by mixing the oxytocic hormone, pentoxifylline and edible oil in normal saline, wherein the dosage of the domperidone maleate is between 100 and 300mg / kg, the dosage of the luteotropin releasing hormone d-ala analog is between 200 and 600mu g / kg, the dosage of the chorionic gonadotropin is between 15,000 and 30,000IU / kg, and the dosage of the normal saline is between 0.2 and 0.3kg / kg; and when the loach parent is soaked, the temperature of the soaking solution is controlled to be between 21 and 29 DEG C, and the soaking time is controlled to be between 0.5 and 8 hours. The aim of inducing the loach parent can be realized through soaking instead of injection.
Owner:ZHEJIANG INST OF FRESH WATER FISHERIES
Pentoxifylline injection composition and preparation method thereof
ActiveCN106309362AGood quality and stabilityImprove stabilityNervous disorderAntipyreticMedicineTheobromine
The invention relates to a pentoxifylline injection composition and a preparation method thereof, belonging to the technical field of medical preparations. The injection composition is prepared from pentoxifylline, sodium dihydrogen phosphate, taurate, vitamin B6, disodium edetate and the like. The pentoxifylline injection composition is simple and feasible in technique. After the pentoxifylline injection composition is placed under accelerated test conditions for 6 months, the solution is still colorless, the pH value is basically unchanged, the increase of related substances is not obvious, the pentoxifylline content decrease is not obvious, and the product has obviously higher quality and stability than the control product.
Owner:CSPC OUYI PHARM CO LTD
Methods and compositions for the treatment of vascular depression
Methods and compositions are provided for treating vascular depression. The methods involve administering to a subject in need thereof a xanthine derivative in a therapeutically effective amount to treat vascular depression, particularly the xanthine derivatives pentoxifylline or propentofylline. The methods may further include administration of an additional therapeutic agent in combination with the xanthine derivative selected from the group consisting of a selective serotonin reuptake inhibitor (SSRI), a serotonin-norepinephrine reuptake inhibitor (SNRI), and a drug used in the treatment of cerebrovascular disease. Compositions of the invention include pharmaceutical compositions and kits for treating vascular depression in a subject in need thereof that include therapeutically effective amounts of a xanthine derivative and an additional therapeutic agent selected from the group consisting of an SSRI, an SNRI, and a drug used in the treatment of cerebrovascular disease.
Owner:DUKE UNIV
Substituted xanthine derivatives
Owner:SUN PHARMA IND INC
Method and composition for inhibiting post-surgical adhesions
A method and compositions for reducing post-surgical adhesion formation / reformation in mammals following surgical injury to a body cavity or organs situated therein. The aqueous compositions comprising pentoxifylline and a polyoxyalkylene block copolymer are applied to the injured areas subsequent to surgical injury.
Owner:REEVE LORRAINE E +1
Refining method for pentoxifylline recycling products
The invention discloses a refining method for pentoxifylline recycling products. The content of impurities in the pentoxifylline recycling products can be reduced, after the recycling products are refined, the maximum single impurities are reduced to be 0.1% or below, the total impurities are 0.5% or below, the standard of finished pentoxifylline is met, the integral yield is improved, and the reaction cost is reduced. The refining method comprises the following steps: (1) adding water in the pentoxifylline recycling products under the condition of heating, dissolving, adding alkaline liquid to regulate a pH value to be 10-14, adding a reducing agent, insulating, and then cooling and filtering to obtain an alkaline solution, wherein the single impurities in the pentoxifylline recycling products are less than 5%, and the total impurities in the pentoxifylline recycling products are less than 10%; (2) fully mixing the alkaline solution obtained in step (1) with an organic solvent A, thenstanding for layering, and removing an organic phase by evaporation to obtain viscous liquid; and (3) adding an organic solvent B to dissolve the viscous liquid, adding activated carbon, after insulating, filtering the activated carbon, cooling filtrate to the temperature of 20 DEG C or below, and filtering and drying to obtain pentoxifylline.
Owner:CSPC INNOVATION PHARMA
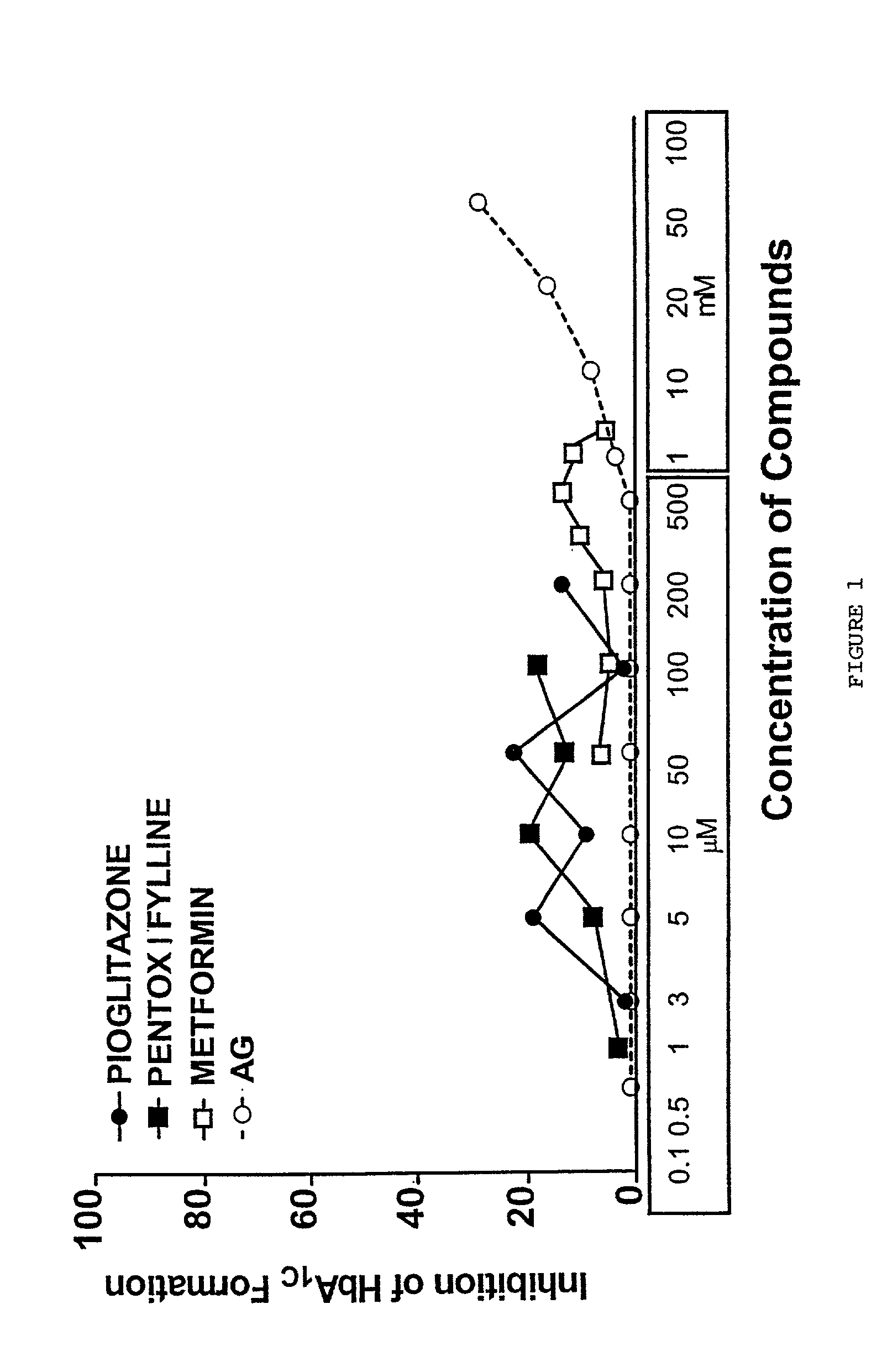
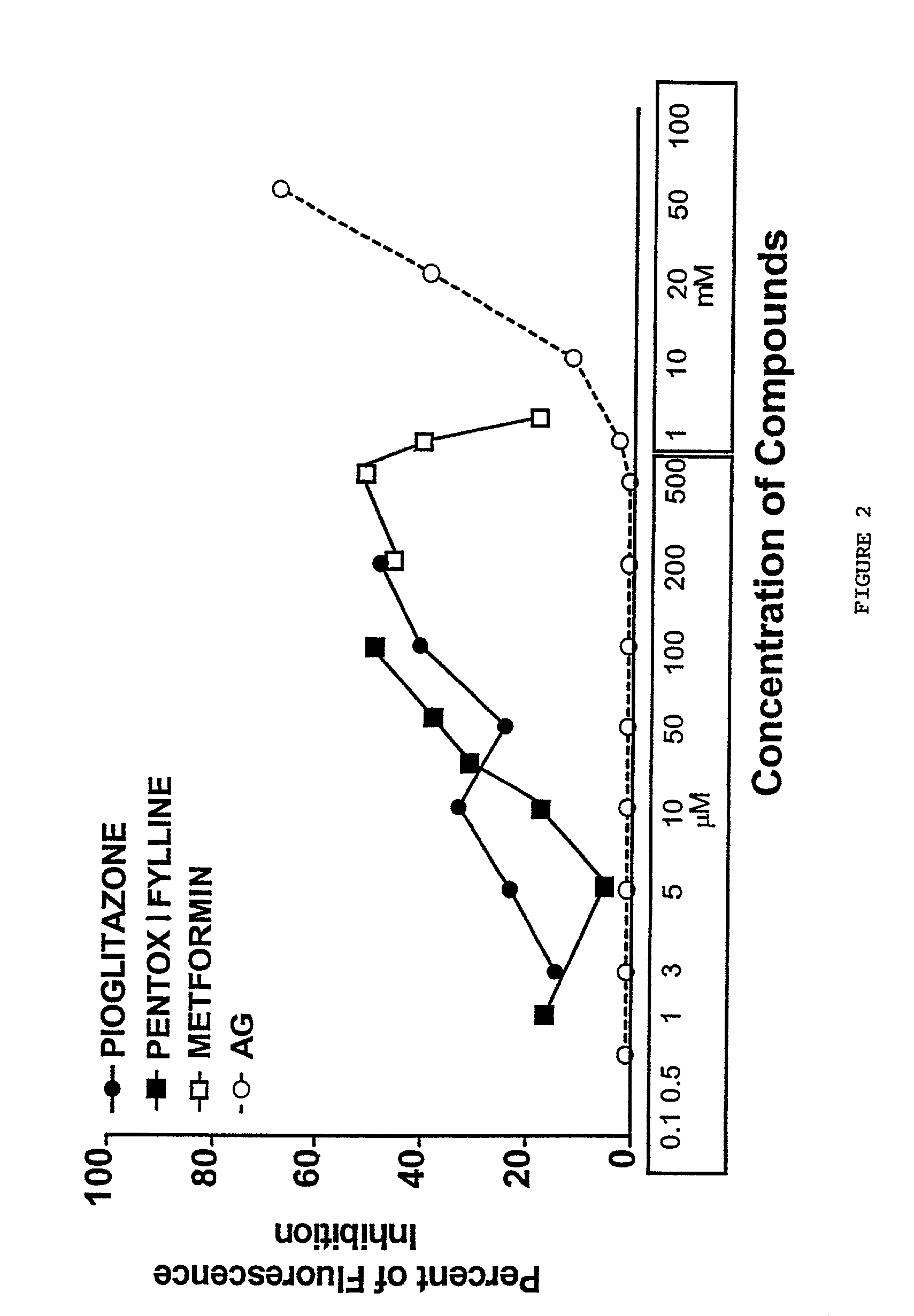
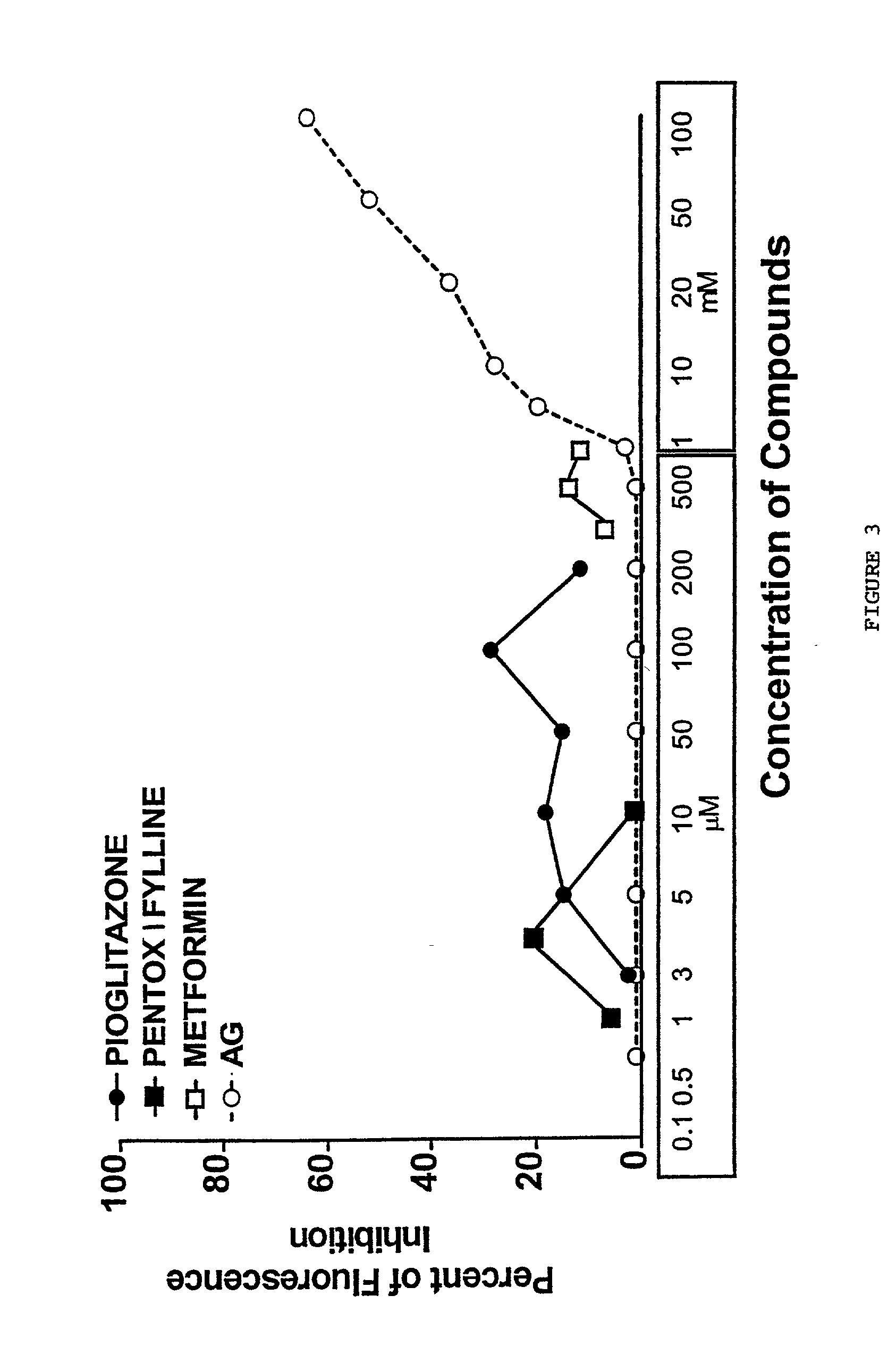
Pentoxifylline, pioglitazone and metformin are inhibitors of formation of advanced glycation endproducts (AGE's)
InactiveUS20020128278A1Inhibit nonenzymatic glycationIll effectBiocideNervous disorderDiseasePremature aging
Pentoxifylline, pioglitazone and metformin have been found to inhibit the nonenzymatic glycation of proteins which often results in formation of advanced glycation endproducts and crosslinks. The nonenzymatic glycation and crosslinking of proteins is a part of the aging process with the glycation endproducts and crosslinking of long-lived proteins increasing with age. This process is increased at elevated concentrations of reducing sugars in the blood and in the intracellular environment such as occurs with diabetes. The structural and functional integrity of the affected molecules become perturbed by these modifications and can result in severe consequences. The compounds of the present invention can be used to inhibit this process of nonenzymatic glycation and therefore to inhibit some of the ill effects caused by diabetes or by aging. The compounds are also useful for preventing premature aging, rheumatoid arthritis, Alzheimer's disease, uremia, neurotoxicity, atherosclerosis and spoilage of proteins in food and can prevent discoloration of teeth.
