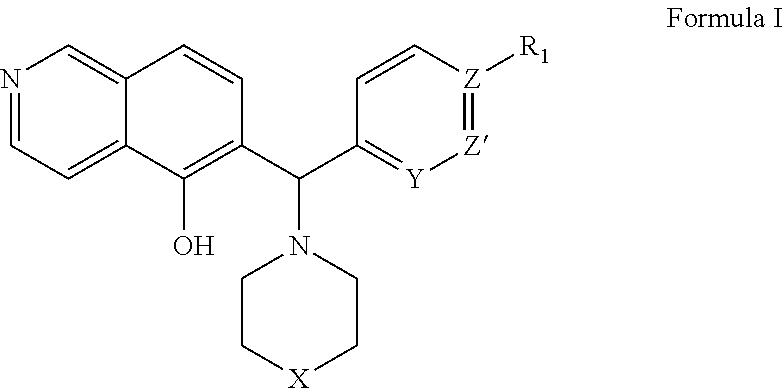
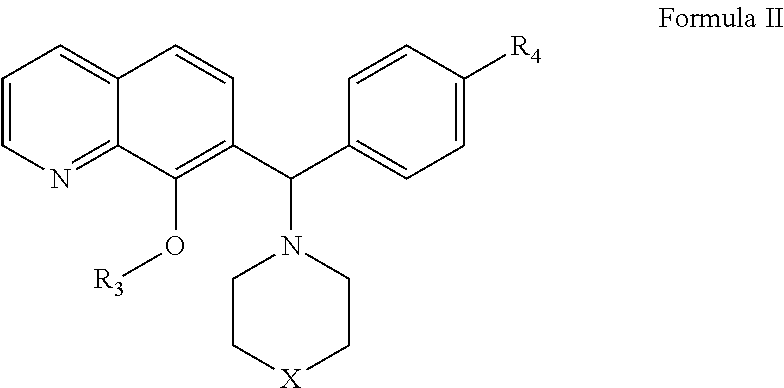
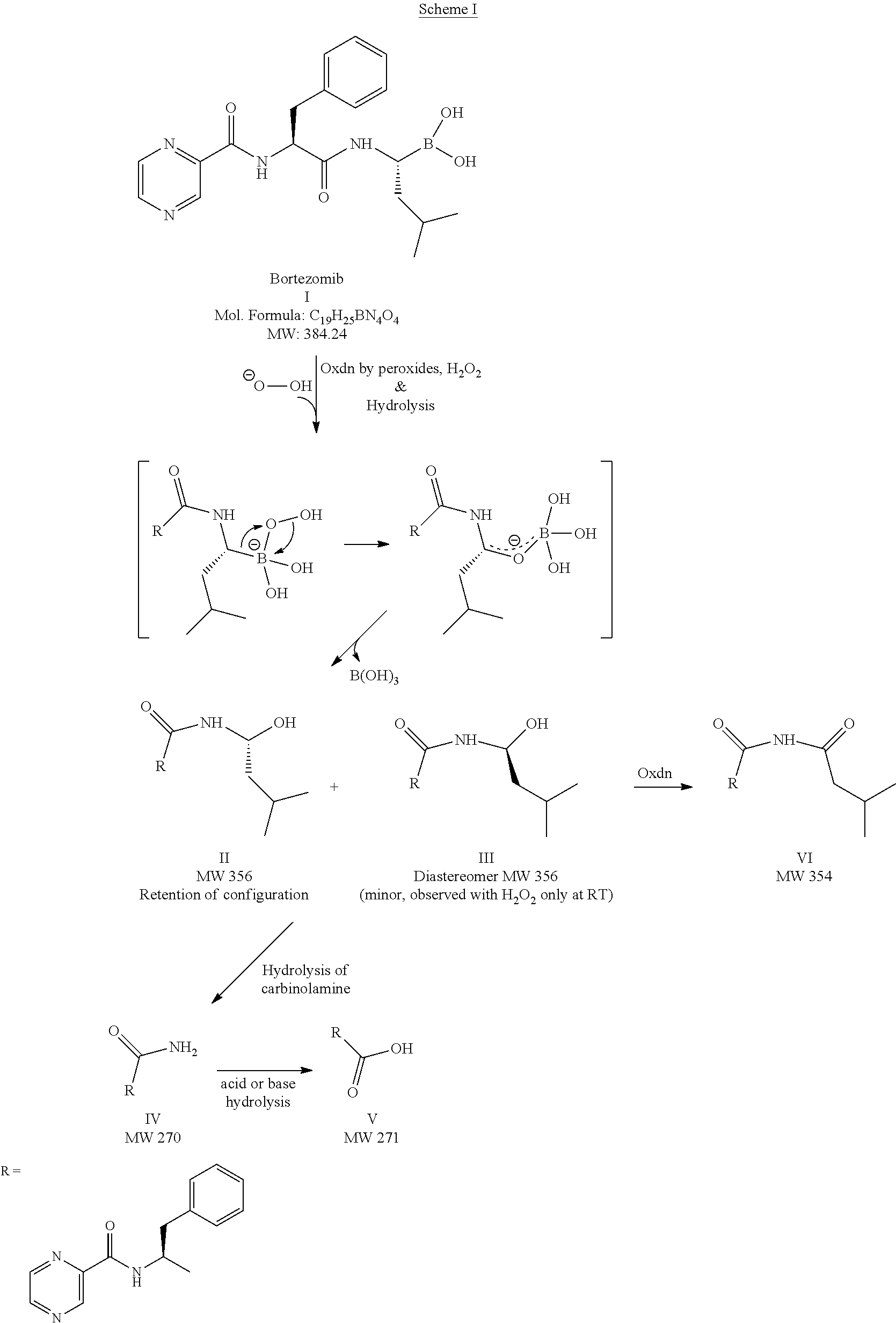
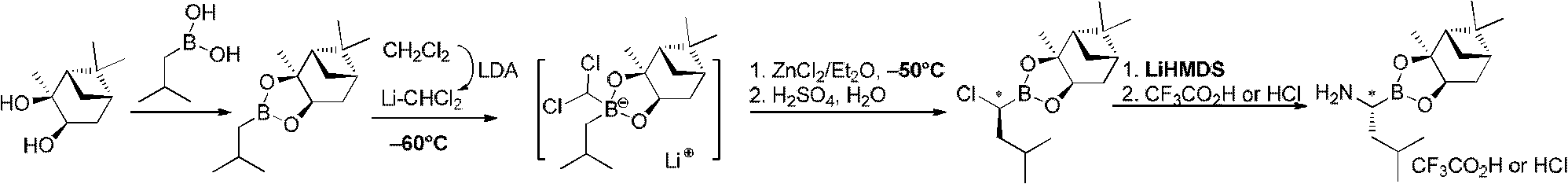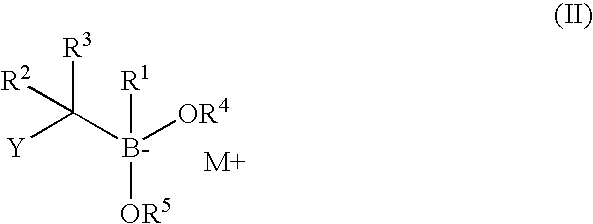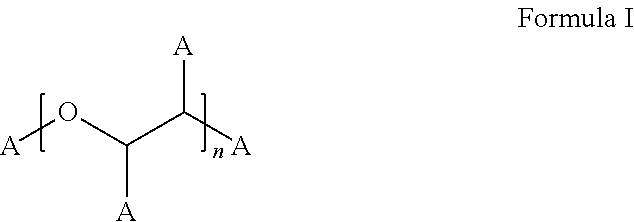Patents
Literature
272 results about "Bortezomib" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
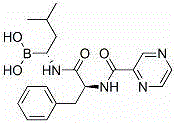
This medication is used to treat certain types of cancer (such as multiple myeloma, mantle cell lymphoma).
Compositions and methods useful for treating diseases
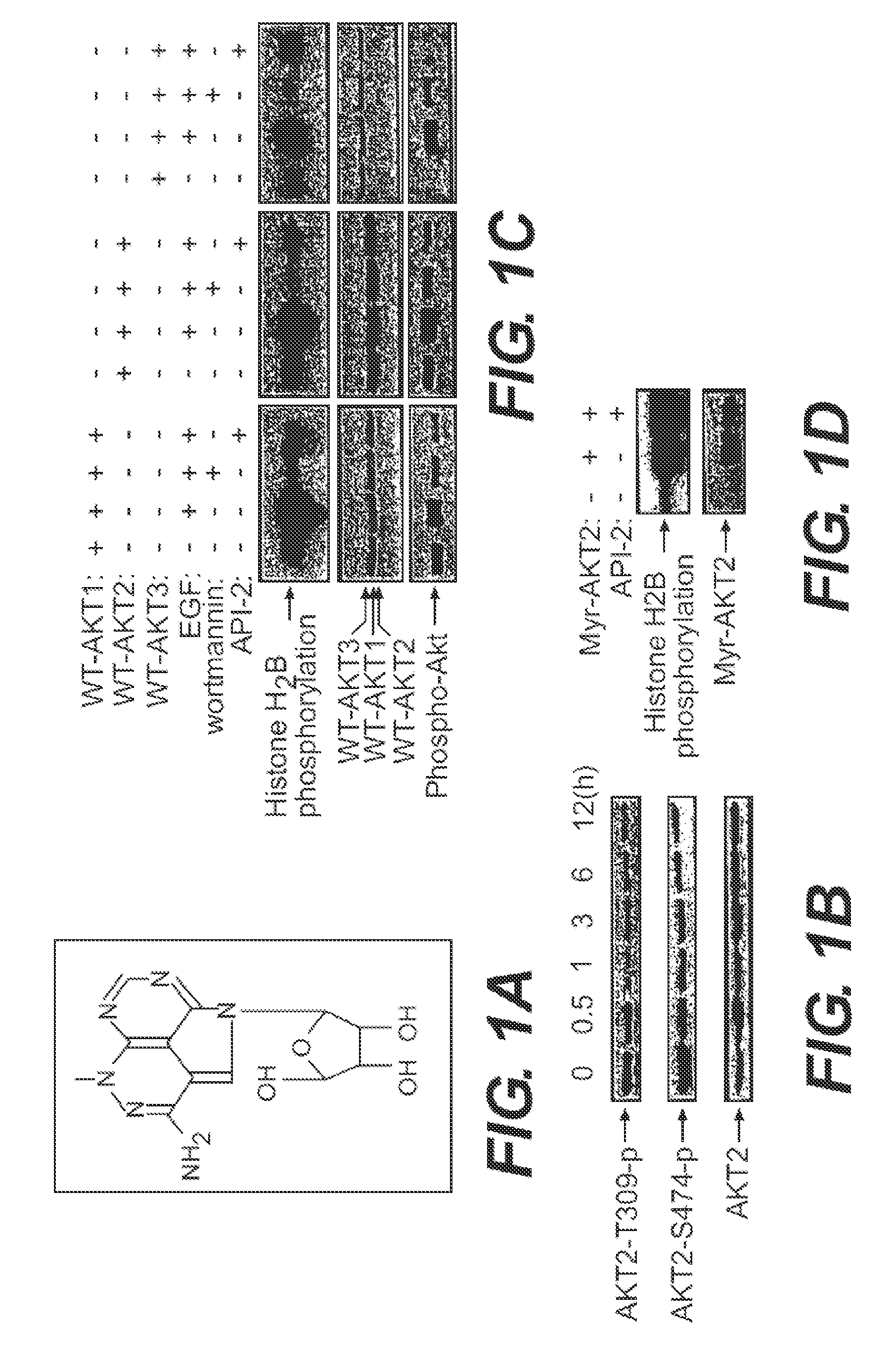
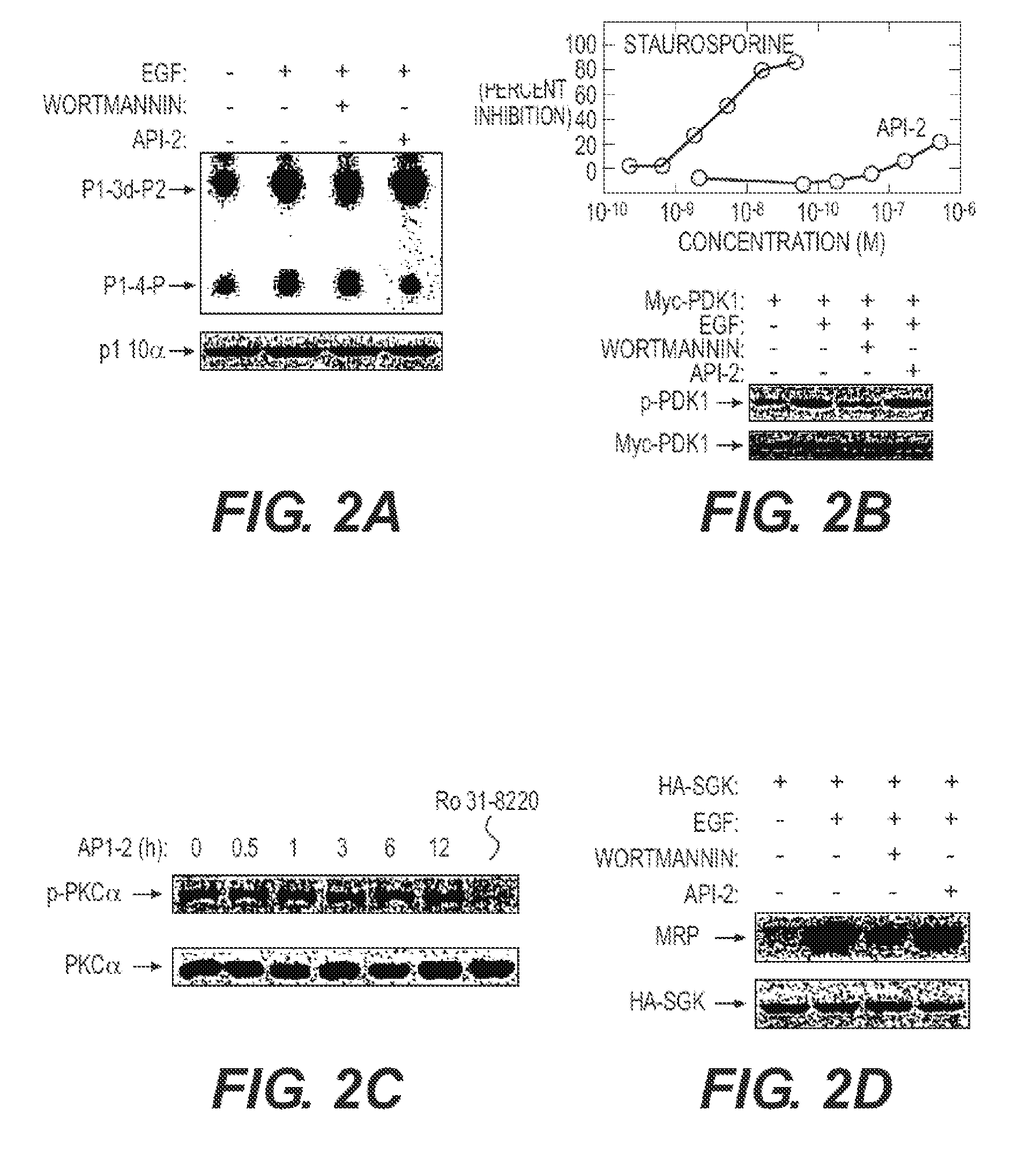
ActiveUS20120225851A1Blocking unwanted cell survival activityBiocideOrganic chemistryDiseaseAutoimmune disease
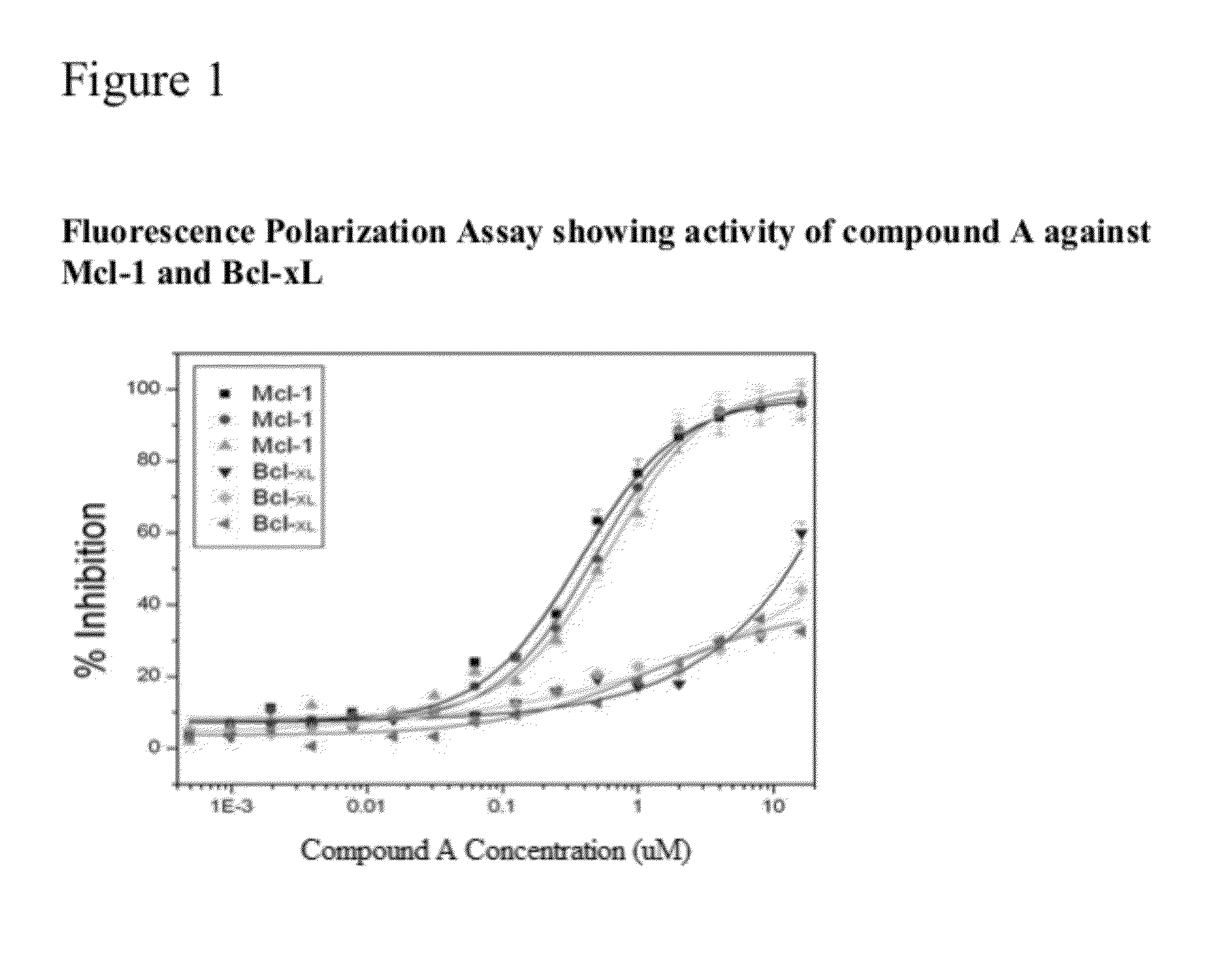
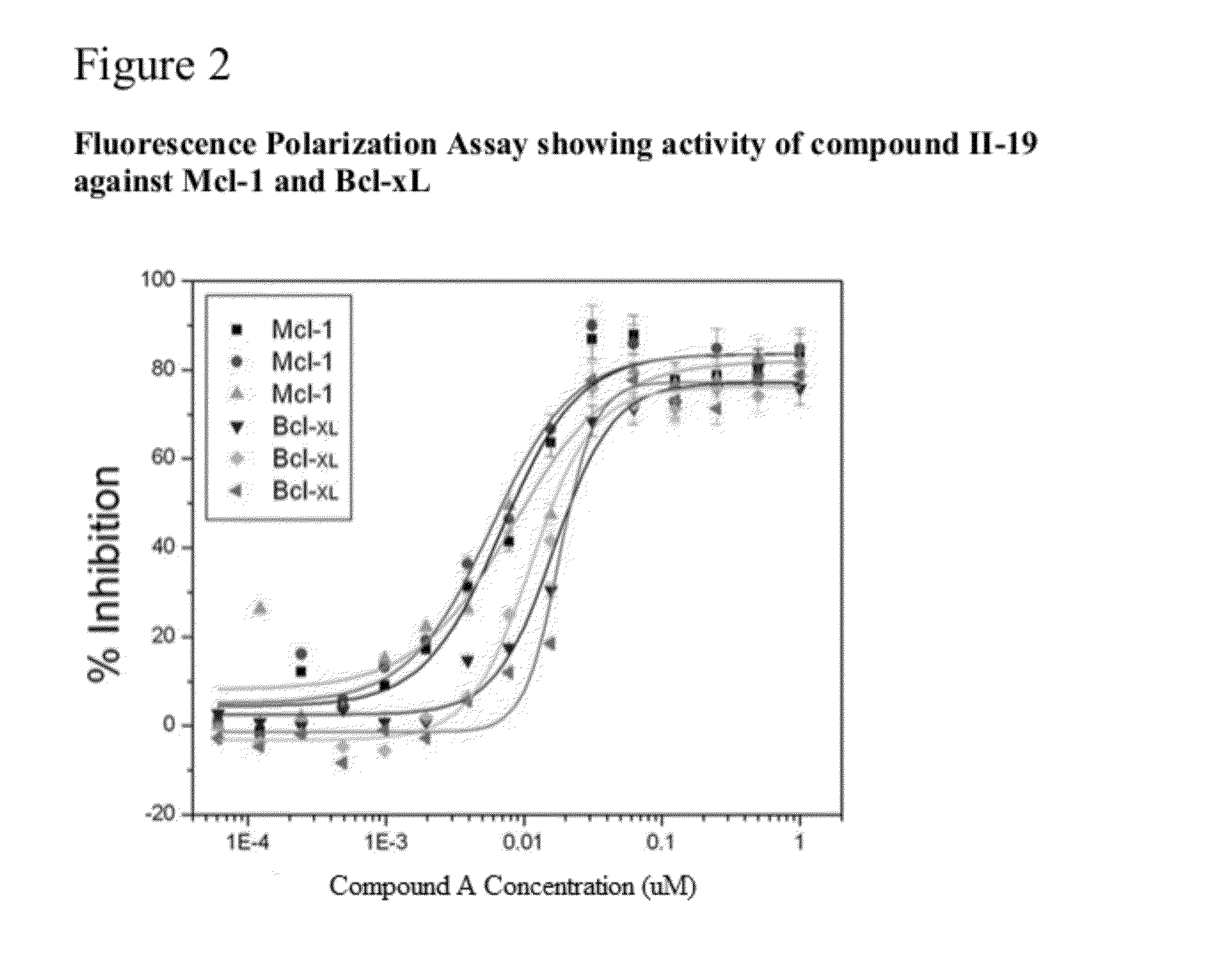
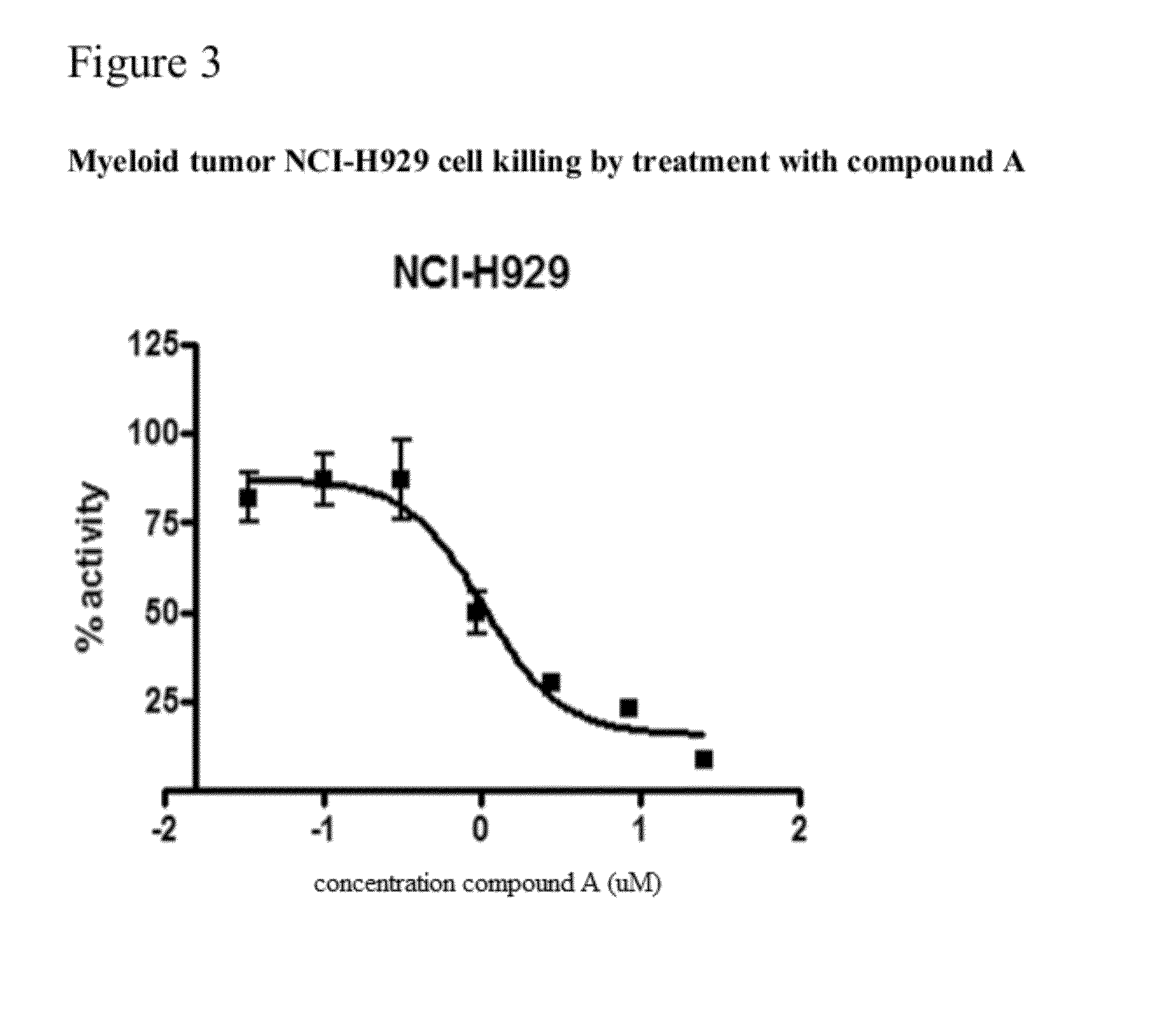
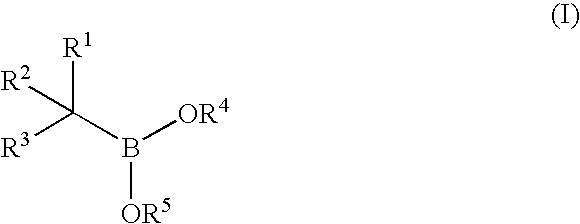
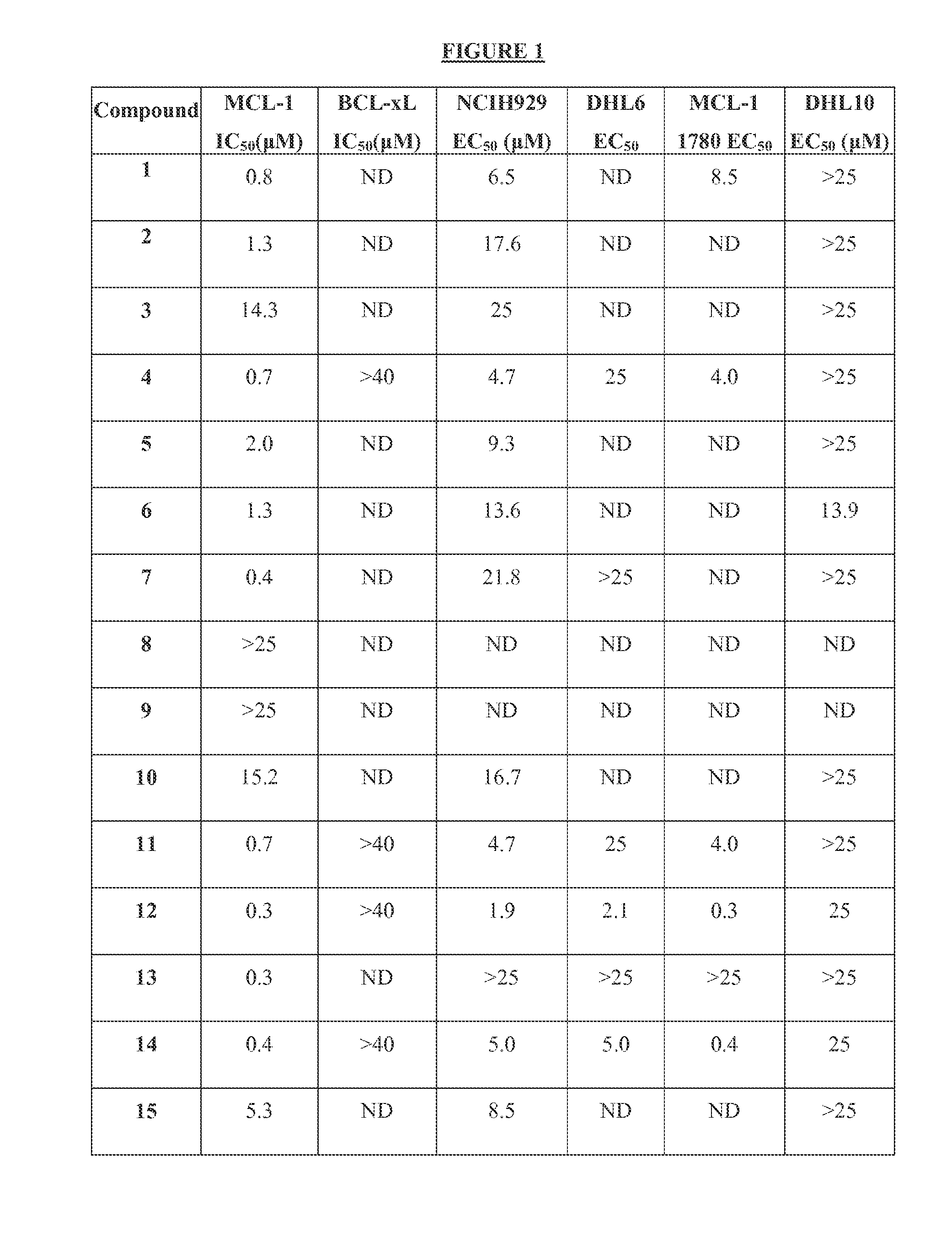
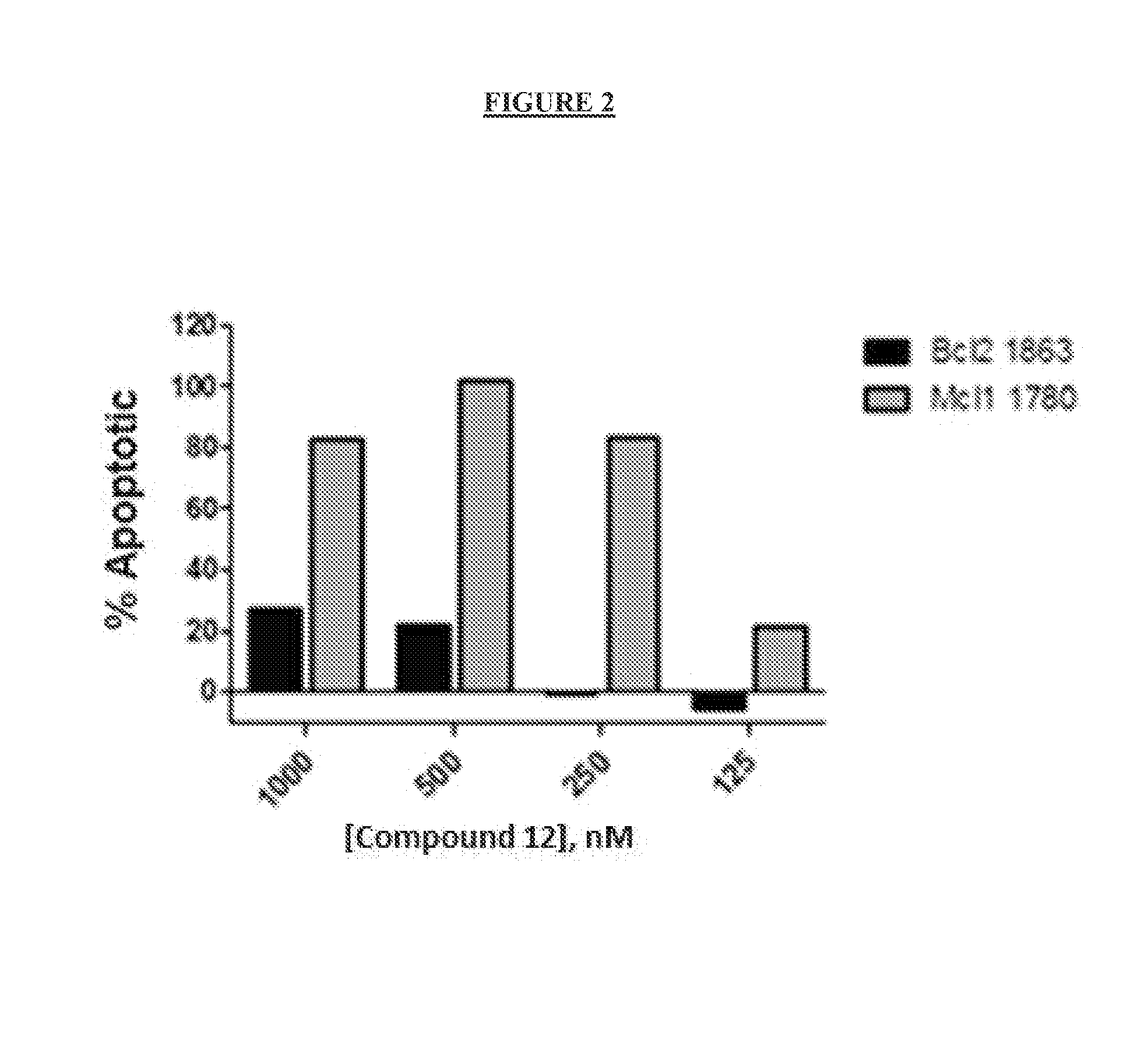
The present invention relates to a chemotherapeutic cancer treatment in which compounds of Formula Ia′, Ib′, Ic′, or II′ (referred to as a group as BH3Is) are administered to a mammal for the treatment of B-cell Lymphoma or other hematopoietic cancers, including diseases associated with MCL-1. In another aspect, the invention provides a method for treating particular types of hematopoietic cancers, such as B-cell lymphoma, using a combination of one or more compounds selected from the group consisting of compounds or Formula Ia, Ib, Ic, or II in combination with other therapies, for example, a class of therapeutics known as 26S proteosome inhibitors, such as, for example, Bortezomib. In another aspect the present invention relates to autoimmune treatment with pharmaceutical compositions comprising one or more compounds of Formula Ia′, Ib′, Ic′, or II′. In another aspect, this invention relates to methods for identifying compounds, for example, compounds of the BH3 mimic class, that have unique in vitro properties that predict in vivo efficacy against B-cell lymphoma tumors and other cancers as well as autoimmune disease.
Owner:EUTROPICS PHARMA
Compositions and methods for treating neoplastic diseases
InactiveUS20070225350A1Increased apoptosisReduce cell viabilityBiocideBoron compound active ingredientsDiseaseCancer research
Disclosed herein are compositions and methods for treating neoplastic diseases. Included are compositions and methods that are effective against multiple myeloma cells resistant to conventional and bortezomib treatment. Furthermore, combination treatment with two different proteosome inhibitors is shown to be synergistic for treating multiple myeloma.
