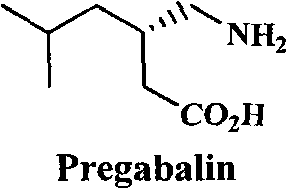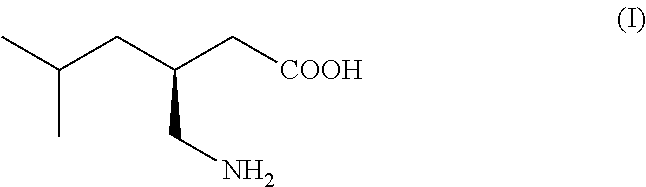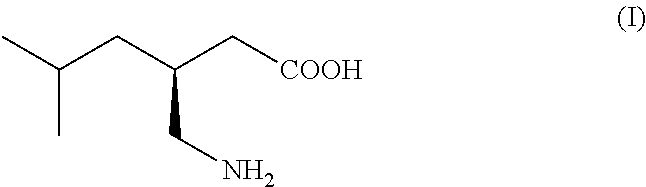Patents
Literature
307 results about "Pregabalin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
This medication is used to treat pain caused by nerve damage due to diabetes or shingles (herpes zoster) infection..
Sustained release pharmaceutical compositions comprising pregabalin
InactiveUS20130280324A1Increase in plasma levelImprove the level ofBiocideOrganic active ingredientsSustained release drugImmediate release
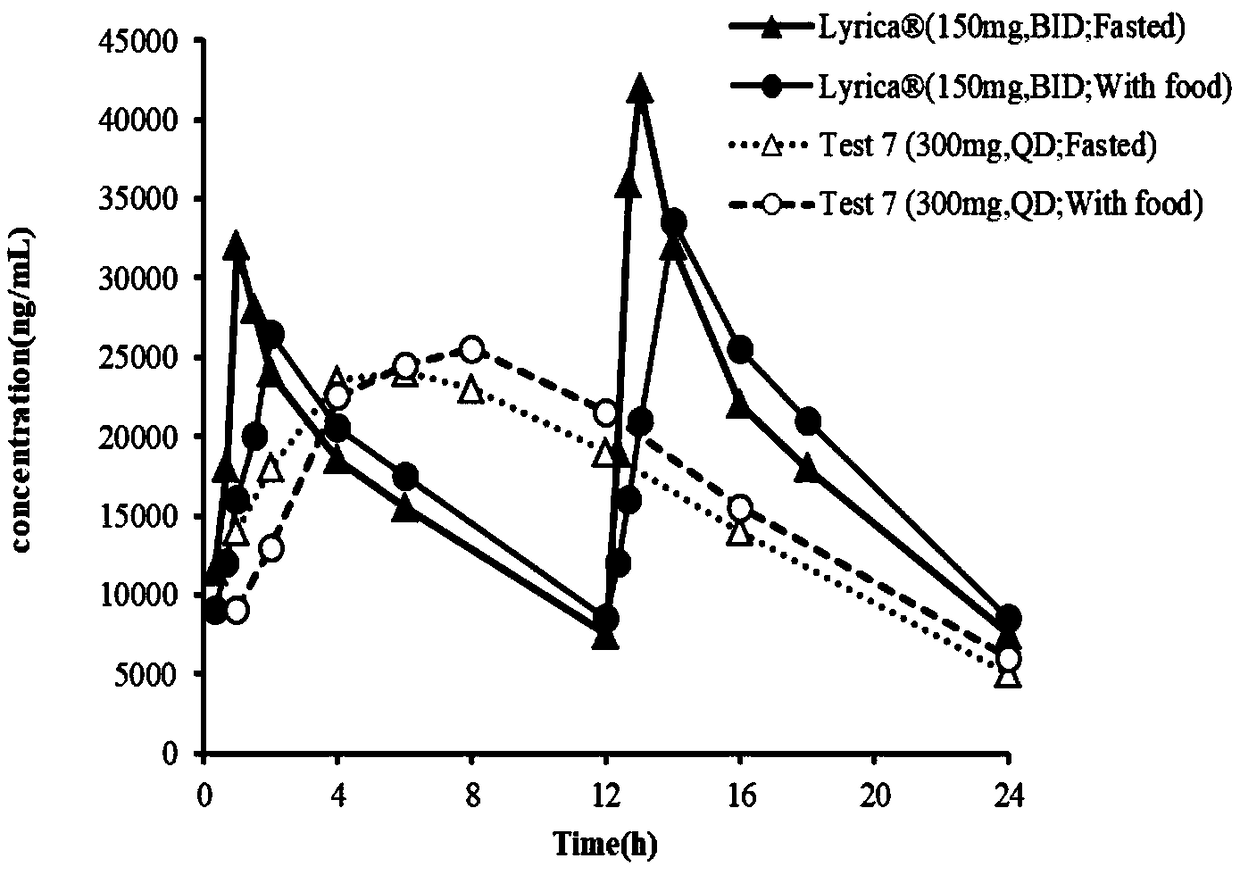
The present invention relates to stable once daily sustained release pharmaceutical compositions comprising pregabalin or pharmaceutically acceptable salts thereof and a pharmaceutically acceptable excipient wherein pharmaceutical composition is bioequivalent to conventional immediate release formulation of pregabalin administered twice daily. The present invention further relates to a composition comprising pregabalin and sugar esters as release retarding agent for maintaining uniform release rate of the drug and process for the preparation of such oral sustained release formulations.
Owner:PANACEA BIOTEC
Sodium channel blocker compositions and the use thereof
Methods of treating or preventing chronic pain or convulsion are disclosed by administering to an animal a sodium channel blocker and at least one of gabapentin and pregabalin. Also disclosed are pharmaceutical compositions and kits for the treatment or prevention of chronic pain or convulsion.
Owner:EURO-CELTIQUE SA
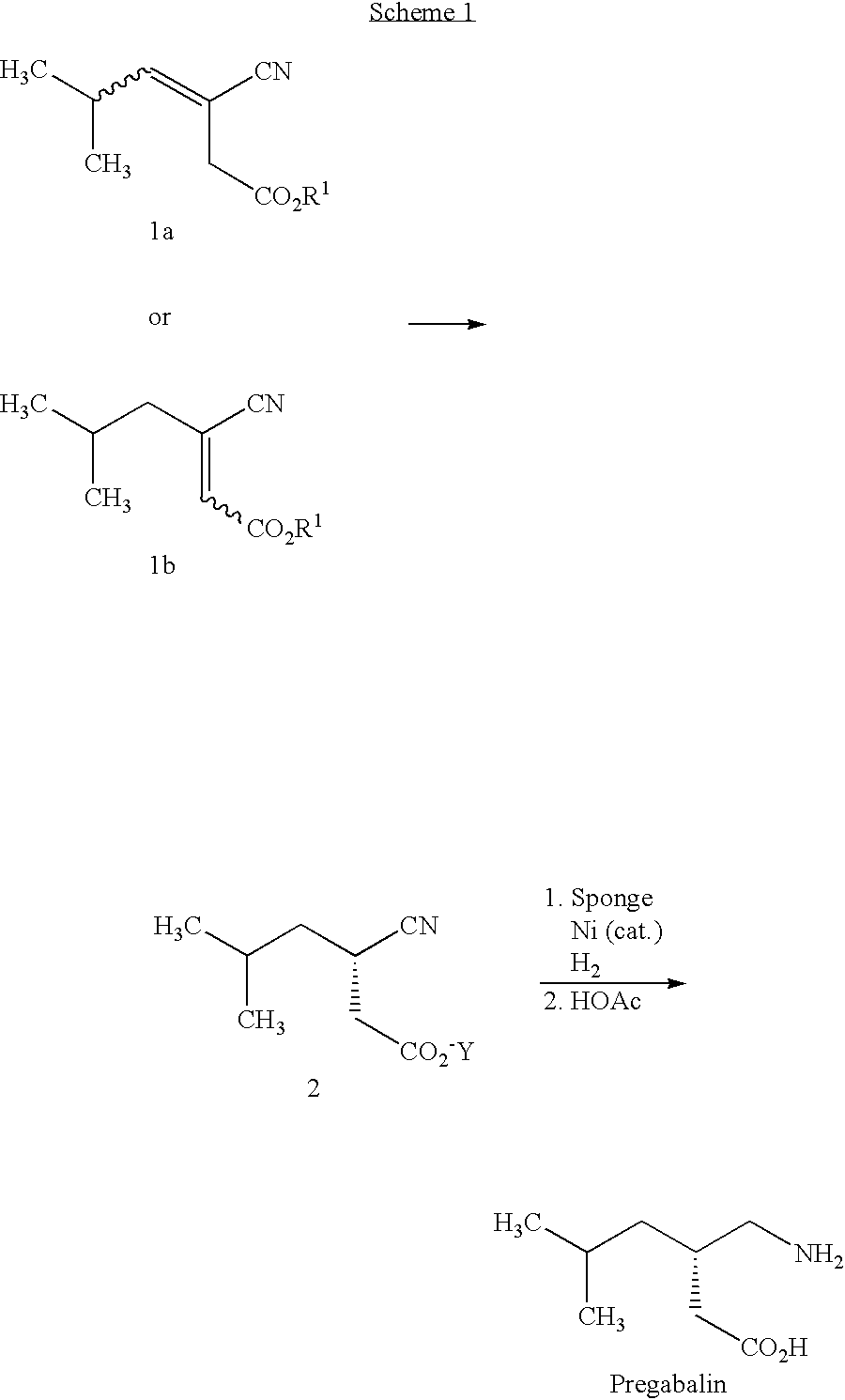
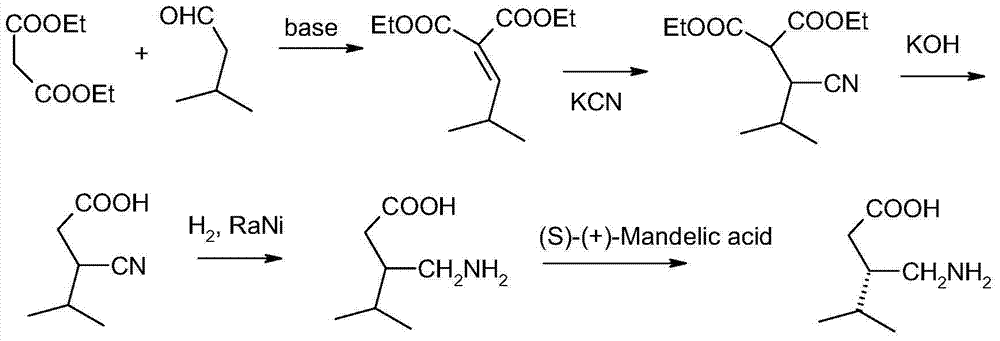
Asymmetric synthesis of pregabalin
InactiveUS6891059B2Effective approachDisposal of minimizedNervous disorderCarboxylic acid nitrile preparationPregabalinAsymmetric hydrogenation
