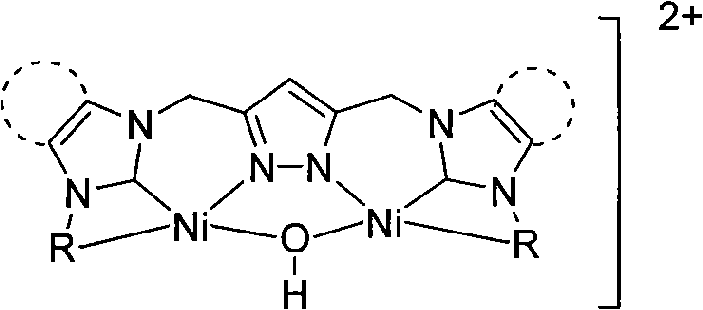
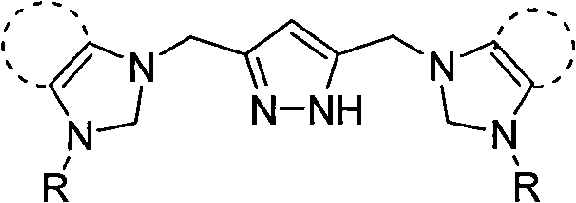
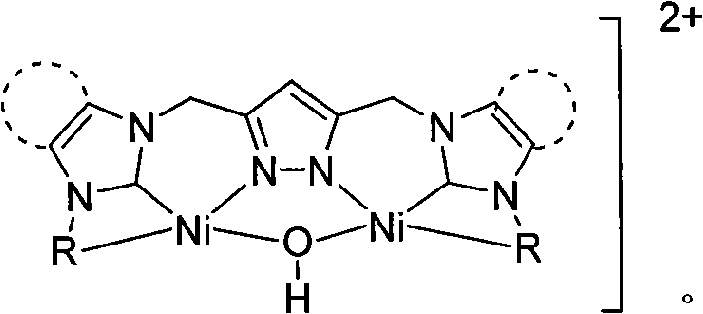
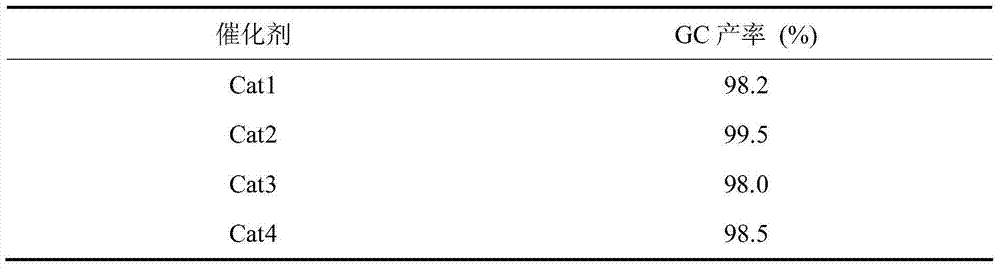
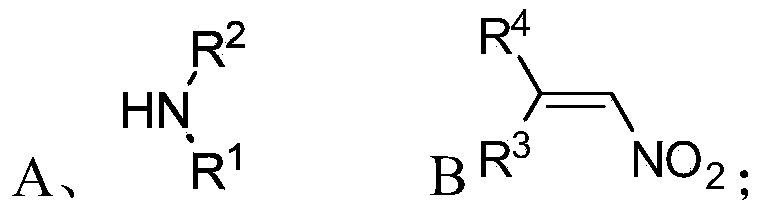Patents
Literature
180 results about "Carbine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
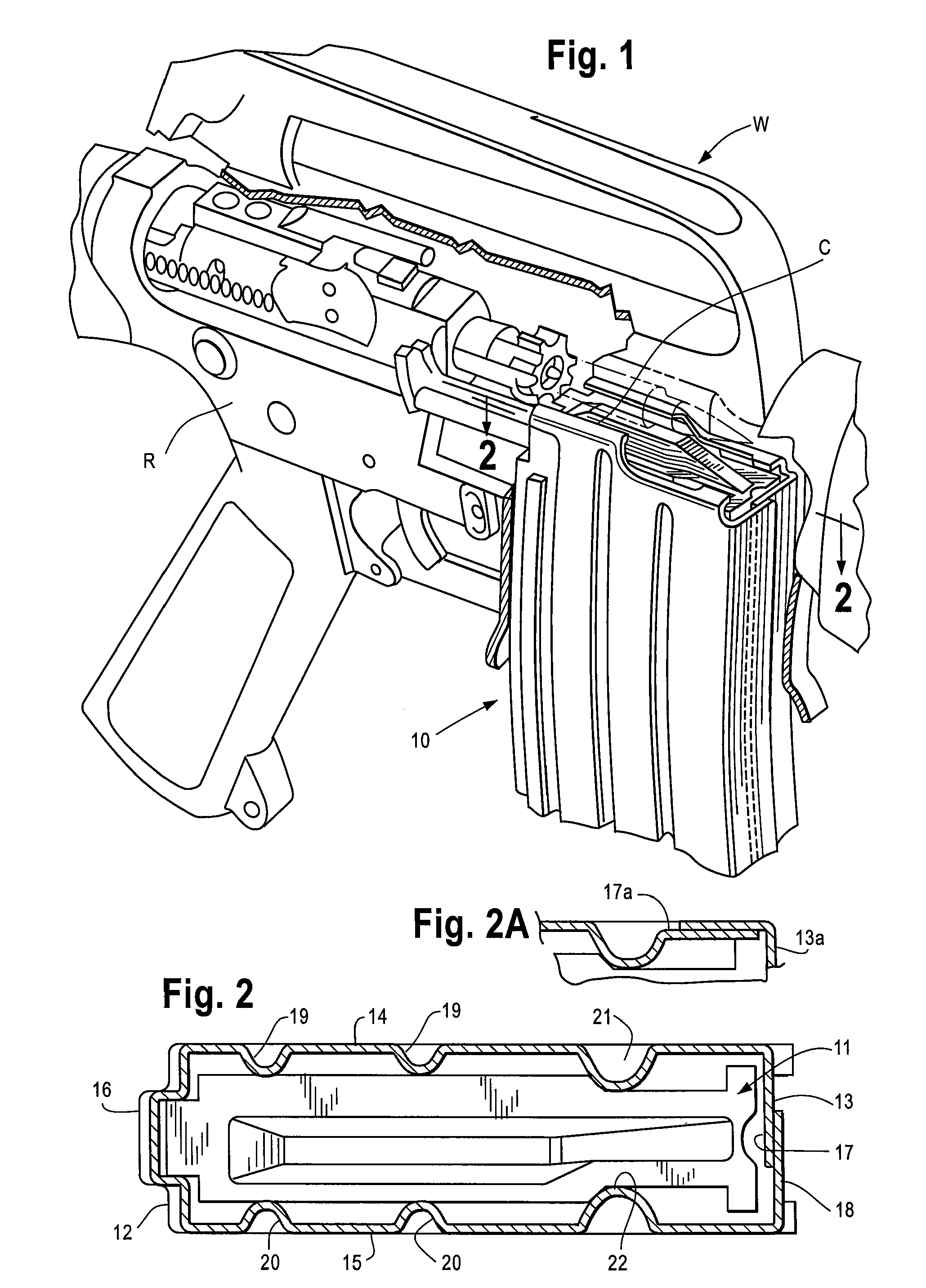
A carbine (/ˈkɑːrbiːn/ or /ˈkɑːrbaɪn/), from French carabine, is a long gun firearm but with a shorter barrel than a rifle or musket. Many carbines are shortened versions of full-length rifles, shooting the same ammunition, while others fire lower-powered ammunition, including types designed for pistols.
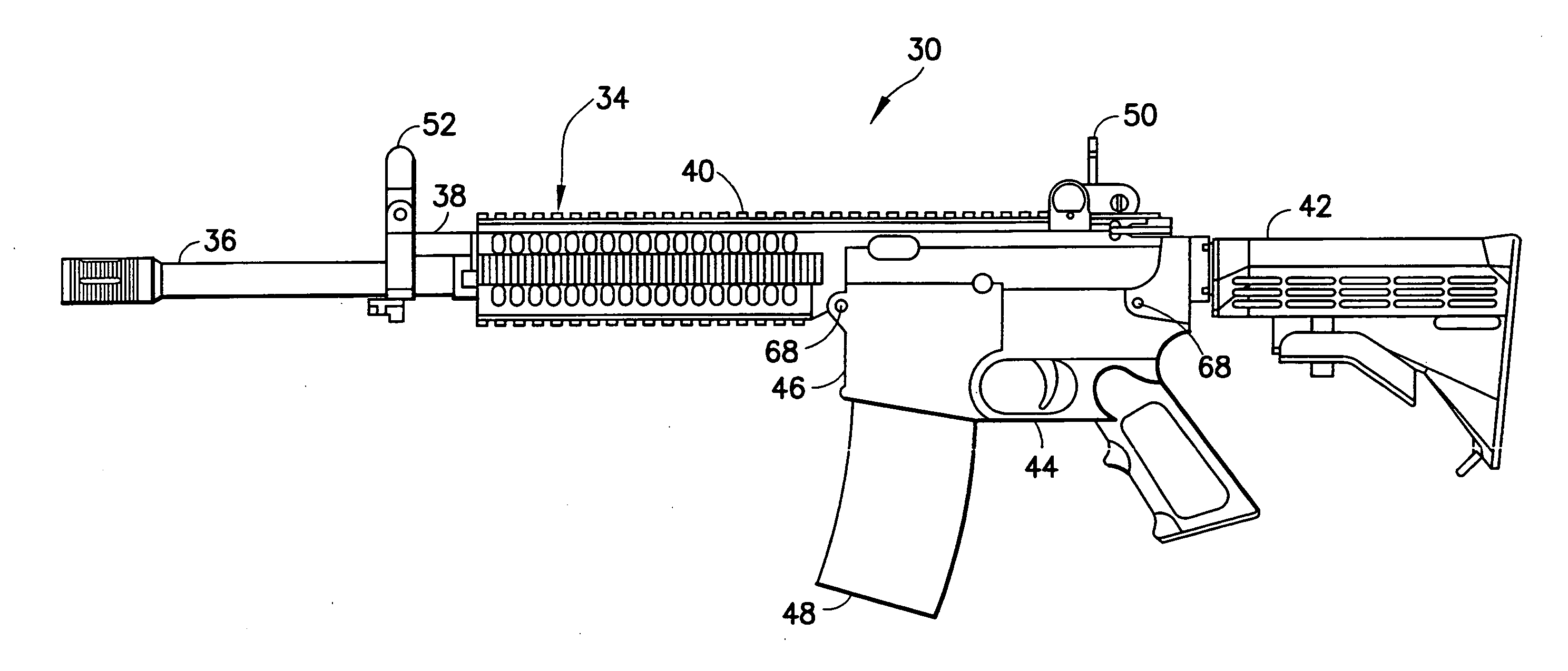
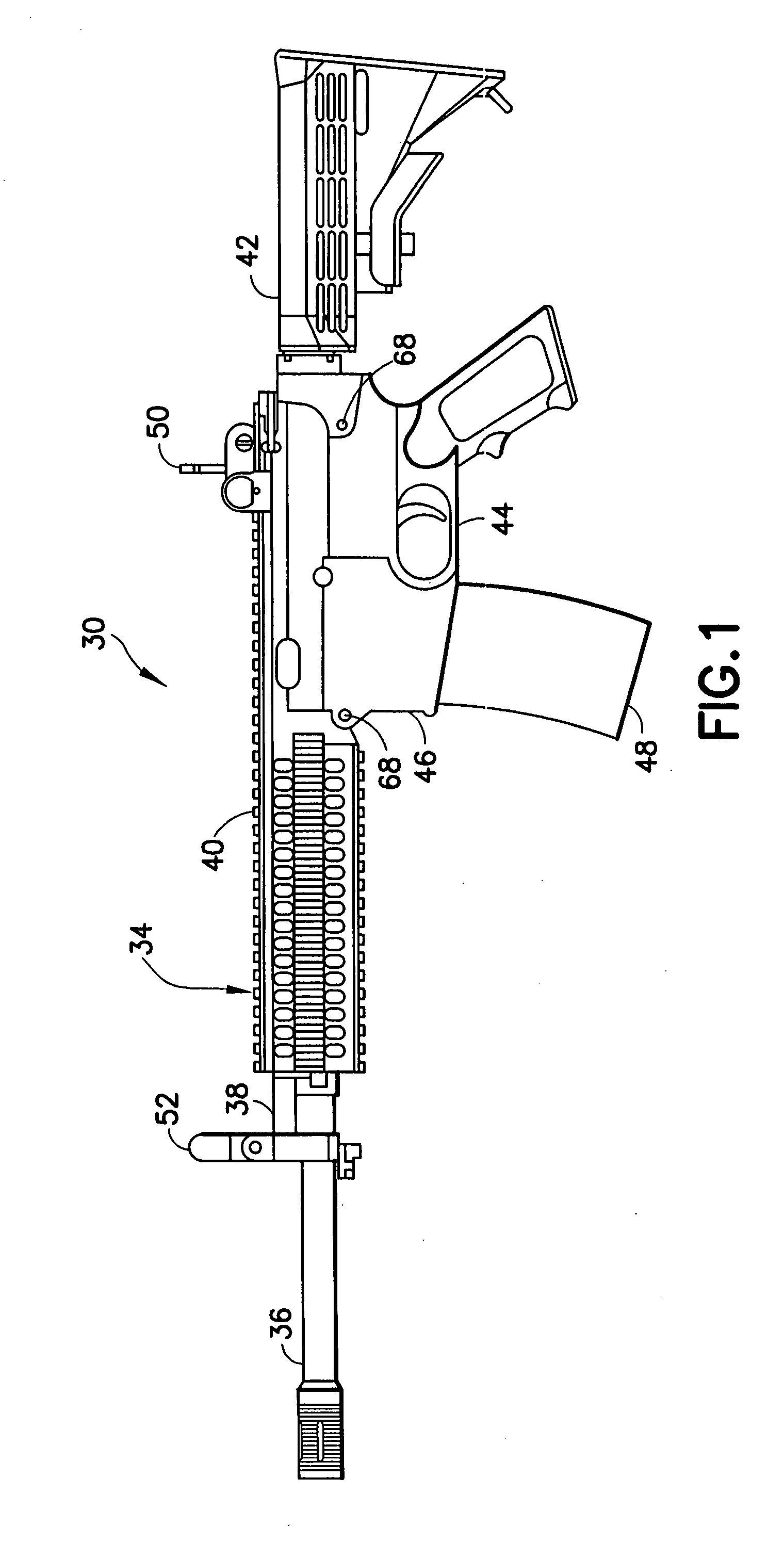
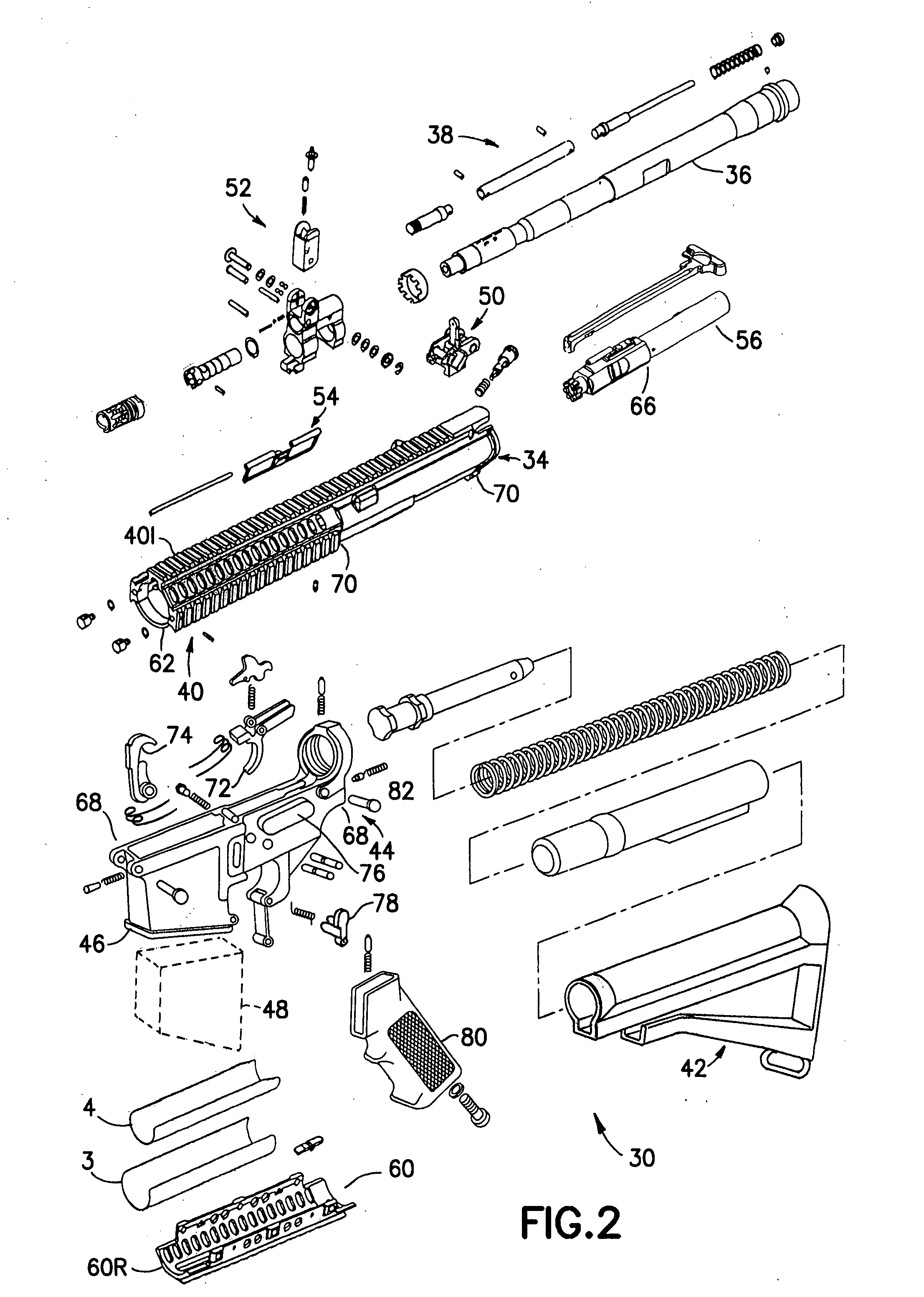
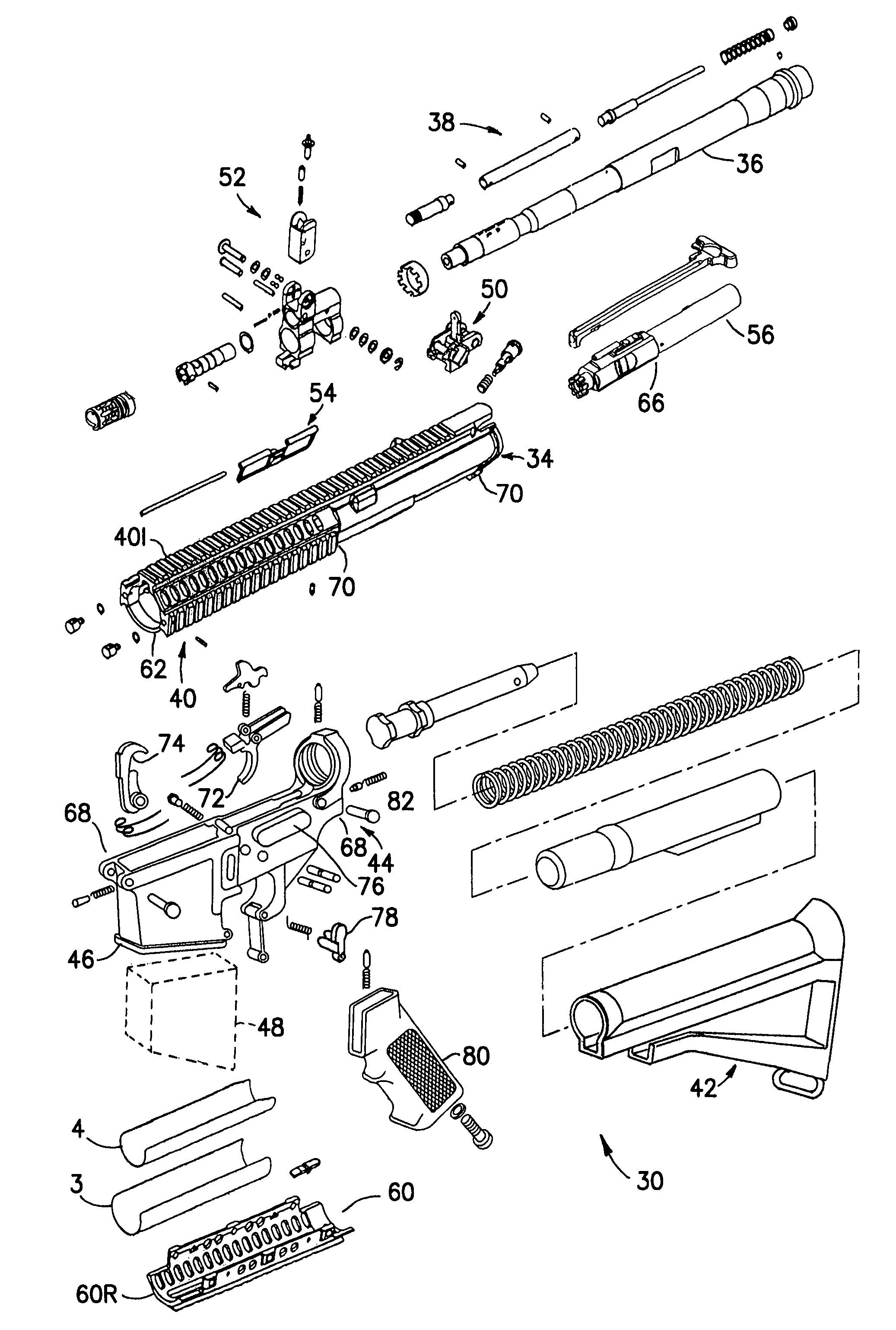
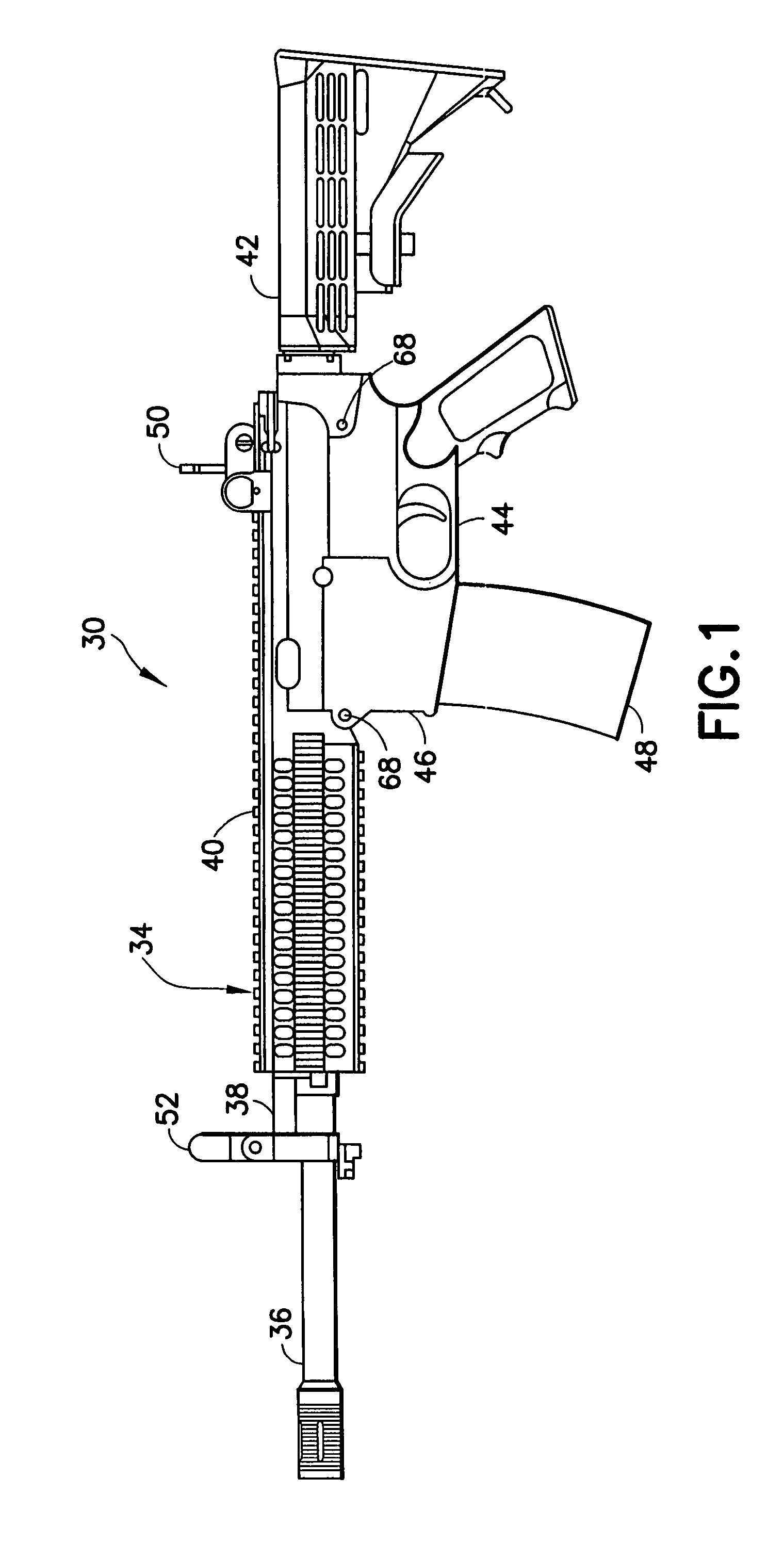
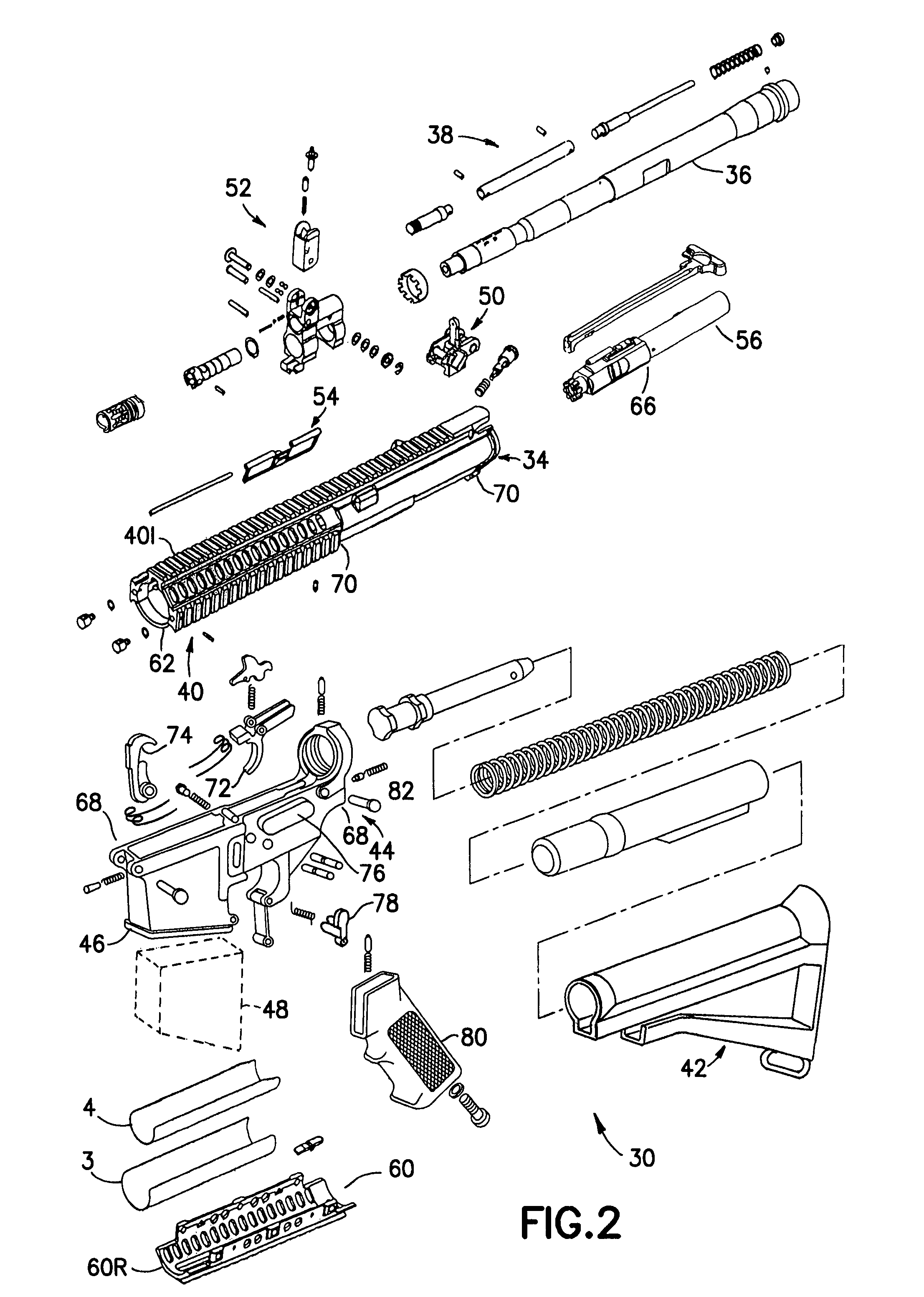