Owner:CITY OF HOPE
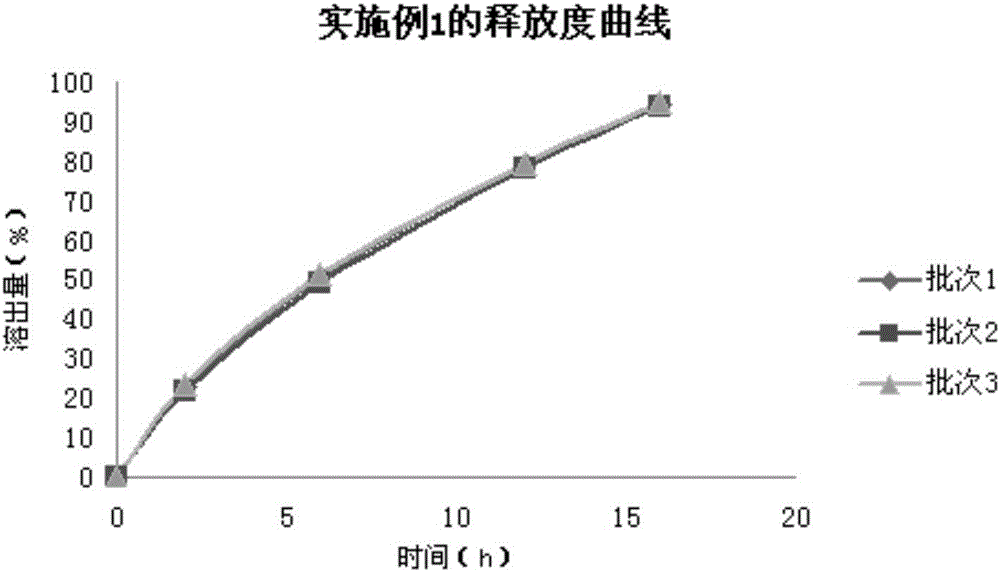
Pentoxifylline slow-release tablet and preparation method thereof
ActiveCN106138004AImprove uniformityReleaseNervous disorderMetabolism disorderMedical prescriptionPentoxifylline
The invention relates to a pentoxifylline slow-release tablet and a preparation method thereof, and belongs to the technical field of medicine preparations. The pentoxifylline slow-release tablet consists of a tablet core and a coating layer, wherein the tablet core is prepared from the following components of pentoxifylline, slow-release material and the like. The pentoxifylline slow-release tablet has the advantages that the type of auxiliary material is fewer, the formula technology is simple, the slow-release effect is good, the medicine-release reproducibility is good, the product stability is high, and the like.
Owner:CSPC OUYI PHARM CO LTD
Medicinal composition containing pentoxifylline and prucalopride and medical application thereof
ActiveCN103356630AFully functionalSmall toxicityDigestive systemHeterocyclic compound active ingredientsTreatment effectSphincter
The invention discloses a medicinal composition for treating constipation. According to the medicinal composition, pentoxifylline and prucalopride or medicinally usable salts thereof are used as main active ingredients. The medicinal composition can obviously improve the intestinal transmission function and reduce the pressure of sphincter ani; and an obvious synergistic effect is achieved after the two medicaments are combined. The medicinal composition has clear ingredients, a clear curative effect and a treatment effect of treating both symptoms and root causes of chronic constipation, particularly functional constipation, and thus has a broad medical application prospect.
Owner:LUNAN PHARMA GROUP CORPORATION
Pharmaceutical composition for treating or preventing alcoholic liver diseases, containing cilostazol as active ingredient
InactiveUS20130267558A1Suppressed expression levelGood effectBiocideOrganic chemistryPentoxifyllineFatty Acid Synthetases
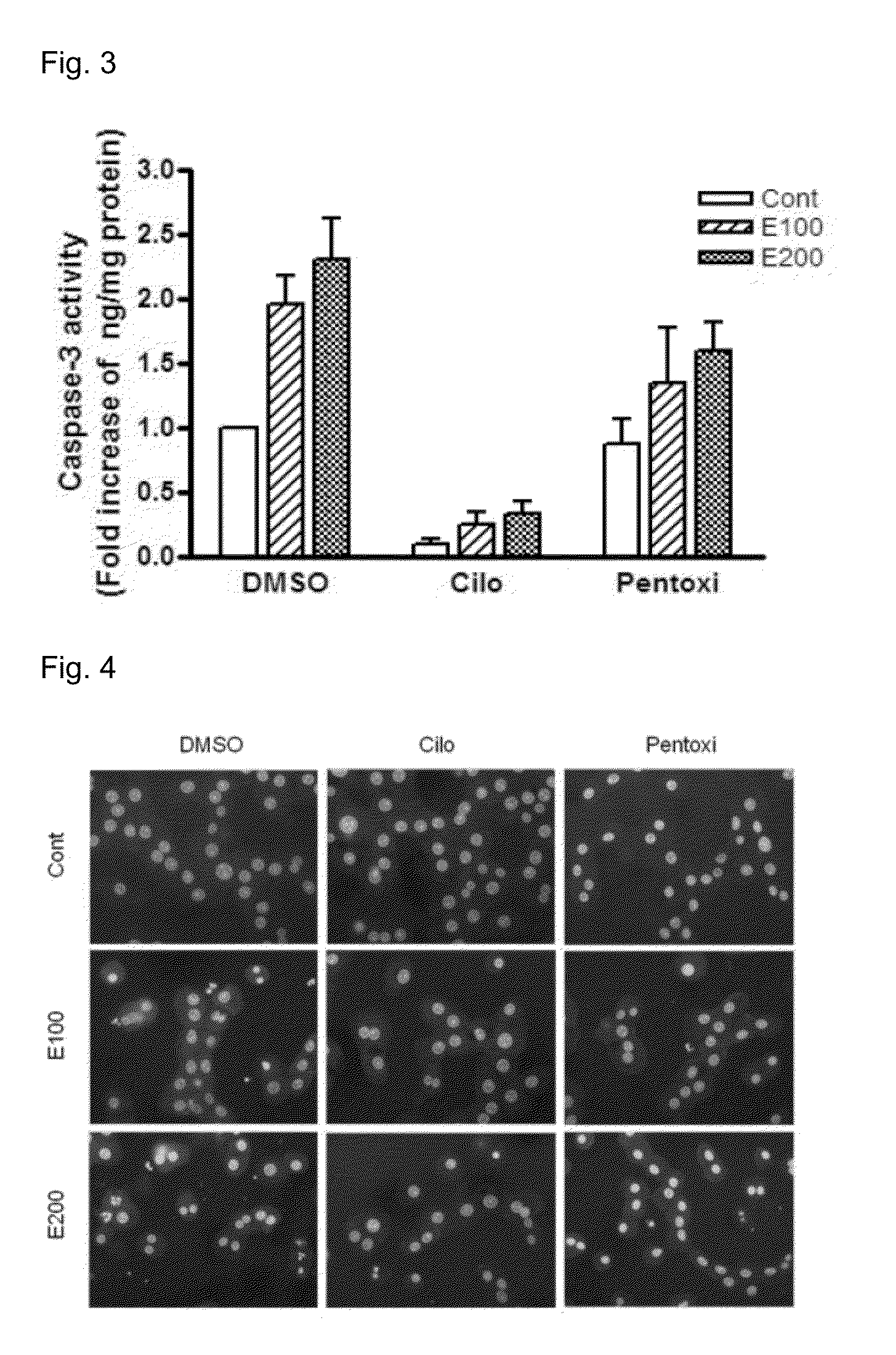
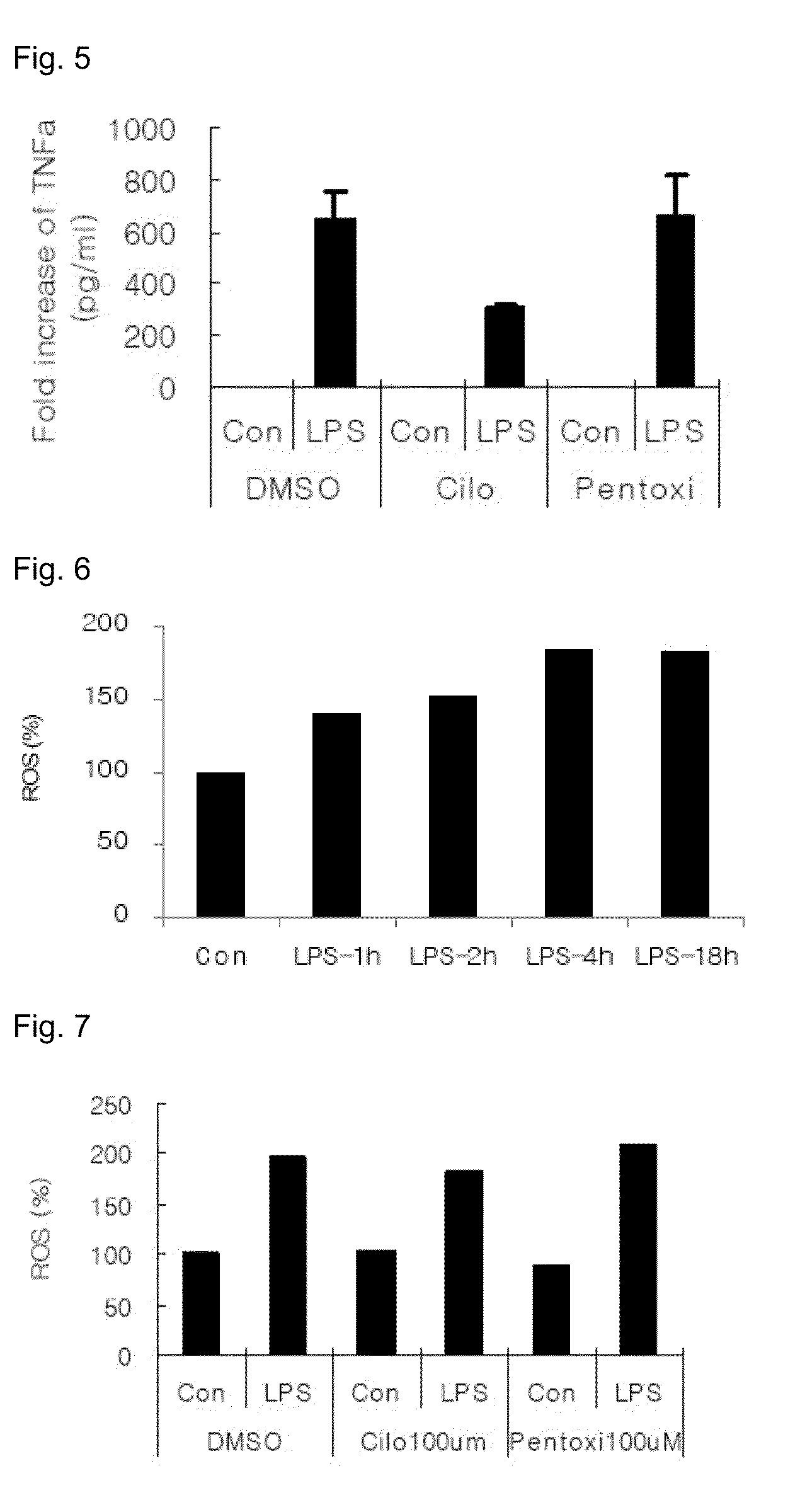
Provided is a pharmaceutical composition for the treatment and prevention of alcoholic liver diseases, including cilostazol as an active ingredient. Cilostazol inhibits expression levels of TNF-α and FAS (fatty acid synthase) gene in a concentration-dependent manner, and also significantly inhibits the activity of caspase-3. Accordingly, cilostazol shows superior effects for the treatment or prevention of alcoholic liver diseases, in particular, alcoholic hepatitis compared to pentoxifylline which is conventionally used as a therapeutic agent for the treatment for alcoholic hepatitis. Thus, cilostazol is suitable for use as a drug for the treatment or prevention of alcoholic hepatitis.