This invention provides a method of making (S)-(+)-3-(aminomethyl)-5-methylhexanoic acid (pregabalin) or a salt thereof via an asymmetric hydrogenation synthesis. Pregabalin is useful for the treatment and prevention of seizure disorders, pain, and psychotic disorders. The invention also provides intermediates useful in the production of pregabalin.
Owner:WARNER-LAMBERT CO
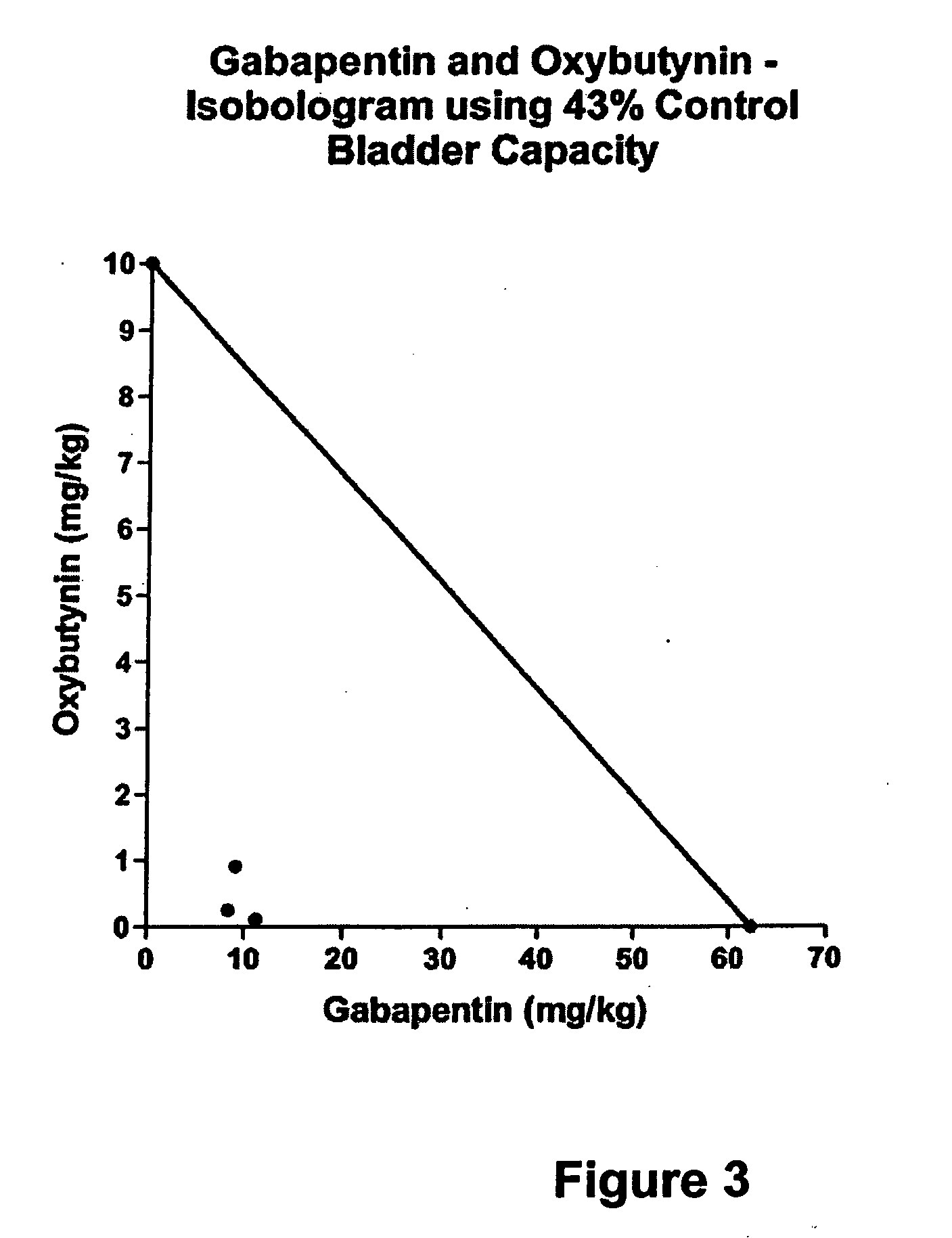
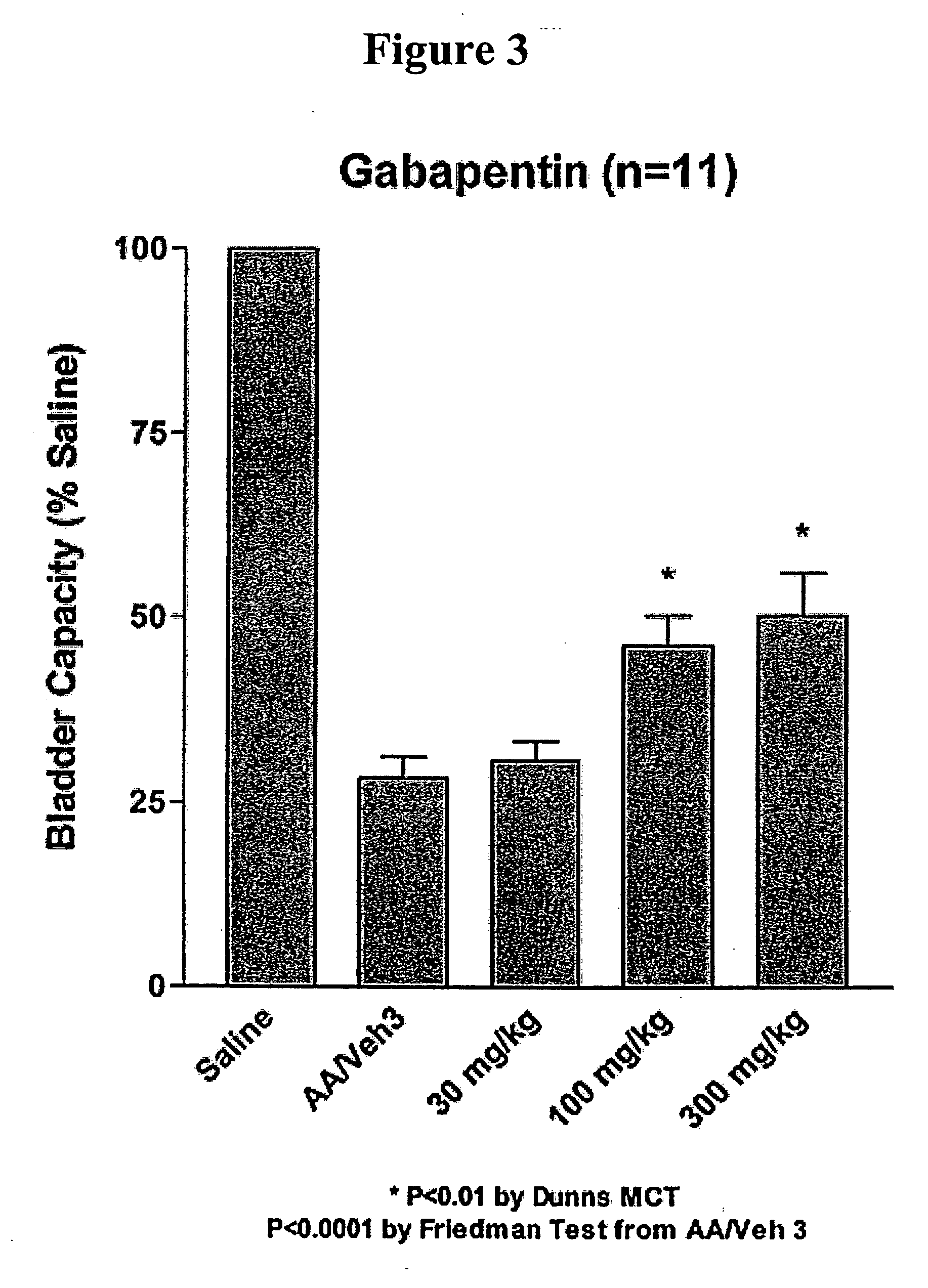
Methods for treating pain using smooth muscle modulators and a2 subunit calcium channel modulators
InactiveUS20060264509A1Limited efficacyReduce patient complianceBiocideOrganic active ingredientsGabapentinAdrenergic receptor agonists
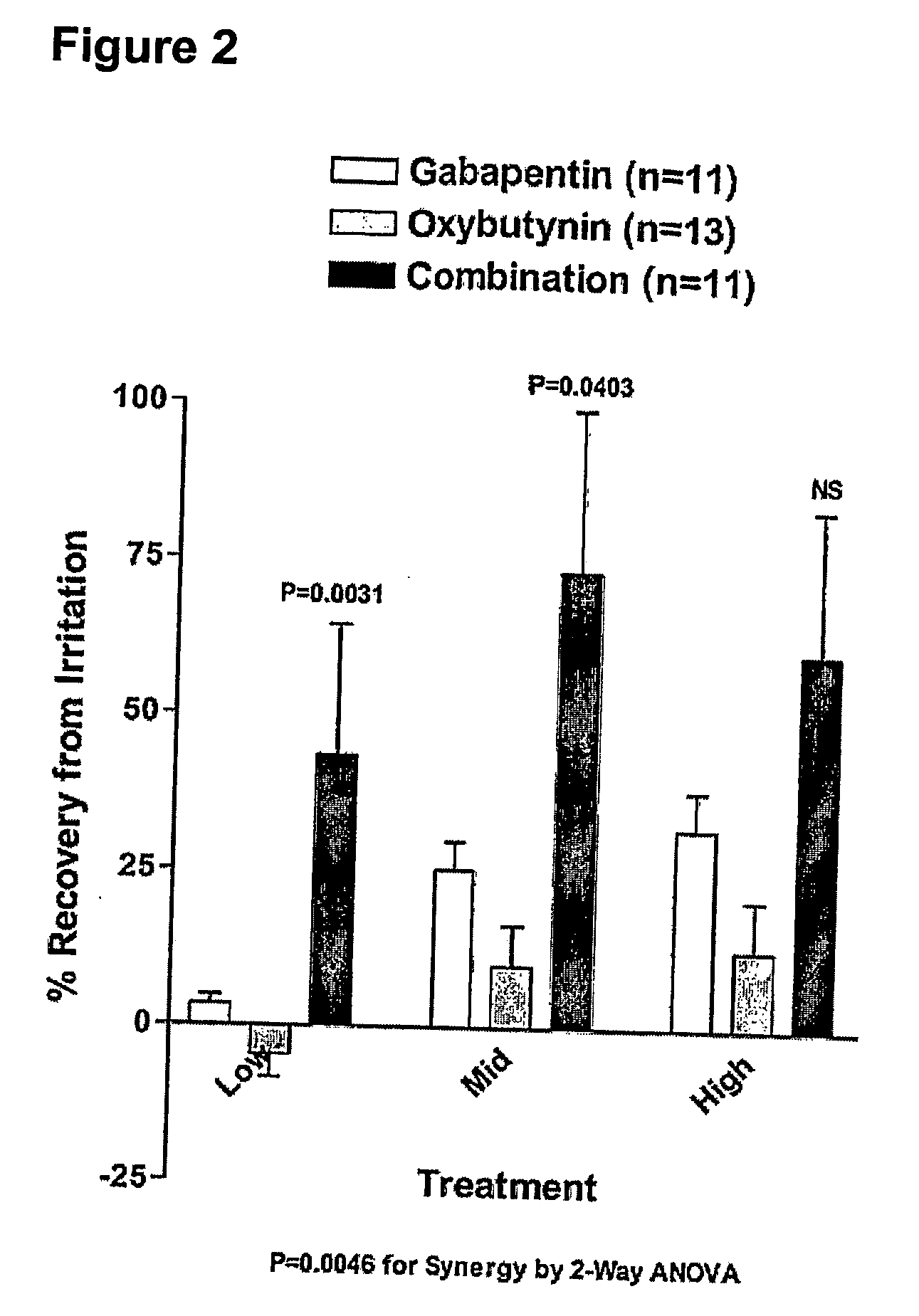
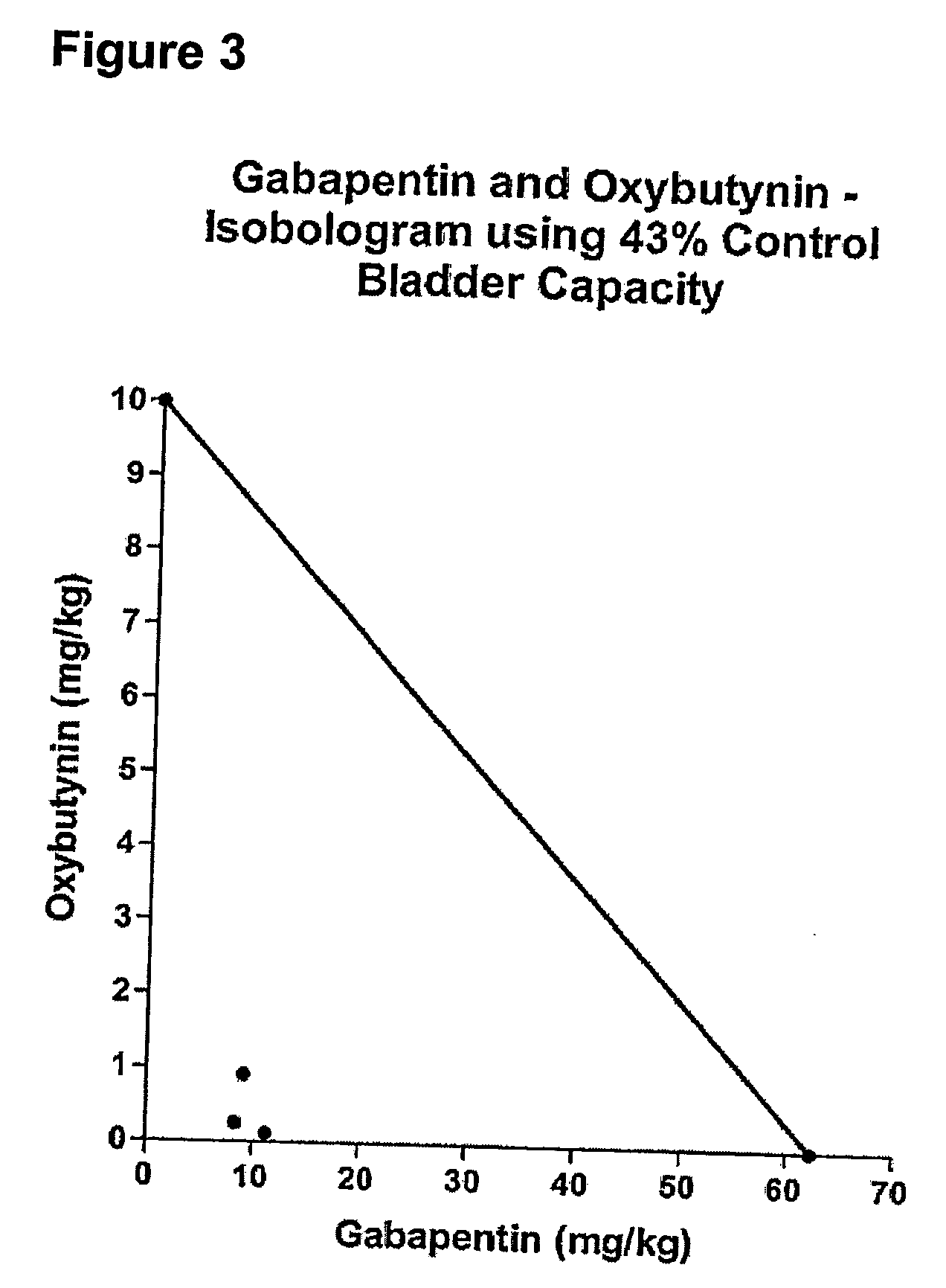
A method is provided for using α2δ subunit calcium channel modulators or other compounds that interact with the α2δ calcium channel subunit in combination with one or more compounds with smooth muscle modulatory effects to treat pain. According to the present invention, α2δ subunit calcium channel modulators include GABA analogs (e.g., gabapentin and pregabalin), fused bicyclic or tricyclic amino acid analogs of gabapentin, and amino acid compounds. Compounds with smooth muscle modulatory effects include antimuscarinics, β3 adrenergic agonists, spasmolytics, neurokinin receptor antagonists, bradykinin receptor antagonists, and nitric oxide donors.
Owner:DYNOGEN PHARM INC
Sodium Channel Blocker Compositions and the Use Thereof
Methods of treating or preventing chronic pain or convulsion are disclosed by administering to an animal a sodium channel blocker and at least one of gabapentin and pregabalin. Also disclosed are pharmaceutical compositions and kits for the treatment or prevention of chronic pain or convulsion.