Owner:TRIPHASE RES & DEV I +1
Synthesis of boronic ester and acid compounds
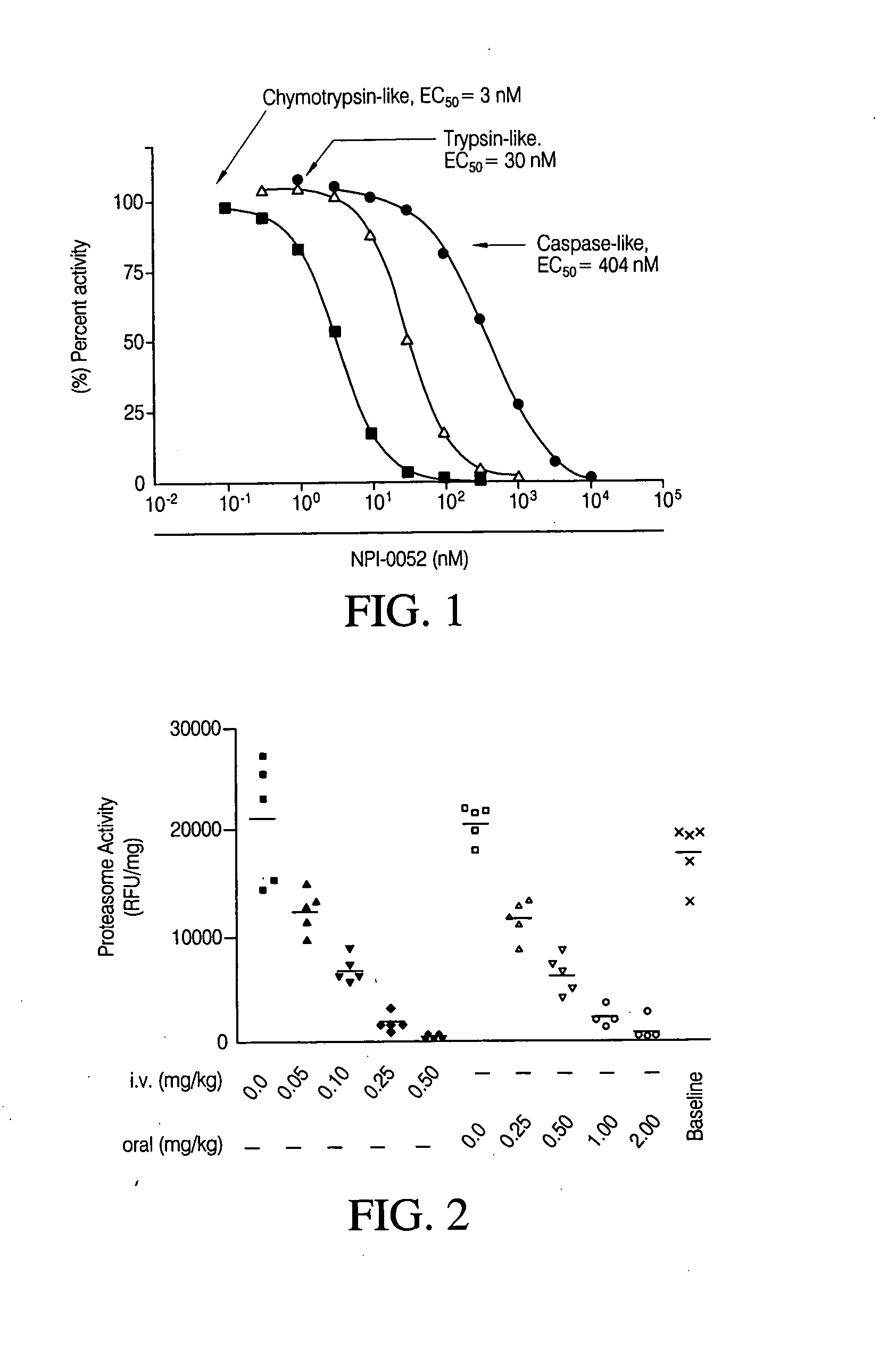
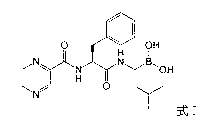
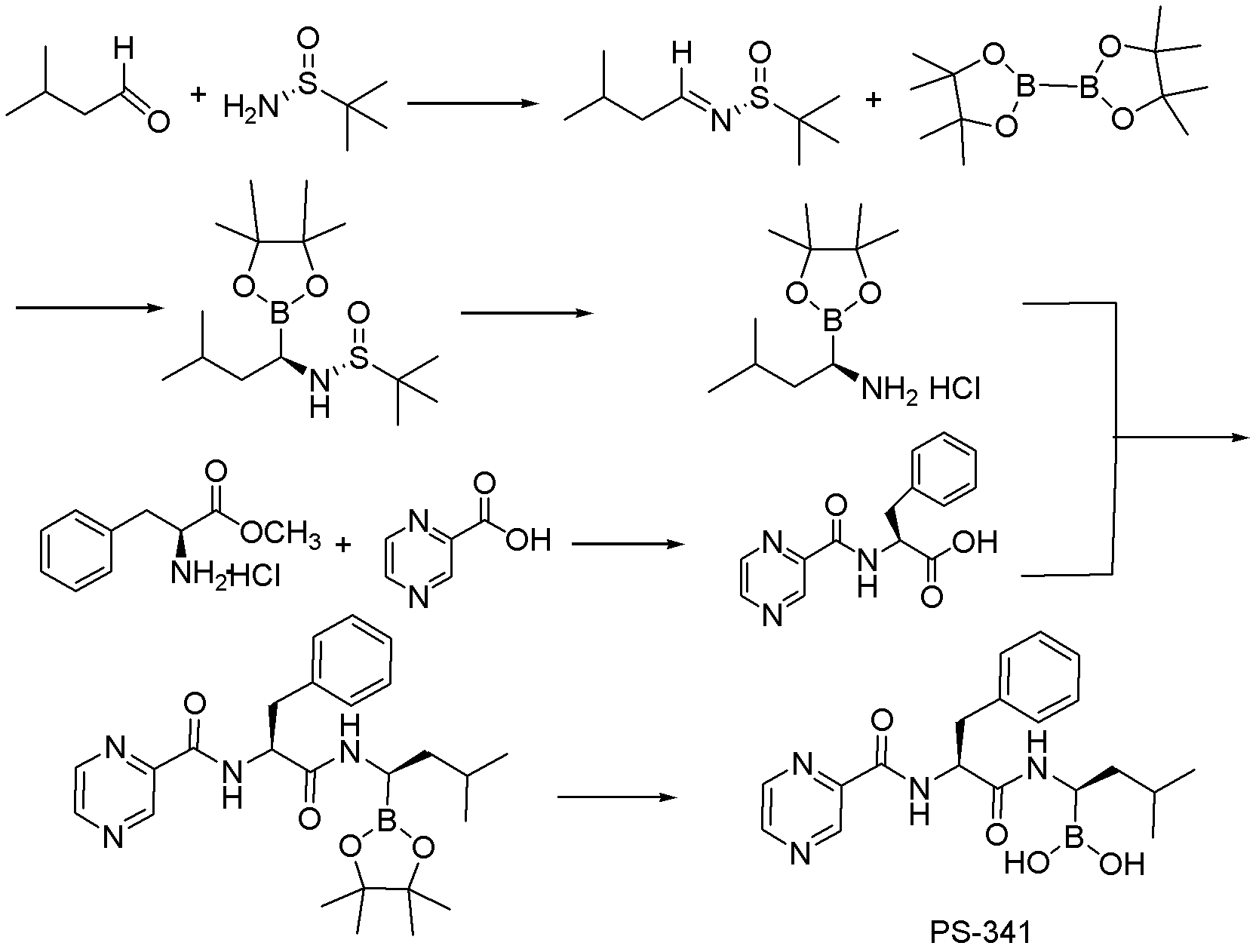
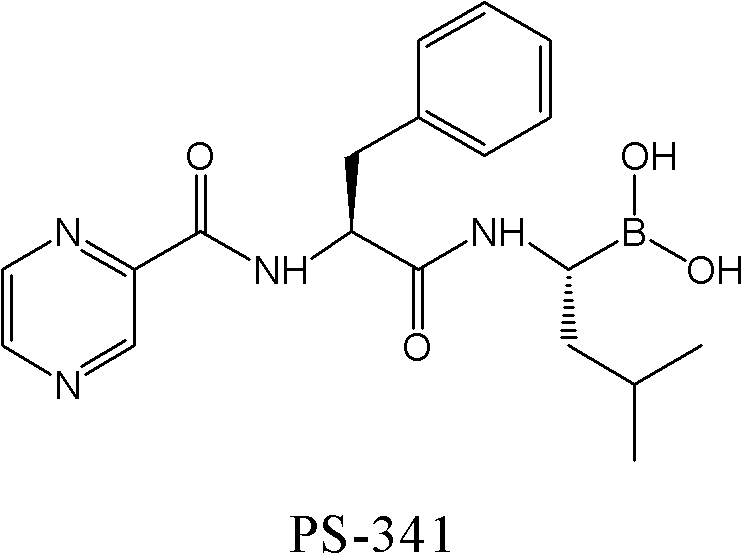
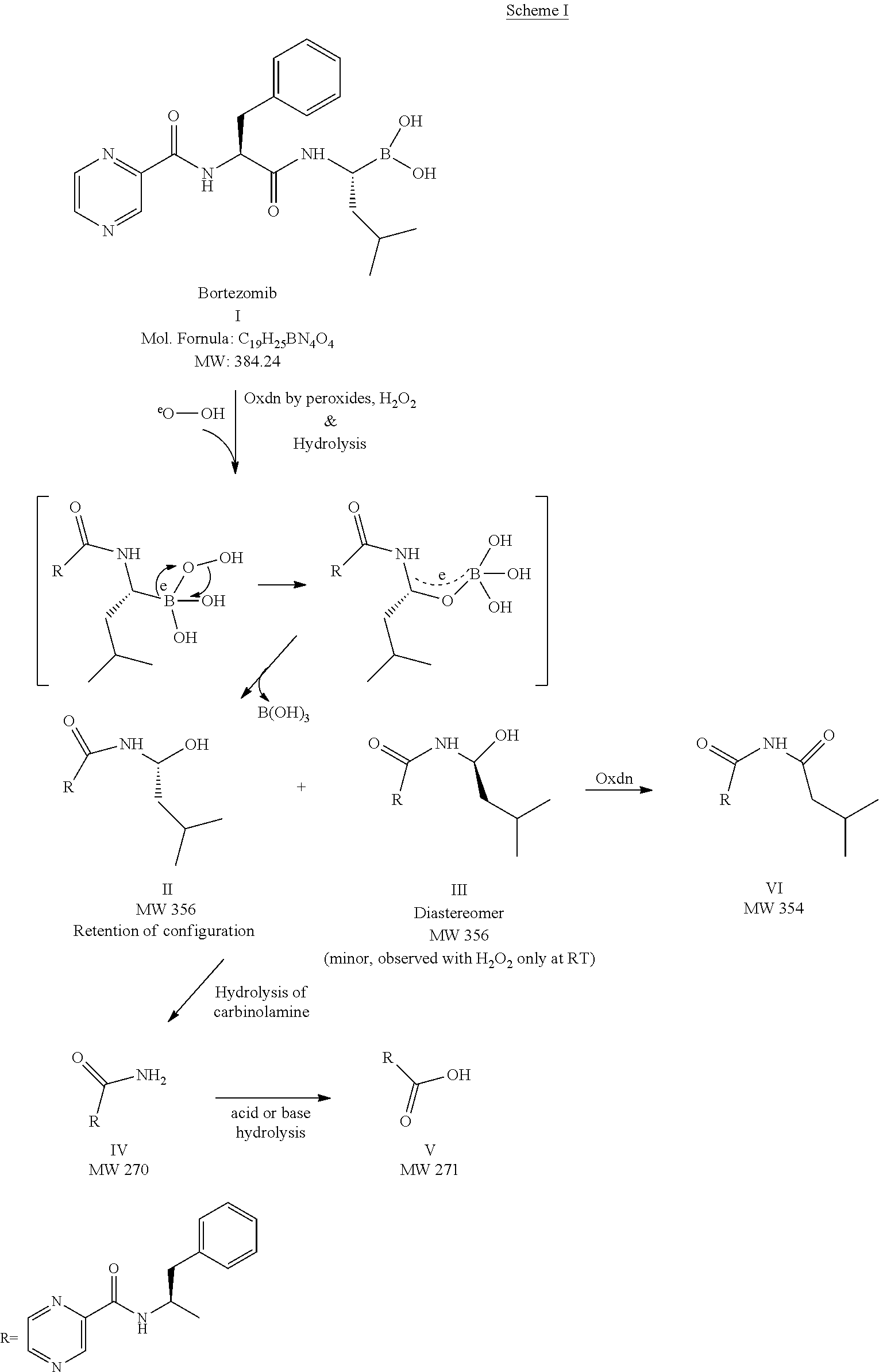
The invention relates to the synthesis of boronic ester and acid compounds. More particularly, the invention provides improved synthetic processes for the large-scale production of boronic ester and acid compounds, including the peptide boronic acid proteasome inhibitor bortezomib.
Owner:MILLENNIUM PHARMA INC
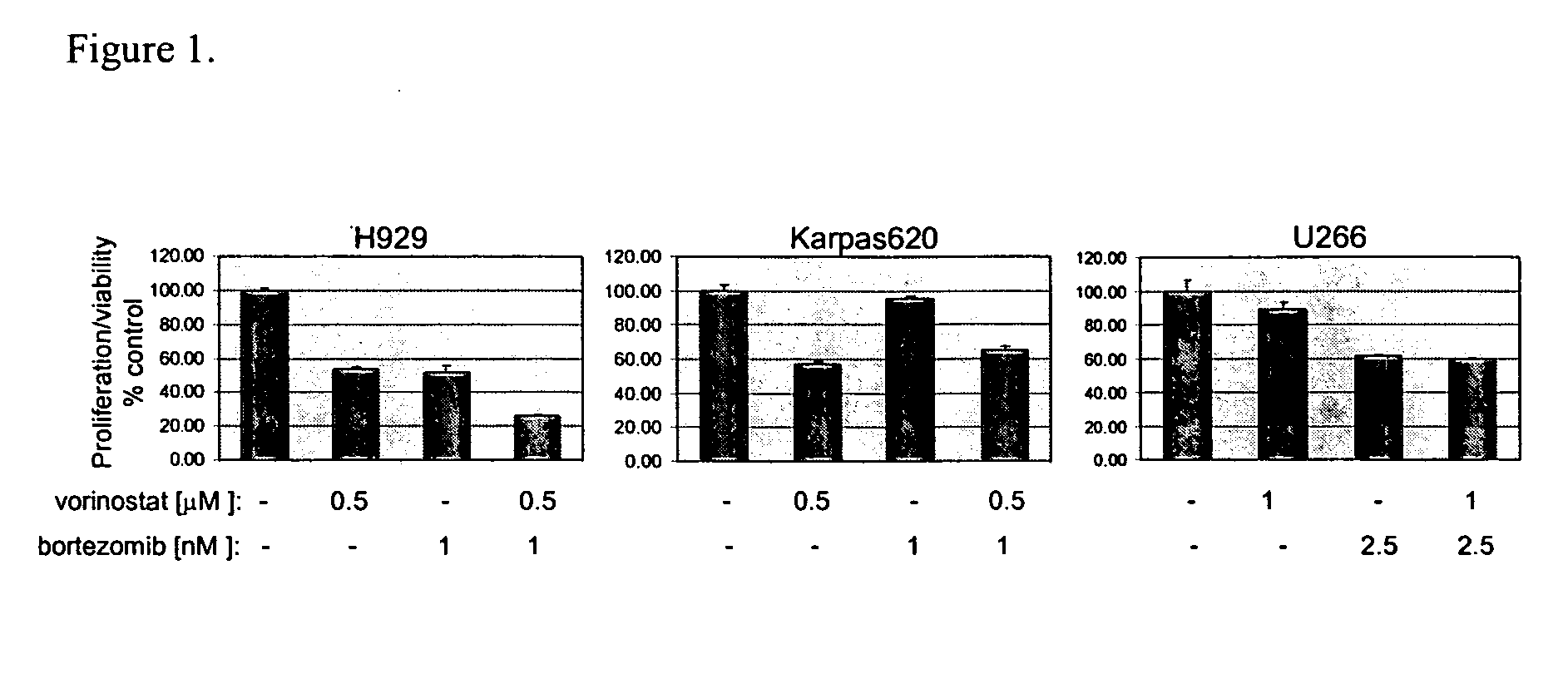
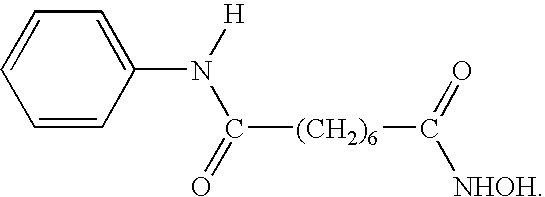
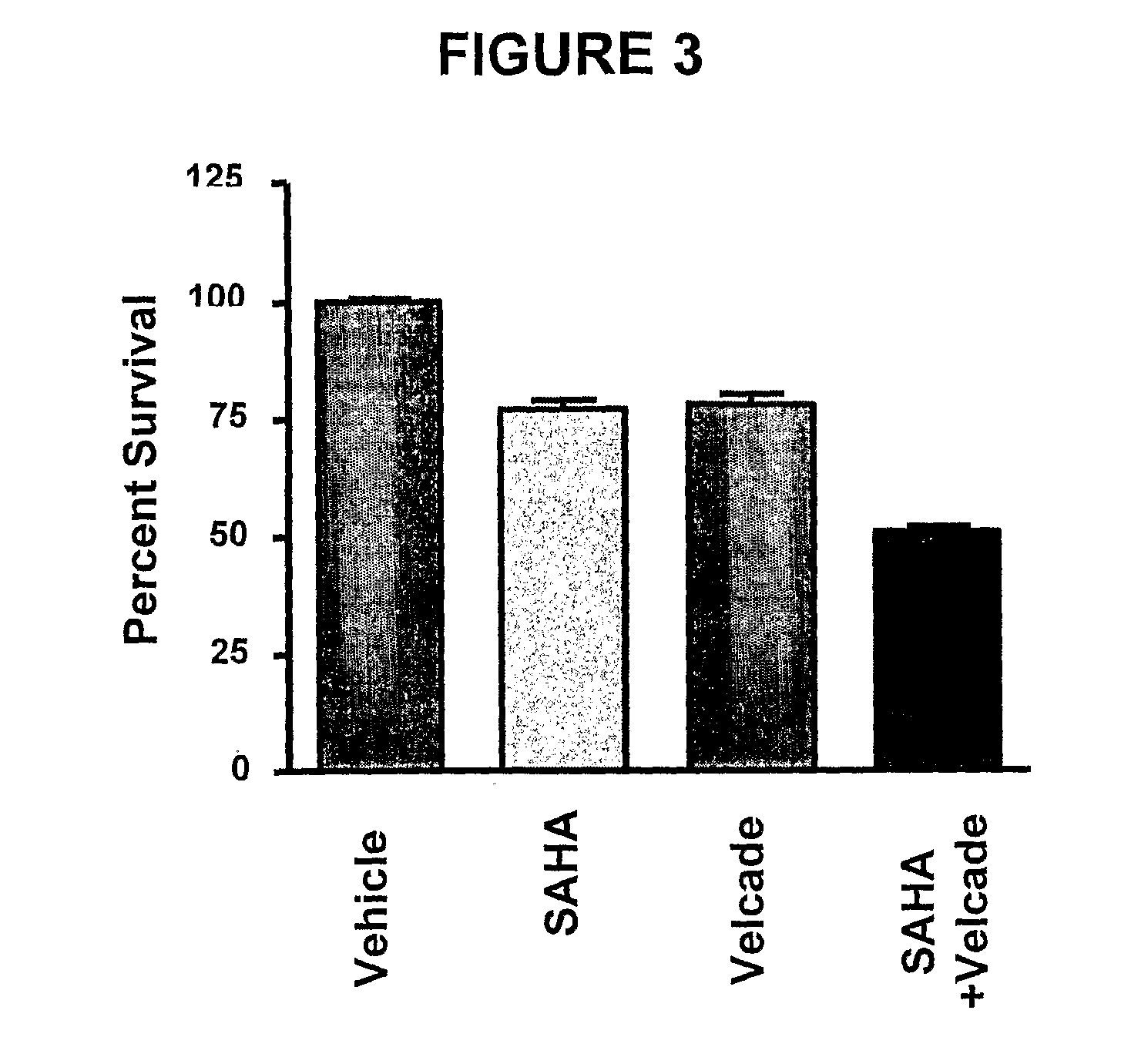
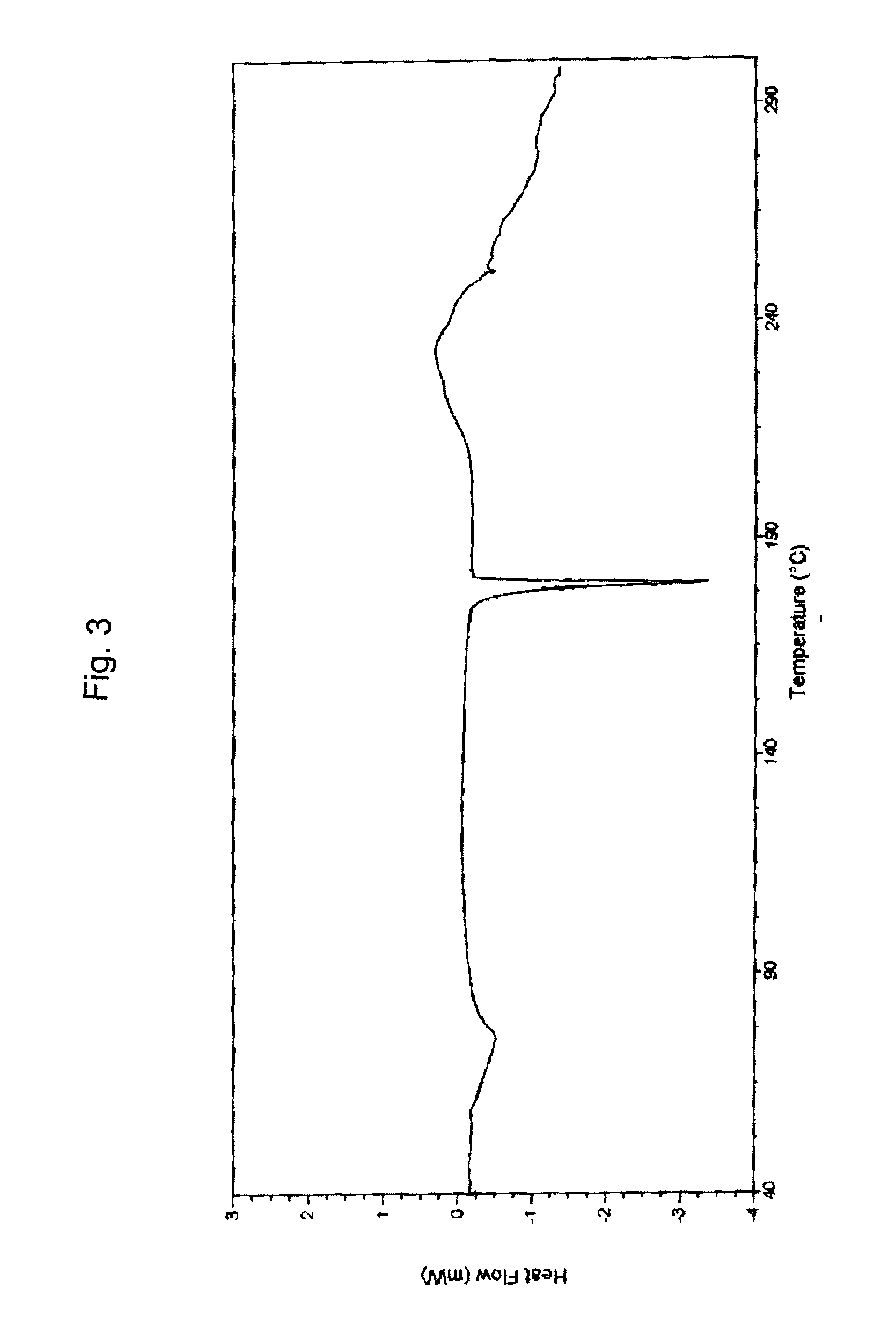
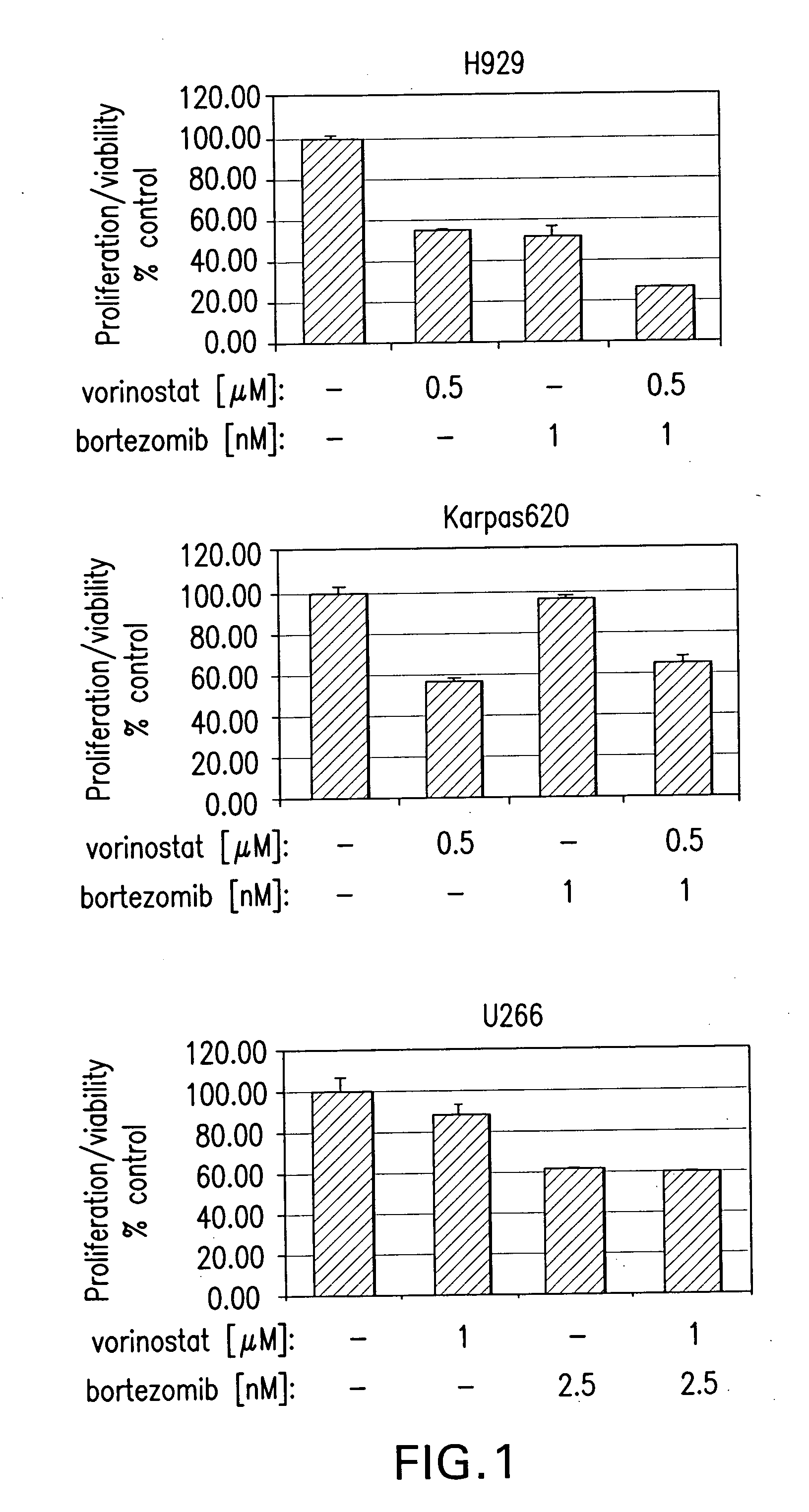
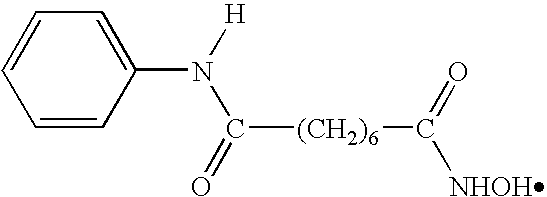
Methods of using SAHA and Bortezomib for treating cancer
The present invention relates to a method of treating cancer in a subject in need thereof, by administering to a subject in need thereof a first amount of a histone deacetylase (HDAC) inhibitor such as suberoylanilide hydroxamic acid (SAHA), or a pharmaceutically acceptable salt or hydrate thereof, and a second amount of one or more anti-cancer agents, including Bortezomib. The HDAC inhibitor and the anti-cancer agent may be administered to comprise therapeutically effective amounts. In various aspects, the effect of the HDAC inhibitor and the anti-cancer agent may be additive or synergistic.
Owner:MERCK & CO INC
