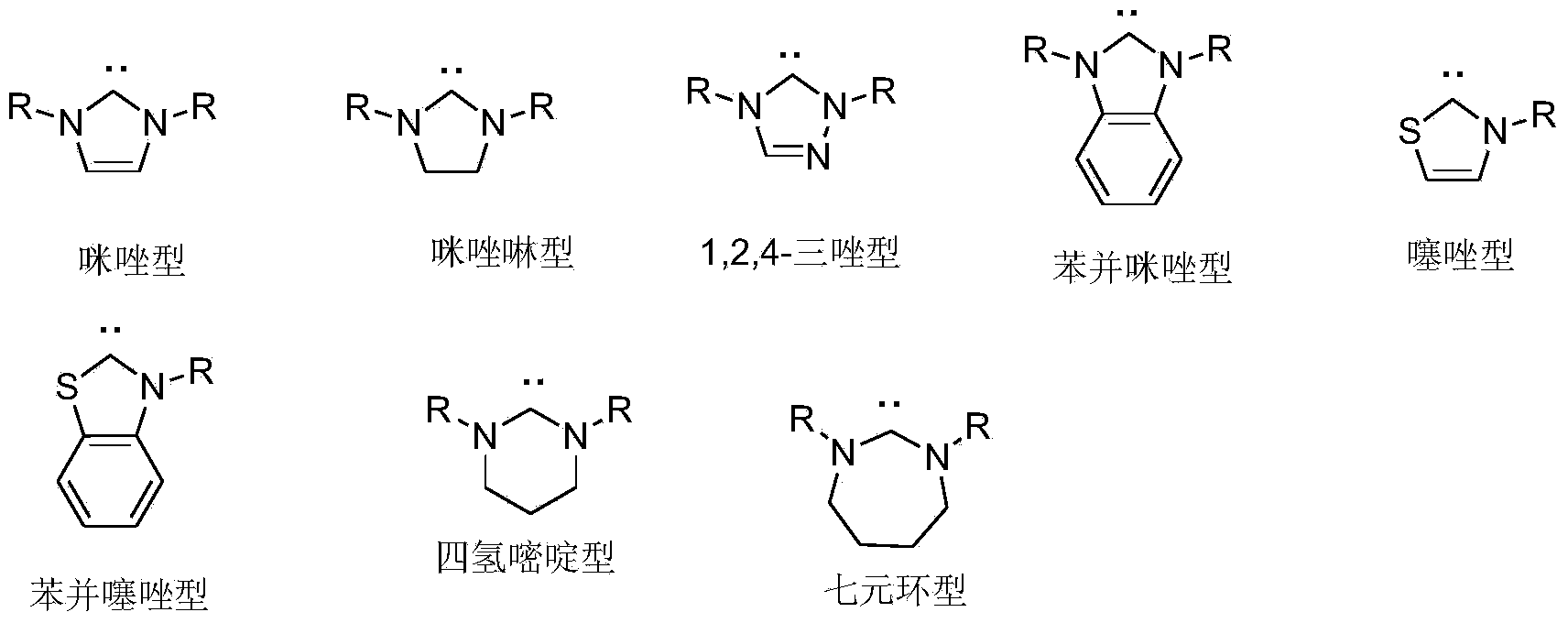
Chiral hexahydroxy n-heterocyclic carbine precursor compound as well as preparation method and application thereof
A technology for nitrogen heterocyclic carbene and precursor compounds, which is applied in the field of preparation of chiral six-membered nitrogen heterocyclic carbene precursor compounds, and can solve the problem of less research on chiral six-membered ring nitrogen heterocyclic carbene metal complexes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0083] Preparation and characterization of compound I-1:
[0084]
[0085] Dissolve 4.0g (20.3mmol) of compound I' and 3.84g of tert-butylsulfinamide in 100mlTHF, add 40mmol of tetraisopropyl titanate, monitor the reaction progress with TLC, use general method 1 to react, and heat at 86°C Stir for 12hr, after the reaction finishes, the reaction solution is poured into NaHCO 3 (200ml) solution, a large number of yellow solids precipitated, suction filtered, the solution was extracted three times with ethyl acetate (300ml), and the organic phase was washed with anhydrous Na 2 SO 4 Drying, suction filtration, spin-drying, and column chromatography separation (petroleum ether: ethyl acetate = 10:1) yielded 5.4 g of compound I-1 with a yield of 89% and a melting point of 140-142°C; 1 H NMR (400MHz, CDCl 3 ,ppm)δ:7.49(s,3H),7.24-7.26(m,2H),7.29-7.13(m,3H),6.73-6.91(m,4H),6.51(s,4H),1.28(s, 9H); 13 C NMR (100MHz, DMSO-d 6 ,δ):156.3,141.5,128.5,128.1,128.0,127.1,126.2,111.5,5...
Embodiment 2
[0087] Preparation and characterization of compound I-2:
[0088]
[0089] The preparation conditions are the same as in Example 1, the yield is 88%, and the melting point is 138~140°C; 1 H NMR (400MHz, CDCl 3 ,ppm)δ:7.50(s,3H),7.26(s,1H),7.16(d,J=7.6Hz,1H),6.84(s,1H),6.71(d,J=8.8Hz,1H), 1.27(s,9H); 13 C NMR (100MHz, CDCl 3 ,δ):154.3,141.8,128.5,128.1,128.0,127.1,126.2,111.3,57.5,55.8,22.7; MS(ESI-TOF)m / z:335.2[M+H] + .
Embodiment 3
[0091] Preparation and characterization of compound I-3:
[0092]
[0093] The preparation conditions are the same as in Example 1, the yield is 86%, and the melting point is 136~138° C.; 1 H NMR (400MHz, CDCl 3 ,ppm)δ:7.51-7.53(m,1H),7.15-7.30(m,3H),6.71(d,J=8.8Hz,1H),1.27(s,9H); 13 C NMR (100MHz, CDCl 3 ,δ):152.1,141.9,128.6,128.3,128.1,127.5,126.4,111.6,57.5,55.8,22.8; MS(ESI-TOF)m / z:353.1[M+H] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com