Owner:PURDUE PHARMA LP
Solid pharmaceutical compositions containing pregabalin
InactiveUS20070269511A1Absorption windowPromote undesirable lactam formationBiocideOrganic active ingredientsCross-linkOral medication
Owner:WARNER-LAMBERT CO
Chiral 3-carbamoylmethyl-5-methyl hexanoic acids, key intermediates for the synthesis of (S)-Pregabalin
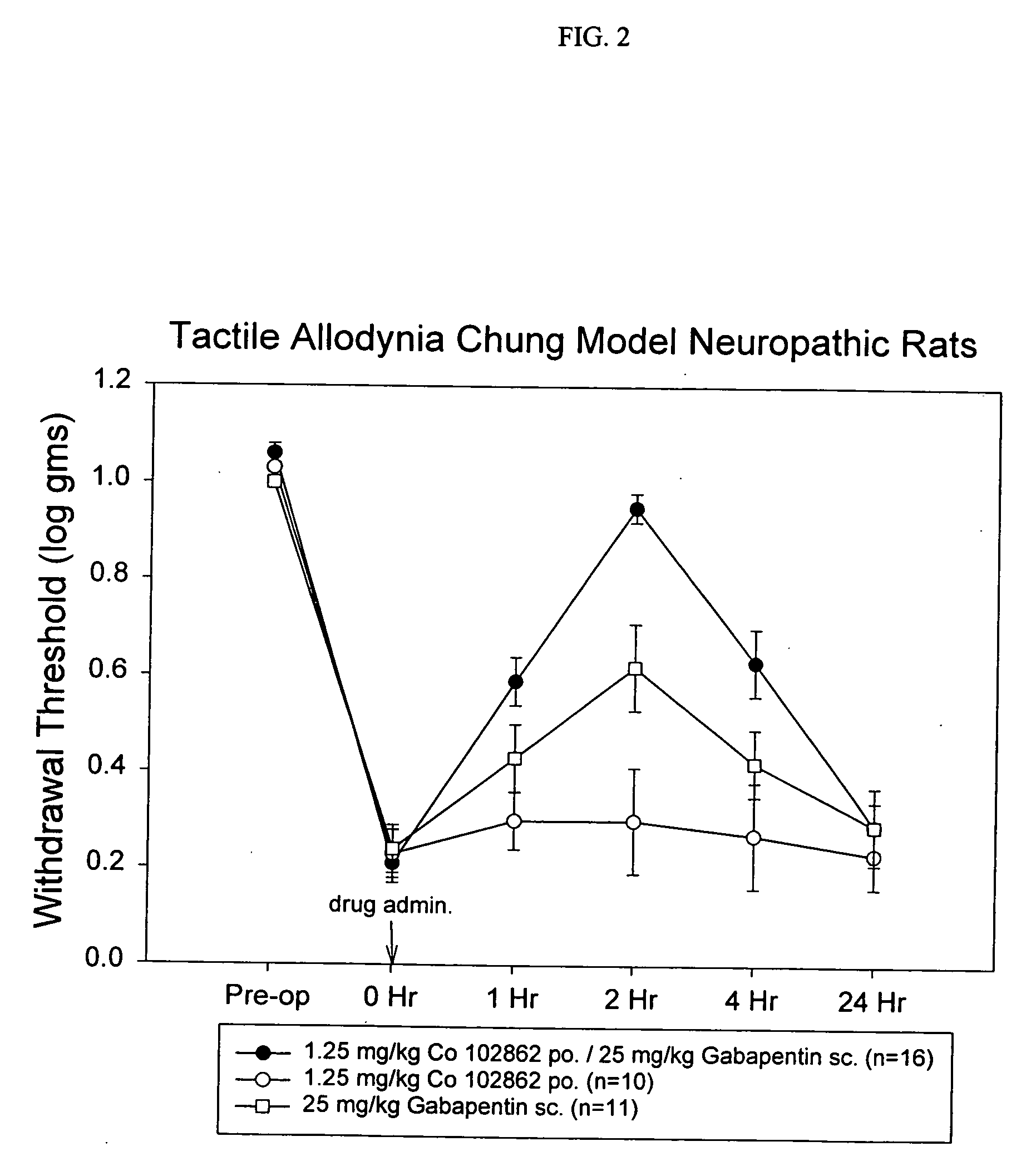
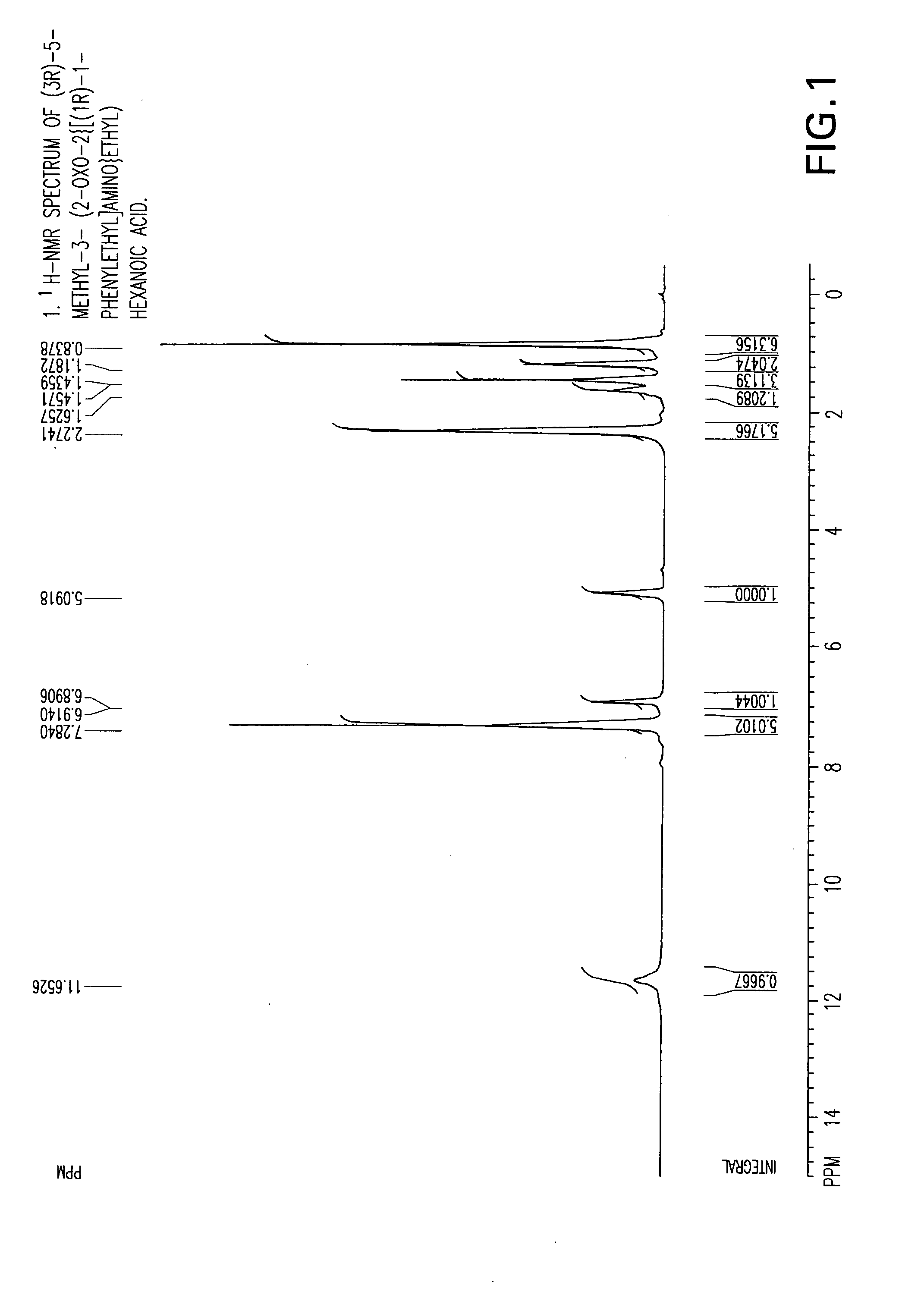
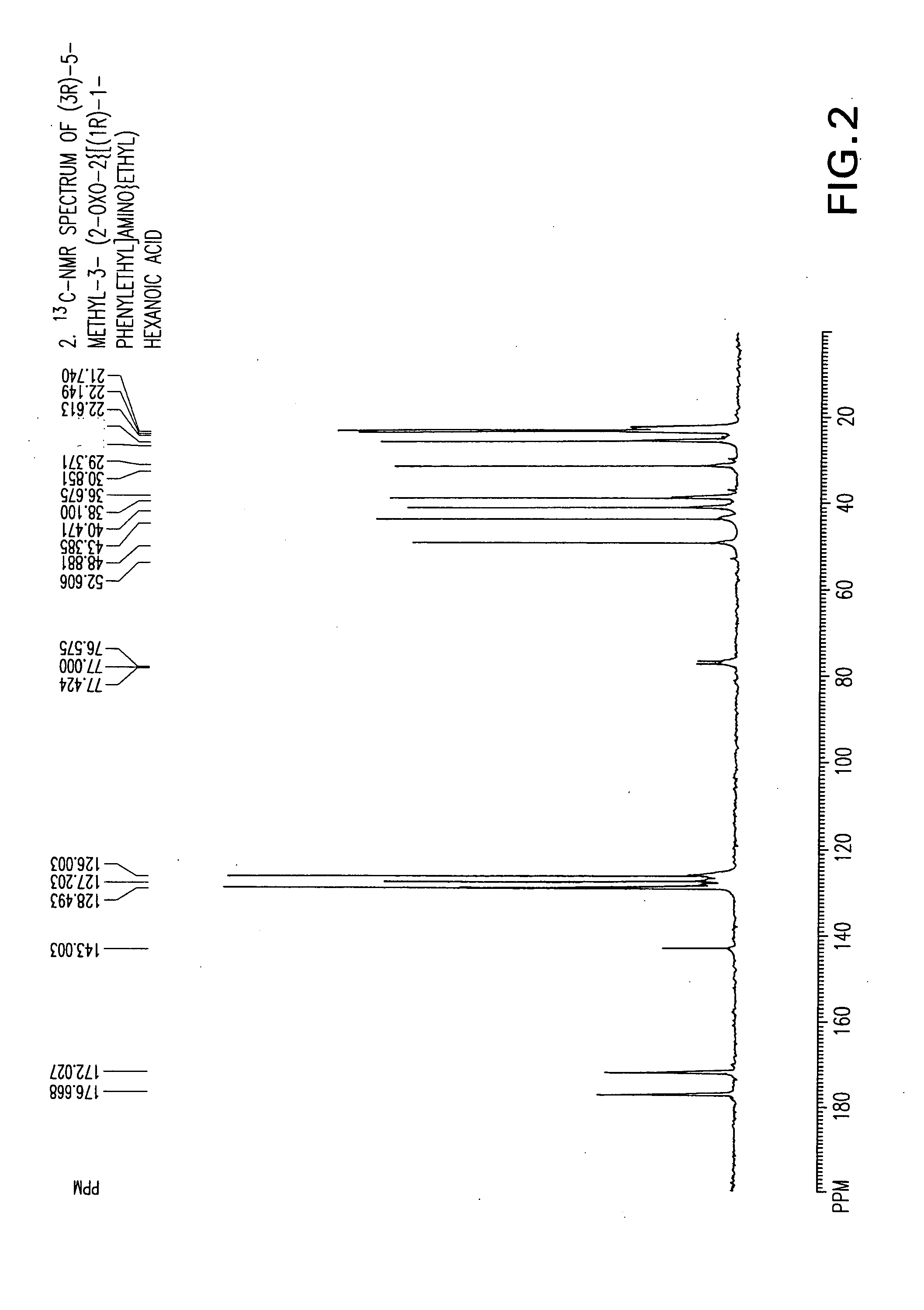
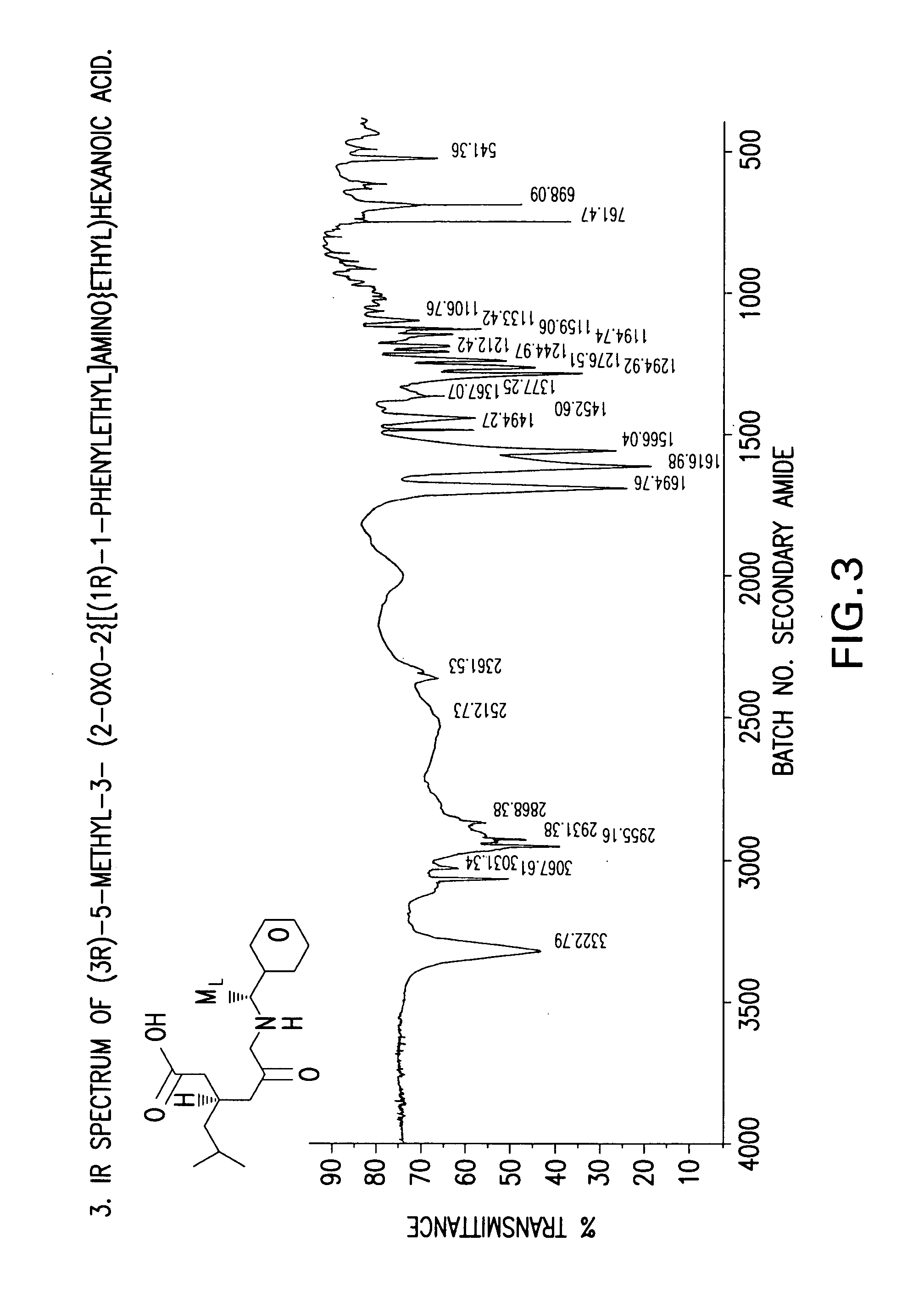
The invention encompasses the synthesis of (S)-(+)-3-(aminomethyl)-5-methylhexanoic acid, (S)-Pregabalin, via the intermediate, (3R)-5-methyl-3-(2-oxo-2{[(1R)-1-phenylethyl]amino}ethyl)hexanoic acid.
Owner:TEVA PHARM USA INC
Methods for treating lower urinary tract disorders using alpha2delta subunit calcium channel modulators with smooth muscle modulators
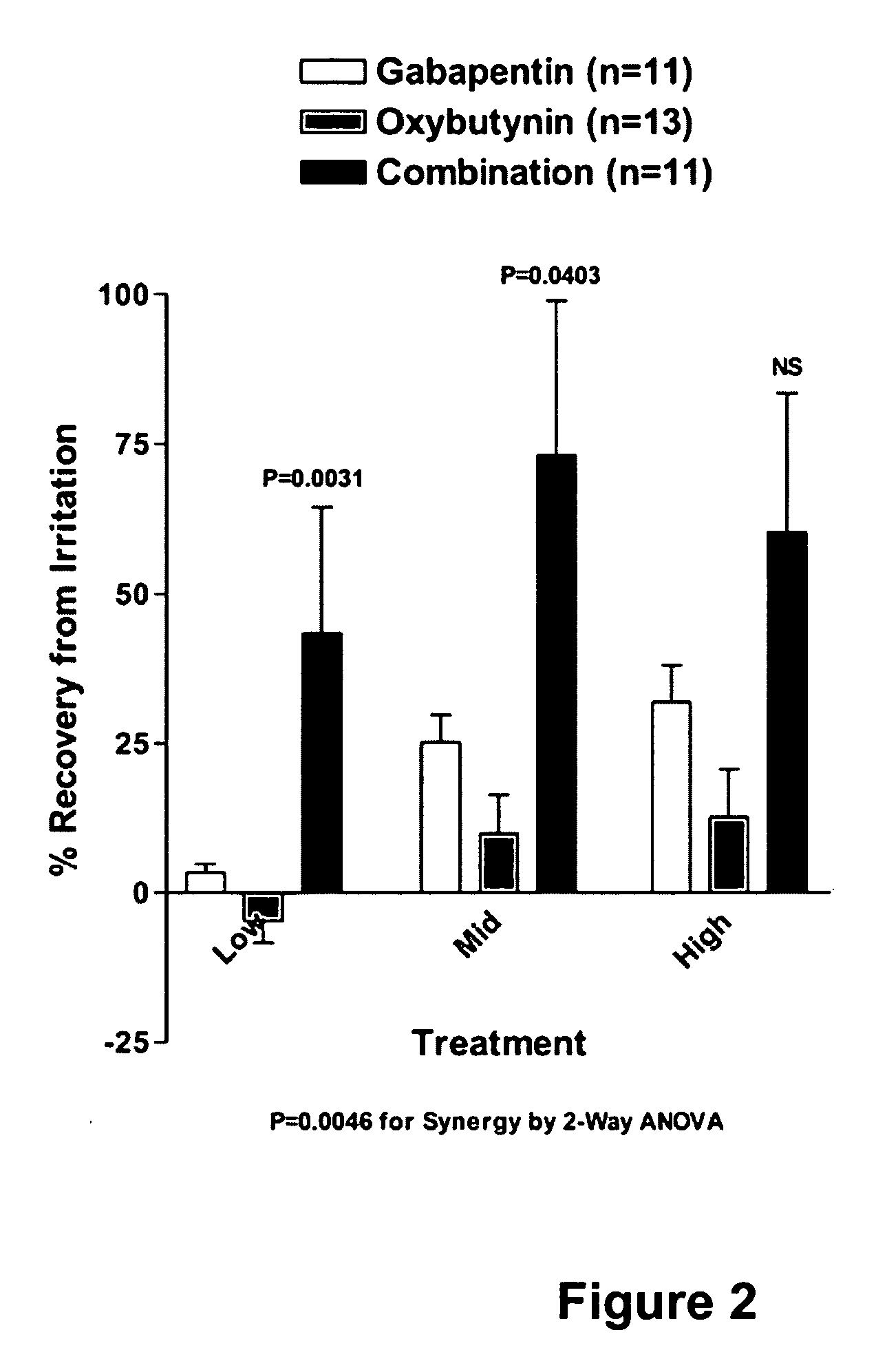
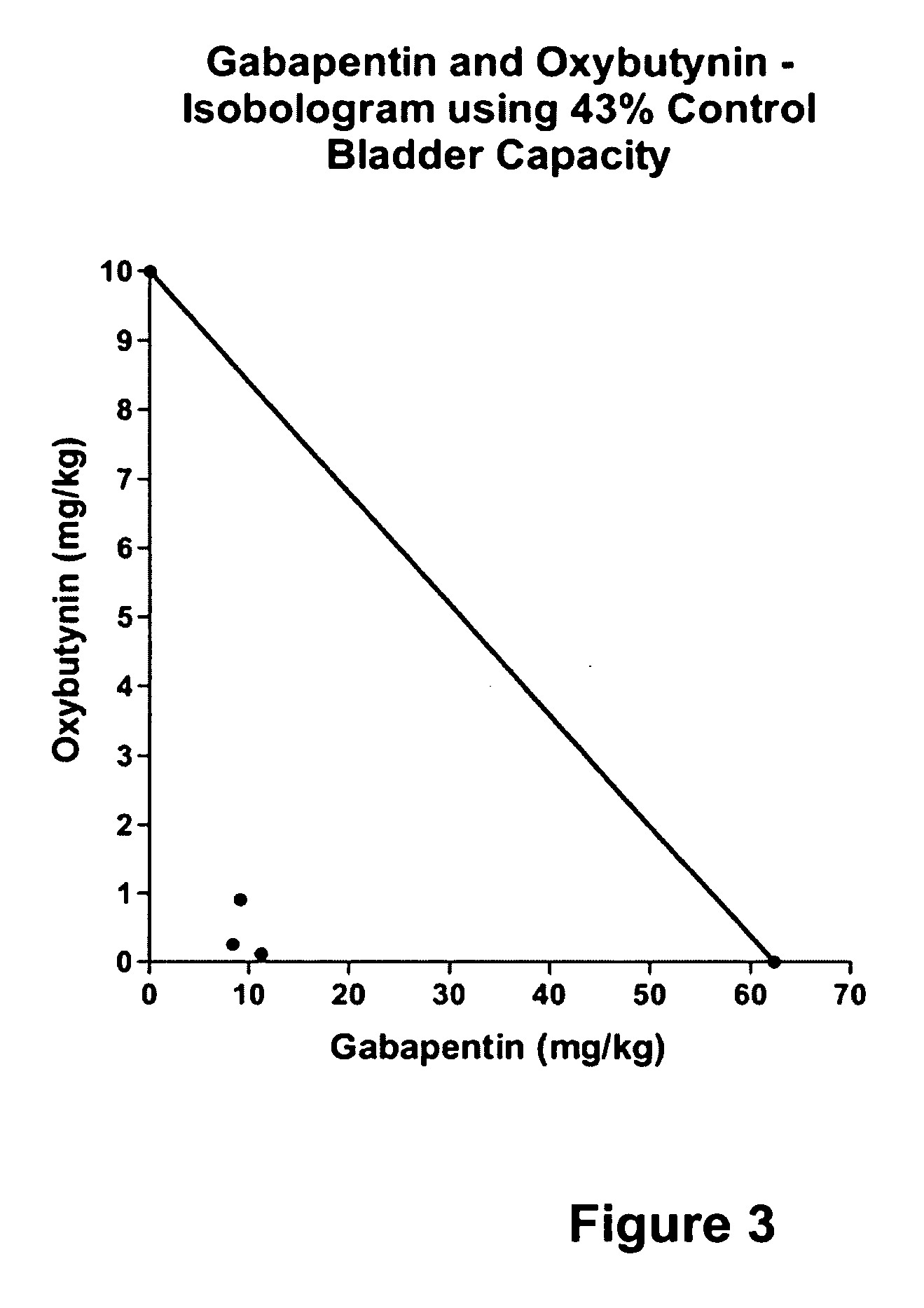
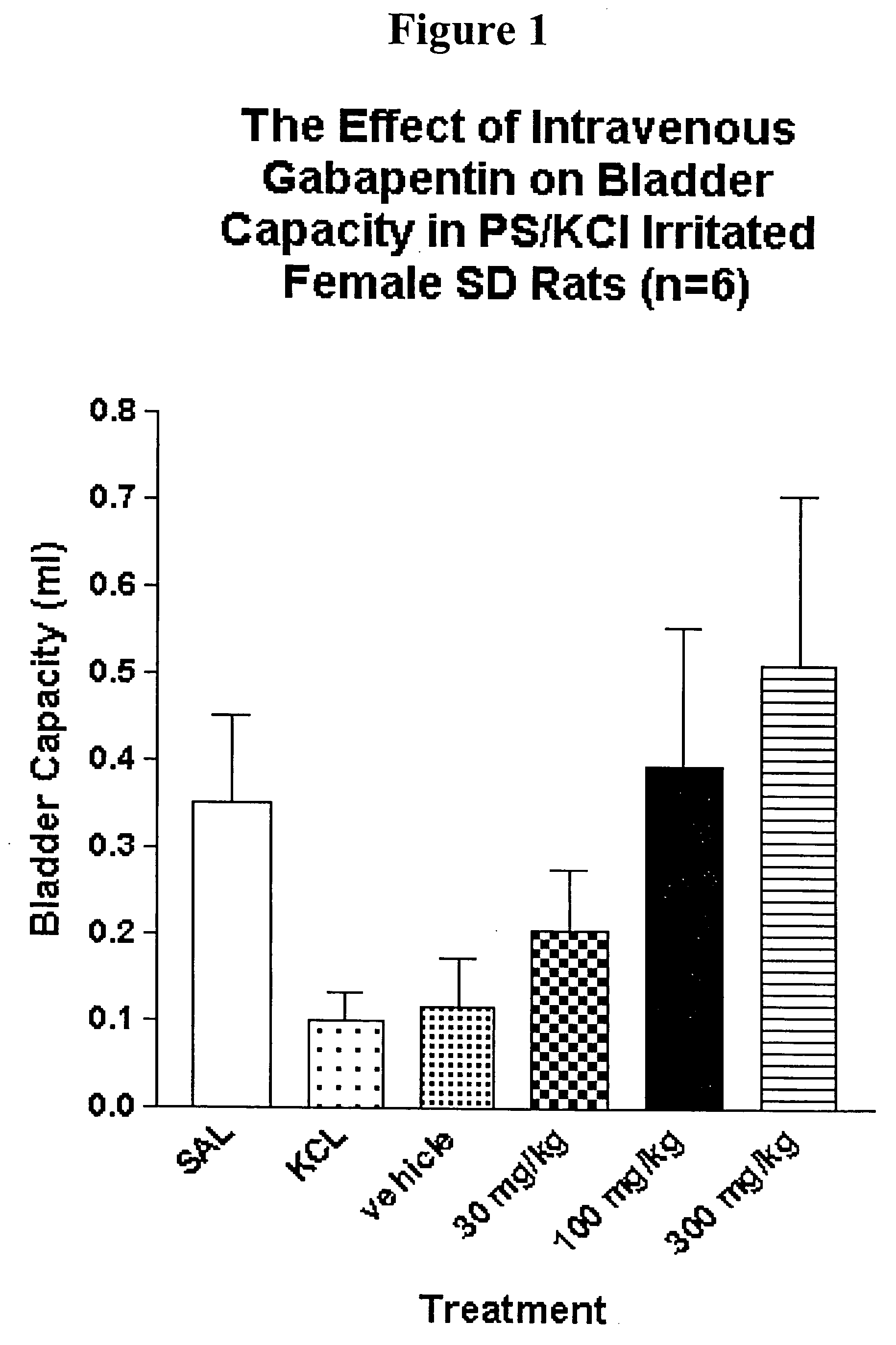
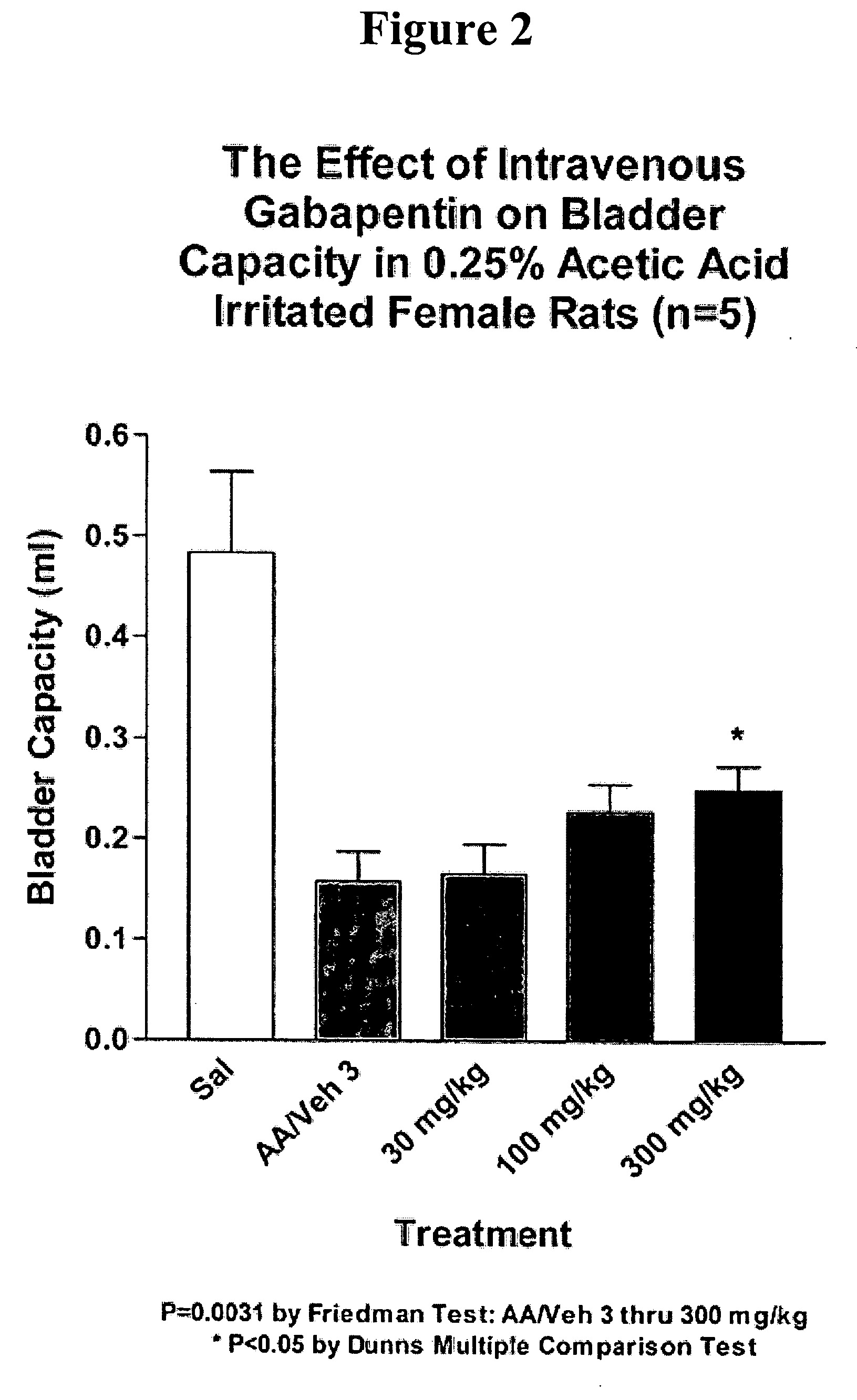
A method is provided for using α2δ subunit calcium channel modulators or other compounds that interact with the α2δ calcium channel subunit in combination with one or more compounds with smooth muscle modulatory effects to treat and / or alleviate the symptoms associated with painful and non-painful lower urinary tract disorders in normal and spinal cord injured patients. According to the present invention, α2δ subunit calcium channel modulators include GABA analogs (e.g. gabapentin and pregabalin), fused bicyclic or tricyclic amino acid analogs of gabapentin, and amino acid compounds. Compounds with smooth muscle modulatory effects include antimuscarinics, β3 adrenergic agonists, spasmolytics, neurokinin receptor antagonists, bradykinin receptor antagonists, and nitric oxide donors.
