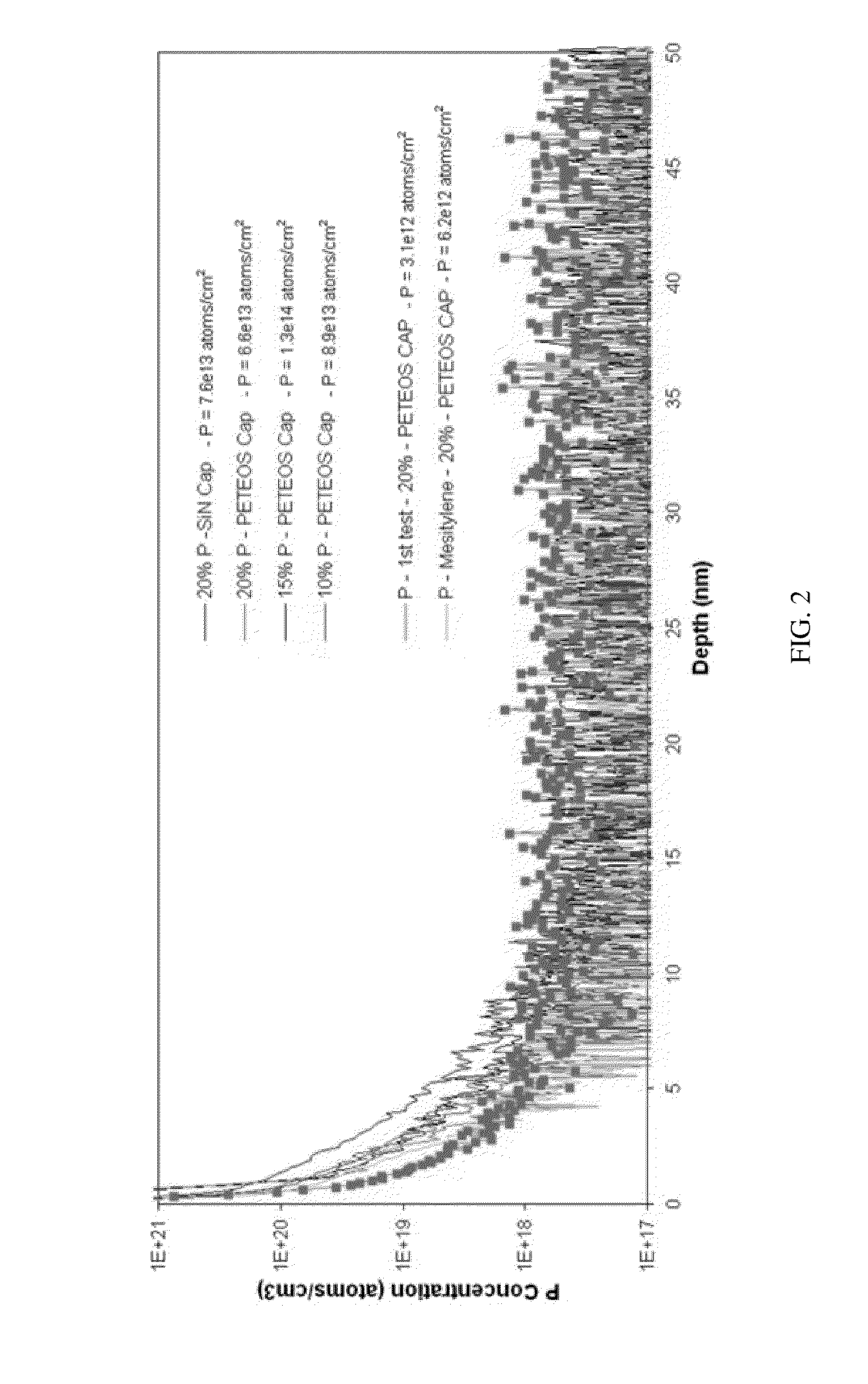
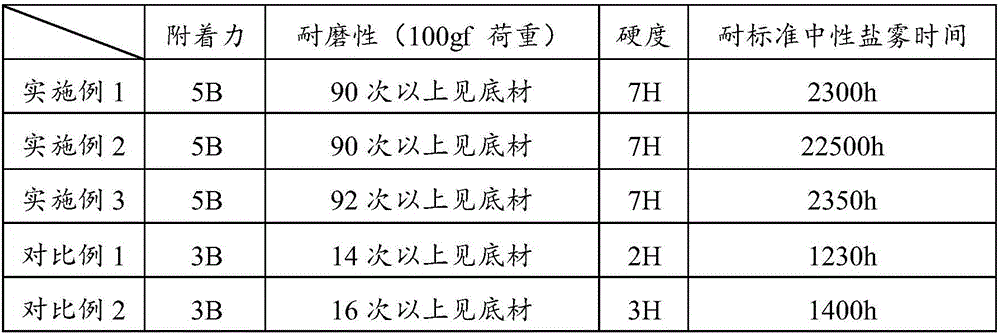
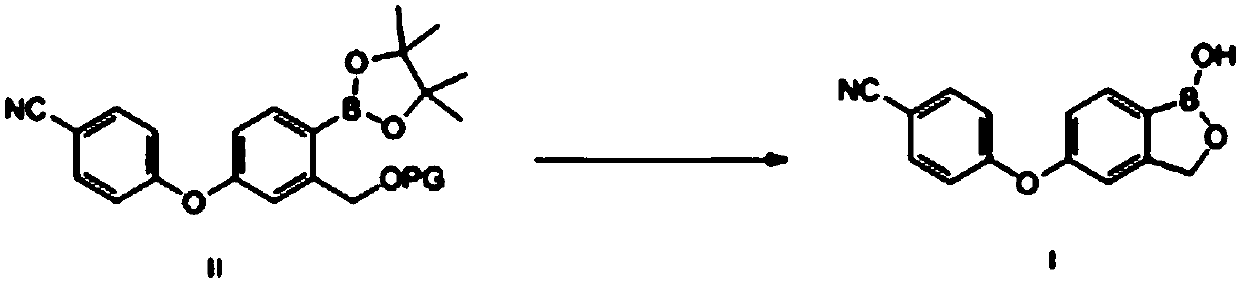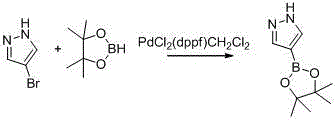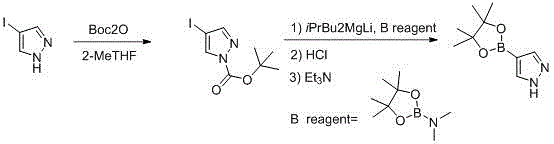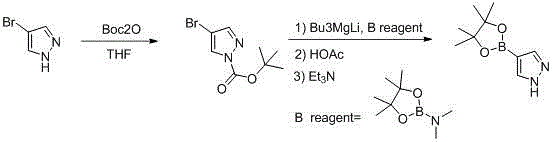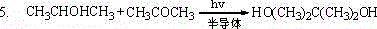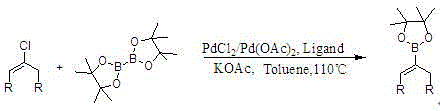Patents
Literature
353 results about "Pinacol" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
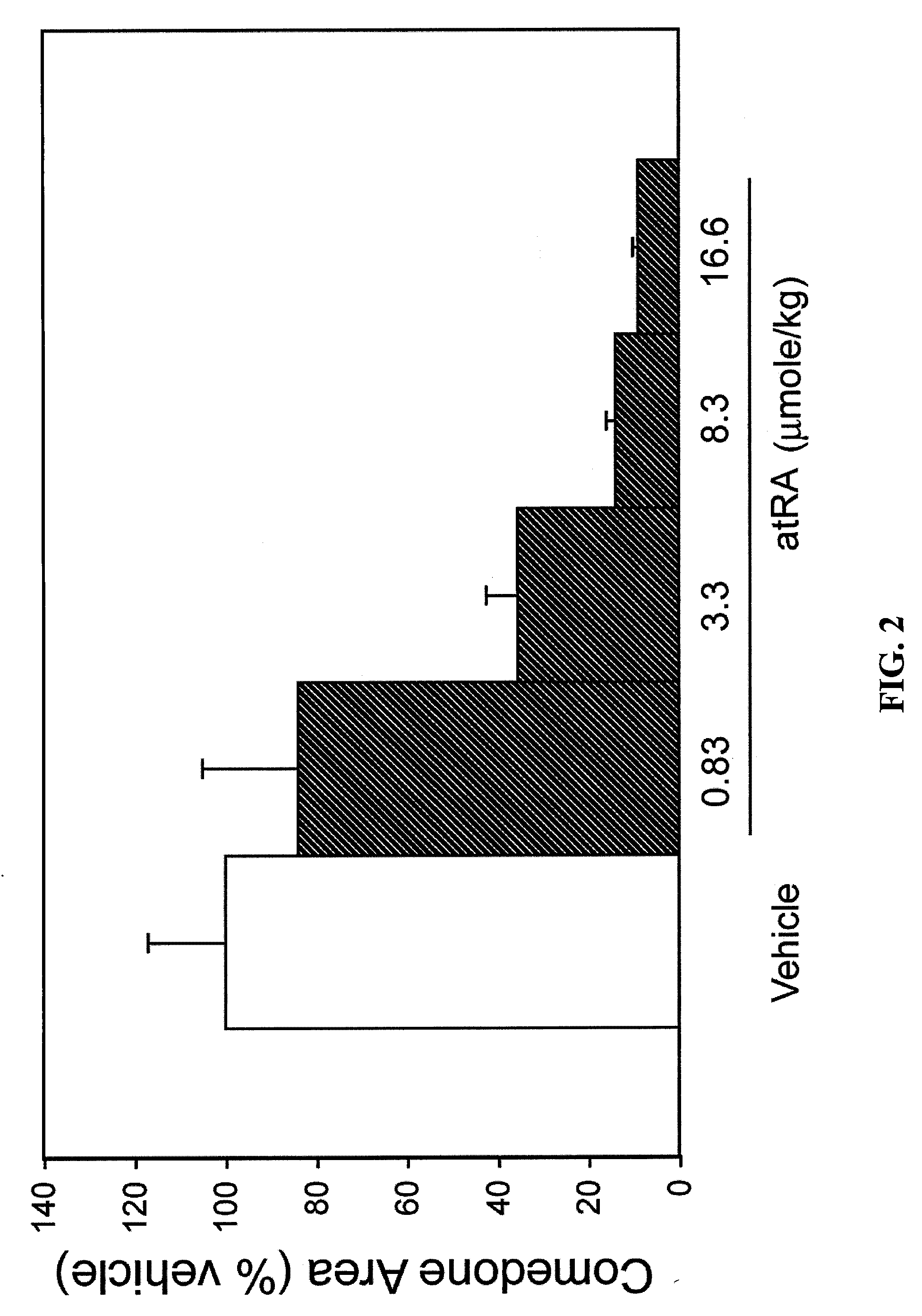
Pinacol is a white solid organic compound. It is a diol that has hydroxyl groups (-OH) on vicinal carbon atoms.
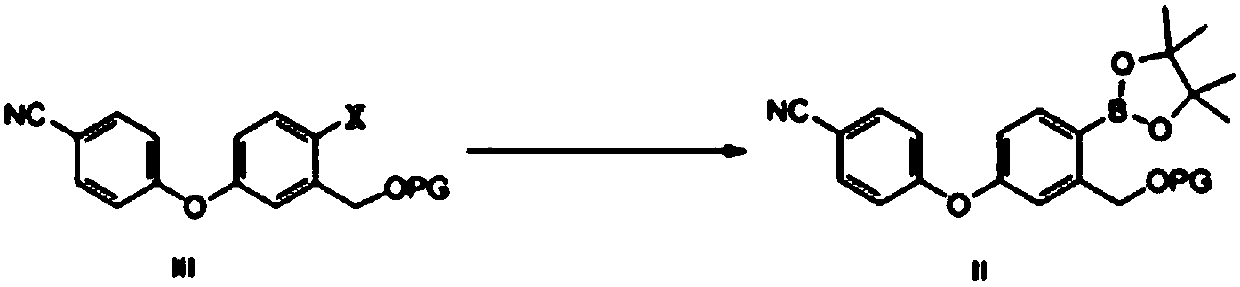
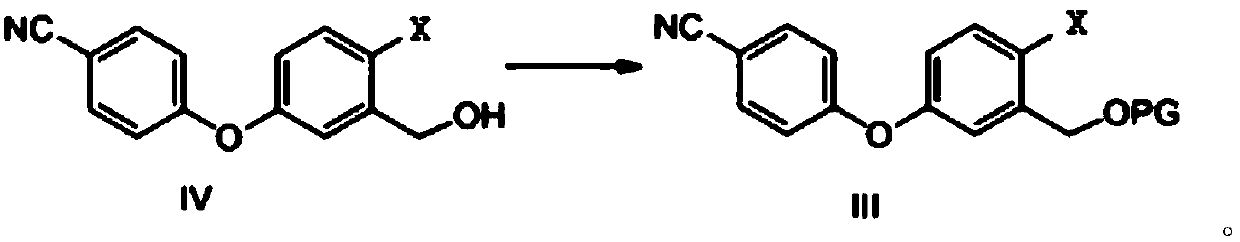
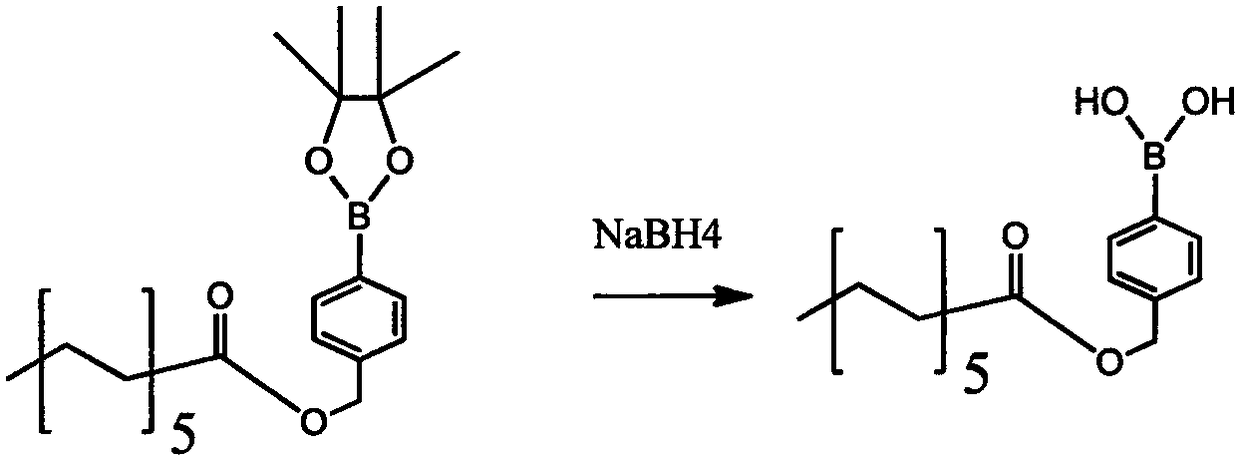
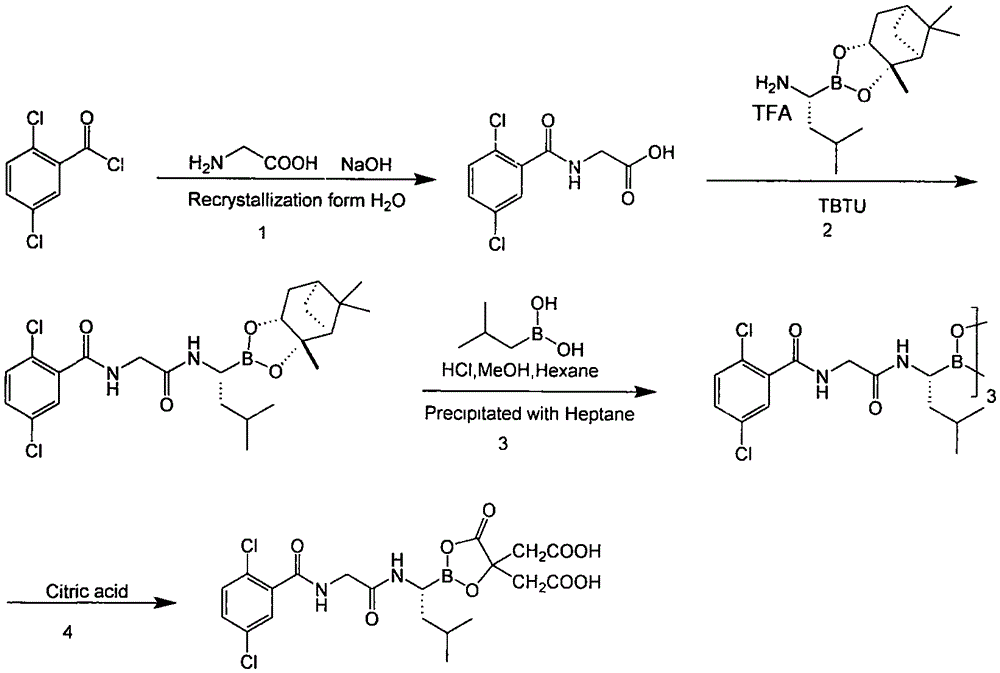
Method for preparing crisaborole
ActiveCN108047261AMild reaction conditionsEasy to operateGroup 3/13 element organic compoundsBulk chemical productionPhenylboronic acidOrganic solvent
The invention discloses a method for preparing crisaborole and an intermediate for preparing crisaborole. The method comprises the following steps: (1) enabling components such as 2-halogen-5-(4-cyanphenoxy) benzyl acetate and bis(pinacolato)diboron to react in the presence of an organic solvent and a palladium catalyst under an alkali condition so as to obtain components such as 2-acetoxyl methyl-4-(4-cyan phenoxy) phenylboronic acid pinacol ester; (2) enabling components such as the 2-acetoxyl methyl-4-(4-cyan phenoxy) phenylboronic acid pinacol ester to react in a solvent under an acid oralkali condition, thereby obtaining crisaborole. The method is gentle in reaction condition, easy to operate, high in reaction yield, simple in aftertreatment, relatively low in cost and applicable toindustrial production.
