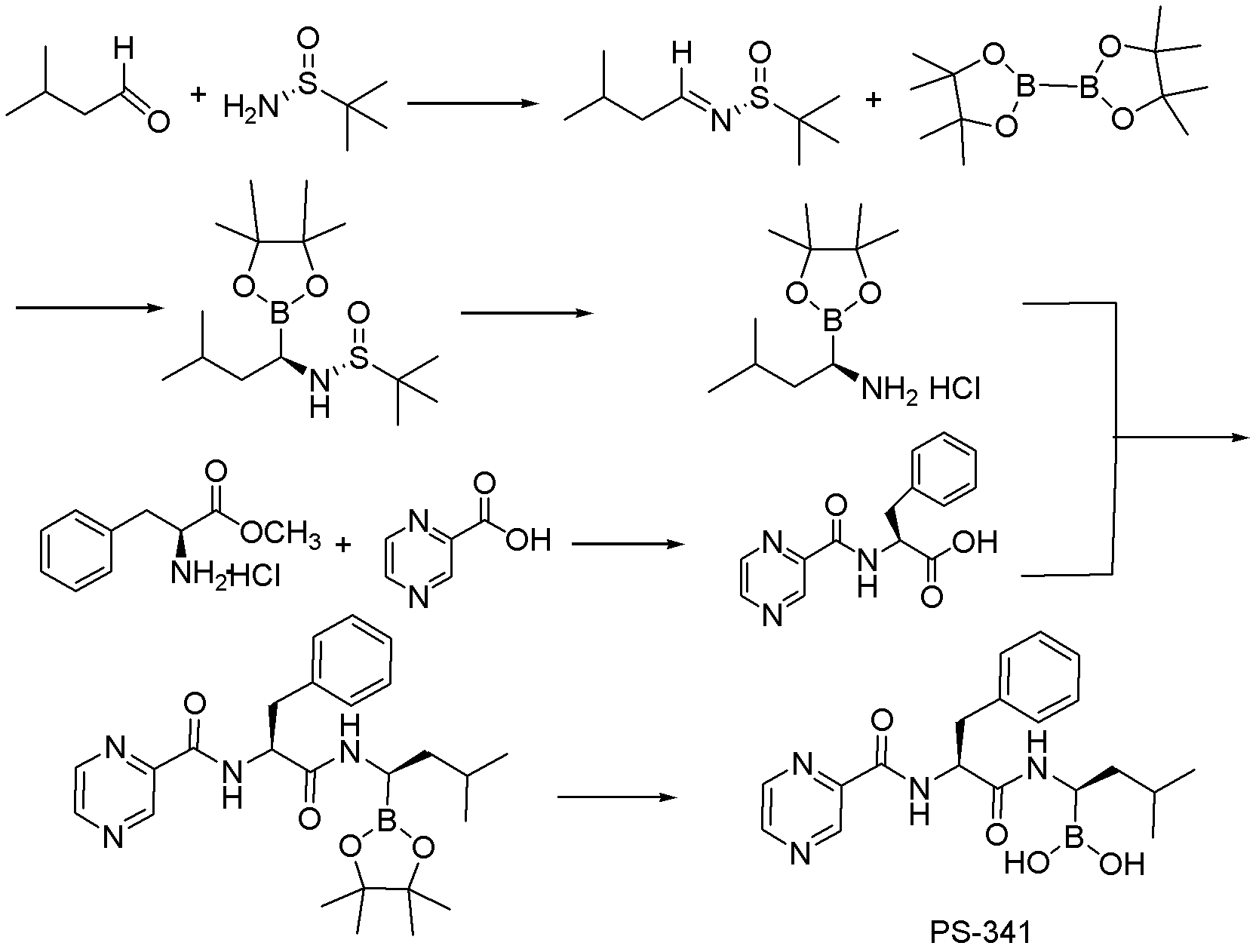
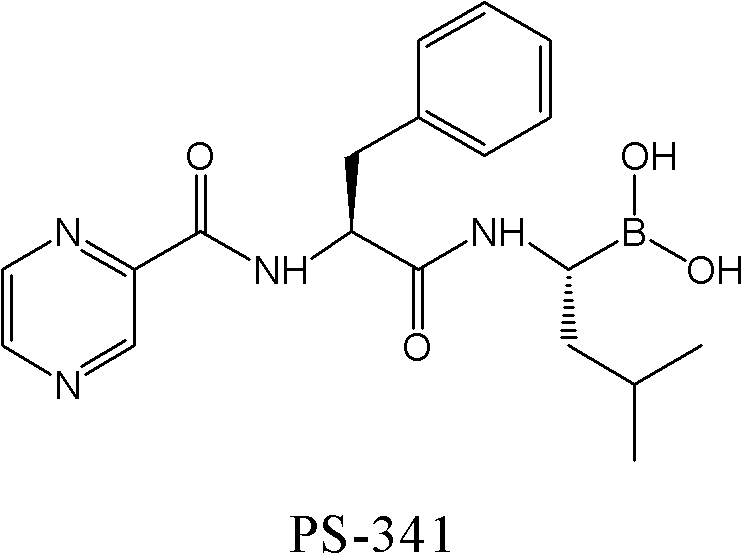
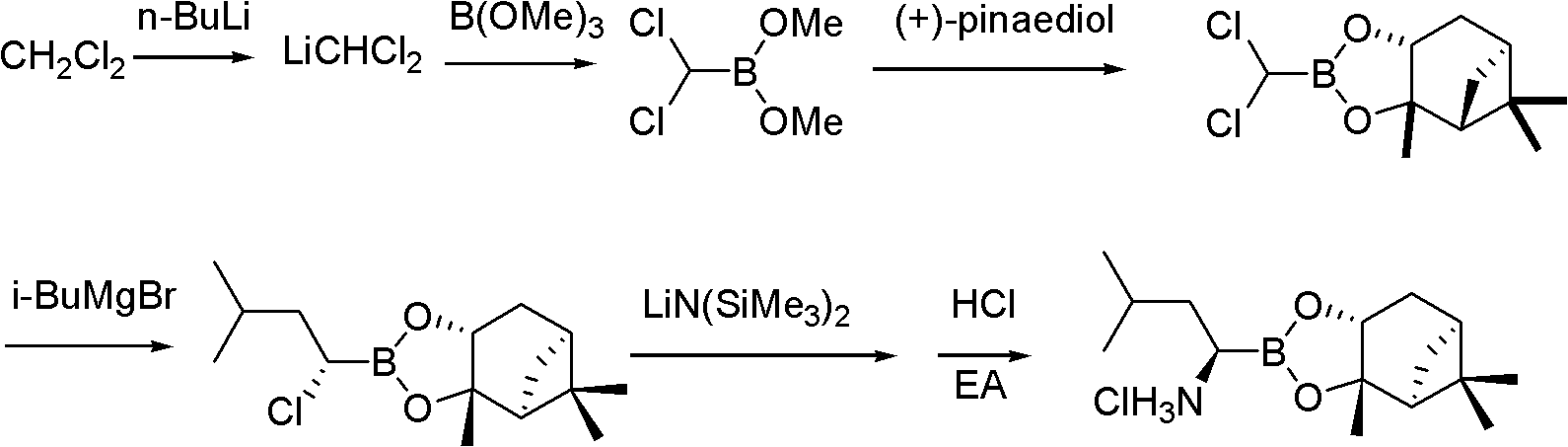
Synthetic method of proteasome inhibitor bortezomib and analogs
A technology of bortezomib and its analogues, applied in the field of new chemical synthesis, can solve the problems of high cost, limited use, difficult synthesis, etc., and achieve the effect of reducing the cost of synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0072] 2. Preparation of catalyst
[0073]
[0074] First complete ICy·HCl synthesis according to other documents (CN1216536A);
[0075]
[0076] In the existing literature, the synthesis of the target product (ICy)CuOt-Bu is completed in two steps, that is, the first step is to convert ICy·HCl into (ICy)CuCl, and then convert it into the desired product under the action of sodium tert-butoxide catalyst. In the present invention, the target product is obtained by directly using 2 times the amount of sodium tert-butoxide in one step, and the catalyst (ICy)CuOt-Bu can still be obtained well, which not only shortens the reaction time, but also simplifies the reaction steps.
[0077] 3. Catalytic addition and deprotection of boric acid pinacol ester
[0078]
[0079] The catalyst obtained in the previous step is directly dropped into the addition reaction system for catalysis after being dissolved in toluene, benzene, tetrahydrofuran, dioxane, etc. (preferably toluene) ...
Embodiment 1
[0093] (R)-N-tert-butylsulfinyl-3-methyl-1-butylimine
[0094]