Owner:EDUSA PHARMA
Novel Pharmaceutical Compositions for Treating Chronic Pain and Pain Associated with Neuropathy
InactiveUS20130189354A1Reduced plasma concentrationEfficient managementOrganic active ingredientsBiocideGabapentinChronic pain
The present invention relates to compositions and methods for treating pain wherein the compositions comprise a combination of tramadol or a pharmaceutically acceptable salt thereof, magnesium or a pharmaceutically acceptable salt thereof; and gabapentin or pregabalin. The therapeutic combination can further contain capsaicin or an ester of capsaicin.
Owner:TRINITY LAB INC
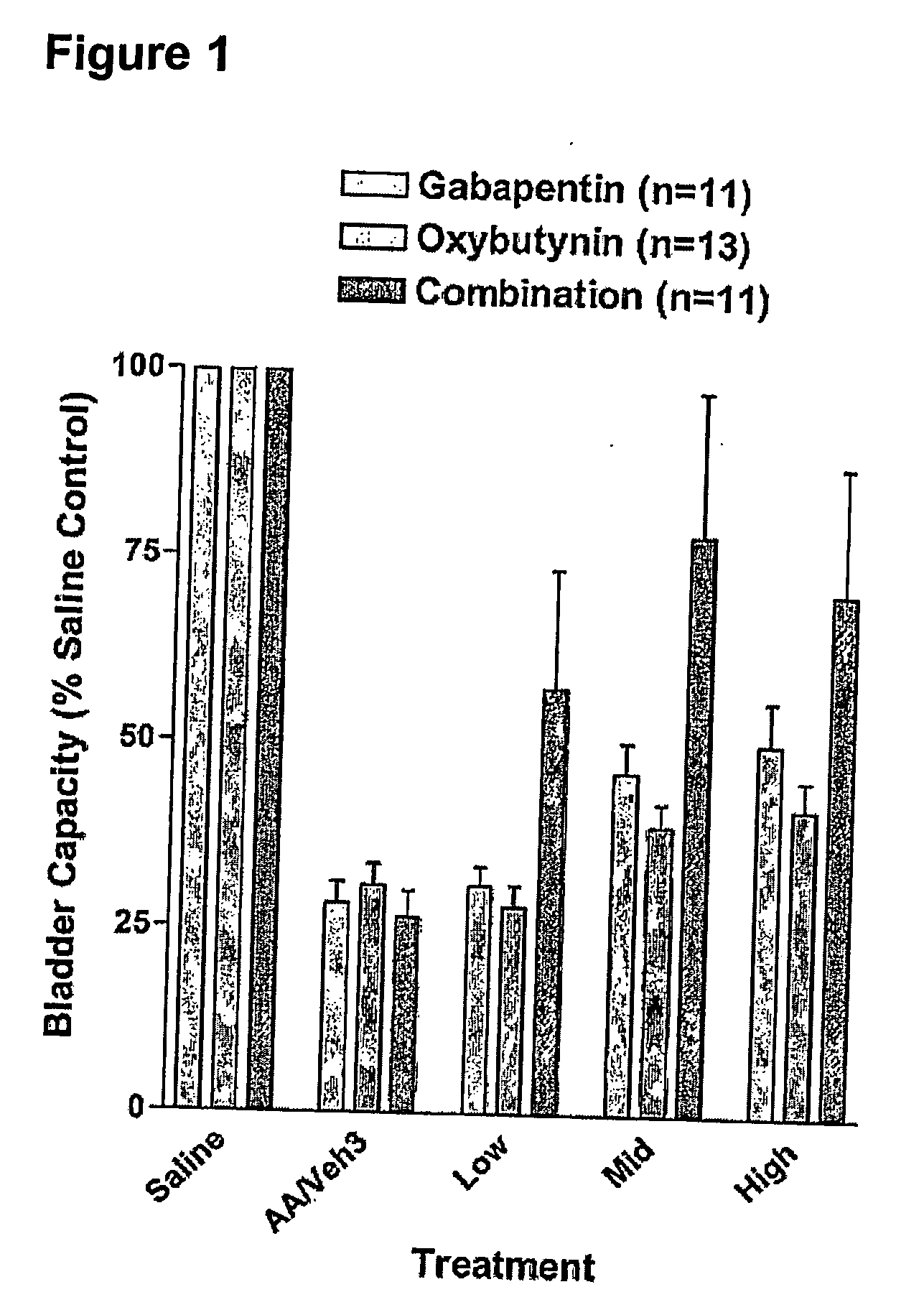
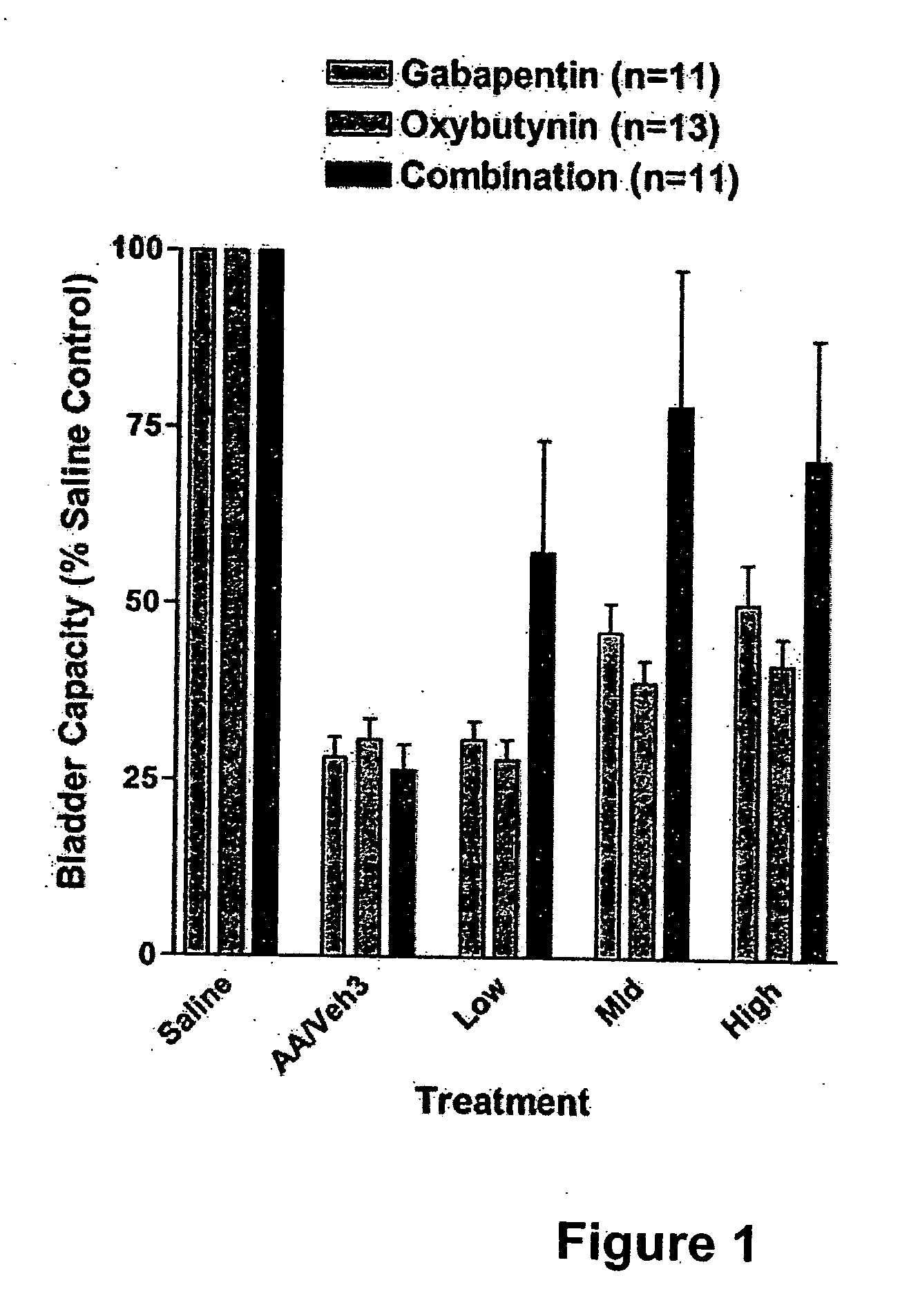
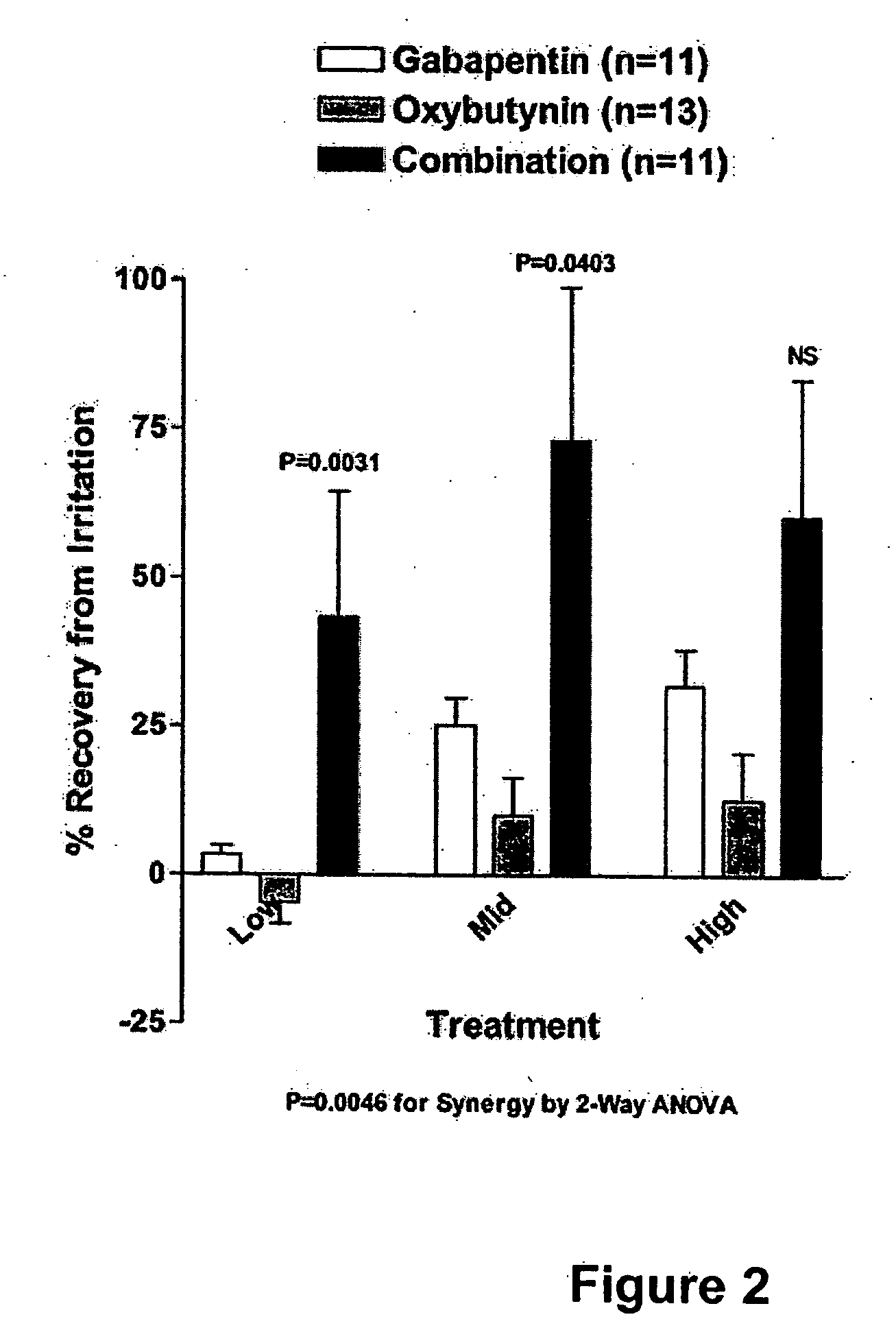
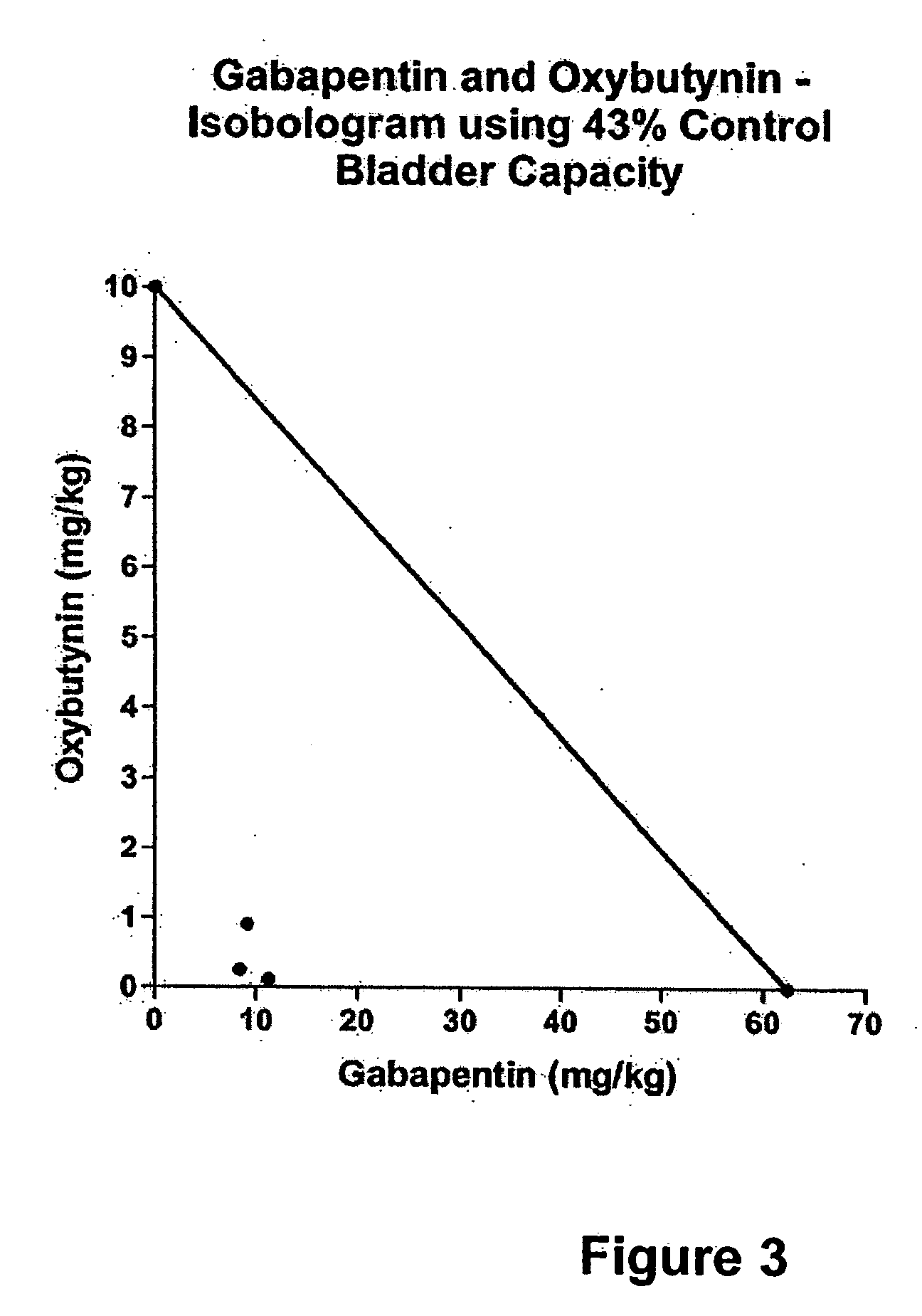
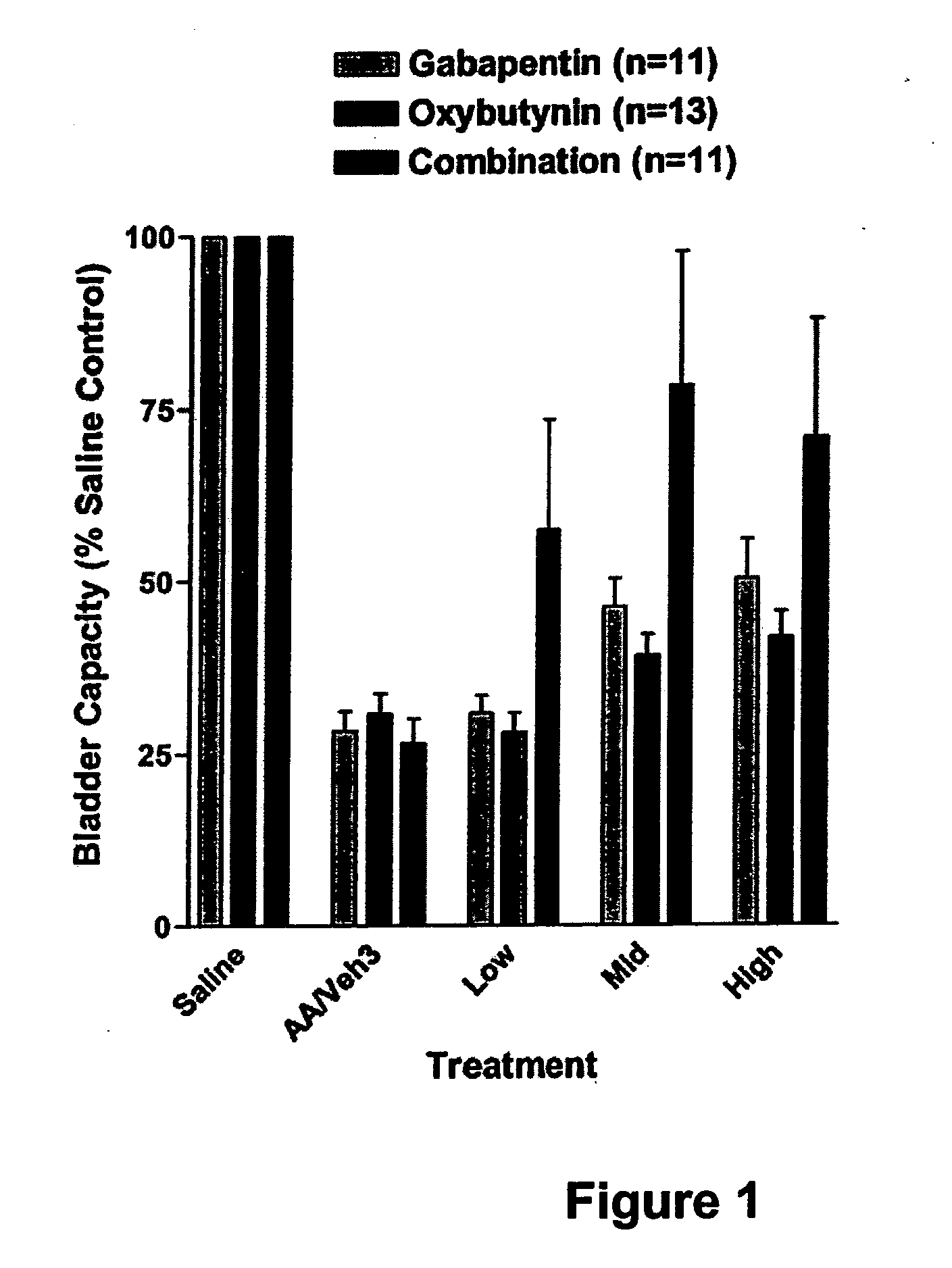
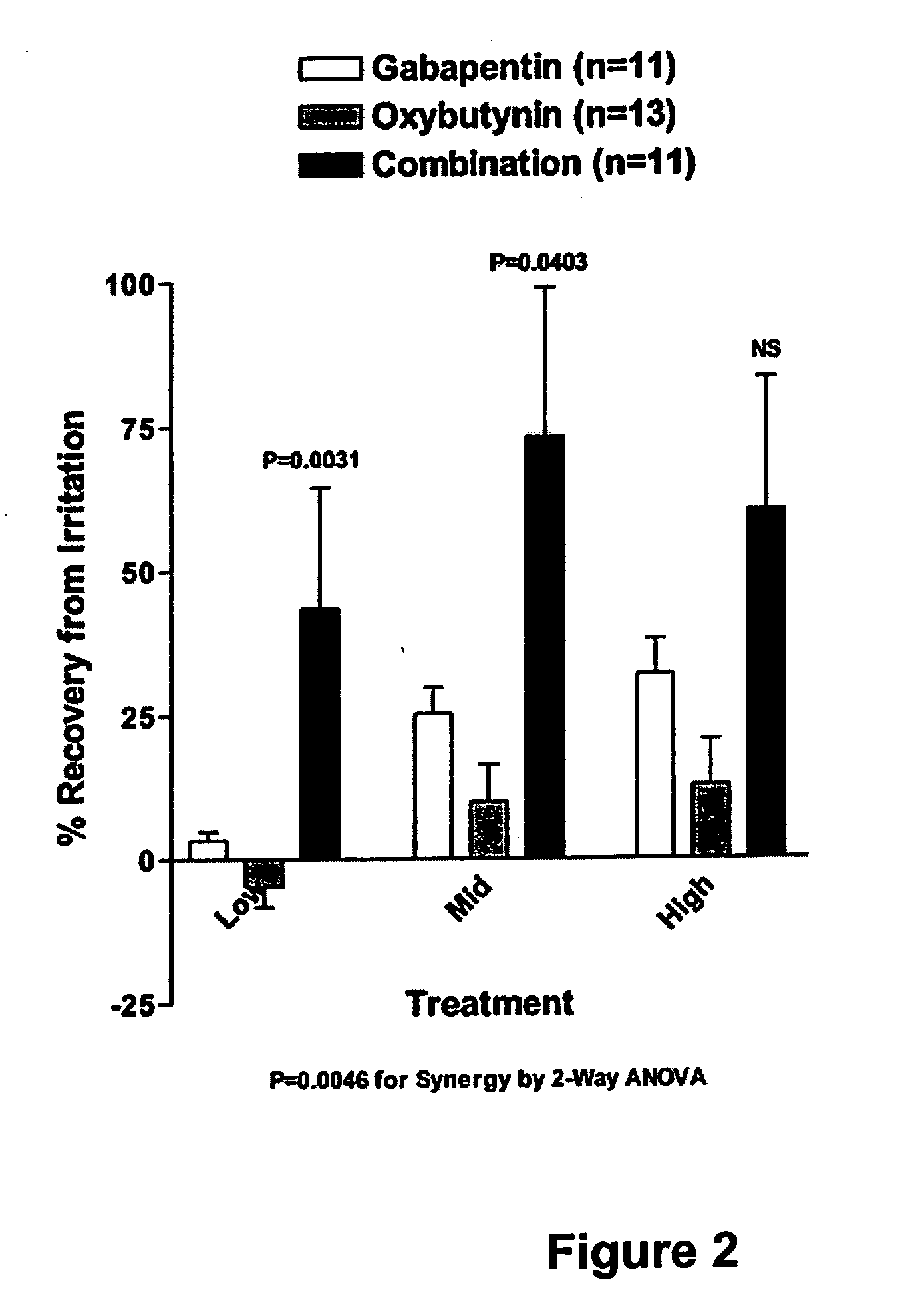
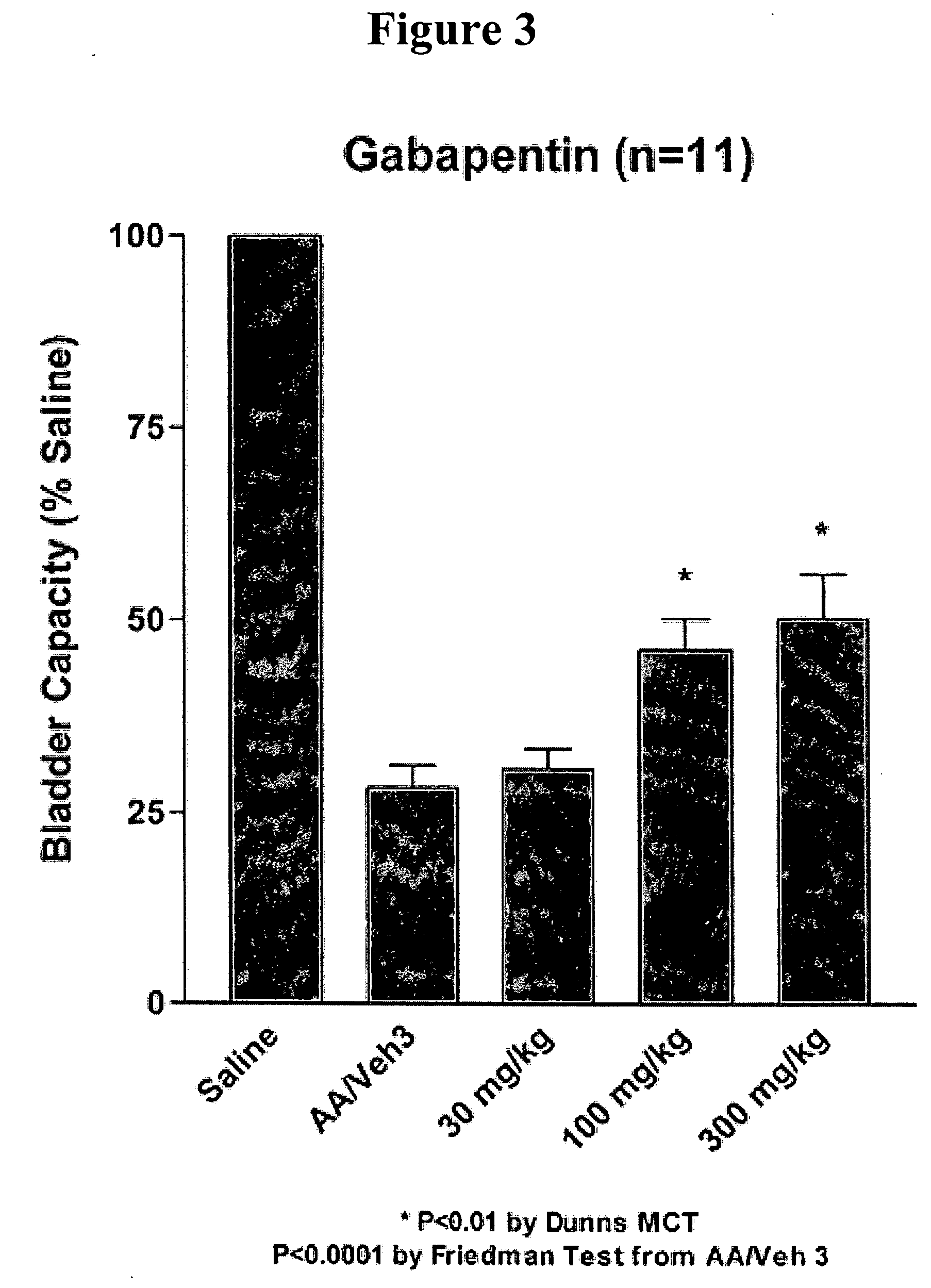
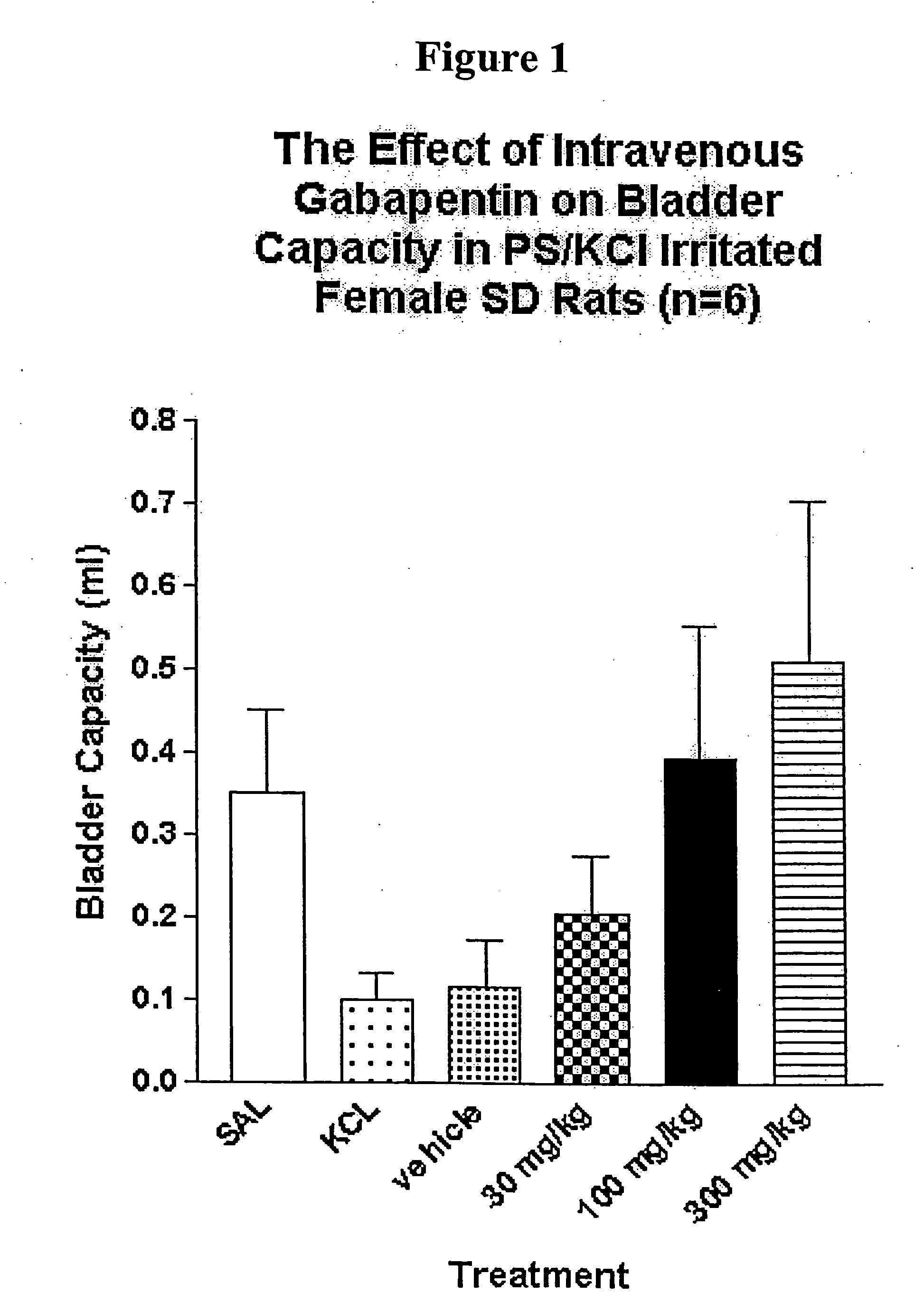
Methods for decreasing detrusor muscle overactivity
InactiveUS20050239890A1Limited efficacyReduce patient complianceBiocideOrganic active ingredientsDiseaseGabapentin
A method is provided for using α2δ subunit calcium channel modulators or other compounds that interact with the α2δ calcium channel subunit in combination with one or more compounds with smooth muscle modulatory effects to treat and / or alleviate the symptoms associated with painful and non-painful lower urinary tract disorders in normal and spinal cord injured patients. According to the present invention, α2δ subunit calcium channel modulators include GABA analogs, e.g., gabapentin and pregabalin, fused bicyclic or tricyclic amino acid analogs of gabapentin, and amino acid compounds. Compounds with smooth muscle modulatory effects include antimuscarinics, β3 adrenergic agonists, spasmolytics, neurokinin receptor antagonists, bradykinin receptor antagonists, and nitric oxide donors.