Owner:SUZHOU VIGONVITA LIFE SCIENCES CO LTD +1
Methods and compositions for doping silicon substrates with molecular monolayers
InactiveUS20120003826A1Semiconductor/solid-state device manufacturingDopantTetraethylene glycol dimethyl ether
Compositions and methods for doping silicon substrates by treating the substrate with a diluted dopant solution comprising tetraethylene glycol dimethyl ether (tetraglyme) and a dopant-containing material and subsequently diffusing the dopant into the surface by rapid thermal annealing. Diethyl-1-propylphosphonate and allylboronic acid pinacol ester are preferred dopant-containing materials, and are preferably included in the diluted dopant solution in an amount ranging from about 1% to about 20%, with a dopant amount of 4% or less being more preferred.
Owner:VERSUM MATERIALS US LLC
Graphene anti-corrosion paint
ActiveCN106479313AImprove stabilityMaximum corrosion resistanceAnti-corrosive paintsEpoxy resin coatingsEpoxySilanes
The invention discloses a graphene anti-corrosion paint. The paint comprises, by weight, 76-87 parts of DER671 type Dow solid epoxy resin, 22-25 parts of 582 type melamine formaldehyde resin, 27-28 parts of stearalkonium hectorite, 6-7 parts of hydroxy-1-propenylboronic acid pinacol ester, 0.5-0.7 part of oxidative modified graphene precursor, 1-3 parts of gamma-amino propyl triethoxy silane, 5-7 parts of nanoscale titanium dioxide, 3-5 parts of Y-311SN type blocked isocyanate curing agent, 12-15 parts of calcined kaolin, 2-3 parts of zinc chromate, 32-35 parts of solvent, and 0.2-0.4 part of paraformaldehyde. The method uses the second time oxidative modification of the graphene to increase the graphene stability, thus benefiting the maximum exertion of the inherent anti-corrosion performance of the graphene, making the prepared paint have excellent adhesive force, hardness, anti-wear ability and resistance to the corrosion caused by neutral salt mist.
Owner:常州市大使涂料有限公司
Method for preparing baricitinib
ActiveCN105294699ARaw materials are easy to getSimple processOrganic chemistryCyclobutaneCyanomethylidyne
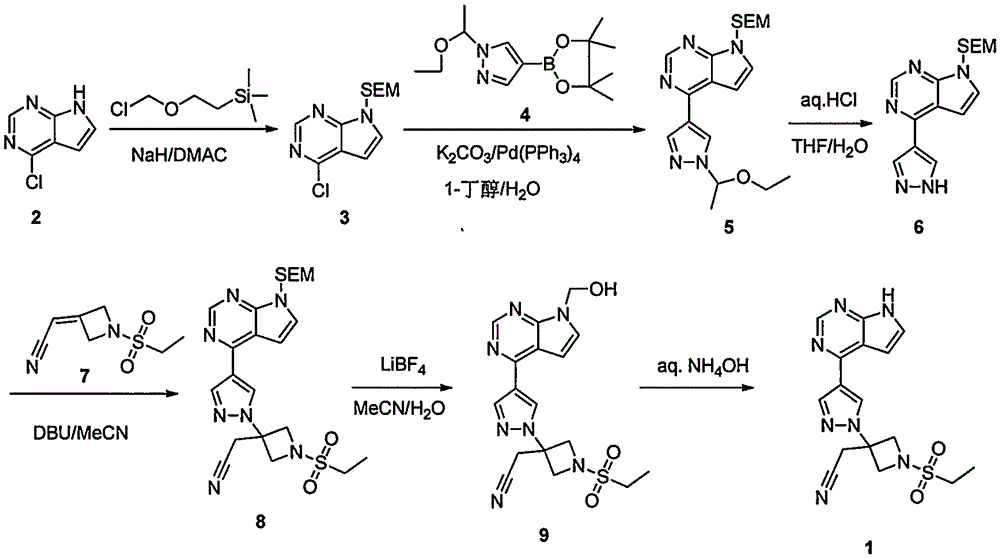
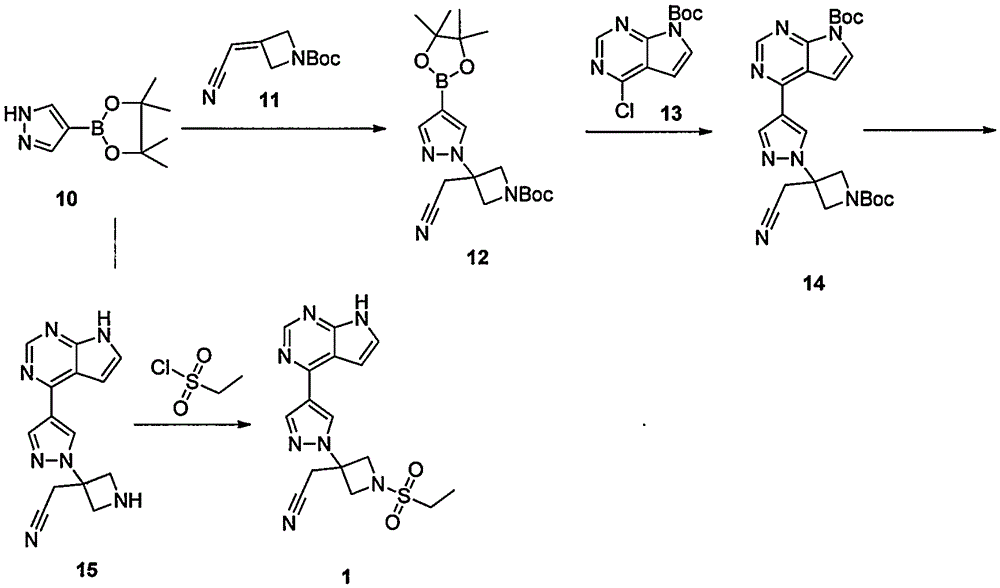
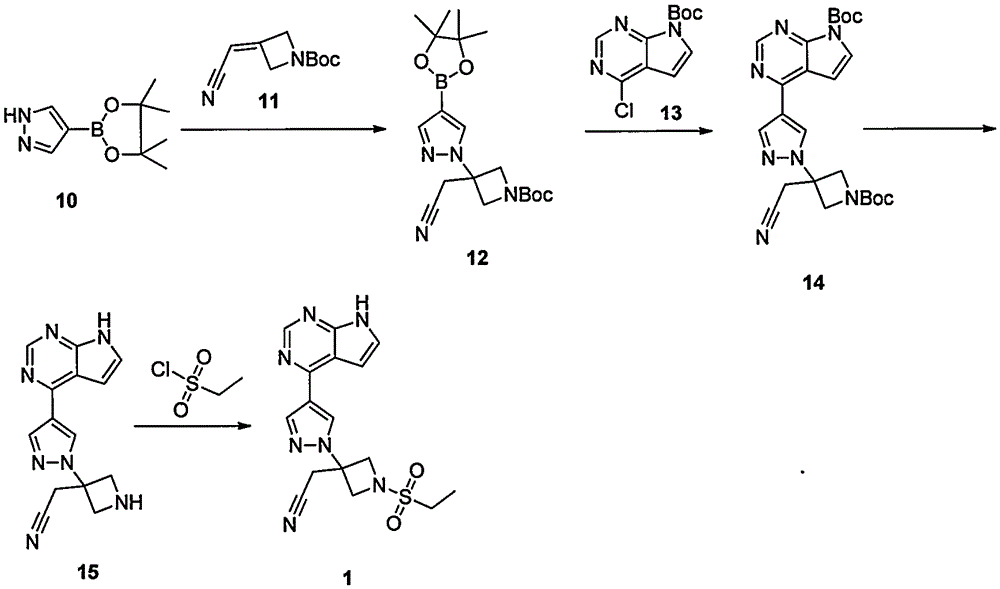
The invention provides a method for preparing baricitinib. The method comprises the following steps: by taking 4-pyrazol boric acid pinacol ester (10) as an initial raw material, performing Michael addition reaction on the initial raw material and 3-(icyanomethylene) azacyclo-cyclobutane-1-tert-butyl formate (11) so as to prepare an intermediate 12, and performing catalytic coupling reaction on 12 and 13, thereby preparing an intermediate 14; removing two-molecule tert-butyl formate of the intermediate 14, thereby preparing an intermediate 15; performing sulfamide reaction on the intermediate 15 and ethanesulfonyl chloride in an organic solvent, thereby obtaining a final product baricitinib (1). The method for preparing baricitinib has the advantages that the raw materials are easy to obtain, the process is simple, the operation is convenient, the reaction yield is high when being compared with that of document records, the atom utilization rate is high, industrial production can be easily achieved, and the like. The reaction general formula is as shown in the specification.
Owner:SHANGHAI XUNHE PHARMA TECH CO LTD
Organic substituted boric acid ester, boron affinity functional material using organic substituted boric acid ester as functional monomer as well as preparation and application of organic substituted boric acid ester
The invention provides organic substituted boric acid ester which is 4-amino-2-(dimethylaminomethyl) phenylboric pinacol ester. The organic substituted boric acid ester has the following structural formula described in the specification and is characterized in that boron-nitrogen coordination exists in molecules of the organic substituted boric acid ester and the organic substituted boric acid ester contains functional groups capable of having chemical effects on the surface of a carrier material. A novel boron affinity functional material prepared by using the invention can adapt to working environments from moderate acidity to alkalinity so as to make up the defect of narrow working acidity range of a traditional boron affinity material and can be applied to the aspects like identification, immobilization, enrichment, separation, detection and the like of cis-o-dihydroxyl biomolecules under the condition of wide pH value range.
Owner:NANJING UNIV
Electrolyte, positive electrode, preparation method thereof, and lithium ion battery
ActiveCN107331892ABlocking side effectsProtect from oxidative decompositionNon-aqueous electrolyte accumulator electrodesSecondary cells servicing/maintenanceSolventBoric acid
The invention provides an electrolyte, a positive electrode, a preparation method thereof, and a lithium ion battery. The electrolyte comprises lithium salts, an electrolyte solvent, and an additive. The additive is boric acid pinacol ester provided by the invention. The electrolyte has the advantages that boric acid pinacol ester is taken as the special additive, can protect the positive electrode, and at the same time, protects the electrolyte solvent from being oxidized and decomposed under a high potential so as to avoid excess consumption. The service life of battery is prolonged under a high voltage.
Owner:BYD CO LTD
Method for preparing cyclopentene/cyclohexene-1-boronic acid pinacol ester
InactiveCN103044469AReduce dosageLow costGroup 3/13 element organic compoundsCyclopentenePtru catalyst
The invention discloses a method for preparing cyclopentene / cyclohexene-1-boronic acid pinacol ester. The method comprises the step of carrying out coupling reaction on 1-chlorine-cyclopentene / cyclohexene serving as a raw material in an organic solvent A in the presence of monophosphate ligand and a palladium catalyst based on potassium acetate as alkali so as to prepare the cyclopentene / cyclohexene-1-boronic acid pinacol ester. The method is characterized in that the physicochemical reaction at the n-butyllithium and ultralow temperature condition in the literature is avoided, and the palladium catalyst is adopted for catalyzing, so that the amount of the used catalyst is reduced, and the cyclopentene / cyclohexene-1-boronic acid pinacol ester can be prepared in relatively high yield at the acceptable temperature; the boronizing agent n-butyllithium / isopropoxy boronic acid pinacol ester system is replaced by the boronizing agent palladium catalyst / bis(pinacolato)diboron system, and therefore, the cost of the raw materials is greatly reduced, the reaction condition is relatively easy to realize, amplification is easy, and the technical operation is simpler and more convenient.