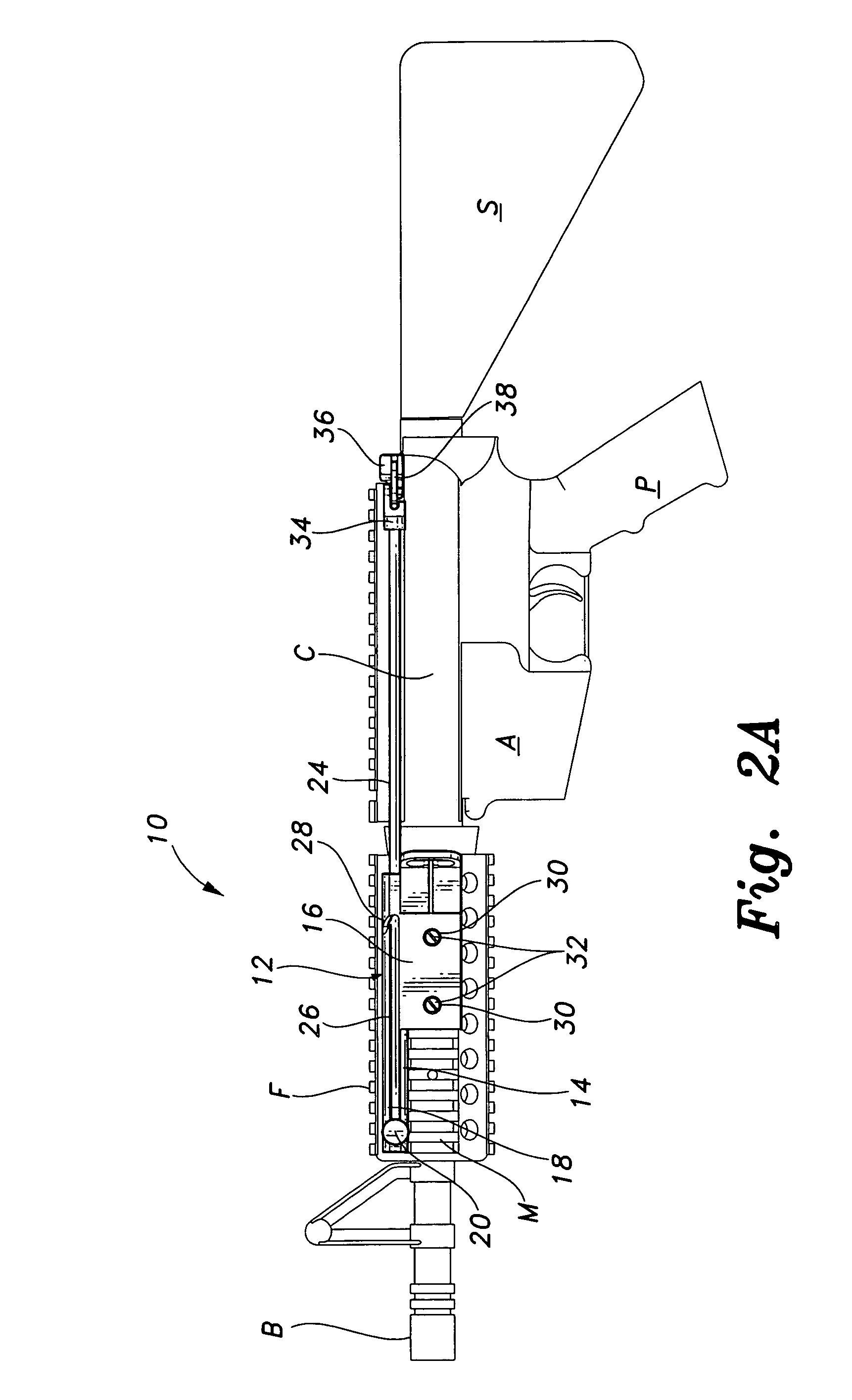
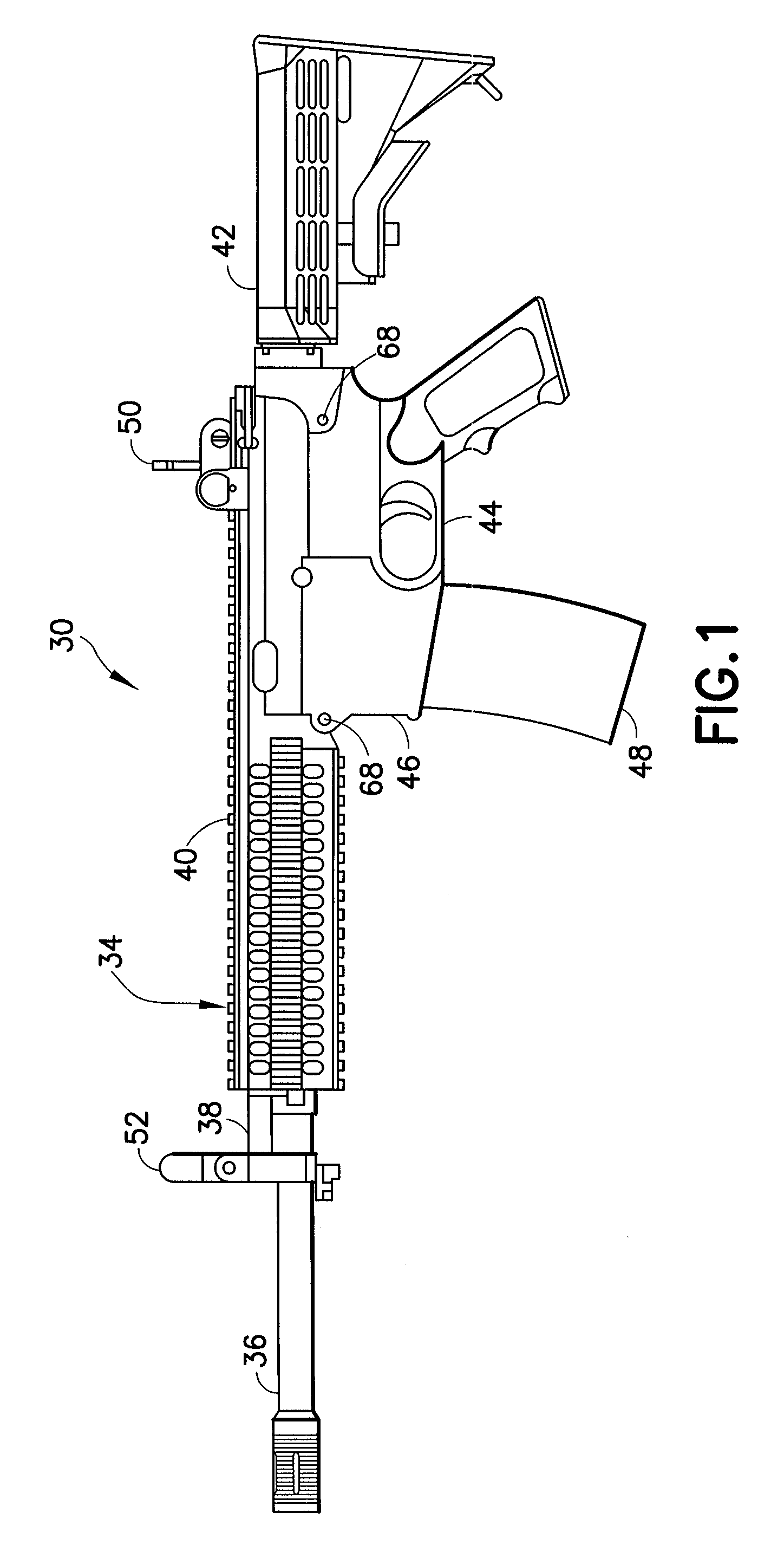
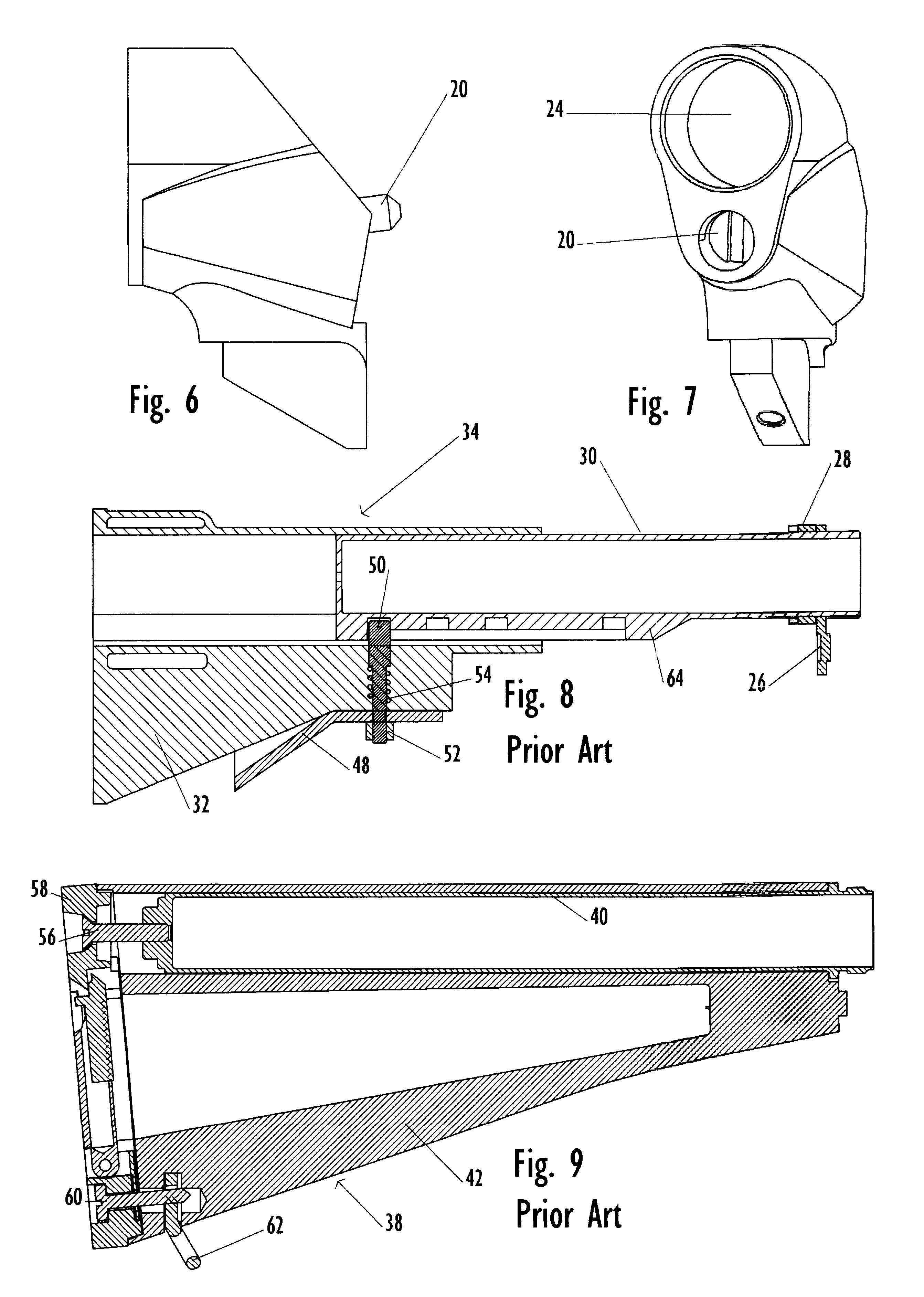
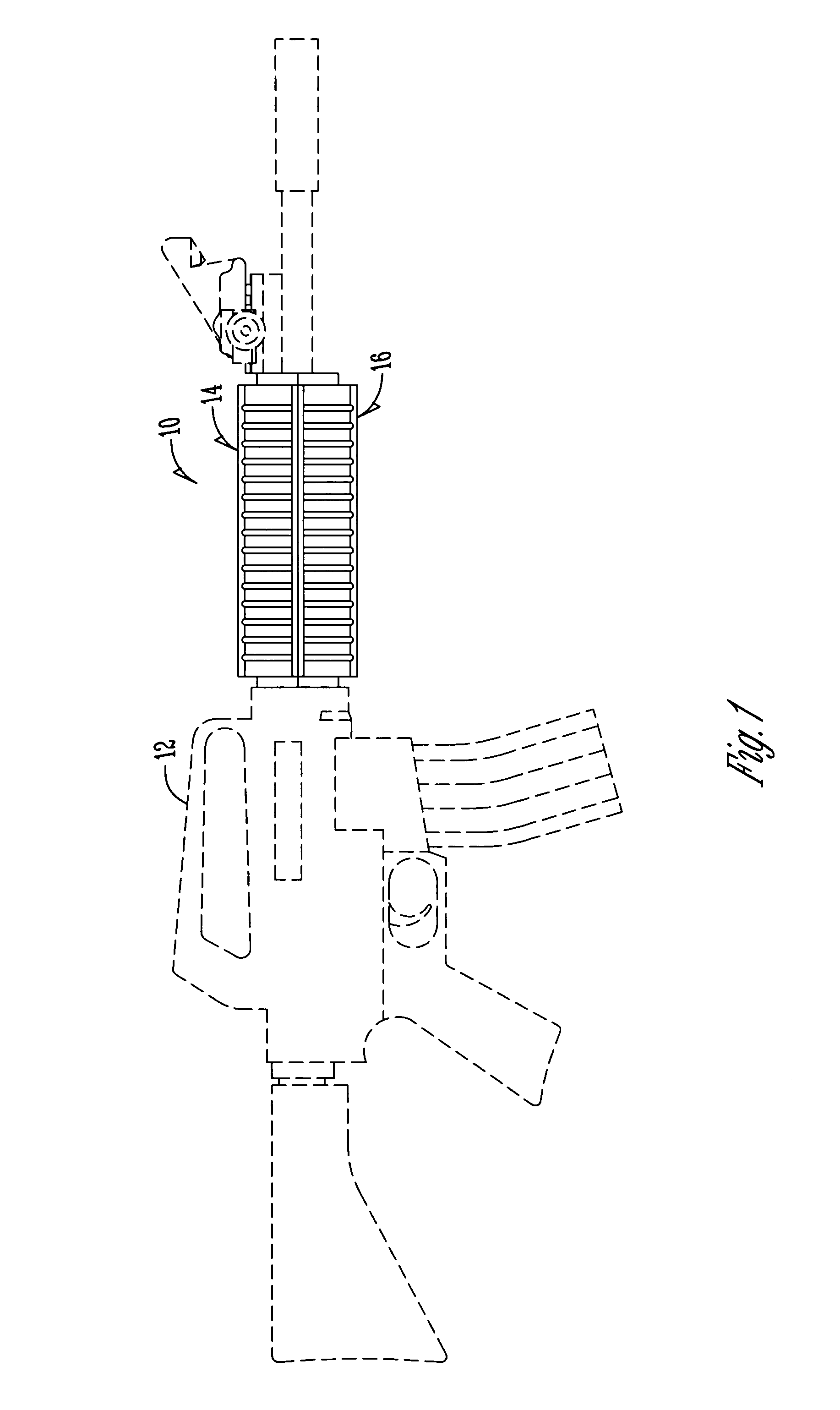
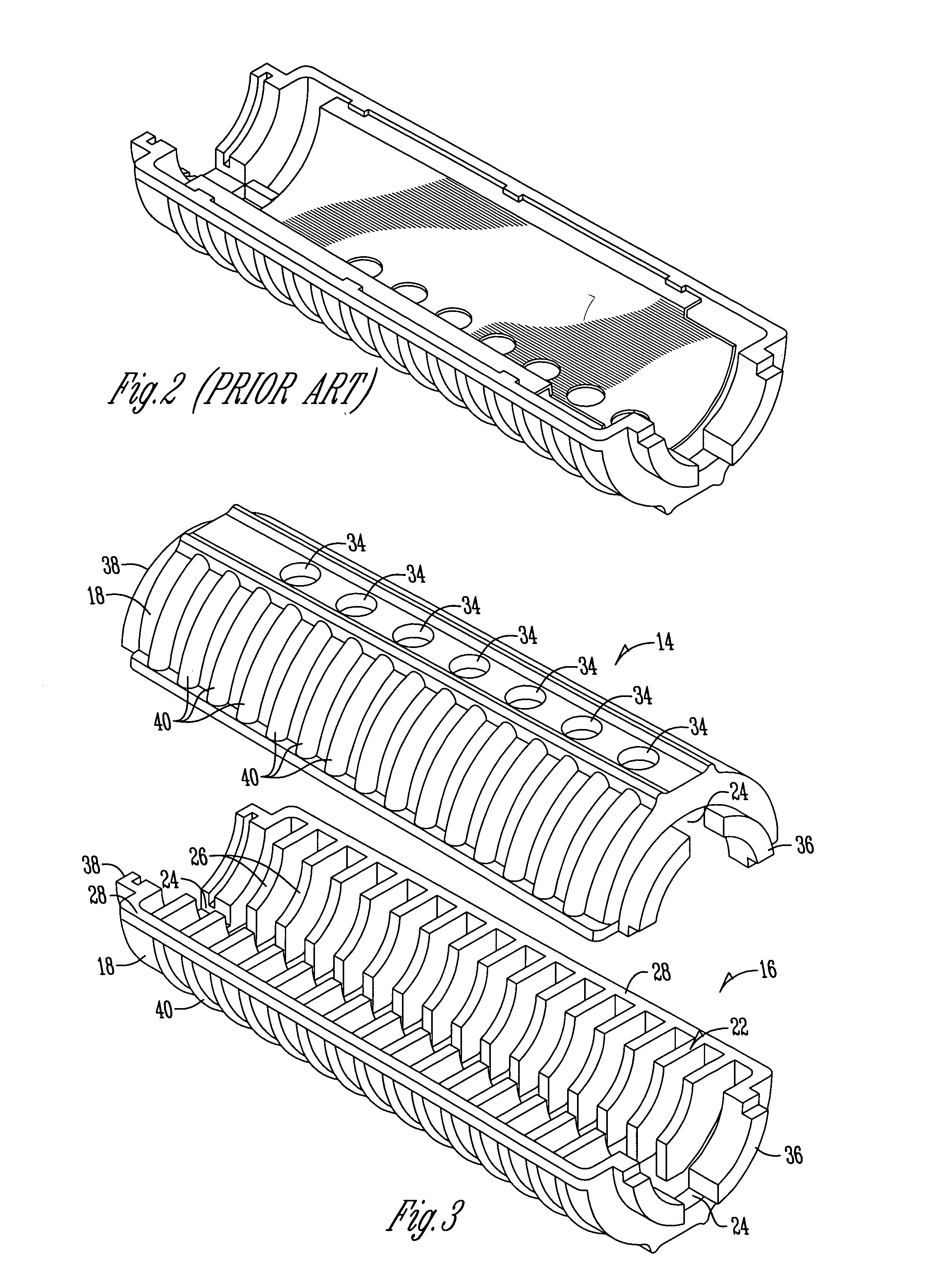
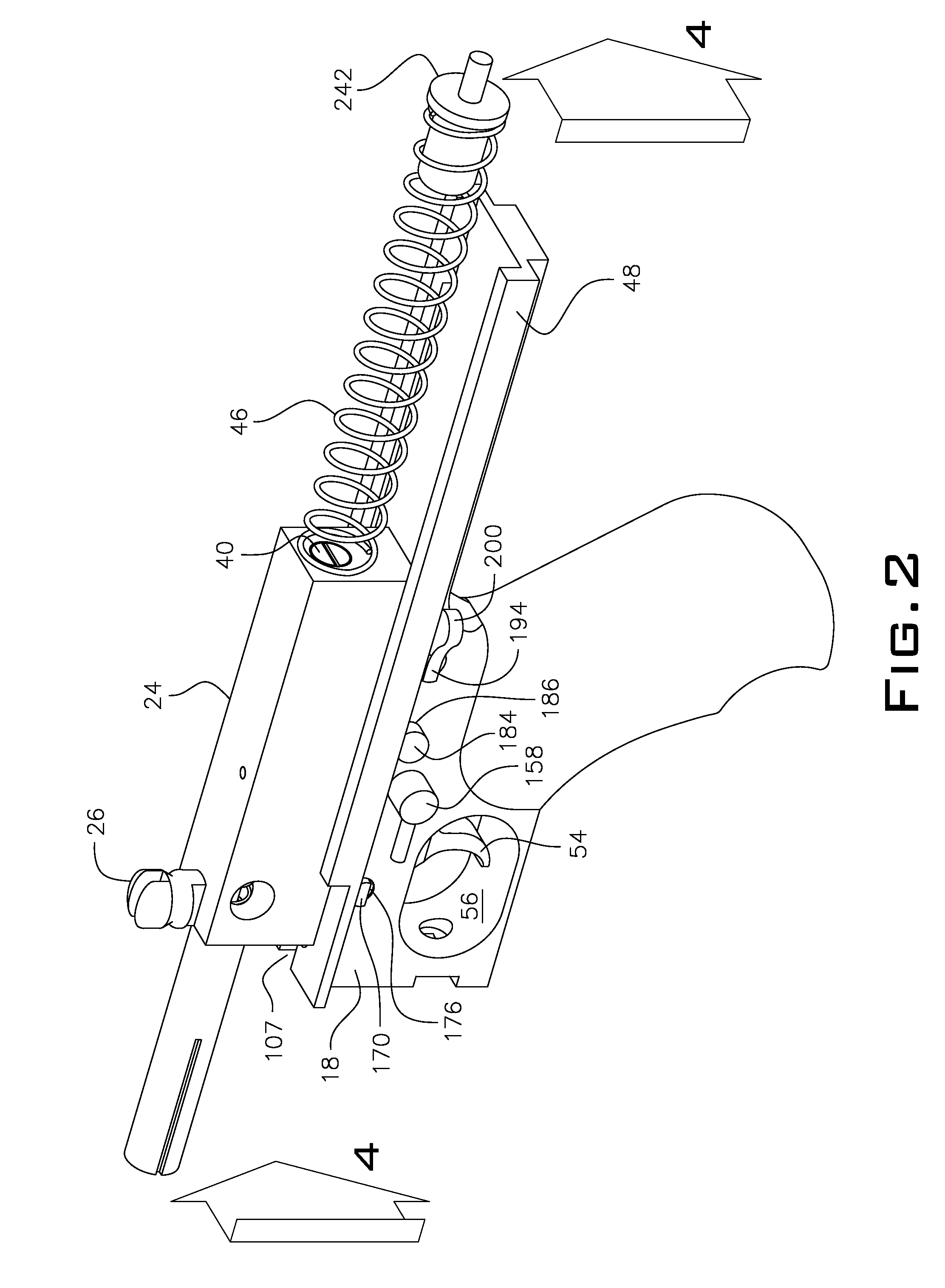
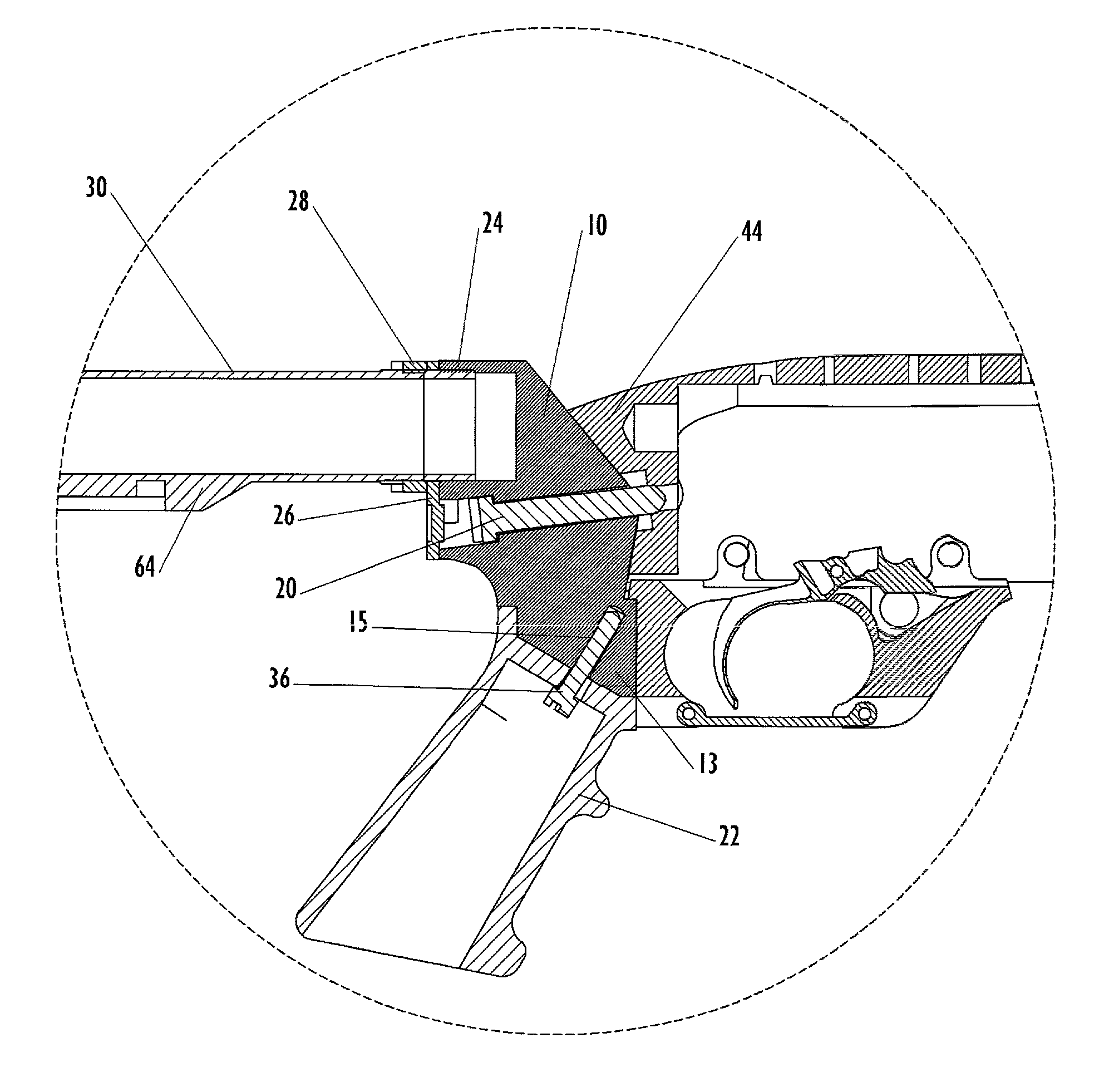
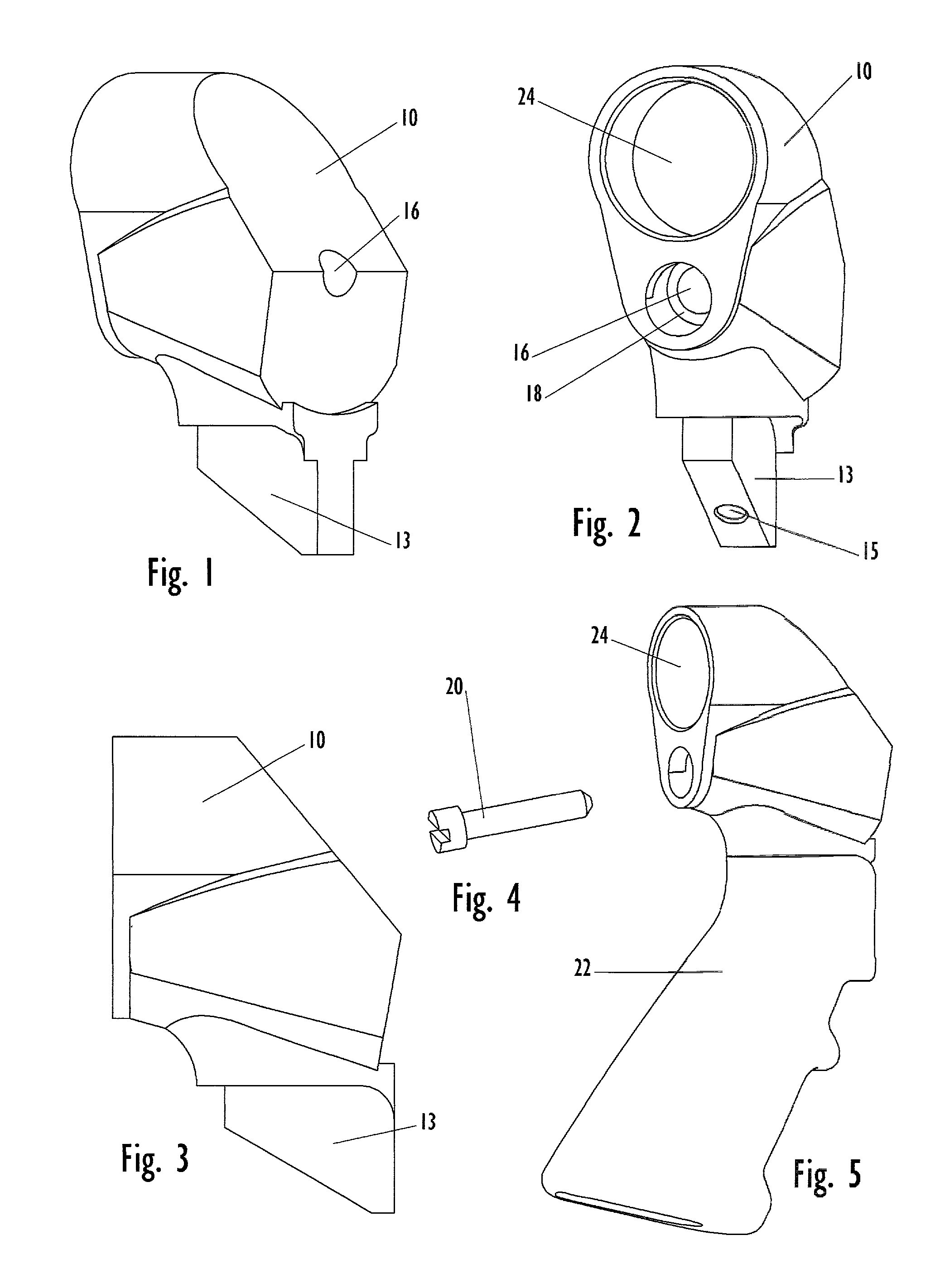
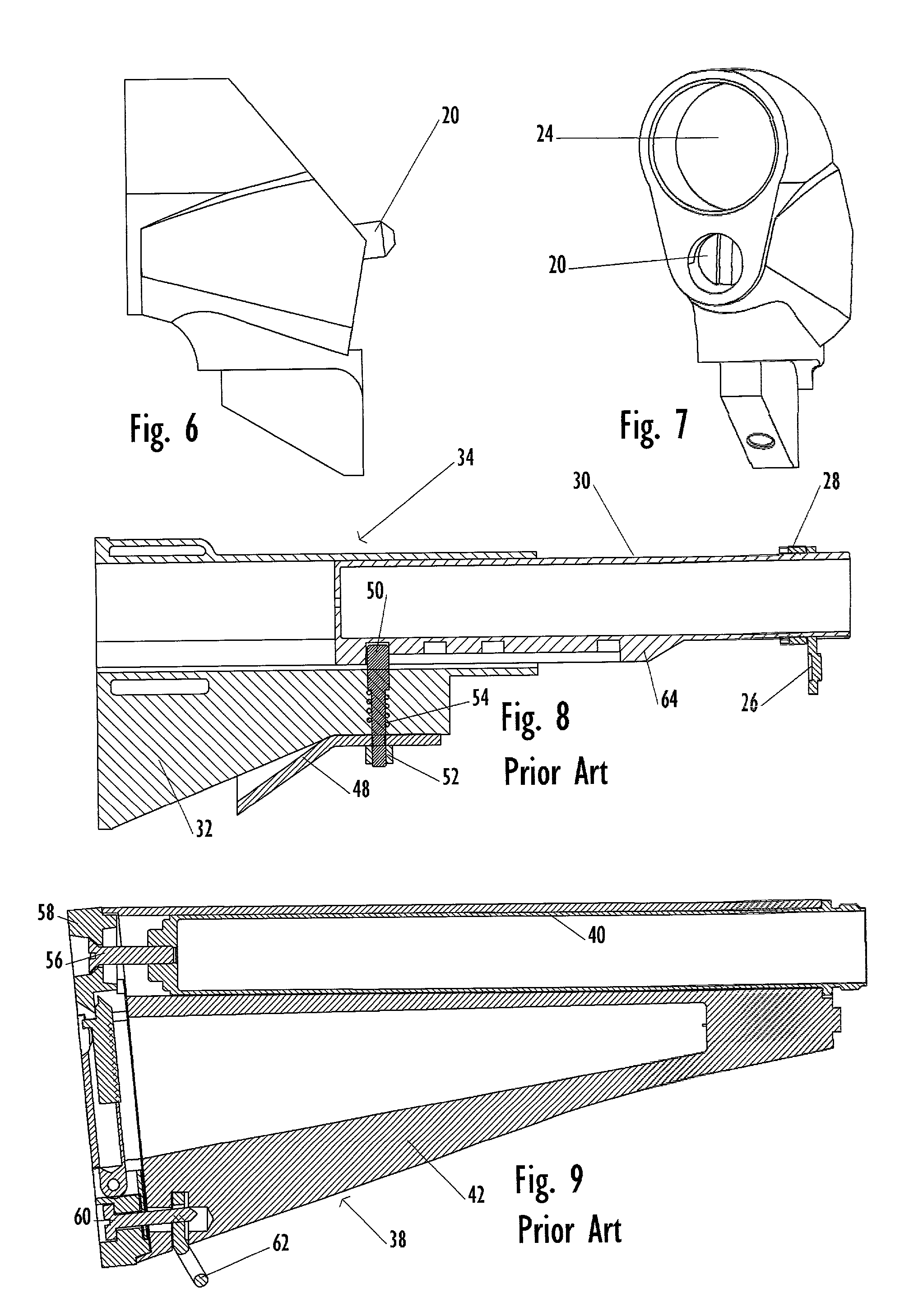
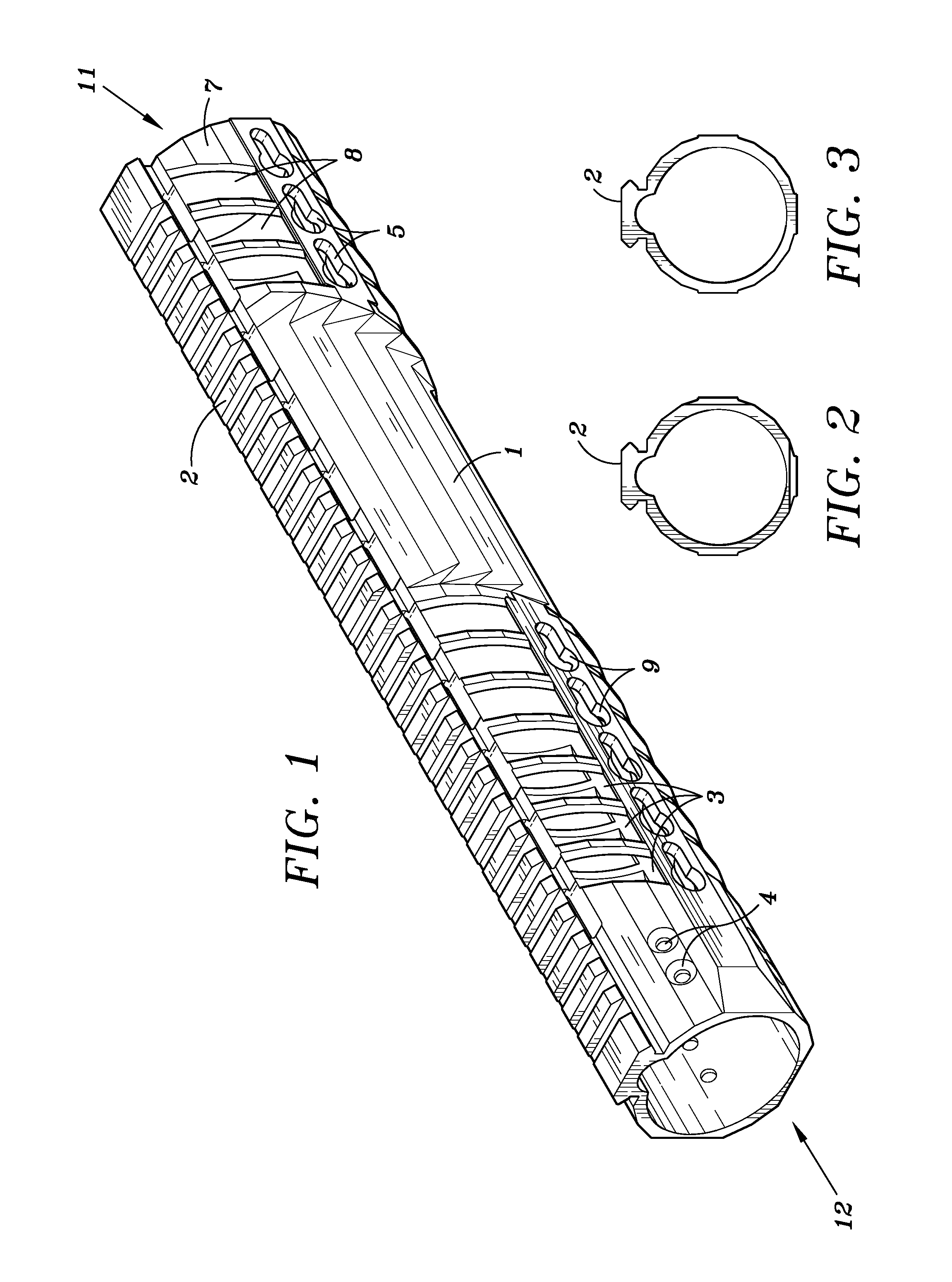
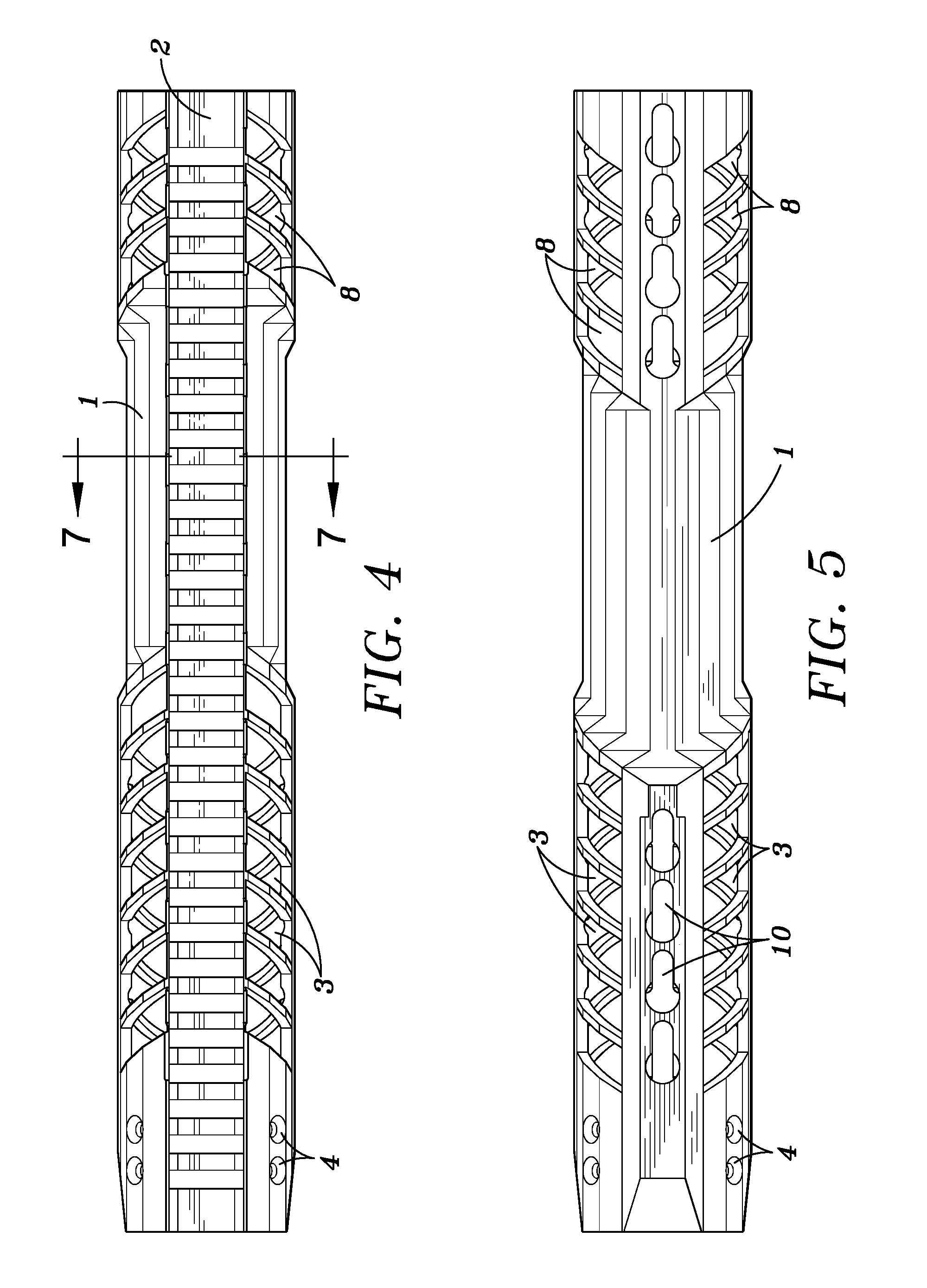
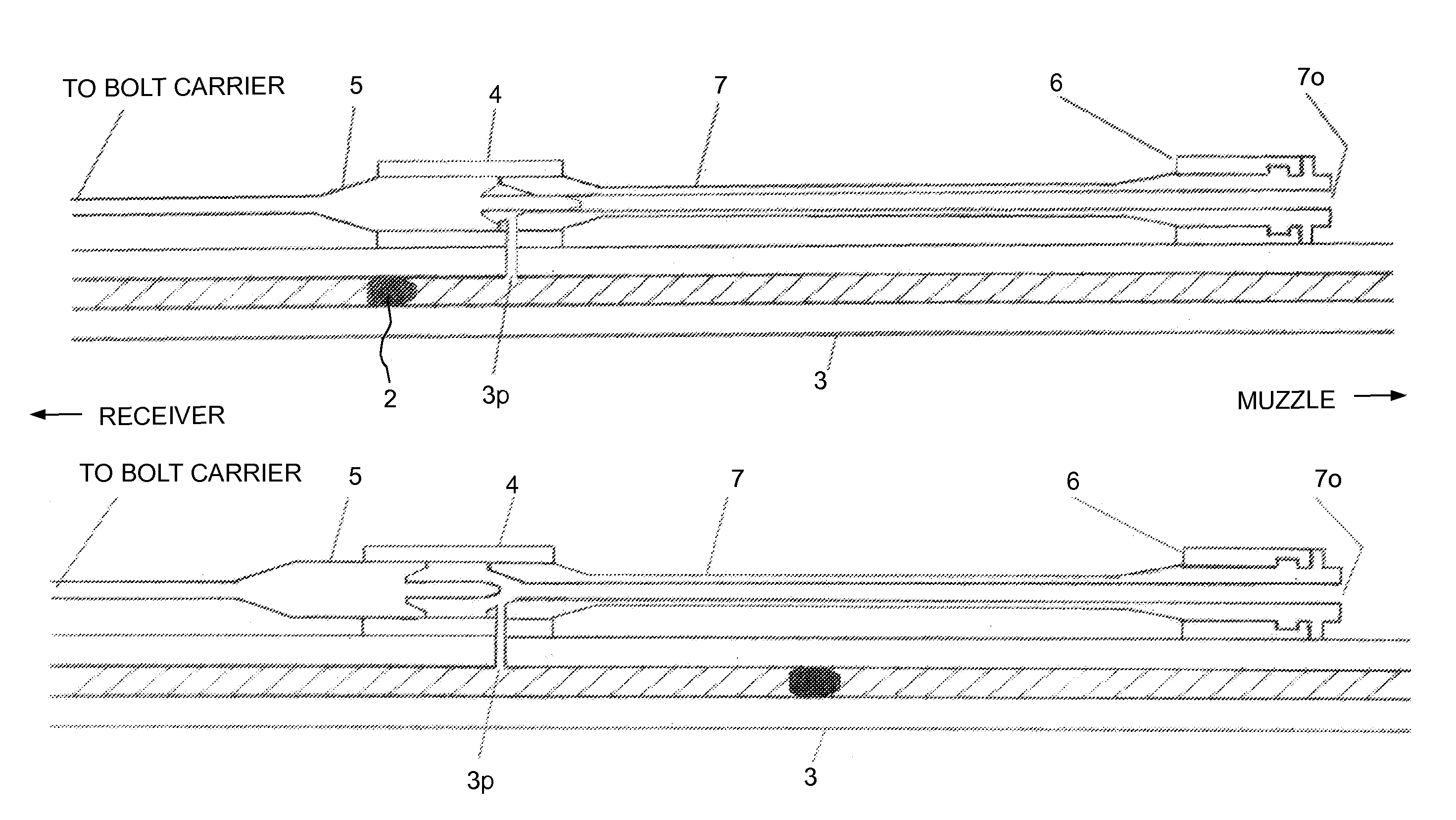
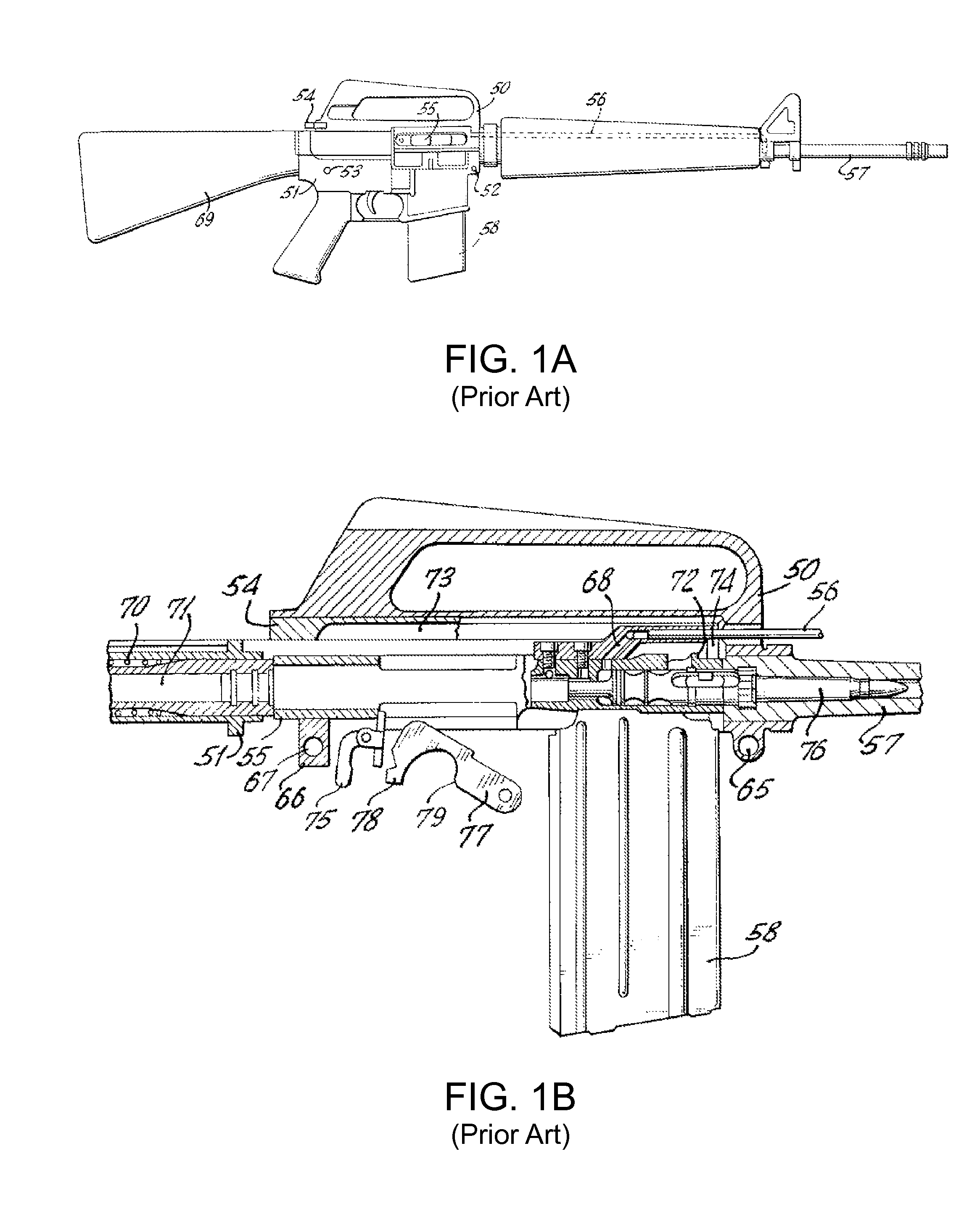
Law enforcement carbine with one piece receiver
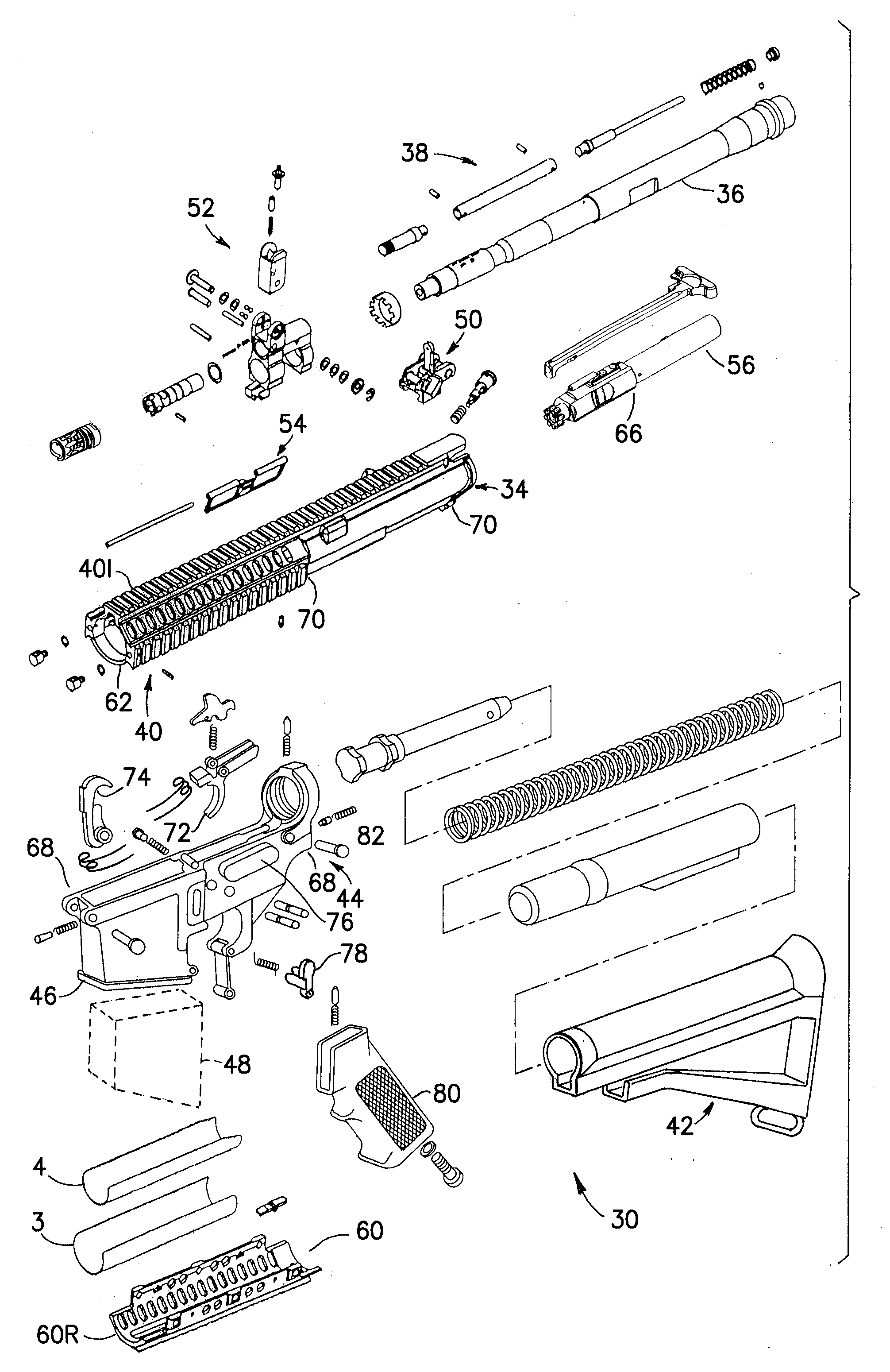
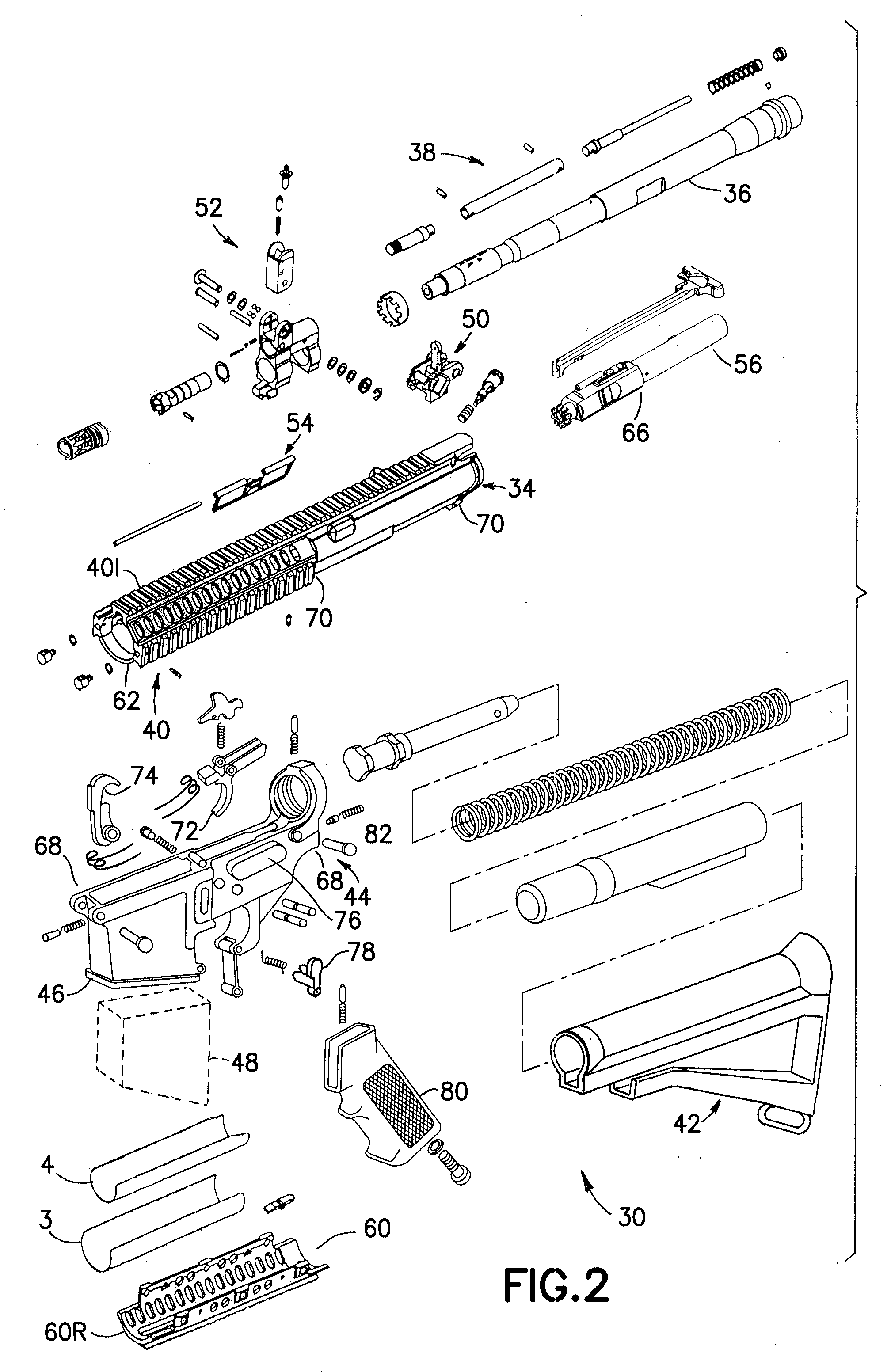
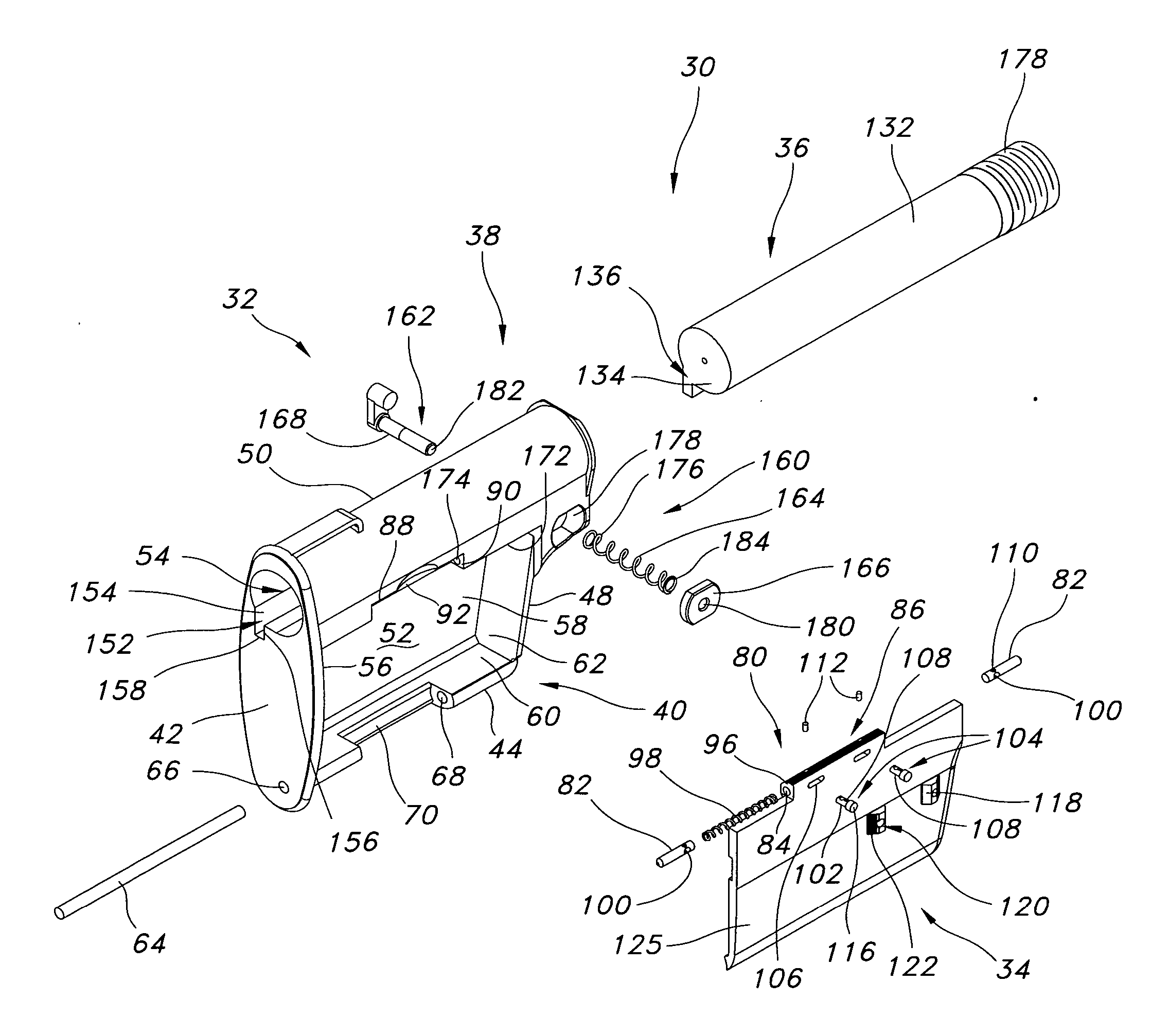
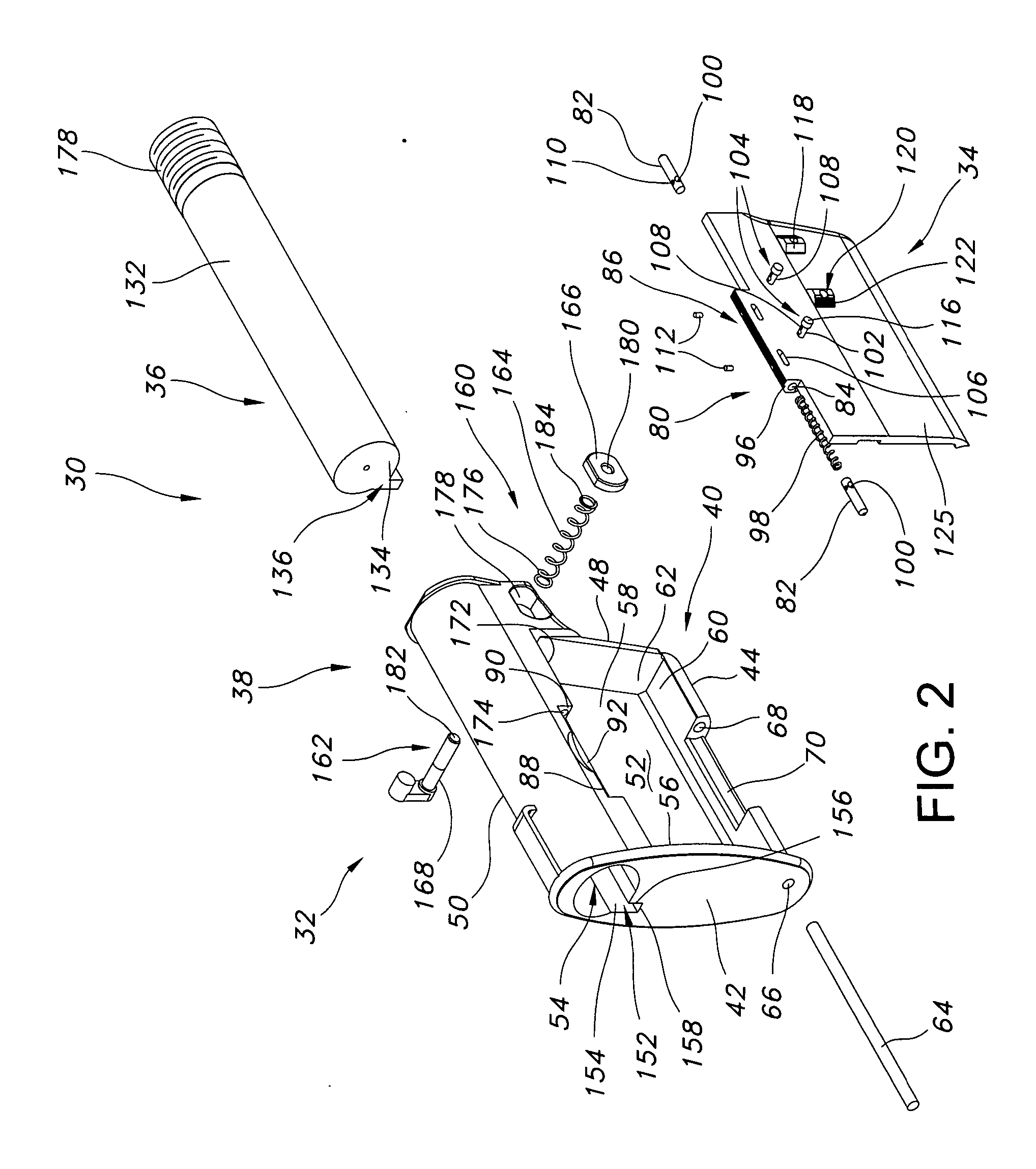
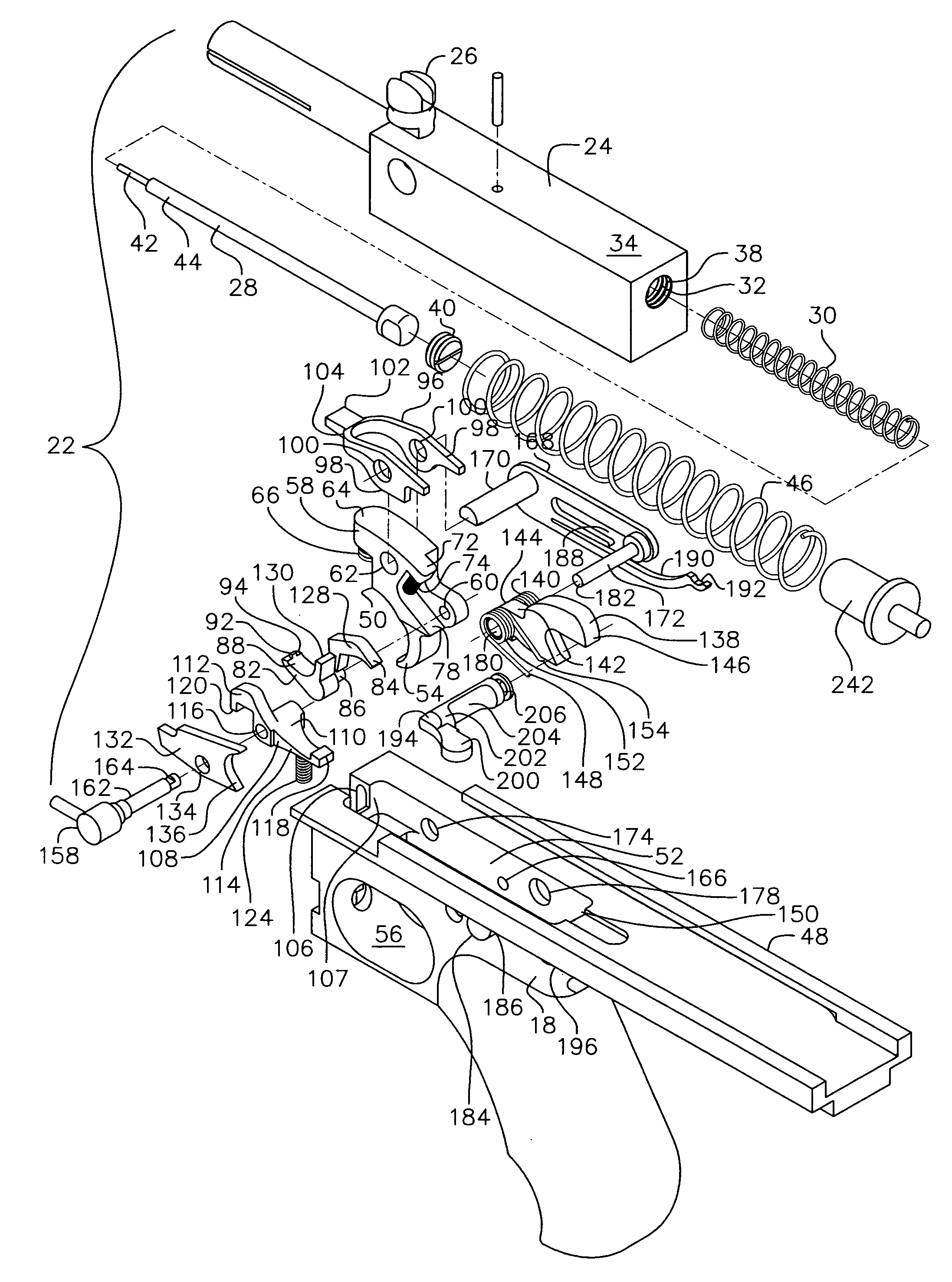
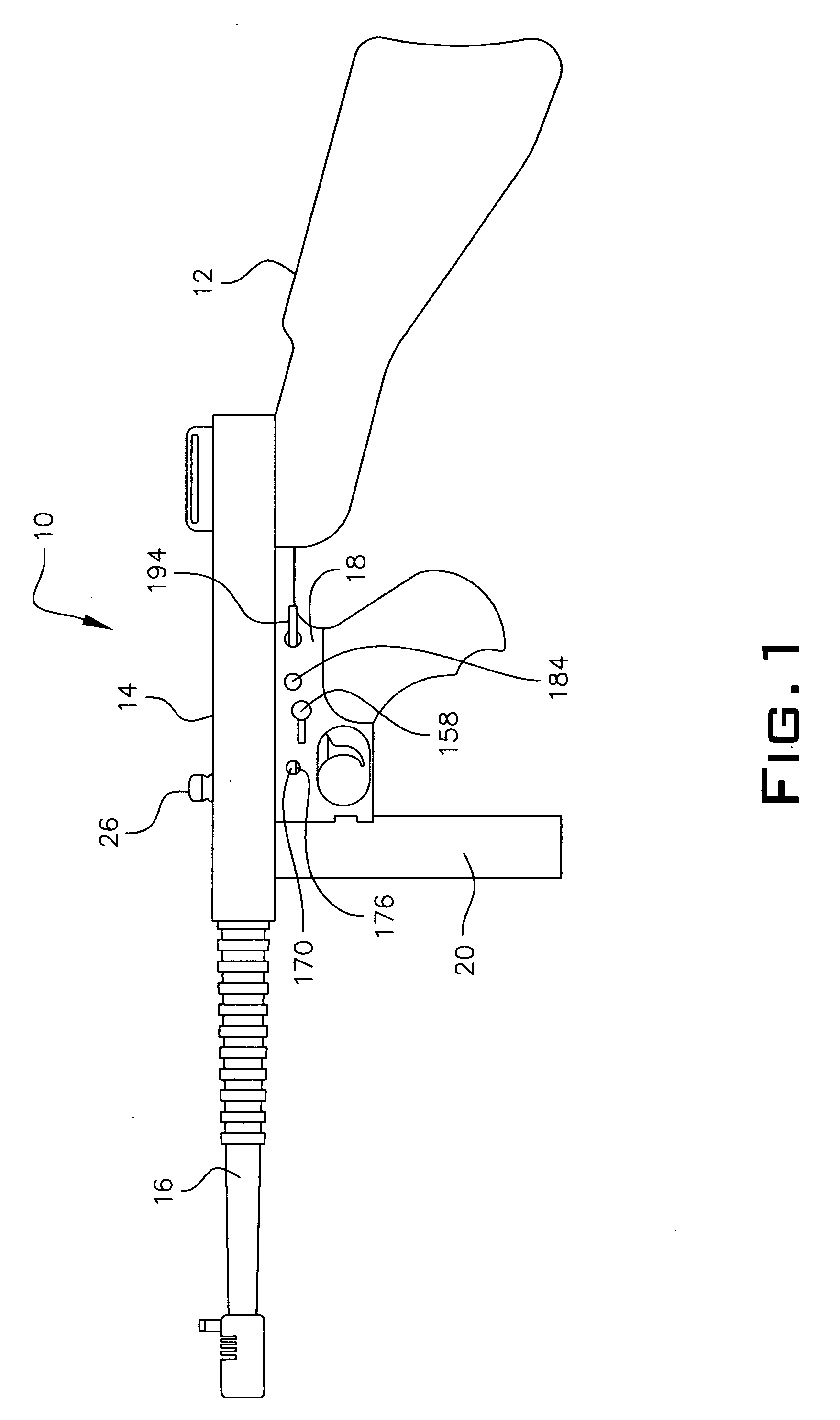
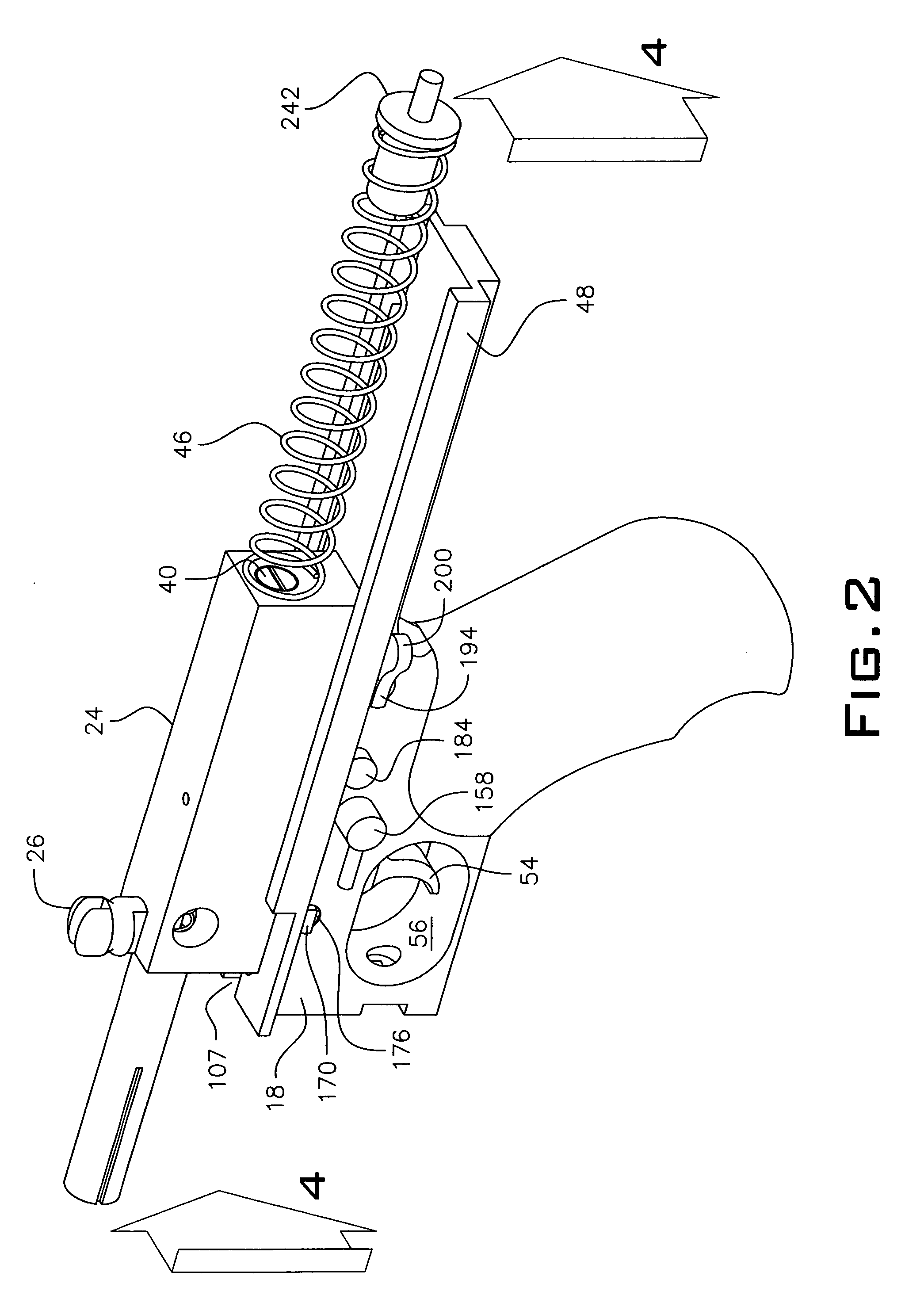
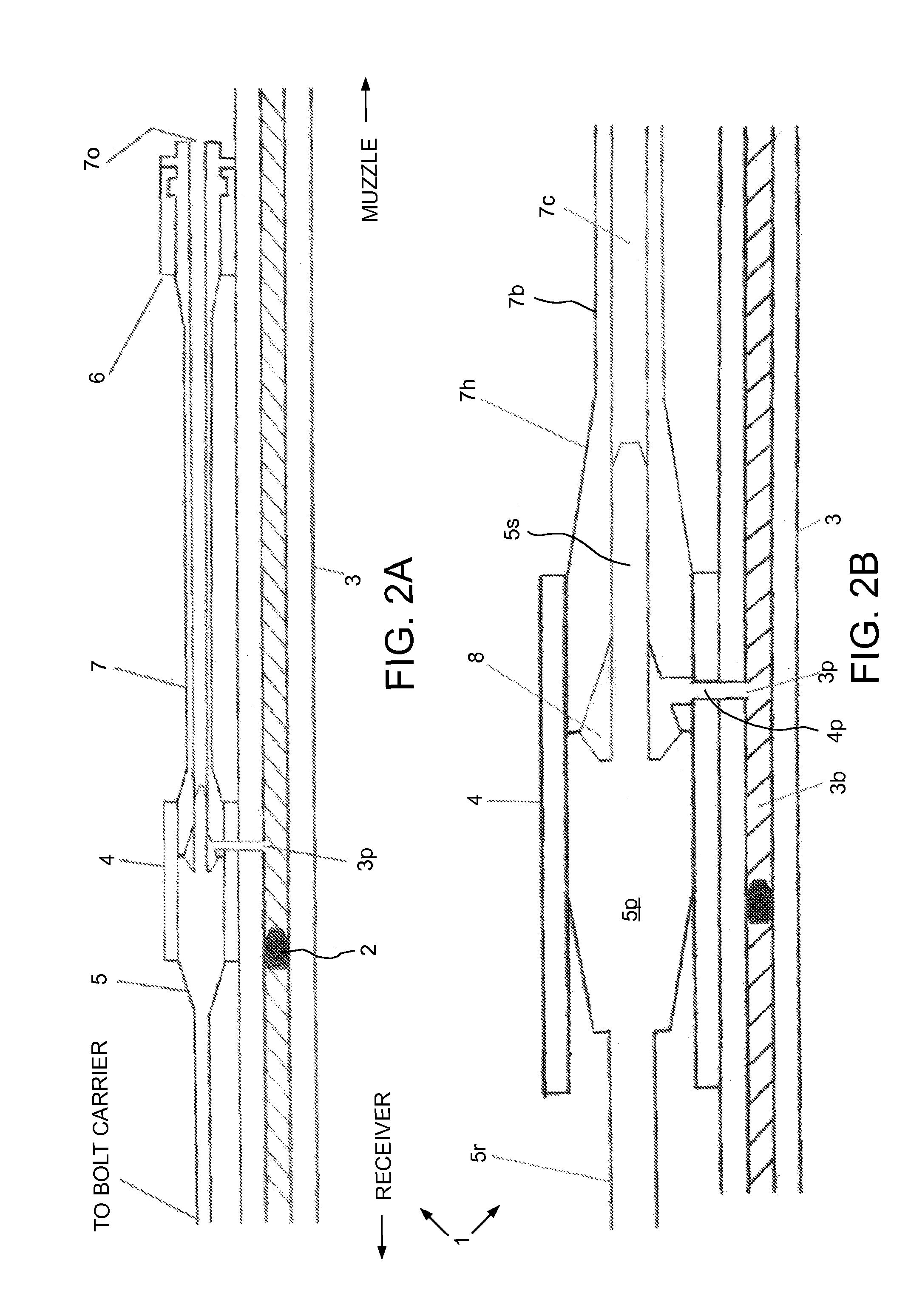
