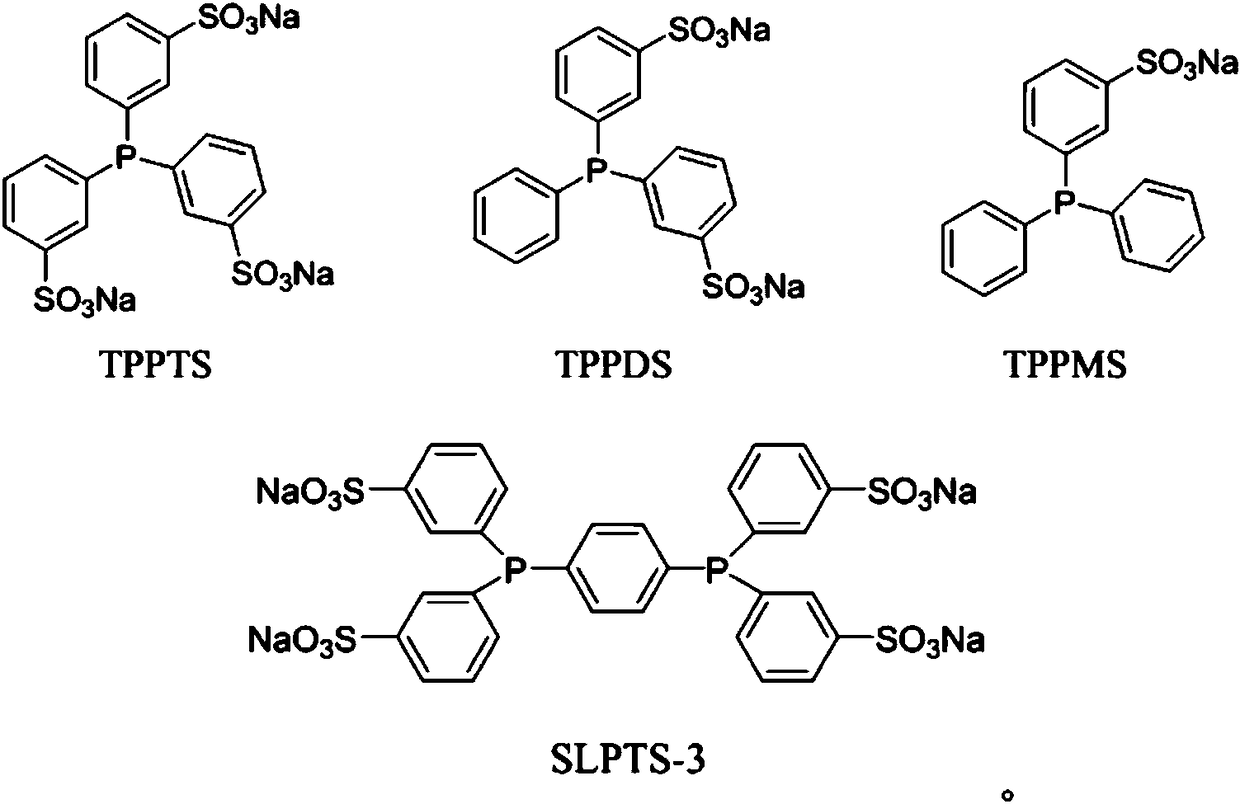
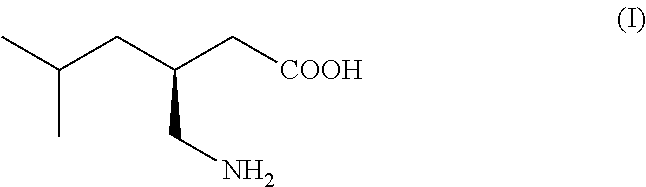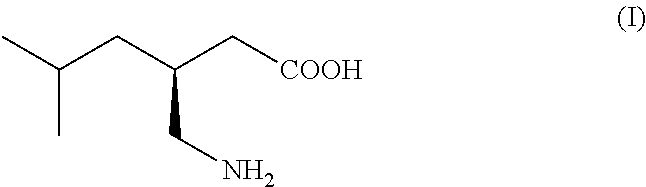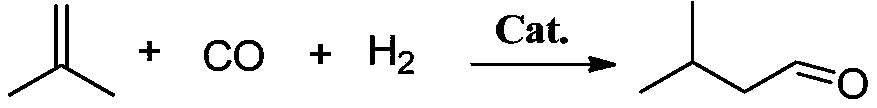Patents
Literature
124 results about "Isovaleraldehyde" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Isovaleraldehyde organic compound, also known as 3-methylbutanal, with the formula (CH₃)₂CHCH₂CHO. It is an aldehyde, a colorless liquid at STP, and found in low concentrations in many types of food. It can be produced commercially and is used as a reagent for the production of pharmaceuticals and pesticides.
Method for producing epoxy cyclohexane
The epoxy cyclohexane producing process includes using cyclohexene as main material, molecular oxygen as oxygen source, n-valeraldehyde or isovaleraldehyde or isobutylaldehyde as intermediate; adopting re-compounded catalyst including at least one oxide of Mn, Fe, Co and Ni, at least one oxide of Mo and W, and at least one oxo acid of N, P and As; and reaction at 30-80 deg.c for 2-12 hr. The re-compounded catalyst can oxidize aldehyde into per-acid in high selectivity and catalyze the reaction between per-acid with cyclohexene in high selectivity to obtain epoxy cyclohexane as the destination product in the same reactor, with the single-path cyclohexene converting rate reaching 23 % and selectivity reaching 98 %. The reaction process uses no solvent and the metal oxide in the re-compounded catalyst may be reused.
Owner:BALING PETRO CHEM CO LTD SINOPEC
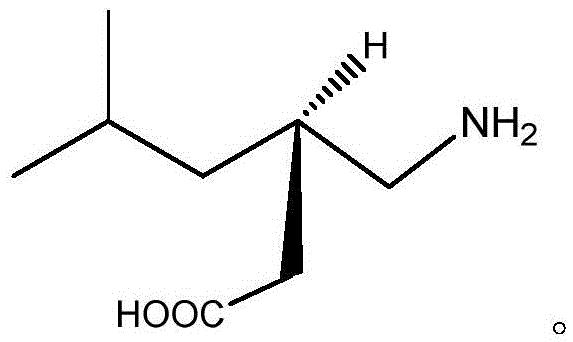
Synthetic method of pregabalin
InactiveCN104496832AImprove conversion rateShort reaction timeOrganic compound preparationAmino-carboxyl compound preparationPregabalinHydrolysis
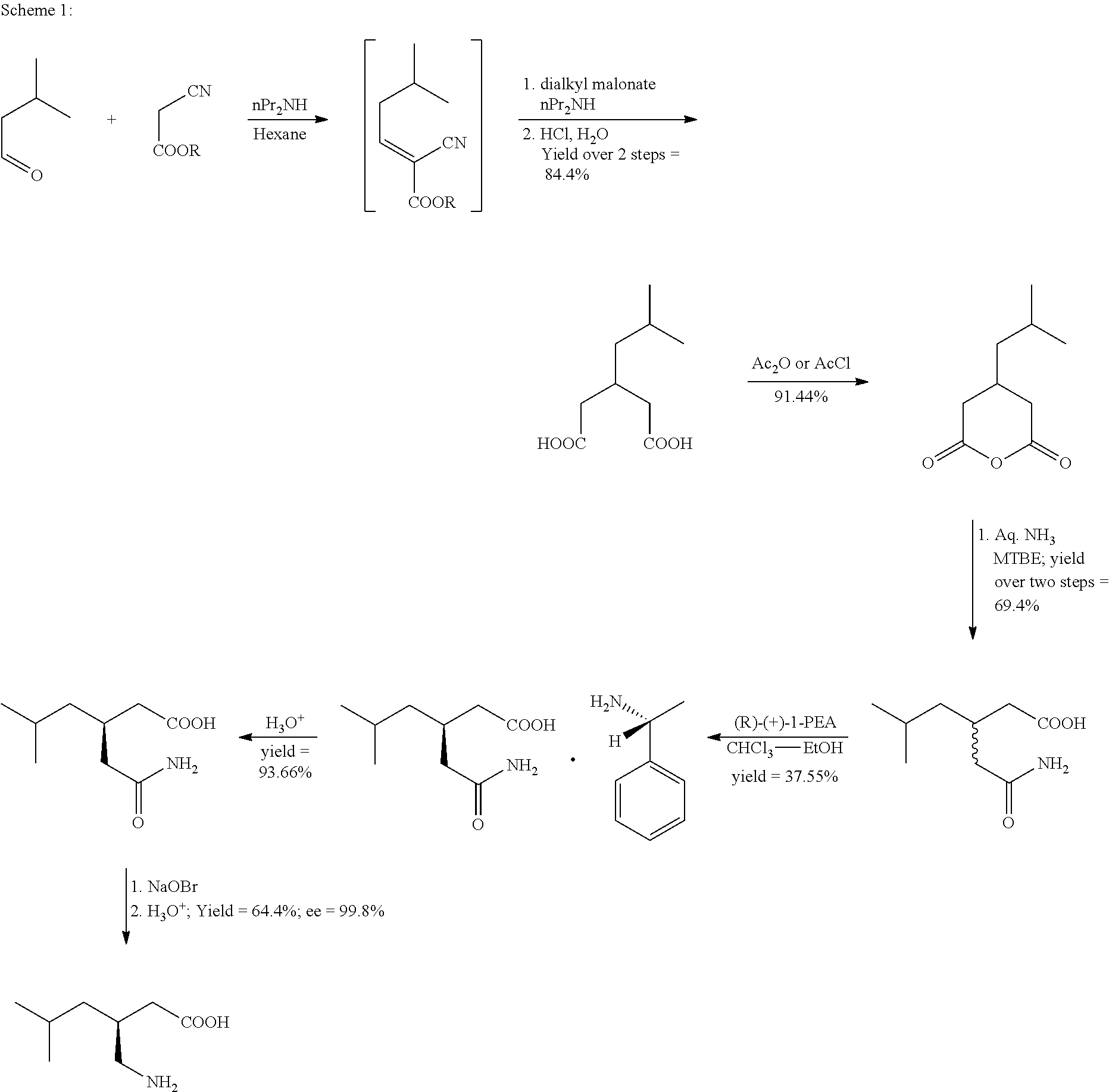
The invention discloses a synthetic method of pregabalin. According to the synthetic method, isovaleraldehyde and cyanoacetic alkyl ester are used as starting materials; condensation reaction, Michael addition reaction, hydrolysis reaction, amidation reaction and the like are carried out successively to obtain 3-(carbamoyl methyl)-5-methyl-hexanoic acid; and resolution and Hofmann elimination are carried out to obtain pregabalin. The greatest improvement of the synthetic method is to carry out the hydrolysis reaction and the amidation reaction under the condition of near-critical water. Thus, addition of a catalyst is avoided, and yield of the reaction is raised. In addition, a flow reactor can be applied to the reaction so as to obtain a better reaction effect.