Owner:RES COOPERATION FOUND OF YEUNGNAM UNIV
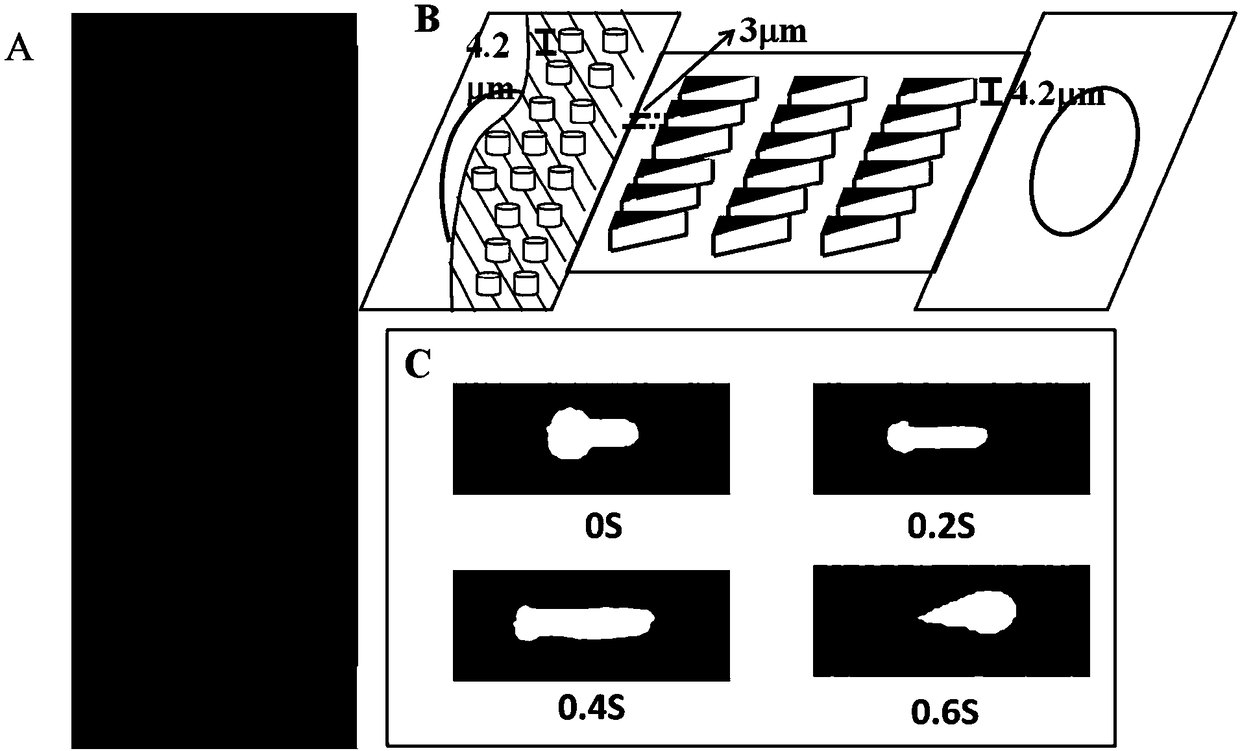
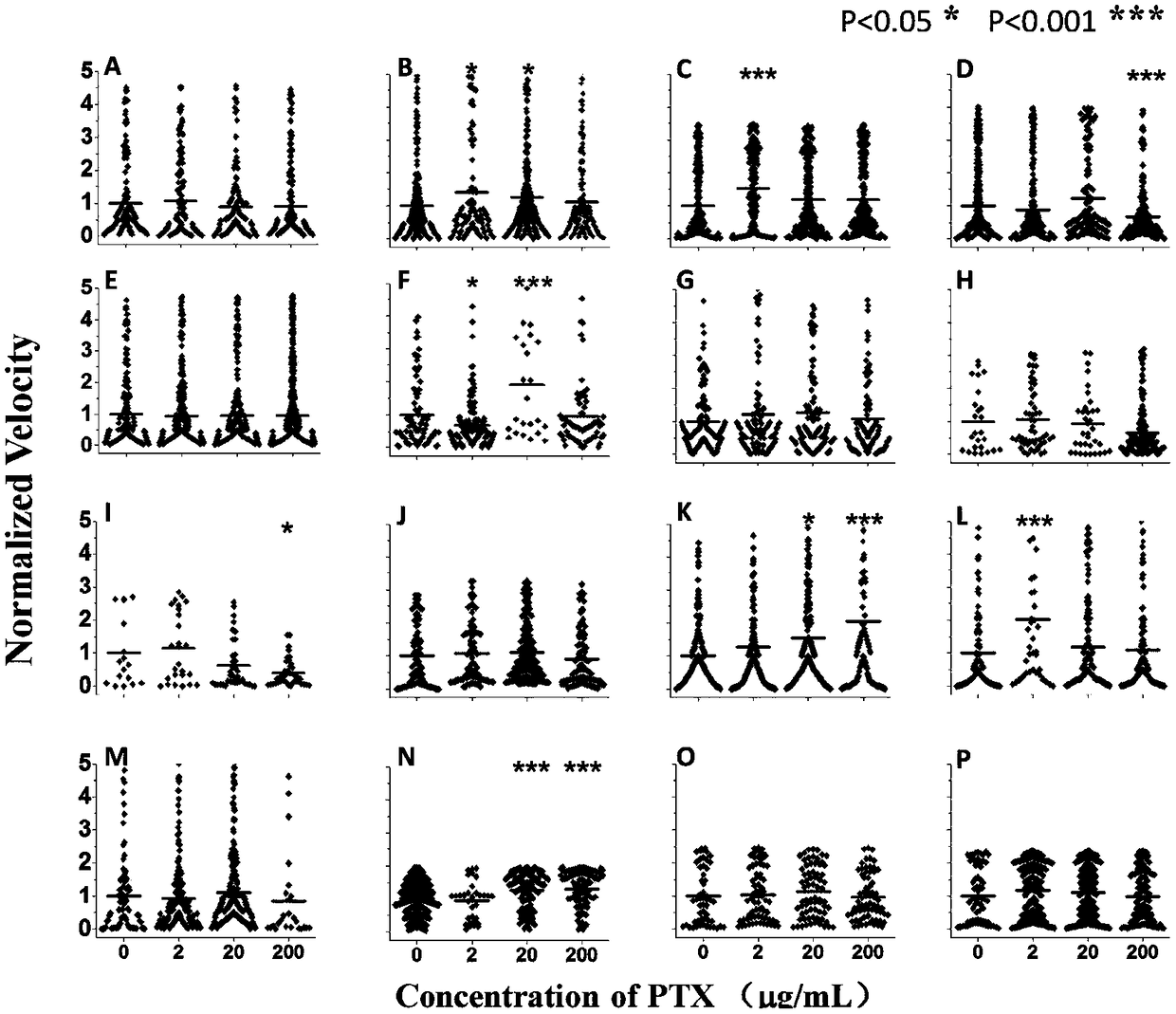
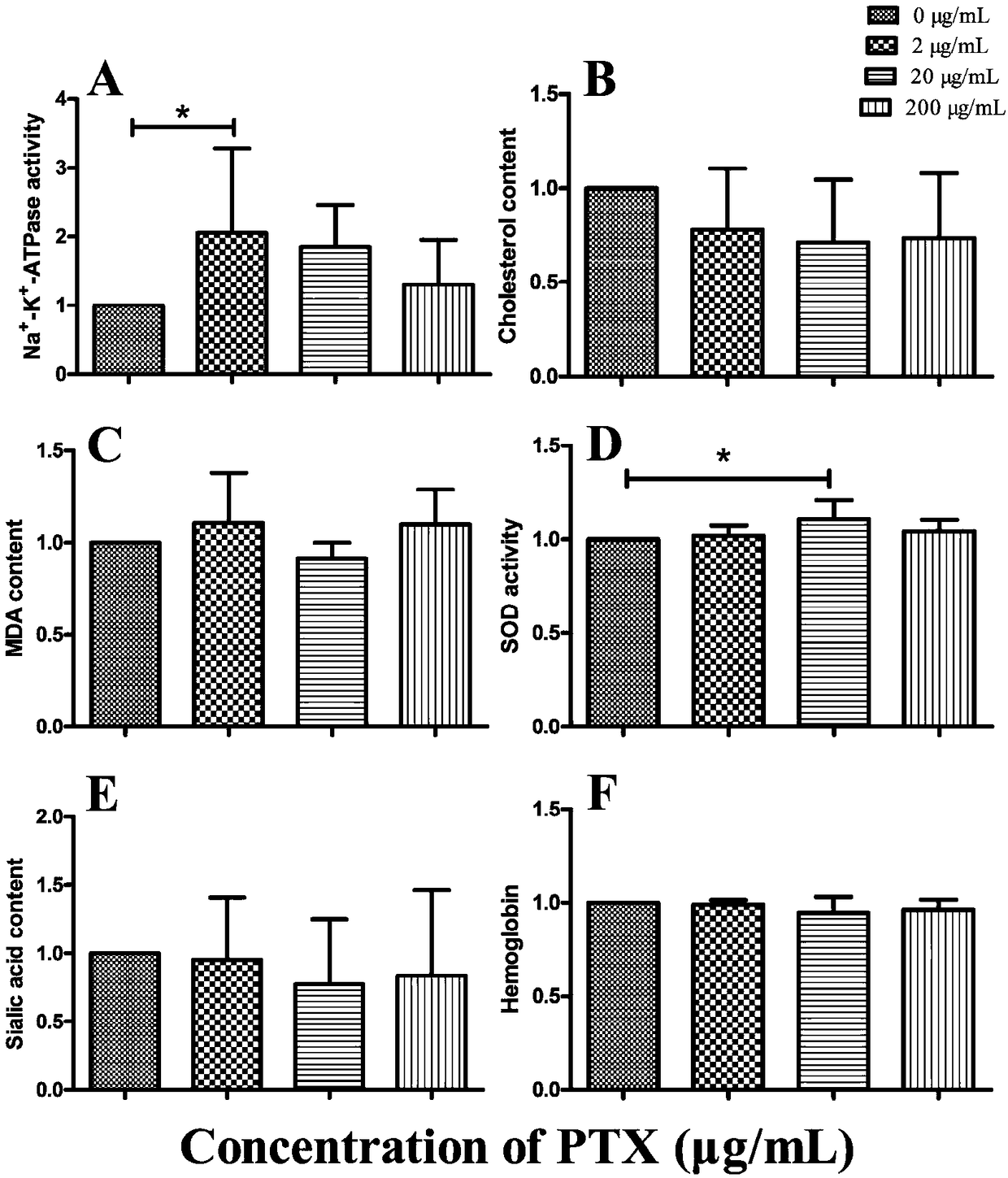
Microfluidic chip-based detection of influence of pentoxifylline on erythrocyte deformability and biochemical indexes in patients with coronary heart disease
InactiveCN108445200AImprove deformation abilityDynamic monitoring of deformabilityInvestigating moving fluids/granular solidsBiological testingATPaseCoronary artery disease
The invention discloses microfluidic chip-based detection of influence of pentoxifylline on erythrocyte deformability and biochemical indexes in patients with coronary heart disease. The individualized detection of the influence of the pentoxifylline on the erythrocyte deformability is realized based on a microfluidic chip, detection and screening of innovative drugs for improving the erythrocytedeformability can be realized, and three main effective biochemical indicators of Na<+>-K<+>-ATPase, superoxide dismutase activity and cholesterol content are found to be important in the change of the erythrocyte deformability in the patients with coronary heart disease.
Owner:SOUTHERN MEDICAL UNIVERSITY
Substituted xanthine derivatives
This invention relates to novel compounds that are substituted xanthine derivatives and pharmaceutically acceptable salts thereof. For example, this invention relates to novel substituted xanthine derivatives that are derivatives of pentoxifylline. This invention also provides compositions comprising one or more compounds of this invention and a carrier and the use of the disclosed compounds and compositions in methods of treating diseases and conditions for which pentoxifylline and related compounds are beneficial.
Owner:CONCERT PHARMA INC
Pharmaceutical composition containing pentoxifylline and carbazochrome sodium sulfonate and application thereof
ActiveCN102125559AImprove microcirculationTreatment fitCardiovascular disorderHeterocyclic compound active ingredientsCarbazochrome Sodium SulfonateActive component
The invention belongs to the field of medicine, and relates to a pharmaceutical composition containing pentoxifylline and carbazochrome sodium sulfonate and application thereof. The pharmaceutical composition contains the active components pentoxifylline and carbazochrome sodium sulfonate, has the efficacies of diminishing inflammation, arresting bleeding, improving the vascular permeability and the like in treating hemorrhagic hemorrhoids, is obviously superior to the single drugs of pentoxifylline and carbazochrome sodium sulfonate in therapeutic action, and has a synergistic action.