An indirect gas operating system for an M4 type automatic or semi-automatic firearm. The indirect gas operating system has a gas block having a cylinder. The gas block is fitted to a barrel assembly having a bore with the cylinder in communication with the bore. A piston having a piston end and a striking end has the piston end fitted to the cylinder. A bolt assembly having a striking surface is provided. When a cartridge is fired, gas displaces the piston end and causes the striking end to strike the striking surface displacing the bolt assembly. The cylinder and the piston are together removable as an assembly from the firearm without removal of the gas block.
Owner:COLTS MFG IP HLDG COMPANY
Law enforcement carbine with one piece receiver
Owner:COLTS MFG IP HLDG COMPANY
Firearm modification assembly
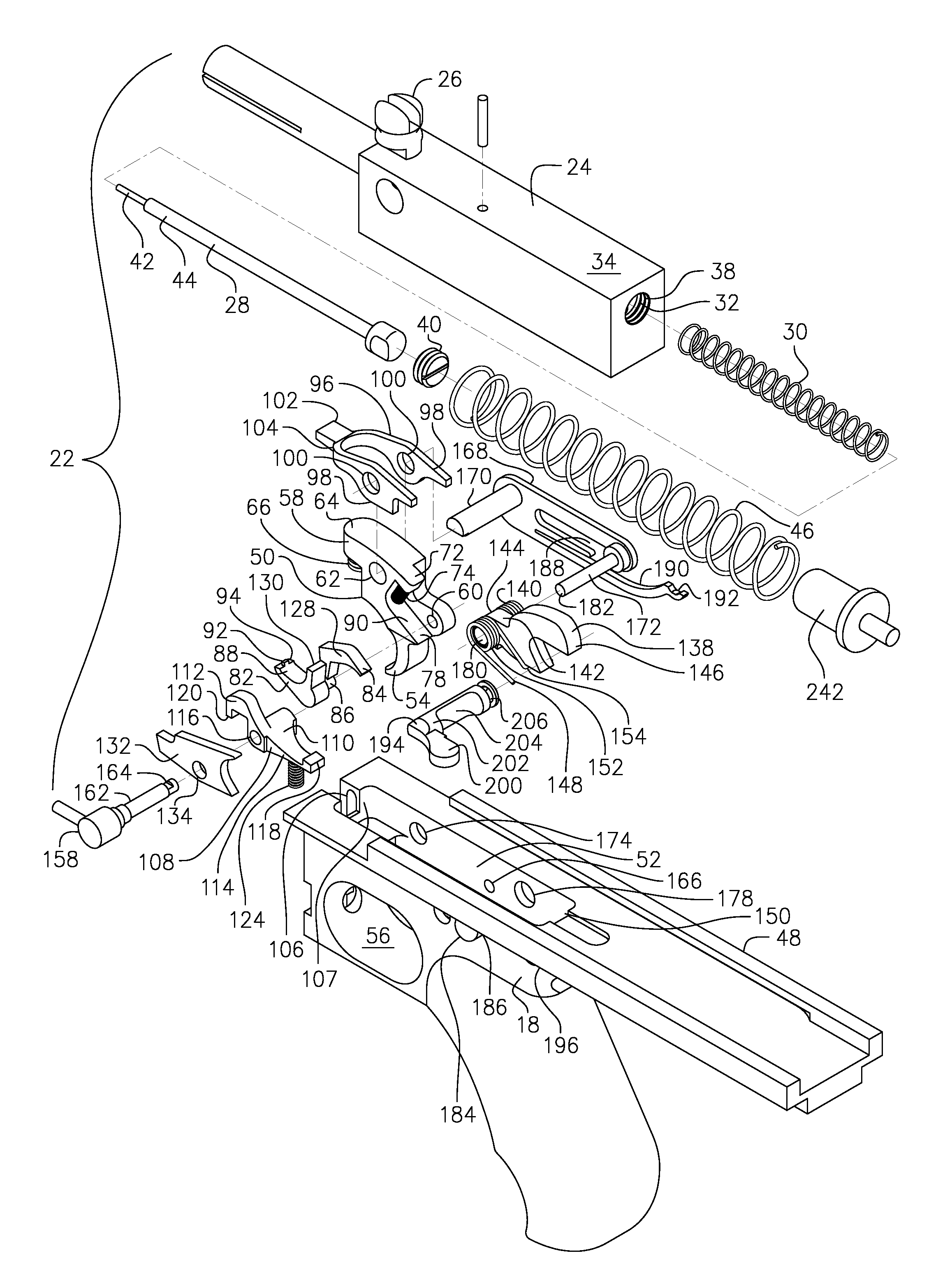
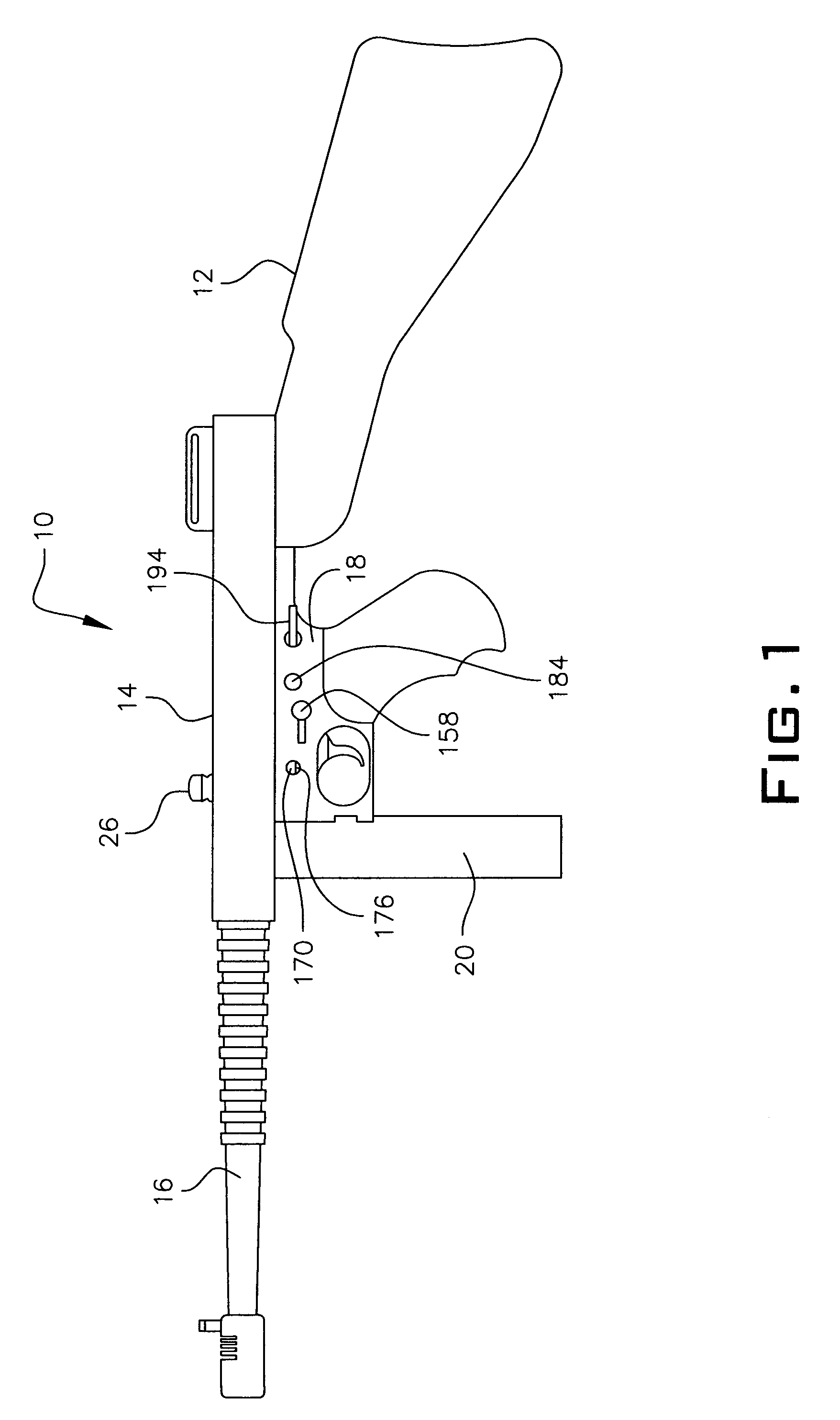