Owner:ZHEJIANG MENOVO PHARMA
Method for synthesizing valeraldehyde through butene hydroformylation
ActiveCN108069842AEasy to separateLess investmentOrganic-compounds/hydrides/coordination-complexes catalystsPreparation by carbon monoxide reactionButeneFormylation reaction
The invention relates to a method for synthesizing valeraldehyde through butene hydroformylation, and particularly provides a phosphorus-containing organic polymer supported heterogeneous catalysis method for synthesizing valeraldehyde through butene hydroformylation. The method is characterized by comprising steps as follows: liquid butene is metered by an electronic metering balance and continuously enters a reactor with synthesis gas, a hydroformylation reaction is performed under the action of a phosphorus-containing organic polymer supported heterogeneous catalyst, and a valeraldehyde product continuously flows out of the reactor and then is separated from the catalyst and produced continuously. The method has the characteristics of high catalytic activity and good product selectivity, almost no butane is contained in the product, and a molar ratio of n-valeraldehyde to isovaleraldehyde can reach 2-65; besides, the catalyst is good in stability, the product and the catalyst can achieve the excellent-performance characteristics of being simple to separate and the like, then industrial low-cost production of a butene hydroformylation product is realized, and new idea and technical guidance are provided for industrialization of the butene hydroformylation.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Synthetic method of bortezomib
The invention discloses a synthetic method of bortezomib, which comprises the following steps of: taking isovaleraldehyde as an initial raw material, taking (R)-methylpropane-2-sulfinamide as a chiral reagent, generating (R,E)-2-methyl-N-(3-methyl butylidene) propane-2-sulfinamide by a condensation and dehydration reaction, then carrying out a nucleophilic addition reaction with pinacol diboron so as to generate (R)-1-N-methylpropane sulfinyl-3-methyl butane-1-pinacol borate ester, afterwards hydrolyzing under an acidic condition so as to obtain pinacol-(R)-1-amino-3-methyl butane-1-borate ester hydrochloride, then reacting with (S)-3-phenyl-2-(pyrazine-2-formamido) propionic acid under the existence of a coupling agent and also hydrolyzing under the action of isobutyl borate so as to generate a final product of the bortezomib. According to the synthetic method of the bortezomib, the (R)-methylpropane-2-sulfinamide which is easy to obtain is used as the chiral induction reagent, so that an obtained intermediate enantiomorph has higher purity, and a bulk drug which is finally obtained has better quality.
Owner:HEFEI UNIV OF TECH
Loaded complex catalyst for isobutylene hydrogen formylation reaction
InactiveCN1781603AOrganic-compounds/hydrides/coordination-complexes catalystsHydrocarbon by hydrogenationHydrogenFormylation reaction
The present invention features that loaded rhodium and cobalt bimetal complex catalyst has the advantages of high activity of rhodium catalyst and low cost of cobalt catalyst combined and excellent reaction effect. Through reaction at the reaction condition of 2.5 MPa for 5 hr, isobutylene converting rate can reach 62%, isovaleraldehyde selectivity can reach 89%, optimized isovaleraldehyde producing rate may reach 1257 mol / mol-Rh each hour, and no side product tert-valeraldehyde is produced. In the same time, the catalyst preparing process has been optimized.
Owner:SICHUAN UNIV
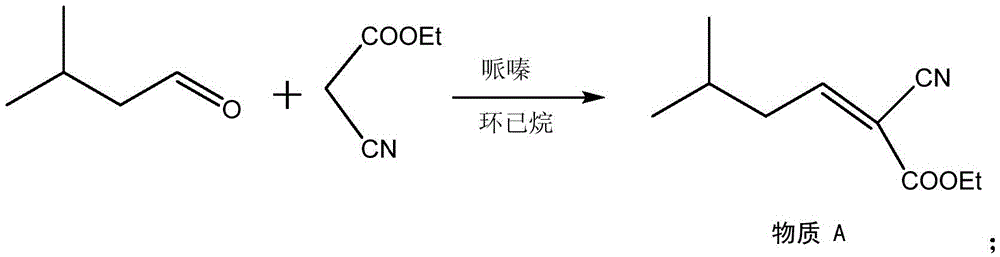
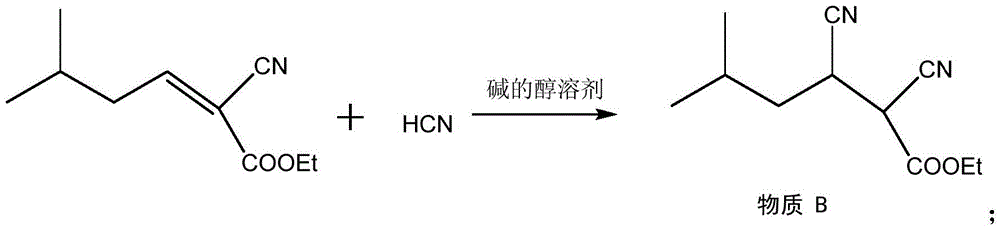
Process for the preparation of pregabalin
ActiveUS20150344919A1Economical and simpleEasy to implementOrganic compound preparationAmino-carboxyl compound preparationAlkaline earth metalMethyl group
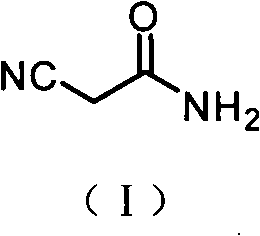
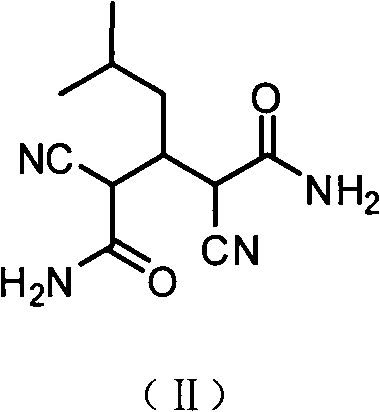
The present invention provides an improved process for the preparation of a compound of formula (I), which comprises the steps of: formula (I), (a) reacting isovaleraldehyde of formula (II) and alkyl cyanoacetate of formula (III) optionally in presence of salts of weak acid and weak base or weak base in a suitable solvent to get 2-cyano-5-methyl-hex-2-enoic acid alkyl ester of formula (IV); (b) reacting 2-cyano-5-methyl-hex-2-enoic acid alkyl ester of formula (IV) with a suitable cyanide source in water or in an organic solvent or mixture thereof to get 2-isobutylsuccinonitrile of formula (V); (c) obtaining optionally 2-isobutylsuccinonitrile of formula (V) by reacting isovaleraldehyde of formula (II) and alkyl cyanoacetate of formula (III) in presence of suitable cyanide source in water or in an organic solvent or mixture thereof in single step; (d) converting 2-isobutylsuccinonitrile of formula pa (V) to racemic 3-cyano-5-methyl-hexanoic acid or salt thereof of formula (VI) with a genetically modified nitrilase enzyme (Nit pt 9N_56_2) in water or optionally with an organic co-solvent at appropriate pH and temperature; (e) converting racemic 3-cyano-5-methyl-hexanoic acid or salt thereof of formula (VI) to racemic alkyl 3-cyano-5-methyl-hexanoate of formula (VII) by treatment with alcohol (R3OH) and acidic catalyst or alkyl halide (R3X) in presence of a base in a suitable solvent or a mixture of solvents thereof; (f) obtaining (S)-alkyl 3-cyano-5-methyl-hexanoate of formula (VIII) and (R)-3-cyano-5-methyl-hexanoic acid or salt thereof of formula (X) by enzymatic enantioselective hydrolysis in water or organic solvent or a mixture thereof from racemic alkyl 3-cyano-5-methyl-hexanoate of formula (VII); (g) obtaining optionally the compound of formula (VII) by racemizing unwanted (R)-3-cyano-5-methyl-hexanoic acid or salt thereof of formula (X) or substantially enriched (R)-3-cyano-5-methyl-hexanoic acid salt thereof of formula (X) in presence of a base in organic solvent or a mixture thereof; (h) converting (S)-alkyl 3-cyano-5-methyl-hexanoate of formula (VIII) to pregabalin of formula (I) by hydrolyzing ester group with suitable alkali or alkaline earth metal base followed by hydrogenation optionally in one pot in a solvent selected from water or other organic solvents or a mixture thereof in presence of a suitable hydrogenation catalyst.