Owner:LUNAN PHARMA GROUP CORPORATION
Pentoxifylline tablet controlled relasease tablet
InactiveCN1444943AMaintain blood levelsMaintain effective plasma concentrationOrganic active ingredientsNervous disorderDiseaseCellulose acetate
A slow-releasing torental table for treating peripheral obliteration vasculitis, sequelae of arteriosclerosis, and local ischemia is prepared from torental (20-70 wt.%), slow releasing agent (cellulose acetate and / or ethyl cellulose) (30-80 wt.%), and other assistants. Its advantages are high curative effect, durable action and low by-effect.
Owner:SHENYANG PHARMA UNIVERSITY
Pentoxifilin-based dermatological pharmaceutical composition, for topical application, in cream, gel, solution, emulsion, liposome and microcapsule form
InactiveUS20110159077A1Reduce the possibilityReduce complicationsBiocideOintment deliveryDiseaseUnguent
A pharmaceutical dermatological composition based on Pentoxifylline, to be applied on the skin in the form of a cream, gel, unguent, solution, in an emulsion, in liposomes and in microcapsules, being selective for the vascular disorders of rosacea and disorders with photosensitivity, reducing the blood vessels and the reactions of reddening of the skin, CHARACTERIZED in that said composition consists of: from 1% to 99% of Pentoxifylline, from 0.1% to 80% of base cream and from 0.1% to 75% of liposomes.
Owner:FIGUEROA LIZAMA PATRICIO ROBERTO
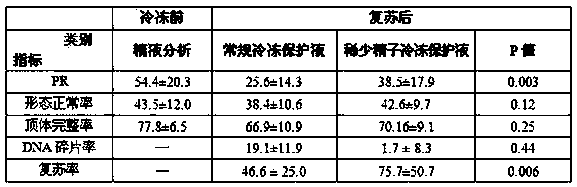
Human testicular tissue suspension cryoprotectant and preparation method thereof
InactiveCN111345284AMake fastImprove freezing and recovery effectDead animal preservationGerm cellsSodium bicarbonateSodium lactate
