Synthetic method of pregabalin
A kind of technology of pregabalin and synthesis method, applied in the field of pharmaceutical intermediate synthesis, can solve the problems of side reaction, insufficient time and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-5
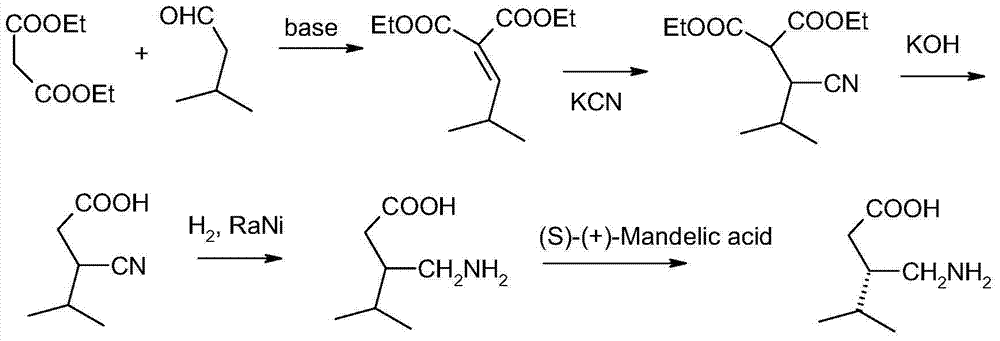
[0067] Step 1: Preparation of (±)-3-(carbamoylmethyl)-5-methyl-hexanoic acid (6)
[0068] Add 110g of ethyl cyanoacetate and 1mL of piperidine into a 1000ml flask, and stir until evenly distributed. Slowly add 86g of isovaleraldehyde dropwise, and stir at 20-30°C for 6-10h. After the dropwise addition was completed, when TLC showed that the reaction was qualified, 164 g of diethyl malonate was added, and 10 g of DBU was added and stirred at room temperature for 2 hours. LCMS showed major [M-H] - The ion peak is 258.1250, and the chemical formula is C 12 h 20 NO 5 . Then 350mL of water was added to the organic phase, then transferred to a 1000mL autoclave, heated to 98-102°C, maintained at this temperature and evacuated for 5 minutes, then sealed the autoclave, continued to heat to 260°C and maintained at this temperature After 8 hours, cool to below 40°C, and then pour ammonia water (1.5 equivalents) into the system. Reheat to 180°C and maintain at this temperature for ...
Embodiment 6
[0077] Embodiment 6: Step 1 reacts under flow reactor
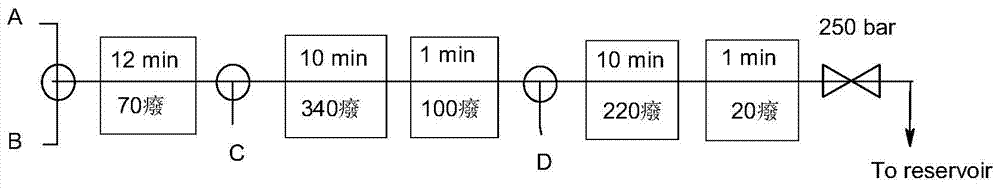
[0078] Add 110 g of ethyl cyanoacetate (1.03 mol) and 1 mL of piperidine (as a catalyst) into the flask, and stir until evenly distributed. 86 g (1 mol) of isovaleraldehyde was slowly added dropwise, the dropping temperature was 20°C, and the dropping time was 30 minutes, then stirred at 20-30°C for 6-10 hours until TLC showed that the reaction was complete. The reaction mixture was pumped proportionally into the T-mixer of the first SS capillary reactor as stream A, while stream B consisting of 164 g (1,02 mol) diethyl malonate and 10 g DBU (as catalyst) was proportionally into the T-blender. The length of the reactor is related to the fluid pumping speed, and the reaction liquid is controlled to react at 70° C. for 12 minutes. The obtained reaction fluid continues to enter the second T-mixer, and the T-mixer is pumped into the fluid C (350mL 90°C hot water) in proportion, and then the temperature is controlled at 340°...
Embodiment 7
[0079] Example 7: Step 1 uses cyanoacetamide
[0080] Add (0.22mol) 185g cyanoacetamide, 500mL water and 5gDMF to the flask, cool to 5-15°C, add 86g (0.1mol) 3-methylbutyraldehyde and then stir. After reacting at 5-15° C. for 8 hours, it was cooled, and the reaction mixture was heated in an autoclave. The subsequent steps were the same as the first step in Example 1. The yield was 88%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com