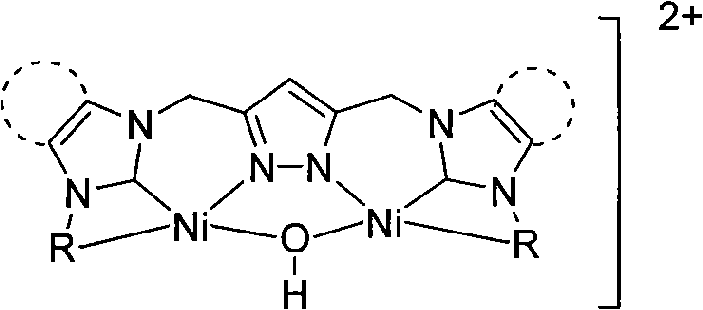
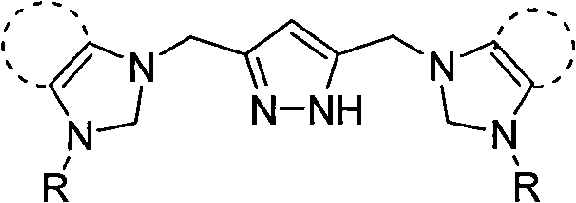
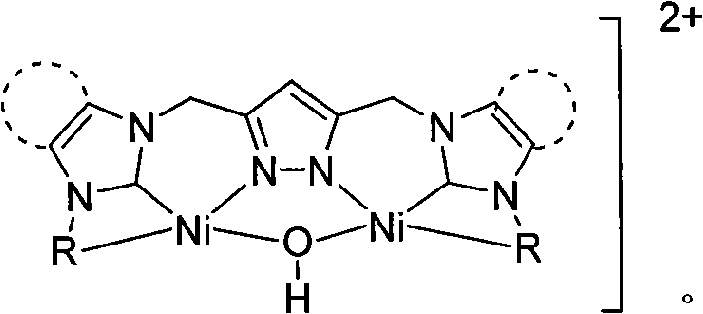
Dinuclear nickel cross-coupling reaction catalyst supported by nitrogen heterocycle carbine ligand and preparation method thereof
A technology for cross-coupling reaction and nitrogen heterocyclic carbene, which is applied in the field of dual-nuclear nickel cross-coupling reaction catalyst and preparation, can solve the problems of high price, limited industrial application, unsatisfactory effect of chlorinated aryl compounds, etc., and can be widely used Prospect, high catalytic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016]
[0017] At room temperature, add ligand L1702mg (1mmol), acetonitrile 20mL, silver oxide 696mg (3mmol), react for 10 hours, add Ni(DME)Cl 2 574mg (2mmol), filtered, the filtrate was concentrated, and diethyl ether was added to precipitate a yellow solid. The yellow solid was washed twice with ethanol and diethyl ether, then dissolved in acetonitrile, and diethyl ether was slowly added to crystallize to obtain 424mg of dinuclear nickel supported by azacyclic carbene ligands. Cross-coupling reaction catalyst 1, yield 51%. 1 H NMR (400MHz, d 6 -DMSO): δ9.03 (d, J=5.6Hz, 2H), 8.11 (t, J=7.6Hz, 2H), 7.71 (d, J=5.6Hz, 2H), 7.62 (d, J=2.0Hz , 2H), 7.59(t, J=6.4, 7.6Hz, 2H), 7.55(d, J=2.0Hz, 2H), 6.36(s, 1H), 5.62(s, 4H), 5.37(s, 4H) , 1.90(s, 1H)ppm. 13 C NMR (400MHz, d 6 -DMSO): δ153.5 (Ni-C), 152.0, 149.7, 145.4, 140.7, 125.4, 125.3, 123.1, 123.1, 102.4, 52.4, 46.6ppm.
Embodiment 2
[0019]
[0020] At room temperature, add ligand L1702mg (1mmol), acetonitrile 20mL, silver oxide 1.392g (6mmol), react for 10 hours, add Ni(DME)Cl 2 574mg (2mmol), filtered, the filtrate was concentrated, and diethyl ether was added to precipitate a yellow solid. The yellow solid was washed twice with ethanol and diethyl ether, then dissolved in acetonitrile, and diethyl ether was slowly added to crystallize to obtain 424mg of dinuclear nickel supported by azacyclic carbene ligands. Cross-coupling reaction catalyst 1, yield 51%. 1 H NMR (400MHz, d 6 -DMSO): δ9.03 (d, J=5.6Hz, 2H), 8.11 (t, J=7.6Hz, 2H), 7.71 (d, J=5.6Hz, 2H), 7.62 (d, J=2.0Hz , 2H), 7.59(t, J=6.4, 7.6Hz, 2H), 7.55(d, J=2.0Hz, 2H), 6.36(s, 1H), 5.62(s, 4H), 5.37(s, 4H) , 1.90(s, 1H)ppm. 13 C NMR (400MHz, d 6 -DMSO): δ153.5 (Ni-C), 152.0, 149.7, 145.4, 140.7, 125.4, 125.3, 123.1, 123.1, 102.4, 52.4, 46.6ppm.
Embodiment 3
[0022]
[0023] At room temperature, add ligand L1702mg (1mmol), acetonitrile 20mL, silver oxide 696g (3mmol), react for 10 hours, add Ni(DME)Cl 2 574mg (2mmol), filtered, the filtrate was concentrated, and diethyl ether was added to precipitate a yellow solid. The yellow solid was washed with ethanol and diethyl ether three times in turn, then dissolved in acetonitrile, and diethyl ether was slowly added to crystallize to obtain 424mg of binuclear nickel supported by azacyclic carbene ligands. Cross-coupling reaction catalyst 1, yield 51%. 1 H NMR (400MHz, d 6 -DMSO): δ9.03 (d, J=5.6Hz, 2H), 8.11 (t, J=7.6Hz, 2H), 7.71 (d, J=5.6Hz, 2H), 7.62 (d, J=2.0Hz , 2H), 7.59(t, J=6.4, 7.6Hz, 2H), 7.55(d, J=2.0Hz, 2H), 6.36(s, 1H), 5.62(s, 4H), 5.37(s, 4H) , 1.90(s, 1H)ppm. 13 C NMR (400MHz, d 6 -DMSO): δ153.5 (Ni-C), 152.0, 149.7, 145.4, 140.7, 125.4, 125.3, 123.1, 123.1, 102.4, 52.4, 46.6ppm.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com