The invention discloses a human testicular tissue suspension cryoprotectant and a preparation method thereof. The human testicular tissue suspension cryoprotectant comprises the following raw materials: sodium chloride, potassium chloride, calcium chloride, magnesium chloride, monopotassium phosphate, sodium bicarbonate, HEPES, caffeine, sodium pyruvate, pentoxifylline, glycine, glucose and sucrose. The human testicular tissue suspension cryoprotectant also comprises sodium lactate and glycerol. The invention further discloses the preparation method of the human testicular tissue suspension cryoprotectant and a freezing method adopting the human testicular tissue suspension cryoprotectant. The cryoprotectant is specially designed for a testicular tissue suspension, the testicular sperm cryopreservation and resuscitation effect can be improved, the problems that potential safety hazards exist, the cryopreservation and resuscitation effect is poor, and movable testicular sperms are not easy to find are effectively solved, and the human testicular tissue suspension cryoprotectant has quite important clinical application value.
Owner:THE WEST CHINA SECOND UNIV HOSPITAL OF SICHUAN
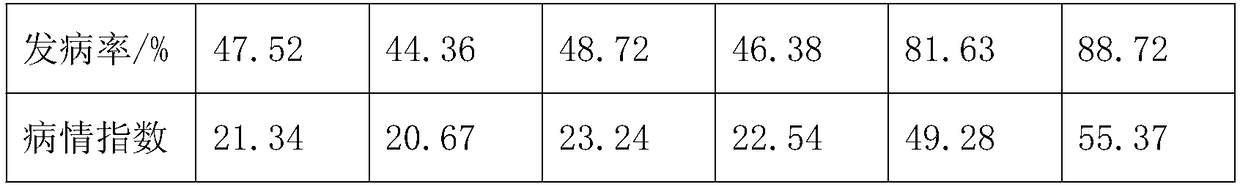
Nutritional solution formula of aeroponic-cultured lettuces and preparation method thereof
InactiveCN108440153AWide variety of sourcesReduce morbidityBio-organic fraction processingMagnesium fertilisersDiseaseTheobromine
The invention discloses a nutritional solution formula of aeroponic-cultured lettuces and a preparation method thereof. The nutritional solution formula is prepared from the following raw materials inparts by weight: 0.36-0.88 part of magnesium sulfate, 0.25-0.46 part of soy peptone, 1.8-3.3 parts of fructooligosaccharide, 0.1-0.27 part of pentoxifylline, 0.06-0.15 part of monopotassium phosphate, 0.05-0.12 part of urea, 3.6-5.5 parts of carrot, 2.4-4.2 parts of fish bone meal, 5-8 parts of rhizoma acori graminei, 960-1050 parts