Owner:HIKAL
Preparation technique for florosa
InactiveCN104803958AHigh yieldLess investmentOrganic chemistry3-methyl-2-buten-1-olOrganic chemistry
The invention belongs to the technical field of fine chemical engineering, relates to florosa, and particularly relates to a preparation technique of florosa. The technical scheme provided by the invention is as follows: continuously putting isovaleraldehyde, 3-methyl-2-buten-1-ol and an acid catalyst into a jet mixing reactor for reaction according to a certain molar ratio under a certain temperature condition; meanwhile, continuously outputting reaction mixtures according to the feeding proportion so as to prepare florosa. The jet mixing reactor is used to carry out the continuous synthesis of florosa, and has better heat and mass transfer performance as compared with the conventional kettle-type batch reactor and the fixed reactor, and the reaction efficiency can be effectively improved; by adopting an acid compound as the catalyst, the conversion rate is greatly improved, the yield is up to 75-85%, and the conversion rate is up to 90% or above; meanwhile, during industrial application, by adopting the efficient jet mixing reactor, the hardware equipment investment in reaction units can be effectively reduced.
Owner:江苏绿源精细化工有限公司
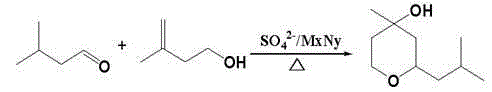
Method for compounding lily-of-the-valley pyran through reaction under static bed loaded solid superacid catalysis
ActiveCN105175372AHigh yieldGood choiceOrganic chemistryPhysical/chemical process catalystsPtru catalystAcid catalysis
The invention provides a preparation method of lily-of-the-valley pyran by using a static bed loaded with a solid superacid catalyst. The method comprises the steps of selection of a static bed, weighing of raw materials, feeding of the raw materials and reaction in the static bed; in the step of weighing of the raw materials, the mass ratio of isovaleraldehyde to allylic alcohols is 1 to 0.93-1; in the step of reaction in the static bed, for the static bed loaded solid superacid catalyst, the length ranges from 80 cm to 280 cm, the gas flow rate ranges from 0.2 m / s to 3 m / s, and the pressure ranges from 0.015 MPa to 0.3 MPa. According to the preparation method, static bed loaded solid superacid serves as the catalyst to prepare the lily-of-the-valley pyran for the first time, the product yield is high, according to the allylic alcohols, the yield ranges from 95.1% to 96.3%, and according to the isovaleraldehyde, the yield ranges from 80.2% to 86.2%; the content of a gas phase of a product is over 99%; the method has the advantages of being good in selectivity, high in reaction efficiency, long in service life, less in amount of three wastes and the like, and the raw material product is prone to separation.