Liposome formulations of boronic acid compounds
A liposome composition comprised of liposomes having the peptide boronic acid proteasome inhibitor compound bortezomib entrapped in the liposomes is described. The boronic acid compound is entrapped in the liposomes in the form of a boronate ester, subsequent to interaction with a liposome-entrapped polyol. In one embodiment, the liposomes have an outer coating of hydrophilic polymer chains and are used to treat a solid tumor in a subject.
Owner:ALZA CORP
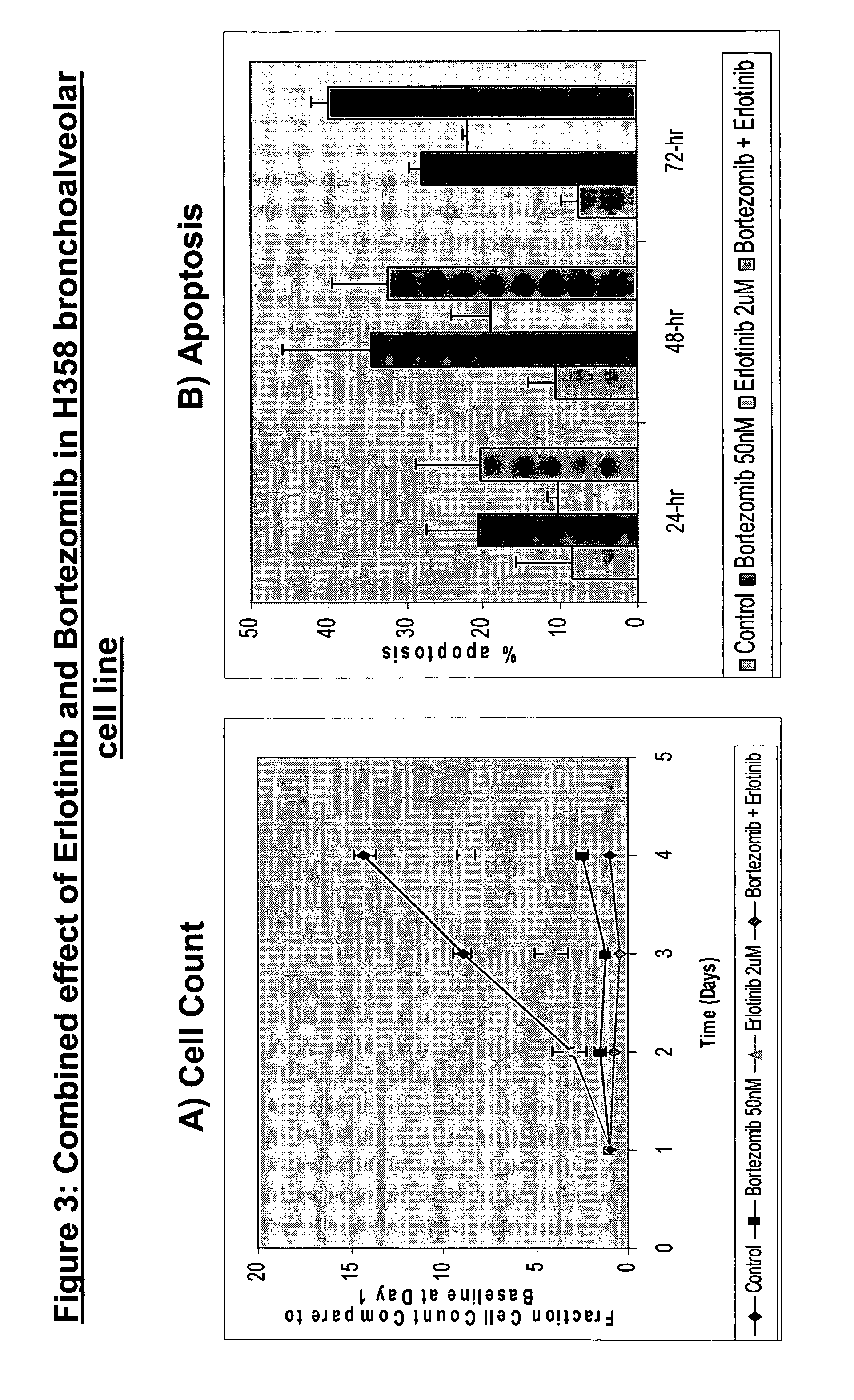
Combined treatment with bortezomib and an epidermal growth factor receptor kinase inhibitor
InactiveUS20060084691A1BiocideBoron compound active ingredientsAbnormal tissue growthEpidermal Growth Factor Receptor Kinase
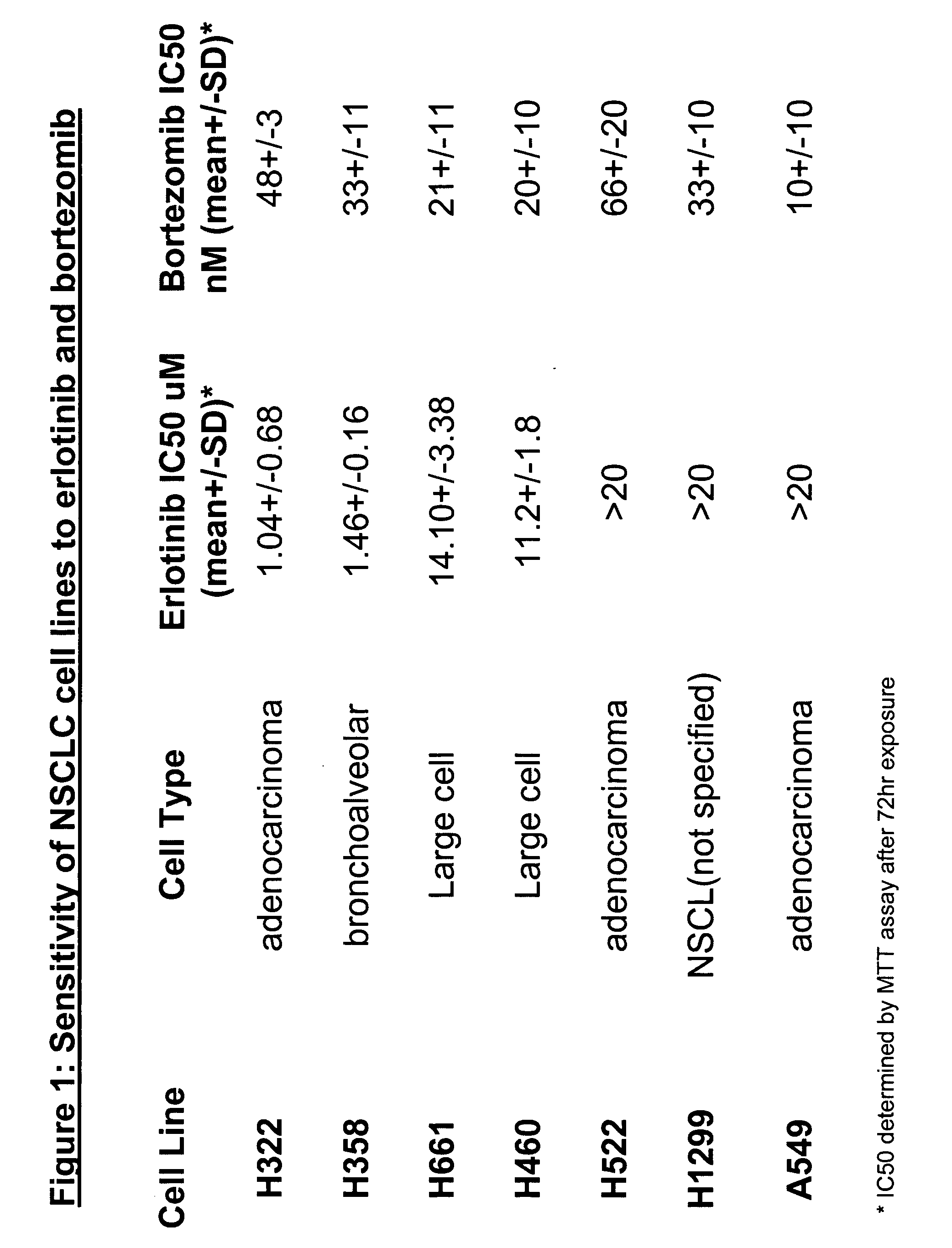
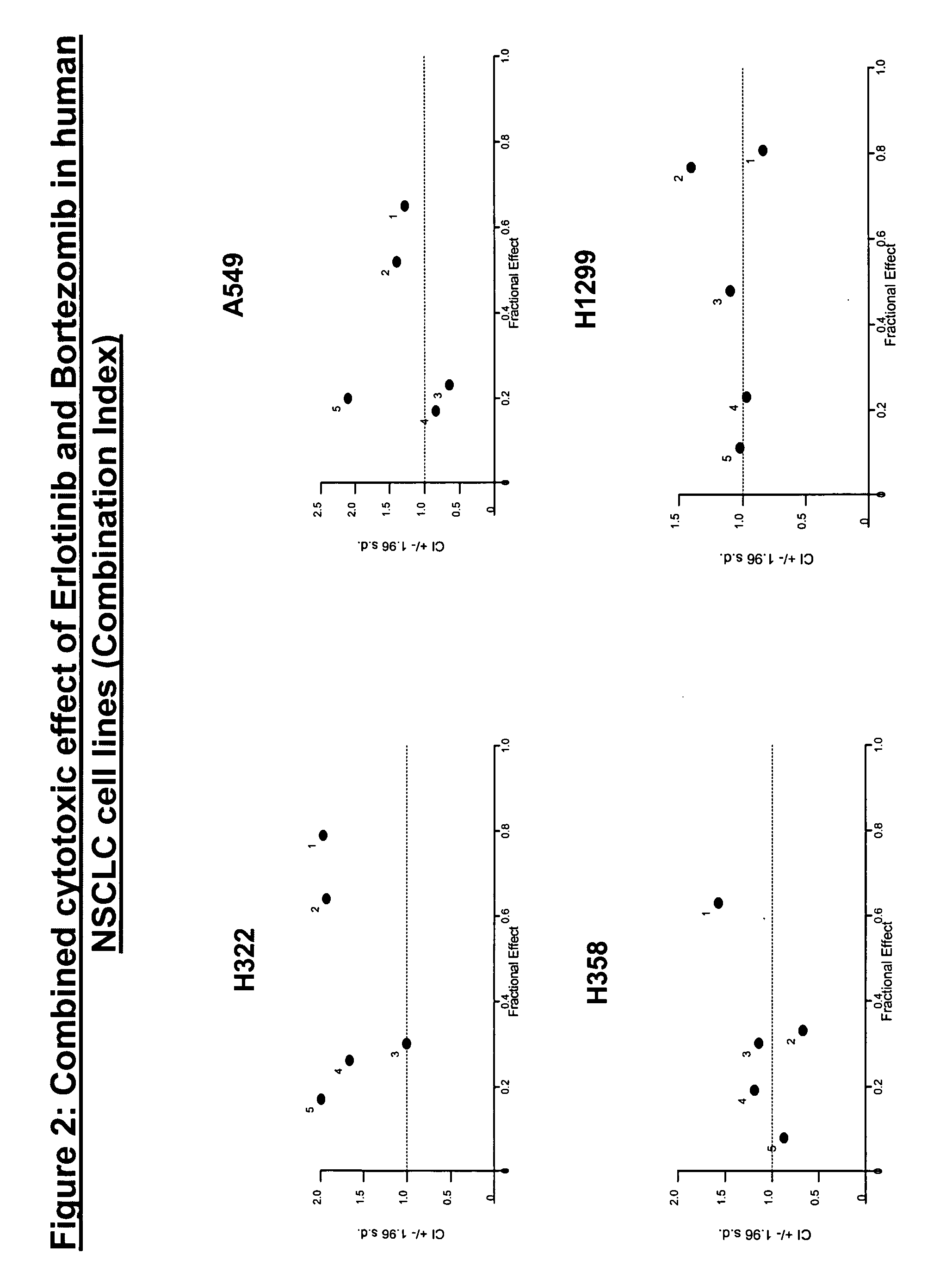
The present invention provides a method for treating tumors or tumor metastases in a patient, comprising administering to the patient simultaneously or sequentially a therapeutically effective amount of an EGFR kinase inhibitor and bortezomib combination, with or without additional agents or treatments, such as other anti-cancer drugs or radiation therapy. The invention also encompasses a pharmaceutical composition that is comprised of an EGFR kinase inhibitor and bortezomib combination in combination with a pharmaceutically acceptable carrier. A preferred example of an EGFR kinase inhibitor that can be used in practicing this invention is the compound erlitinib HCl (also known as Tarceva™).