Owner:DALIAN NETCHEM CHIRAL TECH
Hole-transporting type blue luminescent material as well as preparation and uses thereof
InactiveCN101225298AHigh fluorescence quantum yieldImprove thermal stabilitySolid-state devicesSemiconductor/solid-state device manufacturingSolventNitrogen gas
The invention relates to a hole-transporting blue color compound, which is a fluorine derivative based on 9, 9-bi-(4-(bi-p-methypheny) aminophenyl). The preparation method is to first mix 9, 9-bi-[4-(N, N-bi-p-toluidine) phenyl]-2-dibromofluorine and boric acid pinacol ester of conjugate radicle, or to mix 9, 9-bi-[4-(N, N-bi-p-tolyl-amino) phenyl] fluorine -2, 7-bi-boric acid pinacol ester and bromide of conjugate radicle; and then add in toluene, potash solution, aliquat336 and catalyst tetrakis (triphenylphosphine) palladium, and heat and reflow for 10 to 30 hours in nitrogen environment; when the reaction is done, decompress, remove the solution and get the target product, the derivative based on 9, 9-bi-(4-(bi-p-methypheny) aminophenyl) fluorine substituted by unilateral conjugate radical. The compound can be used as the hole-transporting layer and the luminescent layer as well in electroluminescent devices.
Owner:INST OF CHEM CHINESE ACAD OF SCI +1
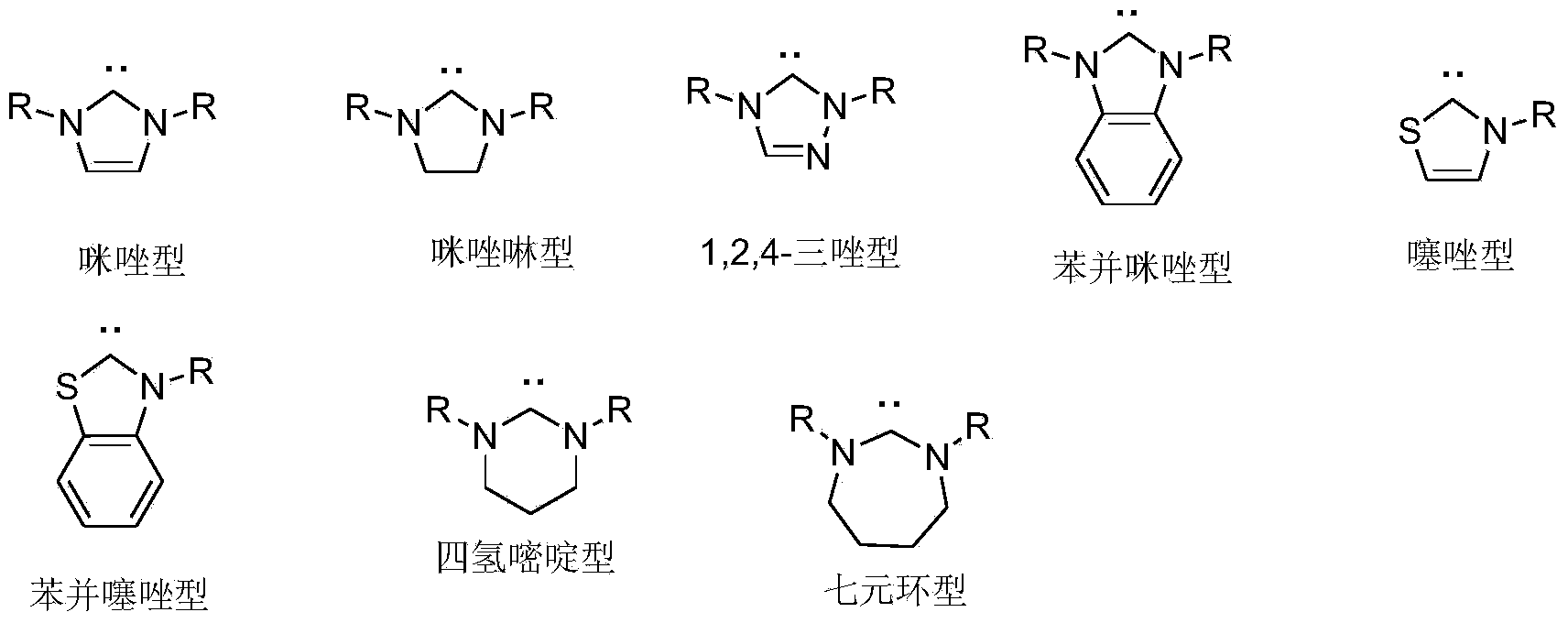
Chiral hexahydroxy n-heterocyclic carbine precursor compound as well as preparation method and application thereof
InactiveCN103772297AOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsOrganic synthesisKetone
The invention relates to the field of organic synthesis, and particularly relates to a preparation method and an application of a chiral hexahydroxy n-heterocyclic carbine precursor compound. The compound has a structure as shown in a formula (V) in descriptions. The chiral hexahydroxy n-heterocyclic carbine precursor compound can be used for catalyzing multiple chiral reactions such as an addition reaction of unsaturated esters, alpha,beta-unsaturated imine and diborane pinacol borate, or a condensation reaction of aldehyde and boric acid compounds or a reduction reaction of ketone and has relatively good catalytic efficiency and enantioselectivity. (imgfile='DDA0000462319780000011.T'IF wi='392 he='496' / ).
Owner:SHANGHAI UNIV OF ENG SCI
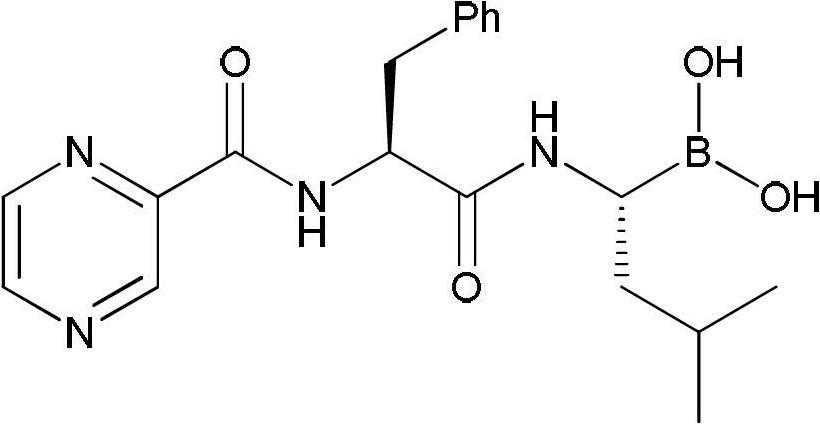
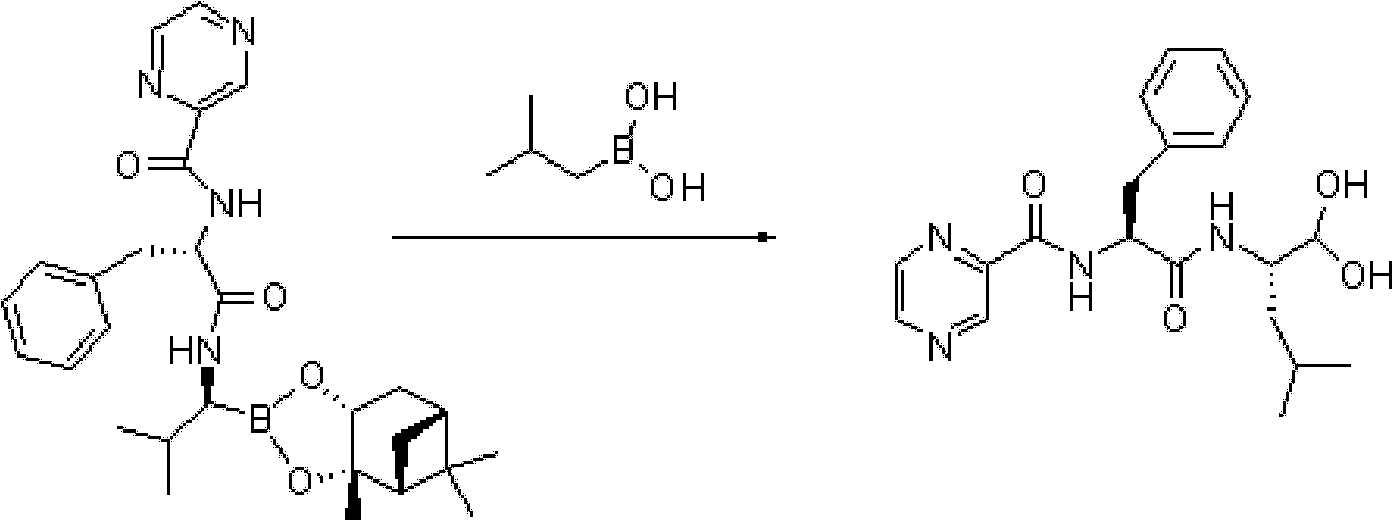
Synthetic method of bortezomib
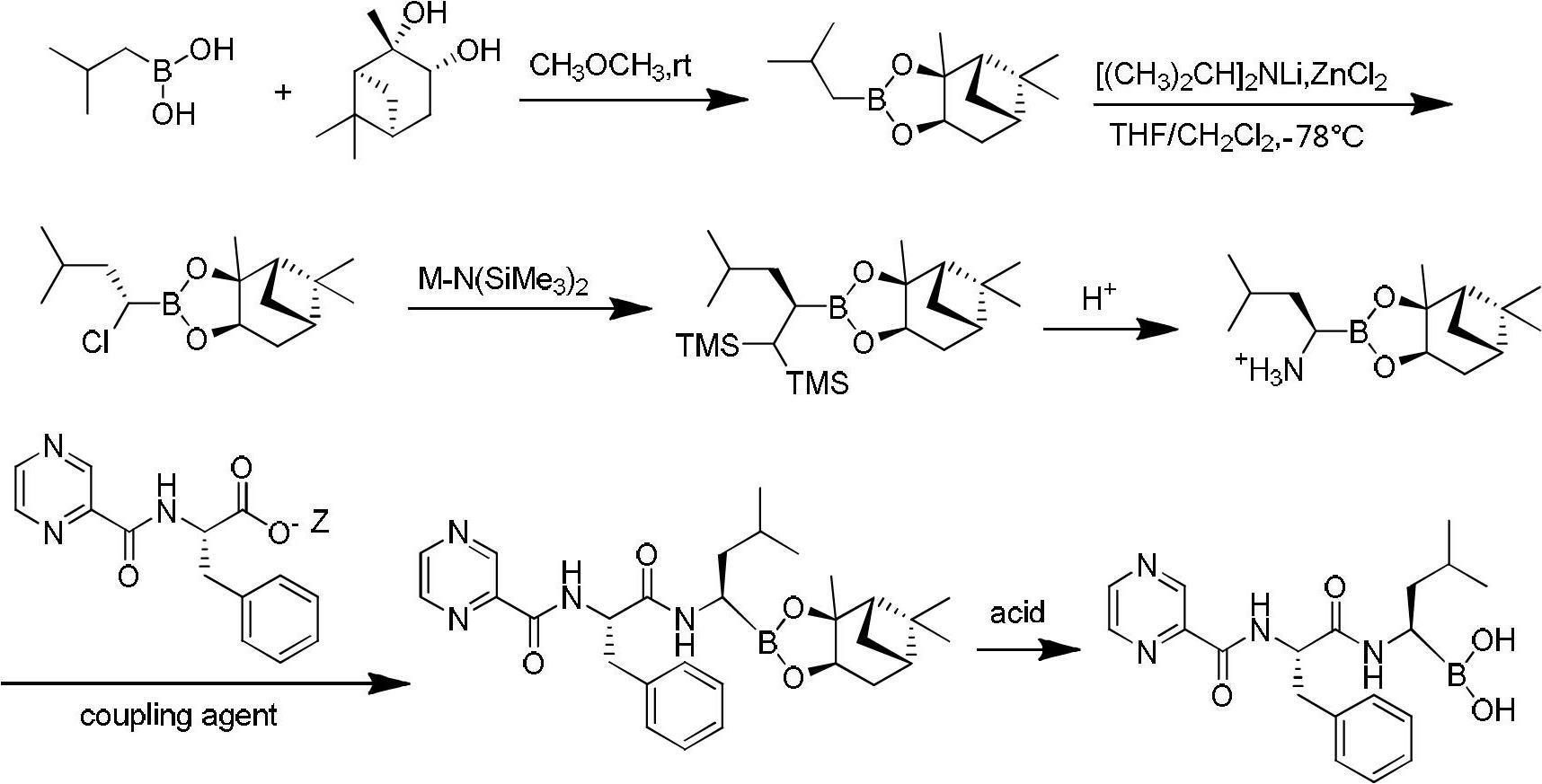
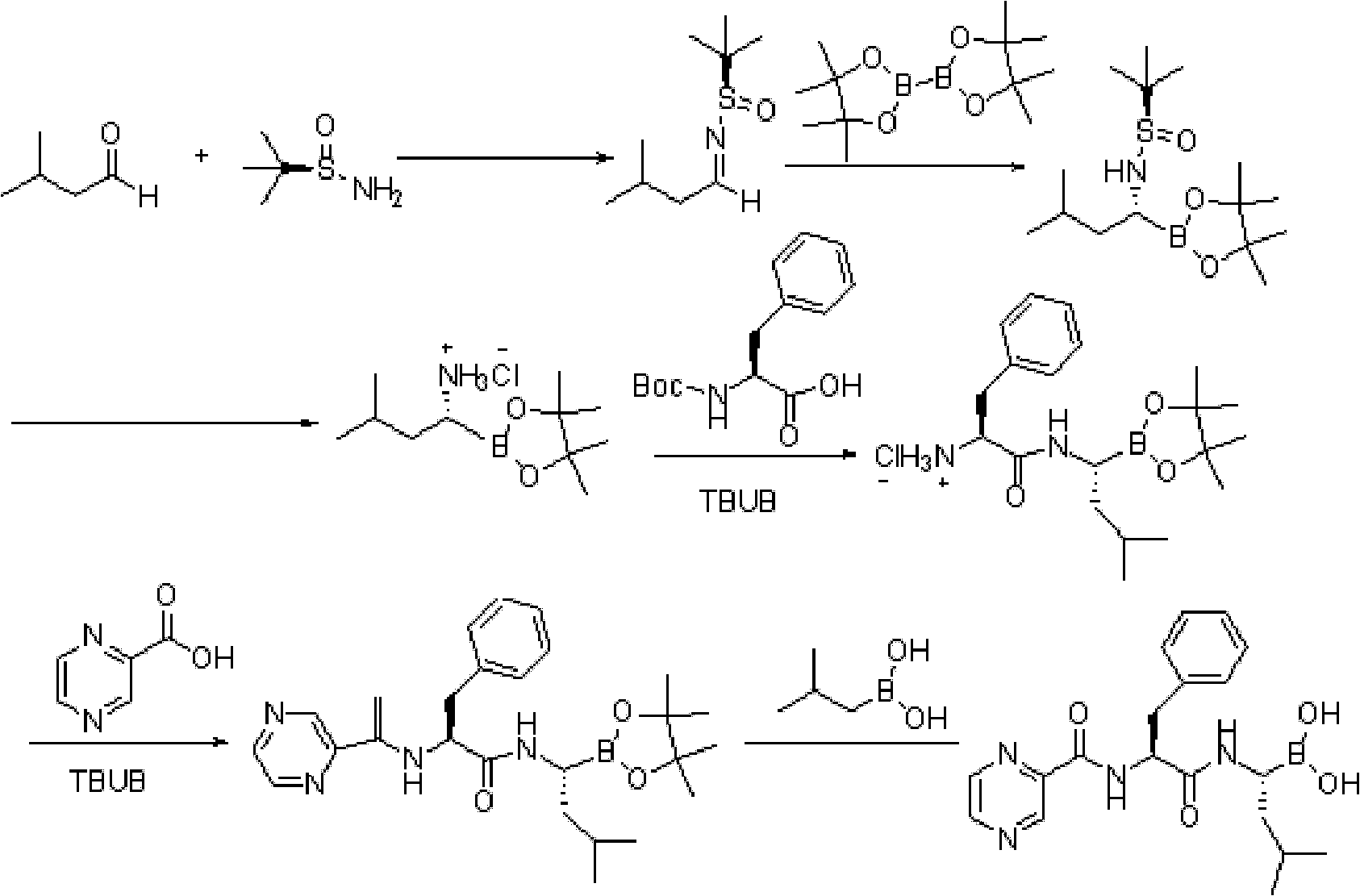
The invention discloses a synthetic method of bortezomib, which comprises the following steps of: taking isovaleraldehyde as an initial raw material, taking (R)-methylpropane-2-sulfinamide as a chiral reagent, generating (R,E)-2-methyl-N-(3-methyl butylidene) propane-2-sulfinamide by a condensation and dehydration reaction, then carrying out a nucleophilic addition reaction with pinacol diboron so as to generate (R)-1-N-methylpropane sulfinyl-3-methyl butane-1-pinacol borate ester, afterwards hydrolyzing under an acidic condition so as to obtain pinacol-(R)-1-amino-3-methyl butane-1-borate ester hydrochloride, then reacting with (S)-3-phenyl-2-(pyrazine-2-formamido) propionic acid under the existence of a coupling agent and also hydrolyzing under the action of isobutyl borate so as to generate a final product of the bortezomib. According to the synthetic method of the bortezomib, the (R)-methylpropane-2-sulfinamide which is easy to obtain is used as the chiral induction reagent, so that an obtained intermediate enantiomorph has higher purity, and a bulk drug which is finally obtained has better quality.