Owner:SHANDONG NHU PHARMA
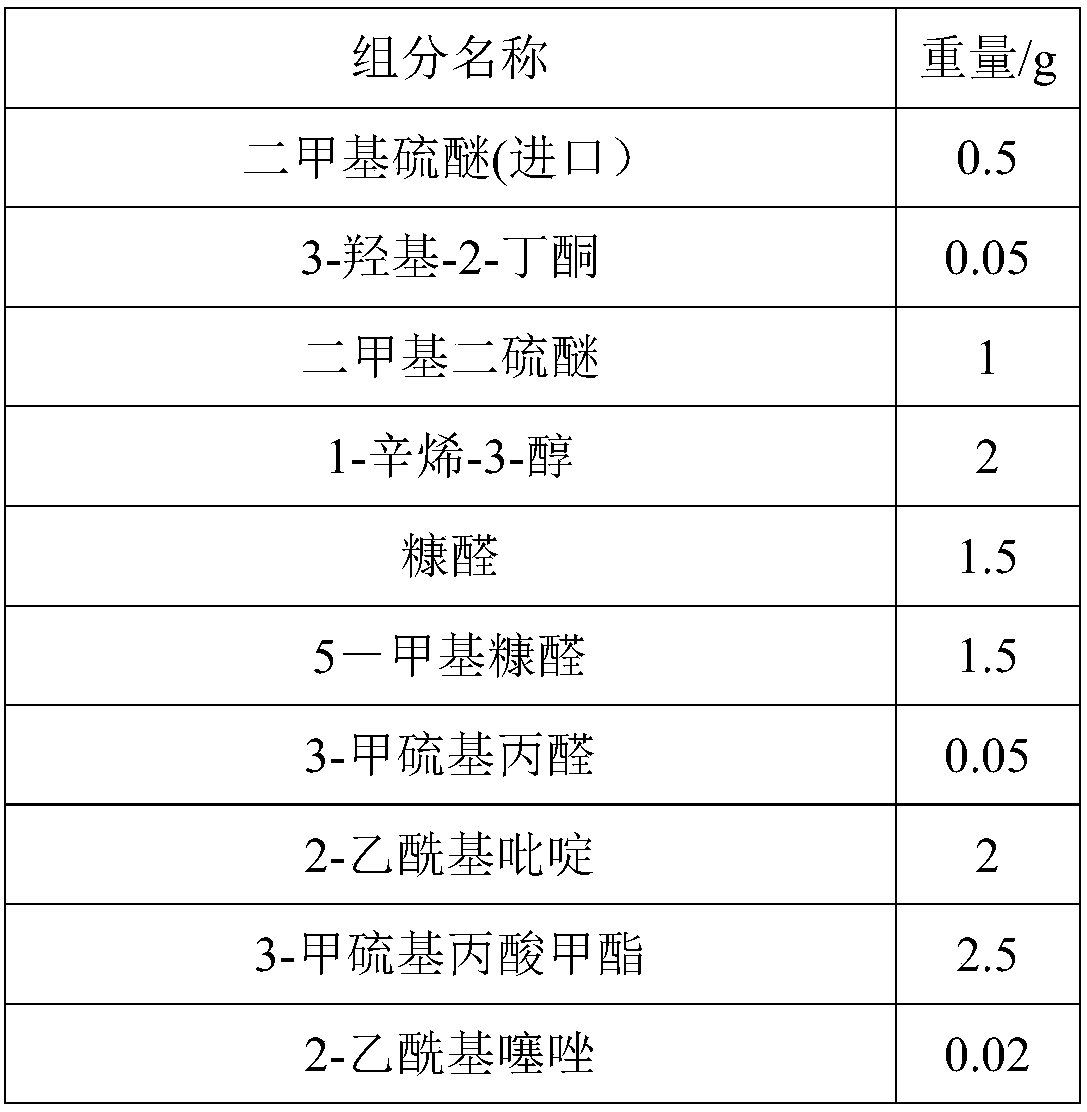
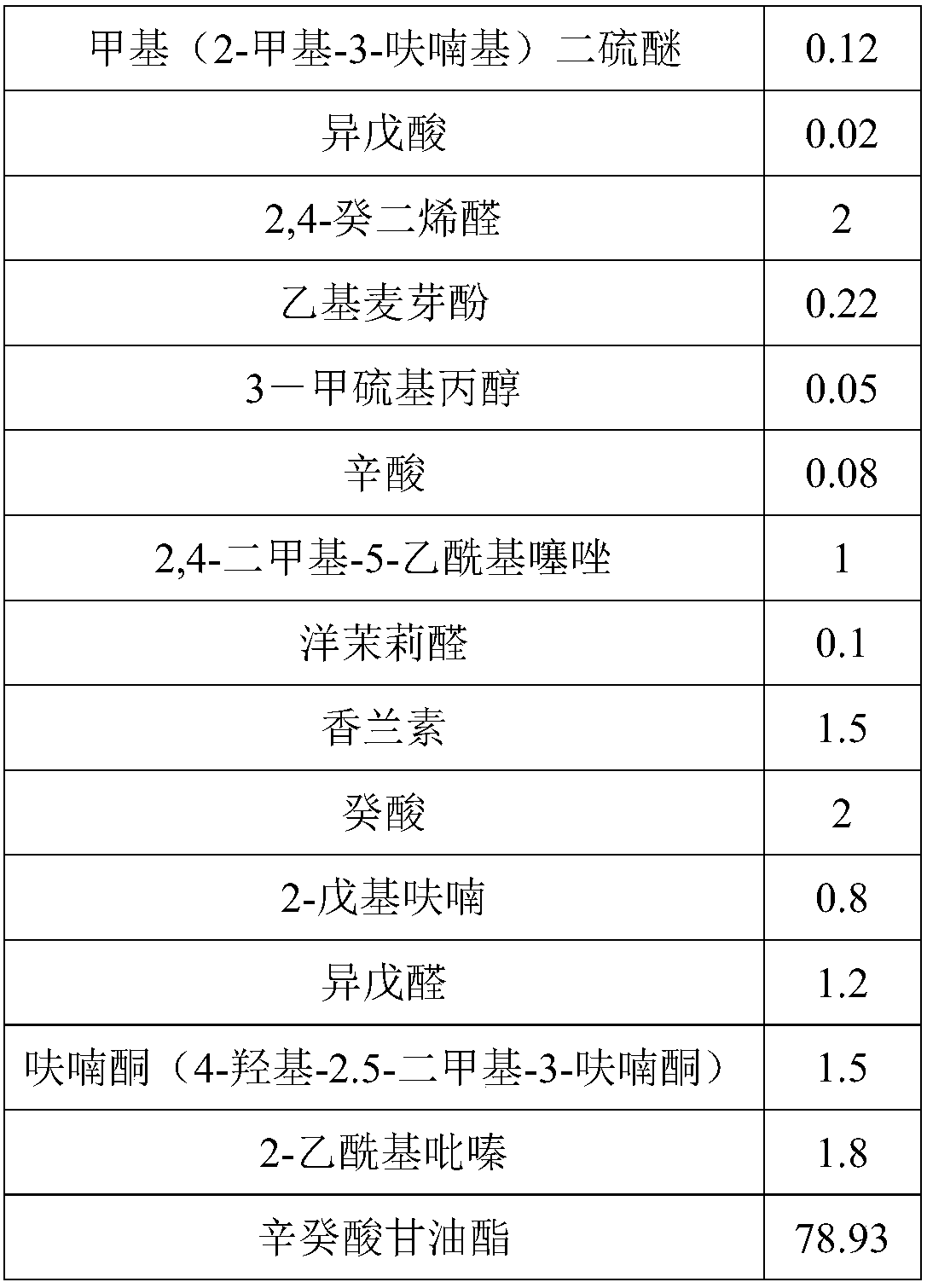
Salted egg yolk essence
ActiveCN108618097AFragrance aroma is pure and fullIncrease the fragranceFood scienceFood additiveFurfural
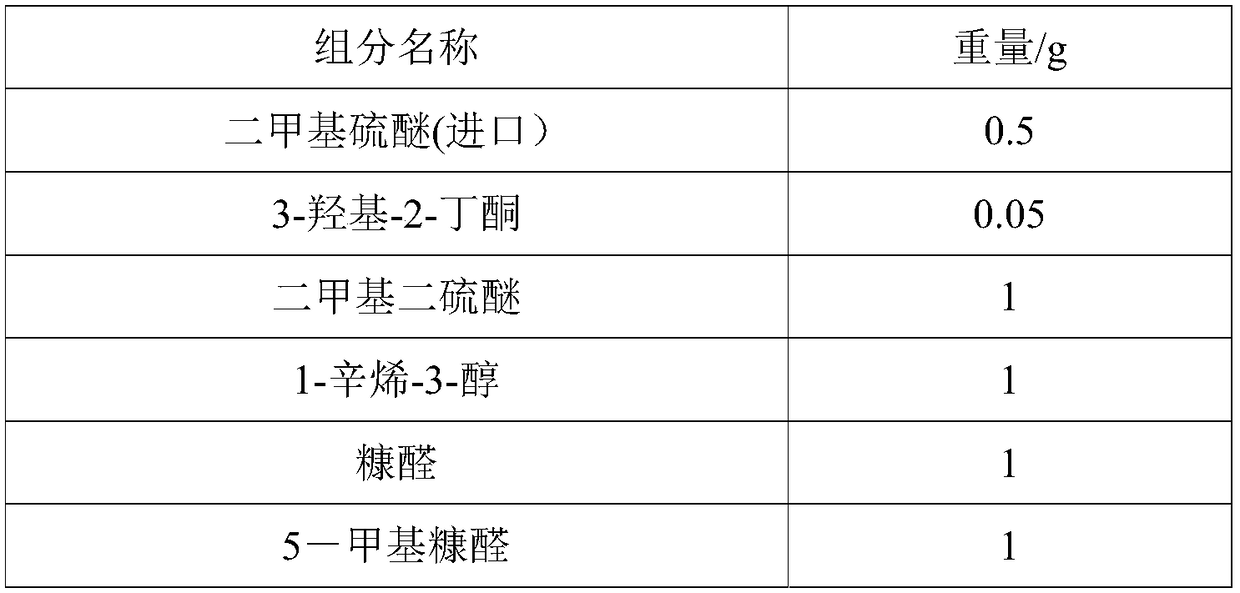
The invention discloses salted egg yolk essence which is composed of caprylic capric glyceride, dimethyl sulfide, 3-hydroxy-2-butanone, dimethyl disulfide, 1-octylene-3-alcohol, furfural, 5-methyl furfural, 3-methylthiopropanal, 2-acetylpyridine, 3-methyl methylthiopropionate, 2-acetylthiazole, methyl(2-methyl-3-furyl)disulfide, isovaleric acid, 2, 4-decadiene aldehyde, ethyl maltol, 3-methylthiopropanol, caprylic acid, 2, 4-dimethyl-5-acetylthiazole, heliotropin, vanillin, capric acid, 2-pentylfuran, isovaleraldehyde, furanone and 2-acetylpyrazine. The salted egg yolk essence is authentic andfull in fragrance, low in cost, good in fragrance lasting and penetrating in fragrance, has the advantage of being delicious, fragrant and oily and can serve as a food additive to be applied in producing food like bake and beverages.
Owner:GUANGZHOU HUABAO FOOD CO LTD
Method for preparing 3-methyl butyraldehyde from isobutylene
ActiveCN106008184AImprove conversion rateHigh selectivityPreparation by carbon monoxide reactionSolventSide reaction
The invention discloses a method for preparing 3-methyl butyraldehyde from isobutylene. According to the method, the isobutylene and synthesis gas are utilized as raw materials, acetylacetonatocarbonyltriphenylp hosphinerhodium(I) is utilized as the catalyst, triphenylphosphine is utilized as a ligan, and n-decane is utilized as a solvent, thereby performing a hydroformylation reaction. According to the method for preparing the 3-methyl butyraldehyde from the isobutylene, performances of the catalyst can be greatly exerted, the conversion rate of the isobutylene is high, and the selectivity of the isovaleraldehyde is high; and side reactions are a few, side products, such as pivaldehyde, isoamyl alcohol and the like, are not detected in products; furthermore, subsequent separation process for the products is simplified.