An assembly for modifying an automatic firearm such as a carbine rifle to relocate operation of the firearm charge handle. The assembly includes a fore-grip bracket, a drive rod movable in the foregrip bracket and attached to the charging handle release lever, and a cocking handle attached to the drive rod, the rod being attached to the charge handle for alternately releasing and latching the charge handle for clearing the firearm. In one embodiment, the foregrip bracket is attached to a removable rail attached to the foregrip by a jaw type device. Another embodiment attaches to the foregrip by attaching the foregrip bracket by screws. Another embodiment attaches to a foregrip having no rail by means of a groove cut in the grip, having a pair of spaced apertures into which hooks of foregrip mount, the bracket being held in place by a rod receiver and rotatable latch.
Owner:GAUNY JUSTIN A +1
Law enforcement carbine with one piece receiver
A semi-automatic or automatic rifle is provided. The semi-automatic or automatic rifle includes a receiver having an integral hand guard portion, a barrel having a bore. The bore is removably connected to the receiver, the hand guard portion extending over and surrounding the barrel, an indirect gas operating system has a gas block and a piston. The gas block has a cylinder, the gas block is fitted to the barrel, the cylinder is in communication with the bore. The piston has a piston end and a striking end, the piston end is fitted to the cylinder and has a bolt carrier having a striking surface. When a cartridge is fired, gas displaces the piston end and causes the striking end to strike the striking surface displacing the bolt assembly, wherein, the cylinder and the piston are together removable as an assembly from the firearm without disassembly of the rifle.