Owner:DYNOGEN PHARM INC
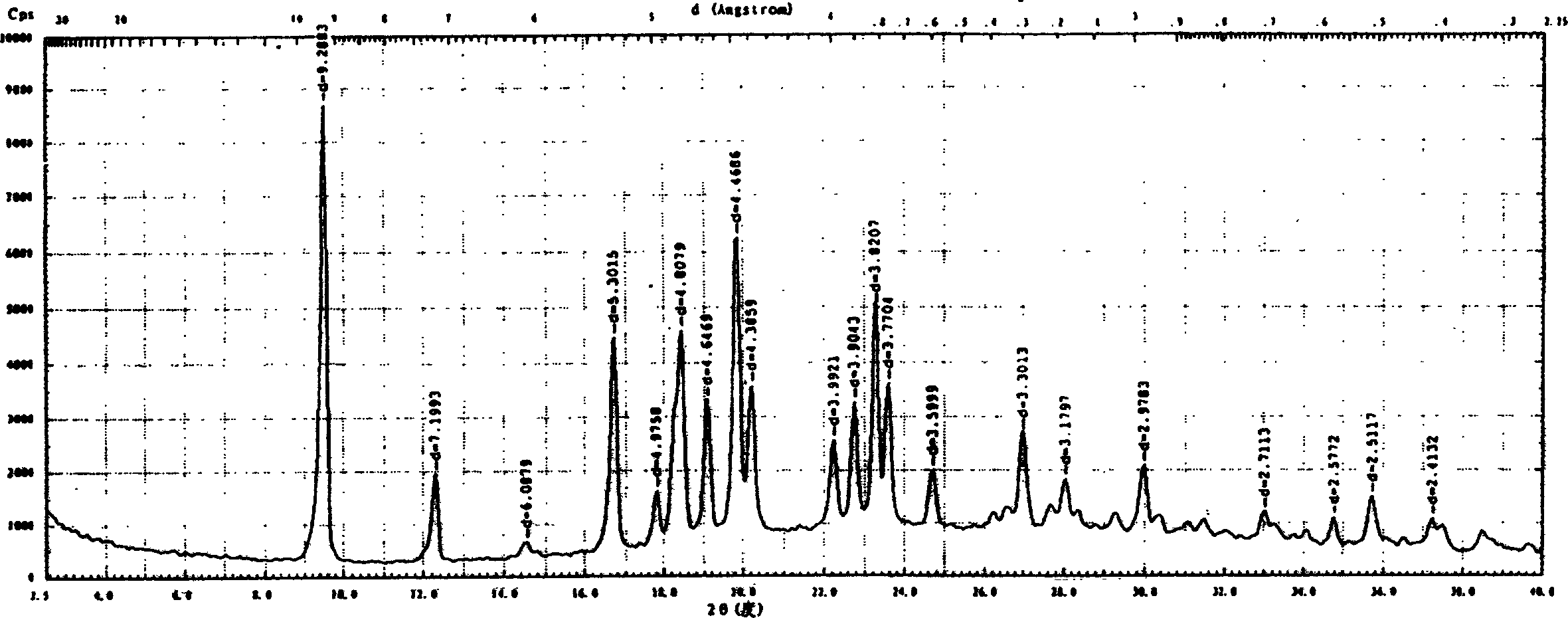
Novel pregabalin crystalline form and its preparing process
InactiveCN1634869AImprove stabilitySuitable for mass productionOrganic active ingredientsNervous disorderPregabalinMedicine
The invention relates to a novel crystalline form of pregabalin drug for treating epilepsy and neuropathic pain and its preparation method. Compared to others pregabalin with agraphitic form and other forms, pregabalin with the novel crystalline form has good stability and is suitable for mass production. Pregabalin with the novel crystalline form uses Cu-Ka radiation and its X-ray powder diffraction has characteristic peak at 9.5(9.3), 12.3(7.2), 16.7(5.3), 18.4(4.8), 19.9(4.5) and 23.3(3.8) with the coordinate of 2-theta and interplanar spacing (d).
Owner:FUKANGREN BIO PHARMA
Sustained release tablet comprising pregabalin through two-phase release-controlling system
ActiveCN103702664AExtended release profileIncrease buoyancyOrganic active ingredientsNervous disorderSustained Release TabletPolyethylene oxide
The invention provides a sustained release tablet having two-phase release-controlling system, which consists of a first release-controlling phase comprising pregabalin or its salt and hydroxypropyl methylcellulose; and a second release-controlling phase comprising polyethylene oxide as a swelling polymer, the first release-controlling phase being homogeneously dispersed in the second release-controlling phase.
Owner:YUHAN
Methods for treating lower urinary tract disorders using alpha2delta subunit calcium channel modulators with smooth muscle modulators
InactiveUS20060247311A1Need lessLimited efficacyBiocidePeptide/protein ingredientsDiseaseGabapentinoid
A method is provided for using α2δ subunit calcium channel modulators or other compounds that interact with the α2δ calcium channel subunit in combination with one or more compounds with smooth muscle modulatory effects to treat and / or alleviate the symptoms associated with painful and non-painful lower urinary tract disorders in normal and spinal cord injured patients. According to the present invention, α2δ subunit calcium channel modulators include GABA analogs (e.g. gabapentin and pregabalin), fused bicyclic or tricyclic amino acid analogs of gabapentin, and amino acid compounds. Compounds with smooth muscle modulatory effects include antimuscarinics, β3 adrenergic agonists, spasmolytics, neurokinin receptor antagonists, bradykinin receptor antagonists, and nitric oxide donors.
Owner:DYNOGEN PHARM INC
Methods for decreasing detrusor
A method is provided for treatment of non-painful bladder disorders, particularly non-painful overactive bladder without loss of urine. The method comprises administration of an α2δ subunit calcium channel modulator, including gabapentin, pregabalin, GABA analogs, fused bicyclic or tricyclic amino acid analogs of gabapentin, amino acid compounds, and other compounds that interact with the α2δ calcium channel subunit.
Owner:DYNOGEN PHARM INC
Synthetic method of pregabalin
InactiveCN104496832AImprove conversion rateShort reaction timeOrganic compound preparationAmino-carboxyl compound preparationPregabalinHydrolysis
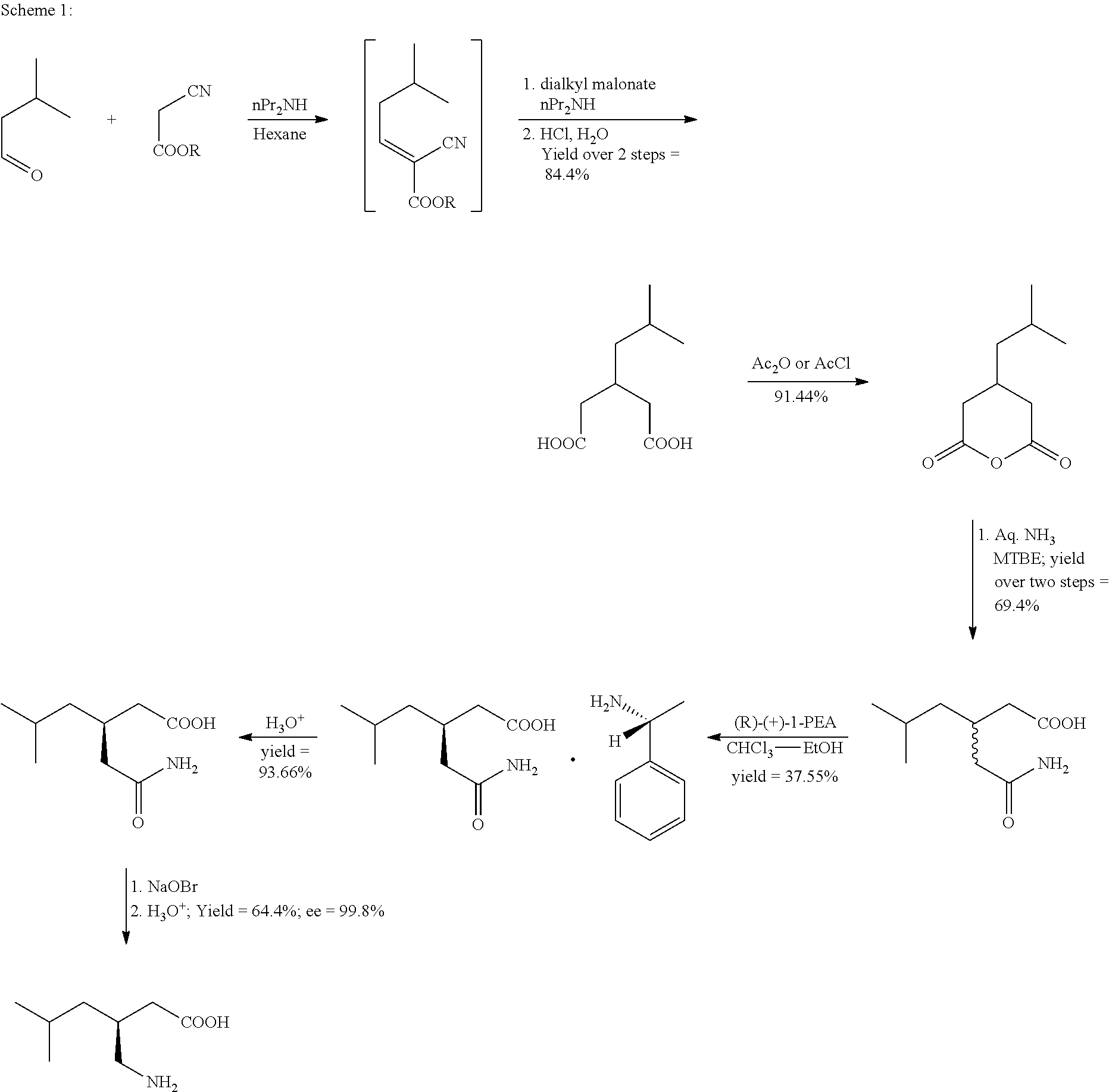
The invention discloses a synthetic method of pregabalin. According to the synthetic method, isovaleraldehyde and cyanoacetic alkyl ester are used as starting materials; condensation reaction, Michael addition reaction, hydrolysis reaction, amidation reaction and the like are carried out successively to obtain 3-(carbamoyl methyl)-5-methyl-hexanoic acid; and resolution and Hofmann elimination are carried out to obtain pregabalin. The greatest improvement of the synthetic method is to carry out the hydrolysis reaction and the amidation reaction under the condition of near-critical water. Thus, addition of a catalyst is avoided, and yield of the reaction is raised. In addition, a flow reactor can be applied to the reaction so as to obtain a better reaction effect.
Owner:ZHEJIANG MENOVO PHARMA
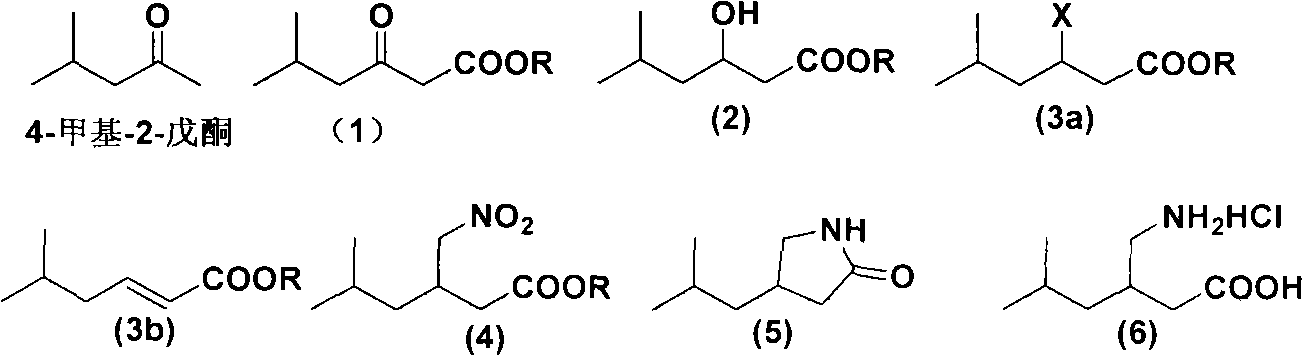
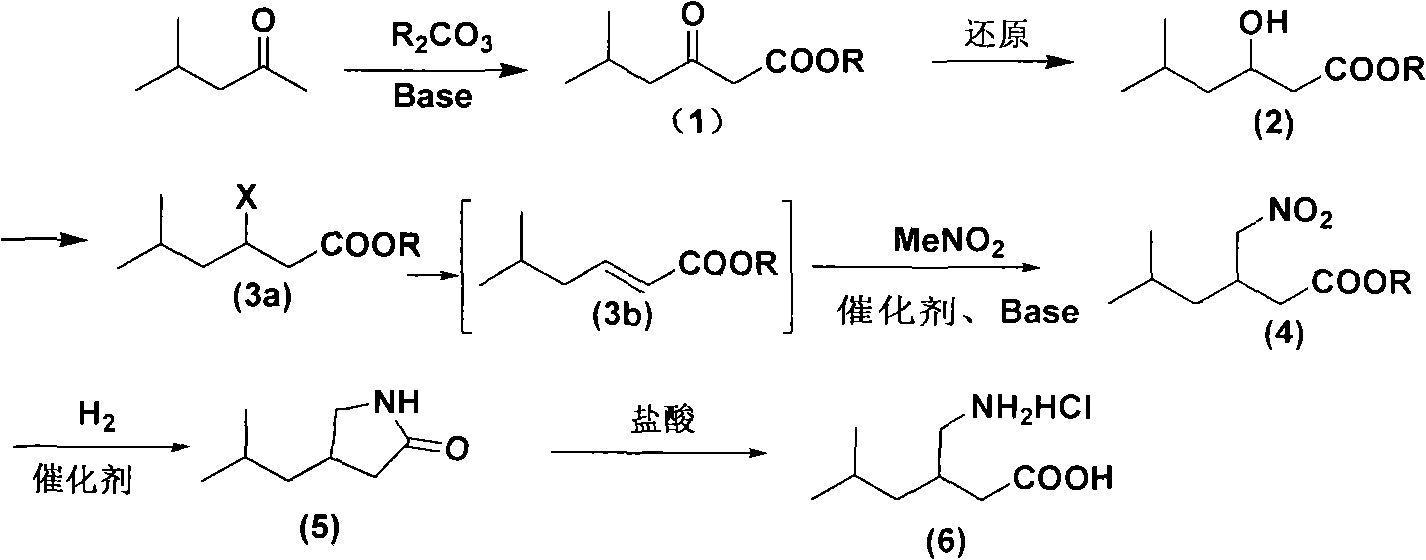
Novel method for preparing pregabalin raceme hydrochloride
ActiveCN102115449AOrganic compound preparationAmino-carboxyl compound preparationPregabalinMethyl group
The invention discloses a route and method for preparing 3-aminomethyl-5-methylhexanoic acid, namely pregabalin raceme hydrochloride. The method comprises the following steps of: performing condensation on 4-methyl-2-pentanone to obtain a compound in a formula (1), and performing reduction to obtain a compound in a formula (2); performing halogenation or esterification to obtain a compound in a formula (3a), and performing substitution to obtain a compound in a formula (4), or performing elimination on the compound in the formula (3a) to obtain a compound in a formula (3b) and performing addition to obtain a compound in the formula (4); and performing reduction and acidolysis on the compound in the formula (4) to obtain a pregabalin hydrochloride compound in a formula (6). The method is easy to operate, mild in reaction conditions, and low in cost, and is suitable for large-scale industrial production.
Owner:ZHEJIANG HUAHAI PHARMA CO LTD +1
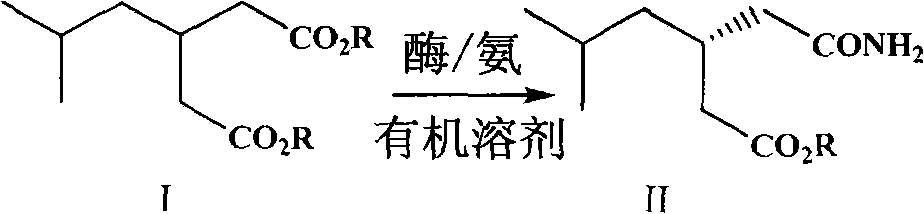
Preparation of pregabalin chiral intermediate with bio-enzyme method
The invention relates to the technical field of preparation of a pregabalin chiral intermediate with a bio-enzyme method, in particular to the preparation of the pregabalin chiral intermediate with the bio-enzyme method. According to a preparation method, a compound of isobutyl glutarate shown as a formula (I) is used as a raw material and used for generating a compound of (S)-3-(carbamoyl methyl)-5-methylcaproate shown as a formula (II) in an organic solvent under the action of bio-enzyme and ammonia, wherein R is preferentially direct-chain or side-chain alkyl of C1-C4.
