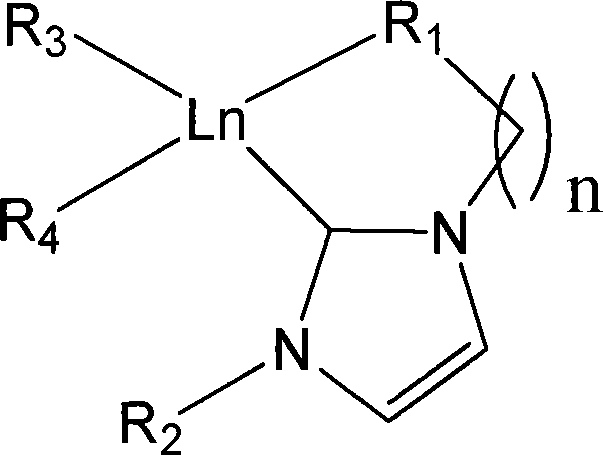
Aza cyclic carbine rear earth catalyst for crystallinity 3,4-polyisoprene
A nitrogen-heterocyclic carbene and polyisoprene technology, which is applied in the field of nitrogen-heterocyclic carbene rare earth catalysts, can solve the problems of wear resistance degradation and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation Embodiment 1
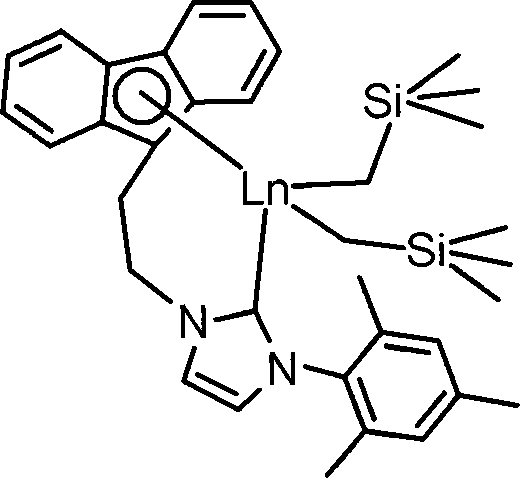
[0039] Preparation Example 1 Complex 1 ((Flu-NHC) Y(CH 2 SiMe 3 ) 2 ) preparation, its process is as follows:
[0040]
[0041] In the glove box, place 0.035 g of LiCH 2 SiMe 3 Dissolve in 30mL of toluene, and add 0.168g of ligand (Flu-H-NHC-H)Br to it, react at room temperature for 3h, add the reaction solution to 20ml, 0.181g of Y(CH 2 SiMe 3 ) 3 (THF) 2 The reaction was continued for 5 h in a toluene solution, the toluene solution was concentrated to 2 ml, 2 ml of n-hexane was added, and recrystallized in a refrigerator at -30°C overnight to obtain complex 1 as yellow crystals. Wash with n-hexane and dry in vacuum for 2 hours to obtain 0.145 g. The yield was 61.7%. deuterated benzene (C 6 D. 6 ) was used as a reagent to characterize the structure of complex 1 by H-NMR spectroscopy and elemental analysis. 1 H NMR (400MHz, C 6 D. 6 , 25°C): δ-1.90, -1.59 (AB, 2 J H-H =10.8 Hz, 4H, Y-CH 2 SiMe 3 ), 0.22(s, 18H, Y-CH 2 SiMe 3 ), 1.85(s, 6H, C 6 h 2 Me ...
preparation Embodiment 2
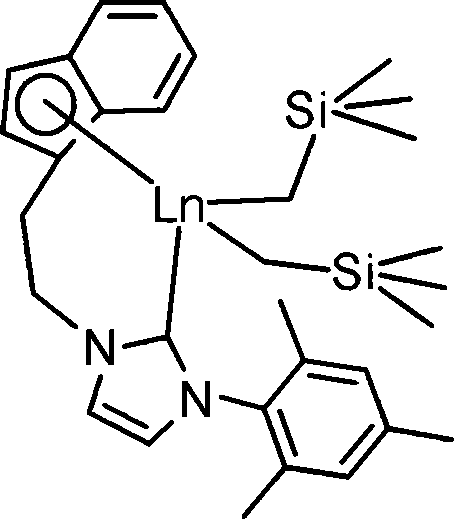
[0042] Preparation Example 2 Complex 2 ((Flu-NHC)Ho(CH 2 SiMe 3 ) 2 ) preparation, its process is as follows:
[0043]
[0044] In the glove box, place 0.035 g of LiCH 2 SiMe 3 Dissolved in 30mL of toluene, and 0.168g ligand (Flu-H-NHC-H)Br was added to it, after 3h reaction at room temperature, the reaction solution was added to 30ml, 0.209g Ho(CH 2 SiMe 3 ) 3 (THF) 2 In the toluene solution, the reaction was continued for 6h, the toluene solution was concentrated to 2ml, 2ml of n-hexane was added, and recrystallized in a -30°C refrigerator overnight to obtain complex 2 as yellow crystals. Wash with n-hexane and dry in vacuo for 2 hours to obtain 0.164 g. The yield was 62.4%. The structure of complex 2 was characterized by elemental analysis, and its molecular formula is C 35 h 47 HoN 2 Si 2 . Among them, the carbon content is 57.53; the hydrogen content is 6.81; the nitrogen content is 3.16.
preparation Embodiment 3
[0045] Preparation Example 3 Complex 3 ((Flu-NHC)Lu(CH 2 SiMe 3 ) 2 ) preparation, its process is as follows:
[0046]
[0047] In the glove box, place 0.035 g of LiCH 2 SiMe 3 Dissolve in 30mL of toluene, and 0.168g ligand (Flu-H-NHC-H)Br was added to it, after reacting at room temperature for 6h, the reaction solution was added to 30ml, 0.213g Lu(CH 2 SiMe 3 ) 3 (THF) 2 The reaction was continued for 6 hours in a toluene solution, the toluene solution was concentrated to 2ml, 2ml of n-hexane was added, and recrystallized in a -30°C refrigerator overnight to obtain complex 3 as yellow crystals. Wash with n-hexane and dry to obtain 0.180g. The yield was 67.7%. deuterated benzene (C 6 D. 6 ) was used as a reagent to characterize the structure of complex 3 by H NMR spectroscopy and elemental analysis. 1 H NMR (400MHz, C 6 D. 6 , 25°C): δ-2.18, -1.90 (AB, 2 J H-H =10.8Hz, 4H, Lu-CH 2 SiMe 3 ), 0.22(s, 18H, Lu-CH 2 SiMe 3 ), 1.85(s, 6H, C 6 h 2 Me 3 ), 2....
PUM
| Property | Measurement | Unit |
|---|---|---|
| glass transition temperature | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com