Owner:COLTS MFG IP HLDG COMPANY
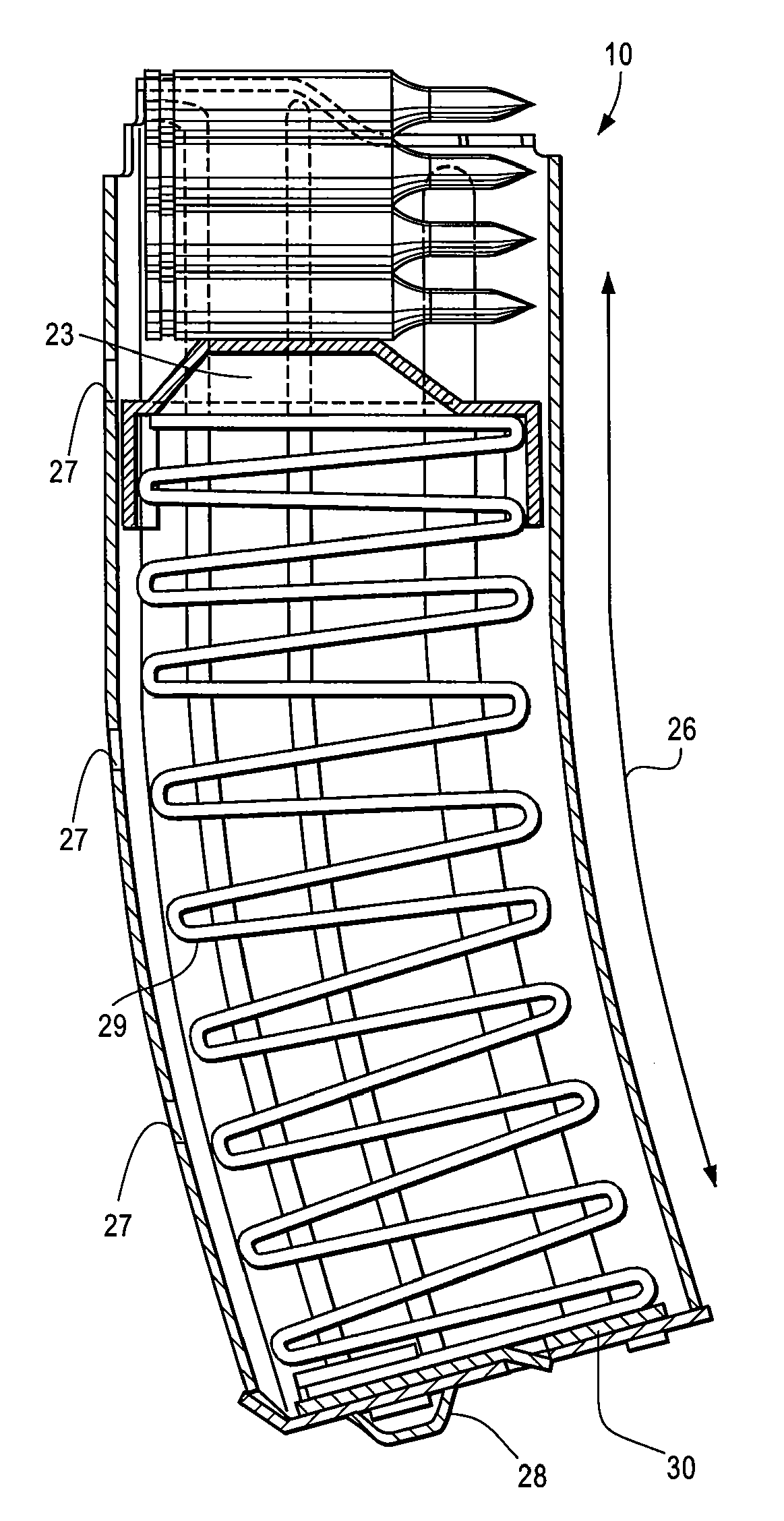
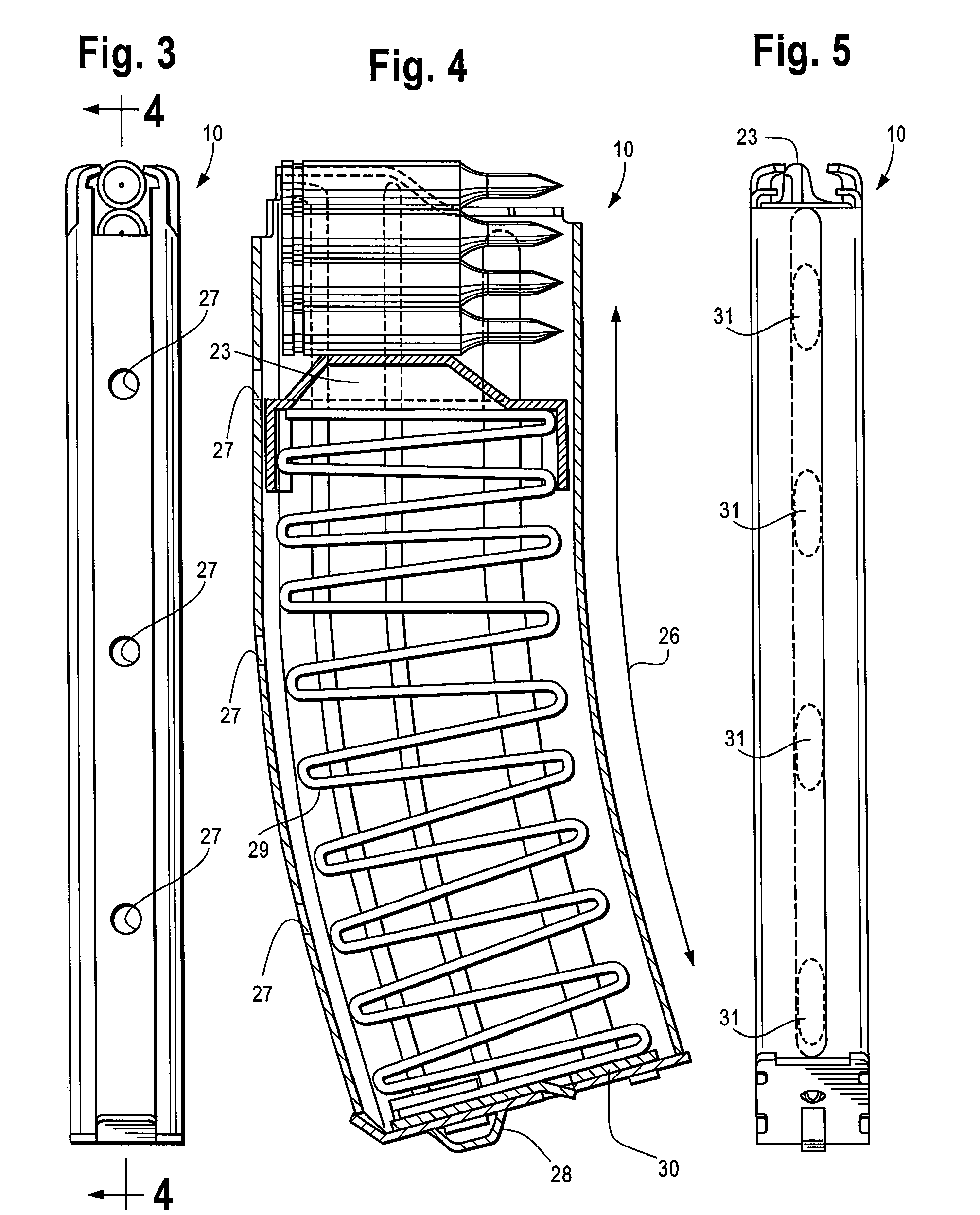
Collapsible carbine stock with spare magazine storage enclosure
A collapsible buttstock which is adapted primarily for use with military and tactical carbines, is supported by an extension tube which is connectable to the receiver of a carbine on which the collapsible buttstock is to be mounted. A storage enclosure is formed in the body of the buttstock and is sized and dimensioned so that it can receive a clip or magazine, typically able to carry 20 rounds, that is usable with the carbine on which the collapsible buttstock is mounted. The enclosure is provided with an openable cover that is hingably attached to the buttstock body. An easily operable cover latch assembly is usable to secure the cover in a closed position while facilitating expeditious opening of the cover to afford access to the spare magazine storage enclosure.
Owner:KENGS FIREARMS SPECIALTY
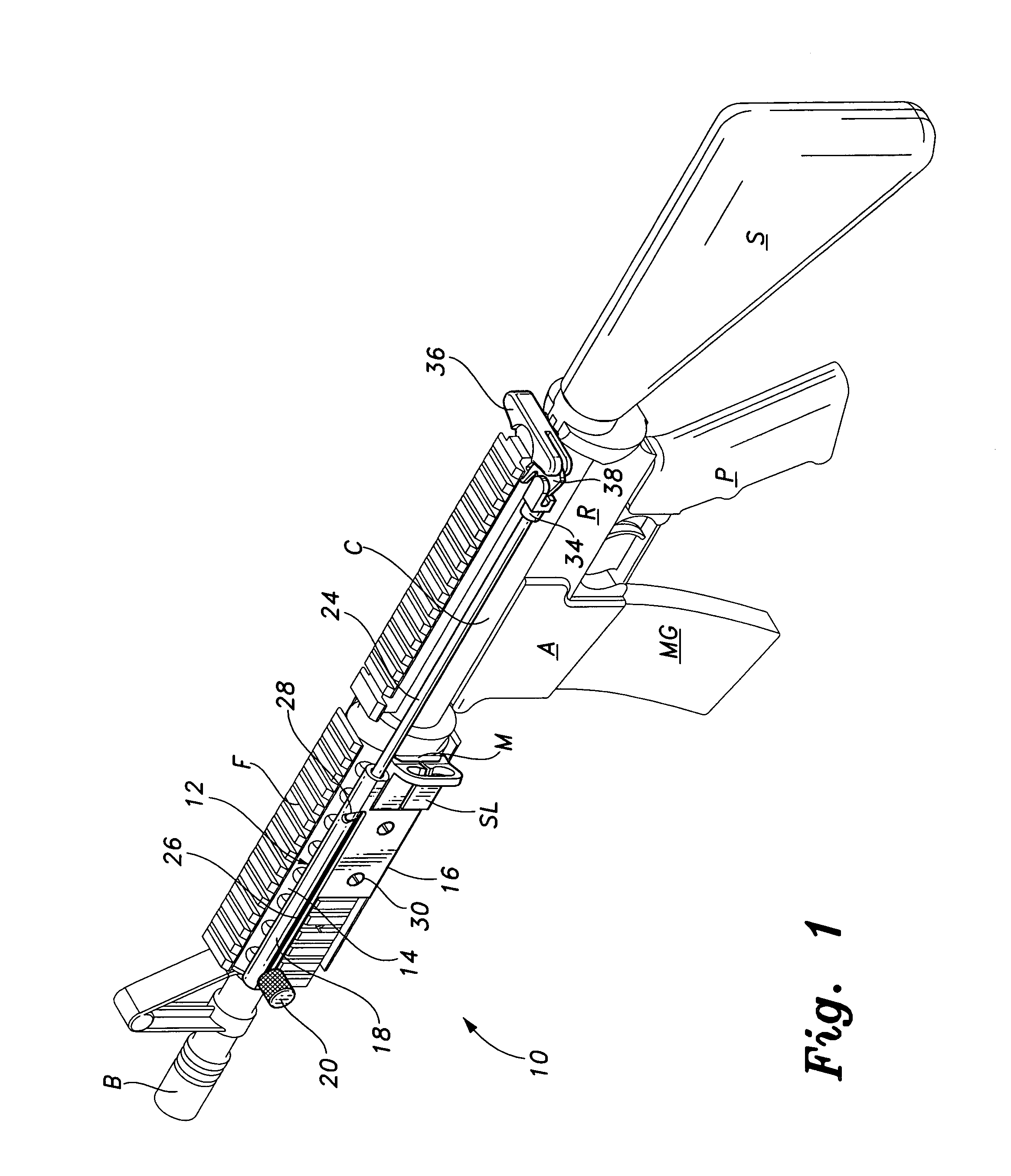
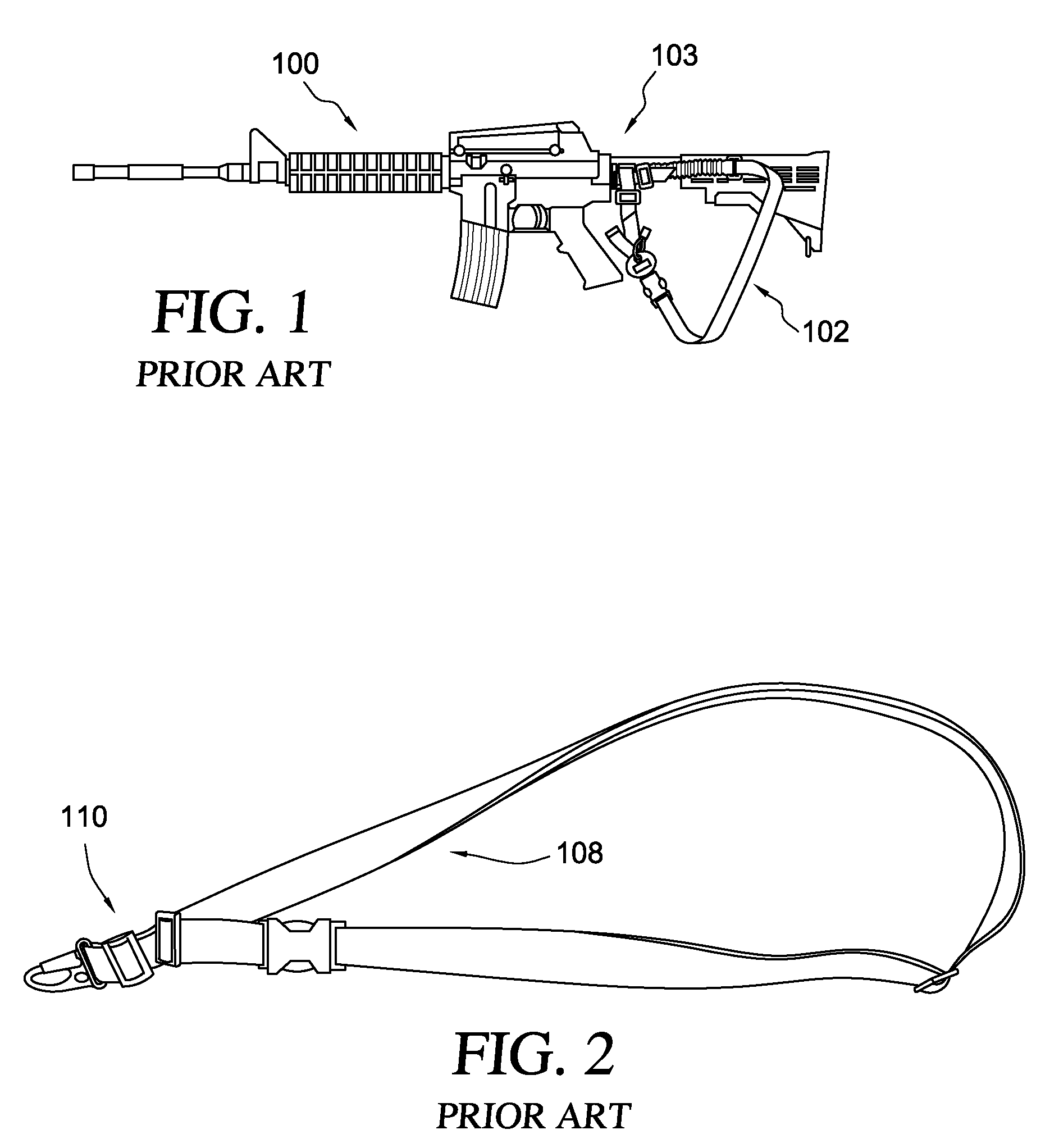
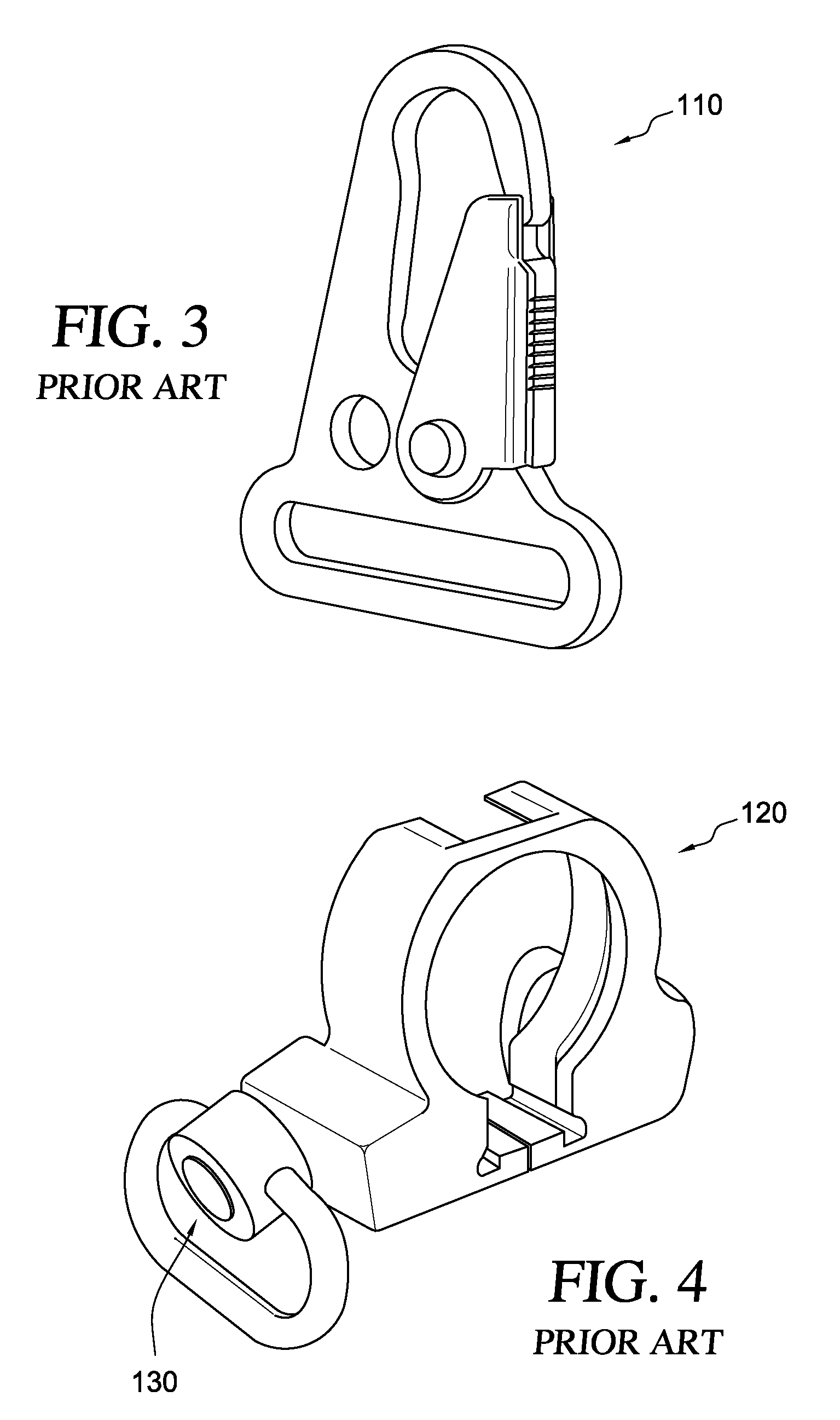
Loop-shaped Sling Adapter for use on Buffer Tube Assembly or Rifle Stock
InactiveUS20100287808A1Convenient and flexible and inexpensiveAwkward to handleButtsEngineeringRifle
A clamping sling attachment is configured for use on a carbine or rifle such as the M4 having it's a butt stock mounted on a cylindrical proximally projecting buffer tube, where a distal end of the buffer tube is threaded into the rear of the rifle's receiver. The sling attachment is releasably mountable on the buffer tube as an openable spring-clamp like loop which can be opened enough to go around the buffer tube's cylindrical outer wall. The sling attachment also has a laterally or transversely projecting sling mount defining a substantially vertical, transverse sling-hook receiving through-bore on one side to which the lower end of a weapon sling is releasably attachable. The buffer tube's central axis is the same as the sling attachment loop's central axis and is substantially perpendicular to the sling attachment's transverse sling-hook receiving through-bore.
Owner:SUPERIOR SHOOTING SYSTEMS INC
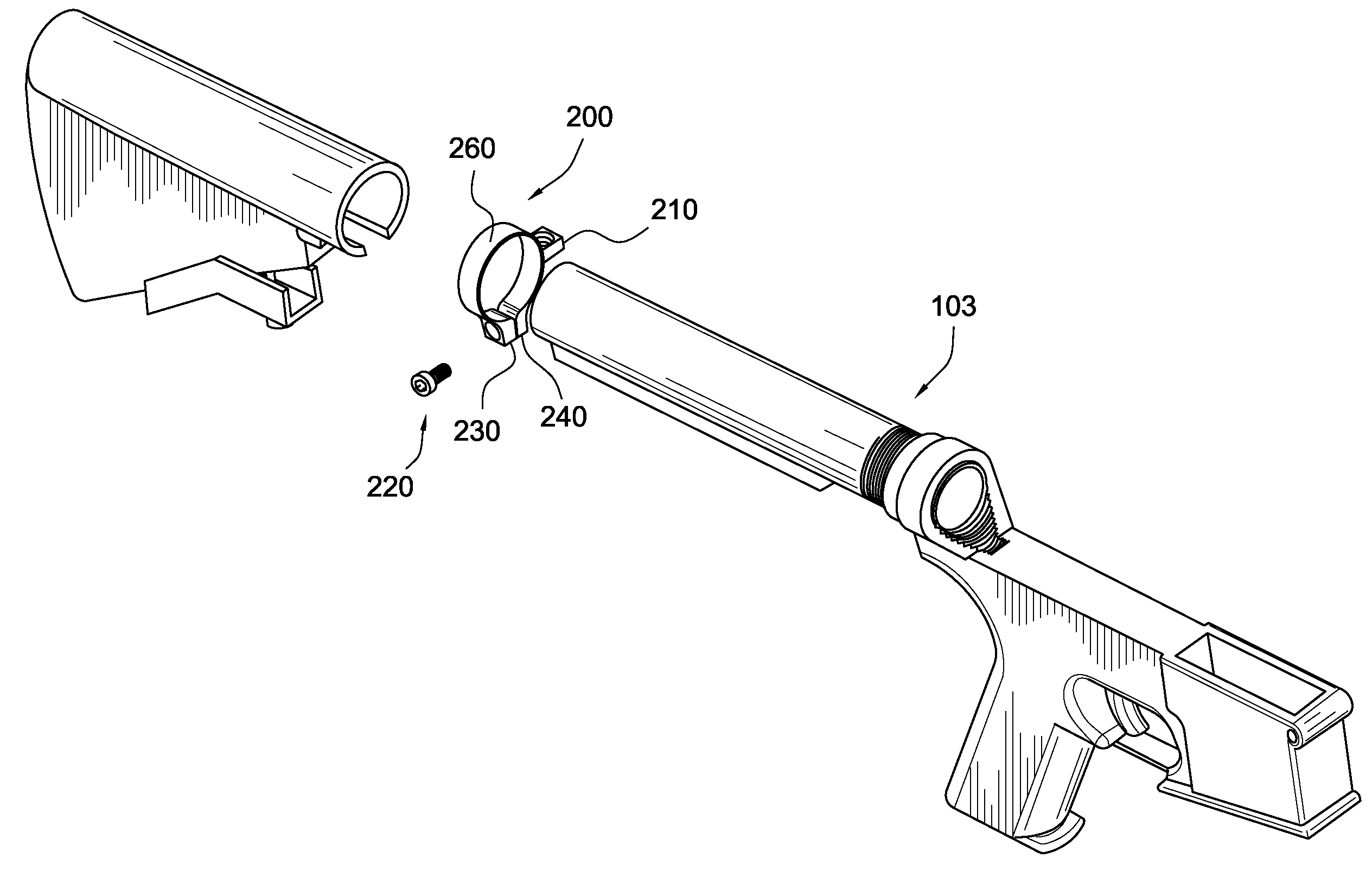
Firearm interface for a buttstock and pistol grip
A firearm interface for a buttstock and pistol grip that provides a means to attach a pistol grip or a buttstock assembly to a firearm, and shotguns in particular. It can be used for either modality individually or both in combination. It allows the utilization of an M16 / M4 style pistol grip and the adjustable, as well as the fixed buttstocks of M4 / M16 style rifles and carbines. The buttstock and pistol grip interface confers the ability to configure the firearm to different requirements and to the ergonometric needs of diverse individuals. By facilitating the use of M4 / M16 style receiver extensions and M16 / M4 style pistol grips the buttstock and pistol grip interface brings a vast array of options to the firearms to which it is affixed. For those individuals with military experience the use of M16 / M4 style components imparts a degree of familiarity and utility.
Owner:KAY IRA
Weapon sling and attachments
A lower sling attachment adapter for use in the M-16 rifle and M-4 carbine and their equivalents utilizing a rear or butt stock mounted on a tube, one end of which tube is threaded into the rear of the receiver of the weapon, the improvement comprising a lower sling attachment means mounted on said threaded tube near the receiver and having a hole through which the said threaded end of said tube is passed, said lower sling attachment means having sling mount means on at least one side thereof to which the lower end of a weapon sling is attached.
Owner:LINDSEY FORREST R
Closed bolt system with tigger assembly for converting afully automatic submachine gun into a semi-automatic carbine
A closed bolt system with a trigger assembly for converting an open bolt, blowback type submachine gun into a single firing carbine is provided. The closed bolt system with trigger assembly includes a tensioned trigger member supporting a tensioned disconnector system. A tensioned sear interacts with the disconnector system and a tensioned hammer. The hammer strikes a firing pin in the bolt when it is released from the sear. The blowback of the bolt, as a result of expanding gases from the exploding and exiting round, re-cocks the hammer by re-engaging the sear with the hammer and disengages the sear from the disconnector system. Only after releasing the trigger will the sear re-engage with the disconnector system and thereby permit another round to be fired. A receiver having a cavity encloses the bolt and prohibits a fully automatic bolt to be used therewith.