Owner:PIPERDI BILAL
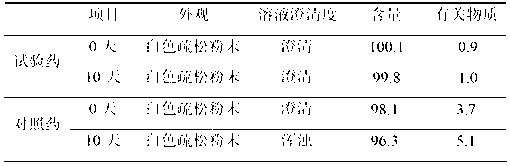
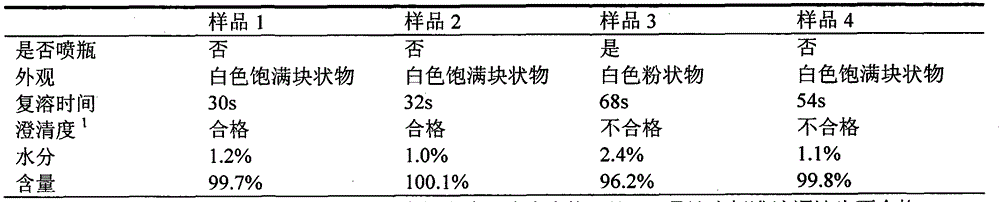
Freeze-dried composition containing bortezomib and preparation method of freeze-dried composition
ActiveCN103070835AReduce preparation timeEasy to producePowder deliveryDipeptide ingredientsMANNITOL/SORBITOLFreeze-drying
The invention belongs to the technical field of medicine, in particular to the field of chemical pharmacy, and particularly relates to a freeze-dried composition containing bortezomib and a preparation method of the freeze-dried composition. The freeze-dried composition and the preparation method aim at overcoming main medicine dissolution difficulty and oxygen environment sensitivity in an environment. A mixed solvent comprising mannitol and tert butyl alcohol is used, so that a dissolution rate of bortezomib is increased significantly, and the dissolution rate of bortezomib can be increased further through an addition sequence of materials. A nitrogen filled environment is used, so that liquid preparation time is shortened greatly, the contact of main medicine components with an aerobic environment is avoided effectively, and contents of relevant substances and total impurities in a final finished product are reduced.
Owner:JIANGSU AOSAIKANG PHARMA CO LTD
Synthetic method of proteasome inhibitor bortezomib and analogs
ActiveCN103030656ALower synthesis costEasy to manufactureGroup 3/13 element organic compoundsProteasome inhibitorChemistry
Owner:PEKING UNIV
Compositions including triciribine and bortezomib and derivatives thereof and methods of use thereof
InactiveUS20100009929A1Reduce systemic toxicityMinimize ToxicityBiocideDipeptide ingredientsTriciribineBortezomib
This application relates to combination therapies including triciribine and related compounds and bortezomib and derivatives thereof analogs and compositions with reduced toxicity for the treatment and prevention of tumors, cancer, and other disorders associated with abnormal cell proliferation.
Owner:UNIV OF SOUTH FLORIDA
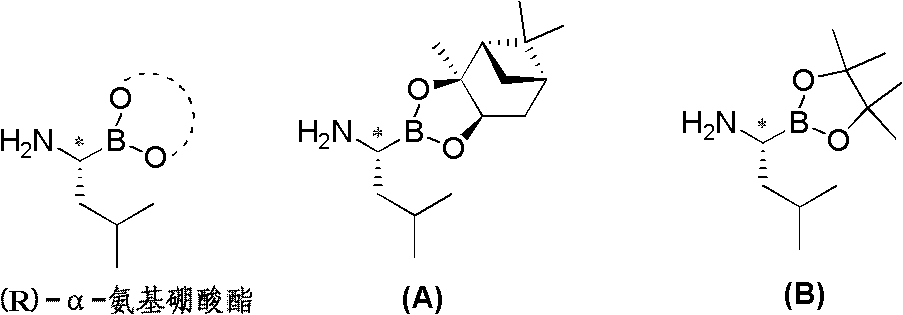
Chiral alpha-amino boric acid esters, a preparation method and an application in the synthesis of bortezomib thereof
InactiveCN103204867AIncrease manufacturing costStable in natureGroup 3/13 element organic compoundsAsymmetric synthesesBoric acidBortezomib
Owner:CHENGDU AIQUN TECH
Medicinal composition for treating non-small cell lung cancer and application thereof
InactiveCN103948689AReduce dosageLow toxicityAntineoplastic agentsHeavy metal compound active ingredientsSalvia miltiorrhizaCarboplatin
The invention discloses a medicinal composition for treating non-small cell lung cancer. The medicinal composition comprises a target medicament, a chemotherapeutic medicament and a traditional Chinese medicament, wherein the target medicament is one or more of bortezomib, imatinib, gefitinib and sunitinib; the chemotherapeutic medicament is one or more of 5-fluorouracil, carboplatin, epirubicin, adriamycin and fludarabine; and the traditional Chinese medicament is one or more of salvia miltiorrhiza, astragalus membranaceus, sappanwood, Chinese pulsatilla root and portulaca oleracea. The medicinal composition has a remarkable synergetic treatment effect when being used for treating the non-small cell lung cancer, and can be used for remarkably strengthening the cancer-inhibition effect compared with treatment of a single medicament, so that the medicament dosage can be reduced, and the toxic and side effects of chemotherapeutic medicaments can be reduced.
Owner:NORTHWEST A & F UNIV
Synthesis of boronic ester and acid compounds
The invention relates to the synthesis of boronic ester and acid compounds. More particularly, the invention provides improved synthetic processes for the large-scale production of boronic ester and acid compounds, including the peptide boronic acid proteasome inhibitor bortezomib.
Owner:MILLENNIUM PHARMA INC
Methods and compositions useful for treating diseases involving bcl-2 family proteins with quinoline derivatives
The present invention relates to compositions and methods for cancer treatment comprising compounds of Formulae I, II, and III. In some aspects, the invention relates to the treatment of B-cell Lymphoma or other hematopoietic cancers. In other aspects, the invention provides methods for treating particular types of hematopoietic cancers, such as, for example, B-cell lymphoma, using a combination of one or more compounds of Formulae I, II, and III. Combination therapy with, for example, 26S proteasome inhibitors, such as, for example, Bortezomib, are also included. In another aspect the present invention relates to autoimmune treatment with compounds of Formulae I, II, and III. In another aspect, this invention relates to methods for identifying compounds, for example, compounds of the BH3 mimic class, that have in vitro properties that predict in vivo efficacy against B-cell lymphoma tumors and other cancers as well as autoimmune disease.
Owner:EUTROPICS PHARMA
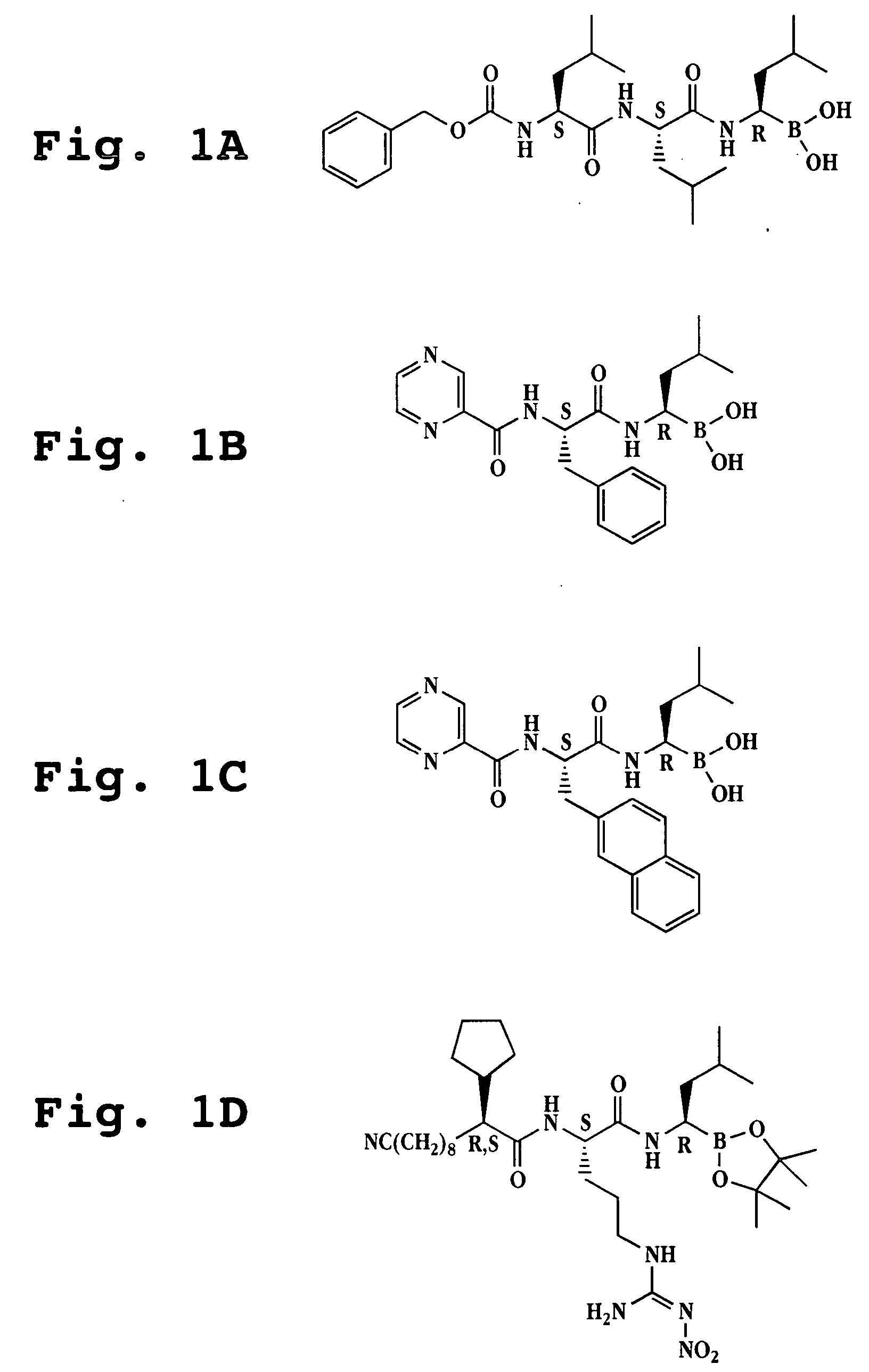
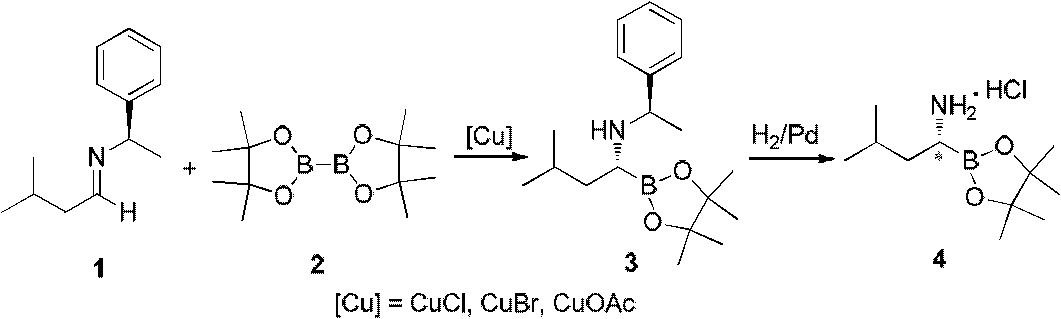
Method for synthesizing bortezomib
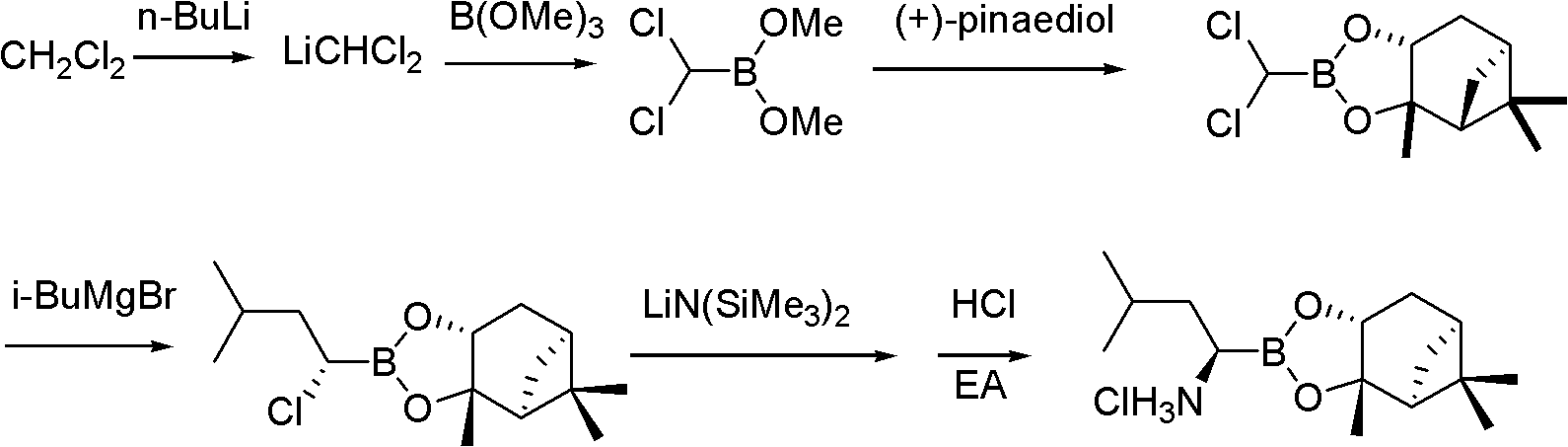
The invention belongs to the field of synthesizing medicaments, and discloses a method for synthesizing bortezomib. In the method, 3-methyl butyraldehyde and R-(+)-1-phenylethylamine are used as initiative materials, and the [(1R)-3-methyl-1-[[(2S)-1-oxygen-3-phenyl-2[(pyrazine formyl) amino] propyl]amino]butyl]-boric acid is obtained by condensation, selective boric acid ester addition, hydrogenation deprotection, chiral condensation with L-phenylalanine, condensation with 2-carboxyl-piperazine and boric acidification. The synthesis method has the advantages of readily available raw materials, higher yield of the whole reaction route, mild reaction conditions, easy operation, lower production cost and the suitability for industrialized production.
Owner:CHANGZHOU YABANG PHARMA
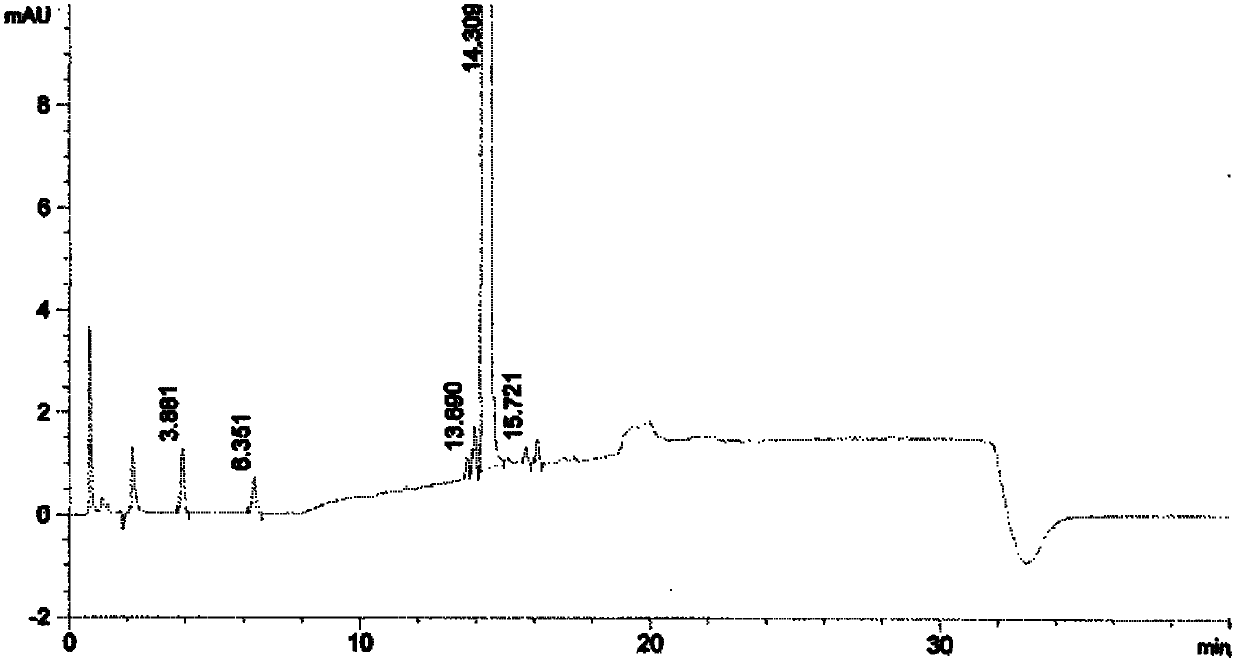
Synthetic method of high-purity bortezomib and intermediate thereof
The invention belongs to the pharmaceutical and chemical fields and particularly relates to a synthetic method of high-purity bortezomib and an intermediate of the high-purity bortezomib. According to the invention, N-(2- pyrazine carbonyl)-L-phenylalanine benzyl ester is obtained from condensation reaction between 2-pyrazine carboxylic acid and L-phenylalanine benzyl ester; then the product is catalyzed and hydrogenated; and then the product is condensed and hydrolyzed with the hydrochloride of (aR,3aS,4S,6S,7aR)-hexahydro-3a,8,8-trimethyl-alpha-(2-methyl propyl)-4,6-methano-1,3,2- benzodioxoborane-2-methylamine or trifluoroacetate so that the bortezomib is obtained. The preparation process disclosed by the invention has the advantages of simplicity in operation, high purity and low cost. The bortezomib obtained through the method disclosed by the invention is in the form of white powder or crystals, the content is 99.8% or higher, and the total content of SS- and RR-isomers is not greater than 0.1%.