Owner:HEFEI UNIV OF TECH
Water-soluble photocuring 3D printing material
ActiveCN108383948AGood dimensional stabilitySmall shrinkageAdditive manufacturing apparatusPolymer chemistryAcrylic monomers
The invention relates to a water-soluble photocuring 3D printing material. The printing material comprises the following components in parts by weight: 100 parts of a polyacrylate prepolymer, 10 to 30parts of water-soluble urethane acrylate, 20 to 50 parts of an acrylic monomer thinner and 0.1 to 5 parts of an initiator, wherein the urethane acrylate is prepared from an acrylic acid polymer and isocyanate; the acrylic acid polymer is formed through copolymerization of acrylic monomers; the acrylic monomers at least comprise polyoxyalkylene acrylate monomers, hydroxy acrylic acid monomers andboric acid pinacol ester monomers; and the boric acid pinacol ester monomers are a compound comprising boric acid pinacol ester and unsaturated bonds. The prepared and obtained 3D printing material has extremely low shrinking percentage.
Owner:HUZHOU JIFU NEW MATERIALS SCI & TECH CO LTD
Active oxygen-responsive polymer support and preparation method thereof
ActiveCN107082828AEasy to prepareGood water solubilityOrganic active ingredientsAntipyreticSolubilityFreeze-drying
The invention discloses an active oxygen-responsive polymer support and a preparation method thereof. The preparation method comprises the following steps: mixing 4-(hydroxymethyl) phenylboronic acid pinacol ester with organic alkali and nitrophenyl chloroformate, and stirring to react to obtain NBC; dissolving a water-soluble polymer containing amino into water, adding an organic solvent, so as to form a solution A; dissolving NBC into the organic solvent, so as to obtain a solution B; adding alkali and the solution A into the solution B; stirring to react, and adjusting the pH value of the reaction system to 6.5-7.0, so as to obtain a modified polymer crude product; and purifying crude product, and carrying out freeze-drying, so as to obtain an active oxygen-responsive polymer support pure product. The preparation method of the active oxygen-responsive polymer support is simple, and the prepared arylboronic acid modified polymer support is easy to be chemically coupled with polyphenol active substances containing a catechol structure, so that the water solubility and stability of the support are improved, and the oxygen-responsive release of the support can be realized; and the support has important application prospects in the fields of anti-inflammation, anti-cancer treatment and the like.
Owner:JINAN UNIVERSITY
Compounds, compositions, kits and methods of use to orally and topically treat acne and other skin conditions by administering a 19-nor containing vitamin d analog with or without a retinoid
Oral and topical pharmaceutical compositions, kits and methods of treatment thereof for treating various skin disorder including acne, psoriasis, ichthyosis, photoaging, photodamaged skin, and, skin cancer. Exemplary vitamin D analogs as active pharmaceutical ingredients include 2-methylene-19-nor-20(S)-1α-hydroxy-bishomopregnacalciferol, 19-nor-26,27-dimethylene-20(S)-2-methylene-1α,25-dihydroxyvitamin D3, 2-methylene-1α,25-dihydroxy-(17E)-17(20)-dehydro-19-nor-vitamin D3, 2-methylene-19-nor-(24R)-1α,25-dihydroxyvitamin D2, 2-methylene-(20R,25S)-19,26-dinor-1α,25-dihydroxyvitamin D3, 2-methylene-19-nor-1α-hydroxy-pregnacalciferol, 1α-hydroxy-2-methylene-19-nor-homopregnacalciferol, (20R)-1α-hydroxy-2-methylene-19-nor-bishomopregnacalciferol, 2-methylene-19-nor-(20S)-1α-hydroxy-trishomopregnacalciferol, 2-methylene-23,23-difluoro-1α-hydroxy-19-nor-bishomopregnacalciferol, 2-methylene-(20S)-23,23-difluoro-1α-hydroxy-19-nor-bishomopregnancalciferol, (2-(3′hydroxypropyl-1′,2′-idene)-19,23,24-trinor-(20S)-1α-hydroxyvitamin D3, 2-methylene-18,19-dinor-(20S)-1α,25-dihydroxyvitamin D3, a stereoisomer thereof, a prodrug thereof in oral compositions, a salt thereof, and / or a solute thereof. Compounds that activate retinoic acid receptors, such as retinoyls and retinoyl esters, include 13-cis-retinoic acid, all-trans-retinoic acid, (2E,4E,6Z,8E)-3,7-dimethyl-9-(2,6,6-trimethyl-1-cyclohexeneyl)nona-2,4,6,8-tetraenoic acid, 9-(4-methoxy-2,3,6-trimethyl-phenyl)-3,7-dimethyl-nona-2,4,6,8-tetraenoic acid, 6-[3-(1-adamantyl)-4-methoxyphenyl]-2-napthoic acid, 4-[1-(3,5,5,8,8-pentamethyl-tetralin-2-yl)ethenyl]benzoic acid, retinobenzoic acid, ethyl 6-[2-(4,4-dimethylthiochroman-6-yl)ethynyl]pyridine-3-carboxylate, retinoyl t-butyrate, retinoyl pinacol, retinoyl cholesterol, an isomer thereof, a prodrug thereof for oral compositions, an ester thereof, a salt thereof, and / or, a solute thereof. Combinations of such active ingredients demonstrate synergistic efficacy.
Owner:WISCONSIN ALUMNI RES FOUND
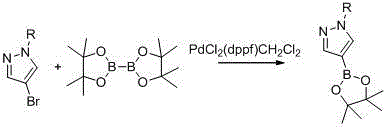
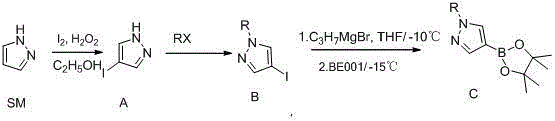
Synthetic method of 1-alkylpyrazole-4-boronic acid pinacol ester
ActiveCN103601749ALow costMild reaction conditionsGroup 3/13 element organic compoundsChemical synthesisIsopropyl
The invention belongs to the field of organic chemical synthesis and provides a synthetic method of 1-alkylpyrazole-4-boronic acid pinacol ester. The synthetic method comprises the following three steps: 1. reacting pyrazole with iodine and hydrogen peroxide to generate 4-iodopyrazole A; 2. reacting the 4-iodopyrazole with alkyl halide to obtain an intermediate B; 3. preparing a Grignard reagent of the raw material by using 1-alkyl-4-iodopyrazole as a raw material and adopting an isopropyl Grignard reagent exchange method at 0-30 DEG C, with BE001 as a boron reagent, and reacting to obtain the final product. The technological method is accessible in raw materials, simple and convenient to operate and lower in cost and is a proper method for preparing 1-alkylpyrazole-4-boronic acid pinacol ester compounds.
Owner:DALIAN NETCHEM CHIRAL TECH
New synthesis process of oxazolinone antibiotic
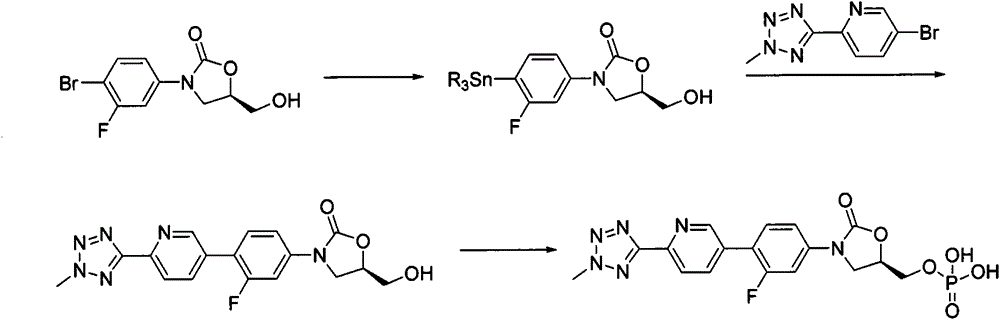
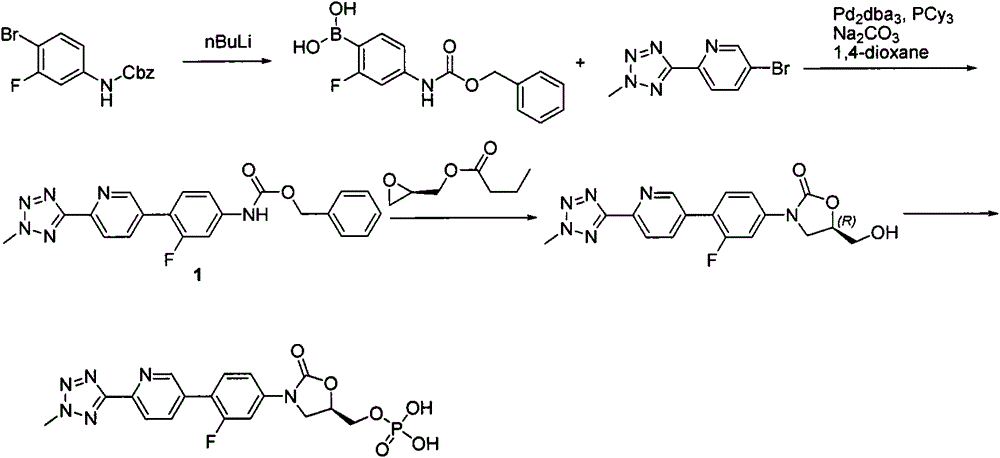
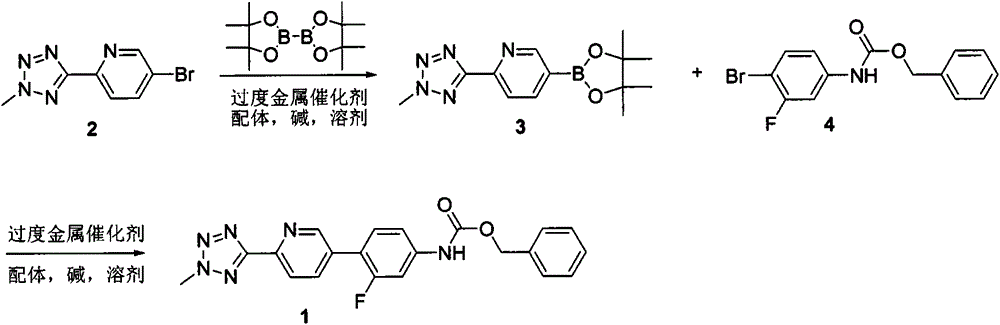
According to the method of the present invention, a methyl tetrazole pyridine bromide (2) and pinacol diboron are subjected to a reaction under catalysis of a transition metal to obtain a boronic acid pinacol ester (3), the compound (3) is separated or is not separated, and the separated compound (3) or the un-separated compound (3) and Cbz-protected bromobenzene (4) are subjected to a reaction under catalysis of a transition metal to obtain a key intermediate (1) of tedizolid. According to the present invention, the compound (3) is not subjected to separation purification, and reacts with the compound (4) in a kettle to generate the compound (1).