of deionized water and 13-19.4 parts of mushroom leftover. The nutritional solution formula and the preparation method disclosed by the invention have the beneficial effects that different preparation processes are adopted for different raw materials to obtain different products, then the different products are primarily fermented and secondarily fermented, and the obtained finished product can cause great reduction of both the disease attack rate and the disease attack index of the aeroponic-cultured lettuces, so that the monetary cost and the labor cost of producers are reduced and the economic benefit is improved.
Owner:JILIN ACAD OF AGRI MACHINERY
Substituted xanthine derivatives
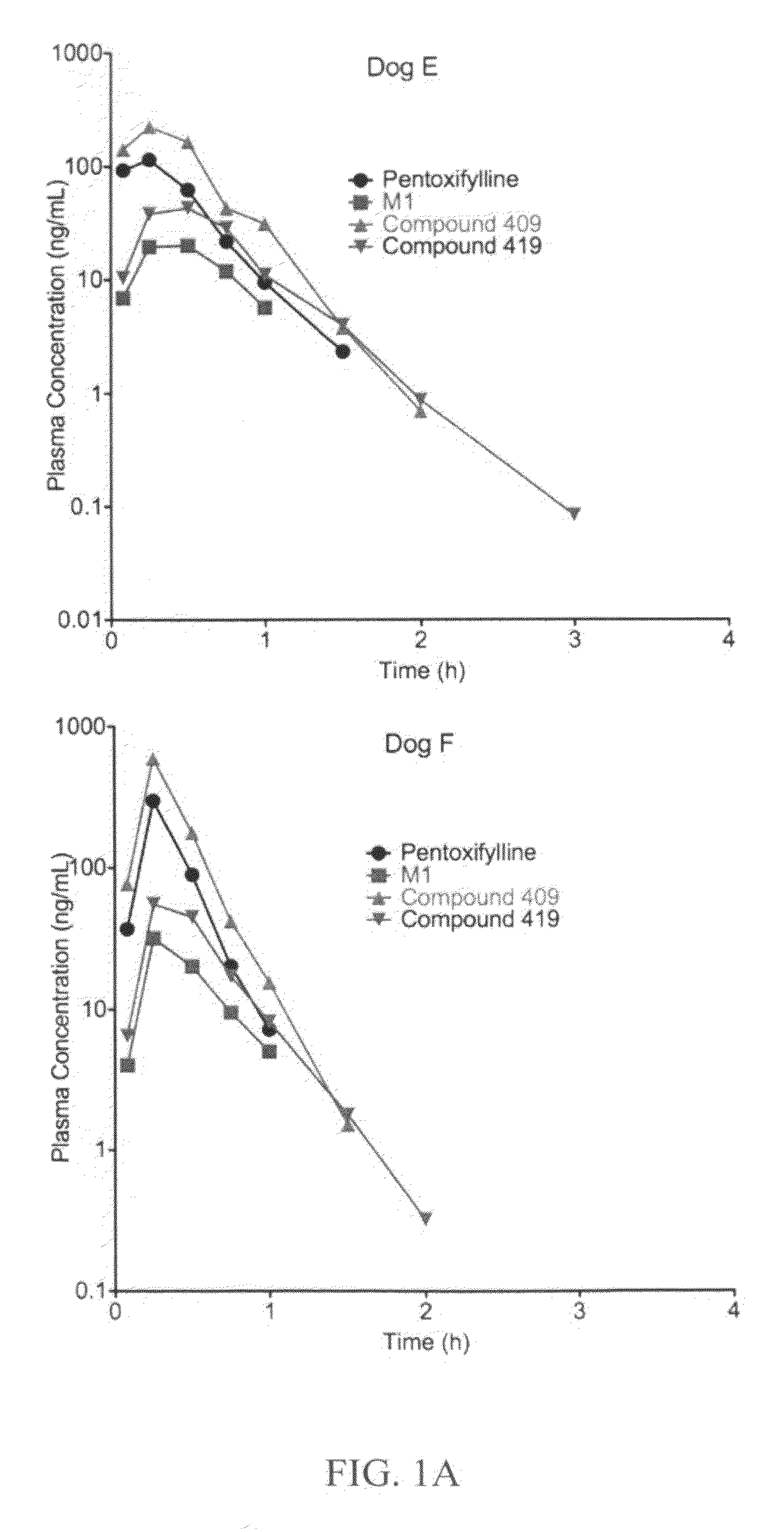
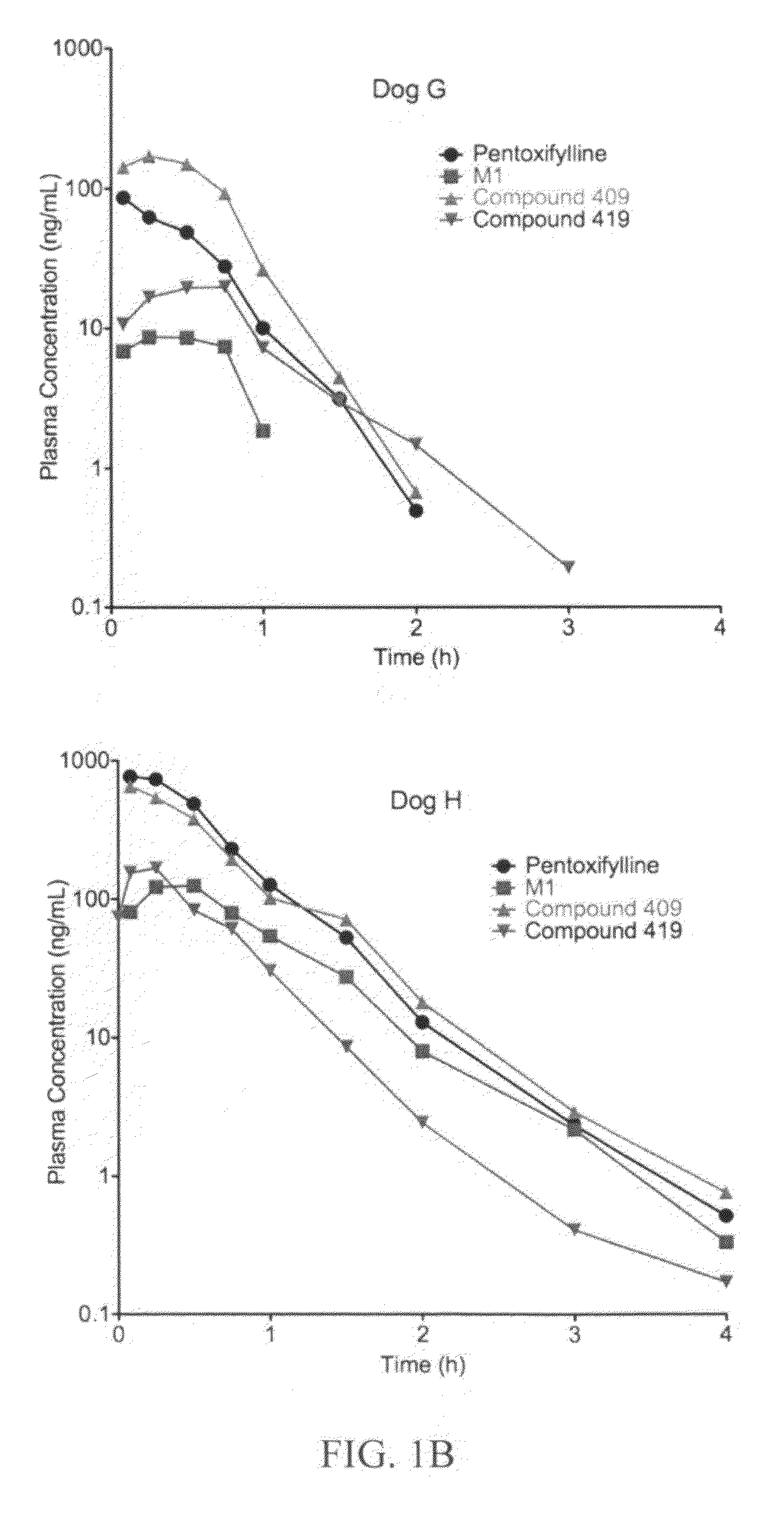
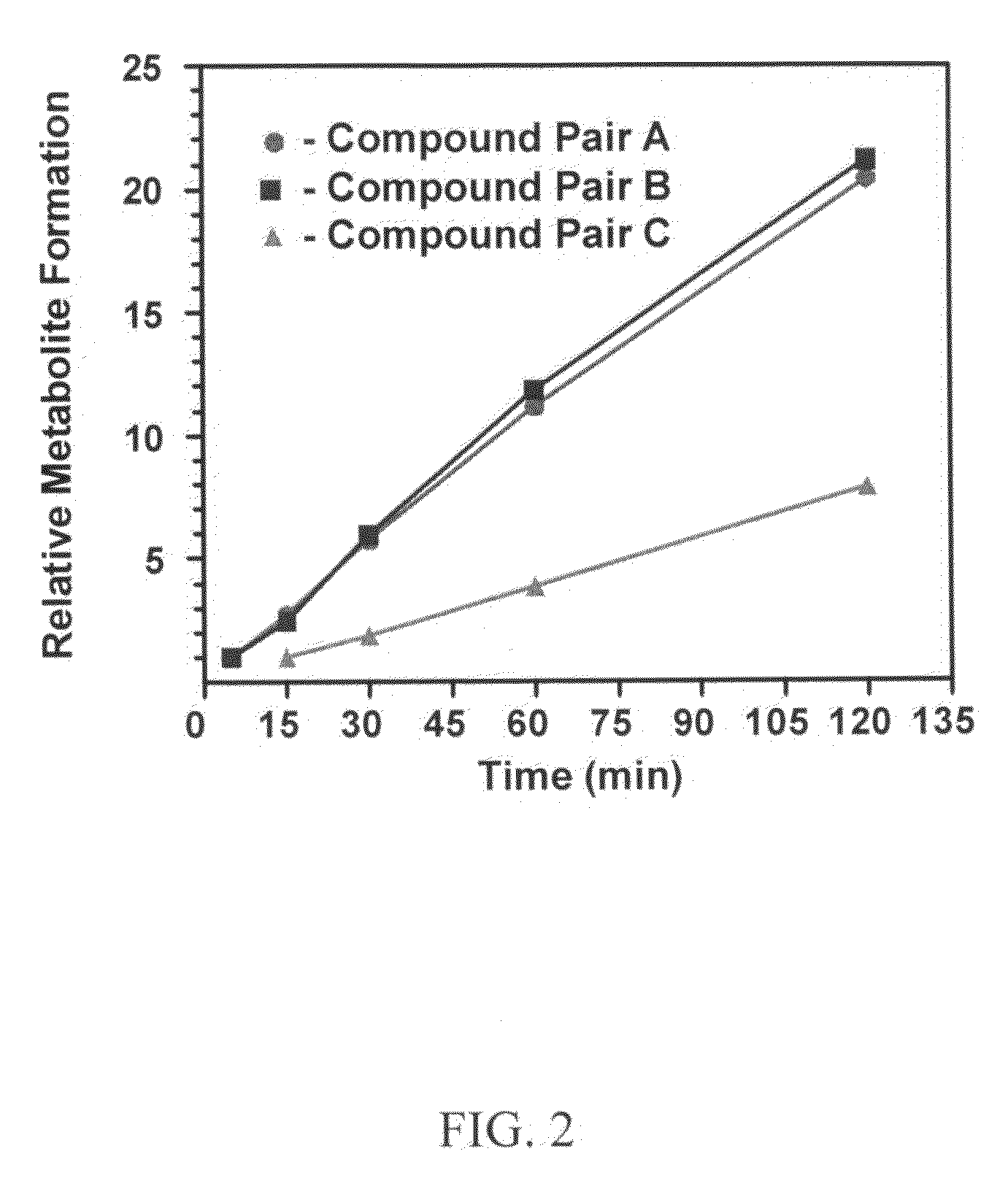
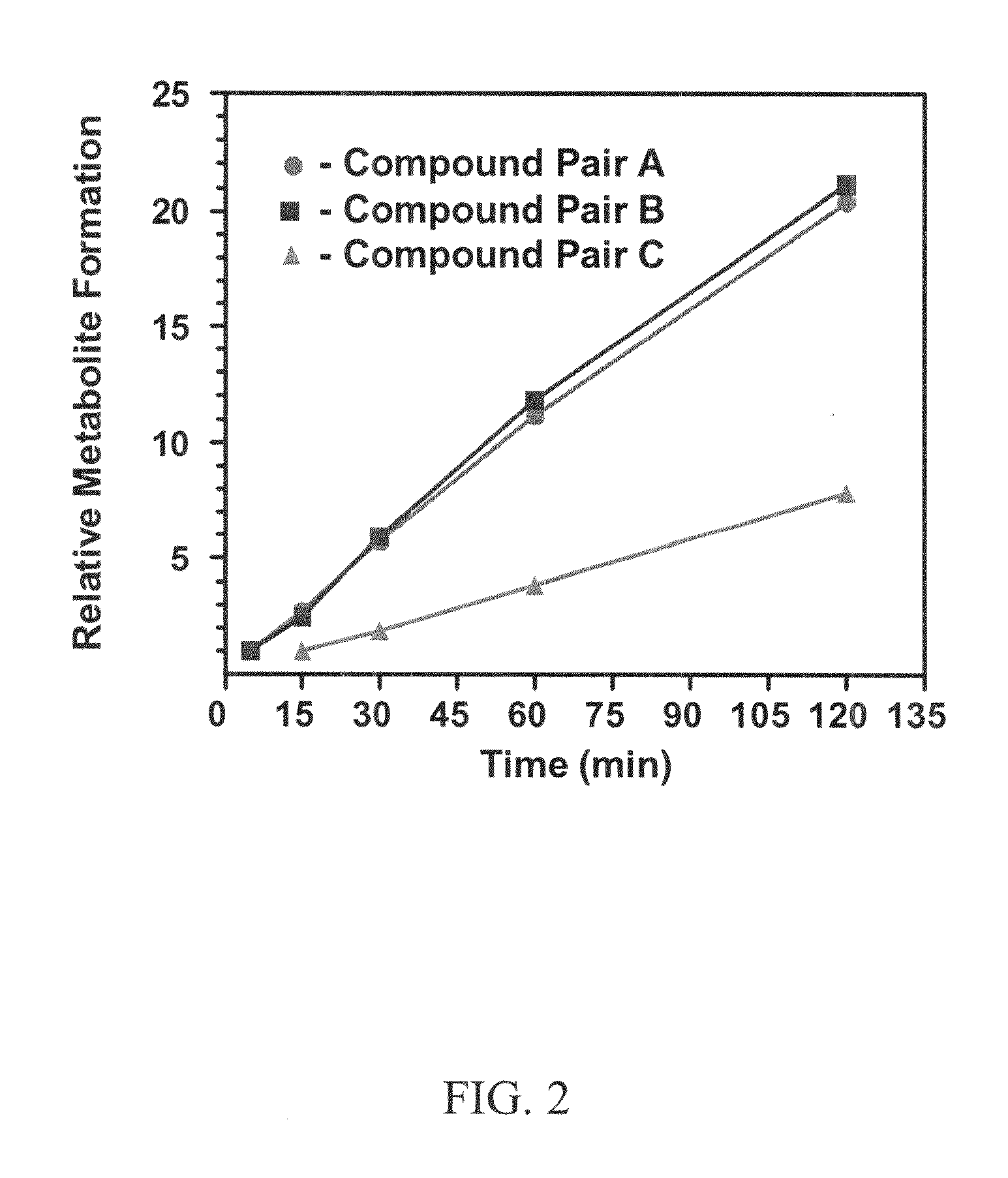
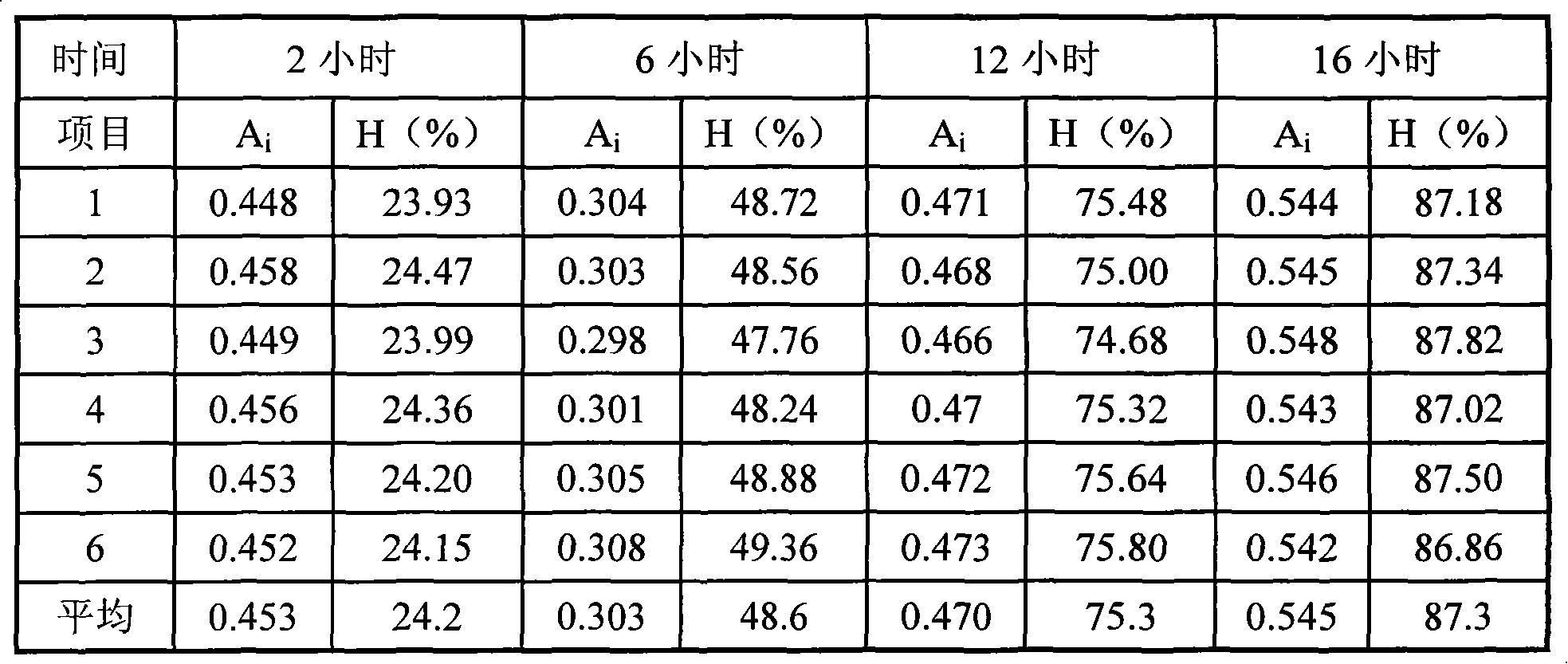
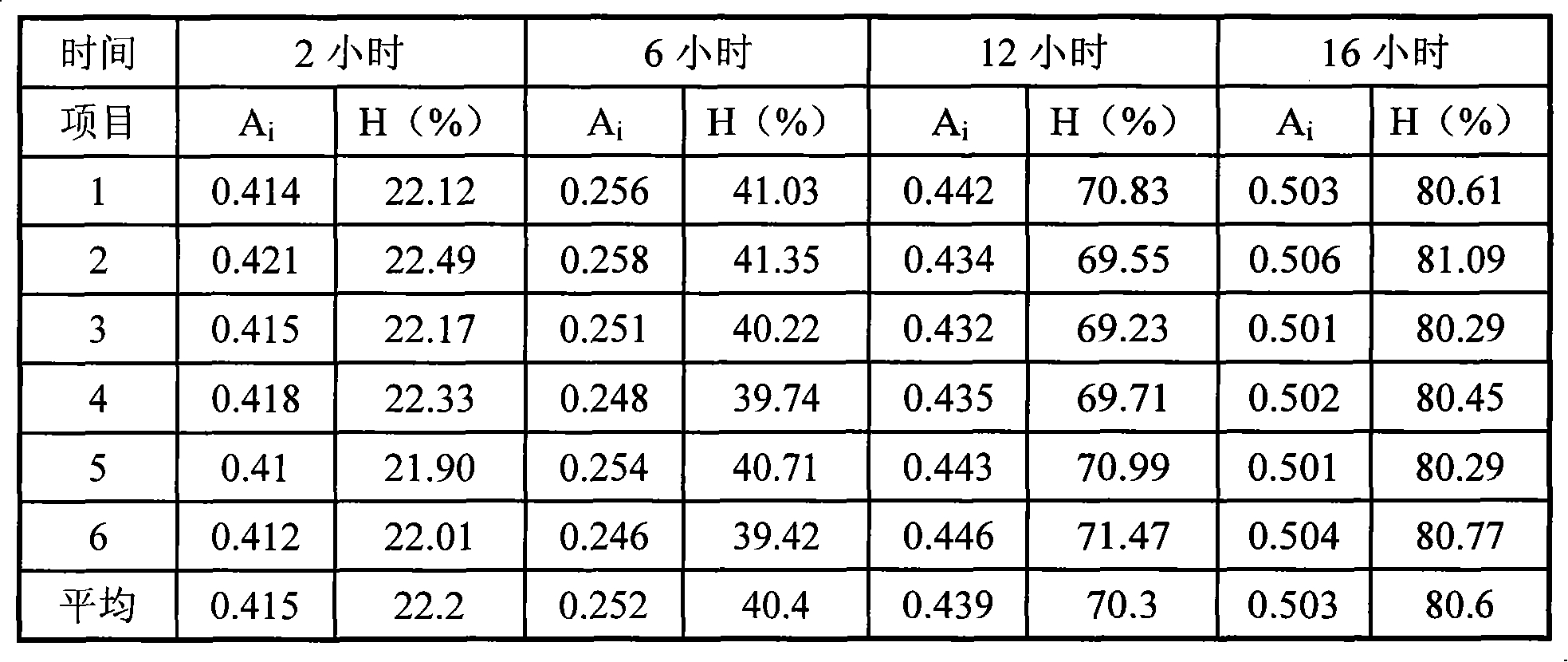
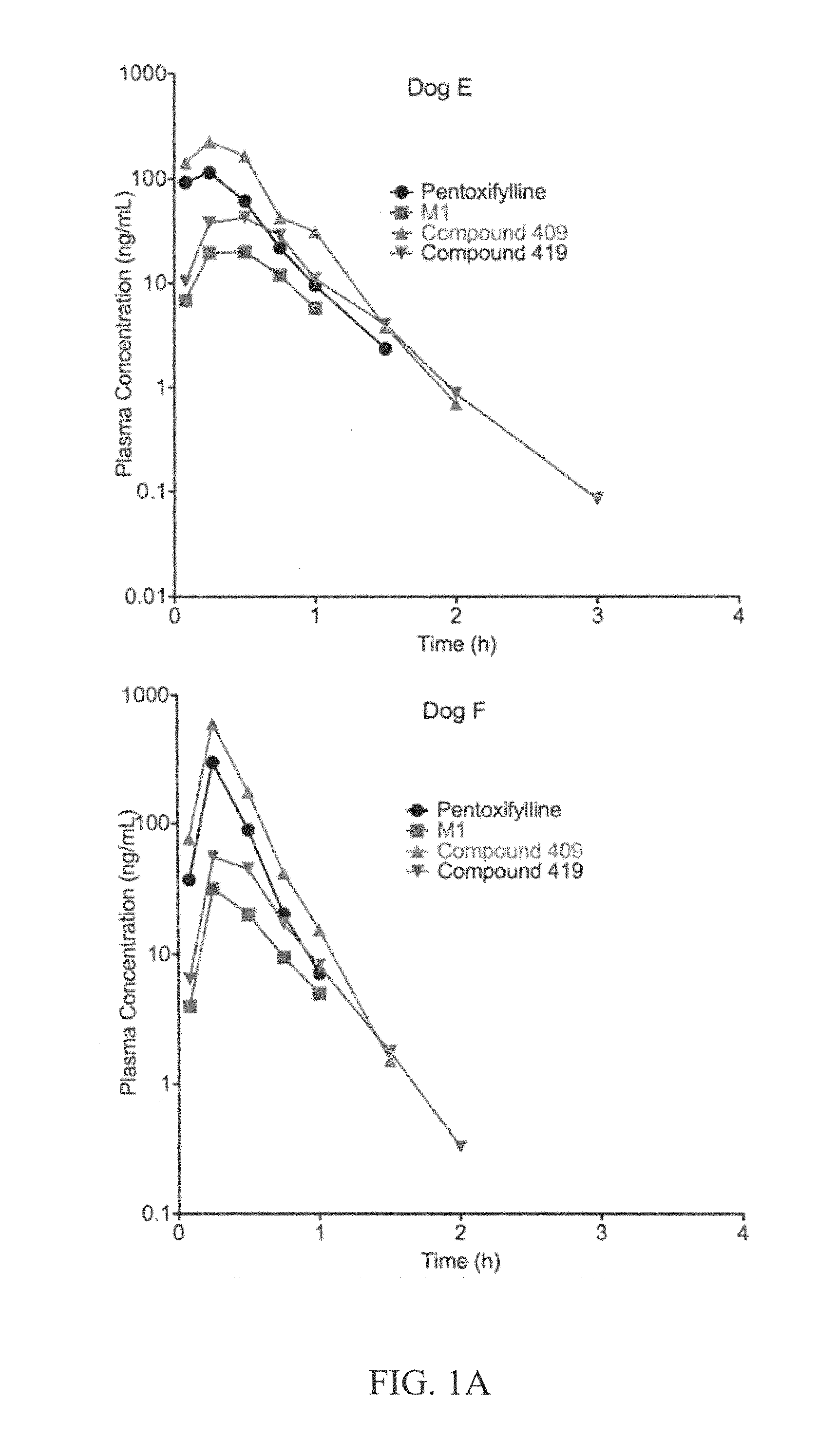
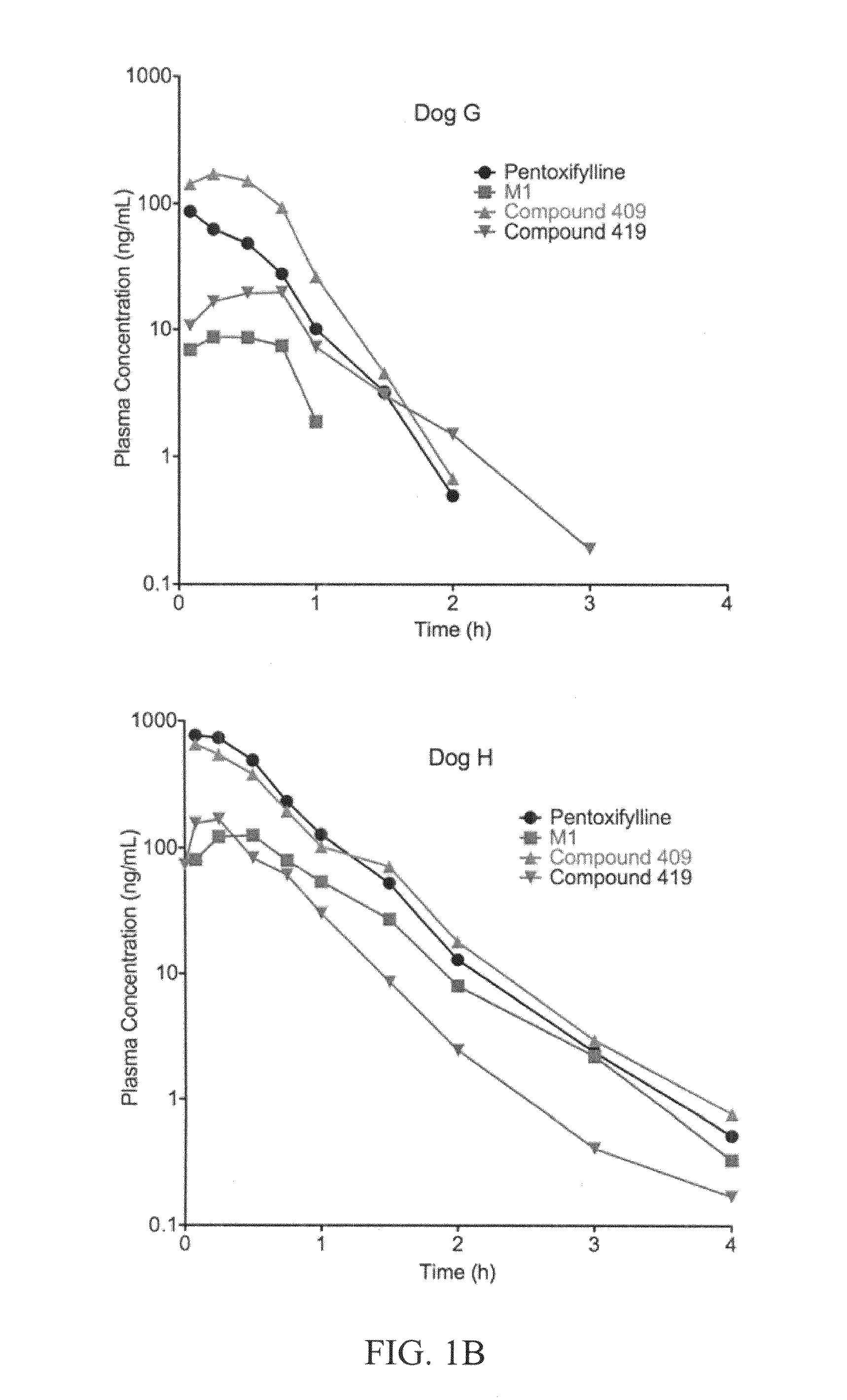
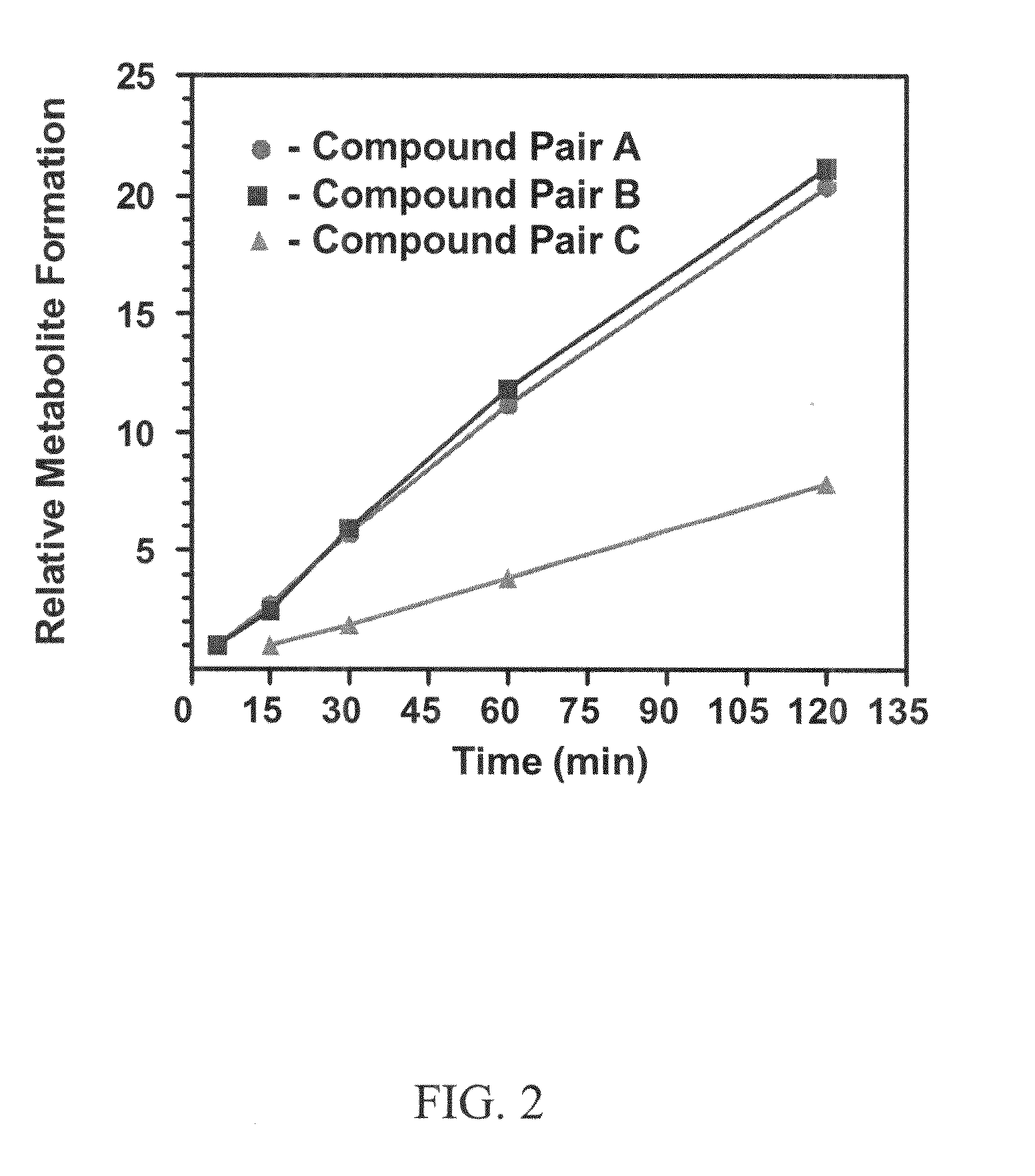
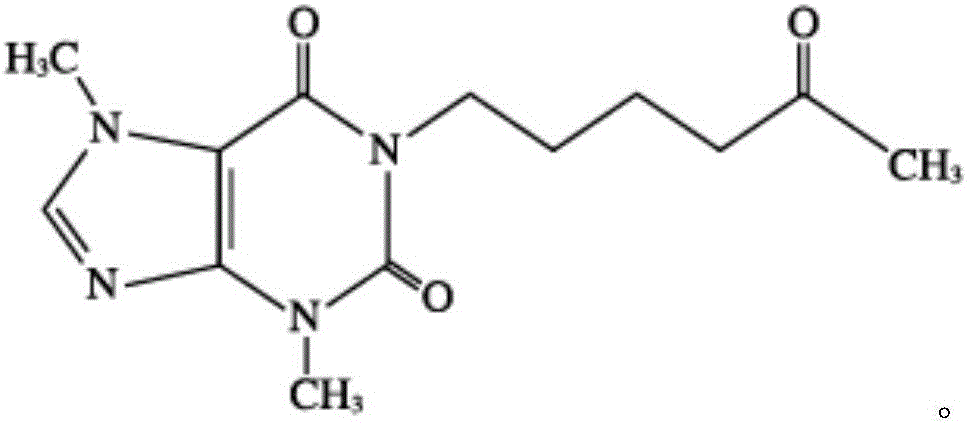
This invention relates to novel deuterated compounds that are substituted xanthine derivatives and pharmaceutically acceptable salts thereof. In particular, this invention relates to novel substituted xanthine derivatives that are deuterated derivatives of a pentoxifylline metabolite. This invention also provides compositions comprising one or more compounds of this invention and a carrier and the use of the disclosed deuterated compounds and compositions in methods of treating diseases and conditions for which pentoxifylline and related compounds are beneficial. The compounds of the invention are represented by the following structural formula:wherein the values of R1, R2, R3, R4, R5, Y1 and Y2 are described herein.