Owner:DAQING HI TECH LIHUA ENVIRONMENTAL PROTECTION TECH CO LTD +1
High temperature resistance chocolate essence and preparation method thereof
The invention relates to a high temperature resistance chocolate essence and a preparation method thereof. The present invention discloses a high temperature resistance chocolate essence, which comprises a chocolate essence base material and a whole chocolate modifying fragrance group, wherein the chocolate essence base material is prepared from the following raw materials: amino acids, a yeast extract, glucose, D-xylose, D-ribose, vanillin, ethyl vanillin, ethyl maltol, a cocoa extract, and the balance of propylene glycol, and the whole chocolate modifying fragrance group comprises the following raw materials: furanone, vanillin, ethyl vanillin, ethyl maltol, 2,3,5-trimethyl pyrazine, 2-acetyl furan, 5-methyl-2-acetyl furan, gamma-decalactone, isovaleraldehyde, 4,5-dimethyl-2-isobutyl-3-thiazoline, 5-methyl furfural, phenethyl alcohol and dimethyl sulfide. According to the present invention, the high temperature resistance chocolate essence has characteristics of natural, rich, pure and mellow aroma, and good high-temperature resistance, and the preparation method is simple.
Owner:ZENGCHENG HANDYWARE SEASONING
Coffee essence
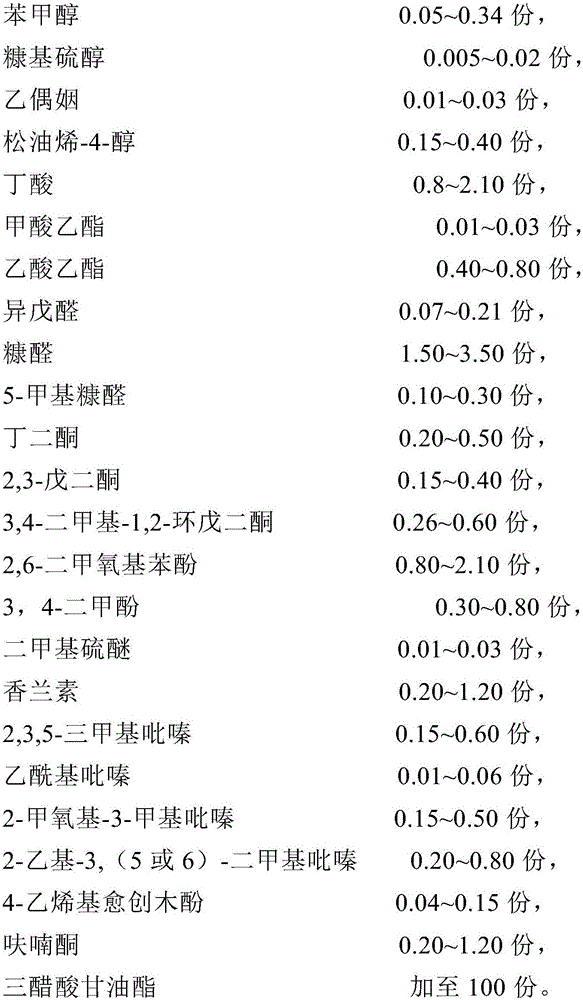
The invention relates to coffee essence. The coffee essence is prepared from an essence base, solvent, surfactant, auxiliary surfactant, 10-50 parts of coffee extracts and a carrier. The essence base is prepared from benzyl alcohol, furfuryl mercaptan, acetoin, terpinen-4-ol, butyric acid, ethyl formate, ethyl acetate, isovaleraldehyde, furfural, 5-methylfurfural, butanedione, 2,3-pentanedione, 3,4-dimethyl-1,2-cyclopentanedione, 2,6-dimethoxy benzene, 3,4-xylenol, dimethyl sulfide, vanillin, 2,3,5-trimethyl pyrazine, 2-acetylpyrazine, 2-methoxy-3-methylpyrazine, 2-ethyl-3,(5 or 6)-dimethyl pyrazine, 4-vinyl guaiacol, furanone and glycerol triacetate. Spices with natural equivalent components having high affinity on coffee beverages are adopted as raw materials of the coffee essence formula, so the natural feeling of beverages and other food containing the coffee essence is enhanced, and the coffee essence has natural aroma and taste.
Owner:SHANGHAI BAIRUN INVESTMENT HLDG GRP CO LTD
Method for preparing fatty aldehyde by means of a hydroformylation reaction
InactiveCN109456154ATake advantage ofGood choiceOrganic compound preparationPreparation by carbon monoxide reactionFormylation reactionDiphosphines
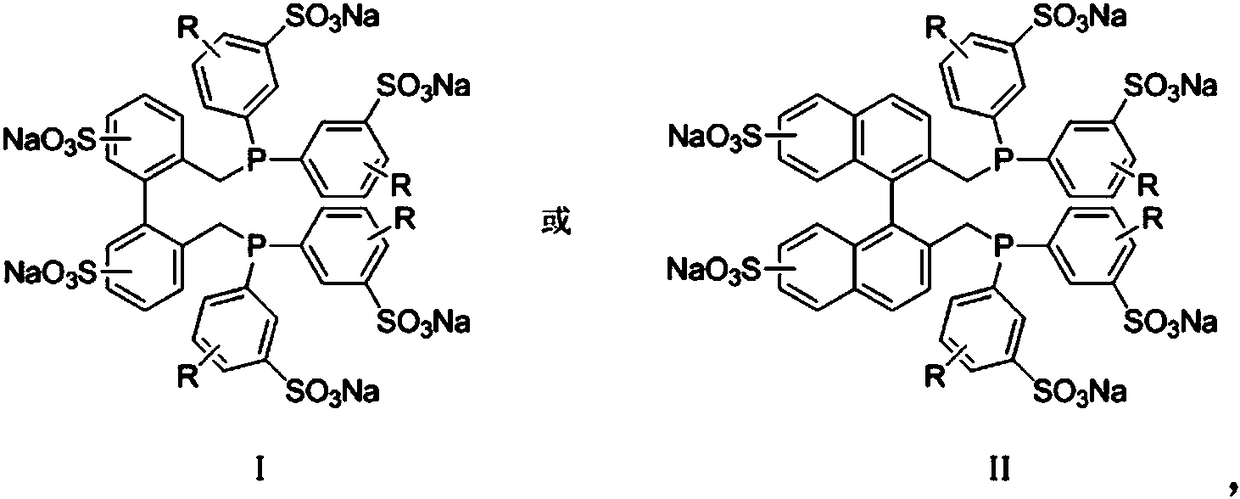
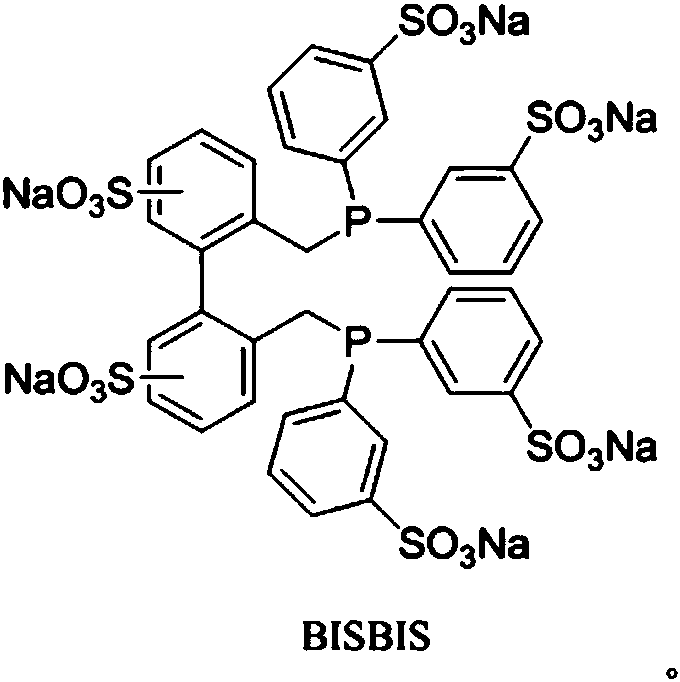
The invention discloses a method for preparing fatty aldehyde by means of a hydroformylation reaction. A catalyst system consisting of a water-soluble rhodium phosphine complex and a diphosphine ligand is used in water / organic two phases for catalyzing 1-butene, 2-butene and a mixture of the 1-butene to be subjected to the hydroformylation reaction so as to prepare n-valeraldehyde; due to the combination of the water-soluble bisphosphine ligand BISBIS and organic additives, the hydroformylation reaction of the butene is accelerated, and the molar ratio of the produced n-valeraldehyde / isovaleraldehyde is greater than 93 to 7. A catalyst aqueous solution is simple and convenient to separate from the product; furthermore, the catalytic performance is stable, the reaction conditions are mild,the service life of a catalyst is long, and the production cost of the n-valeraldehyde is remarkably lowered.