Owner:JIANGSU AOSAIKANG PHARMA CO LTD
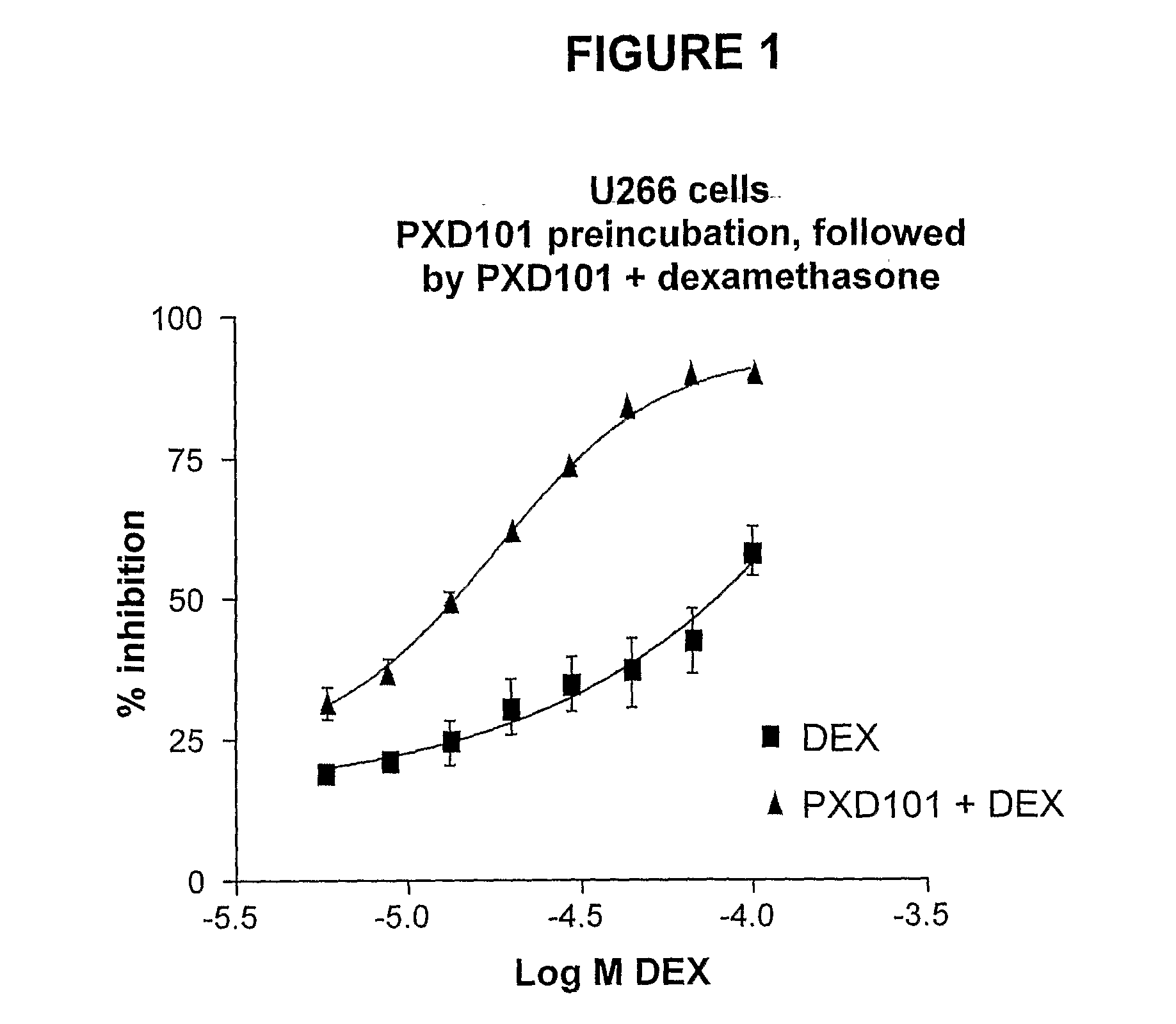
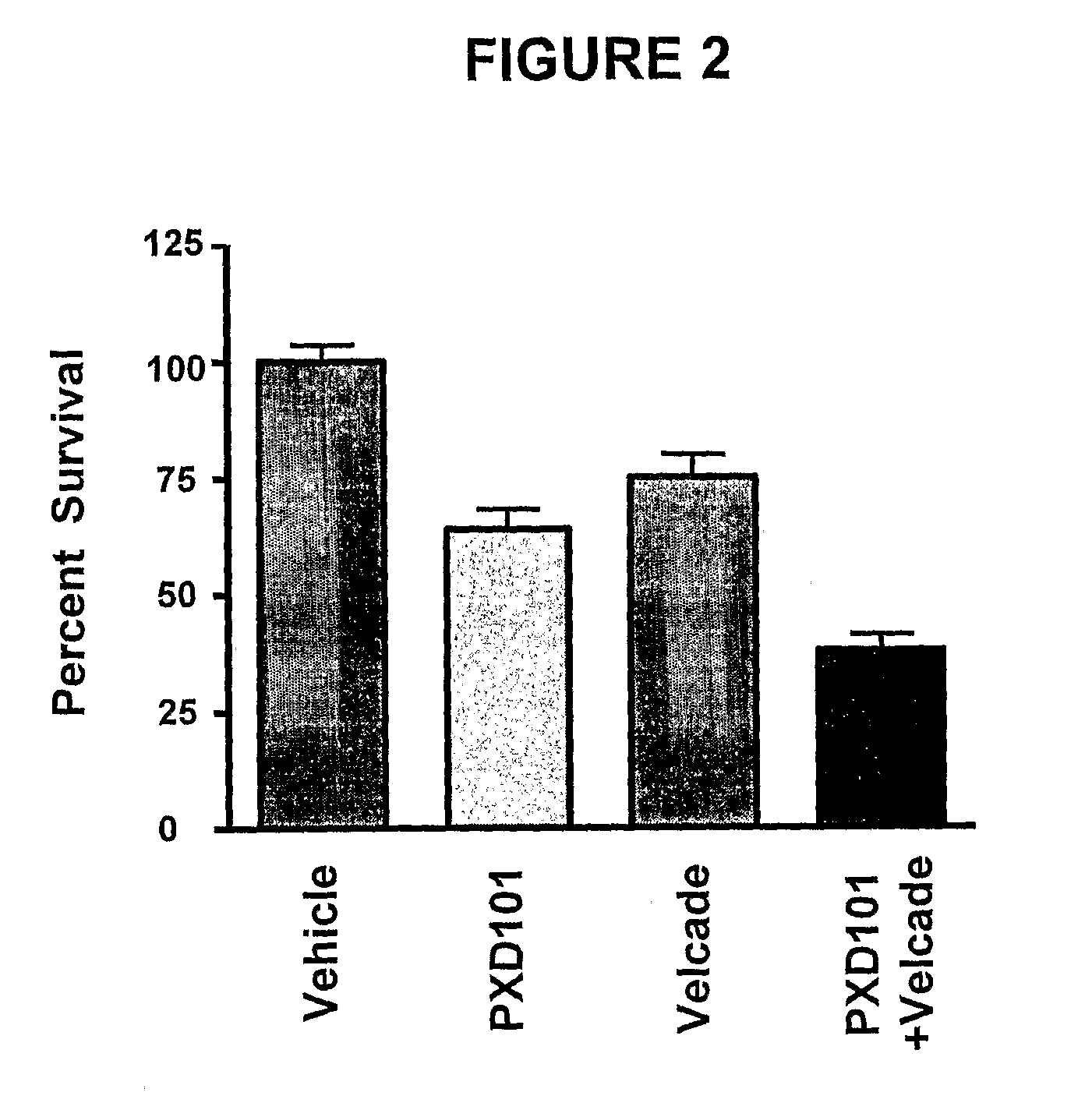
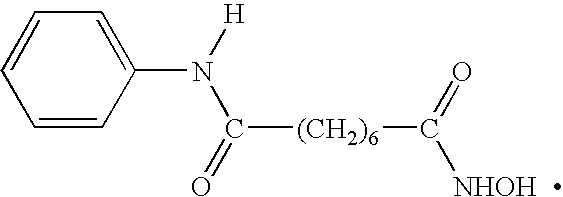
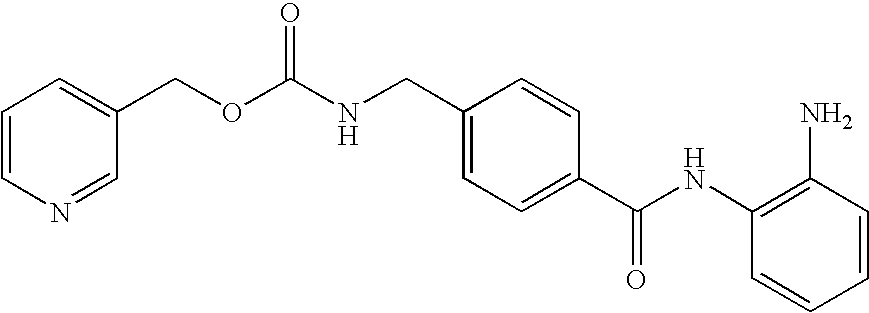
Histone Deacetylase (Hdac) Inhibitors (Pxd101) for the Treatment of Cancer Alone or in Combination With Chemotherapeutic Agent
ActiveUS20080274120A1Reduced characteristicsReduced viabilityBiocidePeptide/protein ingredientsVincristineLeukemia
The present invention relates generally to methods for treating cancer. In one respect, the present invention relates to a method of treating a hematological cancer (e.g., multiple myeloma, leukemia, lymphoma) comprising administering to a patient in need thereof a therapeutically effective amount of a histone deacetylase inhibitor, for example, a histone deacetylase (HDAC) inhibitor as described herein, for example, PXD-101. In another respect, the present invention relates to a method of treating cancer (e.g., solid tumour cancer, e.g., rectal cancer, colon cancer, ovarian cancer; hematological cancer, e.g., multiple myeloma, leukemia, lymphoma) comprising administering to a patient in need thereof, a first amount of a histone deacetylase (HDAC) inhibitor, for example, a histone deacetylase inhibitor as described herein, for example, PXD-101, and a second amount of an other chemotherapeutic agent, for example, an other chemotherapeutic agent selected from: an antibody against VEGF, Avastin®, an antibody against CD20, rituximab, bortezomib, thalidomide, dexamethasone, vincristine, doxorubicin, and melphalan, wherein the first and second amounts together comprise a therapeutically effective amount.
Owner:TOPOTARGET UK LTD
Stable bortezomib formulations
Multi-dose formulations for bortezomib are presented in which bortezomib has significantly improved stability. Especially preferred formulations include those in which bortezomib is in a liquid form suitable for injection, wherein the solvent system predominantly comprises propylene glycol. In other preferred aspects, bortezomib is present as a Lewis donor-acceptor complex with a hetero-bifunctional Lewis base.
Owner:INNOPHARMA
Bortezomib pharmaceutic composition for injection
ActiveCN103142509AImprove solubilityImprove stabilityPowder deliveryDipeptide ingredientsPharmaceutical drugBiology
The invention provides a bortezomib pharmaceutic composition, and aims to solve the technical problem that the stability and dissolubility of the bortezomib are poor. The pharmaceutic composition contains bortezomib and amino acid in the mass ratio of 1:(2-100). By using the bortezomib pharmaceutic composition for injection, the dissolubility of the bortezomib can be effectively improved, and the effect is equivalent or superior to that of the products in the market. The bortezomib pharmaceutic composition is safe and non-irritant, the preparation cost is low, the preparation method is simple, and the stability is superior to that of the existing bortezomib preparation. The bortezomib pharmaceutic composition is favorable for industrial production.
Owner:CSPC ZHONGQI PHARM TECH (SHIJIAZHUANG) CO LTD +1
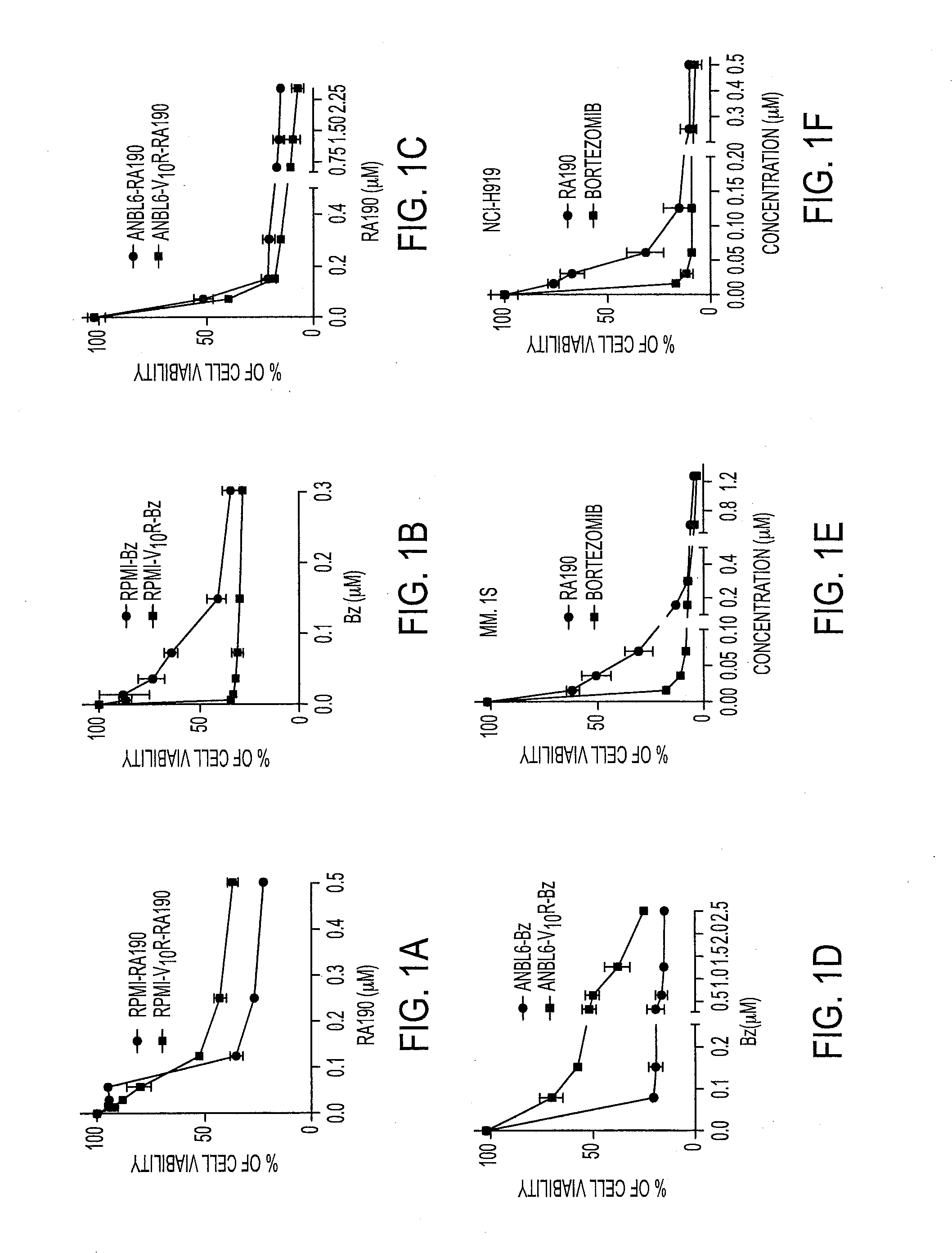
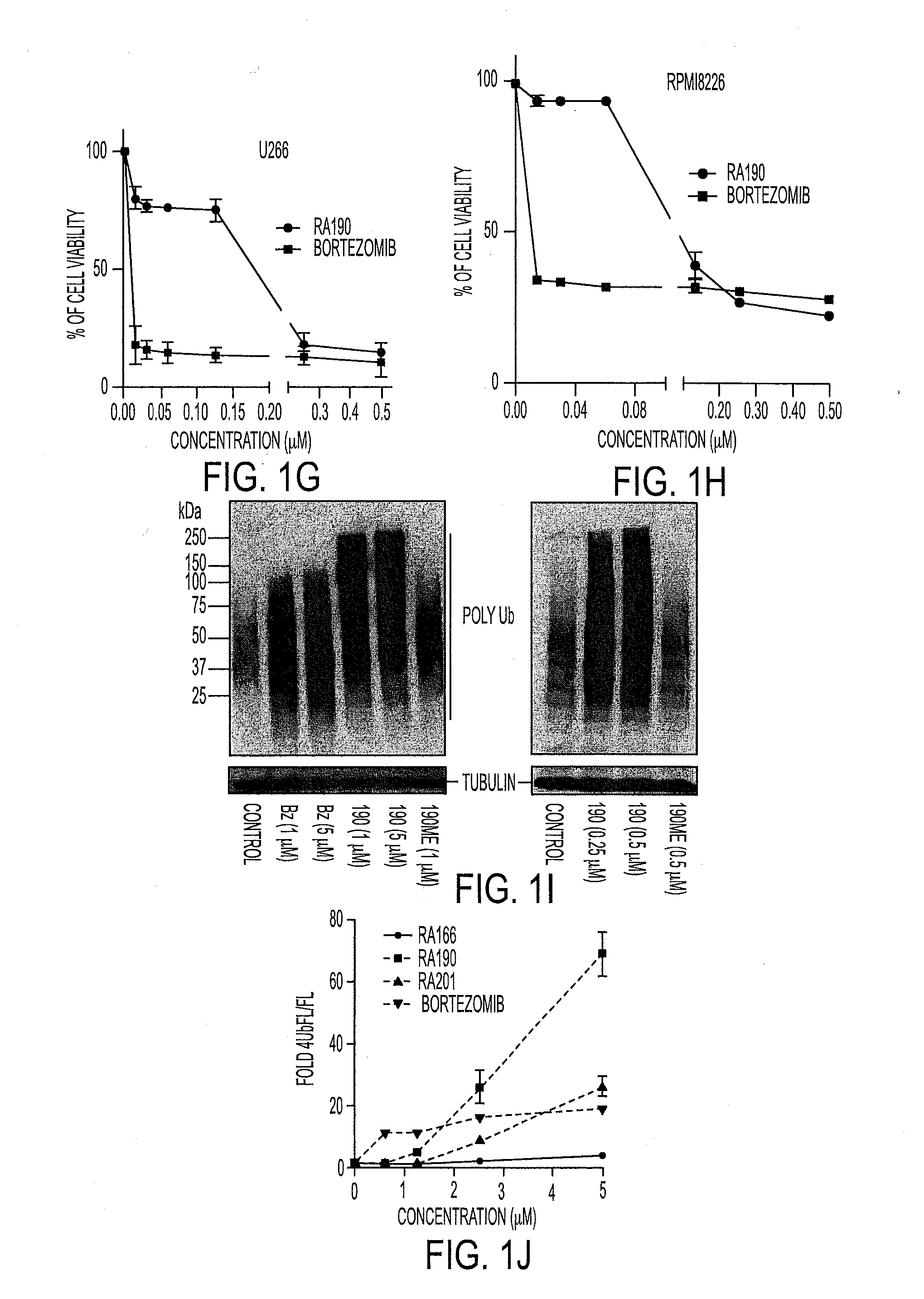
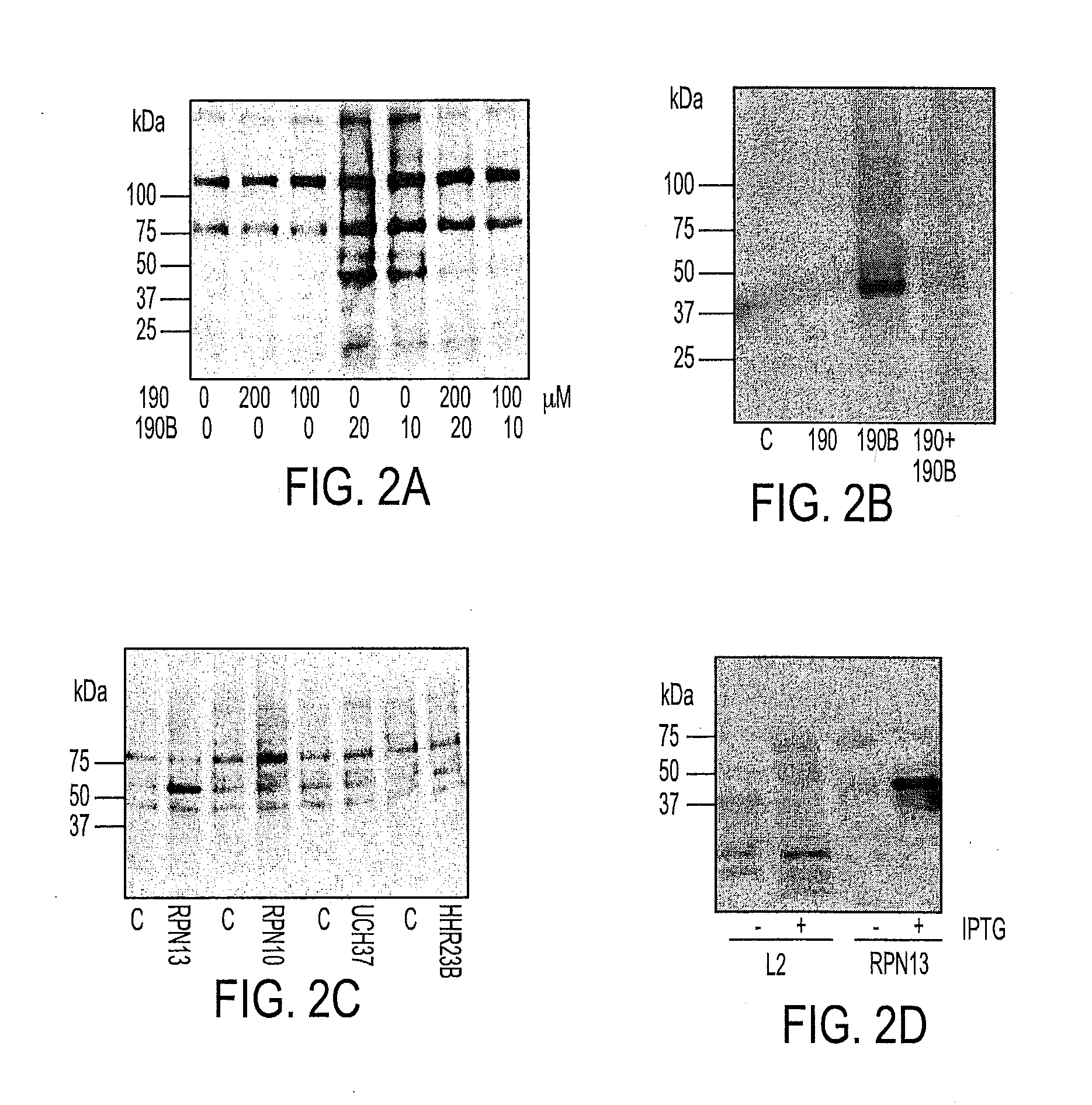
Novel bis-Benzylidine Piperidone Proteasome Inhibitor with Anticancer Activity
ActiveUS20160106725A1Reduced potencyReducing potencyBiocideOrganic chemistryHuman papillomavirus19S regulatory particle
We describe a bis-benzylidine piperidone, RA190, which covalently binds to the ubiquitin receptor RPN13 (ADRM1) in the 19S regulatory particle and inhibits proteasome function, triggering rapid accumulation of polyubiquitinated proteins. Multiple myeloma lines, even those resistant to bortezomib, were sensitive to RA190 via ER stress-related apoptosis. RA190 stabilized targets of human papillomavirus (HPV) E6 oncoprotein, and preferentially killed HPV-transformed cells. After p.o. or i.p. dosing of mice, RA190 distributed to plasma and major organs excepting brain, and potently inhibited proteasome function in skin and muscle. RA190 administration i.p. profoundly reduced growth of multiple myeloma and ovarian cancer xenografts, and oral RA190 treatment retarded HPV+ syngeneic mouse tumor growth, without impacting spontaneous HPV-specific CD8+ T cell responses, suggesting its therapeutic potential. The bis-benzylidine piperidone RA190 is a new orally-available proteasome inhibitor. Multiple myeloma, cervical and ovarian cancers are particularly sensitive to RA190.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