Owner:SUN PHARMA IND INC
Compositions and methods of administering a colchicine based topical composition for the prevention of radiation fibrosis
ActiveUS20170348211A1Avoid scaringInhibition formationCosmetic preparationsToilet preparationsChemical compositionRadiation induced fibrosis
A colchicine-containing composition comprising colchicine as the active ingredient or colchicine in combination with pentoxifylline and tocopherol (Vitamin E) which are formulated for topical use in the prevention and treatment of radiation-induced fibrosis. And methods of making and administering the colchicine-containing compositions. The compositions can be used as topical applications for the prevention and treatment of radiation-induced fibrosis, commonly known as scarring, that can be debilitating, and can occur as a late and permanent complication of radiation therapy
Owner:COLRADEL LLC
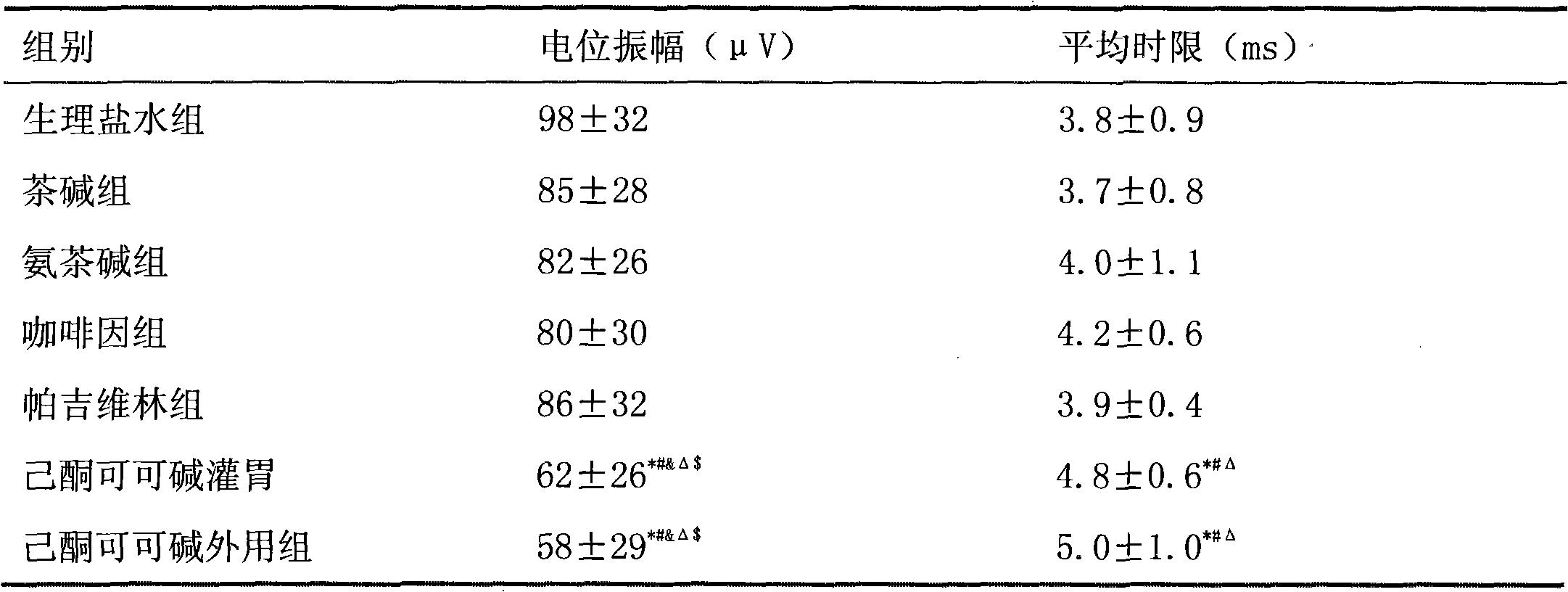
Medicinal composition for treating or preventing neuropathic pain
ActiveCN102648915AGood treatment effectGood synergyNervous disorderHeterocyclic compound active ingredientsDiabetes mellitusTheobromine
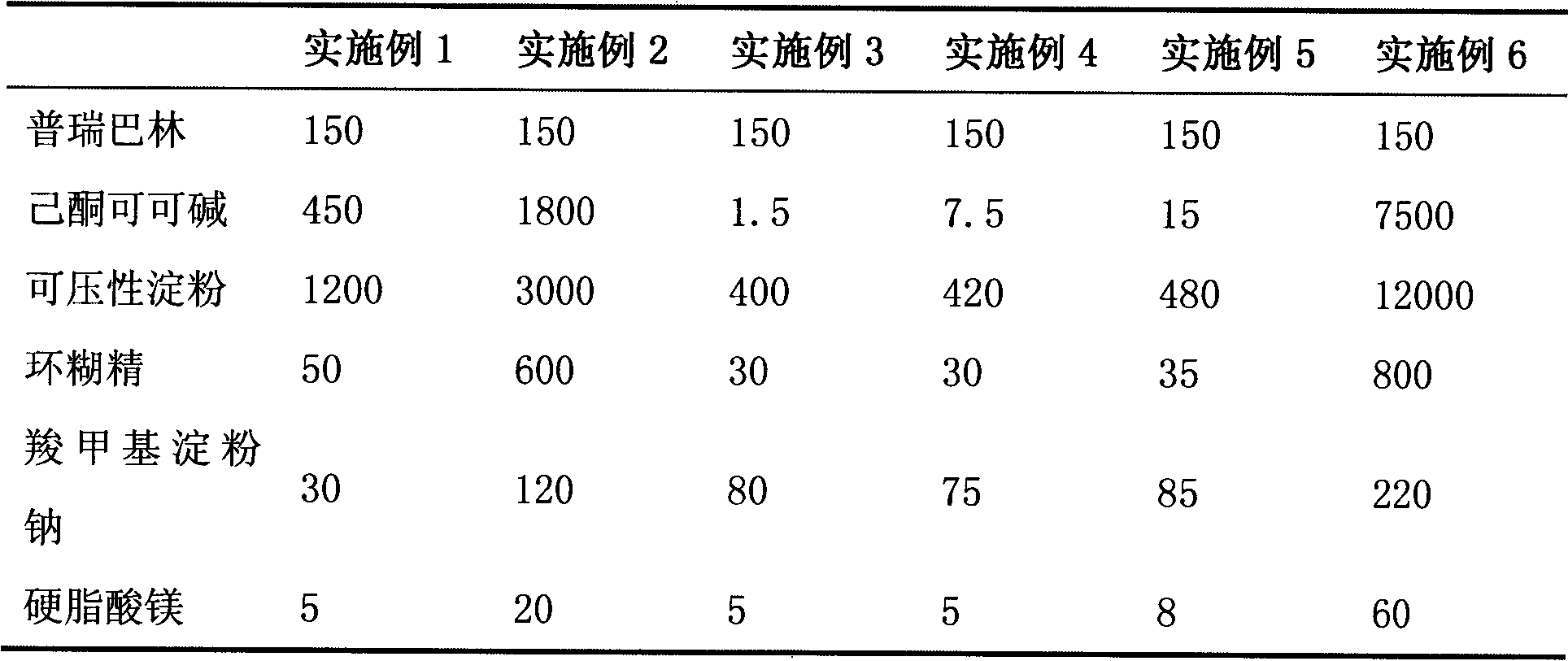
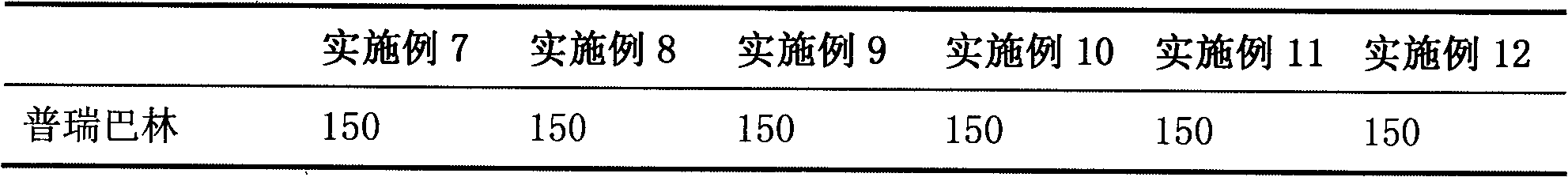
The invention belongs to the field of medicine, and particularly relates to a medicinal composition for treating or preventing neuropathic pain. At present, an effective medicament for treating neuropathic pain is unavailable. The invention provides a medicinal composition for treating neuropathic pain. The medicinal composition contains pregabalin and pentoxifylline, and can further contain diclofenac or a pharmaceutical salt thereof. Compared with separate application of pentoxifylline, pregabalin or diclofenac, the medicinal composition which contains pregabalin and pentoxifylline and further contains diclofenac has a synergistic action on the aspect of treatment of neuropathic pain and a particularly better effect on neuropathic pain caused by diabetes mellitus.
Owner:LUNAN PHARMA GROUP CORPORATION
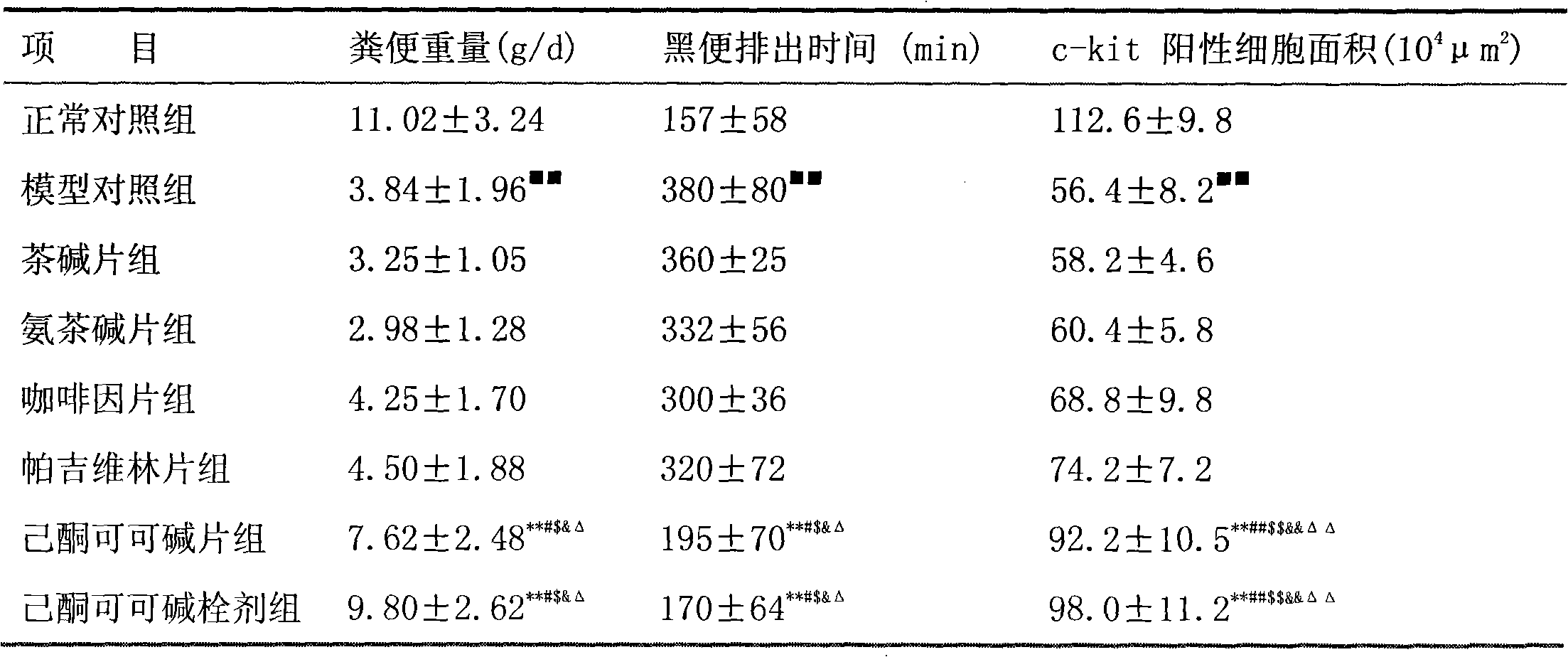
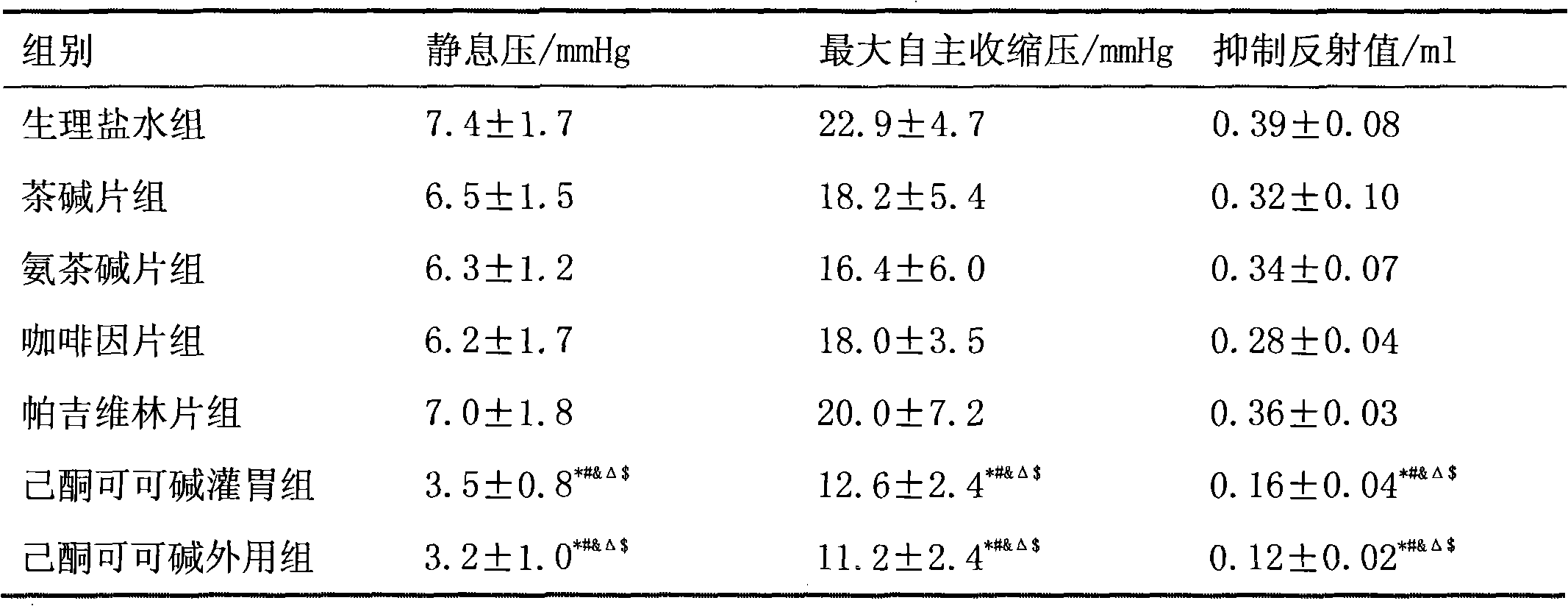
Application of pentoxifylline to preparing medicaments for preventing or treating functional constipation
ActiveCN102145004AFunction increasePlay a role in mechanical protectionDigestive systemCoatingsMedicinePentoxifylline
The invention belongs to the field of medicines and relates to a new application of pentoxifylline, in particular to the application of pentoxifylline to preparing medicaments for preventing or treating functional constipation. Therefore, the invention provides a pentoxifylline-contained pharmaceutical composition for treating functional constipation. Shown by tests, the pentoxifylline has marvelous effects in the aspect of treating the functional constipation.