Owner:成都欣华源科技有限责任公司
Novel methyl heptanone synthesis method
ActiveCN105037120AAvoid condensation reactionsHigh selectivityOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsSynthesis methodsDistillation
The present invention relates to an organic matter synthesis method, particularly to a novel methyl heptanone synthesis method, wherein isovaleraldehyde and acetone are adopted as raw materials and are subjected to an aldol condensation reaction and a hydrogenation reaction through a one-pot method under catalysis of catalysts such as triethylamine and palladium carbon to synthesize the methyl heptanone, a small amount of the by-product methyl isobutyl ketone is subjected to distillation recovery, the recovered product is supplied for other positions, and the catalyst used in the reaction process can be repeatedly applied after being recovered. According to the present invention, the method has characteristics of easy operation, high reaction yield, energy saving and environmental protection, and is suitable for industrial production.
Owner:JILIN BEISHA PHARMA
Process for making isopentyl aldehyde from isobutene
InactiveCN1569790AAbundant resourcesNo isomerismPreparation by carbon monoxide reactionHydrogenFormylation reaction
The invention relates to a process for making isopentyl aldehyde from isobutene which consists of, using isobutene as starting raw material, venting inert gas to remove the air in the system at the presence of rhodium catalyst, then letting in gaseous mixture of CO and H2 with the volume ratio of 1:1 for hydrogen formylation reaction.
Owner:SHANDONG NHU PHARMA +1
Preparation method of antiepileptic drug intermediate
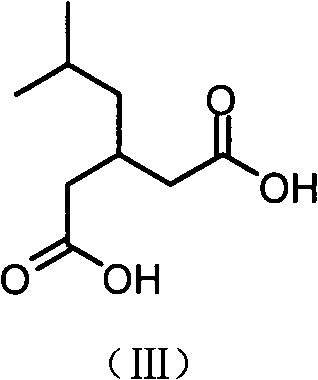
InactiveCN102863326APromote precipitationEasy to operatePreparation from carboxylic acid esters/lactonesGlutaric acidCyanoacetic acid
The invention relates to a preparation method of an antiepileptic drug intermediate 3-isobutyl glutaric acid. The preparation method includes that cyanoacetic acid ester ammonia is decomposed to obtain cyanoacetamide, condensation is performed on the cyanoacetamide and isovaleraldehyde to generate 2, 4-dicyan-3-isobutyl glutaric acid amide, the 2, 4-dicyan-3-isobutyl glutaric acid amide is hydrolysed and decarboxylated to obtain the 3-isobutyl glutaric acid, and total yield in the three steps can reach 77%. The preparation method of the antiepileptic drug intermediate is simple to operate, intermediate products and end products can be separated out easily from reaction, and the preparation method is low in cost and suitable for industrial production.
Owner:CHANGZHOU PHARMA FACTORY
Formula of fresh and sweet tobacco flavoring essence
ActiveCN102304427AGreat tasteAdd natural sweetnessTobacco preparationEssential-oils/perfumesKetoneFurfural
The invention discloses a formula of fresh and sweet tobacco flavoring essence. According to the formula, the fresh and sweet tobacco flavoring essence consists of the following components in percentage by mass: 0.01 to 1.05 percent of ethyl caprylate, 0.1 to 0.3 percent of damascenone, 0.2 to 0.8 percent of isovaleraldehyde propylene glycol acetal, 0.2 to 0.5 percent of ethyl laurate, 0.05 to 0.15 percent of caproic acid, 0.08 to 0.2 percent of 5-methylfurfurol, 0.3 to 0.8 percent of octoic acid, 0.1 to 0.4 percent of 6-methyl-3,5-heptadiene-2-ketone, 0.5 to 1.2 percent of dihydro-beta-irisone, 0.5 to 1.5 percent of phenethyl ethanol, 1 to 2.5 percent of furfural, 25 to 40 percent of propylene glycol and 50 to 70 percent of edible ethanol. By the formula, tobacco produces fresh and sweetfragrance, the tobacco fragrance can be increased and the taste of the tobacco can be improved, so that the taste of the tobacco is improved, the natural sweet fragrance of the tobacco is enhanced and dry, astringent and bitter mouthfeel of customers is improved and requirements of the customers are met.
Owner:GUANGZHOU AOJIAN PERFUME
Black soybean milk flavor and preparation method and application thereof
The invention discloses a black soybean milk flavor and a preparation method and an application thereof. The flavor comprises isovaleric aldehyde, methyl alcohol, isopentyl alcohol, hexanal, 2-methylpyrazine, furfural, trans-2-hexenal, furfuryl alcohol, hexyl alcohol, 2-heptanone, heptanal, 2-acetylfuran, 2, 5-methylpyrazine, 2, 3-dimethyl pyrazine, trans-2-heptenal, 5-methyl-furfural, benzaldehyde, heptanol, 1-octen-3-alcohol, 2-pentyl-furan, caprylaldehyde, 2-acetyl thiazole, trans-2-octenyl aldehyde, octanol, nonanal, capraldehyde, trans,trans-2,4-nonadienal, trans-2-decenal, 2-undecanone,trans,trans-2,4-heptadienal, trans-2-undecenal and propylene glycol. The method comprises the following steps: evenly mixing the above constituents, stewing and curing for 15 days, subpackaging to obtain the black soybean milk flavor. The flavor has convenient raw materials sources, mild fragrance and high naturalness, and is applied to preparing the beans products, is especially used for preparing black soybean milk beverage, not only can enhance mouthfeel, but also can better cover soil mildewed flavor brought by beans in basic materials.
Owner:广州市凯虹香精香料有限公司
Method for synthesizing pregabalin with isobutyl butanedinitrile as intermediate
InactiveCN105463037AGuaranteed yieldGuaranteed purityOrganic compound preparationOrganic chemistry methodsCyanide hydrataseSolvent
The invention discloses a method for synthesizing pregabalin with isobutyl butanedinitrile as the intermediate. The method includes the steps of conducting the Knoevenagel condensation reaction on isovaleraldehyde and ethyl cyanoacetate in cyclohexane solvent with piperazine as the catalyst, conducting Michael addition on the product obtained in the first step and cyanic acid in alkaline alcohol solvent, conducting the decarboxylic reaction on the product obtained in the second step in isopropanol solvent under the heating condition to obtain the isobutyl butanedinitrile solvent, conducting hydrolysis on the intermediate under catalysis of cyanide hydratase AtNiTl, and conducting catalytic hydrogenation with raney nickel as the catalyst. According to the method, isoamyl aldehyde and ethyl cyanoacetate which are low in price and easy to obtain are used as raw materials, and the isobutyl butanedinitrile intermediate is obtained through the Knoevenagel condensation reaction, Michael addition and the decarboxylic reaction. The intermediate is then catalyzed, hydrolyzed and reduced through cyanide hydratase AtNiTl, and pregabalin is obtained. The reaction route is simple, the yield of each step of reaction is high, and therefore the final total recovery and purity of pregabalin are ensured.
Owner:TAICANG YUNTONG BIOCHEM ENG
Luring and killing device for liriomyza sativae pests
ActiveCN102239824AGuarantee green qualityEasy to useBiocidePest attractantsIsoeugenolPhenylacetic acid
The invention relates to a luring and killing device for liriomyza sativae pests, belonging to the field of pest control. A mixture is prepared by the following components in percent by mass: 0.1-5% of isovaleraldehyde, 20-60% of hexenal, 4-25% of hexenol, 0.01%-8% of isoeugenol, 0.01-10% of matsutake alcohol, 10-60% of hexenol acetic ester, 0.1-8% of phenylacetic acid, 1-20% of ionone, 0.1-4% ofphenethyl alcohol and 0.01-5% of carvol; the mixture prepared from the components is dissolved in a normal hexane or dichloromethane dissolvent; the dissolvent is dropwise added on a rubber carrier; after the dissolvent is completely volatilized, a liriomyza sativae lure is formed; and the liriomyza sativae pheromone lure is pasted on a yellow insect-attracting plate manufactured by pest stickingglue and a base plate. The luring and killing device is mainly used for the prevention of liriomyza sativae and has the advantages of convenience in use, good inducing effect, long effective duration, safety, environment friendliness and the like.