Owner:DREHSEN SUSAN +2
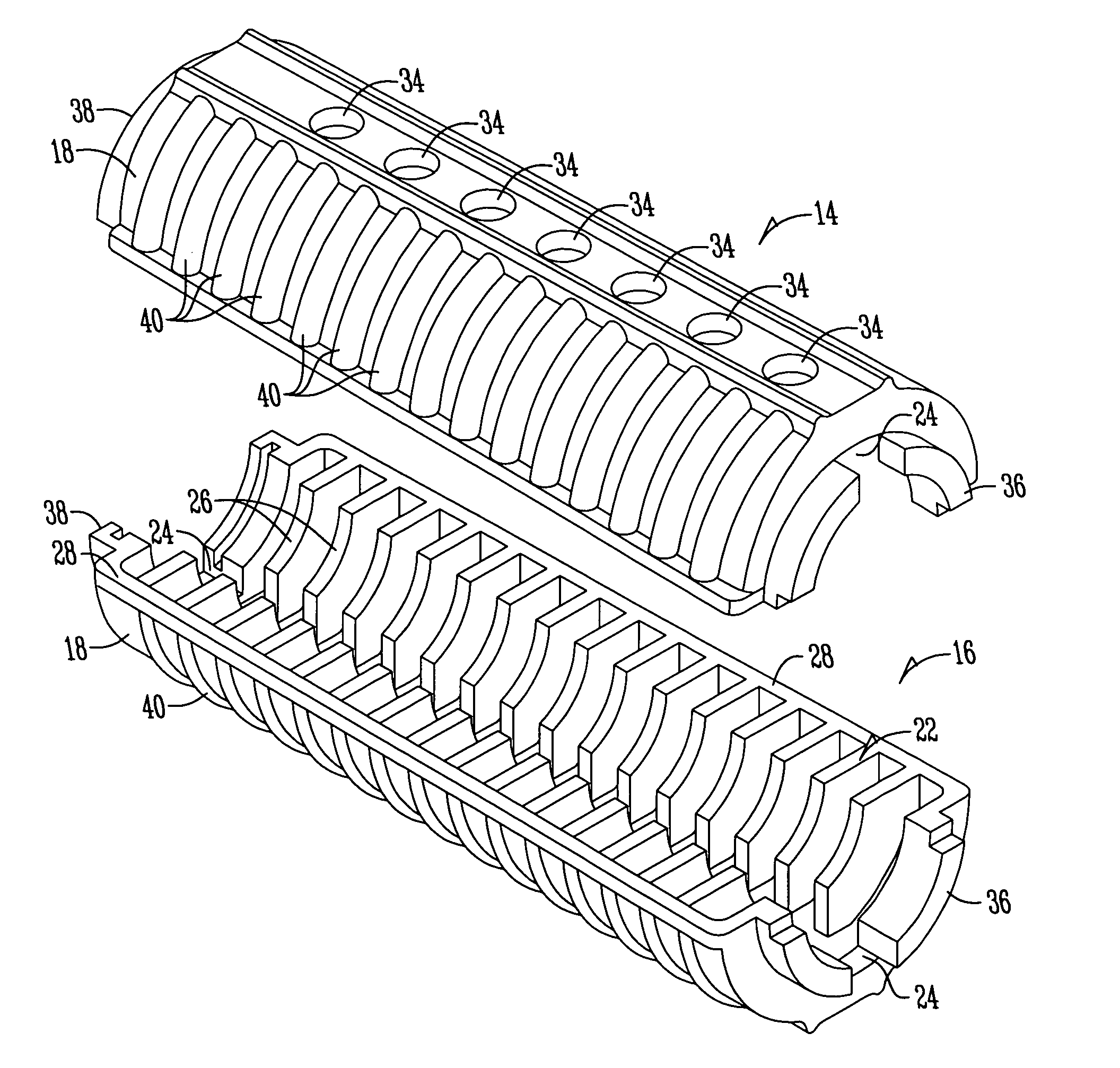
Finned carbine handguard assembly
ActiveUS6945154B1Improve impact resistanceHigh strengthVentillation systemsWeapon cleaningPlastic materialsEngineering
A gun barrel handguard includes half sections having longitudinally spaced apart fins of plastic material positioned around the exterior of the gun barrel for absorbing heat but preventing it from being quickly transmitted to the exterior of the handguard.
Owner:JJE BRANDS LLC
Closed bolt system with trigger assembly for converting a fully automatic submachine gun into a semi-automatic carbine
A closed bolt system with a trigger assembly for converting an open bolt, blowback type submachine gun into a single firing carbine is provided. The closed bolt system with trigger assembly includes a tensioned trigger member supporting a tensioned disconnector system. A tensioned sear interacts with the disconnector system and a tensioned hammer. The hammer strikes a firing pin in the bolt when it is released from the sear. The blowback of the bolt, as a result of expanding gases from the exploding and exiting round, re-cocks the hammer by re-engaging the sear with the hammer and disengages the sear from the disconnector system. Only after releasing the trigger will the sear re-engage with the disconnector system and thereby permit another round to be fired. A receiver having a cavity encloses the bolt and prohibits a fully automatic bolt to be used therewith.
Owner:DREHSEN SUSAN +2
Firearm interface for a buttstock and pistol grip
A firearm interface for a buttstock and pistol grip that provides a means to attach a pistol grip or a buttstock assembly to a firearm, and shotguns in particular. It can be used for either modality individually or both in combination. It allows the utilization of an M16 / M4 style pistol grip and the adjustable, as well as the fixed buttstocks of M4 / M16 style rifles and carbines. The buttstock and pistol grip interface confers the ability to configure the firearm to different requirements and to the ergonometric needs of diverse individuals. By facilitating the use of M4 / M16 style receiver extensions and M16 / M4 style pistol grips the buttstock and pistol grip interface brings a vast array of options to the firearms to which it is afixed. For those individuals with military experience the use of M16 / M4 style components imparts a degree of familiarity and utility.
Owner:KAY IRA
Polydicyclopentadiene composite material and single material reaction injection molding technology thereof
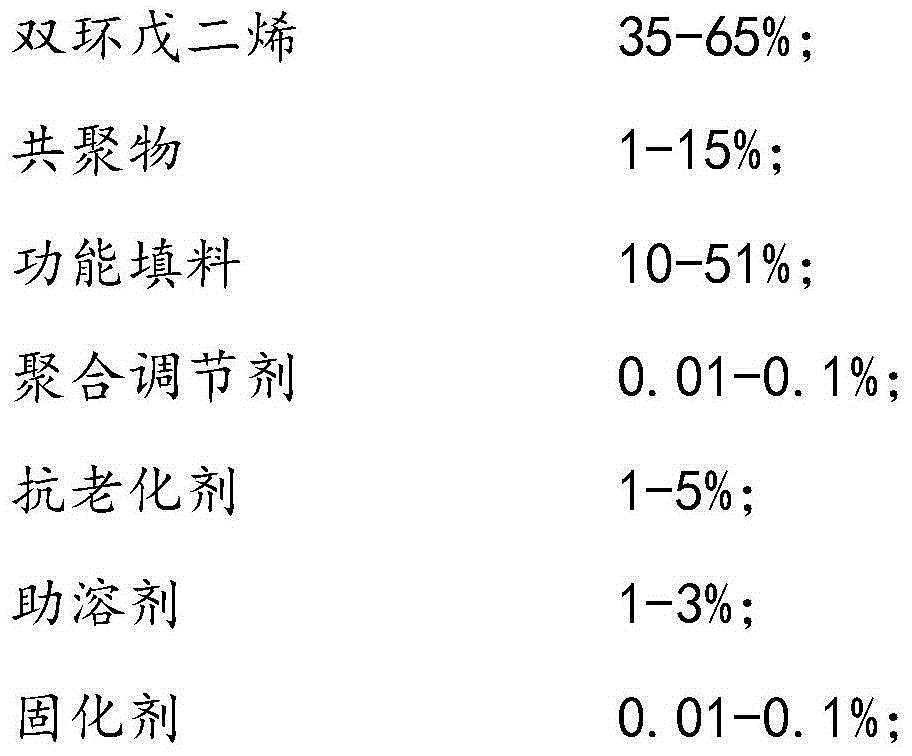
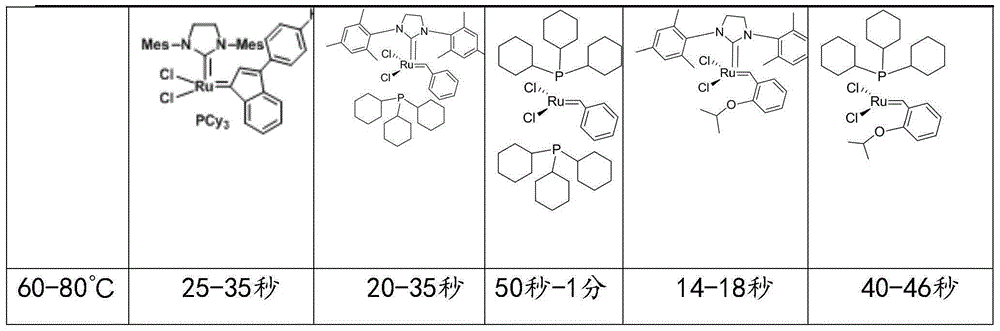
The invention discloses a formula of a polydicyclopentadiene (PDCPD) composite material and a single material reaction injection molding (RIM) technology thereof. The composite material is prepared from 35-65% of dicyclopentadiene, 1-15% of copolymers, 10-51% of functional filler, 0.01-0.1% of polymerization regulator, 1-5% of antiaging agents, 1-3% of cosolvent and 0.01-0.1% of curing agent, and the curing agent is a ruthenium-carbine catalyst. The single material reaction injection molding technological method includes the steps that the formula materials are mixed and evenly dispersed and form mixed slurry with a curing agent solution, and the mixed slurry is injected into a molding mold at the speed of 10-100 ml / s with the temperature kept below 30 DEG C; the mold is heated to 60 DEG C-80 DEG C, curing is carried out fast within one minute, and then demolding is carried out. The PDCPD composite material is good in mechanical property, the curing time is short, and the process is simple.
Owner:克琴新材料科技(上海)有限公司
Sleeve piston for actuating a firearm bolt carrier
The embodiments described herein relate generally to a sleeve piston for operating the bolt carrier of a firearm. The sleeve piston can comprise a monolithic sleeve piston or it can comprise two or more sleeve-piston parts, thereby evenly distributing the reciprocating mass of an auto-loading firearm about its barrel, minimizing muzzle rise. The sleeve piston can further be coupled to the bolt carrier by two or more operating rods, thereby reducing the tipping force or off-axis torque experienced by the bolt carrier during firearm operation. The sleeve piston can also reciprocate in a sleeve gas block coupled to a barrel, thereby helping to transfer heat away from the barrel. The sleeve piston embodiments of the present invention provide a balanced and compact operating mechanism that is ideally suited for rifles, carbines, and personal-defense weapons.
Owner:JACKSON JASON STEWART
Nano-scale wolfram carbine composite powder and method of manufacturing the same
The invention provides a nanometer carbonized tungsten compound powder, which includes carbonized tungsten, vanadium carbide or / and chrome carbide, and a metallic element without binding phase exists in the compound powder. The preparation method of the nanometer carbonized tungsten compound powder is characterized in that the powdery ammonium tungstate, the carbonaceous reducing agent and the inhibitor are adopted as raw materials, the raw materials are dissolved in deionized water or distilled water and evenly stirred so as to obtain a solution, then the solution is heated and dried, the precursor powder is obtained finally, the precursor powder is put into a high-temperature reaction furnace, under the vacuum, argon or hydrogen atmosphere protection condition and the condition of 1,000 to 1,200 DEG C and 30 to 60 min, the carbonized tungsten compound powder with the average particle diameter less than 100 nm and even particle size distribution is obtained through carbonization. The method has the characteristics of low reaction temperature, short reaction time, low manufacturing cost, simple process and the like, which is suitable for the industrial production and used for the ultrafine hard alloy nanometer carbonized tungsten compound powder.
Owner:SICHUAN UNIV
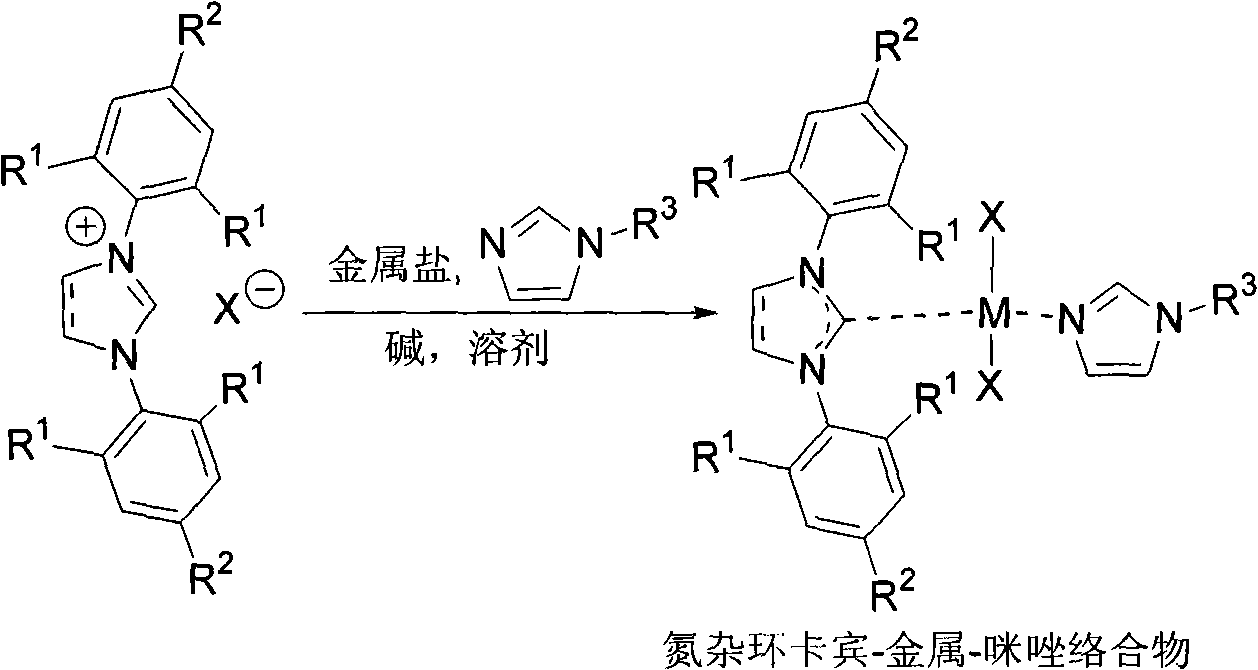
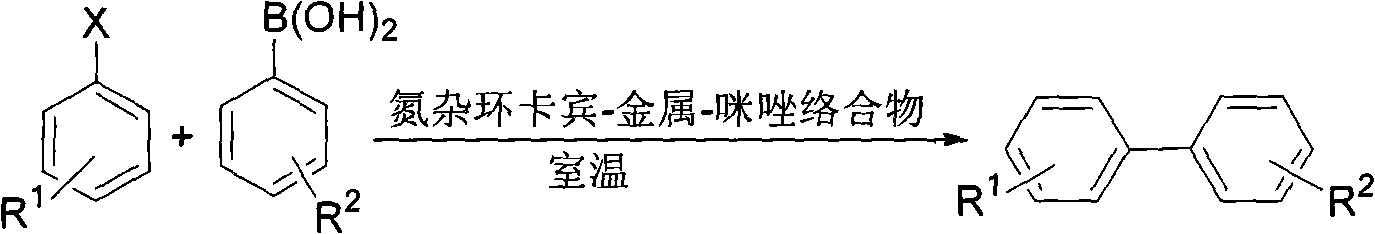
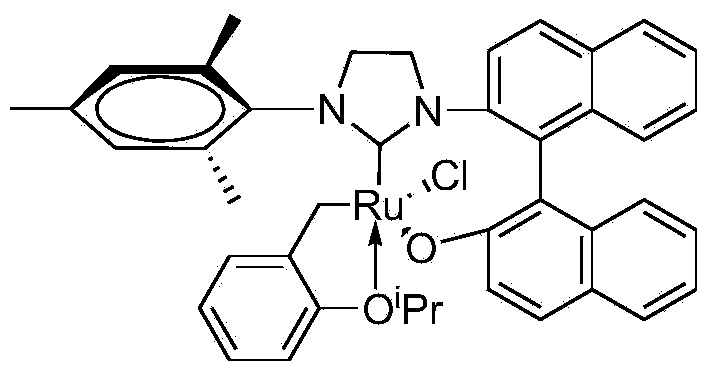
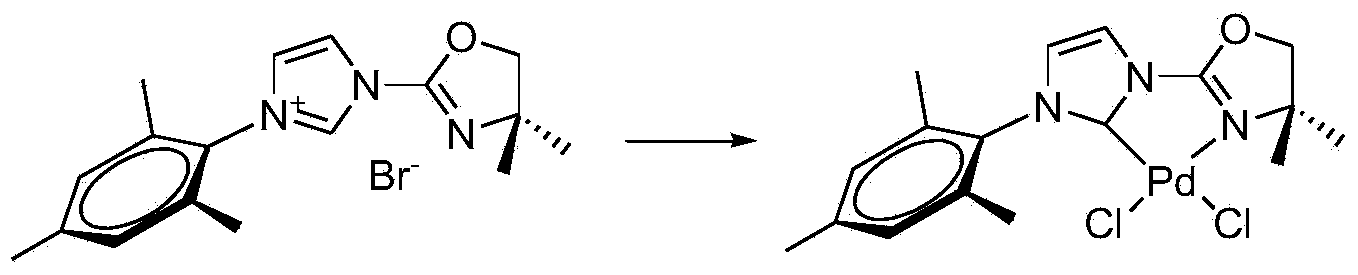
Suzuki-Miyaura coupling reaction of catalyzing aryl chloride by N-heterocyclic carbine-palladium-imidazole complex at room temperature under condition of water phase
InactiveCN102153592AEasy to manufactureLow priceOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsBoronic acidCarbene
The invention provides a reaction method which is as follows: starting from simple and available imidazole salts, corresponding N-heterocyclic carbine-metal-imidazole complexes are synthesized; and then a Suzuki-Miyaura coupling reaction of aryl or heteroaryl boronic acid and aryl chloride by utilizing the complexes at room temperature under the condition of a water phase is carried out. The centers of metals related in the metal salts in the invention can be iron, copper, silver, nickel, palladium, cobalt, rhodium, ruthenium and the like. A catalyst used in the invention is easy to prepare, can be quantitatively synthesized and is stable to air and moisture. By using the catalyst, the corresponding coupling reaction is achieved under the condition that catalytic amount is only 0.01%, and yield is excellent. Compared with other systems reported in documents, the catalytic system is cheap in price, reaction is easy to operate and can be smoothly carried out at room temperature, and the requirement on reaction conditions is extremely low, thus the catalytic system has a good industrial application prospect.
Owner:WENZHOU UNIVERSITY
Arcuate magazine for a firearm and a method for making the same
An arcuate magazine for a firearm, such as a rifle or carbine, which has a keyway and follower assembly for delivering rounds of ammunition one at a time to the firing area of the firearm and which may be fabricated from a single piece of metal and have a smooth interior free of welds and rough spots. The invention also includes a method of making such a magazine which includes the steps of deep drawing and wiping the magazine body under substantial pressure using a progressive die having numerous stations.
