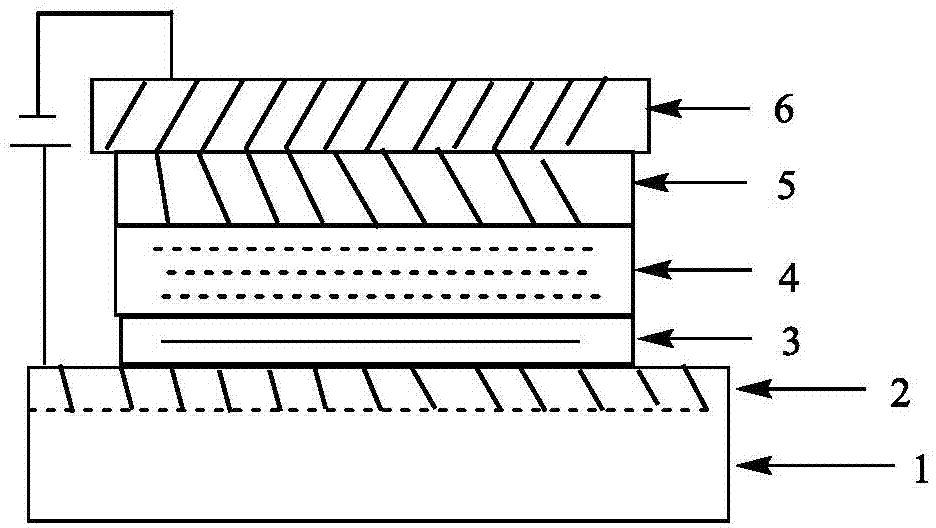
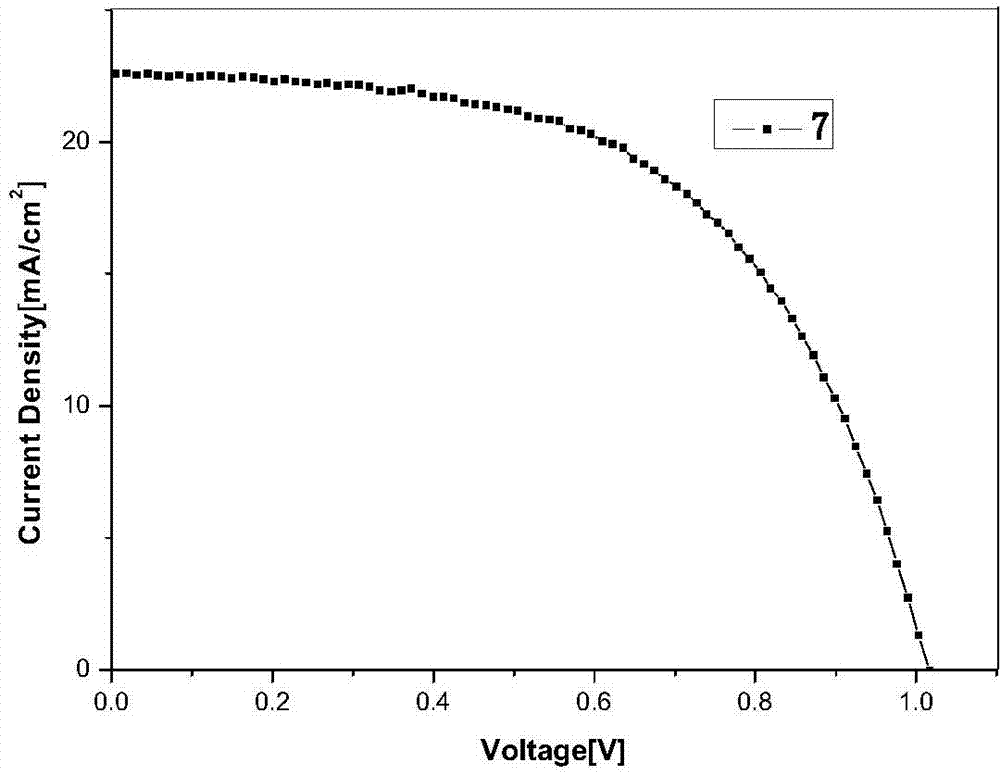
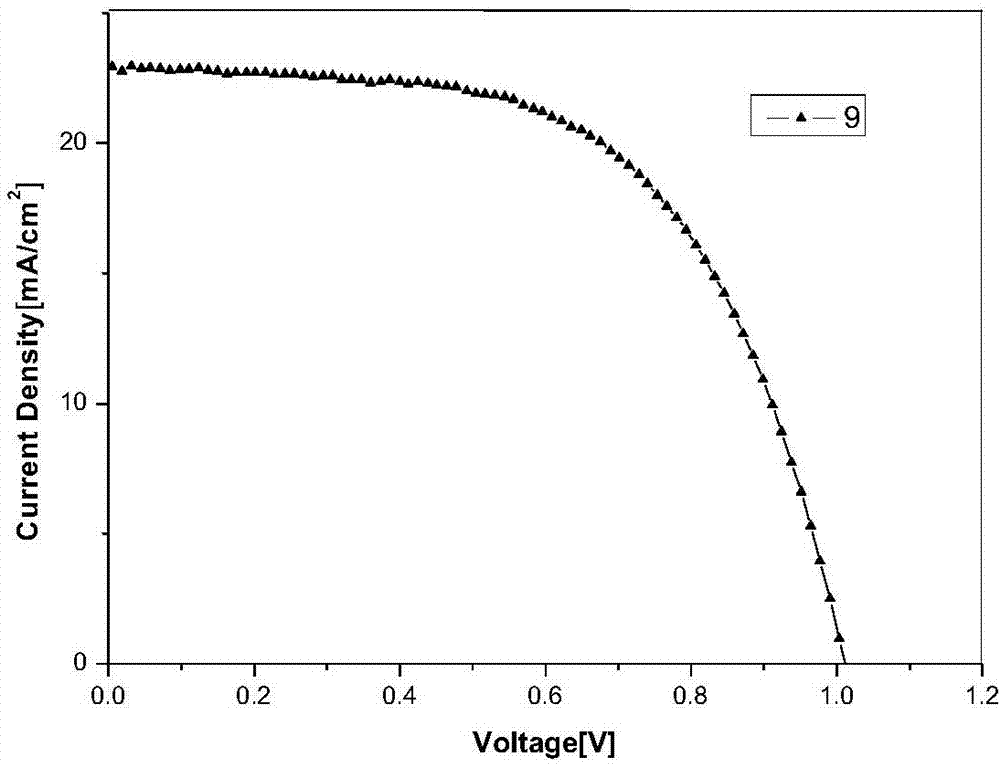
Deprotection 9,9'-spirobifluorene dendritic compound and preparation method and application
A compound, spirobifluorene technology, applied in the field of solar cells, can solve the problems of high cost, decomposition and high manufacturing cost of high-purity silicon
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] The preparation of embodiment one compound 1
[0054] The reaction scheme is as follows:
[0055]
[0056] This can be achieved through the following three options:
[0057] Scheme 1: Add 20g (0.062mol) 4,4'-dibromodiphenylamine and 20.3g (0.093mol) (BOC) to a three-necked flask with a volume of 250mL 2 O, mix well to obtain mixture I, add 80g THF (tetrahydrofuran) to the above mixture I, nitrogen protection, mechanical stirring, the system is completely dissolved, and the system is brown and clear at this time. Slowly add 1.5g (0.012mol) of DMAP (4-dimethylaminopyridine) to the system, a large number of bubbles are generated, and the system turns brownish yellow and clear. At this time, the temperature of the oil bath is raised to 70°C, and the system is refluxed for 2 hours. After the reaction was completed, the temperature was lowered to room temperature, and the solvent was removed under reduced pressure to obtain 33 g of brown oil. Filter through a silica gel...
Embodiment 2
[0060] The preparation of embodiment two compound 2
[0061] The reaction scheme is as follows:
[0062]
[0063] This can be achieved through the following three options:
[0064] Scheme 1: Add 23.7g (0.10mol) 4,4'-dimethoxydiphenylamine, 20g (0.047mol) compound 1 (prepared by Scheme 1 of Example 1), 0.86g ( 9.4×10 -4 mol)Pd 2 (dba) 3 , 0.54g (1.88×10 -3 mol)(t-Bu) 3 P·BF 4 and 15.8g (0.141mol) of potassium tert-butoxide, mix uniformly to obtain mixture II, add 400g toluene to mixture II, pass nitrogen protection, start mechanical stirring, stir evenly, the system becomes black and turbid, heat up to 115°C, reflux Reaction 4h. After the reaction was complete, the system was cooled to room temperature, quenched by pouring into 200g of water, separated, the aqueous phase was extracted with (50mL×2) ethyl acetate, the organic phases were combined, dried over anhydrous sodium sulfate, filtered, and the solvent was removed under reduced pressure to obtain 36.4 g of dar...
Embodiment 3
[0067] The preparation of embodiment three compound 3
[0068] The reaction scheme is as follows:
[0069]
[0070] This can be achieved through the following three options:
[0071] Scheme 1: Add 4.1g (5.67×10 -3 mol) Compound 2 (prepared by Scheme 1 of Example 2), 20 g of dichloromethane, and start stirring. After complete dissolution, 41 g of TFA (trifluoroacetic acid) was added to the system, and reacted at room temperature for 10 min. After the reaction was complete, the solvent was removed under reduced pressure to obtain 5.1 g of a black solid. Add 50g ethyl acetate and 50g mass fraction of 10% NaOH aqueous solution to the system, stir for 0.5h, separate the liquids, extract the aqueous phase with 20mL ethyl acetate, combine the organic phases, add 20g anhydrous sodium sulfate to dry, desolventize, and oxidize Aluminum was filtered, and dichloromethane was rinsed to obtain 3.1 g of compound 3. Yield 87.8%. 1 H NMR (400MHz, acetone-d6) δ / ppm: 7.38 (d, J = 9.0Hz,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com