Owner:北京依科曼生物技术股份有限公司
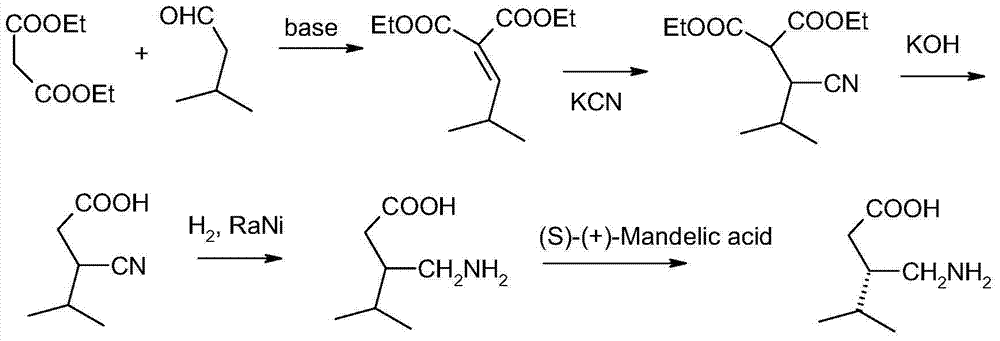
Process for preparing (+/-)-3-(Carbamoymethyl)-5-methylhexanoic acid
ActiveCN102964263AHigh yieldLow costOrganic compound preparationCarboxylic acid amides preparationAcetic anhydridePtru catalyst
The invention relates to a process for preparing (+ / -)-3-(Carbamoymethyl)-5-methylhexanoic acid. The process comprises the steps: (1) preparing 3-isobutylglutaric acid, namely, reacting cyanoacetamide with isovaleric aldehyde in the presence of a catalyst, and then adding concentrated sulfuric acid for reaction to obtain 3-isobutylglutaric acid; (2) reacting 3-isobutylglutaric acid with acetic anhydride to obtain 3-isobutylglutaric anhydride; and (3) reacting 3-isobutylglutaric anhydride with ammonia to produce (+ / -)-3-(Carbamoymethyl)-5-methylhexanoic acid. According to the process, the raw materials adopted by the process are available, the operation is simple, the yield of each reaction is higher than 80 percent, the total yield of the target product is high, and the cost is low.
Owner:太仓市茜泾化工有限公司
Hydroformylation catalyst as well as preparation method and application thereof
ActiveCN111085198AReduce usageGuaranteed stabilityMolecular sieve catalystsPreparation by carbon monoxide reactionPtru catalystFormylation reaction
The invention relates to a hydroformylation catalyst. The catalyst comprises an active component and a carrier for bearing the active component. The active components comprise a first active component, a second active component and a third active component; wherein the first active component is Rh and / or an oxide of Rh, the second active component is at least one metal of Ir, Ru, Os, Pt and Pd and / or an oxide of the metal, and the third active component is at least one metal of Mo, Zn, Mn, Fe, Co, V and Cu and / or an oxide of the metal. The invention also relates to a preparation method of thehydroformylation catalyst. The invention further relates to application of the hydroformylation catalyst in production of isovaleraldehyde through hydroformylation reaction of isobutene.
Owner:SHANDONG NHU VITAMIN CO LTD +1
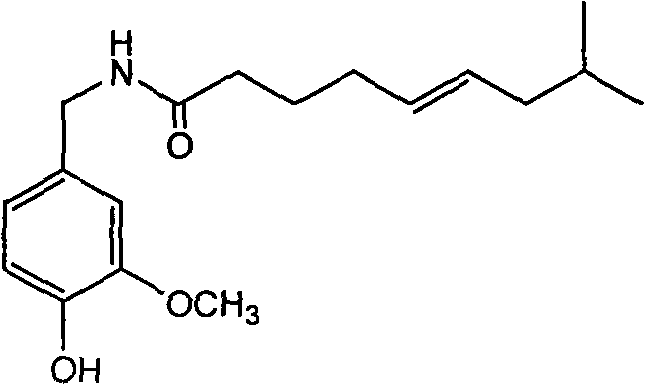
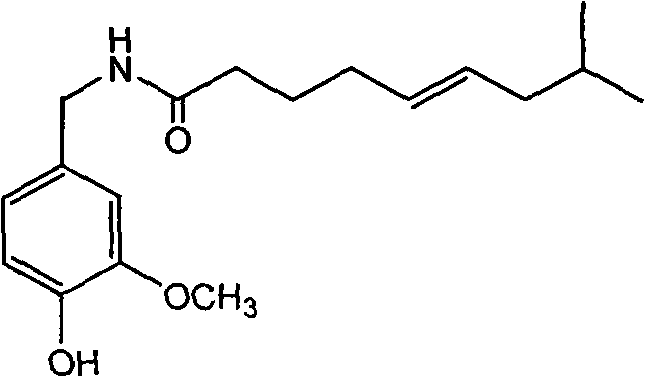
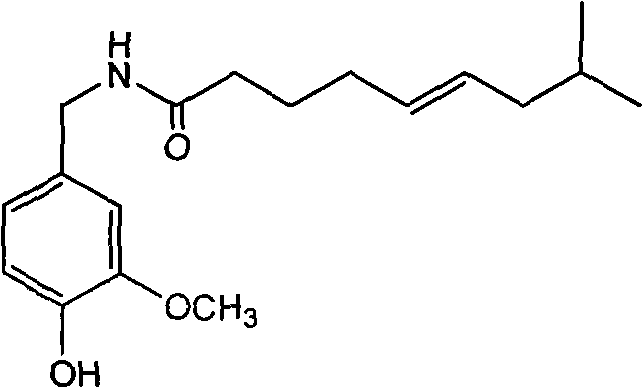
Trans-N-(4- hydroxyl-3-methoxybenzy)-8-methyl-5-nonenamide and preparation method thereof
InactiveCN101774939ASimple preparation processLow costOrganic compound preparationCarboxylic acid amides preparationCapsaicinStructural formula
The invention discloses trans-N-(4-hydroxyl-3-methoxybenzy)-8-methyl-5-nonenamide which has a structural formula as shown in a formula (1). The trans-N-(4-hydroxyl-3-methoxybenzy)-8-methyl-5-nonenamide is prepared by the following steps of: carrying out a single bromination reaction on pentanediol to generate 5-bromo-pentanol; oxidizing the 5-bromo-pentanol to generate 5-bromo-valeric acid; reacting the 5-bromo-valeric acid with triphenylphosphorus to generate (5-carboxyl-amyl)triphenylbromophosphine; reacting the (5-carboxyl-amyl)triphenylbromophosphine with isovaleraldehyde to generate cis-8-methyl-5-nonenoic acid; reacting the cis-8-methyl-5-nonenoic acid with a sodium nitrite / nitric acid solution to obtain trans-8-methyl-5-nonenoic acid; reacting the trans-8-methyl-5-nonenoic acid with an acetylation reagent to obtain trans-8-methyl-5-enoic acid chloride; reacting vanillin with ammonium formate to obtain vanillic amine; acidizing the vanillic amine and neutralizing with alkali liquor; and then reacting the mixture with the trans-8-methyl-5-enoic acid chloride to obtain the trans-N-(4-hydroxyl-3-methoxybenzy)-8-methyl-5-nonenamide. The invention has the advantages of simple and convenient process and mild reaction conditions; and the prepared product provides a new development space for the research on capsaicin substances. The formula (1) is described in specification.
Owner:ZHEJIANG UNIV
Preparation method of isovaleraldehyde
InactiveCN106117024AReduce energy consumptionReduce manufacturing costMolecular sieve catalystsOrganic compound preparationManufacturing cost reductionOrganic layer
The invention discloses a preparation method of isovaleraldehyde. The preparation method comprises the following steps: heating isopentanol till vaporization, then adding a catalyst to perform high-temperature dehydrogenation reaction, and condensing and separating gas generated by reaction to obtain semi-finished isovaleraldehyde; taking the semi-finished isovaleraldehyde, adding sodium hydroxide into the semi-finished isovaleraldehyde, vibrating and shaking, stewing and layering, abandoning a water layer, filtering an organic layer, retaining a filtrate, introducing an absorbent column filled with aluminum oxide into the filtrate for absorption treatment and then rectifying the absorbed isovaleraldehyde to obtain the isovaleraldehyde with a purity of 99.9 percent. With the adoption of the technical scheme, the preparation method disclosed by the invention has the advantages that the conversion rate of the isopentanol can reach 99.9 percent, the purity of the obtained isovaleraldehyde can reach 99.9 percent, the energy consumption is low, the manufacturing cost can be reduced, no corrosion is caused to equipment on the premise of ensuring the conversion rate and the purity, the pollution is less, and the safety is high.
Owner:SHANDONG CHENGTAI CHEM IND
Soy sauce essence and preparation method thereof
Owner:TIANJIN CHUNFA BIO TECH GRP
Nut-flavored cigarette midline adhesive essence, and preparation method and application thereof
The invention provides a nut-flavored cigarette midline adhesive essence. The cigarette midline adhesive essence is prepared by mixing ethyl vanillin, 4-Methyl-5-beta-hydroxyethyl thiazole, ethyl maltol, vanillin, 2-ethy-3,5-dimethylpyrazine of which the mass concentration is 10%, methylmethoxypyrazine of which the mass concentration is 10%, 2-methylbutyraldehyde of which the mass concentration is 10%, isovaleraldehyde of which the mass concentration is 10%, decanoic acid of which the mass concentration is 10%, 2,5-dimethylpyrazine of which the mass concentration is 10%, 2,3,5-trimethylpyrazine of which the mass concentration is 10%, gamma-Nonanolactone, benzaldehyde, 5-methylfurfural, furfural, phenylcarbinol, nut shell extract, peanut shell extract and alcohol. The essence prepared by the invention has rich aroma, is coordinated with the original aroma of tobacco, and can endow a cigarette with unique external aroma; and when a small amount of essence is added into a cigarette midline adhesive, outstanding and long lasting aroma can be brought to the cigarette, and the aroma loss caused by tar reduction and harm lessening can be compensated for.