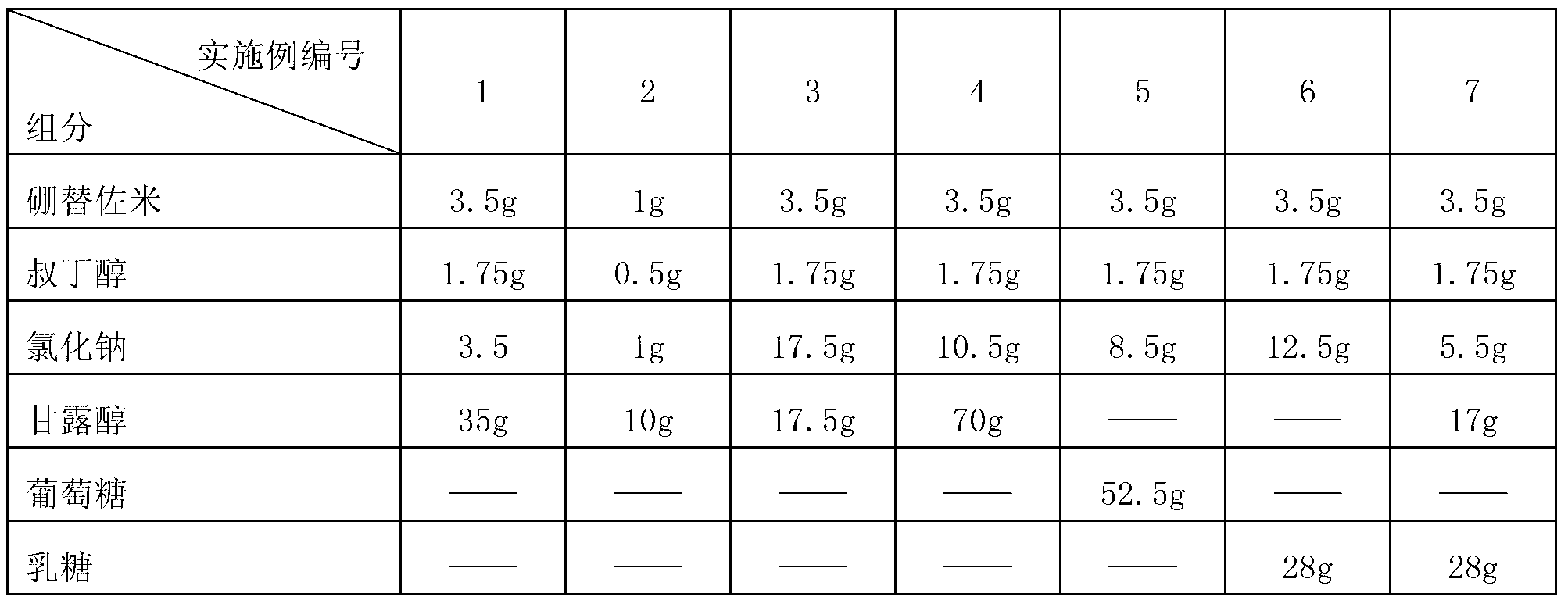
Drug composition of bortezomib and preparation method thereof
InactiveCN103212055AAccelerate the dissolution rateLess insoluble particlesPowder deliveryDipeptide ingredientsMannitolExcipient
The invention belongs to the technical field of medicines and particularly relates to a drug composition of bortezomib and a preparation method thereof. The drug composition disclosed by the invention contains bortezomib, tert butyl alcohol, sodium chloride and an excipient in the mass ratio of 1:0.5:(1-5):(5-20). The drug composition adopts freeze-dried powder injection or water injection, preferably the freeze-dried powder injection. The drug composition contains tert butyl alcohol and sodium chloride besides the conventional excipient; through adding tert butyl alcohol, bortezomib can be rapidly dissolved and rapidly reacted with polyalcohol excipients such as mannitol to form more stable borate, so that the stability problem of the bortezomib freeze-dried powder injection per se is solved; and through adding the drug active constituent i.e. sodium chloride, the normal physiological and biochemical activities and functions in vivo are fully guaranteed.
Owner:HAINAN JINRUI PHARMA
Nanometer drug-loading system as well as preparation method and application thereof
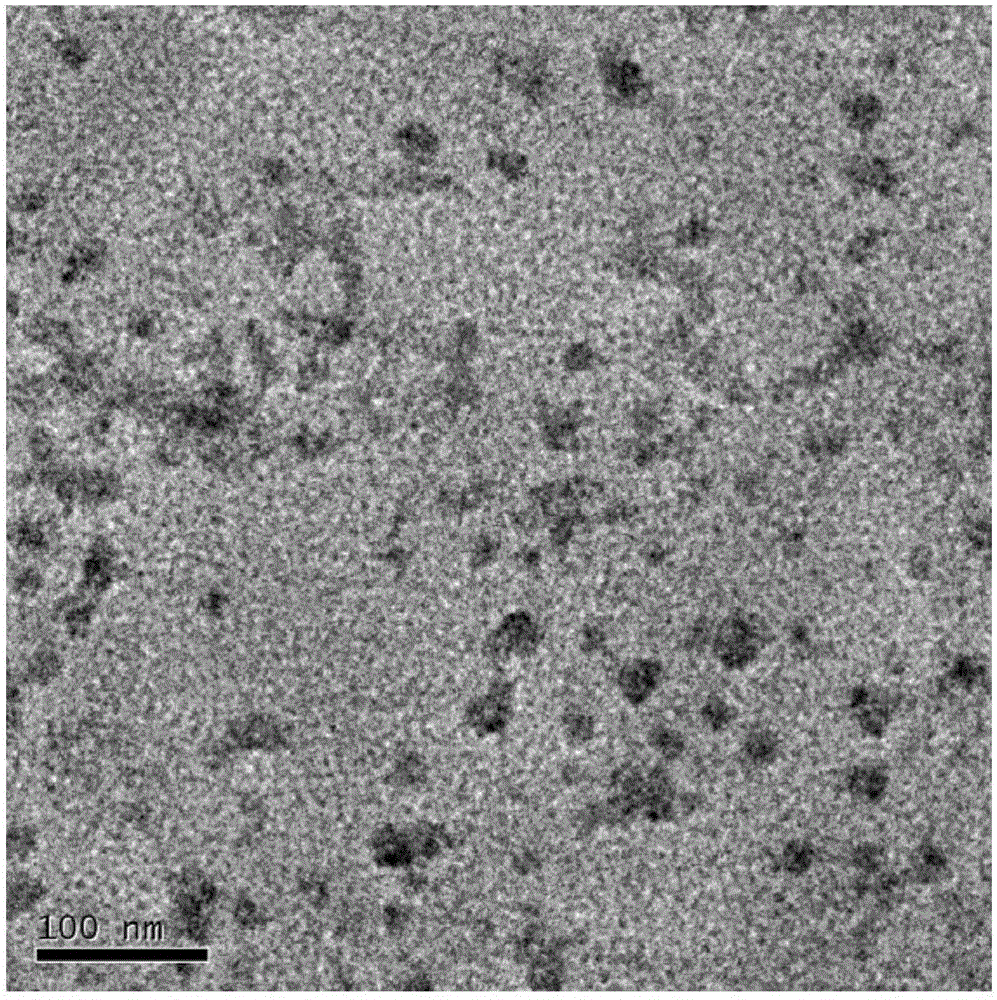
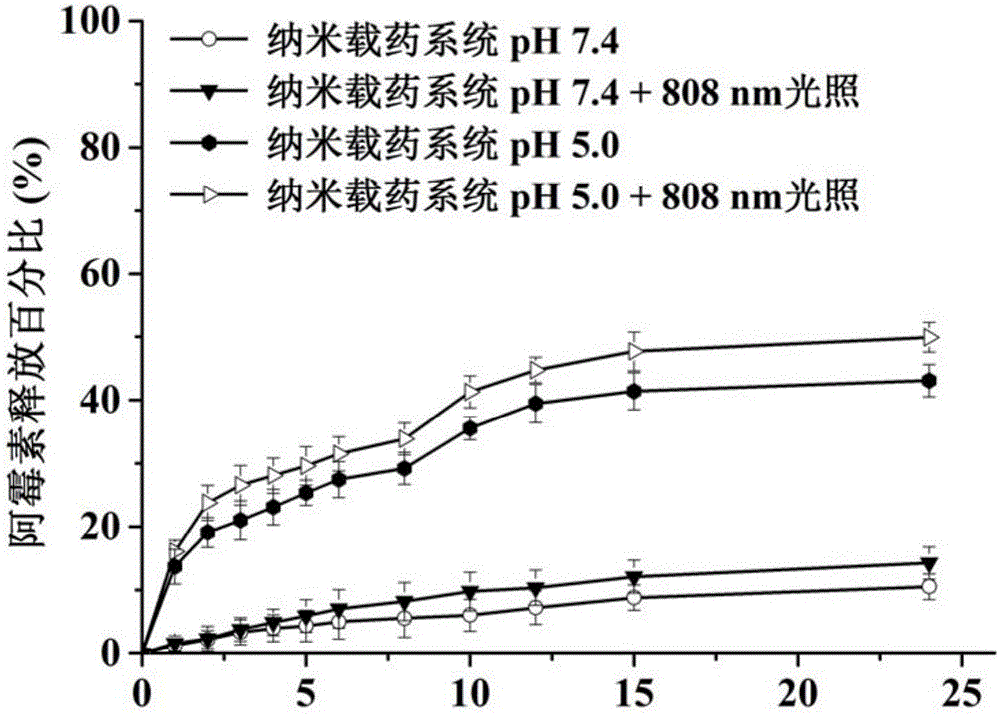
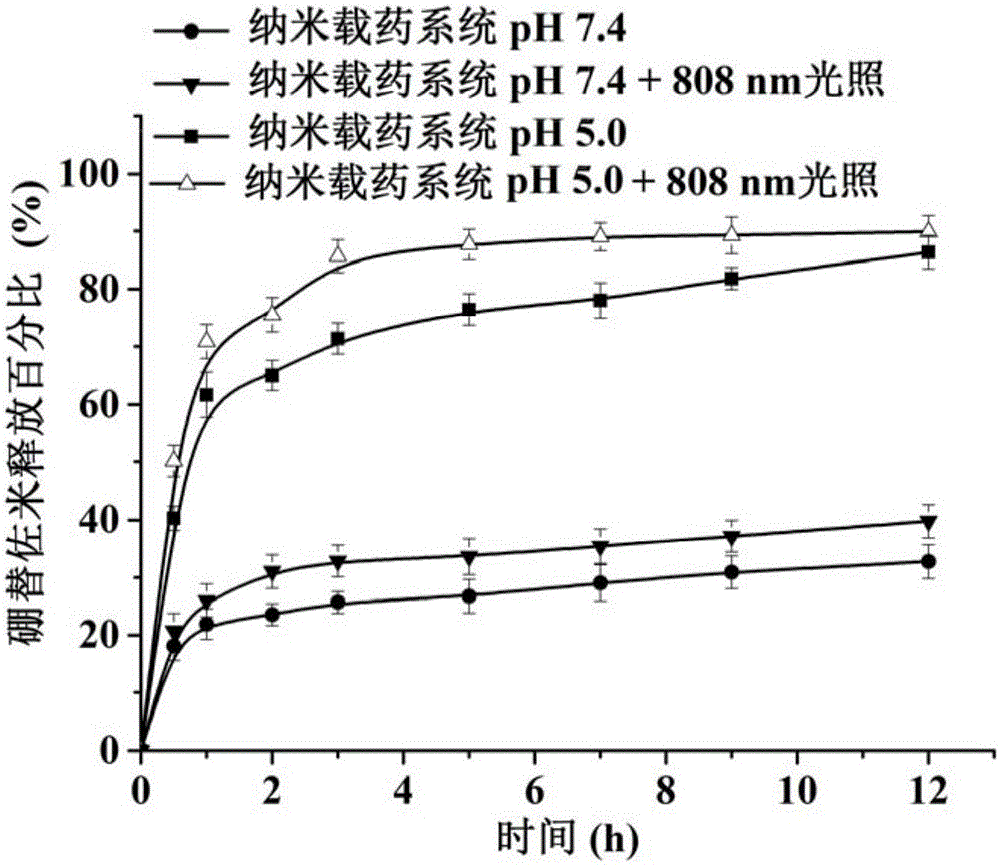
ActiveCN105030795AImprove stabilityTumor enrichmentHeavy metal active ingredientsDipeptide ingredientsMedicinePolyethylene glycol
The invention provides a nanometer drug-loading system as well as a preparation method and an application thereof. The nanometer drug-loading system comprises a drug-loading nano-micelle core, a poly-dopamine shell, and bortezomib connected to the poly-dopamine shell, wherein drug-loading nano-micelles are nano-micelles formed by encapsulating chemical anti-cancer drugs by polyethylene glycol-distearoyl phosphoethanolamine. The preparation method of the nanometer drug-loading system comprises the following steps: forming the drug-loading nano-micelles; forming the poly-dopamine shell outside the drug-loading nano-micelles; and connecting bortezomib to the poly-dopamine shell. The nanometer drug-loading system has the grain diameter smaller than 50nm, is high in stability, has a tumor enrichment effect, and realizes simultaneous delivery of bortezomib and other chemical anti-cancer drugs; poly-dopamine has a photothermal effect, can assist chemotherapeutic medicines in treatment, has the synergy of combined treatment of chemotherapy and thermal therapy, and therefore, the nanometer drug-loading system has a wide medical application prospect.
Owner:THE NAT CENT FOR NANOSCI & TECH NCNST OF CHINA
Methods and compositions useful for treating diseases involving bcl-2 family proteins with isoquinoline and quinoline derivatives
InactiveUS20160038503A1BiocideBoron compound active ingredientsAbnormal tissue growthAutoimmune condition
The present invention relates to a compositions for and methods for cancer treatment, for example, hematopoietic cancers (e.g. B-cell Lymphoma). In other aspects, the invention provides methods for treating particular types of hematopoietic cancers, such as B-cell lymphoma, using a combination of one or more of the disclosed compounds and, for example, 26S proteasome inhibitors, such as, for example, Bortezomib. In another aspect the present invention relates to autoimmune treatment with the disclosed compounds. In another aspect, this invention relates to methods for identifying compounds, for example, compounds of the BH3 mimic class, that have unique in vitro properties that predict in vivo efficacy against B-cell lymphoma tumors and other cancers as well as autoimmune disease.
Owner:EUTROPICS PHARMA
Stable Bortezomib Formulations
Multi-dose formulations for bortezomib are presented in which bortezomib has significantly improved stability. Especially preferred formulations include those in which bortezomib is in a liquid form suitable for injection, wherein the solvent system predominantly comprises propylene glycol. In other preferred aspects, bortezomib is present as a Lewis donor-acceptor complex with a hetero-bifunctional Lewis base.