Owner:BEIJING CHEMPION BIOTECHNOLOGY CO LTD
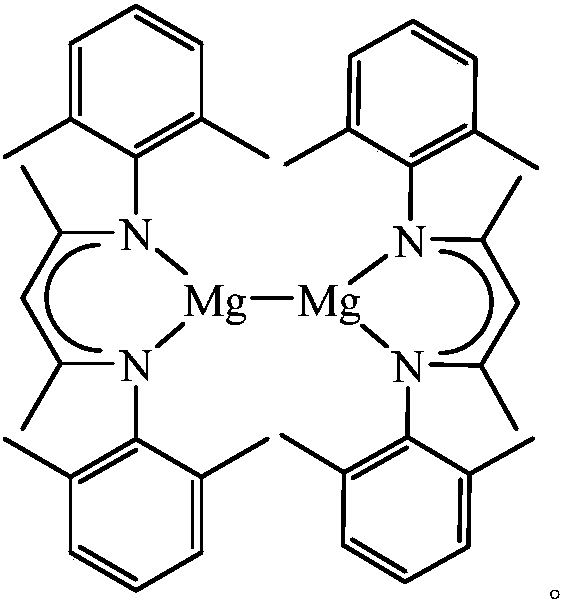
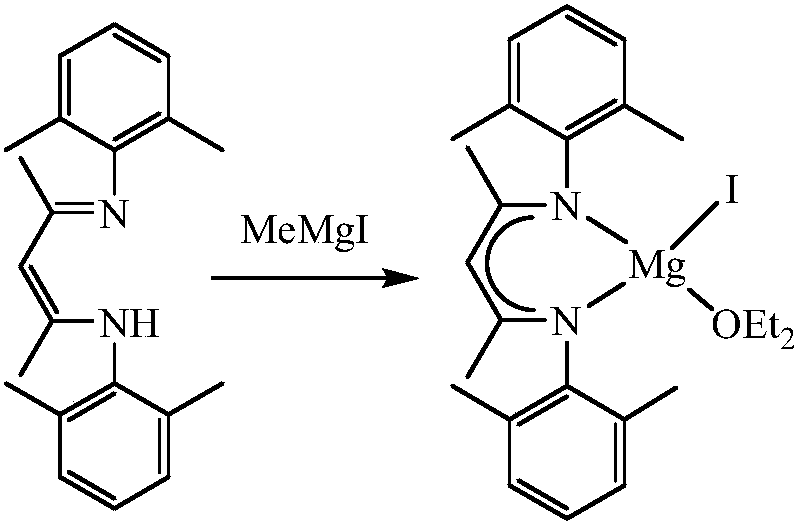
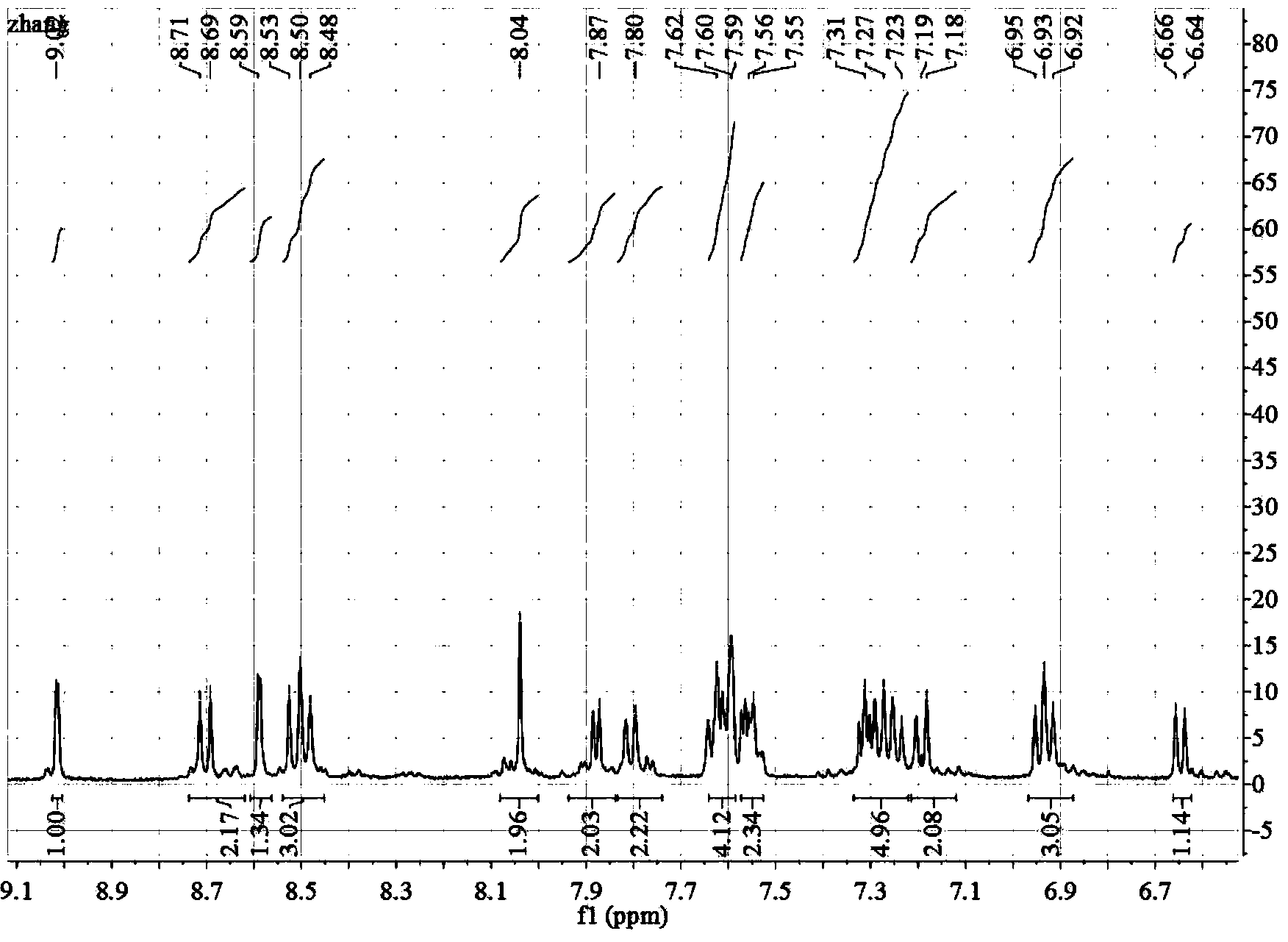
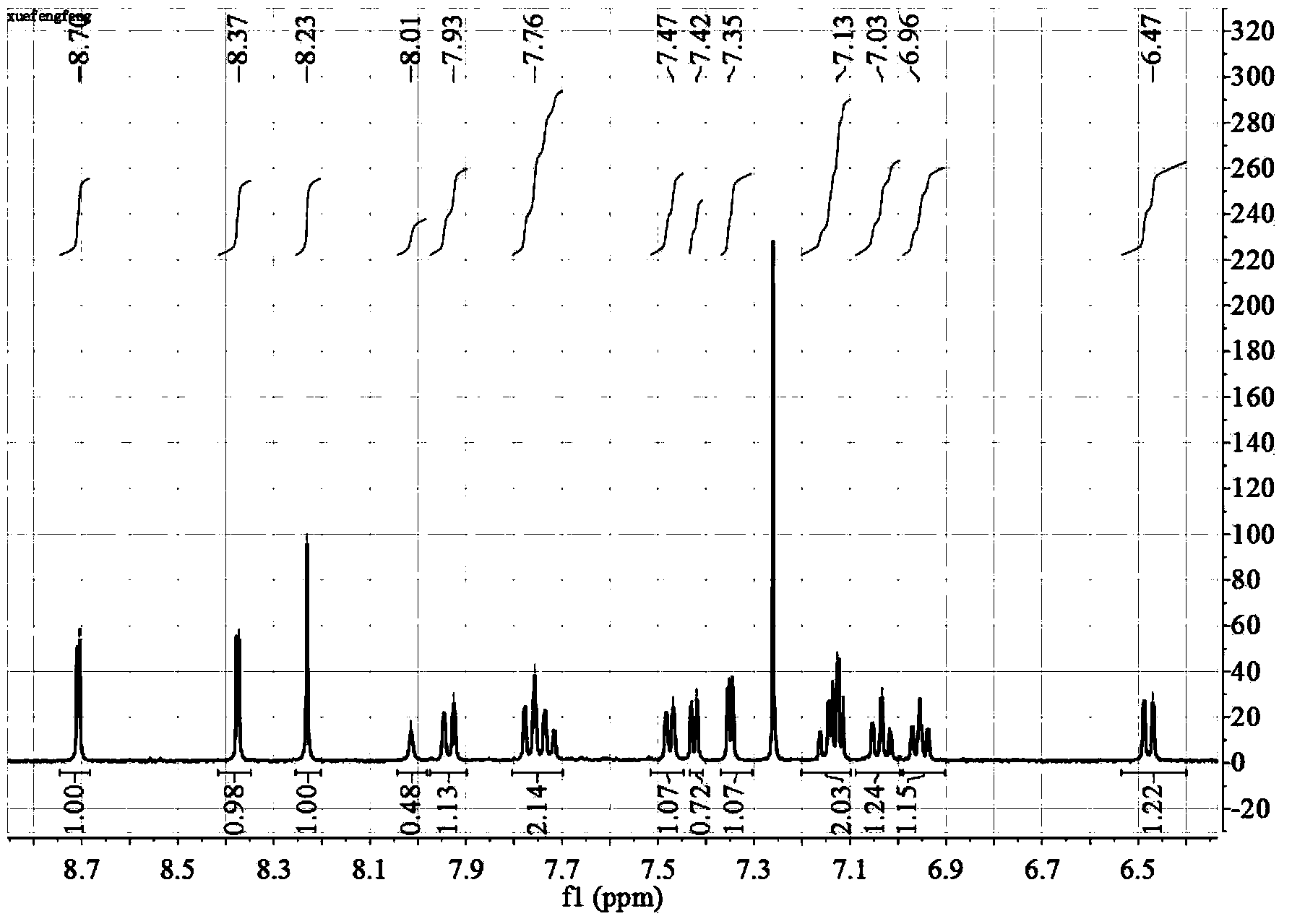
Beta-diimide monovalent magnesium compound, preparation method thereof and application of beta-diimide monovalent magnesium compound in hydroboration of aldehyde or ketone
ActiveCN107602595AEasy to purifyLow toxicityCarboxylic acid nitrile preparationOrganic compound preparationDiimineIodide
The invention discloses a beta-diimide monovalent magnesium compound, a preparation method thereof and an application of the beta-diimide monovalent magnesium compound in hydroboration of aldehyde orketone. The preparation method comprises the following steps: under anhydrous and anaerobic conditions, a beta-diimine ligand reacts with a Grignard reagent, iodide of magnesium is generated and reduced by sodium, and a yellow crystal is obtained and is the beta-diimide monovalent magnesium compound. The beta-diimide monovalent magnesium compound is simple to synthesize, convenient to separate andpurify, clear in structure and high in yield; activity of the compound as a catalyst in catalysis of a reaction of aldehyde or ketone with pinacolborane is high, and the substrate universality is broad.
Owner:厦门欧瑞捷生物科技有限公司
Thienyl phosphorescent iridium complex as well as preparation method and application thereof
InactiveCN103881700AImprove stabilityEasy to prepareEnergy modified materialsGroup 8/9/10/18 element organic compoundsSinglet oxygenPhenanthroline
The invention discloses a thienyl phosphorescent iridium complex. A preparation method for the thienyl phosphorescent iridium complex comprises the following steps: weighing 3,8-dibromoiridium phenanthroline-2-phenylpyridine complex (or 3,8-dibromoiridium phenanthroline-2-phenylquinoline complex) and 2-thiophene pinacol borate at a molar ratio of 1:(0.1-12), and adding tetra(triphenyl phosphine) palladium with the molar percentage of 1-20%; adding sodium carbonate aqueous solution with a proper concentration of 2 M, adding an appropriate amount of DMF (dimethyl formamide), and stirring at a temperature of 25-75 DEG C for 1-75 hours; after the reaction is finished, cooling the reaction solution to a room temperature, pouring the reaction solution in water, and stirring for 1-30 minutes; extracting a water phase by use of dichloromethane, combining an organic phase, and drying the organic phase by use of anhydrous magnesium sulphate; filtering the solution, removing the filtrate, and removing a solvent in vacuum to obtain a crude product. The iridium complex obtained by the preparation method disclosed by the invention is used for generating singlet oxygen from a steady-state near-infrared light, good in stability, and expected to be applied in the aspect of near-infrared photodynamic therapy.
Owner:SHANGHAI NORMAL UNIVERSITY
Synthesis method of pyrazol-4-boronic acid pinacol ester
ActiveCN106188116AAvoid decompositionEasy to operateGroup 3/13 element organic compoundsLithiumSynthesis methods
The invention discloses a synthesis method of pyrazol-4-boronic acid pinacol ester. The method consists of: reacting 4-halogenopyrazole with BOC2O to obtain N-BOC-4-halogenopyrazole, then carrying out reaction with alkyl magnesium lithium, adding aminopinacol borate, conducting acid quenching deprotection, then carrying out rotary evaporation filtering to obtain a solid, and adding organic alkali to conduct dissociation so as to obtain a product. The synthesis method has the advantages of simple operation, good reproducibility and high product purity, and is suitable for large-scale production.
Owner:CANGZHOU PURUI DONGFANG SCI & TECH
Mazarine electroluminescent compound, and preparation method and application thereof
ActiveCN105237519ASolve the problem of excessive starting voltageOrganic chemistrySolid-state devicesQuantum efficiencyHalogen
The invention discloses a mazarine electroluminescent compound, and a preparation method and application thereof. The mazarine electroluminescent compound has a structure as shown in a formula I which is described in the specification. In the formula, X is O or S; R1 is H, halogen, a cyano group, an alkyl group, an alkyloxy group or a phosphate radical; and R2 is H, a diphenylphosphine oxide radical, a diphenylamino group or a carbazolyl group. The compound as shown in the formula I is prepared by preparing a dibenzofuran(or dibenzothiophene)-4-pinacol borate precursor and a phenanthroimidazole precursor at first and then carrying out a coupling reaction. The preparation method for the compound is easy to operate and has high yield; the compound has mazarine fluorescence and good monochromaticity; and an OLED device prepared with the compound as a luminescent material has mazarine emission, starting voltage of 3.6 V and maximum external quantum efficiency of 1.63%.
Owner:PEKING UNIV
Glucan polymer, polymer micelle and medicine carrier system
The invention relates to a glucan polymer, a polymer micelle and a medicine carrier system. The glucan polymer is prepared from glucan, 4-(hydroxymethyl)benzeneboronic acid pinacol ester and polyol fatty acid through reaction, wherein the glucan and the polyol fatty acid provide hydrophilic and hydrophobic units, so that the polymer can automatically form the micelle; the polymer formed by the glucan, the 4-(hydroxymethyl)benzeneboronic acid pinacol ester and the polyol fatty acid through chemical reaction is sensitive to hydrogen peroxide; the response can be fast generated on hydrogen peroxide in an environment of ROS high expression diseases (such as myocardial ischemial injury, atherosclerosis, intestinal inflammation and tumor); the micelle disassembly is caused, so that the medicinemolecules are fast released.
Owner:THE SECOND HOSPITAL AFFILIATED TO WENZHOU MEDICAL COLLEGE
Method for preparing pinacol by dehydrogenation of photocatalytic isopropanol dehydrogenation and hydrogenation coupling of acetone
ActiveCN102976896AEasy to operateNo secondary pollutionOrganic compound preparationHydroxy compound preparationDehydrogenationReaction temperature
The invention discloses a method for preparing pinacol by dehydrogenation of photocatalytic isopropanol and hydrogenation coupling of acetone, comprising the following steps of: adding a semiconductor photocatalyst in a reaction solution which is composed of acetone, isopropanol and a solvent in a ratio of the reaction solution to the semiconductor photocatalyst of (100 to 500 ml): (0.1 to 3.5 g); magnetically stirring; illuminating at the inert atmosphere to react for 0.5-180 hours at the reaction temperature of 5-55 DEG C by dehydrogenation of photocatalytic isopropanol dehydrogenation and hydrogenation coupling of acetone; after the reaction is concluded, separating to obtain a catalyst and a solution; and distilling the solution to obtain the product, namely, pinacol. The method disclosed by the invention has the advantages of high conversion rate and good selectivity.