[0095]Add 40mL of toluene to a 100ml flask, add 1.2ml (11mmol) of isovaleraldehyde, 1.2g (10mmol) of tert-butylsulfinamide and 4g (27mmol) of anhydrous sodium sulfate at room temperature, and stir for more than 48h. The post-treatment is to filter the excess content through diatomaceous earth, spin the organic phase to dryness and direct column chromatography (PE200ml for PE: EA = 20: 1 to complete) to obtain the product as 1.77g of colorless oily substance with special The sweet smell of hair, yield 95%. 1 H NMR (400MHz, CDCl 3 )δ8.06(t, J=5.1Hz, IH), 2.42(t, J=5.9Hz, 2H), 2.07(dp, J=13.6, 6.8Hz, IH), 1.21(d, J=7.8Hz, 9H), 1.00(d, J=6.5Hz, 6H).
Embodiment 2
[0097] 2-Chloro-1,3-dicyclohexyl-2,3-dihydro-1H-imidazole (ICy·HCl)
[0098]
[0099] Inject 100 ml of toluene into a 500 ml round bottom flask, add 9.92 g (100 mmol) of cyclohexylamine and 3 g (100 mmol) of paraformaldehyde while vigorously stirring. After reacting at room temperature for 30 minutes, cool it down to 0° C., further add 9.92 g (100 mmol) of cyclohexylamine, and slowly add 30 ml of 3.3 mol / L HCl aqueous solution (100 mmol) dropwise under cooling and vigorous stirring. Then remove the cooling device, slowly add 14.5 milliliters of 40% glyoxal aqueous solution (100mmol), and stir overnight at 50°C to obtain the desired compound, yield: 60-80%, melting point: 256-257°C . 1 H NMR (400MHz, CDCl 3 )δ10.98(s, IH), 7.46(s, 2H), 4.57(tt, J=11.8, 3.6Hz, 2H), 2.22(d, J=11.0Hz, 4H), 2.03-1.65(m, 10H ), 1.57-1.17(m, 6H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com