Owner:HUBEI CHINA TOBACCO IND
Alcohol gas phase dehydrogenation catalyst, preparation method thereof and application
ActiveCN102500420AAvoid chalkingAvoid cokingOrganic-compounds/hydrides/coordination-complexes catalystsCarbonyl compound preparation by oxidationGas phaseDehydrogenation
The invention discloses an alcohol gas phase dehydrogenation catalyst, a preparation method thereof and application. The catalyst is composed of cuprous oxide, zinc oxide, silicon dioxide, metal oxides, graphite and magnesium stearate according a certain ratio. The preparation method of the catalyst comprises first preparing silicon dioxide composite powder where cuprous oxide and zinc oxide are loaded, adding the metal oxides, graphite and magnesium stearate into the prepared composite powder, and carrying out mixed preforming. The catalyst enables an alcohol gas phase dehydrogenation reaction to be carried out at a low temperature, energy consumption is reduced, and the problems of chalking and coking of the catalyst caused by high temperature are solved. Besides, under the catalysis of the catalyst, a reaction for preparing isovaleraldehyde by isoamyl alcohol gas phase dehydrogenation can achieve selectivity of 98%-99%, conversion rate of 96%-99% and product purity of 95%-97%, operation of isovaleraldehyde can be simplified, preparation cost of isovaleraldehyde is reduced, and the requirements for industrialized batch production of isovaleraldehyde are fully met.
Owner:DAFENG HEGNO PHARMA +2
Compound preparation for preventing and treating respiratory diseases of beasts and birds
InactiveCN103784700AGood treatment effectEffective controlPowder deliveryClimate change adaptationFuranTreatment effect
The invention discloses a compound preparation for preventing and treating respiratory diseases of beasts and birds. The compound preparation comprises, by weight, auxiliary materials 1-2 parts, traditional Chinese medicine preparation 30-50 parts and chemical synergist 10-20 parts. The auxiliary materials include isovaleraldehyde 5-10 parts, 2-pentyl-furan 5-10 parts, vanillin 5-10 parts and xanthan gum 2-5 parts. The chemical synergist comprises aminoglycosides 5-10 parts, florfenicol 5-10 parts, amoxicillin 5-10 parts, erythromycin thiocyanate 5-10 parts and ferrous fumarate 2-4 parts. Substance having various physiological activities in a traditional Chinese medicine formula having synergistic effect is extracted and compatible with the chemical synergist through Chinese herbal compound preparation, so that the compounded medicine has good treatment effect on the respiratory diseases and can effectively prevent and treat the diseases. In addition, main medicine is a traditional Chinese medicine extract and can effectively prevent drug resistance, and the medication safety is also improved.
Owner:SICHUAN TIANHONG ANIMAL HUSBANDRY
Method for preparing isoamyl aldehyde from 3-methyl-3-butenyl-1 alcohol
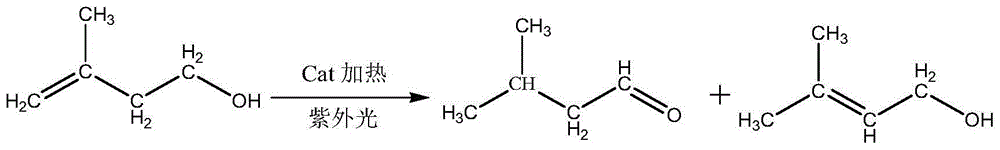
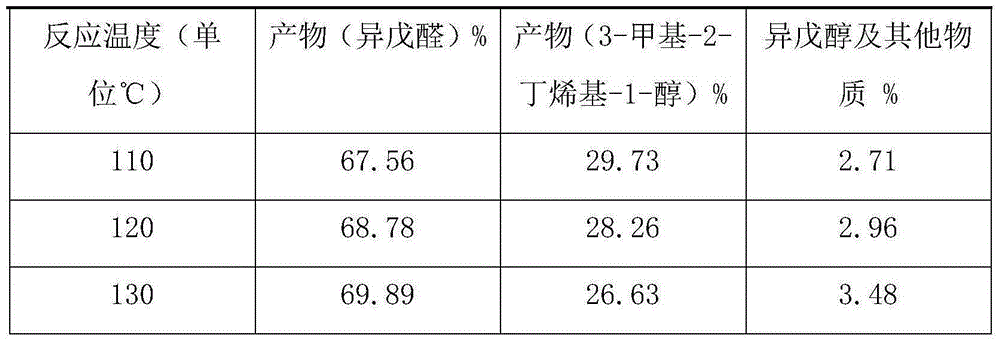
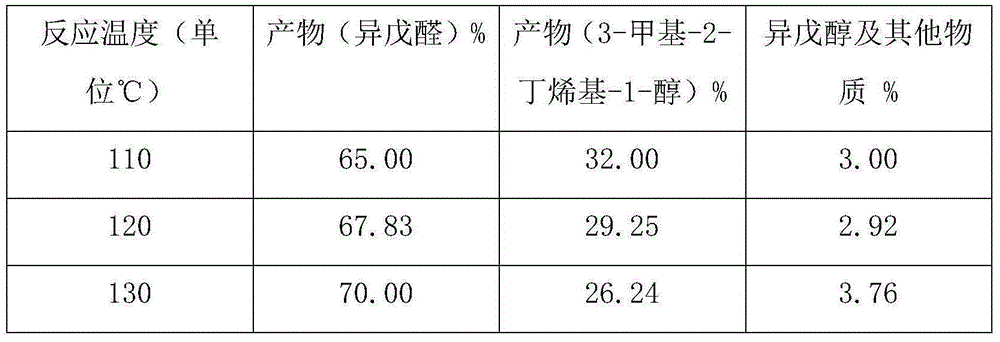
InactiveCN104926631ASufficient sourceImprove conversion ratePreparation by isomerisationOrganic compound preparationPhotocatalytic reactionReaction temperature
The invention discloses a method for preparing isoamyl aldehyde from 3-methyl-3-butenyl-1 alcohol. The method is characterized by comprising the following steps: putting raw materials comprising 98-98.5 percent (in percentage by mass) of 3-methyl-3-butenyl-1 alcohol and 1.5-2 percent of an organic metal compound catalyst into a reactor; putting the reactor into an oil bath pan of 140 DEG C under normal pressure to carry out heating reaction at a temperature of 110-130 DEG C; under a N2 atmosphere anoxic condition, continuously separating part of isoamyl aldehyde generated in a photo-catalytic reaction process by distilling, promoting reaction for generating isoamyl aldehyde to further positively carry out, wherein the total reaction time is 3-4 hours; finally, obtaining high-purity isoamyl aldehyde, 3-methyl-3-butenyl-1 alcohol and a little isoamyl alcohol by carrying out separation, rectification and purification on all crude product. The method is scientific and reasonable, low in reaction temperature and pressure, high in effective product conversion rate, energy-saving and low in production cost.
Owner:吉林众鑫化工集团有限公司
Preparation method of salty beverage
The invention relates to a preparation method of a salty beverage. The preparation method includes the following steps: the salty beverage comprises, by weight, 90-98 parts of water, 0.5-1 part of salt and 0.2-0.5 part of sauce-flavor essence, and the water, the salt and the sauce-flavor essence are mixed evenly, sterilized and filled to obtain the salty beverage. The sauce-flavor essence is obtained by evenly mixing ethyl acetate, 2-acetylfuran, ethyl lactate, diethyl malonate, ethyl butyrate, ethyl levulinate, ethyl pelargonate, ethyl benzoate, soy fouranone, isovaleraldehyde, furfural, 5-hydroxymethyl furfural, 3-methylmercapto-propionaldehyde, phenethyl alcohol, 2, 3-butanedione, 3-hydroxy-2-butanone, benzaldehyde, 2, 3, 5-trimethyl pyrazine, 4-guaethol, 2-acetylpyrrole, 10% difurfuryl sulfide and propylene glycol. The salty beverage manufactured by the preparation method can supplement water, salinity and nutrient substances.
Owner:TIANJIN CHUNFA BIO TECH GRP
Popular searches
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com