Owner:SHANXI INST OF COAL CHEM CHINESE ACAD OF SCI
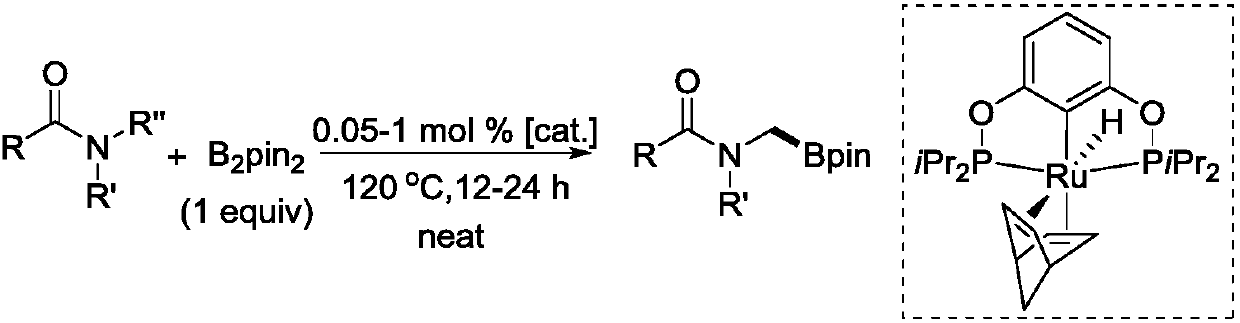
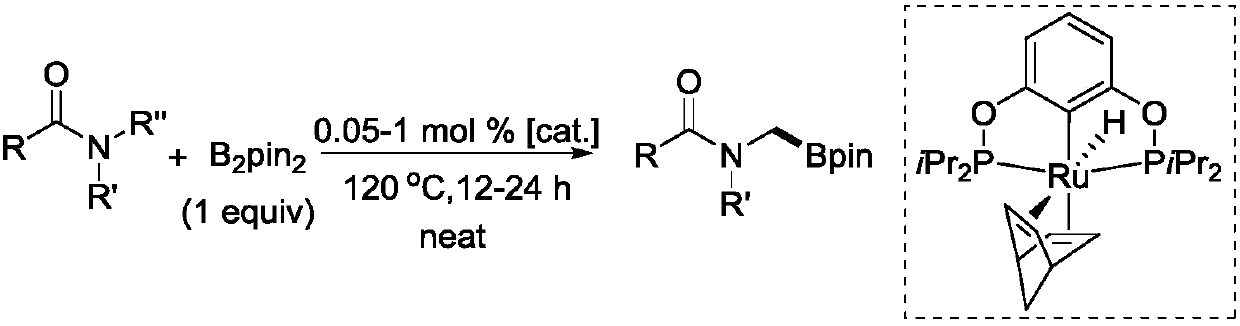
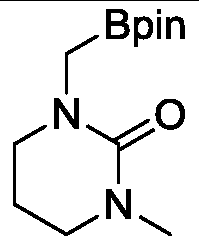
Novel method for ruthenium-catalyzed selective boronation reaction of amides
ActiveCN107892698AGroup 3/13 element organic compoundsFunctional group formation/introductionOrganic synthesisOrtho position
The present invention relates to a novel method for ruthenium-catalyzed selective boronation reaction of amides. According to the method, a hexacoordinated metal ruthenium complex containing a norbornadiene (NBD) ligand is taken as a catalyst, an N,N-disubstituted amide and bis(pinacolato)diboron are taken as reaction substrates, and in the absence of reaction solvents and under mild reaction conditions, a carbon-hydrogen bond of methylene at the ortho position of a nitrogen atom in the N,N-disubstituted amide is selectively subjected to a boronation reaction under efficient catalysis, so thata corresponding amide borate product is obtained. Compared with currently reported methods, the novel method generally has the advantages of wide substrate applicability, low catalyst usage amount, simple operation and the like. The method of the present invention realizes for the first time a selective C(sp3)-H boronation reaction of N,N-dimethyl substituted aromatic amide derivatives. In addition, the method realizes for the first time a metal-ruthenium-catalyzed selective dehydro-boronation reaction of N,N-disubstituted amides, and provides a completely new reaction strategy for organic synthetic intermediates of amide borates.
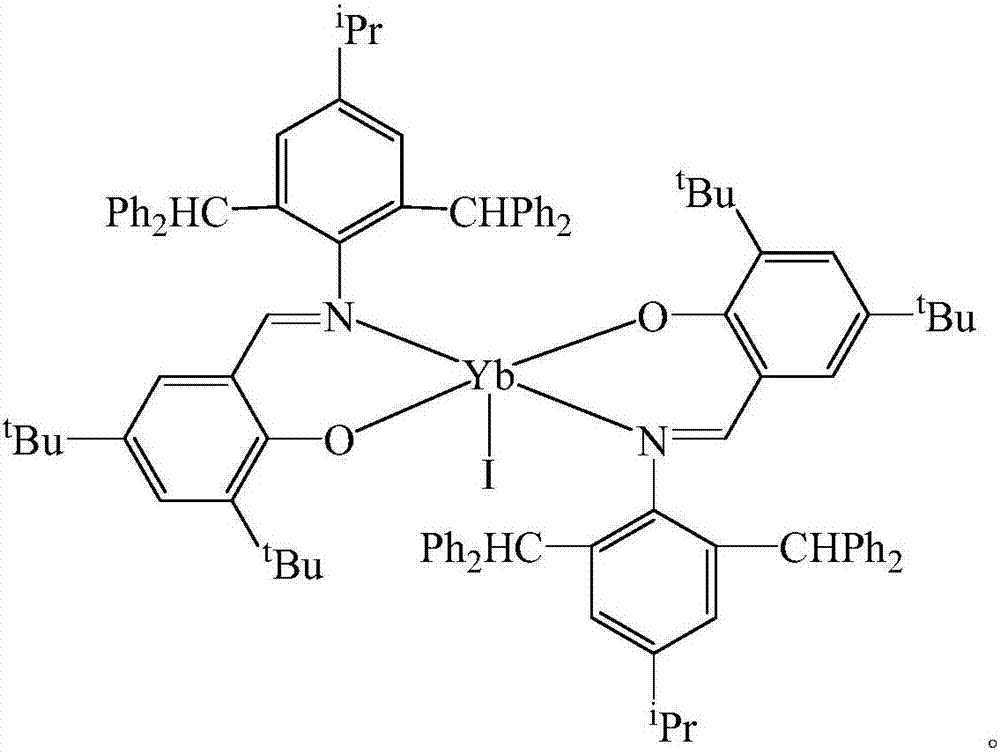
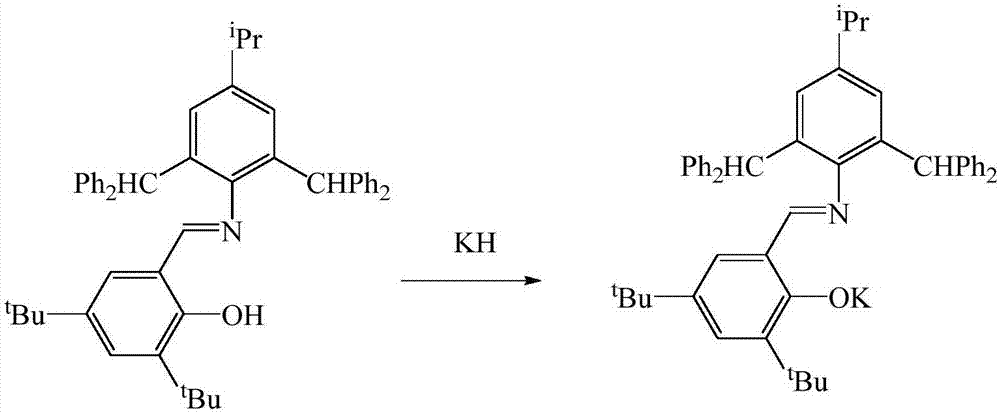
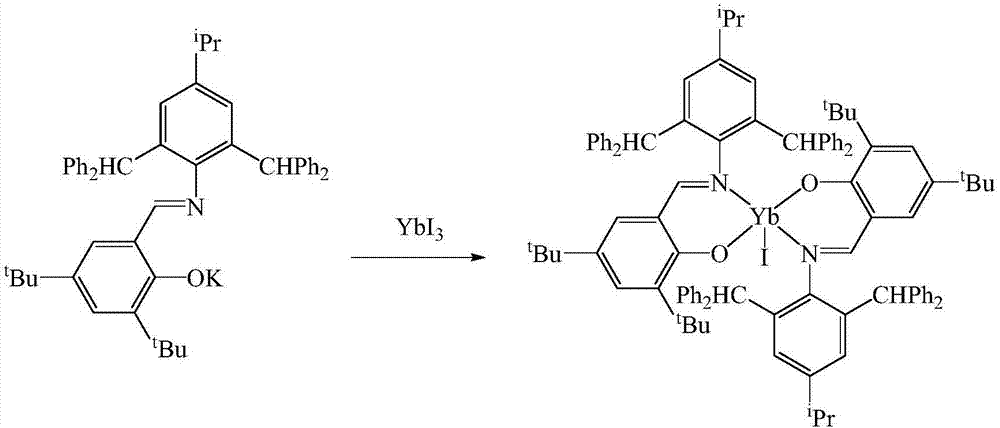
Schiff base rare earth ytterbium iodide, and preparation method and application thereof
InactiveCN107501309AEasy to purifyLow toxicityCarboxylic acid nitrile preparationOrganic compound preparationIodideRare earth
The invention discloses Schiff base rare earth ytterbium iodide, and a preparation method and the application thereof. The Schiff base rare earth ytterbium iodide is N-(4-isopropyl-2,6-di(diphenylmethyl)phenyl)-3,5-ditert-butyl-2-oxygen Schiff base rare earth ytterbium iodide. The preparation method comprises the following steps: performing reaction on N-(4-isopropyl-2,6-di(diphenylmethyl)phenyl)-3,5-ditert-butyl-2-hydroxybenzaldimine and potassium hydride to obtain corresponding potassium salt under the conditions of no water and no oxygen, and performing reaction on the potassium salt and ytterbium iodide to obtain a yellow crystal, namely the N-(4-isopropyl-2,6-di(diphenylmethyl)phenyl)-3,5-ditert-butyl-2-oxygen Schiff base rare earth ytterbium iodide. The Schiff base rare earth ytterbium iodide is simple in synthesis, convenient in separation and purification, definite in structure and high in yield; the Schiff base rare earth ytterbium iodide serving as a catalyst has high activity of catalyzing reaction of aldehyde or ketone and pinacolborane and wide substrate universality.
Owner:NANJING FORESTRY UNIV +1
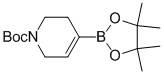
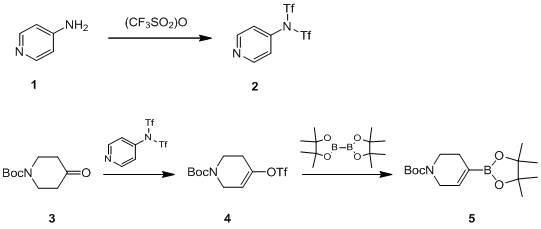
Method for synthesizing N-tert-butoxycarbonyl-1,2,5,6-tetrahydropyridine-4-boronic acid pinacol ester
InactiveCN102153579AShort process routeThe synthesis process is simpleGroup 3/13 element organic compoundsTrifluoromethanesulfonic anhydrideChemical synthesis
The invention provides a novel method for synthesizing N-tert-butoxycarbonyl-1,2,5,6-tetrahydropyridine-4-boronic acid pinacol ester, which belongs to the technical field of chemical synthesis. The method comprises the following steps of: obtaining a target product, namely the N-tert-butoxycarbonyl-1,2,5,6-tetrahydropyridine-4-boronic acid pinacol ester through a three-step reaction by taking N-(tert-butoxycarbonyl)-4-piperidone, 4-aminopyridine, trifluoromethanesulfonic anhydride, n-butyl lithium, bis(pinacolato)diboron, triethylamine, diisopropylamine and potassium acetate as raw materials,dichloromethane, tetrahydrofuran and dioxane as solvents, and [1,1'-bis(diphenylphosphino)ferrocene]dichloropalladium(II) as a catalyst; and characterizing data through liquid chromatogram, nuclear magnetic spectrum and mass spectrum. By the method, the production period is short, the synthetic cost is low, a synthetic process is safe and reliable, and a post-treatment method is simple, convenient and quick; and the yield of a product is high (51 to 58 percent) and the purity of the product is high (98.2 to 99.6 percent).