Owner:AIRTRONIC USA
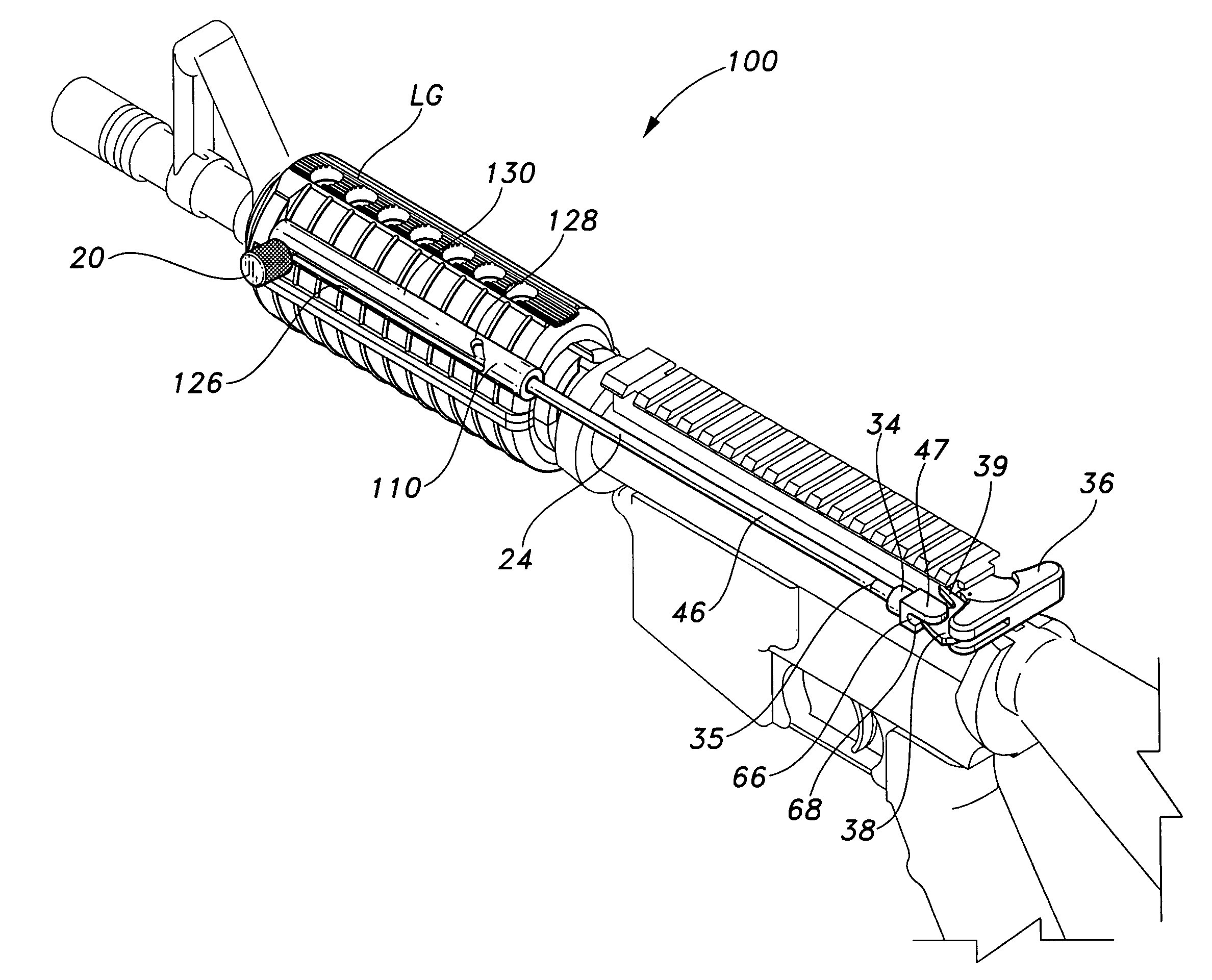
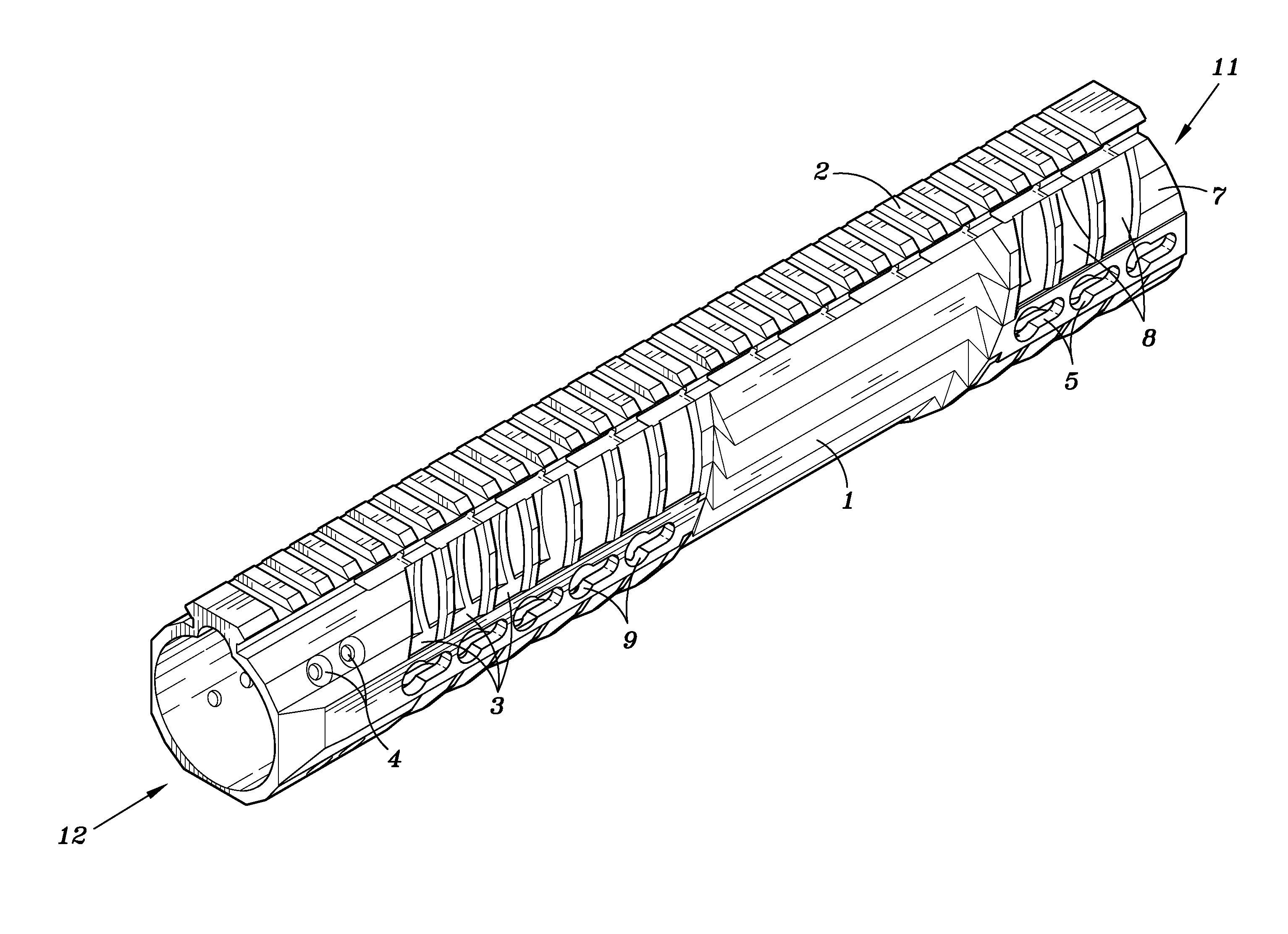
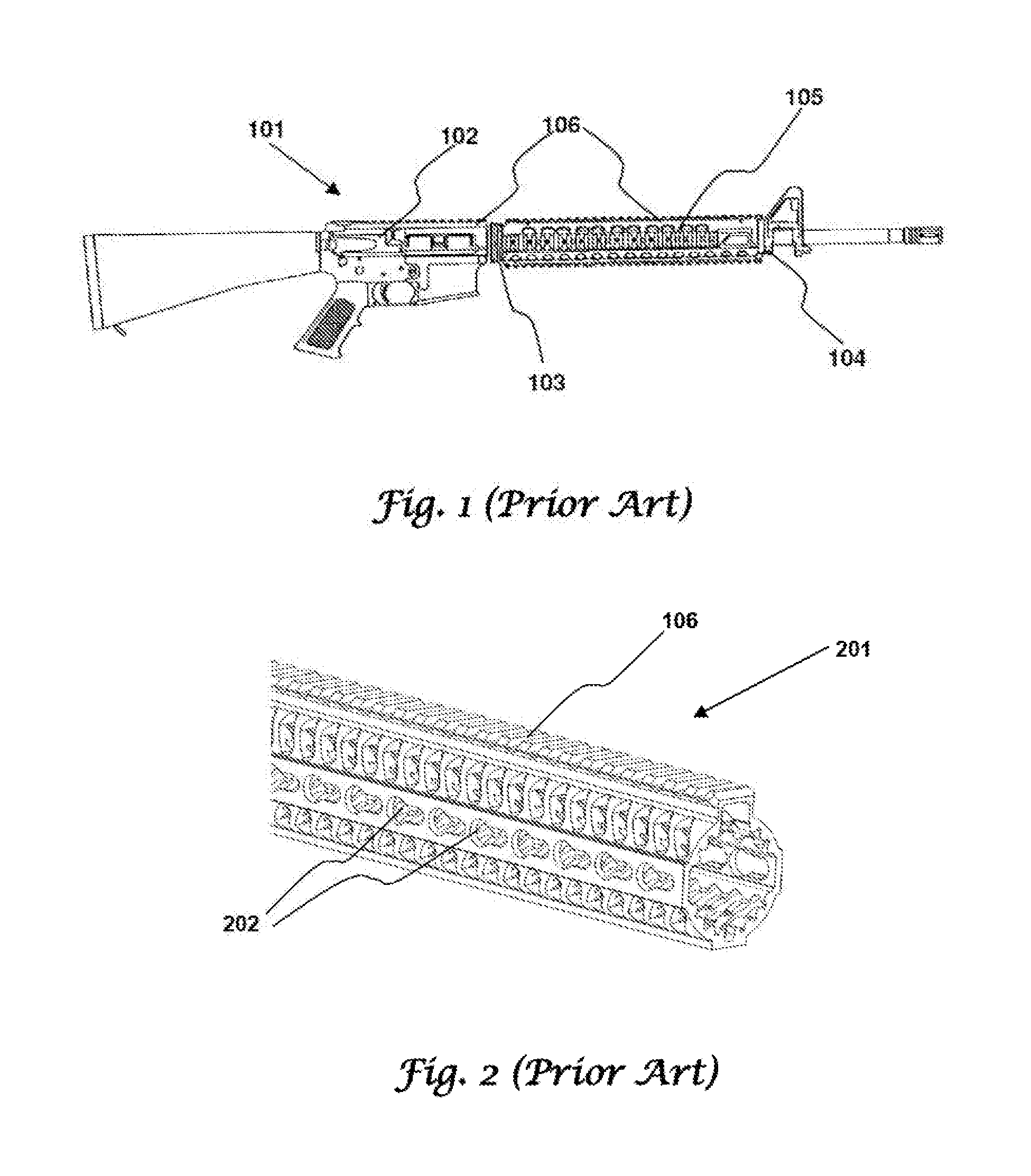
Rifle handguard with grip
This invention relates to a forearm “free floating” handguard for high rate of fire semiautomatic rifles and carbines such as the M16 family. More particularly, a handguard is disclosed that includes an integral gripping section and ventilation cut-outs, providing optimal grip strength, improved convective barrel cooling, while minimizing weight of the firearm.
Owner:F 1 RESERACH LLC
Chiral center nitrogen heterocyclic carbine precursor salt with quadrol skeleton, synthetic method and application
ActiveCN102153557AEfficient manufacturingThe synthesis method is simpleAsymmetric synthesesEthylenediamineBenzopyrone
The invention provides a chiral center nitrogen heterocyclic carbine precursor salt with a quadrol skeleton, a synthetic method and application. The precursor salt has a structural formula shown in the specifications, and can be prepared from cheap and readily-available chiral substituted diamine as an initial raw material by three-step synthesis, well applied to a polarity inversion reaction of catalytic aldehyde compounds, and used for preparing chiral benzopyrone compounds.
Owner:SHANGHAI INST OF ORGANIC CHEM CHINESE ACAD OF SCI
Aza cyclic carbine rear earth catalyst for crystallinity 3,4-polyisoprene
ActiveCN101157737AHigh molecular weightNarrow molecular weight distributionChlorobenzeneReaction temperature
The present invention relates to a nitrogen heterocyclic carbene rare earth complex, a catalyst system which consists of the present invention, aluminum alkyl and an organoboron salt can catalyse the isoprene to carry out the solution polymerization, so as to prepare the polyisoprene with the crystallization property, high 3, 4-structure and high glass transition temperature (Tg). The molar ratio of aluminum alkyl and the rare earth complex is within the scope of 1 to 100, the molar ratio of the organoboron salt and the rare earth complex is within the scope of 0.5 to 2.0; the solvent which is used for polymerization can be toluene, bromobenzene, n-hexane, dichloromethane or chlorobenzene; the polymerization temperature range is -20 DEG C to 80 DEG C, the reaction time of polymerization at minus 20 DEG C is 36 hours, the reaction time of polymerization at 80 DEG C is 1 hour, and the monomer conversion rate can be up to 100 percent. The reaction is characterized by active polymerization, the molecular weight of the product can be controlled by the molar ratio of the monomer and the catalyst, the molecular weight of the polyisoprene can achieve 360,000 at most, and the glass transition temperature Tg is equal to 5 DEG C to 50 DEG C. The content of the 3, 4 structure of the polyisoprene is affected by the spatial effect and the electronic effect of the rare earth complex, the solvent type for polymerization reaction and the polymerization reaction temperature and so on, which is changed between 76 percent and 99 percent.
Owner:CHANGCHUN INST OF APPLIED CHEMISTRY - CHINESE ACAD OF SCI
Wolfram carbine abrasion-proof core deposit welding rod
InactiveCN101116932AHigh hardnessImprove wear resistanceWelding/cutting media/materialsSoldering mediaCrazingWear resistant
A tungsten carbide wear resistant drug core resurfacing welding rod comprises a drug core and a steel sheet for wrapping the drug core. A drug fur powder material is coated outside the steel sheet. The weights of the drug core, the steel sheet and the drug fur powder material separately in the total weight of the welding rod is that is the drug core is ranged from 32 percent to 36 percent, the steel sheet is ranged from 32 percent to 36 percent, the drug fur powder material is ranged from 30 percent to 34 percent, the drug core tungsten carbide is ranged from 96 percent to 98 percent and nickel is ranged from 2 percent to 4 percent. The weight of drug fur is distributed according to percent that marble is ranged from 33 percent to 35 percent, fluorite is ranged from 35 percent to 38 percent, tungsten carbide is ranged from 10 percent to 14 percent, high carbon ferromanganese is ranged from 2 percent to 6 percent, titanium iron is ranged from 8 percent to12 percent, rare earths silicon is ranged from 1 percent to 3 percent and calcined soda is ranged from 1 percent to 3 percent. The resistant alloy is equably bridged to the resurfacing welding metal through welding core. The invention has the advantages that the welding technics performance is good and the welding line ingredient is even. The connecting strength is high, thereby, avoiding gas hole and flaw and with good detachability. The invention has a higher hardness and wear resistant performance. The making technics is simple and the producing cost is lower. The welding rod structure is novel and the welding shape is good to suit in full position resurfacing welding of wearing parts in the engineering machinery in metallurgy and mine.
Owner:WUHAN TEMO WELDING CONSUMABLES CO LTD
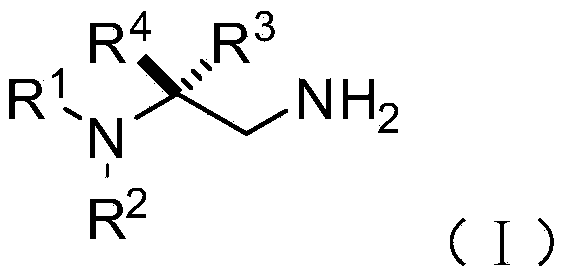
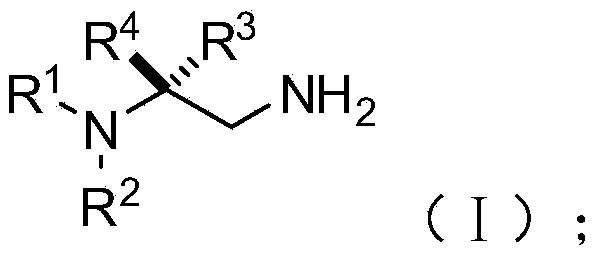
Chiral 1,2-diamine compound and preparation method and application thereof
ActiveCN105367427AAchieve strict metal-freeThe reaction process is safe and controllableOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsCompound aNitroalkene
The invention discloses a chiral 1,2-diamine compound and a preparation method and application thereof. The molecular structure general formula of the chiral 1,2-diamine compound is shown as the general formula (I) in the specification. The preparation method of the chiral 1,2-diamine compound comprises the steps that an amine compound A and a nitroolefin compound B are added to a reaction system containing an n-heterocyclic carbine catalyst, an alkali reagent, a proton additive and a dewatering reagent for reacting and other steps. The chiral 1,2-diamine compound comprises chiral quaternary carbon atoms containing electrondrawing groups and amino groups, and can be widely used for synthesizing drug intermediates, especially heterocyclic ring structure compounds, and preparing functional materials. The preparation method is simple in process and low in requirement for reaction conditions, the reaction process is safe and controllable, the atom utilization rate and production efficiency are high, meanwhile, the enantioselectivity of products is efficiently guaranteed, and the environment pollution pressure of the methodology is low through the introduction of the small organic molecule asymmetric catalysis concept.
Owner:PEKING UNIV SHENZHEN GRADUATE SCHOOL
Chiral hexahydroxy n-heterocyclic carbine precursor compound as well as preparation method and application thereof
InactiveCN103772297AOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsOrganic synthesisKetone
The invention relates to the field of organic synthesis, and particularly relates to a preparation method and an application of a chiral hexahydroxy n-heterocyclic carbine precursor compound. The compound has a structure as shown in a formula (V) in descriptions. The chiral hexahydroxy n-heterocyclic carbine precursor compound can be used for catalyzing multiple chiral reactions such as an addition reaction of unsaturated esters, alpha,beta-unsaturated imine and diborane pinacol borate, or a condensation reaction of aldehyde and boric acid compounds or a reduction reaction of ketone and has relatively good catalytic efficiency and enantioselectivity. (imgfile='DDA0000462319780000011.T'IF wi='392 he='496' / ).
Owner:SHANGHAI UNIV OF ENG SCI
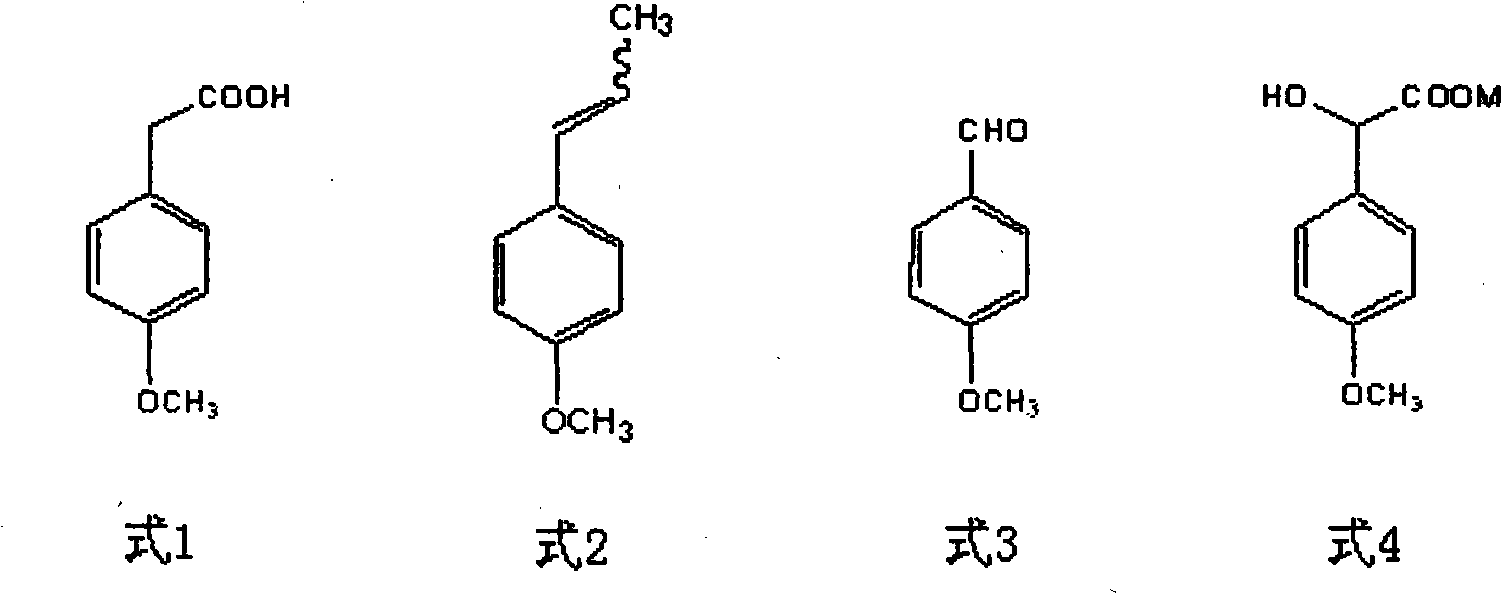
Method for preparing p-methoxypheny-lethyl acid from natural anethole
InactiveCN101298416ANo pollution in the processSimple reaction conditionsOrganic compound preparationCarboxylic preparation by oxidationP-methoxyphenylacetic acidPetrochemical
The invention discloses a method for preparing methoxyphenylacetic acid by natural anethole; the natural anethole is taken as a raw material and generated into the anisicaldehyde by oxidation reaction, then anisic mandelic acid (salt) is generated by the insertion reaction of carbine; finally the methoxyphenylacetic acid is obtained by reduction. The method has the advantages of reproducible raw material, simple operation and high yield, etc.; furthermore, the method can prepare the methoxyphenylacetic acid which can replace the source of petrochemical industry.
Owner:GUANGZHOU INST OF GEOCHEMISTRY - CHINESE ACAD OF SCI
Gas piston system for M16/AR15 rifle or M4 carbine systems
A gas piston system for a firearm includes a gas block having a port in communication with the barrel port and an exhaust tube. The exhaust tube has: a head at least partially disposed in the gas block and having a port in fluid communication with the gas block port; a body extending from the head toward a muzzle of the firearm; and a channel extending from the exhaust tube port through the body. The gas piston system further includes a driver movable relative to the gas block between a forward and rearward position and having: a piston slidable along the gas block; a stinger closing the channel in the forward position and opening the channel in the rearward position, and an operating rod operable to push the bolt carrier assembly away from the barrel.
Owner:KRAMER LAWRENCE S
Grip/cover for key lock system
Covers for key lock mounting systems on small arms such as the AR or M4 family of rifles and carbines can provide protection for the firearm, comfort for the operator, and electrical power to firearm accessories. The cover has keyhole grips that are pressed into the key lock mounting points in mounting system. The cover is held seated against the surface of the mounting system by the shape and resilience of keyhole grips. V-notch keyhole grips can attach to keyhole shaped mounting points and slot shaped mounting points. The covers have connectors and internal circuitry such that covers lined up edge to edge are electrically connected. Firearm accessories can be electrically powered the connectors.
Owner:FALCON INDS
Modular scope mount assembly
Implementations of a modular scope mount assembly are provided. In some implementations, the modular scope mount assembly may be used to secure a telescopic sight to a firearm (e.g., a rifle and / or a carbine). In some implementations, the modular scope mount assembly may be used to co-mount a telescopic sight and a reflex sight to a firearm. In some implementations, the modular scope mount assembly may be configured to place a reflex sight on the right side and / or left side of a co-mounted telescopic sight. In some implementations, the modular scope mount assembly may be configured to allow a user to change (increase or decrease) the eye relief between the user and the co-mounted reflex sight(s).
Owner:ZIMMER TRENT
Dinuclear nickel cross-coupling reaction catalyst supported by nitrogen heterocycle carbine ligand and preparation method thereof
InactiveCN101284247AImprove catalytic performanceGood catalyticOrganic-compounds/hydrides/coordination-complexes catalystsNickel organic compoundsShortest distanceDual core
The invention discloses a dual-core nickel cross-coupling reaction catalyst supported by N-heterocyclic carbine and a preparation method. Acetonitrile is taken as a solvent, N-heterocyclic carbine ligand and silver oxide in the molar ratio of 1: 3 to 1: 6 are added and stirred, the reaction is carried out for 10 to 15 hours away from light; Ni(DME)Cl2 or Ni(PPh3)2Cl2, Ni(DME)Cl2 or Ni(PPH3)2Cl2 and the N-heterocyclic carbine ligand in the molar ratio of 2: 1 to 3: 1 are added and filtered, the filtrate is condensed, ether is added for precipitation of yellow solids, the yellow solids are sequentially washed by ethanol and ether for 2 to 3 times, the acetonitrile is then used for dissolution, the ether is slowly added, and the dual-core nickel cross-coupling reaction catalyst supported by the N-heterocyclic carbine is obtained by crystallization. The novel dual-core nickel catalyst synthesized by the invention can play the synergy due to the shorter distance between two nickel atoms, the catalytic effect thereof is higher than the common palladium catalyst, thus having very ideal catalytic effect on chlorinated aryl compounds with cheap price and wide application prospect in fine chemical and pharmaceutical industries and being environment-friendly.
Owner:ZHEJIANG UNIV
Mesoporous carbon supported N-heterocyclic carbene-palladium catalyst as well as preparation and application thereof
InactiveCN104511310AHigh catalytic activityEasy to useOrganic-compounds/hydrides/coordination-complexes catalystsOrganic substitutionPalladium catalystCarbene
The invention discloses a mesoporous carbon supported N-heterocyclic carbene-palladium catalyst as well as a preparation method and application thereof. The catalyst carrier is hydroxylated mesoporous carbon; the ligand is N-heterocyclic carbine; the active ingredient is palladium chloride; the load capacity of the palladium in the active ingredient is 5.0-10.0% of the total mass of the catalyst; the surface hydroxyl group content of the hydroxylated mesoporous carbon is 1.9-2.2 mmol / g; the BET specific surface area of the hydroxylated mesoporous carbon is 700-1000m<2> / g; the BJH pore diameter is 5-7 nm; the BJH pore volume is 0.8-1.8 ml / g. According to the mesoporous carbon supported N-heterocyclic carbene-palladium catalyst, the palladium can be prevented from loss in the catalytic process; the mesoporous carbon has an ordered mesoporous structure, so that the surface dispersing performance of the palladium nano-particles can be improved to prevent the palladium from gathering in the catalytic process and improve the catalytic activity of the supported catalyst. The mesoporous carbon supported N-heterocyclic carbene-palladium catalyst has relatively high catalytic activity and relatively good reusability.
Owner:EAST CHINA UNIV OF TECH
N-heterocyclic carbine palladium compound containing pyridyl-2-formate or pyridyl-2,6-diformate ligand, preparation thereof and application thereof
InactiveCN102627672AGroup 8/9/10/18 element organic compoundsOrganic-compounds/hydrides/coordination-complexes catalystsCarbon numberFormate
The invention relates to a preparation method and an application of an N-heterocyclic carbine palladium compound containing a pyridyl-2-formate or pyridyl-2,6-diformate ligand. The N-heterocyclic carbine palladium compound containing the pyridyl-2-formate or pyridyl-2,6-diformate ligand is generated by reacting initial raw materials of a substituted imidazolium or imidazolium salt, palladium chloride and 2-picolinic acid or 2,6-dipicolinic acid, or the N-heterocyclic carbine palladium compound is generated in a high yield manner by using a corresponding N-heterocyclic carbine palladium chloride dipolymer and 2-picolinic acid or 2,6-dipicolinic acid as initial raw materials. The N-heterocyclic carbine palladium compound of the invention has a very high catalytic activity when being used in a C-N cross-coupling reaction of a halogenated arene and an organic amine as a catalyst. The chemical structural formula of the N-heterocyclic carbine palladium compound containing the pyridyl-2-formate or pyridyl-2,6-diformate ligand is shown in the specification. In the chemical structural formula, m is 0-4, and n is 0-3; and carbon numbers of R<1>, R<2>, R<3>, R<4> and R<5> are defined in the claim 1.
Owner:NANKAI UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com