Owner:LUNAN PHARMA GROUP CORPORATION
Preparation method of novel goat sperm in-vitro agonist
The invention relates to a preparation method of a novel goat sperm in-vitro agonist. The method is characterized by comprising the following steps: (1) cleaning adult goat testes; (2) cutting the tunica albuginea of the testes; (3) using tweezers to clamp out tissues for washing; (4) adding a DMEM basic culture solution after digestion; (5) performing filtering and centrifuging, adding a buffer solution, and performing centrifuging; (6) adding a DMEM basic culture solution, taking cell suspension, performing centrifuging, and collecting cells; (7) adding PBS, performing centrifuging, and then adding a DMEM basic culture solution for culture; (8) carrying out cell passage, performing culturing for 8 hours, and performing storing to obtain a basic liquid I; (9) adding bill goat serum (EGS), calcium ionophore (IA), caffeine, pentoxifylline (PF), 17alpha-20beta-dihydroxyprogesterone (DHP) and heparin into a DMEM basic culture solution, and performing full and uniform mixing to obtain basic liquid II; and (10) uniformly mixing the basic liquid I with the basic liquid II according to a ratio of 5:1 to obtain the novel goat sperm in-vitro agonist. The agonist has the advantages of good application effect, low cost and easy processing and production.
Owner:QINGDAO AGRI UNIV
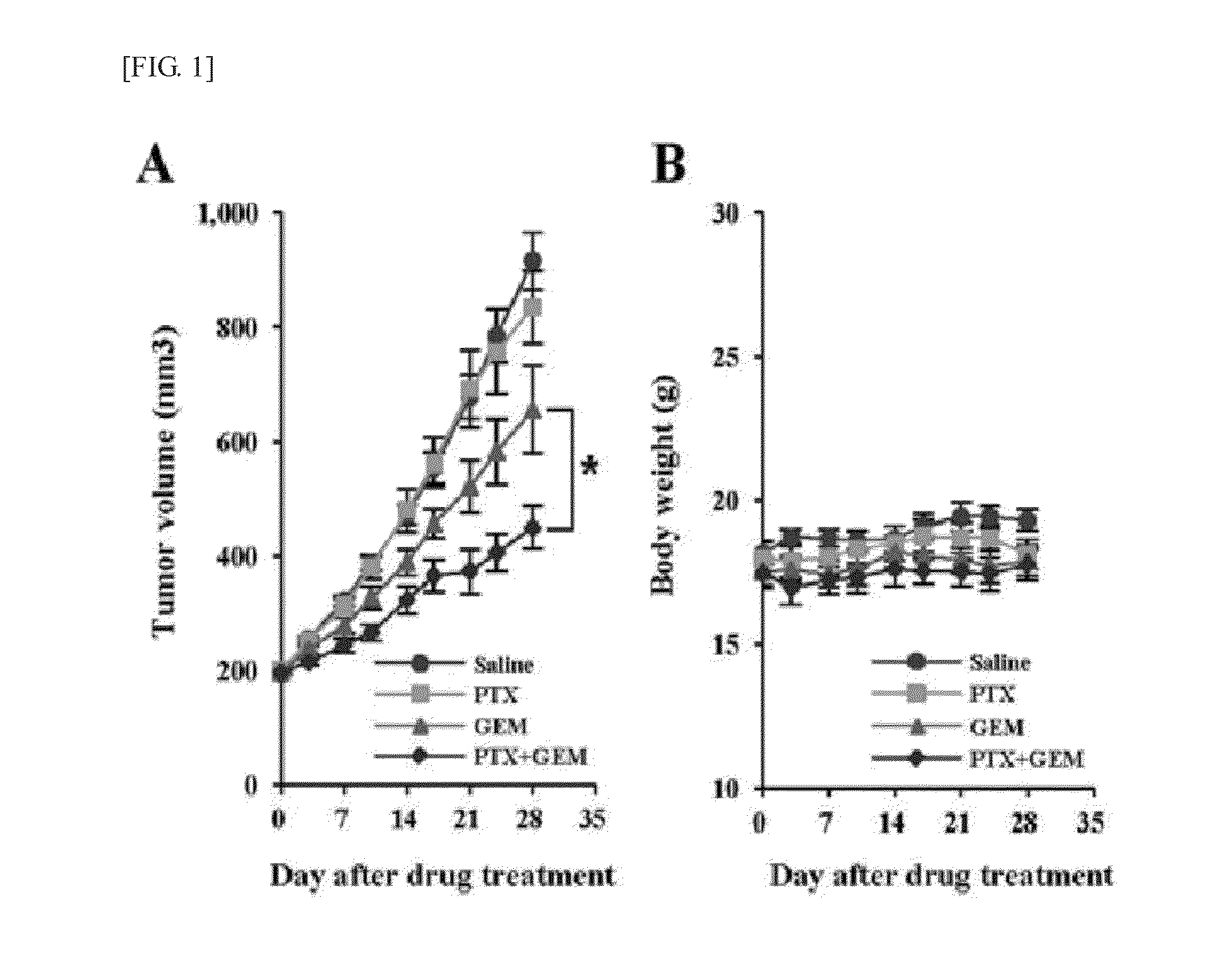
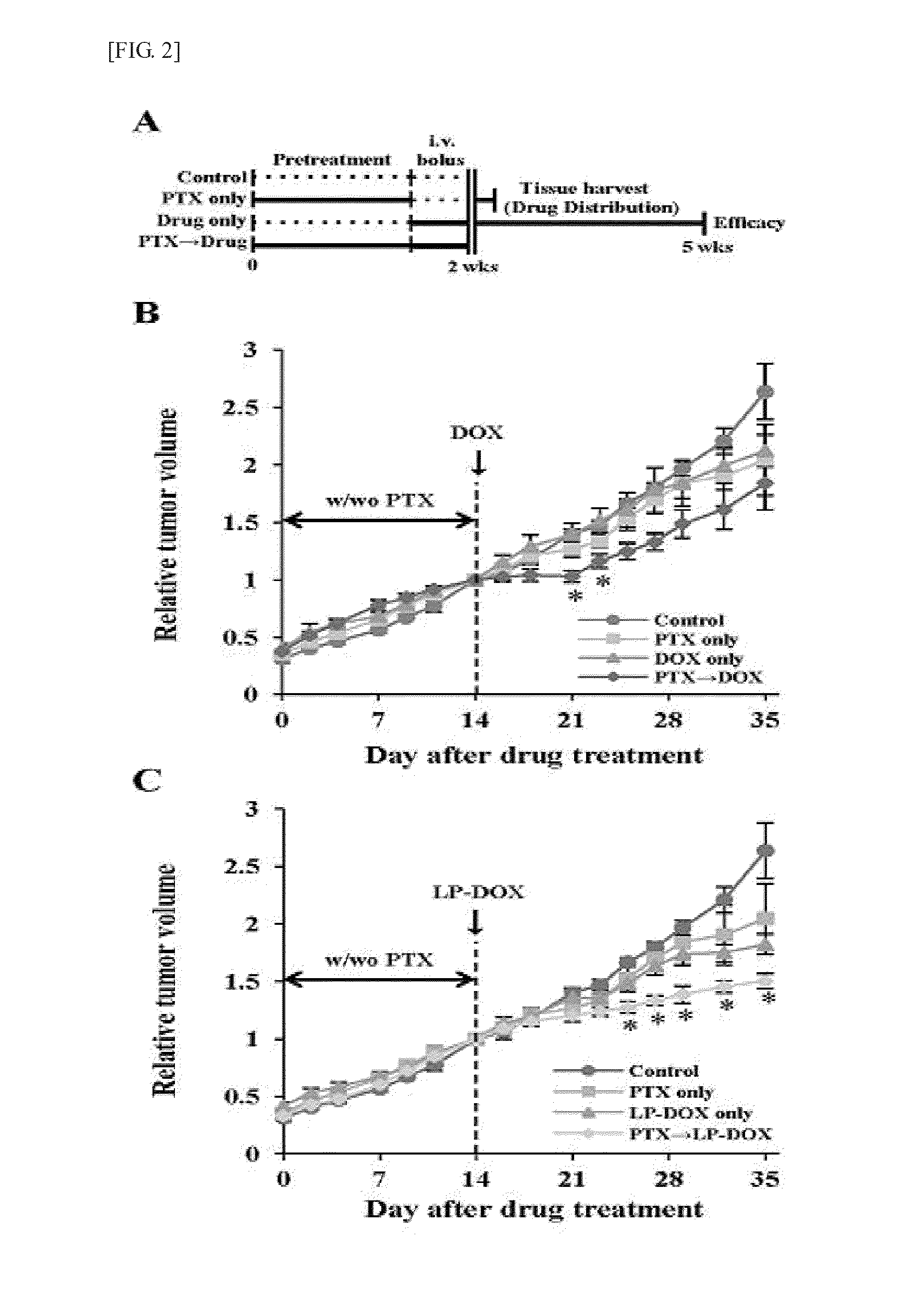
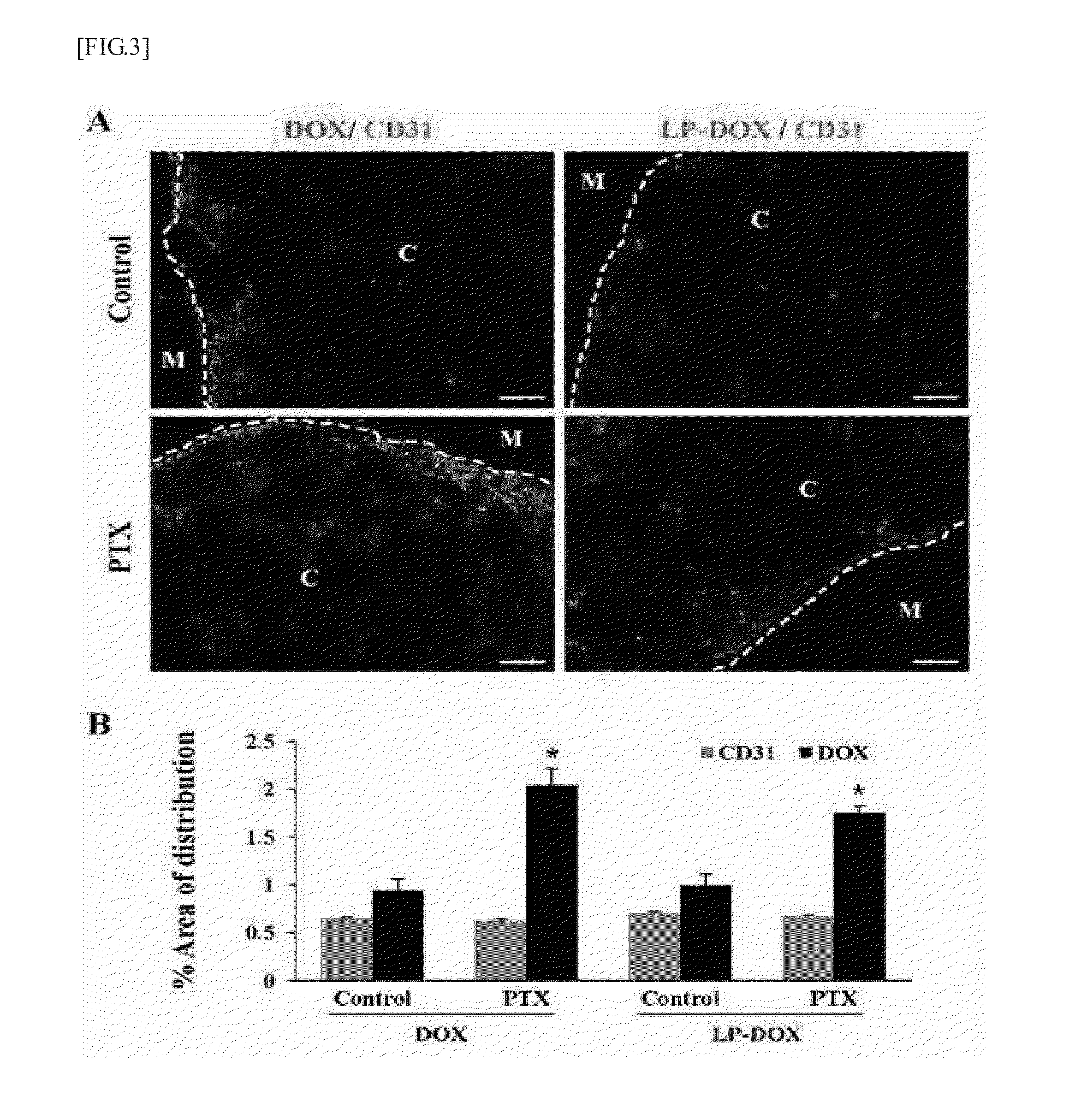
Anticancer adjuvant containing pentoxifylline
InactiveUS20150374702A1Good treatment effectInhibit synthesisBiocideOrganic chemistryCancer cellAdjuvant
The present invention provides an anticancer adjuvant containing pentoxifylline. Pentoxifylline inhibits a collagen synthesis in tumors and consequently increases the distribution of an anticancer drug or sensitivity of cancer cells, which thus provides an improved anticancer treatment effect.
Owner:THE CATHOLIC UNIV OF KOREA IND ACADEMIC COOPERATION FOUND
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com