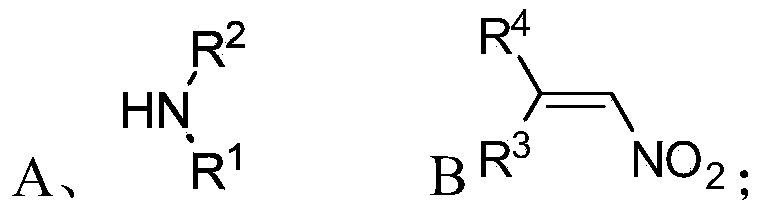Chiral 1,2-diamine compound and preparation method and application thereof
A compound and diamine technology, applied in the field of organic compound synthesis, can solve the problems of limited application range of 1,2-diamine compounds, harsh preparation conditions, complicated processes, etc., and meet the requirements of safe and controllable reaction process and reaction conditions. Low, simple process effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
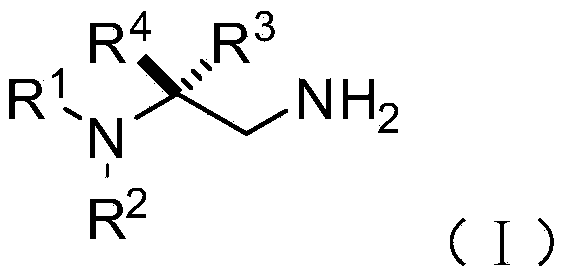
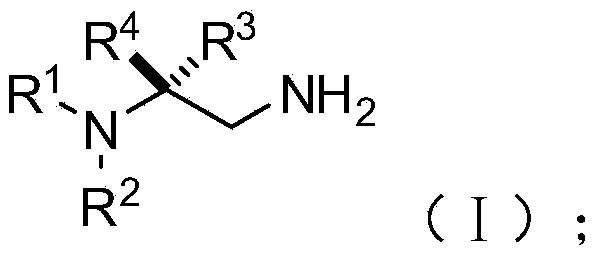
[0074] On the other hand, on the basis of the above-mentioned chiral 1,2-diamine compound of the present invention, the embodiment of the present invention also provides the chiral 1,2-diamine compound of the above molecular structure general formula (I). A preparation method of amine compound. The method comprises the steps of:
[0075] S01: respectively provide the fatty chain amine compound A and the nitroalkene compound B represented by the following structural formula:
[0076]
[0077] S02: adding the aliphatic chain amine compound A and the nitroalkene compound B into a reaction system containing an azacarbene catalyst, a proton additive, an alkali reagent and a water-absorbing additive to react at a temperature of -80-25°C to obtain The following structural general formula is a chiral 1,2-diamine compound shown in (I),
[0078]
[0079] Specifically, in the above step S01, R in the molecular structural formula of fatty chain amine compound A 1 , R 2 The repre...
Embodiment 1
[0100] This example provides (R)-N-benzyl-3,3,3-trifluoro-2-phenylpropyl-1,2-diamine and its preparation method. The structural formula of the (R)-N-benzyl-3,3,3-trifluoro-2-phenylpropyl-1,2-diamine is shown in the following molecular structural formula I1:
[0101]
[0102] Its preparation steps are as follows:
[0103] In a dry 10 mL test tube, add mesitylene-substituted indenol-derived triazole carbene catalyst (0.01 mmol, 0.2 eq), 100 mg of pre-activated powdered molecular sieves and 0.4 mL of anhydrous toluene, replace with argon three times, and add 0.01 mmol of LiHMDS , replaced with argon three times again, added 2.2uL hexafluoroisopropanol (0.02mmol, 0.4eq), sealed the reaction tube and stirred at room temperature for 0.5h. β-trifluoromethyl substituted nitrostyrene (0.05mmol, 1.0eq) was dissolved in 0.2mL toluene and slowly added to the reaction system, and stirred at -78°C for 0.5 hours. Aliphatic chain amine reagent (0.1mmol, 2.0eq) was dissolved in 0.6mL and ...
Embodiment 2
[0105] Embodiment 2 (chiral diamine precursor-nitro compound)
[0106] This example provides a kind of (R)-1,1,1-trifluoro-N-(2-methylphenyl)-3-nitro-2-phenylpropyl-2-amine and its preparation method . The structural formula of the (R)-1,1,1-trifluoro-N-(2-methylbenzyl)-3-nitro-2-phenylpropyl-2-amine is shown in the following molecular structural formula I2:
[0107]
[0108] Its preparation method refers to the preparation method of (R)-N-benzyl-1,1,1-trifluoro-3-nitro-2-phenylpropyl-2-amine in Example 1, the difference is that 2 - Methylbenzylamine (0.1 mmol) instead of benzylamine. The reaction solution was filtered through a glass dropper containing silica gel, rinsed with ether, the filtrate was spin-dried, and separated by column chromatography to obtain the target product precursor, a colorless oily liquid, with a yield of 67% and an ee value of 91%.
[0109] The product I2 prepared is subjected to characterization data analysis, and the result is: 1 HNMR (400MHz...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com