Owner:LANZHOU MINUO BIOLOGICAL TECH
Dendritic hyperbranched polymer as well as preparation method and use thereof
ActiveCN103159940AWeaken electrostatic interactionsOrdered Noncentrosymmetric ArrangementNon-linear opticsPolymer scienceChemical reaction
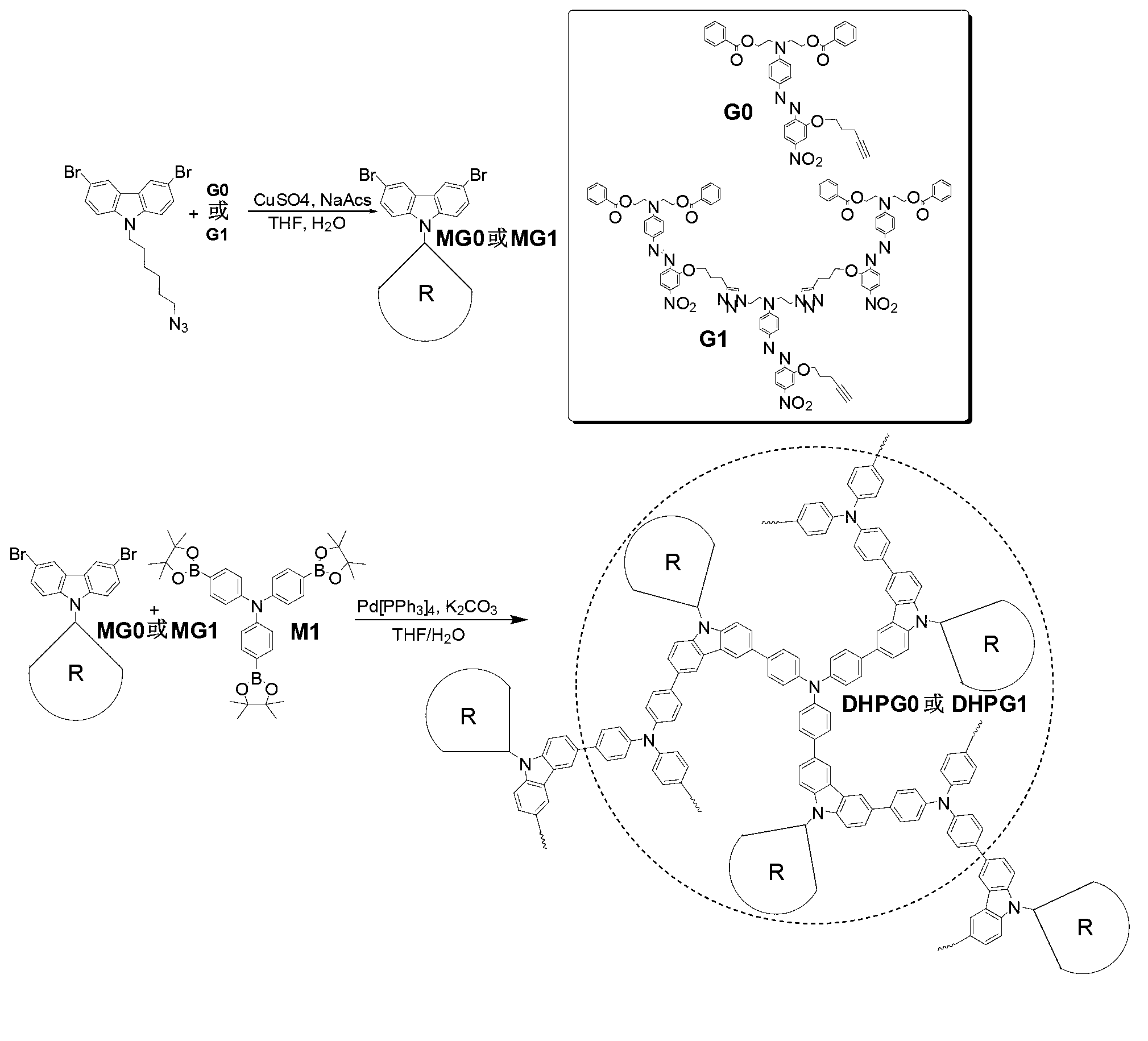
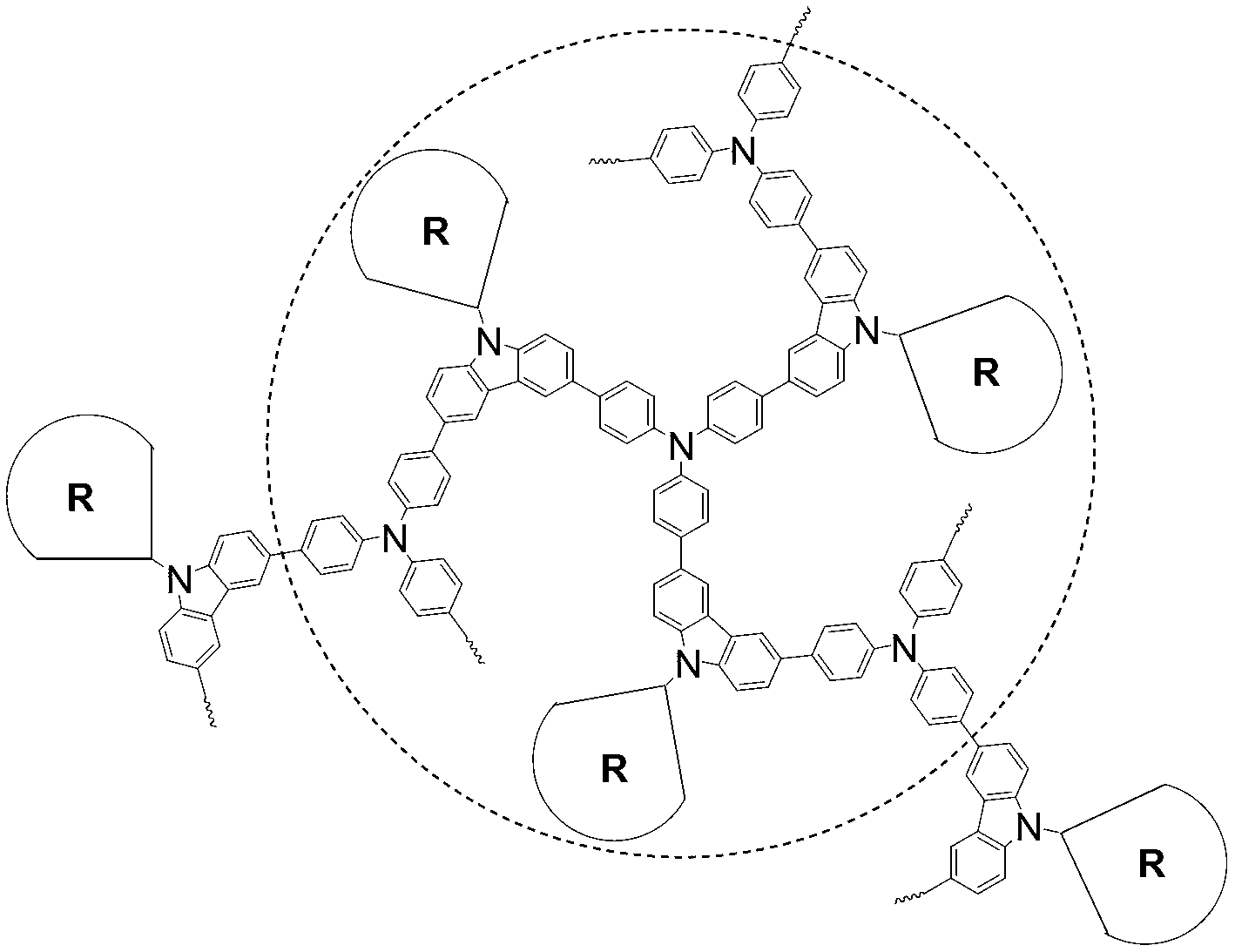
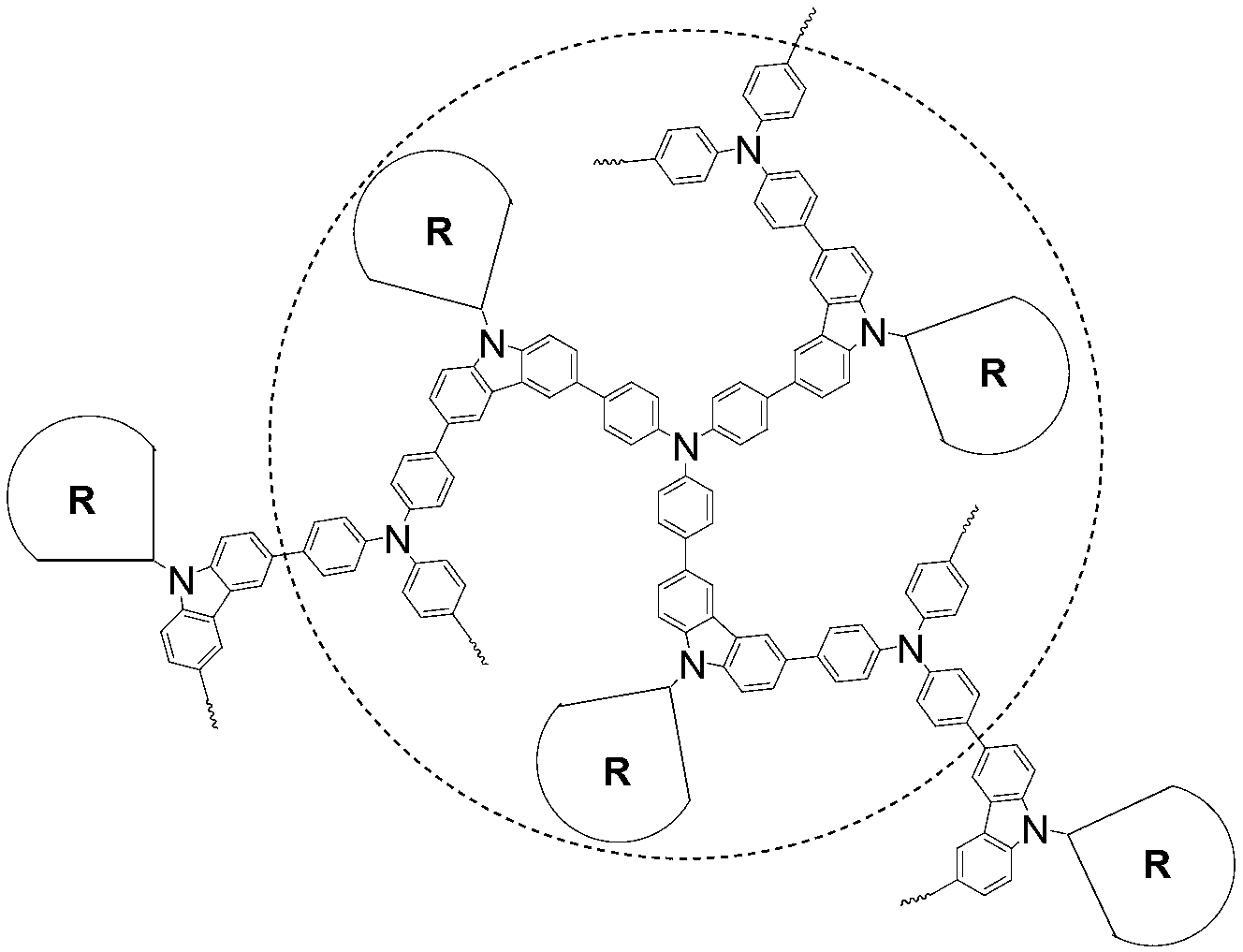
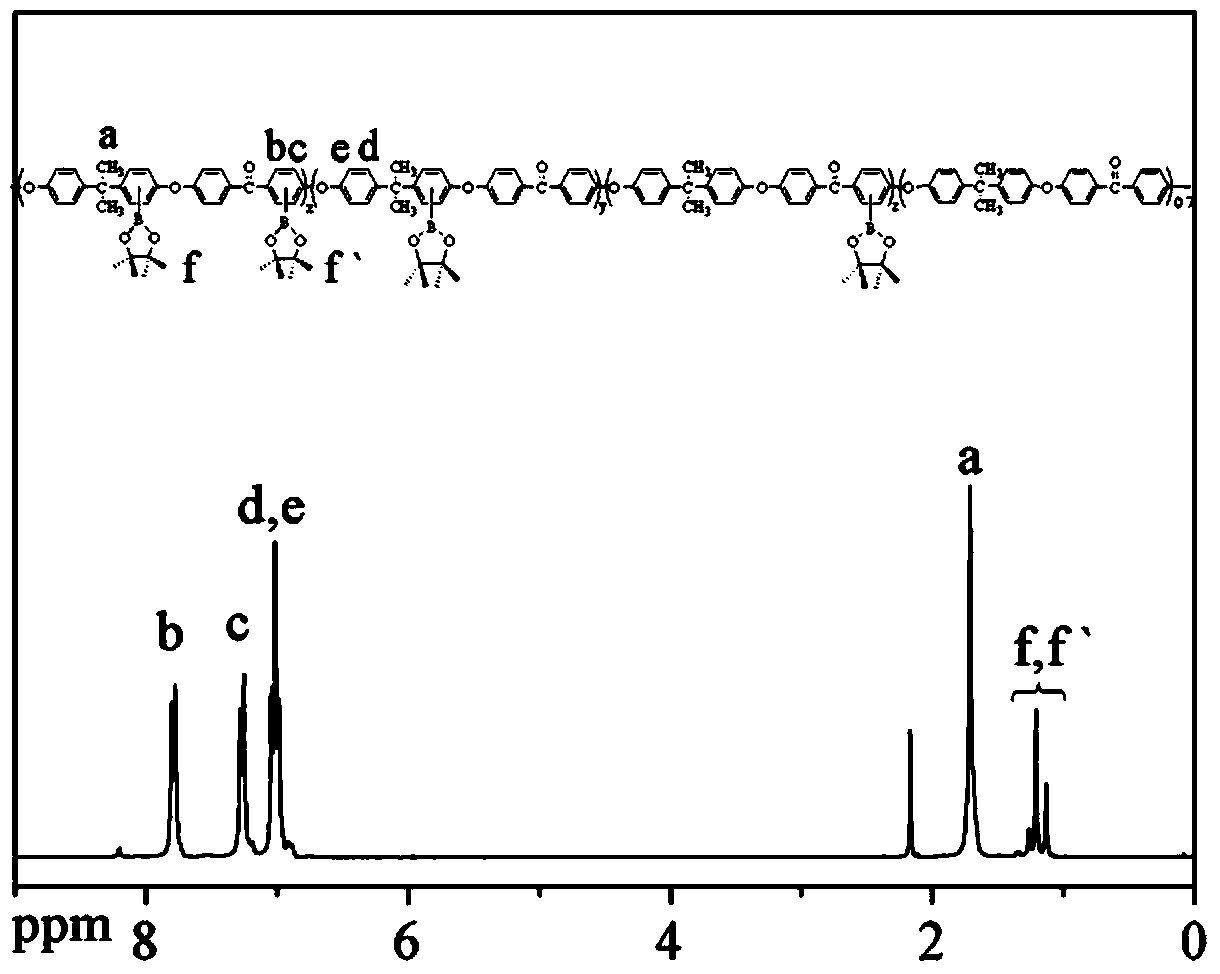
The invention discloses a dendritic hyperbranched polymer as well as a preparation method and a use thereof. The structural formula of the polymer is shown in the specification, wherein R is a low-algebraic dendritic macromolecule based on azobenzene chromophore. The dendritic hyperbranched polymer is prepared through substitution reaction, click chemical reaction and suzuki reaction of 9-(6-azidohexyl)-3,6-dibromocarbazole, triphenylamine tri-band pinacol cyclic ester as well as the low-algebraic dendritic macromolecule based on azobenzene chromophore. The polymer disclosed by the invention is gentle in polymerization reaction conditions, high in yield, large in molecular weight and low in degree of dispersion. The polymer disclosed by the invention has good second-order nonlinear optical performance and thermal stability, and can be used as a second-order nonlinear optical material for being practically applied in the aspects of remote communication, data storage, data conversion, phase conjugation, signal modulation and the like.