Owner:瑞博(杭州)医药科技有限公司
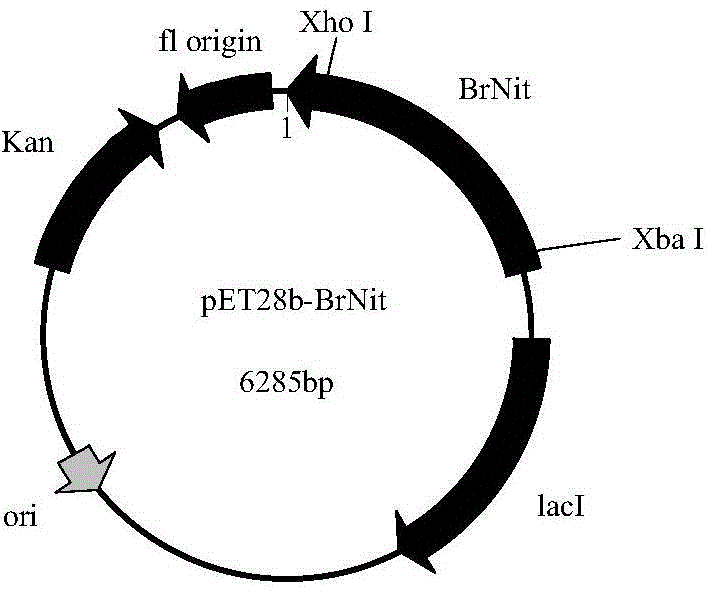
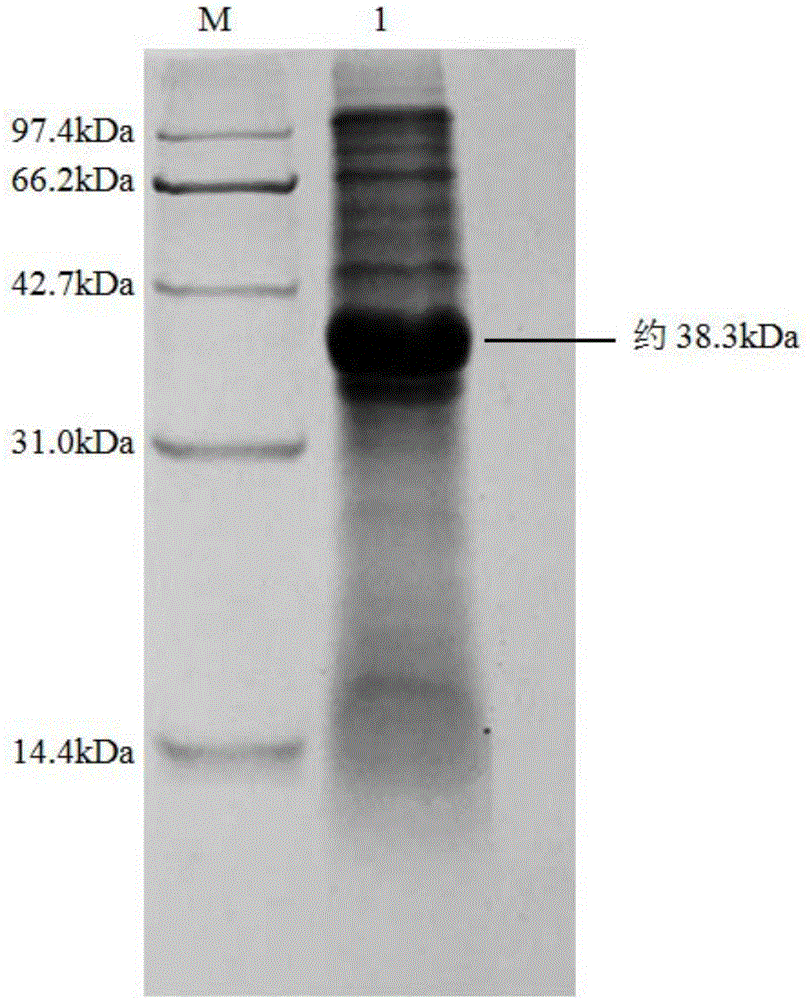
Nitrilase, encoding genes, carrier and application
InactiveCN104962540AAtom economy is highHigh potential for industrial applicationHydrolasesFermentationBrassica rapaBirdsrape Mustard
The invention discloses nitrilase from brassica rapa, encoding genes and an application of the encoding genes to the preparation of pregabalin chiral intermediate (S)-3-cyan-5-methylhexanoic acid through biocatalysis, and an amino acid sequence of the nitrilase is shown as SEQ ID NO:1. The invention provides novel nitrilase for preparing the (S)-3-cyan-5-methylhexanoic acid with high region and high stereoselectivity through hydrolysis, the concentration of hydrolysis substrates of the nitrilase can reach more than 1 M, and an ee (enantiomeric excess) value is kept more than 99 percent. The pregabalin important chiral intermediate (S)-3-cyan-5-methylhexanoic acid can be prepared through the nitrilase; a preparation method of the (S)-3-cyan-5-methylhexanoic acid has the advantages of high atom economy, mild conditions, environmental friendliness and the like and has high industrial application potential.
Owner:ZHEJIANG UNIV OF TECH
Pregabalin controlled release preparation, and preparation method thereof
InactiveCN103585098AExtended stayProlong the action timeOrganic active ingredientsNervous disorderSodium bicarbonateCellulose
The invention relates to a pregabalin controlled release preparation, and a preparation method thereof. The pregabalin controlled release preparation comprises following ingredients, by weight, 30 to 75% of pregabalin, 5 to 40% of a filling material, 5 to 30% of a sustained-release material, 1 to 10% of an adhesive, and the balance an auxiliary material. The auxiliary material is one or more selected from magnesium stearate, talc, superfine silica powder, cetanol, octadecanol, liquid paraffin, stearic acid, sodium bicarbonate, magnesium carbonate and calcium carbonate; the sustained-release material is one or a mixture of more selected from ethyl cellulose, carbomer, polycarbophil, alginate, hydroxypropyl methyl cellulose, polyacrylic resin, and carnauba wax. The pregabalin controlled release preparation is capable of prolonging residence time of medicine in stomach, releasing long-lasting release and absorption of medicine, and further increasing bioavailability of medicine.
Owner:HANGZHOU SHARPLY PHARM R&D INSTIT +2
Pharmaceutical composition containing gabapentin or pregabalin and N-type calcium channel antagonist
The present invention relates to a pharmaceutical composition useful for preventing / treating pain, which comprises combination of gabapentin or pregabalin, or pharmaceutically acceptable salts thereof and N-type calcium channel antagonists or pharmaceutically acceptable salts thereof such as a compound having the following structure.
Owner:AJINOMOTO CO INC
Diagnosis and treatment of kawasaki disease
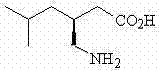
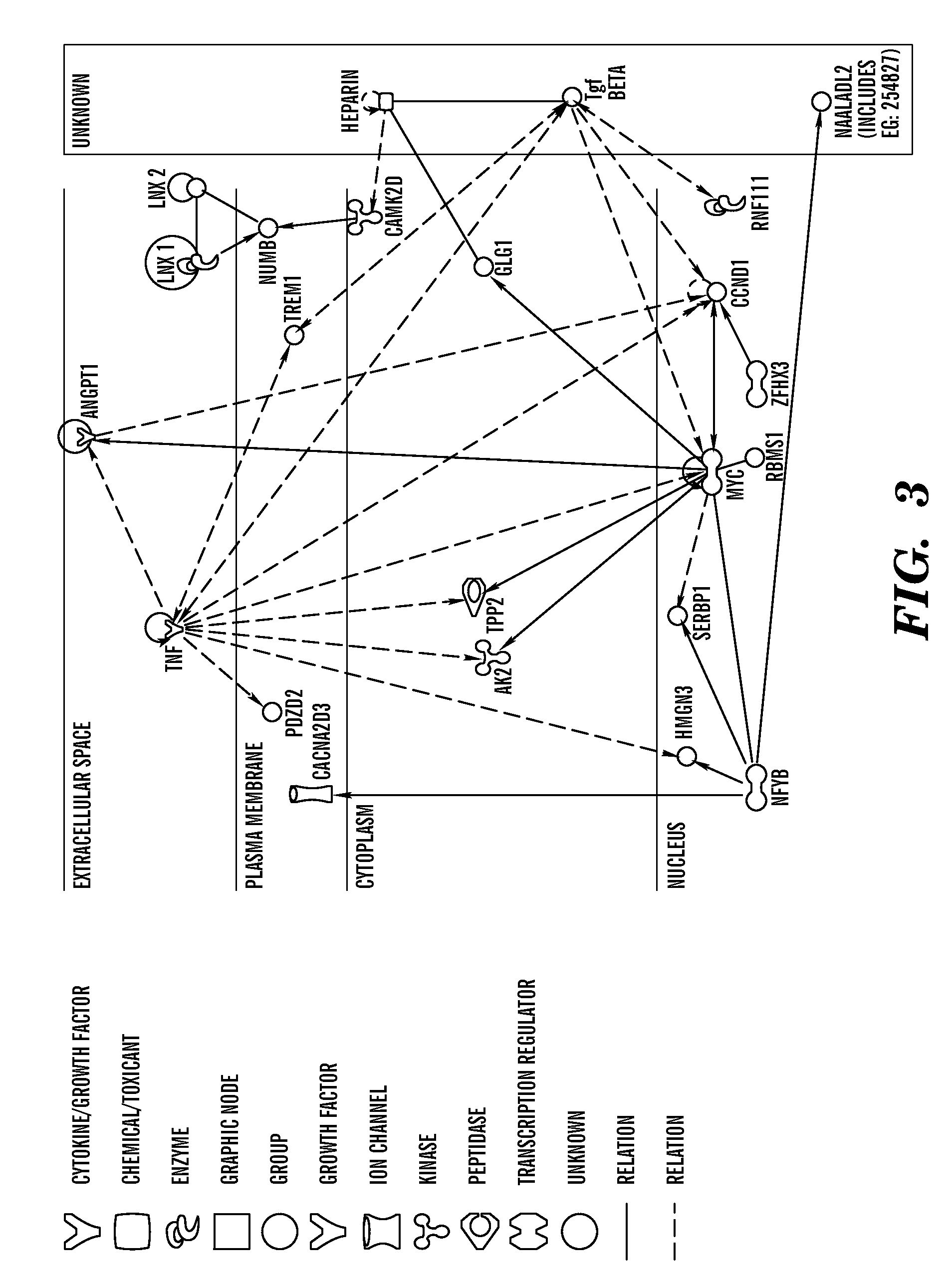
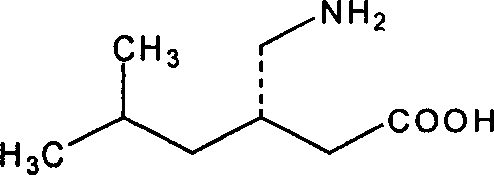
A molecule for use in a method of treatment or diagnosis of Kawasaki Disease in an individual, the molecule comprising: (a) CACNA2D3; (b) CAMK2D; (c) KCNIP4; (d) ANGPT1; (e) NAALADL2; (f) ZFHX3; (g) MPHOSPH10; (h) a sequence having at least 90% sequence identity to any of (a) to (g); or (i) a modulator of any of (a) to (e). We also describe the use of Pregabalin ((3S)-3-(aminomethyl)-5-methyl-hexanoic acid) in the treatment or prevention of Kawasaki Disease in an individual.
Owner:UNIV OF WESTERN AUSTRALIA +2
Medicine composition containing active ingredients of pregabalin
ActiveCN104840443APromote absorptionReduce the number of dosesOrganic active ingredientsNervous disorderTherapeutic effectCarvacryl acetate
The invention belongs to the field of medicinal preparations, and particularly relates to a pregabalin slow release medicine composition. The pregabalin slow release medicine composition comprises active ingredients and excipients, wherein the active ingredients are pregabalin or pharmaceutically acceptable salts of the pregabalin; the excipients comprise a matrix forming agent and a swelling agent; the matrix forming agent is a mixture of polyvinyl acetate and polyvinylpyrrolidone; the swelling agent is selected from one or any combination from croscarmellose sodium, sodium carboxymethyl starch, low-substituted hydroxypropyl cellulose and polyoxyethylene. The medicine composition is suitable for oral administration once in each day; the number of medication times is reduced; the blood concentration peak-to-valley ratio is reduced; meanwhile, the proper swelling agent is selected, so that the gastric retention can be reached, and the problem of stability caused by peroxides can also be avoided; the product stability is improved; the therapy effect can be achieved through once medication in each day; the effect of the medicine composition is equivalent to that of quick release preparations. The medicine composition is manly used for treating epilepsy, neuropathic pain and the like.
Owner:QILU PHARMA HAINAN
Process for separating and determining pregabalin/Lyrica chiral isomer
InactiveCN1786703AHigh analytical sensitivityOvercoming weak UV absorptionComponent separationOther chemical processesReaction rateChromatography column
The invention belongs to analytical chemistry region, and relates to the method of measuring chiral isomer of by high efficiency liquid chromatography after pregabalin deriving. The method of precolumn derivation supplied by the invention has advantages of geniality reaction conditions, steady derivation offspring, fast reaction rate, little subsidiary reaction, etc. It could realize separation and measuration for pregabalin and chiral isomer containing pregabalin preparation.
Owner:CHONGQING PHARMA RES INST +1
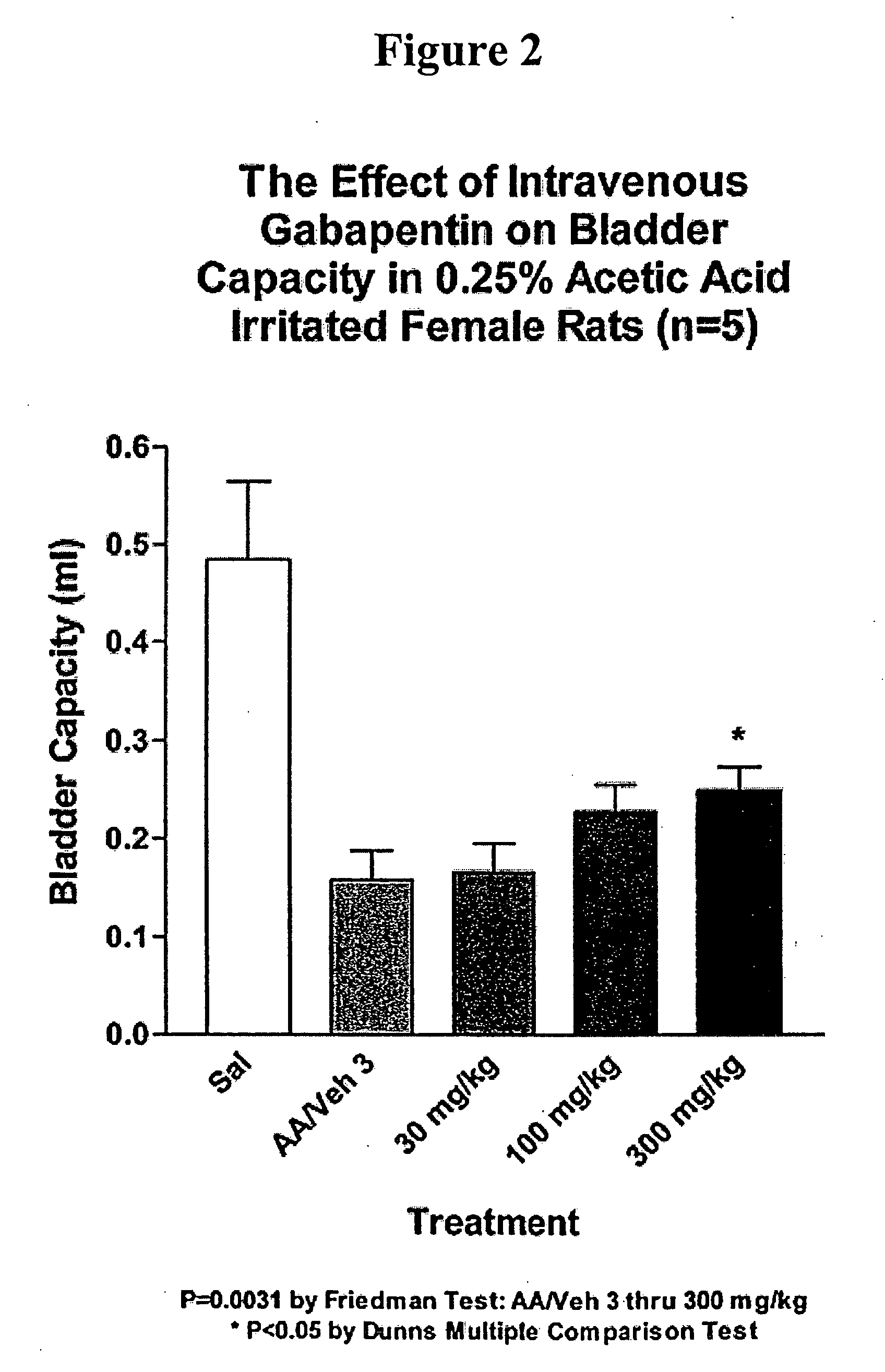
Methods of treating non-painful bladder disorders using alpha2delta subunit calcium channel modulators
A method is provided for treatment of non-painful bladder disorders, particularly non-painful overactive bladder without loss of urine. The method comprises administration of an α2δ subunit calcium channel modulator, including gabapentin, pregabalin, GABA analogs, fused bicyclic or tricyclic amino acid analogs of gabapentin, amino acid compounds, and other compounds that interact with the α2δ calcium channel subunit.
Owner:DYNOGEN PHARM INC
Pharmaceutical composition making pregabalin as active ingredient, its preparation method and uses
The invention relates to a medicinal composition with pregabalin as the active constituent, its preparing process and use, which comprises pregabalin as the medicinal active constituent, as well as pharmaceutically acceptable auxiliary materials, The composition can be used for treating peripheral nerve pain and epilepsy, and can be prepared into the forms of tablets, capsules, dispersible tablets, granules, soft capsules, chewable tablets, buccal tablets, buccal tablets or other oral preparations.
Owner:FUKANGREN BIO PHARMA
Preparation method of pregabalin
InactiveCN104140375AHigh purityEasy to operateOrganic compound preparationAmino-carboxyl compound preparationPregabalinDissociation reaction
The invention provides a preparation method of pregabalin. The preparation method comprises the following steps: performing chiral separation reaction, dissociation reaction and Hofmann degradation reaction with 3-(carbamoyl methyl)-5-methylhexanoic acid serving as raw material to prepare pregabalin, wherein a pregabalin chiral isomer generated in the process reacts under the action of an alkali reagent so as to obtain a hydrolysis product, and the hydrolysis product is hydrolyzed further and recycled to obtain the raw material 3-(carbamoyl methyl)-5-methylhexanoic acid. Compared with the prior art, the preparation method is convenient to carry out; the prepared pregabalin is high in purity; the used raw material can be recycled, so that the production cost is greatly reduced; the preparation method is favorable for large-scale industrial production.
Owner:NANTONG CHANGYOO PHARMATECH CO LTD
Pregabalin stomach floating-type slow-release tablet and preparation method thereof
ActiveCN109044981AProlong gastric residence timePromote absorptionOrganic active ingredientsNervous disorderBlood concentrationAcrylic resin
The invention discloses a pregabalin stomach floating-type slow-release tablet. The pregabalin stomach floating-type slow-release tablet comprises an active ingredient, a framework material, a swelling agent and an excipient. The active ingredient is pregabalin and pharmacologically acceptable salt, a solvate, a hydrate or a complex thereof. The framework material is combination of any one or moreof hydroxypropyl methylcellulose, hydroxypropylcellulose, acrylic resin and derivatives thereof, wherein the active ingredient accounts for 7-33% of a total weight of the slow-release tablet, the framework material accounts for 5-50% of the total weight of the slow-release tablet, the swelling agent accounts for 5-55% of the total weight of the slow-release tablet, and the balance of the excipient. The pregabalin stomach floating-type slow-release tablet is capable of, through selecting the suitable combination of the framework material and the swelling agent, achieving the following purposes: 1) prolonging residence time of the slow-release tablet in a stomach, enabling a drug to be continuously released and absorbed, and reducing fluctuation of blood concentration; and 2) reducing the physiological condition effect of a patient, and enabling the efficacy of the slow-release tablet to have a smaller individual difference.