Owner:INNOPHARMA
Bortezomib Formulations
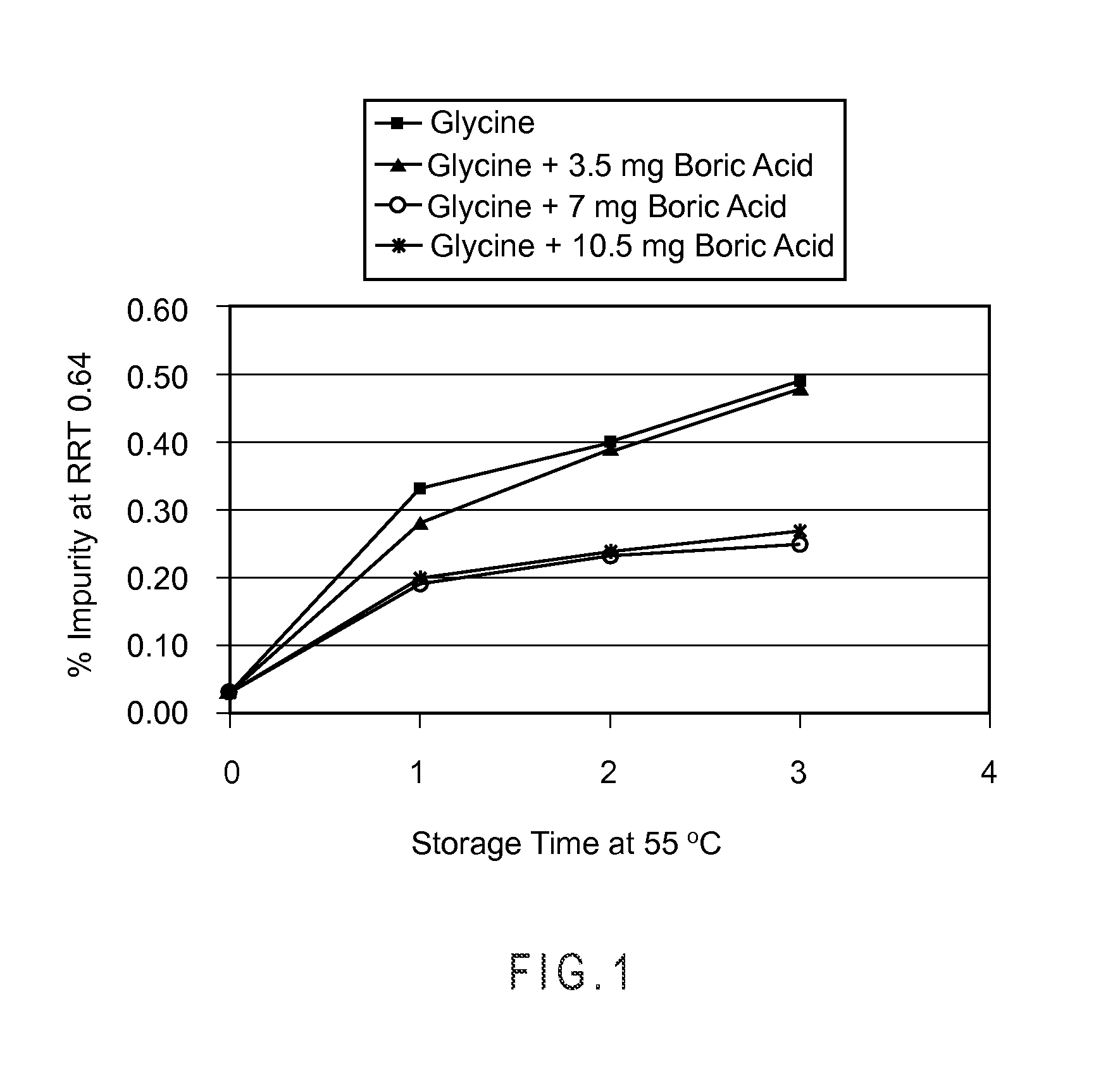
ActiveUS20120083457A1Low pour pointInorganic boron active ingredientsDipeptide ingredientsBoric acidSolvent
A bortezomib composition includes bortezomib and boric acid in a mass ratio of boric acid to bortezomib is from 1:1 to 10:1. The composition is a solid, and may be prepared by forming a liquid mixture including a solvent, bortezomib and boric acid, and lyophilizing the liquid mixture.
Owner:FRESENIUS KABI USA LLC
Process for Preparing and Purifying Bortezomib
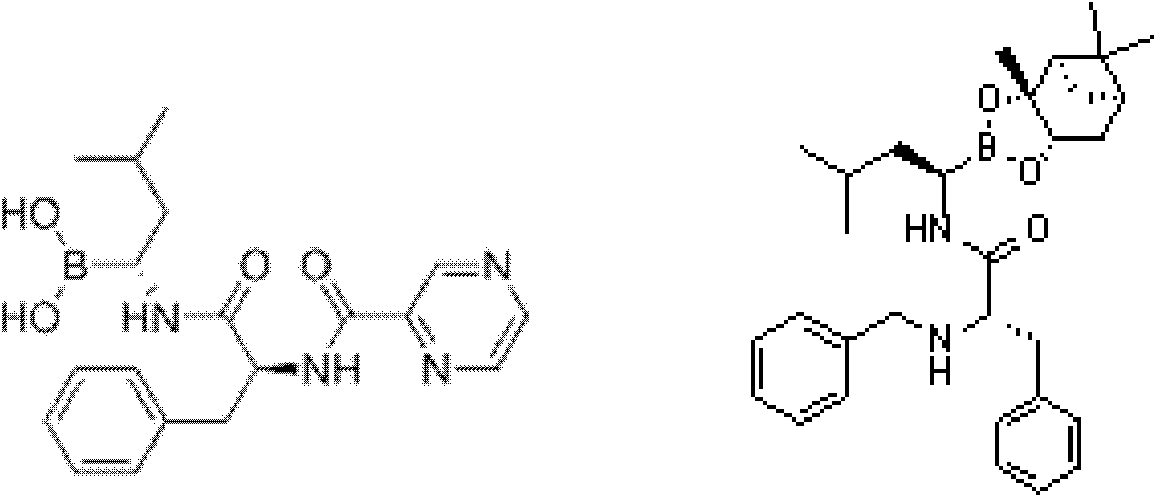
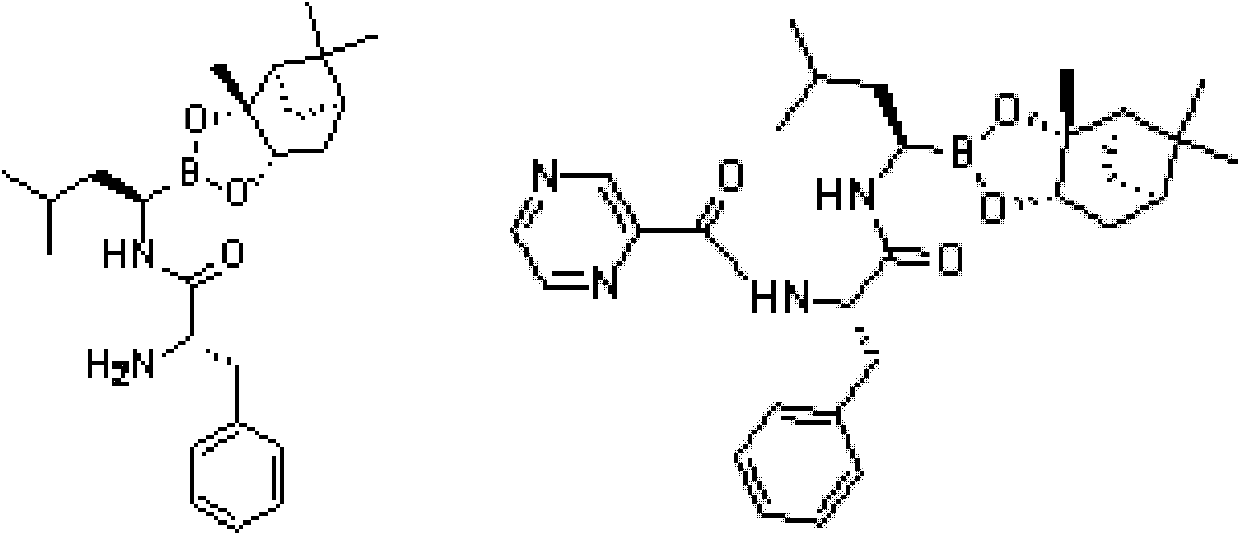
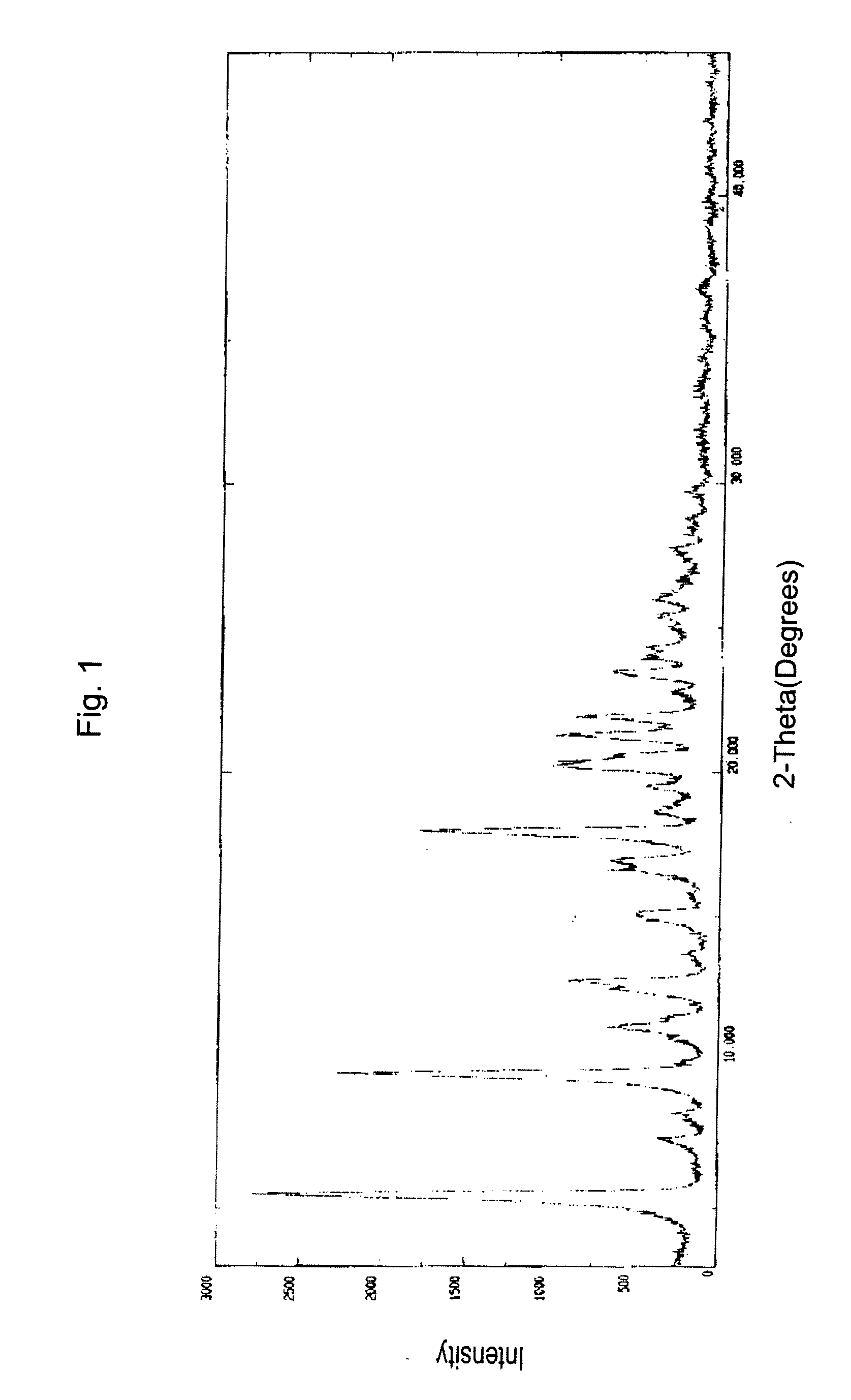
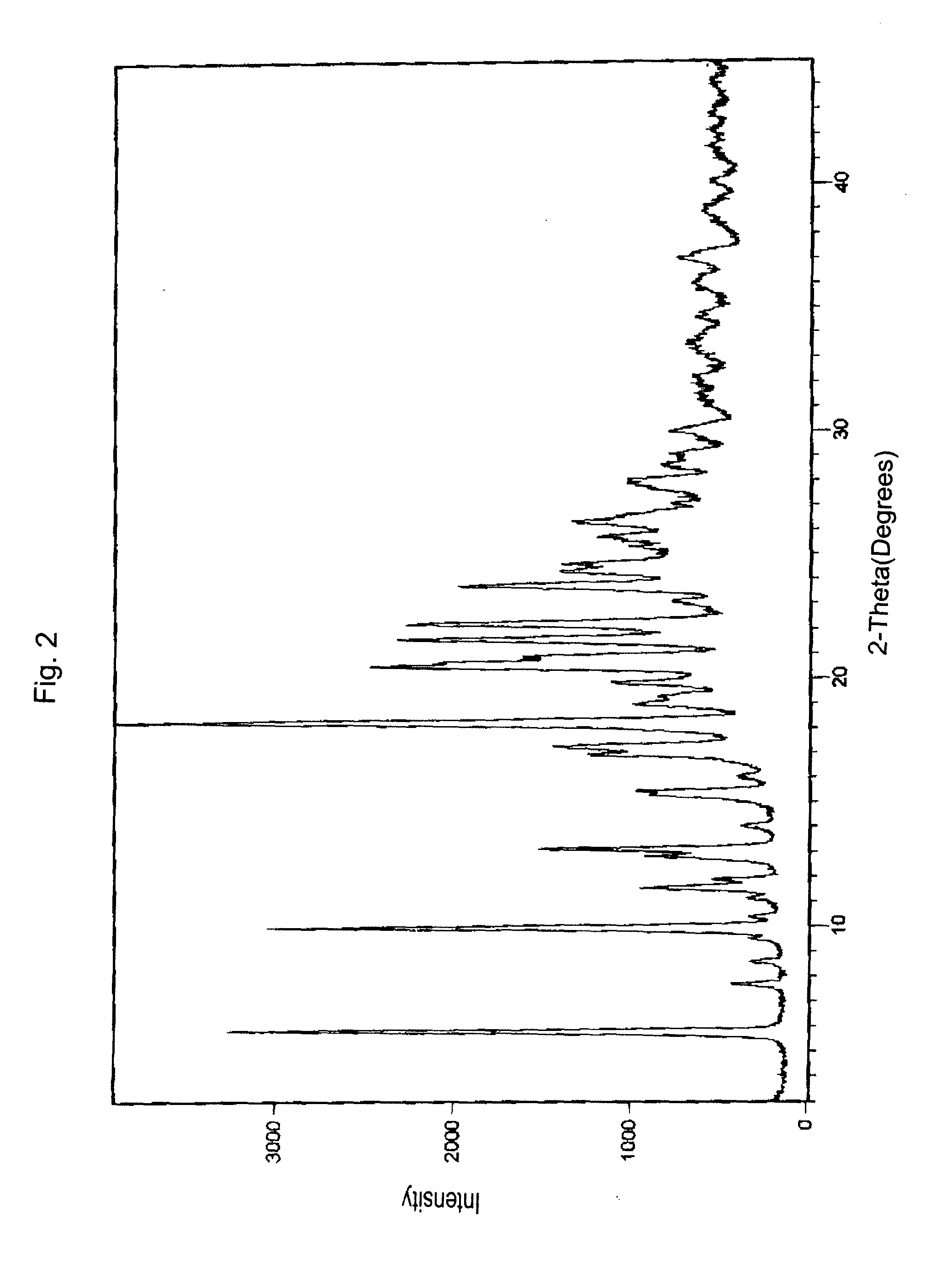
InactiveUS20120289699A1Low cost of reagentsReduce sizeSilicon organic compoundsGroup 3/13 element organic compoundsMedicinal chemistryCrystallization
The present invention provides a synthetic process for producing bortezomib using a novel intermediate. The present invention also provides a process for purifying bortezomib anhydride, and a new crystalline polymorph of bortezomib anhydride.
Owner:SCINOPHARM TAIWAN LTD
Methods of using saha and bortezomib for treating multiple myeloma
InactiveUS20100113392A1Additive and synergistic therapeutic effectBiocideBoron compound active ingredientsAnticarcinogenHDAC inhibitor
The present invention relates to a method of treating cancer in a subject in need thereof, by administering to a subject in need thereof a first amount of a histone deacetylase (HDAC) inhibitor such as suberoylanilide hydroxamic acid (SAHA), or a pharmaceutically acceptable salt or hydrate thereof, and a second amount of one or more anti-cancer agents, including Bortezomib. The HDAC inhibitor and the anti-cancer agent may be administered to comprise therapeutically effective amounts. In various aspects, the effect of the HDAC inhibitor and the anti-cancer agent may be additive or synergistic.
Owner:UNIV OF MARYLAND
Freeze-drying process for preparation of bortezomib for injection
InactiveCN106310217AGood resolubilityClarity qualifiedPowder deliveryDipeptide ingredientsSolubilityFreeze-drying
The invention provides a freeze-drying process for preparation of bortezomib for injection, and according to the process, after subpackage of a bortezomib liquor, freeze-drying is performed, main features of a pre freezing stage comprise two times of annealing, in a sublimation drying stage, drying is kept for 30-40h at -30 DEG C. The bortezomib for injection prepared by the process is not spurted from a bottle, has full and complete appearance, good solubility, qualified clear degree and high safety, and is suitable for industrial production.
Owner:BEIJING JIMEITANG MEDICINE RES CO LTD
Method for synthesizing bortezomib
ActiveCN102351890APurity effectHigh purityGroup 3/13 element organic compoundsChemical synthesisToluene
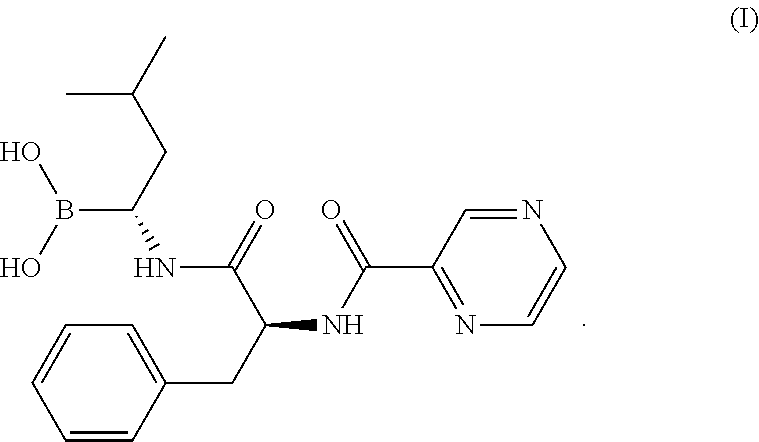
The invention relates to the field of chemical synthesis, in particular to a method for synthesizing bortezomib shown as a structural formula I. The method comprises the followings steps of: performing condensation, debenzylation, condensation and oxidation deprotection on raw materials; and purifying with acetone, toluene and methyl tert-butyl ether to obtain bortezomib. The method for synthesizing the bortezomib provided by the invention has the advantages of low reagent price, a small number of reaction steps, mild reaction condition, increase in the purity of bortezomib tablets and contribution to industrial production. The formula I is shown in the specifications.
Owner:CHONGQING SINTAHO PHARM CO LTD
Bortezomib and process for producing same
InactiveUS20100226597A1Reducing and eliminating drug instabilityPrevent degradationSilicon organic compoundsBagsCrystallizationStereochemistry
The present application provides a process for the preparation of Bortezomib, its intermediates and process for crystalline forms of Bortezomib.
Owner:DR REDDYS LAB LTD +1
Methods of using saha and bortezomib for treating cancer
The present invention relates to a method of treating cancer in a subject in need thereof, by administering to a subject in need thereof a first amount of a histone deacetylase (HDAC) inhibitor such as suberoylanilide hydroxamic acid (SAHA), or a pharmaceutically acceptable salt or hydrate thereof, and a second amount of one or more anti-cancer agents, including Bortezomib. The HDAC inhibitor and the anti-cancer agent may be administered to comprise therapeutically effective amounts. In various aspects, the effect of the HDAC inhibitor and the anti-cancer agent may be additive or synergistic.
Owner:MERCK SHARP & DOHME CORP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com