Owner:WUHAN UNIV
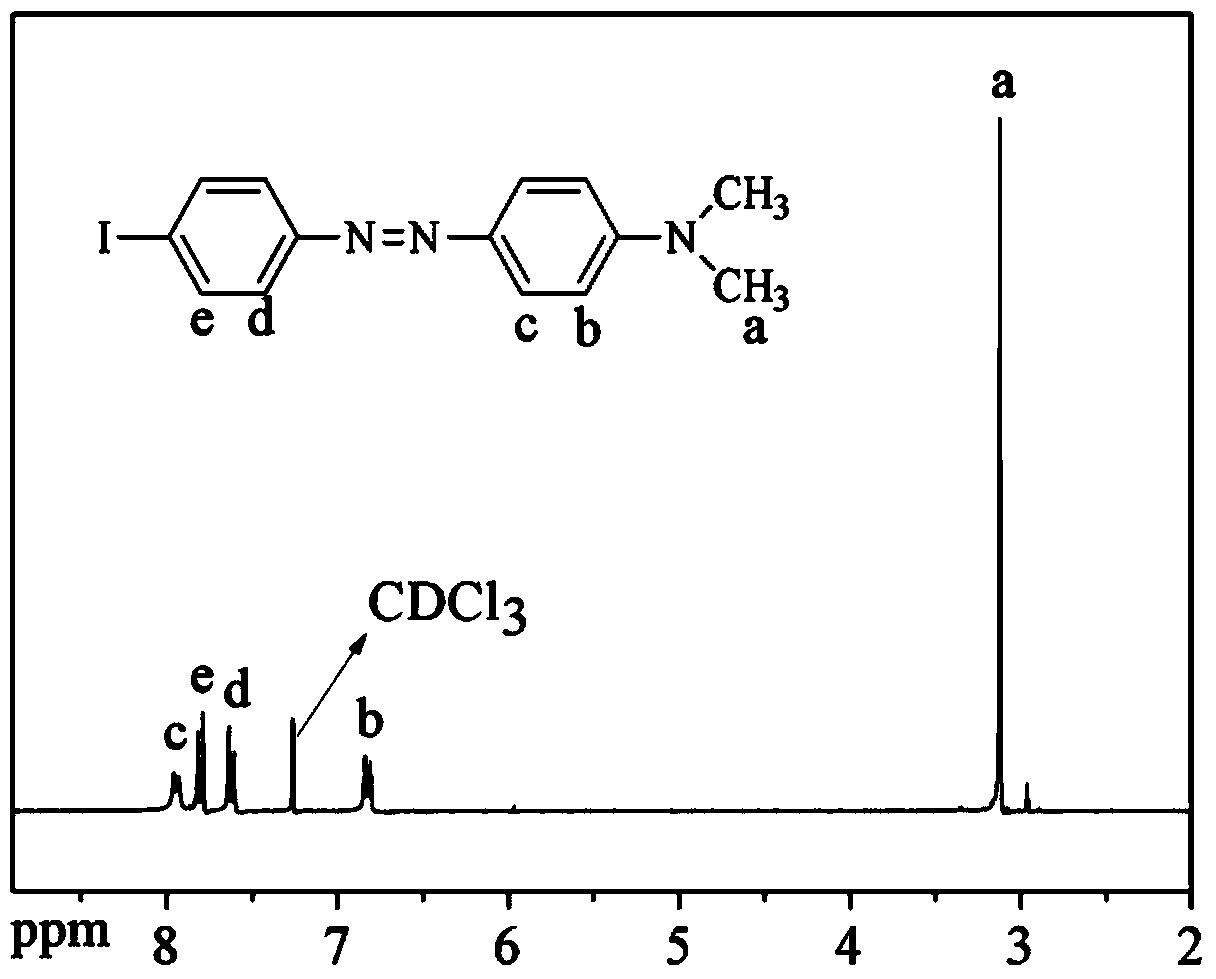
Polyaryletherketone containing boric acid ester, azo polyaryletherketone and preparation method thereof
The invention discloses polyaryletherketone containing a boric acid ester, azo polyaryletherketone and a preparation method thereof, and belongs to the technical field of preparation of a high polymer material. The method comprises the following steps: firstly, synthetizing bisphenol A polyaryletherketone, and carrying out catalytic boriding reaction under a 1,5-cyclooctadiene chloride iridium dipolymer by using the polyaryletherketone and boron pinacol ester; secondly, synthetizing a 4-iodine-4'-(N,N-dimethyl amine) azobenzene monomer; finally, reacting with the 4-iodine-4'-(N,N-dimethyl amine) azobenzene monomer under catalysis of tetrakispalladium by using a polyaryletherketone material containing the boric acid ester, so as to prepare the azo polyaryletherketone. By adopting the method, an azo monomer can be successfully led to the polyaryletherketone, but the performance of the polyaryletherketone is not affected, biphenyl structure azo polyaryletherketone with stable performance also can be generated, and the storage stability of the azo material can be improved. Therefore, the method is expected to be applied in the aspect of preparation of a novel azo polymer material.
Owner:JILIN UNIV
Synthesis method of cycloalkene-1-boronic acid pinacol ester
InactiveCN104478918AAvoid distillationAvoid the problem of impurity generation that is difficult to control when the temperature risesGroup 3/13 element organic compoundsCycloalkeneBiochemical engineering
The invention discloses a synthesis method of cycloalkene-1-boronic acid pinacol ester. The synthesis method comprises three reaction steps of enabling cycloketone to react with phosphorus pentachloride to obtain 1-cycloolefin chloride, preparing cycloalkene-1-boronic acid through 1-cycloolefin chloride, lithium metal and a boronizing agent by the one-step method, and enabling cycloalkene-1-boronic acid to react with pinacol to obtain the final product. According to the method, the reaction is under mild conditions, 1-cycloolefin chloride can directly enter the next reaction without distilling and purifying, and intermediates and products obtained in each step do not need distilling, so that the operation is simple, and moreover, the raw materials are low in cost and easy to obtain, and as a result, mass production can be performed.
Owner:DALIAN NETCHEM CHIRAL TECH
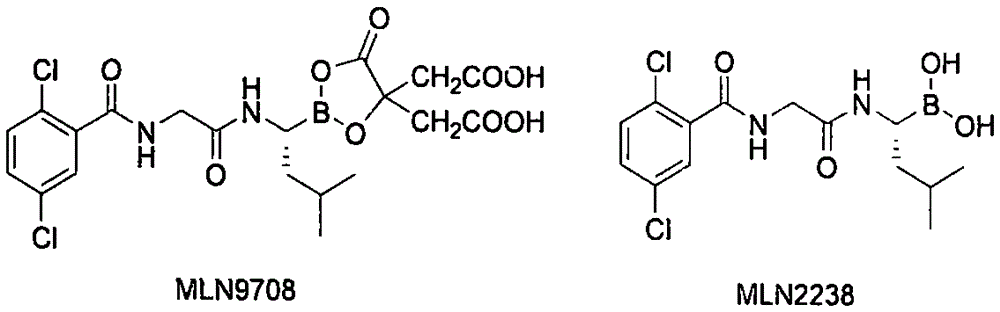
Synthetic method of proteasome inhibitor MLN9708
InactiveCN106608883AReduce usageEasy to operateGroup 3/13 element organic compoundsBulk chemical productionBenzoic acidProteasome Inhibition
The invention provides a synthetic method of a proteasome inhibitor MLN9708. The method comprises: taking 2,5-dichloro benzoic acid as an initial raw material, and performing condensation and saponification to prepare N-(2,5-dichlorobenzoyl) glycine; joining N-(2,5-dichlorobenzoyl) glycine to L-leucine boronic acid pinacol ester hydrochloride; purifying the obtained product through forming a complex with diethanolamine and performing hydrolysis for deprotection to obtain corresponding free boric acid; and reacting the obtained product with citric acid to obtain MLN9708. The method is cheap and available in raw materials, simple and convenient to operate, mild in reaction conditions and easy for industrial production.
Owner:PEKING UNIV
Synthesis method for 1-substitution-1H-pyrazol-4-boric acid pinacol ester
InactiveCN104478917AThe reaction is easy to operateLow costGroup 3/13 element organic compoundsAcetic acidLithium hydroxide
The invention discloses a synthesis method for 1-substitution-1H-pyrazol-4-boric acid pinacol ester. According to the method, 1-substitution-4-bromopyrazole, boric acid ester and n-hexylithium serving raw materials have an reaction and are boronized with a one-pot method, so 1-substitution-1H-pyrazol-4-boric acid ester and lithium hydrate are generated, then pinacol is used for exchange to generate 1-substitution-1H-pyrazol-4-boric acid pinacol ester and lithium hydrate, finally lithium hydrate is hydrolyzed by acetic acid, and 1-substitution-1H-pyrazol-4-boric acid pinacol ester is obtained. The raw materials used in the method are easy to obtain, and the method is easy and convenient to conduct, and is suitable for preparing 1-substitution-1H-pyrazol-4-boric acid pinacol ester compounds.
Owner:DALIAN NETCHEM CHIRAL TECH
Method for preparing intermediate used for synthesizing bortezomib
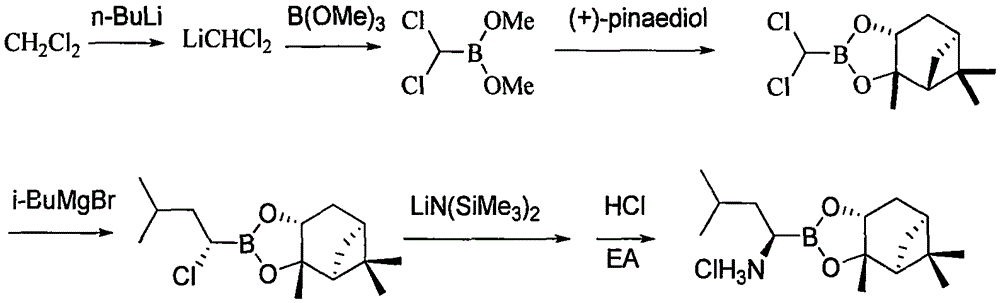
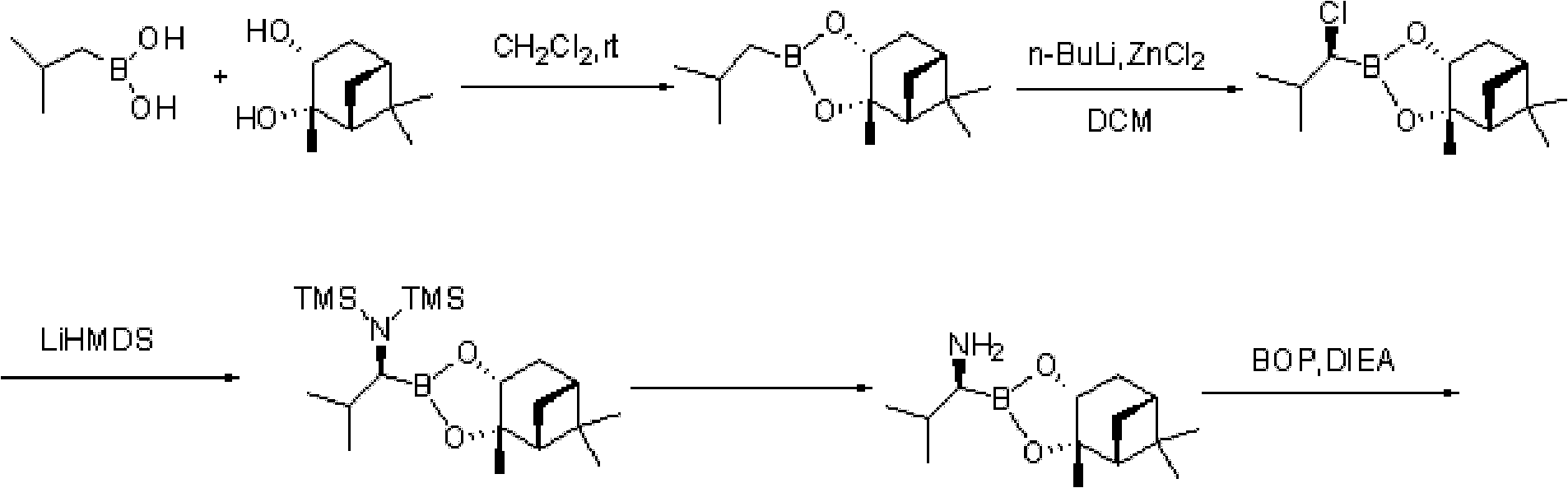
ActiveCN103044467ARaw materials are cheap and easy to getEasy to operateGroup 3/13 element organic compoundsBoronic acidMethyl group
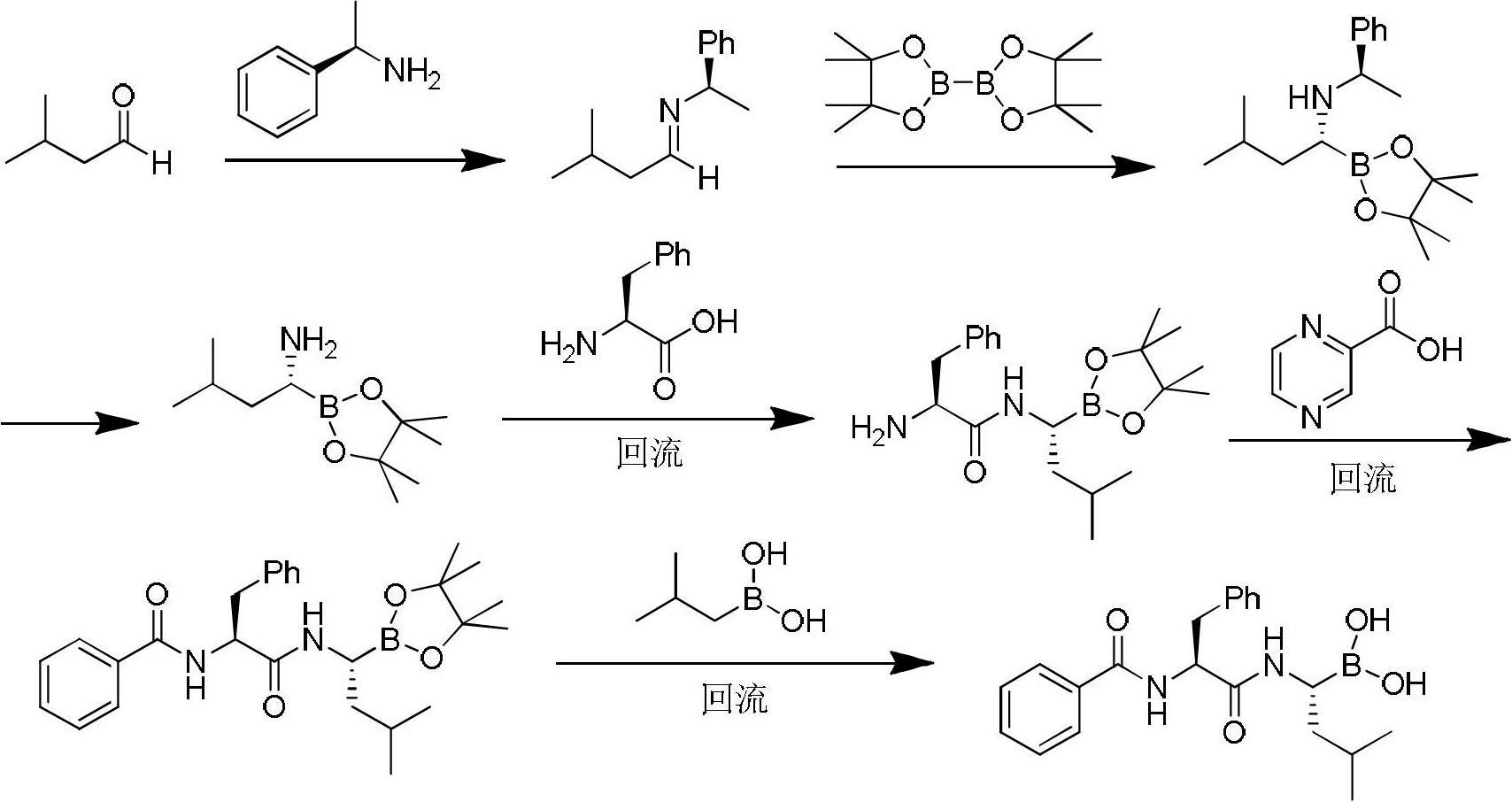
The invention discloses a method for preparing an intermediate used for synthesizing bortezomib, comprising the following steps of: carrying out an addition reaction on 3-methyl butyraldehyde and bis(pinacolaton)diboron, then carrying out sulfonylation or halogenating reaction, carrying out an amination reaction with R-(+)-1-phenylethylamine, carrying out catalytic hydrogenation a debenzylation reaction, and finally carrying out enantiomer resolution. Compared with the prior art, the preparation method of the R-(1-amino-3-methyl) butyl boronic acid pinacol cyclic ester intermediate, provided by the invention, has the advantages that raw materials are cheap and easy to get, operation is easy, reaction conditions are mild and optical purity is high, and especially post-processing is simple and the high-optical-purity bortezomib can be easily obtained without column chromatography separation when the intermediate prepared by the invention is used for synthesizing the bortezomib, so that industrial production requirement of the bortezomib is met and the preparation method provided by the invention has obvious effect and economic practicability.
Owner:重庆瑞泊莱制药有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com