Owner:AC PHARMA CO LTD
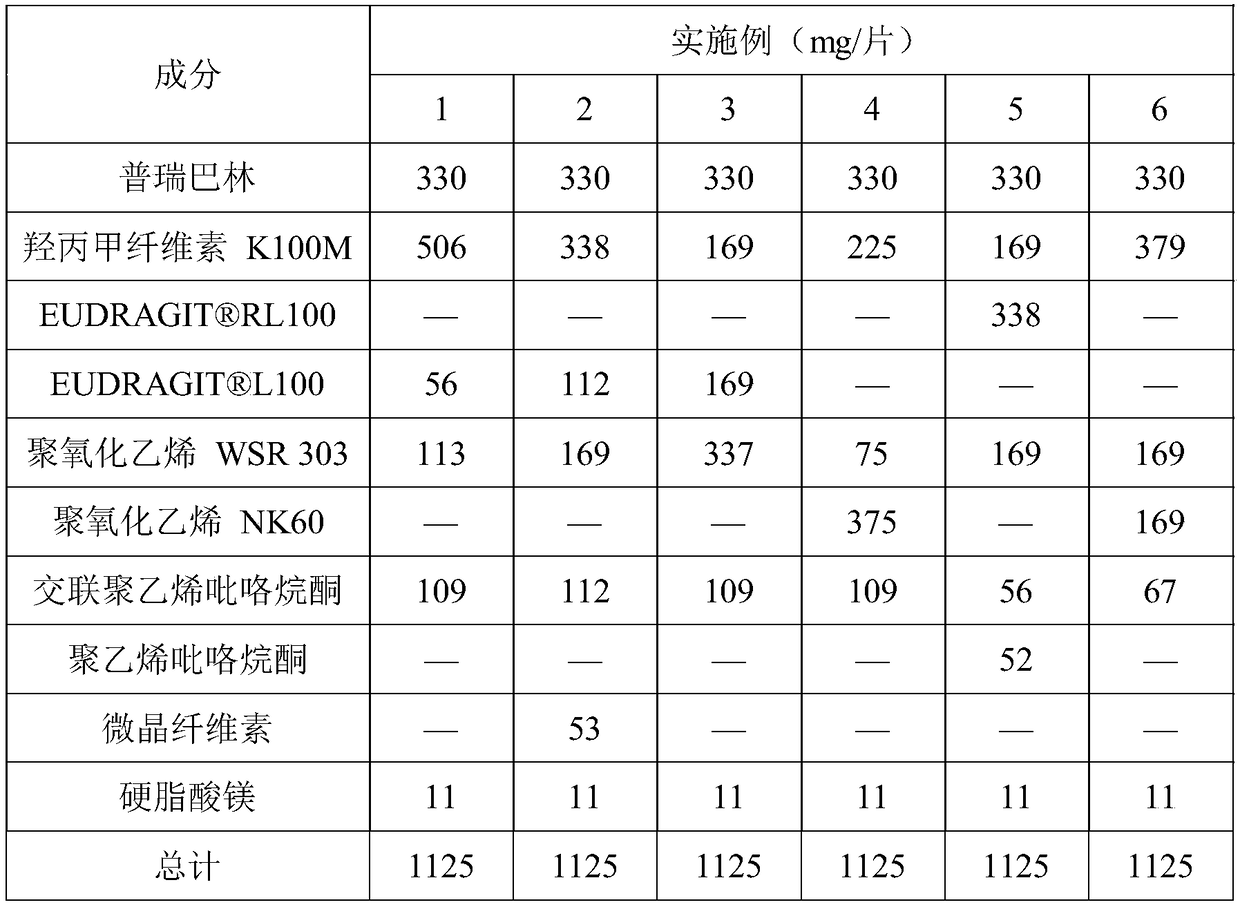
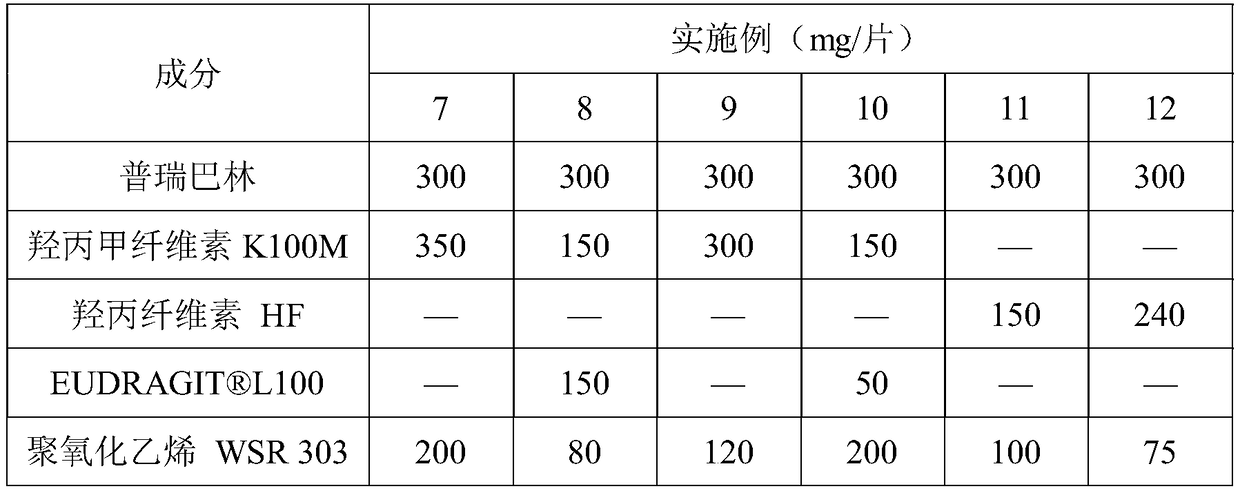
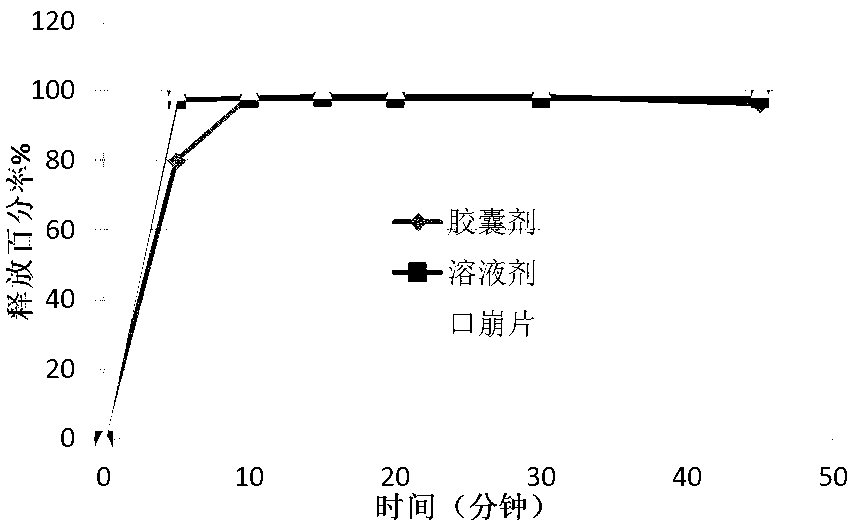
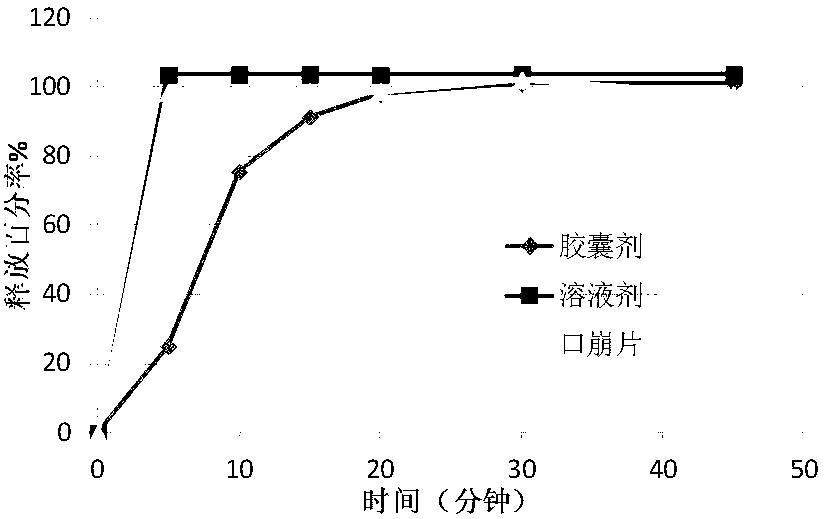
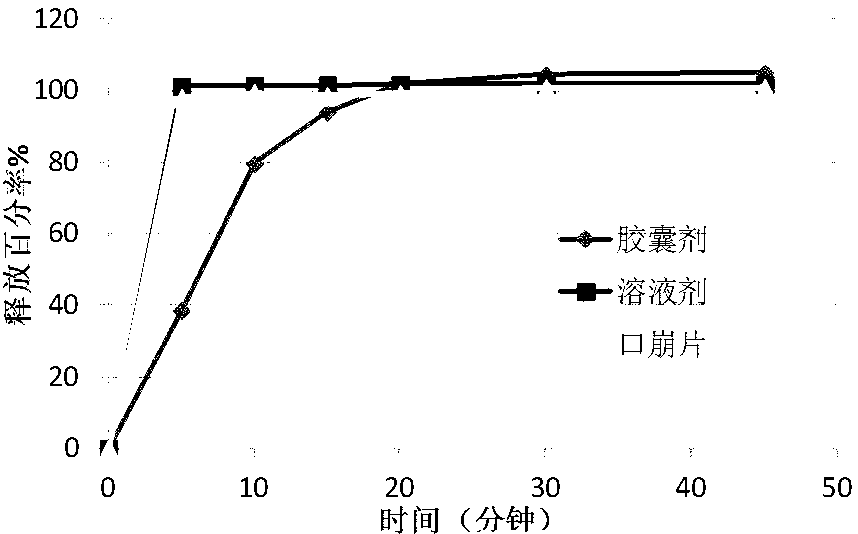
Pregabalin orally disintegrating tablet and dispersible tablet and preparation method thereof
InactiveCN103271888AImprove stabilityOrganic active ingredientsNervous disorderOrally disintegrating tabletPregabalin
The invention belongs to technical field of medicine, and particularly relates to a pregabalin orally disintegrating tablet and a dispersible tablet and the preparation method thereof. A prescription of the pregabalin orally disintegrating tablet and dispersible tablet contains at least one disintegrating agent and insoluble auxiliary material at a certain ratio, so that the disintegration time of six pregabalin orally disintegrating tablets is less than three minutes in one hundred ml of water at the temperature of 2o DEG C, while the disintegration time of pregabalin dispersible table is less than 30 seconds in 2 ml of water at the temperature of 24 DEG C. The bulk pharmaceutical chemicals of pregabalin orally disintegrating tablet and dispersible tablet are controlled in smaller grain size (74 to 250 microns). Combined with the technology of wet granulation, the problem of poor compressibility of pregabalin powder is solved. The solubility of pregabalin orally disintegrating tablet and dispersible tablet can reach more than 85 percent within 15 minutes in different PH mediums.
Owner:SHANGHAI AUCTA PHARMA CO LTD
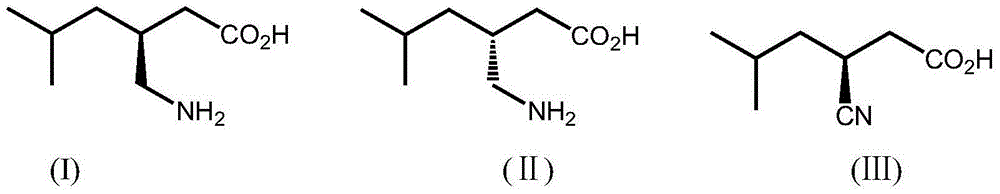
Stereoselective bioconversion of aliphatic dinitriles into cyano carboxylic acids
The present invention is directed to a regio- and stereoselective bioconversion of selected aliphatic dinitriles into corresponding cyanocarboxylic acids. More particularly, the present invention provides methods for the conversion of 2-isobutyl-succinonitrile into (S)-3 cyano-5-methylhexanoic acid, which is a useful intermediate in the synthesis of (S)-3(aminomethyl)-5-methylhexanoic acid (pregabalin). Pregabalin can be used for treating certain cerebral diseases, for example, in the treatment and prevention of seizure disorders, pain, and psychotic disorders.
Owner:PFIZER INC
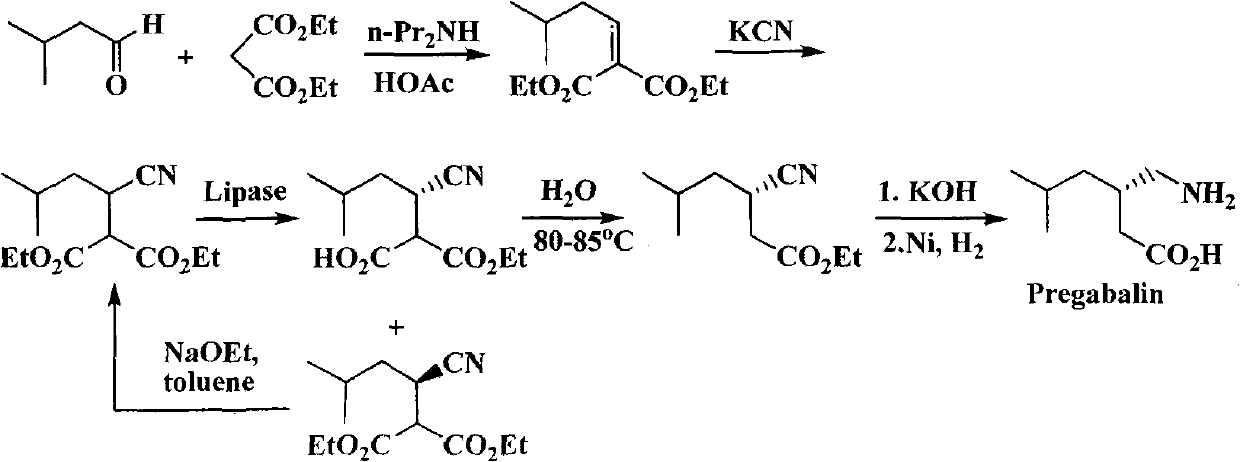
Process for the preparation of pregabalin
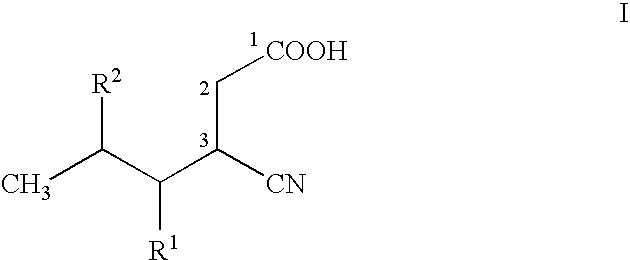
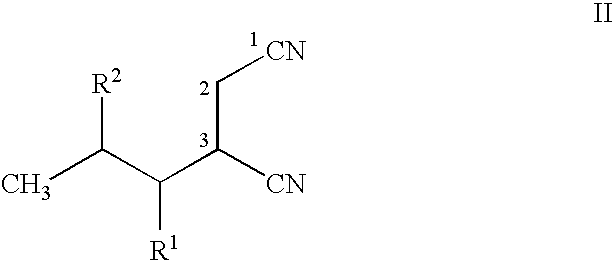
ActiveUS20150344919A1Economical and simpleEasy to implementOrganic compound preparationAmino-carboxyl compound preparationAlkaline earth metalMethyl group
The present invention provides an improved process for the preparation of a compound of formula (I), which comprises the steps of: formula (I), (a) reacting isovaleraldehyde of formula (II) and alkyl cyanoacetate of formula (III) optionally in presence of salts of weak acid and weak base or weak base in a suitable solvent to get 2-cyano-5-methyl-hex-2-enoic acid alkyl ester of formula (IV); (b) reacting 2-cyano-5-methyl-hex-2-enoic acid alkyl ester of formula (IV) with a suitable cyanide source in water or in an organic solvent or mixture thereof to get 2-isobutylsuccinonitrile of formula (V); (c) obtaining optionally 2-isobutylsuccinonitrile of formula (V) by reacting isovaleraldehyde of formula (II) and alkyl cyanoacetate of formula (III) in presence of suitable cyanide source in water or in an organic solvent or mixture thereof in single step; (d) converting 2-isobutylsuccinonitrile of formula pa (V) to racemic 3-cyano-5-methyl-hexanoic acid or salt thereof of formula (VI) with a genetically modified nitrilase enzyme (Nit pt 9N_56_2) in water or optionally with an organic co-solvent at appropriate pH and temperature; (e) converting racemic 3-cyano-5-methyl-hexanoic acid or salt thereof of formula (VI) to racemic alkyl 3-cyano-5-methyl-hexanoate of formula (VII) by treatment with alcohol (R3OH) and acidic catalyst or alkyl halide (R3X) in presence of a base in a suitable solvent or a mixture of solvents thereof; (f) obtaining (S)-alkyl 3-cyano-5-methyl-hexanoate of formula (VIII) and (R)-3-cyano-5-methyl-hexanoic acid or salt thereof of formula (X) by enzymatic enantioselective hydrolysis in water or organic solvent or a mixture thereof from racemic alkyl 3-cyano-5-methyl-hexanoate of formula (VII); (g) obtaining optionally the compound of formula (VII) by racemizing unwanted (R)-3-cyano-5-methyl-hexanoic acid or salt thereof of formula (X) or substantially enriched (R)-3-cyano-5-methyl-hexanoic acid salt thereof of formula (X) in presence of a base in organic solvent or a mixture thereof; (h) converting (S)-alkyl 3-cyano-5-methyl-hexanoate of formula (VIII) to pregabalin of formula (I) by hydrolyzing ester group with suitable alkali or alkaline earth metal base followed by hydrogenation optionally in one pot in a solvent selected from water or other organic solvents or a mixture thereof in presence of a suitable hydrogenation catalyst.
Owner:HIKAL
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com