Suzuki-Miyaura coupling reaction of catalyzing aryl chloride by N-heterocyclic carbine-palladium-imidazole complex at room temperature under condition of water phase
A nitrogen-heterocyclic carbene and imidazole complex technology, applied in the field of Suzuki coupling reaction, to achieve the effects of easy preparation, low price, and low requirements for reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
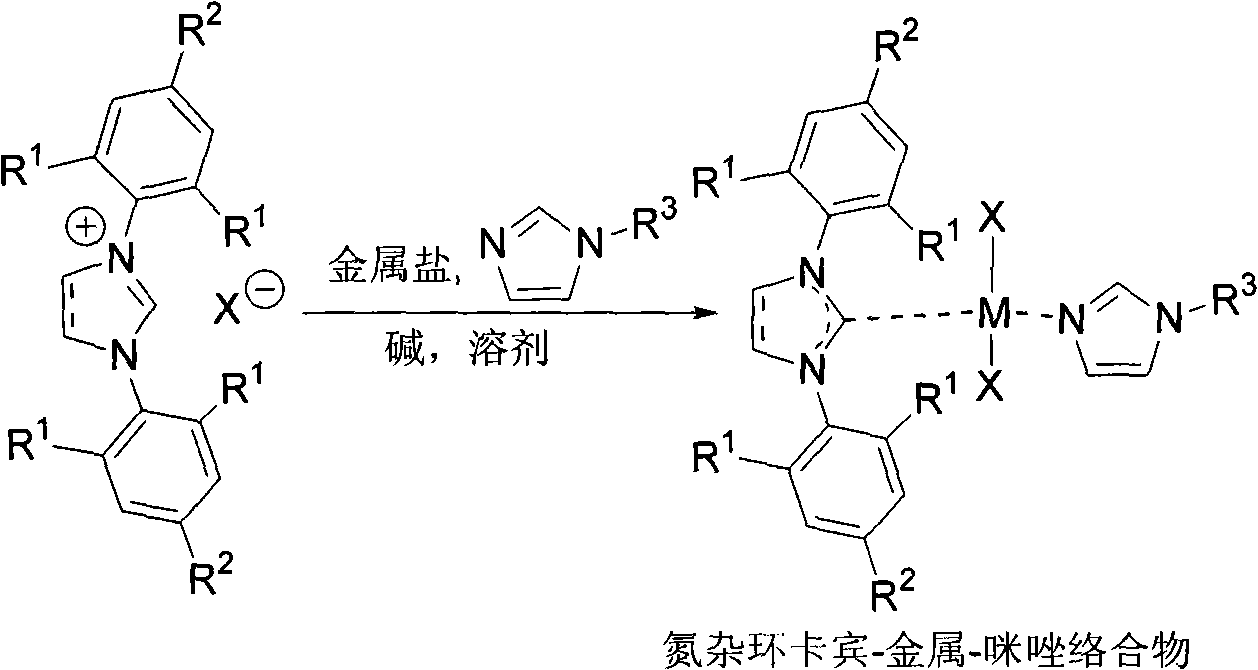
[0025] Synthesis of Nitrocyclic Carbene-Pd(II)-Imidazole Complexes
[0026]
[0027] Under nitrogen protection, add IPr-HCl (2.2304g, 5.2mmol), PdCl 2 (0.8863g, 5.0mmol), K 2 CO 3 (0.6931 g, 5.0 mmol), tetrahydrofuran (30.0 mL) and 1-methylimidazole (1.6 mL, 20 mmol). The reaction mixture was heated to reflux for 20h. Flash column chromatography gave Cat. (2.9534 g, 91%).
example 2
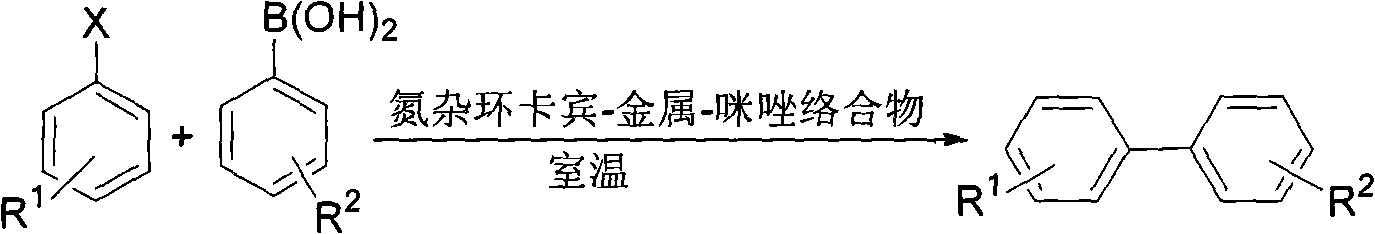
[0029] Reaction of Chlorobenzene with 4-Methoxyphenylboronic Acid
[0030]
[0031] Under nitrogen protection, 4-methoxyphenylboronic acid (91.2mg, 0.6mmol), potassium phosphate trihydrate (266.3mg, 1.0mmol), catalyst (3.2mg, 1.0mol%), water (2.0mL ), tetrahydrofuran (1.0 mL), chlorobenzene (52 μL, 0.5 mmol). The reaction mixture was stirred at room temperature for 24 hours. It was extracted with ethyl acetate, dried, filtered, and flash column chromatographed to obtain 4-methoxybiphenyl in a yield of 99%. 1 H NMR (CDCl 3 , 300MHz, TMS) δ3.79(s, 3H, OCH 3 ), 6.94(d, J=8.4Hz, 2H, Ar), 7.27(t, J=6.9Hz, 1H, Ar), 7.39(t, J=7.5Hz, 2H, Ar), 7.49-7.54(m, 4H, Ar). 13 C NMR (CDCl 3 , 75MHz, TMS) δ55.2, 114.1, 126.6, 126.7, 128.1, 128.7, 133.6, 140.7, 159.1.
example 3
[0033] Reaction of 4-Methoxychlorobenzene with Phenylboronic Acid
[0034]
[0035] Under nitrogen protection, phenylboronic acid (73.2mg, 0.6mmol), potassium phosphate trihydrate (266.4mg, 1.0mmol), catalyst (3.2mg, 1.0mol%), water (2.0mL), THF ( 1.0 mL), 4-methoxychlorobenzene (62 μL, 0.5 mmol). The reaction mixture was stirred at room temperature for 24 hours. Then it was extracted with ethyl acetate, dried, filtered, and flash column chromatographed to obtain 4-methoxybiphenyl in a yield of 93%. 1 H NMR (CDCl 3 , 300MHz, TMS) δ3.79(s, 3H, OCH 3 ), 6.94(d, J=8.4Hz, 2H, Ar), 7.27(t, J=6.9Hz, 1H, Ar), 7.39(t, J=7.5Hz, 2H, Ar), 7.49-7.54(m, 4H, Ar). 13 C NMR (CDCl 3 , 75MHz, TMS) δ55.2, 114.1, 126.6, 126.7, 128.1, 128.7, 133.6, 140.7, 159.1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com